Supplemental Digital Content is available in the text
Keywords: Acylcarnitines, metabolic screening, newborn, perinatal asphyxia, therapeutic hypothermia
Abstract
Optimal prognostic markers evaluating early neuroprotective interventions in neonatal hypoxic-ischemic encephalopathy (HIE) are lacking. This study was designed to assess the prognostic value of acylcarnitines in neonatal HIE.
An observational cohort study was conducted over 10 years in 67 HIE. Variables analyzed included sex, blood cord pH, Apgar score, hypothermia treatment (yes/no), neuron-specific enolase (NSE) levels, and clinical outcome (neurological examination, brain magnetic resonance imaging [MRI], and electroencephalogram) before discharge and at 6 months. Acylcarnitine profiles were analyzed by tandem-mass spectrometry on dried-blood spots collected on day 3 for newborn screening. A cohort of healthy newborns was used as control group.
HIE patients had significantly increased C4, C5, C5:1, C6, C6-OH, C8 levels (all P < .01) and decreased long-chain acylcarnitine levels (P < .03). Hypothermia treatment was associated with a decrease in C4 levels (p = 0.005) and an increase in most long-chain acylcarnitine levels (P < .01). A significant association was found between C4 levels and NSE on day 1 of hypothermia treatment (P = .002) and abnormal brain magnetic resonance imaging (MRI) at discharge (P = .037). In the hypothermia group, C4 levels decreased in patients with favorable outcomes but remained high in those who progressed unfavorably.
C4 appears to be a good prognostic marker in HIE, as blood levels correlated with NSE levels and abnormal MRI findings. Furthermore, hypothermia did not lead to decreased levels in patients with adverse outcomes.
1. Introduction
Acylcarnitines are analyzed primary to detect inborn errors of metabolism in the neonatal screening. Impaired acylcarnitines profile not only may be a consequence of inherited metabolic diseases (IMD), but also might be the reflection of different pathologies such as sepsis, malnutrition, type I and II diabetes, obesity, and intrauterine growth restriction.[1–6] This versatility is due to the fact that the free hydroxyl group of LC—a semi-essential nutrient for the newborn[7–9]—can react with a wide range of molecules resulting in a broad spectrum of molecule structures, and in its capability to transfer esterified metabolites.[10]
Fatty acid beta-oxidation takes place in the mitochondria and there are at least 31 enzymes or carriers that participate in it. Short-chain acyl-CoA dehydrogenase (SCAD) is the most vulnerable enzyme, which catalyzes the first step in the mitochondrial β-oxidation of fatty acids. Elevated butyrylcarnitine is the result of the dysfunction of this enzyme.[11] Acylcarnitines have been attributed a multifactorial neuroprotective role in relation to their contribution to lipid synthesis, neuronal membrane modification and stabilization, protein and gene expression modulation, mitochondrial function optimization, increased antitoxic activity, and increased cholinergic neurotransmitters levels.[12–14]
Hypoxic-ischemic encephalopathy (HIE) affects between 1 and 6 of every 1000 newborns in developed countries. HIE is the leading cause of death and severe neurological morbidity in newborn infants worldwide and is responsible for 20% of all cerebral palsy cases in children.[15] The hypoxic insult that characterizes HIE triggers a complex response that leads to energy failure, disruption of cellular homeostasis, morphologic changes in microglial cells, and mitochondrial failure.[16] Therapeutic hypothermia is the only treatment to date that has been proven to minimize HIE damage, but up to 40% of neonates treated still die or develop severe neurological impairment.[16–18] The prognostic value of clinical biomarkers such as S100B protein in serum and urine and neuron-specific enolase (NSE) in serum and cerebrospinal fluid is not yet clear,[19,20] although a recent study by our group found that urinary S100B levels measured in the first days of life predicted neonatal death and short-term prognosis in asphyxiated newborns treated with hypothermia.[21]
Knowledge of how hypoxia-ischemia affects ACP is limited,[22–24] but evidence of alterations in different ischemic tissues such as the myocardium[25] underlines the need for a better understanding of how these metabolic pathways respond to hypoxic insult. The main aim of the current study was to characterize postnatal ACP in infants with HIE compared with a control group and to correlate results with clinical outcomes. As a secondary aim, we evaluated the influence of therapeutic hypothermia on postnatal ACP in HIE.
2. Methods
2.1. Study design and population
We conducted a retrospective observational cohort study of infants with early HIE from two tertiary care university hospitals in Galicia (northwest Spain) between February 2006 and January 2016. This 10-year period was divided into two subperiods to distinguish between patients born before and after the establishment of therapeutic hypothermia treatment (availability of the equipment) in the neonatal units of our hospitals: 2006–2010 (pre-hypothermia period) and 2011–2016 (hypothermia period). We considered the HIE patients included in the pre-hypothermia subperiod as control group for the post-hypothermia ones. The neonatal population attended by the two hospitals is 7000 births/year. The study was approved by the Santiago Research Ethics Committee (2017/098).
Early HIE was considered in neonates in poor condition at birth (5-minute Apgar score <5 and/or need for prolonged major resuscitation, arterial cord blood pH ≤7, and neonatal encephalopathy) and presence of sentinel events of fetal distress such as altered monitoring, placental abruption, and instrumental delivery. Neonatal encephalopathy was evaluated using the Sarnat Grading Scale[26] based on a neurological examination performed within 6 h of birth and before initiation of hypothermia.
Inclusion and exclusion criteria: All patients with early HIE admitted to the neonatal unit during the study period were candidates for inclusion. Exclusion criteria were gestational age <37 weeks; birth <2500 g and diagnosis of an IMD.
The following variables were collected for each patient: birth weight, sex, 5-minute Apgar score, birth pH (determined in blood cord), therapeutic hypothermia (yes/no), and results of clinical neurological exam, electroencephalography (EEG), and brain magnetic resonance imaging (MRI) before discharge. The ACP was determined by analyzing dried-blood spots collected for newborn metabolic screening on the third day of life. In patients treated with hypothermia, the rewarming period should have been started or finished before the samples have been taken. This procedure should be repeated at 12–15 days of life, according to the recommendation for newborn screening in critically ill infants in our protocol. NSE levels (normal range, 0–12.5 ng/mL) were measured on the 3 days of hypothermia treatment in the 27 patients who received this treatment. All patients received both parenteral nutrition and trophic enteral feeding with human milk (donated and/or own) from the first 24 h of life. The neonates were divided into two groups according to short-term outcome, which was classified as favorable (normal neurological evaluation before discharge) or unfavorable (death or abnormal neurological evaluation before discharge). A follow-up clinical neurological exam was carried out in all patients still alive at 6 months of age.
The control group consisted of healthy term neonates who met the following conditions: absence of maternal disease, natural vaginal delivery, collection of samples for metabolic screening at day 3 of life, birth weight between the 10 and 90th percentiles for gestational age, maternal breastfeeding, and absence of hypoglycemia.
2.2. Methods
Therapeutic hypothermia was administered using the Tecotherm Neo (Inspiration Healthcare Ltd, Leicestershire, UK) total body cooling and warming device for systemic hypothermia. The newborns received whole-body cooling to an esophageal temperature of 33.5 °C for 72 h. After 72 h, they were slowly rewarmed to 36.5 °C in increments of 0.5°C per hour.
ACP were analyzed by electrospray ionization tandem mass spectrometry on a triple quadrupole analyzer (ESI-MS/MS API 2000, Applied Biosystems Sciex, Toronto) coupled to a high-performance liquid chromatography system (Series 200, Perkin-Elmer) following a recognized methodology.[27] The following acylcarnitines were included in the analysis, with the reference range for neonatal period (in μmol/L) expressed in parentheses: short-chain acylcarnitines: acetyl- (C2, 5–51 μmol/L), propionyl- (C3, <4.71 μmol/L), butyryl- (C4, <0.95 μmol/L), 3-OH-butyryl- (C4-OH, <0.60 μmol/L), isovaleryl- (C5, <0.96 μmol/L), and tiglylcarnitine (C5:1, <0.22 μmol/L); medium-chain acylcarnitines: hexanoyl- (C6, <0.32 μmol/L), malonyl- (C3DC, <0.18 μmol/L), octanoyl- (C8, <0.26 μmol/L), methylmalonyl- (C4DC, <0.57 μmol/L), decanoyl- (C10, <0.33 μmol/L), decenoyl- (C10:1, <0.22 μmol/L), dodecanoyl- (C12, <0.87 μmol/L); long-chain acylcarnitines: myristoyl- (C14, <0.52 μmol/L), myristoleyl- (C14:1, <0.47 μmol/L), hydroxymyristoyl- (C14-OH, <0.11 μmol/L), 3-methyl-glutaryl (<0.26 μmol/L), palmitoyl- (C16, 2–6 μmol/L), hexadecenoyl- (C16:1, <0.39 μmol/L), 3-hydroxi-hexadecanoyl- (C16-OH, <0.15 μmol/L), 3-hydroxypalmitoleyl- (C16:1-OH, <0.12 μmol/L), stearoyl- (C18, 1–2 μmol/L), oleyl- (C18:1, <2.94 μmol/L), linoleyl- (C18:2, <0.60 μmol/L), hydroxyoleyl- (C18:1-OH, <0.09 μmol/L) and 3-hydroxy-linoleyl-carnitine (C18:2-OH, <0.66 μmol/L). Neonatal carnitine deficiency was defined as a free to total carnitine ratio of less than 0.54.[10]
For the NSE analysis, serum samples were collected in the first 6 h of life and on the second and third days; they were centrifuged at 3000 rpm for at least 5 minutes and transferred to standard and control test tubes after diluting with 1 mL distilled water. The dilutions were kept stable at −20°C and were analyzed within 18 hours of collection. NSE levels were determined using a Human NSE ELISA kit (DiaMetra S.r.l., cat. #: DKO073, Z.I. Paciano, Italy) and results are expressed as ng/mL.[21]
Clinical neurological evaluation was performed using the Dubowitz neurological examination of the full-term newborn.[28]
Brain MRI was performed using a 1.5 Tesla Scanner (Siemens Sonata). An abnormal MRI was defined as any of the following three patterns of hypoxic–ischemic lesions: periventricular leukomalacia, basal ganglia and/or thalamus lesions, and multicystic encephalopathy accompanied by injury to the basal ganglia, thalamus, and/or cerebral cortex.[29]
EEG was performed using the Neuro-DMS software program (Nihon Kohden). An abnormal EEG was defined as any of the following patterns of electrical alteration: generalized slowing, periodic patterns (e.g., burst suppression), background suppression, electrocerebral inactivity, and less common patterns (alpha coma, beta coma, spindle coma, triphasic waves).[30]
With regard to the data analysis, Z-scores were used to compare acylcarnitine values with reference values from the metabolic laboratory in Santiago de Compostela. The Mann–Whitney U test was used to compare quantitative and qualitative variables included associations between MRI results and biomarkers, and the Wilcoxon test was used for two-level quantitative variables. Correlations between normally distributed paired variables were assessed using the Pearson correlation test. Categorical variables were compared using the Chi-square test.
The P-values obtained were adjusted with the Benjamini–Hochberg procedure and statistical significance was established at P < .05. The data were analyzed with the statistical software SPSS v20.
3. Results
Sixty-seven term neonates with HIE (42 males, 62.7%) were included in this study, with a similar distribution of severity between the two groups: pre-hypothermia and hypothermia. They had a mean gestational age of 39 ± 0.8 weeks and a mean birth weight of 3200 ± 300 g. Forty-seven of the neonates (70%) received therapeutic hypothermia, as they were born on the post-hypothermia subperiod. Of these, 29 (61.7%) had an unfavorable short-term outcome, including two deaths due to limitation of therapeutic effort. At the 6-month follow-up visit, 18 patients still had an abnormal clinical neurological exam. Of the 20 patients not treated with hypothermia (pre-hypothermia subperiod), 15 (75%) had an unfavorable outcome, including one death following limitation of therapeutic effort. Eight of these had an abnormal clinical neurological exam at the 6-month follow-up (supplementary information). NSE levels were high on the 3 days of hypothermia treatment: 90.7 ± 42.6 ng/mL on day 1, 80.2 ± 48.1 ng/mL on day 2, and 85.7 ± 65.5 ng/mL on day 3. There was a significant correlation between levels on day 2 and unfavorable short- and long-term outcomes (P = .029). A second dried-blood spot analysis was performed at 12–15 days of life in 44 neonates (34 of whom were in the hypothermia treatment group). Complete information about patients is shown on Additional Information, Table, and Figure 1.
Figure 1.
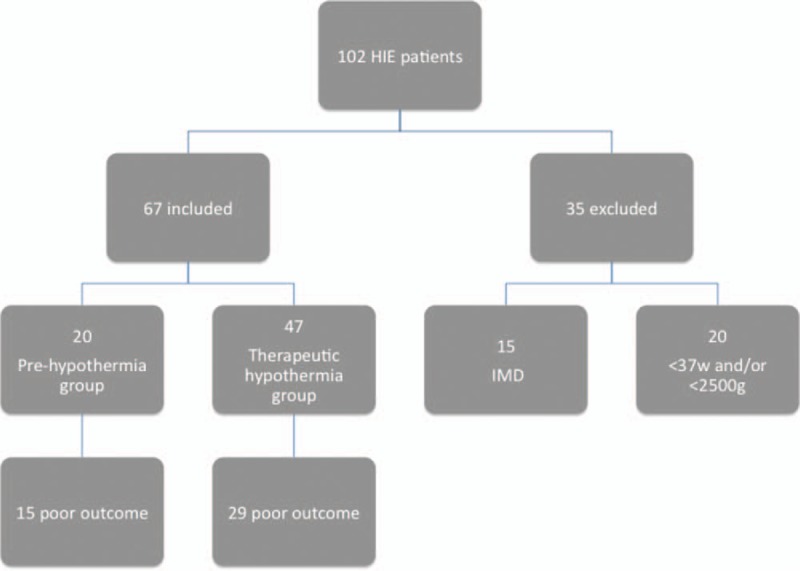
Flow diagram of patients evaluated on the study period. IMD: inherited metabolic diseases.
The healthy control group consisted of 827 newborns (58% male) with a mean gestational age of 39 ± 0.7 weeks and a mean birth weight of 3225 ± 250 g. There were no significant differences between HIE patients and controls in terms of sex or weight.
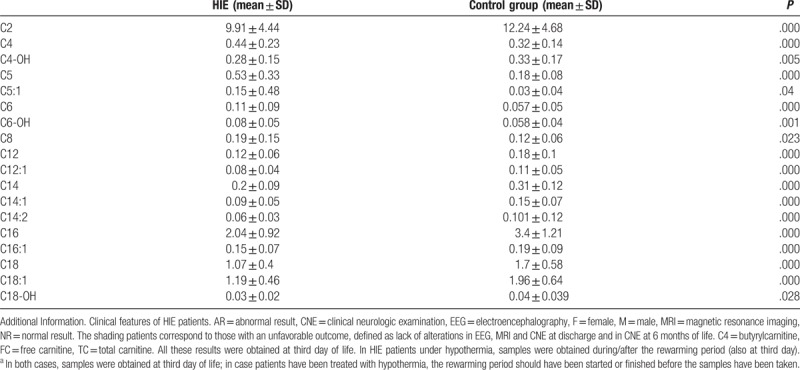
Although no significant differences were observed between patients with and without HIE for free carnitine levels (30.5 ± 11.7 μmol/L vs 27.2 ± 11.6 μmol/L; P = .3 or for the free carnitine/total carnitine ratio (0.780 ± 0.268 μmol/L vs 0.55 ± 0.1 μmol/L; P = .08), the ratio was indicative of neonatal carnitine deficiency in 80% of HIE patients. The following acylcarnitines were significantly lower in HIE patients than in controls: C2, C4-OH, C12:1, C14, C14:1, C14:2, C16, C16:1, C18, C18:1, and C18-OH (all P < .05). Significantly higher levels, however, were observed for C4 (P < .001), C5, C5:1, C6, C6-OH, C8, and C12 (all P < .05) (Table 1). All the patients recruited underwent brain MRI and 28 of them showed abnormal MRI findings at discharge. We observed a marked difference in ACP between patients with favorable and adverse outcomes, in addition to a significant association between C4 levels and abnormal brain MRI at discharge (P = .037).
Table 1.
Acylcarnitines profile in HIE compared to the control groupa.
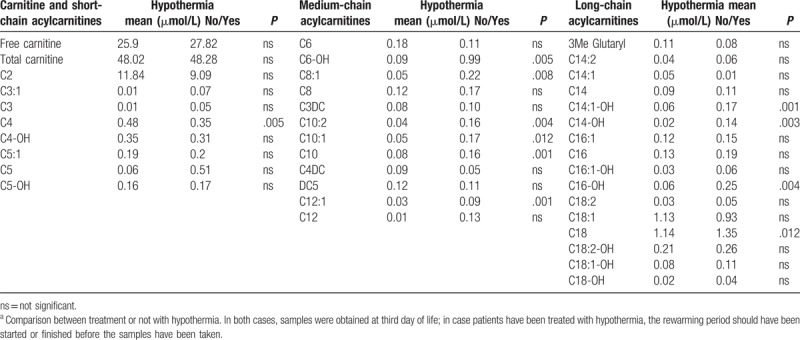
On comparing patients who received therapeutic hypothermia (post-hypothermia) and those who did not (pre-hypothermia), the former had a persistent elevation in C4 (P = .019) and C6-OH (P = .005) and a more marked deficit of C18 (P = .012). The post-hypothermia group also had significantly higher levels of the following medium- and long-chain acylcarnitines: C8:1, C10, C10:1, C10:2, C12:1, C14:1-OH, C14:OH, and C16:OH (all P < .05) (Table 2).
Table 2.
Acylcarnitines profile in HIE patientsa.
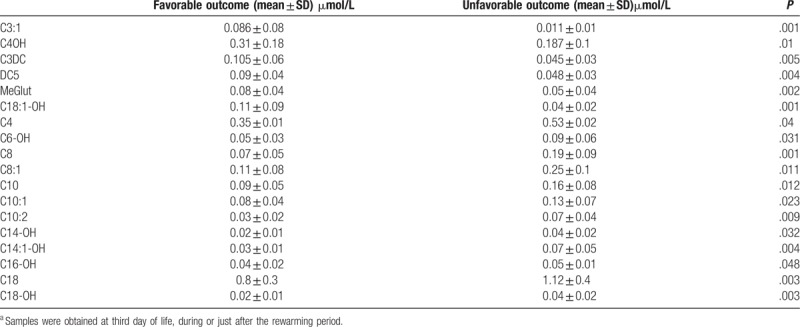
In addition, those patients in the post-hypothermia group who responded unfavorably had significantly higher levels of C6-OH, C8, C8:1, C10:2, C10:1, C10, C14:1-OH, C14-OH, C16-OH, C18, and C18-OH (all P < .05) than those who responded favorably. This latter group of patients had significantly higher levels of C3:1, C4OH, C3DC, DC5, MeGlut, and C18:1-OH (all P < .01) and significantly lower levels of C4 (P = .04) (Table 3).
Table 3.
Acylcarnytine profile in patients under therapeutic hypothermia comparing favorable/unfavorable outcomea.
There was a positive correlation between C4 and NSE levels on the first day of hypothermia treatment (Pearson correlation = 0.57; P = .002). Specifically, C4 levels increased with increasing NSE levels (Fig. 2), highlighting the role of C4 as a marker of neuronal insult.
Figure 2.
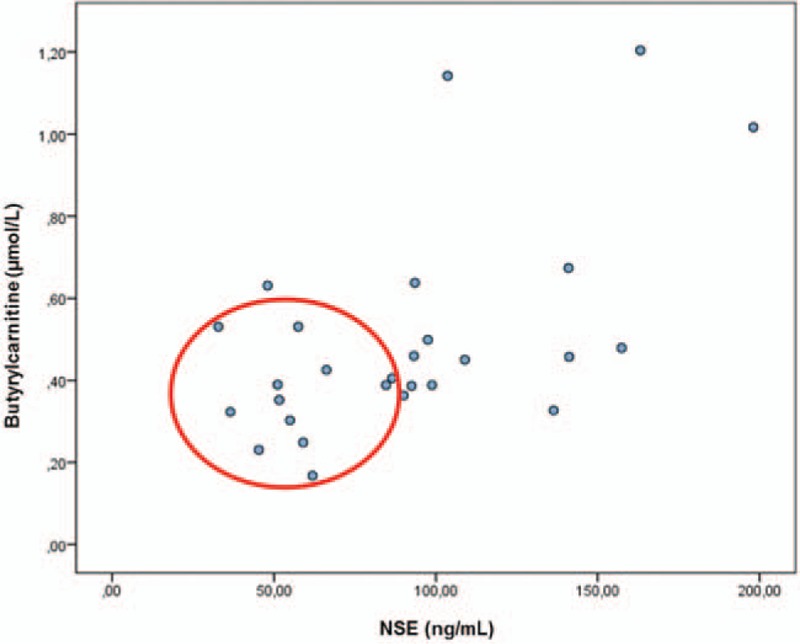
Positive correlation between Butyrylcarnitine and NSE determinations in patients under hypothermia treatment. The figure refers to Butyrylcarnitine samples taken at third day of life and NSE samples determined on 27 patients on the first day of therapeutic hypothermia. We found a significant positive correlation (Pearson Correlation = 0.57; P = .002) between Butyrylcarnitine and NSE, which means that at a higher level of NSE, a higher increase in Butyrylcarnitine levels, which would highlight this metabolite as a marker of neuronal insult, since NSE is considered one of the best prognostic biomarkers for HIE. The red circle marks the patients with good outcome.
Finally, the second analysis of dried-blood spots performed at 12–15 days of life showed that C4 levels had decreased in post-hypothermia group (0.29 ± 0.1 μmol/L) but remained the same in the pre-hypothermia group (0.48 ± 0.1 μmol/L). The difference between the two groups was significant (P = .031).
4. Discussion
In this study, we have evaluated alterations to ACP in neonates with perinatal asphyxia, investigated the possible effect of therapeutic hypothermia on these patterns, and analyzed the potential prognostic role of individual acylcarnitines in HIE. Previous studies that have assessed carnitine or acylcarnitine profiles using blood samples collected from patients with HIE in the perinatal period (days 1–7 of life) have confirmed decreased total and free carnitine levels, increased acylcarnitine levels,[31,32] and an increased acylcarnitine:total carnitine ratio.[25] Findings from animal models suggest that these alterations could be linked to a decrease in carnitine-palmitoyl transferase I and II and a concomitant increase in the acylcarnitine:free carnitine ratio.[33]
Although our results showed acylcarnitine deficiency in 80% of patients with HIE, the free carnitine levels observed were comparable to those seen in the control population. We did, however, observe a significant increase in short- and medium-chain acylcarnitines (C4, C5, C5:1, C6, C6-OH, and C8) and, contrasting with findings by Walsh et al,[22] a significant decrease in the levels of C2, C4-OH, and most long-chain acylcarnitines. Walsh et al studied the metabolomic profile of umbilical cord blood from 100 newborns with HIE and found alterations in 29 metabolites from three groups: amino acids, acylcarnitines, and glycerophospholipids. In the case of acylcarnitines, they found increased levels of C2, C4, C4-OH, C6, C12, C14, C14:1, C14:2, C16, C16:1, C18, C18:1, and C18:2. One important difference with our study is that Walsh et al analyzed umbilical cord blood drawn at birth, while we analyzed dried-blood spots taken at 3 days of life and, in 70% of cases, during hypothermia treatment. Those differences could reflect an evolution on the ACP profile changes with time, after the hypoxic insult has occurred, and as a consequence of resuscitation procedures, treatments, nutrition, etc.
Detailed analysis of ACP showed that compared with controls, HIE patients had significantly lower levels of C2 (P < .001) and long-chain acylcarnitines (P < .01). Decreases in C2 levels are important as this metabolite is known to exert a neuroprotective effect on the developing brain,[34] providing both carnitine for the transport of fatty acids across mitochondrial membranes and acetyl-CoA that can be incorporated into lipids for myelination and cell growth, oxidized in the tricarboxylic acid cycle to produce energy,[35] used as a precursor for acetylcholine,[36] or incorporated into the carbon skeleton of γ-aminobutyric acid and glutamate neurotransmitters.[35] Models of hypoxic brain damage have highlighted several potential therapeutic effects of acetylcarnitine, such as reduced oxidative stress, improved energy status, preserved structural integrity, prevention of cell death, decreased hypoxic lesion size, and improved morphological and functional outcomes.[37–39]
One of the principles underlying the use of therapeutic hypothermia is that the reduction of metabolic rate prevents a rapid decline in ATP levels, improving thus the chances of organ preservation and patient survival and prognosis.[40,41] In our series, acylcarnitine alterations were largely not reversed by therapeutic hypothermia. The treatment, however, did lead to two significant changes: a decrease in C4 levels and, in agreement with findings from animal studies,[42] a decrease in certain medium- and long-chain acylcarnitines (particularly those with 10 and 14 carbon atoms). C4 levels were significant lower in patients who received therapeutic hypothermia and had a favorable rather than an unfavorable outcome. In addition, levels decreased even further after treatment, as shown by the test results at 12–15 days of life.
Our aim in studying ACP in HIE was not so much to find an early marker of damage, as proposed by Walsh et al,[22] but rather to find a marker that would support other prognostic tools (e.g., EEG, cerebral MRI, NSE). Acylcarnitine markers would have an additional advantage in that because they are already included in many newborn screening programs, they would not entail an extra cost. The most consistent prognostic marker identified in our study was C4, as it was significantly higher in patients with HIE compared with healthy controls (P < .001), and correlated positively with other markers of severe disease or poor prognosis. In addition, its levels fell significantly after therapeutic hypothermia (post-hypothermia group) in patients who had favorable outcomes compared with those who did not (P = .04).
One of the best predictors of medium- to long-term disability in post-hypothermia patients is an abnormal early brain MRI. NSE also has potential as a biochemical marker, as shown recently by our group on observing significantly lower levels in HIE patients who received therapeutic hypothermia.[21] Based on the findings of the current study, C4 is another potentially interesting biomarker, as higher levels in the early postnatal period were positively correlated with NSE levels (Fig. 2) and with an abnormal MRI scan at discharge.
In our opinion, C4 could be considered as a biochemical marker of severe hypoxic-ischemic insult, and its decrease during treatment with hypothermia could be a marker of good response to this treatment and favorable prognosis.
Our study has some limitations, including its relatively small sample size and its retrospective design, which prevented us from controlling for intercurrent variables (drugs, diet, etc.) and the duration of the follow-up period, too short to obtain conclusions about the long-term prognosis. The ACPs identified in this series of patients with HIE are, however, consistent with reports in the literature. Of particular note is the significant increase observed in C4 levels and the correlations with abnormal MRI findings and NSE levels. Our findings highlight the importance of studying the potential of these metabolites as predictors of disease severity, disease course, and response to treatment.
Author contributions
MLC, JRFL, and OLS reviewed the literature and conceived the study. OLS, ACG, JAC, and PSP were involved in patient selection, monitoring and data collection. OLS and MLC interpreted the results, drafted the manuscript, and were involved in the statistical analysis. M.L.C. reviewed and edited the manuscript. All authors critically revised the manuscript and approved the final version.
Conceptualization: Olalla López-Suárez, Paula Sánchez-Pintos, María-Luz Couce.
Data curation: Olalla López-Suárez, Ana Concheiro-Guisán.
Formal analysis: Olalla López-Suárez, Ana Concheiro-Guisán, Jose A. Cocho.
Funding acquisition: María-Luz Couce.
Investigation: Paula Sánchez-Pintos.
Methodology: Olalla López-Suárez, Ana Concheiro-Guisán, Paula Sánchez-Pintos, Jose A. Cocho.
Supervision: Paula Sánchez-Pintos, José R. Fernández Lorenzo MD, María-Luz Couce.
Validation: José R. Fernández Lorenzo MD, María-Luz Couce.
Writing – original draft: Olalla López-Suárez.
Writing – review & editing: Olalla López-Suárez, Ana Concheiro-Guisán, Paula Sánchez-Pintos, Jose A. Cocho, José R. Fernández Lorenzo MD, María-Luz Couce.
Supplementary Material
Footnotes
Abbreviations: ACP = acylcarnitine profile, C4 = butyrylcarnitine, EEG = electroencephalography, HIE = hypoxic–ischemic encephalopathy, IMD = inherited metabolic diseases, MRI = magnetic resonance imaging, NSE = neuron-specific enolase, SCAD = short-chain acyl-CoA dehydrogenase.
Source of Support. Article-processing charge was covered by the Fundación IDIS-C012.
Conflict of interest: The authors have indicated they have no potential conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Fernández-Lainez C, Aguilar-Lemus JJ, Vela-Amieva M, et al. Tandem mass spectrometry newborn screening for inborn errors of intermediary metabolism: abnormal profile interpretation. Curr Med Chem 2012;19:4511–22. [DOI] [PubMed] [Google Scholar]
- [2].Neugebauer S, Giamarellos-Bourboulis EJ, Pelekanou A, et al. Metabolite profiles in sepsis: developing prognostic tools based on the type of infection. Crit Care Med 2016;44:1649–62. [DOI] [PubMed] [Google Scholar]
- [3].Bartz S, Mody A, Hornik C, et al. Severe acute malnutrition in childhood: hormonal and metabolic status at presentation, response to treatment, and predictors of mortality. J Clin Endocrinol Metab 2014;99:2128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mihalik SH, Goodpaster BH, Kelley DE, et al. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity 2010;18:1695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bene J, Márton M, Mohás M, et al. Similarities in serum acylcarnitine patterns in type 1 and type 2 diabetes mellitus and in metabolic syndrome. Ann Nutr Metab 2013;62:80–5. [DOI] [PubMed] [Google Scholar]
- [6].Liu J, Chen XX, Li XW, et al. Metabolomic research on newborn infants with intrauterine growth restriction. Medicine (Baltimore) 2016;95:e3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Crill CM, Christensen ML, Storm MC, et al. Relative bioavailability of carnitine supplementation in premature neonates. J Parenter Enteral Nutr 2006;30:421–5. [DOI] [PubMed] [Google Scholar]
- [8].Whitfield J, Smith T, Sollohub H, et al. Clinical effects of L-carnitine supplementation on apnea and growth in very low birth weight infants. Pediatrics 2003;111:477–82. [DOI] [PubMed] [Google Scholar]
- [9].Pande S, Brion LP, Campbell DE, et al. Lack of effect of L-carnitine supplementation on weight gain in very preterm infants. J Perinatol 2005;25:470–7. [DOI] [PubMed] [Google Scholar]
- [10].Sánchez-Pintos P, Pérez-Muñuzuri A, Cocho JA, et al. Evaluation of carnitine deficit in very low birth weight preterm newborns small for their gestational age. J Matern Fetal Neonatal Med 2016;29:933–7. [DOI] [PubMed] [Google Scholar]
- [11].Wolfe L, Jethva R, Oglesbee D, Vockley J. Short-Chain Acyl-CoA Dehydrogenase Deficiency. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H, Stephens K., Amemiya A., editors. SourceGeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-–2018. [PubMed] [Google Scholar]
- [12].Sylvester KG, Kastenberg ZJ, Moss RL, et al. Acylcarnitine profiles reflect metabolic vulnerability for necrotizing enterocolitis in newborns born premature. J Pediatr 2017;181:80–5e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jones LL, McDonald DA, Borum PR. Acylcarnitines: role in brain. Proq Lipid Res 2010;49:61–75. [DOI] [PubMed] [Google Scholar]
- [14].Alves E, Binienda Z, Carvalho F, et al. Acetyl-L-carnitine provides effective in vivo neuroprotection over 3,4-methylendioximethamphetamine-induced mitochondrial neurotoxicity in the adolescent rat brain. Neuroscience 2009;158:514–23. [DOI] [PubMed] [Google Scholar]
- [15].Lai MC, Yang SN. Perinatal hypoxic-ischemic encephalopathy. J Biomed Biotechnol 2011;2011:609813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Azzopardi DV, Strohm B, Edwards AD, et al. TOBY Study Group. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009;361:1349–58. [DOI] [PubMed] [Google Scholar]
- [17].Shah PS. Hypothermia: a systematic review and meta-analysis of clinical trials. Semin Fetal Neonatal Med 2010;15:238–46. [DOI] [PubMed] [Google Scholar]
- [18].Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663070. [DOI] [PubMed] [Google Scholar]
- [19].Giuseppe D, Sergio C, Pasqua B, et al. Perinatal asphyxia in preterm neonates leads to serum changes in protein S-100 and neuron specific enolase. Curr Neurovasc Res 2009;6:110–6. [DOI] [PubMed] [Google Scholar]
- [20].Ruetzler K, Bührer C, Grimmer I, et al. Urinary S-100B concentrations in term and preterm infants at risk for brain damage. Biol Neonate 2006;89:260–4. [DOI] [PubMed] [Google Scholar]
- [21].Alshweki A, Pérez-Muñuzuri A, López-Suárez O, et al. Relevance of urinary S100B protein levels as a short-term prognostic biomarker in asphyxiated infants treated with hypothermia. Medicine (Baltimore) 2017;96:e8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Walsh BH, Broadhurst DI, Mandal R, et al. The metabolomic profile of umbilical cord blood in neonatal hypoxic ischaemic encephalopathy. PLoS One 2012;7:e50520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].El-Farghali OG, El-Chimi MS, El-Abd HS, et al. Amino acid and acylcarnitine profiles in perinatal asphyxia: a case-control study. J Matern Fetal Neonatal Med 2017;5:1–8. [DOI] [PubMed] [Google Scholar]
- [24].Blaise BJ, Schwendimann L, Chhor V, et al. Persistently altered metabolic phenotype following perinatal excitotoxic brain injury. Dev Neurosci 2017;39:182–91. [DOI] [PubMed] [Google Scholar]
- [25].Liepinsh E, Makrecka-Kuka M, Volska K, et al. Long-chain acylcarnitines determine ischaemia/reperfusion-induced damage in heart mitochondria. Biochem J 2016;473:1191–202. [DOI] [PubMed] [Google Scholar]
- [26].Sarnat H, Sarnat M. Neonatal encephalopathy following distress. Arch Neurol 1976;33:695–705. [DOI] [PubMed] [Google Scholar]
- [27].Millington DS, Kodo N, Norwood DL, et al. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inherit Metab Dis 1990;13:321–4. [DOI] [PubMed] [Google Scholar]
- [28].Dubowitz L, Ricci D, Mercuri E. The Dubowitz neurological examination of the full-term newborn. Ment Retard Dev Disabil Res Rev 2005;11:52–60. [DOI] [PubMed] [Google Scholar]
- [29].Shankaran S, McDonald SA, Laptook AR, et al. Neonatal magnetic resonance imaging pattern of brain injury as a biomarker of childhood outcomes following a trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr 2015;167: 987–93.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dereymaeker A, Matic V, Vervisch J, et al. Automated EEG background analysis to identify neonates with hypoxic–ischemic encephalopathy treated with hypothermia at risk for adverse outcome: a pilot study. Pediatr Neonatol 2018;Apr 4. pii: S1875-9572(17)30379-0. doi: 10.1016/j.pedneo.2018.03.010. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cam H, Yildirim B, Aydin A, et al. Carnitine levels in neonatal hypoxia. J TropPediatr 2005;51:106–8. [DOI] [PubMed] [Google Scholar]
- [32].Ezgu FS, Atalay Y, Hasanoglu A, et al. Serum carnitine levels in newborns with perinatal asphyxia and relation to neurologic prognosis. Nutr Neurosci 2004;7:351–6. [DOI] [PubMed] [Google Scholar]
- [33].Rau TF, Lu Q, Sharma S, et al. Oxygen glucose deprivation in rat hippocampal slice cultures results in alterations in carnitine homeostasis and mitochondrial dysfunction. PLos One 2012;7:e40881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ferreira GC, McKenna MC. L-Carnitine and acetyl-l-carnitine roles and neuroprotection in developing brain. Neurochem Res 2017;42:1661–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Scafidi S, Fiskum G, Lindauer SL, et al. Metabolism of acetyl-l-carnitine for energy and neurotransmitter synthesis in the immature rat brain. J Neurochem 2010;114:820–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].White HL, Scates PW. Acetyl-l-carnitine as a precursor of acetylcholine. Neurochem Res 1990;15:597–601. [DOI] [PubMed] [Google Scholar]
- [37].Hota KB, Hota SK, Chaurasia OP, et al. Acetyl-L-carnitine-mediated neuroprotection during hypoxia is attributed to ERK1/2-Nrf2-regulated mitochondrial biosynthesis. Hippocampus 2012;22:723–36. [DOI] [PubMed] [Google Scholar]
- [38].Xu S, Waddell J, Zhu W, et al. In vivo longitudinal proton magnetic resonance spectroscopy on neonatal hypoxic–ischemic rat brain injury—neuroprotective effects of acetyl-L-carnitine. Magn Reson Med 2015;74:1530–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tang S, Xu S, Lu X, et al. Neuroprotective effects of acetyl-l-carnitine on neonatal hypoxia ischemia-induced brain injury in rats. Dev Neurosci 2016;38:384–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Boutilier RG. Mechanisms of cell survival in hypoxia and hypothermia. J Exp Biol 2001;204:3171–81. [DOI] [PubMed] [Google Scholar]
- [41].Gorr TA. Hypometabolism as the ultimate defence in stress response: how the comparative approach helps understanding of medically relevant questions. Acta Physiol (Oxf) 2017;219:409–40. [DOI] [PubMed] [Google Scholar]
- [42].Bruder ED, Raff H. Cardiac and plasma lipid profiles in response to acute hypoxia in neonatal and young adult rats. Lipids Health Dis 2010;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


