Abstract
Background
Bronchopulmonary dysplasia (BPD) remains a major complication of prematurity and currently lacks efficient treatments. Mesenchymal stem/stromal cells (MSCs) have been extensively explored as a potential therapy in several preclinical and clinical settings. Human and animal MSCs have been shown to prevent and treat lung injury in various preclinical models of lung diseases, including experimental BPD.
Objectives
To determine if MSCs, administered intravenously or endotracheally, are safe and effective in preventing or treating BPD, or both, in preterm infants.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 10), MEDLINE via PubMed (1966 to 6 November 2016), Embase (1980 to 6 November 2016), and CINAHL (1982 to 6 November 2016). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomized controlled trials (RCTs) and quasi‐RCTs.
Selection criteria
We considered RCTs and quasi‐RCTs investigating prevention or treatment of BPD, or both, in preterm infants.
Data collection and analysis
Two review authors independently assessed trial quality according to prespecified criteria.
Main results
We found no RCTs or quasi‐RCTs addressing the use of MSCs for prevention or treatment of BPD in premature infants. Two RCTs are currently registered and ongoing.
Authors' conclusions
There is insufficient evidence to determine the safety and efficacy of MSCs in the treatment or prevention of BPD in premature infants. The results of the ongoing trials addressing this issue are expected in the near future.
Plain language summary
Stem cells for the prevention and treatment of bronchopulmonary dysplasia in preterm infants
Background
Bronchopulmonary dysplasia (BPD) is a chronic lung disease that often complicates the course of babies who are born too early. Bronchopulmonary dysplasia can lead to serious health issues during childhood and later in life. There is currently no effective and safe treatment for BPD. Mesenchymal stem cells (MSCs), cells that can multiply and turn into a different type of cell, can protect the damage to newborn lungs in experimental models of BPD. Mesenchymal stem cells may bring new hope for untreatable health issues in babies born too early, including BPD, and thus improve their survival and quality of life.
Review question
Are MSCs, administered intravenously or endotracheally, safe and effective in preventing or treating BPD, or both, in preterm infants?
Study characteristics
We found no clinical trials that addressed the use of MSCs for prevention or treatment of BPD in premature infants. However, some studies are currently underway.
Key results
There is insufficient evidence to determine the safety and efficacy of MSCs for the treatment or prevention of BPD in premature infants. The results of the ongoing trials are expected in the near future.
Background
Description of the condition
Bronchopulmonary dysplasia (BPD) is considered one of the major complications of preterm birth (Farstad 2011; Jobe 2001). Bronchopulmonary dysplasia develops as a consequence of impaired lung development, exacerbated by the imbalance between pro‐inflammatory stimuli and anti‐inflammatory defense mechanisms typical of the preterm infant (Jobe 2001; Speer 2006).
The definition of BPD has been evolving since its first description as 28 days of oxygen exposure with characteristic radiographic changes (NIH 1979). Subsequently, oxygen dependency at 36 weeks' postmenstrual age was shown to better predict long‐term respiratory outcomes (Shennan 1988). The current definition of BPD stratifies infants below 32 weeks requiring supplemental oxygen for at least 28 days into three severity groups (mild, moderate, and severe), depending on the presence and the amount of supplemental oxygen and the mode of respiratory support at 36 weeks' postmenstrual age (Ehrenkranz 2005; Jobe 2001). The ‘physiologic’ definition of BPD was proposed in an attempt to address the significant intercenter variability in oxygen administration (Lapcharoensap 2015). At 36 weeks' postmenstrual age, infants receiving less than 30% supplemental oxygen are challenged by reducing the fraction of administered oxygen during a standardized test. Infants who are unable to maintain saturations above 90% during the test are diagnosed with BPD (Walsh 2003). The incidence of BPD varies depending on the definition, complicating the course of up to 40% of the infants born between 22 and 28 weeks' gestation (Stoll 2010). It must be noted that, although by definition BPD cannot be diagnosed before 28 days of life, a respiratory disease defined as oxygen and/or ventilator‐dependency from 7 to 28 days of life represents the initial phase of the chronic process leading to BPD, thus classified as "evolving BPD" (Walsh 2006). Infants suffering from BPD are at increased risk of death and long‐term pulmonary and neurodevelopmental morbidities (Anderson 2006; Bhandari 2006). Several treatments have been used in an attempt to prevent or treat BPD. Unfortunately, even the most promising strategies have not been able to confirm the initial enthusiasm in robust randomised controlled trials (RCTs). A recent meta‐analysis combining all the available pharmacological options to prevent BPD found that only five out of the 21 drugs tested (vitamin A, caffeine citrate, dexamethasone, inositol, and clarithromycin) in RCTs may reduce the incidence of BPD. Among these, meta‐analysis could confirm the data only for vitamin A and dexamethasone, due to the lack of multiple trials for the other drugs (Beam 2014). Moreover, vitamin A showed only a very modest effect (Darlow 2011), while the use of dexamethasone is limited in preterm infants by its well‐known long‐ and short‐term side effects (Watterberg 2010). Despite the continuous advance of neonatal care, BPD remains a significant burden for the preterm population, lacking a safe and effective treatment.
Description of the intervention
Stem cells are primitive cells capable of extensive self renewal with the potential to give rise to multiple differentiated cellular phenotypes (Blau 2001). Among stem cells, mesenchymal stem cells (MSCs) have been largely explored as a potential regenerative therapy in several preclinical and clinical settings. Mesenchymal stem cells are defined mainly by three criteria:
adherence to plastic in standard culture conditions;
expression and lack of specific surface markers;
multipotent differentiation potential along the osteogenic, chondrogenic, and adipogenic lineages (Dominici 2006; Krampera 2013).
Although bone marrow is the major source of isolation, MSCs can be obtained from any tissue. Although MSCs have been isolated from any tissue (da Silva Meirelles 2006), most of the sites are not clinically relevant due to the difficulty in harvesting. Bone marrow and adipose tissue are the most utilized adult sources of MSCs (Dmitrieva 2012). Lately, MSCs obtained from perinatal tissues and umbilical cord blood have attracted particular interest thanks to their unique advantages in terms of availability, reduced immunogenicity, and pronounced differentiating potential (Batsali 2013; Sullivan 2008).
Beyond the cell source, several factors involving the manufacturing of the final stem cell product can affect the efficacy of MSC therapy. Among these, the aging of the cells with increasing passages may have significant consequences on their function (Bellayr 2014; Wagner 2008). A passage is the process of removing cells from a culture flask and plating them into more culture flasks. Passaging and expansion is necessary to obtain a sufficient number of cells for transplantation. However, early‐passage cells may have superior therapeutic potential and thus improve the chances of success of the stem cell therapy in clinical trials.
The type of transplant (autologous or allogeneic) may yield different results (Alagesan 2014). Autologous transplantation of MSCs (from the same patient) is particularly appealing in terms of lower risks for infections and immune rejection. However, MSCs are characterized by low immunogenicity, although immune rejection is still theoretically possible. On the other hand, allogeneic transplantation (from a donor) offers significant practical advantages.
Human and animal bone marrow‐derived and cord‐derived MSCs have been shown to prevent and treat lung injury in various preclinical models of lung diseases, including experimental BPD (Baker 2014). Their broad‐spectrum clinical potential in the respiratory field is currently under investigation in numerous clinical trials (Antunes 2014). Possible side effects of MSC administration are immune rejection and tumor formation, although these are rather hypothetical possibilities. Mesenchymal stem cell safety has been documented in several clinical trials and confirmed in a recent meta‐analysis in adult patients (Lalu 2012). In preclinical models of BPD, MSC administration in the first days of life was not associated with long‐term adverse lung effects or tumor formation at the total‐body computed tomography scan (Pierro 2013). A recent phase I study showed that the intratracheal transplantation of human cord‐derived MSCs in preterm infants at high risk for BPD seems to be safe and feasible (Chang 2014).
How the intervention might work
Mesenchymal stem cells represent a perfect candidate for allogeneic transplantation, thanks to their paramount immunomodulatory properties, which allow them to reduce the risk for immune rejection (Gebler 2012). The mechanism of action of MSCs is still under investigation. However, MSCs seem to exert their therapeutic effects thanks to the paracrine secretion of anti‐inflammatory, antioxidant, anti‐apoptotic, trophic, and pro‐angiogenic factors (Murphy 2013).
Mesenchymal stem cells and their products seem to ameliorate many critical aspects of BPD pathogenesis in preclinical models, by mitigating lung inflammation, inducing vascular and alveolar growth, and inhibiting lung fibrosis (Aslam 2009; Chang 2011; van Haaften 2009). These effects are confirmed by the significant improvement of lung function tests and long‐term exercise tolerance in MSC‐treated animals (Pierro 2013).
Why it is important to do this review
Bronchopulmonary dysplasia represents a significant burden for the preterm population and lacks an effective treatment. Stem cells, particularly MSCs, may have the potential to regenerate lung tissue and substantially improve the outcome of this disease. To our knowledge this is the first review addressing MSC treatment in patients affected or at risk for BPD.
Objectives
To determine if MSCs, administered intravenously or endotracheally, are safe and effective in preventing or treating BPD, or both, in preterm infants.
We performed the following specific comparisons.
Prevention: to determine if MSCs administered intravenously or endotracheally within the first week of life can prevent BPD in extremely preterm infants less than 26 weeks' gestation.
Treatment of evolving BPD: to determine if MSCs administered intravenously or endotracheally at more than one week of age but less than 36 weeks' postmenstrual age can prevent BPD in preterm infants on supplemental oxygen or respiratory support.
Treatment of established BPD: to determine if MSCs administered intravenously or endotracheally at ≥ 36 weeks' postmenstrual age can reduce mortality in preterm infants on respiratory support and/or supplemental oxygen > 30%.
We would also conduct subgroup analyses including gestational age, source of stem cells, type of graft, route of administration, MSC dose, number of doses, passages, disease severity at time of study entry, and exposure to postnatal steroids (Subgroup analysis and investigation of heterogeneity).
Methods
Criteria for considering studies for this review
Types of studies
We considered RCTs and quasi‐RCTs.
Types of participants
Prevention studies
High‐risk preterm infants: extremely preterm infants (< 26 weeks' gestation) ≤ one week of age.
Treatment studies
Preterm infants with evolving BPD: preterm infants > one week of age but < 36 weeks' postmenstrual age on supplemental oxygen and invasive (conventional mechanical ventilation or high frequency oscillatory ventilation) or non‐invasive (nasal continuous positive airway pressure, bilevel positive airway pressure, non‐invasive positive pressure ventilation, high flow nasal cannula) respiratory support.
Preterm infants with established BPD: preterm infants ≥ 36 weeks' postmenstrual age on invasive or non‐invasive respiratory support and/or supplemental oxygen > 30%.
Types of interventions
Mesenchymal stem cells compared to control (placebo, steroids, or no treatment), including all types of transplantation regardless of cell source (bone marrow, cord blood versus Wharton's jelly, placenta, adipose tissue, peripheral blood), type of graft (autologous or allogeneic), route of cell administration (endotracheal or intravenous), and dose.
Types of outcome measures
Primary outcomes
-
All comparisons (prevention and treatment trials)
Mortality prior to hospital discharge (from any cause)
-
Prevention trials
Neonatal mortality (mortality < 28 days of age) from any cause
-
Chronic lung disease/BPD
Oxygen requirement at 28 to 30 days of age
Oxygen requirement at 36 weeks' postmenstrual age
-
Death or chronic lung disease
Death or oxygen requirement at 28 to 30 days of age
Death or oxygen requirement at 36 weeks' postmenstrual age
-
Treatment of evolving BPD
Oxygen requirement at 36 weeks' postmenstrual age
Death or oxygen requirement at 36 weeks' postmenstrual age
Secondary outcomes
Pneumothorax
Air leak syndromes (including pulmonary interstitial emphysema, pneumothorax, pneumomediastinum)
Pulmonary hemorrhage
Patent ductus arteriosus (that has been treated with cyclo‐oxygenase inhibitor or surgery)
Culture‐confirmed bacterial sepsis
Culture‐confirmed fungal sepsis
Necrotizing enterocolitis (defined as Bell stage II or greater) (Bell 1978)
Periventricular leukomalacia
Retinopathy of prematurity in infants examined (all stages and severe (stage 3 or greater)) (ICCROP 2005)
Intraventricular hemorrhage (any grade and severe (grade 3 to 4)) (Papile 1978)
Pulmonary hypertension (defined by Doppler ultrasound)
Days on assisted ventilation
Days on supplemental oxygen
Length of hospital stay (days)
Cerebral palsy at 18 to 24 months' corrected age
-
Neurodevelopmental outcome at approximately two years' corrected age (acceptable range 18 months to 28 months) including: cerebral palsy, delayed neurodevelopment (Bayley Scales of Infant Development Mental Developmental Index < 70), legal blindness (< 20/200 visual acuity), and hearing deficit (aided or < 60 dB on audiometric testing)
We defined the composite outcome 'neurodevelopmental impairment' as having any one of the aforementioned deficits.
Rehospitalization in the first two years of life
Safety outcomes defined as tumor formation, immune‐rejection, or any serious adverse event (certain, probable, or possible according to the World Health Organization probability scale)
We considered post hoc analyses for any unexpected adverse effects reported by the studies.
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register).
Electronic searches
We conducted a comprehensive search including the Cochrane Central Register of Controlled Trials (CENTRAL) (2016, Issue 10) in the Cochrane Library, MEDLINE via PubMed (1966 to 6 November 2016), Embase (1980 to 6 November 2016), and CINAHL (Cumulative Index to Nursing and Allied Health Literature) (1982 to 6 November 2016) using the following search terms: (stem cell OR Mesenchymal) AND (BPD OR bronchopulmonary dysplasia OR lung disease OR CLD OR chronic lung disease OR respiratory distress syndrome), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We did not apply language restrictions.
We searched the bibliography cited in each publication obtained in order to identify additional relevant articles.
Searching other resources
We handsearched the abstracts of the Society for Pediatric Research for the years 1985 to 1999 using the keyword 'stem cells'. We electronically searched the abstracts of the Pediatric Academic Societies from 2000 to 2014 through the Pediatric Academic Societies' 2000 to 2014 Archive Abstracts2View site (www.abstracts2view.com/pasall/).
We checked references and cross‐references from identified studies. We handsearched abstracts from the proceedings of the Pediatric Academic Societies Meetings (from January 1990 to present). We did not impose any language restrictions.
We searched clinical trials registries (6 November 2016) for ongoing or recently completed trials: US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov), the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/en/), and the ISRCTN registry (www.isrctn.com/).
Data collection and analysis
Selection of studies
Two review authors (MP, BT) independently searched the literature as described above. We considered only RCTs and quasi‐RCTs fulfilling the above criteria for inclusion in the review. We excluded studies published only in abstract form unless the final results of the trial were reported and all necessary information could be ascertained from the abstract or authors, or both. Two review authors (MP, BT) selected studies separately. Any disagreements were resolved by discussion involving all review authors.
Data extraction and management
Two review authors (MP, BT) independently extracted, assessed, and coded data using a form designed specifically for this review.
We collected information regarding the method of randomization, blinding, drug intervention, stratification, and whether the trial was single‐center or multicenter for each included study. We noted information regarding trial participants including gestational age criteria, birth weight criteria, and other inclusion or exclusion criteria. We extracted information on primary and secondary outcomes (Primary outcomes; Secondary outcomes). When any questions arose or additional data were required, we contacted the authors.
For each study, one review author (MP) entered data into Cochrane's statistical software Review Manager 5 (RevMan 2014), and a second review author (BT) checked the entered data. We planned to replace any standard error of the mean by the corresponding standard deviation. We resolved any disagreements by discussion.
Assessment of risk of bias in included studies
We planned to assess risk of bias according to selection bias (quality of randomization, allocation concealment/blinding of randomization), performance bias (blinding of intervention), attrition bias (completeness of follow‐up), and detection bias (blinding of outcome measurement), selective reporting bias, or other bias using Cochrane's tool for assessing risk of bias (Higgins 2011). We planned to assess each domain as 'low risk,' 'high risk,' or 'unclear risk.' Any disagreements would be resolved by discussion involving all review authors.
We planned to evaluate the following issues and enter the information into a 'Risk of bias' table (Higgins 2011).
-
Sequence generation (checking for possible selection bias).
Was the allocation sequence adequately generated?
-
For each included study, we planned to categorize the method used to generate the allocation sequence as:
adequate (any truly random process, e.g. random number table, computer random number generator);
inadequate (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number);
unclear.
-
Allocation concealment (checking for possible selection bias).
Was allocation adequately concealed?
-
For each included study, we planned to categorize the method used to conceal the allocation sequence as:
adequate (e.g. telephone or central randomization, consecutively numbered, sealed, opaque envelopes);
inadequate (open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth);
unclear.
-
Blinding (checking for possible performance bias).
Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment?
For each included study, we planned to categorize the methods used to blind study participants and personnel from knowledge of which intervention a participant received.
We would assess blinding separately for different outcomes or classes of outcomes.
In some situations there might have been partial blinding, for example where outcomes were self reported by unblinded participants, but they were recorded by blinded personnel without knowledge of group assignment. Where needed, we would add 'partial' to the list of options for assessing quality of blinding.
-
We planned to categorize the methods as:
adequate, inadequate, or unclear for participants;
adequate, inadequate, or unclear for personnel;
adequate, inadequate, or unclear for outcome assessors.
-
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations).
Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we would describe the completeness of data including attrition and exclusions from the analysis.
We planned to note whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes.
Where sufficient information was reported or supplied by the trial authors, we would re‐include missing data in the analyses.
-
We planned to categorize the methods as:
adequate (< 20% missing data);
inadequate (≥ 20% missing data);
unclear.
-
Selective reporting bias.
Were reports of the study free of the suggestion of selective outcome reporting?
-
For each included study, we planned to describe how we investigated the possibility of selective outcome reporting bias and what we found. We planned to assess the methods as:
adequate (where it was clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review had been reported);
inadequate (where not all of the study's prespecified outcomes had been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; the study failed to include the results of a key outcome that would have been expected to be reported);
unclear.
-
Other sources of bias.
Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we planned to describe any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early owing to some data‐dependent process).
-
We planned to assess whether each study was free of other problems that could put it at risk of bias as:
yes;
no;
unclear.
We planned to explore the impact of the level of bias through undertaking sensitivity analyses if needed.
Measures of treatment effect
We planned to use risk ratios (RRs), risk differences (RDs), numbers needed to treat for an additional beneficial outcome (NNTB) or numbers needed to treat for an additional harmful outcome (NNTH) for categorical variables, and weighted mean differences (WMDs) for continuous variables. We planned to replace any within‐group standard error of the mean (SEM) reported in a trial by its corresponding standard deviation (SD) using the formula SD = SEM x √N. We planned to report 95% confidence intervals (CIs) for each statistic.
We planned to perform the statistical analyses using Review Manager 5 (RevMan 2014). We planned to analyze categorical data using RRs and RDs. For statistically significant outcomes, we would calculate NNTB or NNTH. We planned to analyze continuous data using WMDs and standardized mean differences (SMDs). We planned to report the 95% CIs on all estimates.
Unit of analysis issues
We planned to include all RCTs and quasi‐RCTs in which the unit of allocation was the individual infant.
Dealing with missing data
When any questions arose or additional data were required, we contacted the authors.
Assessment of heterogeneity
We planned to assess the magnitude of heterogeneity of treatment effects using the I2 statistic. We planned to consider an I2 value of greater than 60% as indicative of high heterogeneity. We also planned to inspect each forest plot carefully for heterogeneity, as indicated by lack of overlapping CIs of individual trials.
We planned to estimate the treatment effects of individual trials and examined heterogeneity among trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I2 statistic. We would grade the degree of heterogeneity as follows:
less than 25%: no heterogeneity;
25% to 49%: low heterogeneity;
50% to 75%: moderate heterogeneity;
greater than 75%: substantial heterogeneity.
In case of statistical heterogeneity (I2 > 50%), we would explore the possible causes (e.g. differences in study quality, participants, intervention regimens, or outcome assessments).
Assessment of reporting biases
We planned to examine the possibility of within‐study selective outcome reporting for each study included in the review. We searched for trial protocols of included trials on electronic sources such as PubMed, ClinicalTrials.gov, and the WHO ICTRP in order to assess whether outcome reporting seemed to be sufficiently complete and transparent. We planned to investigate publication bias using funnel plots if we included at least 10 clinical trials in the review (Egger 1997; Higgins 2011).
Data synthesis
We planned to use a fixed‐effect model to pool data for meta‐analyses.
If we identified multiple studies that we thought to be sufficiently similar, we planned to perform meta‐analysis using Review Manager 5 (RevMan 2014). For categorical outcomes, we would calculate the typical estimates of RRs and RDs with their corresponding 95% CIs; for continuous outcomes we planned to calculate MDs or a summary estimate for SMDs with their respective 95% CIs. When we judged meta‐analysis to be inappropriate, we planned to analyze and interpret individual trials separately.
Quality of evidence
We planned to use the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes:
neonatal mortality (mortality < 28 days of age) from any cause;
chronic lung disease/BPD defined as oxygen requirement at 28 to 30 days of age;
chronic lung disease/BPD defined as oxygen requirement at 36 weeks' postmenstrual age;
death or oxygen requirement at 36 weeks' postmenstrual age;
cerebral palsy at 18 to 24 months' corrected age;
neurodevelopmental outcome at approximately two years' corrected age (acceptable range 18 months to 28 months) including cerebral palsy, delayed neurodevelopment (Bayley Scales of Infant Development Mental Developmental Index < 70), legal blindness (< 20/200 visual acuity), and hearing deficit (aided or < 60 dB on audiometric testing).
Two review authors would independently assess the quality of the evidence for each of the outcomes above. We planned to consider evidence from RCTs as high quality, but downgrade the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We planned to use the GRADEpro Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence (GRADEpro GDT).
The GRADE approach results in an assessment of the quality of a body of evidence in one of the following four grades.
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it was substantially different.
Low: Our confidence in the effect estimate is limited: the true effect could be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: the true effect was likely to be substantially different from the estimate of the effect.
Subgroup analysis and investigation of heterogeneity
Gestational age: < 26 weeks; 26 to 30 weeks; > 30 weeks
Source of stem cells: bone marrow; cord blood; Wharton's jelly; placenta; adipose tissue; peripheral blood
Type of graft: autologous; allogeneic
Route of administration: intravenous; endotracheal
MSC dose: 1 to 9 x 106/kg; 1 to 9 x 107/kg
Number of doses: multiple or single administration
Passage: < 3 versus 3 to 6 versus > 6
-
Disease severity at time of study entry
Prevention: invasive respiratory support with fraction of inspired oxygen (FiO2) > 0.3 versus invasive respiratory with FiO2 < 0.3 versus non‐invasive respiratory support
Treatment of evolving BPD: invasive respiratory support with FiO2 > 0.3 versus invasive respiratory with FiO2 < 0.3 versus non‐invasive respiratory support
Treatment of established BPD: invasive respiratory support with FiO2 > 0.3 versus invasive respiratory with FiO2 < 0.3 versus non‐invasive respiratory support versus FiO2 > 0.3 on spontaneous breathing
Infants who had previously received postnatal steroids for prevention or treatment of chronic lung disease (dexamethasone, hydrocortisone)
Sensitivity analysis
Differences in study design of included trials might affect the results of the systematic review. We planned to perform a sensitivity analysis to compare the effects of stem cells in truly randomized trials as opposed to quasi‐randomized trials.
Results
Description of studies
Results of the search
From an initial search of 1349 citations, we extracted 2 studies for further examination. We excluded both studies from the review (see Characteristics of excluded studies table and PRISMA study flow diagram Figure 1).
1.
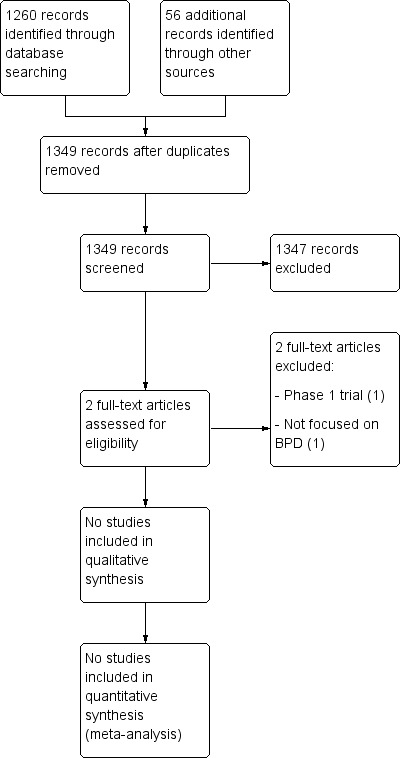
Study flow diagram.
Our search of ClinicalTrials.gov identified 10 registered studies, of which 2 protocols were randomized trials addressing the use of MSCs for prevention or treatment of BPD in premature infants (see Characteristics of ongoing studies table). Three non‐randomized phase 1 trials are also currently registered on our targeted patient group.
Included studies
No studies were identified.
Excluded studies
Two studies were identified but excluded.
Chang 2014 (ClinicalTrials.gov identifier NCT01297205): this is the first published trial on the use of MSCs for BPD in preterm infants with evolving BPD (5 to 14 days requiring continuous ventilatory support). It is dose‐escalation trial, assessing safety and feasibility of a single, intratracheal administration of umbilical cord blood‐derived MSCs, starting from a low dose of 1 x 107 cells/kg for the first three patients, up to the higher dose of 2 x 107 cells/kg for the next six patients. Infants were enrolled between February and September 2011. The authors reported no serious adverse effects or dose‐limiting toxicity. Levels of pro‐inflammatory cytokines (interleukin‐6, interleukin‐8, matrix metalloproteinase‐9, tumor necrosis factor‐a, and transforming growth factor‐b 1) in tracheal aspirates were reduced after MSC transplantation. The enrolled infants were also compared with historical controls (between January 2009 and November 2011). Bronchopulmonary dysplasia severity appeared to be lower in the transplant recipients, and rates of other adverse outcomes did not differ between the comparison group and transplant recipients. We excluded this study because it was a phase 1 trial and, as such, did not meet the inclusion criteria.
Rudnicki 2015 (ClinicalTrials.gov identifier NCT02050971): this is a prospective single‐center study in which preterm infants born below 32 weeks of gestation were randomized to either autologous cord blood transfusion or allogeneic red blood cell transfusion, in order to treat anemia of prematurity. We excluded this study because it was not focused on MSCs and BPD.
Ongoing studies
Our search of PubMed, ClinicalTrials.gov, and the WHO ICTRP revealed two protocols of ongoing randomized trials on our targeted patient group (see Characteristics of ongoing studies table).
NCT01207869: the study 'Intratracheal umbilical cord‐derived mesenchymal stem cells for severe bronchopulmonary dysplasia' is a phase 2, double‐blind, randomized trial on established BPD. Infants up to six months of age with severe BPD are randomized to an intratracheal administration of either MSCs or normal saline. The status of this Taiwanese study is unknown, having not been verified in the past seven years. We were unable to retrieve information on this study.
NCT01828957: the study 'Efficacy and safety evaluation of Pneumostem versus a control group for treatment of BPD in premature infants' is a double‐blind, randomized trial on evolving BPD. Preterm infants less than 29 weeks of gestation are randomized to an intratracheal single dose of allogeneic cord blood‐derived MSCs or normal saline, if they are still ventilated (FiO2 > 0.25) from 5 to 14 days of life. The Korean study includes a registered follow‐up to 60 months (NCT01897987). We contacted the researchers, who are currently analyzing the data and have also completed the follow‐up enrollment. This study constitutes the phase 2 of the previously published phase 1 trial (Chang 2014).
Two non‐randomized trials are also currently registered on our targeted patient group.
NCT02381366: this is a US phase 1 safety study on evolving BPD. In this dose‐escalation trial, the researchers are testing two different intratracheal doses (10 and 20 millions of cells) of human allogeneic umbilical cord blood‐derived MSCs in preterm infants born between 23 and 28 weeks' gestation, on mechanical ventilation (FiO2 > 0.25) between 5 and 14 days of life. The cells are frozen and administered within 24 hours from thawing. Enrollment of the study has been completed and the researchers are currently analyzing the data.
NCT02443961: this is a Spanish phase 1 safety trial on infants with evolving BPD. Three doses of 5 million umbilical cord‐derived MSCs are administered to infants born below 28 weeks' gestation, if they are still on mechanical ventilation (FiO2 > 0.4) at 14 ± 2 days of life. Cells are thawed one week before administration, expanded to passage 6 to 10, and administered intravenously in three weekly doses.
Studies in other neonatal populations
NCT01284673: 'Characterization of the cord blood stem cell in situation of neonatal asphyxia'
NCT02274428: 'Phase 1 clinical trial of PNEUMOSTEM treatment in premature infants with intraventricular hemorrhage'
Risk of bias in included studies
We found no studies meeting the inclusion criteria for this review.
Effects of interventions
We found no studies meeting the inclusion criteria for this review.
Discussion
We found no published RCTs or quasi‐RCTs addressing the effect of MSC administration for prevention or treatment of BPD in premature infants. However, cell‐based therapy is a burgeoning field, and the coming years will likely mark a turning point, since promising animal data have already prompted the clinical translation of MSCs for numerous diseases, including BPD.
The first trial on the use of MSCs in preterm infants was published in 2014 (Chang 2014). This phase 1 dose‐escalation trial showed the safety and feasibility of a single, intratracheal administration of allogeneic umbilical cord blood‐derived MSCs in premature infants with evolving BPD (5 to 14 days of life with deteriorating respiratory conditions). The authors reported no serious adverse effects or dose‐limiting toxicity. We excluded this study from our review because it is a phase 1 trial and, as such, did not meet our inclusion criteria. The follow‐up of this trial was recently published, and no significant adverse respiratory, growth, or neurodevelopmental effects were detected in the MSC‐treated infants up to 24 months (Ahn 2017). This same group also has completed the enrollment for the first RCT on the same patient population (NCT01828957). The primary outcomes in this phase 2 trial are incidence of BPD (moderate to severe) and mortality at 36 weeks' postmenstrual age. A US phase 1 safety and feasibility study testing the effects of a single intratracheal dose of allogeneic cord blood‐derived MSCs in premature infants with evolving BPD has completed participant enrollment, and the results will be available in the next few months (NCT02381366). A Spanish phase 1 trial is expected to start enrollment in 2017. Infants with evolving BPD (≥ 14 days of life) will be treated intravenously with cord‐derived MSCs (NCT02443961).
Phase 1 and 2 trials are currently focusing on evolving BPD, which seems at this point to be the most appropriate target population. However, evolving BPD is not a well‐defined entity, and it carries a wide range of morbidities and mortality. In the group of infants enrolled in the ongoing studies, previous epidemiological data have reported a mortality rate ranging from 3% to 75% and a risk of moderate to severe BPD from 39% to 100%, depending on the severity of the disease and patient characteristics including sex, race, and birth weight (Lughon 2011). Stratification at randomization, based on outcome predictors or severity of the disease at study entry, would prevent inconsistency in the comparison groups, improving the design of future phase 2 studies.
It must be noted that factors including trial design (i.e. timing and route of administration) and manufacturing procedures greatly affect MSC activity, requiring careful interpretation in data analysis or combining results in meta‐analysis. Animal studies have shown that early (as opposed to rescue) (Pierro 2013), intratracheal (as opposed to intravenous) (Chang 2009), MSC administration seems to be most efficient in the treatment of experimental BPD. As a result of the knowledge gained from these preclinical data, all the studies (phase 1 and 2) are currently assessing the administration of allogeneic cord‐derived or cord blood‐derived MSCs to preterm infants with evolving BPD (early administration). In the Spanish study, umbilical cord‐derived cells are thawed one week before administration, expanded to passage 6 to 10, and administered intravenously in three weekly doses. The Korean and US studies are testing intratracheally administered umbilical cord blood‐derived MSCs. The products are frozen and administered within 24 hours from thawing, and the cells are passage 3 to 6. These two studies are using the same pharmaceutical product. The similarities in the design of the ongoing studies should theoretically facilitate the combination of study results into a meta‐analysis, which would be unreliable if the study conditions were too different. In addition to the trial design, numerous and more complex variables specifically related to the manufacturing of the product need to be taken into account. The manufacturing process of MSCs includes: cell source selection, isolation, expansion/culture conditions, cryostorage, thawing procedure, and lot release. With regard to cell source, perinatal tissue (fetal, placental, umbilical cord, and amniotic) may provide MSCs with greater potency and self renewal than older adult sources (bone marrow, adipose tissue) (Batsali 2013). Among perinatal tissues, cord‐derived cells can rely on the most robust protocol of harvesting. However, even when using the same source and complying with good tissue practice (cGTP) and good manufacturing practice (cGMP) requirements (FDA 2011; Sensebe 2011), the protocols currently in use for MSC production are quite dissimilar (Ikebe 2014). Even small variation in the processing methods, such as starting material, plating density, devices used for MSC culture and separation, culture media, media supplements or growth factors, oxygen concentration during culture, passage number, and substances added for cryopreservation, may change the final characteristics and the functionality of the MSC‐based product (Panchalingam 2015; Waszak 2012). The use of potency assays may improve the characterization of MSC behavior and the prediction of their clinical efficacy, but these are currently not available. Since one single assay would only marginally ascertain the product peculiarities, the development of multiple complementary assays (assay matrix), may be better suited to describe different biological, immunological, and analytical MSC features (Galipeau 2015). Ideally, the release of an MSC lot should always include disclosure of its 'strength' in terms of tissue repair and immunomodulation based on potency assays. The definition of standardized protocols for MSC production and the expansion of potency optimal requirements are to be considered a prerequisite for scale‐up of MSC therapies. Unfortunately, despite the availability of several manufacturing systems, a mutual reference platform does not yet exist. In order to allow for eventual subgroup analysis, all the manufacturing protocols should be detailed in the methods section. The editors and reviewers should possibly ask for comprehensive checklists, available for readers, describing in minute detail each step of MSC manufacturing.
The purity of the MSC lot is another crucial issue. After sample collection, other cells, in particular fibroblasts, which share some phenotypic features with MSCs, should be removed. One way to obtain a pure MSC product is based on cell selection, conformed to the expression or lack of surface markers (Dominici 2006). However, the definition of MSCs and their characterization is still evolving (Galipeau 2015; Mendicino 2014), with new markers continuing to be identified (Shen 2015). More advanced and specific characterization protocols are needed to improve MSC quality and therapeutic potential. It is possible that MSC lots considered to be pure according to current knowledge will be proven to express different degrees of purity, if newer or more numerous markers are applied. Moreover, although only circumstantial so far, the evidence that donor‐related MSC characteristics are likely to have a role on the final product is emerging. As an example, female cord‐derived MSCs show better lung protection compared to male cord‐derived MSCs in a rodent model of neonatal hyperoxia‐induced lung injury (Sammour 2016). Similarly, it is possible that other factors comprising, but not limited to, gestational age at harvesting, placental diseases, chorioamnionitis, and maternal or fetal factors, may alter the efficacy of the cells. It would be ideal to store a sample of each MSC lot and record all possible clinical details on the donor, allowing for subsequent analysis as new knowledge arises.
Safety of MSC is a pivotal issue. Few meta‐analyses, either combining the data obtained in any clinical setting ((Lalu 2012) or including studies specific to a certain group of diseases, such as adult respiratory health issues (Zhao 2017), showed no serious adverse effects after MSC administration. However, the level of evidence is low, and the safety of stem cells needs confirmation by more robust and compelling data. Moreover, some cases of adverse events linked to MSC administration have been reported (Ning 2008; Song 2015). The US Food and Drug Administration strongly recommends that scientists and pharmaceutical companies be committed to the principles of adequate evidence generation when dealing with regenerative medicine, in order to ensure that the eventual adoption of this therapy has a favorable and well‐defined risk–benefit balance (Marks 2017). Mesenchymal stem cells are thought to sense the pathological signals sent by damaged tissues and act consequently to repair the defect. However, it cannot be excluded that some signals could be misinterpreted by stem cells, leading to unexpected outcomes. As consequence, RCTs and meta‐analysis are more likely to provide functional information when addressing the issue of safety in one specific disease.
In conclusion, resources should be organized in order to comprise proper long‐term follow‐up, possibly up to school age, for safety and efficacy endpoints.
Authors' conclusions
Implications for practice.
There is insufficient evidence to determine the safety and efficacy of mesenchymal stem cells (MSCs) for the treatment or prevention of BPD in premature infants.
Implications for research.
Continuous research and communication between basic science and the pharmaceutical industry are paramount for the future of regenerative medicine. Three distinct research targets are likely to simultaneously and independently change the face of stem cell therapy in the near future: (i) newer insight into characterization of MSCs and standardization of potency assays; (ii) well‐designed preclinical studies to provide strong rationale for clinical trials; and (iii) rigorous clinical trials to put the current preclinical evidence to the test and yield the required knowledge. These three separate but interrelated levels of research should continuously influence and redirect each other. While more knowledge is developed, ongoing and future trials will need to provide for possible retrospective re‐evaluation (through registries of patients included in MSC trials and storage of MSC lots) of the results based on future unpredictable discoveries.
Long‐term follow‐up is paramount, and all treated infants should be enrolled in a registry.
Appendices
Appendix 1. Standard search methodology
PubMed: ((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh])) Cinahl: (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) Embase: (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial) Cochrane Library: No additional limiters
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chang 2014 | Phase 1 dose‐escalation trial to assess the safety and feasibility of a single, intratracheal transplantation of human umbilical cord blood‐derived mesenchymal stem cells in 9 preterm infants at high risk for bronchopulmonary dysplasia. ClinicalTrials.gov identifier NCT01297205 |
| Rudnicki 2015 | Not focused on mesenchymal stem cells and bronchopulmonary dysplasia. Single‐center, prospective study of the feasibility, safety, and tolerability of autologous whole cord blood transplant in preterm infants born at less than 32 weeks of gestational age who developed anemia of prematurity. ClinicalTrials.gov identifier NCT02050971 |
Characteristics of ongoing studies [ordered by study ID]
NCT01207869.
| Trial name or title | Intratracheal umbilical cord‐derived mesenchymal stem cells for severe bronchopulmonary dysplasia |
| Methods | Randomized double‐blind controlled trial Recruitment: active, not recruiting |
| Participants | Infants affected by severe BPD up to 6 months of life |
| Interventions | Interventions: umbilical cord‐derived MSCs (3 × 106 cells per kg of the infant's weight), instilled through a 6 French end‐hole catheter inserted in the endotracheal tube. Placebo comparator: normal saline (same amount of MSCs suspension) instilled through a 6 French end‐hole catheter inserted in the endotracheal tube. |
| Outcomes | Primary outcome measures: the correlation between the cytokine concentrations in the bronchoalveolar fluid and pulmonary arterial pressure. Secondary outcome measures: BPD severity score ranging from 0 to 6 on the serial chest radiographs. |
| Starting date | June 2010 |
| Contact information | Bai‐Horng Su, MD, PhD, Chairman of Department of Pediatrics, China Medical University Hospital |
| Notes | Recruitment: active, not recruiting Study results: no results available Last update: September 2010 clinicaltrials.gov/show/NCT01207869 Study has passed its completion date and status has not been verified in more than 2 years. |
NCT01284673.
| Trial name or title | Characterization of the cord blood stem cell in situation of neonatal asphyxia |
| Methods | Descriptive, bicentre study on 10 cord blood samples from newborn infants with neonatal asphyxia (5) according to predefined criteria, compared to healthy neonates (5). The total duration of the study will be 2 years. Parents will be informed and a signed parental consent will be asked in the hours following birth before the in vitro study. |
| Participants | Conditions: respiratory distress syndrome |
| Interventions | In vitro characterization of the cord blood stem cell |
| Outcomes | Biological analysis will include elementary analyses for cell quality control, endothelial progenitor exploration, and investigation of MSC function and of their neuronal differentiation potential (on fresh and frozen samples). |
| Starting date | Recruitment: completed Study results: no results available |
| Contact information | Sponsor: Assistance Publique Hopitaux De Marseille |
| Notes | clinicaltrials.gov/show/NCT01284673 Study has passed its completion date and status has not been verified in more than 2 years. |
NCT01632475.
| Trial name or title | Follow‐up study of safety and efficacy of Pneumostem in premature infants with bronchopulmonary dysplasia |
| Methods | |
| Participants | Conditions: bronchopulmonary dysplasia |
| Interventions | Interventions: biological: Pneumostem |
| Outcomes | |
| Starting date | Recruitment: active, not recruiting Study results: no results available |
| Contact information | |
| Notes | clinicaltrials.gov/show/NCT01632475 Study has passed its completion date and status has not been verified in more than 2 years. |
NCT01828957.
| Trial name or title | Efficacy and safety evaluation of Pneumostem versus a control group for treatment of BPD in premature infants |
| Methods | Randomized double‐blind controlled trial |
| Participants | Preterm infants < 29 weeks’ gestation with evolving BPD (intubated and ventilated with deteriorating respiratory conditions) from 5 to 14 days of life |
| Interventions | Interventions: a single intratracheal administration of allogeneic cord blood‐derived MSCs. Cells are passage 3 to 6 at a dose of 1 x 107 cells/kg. The product is frozen and thawed overnight before administration. Placebo comparator: normal saline |
| Outcomes | Primary outcome measures: incidence of BPD (moderate to severe) or mortality at 36 weeks' postmenstrual age. Secondary outcome measures: complications of prematurity, days of oxygen/respiratory support, postnatal steroids, length of stay, body growth. |
| Starting date | April 2013 |
| Contact information | Won‐Soon Park, MD, PhD, Department of Pediatrics, Samsung Medical Center Ai‐Rhan Kim, Department of Neonatology, Asan Medical Center |
| Notes | Recruitment: active, not recruiting clinicaltrials.gov/show/NCT01828957 Study has passed its completion date and status has not been verified in more than 2 years. |
NCT01897987.
| Trial name or title | Follow‐up safety and efficacy evaluation on subjects who completed PNEUMOSTEM phase‐II clinical trial |
| Methods | Recruitment: recruiting Study results: no results available |
| Participants | Conditions: bronchopulmonary dysplasia |
| Interventions | Interventions: biological: Pneumostem Placebo comparator: normal saline |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | clinicaltrials.gov/show/NCT01897987 Study has passed its completion date and status has not been verified in more than 2 years. |
NCT02023788.
| Trial name or title | Long‐term safety and efficacy follow‐up study of PNEUMOSTEM in patients who completed PNEUMOSTEM phase‐I study |
| Methods | Recruitment: active, not recruiting Study results: no results available |
| Participants | Conditions: bronchopulmonary dysplasia; respiratory tract infections; premature birth of newborn |
| Interventions | Interventions: biological: PNEUMOSTEM |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | clinicaltrials.gov/show/NCT02023788 Study has passed its completion date and status has not been verified in more than 2 years. |
NCT02274428.
| Trial name or title | Phase 1 clinical trial of PNEUMOSTEM treatment in premature infants with intraventricular hemorrhage |
| Methods | Single group assignment; not a randomized controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Drug: Pneumostem |
| Outcomes | Primary outcome measures: unsuspected death or anaphylactic shock within 6 hours after Pneumostem transplantation. Secondary outcome measures: death or hydrocephalus requiring shunt operation. |
| Starting date | |
| Contact information | |
| Notes | clinicaltrials.gov/ct2/show/NCT02274428 Study has passed its completion date and status has not been verified in more than 2 years. |
NCT02381366.
| Trial name or title | Safety and efficacy of two dose levels of PNEUMOSTEM in premature infants at high risk for bronchopulmonary dysplasia (BPD) ‐ a US study |
| Methods | Recruitment: recruiting Study results: no results available |
| Participants | Conditions: bronchopulmonary dysplasia |
| Interventions | Interventions: biological: human umbilical cord blood‐derived MSCs |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | clinicaltrials.gov/show/NCT02381366 Study has passed its completion date and status has not been verified in more than 2 years. |
NCT02443961.
| Trial name or title | Mesenchymal stem cell therapy for bronchopulmonary dysplasia in preterm babies |
| Methods | Recruitment: not yet recruiting Study results: no results available |
| Participants | Conditions: bronchopulmonary dysplasia |
| Interventions | Interventions: biological: mesenchymal stem cell therapy |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | clinicaltrials.gov/show/NCT02443961 Study has passed its completion date and status has not been verified in more than 2 years. |
NCT02673788.
| Trial name or title | Follow‐up study of safety and efficacy of Pneumostem in premature infants with intraventricular hemorrhage |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | clinicaltrials.gov/show/NCT02673788 Study has passed its completion date and status has not been verified in more than 2 years. |
BPD: bronchopulmonary dysplasia MSC: mesenchymal stem cell
Differences between protocol and review
We excluded the separate search for long‐term neurodevelopmental sequelae, as we believe the initial search is comprehensive.
Contributions of authors
Maria Pierro (MP), Bernard Thébaud (BT), and Roger Soll (RS) all participated in the conception and drafting of the protocol. Two review authors (MP, BT) independently searched the literature and assessed study eligibility as per inclusion criteria. MP, BT, and RS all participated in the writing of manuscript.
Declarations of interest
Maria Pierro is a funded researcher in the field of mesenchymal stem cells.
Bernard Thébaud's work on mesenchymal stem cells is supported by the Canadian Institute for Health Research, the Canadian Stem Cell Network, the Canadian Thoracic Society, the Ottawa Hospital Research Institute and the Children's Hospital of Eastern Ontario Research Institute, and the Ontario Institute of Regenerative Medicine.
Roger Soll is the Co‐ordinating Editor of the Cochrane Neonatal Review Group, but played no part in determining if this review was acceptable for publication.
New
References
References to studies excluded from this review
Chang 2014 {published data only}
- Chang YS, Ahn SY, Yoo HS, Sung SI, Choi SJ, Oh WI, et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose‐escalation clinical trial. Journal of Pediatrics 2014;164(5):966‐72.e6. [DOI: 10.1016/j.jpeds.2013.12.011; PUBMED: 24508444] [DOI] [PubMed] [Google Scholar]
Rudnicki 2015 {published data only}
- Rudnicki J, Kawa MP, Kotowski M, Michalczyk B, Ustianowski P, Czajka R, et al. Clinical evaluation of the safety and feasibility of whole autologous cord blood transplant as a source of stem and progenitor cells for extremely premature neonates: preliminary report. Experimental and Clinical Transplantation 2015;13(6):563‐72. [DOI: 10.6002/ect.2015.0081; PUBMED: 26643677] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01207869 {published data only}
- NCT01207869. Intratracheal umbilical cord‐derived mesenchymal stem cells for severe bronchopulmonary dysplasia [Intratracheal instillation of umbilical cord‐derived mesenchymal stem cells as a rescue treatment for severe bronchopulmonary dysplasia]. clinicaltrials.gov/show/NCT01207869 (first received 11 September 2010).
NCT01284673 {published data only}
- NCT01284673. Characterization of the cord blood stem cell in situation of neonatal asphyxia. clinicaltrials.gov/show/NCT01284673 (first received 16 April 2010).
NCT01632475 {published data only}
- NCT01632475. Follow‐up study of safety and efficacy of Pneumostem in premature infants with bronchopulmonary dysplasia [Long term follow‐up study of the safety and exploratory efficacy of Pneumostem in premature infants with bronchopulmonary dysplasia]. clinicaltrials.gov/show/NCT01632475 (first received 27 June 2012).
NCT01828957 {published data only}
- NCT01828957. Efficacy and safety evaluation of Pneumostem versus a control group for treatment of BPD in premature infants [Randomized, double‐blind, multi‐center, phase II clinical trial to evaluate the efficacy and safety of Pneumostem versus a control group for treatment of bronchopulmonary dysplasia in premature infants]. clinicaltrials.gov/show/NCT01828957 (first received 2 April 2013).
NCT01897987 {published data only}
- NCT01897987. Follow‐up safety and efficacy evaluation on subjects who completed PNEUMOSTEM phase‐II clinical trial [Follow‐up safety and efficacy evaluation on subjects who completed the initial stage of PNEUMOSTEM phase‐II clinical trial]. clinicaltrials.gov/show/NCT01897987 (first received 4 July 2013).
NCT02023788 {published data only}
- NCT02023788. Long‐term safety and efficacy follow‐up study of PNEUMOSTEM in patients who completed PNEUMOSTEM phase‐I study. clinicaltrials.gov/show/NCT02023788 (first received 17 December 2013).
NCT02274428 {published data only}
- NCT02274428. Phase 1 clinical trial of PNEUMOSTEM treatment in premature infants with intraventricular hemorrhage. clinicaltrials.gov/show/NCT02274428 (first received 2 October 2014).
NCT02381366 {published data only}
- NCT02381366. Safety and efficacy of PNEUMOSTEM in premature infants at high risk for bronchopulmonary dysplasia (BPD) ‐ a US study [A phase I/II, open‐label dose escalation trial to evaluate the safety and efficacy of two dose levels of PNEUMOSTEM in premature infants at high risk for bronchopulmonary dysplasia (BPD)]. clinicaltrials.gov/show/NCT02381366 (first received 15 February 2015).
NCT02443961 {published data only}
- NCT02443961. Mesenchymal stem cell therapy for bronchopulmonary dysplasia in preterm babies [Clinical trial: security and feasibility of mesenchymal stem cell therapy in treatment and prevention of bronchopulmonary dysplasia in preterm babies]. clinicaltrials.gov/show/NCT02443961 (first received 4 May 2015). [Google Scholar]
NCT02673788 {published data only}
- NCT02673788. Follow‐up study of safety and efficacy of Pneumostem in premature infants with intraventricular hemorrhage. clinicaltrials.gov/show/NCT02673788 (first received 26 January 2016).
Additional references
Ahn 2017
- Ahn SY, Chang YS, Kim JH, Sung SI, Park WS. Two‐year follow‐up outcomes of premature infants enrolled in the phase I trial of mesenchymal stem cells transplantation for bronchopulmonary dysplasia. Journal of Pediatrics 2017;185:49–54.e2. [DOI: 10.1016/j.jpeds.2017.02.061; NCT01632475; PUBMED: 28341525] [DOI] [PubMed] [Google Scholar]
Alagesan 2014
- Alagesan S, Griffin MD. Autologous and allogeneic mesenchymal stem cells in organ transplantation: what do we know about their safety and efficacy?. Current Opinion in Organ Transplantation 2014;19(1):65‐72. [DOI: 10.1097/MOT.0000000000000043; PUBMED: 24370985] [DOI] [PubMed] [Google Scholar]
Anderson 2006
- Anderson PJ, Doyle LW. Neurodevelopmental outcome of bronchopulmonary dysplasia. Seminars in Perinatology 2006;30(4):227‐32. [DOI: 10.1053/j.semperi.2006.05.010; PUBMED: 16860163] [DOI] [PubMed] [Google Scholar]
Antunes 2014
- Antunes MA, Laffey JG, Pelosi P, Rocco PR. Mesenchymal stem cell trials for pulmonary diseases. Journal of Cellular Biochemistry 2014;115(6):1023‐32. [DOI: 10.1002/jcb.24783; PUBMED: 24515922] [DOI] [PubMed] [Google Scholar]
Aslam 2009
- Aslam M, Baveja R, Liang OD, Fernandez‐Gonzalez A, Lee C, Mitsialis SA, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. American Journal of Respiratory and Critical Care Medicine 2009;180(11):1122‐30. [DOI: 10.1164/rccm.200902-0242OC; PUBMED: 19713447] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2014
- Baker CD, Alvira CM. Disrupted lung development and bronchopulmonary dysplasia: opportunities for lung repair and regeneration. Current Opinion in Pediatrics 2014;26(3):306‐14. [DOI: 10.1097/MOP.0000000000000095; PUBMED: 24739494] [DOI] [PMC free article] [PubMed] [Google Scholar]
Batsali 2013
- Batsali AK, Kastrinaki MC, Papadaki HA. Mesenchymal stem cells derived from Wharton's Jelly of the umbilical cord: biological properties and emerging clinical applications. Current Stem Cell Research & Therapy 2013;8(2):144‐55. [PUBMED: 23279098] [DOI] [PubMed] [Google Scholar]
Beam 2014
- Beam KS, Aliaga S, Ahlfeld SK, Cohen‐Wolkowiez M, Smith PB, Laughon MM. A systematic review of randomized controlled trials for the prevention of bronchopulmonary dysplasia in infants. Journal of Perinatology 2014;34(9):705‐10. [DOI: 10.1038/jp.2014.126; PUBMED: 25010224] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1‐7. [PUBMED: 413500] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bellayr 2014
- Bellayr IH, Catalano JG, Lababidi S, Yang AX, Lo Surdo JL, Bauer SR, et al. Gene markers of cellular aging in human multipotent stromal cells in culture. Stem Cell Research & Therapy 2014;5(2):59. [DOI: 10.1186/scrt448; PUBMED: 24780490] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhandari 2006
- Bhandari A, Panitch HB. Pulmonary outcomes in bronchopulmonary dysplasia. Seminars in Perinatology 2006;30(4):219‐26. [DOI: 10.1053/j.semperi.2006.05.009; PUBMED: 16860162] [DOI] [PubMed] [Google Scholar]
Blau 2001
- Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function?. Cell 2001;105(7):829‐41. [PUBMED: 11439179] [DOI] [PubMed] [Google Scholar]
Chang 2009
- Chang YS, Oh W, Choi SJ, Sung DK, Kim SY, Choi EY, et al. Human umbilical cord blood‐derived mesenchymal stem cells attenuate hyperoxia‐induced lung injury in neonatal rats. Cell Transplant 2009;18(8):869–86. [DOI: 10.3727/096368909X471189; PUBMED: 19500472] [DOI] [PubMed] [Google Scholar]
Chang 2011
- Chang YS, Choi SJ, Sung DK, Kim SY, Oh W, Yang YS, et al. Intratracheal transplantation of human umbilical cord blood‐derived mesenchymal stem cells dose‐dependently attenuates hyperoxia‐induced lung injury in neonatal rats. Cell Transplantation 2011;20(11‐12):1843‐54. [DOI: 10.3727/096368911X565038; PUBMED: 23167961] [DOI] [PubMed] [Google Scholar]
da Silva Meirelles 2006
- Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post‐natal organs and tissues. Journal of Cell Science 2006;119(Pt 11):2204–13. [DOI: 10.1242/jcs.02932; PUBMED: 16684817] [DOI] [PubMed] [Google Scholar]
Darlow 2011
- Darlow BA, Graham PJ. Vitamin A supplementation to prevent mortality and short‐ and long‐term morbidity in very low birthweight infants. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD000501.pub3] [DOI] [PubMed] [Google Scholar]
Dmitrieva 2012
- Dmitrieva RI, Minullina IR, Bilibina AA, Tarasova OV, Anisimov SV, Zaritskey AY. Bone marrow‐ and subcutaneous adipose tissue‐derived mesenchymal stem cells: differences and similarities. Cell Cycle 2012;11(2):377–83. [DOI: 10.4161/cc.11.2.18858; PUBMED: 22189711] [DOI] [PubMed] [Google Scholar]
Dominici 2006
- Dominici M, Blanc K, Mueller I, Slaper‐Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8(4):315‐7. [DOI: 10.1080/14653240600855905; PUBMED: 16923606] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehrenkranz 2005
- Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. National Institutes of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005;116(6):1353‐60. [DOI: 10.1542/peds.2005-0249; PUBMED: 16322158] [DOI] [PubMed] [Google Scholar]
Farstad 2011
- Farstad T, Bratlid D, Medbo S, Markestad T, Norwegian Extreme Prematurity Study Group. Bronchopulmonary dysplasia ‐ prevalence, severity and predictive factors in a national cohort of extremely premature infants. Acta Paediatrica 2011;100(1):53‐8. [DOI: 10.1111/j.1651-2227.2010.01959.x; PUBMED: 20653607] [DOI] [PubMed] [Google Scholar]
FDA 2011
- U.S. Department of Health and Human Services, Food, Drug Administration. Center for Biologics Evaluation and Research. Guidance for industry: current good tissue practice (CGTP) and additional requirements for manufacturers of human cells, tissues, and cellular and tissue‐based products (HCT/Ps). www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/tissue/ucm285223.pdf 2011 (accessed prior to 23 October 2017).
Galipeau 2015
- Galipeau J, Krampera M. The challenge of defining mesenchymal stromal cell potency assays and their potential use as release criteria. Cytotherapy 2015;17(2):125‐7. [DOI: 10.1016/j.jcyt.2014.12.008; PUBMED: 25593076] [DOI] [PubMed] [Google Scholar]
Gebler 2012
- Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends in Molecular Medicine 2012;18(2):128‐34. [DOI: 10.1016/j.molmed.2011.10.004; PUBMED: 22118960] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version (accessed 5 July 2017). Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Higgins 2011
- Higgins A, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
ICCROP 2005
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Archives of Ophthalmology 2005;123(7):991‐9. [DOI: 10.1001/archopht.123.7.991; PUBMED: 16009843] [DOI] [PubMed] [Google Scholar]
Ikebe 2014
- Ikebe C, Suzuki K. Mesenchymal stem cells for regenerative therapy: optimization of cell preparation protocols. BioMed Research International 2014;2014:951512. [DOI: 10.1155/2014/951512; PUBMED: 24511552] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jobe 2001
Krampera 2013
- Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L, MSC Committee of the International Society for Cellular Therapy (ISCT). Immunological characterization of multipotent mesenchymal stromal cells ‐ The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 2013;15(9):1054‐61. [DOI: 10.1016/j.jcyt.2013.02.010; PUBMED: 23602578] [DOI] [PubMed] [Google Scholar]
Lalu 2012
- Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, et al. Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta‐analysis of clinical trials. PLoS ONE 2012;7(10):e47559. [DOI: 10.1371/journal.pone.0047559; PUBMED: 23133515] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lapcharoensap 2015
- Lapcharoensap W, Gage SC, Kan P, Profit J, Shaw GM, Gould JB, et al. Hospital variation and risk factors for bronchopulmonary dysplasia in a population‐based cohort. JAMA Pediatrics 2015;169(2):e143676. [DOI: 10.1001/jamapediatrics.2014.3676; PUBMED: 25642906] [DOI] [PubMed] [Google Scholar]
Lughon 2011
- Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. American Journal of Respiratory and Critical Care Medicine 2011;183(12):1715‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marks 2017
- Marks PW, Witten CM, Califf RM. Clarifying stem‐cell therapy’s benefits and risks. New England Journal of Medicine 2017;376(11):1007‐9. [DOI] [PubMed] [Google Scholar]
Mendicino 2014
- Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. MSC‐based product characterization for clinical trials: an FDA perspective. Cell Stem Cell 2014;14(2):141‐5. [DOI: 10.1016/j.stem.2014.01.013; PUBMED: 24506881] [DOI] [PubMed] [Google Scholar]
Murphy 2013
- Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Experimental & Molecular Medicine 2013;45:e54. [DOI: 10.1038/emm.2013.94; PUBMED: 24232253] [DOI] [PMC free article] [PubMed] [Google Scholar]
NIH 1979
- National Institutes of Health. Report of workshop on bronchopulmonary dysplasia. Washington, DC: National Institutes of Health; 1979. NIH Publication No. 80‐1660.
Ning 2008
- Ning H, Yang F, Jiang M, Liangding H, Feng K, Zhang J, et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia 2008;22(3):593–9. [DOI] [PubMed] [Google Scholar]
Panchalingam 2015
- Panchalingam KM, Jung S, Rosenberg L, Behie LA. Bioprocessing strategies for the large‐scale production of human mesenchymal stem cells: a review. Stem Cell Research & Therapy 2015;6:225. [DOI: 10.1186/s13287-015-0228-5; PUBMED: 26597928] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [PUBMED: 305471] [DOI] [PubMed] [Google Scholar]
Pierro 2013
- Pierro M, Ionescu L, Montemurro T, Vadivel A, Weissmann G, Oudit G, et al. Short‐term, long‐term and paracrine effect of human umbilical cord‐derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax 2013;68(5):475‐84. [DOI: 10.1136/thoraxjnl-2012-202323; PUBMED: 23212278] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sammour 2016
- Sammour I, Somashekar S, Huang J, Batlahally S, Breton M, Valasaki K, et al. The effect of gender on mesenchymal stem cell (MSC) efficacy in neonatal hyperoxia‐induced lung injury. PLoS ONE 2016;11(10):e0164269. [DOI: 10.1371/journal.pone.0164269; PUBMED: 27711256] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, (editors). GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group. www.guidelinedevelopment.org/handbook Updated October 2013 (accessed prior to 23 October 2017).
Sensebe 2011
- Sensebe L, Bourin P, Tarte K. Good manufacturing practices production of mesenchymal stem/stromal cells. Human Gene Therapy 2011;22(1):19–26. [DOI: 10.1089/hum.2010.197; PUBMED: 21028982] [DOI] [PubMed] [Google Scholar]
Shen 2015
- Shen SP, Liu WT, Lin Y, Li YT, Chang CH, Chang FW, et al. EphA2 is a biomarker of hMSCs derived from human placenta and umbilical cord. Taiwanese Journal of Obstetrics & Gynecology 2015;54(6):749‐56. [DOI: 10.1016/j.tjog.2015.10.012; PUBMED: 26700997] [DOI] [PubMed] [Google Scholar]
Shennan 1988
- Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82(4):527–32. [PUBMED: 3174313] [PubMed] [Google Scholar]
Song 2015
- Song K, Li W, Li M. Acute promyelocytic leukemia following autologous bone marrow‐derived mesenchymal stem cell transplantation for traumatic brain injury: a case report. Oncology Letters 2015;10(5):2905‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Speer 2006
- Speer CP. Pulmonary inflammation and bronchopulmonary dysplasia. Journal of Perinatology 2006;26(Suppl 1):S57‐62. [DOI: 10.1038/sj.jp.7211476; PUBMED: 16625227] [DOI] [PubMed] [Google Scholar]
Stoll 2010
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126(3):443‐56. [DOI: 10.1542/peds.2009-2959; PUBMED: 20732945] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sullivan 2008
- Sullivan MJ. Banking on cord blood stem cells. Nature reviews. Cancer 2008;8(7):555‐63. [DOI: 10.1038/nrc2418; PUBMED: 18548085] [DOI] [PubMed] [Google Scholar]
van Haaften 2009
- Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. American Journal of Respiratory and Critical Care Medicine 2009;180(11):1131‐42. [DOI: 10.1164/rccm.200902-0179OC; PUBMED: 19713449] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagner 2008
- Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS ONE 2008;3(5):e2213. [DOI: 10.1371/journal.pone.0002213; PUBMED: 18493317] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2003
- Walsh MC, Wilson‐Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. Journal of Perinatology 2003;23(6):451–6. [DOI: 10.1038/sj.jp.7210963; PUBMED: 13679930] [DOI] [PubMed] [Google Scholar]
Walsh 2006
- Walsh MC, Szefler S, Davis J, Allen M, Marter L, Abman S, et al. Summary proceedings from the bronchopulmonary dysplasia group. Pediatrics 2006;117(3 Pt 2):S52‐6. [DOI: 10.1542/peds.2005-0620I; PUBMED: 16777823] [DOI] [PubMed] [Google Scholar]
Waszak 2012
- Waszak P, Alphonse R, Vadivel A, Ionescu L, Eaton F, Thébaud B. Preconditioning enhances the paracrine effect of mesenchymal stem cells in preventing oxygen‐induced neonatal lung injury in rats. Stem Cells and Development 2012;21(15):2789‐97. [DOI: 10.1089/scd.2010.0566; PUBMED: 22533467] [DOI] [PubMed] [Google Scholar]
Watterberg 2010
- Watterberg KL, American Academy of Pediatrics. Committee on Fetus and Newborn. Policy statement ‐ postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics 2010;126(4):800‐8. [DOI: 10.1542/peds.2010-1534; PUBMED: 20819899] [DOI] [PubMed] [Google Scholar]
Zhao 2017
- Zhao R, Su Z, Wu J, Ji HL. Serious adverse events of cell therapy for respiratory diseases: a systematic review and meta‐analysis. Oncotarget 2017;8(18):30511‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]


