Abstract
Rationale:
Reports of acute kidney injury (AKI) associated with benzbromarone use in patients with hyperuricemia (HUA) are rare so far.
Patient concerns:
We describe 2 unique clinical patterns in which benzbromarone was a possible cause of AKI following self-medication for HUA. In case 1, a 45-year-old man developed AKI after taking 100 mg of benzbromarone. His serum creatinine (Scr) increased to 2.3 mg/dL on day 2 after benzbromarone administration. Ultrasound showed multiple small stones in both kidneys, and the 24-hour urine uric acid level was 3128 mg. In case 2, a 17-year-old male student presented with AKI after self-administration of 50 mg of benzbromarone. His Scr increased to 6.8 mg/dL on day 3 after benzbromarone administration. Ultrasound showed multiple stones in the left kidney.
Diagnosis:
Both patients underwent renal biopsy, with findings of acute tubular interstitial nephropathy in case 1 and acute tubular damage in case 2. Drug-induced AKI was considered.
Interventions:
Both cases were treated supportively with intravenous hydration only. In both patients, the Scr level recovered within 0.5 months and renal function was normal 3 months after discharge.
Lessons:
Oral benzbromarone is widely used in Asian counties to treat HUA and the adverse effects are mostly mild. However, clinicians should be alert for benzbromarone-induced AKI. Moreover, uricosuric drugs should only be used after exclusion of urolithiasis and other contraindications.
Keywords: acute kidney injury, benzbromarone, hyperuricemia, urolithiasis
1. Introduction
The prevalence rate of hyperuricemia (HUA) has gradually increased in China. In economically developed cities, the prevalence rate has been 8.4% to 25.3%.[1–4] Dincer et al suggested that 90% of HUA was caused by a decrease in kidney excretion of uric acid.[5] The HLA-B∗5801 allele is highly prevalent in Han Chinese (12–23%),[6,7] and is strongly associated with severe, allopurinol-induced adverse cutaneous reactions. Although benzbromarone was withdrawn from the European market in 2003 due to reports of serious hepatotoxicity, it is still commonly prescribed in several countries in Asia, including China.
Benzbromarone-associated hepatotoxicity has been widely recognized, but the association with renal toxicity has not been adequately investigated. Some patients with HUA self-medicate with benzbromarone, despite possible contraindications such as urolithiasis and high 24-hour urine uric acid excretion, triggering a series of complications. We report 2 cases of benzbromarone-induced acute kidney injury (AKI) in patients with urolithiasis, in whom renal function returned to normal without special treatment.
1.1. Consent statement
The patients provided informed consent for the publication of this study. This study was performed in accordance with the Helsinki Declaration and was approved by the Ethics Committee of the China-Japan Friendship Hospital, Beijing.
2. Case report
2.1. Case 1
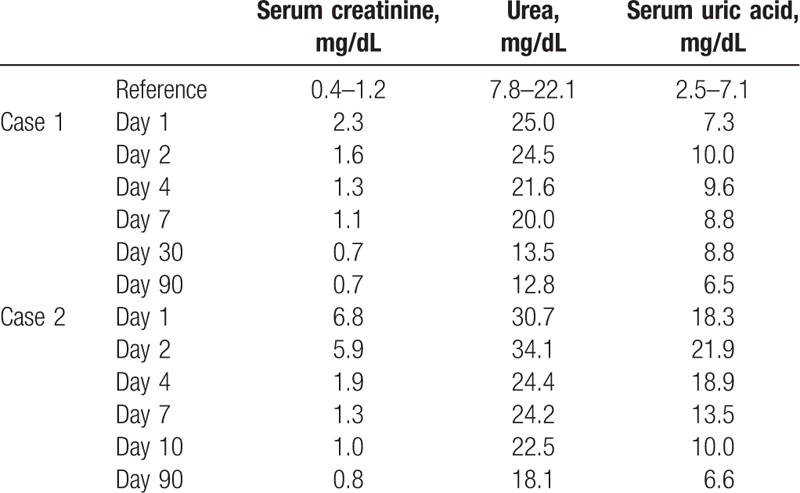
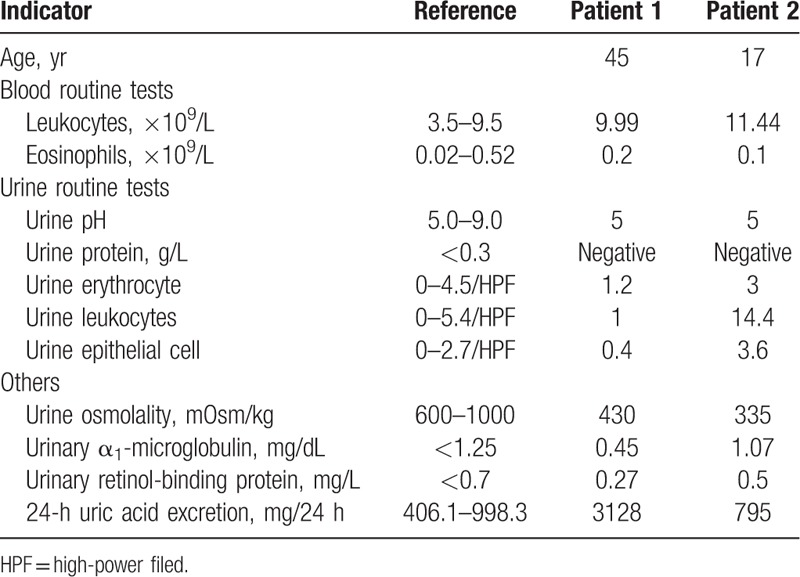
A 45-year-old man was admitted to the hospital due to severe kidney injury. The patient had no prior significant disease, surgical, or medication history. Four days prior to admission, his serum uric acid level was found to be 9.4 mg/dL (reference range: 2.5–7.1 mg/dL), with serum creatinine (Scr) of 0.6 mg/dL (reference range: 0.4–1.2 mg/dL) through physical examinations. The patient self-medicated with 100 mg of benzbromarone from a drugstore in the morning, and then drank 2000 mL of soda water. He did not take any other medication or vitamin C. Renal colic developed that night, but the symptoms improved with hot compresses. Tests the following morning revealed that Scr had risen to 2.3 mg/dL. On ultrasound, the right kidney measured 12.6 cm × 6.6 cm × 6.1 cm, and the left measured 12.0 cm × 6.4 cm × 6.0 cm, with multiple small stones in both kidneys. Urinalysis was negative for leukocytes, erythrocytes, and urine protein, with urine pH 5. Urine output showed mild reduction on the day of disease onset, but increased to 2200 mL/d by the next day. AKI was diagnosed. Admission examination did not reveal pain on percussion over the kidney area. Laboratory tests demonstrated urinary retinol binding protein, 0.27 mg/L; urinary α1-microglobulin, 0.45 mg/dL; and 24-hour urine uric acid excretion, 3128 mg. Renal biopsy revealed acute tubular interstitial nephropathy (Fig. 1). Renal function recovered well (Table 1) with intravenous glucose and sodium chloride infusion (500 mL/d) and sodium bicarbonate tablets (1 tablet, 3 times/d). After 1 month, the Scr returned to a baseline of 0.7 mg/dL and ultrasound did not detect urolithiasis after 3 months.
Figure 1.
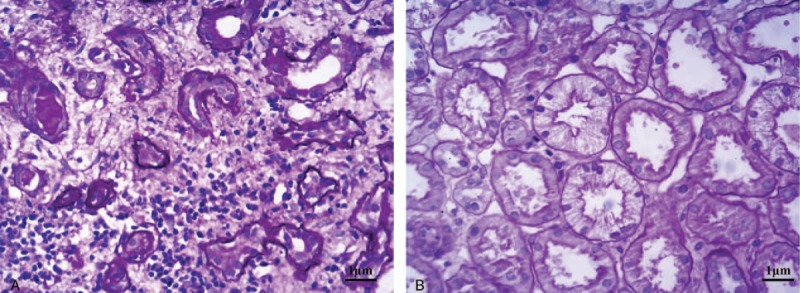
(A) Kidney tubular epithelial cells showed vacuolar and granular degeneration. Some epithelial cells had denuded brush borders and became flattened, with part of the basement membrane exposed. Focal atrophy and cellular debris were visible in the lumen (hematoxylin and eosin [HE], 100×). (B) Renal interstitial edema and focal infiltration of lymphocytes, monocytes, and eosinophils, with partial invasion of kidney tubules. Thickening of small arterial walls is present (HE, 400×).
Table 1.
Renal functional outcomes after medication of both patients.
2.2. Case 2
A 17-year-old Asian male student was admitted to the hospital due to severe kidney injury. He had a history of gout for 2 years, with poor control as he inconsistently self-medicated with uric-acid-lowering drugs. He reported insufficient time to follow-up with a doctor. Three days prior to admission, his serum uric acid level was found to be 18.5 mg/dL. The patient self-medicated with 50 mg of benzbromarone purchased from a drugstore 1 month prior. He did not take sufficient water, and took no other medication or vitamin C. He had nausea without vomiting that night, with worsening the following day. A local hospital diagnosed gastroenteritis, and initiated fluids and antibiotic therapy for 2 consecutive days, but vomiting was not relieved. One day prior to admission, urine output decreased to 200 mL/d, and Scr increased to 6.8 mg/dL. Laboratory studies at that time showed urine pH 5.0, urine protein 2+, occult blood 2+, ketone 1+, erythrocytes 289/μL, and leukocytes 201/μL. Ultrasound showed multiple stones in the left kidney. The patient was admitted with a presumptive diagnosis of drug-induced AKI. Family history was negative for kidney disease. On examination, he appeared fatigued, but was not dehydrated, as his blood pressure was 125/85 mm Hg. He was treated supportively with intravenous glucose and sodium chloride infusion (500 mL/d) and sodium bicarbonate tablets (2 tablets, 3 times/d). Renal function improved, with Scr decreased to 5.9 mg/dL and urine output increased to 2000 mL/d following admission. Laboratory results showed 24-hour urine uric acid excretion of 795 mg (urine output 2400 mL/d). Ultrasound showed the left kidney urolithiasis. Renal biopsy confirmed the presence of acute tubular injury (Fig. 2). Renal function returned to normal, and kidney ultrasound revealed no urolithiasis 3 months later. Recovery of renal function in both patients is shown in Table 1. Other indicators of AKI are presented in Table 2.
Figure 2.
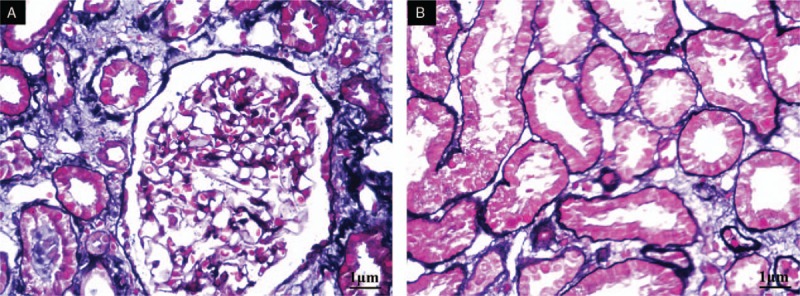
(A) Kidney tubular epithelial cells showed vacuolar and granular degeneration. Some epithelial cells had denuded brush borders and became flattened while some had regenerated. Focal atrophy was present, and cellular debris in the lumen was visible (periodic acid-Schiff, 100×). (B) Renal interstitium showed edema with small foci of infiltrates of lymphocytes and mononuclear cells, fibrosis, and thickening of small arterial walls (HE, 200×).
Table 2.
Acute renal injury related indicators of the 2 patients.
3. Discussion
The most frequently used pharmacological urate-lowing therapy involves inhibition of uric acid synthesis by xanthine oxidase inhibitors (XOIs) and enhancement of urate excretion by uricosuric agents. XOIs, such as allopurinol and febuxostat, are recommended as 1st-line drugs for gout in the American College of Rheumatology guidelines (2012). However, use of allopurinol has been associated with severe adverse cutaneous reactions.[6,7] Febuxostat is more expensive. Although benzbromarone was withdrawn from the European market in 2003 due to reports of serious hepatotoxicity, it is still widely prescribed in Asia, including China.
The classic 4-compartment model,[8] although not validated,[9] indicates that nearly all uric acid is filtered by the glomeruli; 99% of the filtered uric acid is then absorbed in the proximal tubules (S1 segment). The proximal tubules secrete 45% to 50% of the absorbed uric acid in the S1 and S2 segments, followed by postsecretory reabsorption (40–45%) in the S3 segment.[9] Benzbromarone was introduced as a uricosuric drug in the 1970s. It has performed well at inhibiting reabsorption of uric acid by impeding human organic anion transporter 4 (OAT4) and urate transporter 1 (URAT1) in the proximal tubule, and thus reduces blood uric acid levels.[10] Plasma concentration reaches a peak 2 to 3 hours after drug administration. Uric acid clearance rate reaches a maximum after 4 to 5 hours, with a half-life of 12 to 13 hours. These 2 patients developed symptoms 8 to 11 hours after medication administration, and it is likely that crystal precipitation (urolithiasis) occurred within hours of ingestion of the offending drug.
The AKI caused by benzbromarone has not been reported. Benzbromarone reportedly causes uric acid urolithiasis and renal colic.[11] Therefore, its use should be avoided in patients with moderate or severe renal insufficiency (glomerular filtration rate <20 mL/min according to Chinese guidelines), uric acid urolithiasis (kidney stone patients in the benzbromarone [Narcaricin Mite] instructions), or 24-hour urine uric acid excretion >700 mg. In addition, some studies showed that daily uric acid excretion >1100 mg (6.5 mmol) is associated with a nearly 50% incidence of urinary tract stones; consequently, the use inhibitors of uric acid production rather than uricosuric drugs was recommended for these patients.[12,13]
Moreover, with use of uricosuric drugs, the urine should be alkalinized to increase the solubility of uric acid. Uric acid is a weak organic acid and exists in 1 of 2 forms in biological systems, depending upon the prevailing pH. Uric acid solubility in the urine decreases from approximately 200 mg/dL (1.2 mmol/L) at a urine pH of 7 (a setting in which 95% of uric acid is present as the more soluble urate anion) to 15 mg/dL (0.09 mmol/L) at a urine pH of 5 (a setting in which most of the uric acid is the less soluble, undissociated acid).[14,15] As a result, sodium bicarbonate or citric acid mixture should be taken to adjust the pH from 6.5 to 6.8. Concurrently, increased fluid intake should be prescribed (not <1.5–2 L) to avoid crystal formation in excreted urine due to excessive uric acid.
The incidence of drug-induced AKI may increase when benzbromarone is taken despite contraindications such as urolithiasis and 24-hour urine uric acid excretion >700 mg, and when urine alkalinization is not performed or fluid intake is insufficient.
Both patients had contraindications to use of benzbromarone. Both had urolithiasis and 24-hour urine uric acid excretion >700 mg. The 24-hour uric acid excretion in case 1 was 3128 mg, greatly exceeding the normal level. Additionally, the urine pH was as low as 5.0, thus decreasing uric acid solubility, which easily induced uric acid crystal formation, and increased the likelihood of AKI. Although case 1 had taken adequate soda water, urine output decreased after benzbromarone dosing. Hot compresses relieved the renal colic and increased urine output. Laboratory examination revealed 24-hour urine uric acid excretion of 3128 mg, possibly because benzbromarone induced a sharp rise in uric acid excretion. On the contrary, case 2 had dehydration symptoms before benzbromarone dosing. The urine was not alkalinized and the benzbromarone was taken without adequate fluid. Therefore, kidney tubular injury caused by transient obstruction with uric acid crystals was possible. Pathology results were consistent with AKI. Urolithiasis disappeared in both patients after the serum uric acid returned to a normal level, suggesting the formation of uric acid urolithiasis during AKI.
Several drugs can cause AKI. Kidney damage caused by antibiotics such as sulfadiazine-trimethoprim,[16] nonsteroidal anti-inflammatory drugs, or acetaminophen[17] is commonly reported. Neither patient had a history of chronic kidney disease or took drugs other than benzbromarone. Acute renal failure occurred on the day of benzbromarone dosing. The kidneys were either normal in size or somewhat larger, and anemia was not present. After a single dose of benzbromarone, kidney function gradually returned to normal without special treatment. Pathological findings supported acute tubular or interstitial damage.
Most HUA are caused by a decrease in renal uric acid excretion. Benzbromarone is inappropriately used in China due to unrestricted medication purchasing and self-administration. Even some doctors prescribe benzbromarone in the absence of kidney ultrasound results or measurement of 24-hour uric acid excretion. A recent meta-analysis by Kydd et al did not find reports of benzbromarone-induced kidney injury.[18] However, both patients in this report developed AKI after only a single dose. Therefore, physicians should be more aware of benzbromarone-associated adverse kidney reactions, especially in those with contraindications.
4. Conclusion
The most popular antihyperuricemic drugs are XOIs and uricosuric agents. Uricosuric drugs can only be used after exclusion of urolithiasis and other contraindications. Benzbromarone should be initiated at a small dose, and urinary alkalinization and increased fluid intake should be prescribed to reduce the incidence of adverse reactions. We should be more aware of the risk of benzbromarone-associated AKI.
Author contributions
Formal analysis: Xiaolan Ye, Jian Wu.
Funding acquisition: Xiaolan Ye.
Investigation: Xiaolan Ye, Jian Wu, Kun Tang, Wenge Li, Li Zhuo.
Methodology: Xiaolan Ye.
Validation: Xiaolan Ye, Li Zhuo.
Writing – original draft: Xiaolan Ye, Jian Wu, Cunquan Xiong.
Writing – review & editing: Jian Wu, Cunquan Xiong, Li Zhuo.
Footnotes
Abbreviations: AKI = acute kidney injury, HUA = hyperuricemia, OAT4 = organic anion transporter 4, Scr = serum creatinine, URAT1 = urate transporter 1, XOIs = xanthine oxidase inhibitors.
XY and JW contributed equally to this work.
This work was supported by the China-Japan Friendship Hospital Youth Science and Technology Excellence Project (2015-QNYC-B-12) and Special Research Fund of Zhejiang Pharmaceutical Association Hospital Pharmacy (2018ZYY29).
The authors have no conflicts of interest to disclose.
References
- [1].Nan H, Qiao Q, Dong Y, et al. The prevalence of hyperuricemia in a population of the coastal city of Qingdao, China. J Rheumatol 2006;7:1346–50. [PubMed] [Google Scholar]
- [2].Liu H, Zhang XM, Wang YL, et al. Prevalence of hyperuricemia among Chinese adults: a national cross-sectional survey using multistage, stratified sampling. J Nephrol 2014;6:653–8. [DOI] [PubMed] [Google Scholar]
- [3].Liu R, Han C, Wu D, et al. Prevalence of hyperuricemia and gout in Mainland China from 2000 to 2014: a systematic review and meta-analysis. Biomed Res Int 2015;762820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yu S, Yang H, Guo X, et al. Prevalence of hyperuricemia and its correlates in rural Northeast Chinese population: from lifestyle risk factors to metabolic comorbidities. Clin Rheumatol 2016;5:1207–15. [DOI] [PubMed] [Google Scholar]
- [5].Dincer HE, Dincer AP, Levinson DJ. Asymptomatic hyperuricemia: to treat or not to treat. Cleve Clin J Med 2002;8:594597, 600-602 passim. [DOI] [PubMed] [Google Scholar]
- [6].Cao ZH, Wei ZY, Zhu QY, et al. HLA-B∗58:01 allele is associated with augmented risk for both mild and severe cutaneous adverse reactions induced by allopurinol in Han Chinese. Pharmacogenomics 2012;10:1193–201. [DOI] [PubMed] [Google Scholar]
- [7].Diamond HS, Sharon E. Evidence for a post-secretory reabsorptive site for uric acid in man. Adv Exp Med Biol 1974;41:745–9. [DOI] [PubMed] [Google Scholar]
- [8].Hung SI, Chung WH, Liou LB, et al. HLA-B∗5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A 2005;11:4134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hagos Y, Stein D, Ugele B, et al. Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J Am Soc Nephrol 2007;2:430–9. [DOI] [PubMed] [Google Scholar]
- [10].Mount DB. Molecular physiology and the four-component model of renal urate transport. Curr Opin Nephrol Hypertens 2005;5:460–3. [DOI] [PubMed] [Google Scholar]
- [11].Wagayama H, Shiraki K, Sugimoto K, et al. Fatal fulminant hepatic failure associated with benzbromarone. J Hepatol 2000;5:874. [DOI] [PubMed] [Google Scholar]
- [12].Bellomo G. Uric acid and chronic kidney disease: a time to act? World J Nephrol 2013;2:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Arai M, Yokosuka O, Fujiwara K, et al. Fulminant hepatic failure associated with benzbromarone treatment: a case report. J Gastroenterol Hepatol 2002;5:625–6. [DOI] [PubMed] [Google Scholar]
- [14].Coe FL. Uric acid and calcium oxalate nephrolithiasis. Kidney Int 1983;3:392–403. [DOI] [PubMed] [Google Scholar]
- [15].Seegmiller JE. Xanthine stone formation. Am J Med 1968;5:780–3. [DOI] [PubMed] [Google Scholar]
- [16].Billings FTt, Petracek MR, Roberts LJ, 2nd, et al. Perioperative intravenous acetaminophen attenuates lipid peroxidation in adults undergoing cardiopulmonary bypass: a randomized clinical trial. PLoS One 2015;2:e0117625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Garvey JP, Brown CM, Chotirmall SH, et al. Trimethoprim-sulfamethoxazole induced acute interstitial nephritis in renal allografts; clinical course and outcome. Clin Nephrol 2009;5:331–6. [DOI] [PubMed] [Google Scholar]
- [18].Kydd AS, Seth R, Buchbinder R, et al. Uricosuric medications for chronic gout. Cochrane Database Syst Rev 2014;11:Cd010457. [DOI] [PMC free article] [PubMed] [Google Scholar]


