Abstract
The incidence of inflammatory bowel disease (IBD) is increasing and the pathogenesis is still not completely understood. Micronutrients like vitamin D [25 (OH)D] and zinc play an important role in enzyme activities and the immune system. As the 25 (OH)D-receptor has been shown to be downregulated in patients with IBD, 25 (OH)D may emerge as a predictive marker for disease improvement. Studies on relationship of both micronutrients in IBD patients are lacking.
We retrospectively evaluated serum levels of 25(OH)D and zinc together with baseline characteristics of 232 IBD patients. Uni- and multivariate analyses were performed for association between serum levels of 25(OH)D and zinc with clinical and deep remission (CR and DR).
155 Crohn's disease (CD) and 77 ulcerative colitis (UC) patients were included. 54% (n = 125) and 6% (n = 14) of IBD patients showed deficient serum 25(OH)D levels below 20 ng/mL and zinc levels below 7 μmol/L. Serum 25(OH)D levels were significantly higher in IBD patients with CR (P = .02) and DR (P < .001) but not serum zinc levels, respectively. Serum 25(OH)D levels (P = .008), anti-tumor-necrosis-factor-α-trough-concentration (anti-TNF-α-TC) (P = .02) and CRP level (P = .02) were independently associated with CR in CD patients. Serum 25(OH)D threshold of 19 ng/mL discriminated CD patients with or without CR, having an area under the receiver operating curve analysis (AUROC) of 0.77 [95%-confidence interval (CI): 0.68–0.85]. In multivariate analysis serum 25(OH)D levels (P = .04) and anti-TNF-α-TC (P = .04) were associated with DR in CD patients. Serum 25(OH)D threshold of 26 ng/mL discriminated CD patients with or without DR, having an AUROC of 0.75 (95%-CI: 0.68–0.83).
Serum 25(OH)D (P = .04) and fecal calprotectin levels (P = .04) were independently correlated with CR in UC patients. Serum 25(OH)D threshold of 32 ng/mL discriminated UC patients in CR with an AUROC of 0.83 (95%-CI: 0.71–0.95). Zinc levels did not correlate with disease activity status in CD or UC patients either.
In conclusion, beside CRP and fecal calprotectin, serum 25(OH)D levels, but not serum zinc levels, may be an additional useful and noninvasive marker for characterizing different disease activity status of IBD patients. Measurement of serum 25(OH)D in IBD patients may be warranted. 25(OH)D supplementation in deficient IBD patients is recommended.
Keywords: 25(OH)D, clinical remission, Crohn's disease, deep remission, ulcerative colitis, vitamin D, zinc
1. Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing disorder that is represented by Crohn's disease (CD) and ulcerative colitis (UC). CD can affect any segment of the gastrointestinal tract with transmural inflammation. Patients with CD often clinically present with diarrhea, abdominal pain or signs of bowel obstruction. Inflammation in UC is restricted to the mucosal surface, it starts from the rectum and can continuously extend to the proximal colon. It is believed that both diseases are caused by an inappropriate activation of the mucosal immune system by altered intestinal microbiota in genetically predisposed individuals.[1,2] The first goal of IBD therapy is to achieve clinical remission (CR). Subsequently, endoscopic and/or deep remission (DR) is desired and defined by CR and biochemical or endoscopic remission.[3] Various agents are currently available to achieve these goals such as 5-aminosalicylic acid (5-ASA), corticoids, immunomodulators (IMM), anti-tumor-necrosis-factor-α (TNF-α), integrin inhibitors, or antibodies against interleukin 12 and 23.
Around 85% of IBD patients show signs of malnutrition.[4] Malnutrition can be classified as macro- or micronutrient deficiency. While macronutrient deficiency is defined by protein energy malnutrition and usually occurs in IBD patients with severe active disease, micronutrient deficiency can already occur in mild active disease.[4,5] Therefore, micronutrient status evaluation such as measurement of different vitamins, especially vitamin D (25(OH)D), zinc or iron is recommended at least once per year.[6] However, most clinicians pay little attention on nutritional support in patients with IBD.
Under physiological conditions, 25(OH)D controls calcium and bone homeostasis. In addition, 25(OH)D regulates the immune system by promoting anti-inflammatory T-cell pathways and stimulating the antimicrobial effects of macrophages.[7] Interestingly, IBD patients are at an increased risk for 25(OH)D deficiency.[8] Moreover, studies of IBD patients have shown that 25(OH)D deficiency is linked to an increased risk of complication rates, such as increased hospitalization, structuring disease, surgical resection, pancolitis in UC and the use of steroids earlier after diagnosis, giving it an important clinical prognosis.[9,10]
Zinc is an essential micronutrient and is absorbed in the small intestine. Zinc serves as a cofactor for various enzymes involved in immune function, growth, and tissue repair.[11] Zinc deficiency increases the number of pro-inflammatory cells and modulates inflammatory cytokine response leading to intestinal inflammation by aggravation of mucosa leakage.[12] Zinc deficiency seems to be common in young and adolescent IBD patients during remission and active disease status with a prevalence rate of 15% to 40%.[13,14] Epidemiological data on adult IBD patients are lacking. Recently, Siva et al[15] showed that IBD patients with zinc deficiency seem to have more disease-specific adverse events, suggesting a potential clinical significance in IBD. To our knowledge, studies that examined the association between serum 25(OH)D and zinc level in IBD patients, irrespective of their therapy regimen or disease status, are lacking.
Therefore, we examined the serum 25(OH)D and zinc levels of IBD patients from a tertiary referral center and correlated results to CR and DR status.
2. Methods and materials
2.1. Study population
This retrospective study consisted of 232 IBD patients (155 CD and 77 UC diagnosed using established criteria). We retrospectively interrogated a prospectively maintained database of IBD patients maintained in the Department of Gastroenterology and gastrointestinal Oncology at the University Medical Center of Göttingen, Germany from 2017 to 2018. Demographic, clinical, disease activity, endoscopic, and laboratory data were available for all enrolled patients. All patients with measurement of serum 25(OH)D and zinc were included. Patients were excluded if there was no 25(OH)D and zinc level measurement during the study period or if they had a diagnosis of unclassified IBD.
2.2. Clinical data collection
Baseline disease characteristics, including disease type, duration of disease, disease location and behavior, according to Montreal Classification[16] at initial endoscopic or radiographic encounter and history of IBD-related surgery were also collected. Disease activity was evaluated using disease activity indices [Harvey–Bradshaw index (HBI)] for CD[17] and (partial) Mayo score for UC,[18] and biochemical inflammatory markers [C-reactive protein (CRP) and fecal calprotectin] which were collected prospectively at each outpatient visit. CR was defined by an HBI < 5 points in CD and by a (partial) Mayo score <2 points in UC patients. DR was defined by the association of CR with endoscopic remission and/or CRP level <5 mg/L and calprotectin level <50 mg/kg stool.
IBD medication exposure was determined for 5-ASA compounds (sulfasalazine, mesalamine either orally or topically), prednisolone or enteric steroids (oral budesonide and per rectum corticosteroid preparations), IMM agents (azathioprine, methotrexate, 6-mercaptopurine) and anti-tumor-necrosis-factor-α anti-(TNF)-α therapy [infliximab (IFX), adalimumab (ADA)].
All anti-TNF-α treated patients had responded initially to standard induction therapy and were on maintenance therapy.
Serum anti-TNF-α trough concentrations (TC) were measured during patients’ maintenance phase of anti-TNF-α treatment. The included samples were obtained as trough levels. No distinction was made between those who were in remission and those who showed signs of disease activity. Biochemical parameters of disease activity were measured, including CRP (normal <5 mg/L) and calprotectin (normal <50 mg/kg) at the time of serum 25(OH)D and zinc measurement.
IFX was administered at a dose of 5 mg/kg every 8 weeks. In patients with a loss of response the IFX dose was increased to 10 mg/kg. ADA was administered at standard doses of 40 mg every other week. Dose intensification was defined as administration of 40 mg every week.
Serum 25(OH)D was determined as described previously.[19] A serum 25(OH)D level below 20 ng/mL was considered as deficiency. The serum zinc levels were quantified by utilizing an atomic absorption Perkin Elmer spectrophotometer 900T according to the manufacturer's recommendations in the Central Laboratory of the Department of Clinical Chemistry at University Medical Center Göttingen. Serum zinc levels below 7 μmol/L were considered as deficiency.[20]
The serum concentration of CRP was determined by utilizing the automated systems of the Central Laboratory of the Department of Clinical Chemistry at University Medical Center Göttingen. Fecal calprotectin levels were measured with RIDASCREEN Calprotectin according to the manufacturers’ instructions.
Anti-TNF-α-TC was measured as follows: the quantification of IFX and ADA in serum samples was performed using 2 different enzymes immunoassays (IDKmonitor IFX drug level ELISA and IDKmonitor ADA drug level ELISA, Immundiagnostik AG, Germany) according to the manufacturer's recommendations. Briefly, the therapeutic antibody was bound to specific monoclonal anti-IFX or anti-ADA antibodies coated on the plate. Following a washing step, peroxidase-labeled antibodies were added and finally the conversion of tetramethylbenzidine was monitored photometrically. The intensity of the yellow color was directly proportional to the concentration of IFX or ADA. Low trough levels for IFX were defined as <3 μg/mL and high trough levels >7 μg/mL. Low trough level for ADA was defined as <5 μg/mL and high trough levels >7.5 μg/mL.[21]
2.3. Statistical analysis
Descriptive measures of interest were calculated using median and interquartile range (IQR) for continuous variables. Mann–Whitney-U test was utilized to determine significant association for continuous parameters. Categorical variables were expressed as proportions. Pearson χ2 or Fisher's exact tests were used to compare dichotomous variables. When considering serum 25(OH)D and CRP, or calprotectin levels receiver operating characteristic (ROC) curve analysis was performed using therapeutic outcomes as classification variables to calculate the sensitivity, specificity and area under the ROC curve (AUROC) with the corresponding P values. The best threshold levels of all 3 parameters were identified using maximized Youden's index. All reported P values were 2 sided, and P <.05 was considered as statistically significant.
A binary logistic regression analysis with the backward stepwise selection was performed to estimate odds ratios of CR and DR. All variables with a P value <0.1 in the univariate analysis were included in the logistic regression analysis model. Statistical analysis was performed using the SPSS 25 software for Mac OS (SPSS, Chicago, Illinois, USA).
2.4. Ethical considerations
The study complies with the principles of the Declaration of Helsinki. The study protocol was approved by the ethics committee of the University Medical Center of Göttingen (approval number 5/4/18).
3. Results
Demographical, clinical, laboratory, and endoscopic data of 232 IBD patients (155 with CD and 77 with UC) were included in the analysis as summarized in Table 1. Around 67% had CD, the median age was 40 years and 53% were female and 22% smoked and median BMI was 24 kg/m2. The median disease duration was 8 years, 60% of CD patients had either structuring or fistulizing disease course. 40% of UC patients had E3 disease course according to the Montreal classification. About 50% were treated with an anti-TNF-α-therapy, 12% had a combination therapy consisting of anti-TNF-α and an IMM. Around 53% and 27% of the IBD cohort were in CR and DR, respectively and 33% had a prior history of intestinal surgery (n = 72 of CD and n = 5 of UC patients).
Table 1.
Patients’ demographic and clinical characteristics.
Median 25(OH)D and zinc levels of all IBD patients were 18 ng/mL and 10.6 μmol/L, respectively. Around 54% of IBD patients had 25(OH)D concentration below 20 ng/mL, 26% below 30 ng/mL and 20% with normal concentration. 6% of IBD patients showed zinc levels below 7 μmol/L and 94% had normal zinc levels.
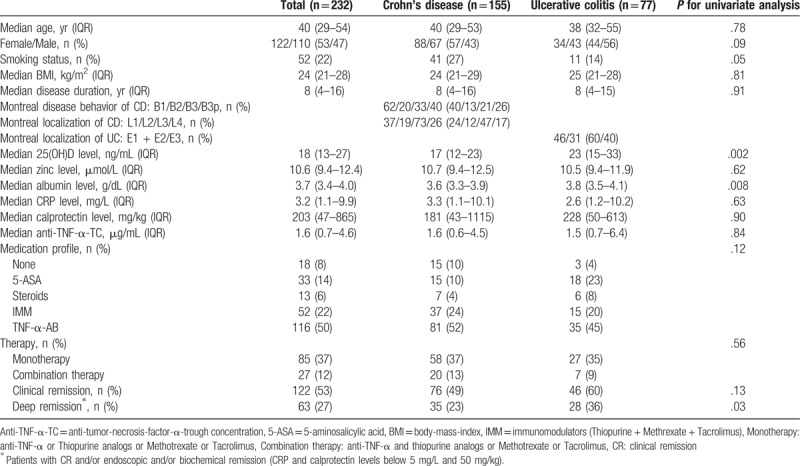
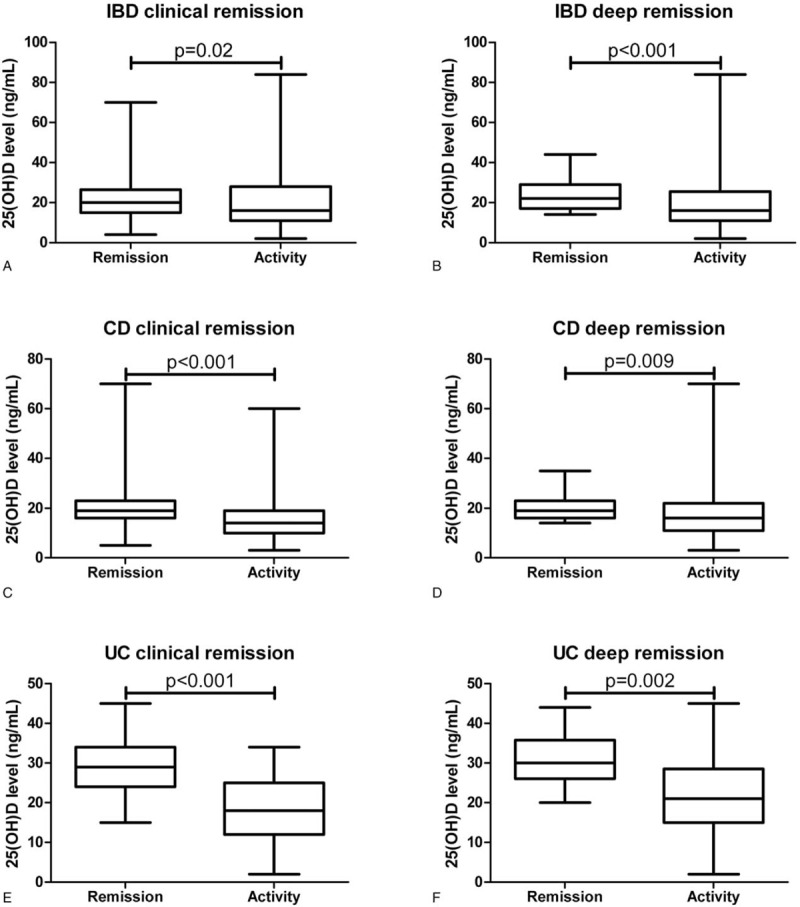
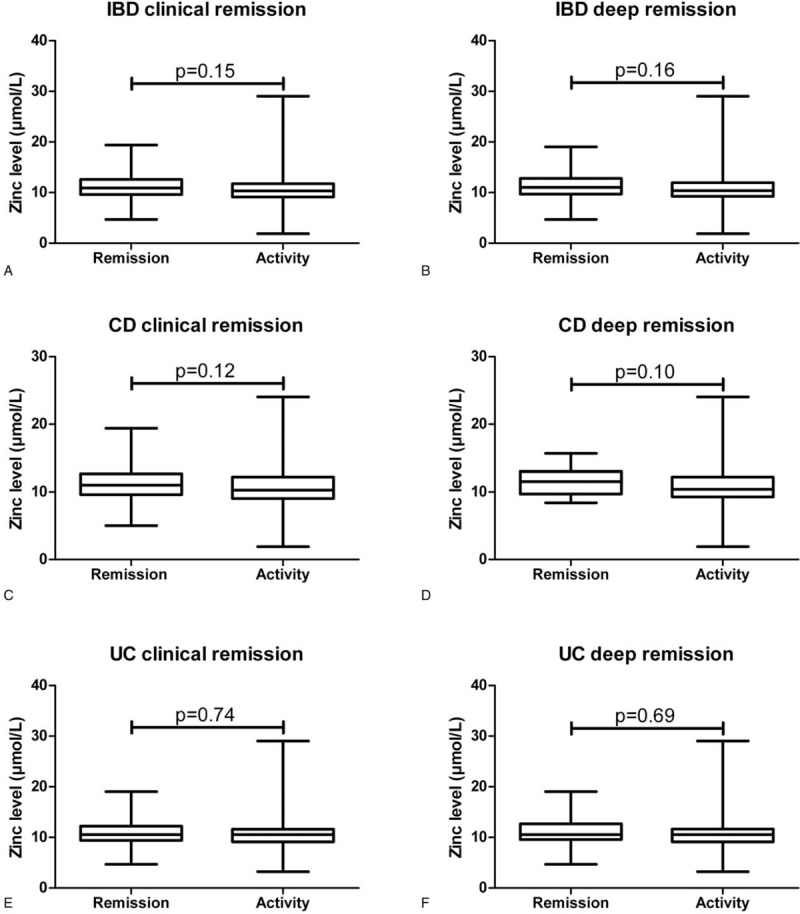
IBD patients in CR (P = .02) and DR (P<.001) showed significant higher levels of 25(OH)D compared to patients with disease activity (Fig. 1A and B). Zinc levels were neither associated with CR (P = .15) nor with DR (P = .16) in IBD patients (Fig. 2A and B). IFX- and ADA-TC of 77 and 35 IBD patients treated with an anti-TNF-α antibody were available. The median IFX- and ADA-TC were 1.5 and 4.9, respectively.
Figure 1.
Association of serum 25(OH)D level with CR (A) or DR (B) in IBD patients, with CR (C) or DR (D) in CD patients, as well as with CR (E) or DR (F) in UC patients.
Figure 2.
Association of serum zinc levels with CR (A) or DR (B) in IBD patients, with CR (C) or DR (D) in CD patients, as well as with CR (E) or DR (F) in UC patients.
More CD patients were female and smoked compared to UC patients, although this did not reach statistically significance (Table 1). Median 25(OH)D (P = .002) and albumin (P = .008) levels of the CD cohort were significantly lower compared to the UC cohort (Table 1). Only 23% of CD patients were in DR compared to 36% of UC patients (P = .03).
Significantly more CD patients had 25(OH)D levels below 20 ng/mL compared to UC patients (60% versus 30%, P<.001), respectively. Around 7% of CD and 6% of UC patients had zinc levels below 7 μmol/L, respectively (P = .86).
Neither the IFX- nor the ADA-TC of CD patients compared to UC patients differed [IFX: 1.6 (IQR: 0.6–3.4] versus 1.2 (IQR: 0.9–2.5) μg/mL, P = .77; ADA: 4.9 (IQR: 0.6–8.8) versus 8.6 (IQR: 0.4–12.0) μg/mL, P = .24).
3.1. Association of variables of CD patients with regard to CR and DR
Around 49% (n = 76) of CD patients were in CR (Table 1). CD patients had significantly higher levels of 25(OH)D (P<.001) compared to those without CR (Fig. 1C). Zinc levels were not associated with CR in CD patients (Fig. 2C).
Univariate analysis revealed that disease duration (P = .01), anti-TNF-α-TC (P = .001), 25(OH)D (P<.001), albumin (P = .001) and CRP levels (P = .001) were associated with CR. Fecal calprotectin level (P = .09) and smoking status tended to be associated (P = .07) with CR. In multivariable analysis, anti-TNF-α-TC (P = .02), 25(OH)D (P = .008) and CRP (P = .02) levels were associated independently with CR (Table 2). The odds ratio (OR) of CD patients for CR was 1.4 (95%-confidence interval (CI): 1.1–1.8) for anti-TNF-α-TC, 1.2 (95%-CI: 1.1–1.3) for 25(OH)D and 0.8 (95%-CI: 0.7–0.9) for CRP levels, respectively. We further analyzed for 25(OH)D and CRP levels the optimal cutoff levels and their diagnostic accuracy utilizing ROC curves. For 25(OH)D and CRP levels, the threshold values to discriminate patients with CR or active disease were 19 ng/mL and 4.9 mg/L with an AUROC of 0.77 (95%-CI: 0.68–0.85) and 0.81 (95%-CI: 0.74–0.87), respectively (Fig. 3A). The sensitivity, specificity, positive and negative predictive values for 25(OH)D cutoff value were 68%, 71%, 69% and 70%, respectively (Table 3).
Table 2.
Variables associated (P < .1) with CR (A) und DR (B) in CD patients.
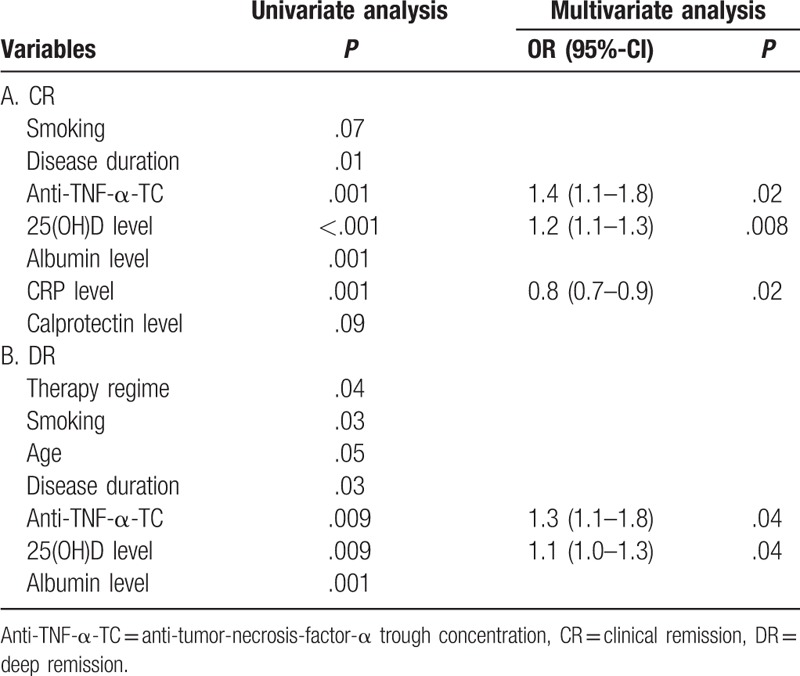
Figure 3.
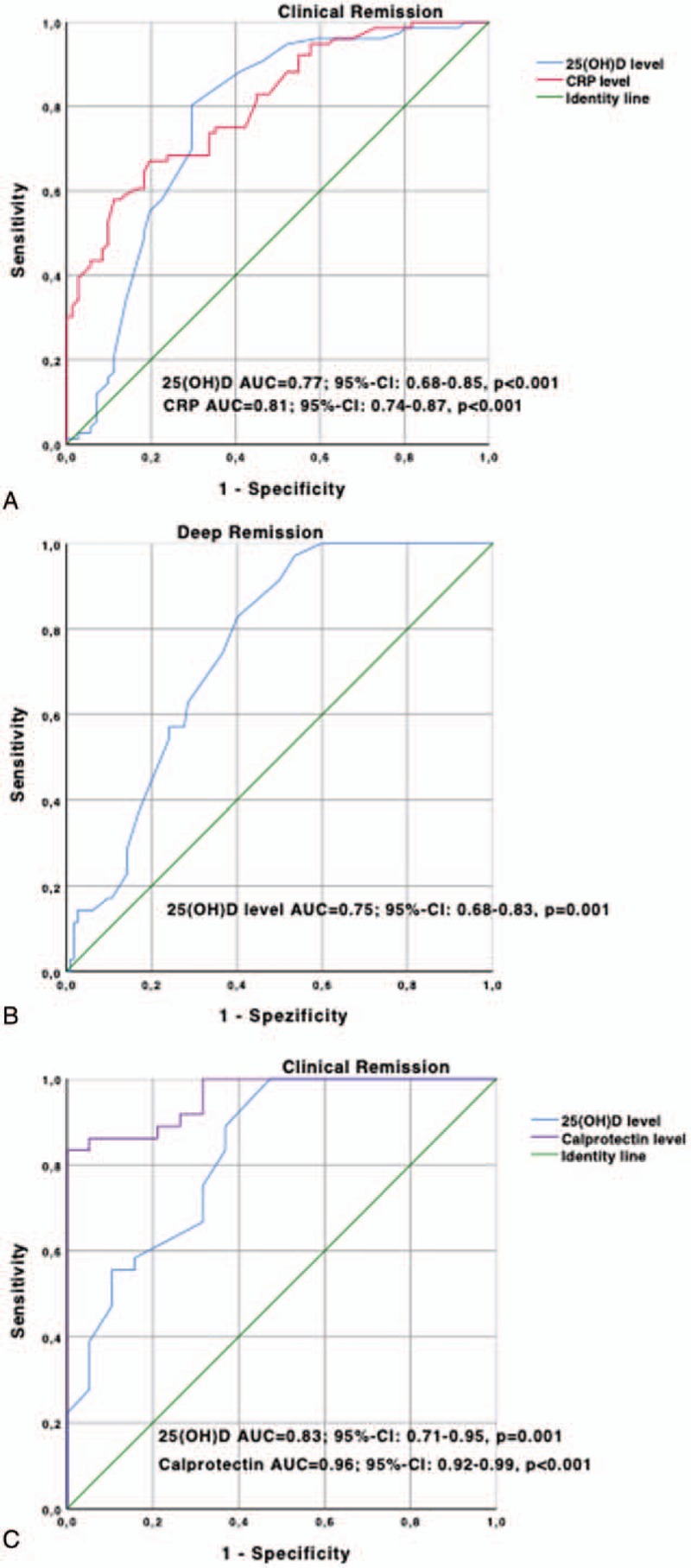
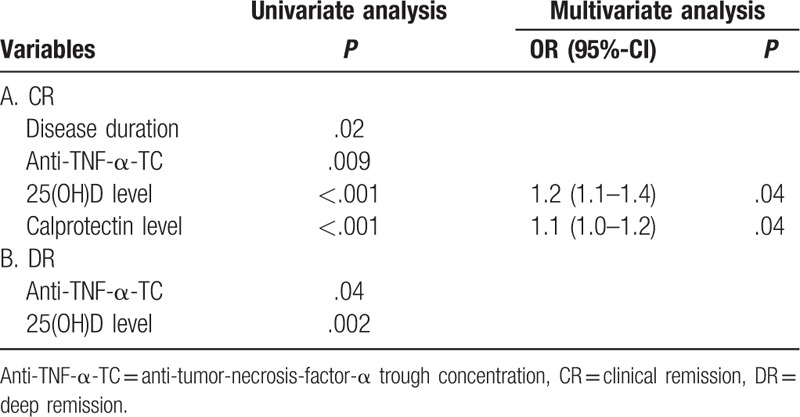
ROC analysis regarding CR in CD patients for 25(OH)D (cut-off level 19 ng/mL) and CRP (cut-off level 4.9 mg/L) (A), regarding DR in CD patients for 25(OH)D (cut-off level 26 ng/mL) (B) and regarding CR in UC patients for 25(OH)D (cut-off level 32 ng/mL) and calprotectin (cut-off level 223 mg/L) (C).
Table 3.
Diagnostic accuracy with regard to cutoff levels of independent parameters associated with CR (A) in CD, CR (B) in UC und DR (C) in CD cohorts of patients.
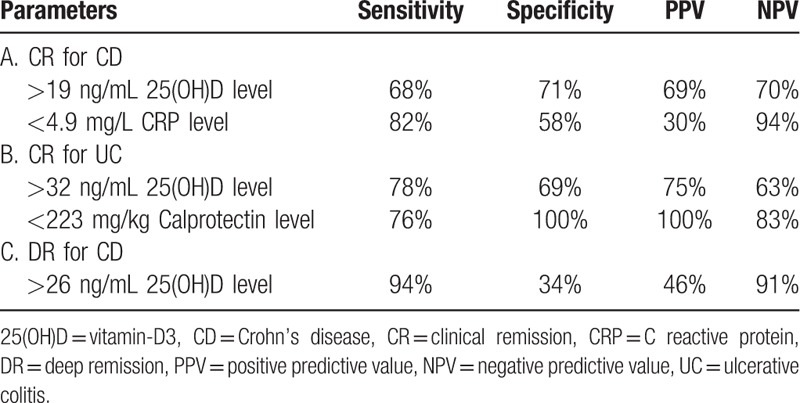
23% (n = 35) of CD patients were in DR (Table 1). CD patients in DR had higher levels of 25(OH)D (P = .009) compared to those without (Fig. 1D). Zinc levels (P = .10) did not differ in CD patients with or without DR (Fig. 2D).
DR was defined as having CR with CRP and calprotectin normalization and/or endoscopic remission. Therefore, we did not include CRP and calprotectin levels in the logistic regression analysis. Univariate analysis revealed that therapy regime (P = .04), smoking status (P = .03), age (P = .05), disease duration (P = .03), anti-TNF-α-TC (P = .009), 25(OH)D (P = .009) and albumin levels (P = .001) were associated with DR. In multivariable analysis, anti-TNF-α-TC (P = .04) and 25(OH)D (P = .04) level were associated independently with DR (Table 2). The OR for anti-TNF-α-TC and 25(OH)D levels were 1.3 (95%-CI: 1.1–1.8) and 1.1 (95%-CI: 1.0–1.3), respectively. For 25(OH)D the threshold value to discriminate patients with DR or active disease were 26 ng/mL with an AUROC of 0.75 (95%-CI: 0.68–0.83) (Fig. 3B). The sensitivity, specificity, positive, and negative predictive values for 25(OH)D cutoff values were 94%, 34%, 46% and 91%, respectively (Table 3).
3.2. Association of variables of UC patients with regard to CR and DR
Around 60% (n = 46) of UC patients were in CR (Table 1). UC patients had significantly higher levels of 25(OH)D (P<.001) compared to those without CR (Fig. 1E). Zinc levels were not associated with CR in UC patients (Fig. 2E).
Univariate analysis revealed that disease duration (P = .02), anti-TNF-α-TC (P = .009), 25(OH)D (P = .001) and calprotectin levels (P<.001) were associated with CR. In multivariable analysis, 25(OH)D (P = .04) and calprotectin (P = .04) levels were associated independently with CR (Table 4). The OR of UC patients were 1.2 (95%-CI: 1.1–1.4) for 25(OH)D and 1.1 (95%-CI: 1.0–1.2) for calprotectin levels, respectively. For 25(OH)D and calprotectin levels, the threshold values to discriminate patients with CR or active disease were 32 ng/mL and 223 mg/L with an AUROC of 0.83 (95%-CI: 0.71–0.95) and 0.96 (95%-CI: 0.92–0.99), respectively (Fig. 3C). Calprotectin showed the best diagnostic accuracy for diagnosing UC patients with CR (Table 3).
Table 4.
Variables associated (P < .1) with CR (A) und DR (B) in UC patients.
About 36% (n = 28) of UC patients were in DR (Table 1). UC patients in DR had a higher levels of 25(OH)D (P = .002) compared to those without (Fig. 2F). Zinc levels (P = .69) did not differ in UC patients with or without DR (Fig. 2F).
Univariate analysis revealed that anti-TNF-α-TC (P = .04) and 25(OH)D (P = .002) levels were associated with DR. No variable was associated with DR in UC cohort in multivariable analysis (Table 4).
4. Discussion
Currently, there is little evidence for routine evaluation of micronutrients in IBD patients. Only few data address the importance of micronutrient in IBD patients.[22] Micronutrient deficiencies in IBD patients, especially 25(OH)D deficiency, was found to be prevalent in patients with IBD[23,24] as well as in the general population.[25] To date, there is little information available regarding zinc deficiency in patients with IBD. Some studies reported prevalence rates of 15% to 40% for zinc deficiency in young and adolescent IBD patients.[6,26]
In this single-center study, we found for the first time an independent association between 25(OH)D level and CR as well as DR in CD patients. In UC patients, a significant association was found only between 25(OH)D levels and CR but not regarding DR. Specifically, CD and UC patients who had high 25(OH)D levels had an increased probability of being in remission. This observation is supported by previously published studies suggesting associations between 25(OH)D levels and disease severity as well as complications of IBD.[27–29] Furthermore, animal models of colitis showed that 25(OH)D supplementation ameliorate intestinal inflammation.[30] Human studies with 25(OH)D supplementation showed decreased risk of relapse[31], decreased risk of IBD-related surgery,[9] and decrease of peripheral inflammatory markers and Crohn's disease activity index.[32] However, it is still a matter of debate which 25(OH)D dose is appropriate to substitute IBD patients. A study of Garg et al[33] could show that a close control of 25(OH)D levels and careful dose adaptation thereafter, could improve symptom-based activity scores in IBD patients. One prospective randomized study could surprisingly show that patients with CD, who received IFX as induction therapy and had low levels of 25(OH)D, reached higher CR rates than those with normal 25(OH)D levels.[34] The results of this study further support the hypothesis that 25(OH)D status may contribute to disease activity in IBD patients, suggesting that the 25(OH)D deficiency is not only a consequence of the IBD activity, but the exact molecular pathways still need to be elucidated.
Serum zinc levels are maintained by a balance of intake in the proximal gastrointestinal tract, fecal losses, and slow release from tissue stores.[35] Animal and human studies have demonstrated that serum zinc is as useful and accurate as tissue concentrations of zinc accumulation in solid organs.[36] Moreover, serum zinc levels may reflect zinc status better during states of acute zinc depletion due to the slow equilibration of total zinc pools.[35] Zinc also has an important function in wound healing, and one study showed that fistulizing CD patients had lower levels compared to those without fistulas.[37] Zinc deficiency was also associated with acrodermatitis enteropatica, an extraintestinal skin manifestation of CD.[38]
Regarding zinc status, 7% of the CD and 6% of the UC cohort showed zinc deficiency. Zinc level neither differed between CD and UC cohort of patients nor was associated with CR or DR in both cohorts either. First, these results are in contrast to the known prevalence rates of 15% to 40% of zinc deficiency mentioned above. Nevertheless, this is the first study that shows zinc deficiency rates in adult IBD patients. Second, to date only one study is published so far and showed that serum zinc deficiency in IBD patients is more likely associated with adverse disease-specific outcomes such as increased risk of subsequent hospitalizations, surgeries, and disease-related complications.[15] Nevertheless, this study did not analyze an association between serum zinc level and disease activity status at the time of measurement. Therefore, the results of the present study do not further support the hypothesis that zinc deficiency may contribute to the disease status of IBD patients.
In our opinion, it is important to obtain new markers to evaluate IBD disease activity status because its pathophysiology is still not completely understood. Moreover, new markers are bringing new insights into the perspectives of IBD therapy. The 2 investigated parameters serum 25(OH)D and zinc are micronutrients with important metabolic implications. Their deficiency could lead to severe forms of extraintestinal manifestations like osteoporosis and fractures. In case of deficiency both micronutrients can be easily substituted, preventing important complications. Possibly, after supplementation of 25(OH)D better remission rates could be achieved, giving a clinical importance.[33]
Further variables associated with disease status were anti-TNF-α-TC, CRP, and calprotectin levels. Anti-TNF-α-TC were independently associated with CR and DR in CD patients and CR in UC patients treated with an TNF-α-inhibitors as known in previously published studies.[39–41] Both, CRP and calprotectin are well known biochemical markers associated with different disease status of patients with CD and UC.[42]
Our study has certain limitations, including a mixture of patients with CD and UC and different therapy regimens. Furthermore, the study is a single-center study and was conducted at a tertiary care center, which could have caused bias, especially for more severe disease manifestations. We could not measure disease activity for some patients directly by endoscopy or radiological examinations because of the study design. Instead, we tried to assess disease severity by measuring serum CRP and fecal calprotectin levels. Furthermore, the lack of dietary intake monitoring could affect the micronutrient status of the IBD patients included. Nonetheless, we do believe that our “real world” assessment of these measures and outcomes have clinical value.
In conclusion, 25(OH)D deficiency but not zinc deficiency is common in IBD patients. Serum 25 (OH)D levels were independently associated with CR and DR in CD patients and with CR in UC patients but not serum zinc level. Therefore, beside CRP and fecal calprotectin serum 25(OH)D levels may be a further useful noninvasive marker for characterizing different disease activities status in IBD patients. Measurement of serum 25(OH)D levels in IBD patients may thus be warranted. 25(OH)D supplementation in deficient IBD patients is recommended.
Author contributions
Conceptualization: Nicolae-Catalin Mechie, Albrecht Neesse, Ahmad Amanzada.
Data curation: Nicolae-Catalin Mechie, Eirini Mavropoulou.
Formal analysis: Golo Petzold, Ahmad Amanzada.
Funding acquisition: Ahmad Amanzada.
Investigation: Nicolae-Catalin Mechie, Albrecht Neesse, Ahmad Amanzada.
Methodology: Steffen Kunsch, Ahmad Amanzada.
Project administration: Ahmad Amanzada.
Software: Ahmad Amanzada.
Supervision: Volker Ellenrieder.
Validation: Nicolae-Catalin Mechie, Ahmad Amanzada.
Visualization: Ahmad Amanzada.
Writing – original draft: Nicolae-Catalin Mechie, Ahmad Amanzada.
Writing – review & editing: Eirini Mavropoulou, Volker Ellenrieder, Golo Petzold, Steffen Kunsch, Albrecht Neesse, Ahmad Amanzada.
Footnotes
Abbreviations: 25 (OH)D = vitamin D, anti-TNF-α-TC = anti-tumor necrosis-factor-α-trough-concentration, AUROC = area under the receiver operating curve analysis, BR = biological remission, CD = Crohn's disease, CI = confidence interval, CR = clinical remission, CRP = C-reactive protein, IBD = inflammatory bowel disease, UC = ulcerative colitis.
The authors acknowledge support by the Open Access Publication Funds of the Göttingen University.
The authors have no conflicts of interest to disclose.
References
- [1].Mayer L. Evolving paradigms in the pathogenesis of IBD. J Gastroenterol 2010;45:9–16. [DOI] [PubMed] [Google Scholar]
- [2].Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008;134:577–94. [DOI] [PubMed] [Google Scholar]
- [3].Colombel JF, Louis E, Peyrin-Biroulet L, et al. Deep remission: a new concept? Dig Dis 2012;30suppl 3:107–11. [DOI] [PubMed] [Google Scholar]
- [4].Weisshof R, Chermesh I. Micronutrient deficiencies in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care 2015;18:576–81. [DOI] [PubMed] [Google Scholar]
- [5].Hwang C, Ross V, Mahadevan U. Popular exclusionary diets for inflammatory bowel disease: the search for a dietary culprit. Inflamm Bowel Dis 2014;20:732–41. [DOI] [PubMed] [Google Scholar]
- [6].Han YM, Yoon H, Lim S, et al. Risk factors for vitamin D, zinc, and selenium deficiencies in Korean patients with inflammatory bowel disease. Gut Liver 2017;11:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Baeke F, Takiishi T, Korf H, et al. Vitamin D: modulator of the immune system. Curr Opin Pharmacol 2010;10:482–96. [DOI] [PubMed] [Google Scholar]
- [8].Del Pinto R, Pietropaoli D, Chandar AK, et al. Association between inflammatory bowel disease and vitamin D deficiency: a systematic review and meta-analysis. Inflamm Bowel Dis 2015;21:2708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ananthakrishnan AN. Environmental triggers for inflammatory bowel disease. Curr Gastroenterol Rep 2013;15:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Reich KM, Fedorak RN, Madsen K, et al. Vitamin D improves inflammatory bowel disease outcomes: basic science and clinical review. World J Gastroenterol 2014;20:4934–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Prasad AS. Effects of zinc deficiency on Th1 and Th2 cytokine shifts. J Infect Dis 2000;182suppl 1:S62–8. [DOI] [PubMed] [Google Scholar]
- [12].Wong CP, Rinaldi NA, Ho E. Zinc deficiency enhanced inflammatory response by increasing immune cell activation and inducing IL6 promoter demethylation. Mol Nutr Food Res 2015;59:991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ojuawo A, Keith L. The serum concentrations of zinc, copper and selenium in children with inflammatory bowel disease. Cent Afr J Med 2002;48:116–9. [PubMed] [Google Scholar]
- [14].Alkhouri RH, Hashmi H, Baker RD, et al. Vitamin and mineral status in patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2013;56:89–92. [DOI] [PubMed] [Google Scholar]
- [15].Siva S, Rubin DT, Gulotta G, et al. Zinc deficiency is associated with poor clinical outcomes in patients with inflammatory bowel disease. Inflamm Bowel Dis 2017;23:152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19suppl A:5A–36A. [DOI] [PubMed] [Google Scholar]
- [17].Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet 1980;1:514. [DOI] [PubMed] [Google Scholar]
- [18].Lewis JD, Chuai S, Nessel L, et al. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis 2008;14:1660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Amanzada A, Goralczyk AD, Moriconi F, et al. Vitamin D status and serum ferritin concentration in chronic hepatitis C virus type 1 infection. J Med Virol 2013;85:1534–41. [DOI] [PubMed] [Google Scholar]
- [20].Livingstone C. Zinc: physiology, deficiency, and parenteral nutrition. Nutr Clin Pract 2015;30:371–82. [DOI] [PubMed] [Google Scholar]
- [21].Vande Casteele CN, Herfarth H, Katz J, et al. American Gastroenterological Association Institute Technical Review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology 2017;153:835–57. [DOI] [PubMed] [Google Scholar]
- [22].Massironi S, Rossi RE, Cavalcoli FA, et al. Nutritional deficiencies in inflammatory bowel disease: therapeutic approaches. Clin Nutr 2013;32:904–10. [DOI] [PubMed] [Google Scholar]
- [23].Ulitsky A, Ananthakrishnan AN, Naik A, et al. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr 2011;35:308–16. [DOI] [PubMed] [Google Scholar]
- [24].Leslie WD, Miller N, Rogala L, et al. Vitamin D status and bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD Cohort Study. Am J Gastroenterol 2008;103:1451–9. [DOI] [PubMed] [Google Scholar]
- [25].Mithal A, Wahl DA, Bonjour JP, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 2009;20:1807–20. [DOI] [PubMed] [Google Scholar]
- [26].Vagianos K, Bector S, McConnell J, et al. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr 2007;31:311–9. [DOI] [PubMed] [Google Scholar]
- [27].Schaffler H, Schmidt M, Huth A, et al. Clinical factors are associated with vitamin D levels in IBD patients: a retrospective analysis. J Dig Dis 2018;19:24–32. [DOI] [PubMed] [Google Scholar]
- [28].Frigstad SO, Hoivik M, Jahnsen J, et al. Vitamin D deficiency in inflammatory bowel disease: prevalence and predictors in a Norwegian outpatient population. Scand J Gastroenterol 2017;52:100–6. [DOI] [PubMed] [Google Scholar]
- [29].Winter RW, Collins E, Cao B, et al. Higher 25-hydroxyvitamin D levels are associated with greater odds of remission with anti-tumour necrosis factor-alpha medications among patients with inflammatory bowel diseases. Aliment Pharmacol Ther 2017;45:653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhu Y, Mahon BD, Froicu M, et al. Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol 2005;35:217–24. [DOI] [PubMed] [Google Scholar]
- [31].Jorgensen SP, Agnholt J, Glerup H, et al. Clinical trial: vitamin D3 treatment in Crohn's disease—a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther 2010;32:377–83. [DOI] [PubMed] [Google Scholar]
- [32].Miheller P, Muzes G, Hritz I, et al. Comparison of the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn's disease patients. Inflamm Bowel Dis 2009;15:1656–62. [DOI] [PubMed] [Google Scholar]
- [33].Garg M, Rosella O, Rosella G, et al. Evaluation of a 12-week targeted vitamin D supplementation regimen in patients with active inflammatory bowel disease. Clin Nutr 2018;37:1375–82. [DOI] [PubMed] [Google Scholar]
- [34].Reich KM, Fedorak RN, Madsen K, et al. Role of vitamin D in infliximab-induced remission in adult patients with Crohn's disease. Inflamm Bowel Dis 2016;22:92–9. [DOI] [PubMed] [Google Scholar]
- [35].King JC, Shames DM, Lowe NM, et al. Effect of acute zinc depletion on zinc homeostasis and plasma zinc kinetics in men. Am J Clin Nutr 2001;74:116–24. [DOI] [PubMed] [Google Scholar]
- [36].Coyle P, Philcox JC, Carey LC, et al. Metallothionein: the multipurpose protein. Cell Mol Life Sci 2002;59:627–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kruis W, Rindfleisch GE, Weinzierl M. Zinc deficiency as a problem in patients with Crohn's disease and fistula formation. Hepatogastroenterology 1985;32:133–4. [PubMed] [Google Scholar]
- [38].Kruis W, Phuong NG. Iron deficiency, zinc, magnesium, vitamin deficiencies in Crohn's disease: substitute or not? Dig Dis 2016;34:105–11. [DOI] [PubMed] [Google Scholar]
- [39].St Clair EW, Wagner CL, Fasanmade AA, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2002;46:1451–9. [DOI] [PubMed] [Google Scholar]
- [40].Ordas I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol 2012;10:1079–87. [DOI] [PubMed] [Google Scholar]
- [41].Ward MG, Warner B, Unsworth N, et al. Infliximab and adalimumab drug levels in Crohn's disease: contrasting associations with disease activity and influencing factors. Aliment Pharmacol Ther 2017;46:150–61. [DOI] [PubMed] [Google Scholar]
- [42].Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol 2015;110:802–19. [DOI] [PubMed] [Google Scholar]


