Abstract
Background
Multiple pregnancy is a strong risk factor for preterm birth, and more than 50% of women with a twin pregnancy will give birth prior to 37 weeks' gestation. Infants born preterm are recognised to be at increased risk of many adverse health outcomes, contributing to more than half of overall perinatal mortality. Progesterone is produced naturally in the body and has a role in maintaining pregnancy, although it is not clear whether administering progestogens to women with multiple pregnancy at high risk of early birth is effective and safe.
Objectives
To assess the benefits and harms of progesterone administration for the prevention of preterm birth in women with a multiple pregnancy.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (1 November 2016) and reference lists of retrieved studies.
Selection criteria
We included randomised controlled trials examining the administration of a progestogen by any route for the prevention of preterm birth in women with multiple pregnancy. We did not include quasi‐randomised or cross‐over studies.
Data collection and analysis
Two review authors independently assessed reports identified by the search for eligibility, extracted data, assessed risk of bias and graded the quality of the evidence.
Main results
We included 17 trials, which all compared either vaginal or intramuscular (IM) progesterone with a placebo or no treatment, and involved a total of 4773 women. The risk of bias for the majority of included studies was low, with the exception of four studies that had inadequate blinding, or significant loss to follow‐up or both, or were not reported well enough for us to make a judgement. We graded the evidence low to high quality, with downgrading for statistical heterogeneity, design limitations in some of the studies contributing data, and imprecision of the effect estimate.
1 IM progesterone versus no treatment or placebo
More women delivered at less than 34 weeks' gestation in the IM progesterone group compared with placebo (risk ratio (RR) 1.54, 95% confidence interval (CI) 1.06 to 2.26; women = 399; studies = 2; low‐quality evidence). Although the incidence of perinatal death in the progesterone group was higher, there was considerable uncertainty around the effect estimate and high heterogeneity between studies (average RR 1.45, 95% CI 0.60 to 3.51; infants = 3089; studies = 6; I2 = 71%; low‐quality evidence). No studies reported maternal mortality or major neurodevelopmental disability at childhood follow‐up.
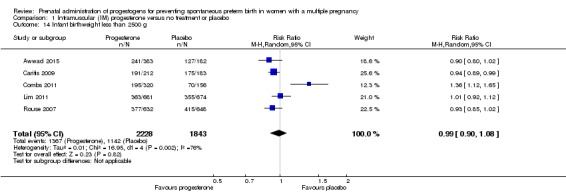
There were no clear group differences found in any of the other maternal or infant outcomes (preterm birth less than 37 weeks (RR 1.05, 95% CI 0.98 to 1.13; women = 2010; studies = 5; high‐quality evidence); preterm birth less than 28 weeks (RR 1.08, 95% CI 0.75 to 1.55; women = 1920; studies = 5; moderate‐quality evidence); infant birthweight less than 2500 g (RR 0.99, 95% CI 0.90 to 1.08; infants = 4071; studies = 5; I2 = 76%, moderate‐quality evidence)). No childhood outcomes were reported in the trials.
2 Vaginal progesterone versus no treatment or placebo by dose
There were no clear group differences in incidence of preterm birth before 34 weeks (average RR 0.83, 95% CI 0.63 to 1.09; women = 1727; studies = 6; I2 = 46%; low‐quality evidence). Although fewer births before 34 weeks appeared to occur in the progesterone group, the CIs crossed the line of no effect. Incidence of perinatal death was higher in the progesterone group, although there was considerable uncertainty in the effect estimate and the quality of the evidence was low for this outcome (RR 1.23, 95% CI 0.74 to 2.06; infants = 2287; studies = 3; low‐quality evidence). No studies reported maternal mortality or major neurodevelopmental disability at childhood follow‐up.
There were no clear group differences found in any of the other maternal or infant outcomes (preterm birth less than 37 weeks (average RR 0.97, 95% CI 0.89 to 1.06; women = 1597; studies = 6; moderate‐quality evidence); preterm birth less than 28 weeks (RR 1.22, 95% CI 0.68 to 2.21; women = 1569; studies = 4; low‐quality evidence); infant birthweight less than 2500 g (RR 0.95, 95% CI 0.88 to 1.03; infants = 3079; studies = 4; I2 = 49%, moderate‐quality evidence)). No childhood outcomes were reported in the trials.
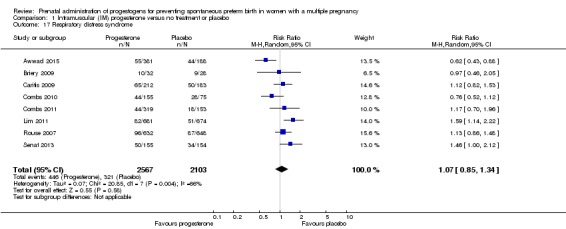
For secondary outcomes, there were no clear group differences found in any of the other maternal outcomes except for caesarean section, where women who received vaginal progesterone did not have as many caesarean sections as those in the placebo group, although the difference between groups was not large (7%) (RR 0.93, 95% CI 0.88 to 0.98; women = 2143; studies = 6; I2 = 0%). There were no clear group differences found in any of the infant outcomes except for mechanical ventilation, which was required by fewer infants whose mothers had received the vaginal progesterone (RR 0.61, 95% CI 0.48 to 0.77; infants = 3134; studies = 5).
Authors' conclusions
Overall, for women with a multiple pregnancy, the administration of progesterone (either IM or vaginal) does not appear to be associated with a reduction in risk of preterm birth or improved neonatal outcomes.
Future research could focus on a comprehensive individual participant data meta‐analysis including all of the available data relating to both IM and vaginal progesterone administration in women with a multiple pregnancy, before considering the need to conduct trials in subgroups of high‐risk women (for example, women with a multiple pregnancy and a short cervical length identified on ultrasound).
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Pregnancy, Multiple; Prenatal Care; Administration, Intravaginal; Infant, Low Birth Weight; Infant, Premature; Injections, Intramuscular; Perinatal Mortality; Premature Birth; Premature Birth/prevention & control; Progesterone; Progesterone/administration & dosage; Progestins; Progestins/administration & dosage; Randomized Controlled Trials as Topic
Prenatal progestogens for preventing preterm birth in women with a multiple pregnancy
What is the issue?
More than half of women with a twin pregnancy give birth before the 37th week of pregnancy (preterm), and women expecting triplets are even more likely to have a preterm birth. Infants born preterm are more likely to die or have health problems compared with babies born at term. Progesterone is produced naturally in the body and is thought to help to maintain pregnancy.
Why is this important?
It is not known whether giving progesterone (by injection, orally or by vaginal suppositories or gels) to women with multiple pregnancy during pregnancy is beneficial or harmful to the woman and her babies.
What evidence did we find?
We searched for evidence on 1 November 2016 and identified 17 randomised controlled trials involving 4773 women for inclusion in the review.
In studies where women received progesterone by injection into the muscle compared with placebo (dummy treatment) more women gave birth before the 34th week of pregnancy in the progesterone group (low‐quality evidence). There was no clear difference between the groups in the likelihood of the baby dying before or soon after the birth (low‐quality evidence). No studies reported whether any women died or whether any babies had longer‐term developmental problems or disability. There seems to be little or no difference between women receiving progesterone or placebo for other important outcomes, such as preterm birth before 37 weeks (high‐quality evidence); preterm birth before 28 weeks (moderate‐quality evidence) or infant birthweight less than 2500 grams (moderate‐quality evidence). No childhood outcomes were reported in the trials.
In studies where women received vaginal progesterone there may be little or no difference between women receiving progesterone or placebo in preterm birth before 34 weeks (low‐quality evidence); although fewer births before 34 weeks occurred in the progesterone group, this finding may have occurred by chance. The number of infant deaths before or soon after birth was similar in both groups (low‐quality evidence). No studies reported maternal death or longer‐term outcomes for babies. There may be little or no difference between groups receiving vaginal progesterone versus placebo in any other important outcomes (preterm birth before 37 weeks (moderate‐quality evidence); preterm birth before 28 weeks (low‐quality evidence); or infant birthweight less than 2500 grams (moderate‐quality evidence)). No childhood outcomes were reported in the trials. For other outcomes, we found no clear group differences, except for caesarean section where women who received vaginal progesterone did not have as many caesarean sections as those in the placebo group (although the difference between groups was not large (7%)). Fewer infants whose mothers had received the vaginal progesterone needed mechanical help with breathing.
We did not find any studies looking at progesterone taken by mouth.
What does this mean?
Overall, for women with a multiple pregnancy, treatment with progesterone (either intramuscular or vaginal) does not appear to reduce the likelihood of preterm birth or improve outcomes for babies.
Future research could focus on looking at information about individual women taking part in studies, so that everything available about both intramuscular and vaginal progesterone treatments in women with a multiple pregnancy can be considered together.
Summary of findings
Summary of findings for the main comparison.
Intramuscular (IM) progesterone compared to no treatment or placebo for preventing spontaneous preterm birth in women with a multiple pregnancy
| Intramuscular (IM) progesterone compared to no treatment or placebo for preventing spontaneous preterm birth in women with a multiple pregnancy | ||||||
| Patient or population: Women with a multiple pregnancy Setting: Obstetric clinics in Finland, France, Lebanon, the Netherlands, and the USA Intervention: Intramuscular (IM) progesterone Comparison: No treatment or placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no treatment or placebo | Risk with intramuscular (IM) progesterone | |||||
| Perinatal death | Study population | RR 1.45 (0.60 to 3.51) | 3089 (6 RCTs) | ⊕⊕⊝⊝ LOW 1, 2 |
‐ | |
| 34 per 1000 | 49 per 1000 (20 to 120) | |||||
| Preterm birth less than 34 weeks | Study population | RR 1.54 (1.06 to 2.26) | 399 (2 RCTs) | ⊕⊕⊝⊝ LOW 3, 4 | ‐ | |
| 191 per 1000 | 298 per 1000 (204 to 436) | |||||
| Major neurodevelopmental disability at childhood follow‐up | Study population | ‐ | (0 studies) | ‐ | None of the included trial reported this outcome | |
| see comment | see comment | |||||
| Infant birthweight less than 2500 g | Study population | RR 0.99 (0.90 to 1.08) | 4071 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ‐ | |
| 620 per 1000 | 613 per 1000 (558 to 669) | |||||
| Preterm birth less than 28 weeks | Study population | RR 1.08 (0.75 to 1.55) | 1920 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | ‐ | |
| ‐58 per 1000 | 62 per 1000 (43 to 89) | |||||
| Preterm birth less than 37 weeks | Study population | RR 1.05 (0.98 to 1.13) | 2010 (5 RCTs) | ⊕⊕⊕⊕ HIGH | ‐ | |
| 614 per 1000 | 639 per 1000 (602 to 688) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Statistical heterogeneity (I2 > 60%). Variation in size and direction of effect (‐1). 2Wide confidence interval crossing the line of no effect. (‐1). 3Study with design limitations (lack of blinding) contributing data (64.2% weight) (‐1). 4Wide confidence interval (‐1).
Summary of findings 2.
Vaginal progesterone compared to no treatment or placebo for preventing spontaneous preterm birth in women with a multiple pregnancy
| Vaginal progesterone compared to no treatment or placebo for preventing spontaneous preterm birth in women with a multiple pregnancy | ||||||
| Patient or population: Women with a multiple pregnancy Setting: Obstetric clinics in Austria, Brazil, Canada, Denmark, Egypt, Spain, Turkey and UK Intervention: Vaginal progesterone Comparison: No treatment or placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no treatment or placebo | Risk with vaginal progesterone | |||||
| Perinatal death | Study population | RR 1.23 (0.74 to 2.06) | 2287 (3 RCTs) | ⊕⊕⊝⊝ LOW 1, 2 | ‐ | |
| 23 per 1000 | 28 per 1000 (17 to 47) | |||||
| Preterm birth less than 34 weeks | Study population | RR 0.83 (0.63 to 1.09) | 1727 (6 RCTs) | ⊕⊕⊝⊝ LOW 2, 3 | ‐ | |
| 227 per 1000 | 188 per 1000 (143 to 247) | |||||
| Major neurodevelopmental disability at childhood follow‐up | Study population | ‐ | (0 study) | ‐ | None of the included trial reported this outcome. | |
| see comment | see comment | |||||
| Infant birthweight less than 2500 g | Study population | RR 0.95 (0.88 to 1.03) | 3079 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 4 | ‐ | |
| 604 per 1000 | 574 per 1000 (532 to 622) | |||||
| Preterm birth less than 37 weeks | Study population | RR 0.97 (0.89 to 1.06) | 1597 (6 RCTs) | ⊕⊕⊕⊝ MODERATE 5 | ‐ | |
| 559 per 1000 | 547 per 1000 (503 to 598) | |||||
| Preterm birth less than 28 weeks | Study population | RR 1.22 (0.68 to 2.21) | 1569 (4 RCTs) | ⊕⊕⊝⊝ LOW 2, 6 | ‐ | |
| 26 per 1000 | 31 per 1000 (18 to 57) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1One study contributing data with design limitations (weight of 52.1%) (‐1). 2Wide confidence interval crossing the line of no effect (‐1). 3Two studies contributing data with design limitations (combined weight 48.5%) (‐1). 4Most of the pooled effect was provided by studies with design limitations (combined weight 54.4%) (‐1). 5One study contributing data with design limitations (weight of 33.9%) (‐1). 6Most of the pooled effect was provided by studies with design limitations (combined weight 57.4%) (‐1).
Background
Description of the condition
The rates of multiple pregnancies that occur naturally vary in different maternal age and ethnic groups; however, since the early 1980s the development of assisted reproduction techniques have led to a large increase in multiple births in high‐resource settings (Collins 2007; Umstad 2013). For example, in the 1980s in England and Wales twin pregnancies accounted for approximately 0.9% of births, but by the late 1990s this had increased to 1.4% (Smith 2014). In Australia in 2010, multiple births accounted for 3.1% of all births (Umstad 2013). These trends have been reflected in many settings across the globe, although in many countries recent policies to restrict the number of embryos transferred during assisted conception may reverse this upward trend (Collins 2007; Umstad 2013).
Multiple pregnancy is a strong risk factor for preterm birth. A woman with a multiple pregnancy is likely to have an over‐distended uterus in addition to any other risk factors which may occur in women with a singleton pregnancy. The risk of early birth before 37 weeks for women with a singleton pregnancy is 7.5% compared with 100% for women with a triplet pregnancy (AIHW 2014). More than 50% of women with a twin pregnancy will give birth prior to 37 weeks' gestation (AIHW 2014).
Infants born preterm are recognised to be at increased risk of many adverse health outcomes, contributing to more than 50% of overall perinatal mortality (AIHW 2003), as well as being at greater risk of dying in their first year of life (Martin 2015). For those preterm infants who initially survive the neonatal period, there is an increased risk of death during childhood due to increased risks of infection and other illnesses (Blencowe 2013; Howson 2013). In addition, infants born preterm are at increased risk of repeated admission to hospital (Elder 1999) and adverse outcomes, including blindness, hearing impairment, chronic lung disease, cerebral palsy and long‐term disability (Blencowe 2013; Hack 1999; Stanley 1992), creating a significant burden upon the community (McCormick 2011). Even accounting for gestational age at birth, infants of a twin pregnancy are at greater risk of complications relating to prematurity than are singleton infants born at the same gestation. For example, the risk of cerebral palsy in all pregnancies is approximately 2/1000, but for twins this increases to 9/1000 and to 31/1000 for triplets (Bromer 2011).
Description of the intervention
Progestogens are a group of hormones that act by binding to and activating the progesterone receptor, and are described as naturally occurring or synthetic agents (Schindler 2008). Progesterone and its metabolite, 17‐hydroxyprogesterone, is naturally occurring, and is produced by the body during pregnancy in high concentrations (Feghali 2014). In contrast, 17‐hydroxyprogesterone caproate is a synthetic progestin that is protein‐bound and lipophilic, and requires metabolism by the liver (Feghali 2014). The metabolites of 17‐hydroxyprogesterone caproate also differ from those of both progesterone and 17‐hydroxyprogesterone (Feghali 2014).
Progestogen compounds may be administered in various forms and by various routes, with different formulations and mode of administration affecting absorption and therefore conferring potentially different bio‐effects (Feghali 2014). For example, 17‐hydroxyprogesterone caproate is administered by intramuscular injection, and has a half‐life of 16 days, with the drug remaining detectable several weeks after intramuscular injection (Caritis 2012). In contrast, progesterone, when administered orally, undergoes significant first‐pass metabolism within the liver, although vaginal administration reduces this effect, with a half‐life of the order of 16 to 18 hours (Stanczyk 2013).
A number of case‐control studies have not identified an increased risk of congenital anomalies following the use of natural progesterone (Raman‐Wilms 1995; Schardein 1980), or 17‐hydroxyprogesterone caproate (Michaelis 1983; Resseguie 1985; Varma 1982) in pregnancy. However, a large population‐based study evaluating the use of progesterone prior to conception indicates an association with some childhood cancers (Hargreave 2015).
Maternal side‐effects from progesterone therapy include headache, breast tenderness, nausea, cough and local irritation if administered intramuscularly. At present, there is little information available about the optimal dose of progesterone, mode of administration, gestational age at which to begin therapy, or duration of therapy (Greene 2003; Iams 2003).
How the intervention might work
Progesterone has a role in maintaining pregnancy (Haluska 1997; Peiber 2001; Pepe 1995), and is thought to act by suppressing smooth muscle activity in the uterus (Astle 2003; Grazzini 1998). In many animal species, there is a reduction in the amount of circulating progesterone before the onset of labour. While these changes have not been shown to occur in women (Astle 2003; Block 1984; Lopez‐Bernal 2003; Peiber 2001; Smit 1984), it has been suggested that there is a 'functional' withdrawal of progesterone related to changes in the expression of progesterone receptors in the uterus (Astle 2003; Condon 2003; Haluska 2002; Peiber 2001). There have been relatively recent reports in the literature advocating the use of progesterone to reduce the risk of preterm birth (Da Fonseca 2003; Meis 2003a), rekindling interest that dates back to the 1960s (Le Vine 1964), although no progestogen deficiency state has been described in women delivering preterm, either with singletons or multiple pregnancy.
Why it is important to do this review
Preterm birth and its consequences for women and their babies is a significant health problem in pregnancy and childbirth. While the suppression or prevention of preterm labour should lead to improved survival through a lower incidence of premature birth, there are theoretical reasons why a fetus may not survive without disability. It is possible that an intrauterine mechanism that would trigger preterm labour could also cause neurological injury to the fetus and that progesterone may prevent labour, but not fetal injury. The purpose of this review is to assess the benefits and harms of progesterone administration for the prevention of preterm birth for both women and their infants, when considering the risk factors present for preterm birth.
An existing Cochrane Review examined the prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth (Dodd 2013). This review included women considered at high risk because of multiple pregnancy, as well as women with singleton pregnancies considered at high risk for various clinical reasons (history of preterm birth, short cervix, threatened preterm labour and other risk factors). The review included 36 trials, with several trials recruiting only women with multiple pregnancies. Results of the review may be easier to interpret and more clinically relevant if the results for women with multiple and singleton pregnancy are assessed and reported separately. Consequently, the review has been divided into two reviews, with this review focusing on women with a multiple pregnancy and the other examining the effects of progesterone in women with singleton pregnancies considered to be at high risk of preterm birth.
Objectives
To assess the benefits and harms of progesterone administration for the prevention of preterm birth in women with a multiple pregnancy.
Methods
Criteria for considering studies for this review
Types of studies
We included all published and unpublished randomised controlled trials (including those using a cluster‐randomised design), in which a progestogen was administered for the prevention of preterm birth in women with multiple pregnancies. We included studies published as abstracts or brief reports, provided there was sufficient information available to assess risks of bias.
Trials were excluded if:
a quasi‐randomised methodology or cross‐over design was used;
a progestogen was administered for the acute treatment of actual or threatened preterm labour (that is, where progesterone was administered as an acute tocolytic medication); or
a progestogen was administered in the first trimester of pregnancy only for preventing miscarriage.
Types of participants
Pregnant women considered to be at increased risk of preterm birth because of a multiple pregnancy. Women with multiple pregnancy may also have additional risk factors such as short cervix, and we have included studies which include women with multiple risk factors.
We planned to include studies which recruited women with either a singleton or multiple pregnancy who were considered to be at high risk of preterm birth for other obstetric reasons, provided that randomisation was stratified by plurality of the pregnancy and that findings for women with multiple pregnancies were reported separately, or could be obtained from trial authors.
Types of interventions
Administration of a progestogen by any route (intravenous (IV), intramuscular (IM), oral or vaginal) for the prevention of preterm birth compared with placebo or no treatment. Where data were available, we have presented results separately according to route of administration, as progestogens administered by different routes may have a different effect.
Types of outcome measures
Primary outcomes
Maternal
Maternal mortality
Preterm birth (less than 34 weeks' gestation)
Infant
Perinatal mortality
Major neurodevelopmental disability at childhood follow‐up
Secondary outcomes
Maternal
Preterm birth less than 37 weeks
Preterm birth less than 28 weeks
Mean gestational age at birth
Threatened preterm labour (as defined by trial authors)
Prelabour spontaneous rupture of membranes
Adverse drug reaction
Pregnancy prolongation (interval between randomisation and birth)
Mode of birth
Number of antenatal hospital admissions
Satisfaction with the therapy
Use of tocolysis
Maternal infection
Antenatal corticosteroids
Maternal quality of life
Infant
Birthweight less than the third centile for gestational age
Birthweight less than 2500 g
Mean birthweight
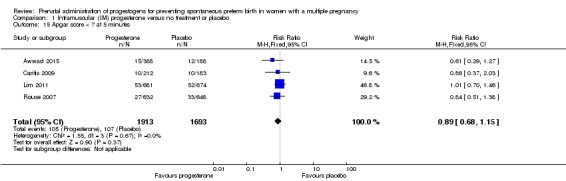
Apgar score of less than seven at five minutes
Respiratory distress syndrome
Use of mechanical ventilation
Duration of mechanical ventilation
Intraventricular haemorrhage ‐ grades III or IV
Periventricular leucomalacia
Retinopathy of prematurity
Retinopathy of prematurity ‐ grades III or IV
Chronic lung disease
Necrotising enterocolitis
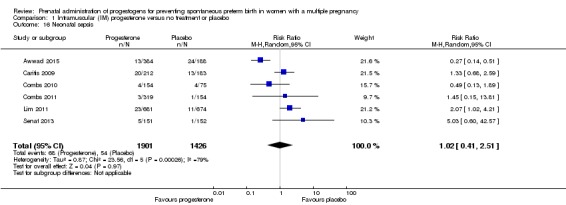
Neonatal sepsis
Fetal death
Neonatal death
Admission to neonatal intensive care unit
Neonatal length of hospital stay
Teratogenic effects (including virilisation in female infants)
Patent ductus arteriosus
Child
Major sensorineural disability (defined as any of legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, or developmental delay or intellectual impairment (defined as developmental quotient or intelligence quotient less than ‐2 standard deviations below mean))
Developmental delay (however defined by the authors)
Intellectual impairment
Motor impairment
Visual impairment
Blindness
Deafness
Hearing impairment
Cerebral palsy
Child behaviour
Child temperament
Learning difficulties
Growth assessments at childhood follow‐up (weight, head circumference, length, skin‐fold thickness)
Search methods for identification of studies
The following Methods section of this protocol is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (1 November 2016).
The Register is a database containing over 22,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Two people screen the search results and review the full text of all relevant trial reports identified through the searching activities described above. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (1 November 2016) for unpublished, planned and ongoing trial reports using the search methods in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
The following Methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the studies we identified as a result of the search strategy. We resolved any disagreement through discussion or consulted a third review author.
We created a study flow diagram to map out the number of records identified, included and excluded (see Figure 1).
Data extraction and management
We designed a form to extract the data, used by two review authors for eligible studies. We resolved discrepancies through discussion or, if required, consulted a third member of the review team. We entered data into Review Manager 5 software (RevMan 2014) and checked them for accuracy. When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risks of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
For each included study we assessed the method as being at:
low risk of bias (any truly random process, e.g. random number table; computer random‐number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We assessed the methods as being at:
low risk of bias (e.g. telephone or central randomisation; consecutively‐numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as being at:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as being at:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses that we undertook.
We assessed methods as being at:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as being at:
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For each included study we described any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it was likely to have an impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses (Sensitivity analysis).
Assessment of the quality of the evidence using GRADE
We assessed the overall quality of the evidence using the GRADE approach, as outlined in the GRADE handbook for the main comparison: administration of progesterone by any route for the prevention of preterm birth compared with placebo or no treatment.
We assessed the quality of the evidence for the following outcomes:
Perinatal mortality
Preterm birth (less than 34 weeks' gestation)
Major neurodevelopmental disability at childhood follow‐up
Infant birthweight less than 2500 g
Preterm birth less than 37 weeks
Preterm birth less than 28 weeks
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5 (RevMan 2014) in order to create ’Summary of findings’ tables. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes using GRADE methodology. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments of risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we present results as a summary risk ratio (RR) with a 95% confidence interval (CI).
Continuous data
For continuous data, we have used the mean difference (MD) if outcomes were measured in the same way between trials. We planned to use the standardised mean difference (SMD) to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
There were no cluster‐randomised trials identified during the search. In future updates of this review, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We plan to adjust their sample sizes using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and we consider the interaction between the effect of intervention and the choice of randomisation unit to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Multiple pregnancy
Special methods are needed when carrying out analysis of outcomes for babies from multiple pregnancies (Gates 2004). Outcomes in babies from multiple pregnancies are not independent. For many outcomes there will be a higher correlation between babies from the same pregnancy than between babies from different pregnancies. The degree of non‐independence of outcomes for babies from multiple pregnancies will vary considerably, depending on the outcome and the type of multiple pregnancy; for some outcomes an adverse event in one twin will almost invariably be associated with the same event in the other (e.g. preterm birth); for other outcomes the degree of correlation will be lower (e.g. fetal death), but still higher than for babies from different pregnancies. In view of this non‐independence, we treated babies from the same pregnancy as clusters and adjusted the data. We planned to obtain ICCs from the trials, or use ICCs from similar studies. However, published ICCs for multiple pregnancies were not available. We therefore estimated ICCs (based on clinical knowledge and data from observational studies) and carried out sensitivity analysis. We tested the effect of using two extremes of ICC. The first assumed complete dependence between twin infants; effectively we divided the number of events and the sample size by two (i.e. to reduce the sample size to the number of women rather than the number of infants). A second sensitivity analysis imagined a very low rate of dependence (1%) between twins; for this analysis we adjusted the events and sample sizes by dividing each by 1.01.
Cross‐over trials
Cross‐over trials are not a suitable design for this type of intervention and have not been included.
Dealing with missing data
For included studies, we have noted levels of attrition. If sufficient data had been available, we would have explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau2, I2 and Chi2 statistics. We regarded heterogeneity as substantial if the I2 was greater than 30% and either the Tau2 was greater than zero, or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will seek statistical advice on further analysis. We will also report whether the trial was prospectively registered and check that outcomes in the trial registration and subsequent publications are the same.
Data synthesis
We carried out statistical analysis using Review Manager 5 software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where trials were examining the same intervention, and we judged the trials’ populations and methods to be sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if we found substantial statistical heterogeneity, we used random‐effects meta‐analysis to produce an overall summary, if we considered an average treatment effect across trials clinically meaningful. We treated the random‐effects summary as the average of the range of possible treatment effects and we have discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
Where we used random‐effects analyses, the results are presented as the average treatment effect with a 95% confidence interval, and the estimates of Tau2 and I2.
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
We carried out, where possible, the following subgroup analyses:
Time of treatment beginning (before 20 weeks' gestation versus after 20 weeks' gestation)
Different dosage regimens (divided arbitrarily into a cumulative dose of less than 500 mg per week versus a dose greater than or equal to 500 mg per week)
We used the following outcomes, where possible, in subgroup analysis:
Perinatal mortality
Preterm birth (less than 34 weeks' gestation)
Major neurodevelopmental disability at childhood follow‐up
We assessed subgroup differences by interaction tests available within Review Manager 5 (RevMan 2014). We reported the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I2 value.
Sensitivity analysis
For perinatal death we carried out sensitivity analysis by testing the effect of using two extremes of ICC. The first assumed complete dependence between twin infants; effectively we divided all events and the sample size by two to reduce the sample size to the number of women rather than the number of infants. A second sensitivity analysis assumed a very low rate of dependence (1%) between twins; for this analysis we adjusted the events and sample sizes by dividing each by 1.01.
For our primary outcomes we planned to carry out sensitivity analysis examining the impact of risk of bias on results; studies that were at high risk of bias due to high sample attrition (> 20% at childhood follow‐up) were to be temporarily excluded from the analysis. Where we have conducted this sensitivity analysis, we have reported the result in the text for our primary analysis in Comparison 1.
Results
Description of studies
Results of the search
See: Figure 1.
Figure 1.
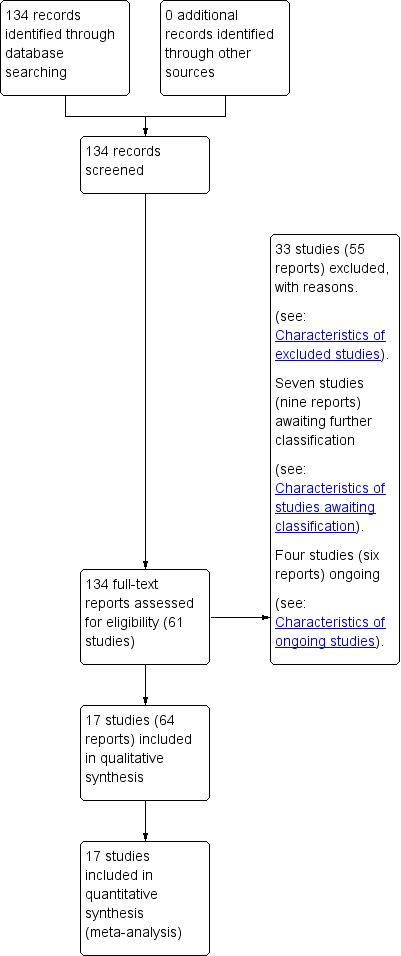
Study flow diagram.
Our search strategy identified 61 studies for consideration (some studies published multiple reports). We include 17 randomised trials in this review (Aboulghar 2012; Awwad 2015; Briery 2009; Brizot 2015; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; El‐Refaie 2016; Hartikainen‐Sorri 1980; Lim 2011; Norman 2009; Rode 2011; Rouse 2007; Senat 2013; Serra 2013; Wood 2012). We excluded 33 studies, seven are awaiting further assessment and four studies are ongoing.
Included studies
Design
All 17 randomised trials included in this review were placebo‐controlled and double‐blind, with the exception of two unblinded studies (El‐Refaie 2016; Senat 2013). All trials compared progesterone with placebo or no treatment; Serra 2013 conducted a three‐arm trial comparing two different doses of progesterone with placebo.
Sample sizes
Seventeen included trials randomised 4773 women with a multiple pregnancy. Sample sizes from the individual trials ranged from n = 30 (Briery 2009) to n = 677 (Rode 2011), with a median of n = 225 participants.
Setting
Trials took place in hospital clinics in the following countries: Austria, Brazil, Canada, Denmark, Egypt (two), Finland, France, Lebanon, Netherlands, Spain, Turkey, the UK and the USA (five). One trial took place in Austria and Denmark (Rode 2011). Several additional trials were conducted at multiple sites (Caritis 2009; Combs 2011; Lim 2011; Norman 2009; Rode 2011; Rouse 2007; Senat 2013; Wood 2012). Women receiving intramuscular (IM) injections often had these administered weekly following presentation to an antenatal clinic. Women allocated to daily progesterone suppositories or gels often self‐administered this medication at home.
Dates of trials, funding and conflicts of interest
Women were recruited to trials between 2004 to 2011, except for El‐Refaie 2016, when recruitment was at a later date (2012 to 2014), and in Briery 2009 and Hartikainen‐Sorri 1980, where dates of recruitment were not clear.
Four trials did not report whether or not trialists had any conflicts of interest (Cetingoz 2011; Combs 2010; Combs 2011; Hartikainen‐Sorri 1980). All remaining trials reported that there were no conflicts of interest.
Funding sources were not reported in four trials (Aboulghar 2012; Brizot 2015; Cetingoz 2011; Hartikainen‐Sorri 1980). Briery 2009 and Wood 2012 reported that pharmaceutical companies had supplied the study drugs, and Combs 2010, Combs 2011, and Serra 2013 appeared to be supported by grants from pharmaceutical companies. All remaining trials appeared to be funded by grants from university or government research funds.
Participants
One trial recruited only women with a triplet pregnancy (Combs 2010). Women with a triplet pregnancy were also eligible for inclusion in Caritis 2009, Lim 2011 and Wood 2012. All remaining trials were of women with a twin pregnancy. Most studies involving women with a twin pregnancy specifically excluded monochorionic twins or women at risk of twin‐transfusion syndrome. However, Lim 2011 included some women with a monochorionic twin pregnancy. Most trials excluded pregnant women with medical conditions, ruptured membranes, the presence of a cervical cerclage, or women who presented with symptoms or signs of labour. All trials excluded pregnant women where a fetal anomaly had been identified.
Assessment of risk of preterm birth varied across trials. Aboulghar 2012 recruited women who conceived following assisted reproduction (predominantly through IVF or ICSI). Cetingoz 2011 recruited pregnant women with a history of one previous spontaneous preterm birth. El‐Refaie 2016 recruited pregnant women with an ultrasound‐identified short cervix (defined as < 25 mm) between 20 and 24 weeks' gestation; approximately 24% of women in this trial had also had a previous preterm birth. Senat 2013 recruited women with an ultrasound‐identified short cervix (defined as < 25 mm), between 24 and 31 weeks' gestation. In contrast, Lim 2011 excluded women with a previous spontaneous preterm birth prior to 34 weeks' gestation, and Brizot 2015 recruited only women who conceived twins spontaneously, and with no history of preterm birth before 37 weeks.
Gestational age at the time of trial entry varied across the included trials. Awwad 2015, Caritis 2009, Lim 2011, Rouse 2007 and Wood 2012 all randomised women at between 16 and 20 weeks' gestation. Combs 2010 randomised women between 16 and 22 weeks, while Combs 2011 included women between 15 and 23 weeks' gestation. Aboulghar 2012, Cetingoz 2011, El‐Refaie 2016, Rode 2011 and Serra 2013 included pregnant women from between 18 or 20 weeks' and 24 weeks' gestation. The remaining trials randomised pregnant women at later gestational ages: Briery 2009 between 20 and 30 weeks; Cetingoz 2011 between 24 and 34 weeks; Senat 2013 between 24 and 31 weeks; Hartikainen‐Sorri 1980 between 28 and 37 weeks; and Norman 2009 between 24 and 34 weeks' gestation.
Interventions and comparisons
Vaginal progesterone
Eight trials (Aboulghar 2012; Brizot 2015; Cetingoz 2011; El‐Refaie 2016; Norman 2009; Rode 2011; Serra 2013; Wood 2012) evaluated vaginal progesterone suppositories, ovules or gel. Daily doses ranged from 90 mg per day (Norman 2009; Wood 2012) up to 400 mg per day (Aboulghar 2012; El‐Refaie 2016).
IM progesterone
Nine trials (Awwad 2015; Briery 2009; Caritis 2009; Combs 2010; Combs 2011; Hartikainen‐Sorri 1980; Lim 2011; Rouse 2007; Senat 2013) evaluated weekly IM injection of 17‐hydroxyprogesterone caproate. All used a single weekly dose of 250 mg, with the exception of Senat 2013, which used twice‐weekly administration of 500 mg.
Outcomes
All included trials contributed data to the meta‐analyses of the prespecified outcomes in the review.
Reporting of the primary outcome varied across the individual trials, although most identified preterm birth prior to 34 weeks (Aboulghar 2012; Brizot 2015; Cetingoz 2011; Combs 2011; El‐Refaie 2016; Rode 2011; Senat 2013; Serra 2013), 35 weeks (Briery 2009; Caritis 2009), or 37 weeks gestation (Aboulghar 2012; Awwad 2015; Brizot 2015; Cetingoz 2011; Combs 2011; Lim 2011; Rode 2011; Rouse 2007; Senat 2013; Serra 2013; Wood 2012). Five trials used a composite primary outcome, including death or birth prior to 34 weeks' gestation (Norman 2009), death or birth prior to 35 weeks' gestation (Rouse 2007), or a composite of neonatal adverse outcomes (Combs 2010; Combs 2011; Lim 2011) (seeTable 8). Gestational age at birth was the primary outcome for three additional trials (Brizot 2015; Caritis 2009; Wood 2012), and one trial reported the interval from randomisation to birth (Senat 2013). The primary outcome for the trial by Hartikainen‐Sorri 1980 was unclear.
Table 1.
Reporting of fetal, neonatal and perinatal death
| Trial | Fetal death (FD) | Intrapartum death | Stillbirth | Neonatal death (NND) | Perinatal death (PND) | Included in Dodd 2013? | Decision for PND for multiples review? |
| Aboulghar 2012 | ‐ | ‐ | ‐ | Reported as maternal outcome. From text appears as if at least one pregnancy affected by demise of both twins | ‐ | Yes | Cannot reliably convert maternal denominator for neonatal outcome. Data not included |
| Awwad 2015 | Yes | ‐ | ‐ | ‐ | Yes, as a baby outcome. | N/A | Include |
| Cetingoz 2011 | No death data | ‐ | ‐ | ‐ | ‐ | No PND reported | N/A |
| El‐Refaie 2016 | No death data | ‐ | ‐ | ‐ | ‐ | No PND reported | N/A |
| Serra 2013 | Fetal death reported as maternal outcome and only as a single co‐twin demise outcome. Unsure if any pregnancies where both twins died | ‐ | ‐ | Yes, as a baby outcome | Not reported. Cannot convert fetal death into a baby outcome because only reported if single twin demise likely to underestimate | Yes | Data not included. |
| Norman 2009 | Reported as a maternal outcome and denominator not clear | ‐ | ‐ | Yes | No | No | No |
| Rode 2011 | Yes. as maternal outcome but specifies only 1 twin affected in each of those pregnancies | ‐ | ‐ | Yes | In text | Yes | Yes |
| Wood 2012 | ‐ | ‐ | ‐ | ‐ | Yes, as infant outcome | N/A | Yes |
| Awwad 2015 | ‐ | ‐ | Yes | Yes | Yes, as infant outcome | N/A | Yes |
| Briery 2009 | ‐ | ‐ | ‐ | Yes | ‐ | No PND reported | N/A |
| Combs 2010 triplets | ‐ | ‐ | ‐ | Yes | Yes | Yes | Yes |
| Combs 2011 twins | ‐ | ‐ | ‐ | Yes | Yes | Yes | Yes |
| Hartikainen‐Sorri 1980 | ‐ | ‐ | ‐ | ‐ | Yes in text | Yes | Yes |
| Lim 2011 | ‐ | 1 or more died during delivery after 24 wks and also any IUD before onset labour or onset delivery | Reported “all live births” | ‐ | No, and cannot be reliably added up from data presented | Yes | Data not included |
| Rouse 2007 | Yes | ‐ | ‐ | Yes | Yes, from text (not in table) | No ‐ not sure why data not included | Yes |
| Senat 2013 | Yes | Yes | ‐ | Yes | Can add NND and FD (IP and IU); all Ns clear | No ‐ not sure why data not included | Yes. Extrapolated from text and checked |
| Caritis 2009 | Yes, as a maternal outcome | ‐ | ‐ | Yes | Cannot add FD and NND because FD reported as a maternal outcome | No | Data not included |
IP: intrapartum IU: intra‐uterine IUD: intra‐uterine death N/A: not applicable wk: week
Excluded studies
Most trials were excluded as they did not include women with a multiple pregnancy, or where the methodology adopted was clearly not randomised (e.g. secondary analysis or quasi‐randomisation). We also excluded trials if progesterone was intended as a tocolytic or used solely in the first trimester to prevent miscarriage. Please see the Excluded studies table for further details.
Risk of bias in included studies
Allocation
An adequate process of random sequence generation was described for most included trials, although risk of bias was unclear in the trial conducted by Hartikainen‐Sorri 1980. We rated allocation concealment at low risk of bias for all trials; trialists described using sealed opaque envelopes (Aboulghar 2012; Awwad 2015; Briery 2009; El‐Refaie 2016), a centralised allocation process (Cetingoz 2011; Senat 2013), or the use of identical‐appearing treatment packs (Brizot 2015; Caritis 2009; Combs 2010; Combs 2011; Hartikainen‐Sorri 1980; Lim 2011; Norman 2009; Rode 2011; Rouse 2007; Serra 2013; Wood 2012) to conceal allocation.
Blinding
Most of the trials were placebo‐controlled and we assessed them at low risk of performance and outcome detection bias. Blinding of participants, caregivers and staff was not achieved in El‐Refaie 2016 and Senat 2013, and was unclear for Hartikainen‐Sorri 1980. Blinding of outcome assessors was unclear in El‐Refaie 2016, Hartikainen‐Sorri 1980, and Senat 2013.
Incomplete outcome data
There were 10% or less missing outcome data for most of the included trials (Aboulghar 2012; Awwad 2015; Briery 2009; Caritis 2009; Cetingoz 2011; Combs 2010; Combs 2011; Hartikainen‐Sorri 1980; Lim 2011; Norman 2009; Rode 2011; Rouse 2007; Senat 2013; Serra 2013; Wood 2012). Missing outcome data were 10.4% in El‐Refaie 2016, and more than 20% in Brizot 2015.
Selective reporting
We judged six trials (Aboulghar 2012 ; Briery 2009; Cetingoz 2011; Combs 2011; El‐Refaie 2016; Hartikainen‐Sorri 1980) to be at high risk of selective outcome reporting, as the study was either registered retrospectively (Aboulghar 2012; El‐Refaie 2016) or was not registered and did not have a published protocol (Briery 2009; Cetingoz 2011; Combs 2011; Hartikainen‐Sorri 1980). We rated Serra 2013 at unclear risk and the remaining trials at low risk of bias for this domain.
Other potential sources of bias
There was no clear evidence of other potential sources of bias, although some trials provided limited information on methods.
See Figure 2 for an overall summary of risk of bias assessments.
Figure 2.
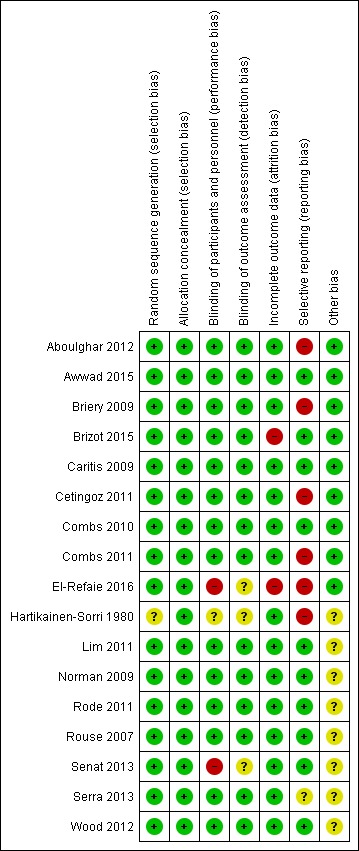
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
For a summary of main findings with an assessment of the quality of the evidence for key outcomes for the main comparisons (1) IM progesterone versus placebo or no treatment, and (2) vaginal progesterone versus placebo or no treatment, please seeTable 1 and Table 2.
Outcomes are presented for the following comparisons.
IM progesterone versus placebo (subgroup by weekly dose and subgroup by timing of start of therapy)
Vaginal progesterone versus placebo (subgroup by weekly dose and subgroup by timing of start of therapy)
IM progesterone versus no treatment (multiple pregnancy and short cervix)
Vaginal progesterone versus placebo (multiple pregnancy and short cervix)
Vaginal progesterone versus placebo (multiple pregnancy and other risk factor)
We report the results for each subgroup. Where there is evidence of subgroup differences, we report the results of the interaction tests and the effect estimates in subgroups.
Comparison 1: Intramuscular (IM) progesterone versus placebo
Subgroup by weekly dose (≤ 250 mg per week OR > 250 mg per week)
Subgroup by timing of start of therapy (< 20 weeks versus > 20 weeks versus mixed gestational age)
Primary outcomes
1.1 Maternal mortality
There were no trials included in this review which reported maternal mortality.
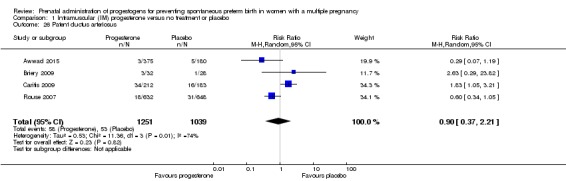
1.2 Preterm birth less than 34 weeks
IM progesterone was associated with an increase in risk of preterm birth prior to 34 weeks' gestation (risk ratio (RR) 1.54, 95% confidence interval (CI) 1.06 to 2.26; women = 399; studies = 2; I2 = 0%; Analysis 1.1, low‐quality evidence) when compared with placebo or no treatment, reflecting the increased risk of preterm birth observed in Senat 2013, which used a higher weekly dose of 500 mg (RR 1.67, 95% CI 1.04 to 2.68; women = 161; studies = 1; Analysis 1.2). There were no clear group differences relating to the timing of the start of IM progesterone therapy for the risk of preterm birth before 34 weeks' gestation (test for subgroup differences: Chi2 = 0.24, df = 1 (P = 0.62), I2 = 0%; Analysis 1.3).
Analysis 1.1.

Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 1 Preterm birth less than 34 weeks.
Analysis 1.2.
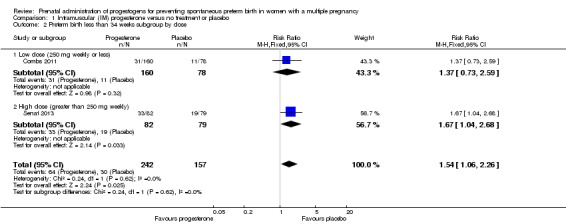
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 2 Preterm birth less than 34 weeks subgroup by dose.
Analysis 1.3.
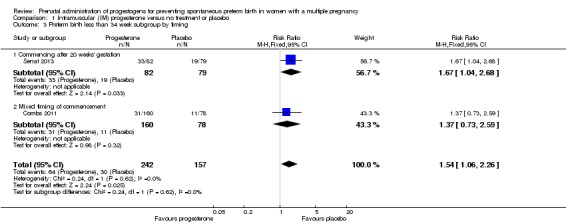
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 3 Preterm birth less than 34 week subgroup by timing.
1.3 Perinatal death
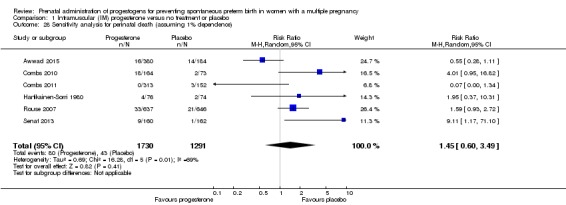
There was no clear evidence that the use of IM progesterone was protective against perinatal death (average RR 1.45, 95% CI 0.60 to 3.51; infants = 3089; studies = 6; I2 = 71%; low‐quality evidence;Analysis 1.4) when compared with placebo or no treatment. Subgroup analysis by dose did not show a clear difference between high‐ and low‐dose subgroups; only one trial with a relatively small sample size used a higher weekly dose of progesterone (Senat 2013) Analysis 1.5) (test for subgroup differences: Chi2 = 3.29, df = 1 (P = 0.07), I2 = 69.6%). There were no apparent subgroup differences relating to the timing of the start of IM progesterone therapy and risk of perinatal death (test for subgroup differences: Chi2 = 2.26, df = 2 (P = 0.32), I2 = 11.6%; Analysis 1.6). (Sensitivity analysis assuming either complete dependence between multiples from the same pregnancy, or a low correlation between outcomes for multiples corresponded closely with the main analysis; Analysis 1.27; Analysis 1.28).
Analysis 1.4.
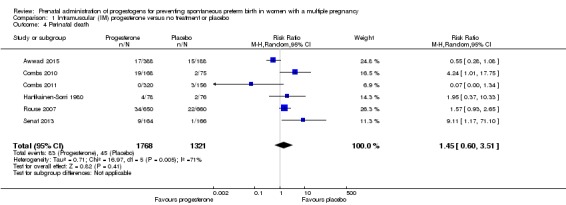
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 4 Perinatal death.
Analysis 1.5.
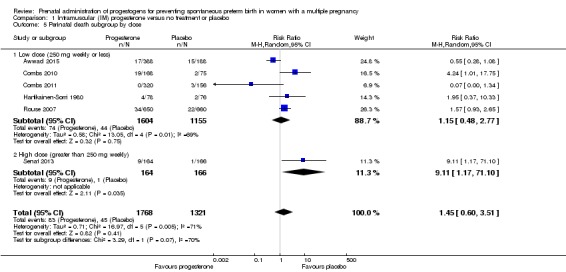
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 5 Perinatal death subgroup by dose.
Analysis 1.6.
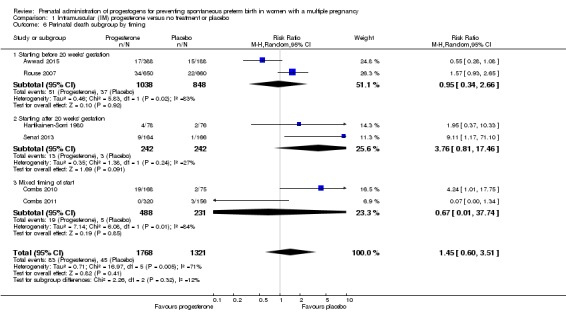
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 6 Perinatal death subgroup by timing.
Analysis 1.27.
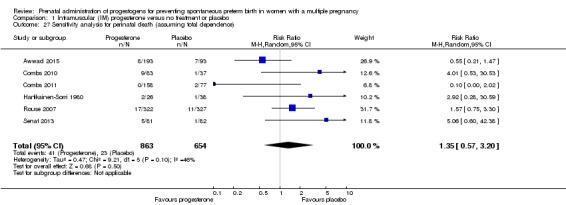
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 27 Sensitivity analysis for perinatal death (assuming total dependence).
Analysis 1.28.
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 28 Sensitivity analysis for perinatal death (assuming 1% dependence).
1.4 Major neurodevelopmental disability at childhood follow‐up
There were no trials included in this review which reported childhood neurodevelopmental outcome.
Secondary outcomes ‐ Maternal
Prelabour ruptured membranes
Women who received IM progesterone, placebo or no treatment had similar rates of prelabour ruptured membranes (RR 1.17, 95% CI 0.84 to 1.63; women = 1257; studies = 6; I2 = 0%; Analysis 1.7).
Analysis 1.7.
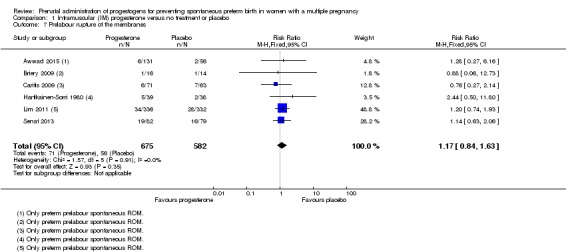
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 7 Prelabour rupture of the membranes.
Preterm birth less than 37 weeks
Women who received IM progesterone, placebo or no treatment had similar rates of preterm birth before 37 weeks' gestation (RR 1.05, 95% CI 0.98 to 1.13; women = 2010; studies = 5; I2 = 0%; high‐quality evidence;Analysis 1.8).
Analysis 1.8.
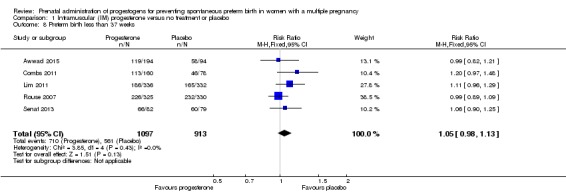
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 8 Preterm birth less than 37 weeks.
Preterm birth less than 28 weeks
Women who received IM progesterone, placebo or no treatment had similar rates of risk of preterm birth before 28 weeks' gestation (RR 1.08, 95% CI 0.75 to 1.55; women = 1920; studies = 5; I2 = 0%; moderate‐quality evidence;Analysis 1.9).
Analysis 1.9.
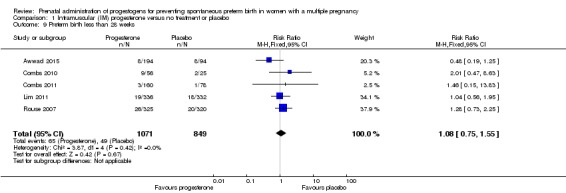
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 9 Preterm birth less than 28 weeks.
Adverse drug reaction
There were no clear group differences between women who received IM progesterone and those women who did not, in the experience of adverse effects relating to drug administration (average RR 0.91, 95% CI 0.63 to 1.32; women = 1316; studies = 2; I2 = 81%; Analysis 1.10).
Analysis 1.10.
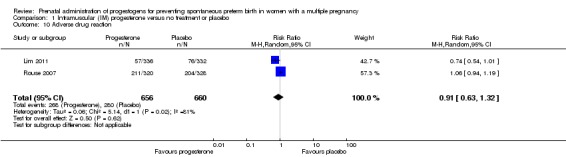
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 10 Adverse drug reaction.
Caesarean birth
Women who received IM progesterone, placebo or no treatment had similar rates of caesarean birth (RR 1.01, 95% CI 0.95 to 1.08; women = 2222; studies = 7; I2 = 0%; Analysis 1.11).
Analysis 1.11.
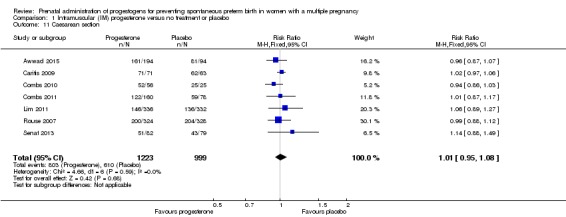
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 11 Caesarean section.
Antenatal tocolysis
There were no clear differences between women who received IM progesterone and those women who did not, in their need for antenatal tocolysis (RR 0.97, 95% CI 0.85 to 1.10; women = 2218; studies = 7; I2 = 19%; Analysis 1.12).
Analysis 1.12.
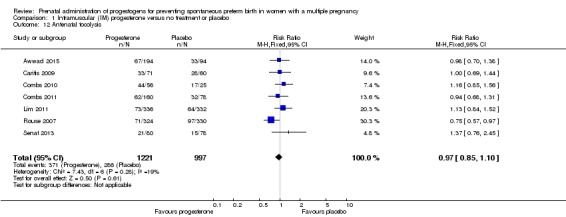
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 12 Antenatal tocolysis.
Antenatal corticosteroids
There were no clear differences between women who received IM progesterone and those women who did not, in their need for antenatal corticosteroid administration (RR 0.99, 95% CI 0.88 to 1.11; women = 2221; studies = 7; I2 = 0%; Analysis 1.13).
Analysis 1.13.
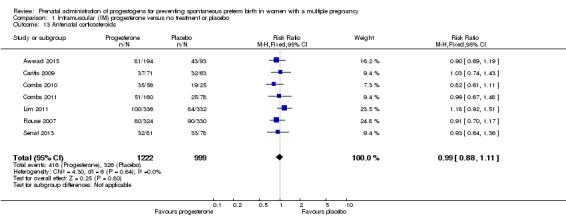
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 13 Antenatal corticosteroids.
Secondary outcomes ‐ Infant
Infant birthweight less than 2500 g
Infants born to women who received IM progesterone and those who did not had similar rates of birthweight less than 2500 g (average RR 0.99, 95% CI 0.90 to 1.08; infants = 4071; studies = 5; I2 = 76%; moderate‐quality evidence; Analysis 1.14).
Analysis 1.14.
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 14 Infant birthweight less than 2500 g.
Apgar score less than seven at five minutes of age
Infants born to women who received IM progesterone and those who did not had similar rates of Apgar score less than seven at five minutes of age (RR 0.89, 95% CI 0.68 to 1.15; infants = 3606; studies = 4; I2 = 0%; Analysis 1.15).
Analysis 1.15.
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 15 Apgar score < 7 at 5 minutes.
Neonatal sepsis
Infants born to women who received IM progesterone and those who did not had similar rates of neonatal sepsis (average RR 1.02, 95% CI 0.41 to 2.51; infants = 3327; studies = 6; I2 = 79%; Analysis 1.16).
Analysis 1.16.
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 16 Neonatal sepsis.
Respiratory distress syndrome
Infants born to women who received IM progesterone and those who did not had similar rates of respiratory distress syndrome (average RR 1.07, 95% CI 0.85 to 1.34; participants = 4670; studies = 8; I² = 66%; Analysis 1.17).
Analysis 1.17.
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 17 Respiratory distress syndrome.
Use of mechanical ventilation
Infants born to women who received IM progesterone and those who did not had similar rates of mechanical ventilation (average RR 0.90, 95% CI 0.69 to 1.17; infants = 2233; studies = 3; I2 = 43%; Analysis 1.18).
Analysis 1.18.
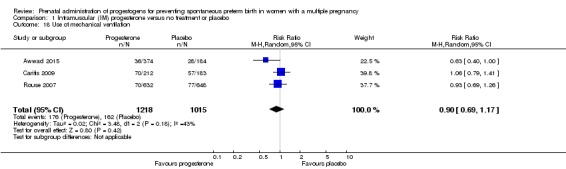
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 18 Use of mechanical ventilation.
Intraventricular haemorrhage
There were no group differences between infants born to women who received IM progesterone and those who did not, for the risk of intraventricular haemorrhage (RR 1.98, 95% CI 0.36 to 10.77; infants = 1355; studies = 1; Analysis 1.19), reported in a single study only.
Analysis 1.19.
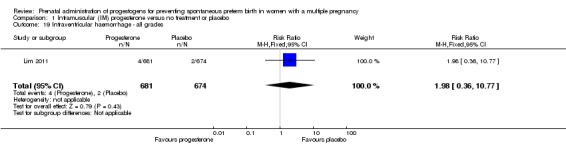
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 19 Intraventricular haemorrhage ‐ all grades.
Retinopathy of prematurity
Infants born to women who received IM progesterone were at reduced risk of retinopathy of prematurity, although event rates were fairly low for this outcome (RR 0.34, 95% CI 0.16 to 0.74; infants = 2807; studies = 5; I2 = 0%; Analysis 1.20).
Analysis 1.20.
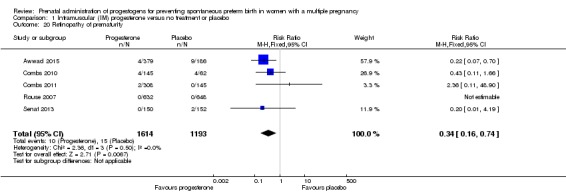
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 20 Retinopathy of prematurity.
Chronic lung disease
There were no clear group difference between infants born to women who received IM progesterone and those who did not for the risk of chronic lung disease (average RR 1.91, 95% CI 0.13 to 27.80; infants = 681; studies = 2; I2 = 71%; Analysis 1.21).
Analysis 1.21.
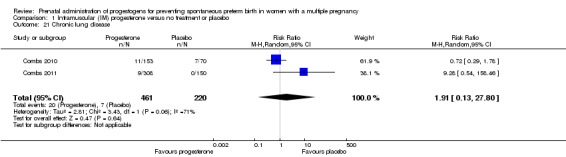
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 21 Chronic lung disease.
Necrotising enterocolitis
There was no clear difference in the rate of necrotising enterocolitis comparing infants born to women who received IM progesterone and those who did not (RR 0.74, 95% CI 0.36 to 1.51; infants = 2610; studies = 5; I2 = 0%; Analysis 1.22).
Analysis 1.22.
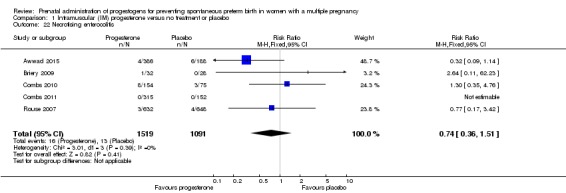
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 22 Necrotising enterocolitis.
Fetal death
There was no clear difference in the rate of fetal death comparing infants born to women who received IM progesterone and those who did not (average RR 0.93, 95% CI 0.39 to 2.20; infants = 3536; studies = 4; I2 = 56%; Analysis 1.23).
Analysis 1.23.
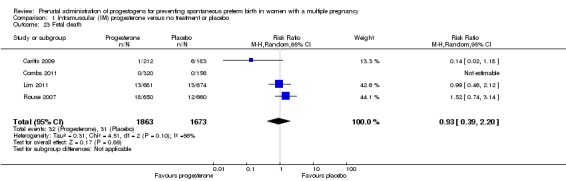
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 23 Fetal death.
Neonatal death
There was no clear difference in the rate of neonatal death comparing infants born to women who received IM progesterone with those who did not (average RR 0.92, 95% CI 0.44 to 1.91; infants = 3399; studies = 7; I2 = 35%; Analysis 1.24).
Analysis 1.24.
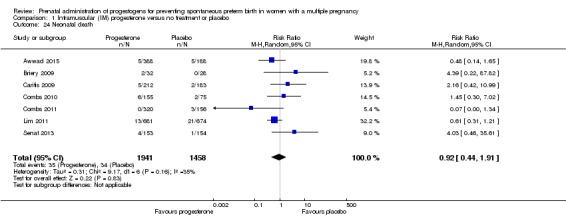
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 24 Neonatal death.
Admission to neonatal intensive care unit
Infants born to women who received IM progesterone were more likely to require admission to the neonatal intensive care unit compared with infants born to women who did not (RR 1.33, 95% CI 1.13 to 1.58; infants = 1668; studies = 2; I2 = 0%; Analysis 1.25).
Analysis 1.25.
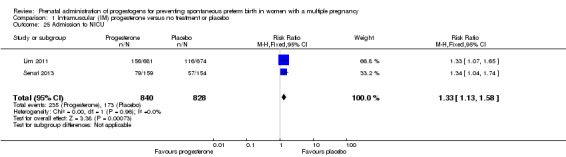
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 25 Admission to NICU.
Patent ductus arteriosus
Infants born to women who received IM progesterone and those who did not had a similar rate of patent ductus arteriosus (average RR 0.90, 95% CI 0.37 to 2.21; infants = 2290; studies = 4; I2 = 74%; Analysis 1.26).
Analysis 1.26.
Comparison 1 Intramuscular (IM) progesterone versus no treatment or placebo, Outcome 26 Patent ductus arteriosus.
Secondary outcomes ‐ Child
None of the included studies evaluating IM progesterone reported childhood outcomes.
Comparison 2: Vaginal progesterone versus placebo
Subgroup by daily dose (≤ 200 mg per day versus > 200 mg per day)
Subgroup by timing of start of therapy (< 20 weeks versus > 20 weeks versus mixed gestational age)
Primary outcomes
2.1 Maternal mortality
There were no trials included in this review reporting maternal mortality.
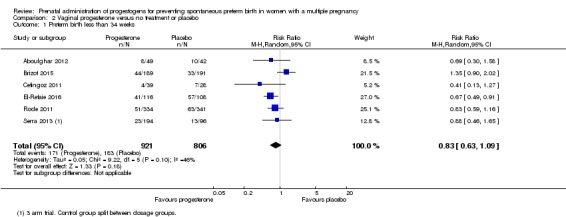
2.2 Preterm birth less than 34 weeks
Women who received vaginal progesterone and those who did not had a similar risk of preterm birth before 34 weeks' gestation (average RR 0.83, 95% CI 0.63 to 1.09; women = 1727; studies = 6; I2 = 46%; low‐quality evidence, Analysis 2.1). We carried out subgroup analysis by higher and lower weekly dose (Analysis 2.2), with the subgroup interaction test suggesting no meaningful differences between subgroups (test for subgroup differences: Chi2 = 1.66, df = 1 (P = 0.20), I2 = 39.7%). Starting vaginal progesterone after 20 weeks' gestation was associated with a reduction in preterm birth before 34 weeks' gestation, compared with starting prior to 20 weeks' gestation, or at mixed gestational age (RR 0.69, 95% CI 0.30 to 1.58; women = 91; studies = 1; Analysis 2.3). However, although the interaction test suggested differences between subgroups, only one study contributed data to the 'before 20 weeks'' subgroup (test for subgroup differences: Chi2 = 7.02, df = 2 (P = 0.03), I2 = 71.5%).
Analysis 2.1.
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 1 Preterm birth less than 34 weeks.
Analysis 2.2.
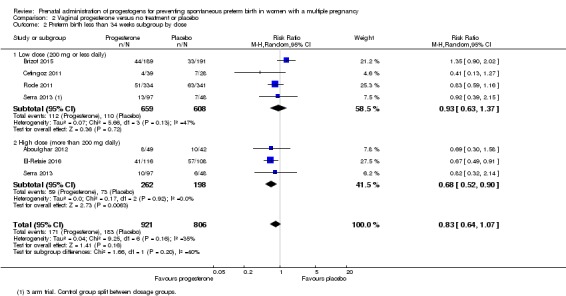
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 2 Preterm birth less than 34 weeks subgroup by dose.
Analysis 2.3.
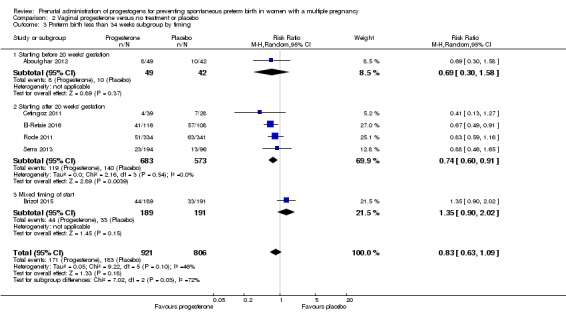
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 3 Preterm birth less than 34 weeks subgroup by timing.
2.3 Perinatal death
There was no clear evidence to suggest that the use of vaginal progesterone was protective against perinatal death (RR 1.23, 95% CI 0.74 to 2.06; infants = 2287; studies = 3; I² = 0%; low‐quality evidence;Analysis 2.4), with all studies reporting this outcome using a daily dose of vaginal progesterone of 200 mg or less. There was no evidence of a different effect relating to the timing of starting progesterone therapy (Analysis 2.6) (test for subgroup differences: Chi2 = 0.23, df = 2 (P = 0.89), I2 = 0%). Sensitivity analysis assuming either complete dependence between multiples from the same pregnancy, or a low correlation between outcomes for multiples corresponded closely with the main analysis; Analysis 2.27; Analysis 2.28).
Analysis 2.4.
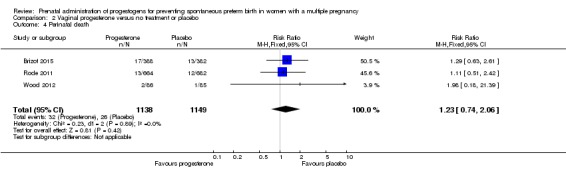
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 4 Perinatal death.
Analysis 2.6.
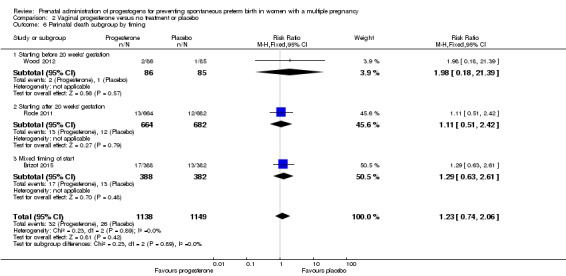
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 6 Perinatal death subgroup by timing.
Analysis 2.27.
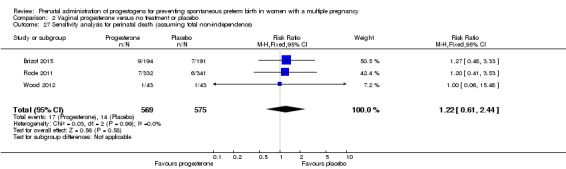
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 27 Sensitivity analysis for perinatal death (assuming total non‐independence).
Analysis 2.28.
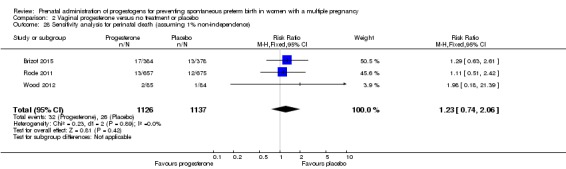
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 28 Sensitivity analysis for perinatal death (assuming 1% non‐independence).
2.4 Major neurodevelopmental disability at childhood follow‐up
There were no trials included in this review reporting childhood neurodevelopmental outcomes.
Secondary outcomes ‐ Maternal
Prelabour ruptured membranes
Women who received vaginal progesterone, placebo or no treatment had similar rates of prelabour ruptured membranes (RR 0.61, 95% CI 0.23 to 1.60; women = 514; studies = 2; I2 = 0%; Analysis 2.7).
Analysis 2.7.
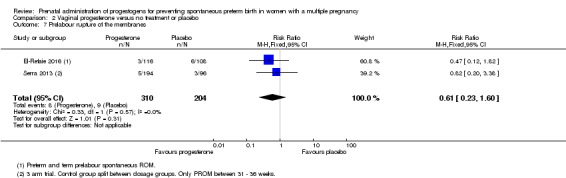
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 7 Prelabour rupture of the membranes.
Preterm birth less than 37 weeks
Women who received vaginal progesterone, placebo or no treatment had similar rates of preterm birth before 37 weeks' gestation (RR 0.97, 95% CI 0.89 to 1.06; women = 1597; studies = 6; I2 = 0%; moderate‐quality evidence;Analysis 2.8).
Analysis 2.8.
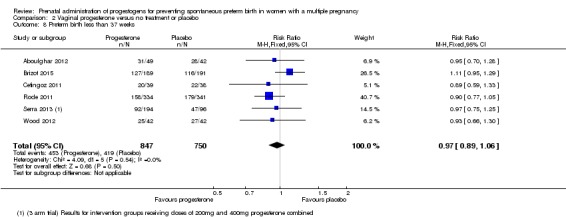
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 8 Preterm birth less than 37 weeks.
Preterm birth less than 28 weeks
Women who received vaginal progesterone, placebo or no treatment had similar rates of preterm birth before 28 weeks' gestation (RR 1.22, 95% CI 0.68 to 2.21; women = 1569; studies = 4; I2 = 0%; low‐quality evidence;Analysis 2.9).
Analysis 2.9.
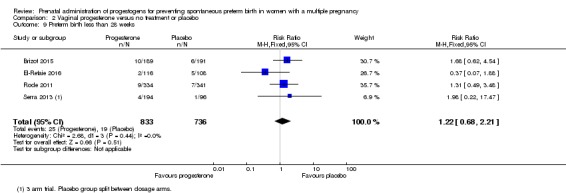
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 9 Preterm birth less than 28 weeks.
Adverse drug reaction
There were no group differences in the reporting of adverse effects relating to drug administration between women who received vaginal progesterone and those who did not (RR 0.99, 95% CI 0.90 to 1.09; women = 562; studies = 2; I2 = 16%; Analysis 2.10).
Analysis 2.10.
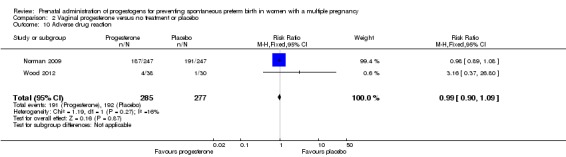
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 10 Adverse drug reaction.
Caesarean birth
Women who received vaginal progesterone were less likely to give birth by caesarean section compared with women who did not (RR 0.93, 95% CI 0.88 to 0.98; women = 2143; studies = 6; I2 = 0%; Analysis 2.11).
Analysis 2.11.
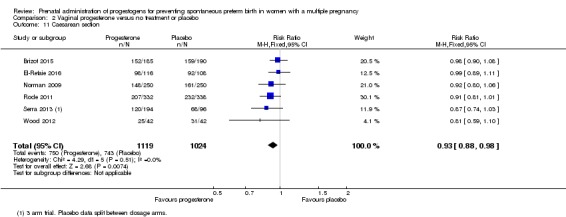
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 11 Caesarean section.
Maternal satisfaction with therapy
There was one study that reported a similar degree of satisfaction between women who received vaginal progesterone and those who did not (mean difference (MD) 0.00, 95% CI ‐0.35 to 0.35; women = 494; studies = 1; Analysis 2.12; Norman 2009).
Analysis 2.12.
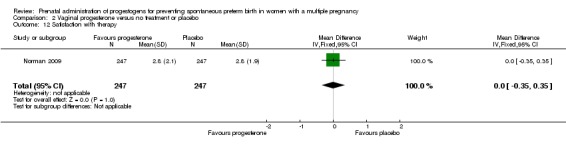
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 12 Satisfaction with therapy.
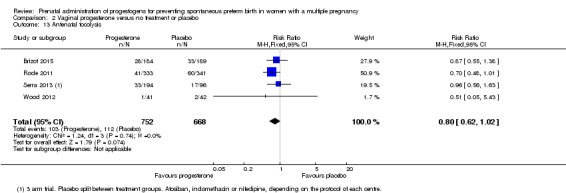
Antenatal tocolysis
Women who received vaginal progesterone, placebo or no treatment had similar rates of antenatal tocolysis (RR 0.80, 95% CI 0.62 to 1.02; women = 1420; studies = 4; I2 = 0%; Analysis 2.13).
Analysis 2.13.
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 13 Antenatal tocolysis.
Antenatal corticosteroids
Women who received vaginal progesterone, placebo or no treatment had similar rates of antenatal corticosteroid administration (RR 0.87, 95% CI 0.71 to 1.06; women = 1422; studies = 4; I2 = 26%; Analysis 2.14).
Analysis 2.14.
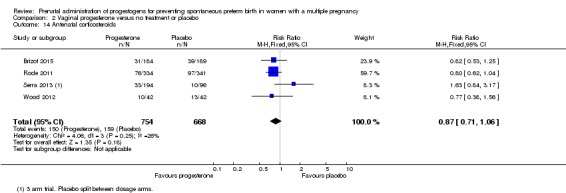
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 14 Antenatal corticosteroids.
Secondary outcomes ‐ Infant
Infant birthweight less than 2500 g
Infants born to women who received vaginal progesterone compared to those who did not had similar rates of birthweight less than 2500 g (average RR 0.95, 95% CI 0.88 to 1.03; infants = 3079; studies = 4; I2 = 49%; moderate‐quality evidence;Analysis 2.15).
Analysis 2.15.
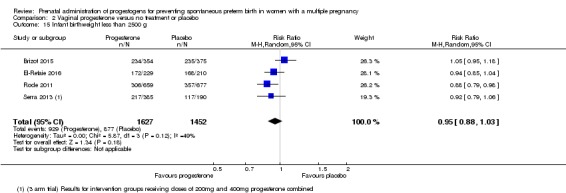
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 15 Infant birthweight less than 2500 g.
Apgar score less than seven at five minutes of age
Infants born to women who received vaginal progesterone had similar rates of Apgar score less than seven at five minutes of age compared with those born to women who did not receive vaginal progesterone (RR 0.65, 95% CI 0.35 to 1.19; infants = 2410; studies = 3; I2 = 0%; Analysis 2.16).
Analysis 2.16.
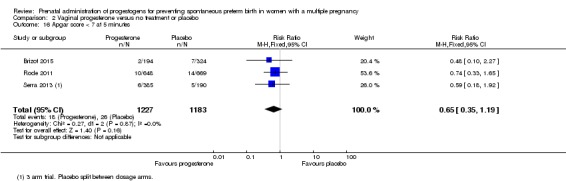
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 16 Apgar score < 7 at 5 minutes.
Respiratory distress syndrome
There were no clear differences between infants born to women who received vaginal progesterone and those who did not, for risk of respiratory distress syndrome (average RR 0.84, 95% CI 0.64 to 1.10; infants = 2560; studies = 4; I2 = 59%; Analysis 2.17).
Analysis 2.17.
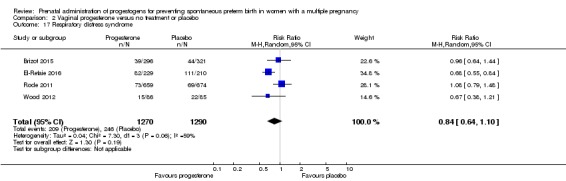
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 17 Respiratory distress syndrome.
Use of mechanical ventilation
Infants born to women who received vaginal progesterone were less likely to require mechanical ventilation than infants born to women who did not (RR 0.61, 95% CI 0.48 to 0.77; infants = 3134; studies = 5; I2 = 0%; Analysis 2.18).
Analysis 2.18.
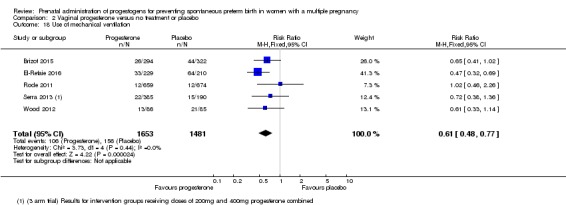
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 18 Use of mechanical ventilation.
Intraventricular haemorrhage
Infants born to women who received vaginal progesterone compared to those who did not had similar rates of intraventricular haemorrhage (RR 1.70, 95% CI 0.62 to 4.66; infants = 1333; studies = 1; Analysis 2.19).
Analysis 2.19.
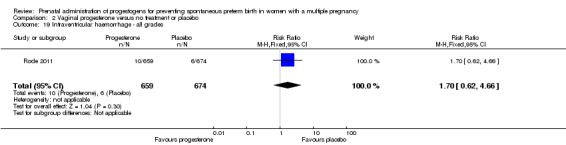
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 19 Intraventricular haemorrhage ‐ all grades.
Retinopathy of prematurity
Infants born to women who received vaginal progesterone compared to those who did not had similar rates of retinopathy of prematurity (RR 1.07, 95% CI 0.45 to 2.54; infants = 1945; studies = 2; I2 = 0%; Analysis 2.20).
Analysis 2.20.
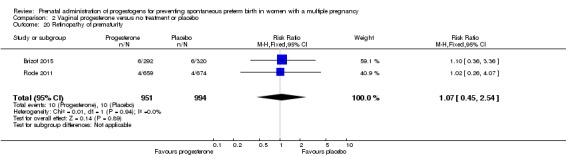
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 20 Retinopathy of prematurity.
Necrotising enterocolitis
Infants born to women who received vaginal progesterone compared to those who did not had similar rates of necrotising enterocolitis (RR 0.52, 95% CI 0.13 to 2.06; infants = 2117; studies = 3; I2 = 0%; Analysis 2.21).
Analysis 2.21.
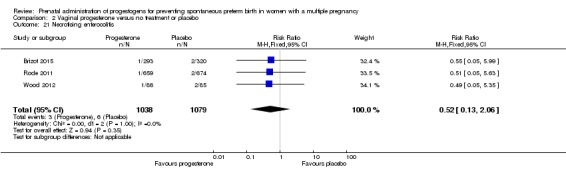
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 21 Necrotising enterocolitis.
Neonatal sepsis
There were no clear differences between infants born to women who received vaginal progesterone and those who did not, for risk of neonatal sepsis (RR 1.41, 95% CI 0.86 to 2.33; infants = 1944; studies = 2; I2 = 19%; Analysis 2.22).
Analysis 2.22.
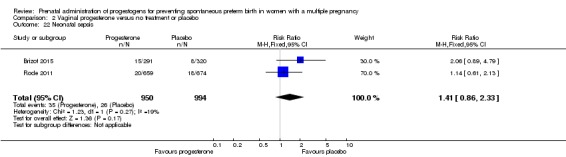
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 22 Neonatal sepsis.
Fetal death
There were no clear differences in the rate of fetal death between infants born to women who received vaginal progesterone and those who did not (RR 1.38, 95% CI 0.65 to 2.90; participants = 2328; studies = 3; I2 = 0%; Analysis 2.23).
Analysis 2.23.
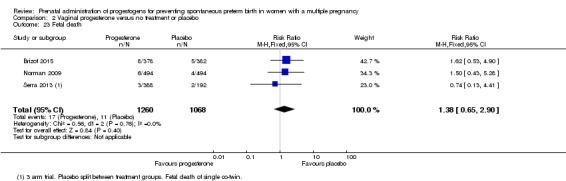
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 23 Fetal death.
Neonatal death
There were no clear differences in the rate of neonatal death between infants born to women who received vaginal progesterone and those who did not (RR 1.53, 95% CI 0.75 to 3.15; infants = 2905; studies = 3; I2 = 0%; Analysis 2.24).
Analysis 2.24.
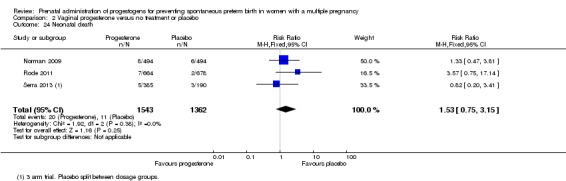
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 24 Neonatal death.
Admission to neonatal intensive care unit (NICU)
There were no clear differences between infants born to women who received vaginal progesterone and those did not, for admission to the neonatal intensive care unit (RR 0.93, 95% CI 0.87 to 1.00; infants = 4052; studies = 5; I2 = 25%; Analysis 2.25).
Analysis 2.25.
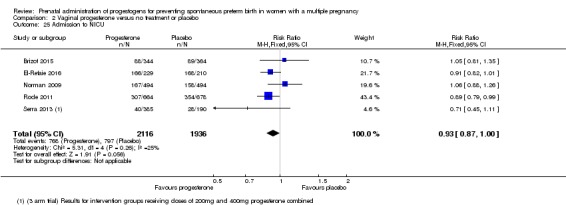
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 25 Admission to NICU.
Patent ductus arteriosus
There were no clear differences between infants born to women who received vaginal progesterone for patent ductus arteriosus, compared with infants born to women who did not (RR 0.76, 95% CI 0.47 to 1.22; infants = 1946; studies = 2; I2 = 0%; Analysis 2.26).
Analysis 2.26.
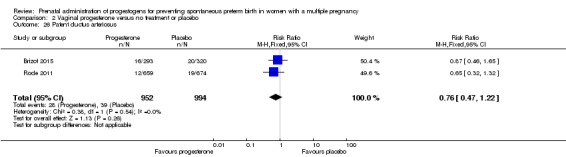
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 26 Patent ductus arteriosus.
Secondary outcomes ‐ Child
None of the included studies evaluating vaginal progesterone reported childhood outcomes.
Further analysis by indication
All of the trials included in this review recruited and reported results for women with multiple pregnancy. However, in some trials there were additional clinical indications for the administration of progesterone, such as short cervix, or trials included only women from a particular population subgroup, such as women undergoing IVF. We therefore looked separately at trials where there were other indications, in comparisons 3 to 5; as in the main analysis, we examined IM and vaginal progesterone administration in separate comparisons.
Comparison 3: IM progesterone versus no treatment: multiple pregnancy and short cervix
A single trial (Senat 2013) contributed data to this comparison. In Senat 2013 165 women with twin pregnancy and short cervix (25 mm or less) were recruited and treatment began at between 24 and 31+6 weeks' gestation; 500 mg of IM 17‐alpha‐hydroxyprogesterone caproate was administered twice weekly until 36 weeks or preterm delivery, whichever occurred first (high dose).
Preterm birth less than 34 weeks
IM progesterone appeared to increase the risk of preterm birth before 34 weeks, although 95% CIs were wide (RR 1.67, 95% CI 1.04 to 2.68; women = 161; studies = 1; Analysis 3.1).
Analysis 3.1.
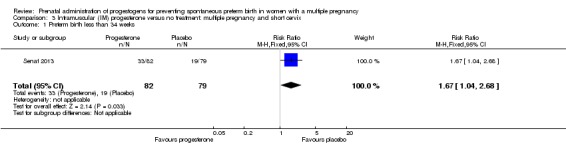
Comparison 3 Intramuscular (IM) progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 1 Preterm birth less than 34 weeks.
Perinatal death
Perinatal death was also increased in the progesterone group in this trial with 9/164 and 1/166 deaths in the intervention and control groups respectively (RR 9.11, 95% CI 1.17 to 71.10; infants = 330; studies = 1; Analysis 3.2).
Analysis 3.2.
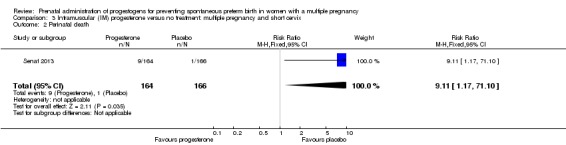
Comparison 3 Intramuscular (IM) progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 2 Perinatal death.
Prelabour rupture of the membranes
There was no clear difference in the risk of prelabour rupture of the membranes between the women who received progesterone compared with women who received placebo (RR 1.14, 95% CI 0.63 to 2.06; women = 161; studies = 1; Analysis 3.3).
Analysis 3.3.
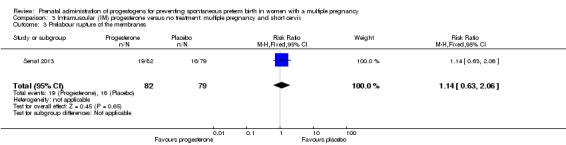
Comparison 3 Intramuscular (IM) progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 3 Prelabour rupture of the membranes.
Preterm birth less than 37 weeks
Women who received IM progesterone had similar rates of preterm birth before 37 weeks' gestation compared with women who received placebo (RR 1.06, 95% CI 0.90 to 1.25; women = 161; studies = 1; Analysis 3.4).
Analysis 3.4.
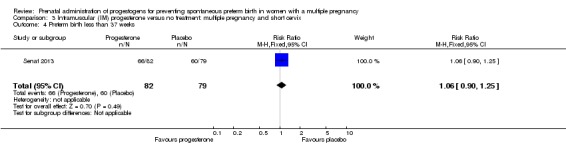
Comparison 3 Intramuscular (IM) progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 4 Preterm birth less than 37 weeks.
Caesarean section
Women who received IM progesterone had similar rates of caesarean birth compared with women who received placebo (RR 1.14, 95% CI 0.88 to 1.49; women = 161; studies = 1; Analysis 3.5).
Analysis 3.5.
Comparison 3 Intramuscular (IM) progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 5 Caesarean section.
Antenatal tocolysis
There was no clear difference in the numbers of women who required antenatal tocolysis between those who received IM progesterone compared with those who did not (RR 1.36, 95% CI 0.76 to 2.45; women = 158; studies = 1; Analysis 3.6).
Analysis 3.6.
Comparison 3 Intramuscular (IM) progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 6 Antenatal tocolysis.
Antenatal corticosteroids
There was no clear difference in the numbers of women who required antenatal corticosteroids between those who received IM progesterone compared with those who did not (RR 0.93, 95% CI 0.64 to 1.36; women = 159; studies = 1; Analysis 3.7).
Analysis 3.7.
Comparison 3 Intramuscular (IM) progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 7 Antenatal corticosteroids.
Neonatal sepsis
Infants born to women who received progesterone had a similar incidence of sepsis to infants of women who did not receive progesterone (RR 5.03, 95% CI 0.60 to 42.57; infants = 303; studies = 1; Analysis 3.8).
Analysis 3.8.
Comparison 3 Intramuscular (IM) progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 8 Neonatal sepsis.
Respiratory distress syndrome
Infants born to women who received progesterone were slightly more likely to have respiratory distress syndrome compared with infants of women who did not receive progesterone (RR 1.46, 95% CI 1.00 to 2.12; infants = 309; studies = 1; Analysis 3.9).
Analysis 3.9.
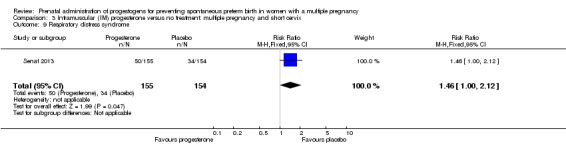
Comparison 3 Intramuscular (IM) progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 9 Respiratory distress syndrome.
Retinopathy of prematurity
There was no clear difference in the number of infants with retinopathy of prematurity when comparing infants of women who received progesterone with infants of women who did not (RR 0.20, 95% CI 0.01 to 4.19); infants = 302; studies = 1; Analysis 3.10).
Analysis 3.10.
Comparison 3 Intramuscular (IM) progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 10 Retinopathy of prematurity.
Neonatal death
There was no clear difference in the risk of death in the neonatal period for infants of women who received progesterone compared with infants of women who did not (RR 4.03, 95% CI 0.46 to 35.61; infants = 307; studies = 1; Analysis 3.11).
Analysis 3.11.
Comparison 3 Intramuscular (IM) progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 11 Neonatal death.
Admission to neonatal intensive care unit
There were more infants of women who received progesterone admitted to the neonatal intensive care unit compared with infants of women who did not receive progesterone (RR 1.34, 95% CI 1.04 to 1.74; infants = 313; studies = 1; Analysis 3.12).
Analysis 3.12.
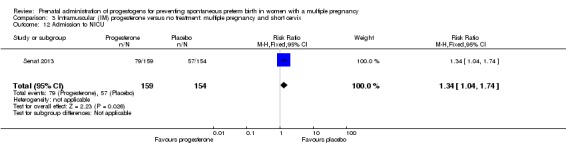
Comparison 3 Intramuscular (IM) progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 12 Admission to NICU.
For perinatal death we carried out sensitivity analyses assuming total dependence and low dependence of outcomes for babies from the same pregnancy. If total dependence is assumed (i.e. all babies from the same pregnancy either survive or die) the evidence of a difference between groups for perinatal death was no longer statistically significant (RR 5.06, 95% CI 0.60 to 42.38; Analysis 3.14).
Analysis 3.14.
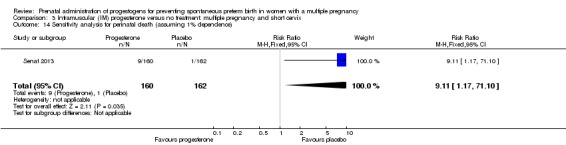
Comparison 3 Intramuscular (IM) progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 14 Sensitivity analysis for perinatal death (assuming 1% dependence).
Comparison 4: Vaginal progesterone versus no treatment: multiple pregnancy and short cervix.
A single study (El‐Refaie 2016) recruiting 225 women with multiple pregnancy and short cervix contributed data to this comparison. In this study the intervention group received vaginal progesterone suppositories (400 mg daily, high dose) starting at 20 to 24 weeks' gestation until 37 weeks, while women in the control group received standard antenatal care.
For our primary outcomes, maternal and perinatal mortality were not reported.
Preterm birth less than 34 weeks
For women receiving vaginal progesterone, there appeared to be a decrease in the risk of preterm birth before 34 weeks compared with women who received placebo (RR 0.67, 95% CI 0.49 to 0.91: women = 224; studies = 1; Analysis 4.1).
Analysis 4.1.
Comparison 4 Vaginal progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 1 Preterm birth less than 34 weeks.
Prelabour rupture of the membranes
There was no clear difference in the risk of prelabour rupture of the membranes between the women who received progesterone compared with women who received placebo (RR 0.47, 95% CI 0.12 to 1.82; women = 224; studies = 1; Analysis 4.2).
Analysis 4.2.
Comparison 4 Vaginal progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 2 Prelabour rupture of the membranes.
Preterm birth less than 28 weeks
Women who received vaginal progesterone had similar rates of preterm birth before 28 weeks' gestation compared with women who received placebo (RR 0.37, 95% CI 0.07 to 1.88; women = 224; studies = 1; Analysis 4.3).
Analysis 4.3.
Comparison 4 Vaginal progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 3 Preterm birth less than 28 weeks.
Caesarean section
Women who received vaginal progesterone had similar rates of caesarean birth compared with women who did not receive progesterone (RR 0.99, 95% CI 0.89 to 1.11; women = 224; studies = 1; Analysis 4.4).
Analysis 4.4.
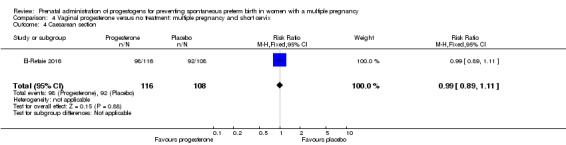
Comparison 4 Vaginal progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 4 Caesarean section.
Infant birthweight less than 2500 g
There was no clear difference in the risk of infant birthweight less than 2500 g between infants of women who received vaginal progesterone and infants of those who did not (RR 0.94, 95% CI 0.85 to 1.04; infants = 439; studies = 1; Analysis 4.5).
Analysis 4.5.
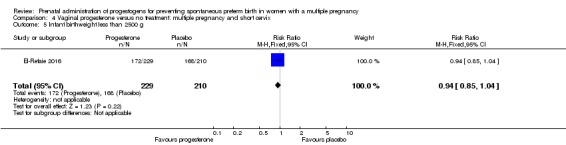
Comparison 4 Vaginal progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 5 Infant birthweight less than 2500 g.
Respiratory distress syndrome
Infants born to women who received vaginal progesterone were less likely to have respiratory distress syndrome compared with infants of those who did not receive progesterone (RR 0.68, 95% CI 0.55 to 0.84; infants = 439; studies = 1; Analysis 4.6).
Analysis 4.6.
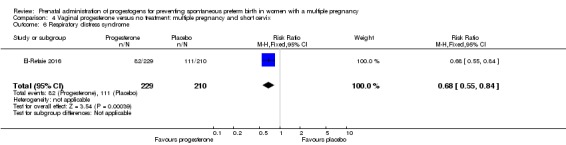
Comparison 4 Vaginal progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 6 Respiratory distress syndrome.
Use of mechanical ventilation
Infants born to women who received vaginal progesterone were less likely to require mechanical ventilation compared with infants of those who did not receive progesterone (RR 0.47, 95% CI 0.32 to 0.69; infants = 439; studies = 1; Analysis 4.7).
Analysis 4.7.
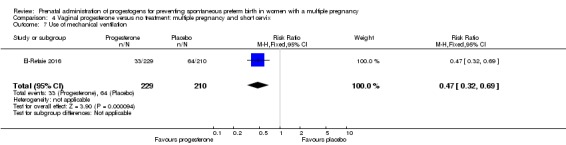
Comparison 4 Vaginal progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 7 Use of mechanical ventilation.
Admission to neonatal intensive care unit
There were no clear differences in the number of infants admitted to intensive care between infants of women who received vaginal progesterone and infants of those who did not (RR 0.91, 95% CI 0.82 to 1.01; infants = 439; studies = 1; Analysis 4.8).
Analysis 4.8.
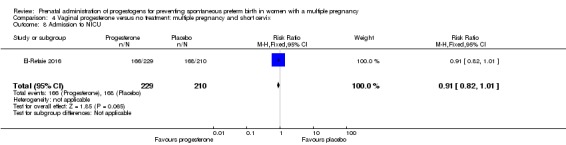
Comparison 4 Vaginal progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 8 Admission to NICU.
Comparison 5: Vaginal progesterone versus placebo: multiple pregnancy with another risk factor
Two studies are included in this comparison. Aboulghar 2012 included 313 women at high risk of preterm birth, including 91 with twin pregnancy, with pregnancies conceived by IVF or ICSI. Women in the intervention group received vaginal progesterone 200 mg twice daily from randomisation until delivery or 37 weeks’ gestation, while controls received placebo. Cetingoz 2011 recruited women with twin pregnancies with other risk factors (previous history of preterm birth or uterine malformation or both (results not separated)). Women in the intervention group received micronised progesterone (100 mg) administered daily by vaginal suppository between 24 and 34 weeks of gestation; controls received placebo.
Only two of our prespecified outcomes were reported in these studies: preterm birth at less than 34 and 37 weeks. There were no clear differences between groups in these studies, either individually or pooled, for either of these outcomes (preterm birth less than 34 weeks: RR 0.57, 95% CI 0.29 to 1.10; preterm birth less than 37 weeks: RR 0.92, 95% CI 0.72 to 1.18).
Discussion
Summary of main results
Seventeen studies met our criteria for inclusion in the review; all of the identified trials contributed data to the analyses, with a combined sample size of 4773 women. Studies examined two main comparisons: intramuscular (IM) or vaginal progesterone versus placebo or no treatment. We also examined outcomes in women with additional risk factors for preterm birth, including short cervical length measured by ultrasound, and other risk factors.
Overall across all comparisons, there were few clear differences between women receiving progesterone and women in the control groups, reflecting in part the small number of studies contributing data.
In studies where women received IM progesterone compared with placebo, more women gave birth before the 34th week of pregnancy in the progesterone group than in the placebo group (low‐quality evidence). There was no clear difference in the incidence of perinatal death between the groups (low‐quality evidence). No studies reported whether any women died or whether the babies had longer‐term developmental problems or disability. There were no clear differences between women receiving progesterone or placebo for other important outcomes such as preterm birth less than 37 weeks (high‐quality evidence); preterm birth less than 28 weeks (moderate‐quality evidence) or infant birthweight less than 2500 g (moderate‐quality evidence). None of the prespecified childhood outcomes were reported in the trials.
In studies where women received vaginal progesterone there were no clear differences between women receiving progesterone or placebo in preterm birth less than 34 weeks (low‐quality evidence). Although there seemed to be fewer births before 34 weeks in the progesterone group, this finding may have occurred by chance. Incidence of perinatal death was similar in both groups (low‐quality evidence). No studies reported maternal death or longer‐term outcomes in the babies. There were no clear differences between groups receiving vaginal progesterone versus placebo in any other important outcomes (preterm birth less than 37 weeks (moderate‐quality evidence); preterm birth less than 28 weeks (low‐quality evidence); infant birthweight less than 2500 g (moderate‐quality evidence)). None of the prespecified childhood outcomes were reported in the trials. For other outcomes, there were no clear group differences found except for caesarean section, where women who received vaginal progesterone did not have as many caesarean sections as those in the placebo group, although the difference between groups was not large (7%). Fewer infants whose mothers had received vaginal progesterone needed mechanical ventilation.
In summary, for women with a multiple pregnancy, IM progesterone was associated with an increase in the risk of preterm birth prior to 34 weeks' gestation when compared to placebo or no treatment. For this comparison, where data were present (for secondary maternal and infant outcomes), there were no other differences identified. Vaginal progesterone was associated with similar risks of all relevant outcomes when compared with placebo or no treatment.
For women with a multiple pregnancy and a short cervix, IM progesterone was associated with an apparent increase in the risk of preterm birth at less than 34 weeks, perinatal death and neonatal intensive care unit admission. In contrast, however, for women with a multiple pregnancy and a short cervix who received vaginal progesterone therapy, there appeared to be a reduced risk of preterm birth before 34 weeks, and a reduction in the risk of respiratory distress syndrome. However, these findings should be interpreted with considerable caution, based as they are on a single trial in each case.
Long‐term follow‐up was lacking in most of the included trials, and will be necessary to inform any impact on outcomes beyond the immediate neonatal period.
Overall completeness and applicability of evidence
The applicability of findings from this systematic review and meta‐analysis in women with a multiple pregnancy is broadly consistent with the findings reported in an individual participant data meta‐analysis (IPD‐MA) (Schuit 2014). This individual participant review included data from 13 randomised trials, involving 3768 women and 7536 infants, where women were administered either IM or vaginal progesterone, or placebo. Overall, progesterone administration was not associated with any improvements in infant outcomes or reduction in the risk of preterm birth (Schuit 2014).
Outcomes for women with a triplet pregnancy remain under‐represented in this systematic review. Women with a triplet pregnancy were recruited exclusively in a single trial (Combs 2010). While three trials (Caritis 2009; Lim 2011; Wood 2012) included women with both a twin or a triplet pregnancy, outcome data were not reported separately according to plurality of the pregnancy, precluding further detailed assessment of the role of progesterone in this setting.
An IPD‐MA has been performed in women with a triplet pregnancy, who received IM 17‐hydroxyprogesterone caproate or placebo (Combs 2016). This IPD‐MA sourced data from three trials (Caritis 2009; Combs 2010; Lim 2011), involving 232 women and 969 infants. Findings from this analysis did not indicate any beneficial effect of IM progesterone for risk of preterm birth prior to 34 (IM progesterone 86/136 (63%) versus placebo 64/96 (67%); risk ratio (RR) 0.95, 95% confidence interval (CI) 0.78 to 1.2) or 28 weeks' gestation (IM progesterone 15/136 (11%) versus placebo 12/96 (12%); RR 0.88, 95% CI 0.43 to 1.8), or in the occurrence of an adverse perinatal composite outcome comprising perinatal death, respiratory distress syndrome, bronchopulmonary dysplasia, intraventricular haemorrhage, necrotising enterocolitis or neonatal sepsis (IM progesterone 140/408 (34%) versus placebo 101/288 (35%); RR 0.98, 95% CI 0.79 to 1.2) (Combs 2016).
It was difficult to assess any additional contribution to the risk of preterm birth for women with a multiple pregnancy, due to the presence of further clinical risk factors, reflecting variable reporting in the original trials. A single trial (Senat 2013) specifically recruited women with a multiple pregnancy and a short cervical length identified by ultrasound assessment (less than 25 mm). While other trials included women with a short cervical length (El‐Refaie 2016) or the presence of a cervical cerclage (Brizot 2015), most trials specifically excluded women with evidence of cervical dilatation, or planned or current placement of a cervical suture (Awwad 2015; Briery 2009; Caritis 2009; Cetingoz 2011; Combs 2010; El‐Refaie 2016; Lim 2011; Norman 2009; Rouse 2007; Serra 2013). Furthermore, El‐Refaie 2016 did not specifically present data according cervical length at trial entry.
The IPD‐MA by Schuit 2014, while identifying no apparent benefit following progesterone therapy overall, did identify a suggestion of benefit from the subgroup of women with a multiple pregnancy and cervical length below 25 mm on ultrasound examination at the time of randomisation following vaginal progesterone administration, with a reduction in the risk of adverse perinatal outcome (vaginal progesterone 15/56 versus placebo 22/60; RR 0.57, 95% CI 0.47 to 0.70) (Schuit 2014). In a subsequent updated IPD‐MA involving data from five randomised trials (Brizot 2015; Cetingoz 2011; El‐Refaie 2016; Rode 2011; Serra 2013) specifically recruiting women with a multiple pregnancy, and additional data from a trial involving a small number of women with a multiple pregnancy and short cervix (Fonseca 2007), data were available from 303 women with a multiple pregnancy and their 606 infants (Romero 2017). Women who received vaginal progesterone therapy with a short cervical length appeared less likely to give birth before 34 weeks' gestation (vaginal progesterone 63/159 versus placebo 78/144; RR 0.71, 95% CI 0.56 to 0.91), with a reduction in risk of a composite adverse perinatal outcome (vaginal progesterone 23/84 versus placebo 28/70; RR 0.61, 95% CI 0.34 to 0.98), accounting for non‐independence of outcomes between infants of a multiple pregnancy (Romero 2017). Some of the significant findings reported in this IPD‐MA (namely, preterm birth prior to 33 weeks' gestation, and neonatal death) became statistically non‐significant when accounting for trial quality and blinding of participants, staff and outcome assessors (Romero 2017).
While IPD‐MA can be used to identify particular subgroups for whom an intervention may be effective (Stewart 2011), interpretation of findings should consider the overall impact of the intervention, recognising the implications of the relatively small sample size and issues relating to adequate statistical power (Sun 2014; Yusuf 1991). The two IPD reviews have included a relatively small subgroup of women with a multiple pregnancy who received progesterone therapy, and while there is a suggestion of benefit, results should be interpreted with caution. Although preterm birth is recognised to be a heterogeneous condition (Romero 2006), consideration should also be given to the possible biological mechanism whereby benefit is only observed in a very specific subgroup of women.
The longer‐term effects of exposure to progesterone during pregnancy have so far been reported in a limited number of studies (McNamara 2015; Vedel 2016), although the available evidence does not suggest an increased risk of harms extending into childhood. In the follow‐up of the STOPPIT trial (McNamara 2015), record linkage studies were performed to assess outcomes at three to six years of age, with data available for 97% of participants. Using these data, there were no differences in risk of death, hospitalisation, congenital anomalies, or outcomes at routine childhood health assessments (McNamara 2015). Follow‐up of the Rode 2011 trial (Vedel 2016) performed neurophysiological assessment at 48 or 60 months of age. There were no apparent differences in the number of hospital admissions or risk of low score using the Ages and Stages Questionnaire to screen for neurodevelopment (Vedel 2016). Further data relating to childhood follow‐up from other randomised trials would be beneficial.
Quality of the evidence
Overall, we rated the included studies at low risk of bias, although six studies were assessed at high risk of bias for selective outcome reporting (Aboulghar 2012; Briery 2009; Cetingoz 2011; Combs 2011; El‐Refaie 2016; Hartikainen‐Sorri 1980). We judged sequence generation to be adequate in most of the included studies, and appropriate blinding was also achieved. Most included studies had less than 10% sample attrition.
We used GRADE to assess the outcomes of perinatal mortality, preterm birth less than 34 weeks, major neurodevelopmental disability at childhood follow‐up, infant birthweight less than 2500 g, birth before 37 completed weeks and birth before 28 completed weeks. The 'Summary of findings' tables show the quality of evidence across these critical outcomes to be low to moderate. The main reason for downgrading the quality of the evidence was due to imprecision in the effect estimates, and for some outcomes design limitations in some of the studies contributing data.
Potential biases in the review process
The inclusion criteria for this review were reasonably broad, in order to evaluate the available evidence, which always includes trials with a range of inclusion criteria. The individual trial characteristics highlight the variation in inclusion criteria, the timing of starting progesterone therapy, the route of progesterone administration and the dose of progesterone given. The available information for specific subgroups of women with a multiple pregnancy are inevitably limited by the characteristics of the included studies.
We acknowledge that there is the potential for bias at all stages of performing a systematic review. We attempted to minimise bias in a number of ways; for example, two review authors independently carried out data extraction and assessed risk of bias.
Agreements and disagreements with other studies or reviews
As highlighted above, the findings of our review are broadly consistent with the IPD‐MA reported by Schuit 2014, derived from a smaller number of included trials and participants, but concluding that the overall administration of progesterone to women with a multiple pregnancy was not associated with any improvements in infant outcomes or reduction in risk of preterm birth. Furthermore, the evidence presented by Combs 2016 does not suggest that there is a benefit associated with IM progesterone, specifically in women with a triplet pregnancy. The effect of vaginal progesterone in women with a triplet pregnancy has so far been underevaluated. While there is a suggestion that vaginal progesterone may be associated with a reduction in risk of preterm birth and improved neonatal outcomes in women with a multiple pregnancy and short cervical length (Romero 2017; Schuit 2014), these IPD‐MAs reflect subgroups of women only, and have involved relatively small numbers of participants.
Authors' conclusions
Overall, for women with a multiple pregnancy, the administration of progesterone (either intramuscular or vaginal) does not appear to be associated with a reduction in risk of preterm birth or improved neonatal outcomes. While there is some suggestion that vaginal progesterone may reduce risk of preterm birth and improve neonatal outcomes in women with a multiple pregnancy and a short cervix identified on ultrasound, the number of participants involved is small, and caution is warranted in the interpretation of findings relating to this relatively small subgroup of women.
Future research could focus on a comprehensive individual participant data meta‐analysis including all of the available data relating to both intramuscular and vaginal progesterone administration in women with a multiple pregnancy, before considering the need to conduct specific trials in subgroups of high‐risk women (for example, women with a multiple pregnancy and a short cervical length identified on ultrasound).
Acknowledgements
As part of the prepublication editorial process, this protocol has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, through Cochrane Infrastructure and Cochrane Programme Grant funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Therese Dowswell's work is supported by an NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines.
Appendices
Appendix 1. Search terms for ClinicalTrials.gov and ICTRP
We ran each line separately
progesterone AND pregnancy AND multiple
progesterone AND pregnancy AND twin(s)
progestogen(s) AND pregnancy AND multiple
progestogen(s) AND pregnancy AND twin(s)
progesterone AND preterm AND multiple
progesterone AND preterm AND twin(s)
Data and analyses
Comparison 1.
Intramuscular (IM) progesterone versus no treatment or placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth less than 34 weeks | 2 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.06, 2.26] |
| 2 Preterm birth less than 34 weeks subgroup by dose | 2 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.06, 2.26] |
| 2.1 Low dose (250 mg weekly or less) | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.73, 2.59] |
| 2.2 High dose (greater than 250 mg weekly) | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.04, 2.68] |
| 3 Preterm birth less than 34 week subgroup by timing | 2 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.06, 2.26] |
| 3.1 Commencing after 20 weeks' gestation | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.04, 2.68] |
| 3.2 Mixed timing of commencement | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.73, 2.59] |
| 4 Perinatal death | 6 | 3089 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.60, 3.51] |
| 5 Perinatal death subgroup by dose | 6 | 3089 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.60, 3.51] |
| 5.1 Low dose (250 mg weekly or less) | 5 | 2759 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.48, 2.77] |
| 5.2 High dose (greater than 250 mg weekly) | 1 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 9.11 [1.17, 71.10] |
| 6 Perinatal death subgroup by timing | 6 | 3089 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.60, 3.51] |
| 6.1 Starting before 20 weeks' gestation | 2 | 1886 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.34, 2.66] |
| 6.2 Starting after 20 weeks' gestation | 2 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 3.76 [0.81, 17.46] |
| 6.3 Mixed timing of start | 2 | 719 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.01, 37.74] |
| 7 Prelabour rupture of the membranes | 6 | 1257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.84, 1.63] |
| 8 Preterm birth less than 37 weeks | 5 | 2010 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.98, 1.13] |
| 9 Preterm birth less than 28 weeks | 5 | 1920 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.75, 1.55] |
| 10 Adverse drug reaction | 2 | 1316 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.63, 1.32] |
| 11 Caesarean section | 7 | 2222 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.08] |
| 12 Antenatal tocolysis | 7 | 2218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.85, 1.10] |
| 13 Antenatal corticosteroids | 7 | 2221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.88, 1.11] |
| 14 Infant birthweight less than 2500 g | 5 | 4071 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.90, 1.08] |
| 15 Apgar score < 7 at 5 minutes | 4 | 3606 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.68, 1.15] |
| 16 Neonatal sepsis | 6 | 3327 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.41, 2.51] |
| 17 Respiratory distress syndrome | 8 | 4670 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.85, 1.34] |
| 18 Use of mechanical ventilation | 3 | 2233 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.69, 1.17] |
| 19 Intraventricular haemorrhage ‐ all grades | 1 | 1355 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.36, 10.77] |
| 20 Retinopathy of prematurity | 5 | 2807 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.16, 0.74] |
| 21 Chronic lung disease | 2 | 681 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.13, 27.80] |
| 22 Necrotising enterocolitis | 5 | 2610 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.36, 1.51] |
| 23 Fetal death | 4 | 3536 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.39, 2.20] |
| 24 Neonatal death | 7 | 3399 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.44, 1.91] |
| 25 Admission to NICU | 2 | 1668 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.13, 1.58] |
| 26 Patent ductus arteriosus | 4 | 2290 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.37, 2.21] |
| 27 Sensitivity analysis for perinatal death (assuming total dependence) | 6 | 1517 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.57, 3.20] |
| 28 Sensitivity analysis for perinatal death (assuming 1% dependence) | 6 | 3021 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.60, 3.49] |
Comparison 2.
Vaginal progesterone versus no treatment or placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth less than 34 weeks | 6 | 1727 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.09] |
| 2 Preterm birth less than 34 weeks subgroup by dose | 6 | 1727 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.64, 1.07] |
| 2.1 Low dose (200 mg or less daily) | 4 | 1267 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.63, 1.37] |
| 2.2 High dose (more than 200 mg daily) | 3 | 460 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.52, 0.90] |
| 3 Preterm birth less than 34 weeks subgroup by timing | 6 | 1727 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.09] |
| 3.1 Starting before 20 weeks' gestation | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.30, 1.58] |
| 3.2 Starting after 20 weeks' gestation | 4 | 1256 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.60, 0.91] |
| 3.3 Mixed timing of start | 1 | 380 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.90, 2.02] |
| 4 Perinatal death | 3 | 2287 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.74, 2.06] |
| 5 Perinatal death subgroup by dose | 3 | 2287 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.74, 2.06] |
| 5.1 Low dose (200 mg or less daily) | 3 | 2287 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.74, 2.06] |
| 6 Perinatal death subgroup by timing | 3 | 2287 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.74, 2.06] |
| 6.1 Starting before 20 weeks' gestation | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.18, 21.39] |
| 6.2 Starting after 20 weeks' gestation | 1 | 1346 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.51, 2.42] |
| 6.3 Mixed timing of start | 1 | 770 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.63, 2.61] |
| 7 Prelabour rupture of the membranes | 2 | 514 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.23, 1.60] |
| 8 Preterm birth less than 37 weeks | 6 | 1597 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.06] |
| 9 Preterm birth less than 28 weeks | 4 | 1569 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.68, 2.21] |
| 10 Adverse drug reaction | 2 | 562 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.90, 1.09] |
| 11 Caesarean section | 6 | 2143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.88, 0.98] |
| 12 Satisfaction with therapy | 1 | 494 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.35, 0.35] |
| 13 Antenatal tocolysis | 4 | 1420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.62, 1.02] |
| 14 Antenatal corticosteroids | 4 | 1422 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.06] |
| 15 Infant birthweight less than 2500 g | 4 | 3079 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.03] |
| 16 Apgar score < 7 at 5 minutes | 3 | 2410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.35, 1.19] |
| 17 Respiratory distress syndrome | 4 | 2560 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.64, 1.10] |
| 18 Use of mechanical ventilation | 5 | 3134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.48, 0.77] |
| 19 Intraventricular haemorrhage ‐ all grades | 1 | 1333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.62, 4.66] |
| 20 Retinopathy of prematurity | 2 | 1945 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.45, 2.54] |
| 21 Necrotising enterocolitis | 3 | 2117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.13, 2.06] |
| 22 Neonatal sepsis | 2 | 1944 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.86, 2.33] |
| 23 Fetal death | 3 | 2328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.65, 2.90] |
| 24 Neonatal death | 3 | 2905 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.75, 3.15] |
| 25 Admission to NICU | 5 | 4052 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 1.00] |
| 26 Patent ductus arteriosus | 2 | 1946 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.47, 1.22] |
| 27 Sensitivity analysis for perinatal death (assuming total non‐independence) | 3 | 1144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.61, 2.44] |
| 28 Sensitivity analysis for perinatal death (assuming 1% non‐independence) | 3 | 2263 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.74, 2.06] |
Analysis 2.5.
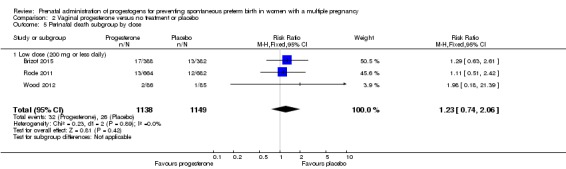
Comparison 2 Vaginal progesterone versus no treatment or placebo, Outcome 5 Perinatal death subgroup by dose.
Comparison 3.
Intramuscular (IM) progesterone versus no treatment: multiple pregnancy and short cervix
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth less than 34 weeks | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.04, 2.68] |
| 2 Perinatal death | 1 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.11 [1.17, 71.10] |
| 3 Prelabour rupture of the membranes | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.63, 2.06] |
| 4 Preterm birth less than 37 weeks | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.90, 1.25] |
| 5 Caesarean section | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.88, 1.49] |
| 6 Antenatal tocolysis | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.76, 2.45] |
| 7 Antenatal corticosteroids | 1 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.64, 1.36] |
| 8 Neonatal sepsis | 1 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.03 [0.60, 42.57] |
| 9 Respiratory distress syndrome | 1 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.00, 2.12] |
| 10 Retinopathy of prematurity | 1 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.19] |
| 11 Neonatal death | 1 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.03 [0.46, 35.61] |
| 12 Admission to NICU | 1 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.04, 1.74] |
| 13 Sensitivity analysis for perinatal death (assuming total dependence) | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.06 [0.60, 42.38] |
| 14 Sensitivity analysis for perinatal death (assuming 1% dependence) | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.11 [1.17, 71.10] |
Analysis 3.13.
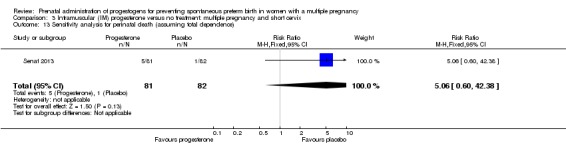
Comparison 3 Intramuscular (IM) progesterone versus no treatment: multiple pregnancy and short cervix, Outcome 13 Sensitivity analysis for perinatal death (assuming total dependence).
Comparison 4.
Vaginal progesterone versus no treatment: multiple pregnancy and short cervix
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth less than 34 weeks | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.49, 0.91] |
| 2 Prelabour rupture of the membranes | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.12, 1.82] |
| 3 Preterm birth less than 28 weeks | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.07, 1.88] |
| 4 Caesarean section | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.89, 1.11] |
| 5 Infant birthweight less than 2500 g | 1 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.85, 1.04] |
| 6 Respiratory distress syndrome | 1 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.55, 0.84] |
| 7 Use of mechanical ventilation | 1 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.32, 0.69] |
| 8 Admission to NICU | 1 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.82, 1.01] |
Comparison 5.
Vaginal progesterone versus placebo: multiple pregnancy and another risk factor
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth less than 34 weeks | 2 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.29, 1.10] |
| 2 Preterm birth less than 37 weeks | 2 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.72, 1.18] |
Analysis 5.1.
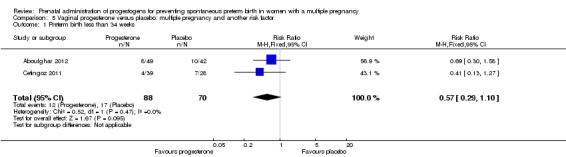
Comparison 5 Vaginal progesterone versus placebo: multiple pregnancy and another risk factor, Outcome 1 Preterm birth less than 34 weeks.
Analysis 5.2.
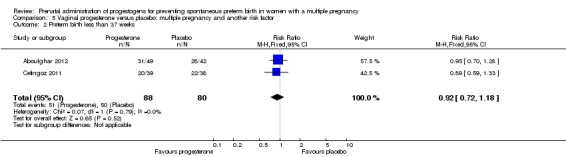
Comparison 5 Vaginal progesterone versus placebo: multiple pregnancy and another risk factor, Outcome 2 Preterm birth less than 37 weeks.
Differences between protocol and review
In the protocol we stated that we would carry out subgroup analysis by route of administration. After consideration, we decided that drugs administered by different routes have different uptake and the effects of different types of progestogens (administered by different routes) are likely to be different. Therefore, in this review we have not carried out pooled analysis, but rather we have set out results for progestogens administered by different routes in separate comparisons.
In the original protocol we had not planned subgroup analysis by short cervix. We have added this so that this review is compatible with a related review examining progestogens in singleton pregnancy, and to reflect increasing interest in interventions in women with multiple risk factors.
In the review, the following outcomes are now listed as maternal, rather than as infant outcomes:
Preterm birth (less than 34 weeks' gestation)
Birth before 37 completed weeks
Birth before 28 completed weeks
Mean gestational age at birth
In the review, we have removed the following outcomes from the GRADE methods:
Adverse drug reaction
Prelabour rupture of membranes (PROM)
We have added the following outcome to the GRADE methods:
Infant birthweight less than 2500 g
This was changed because for multiple pregnancy infant birthweight less than 2500 g is a more clinically relevant and meaningful outcome than either drug reaction or PROM.
We changed the following outcomes:
Birth before 37 completed weeks
Birth before 28 completed weeks
to:
Preterm birth less than 37 weeks
Preterm birth less than 28 weeks
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Single‐centre, prospective, placebo‐controlled randomised clinical trial. The study took place in an IVF Center, Cairo, Egypt between August 2008 and March 2010 | |
| Participants | 313 women at high risk of preterm birth, including 91 with twin pregnancy, with pregnancies conceived by IVF or ICSI Inclusion criteria: healthy pregnant women who conceived after IVF/ICSI between 18 to 24 weeks of gestation, with a first pregnancy, singleton or dichorionic twins, normal uterine and cervical anatomy, and normal fetal anatomy Exclusion criteria: previous pregnancy, serious fetal anomalies for which termination may be considered such as major heart anomaly or major CNS anomaly All women received progesterone injections as luteal phase support which they continued if pregnant until the day of the first ultrasound |
|
| Interventions |
Intervention group: vaginal progesterone 200 mg twice daily from randomisation until delivery or 37 weeks’ gestation. Total number randomised: n = 161 women (161 analysed, 210 babies) Control group: placebo vaginal suppositories from randomisation until 37 weeks’ gestation. Total number randomised: n = 152 women (145 women analysed, 187 babies) |
|
| Outcomes | Primary outcomes: preterm birth of singleton and twin pregnancies before 37 completed weeks and before 34 completed weeks Secondary outcomes: neonatal morbidity and mortality (live‐born children who died < 28 days after delivery) and take‐home baby rate (live‐birth rate per woman). Birthweight > 2500 g; 1500 ‐ 2500 g; < 1500 g; NICU admissions | |
| Notes | Funding sources: none reported. Declarations of interest: the authors report no financial or commercial conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States “Dark, sealed envelopes containing the intervention taken from a table of numbers” ‐ assume random as randomised study |
| Allocation concealment (selection bias) | Low risk | Refers to “dark, sealed, sequentially numbered envelopes” and the envelopes were picked by a nurse not involved in the study. The envelopes had been created by a third party not involved in the allocation process |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States “single blinding” and that “the patient was informed about the allocated arm” so presumably the clinician/personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not clear, but probably low risk. Placebo‐controlled trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study flow diagram clearly displays participant flow in the study 410 women recruited, 313 randomised; none lost to follow‐up in progesterone group and 6 lost to follow‐up in placebo group, and 1 excluded because of termination of pregnancy after diagnosis of trisomy 21. States “Intention‐to‐treat principle was followed during data analysis” |
| Selective reporting (reporting bias) | High risk | Trial registered after recruitment had started |
| Other bias | Low risk | Sample size calculation met. ITT analysis undertaken |
| Methods | Single‐centred, controlled, double‐blind trial with randomisation into 1 of 2 parallel groups, with a treatment‐to‐placebo ratio of 2:1. The study took place in Beruit, Lebanon between September 2006 and December 2011 | |
| Participants | 293 women aged 18 years or more, with an ultrasound‐diagnosed twin pregnancy Exclusion criteria: ultrasonographically‐diagnosed fetal anomalies; elective cervical cerclage before 14 weeks' gestation; hypertension; diabetes; mellitus; asthma; history of deep vein thrombosis; history of hepatic disease or abnormal liver enzymes; pre‐existing renal disease or abnormal kidney function; and seizure disorders |
|
| Interventions |
Intervention group: participants received weekly injections of 250 mg 17‐hydroxyprogesterone caproate from 16 ‐ 20 weeks to 36 weeks of gestation Control group: participants received weekly placebo from 16 ‐ 20 weeks to 36 weeks of gestation |
|
| Outcomes |
Primary outcome: preterm birth prior to 37 weeks of gestation Secondary clinical outcomes measures included: early preterm birth (prior to 32 and 28 weeks of gestation); low birthweight < 2500 g or very low birthweight < 1500 g or extremely low birthweight < 1000 g; neonatal morbidity; perinatal mortality; and maternal morbidity. Neonatal morbidity defined as any of the following: respiratory distress syndrome; pneumonia; culture‐confirmed sepsis; intraventricular haemorrhage grade III or IV; necrotising enterocolitis; periventricular leukomalacia; retinopathy of prematurity; patent ductus arteriosus; seizures; and/or bronchopulmonary dysplasia. Maternal morbidity included any of the following maternal complications occurring during the course of pregnancy: gestational diabetes mellitus; hypertensive disorders; and preterm premature rupture of the membranes Safety outcome measures: local side effects and systemic adverse events |
|
| Notes | Funding sources: This study was funded by a grant from the Medical Practice Plan at the American University of Beirut, Beirut, Lebanon (principal investigator: Anwar H. Nassar, MD). Declarations of interest: none of the authors of this article had any conflicts of interest to report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permutated block randomisation method. Random sequence generation used random‐number tables |
| Allocation concealment (selection bias) | Low risk | Randomisation envelopes prepared in pharmacy department. Research assistants opened the next opaque envelope following recruitment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treating doctors, investigators, ancillary personnel, and participants were all blinded to treatment assignment for the duration of the trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators and ancillary personnel blinded for the duration of the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data reported for all randomised participants |
| Selective reporting (reporting bias) | Low risk | Stated outcomes reported |
| Other bias | Low risk | Sample size calculation met. ITT analysis undertaken. Ethics approval obtained |
| Methods | Placebo‐controlled, double‐blind randomised controlled trial. Participants were recruited from University of Mississippi Obstetric Clinics or Antenatal Diagnostic Unit, Mississippi. Dates of study not reported | |
| Participants | 30 women with twin gestations were randomised Inclusion criteria: between 20 – 30 weeks’ gestation, intact membranes, ability to understand and sign the consent form Exclusion criteria: severe medical disorders such as sickle cell disease, insulin‐dependent diabetes mellitus, chronic hypertension, cervical dilatation 1 cm, intrauterine growth restriction (10th percentile), growth discordancy between twins (20%), cerclage, uterine abnormalities or unwillingness to participate in the study protocol |
|
| Interventions |
Intervention group: participants received weekly injections of 250 mg 17‐alphahydroxyprogesterone from the time of randomisation until 34 weeks' gestation or delivery (whichever came first) Control group: participants received weekly injections of placebo (castor oil) from the time of randomisation until 34 weeks' gestation or delivery (whichever came first) |
|
| Outcomes |
Primary outcome: delivery before 35 completed weeks’ of gestation Preselected secondary outcomes: development of preterm labour, preterm rupture of the membranes and gestational age at delivery Selected infant data, including birthweight, Apgar score, total days in the NICU and occurrence of neonatal morbidity such as RDS, PDA, IVH, or NEC were also recorded. Those infants who died or were discharged with a neurologic handicap were also noted |
|
| Notes | PharmaAmerica donated the 17‐hp Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Women who met the above criteria were randomised when they presented to our outpatient facility by the selection of sequentially numbered, sealed, opaque envelopes generated and opened by a disinterested third party (UMC Pharmacy)" Assume random sequence generation. |
| Allocation concealment (selection bias) | Low risk | "Sequentially numbered, sealed, opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "An order was written by the treating physician that the patient was participating in the Twins‐progesterone trial. This order was submitted to pharmacy and an opaque, number‐coded syringe was returned to the treatment area." ...."The participating women, as well as research personnel and physicians/nurses, were unaware of the study group assessment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "research personnel and physicians/nurses, were unaware of the study group assessment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data available for all women who were randomised |
| Selective reporting (reporting bias) | High risk | Trial was not registered and no published protocol |
| Other bias | Low risk | Sample size calculation met. ITT not stated. IRB (ethics) approval for study obtained. |
| Methods | Double‐blind, placebo‐controlled randomised trial conducted at the Multiple Pregnancy Unit of the Obstetrics Department at Sao Paulo University of Medicine, Brazil From June 2007 until October 2013 | |
| Participants | 390 women with naturally‐conceived diamniotic twin pregnancies. Inclusion criteria: no history of preterm delivery (< 37 weeks’ gestation), gestational age of 18 to 21 weeks and 6 days at random assignment, absence of major fetal abnormalities (such as neural tube defects, abdominal wall defects, cardiac defects, hydrocephalus, and malformations that are associated with polyhydramnios) at the anomaly scan, no allergies to progesterone or peanuts (peanut oil is an excipient that is used in vaginal ovules), absence of hepatic dysfunction, porphyria, otosclerosis, malignant disease, severe depressive state, current or previous thromboembolic disease, uterine malformation, and prophylactic cerclage Exclusion criteria: subsequent diagnosis of major fetal abnormalities, the presence of ovular infection, or being lost to follow‐up |
|
| Interventions |
Intervention group: vaginal progesterone ovules (200 mg of natural micronised progesterone that also contained excipients such as peanut oil, soybean lecithin, glycerol, and titanium dioxide). Control group: placebo ovules |
|
| Outcomes |
Primary outcome: difference in mean gestational age at delivery Secondary outcomes included: spontaneous delivery at < 34 weeks’ gestation and the postnatal data until hospital discharge: birthweight, Apgar score < 7 at 5 minutes, hypoglycaemia, IVH grade 3, jaundice, NEC, PDA, retinopathy, septicaemia, admission to the NICU, RDS, the need for mechanical ventilation, death before hospital discharge, and composite neonatal outcome (defined as the occurrence of any of the following events: IVH, NEC, RDS, sepsis, and death before hospital discharge). |
|
| Notes |
Agra 2016 reports secondary analysis Funding sources: none reported. Declarations of interest: the authors report no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment was performed with a computer‐generated system with balanced blocks of 20 patients in each block |
| Allocation concealment (selection bias) | Low risk | The hospital's pharmacy department was responsible for packing and labelling the ovules (A and B); random assignment code was kept secret until data analysis |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients, researchers, and clinicians who were involved in clinical and ultrasonographic evaluations were blinded to the treatment assignment for the duration of the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers blinded for duration of study. Code was kept secret until data analysis |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data missing for > 20% of neonates as they were born at other hospitals |
| Selective reporting (reporting bias) | Low risk | The trial was registered prospectively and expected outcomes were reported. (ClinicalTrials.gov Identifier: NCT01031017) |
| Other bias | Low risk | Sample size calculation met. No baseline differences in characteristics ITT analysis undertaken Ethics approval obtained |
| Methods | Randomised, double‐blinded, placebo‐controlled trial 14 centres, USA Starting in April 2004 and completed in September 2006 | |
| Participants | 134 women with multiple pregnancies Inclusion criteria: < 21 weeks of gestation when randomised, pregnant women with triplets were eligible if their gestational age was at least 16 weeks and no more than 20 weeks Exclusion criteria: serious fetal anomalies, 2 or more fetuses in 1 amniotic sac, suspected twin‐to‐twin transfusion syndrome, marked ultrasonographic growth discordance, planned non‐study progesterone therapy after 16 weeks, in‐place or planned cerclage, major uterine anomaly, unfractionated heparin therapy at any dose, and major chronic medical diseases |
|
| Interventions | Intervention group: weekly injections of 17‐OHPC (250 mg in 1 mL castor oil) starting at 16 ‐ 20 + 6 weeks and ending at delivery or 35 weeks’ gestation Control/Comparison group: weekly injections of placebo (1 mL castor oil) starting at 16 ‐ 20 + 6 weeks and ending at delivery or 35 weeks’ gestation | |
| Outcomes |
Primary outcomes: composite of delivery or fetal loss before 35 completed weeks of gestation (245 days) ‐ fetal loss included:miscarriage, termination, or stillbirth occurring any time after randomisation. Secondary outcomes: selected individual maternal and neonatal outcomes and a composite of serious adverse neonatal outcomes, including: neonatal death, RDS, culture‐proven sepsis, NEC stage II or III, bronchopulmonary dysplasia, IVH grade III or IV, or periventricular leucomalacia or severe retinopathy of prematurity stage III or higher |
|
| Notes | ClincialTrials.gov: NCT00099164 Funding sources: supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, (HD21410; HD27869; HD40512; HD27915; HD40485; HD34208; HD40500; HD34116; HD40560; HD40544; HD27917; HD27860; HD40545; HD53097; HD36801; HD34136) Declarations of interest: the authors disclosed no potential conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The simple urn‐method of randomization with stratification according to clinical center, was used to create a randomization sequence for each center.” |
| Allocation concealment (selection bias) | Low risk | The injections were prepared by a research pharmacy and boxes of 17‐OHPC and placebo were packaged for each centre according to randomisation sequences ‐ so appears to be central allocation ‐ pharmacy controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The participating women, their caregivers, and the research personnel were not aware of the study group assignment”. Also described as “double‐blinded” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Outcome data were available for 100% of the assigned women, and for all of the 402 fetuses.” No exclusions apparent ITT stated in statistical methods |
| Selective reporting (reporting bias) | Low risk | All expected outcomes appear to have been reported |
| Other bias | Low risk | No group differences in baseline characteristics. Sample size calculation met. ITT analysis undertaken |
| Methods | Randomised placebo‐controlled double‐blind study Department of Obstetrics and Gynecology, Istanbul, Turkey From December 2004 to February 2007 |
|
| Participants | 170 women recruited (n = 160 randomised): 84 allocated to intervention and 76 allocated to placebo. Inclusion criteria: high‐risk pregnant women: twin pregnancies; pregnancies with at least 1 spontaneous preterm birth; uterine malformation; randomisation at 24 weeks' gestation Exclusion criteria: not stated. 2 abortions, 7 deliveries between 20 ‐ 24 weeks and 1 woman with prophylactic cerclage were excluded |
|
| Interventions | Intervention group: micronised progesterone (100 mg) administered daily by vaginal suppository between 24 and 34 weeks of gestation Control/Comparison group: placebo (100 mg) administered daily by vaginal suppository between 24 and 34 weeks of gestation | |
| Outcomes | Delivery < 37 weeks Delivery < 34 weeks Preterm labour admission NICU admission Neonatal death | |
| Notes | Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random‐number list ‐ “Patients were allocated according to randomised number table”. |
| Allocation concealment (selection bias) | Low risk | Random‐number list generated centrally by research hospital pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The participating women, their caregivers, and the research personnel were unaware of the woman’s study‐group assignments.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Treatment assignment blinded until delivery of last pregnant woman |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 160 women were randomised ‐ 10 lost during follow‐up, 6 from the placebo group and 4 from intervention group 150 women analysed. |
| Selective reporting (reporting bias) | High risk | Trial was not registered and no published protocol |
| Other bias | Low risk | Sample size calculation met. No baseline group differences. ITT analysis undertaken Ethics approval obtained |
| Methods | Double‐blind, randomised clinical trial Multicentre, Obstetrix Collaborative Research Network, USA Recruitment took place from November 2004 through June 2008 | |
| Participants | 81 women randomised: 56 allocated to 17‐hp and 25 to placebo. Inclusion criteria: mothers carrying trichorionic‐triamniotic triplets ‐ confirmed at 15 ‐ 23 weeks detailed second‐trimester ultrasound examination, showing normal amniotic fluid volume and no major fetal anomalies Exclusion criteria: women with symptomatic uterine contractions, rupture of fetal membranes, any contraindication to interventions intended to prolong the pregnancy, a pre‐existing medical condition that might be worsened by progesterone, or a pre‐existing medical condition carrying a high risk of preterm delivery. Women less than 18 years of age, had an allergy to 17‐hp or the oil vehicle, had taken any progesterone‐derivative medication after 15 weeks of gestation, or had undergone placement of cervical cerclage for treatment of cervical change in the current pregnancy |
|
| Interventions | Intervention group: 17‐alpha‐hydroxyprogesterone caproate (250 mg in 1 mL castor oil) ‐ weekly injections starting at 16 ‐ 22 weeks and continued until 34 weeks or delivery. Weekly repeat injections were carried out at the site or at home with partner administering after training. Injection diary for partner injections and measurement of unused medication returned by participant used to assess compliance with home administration Control/Comparison group: identical‐appearing placebo (in 1 mL castor oil) | |
| Outcomes |
Primary outcomes: composite neonatal morbidity defined as 1 or more of: perinatal death (stillbirth, neonatal death, miscarriage); RDS; use of oxygen therapy at 28 days of life; neonatal sepsis proven by blood culture; pneumonia; IVH (grade III or IV); periventricular leucomalacia; NEC requiring surgery; retinopathy of prematurity; newborn asphyxia Secondary outcomes: individual neonatal morbidities listed above; gestational age at delivery; birthweight; maternal side effects Other outcomes reported: mean weeks of gestation; delivery before 28, 32 or 35 weeks of gestation; reason for delivery before 32 weeks (spontaneous; indicated); reason for delivery, all deliveries (spontaneous; indicated); caesarean delivery; tocolysis used; antenatal corticosteroids; maternal complications; pre‐eclampsia or gestational hypertension; gestational diabetes; chorioamnionitis; sepsis; postpartum endometritis Neonatal outcomes include: birthweight; head circumference; total hospital stay; NICU admission and intermediate care |
|
| Notes | The trial was conducted under Investigational New Drug (IND) approval Number 69‐536, assigned by the United States Food and Drug Administration (FDA). Clinicaltrials.gov: NCT00163020 An independent Data and Safety Monitoring Board (DSMB) supervised the trial, reviewed adverse event reports, and conducted an interim analysis of efficacy Funding sources: supported by a grant from the Center for Research and Education, Pediatrix Medical Group, Sunrise, FL Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated scheme. Random‐number generated centrally by pharmacy |
| Allocation concealment (selection bias) | Low risk | Random‐number generated centrally by pharmacy. “Progesterone or identical‐appearing placebo was compounded by pharmacy and shipped in advance to each study site in coded pre‐numbered kits. To randomise the research nurse contacted the central pharmacy by telephone or fax to obtain the code number for the kit assigned to that patient.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Subjects, physicians, and study personnel remained blinded as to group assignment until after completion of the trial.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Data were abstracted by study personnel who remained blinded to each subject’s group assignment.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 248 women identified with triplets, 147 eligible for trial inclusion. Of these 89 gave consent (61%) and were given trial injection. 81 (91%) returned for randomisation No loss ‐ 81 women randomised and outcome data available for all 81 mothers and 243 offspring. “Analysis was by the “intention‐to‐treat” principle. Accordingly, outcomes for each patient were tabulated according to the assigned group (17P vs placebo) regardless of her compliance.” |
| Selective reporting (reporting bias) | Low risk | Yes ‐ all expected outcomes reported |
| Other bias | Low risk | Sample size calculation undertaken, but power based on number of neonates and so underpowered to detect differences in maternal outcomes. No baseline group differences. ITT analysis undertaken |
| Methods | Double‐blind, randomised clinical trial Multicentre ‐ 18 sites, Obstetrix Collaborative Research Network, USA Recruitment from November 2004 through August 2009. | |
| Participants | 240 women randomised: 160 allocated to 17‐hp and 80 to placebo. Inclusion criteria: women were eligible if they had a dichorionic‐diamniotic twin pregnancy at 15 ‐ 23 weeks’ gestation and if they had completed a detailed ultrasound examination, showing no major fetal anomalies Exclusion criteria: women < 18 years old, taken any progestins > 15 weeks of gestation, had symptomatic uterine contractions, rupture of the fetal membranes, any contraindication to prolonging the pregnancy, any pre‐existing condition that might be worsened by progesterone, or a pre‐existing medical condition carrying a high risk of preterm delivery |
|
| Interventions |
Intervention group: 17 alpha‐hydroxyprogesterone caproate (250 mg in 1 mL castor oil) ‐ weekly injections starting at 16 ‐ 24 weeks and continued until 34 weeks or delivery. Weekly repeat injections were carried out at the site or at home with partner administering after training. Injection diary for partner injections and measurement of unused medication returned by participant used to assess compliance with home administration Control/Comparison group: identical‐appearing placebo (in 1 mL castor oil) |
|
| Outcomes | Primary outcomes: composite neonatal morbidity defined as 1 or more of: perinatal death (stillbirth, neonatal death, miscarriage); RDS; use of oxygen therapy at 28 days of life; neonatal sepsis proven by blood culture; pneumonia; IVH (grade III or IV); periventricular leucomalacia; NEC requiring surgery; retinopathy of prematurity; newborn asphyxia Secondary outcomes: individual neonatal morbidities listed above; gestational age at delivery; birthweight; maternal side effects. Other outcomes reported: mean weeks of gestation; delivery before 28, 32 or 34 or 37 weeks; reason for delivery before 37 weeks (spontaneous; indicated); caesarean delivery; tocolysis used; antenatal corticosteroids. Maternal complications: pre‐eclampsia or gestational hypertension; gestational diabetes; chorioamnionitis; sepsis; postpartum endometritis. Neonatal outcomes: birthweight; birthweight < 2500 g, < 1500 g and birthweight < 1000 g; small‐for‐gestational age | |
| Notes | Funding sources: supported by a grant from the Center for Research, Education, and Quality, Pediatrix Medical Group, Mednax Inc, Sunrise, FL (groups of clinicians) Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated scheme. Random‐number generated centrally by pharmacy |
| Allocation concealment (selection bias) | Low risk | Random‐number generated centrally by pharmacy. “Progesterone or identical‐appearing placebo was compounded by pharmacy and shipped in advance to each study site in coded pre‐numbered kits. To randomise the research nurse contacted the central pharmacy by telephone or fax to obtain the code number for the kit assigned to that patient.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Subjects, physicians, and study personnel remained blinded as to group assignment until after completion of the trial.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study personnel remained blinded until after completion of the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss in progesterone group ‐ 160 women allocated, 160 mothers delivered and 320 perinates with known outcome. 80 women allocated to placebo ‐ 2 lost to follow‐up ‐ 78 women delivered and 156 perinates with known outcome “Outcomes for each patient were tabulated according to assigned group regardless of her compliance.” |
| Selective reporting (reporting bias) | High risk | Trial was not registered and no published protocol |
| Other bias | Low risk | Sample size calculation met. Interim analysis undertaken when 50% of data collected; primary outcome adjusted for this. No baseline group differences. Compliance 96.4% in the 17‐ph group and 98.7% in the placebo group (P .07) |
| Methods | Randomised controlled study. Mansoura University Hospital and private practice settings in Mansoura, Egypt Participants were recruited from June 2012 until November 2014 |
|
| Participants | 225 women were recruited. Data for 116 intervention group and 108 controls Women with previous preterm birth were included (approximately 25% of each arm) Inclusion criteria: women aged 20 – 35 years old with dichorionic twin pregnancy were selected for measurement of cervical length by transvaginal sonography (TVS) at 20 – 24 weeks of gestation; cervical length of 20 – 25 mm with no symptoms or signs of impending preterm labour Exclusion criteria: known allergy or contraindication (relative or absolute) to progesterone therapy, monochorionic twins, known major fetal structural or chromosomal abnormality, single fetal demise, fetal reduction in current pregnancy, cervical cerclage in current pregnancy, medical conditions that may lead to preterm labour, rupture of membranes, vaginal bleeding |
|
| Interventions |
Intervention group: received vaginal progesterone suppositories (Cyclogest®, Actavis, Barnstaple, EX32 8NS, United Kingdom) in a dose of 400 mg daily, beginning 20 ‐ 24 weeks of gestation until 37 weeks of gestation Contol/comparison group: women received standard antenatal care |
|
| Outcomes |
Primary outcome: preterm labour before 34 weeks of gestation Secondary outcomes: neonatal RDS, early neonatal death (END) (not defined). |
|
| Notes | Funding sources: not reported Declarations of interest: no conflicts of interests |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Sealed unlabeled, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants, caregivers and investigators were not blinded to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9 of 125 (7%) women were lost to follow‐up in the intervention group and 17 of 125 (14%) in the control group. In addition to these 26, 42 women discontinued treatment due to noncompliance or perinatal complications. 182 women received the full course of treatment. Data for 224 |
| Selective reporting (reporting bias) | High risk | Trial was not registered and no published protocol |
| Other bias | Low risk | Sample size calculation met. No baseline group differences. ITT not stated |
| Methods | Setting: Finland, dates unclear Method of randomisation: stated to be “placebo controlled and double blind”. Data available for 77 women |
|
| Participants | 77 women randomised; 39 women received 17‐hp and 38 received placebo Inclusion criteria: women with a twin pregnancy, between 28 and 33 weeks of gestation, no signs of preterm labour. |
|
| Interventions |
Intervention group: weekly intramuscular injections of 250 mg 17‐alpha hydroxyprogesterone caproate from 28 weeks until 37 weeks of gestation or birth, whichever came first. Comparison/control group: placebo in an equivalent volume from 28 weeks until 37 weeks of gestation or birth, whichever came first. 71 of 77 women had prophylactic bed rest from the 32nd week to the 36th gestational week |
|
| Outcomes | Clinical outcomes included preterm birth before 37 weeks of gestation and perinatal mortality | |
| Notes | Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | 'Medication code' specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data available on all women randomised |
| Selective reporting (reporting bias) | High risk | Trial was not registered and no published protocol |
| Other bias | Unclear risk | No baseline group differences. Sample size calculation not described. ITT not stated |
| Methods | Multicentre, double‐bind, placebo‐controlled randomised trial 55 obstetric clinics in Netherland Between July 2006 and August 2009 |
|
| Participants | 671 women randomised: 336 allocated to progesterone and 335 allocated to placebo. Inclusion criteria: women with a multiple pregnancy and gestational age between 15 and 19 weeks Exclusion criteria: women with a previous spontaneous preterm birth before 34 weeks, serious congenital defects or death of 1 or more fetuses, early signs of twin‐to‐twin transfusion syndrome, or primary cerclage were excluded from participation |
|
| Interventions |
Intervention group: 1 mL 17‐hydoxyprogesterone caproate (250 mg/mL in castor oil) ‐ starting between 16 and 20 weeks and continuing to 36 weeks. Injections were administered at the clinic, by a general practitioner or, if the participant or a family member had a background in medical practice, at the participant’s home Control/Comparison group: 1 mL placebo (castor oil) ‐ study medication and placebo were identical in packaging, colour and consistency |
|
| Outcomes |
Primary outcomes: composite adverse neonatal outcome ‐ severe RDS; bronchopulmonary dysplasia; IVH grade II B or worse; NEC; proven sepsis; death before discharge Secondary outcomes: side effects (soreness, itching, and swelling; gestational age at delivery; preterm birth before 28, 32 and 37 weeks; length of admission to the NICU; maternal morbidity; hospitalisation of the mother due to (threatened) preterm labour; costs |
|
| Notes | Funding sources: Funded by ZonMw, the Netherlands organization for health research and development (grant number 62200019) Declarations of interest: The authors did not report any potential conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “An independent data manager rendered a computer‐generated list that was stratified by chorionicity, parity, and number of multiples, using random blocks of maximum block size.” |
| Allocation concealment (selection bias) | Low risk | Web‐based randomisation ‐ “Randomization was accessible through a website” and “Allocation code was known only to ACE Pharmaceuticals" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The participants, caregivers, and data collectors were all blinded to allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data collectors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 4 infants lost to follow‐up States that “all analyses were based on the intention‐to‐treat principle” |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Other bias not apparent |
| Methods | Double‐blind randomised placebo‐controlled trial Multicentre, 9 UK NHS hospitals ‐ STOPPIT study (Study Of Progesterone for the Prevention of Preterm Birth In Twins), UK. Protocol states trial planned to run November 2004 to October 2007; actual study dates unclear | |
| Participants | 500 women randomised: 250 allocated to progesterone and 250 allocated to placebo Inclusion criteria: women with twin pregnancy, with gestation and chorionicity established by scan before 20 weeks’ gestation and attending the antenatal clinic during the recruitment period Exclusion criteria: pregnancy complicated by a recognised structural or chromosomal fetal abnormality at the time of recruitment, or if they had contraindications to progesterone, planned cervical suture, planned elective delivery before 34 weeks’ gestation, or planned intervention for twin‐to‐twin transfusion before 22 weeks’ gestation. Women with higher multiple pregnancy were also excluded |
|
| Interventions |
Intervention group: daily vaginal progesterone gel 90 mg starting at 24 weeks and 0 days of gestation. Each applicator of intervention contained 1.45 g of gel and delivered 1.125 g of gel containing 8% progesterone Control/Comparison group: placebo gel ‐ administered in the same way as active treatment, daily from 24 weeks’ gestation. Each applicator of intervention contained 1.45 g of gel and delivered 1.125g of gel containing 8% excipients |
|
| Outcomes |
Primary outcome: delivery or intrauterine death before 34 weeks and 0 days of gestation. Delivery of the first twin was used to define the time of delivery. If 1 twin died in utero before 34 weeks and the other was born alive after 34 weeks, intrauterine fetal death was defined as occurring before 34 weeks. The gestational age was calculated from ultrasound scan done before 20 weeks Maternal secondary outcomes: gestation at delivery, method of delivery (spontaneous vaginal delivery, vaginal breech, forceps or ventouse, or caesarean section), duration of each stage of labour, and safety outcomes such as duration of stay in hospital. Neonatal secondary outcomes were neonatal unit admission and duration of neonatal unit care. Maternal satisfaction by questionnaire |
|
| Notes | Funding sources: the authors disclosed receipt of the following financial support for the research and/or authorship of this article: grants CZB/4/408 from Chief Scientist Office (www.cso.scot.nhs.uk), Scottish Government; grant SP4068 from Action Medical Research (www.action.org.uk) and grants from Wellcome ‘‘Value in People’’ (www.wellcome.ac.uk) and the Jennifer Brown Research Laboratory (www.piggybankkids. org). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article Declarations of interest: the authors do not declare any conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “We used a randomisation schedule with permuted blocks of randomly mixed sizes to make up treatment packs (either active or placebo) for every patient, which were held in individual hospital pharmacies until use.” |
| Allocation concealment (selection bias) | Low risk | Central allocation from research network ‐ local researcher telephoned the interactive voice response randomisation application at the UK Clinical Research Network registered trials unit to be given a participant number that corresponded to a specific treatment pack |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “All study personnel and participants were masked to treatment assignment for the duration of the study.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study personnel masked to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 6 women of 500 (3 from each treatment group) lost to follow‐up from 500 randomised participants. Analysis was ITT |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Unclear risk | Other bias not apparent |
| Methods | Randomised, double‐blind, placebo‐controlled trial Multicentre, 17 centres in Denmark and Austria Between 1 June 2006 and 31 October 2008 |
|
| Participants | 677 women were randomised: 334 allocated to progesterone and 343 allocated to placebo Inclusion criteria: women with a live, diamniotic twin pregnancy and chorionicity assessed by ultrasound before 16 weeks’ gestation were eligible for recruitment Exclusion criteria: age < 18 years; known allergy to progesterone or peanuts (active treatment contained peanuts); history of hormone‐associated thromboembolic disorders; rupture of membranes; treatment for or signs of twin‐to‐twin transfusion syndrome; intentional fetal reduction; known major structural or chromosomal fetal abnormality; known or suspected malignancy in genitals or breasts; known liver disease; women with higher‐order multiple pregnancies; women who did not speak and understand Danish or German |
|
| Interventions | Intervention group: vaginal micronised progesterone pessaries (200 mg) ‐ self‐administered daily by participants ‐ starting from 20 ‐ 24 weeks until 34 weeks’ gestation Control/Comparison group: vaginal placebo pessaries (200 mg) ‐ self‐administered daily by participants ‐ starting from 20 ‐ 24 weeks until 34 weeks’ gestation | |
| Outcomes |
Primary outcome: incidence of delivery before 34 + 0 weeks' gestation Prespecified secondary outcomes: delivery before 22, 28 and 32 weeks' gestation, number of liveborn infants, treatment with tocolytics and corticosteroids, birthweight, selected neonatal complications, neurophysiological development 6 and 18 months after the estimated date of delivery |
|
| Notes | Funding sources: funding was provided by The Danish Medical Research Council, The Fetal Medicine Foundation, The Copenhagen University Hospital’s Research Fund, The Aase and Ejnar Danielsens Fund, The Augustinus Fund, The Ivan Nielsen Fund, The Doctor Sofus Carl Emil Friis and wife Olga Doris Friis’ Fund, The Simon Fougner Hartmanns Family Fund, The Danish Medical Society in Copenhagen and The A.P. Moeller Foundation Declarations of interest: the authors declare no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random‐number sequence was used by the trial statistician to generate a randomisation code |
| Allocation concealment (selection bias) | Low risk | The boxes of progesterone and placebo were packed and labelled by Bilcare (Waller House, Wales, UK) according to this randomisation sequence and shipped to Copenhagen University Hospital, from where the study medication was distributed to the participating departments. Each local researcher telephoned the randomisation system, entered the participant's social security number and chorionicity, and was given a randomisation number that corresponded to a specific treatment box from a given batch |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants and study personnel were blinded to treatment assignment for the duration of the trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The randomisation code was not broken before all data had been collected |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women of 675 lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Stated outcomes reported |
| Other bias | Unclear risk | Other bias not apparent |
| Methods | Placebo‐controlled double‐blind randomised trial Trial conducted in 14 centres by the Maternal‐Fetal Medicine Network, USA From April 2004 until February 2006 |
|
| Participants | 661 women with a twin pregnancy were randomised. Inclusion criteria: women carrying twins with a gestational age of at least 16 weeks and no more than 20 weeks and 3 days Exclusion criteria: known fetal anomaly, spontaneous fetal death of a fetus after 12 weeks, presumed mono‐amniotic placenta, suspected twin‐twin transfusion syndrome, marked ultrasonographic growth discordance, progesterone or heparin treatment during pregnancy, current or planned cervical cerclage, hypertension, insulin‐dependent diabetes, and twin pregnancies that were the result of intentional fetal reduction |
|
| Interventions |
Intervention group: weekly intramuscular injection of 250 mg 17‐hydroxyprogesterone caproate from 16 ‐ 20 + 3 weeks until 34 completed weeks’ gestation, or birth if earlier Control group: weekly intramuscular injection of placebo (castor oil) from 16 ‐ 20 + 3 weeks until 34 completed weeks’ gestation, or birth if earlier |
|
| Outcomes |
Primary outcome: composite of delivery or death prior to 35 weeks’ gestation Secondary outcomes: randomisation to delivery interval; composite adverse outcomes (retinopathy of prematurity, RDS, sepsis, NEC, bronchopulmonary dysplasia, grade III or IV IVH, periventricular leucomalacia), birthweight (less than 2500 g and less than 1500 g), 5‐minute Apgar score < 7, PDA, pneumonia, mechanical ventilation, seizures. Pretermbirth before 37 weeks’ gestation; birthweight less than 2.5 kg; stillbirth; neonatal death; IVH; RDS; bronchopulmonary dysplasia; sepsis; NEC; retinopathy of prematurity |
|
| Notes | Funding sources: supported by grants (HD27869, HD21410, HD40512, HD34136, HD34208, HD40485, HD27915, HD40544, HD40560, HD27917, HD40500, HD34116, HD40545, HD27860, and HD36801) from the National Institute of Child Health and Human Development Declarations of interest: no potential sources of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The simple urn method of randomisation with stratification according to clinical center was used by the George Washington University Biostatistical Co‐ordinating Center to create a randomization sequence for each center...” |
| Allocation concealment (selection bias) | Low risk | Identical‐appearing treatment packs |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women, caregivers and outcome assessors blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Women, caregivers and outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data available for 655 of 661 women (less than 1% loss to follow‐up) |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported (delivery or fetal death before 35 weeks’ gestation; other obstetric and neonatal outcomes) |
| Other bias | Unclear risk | Other bias not apparent |
| Methods | Open‐label multicentre, randomised controlled trial France ‐ 13 French University Hospitals Between June 2006 and January 2010 |
|
| Participants | 165 women randomised, 82 women randomised to the treatment group and 83 women randomised to the no‐treatment group Inclusion criteria: women older than 18 years, carrying twins, asymptomatic, and with a cervical length of 25 mm or less measured in the sagittal plane by routine transvaginal ultrasound according to the standard technique were eligible for inclusion. Women were 24+0 to 31+6 weeks' gestation Exclusion: cervical dilatation greater than 3 cm, premature rupture of the membranes, placenta previa, monochorial mono‐amniotic pregnancy, signs of twin‐to‐twin transfusion syndrome, severe intrauterine growth restriction of at least 1 fetus, known major structural or chromosomal fetal abnormality, death of 1 fetus, any maternal or fetal disease requiring preterm birth, progesterone therapy before inclusion, ongoing anticonvulsant treatment, or participation in any other treatment trial. Twin gestations resulting from intentional fetal reduction were also excluded |
|
| Interventions |
Intervention group: 500 mg of intramuscular 17‐alpha‐hydroxyprogesterone caproate, to be repeated twice weekly until 36 weeks or preterm delivery, whichever occurred first Control group: no treatment |
|
| Outcomes |
Primary outcome: time from randomisation to delivery Prespecified secondary outcomes: (1) obstetric criteria: rates of preterm birth before 37, 34, and 32 weeks and number of readmissions for preterm labour; (2) neonatal criteria: birthweight, transfer to the NICU, RDS, bronchopulmonary dysplasia, NEC, periventricular leukomalacia, and death; and (3) safety criteria: any severe maternal or neonatal adverse effects (congenital anomalies or other ill effects) |
|
| Notes | Funding sources: this study was supported by a research grant from the Département à la Recherche Clinique Ile‐de‐France, Assistance Publique–Hôpitaux de Paris, which also sponsored the study (PHRC AOM 04038) Declarations of interest: the authors declare no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent computer‐generated randomisation sequence was used for this allocation, based on a randomisation list established by the study statistician, according to a permutated block method |
| Allocation concealment (selection bias) | Low risk | States ‐ central randomisation. “A centralised, computer generated randomised process in a 1:1 ratio.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data available on all participants |
| Selective reporting (reporting bias) | Low risk | Stated outcomes reported |
| Other bias | Unclear risk | Other bias not apparent |
| Methods | 3‐arm randomised controlled trial 5 University hospital centres in Spain Between December 2005 and January 2008. |
|
| Participants | 294 women Inclusion criteria: women were recruited at 11 ‐ 13 weeks’ gestation. If they had previously been treated with vaginal progesterone it was stopped. Women were 18 years or more, dichorionic, diamniotic twin pregnancy Exclusion criteria: singleton pregnancy; monochorionic twin pregnancies; triplets or higher multiple pregnancies; elective cervical cerclage before 14 weeks’ gestation; history of hepatic problems; pregnancy cholestasis; abnormal liver or kidney function; allergy to peanuts or study medication; recurrent vaginal bleeding or infection; fetal anomalies; alcohol or illicit drug use and smoking more than 10 cigarettes per day |
|
| Interventions | Intervention: 1. 200 mg vaginal progesterone self‐inserted daily at bedtime (98 women) 2. 400 mg vaginal progesterone self‐inserted daily at bedtime (98 women) 3. (control) placebo vaginal pessaries self‐inserted daily at bedtime (98 women) All women were provided with specially manufactured identical‐looking pessaries, 2 to be administered each evening from 20 weeks to 34 weeks of gestation or birth, whichever came first | |
| Outcomes | Preterm birth rate < 37 weeks of gestation; early preterm birth rate < 34, 32, 28 weeks of gestation; need for tocolytic treatment; steroid treatment; rate of preterm premature rupture of membranes < 37 weeks of gestation; cervical length measurements at 20, 24, 28 weeks of gestation; perinatal mortality and morbidity; caesarean section. Local tolerance to the treatment; number of serious systemic adverse effects Perinatal outcomes: short‐term neonatal morbidity (RDS; pneumonia; early onset sepsis; seizures; graded III ‐ IV IVH; stage IIII NEC; and/or PDA). Long‐term neonatal morbidity included: broncho‐pulmonary dysplasia; periventricular leucomalacia; and/or severe retinopathy of prematurity, birthweight < 2500 g; 5 minute Apgar score; major congenital malformation; admission to NICU; mechanical ventilation; neonatal death |
|
| Notes | Funding sources: the trial was funded by grant EF489‐2004/1 from Laboratorios Effik S.A. (Madrid, Spain) Declarations of interest: the authors declare no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation was performed by computer (SPSS Random Number Generator, using a randomisation sequence 1:1:1 ratio (blocks of nine, with no stratification).” |
| Allocation concealment (selection bias) | Low risk | Central allocation “An external monitoring centre provided a randomisation code number for each pregnant woman” “The medication was given at each visit by the hospital pharmacy department”. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women and staff were blinded. Medication packs were coded and contained identical‐appearing pessaries |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It was reported that all study personnel were blind to treatment allocation for the duration of the project |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was very little loss to follow‐up It was stated that an ITT analysis was carried out |
| Selective reporting (reporting bias) | Unclear risk | Most expected outcomes reported upon. However ‐ individual outcome results for short‐term and long‐term neonatal morbidity were not reported, e.g. RDS, periventricular leucomalacia. |
| Other bias | Unclear risk | Other bias not apparent. |
| Methods | Double‐blinded, placebo‐controlled randomised trial Antenatal clinics at a tertiary care centre and an academic community hospital in Calgary, AB, Canada June 2006 and October 2010. |
|
| Participants | 84 women were randomised Inclusion criteria: 2 or more live fetuses confirmed at their 16‐ to 18‐week ultrasound and were between 16 + 0 and 20 + 6 weeks gestation at the time of randomisation. Pregnancies reduced from higher‐order multiples to twins were also included if the reduction was carried out before 13 weeks gestation Exclusion: placenta previa, pre‐existing hypertension, known major fetal anomaly detected on ultrasound, monoamniotic monozygotic multiple pregnancies, maternal seizure disorder, active or history of thromboembolic disease, maternal liver disease, known or suspected breast malignancy or pathology, known or suspected progesterone‐dependent neoplasia, plans to move to another city during pregnancy, previous participation in this trial or other perinatal clinical trials during this pregnancy, or known sensitivity to progesterone |
|
| Interventions |
Intervention group: received daily doses of 90 mg progesterone 8% vaginal gel Control/Comparison group: daily doses of identical applicators containing gel without progesterone |
|
| Outcomes |
Primary outcome: gestational age at delivery Maternal secondary outcomes: preterm birth before 35 weeks ’ gestation; preterm birth before 37 weeks ’ gestation; the proportion of women who had a spontaneous delivery; length of hospital stay; the proportion of women who received tocolytic therapy; and compliance with treatment as measured by diary self‐report and return of unused applicators Infant secondary outcomes were length of hospital stay; RDS, defined as requiring assisted ventilation via endotracheal tube and supplemental oxygen both within the first 24 hours of life and for duration of ≥ 24 hours and either an X‐ray compatible with RDS or surfactant given between the first 2 and 24th hour of life; BPD, defined as requiring oxygen at postnatal GA of 36 completed weeks and X‐ray compatible with BPD; IVH grade III or IV diagnosed by cranial ultrasound or at autopsy; NEC, defined as perforation of the intestine, pneumatosis intestinalis, or air in the portal vein, diagnosed by X‐ray, surgery, or at autopsy; number of days of ventilator therapy; birthweight; stillbirth; and neonatal death. Any possible maternal or infant serious adverse events up to 28 days after delivery. |
|
| Notes | Funding sources: This study was funded by the Calgary Health Region Perinatal Funding Competition (peer reviewed funding). We are grateful to Columbia Laboratories (Livingston, NJ, USA) who donated blinded active treatment and placebo gels Declarations of interest: the authors declared no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a random‐number generator with random block sizes of 2 or 4 |
| Allocation concealment (selection bias) | Low risk | The allocation sequence generated by the trial statistician was provided to the dispensing pharmacy. Once a woman consented, the pharmacy dispensed treatment according to the next randomisation allocation from the stratum to which the woman belonged |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research nurse assessing dates was blinded to allocation. Assume true for outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised women and their infants |
| Selective reporting (reporting bias) | Low risk | Stated outcomes are reported |
| Other bias | Unclear risk | Other bias not apparent |
17‐hp: 17‐alphahydroxyprogesterone 17‐OHPC: 17 alpha‐hydroxyprogesterone caproate BPD: bronchopulmonary dysplasia ITT: intention to treat IVH: intraventricular haemorrhage NEC: necrotising enterocolitis NICU: neonatal intensive care unit PDA: patent ductus arteriosus RDS: respiratory distress syndrome
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbott 2012 | The comparison was not relevant (progesterone vs cerclage) |
| ACTRN12616000875404 | Appears to be trial registration for a study examining effect of cervical pessary on preterm birth in twins, so not looking at progesterone |
| Borna 2008 | No multiple pregnancies included |
| Breart 1979 | 2 different types of progesterone compared |
| Brenner 1962 | Women in this study were already at term (36 ‐ 38 weeks) and no separate data for multiples |
| Chandiramani 2012 | Brief abstract ‐ comparison of progesterone with cerclage |
| Coomarasamy 2015 | Women included in this study were at risk of preterm birth because of recurrent miscarriage and not because of multiple pregnancy |
| Facchinetti 2006 | This study included singletons only |
| Grobman 2013 | This study included singletons only |
| Gyamfi‐Bannerman 2015 | It was not clear that this was a randomised trial |
| Hauth 1983 | This study included singletons only |
| Hobel 1986 | It was not clear that this was a randomised trial |
| Ionescu 2012 | Comparison of progesterone versus cerclage ‐ not a relevant comparison for this review |
| Johnson 1975 | The criteria for high risk of preterm birth in this study did not include multiple pregnancy in current pregnancy |
| LeVine 1964 | This was not a randomised controlled trial. Alternate allocation |
| Manuck 2008 | Secondary analysis |
| Mardy 2016 | Abstract only, secondary analysis |
| Martinez de Tejada 2014 | This study included singletons only |
| McKay 2014 | This is secondary analysis for a trial that excluded multiple pregnancies |
| Meints 2016 | This study examines the effects of type of conception on outcomes in twins |
| Meis 2003b | This study included singletons only |
| Moghtadei 2008 | This is a study focusing on women at risk of preterm birth because of age |
| NCT00099164 | Insufficient information to assess eligibility for inclusion |
| NCT02350231 | This is a trial registration for a trial that has now been completed. The study examined relevant progesterone versus tocolytics and was aimed at delaying birth in preterm labour |
| NCT02623881 | Trial registration for a study comparing progesterone with pessary device (not relevant comparison) |
| O'Brien 2009 | This study included singletons only |
| Palacio 2013 | This study included singletons only |
| Papiernik 1970 | This was a study of women in labour (or with symptoms of preterm labour) |
| Rozenberg 2007 | This study included singletons only |
| Rust 2006 | Trial of progesterone versus cerclage which is not a relevant comparison for this review |
| Suvonnakote 1986 | It was not clear that this was a randomised controlled trial |
| Turner 1966 | This was not a randomised controlled trial (alternate allocation) |
| Walch 2005 | This study examines the use of progesterone to prevent miscarriage |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT |
| Participants | Women with twin pregnancies |
| Interventions | 17OHP |
| Outcomes | Specifically looking at effectiveness in overweight and obese women |
| Notes | This may be secondary analysis of Meis 2003b but uncertain, and published only as an abstract. Awaiting assessment pending publication of fuller report. |
| Methods | Probably RCT ‐ women were "divided into 2 groups" |
| Participants | 100 women with twins |
| Interventions | Vaginal progesterone 200 mg daily from 24 ‐ 34 weeks versus placebo |
| Outcomes | Spontaneous preterm labour |
| Notes | Methods and outcomes unclear. We have been unable to find an email address for the trial authors. Awaiting assessment pending further information becoming available |
| Methods | RCT |
| Participants | Women with a short cervix as assessed by TV ultrasound at 22 weeks |
| Interventions | Vaginal progesterone |
| Outcomes | Spontaneous preterm birth less than 34 weeks, plus neonatal morbidity and adverse effects |
| Notes | Potentially eligible but twin outcomes not reported separately. Insufficient information to include |
| Methods | RCT |
| Participants | Twin pregnancies |
| Interventions | Progesterone vaginal gel |
| Outcomes | Preterm birth |
| Notes | Awaiting assessment pending further publications; no results are available yet |
| Methods | RCT |
| Participants | Women with DCDA twin pregnancies and short cervix |
| Interventions | Vaginal progesterone |
| Outcomes | Primary outcome = delivery before 37 weeks |
| Notes | Only trial registration available. Awaiting assessment pending further publications |
| Methods | RCT |
| Participants | 121 pregnant women at high risk for preterm delivery, inpatient? |
| Interventions | IM progesterone, oral progesterone versus oral (3 groups) |
| Outcomes | Abstract is not reported in a way to ascertain what outcomes were collected |
| Notes | Insufficient information to include/exclude as yet |
| Methods | Interventional study |
| Participants | 100 pregnant women |
| Interventions | IM 17OHP versus placebo |
| Outcomes | Gestational age, birthweight |
| Notes | This is potentially relevant and multiples are not explicitly excluded, there are no data for multiples; insufficient information to include |
17OHP: 17 alpha‐hydroxyprogesterone DCDA: dichorionic diamniotic IM: intramuscular RCT: randomised controlled trial TV: transvaginal
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Progesterone after previous preterm birth for prevention of neonatal respiratory distress syndrome: the PROGRESS trial |
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | 787 women recruited Women were eligible if they had a live fetus (singleton or twins), between 18 and 23 + 6 weeks' gestation and a history of prior preterm birth at < 37 weeks' gestation in the immediately preceding pregnancy (where the onset of labour occurred spontaneously, or in association with cervical incompetence, or following preterm prelabour ruptured membranes) |
| Interventions |
Intervention: nightly vaginal pessaries of 100 mg progesterone from 20 weeks' gestation until birth or 34 weeks' gestation Control: nightly vaginal pessaries of similar‐appearing placebo, from 20 weeks' gestation until birth or 34 weeks' gestation |
| Outcomes | Preterm birth Infant respiratory distress syndrome |
| Starting date | Not clear |
| Contact information | Caroline Crowther caroline.crowther@adelaide.edu.au |
| Notes | Waiting for trial to be published. Will be included when results are available |
| Trial name or title | Prevention of preterm birth in twin pregnancies ‐ “Randomised trial of progesterone versus placebo” |
| Methods | Multicentre, double‐blind, placebo‐controlled, randomised trial |
| Participants | Target number of women: 1180 Inclusion criteria: women with a twin pregnancy attending for their routine first trimester scan, 18 or over, DCDA or MCDA twin pregnancies, live fetuses at 11 ‐ 13 weeks of gestation, English‐ or Spanish‐speaking (otherwise interpreters will be used) Exclusion criteria: pregnancies complicated by major fetal abnormality identified at the 11‐13 weeks assessment, including nuchal translucency thickness > 3.5 mm, in MCDA twin pregnancies there are early signs of twin‐to‐twin transfusion syndrome (TTTS) (20% discordance in CRL and/or nuchal translucency), women who are unconscious or severely ill, those with learning difficulties, or serious mental illness, hypersensitivity to progesterone, concurrent participation in another drug trial or at any time within the previous 28 days, any other reason the clinical investigators think will prevent the potential participant from complying with the trial protocol |
| Interventions | Intervention: participants are required to insert a 300 mg progesterone suppository twice daily until 34 weeks’ gestation, or earlier in the event of preterm delivery Control: participants are required to insert a 300 mg placebo suppository twice daily until 34 weeks’ gestation, or earlier in the event of preterm delivery |
| Outcomes |
Primary outcome: Incidence of spontaneous delivery before 34 weeks (238 days) of gestation Secondary outcomes: 1. The incidence of spontaneous preterm birth < 37 weeks (259 days) of gestation 2. Birthweight below the 3rd, 5th and 10th centile 3. Rate of stillbirth or neonatal death due to any cause 4. Major adverse outcomes before discharge from the hospital (IVH, RDS, retinopathy of prematurity, or NEC) 5. Need for neonatal special care (admission to a NICU, ventilation, phototherapy, treatment for proven or suspected sepsis, or blood transfusion) |
| Starting date | April 2016 |
| Contact information | Dr Catalina De Paco Fetal Medicine Unit Hospital Universitario “Virgen de la Arrixaca” Murcia 30120 Spain |
| Notes |
| Trial name or title | Comparing double dose of vaginal progesterone to no treatment for prevention of preterm birth in twins and short cervix |
| Methods | Open‐label, parallel, randomised trial |
| Participants | Estimated enrolment: 214 Inclusion criteria: twin gestation, certain dating (documented first trimester ultrasound, or a reliable menstrual date confirmed by an ultrasound performed before 20 weeks of gestation), age > 18 years, gestational age 16 ‐ 26, cervical length < 25 mm, intact membranes, informed consent Exclusion criteria: major malformation or chromosomal abnormality to at least 1 fetus, higher order pregnancy, mocochorional‐monoamniotic twin, death of 1 fetus, cervical dilatation > 3 cm, chronic medical conditions that would interfere with study participation or evaluation of the treatment (e.g. seizures, psychiatric disorders, uncontrolled chronic hypertension, congestive heart failure, chronic renal failure, uncontrolled diabetes mellitus with end‐organ dysfunction, active thrombophlebitis or a thromboembolic disorder, history of hormone‐associated thrombophlebitis or thromboembolic disorders, active liver dysfunction or disease, known or suspected malignancy of the breast or genital organs) |
| Interventions | Intervention: treatment with 400 mg micronised progesterone (Utrogestan) daily up to 36 weeks of gestation Control: no treatment. Regular follow‐up |
| Outcomes | Preterm delivery (time frame: up to 25 weeks from randomisation) Rate of preterm delivery before 37 weeks |
| Starting date | January 2015 |
| Contact information | Noah Zafran noah_za@clalit.org.il |
| Notes |
| Trial name or title | A randomised trial of Pessary and Progesterone for Preterm Prevention in Twin Gestation With a Short Cervix (PROSPECT) |
| Methods | 3‐armed, double‐blind, parallel, randomised trial |
| Participants | 600 women randomised Inclusion criteria: women with twin pregnancy, cervical length of < 30 millimetres, gestation 16 ‐ 24 weeks Exclusion criteria: cervical dilation (internal os) 2 cm or greater on digital examination or evidence of prolapsed membranes beyond the external cervical os, monoamniotic gestation, twin‐twin transfusion syndrome, evidence of severe IUGR, fetal anomaly in either twin or imminent fetal demise, placenta previa, active vaginal bleeding greater than spotting at the time of randomisation, symptomatic, untreated vaginal or cervical infection, rupture of membranes, more than 6 contractions per hour, known major Mullerian anomaly of the uterus, any fetal/maternal condition which would require invasive in‐utero assessment or treatment, major maternal medical illness associated with increased risk for adverse pregnancy outcome or indicated preterm birth, planned cerclage or cerclage already in place, planned indicated delivery prior to 35 weeks, planned or actual progesterone treatment of any type or form after 14 weeks 6 days during the current pregnancy, allergy to progesterone or excipients in the study drug or placebo, participation in another interventional study that influences gestational age at delivery or neonatal morbidity or mortality, participation in this trial in a previous pregnancy, prenatal care or delivery planned elsewhere |
| Interventions |
Intervention 1: progesterone‐ 200 mg micronised vaginal progesterone soft gel capsule, daily from randomisation to < 35 weeks Intervention 2: Arabin pessary placement management from randomisation to < 35 weeks Control: placebo soft gel capsule, daily from randomisation to < 35 weeks |
| Outcomes |
Primary outcome: Delivery prior to 35 weeks or fetal loss Secondary outcomes: 1. Randomisation to delivery interval 2. Gestational age at delivery 3. Neonatal morbidity and mortality 4. Lower genital tract or urinary tract infection 5. Physician interventions |
| Starting date | October 2015 |
| Contact information | Uma Reddy reddyu@mail.nih.gov |
| Notes |
CRL: crown‐rump length DCDA: dichorionic diamniotic IUGR: intra‐uterine growth retardation NICU: neonatal intensive care unit OVH: intraventricular haemorrhage MCDA: monochorionic diamniotic NEC: necrotising enterocolitis RDS: respiratory distress syndrome
Contributions of authors
Jodie M Dodd drafted the original text of the protocol. Therese Dowswell contributed to the text and commented on drafts. Rosalie M Grivell, Cecelia M OBrien and Andrea R Deussen commented on drafts of the protocol.
Sources of support
Internal sources
(TD) Cochrane Pregnancy and Childbirth Group, Department of Women's and Children's Health, The University of Liverpool, Liverpool, UK.
External sources
-
National Institute for Health Research (NIHR), UK.
NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines
Declarations of interest
Jodie M Dodd is an investigator on the PROGRESS randomised trial, which may contribute data to this review. Rosalie M Grivell: No conflicts of interest. Cecelia M OBrien: No conflicts of interest. Andrea R Deussen: No conflicts of interest.
Therese Dowswell is paid from a grant from her institution to work on this and other Cochrane Reviews. In the last 36 months she has been paid by WHO for work on other reviews.
New
References
References to studies included in this review
- Aboulghar M, Aboulghar M, Amin Y, Al Inany H, Serour G, Mansour R. The use of vaginal natural progesterone for prevention of preterm birth in IVF/ICSI pregnancies. Ultrasound in Obstetrics & Gynecology 2012;40(Suppl 1):19. [Google Scholar]; Aboulghar MM, Aboulghar MA, Amin YM, Al‐Inany HG, Mansour RT, Serour GI. The use of vaginal natural progesterone for prevention of preterm birth in IVF/ICSI pregnancies. Reproductive BioMedicine Online 2012;25(2):133‐8. [DOI] [PubMed] [Google Scholar]
- Awwad J, Usta IM, Ghazeeri G, Yacoub N, Succar J, Hayek S, et al. A randomised controlled double‐blind clinical trial of 17‐hydroxyprogesterone caproate for the prevention of preterm birth in twin gestation (PROGESTWIN): evidence for reduced neonatal morbidity. BJOG: an international journal of obstetrics and gynaecology 2015;122:71‐9. [DOI] [PubMed] [Google Scholar]; NCT00141908. Prevention of preterm delivery in twin pregnancies by 17 alpha hydroxyprogesterone caproate. clinicaltrials.gov/ct2/show/NCT00141908 (first received 1 September 2005). ; Nassar A, Awwad J, Succar J, Saassouh W, Khalife T, Hayek S, et al. Incidence of GDM in twin pregnancies of women receiving prophylactic 17‐A OH progesterone caproate. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(S1):307‐8. [Google Scholar]
- Briery C, Veillon E, Klauser CK, Martin R, Chauhan S, Magann EF, et al. Women with prolonged premature rupture of the membrane do not benefit from weekly progesterone: a randomized clinical trial. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S189. [DOI] [PubMed] [Google Scholar]; Briery CM, Veillon EW, Klauser CK, Martin RW, Chauhan SP, Magann EF, et al. Progesterone does not prevent preterm births in women with twins. Southern Medical Journal 2009;102(9):900‐4. [DOI] [PubMed] [Google Scholar]
- Agra IK, Brizot ML, Miyadahira MY, Carvalho MH, Francisco RP, Zugaib M. The effect of prenatally administered vaginal progesterone on uterine artery doppler in asymptomatic twin pregnancies. European Journal of Obstetrics & Gynecology, and Reproductive Biology2016; Vol. 205:11‐4. [DOI] [PubMed]; Brizot ML, Hernandez W, Liao AW, Bittar RE, Francisco RP, Krebs VL, et al. Vaginal progesterone for the prevention of preterm birth in twin gestations: a randomized placebo‐controlled double‐blind study. American Journal of Obstetrics and Gynecology 2015;213:82.e1‐9. [DOI] [PubMed] [Google Scholar]; Oliveira LA, Brizot ML, Liao AW, Bittar RE, Francisco RP, Zugaib M. Prenatal administration of vaginal progesterone and frequency of uterine contractions in asymptomatic twin pregnancies. Acta Obstetricia et Gynecologica Scandinavica 2016;95(4):436‐43. [DOI] [PubMed] [Google Scholar]
- Caritis SN, Rouse DJ, Peaceman AM, Sciscione A, Momirova V, Spong CY, et al. Prevention of preterm birth in triplets using 17 alpha‐hydroxyprogesterone caproate: a randomized controlled trial. Obstetrics & Gynecology 2009;113(2 Pt 1):285‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetingoz E, Cam C, Sakalli M, Karateke A, Celik C, Sancak A. Progesterone effects on preterm birth in high‐risk pregnancies: a randomized placebo‐controlled trial. Archives of Gynecology and Obstetrics 2011;283(3):423‐9. [DOI] [PubMed] [Google Scholar]
- Combs CA, Garite T, Maurel K, Das A, Porto M, for the OCRN. Failure of 17‐hydroxyprogesterone to reduce neonatal morbidity or prolong triplet pregnancy: a double‐blind, randomized clinical trial. American Journal of Obstetrics and Gynecology 2010;203(3):248.e1‐248.e9. [DOI] [PubMed] [Google Scholar]; Combs CA, Garite T, Porto M, Maurel K, for the OCRN. 17‐hydroxyprogesterone for triplet pregnancy does not reduce prematurity or neonatal morbidity, may increase midtrimester loss. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S168. [Google Scholar]; NCT00163020. 17OHP for reduction of neonatal morbidity due to preterm birth (PTB) in twin and triplet pregnancies. clinicaltrials.gov/ct2/show/NCT00163020 (first received 9 September 2005).
- Combs CA, Garite J, Maurel K, Das A. Fetal fibronectin versus cervical length as predictors of preterm birth in twin pregnancy with or without 17‐hydroxyprogesterone caproate. American Journal of Perinatology 2014;31(12):1023‐30. [DOI] [PubMed] [Google Scholar]; Combs CA, Garite T, Maurel K, Das A. Fetal fibronectin and cervical length as predictors of preterm birth in twin pregnancies, with or without 17‐hydroxyprogesterone caproate. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl 1):S205. [Google Scholar]; Combs CA, Garite T, Maurel K, Das A, Porto M, for the Obstetrix Collaborative Research Network. 17‐hydroxyprogesterone caproate for twin pregnancy: a double‐blind, randomized clinical trial. American Journal of Obstetrics and Gynecology 2011;204:221.e1‐221.e8. [DOI] [PubMed] [Google Scholar]; Combs CA, Garite TJ, Maurel K, Cebrik D. 17‐hydroxyprogesterone caproate for women with history of preterm birth in a prior pregnancy and twins in the current pregnancy. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S213. [Google Scholar]; Combs CA, Maurel K, Garite T, for the Obstetric Collaborative Research Network. 17‐hydroxyprogesterone for twin pregnancy: no reduction in prematurity or neonatal morbidity. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S7. [DOI] [PubMed] [Google Scholar]; Heitmann E, Lu G, Combs CA, Garite TJ, Maurel K. The impact of maternal weight upon the effectiveness of 17‐hydroxyprogesterone in preventing preterm birth among twin gestations. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S98. [Google Scholar]; Horton A, Gyamfi C. 17‐alpha hydroxyprogesterone caproate does not increase the risk of gestational diabetes in singleton and twin pregnancies. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S197. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT01812239. Vaginal progesterone to prevent early preterm birth in twin pregnancy with short cervix. Double blind, placebo‐controlled, randomized clinical trial. clinicaltrials.gov/ct2/show/NCT01812239. NCT01812239 (first received 12 March 2013).
- El‐Refaie W, Abdelhafez MS, Badawy A. Vaginal progesterone for prevention of preterm labor in asymptomatic twin pregnancies with sonographic short cervix: a randomized clinical trial of efficacy and safety. Archives of Gynecology and Obstetrics 2016;293(1):61‐7. [DOI] [PubMed] [Google Scholar]
- Hartikainen‐Sorri AL, Kauppila A, Tuimala R. Inefficacy of 17alpha‐hydroxyprogesterone caproate in the prevention of prematurity in twin pregnancy. Obstetrics & Gynecology 1980;56:692‐5. [PubMed] [Google Scholar]; Hartikainen‐Sorri AL, Kauppila A, Tuimala R. Management of twin pregnancy with 17 alpha‐ hydroxyprogesterone caproate [abstract]. 9th World Congress of Gynecology and Obstetrics; 1979 October 26‐31; Tokyo, Japan. 1979:298‐9. [Google Scholar]
- ISRCTN40512715. 17‐alpha hydroxyprogesterone in multiple pregnancies to prevent handicapped infants. isrctn.com/ISRCTN40512715 (first received 2 November 2006). ; Lim AC. The effect of 17‐alpha hydroxyprogesterone caproate on cervical length in multiple pregnancies. Reproductive Sciences 2010;17(3 Suppl 1):282A. [Google Scholar]; Lim AC, Bloemenkamp KW, Boer K, Duvekot JJ, Erwich JJ, Hasaart TH, et al. Progesterone for the prevention of preterm birth in women with multiple pregnancies: the AMPHIA trial. BMC Pregnancy & Childbirth 2007;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lim AC, Schuit E, Bloemenkamp K, Bernardus RE, Duvekot JJ, Erwich JJ, et al. 17alpha‐hydroxyprogesterone caproate for the prevention of adverse neonatal outcome in multiple pregnancies: A randomized controlled trial. Obstetrics and Gynecology 2011;118(3):513‐20. [DOI] [PubMed] [Google Scholar]; Lim AC, Schuit E, Papatsonis D, Eyck J, Porath MM, Oirschot CM, et al. Effect of 17‐alpha hydroxyprogesterone caproate on cervical length in twin pregnancies. Ultrasound in Obstetrics & Gynecology 2012;40(4):426‐30. [DOI] [PubMed] [Google Scholar]; Lim AC, for the Amphia Study Group. Is second trimester cervical length a predictor for an effect of 17‐alpha hydroxyprogesterone caproate on neonatal morbidity in multiple pregnancies? Results from the AMPHIA trial (ISRCTN40512715). Reproductive Sciences 2010;17(3 Suppl 1):282A. [Google Scholar]; Mulder EJ, Versteegh EM, Bloemenkamp KW, Lim AC, Mol BW, Bekedam DJ, et al. Does 17‐alpha‐hydroxyprogesterone caproate affect fetal biometry and birth weight in twin pregnancy?. Ultrasound in Obstetrics & Gynecology 2013;42(3):329‐34. [DOI] [PubMed] [Google Scholar]; Willekes C, Lim A, Vijgen S, Mol B, Papatsonis D, Hasaart T, et al. Mid‐pregnancy cervical length as a predictor of preterm birth in multiple pregnancies. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S54. 21640231 [Google Scholar]
- Eddama O, Petrou S, Regier D, Norrie J, MacLennan G, MacKenzie F, et al. Study of progesterone for the prevention of preterm birth in twins (STOPPIT): findings from a trial‐based cost‐effectiveness analysis. International Journal of Technology Assessment in Health Care 2010;26(2):141‐8. [DOI] [PubMed] [Google Scholar]; ISRCTN35782581. Double blind randomised placebo controlled study of progesterone for the prevention of preterm birth in twins (STOPPIT) (ongoing trial). www.isrctn.com/ISRCTN35782581 (first received 10 June 2005). ; McNamara HC, Wood R, Chalmers J, Marlow N, Norrie J, MacLennan G, et al. STOPPIT baby follow‐up study: the effect of prophylactic progesterone in twin pregnancy on childhood outcome. Plos One 2015;10(4):e0122341. [DOI] [PMC free article] [PubMed] [Google Scholar]; Norman JE, MacKenzie F, Owen P, Mactier H, Hanretty K, Cooper S, et al. A randomized, double‐masked, placebo‐controlled study of progesterone for the prevention of preterm birth of twins (STOPPIT). Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(Suppl 1):Fa8. [Google Scholar]; Norman JE, Mackenzie F, Owen P, Mactier H, Hanretty K, Cooper S, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double‐blind, placebo‐controlled study and meta‐analysis. Lancet 2009;373(9680):2034‐40. [DOI] [PubMed] [Google Scholar]; Norman JE, Yuan M, Anderson L, Howie F, Harold G, Young A, et al. Effect of prolonged in vivo administration of progesterone in pregnancy on myometrial gene expression, peripheral blood leukocyte activation, and circulating steroid hormone levels. Reproductive Sciences 2011;18(5):435‐46. [DOI] [PubMed] [Google Scholar]
- Klein K, Rode L, Nicolaides K, Krampl‐Bettelheim E, Larsen H, Holmskov A, et al. Vaginal progesterone and the risk of preterm delivery in high‐risk twin gestations ‐ secondary analysis of a placebo‐controlled randomized trial. Ultrasound in Obstetrics & Gynecology 2011;38(S1):11. [DOI] [PubMed] [Google Scholar]; Klein K, Rode L, Nicolaides KH, Krampl‐Bettelheim E, Tabor A, PREDICT Group. Vaginal micronized progesterone and risk of preterm delivery in high‐risk twin pregnancies: secondary analysis of a placebo‐controlled randomized trial and meta‐analysis. Ultrasound in Obstetrics & Gynecology 2011;38(3):281‐7. [DOI] [PubMed] [Google Scholar]; Rode L, Klein K, Larsen H, Holmskov A, Andreasen KR, Uldbjerg N, et al. Cytokines and the risk of preterm delivery in twin pregnancies. Obstetrics and Gynecology 2012;120(1):60‐8. [DOI] [PubMed] [Google Scholar]; Rode L, Klein K, Nicolaides K, Krampl‐Bettelheim E, Vogel I, Larsen H, et al. Prevention of preterm delivery in twin gestations (PREDICT): a multicentre randomised placebo‐controlled trial on the effect of vaginal micronised progesterone. Ultrasound in Obstetrics & Gynecology 2011;38(Suppl 1):1. [DOI] [PubMed] [Google Scholar]; Rode L, Klein K, Nicolaides KH, Krampl‐Bettelheim E, Tabor A, PREDICT Group. Prevention of preterm delivery in twin gestations (PREDICT): a multicenter, randomized, placebo‐controlled trial on the effect of vaginal micronized progesterone. Ultrasound in Obstetrics & Gynecology 2011;38(3):272‐80. [DOI] [PubMed] [Google Scholar]; Vedel C, Larsen H, Holmskov A, Andreasen KR, Uldbjerg N, Ramb J, et al. Long‐term effects of prenatal progesterone exposure: Neurophysiological development and hospital admissions in twins up to 8 years of age. Ultrasound in Obstetrics & Gynecology 2016;48(3):382‐9. [DOI] [PubMed] [Google Scholar]
- Caritis S, Rouse D. A randomized controlled trial of 17‐hydroxyprogesterone caproate (17‐OHPC) for the prevention of preterm birth in twins. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S2. [Google Scholar]; Caritis SN, Simhan H. Relationship of 17‐alpha hydroxyprogesterone caproate (17‐OHPC) concentrations and gestational age at delivery in twins. 55th Annual Meeting of the Society of Gynecologic Investigation; 2008 March 26‐29; San Diego, USA2008:Abstract no: 139. [DOI] [PMC free article] [PubMed]; Caritis SN, Simhan HN, Zhao Y, Rouse DJ, Peaceman AM, Sciscione A, et al. Relationship between 17‐hydroxyprogesterone caproate concentrations and gestational age at delivery in twin gestation. American Journal of Obstetrics and Gynecology 2012;207(5):396.e1‐396.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Caritis SN, Venkat R. Impact of body mass index (BMI) on plasma concentrations of 17‐alpha hydroxyprogesterone caproate (17‐OHPC). 55th Annual Meeting of the Society of Gynecologic Investigation; 2008 March 26‐29; San Diego, USA2008:Abstract no: 138. ; Durnwald C. The impact of cervical length on risk of preterm birth in twin gestations. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S10. [Google Scholar]; Durnwald CP, Momirova V, Rouse DJ, Caritis SN, Peaceman AM, Sciscione A, et al. Second trimester cervical length and risk of preterm birth in women with twin gestations treated with 17‐ hydroxyprogesterone caproate. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(12):1360‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gyamfi C, Horton AL, Momirova V, Rouse DJ, Caritis SN, Peaceman AM, et al. The effect of 17‐alpha hydroxyprogesterone caproate on the risk of gestational diabetes in singleton or twin pregnancies. American Journal of Obstetrics and Gynecology 2009;201(4):392.e1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Refuerzo JS, Momirova V, Peaceman AM, Sciscione A, Rouse DJ, Caritis SN, et al. Neonatal outcomes in twin pregnancies delivered moderately preterm, late preterm, and term. American Journal of Perinatology 2010;27(7):537‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rouse DJ, Caritis SN, Peaceman AM, Sciscione A, Thom EA, Spong CY, et al. A trial of 17 alpha‐hydroxyprogesterone caproate to prevent prematurity in twins. New England Journal of Medicine 2007;357(5):454‐61. [DOI] [PubMed] [Google Scholar]; Simhan HN, Caritis SN. The effect of 17‐alpha hydroxyprogesterone caproate (17‐OHPC) on maternal plasma CRP levels in twin pregnancies. 55th Annual Meeting of the Society of Gynecologic Investigation; 2008 March 26‐29; San Diego, USA2008:Abstract no: 140.
- Senat MV, Deruelle P, Winer N, Rozenberg P. Prevention of preterm delivery by 17 alpha‐hydroxyprogesterone caproate in asymptomatic twin pregnancies with a short cervix: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl):S3. [DOI] [PubMed] [Google Scholar]; Senat MV, Porcher R, Winer N, Vayssiere C, Deruelle P, Capelle M, et al. Prevention of preterm delivery by 17 alpha‐hydroxyprogesterone caproate in asymptomatic twin pregnancies with a short cervix: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2013;208(3):194.e1‐194.e8. [DOI] [PubMed] [Google Scholar]; Senat MV, Winer N, Porcher R, Rozenberg P. Prevention of preterm delivery in asymptomatic women with twin pregnancy by 17 alpha‐Hydroxyprogesterone caproate: a randomised controlled trial. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(S2):14. [Google Scholar]
- NCT00480402. Natural progesterone and preterm birth in twins. clinicaltrials.gov/ct2/show/NCT00480402 (first received 28 May 2007). ; Serra V, Perales A, Meseguer J, Parrilla J, Lara C, Bellver J, et al. Increased doses of vaginal progesterone for the prevention of preterm birth in twin pregnancies: a randomised controlled double‐blind multicentre trial. BJOG: an international journal of obstetrics and gynaecology 2013;120(1):50‐7. [DOI] [PubMed] [Google Scholar]
- NCT00343265. Vaginal progesterone versus placebo in multiple pregnancy. clinicaltrials.gov/ct2/show/NCT00343265 (first received 20 June 2006). ; Swaby C. Pilot randomized controlled trial of vaginal progesterone to prevent preterm birth in multiple pregnancy. JOGC: Journal of Obstetrics and Gynaecology Canada 2007;29(6 Suppl 1):S47. [Google Scholar]; Wood S, Ross S, Tang S, Miller L, Sauve R, Brant R. Vaginal progesterone to prevent preterm birth in multiple pregnancy: A randomized controlled trial. Journal of Perinatal Medicine 2012;40(6):593‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Abbott D, Chin‐Smith E, Seed P, Chandiramani M, Shennan A, Tribe R. Relationship between cervical‐vaginal fluid elafin concentrations and subsequent cervical shortening in women at high risk of spontaneous preterm birth. Reproductive Sciences 2012;19(3 Suppl):73A. [Google Scholar]
- ACTRN12616000875404. Evaluating the effectiveness of a cervical pessary to improve neonatal outcome by preventing preterm birth in women with a twin pregnancy and a short cervix. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616000875404 (first received 5 June 2016).
- Borna S, Shakoie S, Borna H. Progesterone for maintenance tocolytic therapy after threatened preterm labor. randomized controlled trial. Journal of Maternal‐Fetal and Neonatal Medicine 2008;21(Suppl 1):151‐2. [Google Scholar]
- Breart G, Lanfranchi M, Chavigny C, Rumeau‐Rouquette C, Sureau C. A comparative study of the efficiency of hydroxyprogesterone caproate and of chlormadinone acetate in the prevention of premature labor. International Journal of Gynecology & Obstetrics 1979;16:381‐4. [DOI] [PubMed] [Google Scholar]
- Brenner WE, Hendricks CH. Effect of medroxyprogesterone acetate upon the duration and characteristics of human gestation and labor. American Journal of Obstetrics and Gynecology 1962;83:1094‐8. [DOI] [PubMed] [Google Scholar]
- Chandiramani M, Seed PT, Bennett PR, Shennan AH, Tribe RM. Serum progesterone concentrations in women with a previous preterm birth treated with vaginal progesterone supplementation. Reproductive Sciences 2012;19(3 Suppl):189A. [Google Scholar]
- Coomarasamy A, Williams H, Truchanowicz E, Seed PT, Small R, Quenby S, et al. A randomized trial of progesterone in women with recurrent miscarriages. New England Journal of Medicine 2015;373(22):2141‐8. [DOI] [PubMed] [Google Scholar]; ISRCTN92644181. First trimester progesterone therapy in women with a history of unexplained recurrent miscarriages: a randomised double‐blind placebo‐controlled multi‐centre trial (The PROMISE [PROgesterone in recurrent MIScarriagE] Trial). isrctn.com/ISRCTN92644181 (first received 10 March 2009). [DOI] [PMC free article] [PubMed]
- Facchinetti F, Paganelli S, Venturini P, Dante G. 17 alpha hydroxy‐progesterone caproate (17P) treatment reduced cervical shortening inhibiting cervical interleukin‐1 secretion. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S5. [Google Scholar]
- Grobman W. Short cervix and activity restriction. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl 1):S227‐S228. [Google Scholar]
- Gyamfi‐Bannerman C, Miller RS. The role of 17‐OHPC in preventing recurrent preterm birth in a current twin gestation. Reproductive Sciences (Thousand Oaks, Calif.) 2015;22(Suppl 1):151A. [Google Scholar]
- Hauth JC, Gilstrap LC, Brekken AL, Hauth JM. The effect of 17alpha‐hydroxyprogesterone caproate on pregnancy outcome in an active‐duty military population. American Journal of Obstetrics and Gynecology 1983;146:187‐90. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Bemis RL. West Area Los Angeles prematurity prevention demonstration project. In: Papiernik E, Breart G, Spira N editor(s). Prevention of Preterm Birth. Vol. 138, Paris: INSERM, 1986:205‐22. [Google Scholar]; Hobel CJ, Bragonier R, Ross M, Bear M, Bemis R, Mori B. West Los Angeles premature prevention program: significant impact. Journal of Perinatal Medicine 1987;15:112. [Google Scholar]; Hobel CJ, Ross MG, Bemis RL, Bragonier JR, Bear M, Mori B. West Los Angeles preterm birth prevention project (LAPPP): program impact. American Journal of Obstetrics and Gynecology 1992;166:363. [DOI] [PubMed] [Google Scholar]; Hobel CJ, Ross MG, Bemis RL, Bragonier JR, Nessim S, Sandhu M, et al. The West Los Angeles preterm birth prevention project: I. program impact on high‐risk women. American Journal of Obstetrics and Gynecology 1994;170:54‐62. [DOI] [PubMed] [Google Scholar]
- Ionescu AC, Gheorghiu D, Pacu I, Davitoiu B, Dimitriu M, Haradja H. Randomized trial of cerclage and progesterone to prevent spontaneous preterm birth in high‐risk women with a short cervix. Journal of Perinatal Medicine 2012;39 Suppl:Abstract no. 008. [Google Scholar]
- Johnson JW, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17alpha‐hydroxyprogesterone caproate in the prevention of premature labor. New England Journal of Medicine 1975;293(14):675‐80. [DOI] [PubMed] [Google Scholar]
- LeVine L. Habitual abortion. A controlled clinical study of progestational therapy. Western Journal of Surgery, Obstetrics, and Gynecology 1964;72:30‐6. [PubMed] [Google Scholar]
- Manuck T, for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The relationship between polymorphisms in the human progesterone receptor and clinical response to 17 alpha‐hydroxyprogesterone caproate for the prevention of recurrent spontaneous preterm birth. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S18. [Google Scholar]
- Mardy AH, Pan S, Gyamfi‐Bannerman C. The effect of BMI on changes in salivary progesterone and estriol concentrations in pregnant patients receiving 17 a‐hydroxyprogesterone caproate versus placebo. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S143, Abstract no: 243. [Google Scholar]
- Martinez de Tejada B, Karolinski A, Othenin‐Girard V, Bertolino V, Wainer V, Ocampo C, et al. Prevention of preterm delivery with vaginal progesterone in women with arrested preterm labor: secondary analysis of the 4P trial. American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S378. [Google Scholar]; NCT00536003. Vaginal progesterone to prevent preterm delivery in women with preterm labor. clinicaltrials.gov/show/NCT00536003 (first received 24 September 2007).
- McKay LA, Holford TR, Bracken MB. Re‐analysis of PREGNANT trial confirms that vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix. Ultrasound in Obstetrics and Gynecology 2014;43:596‐9. [DOI] [PubMed] [Google Scholar]
- Meints L, Akers D. Effect of conception type on pregnancy outcomes in twin gestations. Reproductive Sciences 2016;23(Suppl 1):271A. [Google Scholar]
- Haidar ZA, Moussa HN, Sibai BM. The effect of partial compliance on the prevention of recurrent preterm birth in women receiving weekly 17 alphahydroxyprogesterone caproate injections. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S288, Abstract no: 533. [Google Scholar]; Heyborne K, Allshouse AA. Smoking and preterm birth: A novel effect of 17 alphahydroxyprogesterone caproate (17OHP‐C). American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S220, Abstract no: 401. [Google Scholar]; Klebanoff M, for the NICHDMFMUN. Impact of 17alpha hydroxyprogesterone caproate administration on salivary progesterone and estriol [abstract]. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S140. [Google Scholar]; Klebanoff MA, Meis PJ, Dombrowski MP, Zhao Y, Moawad AH, Northen A, et al. Salivary progesterone and estriol among pregnant women treated with 17‐alpha‐hydroxyprogesterone caproate or placebo. American Journal of Obstetrics and Gynecology 2008;199(5):506.e1‐506.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Koontz G. Does gestational age at randomization affect the efficacy of alpha hydroxyprogesterone caproate (17‐OHCP) in preventing recurrent preterm delivery? [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S55. [Google Scholar]; Meis P, NICHD MFMUN. More than one previous preterm delivery and the risk of preterm birth in women treated with 17 alpha‐hydroxyprogesterone (17P) [abstract]. American Journal of Obstetrics and Gynecology 2003;189(6 Suppl 1):S168. [Google Scholar]; Meis PJ, Klebanoff M, Dombrowski MP, Sibai BM, Leindecker S, Moawad AH, et al. Does progesterone treatment influence risk factors for recurrent preterm delivery?. Obstetrics & Gynecology 2005;106(3):557‐61. [DOI] [PubMed] [Google Scholar]; Meis PJ, NICHD MFMUN. 17 alphahydroxyprogesterone caproate prevents recurrent preterm birth [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S54. 12516600 [Google Scholar]; Northen A. 4‐year follow‐up of children exposed to 17alpha hydroxyprogesterone caproate (17P) in utero. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S6. [Google Scholar]; Northen AT, Norman GS, Anderson K, Moseley L, Divito M, Cotroneo M, et al. Follow‐up of children exposed in utero to 17 alpha‐hydroxyprogesterone caproate compared with placebo. Obstetrics & Gynecology 2007;110(4):865‐72. [DOI] [PubMed] [Google Scholar]; Sibai B. Plasma CRH levels at 16‐20 weeks do not predict preterm delivery in women at high‐risk for preterm delivery [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S114. [DOI] [PubMed] [Google Scholar]; Sibai B, Meis PJ, Klebanoff M, Dombrowski MP, Weiner SJ, Moawad AH, et al. Plasma CRH measurement at 16 to 20 weeks' gestation does not predict preterm delivery in women at high‐risk for preterm delivery. American Journal of Obstetrics and Gynecology 2005;193(3 Pt 2):1181‐6. [DOI] [PubMed] [Google Scholar]
- Moghtadaei P, Sardari F, Latifi M. Progesterone for prevention of preterm birth and improvement in pregnancy outcomes among primiparae of advanced maternal age. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(Suppl 1):Fa71. [Google Scholar]; Moghtadei P, Sardari F, Latifi M. Progesterone for prevention of preterm birth and improvement pregnancy outcomes among primiparae of advanced maternal age. Journal of Maternal‐Fetal and Neonatal Medicine 2008;21(Suppl 1):122. [Google Scholar]
- NCT00099164. Trial of progesterone in twins and triplets to prevent preterm birth. clinicaltrials.gov/ct2/show/NCT00099164 (first received 8 December 2004).
- NCT02350231. Progesterone vaginal pessary for prevention of preterm twin birth. clinicaltrials.gov/ct2/show/NCT02350231 (first received 5 January 2015).
- NCT02623881. The effectiveness of cervical pessary versus vaginal progesterone for preventing premature birth in IVF twin pregnancies: a randomized controlled trial. clinicaltrials.gov/ct2/show/record/NCT02623881 (first received: 30 November 2015).
- Defranco E, O'Brien J, Adair J, Lewis DF, Hall D, Phillips J, et al. Is there a racial disparity of progesterone to prevent preterm birth. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S200, Abstract no: 702. [Google Scholar]; O'Brien J, Defranco E, Hall D, Creasy G. Natural progesterone administration and the risk of medical complications of pregnancy: secondary analysis from a multinational, randomized, double blind, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S139. [Google Scholar]; O'Brien J, Defranco E, Hall D, Phillips J, Creasy G. Do other elements of the obstetrical history provide a possible indication for progesterone supplementation? Secondary analysis from the progesterone vaginal gel trial. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S42. [Google Scholar]; O'Brien JM, Defranco EA, Adair CD, Lewis DF, Hall DR, How H, et al. Effect of progesterone on cervical shortening in women at risk for preterm birth: secondary analysis from a multinational, randomized, double‐blind, placebo‐controlled trial. Ultrasound in Obstetrics & Gynecology 2009;34(6):653‐9. [DOI] [PubMed] [Google Scholar]; O'Brien JM, Steichen JJ, Phillips JA, Creasy GW. Two year infant outcomes for children exposed to supplemental intravaginal progesterone gel in utero: secondary analysis of a multicenter, randomized, double‐blind, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S223. [Google Scholar]; Spong CY. Progesterone for prevention of recurrent preterm birth: impact of gestational age at prior delivery [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S11. [DOI] [PubMed] [Google Scholar]
- Palacio M, Cobo T, Antolin E, Ramirez M, Cabrera F, Rosales FM, et al. Vaginal progesterone as maintenance after an episode of preterm labor (PROMISE Study): a randomized, double blinded, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl):S10‐S11. [Google Scholar]
- Papiernik E. Double blind study of an agent to prevent preterm delivery among women at increased risk. Edition Schering, Serie IV, fiche 3.1970:65‐8.
- Rozenberg P. Efficacy of 17 alpha‐hydroxyprogesterones caproate for the prevention of preterm delivery. Current Controlled Trials (www.controlled‐trials.com/) (accessed 5 October 2007). NCT00331695 2007.
- Rust O, Larkin R, Roberts W, Quinones J, Rochon M, Reed J, et al. A randomized trial of cerclage versus 17‐hydroxyprogesterone (17p) for the treatment of short cervix [abstract]. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S112. [Google Scholar]
- Suvonnakote T. Prevention of pre‐term labour with progesterone. Journal of the Medical Association of Thailand 1986;69(10):538‐42. [PubMed] [Google Scholar]
- Turner SJ, Mizock GB, Feldman GL. Prolonged gynecologic and endocrine manifestations subsequent to administration of medroxyprogesterone acetate during pregnancy. American Journal of Obstetrics and Gynecology 1966;95:222‐7. [DOI] [PubMed] [Google Scholar]
- Walch K, Hefler L, Nagele F. Oral dydrogesterone treatment during the first trimester of pregnancy: the prevention of miscarriage study (PROMIS). A double‐blind, prospectively randomized, placebo‐controlled, parallel group trial. Journal of Maternal‐Fetal & Neonatal Medicine 2005;18(4):265‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Do S, O’Malley K, Yeaton‐Massey A, Judy AE, Moore GS. Does the rate of preterm delivery in twin pregnancies differ by body mass index in women exposed to 17 alphahydroxyprogesterone?. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S288, Abstract no: 534. [Google Scholar]; Do SC, Yeaton‐Massey A, Judy AE, O’Malley K, Moore GS. Effectiveness of intramuscular progesterone for the prevention of preterm birth in twin pregnancies based on body mass index. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S333‐S334, Abstract no: 625. [Google Scholar]
- Elsheikhah AZ, Dahab S, Negm S, Ebrashy A. Effect of prophylactic progesterone on incidence of preterm labour in spontaneous twin pregnancy, randomized controlled study. Ultrasound in Obstetrics and Gynecology 2010;36(Suppl 1):108. [Google Scholar]
- Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH, for the Fetal Medicine Foundation Second Trimester Screening Group. Progesterone and the risk of preterm birth among women with a short cervix. New England Journal of Medicine 2007;357(5):462‐9. [DOI] [PubMed] [Google Scholar]
- NCT01927029. Preterm delivery prevention in twins with progesterone [Prevención de Parto Prematuro en Gemelares: Ensayo Aleatorio Con Progesterona Vaginal]. clinicaltrials.gov/ct2/show/NCT01927029 (first received 19 August 2013).
- NCT02697331. Evaluation of the role of vaginal progesterone in prevention of preterm labor in twin gestation with short cervix: randomised controlled trial. clinicaltrials.gov/ct2/show/record/NCT02697331 (first received 27 February 2016).
- Ndoni E, Bimbashi A, Dokle A, Kallfa E. Treatment with different types of progesterone in prevention of preterm delivery. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(S1):305. [Google Scholar]
- Saghafi N, Khadem N, Mohajeri T, Shakeri MT. Efficacy of 17alpha‐hydroxyprogesterone caproate in prevention of preterm delivery. Journal of Obstetrics and Gynaecology Research 2011;37(10):1342‐5. [DOI] [PubMed] [Google Scholar]; Saghafi N, Khadem N, Mohajeri T, Shakeri MT, Amini M. Efficacy of 17alpha‐hydroxyprogesterone caproate in preterm delivery prevention. Iranian Journal of Obstetrics, Gynecology and Infertility 2011;14(2):28‐33. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Armson BA, Dodd J, for the POPPICTG. POPPI: prevention of problems of preterm birth with progesterone in women at increased risk: a multicentre randomised controlled trial [abstract]. Journal of Paediatrics and Child Health 2007;43(Suppl 1):A29. [Google Scholar]; Crowther CA, Ashwood PJ, Dodd JM, Yelland L, McPhee AJ, Flenady V, et al. Progesterone after previous preterm birth for prevention of neonatal respiratory distress syndrome: The PROGRESS trial. Journal of Paediatrics and Child Health 2013;49(Suppl 2):40‐1. [Google Scholar]; Dodd JM, Crowther CA, McPhee AJ, Flenady V, Robinson JS. Progesterone after previous preterm birth for prevention of neonatal respiratory distress syndrome (PROGRESS): a randomised controlled trial. BMC Pregnancy Childbirth 2009;9(6). [DOI: 10.1186/1471-2393-9-6] [DOI] [PMC free article] [PubMed] [Google Scholar]; ISRCTN20269066. Australasian collaborative trial of vaginal progesterone therapy. isrctn.com/ISRCTN20269066 (first received 1 August 2005).
- ISRCTN66445401. Prevention of preterm birth in twin pregnancies ‐ “Randomised trial of progesterone versus placebo”. isrctn.com/ISRCTN66445401 (first received 11 October 2015).
- NCT02329535. Comparing a double dose of vaginal progesterone to no treatment for the prevention of preterm birth in twins pregnancy and short cervix. clinicaltrials.gov/ct2/show/NCT02329535. NCT02329535 (first received 30 November 2014).
- NCT02518594. A randomized trial of pessary and progesterone for preterm prevention in twin gestation with a short cervix. clinicaltrials.gov/ct2/show/NCT02518594 (first received 18 March 2015).
Additional references
- Agra IK, Brizot ML, Miyadahira MY, Carvalho MH, Francisco RP, Zugaib M. The effect of prenatally administered vaginal progesterone on uterine artery doppler in asymptomatic twin pregnancies. European Journal of Obstetrics, Gynecology, and Reproductive Biology2016; Vol. 205:11‐4. [DOI] [PubMed]
- Australian I of H, W. Australia's Mothers and Babies 2000. National Perinatal Statistics Unit, 2003. [Google Scholar]
- AIHW 2014. Australia's Health 2014. Australia's Health Series no. 14. Cat. no. AUS 178. Canberra: AIHW, 2014. [Google Scholar]
- Astle S, Slater DM, Thornton S. The involvement of progesterone in the onset of human labour. European Journal of Obstetrics & Gynecology and Reproductive Biology 2003;108(2):177‐81. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller A, et al. Born too soon: the global epidemiology of 15 million preterm births. Reproductive Health 2013;10 Suppl 1:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block BS, Liggins GC, Creasy RK. Preterm delivery is not predicted by serial plasma estradiol or progesterone concentration measurements. American Journal of Obstetrics and Gynecology 1984;150(6):716‐22. [DOI] [PubMed] [Google Scholar]
- Bromer JG, Ata B, Seli M, Lockwood CJ, Seli E. Preterm deliveries that result from multiple pregnancies associated with assisted reproductive technologies in the USA: a cost analysis. Current Opinion in Obstetrics & Gynecology 2011;23(3):168‐73. [DOI] [PubMed] [Google Scholar]
- Caritis SN, Sharma S, Venkataramanan R, Hankins GD, Miodovnik M, Hebert MF, et al. Pharmacology and placental transport of 17‐hydroxyprogesterone caproate in singleton gestation. American Journal of Obstetrics and Gynecology 2012;207(5):398.e1‐398.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. Global epidemiology of multiple birth. Reproductive Biomedicine Online 2007;15 Suppl 3:45‐52. [DOI] [PubMed] [Google Scholar]
- Combs CA, Schuit E, Caritis SN, Lim AC, Garite TJ, Maurel K, et al. 17‐Hydroxyprogesterone caproate in triplet pregnancy: an individual patient data meta‐analysis. BJOG: an international journal of obstetrics and gynaecology 2016;123(5):682‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proceedings of the National Academy of Sciences of the United States of America 2003;100(16):9518‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo‐controlled double‐blind study. American Journal of Obstetrics and Gynecology 2003;188(2):419‐24. [DOI] [PubMed] [Google Scholar]
- Elder DE, Haga R, Evans SF, Benninger HR, French NP. Hospital admissions in the first year of life in very preterm infants. Journal of Paediatrics and Child Health 1999;35(2):145‐50. [DOI] [PubMed] [Google Scholar]
- Feghali M, Venkataramanan R, Caritis S. Prevention of preterm delivery with 17‐hydroxyprogesterone caproate: pharmacologic considerations. Seminars in Perinatology 2014;48:516‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates S, Brocklehurst P. How should randomised trials including multiple pregnancies be analysed?. BJOG: an international journal of obstetrics and gynaecology 2004;111(3):213‐9. [DOI] [PubMed] [Google Scholar]
- Grazzini E, Guillon G, Mouillac B, Zingg HH. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature 1998;392(6675):509‐12. [DOI] [PubMed] [Google Scholar]
- Greene MF. Progesterone and preterm delivery ‐ deja vu all over again. New England Medical Journal 2003;348(24):2453‐5. [DOI] [PubMed] [Google Scholar]
- Hack M. Consideration of the use of health status, functional outcome, and quality‐of‐life to monitor neonatal intensive care practice. Pediatrics 1999;103(1 Suppl E):319‐28. [PubMed] [Google Scholar]
- Haluska GJ, Cook MJ, Novy MJ. Inhibition and augmentation of progesterone production during pregnancy: effects on parturition in rhesus monkeys. American Journal of Obstetrics and Gynecology 1997;176(3):682‐91. [DOI] [PubMed] [Google Scholar]
- Haluska GJ, Wells TR, Hirst JJ, Brenner RM, Sadowsky DW, Novy MJ. Progesterone receptor localisation and isoforms in myometrium, decidua, and fetal membranes from rhesus macaques: evidence for functional progesterone withdrawal at parturition. Journal of the Society for Gynecological Investigation 2002;9(3):125‐36. [PubMed] [Google Scholar]
- Hargreave M, Jensen A, Nielsen TS, Colov EP, Andersen KK, Pinborg A, et al. Maternal use of fertility drugs and risk of cancer in children‐‐a nationwide population‐based cohort study in Denmark. International Journal of Cancer 2015;136(8):1931‐9. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Howson CP, Kinney MV, McDougall L, Lawn JE, Born Too Soon Preterm Birth Action Group. Born too soon: preterm birth matters. Reproductive Health 2013;10 Suppl 1:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iams JD. Supplemental progesterone to prevent preterm birth. American Journal Obstetrics and Gynecology 2003;188(2):303. [DOI] [PubMed] [Google Scholar]
- Vine L. Habitual abortion. A controlled clinical study of progestational therapy. Western Journal of Surgical Obstetrics and Gynecology 1964;72:30‐6. [PubMed] [Google Scholar]
- Lopez‐Bernal A. Mechanism of labour ‐ biochemical aspects. BJOG: an international journal of obstetrics and gynaecology 2003;110(Suppl 20):39‐45. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJK. Births: Final data for 2013. National Vital Statistics Reports. Hyattsville, MD: National Center for Health Statistics, 2015; Vol. 64, issue 1:1‐30. [PubMed]
- McCormick MC, Litt JS, Smith VC, Zupancic JA. Prematurity: an overview and public health implications. Annual Review of Public Health 2011;32:367‐79. [DOI] [PubMed] [Google Scholar]
- McNamara HC, Wood R, Chalmers J, Marlow N, Norrie J, MacLennan G, et al. STOPPIT Baby Follow‐up Study: the effect of prophylactic progesterone in twin pregnancy on childhood outcome. PloS One 2015;10(4):e0122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm delivery by 17 alpha‐hydroxyprogesterone caproate. New England Journal of Medicine 2003;348(24):2379‐85. [DOI] [PubMed] [Google Scholar]
- Michaelis J, Michaelis H, Gluck E, Koller S. Prospective study of suspected associations between certain drugs administered during early pregnancy and congenital malformations. Teratology 1983;27(1):57‐64. [DOI] [PubMed] [Google Scholar]
- Peiber D, Allport VC, Hills F, Johnson M, Bennett PR. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Molecular Human Reproduction 2001;7(9):875‐9. [DOI] [PubMed] [Google Scholar]
- Pepe GJ, Albrecht ED. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocrine Review 1995;16(5):608‐48. [DOI] [PubMed] [Google Scholar]
- Raman‐Wilms L, Tseng AL, Wighardt S, Einarson TR, Koren G. Fetal genital effects of first‐trimester sex hormone exposure: a meta‐analysis. Obstetrics and Gynecology 1995;85(1):141‐9. [DOI] [PubMed] [Google Scholar]
- Resseguie LJ, Hick JF, Bruen JA, Noller KL, O׳Fallon WM, Kurland LT. Congenital malformations among offspring exposed in utero to progestins, Olmsted County, Minnesota, 1936–1974. Fertility and Sterility 1985;43(4):514–9. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG: an international journal of obstetrics and gynaecology 2006;113(Suppl 3):17‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Conde‐Agudelo A, El‐Refaie W, Rode L, Brizot ML, Cetingoz E, et al. Vaginal progesterone decreases preterm birth and neonatal morbidity and mortality in women with a twin gestation and a short cervix: an updated meta‐analysis of individual patient data. Ultrasound in Obstetrics & Gynecology 2017;49(3):303‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardein J. Congenital abnormalities and hormones. Teratology 1980;22:251. [DOI] [PubMed] [Google Scholar]
- Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, et al. Classification and pharmacology of progestins. Maturitas 2008;61(1‐2):171‐80. [DOI] [PubMed] [Google Scholar]
- Schuit E, Stock S, Rode L, Rouse DJ, Lim AC, Norman JE, et al. Effectiveness of progestogens to improve perinatal outcome in twin pregnancies: an individual participant data meta‐analysis. BJOG: an international journal of obstetrics and gynaecology 2015;122(1):27‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit DA, Essed GG, deHaan J. Predictive value of uterine contractility and the serum levels of progesterone and oestrogens with regard to preterm labour. Gynecologic and Obstetric Investigation 1984;18(5):252‐63. [DOI] [PubMed] [Google Scholar]
- Smith LK, Manktelow BN, Draper ES, Boyle EM, Johnson SJ, Field DJ. Trends in the incidence and mortality of multiple births by socioeconomic deprivation and maternal age in England: population‐based cohort study. BMJ Open 2014;4:e004514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczyk FZ, Hapgood JP, Winer S, Mishell DR Jr. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocrine Reviews 2013;34(2):171‐208. [10.1210/er.2012‐1008. Epub 2012 Dec 13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley F. Survival and cerebral palsy in low birthweight infants: implications for perinatal care. Paediatric and Perinatal Epidemiology 1992;6(2):298‐310. [DOI] [PubMed] [Google Scholar]
- Stewart LA, Tierney JF, Clarke, M, Cochrane Individual Patient Data Meta‐analysis Methods Group. Chapter 18: Reviews of individual patient data. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Sun X, Ioannidis JP, Agoritsas T, Alba AC, Guyatt G. How to use a subgroup analysis: users' guide to the medical literature. JAMA 2014;311(4):405‐11. [DOI] [PubMed] [Google Scholar]
- Umstad MP, Hale L, Wang YA, Sullivan EA. Multiple deliveries: the reduced impact of in vitro fertilisation in Australia. Australian & New Zealand Journal of Obstetrics & Gynaecology 2013;53(2):158‐64. [DOI] [PubMed] [Google Scholar]
- Varma TR, Morsman J. Evaluation of the use of Proluton‐Depot (hydroxyprogesterone hexanoate) in early pregnancy. International Journal of Gynaecology and Obstetrics 1882;20(1):13‐7. [DOI] [PubMed] [Google Scholar]
- Vedel C, Larsen H, Holmskov A, Andreasen KR, Uldbjerg N, Ramb J, et al. Long‐term effects of prenatal progesterone exposure: neurophysiological development and hospital admissions in twins up to 8 years of age. Ultrasound in Obstetrics & Gynecology 2016;48(3):382‐9. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA 1991;266(1):93‐8. [PubMed] [Google Scholar]
References to other published versions of this review
- Dodd JM, Jones L, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD004947.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd JM, Grivell RM, OBrien CM, Dowswell T, Deussen AR. Prenatal administration of progestogens for preventing preterm birth in women with a multiple pregnancy. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD012024] [DOI] [Google Scholar]


