Abstract
Context
Patellofemoral pain (PFP) is a chronic condition that presents with lower extremity muscle weakness, decreased flexibility, subjective functional limitations, pain, and decreased physical activity. Patterned electrical neuromuscular stimulation (PENS) has been shown to affect muscle activation and pain after a single treatment, but its use has not been studied in a rehabilitation trial.
Objective
To determine the effects of a 4-week impairment-based rehabilitation program using PENS on subjective function, pain, strength, range of motion, and physical activity in individuals with PFP.
Design
Randomized controlled trial.
Setting
Laboratory.
Patients or Other Participants
A total of 21 patients with PFP (5 males, 16 females; age = 23.4 ± 7.6 years, height = 168.0 ± 7.5 cm, mass = 69.0 ± 19.5 kg).
Intervention(s)
Participants completed a 4-week supervised rehabilitation program in conjunction with random assignment to receive PENS or sham treatments.
Main Outcome Measure(s)
Subjective function, pain, strength, range of motion, and physical activity levels were assessed prerehabilitation and postrehabilitation. Subjective function and pain were also assessed at 6 and 12 months postrehabilitation. Repeated-measures analyses of variance and Tukey post hoc testing were conducted with α ≤ .05. We calculated Cohen d effect sizes with 95% confidence intervals.
Results
Both groups had statistically and clinically meaningful differences in subjective function, pain, strength, range of motion, and activity level after 4 weeks of impairment-based rehabilitation. Improved subjective function was observed in both groups at 6 and 12 months after the interventions. The PENS group had improvements in current pain for all 3 postrehabilitation times compared with baseline measures.
Conclusions
An impairment-based intervention effectively improved subjective function, pain, strength, range of motion, and physical activity levels in individuals with PFP. Participants who received PENS in addition to the rehabilitation program had improved current pain at 6 and 12 months postrehabilitation compared with baseline scores.
Trial Registration
ClinicalTrials.gov identifier: NCT02441712
Keywords: knee, lower extremity, anterior knee pain
Key Points
Patterned electrical neuromuscular stimulation used with a 4-week impairment-based rehabilitation program did not improve clinical measures more than a sham treatment.
Current pain improved from prerehabilitation to postrehabilitation in patients receiving patterned electrical neuromuscular stimulation.
An impairment-based rehabilitation program improved subjective and objective function in individuals with patellofemoral pain.
Clinicians should focus on progressive rehabilitation targeting individual impairments to improve subjective and objective function in individuals with patellofemoral pain.
Patellofemoral pain (PFP) is one of the most commonly treated pathologic knee conditions seen in the general population,1 active individuals,2 and military personnel.3 It accounts for 7% of all diagnoses in patients seeking medical care and up to 25% of all treatment for knee-related injuries in sports medicine clinics.1,2 Whereas PFP is frequently seen in clinical practice, the cause of this chronic condition is unknown. Patients with PFP have retropatellar or peripatellar pain during common functional tasks, such as prolonged sitting, kneeling, jumping, and squatting. These tasks often produce pain due to increased stress placed on the patellofemoral joint.4
Individuals with a diagnosis of PFP often experience debilitating consequences that negatively affect their daily activities. Decreased physical activity due to pain is commonly seen in the short term after diagnosis.5 Muscle weakness and soft tissue restrictions have been noted in cohorts of individuals with PFP when compared with healthy counterparts.6–9 Weakness in the quadriceps and gluteus medius muscles have been documented during strength assessment and theorized to contribute to altered movement in a variety of functional tasks.6,10 These symptoms also negatively influence subjective function, as a linear relationship between decreased strength and increased pain for self-reported knee function has been found.11
The long-term consequences of this pathologic condition are one of the challenges in treating PFP. Up to 90% of all individuals with PFP present with chronic subjective impairments and pain for up to 16 years after the initial diagnosis.12,13 The chronicity of this condition may be a predisposing factor for developing patellofemoral osteoarthritis.14 These alarming recurrence rates suggest that current treatment interventions might not be sufficient to improve long-term outcomes for PFP, possibly due to the heterogeneous presentation of PFP symptoms.
Interventions to address muscle weakness and soft tissue restriction are frequently used in clinical practice.15–20 Strengthening exercises often focus on the quadriceps and gluteus medius muscles with a variety of knee-extension, hip-abduction, and hip–external-rotation tasks.18–20 One concern when strengthening these muscles in patients with PFP is inhibition,21 which prevents patients from reaching their full capacity for muscular contraction. Neuromuscular electrical stimulation (NMES) is a treatment that clinicians use to overcome this muscular inhibition and promote neuromuscular reeducation of the atrophied or weakened muscles. Whereas NMES has been used with rehabilitation for individuals with PFP, this intervention typically targets only the quadriceps.22,23 In addition to this focus on a singular muscle, NMES has been associated with patient discomfort, muscle fatigue, and lack of functional applications.24
To overcome these concerns, novel forms of electrical stimulation have been developed to improve clinical outcomes. Patterned electrical neuromuscular stimulation (PENS) is a treatment that delivers a rhythmic stimulus targeting the vastus medialis oblique and gluteus medius, key muscles in PFP rehabilitation programs.25 The precise stimulus to these muscles has been theorized to replicate normal agonist-antagonist-agonist firing patterns based on healthy electromyographic activity.25 After a single session of PENS intervention among individuals with PFP, researchers26,27 found improvements in lower extremity electromyographic activity, lower extremity kinematics, and pain. However, using PENS in combination with a rehabilitation program has not been evaluated among this population. Therefore, the purpose of our study was to evaluate changes in subjective assessments of function and pain, lower extremity strength, range of motion (ROM), and activity levels immediately after a 4-week impairment program, with or without PENS, among individuals with PFP. We also evaluated subjective function and pain at 6 and 12 months postintervention to assess long-term outcomes in these patients with PFP. We hypothesized that improvements in subjective and objective function after the impairment-based rehabilitation program would be present in both groups, with greater improvements in those who also received the PENS treatment.
METHODS
Participants
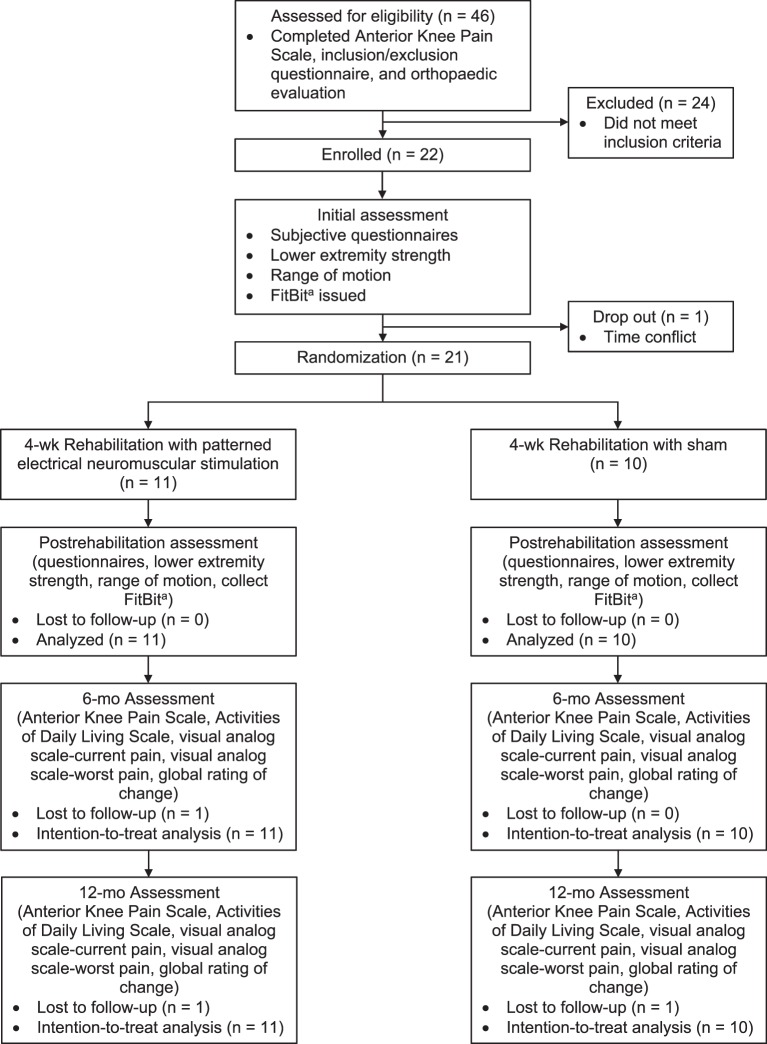
Volunteers were 21 patients with PFP (5 males, 16 females; age = 23.4 ± 7.6 years, height = 168.0 ± 7.5 cm, mass = 69.0 ± 19.5 kg) who were recruited from the local university, community, and orthopaedic clinics. The diagnosis of PFP was determined during the study screening via a score of less than 85% on the Anterior Knee Pain Scale and evaluation by a certified athletic trainer (AT) to assess whether volunteers met the inclusion or exclusion criteria28 (Table 1). Volunteers were also screened for contraindications to electrical stimulation: biomedical device implants, history of neuropathy, hypersensitivity to electrical stimulation, lower extremity muscular abnormality, or active infection in the lower limb. If they presented with bilateral PFP, the limb they identified as having the more affected knee was selected for all testing, interventions, and analyses. Participants were randomized to a PENS (n = 11) or sham (n = 10) group. All participants provided written informed consent, and the University of Virginia Institutional Review Board for Health Science Research approved the study.
Table 1.
Inclusion and Exclusion Criteria
| Criteria |
| Inclusion |
| Nontraumatic peripatellar or retropatellar pain >3 mo |
| Worst pain over last week >3/10 assessed by visual analog scale |
| Pain with ≥2 of the following activities: |
| Stair ambulation |
| Running |
| Jumping |
| Prolonged sitting |
| Quadriceps contraction |
| Kneeling |
| Pressure over the patella |
| Exclusion |
| Previous knee surgery |
| Ligamentous instability defined by orthopaedic special tests (anterior and posterior drawer, valgus and varus stress test) |
| Additional source of anterior knee pain (eg, tendinitis, bursitis, patellar subluxation) |
| Lower extremity or back injury or concussion in the year before the study |
Instruments
We administered PENS treatments using the Omnistim FX2 (Accelerated Care Plus, Reno, NV). The device uses a 50-Hz pulse frequency, 70-μs phase duration, and 200-millisecond stimulus train with an asymmetric biphasic square-waved stimulus. Alternating rhythmic contractions were generated using 2 stimulation patterns to target the agonist muscles (vastus medalis oblique and gluteus medius) and antagonist muscles (hamstrings and adductors; Figure 1). Four 3- × 5-in (7.62- × 12.70-cm) self-adherent electrodes were placed over these muscles, as suggested by the manufacturer, to deliver a 200-millisecond stimulus to the agonist muscles, a 200-millisecond stimulus to the antagonist muscles, and a 120-millisecond stimulus to the agonist muscles. Using concealed envelopes, we randomized participants into a true PENS group or a sham group. To achieve a strong motor response during the treatment, we increased the stimulus intensity for the PENS group. The sham-group participants received a minimal stimulation treatment (1 mA) during which all the machine's lights and timers were operating and visible to the participants, and they were informed that they would receive a subsensory stimulation treatment.26,27 Treatment setups for all participants were identical, and 15 minutes of the intervention were administered before therapeutic exercise during each rehabilitation session.
Figure 1.
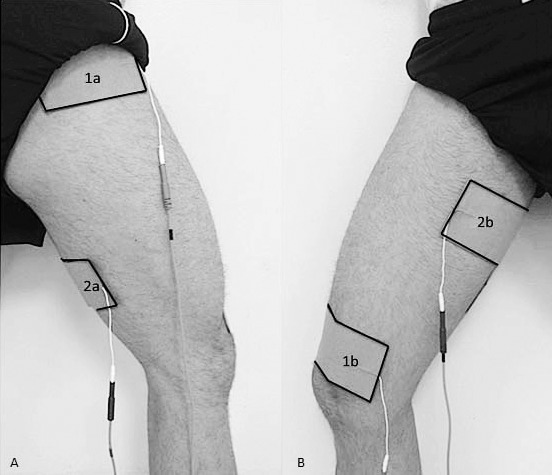
Patterned electrical neuromuscular stimulation electrode setup. Channel 1: 1a, gluteus medius; 1b, vastus medialis oblique. Channel 2: 2a, hamstrings muscle group; 2b, adductor muscle group. A, Posterolateral view. B, Anteromedial view. Reprinted with permission. Glaviano NR, Saliba SA. Immediate effect of patterned electrical neuromuscular stimulation on pain and muscle activation in individuals with patellofemoral pain. J Athl Train. 2016;51(2):118–128.
Procedures
Our participants were part of a larger double-blinded randomized controlled study on neuromuscular and gait factors in patients with PFP. Participants eligible for study enrollment attended an initial laboratory assessment for prerehabilitation data collection. A single researcher (N.R.G.) conducted all prerehabilitation and postrehabilitation assessments of subjective function, strength, ROM, and activity level but was blinded to the allocation and treatment intervention.
Subjective Function
During the initial session, patient-reported outcome measures were collected before the objective measurements (Figure 2). The questionnaires (Anterior Knee Pain Scale [AKPS], Activities of Daily Living Scale [ADLS], Fear-Avoidance Beliefs Questionnaire-Knee [FABQ], Lower Extremity Functional Scale [LEFS], visual analog scale (VAS) for current [VAS-C] and worst [VAS-W] pain in the last week were collected to evaluate the function and impairments of the participants. These scales have been shown to be valid and reliable assessments among patients with PFP.29,30 The VAS-C and VAS-W in the last 24 hours were also collected on separate pieces of paper. No pain and Worst pain imaginable were at opposite ends of a 10-cm line, and participants were instructed to use a pen to make vertical marks for their pain levels.
Figure 2.
Study flow chart. a FitBit Charge HR (FitBit Inc, San Francisco, CA).
Strength
Lower extremity strength of the hip and knee was assessed using a handheld dynamometer (Accelerated Care Plus). Force measurements were collected over three 5-second maximal voluntary isometric contractions for knee flexion, knee extension, hip abduction, hip internal and external rotation, hip extension with knee extension, and hip extension with knee flexion using standard methods.31,32 The “make” methods were used for all strength testing, with participants generating a maximal contraction over 2 seconds before the 5-second maximum hold for each muscle of interest. Participants were provided oral instructions and a practice trial. The average force in newtons of the 3 trials was averaged and normalized to the participant's body mass in kilograms. Excellent reliability (intraclass correlation coefficient [3,3] = 0.96–0.98) has been established for lower extremity strength testing.33
Range of Motion
Range of motion of the hip, knee, and ankle was measured using both a 12-in (30.48-cm) International Standards of Measurement goniometer and a bubble inclinometer (Fabrications Enterprises Inc, White Plains, NY). Data from an average of 2 trials were collected for all assessments. Dorsiflexion was assessed via goniometer with the participant in a straight-knee, long-sitting position. Hamstrings flexibility was assessed during a supine straight-leg–raise test using the bubble inclinometer on the distal tibia.29 With participants positioned prone, we evaluated quadriceps flexibility using a knee-flexion movement with the bubble inclinometer placed on the distal tibia. For the iliotibial (IT) band examination, participants were positioned side lying, and a bubble inclinometer was placed on the lateral knee joint.29 Excellent reliability (intraclass correlation coefficient = 0.91–0.97) has been established for assessing ROM in individuals with PFP.34
Activity Level
Participants were provided a FitBit Charge HR (FitBit Inc, San Francisco, CA) activity band at the end of the initial assessment. They were instructed to wear the band on their self-reported nondominant wrist at all times during the study, except while charging and showering, and to not alter their normal activity levels.35 Each activity monitor was assigned an individual account user name that was accessible only by the research team. The FitBit was synchronized each week via Bluetooth (Bluetooth SIG, Inc, Kirkland, WA) using the FitBit Connect application. Activity levels for each participant were exported from the FitBit Web site each week.
Rehabilitation Protocol
Rehabilitation was initiated within 96 hours after the initial assessment. Participants completed 12 rehabilitation sessions, which were administered as 3 sessions of supervised rehabilitation per week for 4 weeks. A single certified AT (A.N.M.) with more than 7 years of clinical experience supervised all rehabilitation sessions with the PENS or sham PENS treatment for the duration of the study. Participants scheduled their rehabilitation sessions with this research team member for 3 sessions per week, with no more than 2 consecutive sessions. We instructed all participants to complete strengthening exercises of the knee, hip, and core and then assessed individual impairments for ROM, patellar mobility, and pronated foot.36 Individual ROM, mobility, and foot-pronation impairments were defined by the objective thresholds reported by Selfe et al.33 Patients with individual objective measurements that were less than the defined thresholds were included within the impairment-based programs. The duration of treatment was approximately 1 hour per rehabilitation session and consistent among treatment groups.
The lone difference in the rehabilitation sessions was the administration of the PENS or sham treatment, which was conducted before each session's exercises. Before study enrollment, we used a random number generator (Excel; Microsoft Corp, Redmond, WA) to randomize the assignment of PENS or sham treatments for all participants. A 4-block randomization scheme was performed with group allocation concealed in envelopes.
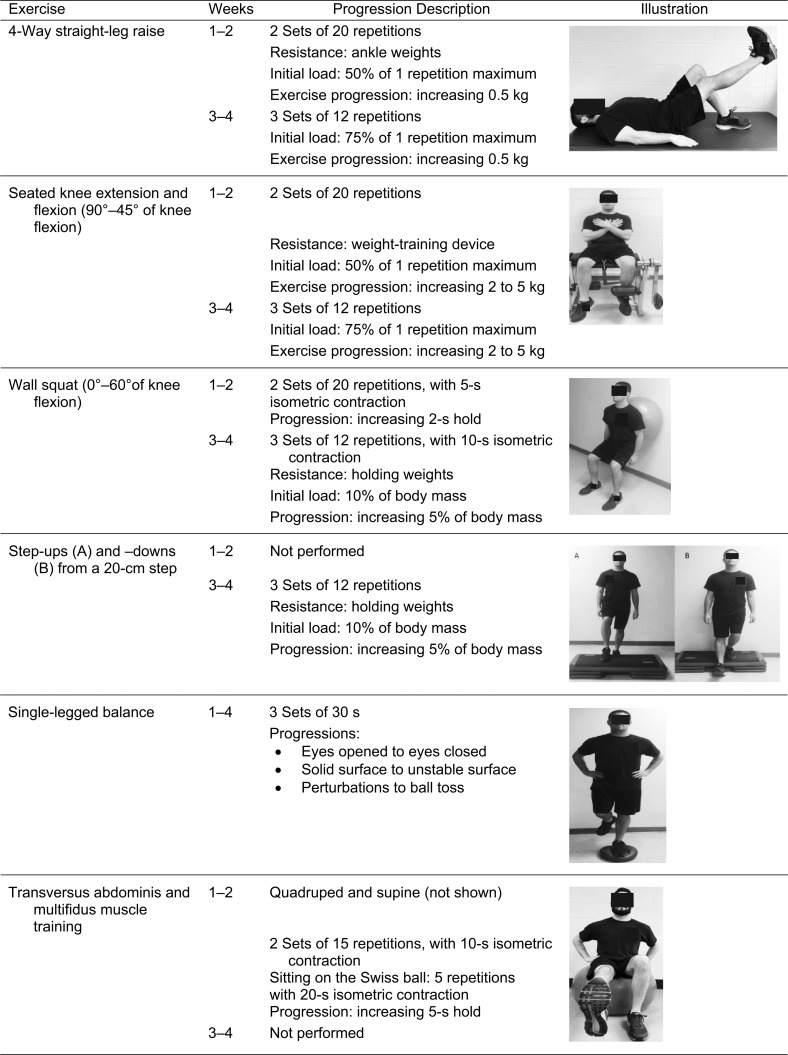
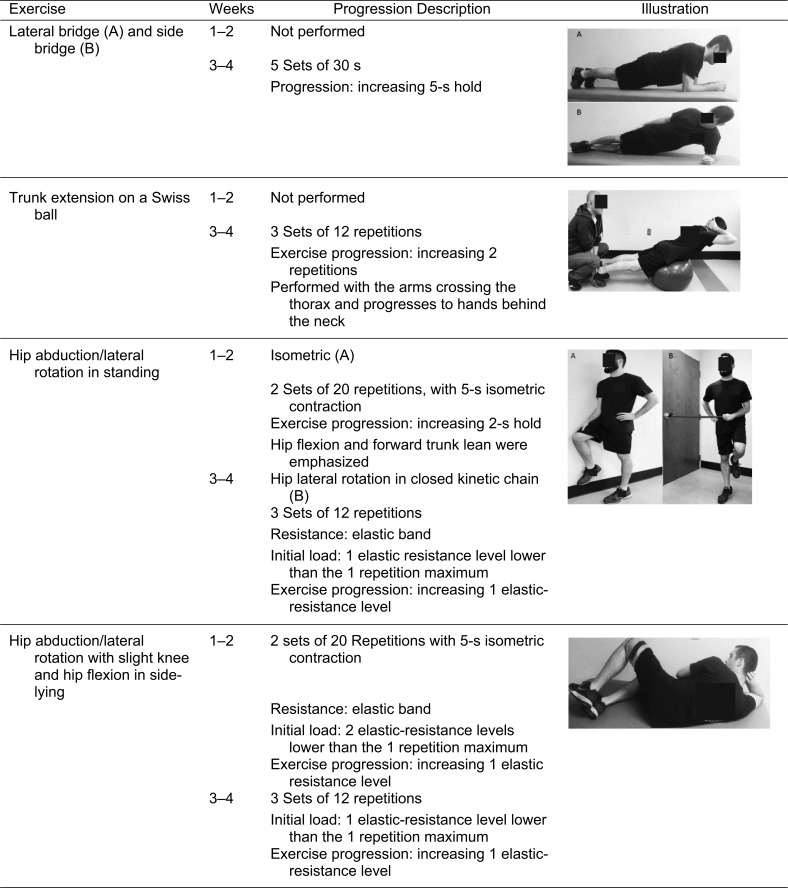
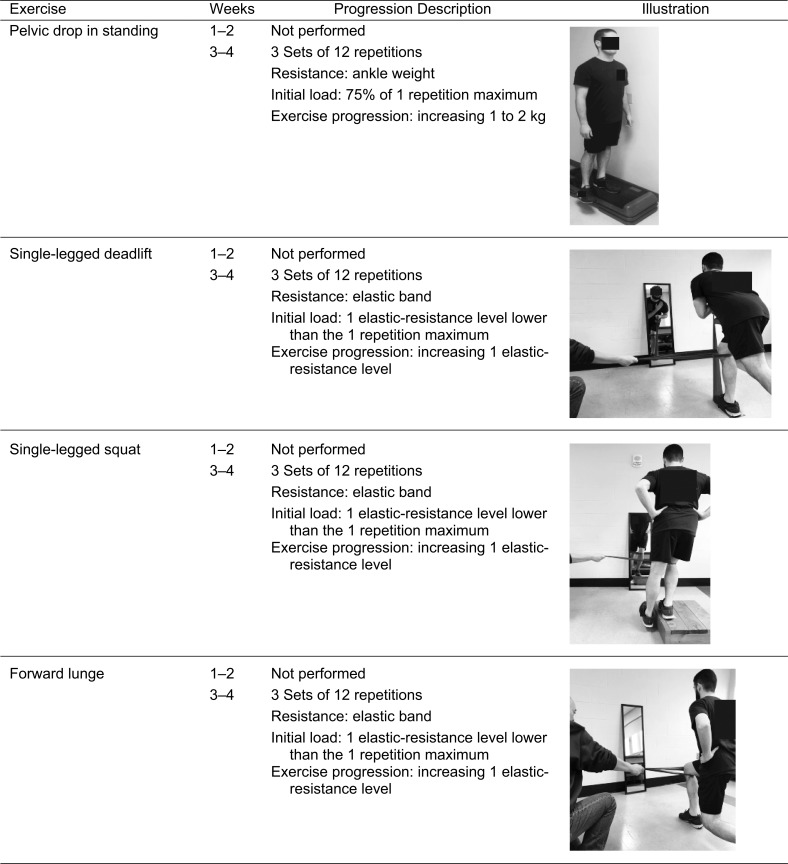
The rehabilitation program was a modification of exercises previously described for individuals with PFP.18 Strengthening exercises and balance training were completed throughout the study, whereas functional retraining tasks were introduced on the seventh visit and performed for the remainder of the study (Table 2). Participants were instructed to perform strengthening exercises for a total of 4 seconds: 2 seconds each for the concentric and eccentric portions. They rested for 1 minute between sets and approximately 2 minutes between exercises. All strengthening exercises were individualized to a percentage of the maximal strength measure collected during the initial testing session (Appendix). This protocol was designed to challenge all participants from the start of the rehabilitation program depending on their lower extremity function. All exercises were progressed throughout the rehabilitation program based on the clinical judgment of the AT, with the goal of repetition to failure without increased pain. We assessed pain during each rehabilitation session to provide additional insight into daily modifications of the program to mimic clinical practice. Rehabilitation compliance and use of the activity band were also monitored 3 times per week during the sessions and recorded by the clinician.
Table 2.
Rehabilitation Program
| Weeks |
Exercise |
Sets |
No. of Repetitions or Time |
| 1–2 | 4-way Straight-leg raise | 2 | 10 repetitions |
| Seated knee flexion and extension | 2 | 10 repetitions | |
| Wall squats | 2 | 10 repetitions | |
| Isometric hip abduction and external rotation | 2 | 10 repetitions | |
| Clam shells | 2 | 10 repetitions | |
| Pelvic tilt prone | 2 | 20 s | |
| Pelvic tilt on Swiss ball | 2 | 20 s | |
| Single-legged balance (eyes open) | 3 | 30 s | |
| Single-legged balance (eyes closed) | 3 | 30 s | |
| 3–4 | 4-way Straight-leg raise | 3 | 10 repetitions |
| Seated knee flexion and extension | 3 | 10 repetitions | |
| Wall squats | 3 | 10 repetitions | |
| Step-ups and step-downs | 3 | 10 repetitions | |
| Lateral rotation in closed kinetic chain | 3 | 10 repetitions | |
| Pelvic drops | 3 | 10 repetitions | |
| Clam shells | 3 | 10 repetitions | |
| Planks (anterior and lateral) | 3 | 30 s | |
| Trunk extension on Swiss ball | 3 | 10 repetitions | |
| Single-legged balance (eyes open) | 3 | 30 s | |
| Single-legged balance (eyes closed) | 3 | 30 s | |
| Single-legged squat with mirror training | 3 | 10 repetitions | |
| Lunge with mirror training | 3 | 10 repetitions | |
| Single-legged deadlift with mirror training | 3 | 10 repetitions |
Follow-Up Testing
Participants returned to the laboratory within 72 hours of the final rehabilitation session. They completed the same subjective assessment consisting of the patient-reported outcome measures, lower extremity strength, and ROM. Activity levels during the 4-week rehabilitation period were collected from the FitBit. In addition, the global rating of change (GROC) questionnaire was administered to all patients to evaluate the change in their knee function since beginning the rehabilitation study. Printed handouts of the exercises performed during the intervention study were provided to all participants. Participants were contacted at 6 and 12 months after their last rehabilitation sessions to assess subjective function on the AKPS, ADLS, and VAS questionnaires and the GROC. They were also asked if they were still performing rehabilitation exercises and if so, the estimated frequency, and if they had received additional medical care for their PFP.
Statistical Analysis
Baseline anthropometric, subjective, and objective characteristics were compared between groups. Dependent variables were evaluated for normality with skewness, kurtosis, and the Levene test for normal variance. We conducted a mixed-model analysis of variance with repeated measures for self-reported function, pain, ROM, strength, and activity level. Tukey post hoc testing was performed to identify interactions between groups at the 2 time points. The within-subjects factor was time (prerehabilitation and postrehabilitation), and the between-subjects factor was group (PENS and sham). We calculated Cohen d effect sizes to examine the magnitude of change in dependent variables for the pooled postrehabilitation means of both groups compared with the pooled prerehabilitation means of both groups. Thresholds for effect sizes were set at trivial (<0.20), small (0.20–0.49), moderate (0.50–0.79), and large (≥0.80).37 An intention-to-treat analysis was undertaken for the longitudinal data, using a last-value-carried-forward technique to account for missing data.38 The α level was set a priori at ≤.05 for all statistical analyses. Data were analyzed using SPSS (version 20.0; IBM Corp, Armonk, NY).
RESULTS
The dependent variables, with the exception of hamstrings flexibility and duration of symptoms, were normally distributed based on skewness, kurtosis, and normal variance as assessed by the Levene test (P > .05). Between-groups comparisons of baseline anthropometric, subjective, and objective characteristics are presented in Table 3. All participants completed all 12 supervised rehabilitation sessions, as allocated with or without PENS, over a 4-week period (32.5 ± 2.4 days). Impairments assessed during the 4-week impairment-based program for all participants involved stretching (76%, n = 16), joint mobility (10%, n = 2), and a pronated foot (0%, n = 0). Individual muscles stretched included the quadriceps (63%, n = 9), hamstrings (75%, n = 12), IT band (50%, n = 8), and gastrocnemius (94%, n = 15).
Table 3.
Baseline Anthropometric, Subjective, and Objective Characteristics
| Characteristic |
Group |
P Value |
|
| Patterned Electrical Neuromuscular Stimulation (n = 11) |
Sham (n = 10) |
||
| Male/Female |
|||
| Sex, no. | 3/8 | 2/8 | Not applicable |
| Mean ± SD |
|||
| Age, y | 23.8 ± 5.6 | 23.0 ± 3.7 | .70 |
| Height, cm | 169.1 ± 7.3 | 166.7 ± 7.8 | .48 |
| Mass, kg | 68.2 ± 11.4 | 69.8 ± 19.0 | .81 |
| Duration, mo | 26.3 ± 26.3 | 23.0 ± 27.8 | .77 |
| Activity level, average No. of steps per day | 7963.7 ± 2949.0 | 8970.7 ± 1968.7 | .37 |
Subjective Function
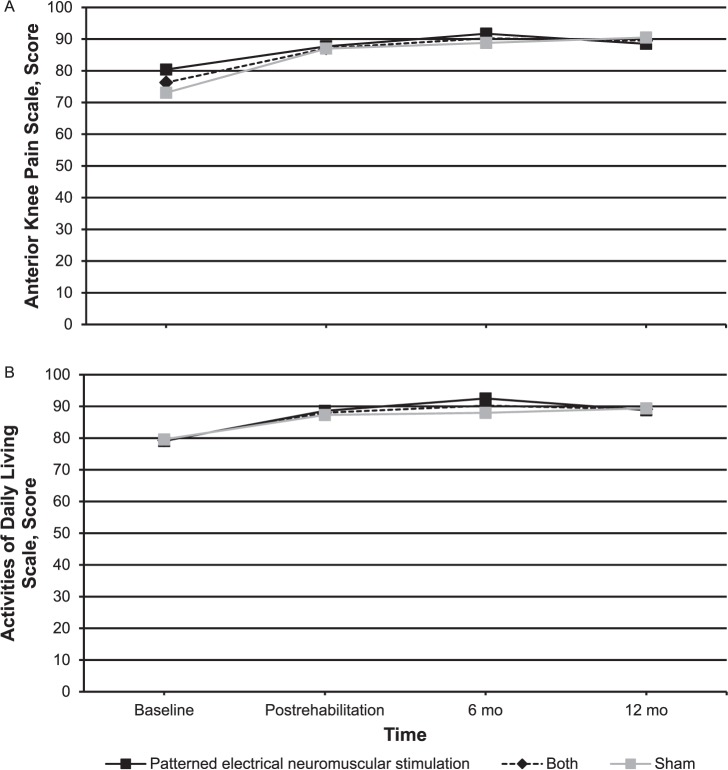
No group main effect or group-by-time interactions were identified on the AKPS, ADLS, FABQ, LEFS, or VAS-C (Table 4). We observed a main effect of time in the combined groups' subjective function postrehabilitation: AKPS (prerehabilitation = 76.3 ± 7.5, postrehabilitation = 87.1 ± 7.7; P < .001), ADLS (prerehabilitation = 79.3 ± 10.0, postrehabilitation = 88.0 ± 5.5; P = .001), FABQ (prerehabilitation = 13.3 ± 4.4, postrehabilitation = 10.0 ± 4.7; P = .004), LEFS (prerehabilitation = 65.6 ± 8.5, postrehabilitation = 73.1 ± 4.6; P < .001),VAS-W (prerehabilitation = 4.9 ± 1.7, postrehabilitation = 2.1 ± 1.6; P < .001), and VAS-C (prerehabilitation = 1.3 ± 1.5, postrehabilitation = 0.6 ± 0.6; P = .04). We noted large effect sizes on the AKPS, ADLS, LEFS, and VAS-W scores and moderate effect sizes on the FABQ and VAS-C scores (Table 4). The average GROC score immediately postrehabilitation for the combined groups was 4.4 ± 1.8, which equates to moderately better.
Table 4.
Prerehabilitation and Postrehabilitation Self-Reported Subjective Function and Cohen d Effect Sizes With 95% CIs
| Patient-Reported Outcome Measure |
Group, Mean ± SD |
P Value |
Pooled Prerehabilitation- Postrehabilitation Cohen d Effect Size (95% CI)a |
|||||
| Patterned Electrical Neuromuscular Stimulation |
Sham |
|||||||
| Prerehabilitation |
Postrehabilitation |
Prerehabilitation |
Postrehabilitation |
Time Main Effect |
Group Main Effect |
Group × Time Interaction |
||
| Visual analog scale-current pain in the last week score | 0.8 ± 0.8 | 0.5 ± 0.6 | 1.8 ± 2.0 | 0.7 ± 0.6 | .03b | .16 | .14 | −0.58 (−1.21, 0.04) |
| Visual analog scale-worst pain in the last week score | 4.2 ± 1.1 | 1.9 ± 2.0 | 5.6 ± 1.2 | 2.2 ± 1.2 | <.001b | .09 | .39 | 1.92 (1.17, 2.67) |
| Anterior Knee Pain Scale score | 80.4 ± 5.0 | 87.2 ± 9.7 | 73.1 ± 7.9 | 87.0 ± 5.6 | <.001b | .20 | .05 | 1.40 (0.74, 2.11,) |
| Activities of Daily Living Scale score | 79.1 ± 8.5 | 88.6 ± 5.9 | 79.6 ± 12.0 | 87.3 ± 5.2 | .002b | .80 | .81 | 1.07 (0.42, 1.73) |
| Fear-Avoidance Beliefs Questionnaire-Knee score | 12.4 ± 5.3 | 8.6 ± 5.2 | 14.4 ± 3.6 | 11.4 ± 3.8 | .005b | .19 | .71 | −0.73 (−1.36, −0.09) |
| Lower Extremity Functional Scale score | 67.0 ± 5.9 | 72.7 ± 4.9 | 64.2 ± 10.7 | 73.5 ± 4.6 | <.001b | .71 | .28 | 1.09 (0.43, 1.75) |
| Global rating of change score | 4.6 ± 1.8 | 4.2 ± 1.8 | .63 | |||||
Abbreviation: CI, confidence interval.
Effect sizes were calculated to compare the pooled group's prerehabilitation and postrehabilitation scores; a positive value denotes an increase in subjective function postrehabilitation.
Indicates difference (P ≤ .05).
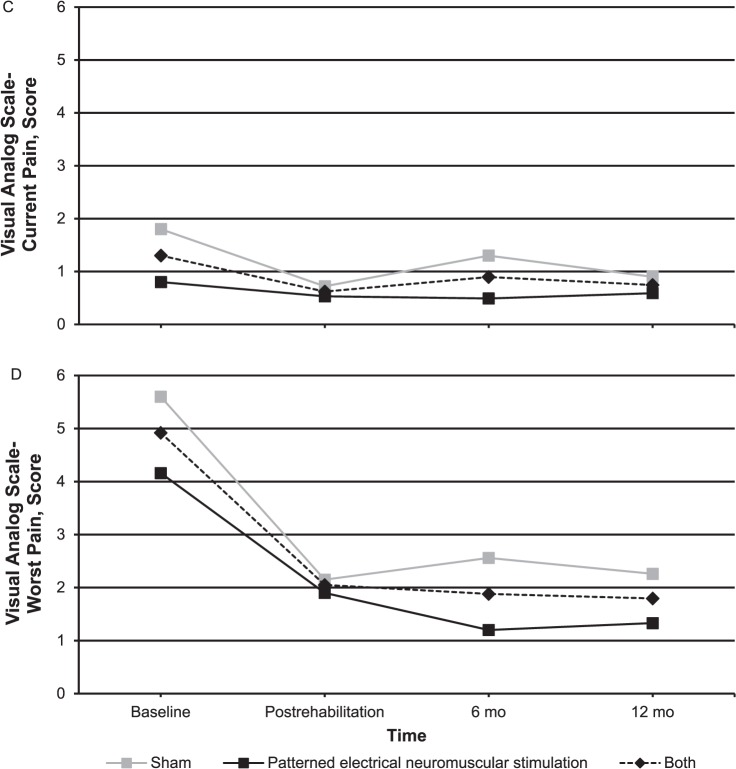
At the 6- and 12-month follow-ups, 95% and 90% of participants, respectively, responded. One participant in the PENS group did not respond at either follow-up time, and 1 participant from the sham group did not respond at the 12-month point. Both groups had improved in subjective function as assessed by the AKPS and ADLS at both 6 and 12 months compared with their baseline measurements (Figure 3). The PENS group had statistically different and clinically important improvements in their VAS-C from baseline (baseline = 1.4 ± 0.7) to postrehabilitation (0.5 ± 0.7; P = .02, Cohen d [95% confidence interval (CI)] = 1.21 [0.26, 2.17]), 6 months (0.5 ± 0.5; P = .005, Cohen d [95% CI] = 1.46 [0.47, 2.44]), and 12 months (0.6 ± 0.7; P = .02, Cohen d [95% CI] = 1.13 [0.18, 2.07]). Pain in the sham group improved immediately postrehabilitation but not at the 6- and 12-month follow-ups. At 6 months, only 4 individuals would have met the inclusion criteria based on their AKPS scores: 1 in the PENS group and 3 in the sham group. At 6 months, only 4 individuals reported occasionally performing some of the exercises conducted during the intervention: 1 in the PENS group and 3 in the sham group. Six individuals would have met the inclusion criteria at 12 months: 3 individuals in each group. At that time, only 1 participant in the sham group reported performing exercises and only when having knee pain.
Figure 3.
Long-term subjective function follow-up for the patterned electrical neuromuscular stimulation group, sham group, and both groups. A, Anterior Knee Pain Scale. B, Activities of Daily Living Scale. C, Visual analog scale-current pain. D, Visual analog scale-worst pain. Continued on next page.
Figure 3.
Continued from previous page.
Strength
Main effects of time demonstrated improvements in knee-flexion, hip-abduction, hip external-rotation, hip internal-rotation, and hip-extension strength (Table 5). No main effects of group were found for any strength measures. We observed group-by-time interactions, with an increase in hip internal-rotation strength and a trend toward increased knee-flexion strength in the sham group. Combined strength differences between prerehabilitation and postrehabilitation were present in hip abduction (prerehabilitation = 2.9 ± 0.8 N/kg, postrehabilitation = 4.5 ± 2.5 N/kg; P = .006), hip internal rotation (prerehabilitation = 1.4 ± 0.5 N/kg, postrehabilitation = 1.7 ± 0.4 N/kg; P = .007), hip external rotation (prerehabilitation = 1.5 ± 0.4 N/kg, postrehabilitation = 3.2 ± 3.5 N/kg; P = .03), and hip extension (prerehabilitation = 3.5 ± 1.3 N/kg, postrehabilitation = 4.5 ± 1.3 N/kg; P = .001). We observed trends toward differences in both knee flexion (prerehabilitation = 2.2 ± 0.6 N/kg, postrehabilitation = 2.4 ± 0.7 N/kg; P = .06) and extension (prerehabilitation = 3.9 ± 1.5 N/kg, postrehabilitation = 4.9 ± 2.9 N/kg; P = .06).
Table 5.
Prerehabilitation and Postrehabilitation Strength for the Patterned Electrical Neuromuscular Stimulation and Sham Groups and Cohen d Effect Sizes With 95% CIs
| Strength Measure |
Group, N/kg (Mean ± SD) |
P Value |
Pooled Prerehabilitation- Postrehabilitation Cohen d Effect Size (95% CI)a |
|||||
| Patterned Electrical Neuromuscular Stimulation |
Sham |
|||||||
| Prerehabilitation |
Postrehabilitation |
Prerehabilitation |
Postrehabilitation |
Time Main Effect |
Group Main Effect |
Group × Time Interaction |
||
| Knee extension | 4.3 ± 1.3 | 5.5 ± 3.6 | 3.7 ± 1.7 | 4.3 ± 1.9 | .07 | .36 | .48 | 0.41 (−0.21, 1.03,) |
| Knee flexion | 2.5 ± 0.6 | 2.5 ± 0.7 | 1.7 ± 0.4 | 2.4 ± 0.6 | .045b | .26 | .05 | 0.31 (−0.30, 0.93) |
| Hip abduction | 3.0 ± 0.8 | 4.6 ± 2.9 | 2.9 ± 0.8 | 4.3 ± 2.3 | .007b | .74 | .84 | 0.85 (0.21, 1.49) |
| Hip external rotation | 1.7 ± 0.4 | 3.7 ± 4.4 | 1.4 ± 0.5 | 2.8 ± 2.5 | .04b | .50 | .67 | 0.66 (0.03, 1.29) |
| Hip internal rotation | 1.7 ± 0.5 | 1.7 ± 0.4 | 1.2 ± 0.4 | 1.7 ± 0.4 | .002b | .23 | .006b | 0.67 (0.04, 1.30) |
| Hip extension | 3.5 ± 1.1 | 4.2 ± 1.1 | 3.7 ± 1.5 | 4.8 ± 1.4 | <.001b | .45 | .47 | 0.70 (0.08, 1.34) |
Abbreviation: CI, confidence interval.
Effect sizes were calculated to compare the pooled group's prerehabilitation and postrehabilitation scores; a positive value denotes an increase in strength postrehabilitation.
Indicates difference (P ≤ .05).
Range of Motion
Range of motion of the quadriceps, hamstrings, IT band, and gastrocnemius showed main effects of time (Table 6). No main effects of group or group-by-time interactions were found in any ROM measures. We observed improvements in lower extremity ROM in the combined groups from prerehabilitation to postrehabilitation: quadriceps (prerehabilitation = 134.8 ± 2.5, postrehabilitation = 138.8 ± 2.1; P = .03), hamstrings (prerehabilitation = 81.8 ± 7.2, postrehabilitation = 94.8 ± 4.2; P = .01), IT band (prerehabilitation = 28.0 ± 4.0, postrehabilitation = 34.2 ± 3.4; P = .044), and gastrocnemius (prerehabilitation = 14.4 ± 2.2, postrehabilitation = 17.7 ± 1.5; P = .04).
Table 6.
Prerehabilitation and Postrehabilitation Range-of-Motion Measurements and Cohen d Effect Sizes With 95% CIs
| Muscle |
Group, ° (Mean ± SD) |
P Value |
Pooled Prerehabilitation- Postrehabilitation Cohen d Effect Size (95% CI)a |
|||||
| Patterned Electrical Neuromuscular Stimulation |
Sham |
|||||||
| Prerehabilitation |
Postrehabilitation |
Prerehabilitation |
Postrehabilitation |
Time Main Effect |
Group Main Effect |
Group × Time Interaction |
||
| Quadriceps | 134.9 ± 8.6 | 141.2 ± 3.6 | 135.0 ± 7.8 | 136.5 ± 8.9 | .03b | .45 | .17 | 0.54 (−0.08, 1.16) |
| Hamstrings | 81.6 ± 29.7 | 97.7 ± 13.6 | 83.9 ± 14.8 | 91.8 ± 13.3 | .01b | .81 | .34 | 0.71 (0.09, 1.35) |
| Iliotibial band | 31.4 ± 10.2 | 32.4 ± 9.6 | 23.0 ± 15.2 | 35.9 ± 4.4 | .03b | .51 | .07 | 0.56 (−0.06, 1.19) |
| Gastrocnemius | 14.0 ± 5.8 | 15.4 ± 4.6 | 14.1 ± 7.9 | 20.0 ± 4.3 | .03b | .28 | .18 | 0.55 (−0.07, 1.18) |
Abbreviation: CI, confidence interval.
Effect sizes were calculated to compare the pooled group's prerehabilitation and postrehabilitation scores; a positive value denotes an increase in range of motion after rehabilitation.
Indicates difference (P ≤ .05).
Activity Level
A main effect of time (both P = .040) was identified in steps per week for the PENS (prerehabilitation = 8660.2 ± 1932.4, postrehabilitation = 9593.6 ± 2350.5) and sham (prerehabilitation = 8970.7 ± 1968.7, postrehabilitation = 10 128.6 ± 2987.7) groups. No main effects of group (P = .69) or group-by-time interactions (P = .56) were observed.
DISCUSSION
The purpose of our study was to evaluate the effects of a 4-week impairment-based rehabilitation program with or without PENS on subjective and objective measures in individuals with PFP. We did not observe differences in subjective function, strength, ROM, or activity levels between the PENS and sham groups. However, all individuals with PFP demonstrated improvements in subjective function, pain, knee and hip strength, lower extremity ROM, and activity level after the impairment-based rehabilitation. Individuals who received an impairment-based rehabilitation program with PENS had improved pain at 6 and 12 months postrehabilitation.
Subjective Function
At postrehabilitation, we found improvements in the AKPS,17,39 ADLS,40 and LEFS39,41 scores similar to other rehabilitation programs for individuals with PFP. Subjective function was not different between groups over the 4-week rehabilitation program. However, combined subjective function improved on all 3 scales: AKPS (10.8 points), ADLS (8.7 points), and LEFS (7.5 points). These values improved and were greater than the minimal clinically important change threshold of the AKPS (8 points) and ADLS (7 points) but not the LEFS (9 points).30,39 The clinically meaningful improvements and large effect sizes that did not cross zero suggested that both interventions effectively improved subjective function in individuals with PFP. Whereas we expected PENS to improve the ability to gain strength and enhance self-reported function, perhaps the magnitude of change from the exercises made it difficult to detect the effect of any additional treatment.
Although the AKPS and LEFS are used more conventionally in the PFP literature, the ADLS produced some of the strongest psychometric properties both for evaluating the presence of PFP and assessing responsiveness to the interventions. Esculier et al40 reported that runners with PFP who completed an 8-week rehabilitation program improved their ADLS scores by 17.8 points, compared with 8.7 points in our participants. Whereas a multimodal rehabilitation program was used in both studies to address the individual needs of patients, our rehabilitation program lasted only half as long as the rehabilitation program for runners. In addition to pursuing the longer rehabilitation program, the runners reported greater subjective impairments (15 points less on the ADLS), which may have provided the potential for greater improvements during rehabilitation.
An impairment-based program with or without PENS also produced clinically important improvements in subjective function at 6 and 12 months. Changes in the AKPS and ADLS scores were greater than the minimal clinically important change threshold at 6 and 12 months. We observed improvements that were almost identical to those reported by Hamstra-Wright et al,42 who compared AKPS and VAS scores after knee-focused or hip-focused rehabilitation for patients with PFP immediately and at 6 months postintervention. Fukada et al38 found similar improvements at 6 and 12 months in subjective function and decreased pain in patients with PFP who completed a combined hip and knee intervention but not an isolated knee-strengthening program. Comparing our results with those of Fukada et al,38 a more comprehensive rehabilitation program that targeted multiple muscles appeared to more effectively improve subjective function and pain 1 year postrehabilitation.
Using PENS with an impairment-based program effectively improved long-term pain levels in those with PFP. Whereas we evaluated only subjective function and pain at the 6- and 12-month time points, greater improvements in pain levels may allow these patients to be more physically active and improve their overall quality of life. Researchers26,27 demonstrated that using PENS in individuals with PFP resulted in immediate improvements in pain, gluteus medius muscle activity, and lower extremity kinematics. One potential hypothesis for long-term improvements in the PENS group may be the cumulative effect of PENS on lower extremity muscle activity over the 4-week intervention. Currently, PFP is attributed to increased stress on the joint, so improved gluteal muscle activity could result in improved frontal-plane motion and decreased joint stress. However, this is speculative, as we did not assess lower extremity biomechanics.
Strength
We observed baseline strength values similar to those reported by Ferber et al15; however, we saw greater improvements in postrehabilitation strength of the knee and hip muscles. A few reasons may exist for the difference between our findings and those of Ferber et al,15 whose patients completed 6-week knee- or hip-strengthening programs. Whereas our rehabilitation program was 2 weeks shorter, we administered more exercises during each rehabilitation session than Ferber et al,15 who administered as few as 3 exercises per session. Our study design was intended to improve the external validity by mimicking clinical practice. All of our participants completed lower extremity strength-training exercises for the duration of the protocol, as such training is considered the standard of care for patients with PFP. Our protocol was developed to individualize treatment, with a percentage of the participants' baseline strength-assessment values selected to challenge them from their initial visits.
Range of Motion
Improvements in ROM were observed for the quadriceps, hamstrings, IT band, and gastrocnemius independent of group intervention. Lower extremity ROM is commonly prescribed in rehabilitation programs20,43,44 but is rarely evaluated as a dependent variable. We noted the largest soft tissue restriction in the hamstrings, as reflected by decreased hip-flexion ROM that improved by more than 10° postrehabilitation. The hamstrings play an important role in PFP: decreased flexibility has been theorized to increase quadriceps force production to complete functional tasks, which may increase stress on the patellofemoral joint.29 Improvements in quadriceps, gastrocnemius, and IT band ROM were identified after this targeted intervention program. Improvements in these motions may also help decrease the stress placed on the patellofemoral joint, decreasing pain and improving subjective function.29
Avraham et al43 were among the few researchers to compare the effectiveness of various stretching and strengthening combinations for PFP treatment. They found that, whereas stretching improved both pain and function, greater improvement was seen in conjunction with strengthening programs. Combining multiple treatment options for the individual patient appears to improve impairments.
Activity Levels
Patients with PFP had decreased physical activity, which negatively relates to their subjective function.35 We observed that 4 weeks of rehabilitation improved weekly physical activity in individuals with PFP by more than 1000 steps per day. Although this improvement appeared positive, a large discrepancy still existed between this postrehabilitation activity level and the activity levels in healthy individuals.35 Physical activity has many health benefits, and investigators45 have reported evidence of its ability to delay the development of osteoarthritis. Given that PFP may be a risk factor for patellofemoral osteoarthritis, interventions to improve activity levels in individuals with PFP should be evaluated to determine their effects on short- and long-term outcomes.
Limitations
Our study had limitations. First, we enrolled a relatively small number of participants, potentially decreasing the generalizability of the findings. Post hoc power was moderate; however, a study with a larger sample size should be conducted to further examine the effect of PENS on lower extremity clinical measures in a population with PFP. This would also allow for more advanced statistical analyses to determine which improvements may be more valuable measures for clinicians who commonly treat this condition. Second, we conducted only 4 weeks of rehabilitation, which would explain neuromuscular adaptations. Longer rehabilitation programs may be required to produce more hypertrophic gains. Third, whereas we had a true intervention group and a sham group, having a true blinded treatment for electrical stimulation is difficult in randomized trials. We could have studied a true control group, but due to the chronicity of PFP impairments, it is a safe assumption that clinical measures will not change in individuals who do not receive any treatment.
CONCLUSIONS
Using PENS in conjunction with a 4-week impairment-based rehabilitation program did not improve clinical measures more than the sham treatment. However, clinical improvements in subjective and objective function were seen after the impairment-based rehabilitation program. Clinicians should focus on progressive rehabilitation that targets individual impairments to improve subjective and objective function in patients with PFP.
ACKNOWLEDGMENTS
This study was funded by the Mid-Atlantic Athletic Trainers' Association and Accelerated Care Plus (Reno, NV; Dr Saliba).
Appendix.
Rehabilitation exercises.
REFERENCES
- 1.Glaviano NR, Kew M, Hart JM, Saliba S. Demographic and epidemiological trends in patellofemoral pain. Int J Sports Phys Ther. 2015;10(3):281–290. [PMC free article] [PubMed] [Google Scholar]
- 2.Taunton JE, Ryan MB, Clement DB, McKenzie DC, Lloyd-Smith DR, Zumbo BD. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med. 2002;36(2):95–101. doi: 10.1136/bjsm.36.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boling M, Padua D, Marshall S, Guskiewicz K, Pyne S, Beutler A. Gender differences in the incidence and prevalence of patellofemoral pain syndrome. Scand J Med Sci Sports. 2010;20(5):725–730. doi: 10.1111/j.1600-0838.2009.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powers CM. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33(11):639–646. doi: 10.2519/jospt.2003.33.11.639. [DOI] [PubMed] [Google Scholar]
- 5.Blond L, Hansen L. Patellofemoral pain syndrome in athletes: a 5.7-year retrospective follow-up study of 250 athletes. Acta Orthop Belg. 1998;64(4):393–400. [PubMed] [Google Scholar]
- 6.Nakagawa TH, Moriya ET, Maciel CD, Serrao FV. Trunk, pelvis, hip, and knee kinematics, hip strength, and gluteal muscle activation during a single-leg squat in males and females with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2012;42(6):491–501. doi: 10.2519/jospt.2012.3987. [DOI] [PubMed] [Google Scholar]
- 7.Bolgla LA, Malone TR, Umberger BR, Uhl TL. Hip strength and hip and knee kinematics during stair descent in females with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2008;38(1):12–18. doi: 10.2519/jospt.2008.2462. [DOI] [PubMed] [Google Scholar]
- 8.Lankhorst NE, Bierma-Zeinstra SM, van Middelkoop M. Factors associated with patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013;47(4):193–206. doi: 10.1136/bjsports-2011-090369. [DOI] [PubMed] [Google Scholar]
- 9.Piva SR, Goodnite EA, Childs JD. Strength around the hip and flexibility of soft tissues in individuals with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2005;35(12):793–801. doi: 10.2519/jospt.2005.35.12.793. [DOI] [PubMed] [Google Scholar]
- 10.Souza RB, Powers CM. Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sports Phys Ther. 2009;39(1):12–19. doi: 10.2519/jospt.2009.2885. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa TH, Baldon Rde M, Muniz TB, Serrao FV. Relationship among eccentric hip and knee torques, symptom severity and functional capacity in females with patellofemoral pain syndrome. Phys Ther Sport. 2011;12(3):133–139. doi: 10.1016/j.ptsp.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Price AJ, Jones J, Allum R. Chronic traumatic anterior knee pain. Injury. 2000;31(5):373–378. doi: 10.1016/s0020-1383(00)00006-1. [DOI] [PubMed] [Google Scholar]
- 13.Stathopulu E, Baildam E. Anterior knee pain: a long-term follow-up. Rheumatology (Oxford) 2003;42(2):380–382. doi: 10.1093/rheumatology/keg093. [DOI] [PubMed] [Google Scholar]
- 14.Utting MR, Davies G, Newman JH. Is anterior knee pain a predisposing factor to patellofemoral osteoarthritis? Knee. 2005;12(5):362–365. doi: 10.1016/j.knee.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Ferber R, Bolgla L, Earl-Boehm JE, Emery C, Hamstra-Wright K. Strengthening of the hip and core versus knee muscles for the treatment of patellofemoral pain: a multicenter randomized controlled trial. J Athl Train. 2015;50(4):366–377. doi: 10.4085/1062-6050-49.3.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferber R, Kendall KD, Farr L. Changes in knee biomechanics after a hip-abductor strengthening protocol for runners with patellofemoral pain syndrome. J Athl Train. 2011;46(2):142–149. doi: 10.4085/1062-6050-46.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Earl JE, Hoch AZ. A proximal strengthening program improves pain, function, and biomechanics in women with patellofemoral pain syndrome. Am J Sports Med. 2011;39(1):154–163. doi: 10.1177/0363546510379967. [DOI] [PubMed] [Google Scholar]
- 18.Baldon Rde M, Serrão FV, Scattone Silva R, Piva SR. Effects of functional stabilization training on pain, function, and lower extremity biomechanics in females with patellofemoral pain: a randomized clinical trial. J Orthop Sports Phys Ther. 2014;44(4):240–A8. doi: 10.2519/jospt.2014.4940. [DOI] [PubMed] [Google Scholar]
- 19.Khayambashi K, Mohammadkhani Z, Ghaznavi K, Lyle MA, Powers CM. The effects of isolated hip abductor and external rotator muscle strengthening on pain, health status, and hip strength in females with patellofemoral pain: a randomized controlled trial. J Orthop Sports Phys Ther. 2012;42(1):22–29. doi: 10.2519/jospt.2012.3704. [DOI] [PubMed] [Google Scholar]
- 20.Boling MC, Bolgla LA, Mattacola CG, Uhl TL, Hosey RG. Outcomes of a weight-bearing rehabilitation program for patients diagnosed with patellofemoral pain syndrome. Arch Phys Med Rehabil. 2006;87(11):1428–1435. doi: 10.1016/j.apmr.2006.07.264. [DOI] [PubMed] [Google Scholar]
- 21.Hart JM, Pietrosimone B, Hertel J, Ingersoll CD. Quadriceps activation following knee injuries: a systematic review. J Athl Train. 2010;45(1):87–97. doi: 10.4085/1062-6050-45.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Callaghan MJ, Oldham JA, Winstanley J. A comparison of two types of electrical stimulation of the quadriceps in the treatment of patellofemoral pain syndrome: a pilot study. Clin Rehabil. 2001;15(6):637–646. doi: 10.1191/0269215501cr457oa. [DOI] [PubMed] [Google Scholar]
- 23.Bily W, Trimmel L, Modlin M, Kaider A, Kern H. Training program and additional electric muscle stimulation for patellofemoral pain syndrome: a pilot study. Arch Phys Med Rehabil. 2008;89(7):1230–1236. doi: 10.1016/j.apmr.2007.10.048. [DOI] [PubMed] [Google Scholar]
- 24.Glaviano NR, Saliba S. Can the use of neuromuscular electrical stimulation be improved to optimize quadriceps strengthening? Sports Health. 2016;8(1):79–85. doi: 10.1177/1941738115618174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooke JD, Brown SH. Movement-related phasic muscle activation: II. Generation and functional role of the triphasic pattern. J Neurophysiol. 1990;63(3):465–472. doi: 10.1152/jn.1990.63.3.465. [DOI] [PubMed] [Google Scholar]
- 26.Glaviano NR, Saliba SA. Immediate effect of patterned electrical neuromuscular stimulation on pain and muscle activation in individuals with patellofemoral pain. J Athl Train. 2016;51(2):118–128. doi: 10.4085/1062-6050-51.4.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glaviano NR, Huntsman S, Dembeck A, Hart JM, Saliba S. Improvements in kinematics, muscle activity and pain during functional tasks in females with patellofemoral pain following a single patterned electrical stimulation treatment. Clin Biomech (Bristol, Avon) 2015;32:20–27. doi: 10.1016/j.clinbiomech.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Willson JD, Davis IS. Lower extremity strength and mechanics during jumping in women with patellofemoral pain. J Sport Rehabil. 2009;18(1):76–90. doi: 10.1123/jsr.18.1.76. [DOI] [PubMed] [Google Scholar]
- 29.Piva SR, Fitzgerald GK, Irrgang JJ, et al. Associates of physical function and pain in patients with patellofemoral pain syndrome. Arch Phys Med Rehabil. 2009;90(2):285–295. doi: 10.1016/j.apmr.2008.08.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esculier JF, Roy JS, Bouyer LJ. Psychometric evidence of self-reported questionnaires for patellofemoral pain syndrome: a systematic review. Disabil Rehabil. 2013;35(26):2181–2190. doi: 10.3109/09638288.2013.774061. [DOI] [PubMed] [Google Scholar]
- 31.Kelln BM, McKeon PO, Gontkof LM, Hertel J. Hand-held dynamometry: reliability of lower extremity muscle testing in healthy, physically active, young adults. J Sport Rehabil. 2008;17(2):160–170. doi: 10.1123/jsr.17.2.160. [DOI] [PubMed] [Google Scholar]
- 32.Ireland ML, Willson JD, Ballantyne BT, Davis IM. Hip strength in females with and without patellofemoral pain. J Orthop Sports Phys Ther. 2003;33(11):671–676. doi: 10.2519/jospt.2003.33.11.671. [DOI] [PubMed] [Google Scholar]
- 33.Selfe J, Callaghan M, Witvrouw E, et al. Targeted interventions for patellofemoral pain syndrome (TIPPS): classification of clinical subgroups. BMJ Open. 2013;3(9):e003795. doi: 10.1136/bmjopen-2013-003795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piva SR, Fitzgerald K, Irrgang JJ, et al. Reliability of measures of impairments associated with patellofemoral pain syndrome. BMC Musculoskelet Disord. 2006;7:33. doi: 10.1186/1471-2474-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glaviano NR, Baellow A, Saliba S. Physical activity levels in individuals with and without patellofemoral pain. Phys Ther Sport. 2017;27:12–16. doi: 10.1016/j.ptsp.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Glaviano NR, Saliba S. Impairment based rehabilitation for patellofemoral pain patients. Phys Sportsmed. 2016;44(3):311–323. doi: 10.1080/00913847.2016.1200443. [DOI] [PubMed] [Google Scholar]
- 37.Glaviano NR, Saliba S. Relationship between lower-extremity strength and subjective function in individuals with patellofemoral pain. J Sport Rehabil. 2018;27(4):327–333. doi: 10.1123/jsr.2016-0177. [DOI] [PubMed] [Google Scholar]
- 38.Fukuda TY, Melo WP, Zaffalon BM, et al. Hip posterolateral musculature strengthening in sedentary women with patellofemoral pain syndrome: a randomized controlled clinical trial with 1-year follow-up. J Orthop Sports Phys Ther. 2012;42(10):823–830. doi: 10.2519/jospt.2012.4184. [DOI] [PubMed] [Google Scholar]
- 39.Fukuda TY, Rossetto FM, Magalhaes E, Bryk FF, Lucareli PR, de Almeida Aparecida Carvalho N. Short-term effects of hip abductors and lateral rotators strengthening in females with patellofemoral pain syndrome: a randomized controlled clinical trial. J Orthop Sports Phys Ther. 2010;40(11):736–742. doi: 10.2519/jospt.2010.3246. [DOI] [PubMed] [Google Scholar]
- 40.Esculier JF, Bouyer LJ, Roy JS. The effects of a multimodal rehabilitation program on symptoms and ground-reaction forces in runners with patellofemoral pain syndrome. J Sport Rehabil. 2016;25(1):23–30. doi: 10.1123/jsr.2014-0245. [DOI] [PubMed] [Google Scholar]
- 41.Dolak KL, Silkman C, Medina McKeon J, Hosey RG, Lattermann C, Uhl TL. Hip strengthening prior to functional exercises reduces pain sooner than quadriceps strengthening in females with patellofemoral pain syndrome: a randomized clinical trial. J Orthop Sports Phys Ther. 2011;41(8):560–570. doi: 10.2519/jospt.2011.3499. [DOI] [PubMed] [Google Scholar]
- 42.Hamstra-Wright KL, Aydemir B, Earl-Boehm J, Bolgla L, Emery C, Ferber R. Lasting improvement of patient-reported outcomes 6 months post patellofemoral pain rehabilitation. J Sport Rehabil. 2017;26(4):223–233. doi: 10.1123/jsr.2015-0176. [DOI] [PubMed] [Google Scholar]
- 43.Avraham F, Aviv S, Ya'akobi P, et al. The efficacy of treatment of different intervention programs for patellofemoral pain syndrome: a single blinded randomized clinical trial. Pilot study. ScientificWorldJournal. 2007;7:1256–1262. doi: 10.1100/tsw.2007.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark DI, Downing N, Mitchell J, Coulson L, Syzpryt EP, Doherty M. Physiotherapy for anterior knee pain: a randomised controlled trial. Ann Rheum Dis. 2000;59(9):700–704. doi: 10.1136/ard.59.9.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hubbard-Turner T, Guderian S, Turner MJ. Lifelong physical activity and knee osteoarthritis development in mice. Int J Rheum Dis. 2015;18(1):33–39. doi: 10.1111/1756-185X.12291. [DOI] [PubMed] [Google Scholar]