Abstract
Rationale:
Sclerosing pneumocytoma is a rare benign lung neoplasm seen in middle aged adults with a female predominance. Originally thought to be vascular in origin, this rare entity is now understood to be epithelial in nature. On imaging, sclerosing pneumocytoma manifests as a well circumscribed nodule or mass, often juxtapleural in location. On histopathology, sclerosing pneumocytoma is composed of cuboidal “surface cells” and round “stromal cells,” both of which show nuclear staining for thyroid transcription factor-1 (TTF-1). Here we review the existing literature on sclerosing pneumocytoma and present a case of sclerosing pneumocytoma in a highly unusual endobronchial location.
Patient concerns:
This case is a 43 year old woman who presented with chronic cough.
Diagnosis:
Imaging revealed a right upper lobe nodule with an endobronchial component.
Interventions and outcomes:
Endoscopic biopsy was performed, and pathologic diagnosis was confirmed.
Lessons:
Although extremely rare, endobronchial presentation of sclerosing pneumocytoma is possible, and should remain on the differential for patients with endobronchial pulmonary lesions. Pathologic tissue analysis is necessary to confirm this uncommon diagnosis.
Keywords: endobronchial, sclerosing hemangioma, Sclerosing pulmonary pneumocytoma, thyroid transcription factor-1 (TTF-1)
1. Introduction
Sclerosing pneumocytoma, an entity that was first described as sclerosing hemangioma,[1] is a rare benign lung neoplasm that commonly affects middle aged adults over the age of 50. There is a marked female predominance.[2,3] This neoplasm was originally thought to be vascular in origin, hence the term “sclerosing hemangioma.” However, based on immunohistochemical studies, it is now thought to be epithelial in nature, and was therefore named sclerosing pneumocytoma by the World Health Organization in 2015.[3,4]
On imaging, sclerosing pneumocytoma manifests as a well circumscribed nodule or mass, often juxtapleural in location. There may be surrounding ground-glass opacities. Fluorodeoxyglucose (FDG) positron emission tomography (PET) uptake is hypometabolic.[5,6] The lesion often shows homogeneous enhancement.[7,8] These imaging features are not specific, precluding a definitive diagnosis based on radiology alone.[9]
On histopathology, sclerosing pneumocytoma is composed of cuboidal “surface cells” and round “stromal cells”, both of which show nuclear staining for thyroid transcription factor-1 (TTF-1).[4,10,11] Characteristically, there are several growth patterns within the same neoplasm, including papillary, sclerotic, solid, and hemorrhagic. The purpose of this report is to present a case of sclerosing pneumocytoma in a highly unusual location.
2. Case presentation
A 43-year-old woman with a history of migraine headaches (controlled with sumatriptan) presented with chronic cough for 1 year, which had previously been productive of thick, yellow sputum with associated chest tightness. She denied hemoptysis, dyspnea, fevers, chills, night sweats, or weight loss. She was up to date on her vaccinations and age-appropriate cancer screening. She was a never-smoker. She worked as a school teacher, and had no identifiable risk factors for tuberculosis. Her family history was negative for significant lung disease or lung cancer.
On physical examination, her vital signs were normal and she was well appearing, without signs of respiratory distress. Chest examination revealed clear vesicular breath sounds bilaterally without adventitious breath sounds. Her laboratory workup showed mild anemia (hemoglobin: 11.5 g/dl) with a normal white blood cell count and normal comprehensive metabolic profile. A chest X-ray was obtained, which showed a nodular opacity in the right upper lobe. She subsequently underwent a computed tomography (CT) scan of the chest that demonstrated a 2.4-cm soft tissue nodule in the right upper lobe with an endobronchial component (Fig. 1). There was no hilar or mediastinal lymphadenopathy. She then underwent further workup with PET/CT scan, which demonstrated mild PET avidity (standard uptake value [SUV]: 3.5) within the right upper lobe lesion (Fig. 2). The nodule was favored to be benign (e.g. hamartoma) given the patient's young age and the absence of risk factors such as smoking. However, a carcinoid tumor or salivary gland neoplasm (such as mucoepidermoid carcinoma) could not be completely excluded. The patient was counseled on her options, including continued surveillance imaging versus bronchoscopy with biopsy of the lesion. The patient chose to proceed with biopsy.
Figure 1.
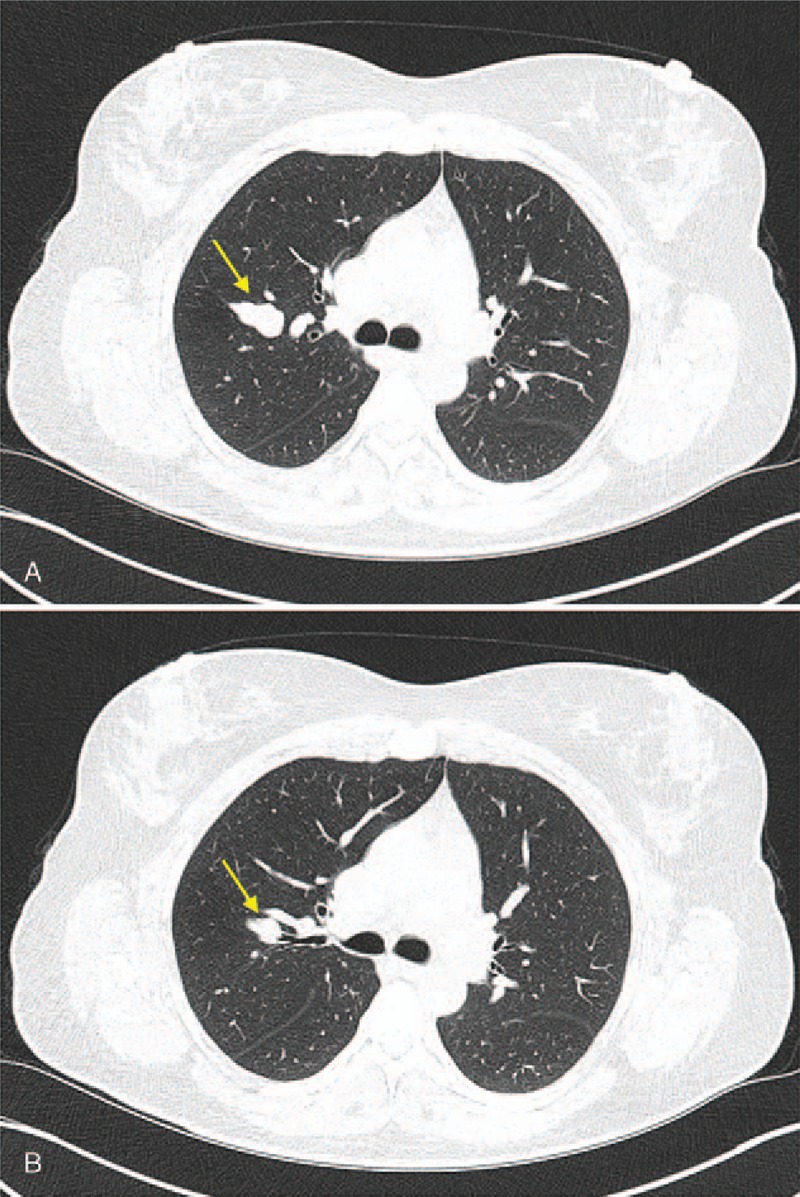
CT Chest demonstrating (A) 2 cm smoothly marginated nodule in the right upper lobe posterior segment. This lesion was found to have radiographic evidence of an (B) endobronchial component (yellow arrow).
Figure 2.
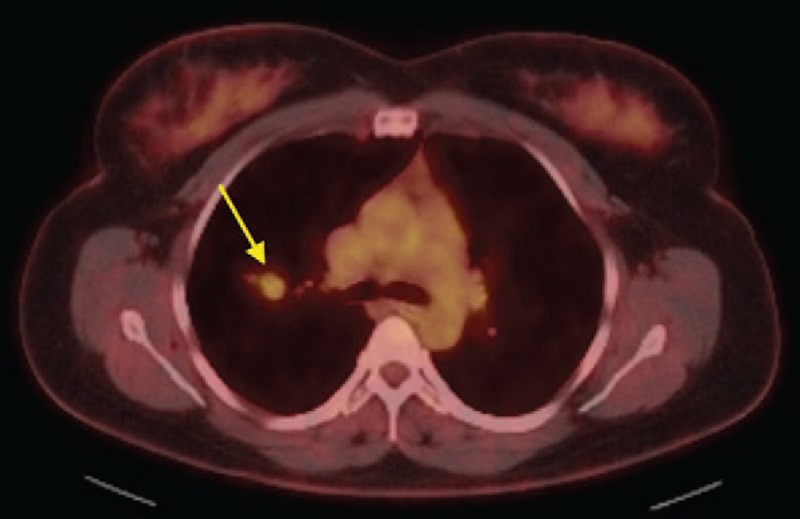
PET/CT showing mild PET avidity associated with right upper lobe nodule (yellow arrow).
Flexible bronchoscopy was performed using an Olympus BF-P190 slim bronchoscope, which was chosen for its smaller outer diameter (4.2 mm) in order to navigate into the distal airways. The right upper lobe bronchial segments and sub-segments were examined systematically. A polypoid endobronchial lesion was found in an anterior sub-segment of the posterior right upper (RB2b), and was biopsied using biopsy forceps. There was minor bleeding, which was controlled with instillation of ice-cold saline.
Pathologic examination showed a cytologically bland epithelioid neoplasm in the bronchial mucosa (Fig. 3). The “surface cells” were positive for keratin AE1/AE3, TTF-1 and napsin A and the “stromal cells” were positive for epithelial membrane antigen (EMA) and TTF-1 but negative for napsin A, chromogranin and smooth muscle actin (SMA), supporting the diagnosis of sclerosing pneumocytoma. The case was reviewed at our multidisciplinary tumor board. Some of the prior case reports of sclerosing pneumocytoma have been managed with surgical resection. However, the indication for resection in prior reports has been to establish a tissue diagnosis in lesions that were otherwise not amenable to endoscopic or percutaneous biopsy,[21] to relieve local compression of adjacent structures,[22] or in the setting of mixed histology of sclerosing pneumocytoma combined with another more aggressive tumor.[23] In this case, due to the rare endobronchial anatomic location, the tumor was amenable to adequate tissue sampling through bronchoscopy, thus resection was not required to establish the diagnosis. Further, although this patient was mildly symptomatic with productive cough, her lesion did not cause compression of any nearby vital structures, thus resection was not imperative. Finally, her histology was consisted with pure sclerosing pneumocytoma without any features to suggest any other histologies. Given the indolent nature of this neoplasm, the decision was made by the multidisciplinary tumor board to continue imaging surveillance, with repeat CT imaging in 6 months. At the time of final manuscript submission, the patient had not yet completed her repeat imaging. The patient provided written informed consent for this case report. Institutional review board approval was not required due to the retrospective case report nature of this manuscript, and for which the patient provided written informed consent.
Figure 3.
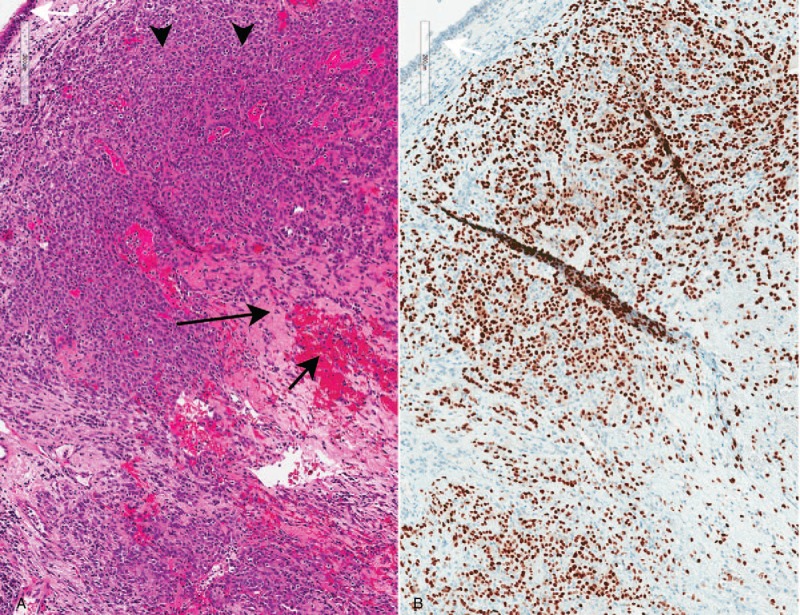
Pathologic findings: sclerosing pneumocytoma in an endobronchial biopsy. A. Sheets of cytologically bland neoplastic cells fill the bronchial mucosa (arrowheads, hematoxylin-eosin, 10×). Sclerotic (long black arrow) and hemorrhagic (short black arrow) areas are appreciable, even in this small sample. White arrow: respiratory epithelium. B. The neoplastic cells are positive for TTF-1 (10×). White arrow: respiratory epithelium.
3. Discussion
This case demonstrates that sclerosing pneumocytoma, which typically presents as a peripheral lung nodule, can rarely be found in an endobronchial location. Although endobronchial examples have been previously reported, not all of these reports represent true endobronchial disease. Shiina et al reported a case that was diagnosed by endobronchial ultrasound with transbronchial needle aspiration.[12] However, this case described a large right upper lobe mass that was abutting the trachea and allowed for needle aspiration via the trachea, but was not truly endobronchial. Wang et al reported the rare occurrence of concurrent sclerosing pneumocytoma with bronchial papilloma.[13] However, in that case, the sclerosing pneumocytoma was parenchymal and entirely distinct from the endobronchial pathology, which was a bronchial papilloma. Indeed, true endobronchial sclerosing pneumocytoma has only been reported in a handful of prior cases,[2,14–19] attesting to the exceedingly rare nature of this variant.
Central or endobronchial location of sclerosing pneumocytoma is considered extremely unusual, dating back to the original research studies that described the classic CT findings of sclerosing pneumocytoma. In a small study with 8 patients in 1994, Im et al, described sclerosing pneumocytoma lesions as being well defined and juxtapleural, with enhancement and possible calcification.[5] However, a newer study by Shin et al included 76 patients and found only 44.7% of the tumors to be juxtapleural or juxtafissural, with 55.3% of tumors being enveloped by the lungs.[6] Therefore, a non-pleura-based location of a nodule/mass does not rule out the possibility of sclerosing pneumocytoma.
Our case also reported hypometabolic uptake, with an SUV of 3.5. Lin et al analyzed FDG/PET uptake in biopsy-proven sclerosing pneumocytoma and reported low to moderate uptake, the mean standard uptake being 2.45 (range, 1.8–3.93).[20] These authors also found that larger tumors tend to have higher FDG uptake, making the distinction from malignancy more difficult.[20]
In addition to FDG avidity, Shin et al reported other imaging features that could be suggestive of sclerosing pneumocytoma, including the marginal pseudo-capsule sign, the overlying vessel sign, the air-gap sign, and the halo sign.[6] Although these features are non-specific, the combination of CT findings, female gender and hypometabolic PET uptake should raise the possibility of sclerosing pneumocytoma.
Pathologists should be aware that they may rarely encounter these tumors in bronchoscopic biopsies. This tumor should always be considered when a cytologically bland neoplasm is encountered in a biopsy from a young woman. Immunohistochemistry is very helpful for confirming the diagnosis.
Sclerosing pneumocytoma is a benign entity with excellent prognosis.[2] Patients typically undergo surgical resection and have good outcomes.
4. Conclusion
Sclerosing pneumocytoma is a rare benign lung neoplasm that typically affects middle aged women of Asian descent. We describe a case of biopsy-proven sclerosing pneumocytoma in a rare endobronchial location. In patients who fit the demographic profile of this tumor and have other CT signs suggestive of sclerosing pneumocytoma, central location or hypometabolic PET uptake should not rule out the possibility of this neoplasm.
Author contributions
Formal analysis: Akriti Khanna.
Investigation: Akriti Khanna, Khaled Alshabani.
Methodology: Louis Lam, Khaled Alshabani.
Resources: Louis Lam, Khaled Alshabani.
Supervision: Subha Ghosh.
Writing – original draft: Akriti Khanna.
Writing – review & editing: Akriti Khanna, Sanjay Mukhopadhyay, Subha Ghosh.
Footnotes
Abbreviations: CT = computed tomography, EMA = epithelial membrane antigen, FDG = fluorodeoxyglucose, PET = positron emission tomography, SMA = smooth muscle actin, SUV = standard uptake value, TTF-1 = thyroid transcription factor-1.
The authors have no conflicts of interest to disclose.
References
- [1].Liebow AA, Hubbell DS. Sclerosing hemangioma (histiocytoma, xanthoma) of the lung. Cancer 1956;9:53–75. http://www.ncbi.nlm.nih.gov/pubmed/13284701http://www.ncbi.nlm.nih.gov/pubmed/13284701. Accessed March 24, 2018. [DOI] [PubMed] [Google Scholar]
- [2].Devouassoux-Shisheboran M, Hayashi T, Linnoila RI, et al. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF-1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am J Surg Pathol 2000;24:906–16. http://www.ncbi.nlm.nih.gov/pubmed/10895813http://www.ncbi.nlm.nih.gov/pubmed/10895813. Accessed March 14, 2018. [DOI] [PubMed] [Google Scholar]
- [3].Illei PB, Rosai J, Klimstra DS. Expression of thyroid transcription factor-1 and other markers in sclerosing hemangioma of the lung. Arch Pathol Lab Med 2001;125:1335–9. doi:10.1043/0003-9985(2001)125<1335:EOTTFA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- [4].Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243–60. doi:10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- [5].Im JG, Kim WH, Han MC, et al. Sclerosing hemangiomas of the lung and interlobar fissures: CT findings. J Comput Assist Tomogr 1994;181:34–8. http://www.ncbi.nlm.nih.gov/pubmed/8282879http://www.ncbi.nlm.nih.gov/pubmed/8282879. Accessed March 23, 2018. [DOI] [PubMed] [Google Scholar]
- [6].Shin SY, Kim MY, Oh SY, et al. Pulmonary sclerosing pneumocytoma of the lung: CT characteristics in a large series of a tertiary referral center. Medicine (Baltimore) 2015;94:e498doi:10.1097/MD.0000000000000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chung MJ, Lee KS, Han J, et al. Pulmonary sclerosing hemangioma presenting as solitary pulmonary nodule: dynamic CT findings and histopathologic comparisons. AJR Am J Roentgenol 2006;187:430–7. doi:10.2214/AJR.05.0460. [DOI] [PubMed] [Google Scholar]
- [8].Xie R, Zhou X, Lü P, et al. Diagnosis of pulmonary sclerosing hemangioma with incremental dynamic CT: analysis of 20 cases. Zhonghua Jie He He Hu Xi Za Zhi 2003;26:7–9. http://www.ncbi.nlm.nih.gov/pubmed/12775260http://www.ncbi.nlm.nih.gov/pubmed/12775260. Accessed March 26, 2018. [PubMed] [Google Scholar]
- [9].Wang Q-B, Chen Y-Q, Shen J-J, et al. Sixteen cases of pulmonary sclerosing haemangioma: CT findings are not definitive for preoperative diagnosis. Clin Radiol 2011;66:708–14. doi:10.1016/j.crad.2011.03.002. [DOI] [PubMed] [Google Scholar]
- [10].Chan AC, Chan JK. Pulmonary sclerosing hemangioma consistently expresses thyroid transcription factor-1 (TTF-1): a new clue to its histogenesis. Am J Surg Pathol 2000;24:1531–6. http://www.ncbi.nlm.nih.gov/pubmed/11075855http://www.ncbi.nlm.nih.gov/pubmed/11075855. Accessed March 14, 2018. [DOI] [PubMed] [Google Scholar]
- [11].Zhou J, Covinsky MH. Sclerosing pneumocytoma: a carcinoma mimicker. a case report and literature review. Ann Clin Lab Sci 2017;47:103–5. http://www.ncbi.nlm.nih.gov/pubmed/28249927http://www.ncbi.nlm.nih.gov/pubmed/28249927. Accessed March 14, 2018. [PubMed] [Google Scholar]
- [12].Shiina Y, Sakairi Y, Wada H, et al. Sclerosing pneumocytoma diagnosed by preoperative endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). Surg case reports 2018;4:20doi:10.1186/s40792-018-0429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang J-M, You Q-H, Niu C-C. Co-occurrence of bronchial papilloma and pulmonary sclerosing hemangioma in a male. Springerplus 2016;5:1818doi:10.1186/s40064-016-3493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wani Y, Notohara K, Tsukayama C, et al. Sclerosing hemangioma with florid endobronchial and endobronchiolar growth. Virchows Arch 2007;450:221–3. doi:10.1007/s00428-006-0339-6. [DOI] [PubMed] [Google Scholar]
- [15].Kim SH, Seon HJ, Song J-H, et al. Radiological-pathological findings of central sclerosing hemangioma initially misdiagnosed as papillary adenoma by bronchoscopic biopsy: a case report. J Korean Soc Radiol 2013;69:283–6. [Google Scholar]
- [16].Boudaya MS, Falcoz PE, Alifano M, et al. Endobronchial sclerosing hemangioma: a rare presentation of a parenchymal tumor. Asian Cardiovasc Thorac Ann 2008;16:57–8. doi:10.1177/021849230801600114. [DOI] [PubMed] [Google Scholar]
- [17].Devouassoux-Shisheboran M, de la Fouchardière A, Thivolet-Béjui F, et al. Endobronchial variant of sclerosing hemangioma of the lung: histological and cytological features on endobronchial material. Mod Pathol 2004;17:252–7. doi:10.1038/modpathol.3800045. [DOI] [PubMed] [Google Scholar]
- [18].Latif MJ, Rahman GF, Connery CP. Respiratory arrest caused by endobronchial sclerosing hemangioma of the left main bronchus. J Bronchol Interv Pulmonol 2009;16:188–90. doi:10.1097/LBR.0b013e3181ab3198. [DOI] [PubMed] [Google Scholar]
- [19].Ekinci GH, Haciömeroğlu O, Ersev A, et al. Pulmonary sclerosing hemangioma (pneumocytoma): an analysis of 8 cases. Eurasian J Pulmonol 2015;17:159–62. [Google Scholar]
- [20].Lim J-H, Lee N, Choi D-W, et al. Pulmonary sclerosing pneumocytoma mimicking lung cancer: case report and review of the literature. Thorac cancer 2016;7:508–11. doi:10.1111/1759-7714.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lovrenski A, Vasilijević M, Panjković M, et al. Sclerosing pneumocytoma: a ten-year experience at a Western Balkan University Hospital. Medicina (Kaunas) 2019;55: doi:10.3390/medicina55020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ikeda M, Okada Y, Hagiwara K, et al. A case of pulmonary sclerosing pneumocytoma in the hilar lesion. Gen ThoracCardiovasc Surg 2018; 10.1007/s11748-018-1043-6 10.1007/s11748-018-1043-6. Accessed January 18, 2019. [DOI] [PubMed] [Google Scholar]
- [23].Wang Z, Yang M-Q, Huang W-J, et al. Sclerosing pneumocytoma mixed with a typical carcinoid tumor: a case report and review of literature. Medicine (Baltimore) 2019;98:e14315doi:10.1097/MD.0000000000014315. [DOI] [PMC free article] [PubMed] [Google Scholar]


