Abstract
The relationship of hypothyroidism and Menière's disease (MD) has been discussed before, yet not well documented. Our study aims to investigate the correlation of both diseases.
This is a retrospective cohort study based on data from the LHID2000 (Longitudinal Health Insurance Database 2000), a subset of the Taiwan National Research Health Insurance Database that contains claims data for the 2000 to 2011 period. A total of 27,050 patients were included in this study, 5410 of whom had received a hypothyroidism diagnosis. The prevalence of MD was high in patients with hypothyroidism (95% confidence interval [CI]: 1.14–1.51), especially in those older than 50 years old (P < .001). Although comorbidities such as hypertension or cirrhosis are significant risk factors for Menière's disease (P < .001, P < .05), the incidence rate of Menière's disease in patients with hypothyroidism differs significantly between groups without these comorbidities (95% CI: 1.14–1.95). Regarding the timing for the occurrence of Menière's disease in patients with hypothyroidism, there was a significant time interval of <5 years (P < .05). The risk of MD decreased after treatment with thyroxine and did not differ from that of the nonhypothyroidism cohort (adjusted HR [aHR] = 0.85, 95% CI: 0.66–1.11).
The study demonstrates a significant association between hypothyroidism and Menière's disease, especially in elderly female patients. Physicians should consider verifying the thyroid function when encountering these patients.
Keywords: endocrine disorder, hypothyroidism, Menière's disease, vertigo
1. Introduction
Menière's disease (MD) is a disabling syndrome characterized by episodic vertigo, fluctuating sensorineural hearing loss, aural fullness, and tinnitus.[1] In 1861, Prosper Menière first described the symptom complex bearing his name as an inner ear labyrinthine dysfunction rather than a central neurological disorder.[2] In Asia, a previous study revealed that the mean annual prevalence and incidence were 34.5 and 5.0 per 100,000 persons, respectively.[3]
Despite the well-known histopathological lesion (endolymphatic hydrops, dilation of the membranous labyrinth of the inner ear),[4] its etiopathogenesis remains uncertain and multifactorial. Autoimmune factors,[5,6] trauma,[7,8] viral infection,[9,10] genetic predisposition,[11,12] hormonal disorder,[13] and metabolic factors[14,15] might contribute to the genesis of MD.
The inner ear is an elegant but extremely sensitive organ. It relies on continuous blood flow for oxygen and to remove metabolic waste. Nevertheless, auditory and vestibular function can sometimes be disrupted by metabolic disorders. The hearing impairment in patients with goiter was first described by Bircher in 1883 and confirmed in 1888 when the Myxoedema Committee of the Clinical Society of London found auditory dysfunction in 38 out of 69 myxoedematous patients.[16] Hearing disorders were found not only in patients with Pendred syndrome but also in those with acquired hypothyroidism.[17] According to another study, the incidence of hearing impairment was 43% in hypothyroid individuals. Tinnitus was found in 7% of cases and vertigo in 29.1%. The incidence of these symptoms correlated linearly with the severity of hypothyroidism. There was a substantial improvement in patients’ perceived symptoms when they became euthyroid, but this has not been confirmed by audiometry.[18]
However, most of these studies had a small sample and cross-sectional design. Therefore, we conducted a population-based cohort study to analyze the interrelationship between hypothyroidism and consequent MD.
2. Methods
2.1. Data source
The data for this population-based retrospective cohort study were sourced from the Longitudinal Health Insurance Database 2000 (LHID2000), which contains the original claims data for 1,000,000 beneficiaries randomly sampled from the 2000 Registry of Beneficiaries of the National Health Insurance Research Database. Instituted in 1995, Taiwan's National Health Insurance (NHI) program is a compulsory social insurance program that covers nearly 99% of residents.[19] Details of the NHI program and LHID2000 have been thoroughly covered in previous studies.[20,21] In the database, diseases are coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The study was approved by the Institutional Review Board of China Medical University Hospital, Taiwan (CMUH-104-REC2–115).
2.2. Sampled participants
The hypothyroidism cohort comprised subjects aged ≥20 years who received a new diagnosis of hypothyroidism (ICD-9-CM codes 243, 244.0, 244.1, 244.3, 244.8, 244.9) between 2000 and 2010. The date of hypothyroidism diagnosis was defined as the index date. A nonhypothyroidism cohort was frequency-matched at a 1:4 ratio with randomly selected subjects aged ≥20 years and without hypothyroidism. Subjects with a previous diagnosis of MD (ICD-9-CM codes 386.0, 386.00, 386.01, 386.02, 386.03, 386.04) before the index date were excluded. Both cohorts were matched by age (at 5-year intervals), sex, and index year. Subjects in both cohorts were followed up until December 31, 2011, unless they received a new diagnosis of MD or withdrew from the NHI program. The baseline comorbid diseases including diabetes, hypertension, hyperlipidemia, stroke, ischemic heart disease, cirrhosis, and chronic kidney disease were identified according to their diagnoses in the medical records prior to the index date.
2.3. Statistical analysis
The chi-square test and Student t test were used to examine the differences in categorical and continuous variables between the 2 cohorts. Cumulative incidence curves of MD were computed using the Kaplan–Meier method, and differences in curves between the 2 cohorts were tested using a log-rank test. The incidence density rates of MD were estimated by dividing the number of MD occurrences by the number of person-years for different risk factors and stratified by sex, age, comorbidity, and follow-up period. Univariable and multivariable Cox proportional hazards regression models were employed to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for developing MD. The multivariable Cox models were adjusted for age, sex, and comorbidities of hypertension, hyperlipidemia, stroke, ischemic heart disease, cirrhosis, and chronic kidney disease. Stratified by age, sex, comorbidity, and follow-up period, the relative risk of MD in patients with hypothyroidism relative to the nonhypothyroidism cohort was also analyzed using the Cox model. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). The two-sided significance level was set at P < .05.
3. Result
3.1. Demographic characteristics and comorbidities
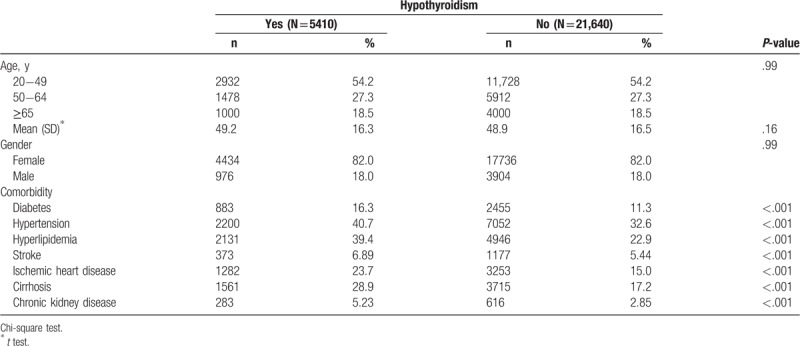
This study analyzed 5410 hypothyroidism cases frequency-matched with 21,640 nonhypothyroidism cases. The distributions of age (54.2% aged 20–49 years) and sex (82% female) were similar in the hypothyroidism (mean, 49.2 ± 16.3 years) and nonhypothyroidism (mean, 48.9 ± 16.5 years) cohorts (Table 1). Compared with the nonhypothyroidism cohort, individuals with hypothyroidism exhibited more comorbidities of diabetes, hypertension, hyperlipidemia, stroke, ischemic heart disease, cirrhosis, and chronic kidney disease at the baseline (P < .001). The mean follow-up period was 5.79 ± 3.28 years for the hypothyroidism cohort and 5.85 ± 3.25 years for the nonhypothyroidism cohort.
Table 1.
Characteristics of subjects with versus without hypothyroidism.
3.2. Cox model with hazard ratios and 95% CI of MD associated with hypothyroidism and covariates
The overall incidence of MD was significantly higher in the hypothyroidism cohort (8.65 vs 6.38 per 1000 person-years) with a crude HR (cHR) of 1.36 (95% CI = 1.18–1.56) (Table 2). After adjustments for age, sex, and comorbidities of hypertension, hyperlipidemia, ischemic heart disease, cirrhosis, and chronic kidney disease the risk of MD was 1.31-fold in the hypothyroidism cohort than that in the nonhypothyroidism cohort, with an adjusted HR (aHR) of 1.31 (95% CI = 1.14–1.51).
Table 2.
Incidence density rates and risk factors for MD.
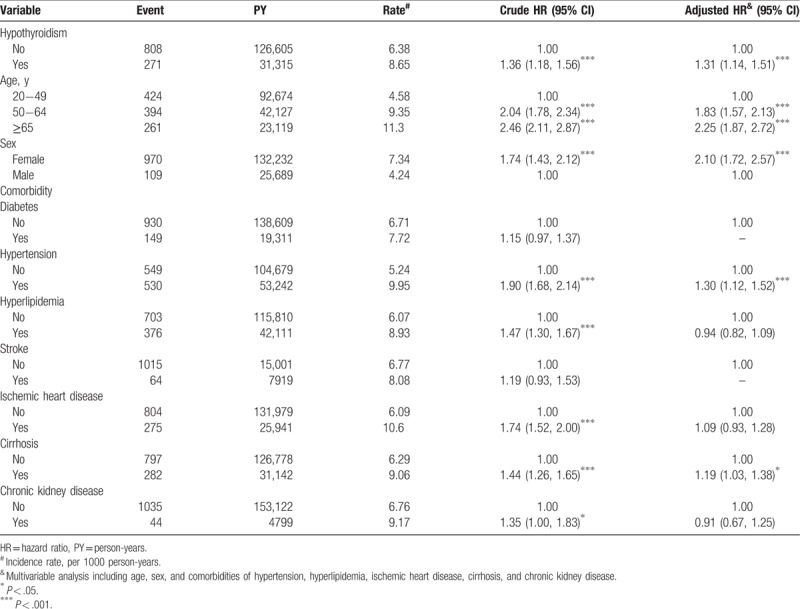
Relative to individuals aged 20 to 49 years, the risk of MD was 1.83 times higher in those aged 50 to 64 years (95% CI = 1.57–2.13) and 2.25 times higher among those aged ≥65 years (95% CI = 1.87–2.72). Multivariable models showed that the risk of MD was 2.10 times higher for women than for men (95% CI = 1.72–2.57). Patients with certain comorbidities were at a greater risk of MD, particularly those with hypertension (aHR = 1.30, 95% CI = 1.12–1.52) or cirrhosis (aHR = 1.19, 95% CI = 1.03–1.38). A previous study posited that hyperlipidemia is related to MD,[26] but the present study has no significant findings supporting this claim.
3.3. Comparison of risks of MD stratified by sex, age, comorbidity, and follow-up period
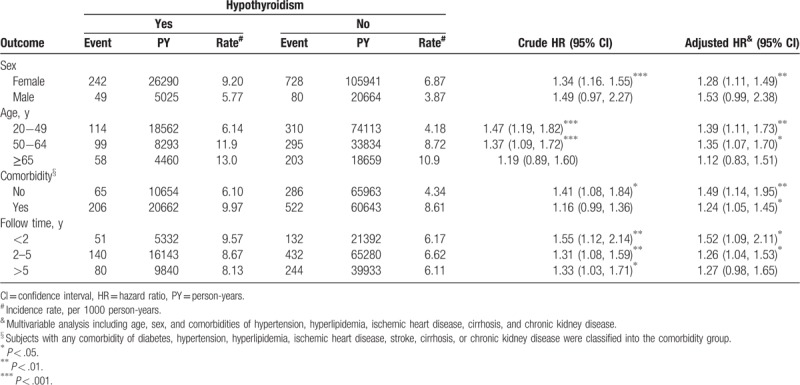
In both cohorts, the incidence density rate of MD was significantly higher in women than men. The sex-specific aHR of MD for hypothyroidism relative to nonhypothyroidism was significant for women (aHR = 1.28, 95% CI = 1.11–1.49) (Table 3). Except for the group aged ≥65 years, patients with hypothyroidism were associated with a significantly higher risk of MD than those without hypothyroidism (20−49 years: aHR = 1.39, 95% CI = 1.11–1.73; 50–64 years: aHR = 1.35, 95% CI = 1.07–1.70). The incidence density rate of MD was significantly higher for patients with comorbidity than those without comorbidity; however, the risk of MD was higher for patients without comorbidity (aHR = 1.49, 95% CI = 1.14–1.95) than with comorbidity (aHR = 1.24, 95% CI = 1.05–1.45). Stratified by follow-up period, the hypothyroidism cohort exhibited a higher risk of MD than nonhypothyroidism cohort, which was significantly higher for the period of <2 years (aHR = 1.52, 95% CI = 1.09–2.11) and for the period of 2 to 5 years (aHR = 1.26, 95% CI = 1.04–1.53).
Table 3.
Incidence density rates and HRs of MD (with vs without hypothyroidism).
3.4. The effects of thyroxine treatment on the risks of MD development
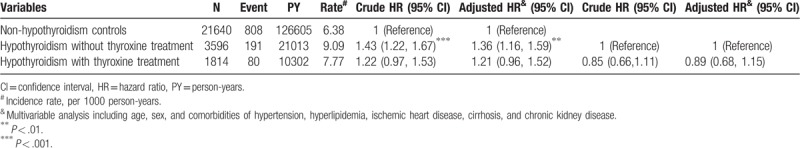
The effects of thyroxine treatment related to MD risk are shown in Table 4. Compared with the nonhypothyroidism cohort, hypothyroidism without treatment was associated with a higher risk of MD (aHR = 1.36, 95% CI = 1.16–1.59). By contrast, the risk of MD decreased after treatment with thyroxine and did not differ from that of the nonhypothyroidism cohort (aHR = 0.85, 95% CI = 0.66–1.11).
Table 4.
Incidence density rates and HRs of MD for the hypothyroidism cohort (with and without thyroxine treatment) relative to the nonhypothyroidism cohort.
3.5. Cumulative incidence of MD in the hypothyroidism and nonhypothyroidism cohorts
The Kaplan–Meier graph shows that the cumulative incidence of MD was higher in the hypothyroidism cohort (log-rank test P < .001) (Fig. 1).
Figure 1.
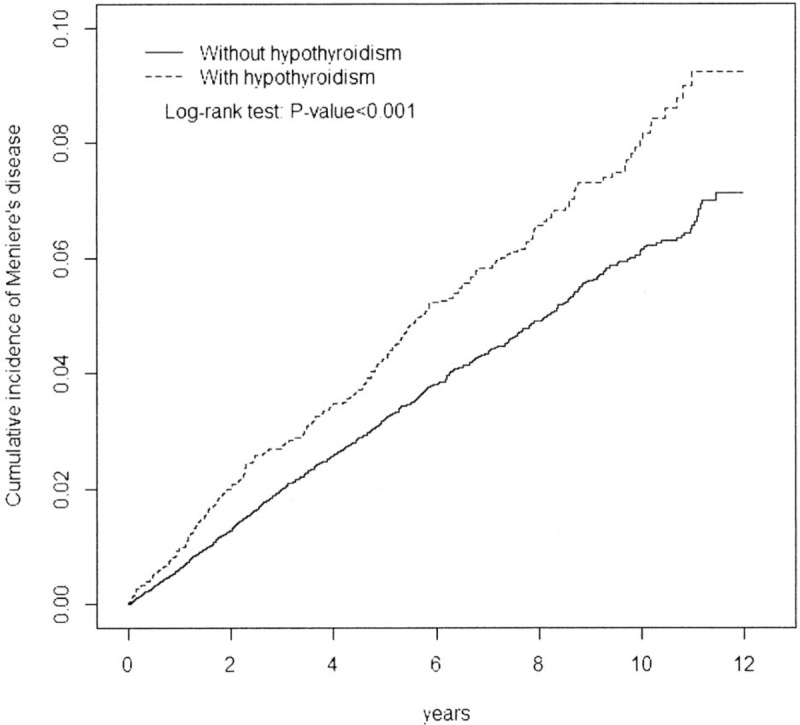
Cumulative incidence of MD (with vs without hypothyroidism). MD = Menière's disease.
4. Discussion
The literature reports numerous discussions about the correlation of hypothyroidism and vestibular system dysfunction. Powers reported on 98 patients with MD, 17% of whom had clinically significant hypothyroidism.[22] Studies performed in Japan on 13 patients with congenital hypothyroidism revealed a high incidence of vestibular dysfunction.[23] The essential factors for distinguishing the peripheral or central vestibular abnormalities were the initial serum thyroxine level and the time of initiation of the thyroxine supplement. Vestibulocerebellar impairment was associated with a severe and prolonged deficiency of thyroid hormone, whereas peripheral vestibular impairment was associated with mild hypothyroidism.[23]
However, testing the hypothesis of hypothyroidism and resultant MD has produced conflicting results. A retrospective review conducted by Meyerhoff revealed that of 211 patients with classic MD and 208 patients tested for hypothyroidism, only one had an abnormal result. This finding led them to conclude that in the absence of myxedema, routine screening of thyroid function was unnecessary.[24]
To the best of our knowledge, this is the first nationwide, population-based retrospective cohort study to evaluate incident MD in patients with hypothyroidism. We found that hypothyroid patients had a significantly elevated risk of MD than those without hypothyroidism. Those who were women, elderly, hypertensive, or cirrhotic accounted for a higher proportion of MD cases. The incidence of MD was higher in elderly individuals, although hypothyroid patients have a higher probability of developing MD when they were young. Previous studies have shown that diabetes mellitus[25] and hyperlipidemia[26] are related to MD. According to our study, however, the increased risk of MD was in patients with hypertension and cirrhosis. The relationship between these diseases remains unclear and requires further research, although some studies have begun to explore the association.[27]
The improvement of MD after thyroxine supplement is controversial. Powers found clinically significant hypothyroidism in 17% of 98 patients with MD, but only 3 of them had their symptoms under control after thyroxine treatment.[22] In another study, 12 among 35 hypothyroid patients were found to have MD, and all 12 reported subjective improvements in their symptoms after 12 weeks of thyroxine treatment.[28] According to our study, the overall incidence of MD was lower in hypothyroid patients with treatment compared with those without treatment, yet the difference was nonsignificant.
Our study has several limitations. First, hypothyroidism can be divided into multiple subgroups and complex etiologies exist in each subgroup. Additional analysis and many other variables are required to confirm our study. Second, the increased risk of MD was found in hypothyroid patients with comorbid hypertension or cirrhosis. But the relationship of these diseases remained unclear and requires further research. It will be helpful if objective measurement such as audiometry or blood pressure monitoring is included. Third, our study had shown that the overall incidence of MD was lower in hypothyroid patients with treatment compared with those without treatment, yet the difference was nonsignificant. Considering the patients’ satisfaction with treatment and the promotion of life quality, further prospective studies should be performed.
5. Conclusion
Our study suggests that patients with hypothyroidism are at a greater risk of incident MD than those without hypothyroidism. Clinical screening for thyroid function should be considered for individuals with MD, especially in elderly women. Furthermore, thyroxine might benefit such patients.
Author contributions
Data curation: Hang Cheng Chen.
Formal analysis: Hang Cheng Chen.
Investigation: Hang Cheng Chen.
Methodology: Tai-Yi Hsu.
Project administration: Tai-Yi Hsu.
Resources: Chih-Yu Chen.
Software: Chih-Yu Chen, Cheng-Li Lin.
Supervision: Chih-Yu Chen, Cheng-Li Lin, Wei-Kung Chen.
Validation: Cheng-Li Lin, Wei-Kung Chen.
Writing – Original Draft: Wen-Ling Lin.
Writing – Review & Editing: Wen-Ling Lin.
Hang Cheng Chen orcid: 0000-0002-9418-6277.
Tai-Yi Hsu orcid: 0000-0003-3095-1248.
Chih-Yu Chen orcid: 0000-0001-9986-8413.
Wen-Ling Lin orcid: 0000-0002-3148-6432.
Footnotes
Abbreviations: aHR = adjusted HR, cHR = crude HR, CI = confidence interval, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, LHID2000 = Longitudinal Health Insurance Database 2000, MD = Menière's disease, NHI = Taiwan's National Health Insurance.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW108-TDU-B-212-133004), China Medical University Hospital, Academia Sinica Stroke Biosignature Project (BM10701010021), MOST Clinical Trial Consortium for Stroke (MOST 107-2321-B-039 -004-), Tseng-Lien Lin Foundation, Taichung, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan.
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- [1].Sajjadi H, Paparella MM. Meniere's disease. Lancet 2008;372:406–14. [DOI] [PubMed] [Google Scholar]
- [2].Meniere P. Sur une forme de surdite grave dependant d’une lesion de l’oreille interne. Gaz M’ed Paris 1861;16:29. [Google Scholar]
- [3].Shojaku H, Watanabe Y, Fujisaka M, et al. Epidemiologic characteristics of definite Ménière's disease in Japan. A long-term survey of Toyama and Niigata prefectures. ORL J Otorhinolaryngol Relat Spec 2005;67:305–9. [DOI] [PubMed] [Google Scholar]
- [4].Ishiyama G, Lopez IA, Sepahdari AR, et al. Meniere's disease: histopathology, cytochemistry, and imaging. Ann N Y Acad Sci 2015;1343:49–57. [DOI] [PubMed] [Google Scholar]
- [5].Kim SH, Kim JY, Lee HJ, et al. Autoimmunity as a candidate for the etiopathogenesis of Meniere's Disease: detection of autoimmune reactions and diagnostic biomarker candidate. PLoS One 2014;9:e111039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fattori B, Nacci A, Dardano A, et al. Possible association between thyroid autoimmunity and Menière's disease. Clin Exp Immunol 2008;152:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chung J, Jung HJ, Kim CS, et al. A case of post-traumatic Meniere's disease. Korean J Audiol 2014;18:41–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Toglia JU, Rosenberg PE, Ronis ML. Posttraumatic dizziness. Arch Otolaryngol 1970;92:485–92. [DOI] [PubMed] [Google Scholar]
- [9].Selmani Z, Marttila T, Pyykkö I. Incidence of virus infection as a cause of Meniere's disease or endolymphatic hydrops assessed by electrocochleography. Eur Arch Otorhinolaryngol 2005;262:331–4. [DOI] [PubMed] [Google Scholar]
- [10].Vrabec JT. Herpes simplex virus and Meniere's disease. Laryngoscope 2003;113:1431–8. [DOI] [PubMed] [Google Scholar]
- [11].Lee JM, Kim MJ, Jung J, et al. Genetic aspects and clinical characteristics of familial Meniere's disease in a South Korean population. Laryngoscope 2015;125:2175–80. [DOI] [PubMed] [Google Scholar]
- [12].Requena T, Cabrera S, Martín-Sierra C, et al. Identification of two novel mutations in FAM136A and DTNA genes in autosomal-dominant familial Meniere's disease. Hum Mol Genet 2015;24:1119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rubin W. Biochemical evaluation of the patient with dizziness. Semin Hear 1989;10:151–9. [Google Scholar]
- [14].Leonard PR. Metabolic disorders of the vestibular system. Otolaryngol Head Neck Surg 1995;112:128–32. [DOI] [PubMed] [Google Scholar]
- [15].Aoki M, Wakaoka Y, Hayashi H, et al. The relevance of hypothalamus-pituitary-adrenocortical axis-related hormones to the cochlear symptoms in Ménière's disease. Int J Audiol 2011;50:897–904. [DOI] [PubMed] [Google Scholar]
- [16].Bircher H. Der Endemische Kropf. Basel 1883. Quoted by Stephens, S.D.G. [Google Scholar]
- [17].Ritter FN. The effects of hypothyroidism upon the ear, nose and throat. A clinical and experimental study. Laryngoscope 1967;77:1427–79. [DOI] [PubMed] [Google Scholar]
- [18].Bhatia PL, Gupta OP, Agrawal MK, et al. Audiological and vestibular function tests in hypothyroidism. Laryngoscope 1977;87:2082–9. [DOI] [PubMed] [Google Scholar]
- [19].Database NHIR. Taiwan; 2015. Available at: http://nhird.nhri.org.tw/en/index.html Accessed February 16, 2017. [Google Scholar]
- [20].Hsu CL, Wang TC, Shen TC, et al. Risk of depression in patients with chronic rhinosinusitis: a nationwide population-based retrospective cohort study. J Affect Disord 2016;206:294–9. [DOI] [PubMed] [Google Scholar]
- [21].Hu WS, Lin CL. Association between cataract and risk of incident atrial fibrillation: a nationwide population-based retrospective cohort study. Mayo Clin Proc 2017;92:370–5. [DOI] [PubMed] [Google Scholar]
- [22].Powers WH. Metabolic aspects of Meniere's disease. Laryngoscope 1978;88:122–9. [DOI] [PubMed] [Google Scholar]
- [23].Sato T, Ishiguro C, Watanabe Y, et al. Quantitative analysis of cerebello-vestibular function in congenital hypothyroidism. Acta Paediatr Jpn 1987;29:121–9. [DOI] [PubMed] [Google Scholar]
- [24].Meyerhoff WL, Paparella MM, Gudbrandsson FK. Clinical evaluation of Meniere's disease. Laryngoscope 1981;91:1663–8. [DOI] [PubMed] [Google Scholar]
- [25].Proctor B, Proctor C. Metabolic management in Meniere's disease. Ann Otol Rhinol Laryngol 1981;90:615–8. [DOI] [PubMed] [Google Scholar]
- [26].Spencer JT., Jr Hyperlipoproteinemias in the etiology of inner ear disease. Laryngoscope 1973;83:639–78. [DOI] [PubMed] [Google Scholar]
- [27].Kumar A, Kaur H, Devi P, et al. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome. Pharmacol Ther 2009;124:259–68. [DOI] [PubMed] [Google Scholar]
- [28].Santosh UP, Rao MS. Incidence of hypothyroidism in Meniere's disease. J Clin Diagn Res 2016;10:MC01–3. [DOI] [PMC free article] [PubMed] [Google Scholar]


