Abstract
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews on 'Single dose oral aspirin for acute pain'. Aspirin has been known for many years to be an effective analgesic for many different pain conditions. Although its use as an analgesic is now limited in developed countries, it is widely available, inexpensive, and remains commonly used throughout the world.
Objectives
To assess the analgesic efficacy and associated adverse events of single dose oral aspirin in acute postoperative pain.
Search methods
For the earlier review, we identified randomised trials by searching CENTRAL (The Cochrane Library) (1998, Issue 1), MEDLINE (1966 to March 1998), EMBASE (1980 to January 1998), and the Oxford Pain Relief Database (1950 to 1994). We updated searches of CENTRAL, MEDLINE, and EMBASE to January 2012.
Selection criteria
Single oral dose, randomised, double‐blind, placebo‐controlled trials of aspirin for relief of established moderate to severe postoperative pain in adults.
Data collection and analysis
We assessed studies for methodological quality and two review authors extracted the data independently. We used summed total pain relief (TOTPAR) over four to six hours to calculate the number of participants achieving at least 50% pain relief. We used these derived results to calculate, with 95% confidence intervals, the relative benefit compared to placebo, and the number needed to treat (NNT) for one participant to experience at least 50% pain relief over four to six hours. We sought numbers of participants using rescue medication over specified time periods, and time to use of rescue medication, as additional measures of efficacy. We collected information on adverse events and withdrawals.
Main results
We included 68 studies in which aspirin was used at doses from 300 mg to 1200 mg, but the vast majority of participants received either 600/650 mg (2409 participants, 64 studies) or 990/1000 mg (380 participants, eight studies). There was only one new study.
Studies were overwhelmingly of adequate or good methodological quality. NNTs for at least 50% pain relief over four to six hours were 4.2 (3.9 to 4.8), 3.8 (3.0 to 5.1), and 2.7 (2.0 to 3.8) for 600/650 mg, 900/1000 mg, and 1200 mg respectively, compared with placebo. Type of pain model had no significant impact on the results. Lower doses were not significantly different from placebo. These results do not differ from those of the earlier review.
Fewer participants required rescue medication with aspirin than with placebo over four to eight hours postdose, but by 12 hours there was no difference. The number of participants experiencing adverse events was not significantly different from placebo for 600/650 mg aspirin, but for 900/1000 mg the number needed to treat to harm was 7.5 (4.8 to 17). The most commonly reported events were dizziness, drowsiness, gastric irritation, nausea, and vomiting, nearly all of which were of mild to moderate severity.
Authors' conclusions
Aspirin is an effective analgesic for acute pain of moderate to severe intensity. High doses are more effective, but are associated with increased adverse events, including drowsiness and gastric irritation. The pain relief achieved with aspirin was very similar milligram for milligram to that seen with paracetamol. There was no change to the conclusions in this update.
Plain language summary
Single dose oral aspirin for acute postoperative pain in adults
Aspirin is commonly used throughout the world as an over the counter (OTC) analgesic medication used to treat various painful conditions and to reduce fever. This review assessed both the pain‐relieving effectiveness and the adverse effects of a single dose of aspirin in acute postoperative pain of moderate to severe intensity. We included 67 studies, with 3111 participants given aspirin in comparisons with 2632 given placebo. Most of the information was for a 600 mg or 650 mg dose. The results confirm that in patients with moderate to severe postoperative pain, about 40% of those treated with aspirin 600/650 mg will experience good levels of pain relief, compared with about 15% treated with placebo. This level of pain relief is comparable to that experienced with the same dose of paracetamol. In these single dose studies there was no significant difference between aspirin 600/650 mg and placebo for the number of participants experiencing adverse events, but at 900/1000 mg, twice as many did so, with dizziness, drowsiness, gastric irritation, nausea, and vomiting being the most common events reported.
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews on 'Single dose oral aspirin for acute pain' (Edwards 2000). The title now states that the review is limited to postoperative pain in adults.
Description of the condition
Acute pain occurs as a result of tissue damage either accidentally due to an injury or as a result of surgery. Acute postoperative pain is a manifestation of inflammation due to tissue injury. The management of postoperative pain and inflammation is a critical component of patient care.
This is one of a series of reviews whose aim is to increase awareness of the range of analgesics that are potentially available, and present evidence for relative analgesic efficacy through indirect comparisons with placebo, in very similar trials performed in a standard manner, with very similar outcomes, and over the same duration. Such relative analgesic efficacy does not in itself determine choice of drug for any situation or patient, but guides policy‐making at the local level. The series covers all analgesics licensed for acute postoperative pain in the UK, and dipyrone, which is commonly used in Spain, Portugal, and Latin‐American countries. The results have been examined in an overview (Moore 2011), and important individual reviews include ibuprofen (Derry 2009), paracetamol (Toms 2008), and etoricoxib (Clarke 2009).
Description of the intervention
Acute pain trials
Single dose trials in acute pain are commonly short in duration, rarely lasting longer than 12 hours. The numbers of participants are small, allowing no reliable conclusions to be drawn about safety. To show that the analgesic is working, it is necessary to use placebo (McQuay 2005). There are clear ethical considerations in doing this. These ethical considerations are answered by using acute pain situations where the pain is expected to go away, and by providing additional analgesia, commonly called rescue analgesia, if the pain has not diminished after about an hour. This is reasonable, because not all participants given an analgesic will have significant pain relief. Approximately 18% of participants given placebo will have significant pain relief (Moore 2006), and up to 50% may have inadequate analgesia with active medicines. The use of additional or rescue analgesia is hence important for all participants in the trials.
Clinical trials measuring the efficacy of analgesics in acute pain have been standardised over many years. Trials have to be randomised and double‐blind. Typically, in the first few hours or days after an operation, patients develop pain that is moderate to severe in intensity, and will then be given the test analgesic or placebo. Pain is measured using standard pain intensity scales immediately before the intervention, and then using pain intensity and pain relief scales over the following four to six hours for shorter‐acting drugs, and up to 12 or 24 hours for longer‐acting drugs. Pain relief of half the maximum possible pain relief or better (at least 50% pain relief) is typically regarded as a clinically useful outcome. For patients given rescue medication it is usual for no additional pain measurements to be made, and for all subsequent measures to be recorded as initial pain intensity or baseline (zero) pain relief (baseline observation carried forward). This process ensures that analgesia from the rescue medication is not wrongly ascribed to the test intervention. In some trials the last observation is carried forward, which gives an inflated response for the test intervention compared to placebo, but the effect has been shown to be negligible over four to six hours (Moore 2005). Patients usually remain in the hospital or clinic for at least the first six hours following the intervention, with measurements supervised, although they may then be allowed home to make their own measurements in trials of longer duration.
Knowing the relative efficacy of different analgesic drugs at various doses can be helpful. An example is the relative efficacy in the third molar extraction pain model (Barden 2004).
Aspirin
Aspirin (acetylsalicylic acid, (ASA)) is an analgesic and a non‐steroidal anti‐inflammatory drug (NSAID); it also has antipyretic (reducing fever) and anti‐inflammatory effects. Aspirin has been known to be an effective analgesic for many years. Rheumatism has affected man since the great river cultures of the Middle East. Clay tablets from the Sumerian period described the use of willow leaves to treat it. The Egyptians were also aware of the pain‐relieving effects of potions made from myrtle or willow leaves. Edward Stone, a vicar from Chipping Norton in Oxfordshire, is generally recognised as the man who gave the first scientific description of the effects of willow bark. In 1763 he wrote a letter to the Earl of Macclesfield, then President of the Royal Society in London, in which he described treating patients suffering from ague (fever) with 20 grains (approximately a 1000 mg) of powdered willow bark in a dram of water every four hours. Today aspirin is commonly used throughout the world for many different pain conditions.
When used as an analgesic for adults, aspirin is given orally at a dose of 300 to 1000 mg every four to six hours, with a maximum of 4000 mg daily. In some countries/the UK it is contraindicated in children under 16 years (due to its association with Reye's syndrome), except in certain conditions, such as Kawasaki disease. Much lower doses (typically 75 mg or 81 mg daily) are used for antiplatelet effects to prevent cardiovascular events.
Aspirin use is associated with a high incidence of gastrointestinal irritation and occult (hidden) blood loss, and occasional overt gastrointestinal bleeding, even with short‐term use. Dispersible and enteric coated formulations have been developed to limit these gastrointestinal adverse effects. The availability of alternative drugs with better tolerability has reduced the use of aspirin for pain relief in many countries in recent years. In the UK in 2010 just over 4 million prescriptions for aspirin were written in primary care, mostly for 300 mg dispersible or enteric coated tablets (PCA 2011); in 2009 the number was almost 4.6 million, while in 2006 it was just over 6 million. In other parts of the world, where newer alternatives are either not available or are too expensive, aspirin may still be the main analgesic in use.
How the intervention might work
Clinicians prescribe NSAIDs on a routine basis for a range of mild‐to‐moderate pain. NSAIDs are the most commonly prescribed analgesic medications worldwide, and their efficacy for treating acute pain has been well demonstrated (Moore 2003). They reversibly inhibit cyclooxygenase (prostaglandin endoperoxide synthase), the enzyme mediating production of prostaglandins and thromboxane A2 (Fitzgerald 2001). Prostaglandins mediate a variety of physiological functions such as maintenance of the gastric mucosal barrier, regulation of renal blood flow, and regulation of endothelial tone. They also play an important role in inflammatory and nociceptive processes. Aspirin's main mechanism of action is irreversible inhibition of the enzyme cyclooxygenase 1 (COX‐1) and modification of cyclooxygenase 2 (COX‐2), which interferes with thromboxane and prostaglandin synthesis, and increases production of anti‐inflammatory lipoxins. Other non‐steroidal anti‐inflammatory drugs cause reversible inhibition of the cyclooxygenases.
Why it is important to do this review
Aspirin is an inexpensive and effective analgesic available worldwide, and therefore an important drug for treating pain.
Objectives
To assess the efficacy and adverse effects of single dose oral aspirin for acute postoperative pain using methods that permit comparison with other analgesics evaluated in standardised trials using almost identical methods and outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Included studies were double‐blind trials of single dose oral aspirin compared with placebo for the treatment of moderate to severe postoperative pain in adults, with at least 10 participants randomly allocated to each treatment group. We included multiple dose studies if appropriate data from the first dose were available, and cross‐over studies provided that data from the first arm were presented separately.
We excluded the following:
review articles, case reports, and clinical observations;
studies of experimental pain;
studies where pain relief is assessed only by clinicians, nurses, or carers (i.e. not patient‐reported);
studies of less than four hours duration or studies that fail to present data over four to six hours postdose.
For postpartum pain, we included studies if the pain investigated was due to episiotomy or Caesarean section irrespective of the presence of uterine cramps; we excluded studies investigating pain due to uterine cramps alone.
Types of participants
We included studies of adult participants (> 15 years) with established postoperative pain of moderate to severe intensity following day surgery or in‐patient surgery. For studies using a visual analogue scale (VAS), we considered that pain intensity of greater than 30 mm equated to pain of at least moderate intensity (Collins 1997).
Types of interventions
Aspirin or matched placebo administered as a single oral dose for postoperative pain.
Types of outcome measures
Data collected included the following if available:
participant characteristics;
patient reported pain at baseline (physician, nurse, or carer reported pain was not included in the analysis);
patient reported pain relief expressed at least hourly over four to six hours using validated pain scales (pain intensity and pain relief in the form of VAS or categorical scales, or both);
patient global assessment of efficacy (PGE), using a standard categorical scale;
time to use of rescue medication;
number of participants using rescue medication;
number of participants with one or more adverse events;
number of participants with serious adverse events;
number of withdrawals (all‐cause, adverse events).
Search methods for identification of studies
Electronic searches
We searched the following databases for the original review:
the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library) (1998, Issue 1);
MEDLINE (1966 to March 1998);
EMBASE (1980 to January 1998);
Oxford Pain Relief Database (1950 to 1994; Jadad 1996a).
We updated searches using the following electronic databases:
the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library) (issue 1, 2012);
MEDLINE via Ovid (25 January 2012);
EMBASE via Ovid (25 January 2012).
See Appendix 1 for the MEDLINE search strategy, Appendix 2 for the EMBASE search strategy and Appendix 3 for the CENTRAL search strategy.
Language
We did not limit the searches by language.
Searching other resources
We sought additional studies in reference lists of retrieved articles and reviews. We did not attempt to contact manufacturers since aspirin is so widely available in generic formulations.
Unpublished studies
We did not contact any manufacturing pharmaceutical company producing this drug for unpublished trial data.
Data collection and analysis
Selection of studies
Two review authors independently assessed and agreed the search results for studies that might be included in the review. Disagreements were resolved by consensus or referral to a third review author.
Quality assessment
Two review authors independently assessed the included studies for quality using a five‐point scale (Jadad 1996b) that considers randomisation, blinding, and study withdrawals and dropouts.
The scale used is as follows.
Is the study randomised? If yes give one point.
Is the randomisation procedure reported and is it appropriate? If yes add one point, if no deduct one point.
Is the study double‐blind? If yes then add one point.
Is the double‐blind method reported and is it appropriate? If yes add 1 point, if no deduct one point.
Are the reasons for patient withdrawals and dropouts described? If yes add one point.
The results are described in the 'Risk of bias in included studies' section below, and 'Characteristics of included studies' table.
Data management
Two review authors extracted data and recorded them on a standard data extraction form. We entered data suitable for pooling into RevMan 5.1 (RevMan 2011).
Data analysis
For each study we converted the mean summed total pain relief (TOTPAR), summed pain intensity difference (SPID), VAS TOTPAR or VAS SPID (Appendix 4) values for active and placebo to %maxTOTPAR or %maxSPID by division into the calculated maximum value (Cooper 1991), and calculated the proportion of participants in each treatment group who achieved at least 50%maxTOTPAR using verified equations (Moore 1996; Moore 1997a; Moore 1997b). We then converted these proportions into the number of participants achieving at least 50%maxTOTPAR by multiplying by the total number of participants in the treatment group. We used this information on the number of participants with at least 50%maxTOTPAR for active and placebo to calculate relative benefit or relative risk, and number needed to treat to benefit (NNT).
We accepted the following pain measures for the calculation of TOTPAR or SPID:
five‐point categorical pain relief (PR) scales with comparable wording to "none, slight, moderate, good or complete";
four‐point categorical pain intensity (PI) scales with comparable wording to "none, mild, moderate, severe";
VAS for pain relief;
VAS for pain intensity.
If none of these measures were available, we used the number of participants reporting "very good or excellent" on a five‐point categorical global scale with the wording "poor, fair, good, very good, excellent" for the number of participants achieving at least 50% pain relief (Collins 2001).
For each treatment group we extracted the number of participants reporting treatment‐emergent adverse effects, and calculated relative benefit and risk estimates with 95% confidence intervals (CI) using a fixed‐effect model (Morris 1995). We calculated NNT and number needed to treat to harm (NNH) and 95% CI using the pooled number of events using the method devised by Cook and Sackett (Cook 1995). We assumed a statistically significant difference from control when the 95% CI of the relative risk (RR) or relative benefit (RB) did not include the number one. We examined homogeneity visually using L'Abbé plots (L'Abbé 1987).
We planned subgroup analyses to determine the effect of dose and presenting condition (pain model), and sensitivity analyses for high versus low (two or fewer versus three or more) quality trials. A minimum of two studies and 200 participants had to be available in any subgroup or sensitivity analysis (Moore 1998), which was restricted to the primary outcome (50% pain relief over four to six hours) and the dose with the greatest amount of data (600/650 mg).
Results
Description of studies
Included studies
We identified 72 publications, reporting on 67 studies with 5743 participants, that satisfied our inclusion criteria. We included two studies that were not in the earlier review: Gaston 1984 had been excluded because of non‐standard labelling of the pain scale, but an adjustment could be made for this, and Seymour 2003 was published after the earlier review. We identified three further studies, Beaver 1983, Cooper 1983, and Lindenmuth 1989, as reporting on the same studies as Forbes 1982, Mardirossian 1985, and Clark 1989 respectively, and these are now linked to the parent IDs.
Aspirin was used at doses from 300 mg to 1200 mg: 77 participants took a 300 mg dose (two studies), 135 took 500 mg (two studies), 2379 took 600 mg or 650 mg (63 studies), 380 took 900 mg or 1000 mg (eight studies), 140 took 1200 mg (five studies), and 2632 took placebo (68 studies). Three studies (Holland 1988; Seymour 1986; Seymour 2003) specified using a soluble formulation, and five (Kempf 1987; Markowitz 1985; Parkhouse 1969; Rowe 1985; Seymour 1992) specified using a buffered formulation. The remaining studies are assumed to have used a standard formulation tablet.
Most studies included one or more active comparators, so that a large number of other drugs were used: amfenac, aspirin plus caffeine, aspirin plus codeine, aspirin plus chlorphenasin, aspirin plus mefenamic acid, aspirin plus pentazocine, bicifadine, bromfenac, caffeine, carprofen, chlorphenasin, codeine, codeine plus ibuprofen, codeine plus paracetamol, codeine plus naproxen, diclofenac, diflunisal, dipyrone, etodolac, fenbufen, fendosal, fluproquazone, fluradoline, flurbiprofen, FS205‐397, ibuprofen, indoprofen, ketorolac, lornoxicam, meclofenamate, naproxen, oxaprozin, paracetamol, paracetamol plus phenyltoloxamine, piroxicam, propoxyphene, propiram fumarate, proquazone, suprofen, and zomepirac. There were insufficient data to compare a given dose of aspirin with a given dose of any active comparator.
Forty‐eight studies treated participants with pain following dental surgery, 12 following episiotomy or other gynaecological surgery, three following orthopaedic surgery, one following urological surgery, and four included participants who had undergone a mixture of types of surgery, or the type of surgery was not specified.
Further details of included studies are in the 'Characteristics of included studies' table.
Excluded studies
We excluded five studies that were included in the earlier review because they included participants with pain due to trauma (Herrmann 1980c; Herrmann 1980d), fracture (Kantor 1965; Wang 1979), or fracture and musculoskeletal pain (Okun 1979), which are outside the scope of this update.
In total we excluded 102 studies after reading the full report. Reasons for exclusion are in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
All included studies were randomised and double‐blind. Twenty studies scored 5/5, 25 scored 4/5, 19 scored 3/5, and three scored 2/5 on the Oxford Quality Scale. Points were lost mostly for failure to describe the methods used to ensure randomisation and blinding adequately, but 14 studies failed to report adequately on withdrawals (Boraks 1987; Breivik 1984; Clark 1989; Coutinho 1976; Herbertson 1994; Herrmann 1980a; Herrmann 1980b; London 1983b; Mardirossian 1985; Nelson 1994b; Rowe 1985; Seymour 1986; Seymour 1992; Wang 1982). Details of scores in individual studies are in the 'Characteristics of included studies' table.
We also drew up a 'Risk of bias' table to consider randomisation, allocation, and blinding. We considered no studies at high risk of bias, but the majority did not adequately report the methods used for randomisation and allocation concealment. This may reflect older reporting of methods rather than actual poor study quality (Figure 1).
1.
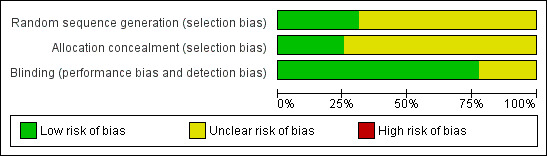
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
Participants with at least 50% pain relief
Aspirin 300/325 mg versus placebo
There were insufficient data for analysis of aspirin 300/325 mg.
Aspirin 500 mg versus placebo
Two studies (213 participants) compared aspirin 500 mg with placebo (Nelson 1994a; Seymour 1992).
The proportion of participants with ≥ 50% pain relief with aspirin 500 mg was 34% (45/135, range 31% to 36%).
The proportion of participants with ≥ 50% pain relief with placebo was 26% (20/78, range 20% to 32%).
The relative benefit of treatment compared with placebo was 1.3 (0.82 to 2.0), showing no significant benefit. We did not calculate the number needed to treat (NNT) (Analysis 1.1).
1.1. Analysis.
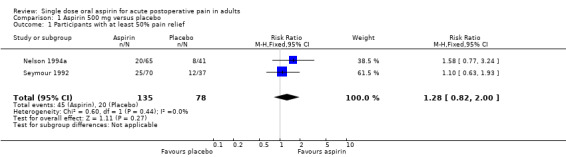
Comparison 1 Aspirin 500 mg versus placebo, Outcome 1 Participants with at least 50% pain relief.
Aspirin 600/650 mg versus placebo
Sixty studies (4659 participants) compared aspirin 600 or 650 mg with placebo.
The proportion of participants with ≥ 50% pain relief with aspirin 600/650 mg was 39% (905/2339, range 12% to 90%).
The proportion of participants with ≥ 50% pain relief with placebo was 15% (352/2320, range 0% to 46%).
The relative benefit of treatment compared with placebo was 2.5 (2.2 to 2.7), giving a NNT of 4.2 (3.9 to 4.8) (Analysis 2.1; Figure 2; Figure 3).
2.1. Analysis.

Comparison 2 Aspirin 600 or 650 mg versus placebo, Outcome 1 Participants with at least 50% pain relief.
2.

Forest plot of comparison: 2 Aspirin 600 or 650 mg versus placebo, outcome: 2.1 Participants with at least 50% pain relief.
3.

L'Abbé plot showing ≥ 50 % pain relief for all studies using 600/650 mg aspirin versus placebo. Each circle represents a different study. Size of circle is proportional to size of study.
Subgroup analysis for pain model
For the 43 studies in dental pain only (3433 participants) the relative benefit (RB) was 2.5 (2.2 to 2.9), giving a NNT of 4.6 (4.1 to 5.3), while for the 17 studies in other types of surgery (1211 participants), the RB was 2.3 (1.9 to 2.8), giving a NNT of 3.6 (3.0 to 4.5). There was no significant difference between the different types of surgery in these studies (Analysis 2.1).
Sensitivity analysis for study quality
For the 57 studies scoring ≥ 3/5 on the Oxford Quality Score (4373 participants), the RB was 2.4 (2.2 to 2.7), giving a NNT of 4.3 (3.8 to 4.8), while for the three studies scoring only 2/5 (257 participants), the RB was 2.9 (1.9 to 4.6), giving a NNT of 3.4 (2.5 to 5.4). There was no significant difference between studies scoring the minimum for inclusion in this review (2/5), and those with higher scores.
Aspirin 1000 mg versus placebo
Six studies (618 participants) compared aspirin 1000 mg with placebo (Forbes 1990a; Herrmann 1980a; Herrmann 1980b; Lehnert 1990; Seymour 1992; Seymour 2003).
The proportion of participants with ≥ 50% pain relief with aspirin 1000 mg was 41% (138/340, range 24% to 64%).
The proportion of participants with ≥ 50% pain relief with placebo was 14% (40/278, range 0% to 32%).
The relative benefit of treatment compared with placebo was 2.7 (2.0 to 3.7), giving a NNT of 4.2 (3.8 to 4.6) (Analysis 3.1).
3.1. Analysis.

Comparison 3 Aspirin 900 or 1000 mg versus placebo, Outcome 1 Participants with at least 50% pain relief.
Aspirin 1200 mg versus placebo
Three studies (249 participants) compared aspirin 1000 mg with placebo (Holland 1988; London 1983b; Seymour 1986).
The proportion of participants with ≥ 50% pain relief with aspirin 1200 mg was 61% (85/140, range 53% to 70%).
The proportion of participants with ≥ 50% pain relief with placebo was 23% (25/109, range 7% to 36%).
The relative benefit of treatment compared with placebo was 2.9 (2.0 to 4.2), giving a NNT of 2.7 (2.0 to 3.8) (Analysis 4.1).
4.1. Analysis.

Comparison 4 Aspirin 1200 mg versus placebo, Outcome 1 Participants with at least 50% pain relief.
| Summary of results A: Number of participants with ≥ 50% pain relief over 6 hours | ||||||
| Dose | Studies | Participants | Aspirin (%) | Placebo (%) | Relative benefit (95% CI) | NNT (95% CI) |
| 500 mg | 2 | 213 | 33 | 26 | 1.3 (0.82 to 2.0) | not calculated |
| 600/650 mg | 60 | 4630 | 39 | 15 | 2.5 (2.2 to 2.7) | 4.2 (3.9 to 4.8) |
| 900/1000 mg | 6 | 618 | 41 | 14 | 2.7 (2.0 to 3.7) | 3.8 (3.0 to 5.1) |
| 1200 mg | 3 | 249 | 61 | 23 | 2.9 (2.0 to 4.2) | 2.7 (2.0 to 3.8) |
Results for the different doses are compatible with a dose response, with the 1200 mg dose being significantly superior to the 600/650 mg dose (z = 2.416, P = 0.016).
Participants using rescue medication at four to five hours
Aspirin 600/650 mg versus placebo
Eleven studies (982 participants) compared aspirin 600 or 650 mg with placebo (Boraks 1987; Calimlim 1977; Frame 1986; Jain 1985b; Jain 1986a; London 1983b; Mehlisch 1984; Mehlisch 1994; Parkhouse 1969; Sunshine 1983b; Sunshine 1983c).
The proportion of participants using rescue medication at four or five hours with aspirin 600/650 mg was 27% (144/530, range 2% to 71%).
The proportion of participants using rescue medication at four or five hours with placebo was 47% (211/452, range 5% to 89%).
The relative benefit of treatment compared with placebo was 0.65 (0.56 to 0.76), giving a number needed to treat to prevent one event (NNTp) of 5.1 (3.9 to 7.4) (Analysis 2.2).
2.2. Analysis.

Comparison 2 Aspirin 600 or 650 mg versus placebo, Outcome 2 Participants using rescue medication at 4 to 5 h.
Aspirin 1000 mg versus placebo
Five studies (501 participants) compared aspirin 900/1000 mg with placebo (Herrmann 1980a; Herrmann 1980b; London 1983b; Seymour 1992; Seymour 2003).
The proportion of participants using rescue medication at four or five hours with aspirin 900/1000 mg was 46% (121/264, range 3% to 81%).
The proportion of participants using rescue medication at four or five hours with placebo was 67% (158/237, range 26% to 91%).
The relative benefit of treatment compared with placebo was 0.64 (0.56 to 0.74), giving a NNTp of 4.8 (3.4 to 8.1) (Analysis 3.2).
3.2. Analysis.

Comparison 3 Aspirin 900 or 1000 mg versus placebo, Outcome 2 Participants using rescue medication at 4 to 5 h.
There were insufficient data for analysis of other doses of aspirin for use of rescue medication at four or five hours.
Participants using rescue medication at six to eight hours
Aspirin 600/650 mg versus placebo
Twenty studies (1838 participants) compared aspirin 600 or 650 mg with placebo.
The proportion of participants using rescue medication at six or eight hours with aspirin 600/650 mg was 55% (530/955, range 3% to 98%).
The proportion of participants using rescue medication at six or eight hours with placebo was 75% (664/883, range 37% to 97%).
The relative benefit of treatment compared with placebo was 0.77 (0.73 to 0.82), giving a NNTp of 5.1 (4.2 to 6.5) (Analysis 2.3).
2.3. Analysis.

Comparison 2 Aspirin 600 or 650 mg versus placebo, Outcome 3 Participants using rescue medication at 6 h.
Aspirin 1000 mg versus placebo
Two studies (233 participants) compared aspirin 1000 mg with placebo (Forbes 1990a; Lehnert 1990).
The proportion of participants using rescue medication at six or eight hours with aspirin 1000 mg was 67% (78/116, range 51% to 77%).
The proportion of participants using rescue medication at six or eight hours with placebo was 83% (97/117, range 64% to 93%).
The relative benefit of treatment compared with placebo was 0.82 (0.71 to 0.95), giving a NNTp of 6.4 (3.8 to 21) (Analysis 3.3).
3.3. Analysis.

Comparison 3 Aspirin 900 or 1000 mg versus placebo, Outcome 3 Participants using rescue medication at 6 h.
There were insufficient data for analysis of other doses of aspirin for use of rescue medication at six or eight hours.
Participants using rescue medication at 12 hours
Aspirin 600/650 mg versus placebo
Four studies (291 participants) compared aspirin 600 or 650 mg with placebo (Clark 1989; Forbes 1982; Forbes 1983; Forbes 1984).
The proportion of participants using rescue medication at 12 hours with aspirin 600/650 mg was 81% (117/145, range 65% to 97%).
The proportion of participants using rescue medication at 12 hours with placebo was 86% (125/146, range 76% to 90%).
The relative benefit of treatment compared with placebo was 0.95 (0.86 to 1.05). There was no significant difference between treatment groups for use of rescue medication by 12 hours (Analysis 2.4).
2.4. Analysis.

Comparison 2 Aspirin 600 or 650 mg versus placebo, Outcome 4 Participants using rescue medication at 12 h.
There were insufficient data for analysis of other doses of aspirin for use of rescue medication at 12 hours.
| Summary of results B: Number of participants using rescue medication | ||||||
| Dose | Studies | Participants | Aspirin (%) | Placebo (%) | Relative benefit (95% CI) | NNTp (95% CI) |
| at 4 to 5 hours | ||||||
| 600/650 mg | 11 | 1067 | 27 | 47 | 0.58 (0.50 to 0.67) | 4.9 (3.9 to 6.8) |
| 1000 mg | 5 | 501 | 46 | 67 | 0.64 (0.56 to 0.74) | 4.8 (3.4 to 8.1) |
| at 6 to 8 hours | ||||||
| 600/650 mg | 20 | 1838 | 55 | 75 | 0.77 (0.73 to 0.82) | 5.1 (4.2 to 6.5) |
| 1000 mg | 2 | 233 | 67 | 83 | 0.82 (0.71 to 0.95) | 6.4 (3.8 to 21) |
| at 12 hours | ||||||
| 600/650 mg | 4 | 291 | 81 | 86 | 0.95 (0.86 to 1.05) | not calculated |
Time to use of rescue medication
Aspirin 600/650 mg versus placebo
Eighteen studies reported the mean time to use of rescue medication following 600 or 650 mg aspirin (Cooper 1982; Cooper 1983; Cooper 1986; Cooper 1988; Cooper 1991; Desjardins 1984; Forbes 1980; Forbes 1983; Forbes 1984; Forbes 1986; Forbes 1989; Forbes 1990a; Forbes 1990b; Forbes 1991; Forbes 1992; Jain 1986a; Mardirossian 1985; Mehlisch 1990). The weighted mean of the mean time to use was 3.8 hours with aspirin 600/650 mg, and 3.0 hours with placebo.
A further 11 studies reported the median time to use of rescue medication following 600 or 650 mg aspirin (Clark 1989; Cooper 1992; Forbes 1982; Frame 1986; Herbertson 1994; McQuay 1987; Mehlisch 1994; Nelson 1994b; Olsen 1997; Parkhouse 1969; Sunshine 1988). The weighted mean of the median time to use was 5.2 hours with aspirin 600/650 mg, and 3.4 hours with placebo.
Aspirin 900/1000 mg versus placebo
Only one study (Forbes 1990a; 146 participants) reported the mean time to use of rescue medication following 1000 mg aspirin, which was 3.9 hours with aspirin and 2.8 hours with placebo. Two studies reported the mean time to use of rescue medication following 900 or 1000 mg aspirin (Seymour 1992; Seymour 2003; 203 participants). The weighted mean of the mean time to use was 1.9 hours with aspirin 900/1000 mg, and 1.2 hours with placebo.
There were insufficient data for analysis of other doses of aspirin for this outcome.
Participants with any adverse event
The most frequently reported effects were dizziness, drowsiness, gastric irritation, nausea, and vomiting. Nearly all effects were of mild or moderate severity and only one study (Herbertson 1994) reported any withdrawals (8/217) due to "medical problems". In this study one event was considered possibly related to study medication (nausea and vomiting with the active comparator, diclofenac), while the other seven were most likely due to the surgical procedure itself.
Aspirin 600/650 mg versus placebo
Forty‐six studies (3633 participants) compared aspirin 600 or 650 mg with placebo.
The proportion of participants experiencing any adverse event with aspirin 600/650 mg was 11% (205/1791, range 0% to 33%).
The proportion of participants experiencing any adverse event with placebo was 9.5% (175/1842, range 0% to 38%).
The relative harm of treatment compared with placebo was 1.2 (1.0 to 1.4). There was no difference between treatment groups, and we did not calculate the number needed to treat to harm (NNH) (Analysis 2.5).
2.5. Analysis.

Comparison 2 Aspirin 600 or 650 mg versus placebo, Outcome 5 Any adverse event.
Aspirin 900/1000 mg versus placebo
Four studies (404 participants) compared aspirin 900 or 1000 mg with placebo (Forbes 1990a; Holland 1988; Lehnert 1990; Seymour 2003).
The proportion of participants experiencing any adverse event with aspirin 900/1000 mg was 26% (55/215, range 0% to 75%).
The proportion of participants experiencing any adverse event with placebo was 12% (23/189, range 0% to 41%).
The relative harm of treatment compared with placebo was 1.6 (1.1 to 2.3), giving a NNH of 7.5 (4.8 to 17) (Analysis 3.4).
3.4. Analysis.

Comparison 3 Aspirin 900 or 1000 mg versus placebo, Outcome 4 Any adverse event.
There were insufficient data for analysis of other doses of aspirin for participants experiencing any adverse event.
Discussion
Summary of main results
This updated review included essentially the same studies as the earlier review, adding one more recent study with 153 participants in comparisons of aspirin 900 mg with placebo and excluding five studies that were included in the earlier review because they included participants with pain due to trauma (Herrmann 1980c; Herrmann 1980d), fracture (Kantor 1965; Wang 1979), or fracture and musculoskeletal pain (Okun 1979), all outside the scope of this update. Results for the primary outcome of at least 50% total pain relief over four to six hours are unchanged for doses of 600/650 mg (number needed to treat (NNT) 4.2), 900/1000 mg (3.8), and 1200 mg (2.7), compared with placebo. There was no statistically significant difference between doses of 600/650 mg and 900/100 mg, or between 900/1000 mg and 1200 mg, but 1200 mg was significantly better than 600/650 mg (z = 2.416, P = 0.016) for this outcome, based on limited numbers of participants at the higher dose; dose response for analgesics is consistently difficult to determine except in direct comparisons (McQuay 2007). Doses below 500 mg were not significantly better than placebo.
Indirect comparison with other common analgesics, evaluated in the same way, indicate that aspirin 600/650 mg has comparable efficacy for at least 50% pain relief over four to six hours, to celecoxib 200 mg (NNT 4.2 (3.4 to 5.6); Derry 2008), ibuprofen 100 mg (4.3 (3.2 to 6.4); Derry 2009), and paracetamol 600/650 mg (4.6 (3.9 to 5.5); Toms 2008). This 'mg for mg' equivalence between 600 mg of aspirin and 600 mg paracetamol was also seen in a randomised direct comparison in 1965 (Houde 1965).
This review also considered number of participants requiring rescue medication within defined time periods, and time to use of rescue medication, as additional measures of analgesic efficacy. The NNT to prevent remedication was about 5 over four to eight hours after dosing for both 600/650 mg and 900/1000 mg doses, and not significantly different from placebo by 12 hours for the 600/650 mg dose. In most studies, participants were asked to wait for two hours after taking study medication before using rescue medication; the weighted mean of the median time to use of rescue medication was about five hours for 600/650 mg aspirin and three hours for placebo. The time to remedication with the 900/1000 mg dose was unexpectedly much shorter, at 1.9 hours for aspirin and 1.2 hours for placebo. The reason for much earlier remedication in these participants is unclear, but in one of the studies they could take rescue medication at any time, and in the other they were encouraged to wait for one hour.
There was no difference in the number of participants reporting any adverse event for aspirin 600/650 mg compared with placebo, but for 900/1200 mg significantly more aspirin than placebo treated participants experienced adverse events, with a number needed to treat to harm (NNH) of 7.5. Most events were mild or moderate in severity and transient (self limiting, did not require treatment), with only one study reporting any adverse event ("medical problems") withdrawals. Gastrointestinal events (e.g. gastric irritation) and central nervous system events (e.g. nausea) were more common with aspirin, but many of the events reported may be due to the anaesthetic and surgery, rather than the test drug.
Overall completeness and applicability of evidence
We carried out extensive bibliographic searches for published studies, and identified a large body of data. Studies followed standard methods and the great majority reported data for most of our prespecified outcomes. Single dose studies can tell us whether a drug is an effective analgesic, but do not tell us about the best dosing regimens, or how the drug performs or is tolerated in the longer term.
Adverse events were collected using various methods (spontaneous reporting, observed at clinic visits, questioning, patient diary) over different periods of time. This may have included periods after the use of rescue medication, which may cause its own adverse events. Poor reporting of adverse events in acute pain trials have been noted before (Edwards 1999a). The usefulness of single dose studies for assessing adverse events is questionable, but it is nonetheless reassuring that no serious adverse events were reported, and only one study reported withdrawals due to "medical problems". Long‐term multiple dose studies should be used for meaningful analysis of adverse events since, even in acute pain settings, analgesics are likely to be used in multiple doses. The difficulty will be that the postoperative setting is one in which there are many sequelae of surgery and anaesthesia that manifest as adverse events, like nausea, vomiting, or abdominal discomfort, while others, like headache, can be caused by acute caffeine withdrawal over the postoperative period. The main issue is that of rare but serious adverse events, and these are more likely to be found in large observational studies.
Quality of the evidence
Many of the studies were older, with only one published in the last 10 years, but 65/68 satisfied minimum criteria for adequate quality (≥ 3/5 on the Oxford Quality Scale), and 45/68 scored 4 or 5/5. All studies were randomised and double‐blind, with points lost due to failure to report sufficient detail of the methods of randomisation and blinding, which may reflect older reporting of methods rather than actual poor study quality. The variability seen between individual studies (Figure 3) is that to be expected for their size (Moore 1998).
Potential biases in the review process
We identified a large amount of information in studies that satisfied out inclusion criteria, but four studies could not be obtained in full copy in the UK, and we excluded a number of studies either because they did not describe their methods in sufficient detail to determine eligibility, or because they used non‐standard methods. It is also possible that there are data that we failed to identify, for example because they are unpublished. However, we think it unlikely that the amount of missing data is large enough to significantly affect the primary outcome, particularly for the 600/650 mg dose. An additional 4000 participants would have to have been involved in unpublished trials with zero treatment effects for the NNT for at least 50% pain relief to increase above 8, a level we consider to be the limit of clinical utility for this outcome (Moore 2008).
Agreements and disagreements with other studies or reviews
This review is in agreement with the earlier review (Edwards 1999b; Edwards 2000) for the outcomes they have in common.
Authors' conclusions
Implications for practice.
This updated review confirms that aspirin is an effective analgesic for acute postoperative pain of moderate to severe intensity. The 600/650 mg dose has comparable efficacy to the same dose of paracetamol, and a 1200 mg gives a better response. However, even in these single dose studies, adverse events such as gastric irritation and nausea were more common with aspirin than placebo at higher does.
Implications for research.
It is unlikely that further studies of this sort will be carried out for aspirin, and we have a sufficiently large body of evidence to be confident with the results for the 600/650 mg dose. In many parts of the world, use of aspirin as an analgesic is falling because of its known gastrointestinal effects.
What's new
| Date | Event | Description |
|---|---|---|
| 29 May 2019 | Amended | Contact details updated. |
| 11 October 2017 | Review declared as stable | No new studies likely to change the conclusions are expected. |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 3, 2000
| Date | Event | Description |
|---|---|---|
| 16 April 2015 | Review declared as stable | To be assessed for further updating in 2020. |
| 25 January 2012 | New citation required but conclusions have not changed | New search with slightly more restrictive inclusion criteria, which excluded a small number of studies that included participants with pain following trauma rather than surgery. One new study identified, and included (Seymour 2003). New outcomes relating to use of rescue medication added: number of participants using rescue medication within specified times, and mean or median time to use of rescue medication. These are additional measures of efficacy, providing information on duration of analgesia, that have been identified as clinically useful by healthcare workers. We excluded five studies that were included in the earlier review because they included participants with pain due to trauma (Herrmann 1980c; Herrmann 1980d), fracture (Kantor 1965; Wang 1979) or fracture and musculoskeletal pain (Okun 1979), which are outside the scope of this update. Results for participants with at least 50% pain relief over 4 to 6 hours, and participants experiencing adverse events, were not changed. The median time to use of rescue medication was about 5 hours with 600/650 mg aspirin, and the number needed to treat to prevent one participant needing rescue medication was 5 over 4 to 8 hours. |
| 25 January 2012 | New search has been performed | The search was brought up to date to January 2012. |
| 8 February 2011 | Amended | Contact details updated. |
| 24 September 2010 | Amended | Contact details updated. |
| 4 July 2010 | Amended | Jayne Rees reverted to Jayne Edwards so that Cochrane Library citations will match bibliographic databases outside Cochrane |
| 5 November 2008 | Amended | Minor amendment to what's new and to rest of text |
| 21 April 2008 | Amended | Converted to new review format and synopsis included. |
Acknowledgements
Jayne Edwards, Anna Oldman, Lesley A Smith, Sally L Collins, Dawn Carroll, Philip J Wiffen, Henry J McQuay, R Andrew Moore were authors on the original 1999 review.
Appendices
Appendix 1. Search strategy
Aspirin/
(aspirin OR acetylsalicylic acid).ti,ab,kw.
OR/1‐2
Postoperative pain/
((postoperative adj4 pain$) or (post‐operative adj4 pain$) or post‐operative‐pain$ or (post$ NEAR pain$) or (postoperative adj4 analgesi$) or (post‐operative adj4 analgesi$) or ("post‐operative analgesi$")).ti,ab,kw.
((post‐surgical adj4 pain$) or ("post surgical" adj4 pain$) or (post‐surgery adj4 pain$)).ti,ab,kw.
(("pain‐relief after surg$") or ("pain following surg$") or ("pain control after")).ti,ab,kw.
(("post surg$" or post‐surg$) AND (pain$ or discomfort)).ti,ab,kw.
((pain$ adj4 "after surg$") or (pain$ adj4 "after operat$") or (pain$ adj4 "follow$ operat$") or (pain$ adj4 "follow$ surg$")).ti,ab,kw.
((analgesi$ adj4 "after surg$") or (analgesi$ adj4 "after operat$") or (analgesi$ adj4 "follow$ operat$") or (analgesi$ adj4 "follow$ surg$")).ti,ab,kw.
OR/4‐10
clinical trials.sh.
controlled clinical trials.sh.
randomized controlled trial.sh.
double‐blind procedure.sh.
(clin$ adj25 trial$).ab.
((doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ab.
placebo$.ab.
random$.ab.
OR/12‐19
3 AND 11 AND 20
Appendix 2. Search strategy for EMBASE (via OVID)
Acetylsalicylic acid/
(aspirin OR acetylsalicylic acid).ti,ab,kw.
OR/1‐2
Postoperative pain/
((postoperative adj4 pain$) or (post‐operative adj4 pain$) or post‐operative‐pain$ or (post$ NEAR pain$) or (postoperative adj4 analgesi$) or (post‐operative adj4 analgesi$) or ("post‐operative analgesi$")).ti,ab,kw.
((post‐surgical adj4 pain$) or ("post surgical" adj4 pain$) or (post‐surgery adj4 pain$)).ti,ab,kw.
(("pain‐relief after surg$") or ("pain following surg$") or ("pain control after")).ti,ab,kw.
(("post surg$" or post‐surg$) AND (pain$ or discomfort)).ti,ab,kw.
((pain$ adj4 "after surg$") or (pain$ adj4 "after operat$") or (pain$ adj4 "follow$ operat$") or (pain$ adj4 "follow$ surg$")).ti,ab,kw.
((analgesi$ adj4 "after surg$") or (analgesi$ adj4 "after operat$") or (analgesi$ adj4 "follow$ operat$") or (analgesi$ adj4 "follow$ surg$")).ti,ab,kw.
OR/4‐10
clinical trials.sh.
controlled clinical trials.sh.
randomized controlled trial.sh.
double‐blind procedure.sh.
(clin$ adj25 trial$).ab.
((doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ab.
placebo$.ab.
random$.ab.
OR/12‐19
3 AND 11 AND 20
Appendix 3. Search strategy for CENTRAL
MESH descriptor Aspirin
(aspirin OR acetylsalicylic acid).ti,ab,kw.
OR/1‐2
MESH descriptor Pain, Postoperative
((postoperative adj4 pain$) or (post‐operative adj4 pain$) or post‐operative‐pain$ or (post$ NEAR pain$) or (postoperative adj4 analgesi$) or (post‐operative adj4 analgesi$) or ("post‐operative analgesi$")):ti,ab,kw.
((post‐surgical adj4 pain$) or ("post surgical" adj4 pain$) or (post‐surgery adj4 pain$)):ti,ab,kw.
(("pain‐relief after surg$") or ("pain following surg$") or ("pain control after")):ti,ab,kw.
(("post surg$" or post‐surg$) AND (pain$ or discomfort)):ti,ab,kw.
((pain$ adj4 "after surg$") or (pain$ adj4 "after operat$") or (pain$ adj4 "follow$ operat$") or (pain$ adj4 "follow$ surg$")):ti,ab,kw.
((analgesi$ adj4 "after surg$") or (analgesi$ adj4 "after operat$") or (analgesi$ adj4 "follow$ operat$") or (analgesi$ adj4 "follow$ surg$")):ti,ab,kw.
OR/4‐10
Clinical trials:pt.
Controlled Clinical Trial:pt.
Randomized Controlled Trial.pt.
MESH descriptor Double‐Blind Method
(clin$ adj25 trial$):ti,ab,kw.
((doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)):ti,ab,kw.
placebo$:ti,ab,kw.
random$:ti,ab,kw.
OR/12‐19
3 AND 11 AND 20
Appendix 4. Glossary
Categorical rating scale: the commonest is the five category scale (none, slight, moderate, good or lots, and complete). For analysis numbers are given to the verbal categories (for pain intensity, none = 0, mild = 1, moderate = 2, and severe = 3, and for relief none = 0, slight = 1, moderate = 2, good or lots = 3, and complete = 4). Data from different subjects are then combined to produce means (rarely medians) and measures of dispersion (usually standard errors of means). The validity of converting categories into numerical scores was checked by comparison with concurrent visual analogue scale measurements. Good correlation was found, especially between pain relief scales using cross‐modality matching techniques. Results are usually reported as continuous data, mean or median pain relief or intensity. Few studies present results as discrete data, giving the number of participants who report a certain level of pain intensity or relief at any given assessment point. The main advantages of the categorical scales are that they are quick and simple. The small number of descriptors may force the scorer to choose a particular category when none describes the pain satisfactorily.
VAS: visual analogue scale: lines with left end labelled "no relief of pain" and right end labelled "complete relief of pain", seem to overcome this limitation. Patients mark the line at the point which corresponds to their pain. The scores are obtained by measuring the distance between the no relief end and the patient's mark, usually in millimetres. The main advantages of VAS are that they are simple and quick to score, avoid imprecise descriptive terms and provide many points from which to choose. More concentration and co‐ordination are needed, which can be difficult postoperatively or with neurological disorders.
TOTPAR: total pain relief (TOTPAR) is calculated as the sum of pain relief scores over a period of time. If a patient had complete pain relief immediately after taking an analgesic, and maintained that level of pain relief for six hours, they would have a six‐hour TOTPAR of the maximum of 24. Differences between pain relief values at the start and end of a measurement period are dealt with by the composite trapezoidal rule. This is a simple method that approximately calculates the definite integral of the area under the pain relief curve by calculating the sum of the areas of several trapezoids that together closely approximate to the area under the curve.
SPID: summed pain intensity difference (SPID) is calculated as the sum of the differences between the pain scores over a period of time. Differences between pain intensity values at the start and end of a measurement period are dealt with by the trapezoidal rule.
VAS TOTPAR and VAS SPID are visual analogue versions of TOTPAR and SPID.
See 'Measuring pain' in Bandolier's Little Book of Pain, Oxford University Press, Oxford. 2003; pp 7‐13 (Moore 2003).
Appendix 5. Summary of outcomes: analgesia and rescue medication
| Analgesia | Rescue medication | ||||
| Study ID | Treatment | PI or PR | Number with 50% PR | Median time to use (h) | % using |
| Bloomfield 1967 | (1) aspirin 600 mg, n = 16 (2) placebo, n = 18 Also 3 doses of chlorphenesin (n = 50) | SPID 6 (1) 6.71 (2) 4.29 |
(1) 9/16 (2) 6/18 |
No data | No data |
| Boraks 1987 | (1) aspirin 650 mg, n = 41 (2) placebo, n = 39 Also flurbiprofen and dipyrone | TOTPAR 6 (1) 12.39 (2) 6.89 |
(1) 23/41 (2) 10/39 |
No data | Over 4 h: (1) 2.4% (2) 51% |
| Breivik 1984 | (1) aspirin 650 mg, n = 29 (2) placebo, n = 30 Also 2 doses of piroxicam | SPID 6 (1) 3.61 (2) 1.98 |
(1) 9/29 (2) 5/30 |
No data | Over 6 h: (1) 41% (2) 67% |
| Calimlim 1977 | (1) aspirin 650 mg, n = 23 (2) placebo, n = 26 Also 2 doses of pentazocine plus aspirin | TOTPAR 5 (1) 12.11 (2) 9.24 |
(1) 16/23 (2) 13/26 |
No data | Over 5 h: (1) 35% (2) 69% |
| Clark 1989 | (1) aspirin 600 mg, n = 40 (2) placebo, n = 40 Also 3 doses of carprofen, 1 of diflunisal | TOTPAR 6 (1) 7.5 (2) 4.4 |
(1) 12/40 (2) 5/40 |
(1) 4.7 (2) 3.8 | Over 6 h: (1) 55% (2) 65%, Over 8 h: (1) 60% (2) 78% Over 12 h: (1) 65% (2) 87% |
| Cooper 1977 | (1) aspirin 325 mg, n = 37 (2) aspirin 650 mg, n = 37 (3) placebo, n = 40 Also 2 doses ibuprofen | TOTPAR 4 (1) 5.35 (2) 3.36 |
(1) 13/37 (2) 7/40 |
No data | No data |
| Cooper 1979a | Study 2: (1) aspirin 600 mg, n = 47 (2) placebo, n = 58 Also 2 doses indoprofen (n = 96) | TOTPAR 4 (1) 8.4 (2) 4.3 |
(1) 27/47 (2) 14/58 |
No data | No data |
| Cooper 1982 | (1) aspirin 650 mg, n = 38
(2) placebo, n = 46
Also codeine, codeine plus aspirin, ibuprofen, codeine plus ibuprofen (n = 165) |
TOTPAR 4 (1) 5.66 (2) 2.65 |
(1) 14/38 (2) 5/46 |
Mean (1) 2.97 (2) 2.38 | No data |
| Cooper 1983 | (1) aspirin 650 mg, n = 43 (2) placebo, n = 44 Also 2 doses of suprofen (n = 89) | TOTPAR 6 (1) 11.0 (2) 7.27 |
(1) 21/43 (2) 13/44 |
Mean (1) 242 min (2) 194 min | No data |
| Cooper 1986a | Study C (1) aspirin 650 mg, n = 40 (2) placebo, n = 41 Also aspirin plus codeine and 2 doses of suprofen (n = 123) | TOTPAR 6 (1) 8.13 (2) 3.59 |
(1) 13/40 (2) 3/41 |
Mean (1) 165 min (2) 136 min | No data |
| Cooper 1988 | (1) aspirin 600 mg, n = 29 (2) placebo, n = 33 Also 3 doses of flurbiprofen (n = 89) | TOTPAR 6 (1) 4.61 (2) 2.60 |
(1) 4/29 (2) 1/33 |
Mean (1) 203 min (2) 142 min | No data |
| Cooper 1991b | (1) aspirin 650 mg, n = 46 (2) placebo, n = 48 Also 2 doses of meclofenamate | TOTPAR 6 (1) 6.22 (2) 3.79 |
(1) 11/46 (2) 5/48 |
Mean (1) 3.1 (2) 2.9 | No data |
| Cooper 1992 | (1) aspirin 650 mg, n = 28 (2) placebo, n = 26 Also 2 doses oxaprozin (n = 50) | SPID 6 (1) 1.67 (2) 0.19 |
(1) 4/28 (2) 0/26 |
(1) 2.8 (2) 1.8 | Over 6 h: (1) 80% (2) 88%, Over 8 h: (1) 92% (2) 92% |
| Coutinho 1976 | (1) aspirin 650 mg, n = 15 (2) placebo, n = 15 Also codeine, propoxyphene, 2 doses fenbufen (n = 60) | SPID 5 (1) 6.8 (2) 3.3 |
(1) 13/15 (2) 6/15 |
No data | No data |
| De Vroey 1977 | (1) aspirin 650 mg, n = 32 (2) placebo, n = 31 Also 3 doses diflunisal | SPID 6 (1) 8.1 (2) 2.87 |
(1) 19/32 (2) 1/31 |
No data | No data |
| Desjardins 1983a | (1) aspirin 650 mg, n = 40 (2) placebo, n = 39 Also codeine and propiram fumarate (n = 80) | TOTPAR 6 (1) 9.65 (2) 3.23 |
(1) 17/40 (2) 2/39 |
Mean (1) 184 min (2) 117 min | No data |
| Fliedner 1984 | (1) aspirin 650 mg, n = 83 (2) placebo, n = 87 Also 3 doses etodolac (n = 210) | TOTPAR 6 (1) 7.5 (2) 4.0 |
(1) 25/83 (2) 9/87 |
Aspirin longer than placebo | No data |
| Forbes 1980 | (1) aspirin 650 mg, n = 38 (2) placebo, n = 43 Also 3 doses proquazone (n = 129) | TOTPAR 4 (1) 6.08 (2) 2.4 |
(1) 15/38 (2) 4/43 |
Mean (1) 3.1 (2) 2.4 | No data |
| Forbes 1982 | (1) aspirin 650 mg, n = 42 (2) placebo, n = 38 | TOTPAR 4 (1) 6.26 (2) 4.21 |
(1) 17/42 (2) 9/38 |
Mean (1) 3.1 (2) 2.4 | Over 12 h: (1) 74% (2) 76% |
| Forbes 1983 | (1) aspirin 650 mg, n = 39 (2) placebo, n = 40 Also zomepirac and 2 doses of diflunisal (n = 120) | TOTPAR 6 (1) 7.46 (2) 4.07 |
(1) 12/39 (2) 4/40 |
Mean (1) 5.0 (2) 4.6 | Over 6 h: (1) 79% (2) 80% Over 8 h: (1) 90% (2) 83% Over 12 h: (1) 97% (2) 90% |
| Forbes 1984 | (1) aspirin 650 mg, n = 24 (2) placebo, n = 28 Also ibuprofen and fendosal (n = 57) | TOTPAR 6 (1) 6.67 (2) 3.79 |
(1) 6/24 (2) 3/28 |
Mean (1) 5.3 (2) 4.5 | Over 12 h: (1) 92% (2) 89% |
| Forbes 1986 | (1) aspirin 650 mg, n = 36 (2) placebo, n = 42 Also naproxen, codeine and naproxen plus codeine (n = 158) | TOTPAR 6 (1) 6.89 (2) 4.07 |
(1) 10/36 (2) 5/42 |
Mean (1) 5.4 (2) 5.3 | No data |
| Forbes 1989 | (1) aspirin 600 mg, n = 31 (2) placebo, n = 33 Also 3 doses flurbiprofen (n = 100) | TOTPAR 6 (1) 7.12 (2) 3.49 |
(1) 9/31 (2) 3/33 |
Mean (1) 5.1 (2) 3.3 | Over 8 h: (1) 77% (2) 94% |
| Forbes 1990a | (1) aspirin 650 mg, n = 68 (2) aspirin 1000 mg, n = 71 (3) placebo, n = 75 Also aspirin plus caffeine (n = 66) | TOTPAR 6 (1) 6.57 (2) 6.35 (3) 1.99 |
(1) 17/68 (2) 17/71 (3) 0/75 |
Mean (1) 3.9 (2) 3.9 (2) 2.8 | Over 6 h: (1) 65% (2) 77% (3) 93% |
| Forbes 1990b | (1) aspirin 650 mg, n = 32 (2) placebo, n = 32 Also ketorolac and paracetamol plus codeine (n = 64) | TOTPAR 6 (1) 6.5 (2) 2.91 |
(1) 8/32 (2) 1/32 |
Mean (1) 4.2 (2) 3.1 | Over 6 h: (1) 81% (2) 84% |
| Forbes 1991 | (1) aspirin 650 mg, n = 41 (2) placebo, n = 39 Also 3 doses bromfenac | TOTPAR 6 (1) 5.15 (2) 2.49 |
(1) 7/41 (2) 1/39 |
Mean (1) 3.5 (2) 2.8 | Over 8 h: (1) 98% (2) 97% |
| Forbes 1992 | (1) aspirin 650 mg, n = 38 (2) placebo, n = 38 Also ibuprofen and 3 doses bromfenac (n = 204) | TOTPAR 6 (1) 5.81 (2) 2.06 |
(1) 8/38 (2) 0/38 |
Mean (1) 3.9 (2) 2.7 | Over 8 h: (1) 97% (2) 97% |
| Frame 1986 | (1) aspirin 600 mg, n = 25 (2) placebo, n = 26 Also 3 doses ibuprofen plus codeine (n = 4) | TOTPAR 5 (1) 10.06 (2) 8.7 |
(1) 14/25 (2) 12/26 |
(1) 2.9 (2) 2.6 | Over 5 h: (1) 64% (2) 78% |
| Gaston 1984 | (1) aspirin 650 mg, n = 40 (2) placebo, n = 42 | TOTPAR 6 (1) 5.99 (2) 4.8 | (1) 9/40 (2) 6/42 |
No data | No data |
| Gaston 1986 | (1) aspirin 650 mg, n = 38 (2) placebo, n = 38 Also 3 doses etodolac (n = 113) | TOTPAR 6 (1) 5.8 (2) 3.9 | (1) 8/38 (2) 4/38 |
No data | No data |
| Herbertson 1994 | (1) aspirin 650 mg, n = 53 (2) placebo, n = 52 Also 2 doses diclofenac | TOTPAR 6 (1) 10.2 (2) 3.54 | (1) 23/50 (2) 4/47 |
(1) 4.4 (2) 1.4 | Over 6 h: (1) 70% (2) 80% Over 8 h: (1) 90% (2) 80% |
| Herrmann 1980a Study 1 | (1) aspirin 1000 mg, n = 50 (2) placebo, n = 50 Also 2 doses fluproquazone (n = 103) | TOTPAR 4 (1) 9.2 (2) 5.3 | (1) 32/50 (2) 16/50 |
No data | Over 4 h: (1) 20% (2) 50% |
| Herrmann 1980b Study 2 | (1) aspirin 1000 mg, n = 40 (2) placebo, n = 42 Also 2 doses fluproquazone (n = 86) | TOTPAR 4 (1) 7.0 (2) 2.62 | (1) 19/40 (2) 4/42 |
No data | Over 4 h: (1) 10% (2) 67% |
| Holland 1988 | (1) aspirin 600 mg, n = 20
(2) sol aspirin 600 mg, n = 20
(3) aspirin 900 mg, n = 20
(4) sol aspirin 900 mg, n = 20
(5) aspirin 1200 mg, n = 20
(6) sol aspirin 1200 mg, n = 20
(7) placebo, n = 20 (8) sol placebo, n = 20 |
VAS SPID 5 (1) 107.4 (2) 200.8 (3) 123.9 (4) 215.0 (5) 194.8 (6) 226.7 (7) 75.1 (8) 69.4 |
(1) 6/20 (2) 12/20 (3) 8/20 (4) 14/20 (5) 13/20 (6) 15/20 (7) 4/20 (8) 4/20 |
No data | No data |
| Honig 1978a | (1) aspirin 600 mg, n = 30 (2) placebo, n = 28 Also 3 doses diflunisal (n = 151) | SPID 6 (1) 3.74 (2) 0.96 |
(1) 9/30 (2) 1/28 |
No data | No data |
| Jain 1985a | (1) aspirin 600 mg, n = 30 (2) placebo, n = 30 Also 2 doses indoprofen (n = 60) | TOTPAR 5 (1) 11.1 (2) 4.0 | (1) 19/30 (2) 4/30 |
No data | No data |
| Jain 1985b | (1) aspirin 600 mg, n = 29 (2) placebo, n = 29 Also 3 doses indoprofen (n = 88) | TOTPAR 4 (1) 9.4 (2) 6.2 | (1) 19/29 (2) 12/29 |
No data | Over 4 h: (1) 10% (2) 41% |
| Jain 1986a | (1) aspirin 600 mg, n = 38 (2) placebo, n = 40 Also amfenac (n = 40) | TOTPAR 4 (1) 6.94 (2) 4.79 | (1) 17/37 (2) 11/39 |
No data | Over 4 h: (1) 37% (2) 60% |
| Jain 1986b | (1) aspirin 650 mg, n = 45 (2) placebo, n = 47 Also 3 doses ibuprofen (n = 136) | SPID 6 (1) 2.11 (2) ‐1.73 | (1) 6/45 (2) 0/47 |
Mean (1) 240 min (2) 121 min | Over 6 h: (1) 73% (2) 96% |
| Kempf 1987 | (1) aspirin buffered 600 mg, n = 24 (2) placebo, n = 24 Also 2 doses meclofenamate (n = 50) | TOTPAR 4 (1) 4.54 (2) 2.3 | (1) 6/24 (2) 2/23 |
No data | No data |
| Lehnert 1990 | (1) aspirin 1000 mg, n = 45 (2) placebo, n = 42 Also paracetamol 1000 mg (n = 49) | SPID 6 (1) 5.0 (2) 1.5 |
(1) 20/45 (2) 5/42 |
No data | Over 6 h: (1) 50% (2) 65% |
| London 1983a | (1) aspirin 650 mg, n = 40 (2) placebo, n = 40 Also 2 doses fluproquazone (n = 80) | PGE very good or excellent (1) 12/40 (2) 7/40 |
(1) 12/40 (2) 7/40 |
No data | Over 4 h: (1) 15% (2) 40% Over 6 h: (1) 28% (2) 63% |
| London 1983b | (1) aspirin 300 mg, n = 40 (2) aspirin 600 mg, n = 41 (3) aspirin 1200 mg, n = 40 (4) placebo, n = 39 | SPID 4 (1) 3.29 (2) 3.73 (3) 4.38 (4) 2.61 |
(1) 19/40 (2) 22/41 (3) 25/40 (4) 14/39 |
No data | Over 4 h: (1) 5% (2) 7% (3) 3% (4) 25% |
| Mardirossian 1985 | (1) aspirin 650 mg, n = 40 (2) placebo, n = 42 Also 2 doses of flurbiprofen (n = 82) | TOTPAR 6 (1) 7.15 (2) 3.29 | (1) 11/40 (2) 3/42 |
Mean (1) 216 min (2) 175 min | No data |
| Markowitz 1985 | (1) aspirin buffered 600 mg, n = 50 (2) placebo, n = 53 Also 2 doses meclofenamate (n = 102) | TOTPAR 6 (1) 4.74 (2) 1.87 | (1) 5/47 (2) 0/53 |
No data | No data |
| McQuay 1987 | (1) aspirin 650 mg, n = 30 (2) placebo, n = 30 Also 2 doses fluradoline (n = 60) | TOTPAR 6 (1) 5.62 (2) 2.57 | (1) 6/30 (2) 1/30 |
(1) 3.5 (2) 2.0 | Over 6 h: (1) 70% (2) 87% |
| Mehlisch 1984 | (1) aspirin 650 mg, n = 49 (2) placebo, n = 55 Also paracetamol (n = 58) | TOTPAR 6 (1) 5.51 (2) 1.75 | (1) 9/49 (2) 0/55 |
No data | Over 4 h: (1) 71% (2) 89% Over 6 h: (1) 76% (2) 95% |
| Mehlisch 1990 | (1) aspirin 650 mg, n = 40 (2) placebo, n = 41 Also 2 doses FS 205‐397 (n = 80) | TOTPAR 6 (1) 13.28 (2) 9.32 | (1) 25/40 (2) 16/41 |
Mean (1) 3.5 (2) 2.4 | No data |
| Mehlisch 1994 | (1) aspirin 650 mg, n = 51 (2) placebo, n = 52 Also 2 doses diclofenac (n = 105) | TOTPAR 6 (1) 8.53 (2) 3.09 | (1) 18/51 (2) 3/52 |
(1) 4.0 (2) 1.8 | Over 4 h: (1) 60% (2) 80% Over 6 h: (1) 75% (2) 87% Over 6 h: (1) 86% (2) 94% |
| Nelson 1985 | (1) aspirin 650 mg, n = 40 (2) placebo, n = 39 Also 3 doses etodolac (n = 122) | TOTPAR 6 (1) 6.13 (2) 3.28 | (1) 9/40 (2) 3/39 |
No data | No data |
| Nelson 1994a | (1) aspirin 500 mg, n = 65 (2) placebo, n = 41 Also ibu lysine (n = 77) | TOTPAR 6 (1) 7.65 (2) 5.56 | (1) 20/65 (2) 8/41 |
(1) 235 min (2) 174 min | Over 6 h: (1) 66% (2) 70% |
| Nelson 1994b | (1) aspirin 650 mg, n = 51 (2) placebo, n = 51 Also 3 doses diclofenac (153) | TOTPAR 6 (1) 11.3 (2) 3.65 | (1) 26/50 (2) 4/50 |
(1) 8.0 (2) 2.7 | Over 8 h: (1) 51% (2) 76% |
| Olsen 1997 | (1) aspirin 650 mg, n = 40 (2) placebo, n = 90 | TOTPAR 4 (1) 8.0 (2) 4.9 | (1) 27/50 (2) 15/52 |
(1) 3.5 (2) 2.3 | At 6 h: (1) 10% (2) 37% |
| Or 1988 | (1) aspirin 650 mg, n = 27 (2) placebo, n = 27 Also mefenamic acid and mefenamic acid plus aspirin (n = 54) | TOTPAR 4 (1) 8.3 (2) 5.1 | (1) 16/27 (2) 8/27 |
No data | No data |
| Parkhouse 1969 | (1) aspirin 600 mg tab, n = 82 (2) aspirin 600 mg fast buffered, n = 87 (2) placebo, n = 85 | TOTPAR 6 (1) 13.0 (2) 9.1 (3) 8.1 |
(1) 31/82 (2) 54/87 (3) 29/85 |
> 6 h for all treatments | Over 4 h: (1) 17% (2) 17% (3) 28% Over 6 h: (1) 28% (2) 28% (3) 40% |
| Patel 1991 | (1) aspirin 650 mg, n = 30 (2) placebo, n = 30 Also 3 doses lornoxicam (n = 90) | TOTPAR 6 (1) 8.9 (2) 7.8 | (1) 11/30 (2) 10/30 |
No data | No data |
| Rowe 1985 | (1) aspirin 600 mg, n = 43 (2) placebo, n = 41 Also 2 doses meclofenate | TOTPAR 6 (1) 5.84 (2) 3.47 | (1) 9/43 (2) 3/41 |
No data | No data |
| Seymour 1986 | (1) aspirin sol 1200 mg, n = 30 (2) aspirin tabs 1200 mg, n = 30 (3) placebo, n = 30 | SPID 5 | (1) 32/60 (2) 2/30 |
No data | No data |
| Seymour 1992 | (1) aspirin buffered 500 mg, n = 35 (2) aspirin buffered 1000 mg, n = 38 (3) aspirin tabs 500 mg, n = 35 (4) aspirin tabs 1000 mg, n = 37 (5) placebo, n = 37 | VAS SPID 5 (1) 65.0 (2) 72.25 (3) 117.5 (4) 91.5 (5) 88.25 |
(1)+(3) 25/70 (2)+(4) 25/75 (5) 12/37 |
(1) 135 min (2) 135 min (3) 100 min (4) 95 min (5) 70 min | Over 5 h: (1) 30/35 (2) 28/38 (3) 25/35 (4) 30/37 (5) 33/37 |
| Seymour 2003 | (1) aspirin 900 mg, n = 59 (2) placebo, n = 32 | VAS SPID 4 (1) 87.3 (2) 22.6 |
(1) 25/59 (2) 3/32 |
1) 1.9 2) 1.1 | At 4 hrs: 1) 81% 2) 91% |
| Sunshine 1983a | (1) aspirin 650 mg, n = 41 (2) placebo, n = 40 Also aspirin plus codeine, and 2 doses suprofen | TOTPAR 4 (1) 12.25 (2) 6.67 |
(1) 27/30 (2) 11/26 |
No data | Over 4 h: (1) 7% (2) 48% Over 6 h: (1) 7% (2) 48% |
| Sunshine 1983b | (1) aspirin 600 mg, n = 29 (2) placebo, n = 31 Also 3 doses flurbiprofen (n = 92) | TOTPAR 6 (1) 10.6 (2) 2.3 |
(1) 14/29 (2) 0/31 |
No data | Over 4 h: (1) 0% (2) 23% Over 6 h: (1) 3% (2) 45% |
| Sunshine 1983c | (1) aspirin 600 mg, n = 30 (2) placebo, n = 30 Also zomepirac and ibuprofen (n = 60) | TOTPAR 6 (1) 7.18 (2) 1.55 |
(1) 14/30 (2) 0/30 |
No data | Over 4 h: (1) 13% (2) 17% |
| Sunshine 1988 | (1) aspirin 648 mg, n = 15 (2) placebo, n = 15 Also 2 doses piroxicam (n = 30) | TOTPAR 6 (1) 15.3 (2) 7.3 |
(1) 11/15 (2) 4/15 |
(1) 9.1 (2) 5.6 | Over 24 h: (1) 87% (2) no data |
| Wang 1982 | (1) aspirin 650 mg, n = 2 (2) placebo, n = 25 Also 2 doses bicifadine (n = 50) | TOTPAR 6 (1) 8.95 (2) 3.83 |
(1) 10/25 (2) 2/25 |
No data | No data |
| Winter 1983a | (1) aspirin 650 mg, n = 37 (2) placebo, n = 35 Also oxaprozin (n = 33) | SPID 6 (1) 7.9 (2) 4.3 |
(1) 24/37 (2) 12/35 |
No data | Over 8 h: (1) 41% (2) 60% |
| Winter 1983b | (1) aspirin 650 mg, n = 42 (2) placebo, n = 44 Also phenyltoloxamine plus paracetamol (n = 41) | TOTPAR 4 (1) 6.19 (2) 4.76 |
(1) 17/42 (2) 12/44 |
No data | No data |
PGE = patient global assessment of efficacy; PI = pain intensity; PR = pain relief; SPID = summed pain intensity difference; TOTPAR = summed total pain relief; VAS = visual analogue scale
Appendix 6. Summary of outcomes: adverse events and withdrawals
| Adverse events | Withdrawals | ||||
| Study ID | Treatment | Any | Serious | Adverse event | Other |
| Bloomfield 1967 | (1) aspirin 600 mg, n = 16 (2) placebo, n = 18 Also 3 doses of chlorphenesin (n = 50) | (1) 3/16 (2) 1/18 | No data | No data | 4 pts randomised, but did not complete study and excluded from analyses |
| Boraks 1987 | (1) aspirin 650 mg, n = 41 (2) placebo, n = 39 Also flurbiprofen and dipyrone | No usable data | No data | No data | No data |
| Breivik 1984 | (1) aspirin 650 mg, n = 29 (2) placebo, n = 30 Also 2 doses of piroxicam | No GI discomfort or nausea, or other symptom attributable to study medication | None | None reported | None |
| Calimlim 1977 | (1) aspirin 650 mg, n = 23 (2) placebo, n = 26 Also 2 doses of pentazocine plus aspirin | No usable data | No data | None reported | 2 pts randomised but did not provide data (not linked to study med) |
| Clark 1989 | (1) aspirin 600 mg, n = 40 (2) placebo, n = 40 Also 3 doses of carprofen, 1 of diflunisal | (1) 9/40 (2) 4/40 Most mild or moderate | None reported | None | 30 pts did not take medication, 11 pts had invalid data due to protocol violations |
| Cooper 1977 | (1) aspirin 325 mg, n = 37 (2) aspirin 650 mg, n = 37 (3) placebo, n = 40 Also 2 doses ibuprofen | No significant difference between treatment groups | None | None | 53 pts excluded for uninterpretable form (17), confounding medication (12), loss to follow‐up (10), inadequate baseline pain (9), asleep after 1 h (5) |
| Cooper 1979a | Study 2: (1) aspirin 600 mg, n = 47 (2) placebo, n = 58 Also 2 doses indoprofen (n = 96) | (1) 8/54 (2) 0/61 All mild | None | None | 27 pts excluded for falling asleep (10), inaccurate timing (8), incorrect medication (7), confounding medication (2) |
| Cooper 1982 | (1) aspirin 650 mg, n = 38 (2) placebo, n = 46 Also codeine, codeine plus aspirin, ibuprofen, codeine plus ibuprofen (n = 165) | (1) 9/38 (2) 5/46 Most mild or moderate | None reported | None | 67 pts originally randomised excluded for loss to follow‐up (30), incorrect administration of medication (31), missing observations (6) |
| Cooper 1983 | (1) aspirin 650 mg, n = 43 (2) placebo, n = 44 Also 2 doses of suprofen (n = 89) | (1) 3/43 (2) 5/44 Generally "minor" | None reported | None | 60 pts originally randomised excluded for loss to follow‐up (3), did not take medication (37), took rescue medication < 1 h (7), other protocol violations (13) |
| Cooper 1986a | Study C (1) aspirin 650 mg, n = 40 (2) placebo, n = 41 Also aspirin plus codeine and 2 doses of suprofen (n = 123) | (1) 4/40 (2) 5/41 | None reported | None | 7 pts randomised, but excluded from analyses for loss to follow‐up (4), took rescue medication < 1 h (3) |
| Cooper 1988 | (1) aspirin 600 mg, n = 29 (2) placebo, n = 33 Also 3 doses of flurbiprofen (n = 89) | (1) 10/30 (2) 12/33 No unexpected events, none could be directly linked to study drugs | None reported | None | 8 pts randomised but excluded from analyses for loss to follow‐up, rescue medication < 1 h, asleep during observation times, uninterpretable responses, surgical complication, confounding medication, inappropriate administration, vomiting soon after taking medication |
| Cooper 1991b | (1) aspirin 650 mg, n = 46 (2) placebo, n = 48 Also 2 doses of meclofenamate | (1) 8/46 (2) 6/48 Most mild to moderate | None reported | None | 7 pts randomised, but excluded from analyses for protocol violations ‐ missing observations, early remedication |
| Cooper 1992 | (1) aspirin 650 mg, n = 28 (2) placebo, n = 26 Also 2 doses oxaprozin (n = 50) | (1) 3/28 (2) 3/26 All mild and transient | None | None | 8 pts randomised but excluded from analyses for loss to follow‐up, rescue medication < 1 h, insufficient baseline pain |
| Coutinho 1976 | (1) aspirin 650 mg, n = 15 (2) placebo, n = 15 Also codeine, propoxyphene, 2 doses fenbufen (n = 60) | (1) 4/15 (2) 0/15 But this assumes nobody had > 1 event | None | None reported | 21 pts did not complete trial ‐ no further details |
| De Vroey 1977 | (1) aspirin 650 mg, n = 32 (2) placebo, n = 31 Also 3 doses diflunisal | (1) 1/32 (2) 0/31 | None | None | LoE: (1) 0/32 (2) 3/31 |
| Desjardins 1983a | (1) aspirin 650 mg, n = 40 (2) placebo, n = 39 Also codeine and propiram fumarate (n = 80) | (1) 14/40 (2) 7/39 | None | None | 1 pt randomised to placebo was lost to follow up |
| Fliedner 1984 | (1) aspirin 650 mg, n = 83 (2) placebo, n = 87 Also 3 doses etodolac (n = 210) | (1) 13/85 (2) 9/87 Most mild and self limiting | None | None | 4 pts randomised, but excluded from analyses for loss to follow‐up and confounding medication LoE: 1 pt (etodolac) |
| Forbes 1980 | (1) aspirin 650 mg, n = 38 (2) placebo, n = 43 Also 3 doses proquazone (n = 129) | (1) 2/38 (2) 2/43 (before rescue medication) All transitory | None | None | 4 pts lost to follow‐up, 15 did not need analgesic, 20 pts had invalid efficacy data (fell asleep, early remedication, code broken, invalid entry, vomiting soon after taking study medication) |
| Forbes 1982 | (1) aspirin 650 mg, n = 42 (2) placebo, n = 38 | (1) 0/42 (2) no usable data All transient | None | None | 11 pts from all groups excluded from efficacy analyses due to protocol violations |
| Forbes 1983 | (1) aspirin 650 mg, n = 39 (2) placebo, n = 40 Also zomepirac and 2 doses of diflunisal (n = 120) | (1) 4/42 (2) 9/42 All transitory | None | None | 2 pts did not return forms, 11 did not need analgesic, 10 had invalid efficacy data (early remedication, did not follow instructions) |
| Forbes 1984 | (1) aspirin 650 mg, n = 24 (2) placebo, n = 28 Also ibuprofen and fendosal (n = 57) | (1) 3/25 (2) 3/30 All transitory, did not require treatment | None | None | 21 pts did not need analgesic, 6 had invalid efficacy data |
| Forbes 1986 | (1) aspirin 650 mg, n = 36 (2) placebo, n = 42 Also naproxen, codeine, and naproxen plus codeine (n = 158) | (1) 3/41 (2) 7/46 (before rescue medication) All transitory | None | None | 46 pts did not need analgesic, 24 had invalid efficacy data (inappropriate medication, incomplete evaluations, surgical complication) |
| Forbes 1989 | (1) aspirin 600 mg, n = 31 (2) placebo, n = 33 Also 3 doses flurbiprofen (n = 100) | (1) 5/38 (2) 1/39 (before rescue medication) All transitory | None | None | 26 pts did not need analgesic, 24 had invalid efficacy data (inappropriate medication or rescue medication, incomplete evaluations) |
| Forbes 1990a | (1) aspirin 650 mg, n = 68 (2) aspirin 1000 mg, n = 71 (3) placebo, n = 75 Also aspirin plus caffeine (n = 66) | (1) 8/68 (2) 5/71 (3) 6/75 (before rescue medication) All transitory, did not require treatment | None | None | 3 pts lost to follow‐up, 43 did not need analgesic, 51 had invalid efficacy data (inappropriate medication or rescue medication, inconsistent data, vomiting soon after taking medication) |
| Forbes 1990b | (1) aspirin 650 mg, n = 32 (2) placebo, n = 32 Also ketorolac and paracetamol plus codeine (n = 64) | (1) 4/38 (2) 5/34 (before 2nd dose) | None | None | 19 pts did not need analgesic, 14 had invalid efficacy data (inappropriate medication or rescue medication, vomiting soon after taking study medication) |
| Forbes 1991 | (1) aspirin 650 mg, n = 41 (2) placebo, n = 39 Also 3 doses bromfenac | (1) 3/46 (2) 3/47 (before rescue medication) All transitory, did not require treatment | None | None | 7 pts lost to follow up, 12 did not need analgesic, 28 had invalid efficacy data (inappropriate medication or rescue med, inconsistent or incomplete data) |
| Forbes 1992 | (1) aspirin 650 mg, n = 38 (2) placebo, n = 38 Also ibuprofen and 3 doses bromfenac (n = 204) | (1) 5/44 (2) 2/46 (before rescue medication) All transitory, did not require treatment | None | None | 3 pts lost to follow‐up, 14 did not need analgesic, 41 had invalid efficacy data (inappropriate rescue medication, incomplete or inconsistent data) |
| Frame 1986 | (1) aspirin 600 mg, n = 25 (2) placebo, n = 26 Also 3 doses ibuprofen plus codeine (n = 4) | (1) 2/25 (2) 1/26 | None | None | 6 pts did not need analgesic, 12 took medication incorrectly, 12 lost to follow‐up or incomplete data |
| Gaston 1984 | (1) aspirin 650 mg, n = 40 (2) placebo, n = 42 | (1) 1/40 (2) 0/42 | None | None | None reported |
| Gaston 1986 | (1) aspirin 650 mg, n = 38 (2) placebo, n = 38 Also 3 doses etodolac (n = 113) | (1) 3/38 (2) 3/38 Most mild to moderate, all self limiting | None | None | All pts who took drug were included in analyses |
| Herbertson 1994 | (1) aspirin 650 mg, n = 53 (2) placebo, n = 52 Also 2 doses diclofenac | "medical problems" (1) 5/54 (2) 2/54 All considered possibly drug‐related All mild or moderate | None | 8 pts withdrew due to "medical problems", only 1 was drug‐related (nausea + vomiting with diclofenac) | None reported |
| Herrmann 1980a Study 1 | (1) aspirin 1000 mg, n = 50 (2) placebo, n = 50 Also 2 doses fluproquazone (n = 103) | Data are provided for 4 studies combined, 2 of which are excluded studies | |||
| Herrmann 1980b Study 2 | (1) aspirin 1000 mg, n = 40 (2) placebo, n = 42 Also 2 doses fluproquazone (n = 86) | ||||
| Holland 1988 | (1) aspirin 600 mg, n = 20
(2) sol aspirin 600 mg, n = 20
(3) aspirin 900 mg, n = 20
(4) sol aspirin 900 mg, n = 20
(5) aspirin 1200 mg, n = 20
(6) sol aspirin 1200 mg, n = 20
(7) placebo, n = 20 (8) sol placebo, n = 20 |
(3) 1/20 (6) 2/20 Mild and transient | None | None | None |
| Honig 1978a | (1) aspirin 600 mg, n = 30 (2) placebo, n = 28 Also 3 doses diflunisal (n = 151) | (1) 0/30 (2) 0/28 | None | None | 5 pts randomised but excluded from efficacy due to failing to meet inclusion criteria (3) or reasons unrelated to study (2) LoE (very severe pain): (1) 5/30 (2) 10/28 |
| Jain 1985a | (1) aspirin 600 mg, n = 30 (2) placebo, n = 30 Also 2 doses indoprofen (n = 60) | None | None | None | None |
| Jain 1985b | (1) aspirin 600 mg, n = 29 (2) placebo, n = 29 Also 3 doses indoprofen (n = 88) | (1) 1/29 (2) 0/30 | None | None | 5 pts randomised but excluded from analyses due to early remedication (2), confounding medication (2), failure to follow instructions |
| Jain 1986a | (1) aspirin 600 mg, n = 38 (2) placebo, n = 40 Also amfenac (n = 40) | (1) 0/39 (2) 5/41 | None | None | 2 pts in (1) used confounding medication so excluded from all analyses 1 pt in each group had missing data so excluded from efficacy analyses |
| Jain 1986b | (1) aspirin 650 mg, n = 45 (2) placebo, n = 47 Also 3 doses ibuprofen (n = 136) | (1) 6/52 (2) 12/52 | None | None | 33 pts randomised but excluded from efficacy analyses due to early remedication (10), did not need analgesic or loss to follow‐up (19), missing data, confounding medication, mild baseline pain |
| Kempf 1987 | (1) aspirin buffered 600 mg, n = 24 (2) placebo, n = 24 Also 2 doses meclofenamate (n = 50) | (1) 3/26 (2) 1/26 | None | None | 7 pts had missing data for PI, 11 for PR ‐ excluded from efficacy analyses |
| Lehnert 1990 | (1) aspirin 1000 mg, n = 45 (2) placebo, n = 42 Also paracetamol 1000 mg (n = 49) | (1) 5/45 (2) 4/42 | None | None | 17 pts excluded from analyses due to loss to follow‐up (3), did not need analgesic (11), violation of procedures (3) |
| London 1983a | (1) aspirin 650 mg, n = 40 (2) placebo, n = 40 Also 2 doses fluproquazone (n = 80) | (1) 2/40 (2) 3/40 All mild or moderate | None | None | 6 pts randomised but excluded from analyses since did not follow protocol |
| London 1983b | (1) aspirin 300 mg, n = 40 (2) aspirin 600 mg, n = 41 (3) aspirin 1200 mg, n = 40 (4) placebo, n = 39 | None | None | None | No data |
| Mardirossian 1985 | (1) aspirin 650 mg, n = 40 (2) placebo, n = 42 Also 2 doses of flurbiprofen (n = 82) | (1) 13/40 (2) 16/42 No unexpected events, none could be directly linked to study drugs | None | None | 7 pts randomised but excluded from analyses for loss to follow‐up (2), inappropriate remedication (2), confounding medication (1), broke code (1), did not take medication (1) |
| Markowitz 1985 | (1) aspirin buffered 600 mg, n = 50 (2) placebo, n = 53 Also 2 doses meclofenamate (n = 102) | (1) 1/50
(2) 0/53 Generally mild |
None | None | 23 pts randomised but excluded from analyses due to loss to follow‐up, deemed unreliable, or taking confounding medication |
| McQuay 1987 | (1) aspirin 650 mg, n = 30 (2) placebo, n = 30 Also 2 doses fluradoline (n = 60) | (1) 9/30 (2) 11/30 | None | None | None |
| Mehlisch 1984 | (1) aspirin 650 mg, n = 49 (2) placebo, n = 55 Also paracetamol (n = 58) | No usable data No significant difference between aspirin and placebo | No data | None | 12 pts randomised but excluded from analyses because loss to follow‐up (3), did not comply with protocol (9) |
| Mehlisch 1990 | (1) aspirin 650 mg, n = 40 (2) placebo, n = 41 Also 2 doses FS 205‐397 (n = 80) | (1) 6/40 (2) 7/41 All mild or moderate | None | None | None |
| Mehlisch 1994 | (1) aspirin 650 mg, n = 51 (2) placebo, n = 52 Also 2 doses diclofenac (n = 105) | (1) 7/51 (2) 4/52 All mild or moderate | None | None | None |
| Nelson 1985 | (1) aspirin 650 mg, n = 40 (2) placebo, n = 39 Also 3 doses etodolac (n = 122) | No usable data No significant difference between aspirin and placebo | No data | None | 6 pts randomised but excluded from analyses because 3 lost to follow‐up, 3 dropped out before 1 h evaluation |
| Nelson 1994a | (1) aspirin 500 mg, n = 65 (2) placebo, n = 41 Also ibu lysine (n = 77) | (1) 13/65 (2) 11/41 | None | None | 3 pts randomised but excluded from efficacy analyses due to early remedication (2), failure to record baseline pain (1) |
| Nelson 1994b | (1) aspirin 650 mg, n = 51 (2) placebo, n = 51 Also 3 doses diclofenac (153) | (1) 4/51 (2) 6/51 | None | None | 3 pts excluded from efficacy analyses ‐ no reason given |
| Olsen 1997 | (1) aspirin 650 mg, n = 40 (2) placebo, n = 90 | Probably judged treatment‐related (1) 1/50 (2) 1/52 | None | None | None |
| Or 1988 | (1) aspirin 650 mg, n = 27 (2) placebo, n = 27 Also mefenamic acid and mefenamic acid plus aspirin (n = 54) | (1) 5/27 (2) 1/27 All mild and transient | None | None | 12 pts had invalid efficacy data, 8 did not follow protocol, 4 took confounding medication |
| Parkhouse 1969 | (1) aspirin 600 mg tab, n = 82 (2) aspirin 600 mg fast buffered, n = 87 (2) placebo, n = 85 | No usable data Most mild or moderate | No data | No data | No data |
| Patel 1991 | (1) aspirin 650 mg, n = 30 (2) placebo, n = 30 Also 3 doses lornoxicam (n = 90) | (1) 2/30 (2) 2/30 | None | None | Data reported for those who completed trial according to protocol |
| Rowe 1985 | (1) aspirin 600 mg, n = 43 (2) placebo, n = 41 Also 2 doses meclofenate | No usable data | None reported | None | Only LoE reported |
| Seymour 1986 | (1) aspirin sol 1200 mg, n = 30 (2) aspirin tabs 1200 mg, n = 30 (3) placebo, n = 30 | No data | No data | No data | No data |
| Seymour 1992 | (1) aspirin buffered 500 mg, n = 35 (2) aspirin buffered 1000 mg, n = 38 (3) aspirin tabs 500 mg, n = 35 (4) aspirin tabs 1000 mg, n = 37 (5) placebo, n = 37 | No data | No data | No data | No data |
| Seymour 2003 | (1) aspirin 900 mg, n = 59 (2) placebo, n = 32 | At 4 h: 1) 51/59 2) 28/32 | None | None | 14 pts enrolled but not randomised: 10 ‐ insufficient pain 2 ‐ withdrew consent 1 ‐ adverse reaction to anaesthetic 1‐ protocol violator |
| Sunshine 1983a | (1) aspirin 650 mg, n = 41 (2) placebo, n = 40 Also aspirin plus codeine, and 2 doses suprofen | (1) 3/42 (2) 1/40 | None | None | 46 pts participated in study twice. Results not used for efficacy, but included in safety. 9 pts excluded from efficacy analyses for early remedication |
| Sunshine 1983b | (1) aspirin 600 mg, n = 29 (2) placebo, n = 31 Also 3 doses flurbiprofen (n = 92) | No data | No data | None | 16 pts excluded from all analyses due to confounding medication |
| Sunshine 1983c | (1) aspirin 600 mg, n = 30 (2) placebo, n = 30 Also zomepirac and ibuprofen (n = 60) | None | None | None | None |
| Sunshine 1988 | (1) aspirin 648 mg, n = 15 (2) placebo, n = 15 Also 2 doses piroxicam (n = 30) | (1) 8/15 (2) 5/15 | None reported | None | 1 pt excluded due to history of drug abuse |
| Wang 1982 | (1) aspirin 650 mg, n = 2 (2) placebo, n = 25 Also 2 doses bicifadine (n = 50) | No data | No data | No data | No data |
| Winter 1983a | (1) aspirin 650 mg, n = 37 (2) placebo, n = 35 Also oxaprozin (n = 33) | No data | No data | None | 22 pts randomised but did not experience moderate/severe pain |
| Winter 1983b | (1) aspirin 650 mg, n = 42 (2) placebo, n = 44 Also phenyltoloxamine plus paracetamol (n = 41) | No usable data | None | None | 34 pts randomised but excluded from analyses due to lost to follow‐up, did not need analgesic, early remedication, fell asleep, confused answers |
Data and analyses
Comparison 1. Aspirin 500 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief | 2 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.82, 2.00] |
Comparison 2. Aspirin 600 or 650 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief | 59 | 4644 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [2.22, 2.72] |
| 1.1 Dental surgery | 43 | 3433 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [2.23, 2.88] |
| 1.2 Non‐dental surgery | 17 | 1211 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [1.93, 2.75] |
| 2 Participants using rescue medication at 4 to 5 h | 11 | 982 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.50, 0.67] |
| 3 Participants using rescue medication at 6 h | 20 | 1923 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.73, 0.82] |
| 4 Participants using rescue medication at 12 h | 4 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.05] |
| 5 Any adverse event | 46 | 3633 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.00, 1.44] |
Comparison 3. Aspirin 900 or 1000 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief | 6 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [2.00, 3.64] |
| 2 Participants using rescue medication at 4 to 5 h | 5 | 501 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.56, 0.74] |
| 3 Participants using rescue medication at 6 h | 2 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.71, 0.95] |
| 4 Any adverse event | 4 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.05, 2.30] |
Comparison 4. Aspirin 1200 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief | 3 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [1.95, 4.20] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bloomfield 1967.
| Methods | RCT, DB, single oral dose, parallel groups. Medication administered when pain was moderate or severe Assessed at 0, 1, 2, 3, 4, 5, 6 h |
|
| Participants | Episiotomy N = 100 (88 moderate/severe pain) Age 18 to 39 years |
|
| Interventions | Aspirin 600 mg, n = 17 Chlorphenesin 400 mg, n = 18 Chlorphenesin 800 mg, n = 17 Chlorphenesin 400 mg + aspirin 600 mg, n = 18 Placebo, n = 18 |
|
| Outcomes | PI: standard 4‐point scale Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" but method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identical black capsules" |
Boraks 1987.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 0.5, 1 h then hourly to 6 h | |
| Participants | Dental extraction N = 159 Age ≥ 16 years | |
| Interventions | Aspirin 650 mg, n = 41 Flurbiprofen 50 mg, n = 40 Dipyrone 500 mg, n = 39 Placebo, n = 39 Rescue medication allowed after 1 h (LOCF) |
|
| Outcomes | PI: standard 4‐point scale PR: 5‐point scale Global assessment Use of rescue medication |
|
| Notes | Language: Portuguese Oxford Quality Score: R = 1, DB = 1, W = 0. Total = 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
Breivik 1984.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 0.5, 1 h then hourly to 8 h | |
| Participants | Orthopaedic surgery (knee, ankle etc) N = 120 Age 18 to 65 years | |
| Interventions | Aspirin 650 mg, n = 29 Piroxicam 5 mg, n = 30 Piroxicam 10 mg, n = 31 Placebo, n = 30 Rescue medication allowed after 2 h (paracetamol plus codeine) |
|
| Outcomes | PI: standard 4‐point scale
PI: VAS (none to unbearable pain)
PR: standard 5‐point scale
Global assessment: 5‐point scale Use of rescue medication Adverse events |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 0. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double dummy |
Calimlim 1977.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 0.5, 1 h then hourly to 5 h | |
| Participants | Postsurgical patients (exact type of surgery not reported) N = 100 (98 included, but only 74 with global ratings) Age 21 to 65 years | |
| Interventions | Aspirin 650 mg, n = 23
Aspirin 325 mg + pentazocine 25 mg, n = 25
Aspirin 650 mg + pentazocine 50 mg, n = 50 Placebo, n = 26 Rescue medication generally not allowed before 3 h (standard analgesic) (BOCF) |
|
| Outcomes | PI: non‐standard 5‐point scale and VAS 20 cm (no pain at all ‐ worst pain ever experienced)
PR: standard 5‐point scale
Global assessment: 5‐point scale Use of rescue medication Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 1, W = 1. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
Clark 1989.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 12 h | |
| Participants | Oral surgery N = 248 Age 15 to 63 years | |
| Interventions | Aspirin 600 mg, n ˜40 Carprofen 75 mg, n = ˜40 Carprofen 100 mg, n = ˜40 Carprofen 150 mg, n = ˜40 Placebo, n ˜40 Rescue medication allowed after 1 h |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Use of rescue medication Adverse events |
|
| Notes | Oxford Quality Score: R = 1, DB = 1, W = 0. Total = 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
Cooper 1977.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 and hourly to 4 h | |
| Participants | Oral surgery ‐ impacted teeth N = 245 Age 16 to 35 years | |
| Interventions | Aspirin 325 mg, n = 37
Aspirin 650 mg, n = 37 Ibuprofen 200 mg, n = 38 Ibuprofen 400 mg, n = 40 Placebo, n = 40 Rescue medication allowed after 2 h (routine clinic medication) (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Serious adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Medications assigned using a card‐shuffling technique" |
| Allocation concealment (selection bias) | Low risk | "tablets in an envelope identified only by a sequential number" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "two identically appearing tablets" |
Cooper 1979a.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 4 h Study 2 | |
| Participants | Oral surgery N = 254 (201 for efficacy analyses) Age adult | |
| Interventions | Aspirin 600 mg, n = 47 Indoprofen 100 mg, n = 46 Indoprofen 200 mg, n = 50 Placebo, n = 58 Rescue medication allowed after 1 h (did not report data handling) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated codes" |
| Allocation concealment (selection bias) | Low risk | "medication was placed in an envelope identified only by a sequential number code" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identically‐appearing capsules" |
Cooper 1982.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 4 h | |
| Participants | Dental impaction N = 316 (249 for efficacy analyses) Age 16 to 65 years | |
| Interventions | Aspirin 650 mg, n = 38 Ibuprofen 400 mg, n = 38 Codeine 60 mg, n = 41 Aspirin 650 mg + codeine 60 mg, n = 45 Ibuprofen 400 mg + codeine 60 mg, n = 45 Placebo, n = 46 Rescue medication allowed after 1 h (LOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Tablets "appeared identical for every patient" |
Cooper 1983.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 0.5, 1 h then hourly to 6 h | |
| Participants | Peridontal treatment N = 199 (176 for efficacy analyses) Age adults | |
| Interventions | Aspirin 650 mg, n = 43 Suprofen 200 mg, n = 44 Suprofen 400 mg, n = 45 Placebo, n = 44 Rescue medication allowed after 1 h (LOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 1, W = 1. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
Cooper 1986.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0.5, 1 h then hourly to 6 h Study C | |
| Participants | Dental extraction (third molar) N = 211 (204 for efficacy analyses) Age 18 to 46 | |
| Interventions | Aspirin 650 mg, n = 40 Aspirin 650 mg + codeine 60 mg, n = 39 Suprofen 200 mg, n = 42 Suprofen 400 mg, n = 42 Placebo, n = 41 Rescue medication allowed after 1 h (paracetamol + codeine) (LOCF) |
|
| Outcomes | PI standard 4‐point scale
PR standard 5‐point scale
Global standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated" |
| Allocation concealment (selection bias) | Low risk | Identical bottles bearing sequential number |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐dummy technique" |
Cooper 1988.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 0.5, 1 h then hourly to 8 h | |
| Participants | Dental surgery (impacted third molars) N = 159 (151 for efficacy analyses) Age adult | |
| Interventions | Aspirin 600 mg, n = 29 Flurbiprofen 50 mg, n = 30 Flurbiprofen 100 mg, n = 30 Flurbiprofen 150 mg, n = 29 Placebo, n = 33 Rescue medication allowed after 1 h (LOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 1, W = 1. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Remote allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
Cooper 1991.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 0.5, 1 h then hourly to 6 h | |
| Participants | Dental surgery (impacted third molar) N = 189 (182 for efficacy) Age ≥ 16 years | |
| Interventions | Aspirin 650 mg, n = 46 Meclofenamate 50 mg, n = 40 Meclofenamate 100 mg, n = 48 Placebo, n = 48 Rescue medication allowed after 1 h (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization code" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identically appearing single doses" |
Cooper 1992.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0.5, 1 h then hourly to 8 h | |
| Participants | Dental surgery (impacted third molar) N = 112 (104 for efficacy analyses) Age 16 to 59 years | |
| Interventions | Aspirin 650 mg, n = 28 Oxaprozin 600 mg, n = 28 Oxaprozin 1200 mg, n = 22 placebo n = 26 |
|
| Outcomes | PI: standard 4‐point scale
PR: non‐standard 6‐point scale
Global assessment: 5‐point standard Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "tablets and their packaging were identical" |
Coutinho 1976.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 0.5, 1 h then hourly to 5 h | |
| Participants | Urogenital surgery (various) N = 90 Age 23 to 64 years | |
| Interventions | Aspirin 600 mg n = 15 Fenbufen 400 mg, n = 15 Fenbufen 800 mg, n = 16 Codeine 30 mg, n = 14 Propoxyphene 65 mg, n = 15 Placebo n = 15 Rescue medication allowed after 4 h (coded ‐ aspirin or fenbufen) (LOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale Adverse events |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 0. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization list" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identically‐appearing capsules, administered in a single oral dose" |
De Vroey 1977.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0 h then hourly for 6 to 8 h | |
| Participants | Episiotomy N = 156 Age 16 to 40 years | |
| Interventions | Aspirin 600 mg, n = 32 Diflunisal 125 mg, n = 33 Diflunisal 250 mg, n = 30 Diflunisal 500 mg, n = 30 Placebo, n = 31 Rescue medication allowed ‐ no further details (PI = 4 carried forward) |
|
| Outcomes | PI: standard 4‐point scale Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total =4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "individual patient‐coded vials" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "two identically appearing capsules" |
Desjardins 1984.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 0.5, 1 h then hourly to 6 h | |
| Participants | Dental extraction (impacted third molar) N = 160 (159 for efficacy analyses) Age 18 to 68 years | |
| Interventions | Aspirin 650 mg, n = 40 Propiram fumarate 50 mg, n = 40 Codeine 60 mg, n = 40 Placebo, n = 40 Rescue medication allowed ‐ participants asked to wait as long as possible |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "medications identical in appearance (two capsules)" |
Fliedner 1984.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0.5, 1 h then hourly to 12 h | |
| Participants | Dental extraction (impacted third molar) N = 384 (380 for efficacy analyses) Age not reported | |
| Interventions | Aspirin 650 mg, n = 83 Etodolac 50 mg, n = 37 Etodolac 100 mg, n = 87 Etodolac 200 mg, n = 86 Placebo n=87 Rescue medication allowed (no further details) (BOCF) |
|
| Outcomes | PI: non‐standard 5‐point scale
PR: 5‐point scale
Global assessment: non‐standard 4‐point scale Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 1, W = 1. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
Forbes 1980.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 4 h | |
| Participants | Dental surgery (impacted third molar) N = 247 Age ≥ 15 years | |
| Interventions | Aspirin 650 mg, n = 38 Proquazone 75 mg, n = 40 Proquazone 150 mg, n = 44 Proquazone 300 mg, n = 45 placebo n = 43 Rescue medication allowed after 1 h (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "tablets ..... packaged in envelopes identified only be individual numerical code" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All tablets were identical in appearance" |
Forbes 1982.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 12 h | |
| Participants | Dental surgery (multiple mandibular or impacted third molar) N = 212 (201 for efficacy analyses) Age ≥ 15 years | |
| Interventions | Aspirin 650 mg, n = 42 Diflunisal 250 mg, n = 39 Diflunisal 500 mg, n = 41 Diflunisal 1000 mg, n = 41 Placebo, n = 38 Rescue medication allowed after 2 h (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers list |
| Allocation concealment (selection bias) | Low risk | "treatments ... packaged in envelopes identified only by individual numerical code" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "treatments were identical in appearance" |
Forbes 1983.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 12 h | |
| Participants | Dental surgery (impacted third molar) N = 211 (199 for efficacy analyses) Age ≥ 15 years | |
| Interventions | Aspirin 650 mg, n = 39 Diflunisal 500 mg, n = 39 Diflunisal 1000 mg, n = 40 Zomepirac 100 mg, n = 41 Placebo n = 40 Rescue medication (Tylenol) allowed after 2 h (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers list |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double dummy technique" |
Forbes 1984.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 12 h | |
| Participants | Dental surgery (impacted third molar) N = 115 (109 for efficacy analyses) Age ≥ 15 years | |
| Interventions | Aspirin 650 mg n = 24 Fendosal 200 mg, n = 29 Ibuprofen 400 mg, n = 28 Placebo, n = 28 Rescue medication allowed after 2 h (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers list |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
Forbes 1986.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 12 h | |
| Participants | Dental surgery (impacted third molar) N = 222 (198 for efficacy analyses) Age ≥ 15 years | |
| Interventions | Aspirin 650 mg, n = 36 Naproxen 550 mg, n = 38 Codeine 60 mg, n = 44 Naproxen 550 mg + codeine 60 mg, n = 38 Placebo, n = 42 Rescue medication (paracetamol + codeine) allowed after 2 h (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list |
| Allocation concealment (selection bias) | Low risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
Forbes 1989.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 8 h | |
| Participants | Dental surgery (impacted third molar) N = 191 (164 for efficacy) Age ≥ 15 years | |
| Interventions | Aspirin 600 mg, n = 31 Flurbiprofen 25 mg, n = 31 Flurbiprofen 50 mg, n = 33 Flurbiprofen 100 mg, n = 36 Placebo n = 33 Rescue medication (paracetamol + codeine) allowed after 2 h (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "all tablets were identical in appearance" |
Forbes 1990a.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 6 | |
| Participants | Dental surgery (impacted third molar) N = 404 (350 for efficacy analyses) Age ≥ 15 years | |
| Interventions | Aspirin 650 mg, n = 68
Aspirin 1000 mg, n = 71 Caffeine 65 mg, n = 70 Aspirin 650 mg + caffeine 65 mg, n = 66 Placebo n = 75 Rescue medication allowed after 2 h (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "all tablets were identical in appearance" |
Forbes 1990b.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 6 h | |
| Participants | Dental surgery (impacted third molar) N = 153 (128 for efficacy analyses) Age ≥ 15 years | |
| Interventions | Aspirin 650 mg, n = 32 Ketorolac 10 mg, n = 37 Paracetamol 600 mg + codeine 60 mg, n = 27 Placebo n = 32 Rescue medication (Tylenol) allowed after 2 h (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "capsules were identical in appearance" |
Forbes 1991.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 8 h | |
| Participants | Dental surgery (impacted third molar) N = 276 (241 for efficacy analyses) Age 15 to 44 years | |
| Interventions | Aspirin 650 mg, n = 41 Ibuprofen 400 mg, n = 37 Bromfenac 5 mg, n = 39 Bromfenac 10 mg, n = 43 Bromfenac 25 mg, n = 42 Placebo n = 39 Rescue medication (Vicodin) allowed after 2 h (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "all capsules were identical in appearance" |
Forbes 1992.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 8 h | |
| Participants | Dental surgery (impacted third molar) N = 324 (280 for efficacy analyses) Age 15 to 56 years | |
| Interventions | Aspirin 650 mg, n = 38 Ibuprofen 400 mg, n = 38 Bromfenac 10 mg, n = 43 Bromfenac 25 mg, n = 41 Bromfenac 50 mg, n = 42 Bromfenac 100 mg, n = 40 Placebo n = 38 Rescue medication (Vicodin) allowed after 2 h (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "all capsules were identical in appearance" |
Frame 1986.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 5 h | |
| Participants | Dental surgery (impacted third molar) N = 159 (141 for efficacy analyses) Mean age 24 years | |
| Interventions | Aspirin 600 mg, n = 25 Ibuprofen 200 mg + codeine 15 mg, n = 32 Ibuprofen 400 mg + codeine 30 mg, n = 32 Ibuprofen 800 mg + codeine 60 mg, n = 26 Placebo n = 26 Rescue medication (paracetamol) allowed after 2 h |
|
| Outcomes | PI: non‐standard 9‐point scale
PR: standard 5‐point scale
Global assessment (no scale reported) Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Sealed sachets |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "aspirin specially formulated to match the other drugs" |
Gaston 1984.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 0.5, 1 h then hourly to 8 h | |
| Participants | Oral surgery N = 161 Mean age 27 years |
|
| Interventions | Aspirin 650 mg, n = 40 Etodolac 50 mg, n = 39 Etodolac 200 mg, n = 40 Placebo, n = 42 Rescue medication allowed after 1 h (BOCF) |
|
| Outcomes | PI: non‐standard 5‐point scale PR: 5‐point scale Global assessment: non‐standard 4‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 1, W = 1. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
Gaston 1986.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 0.5, 1 h then hourly to 12 h | |
| Participants | Dental surgery (impacted third molar) N = 189 Age 18 to 48 years | |
| Interventions | Aspirin 650 mg, n = 38 Etodolac 50 mg, n = 37 Etodolac 100 mg, n = 38 Etodolac 200 mg, n = 38 Placebo n = 38 Rescue medication allowed after 1 h (BOCF) |
|
| Outcomes | PI: non‐standard 5‐point scale
PR: 5‐point scale
Global assessment: non‐standard 4‐point scale
Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "a randomization code was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | Patients allocated sequential code numbers based on order of admission to study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All medications were in the form of identical tablets" |
Herbertson 1994.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 0.5, 1 h then hourly to 8 h | |
| Participants | Gynaecological surgery N = 217 (209 for efficacy analyses) Mean age 43 years | |
| Interventions | Aspirin 650 mg, n = 53 Diclofenac 50 mg, n = 52 Diclofenac 100 mg, n = 52 Placebo n = 52 Rescue medication allowed after 1 h |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 1, W = 0. Total = 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
Herrmann 1980a.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1, 2, 3, 4 h Study 1 | |
| Participants | Episiotomy (not uterine cramps) N = 203 Mean age 27 years | |
| Interventions | Aspirin 1000 mg, n= 50 Fluproquazone 75 mg, n = 52 Fluproquazone 150 mg, n = 51 Placebo n = 50 Rescue medication allowed after 1 h (LOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale Adverse events (combined for 4 studies) |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 0. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | All medication "prepackaged in code‐numbered individual envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All capsules were identical in appearance" |
Herrmann 1980b.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1, 2, 3, 4 h Study 2 | |
| Participants | Episiotomy (not uterine cramps) N = 168 Mean age 27 years | |
| Interventions | Aspirin 1000 mg, n = 40 Fluproquazone 100 mg, n = 42 Fluproquazone 200 mg, n = 44 Placebo, n = 42 |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale Adverse events (combined for 4 studies) |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 0. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | All medication "prepackaged in code‐numbered individual envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All capsules were identical in appearance" |
Holland 1988.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 0.25, 0.5, 0.75, 1, 1.5, 2 h then hourly to 5 h | |
| Participants | Dental surgery (impacted third molar) N = 140 Age 18 to 35 years | |
| Interventions | Soluble aspirin 600 mg, n = 20
Soluble aspirin 900 mg, n = 20
Soluble aspirin 1200 mg, n = 20
Aspirin 600 mg, n = 20
Aspirin 900 mg, n = 20
Aspirin 1200 mg, n = 20
Placebo, n = 20 Rescue medication (paracetamol) allowed ‐ no further details |
|
| Outcomes | PI: 100 mm VAS Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double dummy technique" |
Honig 1978.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 8 h | |
| Participants | Meniscectomy N = 145 Mean age ˜30 years | |
| Interventions | Aspirin 600 mg, n = 30 Diflunisal 125 mg, n = 28 Diflunisal 250 mg, n = 29 Diflunisal 500 mg, n = 30 Placebo n = 28 Rescue medication allowed (time not specified) (participants assigned PI = severe for analysis) |
|
| Outcomes | PI: standard 4‐point scale
Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Pre‐packaged in individual patient‐coded vials |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All capsules were identical in appearance" |
Jain 1985a.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 0.5, 1 h then hourly to 5 h | |
| Participants | Episiotomy N = 120 Mean age 23 years | |
| Interventions | Aspirin 600 mg, n = 30 Indoprofen 50 mg, n = 30 Indoprofen 100 mg, n = 30 Placebo n = 30 Rescue medication allowed (time not specified) (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: non‐standard 4‐point scale
Rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "matching capsules" |
Jain 1985b.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 0.5, 1 h then hourly to 4 h | |
| Participants | Dental surgery (impacted third molar) N = 151 (146 for efficacy analyses) Mean age 34 years | |
| Interventions | Aspirin 600 mg, n = 29 Indoprofen 50 mg, n = 29 Indoprofen 100 mg, n = 30 Indoprofen 200 mg, n = 29 Placebo n = 29 Rescue medication allowed after 2 h (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 1, W = 1. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
Jain 1986a.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1, 2, 3, 4 h | |
| Participants | Dental surgery (impacted third molar) N = 120 Mean age 24 years | |
| Interventions | Aspirin 600 mg, n = 38 Amfenac 100 mg, n = 40 Placebo n = 40 Rescue medication allowed after 2 h (BOCF) |
|
| Outcomes | PI: standard 4‐point scale and 10 cm VAS
PR: standard 5‐point scale
Global assessment: non‐standard 4‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 1, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random number" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
Jain 1986b.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 6 h | |
| Participants | Dental surgery (impacted third molar) N = 241 (227 for efficacy analyses) Mean age 23 years | |
| Interventions | Aspirin 650 mg, n = 45 Ibuprofen 100 mg, n = 39 Ibuprofen 200 mg, n = 47 Ibuprofen 400 mg, n = 49 Placebo n = 47 Rescue medication allowed after 1 h (LOCF) |
|
| Outcomes | PI: standard 4‐point scale, 100 mm VAS
PR: non‐standard 6‐point scale
Global assessment: 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list |
| Allocation concealment (selection bias) | Low risk | Bottle identified only by a sequential number |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identically appearing tablets" |
Kempf 1987.
| Methods | RCT, DB, single oral dose (followed by multiple dose phase), parallel groups Assessed at 0, 0.5, 1 hourly to 6 | |
| Participants | Dental surgery (impacted third molar) N = 105 (98 for efficacy analysis) Age 18 to 60 years | |
| Interventions | Buffered aspirin 600 mg, n = 24 Meclofenamate 100 mg, n = 24 Meclofenamate 200 mg, n = 26 Placebo, n = 24 Second dose allowed for inadequate response after 4 h Rescue medication (paracetamol + codeine) allowed after 1 h (LOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: non‐standard 3‐point scale
Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identically appearing capsules" |
Lehnert 1990.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0.5, 1 h then hourly to 6 h | |
| Participants | Dental surgery (impacted third molar) N = 150 (139 for efficacy analyses) Age 17 to 54 years | |
| Interventions | Aspirin 1000 mg, n = 45 Paracetamol 1000 mg, n = 49 Placebo, n = 42 Rescue medication allowed (no further details) (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: non‐standard 4‐point scale
Global assessment: non‐standard 4‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Language: German Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All capsules were identical in appearance |
London 1983a.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 6 h | |
| Participants | Episiotomy N = 166 (160 for efficacy) Age 18 to 40 | |
| Interventions | Aspirin 650 mg, n = 40 Fluproquazone 100 mg, n = 41 Fluproquazone 200 mg, n = 39 Placebo, n = 40 Rescue medication allowed ‐ no further details |
|
| Outcomes | Global assessment: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identical capsules" |
London 1983b.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0.5, 1 h then hourly to 4 h | |
| Participants | Episiotomy N = 160 Age 18 to 36 years | |
| Interventions | Aspirin 300 mg, n = 40
Aspirin 600 mg, n = 41
Aspirin 1200 mg, n = 40
Placebo, n = 39 Rescue medication allowed, data up to point of remedication used for analysis |
|
| Outcomes | PI: standard 4‐point scale Use of rescue medication Adverse events |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 0. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Study medication "individually packaged and identified only by study number and patient number" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "all tablets appeared identical" |
Mardirossian 1985.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0.5, 1 h then hourly to 6 h | |
| Participants | Dental surgery (impacted third molar) N = 171 (164 analysed) Mean age 24 years | |
| Interventions | Aspirin 650 mg, n = 40 Flurbiprofen 25 mg, n = 39 Flurbiprofen 50 mg, n = 43 Placebo, n = 42 Rescue medication allowed after 1 h (LOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: non‐standard 4‐point scale Use of rescue medication Adverse events |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Remote allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identically appearing medications" |
Markowitz 1985.
| Methods | RCT, DB, single oral dose (followed by multiple dose phase), parallel groups Assessed at 0.5, 1 h then hourly to 6 h | |
| Participants | Dental surgery (impacted third molar) N = 225 (205 analysed) Mean age 24 years | |
| Interventions | Buffered aspirin 600 mg, n = 50 Meclofenamate 100 mg, n = 51 Meclofenamate 200 mg, n = 51 Placebo, n = 53 Rescue medication allowed after 1 h (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: non‐standard
Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 1, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization code" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
McQuay 1987.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0.5, 1, 1.5, 2, h then hourly to 6 h | |
| Participants | Orthopaedic surgery N = 120 Age 18 to 70 years | |
| Interventions | Aspirin 650 mg, n = 30 Fluradoline 150 mg, n = 30 Fluradoline 300 mg, n = 30 Placebo, n = 30 Rescue medication allowed after 1 h (BOCF) |
|
| Outcomes | PI: standard 4‐point scale, 100 mm VAS
PR: standard 5‐point scale, 100 mm VAS
Global assessment: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All capsules "identical in external appearance" |
Mehlisch 1984.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0.5, 1 h then hourly to 6 h | |
| Participants | Dental surgery (impacted third molar) N = 162 Age 16 to 78 years | |
| Interventions | Aspirin 650 mg, n = 49 Paracetamol 1000 mg, n = 58 Placebo, n = 55 Rescue medication allowed after 1 h (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Use of rescue medication Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "capsules were identical in appearance" |
Mehlisch 1990.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0.5, 1 h then hourly to 6 h | |
| Participants | Dental surgery (impacted third molar) N = 161 Mean age 24 years | |
| Interventions | Aspirin 650 mg, n = 40 FS205‐397 250 mg, n = 40 FS205‐397 500 mg, n = 40 Placebo, n = 41 |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐derived randomization schedule" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identically appearing two‐capsule doses" |
Mehlisch 1994.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0.5, 1 h then hourly to 8 h | |
| Participants | Dental surgery (impacted third molar) N = 208 Age 20 to 90 years | |
| Interventions | Aspirin 650 mg, n = 51 Diclofenac 50 mg, n = 53 Diclofenac 100 mg, n = 52 placebo, n = 52 |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale Global assessment: non‐standard 3‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 1, W = 1. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
Nelson 1985.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0.5, 1 h then hourly to 12 h | |
| Participants | Dental surgery (impacted third molar) N = 207 (201 for analyses) Age 17 to 45 years | |
| Interventions | Aspirin 650 mg, n = 40 Etodolac 50 mg, n = 41 Etodolac 100 mg, n = 42 Etodolac 200 mg, n = 39 Placebo, n = 39 Rescue medication allowed (time not specified) (BOCF) |
|
| Outcomes | PI: non‐standard 5‐point scale PR: 5‐point scale Global assessment: non‐standard 4‐point scale Withdrawals | |
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "two tablets which appeared identical" |
Nelson 1994a.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0.25, 0.5, 0.75, 1, 1.5, 2 h then hourly to 6 h | |
| Participants | Dental surgery (impacted third molar) N = 183 (180 for efficacy analyses) Age 15 to 52 years | |
| Interventions | Aspirin 500 mg, n = 65 Ibuprofen lysine 200 mg, n = 77 Placebo, n = 41 Rescue medication allowed after 1 h (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "allocation schedule of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy method |
Nelson 1994b.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0.5, 1 h then hourly to 8 h | |
| Participants | Dental surgery (impacted third molar) N = 255 (252 for analyses) Age 16 to 70 years | |
| Interventions | Aspirin 650 mg, n = 51 Diclofenac 25 mg, n = 51 Diclofenac 50 mg, n = 51 Diclofenac 100 mg, n = 51 Placebo, n = 51 Rescue medication allowed after 1 h ‐ no further details |
|
| Outcomes | PI: standard 4‐point scale
PR: 5‐point scale Use of rescue medication Adverse events |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 0. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐dummy technique" |
Olsen 1997.
| Methods | RCT, DB, single oral dose, parallel groups. Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 0.5, 1 h, then hourly to 8 h |
|
| Participants | Episiotomy N = 255 All F Mean age 24 years |
|
| Interventions | Aspirin 650 mg, n = 50 Diclofenac K 25 mg, n = 52 Diclofenac K 50 mg, n = 50 Diclofenac K 100 mg, n = 51 Placebo, n = 52 Rescue medication (non‐study analgesic) allowed after 1 h |
|
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale Global assessment: non‐standard 4‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
Or 1988.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1, 2, 3, 4 h | |
| Participants | Dental surgery (impacted third molar) N = 120 Mean age 26 years | |
| Interventions | Aspirin 650 mg, n = 27 Mefenamic acid 250 mg, n = 27 Mefenamic acid 250 mg plus aspirin 650 mg, n = 27 Placebo, n = 27 |
|
| Outcomes | PI: non‐standard 5‐point scale, 100 mm VAS
PR: standard 5‐point scale
Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identically‐appearing two‐capsule doses" |
Parkhouse 1969.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0. 0.5, 1 h then hourly to 6 h |
|
| Participants | Postoperative pain | |
| Interventions | Hospital A Aspirin fast‐buffered 600 mg, n = 45 Aspirin standard tablets 600 mg, n = 45 Placebo, n = 46 Hospital B Aspirin fast‐buffered 600 mg, n = 42 Aspirin standard tablets 600 mg, n = 37 Placebo, n = 39 Rescue medication allowed at any time |
|
| Outcomes | PI: standard 4‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identically formulated" |
Patel 1991.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0.5, 1 h then hourly to 8 h | |
| Participants | Dental surgery (impacted third molar) N = 150 Mean age 24 years | |
| Interventions | Aspirin 650 mg, n = 30 Lornoxicam 2 mg, n = 30 Lornoxicam 4 mg, n = 30 Lornoxicam 8 mg, n = 30 Placebo, n = 30 Rescue medication allowed at any time (LOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale
Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 1, W = 1. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
Rowe 1985.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0.5, 1 h then hourly to 6 h | |
| Participants | Dental surgery (impacted third molar) N = 174 (167 for efficacy analyses) Age 17 to 58 years | |
| Interventions | Buffered aspirin 600 mg, n = 43 Meclofenamate 100 mg, n = 44 Meclofenamate 200 mg, n = 39 Placebo, n = 41 Rescue medication allowed after 1 h (no further details) |
|
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale Global assessment: scale not reported Adverse events | |
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 0. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identical appearing medications" |
Seymour 1986.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0.25, 0.5, 0.45, 1, 1.5, 2 h then hourly to 5 h | |
| Participants | Dental surgery (impacted third molar) N = 90 Age 17 to 40 years | |
| Interventions | Soluble aspirin 1200 mg, n = 30 Aspirin tablets 1200 mg, n = 30 Placebo, n = 30 | |
| Outcomes | PI: 100 mm VAS | |
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 0. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐dummy technique" |
Seymour 1992.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0.25, 0.5, 0.75, 1, 1.5, 2 h then hourly to 5 h | |
| Participants | Dental surgery (impacted third molar) N = 182 Mean age 26 years | |
| Interventions | Buffered aspirin 500 mg, n = 35 Buffered aspirin 1000 mg, n = 38 Aspirin tablets 500 mg, n = 35 Aspirin tablets 1000 mg, n = 37 Placebo, n = 37 Rescue medication allowed (LOCF) |
|
| Outcomes | PI: 100 mm VAS
Global assessment: 5‐point scale Use of rescue medication |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 0. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐dummy technique" |
Seymour 2003.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 0.08, 0.17, 0.25, 0.33, 0.5, 0.75, 1, 1.5, 2 h | |
| Participants | Dental surgery (impacted third molar) N = 153 Mean age 25 years | |
| Interventions | Soluble aspirin 900 mg, n = 59 Paracetamol tablets 1000 mg, n = 62 Placebo, n = 32 Rescue medication (ibuprofen 400 mg) allowed after 1 h (LOCF) |
|
| Outcomes | PI: 100 mm VAS Global assessment: 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐dummy method" |
Sunshine 1983a.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 0.5, 1 h then hourly to 6 h | |
| Participants | Dental surgery (impacted third molars) N = 148 Mean age 23 years | |
| Interventions | Aspirin 650 mg, n = 41 Aspirin 650 mg + codeine 60 mg, n = 41 Suprofen 200 mg, n = 40 Suprofen 400 mg, n = 41 Placebo, n = 40 Rescue medication allowed after 2 h (LOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identically‐appearing capsules" |
Sunshine 1983b.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 6 h | |
| Participants | Episiotomy N = 168 (152 for analysis) Mean age 24 years | |
| Interventions | Aspirin 600 mg, n = 29 Flurbiprofen 25 mg, n = 32 Flurbiprofen 50 mg, n = 29 Flurbiprofen 100 mg, n = 31 Placebo, n = 31 Rescue medication allowed after 1 h (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale Use of rescue medication Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Tablets were "identical in appearance" |
Sunshine 1983c.
| Methods | RCT, DB, single oral dose, parallel groups
Assessed at 0, 0.5, 1 h then hourly to 4 h Medication administered when pain severe |
|
| Participants | Episiotomy N = 120 Mean age 24 years | |
| Interventions | Aspirin 600 mg, n = 30 Ibuprofen 400 mg, n = 30 Zomepirac 100 mg, n = 30 Placebo, n = 30 Rescue medication allowed after 1 h (LOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: 5‐point scale
Global assessment: non‐standard 7‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐dummy technique" |
Sunshine 1988.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 12 h | |
| Participants | Study 2 Mixed general surgery (35%) and gynaecological surgery 65%) N = 61 (60 for analysis) Age 31 to 33 years |
|
| Interventions | Aspirin 648 mg, n = 15 Piroxicam 20 mg, n = 15 Piroxicam 40 mg, n = 15 Placebo, n = 15 Rescue medication allowed (time not specified) (BOCF) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 1, W = 1. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
Wang 1982.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0.5, 1 h then hourly to 6 h | |
| Participants | Mixed general and orthopaedic surgery N = 100 Age 18 to 61 years | |
| Interventions | Aspirin 650 mg, n = 25 Bicifadine 75 mg, n = 25 Bicifadine 150 mg, n = 25 Placebo n = 25 Rescue medication allowed (time not specified) (BOCF) |
|
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale | |
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 0. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "coded single‐dose envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identically‐appearing capsules" |
Winter 1983a.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0, 1 h then hourly to 8 h | |
| Participants | Dental surgery (complicated or multiple extractions or impactions) N = 105 Age 18 to 65 years | |
| Interventions | Aspirin 650 mg, n = 37 Oxaprozin 1200 mg, n = 33 Placebo, n = 35 Rescue medication (paracetamol plus codeine) allowed after 2 h (data handling not reported) |
|
| Outcomes | PI: standard 4‐point scale
PR: non‐standard scale
Global assessment: non‐standard 4‐point scale Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 1, W = 1. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
Winter 1983b.
| Methods | RCT, DB, single oral dose, parallel groups Assessed at 0.5, 1 h then hourly to 4 h | |
| Participants | Dental surgery (complicated or multiple extractions or impactions) N = 161 Mean age 32 years | |
| Interventions | Aspirin 650 mg, n = 42 Phenyltoloxamine 60 mg + paracetamol 1000 mg, n = 41 Placebo, n = 44 Rescue medication allowed after 2 h (data handling not reported) |
|
| Outcomes | PI: standard 4‐point scale
PR: standard 5‐point scale
Global assessment: standard 5‐point scale
Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identically‐appearing 2‐capsule single doses" |
BOCF = baseline observation carried forward; DB = double‐blind; h = hour; LOCF = last observation carried forward; N = number of participants in study; n = number of participants in treatment arm; PI = pain intensity; PR = pain relief; RCT = randomised controlled trial; VAS = visual analogue scale; W = withdrawals
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahlstrom 1968 | No extractable data |
| Ahlstrom 1974 | No extractable data |
| Antonsson 1985 | No placebo |
| Aromaa 1978 | No placebo |
| Barrenechea 1981 | Not randomised |
| Bella 1987 | No placebo |
| Bhounsule 1990 | Did not state baseline pain |
| Bloomfield 1974 | No extractable data |
| Bloomfield 1976 | Baseline pain intensity not given so no extractable data |
| Boerlin 1986 | Not randomised (MEDLINE indexed as RCT) |
| Burguet 1989 | No placebo |
| Campos 1980 | Included patients with somatic pain |
| Capuano 1990 | No placebo |
| Carstens 1987 | Unable to obtain the paper locally or through the British Library. Not indexed in MEDLINE as randomised controlled trial |
| Cooper 1976 | Only 3 hours observation time |
| Cooper 1979b | Inappropriate formulation ?? Probably not randomised (not indexed RCT) and probably no placebo |
| Cooper 1980a | Inappropriate study design ?? Probably not randomised (not indexed RCT) |
| Cooper 1980b | Duplicate publication of Cooper 1980a (excluded) |
| Cordero 1985 | Unable to obtain the paper locally or through the British Library |
| Dahl 1985 | No placebo |
| Desjardins 1983 | Inappropriate study design ?? |
| Di Blasi 1980a | No placebo, includes children (< 12 years) |
| Di Blasi 1980b | No placebo, includes children (< 12 years) |
| Feldmeier 1982 | Non‐standard pain measures |
| Fink 1978 | Included patients with 'slight' baseline pain |
| Fleiss 1979 | No extractable data |
| Friedrich 1983 | Non‐standard pain measures |
| Fuccella 1977 | Inappropriate study design ?? |
| Gallardo 1982 | Observation period only 3 hours |
| Gallardo 1984 | Observation period only 3 hours |
| Gallardo 1990 | Not randomised |
| Gallardo 1992 | Observation period only 3 hours |
| Gammelgaard 1981 | No extractable data |
| Giglio 1984 | Medication administered preoperatively ‐ inadequate baseline pain |
| Giles 1986 | Inappropriate study design ‐ data from patients remedicating were not handled correctly |
| Green 1978 | Not randomised |
| Grippaudo 1981 | No placebo |
| Happonen 1987 | No placebo |
| Heimdahl 1979 | Inadequate baseline pain |
| Henrikson 1979 | Intervention given immediately after surgery without regard to baseline pain |
| Hepso 1976 | Not double‐blind (title says double‐blind) |
| Herrmann 1980c | Includes participants with pain due to trauma |
| Herrmann 1980d | Includes participants with pain due to trauma |
| Hill 1969 | Inadequate baseline pain |
| Hutton 1983 | No extractable data |
| Ihalainen 1980 | No placebo |
| Irwin 1984 | No placebo |
| Izquierdo 1995 | No placebo, intravenous administration |
| Jain 1978a | Study 1: combined data for episiotomy and uterine cramps. Study 2: non‐standard pain scales |
| Jain 1978b | No usable data |
| Joubert 1982 | Not randomised |
| Kamiyama 1980 | No placebo |
| Kantor 1965 | Includes participants with pain due to fracture |
| Kristensen 1986 | No placebo |
| Kristensen 1988 | No placebo |
| Lamphier 1974 | Multiple dose study ‐ 1 tablet every 3 hours |
| Lobo 1983 | Observation period only 3 hours long |
| Maeglin 1979 | Observation period only 3 hours long |
| Mahler 1976 | Not randomised, no placebo |
| Mandujano | Unable to obtain the paper locally or through the British Library |
| Mehta 1986 | No extractable data |
| Mitchell 1985 | Baseline pain not of moderate to severe intensity |
| Mukherjee 1980 | No extractable data |
| Okun 1979 | Includes participants with musculoskeletal pain and fractures |
| Okun 1982 | Non‐standard pain scales therefore no extractable data |
| Or 1985 | Unable to obtain the paper locally or through the British Library. Probably no placebo |
| Ormiston 1981 | No placebo |
| Parker 1986 | Baseline pain and age not reported. Dose of aspirin unclear |
| Parkhouse 1967a | Not double‐blind |
| Parkhouse 1967b | Inadequate baseline pain |
| Parkhouse 1968 | Baseline pain not reported |
| Perotti 1984 | No placebo |
| Reines 1986 | Inadequate baseline pain |
| Rowe 1981 | Inadequate baseline pain |
| Rye 1978 | Duplicate publication; data already included in studies published by Cooper |
| Sechzer 1977 | Included patients with postpartum uterine cramps |
| Seymour 1982 | Inadequate baseline pain |
| Seymour 1983a | Not randomised |
| Seymour 1983b | Inadequate baseline pain |
| Seymour 1984 | Inadequate baseline pain |
| Sindet 1986 | No placebo |
| Skjelbred 1977 | No placebo |
| Skjelbred 1984 | Inadequate baseline pain |
| Slatis 1980 | No placebo |
| Spivach 1984 | No placebo |
| Sunshine 1975 | Included patients with somatic pain |
| Sunshine 1978a | Included patients with somatic pain |
| Sunshine 1978b | Included patients with somatic pain |
| Sunshine 1986 | Included patients with postpartum uterine cramps |
| Sveen 1975 | Inadequate baseline pain |
| Szmyd 1959 | Inadequate baseline pain |
| Todorovic 1982 | No placebo |
| Trop 1983 | Non‐standard pain scales therefore no extractable data |
| Vaidya 1974 | Non‐standard pain scales therefore no extractable data |
| Versichelen 1982 | No extractable data |
| Von Graffenried 1980 | Observation period only 3 hours long |
| Walker 1960 | Not randomised |
| Wang 1979 | Includes participants with fracture. No separate results for postoperative pain |
| Wilkinson 1960 | No placebo |
| Winter 1978 | Observation period only 3 hours long |
| Wojcicki 1977 | Observation period only 1 hour long |
| Zederfeldt 1977 | Inadequate baseline pain |
RCT = randomised controlled trial
Differences between protocol and review
There are no differences between protocol and review. The original review was conducted before the need for protocols, but it conforms in all essentials with protocols for recent reviews in this series for which protocols were prepared.
Contributions of authors
RAM and SD both carried out searches and reassessed the original trials for inclusion and new outcomes. SD entered the data into RevMan. Both authors were involved in analyses and writing. RAM and SD are responsible for updates.
Sources of support
Internal sources
Pain Research Funds, UK.
External sources
-
NHS R&D Health Technology Evaluation programmes (#93/31/4 and #94/11/4), UK.
For original review
-
European Union Biomed 2 BMH4 CT95 0172, UK.
For original review
-
SmithKline Beecham Consumer Healthcare, UK.
For original review
-
Biotechnology and Biological Sciences Research Council, UK.
For original review
Declarations of interest
RAM has consulted for various pharmaceutical companies and received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. RAM and SD have received research support from charities, government and industry sources at various times. Support for this review came from Oxford Pain Research Funds.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Bloomfield 1967 {published data only}
- Bloomfield SS, Gaffney TE, Howett M. Comparative analgesic efficacy of chlorphenesin carbamate and acetylsalicylic acid after episiotomy. Anesthesia and Analgesia 1967;46(5):515‐20. [PubMed] [Google Scholar]
Boraks 1987 {published data only}
- Boraks S. Flurbiprofen in low dosage compared with metamizole sodium (dipyrone), acetylsalicylic acid and placebo in treatment of pain following dental extractions [Flurbiprofen em dose baixa comparado a dipirona, acido acetilsalicilico e placebo no tratamento da dor pos‐extracao dentaria]. Arquivos Brasileiros de Medicina 1987;61(6):424‐30. [Google Scholar]
Breivik 1984 {published data only}
- Breivik H, Stenseth R, Apalseth K, Spilsberg AM. Piroxicam, acetylsalicylic acid and placebo for postoperative pain. Acta Anaesthesiologica Scandinavica 1984;28:37‐9. [DOI] [PubMed] [Google Scholar]
Calimlim 1977 {published data only}
- Calimlim JF, Wardell WM, Davis HT, Lasagna L, Gillies AJ. Analgesic efficacy of an orally administered combination of pentazocine and aspirin. With observations on the use and statistical efficiency of GLOBAL subjective efficacy ratings. Clinical Pharmacology and Therapeutics 1977;21(1):34‐43. [DOI] [PubMed] [Google Scholar]
Clark 1989 {published data only}
- Clark MS, Lindenmuth JE, Silverstone LM, Fryer GE Jr. A double blind single dose evaluation of the relative analgesic efficacy and safety of carprofen in the treatment of postoperative pain after oral surgery. Oral Surgery Oral Medicine and Oral Pathology 1989;68(3):273‐8. [DOI] [PubMed] [Google Scholar]
- Lindenmuth J, Clark M, Fryer GJ. Evaluation of the analgesic efficacy and safety of carprofen. Anesthesia Progress 1989;36(4‐5):206‐9. [PMC free article] [PubMed] [Google Scholar]
Cooper 1977 {published data only}
- Cooper SA, Needle SE, Kruger GO. Comparative analgesic potency of aspirin and ibuprofen. Journal of Oral Surgery 1977;35:898‐903. [PubMed] [Google Scholar]
Cooper 1979a {published data only}
- Cooper SA, Breen JF, Giuliani RL. Replicate studies comparing the relative efficacies of aspirin and indoprofen in oral surgery outpatients. Journal of Clinical Pharmacology 1979;19(2‐3):151‐9. [DOI] [PubMed] [Google Scholar]
Cooper 1982 {published data only}
- Cooper SA, Engel J, Ladov M, Precheur H, Rosenheck A, Rauch D. Analgesic efficacy of an ibuprofen‐codeine combination. Pharmacotherapy 1982;2:162‐7. [DOI] [PubMed] [Google Scholar]
Cooper 1983 {published data only}
- Cooper SA, Wagenberg B, Eskow R, Zissu J. Double blind evaluation of suprofen and aspirin in the treatment of periodontal pain. Pharmacology 1983;27(Suppl 1):23‐30. [DOI] [PubMed] [Google Scholar]
Cooper 1986 {published data only}
- Cooper SA, Wagenberg B, Zissu J, Kruger GO, Reynolds DC, Gallegos LT, et al. The analgesic efficacy of suprofen in periodontal and oral surgical pain. Pharmacotherapy 1986;6(5):267‐76. [DOI] [PubMed] [Google Scholar]
Cooper 1988 {published data only}
- Cooper SA, Mardirossian G, Milles M. Analgesic relative potency assay comparing flurbiprofen 50, 100, and 150 mg, aspirin 600 mg, and placebo in postsurgical dental pain. Clinical Journal of Pain 1988;4:175‐81. [Google Scholar]
Cooper 1991 {published data only}
- Cooper SA, Hutton C, Reynolds DC, Gallegos LT, Allen C, Marriott JG, et al. Dose‐response analgesic activity of meclofenamate sodium in dental pain. Advances in Therapy 1991;8(4):157‐65. [Google Scholar]
Cooper 1992 {published data only}
- Cooper SA, Itkin A, Zweig B. Comparison of oxaprozin, aspirin, and placebo in a dental impaction pain model. Advances in Therapy 1992;9(3):184‐94. [Google Scholar]
Coutinho 1976 {published data only}
- Coutinho A, Bonelli J, Nanci de Carvelho P. A double‐blind comparative study of the analgesic effects of fenbufen, codeine, aspirin, propoxyphene and placebo. Current Therapeutic Research 1976;19:58‐65. [PubMed] [Google Scholar]
Desjardins 1984 {published data only}
- Desjardins PJ, Cooper SA, Gallegos TL, Allwein JB, Reynolds DC, Kruger GO, et al. The relative analgesic efficacy of propiram fumarate, codeine, aspirin, and placebo in post impaction dental pain. Journal of Clinical Pharmacology 1984;24(1):35‐42. [DOI] [PubMed] [Google Scholar]
De Vroey 1977 {published data only}
- Vroey P. A double blind comparison of diflunisal and aspirin in the treatment of post operative pain after episiotomy. Current Medical Research and Opinion 1978;5(7):544‐7. [DOI] [PubMed] [Google Scholar]
- DeVroey P, Steelman SL, Caudron J, Verhaest L, Besselaar GH, Buntinx A. A double‐blind, placebo‐controlled, single‐dose study comparing three‐dose levels of diflunisal with aspirin and placebo in patients with pain due to episiotomy. Acta Therapeutica 1977;3:205‐15. [Google Scholar]
Fliedner 1984 {published data only}
- Fliedner L, Levsky M, Kechejian H, Berger J, Gaston G, Hutton CE. Analgesia with etodolac in oral postsurgical pain. Current Therapeutic Research 1984;36(1):33‐45. [Google Scholar]
Forbes 1980 {published data only}
- Forbes JA, White RW, White EH, Hughes MK. An evaluation of the analgesic efficacy of proquazone and aspirin in postoperative dental pain. Journal of Clinical Pharmacology 1980;20(7):465‐74. [DOI] [PubMed] [Google Scholar]
Forbes 1982 {published data only}
- Beaver WT, Forbes JA, Shackleford RW. A method for the 12‐hour evaluation of analgesic efficacy in outpatients with postoperative oral surgery pain. Three studies of diflunisal. Pharmacotherapy 1983;3(2):23S‐37S. [PubMed] [Google Scholar]
- Forbes JA, Calderazzo JP, Bowser MW, Foor VM, Shackleford RW, Beaver WT. A 12 hour evaluation of the analgesic efficacy of diflunisal, aspirin, and placebo in postoperative dental pain. Journal of Clinical Pharmacology 1982;22(2‐3):89‐96. [DOI] [PubMed] [Google Scholar]
Forbes 1983 {published data only}
- Forbes JA, Butterworth GA, Burchfield WH, Beaver WT, Shackleford RW. A 12 hour evaluation of the analgesic efficacy of diflunisal, zomepirac sodium, aspirin, and placebo in postoperative oral surgery pain. Pharmacotherapy 1983;3(2):38S‐46S. [PubMed] [Google Scholar]
Forbes 1984 {published data only}
- Forbes J, Barkaszi B, Ragland R, Hankle J. Analgesic effect of fendosal, ibuprofen and aspirin in postoperative oral surgery pain. Pharmacotherapy 1984;4:385‐91. [DOI] [PubMed] [Google Scholar]
Forbes 1986 {published data only}
- Forbes JA, Keller CK, Smith JW, Zeleznock JR, Sevelius H, Beaver WT. Analgesic effect of naproxen sodium, codeine, a naproxen codeine combination and aspirin on the postoperative pain of oral surgery. Pharmacotherapy 1986;6(5):211‐8. [DOI] [PubMed] [Google Scholar]
Forbes 1989 {published data only}
- Forbes JA, Yorio CC, Selinger LR, Rosenmertz SK, Beaver WT. An evaluation of flurbiprofen, aspirin, and placebo in postoperative oral surgery pain. Pharmacotherapy 1989;9(2):66‐73. [DOI] [PubMed] [Google Scholar]
Forbes 1990a {published data only}
- Forbes JA, Jones KF, Kehm CJ, Smith WK, Gongloff CM, Zeleznock JR, et al. Evaluation of aspirin, caffeine, and their combination in postoperative oral surgery pain. Pharmacotherapy 1990;10(6):387‐93. [PubMed] [Google Scholar]
Forbes 1990b {published data only}
- Forbes JA, Butterworth GA, Burchfield WH, Beaver WT. Evaluation of ketorolac, aspirin, and an acetaminophen‐codeine combination in postoperative oral surgery pain. Pharmacotherapy 1990;10(6):77S‐93S. [PubMed] [Google Scholar]
Forbes 1991 {published data only}
- Forbes J, Edquist I, Smith F, Schwartz M, Beaver W. Evaluation of bromfenac, aspirin, and ibuprofen in postoperative oral surgery pain. Pharmacotherapy 1991;11:64‐70. [PubMed] [Google Scholar]
Forbes 1992 {published data only}
- Forbes JA, Beaver WT, Jones KF, Edquist IA, Gongloff CM, Smith WK, et al. Analgesic efficacy of bromfenac, ibuprofen, and aspirin in postoperative oral surgery pain. Clinical Pharmacology and Therapeutics 1992;51(3):343‐52. [DOI] [PubMed] [Google Scholar]
Frame 1986 {published data only}
- Frame J, Fisher S, Pickvance N, Skene A. A double blind placebo controlled comparison of three ibuprofen combinations and aspirin. British Journal of Oral and Maxillofacial Surgery 1986;24:122‐9. [DOI] [PubMed] [Google Scholar]
Gaston 1984 {published data only}
- Gaston GW, Mallow RD, Frank JE. The efficacy of etodolac for patients with pain following oral surgery. Journal of Oral and Maxillofacial Surgery 1984;42(6):362‐6. [DOI] [PubMed] [Google Scholar]
Gaston 1986 {published data only}
- Gaston GW, Mallow RD, Frank JE. Comparison of etodolac, aspirin and placebo for pain after oral surgery. Pharmacotherapy 1986;6(5):199‐205. [DOI] [PubMed] [Google Scholar]
Herbertson 1994 {published data only}
- Herbertson R, Storey N. The comparative efficacy of diclofenac potassium, aspirin, and placebo in the treatment of patients with pain following gynecologic surgery. Today's Therapeutic Trends 1994;12(S1):33‐45. [Google Scholar]
Herrmann 1980a {published data only}
- Herrmann U, Laube W, Roth F, Schultheiss HR, Berger M, Schürmann P, et al. Analgesic effect of fluproquazone in postoperative patients. Clinical Pharmacology and Therapeutics 1980;27(3):379‐85. [DOI] [PubMed] [Google Scholar]
Herrmann 1980b {published data only}
- Herrmann U, Laube W, Roth F, Schultheiss HR, Berger M, Schürmann P, et al. Analgesic effect of fluproquazone in postoperative patients. Clinical Pharmacology and Therapeutics 1980;27(3):379‐85. [DOI] [PubMed] [Google Scholar]
Holland 1988 {published data only}
- Holland IS, Seymour RA, Ward Booth RP, Ord RA, Lim KL, Hoare RC. An evaluation of different doses of soluble aspirin and aspirin tablets in postoperative dental pain. British Journal of Clinical Pharmacology 1988;26(4):463‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Honig 1978 {published data only}
- Honig WJ. The use of diflunisal in post operative pain: a report of double blind comparative trials in patients after meniscectomy. Current Medical Research and Opinion 1978;5(7):536‐43. [DOI] [PubMed] [Google Scholar]
- Honig WJ, Cremer CW, Manni JG. A single dose study comparing the analgesic effects of diflunisal, acetylsalicylic acid, and placebo in pain following meniscectomy. Journal of International Medical Research 1978;6(3):172‐9. [DOI] [PubMed] [Google Scholar]
Jain 1985a {published data only}
- Jain AK, McMahon FG, Ryan JR, Smith GB. Analgesic efficacy of indoprofen in postpartum episiotomy pain. Current Therapeutic Research 1985;38(5):677‐81. [Google Scholar]
Jain 1985b {published data only}
- Jain AK, McMahon FG, Ryan JR. An analgesic study of indoprofen, aspirin and placebo in dental surgery patients. Current Therapeutic Research 1985;38(5):682‐8. [Google Scholar]
Jain 1986a {published data only}
- Jain AK, Hunley CC, Kuebel J, McMahon FG, Ryan JJ. Analgesic efficacy of amfenac, aspirin and placebo after extraction of impacted teeth. Pharmacotherapy 1986;6(5):236‐40. [DOI] [PubMed] [Google Scholar]
Jain 1986b {published data only}
- Jain A, Ryan J, McMahon F, Kuebel J, Walters P, Noveck C. Analgesic efficacy of low dose ibuprofen in dental extraction pain. Pharmacotherapy 1986;6:318‐22. [DOI] [PubMed] [Google Scholar]
Kempf 1987 {published data only}
- Kempf K, Konzelman J, Schultz R, Turner J. Comparison of meclofenamate sodium with buffered aspirin and placebo for the relief of postoperative dental pain. Clinical Therapy 1987;9(6):594‐601. [PubMed] [Google Scholar]
Lehnert 1990 {published data only}
- Lehnert S, Reuther J, Wahl G, Brarthel K. The efficacy of paracetamol (Tylenol) and acetyl salicylic acid (aspirin) in treating postoperative pain [Wirksamkeit von paracetamol (tylenol) und acetylsalizylure (aspirin) bei postperativen schmerzen]. Deutsche zahnarztliche Zeitschrift 1990;45:23‐6. [PubMed] [Google Scholar]
London 1983a {published data only}
- London R, Sundaram G, Feldman S, Goldstein P. Episiotomy pain:efficacy and safety of fluproquazone compared to aspirin and placebo. International Journal of Gynaecology and Obstetrics 1983;21(3):251‐5. [DOI] [PubMed] [Google Scholar]
London 1983b {published data only}
- London R, Sundaram G, Feldman S, Goldstein P. Aspirin in the treatment of episiotomy pain. Southern Medical Journal 1983;76(7):844‐5. [DOI] [PubMed] [Google Scholar]
Mardirossian 1985 {published data only}
- Cooper SA, Mardirossian G. Comparison of flurbiprofen and aspirin in the relief of postsurgical pain using the dental pain model. American Journal of Medicine 1986;80(3A):36‐40. [DOI] [PubMed] [Google Scholar]
- Mardirossian G, Cooper S. Comparison of the analgesic efficacy of flurbiprofen and aspirin for postsurgical dental pain. Journal of Oral and Maxillofacial Surgery 1985;43(2):106‐9. [DOI] [PubMed] [Google Scholar]
Markowitz 1985 {published data only}
- Markowitz N, Young S, Rohrer M, Turner J. Comparison of meclofenamate sodium with buffered aspirin and placebo in the treatment of postsurgical dental pain. Journal of Oral and Maxillofacial Surgery 1985;43(7):517‐22. [DOI] [PubMed] [Google Scholar]
McQuay 1987 {published data only}
- McQuay HJ, Carroll D, Poppleton P, Summerfield RJ, Moore RA. Fluradoline and aspirin for orthopedic postoperative pain. Clinical Pharmacology and Therapeutics 1987;41(5):531‐6. [DOI] [PubMed] [Google Scholar]
Mehlisch 1984 {published data only}
- Mehlisch DR, Frakes LA. A controlled comparative evaluation of acetaminophen and aspirin in the treatment of postoperative pain. Clinical Therapeutics 1984;7(1):89‐97. [PubMed] [Google Scholar]
Mehlisch 1990 {published data only}
- Mehlisch DR, Sterling WR, Mazza FA, Singer JM. A single dose study of the efficacy and safety of FS 205 397 (250 mg or 500 mg) versus aspirin and placebo in the treatment of postsurgery dental pain. Journal of Clinical Pharmacology 1990;30(9):815‐23. [DOI] [PubMed] [Google Scholar]
Mehlisch 1994 {published data only}
- Mehlisch D, Brown P. Single‐dose therapy with diclofenac potassium, aspirin, or placebo following dental impaction surgery. Today's Therapeutic Trends 1994;12(S1):15‐31. [Google Scholar]
Nelson 1985 {published data only}
- Nelson SL, Bergman SA. Relief of dental surgery pain: a controlled 12 hour comparison of etodolac, aspirin, and placebo. Anesthesia Progress 1985;32(4):151‐6. [PMC free article] [PubMed] [Google Scholar]
Nelson 1994a {published data only}
- Nelson SL, Brahim JS, Korn SH, Greene SS, Suchower LJ. Comparison of single‐dose ibuprofen lysine, acetylsalicylic acid, and placebo for moderate‐to‐severe postoperative dental pain. Clinical Therapeutics 1994;16(3):458‐65. [PubMed] [Google Scholar]
Nelson 1994b {published data only}
- Nelson S, Brahim J. An evaluation of the analgesic efficacy of diclofenac potassium, aspirin, and placebo in postoperative dental pain. Today's Therapeutic Trends 1994;12(S1):3‐14. [Google Scholar]
Olsen 1997 {published data only}
- Olsen NZ, Sunshine A, Zighelboim I, DeCastro A. Onset and duration of analgesia of diclofenac potassium in the treatment of postepisiotomy pain. American Journal of Therapeutics 1997;4:239‐46. [DOI] [PubMed] [Google Scholar]
Or 1988 {published data only}
- Or S, Bozkurt A. Analgesic effect of aspirin, mefenamic acid and their combination in post operative oral surgery pain. Journal of International Medical Research 1988;16(3):167‐72. [DOI] [PubMed] [Google Scholar]
Parkhouse 1969 {published data only}
- Parkhouse J, Heppenstall C, Smith E, Wright V. An attempt to evaluate a rapidly acting oral analgesic. Canadian Medical Association Journal 1969;100:1057‐8. [PMC free article] [PubMed] [Google Scholar]
Patel 1991 {published data only}
- Patel A, Skelly AM, Kohn H, Preiskel HW. Double‐blind placebo‐controlled comparison of the analgesic effects of single doses of lornoxicam and aspirin in patients with postoperative dental pain. British Dental Journal 1991;170(8):295‐9. [DOI] [PubMed] [Google Scholar]
Rowe 1985 {published data only}
- Rowe NH, Aseltine LF, Turner JL. Control of pain with meclofenamate sodium following removal of an impacted molar. Oral Surgery Oral Medicine and Oral Pathology 1985;59(5):446‐8. [DOI] [PubMed] [Google Scholar]
Seymour 1986 {published data only}
- Seymour RA, Williams FM, Luyk NM, Boyle MA, Whitfield PM, Nicholson E, et al. Comparative efficacy of soluble aspirin and aspirin tablets in postoperative dental pain. European Journal of Clinical Pharmacology 1986;30(4):495‐8. [DOI] [PubMed] [Google Scholar]
Seymour 1992 {published data only}
- Seymour RA, Weldon M, Kelly P, Nicholson E, Hawkesford JE. An evaluation of buffered aspirin and aspirin tablets in postoperative pain after third molar surgery. British Journal of Clinical Pharmacology 1992;33(4):395‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seymour 2003 {published data only}
- Seymour RA, Hawkesford JE, Sykes J, Stillings M, Hill CM. An investigation into the comparative efficacy of soluble aspirin and solid paracetamol in postoperative pain after third molar surgery. British Dental Journal 2003;194(3):153‐7. [DOI] [PubMed] [Google Scholar]
Sunshine 1983a {published data only}
- Sunshine A, Marrero I, Olson NZ, Laska EM, McCormick N. Oral analgesic efficacy of suprofen compared to aspirin, aspirin plus codeine, and placebo in patients with postoperative dental pain. Pharmacology 1983;27(1):31‐40. [DOI] [PubMed] [Google Scholar]
Sunshine 1983b {published data only}
- Sunshine A, Olson NZ, Laska EM, Zighelboim I, Castro A, Sarrazin C. Analgesic effect of graded doses of flurbiprofen in post episiotomy pain. Pharmacotherapy 1983;3(3):177‐81. [DOI] [PubMed] [Google Scholar]
Sunshine 1983c {published data only}
- Sunshine A, Olson N, Laska E, Zighelboim I, Castro A, Sarrazin C. Ibuprofen, zomepirac, aspirin, and placebo in the relief of postepisiotomy pain. Clinical Pharmacological Therapeutics 1983;34(2):254‐8. [DOI] [PubMed] [Google Scholar]
Sunshine 1988 {published data only}
- Sunshine A, Roure C, Colon A, Olson NZ, Gonzalez L, Siegel C, et al. Analgesic efficacy of piroxicam in the treatment of postoperative pain. American Journal of Medicine 1988;84(5A):16‐22. [DOI] [PubMed] [Google Scholar]
Wang 1982 {published data only}
- Wang RIH, Johnson RP, Lee JCM, Waite EM. The oral analgesic efficacy of bicifadine hydrochloride in postoperative pain. Journal of Clinical Pharmacology 1982;22(4):160‐4. [DOI] [PubMed] [Google Scholar]
Winter 1983a {published data only}
- Winter LJ, Post A. Double blind comparison of single oral doses of oxaprozin, aspirin, and placebo for relief of post operative oral surgery pain. Journal of International Medical Research 1983;11(5):308‐14. [DOI] [PubMed] [Google Scholar]
Winter 1983b {published data only}
- Winter LJr, Appleby F, Ciccone PE, Pigeon JG. A comparative study of an acetaminophen analgesic combination and aspirin in the treatment of post‐operative oral surgery pain. Current Therapeutic Research 1983;33(2):200‐13. [Google Scholar]
References to studies excluded from this review
Ahlstrom 1968 {published data only}
- Ahlstrom U, Lantz B. A comparison between dextropropoxyphene hydrochloride and acetylsalicylic acid as analgesics after oral surgery. Odontologisk Revy 1968;19:55‐63. [PubMed] [Google Scholar]
Ahlstrom 1974 {published data only}
- Ahlstrom U, Kahnberg KE, Roos BE. Pentazocine and aspirin for pain following oral surgery. Acta Pharmacologica et Toxicologica (Copenhagen) 1974;35(4):325‐36. [DOI] [PubMed] [Google Scholar]
Antonsson 1985 {published data only}
- Antonsson J, Carlstedt A, Lofgren B, Solhaug JH, Spangen L. The analgesic effects of acetylsalicylic acid combined with codeine versus given separately to patients with postoperative pain. Opmear 1985;30(3):90‐2. [Google Scholar]
Aromaa 1978 {published data only}
- Aromaa U, Asp K. A comparison of naproxen, indomethacin and acetylsalicylic acid in pain after varicose vein surgery. Journal of International Medical Research 1978;6(2):152‐6. [DOI] [PubMed] [Google Scholar]
Barrenechea 1981 {published data only}
- Barrenechea Arana J, Berrazueta Fernandez M, Pascual Borrego A, Castillo EscAndon R. Diflunisal in the treatment of post operative pain in oral surgery. Double blind study [Diflunisal en el tratamiento del dolor postoperatorio en cirugia oral. Estudio doble ciego]. Revista Espanola de Estomatologia 1981;29(3):171‐8. [PubMed] [Google Scholar]
Bella 1987 {published data only}
- Bella G, Russo S, Messina G. Clinical trial of a new non steroidal analgesic (diflunisal) in oral surgery [Ricerche di sperimentazione clinica su un nuovo analgesico non steroideo (diflunisal) in chirurgia orale]. Minerva Stomatologica 1987;36(4):297‐300. [PubMed] [Google Scholar]
Bhounsule 1990 {published data only}
- Bhounsule SA, Nevreker PR, Agshikar NV, Pal MN, Dhume VG. A comparison of four analgesics in post‐episiotomy pain. Indian Journal of Physiology and Pharmacology 1990;34(1):34‐8. [PubMed] [Google Scholar]
Bloomfield 1974 {published data only}
- Bloomfield SS, Barden TP, Mitchell J. Comparative efficacy of ibuprofen and aspirin in episiotomy pain. Clinical Pharmacology and Therapeutics 1974;15(6):565‐70. [DOI] [PubMed] [Google Scholar]
Bloomfield 1976 {published data only}
- Bloomfield SS, Barden TP, Mitchell J. Aspirin and codeine in two postpartum pain models. Clinical Pharmacology and Therapeutics 1976;20(4):499‐503. [DOI] [PubMed] [Google Scholar]
Boerlin 1986 {published data only}
- Boerlin V, Maeglin B, Hagler W, Kuhn M, Nuesch E. Analgesic activity of propyphenazone in patients with pain following oral surgery. European Journal of Clinical Pharmacology 1986;31(2):127‐31. [DOI] [PubMed] [Google Scholar]
Burguet 1989 {published data only}
- Burguet J, Kupperberg E. Double‐blind therapeutic study comparing tiaprofenic acid and acetylsalicylic acid in dentistry. Journal of Drug Development 1989;2(2):115‐7. [Google Scholar]
Campos 1980 {published data only}
- Campos VM, Solis EL. The analgesic and hypothermic effects of nefopam, morphine, aspirin, diphenhydramine, and placebo. Journal of Clinical Pharmacology 1980;20(1):42‐9. [DOI] [PubMed] [Google Scholar]
Capuano 1990 {published data only}
- Capuano A, Valerio P, Bonucci I, Rosadini F. Use of NSAIDs in dentistry: double blind controlled study of Diflunisal versus ASA [Uso dei fans in odontostomatologia: studio controllato in doppio cieco Diflunisal versus ASA]. Giornale di Anestesia Stomatologica 1990;19(2):27‐36. [PubMed] [Google Scholar]
Carstens 1987 {published data only}
- Carstens M, Ligueros O, Lobo R, Pauz M, Gallardo F. Comparative double blind study of the analgesic and anti inflammatory activity of glucamethacin (Indicin), aspirin and placebo in oral surgery [Estudio comparativo doble‐ciego de la actividad analgesica y antiiflamatoria de la glucametacina (indicin), aspirina y placebo en cirugia oral]. Odontologia Chilena 1987;35(1):31‐9. [PubMed] [Google Scholar]
Cooper 1976 {published data only}
- Cooper SA, Beaver WT. A model to evaluate mild analgesics in oral surgery outpatients. Clinical Pharmacology and Therapeutics 1976;20(2):241‐50. [DOI] [PubMed] [Google Scholar]
Cooper 1979b {published data only}
- Cooper SA, Breen JF, Giuliani RL. Replicate studies comparing the relative efficacies of aspirin and indoprofen in oral surgery outpatients. Journal of Clinical Pharmacology 1979;19(2‐3):151‐9. [DOI] [PubMed] [Google Scholar]
Cooper 1980a {published data only}
- Cooper SA, Reynolds DC, Kruger GO, Gottlieb S. An analgesic relative potency assay comparing zomepirac sodium and aspirin. Journal of Clinical Pharmacology 1980;20(2‐3):98‐106. [DOI] [PubMed] [Google Scholar]
Cooper 1980b {published data only}
- Cooper S. Efficacy of zomepirac in oral surgical pain. Journal of Clinical Pharmacology 1980;20(4):230‐42. [DOI] [PubMed] [Google Scholar]
Cordero 1985 {published data only}
- Cordero R. A comparative study of Lagundi and aspirin as analgesics for post‐extraction pain. Journal of the Philippine Dental Association 1985;35(1):15‐8. [PubMed] [Google Scholar]
Dahl 1985 {published data only}
- Dahl E, Feldmann G, Jonsson E. Acetylsalicylic acid compared with acetylsalicylic acid plus codeine as postoperative analgesics after removal of impacted mandibular third molars. Swedish Dental Journal 1985;9(5):207‐12. [PubMed] [Google Scholar]
Desjardins 1983 {published data only}
- Desjardins PJ, Cooper SA, Ruderman CM, Gallegos LT, Reynolds DC, Kruger GO. The effects of fendosal, aspirin and placebo on postoperative dental pain. A dose ranging and efficacy study. Pharmacotherapy 1983;3(1):52‐7. [DOI] [PubMed] [Google Scholar]
Di Blasi 1980a {published data only}
- Blasi F, Gnudi A. Use of floctafenine for dental pain of adults and children: a controlled study 1 [Impiego della floctafenina nei dolori odontostomatologici degli adulti e dei bambini:studio controllato ‐ Prima parte]. Minerva Stomatologica 1980;29(4):265‐80. [PubMed] [Google Scholar]
Di Blasi 1980b {published data only}
- Blasi F, Gnudi A. Use of floctafenine in toothaches in adults and children: a controlled study II [Impiego della floctafenina nei dolori odontostomatologici degli adulti e dei bambini (studio controllato). Seconda parte]. Minerva Stomatologica 1980;29(5):345‐58. [PubMed] [Google Scholar]
Feldmeier 1982 {published data only}
- Feldmeier C. The analgesic effect of carprofen in acute pain: results of two clinical studies. European Journal of Rheumatology and Inflammation 1982;5(4):522‐6. [PubMed] [Google Scholar]
Fink 1978 {published data only}
- Fink S, Nielsen KO. Comparison of a new analgesic, rimazolium with acetylsalicylic acid and placebo in postoperative pain. A randomised clinical trial. Current Therapeutic Research 1978;24:900‐4. [Google Scholar]
Fleiss 1979 {published data only}
- Fleiss JL, Chilton NW, Wallenstein S. Ridit analysis in dental clinical studies. Journal of Dental Research 1979;58(11):2080‐4. [DOI] [PubMed] [Google Scholar]
Friedrich 1983 {published data only}
- Friedrich E. A comparison of etodolac (Ultradol) with aspirin and placebo in patients with episiotomy pain. Current Therapeutic Research 1983;33(1):100‐7. [Google Scholar]
Fuccella 1977 {published data only}
- Fuccella LM, Corvi G, Gorini F, Mandelli V, Mascellani G, Nobili F, et al. Application of nonparametric procedure for bioassay data to the evaluation of analgesics in man. Journal of Clinical Pharmacology 1977;17(4):177‐84. [DOI] [PubMed] [Google Scholar]
Gallardo 1982 {published data only}
- Gallardo F, Rossi E, Cisscutti V. Analgesic efficacy of ketoprofen on postoperative pain following periodontal surgery. IRCS Medical Science 1982;10(12):1036‐7. [Google Scholar]
Gallardo 1984 {published data only}
- Gallardo F, Rossi VE, Lobos TM. Indoprofen and aspirin for the control of postoperative pain following peridontal surgery. Acta Odontologica Latinoamericana 1984;1:87‐91. [Google Scholar]
Gallardo 1990 {published data only}
- Gallardo F, Carstens M, Ayarza M. Analgesic and anti inflammatory effects of glucamethacin (a nonsteroidal anti inflammatory analgesic) after the removal of impacted third molars. Oral Surgery, Oral Medicine and Oral Pathology 1990;69(2):157‐60. [DOI] [PubMed] [Google Scholar]
Gallardo 1992 {published data only}
- Gallardo F, Rossi E. Effects of sodium meclofenamate on postoperative pain following periodontal surgery. Journal of Periodontology 1992;63(3):166‐8. [DOI] [PubMed] [Google Scholar]
Gammelgaard 1981 {published data only}
- Gammelgaard NP, Juul A, Larsen K, Ohrt Mikkelsen B. Comparison of a new analgesic, Ro 11‐4337, with acetylsalicylic acid and placebo in pain following tonsillectomy: a randomized clinical trial. Current Therapeutic Research 1981;30(2):151‐5. [Google Scholar]
Giglio 1984 {published data only}
- Giglio JA, Campbell RL. The prophylactic use of flurbiprofen to prevent post‐extraction dental pain. Anesthesia Progress 1984;31(2):74‐6. [PMC free article] [PubMed] [Google Scholar]
Giles 1986 {published data only}
- Giles AD, Hill CM, Shepherd JP, Stewart DJ, Pickvance NJ. A single dose assessment of an ibuprofen/codeine combination in postoperative dental pain. International Journal of Oral and Maxillofacial Surgery 1986;15(6):727‐32. [DOI] [PubMed] [Google Scholar]
Green 1978 {published data only}
- Green AE. A clinical trial of pentazocine and aspirin following minor oral surgery. British Dental Journal 1976;141(8):247‐50. [DOI] [PubMed] [Google Scholar]
Grippaudo 1981 {published data only}
- Grippaudo G, Amato R, Giordano A, Sarzani R, Segatore I, Addessi G. Analgesic treatment of trigeminal nerve pain following dental extractions [Terapia analgesica nel polore postestrattivo dell incluso]. Clin Europ 1981;20(4):661‐6. [Google Scholar]
Happonen 1987 {published data only}
- Happonen RP, Oksala E, Ylipaavalniemi P. A combination of acetylsalicylic acid and codeine phosphate versus acetylsalicylic acid as postoperative analgesics after mandibular third molar surgery. Proceedings of the Finnish Dental Society 1987;83(1):32‐5. [PubMed] [Google Scholar]
Heimdahl 1979 {published data only}
- Heimdahl A, Dahlstrom H. A double blind single dose comparison between two analgesics, rimazolium and acetylsalicylic acid in oral surgery outpatients. Swedish Dental Journal 1979;3(2):57‐61. [PubMed] [Google Scholar]
Henrikson 1979 {published data only}
- Henrikson PA, Tjernberg A, Ahlstrom U, Peterson LE. Analgesic efficacy and safety of Fenbufen following surgical removal of a lower wisdom tooth: a comparison with acetylsalicylic acid and placebo. Journal of International Medical Research 1979;7(2):107‐16. [DOI] [PubMed] [Google Scholar]
Hepso 1976 {published data only}
- Hepso HU, Lokken P, Bjornson J, Godal HC. Double blind crossover study of the effect of acetylsalicylic acid on bleeding and post‐operative course after bilateral oral surgery. European Journal of Clinical Pharmacology 1976;10:217‐25. [Google Scholar]
Herrmann 1980c {published data only}
- Herrmann U, Laube W, Roth F, Schultheiss HR, Berger M, Schürmann P, et al. Analgesic effect of fluproquazone in postoperative patients. Clinical Pharmacology and Therapeutics 1980;27(3):379‐85. [DOI] [PubMed] [Google Scholar]
Herrmann 1980d {published data only}
- Herrmann U, Laube W, Roth F, Schultheiss HR, Berger M, Schürmann P, et al. Analgesic effect of fluproquazone in postoperative patients. Clinical Pharmacology and Therapeutics 1980;27(3):379‐85. [DOI] [PubMed] [Google Scholar]
Hill 1969 {published data only}
- Hill RC, Turner P. Importance of initial pain in post operative assessment of analgesic drugs. Journal of Clinical Pharmacology and the Journal of New Drugs 1969;9(5):321‐7. [PubMed] [Google Scholar]
Hutton 1983 {published data only}
- Hutton CE. The effectiveness of 100 and 200 mg etodolac (Ultradol), aspirin, and placebo in patients with pain following oral surgery. Oral Surgery, Oral Medicine and Oral Pathology 1983;56(6):575‐80. [DOI] [PubMed] [Google Scholar]
Ihalainen 1980 {published data only}
- Ihalainen U, Rissanen H, Oikarinen VJ. The efficacy and tolerability of diflunisal and ASA in the relief of postoperative pain in oral surgery. Proceedings of the Finnish Dental Society 1980;76(5‐6):262‐6. [PubMed] [Google Scholar]
Irwin 1984 {published data only}
- Irwin BC, Acharya KB. A comparative study of benzydamine hydrochloride 0.15% w/v ('Difflam' oral rinse) and acetyl salicylic acid as analgesics following tonsillectomy. Journal of International Medical Research 1984;12(3):216‐8. [DOI] [PubMed] [Google Scholar]
Izquierdo 1995 {published data only}
- Izquierdo E, Fabregas N, Valero R, Salvador L, Soley R, Nalda MA. Postoperative analgesia in herniated disk surgery. Comparative study of diclofenac, lysine acetylsalicylate and ketorolac [Analgesia postoperatoria en la cirugia de hernia discal. Estudio comparativo de diclofenaco, acetilsalicilato de lisina y ketorolaco]. Revista Espanola de Anestesiologia y Reanimacion 1995;42(8):316‐9. [PubMed] [Google Scholar]
Jain 1978a {published data only}
- Jain AK, McMahon FG, Ryan JR, Unger D, Richard W. Aspirin and aspirin‐caffeine in postpartum pain relief. Clinical Pharmacology and Therapeutics 1978;24(1):69‐75. [DOI] [PubMed] [Google Scholar]
Jain 1978b {published data only}
- Jain AK, McMahon FG, Ryan JR. Piroxicam, a novel analgesic in postpartum pain. European Journal of Rheumatology and Inflammation 1978;1(3):356‐9. [Google Scholar]
Joubert 1982 {published data only}
- Joubert L, Mullane JF, Merlo M, Martel R, Arnold J, Rolly G, et al. Clinical pharmacological profile of Ultradol registered, a new nonsteroidal anti‐inflammatory drug. Current Therapeutic Research 1982;32(1):74‐88. [Google Scholar]
Kamiyama 1980 {published data only}
- Kamiyama Y, Takahashi Y, Hagiwara H, Horiuchi H, Tokusu S. Effects of flurbiprofen (FP 70) on pain in periodontics: double blind methods in comparison with aspirin. Shikai Tenbo 1980;56(5):849‐58. [PubMed] [Google Scholar]
Kantor 1965 {published data only}
- Kantor TG, Sunshine A, Laska E, Meisner M, Hopper M. Oral analgesic studies: pentazocine hydrochloride, codeine, aspirin, and placebo and their influence on response to placebo. Clinical Pharmacology and Therapeutics 1965;7(4):447‐54. [DOI] [PubMed] [Google Scholar]
Kristensen 1986 {published data only}
- Kristensen S, Tveteras K, Outzen K, Poulsen H. Treatment of pain after tonsillectomy. Comparison between naproxen (Naprosyn) and acetylsalicylic acid (Kalcatyl) [Smertebehandling efter tonsillektomi. En sammenlignende undersogelse af naproxen (Naprosyn) og acetylsalicylsyre (Kalcatyl)]. Ugeskrift for Laeger 1986;148(44):2832‐5. [PubMed] [Google Scholar]
Kristensen 1988 {published data only}
- Kristensen S, Tveteraas K, Hein P, Poulsen H, Outzen K. Relief of pain and trismus in patients treated with naproxen or acetylsalicylic acid after tonsillectomy. Journal of Laryngology and Otology 1988;102(1):39‐42. [DOI] [PubMed] [Google Scholar]
Lamphier 1974 {published data only}
- Lamphier T, Rodriguez A, Richards D. Double blind study of the analgesic effect of ethoheptazine aspirin. Current Therapeutic Research 1974;16(7):718‐33. [PubMed] [Google Scholar]
Lobo 1983 {published data only}
- Lobo R, Gallardo F, Henriquez E, Iriarte E. Analgesic activity of ketoprofen in post‐operative oral surgery pain. IRCS Medical Science 1983;11(7):639‐40. [Google Scholar]
Maeglin 1979 {published data only}
- Maeglin B, Hagler W, Kuhn M, Graffenried B, Nüesch E. Analgesic effect of fluproquazone in oral surgery outpatients. Current Therapeutic Research 1979;26(3):284‐94. [Google Scholar]
Mahler 1976 {published data only}
- Mahler D, Forrest WJ, Brown C, Shroff PF, Gordon HE, Brown BW Jr, et al. Assay of aspirin and naproxen analgesia. Clinical Pharmacology and Therapeutics 1976;19(1):18‐23. [DOI] [PubMed] [Google Scholar]
Mandujano {published data only}
- Mandujano VM. Comparative study of the floctafenina and acid acetisalicilo in a cancer services [Estudio comparativo del floctafenina y acido acetisalicilo en un servico ocorinolaringolgia]. Investigation Medico International [Date not given];3/6:379. [Google Scholar]
Mehta 1986 {published data only}
- Mehta SD. A randomized double‐blind placebo‐controlled study of dipyrone and aspirin in post‐operative orthopaedic patients. Journal of International Medical Research 1986;14:63‐6. [DOI] [PubMed] [Google Scholar]
Mitchell 1985 {published data only}
- Mitchell DA, Ward BP, Seymour RA. A comparative study of the efficacy of aspirin and an ibuprofen/codeine combination in patients treated pre operatively with methylprednisolone acetate. British Dental Journal 1985;159(3):78‐81. [DOI] [PubMed] [Google Scholar]
Mukherjee 1980 {published data only}
- Mukherjee S, Sood S. A controlled evaluation of orally administered aspirin, dipyrone and placebo in patients with post operative pain. Current Medical Research and Opinion 1980;6(9):619‐23. [DOI] [PubMed] [Google Scholar]
Okun 1979 {published data only}
- Okun R, Green J, Shackleford R. An analgesic comparison study of indoprofen versus aspirin and placebo in surgical pain. Journal of Clinical Pharmacology 1979;19(8‐9 Pt 1):487‐92. [DOI] [PubMed] [Google Scholar]
Okun 1982 {published data only}
- Okun R. Evaluation of the analgesic effect of Fendosal in patients with postpartum uterine cramp or episiotomy pain. Current Therapeutic Research 1982;31(1):65‐73. [Google Scholar]
Or 1985 {published data only}
- Or S, Bozkurt A. Evaluation of the analgesic effects of various aspirin combinations in oral surgery [Agiz cerrahisi pratigindeki postoperatif agrida bazi aspirin kombinasyonlarinin analjezik etikilerinin arastirilmasi]. Ankara Universitesi Dis Hekimligi Fakultesi Dergisi 1985;12(2):321‐32. [PubMed] [Google Scholar]
Ormiston 1981 {published data only}
- Ormiston MC, Vaughton KC, Thornton EJ, Coste JJ, Milroy E. The comparative effectiveness of tiaprofenic acid and aspirin in the treatment of post‐prostatectomy pain. British Journal of Clinical Practice 1981;35(10):360‐2. [PubMed] [Google Scholar]
Parker 1986 {published data only}
- Parker D, Gibbin K, Noyelle R. Syrup formulations for post tonsillectomy analgesia: a double blind study comparing ibuprofen, aspirin and placebo. Journal of Laryngology and Otology 1986;100(9):1055‐60. [DOI] [PubMed] [Google Scholar]
Parkhouse 1967a {published data only}
- Parkhouse J, Collie JA, Wood V. A study of aspirin and nepenthe. British Journal of Anaesthesia 1967;39:38‐49. [DOI] [PubMed] [Google Scholar]
Parkhouse 1967b {published data only}
- Parkhouse J, Hallinon P. A comparison of aspirin and paracetamol. British Journal of Anaesthesia 1967;39:146‐54. [DOI] [PubMed] [Google Scholar]
Parkhouse 1968 {published data only}
- Parkhouse J, Rees‐Lewis M, Skolinik M, Peters H. The clinical dose response to aspirin. British Journal of Anaesthesia 1968;40(6):433‐41. [DOI] [PubMed] [Google Scholar]
Perotti 1984 {published data only}
- Perotti R. Diflunisal and acetylsalicylic acid: a double blind study of their effect on post‐extraction pain [Diflunisal ed ASA: studio in doppio cieco, sull'azione del dolore post‐estrattivo]. Minerva Stomatologica 1984;33(4):645‐50. [PubMed] [Google Scholar]
Reines 1986 {published data only}
- Reines HD, Hunt P, Rambo W, Loadholt CB. Oral ciramadol: a new analgesic for postoperative pain. Journal of Clinical Pharmacology 1986;26(2):111‐4. [DOI] [PubMed] [Google Scholar]
Rowe 1981 {published data only}
- Rowe N, Cudmore C, Turner J. Control of pain by mefenamic acid following removal of impacted molar. A double blind, placebo controlled study. Oral Surgery, Oral Medicine and Oral Pathology 1981;51(6):575‐80. [DOI] [PubMed] [Google Scholar]
Rye 1978 {published data only}
- Rye L, Siegel S. A study of factors influencing the qualitative response and/or the time interval of response to mild analgesics for postoperative pain. Georgetown Dental Journal 1978;62(2):36‐41. [PubMed] [Google Scholar]
Sechzer 1977 {published data only}
- Sechzer P. Evaluation of fenoprofen as a postoperative analgesic. Current Therapeutic Research 1977;21(2):137‐48. [PubMed] [Google Scholar]
Seymour 1982 {published data only}
- Seymour RA, Rawlins MD. Efficacy and pharmacokinetics of aspirin in post operative dental pain. British Journal of Clinical Pharmacology 1982;13(6):807‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seymour 1983a {published data only}
- Seymour RA, Blair GS, Wyatt FAR. Post‐operative dental pain and analgesic efficacy. British Journal of Oral Surgery 1983;21:298‐303. [DOI] [PubMed] [Google Scholar]
Seymour 1983b {published data only}
- Seymour RA. Analgesic efficacy and plasma concentration of three analgesics in pain after lower third molar removal. SAAD Digest 1983;5(7):172‐88. [PubMed] [Google Scholar]
Seymour 1984 {published data only}
- Seymour RA, Williams FM, Ward A, Rawlins MD. Aspirin metabolism and efficacy in postoperative dental pain. British Journal of Clinical Pharmacology 1984;17(6):697‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sindet 1986 {published data only}
- Sindet PS, Petersen J, Gotzsche P, Christensen H. A double blind, randomized study of naproxen and acetylsalicylic acid after surgical removal of impacted lower third molars. International Journal of Oral and Maxillofacial Surgery 1986;15(4):389‐94. [DOI] [PubMed] [Google Scholar]
Skjelbred 1977 {published data only}
- Skjelbred P, Album B, Lokken P. Acetylsalicylic acid vs paracetamol:effects on postoperative course. European Journal of Clinical Pharmacology 1977;12:257‐64. [DOI] [PubMed] [Google Scholar]
Skjelbred 1984 {published data only}
- Skjelbred P. The effects of acetylsalicylic acid on swelling, pain and other events after surgery. British Journal of Clinical Pharmacology 1984;17(4):379‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Slatis 1980 {published data only}
- Slatis P, Janhunen L, Quiding H. Multiple dose evaluation of analgesics, a comparison between two dextropropoxyphene compounds and acetylsalicylic acid in pain following meniscectomy. Current Therapeutic Research 1980;27:595‐600. [Google Scholar]
Spivach 1984 {published data only}
- Spivach A, Zamborlini F, Piccoli‐Briganti F. Appraisal of the activity of one analgesic and tolerability of a drug of cecita association a controlled clinical study [Valutazione dell'attivita analgescia e della tollerabilita di un farmaco di associazione studio clinico controllato in doppia cecita]. Archivo Di Medicina Internationali 1984;36(3):151‐65. [Google Scholar]
Sunshine 1975 {published data only}
- Sunshine A. Analgesic value of fenbufen in postoperative patients. A comparative oral analgesic study of fenbufen, aspirin, and placebo. Journal of Clinical Pharmacology 1975;15(8‐9):591‐7. [DOI] [PubMed] [Google Scholar]
Sunshine 1978a {published data only}
- Sunshine A, Slafta J, Gruber CJr. A comparative analgesic study of propoxyphene, fenoprofen, the combination of propoxyphene and fenoprofen, aspirin, and placebo. Journal of Clinical Pharmacology 1978;18(11‐12):556‐63. [DOI] [PubMed] [Google Scholar]
Sunshine 1978b {published data only}
- Sunshine A, Laska E, Slafta J. Oral nefopam and aspirin. Clinical Pharmacology and Therapeutics 1978;24(5):555‐9. [DOI] [PubMed] [Google Scholar]
Sunshine 1986 {published data only}
- Sunshine A, Zighelboim I, Laska E, Siegel C, Olson NZ, Castro A. A double blind, parallel comparison of ketoprofen, aspirin, and placebo in patients with postpartum pain. Journal of Clinical Pharmacology 1986;26(8):706‐11. [DOI] [PubMed] [Google Scholar]
Sveen 1975 {published data only}
- Sveen K, Gilhuus MO. Paracetamol/codeine in relieving pain following removal of impacted mandibular third molars. International Journal of Oral Surgery 1975;4(6):258‐66. [DOI] [PubMed] [Google Scholar]
Szmyd 1959 {published data only}
- Szmyd L, McCall CM, Porreca AL. Aspirin and placebo in oral surgery. Journal of the American Dental Association 1959;59:84‐7. [DOI] [PubMed] [Google Scholar]
Todorovic 1982 {published data only}
- Todorovic L, Petrovic V, Kafedziska V. Clinical investigation of analgesic and anti inflammatory effects of niflumic acid following oral surgery [Klinicka ispitivanja analgetskog i antiinflamatornog efekta niflumicne kiseline posle oralno‐hirurskih intervencija]. Stomatoloski Glasnik Srbije 1982;29(5):325‐30. [PubMed] [Google Scholar]
Trop 1983 {published data only}
- Trop D, Nucci C, Elie R, Gareau J. Double‐blind comparative evaluation of tiaprofenic acid (Surgam®) versus acetylsalicylic acid (ASA) in relieving pain following episiotomy. Current Therapeutic Research 1983;34(2I):274‐9. [Google Scholar]
Vaidya 1974 {published data only}
- Vaidya A, Sheth M, Manghani K, Shroff P, Vora K, Sheth U. Double blind trial of mefenamic acid, aspirin and placebo in patients with post‐operative pain. Indian Journal of Medical Sciences 1974;28(12):532‐6. [PubMed] [Google Scholar]
Versichelen 1982 {published data only}
- Versichelen L, Bilsback P, Rolly G, Merlo M, Joubert L. Etodolac in postsurgical pain: a double blind dose ranging efficacy study with aspirin and placebo. International Journal of Clinical Pharmacology Therapeutics and Toxicology 1982;20(5):236‐9. [PubMed] [Google Scholar]
Von Graffenried 1980 {published data only}
- Graffenried B, Nuesch E, Maeglin B, Hagler W, Kuhn M. Assessment of analgesics in dental surgery outpatients. European Journal of Clinical Pharmacology 1980;18(6):479‐82. [DOI] [PubMed] [Google Scholar]
Walker 1960 {published data only}
- Walker DE, Grimm DH, Way EL. An evaluation of the analgesic properties of a salicylamide derivative in comparison with a placebo and aspirin. Oral Surgery, Oral Medicine and Oral Pathology 1960;13(10):1214‐7. [DOI] [PubMed] [Google Scholar]
Wang 1979 {published data only}
- Wang RI, Waite EM. The clinical analgesic efficacy of oral nefopam hydrochloride. Journal of Clinical Pharmacology 1979;19(7):395‐402. [DOI] [PubMed] [Google Scholar]
Wilkinson 1960 {published data only}
- Wilkinson FC, Howe GL. The relative values of 'Antidol' and calcium aspirin as an analgesic. Practitioner 1960;185:316‐20. [PubMed] [Google Scholar]
Winter 1978 {published data only}
- Winter LJ, Bass E, Recant B, Cahaly J. Analgesic activity of ibuprofen (Motrin) in postoperative oral surgical pain. Oral Surgery, Oral Medicine and Oral Pathology 1978;45:159‐66. [DOI] [PubMed] [Google Scholar]
Wojcicki 1977 {published data only}
- Wojcicki J, Samochowiec L, Lawczynski L, Szwed G, Olszewska M. A double blind comparative evaluation of aspirin, paracetamol and paracetamol + caffeine (Finimal) for their analgesic effectiveness. Archivum Immunologiae et Therapiae Experimentalis 1977;25(2):175‐9. [PubMed] [Google Scholar]
Zederfeldt 1977 {published data only}
- Zederfeldt B, Borg I, Haeger K. Efficacy and tolerance of Flunixin (SCH 14714) in the treatment of postoperative pain, with observations on the methodology of postoperative pain studies. British Journal of Anaesthetics 1977;49(5):467‐71. [DOI] [PubMed] [Google Scholar]
Additional references
Barden 2004
- Barden J, Edwards JE, McQuay HJ, Wiffen PJ. Relative efficacy of oral analgesics after third molar extraction. British Dental Journal 2004;197(7):407‐11. [DOI] [PubMed] [Google Scholar]
Clarke 2009
- Clarke R, Derry S, Moore RA, McQuay HJ. Single dose oral etoricoxib for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004309.pub2] [DOI] [PubMed] [Google Scholar]
Collins 1997
- Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres?. Pain 1997;72:95‐7. [DOI] [PubMed] [Google Scholar]
Collins 2001
- Collins SL, Edwards J, Moore RA, Smith LA, McQuay HJ. Seeking a simple measure of analgesia for mega‐trials: is a single global assessment good enough?. Pain 2001;91(1‐2):189‐94. [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310:452‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2008
- Derry S, Moore RA, McQuay HJ. Single dose oral celecoxib for acute postoperative pain. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004233] [DOI] [PubMed] [Google Scholar]
Derry 2009
- Derry C, Derry S, Moore RA, McQuay HJ. Single dose oral ibuprofen for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001548.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 1999a
- Edwards JE, McQuay HJ, Moore RA, Collins SL. Reporting of adverse effects in clinical trials should be improved ‐ lessons from acute pain. Journal of Pain and Symptom Management 1999;18:427‐37. [DOI] [PubMed] [Google Scholar]
Fitzgerald 2001
- FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase‐2. New England Journal of Medicine 2001;345(6):433‐42. [DOI] [PubMed] [Google Scholar]
Houde 1965
- Houde RW, Wallenstein SL, Beaver WT. Clinical measurement of pain. In: Stevens G editor(s). Analgetics. New York and London: Academic Press, 1965:75‐122. [Google Scholar]
Jadad 1996a
- Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66:239‐46. [DOI] [PubMed] [Google Scholar]
Jadad 1996b
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. [DOI] [PubMed] [Google Scholar]
McQuay 2005
- McQuay HJ, Moore RA. Placebo. Postgraduate Medical Journal 2005;81:155‐60. [DOI: 10.1136/pgmj.2004.024737] [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 2007
- McQuay HJ, Moore RA. Dose‐response in direct comparisons of different doses of aspirin, ibuprofen and paracetamol (acetaminophen) in analgesic studies. British Journal of Clinical Pharmacology 2007;63(3):271‐8. [DOI: 10.1111/j.1365-2125.2006.02723.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 1996
- Moore RA, McQuay HJ, Gavaghan DJ. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain 1996;66:229‐37. [DOI] [PubMed] [Google Scholar]
Moore 1997a
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: verification from independent data. Pain 1997;69:127‐30. [DOI] [PubMed] [Google Scholar]
Moore 1997b
- Moore A, Moore O, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: use of pain intensity and visual analogue scales. Pain 1997;69:311‐5. [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramer MR, Collins SL, McQuay HJ. Size is everything‐large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI: 10.1016/S0304-3959(98)00140-7] [DOI] [PubMed] [Google Scholar]
Moore 2003
- Moore RA, Edwards J, Barden J, McQuay HJ. Bandolier's Little Book of Pain. Oxford: Oxford University Press, 2003. [ISBN: 0‐19‐263247‐7] [Google Scholar]
Moore 2005
- Moore RA, Edwards JE, McQuay HJ. Acute pain: individual patient meta‐analysis shows the impact of different ways of analysing and presenting results. Pain 2005;116(3):322‐31. [DOI: 10.1016/j.pain.2005.05.001] [DOI] [PubMed] [Google Scholar]
Moore 2006
- Moore A, McQuay H. Bandolier's Little Book of Making Sense of the Medical Evidence. Oxford: Oxford University Press, 2006. [ISBN: 0‐19‐856604‐2] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐23. [ISBN: 978‐0‐931092‐69‐5] [Google Scholar]
Moore 2011
- Moore RA, Derry S, McQuay HJ, Wiffen PJ. Single dose oral analgesics for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD008659.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 1995
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratio and standardised ratios and rates. In: Gardner MJ, Altman DG editor(s). Statistics with Confidence ‐ Confidence Intervals and Statistical Guidelines. London: British Medical Journal, 1995:50‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
PCA 2011
- The NHS Information Centre, Prescribing Support Unit. Prescription Cost Analysis England 2010. The Health and Social Care Information Centre 2011.
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Toms 2008
- Toms L, McQuay HJ, Derry S, Moore RA. Single dose oral paracetamol (acetaminophen) for postoperative pain in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004602] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Edwards 1999b
- Edwards JE, Oldman A, Smith L, Carroll D, Wiffen PJ, McQuay HJ, et al. Oral aspirin in postoperative pain: a quantitative systematic review. Pain 1999;81:289‐97. [DOI] [PubMed] [Google Scholar]
Edwards 2000
- Edwards JE, Oldman A, Smith L, Collins SL, Carroll D, Wiffen PJ, et al. Single dose oral aspirin for acute pain. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002067] [DOI] [PubMed] [Google Scholar]


