Abstract
The aim of this study was to determine what lifestyle changes can predict acute onset hypertension in the normotensive community-dwelling elderly.
This study targeted elderly people enrolled in National Health Insurance in Fukushima Prefecture, Japan. The subjects were 24,490 people who took all of the specific health examination conducted by National Health Insurance in fiscal years 2013, 2014, and 2015 continuously and had a recorded systolic blood pressure (BP) <130 mm Hg and diastolic BP <85 mm Hg in the first 2 fiscal years. We examined their lifestyle changes for the first 2 fiscal years using the questionnaires given at the health examination. Multivariate Poisson regression analysis was conducted to examine the relationship between new-onset hypertension observed at the last examination and unhealthy lifestyle changes.
The mean age of the subjects was 61.5 ± 8.2 years old at baseline. We observed new-onset hypertension in 1.062 subjects at the last examination. Of the study subjects, 12,027 (49.1%) answered to having at least one of the items of unhealthy lifestyle change in the questionnaire. In the multivariate logistic regression, eating supper before bedtime showed a significant increase in the risk ratio for acute onset hypertension (risk ratio 1.27, 95% confidence interval, 1.01–1.58).
This study indicated that eating before bedtime is a risk factor of new-onset hypertension in the normotensive community-dwelling elderly. Adequate health guidance to avoid unhealthy lifestyle changes is required even in normotensive people as this hypertension is preventable.
Keywords: community-dwelling elderly, health examination, hypertension, unhealthy lifestyle change
1. Introduction
Hypertension is one of the most common chronic diseases in the world and is widely known as a major risk factor for myocardial infarction, stroke, heart failure, and renal failure.[1–3] It is estimated that 10 million individuals have hypertension in Japan,[4] and hypertension and related diseases amount to large medical expenditure.[5] The disease and its related complications annually account for 5.9 trillion yen.[6] In Japan, the total medical expenditure is increasing due to the rapidly aging population and cardiovascular disease; thus, concerns regarding this issue have been raised.[5] The prevalence of hypertension increases with aging, and Japan has a rapidly increasing elderly population. A preventive strategy for hypertension is important in avoiding an increase in future medical expenditure.
The classification of blood pressure (BP) was changed in the 2017 guidelines by the American Heart Association (AHA).[7] A BP level of 130 to 139/80 to 89 mm Hg that had been classified as high-normal was newly categorized as stage 1 hypertension.[7,8] The importance of nonpharmacological intervention is emphasized in the new guidelines, especially for stage 1 hypertension. Lifestyle modification is an important nonpharmacological intervention strategy for hypertension. Modifications such as increasing exercise and decreasing salt intake can improve BP,[9,10] whereas an unhealthy lifestyle such as fast eating and a lack of regular exercise is strongly related to hypertension.[11,12] In Japan, all residents over 40 years of age can take a specific health examination and receive health guidance every year, according to the Health and Medical Service Law for the Aged.[13] This system is primary prevention aiming at self-management and prolonging the onset of lifestyle-related diseases.
Lifestyle modification strategies mainly target prehypertension[7,8]; however, we hypothesized that if we could identify what unhealthy lifestyle changes in normotensive people are associated with acute impairment of BP, a more effective primary prevention strategy could be established. The aim of this study was, therefore, to analyze what unhealthy lifestyle changes can predict acute onset hypertension in the normotensive community-dwelling elderly.
2. Method
2.1. Study design
This study was conducted as a retrospective cohort study. We used data on specific health examinations of National Health Insurance (NHI) in Fukushima Prefecture, Japan, that belonged to Fukushima National Health Insurance Organization. The specific health examination is an annual health examination that is conducted by the health insurance.[14,15] The NHI, one of the health insurances, is mainly for farmers, self-employed persons, retired persons, and their nonworking dependents aged <75 years old.[16]
2.2. Study participants
We obtained data from 92,279 subjects who had taken all of their specific health examination annually from fiscal year (FY) 2013 (from April 2013 to March 2014) to FY 2015 continuously. We used the data on age, sex, height (cm), weight (kg), body mass index (BMI) (kg/m2), waist circumference (cm), systolic BP (mm Hg), diastolic BP (mm Hg), self-reported medication (hypertension, diabetes, and dyslipidemia), a self-reported questionnaire about lifestyle (current smoker [yes, no], alcohol drinking [everyday, sometimes, rarely, never], weight gain or loss of ≥3 kg over the previous year [yes, no], regular physical exercise [30 min/time, twice a week] [yes, no], daily walking or equivalent physical activity [1 hour] [yes, no], fast walking speed [yes, no], eating speed [fast, moderate, slow], eating supper before bedtime [within 2 hours, 3 times/wk] [yes, no], eating a snack after supper [3 times/week] [yes, no], skipping breakfast [3 times/wk] [yes, no], subjectively sufficient sleep [yes, no], wanting to improve their own life habits [no plan, start within 6 months, start within a month, already started <6 months ago, already started ≥6 months ago], and a self-reported past medical history). The self-reported questionnaire was verified by nurses.
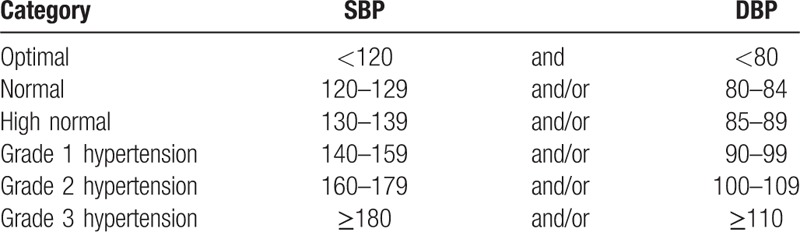
The criteria of selecting study subjects are shown in Figure 1. First, individuals with missing data (n = 4332) were excluded. Second, from the data on the FY 2013 health checkup of the remaining 87,947 subjects, we excluded individuals with a past medical history of hypertension, cardiac disease (myocardial infarction, atrial defibrillation, bundle branch block, arrhythmia, or other cardiac diseases), and/or cerebrovascular disease (cerebral infarction, cerebral hemorrhage, subarachnoid hemorrhage, or cerebral stroke) (n = 29,341) and those with a BP level of high-normal, hypertension, and taking antihypertensive medication (n = 27,015) were excluded. In the FY 2014 health examination, subjects with a BP level of high-normal and hypertension, and taking antihypertensive medication (n = 7101), were also excluded. Finally, 24,490 remained as study subjects. In the specific health examination, BP was measured once by trained public health nurses using a standard mercury sphygmomanometer on the right arm of seated participants based on the standardized health examination protocol. BP level was categorized according to the definition of the European Society of Hypertension and the European Society of Cardiology (ESC) (Table 1).[17]
Figure 1.
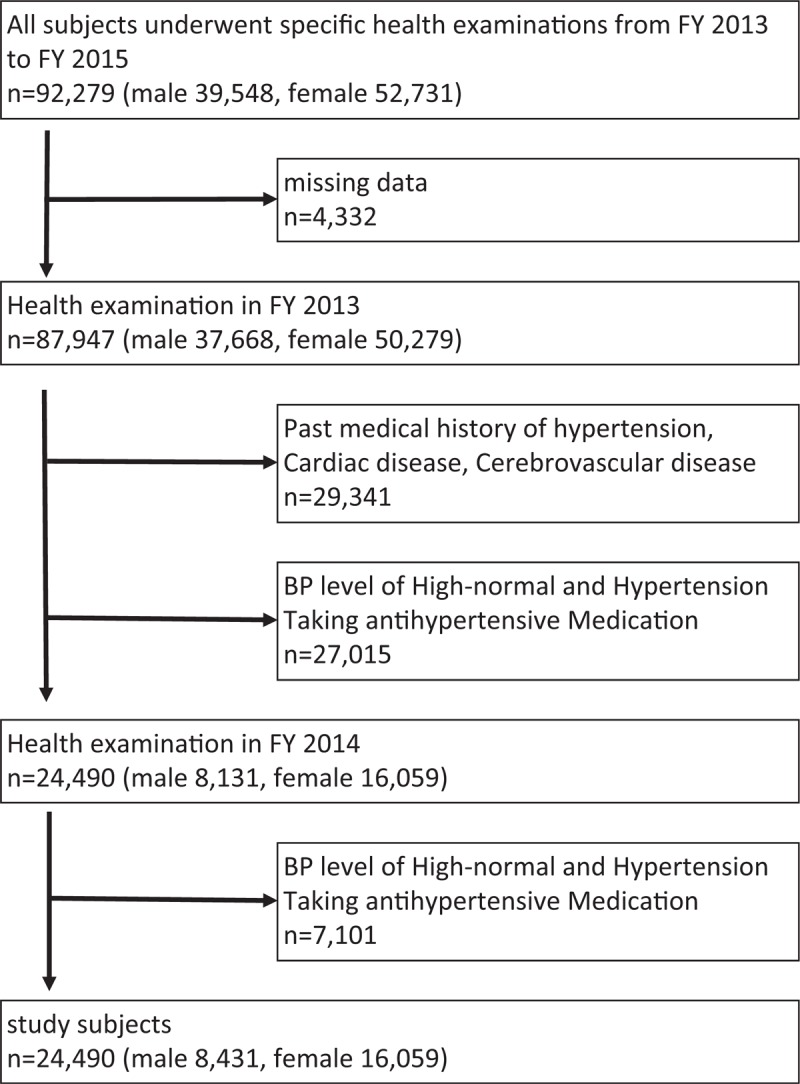
Subjects included in the study, excluded from the study and follow-up criteria.
Table 1.
Classification of blood pressure levels (mm Hg).
2.3. Definition of new-onset hypertension
New-onset hypertension was defined following the definition of ESC and/or taking antihypertensive medication in FY 2015. The validation of self-reported antihypertensive medication was high. The comparison of the check-up month with the previous 2 months yielded a sensitivity, specificity, and κ statistic of 92.4, 86.4, and 70.9%, respectively.[18]
2.4. Unhealthy lifestyle changes
We compared the self-reported lifestyle questionnaires answered in FY 2013 and FY 2014. Unhealthy lifestyle changes were determined as follows: for the 2 choice questions; current smoker, from “no’ to “yes’; weight gain or loss of ≥3 kg over the previous year, from “no” to “yes”; regular physical exercise, from “yes” to “no”; daily walking or equivalent physical activity, from “yes” to “no”; fast walking speed, from “yes” to “no”; eating before bedtime, from “no” to “yes”; eating a snack after supper, from “no” to “yes”; skipping breakfast, from “no” to “yes”; subjectively sufficient sleep, from “yes” to “no.” For the multiple choice questions, frequency of alcohol drinking, from “rarely” or “sometimes” to “every day”; eating speed, from “slow” or “moderate” to “fast.”[19]
2.5. Statistical analysis
The baseline data on the subjects were analyzed. Continuous variables are expressed as means (standard deviations [SDs]) and categorical variables as percentages. We also compared them between the 2 groups, subjects with new-onset hypertension and the others. As the normality of all continuous variables was rejected by the Kolmogorov–Smirnov test, the Mann–Whitney U test was used for continuous variables and the chi-square test was used for categorical variables. We compared the rate of unhealthy lifestyle changes between the 2 groups.
Poisson regression analysis was performed to estimate the risk ratio (RR) and 95% confidence interval (CI) of new-onset hypertension for each lifestyle change. Age (<45 [reference], 45–<50, 50–<55, 55–<60, 60–<65, 65–<70, ≥70), sex, BMI (<18.5, 18.5–<22 [reference], 22–<25, ≥25), and central obesity (waist circumference ≥85 cm for males, and ≥90 cm for females) were used as confounding factors. We also used smoking status in FY 2013 as a confounding factor because the number of subjects who changed smoking status from FY 2013 to FY 2014 was only 393 (1.6%). Age and sex were adjusted for each lifestyle-questionnaire item. Age, sex, BMI, central obesity, medication (diabetes and dyslipidemia), current smoker (in FY 2013), and 2 items of unhealthy lifestyle change (daily walking or equivalent physical activity and eating before bedtime), which showed a significant relationship in the age–sex-adjusted analysis, were included in the multiple-adjusted analysis.
We used the statistical software package R, version 3.4.4 (the R Foundation for Statistical Computing, Vienna, Austria).
2.6. Ethics
All data were anonymized by the Fukushima National Health Insurance Organization. Fukushima Medical University received anonymous data and analyzed it for the present study. This study was approved by the Ethics Committees of Fukushima Medical University (Application No. 2974).
3. Results
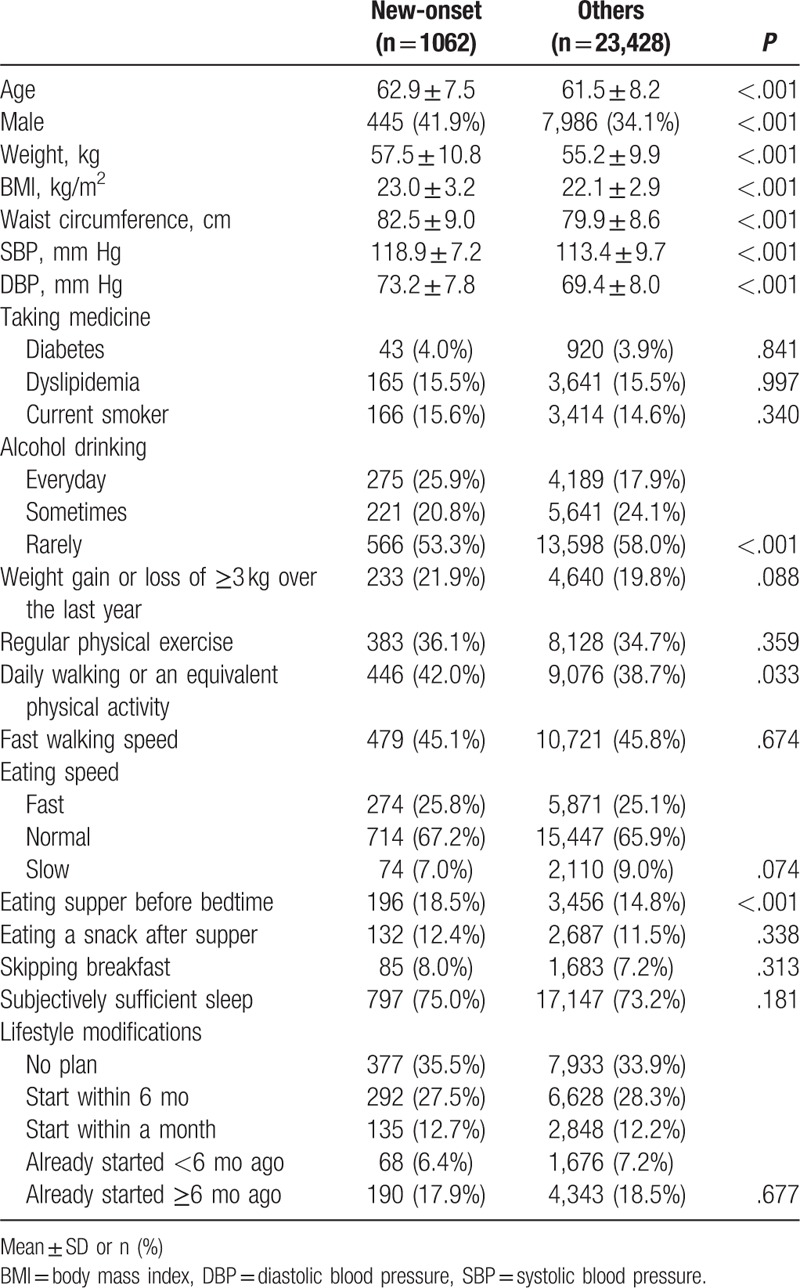
We described the baseline data on the subjects in Table 2. In FY 2015, 1062 (4.3%) of the subjects were detected to have new-onset hypertension. The mean age of the study subjects was 61.5 ± 8.2 years old and 10,635 (43.4%) subjects were 65 years or older. Age, sex, weight, BMI, waist, SBP, DBP, alcohol drinking, daily walking or equivalent physical activity, and eating before bedtime showed a significant difference between the 2 groups. More subjects in the new-onset group engaged in daily walking or equivalent physical activity (42.0% in the new-onset group and 38.7% in the others, P = .033).
Table 2.
Characteristics in 2013 between the 2 groups.
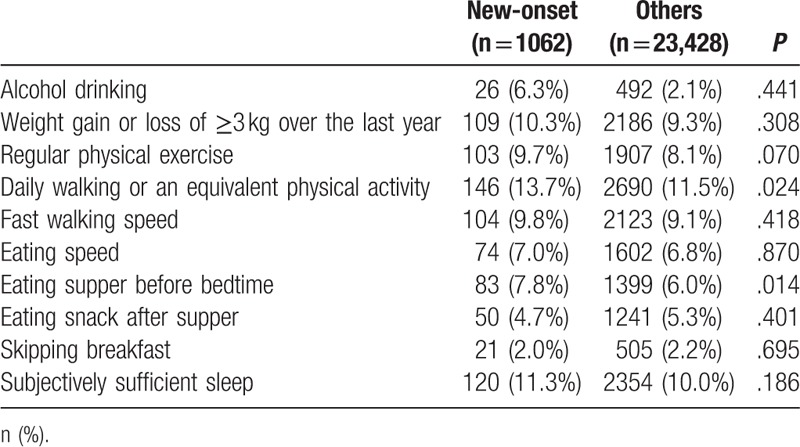
In FY 2014, 9903 (40.5%) subjects reported that they would be willing to change their lifestyle for the better and 6277 (29.6%) subjects reported to have made healthy lifestyle changes. On the contrary, 12,027 (49.1%) subjects reported that they had made at least one of the 11 items of unhealthy lifestyle change. For physical activity, 2010 (8.2%) subjects discontinued regular physical exercise; 2836 (11.6%) subjects discontinued daily walking or equivalent physical activity; and 2295 (9.1%) subjects slowed their walking speed. For eating habits, 1676 (6.8%) subjects increased their eating speed, 1482 (6.1%) subjects started eating supper before bedtime, 1291 (5.3%) subjects started eating snack after supper, and 526 (2.1%) subjects started skipping breakfast. In the new-onset group, significantly more subjects made unhealthy changes such as cessation of daily walking or equivalent physical activity and commencement of eating before bedtime (Table 3). In both groups, almost the same number of subjects made healthy changes by starting daily walking or equivalent physical activity or by stopping eating before bedtime.
Table 3.
Undesirable lifestyle change between the 2 groups.
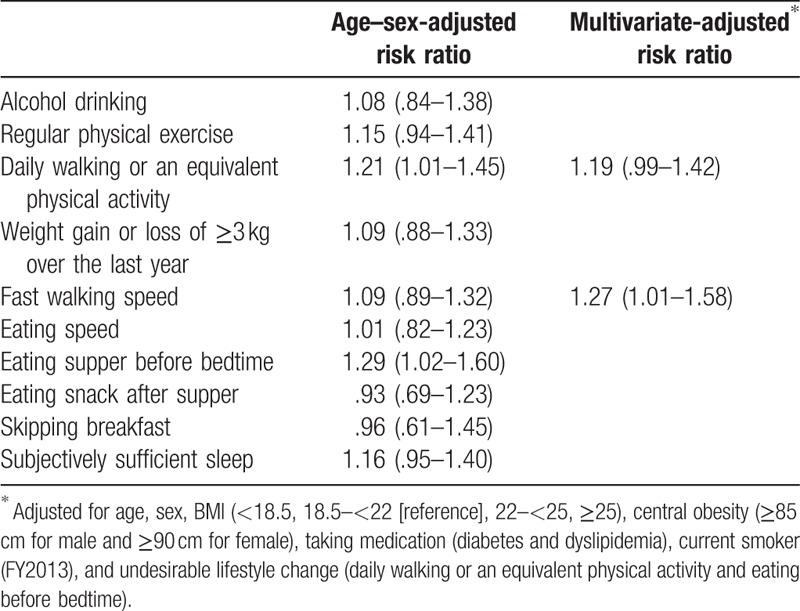
The results of Poisson regression analysis are shown in Table 4. In the age–sex-adjusted model, daily walking (RR 1.21, 95% CI, 1.01–1.45) or equivalent physical activity and eating before bedtime (RR 1.29, 95% CI, 1.02–1.60) showed a significant increase in RR of new-onset hypertension. In the multivariate adjusted model, eating before bedtime also showed a significant increase in RR of new-onset hypertension (RR 1.29, 95% CI, 1.01–1.61), but daily walking or equivalent physical activity did not show a significant increase in RR for new-onset hypertension (RR 1.19, 95% CI, 0.99–1.58).
Table 4.
Odds ratio of new-onset hypertension for undesirable lifestyle change.
4. Discussion
We followed up 24,490 normotensive community dwelling elderly people for 3 years and revealed that an increase in frequency of eating supper before bedtime was significantly related to new-onset hypertension. The subjects, who recorded a normotensive BP but started to eat supper before bedtime during FY 2013 and 2014, had acute BP increase and developed new-onset hypertension in FY 2015. Because BP increases gradually with aging, the prevalence of hypertension increases with aging.[2,14,20,21] Conventionally, the hypertension prevention strategy was mainly targeted at prehypertension. Our results implicate that unhealthy lifestyle change, especially an increase in frequency of eating before bedtime in people with a normal BP, may cause acute BP impairment, and a hypertension prevention strategy is required even for normotensive people.
In this study, the rate of new-onset hypertension was 4.3%, which is quite lower than past studies, where annual incidence rates of new-onset hypertension among 40 to 75-year-old community-dwelling people were 3.7% to 16.0% for optimal BP levels and 12.6% to 25.5% for normal BP levels.[21–23] In this study, the subjects were those who recorded optimal or normal BP levels continuously for the 2 years. Thus, this study might have shown a lower incidence rate of new-onset hypertension compared to past studies.
Increased frequency of eating before bedtime was significantly related to new-onset of hypertension. For the dietary strategy for hypertension, salt intake is widely considered as a major risk factor.[24,25] In addition, dinner time was also indicated as a risk factor. In Japan, the period between dinner time and bedtime showed a significant dose–response relationship with hypertension in workers.[26] Eating dinner late causes obesity and metabolic syndrome.[27–29] Eating dinner late is currently considered a night eating disorder (NES) involving many habitual problems such as excess energy intake, sleep disorder, and morning anorexia.[29,30] Among NES patients, 55.7% have major depression and 66.0% have sleep onset disorder.[31] Morning anorexia is one of the diagnostic criteria of NES, which leads to skipping breakfast.[30,31] Creating a strategy for NES is difficult because sometimes those who suffer are unaware of having NES, and eating is not the primary reason for getting up at night, but rather a way to kill time.[31] Night time eating does not always have bad health effects.[29,32] Some studies suggest that the guidance to taking protein-rich food late at night may improve health.[32,33] NES with mental problems requires another strategy. In addition, some lifestyle-related problems such as obesity, stress, and sleep are risk factors of nonalcoholic fatty liver disease (NAFLD).[34] As a result of eating before bedtime, the risk of NAFLD increases. NAFLD is an independent risk factor of hypertension.[35] Thus, healthcare providers should offer examinations for NAFLD for subjects who reported increased frequency of eating supper before bedtime.
In the present study, cessation of daily walking or equivalent physical exercise showed a significant relationship to new-onset hypertension in the age–sex-adjusted model, but did not show a significant relationship in the multivariate model. Increased physical exercise and physical activity have been shown to improve BP.[36,37] At the baseline, the rate of individuals who ceased daily walking or equivalent physical exercise was significantly higher in the new-onset group, but the rate of unhealthy lifestyle changes was also significantly higher. These results indicate that an acute cessation of physical exercise is strongly related to increased BP. Decreased physical activity might cause physical problems such as orthopedic diseases or social problems.
Health guidance for lifestyle modification must look at both work and daily living. AHA Guidelines recommend 6 lifestyle changes for nonpharmacological antihypertensive interventions; weight loss, a heart-healthy diet, sodium reduction, potassium supplementation, increased physical activity, and moderate alcohol consumption.[7] More than half of the subjects in the present study were at working ages, over 65 years or older. The occupations of those enrolled NHI are mainly agriculture, fishery, self-employed, or temporary part-time work. These occupations are thought to be vulnerable and low discretion job,[38] and their working style may cause unhealthy lifestyle changes.[39,40] There are several studies that have shown the difficulty of lifestyle modification in community-dwelling people.[41,42] Unhealthy lifestyle changes may also be caused by mental problems related to inevitable factors such as nursing care of their family, severe disease of themselves, and stress for their jobs. The healthcare provider should take care of the background of unhealthy lifestyle changes.
Past studies on lifestyle and lifestyle-related diseases used data only on lifestyle at the baseline[11] or were cross-sectional studies.[12] The present study, which focused on unhealthy lifestyle changes, indicated that many people go through lifestyle change, so it is important to focus on lifestyle change in health guidance.
5. Limitations
On the contrary, there are some limitations in this study. First, target subjects were those who attended all medical examinations continuously for 3 years. We could not estimate the attendance rate for the health examinations. Second, these subjects are enrolled in the NHI. Although 45% of the census population aged 40 to 74 years in these communities are enrolled in the NHI, the present results may not be generalizable to the entire population. Thus, these results include selection bias.
5.1. Future directions
Follow-up study of the subjects is required to investigate the longitudinal effect of lifestyle changes. In addition, to support the follow-up study, this study can be expanded to cover all people who undergo the specific health examination conducted by NHI.
6. Conclusion
This study indicated that eating before bedtime is a risk factor of new-onset hypertension in the normotensive community-dwelling elderly. Adequate health guidance is required even in normotensive people as this hypertension is preventable.
Acknowledgment
We thank the Fukushima National Health Insurance Organization for data collection.
Author contributions
Conceptualization: Takeyasu Kakamu, Tomoo Hidaka, Tomohiro Kumagai, Yusuke Masuishi, Hideaki Kasuga, Shota Endo, Sei Sato, Akiko Takeda, Makoto Koizumi, Tetsuhito Fukushima.
Formal analysis: Hideaki Kasuga, Shota Endo, Sei Sato.
Investigation: Takeyasu Kakamu, Tomoo Hidaka, Akiko Takeda, Makoto Koizumi.
Methodology: Takeyasu Kakamu, Tomoo Hidaka, Tomohiro Kumagai, Akiko Takeda, Makoto Koizumi.
Project administration: Takeyasu Kakamu, Tomoo Hidaka, Yusuke Masuishi, Hideaki Kasuga, Sei Sato.
Resources: Akiko Takeda, Makoto Koizumi.
Software: Takeyasu Kakamu.
Supervision: Tomohiro Kumagai, Tetsuhito Fukushima.
Writing – original draft: Takeyasu Kakamu.
Writing – review & editing: Tomoo Hidaka, Tomohiro Kumagai, Yusuke Masuishi, Hideaki Kasuga, Shota Endo, Sei Sato, Akiko Takeda, Makoto Koizumi, Tetsuhito Fukushima.
Takeyasu Kakamu orcid: 0000-0001-6920-8457.
Footnotes
Abbreviations: AHA = American Heart Association, BMI = body mass index, BP = blood pressure, CI = confidence interval, ESC = European Society of Cardiology, FY = fiscal year, NES = night eating syndrome, NHI = National Health Insurance, OR = odds ratio, SD = standard deviation.
AT and MK are employed in Fukushima National Health Insurance Organization and they used the data as their work.
The authors have no funding to disclose.
References
- [1].Okayama A, Kadowaki T, Okamura T, et al. Age-specific effects of systolic and diastolic blood pressures on mortality due to cardiovascular diseases among Japanese men (NIPPON Data80∗). J Hypertens 2006;24:459–62. [DOI] [PubMed] [Google Scholar]
- [2].Ikeda A, Iso H, Yamagishi K, et al. Blood pressure and the risk of stroke, cardiovascular disease, and all-cause mortality among Japanese: the JPHC Study. Am J Hypertens 2009;22:273–80. [DOI] [PubMed] [Google Scholar]
- [3].Iyer AS, Ahmed MI, Filippatos GS, et al. Uncontrolled hypertension and increased risk for incident heart failure in older adults with hypertension: findings from a propensity-matched prospective population study. J Am Soc Hypertens JASH 2010;4:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ministry of Health, Labour and Welfare, Japan. Patient Survey 2014. Available at: http://www.mhlw.go.jp/toukei/saikin/hw/kanja/14/index.html Accessed February 7, 2018. [Google Scholar]
- [5].Nakamura K. Impact of cardiovascular risk factors on medical expenditure: evidence from epidemiological studies analysing data on health checkups and medical insurance. J Epidemiol 2014;24:437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ministry of Health, Labour and Welfare, Japan. Estimates of National Medical Care Expenditure 2014. Available at: http://www.mhlw.go.jp/toukei/saikin/hw/k-iryohi/14/index.html Accessed February 7, 2018. [Google Scholar]
- [7].Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- [8].ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens 2013;31:1925–38. [DOI] [PubMed] [Google Scholar]
- [9].Michishita R, Ohta M, Ikeda M, et al. Effects of lifestyle modification on an exaggerated blood pressure response to exercise in normotensive females. Am J Hypertens 2017;30:999–1007. [DOI] [PubMed] [Google Scholar]
- [10].Kitaoka K, Kitade A, Nagaoka J, et al. Lifestyle intervention might easily improve blood pressure in hypertensive men with the C genotype of angiotensin II type 2 receptor gene. Nutr Res Pract 2015;9:385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tsutatani H, Funamoto M, Sugiyama D, et al. Association between lifestyle factors assessed by standard question items of specific health checkup and the incidence of metabolic syndrome and hypertension in community dwellers: a five-year cohort study of National Health Insurance beneficiaries in Habikino City. Jpn J Public Health 2017;64:258–69. [DOI] [PubMed] [Google Scholar]
- [12].Hattori T, Konno S, Munakata M. Gender differences in lifestyle factors associated with metabolic syndrome and preliminary metabolic syndrome in the general population: the Watari Study. Intern Med Tokyo Jpn 2017;56:2253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ministry of Health, Labour, and Welfare, Japan. Standard program of health checkup and health guidance. Available at: http://www.mhlw.go.jp/bunya/shakaihosho/iryouseido01/info03a.html Accessed February 7, 2018. [Google Scholar]
- [14].Ong KL, Cheung BMY, Man YB, et al. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999-2004. Hypertens 2007;49:69–75. [DOI] [PubMed] [Google Scholar]
- [15].Kohro T, Furui Y, Mitsutake N, et al. The Japanese national health screening and intervention program aimed at preventing worsening of the metabolic syndrome. Int Heart J 2008;49:193–203. [DOI] [PubMed] [Google Scholar]
- [16].Ministry of Health, Labour and Welfare, Japan. Annual Health, Labour and Welfare Report 2016. Available at: http://www.mhlw.go.jp/english/wp/wp-hw10/index.html Accessed February 7, 2018. [Google Scholar]
- [17].Ross S, Walker A, MacLeod MJ. Patient compliance in hypertension: role of illness perceptions and treatment beliefs. J Hum Hypertens 2004;18:607–13. [DOI] [PubMed] [Google Scholar]
- [18].Fujita M, Sato Y, Nagashima K, et al. Validity assessment of self-reported medication use by comparing to pharmacy insurance claims. BMJ Open 2015;5:e009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ministry of Health, Labour, and Welfare, Japan. Standard questionnaire. Available at: http://www.mhlw.go.jp/seisakunitsuite/bunya/kenkou_iryou/kenkou/seikatsu/dl/hoken-program2_02.pdf Accessed February 7, 2018. [Google Scholar]
- [20].Whelton PK. The elusiveness of population-wide high blood pressure control. Annu Rev Public Health 2015;36:109–30. [DOI] [PubMed] [Google Scholar]
- [21].Vasan RS, Larson MG, Leip EP, et al. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet Lond Engl 2001;358:1682–6. [DOI] [PubMed] [Google Scholar]
- [22].Yano Y, Fujimoto S, Sato Y, et al. New-onset hypertension and risk for chronic kidney disease in the Japanese general population. J Hypertens 2014;32:2371–7. [DOI] [PubMed] [Google Scholar]
- [23].Kim J, Kim E, Yi H, et al. Short-term incidence rate of hypertension in Korea middle-aged adults. J Hypertens 2006;24:2177–82. [DOI] [PubMed] [Google Scholar]
- [24].Ohta Y, Ohta K, Ishizuka A, et al. Awareness of salt restriction and actual salt intake in hypertensive patients at a hypertension clinic and general clinic. Clin Exp Hypertens 2015;37:172–5. [DOI] [PubMed] [Google Scholar]
- [25].Takada T, Imamoto M, Sasaki S, et al. Effects of self-monitoring of daily salt intake estimated by a simple electrical device for salt reduction: a cluster randomized trial. Hypertens Res. 2018;41:524–530. [DOI] [PubMed] [Google Scholar]
- [26].Nakamoto M, Sakai T, Shuto E, et al. Period between dinner and bedtime is related to hypertension. J Jpn Soc Nutr Food Sci 2013;66:185–93. [Google Scholar]
- [27].Kutsuma A, Nakajima K, Suwa K. Potential association between breakfast skipping and concomitant late-night-dinner eating with metabolic syndrome and proteinuria in the Japanese population. Scientifica 2014;2014:253581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Navia B, López-Sobaler AM, Villalobos T, et al. Breakfast habits and differences regarding abdominal obesity in a cross-sectional study in Spanish adults: the ANIBES study. PloS One 2017;12:e0188828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kinsey AW, Ormsbee MJ. The health impact of nighttime eating: old and new perspectives. Nutrients 2015;7:2648–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Birketvedt GS, Florholmen J, Sundsfjord J, et al. Behavioral and neuroendocrine characteristics of the night-eating syndrome. JAMA 1999;282:657–63. [DOI] [PubMed] [Google Scholar]
- [31].de Zwaan M, Roerig DB, Crosby RD, et al. Nighttime eating: a descriptive study. Int J Eat Disord 2006;39:224–32. [DOI] [PubMed] [Google Scholar]
- [32].Madzima TA, Panton LB, Fretti SK, et al. Night-time consumption of protein or carbohydrate results in increased morning resting energy expenditure in active college-aged men. Br J Nutr 2014;111:71–7. [DOI] [PubMed] [Google Scholar]
- [33].Figueroa A, Wong A, Kinsey A, et al. Effects of milk proteins and combined exercise training on aortic hemodynamics and arterial stiffness in young obese women with high blood pressure. Am J Hypertens 2014;27:338–44. [DOI] [PubMed] [Google Scholar]
- [34].Ullah R, Rauf N, Nabi G, et al. Role of nutrition in the pathogenesis and prevention of non-alcoholic fatty liver disease: recent updates. Int J Biol Sci 2019;15:265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Oikonomou D, Georgiopoulos G, Katsi V, et al. Non-alcoholic fatty liver disease and hypertension: coprevalent or correlated? Eur J Gastroenterol Hepatol 2018;30:979–85. [DOI] [PubMed] [Google Scholar]
- [36].Arija V, Villalobos F, Pedret R, et al. Effectiveness of a physical activity program on cardiovascular disease risk in adult primary health-care users: the “Pas-a-Pas” community intervention trial. BMC Public Health 2017;17:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lundqvist S, Börjesson M, Larsson MEH, et al. Physical Activity on Prescription (PAP), in patients with metabolic risk factors. A 6-month follow-up study in primary health care. PloS One 2017;12:e0175190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Honjo K, Iso H, Ikeda A, et al. JACC Study Group. Employment situation and risk of death among middle-aged Japanese women. J Epidemiol Community Health 2015;69:1012–7. [DOI] [PubMed] [Google Scholar]
- [39].Inoue M, Minami M, Yano E. Body mass index, blood pressure, and glucose and lipid metabolism among permanent and fixed-term workers in the manufacturing industry: a cross-sectional study. BMC Public Health 2014;14:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Virtanen P, Vahtera J, Broms U, et al. Employment trajectory as determinant of change in health-related lifestyle: the prospective HeSSup study. Eur J Public Health 2008;18:504–8. [DOI] [PubMed] [Google Scholar]
- [41].Volker N, Williams LT, Davey RC, et al. Community-based lifestyle modification workforce: an underutilised asset for cardiovascular disease prevention. Aust J Prim Health 2016;22:327–31. [DOI] [PubMed] [Google Scholar]
- [42].Robare JF, Milas NC, Bayles CM, et al. The key to life nutrition program: results from a community-based dietary sodium reduction trial. Public Health Nutr 2010;13:606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


