Abstract
Background
Gastro‐oesophageal reflux (GOR) is common in infants, and feed thickeners are often used to manage it in infants as they are simple to use and perceived to be harmless. However, conflicting evidence exists to support the use of feed thickeners.
Objectives
To evaluate the use of feed thickeners in infants up to six months of age with GOR in terms of reduction in a) signs and symptoms of GOR, b) reflux episodes on pH probe monitoring or intraluminal impedance or a combination of both, or c) histological evidence of oesophagitis.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 2), MEDLINE via PubMed (1966 to 22 November 2016), Embase (1980 to 22 November 2016), and CINAHL (1982 to 22 November 2016). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials.
Selection criteria
We included randomised controlled trials if they examined the effects of feed thickeners as compared to unthickened feeds (no treatment or placebo) in treating GOR in term infants up to six months of age or six months of corrected gestational age for those born preterm.
Data collection and analysis
Two review authors independently identified eligible studies from the literature search. Two review authors independently performed data extraction and quality assessments of the eligible studies. Differences in opinion were resolved by discussion with a third review author, and consensus was reached among all three review authors. We used the GRADE approach to assess the quality of the evidence.
Main results
Eight trials recruiting a total of 637 infants met the inclusion criteria for the systematic review. The infants included in the review were mainly formula‐fed term infants. The trials were of variable methodological quality. Formula‐fed term infants with GOR on feed thickeners had nearly two fewer episodes of regurgitation per day (mean difference ‐1.97 episodes per day, 95% confidence interval (CI) ‐2.32 to ‐1.61; 6 studies, 442 infants, moderate‐certainty evidence) and were 2.5 times more likely to be asymptomatic from regurgitation at the end of the intervention period (risk ratio 2.50, 95% CI 1.38 to 4.51; number needed to treat for an additional beneficial outcome 5, 95% CI 4 to 13; 2 studies, 186 infants, low‐certainty evidence) when compared to infants with GOR on unthickened feeds. No studies reported failure to thrive as an outcome. We found low‐certainty evidence based on 2 studies recruiting 116 infants that use of feed thickeners improved the oesophageal pH probe parameters of reflux index (i.e. percentage of time pH < 4), number of reflux episodes lasting longer than 5 minutes, and duration of longest reflux episode. No major side effects were reported with the use of feed thickeners. Information was insufficient to conclude which type of feed thickener is superior.
Authors' conclusions
Gastro‐oesophageal reflux is a physiological self resolving phenomenon in infants that does not necessarily require any treatment. However, we found moderate‐certainty evidence that feed thickeners should be considered if regurgitation symptoms persist in term bottle‐fed infants. The reduction of two episodes of regurgitation per day is likely to be of clinical significance to caregivers. Due to the limited information available, we were unable to assess the use of feed thickeners in infants who are breastfeeding or preterm nor could we conclude which type of feed thickener is superior.
Plain language summary
Feed thickener for infants up to six months of age with gastro‐oesophageal reflux
Review question
We reviewed the evidence for the effect of feed thickener on gastro‐oesophageal reflux (GOR) in babies up to six months of age.
Background
Gastro‐oesophageal reflux is a common condition in babies. It occurs when the stomach contents (milk feeds and acid) come back up into the gullet or mouth. While this normally improves as babies grow older, it can sometimes become troublesome and treatment may be needed. Thickening the milk feeds is a simple method that is commonly used to treat GOR. However, it is unclear if using feed thickeners improves GOR.
Study characteristics
We examined the research published up to 22 November 2016. We found 8 clinical trials recruiting 637 babies up to 6 months of age who presented with symptoms of GOR. The recruited babies were mainly 'healthy' term babies (i.e. babies born within three weeks of the due date) who were bottle feeding. Three of the studies were funded by a pharmaceutical company, hence the quality of the evidence presented must be interpreted with caution.
Key results
We found that term babies with GOR given feed thickeners had nearly two fewer reflux episodes per day. Babies with GOR were also 2.5 times more likely to have no reflux symptoms if feed thickeners were used. No studies reported information on failure to thrive (i.e. poor growth). We found that babies with GOR given feed thickeners showed an improvement in an important measure of acid reflux obtained from pH study. Reflux index (i.e. percentage of time of acidic reflux of pH < 4) was 5% lower in babies given feed thickeners. No major harms were reported in the eight studies.
Quality of evidence
Due to study design limitations, we are moderately confident in the evidence for the reduction of two reflux episodes per day. Hence, feed thickeners can be useful in term babies who are bottle feeding and have troublesome GOR.
We rated the quality of the evidence for the other outcomes as low due to the small number of studies with small numbers of babies recruited. Further research is needed to determine which type of feed thickener is better and whether feed thickeners are useful in babies with GOR who are breastfeeding or preterm.
Summary of findings
Summary of findings for the main comparison. Feed thickener compared to control for infants up to 6 months of age with gastro‐oesophageal reflux.
| Feed thickener compared to control for infants up to 6 months of age with gastro‐oesophageal reflux | ||||||
| Patient or population: Formula‐fed healthy term infants up to 6 months of age with gastro‐oesophageal reflux Intervention: Feed thickener Comparison: Control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with feed thickener | |||||
| Number of episodes of regurgitation or vomiting per day assessed with parental report of symptoms Follow‐up: range 2 to 8 weeks | The mean number of episodes of regurgitation or vomiting per day was 3 episodes per day. | MD 1.97 episodes per day lower (2.32 lower to 1.61 lower) | ‐ | 442 (6 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Change from baseline value was used for 5 studies. Endpoint value was used for the remaining study due to insufficient data (Chao 2007). Frequency of regurgitation value was used in preference to frequency of vomiting in 1 study (Xinias 2005). |
| Proportion of infants without regurgitation or vomiting at the end of intervention period (asymptomatic infants) assessed with parental report of symptoms Follow‐up: range 1 to 8 weeks | Study population | RR 2.50 (1.38 to 4.51) | 186 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 | ||
| 128 per 1000 | 319 per 1000 (176 to 576) | |||||
| Reflux index (percentage of time pH < 4) assessed with oesophageal pH probe study Follow‐up: range 1 to 4 weeks | The mean reflux index was 12%. | MD 5.08% lower (8.89 lower to 1.28 lower) | ‐ | 116 (2 RCTs) | ⊕⊕⊝⊝ LOW 3 | Higher reflux index indicates higher percentage of total time that oesophageal pH is less than 4. |
| Number of reflux episodes lasting > 5 minutes assessed with oesophageal pH probe study Follow‐up: range 1 to 4 weeks | The mean number of reflux episodes lasting > 5 minutes was 6 episodes. | MD 3.4 episodes lower (5.44 lower to 1.36 lower) | ‐ | 116 (2 RCTs) | ⊕⊕⊝⊝ LOW 3 | |
| Duration of longest reflux episode assessed with oesophageal pH probe study Follow‐up: range 1 to 4 weeks | The mean duration of longest reflux episode was 20 minutes. | MD 12.41 minutes lower (23.25 lower to 1.58 lower) | ‐ | 116 (2 RCTs) | ⊕⊕⊝⊝ LOW 3 | |
| Diarrhoea assessed with parental report Follow‐up: range 2 to 8 weeks | ‐ | ‐ | 511 (6 RCTs) | ⊕⊕⊝⊝ LOW 4 | Insufficient data to perform analysis. No difference in diarrhoea incidence or stooling frequency in 4 studies. 17% of infants in the intervention group in Iacono 2002 and 10% of total infants in Hegar 2008 withdrew due to diarrhoea. | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | ||||||
1Downgraded one level for serious study limitation. There was unclear risk of bias for allocation concealment and high risk of bias for blinding, as frequency of regurgitation was dependent on parental report, who were likely to note the higher viscosity of the thickened formula. 2Downgraded two levels for: i) serious study limitations (unclear risk of bias for allocation concealment and high risk of bias for blinding, as frequency of regurgitation was dependent on parental report, who were likely to note the higher viscosity of the thickened formula) and ii) serious imprecision (analysis was derived from two studies which both contained incomplete reporting of all measurements but together could be combined). 3Downgraded by two levels for: i) serious study limitation (unclear/high risk of bias for randomisation and allocation concealment) and ii) serious imprecision (limited number of studies with small sample size and wide confidence interval). 4Downgraded by two levels for: i) serious study limitations (unclear risk of bias for allocation concealment and high risk of bias for blinding, as diarrhoea/side effect was dependent on parental report, who were likely to note the higher viscosity of the thickened formula) and ii) serious publication bias (none of the studies was designed to measure side effect/diarrhoea).
Background
Description of the condition
Gastro‐oesophageal reflux (GOR) is an involuntary retrograde movement of gastric content into the oesophagus with or without regurgitation (Falconer 2010; Horvath 2008; Vandenplas 2009). This is a normal physiological, self limiting occurrence in healthy infants causing little or no symptoms (Czinn 2013; Ferreira 2014; Vandenplas 2009). Prevalence of regurgitation or GOR in infants peaks at 4 months of age, with 50% to 85% of infants reported to have regurgitation at least once a day (Czinn 2013; Nelson 1997; Neu 2012; Puntis 2015; Sherman 2009).
Transient inappropriate relaxation of the lower oesophageal sphincter is the main mechanism leading to GOR in most age groups (Czinn 2013). This was found to occur in preterm infants as early as 26 weeks' gestation (Omari 2002). Pharyngeal stimulation, gastric distension, and raised intra‐abdominal pressure were found to trigger the relaxation of the lower oesophageal sphincter. The role of delayed gastric emptying, another potential mechanism, on the pathophysiology of GOR is unclear (Czinn 2013; Omari 2002).
With increasing frequency and severity, GOR eventually becomes pathological, although this differentiation is difficult to define. The joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) defined gastro‐oesophageal reflux disease (GORD) as reflux of gastric contents causing troublesome symptoms or complication, or both (Vandenplas 2009). The definition of ‘troublesome’ symptoms in infants is vague due to the non‐specific and pervasive nature of GOR symptoms, reliance on parents or caregiver to report the symptoms, and the lack of an objective gold standard. Symptoms such as regurgitation or vomiting and irritable or disordered sleep can significantly impair quality of life. In an attempt to provide some objectivity, some authors have suggested that regurgitation more than four times daily over more than a two‐week period in infants between three weeks and six months old should be designated as "troublesome" (Vandenplas 2015).
Newborn infants (defined as infants up to 28 days of life) are an interesting group to investigate for GOR, as there are wide variations in the reported prevalence and clinical course in this age group. Neonates normally present with GOR at two to three weeks of age (Meunier 2014), and suffer more frequently probably due to the liquid diet and supine posture, as compared to older infants (Ferreira 2014). This is further exaggerated in preterm infants who also have a shorter and immature oesophagus with non‐peristaltic oesophageal motility (Corvaglia 2013; Neu 2012; Omari 1995), leading to poor clearance of reflux material from the oesophagus. Other groups of neonates at higher risk of GOR are those with chronic lung disease, neurological impairment, genetic conditions, or gastrointestinal tract abnormalities such as tracheo‐oesophageal fistula (Ferreira 2014). Generally, newborn infants have a more benign and self limiting clinical course with improvement in symptoms at 6 months old (Nelson 1997; Puntis 2015), and spontaneous resolution by 12 to 14 months old as they move to a more solid diet and acquire neurodevelopmental maturation, achieving a more upright posture (Ferreira 2014).
However, GOR and GORD are difficult to diagnose in newborn infants. Unlike with older children and adolescents where GOR is often diagnosed based on verbally reported symptoms like heartburn, clinical presentations are more variable in newborn infants. Regurgitation and irritability are the most common symptoms described by caregivers (Czinn 2013; Neu 2012). Other presentations are oesophagitis, haematemesis, apnoea, desaturations, bradycardia, and poor growth (Czinn 2013; Orenstein 1993; Puntis 2015; Sherman 2009). A 12‐item caregiver‐completed Infant Gastroesophageal Reflux Questionnaire Revised (I‐GERQ‐R) is one of the most commonly used questionnaires validated for evaluating treatment response in infants with GOR (Kleinman 2006; Orenstein 1996). However, studies using the I‐GERQ‐R for diagnosing infants with GORD have revealed puzzling results (Vandenplas 2009). The questionnaire was found to lack specificity in differentiating infants with GORD with symptomatic infants without GORD (Orenstein 2010). Hence, there is no one symptom or cluster of symptoms that can predict complications of GOR or treatment response in newborn infants (Vandenplas 2009). Diagnosis is inferred in an infant when investigation demonstrates a high frequency or duration of reflux episodes as well as a clear association between symptoms and milk reflux without an alternative diagnosis.
Lower oesophageal pH monitoring with or without multiple intraluminal impedance (MII) is used to investigate GORD. Potential of hydrogen monitoring is an objective and sensitive measure of acidic oesophageal reflux, with established normal ranges based on pH cut‐off value of 4 (Czinn 2013; Vandenplas 2007). The commonly used measures in pH monitoring are reflux index (percentage of entire record with oesophageal pH < 4), total number of acidic reflux episodes, number of acid reflux episodes lasting more than 5 minutes, and the duration of the longest acidic reflux episode. However, pH monitoring is insensitive to weak‐acid or non‐acid reflux, which may pose an issue in newborn infants, as their milk diet can buffer the acidic gastric content. Multiple intraluminal impedance measures changes in electrical impedance caused by movement of liquid, solid, gas, or mix bolus along the oesophagus. Hence, the combined MII and pH monitoring offers the ability to measure reflux regardless of pH as well as to determine the direction, velocity, and height of reflux. However, normal ranges of MII measures for all age groups are not well established and are not consistently reproducible (Corvaglia 2013; Czinn 2013; Vandenplas 2009). These investigation techniques may also detect normal variations and are unable to predict severity of symptoms, prognosis, progression, as well as response to treatment (Horvath 2008; Sherman 2009; Vandenplas 2009). Evidence is insufficient for using other investigations like histology, endoscopy, manometry, barium studies, or nuclear scintigraphy in diagnosing or excluding GORD (Carroll 2002; Vandenplas 2009).
Description of the intervention
Current guidelines suggest an expectant management strategy with reassurance and parental education for the management of uncomplicated GOR (Puntis 2015; Vandenplas 2009; Vandenplas 2015). Pharmacological management is usually reserved for infants who fail to respond to conservative management or have complicated GORD, or both (Corvaglia 2013).
Thickening infant feeds is a simple and commonly used method in managing regurgitation and GOR in newborn infants (Dhillon 2004; Madhoun 2015). Commonly used feed thickeners include cereal‐based thickeners made from rice or maize, gum‐based thickeners from guar or locust bean, and carboxymethyl cellulose (Vandenplas 2005; Vandenplas 2015). However, there is variability in practices among healthcare professionals in the prescription, preparation, and presentation of feed thickening (Madhoun 2015). Apart from the conventional way of thickening the feed by adding the feed thickeners into the infant’s milk or liquid, there are now commercially prepared antiregurgitation formulae that help to reduce variations in preparation. Commercially prepared antiregurgitation formulae are designed to provide energy density, osmolality, and nutritional values appropriate to the needs of infants, unlike when thickeners are added to formula feeds (Horvath 2008; Vandenplas 2005; Vandenplas 2015).
The thickening of infant feeds is generally thought to be innocuous. However, there are case series reporting the development of necrotising enterocolitis in preterm infants prescribed xanthan preparations, Beal 2012; Woods 2012, or locust bean gum thickeners (Clarke 2004). Concerns regarding nutritional consequences have been raised that include impaired absorption of nutrients from feeds thickened with indigestible carbohydrate (Bosscher 2000), and excessive weight gain in infants using rice cereal as thickener due to higher carbohydrate and lower protein and fat content in such compositions (Horvath 2008). Gastrointestinal symptoms like abdominal pain, diarrhoea, and constipation are reported in infants using thickened feed (Corvaglia 2013; Mascarenhas 2005; Vandenplas 2015).
Other conservative management strategies such as small, frequent feeds may be impractical. This approach may also increase weak‐acid and non‐acid GOR episode frequency due to shorter postprandial periods (Corvaglia 2013). Positioning the infant on a head‐up slope of up to 45 degrees is commonly used in clinical practice to manage GOR (Dhillon 2004). However, the evidence for it reducing acid GOR is lacking (Corvaglia 2013). Positioning the infant in the prone or left lateral position has been found to improve GOR but cannot be recommended in infants without cardiorespiratory monitoring due to the well‐established association between the risk of sudden infant death syndrome and prone positioning (Corvaglia 2013).
How the intervention might work
Feed thickener is thought to prevent reflux of gastric content into the oesophagus by increasing the ‘stickiness’ and weight of the liquid, hence retaining the feed in the stomach (Orenstein 1987). The maintenance of appropriate viscosity and consistency of thickened feed is challenging as viscosity varies depending on the type of liquid into which the feed thickener is added (Almeida 2011), the dwell time, temperature of liquid, and amount of saliva (Almeida 2011; Hanson 2012).
In contrast, addition of feed thickeners may increase the energy density and osmolarity of feeds, leading to an increase in the frequency of relaxation of lower oesophageal sphincter and a delay in gastric emptying (Vandenplas 2015), thus worsening GOR (Minami 1984).
Why it is important to do this review
Gastro‐oesophageal reflux is a frequent problem in infants, and feed thickeners, which are simple to use and perceived to be harmless, continue to be prescribed despite the lack of good‐quality scientific evidence to support their use. The previous version of this review, conducted nearly 14 years ago, located no suitable studies for inclusion. This review was restricted to infants up to 28 days of age. We have expanded the age of inclusion to six months (or six months corrected gestational age for those born preterm), as GOR peaks at four months of age, and GORD continues to be a problem throughout the first half of infancy.
Objectives
To evaluate the use of feed thickeners in infants up to six months of age with GOR in terms of reduction in a) signs and symptoms of GOR, b) reflux episodes on pH probe monitoring or intraluminal impedance or a combination of both, or c) histological evidence of oesophagitis.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials that examined the effects of thickening infant formula as compared to unthickened feeds (no treatment or placebo) on GOR in infants up to six months of age (six months corrected gestational age for those born preterm). We included trials reported as abstracts provided we were able to obtain sufficient information from the abstract or by contacting the author.
We excluded cross‐over studies. Gastro‐oesophageal reflux disease is a condition that spontaneously resolves with age, and therefore results of cross‐over trials are likely to be confounded by the natural resolution of the condition. Some cross‐over studies guarded against this by using feed thickener and control intervention alternately. However, residual thickened feeds may still be present in the gastric region due to insufficient washout period.
Types of participants
We included healthy infants with signs or symptoms suggestive of GOR, or those with a diagnosis of GOR based on 24‐hour ambulatory pH monitoring or oesophagitis on biopsy, or both in the review.
For this update we included full‐term infants less than six months old or preterm infants up to six months of age corrected for prematurity at the time of inclusion in the trial. We excluded trials including combined populations of infants within and above this age criteria if results for infants within this specified age range were not reported separately from infants above our age criteria. We attempted to contact the author for this information prior to exclusion.
Previous versions of this review were restricted to including only studies in full‐term infants up 28 days old and preterm infants up to 44 weeks corrected gestational age. We have expanded the age range, as the majority of infants present with GOR after the first two to three weeks of life (Meunier 2014; Vandenplas 2009; Vandenplas 2015). Reassurance and parental education are the usual first‐line management for uncomplicated reflux (Vandenplas 2015). Hence, feed thickeners are rarely started by the first 28 days of life. In addition, the prevalence of GOR peaks at four months of age, and GORD commonly continues to be a problem until six months of age, when most infants start having solids in their diet and acquire the neurological maturity to sit with support in a more upright posture (Nelson 1997).
We excluded trials whose populations were restricted to infants with neurological, congenital, or anatomical gastrointestinal tract abnormalities.
Types of interventions
We included trials reporting the use of thickeners of all types added to all types of milk, including formula and human milk. This included rice‐, gum‐, or flour‐based thickeners, as well as commercially available pre‐prepared thickened formulae, which are commonly described as antiregurgitation formula. We included trials reporting the use of Gaviscon Infant (alginate preparation without antacid). We excluded other preparations of Gaviscon or alginate with antacid component because they may have a dual mechanism of action.
Comparators of interest included no treatment or placebo interventions that are thought not to affect GOR.
Types of outcome measures
Primary outcomes
1. Symptoms or signs of GOR including:
a) regurgitation, posseting, or vomiting,
b) failure to thrive.
We noted each symptom or sign as a dichotomous and separate outcome. We note that no sign or symptom is sensitive or specific to GOR.
We noted number of episodes per day of regurgitation, posseting, or vomiting separately as continuous outcomes. We used change in measurement from baseline to the end of the intervention period in preference to final measurement value. In trials where multiple measurements were performed, we used only the measurement at the end of the intervention period.
2. Measures of gastric and oesophageal acidity based on pH monitoring. We included pH probe study parameters of reflux index (i.e. percentage of time pH < 4) as quantitative discrete variables.
Secondary outcomes
3. Other symptoms or signs of GOR:
a) irritability,
b) disturbed sleep,
c) respiratory symptoms (cough, apnoeas, and recurrent oxygen desaturation),
d) episodes of bradycardia.
We noted each symptom or sign as a dichotomous and separate outcome. We noted number of episodes per day of: i) respiratory symptoms (cough, apnoeas, and recurrent oxygen desaturation) and ii) bradycardias separately as continuous outcomes. We used change in measurement from baseline to the end of the intervention period in preference to final measurement value. In trials where multiple measurements were performed, we used only the measurement at the end of the intervention period.
4. Other pH probe study parameters included as quantitative discrete variables were:
a) number of reflux episodes per hour,
b) number of reflux episodes lasting > 5 minutes,
c) duration of longest reflux episode.
5. Measure of intraoesophageal intraluminal electrical impedance. Parameters to be included as discrete quantitative variables were:
a) number of reflux episodes,
b) height of refluxate in the oesophagus,
c) mean GOR duration of reflux episode.
6. Microscopic evidence of oesophagitis on tissue biopsy. We considered this as a dichotomous outcome based on the presence or absence of inflammation.
7. Significant side effects of the therapy, including:
a) bowel obstruction,
b) diarrhoea,
c) aspiration,
d) colic.
Search methods for identification of studies
Electronic searches
For this update we used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group.
We conducted a comprehensive search including: the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 10) in the Cochrane Library; MEDLINE via PubMed (1966 to 22 November 2016); Embase (1980 to 22 November 2016); and CINAHL (Cumulative Index to Nursing and Allied Health Literature) (1982 to 22 November 2016) using the following search terms: ("Gastroesophageal Reflux"[Mesh] OR Gastroesophageal Reflux OR gastro‐oesophageal reflux) AND (thick* OR diet OR antiregurg* OR conserv* OR non‐pharmaco* OR nonpharmaco*), plus database‐specific limiters for randomised controlled trials and neonates (see Appendix 1 for the full search strategies for each database). We did not apply any language restrictions.
We searched clinical trials registries for ongoing or recently completed trials (US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov/), the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/search/en/), and the ISRCTN registry (www.isrctn.com/)). We also searched conference proceedings for the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) from 1994 to 2016.
The previous versions of this review used text word terms 'gastro‐oesophageal reflux' or 'gastro‐esophageal reflux', or the MeSH term 'gastroesophageal reflux', and the MeSH term 'exp infant, newborn'. We searched the Cochrane Central Register of Controlled Trials (CENTRAL Issue 2, 2004) in the Cochrane Library, MEDLINE from 1966 to March 2004, CINAHL from 1982 to December 2001, and conference and symposia proceedings published in Pediatric Research 1990 to 1994. We also searched the conference proceedings for the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) from 1994 to December 2001. The searches were not restricted to the English language.
Searching other resources
We searched the reference list of reviews and studies included in the review.
Data collection and analysis
Selection of studies
Two review authors (TK, SO) independently reviewed all the titles and abstracts retrieved from the search for eligibility against our inclusion criteria using Covidence (Covidence 2015). We obtained full‐text copies of all potentially eligible studies, which both review authors independently reviewed.
Differences were resolved by discussion and consensus of the review authors (TK, SO, JD). If necessary, we contacted authors of the studies for additional data or information.
Data extraction and management
Two review authors (TK, SO) independently extracted data using a standard data extraction form on Covidence (Covidence 2015). Differences were resolved by discussion and consensus of the review authors (TK, SO, JD). If necessary, we contacted authors of the studies for additional data or information.
Assessment of risk of bias in included studies
Two review authors (TK, SO) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool for the following domains (Higgins 2011).
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Any other bias
Any disagreements were resolved by discussion or by a third review author (JD). See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We reported risk ratios with 95% confidence intervals (CIs) for dichotomous variables, and mean differences with 95% CIs for continuous variables. We reported standardised mean differences with 95% CIs if different measures were used for the continuous variables. We preferred using change from baseline measurements to endpoint measurements for continuous variables. We used Review Manager 5 to assess treatment effect of outcome measures (RevMan 2014).
Unit of analysis issues
For randomised controlled trials, the infant was designated as the unit of analysis. For cluster randomised trials, we carried out analyses adjusting for clustering as described in the Cochrane Handbook for Systematic Reviews of Interventions using the intracluster correlation coefficient derived from the trial or other sources (Higgins 2011).
For multi‐arm randomised trials, we pooled together arms reporting feed thickeners of different type or different dosage. Arms reporting a non‐feed thickener intervention were ignored. During meta‐analysis, we would consider an infant only once in the analysis (i.e. there was no double counting of any groups, including those infants in the control group) as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We contacted study authors regarding any missing data or uncertainty about specifics of the study that were vital to the meta‐analysis, and described the contribution of the authors. We derived missing standard deviations from reported confidence intervals, P values, or standard errors. If data were still missing, we did not assume values in order to present the analyses, instead presenting the information in a descriptive manner.
Assessment of heterogeneity
We assessed heterogeneity between effect size of in the included studies by visual inspection of the forest plot and Chi2 test for heterogeneity with a P value of less than 0.1. Inconsistency across studies and its impact on the meta‐analysis were quantified using I2 statistics and described as percentage of variability in effect estimates that is due to heterogeneity rather than sampling error (Higgins 2011). We used Cochrane Neonatal Review Group guidelines to interpret the I2 statistics as follows.
less than 25%: no heterogeneity
25% to 49%: low heterogeneity
50% to 74%: moderate heterogeneity
greater than 75%: high heterogeneity
If we detected moderate or high heterogeneity (I2 greater than 50%), we explored possible reasons (e.g. differences in study design, trial participants, interventions, or completeness of outcome measures) using sensitivity analysis.
Assessment of reporting biases
We assessed potential reporting bias using funnel plots if an individual meta‐analysis contained more than 10 studies.
Data synthesis
We used a fixed‐effect model unless there was substantial heterogeneity (I2 greater than 50%) (Higgins 2011). For meta‐analyses with substantial heterogeneity, we explored possible causes of the heterogeneity using sensitivity analyses.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes:
number of episodes of regurgitation or vomiting per day,
proportion of infants without regurgitation or vomiting at the end of the intervention period,
reflux index (percentage of time pH < 4),
number of reflux episodes lasting > 5 minutes,
duration of longest reflux episodes,
diarrhoea.
Two review authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality, but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEpro Guideline Development Tool to create a ‘Summary of findings’ table (Table 1) to report the quality of the evidence (GRADEpro GDT).
The GRADE approach results in an assessment of the quality of a body of evidence in one of the following four grades.
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect.
Subgroup analysis and investigation of heterogeneity
To determine if results differed for term versus preterm infants or by type of feed thickener, we performed planned a priori subgroup analyses by inspection of the forest plot for the overlap of confidence intervals and tests for subgroup differences.
Sensitivity analysis
We performed multiple sensitivity analyses to explore the effects of the methodology of the included trials, excluding trials with high risk of bias (defined as having more than one domain assessed at high risk of bias in the 'Risk of bias' table).
Results
Description of studies
Results of the search
Our initial search yielded 405 reports and conference proceedings. Based on their abstracts, we examined the full‐text reports of 39 promising publications. Of these 39 publications, we excluded three that were letters or expert opinions on the topic (Craig 2002; EBP 2004; Savino 2008). There were two publications from the same study (Gouyon 1988). Hence, we found 35 possibly eligible studies. Of these, eight matched the inclusion criteria for study design and types of participants. These are depicted in Figure 1.
1.
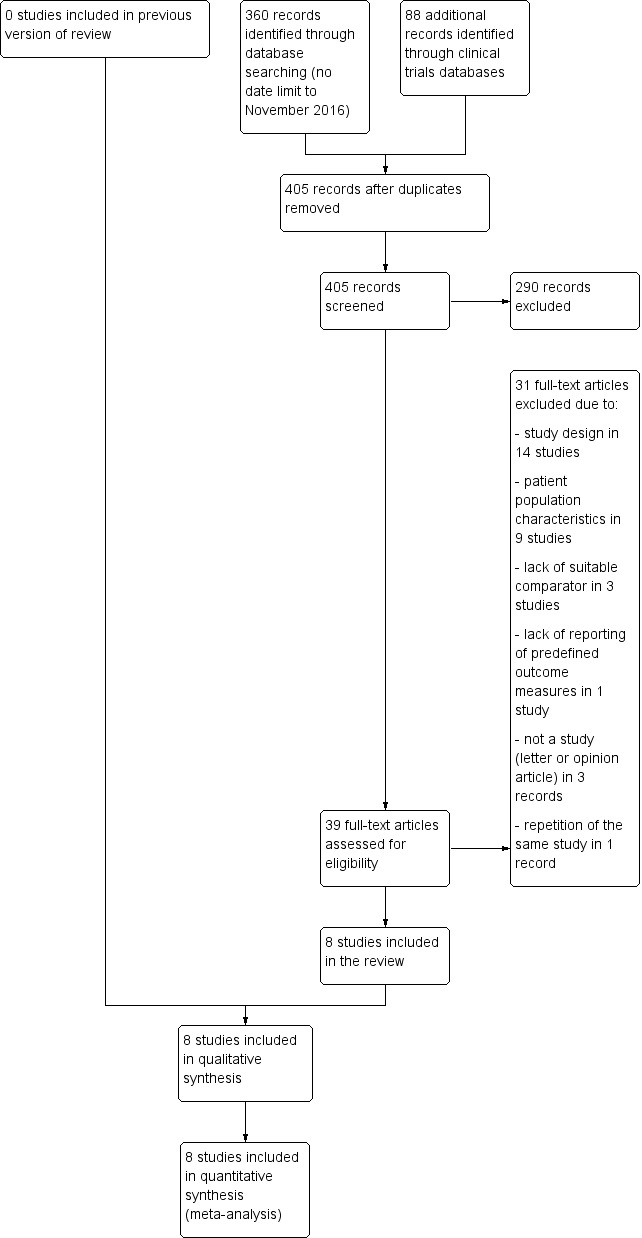
Study flow diagram.
Included studies
We identified eight suitable studies for inclusion in the updated review after expanding the age criteria to include infants up to six months old (see Characteristics of included studies table) (Chao 2007; Hegar 2008; Iacono 2002; Miller 1999; Moya 1999; Vandenplas 1994; Vanderhoof 2003; Xinias 2005). Five of the trials were carried out in North America and Europe in the 1990s (Iacono 2002; Miller 1999; Moya 1999; Vandenplas 1994; Vanderhoof 2003). These included 3 multicentre trials involving 25 general practices in the UK (Miller 1999), 6 children's outpatient centres in Italy (Iacono 2002), and 6 North American paediatric centres (Vanderhoof 2003). Two trials were carried out in Taiwan and Indonesia, respectively, in the 2000s (Chao 2007; Hegar 2008), while Xinias 2005 was a multinational clinical trial recruiting 96 infants from Greece, France, Belgium, and Morocco, also in the 2000s.
Population
A total of 637 infants participated in the included trials (range 20 to 166). The largest of these, Iacono 2002, was published as a correspondence to the editor only. Most trials included formula‐feeding 'healthy' term infants presenting with symptoms of GOR or abnormal oesophageal pH probe parameters, except for Miller 1999, which included formula‐feeding and breastfeeding term infants. It was unclear in two studies if preterm infants were included as well (Chao 2007; Iacono 2002). Generally, infants with complicated GORD (e.g. haematemesis, melena), mechanical obstruction, atopy, or previous treatment with thickened feeds or antireflux medication were excluded.
The age criteria specified in the included trials were as follows.
Up to 3 months: Hegar 2008.
Up to 4 months: Chao 2007; Iacono 2002; Moya 1999; Vandenplas 1994; Vanderhoof 2003.
Up to 12 months: Miller 1999. We decided to include this study as the spread of infants was likely to be between 3 to 6 months based on the reported mean and standard deviation value of 4 ± 0.4 months.
Not stated: Xinias 2005. No age inclusion criteria were reported. The mean age of recruited infants was 3.1 months (93 days).
The criteria for frequency or symptoms of regurgitation or vomiting were as follows.
≥ 2 episodes per day or persistent unmanageable regurgitation/vomiting: Miller 1999.
≥ 3 episodes per day: Chao 2007.
≥ 4 episodes per day: Hegar 2008.
> 5 episodes per day: Iacono 2002; Moya 1999; Vandenplas 1994; Vanderhoof 2003.
Excessive regurgitation or vomiting: Iacono 2002; Xinias 2005.
Two trials specified abnormal oesophageal pH probe parameters as an inclusion criterion.
Reflux index > 5%: Xinias 2005.
Reflux index 10% to 30%: Vandenplas 1994.
Intervention and comparison
Intervention
Four trials used antiregurgitation formula thickened with carob bean gum (Hegar 2008; Iacono 2002; Moya 1999; Vandenplas 1994). Hegar 2008 had two intervention arms; infants in the other intervention arm were given standard formula with 5 grams rice cereal per 100 mL. Vanderhoof 2003 also used pre‐prepared antiregurgitation formula thickened with rice cereal. Three trials used cornstarch‐thickened antiregurgitation formula (Chao 2007; Moya 1999; Xinias 2005). As noted, Moya 1999 had two intervention arms: one with carob bean gum and the other with cornstarch‐thickened formula.
One trial used alginate without antacid as feed thickener (Miller 1999). Infants weighing more than 4.54 kg were given one sachet of alginate (0.65 grams alginate). Infants weighing ≥ 4.54 kg or taking solids were given two sachets of alginate (1.3 grams alginate).
Control
Standard formula without feed thickeners was used as the control in trials that used carob bean gum, cornstarch, and rice cereals as feed thickener. One study used 25% strengthened formula as their control (Chao 2007), where five measurements of formula were added to 120 mL of water instead of the recommended four measurements. Matching placebo was used in the study that used alginate as feed thickener (Miller 1999).
Co‐intervention
Co‐interventions were reported in both intervention and control groups in three studies.
Parental reassurance: Hegar 2008; Moya 1999; Vandenplas 1994.
Positional therapy: Vandenplas 1994.
Adjustment of amount and frequency of feeds: Moya 1999.
Duration of intervention
1 week: Vandenplas 1994.
2 weeks: Miller 1999; Moya 1999.
4 weeks: Hegar 2008; Xinias 2005.
5 weeks: Vanderhoof 2003.
8 weeks: Chao 2007; Iacono 2002.
Outcome
Six trials reported number of episodes of regurgitation or vomiting per day (Chao 2007; Hegar 2008; Miller 1999; Moya 1999; Vanderhoof 2003; Xinias 2005), whereas the remaining two trials reported the proportion of infants without regurgitation or vomiting at the end of the intervention (Iacono 2002; Vandenplas 1994). Two trials reported oesophageal pH probe parameters (Vandenplas 1994; Xinias 2005). The other reported outcomes included non‐regurgitation symptoms and signs (cough, sleep disturbance, and irritability) and side effects (diarrhoea, constipation, colic, respiratory).
Excluded studies
We excluded 27 studies (see Characteristics of excluded studies table). We excluded eight studies that recruited infants above six months old and it was not possible to analyse the group of infants less than six months old separately (Bailey 1987; Borrelli 1997; Chevallier 1998; Fabiani 2000; Khoshoo 2000; Penna 2003; Tolia 1999; Ummarino 2013). We excluded 14 studies that were not randomised controlled trials; 10 that were cross‐over studies (Corvaglia 2006; Corvaglia 2011; Corvaglia 2011a; Corvaglia 2012; Gouyon 1988; Miyazawa 2006; Miyazawa 2007; Moukarzel 2007; Orenstein 1992; Wenzl 2003); and four that were cohort studies (Atasay 2010; Chevallier 2009; Dupont 2016; Xinias 2003). Two trials did not have any suitable controls: Chao 2007a used infants receiving postural therapy as control, while all three arms in Georgieva 2016 were formula thickened with carob bean gum galactomannans in different dosage or temperature. Lasekan 2014 recruited healthy infants without signs or symptoms of GOR. Ostrom 2006 investigated the effect of soy protein‐based thickened formula versus whey protein‐based unthickened formula; the results of this trial are therefore confounded by the effect of replacement of cow's milk protein with soy protein. Toporovski 2013 published their study as an abstract without any mention of the outcome measures specified in this review, and attempts to contact the authors were unsuccessful.
Of the 27 excluded studies, 8 studies recruited infants that were exclusively within the neonatal age range defined in the previous version of the review (Huang 2002), which is full‐term infants less than 28 days or preterm infants up to 44 weeks corrected age. However, we excluded these studies as five were cross‐over studies (Corvaglia 2006; Corvaglia 2011; Corvaglia 2011a; Corvaglia 2012; Gouyon 1988); one was a cohort study (Atasay 2010); one study recruited infants without signs or symptoms of GOR (Lasekan 2014); and one study investigated the co‐intervention of thickened and soy protein‐based formula (Ostrom 2006).
Risk of bias in included studies
The trials were of variable methodological quality, as described in the Characteristics of included studies table and summarised in Figure 2.
2.
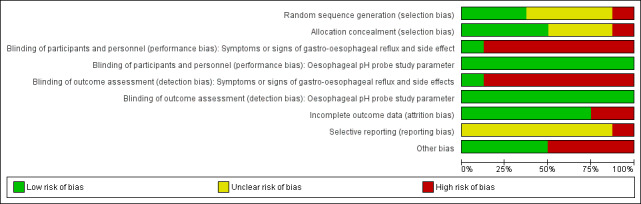
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We assessed the randomisation method described in two trials to be at low risk for selection bias (Moya 1999; Vanderhoof 2003). It was unclear if the randomisation method described adequately prevented selection bias in five trials (Chao 2007; Hegar 2008; Iacono 2002; Miller 1999; Xinias 2005). We judged Vandenplas 1994 to be at high risk of selection bias, as infants were randomised to thickened or unthickened formula in an alternate manner based on order of presentation to the clinic, which is not true randomisation.
Blinding
We found blinding of parents and outcome assessors to be adequate in the alginate study (Miller 1999), where a matching placebo was used.
For the remaining studies using rice cereal, cornstarch, and carob bean gum feed thickeners, the assessment of symptoms or signs of GOR and side effects depended on parental report. Although blinding of parents and outcome assessors was attempted, parents were likely to note the higher viscosity of the thickened formula. This may have affected the parental perception of symptoms, signs, or side effects.
Two studies reported oesophageal pH probe parameters (Vandenplas 1994; Xinias 2005). The difficulty in blinding parents to the different viscosity of thickened formula was unlikely to have affected the objective measure of the pH probe study, hence we assessed these studies as low risk.
Incomplete outcome data
Five trials achieved complete follow up‐assessment. Although 20 infants (22%) withdrew from the Miller 1999 study, their outcome data were included in the final analyses, achieving over 90% follow‐up assessment. However, the results excluded two infants from the control (placebo) group, as they did not receive any study medication. In two trials (Chao 2007; Vanderhoof 2003), only 81% and 78% of infants completed the eight and five weeks' follow‐up, respectively. However, the outcome measures at the end of study participation in the latter study, Vanderhoof 2003, were reported in 93% of the infants. This was because the last available data prior to discontinuation from the study were entered. Chao 2007 excluded 19 infants, including 8 who developed marked diarrhoea or enteritis, and it was not possible to discern whether the excluded infants were in the feed thickener or the control group.
Selective reporting
The largest included study was only published as correspondence to the editor (Iacono 2002). Hence, not all values for the measured outcome and baseline characteristics of the infants were reported. We assessed reporting bias to be unclear in the remaining trials, as the study protocol or trial registration number, or both were not available.
The study protocol or trial registration details were not available for any of the included studies.
Other potential sources of bias
Three trials declared that they received sponsorship or funding from pharmaceutical companies (Miller 1999; Vanderhoof 2003; Xinias 2005). Infants in the intervention group in these three trials had a higher number of vomiting episodes per day or larger measure of regurgitation volume than the infants in the control group. Infants in the intervention group in the Moya 1999 study were older than in the control group. These differences may have introduced bias in the final analyses.
Effects of interventions
See: Table 1
Primary outcomes
Symptoms or signs or gastro‐oesophageal reflux (Outcome 1)
Regurgitation, posseting, or vomiting (Outcome 1a)
Vandenplas 1994 graded the severity of regurgitation using the Vandenplas scoring system (Vandenplas 1994). Although the improvement in the score of severity of regurgitation was found to be greater with the Saint John's bread (carob bean gum) thickened antiregurgitation formula than the control, it did not achieve statistical significance (mean difference (MD) ‐1.30 episodes per day, 95% confidence interval (CI) ‐2.68 to 0.08). Twenty per cent of infants were found to be asymptomatic in the intervention group compared to none in the control group at the end of the one‐week intervention (typical risk ratio (RR) 5.00, 95% CI 0.27 to 92.62).
Moya 1999 found that frequency of regurgitation decreased significantly in the control group as well as in both intervention groups of cornstarch‐ and carob flour‐thickened formula after two weeks of intervention. However, the difference in reduction of frequency was not statistically significant between the control and either intervention groups of cornstarch‐ (MD ‐0.60 episodes per day, 95% CI ‐5.45 to 4.25) or carob flour‐thickened formula (MD ‐1.20 episodes per day, 95% CI ‐6.35 to 3.95).
Miller 1999 found a statistically significant greater reduction in frequency of vomiting or regurgitation in the previous 24 hours in the alginate group when compared to the control group (median difference ‐5.5 versus ‐2 episodes per day, P = 0.009) after two weeks of intervention. The alginate group also reported a lower severity of vomiting or regurgitation, but this difference did not achieve statistical significance (P = 0.061). The severity of vomiting or regurgitation was assessed based on the most severe event recorded in the previous 24 hours on a 4‐point Likert scale (none, mild, moderate, or severe).
Iacono 2002 found that two‐thirds of infants were asymptomatic or improved in both the control and carob flour‐thickened antiregurgitation formula groups after eight weeks of intervention. However, 34% of infants were found to be asymptomatic after eight weeks of intervention as compared to 14% in the control group (typical RR 2.39, 95% CI 1.31 to 4.37).
Vanderhoof 2003 found no statistically significant difference in the reduction of frequency of regurgitation expressed as number of episodes per day between the control and rice cereal‐thickened formula group (MD ‐2.00 episodes per day, 95% CI ‐4.77 to 0.77) in the five‐week intervention period. However, the rice cereal‐thickened group had a borderline statistically significant greater reduction in frequency of regurgitation expressed as percentage of feeds with regurgitation (MD ‐14%, 95% CI ‐28% to ‐0.1%). The study assessed the volume of regurgitation using the total regurgitation volume score, which is a summation of the four largest regurgitation scores (1 = teaspoon to tablespoon, 2 = tablespoon to ounce, 3 = ounce to entire feeding, 4 = entire feeding) from each of the day's feedings. The rice cereal‐thickened group was found to display a trend towards greater reduction in the total regurgitation volume score than the control group (MD ‐1.20, 95% CI ‐2.59 to 0.19).
Xinias 2005 reported frequency of regurgitation and vomiting separately based on parental report. Regurgitation was defined as "effortless passage of refluxed gastric contents into the oral pharynx and the mouth with effortless drooling out of the mouth", whereas vomiting was defined as "forceful expulsion of the refluxed gastric contents from the mouth". The cornstarch‐thickened formula group was found to have a greater reduction in the frequency of regurgitation than the control group that achieved statistical significance (MD ‐2.57 episodes per day, 95% CI ‐4.28 to ‐0.86) at the end of the four‐week intervention period. Similiar findings were reported for the frequency of vomiting (MD ‐2.54 episodes per day, 95% CI ‐4.10 to ‐0.98).
Chao 2007 found that infants receiving cornstarch‐thickened antiregurgitation formula had a statistically significant lower frequency of regurgitation or vomiting at the end of the eight‐week intervention period as compared to the 25% strengthened formula control group (MD ‐1.96 episodes per day, 95% CI ‐2.34 to ‐1.58).
Hegar 2008 found a statistically significant decrease in the frequency of regurgitation and vomiting in the control group as well as in the rice cereal‐ and bean gum‐thickened formula groups after four weeks of intervention. Although the reduction in frequency of regurgitation was greater in the intervention groups, the difference in reduction in frequency when compared to the control group was not statistically significant in both the rice cereal (MD ‐0.90 episodes per day, 95% CI ‐3.57 to 1.77) and bean gum groups (MD ‐1.50 episodes per day, 95% CI ‐3.25 to 0.25).
Meta‐analyses of the regurgitation, posseting, or vomiting data
Number of episodes of regurgitation, posseting, or vomiting per day (Outcome 1a)
Sufficient data were reported or obtained from the author to use change of baseline value in five trials. The Xinias 2005 study used frequency of regurgitation rather than vomiting. In another of these five trials, Miller 1999 reported the median number of episodes of regurgitation rather than the mean. As the sample size was more than 25, it was assumed that the median and mean were similar (Hozo 2005), and the standard deviation for the mean difference was obtained using the reported P value (Higgins 2011). There was insufficient information in Chao 2007 to report the change of baseline value, hence the endpoint value was used instead. We felt this would not affect the analysis significantly, as the baseline values were comparable in the intervention and control groups (Higgins 2011).
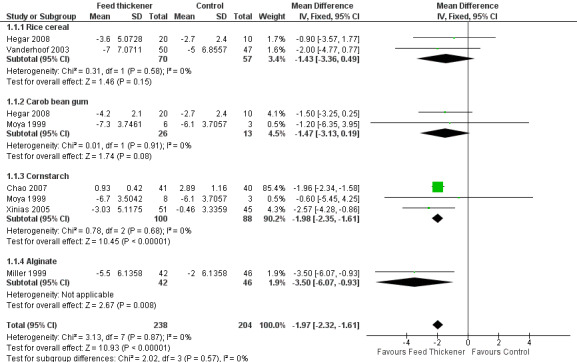
Meta‐analysis found that the reduction in frequency of regurgitation or vomiting was significantly greater in infants using feed thickeners compared to control by nearly 2 episodes per day (MD ‐1.97 episodes per day, 95% CI ‐2.32 to ‐1.61; 6 studies, 442 infants; I2 = 0%) (Analysis 1.1; Figure 3). This finding remained the same even after excluding both the Miller 1999 study, which reported median rather than the mean value, and the Chao 2007 study, which used endpoint value rather than change from baseline value (MD ‐1.79 episodes per day, 95% CI ‐2.78 to ‐0.80; 4 studies, 273 infants; I2 = 0%).
1.1. Analysis.
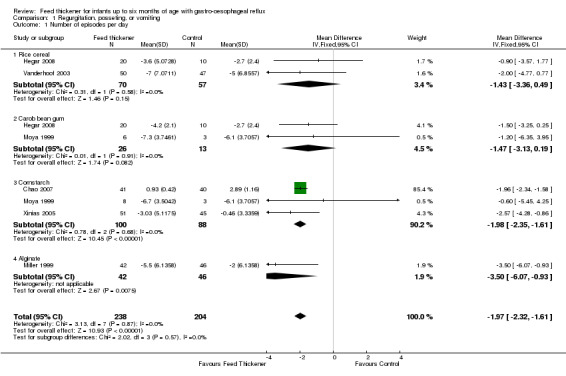
Comparison 1 Regurgitation, posseting, or vomiting, Outcome 1 Number of episodes per day.
3.
Forest plot of comparison: 1 Regurgitation, posseting, or vomiting, outcome: 1.1 Number of episodes per day.
Assumptions
1. There was insufficient information in Chao 2007 to report the change of baseline value, hence we used the endpoint data instead. Change from baseline value was used for the remaining five studies, where P value was used to determine the standard deviation for the change from baseline value.
2. Frequency of regurgitation rather than vomiting was used for the Xinias 2005 study.
3. In Miller 1999, median number of episodes of regurgitation was reported rather than the mean value. As the sample size was more than 25, it was assumed that median and mean were similar (Hozo 2005), and the standard deviation for the mean difference was obtained using the reported P value (Higgins 2011).
4. We halved control groups for Hegar 2008 and Moya 1999, as these were three‐arm trials involving one control and two intervention arms.
Proportions of infants without regurgitation, posseting, or vomiting at the end of the intervention period (Outcome 1a)
Meta‐analysis of data from two trials (186 infants) found that the infants receiving feed thickeners were 2.5 times more likely to be asymptomatic from regurgitation or vomiting at the end of the intervention period when compared to the control. This finding achieved statistical significance (typical RR 2.50, 95% CI 1.38 to 4.51; typical risk difference (RD) 0.20, 95% CI 0.08 to 0.32; number needed to treat for an additional beneficial outcome (NNTB) 5, 95% CI 4 to 13) (Analysis 1.2; Figure 4).
1.2. Analysis.
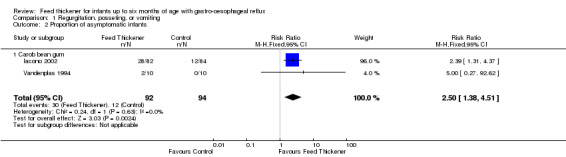
Comparison 1 Regurgitation, posseting, or vomiting, Outcome 2 Proportion of asymptomatic infants.
4.
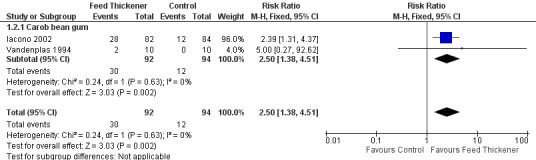
Forest plot of comparison: 1 Regurgitation, posseting, or vomiting, outcome: 1.2 Proportion of asymptomatic infants.
Failure to thrive (Outcome 1b)
No trials formally assessed failure to thrive as an outcome, as infants with failure to thrive due to GOR were usually excluded from the trials.
Oesophageal pH probe study parameters
Two trials performed oesophageal pH probe study (Vandenplas 1994; Xinias 2005).
Reflux index (Outcome 2)
Meta‐analysis of 2 trials (116 infants) detected a statistically significant reduction in reflux index in the feed thickener group by 5% when compared to the control group (MD ‐5.08, 95% CI ‐8.89 to ‐1.28; I2 = 0%) (Analysis 2.1; Figure 5).
2.1. Analysis.
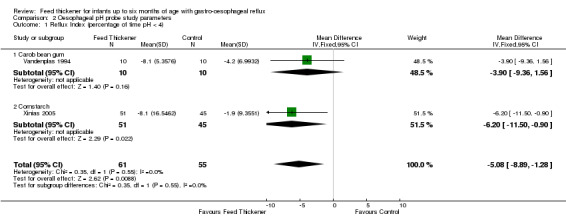
Comparison 2 Oesophageal pH probe study parameters, Outcome 1 Reflux Index (percentage of time pH < 4).
5.
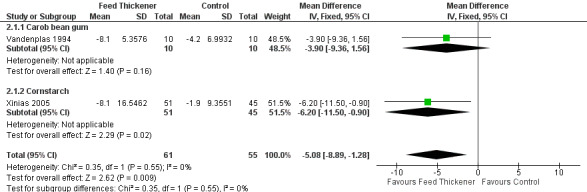
Forest plot of comparison: 2 Oesophageal pH probe study parameters, outcome: 2.1 Reflux index (percentage of time pH < 4).
Secondary outcomes
Other symptoms or signs of gastro‐oesophageal reflux (Outcome 3)
Irritability (Outcome 3a)
Two studies reported the effect of feed thickeners on irritability.
Chao 2007: Statistically significant improvement in the number of infants with irritability in the feed thickener group (typical RR 0.12, 95% CI 0.02 to 0.93; typical RD ‐0.18, 95% CI ‐0.31 to ‐0.04; NNTB 6, 95% CI 4 to 25).
Vanderhoof 2003: No statistically significant improvement in the percentage of feeds followed by pain in the most symptomatic quartile of infants at baseline (MD 12%, CI not given). Percentage of feeds followed by pain was assumed to be a measure of irritability.
Sleep disturbance (Outcome 3b)
Three studies reported the effect of feed thickeners on sleep disturbance.
Chao 2007: No statistically significant improvement in the number of infants with sleep disturbance in the feed thickener group (typical RR 0.49, 95% CI 0.05 to 5.17; typical RD ‐0.03, 95% CI ‐0.11 to 0.06).
Vanderhoof 2003: Statistically significant improvement in the percentage of feeds followed by trouble sleeping in the most symptomatic quartile of infants at baseline (MD 29%, CI not given; P = 0.03).
Hegar 2008: No difference in sleep disturbance was reported between the control and feed thickener groups (no data reported).
Respiratory symptoms (Outcome 3c)
Two studies reported the effect of feed thickeners on cough.
Chao 2007: No statistically significant improvement in the number of infants with cough in the feed thickener group (typical RR 0.20, 95% CI 0.01 to 3.94; typical RD ‐0.05, 95% CI ‐0.13 to 0.03).
Vanderhoof 2003: Borderline statistically significant improvement in the percentage of feeds followed by cough, choke, or gag at the end of the five‐week intervention period (MD and CI not given; P = 0.049).
Bradycardia (Outcome 3d)
No studies reported this outcome.
Other oesophageal pH probe study parameters (Outcome 4)
Number of reflux episodes per hour (Outcome 4a)
Only one study reported the number of reflux episodes per hour using the pH probe study. Xinias 2005 found no statistically significant reduction in number of reflux episodes per hour (MD ‐3.40, 95% CI ‐7.07 to 0.27).
Number of reflux episodes lasting > 5 minutes (Outcome 4b)
Meta‐analysis of 2 trials (116 infants) found a statistically significant reduction by 3 reflux episodes lasting more than 5 minutes using the pH probe study in the feed thickener group when compared to the control group (MD ‐3.40, 95% CI ‐5.44 to ‐1.36; I2 = 0%) (Analysis 2.2; Figure 6).
2.2. Analysis.
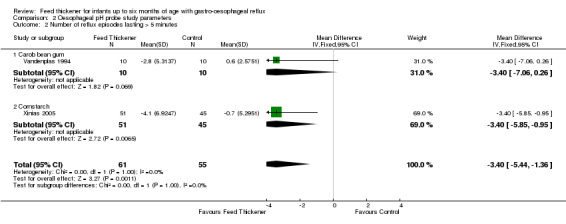
Comparison 2 Oesophageal pH probe study parameters, Outcome 2 Number of reflux episodes lasting > 5 minutes.
6.
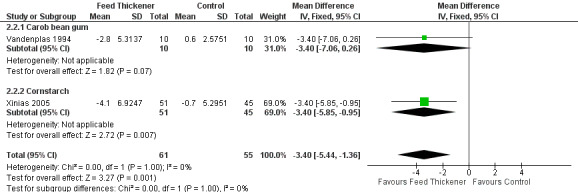
Forest plot of comparison: 2 Oesophageal pH probe study parameters, outcome: 2.2 Number of reflux episodes lasting > 5 minutes.
Duration of longest reflux episode (Outcome 4c)
Meta‐analysis of 2 trials (116 infants) found that infants in the feed thickener group had statistically significant shorter reflux episodes detected by the pH probe study by 12.4 minutes when compared to the control group (MD ‐12.41 minutes, 95% CI ‐23.25 to ‐1.58; I2 = 7%) (Analysis 2.3; Figure 7).
2.3. Analysis.
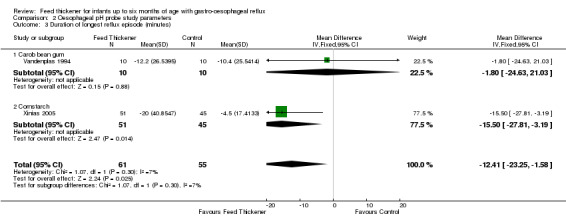
Comparison 2 Oesophageal pH probe study parameters, Outcome 3 Duration of longest reflux episode (minutes).
7.
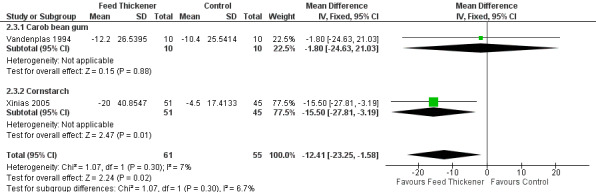
Forest plot of comparison: 2 Oesophageal pH probe study parameters, outcome: 2.3 Duration of longest reflux episode (minutes).
Intraoesophageal intraluminal electrical impedance (Outcome 5)
No studies used intraoesophageal intraluminal electrical impedence.
Microscopic evidence of oesophagitis on tissue biopsy (Outcome 6)
No studies performed endoscopy to obtain tissue biopsy of the oesophagus. Vandenplas 1994 performed upper gastrointestinal endoscopy with biopsies for histology at baseline in 11 infants for whom there was suspicion of peptic oesophagitis. No postintervention endoscopies were performed.
Side effects (Outcome 7)
Two trials reported that there was no recorded side effects in any of the infants participating in the study (Moya 1999; Xinias 2005).
Bowel obstruction (Outcome 7a)
There were no reported incidences of bowel obstruction in any of the eight included studies. Miller 1999 found no difference in the incidence of constipation between the alginate and control groups (typical RR 4.38, 95% CI 0.51 to 37.65; typical RD 0.07, 95% CI ‐0.02 to 0.17).
Diarrhoea (Outcome 7b)
Six trials reported on the effect of intervention on bowel movements.
Miller 1999: No statistically significant difference in the incidence of diarrhoea between the alginate and control groups (typical RR 0.85, 95% CI 0.35 to 2.08; typical RD ‐0.03, 95% CI ‐0.19 to 0.13).
Moya 1999: No statistically significant difference in the reduction in number of stools per day was noted between the control and cornstarch‐thickened group (MD ‐0.22 episodes per day, 95% CI ‐1.33 to 0.89). We could not determine standard deviation for the reduction in frequency of stooling in the carob flour‐thickened group from the information available. However, there were no reported differences in the frequency of stooling before and after starting carob flour‐thickened formula.
Iacono 2002: Intervention was suspended in 14 infants in the carob flour‐thickened group due to diarrhoea. The number of infants in the control group with diarrhoea was not reported.
Xinias 2005: There was no significant difference in the reduction of number of stools per day in the cornstarch‐thickened formula group versus the control group (MD 0.24, 95% CI ‐1.58 to 2.06).
Chao 2007: Eight infants did not complete the eight‐week study due to diarrhoea. The proportion of these infants in the feed thickener or control group was not reported.
Hegar 2008: Consistency and frequency of stools were reported not to differ between the feed thickener and control groups. A majority of infants were reported to have normal soft stools.
Aspiration (Outcome 7c)
No trials reported cases of aspiration during the study period.
Colic (Outcome 7d)
Only one trial reported the effect of feed thickeners on the incidence of colic (Miller 1999), finding no difference in the incidence of colic between the alginate and control groups (typical RR 0.73, 95% CI 0.13 to 4.16; typical RD ‐0.02, 95% CI ‐0.11 to 0.08).
Subgroup analyses
We carried out the following a priori subgroup analyses.
Term or preterm infants
No trials were performed on preterm infants.
Type of feed thickener
-
Number of episodes of regurgitation, posseting, or vomiting per day (Outcome 1a): No significant interaction existed between the subtotal estimates for the type of feed thickener (Chi2 = 2.02, df = 3, P = 0.57) (Analysis 1.1; Figure 3).
Rice cereal: MD ‐1.43 episodes per day, 95% CI ‐3.36 to 0.49; 2 studies, 127 infants; I2 = 0%.
Carob bean gum: MD ‐1.47 episodes per day, 95% CI ‐3.13 to 0.19; 2 studies, 39 infants; I2 = 0%.
Cornstarch: MD ‐1.98 episodes per day, 95% CI ‐2.35 to ‐1.61; 3 studies, 188 infants; I2 = 0%.
Alginate: MD ‐3.50 episodes per day, 95% CI ‐6.07 to ‐0.93; 1 study, 88 infants.
Proprotions of infants with regurgitation, posseting, or vomiting at the end of the intervention period (Outcome 1a): Both studies used carob bean gum feed thickener.
-
Reflux index (Outcome 2): No significant interaction existed between the subtotal estimates for the type of feed thickener subgroups (Chi2 = 0.35, df = 1, P = 0.55) (Analysis 2.1; Figure 5).
Carob bean gum: MD ‐3.90%, 95% CI ‐9.36 to 1.56; 1 study, 20 infants.
Cornstarch: MD ‐6.20%, 95% CI ‐11.50 to ‐0.90; 1 study, 96 infants.
-
Number of reflux episodes lasting > 5 minutes (Outcome 4b): No significant interaction existed between the subtotal estimates for the type of feed thickener (Chi2 = 0.35, df = 1, P = 0.55) (Analysis 2.2; Figure 6).
Carob bean gum: MD ‐3.40, 95% CI ‐7.06 to 0.26; 1 study, 20 infants.
Cornstarch: MD ‐3.40, 95% CI ‐5.85 to ‐0.95; 1 study, 96 infants.
-
Duration of longest reflux episode (Outcome 4c): No significant interaction existed between the subtotal estimates for the type of feed thickener (Chi2 = 1.07, df = 1, P = 0.30) (Analysis 2.3; Figure 7).
Carob bean gum: MD ‐1.80 minutes, 95% CI ‐24.63 to 21.03; 1 study, 20 infants.
Cornstarch: MD ‐15.50 minutes, 95% CI ‐27.81 to ‐3.19; 1 study, 96 infants.
Sensitivity analyses
We carried out the following sensitivity analyses. The first analysis was a predetermined a priori analysis, while the second analysis was performed to explore the effect of using endpoint value rather than change from baseline value in the analysis.
Effect of methodology of trials
As the included trials relied upon parental report for symptoms or signs and side effects, we combined the blinding of participant and personnel with blinding of outcome assessors as one risk of bias. We assessed six trials as having more than one rating of high risk of bias in our analysis.
We re‐performed meta‐analysis of the number of episodes of regurgitation or vomiting per day (Outcome 1a) using data from the remaining two studies (Hegar 2008; Miller 1999), which showed similar results (MD ‐1.86, 95% CI ‐3.13 to ‐0.58; 2 studies, 148 infants; I2 = 10%).
Trials reporting the remaining outcome measures had more than one rating of high risk of bias.
Endpoint value effects
We re‐performed the following five outcome measures using endpoint value. The results we obtained were similar to those previously stated.
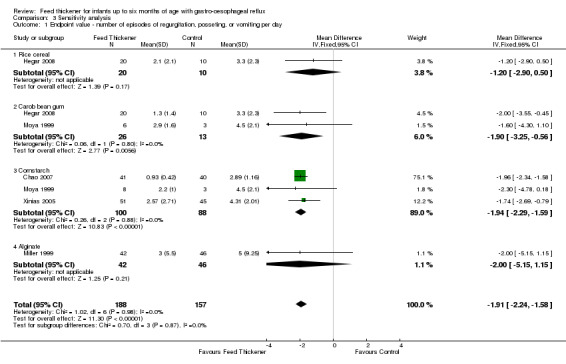
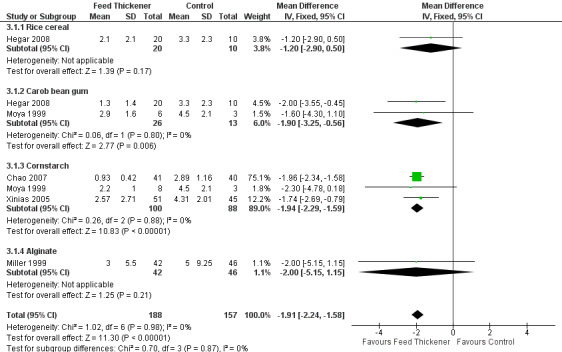
Number of episodes of regurgitation, posseting, or vomiting per day (Outcome 1a): MD ‐1.91 episodes per day, 95% CI ‐2.24 to ‐1.58; 5 studies, 345 infants; I2 = 0% (Analysis 3.1; Figure 8).
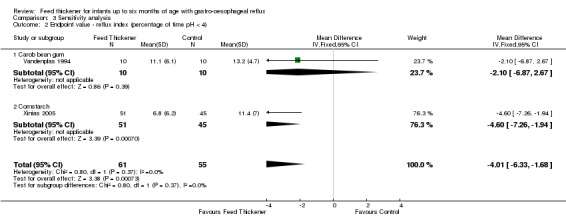
Reflux index (Outcome 2): MD ‐4.01%, 95% CI ‐6.33 to ‐1.68; 2 studies, 116 infants; I2 = 0% (Analysis 3.2).
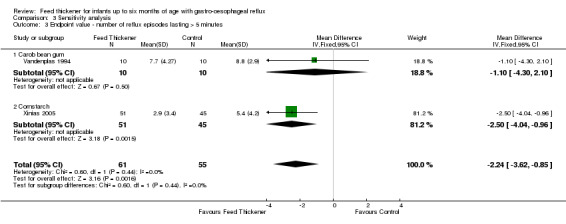
Number of reflux episodes lasting > 5 minutes (Outcome 4b): MD ‐2.24, 95% CI ‐3.62 to ‐0.85; 2 studies, 116 infants; I2 = 0 (Analysis 3.3).
Duration of longest reflux episode (Outcome 4c): MD ‐8.09 minutes, 95% CI ‐11.93 to ‐4.25; 2 studies, 116 infants; I2 = 0% (Analysis 3.4).
3.1. Analysis.
Comparison 3 Sensitivity analysis, Outcome 1 Endpoint value ‐ number of episodes of regurgitation, posseting, or vomiting per day.
8.
Forest plot of comparison: 3 Sensitivity analysis, outcome: 3.1 Endpoint value ‐ number of episodes of regurgitation, posseting, or vomiting per day.
3.2. Analysis.
Comparison 3 Sensitivity analysis, Outcome 2 Endpoint value ‐ reflux index (percentage of time pH < 4).
3.3. Analysis.
Comparison 3 Sensitivity analysis, Outcome 3 Endpoint value ‐ number of reflux episodes lasting > 5 minutes.
3.4. Analysis.
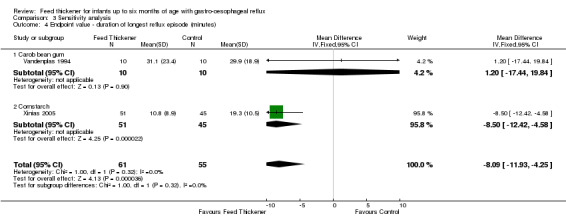
Comparison 3 Sensitivity analysis, Outcome 4 Endpoint value ‐ duration of longest reflux episode (minutes).
Discussion
Summary of main results
Meta‐analysis of the included studies found that usage of feed thickeners reduced the frequency of regurgitation or vomiting by two episodes per day when compared to control. The evidence was of moderate quality due to serious study limitations (Table 1). There was unclear risk of bias in allocation concealment and high risk of bias for blinding, as frequency of regurgitation was dependent on parental report who were likely to note the higher viscosity of the thickened formula. These limitations lowered our confidence in the effect estimate. The 95% confidence interval for the estimate of effect was narrow, consistent with a reduction of between 1.6 to 2.3 episodes per day. Chao 2007 had the highest weight in the meta‐analysis, of 85%, due to its smaller standard deviation and larger sample size compared to the other five studies included in the analysis. However, the smaller standard deviation could be a result of using the endpoint score rather than the change from baseline due to insufficient information in the report. This finding remained after removing two studies (Chao 2007 for the usage of endpoint scores rather than change from baseline score, and Miller 1999 due to the reporting of median rather than mean values), with more equal distribution of weight across the remaining four studies, ranging from 4% to 34%. The repeat meta‐analysis of the remaining four studies found that feed thickeners reduced frequency of regurgitation or vomiting by 1.8 episodes per day with 95% confidence interval of reduction between 0.8 and 2.8 episodes per day. Although only the cornstarch‐ and alginate‐thickened groups showed significant reduction in the frequency of regurgitation or vomiting (Figure 3; Figure 4), we found no significant interaction between the subtotal estimates for the type of feed thickener subgroups. This may because there are very few studies in each feed thickener subgroup.
We also found infants given feed thickeners to be 2.5 times more likely to be asymptomatic from regurgitation or vomiting at the end of the intervention period compared to control infants. However, this evidence was of low quality ( Table 1 ). Besides the serious study limitation mentioned above, there was serious imprecision. This analysis was derived from just two studies, including the largest study in the review recruiting 166 infants (Iacono 2002), which was reported as an abstract only. Results for whether feed thickeners improved non‐regurgitation signs or symptoms were conflicting.
We also found usage of feed thickeners to improve the oesophageal pH probe parameters of reflux index by 5%, the number of reflux episodes lasting more than 5 minutes by 3.4 episodes per day, and the duration of longest reflux episodes by 12.4 minutes when compared to control. However, the evidence for these findings was of low quality due to serious study limitation (unclear/high randomisation and allocation concealment bias) as well as imprecision ( Table 1). The analyses were based on two trials with a total of 116 infants. Only one trial in 96 infants reported the number of reflux episodes per hour detected by oesophageal pH probe, and did not show any difference.
There was no significant difference in the incidence of side effects reported between the control and feed‐thickened groups in the majority of the included studies except for Iacono 2002, where the intervention was suspended in 14 infants (17%) in the carob flour‐thickened group due to diarrhoea. The number of infants in the control group with diarrhoea was not reported in this study. Similarly, the evidence for these findings was of low quality due to serious imprecision and publication bias. None of the studies was designed to measure side effects ( Table 1).
Overall completeness and applicability of evidence
Firstly, these findings are broadly applicable only to formula‐feeding term infants up to six months of age presenting with uncomplicated GOR diagnosed by clinical symptoms, including the frequency of regurgitation. These findings may not be applicable to preterm infants despite reflux being a common problem in this group (Drug and Therapeutics Bulletin 2010). The lack of studies in preterm infants may be due to the difficulty in diagnosing or measuring GOR in preterm infants as well as the lack of feed thickeners that meet the nutritional demands of preterm infants (Corvaglia 2013). The use of alginate in preterm infants was found to reduce the acid oesophageal exposure detected by combined pH and impedence monitoring (pH‐MII) in two case control studies in 28 and 32 preterm infants, respectively (Corvaglia 2011; Corvaglia 2011a), and in a pH probe cohort study in 50 preterm infants (Atasay 2010). However, there was no effect on non‐acid GOR. The impact of alginate in reducing symptoms of GOR in preterm infants is unclear. Corvaglia 2011 found that alginate did not reduce the frequency of GOR‐related apnoea, while Atasay 2010 found improvement in vomiting and weight gain. Corvaglia 2012, a case control study of 28 preterm infants, found that specially designed preterm formula thickened with the digestible carbohydrate amylopectin reduced the number of acid GOR episodes without improving the crucial acid oesophageal exposure and non‐acid GOR detected by pH‐MII. In Corvaglia 2006, a case control study of 5 preterm infants, starch‐thickened expressed human breast milk showed no effect on number of acid and non‐acid GOR episodes detected by pH‐MII.
The findings of this review may also not be applicable to infants with GOR due to neurological impairment or other medical reasons, such as cow's milk intolerance. The use of feed thickeners such as alginates in breastfeeding infants, including their efficacy and impact on breastfeeding rates, have also not been fully investigated in trials to date. This is crucial, as the prevalence of GOR in breastfeeding infants is similar to bottle‐feeding infants (Barak 2006).
Apart from diarrhoea reported in one study (Iacono 2002), the majority of studies reported no significant differences in side effects between the control and feed thickener groups. However, the studies were mainly short term, with follow‐up periods of up to eight weeks, and were not powered to detect side effects. Hence, small but real differences in adverse effects may not be found without a large‐scale randomised controlled trial or observational study. In addition, some reports excluded infants who had side effects from the analysis.
The long‐term impact of excessive weight gain in feed thickener groups noted in some studies is unclear, especially with the use of non‐standard thickened formula when feed thickeners are added to a standard formula at home (Chao 2007; Hegar 2008; Moya 1999; Xinias 2005). This may provide an excessively high caloric density. However, there is increasing evidence that infants regulate the volume of intake depending on calorie density, although the excessive calories added due to feed thickeners are mostly in the form of carbohydrates, and the risk of high‐carbohydrate but low‐protein feeding remains even when the infant self regulates (Agosti 2003; Atkinson 2004; Carver 2001; Cooke 2001). The allergenicity of commercially available thickening agents is also uncertain to date, although none of the studies reported any such events.
The severity of GOR is usually dependent on the frequency and volume of regurgitation or vomiting, or both. Although our meta‐analyses show that feed thickeners reduced the frequency of regurgitation or vomiting, the impact on volume or severity of the events is unclear due to the lack of standardisation in how these variables were reported in the four included studies that reported volume or severity of regurgitation (Iacono 2002; Miller 1999; Vandenplas 1994; Vanderhoof 2003).
Potential biases in the review process
The methodological quality of the included studies varied (Figure 2). The included studies depended on parental report of signs or symptoms. Despite attempts to blind the parents or caregivers from the intervention, and the fact that thickened formula mainly thickens only when it comes in contact with acid in the stomach, it was likely that parents may still have noted the higher viscosity of the thickened formula compared to control unthickened feeds. This could potentially lead to overestimation of the reduction in frequency of regurgitation or vomiting and the incidence of side effects in the feed thickener group. However, this lack of blinding may be inevitable in such studies.
We assessed the randomisation or allocation concealment method as at unclear or high risk of bias in five of the eight included studies. This may have introduced selection bias in our analysis. There was an imbalance in the baseline characteristics of infants in the feed thickener and control groups in four studies. In three trials infants in the intervention group had a higher number of vomiting episodes per day or larger measures of regurgitation volume than infants in the control group (Miller 1999; Vanderhoof 2003; Xinias 2005). Infants in the intervention group in the Moya 1999 study were older than those in the control group. These imbalances may have resulted in an overestimation of the effects of the intervention, as infants in the former three studies had more severe symptoms that were likely to respond better to feed thickeners, while older infants in the latter study were more likely to recover from regurgitation due to spontaneous resolution of the condition with age.
Three studies declared receiving funding from a pharmaceutical company (Miller 1999; Vanderhoof 2003; Xinias 2005). We did not assess publication bias in our analysis as there were fewer than 10 included studies. Given these limitations, the strength of the evidence presented must be interpreted with caution.
Agreements and disagreements with other studies or reviews
Similiar to four previous reviews (Carroll 2002; Craig 2004; Horvath 2008; Neu 2012), our review found moderate‐certainty evidence that feed thickeners reduce the frequency of regurgitation or vomiting per day in formula‐feeding term infants up to six months of age presenting with uncomplicated GOR. Although only the cornstarch‐ and alginate‐thickened groups showed a significant reduction in frequency of regurgitation or vomiting (Figure 3; Figure 4), the evidence in our current analysis was insufficient to conclude that one form of feed thickener is superior to another, which is in concordance with previous reviews. However, unlike in previous reviews, we found that feed thickeners also improved pH probe study parameters of reflux index (measure of oesophageal acid exposure), number of acid reflux episodes over 5 minutes, and duration of longest acid reflux episode, but noted no difference in number of acid reflux episodes per hour. However, the strength of evidence for this is low, as only two included studies reported pH probe study parameters.
Neu 2012 (search date 2002 to 2011) reviewed the literature for full‐text articles in the English language on non‐surgical treatment for symptoms of irritability in infants with GORD up to 23 months of age. Cross‐over trials were included in the review. The review found three studies, and the authors concluded that infants treated with rice‐ and corn‐thickened formula had more reduction in irritability and reflux than the control group.
Horvath 2008 (search date up to May 2008) found that feed thickeners were moderately effective in treating GOR in healthy infants based on 14 included studies recruiting 877 infants. The review included cross‐over trials and infants up to two years of age. The authors found that feed thickener improved regurgitation or vomiting and weight gain symptoms with no serious adverse effects being reported. However, apart from the duration of longest acid reflux episode, they did not find improvement in pH probe study parameters, including the reflux index. They also found no definitive evidence showing that one particular feed thickener is more effective than another.
Craig 2004 (search date up to January 2003) concluded that feed thickeners reduced the frequency and severity of regurgitation of developmentally normal children aged between 1 month and 2 years based on 8 studies recruiting 419 infants. There was no difference in reflux index obtained from pH probe parameter.
Carroll 2002 (search date up to November 2000) found that feed thickeners reduced vomiting symptoms without reducing measurable reflux based on 4 studies recruiting 188 infants.
Authors' conclusions
Implications for practice.
Gastro‐oesophageal reflux (GOR) is a physiological self resolving phenomenon in infants that does not necessarily require any treatment. However, GOR becomes pathological when it causes complications or troublesome symptoms. The aim of management of gastro‐oesophageal reflux disease (GORD) should be to reduce symptoms and prevent complications while maintaining normal growth without side effects of the treatment. However, this distinction between GOR and GORD may be unclear at times. Frequent regurgitation is a common cause of parental anxiety and referral for specialist input. The cornerstone in the management of uncomplicated GOR should be parental education and reassurance with modification of feeding frequency and volume if necessary. However, if regurgitation symptoms persist in term bottle‐feeding infants, feed thickeners may be considered, as they have been found to reduce the frequency of regurgitation or vomiting by two episodes per day when compared to control treatment. The reduction by two episodes of regurgitation or vomiting per day may be of clinical significance to parents or caregivers. This therapeutic benefit of feed thickener was reproducible after removing studies with high risk of bias from our analysis. However, the improvement in pH probe parameters, especially reflux index, with feed thickeners should be interpreted with caution as there was very limited evidence available.
Due to the small number of studies available, our analysis provided insufficient information to conclude whether one type of feed thickener is superior to another. However, it may be prudent to use feed thickeners that have undergone clinical evaluation. Although alginate can be used in breastfeeding infants, so far there is insufficient evidence to show its effectiveness and impact on breastfeeding infants. There is also insufficient evidence to recommend the use of feed thickeners including alginate in preterm infants, as well as reported side effects that include necrotising enterocolitis (Beal 2012; Clarke 2004).
Implications for research.
Despite the widespread use of feed thickeners such as alginates in neonatal units (Dhillon 2004), there is a lack of evidence for the use of feed thickeners in preterm infants. The results of this review suggest that a randomised controlled trial should be carried out in preterm infants up to 44 weeks corrected age to investigate the role of feed thickeners in the treatment of GORD. A randomised controlled trial design is crucial for investigating the efficacy of any treatment for GOR, as it is well accepted that the condition improves and resolves spontaneously as the infant grows. Comparison with a parallel control group, selected by appropriate randomisation, is essential to differentiate the natural progression of the disease from the effect of the intervention. In addition, concerns about serious side effects such as necrotising enterocolitis warrant that there should be a strict safety reporting mechanism, and the intervention should be started only after infants are tolerating full enteral feeds. Feed thickeners should be specially designed to meet the nutritional demands of preterm infants. Such a study should consider objective outcomes such as pH probe study or combined pH and impedence measures, as well as clinical outcomes such as regurgitation frequency and severity, GOR‐related apnoeas and desaturations, and growth to provide clinically relevant conclusions.
For term infants, further parallel‐group randomised controlled trials exploring the effect of different types of feed thickeners would be informative. Comparison with an appropriate control must be included to differentiate between the natural progression of the disease and the effect of the intervention. Although parental reporting of symptoms of GOR may be affected by incomplete blinding, it should still be reported alongside objective measurements such as pH probe study or combined pH and impedence monitoring, as the reported outcomes are more relevant for clinical practice. The use of a validated regurgitation scoring system such as the Infant Gastroesophageal Reflux Questionnaire Revised (I‐GERQ‐R) or the Vandenplas scoring system should be considered to enable comparability between studies (Kleinman 2006; Orenstein 2010; Vandenplas 1994).
What's new
| Date | Event | Description |
|---|---|---|
| 28 June 2017 | New citation required and conclusions have changed | Studies are now eligible for inclusion and to inform conclusions. |
| 22 November 2016 | New search has been performed | We expanded the age criterion to full‐term infants up to six months old and preterm infants up to six months corrected age. |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 3, 2002
| Date | Event | Description |
|---|---|---|
| 21 October 2008 | Amended | Converted to new review format. |
| 17 April 2004 | New search has been performed | This is an update of the review 'Feed thickener for newborn infants with gastro‐oesophageal reflux' published in the Cochrane Library, Issue 3, 2002 (Huang 2002). We searched for additional studies using the same criteria and identified four additional studies, which we excluded from the review. We identified no new trials fulfilling our inclusion criteria. There are therefore no substantive changes in the review update. |
| 11 March 2002 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank the Cochrane Neonatal Review Group for help with literature review. We would also like to thank Prof Vandenplas, Prof Moya, Prof Vanderhoof, Dr Miller, Prof Carroccio, and Dr Tolia for providing further information regarding their trials that was not published in the original manuscript.
Appendices
Appendix 1. Standard search methodology
Review specific terms: ("Gastroesophageal Reflux"[Mesh] OR Gastroesophageal Reflux OR gastro‐oesophageal reflux) AND (thick* OR diet OR antiregurg* OR conserv* OR non‐pharmaco* OR nonpharmaco*)
AND
Database specific terms:
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. 'Risk of bias' tool
We used the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality (to meet the validity criteria) of the trials. For each trial, we sought information regarding the method of randomisation and the blinding and reporting of all outcomes of all infants enrolled in the trial. We assessed each criterion as low, high, or unclear risk. Two review authors independently assessed each study. Any disagreements were resolved by discussion. We added this information to the Characteristics of included studies table. We evaluated the following issues and entered the findings into the 'Risk of bias' table.
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
a. low risk (any truly random process, e.g. random number table, computer random number generator);
b. high risk (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number);
c. unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
a. low risk (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes);
b. high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
c. unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
a. low risk, high risk, or unclear risk for participants;
b. low risk, high risk, or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
a. low risk for outcome assessors;
b. high risk for outcome assessors;
c. unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of the data, including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
a. low risk (< 20% missing data);
b. high risk (≥ 20% missing data);
c. unclear risk.
6. Selective reporting bias. Are reports of the study free of the suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
a. low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
b. high risk (where not all of the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
c. unclear risk.
7. Other sources of bias. Did the study appear to be free of other problems that could put it at high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
a. low risk;
b. high risk;
c. unclear risk.
If needed, we explored the impact of the level of bias by undertaking sensitivity analyses.
Data and analyses
Comparison 1. Regurgitation, posseting, or vomiting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of episodes per day | 6 | 442 | Mean Difference (IV, Fixed, 95% CI) | ‐1.97 [‐2.32, ‐1.61] |
| 1.1 Rice cereal | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐1.43 [‐3.36, 0.49] |
| 1.2 Carob bean gum | 2 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐1.47 [‐3.13, 0.19] |
| 1.3 Cornstarch | 3 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐1.98 [‐2.35, ‐1.61] |
| 1.4 Alginate | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐3.5 [‐6.07, ‐0.93] |
| 2 Proportion of asymptomatic infants | 2 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.38, 4.51] |
| 2.1 Carob bean gum | 2 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.38, 4.51] |
Comparison 2. Oesophageal pH probe study parameters.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reflux Index (percentage of time pH < 4) | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐5.08 [‐8.89, ‐1.28] |
| 1.1 Carob bean gum | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐3.90 [‐9.36, 1.56] |
| 1.2 Cornstarch | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐6.20 [‐11.50, ‐0.90] |
| 2 Number of reflux episodes lasting > 5 minutes | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐5.44, ‐1.36] |
| 2.1 Carob bean gum | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐3.4 [‐7.06, 0.26] |
| 2.2 Cornstarch | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐5.85, ‐0.95] |
| 3 Duration of longest reflux episode (minutes) | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐12.41 [‐23.25, ‐1.58] |
| 3.1 Carob bean gum | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐24.63, 21.03] |
| 3.2 Cornstarch | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐15.5 [‐27.81, ‐3.19] |
Comparison 3. Sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Endpoint value ‐ number of episodes of regurgitation, posseting, or vomiting per day | 5 | 345 | Mean Difference (IV, Fixed, 95% CI) | ‐1.91 [‐2.24, ‐1.58] |
| 1.1 Rice cereal | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.90, 0.50] |
| 1.2 Carob bean gum | 2 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐3.25, ‐0.56] |
| 1.3 Cornstarch | 3 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐1.94 [‐2.29, ‐1.59] |
| 1.4 Alginate | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐5.15, 1.15] |
| 2 Endpoint value ‐ reflux index (percentage of time pH < 4) | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐4.01 [‐6.33, ‐1.68] |
| 2.1 Carob bean gum | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐6.87, 2.67] |
| 2.2 Cornstarch | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐4.60 [‐7.26, ‐1.94] |
| 3 Endpoint value ‐ number of reflux episodes lasting > 5 minutes | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐2.24 [‐3.62, ‐0.85] |
| 3.1 Carob bean gum | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐4.30, 2.10] |
| 3.2 Cornstarch | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐2.50 [‐4.04, ‐0.96] |
| 4 Endpoint value ‐ duration of longest reflux episode (minutes) | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐8.09 [‐11.93, ‐4.25] |
| 4.1 Carob bean gum | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐17.44, 19.84] |
| 4.2 Cornstarch | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐8.5 [‐12.42, ‐4.58] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chao 2007.
| Methods |
Study design: Randomised controlled trial. Study grouping: Parallel group. |
|
| Participants |
Baseline characteristics Control/placebo
Feed thickener
Inclusion criteria: Non‐breastfed infants (age 2 to 4 months) presenting with 3 or more episodes of regurgitation/vomiting per day. Exclusion criteria: Mechanical obstruction such as infantile hypertrophic pyloric stenosis and malrotation were excluded with an upper gastrointestinal barium study before inclusion. Infants with atopic symptoms such as eczema, watery rhinorrhoea, or diarrhoea suspecting of cow's milk allergy were excluded. Pretreatment: There was no difference in demographics and baseline clinical characteristics between intervention and control groups. Study period: 2 years (July 2002 to July 2004). |
|
| Interventions |
Intervention characteristics Control/placebo
Feed thickener
|
|
| Outcomes | Outcomes were reported after 8 weeks of intervention. Number of episodes of regurgitation, posseting, or vomiting per day
Sleep disturbance
Irritability
Cough
Diarrhoea
|
|
| Notes |
Sponsorship source: None declared. Country: Taiwan. Setting: Outpatients. Author name: Yvan Vandenplas. Institution: Universitair Ziekenhuis Brussel Kinderen. Email: yvan.vandenplas@uzbrussel.be Address: Department of Pediatrics, Universitair Ziekenhuis Brussel Kinderen, Vrije Universiteit Brussel, Laarbeeklaan 101, 1090 Brussels, Belgium. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed using an envelope‐drawing system. However, there was no mention of how the random numbers were generated. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed using an envelope‐drawing system. |
| Blinding of participants and personnel (performance bias) Symptoms or signs of gastro‐oesophageal reflux and side effect | High risk | Blinding of intervention (cornstarch‐thickened AR formula) and control (25% strengthened regular infant formula) were unlikely. Frequency of symptoms depended on parental report. |
| Blinding of participants and personnel (performance bias) Oesophageal pH probe study parameter | Low risk | Not applicable. |
| Blinding of outcome assessment (detection bias) Symptoms or signs of gastro‐oesophageal reflux and side effects | High risk | Blinding of intervention (cornstarch‐thickened antiregurgitation formula) and control (25% strengthened regular infant formula) were unlikely. Frequency of symptoms depended on parental report. |
| Blinding of outcome assessment (detection bias) Oesophageal pH probe study parameter | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 100 included infants, 81 infants completed the 2‐month clinical follow‐up. Of the 19 excluded infants, 8 developed marked diarrhoea or enteritis, 5 experienced upper respiratory infection, and 6 did not have regular follow‐up. The proportion of these infants in the feed thickener and control group was not specified. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods section of the study were reported, but the study protocol or trial registration numbers were not available. |
| Other bias | Low risk | There was no difference in the demographics and baseline clinical characteristics between the intervention and control group. |
Hegar 2008.
| Methods |
Study design: Randomised controlled trial. Study grouping: Parallel group. |
|
| Participants |
Baseline characteristics Control/placebo
Feed thickener 1
Feed thickener 2
Inclusion criteria: Healthy term‐born, formula‐fed infants (age 1 to 3 months) presenting with frequent regurgitation or vomiting, or both (4 times/day since at least 1 week before inclusion). All of the infants were exclusively fed with standard infant formula at normal concentration at baseline. Exclusion criteria:
Pretreatment: No baseline characteristics were statistically significant. Study period: Not reported. |
|
| Interventions |
Intervention characteristics Control/placebo
Feed thickener 1
Feed thickener 2
|
|
| Outcomes | Outcomes were reported after 4 weeks of intervention. Number of episodes of regurgitation, posseting, or vomiting per day
Sleep disturbance
Diarrhoea
|
|
| Notes |
Sponsorship source: None. Country: Belgium. Setting: Not specified but likely outpatient. Author name: Yvan Vandenplas. Institution: Universitair Ziekenhuis Brussel Kinderen. Email: yvan.vandenplas@uzbrussel.be Address: Department of Pediatrics, Universitair Ziekenhuis Brussel Kinderen, Vrije Universiteit Brussel, Laarbeeklaan 101, 1090 Brussels, Belgium. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed according to an automated randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) Symptoms or signs of gastro‐oesophageal reflux and side effect | High risk | Parents were blinded to the formulae. However, parents were likely to observe that the thickened formula had a higher viscosity, which could affect parent‐reported symptoms or signs of gastro‐oesophageal reflux and side effect. |
| Blinding of participants and personnel (performance bias) Oesophageal pH probe study parameter | Low risk | Not applicable |
| Blinding of outcome assessment (detection bias) Symptoms or signs of gastro‐oesophageal reflux and side effects | High risk | Parents were blinded to the formulae. However, parents were likely to observe that the thickened formula had a higher viscosity, which could affect parent‐reported symptoms or signs of gastro‐oesophageal reflux and side effect. |
| Blinding of outcome assessment (detection bias) Oesophageal pH probe study parameter | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 60 included infants completed the study and were accounted for. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods section of the study were reported, but the study protocol or trial registration numbers were not available. |
| Other bias | Low risk | No statistically significant difference between intervention and control groups at baseline |
Iacono 2002.
| Methods |
Study design: Randomised controlled trial. Study grouping: Parallel group. |
|
| Participants |
Baseline characteristics Control/placebo
Intervention
Inclusion criteria: Bottle‐fed infants, under 4 months of age, who consecutively presented at the outpatient clinics of 6 paediatric centres with frequent regurgitation/vomiting due to uncomplicated GOR. Exclusion criteria: Patients over 4 months, breast‐ or mixed‐feeding, signs of “complicated” GOR, GOR secondary to food allergy. Pretreatment: No difference in regurgitation score at baseline. Study period: Not reported. |
|
| Interventions |
Intervention characteristics Control/placebo
Intrevention
|
|
| Outcomes | Outcomes were reported after 8 weeks of intervention. Number of infants without regurgitation, posseting, or vomiting at the end of the intervention period
Diarrhoea
|
|
| Notes |
Comments: Published as correspondence to the editor only. We obtained further information from the author. Sponsorship source: None declared. Country: Italy. Setting: Children's outpatient clinics in 6 centres. Comments: Author name: Giuseppe Iacono. Institution: Pediatric Gastroenterology, “Di Cristina” Hospital. Email: stoai@inwind.it Address: Pediatric Gastroenterology, “Di Cristina” Hospital, Palermo, Italy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although the correspondence stated that infants were randomly assigned to 2 treatment regimens, the method of randomisation was not described. |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) Symptoms or signs of gastro‐oesophageal reflux and side effect | High risk | Method of blinding was not described. However, parents were likely to note the higher viscosity of the thickened feed, which could have affected parental report of signs and symptoms. |
| Blinding of participants and personnel (performance bias) Oesophageal pH probe study parameter | Low risk | Not applicable |
| Blinding of outcome assessment (detection bias) Symptoms or signs of gastro‐oesophageal reflux and side effects | High risk | Method of blinding was not described. However, parents were likely to note the higher viscosity of the thickened feed, which could have affected parental report of signs and symptoms. |
| Blinding of outcome assessment (detection bias) Oesophageal pH probe study parameter | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Author did not mention the number of infants excluded prior to randomisation. However, all randomised infants were included in the final analysis. |
| Selective reporting (reporting bias) | High risk | This study was published as a correspondence to the editor only. Not all values for the measured outcome were reported. Baseline characteristics of infants pre‐intervention were not reported. Study protocol or trial registration numbers were not available. |
| Other bias | Low risk | The intergroup comparison of the data did not show any difference in regurgitation score between the group receiving the thickened formula and the control group at baseline. |
Miller 1999.
| Methods |
Study design: Randomised controlled trial. Study grouping: Parallel group. |
|
| Participants |
Baseline characteristics Control/placebo
Feed thickener
Inclusion criteria: Paediatric patients aged between 0 and 12 months at the pretreatment assessment, with symptoms consistent with gastro‐oesophageal reflux (persistent, unmanageable vomiting/regurgitation or vomiting/regurgitation at least twice daily for the 2 days prior to the start of the study). Exclusion criteria: Infants were excluded from the study if they had known or suspected oesophageaI disease, significant gastrointestinal disease, or uncontrolled neurological, cardiac, respiratory, metabolic, hepatic disease or renal impairment; were likely to experience excessive water loss (e.g. fever, diarrhoea); had not yet completed the 37th week of development or weighed less than 2.5 kg; were receiving drugs likely to cause sodium retention; had previously participated in the present study or were currently participating in any other clinical study or had suspected or known sensitivity to alginates. Pretreatment: Patient demographics were similar with respect to age, gender, weight, and ethnic origin between the 2 study groups. 22% of infants had a pre‐existing medical condition upon entry into the study that was comparable between groups. The nature of this condition was not reported. The duration of vomiting/regurgitation was comparable between the 2 treatment groups. However, the number of vomiting/regurgitation episodes in the 24‐hour period prior to the pretreatment assessment was slightly higher in the alginate group (8.5 vs 7 episodes per day), and the number of episodes was not evenly distributed between sex and treatment groups. Study period: 18 months (April 1994 to October 1995). |
|
| Interventions |
Intervention characteristics Control/placebo
Feed thickener
|
|
| Outcomes | Outcomes were reported after 2 weeks of intervention. Number of episodes of regurgitation, posseting, or vomiting per day
Diarrhoea
Colic
Respiratory symptom (acute nasopharyngitis)
Constipation (bowel obstruction)
|
|
| Notes |
Comments: The study included infants up to 12 months of age. The author was contacted and did not have the actual age ranges of infants recruited in the study. However, the reported mean age and standard deviation of infants recruited in the study was 4 ± 0.4 months. Based upon these values, we decided to include the study, as the spread of infants was likely to be between 3 to 5 months. 2 infants were excluded from the analysis of the placebo group as they did not receive the placebo. Sponsorship source: Parexel International Ltd and Reckitt 6 Colman Products Ltd for funding the study. Country: UK. Setting: 25 general practice centres in the UK. Author name: Dr S. Miller. Institution: The New Surgery. Email: thenewsurgeryW12@nhs.net Address: 143a Uxbridge Road, Shepherds Bush, London W12. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The paper stated that the study was randomised, but did not mention how randomisation was performed. |
| Allocation concealment (selection bias) | Unclear risk | No mention of how allocation concealment was performed |
| Blinding of participants and personnel (performance bias) Symptoms or signs of gastro‐oesophageal reflux and side effect | Low risk | This was a double‐blind trial with matching placebo administered. |
| Blinding of participants and personnel (performance bias) Oesophageal pH probe study parameter | Low risk | Not applicable |
| Blinding of outcome assessment (detection bias) Symptoms or signs of gastro‐oesophageal reflux and side effects | Low risk | This was a double‐blind trial with matching placebo administered. |
| Blinding of outcome assessment (detection bias) Oesophageal pH probe study parameter | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 infants were excluded from the analysis of the placebo group as they did not receive the placebo. A further 20 infants withdrew from the study (7 in the alginate group and 13 in the placebo group). However, they were all included in the final analyses as per the intention‐to‐treat protocol. Results from the efficacy evaluable population were similar to those from the intention‐to‐treat protocol. |
| Selective reporting (reporting bias) | Unclear risk | All outcome measures described in the methods section were reported. However, study protocol or trial registration number, or both, were not available. |
| Other bias | High risk | The study was funded by Parexel International Ltd and Reckitt 6 Colman Products Ltd. Infants in the alginate group had a higher number of vomiting/regurgitation episodes per day at baseline as compared to the placebo group. |
Moya 1999.
| Methods |
Study design: Randomised controlled trial. Study grouping: Parallel group. |
|
| Participants |
Baseline characteristics Control/placebo
Feed thickener 1
Feed thickener 2
Inclusion criteria: Infants under 4 months of age (1 to 4 months old) with frequent regurgitations (more than 5 regurgitations per day) without any signs of gastro‐oesophageal reflux disease were included. Exclusion criteria: Preterm, low birth weight, breastfeeding infants, infants with history of previous illness, or infants who had received any antireflux medication were excluded. Pretreatment: The baseline clinical characteristics of infants were similar between the groups, except for the age of infant at entry to the study between infants in the control group (group 1) and infants in the group receiving carob bean gum‐thickened formula (group 3). Infants in group 1 were younger at entry to the study, at 54.5 days (standard deviation 16.8 days), as compared to 82.2 days (standard deviation 24.4 days) in group 3. Study period: Not reported. |
|
| Interventions |
Intervention characteristics Control/placebo
Feed thickener 1
Feed thickener 2
|
|
| Outcomes | Outcomes were reported after 2 weeks of intervention. Number of episodes of regurgitation, posseting, vomiting, or haematemesis per day
Diarrhoea
|
|
| Notes |
Comments: Published in Spanish. Sponsorship source: None mentioned. Country: Spain. Setting: Outpatients. Author name: M Juste. Institution: Universidad Miguel Hernandez. Email: Manuel.Moya@umh.es Address: Servicio de Pediatria, Hospital Universitario San Juan, Universidad Miguel Hernandez, Ctra. Nacional 332, Alicante–Valencia, s/n 03550 San Juan de Alicante. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out by the hospital pharmacy, which was not involved in the trial (information from author). |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed using signed, sealed envelope (information from author). |
| Blinding of participants and personnel (performance bias) Symptoms or signs of gastro‐oesophageal reflux and side effect | High risk | Initials 'AR' appear on the label depending on the type of formula infant received. Parents may notice the higher viscosity of the antiregurgitation formula, which could affect parental report of signs or symptoms. |
| Blinding of participants and personnel (performance bias) Oesophageal pH probe study parameter | Low risk | Not applicable |
| Blinding of outcome assessment (detection bias) Symptoms or signs of gastro‐oesophageal reflux and side effects | High risk | Parents may notice the higher viscosity of the antiregurgitation formula, which could affect parental report of signs or symptoms. |
| Blinding of outcome assessment (detection bias) Oesophageal pH probe study parameter | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants were followed up until the end of the 2‐week study period (information from author). |
| Selective reporting (reporting bias) | Unclear risk | All outcome measures described in the methods section were reported. However, the study protocol or trial registration number, or both, were not available. |
| Other bias | High risk | Infants in the control group were younger on entry to the study than those in the intervention group. This could have resulted in an overestimation of the effect of the intervention, as gastro‐oesophageal reflux normally resolves with time. |
Vandenplas 1994.
| Methods |
Study design: Quasi‐randomised controlled trial. Study grouping: Parallel group. |
|
| Participants |
Baseline characteristics Control/placebo
Feed thickener
Inclusion criteria: Term formula milk‐feeding infants between 1 week and 4 months old, presenting with more than 5 episodes of regurgitations a day and abnormal oesophageal pH monitoring results with percentage of time with pH < 4.0 between 10% and 30%. Exclusion criteria: Infants with secondary gastro‐oesophageal reflux caused by urinary or gastrointestinal infection and food allergy were excluded using appropriate cultures, immunoglobulin E, radioallergosorbent test (cow's milk, betalactoglobulin, lactalbumin, casein), and skin prick test for cow’s milk. Infants who were not thriving or had symptoms suggestive of peptic oesophagitis were excluded. If there was any suspicion, upper gastrointestinal endoscopy with multiple biopsies was performed. Pretreatment: There was no difference in participant characteristics between intervention and control group at baseline. Study period: Not reported. |
|
| Interventions |
Intervention characteristics Control/placebo
Feed thickener
|
|
| Outcomes | Outcomes were reported after 1 week of intervention. Number of infants without regurgitation, posseting, or vomiting at the end of the intervention period
Reflux index
Duration of longest episode
Number of reflux episodes lasting > 5 minutes
|
|
| Notes |
Sponsorship source: None declared. Country: Belgium. Setting: Outpatient clinic. Author name: Yvan Vandenplas. Institution: Loeb Academic Children's Hospital, Free University of Brussels. Email: Yvan.Vandenplas@uzbrussel.be Address: Loeb Academic Children's Hospital, Free University of Brussels, Laarbeeklaan 101, B‐1090 Brussels, Belgium. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Infants were randomised to thickened or unthickened formula in an alternate manner based on order of presentation to the clinic (information provided by author). |
| Allocation concealment (selection bias) | High risk | Infants were randomised to thickened or unthickened formula in an alternate manner based on order of presentation to the clinic (information provided by author). |
| Blinding of participants and personnel (performance bias) Symptoms or signs of gastro‐oesophageal reflux and side effect | High risk | Blinding was performed using anonymous milk tins. Neither parents nor physicians knew the content of the formula during the study period. Parents were informed that the study investigated a 'new' formula rather than a 'thickened' formula (information from author). However, parents could have noticed the higher viscosity of the antiregurgitation formula, which may have affected parental report of signs or symptoms. |
| Blinding of participants and personnel (performance bias) Oesophageal pH probe study parameter | Low risk | Potential of hydrogen probe study parameter is an objective measure of reflux and is unlikely to be affected by blinding bias. |
| Blinding of outcome assessment (detection bias) Symptoms or signs of gastro‐oesophageal reflux and side effects | High risk | Blinding was performed using anonymous milk tins. Neither parents nor physicians knew the content of the formula during the study period. Parents were informed that the study investigated a 'new' formula rather than a 'thickened' formula (information from author). However, parents could have noticed the higher viscosity of the antiregurgitation formula, which may have affected parental report of signs or symptoms. |
| Blinding of outcome assessment (detection bias) Oesophageal pH probe study parameter | Low risk | Potential of hydrogen probe study parameter is an objective measure of reflux and is unlikely to be affected by blinding bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data were reported and no exclusions were mentioned. |
| Selective reporting (reporting bias) | Unclear risk | All outcome measures described in the methods section were reported. However, the study protocol or trial registration number, or both, were not available. |
| Other bias | Low risk | There were no reported differences in participant characteristics at baseline. |
Vanderhoof 2003.
| Methods |
Study design: Randomised controlled trial. Study grouping: Parallel group. |
|
| Participants |
Baseline characteristics Control/placebo
Feed thickener
Inclusion criteria: Infants with more than 5 regurgitations per day for 2 baseline days, age between 14 and 120 days, gestational age at birth > 37 weeks, birth weight > 2500 g, and maternal age > 18 years were included. Exclusion criteria: Disease or congenital anomalies interfering with normal feeding or causing repeated regurgitation, fever or infectious illness at enrolment, clinical diagnosis of milk or soy protein allergy, complicated gastro‐oesophageal reflux disease (oesophagitis, haematemesis, recurrent respiratory symptoms, failure to thrive), previous treatment with thickened formula, or treatment with prokinetic medication within 5 days before the start of the study. Pretreatment: Baseline demographics and clinical parameters were similar between infants in the intervention and control groups except for the total regurgitation volume score, which was worse in the intervention group. The total regurgitation volume score is a measure of the volume of the largest regurgitation after each bottle of the day. 6 randomised infants were excluded as they did not receive the study formula. Study period: 18 months (December 1996 to July 1998). |
|
| Interventions |
Intervention characteristics Control/placebo
Feed thickener
|
|
| Outcomes | Outcomes were reported after 5 weeks of intervention. Number of episodes of regurgitation, posseting, or vomiting per day
|
|
| Notes |
Sponsorship source: This study was supported by a grant from Mead Johnson & Co. to each of the recruitment sites. Country: USA and Canada. Setting: 6 North American paediatric centres. Comments: Author name: Jon A Vanderhoof. Institution: University of Nebraska Medical Center and Creighton University. Email: Jon.Vanderhoof@childrens.harvard.edu Address: Joint Section of Pediatric Gastroenterology and Nutrition, University of Nebraska Medical Center and Creighton University, Omaha, Nebraska. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computerised randomisation schedule was prepared for each study site (information obtained from author). |
| Allocation concealment (selection bias) | Low risk | Each site was supplied with sealed envelopes containing the product code of the formula that the infant was to receive after verifying inclusion/exclusion criteria (information obtained from author). |
| Blinding of participants and personnel (performance bias) Symptoms or signs of gastro‐oesophageal reflux and side effect | High risk | This was a double‐blind trial. The study products were only identified by a randomly generated code, where each study formula group received 2 codes. The products were similar otherwise (information above obtained from author). However, parents were likely to note the higher viscosity of the thickened feed. |
| Blinding of participants and personnel (performance bias) Oesophageal pH probe study parameter | Low risk | Not applicable |
| Blinding of outcome assessment (detection bias) Symptoms or signs of gastro‐oesophageal reflux and side effects | High risk | This was a double‐blind trial. The study products were only identified by a randomly generated code, where each study formula group received 2 codes. The products were similar otherwise (information above obtained from author). However, parents were likely to note the higher viscosity of the thickened feed. |
| Blinding of outcome assessment (detection bias) Oesophageal pH probe study parameter | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 84% of infants in intervention group and 73% in the control group completed the 5‐week study. Outcome measures at the end of the study were reported in 91% and 98% of infants in the intervention and control groups, respectively. However, more than 10% of infants in the intervention and control groups did not complete the study. The data entered in the final outcome measures were based on the last data available prior to discontinuation from the study. |
| Selective reporting (reporting bias) | Unclear risk | All outcome measures described in the methods section were reported. However, study protocol or trial registration number, or both, were not available. |
| Other bias | High risk | This study was supported by a grant from Mead Johnson & Co. to each of the recruitment sites. Infants in the intervention group had a worse average total regurgitation volume score that those in the control group. |
Xinias 2005.
| Methods |
Study design: Randomised controlled trial. Study grouping: Parallel group. |
|
| Participants |
Baseline characteristics Control/placebo
Feed thickener
Inclusion criteria: All infants were term, exclusively formula‐fed, and ‘healthy’, except for excessive regurgitation and/or vomiting and abnormal pH probe study parameters of reflux index > 5%. Exclusion criteria: Infants who were very irritable or had haematemesis, passed black stools, had chronic cough, had episodes of cyanosis, or had any other medical problems were excluded. Infants on a dietetic formula (such as hydrolysed or soy‐based formula) were excluded, as the specialised formula is considered as a therapeutic intervention. Pretreatment: At baseline, infants in the feed thickener group had a higher number of episodes of vomiting per day than the control group (4.34 ± 2.42 vs 3.09 ± 1.24), but no difference in number of episodes of regurgitation per day. Study period: Not reported. |
|
| Interventions |
Intervention characteristics Control/placebo
Feed thickener
|
|
| Outcomes | Outcomes were reported after 4 weeks of intervention. Number of episodes of regurgitation, posseting, or vomiting per day
Reflux index
Number of reflux episodes per hour
Number of reflux episodes lasting > 5 minutes
Duration of longest episode
Side effect
|
|
| Notes |
Sponsorship source: United Pharmaceuticals provided funding of formula Novalac AR for the participating infants up to a maximal period of 3 months. Country: Multinational (Greece, Morocco, France, and Belgium). Setting: Outpatients. Comments: Author name: Yvan Vandenplas. Institution: Paediatric Gastroenterology, Academisch Ziekenhuis Vrije Universiteit Brussel. Email: yvan.vandenplas@az.vub.ac.be Address: Paediatric Gastroenterology, Academisch Ziekenhuis Vrije Universiteit Brussel, Laarbeeklaan 101, 1090 Brussels, Belgium. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not mentioned. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed using sealed envelopes. |
| Blinding of participants and personnel (performance bias) Symptoms or signs of gastro‐oesophageal reflux and side effect | High risk | Parents were blinded to the formulae. However, they were able to observe that the thickened formula had a higher viscosity, which may have affected parent‐reported symptoms or signs of gastro‐oesophageal reflux. |
| Blinding of participants and personnel (performance bias) Oesophageal pH probe study parameter | Low risk | Although parents were blinded to the formulae, they were able to observe that the thickened formula had a higher viscosity. However, this should not affect objective pH probe study parameters. |
| Blinding of outcome assessment (detection bias) Symptoms or signs of gastro‐oesophageal reflux and side effects | High risk | Parents were blinded to the formulae. However, they were able to observe that the thickened formula had a higher viscosity, which may have affected parent‐reported symptoms or signs of gastro‐oesophageal reflux. |
| Blinding of outcome assessment (detection bias) Oesophageal pH probe study parameter | Low risk | Although parents were blinded to the formulae, they were able to observe that the thickened formula had a higher viscosity. However, this should not affect objective pH probe study parameters. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None of the infants dropped out. |
| Selective reporting (reporting bias) | Unclear risk | All outcome measures described in the methods section were reported. However, study protocol or trial registration number, or both, were not available. |
| Other bias | High risk | United Pharmaceuticals provided funding of formula Novalac AR for the participating infants up to a maximal period of 3 months.. At baseline, infants in the feed thickener group had a higher number episodes of vomiting per day than the control group (4.34 ± 2.42 vs 3.09 ± 1.24), but there was no difference in number of episodes of regurgitation per day. Regurgitation was defined as the effortless passage of refluxed gastric contents into the oral pharynx and the mouth with effortless drooling out of the mouth, whereas vomiting was defined as forceful expulsion of the refluxed gastric contents from the mouth. |
GOR: gastro‐oesophageal reflux
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Atasay 2010 | This was a before‐after study design and not a randomised controlled trial. All preterm infants received sodium alginate with potassium bicarbonate. |
| Bailey 1987 | Participant ages ranged from 4 days to 14 months. This was a cross‐over study in which each participant received both thickened and unthickened feeds. |
| Borrelli 1997 | Participant ages ranged from 5 to 11 months. All infants received either a traditional formula thickened with rice flour at a concentration of 5% or antiregurgitation formula Nutrilon AR, which contained locus bean gum as thickener. There was no control group. |
| Chao 2007a | This was a randomised clinical trial comparing cereal‐thickened formula versus postural therapy. There was no placebo or control group. |
| Chevallier 1998 | This was a cohort study where all infants received formula thickened with cornstarch. Participant ages ranged from 2 weeks to 11 months. |
| Chevallier 2009 | This was a open, uncontrolled, multicentre cohort study. All infants received formula thickened with starch. |
| Corvaglia 2006 | This was a cross‐over study. All 5 preterm infants in the study received fortified human milk thickened with starch (70% from maize and 30% from potato). |
| Corvaglia 2011 | This was a cross‐over study. All 28 preterm infants in the study received Gaviscon, which contains both sodium alginate and sodium bicarbonate. |
| Corvaglia 2011a | This was a cross‐over study. All 32 preterm infants in the study received Gaviscon, which contains both sodium alginate and sodium bicarbonate. |
| Corvaglia 2012 | This was a cross‐over study. All 28 preterm infants in the study received preterm formula thickened with amylopectin. |
| Craig 2002 | This is not a study, but rather an expert opinion piece on the topic. |
| Dupont 2016 | This was a before‐after study design and not a randomised controlled trial. All 100 recruited infants received antiregurgitation formula with baseline and postintervention outcomes measured. |
| EBP 2004 | This is not a study, but rather an expert opinion piece on the topic. |
| Fabiani 2000 | This was a cross‐over study investigating the impact of formula thickened with carob seed galactomannans on ultrasonographic evaluation of gastric emptying time. Participant ages ranged from 1 to 12 months. |
| Georgieva 2016 | There was no control group in this randomised clinical trial. Infants in all 3 arms of the trial received formula thickened with carob bean gum galactomannans in varying doses or temperatures. |
| Gouyon 1988 | This was a cross‐over study. All 20 preterm infants in the study received smectite and postural therapy. |
| Khoshoo 2000 | This was a cohort study. All 6 infants received a smaller volume of formula thickened with dry rice cereal and postural therapy. Participant ages ranged from 4 to 10 months. |
| Lasekan 2014 | This was a multicentre randomised controlled trial that recruited healthy term infants without regard to signs or symptoms of gastro‐oesophageal reflux. The intervention was a formula thickened with rice starch that was also lactose free. The control was an unthickened formula with lactose. |
| Miyazawa 2006 | This was not a randomised controlled trial. The first part of the study investigated the impact of formula thickened with carob flour on ultrasonographic evaluation of gastric emptying time. The second part of the study was a cross‐over trial where all 27 infants received formula thickened with carob flour. |
| Miyazawa 2007 | This was a cross‐over study where each infant received both thickened and unthickened feeds. |
| Moukarzel 2007 | This was a cross‐over study where each infant received both thickened and unthickened feeds. |
| Orenstein 1992 | This was a cross‐over study where each infant received both dry rice cereal‐thickened and unthickened feeds. |
| Ostrom 2006 | The intervention in this randomised controlled trial was a soy protein‐based formula thickened with soy fibre, whereas the control was an unthickened whey protein‐based formula. Hence, there was co‐intervention of both thickening and substituting soy protein‐based formula. |
| Penna 2003 | This was not a randomised controlled trial but rather a prospective case control investigating the effect of formula thickened with home‐prepared cornstarch versus antiregurgitation formula. Hence, there was no suitable control. Participant ages ranged from 0 to 12 months. |
| Savino 2008 | This is not a study, but rather an expert opinion piece on the topic. |
| Tolia 1999 | The trial included infants up to 1 year old. |
| Toporovski 2013 | The trial did not report outcome measure specified in the review. |
| Ummarino 2013 | The trial included infants up to 1 year old with a mean age of 5.56 ± 2.34 months. |
| Wenzl 2003 | This was a cross‐over study where each infant received both carob bean gum‐thickened and unthickened feeds. |
| Xinias 2003 | This study is not a randomised controlled trial, but rather a non‐randomised open‐label study. |
Differences between protocol and review
We have made the following changes since the last update of the review in 2002.
We expanded the age criteria for the participants from 'full‐term infants less than 28 days and preterm infants up to 44 weeks corrected age’ to ‘full‐term infants less than six months and preterm infants up to six months corrected age’, because the majority of infants present with uncomplicated reflux only after the first two to three weeks of life (Meunier 2014; Vandenplas 2009; Vandenplas 2015). Reassurance and parental education is the usual first‐line management for uncomplicated reflux (Vandenplas 2015), and feed thickeners are rarely started in the first 28 days of life. We used the six‐month cut‐off due to the natural progression of GOR, which is believed to start improving by six months of age, when the infant starts on a more solid diet and achieves neurodevelopmental maturation to maintain a more upright posture (Nelson 1997).
We included Gaviscon Infant (alginate preparation without antacid) as an eligible intervention for inclusion in the review. We excluded other preparations of Gaviscon or alginate with antacid components. Gaviscon Infant works primarily by thickening the feed and does not have antacid properties, unlike other preparations of Gaviscon. We performed a subgroup analysis to determine if the results differed when trials investigating Gaviscon Infant as feed thickener were excluded.
We streamlined the primary outcomes to three outcome measures that we felt to be important to parents, caregivers, and clinicians. They were: i) regurgitation, posseting, or vomiting; ii) failure to thrive; and iii) reflux index. We analysed the remaining outcome measures as secondary outcome measures.
We also noted number of episodes per day of: i) regurgitation, posseting, vomiting, or haematemesis; ii) respiratory symptoms (cough, apnoeas, and recurrent oxygen desaturation); and iii) bradycardias as continuous and separate outcomes. We used change from baseline measurements in preference to final measurements.
We did not assess cough as a side effect of feed thickener, as it was already considered to be a symptom of GOR.
We carried out sensitivity analyses to explore the effect of change from baseline versus endpoint values on the analysis.
Contributions of authors
T'ng Chang Kwok: undertook literature searching and critical appraisal of studies, wrote review.
Shalini Ojha: undertook literature searching and critical appraisal of studies, co‐wrote review.
Jon Dorling: oversaw the project, resolved differences that occurred during the review, co‐wrote review.
Sources of support
Internal sources
Academic Division of Child Health, University of Nottingham, UK.
External sources
Cochrane Neonatal Review Group, Canada.
-
National Institute for Health Research, UK.
Editorial support for Cochrane Neonatal has been funded by a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service (NHS), the NIHR, or the UK Department of Health.
Declarations of interest
T'ng Chang Kwok: none to declare.
Shalini Ojha: none to declare.
Jon Dorling: none to declare.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Chao 2007 {published data only}
- Chao HC, Vandenplas Y. Comparison of the effect of a cornstarch thickened formula and strengthened regular formula on regurgitation, gastric emptying and weight gain in infantile regurgitation. Diseases of the Esophagus 2007;20(2):155‐60. [DOI: 10.1111/j.1442-2050.2007.00662.x; PUBMED: 17439600] [DOI] [PubMed] [Google Scholar]
Hegar 2008 {published data only}
- Hegar B, Rantos R, Firmansyah A, Schepper J, Vandenplas Y. Natural evolution of infantile regurgitation versus the efficacy of thickened formula. Journal of Pediatric Gastroenterology and Nutrition 2008;47(1):26‐30. [DOI: 10.1097/MPG.0b013e31815eeae9; PUBMED: 18607265] [DOI] [PubMed] [Google Scholar]
Iacono 2002 {published data only}
- Iacono G, Vetrano S, Cataldo F, Ziino O, Russo A, Lorello D, et al. Clinical trial with thickened feeding for treatment of regurgitation in infants. Digestive and Liver Disease 2002;34(7):532‐3. [PUBMED: 12236490] [DOI] [PubMed] [Google Scholar]
Miller 1999 {published data only}
- Miller S. Comparison of the efficacy and safety of a new aluminium‐free paediatric alginate preparation and placebo in infants with recurrent gastro‐oesophageal reflux. Current Medical Research and Opinion 1999;15(3):160‐8. [DOI: 10.1185/03007999909114087; PUBMED: 10621922] [DOI] [PubMed] [Google Scholar]
Moya 1999 {published data only}
- Moya M, Juste M, Cortes E, Auxina A, Ortiz I. Clinical evaluation of the different therapeutic possibilities in the treatment of infant regurgitation [Valoracion clinica de las distintas posibilidades terapeuticas en el manejo de las regurgitaciones del lactante]. Revista Espanola de Pediatria 1999;55(327):219‐23. [EMBASE: 29282513] [Google Scholar]
Vandenplas 1994 {published data only}
- Vandenplas Y, Hachimi‐Idrissi S, Casteels A, Mahler T, Loeb H. A clinical trial with an "anti‐regurgitation" formula. European Journal of Pediatrics 1994;153(6):419‐23. [PUBMED: 8088297] [DOI] [PubMed] [Google Scholar]
Vanderhoof 2003 {published data only}
- Vanderhoof JA, Moran JR, Harris CL, Merkel KL, Orenstein SR. Efficacy of a pre‐thickened infant formula: a multicenter, double‐blind, randomized, placebo‐controlled parallel group trial in 104 infants with symptomatic gastroesophageal reflux. Clinical Pediatrics 2003;42(6):483‐95. [DOI: 10.1177/000992280304200602; PUBMED: 12921449] [DOI] [PubMed] [Google Scholar]
Xinias 2005 {published data only}
- Xinias I, Mouane N, Luyer B, Spiroglou K, Demertzidou V, Hauser B, et al. Cornstarch thickened formula reduces oesophageal acid exposure time in infants. Digestive and Liver Disease 2005;37(1):23‐7. [DOI: 10.1016/j.dld.2004.07.015; PUBMED: 15702855] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Atasay 2010 {published data only}
- Atasay B, Erdeve O, Arsan S, Türmen T. Effect of sodium alginate on acid gastroesophageal reflux disease in preterm infants: a pilot study. Journal of Clinical Pharmacology 2010;50(11):1267‐72. [DOI: 10.1177/0091270009338483; PUBMED: 20489030] [DOI] [PubMed] [Google Scholar]
Bailey 1987 {published data only}
- Bailey DJ, Andres JM, Danek GD, Pineiro‐Carrero VM. Lack of efficacy of thickened feeding as treatment for gastroesophageal reflux. Journal of Pediatrics 1987;110(2):187‐9. [PUBMED: 3806288] [DOI] [PubMed] [Google Scholar]
Borrelli 1997 {published data only}
- Borrelli O, Salvia G, Campanozzi A, Franco MT, Moreira FL, Emiliano M, et al. Use of a new thickened formula for treatment of symptomatic gastrooesophageal reflux in infants. Italian Journal of Gastroenterology and Hepatology 1997;29(3):237‐42. [PUBMED: 9646215] [PubMed] [Google Scholar]
Chao 2007a {published data only}
- Chao HC, Vandenplas Y. Effect of cereal‐thickened formula and upright positioning on regurgitation, gastric emptying, and weight gain in infants with regurgitation. Nutrition 2007;23(1):23‐8. [DOI: 10.1016/j.nut.2006.10.003; PUBMED: 17189087] [DOI] [PubMed] [Google Scholar]
Chevallier 1998 {published data only}
- Chevallier B, Grunberg J, Rives JJ, Egroo LD, Fichot MC. Evaluation of the safety and efficacy of a corn starch‐thickened milk formula in infants with simple regurgitation [Evaluation de la tolerance et de l'efficacite d'un lait epaissi a l'amidon de mais chez les nourrissons presentant des regurgitations simples]. Annales de Pediatrie 1998;45(7):509‐15. [EMBASE: 28459703] [Google Scholar]
Chevallier 2009 {published data only}
- Chevallier B, Fournier V, Logre B, Beck L, Ceccato F, Hui Bon Hoa G, et al. Value of a new thickened formula in infants with regurgitations. Archives de Pediatrie 2009;16(4):343‐52. [DOI: 10.1016/j.arcped.2009.01.001; PUBMED: 19246178] [DOI] [PubMed] [Google Scholar]
Corvaglia 2006 {published data only}
- Corvaglia L, Ferlini M, Rotatori R, Paoletti V, Alessandroni R, Cocchi G, et al. Starch thickening of human milk is ineffective in reducing the gastroesophageal reflux in preterm infants: a crossover study using intraluminal impedance. Journal of Pediatrics 2006;148(2):265‐8. [DOI: 10.1016/j.jpeds.2005.09.034; PUBMED: 16492440] [DOI] [PubMed] [Google Scholar]
Corvaglia 2011 {published data only}
- Corvaglia L, Spizzichino M, Zama D, Aceti A, Mariani E, Legnani E, et al. Sodium alginate (Gaviscon) does not reduce apnoeas related to gastro‐oesophageal reflux in preterm infants. Early Human Development 2011;87(12):775‐8. [DOI: 10.1016/j.earlhumdev.2011.05.013; PUBMED: 21696897] [DOI] [PubMed] [Google Scholar]
Corvaglia 2011a {published data only}
- Corvaglia L, Aceti A, Mariani E, Giorgi M, Capretti MG, Faldella G. The efficacy of sodium alginate (Gaviscon) for the treatment of gastro‐oesophageal reflux in preterm infants. Alimentary Pharmacology & Therapeutics 2011;33(4):466‐70. [DOI: 10.1111/j.1365-2036.2010.04545.x; PUBMED: 21158879] [DOI] [PubMed] [Google Scholar]
Corvaglia 2012 {published data only}
- Corvaglia L, Aceti A, Mariani E, Legnani E, Ferlini M, Raffaeli G, et al. Lack of efficacy of a starch‐thickened preterm formula on gastro‐oesophageal reflux in preterm infants: a pilot study. Journal of Maternal‐Fetal & Neonatal Medicine 2012;25(12):2735‐8. [DOI: 10.3109/14767058.2012.704440; PUBMED: 22725606] [DOI] [PubMed] [Google Scholar]
Craig 2002 {published data only}
- Craig JV. Research & commentary: non‐pharmacological and non‐surgical therapy for reflux. Paediatric Nursing 2002;14(7):12. [Google Scholar]
Dupont 2016 {published data only}
- Dupont C, Vandenplas Y, SONAR Study Group. Efficacy and tolerance of a new anti‐regurgitation formula. Pediatric Gastroenterology, Hepatology, and Nutrition 2016;19(2):104‐9. [DOI: 10.5223/pghn.2016.19.2.104; PUBMED: 27437186] [DOI] [PMC free article] [PubMed] [Google Scholar]
EBP 2004 {published data only}
- Evidence in perspective. Treatment of symptomatic gastroesophageal reflux in infants. Evidence‐based Practice 2004;7(6):9‐10. [Google Scholar]
Fabiani 2000 {published data only}
- Fabiani E, Bolli V, Pieroni G, Corrado G, Carlucci A, Giacomo C, et al. Effect of a water‐soluble fiber (galactomannans)‐enriched formula on gastric emptying time of regurgitating infants evaluated using an ultrasound technique. Journal of Pediatric Gastroenterology and Nutrition 2000;31(3):248‐50. [PUBMED: 10997367] [DOI] [PubMed] [Google Scholar]
Georgieva 2016 {published data only}
- Georgieva M, Manios Y, Rasheva N, Pancheva R, Dimitrova E, Schaafsma A. Effects of carob‐bean gum thickened formulas on infants' reflux and tolerance indices. World Journal of Clinical Pediatrics 2016;5(1):118‐27. [DOI: 10.5409/wjcp.v5.i1.118; PUBMED: 26862511] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gouyon 1988 {published data only}
- Gouyon JB, Boggio V, Fantino M, Gillot I, Schatz B, Vallin A. Smectite reduces gastroesophageal reflux in newborn infants. Developmental Pharmacology and Therapeutics 1989;13(1):46‐50. [PUBMED: 2673691] [DOI] [PubMed] [Google Scholar]
- Gouyon JB, Boggio V, Gillot I, Fantino M, Schatz B, Vallin A. Efficacy of smectite in gastroesophageal reflux in the newborn infant [Efficacite de la smectite sur le reflux gastro‐oesophagien du nouveau‐ne]. Presse Medicale 1988;17(3):123‐4. [PUBMED: 2964601] [PubMed] [Google Scholar]
Khoshoo 2000 {published data only}
- Khoshoo V, Ross G, Brown S, Edell D. Smaller volume, thickened formulas in the management of gastroesophageal reflux in thriving infants. Journal of Pediatric Gastroenterology and Nutrition 2000;31(5):554‐6. [PUBMED: 11144442] [DOI] [PubMed] [Google Scholar]
Lasekan 2014 {published data only}
- Lasekan JB, Linke HK, Oliver JS, Carver JD, Blatter MM, Kuchan MJ, et al. Milk protein‐based infant formula containing rice starch and low lactose reduces common regurgitation in healthy term infants: a randomized, blinded, and prospective trial. Journal of the American College of Nutrition 2014;33(2):136‐46. [DOI: 10.1080/07315724.2013.828578; PUBMED: 24724771] [DOI] [PubMed] [Google Scholar]
Miyazawa 2006 {published data only}
- Miyazawa R, Tomomasa T, Kaneko H, Morikawa A. Effect of formula thickened with locust bean gum on gastric emptying in infants. Journal of Paediatrics and Child Health 2006;42(12):808‐12. [DOI: 10.1111/j.1440-1754.2006.00982.x; PUBMED: 17096718] [DOI] [PubMed] [Google Scholar]
Miyazawa 2007 {published data only}
- Miyazawa R, Tomomasa T, Kaneko H, Arakawa H, Morikawa A. Effect of formula thickened with reduced concentration of locust bean gum on gastroesophageal reflux. Acta Paediatrica 2007;96(6):910‐4. [DOI: 10.1111/j.1651-2227.2007.00279.x; PUBMED: 17537023] [DOI] [PubMed] [Google Scholar]
Moukarzel 2007 {published data only}
- Moukarzel AA, Abdelnour H, Akatcherian C. Effects of a prethickened formula on esophageal pH and gastric emptying of infants with GER. Journal of Clinical Gastroenterology 2007;41(9):823‐9. [DOI: 10.1097/MCG.0b013e31802c2a10; PUBMED: 17881928] [DOI] [PubMed] [Google Scholar]
Orenstein 1992 {published data only}
- Orenstein SR, Shalaby TM, Putnam PE. Thickened feedings as a cause of increased coughing when used as therapy for gastroesophageal reflux in infants. Journal of Pediatrics 1992;121(6):913‐5. [PUBMED: 1447654] [DOI] [PubMed] [Google Scholar]
Ostrom 2006 {published data only}
- Ostrom KM, Jacobs JR, Merritt RJ, Murray RD. Decreased regurgitation with a soy formula containing added soy fiber. Clinical Pediatrics 2006;45(1):29‐36. [DOI: 10.1177/000992280604500105; PUBMED: 16429213] [DOI] [PubMed] [Google Scholar]
Penna 2003 {published data only}
- Penna FJ, Norton RC, Carvalho AS, Pompeu BC, Penna GC, Ferreira MF, et al. Comparison between pre‐thickened and home‐thickened formulas in gastroesophageal reflux treatment. Jornal de Pediatria 2003;79(1):49‐54. [PUBMED: 12973509] [PubMed] [Google Scholar]
Savino 2008 {published data only}
- Savino F, Castagno E. Is cornstarch‐thickened milk formula better than strengthened regular milk formula for infant regurgitation?. Nature Clinical Practice. Gastroenterology & Hepatology 2008;5(2):72‐3. [DOI: 10.1038/ncpgasthep1009; PUBMED: 18059380] [DOI] [PubMed] [Google Scholar]
Tolia 1999 {published data only}
- Tolia V, Wuerth A, Shashidhar HS, Peters JM, Lin CH. A prospective study with an antiregurgitation (AR) formula in infants with gastroesophageal reflux (GER) symptoms. Gastrointestinal Endoscopy 1999;49(4 Pt 2):AB186. [Google Scholar]
Toporovski 2013 {published data only}
- Toporovski MS, Neufeld CB. A comparative study among two different AR formulas and a standard formula in infants with gastroesophageal reflux (GER). Journal of Pediatric Gastroenterology and Nutrition 2013;56(Suppl 2):330. [Google Scholar]
Ummarino 2013 {published data only}
- Ummarino D, Sciorio E, Crocetto F, Miele E, Staiano A. A prospective, comparative, randomized, controlled study on the efficacy of the treatment with magnesium (Mg‐) alginate in infants with gastroesophageal reflux. Digestive and Liver Disease 2013;45:e299‐300. [DOI: 10.1016/j.dld.2013.08.217] [DOI] [Google Scholar]
Wenzl 2003 {published data only}
- Wenzl TG, Schneider S, Scheele F, Silny J, Heimann G, Skopnik H. Effects of thickened feeding on gastroesophageal reflux in infants: a placebo‐controlled crossover study using intraluminal impedence. Pediatrics 2003;111(4 Pt 1):e355‐9. [PUBMED: 12671151] [DOI] [PubMed] [Google Scholar]
Xinias 2003 {published data only}
- Xinias I, Spiroglou K, Demertzidou V, Karatza E, Panteliadis C. An antiregurgitation milk formula in the management of infants with mild to moderate gastroesophageal reflux. Current Therapeutic Research, Clinical and Experimental 2003;64(4):270‐8. [DOI: 10.1016/S0011-393X(03)00039-0; PUBMED: 24944374 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Agosti 2003
- Agosti M, Vegni C, Calciolari G, Marini A, GAMMA Study Group. Post‐discharge nutrition of the very low‐birthweight infant: interim results of the multicentric GAMMA study. Acta Paediatrica 2003;92(441):39‐43. [PUBMED: 14599040] [DOI] [PubMed] [Google Scholar]
Almeida 2011
- Almeida MB, Almeida JA, Moreira ME, Novak FR. Adequacy of human milk viscosity to respond to infants with dysphagia: experimental study. Journal of Applied Oral Science: Revista FOB 2011;19(6):554‐9. [PUBMED: 22230987] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkinson 2004
- Atkinson SA, Pacs B, Saigal S, Hussey T, Lee D. Nutrient‐enriched discharge formula compared to standard formula does not benefit growth, bone mineral accretion or trace element status in preterm small for gestational age (SGA) infants to one year corrected age: a RCT. Pediatric Research 2004;55(4):383A. [Google Scholar]
Barak 2006
- Barak M, Lahav S, Mimouni FB, Dollberg S. The prevalence of regurgitations in the first 2 days of life in human milk‐ and formula‐fed term infants. Breastfeeding Medicine 2006;1(3):168‐71. [DOI: 10.1089/bfm.2006.1.168; PUBMED: 17661594] [DOI] [PubMed] [Google Scholar]
Beal 2012
- Beal J, Silverman B, Bellant J, Young TE, Klontz K. Late onset necrotizing enterocolitis in infants following use of a xanthan gum‐containing thickening agent. Journal of Pediatrics 2012;161(2):354‐6. [DOI: 10.1016/j.jpeds.2012.03.054; PUBMED: 22575248] [DOI] [PubMed] [Google Scholar]
Bosscher 2000
- Bosscher D, Caillie‐Bertrand M, Dyck K, Robberecht H, Cauwenbergh R, Deelstra H. Thickening infant formula with digestible and indigestible carbohydrate: availability of calcium, iron, and zinc in vitro. Journal of Pediatric Gastroenterology and Nutrition 2000;30(4):373‐8. [PUBMED: 10776946] [DOI] [PubMed] [Google Scholar]
Carroll 2002
- Carroll AE, Garrison MM, Christakis DA. A systematic review of nonpharmacological and nonsurgical therapies for gastroesophageal reflux in infants. Archives of Pediatrics & Adolescent Medicine 2002;156(2):109‐13. [PUBMED: 11814369] [DOI] [PubMed] [Google Scholar]
Carver 2001
- Carver JD, Wu PY, Hall RT, Ziegler EE, Sosa R, Jacobs J, et al. Growth of preterm infants fed nutrient‐enriched or term formula after hospital discharge. Pediatrics 2001;107(4):683‐9. [PUBMED: 11335744] [DOI] [PubMed] [Google Scholar]
Clarke 2004
- Clarke P, Robinson MJ. Thickening milk feeds may cause necrotising enterocolitis. Archives of Disease in Childhood 2004;89(3):F280. [PUBMED: 15102738] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooke 2001
- Cooke RJ, Embleton ND, Griffin IJ, Wells JC, McCormick KP. Feeding preterm infants after hospital discharge: growth and development at 18 months of age. Pediatric Research 2001;49(5):719‐22. [DOI: 10.1203/00006450-200105000-00018; PUBMED: 11328958] [DOI] [PubMed] [Google Scholar]
Corvaglia 2013
- Corvaglia L, Martini S, Aceti A, Arcuri S, Rossini R, Faldella G. Nonpharmacological management of gastroesophageal reflux in preterm infants. BioMed Research International 2013;2013:141967. [DOI: 10.1155/2013/141967; PUBMED: 24073393] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence 2015 [Computer program]
- Veritas Heath Innovation. Covidence. Version (accessed 22 November 2017). Melbourne, Australia: Veritas Heath Innovation, 2015.
Craig 2004
- Craig WR, Hanlon‐Dearman A, Sinclair C, Taback S, Moffatt M. Metoclopramide, thickened feedings, and positioning for gastro‐oesophageal reflux in children under two years. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD003502.pub2] [DOI] [PubMed] [Google Scholar]
Czinn 2013
- Czinn SJ, Blanchard S. Gastroesophageal reflux disease in neonates and infants: when and how to treat. Paediatric Drugs 2013;15(1):19‐27. [DOI: 10.1007/s40272-012-0004-2; PUBMED: 23322552] [DOI] [PubMed] [Google Scholar]
Dhillon 2004
- Dhillon AS, Ewer AK. Diagnosis and management of gastro‐oesophageal reflux in preterm infants in neonatal intensive care units. Acta Paediatrica 2004;93(1):88‐93. [PUBMED: 14989446] [PubMed] [Google Scholar]
Drug and Therapeutics Bulletin 2010
- Drug, Therapeutics Bulletin. Managing gastro‐oesophageal reflux in infants. BMJ 2010;341:c4420. [DOI: 10.1136/bmj.c4420; PUBMED: 20802001] [DOI] [PubMed] [Google Scholar]
Falconer 2010
- Falconer J. Gastro‐oesophageal reflux and gastro‐oesophageal reflux disease in infants and children. Journal of Family Health Care 2010;20(5):175‐7. [PUBMED: 21158357] [PubMed] [Google Scholar]
Ferreira 2014
- Ferreira CT, Carvalho E, Sdepanian VL, Morais MB, Vieira MC, Silva LR. Gastroesophageal reflux disease: exaggerations, evidence and clinical practice. Jornal de Pediatria 2014;90(2):105‐18. [DOI: 10.1016/j.jped.2013.05.009; PUBMED: 24184302] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version (accessed 28 June 2017). Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Hanson 2012
- Hanson B, O'Leary MT, Smith CH. The effect of saliva on the viscosity of thickened drinks. Dysphagia 2012;27(1):10‐9. [DOI: 10.1007/s00455-011-9330-8; PUBMED: 21374083] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Horvath 2008
- Horvath A, Dziechciarz P, Szajewska H. The effect of thickened‐feed interventions on gastroesophageal reflux in infants: systematic review and meta‐analysis of randomized, controlled trials. Pediatrics 2008;122(6):e1268‐77. [DOI: 10.1542/peds.2008-1900; PUBMED: 19001038] [DOI] [PubMed] [Google Scholar]
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [DOI: 10.1186/1471-2288-5-13; PUBMED: 15840177] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kleinman 2006
- Kleinman L, Rothman M, Strauss R, Orenstein SR, Nelson S, Vandenplas Y, et al. The infant gastroesophageal reflux questionnaire revised: development and validation as an evaluative instrument. Clinical Gastroenterology and Hepatology 2006;4(5):588‐96. [DOI: 10.1016/j.cgh.2006.02.016; PUBMED: 16678075] [DOI] [PubMed] [Google Scholar]
Madhoun 2015
- Madhoun LL, Siler‐Wurst KK, Sitaram S, Jadcherla SR. Feed‐thickening practices in NICUs in the current era: variability in prescription and implementation patterns. Journal of Neonatal Nursing 2015;21(6):255‐62. [DOI: 10.1016/j.jnn.2015.07.004; PUBMED: 26664251] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mascarenhas 2005
- Mascarenhas R, Landry L, Khoshoo V. Difficulty in defecation in infants with gastroesophageal reflux treated with smaller volume feeds thickened with rice cereal. Clinical Pediatrics 2005;44(8):671‐3. [DOI: 10.1177/000992280504400804; PUBMED: 16211190] [DOI] [PubMed] [Google Scholar]
Meunier 2014
- Meunier L, Garthoff JA, Schaafsma A, Krul L, Schrijver J, Goudoever JB, et al. Locust bean gum safety in neonates and young infants: an integrated review of the toxicological database and clinical evidence. Regulatory Toxicology and Pharmacology: RTP 2014;70(1):155‐69. [DOI: 10.1016/j.yrtph.2014.06.023; PUBMED: 24997231] [DOI] [PubMed] [Google Scholar]
Minami 1984
- Minami H, McCallum RW. The physiology and pathophysiology of gastric emptying in humans. Gastroenterology 1984;86(6):1592‐610. [PUBMED: 6370777] [PubMed] [Google Scholar]
Nelson 1997
- Nelson SP, Chen EH, Syniar GM, Christoffel KK, Pediatric Practice Research Group. Prevalence of symptoms of gastroesophageal reflux during infancy. A pediatric practice‐based survey. Archives of Pediatrics & Adolescent Medicine 1997;151(6):569‐72. [PUBMED: 9193240] [DOI] [PubMed] [Google Scholar]
Neu 2012
- Neu M, Corwin E, Lareau SC, Marcheggiani‐Howard C. A review of nonsurgical treatment for the symptom of irritability in infants with GERD. Journal for Specialists in Pediatric Nursing 2012;17(3):177‐92. [DOI: 10.1111/j.1744-6155.2011.00310.x; PUBMED: 22734872] [DOI] [PMC free article] [PubMed] [Google Scholar]
Omari 1995
- Omari TI, Miki K, Fraser R, Davidson G, Haslam R, Goldsworthy W, et al. Esophageal body and lower esophageal sphincter function in healthy premature infants. Gastroenterology 1995;109(6):1757‐64. [PUBMED: 7498639] [DOI] [PubMed] [Google Scholar]
Omari 2002
- Omari TI, Barnett CP, Benninga MA, Lontis R, Goodchild L, Haslam RR, et al. Mechanisms of gastro‐oesophageal reflux in preterm and term infants with reflux disease. Gut 2002;51(4):475‐9. [PUBMED: 12235066] [DOI] [PMC free article] [PubMed] [Google Scholar]
Orenstein 1987
- Orenstein SR, Magill HL, Brooks P. Thickening of infant feedings for therapy of gastroesophageal reflux. Journal of Pediatrics 1987;110(2):181‐6. [PUBMED: 3806287] [DOI] [PubMed] [Google Scholar]
Orenstein 1993
- Orenstein SR, Cohn JF, Shalaby TM, Kartan R. Reliability and validity of an infant gastroesophageal reflux questionnaire. Clinical Pediatrics 1993;32(8):472‐84. [DOI: 10.1177/000992289303200806; PUBMED: 8403746] [DOI] [PubMed] [Google Scholar]
Orenstein 1996
- Orenstein SR, Shalaby TM, Cohn JF. Reflux symptoms in 100 normal infants: diagnostic validity of the infant gastroesophageal reflux questionnaire. Clinical Pediatrics 1996;35(12):607‐14. [DOI: 10.1177/000992289603501201; PUBMED: 8970752] [DOI] [PubMed] [Google Scholar]
Orenstein 2010
- Orenstein SR. Symptoms and reflux in infants: Infant Gastroesophageal Reflux Questionnaire Revised (I‐GERQ‐R) ‐ utility for symptom tracking and diagnosis. Current Gastroenterology Reports 2010;12(6):431‐6. [DOI: 10.1007/s11894-010-0140-1; PUBMED: 20857238] [DOI] [PubMed] [Google Scholar]
Puntis 2015
- Puntis JW. Gastro‐oesophageal reflux in young babies: who should be treated?. Archives of Disease in Childhood 2015;100(10):989‐93. [DOI: 10.1136/archdischild-2014-306232; PUBMED: 25755169] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, (editors), GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. gdt.gradepro.org/app/handbook/handbook.html Updated October 2013 (accessed prior to 28 October 2017).
Sherman 2009
- Sherman PM, Hassall E, Fagundes‐Neto U, Gold BD, Kato S, Koletzko S, et al. A global, evidence‐based consensus on the definition of gastroesophageal reflux disease in the pediatric population. American Journal of Gastroenterology 2009;104(5):1278‐95. [DOI: 10.1038/ajg.2009.129; PUBMED: 19352345] [DOI] [PubMed] [Google Scholar]
Vandenplas 2005
- Vandenplas Y, Salvatore S, Hauser B. The diagnosis and management of gastro‐oesophageal reflux in infants. Early Human Development 2005;81(12):1011‐24. [DOI: 10.1016/j.earlhumdev.2005.10.011; PUBMED: 16278060] [DOI] [PubMed] [Google Scholar]
Vandenplas 2007
- Vandenplas Y, Salvatore S, Devreker T, Hauser B. Gastro‐oesophageal reflux disease: oesophageal impedance versus pH monitoring. Acta Paediatrica 2007;96(7):956‐62. [DOI: 10.1111/j.1651-2227.2007.00306.x; PUBMED: 17498193] [DOI] [PubMed] [Google Scholar]
Vandenplas 2009
- Vandenplas Y, Rudolph CD, Lorenzo C, Hassall E, Liptak G, Mazur L, et al. North American Society for Pediatric Gastroenterology, Hepatology, Nutrition. European Society for Paediatric Gastroenterology, Hepatology, Nutrition. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN). Journal of Pediatric Gastroenterology and Nutrition 2009;49(4):498‐547. [DOI: 10.1097/MPG.0b013e3181b7f563; PUBMED: 19745761] [DOI] [PubMed] [Google Scholar]
Vandenplas 2015
- Vandenplas Y, Alarcon P. Updated algorithms for managing frequent gastro‐intestinal symptoms in infants. Beneficial Microbes 2015;6(2):199‐208. [DOI: 10.3920/BM2014.0075] [DOI] [PubMed] [Google Scholar]
Woods 2012
- Woods CW, Oliver T, Lewis K, Yang Q. Development of necrotizing enterocolitis in premature infants receiving thickened feeds using SimplyThick. Journal of Perinatology 2012;32(2):150‐2. [DOI: 10.1038/jp.2011.105; PUBMED: 22289705] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Huang 2002
- Huang R‐C, Forbes DA, Davies MW. Feed thickener for newborn infants with gastro‐oesophageal reflux. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003211] [DOI] [PubMed] [Google Scholar]


