Abstract
Background
Access to mobile phones continues to increase exponentially globally, outstripping access to fixed telephone lines, fixed computers and the Internet. Mobile phones are an appropriate and effective option for the delivery of smoking cessation support in some contexts. This review updates the evidence on the effectiveness of mobile phone‐based smoking cessation interventions.
Objectives
To determine whether mobile phone‐based smoking cessation interventions increase smoking cessation in people who smoke and want to quit.
Search methods
For the most recent update, we searched the Cochrane Tobacco Addiction Group Specialised Register in April 2015. We also searched the UK Clinical Research Network Portfolio for current projects in the UK, and the ClinicalTrials.gov register for ongoing or recently completed studies. We searched through the reference lists of identified studies and attempted to contact the authors of ongoing studies. We applied no restrictions on language or publication date.
Selection criteria
We included randomised or quasi‐randomised trials. Participants were smokers of any age who wanted to quit. Studies were those examining any type of mobile phone‐based intervention for smoking cessation. This included any intervention aimed at mobile phone users, based around delivery via mobile phone, and using any functions or applications that can be used or sent via a mobile phone.
Data collection and analysis
Review authors extracted information on risk of bias and methodological details using a standardised form. We considered participants who dropped out of the trials or were lost to follow‐up to be smoking. We calculated risk ratios (RR) and 95% confidence intervals (CI) for each included study. Meta‐analysis of the included studies used the Mantel‐Haenszel fixed‐effect method. Where meta‐analysis was not possible, we presented a narrative summary and descriptive statistics.
Main results
This updated search identified 12 studies with six‐month smoking cessation outcomes, including seven studies completed since the previous review. The interventions were predominantly text messaging‐based, although several paired text messaging with in‐person visits or initial assessments. Two studies gave pre‐paid mobile phones to low‐income human immunodeficiency virus (HIV)‐positive populations ‐ one solely for phone counselling, the other also included text messaging. One study used text messages to link to video messages. Control programmes varied widely. Studies were pooled according to outcomes ‐ some providing measures of continuous abstinence or repeated measures of point prevalence; others only providing 7‐day point prevalence abstinence. All 12 studies pooled using their most rigorous 26‐week measures of abstinence provided an RR of 1.67 (95% CI 1.46 to 1.90; I2 = 59%). Six studies verified quitting biochemically at six months (RR 1.83; 95% CI 1.54 to 2.19).
Authors' conclusions
The current evidence supports a beneficial impact of mobile phone‐based smoking cessation interventions on six‐month cessation outcomes. While all studies were good quality, the fact that those studies with biochemical verification of quitting status demonstrated an even higher chance of quitting further supports the positive findings. However, it should be noted that most included studies were of text message interventions in high‐income countries with good tobacco control policies. Therefore, caution should be taken in generalising these results outside of this type of intervention and context.
Keywords: Adult, Humans, Cell Phone, Smoking Cessation, Text Messaging, Counseling, Counseling/methods, Randomized Controlled Trials as Topic
Can programmes delivered by mobile phones help people to stop smoking?
Background
Mobile phones are being used more to support healthy lifestyles. We wanted to know whether they could be used to support people to stop smoking. We reviewed the evidence on the effect of quit smoking programmes delivered by mobile phones to people who want to stop smoking.
Study characteristics
We found 12 studies up to April 2015 that could be included. These studies included 11,885 people who were monitored to see if they managed to quit smoking and if they were still quit six months later.
Key results
When the information from all the studies were combined, smokers who received the support programmes were around 1.7 times more likely to stay quit than smokers who did not receive the programmes (9.3% quit with programmes compared with 5.6% quit with no programmes). Most of the studies were of programmes relying mainly on text messages.
Quality and completeness of the evidence
We are moderately confident in the findings of this review. However, all studies took place in high‐income countries and mainly used text messages, so these results may not hold true in people from poorer countries or with other types of mobile phone programmes. There were no published trials of smartphone 'apps' to help people stop smoking that met the inclusion criteria.
Summary of findings
Summary of findings for the main comparison.
Mobile phone‐based interventions for smoking cessation
| Mobile phone‐based interventions for smoking cessation | ||||||
| Patient or population: people who smoke Setting: mobile phone technology Intervention: mobile phone smoking cessation interventions Comparison: controls | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed quitters without intervention | Estimated quitters with mobile phone interventions | |||||
| 26‐week smoking cessation | Study population | RR 1.67 (1.46 to 1.90) | 11,885 (12 RCTs) | ⊕⊕⊕⊝ Moderate 1 | There was evidence of moderate heterogeneity across the included studies | |
| 56 per 1000 | 93 per 1000 (81 to 106) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 There was evidence of moderate heterogeneity. Sensitivity analyses around potential explanations for heterogeneity did not make substantial differences to the findings.
Background
This is the second update of a review of the evidence on the effectiveness of mobile phone‐delivered smoking cessation support. Since the previous review, the use of mobile phones globally has continued to increase at an exponential rate, far exceeding access to the Internet or fixed telephone lines in many regions. The International Telecommunications Union (ITU) estimated that there were more than seven billion mobile phone subscriptions in 2015; approximately 96.8 per 100 inhabitants, ranging from 120.6/100 in high‐income countries to 91.8/100 in low‐income countries. Access to mobile broadband is also growing fast, with an estimated 47% of the world's population subscribing to mobile broadband, compared with the 29% who have fixed broadband subscriptions (ITU 2015). The smartphone (mobile phone with a computer operating system) is fast becoming the computer of choice, or at least the most accessible computer, in many countries. It is reported that about 45% of global mobile phone subscriptions are associated with smartphones and that, with 75% of new sales of mobile phones being smartphones, this will continue to increase (Ericsson 2015).
Mobile phones are increasingly useful in health information and healthcare delivery around the world. Text messaging has been used for health service appointment reminders, preventive activities and medication adherence (Free 2013). Mobile phones have also been used in monitoring and the self management of chronic disorders such as diabetes (Holtz 2012). In addition, smartphone applications for health and wellness are proliferating, although there is little published research in this area (Abroms 2011).
Smoking cessation services internationally are using mobile phones to deliver support, particularly as adjuncts to other services. In 2014, the UK's National Health Service rolled out text messaging integrated into routine clinical practice and in 2013 almost half of US quitlines offered text messaging in addition to phone counselling services (Abroms 2015). The potential benefits of mobile phone‐based smoking cessation interventions include: the ease of use anywhere at anytime; cost‐effective delivery and scalability to large populations, regardless of location; the ability to tailor messages to key user characteristics (such as age, sex, ethnicity); the ability to send time‐sensitive messages with an 'always on' device; the provision of content that can distract the user from cravings; and the ability to link the user with others for social support.
It is likely that the use of mobile phones for smoking cessation will continue to grow as they become even more ubiquitous and as technological advances increase the number of applications and functions available. While mobile technology continues to change, it is important to review the body of research on interventions using mobile phones regularly to support people to stop smoking. This is particularly so, given the exponential increase in access to mobile phones in high‐income countries (ITU 2015), where the burden of tobacco‐related morbidity and mortality is predicted to be greatest (Jha 2014).
Objectives
To determine whether mobile phone‐based smoking cessation interventions increase smoking cessation in people who smoke and want to quit.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised trials.
Types of participants
Any smokers who want to quit smoking.
Types of interventions
We included studies that examined any type of mobile phone‐based intervention for smoking cessation. This included any intervention aimed at mobile phone users, based around delivery via mobile phone, and using any functions or applications that could be used or sent via a mobile phone. We excluded trials where mobile phones were seen as an adjunct to face‐to‐face or Internet‐based programmes, such as to remind participants of appointments or where the effects of the various components of a multi‐faceted programme could not be separated.
Types of outcome measures
The primary outcome was smoking abstinence at six months or longer from the start of the intervention. When available, we preferred sustained abstinence to point prevalence abstinence and biochemically validated results to self report.
Search methods for identification of studies
For the present update of the review, we searched the Specialised Register of the Cochrane Tobacco Addiction Review Group in April 2015 using the terms 'mobile phone', 'cell phone', 'txt', 'pxt', 'sms', or 'mms' in the title, abstract or keyword fields. The Specialised Register includes reports of possible controlled trials of smoking cessation interventions identified from sensitive searches of databases. At the time of the search, the Register included the results of searches of the Cochrane Central Register of Controlled trials (CENTRAL; 2015 Issue 3); MEDLINE (via Ovid, to update 13 March 2015), EMBASE (via Ovid, to update week 12 2015) and PsycINFO (via Ovid; to 23 March 2015). See the Cochrane Tobacco Addiction Module in The Cochrane Library for full search strategies and a list of other resources searched. We also searched the UK Clinical Research Network Portfolio for current projects in the UK and the US ClinicalTrials.gov register for ongoing or recently completed studies. We searched through the reference lists of identified studies and attempted to contact the authors of ongoing studies.
We placed no restrictions on language or publication date.
Data collection and analysis
Selection of studies
The Tobacco Addiction Group Trial Search Co‐ordinator pre‐screened the titles and abstracts of records identified from the Register search to exclude reports that had no relevance to the topic and to provide a list of potentially relevant citations. Two review authors (RW, YG) identified potentially eligible studies and obtained full‐text copies. The same review authors independently selected studies to be included against the criteria listed above and resolved any disagreements by discussion, by contacting study authors, or by referring to a third review author (HM) to act as arbiter where required. We recorded reasons for exclusion of studies.
Data extraction and management
We extracted the following methodological details from the included study reports and presented them in the Characteristics of included studies table. Two review authors (RW, YG) independently extracted data using a standardised form. Articles were not blinded for authors, institution and journal, because the review authors who performed the quality assessment were familiar with the literature. If an article did not contain enough information on methodological criteria, that is, if one or more of the risk of bias criteria were scored 'unclear', we contacted the trial authors for additional information.
Characteristics of study participants
Definition of smoking status used in the study.
Age and any other recorded characteristics of study participants.
Inclusion criteria.
Exclusion criteria.
Interventions used
Type and 'dose' of mobile phone intervention used.
Type of control used.
Duration of intervention.
Length of follow‐up.
Assessment of risk of bias in included studies
We also extracted information on the following criteria from included studies.
Method of randomisation.
Presence or absence of blinding to treatment allocation (non‐blinded/open label, single blind, double blind, triple blind).
Quality of allocation concealment (adequate, unclear, inadequate, not used).
Number of participants randomised, excluded and lost to follow‐up.
Whether an intention‐to‐treat (ITT) analysis was carried out.
Whether a power calculation was reported.
Duration, timing and location of the study.
Measures of treatment effect
We recorded the information below where available.
Definition of smoking cessation as used in the study.
Smoking cessation rates at four weeks (self reported abstinence or biochemically verified abstinence, or both).
Smoking cessation at rates at six months (self reported abstinence or biochemically verified abstinence, or both).
Smoking cessation rates at final follow‐up (if follow‐up greater than six months and where these data were available).
We calculated risk ratios (RR) and 95% confidence intervals (CI) for each outcome for each included study.
Dealing with missing data
We regarded those trial participants who dropped out of the trials or were lost to follow‐up as continuing to smoke according to the Cochrane Tobacco Group's guidelines.
Data synthesis
We conducted a meta‐analysis of the included studies, using the Mantel‐Haenszel fixed‐effect method to pool RRs. This pooling method was chosen given the undesirable weighting properties of random‐effects models when small studies are present (Peto 2013). In the presence of substantial statistical heterogeneity as assessed by the I2 statistic (Higgins 2003), we planned to evaluate possible explanations for this heterogeneity using subgroup analyses.
Results
Description of studies
Results of the search
The previous review (published in 2012) included five studies from the 68 initially identified (Borland 2013; Free 2009; Free 2011; Rodgers 2005; Whittaker 2011). For this update of our review, the literature search identified 37 new studies. Many were unrelated and were immediately excluded, leaving 21 potentially relevant papers. Some of these were not focused around delivery via mobile phone (Fraser 2014; Mehring 2014; Peng 2013; Skov‐Ettrup 2013; Stanczyk 2014); one was not randomised (Pechmann 2015); one was a pilot study with only two‐month follow‐up (Bricker 2014); four only followed participants up to three‐months (Buller 2014; Mehring 2014; Shi 2013; Vilaplana 2014); one was investigating gradual reduction of smoking in pregnant women, rather than quitting (Pollak 2013); and one compared tailored with untailored text messages (Skov‐Ettrup 2014) (see Discussion).
Approaching authors of ongoing studies revealed several that were in the process of being finalised or submitted for publication. We were able to get data directly from the authors of five studies ‐ two of which we included (Ferguson 2015; Shelley 2015); however, one study was not eligible, with only three months' follow‐up (Jordan 2015), and a further two were focused on cardiovascular disease secondary prevention rather than smoking cessation (Chow 2012; Dale 2014).
Details of excluded and ongoing studies can be found in the Characteristics of excluded studies and Characteristics of ongoing studies tables.
In this update, our literature search identified seven new randomised controlled trials (RCT) with six‐month outcomes (Abroms 2014; Bock 2013; Ferguson 2015; Gritz 2013; Haug 2013; Naughton 2014; Shelley 2015).
Included studies
Intervention programmes
Almost all of the included trials used text messaging (SMS) as a central component of the intervention. A major exception to this was Gritz 2013 who gave pre‐paid mobile phones to participants, which were used to provide cognitive behavioural and motivational counselling, and access to a reactive telephone helpline. The intervention was based on US guidelines around cognitive‐behavioural and motivational interviewing techniques over the mobile phone (Fiore 2008). Shelley 2015 also gave mobile phones to participants in a three‐arm trial comparing standard pharmacotherapy, with pharmacotherapy plus text messages, and with pharmacotherapy plus text messages and phone counselling. We included this as the pre‐paid mobile phone was specifically provided as part of the study to facilitate the interventions (indicating it could not have been delivered without the mobile phone), and this was very similar in concept to the Gritz 2013 study. Whittaker 2011 sent SMS containing links to theoretically driven video messages from 'ordinary' role models coping with quitting.
Several studies paired SMS with in‐person visits or assessments (Bock 2013; Gritz 2013, Haug 2013; Naughton 2014; Shelley 2015). The remainder were purely text messaging interventions (Abroms 2014; Borland 2013; Ferguson 2015; Free 2009; Free 2011; Rodgers 2005; Whittaker 2011).
Many of the studies stated that their interventions were theory based (Abroms 2014; Bock 2013; Gritz 2013; Naughton 2014; Whittaker 2011; Haug 2009). In Haug 2013, the intervention was said to be based on cognitive behavioural components, stages of change and the social norms approach, with an online assessment that allowed tailoring based on stage of change and other baseline data.
Bock 2013 conducted an initial counselling session then randomised participants to an eight‐week intervention based on national guidelines, social cognitive theory and the stages of change. The programme was tailored to stage of readiness, starting with either 'not ready' or 'prepared to quit', that could change according to text message questions and answers. There were also on‐demand components.
The text messaging intervention in Rodgers 2005 was developed in New Zealand, and later adapted for the UK and tested in a pilot study (Free 2009), and then a large randomised controlled study (Free 2011). Messages commenced prior to quitting and were based on effective brief interventions including quitting advice and motivational messages. Interactive components included the ability to text in for more support (in the instance of cravings or lapses) and an optional Quit Buddy in Rodgers 2005. A cost‐effectiveness analysis was also conducted as part of the Free 2011 trial (Guerriero 2013). This showed that the cost of text‐based support per 1000 enrolled smokers was GBP278 per quitter. When the future health service costs saved (as a result of reduced smoking) were included, text‐based support was considered to be cost saving, with 0.5 quality‐adjusted life years (QALYs) gained per quitter.
In Borland 2013, participants received offers of support via a personalised tailored Internet programme, an SMS programme, both programmes, a choice of all three or a minimal control. The SMS programme provided advice on strategy and motivational messages relevant to their stage of readiness for quitting, plus messages on demand. For the purposes of meta‐analyses, we compared the SMS group with the control group.
In Naughton 2014, the intervention group received the 'usual care' received by the control group (described below), as well as a four‐page tailored advice report and a tailored theoretically based SMS message programme for 90 days, with interactive components (i.e. they could text for help when in difficult situations, or if they had lapsed).
Control programmes
The control programmes across the studies varied from nothing (Haug 2013), to fortnightly (Free 2009; Free 2011; Rodgers 2005; Whittaker 2011) or daily (Bock 2013) text messages, written/Internet untailored materials (Abroms 2014; Ferguson 2015; Gritz 2013), and untailored messages, to standard cessation advice and treatment (Naughton 2014; Shelley 2015).
The control group of Naughton 2014 received support from practice staff who had received smoking cessation training. This support included setting a quit date within 14 days, a prescription for pharmacotherapy, the opportunity for multiple follow‐up visits and routine measurement of carbon monoxide (CO) in expired breath.
Context and participants
The settings and recruitment methods, and therefore the participants, varied considerably across studies. Two studies targeted young people (Haug 2009; Whittaker 2011). Bock 2013 found usual in‐person recruitment methods slow and shifted to online recruitment methods during the study. Borland 2013 and Abroms 2014 also used online recruitment via Internet advertisements. In Abroms 2014, this initially led to some fraudulent participants who were discovered and disqualified, and extra procedures were put in place to prevent this from happening again.
Naughton 2014 was set in primary care practices in the UK with trained smoking cessation advisors providing smoking cessation advice. The Gritz 2013 study recruited in a human immunodeficiency virus (HIV)‐positive, multi‐ethnic, low‐income population. Participants in this study were 76% African American, 79% unemployed, with high levels of depression and other alcohol/drug problems. Shelley 2015 similarly recruited from urban HIV clinics in a different region of the US.
Haug 2013 recruited in vocational schools and differed from the other studies by allowing the inclusion of occasional smokers (at least four cigarettes in the past month or at least one in the preceding week). All other studies used a definition related to daily smoking.
Where recorded, participants in most of the studies had similar degrees of nicotine dependence, although in Whittaker 2011, the 'Hooked on Nicotine Checklist' mean scores of 8 indicated a more highly addicted group (Wellman 2006).
Participants in three trials were younger (mean age 18.2 years in Haug 2013, 22 years in Rodgers 2005, and 27 years in Whittaker 2011) than in the other trials (means ranged from 30.7 years in Bock 2013 to 44.8 years in Gritz 2013). Most trials had slightly more women than men, with the exception of Gritz 2013 with 70% male participants.
The Characteristics of included studies table gives further details on the included studies.
Risk of bias in included studies
Randomisation was adequate in all trials. Haug 2013 was the only cluster randomised trial, and recruited via vocational schools. The vocational school class was the unit of randomisation, stratified by school and with randomly permuted blocks of four cases.
In all trials except for Borland 2013, participants were not blinded to treatment assignment, although research staff were blind to allocation at follow‐up data collection. As seen in Figure 1, all trials but Rodgers 2005 were at low risk of bias in all domains.
Figure 1.
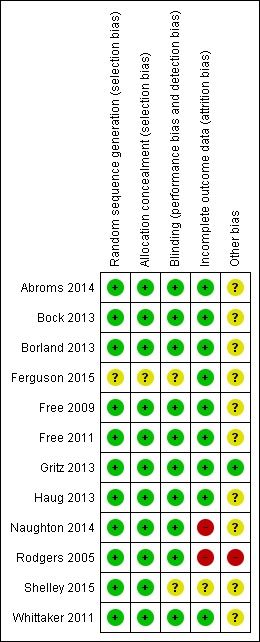
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
In Rodgers 2005, incentives for providing final follow‐up data differed between groups ‐ one month of free text messaging was received by the control group on completion of follow‐up whereas the intervention group had already received their month of free text messaging from their Quit Day and did not receive a further incentive at follow‐up. This may have caused the differential loss to follow‐up seen at six months (69.4% providing data at six months in the active group compared with 79% in the control group), which in turn may have affected the long‐term results of this study. The authors also suggested that some participants in the control group may have thought their month of free text messaging depended on reporting quitting. This could account for an unexpected increase in control group participants reporting quitting from six weeks (109 participants) to six months (202 participants reporting no smoking in the past seven days). Both of these elements may have potentially led to an underestimation of the effect of the intervention.
Two papers stated that they had difficulty recruiting their target sample size (Haug 2013; Whittaker 2011). Both targeted a younger population and, as a result, did not recruit their target sample size.
All studies presented long‐term outcomes at six months, either as self reported point prevalence (no smoking in past seven to 28 days) or repeated measures of point prevalence abstinence (Abroms 2014; Bock 2013; Gritz 2013) (or both), or continuous abstinence, defined as no smoking since quit day, but with up to three lapses (Rodgers 2005), or five cigarettes (Free 2009; Free 2011; Whittaker 2011), allowed.
Seven of the trials sought biochemical verification of self reported six‐month abstinence with salivary cotinine (Abroms 2014; Free 2009; Free 2011; Gritz 2013; Rodgers 2005) or expired CO (Ferguson 2015; Shelley 2015). Those reporting response rates for verification varied from 39% in Rodgers 2005 to 92% in Free 2011, with little difference between intervention and control groups within studies. Of those participants that were tested, 76% in Abroms 2014 and 72% in Free 2011 were verified as abstinent. The proportion was lower in Rodgers 2005 (55% in the intervention group and 33.3% in the control group) and Free 2009 (53% (8/15) in the intervention group and 40% (6/15) in the control group). Naughton 2014 also verified quitting but only at four‐week and not at six‐month follow‐up.
All trials conducted ITT analyses, where participants with missing data were assumed to be smokers. Any differential loss to follow‐up by group can create potential bias when all are inferred to be smokers. Sensitivity analyses were used to test the effects of other potential reasons for drop out. Free 2011 and Haug 2013 used multiple imputation, by using the observed predictors of outcomes and the predictors of loss to follow‐up to impute missing outcome data.
A further potential source of bias could be any differential use of other cessation interventions. In Borland 2013, where use of the studied interventions was low, it is possible that participants were motivated to try other cessation programmes.
The Characteristics of included studies table provides details of risk of bias judgements for each domain of each included study. Figure 1 illustrates judgements for each included study.
Effects of interventions
See: Table 1
Standard ITT analyses are presented here, with all participants lost to follow‐up counted as continuing smokers. This may differ from how results were presented by the individual studies due to variations in primary outcomes and in analytic methods used. For example, Free 2011 used multiple imputation by chained equations, and the Bock 2013 paper reported a significant main effect of a two (treatment groups) x three (time points) generalised estimating equations (GEE) repeated measures analysis with higher odds of point prevalence abstinence compared with a control group (odds ratio (OR) 4.52, 95% CI 1.24 to 16.53). However, individual time point comparisons did not show significant differences.
Although Naughton 2014 did not find a significant difference in their primary outcome (of two‐week point prevalence) at eight weeks (45.2% with intervention programme versus 40.3% with control programme; OR 1.22, 95% CI 0.88 to 1.69), by six months there was an effect on self reported prolonged abstinence (15.1% with intervention programme versus 8.9% with control intervention; OR 1.81, 95% CI 1.09 to 3.01) and using a continuous abstinence measure that included outcomes at four weeks, eight weeks and six months (11.4% with intervention programme versus 6.3% with control programme; OR 1.92, 95% CI 1.07 to 3.45).
We undertook meta‐analyses on the 12 included studies (Abroms 2014; Bock 2013; Borland 2013; Ferguson 2015; Free 2009; Free 2011; Gritz 2013; Haug 2013; Naughton 2014; Rodgers 2005; Shelley 2015; Whittaker 2011). First, we pooled all 12 studies using their most rigorous 26‐week measures of abstinence, giving an RR of 1.67 (95% CI 1.46 to 1.90; 12 studies; 11,885 participants; I2 = 59%) (Analysis 1.1; Figure 2). Both the Free 2009 pilot study and the Whittaker 2011 study were underpowered and individually did not find an effect. When we removed these two studies from the analysis, the results produced an RR of 1.81 (95% CI 1.57 to 2.09; I2 = 32%; 10 studies; 11,459 participants). In addition, we carried out this main analysis, removing Haug 2013, to see if the result was sensitive to the inclusion of this cluster randomised controlled trial. However, this had very little impact on the result (RR 1.69, 95% CI 1.47 to 1.94; I2 = 62%; 11 studies; 11,130 participants). Due to the amount of heterogeneity detected, we also made a post‐hoc decision to re‐calculate the main analysis using a random‐effects model. This resulted in an RR of 1.42 (95% CI 1.11 to 1.83; 12 studies; 11,885 participants), and therefore none of the adjustments had an impact on the interpretation of the results, and none of the sensitivity analyses accounted for the majority of the heterogeneity.
Analysis 1.1.
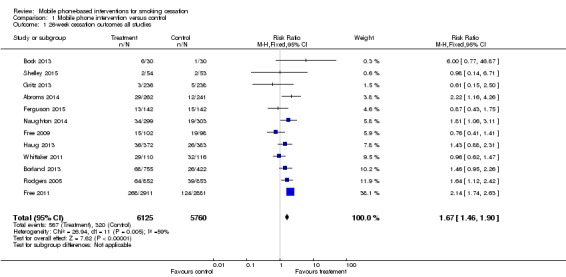
Comparison 1 Mobile phone intervention versus control, Outcome 1 26‐week cessation outcomes all studies.
Figure 2.
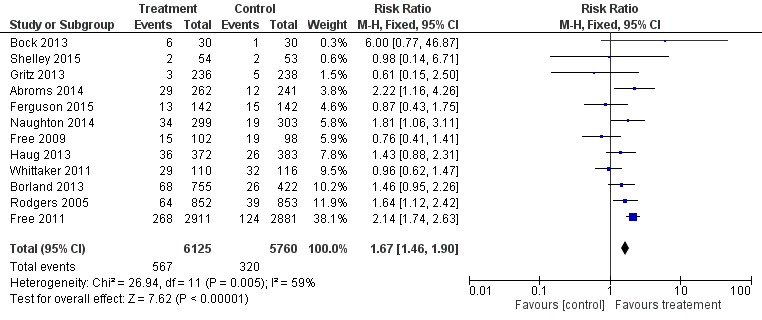
Forest plot of comparison: 1 Mobile phone intervention v ersus control, outcome: 1.1 26‐week cessation outcomes all studies.
Subgroup analyses
We then grouped studies according to definition of abstinence used (continuous abstinence, point prevalence, biochemically verified or not) and by differences in intervention (text messaging alone, text messaging plus some form of personal contact and phone counselling).
Abstinence
Continuous abstinence
We pooled data from the eight studies reporting continuous abstinence, with those reporting repeated measures of point prevalence abstinence used as a proxy for continuous abstinence (Abroms 2014; Borland 2013; Free 2009; Free 2011; Gritz 2013; Naughton 2014; Rodgers 2005; Whittaker 2011). This gave an RR of 1.72 (95% CI 1.50 to 1.98; I2 = 68%; eight studies; 10,679 participants), with moderate heterogeneity.
Point prevalence
We pooled studies presenting point prevalence abstinence measures at six months separately (Abroms 2014; Bock 2013; Ferguson 2015; Gritz 2013; Haug 2013; Rodgers 2005; Shelley 2015). This analysis showed a marginally statistically significant effect of intervention programmes over control programmes (RR 1.18, 95% CI 1.03 to 1.35; I2 = 24%; seven studies; 3,888 participants) (Analysis 1.3).
Analysis 1.3.
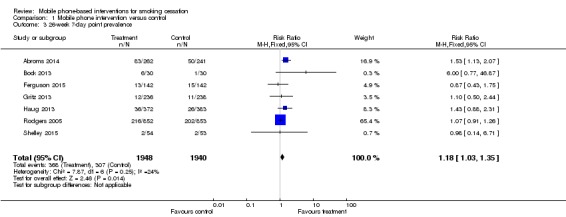
Comparison 1 Mobile phone intervention versus control, Outcome 3 26‐week 7‐day point prevalence.
Bock 2013 was a small study early in the acceptability phase of testing, with a consequently wide CI. Ferguson 2015, Gritz 2013, and Shelley 2015 had little difference between groups. Haug 2013 and Rodgers 2005 favoured the intervention but without statistical significance.
Biochemically verified abstinence
We pooled studies that biochemically verified quitting separately at 26 weeks (Abroms 2014; Ferguson 2015; Free 2009; Free 2011; Gritz 2013; Shelley 2015). This resulted in an RR of 1.83 (95% CI 1.54 to 2.19; I2 = 71%; six studies; 7,360 participants) (Analysis 1.4; Figure 3).
Analysis 1.4.
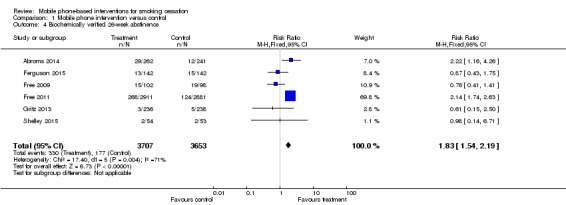
Comparison 1 Mobile phone intervention versus control, Outcome 4 Biochemically verified 26‐week abstinence.
Figure 3.
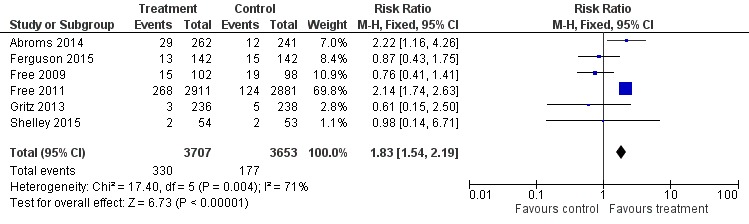
Forest plot of comparison: 1 Mobile phone intervention versus control; outcome: 1.4 26‐week biochemically verified cessation outcomes (six studies).
Differences in interventions
Text message alone interventions
When we removed studies with interventions that included in‐person contacts from the main analysis (all studies 26 week outcomes) in order to examine only those interventions that used text messaging only, there was no difference in the results (RR 1.69, 95% CI 1.46 to 1.95, I2 = 74%; seven studies; 9887 participants) (Analysis 2.1) (Abroms 2014; Borland 2013; Ferguson 2015; Free 2009; Free 2011; Rodgers 2005; Whittaker 2011).
Analysis 2.1.
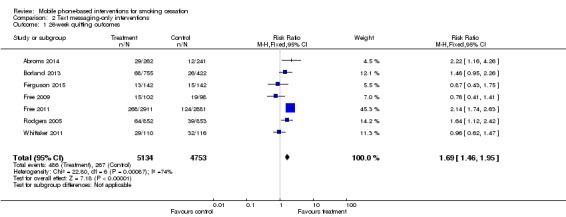
Comparison 2 Text messaging‐only interventions, Outcome 1 26‐week quitting outcomes.
Active versus minimal control
We carried out a sensitivity analysis on the main analysis of all studies' 26 week outcomes (12 studies). We removed studies with more active control programmes (of standard cessation practice ‐ Naughton 2014 and Shelley 2015); however, again this made minimal difference to the overall result of the pooled analysis (RR 1.66, 95% CI 1.45 to 1.91, I2 = 66%; 10 studies; 11,176 participants).
Discussion
Summary of main results
We included seven further studies of mobile phone smoking cessation interventions meeting our inclusion criteria since the previous version of this review, giving a total of 12 included studies. The first systematic review in 2009 showed short‐term benefits, but found no long‐term effects, of mobile phone‐only interventions. The second update, with five studies, showed an overall long‐term benefit of mobile phone interventions for smoking cessation, though there was a high level of statistical heterogeneity in the pooled result. This update of 12 studies also suggested a positive effect of mobile phone interventions on smoking cessation at six months in comparison with 'usual care', although there was still significant unexplained heterogeneity. Our findings appeared to have been strengthened by the highest quality studies, that is, those studies using stricter outcome definitions, including biochemical verification. The benefits were large, and similar in size to those seen using of other effective treatments such as nicotine replacement therapy (Stead 2012).
Overall completeness and applicability of evidence
Our review currently includes 12 studies with 11,885 participants. There has been a steady increase in the number of studies eligible for this review over time. All of the studies included were conducted in high‐income countries with mature tobacco control policies; although two studies specifically recruited from low‐income populations (Gritz 2013; Shelley 2015). This means that it is possible that text messaging interventions may not be appropriate or effective in other contexts, or alternatively they may be even more effective in those settings where cessation information and support is relatively new. Clearly there is a major gap in the current evidence.
We also found 25 ongoing studies. As the body of evidence supporting the effectiveness of text messaging in high‐income countries grows, it is hoped that some of these are being conducted in other contexts or with different populations. It is interesting to note that there were no trials of smartphone 'app'‐based interventions that met our eligibility criteria despite the proliferation of available cessation apps. In 2011, one review of available smoking cessation apps found them to be lacking in adherence to cessation guidelines or theory (Abroms 2011).
There is as yet little research into the different functional components, message content, mediators and moderators of mobile phone programmes, in order to learn what type of programmes work best for whom. Skov‐Ettrup 2014 conducted a study, based on an ongoing online programme, of untailored messages compared with tailored messages. Participants were randomised first and then offered text messages on top of the online programme. Overall, there was no significant difference between groups in long‐term quit rates; however, when restricted to only those who chose to receive the text messages, there was an effect of tailored over untailored messages (OR 1.45, 95% CI 1.01 to 2.08; 1,809 participants). As the tailored messages were also more frequent, it is not clear whether this effect was due to intensity, tailoring or both.
Quality of the evidence
The studies included varied in size from 60 to 5800 participants, but were generally all of a reasonable quality with a low risk of bias. They were all randomised controlled trials, with one cluster randomised trial (the result of analyses were not sensitive to removal if this study), and with similar outcomes measures. Substantial heterogeneity was detected across analyses; however, a post hoc decision to conduct the main analysis using a random‐effects model resulted in no difference in the interpretation of findings. Half of the included studies attempted to biochemically verify self reported quitting outcomes. When we pooled these studies separately, the result was similar, if not more strongly in favour of the intervention.
Two studies reported that they were unable to recruit their target sample sizes, both of which were targeting a younger population (Haug 2013; Whittaker 2011). Both studies found no statistically significant effect of the intervention, but were reported to be slightly underpowered. More research is needed in young adults to determine the acceptability and effectiveness of mobile phone based interventions. Overall, we are moderately confident in the main effect estimate generated through our analysis (Table 1).
Authors' conclusions
At least in high‐income countries with existing tobacco control policies, media and education, text message‐based smoking cessation interventions, either alone or in combination with face‐to‐face assessments or online programmes, appear to be a helpful option to offer to quitters. It is not yet clear whether this translates to other contexts, such as low‐ or middle‐income countries, and younger people; however, many are proceeding to implement such programmes anyway. High‐quality evaluations of these implemented programmes will be valuable.
Research into the effectiveness of mobile phone‐based cessation programmes for young people, in low‐ and middle‐income countries and countries with little active tobacco control policy, is still required. There is also a lack of research into the effectiveness of individual components of programmes, in order to determine what works best for whom. There does not appear to be any rigorous trials of smartphone‐based programmes published as yet. Due to their widespread availability, it would be useful to know if the broader functionality available in apps can be harnessed effectively to support cessation.
Acknowledgements
We acknowledge the assistance of the Cochrane Tobacco Addiction Review Group Editorial base in the preparation of this review.
Appendices
Appendix 1. Tobacco Addiction Group Register search strategy
Searched in Cochrane Register of Studies (CRS) 8 April 2015
#1 Cellular Phone:MH #2 Cell Phones:MH #3 MeSH DESCRIPTOR Cellular Phone #4 MESH DESCRIPTOR Cell Phones #5 MeSH DESCRIPTOR Text Messaging #6 (mobile NEAR2 (phone* OR telephon*)):TI,AB,MH,EMT,XKY,KY,KW #7 (cell* NEAR2 (phone* OR telephon*)):TI,AB,MH,EMT,XKY,KY,KW #8 smartphone*:TI,AB,MH,EMT,XKY,KY,KW #9 text messag*:TI,AB,MH,EMT,XKY,KY,KW #10 (txt OR pxt OR mms OR sms):TI,AB,MH,EMT,XKY,KY,KW #11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
Data and analyses
Comparison 1.
Mobile phone intervention versus control
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 26‐week cessation outcomes all studies | 12 | 11885 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.46, 1.90] |
| 2 26‐week continuous abstinence | 8 | 10679 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.50, 1.98] |
| 3 26‐week 7‐day point prevalence | 7 | 3888 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.03, 1.35] |
| 4 Biochemically verified 26‐week abstinence | 6 | 7360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.54, 2.19] |
Analysis 1.2.
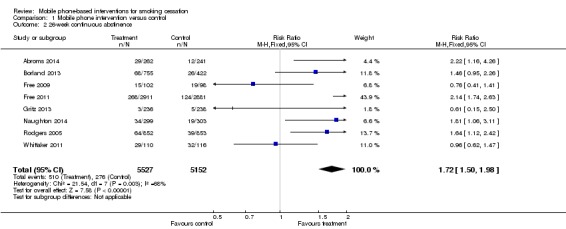
Comparison 1 Mobile phone intervention versus control, Outcome 2 26‐week continuous abstinence.
Comparison 2.
Text messaging‐only interventions
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 26‐week quitting outcomes | 7 | 9887 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.46, 1.95] |
Comparison 3.
Text messaging plus face‐to‐face interventions
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Text message plus face‐to‐face interventions | 5 | 1995 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.12, 2.11] |
Analysis 3.1.
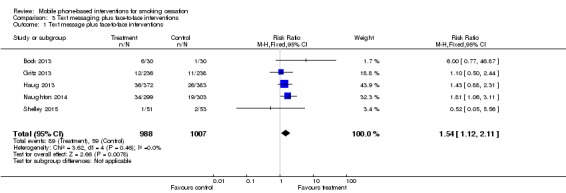
Comparison 3 Text messaging plus face‐to‐face interventions, Outcome 1 Text message plus face‐to‐face interventions.
What's new
| Date | Event | Description |
|---|---|---|
| 1 October 2015 | New search has been performed | Updated 2015, seven new studies added and text updated |
| 1 October 2012 | New search has been performed | Updated 2012, three new studies added and text updated |
| 1 October 2012 | New citation required and conclusions have changed | Three new included studies added, meta‐analysis conducted, conclusions changed (pooled effect statistically significant) |
History
| Date | Event | Description |
|---|---|---|
| 15 July 2008 | Amended | Converted to new review format. |
| 5 September 2006 | New citation required and conclusions have changed | Substantive amendment |
Differences between protocol and review
We have followed the change of policy of the Cochrane Tobacco Addiction Group, and now report our findings using Mantel‐Haenszel fixed‐effect risk ratios rather than as odds ratios. Previously, we had not pooled studies in the presence of substantial statistical heterogeneity as assessed by the I2 statistic, but in this update of the review, we report pooled results due to the homogenous nature of the included studies in terms of design, intervention and outcome.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT in US | |
| Participants | 503 participants aged ≥ 18 years recruited via online advertisements when Internet searching for 'quitting smoking'. 34% men, mean age of 35.7 years, and mean FTND score of 5.3. Eligibility criteria included smoking ≥ 5 cigarettes/day, having an e‐mail address, a mobile phone number with an unlimited SMS plan, an interest in quitting smoking in the next month and not pregnant |
|
| Interventions | Automated bidirectional text messages, personalisation and interactive components, automated unidirectional emails and an Internet portal ‐ has been revised since pilot study Text2Quit: a 6‐month SMS programme with the first 3 months offering both outgoing messages about quitting smoking and on‐demand help using keywords. Outgoing messages peaked in the period just prior to and following the quit date. Participants received 5 messages on their quit date and approximately 2/day in the week after the quit date. Frequency declined in the subsequent weeks to approximately 3/week for the next 2 months and then < 1/week for the remaining portion of the outgoing phase. After the outgoing messages stopped, participants could still text at any time for help through keywords ‐ to reset a quit date (DATE), get help with a craving through a tip or a trivia game (CRAVE); get a summary of their quitting statistics (STATS) and to indicate that they had smoked (SMOKED). The SMS were supplemented by a personalised Internet portal (text2quit.com) and e‐mails Control: sent an Internet link to Smokefree.gov, a leading website with quitting smoking information run by the National Cancer Institute. Later, once the website began to offer an SMS programme, a guidebook on quitting smoking was offered via an Internet link that led participants to a document containing similar advice and information as Smokefree.gov |
|
| Outcomes | Primary outcome: biochemically confirmed repeated point prevalence abstinence, defined as a self report of no smoking in the past 30 days on the 3‐ and 6‐month surveys and a cotinine level ≤ 15 ng/mL at 6 months Secondary outcomes: 7‐ and 30‐day abstinence at 1‐, 3‐, and 6‐month follow‐up and biochemically confirmed abstinence at the 6‐month follow‐up |
|
| Notes | Enrolment procedures were modified after a group of participants was discovered to be fraudulent and disqualified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised via computer system |
| Allocation concealment (selection bias) | Low risk | Recruited and randomised online |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants completed questionnaires online |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 52 in control and 70 in intervention group lost to follow‐up at 6 months but ITT analysis presented |
| Other bias | Unclear risk | 13 participants from the control group (5%) indicated on their 3‐month survey that they had used a texting programme for smoking cessation since enrolling in the study Saliva was collected by mail for participants reporting abstinence at 6 months. There was a low response rate (64.7%) among participants eligible for providing a saliva sample for biochemical verification although this did not differ across the groups. Of those participants who provided a sample, 21 (24.4%) had high levels of cotinine and were coded as smokers in analyses |
| Methods | Pilot RCT in US | |
| Participants | Adults aged ≥ 18 years of age current daily smokers recruited online and eligible if interested in quitting smoking in the next 30 days, with a mobile phone with SMS text messaging capability, and using SMS text messaging at least once monthly. 43% men, mean age 30.7 years (range 18‐52 years), and mean FTND of 4.9 (moderate level of dependence on nicotine) | |
| Interventions | All participants received a single individual 30‐minute smoking cessation counselling session TXT‐2‐Quit: an 8‐week programme with 1‐4 text messages/day (depending on quit stage). Smoking cessation messages were tailored to the participant's stage of smoking cessation, with specialised messages provided on‐demand based on user requests for additional support, and an optional peer‐to‐peer social support network Control: an 8‐week programme of daily non‐smoking related text messages |
|
| Outcomes | Primary outcome: 7‐day point‐prevalence abstinence using a 2 (treatment groups) × 3 (time points) repeated measures design across 3 time points: 8 weeks, 3 months and 6 months; showed a significant main effect for treatment group (P value = 0.02) with higher odds of quitting in the intervention group compared with the control group (odds ratio 4.52, 95% CI 1.24 to 16.53). Although there was no individual time point difference between groups at 6 months (20% with intervention programme vs. 3.6% with control programme; odds ratio 6.75, 95% CI 0.76 to 60.15) it was likely to have been affected by reduced statistical power. Secondary outcome: 24‐hour point prevalence abstinence at 8 weeks, 3 months and 6 months |
|
| Notes | Designed as a small study to develop and provide initial testing of the system During the 6 months' follow‐up, there was a significant improvement in Mood and Physical Symptoms Scale (MPSS) mood symptoms of nicotine withdrawal (P value = 0.03) among the TXT‐2‐Quit participants as compared with the control participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation via computerised random number generator |
| Allocation concealment (selection bias) | Low risk | Assignments in a sealed envelope delivered after completion of the baseline data collection |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants completed questionnaires online. Research assistants and counsellors were blind to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants in control group appeared to be missing at 6 months; however, ITT analysis presented |
| Other bias | Unclear risk | Self report and not biochemically verified |
| Methods | RCT in Australia | |
| Participants | 3530 participants in total (control = 422; onQ = 756; QuitCoach = 809; both = 785; participant choice = 758). 60% female, mean age 42.1 years, and 87.4% were currently smoking a mean of 16.9 cigarettes/day | |
| Interventions | onQ programme: provides a stream of SMS messages to the person that mixes snippets of advice on strategy and motivational messages. The user can interact with it by indicating their stage of quitting so that appropriate stage‐specific messages are sent, and once quit can also call up messages in crisis situations QuitCoach: a personalised, automated tailored cessation programme delivered via the Internet. It generates letters of advice based on answers to an assessment questionnaire, including suggestions about strategy and motivational messages. It also provides further untailored supplementary resources Control: brief information on Internet‐ and phone‐based assistance available in Australia |
|
| Outcomes | Self reported 6‐month sustained abstinence at 7‐month follow‐up Intention‐to‐quit analysis and sensitivity analysis around treatment of missing data |
|
| Notes | Only onQ and control arms used in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generator embedded within the baseline survey |
| Allocation concealment (selection bias) | Low risk | Not a typical RCT as participants were enrolled in a study described to them as being about "the effectiveness of Internet and telephone‐based resources in helping smokers quit", and were only then randomised to a condition that they were offered with no obligation to use |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Not a typical RCT as participants were enrolled in a study described to them as being about "the effectiveness of Internet and telephone‐based resources in helping smokers quit", and were only then randomised to a condition that they were offered with no obligation to use |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 475 (13% total) with similar numbers across groups (control = 66, onQ = 89, QuitCoach = 104, both = 121, participant choice = 95); 2 excluded as reported to have died at 7‐month follow‐up |
| Other bias | Unclear risk | Nothing else described |
| Methods | RCT in Australia | |
| Participants | Participants recruited via advertisements in traditional and social media in Tasmania, Australia. 49% male, mean age 42.1 years and mean FTND of 4.8, and with a high motivation to quit (≥ 75 on 100‐point scale) Eligibility criteria included: daily smokers of > 10 cigarettes/day for past 3 years |
|
| Interventions | Intervention: Self‐help Quit booklet plus 4 or 5 randomly timed text messages/day containing quit smoking advice and encouragement tailored to participants' current quit status (preparing to quit, first week of the quit attempt, second week of attempt etc.). Participants could request additional text messages Control: Self‐help Quit booklet containing tips for quitting and cognitive and behavioural coping mechanisms Study visit days: ‐11 (enrolment/randomisation), ‐7 (commence study group), 0 (QD), day 7, day 28, and day 180 post quit |
|
| Outcomes | Primary outcome: 7‐day point prevalence abstinence verified by expired CO Secondary outcomes: 1‐month abstinence, cigarette consumption by time‐line follow back, mean time to first lapse |
|
| Notes | Not published as yet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 in control and 22 in intervention did not commence study. ITT analysis presented in this meta‐analysis |
| Other bias | Unclear risk | No information |
| Methods | Pilot RCT in UK | |
| Participants | 200 participants aged ≥ 16 years; smoking daily and interested in quitting; current owner of mobile phone. 63% men, median age 36 years, median 20 cigarettes/day, 7% FTND dependence score > 5 | |
| Interventions | 6‐month programme delivered solely over mobile phone based on programme in Rodgers 2005 but messages adapted for UK population. Participant nominates QD and receives regular personalised text messages with advice, support and distraction, with a countdown to QD, intensive 4 weeks of 5 or 6 messages/day then maintenance phase of 1 message/2 weeks. Messages selected from database matched to participant characteristics. Free month of text messaging from QD. Optional Quit Buddy, and Text Crave (messages on demand). Interactive polls and quizzes Control: 1 text message/fortnight | |
| Outcomes | Primary outcome: point prevalence abstinence (no smoking in past 7 days) at 6 weeks post randomisation (approximates 4 weeks post‐QD) Secondary outcomes: point prevalence abstinence and continuous abstinence (< 5 cigarettes) at 26 weeks. Verification with salivary cotinine in quitters at 26 weeks | |
| Notes | Pilot study ‐ full trial is Free 2011 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Concealed until after assignment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single blind (participants not blinded) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: 4 (control) and 1 (intervention) at 4 weeks (98% follow‐up); 8 (control) and 8 (intervention) at 6 months (92% follow‐up) |
| Other bias | Unclear risk | None described |
| Methods | RCT in UK | |
| Participants | 5800 participants aged ≥ 16 years, willing to make an attempt to quit smoking in the next month and owned a mobile phone. 45% women, mean age of 37 years, 89% white and 25% students/unemployed. 60% of participants had an FTND dependence score of ≤ 5 | |
| Interventions | 6‐month programme: delivered solely over mobile phone based on programme in Rodgers 2005. Participants asked to set a QD within 2 weeks of randomisation. They received 5 text messages/day for the first 5 weeks and then 3/week for the next 26 weeks. Intervention included motivational messages and behaviour‐change techniques. The programme was also personalised with an algorithm based on demographic and other information gathered at baseline, such as smoker's concerns about weight gain after quitting. The core programme consisted of 186 messages and the personalised messages were selected from a database of 713 messages. For instance, by texting the word "lapse", participants received a series of 3 text messages that encouraged them to continue with their quit attempt. Participants could also request the mobile phone number of another trial participant so that they could text each other for support. Participants in the intervention group using pay‐as‐you‐go mobile phone schemes were given a £20 top‐up voucher to provide sufficient credit to participate in the intervention Control: fortnightly, simple, short, text messages related to the importance of trial participation |
|
| Outcomes | No more than 5 cigarettes smoked since the start of the abstinence period at 6 months of follow‐up, self reported and verified by postal salivary cotinine testing or a CO test in person | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent telephone randomisation system |
| Allocation concealment (selection bias) | Low risk | Concealed until after assignment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single blind (participants not blinded) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 176 intervention and 92 control lost to follow‐up (< 5% total) |
| Other bias | Unclear risk | None described |
| Methods | RCT in US | |
| Participants | 474 participants aged ≥ 18 years recruited from an HIV clinic in a low‐income multi‐ethnic urban population in Texas, USA. 70% men, mean age 44.8 years, mean FTND 5.8. Inclusion criteria: HIV‐positive, current smoker (≥ 5 cigarettes/day and expired CO ≥ 7 ppm), willing to set a QD within 7 days, and ability to speak English or Spanish |
|
| Interventions | Participants in the mobile phone intervention group received the usual care components plus a mobile phone‐delivered counselling intervention over 3 months and access to a supportive hotline. They were provided with a pre‐paid mobile phone on which a series of 11 proactive counselling sessions were conducted. The phone calls spanned a 3‐month period but were front loaded such that the frequency of the calls was highest near the time of scheduled quit attempt. Counselling session content was primarily drawn from a cognitive‐behavioural foundation emphasising problem solving and skills training techniques Control: participants completed an audio computer‐assisted self interview, then received provider advice to quit smoking. Usual care was provided with targeted written smoking cessation materials (i.e. a "tip sheet" designed to address concerns of HIV‐positive smokers) and instructions on how to obtain nicotine‐replacement therapy in the form of nicotine patches at the clinic |
|
| Outcomes | Primary outcome: self reported repeated measures 7‐day point prevalence at 3, 6 and 12 months Secondary outcomes: 3, 6 and 12 months' smoking abstinence (24 hours, 7 days and 30 days), CO verified quitting, number of quit attempts, length of abstinence (in days), use of nicotine‐replacement therapy, use of other cessation treatments and exposure to other forms of tobacco. Other smoking‐related measures included the FTND, the Reasons for Quitting scale (intrinsic and extrinsic quit motivation) and the 9‐item quitting self efficacy scale. Depressive symptoms: Center for Epidemiologic Studies Depression (CES‐D), State Trait Anxiety Inventory. Quality of life: Medical Outcomes Study HIV Health Survey (MOS‐HIV). Alcohol use: Alcohol Use Disorders Identification Test. A single item was used to assess illicit drug use in the past month |
|
| Notes | Expanded programme based on Vidrine 2006 Varied from the other interventions in using pre‐paid provided mobile phones to provide counselling instead of an SMS intervention. Many smokers were excluded (40%) due to not meeting 5 cigarettes/day and CO ≥ 7 ppm). Low absolute quit rates may be due to high nicotine dependence, high rate of alcohol and drug use, and the substantial burden of mental illness amongst participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised ‐ method not stated |
| Allocation concealment (selection bias) | Low risk | Allocated after baseline data collected |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Risk was minimised with the following measures:
|
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 51 in control and 61 in intervention did not complete 6‐month follow‐up but ITT analysis presented |
| Other bias | Low risk | Expired CO to validate smoking status. Use of a single HIV clinic, a county site with a large patient population |
| Methods | Cluster RCT in Switzerland | |
| Participants | 755 daily or occasional smokers (≥ 4 cigarettes in the preceding month and ≥ 1 cigarette during the preceding week) recruited at 24 vocational schools (178 classes). 48% male, mean age 18.2 years (SD 2.3) | |
| Interventions | SMS‐COACH: a 3‐month programme including a weekly SMS text message assessment of smoking‐related target behaviour, 2 weekly text messages tailored to baseline data and responses to the SMS text message assessments, and an optional further integrated QD preparation and relapse prevention SMS programme. Participants who did not use the integrated programme for QD preparation and relapse prevention received a total of 37 text messages (1 welcome message, 11 assessment messages, 24 tailored feedback messages, 1 goodbye message). Participants, who used the QD preparation and relapse‐prevention programme for the whole period from 1 week before the scheduled quit date until 3 weeks afterwards, received an additional 42 text messages Control: all students in participating classes were invited to participate in an online health screening survey during a regular school lesson reserved for health education. The control group did not receive anything else |
|
| Outcomes | Primary outcome: 7‐day point prevalence smoking abstinence at 6 months Secondary outcomes: 4‐week point prevalence smoking abstinence, the number of cigarettes smoked/day, stage of change and number of attempts to quit smoking |
|
| Notes | The study did not reach the target sample size of 910 participants due to smaller class size than expected and time restrictions. Nicotine dependence was not calculated but number of cigarettes smoked/day used as an indicator and outcome variable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster randomisation with class as the unit of randomisation, stratified by school to control for heterogeneity between schools. Block randomisation with computer‐generated randomly permuted blocks of 4 cases |
| Allocation concealment (selection bias) | Low risk | Students recruited prior to randomisation and informed after baseline |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Baseline and follow‐up assessors blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 111 in control and 85 in intervention were lost to follow‐up at 6 months. ITT analysis conducted |
| Other bias | Unclear risk | Self report outcomes |
| Methods | RCT in UK | |
| Participants | Participants aged 18‐75 years and current smokers (≥ 1 cigarette /day and smoked within previous 7 days) who were willing to quit within 14 days of randomisation, recruited in primary care. Primary care practices were those that had smoking cessation advisors (primary care nurses or healthcare assistants) providing level 2 cessation advice. Participants were self referred or referred by a health professional. 47% men, mean age 41.8 years (range 18‐75 years), able to read English and provide written informed consent, with a mobile phone and familiar with sending and receiving text messages and not enrolled in another formal smoking cessation study or other cessation programme | |
| Interventions | Intervention: usual care plus a tailored advice report and a 90‐day programme of tailored text messages generated by the iQuit system (number of messages sent each day varied from 0 to 2, mean/day over 90 days 1.2). The messages were designed to advise smokers on their quit attempt, provide information about the consequences of smoking and expectations for quitting, provide encouragement, boost self efficacy, maintain motivation to quit and remind smokers how to cope with difficult situations Control: 'usual care' consisted of routine 'level 2' smoking cessation advice delivered by smoking cessation adviser. This included a brief discussion about smoking habits and history, measurement of expired‐air CO, brief advice to quit, setting a QD within the next 14 days, options for pharmacotherapy, a prescription and arranging a follow‐up visit. Usually the opportunity for multiple follow‐up visits was offered |
|
| Outcomes | Primary outcome: self reported 2‐week point prevalence abstinence at 8 weeks Secondary outcomes: CO‐verified abstinence at 4‐week for at least 2 weeks, assessed by a smoking cessation adviser (a CO reading assessed 25‐42 days from QD that was < 10 ppm), self reported 3‐month prolonged abstinence at 6 months, 6‐month prolonged abstinence at 6‐month follow‐up and a strict continuous abstinence measure using all outcome time points: CO‐validated 2‐week point prevalence abstinence at 4 weeks, 4‐week point prevalence abstinence at 8 weeks and 6‐month prolonged abstinence at 6 months |
|
| Notes | Outcomes used in this meta‐analysis were self reported. Control programme was fairly intensive, i.e. smoking cessation advice provided in‐person | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by online programme |
| Allocation concealment (selection bias) | Low risk | Randomised by online programme once baseline data collected |
| Blinding (performance bias and detection bias) All outcomes | Low risk | 6‐month data collected by postal questionnaire or by researchers blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 65 in control and 70 in intervention lost to follow‐up at 6 months but ITT analyses presented |
| Other bias | Unclear risk | Self reported outcomes included |
| Methods | RCT in New Zealand | |
| Participants | 1705 participants aged ≥ 16 years recruited by direct advertising, smoking daily, wanting to quit within the next month, and were able to send and receive text messages on their own mobile phone 58% female, median age 22 years, 20.8% Maori (indigenous population), smoked mean 15 cigarettes/day and mean FTND dependence score 5 | |
| Interventions | 6‐month programme delivered solely over mobile phone. Participant nominated QD and received regular personalised text messages with advice, support and distraction, with a countdown to QD, intensive 4 weeks of 5 or 6 messages/day then maintenance phase of 1 message/2 weeks. Messages selected from database matched to participant characteristics. Free month of text messaging from QD. Optional Quit Buddy and Text Crave (messages on demand). Interactive polls and quizzes Control: 1 text message/fortnight | |
| Outcomes | Primary outcome: point prevalence abstinence (no smoking in past 7 days) at 6 weeks' post‐randomisation (approximates 4 weeks post‐QD). Verification with salivary cotinine in small number of quitters at 6 weeks Secondary outcome: point prevalence abstinence at 12 and 26 weeks, and continuous abstinence at 26 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Concealed until after assignment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single blind (participants not blinded) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up: 35 control (95.9%) and 46 intervention (94.6%) followed up at 6 weeks; but differential loss to follow‐up at 6 months (79% control vs. 69% intervention). Possibly due to incentive being offered to control group for follow‐up, may in turn have affected long‐term results of study (by underestimating effect) |
| Other bias | High risk | The authors suggested that some participants in the control group may have thought their incentive at follow‐up (month of free text messaging) depended on reporting quitting. This could account for an unexpected increase in control group participants reporting quitting from 6 weeks (109 participants) to 6 months (202 participants reporting no smoking in the past 7 days), which could have led to an underestimation of the effect of the intervention |
| Methods | 3‐arm RCT in US | |
| Participants | Participants aged ≥ 18 years recruited from large urban HIV clinics in the USA Inclusion criteria: current patient of the clinics, current or regular smoker (≥ 5 cigarettes/day), CO ≥ 8 ppm, willing to set a QD within the next 2 weeks, willing to use a mobile phone and able to read text messages, and eligible to take varenicline Exclusion criteria: alcohol dependence and active drug abuse, and conditions that would prevent the use of varenicline |
|
| Interventions | Intervention: participants received standard care (see below) and 2 text messages/day for 12 weeks. 1 message reminding them to take their medication and 1 motivational message regarding cessation Control group: received standard care, which consisted of a self help information sheet, tailored to HIV‐positive smokers and an offer of varenicline for 12 weeks according to the standard dosage schedule. Participants needed to return to the clinic each 4 weeks to receive further medication. All participants were provided with a pre‐paid mobile phone ‐ the control group received phones to facilitate their ability to call the quit line and receive text message appointment reminders only A third arm received standard care, text messages, plus behavioural therapy delivered via 7 proactive mobile phone‐delivered counselling sessions over a 6‐week period. These combined cognitive behavioural therapy and motivational interviewing techniques |
|
| Outcomes | Primary outcome: 7‐day point prevalence abstinence verified by CO < 8 ppm at 24 weeks, also measured at 1, 4, 8 and 12 weeks | |
| Notes | Unpublished ‐ we did not have the participant characteristics tables | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule, stratified by people smoking 5‐10 and people smoking > 10 cigarettes/day |
| Allocation concealment (selection bias) | Low risk | After consent and baseline data collected, the research assistant called to receive the assignment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants self complete questionnaires through audio computer‐assisted self interviewing |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 21 participants in the control group, 19 in the text message group (and 24 in the text message + phone counselling group) did not complete 24‐week follow‐up visits. ITT analysis is used in this meta‐analysis |
| Other bias | Unclear risk | None described |
| Methods | RCT in New Zealand | |
| Participants | 226 participants aged ≥ 16 years recruited by advertising if they were current daily smokers ready to quit, and had a video message‐capable phone. Advertising particularly targeted young adults. 47% female, 24% Maori (indigenous population), mean age 27 years and appeared to be highly addicted due to the Hooked on Nicotine Checklist mean scores of 8 (SD 1.9) out of 10 | |
| Interventions | Intervention group: received an automated package of video and text messages over 6 months that was tailored to self selected quit date, role model and timing of messages. Video messages were video diary‐style from a selected 'ordinary' person going through a quit attempt in advance of the participant. Frequency of messages varied from 1/day in the lead up to QD, 2/day from QD for 4 weeks, then reducing to 1 every 2 days for 2 weeks and then 1 every 4 days for about 20 weeks until 6 months after randomisation. Extra messages were available on demand to beat cravings and address lapses. Additional website for intervention group participants to review video messages they had been sent (and rate them if desired), change their selected time periods and change (or add to) their selected role model. Control: also set a QD and received a general health video message sent to their phone every 2 weeks |
|
| Outcomes | Self reported continuous abstinence ‐ no more than 5 cigarettes smoked since the start of the abstinence period at 6 months of follow‐up | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Concealed until after assignment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single blind (participants not blinded) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 32% intervention and 22% control lost to follow‐up at 6 months |
| Other bias | Unclear risk | None described |
CI: confidence interval; CO: carbon monoxide; FTND: Fagerström Test of Nicotine Dependence; HIV: human immunodeficiency virus; ITT: intention to treat; ppm: parts per million; QD: quit day; RCT: randomised controlled trial; SD: standard deviation; SMS: short messaging service.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Applegate 2007 | Abstract describing intervention to increase adherence to the use of nicotine replacement gum in people attempting to quit smoking. Duration 8 weeks |
| Bennett 2011 | Editorial comment on the Free 2011 trial |
| Blasco 2012 | Internet‐based telemonitoring programme for secondary prevention in cardiovascular disease with parameters sent by mobile phone |
| Brendryen 2008a | Mobile phone intervention confounded with Internet intervention (previously included in Whittaker 2009) |
| Brendryen 2008b | Mobile phone intervention confounded with Internet intervention (previously included in Whittaker 2009) |
| Bricker 2014 | Pilot study with 2‐month follow‐up only |
| Buller 2014 | Follow‐up only to 3 months |
| Chow 2012 | Focused on cardiac rehabilitation |
| Dale 2014 | Focused on cardiac rehabilitation |
| Fingrut 2014 | Not focused on delivery by mobile phone |
| Fraser 2014 | Not focused on delivery by mobile phone |
| Haug 2008 | Non‐randomised feasibility study. Duration 12 weeks |
| Haug 2009 | Mainly about acceptability, 3 months' follow‐up |
| Haug 2014 | Protocol for a study on alcohol and smoking in adolescents |
| Jordan 2015 | 3 months' follow‐up |
| Kiselev 2011 | Not focused on smoking cessation |
| Lazev 2004 | Not randomised. No control group. Feasibility study for the programme presented in Vidrine 2006 |
| Mehring 2014 | Not focused on delivery by mobile phone |
| Naughton 2012 | Randomised controlled trial with pregnant smokers, follow‐up to 3 months |
| Obermayer 2004 | Not randomised. No control group |
| Pechmann 2015 | Not randomised |
| Peng 2013 | Not focused on delivery by mobile phone |
| Pollak 2013 | Gradual reduction in pregnant women |
| Riley 2008 | Small non‐randomised study with only 6 weeks' follow‐up |
| Shi 2013 | Follow‐up only 3 months |
| Skov‐Ettrup 2013 | Not focused on delivery by mobile phone |
| Skov‐Ettrup 2014 | Compared tailored with un‐tailored text messages ‐ no control group |
| Snider 2011 | Not a trial |
| Stanczyk 2014 | Not focused on delivery by mobile phone |
| Vidrine 2006 | Randomised trial of phone counselling with mobile phones, follow‐up only 3 months |
| Vilaplana 2014 | Follow‐up only 3 months |
| Wizner 2009 | Not focused on smoking cessation |
| Ybarra 2012 | Pilot RCT, follow‐up only 3 months |
| Ybarra 2013 | Pilot RCT, follow‐up only 3 months |
| Yuhongxia 2011 | Single‐blind RCT, follow‐up 24 weeks, but with no details available on the randomisation method or the intervention content. Abstract only, unable to contact authors |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Assessing the Effect of an Interactive Decision‐Aid Smartphone Smoking Cessation Application (app) on Quit Rates: a Double‐Blind Automated Randomised Control Trial Protocol |
| Methods | RCT |
| Participants | Daily smokers aged ≥ 18 years in Australia, Singapore, the UK and the US |
| Interventions | Smoking cessation app |
| Outcomes | Continuous abstinence at 1 and 6 months |
| Starting date | May 2014 |
| Contact information | Nasser F BinDhim; nbin6641@uni.sydney.edu.au |
| Notes |
| Trial name or title | MiQuit Trial: Tailored Text Messages for Pregnant Women |
| Methods | RCT |
| Participants | Smokers aged ≥ 16 years, pregnant, in UK |
| Interventions | MiQuit is an automated responsive text message support programme lasting 12 weeks |
| Outcomes | Continuous abstinence from 4 weeks after randomisation until follow‐up at the end of pregnancy |
| Starting date | February 2014 |
| Contact information | Tim Coleman, University of Nottingham |
| Notes |
| Trial name or title | Internet and Text Messaging Intervention in Norway |
| Methods | RCT |
| Participants | Current smokers aged ≥ 16 years |
| Interventions | Internet‐based smoking cessation versus Internet plus text messaging |
| Outcomes | Point prevalence abstinence at 1, 3, 6 and 12 months |
| Starting date | 2011 |
| Contact information | Professor Gram, Faculty of Health Sciences, Institute of Community Medicine, University of Tromsø, Norway, inger.gram@uit.no |
| Notes |
| Trial name or title | Abstinence Reinforcement Therapy for Rural Veteran Smokers |
| Methods | RCT |
| Participants | Durham VA enrolees |
| Interventions | Cognitive behavioural telephone counselling, telemedicine clinic for access to nicotine‐replacement therapy, mobile contingency management |
| Outcomes | Quality‐adjusted life years at 12 months |
| Starting date | November 2013 |
| Contact information | Patrick Calhoun, Durham VA Medical Center, Durham NC |
| Notes |
| Trial name or title | Effectiveness of Messages to Mobile Phone in Smoke Cessation |
| Methods | RCT |
| Participants | Current smokers aged > 18 years in Spain |
| Interventions | Quit advice by a doctor and support messages to mobile phones |
| Outcomes | Continuous abstinence at 6 months |
| Starting date | December 2012 |
| Contact information | Raquel Cobos Campos, Basque Health Service |
| Notes |
| Trial name or title | Study of Mobile Phone Support for the DC Tobacco Quitline |
| Methods | RCT |
| Participants | Current smokers aged ≥ 18 years in US |
| Interventions | Internet‐based system using mobile phones to increase the quality, frequency and accessibility of support |
| Outcomes | Point prevalence abstinence at 3, 6 and 9 months |
| Starting date | July 2010 |
| Contact information | Thomas Kirchner, American Legacy Foundation |
| Notes |
| Trial name or title | Web and Mobile Smoking Cessation |
| Methods | RCT |
| Participants | Smokers aged ≥ 18 years in US |
| Interventions | Internet plus mobile compared with Internet only |
| Outcomes | Point prevalence 3 and 6 months |
| Starting date | April 2015 |
| Contact information | Brian Danaher briand@ori.org |
| Notes |
| Trial name or title | A Mindfulness Based Application for Smoking Cessation |
| Methods | RCT |
| Participants | Smokers aged ≥ 18 years in the US |
| Interventions | A mindfulness based smartphone app |
| Outcomes | Number of cigarettes smoked at 6 months |
| Starting date | September 2013 |
| Contact information | Jennifer Penberthy jkp2n@virginia.edu |
| Notes |
| Trial name or title | A Randomised Controlled Trial to Test the Effect of a Smartphone Quit Smoking Intervention on Young Adult Smokers |
| Methods | RCT |
| Participants | Smokers aged 19‐29 years in Canada |
| Interventions | Crush The Crave smartphone application |
| Outcomes | Point prevalence (30 day) at 6 months |
| Starting date | January 2014 |
| Contact information | |
| Notes |
| Trial name or title | Use of Technological Advances to Prevent Smoking Relapse Among Smokers with PTSD |
| Methods | RCT |
| Participants | Smokers aged 18‐70 years in US |
| Interventions | Quit4ever combines counselling sessions, bupropion and nicotine‐replacement therapy, mobile contingency management and the smartphone application Stay Quit Coach |
| Outcomes | Point prevalence abstinence at 3 and 6 months |
| Starting date | December 2013 |
| Contact information | Jean Beckham, Duke University |
| Notes |
| Trial name or title | Korean Youth Smoking Cessation Study |
| Methods | RCT |
| Participants | Smokers aged 14‐19 years Korean/American youth in US |
| Interventions | Tailored interactive cognitive behavioural motivation enhancement therapy delivered through Internet and mobile phones |
| Outcomes | Point prevalence abstinence at 6 months |
| Starting date | June 2016 |
| Contact information | Steve Shoptaw sshoptaw@mednet.ucla.edu |
| Notes |
| Trial name or title | Mobile Mindfulness Training for Smoking Cessation |
| Methods | RCT |
| Participants | Smokers aged 18‐65 years in US |
| Interventions | Smartphone‐based training programme |
| Outcomes | Point prevalence abstinence at 6 months |
| Starting date | August 2015 |
| Contact information | Judson Brewer judson.brewer@yale.edu |
| Notes |
| Trial name or title | Smartphone Application for Smoking Cessation |
| Methods | RCT |
| Participants | Smokers aged 18‐65 years in US |
| Interventions | 3‐week smartphone‐based training programme |
| Outcomes | Point prevalence at 6 months |
| Starting date | October 2014 |
| Contact information | Kathleen Garrison, Yale University |
| Notes |
| Trial name or title | Internet‐Based Medication Adherence Program for Nicotine Dependence Treatment |
| Methods | RCT |
| Participants | Smokers aged 18‐65 years |
| Interventions | My Mobile Advice Program via smartphone or Internet device |
| Outcomes | Point prevalence abstinence at 5 months |
| Starting date | October 2014 |
| Contact information | |
| Notes |
| Trial name or title | A Quit Smoking Study Using Smartphones |
| Methods | RCT |
| Participants | Smokers aged ≥ 18 years in the US |
| Interventions | Mobile games |
| Outcomes | Number of cigarettes/day |
| Starting date | October 2014 |
| Contact information | Tanya Schlam trschlam@ctri.wisc.edu |
| Notes |
| Trial name or title | Social Media Intervention for Young Adult Smokers |
| Methods | RCT |
| Participants | Smokers aged 18‐25 years in the US |
| Interventions | 3 month Facebook intervention |
| Outcomes | Point prevalence abstinence at 6 and 12 months |
| Starting date | October 2014 |
| Contact information | Danielle A Ramo, UCSF |
| Notes |
| Trial name or title | Developing a Smartphone App With Mindfulness Training for Teen Smoking Cessation |
| Methods | RCT |
| Participants | Smokers aged 13‐19 years in US |
| Interventions | Smoking cessation treatment delivered through a smartphone app via mindfulness training |
| Outcomes | Point prevalence abstinence at 3 and 6 months |
| Starting date | September 2014 |
| Contact information | Lori Pbert, University of Massachusetts |
| Notes |
| Trial name or title | Smoking Response Inhibition Training |
| Methods | RCT |
| Participants | Smokers aged 18‐45 years in US |
| Interventions | Smoking‐specific response inhibition training programme in the context of a quit attempt. The task is based on a modified stop‐signal task |
| Outcomes | Smoking relapse at 6 months |
| Starting date | September 2014 |
| Contact information | Robert D Dvorak, North Dakota State University |
| Notes |
| Trial name or title | Harnessing the Power of Technology: MoMba for Postpartum Smoking |
| Methods | RCT |
| Participants | Smokers aged 18‐50 years in US |
| Interventions | MoMba Live Long Smartphone application |
| Outcomes | Point prevalence abstinence at 21 months |
| Starting date | February 2016 |
| Contact information | Ruth M Arnold, ruth.arnold@yale.edu |
| Notes |
| Trial name or title | Abstinence Reinforcement Therapy (ART) for Homeless Veteran Smokers |
| Methods | RCT |
| Participants | Smokers aged 18‐75 years |
| Interventions | Nicotine patches plus mobile contingency management (participants upload videos of themselves taking carbon monoxide readings. Any time a participant uploads a video that suggests abstinence, he/she is provided with a monetary reward) |
| Outcomes | Smoking abstinence at 6 months |
| Starting date | October 2014 |
| Contact information | Angela C Kirby, angela.kirby@va.gov |
| Notes |
| Trial name or title | Mobile Media‐Rich Interactive Guideline System (MMRIGS) Pilot Study |
| Methods | RCT |
| Participants | Smokers aged ≥ 18 years and older |
| Interventions | Brief advice to quit smoking (tailored video clips) and an 8‐week automated intervention (interactive text messages and graphical messages) for support in quitting smoking via smartphone |
| Outcomes | Smoking abstinence 3 months |
| Starting date | January 2015 |
| Contact information | Alex Prokhorov, M.D. Anderson Cancer Center |
| Notes |
| Trial name or title | Randomized Clinical Trial to Reduce Harm From Tobacco |
| Methods | RCT |
| Participants | Smokers aged ≥ 18 years |
| Interventions | eCigarettes, nicotine‐replacement therapy and other pharmacotherapy, incentives and a standard programme of support including text messaging |
| Outcomes | Verified abstinence at 6 months |
| Starting date | January 2015 |
| Contact information | Kathryn A Saulsgiver kasau@mail.med.upenn.edu |
| Notes |
| Trial name or title | Penn State TXT2Quit Study |
| Methods | RCT |
| Participants | Smokers aged ≥ 21 years |
| Interventions | Varenicline and motivational text messages |
| Outcomes | Point prevalence at 3 months |
| Starting date | January 2015 |
| Contact information | Jonathan Foulds, Milton S. Hershey Medical Center |
| Notes |
| Trial name or title | Efficacy of a Mobile Application in the Smoking Cessation Among Young People (TOBB_STOP) |
| Methods | RCT |
| Participants | Smokers aged 18‐30 years in Spain |
| Interventions | Mobile phone application for smartphone |
| Outcomes | Smoking cessation at 6 months |
| Starting date | January 2013 |
| Contact information | Empar Valdivieso López evaldivieso.tarte.ics%40gencat.cat |
| Notes |
| Trial name or title | Smoking Cessation for Low‐Income Adults |
| Methods | RCT |
| Participants | Smokers aged ≥ 18 years in US |
| Interventions | Standard care plus mobile phone‐delivered text/graphical messaging component plus 11 mobile phone‐delivered proactive counselling sessions |
| Outcomes | Smoking abstinence at 12 months |
| Starting date | June 2010 |
| Contact information | Alex Prokhorov, MD Anderson Cancer Center |
| Notes |
RCT: randomised controlled trial.
Contributions of authors
RW is the lead author of this review.
For the most recent update:
RW and YG selected studies for inclusion.
HM reviewed the selected studies for inclusion.
RW and YG independently extracted data from the papers and undertook the analysis.
All authors contributed to the writing and editing of the review.
Sources of support
Internal sources
National Institute for Health Innovation (Auckland Uniservices), New Zealand.
Cancer Council Victoria, Australia.
External sources
No sources of support supplied
Declarations of interest
RW was co‐author of one paper on one of the included studies (Bramley 2005). She was a co‐investigator on two included studies (Free 2009; Free 2011), and principle investigator of a further included study (Whittaker 2011).
CB and HM were co‐authors of Whittaker 2011.
RB was co‐author of one of the trials (Borland 2013), and he led the development of the intervention.
AR was a lead author (Rodgers 2005), and a co‐author (Free 2009; Free 2011; Whittaker 2011), on included studies.
HM received honoraria from Johnson & Johnson and Pfizer for speaking at educational events and attending advisory group meetings. He has also received investigator initiated research funding from Pfizer.
RW's institution has received grant money to cover the costs of providing the text messaging intervention for the study described in Free 2011, and there is a grant pending to develop this intervention further for a different audience. The institution has also licensed the STOMP intervention described in Rodgers 2005 to HSAGlobal.
All other authors had no other known conflicts of interest.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
- Abroms L, Ahuja M, Kodl Y, Thaweethai L, Sims J, Winickoff J, et al. Text2Quit: results from a pilot test of a personalised, interactive mobile health smoking cessation program. Journal of Health Communication 2012;17(Suppl 1):44‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]; Abroms LC, Boal AL, Simmens SJ, Mendel JA, Windsor RA. A randomized trial of Text2Quit: a text messaging program for smoking cessation. American Journal of Preventive Medicine 2014;47(3):242‐50. [CENTRAL: 1002545; CRS: 9400129000003040; EMBASE: 2014941087] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock B, Heron K, Jennings E, Morrow K, Cobb V, Magee J, et al. A text message delivered smoking cessation intervention: the initial trial of TXT‐2‐Quit: randomized controlled trial. JMIR mHealth and uHealth 2013;1(2):e17. [CENTRAL: 1015646; CRS: 9400129000003441] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmford J, Borland R, Benda P, Howard S. Factors associated with use of automated smoking cessation interventions: findings from the eQuit study. Health Education Research 2013;28(2):288‐99. [CENTRAL: 921007; CRS: 9400107000001543; PUBMED: 23107931] [DOI] [PubMed] [Google Scholar]; Borland R, Balmford J, Benda P. Population‐level effects of automated smoking cessation help programs: a randomized controlled trial. Addiction (Abingdon, England) 2013;108(3):618‐28. [CENTRAL: 921001; CRS: 9400107000000767; PUBMED: 22994457] [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Walters JAE. The effect of mobile phone text messages on short and long term quitting in motivated smokers: a randomised controlled trial. Proceedings of the Society for Research on Nicotine and Tobacco 21st Annual Meeting; 2015 Feb 25‐28 Philadelphia. 2015:252 (POS3‐80). [CRS: 9400131000001072] [Google Scholar]; Schuz N, Walters JAE, Frandsen M, Bower J, Ferguson SG. Compliance with an EMA monitoring protocol and its relationship with participant and smoking characteristics. Nicotine & Tobacco Research 2014;16(Suppl 2):S88‐92. [CRS: 9400129000001511; EMBASE: 2014262309] [DOI] [PubMed] [Google Scholar]
- Free C, Whittaker R, Knight R, Abramsky T, Rodgers A, Roberts IG. Txt2stop: a pilot randomised controlled trial of mobile phone‐based smoking cessation support. Tobacco Control 2009;18:88‐91. [DOI] [PubMed] [Google Scholar]
- Douglas N, Free C. 'Someone batting in my corner': experiences of smoking‐cessation support via text message. British Journal of General Practice 2013;63(616):e768‐76. [CENTRAL: 1000333; CRS: 9400129000002807; PUBMED: 24267860] [DOI] [PMC free article] [PubMed] [Google Scholar]; Free C, Hoile E, Robertson S, Knight R. Three controlled trials of interventions to increase recruitment to a randomized controlled trial of mobile phone based smoking cessation support. Clinical Trials 2010;7(3):265‐73. [DOI] [PubMed] [Google Scholar]; Free C, Knight R, Robertson S, Whittaker R, Edwards P, Zhou W, et al. Smoking cessation support delivered via mobile phone text messaging (Txt2stop): a single‐blind, randomised trial. Lancet 2011;378:49‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]; Guerriero C, Cairns J, Roberts I, Rodgers A, Whittaker R, Free C. The cost‐effectiveness of smoking cessation support delivered by mobile phone text messaging: Txt2stop. European Journal of Health Economics 2013;14(5):789‐97. [CENTRAL: 986154; CRS: 9400129000001252; PUBMED: 22961230] [DOI] [PMC free article] [PubMed] [Google Scholar]; Michie S, Free C, West R. Characterising the 'Txt2Stop' smoking cessation text messaging intervention in terms of behaviour change techniques. Journal of Smoking Cessation 2012;7(1):55‐60. [CRS: 9400123000018452] [Google Scholar]; Severi E, Free C, Knight R, Robertson S, Edwards P, Hoile E. Two controlled trials to increase participant retention in a randomized controlled trial of mobile phone‐based smoking cessation support in the United Kingdom. Clinical Trials 2011;8(5):654‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritz ER, Danysh HE, Fletcher FE, Tami‐Maury I, Fingeret MC, King RM, et al. Long‐term outcomes of a cell phone‐delivered intervention for smokers living with HIV/AIDS. Clinical Infectious Diseases 2013;57(4):608‐15. [CENTRAL: 871211; CRS: 9400107000001912; EMBASE: 2013482868; PUBMED: 23704120] [DOI] [PMC free article] [PubMed] [Google Scholar]; Gritz ER, Vidrine DJ, Marks RM, Arduino RC. A randomized trial of an innovative cell phone intervention for smokers living with HIV/AIDS [SYM 10A]. Proceedings of the Society for Research on Nicotine & Tobacco 17th Annual Meeting; 2011 Feb 16‐19, Toronto. 2011:13. [CENTRAL: 790955; CRS: 9400123000006041] [Google Scholar]; NCT00502827. An innovative telephone intervention for HIV‐positive smokers, 2007. ClinicalTrials.gov/ct2/show/record/NCT00502827 (accessed 5 April 2016). ; Tami‐Maury I, Vidrine DJ, Fletcher FE, Danysh H, Arduino R, Gritz ER. Poly‐tobacco use among HIV‐positive smokers: implications for smoking cessation efforts. Nicotine & Tobacco Research 2013;15(12):2100‐6. [CENTRAL: 915428; CRS: 9400129000000258; EMBASE: 2013717003] [DOI] [PMC free article] [PubMed] [Google Scholar]; Vidrine DJ, Kypriotakis G, Li L, Arduino RC, Fletcher FE, Tami‐Maury I, et al. Mediators of a smoking cessation intervention for persons living with HIV/AIDS. Drug and Alcohol Dependence 2015;147:76‐80. [CRS: 9400131000000661; EMBASE: 2014625524; PUBMED: 25542824] [DOI] [PMC free article] [PubMed] [Google Scholar]; Vidrine DJ, Marks RM, Arduino RC, Gritz ER. Efficacy of cell phone‐delivered smoking cessation counseling for persons living with HIV/AIDS: 3‐month outcomes. Nicotine & Tobacco Research 2012;14(1):106‐10. [CENTRAL: 814356; CRS: 9400123000013048; PUBMED: 21669958] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug S, Meyer C, Dymalski A, Lippke S, John U. Efficacy of a text messaging (SMS) based smoking cessation intervention for adolescents and young adults: study protocol of a cluster randomised controlled trial. BMC Public Health 2012;12:51. [CENTRAL: 814353; CRS: 9400123000011961; EMBASE: 22260736; PUBMED: 22260736] [DOI] [PMC free article] [PubMed] [Google Scholar]; Haug S, Schaub MP, Venzin V, Meyer C, John U. Efficacy of a text message‐based smoking cessation intervention for young people: a cluster randomized controlled trial. Journal of Medical Internet Research 2013;15(8):142‐55. [CENTRAL: 980800; CRS: 9400129000000569; PUBMED: 23956024] [DOI] [PMC free article] [PubMed] [Google Scholar]; Haug S, Schaub MP, Venzin V, Meyer C, John U. Moderators of outcome in a text messaging (SMS)‐based smoking cessation intervention for young people [Differenzielle Wirksamkeit eines Short Message Service (SMS)‐basierten Programms zur Forderung des Rauchstopps bei Jugendlichen]. Psychiatrische Praxis 2013;40(6):339‐46. [CENTRAL: 875575; CRS: 9400126000000210; EMBASE: 2013576012; PUBMED: 24008683] [DOI] [PubMed] [Google Scholar]; Haug S, Schaub Michael P, Schmid H. Predictors of adolescent smoking cessation and smoking reduction. Patient Education and Counseling 2014;95(3):378‐83. [CRS: 9400129000001807; PUBMED: 24674150] [DOI] [PubMed] [Google Scholar]
- Naughton F, Jamison J, Boase S, Sloan M, Gilbert H, Prevost AT, et al. Randomized controlled trial to assess the short‐term effectiveness of tailored web‐ and text‐based facilitation of smoking cessation in primary care (iQuit in Practice). Addiction (Abingdon, England) 2014;109(7):1184‐93. [CENTRAL: 1051137; CRS: 9400129000001642; PUBMED: 24661312] [DOI] [PMC free article] [PubMed] [Google Scholar]; Sutton S, Smith S, Jamison J, Boase S, Mason D, Prevost AT, et al. Study protocol for iQuit in Practice: a randomised controlled trial to assess the feasibility, acceptability and effectiveness of tailored web‐ and text‐based facilitation of smoking cessation in primary care. BMC Public Health 2013;13:324. [CENTRAL: 873088; CRS: 9400126000000015; PUBMED: 23575031] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramley D, Riddell T, Whittaker R, Corbett T, Lin R‐B, Wills M. Smoking cessation using mobile phone text messaging is as effective in Maori as non‐Maori. New Zealand Medical Journal 2005;118(1216):1494‐504. [PubMed] [Google Scholar]; Rodgers A, Corbett T, Bramley D, Riddell T, Wills M, Lin R‐B, et al. Do u smoke after txt? Results of a randomised trial of smoking cessation using mobile phone text messaging. Tobacco Control 2005;14:255‐61. [doi: 10.1136/tc.2005.011577] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01898195. Improving adherence to smoking cessation medication among PLWHA, 2013. clinicaltrials.gov/show/NCT01898195 (accessed 5 April 2016). [CRS: 9400129000001221] ; Shelley D, Krebs P, Schoenthaler A, Urbina A, Gonzalez M, Tseng T‐Y, et al. Correlates of adherence to varenicline among HIV+ smokers: a path analysis. Proceedings of the Society for Research on Nicotine and Tobacco 21st Annual Meeting; 2015 Feb 25‐28; Philadelphia. 2015:49 (PA14‐2). [CRS: 9400131000001079] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker R, Dorey E, Bramley D, Bullen C, Denny S, Elley C, et al. A theory‐based video messaging mobile phone intervention for smoking cessation: randomised controlled trial. Journal of Medical Internet Research 2011;13(1):e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Applegate BW, Raymond C, Collado‐Rodriguez A, Riley WT, Schneider NG. Improving adherence to nicotine gum by sms text messaging: a pilot study (RPOS3‐57). Proceedings of the Society for Research on Nicotine and Tobacco, 13th Annual Meeting; 2007 Feb 21‐24; Austin, Texas. 2007:14. [Google Scholar]
- Bennett DA, Emberson JR. Text messaging in smoking cessation: the Txt2stop trial [Comment]. Lancet 2011;378(9785):6‐7. [DOI] [PubMed] [Google Scholar]
- Blasco A, Carmona M, Fernandez‐Lozano I, Salvador CH, Pascual M, Sagredo PG, et al. Evaluation of a telemedicine service for the secondary prevention of coronary artery disease. Journal of Cardiopulmonary Rehabilitation and Prevention 2012;32(1):25‐31. [CENTRAL: 814645; CRS: 9400123000012404; 6831; PUBMED: 22113368] [DOI] [PubMed] [Google Scholar]
- Brendryen H, Kraft P. Happy Ending: a randomized controlled trial of a digital multi‐media smoking cessation intervention. Addiction 2008;103:478‐84. [doi:10.1111/j.1360‐0443.2007.02119.x] [DOI] [PubMed] [Google Scholar]
- Brendryen H, Drozd F, Kraft P. A digital smoking cessation program delivered through internet and cell phone without nicotine replacement (Happy Ending): randomized controlled trial. Journal of Medical Internet Research 2008;10(5):e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker JB, Mull KE, Kientz JA, Vilardaga R, Mercer LD, Akioka KJ, et al. Randomized, controlled pilot trial of a smartphone app for smoking cessation using acceptance and commitment therapy. Drug and Alcohol Dependence 2014;143(1):87‐94. [CENTRAL: 1047686; CRS: 9400050000000151; EMBASE: 2014721228; PUBMED: 25085225] [DOI] [PMC free article] [PubMed] [Google Scholar]; Heffner JL, Vilardaga R, Mercer LD, Kientz JA, Bricker JB. Feature‐level analysis of a novel smartphone application for smoking cessation. American Journal of Drug and Alcohol Abuse 2015;41(1):68‐73. [CENTRAL: 1036931; CRS: 9400129000003871; EMBASE: 2014973943] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller DB, Borland R, Bettinghaus EP, Shane JH, Zimmerman DE. Randomized trial of a smartphone mobile application compared to text messaging to support smoking cessation. Telemedicine Journal and E‐health 2014;20(3):206‐14. [CENTRAL: 1053488; CRS: 9400129000003463] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CK, Redfern J, Thiagalingam A, Jan S, Whittaker R, Hackett M, et al. Design and rationale of the tobacco, exercise and diet messages (TEXT ME) trial of a text message‐based intervention for ongoing prevention of cardiovascular disease in people with coronary disease: a randomised controlled trial protocol. BMJ Open 2012;2(1):e000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale LP, Whittaker R, Jiang Y, Stewart R, Rolleston A, Maddison R. Improving coronary heart disease self‐management using mobile technologies (Text4Heart): a randomised controlled trial protocol. Trials 2014;15:71. [CENTRAL: 984614; CRS: 9400129000001546; EMBASE: 2014230967; PUBMED: 24588893] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingrut W, Stewart L, Cheung K. Choice of smoking cessation counselling via phone, text, or email in emergency department patients. Canadian Journal of Emergency Medicine 2014;16:S86. [CENTRAL: 1009852; CRS: 9400129000003122; EMBASE: 75007022] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D, Kobinsky K, Smith SS, Kramer J, Theobald WE, Baker TB. Five population‐based interventions for smoking cessation: a MOST trial. Translational Behavioral Medicine 2014;4(4):382‐90. [CENTRAL: 1040081; CRS: 9400129000003838; EMBASE: 2015657527] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug S, Meyer C, Gross B, Schorr G, Thyrian JR, Kordy H, et al. Continuous individual support of smoking cessation in socially deprived young adults via mobile phones ‐ results of a pilot study. Gesundheitswesen 2008;70(6):364‐71. [DOI] [PubMed] [Google Scholar]
- Haug S, Meyer C, John U. Individual support of smoking cessation using text messaging: results of two pilot studies. Sucht 2008;54(5):316. [CENTRAL: 794115; CRS: 9400123000006096] [Google Scholar]; Haug S, Meyer C, Schorr G, Bauer S, John U. Continuous individual support of smoking cessation using text messaging: a pilot experimental study. Nicotine & Tobacco Research 2009;11(8):915‐23. [DOI] [PubMed] [Google Scholar]
- Haug S, Paz Castro R, Filler A, Kowatsch T, Fleisch E, Schaub MP. Efficacy of an internet and SMS‐based integrated smoking cessation and alcohol intervention for smoking cessation in young people: study protocol of a two‐arm cluster randomised controlled trial. BMC Public Health 2014;14:1140. [CENTRAL: 1053487; CRS: 9400129000003374] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P, Evers K, Levesque D, Prochaska J. Tailored texting to enhance a stage‐based online tailored program for veterans who smoke: a randomized breakthrough and benchmark trial. Personal communication. ; NCT01454999. Cell phone‐based expert systems for smoking cessation, 2011. clinicaltrials.gov/ct2/show/NCT01454999 (accessed 5 April 2016).
- Kiselev AR, Shvarts VA, Posnenkova OM, Gridnev VI, Dovgalevskii P, Oshchepkova EV, et al. Outpatient prophylaxis and treatment of arterial hypertension with application of mobile telephone systems and Internet techniques [In Russian]. Terapevticheskiĭ arkhiv 2011;83(4):46‐52. [PubMed] [Google Scholar]
- Lazev A, Vidrine D, Arduino R, Gritz E. Increasing access to smoking cessation treatment in a low‐income, HIV‐positive population: the feasibility of using cellular telephones. Nicotine & Tobacco Research 2004;6(2):281‐6. [doi: 10.1080/4622200410001676314] [DOI] [PubMed] [Google Scholar]
- Mehring M, Haag M, Linde K, Wagenpfeil S, Schneider A. Effects of a guided web‐based smoking cessation program with telephone counseling: a cluster randomized controlled trial. Journal of Medical Internet Research 2014;16(9):e218. [CENTRAL: 1051138; CRS: 9400129000003393] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton F, Prevost A, Gilbert H, Sutton S. Randomized controlled trial evaluation of a tailored leaflet and SMS text message self‐help intervention for pregnant smokers (MiQuit). Nicotine & Tobacco Research 2012;14(5):569‐77. [DOI] [PubMed] [Google Scholar]
- Obermayer J, Riley W, Asif O, Jean‐Mary J. College smoking cessation using cell phone text messaging. Journal of American College Health 2004;53(2):71‐8. [DOI] [PubMed] [Google Scholar]
- Pechmann C, Pan L, Delucchi K, Lakon CM, Prochaska JJ. Development of a Twitter‐based intervention for smoking cessation that encourages high‐quality social media interactions via automessages. Journal of Medical Internet Research 2015;17(2):e50. [CRS: 9400131000001029; PUBMED: 25707037] [DOI] [PMC free article] [PubMed] [Google Scholar]; Prochaska JJ, Pechmann C, Lakon C, Delucchi K. Randomized controlled trial of tweet2quit for smoking cessation. Proceeding of the Society for Research on Nicotine and Tobacco 21st Annual Meeting; 2015 Feb 25‐28; Philadelphia. 2015:82 (PA21‐2). [CRS: 9400131000001026] [Google Scholar]
- Peng WB, Schoech D. Evaluation of a web‐phone intervention system in changing smoking behavior ‐ a randomized controlled trial. Journal of Technology in Human Services 2013;31(3):248‐68. [CENTRAL: 1053486; CRS: 9400129000000455] [Google Scholar]
- Pollak KI, Lyna P, Bilheimer A, Farrell D, Gao X, Swamy GK, et al. A pilot study testing SMS text delivered scheduled gradual reduction to pregnant smokers. Nicotine & Tobacco Research 2013;15(10):1773‐6. [CENTRAL: 982524; CRS: 9400129000000412; EMBASE: 2014131762; PUBMED: 23569007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley W, Obermayer J, Jean‐Mary J. Internet and mobile phone text messaging intervention for college smokers. Journal of American College Health 2008;57(2):245‐8. [DOI] [PubMed] [Google Scholar]
- Shi HJ, Jiang XX, Yu CY, Zhang Y. Use of mobile phone text messaging to deliver an individualized smoking behaviour intervention in Chinese adolescents. Journal of Telemedicine and Telecare 2013;19(5):282‐7. [CENTRAL: 984362; CRS: 9400126000000380; PUBMED: 24163238] [DOI] [PubMed] [Google Scholar]
- Skov‐Ettrup LS, Dalum P, Ekholm O, Tolstrup JS. Reach and uptake of Internet‐ and phone‐based smoking cessation interventions: results from a randomized controlled trial. Preventive Medicine 2014;62:38‐43. [CENTRAL: 1001289; CRS: 9400129000000774; EMBASE: 2014140700] [DOI] [PubMed] [Google Scholar]; Skov‐Ettrup LS, Dalum P, Tolstrup JS. Internet‐based intervention and telephone counselling for smoking cessation: results from a 4‐arm randomised controlled trial. European Journal of Epidemiology 2013;28(1 Suppl 1):S48‐9. [CENTRAL: 1011884; CRS: 9400130000000339; EMBASE: 71300831] [Google Scholar]
- Skov‐Ettrup LS, Ringgaard LW, Dalum P, Flensborg‐Madsen T, Thygesen LC, Tolstrup JS. Comparing tailored and untailored text messages for smoking cessation: a randomized controlled trial among adolescent and young adult smokers. Health Education Research 2014;29(2):195‐205. [CRS: 9400129000003462; PUBMED: 24399268] [DOI] [PubMed] [Google Scholar]
- Snider J. Cell phone text messaging may boost smoking quit rates. Journal of the American Dental Association 2011;142(8):901‐2. [DOI] [PubMed] [Google Scholar]
- Stanczyk N, Bolman C, Adrichem M, Candel M, Muris J, Vries H. Comparison of text and video computer‐tailored interventions for smoking cessation: randomized controlled trial. Journal of Medical Internet Research 2014;16(3):e69. [CENTRAL: 1046762; CRS: 9400129000002402] [DOI] [PMC free article] [PubMed] [Google Scholar]; Stanczyk NE, Bolman C, Muris JW, Vries H. Study protocol of a Dutch smoking cessation e‐health program. BMC Public Health 2011;11:847. [CENTRAL: 814250; CRS: 9400100000000130; PUBMED: 22059446] [DOI] [PMC free article] [PubMed] [Google Scholar]; Stanczyk NE, Crutzen R, Bolman C, Muris J, Vries H. Influence of delivery strategy on message‐processing mechanisms and future adherence to a Dutch computer‐tailored smoking cessation intervention. Journal of Medical Internet Research 2013;15(2):e28. [CENTRAL: 873085; CRS: 9400107000001436; PUBMED: 23388554] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine D, Arduino R, Gritz E. Impact of a cell phone intervention on mediating mechanisms of smoking cessation in individuals living with HIV/AIDS. Nicotine & Tobacco Research 2006;8(Suppl 1):S103‐8. [DOI] [PubMed] [Google Scholar]; Vidrine D, Arduino R, Lazev A, Gritz E. A randomized trial of a proactive cellular telephone intervention for smokers living with HIV/AIDS. AIDS 2006;20:253‐60. [ISSN 0269‐9370] [DOI] [PubMed] [Google Scholar]
- Vilaplana J, Solsona F, Abella F, Cuadrado J, Alves R, Mateo J. S‐PC: an e‐treatment application for management of smoke‐quitting patients. Computer Methods and Programs in Biomedicine 2014;115(1):33‐45. [CENTRAL: 988111; CRS: 9400050000000047; EMBASE: 2014296941; PUBMED: 24742965] [DOI] [PubMed] [Google Scholar]
- Wizner B, Gaciong Z, Narkiewicz K, Grodzicki T. Education using SMS increases efficacy of treatment of hypertensive patients [Zwiększenie skuteczności terapii hipotensyjnej u pacjentów z nadciśnieniem tętniczym dzięki edukacji przez SMS]. Nadcisnienie Tetnicze 2009;13(3):147‐57. [Google Scholar]
- Ybarra M, Bagci Bosi AT, Korchmaros J, Emri S. A text messaging‐based smoking cessation program for adult smokers: randomized controlled trial. Journal of Medical Internet Research 2012;14(6):e172. [CENTRAL: 863878; CRS: 9400107000000182; PUBMED: 23271159] [DOI] [PMC free article] [PubMed] [Google Scholar]; Ybarra ML, Holtrop JS, Bagci Bosi AT, Emri S. Design considerations in developing a text messaging program aimed at smoking cessation. Journal of Medical Internet Research 2012;14(4):e103. [CRS: 9400123000016823; PUBMED: 22832182] [DOI] [PMC free article] [PubMed] [Google Scholar]; Ybarra ML, Holtrop JS, Bosi ATB, Bilir N, Korchmaros JD, Emri AKS. Feasibility and acceptability of a text messaging‐based smoking cessation program in Ankara, Turkey. Journal of Health Communication 2013;18(8):960‐73. [CRS: 9400126000000575; PUBMED: 23627304] [DOI] [PubMed] [Google Scholar]
- Ybarra M, Hotrop J, Prescott T, Rahbar M, Strong D. Pilot RCT results of Stop My Smoking (SMS) USA: a text messaging‐based smoking cessation program for young adults. Nicotine & Tobacco Research 2013;15(8):1388‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ybarra ML, Holtrop JS, Prescott TL, Strong D. Process evaluation of a mHealth program: lessons learned from Stop my Smoking USA, a text messaging‐based smoking cessation program for young adults. Patient Education and Counseling 2014;97(2):236‐43. [CRS: 9400131000000800; PUBMED: 25103183] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuhongxia L. The compliance of varenicline usage and the smoking abstinence rate via mobile phone text messaging combine with varenicline: a single‐blind, randomised control trial. Respirology 2011;16 :46‐7. [Google Scholar]
References to ongoing studies
- BinDhim NF, McGeechan K, Trevena L. Assessing the effect of an interactive decision‐aid smartphone smoking cessation application (app) on quit rates: a double‐blind automated randomised control trial protocol. BMJ Open 2014;4(7):e005371. [CENTRAL: 1041052; CRS: 9400129000003073] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Foster K, Naughton F, Leonardi‐Bee J, Sutton S, Ussher M, et al. Pilot study to evaluate a tailored text message intervention for pregnant smokers (MiQuit): study protocol for a randomised controlled trial . Trials 2015;16(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01103427. Mobile text messaging as an adjunct function to an Internet‐based smoking cessation intervention implemented in the general population and in a health care setting, 2010. clinicaltrials.gov/show/NCT01103427 (accessed 5 April 2016). [CRS: 9400131000001046]
- NCT01723163. Abstinence reinforcement therapy (ART) for rural veteran smokers, 2013. clinicaltrials.gov/show/NCT01723163 (accessed 5 April 2016). [CRS: 9400131000001061]
- NCT01746069. Evaluation of a reinforcement program using text messages through mobile phone in smoking cessation programs in primary care, 2013. clinicaltrials.gov/show/NCT01746069 (accessed 5 April 2016). [CRS: 9400131000001033]
- NCT01817842. Comparative effectiveness of web‐based mobile support for the DC Tobacco Quitline, 2010. clinicaltrials.gov/show/NCT01817842 (accessed 5 April 2016). [CRS: 9400131000001040]
- NCT01952236. Web and mobile smoking cessation interventions, 2015. clinicaltrials.gov/show/NCT01952236 (accessed 5 April 2016). [CRS: 9400131000001031]
- NCT01982110. A mindfulness based application for smoking cessation, 2013. clinicaltrials.gov/show/NCT01982110 (accessed 5 April 2016). [CRS: 9400131000001042]
- Baskerville NB, Struik LL, Hammond D, Guindon GE, Norman CD, Whittaker R, et al. Effect of a mobile phone intervention on quitting smoking in a young adult population of smokers: randomized controlled trial study protocol. JMIR Research Protocols 2015;1:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT01983150. Effect of a smartphone intervention on quitting smoking in a young adult population of smokers: a randomized controlled trial, 2014. clinicaltrials.gov/show/NCT01983150 (accessed 5 April 2016). [CRS: 9400131000001056]
- NCT01990079. Use of technological advances to prevent smoking relapse among smokers with PTSD, 2013. clinicaltrials.gov/show/NCT01990079 (accessed 5 April 2016). [CRS: 9400131000001036]
- NCT02021175. Adaptation and development of a web and cell phone quit smoking treatment for Korean youth, 2014. clinicaltrials.gov/show/NCT02021175 (accessed 5 April 2016). [CRS: 9400131000001091]
- NCT02037360. Mobile mindfulness training for smoking cessation, 2015. clinicaltrials.gov/show/NCT02037360 (accessed 5 April 2016). [CRS: 9400131000001048]
- Garrison KA, Pal P, Rojiani R, Dallery J, O'Malley SS, Brewer JA. A randomized controlled trial of smartphone‐based mindfulness training for smoking cessation: a study protocol. BMC Psychiatry 2015;15:83. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT02134509. Smartphone application for smoking cessation, 2014. clinicaltrials.gov/show/NCT02134509 (accessed 5 April 2016). [CRS: 9400131000001089]
- NCT02136498. Internet‐based medication adherence program for nicotine dependence treatment, 2014. clinicaltrials.gov/show/NCT02136498 (accessed 5 April 2016). [CRS: 9400131000001052]
- NCT02164383. A quit smoking study using smartphones, 2014. clinicaltrials.gov/show/NCT02164383 (accessed 5 April 2016). [CRS: 9400131000001038]
- NCT02207036. Social media intervention for young adult smokers, 2014. clinicaltrials.gov/show/NCT02207036 (accessed 5 April 2016). [CRS: 9400131000001071] ; Ramo DE, Thrul J, Delucchi KL, Ling PM, Hall SM, Prochaska JJ. The Tobacco Status Project (TSP): study protocol for a randomized controlled trial of a Facebook smoking cessation intervention for young adults. BMC Public Health2015:897. [DOI] [PMC free article] [PubMed]
- NCT02218281. Developing a smartphone app with mindfulness training for teen smoking cessation, 2014. clinicaltrials.gov/show/NCT02218281 (accessed 5 April 2016). [CRS: 9400131000001087]
- NCT02218944. Response inhibition training in smoking cessation, 2014. clinicaltrials.gov/show/NCT02218944 (accessed 5 April 2016). [CRS: 9400131000001067]
- NCT02237898. SCH: INT: harnessing the power of technology: MOMBA for postpartum smoking, 2014. clinicaltrials.gov/show/NCT02237898 (accessed 5 April 2016). [CRS: 9400131000001085]
- NCT02245308. Abstinence reinforcement therapy (ART) for homeless veteran smokers, 2014. clinicaltrials.gov/show/NCT02245308 (accessed 5 April 2016). [CRS: 9400131000001050]
- NCT02302859. Mobile smoking cessation treatment for underserved smokers: a pilot and feasibility study, 2015. clinicaltrials.gov/show/NCT02302859 (accessed 5 April 2016). [CRS: 9400131000001044]
- NCT02328794. Randomized clinical trial to reduce harm from tobacco, 2015. clinicaltrials.gov/show/NCT02328794 (accessed 5 April 2016). [CRS: 9400131000001081]
- NCT02367391. Pilot randomized trial of an automated smoking cessation intervention via mobile phone text messages as an adjunct to varenicline in primary care, 2015. clinicaltrials.gov/show/NCT02367391 (accessed 5 April 2016). [CRS: 9400131000001054] [DOI] [PMC free article] [PubMed]
- Valdivieso‐Lopez E, Flores‐Mateo G, Molina‐Gomez JD, Rey‐Renones C, Barrera Uriarte ML, Duch J, et al. Efficacy of a mobile application for smoking cessation in young people: study protocol for a clustered, randomized trial. BMC Public Health 2013;13:704. [CENTRAL: 873091; CRS: 9400126000000024; PUBMED: 23915067] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00948129. Enhancing cancer outreach for low‐income adults with innovative smoking cessation, 2010. clinicaltrials.gov/show/NCT00948129 (accessed 5 April 2016). [CRS: 9400123000013543] ; Vidrine DJ, Fletcher FE, Danysh HE, Marani S, Vidrine JI, Cantor SB, et al. A randomized controlled trial to assess the efficacy of an interactive mobile messaging intervention for underserved smokers: project ACTION. BMC Public Health 2012;12:696. [CENTRAL: 863874; CRS: 9400107000000036; EMBASE: 22920991; PUBMED: 22920991] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
- Abroms LC, Padmanabhan N, Thaweethai L, Phillips T. iPhone apps for smoking cessation: a content analysis. American Journal of Preventitive Medicine 2011;40(3):279‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abroms L, Whittaker R, Free C, Mendel Van Alstyne J, Schindler‐Ruwisch J. Recommended steps for developing and pretesting a text messaging program for health behavior change. JMIR mHealth and uHealth 2015;3(4):e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramley D, Riddell T, Whittaker R, Corbett T, Lin R‐B, Wills M. Smoking cessation using mobile phone text messaging is as effective in Maori as non‐Maori. New Zealand Medical Journal 2005;118(1216):1494‐504. [PubMed] [Google Scholar]
- Ericsson. Ericsson mobility report on the pulse of the networked society, 2015. www.ericsson.com/ericsson‐mobility‐report (accessed 5 April 2016).
- Fiore M, Jaén C, Baker T. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services, Public Health Service, 2008. [Google Scholar]
- Free C, Phillips G, Watson L, Galli L, Felix L, Edwards P, et al. The effectiveness of mobile‐health technologies to improve health care service delivery processes: a systematic review and meta‐analysis. PLoS Medicine 2013;10(1):e1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero C, Cairns J, Roberts I, Rodgers A, Whittaker R, Free C. The cost‐effectiveness of smoking cessation support delivered by mobile phone text messaging: Txt2stop. European Journal of Health Economics 2013;14:789‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz B, Lauckner C. Diabetes management via mobile phones: a systematic review . Telemedicine and e‐Health 2012;18(3):175‐84. [DOI] [PubMed] [Google Scholar]
- International Telecommunications Union. ICT Facts & Figures: The World in 2015. www.itu.int/en/ITU‐D/Statistics/Pages/facts/default.aspx (accessed 5 April 2016).
- Jha P, Peto R. Global effects of smoking, of quitting, and of taxing tobacco. New England Journal of Medicine 2014;370:60‐8. [DOI] [PubMed] [Google Scholar]
- Peto R, Awasthi S, Read S, Clark S, Bundy D. Vitamin A supplementation in Indian children ‐ authors' reply. Lancet 2013;382(9892):594‐6. [DOI] [PubMed] [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Hartmann‐Boyce J, Cahill K, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD000146.pub4] [DOI] [PubMed] [Google Scholar]
- Wellman RJ, Savageau JA, Godiwala S, Savageau N, Friedman K, Hazelton J, et al. A comparison of the Hooked on Nicotine Checklist and the Fagerstrom Test for Nicotine Dependence in adult smokers. Nicotine and Tobacco Research 2006;8(4):575‐80. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Whittaker R, Borland R, Bullen C, Lin RB, McRobbie H, Rodgers A. Mobile phone‐based interventions for smoking cessation. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD006611.pub2] [DOI] [PubMed] [Google Scholar]
- Whittaker R, McRobbie H, Bullen C, Borland R, Rodgers A, Gu Y. Mobile phone‐based interventions for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD006611.pub3] [DOI] [PubMed] [Google Scholar]


