Abstract
Colorectal cancer has high incidence and mortality. Early diagnosis could increase patient survival, but early diagnosis has been poor in China for the past decades. The purpose of this study is to assess the polyp detection rate (PDR) and adenoma detection rate (ADR) by colonoscopy in a Chinese population, and to determine the risk factors for adenoma.
This prospective study at Sichuan Provincial People's Hospital evaluated patients who underwent colonoscopy in September 2017 to February 2018. Basic information, exact insertion and withdrawal times, PDR, and ADR were assessed. Risk factors for colorectal adenoma in the adenoma-positive and adenoma-negative groups (based on pathology) were assessed by multivariable logistic regression analysis.
A total of 1058 procedures with 767 polyps were analyzed. The overall PDR and ADR were 36.96% (391/1058) and 24.67% (261/1058), respectively. Occurrence of adenoma was associated with age, gender, body mass index (BMI), family history of colon cancer, personal history of adenoma, diabetes mellitus, and tobacco use. There was a significant association between withdrawal time and ADR (P < .001). In the multivariable analysis, age (OR = 1.041, 95%CI 1.028–1.055; P < .001), insertion time (OR = 0.999, 95%CI 0.998–1.000; P = .009), withdrawal time (OR = 1.009, 95%CI 1.007–1.011; P < .001), personal history of adenoma (OR = 2.572, 95%CI 1.115–5.932; P = .027), and diabetes mellitus (OR = 2.221, 95%CI 1.084–4.549; P = .029) were risk factors for colorectal adenoma detection.
In a Chinese population, ADR increases with age, withdrawal time, a personal history of adenoma, and diabetes. Age, insertion and withdrawal times, and a personal history of adenoma may independently predict colorectal adenoma detection.
Keywords: colorectal adenoma, detection, prospective study, risk factor
1. Introduction
Colorectal cancer (CRC) is an important public health issue due to its high incidence and mortality rates. Early diagnosis could increase the 5-year relative survival rate, but even in the USA, only 39% of patients with CRC are diagnosed at an early stage.[1] Current evidence shows that factors affecting the detection rate of adenoma include bowel preparation, time of withdrawal, and operator factors. China has a large CRC patient population.[2] Thus screening for high risk individuals is particularly important and accurate risk factors for colon adenoma or colon cancer are urgently needed. Meanwhile, the Chinese ethnicity is different from that of Westerners, which may lead to different risk factors.
Data quality is very important for the accurate determination of risk factors. The American Cancer Society (ACS) Guideline (2018) recommends that adults aged 45 years and older with an average risk of CRC should undergo regular screening.[3] The American Society for Gastrointestinal Endoscopy (ASGE) stipulates that the age at which average-risk Whites should begin to undergo colorectal colonoscopy screening is 50 years, requiring an adenoma detection rate (ADR) for the population to reach 25% in men and 15% in women.[4] In the past decades, colonoscopy screening has been poor in China, and most studies assessing the ADR are uncontrolled retrospective trials, with inherent bias. In addition, the quality of endoscopic procedures and diagnosis are suboptimal. A recent study revealed an ADR in China of about 11.48%, with 18.30% in males and 3.36% in 50–60 years old individuals,[5] which are far below the international levels. Such results may be related to short withdrawal time, operator skills, and standard bowel preparation, among others. Indeed, prolonged observation time is associated with elevated ADR in the colon, especially in the proximal colon.[6]
Since high ADR is considered to be associated with lower risk of CRC and CRC-related death,[7] we hypothesized that identifying factors determining the ADR would be clinically relevant, especially in high risk populations. Therefore, this prospective study used the most standardized endoscopic procedure to assess the risk factors for the ADR based on high-quality data, hoping to define the high risk population and a screening age for colonoscopy in China.
2. Methods
2.1. Patients
This prospective study was conducted at the Endoscopy Center of Sichuan Provincial People's Hospital, China. Outpatients and inpatients ≥14 years of age scheduled for colonoscopy at the Department of Gastroenterology from September 2017 to February 2018 were eligible for enrollment. Patients with a history of inflammatory bowel disease (IBD), polyposis syndromes, CRC, colorectal surgery, or contraindication to biopsy were excluded. In addition, the patients with incomplete colonoscopy procedure (insertion to the cecum), highly suspected adenoma polyposis, or giant advanced CRC masses found during colonoscopy were also excluded for safety purpose during biopsy.
Basic demographic characteristics were recorded before colonoscopy, including gender, age, chief complaint, procedure time (morning/afternoon), and anesthesia or not, as well as risk factors for colon polyps, including diabetes mellitus, coronary artery disease, body mass index (BMI), a family history of colon adenoma or cancer, alcohol use, tobacco use, and use of aspirin, non-steroidal anti-inflammatory drugs, metformin, folic acid, calcium, and hormone replacement therapy.
2.2. Colonoscopy procedure
This was a prospective study involving eight physicians who have passed the unified standardized training and authorization. These doctors performed colonoscopy in the Division of Gastroenterology and included two senior (>20,000 colonoscopy procedures), two mid-level (between 3000 and 10,000 colonoscopy procedures), and four junior (between 100 and 500 colonoscopy procedures) endoscopists.
All investigations were conducted using an Olympus Evis Exera III (CF-H260/CF-Q260) (Olympus, Tokyo, Japan). Intubation time was defined as the time span from scope insertion into the anus to the cecal pole, proximal of the ileocecal valve.[8] Withdrawal time was defined as the time spent for withdrawing the scope from the cecum to scope extraction from the anus. The withdrawal time included the time to observe the polyps and excluded the biopsy time. Time recording was stopped when the biopsy began and was started again at the end of the biopsy.
In order to describe the exact location of the polyps, the colon was divided into six sections: cecum, ascending colon, transverse colon, descending colon, sigmoid colon, and rectum. When a polyp was detected, the nurse helped perform the biopsy for histological examination, while the staff assistant recorded the location, size, and morphological features based on the Paris classification of polyps.[9] All polyps were obtained by biopsy for histological examination.
Routine bowel preparation for each procedure consisted of 4 L of polyethylene glycol, given in split doses. The Boston Bowel Preparation Scale (BBPS)[9] was used to assess bowel preparation. Segmental scores (0–3) from the right, transverse, and left sides of the colon were combined to calculate the composite BBPS (0–9). According to the Boston Bowel Preparation Scale standard, inadequate bowel preparation is reflected by composite BBPS <6 or any segmental score ≤1, while composite BBPS ≥6 or every segmental score >1 is considered to reflect adequate bowel preparation.
Propofol or its combination with fentanyl or etomidate was used for sedation. All patients were monitored for the duration of the procedure. Blood pressure was measured every 5 min. Pulse oximetry was used. Electrocardiography was performed in selected cases with preexisting cardiac disease. All patients received 4 L/min oxygen via a nasal cannula throughout the procedure. After colonoscopy, the patients were disconnected from electronic monitoring and transferred to the recovery ward provided they had gained an adequate level of consciousness.
2.3. Pathological polyp evaluation
All polyps were sent to the Pathology Department for histopathological examination. All pathologists were board-certified. Polyps revealing serrated adenoma histology were considered adenomas in this trial, since the histo-morphology of serrated adenomas is between proliferative polyps and adenomas, but with high risk of malignant transformation.
2.4. Ethics
This study was approved by the Institutional Review Board and ethics committee of the Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital. Written informed consent was obtained from all patients. The trial has been registered with the Chinese Clinical Trial Registry (ChiCTR) under the identifier ChiCTR-DDD-17012221.
2.5. Endpoints
The primary endpoint was polyp detection in the whole colon and rectum. Secondary endpoints were PDR and polyp morphology.
2.6. Statistical analysis
Continuous data were presented as means ± standard deviation (SD). For categorical data, absolute and relative frequencies were used. Univariable and multivariable logistic regression models were performed to identify the risk factors of the ADR. P < .05 was considered statistically significant. Statistical analysis was performed with SPSS 24.0 (IBM, USA).
3. Results
3.1. Patient and procedure characteristics
A total of 1197 patients were screened by colonoscopy; 139 cases did not meet the inclusion criteria: 22 with contraindication to biopsy, 69 with giant advanced CRC masses, three with a history of FAP/HNPCC, 12 with a history of IBD, and 33 not completing the procedure due to poor bowel preparation, stenosis, or other reasons. Thus, a total of 1058 patients were included in the final analysis.
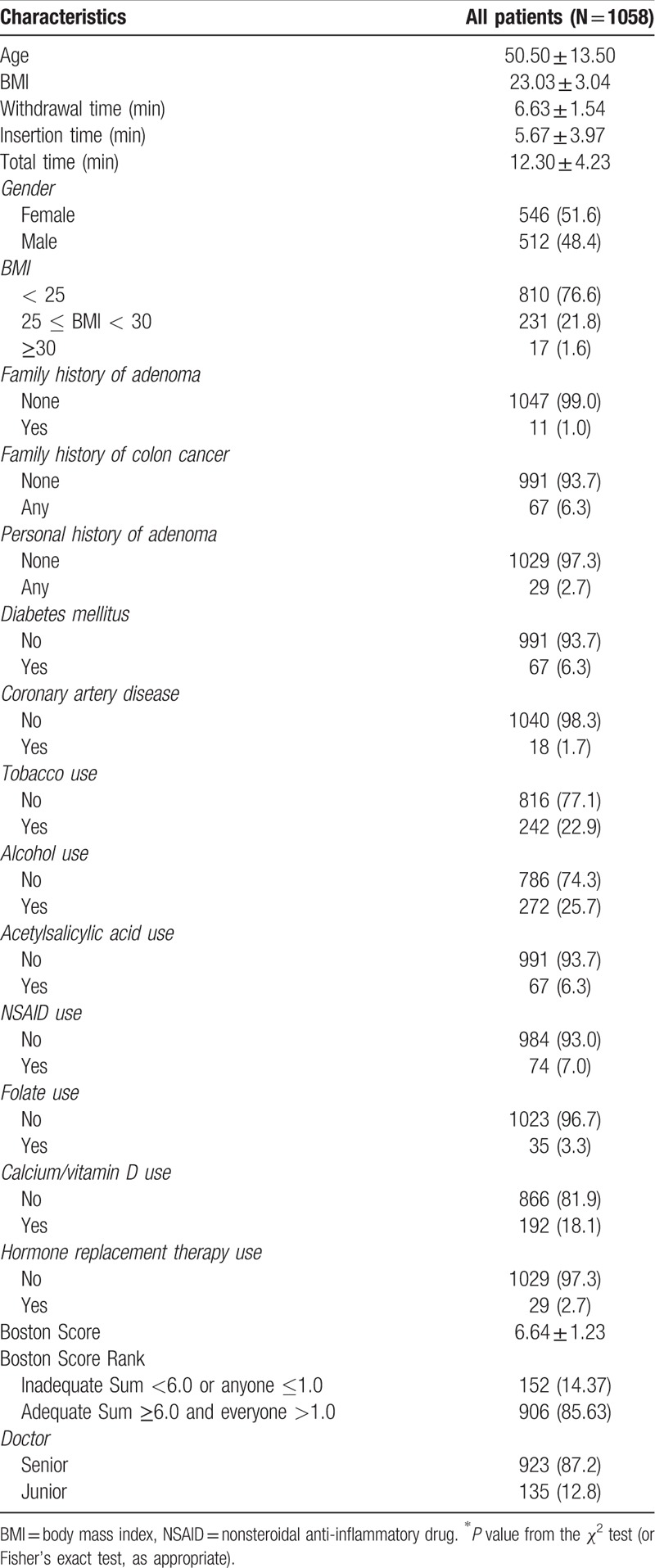
The characteristics of the 1058 cases are shown in Table 1. There were 512 (48.39%) men and 546 (51.61%) women, with a mean age of 50.5 ± 13.5 years. Withdrawal time was 6.63 ± 1.54 min. The proportion of patients with adequate bowel preparation (including excellent and good preparations) was 910 (85.61%). In total, 767 polyps were detected, resulting in PDR and ADR of 36.96% (391 patients) and 24.67% (261 patients), respectively.
Table 1.
Demographic, clinical, and procedure characteristics.
3.2. Characteristics of polyps and adenomas
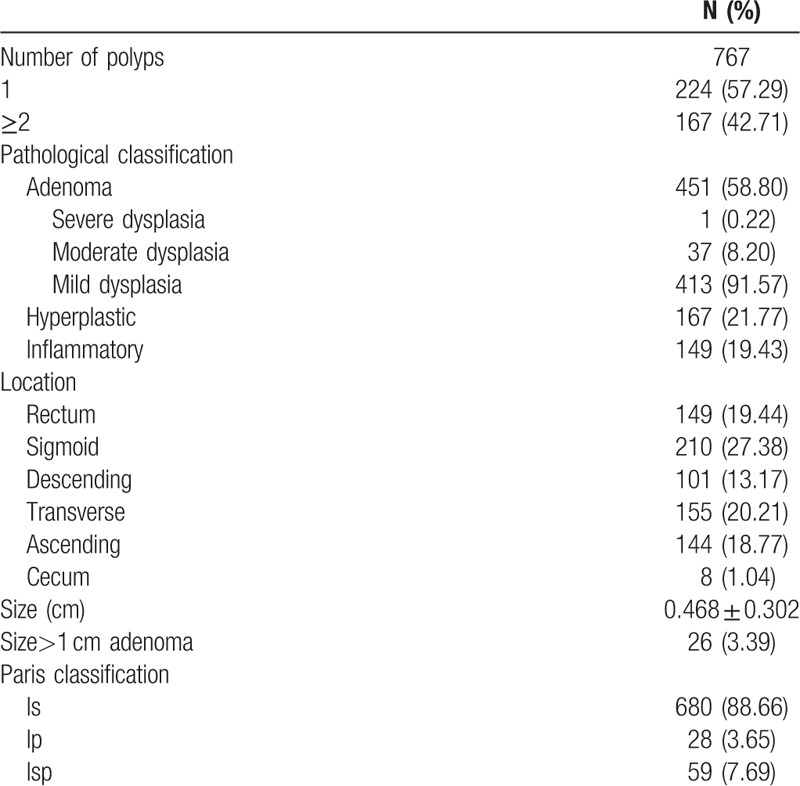
In total, 767 polyps were detected, including 451 (58.80%) adenoma, 167 (21.77%) hyperplastic polyps, and 149 (19.43%) inflammatory polyps. Most adenomas showed mild dysplasia 413 (91.57%), including 680 (88.66%) of Paris classification type Is. The adenomas were more commonly found in the sigmoid colon 210 (27.38%), transverse colon 155 (20.21%), and rectum 149 (19.44%). Location, size, and Paris classification of polyps are shown in Table 2. No malignant samples were recorded.
Table 2.
Characteristics of the polyps.
3.3. Adenoma detection
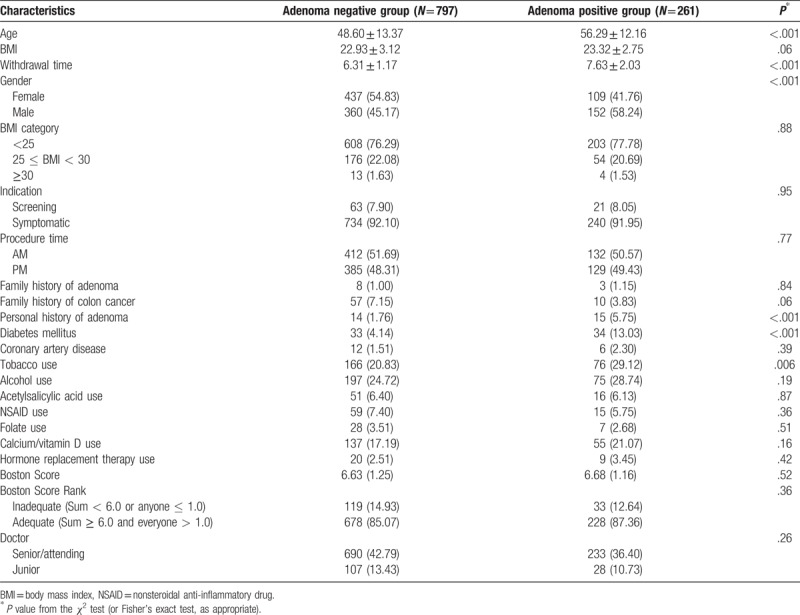
In total, 261 patients had adenomas confirmed by pathological diagnosis. Comparisons between the adenoma-negative and positive groups are shown in Table 3. Patient age was 48.6 ± 13.4 years and 56.3 ± 12.2 years in the two groups, respectively. Males showed more adenomas than females. The proportion of cases with tobacco use was larger in the adenoma positive group (P = .006). The proportions of cases with a personal history of adenoma (P = .001) and diabetes mellitus (P < .001) were higher in the adenoma-positive group compared with cases without adenoma. There were no significant differences in coronary heart disease, BMI, family history of colon cancer/adenoma, and use of acetylsalicylic acid, alcohol, NSAIDS, folate, calcium/vitamin D, and hormone replacement therapy between the two groups. Regarding the colonoscopy factors, there were no significant differences in bowel preparation and doctor experience between the two groups, but withdrawal time in the adenoma-positive group was significantly higher compared with that of patients with no adenoma (6.31 min vs. 7.63 min, P < .001).
Table 3.
Characteristics of the adenoma-positive and -negative groups.
3.4. Impact of withdrawal time on the ADR
In this study, withdrawal time ranged from 2.7 to 18.7 min. There was a significant association of withdrawal time with the ADR. The 2015 edition of the ASGE CRC screening and monitoring guidelines proposed that in colonoscopy, withdrawal time should be greater than 6 min.[10] The results showed that withdrawal time <6.0 min resulted in significantly lower ADR compared with the ≥6.0 min group (P < .001) (Table 4). When the withdrawal time was <6 min, the ADR of the primary and senior physicians was 7.50% and 9.41%, respectively. When the withdrawal time was extended to ≥6 min, the ADR was significantly improved at 26.32% and 35.16%, respectively. The difference between certification grade was not significant (P =.121).
Table 4.
ADR in different withdrawal time categories.

3.5. Risk factors for the adenoma detection rate
Factors affecting the prevalence of adenomas and those affecting the ability of the procedure to detect them were analyzed by logistic regression analysis. The factors independently affecting adenoma occurrence were age (OR = 1.046, 95%CI 1.034–1.059; P < .001), gender (OR = 1.531, 95%CI 1.083–2.166; P = .016), a personal history of adenoma (OR = 2.860, 95%CI 1.304–6.273; P = .009), and diabetes mellitus (OR = 2.307, 95%CI 1.167–4.560; P = .016). The factors independently affecting ADR were insertion time (OR = 0.999, 95%CI 0.998–1.000; P = .007) and withdrawal time (OR = 1.010, 95%CI 1.008–1.012; P < .001).
3.6. Age of colonoscopy screening
Adenoma detection rates were assessed in eight age categories (10-year intervals) (Fig. 1). The ADR increased with age, especially after 40 years, and the difference was statistically significant between the two genders (P < .001). The ADR in males (29.5%) was significantly higher than that in females (20.5%) (P = .001). Compared with women, the ADR in males between 40 and 59 years of age was much higher compared with that of age matched females (P < .05). There were statistically significant differences in overall ADR values between the two genders and various age groups (P < .05). The 2013 guidelines from the National Comprehensive Cancer Network (NCCN) on CRC screening suggest that people with unknown family history should begin screening for CRC at the age of 40.[11] Since CRC screening in China has been very poor in the past decades, family history is often inaccurate. Therefore, most individuals could be considered to have an unknown family history, so we selected a cutoff of 40 years in this study. According to the obtained results, the ADR of patients over 40 years of age (29.56%) was significantly higher than that of subjects that were 40 years old or below (7.63%) (P < .001) (Table 5).
Figure 1.
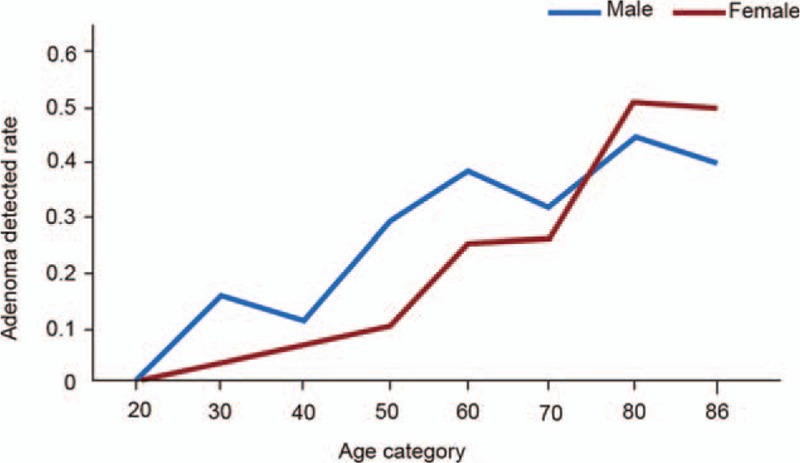
ADR values for various age categories in both genders.
Table 5.
Adenoma detection in various age categories.

3.7. Risk factors for ADR in individuals based on the cutoff age of 40 years
In the subgroup analysis based on age, no parameter was associated with ADR in patients 40 years and below. The multivariable analysis showed that age (OR = 1.038, 95%CI 1.020–1.056; P < .001), a personal history of adenoma (OR = 2.568, 95%CI 1.061–6.212; P = .036), diabetes mellitus (OR = 2.280, 95%CI 1.104–4.707; P = .026), insertion time (OR = 0.999, 95%CI 0.998–1.000; P = .006), and withdrawal time (OR = 1.009, 95%CI 1.007–1.011; p < .001) were risk factors for ADR in individuals above 40 years old.
3.8. Risk factors for ADR based on gender
In the subgroup analysis based on gender, the multivariable analysis showed that age (OR = 1.025, 95%CI 1.008–1.042; P = .003), diabetes mellitus (OR = 3.065, 95%CI 1.448–6.488; P = .003), and withdrawal time (OR = 1.007, 95%CI 1.005–1.010; P < .001) were risk factors for ADR in males. In females, age (OR = 1.064, 95%CI 1.042–1.087; P < .001) and withdrawal time (OR = 1.012, 95%CI 1.008–1.015; P < .001) were risk factors for ADR.
4. Discussion
Adenoma detection is the key task of screening colonoscopy. Through standardized bowel preparation and colonoscopy parameters (e.g., withdrawal time), the ADR has reached the level suggested by the ASGE guidelines[10] in this study. The overall PDR and ADR were 36.96% and 24.67%, respectively, significantly higher than previously reported in China and close to the requirement of the Chinese guidelines.[12]
4.1. Risk factors for colon polyps and PDR
Previous studies have indicated that gender, smoking, alcohol, BMI, diet, physical activity, medication, and/or hormone replacement therapy are risk factors associated with colorectal polyps.[13,14] In contrast, factors such as use of non-steroidal anti-inflammatory drugs or aspirin, high intake of folate, calcium, and fiber were found to significantly decrease the risk of polyp.[13] In a recent large multicenter chemoprevention study, evidence was provided that calcium and vitamin D supplementation increases the risk of SSA/Ps.[15]
As for operator factors, studies of colonoscopists within the same gastroenterology groups consistently demonstrate an ADR variation of 3–6 fold between the best and worst performers.[16–18] When detection is expressed as adenomas per colonoscopy (APC), the differences between the top and bottom performers can even exceed 10 fold.[16]
According to the above results, the PDR was 36.96% and related to age (P < .001), BMI (P = .001), procedure time (including withdrawal [P < .001] and insertion [P = .004] times), gender (P < .001), sedation (P = .023), a personal history of adenoma (P = .004) and diabetes mellitus (P < .001), tobacco (P = .001) and alcohol use (P = .003), and calcium and vitamin D use (P = .013). These findings corroborated reports by Western studies. Nevertheless, no significant associations were found between the PDR and family history of adenoma/colon cancer, coronary artery disease, use of aspirin, NSAIDS, folate and hormone replacement therapy, bowel preparation and operator experience, which may due to the limited samples size, for example, the numbers of patients with coronary artery disease (n = 18), acetylsalicylic acid use (n = 67), folate use (n = 35), and hormone replacement therapy (n = 29) were relatively low. There was no significant correlation between BMI and ADR in our study, which may be related to the narrow range of BMI of the study subjects. Because some patients with cardiovascular and cerebrovascular diseases used antiplatelet and anticoagulant drugs such as aspirin or clopidogrel, biopsy could not be performed. In addition, some patients refused polyp biopsy for economic and conceptual reasons. Furthermore, the rate of colonoscopy in China is low, especially in the previous decades, with deficient CRC screening. Therefore, data regarding family history of CRC or adenoma may not be accurate, which could also lead to discrepant statistical results.
4.2. Risk factors for colorectal adenoma
Multiple studies have assessed the risk factors of colorectal adenoma. It is generally acknowledged that the main risk factors include age, male gender, smoking, drinking, obesity, metabolic syndrome, and family history.[19] It is estimated that 30–50% of the CRC risk is attributable to lifestyle factors such as smoking, high consumption of red and processed meat, obesity, diabetes, and excessive consumption of alcohol.[20] Studies have reported an increased risk associated with long-term cigarette smoking, which may be responsible for 20% of CRC cases. Age as a risk factor is equally relevant in women and men. More than 50% of CRC cases are diagnosed after the age of 70, with only 10% being detected before age 55.[21] Nevertheless, the risk of men developing advanced adenoma or cancer is roughly double that of women.[14,22] A recent study demonstrated that the male gender increases the risk to a similar extent as a positive family history of CRC.[7]
In a Portuguese prospective study, the metabolic syndrome (MS) was shown to be associated with increased prevalence rates of adenoma (43% vs. 25%, P = .004) and CRC (13% vs. 5%, P = .027), compared to patients without MS.[23] A recent meta-analysis[24] of 29 eligible studies confirmed these estimates, indicating an increased risk of CRC in type 2 diabetes (RR = 1.29 for men and 1.34 for women).
Based on the high-quality colonoscopy data from the present prospective study, the multivariable analysis demonstrated that age (P < .001), gender (P = .016), a personal history of adenoma (P = .009), diabetes mellitus (P = .016) are predictors of adenoma incidence. With regard to alcohol use, a family history of adenoma, and a family history of colon cancer, our data failed to show significant differences in adenoma incidence rates, probably due to the limited sample size and the likely inaccuracy of family history mentioned above. In addition, most previous studies were performed in Western countries, and racial and genetic differences may explain the discrepancies described here.
On the other hand, protective factors for adenomas have been reported. Evidence suggests an association of regular aspirin use with reduced risk of adenomatous polyp and CRC.[25] In a Health Technology Assessment report,[26] pooled analysis of two studies evaluating 300 to 1200 mg/day of aspirin indicated a 26% reduction in CRC incidence over a 23-year follow-up period. Furthermore, a meta-analysis suggested that metformin therapy may be associated with a decreased risk of colorectal adenoma and CRC in type 2 diabetes mellitus patients.[27] Another systematic review of four randomized, placebo-controlled trials including 2998 participants indicated a modest chemopreventive effect of calcium supplement against colorectal adenoma (approximately 10%–15% risk reduction; high-quality evidence).[28]
Nevertheless, we found no significant association of these factors with adenoma, including use of acetylsalicylic acid, NSAIDS, folate, metformin, calcium/vitamin D, and hormone replacement therapy. These discrepancies are likely related to racial and genetic differences and the limited number of samples, especially for medication users. Therefore, more studies are needed for confirmation.
4.3. Withdrawal time and age have effects on the ADR
This study showed that the ADR was associated with age, gender, insertion time, withdrawal time, a personal history of adenoma, and diabetes mellitus. With withdrawal time <6 min, the ADR was significantly reduced (P < .001). Our results show that both the junior and the senior physicians have significantly improved ADR when the withdrawal time is >6 min, regardless of the operator's certification. Nevertheless, since the number of junior physicians involved in this study was only 12.8%, this conclusion needs further confirmation. According to the multivariable analysis, age, withdrawal time, a personal history of adenoma, and diabetes mellitus may be independent predictors of the ADR. In subgroup analysis based on age and gender, patients ≤40 years of age showed no parameter was associated with ADR except for withdrawal time, while patients above 40 years old showed similar risk factors with all subjects, and diabetes mellitus showed higher correlation with ADR in male. On the other hand, BMI, anesthesia, a family history of colon cancer, a family history of adenoma, coronary heart disease, tobacco use, drug use (acetylsalicylic acid, NSAIDS, folate calcium, vitamin D, and hormone replacement therapy), and bowel preparation were not directly associated with ADR.
Factors impacting the ADR have been widely studied. Indeed, bowel preparation and colonoscopy time, inspection time, and operator factors are known factors of ADR. The associations of some of these factors have been confirmed with ADR, with their inclusion in the guidelines for colonoscopy as control elements.[18,29–36] The ASGE has listed ADR factors in details in their 2015 edition of CRC screening and monitoring guidelines, and has made corresponding quality control requirements. For example, when colonoscopy is performed, withdrawal time should be >6 min.[37] Operator factors also significantly affect the ADR, for example, observation skills (detection behind the intestinal content and dilated colon), and their predictive potential for the ADR may even exceed the effects of age and gender, through appropriate training that can significantly improve the ADR.[17,38–40]
Good bowel preparation is the basis for ensuring quality colonoscopy. Under poor bowel preparation, “micro adenomas” with diameters <5 mm are easily missed. Such patients need to shorten the screening period to avoid the occurrence of interphase CRC.[41] In this study, due to the limited number of patients with inadequate bowel preparation, we found no significant association of bowel preparation with ADR.
The ADR directly correlates with the incidence and mortality of post-colonoscopy (or interval) CRC. Patients with a history of CRC or a family history of CRC have significantly higher rates of adenoma. As shown above, however, a family history of CRC seemed to have no significant correlation with the ADR, which may be due to the exclusion of cases with giant colonic neoplasms and polyposis in this study.
4.4. Men over 40 may need colonoscopic screening
With the increased incidence of sporadic CRC in young adults, several behavioral and environmental factors affecting CRC have been proposed. The current data showed that about 28.87% of men and 10.45% of women in their 40s have adenomas detected through colonoscopic screening. The NCCN published in 2013 a guideline about CRC screening, mention that people with unknown family history should begin screening for CRC at the age of 40.[11] Since CRC screening in China has been very poor in previous decades, family history is often inaccurate and can be considered an unknown family history. Therefore, a cutoff age of 40 years was selected in this study. The ASGE stipulates that Caucasians at average risk should begin to undergo CRC colonoscopic screening at 50 years of age, requiring that ADR values for the population should reach 25% in men and 15% in women.[5] These values were determined from domestic CRC epidemiology and ADR-related studies in the United States. No such data have been previously reported in China, which makes it very difficult for China to determine the age at which CRC screening should be performed. This study showed that the ADR in males increased gradually after the age of 40. Nevertheless, due to the limited sample size, we failed to analyze age subgroups at 5 year intervals, to provide information about the PDR and ADR in the region. Previous studies showed that the incidence of advanced adenoma in the average risk population in China is lower than that in Europe or America, but higher than the rates in other Asian countries. Accordingly, the screening age could be at 55 years, between 50 years in the USA and 60 years in South Korea. Differently, this study showed that in individuals over 40 years old, the ADR was higher than described by the ASGE, suggesting that CRC screening may occur earlier in China than in the United States, which is quite different from a previous similar study in China. However, an accurate answer to the problem of screening age cutoff requires larger sample size studies and more detailed analysis. Finally, we also found significant differences between ADR values in males and females of the same age groups, suggesting that screening strategies should take gender into consideration.
4.5. Limitations
Several limitations should be mentioned of this study. First, this was a single center study, and the Han nationality in China is large; thus, all the study patients were Han Chinese. Therefore, the current data cannot reflect ethnic differences and the findings may not be generalizable. Second, the sample size was relatively limited. Third, during the procedure, patients with massive CRC were excluded, which may lead to selection bias. Fourth, since social responsibility is a major cultural concern for many Chinese, the rate of adequate preparation was quite high, preventing to observe an effect of bowel preparation on the ADR. Fifth, in the subgroup analysis based on age, no parameter was associated with ADR in patients 40 years and below, but this lack of association can be due to the small number of adenoma-positive patients among those <40 years of age. Sixth, because the examination procedures were carried out in the examination room in the study, the endoscopic physicians could not be blinded. Finally, in China, it is necessary to complete blood routine, coagulation function, pre-transfusion measurements, electrocardiogram, and other preoperative examinations before polypectomy. Therefore, when polyps are found and biopsied, they have to be removed during a second colonoscopy. This difference with Western practice could affect the generalizability of the results.
5. Conclusions
In conclusion, these findings add to several evidences for CRC screening in the Chinese population. ADR increases with age, withdrawal time, a personal history of adenoma, and diabetes mellitus. Age, insertion time, withdrawal time, a personal history of adenoma may be independent predictors of colorectal adenoma detection. This study, combined with European and American guidelines,[3,11] found that 40 years old may be a suitable age for beginning CRC screening in the general population in China. The relevance of the results to routine clinical practice is mainly the determination of the key factors that could suggest the need for CRC screening: male, >40 years of age, history of polyp, diabetes, etc. In addition, this study analyzed the factors related to the colonoscopy itself, in order to improve the detection rate of polyps/adenoma. Those factors include the time for entering and withdrawal of the endoscope, which can help clinicians to improve their ADR performance.
Author contributions
Data curation: Han Wang, Xiaogang Liu, Guangre Xu.
Formal analysis: Han Wang, Pu Wang, Xiaogang Liu, Guangre Xu.
Investigation: Han Wang, Liangping Li, Mengtian Tu.
Methodology: Han Wang, Pu Wang, Liangping Li, Xun Xiao, Di Zhang, Mengtian Tu.
Project administration: Xiaogang Liu, Di Zhang, Yi Li, Guangre Xu, Mengtian Tu, Yan Song.
Resources: Pu Wang, Xun Xiao, Di Zhang, Yi Li, Yan Song.
Software: Peixi Liu, Yi Li, Yan Song.
Supervision: Xun Xiao.
Validation: Peixi Liu.
Visualization: Peixi Liu.
Writing – Original Draft: Han Wang.
Writing – Review & Editing: Pu Wang, Xiaogang Liu, Liangping Li, Xun Xiao, Peixi Liu, Di Zhang, Yi Li, Guangre Xu, Mengtian Tu, Yan Song.
Footnotes
Abbreviations: ADR = adenoma detection rate, APC = adenomas per colonoscopy, ASGE = American Society of Digestive Endoscopy, BBPS = Boston Bowel Preparation Scale, BMI = body mass index, ChiCTR = Chinese Clinical Trial Registry, CRC = colorectal cancer, FAP = familial adenomatous polyposis, HNPCC = hereditary non-polyposis colorectal cancer, IBD = inflammatory bowel disease, NCCN = National Comprehensive Cancer Network, PDR = polyp detection rate, SD = standard deviation.
The author (s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this study.
The author(s) of this work have nothing to disclose.
References
- [1].Burke C, Kaul V, Pohl H. Polyp resection and removal procedures: insights from the 2017 Digestive Disease Week. Gastroenterol Hepatol 2017;13Suppl 2:1–24. [PMC free article] [PubMed] [Google Scholar]
- [2].Chinese Gastroenterology Society consensus: screening, early diagnosis and treatment, comprehensive prevention of large bowel cancer. Chin J Gastroenterol Hepatol 2011;11:979–95. [Google Scholar]
- [3].Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250–81. [DOI] [PubMed] [Google Scholar]
- [4].Williams JE, Holub JL, Faigel DO. Polypectomy rate is a valid quality measure for colonoscopy: results from a national endoscopy database. Gastrointest Endosc 2012;75:576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhao ZY, Li JQ, Shan YQ. Detection rates of colonoscopic polyp and adenoma in average risk population of colorectal cancer and its age distribution: retrospective analysis of data from single tertiary medical center. Chin J Dig Endosc 2014;31:64–8. [Google Scholar]
- [6].Klare P, Phlipsen H, Haller B, et al. Longer observation time increases adenoma detection in the proximal colon – a prospective study. Endosc Int Open 2017;5:E1289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kaminski MF, Wieszczy P, Rupinski M, et al. Increased rate of adenoma detection associates with reduced risk of colorectal cancer and death. Gastroenterology 2017;153:98–105. [DOI] [PubMed] [Google Scholar]
- [8].Pullens HJ, Siersema PD. Quality indicators for colonoscopy: current insights and caveats. World J Gastrointest Endosc 2014;6:571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lai EJ, Calderwood AH, Doros G, et al. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc 2009;69(Pt 2):620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015;81:31–53. [DOI] [PubMed] [Google Scholar]
- [11].Burt RW, Cannon JA, David DS, et al. Colorectal cancer screening. J Natl Compr Canc Netw 2013;11:1538–75. [DOI] [PubMed] [Google Scholar]
- [12].Chinese Society of Digestive Endoscopology, China Anti-Cancer Association, Oncology Endoscopology Specialized Committee. Guidelines for early screening and endoscopic diagnosis and treatment of colorectal cancer in China. Chin J Dig Endosc 2015;32:341–60. [Google Scholar]
- [13].Bailie L, Loughrey MB, Coleman HG. Lifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysis. Gastroenterology 2017;152:92–104. [DOI] [PubMed] [Google Scholar]
- [14].Kolligs FT, Crispin A, Munte A, et al. Risk of advanced colorectal neoplasia according to age and gender. PLoS One 2011;6:e20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Crockett SD, Barry EL, Mott LA, et al. Calcium and vitamin D supplementation and increased risk of serrated polyps: results from a randomised clinical trial. Gut 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 2006;355:2533–41. [DOI] [PubMed] [Google Scholar]
- [17].Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol 2007;102:856–61. [DOI] [PubMed] [Google Scholar]
- [18].Sanchez W, Harewood GC, Petersen BT. Evaluation of polyp detection in relation to procedure time of screening or surveillance colonoscopy. Am J Gastroenterol 2004;99:1941–5. [DOI] [PubMed] [Google Scholar]
- [19].Chubak J. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews, in Aspirin Use for the Prevention of Colorectal Cancer: An Updated Systematic Evidence Review for the U.S. Preventive Services Task Force. Rockville: Agency for Healthcare Research and Quality (US); 2015. [PubMed] [Google Scholar]
- [20].Platz EA, Willett WC, Colditz GA, et al. Proportion of colon cancer risk that might be preventable in a cohort of middle-aged US men. Cancer Causes Control 2000;11:579–88. [DOI] [PubMed] [Google Scholar]
- [21].Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev 2009;18:1688–94. [DOI] [PubMed] [Google Scholar]
- [22].Nguyen SP, Bent S, Chen YH, et al. Gender as a risk factor for advanced neoplasia and colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2009;7: 676-81.e1-3. [DOI] [PubMed] [Google Scholar]
- [23].Trabulo D, Ribeiro S, Martins C, et al. Metabolic syndrome and colorectal neoplasms: an ominous association. World J Gastroenterol 2015;21:5320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Krämer HU, Schöttker B, Raum E, et al. Type 2 diabetes mellitus and colorectal cancer: meta-analysis on sex-specific differences. Eur J Cancer 2012;48:1269–82. [DOI] [PubMed] [Google Scholar]
- [25].U.S. Preventive Services Task Force. Routine aspirin or nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2007;146:361–4. [PubMed] [Google Scholar]
- [26].Cooper K, Squires H, Carroll C, et al. Chemoprevention of colorectal cancer: systematic review and economic evaluation. Health Technol Assess 2010;14:1–206. [DOI] [PubMed] [Google Scholar]
- [27].Liu F, Yan L, Wang Z, et al. Metformin therapy and risk of colorectal adenomas and colorectal cancer in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Oncotarget 2017;8:16017–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bonovas S, Fiorino G, Lytras T, et al. Calcium supplementation for the prevention of colorectal adenomas: a systematic review and meta-analysis of randomized controlled trials. World J Gastroenterol 2016;22:4594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Long MD, Martin C, Sandler RS, et al. Reduced polyp detection as endoscopy shift progresses: experience with screening colonoscopy at a tertiary-care hospital. J Clin Gastroenterol 2011;45:253–8. [DOI] [PubMed] [Google Scholar]
- [30].Kaneshiro M, Ho A, Chan M, et al. Colonoscopy yields fewer polyps as the day progresses despite using social influence theory to reverse the trend. Gastrointest Endosc 2010;72:1233–40. [DOI] [PubMed] [Google Scholar]
- [31].Lebwohl B, Kastrinos F, Glick M, et al. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc 2011;73:1207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc 2003;58:76–9. [DOI] [PubMed] [Google Scholar]
- [33].Froehlich F, Wietlisbach V, Gonvers JJ, et al. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc 2005;61:378–84. [DOI] [PubMed] [Google Scholar]
- [34].Sawhney MS, Cury MS, Neeman N, et al. Effect of institution-wide policy of colonoscopy withdrawal time > or = 7 minutes on polyp detection. Gastroenterology 2008;135:1892–8. [DOI] [PubMed] [Google Scholar]
- [35].Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2002;97:1296–308. [DOI] [PubMed] [Google Scholar]
- [36].Imperiale TF, Glowinski EA, Juliar BE, et al. Variation in polyp detection rates at screening colonoscopy. Gastrointest Endosc 2009;69:1288–95. [DOI] [PubMed] [Google Scholar]
- [37].Chen F, Wang LJ. New technique of colonoscopy and quality control of endoscopy. Chin J Dig Endosc 2018;35: [Google Scholar]
- [38].Rex DK. Colonoscopic withdrawal technique is associated with adenoma miss rates. Gastrointest Endosc 2000;51:33–6. [DOI] [PubMed] [Google Scholar]
- [39].Lee RH, Tang RS, Muthusamy VR, et al. Quality of colonoscopy withdrawal technique and variability in adenoma detection rates (with videos). Gastrointest Endosc 2011;74:128–34. [DOI] [PubMed] [Google Scholar]
- [40].Coe SG, Crook JE, Diehl NN, et al. An endoscopic quality improvement program improves detection of colorectal adenomas. Am J Gastroenterol 2013;108:219–26. quiz 227. [DOI] [PubMed] [Google Scholar]
- [41].Wildi SM, Schoepfer AM, Vavricka SR, et al. Colorectal polypectomy during insertion and withdrawal or only during withdrawal? A randomized controlled trial. Endoscopy 2012;44:1019–23. [DOI] [PubMed] [Google Scholar]


