Abstract
Background
Bacterial infections occurring during labour, childbirth, and the puerperium may be associated with considerable maternal and perinatal morbidity and mortality. Antibiotic prophylaxis might reduce wound infection incidence after an episiotomy, particularly in situations associated with a higher risk of postpartum perineal infection, such as midline episiotomy, extension of the incision, or in settings where the baseline risk of infection after vaginal birth is high. However, available evidence is unclear concerning the role of prophylactic antibiotics in preventing infections after an episiotomy.
Objectives
To assess whether routine antibiotic prophylaxis before or immediately after incision or repair of episiotomy for women with an uncomplicated vaginal birth, compared with either placebo or no antibiotic prophylaxis, prevents maternal infectious morbidities and improves outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth's Trials Register, LILACS, ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform (ICTRP) on 24 July 2017, and screened reference lists of retrieved studies.
Selection criteria
We considered randomised controlled trials, quasi‐randomised trials, and cluster‐randomised trials that compared the use of routine antibiotic prophylaxis for incision or repair of an episiotomy for women with otherwise normal vaginal births, compared with either placebo or no antibiotic prophylaxis.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data, and checked them for accuracy. We only found one quasi‐randomised trial that met the inclusion criteria and was included in the analysis, therefore, we did not perform a meta‐analysis.
Main results
We included one quasi‐RCT (with data from 73 women) in the review. The trial, which was conducted in a public hospital in Brazil, compared oral chloramphenicol 500 mg four times daily for 72 hours after episiotomy repair (N = 34) and no treatment (N = 39). We assessed most of the domains at high risk of bias because women were randomised according to even and odd numbers, allocation concealment was based on protocol number, there was no treatment or placebo administered in the control group, we were unclear about the blinding of outcome assessments, and outcomes were incompletely reported. We considered the other domains to be at low risk of bias. We downgraded the quality of the evidence for very serious design limitations (related to lack of random sequence generation, allocation concealment, and blinding) and imprecision of effect estimates (small sample sizes and wide confidence intervals (CI) of effect estimates).
We found very low‐quality evidence, from one trial of 73 women, that there was no clear indication that prophylactic antibiotics reduced the incidence of episiotomy wound dehiscence with infection (risk ratio (RR) 0.13, 95% CI 0.01 to 2.28), or without infection (RR 0.82, 95% CI 0.29 to 2.34). No cases of other puerperal infections (e.g. endometritis) were reported in either the antibiotic or control group.
The trial did not report on any of the secondary outcomes of interest for this review, including severe maternal infectious morbidity, discomfort or pain at the episiotomy wound site, sexual function postpartum, adverse effects of antibiotics, costs of care, women's satisfaction with care, and individual antimicrobial resistance.
Authors' conclusions
There was insufficient evidence to assess the clinical benefits or harms of routine antibiotic prophylaxis for episiotomy repair after normal birth. The only trial included in this review had several methodological limitations, with very serious limitations in design, and imprecision of effect estimates. In addition, the trial tested an antibiotic with limited application in current clinical practice. There is a need for a careful and rigorous assessment of the comparative benefits and harms of prophylactic antibiotics on infection morbidity after episiotomy, in well‐designed randomised controlled trials, using common antibiotics and regimens in current obstetric practice.
Plain language summary
Routine antibiotic use for episiotomy repair after normal vaginal birth
What is the issue?
Current research evidence favours a hospital policy of restrictive use of episiotomy, rather than routine episiotomy. However, the practice of performing an episiotomy is still very common among women giving birth vaginally, in many parts of the world. Bacterial infections associated with childbirth can cause considerable ill‐health for the mother and her baby, and even death. General infection control measures, such as hand hygiene, aseptic surgical techniques, disinfection of the surgical site, and sterilisation of instruments can help minimise the risk of episiotomy infection. Preventative antibiotics, or prophylaxis, might reduce wound infections after episiotomy, particularly in situations associated with a higher risk of infection, such as extension of the incision during childbirth, or in healthcare settings where the baseline risk of childbirth‐related infections is high.
Why is this important?
Women with an episiotomy may not require the routine use of antibiotics to prevent infection, particularly if general infection control measures have been respected. Inadequate use of antibiotics is associated with poorer outcomes, while still exposing women and their nursing babies to the risk of antibiotic‐related side effects. Healthcare costs may be increased with antibiotic use, and widespread use of antibiotics can lead to the emergence of antibiotic resistance.
What evidence did we find?
The review assessed whether routine use of antibiotics at the time of an episiotomy prevented infection for women with an uncomplicated vaginal birth, compared with either placebo, or no antibiotics. We searched for evidence (24 July 2017) from randomised controlled trials in the medical literature. We only identified one small trial that was conducted in a public hospital in Brazil and provided very low‐quality data from 73 women. The trial showed no clear difference between the groups, with or without antibiotics, of the number of women who experienced infection or breakdown of the episiotomy wound. No women developed infection of the lining of the uterus in either group. The trial did not report on any other outcomes of interest for this review.
What does this mean?
The current evidence on the impact of prophylactic antibiotics for prevention of infection after episiotomy is from one small trial with design limitations. The relatively low incidence of episiotomy infection, when infection control measures are well observed, raises questions about the potential added benefit of antibiotic prophylaxis, particularly when balanced against the risk of antibiotic‐related side effects for the mother, and her baby, and in terms of emerging antibiotic resistance. There is a need for a careful and rigorous assessment of the comparative benefits and harms of prophylactic antibiotics on infection morbidity after episiotomy, in well‐designed randomised controlled trials, using common antibiotics and regimens in current obstetric practice.
Summary of findings
Summary of findings for the main comparison. Antibiotic prophylaxis compared to no treatment for episiotomy repair following vaginal birth.
| Antibiotic prophylaxis compared to no treatment for episiotomy repair following vaginal birth | ||||||
|
Patient or population: women with episiotomy repair following vaginal birth Settings: public hospital, Brazil Intervention: antibiotic prophylaxis with oral chloramphenicol 500 mg four times daily for 72 hours after episiotomy repair Comparison: no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with no treatment | Risk with antibiotic prophylaxis | |||||
| Incidence of episiotomy wound infection with wound dehiscence | Study population | RR 0.13 (0.01 to 2.28) | 73 (1 quasi‐RCT) | ⊕⊝⊝⊝ very low1,2 | ||
| 103 per 1000 | 14 per 1000 (1 to 257) | |||||
| Incidence of episiotomy wound dehiscence without wound infection | Study population | RR 0.82 (0.29 to 2.34) | 73 (1 quasi‐RCT) | ⊕⊝⊝⊝ very low1,2 | ||
| 179 per 1000 | 151 per 1000 (52 to 439) | |||||
| Incidence of puerperal infection (endometritis) | Study population | not estimable | 73 (1 quasi‐RCT) | ⊕⊝⊝⊝ very low1,3 | There were no events in either group. | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Incidence of severe maternal infectious morbidity | Study population | ‐ | 0 (0 RCT) | ‐ | trial did not measure this outcome | |
| ‐ | ‐ | |||||
| Discomfort or pain at episiotomy wound site | ‐ | ‐ | ‐ | 0 (0 RCT) | ‐ | trial did not measure this outcome |
| Women's satisfaction with care | ‐ | ‐ | ‐ | 0 (0 RCT) | ‐ | trial did not measure this outcome |
| Adverse effects of antibiotics | Study population | ‐ | 0 (0 RCT) | ‐ | trial did not measure this outcome | |
| ‐ | ‐ | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 One study with very serious design limitations (‐2) 2 Wide confidence interval crossing the line of no effect, and few events (‐1) 3 No events (‐1)
Background
Bacterial infections occurring during labour, childbirth, and the puerperium might be associated with considerable maternal and perinatal morbidity and mortality. According to the last estimates, the global incidence of maternal puerperal infection is about 4.4% among live births, representing over 5.7 million cases per year, with important geographical variations (WHO 2005). Puerperal infections are still a life‐threatening condition and one of the leading direct causes of maternal mortality worldwide. They account for up to 15% of the total maternal deaths, with most of the deaths recorded in low‐ and middle‐income countries (Say 2014; Van Dillen 2010).
Different effective infection control measures are relevant to prevent infections around the time of childbirth, particularly at the facility level, including the use of prophylactic antibiotics for infection‐prone conditions. In this context, evidence is unclear about the role of prophylactic antibiotics in the prevention of infection after an episiotomy, a common obstetrical procedure.
Description of the condition
Episiotomy is the surgical enlargement of the vaginal orifice by an incision in the perineum during the last part of the second stage of labour. This procedure is performed with sterile scissors or scalpel, and is repaired with sutures (Thacker 1983). Seven different types of episiotomy have been described in the literature, but no standardised practice exists in terms of point of origin, angle of the cut, or the length of the incision (Kalis 2012). The most common angles are mediolateral and midline incisions.
Episiotomy was introduced in obstetric practice with the idea of facilitating vaginal births, and preventing maternal and fetal complications. Common clinical use of episiotomy include operative vaginal deliveries (forceps or vacuum), fetal dystocia (breech or face presentation, occiput posterior position), macrosomia (larger than average baby), shoulder dystocia, rigid perineum or threat of severe perineal rupture, maternal exhaustion, or to expedite childbirth in the case of suspected fetal compromise (fetal distress, fetal abnormal heart rate; Carroli 2009; Graham 2005; Thacker 1983). However, there is no clear consensus, as most of these indications and claimed benefits have not been confirmed by randomised controlled trials (RCTs) or observational studies.
In contrast, current evidence is in favour of restrictive use of episiotomy rather than routine episiotomy. A Cochrane review of eight RCTs showed that a restrictive episiotomy policy resulted in less severe perineal trauma, less suturing, and fewer wound complications than liberal episiotomy policies (Carroli 2009). No clear differences were found between the two policies in terms of severe vaginal or perineal trauma, dyspareunia (painful sexual intercourse), urinary incontinence, or pain outcomes.
The practice of episiotomy varies widely, according to demographic characteristics of the women, type, preferences, and experience of healthcare providers, and characteristics of the healthcare facility (e.g. type and size of the facility; Viswanathan 2005 (AHRQ); Friedman 2015). In spite of evidence suggesting the potential harms of a routine episiotomy policy, the practice is still very common among women giving birth vaginally in many parts of the world, with estimates that vary between and within countries. Reported rates vary between 9.7% in Sweden to 100% in Taiwan between 1990 and 2000 (Graham 2005). Rates seem to be lower in Europe (EURO‐PERISTAT 2013), and North America (Friedman 2015), than in Central and South America, South Africa, and Asia (Graham 2005; Kropp 2005). However, these trends are expected to decrease as restrictive episiotomy policies are implemented around the world (EURO‐PERISTAT 2013; Hartmann 2005).
An episiotomy is considered a clean‐contaminated wound, where the normal vaginal, bowel, or skin flora is presumed to contaminate the wound during the procedure (Tharpe 2008). Other potential sources of contamination by exogenous micro‐organisms include: the birth attendant, a poor surgical technique, and contaminated instruments or surgical environment. An infection of the episiotomy can be clinically defined as the presence of an elevated temperature, local pain, heat, redness, ecchymosis or discharge from the site of incision, the presence of perineal abscess or wound healing complications, or wound breakdown (Tharpe 2008). As with the majority of obstetrical skin and soft tissue infections, episiotomy infections are polymicrobial, and result mainly from contamination with both aerobes, including Gram‐negative bacilli, enterococci, Group B streptococci, and anaerobes (Hussein 2010; Newton 2008).
Data on the incidence of infection after episiotomy are scarce and confounded by several factors, such as lack of standard definition, including variations in diagnostic criteria and timing of infection assessment, and the inclusion of infections of other types of perineal tears. Despite the high risk of contamination with the normal flora of the female genital or gastrointestinal tract, the incidence of episiotomy infection seems to be relatively low, and is estimated between 0.3% and 5%, depending on the setting (Carroli 2009; Newton 2008; Thacker 1983; Tharpe 2008). Other complications potentially related to infection, such as episiotomy wound dehiscence and wound healing problems are more frequent (Carroli 2009). While skin and soft‐tissue infections after episiotomy are rare postpartum complications, they can be associated with high mortality and morbidity, including necrotising fasciitis, septicaemia, and tissue necrosis (Gravett 2012).
Description of the intervention
The goal of antibiotic prophylaxis is to prevent infection by reaching therapeutic tissue levels at the time microbial contamination is most likely to occur (ACOG 2011). Prophylaxis is characterised by the administration of broad‐spectrum antibiotics (e.g. ampicillin, cephalosporin, or a combination of antibiotics) that are effective against the micro‐organism most likely to cause infections, which are given before, during, or immediately after the procedure for a short period of time (single dose or for less than 24 hours), in the absence of any sign of infection (ACOG 2011; Van Schalkwyk 2010).
Antibiotic prophylaxis is recommended to avoid infectious complications of infection‐prone obstetrical procedures, such as caesarean section, manual removal of the placenta, and repair of third‐ or fourth‐degree perineal tears (WHO 2015). Episiotomies are anatomically similar to a second‐degree perineal laceration, involving the vaginal mucosa, connective tissue, and underlying muscles, and might not warrant the routine use of prophylactic antibiotics (ACOG 2011; NICE 2014; Van Schalkwyk 2010; WHO 2015). However, the use of prophylactic antibiotics for episiotomies seems to vary widely. While in high‐income countries there is, to our knowledge, no report on the use of prophylactic antibiotics for episiotomies, and clinical recommendations do not mention their use in the absence of infection (ACOG 2011; NICE 2014; Van Schalkwyk 2010), it seems to be very common practice in some low‐income countries, where the majority of women have episiotomies and receive prophylactic antibiotics (Sharma 2008).
How the intervention might work
General infection control measures, such as hand hygiene, aseptic surgical techniques, disinfection of site, and sterilisation of instruments used to perform episiotomy minimise the risk of episiotomy infection (Hussein 2010; Kamel 2014; Tharpe 2008). The use of different repair techniques and materials also might affect the risk of infection, however, evidence is limited. A systematic review of randomised controlled trials by the Agency for Healthcare Research and Quality suggested that a two‐layer repair (perineal skin not sutured) technique appeared to decrease the risk of wound infections compared to a three‐layer repair (Viswanathan 2005 (AHRQ)). The evidence was unclear on the impact of different materials used for repair. In general, trials found no clear differences between non absorbable or absorbable sutures, or tissue adhesives. Two recently published Cochrane reviews on materials and methods for episiotomy repair did not specifically report on perineal wound infection, however, these reviews showed little differences between groups for infection‐related outcomes, such as wound dehiscence or gaping, when different suture materials or techniques were compared, although authors reported high heterogeneity for these outcomes (Kettle 2010; Kettle 2012).
Antibiotic prophylaxis might reduce the incidence of wound infection after episiotomy by decreasing the bacterial load that might cause postpartum infection. This may apply particularly in situations associated with higher risk of postpartum perineal infection, such as midline episiotomy, extension of the incision, or in settings where the baseline risk of infection after vaginal birth is high (Tharpe 2008). However, evidence is scarce on the use of routine antibiotic prophylaxis for conditions usually associated with the practice of episiotomy, such as operative vaginal deliveries, or complications of episiotomy, such as third‐ or fourth‐degree perineal lacerations. A previous Cochrane review that included one trial involving 393 women, found no evidence supporting the routine use of prophylactic antibiotics to reduce infectious risk after operative vaginal birth (Liabsuetrakul 2014). Evidence from another Cochrane review including one trial involving 147 women, found insufficient evidence supporting the use of routine prophylactic antibiotics in women with a third‐ or fourth‐degree perineal tear postpartum (Buppasiri 2014a).
Advantages of wide‐spread use of prophylactic antibiotics has to be balanced against the disadvantages, such as potential adverse effects for the mother and the baby, including disruption of the normal microbial flora, increased risk of resistant bacterial infections, allergic reactions, and increased healthcare costs (ACOG 2011; Newton 2008). Indiscrimanate use of prophylactic antibiotics might also contribute to the rise in individual and environmental antimicrobial resistance at the facility and community levels in many parts of the world (WHO 2014).
Why it is important to do this review
Available evidence is unclear about the role of prophylactic antibiotics in preventing infections after episiotomy. Indeed, most women requiring perineal repair after an episiotomy present with no complications, but a small number might experience pain and healing complications related to infection (Carroli 2009; Thacker 1983; Tharpe 2008). Along with the infection control measures mentioned above, antibiotic prophylaxis may have the potential to further prevent some cases of infection and to improve maternal outcomes in the puerperium. Reduction of infections of the genital tract after vaginal birth might also contribute to reducing the burden related to treatment of infections, maternal re‐hospitalisations, and long‐term complications (Gravett 2012). It might also minimise the interference of infection‐related morbidities with mother‐infant bonding in the first days after birth.
It is well known that sub‐optimal use of antibiotic prophylaxis is associated with poor outcomes, and increases the risk of antibiotic‐related side effects for the mother and her nursing baby, and healthcare costs (ACOG 2011; Newton 2008). A major concern regarding the routine and indiscriminate use of prophylactic antibiotics is the emergence of antibiotic resistance in obstetric populations, particularly in settings where episiotomy is still common. As part of the global efforts to reduce antimicrobial resistance, and in accordance with the WHO Global Strategy for Containment of Antimicrobial Resistance (WHO 2001), antibiotics should be administered only when there is a clear medical indication, and where the expected benefits outweigh the potential harms.
Objectives
To assess whether routine antibiotic prophylaxis before or immediately after incision or repair of episiotomy for women with an uncomplicated vaginal birth, compared with either placebo or no antibiotic prophylaxis, prevents maternal infectious morbidities and improves outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials, quasi‐randomised trials, and cluster‐randomised trials that compared the use of routine antibiotic prophylaxis, before or immediately after incision or repair of episiotomy for women with otherwise normal vaginal births, with either placebo or no antibiotic prophylaxis. We included studies published in abstract form only, provided sufficient information was available. In future updates, we will include abstracts for which we had insufficient information, once the full publication is available, or the authors provide more information.
We will exclude cross‐over trials, as this design is not appropriate for the intervention of interest.
Types of participants
All pregnant women who underwent an episiotomy for a uncomplicated vaginal birth, regardless of the gestational age at the time of birth. We excluded women who had intrapartum procedures that might have required an episiotomy (vacuum, forceps), or third‐ or fourth‐degree vaginal tears.
Types of interventions
The main comparison of the review was the use of any regimen of routine antibiotic prophylaxis before or immediately after incision or repair of an episiotomy, compared with either placebo or no antibiotic prophylaxis.
Types of outcome measures
Primary outcomes
Incidence of episiotomy wound infection (including oedema, erythema, serosanguinous, or frankly purulent material), as defined by trial authors.
Incidence of episiotomy wound dehiscence (breakdown).
Incidence of puerperal infection (e.g. endometritis with or without myometritis, and with or without salpingitis causing maternal febrile morbidity after an episiotomy), as defined by authors.
Secondary outcomes
Incidence of severe maternal infectious morbidity (septicaemia, septic shock, laparotomy or hysterectomy for infection, or maternal intensive care unit (ICU) admission), as defined by trial authors.
Length of maternal hospital stay.
Discomfort or pain at episiotomy wound site, as defined by author.
Sexual function postpartum (dyspareunia, sexual satisfaction, sexual sensation, time‐to‐resuming sexual intercourse), as defined by trials authors.
Adverse effects of antibiotics (maternal: allergic reaction, nausea, vomiting, diarrhoea, skin rashes, anaphylaxis; neonatal: allergic reaction, diarrhoea, skin rashes, anaphylaxis).
Cost of care, including re‐admission to hospital, cost of antibiotic treatment.
Women's satisfaction with care, as reported by trial authors.
Individual antimicrobial resistance (e.g. no response to first‐line antibiotic treatment, culture of antibiotic‐resistant bacterial strains), as reported by trial authors.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (24 July 2017).
The Register is a database containing over 23,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the review.
In addition to the search carried out by the Information Specialist, we searched LILACS using the search strategy given in Appendix 1 (24 July 2017).
We also searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports using the search terms in Appendix 2 (24 July 2017).
Searching other resources
We searched the reference lists of identified studies for any relevant trial studies.
We did not apply any language or date restrictions.
Data collection and analysis
The methods described below were used to assess risks of bias in included studies, and the quality of the evidence using the GRADE approach, following standard procedures of Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors (MB and CEC) independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion.
Figure 1 shows the study flow diagram, mapping out the number of records identified, included and excluded.
1.
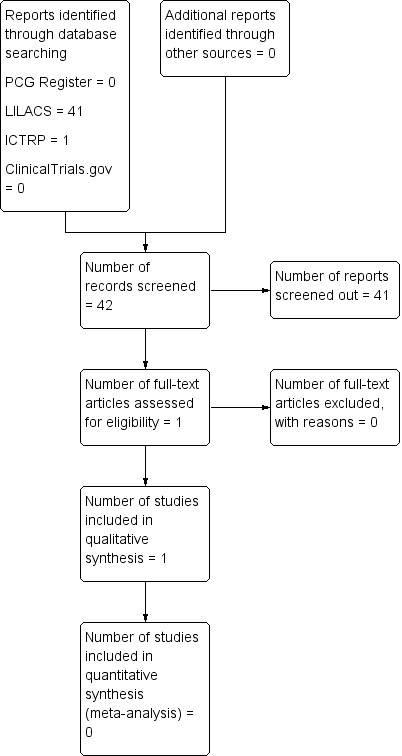
Study flow diagram
Data extraction and management
We designed a form to extract data. For the eligible study, two review authors (MB and CEC) independently extracted the data using the agreed form. We resolved any disagreement through discussion. We entered data into Review Manager 5 software, and checked for accuracy (RevMan 2014). We did not contact the original study authors because the reported information was considered sufficient.
Assessment of risk of bias in included studies
Two review authors (MB and CEC) independently assessed risk of bias for the study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement through discussion.
(1) Random sequence generation (checking for possible selection bias)
For the included study, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table, computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For the included study, we described the method used to conceal allocation to interventions prior to assignment, and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation, consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For the included study, we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For the included study, we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For the included study, and for each outcome or class of outcomes, we described the completeness of data, including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, and the numbers included in the analysis at each stage (compared with the total randomised participants), whether reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. No missing data were re‐included in the analyses undertaken.
We assessed methods as:
low risk of bias (e.g. no missing outcome data or less than 20%, missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups, ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For the included study, we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study’s pre‐specified outcomes were reported, one or more reported primary outcomes were not pre‐specified, outcomes of interest were reported incompletely and so could not be used, study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For the included study, we described any important concerns we had about other possible sources of bias.
We assessed whether the study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether the study was at high risk of bias, according to the criteria given in the Handbook of Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias, and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias by undertaking sensitivity analyses ‐ see Sensitivity analysis.
Assessing the quality of the evidence using GRADE
We assessed the quality of the evidence using the GRADE approach, as outlined in the GRADE Handbook, in order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons (Schünemann 2013).
Incidence of episiotomy wound infection (including oedema, erythema, serosanguinous, or frankly purulent material), as defined by trial authors.
Incidence of episiotomy wound dehiscence (breakdown).
Incidence of puerperal infection (e.g. endometritis with or without myometritis and with or without salpingitis causing maternal febrile morbidity after an episiotomy), as defined by authors.
Incidence of severe maternal infectious morbidity (septicaemia, septic shock, laparotomy, or hysterectomy for infection, or maternal ICU admission), as defined by trial authors.
Discomfort or pain at episiotomy wound site, as defined by author.
Women's satisfaction with care, as reported by trial authors.
Adverse effects of antibiotics (maternal: allergic reaction, nausea, vomiting, diarrhoea, skin rashes, anaphylaxis; neonatal: allergic reaction, diarrhoea, skin rashes, anaphylaxis).
We used the GRADEpro Guideline Development Tool (GRADEpro GDT) to import data from Review Manager 5, in order to create ’Summary of findings’ tables (RevMan 2014). A summary of the intervention effect and a measure of quality for each of the above outcomes were produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratios with 95% confidence intervals.
Continuous data
We did not identify any continuous outcome data. However, if we identify continuous outcome data in future updates of this review, we will use the mean difference if outcomes are measured in the same way between trials. We will use standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials. However, if we identify cluster‐randomised trials in future updates of this review, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Handbook of Systematic Reviews of Interventions, Section 16.3.4 or 16.3.6, using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs, and the interaction between the effect of the intervention and the choice of the randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit, and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We did not plan to include cross‐over trials.
Other unit of analysis issues
We did not identify any trials with more than two treatment groups. However, if we identify trials with more than two treatment groups in future updates of this review, we will consider trials with more than two treatment groups for inclusion if at least one intervention group is relevant to the systematic review. If multiple groups from one study are relevant for inclusion, we plan to combine all relevant experimental intervention groups of the study into a single group, and to combine all relevant control intervention groups into a single control group, if appropriate.
Dealing with missing data
We noted levels of attrition for the only trial included in this review. We were not able to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
We were unable to carry out analysis on an intention‐to‐treat basis because information was not available. The denominator for each outcome in each trial was to be the number randomised minus any participants whose outcomes were known to be missing. In future update, and for all outcomes, we plan to carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we will attempt to include all participants randomised to each group in the analyses, and all participants will be analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We were unable to carry out meta‐analysis in this review. In future update, we will assess statistical heterogeneity in each meta‐analysis using the Tau², I², and Chi² statistics. We will regard heterogeneity as substantial if an I² is greater than 30%, and either Tau² is greater than zero, or P < 0.10 in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager 5 software (RevMan 2014). Meta‐analysis was not possible because we only included one study. In future updates, we will use fixed‐effect meta‐analysis for combining data when it is reasonable to assume that studies are estimating the same underlying treatment effect, i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average of the range of possible treatment effects, and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We did not carry out meta‐analysis in this review, as all women in the intervention arm of the only trial included in this review received antibiotic prophylaxis immediately after episiotomy repair. No information was available on the type of episiotomy. In future updates, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses.
We did not carry out subgroup analyses. In future updates, we plan to carry out the following subgroup analysis.
Type of episiotomy (mediolateral episiotomy versus other type of episiotomy, mediolateral episiotomy versus median episiotomy).
Time of prophylactic antibiotic administration (before episiotomy incision versus antibiotic prophylaxis after incision or repair).
Subgroup analysis will be restricted to the review's primary outcomes.
We will assess subgroup differences by interaction tests available within Review Manager 5. We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
In future updates, we will undertake sensitivity analysis for primary outcomes based on the 'Risk of bias' assessment for allocation concealment and attrition rates. We will conduct sensitivity analyses by removing studies that are at high risk of bias (such as quasi‐randomised studies) for these domains, in order to assess whether this makes any difference to the overall result. If cluster‐randomised trials are included, we will investigate the effect of different values of the ICC.
Results
Description of studies
Results of the search
See: Figure 1.
We did not find any studies during the search of the Cochrane Pregnancy and Childbirth Group’s Trials Register. The search of the LILACS database yielded 41 titles; after independent title and abstract screening by two authors, we retrieved one report for full‐text evaluation. We identified one report during the search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
Included studies
Design
This review includes one randomised controlled trial (Neto 1990).
Sample sizes
Neto 1990 included 80 women with normal births and episiotomies, 73 of whom were followed‐up and included in the analysis, 34 in the intervention arm, and 39 in the non‐treated control arm.
Setting
The trial was conducted in one public hospital in Brazil, between October 1988 and September 1989.
Participants
Neto 1990 included 34 women in the intervention arm and 39 in the control no‐treated arm. They reported no baseline differences between groups regarding race, maternal age, parity, gestational age, length of labour, length of rupture of membranes, or number of vaginal examinations.
Interventions and comparisons
The included trial compared oral chloramphenicol 500 mg four times daily for 72 hours after episiotomy repair versus no treatment.
Outcomes
Neto 1990 measured episiotomy dehiscence (defined as wound rupture without signs of infection), episiotomy infection (defined as pain, heat, redness or purulent discharge, and wound rupture), and puerperal endometritis assessed at 10 days postpartum (defined as two of the following; fever, hypogastric pain, uterine involution, abnormal lochia).
Sources of trial funding and declarations of interest
Neto 1990 did not provide information relating to sources of trial funding or declarations of interest.
Excluded studies
We did not exclude any studies.
Risk of bias in included studies
We summarised the 'Risk of bias' assessment in Figure 2. Overall, we assessed the potential risk of bias in the included study to be high.
2.

Risk of bias summary: review authors' judgements about each 'risk of bias' domain
Allocation
We assessed the risk of selection bias as high for the included trial. The trial used a non‐random sequence generation and allocation concealment approach, according to protocol number (even and odd numbers).
Blinding
We assessed the risk of performance bias as high for the included trial, as the control arm received no treatment. We considered the risk of detection bias to be unclear, as no information was available on whether physicians who evaluated the women at 10 days postpartum were blinded to the treatment allocation.
Incomplete outcome data
We assessed the risk of attrition bias as high, as information was not available for all 80 women randomised in the included trial. Six women in the intervention group and one in the control group were missed for follow‐up at 10 days postpartum when the outcomes were assessed. However, there were no differences reported in baseline characteristics among those followed‐up.
Selective reporting
We assessed the risk of reporting bias as unclear, as Neto 1990 did not provide sufficient information to assess the risk of reporting bias.
Other potential sources of bias
It was unclear if other areas of potential bias existed, based on information available from Neto 1990.
Effects of interventions
See: Table 1
Antibiotic versus no treatment
Primary outcomes
We adjusted the reporting of the outcomes 'wound infection' and 'wound dehiscence', as the included trial reported separately wound dehiscence with or without infection.
Incidence of episiotomy wound infection (including oedema, erythema, serosanguinous, or frankly purulent material)
There was no clear difference between the antibiotic group and the control group on the incidence of episiotomy wound infection with dehiscence (risk ratio (RR) 0.13, 95% confidence interval (CI) 0.01 to 2.28; one trial; 73 women; very low‐quality evidence; Analysis 1.1).
1.1. Analysis.
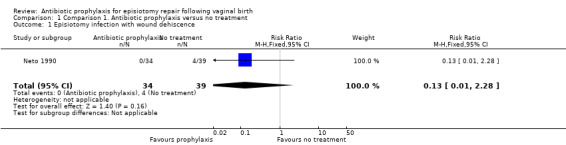
Comparison 1 Comparison 1. Antibiotic prophylaxis versus no treatment, Outcome 1 Episiotomy infection with wound dehiscence.
Incidence of episiotomy wound dehiscence (breakdown)
There was no clear difference between the antibiotic group and the control group on the incidence of episiotomy wound dehiscence without infection (RR 0.82, 95% CI 0.29 to 2.34; one trial; 73 women; very low‐quality evidence; Analysis 1.2). There was no clear difference in the overall risk of wound dehiscence with or without infection when these outcomes were combined (RR 0.52, 95% CI 0.20 to 1.35; one trial; 73 women; very low‐quality evidence; Analysis 1.3).
1.2. Analysis.
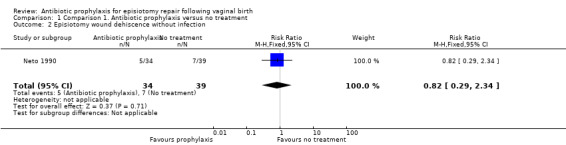
Comparison 1 Comparison 1. Antibiotic prophylaxis versus no treatment, Outcome 2 Episiotomy wound dehiscence without infection.
1.3. Analysis.
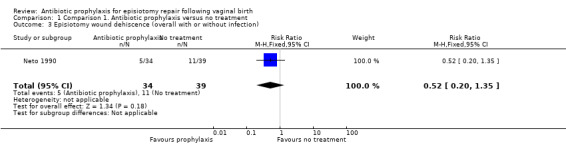
Comparison 1 Comparison 1. Antibiotic prophylaxis versus no treatment, Outcome 3 Episiotomy wound dehiscence (overall with or without infection).
Incidence of puerperal infection (e.g. endometritis with or without myometritis and with or without salpingitis causing maternal febrile morbidity after an episiotomy)
The only additional puerperal infection reported in the included study was endometritis. No cases of postpartum endometritis were reported in either the antibiotic or control group. No other puerperal infection was measured in the included trial (73 women; Analysis 1.4).
1.4. Analysis.
Comparison 1 Comparison 1. Antibiotic prophylaxis versus no treatment, Outcome 4 Incidence of puerperal infection (endometritis).
Secondary outcomes
The only trial we included in this review did not provide data on any of the pre‐specified secondary outcomes. No data were reported on the incidence of severe maternal infectious morbidity (septicaemia, septic shock, laparotomy or hysterectomy for infection, or maternal intensive care unit admission), length of maternal hospital stay, discomfort or pain at episiotomy wound site, sexual function postpartum (dyspareunia, sexual satisfaction, sexual sensation, time‐to‐resuming sexual intercourse), adverse effects of antibiotics (maternal: allergic reaction, nausea, vomiting, diarrhoea, skin rashes, anaphylaxis; neonatal: allergic reaction, diarrhoea, skin rashes, anaphylaxis), cost of care (re‐admission to hospital, cost of antibiotic treatment), women's satisfaction with care, or individual antimicrobial resistance (e.g. no response to first‐line antibiotic treatment, culture of antibiotic‐resistant bacteria strains).
Discussion
Summary of main results
The only study included in this review showed no clear differences between women who received chloramphenicol after repair of an episiotomy and those who received no treatment, in the incidence of postpartum infection, wound dehiscence, wound infection, or postpartum endometritis. The very‐low quality of the evidence limits the interpretation of the results. No data were provided for any of the pre‐specified secondary outcomes.
Overall completeness and applicability of evidence
The included study provided data from a public hospital in one middle‐income country (Brazil). It was a quasi‐RCT with serious design limitations, and used an antibiotic regime (chloramphenicol) with limited use in current practice, given its side effects. This raises questions about any applicability of the results in current obstetric practice.
Quality of the evidence
We only included one small trial with 73 women, and assessed the overall risk of bias as high, since there was inadequate generation of a randomised sequence, concealment of allocation, and blinding.
We graded the level of evidence for all GRADE outcomes (where reported) as very‐low quality, because of very serious design limitations and small sample sizes with wide confidence intervals (Table 1).
Potential biases in the review process
We adhered to the recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We minimised potential review biases by searching the LILACS database for potential relevant trials published in journals from Latin America and the Caribbean region. This additional search led to the inclusion of the only trial in this review, which was not identified by Cochrane Pregnancy and Childbirth’s Trials Register.
Agreements and disagreements with other studies or reviews
A previous systematic review of non‐observational studies found no studies that looked at the use of prophylactic antibiotics after episiotomy to prevent infectious morbidity (WHO 2015). Another Cochrane review (Bonet 2016a) looking at prophylactic antibiotics for prevention of infection after uncomplicated vaginal birth included episiotomy infection as an outcome is currently being prepared.
Authors' conclusions
Implications for practice.
We found insufficient data to assess the clinical benefit of routine administration of antibiotic prophylaxis for episiotomy after normal birth. However, the relatively low incidence of episiotomy infection when infection control measures are well observed, raises questions about the potential added benefits of antibiotic prophylaxis, particularly when balanced against the risk of antibiotic‐related side effects for the mother and baby, and in terms of antibiotic resistance. Indeed, episiotomies are anatomically similar to a second‐degree perineal laceration, involving the vaginal mucosa, connective tissue and underlying muscles, and might not require the routine use of prophylactic antibiotics. In addition, the relatively high proportion of episiotomies in some settings, would mean that an important number of women would be exposed to antibiotics, probably unnecessarily.
Implications for research.
Rigorous research is needed to assess the effect of prophylactic antibiotics on the incidence of infections after episiotomy. This review highlighted the need of well‐designed randomised controlled trials to evaluate the potential benefits and harms of antibiotic prophylaxis for women with episiotomies, specially in settings or populations at a higher risk of infection. These trials could evaluate antibiotics and regimens that are considered safe for use in the obstetric population. Future research may consider reporting on severe maternal infectious morbidity, discomfort or pain at the episiotomy wound site, sexual function postpartum, adverse effects of antibiotics, costs of care, women's satisfaction with care, and individual antimicrobial resistance.
Acknowledgements
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
This work was supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization.
Chioma E Chibueze was financially supported by a grant from the National Center for Child Health and Development, Japan 27B‐10, 26A‐5.
The World Health Organization, Erika Ota, and Chioma E Chibueze retain copyright and all other rights in their respective contributions to the manuscript of this review, as submitted for publication.
As part of the pre‐publication editorial process, this review received comments from three peers (an editor and two referees who are external to the editorial team), a member of Cochrane Pregnancy and Childbirth's international panel of consumers, and the Group's Statistical Adviser.
Appendices
Appendix 1. LILACS search strategy
Search performed in LILACS
Step 1: ((antibiotico) OR (antibiótico) OR (antibiotics))
Step 2: ((episiotomia) OR (episiotomía) OR (episiotomy))
Appendix 2. Search terms for ICTRP and ClinicalTrials.gov
episiotomy AND antibiotics
Data and analyses
Comparison 1. Comparison 1. Antibiotic prophylaxis versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Episiotomy infection with wound dehiscence | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.28] |
| 2 Episiotomy wound dehiscence without infection | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.29, 2.34] |
| 3 Episiotomy wound dehiscence (overall with or without infection) | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.20, 1.35] |
| 4 Incidence of puerperal infection (endometritis) | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Neto 1990.
| Methods | Quasi‐randomised controlled trial | |
| Participants | 80 women with normal labour and episiotomy in 1 public hospital, recruited between October 1988 and September 1989. | |
| Interventions | Intervention arm received oral chloramphenicol 500 mg 4 times daily for 72 hours after episiotomy repair. Control arm received no treatment. | |
| Outcomes | A total of 73 women included in analysis, 34 in intervention arm and 39 in control arm. Outcomes measured included episiotomy dehiscence (wound rupture without signs of infection), episiotomy infection (pain, heat, redness, or purulent discharge and wound rupture), and puerperal endometritis assessed at 10 days postpartum (defined as 2 of the following; fever, hypogastric pain, uterine involution, abnormal lochia). | |
| Notes | Florianopolis, Brazil. Exclusion of 7 women lost to follow‐up at 10 days postpartum. All women were from low socioeconomic class. All women attended by registrars. Funding: not reported. Conflicts of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomised according to protocol number (even and odd numbers) |
| Allocation concealment (selection bias) | High risk | Allocation concealment based on protocol number |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not double‐blinded. Control arm received no treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unknown. No information on whether physicians who evaluated the women at 10 days postpartum were blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 women in intervention group and 1 in control group were missing for follow‐up, but no differences reported in baseline characteristics among those followed‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | No other sources of bias noted. |
Differences between protocol and review
We adjusted the reporting of the outcomes 'wound infection' and 'wound dehiscence', as the included trial reported separately wound dehiscence with or without infection.
There are no other differences between the published protocol for this review and this full review (Bonet 2016b).
Contributions of authors
All review authors were involved in development of the protocol. M Bonet and CE Chibueze assessed relevant trials, extracted, entered, and checked data from trials into Review Manager 5. All authors contributed to the interpretation of the data. M Bonet and CE Chibueze drafted the review. All authors read and approved the final version of the review for publication.
Sources of support
Internal sources
-
The Grant of National Center for Child Health and Development, Japan.
27B‐10, 26A‐5
External sources
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), the Department of Reproductive Health and Research (RHR), World Health Organization, Geneva, Switzerland.
-
Japan Agency for Medical Research and Development, Japan.
The National Research Center for Child Health and Development, Japan receives government funding (AMED No.27300101) from the Clinical Research Program for Child Health and Development, AMED, Japan to support the Cochrane Pregnancy and Childbirth Satellite in Japan.
Declarations of interest
Mercedes Bonet: none known.
Erika Ota: none known.
Chioma E Chibueze contribution to this review was financially supported by a grant from the National Center for Child Health and Development, Japan 27B‐10, 26A‐5.
Olufemi T Oladapo: none known.
New
References
References to studies included in this review
Neto 1990 {published data only}
- Neto S, Goncalves JA, Andrade LF. Clinical evaluation of the chloramphenicol use as a prophylactic antibiotic in the vaginal delivery with episiotomy [Avaliacao clinica do emprego do cloranfenicol como antibiotico profilatico no parto normal com episiotomia]. ACM: Arquivos Catarinenses de Medicina 1990;19(2):97‐102. [Google Scholar]
Additional references
ACOG 2011
- American College of Obstetricians and Gynecologist. ACOG Practice Bulletin No. 120: Use of prophylactic antibiotics in labor and delivery. Obstetrics and Gynecology 2011;117(6):1472‐83. [PUBMED: 21606770] [DOI] [PubMed] [Google Scholar]
Bonet 2016a
- Bonet M, Ota E, Chibueze CE, Oladapo OT. Routine antibiotic prophylaxis after normal vaginal birth for reducing maternal infectious morbidity. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD012137] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buppasiri 2014a
- Buppasiri P, Lumbiganon P, Thinkhamrop J, Thinkhamrop B. Antibiotic prophylaxis for third‐ and fourth‐degree perineal tear during vaginal birth. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD005125.pub4] [DOI] [PubMed] [Google Scholar]
Carroli 2009
- Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD000081.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
EURO‐PERISTAT 2013
- EURO‐PERISTAT Project with SCPE and EUROCAT. European Perinatal Health Report. The health and care of pregnant women and babies in Europe in 2010. www.europeristat.com (accessed 2015) 2013.
Friedman 2015
- Friedman AM, Ananth CV, Prendergast E, D' Alton ME, Wright JD. Variation in and factors associated with use of episiotomy. JAMA 2015;313(2):197‐9. [PUBMED: 25585333] [DOI] [PubMed] [Google Scholar]
Graham 2005
- Graham ID, Carroli G, Davies C, Medves JM. Episiotomy rates around the world: an update. Birth (Berkeley, Calif.) 2005;32(3):219‐23. [PUBMED: 16128977] [DOI] [PubMed] [Google Scholar]
Gravett 2012
- Gravett CA, Gravett MG, Martin ET, Bernson JD, Khan S, Boyle DS, et al. Serious and life‐threatening pregnancy‐related infections: opportunities to reduce the global burden. PLoS Medicine 2012;9(10):e1001324. [PUBMED: 23055837] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann 2005
- Hartmann K, Viswanathan M, Palmieri R, Gartlehner G, Thorp J Jr, Lohr KN. Outcomes of routine episiotomy: a systematic review. JAMA 2005;293(17):2141‐8. [PUBMED: 15870418] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hussein 2010
- Hussein J, Walker L. Chapter 8. Puerperal sepsis in low‐ and middle‐income settings: past, present and future. Maternal and Infant Deaths: Chasing Millennium Development Goals 4 & 5. London: RCOG Press, 2010:131‐47. [Google Scholar]
Kalis 2012
- Kalis V, Laine K, Leeuw JW, Ismail KM, Tincello DG. Classification of episiotomy: towards a standardisation of terminology. BJOG: an international journal of obstetrics and gynaecology 2012;119(5):522‐6. [ PubMed: 22304364] 2012;119(5):522‐6. [PUBMED: 22304364] [DOI] [PubMed] [Google Scholar]
Kamel 2014
- Kamel A, Khaled M. Episiotomy and obstetric perineal wound dehiscence: beyond soreness. Journal of Obstetrics and Gynaecology 2014;34(3):215‐7. [PUBMED: 24484355] [DOI] [PubMed] [Google Scholar]
Kettle 2010
- Kettle C, Dowswell T, Ismail KMK. Absorbable suture materials for primary repair of episiotomy and second degree tears. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD000006.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kettle 2012
- Kettle C, Dowswell T, Ismail KMK. Continuous and interrupted suturing techniques for repair of episiotomy or second‐degree tears. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD000947.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kropp 2005
- Kropp N, Hartwell T, Althabe F. Episiotomy rates from eleven developing countries. International Journal of Gynaecology and Obstetrics 2005;91(2):157‐9. [PUBMED: 16169552] [DOI] [PubMed] [Google Scholar]
Liabsuetrakul 2014
- Liabsuetrakul T, Choobun T, Peeyananjarassri K, Islam QM. Antibiotic prophylaxis for operative vaginal delivery. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD004455.pub3] [DOI] [PubMed] [Google Scholar]
Newton 2008
- Newton ER. Antibiotics in Maternal‐Fetal Medicine. www.glowm.com/section_view/heading/Antibiotics%2520in%2520Maternal‐Fetal%2520Medicine/item/175 (accessed 23 February 2016) 2008.
NICE 2014
- National Institute for Health and Care Excellence. Intrapartum care: care of healthy women and their babies during childbirth. (Clinical Guideline 109). www.nice.org.uk/guidance/cg190 (accessed 2015) 2014.
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Say 2014
- Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet. Global Health 2014;2(6):e323‐33. [PUBMED: 25103301] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors), The GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html Updated October 2013.
Sharma 2008
- Sharma JB, Gupta N, Aggarwal P, Mittal S. A survey of obstetricians' practice of using prophylactic antibiotics in vaginal deliveries and caesarean sections. Journal of the Indian Medical Association 2008;106(3):147‐9. [PUBMED: 18712132] [PubMed] [Google Scholar]
Thacker 1983
- Thacker SB, Banta HD. Benefits and risks of episiotomy: an interpretative review of the English language literature, 1860‐1980. Obstetrical & Gynecological Survey 1983;38(6):322‐38. [PUBMED: 6346168] [PubMed] [Google Scholar]
Tharpe 2008
- Tharpe N. Postpregnancy genital tract and wound infections. Journal of Midwifery & Women's Health 2008;53(3):236‐46. [PUBMED: 18455098] [DOI] [PubMed] [Google Scholar]
Van Dillen 2010
- Dillen J, Zwart J, Schutte J, Roosmalen J. Maternal sepsis: epidemiology, etiology and outcome. Current Opinion in Infectious Diseases 2010;23(3):249‐54. [PUBMED: 20375891] [DOI] [PubMed] [Google Scholar]
Van Schalkwyk 2010
- Schalkwyk J, Eyk N. Antibiotic prophylaxis in obstetric procedures. Journal of Obstetrics and Gynaecology Canada: JOGC 2010;32(9):878‐92. [PUBMED: 21050523] [DOI] [PMC free article] [PubMed] [Google Scholar]
Viswanathan 2005 (AHRQ)
- Viswanathan M, Hartmann K, Palmieri R, Lux L, Swinson T, Lohr KN, et al. The use of episiotomy in obstetrical care: a systematic review. Evidence Report/Technology Assessment No. 112. (Prepared by the RTI‐UNC Evidence‐based Practice Center, under Contract No. 290‐02‐0016). AHRQ Publication No. 05‐E009‐2. Rockville, MD: Agency for Healthcare Research and Quality 2005. [PMC free article] [PubMed]
WHO 2001
- World Health Organization. WHO Global Strategy for Containment of Antimicrobial Resistance. 2001. www.who.int/drugresistance/WHO_Global_Strategy_English.pdf (accessed 21 January 2016).
WHO 2005
- World Health Organization. The World Health Report 2005: Make every mother and child count. apps.who.int/iris/bitstream/10665/43131/1/9241562900.pdf (accessed 21 January 2016).
WHO 2014
- World Health Organization. Antimicrobial Resistance Global Report on Surveillance. 2014. apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf. Geneva: WHO, (accessed 21 January 2016).
WHO 2015
- World Health Organization. WHO Recommendations for Prevention and Treatment of Maternal Peripartum Infections. 2015. apps.who.int/iris/bitstream/10665/186171/1/9789241549363_eng.pdf (accessed 21 January 2016). [PubMed]
References to other published versions of this review
Bonet 2016b
- Bonet M, Ota E, Chibueze CE, Oladapo OT. Antibiotic prophylaxis for episiotomy repair following vaginal birth. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD012136] [DOI] [PMC free article] [PubMed] [Google Scholar]


