Abstract
Background
Migraine is a common, disabling condition and a burden for the individual, health services, and society. Zolmitriptan is an abortive medication for migraine attacks, belonging to the triptan family. These medicines work in a different way to analgesics such as paracetamol and ibuprofen.
Objectives
To determine the efficacy and tolerability of zolmitriptan compared to placebo and other active interventions in the treatment of acute migraine attacks in adults.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and the Oxford Pain Relief Database, together with three online databases (www.astrazenecaclinicaltrials.com, www.clinicaltrials.gov, and apps.who.int/trialsearch) for studies to 12 March 2014. We also searched the reference lists of included studies and relevant reviews.
Selection criteria
We included randomised, double‐blind, placebo‐ or active‐controlled studies, with at least 10 participants per treatment arm, using zolmitriptan to treat a migraine headache episode.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. We used numbers of participants achieving each outcome to calculate risk ratios and numbers needed to treat for an additional beneficial effect (NNT) or harmful effect (NNH) compared with placebo or a different active treatment.
Main results
Twenty‐five studies (20,162 participants) compared zolmitriptan with placebo or an active comparator. The evidence from placebo‐controlled studies was of high quality for all outcomes except 24 hour outcomes and serious adverse events where only limited data were available. The majority of included studies were at a low risk of performance, detection and attrition biases, but did not adequately describe methods of randomisation and concealment.
Most of the data were for the 2.5 mg and 5 mg doses compared with placebo, for treatment of moderate to severe pain. For all efficacy outcomes, zolmitriptan surpassed placebo. For oral zolmitriptan 2.5 mg versus placebo, the NNTs were 5.0, 3.2, 7.7, and 4.1 for pain‐free at two hours, headache relief at two hours, sustained pain‐free during the 24 hours postdose, and sustained headache relief during the 24 hours postdose, respectively. Results for the oral 5 mg dose were similar to the 2.5 mg dose, while zolmitriptan 10 mg was significantly more effective than 5 mg for pain‐free and headache relief at two hours. For headache relief at one and two hours and sustained headache relief during the 24 hours postdose, but not pain‐free at two hours, zolmitriptan 5 mg nasal spray was significantly more effective than the 5 mg oral tablet.
For the most part, adverse events were transient and mild and were more common with zolmitriptan than placebo, with a clear dose response relationship (1 mg to 10 mg).
High quality evidence from two studies showed that oral zolmitriptan 2.5 mg and 5 mg provided headache relief at two hours to the same proportion of people as oral sumatriptan 50 mg (66%, 67%, and 68% respectively), although not necessarily the same individuals. There was no significant difference in numbers experiencing adverse events. Single studies reported on other active treatment comparisons but are not described further because of the small amount of data.
Authors' conclusions
Zolmitriptan is effective as an abortive treatment for migraine attacks for some people, but is associated with increased adverse events compared to placebo. Zolmitriptan 2.5 mg and 5 mg benefited the same proportion of people as sumatriptan 50 mg, although not necessarily the same individuals, for headache relief at two hours.
Plain language summary
Zolmitriptan for acute migraine attacks in adults
Migraine is a complex condition with a wide variety of symptoms. It affects about 1 person in 8, mainly women aged 30 to 50 years. For many people, the main feature is a painful, and often disabling, headache. Other symptoms include feeling sick, vomiting, disturbed vision, and sensitivity to light, sound, and smells.
Zolmitriptan is one of the triptan family of drugs. It is used to treat migraine attacks when they occur, not to prevent attacks occurring. It is available as an oral tablet to swallow whole, an oral tablet to dissolve in the mouth, and a nasal spray. This review looked at 25 studies that involved over 20,000 participants reporting the effects of zolmitriptan on migraine attacks. Most information was for tablets taken by mouth. Overall methodological quality of the included studies was good, and treatment group sizes were large enough to avoid major bias. There were inconsistencies in the way use of rescue medication and adverse events were reported.
A single oral dose of zolmitriptan relieved migraine headache pain in some people. Several different pain outcomes were reported.
One outcome was pain reduced from moderate or severe to no pain at all two hours after taking treatment. An oral zolmitriptan 2.5 mg tablet delivered this outcome to about 3 in 10 people (30%), compared with about 1 in 10 (10%) taking placebo.
Another outcome was pain reduced from moderate or severe to no worse than mild pain two hours after taking treatment (called headache relief). An oral zolmitriptan 2.5 mg tablet delivered this outcome to about 6 in 10 people (61%), compared with 3 in 10 (29%) taking placebo.
Slightly better results were obtained with higher doses of 5 mg or 10 mg oral tablets, but the 10 mg dose was associated with more adverse events, most of which were of short duration and mild or moderate in severity. Results for the 5 mg nasal spray were generally similar to those for the oral tablet, but it was significantly better than the tablet at 1 hour.
People with migraine want treatment that eliminates the headache and any associated symptoms quickly (maximum two hours) and prevents it returning (within 24 hours). Results indicate that with the 5 mg dose only 14% of those treated were pain‐free at 2 hours with no headache recurrence within 24 hours.
Oral zolmitriptan 2.5 mg and 5 mg provided headache relief at two hours to the same proportion of people (2 in 3) as oral sumatriptan 50 mg, with no difference in numbers experiencing adverse events. The individuals who respond to each drug may not be the same.
Summary of findings
for the main comparison.
| Oral zolmitriptan 2.5 mg compared with placebo for migraine headache | ||||||
|
Patient or population: adults with migraine headache ‐ moderate or severe pain Settings: community Intervention: oral zolmitriptan 2.5 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with comparator | Probable outcome with intervention | NNT or NNTH and/or relative effect (95% CI) | No of studies, attacks, events | Quality of the evidence (GRADE) | Comments |
| Pain‐free response at 2 h | 100 in 1000 | 300 in 1000 | NNT 5.0 (4.5 to 5.6) | 11 studies, 5223 attacks, 1157 events | High | Lower NNTs are better than higher NNTs |
| Headache relief at 2 h | 290 in 1000 | 610 in 1000 | NNT 3.2 (3.0 to 3.5) | 11 studies, 4567 attacks, 2184 events | High | Lower NNTs are better than higher NNTs |
| Headache relief at 1 h | 210 in 1000 | 380 in 1000 | NNT 6.0 (5.2 to 7.2) | 9 studies, 4123 attacks, 1273 events | High | Lower NNTs are better than higher NNTs |
| Sustained pain‐free during the 24 h post dose | 60 in 1000 | 190 in 1000 | NNT 7.7 (5.9 to 11) | 2 studies, 984 attacks, 145 events | Moderate | Lower NNTs are better than higher NNTs Downgraded due to small number of studies and events |
| Sustained headache relief during the 24 h post dose | 140 in 1000 | 380 in 1000 | NNT 4.1 (3.5 to 5.0) | 4 studies, 1457 attacks, 451 events | High | Lower NNTs are better than higher NNTs |
| At least one AE | 170 in 1000 | 310 in 1000 | NNH 7.0 (6.1 to 8.2) | 12 studies, 5717 attacks, 1464 events | High | Higher NNHs are better than lower NNTs |
| Serious AE* | 2.0 in 1000 | 3.3 in 1000 | insufficient data to calculate | 10,561 attacks, 30 events | Moderate | *all doses > 1 mg and all formulations combined Downgraded due to small number of events |
|
AE: adverse event;CI: Confidence interval; NNT: number needed to treat; NNH: number needed to harm Note: NNT or NNH is reported when an outcome is statistically different from placebo or comparator. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Migraine is a common, disabling headache disorder, ranked seventh highest among specific causes of disability globally (Steiner 2013), and with considerable social and economic impact (Hazard 2009). Recent reviews found a one‐year prevalence of 15% globally (Vos 2012) and for adults in European countries (Stovner 2010), 13% for all ages in the USA (Victor 2010), 21% in Russia (Ayzenberg 2012), and 9% for adults in China (Yu 2012). Migraine is more prevalent in women than in men (by a factor of two to three), and in the age range 30 to 50 years.
The International Headache Society (IHS) classifies two major subtypes (IHS 2013). Migraine without aura is the most common subtype. It is characterised by attacks lasting 4 to 72 hours that are typically of moderate to severe pain intensity, unilateral, pulsating, aggravated by normal physical activity, and associated with nausea with or without photophobia and phonophobia. Migraine with aura is characterised by reversible focal neurological symptoms that develop over a period of at least 5 minutes and last for less than 60 minutes, followed by headache with the features of migraine without aura. In some cases, the headache may lack migrainous features or be absent altogether (IHS 2013).
A large prevalence study in the USA found that over half of migraineurs had severe impairment or required bed rest during attacks. Despite this high level of disability and a strong desire for successful treatment, only a proportion of people with migraine seek professional advice for the treatment of attacks. The majority were not taking any preventive medication, although one‐third met guideline criteria for being offered or considering it. Nearly all (98%) migraineurs used acute treatments for attacks, with 49% using over‐the‐counter (OTC) medication only, 20% using prescription medication, and 29% using both. OTC medications included aspirin, other non‐steroidal anti‐inflammatory drugs (NSAIDs), paracetamol (acetaminophen), and paracetamol plus caffeine (Bigal 2008; Diamond 2007; Lipton 2007). Similar findings have been reported from other large studies in France and Germany (Lucas 2006; Radtke 2009).
The significant impact of migraine with regard to pain, functional health, and well‐being is well documented (Buse 2011; Leonardi 2005); it is ranked in the top 10 disorders for global years lived with disability (Vos 2012). A cross‐sectional survey of eight European Union (EU) countries (representing 55% of the adult population) has estimated an annual direct and indirect cost of migraine per person of EUR 1222, and a total annual cost for the EU of EUR 111 billion for adults aged 18 to 65 years (Linde 2012). Costs vary between countries, probably due to differences in available therapies and the way they are delivered and structural differences in healthcare systems (Bloudek 2012). In the USA, the mean annual direct cost per person has been estimated at USD 1757 for episodic migraine and USD 7750 for chronic migraine (Munakata 2009). Whatever the exact direct and indirect costs are for each country, it is clear that they are substantial. Successful treatment of acute migraine attacks not only benefits patients by reducing their disability and improving health‐related quality of life, but also has the potential to reduce the need for healthcare resources and increase economic productivity.
Description of the intervention
The symptomatic treatment of migraine advanced significantly with the development of the triptan class of drugs, of which sumatriptan was the first, in 1991. Zolmitriptan (trade names include Zomig, Zomigon, Zomigoro, AscoTop) is a second‐generation triptan, available as a standard tablet to be swallowed whole (2.5 mg), an oral disintegrating tablet (also called a 'melt'; 2.5 mg and 5 mg), and a nasal spray (5 mg). The disintegrating tablet contains aspartame, which can cause headache in some individuals and should be avoided by them. It dissolves rapidly in the mouth and can be swallowed without the need for fluid intake. It confers convenience, and may also provide faster onset of action if it dissolves sufficiently quickly to allow substantial uptake through the buccal mucosa. The nasal spray provides a route of administration that avoids oral ingestion altogether, which may be preferable for those who experience nausea and vomiting with migraine attacks. It also partly avoids the problem of slow absorption due to gastric stasis, which is commonly experienced. Up to 30% of the absorbed drug is taken up across the nasal mucosa, mostly immediately after dosing, and this may also lead to a faster onset of action.
The 'recommended' dose in the United Kingdom (UK) is 2.5 mg, with an optional second 2.5 mg dose for recurrence, and subsequent 5 mg for the next attack if 2.5 mg provides inadequate relief (BNF 2013). The maximum dose is 10 mg in 24 hours. In England in 2011 there were 300,000 prescriptions for zolmitriptan in primary care, almost two‐thirds of which were for the standard 2.5 mg tablet (PCA 2012). In the USA recommended doses are lower (1.25 or 2.5 mg) but as in the UK, the maximum dose in 24 hours is 10 mg.
In order to establish whether zolmitriptan is an effective treatment for migraine at a specified dose in acute migraine attacks, it is necessary to study its effects in circumstances that permit detection of pain relief. Such studies are carried out in individuals with established pain of moderate to severe intensity, using single doses of the interventions. Participants who experience an inadequate response with either placebo or active treatment are permitted to use rescue medication, and the intervention is considered to have failed in those individuals. In clinical practice, however, individuals would not normally wait until pain is of at least moderate severity, and may take a second dose of medication if the first dose does not provide adequate relief. Once efficacy is established in studies using single doses in established pain, further studies may investigate different treatment strategies and patient preferences. These are likely to include treating the migraine attack early while pain is mild, and using a low dose initially, with a second dose if the response is inadequate.
How the intervention might work
Zolmitriptan is a 5‐hydroxytryptamine 1 (5‐HT1) agonist, mainly targeting the 5‐HT (serotonin) 1B and 1D receptors. It has three putative mechanisms of therapeutic action (Ferrari 2002; Goadsby 2007a):
vasoconstriction of dilated meningeal blood vessels;
inhibition of the release of vasoactive neuropeptides from perivascular trigeminal sensory neurons;
reduction of pain signal transmission in the trigeminal dorsal horn.
It is used for acute treatment, having no efficacy in preventing future attacks.
Why it is important to do this review
There are a number of studies investigating the efficacy and tolerability of zolmitriptan for the treatment of acute migraine attacks, but no Cochrane review of these studies. Several treatment options are available to treat acute migraine headaches, including sumatriptan (Derry 2012a; Derry 2012b; Derry 2012c; Derry 2012d) and common OTC analgesics such as aspirin (Kirthi 2013), ibuprofen (Rabbie 2013), and paracetamol (Derry 2013). This is one of a series of reviews planned for acute treatments for migraine headaches.
Objectives
To determine the efficacy and tolerability of zolmitriptan compared to placebo and other active interventions in the treatment of acute migraine headaches in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, double‐blind, placebo‐ or active‐controlled studies using zolmitriptan to treat a migraine headache episode. Studies had to have a minimum of 10 participants per treatment arm and report dichotomous data for at least one of the outcomes specified below. We accepted studies reporting treatment of consecutive headache episodes if outcomes for the first, or each, episode were reported separately; we used first‐attack data preferentially. We accepted cross‐over studies if there was adequate (≥ 48 hours) washout between treatments.
Types of participants
Studies enrolled adults (at least 18 years of age) with episodic migraine. We used the definition of migraine specified by the International Headache Society (IHS) (IHS 1988; IHS 2004; IHS 2013) and excluded studies evaluating treatments for chronic migraine. There were no other restrictions on migraine frequency, duration, or type (with or without aura). We accepted studies that included participants taking stable prophylactic therapy to reduce the frequency of migraine attacks. If reported, details on any prophylactic therapy prescribed or allowed are provided in the Characteristics of included studies table.
Types of interventions
We included studies that used a single dose of zolmitriptan to treat a migraine headache episode when pain was of moderate to severe intensity, or investigated different dosing strategies or timing of the first dose in relation to headache intensity, or both. There were no restrictions on dose or route of administration, provided the medication was self‐administered.
A placebo comparator is essential to demonstrate that zolmitriptan is effective in this condition. We considered active‐controlled trials without a placebo as secondary evidence. We excluded studies designed to demonstrate prophylactic efficacy in reducing the number or frequency of migraine attacks.
Types of outcome measures
In selecting the main outcome measures for this review, we considered scientific rigour, availability of data, and patient preferences. Patients with acute migraine headaches have rated complete pain relief, no headache recurrence, rapid onset of pain relief, and no side effects as the four most important outcomes (Lipton 1999).
In view of these patient preferences, and in line with the guidelines for controlled trials of drugs in migraine issued by the IHS (IHS 2000), we considered the following main outcomes.
Primary outcomes
Pain‐free at two hours, without the use of rescue medication.
Reduction in headache pain ('headache relief') at two hours (pain reduced from moderate or severe to none or mild without the use of rescue medication).
We also collected data for pain‐free and headache relief outcomes at one hour if reported and relevant, for example, if a fast‐acting formulation of the intervention was tested. For the purposes of this review, we considered the nasal spray and oral disintegrating tablet formulations to be potentially fast‐acting.
Secondary outcomes
Sustained pain‐free during the 24 hours postdose (pain‐free within two hours, with no use of rescue medication or recurrence of pain of any intensity within 24 hours).
Sustained headache relief during the 24 hours postdose (headache relief at two hours, with no use of rescue medication or a second dose of study medication, or recurrence of moderate or severe pain within 24 hours).
Adverse events: participants with any adverse event during the 24 hours postdose; serious adverse events; adverse events leading to withdrawal.
Other outcomes
We also collected data for other outcomes, including:
use of rescue medication;
relief of headache‐associated symptoms;
relief of functional disability.
Pain intensity or pain relief had to be measured by the participant (not the investigator or care giver). Pain measures accepted for the main efficacy outcomes were the following.
Pain intensity: 4‐point categorical scale, with wording equivalent to none, mild, moderate and severe; or 100 mm VAS, where < 30 mm was considered equivalent to mild or no pain and ≥ 30 mm equivalent to moderate or severe pain (Collins 1997).
Pain relief: 5‐point categorical scale, with wording equivalent to none, a little, some, a lot, complete; or 100 mm VAS, where < 30 mm was considered equivalent to none or a little, and ≥ 30 mm equivalent to some, a lot or complete.
Definitions of important terms, including all measured outcomes, are provided in Appendix 1.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases:
the Cochrane Central Register of Controlled Trials (CENTRAL), The Cochrane Library (Issue 3 of 12, 2014).
MEDLINE (via Ovid) (1990 to 12 March 2014).
EMBASE (via Ovid) (1990 to 12 March 2014).
Oxford Pain Relief Database, searched on 22 May 2013 (Jadad 1996a).
See Appendix 2, Appendix 3, and Appendix 4 for the search strategies for CENTRAL, MEDLINE (via Ovid), and EMBASE (via Ovid), respectively.
Searches of MEDLINE and EMBASE started in 2009 because we were looking only for randomised controlled trials and these two databases are routinely searched and all controlled trials added to CENTRAL. This may not capture studies that have been published or indexed in the previous year, but searching back to 2009 provided a considerable overlap. We did not apply any language restrictions.
Searching other resources
We searched for additional studies in reference lists of retrieved studies and review articles, and in three clinical trials databases (www.astrazenecaclinicaltrials.com, www.clinicaltrials.gov, and apps.who.int/trialsearch). AstraZeneca, the manufacturer of Zomig, provided a database search of publications relating to zolmitriptan in migraine; no mention of unpublished data was made. No studies, published or unpublished, were identified in the list they provided that were not identified by our searches.
Data collection and analysis
Selection of studies
Two review authors independently carried out the searches and selected studies for inclusion. We viewed the titles and abstracts of all studies identified by the electronic searches on screen and excluded any that clearly did not satisfy the inclusion criteria. We read full copies of the remaining studies to identify those suitable for inclusion. Disagreements were settled by discussion with a third review author.
Data extraction and management
Two review authors independently extracted data from the included studies using a standard data extraction form. We settled disagreements by discussion with a third review author. One review author entered data into Review Manager 5 (RevMan 2012).
Assessment of risk of bias in included studies
We used the Oxford Quality Score (Jadad 1996b) as the basis for inclusion, limiting inclusion to studies that were randomised and double‐blind as a minimum. The scores for each study are reported in the Characteristics of included studies table.
Two review authors independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion. We assessed the following for each study.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, eg random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a non‐random process (eg odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (eg telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation (eg open list).
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study states that it was blinded and describes the method used to achieve blinding, eg identical tablets; matched in appearance and smell); unclear risk of bias (study states that it was blinded but does not provide an adequate description of how it was achieved). We excluded studies that were not double‐blind.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (< 10% of participants provided no data without acceptable reason, eg they were randomised but did not have a qualifying headache). We excluded studies with high data loss.
Size of study (checking for possible biases confounded by small size). We assessed studies as being at low risk of bias (≥ 200 participants per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); high risk of bias (< 50 participants per treatment arm).
Measures of treatment effect
We used risk ratios (relative risk; RR) to establish statistical difference. Numbers needed to treat (NNT) and pooled percentages were used as absolute measures of benefit or harm.
We used the following terms to describe adverse outcomes in terms of harm or prevention of harm:
when significantly fewer adverse outcomes occurred with zolmitriptan than with control (placebo or active), we use the term the number needed to treat to prevent one event (NNTp).
when significantly more adverse outcomes occurred with zolmitriptan compared with control (placebo or active), we use the term the number needed to harm or cause one event (NNH).
Unit of analysis issues
We accepted randomisation to the individual patient only. For analysis of studies with more than one treatment arm contributing to any one analysis (for example two formulations of the same dose of zolmitriptan in the same study with a single placebo group), we would split the placebo group equally between the two treatment arms so as not to double‐count placebo participants.
Where participants treated more than one attack we used first attack data preferentially. When that was not reported we have used data from combined attacks and have considered how this might affect the results.
Dealing with missing data
The most likely source of missing data was in cross‐over studies; we planned to use only the first‐period data where possible, but where that was not provided we treated the results as if they were parallel group results. Where there were substantial missing data in any study, we would comment on this and perform sensitivity analyses to investigate their effect.
For all outcomes we carried out analyses, as far as possible, on a modified intention‐to‐treat (ITT) basis, that is, we included all participants who were randomised and received an intervention. Where sufficient information was reported, we re‐included missing data in the analyses we undertook. We planned to exclude data from outcomes where data from 10% or more of participants were missing with no acceptable reason provided or apparent.
Assessment of heterogeneity
We assessed heterogeneity of response rates using L'Abbé plots, a visual method for assessing differences in the results of individual studies (L'Abbé 1987). Where data could be pooled, we reported the I2 statistic.
Assessment of reporting biases
We assessed publication bias by examining the number of participants in trials with zero effect (relative risk of 1.0) needed for the point estimate of the NNT to increase beyond a clinically useful level (Moore 2008). In this case, we specified a clinically useful level as a NNT of 8 or greater for pain‐free at two hours, and NNT of 6 or greater for headache relief at two hours.
Data synthesis
We analysed studies using a single dose of zolmitriptan in established pain of at least moderate intensity separately from studies in which the medication was taken before pain became well established, or in which a second dose of medication was permitted.
We calculated effect sizes and combined data for analysis only for comparisons and outcomes where there were at least two studies and 200 participants (Moore 1998). Relative risk (RR) of benefit ('relative benefit') or harm ('relative risk') was calculated with 95% confidence intervals (CIs) using a fixed‐effect model (Morris 1995). We calculated NNT, NNTp, and NNH with 95% CIs, where possible, using the pooled number of events by the method of Cook and Sackett (Cook 1995). We assumed a statistically significant difference from control when the 95% CI of the RR of benefit or harm did not include the number one.
We used the z test (Tramer 1997) to determine significant differences between NNT, NNTp, and NNH for different groups in subgroup and sensitivity analyses.
We described data from comparisons and outcomes with only one study or fewer than 200 participants in the summary tables and text, where appropriate, for information and comparison, but we did not analyse these data quantitatively.
Subgroup analysis and investigation of heterogeneity
Issues for potential subgroup analysis were dose, formulation, and route of administration.
Sensitivity analysis
We planned sensitivity analysis for study quality (Oxford Quality Score of 2 versus 3 or more) and for migraine type (with aura versus without aura). A minimum of two studies and 200 participants had to be available for any sensitivity analysis.
Results
Description of studies
Results of the search
Searches of bibliographic databases identified over 800 potentially relevant reports, of which 34 were examined in detail after screening titles and abstracts. We included 25 studies in the review, two of which had published subgroup analyses in three additional reports, and another had a published post‐hoc analysis. We also identified, from the World Health Organization (WHO) international clinical trials registry platform, one additional study with 126 participants (CTRI/2009/091/000196). This study appears to satisfy the inclusion criteria, but has not been published, and the report does not provide sufficient data for us to include it in this review. The report claims that results "are similar to the published studies"; details are in the Characteristics of studies awaiting classification table. Three studies (four reports) were excluded (Figure 1).
1.
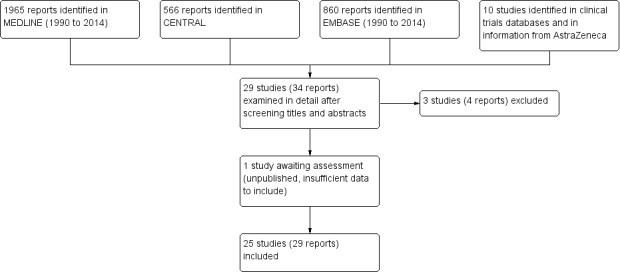
Flow diagram.
Included studies
Twenty‐five studies fulfilled the inclusion criteria for this review (311CIL/0099 2000; Charlesworth 2003; Dahlof 1998; Dib 2002; Dodick 2005; Dowson 2002; Gallagher 2000; Gawel 2005; Geraud 2000; Geraud 2002; Goadsby 2007b; Gruffyd‐Jones 2001; Ho 2008; Klapper 2004; Loder 2005; Pascual 2000; Rapoport 1997; Ryan 2000; Sakai 2002; Solomon 1997; Spierings 2004; Steiner 2003; Tuchman 2006; Tullo 2010; Visser 1996) with contributions from a total of 20,162 participants.
The studies tested the following interventions.
Zolmitriptan standard oral tablet versus placebo (311CIL/0099 2000; Charlesworth 2003; Dahlof 1998; Dib 2002; Geraud 2000; Ho 2008; Klapper 2004; Pascual 2000; Rapoport 1997; Ryan 2000; Sakai 2002; Solomon 1997; Steiner 2003; Tuchman 2006; Visser 1996).
Zolmitriptan oral disintegrating tablet versus placebo (Dowson 2002; Loder 2005; Spierings 2004).
Zolmitriptan nasal spray versus placebo (Charlesworth 2003; Dodick 2005; Gawel 2005).
Zolmitriptan versus ketoprofen (Dib 2002).
Zolmitriptan versus sumatriptan (Gallagher 2000; Geraud 2000; Gruffyd‐Jones 2001).
Zolmitriptan versus acetylsalicylic acid plus metoclopramide (Geraud 2002).
Zolmitriptan versus almotriptan (Goadsby 2007b).
Zolmitriptan versus telcagepant (Ho 2008).
Zolmitriptan versus rizatriptan (Pascual 2000).
Zolmitriptan versus eletriptan (Steiner 2003).
Zolmitriptan versus frovatriptan (Tullo 2010).
Zolmitriptan versus naratriptan (311CIL/0099 2000).
Participants included in all of the studies were diagnosed with migraine with or without aura in accordance with the IHS criteria (IHS 1988; IHS 2004), typically had a history of migraine for more than one year, and suffered from one to six migraine attacks per month, with slight variances. For the most part, the participants recruited into the studies were between 18 and 65 years of age, although Goadsby 2007b included participants aged 16 to 65 years and Solomon 1997 and Rapoport 1997 included participants aged 12 to 65 years. In addition, Pascual 2000 and Gallagher 2000 were not explicit about the age range of those included, but the mean ages were 39 years and 40 years, respectively. Although this review is focused on zolmitriptan for acute migraine solely in adults, we included these studies because we felt that the number of individuals younger than 18 years was small, and because all were ≥ 12 years of age they were likely to require an adult dose. Stable prophylactic medications were allowed in most of the studies with the exception of Visser 1996, in which patients were excluded if they had used prophylactic treatment in the month before the study. Almost all studies stated that study medication was not to be used within 24 hours of taking a triptan or an ergot derivative. All studies included both men and women, except the study concerning menstrual migraine (Tuchman 2006). The participants in Tuchman 2006 were required to have a diagnosis of menstrual migraine (IHS 1988), with migraine occurring within two days of the expected onset of menses to five days after onset, and with > 75% of all menstrual periods associated with migraine attacks.
Twenty‐two of the included studies had a parallel‐group design, and three had a cross‐over design, of which Dib 2002 and Ryan 2000 specified at least 48 hours between treated attacks, and Tullo 2010 did not report a minimum time, but had a 48‐hour outcome, implying that qualifying attacks had to be separated by at least that period. Most studies instructed participants to treat a single migraine attack with the study medication, though in five studies participants treated multiple attacks (Charlesworth 2003; Dodick 2005; Gallagher 2000; Gruffyd‐Jones 2001; Tuchman 2006). Only in Charlesworth 2003 and Dodick 2005 could first‐attack data for certain criteria be extracted, and these data were used where possible.
In all studies rescue medication was allowed if the headache persisted, typically two hours after study medication had been taken. In a number of studies a second dose of study medication was allowed, either if the headache persisted or if it recurred (311CIL/0099 2000; Dowson 2002; Gallagher 2000; Gawel 2005; Geraud 2002; Goadsby 2007b; Gruffyd‐Jones 2001; Ho 2008; Klapper 2004; Rapoport 1997; Ryan 2000; Spierings 2004; Steiner 2003; Tullo 2010; Visser 1996). The administration of zolmitriptan was via an oral tablet (standard or disintegrating) or a nasal spray, with the majority of studies using the standard oral tablet formulation.
In most studies the treated migraine attacks had to be of moderate or severe baseline intensity. Gallagher 2000 did not state pain intensity in the methods, but reported results for reduction from at least moderate to no greater than mild; Gawel 2005 treated any severity, but fewer than 10% were mild, and results were reported separately for attacks of moderate or severe baseline intensity; Loder 2005 treated 'as soon as possible', but reported some outcomes for attacks of moderate or severe baseline intensity; Tullo 2010 treated 'as soon as possible', reporting for mixed baseline pain intensities. Finally, Klapper 2004 treated when pain intensity was mild.
Some studies were inconsistent in the denominators reported, so that the population varied slightly in size for different outcomes or at different time points. Where this variance was not explained in the text, the denominators were changed to the intention‐to‐treat (ITT) number if this gave a more conservative estimate of the efficacy of the drug.
Excluded studies
Five studies were excluded after reading the full report (Dodick 2011; Dowson 2003; Loder 2004; Mauskop 1999; Tepper 1999). The reasons for exclusion are provided in the Characteristics of excluded studies table.
Risk of bias in included studies
The included studies were all randomised and double‐blind. The majority of the studies provided information about withdrawals and dropouts; Goadsby 2007b, Steiner 2003, and Tullo 2010 either made no statement about withdrawals or did not give an adequate explantation for differing denominators. The methodological quality of the trials was determined using the Oxford Quality Scale (Jadad 1996a). Six studies (Charlesworth 2003; Dahlof 1998; Geraud 2002; Gruffyd‐Jones 2001; Ho 2008; Visser 1996) scored 5/5, 11 studies (Dib 2002; Dodick 2005; Dowson 2002; Gawel 2005; Geraud 2000; Klapper 2004; Loder 2005; Rapoport 1997; Ryan 2000; Spierings 2004; Steiner 2003) scored 4/5, seven studies (311CIL/0099 2000; Gallagher 2000; Goadsby 2007b; Pascual 2000; Sakai 2002; Solomon 1997; Tuchman 2006) scored 3/5 and one study (Tullo 2010) scored 2/5. Points were lost mainly due to inadequate description of the method of randomisation or double blinding, and also due to lack of information about withdrawals and drop‐outs. Most studies were performed for registration or other purposes by pharmaceutical companies and would have had to satisfy very strict requirements to randomisation and allocation concealment, and effectiveness of double blinding, although these were not always fully reported in the published studies. Details are provided in the Characteristics of included studies table.
In addition a risk of bias table was created which considered sequence generation, allocation concealment, blinding, incomplete outcome data, and study size (Figure 2). Only one study (Visser 1996) was identified as being at high risk of bias, due to small size, although one other study (Sakai 2002) had group sizes very close to the cut‐off point of 50.
2.
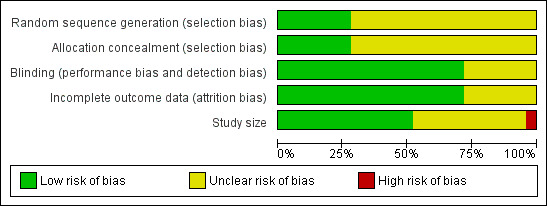
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
See: Table 1
All included studies used a four‐point categorical scale (none, mild, moderate, severe) for rating pain intensity.
In the great majority of studies participants treated attacks when pain was of moderate to severe intensity, or the study reported efficacy results separately for attacks where the baseline pain was moderate to severe. There were sufficient data for at least the primary outcomes of pain‐free and headache relief at two hours for pooled analyses of zolmitriptan versus placebo at doses of 1 mg, 2.5 mg, 5 mg and 10 mg, when used to treat moderate to severe pain. There were insufficient data to allow pooled analysis from studies in which participants treated attacks when pain was mild, or that included mixed baseline intensities. Unless otherwise stated, results reported in this review are for attacks of moderate to severe baseline pain intensity. There were also insufficient data from studies that allowed second or third doses of medication for a single attack in order to allow analysis of these different dosing strategies.
Analyses of other doses of zolmitriptan versus placebo and almost any dose of zolmitriptan versus an active comparator, for any outcome, were not possible because there were insufficient data; it was common for only one study to provide data. We have reported results where analyses were possible in the text and tables in this section, and details of all results in individual studies are available in Appendix 5 (efficacy) and Appendix 6 (adverse events and withdrawals).
For all analysis graphs, studies have used oral tablets unless otherwise noted in the footnotes.
See 'Summary of results A' for results of all efficacy outcomes.
Pain‐free at two hours
Zolmitriptan 1 mg versus placebo
Four studies (1200 attacks) provided data comparing zolmitriptan 1 mg with placebo. Three studies used an oral tablet formulation (Rapoport 1997; Sakai 2002; Visser 1996) and one a nasal spray formulation (Charlesworth 2003).
The proportion of attacks pain‐free at two hours with zolmitriptan 1 mg was 22% (138/621; range 9% to 25%).
The proportion of attacks pain‐free at two hours with placebo was 8.1% (47/579; range 5% to 14%).
The relative benefit of treatment compared with placebo was 2.7 (95% CI 2.0 to 3.7; Analysis 1.1); the NNT was 7.1 (5.5 to 9.9).
For oral treatment alone, the relative benefit of treatment compared with placebo was 1.9 (1.1 to 3.3) and the NNT was 14 (7.4 to 170).
1.1. Analysis.
Comparison 1 Zolmitriptan 1 mg versus placebo, Outcome 1 Pain‐free at 2 h.
Zolmitriptan 2.5 mg versus placebo
Eleven studies (5825 attacks) provided data comparing zolmitriptan 2.5 mg with placebo. Each of the 11 studies used an oral tablet (Charlesworth 2003; Dib 2002; Dowson 2002; Loder 2005; Pascual 2000; Rapoport 1997; Ryan 2000; Sakai 2002; Solomon 1997; Steiner 2003; Tuchman 2006), with one of these studies also providing information on the use of a zolmitriptan 2.5 mg nasal spray (Charlesworth 2003).
The proportion of attacks pain‐free at two hours with zolmitriptan 2.5 mg was 30% (1030/3455; range 19% to 36%).
The proportion of attacks pain‐free at two hours with placebo was 10% (243/2370; range 6% to 17%).
The relative benefit of treatment compared with placebo was 3.0 (2.6 to 3.5) (Figure 3; Analysis 2.1); the NNT was 5.1 (4.7 to 5.7).
For oral treatment alone, the relative benefit of treatment compared with placebo was 3.0 (2.6 to 3.5) and the NNT was 5.0 (4.5 to 5.6).
3.
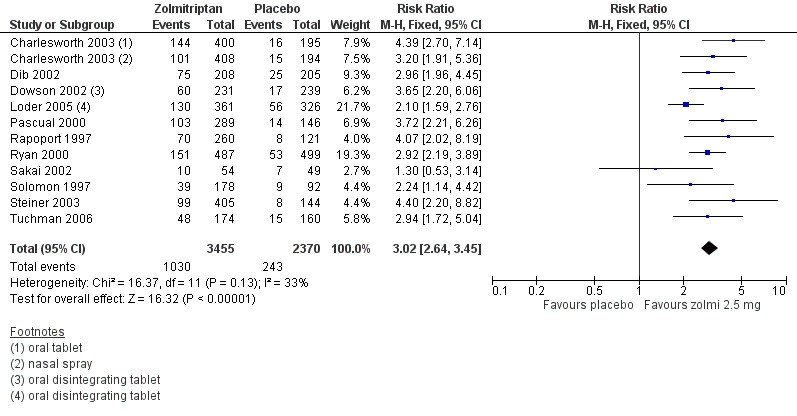
Forest plot of comparison: 2 Zolmitriptan 2.5 mg versus placebo, outcome: 2.1 Pain‐free at 2 h. Studies without footnotes use the standard oral tablet.
2.1. Analysis.
Comparison 2 Zolmitriptan 2.5 mg versus placebo, Outcome 1 Pain‐free at 2 h.
Zolmitriptan 2.5 mg was significantly better than 1 mg (z = 3.933, P = 0.0001). A L'Abbé plot shows the similarity in results between studies (Figure 4).
4.
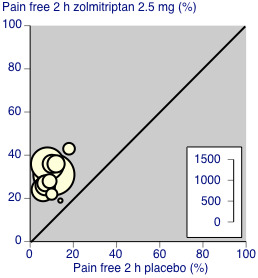
L'Abbé plot showing results for zolmitriptan 2.5 mg versus placebo for pain‐free at 2 hours. Each circle represents a different study; size of circle is proportional to size of study; diagonal is line of equivalence
Klapper 2004 compared zolmitriptan 2.5 mg with placebo to treat headache of mild intensity; 43% (59/138) of participants were pain‐free at two hours with zolmitriptan, while 18% (26/142) experienced this outcome with placebo.
Zolmitriptan 5 mg versus placebo
Eleven studies (9372 attacks) provided data comparing zolmitriptan 5 mg with placebo. Eight studies used an oral tablet formulation (Dahlof 1998; Geraud 2000; Ho 2008; Rapoport 1997; Ryan 2000; Sakai 2002; Spierings 2004; Visser 1996), whilst the other three studies used a nasal spray formulation (Charlesworth 2003; Dodick 2005; Gawel 2005).
The proportion of attacks pain‐free at two hours with zolmitriptan 5 mg was 32% (1616/5024; range 14% to 39%).
The proportion of attacks pain‐free at two hours with placebo was 11% (481/4348; range 2% to 14%).
The relative benefit of treatment compared with placebo was 3.0 (2.7 to 3.2; Analysis 3.1); the NNT was 4.7 (4.4 to 5.1).
For oral treatment alone, the relative benefit of treatment compared to placebo was 3.2 (2.7 to 3.7) and the NNT was 4.8 (4.3 to 5.4), while for nasal spray alone, the relative benefit of treatment compared to placebo was 2.8 (2.5 to 3.2) and the NNT was 4.6 (4.2 to 5.2).
3.1. Analysis.
Comparison 3 Zolmitriptan 5 mg versus placebo, Outcome 1 Pain‐free at 2 h.
There was no statistically significant difference between the two formulations for the outcome of pain‐free at two hours (z = 0.53, P = 0.10).
Zolmitriptan 10 mg versus placebo
Two studies (648 participants) provided data comparing zolmitriptan 10 mg with placebo. Both studies used an oral tablet formulation (Dahlof 1998; Rapoport 1997).
The proportion of participants pain‐free at two hours with zolmitriptan 10 mg was 37% (163/439; range 36% to 39%).
The proportion of participants pain‐free at two hours with placebo was 5% (10/209; range 2% to 7%).
The relative benefit of treatment compared with placebo was 7.8 (4.2 to 14.5; Analysis 4.1); the NNT was 3.1 (2.7 to 3.7).
4.1. Analysis.
Comparison 4 Zolmitriptan 10 mg versus placebo, Outcome 1 Pain‐free at 2 h.
The oral 10 mg dose was significantly better than the oral 5 mg dose for pain‐free at two hours (z = 3.882, P = 0.0001).
Headache relief at two hours
Zolmitriptan 1 mg versus placebo
Four studies (861 participants) provided data comparing zolmitriptan 1 mg with placebo. Three studies used an oral tablet formulation (Rapoport 1997; Sakai 2002; Visser 1996), and one used a nasal spray formulation (Charlesworth 2003).
The proportion of participants experiencing headache relief at two hours with zolmitriptan 1 mg was 55% (333/626; range 27% to 55%).
The proportion of participants experiencing headache relief at two hours with placebo was 31% (183/589; range 15% to 34%).
The relative benefit of treatment compared with placebo was 1.8 (1.5 to 2.1) (Analysis 1.2); the NNT was 4.3 (3.3 to 5.9).
For oral treatment alone the relative benefit of treatment compared to placebo was 1.6 (1.2 to 2.0) and the NNT was 5.6 (3.7 to 12).
1.2. Analysis.
Comparison 1 Zolmitriptan 1 mg versus placebo, Outcome 2 Headache relief at 2 h.
Zolmitriptan 2.5 mg versus placebo
Eleven studies (4904 participants or attacks) provided data comparing zolmitriptan 2.5 mg with placebo. Each of the studies used an oral tablet formulation (311CIL/0099 2000; Charlesworth 2003; Dib 2002; Dowson 2002; Pascual 2000; Rapoport 1997; Ryan 2000; Sakai 2002; Spierings 2004; Tuchman 2006), with one of these studies also providing information on the use of a zolmitriptan 2.5 mg nasal spray (Charlesworth 2003).
The proportion of participants experiencing headache relief at two hours with zolmitriptan 2.5 mg was 60% (1758/2921; range 54% to 67%).
The proportion of participants experiencing headache relief at two hours with placebo was 29% (584/1983; range 21% to 36%).
The relative benefit of treatment compared with placebo was 2.1 (1.9 to 2.2) (Figure 5: Analysis 2.2); the NNT was 3.3 (3.0 to 3.6).
For oral treatment alone the relative benefit of treatment compared to placebo was 2.1 (1.9 to 2.2) and the NNT was 3.2 (3.0 to 3.5).
5.
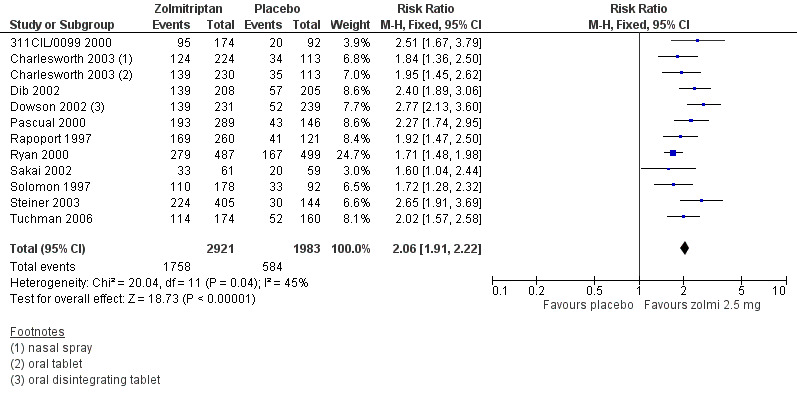
Forest plot of comparison: 2 Zolmitriptan 2.5 mg versus placebo, outcome: 2.2 Headache relief at 2 h. Studies without footnotes use the standard oral tablet.
2.2. Analysis.
Comparison 2 Zolmitriptan 2.5 mg versus placebo, Outcome 2 Headache relief at 2 h.
Zolmitriptan 2.5 mg was significantly better than 1 mg (z = 2.657, P = 0.008). A L'Abbé plot shows the similarity in results between studies (Figure 6).
6.
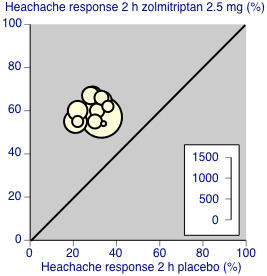
L'Abbé plot showing results for zolmitriptan 2.5 mg versus placebo for headache relief at 2 hours. Each circle represents a different study; size of circle is proportional to size of study; diagonal is line of equivalence
Zolmitriptan 5 mg versus placebo
Eleven studies (7456 participants) provided data comparing zolmitriptan 5 mg with placebo. Eight studies used an oral tablet formulation (Dahlof 1998; Geraud 2000; Ho 2008; Rapoport 1997; Ryan 2000; Sakai 2002; Spierings 2004; Visser 1996), whilst the other three studies used a nasal spray formulation (Charlesworth 2003; Dodick 2005; Gawel 2005).
The proportion of participants experiencing headache relief at two hours with zolmitriptan 5 mg was 63% (2537/4046; range 56% to 69%).
The proportion of participants experiencing headache relief at two hours with placebo was 32% (1078/3410; range 15% to 43%).
The relative benefit of treatment compared with placebo was 2.0 (1.9 to 2.1; Analysis 3.2); the NNT was 3.2 (3.1 to 3.5).
For oral treatment alone, the relative benefit of treatment compared to placebo was 1.9 (1.8 to 2.1) and the NNT was 3.5 (3.2 to 3.9), while for nasal spray alone, the relative benefit of treatment compared to placebo was 2.1 (1.9 to 2.2) and the NNT was 2.9 (2.6 to 3.2).
3.2. Analysis.
Comparison 3 Zolmitriptan 5 mg versus placebo, Outcome 2 Headache relief at 2 h.
The nasal spray formulation was significantly better than oral tablets for the outcome of headache relief at two hours (z = 2.746, P = 0.006).
Zolmitriptan 10 mg versus placebo
Two studies (648 participants) provided data comparing zolmitriptan 10 mg with placebo. Both studies used an oral tablet formulation (Dahlof 1998; Rapoport 1997).
The proportion of participants experiencing headache relief at two hours with zolmitriptan 10 mg was 69% (302/439; range 67% to 71%).
The proportion of participants experiencing headache relief at two hours with placebo was 28% (58/209; range 19% to 34%).
The relative benefit of treatment compared with placebo was 2.5 (2.0 to 3.1; Analysis 4.2); the NNT was 2.4 (2.1 to 3.0).
4.2. Analysis.
Comparison 4 Zolmitriptan 10 mg versus placebo, Outcome 2 Headache relief at 2 h.
The oral 10 mg dose was significantly better than the oral 5 mg dose for headache relief at two hours (z = 2.987, P = 0.003).
Zolmitriptan 2.5 mg versus sumatriptan 50 mg
Two studies (1609 attacks) compared zolmitriptan 2.5 mg with sumatriptan 50 mg (Gallagher 2000; Gruffyd‐Jones 2001). Both studies used oral tablets.
The proportion of attacks with headache relief at two hours with zolmitriptan 2.5 mg was 66% (521/795; range 65% to 67%).
The proportion of attacks with headache relief at two hours with sumatriptan 50 mg was 68% (554/814; range 64% to 71%).
The relative benefit of treatment compared with placebo was 0.96 (0.90 to 1.03; Analysis 5.1). There was no significant difference between treatments.
5.1. Analysis.
Comparison 5 Zolmiriptan 2.5 mg versus sumatriptan 50 mg, Outcome 1 Headache relief at 2 h.
Zolmitriptan 5 mg versus sumatriptan 50 mg
Two studies (1633 attacks) compared zolmitriptan 5 mg with sumatriptan 50 mg (Gallagher 2000; Gruffyd‐Jones 2001). Both studies used oral tablets.
The proportion of attacks with headache relief at two hours with zolmitriptan 5 mg was 67% (545/819; range 65% to 68%).
The proportion of attacks with headache relief at two hours with sumatriptan 50 mg was 68% (554/814; range 64% to 71%).
The relative benefit of treatment compared with placebo was 0.98 (0.92 to 1.1; Analysis 6.1). There was no significant difference between treatments.
6.1. Analysis.
Comparison 6 Zolmitripan 5 mg versus sumatriptan 50 mg, Outcome 1 Headache relief at 2 h.
Headache relief at one hour
Two studies provided data for the 5 mg dose of the nasal spray (potentially fast‐acting) formulation for this outcome (Charlesworth 2003; Dodick 2005). There were insufficient data for analysis of any other dose of the nasal spray, or for any dose of the oral disintegrating tablet.
Zolmitriptan 5 mg nasal spray versus placebo
The proportion of attacks with headache relief at one hour with zolmitriptan 5 mg nasal spray was 56% (763/1362; range 54% to 57%).
The proportion of attacks with headache relief at one hour with placebo was 32% (420/1322; range 26% to 34%).
The relative benefit of treatment compared with placebo was 1.8 (1.6 to 1.9); the NNT was 4.2 (3.6 to 4.9) (Analysis 3.3).
3.3. Analysis.
Comparison 3 Zolmitriptan 5 mg versus placebo, Outcome 3 Headache relief at 1 h.
Zolmitriptan 5 mg oral tablets versus placebo
To investigate whether the nasal spray formulation was better at this early time point than the standard oral tablet, we also analysed the six studies using the same dose of standard oral tablets that reported this outcome (Dahlof 1998; Geraud 2000; Rapoport 1997; Ryan 2000; Sakai 2002; Visser 1996).
The proportion of attacks with headache relief at one hour with zolmitriptan 5 mg standard oral tablets was 38% (558/1477; range 24% to 44%).
The proportion of attacks with headache relief at one hour with placebo was 22% (183/833; range 15% to 27%).
The relative benefit of treatment compared with placebo was 1.8 (1.5 to 2.1); the NNT was 6.3 (5.1 to 8.3) (Analysis 3.3).
Adding the single study that used an oral disintegrating tablet formulation (Spierings 2004) did not change the result (RR 1.8 (1.6 to 1.9), NNT 6.1 (5.2 to 7.5)).
The nasal spray formulation was significantly better than standard oral tablets for the outcome of headache relief at one hour (z = 3.168, P = 0.001).
Sustained pain‐free during the 24 hours postdose
Zolmitriptan 2.5 mg versus placebo
Two studies (984 participants) provided data comparing zolmitriptan 2.5 mg with placebo. The two studies used an oral tablet formulation (Pascual 2000; Steiner 2003).
The proportion of participants experiencing a 24‐hour sustained pain‐free response with zolmitriptan 2.5 mg was 19% (129/694; range 15% to 24%).
The proportion of participants experiencing a 24‐hour sustained pain‐free response with placebo was 6% (16/290; range 4% to 7%).
The relative benefit of treatment compared with placebo was 3.5 (2.1 to 5.8; Analysis 2.3); the NNT was 7.7 (6.0 to 11).
2.3. Analysis.
Comparison 2 Zolmitriptan 2.5 mg versus placebo, Outcome 3 24‐h sustained pain‐free.
Zolmitriptan 5 mg versus placebo
Three studies (4991 attacks) provided data comparing zolmitriptan 5 mg with placebo. One study used an oral tablet formulation (Ho 2008), whilst the other two studies used a nasal spray formulation (Dodick 2005; Gawel 2005).
The proportion of attacks with a 24‐hour sustained pain‐free response with zolmitriptan 5 mg was 14% (346/2516; range 11% to 23%).
The proportion of attacks with a 24‐hour sustained pain‐free response with placebo was 2.9% (73/2475; range 1.6% to 7.1%).
The relative benefit of treatment compared with placebo was 4.7 (3.6 to 5.9; Analysis 3.4); the NNT was 9.3 (8.1 to 11).
For nasal spray treatment alone, the relative benefit of treatment compared to placebo was 4.9 (3.7 to 6.5; Analysis 3.4) and the NNT was 9.6 (8.3 to 11). There was no significant difference between formulations.
3.4. Analysis.
Comparison 3 Zolmitriptan 5 mg versus placebo, Outcome 4 24‐h sustained pain‐free.
Sustained headache relief during the 24 hours postdose
Zolmitriptan 2.5 mg versus placebo
Four studies (2059 attacks) provided data comparing zolmitriptan 2.5 mg with placebo. Each of the four studies used an oral tablet formulation (Charlesworth 2003; Rapoport 1997; Sakai 2002; Steiner 2003), with one also providing information on the use of the zolmitriptan 2.5 mg nasal spray (Charlesworth 2003).
The proportion of attacks with 24‐hour sustained headache relief with zolmitriptan 2.5 mg was 39% (557/1436; range 31% to 46%).
The proportion of attacks with 24‐hour sustained headache relief with placebo was 14% (85/623; range 10% to 22%).
The relative benefit of treatment compared with placebo was 2.9 (2.4 to 3.6; Analysis 2.4); the NNT was 4.0 (3.5 to 4.7).
For oral treatment alone, the relative benefit of treatment compared to placebo was 2.9 (2.2 to 3.7) and the NNT was 4.1 (3.5 to 5.0).
2.4. Analysis.
Comparison 2 Zolmitriptan 2.5 mg versus placebo, Outcome 4 24‐h sustained headache relief.
Zolmitriptan 5 mg versus placebo
Seven studies (7106 attacks) provided data comparing zolmitriptan 5 mg with placebo. Five studies used an oral tablet formulation (Geraud 2000; Ho 2008; Rapoport 1997; Sakai 2002; Spierings 2004), whilst the other two studies used a nasal spray formulation (Charlesworth 2003; Dodick 2005).
The proportion of attacks with 24‐hour sustained headache relief with zolmitriptan 5 mg was 37% (1445/3854; range 35% to 49%).
The proportion of attacks with 24‐hour sustained headache relief with placebo was 12% (375/3252; range 9% to 25%).
The relative benefit of treatment compared with placebo was 2.9 (2.4 to 3.6); the NNT was 3.9 (3.6 to 4.2).
For oral treatment alone, the relative benefit of treatment compared to placebo was 2.4 (2.0 to 2.8) and the NNT was 4.6 (4.0 to 5.3), while for nasal spray alone the relative benefit of treatment compared to placebo was 4.0 (3.4 to 4.6) and the NNT was 3.6 (3.3 to 3.9) (Analysis 3.5).
3.5. Analysis.
Comparison 3 Zolmitriptan 5 mg versus placebo, Outcome 5 24‐h sustained headache relief.
The nasal spray formulation was significantly better than oral tablets for the outcome of sustained headache relief during the 24 hours post dose (z = 2.076, P = 0.039).
| Summary of results A: pain‐free and headache relief | |||||||
| Studies |
Attacks treated |
Treatment (%) |
Placebo or comparator (%) |
Relative benefit (95% CI) |
NNT (95% CI) |
P for difference | |
| Pain‐free at 2 hours | |||||||
| Zolmitriptan 1 mg (all formulations) versus placebo | 4 | 1200 | 22 | 8 | 2.7 (2.0 to 3.7) | 7.1 (5.5 to 9.9) | |
| Zolmitriptan 1 mg oral versus placebo | 3 | 384 | 15 | 8 | 1.9 (1.1 to 3.3) | 14 (7.4 to 170) | 1 mg oral vs 2.5 mg oral z = 3.933, P = 0.001 |
| Zolmitriptan 2.5 mg (all formulations) versus placebo | 12 | 5825 | 30 | 10 | 3.0 (2.6 to 3.5) | 5.1 (4.7 to 5.7) | |
| Zolmitriptan 2.5 mg oral versus placebo | 11 | 5223 | 30 | 10 | 3.0 (2.6 to 3.5) | 5.0 (4.5 to 5.6) | |
| Zolmitriptan 5 mg (all formulations) versus placebo | 11 | 9372 | 33 | 11 | 3.0 (2.7 to 3.2) | 4.7 (4.4 to 5.1) | |
| Zolmitriptan 5 mg oral versus placebo | 8 | 4277 | 31 | 10 | 3.2 (2.7 to 3.7) | 4.8 (4.3 to 5.4) | 5 mg oral versus 5 mg nasal z = 0.53, P = 0.10 |
| Zolmitriptan 5 mg nasal spray versus placebo | 3 | 5095 | 34 | 12 | 2.8 (2.5 to 3.2) | 4.6 (4.2 to 5.2) | |
| Zolmitriptan 10 mg oral versus placebo | 2 | 648 | 37 | 5 | 7.8 (4.2 to 14) | 3.1 (2.7 to 3.7) | 10 mg oral versus 5 mg oral z = 3.882, P = 0.0001 |
| Headache relief at 2 hours | |||||||
| Zolmitriptan 1 mg (all formulations) versus placebo | 4 | 861 | 55 | 31 | 1.8 (1.5 to 2.1) | 4.3 (3.3 to 5.9) | |
| Zolmitriptan 1 mg oral versus placebo | 3 | 399 | 50 | 32 | 1.6 (1.2 to 2.0) | 5.6 (3.7 to 12) | 1 mg oral versus 2.5 mg oral z = 2.657, P = 0.008 |
| Zolmitriptan 2.5 mg (all formulations) versus placebo | 11 | 4904 | 60 | 29 | 2.1 (1.9 to 2.2) | 3.3 (3.0 to 3.6) | |
| Zolmitriptan 2.5 mg oral versus placebo | 10 | 4567 | 61 | 29 | 2.1 (1.9 to 2.2) | 3.2 (3.0 to 3.5) | |
| Zolmitriptan 5 mg (all formulations) versus placebo | 11 | 7456 | 63 | 32 | 2.0 (1.9 to 2.1) | 3.2 (3.0 to 3.5) | |
| Zolmitriptan 5 mg oral versus placebo | 8 | 4292 | 59 | 30 | 1.9 (1.8 to 2.1) | 3.5 (3.2 to 3.9) | 5 mg oral versus 5 mg nasal z = 2.746, P = 0.006 |
| Zolmitriptan 5 mg nasal spray versus placebo | 3 | 3164 | 68 | 33 | 2.1 (1.9 to 2.2) | 2.9 (2.6 to 3.2) | |
| Zolmitriptan 10 mg oral versus placebo | 2 | 648 | 69 | 28 | 2.5 (2.0 to 3.1) | 2.4 (2.1 to 3.0) | 10 mg oral versus 5 mg oral z = 2.987, P = 0.003 |
| Zolmitriptan 2.5 mg versus sumatriptan 50 mg (oral) | 2 | 1609 | 66 | 68 | 0.96 (0.90 to 1.0) | not calculated | |
| Zolmitriptan 5 mg versus sumatriptan 50 mg (oral) | 2 | 1633 | 67 | 68 | 0.98 (0.92 to 1.1) | not calculated | |
| Headache relief at 1 hour | |||||||
| Zolmitriptan 5 mg oral versus placebo | 7 | 3584 | 39 | 22 | 1.8 (1.6 to 2.1) | 6.1 (5.2 to 7.5) | 5 mg oral versus 5 mg nasal z = 3.284, P = 0.001 |
| Zolmitriptan 5 mg nasal spray versus placebo | 2 | 2684 | 56 | 32 | 1.8 (1.6 to 1.9) | 4.1 (3.6 to 4.9) | |
| Sustained pain‐free during the 24 hours post dose | |||||||
| Zolmitriptan 2.5 mg oral versus placebo | 2 | 984 | 19 | 6 | 3.5 (2.1 to 5.8) | 7.7 (5.9 to 11) | |
| Zolmitriptan 5 mg (all formulations) versus placebo | 3 | 4991 | 14 | 4 | 4.7 (3.6 to 5.9) | 9.3 (8.1 to 11) | |
| Zolmitriptan 5 mg nasal spray versus placebo | 2 | 4298 | 13 | 3 | 4.9 (3.7 to 6.5) | 9.6 (8.3 to 11) | |
| Sustained headache relief during the 24 hours post dose | |||||||
| Zolmitriptan 2.5 mg (all formulations) versus placebo | 4 | 2059 | 39 | 14 | 2.9 (2.4 to 3.6) | 4.0 (3.5 to 4.7) | |
| Zolmitriptan 2.5 mg oral versus placebo | 4 | 1457 | 38 | 14 | 2.9 (2.2 to 3.7) | 4.1 (3.5 to 5.0) | |
| Zolmitriptan 5 mg (all formulations) versus placebo | 7 | 7106 | 37 | 12 | 3.2 (2.9 to 3.5) | 3.9 (3.6 to 4.2) | |
| Zolmitriptan 5 mg oral versus placebo | 5 | 2827 | 37 | 15 | 2.4 (2.0 to 2.8) | 4.6 (4.0 to 5.3) | 5 mg oral versus 5 mg nasal z = 2.076, P = 0.038 |
| Zolmitriptan 5 mg nasal spray versus placebo | 2 | 4279 | 38 | 9 | 4.0 (3.4 to 4.6) | 3.6 (3.3 to 3.9) | |
Subgroup analyses of primary outcomes
Subgroup analyses for dose and route of administration have been carried out alongside the main analyses. Three studies (Dowson 2002; Loder 2005; Spierings 2004) used an oral disintegrating tablet (ODT) formulation, but only one study contributed to any analysis, so there were insufficient data for subgroup analysis. Results for ODT formulations fell within the range of the standard tablet formulation.
Sensitivity analyses of the primary outcomes
Only one study (Tullo 2010) scored fewer than 3 points on the Oxford Quality Scale, so no sensitivity analysis could be carried out for methodological quality. Similarly, only one study (Rapoport 1997) reported data separately for participants with and without aura, so no subgroup analysis could be carried out for this subtype.
Three studies (Dib 2002; Ryan 2000; Tullo 2010) used a cross‐over design. Only with Tullo 2010 did there appear to be potential for missing data, but this study did not contribute to any meta‐analyses. Cross‐over designs may introduce other biases (Elbourne 2002; Khan 1996) but removing these studies from any analyses did not affect the results.
None of the studies using a parallel group design had substantial amounts of missing data that might influence the results.
Adverse events
All except one study reported on the number of participants experiencing any adverse events after treatment; Visser 1996 did not report adverse events for the placebo group, so no comparison could be made. Most studies appeared to collect data using spontaneous reports in diary cards and at follow‐up review after the end of treatment. Gallagher 2000, Pascual 2000, Spierings 2004, and Visser 1996 did not provide any details of the method of adverse event data collection. The duration over which data were collected was not always specified and, where it was, there were differences between studies. Most studies probably collected data during the 24 hours following treatment, but Ho 2008 specified 48 hours, Solomon 1997 and Steiner 2003 7 days, and Rapoport 1997 10 days. Spierings 2004 collected data for 24 hours for non‐serious adverse events and 7 days for serious events, while Tullo 2010 reported only events that were considered 'treatment‐related'.
In some studies a second dose of study medication was taken, and in all studies rescue medication was allowed if there was an inadequate response after a given period of time. It was likely that in all cases adverse event data continued to be collected after such additional medication. Ryan 2000 reported adverse event data according to the total dose of study medication taken.
A number of studies treated more than one attack. In Gallagher 2000, first‐attack data were reported for adverse events, while Dodick 2005, Geraud 2002, and Ryan 2000 reported events in all attacks combined. Charlesworth 2003, Gruffyd‐Jones 2001, Spierings 2004, and Tullo 2010 reported events per treatment group, but it was unclear how multiple attacks were combined.
Despite these inconsistencies, we have included as much data as possible in the adverse event analyses in order to be more inclusive and conservative (Appendix 6). See 'Summary of results B' and 'Summary of results C' for adverse event results.
Treatments were generally described as well‐tolerated, with most adverse events being of mild or moderate severity and self‐limiting.
Participants experiencing any adverse event
Zolmitriptan 1 mg versus placebo
Three studies (856 attacks) comparing zolmitriptan 1 mg with placebo provided data. Two studies used an oral tablet (Rapoport 1997; Sakai 2002) and one used a nasal spray formulation (Charlesworth 2003).
The proportion of attacks with adverse events with zolmitriptan 1 mg was 31% (132/431; range 15% to 39%).
The proportion of attacks with adverse events with placebo was 24% (103/425; range 14% to 30%).
The relative harm of treatment compared with placebo was 1.3 (1.01 to 1.6; Analysis 1.3); the NNH was 16 (8.1 to 230).
1.3. Analysis.
Comparison 1 Zolmitriptan 1 mg versus placebo, Outcome 3 Any adverse event.
Zolmitriptan 2.5 mg versus placebo
Twelve studies (6055 attacks) comparing zolmitriptan 2.5 mg with placebo provided data. Each of the 11 studies used an oral tablet formulation (Charlesworth 2003; Dib 2002; Dowson 2002; Klapper 2004; Loder 2005, Pascual 2000; Rapoport 1997; Ryan 2000; Sakai 2002; Solomon 1997; Steiner 2003; Tuchman 2006), with one also using a nasal spray formulation (Charlesworth 2003).
The proportion of attacks with adverse events with zolmitriptan 2.5 mg was 32% (1167/3628; range 16% to 63%).
The proportion of attacks with adverse events with placebo was 17% (422/2427; range 9% to 40%).
The relative harm of treatment compared with placebo was 1.7 (1.6 to 1.9; Analysis 2.5); the NNH was 6.8 (5.9 to 7.9).
2.5. Analysis.
Comparison 2 Zolmitriptan 2.5 mg versus placebo, Outcome 5 Any adverse event.
Zolmitriptan 5 mg versus placebo
Ten studies (9072 attacks) comparing zolmitriptan 5 mg with placebo provided data. Seven studies used an oral tablet formulation (Dahlof 1998; Geraud 2000; Ho 2008; Rapoport 1997; Ryan 2000; Sakai 2002; Spierings 2004), whilst the other three studies used a nasal spray formulation (Charlesworth 2003; Dodick 2005; Gawel 2005).
The proportion of attacks with adverse events with zolmitriptan 5 mg was 41% (2101/5065; range 20% to 61%).
The proportion of attacks with adverse events with placebo was 19% (742/4007; range 9% to 34%).
The relative harm of treatment compared with placebo was 2.2 (2.0 to 2.3; Analysis 3.6); the NNH was 4.4 (4.0 to 4.7).
For oral treatment alone, the relative harm of treatment compared to placebo was 2.0 (1.8 to 2.2) and the NNH was 4.6 (4.2 to 5.3), while for nasal spray alone the relative benefit of treatment compared to placebo was 2.4 (2.1 to 2.6) and the NNH was 4.2 (3.8 to 4.7) (Analysis 3.6).
3.6. Analysis.
Comparison 3 Zolmitriptan 5 mg versus placebo, Outcome 6 Any adverse event.
There was no statistically significant difference between the two formulations for participants experiencing any adverse events.
Zolmitriptan 10 mg versus placebo
Two studies (736 participants) comparing zolmitriptan 10 mg with placebo provided data. Both studies used an oral tablet formulation (Dahlof 1998; Rapoport 1997).
The proportion of participants experiencing adverse events with zolmitriptan 10 mg was 71% (352/499; range 67% to 75%).
The proportion of participants experiencing adverse events with placebo was 32% (75/237; range 30% to 34%).
The relative harm of treatment compared with placebo was 2.2 (1.8 to 2.7; Analysis 4.3); the NNH was 2.6 (2.2 to 3.2).
4.3. Analysis.
Comparison 4 Zolmitriptan 10 mg versus placebo, Outcome 3 Any adverse event.
Zolmitriptan 2.5 mg versus sumatriptan 50 mg
Two studies (1771 attacks) comparing zolmitriptan 2.5 mg with sumatriptan 50 mg provided data. Both studies used oral tablet formulations (Gallagher 2000; Gruffyd‐Jones 2001).
The proportion of attacks with adverse events with zolmitriptan 2.5 mg was 32% (283/878; range 28% to 35%).
The proportion of attacks with adverse events with sumatriptan 50 mg was 28% (251/893; range 18% to 34%).
The relative harm of treatment compared with placebo was 1.1 (0.99 to 1.3; Analysis 5.2). The NNH was not calculated.
5.2. Analysis.
Comparison 5 Zolmiriptan 2.5 mg versus sumatriptan 50 mg, Outcome 2 Any adverse event.
Zolmitriptan 5 mg versus sumatriptan 50 mg
Two studies (1789 attacks) comparing zolmitriptan 5 mg with sumatriptan 50 mg provided data. Both studies used oral tablet formulations (Gallagher 2000; Gruffyd‐Jones 2001).
The proportion of attacks with adverse events with zolmitriptan 5 mg was 31% (280/896; range 21% to 38%).
The proportion of attacks with adverse events with sumatriptan 50 mg was 28% (251/893; range 18% to 34%).
The relative harm of treatment compared with placebo was 1.1 (0.96 to 1.3; Analysis 6.2). The NNH was not calculated.
6.2. Analysis.
Comparison 6 Zolmitripan 5 mg versus sumatriptan 50 mg, Outcome 2 Any adverse event.
| Summary of results B: number of participants experiencing any adverse event (all formulations) | |||||||
| Comparison | Studies | Participants | Zolmitriptan (%) | Comparator (%) | Relative risk (95% CI) | NNH (95% CI) | P for difference |
| Zolmitriptan 1 mg versus placebo | 3 | 856 | 31 | 24 | 1.3 (1.01 to 1.6 ) | 16 (8.1 to 230) | 1 mg v 2.5 mg z = 2.596, P = 0.010 |
| Zolmitriptan 2.5 mg versus placebo | 13 | 5489 | 32 | 17 | 1.7 (1.6 to 1.9) | 6.8 (5.9 to 7.9) | 2.5 mg v 5 mg z = 5.718, P < 0.0001 |
| Zolmitriptan 5 mg versus placebo | 10 | 9072 | 41 | 19 | 2.2 (2.0 to 2.3) | 4.4 (4.0 to 4.7) | 5 mg v 10 mg z = 4.236, P < 0.0001 |
| Zolmitriptan 10 mg versus placebo | 2 | 736 | 71 | 32 | 2.2 (1.8 to 2.7) | 2.6 (2.2 to 3.2) | |
| Zolmitriptan 2.5 mg versus sumatriptan 50 mg | 2 | 1771 | 32 | 28 | 1.1 (0.99 to 1.3) | not calculated | |
| Zolmitriptan 5 mg versus sumatriptan 50 mg | 2 | 1789 | 31 | 28 | 1.1 (0.96 to 1.3) | not calculated | |
There was a clear dose response relationship for zolmitriptan in comparisons with placebo, with significantly more participants experiencing adverse events with each dose increment. Excluding studies that reported over periods greater than 24 hours did not significantly change the results. There was no significant difference between zolmitriptan 5 mg and sumatriptan 50 mg.
Participants experiencing serious adverse events
Three studies did not specifically comment on serious adverse events (Goadsby 2007b; Tullo 2010; Visser 1996), four reported that there were none during the study (Rapoport 1997; Sakai 2002; Steiner 2003; Tuchman 2006), and two reported that there were no drug‐related serious adverse events (Geraud 2000; Solomon 1997). The remaining 17 studies all reported at least one serious adverse event, although most occurred outside the 24‐hour post dose window, and most were judged to be unrelated to any study medication.
In studies reporting occurrence of serious adverse events separately for the zolmitriptan and placebo treatment arms (Charlesworth 2003; Dahlof 1998; Dib 2002; Dodick 2005; Dowson 2002; Gawel 2005; Ho 2008; Klapper 2004; Loder 2005; Pascual 2000; Spierings 2004), or the absence of such events (Rapoport 1997; Sakai 2002; Steiner 2003; Tuchman 2006), the incidence was ≤ 1% in any treatment arm, and overall was 0.33% (22/6647) for all doses ≥ 1 mg (including second doses and rescue medication) and all formulations of zolmitriptan combined, and 0.20% (8/3914) for placebo. There was no evidence of a dose response relationship, and there were too few events to calculate RR or NNH. Further details of individual studies are in Appendix 6.
Withdrawals due to adverse events
Ten studies either did not specifically report on adverse event withdrawals, did not report data for each treatment arm separately, or did not report clearly either whether the percentage referred to patients or attacks, or how multiple attacks were handled (311CIL/0099 2000; Dahlof 1998; Dib 2002; Gallagher 2000; Geraud 2000; Ho 2008; Ryan 2000; Sakai 2002; Solomon 1997; Tullo 2010). The remaining studies reported the number of withdrawals per treatment group.
In studies reporting the occurrence of adverse event withdrawals separately for zolmitriptan and placebo treatment arms (Charlesworth 2003; Dodick 2005; Gawel 2005; Loder 2005; Spierings 2004; Tuchman 2006), or the absence of such withdrawals (Dowson 2002; Klapper 2004; Pascual 2000; Rapoport 1997; Steiner 2003; Visser 1996), the incidence was ≤ 2.1% in any treatment arm, and overall was 0.65% (35/5346) for all doses ≥ 1 mg (including second doses and rescue medication) and formulations of zolmitriptan combined, and 0.20% (7/3428) for placebo. There was no evidence of a dose response relationship, and there were too few events to calculate relative risk or NNH.
Four studies with active comparators reported adverse event withdrawals: Geraud 2002 for zolmitriptan versus aspirin plus metoclopramide, Goadsby 2007b for zolmitriptan versus almotriptan, and Gallagher 2000 and Gruffyd‐Jones 2001 for zolmitriptan versus sumatriptan. There was no significant difference between zolmitriptan 2.5 mg or 5 mg and sumatriptan 50 mg in these two studies that treated up to six attacks.
Other withdrawals
In most studies withdrawals due to reasons other than adverse events were well documented and included protocol violations, loss on follow‐up, withdrawal of consent, lack of efficacy, and lack of qualifying attacks. However, other studies did not provide detailed information or specify withdrawals by group, and in certain cases it was unclear if withdrawal had occurred before or after taking the study medication. The number of withdrawals was not likely to affect estimates of efficacy or harm.
Discussion
Summary of main results
This review included 25 randomised, double‐blind, controlled studies with 20,162 participants. Fourteen studies had only a placebo control, six had both placebo control and an active comparator, and five had an active comparator only. Active comparators were ketoprofen, sumatriptan, acetylsalicylic acid plus metoclopramide, almotriptan, telcagepant, rizatriptan, eletriptan and frovatriptan. Included studies used zolmitriptan at doses of 0.5, 1, 2.5, 5, 10, 15, 20, and 25 mg in an oral tablet (either standard or disintegrating) or nasal spray formulation. Most of the data were for the 2.5 mg and 5 mg doses, and for the standard oral tablet. In most studies participants treated established attacks of moderate to severe intensity, and there were insufficient data for any analysis of treatment when pain was still mild.
For all efficacy outcomes zolmitriptan was superior to placebo, and doses of 2.5 mg or more gave clinically useful NNTs for all outcomes. The remarkably consistent response between studies for the primary outcomes, as illustrated by L'Abbe plots, was not unexpected given the inclusion criteria for the studies and the well‐defined outcomes. There was a trend for lower (better) NNTs at higher doses, with significant differences between 10 mg and 5 mg oral zolmitriptan for pain‐free at two hours, and headache relief at two hours. There were no significant differences between oral zolmitriptan 5 mg and 2.5 mg for any of the primary efficacy outcomes. There was no difference between oral and nasal spray formulation at the 5 mg dose, and insufficient data to compare oral with nasal spray formulations at other doses.
For the IHS preferred outcome of pain‐free at two hours, zolmitriptan 2.5 mg, 5 mg, and 10 mg (oral formulations) compared to placebo gave NNTs of 4.9, 4.8, and 3.1 respectively. About 30% to 40% of participants were responders with zolmitriptan compared to 5% to 10% with placebo. For headache relief at two hours the NNTs were 3.2, 3.5, and 2.4, respectively (60% to 70% responders with zolmitriptan, 30% with placebo). For sustained headache relief during the 24 hours post dose the NNTs for 2.5 mg and 5 mg were about 4 and for sustained pain‐free they were 8 to 9.
Analysis of adverse events was compromised by the fact that some studies did not specify the time period over which the data were collected, and some specified time periods different from the 24‐hour period specified in the protocol. Furthermore, studies allowed use of rescue medication for inadequate response (usually after two hours), and many allowed a second dose of study medication for headache recurrence (or sometimes lack of efficacy), and did not specify whether adverse event data continued to be collected from participants who had taken additional medication. It is likely that they were, so comparisons between doses and with placebo may not be reliable. With these caveats, we chose to pool as much data as possible. More participants experienced adverse events with zolmitriptan than with placebo, and a dose response relationship was seen over the range 1 mg to 10 mg, with NNHs of 16 to 2.6. For the most part adverse events were described as of mild to moderate intensity, and self‐limiting. Serious adverse events were uncommon and only one was reported as related to a study drug, in this case aspirin plus metoclopramide.
Specific adverse events occurred more often with zolmitriptan than with placebo, and for somnolence, asthenia, dizziness or vertigo, paraesthesia, and vasodilation or warm sensation or flush, there were clear dose response relationships, with significant differences between 2.5 mg and 5 mg, and 5 mg and 10 mg for most events. Taste disturbance was a problem with the intranasal formulation, with an NNH of 6.6 during the 24 hours postdose for zolmitriptan 5 mg.
Recurrence of headache following an initial response is perceived to be a problem with triptans, and its impact is probably best judged using outcomes of sustained pain‐free and sustained headache relief during the 24 hours postdose (without use of rescue medication). These outcomes were not reported in all the included studies. Available data indicate that approximately two‐thirds of those who experience headache relief at two hours will sustain that response for 24 hours with zolmitriptan, compared to half with placebo, while half to one‐third who are pain‐free at two hours will sustain that response for 24 hours with zolmitriptan compared to half with placebo. There was no difference between the 2.5 mg and 5 mg doses, and only one study reported findings for 10 mg. Slightly fewer than 40% (2 in 5) of participants experienced sustained headache relief and 14% (1 in 7) sustained pain‐free responses, without use of rescue medication.
Amongst the active comparators, only sumatriptan 50 mg was evaluated in more than one study. There were no significant differences between zolmitriptan 2.5 mg and sumatriptan 50 mg or zolmitriptan 5 mg and sumatriptan 50 mg for headache relief at two hours, any adverse event, or withdrawals due to adverse events.
Additional analyses (Appendix 7) show that almost twice as many participants (63%) treated with placebo used rescue medication than with zolmitriptan 2.5 mg or 5 mg (35%). Data were available for relief of associated symptoms of nausea, photophobia and phonophobia, but vomiting occurred too infrequently for reliable analysis. Zolmitriptan 2.5 mg and 5 mg compared with placebo gave NNTs of about 7 for relief of nausea at 2 hours, and 4 for relief of either photophobia or phonophobia. Approximately half of the participants treated with zolmitriptan achieved relief of these symptoms, compared with one in three or one in four with placebo.
Overall completeness and applicability of evidence
Included participants suffered from migraine in accordance with IHS criteria, with around one to six attacks per month, and a history of attacks for at least six months, and usually one year. In the majority of studies the intensity of treated attacks had to be greater than moderate when medication was taken. In most studies stable prophylactic medication was allowed. However, some papers did not mention the use of prophylactics, and two studies (Dodick 2005; Visser 1996) included only participants who were not taking prophylactics. Geraud 2000 required participants to be triptan‐naïve, Tullo 2010 excluded those who had a previous inadequate response to at least two triptans, and Steiner 2003 excluded people who were consistently resistant to all treatments. Overall there did not appear to be a particular bias towards a certain type of migraine patient, but many studies recruited participants through headache clinics, which may have selected for those with more severe or hard‐to‐treat pain. Individuals were carefully screened before study entry, and those with certain conditions, particularly cardio‐ or cerebrovascular disease, were excluded from the studies. Other exclusions included pregnant or lactating women and individuals with renal or hepatic disease, or who regularly experienced vomiting, together with individuals who suffered from frequent non‐migraine headaches or basilar, ophthalmoplegic or hemiplegic migraine. This may mean that the study population is not a reflection of a less carefully screened general population who may be prescribed zolmitriptan.
While most studies reported IHS preferred outcomes, they did not all report all the outcomes of interest for this review so that numbers of participants in any comparison were usually smaller than the numbers treated.
Although zolmitriptan was compared with a number of different active comparators, most were used in only one study each, so that no analysis was possible except for zolmitriptan 2.5 mg and 5 mg versus sumatriptan 50 mg.
Single‐dose studies provide only limited information about adverse events, and individual studies are generally underpowered to assess harm, but pooling adverse event data from similar studies may allow more robust estimates for short‐term use. In these studies the number of participants who experienced any adverse events was slightly increased with zolmitriptan compared to placebo. However, it is important to remember that in many studies rescue medication or a second dose of study medication was permitted if the study medication did not provide relief, or in the event of recurrence, and this may disproportionately increase the rates of adverse events in the placebo group. Some studies in this review reported data for individual adverse events only if they occurred at a specified rate, which differed between studies (> 1% to ≥ 5%); this inevitably means that some events occurring at lower frequencies were not reported in some studies.
Only three studies (Gawel 2005; Klapper 2004; Loder 2005) allowed participants to treat a migraine attack of mild severity. However, in clinical practice most people treat their headache when it first begins and often this means the pain is mild. There is also some evidence that treating attacks in the early stages is beneficial (Gendolla 2008).
Quality of the evidence
The majority of included studies were of good quality. However, 18 studies did not adequately describe sequence generation, 18 studies did not provide information about allocation concealment, and seven studies did not provide details on the method of blinding. In a number of studies withdrawals and drop‐outs were not reported adequately by treatment group, and for some outcomes, reported denominators differed from the intention‐to‐treat (ITT) population, presumably because some participants failed to record data at that point. Wherever an adequate explanation was not given we have used the ITT denominator if it gave a more conservative estimate; in general, the number of missing participants was not sufficient to significantly alter the results. Thirteen studies had at least 200 participants in each treatment arm, a further 11 had between 50 and 200 in one or more treatment arms (the placebo arm was often smallest with other treatment arms having over 200 participants), and one had fewer than 50 participants in all treatment arms. The overall methodological quality of the included studies was good, and treatment group sizes were sufficiently large to avoid major bias in the results for efficacy.
While most studies used patient diaries and reported some information about adverse events, the outcomes were not always our preferred ones, and the time over which data were collected was frequently not explicit. It is likely that data continued to be collected after intake of rescue medication or a second dose of study medication, so that the total dose over the period assessed is uncertain.
Potential biases in the review process
We identified a large amount of data in comparisons with placebo, particularly for the 2.5 mg and 5 mg doses. Almost 3800 participants would have to have been involved in unpublished trials with zero treatment effects for the NNT for headache relief at two hours to increase to 6 or more (which we considered the limit of clinical utility in this situation) for the 2.5 mg dose (Moore 2008). This is equivalent to 10 studies with 380 participants in each, or 5 with 760 participants. Similarly over 3300 additional participants would be needed in trials with zero effect to increase the NNT for pain‐free at two hours to 8 or more. It is unlikely, but not impossible, that this amount of unidentified data exists. We know of one apparently unpublished cross‐over study, involving 126 participants treated with zolmitriptan nasal spray 5 mg and placebo (CTRI/2009/091/000196), but this study reported that the results were similar to published studies.
The methods of review were such as to minimise bias due to the review process itself, but use of data from both phases of cross‐over studies and from studies reporting combined data from several attacks may introduce unknown biases (Elbourne 2002). For cross‐over studies a 48‐hour period between qualifying attacks should limit the potential for carry‐over effects, and for multiple attacks there is some evidence of consistency of response (in terms of proportion of participants achieving the outcome) for aspirin in migraine (Kirthi 2013) and within some studies in this review (Charlesworth 2003; Dodick 2005; Gruffyd‐Jones 2001).
We specified that a minimum of 200 participants in at least two studies were required before carrying out any pooled analysis, but ideally we would need at least 200 participants in each treatment arm where there is an event rate of 50% to be reasonably confident in the size of an effect (Moore 2010). The magnitude of effect for outcomes with fewer participants or lower event rates should be interpreted with caution.
Agreements and disagreements with other studies or reviews
Oldman 2002 reviewed all pharmacological treatments for acute migraine, including four studies involving zolmitriptan, all of which are included here. Results are presented as the proportion responding, relative risk and NNT, and are broadly consistent with those found in this review for the 5 mg dose: NNTs for headache relief at two hours are very similar, but our estimate for pain‐free at two hours is higher (worse), and we found a significant difference from placebo for sustained headache relief during the 24 hours post dose. Results for the 2.5 mg dose differ more from ours, probably because only two studies (maximum 651 participants) contributed data in the 2002 review. Adverse events were not analysed by Oldman et al. because of poor reporting, on which we have commented in this review.
Ferrari 2002 carried out a meta‐analysis of all triptans for acute migraine, reviewing nine studies (three unpublished at the time of the review) involving zolmitriptan, all of which are included here. Results were presented as absolute or placebo‐subtracted event rates, or both; they are consistent with those found in this review. NNTs were not calculated.
Chen 2007 carried out a meta‐analysis of zolmitriptan for acute migraine, including 24 studies. Three of these studies did not satisfy the inclusion criteria for our review (one was an abstract, one examined a second dose of medication for persistent headache, and one enrolled only adolescents), and we have included four additional studies (Dib 2002; Ho 2008; Tuchman 2006; Tullo 2010). Results were presented as relative risk, calculated using random effects; their results for relative risk are generally slightly lower than those in this review in comparisons with placebo, except for zolmitriptan 5 mg nasal spray, which are significantly higher. NNTs were not reported. With the exception of sumatriptan (two studies), all other active comparisons were made with data from one study only; we chose not to do this since results from only one study are highly susceptible to random chance. Relative risks for adverse events are again similar to this review, except for the 5 mg nasal spray.
None of these earlier reviews reported on use of rescue medication or relief of associated symptoms.
Amanzio 2009 carried out a systematic review of placebo groups in trials of acute migraine treatment where active agents included triptans, NSAIDs, and anticonvulsants. Results for zolmitriptan were not reported separately, and the review did not take account of the complexities and problems of adverse event reporting in migraine trials, commented on above. A multiple treatment comparison meta‐analysis (network meta‐analysis) using the same efficacy outcomes as here concluded that zolmitriptan had the second best efficacy, after rizatriptan (Thorlund 2014). The methods in the network meta‐analysis did not allow comparison with this review directly, but the general thrust of the results was compatible with the results of this review.
Authors' conclusions
Implications for practice.
Zolmitriptan is an effective treatment for some people for the relief of headache pain and other symptoms associated with migraine, with single doses of 2.5 mg or more providing clinically useful levels of relief. People with migraine want treatment that eliminates the headache and any associated symptoms quickly (maximum two hours) and prevents it returning (within 24 hours). Results indicate that with the 5 mg dose only 14% of those treated will be pain‐free at two hours with no headache recurrence within 24 hours.
There was no significant difference in efficacy between 2.5 mg and 5 mg doses for any outcome in these studies, but 10 mg was significantly better for the available outcomes of pain‐free and headache relief at two hours, and at 5 mg the nasal spray formulation was better than oral tablets for headache relief at one and two hours, but not pain‐free at two hours. The occurrence of adverse events was slightly greater in individuals taking zolmitriptan compared to those taking placebo, with significantly more adverse events with 10 mg than with 2.5 mg or 5 mg. Most events were described as mild and of short duration.
Given that 2.5 mg and 5 mg produce the same effect, a 2.5 mg dose would be a sensible starting dose, with increase to 5 mg if there was inadequate response. The intranasal formulation provides more rapid relief of headache pain than oral tablets, but one in seven patients will experience taste disturbances.
Implications for research.
Comparison of zolmitriptan with other active treatments in this review was severely compromised by the small number of available studies that compared zolmitriptan with the same active comparator at the same dose of comparator. Further large, good quality randomised controlled trials making direct comparisons of efficacy and harm between zolmitriptan and other triptans, common analgesics (aspirin, ibuprofen, paracetamol, diclofenac) and ergot derivatives now seem unlikely to be done. We do have large amounts of good quality data that compare different active therapies with placebo in identically designed and conducted studies, using the same outcomes over the same periods of time, and in similar participants; in these circumstances indirect comparison has been legitimised (Song 2003). It may also be possible to use existing study data for network meta‐analysis of very large amounts of high quality, consistently collected data in migraine headache.
New studies should investigate the response to zolmitriptan in individuals who have not responded to other therapies, such as aspirin or sumatriptan, and why some people respond to one triptan but not another.
More complete and consistent reporting of adverse events is necessary to properly assess their impact and make comparisons between different treatments.
Feedback
Comments received, 13 April 2019
Summary
Name: Richard Sheldrake Email Address: richard.sheldrake@nhs.net Affiliation: Primary Integrated Community Services Ltd Role: Senior Clinical Pharmacist
You've included three trials comparing zolmitriptan 5mg nasal spray with placebo in your meta analysis‐ Charlesworth 2003, Dodick 2005 and Gawel 2005. From pooled data you quote 35% of patients taking zolmitriptan 5 mg nasal spray v 12% taking placebo being pain free at 2 hours giving a NNT of 4.3. However it looks like for the Charlesworth paper you have quoted the figures for those patients in the zolmitriptan 2.5 mg tablets arm instead of the 5 mg nasal spray arm i.e. 154/427 v 101/408 being pain free at 2 hours. This erroneously makes the total number of patients across the 3 trials who were pain free on zolmitriptan at 2 hours 919/2598 i.e. 35.37% when actually it was only 866/2579 i.e. 33.58%. This means that as the cumulative placebo arm numbers were 300/2516 i.e. 11.92% the NNT is actually 4.6. Interestingly the authors of "SIGN 155 ‐ Migraine" published in February 2018 have misquoted your NNT figure and used the relative benefit figure of 3.0 rather than your quoted figure of 4.3 that I believe should read 4.6. Given the NNT for sumatriptan 20 mg nasal spray is 4.7 I'm surprised GSK haven't picked up on these apparent errors as they paint their product in a relatively poorer light than it should be. I have also contacted SIGN about that. I look forward to hearing back from you.
Kind Regards, Richard Sheldrake
Reply
We are grateful to have an error in this review pointed out to us. For the comparison of 5 mg zolmitriptan nasal spray for the outcome of pain free at 2 hours we used the data for the 2.5 mg dose instead of for the 5 mg dose for Charlesworth 2003. Because this study contributed only 16% of the total participants in the comparison, the effect of the error is small. The correct result is as follows.
Number pain free at 2 hours with zolmitriptan 5 mg intranasal: 866/2579 (34%)
Number pain free at 2 hours with placebo: 300/2516 (12%)
Risk ratio: 2.8 (95% CI 2.5 to 3.2)
NNT: 4.6 (95% CI 4.2 to 5.2)
Comparison of 5 mg intranasal with 5 mg oral: z = ‐0.53, p = 0.10
The effect is to increase the NNT to 4.6 from the NNT of 4.3 quoted in the review.
We regard this as of small clinical significance. The error has now been corrected, as well as small inconsistencies for all 5 mg data of oral and intranasal routes combined.
Professor Andrew Moore
Contributors
PaPaS Feedback Editor Hayley Barnes, Co‐ordinating Editor Christopher Eccleston, and Managing Editor Anna Erskine.
What's new
| Date | Event | Description |
|---|---|---|
| 9 July 2019 | Review declared as stable | See Published notes. |
| 26 June 2019 | Feedback has been incorporated | See Feedback. |
History
Protocol first published: Issue 7, 2010 Review first published: Issue 5, 2014
| Date | Event | Description |
|---|---|---|
| 29 May 2019 | Amended | Contact details updated. |
| 11 October 2017 | Review declared as stable | No new studies likely to change the conclusions are expected. |
| 27 May 2014 | Review declared as stable | This review will be assessed for updating in 2019. |
Notes
This is not an active area of research and so this review has been stabilised following discussion with the authors and editors. We will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
AstraZeneca, the manufacturer of Zomig, provided a database search of publications relating to zolmitriptan in migraine; no mention of unpublished data was made. No studies, published or unpublished, were identified in the list they provided that were not identified by our searches.
Lifting The Burden: the Global Campaign against Headache and the International Headache Society provided financial support for the editorial process. See Sources of support for details.
Appendices
Appendix 1. Definitions
All terms relating to primary efficacy outcomes are defined according to the effect of the treatment on headache pain, measured using a four‐point pain intensity scale (ranging from 0 to 3; or none, mild, moderate, and severe).
Baseline pain intensity ‐ level of pain participant must be experiencing in order to receive study medication, either 1 (mild pain) or 2/3 (moderate or severe pain).
Pain‐free at two hours ‐ number of participants with a pain intensity of 0 (none) at two hours after administration of study medication, expressed as a fraction of the treated participants with the appropriate baseline pain.
Pain‐free at one hour ‐ number of participants with a pain intensity of 0 (none) at one hour after administration of study medication, expressed as a fraction of the treated participants with the appropriate baseline pain.
Headache relief at two hours ‐ number of participants with a reduction in pain intensity from 2/3 (moderate/severe) to 0/1 (none/mild) at two hours after administration of study medication, expressed as a fraction of the treated participants with grade 2/3 baseline pain.
Headache relief at one hour ‐ number of participants with a reduction in pain intensity from 2/3 (moderate/severe) to 0/1 (none/mild) at one hour after administration of study medication, expressed as a fraction of the treated participants with grade 2/3 baseline pain.
24‐hour sustained headache relief ‐ number of participants with a reduction in pain intensity from 2/3 (moderate/severe) to 0/1 (none/mild) at two hours after administration of study medication which is then sustained between 2 and 24 hours without recurrence of headache or use of additional medication, expressed as a fraction of the treated participants with grade 2/3 baseline pain.
24‐hour sustained pain‐free ‐ number of participants with a pain intensity of 0 (none) at two hours after administration of study medication which is then sustained between 2 and 24 hours without recurrence of headache or use of additional medication expressed as a fraction of the treated participants with the appropriate baseline pain.
Use of rescue medication ‐ number of participants requiring the use of additional medication to treat an inadequate response to study medication, provided that the additional medication is not, or does not include, the study drug.
Relief of associated symptoms ‐ number of participants with an absence of a headache‐associated symptom (nausea, vomiting, photophobia, or phonophobia) at two hours after administration of study medication, expressed as a fraction of the treated participants for whom the symptom was present at baseline.
Complete relief of functional disability ‐ reduction in the level of functional disability, measured using a four‐point scale, from any degree of disability (grade 1/2/3) at baseline to grade 0 (able to work/function normally) at two hours after administration of study medication, expressed as a fraction of the treated participants with any functional disability at baseline.
Appendix 2. Search strategy for CENTRAL
MeSH descriptor Serotonin Agonists (728)
MeSH descriptor Tryptamines (2166)
(zolmitriptan OR Zomog OR Zomigoro OR Zomigon OR AscoTop OR 311C90):it,ab,kw (113)
1 OR 2 OR 3 (2506)
MeSH descriptor Headache explode all trees (1566)
MeSH descriptor Headache Disorders explode all trees (1983)
MeSH descriptor Migraine Disorders explode all trees (1646)
(headach* OR migrain* OR cephalgi* OR cephalalgi*):it,ab,kw (13289)
5 OR 6 OR 7 OR 8 (13289)
4 AND 9 (634)
Limit 10 to Clinical Trials (CENTRAL) (566)
Appendix 3. Search strategy for MEDLINE (via Ovid)
Serotonin Agonists/ OR Tryptamines/ (11776)
(zolmitriptan OR Zomog OR Zomigoro OR Zomigon OR AscoTop OR 311C90).mp. (494)
1 OR 2 (11812)
Headache/ OR exp Headache Disorders/ OR exp Migraine Disorders/ (45132)
(headach* OR migrain* OR cephalgi* OR cephalalgi*).mp. (76199)
4 OR 5 (76237)
randomized controlled trial.pt. (366322)
controlled clinical trial.pt. (87769)
randomized.ab. (265828)
placebo.ab. (143634)
drug therapy.fs. (1674284)
randomly.ab. (189286)
trial.ab. (274923)
groups.ab. (1219659)
OR/7‐14 (3136032)
3 AND 6 AND 15 (1967)
Limit 16 to yr = 1990‐current (1965)
Appendix 4. Search strategy for EMBASE (via Ovid)
Zolmitriptan/ (2356)
(zolmitriptan OR Zomog OR Zomigoro OR Zomigon OR AscoTop OR 311C90).mp. (2394)
1 OR 2 (2394)
exp Headache/ OR exp facial pain/ (127182)
exp Migraine/ (36474)
(headach* OR migrain* OR cephalgi* OR cephalalgi*).mp. (179835)
4 OR 5 OR 6 (184754)
clinical trial.sh. (783675)
controlled clinical trial.sh. (382327)
randomized controlled trial.sh. (322363)
double‐blind procedure.sh. (104865 )
(clin* adj25 trial*).ab. (289739)
((doubl* or trebl* or tripl*) adj25 (blind* or mask*)).ab. (114894)
placebo*.ab. (170650)
random*.ab. (789218)
OR/8‐15 (1523869)
3 AND 7 AND 16 (860)
Limit 17 to py = 1990 ‐ current (860)
Appendix 5. Summary of outcomes: efficacy
| Study ID | Treatment | HR 1 h | HR 2 h | PF 2h | SHR 24 h | SPF 24 h | Use of rescue medication |
| 311CIL/0099 2000 | Part 1 (1) zolmitriptan 2.5 mg, n = 174 (2) naratriptan 2.5 mg, n = 174 (3) placebo, n = 92 |
No usable data | [corrected for baseline pain] (1) 95/174 (2) 81/174 (3) 20/92 |
No usable data | No data | No data | No data |
| Charlesworth 2003 | (1) zolmitriptan 0.5 mg nasal spray, n = 221 (2) zolmitriptan 1 mg nasal spray, n = 236 (3) zolmitriptan 2.5 mg nasal spray, n = 224 (4) zolmitriptan 5 mg nasal spray, n = 235 (5) zolmitriptan 2.5 mg oral, n = 230 (6) placebo, n = 226 |
Attacks 1 and 2: (1) 184/400 (2) 119/398 (3) 162/427 (4) 171/408 (5) 231/427 (6) 101/389 |
1st attack: (1) 254/400 (2) 165/398 (3) 234/427 (4) 239/408 (5) 300/427 (6) 119/389 |
Attacks 1 and 2: (1) 144/400 (2) 40/398 (3) 108/427 (4) 101/408 (5) 154/427 (6) 31/389 |
Attacks 1 and 2: (1) 174/400 (2) no data (3) no data (4) 165/408 (5) 210/427 (6) 53/389 |
No data | Attacks 1 and 2: (1) 133/400 (2) 233/398 (3) 205/427 (4) 160/408 (5) 138/427 (6) 281/389 |
| Dahlof 1998 | (1) zolmitriptan 5 mg, n = 213 (2) zolmitriptan 10 mg, n = 214 (3) zolmitriptan 15 mg, n = 215 (4) zolmitriptan 20 mg, n = 210 (5) placebo, n = 99 |
PP population: (1) 79/179 (2) 76/191 (3) 81/194 (4) 94/187 (5) 14/88 |
PP population: (1) 118/179 (2) 136/191 (3) 134/194 (4) 144/187 (5) 17/88 |
PP population: (1) 70/179 (2) 74/191 (3) 83/194 (4) 88/187 (5) 2/88 |
No usable data‐ does not specify no use of rescue medications | No data | PP population: (1) 95/179 (2) 96/191 (3) 81/194 (4) 77/187 (5) 71/88 |
| Dib 2002 | (1) zolmitriptan 2.5 mg, n = 208 (2) ketoprofen 75 mg, n = 214 (3) ketoprofen 150 mg, n = 211 (4) placebo, n = 205 |
No data | (1) 139/208 (2) 134/214 (3) 130/211 (4) 57/205 |
(1) 75/208 (2) 57/214 (3) 66/211 (4) 25/205 |
No data | No data | (1) 51/208 (2) 63/214 (3) 65/211 (4) 128/205 |
| Dodick 2005 | (1) zolmitriptan 5 mg nasal spray, n = 935 (1745 attacks) (2) placebo, n = 934 (1718 attacks) |
1st attack (1) 532/935 (2) 319/933 |
1st attack (1) 647/935 (2) 351/933 |
All attacks: (1) 621/1745 (2) 235/1718 |
All attacks: (1) 608/1745 (2) 147/1718 |
All attacks: (1) 185/1745 (2) 27/1718 |
All attacks: (1) 537/1745 (2) 1007/1718 |
| Dowson 2002 | (1) zolmitriptan 2.5 mg ODT, n = 231 (2) placebo, n = 239 |
(1) 101/231 (2) 44/239 |
(1) 139/231 (2) 52/239 |
(1) 60/231 (2) 17/239 |
No usable data‐ does not specifically state no use of rescue medications | No data | (1) 131/231 (2) 203/239 |
| Gallagher 2000 | (1) zolmitriptan 2.5 mg, n = 327 (295 for efficacy) (2) zolmitriptan 5 g, n = 337 (305 for efficacy) (3) sumatriptan 25 mg, n = 336 (306 for efficacy) (4) sumatriptan 50 mg, n = 338 (306 for efficacy) |
Pts treated up to 6 attacks (1) 103/295 (2) 114/305 (3) 101/306 (4) 106/306 |
Pts treated up to 6 attacks (1) 198/295 (2) 198/305 (3) 182/306 (4) 195/306 |
No data | No data | No data | No usable data |
| Gawel 2005 | (1) zolmitriptan 5mg nasal spray (2) placebo |
No data for moderate/severe baseline attacks only | (1) 276/426 (2) 98/409 |
(1) 144/426 (2) 34/409 |
No data | (1) 99/426 (2) 29/409 |
(1) 219/461 (2) 362/451 |
| Geraud 2000 | (1) zolmitriptan 5mg oral tablet (2) sumatriptan 100mg (3) placebo |
(1) 163/498 (2) 171/504 (3) 11/56 |
(1) 288/498 (2) 304/504 (3) 24/56 |
(1) 144/498 (2) 150/504 (3) 7/56 |
(1) 180/498 (2) 195/504 (3) 14/56 |
No data | (1) 189/498 (2) 192/504 (3) 32/56 |
| Geraud 2002 | (1) zolmitriptan 2.5mg oral tablet (2) acetylsalicylic acid 900mg plus metoclopramide 10mg |
No data | 1st attack (1) 201/326 (2) 229/340 |
Overall (1) 315/909 (2) 265/949 |
No usable data‐ >50% took second dose | No data | (1) 487/909 (2) 526/949 |
| Goadsby 2007b | (1) zolmitriptan 2.5mg oral tablet (2) almotriptan 12.5mg |
(1) 201/530 (2) 191/531 |
(1) 372/530 (2) 347/531 |
(1) 256/530 (2) 231/531 |
(1) 312/372 (2) 288/347 |
(1) 185/256 (2) 157/231 |
(1) 113/530 (2) 112/531 |
| Gruffyd‐Jones 2001 | (1) zolmitriptan 2.5mg oral tablet (2) zolmitriptan 5mg oral tablet (3) sumatriptan 50mg |
1st attack (1) 208/500 (2) 203/514 (3) 222/508 |
1st attack (1) 323/500 (2) 347/514 (3) 359/508 |
1st attack (1) 160/500 (2) 190/514 (3) 187/508 |
(1) 705/1680 (2) 803/1803 (3) 780/1794 |
1st attack (1) 126/500 (2) 125/514 (3) 138/508 |
(1) 631/1271 (2) 608/2744 (3) 620/2693 |
| Ho 2008 | (1) zolmtriptan 5mg oral tablet (2) telcagepant 150mg (3) telcagepant 300mg (4) placebo |
No data | (1) 193/345 (2) 165/333 (3) 194/354 (4) 95/348 |
(1) 107/345 (2) 57/333 (3) 95/354 (4) 33/348 |
(1) 122/345 (2) 94/333 (3) 132/354 (4) 46/348 |
(1) 62/345 (2) 35/333 (3) 71/354 (4) 17/348 |
No usable data |
| Klapper 2004 | (1) zolmitriptan 2.5mg oral tablet (2) placebo |
No data | No data | (1) 59/138 (2) 26/142 |
No data | No data | (1) 64/138 (2) 101/142 |
| Loder 2005 | (1) zolmitriptan 2.5mg oral tablet (2) placebo |
No data | No data | (1) 130/361 (2) 56/326 |
No data | No usable data ‐ attacks of mild intensity at baseline included | No usable data |
| Pascual 2000 | (1) zolmitriptan 2.5mg oral tablet (2) rizatriptan 10 mg (3) placebo |
(1) 102/289 (2) 124/292 (3) 26/146 |
(1) 193/289 (2) 206/292 (3) 43/146 |
(1) 103/289 (2) 126/292 (3) 14/146 |
No data | (1) 68/289 (2) 94/292 (3) 10/146 |
(1) 126/289 (2) 115/292 (3) 103/146 |
| Rapoport 1997 | (1) zolmitriptan 1mg oral tablet (2) zolmitriptan 2.5 mg oral tablet (3) zolmitriptan 5 mg oral tablet (4) zolmitriptan 10 mg oral tablet (5) placebo |
PP population (1) 45/125 (2) 114/260 (3) 108/245 (4) 126/248 (5) 31/121 |
PP population (1) 66/125 (2) 169/260 (3) 164/245 (4) 166/248 (5) 41/121 |
PP population (1) 20/125 (2) 70/260 (3) 81/245 (4) 89/248 (5) 8/121 |
(1) 20/66 (2) 68/169 (3) 74/164 (4) 83/166 (5) 7/41 |
No data | No usable data |
| Ryan 2000 | (1 )zolmitriptan 2.5 mg oral tablet (2) zolmitriptan 5 mg oral tablet (3) placebo |
(1) 191/487 (2) 185/482 (3) 111/499 |
(1) 279/487 (2) 291/482 (3) 167/499 |
(1) 151/487 (2) 148/482 (3) 53/499 |
No data | No data | (1) 99/485 (2) 93/481 (3) 222/498 |
| Sakai 2002 | (1) zolmitriptan 1 mg oral tablet (2) zolmitriptan 2.5 mg oral tablet (3) zolmitriptan 5 mg oral tablet (4) placebo |
PP population (1) 14/47 (2) 15/54 (3) 18/52 (4) 13/49 |
ITT population (1) 27/52 (2) 33/61 (3) 38/57 (4) 20/59 |
PP population (1) 8/47 (2) 10/54 (3) 12/52 (4) 7/49 |
PP population (1) 18/47 (2) 25/54 (3) 24/52 (4) 11/49 |
No data | PP population (1) 6/47 (2) 3/54 (3) 9/52 (4)13/49 |
| Solomon 1997 | (1) zolmitriptan 2.5 mg oral tablet (2) placebo |
(1) 59/178 (2) 24/92 |
(1) 110/178 (2) 33/92 |
(1) 39/178 (2) 9/92 |
No usable data‐ included participants may have used rescue medications | No data | (1) 77/178 (2) 62/92 |
| Spierings 2004 | (1) zolmitriptan 5 mg oral tablet (2) placebo |
(1) 253/623 (2) 147/651 |
(1) 347/623 (2) 193/651 |
(1) 185/623 (2) 70/651 |
(1) 227/623 (2) 97/651 |
No data | 1st attack (1) 175/329 (2) 258/341 |
| Steiner 2003 | (1) zolmitriptan 2.5 mg oral tablet (2) eletriptan 40 mg (3) eletriptan 80 mg (4) placebo |
(1) 93/405 (2) 101/392 (3) 149/396 (4) 7/144 |
(1) 224/405 (2) 229/392 (3) 265/396 (4) 30/144 |
(1) 99/405 (2) 115/392 (3) 157/396 (4) 8/144 |
(1) 125/405 (2) 151/392 (3) 160/396 (4) 14/144 |
(1) 61/405 (2) 75/392 (3) 100/396 (4) 6/144 |
(1) 101/395 (2) 76/387 (3) 53/390 (4) 81/140 |
| Tuchman 2006 | (1) zolmitriptan 2.5 mg oral tablet (2) placebo |
(1) 71/174 (2) 36/160 |
(1) 114/174 (2) 52/160 |
(1) 48/174 (2) 15/160 |
No data | No data | (1) 74/174 (2) 114/160 |
| Tullo 2010 | (1) zolmitriptan 2.5 mg oral tablet (2) frovatriptan 2.5 mg |
No data | (1) 142/245 (2) 141/247 |
(1) 94/303 (2) 80/308 |
No data | No data | No data |
| Visser 1996 | (1) zolmitriptan 1 mg oral tablet (2) zolmitriptan 5 mg oral tablet 3)zolmitriptan 25 mg oral tablet 4)placebo |
(1) 2/22 (2) 5/21 (3) 9/21 (4) 3/20 |
(1) 6/22 (2) 13/21 (3) 17/21 (4) 3/20 |
(1) 2/22 (2) 3/21 (3) 8/21 (4) 1/20 |
No data | No data | No data |
| ITT ‐ intention to treat; PP ‐ per protocol | |||||||
Appendix 6. Adverse events and withdrawals
| Study ID | Treatment | Any AE | Specific AEs | Serious AEs | AE withdrawal | Other withdrawals/exclusions | |
| 311CIL/0099 2000 | Part 1 (1) zolmitriptan 2.5 mg, n = 174 (2) naratriptan 2.5 mg, n = 174 (3) placebo, n = 92 |
No usable data Most of mild or moderate intensity |
Triptan‐rel: (1) 24/174 (2) 11/174 (3) 5/92 |
None during treatment period (but several before treatment, between phases or outside "treatment period") | 6 pts in total | 16 pts in total (just over 5% in zolmitriptan and placebo groups, 2.3% in naratriptan group) including those due to AEs | |
| Charlesworth 2003 | (1) zolmitriptan 2.5 mg oral tablet (2) zolmitriptan 0.5 mg nasal spray (3) zolmitriptan 1 mg nasal spray (4) zolmitriptan 2.5 mg nasal spray (5) zolmitriptan 5mg nasal spray (6) placebo |
Over 3 attacks: (1) 88/233 (2) 48/224 (3) 69/238 (4) 98/224 (5) 116/236 (6) 54/228 |
Most common (attacks): Taste: (1) 8/571, (2) 19/544, (3) 44/587, (4) 83/574, (5) 91/606, (6) 12/516 Paraesthesia: (1)21/571, (2) 9/544, (3) 15/587, (4) 20/574, (5) 19/606, (6) 18/516 Intranasal paraesthesia: (1) 8/571, (2) 11/544, (3) 10/587, (4) 5/574, (5) 26/606, (6) 6/516 Somnolence: (1)1/571, (2) 0/544, (3) 2/587, (4) 3/574, (5) 15/606, (6) 6/516 Dizziness: (1)7/571, (2) 5/544, (3) 11/587, (4) 19/574 (5) 9/606, (6) 13/516 |
(1) 3/233 (2) 0/224 (3) 2/238 (4) 3/224 (5) 0/236 (6) 1/228 including chest pain in (4) and angina in (1). All occurred several days after taking medication with the exception of chest pain in (4) and colitis in (3). None considered related to study medication |
(1) 3/233 (2) 1/224 (3) 2/238 (4) 0/224 (5) 3/236 (6) 1/228 |
Lack of efficacy: (1) 8/233, (2) 12/224, (3) 13/238, (4) 5/224, (5) 3/236, (6) 25/228
Exclusions: Concurrent illness: 3 Protocol non‐compliance: 9 Consent withdrawn: 4 Lost to follow up: 8 Other: 7 |
|
| Dahlof 1998 | (1) zolmitriptan 5 mg oral tablet (2) zolmitriptan 10 mg oral tablet (3) zolmitriptan 15 mg oral tablet (4) zolmitriptan 20 mg oral tablet (5) placebo |
(1) 130/213 (2) 161/214 (3) 170/215 (4) 159/209 (5) 34/99 |
AE > 5% of patients: Asthenia: (1) 17/213, (2) 30/214, (3) 37/215, (4) 46/209, (5) 5/99 Dizziness: (1) 23/213, (2) 21/214, (3) 28/215, (4) 33/209, (5) 6/99 Paraesthesia: (1) 19/213, (2) 26/214, (3) 22/215, (4) 38/209, (5) 2/99 Heaviness (not chest/neck): (1) 13/213, (2) 19/214, (3) 37/215, (4) 33/209, (5) 1/99 Somnolence: (1) 17/213, (2) 26/214, (3) 19/215, (4) 27/209, (5) 2/99 Nausea: (1) 17/213, (2) 13/214, (3) 32/215, (4) 21/209, (5) 1/99 Warm sensation: (1) 13/213, (2) 17/214, (3) 19/215, (4) 13/209, (5) 3/99 Chest tightness/pain/ heaviness/pressure ‐ incidence low: ≤1% in 5 mg and placebo, ≤4% at higher doses |
1 pt with pre‐existing WPW in group 1 experienced tachycardia. This was considered a typical WPW episode for this individual | Exclusions: 2.6% to 5.2% of each group were randomised but did not take study med or were lost on follow up. 10% to 16% of pts in the safety analysis of each group were excluded from efficacy outcome due to protocol deviations. There was no systematic difference between groups | ||
| Dib 2002 | (1) zolmitriptan 2.5 mg oral tablet (2) ketoprofen 75 mg (3) ketoprofen 150 mg (4) placebo |
(1) 33/210 (2) 13/218 (3) 23/218 (4) 19/209 |
AEs in ≥3 patients: Zolmitriptan: fatigue (5), tight throat (4), paraesthesia (4), asthenia (3), vertigo (3), hot flushes (3) Ketoprofen 75 mg: dyspepsia (4), nausea (3) Ketoprofen 150 mg: none Placebo: nausea (3) |
(1) 0/210 (2) 0/218 (3) 1/218 (pregnancy) (4) 1/209 (GI bleed) Before treatment one pt had a slipped disc and one had suspected cerebral ischaemia |
(1) 1/210 (depression (2) 1/218 (gastric pain and vomiting) (3) 2/218 (pregnancy, depression) (4) 0/209 Before treatment one pt withdrew due to a suspected cerebral ischaemia, one due to gastric pain/vomiting, and one due to anxiety |
Exclusions: Lost to follow‐up: 8 Withdrew consent: 9 Protocol violation requiring w'd: 3 Unrelated medical: 1 |
|
| Dodick 2005 | (1) zolmitriptan 5 mg nasal spray (2) placebo |
Over 2 attacks: (1) 674/1745 (2) 278/1718 |
Most common (attacks): Disguesia: (1) 313/1745, (2) 26/1718 Nasal irritation: (1) 110/1745, (2) 40/1718 Dizziness: (1) 71/1745, (2) 36/1718 Throat irritation: (1) 67/1745, (2) 36/1718 Pharyngolaryngeal pain: (1) 49/1745, (2) 8/1718 Somnolence: (1) 44/1745, (2) 11/1718 Nausea: (1) 35/1745, (2) 18/1718 Paraesthesia: (1) 22/1745, (2) 17/1718 |
(1) 5/935 (2) 3/933 |
(1) 6/935 (2) 3/933 |
Exclusion: 1 pt in (2) did not provide post‐baseline efficacy data Total w'd from ITT pop: (1) 24/935 (2) 33/933 Of which: AE w'd: (1) 6/935, (2) 3/933 Lack of efficacy: (1) 5/935, (2) 16/933 Others not specified |
|
| Dowson 2002 | (1) zolmitriptan 2.5 mg oral tablet (2) placebo |
(1) 63/231 (2) 29/240 |
AEs in >2% of pts: Dizziness: (1) 6/231, (2) 2/240 Asthenia: (1) 8/231, (2) 3/240 Somnolence: (1) 7/321, (2) 4/240 Throat tightness: (1) 6/231, (2) 0/240 Paraesthesia: (1) 5/231, (2) 2/240 Hyperaesthesia: (1) 5/231, (2) 0/240 Nausea: (1) 5/231, (2) 3/240 |
No SAE within 24 h of treating attack. 1 pt had myalgia after zolmitriptan and 1 pt had abdominal pain after placebo but neither was considered drug related | No AE w'd | 1 placebo pt withdrew consent after taking medication. No further details given. | |
| Gallagher 2000 | (1) zolmitriptan 2.5 mg oral tablet (2) zolmitriptan 5 mg oral tablet (3) sumatriptan 25 mg (4) sumatriptan 50 mg |
1st attack only. Some pts took a second dose of medication (1) 91/327 (2) 69/336 (3) 111/337 (4) 60/338 |
Most common: Infection: (1) 19/327, (2) 14/337, (3) 6/336, (4) 19/338 Tightness: (1) 7/327, (2) 22/337, (3) 3/336, (4) 9/338 Nausea: (1) 23/327, (2) 38/337, (3) 14/336, (4) 25/338 Vomiting: (1) 12/327, (2) 14/337, (3) 13/336, (4) 20/338 Dizziness: (1) 20/327, (2) 27/337, (3) 15/336, (4) 17/338 Paraesthesia: (1) 16/327, (2) 27/337, (3) 12/336, (4) 15/338 Somnolence: (1) 14/327, (2) 26/337, (3) 12/336, (4) 13/338 Pharyngitis: (1) 23/327, (2) 26/337, (3) 17/336, (4) 14/338 |
SAEs: 4 with zolmitriptan, 6 with sumatriptan ‐ none considered related to consumption of the study drug | (1) 6/327 (2) 9/336 (3) 12/337 (4) 7/338 |
No other details | |
| Gawel 2005 | (1) zolmitriptan 5 mg nasal spray (2) placebo |
(1) 228/464 (2) 92/451 |
Most common: Dysgeusia: (1) 74/464, (2) 10/451 Nasal irritation: (1) 29/464, (2) 5/451 Dizziness: (1) 28/464, (2) 10/451 Throat irritation: (1) 25/464, (2) 12/451 Fatigue: (1) 24/464, (2) 5/451 Paraesthesia: (1) 14/464, (2) 6/451 |
(1) 1/464 (whiplash injury) (2) 1/451 (headache, depression, drug abuse) |
(1) 7 (2) 2 | Total discontinued:
(1) 18/464
(2) 10/451 Protocol non compliance: 6 Withdrew informed consent: 5 LoE: 2 Protocol violation: 2 Other: 4 |
|
| Geraud 2000 | (1) zolmitriptan 5 mg oral tablet (2) sumatriptan 100 mg (3) placebo |
(1) 287/491 (2) 279/492 (3) 13/56 |
AEs reported in >5% of pts: Asthenia: (1) 53/491, (2) 53/492, (3) 3/56 Dizziness/vertigo: (1) 44/491, (2) 46/492, (3) 1/56 Somnolence: (1) 37/491, (2) 29/492, (3) 2/56 Paraesthesia: (1) 29/491, (2) 33/492, (3) 0/56 Heavyness (not chest/neck): (1) 29/491, (2) 27/492, (3) 0/56 Nausea: (1) 30/491, (2) 35/492, (3) 1/56 Warm sensation: (1) 24/491, (2) 29/492, (3) 1/56 Neck pain: (1) 17/491, (2) 24/492, (3) 1/56 Events of poss cardia origin occurred at 0 to 2% in each group. 1 patient in group 2 had ST depression indicative of an ischaemic episode |
None | None | 28 randomised pts lost to follow up, presumably after they took medication. 19 pts did not provide AE data and no reasons were given. |
|
| Geraud 2002 | (1) zolmitriptan 2.5 mg oral tablet (2) acetylsalicylic acid 900 mg plus metoclopramide 10 mg |
Over 3 attacks: (1) 133/326 (2) 99/340 |
AEs reported in >3% of patients: Vertigo: (1) 22/326, (2) 12/340 Somnolence: (1) 18/326, (2) 17/340 Paraesthesia: (1) 14/326, (2) 5/340 Asthenia: (1) 17/326, (2) 16/340 Tightness: (1) 12/326, (2) 2/340 Nausea: (1) 11/326, (2) 11/340 Abdominal pain: (1) 9/326, (2) 17/340 |
(1) 6/326 (2) 5/340 1 case of phlebitis in the ASA‐MCP group was the only serious adverse event considered to be drug‐related. |
(1) 3/326 (dizziness, somnolence, dizziness + vasodilation) (2) 5/340 ( 2 diarrhoea, palpitations plus asthenia, anxiety + dry mouth, phlebitis |
Numbers and reasons for withdrawals given, but unclear which occurred before medication taken. Most were due to failure to treat sufficient number of headaches with study medication. 2 pts were lost on follow up, 4 pts withdrew to reasons that were not recorded and 20 had protocol non‐compliance. | |
| Goadsby 2007b | (1) zolmitriptan 2.5 mg oral tablet (2) almotriptan 12.5 mg |
(1) 95/530 (2) 88/532 |
All present in < 3%, except fatigue with zolmitriptan.
Most common: Fatigue: (1) 21/530, (2) 11/532 Dizziness: (1) 13/530, (2) 7/532 Nausea: (1) 11/530, (2) 14/532 Chest pain: (1) 3/530, (2) 6/532 Triptan‐associated: (1) 15.7% = 83/530 (2) 11.1% = 59/532 |
None | None | 1 pt in group 2 was excluded from efficacy analysis because they provided no recorded data. 41 randomised pts were not accounted for. They probably did not take medication. | |
| Gruffyd‐Jones 2001 | (1) zolmitriptan 2.5 mg oral tablet (2) zolmitriptan 5 mg oral tablet (3) sumatriptan 50 mg |
(1) 192/551 (2) 211/560 (3) 191/555 |
AEs in >4% of patients: Asthenia: (1) 29/551, (2) 37/560, (3) 25/555 Paraesthesia: (1) 29/551, (2) 29/560, (3) 30/555 Tightness: (1) 19/551, (2) 28/560, (3) 17/555 Dizziness: (1) 19/551, (2) 32/560, (3) 28/555 Somnolence: (1) 17/551, (2) 28/560, (3) 25/555 |
No SAEs occurred within 24 h of taking medication. 28 pts had SAEs outside of this window, including 9 unintended pregnancies. | (1) 14/514 (2) 17/500 (3) 14/508 Most common: pregnancy (6), vomiting (6), dizziness (5) |
Total withdrawals per group were provided with reasons but it was not always clear which withdrew before taking study medication | |
| Ho 2008 | (1) zolmtriptan 5 mg oral tablet (2) telcagepant 150 mg (3) telcagepant 300 mg (4) placebo |
Some patients in groups (2) and (3) took a second dose of active medication (1) 174/345 (2) 95/334 (3) 120/352 (4) 107/349 |
Within 48 h: Dry mouth: (1) 28/345, (2)18/334, (3)21/352, (4) 13/349 Somnolence: (1) 19/345, (2) 15/334, (3)18/352, (4) 14/349 Dizziness: (1) 38/345, (2) 14/334, (3)18/352, (4) 20/349 Nausea: (1) 20/345, (2)13/334, (3)16/352, (4) 13/349 Fatigue: (1) 24/345, (2) 14/334, (3) 15/352, (4) 8/349 Paraesthesia: (1) 18/345, (2) 4/334, (3) 6/352, (4) 5/349 Chest discomfort: (1) 10/345, (2) 1/334, (3) 3/352, (4) 1/349 |
1 SAE in placebo group ‐ no further details | No details | No withdrawals were reported, but denominators vary | |
| Klapper 2004 | (1) zolmitriptan 2.5 mg oral tablet (2) placebo |
(1) 43/138 (2) 16/142 |
Most common: Asthenia: (1) 10/138, (2) 3/142 General heaviness: (1) 5/138, (2) 1/142 Neck tightness: (1) 4/138, (2) 0/142 Dry mouth: (1) 4/138, (2) 3/142 Nausea: (1) 3/138, (2) 0/142 Dizziness: (1) 8/138, (2) 4/142 Hyperasthesia: (1) 4/138, (2) 0/142 Paraesthesia: (1) 6/138, (2) 1/142 Warm sensation: (1) 3/138, (2) 1/142 |
(1) 1/138 (hypersensitivity reaction 10 h after taking zolmitriptan‐ resolved with adrenaline) (2) 0/142 |
None | 4 pts in the placebo group were withdrawn after treatment (reasons unclear) but were included in ITT and safety analyses | |
| Loder 2004 | (1) zolmitriptan (2) placebo |
Over 3 attacks: (1) 72/260 (2) 57/251 Treatment related: (1) 41/260 (2) 23/251 |
Most common: Tightness: (1) 7/260, (2) 5/251 Dizziness: (1) 8/260, (2) 4/251 Paraesthesia: (1) 10/260, (2) 4/251 Somnolence: (1) 9/260, (2) 5/251 |
(1) 3/260 (accidental injury, convulsion >10 days after treatment, bronchitis) (2) 2/251 (accidental injury, increased intracranial pressure) |
No SAE withdrawal.
Other AE w'd: (1) 4/260 (asthenia, dyspnea, tightness, paresthesia ± pressure) (2) 1/251 (arteriospasm 4 days after treatment during a treadmill test) One pt in each group withdrew due to pregnancy |
Lack of efficacy: (1) 3/260, (2) 11/251 Lost to follow up/non‐compliance/withdrew consent/other: (1) 85/260, (2) 83/251 |
|
| Loder 2005 | (1) zolmitriptan 2.5 mg oral tablet (2) placebo |
Over 2 attacks: (1) 92/282 (2) 41/284 |
Most common: Dizziness: (1) 15/282, (2) 7/284 Tightness (chest/neck): (1) 14/282, (2) 1/284 Paraesthesia: (1) 13/282, (2) 3/284 Somnolence: (1) 13/282, (2) 6/284 Heaviness: (1) 7/282, (2) 2/284 |
(1) 3/282 (acute cholcystitis, acute bronchospasm, chest pain with oesophogeal dilation) (2) 0/284 |
(1) 3/282 (2) 1/284 |
Lack of efficacy: (1) 5, (2) 14 Lost to follow up: (1) 8, (2) 5 |
|
| Pascual 2000 | (1) zolmitriptan 2.5 mg oral tablet (2) rizatriptan 10 mg (3) placebo |
(1) 119/304 (2) 95/308 (3) 32/154 Drug related: (1) 85/304 (2) 77/308 (3) 15/154 |
Most common: Dizziness: (1) 18/304, (2) 15/308, (3) 5/154 Asthenia/fatigue: (1) 15/304, (2) 18/308, (3) 5/154 Somnolence: (1) 12/304, (2) 18/308, (3) 5/154 Chest pain/pressure: (1) 12/304, (2) 6/308, (3) 2/154 |
(1) 1/304 (hospitalisation for abdominal pain after 4 days) (2) 1/308 (appendicitis after 13 days) (3) 0/154 |
None | Exclusion of 39 pts who returned their diaries late and this were excluded from efficacy analyses: (1) 15, (2) 16, (3) 8 | |
| Rapoport 1997 | (1) zolmitriptan 1 mg oral tablet (2) zolmitriptan 2.5 mg oral tablet (3) zolmitriptan 5 mg oral tablet (4) zolmitriptan 10 mg oral tablet (5) placebo |
Some pts took a second dose of study medication/rescue medication. (1) 55/141 (2) 131/298 (3) 161/278 (4) 191/285 (5) 41/138 |
Most common: Nausea: (1) 6/141, (2) 24/298, (3) 17/278, (4) 29/285, (5) 7/138 Dizziness: (1) 7/141, (2) 24/298, (3) 36/278, (4) 43/285, (5) 6/138 Somnolence: (1) 8/141, (2) 21/298, (3) 19/278, (4) 29/285 (5) 6/138 Paraesthesia: (1) 7/141, (2) 18/298, (3) 28/278, (4) 29/285, (5) 0/138 Asthenia: (1) 6/141, (2) 9/298, (3) 17/278, (4) 34/285, (5) 4/138 Warm sensation: (1) 6/141, (2) 15/298, (3) 17/278, (4) 17/285, (5) 1/138 Tightness of throat: (1) 1/141, (2) 9/298, (3) 14/278, (4) 14/285, (5) 0/138 Tightness of chest: (1) 1/141, (2) 3/298, (3) 8/278, (4) 17/285, (5) 1/138 No clinically significant effects on lab tests, vital signs, or ECGs |
None | None | Exclusions included 145 due to use of rescue med within <2 h, vomiting within 30 mins, no 2 h assessment, medication taken >12 h after start of attack, baseline pain mild or <48 h since last migraine. | |
| Ryan 2000 | (1) zolmitriptan 2.5 mg oral tablet (2) zolmitriptan 5 mg oral tablet (3) placebo |
Over 3 attacks: (1) 141/867 (2) 182/907 (3) 53/567 |
No AE occurred in >5% of pts. The most common AEs included nausea, dizziness, paraesthesia and somnolence | 9 SAEs in pts receiving study medication ‐ none considered related to medication | In total 25/924 pts withdrew due to adverse events. 20 of these experienced at least 1 adverse event considered to be related to study medication | 165 other pts withdrew after treating 1 attack but the number of withdrawals from each group was not given. withdrawals were due to loss on follow up (15), protocol non compliance (30), withdrawal of consent (20), lack of qualifying attacks (91) and other reasons (9) | |
| Sakai 2002 | (1) zolmitriptan 1 mg oral tablet (2) zolmitriptan 2.5 mg oral tablet (3) zolmitriptan 5 mg oral tablet (4) placebo |
(1) 8/52 (2) 20/61 (3) 25/57 (4) 8/59 |
Most common: Asthenia: (1) 1/52, (2) 1/61, (3) 4/57, (4) 1/59 Hypoasthesia: (1) 1/52, (2) 1/61, (3) 4/57, (4) 0/59 Abdominal pain: (1) 0/52, (2) 1/61, (3) 4/57, (4) 1/59 Paraesthesia: (1) 0/52, (2) 0/61, (3) 3/57, (4) 0/59 Somnolence: (1) 0/52, (2) 2/61, (3) 3/57, (4) 1/59 Hypertension: (1) 0/52, (2) 0/61, (3) 3/57, (4) 0/59 Palpitation: (1) 0/52, (2) 0/61, (3) 3/57, (4) 0/59 Aggravation of migraine: (1) 2/52, (2) 2/61, (3) 3/57, (4) 0/59 |
No SAEs | No details | Exclusions: 27 pts (groups given) for violation of inclusion/exclusion criteria or deviation from protocol. No other withdrawals reported. | |
| Solomon 1997 | (1) zolmitriptan 2.5 mg oral tablet (2) placebo |
(1) 92/200 (2) 29/101 |
Most common: Nausea: (1) 21/200, (2) 6/101 Dizziness: (1) 17/200, (2) 3/101 Paraesthesia: (1) 11/200, (2) 4/101 Chest tightness: (1) 9/200, (2) 1/101 Somnolence: (1) 9/200, (2) 2/101 Vomiting: (1) 3/200, (2) 5/101 |
No SAE judged to be related to zolmitriptan | No details | Exclusions: 19 zolmitriptan and 7 placebo pts did not take study med (23) or were lost on follow up (3) and there were 31 protocol violations. No other withdrawals reported. | |
| Spierings 2004 | (1) zolmitriptan 5 mg oral tablet (2) placebo |
(1) 124/329 (2) 62/342 |
Most common: Tightness: (1) 23/329, (2) 1/342 Dizziness: (1) 22/329, (2) 9/342 Somnolence: (1) 22/329, (2) 11/342 Paraesthesias: (1) 20/329, (2) 2/342 Asthenia: (1) 17/329,(2) 2/342 Nausea: (1) 15/329, (2) 9/342 Myalgia: (1) 12/329, (2) 0/342 Vasodilation: (1) 12/329, (2) 8/342 |
(1) 1/329 (hospitalised for dehydration 13 days after medication‐ judged to be unrelated) (2) 0/342 |
(1) 7/329 (2) 0/342 |
No details | |
| Steiner 2003 | (1) zolmitriptan 2.5 mg oral tablet (2) eletriptan 40 mg (3) eletriptan 80 mg (4) placebo |
(1) 137/405 (2) 117/392 (3) 168/396 (4) 57/144 |
Most common: Asthenia: (1) 10/405, (2) 13/392, (3) 33/396, (4) 0/144 Chest symptoms: (1) 1/405, (2) 9/392, (3) 13/396, (4) 2/144 Dizziness: (1) 7/405, (2) 6/392, (3) 17/396, (4) 2/144 Somnolence: (1) 5/405, (2) 9/392, (3) 12/396, (4) 0/144 |
None | Presume no AE withdrawals | No w'd reported. 25 treated pts had missing baseline data but groups not given. Further, 77 pts did not provide data for 1 h assessment, and 82 for 2 h assessment. | |
| Tuchman 2006 | (1) zolmitriptan 2.5 mg oral tablet (2) placebo |
(1) 110/175 (2) 43/161 |
Most common: Dizziness: (1) 27/175, (2) 6/161 Chest tightness: (1) 20/175, (2) 0/161 Nausea: (1) 17/175, (2) 4/161 Paraesthesia: (1) 16/175, (2) 4/161 Somnolence: (1) 15/175, (2) 3/161 Dry mouth: (1) 13/175, (2) 6/161 Asthenia: (1) 11/175, (2) 6/161 Pain: (1) 10/175, (2) 0/161 Vasodilation: (1) 10/175, (2) 6/161 |
None | (1) 3/175 (2) 0/161 |
Exclusions: 2 pts took medication but did not provide post‐baseline for pain intensity. Other exclusions included: condition worse (1) 3, (2) 6 lost on follow up (1) 8, (2) 0 protocol noncompliance (1) 7, (2) 4 consent withdrawn (1) 9, (2) 2 other (1) 7, (2) 2 | |
| Tullo 2010 | (1) zolmitriptan 2.5 mg oral tablet (2) frovatriptan 2.5 mg |
(1) 5/121 (2) 2/121 |
4 pts in the zolmitriptan group reported angina‐like symptoms (tachycardia, thoracic constriction, pain) vs none in the frovatriptan group Asthenia: (1) 1/121, (2) 2/121 |
No details | No details | No reasons given for discontinuations from the randomised population or groups | |
| Visser 1996 | (1) zolmitriptan 1 mg oral tablet (2) zolmitriptan 5 mg oral tablet (3) zolmitriptan 25 mg oral tablet (4) placebo |
Over 24 h (some pts took > 1 dose ± rescue med) the number of pts reporting AEs rose with increasing cumulative dose of zolmitriptan | Most common: asthenia, somnolence, dry mouth, non‐chest pressure | No details | No details | No details | |
| AE ‐ adverse event; pts ‐ participants; SAE ‐ serious adverse event; w'd ‐ withdrawal; WPW ‐ Wolff‐Parkinson‐White | |||||||
Appendix 7. Other outcomes
Use of rescue medication
All studies asked participants whose symptoms were not adequately controlled to wait, usually for two hours, before taking any additional medication in order to give the test medication enough time to have an effect. Use of rescue or 'escape' medication (usually a different analgesic, or in some studies a second dose of test medication) after that time was reported in most studies and is a measure of treatment failure (lack of efficacy). The time over which use of rescue medication was measured was not always stated, but is likely to have been two to six hours. Results are summarised in Summary of results C.
Zolmitriptan 1 mg versus placebo
Two studies (912 attacks) comparing zolmitriptan 1 mg with placebo provided data. One study used an oral tablet formulation (Sakai 2002) and one used a nasal spray formulation (Charlesworth 2003).
The proportion of attacks requiring rescue medication with zolmitriptan 1 mg was 45% (211/474; range 13% to 48%).
The proportion of attacks requiring rescue medication with placebo was 67% (294/438; range 27% to 72%).
The relative benefit of treatment compared with placebo was 0.66 (0.58 to 0.74; Analysis 1.4); the NNTp was 4.4 (3.5 to 6.1).
1.4. Analysis.
Comparison 1 Zolmitriptan 1 mg versus placebo, Outcome 4 Use of rescue medication.
Zolmitriptan 2.5 mg versus placebo
Ten studies (5020 attacks) comparing zolmitriptan 2.5 mg with placebo provided data. Each of the 10 studies used an oral tablet formulation (Charlesworth 2003; Dib 2002; Dowson 2002; Klapper 2004; Pascual 2000; Ryan 2000; Sakai 2002; Solomon 1997; Steiner 2003; Tuchman 2006), with one also using a nasal spray formulation (Charlesworth 2003).
The proportion of attacks requiring rescue medication with zolmitriptan 2.5 mg was 34% (1019/2960; range 5.6% to 57%).
The proportion of attacks requiring rescue medication with placebo was 63% (1308/2060; range 27% to 85%).
The relative benefit of treatment compared with placebo was 0.54 (0.51 to 0.57; Analysis 2.7); the NNTp was 3.4 (3.2 to 3.8).
2.7. Analysis.
Comparison 2 Zolmitriptan 2.5 mg versus placebo, Outcome 7 Use of rescue medication.
Zolmitriptan 5 mg versus placebo
Eight studies (7762 attacks) comparing zolmitriptan 5 mg with placebo provided data. Five studies used an oral tablet formulation (Dahlof 1998; Geraud 2000; Ryan 2000; Sakai 2002; Spierings 2004), whilst the other three studies used a nasal spray formulation (Charlesworth 2003; Dodick 2005; Gawel 2005).
The proportion of attacks requiring rescue medication with zolmitriptan 5 mg was 37% (1455/4172; range 17% to 53%).
The proportion of attacks requiring rescue medication with placebo was 63% (2246/3590; range 27% to 80%).
The relative benefit of treatment compared with placebo was 0.55 (0.52 to 0.57); the NNTp was 3.6 (3.4 to 3.9).
For oral treatment alone, the relative benefit of treatment compared to placebo was 0.60 (0.55 to o.66) and the NNTp was 4.7 (4.0 to 5.7), while for nasal spray alone the relative benefit of treatment compared to placebo was 0.53 (0.50 to 0.56) and the NNTp was 3.3 (3.0 to 3.6) (Analysis 3.8).
3.8. Analysis.
Comparison 3 Zolmitriptan 5 mg versus placebo, Outcome 8 Use of rescue medication ‐ subgroups.
There was a statistically significant difference between the two formulations with the 5 mg dose for use of rescue medication (z = 3.903, P < 0.0001).
| Summary of results C: use of rescue medication in placebo‐controlled studies | |||||||
| Intervention | Studies | Attacks treated | Treatment (%) | Placebo (%) | Relative benefit (95% CI) | NNTp (95% CI) | P for difference |
| Zolmitriptan 1 mg | 2 | 912 | 45 | 67 | 0.66 (0.58 to 0.74) | 4.4 (3.5 to 6.1) | |
| Zolmitriptan 2.5 mg | 10 | 5020 | 34 | 63 | 0.54 (0.51 to 0.57) | 3.4 (3.2 to 3.8) | |
| Zolmitriptan 5 mg | 8 | 7762 | 35 | 63 | 0.55 (0.52 to 0.57) | 3.6 (3.4 to 3.9) | |
| Zolmitriptan 5 mg oral | 5 | 2571 | 36 | 58 | 0.60 (0.55 to 0.66) | 4.7 (4.0 to 5.7) | 5 mg oral vs 5 mg nasal z = 3.903, P = 0.0001 |
| Zolmitriptan 5 mg nasal spray | 3 | 5191 | 34 | 65 | 0.53 (0.50 to 0.56) | 3.3 (3.0 to 3.6) | |
Zolmitriptan was significantly better than placebo in preventing the use of rescue medication. There was no clear dose response relationship over the dose range studied (1 mg to 5 mg), but for 5 mg, the nasal spray formulation was better than oral tablets for this outcome (z = 3.903, P = 0.0001). For every three or four individuals treated with zolmitriptan, one would not need rescue medication who would have done with placebo.
Relief of associated symptoms
Effects of treatment on relieving (from any intensity at baseline to none at two hours) associated symptoms of nausea, photophobia and phonophobia are presented in Summary of results D. The incidence of vomiting was very low in all studies and where reported did not permit analysis. There were insufficient data on relief of associated symptoms for analysis of comparisons between zolmitriptan and other active treatments.
| Summary of results D: relief of associated symptoms at 2 hours in placebo‐controlled studies | ||||||
| Intervention | Studies | Attacks with symptom present | Treatment (%) | Placebo (%) | Relative risk (95% CI) | NNT |
| Nausea | ||||||
| Zolmitriptan 1 mg | 2 | 194 | 46 | 30 | not calculated | |
| Zolmitriptan 2.5 mg | 7 | 2140 | 53 | 36 | 1.5 (1.4 to 1.7) | 6.0 (4.8 to 8.0) |
| Zolmitriptan 2.5 mg oral | 6 | 1343 | 51 | 31 | 1.7 (1.5 to 2.0) | 5.0 (3.9 to 6.7) |
| Zolmitriptan 5 mg | 6 | 2056 | 51 | 36 | 1.5 (1.4 to 1.7) | 6.7 (5.2 to 9.4) |
| Zolmitriptan 5 mg oral | 5 | 1240 | 45 | 31 | 1.6 (1.4 to 1.9) | 7.0 (5.1 to 11) |
| Zolmitriptan 10 mg | 1 | 195 | 50 | 26 | not calculated | |
| Photophobia | ||||||
| Zolmitriptan 1 mg | 2 | 237 | 37 | 24 | 1.6 (1.1 to 2.4) | 7.5 (4.0 to 60) |
| Zolmitriptan 2.5 mg | 7 | 2680 | 51 | 26 | 2.0 (1.8 to 2.2) | 4.1 (3.6 to 4.8) |
| Zolmitriptan 2.5 mg oral | 6 | 1883 | 52 | 26 | 2.1 (1.8 to 2.4) | 3.9 (3.3 to 4.6) |
| Zolmitriptan 5 mg | 6 | 2690 | 49 | 24 | 2.0 (1.8 to 2.3) | 3.9 (3.5 to 4.6) |
| Zolmitriptan 5 mg oral | 5 | 1874 | 46 | 22 | 1.9 (1.7 to 2.3) | 4.3 (3.6 to 5.2) |
| Zolmitriptan 10 mg | 1 | 306 | 52 | 17 | not calculated | |
| Phonophobia | ||||||
| Zolmitriptan 1 mg | 2 | 209 | 45 | 21 | 2.2 (1.4 to 3.3) | 4.1 (2.7 to 8.1) |
| Zolmitriptan 2.5 mg | 7 | 2437 | 54 | 26 | 2.1 (1.9 to 2.4) | 3.6 (3.2 to 4.2) |
| Zolmitriptan 2.5 mg oral | 6 | 1640 | 54 | 27 | 2.1 (1.8 to 2.4) | 3.7 (3.2 to 4.5) |
| Zolmitriptan 5 mg | 6 | 2512 | 50 | 24 | 2.0 (1.8 to 2.3) | 4.0 (3.5 to 4.6) |
| Zolmitriptan 5 mg oral | 5 | 1696 | 46 | 24 | 1.9 (1.6 to 2.2) | 4.5 (3.7 to 5.6) |
| Zolmitriptan 10 mg | 1 | 268 | 49 | 16 | not calculated | |
Zolmitriptan at doses of 1 mg to 5 mg showed efficacy in relief of the associated symptoms of nausea, photophobia and phonophobia at two hours (Analysis 1.5; Analysis 2.8; Analysis 3.9). There was no obvious dose response relationship. For every four individuals with photophobia or phonophobia, one would experience relief of the symptom within two hours with zolmitriptan who would not have done with placebo, and for every six individuals with nausea, one would experience relief of the symptom within two hours with zolmitriptan who would not have done with placebo.
1.5. Analysis.
Comparison 1 Zolmitriptan 1 mg versus placebo, Outcome 5 Relief of associated symptoms at 2 h.
2.8. Analysis.
Comparison 2 Zolmitriptan 2.5 mg versus placebo, Outcome 8 Relief of associated symptoms at 2 h.
3.9. Analysis.
Comparison 3 Zolmitriptan 5 mg versus placebo, Outcome 9 Relief of associated symptoms at 2 h.
Relief of functional disability at two hours
Few studies reported relief (from any degree at baseline to none at two hours) of functional disability. Analysis was possible for only the 5 mg dose.
Zolmitriptan 5 mg versus placebo
Two studies (3228 attacks) provided data comparing zolmitriptan 5 mg with placebo for attacks of moderate to severe intensity. One study used an oral tablet formulation (Ho 2008) and one study used a nasal spray formulation (Dodick 2005).
The proportion of attacks with improvement in functional disability at two hours with zolmitriptan 5 mg was 36% (581/1631; range 31% to 37%);
The proportion of attacks with improvement in functional disability at two hours with placebo was 15% (235/1597; range 14% to 15%);
The relative benefit of treatment compared with placebo was 2.4 (2.1 to 2.8; Analysis 3.10); the NNT was 4.8 (4.2 to 5.6).
3.10. Analysis.
Comparison 3 Zolmitriptan 5 mg versus placebo, Outcome 10 Free of functional disability at 2 h.
While all studies reporting this outcome individually found zolmitriptan to be superior to placebo for relief of functional disability within two hours, consistent with the pooled analysis for zolmitriptan 5 mg, caution is required in interpreting and extrapolating this limited data.
Participants experiencing specific adverse events
Twenty‐three studies provided information on the incidence of specific adverse events, although data were not consistently reported. Most studies used a threshold incidence for reporting, which ranged from 1% to 5% in any treatment group, but Gallagher 2000 did not specify any threshold and Tullo 2010 reported on all participants in the safety analysis. Clearly the use of any threshold introduces an element of chance as to whether an adverse event occurring at around that frequency gets reported or not, and different threshold values mean that an adverse event that would be reported in one study might not in another, reducing the amount of data available for analysis. The terms used to describe individual events were also inconsistent. For example some studies reported dizziness, others vertigo, others "dizziness/vertigo", and others dizziness and vertigo as discrete events. Similarly, some studies reported tightness (not chest), others tightness without further specification, or including chest tightness. Some studies reported tightness, but not heaviness, others heaviness but not tightness, and a few reported both as discrete events.
'Summary of results E' table shows specific adverse events where there were sufficient data for analysis, combining events where we considered it appropriate. All adverse events occurred more often with zolmitriptan than with placebo. There was a trend for lower (worse) NNHs with higher doses, and this reached statistical significance for somnolence 2.5 mg versus 5 mg and 5 mg versus 10 mg, asthenia 2.5 mg versus 5 mg and 5 mg versus 10 mg, dizziness or vertigo 2.5 mg versus 5 mg and 5 mg versus 10 mg, paresthesia 5 mg versus 10 mg, and vasodilation or warm sensation or flush 2.5 mg versus 5 mg. Taste disturbance was a significant problem with the intranasal formulation.
| Summary of results E: specific adverse events in placebo controlled studies | |||||||
| Studies | Participants or attacks | Treatment (%) | Placebo (%) | Relative risk (95% CI) | NNH (95% CI) | P for difference | |
| Somnolence | |||||||
| Zolmitriptan 2.5 mg | 10 | 5645 | 2.7 | 1.8 | 1.7 (1.2 to 2.4) | 100 (57 to 570) | 2.5 mg versus 5 mg z = 2.914, P = 0.004 |
| Zolmitriptan 5 mg | 10 | 8622 | 4.1 | 1.5 | 2.2 (1.7 to 3.0) | 40 (31 to 55) | 5 mg versus 10 mg z = 2.759, P = 0.006 |
| Zolmitriptan 10 mg | 2 | 736 | 11 | 3.4 | 3.3 (1.6 to 6.8) | 13 (8.9 to 25) | |
| Zolmitriptan ≥10 mg | 4 | 1160 | 11 | 3.4 | 3.3 (1.6 to 7.0) | 13 (9.4 to 22) | |
| Asthenia | |||||||
| Zolmitriptan 2.5 mg | 10 | 4382 | 3.0 | 1.4 | 2.1 (1.4 to 3.3) | 64 (41 to 140) | 2.5 mg versus 5 mg z = 4.063, P < 0.0001 |
| Zolmitriptan 5 mg | 7 | 3122 | 6.4 | 1.6 | 2.4 (1.5 to 3.8) | 21 (16 to 29) | 5 mg versus 10 mg z = 2.043, P = 0.041 |
| Zolmitriptan 10 mg | 2 | 736 | 13 | 3.8 | 3.4 (1.7 to 6.7) | 11 (7.8 to 19) | |
| Zolmitriptan ≥10 mg | 4 | 1160 | 16 | 3.8 | 3.7 (1.9 to 7.2) | 8.2 (6.4 to 11) | |
| Dizziness/vertigo | |||||||
| Zolmitriptan 2.5 mg | 11 | 6224 | 4.3 | 2.1 | 2.1 (1.6 to 2.9) | 45 (32 to 72) | 2.5 mg versus 5 mg z = 2.011, P = 0.044 |
| Zolmitriptan 5 mg | 9 | 8506 | 6.1 | 2.6 | 2.0 (1.6 to 2.5) | 29 (23 to 38) | 5 mg versus 10 mg z = 2.375, P = 0.018 |
| Zolmitriptan 10 mg | 2 | 736 | 14 | 5.1 | 2.7 (1.5 to 4.9) | 12 (7.9 to 22) | |
| Zolmitriptan ≥10 mg | 4 | 1160 | 17 | 5.1 | 3.2 (1.8 to 5.6) | 8.4 (6.4 to 12) | |
| Paresthesia | |||||||
| Zolmitriptan 2.5 mg | 10 | 5337 | 3.8 | 1.5 | 2.2 (1.6 to 3.2) | 45 (32 to 72) | 2.5 mg versus 5 mg z = 0.463, P = 0.646 |
| Zolmitriptan 5 mg | 10 | 8622 | 3.8 | 1.2 | 2.5 (1.8 to 3.4) | 40 (32 to 54) | 5 mg versus 10 mg z = 3.995, P < 0.0001 |
| Zolmitriptan 10 mg | 2 | 736 | 11 | 0.84 | 11 (3.0 to 37) | 9.9 (7.6 to 14) | |
| Zolmitriptan ≥10 mg | 2 | 1160 | 13 | 1.1 | 10 (3.1 to 34) | 8.7 (7.2 to 11) | |
| Vasolidation/warm sensation/flush | |||||||
| Zolmitriptan 2.5 mg | 6 | 2784 | 2.4 | 1.1 | 2.3 (1.2 to 4.2) | 74 (43 to 260) | 2.5 mg versus 5 mg z = 2.423, P = 0.016 |
| Zolmitriptan 5 mg | 6 | 3006 | 4.4 | 1.2 | 2.9 (1.7 to 5.2) | 31 (23 to 49) | 5 mg versus 10 mg z = 1.275, P = 0.204 |
| Zolmitriptan 10 mg | 2 | 736 | 6.8 | 1.7 | 4.0 (1.4 to 11) | 20 (13 to 42) | |
| Zolmitriptan ≥10 mg | 2 | 1160 | 7.2 | 0.84 | 7.9 (1.9 to 32) | 16 (12 to 23) | |
| Taste disturbance | |||||||
| Zolmitriptan 5 mg (nasal spray) | 3 | 5500 | 17 | 1.8 | 9.5 (7.1 to 13) | 6.6 (6.0 to 7.3) | |
| Nausea | |||||||
| Zolmitriptan 2.5 mg | 6 | 2390 | 5.7 | 2.4 | 1.9 (1.3 to 3.0) | 30 (21 to 58) | |
| Zolmitriptan 5 mg | 7 | 7018 | 3.8 | 1.7 | 2.0 (1.4 to 2.7) | 48 (35 to 74) | |
| Zolmitriptan 10 mg | 2 | 736 | 8.4 | 3.4 | 2.5 (1.2 to 5.3) | 20 (12 to 59) | |
| Zolmitriptan ≥10 mg | 2 | 1160 | 10 | 3.4 | 3.3 (1.5 to 7.0) | 14 (10 to 26) | |
| Tightness (not chest) | |||||||
| Zolmitriptan 2.5 mg | 6 | 2919 | 2.6 | 0.08 | 11 (3.6 to 31) | 40 (30 to 61) | |
| Zolmitriptan 5 mg | 5 | 5610 | 2.4 | 0.04 | 23 (7.0 to 76) | 41 (33 to 55) | |
| Heaviness (not chest) | |||||||
| Zolmitriptan 2.5 mg | 2 | 846 | 2.9 | 0.70 | 4.1 (1.2 to 14) | 46 (25 to 270) | |
| Zolmitriptan 5 mg | 4 | 2445 | 4.1 | 0.63 | 4.7 (2.0 to 11) | 29 (22 to 43) | |
| Dry mouth | |||||||
| Zolmitriptan 2.5 mg | 3 | 1182 | 3.5 | 2.4 | 1.5 (0.74 to 2.8) | Not calculated | |
| Zolmitriptan 5 mg | 4 | 2592 | 3.8 | 1.8 | 2.2 (1.4 to 3.6) | 50 (31 to 140) | |
Data and analyses
Comparison 1. Zolmitriptan 1 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 4 | 1200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [2.00, 3.74] |
| 2 Headache relief at 2 h | 4 | 861 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.49, 2.07] |
| 3 Any adverse event | 3 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.01, 1.56] |
| 4 Use of rescue medication | 2 | 912 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.58, 0.74] |
| 5 Relief of associated symptoms at 2 h | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Nausea | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.04, 2.22] |
| 5.2 Photophobia | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.09, 2.39] |
| 5.3 Phonophobia | 2 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.40, 3.31] |
Comparison 2. Zolmitriptan 2.5 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 11 | 5825 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.02 [2.64, 3.45] |
| 2 Headache relief at 2 h | 11 | 4904 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.91, 2.22] |
| 3 24‐h sustained pain‐free | 2 | 984 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.51 [2.12, 5.79] |
| 4 24‐h sustained headache relief | 4 | 2059 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [2.37, 3.61] |
| 5 Any adverse event | 12 | 6055 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.57, 1.90] |
| 6 Specific adverse events | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Somnolence | 10 | 5645 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.17, 2.44] |
| 6.2 Asthenia | 10 | 4382 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [1.36, 3.28] |
| 6.3 Dizziness/vertigo | 11 | 6224 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.57, 2.91] |
| 6.4 Paresthesia | 10 | 5337 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.24 [1.55, 3.24] |
| 6.5 Vasodilation/warm feeling | 6 | 2784 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.18, 4.22] |
| 6.6 Nausea | 6 | 2390 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.26, 2.98] |
| 6.7 Tightness (not chest) | 6 | 2919 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.56 [3.58, 31.16] |
| 6.8 Heaviness (not chest) | 2 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.06 [1.15, 14.28] |
| 6.9 Dry mouth | 3 | 1182 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.74, 2.82] |
| 7 Use of rescue medication | 10 | 5020 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.51, 0.57] |
| 8 Relief of associated symptoms at 2 h | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Nausea | 7 | 2140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.37, 1.69] |
| 8.2 Photophobia | 7 | 2700 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.78, 2.23] |
| 8.3 Phonophobia | 6 | 2068 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.80, 2.30] |
2.6. Analysis.
Comparison 2 Zolmitriptan 2.5 mg versus placebo, Outcome 6 Specific adverse events.
Comparison 3. Zolmitriptan 5 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 11 | 9372 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [2.68, 3.24] |
| 1.1 Oral tablet | 8 | 4277 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [2.69, 3.68] |
| 1.2 Nasal spray | 3 | 5095 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [2.51, 3.18] |
| 2 Headache relief at 2 h | 11 | 7456 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.88, 2.10] |
| 2.1 Oral tablet | 8 | 4292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.78, 2.09] |
| 2.2 Nasal spray | 3 | 3164 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.91, 2.23] |
| 3 Headache relief at 1 h | 8 | 4994 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.64, 1.92] |
| 3.1 Oral tablet ‐ standard | 6 | 2310 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.54, 2.07] |
| 3.2 Nasal spray | 2 | 2684 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.61, 1.94] |
| 4 24‐h sustained pain‐free | 3 | 4991 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.65 [3.64, 5.94] |
| 4.1 Oral tablet | 1 | 693 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.68 [2.20, 6.16] |
| 4.2 Nasal spray | 2 | 4298 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.94 [3.74, 6.52] |
| 5 24‐h sustained headache relief | 7 | 7106 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.18 [2.87, 3.53] |
| 5.1 Oral tablet | 5 | 2827 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [2.02, 2.75] |
| 5.2 Nasal spray | 2 | 4279 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.95 [3.42, 4.55] |
| 6 Any adverse event | 10 | 9072 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [2.02, 2.34] |
| 6.1 Oral tablet | 7 | 4230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.76, 2.19] |
| 6.2 Nasal spray | 3 | 4842 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [2.13, 2.59] |
| 7 Specific adverse events | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Somnolence | 10 | 8622 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.24 [1.67, 3.01] |
| 7.2 Asthenia | 7 | 3122 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [1.50, 3.81] |
| 7.3 Dizziness/vertigo | 9 | 8506 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.61, 2.55] |
| 7.4 Paresthesia | 10 | 8622 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.49 [1.81, 3.43] |
| 7.5 Vasodilation/warm feeling | 6 | 3006 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [1.65, 5.20] |
| 7.6 Taste disturbance | 3 | 5500 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.48 [7.08, 12.68] |
| 7.7 Nausea | 7 | 7018 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.41, 2.70] |
| 7.8 Tightness (not chest) | 5 | 5610 | Risk Ratio (M‐H, Fixed, 95% CI) | 23.06 [7.01, 75.93] |
| 7.9 Heaviness (not chest) | 4 | 2445 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.73 [2.01, 11.15] |
| 7.10 Dry mouth | 4 | 2592 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.36, 3.61] |
| 8 Use of rescue medication ‐ subgroups | 8 | 7762 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.52, 0.57] |
| 8.1 Oral tablet | 5 | 2571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.55, 0.65] |
| 8.2 Nasal spray | 3 | 5191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.50, 0.56] |
| 9 Relief of associated symptoms at 2 h | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Nausea | 6 | 2056 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.36, 1.68] |
| 9.2 Photophobia | 6 | 2690 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.81, 2.29] |
| 9.3 Phonophobia | 6 | 2512 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.81, 2.30] |
| 10 Free of functional disability at 2 h | 2 | 3228 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.42 [2.11, 2.77] |
3.7. Analysis.
Comparison 3 Zolmitriptan 5 mg versus placebo, Outcome 7 Specific adverse events.
Comparison 4. Zolmitriptan 10 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 2 | 648 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.79 [4.19, 14.46] |
| 2 Headache relief at 2 h | 2 | 648 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [1.98, 3.12] |
| 3 Any adverse event | 2 | 736 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.83, 2.71] |
| 4 Specific adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Somnolence | 2 | 736 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [1.58, 6.76] |
| 4.2 Asthenia | 2 | 736 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.37 [1.71, 6.64] |
| 4.3 Dizziness/vertigo | 2 | 736 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [1.49, 4.88] |
| 4.4 Paresthesia | 2 | 736 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.49 [2.99, 36.74] |
| 4.5 Vasoldilation/warm feeling | 2 | 736 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.01 [1.44, 11.13] |
| 4.6 Nausea | 2 | 736 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.51 [1.20, 5.26] |
4.4. Analysis.
Comparison 4 Zolmitriptan 10 mg versus placebo, Outcome 4 Specific adverse events.
Comparison 5. Zolmiriptan 2.5 mg versus sumatriptan 50 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Headache relief at 2 h | 2 | 1609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.90, 1.03] |
| 2 Any adverse event | 2 | 1771 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.99, 1.32] |
| 3 Withdrawals due to adverse events | 2 | 1687 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.52, 1.75] |
5.3. Analysis.
Comparison 5 Zolmiriptan 2.5 mg versus sumatriptan 50 mg, Outcome 3 Withdrawals due to adverse events.
Comparison 6. Zolmitripan 5 mg versus sumatriptan 50 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Headache relief at 2 h | 2 | 1633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.05] |
| 2 Any adverse event | 2 | 1789 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.96, 1.28] |
| 3 Withdrawals due to adverse events | 2 | 1682 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.71, 2.21] |
6.3. Analysis.
Comparison 6 Zolmitripan 5 mg versus sumatriptan 50 mg, Outcome 3 Withdrawals due to adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
311CIL/0099 2000.
| Methods | Multicentre, randomised, double‐blinded, placebo‐controlled, parallel group. Part 1: single dose to treat single attack. [Part 2: participants able to tolerate study medication re‐randomised to active treatments for 3 further attacks] Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0, 0.5, 1, 2 h Second dose of trial medication available after 4 h if necessary |
|
| Participants | Aged 18‐65 years, meeting IHS criteria for migraine with or without aura. Onset < 50 years and ≥ 1 attack/month before start of trial No methysergide or methylergonovine within 2 weeks Excluded participants with previous unacceptable experience with a triptan, or with ischaemic heart or other vascular disease, or severe hepatic or renal disease N = 440 (treated attack and had efficacy data) M 71, F 369 (84%) Mean age not reported, presence of aura not reported Use of prophylactic medication not reported |
|
| Interventions | zolmitriptan 2.5 mg, n = 174 naratriptan 2.5 mg, n = 174 placebo, n = 92 |
|
| Outcomes | Headache relief (at 1 and 2 h) Adverse events Withdrawals |
|
| Notes | Data from on‐line clinical trial summary Oxford Qulaity Score: R1, DB1, W1. Total = 3 Baseline pain not equally distributed between groups ‐ correction made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | drop‐outs described |
| Study size | Unclear risk | group sizes 92 to 174 |
Charlesworth 2003.
| Methods | Multicentre, randomised, double‐blinded, double‐dummy, placebo‐controlled, parallel group. Single dose to treat each of 3 attacks Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0, 0.25, 0.5, 0.75, 1, 2, 4 and 24 h Approved rescue medications were allowed after the 4 h post dose assessment |
|
| Participants | Aged 18‐65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1 year history of migraine with onset < 50 years and an average of 1 to 6 attacks/month for the previous 2 months No MAOI, methysergide or methylergonovine within 2 weeks and no analgesics within 6 h. Excluded participants with uncontrolled hypertension, vascular disease, cardiac arrhythmias N = 1383 (1372 with moderate/severe intensity) M 234, F 1138 (83%) Mean age 41 years Without aura ˜62% |
|
| Interventions | zolmitriptan 0.5 mg nasal spray, n = 221 zolmitriptan 1 mg nasal spray, n = 236 zolmitriptan 2.5 mg nasal spray, n = 224 zolmitriptan 5 mg nasal spray, n = 235 zolmitriptan 2.5 mg oral, n = 230 placebo, n = 226 |
|
| Outcomes | Headache relief (at 1, 2 and 4 h) Pain‐free (at 2 and 4 h) 24‐h sustained headache relief Use of rescue medication Improvement in nausea, photophobia, phonophobia Resumption of normal activities Adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random numbers scheme" |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double dummy method" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | drop‐outs described |
| Study size | Low risk | all groups >200 |
Dahlof 1998.
| Methods | Multicentre, randomised, double‐blind, placebo controlled, parallel group. Single dose to treat single attack Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0, 1, 2, 24 h Rescue medications were allowed after 2 h. Ergot‐derivatives or sumatriptan were not allowed as rescue medication within 12 hours of taking study medication |
|
| Participants | Aged 18‐65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1 year history of migraine with onset < 40 years and an average of 1 to 6 attacks/month Prophylaxis allowed, excluding medications considered psychoactive or active at 5‐HT receptor sites. No sumatriptan or ergot within 72 h or analgesics within 24 h Excluded participants with cardiovascular disease, uncontrolled hypertension and severe renal or hepatic disease N = 951 (840 for efficacy) M 139, F 701 (83%) Mean age 40 years Without aura 69% |
|
| Interventions | zolmitriptan 5 mg, n = 213 zolmitriptan 10 mg, n = 214 zolmitriptan 15 mg, n = 215 zolmitriptan 20 mg, n = 210 placebo, n = 99 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) 24‐h sustained headache relief Use of rescue medication Improvement in nausea, photophobia and phonophobia Adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated numerical sequence" |
| Allocation concealment (selection bias) | Low risk | "assigning the next medication pack in the .... sequence" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "all tablets were identical in appearance" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | drop‐outs described, missing data ≤ 5% |
| Study size | Unclear risk | group sizes 99 to 215 |
Dib 2002.
| Methods | Multicentre, randomised, double‐blind, placebo controlled and active controlled , cross‐over design. Four consecutive attacks treated with single dose of each test medication Medication administered when migraine headache pain was of moderate or severe intensity. Minimum of 48 h between attacks Assessment times not specified Rescue medication permitted after 2 h |
|
| Participants | Aged 18‐65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1 year history of migraine with a frequency of 1 to 6 attacks/month for previous 3 months. Able to recognise early signs of attack No NSAID, triptan or prophylactic ergot (time not specified) Excluded participants who experienced regular vomiting N = 235 M 39, F 196 Mean age 38 years 6% to 11% with aura |
|
| Interventions | zolmitriptan 2.5 mg, n = 208 ketoprofen 75 mg, n = 214 ketoprofen 150 mg, n = 211 placebo, n = 205 |
|
| Outcomes | Headache relief (at 2 h) Pain‐free (at 2 h) Use of rescue medications Improvement in nausea, vomiting, photophobia and phonophobia Work capacity 2 h post‐treatment Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Low risk | remote allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "each treatment was enclosed in opaque soft gelatin capsules" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | drop‐outs described, missing data < 10% |
| Study size | Low risk | all groups >200 |
Dodick 2005.
| Methods | Multicentre, randomised, double‐blind, placebo controlled, parallel group. Single dose to treat up to 2 attacks Study medication to be taken within 15 minutes of pain becoming moderate or severe intensity. Headaches with moderate/severe intensity upon awakening were not to be treated Assessments made at 0, 0.25, 0.5, 1, 2 and 4 h Rescue medication permitted after 4 h |
|
| Participants | Aged 18‐ 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1 year history of migraine, with onset <50 years and 2 to 6 attacks/month. No prophylactics or non‐stable dose of SSRI within 2 months. No MAOI within 2 weeks. No analgesics, ergots or triptans within 24 h. Furthermore, no naratriptan within 36 h and no frovatriptan within 5 days. Excluded participants who had hypertension or any medical or physical condition that might put the patient at risk with exposure to zolmitriptan. N = 1869 (1868 for efficacy) M 248, F 1620 Mean age 41 years Without aura 56% |
|
| Interventions | zolmitriptan 5 mg nasal spray, n = 935 (1745 attacks) placebo, n = 934 (1718 attacks) |
|
| Outcomes | Headache relief (at 1, 2 and 4 h) Pain free (at 2 and 4 h) 24‐h sustained headache relief 24‐h sustained pain‐free Use of rescue medication Improvement in nausea, vomiting, photophobia, phonophobia Return to normal activities Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "matching placebo" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | drop‐outs described |
| Study size | Low risk | both groups >200 |
Dowson 2002.
| Methods | Multicentre, randomised, double‐blind, placebo controlled, parallel group. Single dose to treat single attack Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0, 0.5, 1, 2 and 4 h A 2nd dose of study medication or rescue medication was allowed after 2 h |
|
| Participants | Aged 18‐ 65 years, meeting IHS criteria for migraine (1988) with or without aura. Patients required to have an age of migraine onset of <50 years and at least1 attack/month for the previous 3 months
No MAOI, methysergide, methylergonovine within 2 weeks, no triptans or ergot within 24 h, no opiates within 12 h and no analgesics within 6 h Excluded participants who had uncontrolled hypertension or cardiovascular disease N = 471 F 87% Mean age 42 years With aura 23 % |
|
| Interventions | zolmitriptan 2.5 mg ODT, n = 231 placebo, n = 239 |
|
| Outcomes | Headache relief (at 2 and 4 h) Pain‐free (at 2 and 4 h) Use of rescue medication Improvement in nausea, photophobia and phonophobia Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Low risk | "sealed envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "matched for taste, size and shape" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | drop‐outs described |
| Study size | Low risk | both groups >200 |
Gallagher 2000.
| Methods | Multicentre, randomised, double‐blind, active controlled, parallel group. Single dose to treat each of up to six attacks. Second identical dose was available for recurrence 4 to 24 h Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0, 1, 2, 4 and 24 h Rescue medication permitted after 2 h (but no acute antimigraine treatments) |
|
| Participants | Aged 18‐ 65 years, meeting IHS criteria for migraine (1988) with or without aura. Patients required to have a history of attacks for at least 1 year No MAOI, methysergide, methylergonovine, (dex)fenfluramine Excluded participants with hypertension or cardiovascular problems N = 1338 (1212 treated 2 attacks ‐ 6187 attacks in total) F 87% Mean age 40 years Without aura ˜57% |
|
| Interventions | zolmitriptan 2.5 mg, n = 327 (295 for efficacy) zolmitriptan 5 mg, n = 337 (305 for efficacy) sumatriptan 25 mg, n = 336 (306 for efficacy) sumatriptan 50 mg, n = 338 (306 for efficacy) |
|
| Outcomes | Headache relief (1, 2 and 4 h) 24‐h sustained headache relief Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT population comprised participants treating ≥ 2 attacks |
| Study size | Low risk | all groups >200 |
Gawel 2005.
| Methods | Multicentre, randomised, double‐blind, placebo controlled, parallel group. Single dose to treat single attack, at any time after onset (pain mild/moderate/severe) Assessments at 0, 10, 30 min, and 1, 2, 24 h 2nd dose or rescue medication (not triptan or ergot) permitted after 2 h |
|
| Participants | Aged 18‐65 years, meeting IHS criteria for migraine (1988) with or without aura Participants had history of migraine for at least a year, with at least 1 attack/month for the previous 3 months No MAOI, methysergide, methylergonovine within 2 weeks and no triptans, ergot within 24 h, opiates, analgesics within 12 h Excluded participants with a history, symptoms or significant risk factors for CV disease, uncontrolled hypertension and severe hepatic impairment N = 915 (912 for efficacy) M 114, F 798 (87%) Mean age 41 years Only 73 participants (8%) treated when pain mild |
|
| Interventions | zolmitriptan 5 mg nasal spray, n = 464 placebo, n = 451 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) 24‐h sustained pain‐free Use of rescue medication Impact of normal activities Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "placebo nasal spray device exactly matched zolmitriptan device in terms of appearance, weight, drug volume, and labelling" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | drop‐outs described |
| Study size | Low risk | both groups >200 |
Geraud 2000.
| Methods | Multicentre, randomised, double‐blind (double‐dummy), placebo controlled and active controlled, parallel group. Single dose to treat single attack Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0, 1, 2, 4, 24 h Rescue medication permitted after 2 h if symptoms persisted (no ergot for 12 h, no sumatriptan) |
|
| Participants | Aged 18‐ 65 years, meeting IHS criteria for migraine (1988) with or without aura. Patients required to have a history of migraine for at least 1 year, with an onset at < 50 years and with 1 to 6 attacks/month in the previous 6 months. Triptan naïve participants only Prophyalxis with beta‐blockers, calcium channel blockers (except flunarizine), clonidine and valproic acid was allowed. No psychoactive drugs or drugs with a clinically important action at 5‐HT receptor were permitted in the previous 4 weeks Excluded participants with cardiovascular disease, uncontrolled hypertension and severe renal or hepatic disease N = 1058 M 174, F 884 (84%) Mean age 38 years Without aura ˜73% |
|
| Interventions | zolmitriptan 5 mg, n = 498 sumatriptan 100 mg, n = 504 placebo, n = 56 |
|
| Outcomes | Headache relief (at 1, 2 and 4 h) Pain‐free (at 1, 2 and 4 h) 24‐h sustained headache relief Use of rescue medication Relief from nausea, photophobia, phonophobia Activity impairment Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double dummy technique" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | drop‐outs described, missing data 2% |
| Study size | Unclear risk | group sizes 56 (placebo) to 504 |
Geraud 2002.
| Methods | Multicentre, randomised, double‐blind, placebo controlled, parallel group. Single dose to treat single attack Participants instructed to treat attack when pain intensity is greater than moderate, however a few patients that had mild baseline pain were included Assessments at 0, 1, 2, 4 and 24 h Rescue medication time not specified. 2nd dose available 2‐24 h for persistence or recurrence. Rescue medication permitted 2 h after 2nd dose if still inadequate relief |
|
| Participants | Aged 18‐ 65 years, meeting IHS criteria for migraine (1988) with or without aura Participants required to have a history of symptoms for at least 1 year, with an age of onset < 50 yrs and 1‐6 attacks/month of moderate/severe intensity for 3 months prior to inclusion in study. Excluded patients with non‐migraine headache on > 10 days/month for the preceding 6 months No mention of prophylaxis Excluded patients with basilar, ophthalmoplegic or hemiplegic migraine N = 666 (patients who received at least one dose of trial medication) M 100, F 566 Mean age 41 years |
|
| Interventions | zolmitriptan 2.5 mg, n = 326 acetylsalicylic acid 900 mg + metoclopramide 10 mg, n = 340 |
|
| Outcomes | Headache relief (at 2h) Pain‐free (at 2h) Use of rescue medication Relief from nausea, vomiting, photophobia and phonophobia Adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double dummy placebo" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | drop‐outs described, missing data < 5% |
| Study size | Low risk | both groups >200 |
Goadsby 2007b.
| Methods | Multicentre, randomised, double‐blind, placebo controlled, parallel group. Single dose to treat single attack but a second dose could be taken if symptoms were alleviated but recurred within 24 h Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0, 0.5, 1, 1.5, 2 and 24 h Rescue medication permitted (other than triptan or ergots) but time not specified |
|
| Participants | Aged 18‐ 65 years, meeting IHS criteria for migraine (2004) with or without aura Participants required to have a history of migraine for at least 1 year, with an onset < 50 years and 2 to 6 attacks/month in the previous 2 months No details about washout or prophylactic medication Excluded participants with cardiovascular disease, uncontrolled hypertension and moderate/severe renal or hepatic disease N = 1062 M 160, F 902 (85%) Mean age 40 years (range 18 to 72) 122 major protocol violations: 11 participants had mild baseline pain |
|
| Interventions | zolmitriptan 2.5 mg, n = 530 almotriptan 12.5 mg, n = 532 |
|
| Outcomes | Headache relief (at 2 h) Pain‐free (at 2 h) 24‐h sustained headache relief 24‐h sustained pain‐free Use of rescue medication Functional impairment Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "both agents were encapsulated to ensure treatment blinding" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | drop‐outs described |
| Study size | Low risk | both groups >200 |
Gruffyd‐Jones 2001.
| Methods | Multicentre, randomised, double‐blind, active controlled, parallel group. Single dose to treat each of up to six attacks. 2nd identical dose available for recurrence 2 to 24 h Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0, 1, 2, 4 and 24 h Rescue medication permitted after 2 h (but no ergotamine within 6 h) |
|
| Participants | Aged 18‐ 65 years, meeting IHS criteria of migraine (2004) with or without aura Participants required to have a history of migraine for at least 1 year, with onset < 50 years and 2 to 6 attacks/month in the previous 2 months No MAOI, methysergide or methylergonovine within 2 weeks. No ergot derivative, sumatriptan or opiate within 24 h, other analgesic within 6 h. Other medications (including prophylaxis?) at discretion of investigator Excluded participants with cardiovascular disease, uncontrolled hypertension and moderate or severe renal or hepatic disease N = 1666 (1522 treated ≥ 2 attacks) M 223, F 1299 (85%) Mean age 42 years Without aura 57% |
|
| Interventions | zolmitriptan 2.5 mg, n = 555 (500 treated 2 attacks (ITT), total attacks 2671) zolmitriptan 5 mg, n = 551 (514 treated 2 attacks (ITT), total attacks 2744) sumatriptan 50 mg, n = 560 (508 treated 2 attacks (ITT), total attacks 2693 ) |
|
| Outcomes | Headache relief (at 1, 2 and 4 h) 24‐h sustained headache relief 24‐h sustained pain‐free Relief from nausea, photophobia, phonophobia Adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random numbers scheme" |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double dummy method" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT population comprised participants treating ≥ 2 attacks |
| Study size | Low risk | all groups >200 |
Ho 2008.
| Methods | Multicentre, randomised, double‐blind, parallel controlled and active controlled, parallel group. Single dose to treat single attack, when pain ≥moderate. 2nd dose (blinded) or rescue medication was permitted if there had been no response at 2 h or if headache returned within 48 h. Blinded 2nd dose for zolmitriptan and placebo participants was always placebo, for telcagepant either telcagepant or placebo Assessments made at 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8 and 24 h |
|
| Participants | Aged over 18 years, meeting IHS criteria for migraine (2004) with or without aura Participants were required to have good general health and a history of migraine for at least 1 year, with 1 to 8 attacks (of moderate/severe) per month Patients taking prophylaxis were allowed to enter the study provided that their prescribed daily dose had not changed during the 3 months before screening; ˜55% of included participants were using prophylaxis No potent CYP3A4 inhibitors or inducers, SNRIs, SSRIs, MAO inhibitors or propranolol within 1 month Excluded participants with cardiovascular disease or uncontrolled hypertension N = 1380 F 85% Mean age 42 years |
|
| Interventions | zolmitriptan 5 mg, n = 345 telcagepant 150 mg, n = 333 telcagepant 300 mg, n = 354 placebo, n = 348 |
|
| Outcomes | Headache relief (at 1, 2, 4 h) Pain‐free (at 1, 2, 4 h) 24‐h sustained headache relief 24‐h sustained pain‐free Use of rescue medications Relief from nausea, photophobia, phonophobia Adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomised schedule" |
| Allocation concealment (selection bias) | Low risk | interactive voice response for remote allocation, with numbered containers |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "matching placebo" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | drop‐outs described, missing data < 10% |
| Study size | Low risk | all groups >200 |
Klapper 2004.
| Methods | Multicentre, randomised, double‐blind, placebo controlled, parallel group. Single dose to treat single attack, when pain mild and within 4 h of onset. 2nd dose or rescue medication allowed after 2 h for persistent or recurrent headache Assessments at 0, 0.5, 1, 1.5, 2 and 12 h |
|
| Participants | Aged 18‐ 65 years, meeting IHS criteria for migraine (1988) with or without aura with an onset < 50 years. Participants were required to suffer from 1 attack/month for previous 3 months and the migraines experienced had to be initially mild but progress to moderate/severe intensity. Participants also had to be able to distinguish from other types of headache and have moderate/severe disability (MIDAS) No MAOI, methysergide, methylergonovine (time not specified) Excluded participants with uncontrolled hypertension or cardiovascular disease N = 280 M 39, F 241 Mean age 42 years Without aura 59% |
|
| Interventions | zolmitriptan 2.5 mg, n = 138 placebo, n = 142 |
|
| Outcomes | Pain‐free (at 1 h) Use of further medication/ rescue medication Ability to perform normal activities Reduction in impact on usual activities Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "matching placebo" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | drop‐outs described |
| Study size | Unclear risk | group sizes 138 and 142 |
Loder 2005.
| Methods | Multicentre, randomised, double‐blind, placebo controlled, parallel group. Single dose to treat single attack, as soon as possible (pain mild/moderate/severe) Assessments at 0, 0.5, 1, 1.5, 2 and 24 h 2nd dose or rescue med permitted after 2 h |
|
| Participants | Aged 18‐ 65 years, meeting IHS criteria for migraine (1988) with or without aura Participants were required to have a history of migraine of at least 1 year, with an age of onset of < 50 years and at least 2 attacks/month for the previous 3 months No MAOI, propranolol or cimetidine within 2 weeks Excluded participants with a history or symptoms of IHD or other vascular disease, uncontrolled hypertension or renal or liver impairment N = 566 (565 for efficacy) M 83, F 482 (85%) Mean age 41 years Without aura 72% ˜35% treated when pain mild |
|
| Interventions | zolmitriptan 2.5 mg ODT, n = 282 placebo, n = 284 |
|
| Outcomes | Pain‐free (at 1 and 2 h) 24‐h sustained pain‐free Use of further medication or rescue medication Relief from nausea, photophobia and phonophobia Return to normal activities Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "matching placebo" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | drop‐outs described |
| Study size | Low risk | both groups >200 |
Pascual 2000.
| Methods | Multicentre, randomised, double‐blind, placebo controlled and active controlled. Single dose to treat single attack Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0, 0.5, 1, 1.5, 2, 3, 4 h and 24 h Rescue medication allowed after 2 h |
|
| Participants | Meeting IHS criteria for migraine (1988) with or without aura. Participants required to have a history of migraine for ar least six months and usually experience 1 to 8 attacks/month No MAOI or methysergide within 2 weeks, propranolol within 3 days, triptan, ergot or opiate within 24 h and any other analgesic or antiemetic within 6 h. Other stable prophylaxis permitted Excluded participants with cerebrovascular or cardiovascular disease N = 766 (727 for efficacy) F 83% Mean age 39 years With aura 12% |
|
| Interventions | zolmitriptan 2.5 mg, n = 304 (289 for efficacy) rizatriptan 10 mg, n = 308 (292 for efficacy) placebo, n = 154 (146 for efficacy) |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 2 h) Use of rescue medication Relief from nausea, vomiting, photophobia and phonophobia Functional disability Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | drop‐outs described, missing data 5% |
| Study size | Unclear risk | group sizes 154 (placebo) to 308 |
Rapoport 1997.
| Methods | Multicentre, randomised, double‐blind, placebo controlled, parallel group. Single dose to treat single attack Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0, 0.5, 1, 2, 4 and 24 h 2nd dose or rescue medication permitted after 4 h (but no ergot or sumatriptan for 12 h) |
|
| Participants | Aged 12‐ 65 years, meeting IHS criteria for migraine (1988) with or without aura Participants were required to have a history of migraine for at least a year, with onset <50 years and 1 to 6 attacks/month for the previous 6 months No sumatriptan or ergot within 48 h and analgesics/NSAIDs within 6 h. Prophylaxis was allowed Excluded participants with hypertension or any medical or physical condition that might put the patient at risk with exposure to zolmitriptan N = 1144 (999 for efficacy) M 123, F 876 (˜88%) Mean age 41 years (all groups included at least 1 individual aged 12 or 13) |
|
| Interventions | zolmitriptan 1 mg, n = 125 zolmitriptan 2.5 mg, n = 260 zolmitriptan 5 mg, n = 245 zolmitriptan 10 mg, n = 248 placebo, n = 121 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 2 h) 24‐h sustained headache relief Use of rescue medication Relief from nausea, photophobia and phonophobia Absenteeism from work Impairment of normal activities Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Low risk | sequentially numbered medication packets |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "matching oral placebo or zolmitriptan" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | PP analysis reported. "Results from the all‐treated analysis did not differ ..." |
| Study size | Unclear risk | group sizes 121 (placebo) to 260 |
Ryan 2000.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel group. Single dose of each of three treatments for initial treatment of each of three attacks. Second (R, DB) dose at 4 h to treat recurrence if necessary, or at 8 h to prevent recurrence if rescue medication not used Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0, 0.5, 0.75, 1, 2 and 4 h Rescue medication permitted after 2 h, but asked to wait 4 h if possible |
|
| Participants | Aged 18‐ 65 years, meeting IHS criteria for migraine (1988) with or without aura Pariticpants were required to have a history of migraine for at least 1 year, with onset <50 years and 2 to 6 attacks/month in the previous 2 months Minimum 48 h since last migraine and no sumatriptan or ergot within 24 h. Prophylactic medication and other chronic non‐migraine medications permitted if stable for 2 months Excluded participants with hypertension or any medical or physical condition that might put the patient at risk with exposure to zolmitriptan N = 924 (734 treated 3 attacks) F 86% Mean age 40 years Without aura 60% |
|
| Interventions | zolmitriptan 2.5 mg, n = 546 (487 for efficacy) zolmitriptan 5 mg, n = 542 (482 for efficacy) placebo, n = 282 + 285 (247 + 252 for efficacy) |
|
| Outcomes | Headache relief (at 1, 2 and 4 h) Pain‐free (at 1, 2 and 4 h) Use of rescue medication Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | tablets were "identical in appearance" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT population comprised participants treating 3 attacks |
| Study size | Low risk | all groups >200 |
Sakai 2002.
| Methods | Multicentre, randomised, double‐blind, placebo controlled, parallel group. Single dose to treat single attack Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0, 0.5, 1, 2 and 4 h Rescue medication permitted after 4 h |
|
| Participants | Aged 18‐ 64 years, meeting IHS criteria for migraine (1988) with or without aura Participants required to have a history of migraine for at least 1 year, with onset < 50 years and 1 to 6 attacks/month in the previous 3 months No ergotamine within 48 h and no analgesics, steroids, antidepressants, antiemetics, anticonvulsants, sedatives within 8 h Excluded participants with cardiovascular disease, uncontrolled hypertension and those with severe renal or hepatic disease N = 229 (202 in analysis) M 52, F 150 (74%) Mean age 38 years Without aura 64% |
|
| Interventions | zolmitriptan 1 mg, n = 52 (47) zolmitriptan 2.5 mg, n = 61 (54) zolmitriptan 5 mg, n = 57 (52) placebo, n = 59 (49) |
|
| Outcomes | Headache relief (at 1, 2 and 4 h) Pain‐free (at 2 h) 24‐h sustained headache relief Use of rescue medication Relief from nausea, vomiting, photophobia and phonophobia Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | some outcomes (PF2, HR1, SHR24) reported only for PP population |
| Study size | Unclear risk | group sizes 52 to 61 |
Solomon 1997.
| Methods | Multicentre, randomised, double‐blind, placebo controlled, parallel group. Single dose to treat single attack Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0, 1, 2, 4 and 24 h Rescue med time not specified |
|
| Participants | Aged 12 ‐65 years, meeting IHS criteria for migraine (1988) with or without aura Participants were required to have a history of migraine for a minimum of 1 year, with onset <50 years and 1 to 6 attacks/month for the previous 6 months No MAOI, no NSAID, analgesic, sedative, antiemetic within 6 h and no sumatriptan or ergotamines within 48 h Excluded participants with hypertension or any medical or physical condition that might put the patient at risk with exposure to zolmitriptan N = 270 M 39, F 231 (86%) Mean age 40 years Without aura ˜68% |
|
| Interventions | zolmitriptan 2.5 mg, n = 178 placebo, n = 92 |
|
| Outcomes | Headache relief (1, 2 and 4 h) Pain free (at 1, 2 and 4 h) 24‐h sustained headache relief Use of rescue medication Relief from nausea, photophobia and phonophobia Time absent from work Level of activity impairment Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Low risk | "sequentially numbered medication packet" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | PP analysis reported for efficacy. "Results did not differ from those of the all‐treated group for [HR2]" |
| Study size | Unclear risk | groups sizes 92 (placebo) and 178 |
Spierings 2004.
| Methods | Multicentre, randomised, double‐blind, placebo controlled, parallel group. Single dose to treat each of 2 attacks Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0, 0.5, 1, 2 and 24 h 2nd dose or rescue med after 2 h if necessary |
|
| Participants | Aged 18 ‐ 65 years, meeting IHS criteria for migraine (1988) with or without aura Participants were required to have a history of migraine of at least 1 year, with on age of onset of , 50 years and an average of 2 attacks/month No MAOI or initiation of SSRI within 2 weeks and no concomitant treatment with propranolol or cimetidine Excluded participants with a history or symptoms of IHD or other vascular disease or uncontrolled hypertension N = 671 (670 for efficacy) M 90, F 580 Mean age 42 years Without aura 65% |
|
| Interventions | zolmitriptan 5 mg ODT, n = 329 placebo, n = 341 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐ free (at 1 and 2 h) 24‐h sustained headache relief Use of rescue medication Relief from nausea, photophobia and phonophobia Return to normal activities Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "matching placebo" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | drop‐outs described |
| Study size | Low risk | both groups >200 |
Steiner 2003.
| Methods | Multicentre, randomised, double‐blind (double‐dummy), placebo controlled and active controlled, parallel group. Single dose to treat single attack. 2nd dose available after 4 h for recurrence Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0, 1, 1.5, 2, 4 and 24 h Rescue medication permitted after 2 h |
|
| Participants | Aged 18 ‐ 65 years, meeting IHS criteria for migraine (1988) with or without aura (IHS 1988). Participants were required to experience attacks at least once every 6 weeks No MAOI or CYP3A4 inhibitors within 2 weeks, no analgesic or antiemetic for that attack and no triptan, ergotamine, dihydroergotamine within 48 h Excluded participants if their migraines were consistently resistant to all treatments or if they had any clinically significant medical illness/lab abnormalities, especially those indicative of CHD, HF and hypertension N = 1337 (1312 for efficacy analysis) F 85% Mean age 40 years Without aura ˜73% |
|
| Interventions | zolmitriptan 2.5 mg, n = 405 eletriptan 40 mg, n = 392 eletriptan 80 mg, n = 396 placebo, n = 144 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) 24‐h sustained headache relief 24‐h sustained pain‐free Use of rescue medication Relief from nausea, photophobia and phonophobia Functional recovery (at 1 and 2 h) Adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W0. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated list" |
| Allocation concealment (selection bias) | Low risk | remote allocation. Centre "allocated prenumbered treatments to consecutive patients by next‐number on this list" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | double‐dummy design: matched tablets for eletriptan, identical capsules for zolmitriptan |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | drop‐outs described |
| Study size | Unclear risk | group sizes 144 (placebo) to 405 |
Tuchman 2006.
| Methods | Multicentre, randomised, double‐blind, placebo controlled, parallel group. Single dose to treat each of up to 6 attacks with at least 24 h between treated attacks Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0, 1, 2, 4 and 24 h |
|
| Participants | Aged 18 years and over, meeting IHS criteria for menstrual migraine (1988) with or without aura. Participants were required to have had at least 3 menstrual migraine headaches of moderate/severe intensity within the previous 3 months No MAOI within 2 weeks or SSRI if dose not stabilised. Study medication should not be used for attacks already treated with other acute medication (NSAIDs, paracetamol) Excluded participants with uncontrolled hypertension or cardiovascular disease N = 336 (334 for efficacy) All F Mean age 38 years Without aura ˜72% |
|
| Interventions | zolmitriptan 2.5 mg, n = 174 placebo, n = 160 |
|
| Outcomes | Headache relief (at 1, 2 and 4 h) Pain‐free (at 1, 2 and 4 h) Use of rescue medication Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | drop‐outs described |
| Study size | Unclear risk | group sizes 160 and 174 |
Tullo 2010.
| Methods | Multicentre, randomised, double‐blind, cross‐over. Single dose to treat each of 3 attacks, as soon as possible after onset, in a maximum of 3 months for each treatment period 2nd dose allowed after 2 h if insufficient relief obtained Assessments 2 and 48 h Rescue medication (not triptan, ergot) allowed 1 h after 2nd dose |
|
| Participants | Aged 18 ‐ 65 years, meeting IHS criteria for migraine with or without aura. Participants were required to have at least 1 attack/month for the previous 6 months No MAOI Excluded participants with uncontrolled hypertension or cardiac, vascular, liver or renal impairment. Also excluded those with a history of previous inadequate response to ≥2 triptans N = 121 (107 for efficacy) M 22, F 85 (79%) Mean age 38 years With aura 15% |
|
| Interventions | zolmitriptan 2.5 mg, n = 107 frovatriptan 2.5 mg, n = 107 |
|
| Outcomes | Headache relief at 2 h Pain‐free at 2 h 24‐h sustained pain‐free Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis, but denominators unclear |
| Study size | Unclear risk | Both groups 107 |
Visser 1996.
| Methods | Single centre, inpatient (4 h), R, DB, PC, dose‐finding, parallel group. Single dose to treat single attack. Optional 2nd dose available after 2 h Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0. 0.5, 1, 2 and 24 h (additional assessments for patients taking 2nd dose) Rescue medication permitted after 3 h (for single dose patients) |
|
| Participants | Aged 18 ‐ 55 years, meeting IHS criteria for migraine (1988) with or without aura Participants were required to have a history of migraine of at least 1 year, with an age of onset < 40 years with an average of 1 to 6 attacks/month No prophylactics within 1 month Excluded participants who experienced regular vomiting or had a personal or family history of CAD, peripheral vascular disease, hypertension or renal or liver disease N = 84 M 17, F 67 Mean age 43 years Without aura 63% |
|
| Interventions | zolmitriptan 1 mg, n = 22 zolmitriptan 5 mg, n = 21 zolmitriptan 25 mg, n = 21 placebo, n = 20 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 2 h) Use of rescue medication Relief from nausea, vomiting, photophobia and phonophobia Adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized by computer" |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "indistinguishable tablets" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | drop‐outs described |
| Study size | High risk | group sizes 20 to 22 |
ITT ‐ intention to treat; PP per protocol
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dowson 2003 | No usable data |
| Loder 2004 | Variable dose |
| Mauskop 1999 | No usable data ‐ 2nd dose to treat persistent migraine |
| Tepper 1999 | Same study as Mauskop 1999 |
Characteristics of studies awaiting assessment [ordered by study ID]
CTRI/2009/091/000196.
| Methods | Multicentre, randomised, double‐blind, placebo controlled, cross‐over study. Single dose of medication administered within 15 minutes of migraine headache pain being of moderate or severe intensity |
| Participants | Aged 18 ‐ 65 years, meeting IHS criteria for migraine with or without aura. Participants were required to have a history of migraine of at least 1 year; average of 9 attacks in previous 3 months N = 126 |
| Interventions | Zolmitriptan nasal spray 5 mg Placebo |
| Outcomes | Headache response at 15, 30, 60 minutes and 2, 4 hours Sustained headache response at 24 hours Time to onset of response Pain‐free at "various time points" Sustained pain‐free at 24 hours Return to normal activities Resolution of headache symptoms Use of rescue medication Adverse events |
| Notes | Computer generated randomisation code, pre‐numbered or coded identical containers, participant, investigator and outcome assessor blinded Sponsor: Cipla Ltd "The results of the present study conducted in India are similar to the published studies" |
Differences between protocol and review
After discussion with headache specialists and editorial staff, and in line with Cochrane recommendations, we decided to limit our outcomes for acute migraine headache reviews in order to focus attention on the most important outcomes and to make them more readable for both clinicians and patients. For the majority of interventions we will now include two‐hour pain‐free and headache relief as primary outcomes, and 24‐hour sustained pain‐free, sustained headache relief, and adverse events as secondary outcomes. Pain‐free and headache relief outcomes at earlier time points will be included in special circumstances, if reported and relevant (for example if a 'fast acting' formulation is investigated). We have moved results for use of rescue medication (added post protocol) and relief of headache‐associated symptoms and functional disability to Appendix 7.
We have expanded the risk of bias table; this review uses the new criteria for analysis.
In some studies participants treated more than one attack with the same medication. We have used first attack data when possible, but when it was not reported we have used data from combined attacks rather than omitting the results altogether. We have noted when combined attack data were used.
Contributions of authors
SD and RAM wrote the protocol.
For the full review, SB and SD carried out searches for studies and data extraction. All authors were involved with analyses and writing. RAM acted as arbitrator.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support
External sources
-
Lifting the Burden: the Global Campaign against Headache, UK.
Funding for administrative costs associated with editorial review of the protocol
-
International Headache Society, UK.
Funding for administrative costs associated with editorial and peer review of the full review
Declarations of interest
RAM and SD have received research support from charities, government and industry sources at various times, none of which were related to this review. RAM has consulted for various pharmaceutical companies (including AstraZeneca, the manufacturers of zolmitriptan) and has received lecture fees from other pharmaceutical companies related to analgesics and other healthcare interventions, none of which were related to this review. SB has no interests to declare. AstraZeneca were not in any way involved in carrying out this review.
Stable (no update expected for reasons given in 'What's new'), comment added to review
References
References to studies included in this review
311CIL/0099 2000 {published data only}
- Astra Zeneca. A multicentre, randomised, double‐blind trial to compare the efficacy and safety of ZOMIG 2.5 mg, NARAMIG 2.5 mg and placebo in the acute treatment of adult patients with migraine. www.astrazenecaclinicaltrials.com 2005.
Charlesworth 2003 {published data only}
- Charlesworth BR, Dowson AJ, Purdy A, Becker WJ, Boes‐Hansen S, Färkkilä M. Speed of onset and efficacy of zolmitriptan nasal spray in the acute treatment of migraine: a randomised, double‐blind, placebo‐controlled, dose‐ranging study versus zolmitriptan tablet. CNS Drugs 2003;17(9):653‐67. [DOI] [PubMed] [Google Scholar]
Dahlof 1998 {published data only}
- Dahlöf C, Diener HC, Goadsby PJ, Massiou H, Olesen J, Schoenen J, et al. Zolmitriptan, a 5‐HT1B/1D receptor agonist for the acute oral treatment of migraine: a multicentre, dose‐range finding study. Eurean Journal of Neurology 1998;5(6):535‐43. [DOI] [PubMed] [Google Scholar]
Dib 2002 {published data only}
- Dib M, Massiou H, Weber M, Henry P, Garcia‐Acosta S, Bousser MG, Bi‐Profenid Migraine Study Group. Efficacy of oral ketoprofen in acute migraine: a double‐blind randomized clinical trial. Neurology 2002;58(11):1660‐5. [DOI] [PubMed] [Google Scholar]
Dodick 2005 {published data only}
- Dodick D, Brandes J, Elkind A, Mathew N, Rodichok L. Speed of onset, efficacy and tolerability of zolmitriptan nasal spray in the acute treatment of migraine: a randomised, double‐blind, placebo‐controlled study. CNS Drugs 2005;19(2):125‐36. [DOI] [PubMed] [Google Scholar]
Dowson 2002 {published data only}
- Dowson AJ, MacGregor EA, Purdy RA, Becker WJ, Green J, Levy SL. Zolmitriptan orally disintegrating tablet is effective in the acute treatment of migraine. Cephalalgia 2002;22(2):101‐6. [DOI] [PubMed] [Google Scholar]
Gallagher 2000 {published data only}
- Gallagher RM, Dennish G, Spierings EL, Chitra R. A comparative trial of zolmitriptan and sumatriptan for the acute oral treatment of migraine. Headache 2000;45(1):7‐16. [DOI] [PubMed] [Google Scholar]
Gawel 2005 {published data only}
- Gawel M, Aschoff J, May A, Charlesworth BR, REALIZE Study Team. Zolmitriptan 5 mg nasal spray: efficacy and onset of action in the acute treatment of migraine‐‐results from phase 1 of the REALIZE Study. Headache 2005;45(1):7‐16. [DOI] [PubMed] [Google Scholar]
Geraud 2000 {published data only}
- Geraud G, Olesen J, Pfaffenrath V, Tfelt‐Hansen P, Zupping R, Diener HC, et al. Comparison of the efficacy of zolmitriptan and sumatriptan: issues in migraine trial design. Cephalalgia 2000;20(1):30‐8. [DOI] [PubMed] [Google Scholar]
Geraud 2002 {published data only}
- Geraud G, Compagnon A, Rossi A, COZAM Study Group. Zolmitriptan versus a combination of acetylsalicylic acid and metoclopramide in the acute oral treatment of migraine: a double‐blind, randomised, three‐attack study. European Neurology 2002;47(2):88‐98. [DOI] [PubMed] [Google Scholar]
Goadsby 2007b {published data only}
- Allais G, Acuto G, Cabarrocas X, Esbri R, Benedetto C, Bussone G. Efficacy and tolerability of almotriptan versus zolmitriptan for the acute treatment of menstrual migraine. Neurological Sciences 2006;27 Suppl 2:S193‐7. [DOI: 10.1007/s10072-006-0600-4] [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Massiou H, Pascual J, Diener HC, Dahlöf CG, Mateos V. Almotriptan and zolmitriptan in the acute treatment of migraine. Acta Neurologica Scandinavica 2007;115(1):34‐40. [DOI] [PubMed] [Google Scholar]
Gruffyd‐Jones 2001 {published data only}
- Gruffyd‐Jones K, Kies B, Middleton A, Mulder LJ, Røsjø Ø, Millson DS. Zolmitriptan versus sumatriptan for the acute oral treatment of migraine: a randomized, double‐blind, international study. European Journal of Neurology 2001;8(3):237‐45. [DOI] [PubMed] [Google Scholar]
Ho 2008 {published data only}
- Dodick DW, Kost J, Assaid C, Lines C, Ho TW. Sustained pain freedom and no adverse events as an endpoint in clinical trials of acute migraine treatments: Application to patient‐level data from a trial of the CGRP receptor antagonist, telcagepant, and zolmitriptan. Cephalalgia 2011;31(3):296‐300. [DOI: 10.1177/0333102410385585] [DOI] [PubMed] [Google Scholar]
- Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, et al. Efficacy and tolerability of MK‐0974 (telcagepant), a new oral antagonist of calcitonin gene‐related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo‐controlled, parallel‐treatment trial. Lancet 2008;372(9656):2115‐23. [DOI] [PubMed] [Google Scholar]
Klapper 2004 {published data only}
- Klapper J, Lucas C, Røsjø Ø, Charlesworth B, ZODIAC study group. Benefits of treating highly disabled migraine patients with zolmitriptan while pain is mild. Cephalalgia 2004;24(11):918‐24. [DOI] [PubMed] [Google Scholar]
Loder 2005 {published data only}
- Loder E, Freitag FG, Adelman J, Pearlmand S, Abu‐Shakra S. Pain‐free rates with zolmitriptan 2.5 mg ODT in the acute treatment of migraine: results of a large double‐blind placebo‐ controlled trial. Current Medical Research and Opinion 2005;21(3):381‐9. [DOI] [PubMed] [Google Scholar]
Pascual 2000 {published data only}
- Pascual J, Vega P, Diener HC, Allen C, Vrijens F, Patel K. Comparison of rizatriptan 10 mg vs. zolmitriptan 2.5 mg in the acute treatment of migraine. Rizatriptan‐Zolmitriptan Study Group. Cephalalgia 2000;20(5):455‐61. [DOI] [PubMed] [Google Scholar]
Rapoport 1997 {published data only}
- Rapoport AM, Ramadan NM, Adelman JU, Mathew NT, Elkind AH, Kudrow DB, et al. Optimizing the dose of zolmitriptan (Zomig, 311C90) for the acute treatment of migraine. A multicenter, double‐blind, placebo‐controlled, dose range‐finding study. The 017 Clinical Trial Study Group. Neurology 1997;49(5):1210‐8. [DOI] [PubMed] [Google Scholar]
Ryan 2000 {published data only}
- Ryan RE, Diamond S, Giammarco RAM, Aurora SK, Reed RC, Fletcher PE. Efficacy of zolmitriptan at early time‐points for the acute treatment of migraine and treatment recurrence. A randomised, placebo‐controlled trial. CNS Drugs 2000;13(3):215‐26. [Google Scholar]
Sakai 2002 {published data only}
- Sakai F, Iwata M, Tashiro K, Itoyama Y, Tsuji S, Fukuuchi Y, et al. Zolmitriptan is effective and well tolerated in Japanese patients with migraine: a dose‐response study. Cephalalgia 2002;22(5):376‐83. [DOI] [PubMed] [Google Scholar]
Solomon 1997 {published data only}
- Solomon GD, Cady RK, Klapper JA, Earl NL, Saper JR, Ramadan NM. Clinical efficacy and tolerability of 2.5 mg zolmitriptan for the acute treatment of migraine. The 042 Clinical Trial Study Group. Neurology 1997;49(5):1219‐25. [DOI] [PubMed] [Google Scholar]
Spierings 2004 {published data only}
- Spierings EL, Rapoport AM, Dodick DW, Charlesworth B. Acute treatment of migraine with zolmitriptan 5 mg orally disintegrating tablet. CNS Drugs 2004;18(15):1133‐41. [DOI] [PubMed] [Google Scholar]
Steiner 2003 {published data only}
- Steiner TJ, Diener HC, MacGregor EA, Schoenen J, Muirheads N, Sikes CR. Comparative efficacy of eletriptan and zolmitriptan in the acute treatment of migraine. Cephalalgia 2003;23(10):942‐52. [DOI] [PubMed] [Google Scholar]
Tuchman 2006 {published data only}
- Tuchman M, Hee A, Emeribe U, Silberstein S. Efficacy and tolerability of zolmitriptan oral tablet in the acute treatment of menstrual migraine. CNS Drugs 2006;20(12):1019‐26. [DOI] [PubMed] [Google Scholar]
Tullo 2010 {published data only}
- Allais G, Tullo V, Benedetto C, Zava D, Omboni S, Bussone G. Efficacy of frovatriptan in the acute treatment of menstrually related migraine: analysis of a double‐blind, randomized, multicenter, Italian, comparative study versus zolmitriptan. Neurological Sciences 2011;32 Suppl 1:S99‐104. [DOI: 10.1007/s10072-011-0547-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullo V, Allais G, Curone M, Ferrari MD, Omboni S, Benedetto C, et al. Frovatriptan versus zolmitriptan for the acute treatment of migraine with aura: a subgroup analysis of a double‐blind, randomized, multicenter, Italian study. Neurological Sciences 2012;33 Suppl 1:S61‐4. [DOI: 10.1007/s10072-012-1043-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullo V, Allais G, Ferrari MD, Curone M, Mea E, Omboni S, et al. Frovatriptan versus zolmitriptan for the acute treatment of migraine: a double‐blind, randomized, multicenter, Italian study. Neurological Sciences 2010;31 Suppl 1:S51‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Visser 1996 {published data only}
- Visser WH, Klein KB, Cox RC, Jones D, Ferrari MD. 311C90, a new central and peripherally acting 5‐HT1D receptor agonist in the acute oral treatment of migraine: a double‐blind, placebo‐controlled, dose‐range finding study. Neurology 1996;46(2):522‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Dowson 2003 {published data only}
- Dowson AJ, Charlesworth BR, Purdy A, Becker WJ, Boes‐Hansen S, Färkkilä M. Tolerability and consistency of effect of zolmitriptan nasal spray in a long‐term migraine treatment trial. CNS Drugs 2003;17(11):839‐51. [DOI] [PubMed] [Google Scholar]
Loder 2004 {published data only}
- Loder E, Silberstein SD, Abu‐Shakra S, Mueller L, Smith T. Efficacy and tolerability of oral zolmitriptan in menstrually associated migraine: a randomized, prospective, parallel‐group, double‐blind, placebo‐controlled study. Headache 2004;44(2):120‐30. [DOI] [PubMed] [Google Scholar]
Mauskop 1999 {published data only}
- Mauskop A, Farkkila M, Hering‐Hanit R, Rapoport A, Warner J. Zolmitriptan is effective for the treatment of persistent and recurrent migraine headache. Current Medical Research and Opinion 1999;15(4):282‐9. [DOI] [PubMed] [Google Scholar]
Tepper 1999 {published data only}
- Tepper SJ, Donnan GA, Dowson AJ, Bomhof MA, Elkind A, Meloche J, et al. A long‐term study to maximise migraine relief with zolmitriptan. Current Medical Research and Opinion 1999;15(4):254‐71. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
CTRI/2009/091/000196 {unpublished data only}
- Dr Avanti Biniwale (Principle investigator). A Clinical Trial to evaluate efficacy and safety of zolmitriptan 5 mg nasal spray in acute treatment of migraine. http://ctri.nic.in (accessed 13 November 2013) 2013. [CTRI/2009/091/000196]
Additional references
Amanzio 2009
- Amanzio M, Corazzini LL, Vase L, Benedetti F. A systematic review of adverse events in placebo groups of anti‐migraine clinical trials. Pain 2009;146(3):261‐9. [DOI: 10.1016/j.pain.2009.07.010] [DOI] [PubMed] [Google Scholar]
Ayzenberg 2012
- Ayzenberg I, Katsarava Z, Sborowski A, Chernysh M, Osipova V, Tabeeva G, et al. The prevalence of primary headache disorders in Russia: a countrywide survey. Cephalalgia 2012;32(5):373‐81. [DOI: 10.1177/0333102412438977] [DOI] [PubMed] [Google Scholar]
Bigal 2008
- Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology 2008;71(8):559‐66. [DOI: 10.1212/01.wnl.0000323925.29520.e7] [DOI] [PubMed] [Google Scholar]
Bloudek 2012
- Bloudek LM, Stokes M, Buse DC, Wilcox TK, Lipton RB, Goadsby PJ, et al. Cost of healthcare for patients with migraine in five European countries: results from the International Burden of Migraine Study (IBMS). Journal of Headache and Pain 2012;13(5):361‐78. [DOI: 10.1007/s10194-012-0460-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
BNF 2013
- Zolmitriptan. British National Formulary 2013; Vol. March:Available at http://www.medicinescomplete.com/mc/bnf/current/PHP2859‐zolmitriptan.htm.
Buse 2011
- Buse D, Manack A, Serrano D, Reed M, Varon S, Turkel C, et al. Headache impact of chronic and episodic migraine: results from the American Migraine Prevalence and Prevention study. Headache 2012;52(1):3‐17. [DOI: 10.1111/j.1526-4610.2011.02046.x] [DOI] [PubMed] [Google Scholar]
Chen 2007
- Chen LC, Ashcroft DM. Meta‐analysis of the efficacy and safety of zolmitriptan in the acute treatment of migraine. Headache 2008;48(2):236‐47. [DOI: 10.1111/j.1526-4610.2007.01007.x] [DOI] [PubMed] [Google Scholar]
Collins 1997
- Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres?. Pain 1997;72(1‐2):95‐7. [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310(6977):452‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012a
- Derry CJ, Derry S, Moore RA. Sumatriptan (oral route of administration) for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008615.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012b
- Derry CJ, Derry S, Moore RA. Sumatriptan (subcutaneous route of administration) for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009665] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012c
- Derry CJ, Derry S, Moore RA. Sumatriptan (intranasal route of administration) for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009663] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012d
- Derry CJ, Derry S, Moore RA. Sumatriptan (rectal route of administration) for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009664] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2013
- Derry S, Moore RA, McQuay HJ. Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headaches in adults. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008040.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diamond 2007
- Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache 2007;47(3):355‐63. [DOI: 10.1111/j.1526-4610.2006.00631.x] [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI: 10.1093/ije/31.1.140] [DOI] [PubMed] [Google Scholar]
Ferrari 2002
- Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Triptans (serotonin, 5‐HT1B/1D agonists) in migraine: detailed results and methods of a meta‐analysis of 53 trials. Cephalalgia 2002;22(8):633‐58. [DOI: 10.1046/j.1468-2982.2002.00404.x] [DOI] [PubMed] [Google Scholar]
Gendolla 2008
- Gendolla A. Early treatment in migraine: how strong is the current evidence?. Cephalalgia 2008;28 Suppl 2:28‐35. [DOI: 10.1111/j.1468-2982.2008.01688.x] [DOI] [PubMed] [Google Scholar]
Goadsby 2007a
- Goadsby PJ. Recent advances in understanding migraine mechanisms, molecules and therapeutics. Trends in Molecular Medicine 2007;13(1):39‐44. [DOI: 10.1016/j.molmed.2006.11.005] [DOI] [PubMed] [Google Scholar]
Hazard 2009
- Hazard E, Munakata J, Bigal ME, Rupnow MF, Lipton RB. The burden of migraine in the United States: current and emerging perspectives on disease management and economic analysis. Value in Health 2009;12(1):55‐64. [DOI: 10.1111/j.1524-4733.2008.00404.x] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Altman DG, Sterne JAC editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
IHS 1988
- Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8(Suppl 7):1‐96. [PubMed] [Google Scholar]
IHS 2000
- International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia 2000;20(9):765‐86. [DOI] [PubMed] [Google Scholar]
IHS 2004
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24(Suppl 1):1‐160. [DOI] [PubMed] [Google Scholar]
IHS 2013
- Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33(9):629‐808. [DOI: 10.1177/0333102413485658] [DOI] [PubMed] [Google Scholar]
Jadad 1996a
- Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66(2‐3):239‐46. [DOI] [PubMed] [Google Scholar]
Jadad 1996b
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Khan 1996
- Khan KS, Daya S, Jadad A. The importance of quality of primary studies in producing unbiased systematic reviews. Archives of Internal Medicine 1996;156(6):661‐6. [PubMed] [Google Scholar]
Kirthi 2013
- Kirthi V, Derry S, Moore RA, McQuay HJ. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008041.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107(2):224‐33. [DOI] [PubMed] [Google Scholar]
Leonardi 2005
- Leonardi M, Steiner TJ, Scher AT, Lipton RB. The global burden of migraine: measuring disability in headache disorders with WHO's Classification of Functioning, Disability and Health (ICF). Journal of Headache and Pain 2005;6(6):429‐40. [DOI: 10.1007/s10194-005-0252-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Linde 2012
- Linde M, Gustavsson A, Stovner LJ, Steiner TJ, Barré J, Katsarava Z, et al. The cost of headache disorders in Europe: the Eurolight project. European Journal of Neurology 2012;19(5):703‐11. [DOI: 10.1111/j.1468-1331.2011.03612.x] [DOI] [PubMed] [Google Scholar]
Lipton 1999
- Lipton RB, Stewart WF. Acute migraine therapy: do doctors understand what patients with migraine want from therapy?. Headache 1999;39(Suppl 2):S20‐S26. [Google Scholar]
Lipton 2007
- Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, AMPP Advisory Group, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68(5):343‐9. [DOI] [PubMed] [Google Scholar]
Lucas 2006
- Lucas C, Géraud G, Valade D, Chautard MH, Lantéri‐Minet M. Recognition and therapeutic management of migraine in 2004, in France: results of FRAMIG 3, a French nationwide population‐based survey. Headache 2006;46(5):715‐25. [DOI: 10.1111/j.1526-4610.2006.00430.x] [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5] [Google Scholar]
Moore 2010
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. ‘‘Evidence” in chronic pain – establishing best practice in the reporting of systematic reviews. Pain 2010;150:386–389. [DOI] [PubMed] [Google Scholar]
Morris 1995
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. In: Gardner MJ, Altman DG editor(s). Statistics with confidence ‐ confidence intervals and statistical guidelines. London: British Medical Journal, 1995:50‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munakata 2009
- Munakata J, Hazard E, Serrano D, Klingman D, Rupnow MF, Tierce J, et al. Economic burden of transformed migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache 2009;49(4):498‐508. [DOI: 10.1111/j.1526-4610.2009.01369.x] [DOI] [PubMed] [Google Scholar]
Oldman 2002
- Oldman AD, Smith LA, McQuay HJ, Moore RA. Pharmacological treatments for acute migraine: quantitative systematic review. Pain 2002;97(3):247‐57. [DOI: 10.1016/S0304-3959(02)00024-6] [DOI] [PubMed] [Google Scholar]
PCA 2012
- Anonymous. Prescription cost analysis, England 2011. The NHS Information Centre, Prescribing Support Unit 2012. [ISBN: 978‐1‐84636‐685‐7]
Rabbie 2013
- Rabbie R, Derry S, Moore RA, McQuay HJ. Ibuprofen with or without an antiemetic for acute migraine headaches in adults. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008039.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Radtke 2009
- Radtke A, Neuhauser H. Prevalence and burden of headache and migraine in Germany. Headache 2009;49(1):79‐89. [DOI: 10.1111/j.1526-4610.2008.01263.x] [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Song 2003
- Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta‐analyses. BMJ 2003;326(7387):472. [DOI: 10.1136/bmj.326.7387.472] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steiner 2013
- Steiner TJ, Stovner LJ, Birbeck GL. Migraine: the seventh disabler. Journal of Headache and Pain 2013;14:1. [DOI: 10.1186/1129-2377-14-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stovner 2010
- Stovner LJ, Andree C. Prevalence of headache in Europe: a review for the Eurolight project. Journal of Headache and Pain 2010;11(4):289‐99. [DOI: 10.1007/s10194-010-0217-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2014
- Thorlund K, Mills EJ, Wu P, Ramos E, Chatterjee A, Druyts E. Comparative efficacy of triptans for the abortive treatment of migraine: A multiple treatment comparison meta‐analysis. Cephalalgia 2014;34(4):258‐67. [DOI: 10.1177/0333102413508661] [DOI] [PubMed] [Google Scholar]
Tramer 1997
- Tramèr MR, Reynolds DJM, Moore RA, McQuay HJ. Impact of covert duplicate results on meta‐analysis: a case study. BMJ 1997;315(7109):635‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Victor 2010
- Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life‐span study. Cephalalgia 2010;30(9):1065‐72. [DOI] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163‐96. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2012
- Yu S, Liu R, Zhao G, Yang X, Qiao X, Feng J, et al. The prevalence and burden of primary headaches in China: a population‐based door‐to‐door survey. Headache 2012;52(4):582‐91. [DOI: 10.1111/j.1526-4610.2011.02061.x] [DOI] [PubMed] [Google Scholar]


