Abstract
Background
Infection is one of the most common complications and still remains a significant cause of morbidity and occasionally mortality in patients, especially children with nephrotic syndrome. Many different prophylactic interventions have been used or recommended for reducing the risks of infection in nephrotic syndrome in clinical practice. Whether the existing evidence is scientifically rigorous and which prophylactic intervention can be recommended for routine use based on the current evidence is still unknown.
Objectives
To assess the benefits and harms of any prophylactic intervention for reducing the risk of infection in children and adults with nephrotic syndrome.
Search methods
We searched the Cochrane Renal Group's specialised register, the Cochrane Central Register of Controlled Trials (CENTRAL) (in The Cochrane Library), MEDLINE and Pre‐MEDLINE (from 1966), EMBASE (from 1980), China Biological Medicine Database (1979 to December 2009), Chinese Science and Technique Journals Database (to December 2009), China National Infrastructure (to December 2009), WangFang database (to December 2009), reference lists of nephrology textbooks, review articles, relevant studies and abstracts from nephrology meetings without language restriction.
Date of last search: 6 February 2012
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs comparing any prophylactic interventions (pharmacological or non‐pharmacological) for preventing any infection in children and adults with nephrotic syndrome.
Data collection and analysis
Two authors independently assessed and extracted information. Information was collected on methods, participants, interventions and outcomes (appearance of infection, mortality, quality of life and adverse events). Results were expressed as risk ratios (RR) for dichotomous outcomes or as mean differences (MD) for continuous data with 95% confidence intervals (CI).
Main results
Twelve studies conducted in China, including 762 children with nephrotic syndrome were identified. No studies were identified in adults. All studies compared one kind of prophylactic pharmacotherapy (intravenous immunoglobulin (IVIG), thymosin, oral transfer factor, mannan peptide tablet, Bacillus Calmette‐Guerin (BCG) vaccine injection, polyvalent bacterial vaccine (Lantigen B) and two kinds of Chinese medicinal herbs: a compound of Chinese medicinal herbs (TIAOJINING) and Huangqi (astragalus) granules) plus baseline treatment with baseline treatment alone. No RCTs were identified comparing antibiotics, non‐pharmacological prophylaxis, or pneumococcal vaccination. Four studies showed a significantly beneficial effect of IVIG on preventing nosocomial or unspecified infection in children with nephrotic syndrome (RR 0.47, 95% CI 0.31 to 0.73). Thymosin (RR 0.50, 95% CI 0.26 to 0.97), oral transfer factor (RR 0.51, 95% CI 0.35 to 0.73), BCG vaccine injection (RR 0.68, 95% CI 0.48 to 0.95), Huangqi granules (RR 0.62, 95% CI 0.47 to 0.83) and TIAOJINING (RR 0.59, 95% CI 0.43 to 0.81) were also effective in reducing the risk of infection in children with nephrotic syndrome. However mannan peptide tablet (RR 0.46, 95% CI 0.21 to 1.01) and polyvalent bacterial vaccine (RR 0.24, 95% CI 0.06 to1.00) were not superior to baseline treatment in reducing the risk of infection for nephrotic children. No serious adverse events were reported.
Authors' conclusions
IVIG, thymosin, oral transfer factor, BCG vaccine, Huangqi granules and TIAOJINING may have positive effects on the prevention of nosocomial or unspecified infection with no obvious serious adverse events in children with nephrotic syndrome. However the methodological quality of all studies was poor, the sample sizes small, and all studies were from China, and thus there is no strong evidence on the effectiveness of these interventions.
Keywords: Child; Humans; Astragalus Plant; BCG Vaccine; BCG Vaccine/therapeutic use; Bacterial Infections; Bacterial Infections/prevention & control; China; Cross Infection; Cross Infection/prevention & control; Drugs, Chinese Herbal; Drugs, Chinese Herbal/therapeutic use; Immunoglobulins, Intravenous; Immunoglobulins, Intravenous/therapeutic use; Nephrotic Syndrome; Nephrotic Syndrome/complications; Randomized Controlled Trials as Topic; Thymosin; Thymosin/therapeutic use; Transfer Factor; Transfer Factor/therapeutic use
No strong evidence for any interventions for preventing infection in nephrotic syndrome
Patients with nephrotic syndrome, particularly children, are susceptible to infections. Infections can cause frequent relapses of illness, poor response to therapies (e.g. steroids) and severe infections occasionally lead to death. Oral antibiotics, pneumococcal vaccination, some immunomodulators and Chinese medicinal herbs have been used/recommended for reducing the risk of infection. No studies on antibiotics, pneumococcal vaccination and any other non‐drug prophylaxis were identified. This review found that intravenous immunoglobulin (IVIG), thymosin, oral transfer factor, Bacillus Calmette‐Guerin (BCG) vaccine injection and two kinds of Chinese medicinal herbs (Huangqi granules and TIAOJINING) may help prevent infections in nephrotic children. These studies were methodologically poor. Currently there is no strong evidence for recommending any interventions for preventing infections in nephrotic syndrome. More research is needed.
Background
Infection is one of the most common complications in patients with nephrotic syndrome, especially in nephrotic children. The majority of infections are closely associated with frequent relapses and steroid dependency in children with nephrotic syndrome (Gulati 1996; Guo 2008; Kang 2005; Moorani 2003; Wang 1999; Yap 2001) which result in significant mortality, morbidity and health care costs, especially in developing countries (Choudhary 1977). Hospital‐based, retrospective case series from the 1960s to 1980s had consistently shown the annual incidence of invasive bacterial infection to be about 1% to 2%, and a cumulative risk of 10% to 20% for the 10‐year susceptibility period (McIntyre 1998). In China, many studies have also reported a high incidence rate of nosocomial infection of about 34% to 79% in nephrotic children (Du 1996; Guo 2008; Ma 1996; Rao 2005; Wu 1998; Wu 2000; Wu 2009), and 22% in adults (Li 1996). With the introduction and widespread use of corticosteroids and antibiotics in the management of nephrotic syndrome, death from infective complications has now become rare. Despite this, the International Study Group of Kidney Disease in Children indicated that, of the 10 deaths amongst the nearly 400 children with minimal change disease followed for five to 10 years, six occurred after infection, resulting in a cumulative infection‐related mortality incidence of 1.5% (ISKDC 1984). Therefore infections still remain a significant cause of morbidity and occasionally mortality in nephrotic patients all over the world (Tain 1999).
With respect to the pattern of infection, a variety of infectious complications have been reported in patients with nephrotic syndrome, particularly bacterial infections. Of these, peritonitis and sepsis are the most serious infections in hospitalised nephrotic children, usually caused by encapsulated organisms (Streptococcus pneumonia) and Gram‐negative enteric organisms, predominantly Escherichia coli (E. coli) (McIntyre 1998; Moorani 2003; Tain 1999).
There are several explanations for this increased risk, including oedema (which may predispose to entry and spread of infection), urinary losses of factor B and D of the alternative complement pathway, impaired polymorph phagocytic function and secondary effects of corticosteroids and cytotoxic therapy (Johnson 2000; Shroff 2002).
Many efforts have been made to investigate the effectiveness of various interventions for dealing with this problem worldwide. At present, multiple different prophylactic interventions are used and/or recommended for reducing the risk of infection in patients with nephrotic syndrome in clinical practice. These include avoidance of nephrosis, chemoprophylaxis with antibiotics, pneumococcal vaccines, and immunoglobulin replacement therapies (McIntyre 1998; Shroff 2002). One study from the USA demonstrated that concomitant use of oral acyclovir might prevent serious varicella infection in patients receiving corticosteroids (Goldstein 2000). Another study from the USA showed that varicella vaccine was generally well tolerated and highly immunogenic in children with nephrotic syndrome, including those on low‐dose, alternate‐day prednisone (Furth 2003). One study from the France indicated that there was high serological response to pneumococcal vaccine in nephrotic children at disease onset on high‐dose prednisone (Ulinski 2008). Other studies have reported that serum immunoglobulin (IgG) levels were markedly depressed in nephrotic syndrome with serious infections, and administration of intravenous immunoglobulin (IVIG) may reduce the risk of infection in adult nephrotic syndrome patients with serum IgG levels below 600 mg/dL (Ogi 1994; Tain 1999). In China, several studies have also demonstrated that IVIG, intravenous or intramuscular thymosin (mainly thymosin‐α1) are beneficial for preventing nosocomial infection in children with nephrotic syndrome (Dang 1999; Wu 2009; Zhang 2000). IVIG contains an array of alloantibodies directed against infectious agents. This pooled material also has a broad spectrum of allo‐ and autoantibodies and other substances, which may be key to immune function and regulation (Maramica 2003). With respect to thymosin (mainly thymosin‐α1), it is an immunomodulating agent and may influence T‐cell maturation and antigen recognition, the stimulation of interferon and cytokine production, and the activity of natural killer cell‐mediated cytotoxicity (Chan 2001). Whether the existing evidence is scientifically rigorous and which prophylactic intervention can be recommended for routine use based on the current evidence is still unknown. One narrative review about the prevention of bacterial infection in nephrotic children indicated that there were insufficient data (i.e. controlled studies) for recommending prophylactic use of antibiotics and pneumococcal vaccination (McIntyre 1998). However where penicillin‐resistant pneumococci are common, they recommended the administration of pneumococcal vaccination in paediatric practices and claimed that it would favourably alter the outcome of children with nephrotic syndrome, making treatment with prophylactic antibiotics obsolete. Furthermore, some antibiotics, pneumococcal vaccine and other prophylactic therapies (e.g. IVIG, thymosin) are quite expensive and have some adverse effects. Before these prophylactic interventions can be recommended for routine use in patients with nephrotic syndrome, rigorous randomised evidence should be provided to show its effectiveness.
The aim of this review is to analyse systematically all the randomised controlled trials (RCTs) of prophylactic interventions for reducing the risk of infection in nephrotic syndrome in children and adults.
Objectives
This review aims to assess the benefits and harms of any prophylactic intervention for reducing the risk of infection in children and adults with nephrotic syndrome, regardless of cause or pathologic change.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs (e.g. allocation using alternative, case record numbers, date of birth or day of the week) looking at the benefits and harms of any prophylactic intervention (pharmacological or non‐pharmacological) compared with placebo, no treatment or other pharmacological or non‐pharmacological treatment were eligible for inclusion. The first period of randomised cross‐over studies was also to be included.
Types of participants
Inclusion criteria
Studies that included patients of any age or sex, with any type of nephrotic syndrome (primary or secondary) regardless of pathologic changes were eligible. In the absence of an explicit definition of "nephrotic syndrome", the diagnosis of nephrotic syndrome in adults was based on the excretion of large amount of protein in the urine/d (> 3.5 g/24 h urine/d) and low serum protein (< 30 g/L), and in children defined by proteinuria > 50 mg/kg/24 h and serum albumin ≤ 2.0 g/dL (Roth 2002).
Exclusion criteria
There were no exclusions.
Types of interventions
Studies evaluating any prophylactic therapy, pharmacological or not, administered for patients with nephrotic syndrome to prevent appearance of any types of infection were included (e.g. oral antibiotics; long‐term, low‐dose antibiotics; pneumococcal vaccination).
We intended to display studies as comparisons as follows in this review:
any prophylactic intervention compared to placebo;
any prophylactic intervention compared to no treatment;
prophylactic intervention in addition baseline medication or treatment compared to baseline medication or treatment alone (e.g. steroidal agents and/or adjunctive immunosuppressive agents (e.g. cyclophosphamide) and other supportive therapies);
any prophylactic intervention compared to another treatment.
Studies that assessed the effectiveness of any interventions for treatment of suspected or confirmed infection in patents with nephrotic syndrome were excluded.
Types of outcome measures
Number of patients who develop any type of infection in each group. We used the authors` definition for the diagnosis of infections.
Mortality
Quality of life
Any adverse events
Search methods for identification of studies
Electronic searches
Initial search
Relevant studies were initially obtained from the following sources (see Appendix 1) with no language restriction.
Cochrane Renal Group Specialised Register of Randomised Controlled Trials (January 2003)
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 1, 2003)
MEDLINE and Pre‐MEDLINE (1966 to February 2003) combined with the Cochrane highly sensitive search strategy for identifying RCTs in MEDLINE (Dickersin 1994). Please see Cochrane Renal Group Module for details of these strategies.
EMBASE (1980 to February 2003) Modified MEDLINE search and combines with the Cochrane highly sensitive search strategy for identifying RCTs in EMBASE (Lefebvre 1996).
China Biological Medicine Database (CBMdisc, 1979 to December 2002) which is a Chinese biological research literature registry.
Review update
CENTRAL and the Cochrane Renal Group's specialised register were searched as above for new studies. CENTRAL and the Renal Group's Specialised Register contain the handsearched results of conference proceedings from general and speciality meetings. This is an ongoing activity across the Cochrane Collaboration and is both retrospective and prospective (http://www.cochrane.us/masterlist.asp). Therefore we did not specifically search conference proceedings for any new studies.
Relevant new studies were obtained from the following sources (see Appendix 1) with no language restriction in the updated review.
Cochrane Renal Group Specialised Register of Randomised Controlled Trials (January 2012).
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 1, 2012)
China Biological Medicine Database (CBMdisc, December 2002 to December 2009) which is a Chinese biological research literature registry.
Chinese Science and Technique Journals Database (until December 2009)
China National Infrastructure (until December 2009)
WangFang database (until December 2009)
Searching other resources
Reference lists of nephrology textbooks, review articles and relevant studies.
Reference lists of abstracts from nephrology scientific meetings.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable, however studies and reviews that might include relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and the full text of these studies to determine which studies satisfied our inclusion criteria.
Data extraction and management
Data extraction was carried out independently by the same authors using standard data extraction forms. Studies not in English or Chinese were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used. Where relevant outcomes were only published in earlier versions these data were used. Any discrepancy between published versions was to be highlighted. Any further information required from the original author was requested by written correspondence and any relevant information obtained in this manner was included in the review. Disagreements were resolved in consultation with a third author.
Assessment of risk of bias in included studies
The following items were assessed using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation?
Was allocation adequately concealed?
Was knowledge of the allocated interventions adequately prevented during the study?
Were incomplete outcome data adequately addressed?
Are reports of the study free of suggestion of selective outcome reporting?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (e.g. mortality, appearance of infection, adverse effects), the results were expressed as risk ratios (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used (e.g. quality of life), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales were to be used.
Assessment of heterogeneity
Heterogeneity was analysed using a Chi² test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
If sufficient studies were identified, we planned to examine for publication bias using a funnel plot (Higgins 2011).
Data synthesis
Data were pooled using the random‐effects model but the fixed‐effect model was also used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
We planned to sue subgroup analyses to explore possible sources of heterogeneity (e.g. participants, type of nephrotic syndrome, treatments). Heterogeneity among participants could be related to age, cause of nephrotic syndrome, and renal pathology. Heterogeneity in treatments could be related to the type of nephrotic syndrome (primary or secondary), prior agent(s) used, the agent used, and dose and duration of therapy. Adverse effects were to be tabulated and assessed with descriptive techniques, as they were likely to be different for the various agents used. If possible, the risk difference (RD) with 95% CI was to be calculated for each adverse effect, either compared to no treatment or to another agent.
Sensitivity analysis
If a sufficient number of studies were found, sensitivity analyses were to be undertaken to examine the stability of the results in relation to study quality as follows:
excluding studies with inadequate concealment of allocation (Schulz 1995);
excluding unblinded studies.
Results
Description of studies
Results of the search
A total of 553 articles were initially identified with 215 published in non‐Chinese (English and Polish) and 338 in Chinese. Of these, 537 of articles were excluded for one or more of the following reasons through reading the title, abstract and/or full‐text:
duplicates;
review articles;
non‐clinical studies;
no control or reporting a clinical case series;
including disorders other than nephrotic syndrome;
studies evaluating the treatment of infection.
Sixteen potentially eligible studies were retrieved for further assessment. Of these, four articles were excluded.
Not pertinent to prevention of infection in patients with nephrotic syndrome (Abeyagunawardena 2008; Allison 1969).
Mixed population and the data were not able to be separated (Goldstein 2000)
Not randomised (Grzesiowski 1995).
Finally, twelve studies were eligible for inclusion in this systematic review. For details of the selection process for included studies (see Figure 1).
Figure 1.
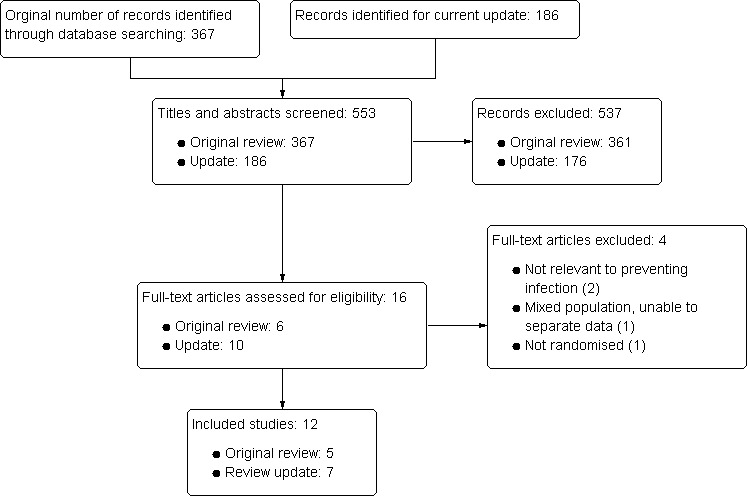
Study flow diagram.
For details see Characteristics of included studies and Characteristics of excluded studies.
Included studies
Of the twelve included studies, five studies were included in the first iteration of this review (Dang 1999; Dou 2000; Li 2000; Tong 1998; Zhang 2000) and seven new studies were included in this update (Chen 2008; Guo 2008; Kang 2003; Kang 2005; Rao 2005; Wu 2009; Ye 2004). The total number of patients enrolled was 762 and the average number of patient enrolled/study was 64 patients.
Participants
In 10 studies the age range was one to 14 years, and more males than females were included (57% to 80%) (Chen 2008; Dang 1999; Dou 2000; Li 2000; Kang 2003; Kang 2005; Rao 2005; Wu 2009; Ye 2004; Zhang 2000). Two studies did not describe the age or sex of the children (Guo 2008; Tong 1998). No studies were conducted in adults. Only two studies reported the range of clinical course (e.g. course of the disease). In Li 2000 this was five days to five months in new‐onset nephrotic syndrome, and 2.5 months to four years in relapsing patients, while in Chen 2008 the range of clinical course was one day to 26 months. All participants were inpatients recruited from paediatric departments. Eight studies only enrolled patients with primary nephrotic syndrome (Chen 2008; Dang 1999; Guo 2008; Kang 2003; Kang 2005; Rao 2005; Ye 2004; Zhang 2000); one study enrolled patients with both primary and secondary nephrotic syndrome (Tong 1998); and in three studies the type of nephrotic syndrome was not specified (Dou 2000; Li 2000; Wu 2009). All studies used "the National Diagnostic Criteria for Nephrotic Syndrome in Children" to confirm the diagnosis of nephrotic syndrome (Jiang 1981), which is similar to "the International Study of Kidney Disease in Children Criteria for Nephrotic Syndrome" (Brodehl 1986). All studies were conducted in China.
From the available data, it was not possible to conduct subgroup analyses based on age, cause and renal pathology.
Interventions
All studies compared one kind of prophylactic pharmacotherapy in addition to baseline medication or treatment with baseline medication or treatment alone. No studies used placebo, no treatment or another prophylactic treatment control. The prophylactic therapies evaluated in this review included the following.
Thymosin (Zhang 2000): a 28‐amino acid polypeptide isolated from thymosin fraction V, a bovine thymus extract containing a number of immunologically active peptides
Oral transfer factor (Rao 2005): an immunomodulating agent, is a kind of polynuclear acid and small molecular polypeptide isolated from human's white blood cells
Mannan peptide tablet (Guo 2008): a new immunomodulating agent with multiple beneficial effects on human's immune functions
BCG vaccine injection (Kang 2003): made from a weakened form of Mycobacterium bovisa bacterium closely related to human, is also an immunomodulating agent
Polyvalent bacterial vaccine (Lantigen B) (Ye 2004): oral product based on bacterial lysates of six different inactivated strains (Streptococcus pneumoniae type 3, Streptococcus pyogenes group A, Branhamella catarrhalis, Staphylococcus aureus, Haemophilus influenzae type B and Klebsiella pneumoniae) commonly involved in respiratory tract infections (Pozzi 2004)
TIAOJINING (Li 2000): a compound of Chinese medicinal herbs including six principle herbs (Shengdi, Zhimu, Zexie, Shanyurou, Xianlinpi, Baihuasheshecao) with some effects on immunomodulation
Huangqi (astragalus) granules (Chen 2008; Kang 2005): astragalus polysaccharides, astragaloside, amino acids and multiple microelements, is an immunomodulating traditional Chinese herb with beneficial effects on the improvement of human's immune functions (Li 1992; Xu 2010).
No studies involved prophylactic antibiotics, pneumococcal vaccination, varicella vaccine or other non‐pharmacological prophylactic interventions for reducing the risk of infection in children or adults with nephrotic syndrome. The baseline medication included steroid agents (e.g. oral prednisone), adjunctive immunosuppressive agents (e.g. cyclophosphamide) and other supportive therapies (e.g. calcium, potassium, heparin).
Outcomes
The most commonly reported outcome was the number of patients developing nosocomial or unspecified infections. The diagnosis of nosocomial infection was based on the diagnostic criteria for nosocomial infection established by both USA and China (Wang 1990). Unspecified infections were based on the authors' definition including clinical symptoms and signs, laboratory examinations and chest X‐ray. Mortality was assessed in only one study (Tong 1998). None of the 12 studies provided any information on quality of life. Adverse events were reported in six studies (Chen 2008; Dang 1999; Kang 2003; Kang 2005; Rao 2005; Tong 1998).
Risk of bias in included studies
Allocation
Of the 12 included studies, the method of sequence generation was inadequate in one study (Chen 2008) the patients with nephrotic syndrome were allocated to the treatment and control groups according to the order of admission. The remaining eleven studies did not describe the method of randomisation in detail.
Allocation concealment was unclear in all included studies.
Blinding
Blinding was not reported in any of the included studies.
Incomplete outcome data
No studies reported a sample size calculation. There was no statement on intention‐to‐treat analysis in any of the included studies. The number of patients randomised equalled that analysed in all included studies. There was no statement on dropouts or withdrawals in any of the included studies.
Selective reporting
Of the 12 included studies, one study was free of selective reporting (Tong 1998), five studies were unclear (Chen 2008; Dang 1999; Kang 2003; Kang 2005; Rao 2005) and six studies were assessed as having a high risk of bias for selective reporting due to the non‐reporting of some clinically important outcomes (e.g. adverse events) (Dou 2000; Guo 2008; Li 2000; Wu 2009; Ye 2004; Zhang 2000).
Other potential sources of bias
We were unable to determine if there were any other potential sources of bias in any of the included studies.
Generally, of the 12 included studies, seven studies were assessed as high risk of bias and five studies were assessed as unclear in risk of bias. See Figure 2 and Figure 3.
Figure 2.
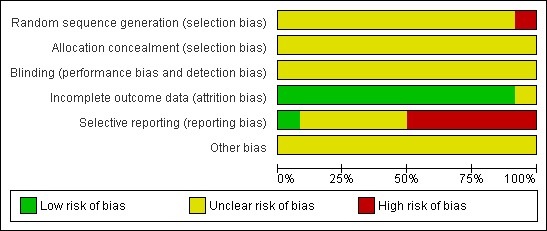
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
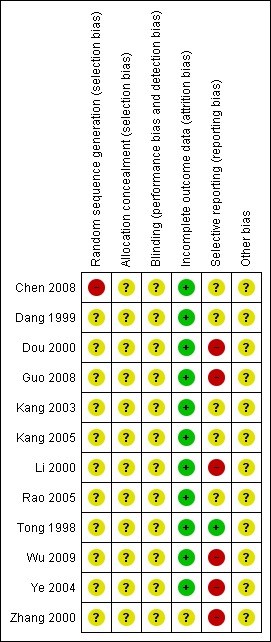
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Number of patients developing nosocomial infection or unspecified infection
Intravenous immunoglobulin
IVIG significantly reduced the number of nosocomial or unspecified infection in children with nephrotic syndrome when compared to baseline treatment (Analysis 1.1 (4 studies, 248 participants): RR 0.47, 95% CI 0.31 to 0.73; P = 0.0006). There was no statistically significant between‐study heterogeneity (I² = 22%).
Analysis 1.1.
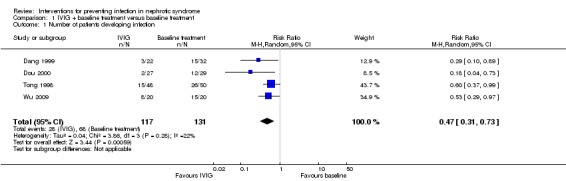
Comparison 1 IVIG + baseline treatment versus baseline treatment, Outcome 1 Number of patients developing infection.
Two studies reported the spectrum of infections and causative microorganisms (Dang 1999; Tong 1998). Of these, one study showed no statistically significant difference in the spectrum of infections between the IVIG and the control groups (P > 0.05) (Tong 1998). Abdominal infection, upper respiratory infection (URI) and urinary tract infection (UTI) were found to be the most common infections in control group and E. coli was the major pathogen in the cultures of ascitic fluid and urine. Data on the difference in the spectrum of infections between groups were not available in Dang 1999, in which respiratory infection and UTI were reported to be the most frequent infections, followed by diarrhoea and skin infection, and E. coli was found to be predominant in the urine cultures. The time to measurement of outcomes was stated clearly in only one study (four to six weeks after the end of treatment) (Dou 2000).
Thymosin
One study (Zhang 2000) reported thymosin reduced the risk of infection in children with nephrotic syndrome at the end of treatment (Analysis 2.1 (1 study, 40 participants): RR 0.50, 95% CI 0.26 to 0.97). The spectrum of infections showed no statistically significant difference between two groups (P > 0.05); however skin infection and peritonitis were only found in the control group. URI and UTI were found to be the most common infections in this study. Data on the spectrum of pathogen were not stated sufficiently where E. coli was proved to be positive in two urine cultures.
Analysis 2.1.

Comparison 2 Thymosin + baseline treatment versus baseline treatment, Outcome 1 Number of patients developing infection.
Oral transfer factor
One study (Rao 2005) reported oral transfer factor reduced the risk of infection in children with simple nephrotic syndrome at the end of follow‐up (one year) (Analysis 3.1 (1 study, 98 participants): RR 0.51, 95% CI 0.35 to 0.73). Data on the difference in the spectrum of infections between groups were not available, in which respiratory infection, urological infection and intestinal infection were reported to be the most frequent infections in this study. Data on the spectrum of pathogen were not stated.
Analysis 3.1.
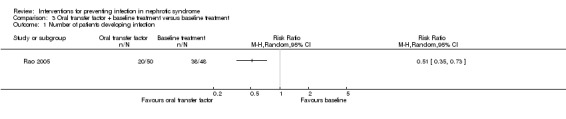
Comparison 3 Oral transfer factor + baseline treatment versus baseline treatment, Outcome 1 Number of patients developing infection.
Mannan peptide
One study (Guo 2008) reported mannan peptide was not superior to the control for preventing secondary infections in children with simple nephrotic syndrome at the end of follow‐up (six months) (Analysis 4.1 (1 study, 67 participants): RR 0.46, 95% CI 0.21 to 1.01). The spectrum of infections showed no statistically significant difference between groups (P > 0.05); however respiratory infection, urological infection and intestinal infection were found to be the most common infections in this study. Data on the spectrum of pathogen were not stated.
Analysis 4.1.
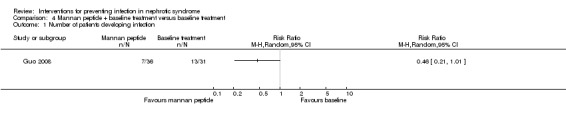
Comparison 4 Mannan peptide + baseline treatment versus baseline treatment, Outcome 1 Number of patients developing infection.
BCG vaccine injection
One study (Kang 2003) reported BCG vaccine prevented secondary infection in children with nephrotic syndrome at the end of follow‐up (three months) (Analysis 5.1 (1 study, 38 participants): RR 0.68, 95% CI 0.48 to 0.95). The spectrum of infections showed no statistically significant difference between groups (P > 0.05); however respiratory infection, urological infection and intestinal infection were found to be the most common infections in this study. Data on the spectrum of pathogen were not stated.
Analysis 5.1.
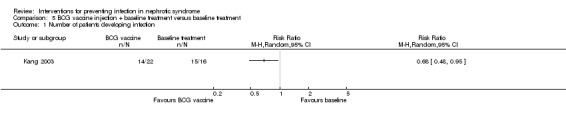
Comparison 5 BCG vaccine injection + baseline treatment versus baseline treatment, Outcome 1 Number of patients developing infection.
Polyvalent bacterial vaccine
One study (Ye 2004) reported Lantigen B was not superior to control in preventing infections for children with primary nephrotic syndrome at the end of treatment (four weeks) (Analysis 6.1 (1 study, 46 participants): RR 0.24, 95% CI 0.06 to1.00). There was a statistically significant difference in the spectrum of infections between groups (P < 0.05). It was reported that there were two patients suffering from URI and skin infection respectively in the treatment group, and four patients with URI, two patients with pneumonia and two patients with UTI in the control group. Data on the spectrum of pathogen were not stated in this study.
Analysis 6.1.
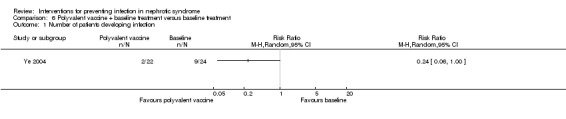
Comparison 6 Polyvalent vaccine + baseline treatment versus baseline treatment, Outcome 1 Number of patients developing infection.
Chinese medicinal herbs
Huangqi granules
Huangqi granules significantly reduced the number of nosocomial infection or unspecified infection in children with nephrotic syndrome (Analysis 7.1 (2 studies, 130 participants): RR 0.62, 95% CI 0.47 to 0.83; P = 0.001). There was no statistically significant between‐study heterogeneity for this outcome (I² = 0%). The spectrum of infections showed no statistically significant difference between groups (P > 0.05) in Kang 2005, however, respiratory infection, urological infection and intestinal infection were found to be the most common infections in this study. Data on the difference in the spectrum of infections between groups were not available in Chen 2008, in which respiratory infection was reported to be the most frequent infection followed by urological infection, intestinal infection and skin infection in both groups. Data on the spectrum of pathogen were not stated in the two studies.
Analysis 7.1.
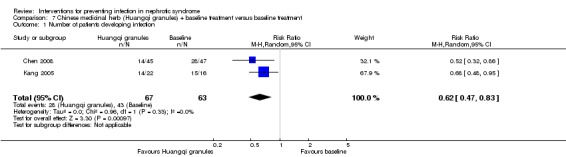
Comparison 7 Chinese medicinal herb (Huangqi granules) + baseline treatment versus baseline treatment, Outcome 1 Number of patients developing infection.
TIAOJINING
One study (Li 2000) reported that TIAOJINING prevented infections in children with nephrotic syndrome at the end of treatment (two months) (Analysis 8.1 (1 study, 60 participants): RR 0.59, 95% CI 0.43 to 0.81). There was no significant difference in the spectrum of infections between two groups (P > 0.05); however acute dysentery, skin infection, UTI and varicella were only found in the control group. URI was the most common infection in this study. Data on causative microorganisms were not stated.
Analysis 8.1.
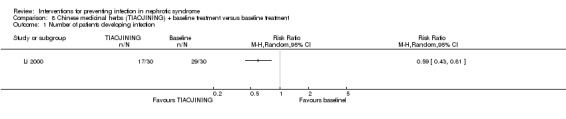
Comparison 8 Chinese medicinal herbs (TIAOJINING) + baseline treatment versus baseline treatment, Outcome 1 Number of patients developing infection.
Mortality
One study (Tong 1998), evaluating IVIG, reported two patients died of toxic dysentery and toxic ascitics respectively in the control group and no patients died in the intervention group. There was no significant difference in mortality between the IVIG and the control group (Analysis 1.2 (1 study, 98 participants): RR 0.21, 95% CI 0.01 to 4.23).
Analysis 1.2.
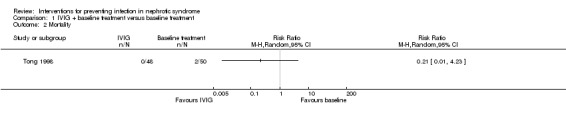
Comparison 1 IVIG + baseline treatment versus baseline treatment, Outcome 2 Mortality.
Quality of life
No studies evaluated quality of life.
Adverse events
Adverse events were reported in six studies (Chen 2008; Dang 1999; Kang 2003; Kang 2005; Rao 2005; Tong 1998) however they did not indicate whether the effects were ascertained through standardised monitoring or voluntary self‐report.
Two studies (Chen 2008; Kang 2005) reported that no obvious adverse effects were found in children with nephrotic syndrome treated with Huangqi granules.
Rao 2005 stated that oral transfer factor showed no obvious adverse effects for children with nephrotic syndrome.
Kang 2003 described an occurrence of local scleroma of the skin in a few of children with nephrotic syndrome after using BCG vaccine intramuscularly for a long time, which was relieved after physical therapy.
Dang 1999 described an occurrence of low fever in one patient with primary nephrotic syndrome during the second intervention with IVIG, and appearance of perspiration and tachycardia in another patient.
Tong 1998 reported one patient given IVIG developed sudden cough with shortness of breath which was relieved immediately after stopping the injection.
No data were reported on adverse events of thymosin, mannan peptide, polyvalent bacterial vaccine and TIAOJINING.
No other serious adverse events were reported in these six studies.
Subgroup analysis and investigation of heterogeneity
It was not possible to perform subgroup analysis in this systematic review because of the limited studies for each intervention.
Sensitivity analysis
It was not possible to perform sensitivity analysis based on risk of bias in this systematic review because of the general poor methodological quality in included studies.
Publication bias
It was not possible to perform a funnel plot to assess the degree of publication bias in this systematic review because of the limited studies for each outcome.
Discussion
Summary of main results
Twelve studies enrolling 762 children with nephrotic syndrome were included in this review. Because all the included studies were conducted in China and no data from other country were available, this review on interventions for preventing infection in patients with nephrotic syndrome is not representative of different racial groups. The main results revealed the following.
Compared with control, IVIG, thymosin, oral transfer factor, BCG vaccine injection, Huangqi granules, and TIAOJINING may have positive effects on the prevention of nosocomial infection or unspecified infection in children with nephrotic syndrome.
Mannan peptide and polyvalent bacterial vaccine were not superior to control on the prevention of nosocomial infection or unspecified infection in children with nephrotic syndrome.
Currently there was no evidence to support the superiority of IVIG, thymosin, oral transfer factor, mannan peptide, BCG vaccine injection, polyvalent bacterial vaccine, Huangqi granules and TIAOJININ over control in the changes of quality of life for children with nephrotic syndrome.
There were no serious adverse events reported in children with nephrotic syndrome using IVIG, oral transfer factor, BCG vaccine injection, and Huangqi granules for reducing the risk of infection.
No studies were identified that used chemoprophylaxis, pneumococcal vaccination, varicella vaccine or any other non‐pharmacological interventions for reducing the risk of infection in children or adults with nephrotic syndrome.
No studies were identified on interventions for preventing infection in adults with nephrotic syndrome.
Overall completeness and applicability of evidence
Validity of treatment
The validity of prophylactic intervention is closely related to its effectiveness. Information on the time to measurement of outcome and duration of follow‐up was not clearly reported in the majority of the included studies. Furthermore, the dosage and treatment period varied across studies involving IgG. Ideally, differences in agents, dosage, duration of therapy and follow‐up time in each study when evaluating the efficacy of prophylactic interventions should be considered. We were limited in this respect by the small number of studies.
Outcomes
The outcome definitions for the appearance of infection varied and were unclearly reported in most of the included studies. It is well known that prophylactic interventions show different effects on a variety of infections. However, data on the spectrum of infection and causative microorganisms were inadequately reported in many studies. Due to the limited number of studies, it was not possible to conduct subgroup analysis for the types of infections. The long‐term goal of prevention of infection in nephrotic syndrome is to reduce mortality and ultimately to prolong survival and improve quality of life. There was a lack of data available on clinically relevant outcomes from long‐term follow‐up such as mortality and quality of life. We were therefore unable to draw conclusions about these important outcomes.
Economic evaluation
In current health care practice, judgments often reflect clinical or social values concerning whether intervention benefits are worth the cost (Napodano 1986). Prophylactic use of IVIG and thymosin‐α1 should be based on a full economic evaluation and a clinical decision analysis that incorporates baseline risk for serious infection. However, no studies in this systematic review performed such analyses. It is well known that both IVIG and thymosin‐α1 are quite expensive, and cost is a burden, even in developed countries. In the US the FDA currently has regulatory control over IVIG as a drug and has approved its use for only six conditions in which efficacy has been proven in well‐controlled clinical studies (idiopathic thrombocytopenic purpura, primary immunodeficiency, secondary immunodeficiency due to chronic lymphocytic leukaemia, paediatric HIV infection, prevention of graft‐versus‐host disease and infection in adult BMT and Kawasaki syndrome) (Maramica 2003). Thus, further cost‐effectiveness evaluation on IVIG and thymosin‐α1 should be considered in clinical decision‐making.
Chemoprophylaxis and pneumococcal vaccination
Although it is well known that children with nephrotic syndrome are susceptible to a variety of infectious complications (McIntyre 1998), no RCTs evaluating prophylactic antibiotics, or pneumococcal vaccines in nephrotic syndrome were identified in this systematic review. A survey of paediatric nephrologists from the USA demonstrated that doctors who did not prescribe prophylactic antibiotics expressed concern about the emergence of resistant organisms (Shroff 2002). In one RCT involving children with sickle cell disease, which shares some of the mechanisms implicated in an increased risk of bacterial infection in nephrotic syndrome, chemoprophylaxis with phenoxymethyl penicillin was proved to be beneficial for reducing the incidence of pneumococcal bacteraemia. There did not appear to be an increased rate of colonization with penicillin‐resistant pneumococci during long‐term administration of prophylactic penicillin (Gaston 1988). With respect to pneumococcal vaccine, some paediatric nephrologists who were reluctant to immunise nephrotic children were concerned over the possibility of provoking relapses, although there were no data to support this (Schnaper 1994). However, a recent report from the UK indicated the relapse rate of nephrotic syndrome in children under age 18 years increased significantly after administration of a conjugate meningococcal C vaccine (Abeyagunawardena 2003). Therefore the decision to vaccinate certain children needs to be carefully considered. Further rigorous research should be done to clarify these issues.
Prevention of Varicella infection
Since many children with nephrotic syndrome are varicella non‐immune, varicella exposure and infection require special consideration. It was reported that concomitant use of oral acyclovir might prevent serious varicella infection in patients receiving corticosteroids (Goldstein 2000). However this is currently a lack of RCTs on prevention of varicella with acyclovir in patients with nephrotic syndrome. In addition, the research from the USA showed that varicella vaccine was generally well tolerated and highly immunogenic in children with nephrotic syndrome, including those on low‐dose, alternate‐day prednisone (Furth 2003). It is reported that once remission is achieved, immunisation with varicella vaccine seems safe and effective for preventing varicella infection in children with nephrotic syndrome, although additional doses may be required to achieve full immunity (Eddy 2003). However, no relevant RCTs were identified in this systematic review.
Other non‐pharmacological interventions are also quite important in the prevention of infections in nephrotic syndrome. No relevant RCTs were identified in this systematic review representing a significant gap in the literature in this field.
Adverse effects
A definite conclusion on adverse events associated with prophylactic interventions involving IVIG, thymosin, oral transfer factor, BCG vaccine injection Huangqi granules and TIAOJINING cannot be drawn from this review due to the limited number of studies identified, the limited duration of treatment and follow‐up, and inadequate recording and reporting of adverse events. Some serious adverse events have been reported to be associated with using these prophylactic interventions (Liang 1994; Maramica 2003; Tomlinson 2000). In clinical studies efficacy and safety should receive equal attention. However, RCTs are often impractical for assessment of rare and/or long‐term adverse effects, so observational studies should be included to identify occasional and severe adverse events in future reviews.
Quality of the evidence
The methodological quality of the included RCTs in this systematic review was very poor. The methodological quality is defined by the internal validity criteria, which refers to characteristics of the study that might be related to (selection, performance, attrition and detection) bias. Many of the studies included in this review were subject to a number of biases. The most remarkable findings of this systematic review were the paucity and the overall poor quality of the studies. The quality of reporting was poor with most studies not describing the procedure of randomisation, blinding, dropouts or withdrawals. Allocation concealment was unclear in all studies. Methodologically less rigorous studies show larger differences between experimental and control groups than do those conducted with greater rigor (Kjaergard 1999; Moher 1998; Schulz 1995). The small number of studies identified, and their general low methodological quality, prohibited meaningful sensitivity analysis to illuminate the robustness of the results of the review. No large scale, multi‐centre RCTs were identified. Nephrotic syndrome is a condition for which many confounding variables exist (e.g. aetiology, types of pathology, age, duration). Sufficient sample size is therefore an essential precondition. It is disappointing that no studies included in this systematic review reported a sample size calculation.
Potential biases in the review process
There were too few studies identified to investigate possible publication bias using a funnel plot in this review. All of the included studies in this review were conducted in China and 11/12 studies were published in Chinese and Dang 1999 was published in both Chinese and English. Vickers 1998 found that some countries, including China, publish unusually high proportions of positive results. Although we have conducted extensive searches for published material, we could not disregard the fact that studies with negative findings remain unpublished.
Evidence for one type of patient population may not necessarily be confidently applied to another. All studies in this systematic review were conducted in China. No eligible studies were conducted in adults with nephrotic syndrome and all participants were hospitalised children. Furthermore, the small number of studies made it impossible to conduct subgroup analysis for age, type of nephrotic syndrome and renal pathology. All these issues can considerably limit the applicability/generalizability of these prophylactic interventions for reducing the risk of infection in nephrotic syndrome in clinical practice. Studies outside of China, conducted in adults and including both inpatients and outpatients are needed.
Currently multiple prophylactic interventions have been used to reduce the morbidity and mortality caused by infection in children with nephrotic syndrome around the world. Although there are no data to support this, advisory bodies in Canada and the US have recommended routine immunisation (pneumococcal vaccination) except for omission of live vaccines if patients are receiving high‐dose corticosteroids or immunosuppressive agents. In this review, four RCTs demonstrated consistent positive effects of IVIG on preventing infection in children with nephrotic syndrome. Thymosin, oral transfer factor, BCG vaccine injection, Huangqi granules and TIAOJINING seem to have better effects on reducing the risk of infection in children with nephrotic syndrome. Prophylactic intervention for reducing the risk of infection in children and adults with nephrotic syndrome therefore warrants further study. Such studies should incorporate the following features: sample size estimated by statistical calculation; clear definition of modality of interventions; usage of standard validated outcome measures; randomisation; subject and assessor blinding; and placebo‐controlled design with adequate description of the procedure of randomisation and allocation concealment.
Authors' conclusions
Based on this systematic review IVIG, thymosin, oral transfer factor, BCG vaccine injection, Huangqi granules and TIAOJINING may have positive effects on the prevention of nosocomial or unspecified infection, with no obvious serious adverse events in children with nephrotic syndrome. Unfortunately due to the low methodological quality of the RCTs and the small number of studies and probable publication bias, there is currently insufficient evidence for determining which of these interventions could be used for preventing infection in children with nephrotic syndrome. No RCTs were identified in adults with nephrotic syndrome.
No RCTs were available on chemoprophylaxis, pneumococcal vaccination, varicella vaccine and any other non‐pharmacological interventions for reducing the risk of infection in children or adults with nephrotic syndrome in this systematic review.
The promising results and the insufficient quality of the available studies warrant further research. Large, properly randomised, placebo‐controlled, blinded studies are needed to confirm (or refute) the available evidence that effects of reducing risks of infection in children or adults with nephrotic syndrome are truly specific. The following features should be addressed in future studies:
sample size estimated by statistical calculation;
detailed reporting of the generation of allocation sequence and the allocation concealment;
application and clear description of blinding;
using placebo as control;
clear description of withdrawal/dropout during the study;
clear definition of modality of prophylactic interventions such as the dosage, the treatment period and duration of follow‐up;
usage of standard validated outcome measures;
reporting of clinically important outcome measures from long‐term follow‐up such as mortality and quality of life.
Adverse events should be critically assessed by standardized monitoring or an effective self‐report system. Attention should be paid to some rare and severe adverse events relevant to prophylactic interventions. Rigorous studies evaluating pneumococcal vaccination and prophylactic non‐pharmacological therapies, conducted outside of china, conducted in adults and including both inpatients and outpatients are needed. Finally the study should be reported according to the CONSORT statement (Begg 1996).
Acknowledgements
We would like to thank:
Narelle Willis and Ruth Mitchell for providing us with relevant studies from the Renal Group's Specialised Register, for help with developing the search strategy
Bin Zhu for providing statistical support, Lina Santaguida and Karen Szala‐Meneok for expert suggestions and corrections
The referees for their comments and feedback during the preparation or this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE and Pre‐MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1.
IVIG + baseline treatment versus baseline treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients developing infection | 4 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.31, 0.73] |
| 2 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2.
Thymosin + baseline treatment versus baseline treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients developing infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3.
Oral transfer factor + baseline treatment versus baseline treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients developing infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 4.
Mannan peptide + baseline treatment versus baseline treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients developing infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 5.
BCG vaccine injection + baseline treatment versus baseline treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients developing infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 6.
Polyvalent vaccine + baseline treatment versus baseline treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients developing infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 7.
Chinese medicinal herb (Huangqi granules) + baseline treatment versus baseline treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients developing infection | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.47, 0.83] |
Comparison 8.
Chinese medicinal herbs (TIAOJINING) + baseline treatment versus baseline treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients developing infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
What's new
| Date | Event | Description |
|---|---|---|
| 6 February 2012 | New citation required and conclusions have changed | New studies identified and included |
History
| Date | Event | Description |
|---|---|---|
| 6 January 2009 | Amended | Search strategy run, no new studies found |
| 9 October 2008 | Amended | Converted to new review format. |
Differences between protocol and review
The risk of bias assessment tool has replaced the quality assessment checklist.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
|
|
| Participants |
Exclusion criteria: NS |
|
| Interventions | Treatment group
Control group
Duration of follow‐up: 8 months |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The patients with nephrotic syndrome were allocated to the treatment and control groups according to the order of admission. |
| Allocation concealment (selection bias) | Unclear risk | The study did not provide the information on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not provide the information on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data in this study. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to make judgement. |
| Other bias | Unclear risk | There was insufficient information to make judgement. |
| Methods |
|
|
| Participants |
Exclusion criteria: NS |
|
| Interventions | Treatment group
Control group
Duration of follow ‐up: NS |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study did not provide the information on the method of random sequence generation, and only presented with the data as "all participants with nephrotic syndrome were randomly allocated to treatment and control groups". |
| Allocation concealment (selection bias) | Unclear risk | The study did not provide the information on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not provide the information on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data in this study. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to make judgement. |
| Other bias | Unclear risk | There was insufficient information to make judgement. |
| Methods |
|
|
| Participants |
Exclusion criteria: NS |
|
| Interventions | Treatment group
Control group
Duration of follow‐up: 4 to 6 weeks |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study did not provide the information on the method of random sequence generation, and only presented with the data as "all participants with nephrotic syndrome were randomly allocated to treatment and control groups". |
| Allocation concealment (selection bias) | Unclear risk | The study did not provide the information on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not provide the information on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data in this study. |
| Selective reporting (reporting bias) | High risk | Free of selective reporting bias was assessed as "No" due to some clinically important outcomes unstated, such as adverse events. |
| Other bias | Unclear risk | There was insufficient information to make judgement. |
| Methods |
|
|
| Participants |
Exclusion criteria: NS |
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Duration of follow‐up: NS |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study did not provide the information on the method of random sequence generation, and only presented with the data as "all participants with nephrotic syndrome were randomly allocated to treatment and control groups". |
| Allocation concealment (selection bias) | Unclear risk | The study did not provide the information on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not provide the information on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data in this study. |
| Selective reporting (reporting bias) | High risk | Free of selective reporting bias was assessed as "No" due to some clinically important outcomes unstated, such as adverse events. |
| Other bias | Unclear risk | There was insufficient information to make judgement. |
| Methods | Study design: parallel RCT Losses to follow‐up/withdrawals: None | |
| Participants |
Exclusion criteria: NS |
|
| Interventions | Treatment group
Control group
Duration of follow‐up: NS |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study did not provide the information on the method of random sequence generation, and only presented with the data as "all participants with nephrotic syndrome were randomly allocated to treatment and control groups". |
| Allocation concealment (selection bias) | Unclear risk | The study did not provide the information on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not provide the information on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data in this study. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to make judgement. |
| Other bias | Unclear risk | There was insufficient information to make judgement. |
| Methods |
|
|
| Participants |
Exclusion criteria: NS |
|
| Interventions | Treatment group
Control group
Duration of follow‐up: NS |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study did not provide the information on the method of random sequence generation, and only presented with the data as "all participants with nephrotic syndrome were randomly allocated to treatment and control groups". |
| Allocation concealment (selection bias) | Unclear risk | The study did not provide the information on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not provide the information on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data in this study. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to make judgement. |
| Other bias | Unclear risk | There was insufficient information to make judgement. |
| Methods |
|
|
| Participants |
Exclusion criteria: NS |
|
| Interventions | Treatment group
Control group
Duration of follow‐up: NS |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study did not provide the information on the method of random sequence generation, and only presented with the data as "all participants with nephrotic syndrome were randomly allocated to treatment and control groups". |
| Allocation concealment (selection bias) | Unclear risk | The study did not provide the information on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not provide the information on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data in this study. |
| Selective reporting (reporting bias) | High risk | Free of selective reporting bias was assessed as "No" due to some clinically important outcomes unstated, such as adverse events. |
| Other bias | Unclear risk | There was insufficient information to make judgement. |
| Methods |
|
|
| Participants |
Exclusion criteria: NS |
|
| Interventions | Treatment group
Control group
Duration of follow‐up: 12 months |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study did not provide the information on the method of random sequence generation, and only presented with the data as "all participants with nephrotic syndrome were randomly allocated to treatment and control groups". |
| Allocation concealment (selection bias) | Unclear risk | The study did not provide the information on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not provide the information on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data in this study. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to make judgement. |
| Other bias | Unclear risk | There was insufficient information to make judgement. |
| Methods |
|
|
| Participants |
Exclusion criteria: NS |
|
| Interventions | Treatment group
Control group
Duration of follow‐up: NS |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study did not provide the information on the method of random sequence generation, and only presented with the data as "all participants with nephrotic syndrome were randomly allocated to treatment and control groups". |
| Allocation concealment (selection bias) | Unclear risk | The study did not provide the information on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not provide the information on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data in this study. |
| Selective reporting (reporting bias) | Low risk | The study was free of selective reporting. |
| Other bias | Unclear risk | There was insufficient information to make judgement. |
| Methods |
|
|
| Participants |
Exclusion criteria: NS |
|
| Interventions | Treatment group
Control group
Duration of follow‐up: NS |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study did not provide the information on the method of random sequence generation, and only presented with the data as "all participants with nephrotic syndrome were randomly allocated to treatment and control groups". |
| Allocation concealment (selection bias) | Unclear risk | The study did not provide the information on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not provide the information on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | High risk | Free of selective reporting bias was assessed as "No" due to some clinically important outcomes unstated, such as adverse effects of IV IgG. |
| Other bias | Unclear risk | There was insufficient information to permit judgement. |
| Methods |
|
|
| Participants |
Exclusion criteria: NS |
|
| Interventions | Treatment group
Control group
Duration of follow‐up: NS |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study did not provide the information on the method of random sequence generation, and only presented with the data as "all participants with nephrotic syndrome were randomly allocated to treatment and control groups". |
| Allocation concealment (selection bias) | Unclear risk | The study did not provide the information on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not provide the information on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data in this study. |
| Selective reporting (reporting bias) | High risk | Free of selective reporting bias was assessed as "No" due to some clinically important outcomes unstated, such as adverse effects of polyvalent vaccine. |
| Other bias | Unclear risk | There was insufficient information to make judgement. |
| Methods |
|
|
| Participants |
Exclusion criteria: NS |
|
| Interventions | Treatment group
Control group
Duration of follow‐up: NS |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study did not provide the information on the method of random sequence generation, and only presented with the data as "all participants with nephrotic syndrome were randomly allocated to treatment and control groups". |
| Allocation concealment (selection bias) | Unclear risk | The study did not provide the information on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not provide the information on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were no missing data in this study. |
| Selective reporting (reporting bias) | High risk | Free of selective reporting bias was assessed as "No" due to some clinically important outcomes unstated, such as adverse effects of thymosin. |
| Other bias | Unclear risk | There was insufficient information to make judgement. |
BCG ‐ Bacillus Calmette‐Guerin; IVIG ‐ intravenous immunoglobulin; NA ‐ not associated; NS ‐ not stated; URI ‐ upper respiratory infection; UTI ‐ urinary tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abeyagunawardena 2008 | Not pertinent to prevention of infection in patients with nephrotic syndrome |
| Allison 1969 | Not pertinent to prevention of infection in patients with nephrotic syndrome |
| Goldstein 2000 | Participants included both nephrotic syndrome and kidney transplantation and the data of nephrotic syndrome were not separated. |
| Grzesiowski 1995 | Not a RCT |
Contributions of authors
Wu HM: developing search strategy, assessment of studies, data extraction, data analysis, data entry, writing of protocol and review
Cao L: developing search strategy, assessment of studies, data extraction, data analysis, data entry, writing of protocol and review
Tang JL; data analysis, Resolution of disagreements, writing protocol and review
Li YP: writing of protocol and review
Sha ZH: data extraction and data entry
Sources of support
Internal sources
Chinese Cochrane Center, China.
Department of Gerontology, West China Hospital, Si Chuan University, China.
External sources
Hong Kong Branch of Chinese Cochrane Center, China.
Hong Kong Croucher Foundation, China.
Department of Family & Community Medicine, The Chinese University of Hong Kong, China.
China Medical Board of New York (Grant 98‐680), USA.
Declarations of interest
Hong Mei Wu: none known
Jin Ling Tang: none known
Li Cao: none known
Zhao Hui Sha: none known
You Ping Li: none known
Edited (conclusions changed)
References
References to studies included in this review
- Chen J, Chen SQ. Preventive effects of Huangqi on infection in children with nephrotic syndrome. Zhong Guo Zhong Xi Yi Jie He Za Zhi [Chinese Journal of Integrated Traditional and Western Medicine] 2008;28(5):467‐9. [Google Scholar]
- Dang X, Yi Z, Wang X, Wu X, Zhang X, He Q. Preventive efficiency of IVIgG on nosocomial infection in the children with nephrotic syndrome. Bulletin of Hunan Medical University 1999;24(3):290‐2. [MEDLINE: ] [PubMed] [Google Scholar]
- Dou ZY, Wang JY, Liu YP. Preventive efficiency of low‐dose IVIgG on infection in nephrotic syndrome. Chinese Journal of Biologicals 2000;13(3):160. [Google Scholar]
- Guo YC, Cheng YY, Zhang LH. Preventive effects of secondary Infection on children simple nephrotic syndrome by mannan peptide. Chinese JournaI of MedicinaI Guide 2008;10(5):715‐6. [Google Scholar]
- Kang GG. Preventive effect of complicated infection of children with nephrotic syndrome on vaccine injection. Chinese Pediatric Emergency Medicine 2003;10(5):299‐301. [Google Scholar]
- Kang GG. Preventive effects of Huangqi on secondary infection in children with simple nephrotic syndrome. Chinese Journal of Integrated Traditional and Western Nephrology 2005;6(12):718‐9. [Google Scholar]
- Li RH, Peng ZP, Wei YL, Liu CH. Clinical observation on Chinese medicinal herbs combined with prednisone for reducing the risks of infection in children with nephrotic syndrome. Information Journal of Chinese Medicine 2000;7(10):60‐1. [Google Scholar]
- Rao XZ. Preventive effect of oral transferin factor on secondary infection in children with simple nephrotic syndrome. Chinese Journal of Coal Industry Medicine 2005;8(3):270. [Google Scholar]
- Tong LZ, Mi LZ. Preventive efficiency of IVIgG on secondary nosocomial infection in nephrotic syndrome. Modern Rehabilitation 1998;2(3):236. [Google Scholar]
- Wu QY, Wu X. Clinical study of gammaglobulin on upper respiratory infection prevention in nephrotic syndrome children. Journal of Hainan Medical College 2009;15(8):879‐80. [Google Scholar]
- Ye QB, Huang T, Li ZH, Wang YQ. The clinical research on using polyvalent bacterial vaccine to prevent the nosocomial infection of children with primary nephritic syndrome. Applied Journal of General Practice 2004;12(2):132‐4. [Google Scholar]
- Zhang YJ, Wang Y, Yang ZW, Li XT. Thymosin for the prevention of infection in children with the idiopathic nephrotic syndrome. Chinese Journal of Contemporary Pediatrics 2000;2(3):197‐9. [Google Scholar]
References to studies excluded from this review
- Abeyagunawardena AS, Trompeter RS. Increasing the dose of prednisolone during viral infections reduces the risk of relapse in nephrotic syndrome: a randomised controlled trial. Archives of Disease in Childhood 2008;93(3):226‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Allison AC, Hendrickse RG, Edington GM, Houba V, Petris S, Adeniyi A. Immune complexes in the nephrotic syndrome of African children. Lancet 1969;1(7608):1232‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Goldstein SL, Somers MJ, Lande MB, Brewer ED, Jabs KL. Acyclovir prophylaxis of varicella in children with renal disease receiving steroids. Pediatric Nephrology 2000;14(4):305‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grzesiowski P, Tanska A, Sieniawska M. The influence of hepatitis B vaccine dose on direct results of hepatitis B vaccination in children with nephrotic syndrome. Pediatria Polska 1995;70(1):25‐8. [EMBASE: 1995078155] [PubMed] [Google Scholar]
Additional references
- Abeyagunawardena AS, Goldblatt D, Andrews N, Trompeter RS. Risk of relapse after meningococcal C conjugate vaccine in nephrotic syndrome. Lancet 2003;362(9382):449‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276(8):637‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brodehl J. Nephrotic syndrome in children: diagnosis and treatment. World Pediatrics and Child Care 1986;1:9‐18. [Google Scholar]
- Chan HL, Tang JL, Tam W, Sung JJ. The efficacy of thymosin in the treatment of chronic hepatitis B virus infection: a meta‐analysis. Alimentary Pharmacology & Therapeutics 2001;15(12):1899‐905. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Choudhary VP, Ghai OP. Peritonitis in nephrotic syndrome. Indian Pediatrics 1977;14(5):405‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309(6964):1286‐91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du PF, Zhu SR, Wang GP. An analysis for nosocomial infection in primary nephrotic syndrome‐ a report of 125 cases. Chinese Journal of Clinical Pediatrics 1996;14(1):35‐6. [Google Scholar]
- Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet 2003;362(9384):629–39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Furth SL, Arbus GS, Hogg R, Tarver J, Chan C, Fivush BA, et al. Varicella vaccination in children with nephrotic syndrome: a report of the Southwest Pediatric Nephrology Study Group. Journal of Pediatrics 2003;142(2):145‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gaston MH, Verter JI, Woods G, Pegelow C, Kelleher J, Presbury G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. New England Journal of Medicine 1988;314(25):1593‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gulati S, Kher V, Arora P, Gupta S, Kale S. Urinary tract infection in nephrotic syndrome. Pediatric Infectious Disease Journal 1996;15(3):237‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Anonymous. Minimal change nephrotic syndrome in children: deaths during the first 5‐15 years` observation. Report of the international Study of Kidney Disease in Children. Pediatrics 1984;73(4):497‐501. [MEDLINE: ] [PubMed] [Google Scholar]
- Jiang X. The revised suggestions on clinical classifications and treatments for glomerular diseases in children. Journal of Chinese Pediatrics 1981;19:241‐3. [Google Scholar]
- Johnson RJ, Feehally J. Introduction to glomerular disease: clinical presentations. Comprehensive Clinical Nephrology. London: Mosby, 2000. [ISBN: 0723431175] [Google Scholar]
- Kjaergard LL, Villumsen J, Gluud C. Quality of randomised clinical trials affects estimates of intervention efficacy. Seventh International Cochrane Colloquium; 1999 Oct; Rome (Italy). 1999:57. [Google Scholar]
- Lefebvre C, McDonald S. Development of a sensitive search strategy for reports of randomized controlled trials in EMBASE. Fourth International Cochrane Colloquium; 1996 Oct 20‐24; Adelaide (Australia). 1996. [Google Scholar]
- Li YK, Jiang MY. Pharmacology of Traditional Chinese Medicine. Beijing: China Press of Traditional Chinese Medicine, 1992. [Google Scholar]
- Li YJ, Xie C, Xia ZM. Risk factors for infection in adult nephrotic syndrome. Chinese Journal of Nephrology 1996;12(2):76‐8. [Google Scholar]
- Liang M. The clinical application of intravenous immunoglobulin gamma. Practical Journal of Chinese Pediatrics 1994;9(5):265‐7. [Google Scholar]
- Ma YW, Yuan XY, Jiang JY. Nosocomial infection and nephrotic syndrome in children. Chinese Journal of Nosocomiology 1996;6(1):12‐4. [Google Scholar]
- Knezevic‐Maramica I, Kruskall MS. Intravenous immune globulins: an update for clinicians. Transfusion 2003;43(10):1460‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- McIntyre P, Craig JC. Prevention of serious bacterial infection in children with nephrotic syndrome. Journal of Paediatrics & Child Health 1998;34(4):314‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Moorani KN, Khan KM, Ramzan A. Infections in children with nephrotic syndrome. Jcpsp, Journal of the College of Physicians & Surgeons ‐ Pakistan 2003;13(6):337‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Napodano RJ. Values in Medical Practice. New York, NY: Human Sciences Press, 1986. [Google Scholar]
- Ogi M, Yokoyama H, Tomosugi N, Hisada Y, Ohta S, Takaeda M, et al. Risk factors for infection and immunoglobulin replacement therapy in adult nephrotic syndrome. American Journal of Kidney Diseases 1994;24(3):427‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pozzi E, Serra C. Efficacy of Lantigen B in the prevention of bacterial respiratory infections. Monaldi Archives for Chest Disease 2004;61(1):19‐27. [MEDLINE: ] [PubMed] [Google Scholar]
- Roth KS, Amaker BH, Chan JC. Nephrotic syndrome: pathogenesis and management. Pediatrics in Review 2002;23(7):237‐48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schnaper HW. Immunization practices in childhood nephrotic syndrome: a survey of North American pediatric nephrologists. Pediatric Nephrology 1994;8(1):4‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shroff A, Frank R, Vergara M, Gauthier B, Trachtman H. Prevention of serious bacterial infections in new‐onset nephrotic syndrome: a survey of current practices. Clinical Pediatrics 2002;41(1):47‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tain YL, Lin G, Cher TW. Microbiological spectrum of septicemia and peritonitis in nephrotic children. Pediatric Nephrology 1999;13(9):835‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tomlinson B, Chan TY, Chan JC, Critchley JA, But PP. Toxicity of complementary therapies: an eastern perspective. Journal of Clinical Pharmacology 2000;40(5):451‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ulinski T, Leroy S, Dubrel M, Danon S, Bensman A. High serological response to pneumococcal vaccine in nephrotic children at disease onset on high‐dose prednisone. Pediatric Nephrology 2008;23(7):1107‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Controlled Clinical Trials 1998;19(2):159‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wang SQ, Zhang BY. Nosocomiology. Chongqing: Science Technological Publishing House, 1990. [Google Scholar]
- Wang K, Chui AD. An analysis of dangerous factors for relapse in primary nephrotic syndrome. Acta Academiae Medicine XuZhou 1999;19(3):227‐8. [Google Scholar]
- Wu LY, Dan XQ. An analysis of risk factors and managements for nosocomial infection in children with nephrotic syndrome. Chinese Journal of Nosocomiology 1998;8(B):34‐5. [Google Scholar]
- Wu B, Xu NF, Yang LP, Chen SQ. Clinical epidemiology study on risk factors for nosocomial infection in children with nephrotic syndrome. Chinese Journal of Nosocomiology 2000;10(3):171‐3. [Google Scholar]
- Xu XY. Pharmacology of Traditional Chinese Medicine. Beijing: China Press of Traditional Chinese Medicine, 2010. [Google Scholar]
- Yap HK, Han EJ, Heng CK, Gong WK. Risk factors for steroid dependency in children with idiopathic nephrotic syndrome. Pediatric Nephrology 2001;16(12):1049‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Wu HM, Tang JL, Sha ZH, Li YP. Interventions for preventing infection in nephrotic syndrome. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003964] [DOI] [PubMed] [Google Scholar]
- Wu HM, Tang JL, Sha ZH, Li Y, Cao I. Interventions for preventing infection in nephrotic syndrome. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD003964.pub2] [DOI] [PubMed] [Google Scholar]


