Abstract
Rationale:
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening hyperinflammatory syndrome that can be caused by bacterial infection. Streptococcus suis (S. suis) is a zoonotic pathogen that can cause severe disease in both pigs and humans. We report the first-ever documented case of HLH secondary to S. suis infection.
Patient concerns:
A 12-year-old girl presented with fever, rash, hepatosplenomegaly, pancytopenia, and elevated levels of ferritin and soluble CD25. Bone marrow examination revealed hemophagocytosis. Blood culture was positive for S. suis.
Diagnosis:
A diagnosis of hemophagocytic syndrome due to S. suis was established.
Interventions:
We treated the patient with intravenous immunoglobulin, intravenous imipenem, and supportive care.
Outcomes:
The patient eventually showed complete recovery.
Lessons:
Inflammatory response plays an important role in S. suis infection. Aberrant inflammatory response to S. suis infection may induce HLH. This case report illustrates that early definitive diagnosis and prompt treatment is a key imperative in patients with suspected S. suis infection.
Keywords: children, hemophagocytic lymphohistiocytosis, infection, pancytopenia, Streptococcus suis
1. Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening hyperinflammatory syndrome that results from a highly stimulated but ineffective immune response to antigens. HLH is of 2 different forms: primary and secondary HLH. The condition is associated with various infections. The infectious triggers include viruses, bacteria, parasites, and fungi.[1–3] Among these, Epstein–Barr virus (EBV) is the most frequent trigger. Streptococcus suis (S. suis) is a zoonotic pathogen that can cause severe disease in both pigs and humans. Till date, there are no case reports on S. suis infection associated with HLH in the published literature. Herein, we describe the first case of HLH secondary to S. suis infection in a previously healthy girl.
2. Case presentation
A 12-year-old previously healthy girl had a 6-day history of fever (Tmax 39.5°C) with chills and did not respond to treatment administered at a local clinic. Skin rash was present 1 day before admission to our hospital. During her illness, a pig reared by her family died from infection, with ecchymosis in its ear. Physical examination of the patient revealed dense, rice grain-sized rash over her face and trunk; however, there was no superficial lymph node enlargement. Slightly hyperemic pharynx and mild swollen tonsils were observed. Abdominal examination revealed hepatosplenomegaly. Patient has provided informed consent for publication of the case.
Laboratory tests showed leukopenia (white blood cell [WBC] count 1.05 × 109/L), neutropenia (neutrophils [N] count 0.68 × 109/L), thrombocytopenia (platelet count 71 × 109/L), and anemia (hemoglobin 96 g/L). Additionally, elevated levels of lactate dehydrogenase, α-hydroxybutyric dehydrogenase, cholinesterase, liver enzymes, ferritin, and C-reactive protein were observed. Blood glucose, triglycerides, prothrombin time, activated partial prothrombin time, erythrocyte sedimentation rate, and reticulocyte counts were normal (Table 1). Serum antibodies for hepatitis B and C, HIV, EBV, and T-SPOT TB test were negative. Serology tests for Widal reaction, Brucella sp., toxoplasmosis and hemorrhagic fever viruses were negative. Autoantibodies (anti-nuclear, anti-endothelial cell, anti-smooth muscle cell antibodies) and Coombs’ tests were also negative. Serum level of soluble CD25 (interleukin [IL]-2 receptor, normal range: 223–710 U/mL) was 3817 U/mL, and low natural killer (NK) cell activity was observed. Chest and abdominal computed tomography revealed mild inflammation in right middle lung, splenomegaly, and scattered lymph nodes in the abdominal cavity and retroperitoneum. Bone marrow examination showed evidence of hemophagocytosis (Fig. 1). Increased proportion of peripheral lymphocytes was observed; the proportion of peripheral blood atypical lymphocytes was 6%, and toxic granules in neutrophils were increased.
Table 1.
Results of laboratory investigations.
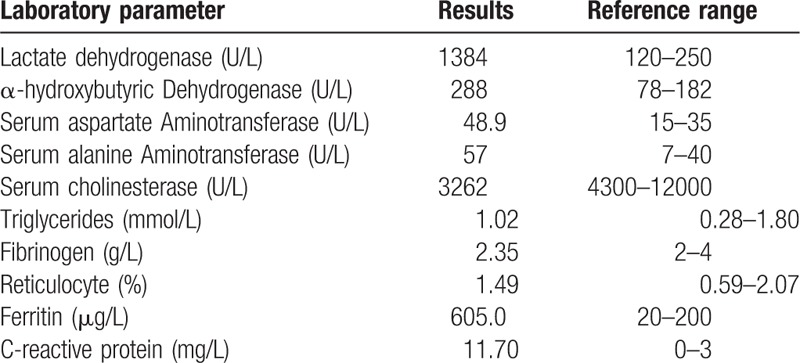
Figure 1.
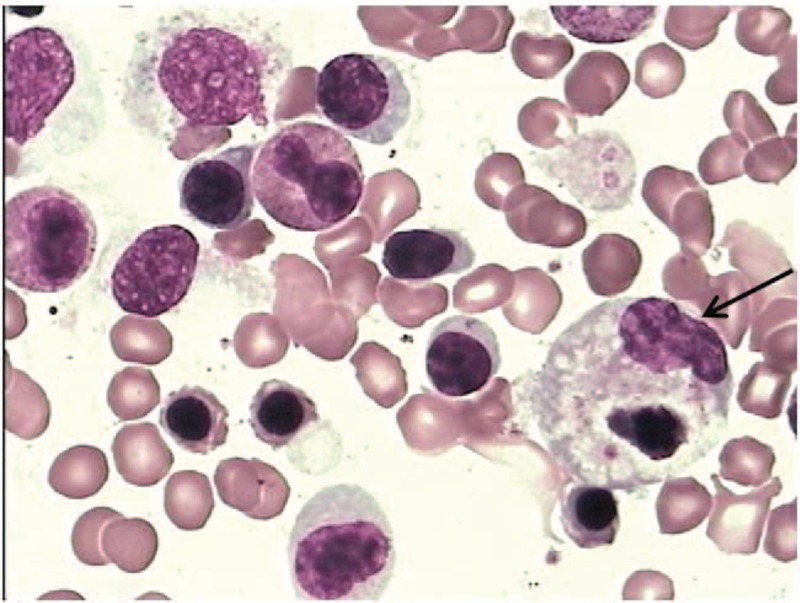
Bone marrow smear of the patient showing hemophagocytosis. Arrow shows a macrophage engulfing blood cell.
A diagnosis of septicemia, pneumonia, hepatic lesion, and HLH was considered and she was managed with azithromycin, cefoperazone/sulbactam, compound glycyrrhizin, and intravenous immunoglobulins for 3 days. However, she failed to respond to treatment. Therefore, cefoperazone/sulbactam was substituted by imipenem. After 2 days, she no longer suffered from fever and the rash was gradually fading. Blood tests indicated WBC count 1.50 × 109/L, N count 0.71 × 109/L, hemoglobin 94 g/L, and platelet count 134 × 109/L. Blood culture was positive for S. suis; antibiotic susceptibility testing showed sensitivity to imipenem. Hence, she continued to receive intravenous imipenem for 10 days. At the time of discharge, she had completely recovered with no fever or rash. The laboratory tests indicated WBC count 2.28 × 109/L, N count 1.15 × 109/L, hemoglobin 108 g/L, platelet count 248 × 109/L, and ferritin 228.8 μg/L. Blood culture was negative. Her blood counts, blood biochemistry, coagulation profile, and soluble CD25 levels were normal on follow-up examination at 1 month and 3 months after discharge.
3. Discussion
A previously healthy girl presented with fever, hepatosplenomegaly, pancytopenia (anemia, leucopenia, and thrombocytopenia), elevated ferritin, low NK cell activity, elevated soluble CD25, and hemophagocytosis in bone marrow. The diagnosis of hemophagocytic syndrome was established as the patient qualified 7 of the 8 criteria proposed by the Histiocyte Society.[4]
HLH usually occurs in association with various underlying genetic and/or acquired conditions. HLH frequently develops in patients with underlying genetic disease. However, in this case, there were no strong indications for a genetic predisposition, such as familial disease or recurrent episodes of HLH. Therefore, the patient was classified as a case of “secondary” HLH. There was no evidence of malignancy (such as leukemias or lymphomas), metabolic or autoimmune disease as the potential trigger for “secondary” HLH. The most common form of “secondary” HLH, that is, infection-associated HLH, was the likely cause of “secondary” HLH. This is supported by the recent history of death of a pig reared by the family, ostensibly due to infection, positive blood culture for S. suis, and complete recovery with intravenous imipenem therapy. Based on the above, a diagnosis of hemophagocytic syndromes secondary to S. suis was established. Other infectious triggers for “secondary” HLH have been described such as EBV, HIV, human herpes virus, cytomegalovirus, varicella zoster, herpes simplex, influenza, and parainfluenza and measles virus; besides these, there have been several bacterial etiologies, such as Mycobacteria, Brucella, Rickettsia, Haemophilus influenza, and Serratia sp.[5–13]
S. suis is an ovoid-shaped gram-positive coccus that forms short chains. To date, 35 serotypes of S. suis have been described, of which, serotype 2 is a major and virulent human and pig pathogen.[14] Schwerk et al[15] reported that S. suis infection is associated with release of pro-inflammatory cytokines and chemokines (e.g., IL6 and IL8). de Greeff et al[16] reported significantly altered expression of macrophage-specific genes (IL-1-β, MIP-2-α, and TNF-α) in the setting of S. suis infection, which suggests that MAP-kinase signaling pathway and NF-κB signaling are associated with the response of porcine alveolar macrophages to S. suis infection. Apparently, the inflammatory response plays an important role in S. suis infection. Hence, S. suis infection may induce HLH owing to aberrant inflammatory response.
Additionally, immunosuppressive conditions can predispose persons to S. suis infection [17]; the 12-year-old girl with a normal immune system may have been susceptible, owing to the generally low immunity during childhood like other children. The most common clinical manifestations of S. suis infection in humans are bacteremia and/or septicemia, meningitis, endocarditis, arthritis, endophthalmitis, and spondylodiscitis;[18] a case of pneumonia has also been reported earlier.[19] Septicemia and pneumonia were observed in our patient. Because of the successful treatment, complications that are associated with high mortality were not found in the girl.
4. Conclusion
This case report describes a previously healthy girl who developed HLH secondary to S. suis infection. She fully recovered with intravenous immunoglobulins, intravenous imipenem, and supportive care, without HLH protocol. Our experience with this patient suggests the need for a high index of suspicion for diagnosis of HLH due to S. suis in HLH patients who have a history of porcine exposure before the onset of symptoms. It also reminds us not to delay treatment while waiting for a definitive diagnosis of HLH, owing to the risk of potentially serious complications such as secondary sepsis with multi organ dysfunction and failure with high mortality. In other words, once S. suis infection is suspected, immediate steps should be taken to make a clear diagnosis and institute prompt treatment.
Author contributions
Conceptualization: Shuang-Shuang Liu.
Data curation: Shuang-Shuang Liu, Yue Wang.
Investigation: Shuang-Shuang Liu.
Methodology: Shuang-Shuang Liu, Yue Wang.
Project administration: Chun-Huai Li.
Writing – Original Draft: Shuang-Shuang Liu.
Writing – Review & Editing: Lu Xue, Cui Ma, Chun-Huai Li.
Footnotes
Abbreviations: CRP = C-reactive protein, EBV = Epstein–Barr virus, HLH = Hemophagocytic lymphohistiocytosis, NK = natural killer.
The authors report no conflicts of interest.
References
- [1].Awan NA, Alkhazraji HA, Taryam E, et al. Hemophagocytic lymphohistiocytosis: a case series of 10 patients from UAE. J Appl Hematol 2013;4:96. [Google Scholar]
- [2].Janka G, Imashuku S, Elinder G, et al. Infection- and malignancy-associated hemophagocytic syndromes. Secondary hemophagocytic lymphohistiocytosis. Hematol Oncol Clin North Am 1998;12:435–44. [DOI] [PubMed] [Google Scholar]
- [3].Risdall RJ, McKenna RW, Nesbit ME, et al. Virus-associated hemophagocytic syndrome: a benign histiocytic proliferation distinct from malignant histiocytosis. Cancer 1979;44:993–1002. [DOI] [PubMed] [Google Scholar]
- [4].Henter JI, Horne A, Arico M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007;48:124–31. [DOI] [PubMed] [Google Scholar]
- [5].Cattaneo C, Oberti M, Skert C, et al. Adult onset hemophagocytic lymphohistiocytosis prognosis is affected by underlying disease and coexisting viral infection: analysis of a single institution series of 35 patients. Hematol Oncol 2017;35:828–34. [DOI] [PubMed] [Google Scholar]
- [6].Atim-Oluk M. Cytomegalovirus associated haemophagocytic lymphohistiocytosis in the immunocompetent adult managed according to HLH-2004 diagnostic using clinical and serological means only. Eur J Microbiol Immunol (Bp) 2013;3:81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Presti MA, Costantino G, Della Torre A, et al. Severe CMV-related pneumonia complicated by the hemophagocytic lymphohistiocytic (HLH) syndrome in quiescent Crohn's colitis: harmful cure? Inflamm Bowel Diseases 2011;17:E145–6. [DOI] [PubMed] [Google Scholar]
- [8].Kanhere S, Bhagat M, Kadakia P, et al. Hemophagocytic lymphohistiocytosis associated with cytomegalovirus infection in an immunocompetent infant: a diagnostic and therapeutic challenge!. Indian J Hematol Blood Transfus 2014;30:299–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cascio A, Fama F, Mondello P, et al. Cytomegalovirus infections, kidney transplantation, and secondary hemophagocytic lymphohistiocytosis. Transpl Infect Dis 2014;16:1039–41. [DOI] [PubMed] [Google Scholar]
- [10].Brito-Zeron P, Bosch X, Perez-de-Lis M, et al. Infection is the major trigger of hemophagocytic syndrome in adult patients treated with biological therapies. Semin Arthritis Rheum 2016;45:391–9. [DOI] [PubMed] [Google Scholar]
- [11].Yaman Y, Gozmen S, Ozkaya AK, et al. Secondary hemophagocytic lymphohistiocytosis in children with brucellosis: report of three cases. J Infect Dev Ctries 2015;9:1172–6. [DOI] [PubMed] [Google Scholar]
- [12].Anoun S, Traoue Y, Ouled Lahcen A, et al. Reactive hemophagocytic syndrome caused by Rickettsial infection: report of a case. Lab Hematol 2012;18:14–6. [DOI] [PubMed] [Google Scholar]
- [13].Saidi W, Gammoudi R, Korbi M, et al. Hemophagocytic lymphohistiocytosis: an unusual complication of leprosy. Int J Dermatol 2015;54:1054–9. [DOI] [PubMed] [Google Scholar]
- [14].Staats JJ, Feder I, Okwumabua O, et al. Streptococcus suis: past and present. Vet Res Commun 1997;21:381–407. [DOI] [PubMed] [Google Scholar]
- [15].Schwerk C, Adam R, Borkowski J, et al. In vitro transcriptome analysis of porcine choroid plexus epithelial cells in response to Streptococcus suis: release of pro-inflammatory cytokines and chemokines. Microbes Infect 2011;13:953–62. [DOI] [PubMed] [Google Scholar]
- [16].de Greeff A, Benga L, Wichgers Schreur PJ, et al. Involvement of NF-kappaB and MAP-kinases in the transcriptional response of alveolar macrophages to Streptococcus suis. Vet Microbiol 2010;141:59–67. [DOI] [PubMed] [Google Scholar]
- [17].Gomez-Zorrilla S, Ardanuy C, Lora-Tamayo J, et al. Streptococcus suis infection and malignancy in man, Spain. Emerg Infect Dis 2014;20:1067–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Huong VT, Ha N, Huy NT, et al. Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg Infect Dis 2014;20:1105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Oh YJ, Song SH. A case of Streptococcus suis infection causing pneumonia with empyema in Korea. Tuberc Respir Dis (Seoul) 2012;73:178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]


