Abstract
The aim of this analysis is to investigate the level of T cell subsets in ankylosing spondylitis (AS) and the effect of low-dose interleukin 2 (IL-2).
This is a retrospective cohort study, we collected basic information, inflammatory markers, BASDAI scores, and T lymphocyte subsets of peripheral blood in patients with AS, baseline analysis, correlation analysis and comparative analysis before and after treatment were performed.
Data from 73 patients (of these, 36 patients were treated with IL-2) and 85 the health were included. The absolute numbers of peripheral CD4+ Treg and CD8+ Treg cells were lower, while the Th17 cell number and the ratio of Th17/CD4+ Treg and CD4+/CD8+ Treg were higher. The CD4+ Treg levels and the ratio of Th17/CD4+ Tregs were correlated with BASDAI. The CD4+ Treg, CD8+ Treg, and Th17 cells were increased after treatment with IL-2 and the ratio of Th17/CD4+ Treg was decreased.
Increase in ratio of Th17/CD4+ Treg involved in the occurrence of AS. At the same time, low-dose IL-2 could decrease the ratio of Th17/CD4+ Treg in short time, low-dose IL-2 can be used to treat autoimmune diseases to avoid adverse reactions caused by immunosuppressants. Further clinical study is needed on the efficacy of IL-2.
Keywords: AS (ankylosing spondylitis), IL-2 (interleukin 2), Th17 cells, Treg
1. Introduction
Ankylosing spondylitis (AS) is a chronic progressive inflammatory disease that mainly invades the spine and affects the ankle joint and surrounding joints to varying degrees. It occurs in young man aged 20 to 30 years. The lesion is characterized by the insolvents of the ankle joint and intervertebral disc. The ligament of the annulus fibrosus often undergoes calcification. If the diagnosis is not timely, spinal rigidity and severe joint deformity may occur. AS hip involvement is common (about 60% in China). Patients with severe hip deformity will be disabled for life, lose labor, and the quality of life will drop significantly. Therefore, actively exploring the pathogenesis of AS has become an urgent research.
So far, studies have shown that the functional changes of T lymphocytes are closely related to the pathogenesis of the disease. Regulatory T (Treg) cells play an important role in maintaining peripheral tolerance and preventing autoimmune diseases. Clinical studies have shown that some autoimmune patients have the reduced numbers and/or dysfunction of Treg cells such as autoimmune myocarditis, multiple sclerosis,[1] systemic lupus erythematosus,[2,3] inflammatory bowel disease.[4] Treg cells prevent the development of immunopathological processes and the onset of autoimmune diseases by inhibiting excessive immune responses against self and foreign antigens.
In addition, studies have found that the surface of Treg cells has interleukin-2 receptor (IL-2R). IL-2R is a high-affinity triplet composed of α (CD25), β (CD122), and ɣ (CD132) chains. The expression of CD25 molecule requires activation of the transcription factor Foxp3,[5] so CD25+Foxp3+ is characteristic of Treg cells. After IL-2 binds to IL-2R on T cells, IL-2 activates T cell JAK/STAT signaling pathway, which leads to phosphorylation of STAT5 transcription factor, and STAT5 binds to Foxp3 promoter to induce Treg cells.[6] Treg cells through a variety of mediators including cytotoxic T lymphocyte antigen 4 (CTLA4), glucocorticoid-induced necrosis factor receptor family-related genes (GITR), lymphocyte activation gene-3, CD25, TGF- β, IL-10, and IL-35 inhibit the activation and subsequent effector function of pathogenic T cells.[7–11] Lee et al[12] demonstrated that IL-2 or IL-2/anti-interleukin-2 monoclonal antibody JES6-1 immune complexes inhibit mouse collagen-induced arthritis (CIA) via the IL-2/STAT5 signaling pathway. In this CIA model, IL-2/JES6-1-induced Treg cell expansion, which express high levels of inhibitory molecules, such as CD25, CTLA4, GITR, and GARP.[13,14] Increased expression levels of CD25 significantly induce strong amplification and activation of Treg cells by further enhancing IL-2 binding.
Due to higher IL-2 binding ability of CD25, low-dose IL-2 can promote the growth of CD25+Foxp3+ Treg. So far, two subgroups of Treg cells have been identified: CD4+ and CD8+ Treg cells according to the molecules on the cell surface. At present, CD4+CD25+FOXP3+ Treg cells (CD4+ Tregs) have been widely studied. Xystrakis et al[15] and others have shown that CD8+ T cells express different levels of Foxp3, similar to CD4+ T cells. Therefore, CD4-CD25+FOXP3+ T cells can be regarded as CD8+ Treg.
This study examined the level of T cell subsets in AS and the effect of low-dose IL-2, aiming to clarify the relation of peripheral Treg cells with the pathogenesis of AS and the effect and safety of low-dose recombinant human IL-2 on the growth of Treg cells in vivo and on treatment of AS.
2. Participants and methods
2.1. Participants
Participants in the trial were 73 rheumatology inpatients in our hospital. The inclusion criteria for enrolment were: met the diagnostic criteria for AS revised in New York in 1984; signed the Bath AS Disease Activity Index (BASDAI) form. Patients were excluded according to the following criteria: pregnant woman; merge with other autoimmune diseases; blood system diseases and malignant tumors; important organ failure; thalidomide, hydroxychloroquine, methotrexate, and leflunomide were used in the past 3 months. At the same time, 85 healthy people from physical examination were selected as healthy group.
2.2. Interventions
We collected 73 hospitalized patients only received low-dose hormone and nonsteroidal anti-inflammatory drugs when they were admitted to hospital. Of these patients, 48 patients received IL-2 (50WIU, subcutaneous injection for 5 days) therapy.
2.3. Methods
We collected basic information (gender, age), inflammatory markers [erythrocyte sedimentation rate (ESR), c-reactive protein (CRP)], BASDAI scores and T lymphocyte subsets [CD4+ Treg, Th17, Th17/CD4+ Treg, CD4-Treg (viewed as CD8+ Treg), CD4+/CD8+ Treg] of peripheral blood in patients with AS, baseline analysis, correlation analysis and comparative analysis before and after treatment were performed. This study was approved by the Medical Ethics Committee of the Second Hospital of Shanxi Medical University (number 2016KY007), which complied with the Declaration of Helsinki.
2.4. Statistical analysis
Statistical analysis was performed using IBM SPSS statistic software, version 20.0. Continuous variables were expressed as mean ± standard deviation with normal distribution and median (range) without normal distribution. The comparison of continuous variables with or without normal distribution was analyzed with Student t test and Wilcoxon rank test, respectively. Two-sided P value of >0.05 was considered significant. For T cell subsets, continuous variables were expressed as mean ± standard deviation with normal distribution. In correlation analysis, it conforms to normal distribution, Pearson correlation analysis is used, and Spearman correlation analysis is used when it does not conform to normal distribution, P < .05 was regarded as statistically significant. Correlation analysis T-cell subsets with BASDAI scores, Pearson correlation analysis is used, correlation analysis T-cell subsets with ESR and CRP, Spearman correlation analysis is used. Figures were made with GraphPad Prism version 6.07 software.
2.5. Result
2.5.1. Baseline level of AS compared with healthy group
We analyzed the absolute counts of T lymphocyte subsets and the ratio between them. This study found that CD4+ Treg and CD8+ Treg cells were lower in AS patients than in healthy controls, while the Th17 cell number and the ratios of Th17/CD4+ Treg and CD4+/CD8+ Treg were higher (Table 1, Fig. 1).CD4+ Treg and CD8+ Treg are below normal levels (Fig. 2A and B). Th17 cells were higher than normal (Fig. 2C), and the ratio of CD4+/CD8+ Treg and Th17/CD4+ Treg were higher than normal (Fig. 2D and E).
Table 1.
Baseline level of AS compared with healthy group.

Figure 1.
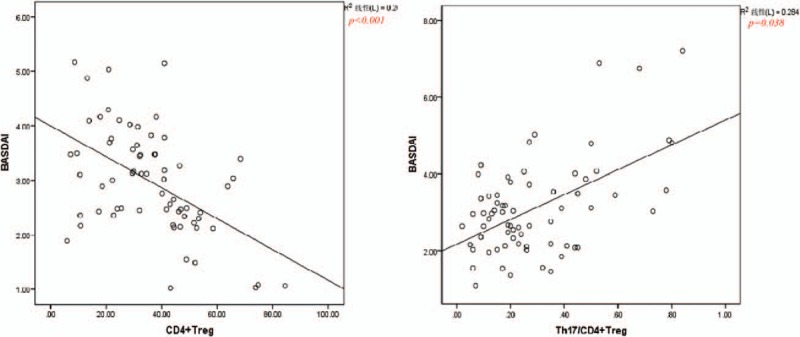
Correlation analysis of CD4+ Treg, Th17, Th17/CD4+ Treg, CD8+ Treg, and CD4+/CD8+ Treg with ESR,CRP and BASDAI in AS group. Correlation analysis T cell subsets with BASDAI scores, Pearson correlation analysis is used, correlation analysis T cell subsets with ESR and CRP, Spearman correlation analysis is used. BASDAI = Bath AS Disease Activity Index, CRP = c-reactive protein, ESR = erythrocyte sedimentation rate.
Figure 2.
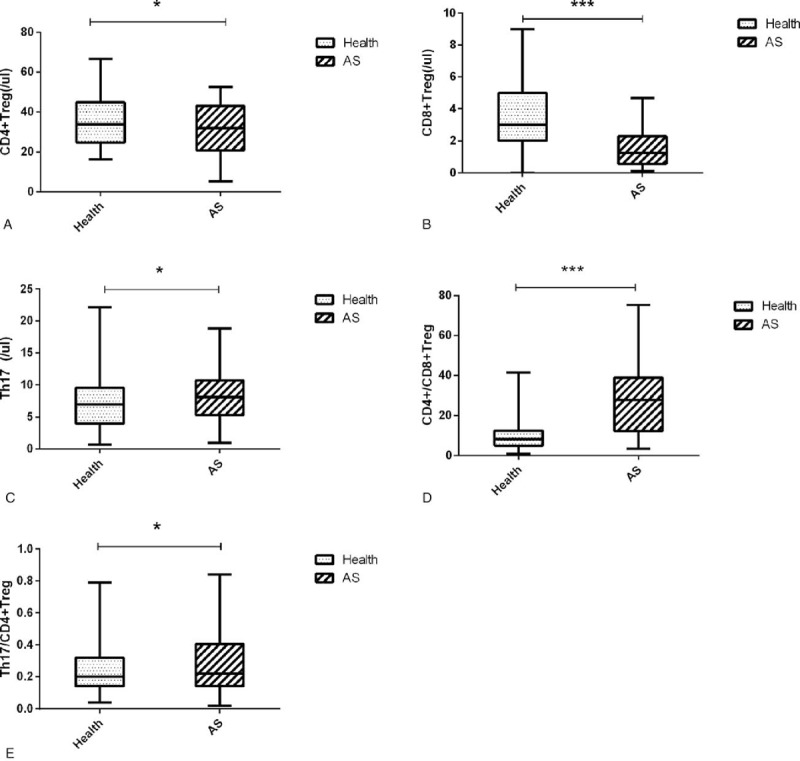
The levels of CD4+ Treg, Th17, Th17/CD4+ Treg, CD8+ Treg, and CD4+/CD8+ Treg in AS patients and healthy group. ankylosing spondylitis = AS. ∗Significant between-group difference P < .05. ∗∗Significant between-group difference P < .01. ∗∗∗Significant between-group difference P < .001.
2.5.2. Correlation analysis
In order to clarify the correlation between the above results and the inflammatory indexes of ESR, CRP and BASDAI, we analyzed the correlation, and found that only CD4+ Treg, Th17 / CD4+ Treg and BASDAI were correlated, the former was negatively correlated, the latter was positively correlated. The levels of other T lymphocyte subsets in AS group were not correlated with ESR and CRP and BASDAI (Fig. 1).
2.5.3. Short time effect of low-dose IL-2
Finally, in order to clarify the short time effect of low-dose IL-2 on the cells detected in the peripheral blood, we found that CD4+ Tregs, CD8+ Tregs, and Th17 cells were increased after treatment with IL-2 (Table 2, Fig. 3). After treatment with IL-2, CD4+ Treg, Th17, CD8+ Treg, CD4+/CD8+ Treg were significantly higher (Fig. 3A–D), Th17 / CD4+ Treg was lower than before IL-2 treatment (Fig. 3E).
Table 2.
Short time effect low-dose interleukin-2.

Figure 3.

Short time effect of low-dose interleukin-2. interleukin 2 = IL-2. ∗Significant between-group difference P < .05. ∗∗Significant between-group difference P < .01. ∗∗∗Significant between-group difference P < .001.
3. Discussion
3.1. Regulatory T cell
Gershon[16] first described the function of naturally occurring T cell subsets and their inhibition of autoimmune responses in the 1970s. Sakaguchi et al[9] mentioned that in addition to the effector lymphocytes used to attack invading microorganisms, the human immune system also has an inhibitory lymphocyte called regulatory T cells, which are Treg cells. These lymphocytes are specifically intended to suppress excessive or harmful immune response. As early as 1905, Josefowicz et al[11] found that goats produced antibodies against other goat blood in the body, and the concept of immune tolerance was proposed when there was no such reaction in their own blood. The immune tolerance is that the body has no resistance to its own ingredients but has rejection of foreign substances entering the body, there are two types of mechanisms of immune tolerance, one refers to the internal mechanism of cells, including the elimination of self-reactive thymocytes or chronic stimulation of peripheral T cell clonal apoptosis or inactivated, the other is a subset of cells that mediate the inhibition of pathogenic immune responses. This subpopulation of cells that inhibit the pathogenic immune response is the T cell subset of Treg cells mentioned above. Treg cells mediate immune tolerance by producing cell surface molecules such as transforming growth factor-beta (TGF- β) that is anti-inflammatory cytokine, by CTLA-4 on inhibitory T cells binds directly to CD80 and CD86 molecules on effector T cells, and by inhibiting the activation of antigen-presenting cells. The inhibitory effect requires inhibitors and effector T cells co-localized, and may involve T cell receptor (TCR) signaling that triggers transcription factors important for regulating effector cell function.[17] At the beginning of 2000, when exploring the X-chromosome-related immune-deficient, multiendocrine disease, and enteropathological (IPEX)-related genes, foreign researchers found FOXP3+ gene encoded by the X chromosome of Treg cells. Mutation of the human FOXP3+ gene leads to the development of autoimmune diseases, allergies, and immunopathology. In 2003, mice and humans with mutations in the Foxp3 gene were found to be susceptible to life-threatening early-stage lymphoproliferative immune-mediated diseases. In a similar study, Fontenot et al[19] revealed that Foxp3 is specifically expressed in Treg cells and can maintain the proliferation and differentiation of Treg and its inhibitory function. Treg cells are divided into natural and inducible types, natural types are produced by the thymus, and inducible forms are transformed by other cells in peripheral tissues. Foxp3+ native Treg cells produced by normal thymus inhibit the activation and expansion of naive T cells and their differentiation into effector T cells, including Th1, Th2 and Th17 cells that mediate various pathological and physiological immune responses.[9] Deletion of Foxp3 or its reduced expression in Treg cells will result in the production of effector T cells and cytokines such as IL-2, IL-4, IL-17 and IFN-γ that promote immune responses. Under the experimental conditions, the natural Treg cell Foxp3 gene was transduced into naive T cells, and the naive T cells were transformed into Treg-like cells. As a result, Foxp3 inhibited the transcription of IL-2-encoding genes and up-regulated Treg cell-associated molecule. Recent studies indicate that Foxp3 binds to other transcription factors such as NFAT (a factor of nucleic acid-activated T cells) and AML1 (acute leukemia-1)/Runx1 (runt-associated transcription factor 1) may be associated with activator protein 1 and nuclear factor kB, the Foxp3/NFAT/Runx1 complex, in combination with other coactivators or co-repressor proteins, inhibits IL-2 and other cytokines, as well as activates CD25, CTLA-4, and GITR.[9] Treg cells inhibit the activation and subsequent effector function of pathogenic T cells through a variety of mediators including CTLA4, GITR, lymphocyte activation gene-3, CD25, TGF-β, IL-10, and IL-35.[7–11]
Although multiple lymphocyte subsets exhibit immunosuppression or immunomodulation, Foxp3+ Treg cells are currently the only lymphocytes that are known to dominate immune tolerance and can directly or indirectly inhibit many cells, including T cells, in vitro and in vivo. B cells, DCs, NK cells and NKT cell activation and proliferation are essential for maintaining immune homeostasis.[11,18]
3.2. CD4+ Treg cells
At present, CD4+ Treg research is more mature and extensive. CD4+ Treg was originally found in mice. The thymus resection in the first 3 days after birth makes CD4+CD25+ cell deficiency leading to the development of various autoimmune diseases.[19] Consumption of adult mouse CD25+ cells also present with various autoimmune diseases such as gastritis and thyroiditis.[20] Recently, a significant number of T cells with the same phenotype and functional properties were found in rats and humans.[21–25] They account for 5% to 10% of all peripheral CD4+ T cells. Subsequent studies in vitro have shown that CD4+CD25+T regulates cells, both immunologically incompetent and immunosuppressive.[26–28] Under in vitro co-culture conditions, CD4+CD25+ Treg inhibited the proliferation of traditional CD25+ T cells and showed inhibition only when their TCRs were activated.[29] Once activated, CD4+CD25+ Treg is nonantigen-specific.[26] In vitro, CD4+CD25+ Treg inhibitory activity is dependent on exposure to CD25+ T cells and is independent of soluble inhibitory cytokines.[30] The exact mechanism by which CD4+CD25+ Treg exerts an inhibitory effect remains unknown. Studies have shown that this inhibition is essentially the inhibition of IL-2 transcription in reactive cells.[26] The inhibitory effect and low response state of CD4+CD25+ Treg can be altered by exogenous IL-2 and IL-15.[21,23,26] In addition, the elimination of rodent CD4+ CD25+ Treg cells produces a variety of autoimmune inflammatory diseases, and the reconstitution of CD4+ CD25+ T cells can inhibit the development of the disease, suggesting that CD4 +CD25+ Treg cells maintain immune homeostasis and play an important role in immune tolerance.[9,11,31–33]
3.3. CD8+ Treg cells
Although CD8+ Treg cells are similarly immunomodulatory as CD4+ Treg cells, there are few studies on CD8+ Treg. Churlaud et al[34] have found, in humans, CD8+ Treg accounts for 0.4% of peripheral blood T cells, and CD4+ Treg cells account for 5–10% of peripheral CD4+ T cells. The number of CD8+ Tregs is less than one-tenth of CD4+ Treg, which makes CD8+ Treg study is more difficult. Xystrakis et al[15] analyzed the spleen lymph node cells of normal LEW rats by flow cytometry, showing that CD8+ T cells can be divided into 2 subgroups according to the expression level of CD45RC molecules, namely CD45RChigh and CD45RClow subpopulations, CD45RChigh represents 80 ± 3% of CD8+ T cells, while the CD45RClow subpopulation typically represents 20 ± 3% of CD8+ T cells. The CD45RClow subpopulation produced the anti-inflammatory cytokines IL-4, IL-10 and IL-13, both of which produced the inflammatory cytokines IL-2 and IFN-ɣ, but the CD45RChigh subpopulation produced more and confirmed CD45RChigh-mediated in an allogeneic response, CD45RChigh subpopulation inhibits proliferation of alloreactive CD4+ T cells in vitro by a cell contact mechanism. So far, CD8+ Treg has been described to have immunosuppressive properties in the pathology of autoimmunity, cancer, infection, and organ transplantation. Impaired number and function of CD8+ Treg are involved in the pathogenesis of autoimmune diseases, graft versus host disease, and transplant rejection. High CD8+ Treg numbers allow cancer cells to evade host immune responses.
In summary, Treg cells are important cells for manipulating immune responses and play an indispensable role in maintaining a nonresponsiveness to autoantigens and inhibiting excessive immune responses to the host. In several animal models of autoimmune diseases and autoimmune experiments, the number and/or function of CD4+ Treg and CD8+ Treg cells was impaired, and the possibility of these cells as targeted immunotherapy was elevated. Many studies turned to seeking a method to promote Treg cell proliferation.
3.4. Proliferation of Treg cells
3.4.1. IL-2
IL-2 is one of the important members of the interleukin family. Its biological function is to stimulate T cell proliferation, so it is also called T cell growth factor. IL-2 is the core substance in the body's immune regulation network. It has synergistic and antagonistic effects with other cytokines, and together completes the balance regulation of the body's immune function. It is widely used in the treatment of diseases such as anti-infection and antitumor. The surface of Treg cells has IL-2R. IL-2R is a high-affinity triplet composed of α, β, and ɣ chains. The α, β, and ɣ chains are CD25, CD122, and CD132 molecules on the surface of Treg, respectively. Expression requires activation of the transcription factor Foxp3.[5] After IL-2 binds to IL-2R on T cells, IL-2 activates T cell JAK/STAT signaling pathway, which leads to phosphorylation of STAT5 transcription factor, and STAT5 binds to Foxp3 promoter to induce Treg cells.[6] Lee et al[12] demonstrated that the IL-2/anti-interleukin-2 monoclonal antibody immune complex inhibits CIA via the IL-2/STAT5 signaling pathway, in this CIA IL-2/JES6–1 induct expansion of Treg cells which express high levels of inhibitory molecules, such as CD25, CTLA4, GITR and GARP,[13,14] increased CD25 significantly induced the strong expansion and activation of Treg cells by further enhancing IL-2 binding. in addition, the upregulation of SOCS1, SOCS3 and Oncostatin M produced by STAT5 inhibits the production of CII-specific antibodies, Th17 cells and the proinflammatory factor IL-17, and finally the arthritis is significantly improved. In the periphery, IL-2 signaling is a necessary pathway for CD4+CD25+ cell expansion. After CD4+CD25+ cells are transfected into IL-2Rα/β- mice, CD4+CD25+ cells are significantly expanded and prevent disease, CD4+CD25+ cells were transfected into IL-2- mice, CD4+CD25+ cells were not expanded, and autoimmune disease or death was not spared, suggesting that IL-2 is required for CD4+CD25+ expansion.[35] IL-2 is pleiotropic in the overall immune response because IL-2 has at least 2 forms of receptors (IL-2Rαβɣ and IL-2Rαβ) that can be recognized by multiple cells, depending on the activation and differentiation of the responding cells. The state mediates the opposite effect, in the sense that IL-2 improves or aggravates arthritis depending on the time of administration,[36] and it is important that IL-2 targets specific T cell subsets at the right time in the immune response. Studies on the role of IL-2 in T cell activation still require a lengthy research process.
4. Conclusion
Tumor necrosis factor-α (TNF-α) is the main inflammatory factor leading to AS. It is the best choice for treating AS and other spinal joint diseases at present. Those who have the conditions should choose as much as possible. However, biological agents are expensive and many patients cannot afford them. In addition, we find that many patients who use biological agents need to use them continuously. Once they stop taking drugs, the disease recurs or even aggravates. This is because TNF-α only inhibit the intermediate process of the pathogenesis of the disease, but they do not inhibit the disease from the source. So it is necessary for us to re-explore the pathogenesis of diseases, find new entry points, find new treatment options, and solve the current situation of expensive drugs or poor efficacy. Treg cells exert immune tolerance to inhibit the occurrence of autoimmune diseases, and Th17 promotes inflammation. Many studies believe that the imbalance between Treg cells and Th17 is the fundamental cause of many autoimmune diseases. Our previous studies have found imbalance in Sjogren's syndrome and the therapeutic effect of low-dose IL-2. The results were published in a letter on ARD in June 2018. This study explores whether there is imbalance in AS. This study found that CD4+ Treg and CD8+ Treg, especially CD8+ Treg, are below normal levels in peripheral blood T lymphocytes of AS patients. Th17 cells were higher than normal, and the ratio of them was higher than normal. It seems that increase in ratio of Th17/CD4+ Treg involved in the occurrence of disease. There was a negative correlation between CD4+ Treg and BASDAI score, but a positive correlation between Th17/CD4+ Treg and BASDAI score. At the same time, the study concluded that IL-2 could enhance the peripheral blood CD4+ Treg, CD8+ Treg, Th17 cells, and the ability of raising Treg was stronger than Th17 cells,as consequence corrected the imbalance. This is the reason why some scholars believe that IL-2 can be used to treat autoimmune diseases and avoid adverse reactions caused by immunosuppressants. Further clinical study is needed on the efficacy of IL-2.
Author contributions
The paper was co-authored by Haizhuan An, Xin Li, Fang Li, Chong Gao, Xiaofeng Li and Jing Luo. Haizhuan An and Xin Li contributed equally to this work and share the first authorship. Haizhuan An and Xin Li involved in the acquisition, analysis of data for the work and drafted the manuscript. Jing Luo conducted the majority of the laboratory work. Fang Li provided substantial contributions to the conception or design of the work. Chong Gao revisied the work critically for important intellectual content. Xiaofeng Li accountable for all aspects of the work and final approval of the version to be published.
Conceptualization: Fang Li, Chong Gao, Xiaofeng Li.
Data curation: Haizhuan An, Jing Luo.
Methodology: Fang Li, Xin Li.
Resources: Chong Gao.
Software: Haizhuan An, Xin Li.
Supervision: Chong Gao, Xiaofeng Li.
Visualization: Jing Luo.
Writing – original draft: Haizhuan An.
Writing – review & editing: Fang Li, Xin Li, Chong Gao, Xiaofeng Li, Jing Luo.
Footnotes
Abbreviations: AML1 = acute leukemia-1, AS = ankylosing spondylitis, BASDAI = Bath AS Disease Activity Index, CIA = collagen-induced arthritis, CRP = c-reactive protein, CTLA4 = cytotoxic T lymphocyte antigen 4, ESR = erythrocyte sedimentation rate, NFAT = factor of nucleic acid-activated T cells, GITR = glucocorticoid-induced necrosis factor receptor family-related genes, IL-2 = interleukin 2, IL-2R = interleukin-2 receptor, Treg = regulatory T, Runx1 = runt-associated transcription factor 1, TCR = T cell receptor, TGF-β = transforming growth factor-beta, TNF-α. = tumor necrosis factor-α.
The authors have passed the ethical review of low-dose interleukin 2 therapy (Admissible number: 2016KY007).
No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Lu L, Cantor H. Generation and regulation of CD8 (+) regulatory T cells. Cell Mol Immunol 2008;5:401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tulunay A, Yavuz S, Direskeneli H, et al. CD8+CD28-, suppressive T cells in systemic lupus erythematosus. Lupus 2008;17:630–7. [DOI] [PubMed] [Google Scholar]
- [3].Zhang L, Bertucci AM, Ramsey-Goldman R, et al. Regulatory T cell (Treg) subsets return in patients with refractory lupus following stem cell transplantation, and TGF-beta-producing CD8+ Treg cells are associated with immunological remission of lupus. J Immunol 2009;183:6346–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brimnes J, Allez M, Dotan I, et al. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol 2005;174:5814–22. [DOI] [PubMed] [Google Scholar]
- [5].Fontenot JD, Rasmussen JP, Gavin MA, et al. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol 2005;6:1142–51. [DOI] [PubMed] [Google Scholar]
- [6].Burchill MA, Yang J, Vogtenhuber C, et al. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol 2007;178:280–90. [DOI] [PubMed] [Google Scholar]
- [7].Fontenot JD, Gavin MA, Rudensky AY. Pillars article: Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003. 4: 330–336. J Immunol 2017;198:986–92. [PubMed] [Google Scholar]
- [8].Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299:1057–61. [DOI] [PubMed] [Google Scholar]
- [9].Sakaguchi S, Powrie F. Emerging challenges in regulatory T cell function and biology. Science 2007;317:627–9. [DOI] [PubMed] [Google Scholar]
- [10].Jonuleit H, Schmitt E. The regulatory T cell family: distinct subsets and their interrelations. J Immunol 2003;171:6323–7. [DOI] [PubMed] [Google Scholar]
- [11].Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity 2009;30:616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee SY, Cho ML, Oh HJ, et al. Interleukin-2/anti-interleukin-2 monoclonal antibody immune complex suppresses collagen-induced arthritis in mice by fortifying interleukin-2/STAT5 signalling pathways. Immunology 2012;137:305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008;322:271–5. [DOI] [PubMed] [Google Scholar]
- [14].Wang R, Kozhaya L, Mercer F, et al. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A 2009;106:13439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xystrakis E, Dejean AS, Bernard I, et al. Identification of a novel natural regulatory CD8 T-cell subset and analysis of its mechanism of regulation. Blood 2004;104:3294–301. [DOI] [PubMed] [Google Scholar]
- [16].Gershon RK. A disquisition on suppressor T cells. Transplant Rev 1975;26:170–85. [DOI] [PubMed] [Google Scholar]
- [17].von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol 2005;6:338–44. [DOI] [PubMed] [Google Scholar]
- [18].Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity 2006;25:195–201. [DOI] [PubMed] [Google Scholar]
- [19].Suri-Payer E, Amar AZ, Thornton AM, et al. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol 1998;160:1212–8. [PubMed] [Google Scholar]
- [20].Sakaguchi S, Sakaguchi N, Asano M, et al. Pillars article: immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995. J Immunol 2011;186:3808–21. [PubMed] [Google Scholar]
- [21].Jonuleit H, Schmitt E, Stassen M, et al. Identification and functional characterization of human CD4 (+)CD25 (+) T cells with regulatory properties isolated from peripheral blood. J Exp Med 2001;193:1285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ng WF, Duggan PJ, Ponchel F, et al. Human CD4 (+)CD25 (+) cells: a naturally occurring population of regulatory T cells. Blood 2001;98:2736–44. [DOI] [PubMed] [Google Scholar]
- [23].Dieckmann D, Plottner H, Berchtold S, et al. Ex vivo isolation and characterization of CD4 (+)CD25 (+) T cells with regulatory properties from human blood. J Exp Med 2001;193:1303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stephens LA, Mottet C, Mason D, et al. Human CD4 (+)CD25 (+) thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol 2001;31:1247–54. [DOI] [PubMed] [Google Scholar]
- [25].Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25- subpopulations. J Immunol 2000;165:3105–10. [DOI] [PubMed] [Google Scholar]
- [26].Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med 1998;188:287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Takahashi T, Kuniyasu Y, Toda M, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol 1998;10:1969–80. [DOI] [PubMed] [Google Scholar]
- [28].Read S, Mauze S, Asseman C, et al. CD38+ CD45RB (low) CD4+ T cells: a population of T cells with immune regulatory activities in vitro. Eur J Immunol 1998;28:3435–47. [DOI] [PubMed] [Google Scholar]
- [29].Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol 2002;2:389–400. [DOI] [PubMed] [Google Scholar]
- [30].Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol 2000;164:183–90. [DOI] [PubMed] [Google Scholar]
- [31].Wu Y, Borde M, Heissmeyer V, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell 2006;126:375–87. [DOI] [PubMed] [Google Scholar]
- [32].Ono M, Yaguchi H, Ohkura N, et al. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature 2007;446:685–9. [DOI] [PubMed] [Google Scholar]
- [33].Churlaud G, Pitoiset F, Jebbawi F, et al. Human and mouse CD8 (+)CD25 (+)FOXP3 (+) regulatory T cells at steady state and during interleukin-2 therapy. Front Immunol 2015;6:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol 2006;24:209–26. [DOI] [PubMed] [Google Scholar]
- [35].Thornton AM, Donovan EE, Piccirillo CA, et al. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol 2004;172:6519–23. [DOI] [PubMed] [Google Scholar]
- [36].Thornton S, Boivin GP, Kim KN, et al. Heterogeneous effects of IL-2 on collagen-induced arthritis. J Immunol 2000;165:1557–63. [DOI] [PubMed] [Google Scholar]


