Abstract
Background
18F‐florbetaben uptake by brain tissue, measured by positron emission tomography (PET), is accepted by regulatory agencies like the Food and Drug Administration (FDA) and the European Medicine Agencies (EMA) for assessing amyloid load in people with dementia. Its added value is mainly demonstrated by excluding Alzheimer's pathology in an established dementia diagnosis. However, the National Institute on Aging and Alzheimer's Association (NIA‐AA) revised the diagnostic criteria for Alzheimer's disease and confidence in the diagnosis of mild cognitive impairment (MCI) due to Alzheimer's disease may be increased when using some amyloid biomarkers tests like 18F‐florbetaben. These tests, added to the MCI core clinical criteria, might increase the diagnostic test accuracy (DTA) of a testing strategy. However, the DTA of 18F‐florbetaben to predict the progression from MCI to Alzheimer’s disease dementia (ADD) or other dementias has not yet been systematically evaluated.
Objectives
To determine the DTA of the 18F‐florbetaben PET scan for detecting people with MCI at time of performing the test who will clinically progress to ADD, other forms of dementia (non‐ADD), or any form of dementia at follow‐up.
Search methods
The most recent search for this review was performed in May 2017. We searched MEDLINE (OvidSP), Embase (OvidSP), PsycINFO (OvidSP), BIOSIS Citation Index (Thomson Reuters Web of Science), Web of Science Core Collection, including the Science Citation Index (Thomson Reuters Web of Science) and the Conference Proceedings Citation Index (Thomson Reuters Web of Science), LILACS (BIREME), CINAHL (EBSCOhost), ClinicalTrials.gov (https://clinicaltrials.gov), and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (http://www.who.int/ictrp/search/en/). We also searched ALOIS, the Cochrane Dementia & Cognitive Improvement Group’s specialised register of dementia studies (http://www.medicine.ox.ac.uk/alois/). We checked the reference lists of any relevant studies and systematic reviews, and performed citation tracking using the Science Citation Index to identify any additional relevant studies. No language or date restrictions were applied to electronic searches.
Selection criteria
We included studies that had prospectively defined cohorts with any accepted definition of MCI at time of performing the test and the use of 18F‐florbetaben scan to evaluate the DTA of the progression from MCI to ADD or other forms of dementia. In addition, we only selected studies that applied a reference standard for Alzheimer’s dementia diagnosis, for example, the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS‐ADRDA) or Diagnostic and Statistical Manual of Mental Disorders‐IV (DSM‐IV) criteria.
Data collection and analysis
We screened all titles and abstracts identified in electronic‐database searches. Two review authors independently selected studies for inclusion and extracted data to create two‐by‐two tables, showing the binary test results cross‐classified with the binary reference standard. We used these data to calculate sensitivities, specificities, and their 95% confidence intervals. Two independent assessors performed quality assessment using the QUADAS‐2 tool plus some additional items to assess the methodological quality of the included studies.
Main results
Progression from MCI to ADD, any other form of dementia, and any form of dementia was evaluated in one study (Ong 2015). It reported data on 45 participants at four years of follow‐up; 21 participants met NINCDS‐ADRDA criteria for Alzheimer’s disease dementia at four years of follow‐up, the proportion converting to ADD was 47% of the 45 participants, and 11% of the 45 participants met criteria for other types of dementias (three cases of FrontoTemporal Dementia (FTD), one of Dementia with Lewy body (DLB), and one of Progressive Supranuclear Palsy (PSP)). We considered the study to be at high risk of bias in the domains of the reference standard, flow, and timing (QUADAS‐2).
MCI to ADD; 18F‐florbetaben PET scan analysed visually: the sensitivity was 100% (95% confidence interval (CI) 84% to 100%) and the specificity was 83% (95% CI 63% to 98%) (n = 45, 1 study). Analysed quantitatively: the sensitivity was 100% (95% CI 84% to 100%) and the specificity was 88% (95% CI 68% to 97%) for the diagnosis of ADD at follow‐up (n = 45, 1 study).
MCI to any other form of dementia (non‐ADD);18F‐florbetaben PET scan analysed visually: the sensitivity was 0% (95% CI 0% to 52%) and the specificity was 38% (95% CI 23% to 54%) (n = 45, 1 study). Analysed quantitatively: the sensitivity was 0% (95% CI 0% to 52%) and the specificity was 40% (95% CI 25% to 57%) for the diagnosis of any other form of dementia at follow‐up (n = 45, 1 study).
MCI to any form of dementia;18F‐florbetaben PET scan analysed visually: the sensitivity was 81% (95% CI 61% to 93%) and the specificity was 79% (95% CI 54% to 94%) (n = 45, 1 study). Analysed quantitatively: the sensitivity was 81% (95% CI 61% to 93%) and the specificity was 84% (95% CI 60% to 97%) for the diagnosis of any form of dementia at follow‐up (n = 45, 1 study).
Authors' conclusions
Although we were able to calculate one estimation of DTA in, especially, the prediction of progression from MCI to ADD at four years follow‐up, the small number of participants implies imprecision of sensitivity and specificity estimates. We cannot make any recommendation regarding the routine use of 18F‐florbetaben in clinical practice based on one single study with 45 participants. 18F‐florbetaben has high financial costs, therefore, clearly demonstrating its DTA and standardising the process of the 18F‐florbetaben modality are important prior to its wider use.
Plain language summary
18F PET with florbetaben for the early diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment
Review question: In people with mild cognitive impairment (MCI), does using a 18F PET scan with florbetaben predict progression to Alzheimer's disease dementia (ADD) and other dementias?
Background Due to global ageing, the number of people with dementia is expected to increase dramatically in the next few decades. Diagnosing dementia at an early stage is desirable, but there is no widespread agreement on the best approach. A range of simple pen and paper tests used by healthcare professionals can assess people with poor memory or cognitive impairment. Whether or not using special PET scans that detect amyloid —one of the hallmarks of Alzheimer's disease— improves our ability to predict the progression from MCI to ADD or other forms of dementia remains unclear. Since these tests are expensive, it is important that they provide additional benefits.
Aim
We aimed to evaluate the accuracy of the 18F‐florbetaben PET scan in identifying those people with MCI who clinically progress to ADD, other types of dementia, or any form of dementia over a period of time.
Study characteristics The evidence is current to May 2017. We found 1 study including 45 participants with MCI with a follow‐up of 4 years; gender was not reported and the median age for those with a PET‐positive scan by quantitative assessment was 73.5 years old. For those with a PET‐negative scan the mean age was 71.8 years old. Participants were mainly recruited from local memory clinics.
Study funding sources: the study was funded by the test manufacturer.
Quality of the evidence The main limitation of this review was that our findings were based on only one study, with not enough details on how the participants were selected. The study was considered to be at high risk of bias, since the final ADD diagnosis was not established separately from the scan results, and due to potential conflicts of interest detected.
Key findings In this review, based on only one study, we found that the 18F‐florbetaben PET scan, as a single test with visual assessment, correctly classified 100% of the participants who will progress to ADD and 83% of the participants who did not progress to ADD at four years follow‐up. This means that in a cohort with 100 participants with MCI, 47 of whom will progress to ADD, we would expect that all those 47 MCI participants would test positive with the 18F‐florbetaben scan and that 0 participants would be falsely negative (i.e. none of the 47 participants would have a negative test and yet progress to ADD). In addition, we would expect 44 of 53 participants who did not progress to ADD to be 18F‐florbetaben‐negative and 9 to be falsely positive (i.e. 9 of the 53 participants would have a positive test but not progress to ADD).
The small size of the included study lowered our confidence on these estimates of accuracy and it is still possible that the test is considerably less accurate than these results suggest.
We conclude that 18F‐florbetaben imaging is a promising test to predict the progression from MCI to ADD; however, we need more studies to clearly demonstrate its accuracy.
Summary of findings
Summary of findings'. 'Diagnostic test accuracy of 18F‐florbetaben to predict the progression to ADD, any other form of dementia (non‐ADD) or any form of dementia in people with MCI.
| What is the diagnostic accuracy of 18F‐florbetaben PET amyloid biomarker for predict progression to ADD or any other form of dementia (non‐ADD) or any form of dementia in people with MCI? | |||||||
| Descriptive | |||||||
| Patient population | Participants diagnosed with MCI at baseline using any of the Petersen criteria or Winblad criteria or CDR = 0.5 or any 16 definitions included by Matthews (Matthews 2008) | ||||||
| Sources of referral | Memory clinic | ||||||
| MCI criteria | Petersen criteria 2004 and Winblad 2004 (Petersen 2004; Winblad 2004) | ||||||
| Sampling procedure | unclear | ||||||
| Prior testing | The only testing prior performing the 18F‐florbetaben PET amyloid biomarker was the application of diagnostic criteria for identifying participants with MCI | ||||||
| Settings | Secondary care | ||||||
| Index test | 18F‐florbetaben PET | ||||||
| Threshold prespecified at baseline | Yes | ||||||
| Threshold interpretation | Visual and quantitative | ||||||
| Threshold | Visual: if any tracer uptake was visible in any of the frontal, parietal, temporal, and posterior cingulate/precuneus cortices SUVR (Standardised Uptake Volume ratio) of ROI: > 1.45 |
||||||
| 18F‐florbetaben retention region | Visual: frontal, parietal, temporal, and posterior cingulate/precuneus cortices Global cortex (SUVR) SUVR: Global cortex |
||||||
| Reference Standard | For Alzheimer’s disease dementia: NINCDS‐ADRDA (McKhann 1984) For Lewy body dementia: McKeith criteria (McKeith 2005) For frontotemporal dementia: Lund criteria (Brun 1994) For progressive supranuclear palsy: Preliminary NINDS criteria (Hauw 1994) |
||||||
| Target condition | Progression from MCI to Alzheimer’s disease dementia or any other forms of dementia or any form of dementia. | ||||||
| Included studies | Prospectively well‐defined cohorts with any accepted definition of MCI (as above). One study (N = 45 participants) was included. Number of participants included in analysis: 45. | ||||||
| Quality concerns | Patient characteristics were poorly reported. Reference standard diagnosis was made with knowledge of the index test. Applicability concerns were high in reference standard. | ||||||
| Limitations | We were not able to calculate a summary of sensitivity and specificity due to insufficient number of studies. Investigation of heterogeneity and sensitivity analysis were not done due to insufficient number of studies. |
||||||
| Test | Studies | Cases/Participants | Sensitivity | Specificity | Consequences in a cohort of 100 | ||
| Proportion converting1 | Missed cases2 | Overdiagnosed | |||||
| Alzheimer's disease dementia | |||||||
| 18F‐florbetaben (visual assessment) | 1 | 21/45 | 100% (95% CI 84% to 100%) | 83% (95% CI 63% to 95%) | 47 | 0 | 9 |
| 18F‐florbetaben (SUVR) | 1 | 21/45 | 100% (95% CI 84% to 100%) | 88% (95% CI 68% to 97%) | 47 | 0 | 6 |
| Any other form of dementia (non‐ADD) | |||||||
| 18F‐florbetaben (visual assessment) | 1 | 5/45 | 0% (95% CI 0% to 52%) | 38% (95% CI 23% to 54%) | 11 | 11 | 55 |
| 18F‐florbetaben (SUVR) | 1 | 5/45 | 0% (95% CI 0% to 52%) | 40% (95% CI 25% to 57%) | 11 | 11 | 53 |
| Any form of dementia | |||||||
| 18F‐florbetaben (visual assessment) | 1 | 26/45 | 81% (95% CI 61% to 93%) | 79% (95% CI 54% to 94%) | 58 | 11 | 9 |
| 18F‐florbetaben (SUVR) | 1 | 26/45 | 81% (95% CI 61% to 93%) | 84% (95% CI 60% to 97%) | 58 | 11 | 7 |
| Investigation of heterogeneity and sensitivity analysis: The planned investigations of heterogeneity or sensitivity analyses were not possible due to a limited number of studies available for each analysis. | |||||||
| Conclusions:18F‐florbetaben PET scan has a good sensitivity achieved especially in predicting the progression from MCI to ADD. The quality of evidence was weak because it was based on only one study (45 participants) and there was high risk of bias due to the knowledge of the reference standard to do the diagnosis at four‐year follow‐up and due to possible conflict of interest detected. There is a need for conducting studies using standardised 18F‐florbetaben PET scan methodology in larger populations. Regarding the aforementioned we do not recommend the use in clinical practice until the DTA performance will be clearly demonstrated. | |||||||
1. Proportion converting to ADD or any other form of dementia (non‐ADD) or any form of dementia in the included study
2. Missed and overdiagnosed numbers were computed using the proportion converting to the target condition.
ADD: Alzheimer's disease dementia CDR: Clinical Dementia Rating MCI: Mild cognitive impairment NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association
NINDS: National Institute of Neurological Disorders and Stroke PET: Positron emission tomography ROI: Region of interest SUVR: Standardised uptake value ratio
Background
Dementia is a syndrome due to a brain disease — usually of a chronic or progressive nature — in which there is disturbance of multiple higher cortical functions, including memory, thinking, orientation, comprehension, calculation, learning capacity, language, and judgement. However, consciousness remains unaffected. See the glossary in Appendix 1. The impairments of cognitive function are commonly accompanied, and occasionally preceded, by a deterioration in emotional control, social behaviour, and motivation, and the impairment is sufficient to interfere with everyday activities. Dementia is a collection of different subtypes distinguished by the underlying pathology. ADD is the most common form of dementia and other important pathologies associated with dementia are vascular disease, Lewy bodies, and frontotemporal pathology (WHO 2012).
Dementia is a serious worldwide public health problem, with a prevalence of 4.7% in adults older than 60 years (6.2% and 6.5% in Europe and the Americas, respectively). Due to its prevalence in older people, it is expected that the number of people with dementia will increase dramatically. Consequently, in the year 2050, an expected number of 115 million people will have dementia. This will result in a considerable economic burden, which currently stands at 1% of the world's Gross National Product (GNP) in direct and indirect costs (WHO 2012). These financial costs are in addition to the devastating personal and social consequences of the condition.
The definition of MCI applies to people without evidence of significant deterioration in activities of daily living, but with subjective memory complaints and cognitive impairment detected by standardised tests. MCI often precedes clinical dementia, but there is no consensus regarding how to operationalise the MCI diagnosis. There are several clinical criteria to define which people have MCI, including the Petersen criteria or Petersen Revised Criteria (Petersen 1999; Petersen 2004; Winblad 2004), Clinical Dementia Rating (CDR = 0.5) (Morris 1993), or 16 other different classifications of MCI (Matthews 2008).
A diagnosis of MCI reputedly allows testing of preventive interventions that would slow the progression of MCI to dementia. If the progression of MCI to dementia could be deferred by five years, the prevalence of dementia would decrease by 43% in 2050 (Alzheimer's Association 2010). MCI has an annual progression rate to ADD from 5% to 15%. However, not every person with MCI develops dementia, and a significant number of people recover or stabilise. Therefore, future research should try to clarify which people with MCI develop dementia in order to be able to focus specifically on people who are at high risk of developing dementia. This may possibly explain the failure of therapy to alter the progression to dementia in people with MCI. Other aspects that may contribute to this failure are the disparity in diagnostic criteria and different settings of the studied participants: community, primary, secondary, and research centres (Bruscoli 2004; Mattsson 2009; Petersen 1999; Petersen 2009).
The definition of Alzheimer's disease pathology is over 100 years old. This pathology includes neuritic plaques that contain deposits of amyloid beta (Aβ) and neurofibrillary tangles (Goedert 2006). This pathology is present in approximately 84% of all people with dementia (Schneider 2007). Furthermore, Alzheimer's disease pathology is found in 88% of people diagnosed with probable ADD (Schneider 2009). Despite this, Alzheimer's disease pathology may be found concomitantly at autopsy in people thought to have other forms of dementia, such as vascular dementia, Lewy body dementia, or frontotemporal dementia (FTD) (Jellinger 2006). Furthermore, at least five common pathologies have been found in the brains of people who died and were thought to have ADD prior to death (White 2009). Also, Alzheimer's disease pathology was found in 42% of community‐dwelling older people without dementia (Schneider 2007). This has generated controversy about the importance of the presence of Alzheimer's disease pathology. The pathology can be associated with aging per se, and, for older people, the relationship between amyloid plaque burden and cognitive impairment diminishes as age progresses (Savva 2009). Thus, this pathology could be an epiphenomenon associated with the presence of dementia, e.g. a by‐product of repair mechanisms by vascular damage (De la Torre 2004; Garcia‐Alloza 2011). On the other hand, this controversy could be because our clinical diagnostic criteria have not had enough accuracy to diagnose Alzheimer's disease that is detected by histopathology in postmortem studies (Hyman 2012). In addition, other researchers think that there is not a real controversy about the amyloid hypothesis, because the amyloid cascade and the Aβ deposition have a primary role in Alzheimer's disease (Selkoe 2016).
More recently, the development of Aβ pathology biomarkers in vivo has been suggested as an important advance as a diagnostic tool in the field of Alzheimer's disease, and has promoted the creation of new diagnostic criteria for people without symptoms (preclinical stages), people with MCI, and people with ADD, based on the presence of biomarkers of Alzheimer's disease. These have included Aβ tracers by positron emission tomography (PET) (Albert 2011; Dubois 2014; McKhann 2011; Sperling 2011). However, uncertainties regarding the usability of biomarkers in the diagnosis of dementia still exist, mainly due to variation between biomarker types, criteria for positivity, and differences in methodology (Noel‐Storr 2013). This prompted an important initiative, the Standards for Reporting of Diagnostic Accuracy Studies in dementia studies (STARDdem) statement (Noel‐Storr 2014). Consequently, clinical properties of dementia biomarkers should not be assumed, and formal systematic evaluations of sensitivity, specificity, and other properties of biomarkers should be performed (Davis 2013).
PET is an imaging technique using compounds labelled with short‐lived positron‐emitting radionuclides. The use of Aβ ligands permits the in vivo detection of amyloid deposition in the brain. 18F‐florbetaben is a stilbene derivative, which was first described 12 years ago, and is characterised by a high affinity for Aβ. 18F‐florbetaben has excellent uptake by brain tissue and washout kinetics in mice (Zhang 2005). 18F‐florbetaben was evaluated in people with ADD, healthy people without ADD (Barthel 2011), and people with other dementias (Villemagne 2011).
The Food and Drug Administration (FDA) and the European Medicines Agency (EMA) approved 18F‐florbetaben for Aβ binding. These agencies have stated that a negative scan indicates sparse or no plaques, which is inconsistent with a diagnosis of ADD, thus effectively excluding this diagnosis. A positive 18F‐florbetaben scan indicates moderate to frequent presence of neuritic amyloid plaques. However, this might also occur in people with other neurological conditions and in older adults with normal cognition. Therefore, it should be combined with other diagnostic evaluations or instruments and cannot be used solely to assess the risk of progression to ADD. Therefore, a positive result of an 18F‐florbetaben scan does not establish the diagnosis of ADD or any other cognitive disorder definitely, and it should be combined with other diagnostic evaluations or instruments. Additionally, the effectiveness and safety of the tests have not been established by predicting development of dementia or other neurological conditions, or by monitoring responses to therapies (EMA 2014; FDA 2014).
Despite not being approved for this purpose by the regulatory agencies, research has been conducted in people with MCI to determine whether biomarkers, such as 18F‐florbetaben for Aβ, increase the risk of developing dementia over time. The evidence for this is uncertain. For this and other reasons, the NIA‐AA in the USA established two different criteria for MCI. Firstly, they established the Core Clinical Criteria for use in all clinical settings, without use of biomarkers, and characterised by concerns regarding a change in cognition with impairment in one or more cognitive domains with preservation of independence in functional abilities, therefore no dementia. Secondly, they established the Clinical Research Criteria, which incorporate the use of biomarkers, such as PET amyloid scans, intended for use exclusively in research settings, including academic centres and clinical trials. This will help determine whether positive scans increase the likelihood of progression from MCI to clinical dementia (Albert 2011). Lastly, it is hoped that people with MCI and positive scans will 'enrich' clinical trials, and more people who will progress to dementia in a shorter time will be included to allow more efficient studies of treatments and prevention strategies of ADD (CMS 2013).
An assumption for some researchers, and one on which this systematic review (SR) is predicated, is that if a person has both MCI and the pathology of Alzheimer's disease and develops clinical ADD subsequently, then the cause of the initial MCI and of the ADD was the Alzheimer’s pathology. Our approach is an example of assessing diagnostic test accuracy (DTA) using delayed verification of diagnosis. Instead of the reference standard being based on pathology, it is based on a clinical standard and the progression from MCI to ADD, or any other form of non‐ADD, or any dementia. Although, for the reasons stated above, a degree of unreliability has been introduced, defining progression has the advantage of being based on what matters most to people with MCI, their families, and clinicians involved in their care.
18F‐florbetaben PET scan is considered the diagnostic marker of interest, and in this SR we assessed the DTA of 18F‐florbetaben Aβ binding in the brain and progression of the following:
From MCI to ADD.
From MCI to any other form of non‐ADD.
From MCI to any form of dementia
This SR belongs to a series of SRs regarding PET biomarkers for Aβ, including 18F‐florbetapir and 18F‐flutemetamol (Martínez 2016).
Target condition being diagnosed
This SR assessed the following three target conditions.
ADD (progression from MCI to ADD).
Any other form of dementia (progression from MCI to any other form of non‐ADD).
Any form of dementia (progression from MCI to any form of dementia).
We compared the index test results obtained at baseline with the results of the reference standards obtained at follow‐up (delayed verification).
Index test(s)
The 18F‐florbetaben scan is an index test for the detection of Aβ deposition in the brain region of interest (ROI). The ROI is a selected brain area that physicians create for further study in various anatomical areas of the brain. 18F‐florbetaben is a molecular biomarker, described as
[18F]BAY 94‐9172, trans‐4‐(N‐methyl‐amino)‐4’‐2‐[2‐(2‐[18F]fluoro‐ethoxy)‐ethoxy]‐ethoxy‐stilbene and also referred to as BAY 94‐9172 or ZK 6013443, which is a polyethylene glycol stilbene derivative (Zhang 2005).
Image Interpretation
Both the FDA and EMA have described the criteria for 18F‐florbetaben Aβ positivity (EMA 2014; FDA 2014).
18F‐florbetaben diagnosis is by PET image assessment, and is defined as positive if the analysis shows the following.
Moderate or smaller area(s) of tracer uptake equal to or higher than that presented in the white matter: extending beyond the white matter rim to the outer cortical margin involving the majority of the slices within the respective region.
Pronounced Aβ deposition (a large confluent area of tracer uptake equal to or higher than that presented in white matter extending beyond the white matter rim to the outer cortical margin and involving the entire region including the majority of slices within the respective region) in the grey matter of the following four brain regions: the temporal lobes, the frontal lobes, the posterior cingulate cortex/precuneus, and the parietal lobes.
Readers trained in PET images with the 18F‐florbetaben should interpret the Aβ PET images made with this ligand (EMA 2014; FDA 2014).
Before the FDA and EMA described the criteria for 18F‐florbetaben scan positivity, the diagnosis of dementia was made using different thresholds. Therefore, we planned to use the FDA or EMA criteria applied in each included study to classify participants as either test‐positive or test‐negative, or, alternatively, if 18F‐florbetaben Aβ uptake and retention exceeded a certain threshold.
We considered the measurement of the 18F‐florbetaben retention (retention ratio): distribution volume ratio (DVR), standardised uptake value ratio (SUVR), or other ratios. DVR refers to the ratio of the 18F‐florbetaben distribution volume in the selected area (ROI) to the distribution volume in the reference area. SUVR is the ratio of the 18F‐florbetaben ligand standardised uptake value in the selected area (ROI) to the standardised uptake value in the reference area.
The unit of analysis of our SR was the participant. We did not include studies that analysed multiple ROIs per person.
Image analysis was not prespecified (e.g. Statistical Parametric Mapping (SPM) or other image analysis techniques).
Administration Instructions and Recommended Dosing
Time between 18F‐florbetaben injection and PET acquisition: images should be acquired in 15 to 20 minutes starting from 45 to 130 minutes after intravenous administration (FDA 2014) or acquired in 20 minutes starting from 90 minutes after intravenous administration (EMA 2014);
Injection dose: the recommended dose for 18F‐florbetaben Aβ PET is 300 MBq (8.1 mCi), maximum 30 mcg mass dose (FDA 2014) or 300 MBq (240 to 360 MBq) as a single slow intravenous bolus (6 sec/mL) in a total volume of up to 10 mL (EMA 2014).
Although it was inevitable that included studies had used different imaging protocols, readers' expertise, and varied parameters, the amyloid PET data in these included studies should be technically adequate and acquired at a fully qualified and certified facility.
Clinical pathway
At this time, the clinical evaluation often has similarities between different countries (Cordella 2013; NICE 2006). It often starts with people experiencing memory complaints detected by themselves or their relatives. Frequently, general practitioners or family physicians are consulted, and they often conduct a medical evaluation using a screening test for cognitive impairment. Whenever this screening test is positive, they complete an assessment with a clinical evaluation conducted with laboratory studies that can rule out a secondary cause of cognitive impairment (e.g. hypothyroidism, renal failure, liver failure, vitamin B12 or folate deficiency, and others). In addition, these people are then referred to medical specialists in cognitive disorders (preferably a geriatrician, psychiatrist, or neurologist) in a secondary centre or directly to memory clinics where further clinical assessment, laboratory studies, and cerebral image studies are conducted to confirm the dementia diagnosis.
People with dementia, or their relatives, often directly consult these specialists or specialised memory clinics in the study of cognitive disorders. Therefore, the performance of the diagnostic tests will probably vary according to whether it is a primary consultation or referral from primary to specialist care, or if the people have different clinical stages of the disease (MCI, mild, moderate, or severe dementia). Due to these differing pathways, the use of 18F‐florbetaben PET ligand for Aβ is mainly used in specialist consultations and memory clinics as an addition to clinical evaluation or other tests, helping in a clinical setting to discard a diagnosis of Alzheimer's dementia with a negative scan in a person with clinical dementia and doubts about the aetiology (e.g. FTD versus ADD). Otherwise, it might be used solely in the research field in people with MCI for the enrichment of clinical trials, for example, enrolling people with MCI and a positive PET scan to study preventive interventions before people develop dementia.
However, in some memory clinics the 18F‐florbetaben PET is used for clinical purposes in people with persistent or progressive unexplained MCI adopting the Johnson criteria (Johnson 2013), criteria without sufficient evidence. Therefore, if the 18F‐florbetaben PET is positive in a person with MCI, this positivity is considered as one of the core histopathological findings of Alzheimer's disease. The person will thus be catalogued as a patient with prodromal Alzheimer's disease or MCI due to Alzheimer's disease.
Alternative test(s)
Currently, there are no standard practice tests available for the clinical diagnosis of Alzheimer's disease dementia. Below, we have listed the alternative tests that we have excluded from this SR. The Cochrane Dementia and Cognitive Improvement Group is in the process of conducting a series of DTA SRs of biomarkers and scales (see list below).
18F PET ligands for Aβ (18F‐florbetapir, 18F‐flutemetamol) (Martínez 2016).
18F‐FDG‐PET (PET F‐fluorodeoxyglucose) (Smailagic 2015).
11C‐PIB‐PET (PET‐Pittsburgh compound B) (Zhang 2014).
Cerebrospinal fluid (CSF) analysis of Aβ and tau (Kokkinou 2014; Ritchie 2013; Ritchie 2014).
Structural magnetic resonance imaging (sMRI) (Filippini 2012).
Neuropsychological tests (Mini‐Mental State Examination (MMSE); MiniCOG; Montreal Cognitive Assessment (MoCA) (Arevalo‐Rodriguez 2015; Chan 2014; Creavin 2016; Davis 2015; Fage 2015; Seitz 2014).
Informant interviews (Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE); AD8) (Harrison 2014; Hendry 2014; Lees 2014; Harrison 2015; Quinn 2014).
APOE‐ϵ4 (Elias‐Sonnenschein 2014a; Elias‐Sonnenschein 2014b; Elias‐Sonnenschein 2014c).
Single‐photon emission computed tomography (SPECT) brain imaging (Archer 2015; McCleery 2015).
Rationale
Accurate and early diagnosis of Alzheimer's disease is crucial for planning in healthcare systems, because the costs of dementia are currently at least 1% of the world's GNP (WHO 2012).
18F‐florbetaben is approved for use in the clinical field mainly in people who are diagnosed clinically with dementia of uncertain aetiology, in which case diagnosis of ADD can be discarded if the test is negative. Even though 18F‐florbetaben is not approved for this purpose, this biomarker test is currently being used in the research field to search for the accurate identification of people with MCI who would progress to ADD or other forms of dementia. Amyloid β tracers by PET have been included in newly diagnostic criteria in the study in people with MCI (Albert 2011; Dubois 2014). However, some uncertainties exist about the generalisability of the DTA results in clinical settings, especially in older people (Richard 2012).
It is currently believed that if the health system can identify which people are at high risk of progressing from MCI to dementia, it can focus on improving opportunities for appropriate contingency planning for them. Proper recognition of the disease may also help prevent inappropriate and potentially harmful admissions to hospital or institutional care (NAO 2007), and enable the development of new treatments designed to delay or prevent progression to more debilitating stages of the disease. Additionally, this may demonstrate a real clinical benefit for people and caregivers, and will reduce health system costs.
This SR assesses the DTA with 18F‐florbetaben Aβ PET in people with MCI.
Objectives
To determine the diagnostic test accuracy (DTA) of 18F‐florbetaben as the index test for detecting people with mild cognitive impairment (MCI) at time of performing the test who would clinically progress to Alzheimer's disease dementia (ADD), or other forms of non‐ADD, or any form of dementia at follow‐up.
Secondary objectives
To investigate the heterogeneity of the DTA in the included studies, by evaluating the spectrum of people, referral centres, clinical criteria of MCI, 18F‐florbetaben techniques, reference standards used, duration of follow‐up, aspects of study quality, and conflicts of interest.
Methods
Criteria for considering studies for this review
Types of studies
We included longitudinal studies that had prospectively defined cohorts with any accepted definition of mild cognitive impairment (MCI), as outlined below, at time of performing the 18F‐florbetaben Aβ scan and a reference standard (see Index tests and Reference standards below). We obtained the results at the follow‐up of the studies. These studies had to employ delayed verification of progression to dementia and were sometimes labelled as 'delayed verification cross‐sectional studies' (Bossuyt 2008; Knottnerus 2002). We included case‐control studies when they incorporate a delayed verification design. This occurred in the context of a cohort study, so these studies were invariably diagnostic‐nested case‐control studies.
Participants
Participants recruited and clinically classified as having MCI at time of performing the test were eligible for inclusion. We established the diagnosis of MCI using the Petersen criteria or revised Petersen criteria (Petersen 1999; Petersen 2004; Winblad 2004), the criteria included in Matthews study (Matthews 2008), CDR = 0.5 (CDR structured interviews collects information from both the collateral source and the subject regarding memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care, where the range of possible scores varies from none = 0 point to severe = 3 points) (Morris 1993), the National Institute on Aging‐Alzheimer's Association (NIA‐AA) core clinical criteria (Albert 2011), or a combination.
We excluded studies that included people with MCI possibly caused by any of the following.
Current or a history of alcohol or drug abuse.
Central nervous system (CNS) trauma (e.g. subdural hematoma), tumour, or infection.
Other neurological conditions (e.g. Parkinson’s or Huntington’s diseases). Regarding Parkinson's disease, many of the studies specifically excluded people with Parkinson's disease from the group with mild cognitive impairment. This specific group of people is complex in both regards to defining neuropathology and in determination of functional decline. For these reasons, this group of people needs to be addressed in specific studies.
Index tests
The index test of this SR was 18F‐florbetaben biomarker test. We used the criteria and cut‐off values for test positivity, as reported in the included studies. We considered positivity for 18F‐florbetaben Aβ scan uptake and retention exceeding a certain threshold.
Target conditions
Three target conditions were included in this SR:
Alzheimer’s disease dementia (ADD) (progression from MCI to ADD).
Any other forms of dementia (progression from MCI to any other forms of non‐ADD).
Any form of dementia (progression from MCI to any form of dementia).
Reference standards
The reference standard was the progression to the target conditions evaluated by a physician with expertise in the dementia field (preferably a geriatrician, psychiatrist, or neurologist). For the purpose of this SR, we accepted several definitions of ADD. We included studies that applied the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDSADRDA) criteria (McKhann 1984), the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria (APA 1987; APA 1994), and the International Classification of Diseases (ICD) (ICD‐10) criteria for ADD. Notably, different iterations of these standards may not be directly comparable over time (e.g. APA 1987 versus APA 1994). Moreover, the validity of the diagnoses may vary with the degree or manner in which the criteria have been operationalised (e.g. individual clinician versus algorithm versus consensus determination). We considered all these issues when we interpreted the results.
Similarly, we accepted differing clinical definitions of other dementias. For Lewy body dementia, the reference standard is the McKeith criteria (McKeith 1996; McKeith 2005); for frontotemporal dementia the Lund criteria (Boxer 2005; Brun 1994; Neary 1998), the DSM criteria (APA 1987; APA 1994), the ICD criteria (ICD‐10), or the International Behavioural Variant FTD Criteria Consortium (Rascovsky 2011); for vascular dementia, the National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l'Enseignement en Neurosciences (NINDS‐AIREN) criteria (Román 1993), the DSM criteria (APA 1987; APA 1994), or the ICD criteria (ICD‐10); and, for progressive supranuclear palsy (PSP), the preliminary NINDS criteria (Hauw 1994).
The time interval over which the progression from MCI to ADD (or other forms of dementia) occurs is very important. We used one year as the minimum period of delay in the verification of the diagnosis (the time between the assessment at which a diagnosis of MCI is made and the assessment at which the diagnosis of dementia is made).
Search methods for identification of studies
Electronic searches
We searched MEDLINE (Ovid SP) from 1946 to May 2017; Embase (Ovid SP) from 1974 to May 2017; PsycINFO (Ovid SP) from 1806 to May 2017; BIOSIS Citation Index (Thomson Reuters Web of Science) from 1922 to May 2017; Web of Science Core Collection, including the Science Citation Index (Thomson Reuters Web of Science) and the Conference Proceedings Citation Index (Thomson Reuters Web of Science) from 1946 to May 2017; LILACS (Bireme); CINAHL (EBSCOhost) from 1980 to May 2017; ClinicalTrials.gov (https://clinicaltrials.gov); and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (http://www.who.int/ictrp/search/en/). We also searched ALOIS, the Cochrane Dementia & Cognitive Improvement Group’s specialized register of dementia studies (http://www.medicine.ox.ac.uk/alois/).
We used two approaches in designing the search. One focused solely on the specifically named index test (including a range of synonyms) and the second, run in parallel covered a more general search, linking broader terms for the index test. It focused on terms describing its diagnostic use and terms for the target condition to try to capture the more difficult to locate studies of a more general nature, where these particular radioligands were included in diagnostic accuracy research but not named specifically in the parts of the electronic bibliographic record that are searchable and therefore would be missed.
See Appendix 2 for details of the sources and search strategies that we used. No language or date restrictions were applied to the electronic searches.
Searching other resources
We examined the reference lists of all relevant studies for additional studies. We also searched the Database of Abstracts of Reviews of Effects (DARE) via the Cochrane Library (www.cochranelibrary.com)), the National Institute for Health Research ‐ Health Technology Assessment Database (NIHR‐HTA) (via the Cochrane Library: www.cochranelibrary.com), the Aggressive Research Intelligence Facility (ARIF) database (www.arif.bham.ac.uk) for other related systematic diagnostic accuracy reviews, and the International Federation of Clinical Chemistry and Laboratory Medicine Committee for Evidence‐based Laboratory Medicine database (C‐EBLM) (http://www.ifcc.org/ifcc‐education‐division/emd‐committees/c‐eblm/evidence‐based‐laboratory‐medicine‐c‐eblm‐base).
We checked the reference lists of any relevant studies and SRs, and performed citation tracking using the Science Citation Index to identify any additional relevant studies.
Data collection and analysis
Selection of studies
Two review authors (GM, RV) independently screened the retrieved titles and abstracts for potentially eligible studies. A third review author (PF) resolved any disagreements between the two review authors. The two review authors (GM, RV) then independently assessed the full‐text articles of the selected studies with the inclusion criteria. They resolved any disagreements through discussion or, where necessary, consulted a third review author (PF) who acted as an arbitrator. When a study did not present all relevant data for creating 2 × 2 table, we contacted the study authors directly to request further information. When more than one article presented data on the same population, we included the primary article, which was the article with the largest number of people or with the most informative data (e.g. longest time of follow‐up in the primary outcome).
Data extraction and management
We planned to extract the following data regarding the study characteristics.
-
Bibliographic details of primary paper:
author, title of study, year, and journal.
-
Basic clinical and demographic details:
number of participants;
clinical diagnosis;
MCI clinical criteria;
age;
gender;
sources of referral;
participant recruitment;
sampling procedures.
-
Details of the index test:
method of the 18F‐florbetaben administration, including those who administered the test;
thresholds used to define positive and negative tests;
other technical aspects as seemed relevant to the review, e.g. brain areas.
-
Details of the reference standard:
definition of ADD and other dementias used in the reference standard;
duration of follow‐up from time of the index test performed to defining ADD and other dementias by the reference standard: one year to less than two years; two years to less than four years; and four years or more. If participants had been followed for varied amounts of time, we recorded a mean follow‐up period for each included study. If possible, we grouped those data into minimum, maximum, and median follow‐up periods, to enable subgroup analyses;
prevalence or proportion of population developing ADD and other dementias, with severity, if described.
We created 2 × 2 tables (cross‐relating index test results of the reference standards) as shown in Appendix 3. For the included study, we recorded the number of participants lost to follow‐up. We also extracted data necessary for the quality assessment, as defined below. Two review authors (GM, RV) independently performed data extraction. We resolved any disagreements regarding data extraction by discussion, or by consulting a third review author (PF), if it was necessary.
Assessment of methodological quality
We assessed the methodological quality of the included studies using the Quality Assessment of Diagnostic Accuracy Studies 2 tool (QUADAS‐2) (Whiting 2011), as recommended by Cochrane (Davis 2013). This tool is comprised of four domains: patient selection, index test, reference standard, and patient flow. Two review authors (GM, RV), who were blinded to each other’s scores, independently performed the QUADAS‐2 assessment. We resolved any disagreements by discussion or, if necessary, consulted a third review author (PF) who acted as an arbitrator. We assessed each domain in terms of risk of bias, and also considered the first three domains in terms of applicability concerns. In Appendix 4, we have detailed the components of each of these domains and provided a rubric that shows how we made judgements concerning risk of bias. Key areas important to quality assessment were participant selection, blinding, and missing data.
We included three additional signalling questions on our checklist.
Was the PET scan interpretation done by a trained reader physician? (We included this under the ’Index test’ domain.)
Was there a clear definition of a positive result? (We included this under the ’Index test’ domain.)
Was the study free of commercial funding? (We included this under the ’flow and timing’ domain.)
We included the item pertaining to the PET scan interpretation and the definition of positive results to take into account the subjective nature of the 18F‐florbetaben Aβ scan image interpretation, which may be based on a variety of different criteria, such as extensive clinical experience, different standardised uptake values (SUV), different morphological features, or a combination of the aforementioned. We included the third additional item in order to record any potential bias resulting from commercial interest in the results due to the potential risk by the manufacturing company leading to more favourable results and conclusions than sponsorship by other sources (Lundh 2017).
We did not use QUADAS‐2 data to form a summary quality score. We produced a narrative summary that described each included study as at high, low, or unclear risk of bias, as well as concerns regarding applicability, which we have described in Appendix 5.
Statistical analysis and data synthesis
We applied the DTA framework for the analysis of a single test and extracted the data from the study into a 2 x 2 table, showing the binary test results cross‐classified with the binary reference standard. We used data from the 2 x 2 tables abstracted from the included study: true positive (TP), false negative (FN), false positive (FP), true negative (TN), and entered these into Review Manager (RevMan) Review Manager 2014 to calculate the sensitivities, specificities, and their 95% confidence intervals. We also presented the study results graphically by plotting estimates of sensitivities and specificities in a forest plot.
However, due to lack of data, we conducted no meta‐analyses. However, we prepared a 'summary of findings table'.
Investigations of heterogeneity
We were able to include only one study, therefore issues of heterogeneity did not arise.
Sensitivity analyses
We found insufficient data to conduct any sensitivity analyses.
Assessment of reporting bias
We did not investigate reporting bias.
Results
Results of the search
The total number of records identified through all databases for this SR was 1382. The PRISMA diagram shows the selection of records through the screening and selection processes (Figure 1). In total, we assessed 79 studies (42 full text papers, 16 conference publications, 9 registered studies in clinicaltrials.gov, and 12 registered in WHO ICTRP) for eligibility in the full‐text screening. We included one study (Ong 2015). Additionally, five references were identified as ongoing studies (EUCTR2013‐004671‐12‐BE; EUCTR2014‐000562‐21‐NL; EUCTR2014‐004244‐35‐IT; NCT01222351, NCT02854033). We excluded 73 studies: 19 studies were multiple publications or duplicated, and the remaining 53 studies were excluded as they did not meet the inclusion criteria: i) not a longitudinal study (n = 13); ii) not MCI participants at baseline (n = 14); iii) index test not a 18F‐florbetaben PET scan (n = 5); iv) discussion or review paper (n = 19); v) wrong outcome or study design (n = 2). One study did not have data suitable for analysis (Characteristics of excluded studies).
1.
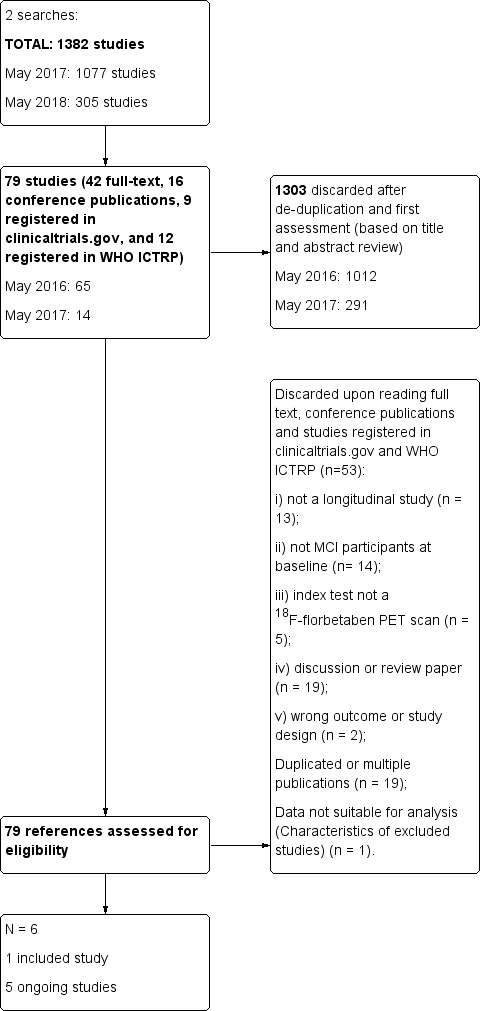
Flow diagram.
Included Study
See Characteristics of included studies.
The study of Ong 2015 was conducted in Australia (Ong 2015). This study included older adult participants who were referred from local memory clinics and who met consensus criteria for MCI at baseline, and were recruited as part of a study to evaluate the 18F‐florbetaben PET positivity scan at baseline and progression from MCI to ADD, and compare an SUVR assessment with visually assessed scans in determining a positive or negative scan. The other objective of this study was to examine whether progressive Aβ accumulation was detectable using the 18F‐florbetaben PET scan at follow‐up.
Ong 2015 included 45 MCI participants and performed follow‐up at two and four years, evaluating progression from MCI to probable ADD. The authors described their 18F‐florbetaben status as positive or negative, using a visual assessment by five readers trained on an electronic training tool and their SUVR > 1.45 as described previously (Ong 2015). We included the data at four years of follow‐up, because, according to the methodology, we included the longest time of follow‐up in the primary outcome. MCI participants fulfilled the Petersen 2004 and Winblad 2004 criteria for MCI. Participants had to be at least 60 years old and to have had at least seven years of formal schooling. They were also required to communicate fluently in English, to have no contraindications to undergoing an MRI scan, and to have a MMSE of > 23 points.
There were 21 participants with 18F‐florbetaben with an SUVR value < 1.45 and 24 participants with a 18F‐florbetaben SUVR value > 1.45; using the visual assessment, there were 20 participants with 18F‐florbetaben negative and 25 participants with 18F‐florbetaben positive. The demographic data provided was based on those classified as positive or negative by SUVR. The age of the participants was 71.8 + 6.1 and 73.5 + 6.9 years, years of education 13.5 + 3.0, and 13.8 + 4.2, and MMSE was 27.9 + 1.4 and 26.7 + 1.9 for those with 18F‐florbetaben < 1.45 or > 1.45, respectively. No demographic data was available for those classified by visual assessment. Of the 45 participants classified with SUVR, at four years follow‐up, 21 of 45 participants (46.7%) had developed ADD, and 5 of 45 (11.1%) had developed another form of dementia. Of the 45 participants classified with visual assessment, at four years follow‐up, 21 of 45 participants (46.7%) had developed ADD, and 5 of 45 (11.1%) had developed another form of dementia. At four years follow‐up, the diagnosis was performed by a neurologist with access to all study results and personal medical records. The reference standard was the NINCDS‐ADRDA criteria for ADD (McKhann 1984) and, for other forms of dementia, the reference standards were McKeith 1996 for Lewy body dementia, Lund criteria for frontotemporal dementia Neary 1998, and Hauw 1994 for PSP.
Potential conflicts of interest were noted. Financial support for the study was provided by the previous and current manufacturer of the test; three authors were employees from the previous manufacturer of 18F‐florbetaben tracer and another three authors were employees from the actual manufacturer of 18F‐florbetaben (Ong 2015).
Ongoing studies
Two studies were found as ongoing studies in clinicaltrials.gov. The first study, NCT01222351, included a sub‐study of the Washington Heights‐Inwood Community Aging Project focused on cognitively normal older adults, older adults with MCI, and older adults with Alzheimer's disease. Participants were selected on the basis of change in plasma amyloid beta levels over prior assessment intervals. The purpose of the study was to examine whether brain amyloid plaque load, which was to be measured with 18F‐florbetaben PET scan, varied as a function of change in plasma levels of amyloid beta and the risk and progression of late onset Alzheimer's disease, MCI, and cognitive decline after three years follow‐up. No further details were provided regarding index test and reference standard(s). This study has been recruiting participants since December 2010 in the United States. No expected date of publication was provided in this record. The second study, NCT02854033, was focused on cognitively normal, mild cognitive impairment, and mild ADD participants. The main objective was to determine the relationships among the clinical, cognitive, imaging, genetic, and biochemical biomarker characteristics and the evolution of the entire spectrum of ADD and try to identify diagnostic and prognostic markers among others. The clinical follow‐up will be five years. No further details were provided regarding the reference standard(s). This study has been recruiting participants since October 2016. No expected date of publication was provided in this record.
Three studies were found as ongoing studies in WHO ICTRP and they belong to the European Union Clinical Trials Register. EUCTR2014‐004244‐35‐IT is a study focused on amnestic MCI participants with long disease duration (range 2 to 10 years) and imaging studies (MRI and/or 18F‐FDG‐PET) suggestive of involvement of the limbic/mesial temporal lobe, with the main objective to define the value of the load of amyloid protein. The secondary outcome was the correlation of the amyloid load with neuropsychological measures, the clinical indices, the values of Aβ42, total tau, and phospho‐tau and with data from MRI and 18F‐FDG‐PET previously acquired for diagnostic purposes and with a clinical follow‐up for at least two years in order to assess the possible clinical progression with basal 18F‐florbetaben. No further details were provided regarding the participants, index test, and reference standard(s). This study has been ongoing since March 2015. No expected date of publication was provided in this record.
One additional Dutch ongoing study has been found that focuses on an unselected patient population of subjects visiting the memory clinic of the VUmc Alzheimer Center (EUCTR2014‐000562‐21‐NL). Its main objective was change after 18F‐florbetaben in diagnosis, change in level of confidence of diagnosis, and the impact on patient healthcare management, and the secondary outcome in those with MCI was the clinical progression to dementia during annual follow‐up (based on follow‐up visits to neurologist and neuropsychologist). No further details were provided regarding the participants, index test, and reference standard(s) and the length of follow‐up. This study has been ongoing since January 2015. Similarly, no expected date of publication was provided in this record.
One additional Belgian ongoing study has been found that focuses on the predictive value of baseline 18F‐florbetaben capture for longitudinal change in amyloid load measured using PET in MCI cases (EUCTR2013‐004671‐12‐BE). A secondary outcome was the comparison of CSF Aß42 and amyloid PET for classification of amyloid‐positive and amyloid‐negative cases, and the comparison of the predictive value of CSF biomarkers Aß42, T‐tau and P‐tau181P with that of amyloid imaging for MCI cases that progressed to Alzheimer's disease dementia. No further details were provided regarding the index test, and reference standard(s). This study has been ongoing since June 2014 and no expected date of publication has been provided.
Methodological quality of included studies
We assessed methodological quality using the QUADAS‐2 tool (Whiting 2011). Review authors’ judgements about each methodological quality item for the included study are presented in the Characteristics of included studies and in Figure 2.
2.
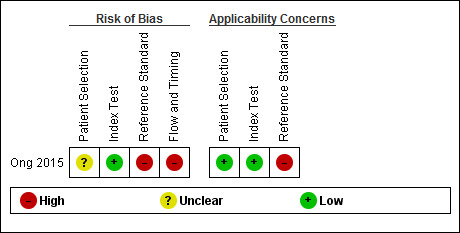
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
In the patient selection domain, we considered the study of Ong 2015 to be at unclear risk of bias due to lack of reporting on sampling procedures and exclusion criteria (Ong 2015). We stated that the included study avoided a case‐control design because we only considered data on performance of the index test to discriminate between people with MCI who converted to dementia and those who remained stable.
In the index test domain, we considered the study of Ong 2015 at low risk of bias because the positive threshold used in both visual and quantitative assessment was prespecified (Ong 2015). Moreover, the index test results were interpreted without knowledge of the results of the reference standard. In our two additional signalling questions, the risk concerning the index test being interpreted by a trained reader physician was low, because they were trained on an electronic training tool to do the amyloid PET visual interpretation. The other signalling question was rated as low risk, because there was a clear definition of a positive result.
In the reference standard domain, we considered the study to be at high risk of bias because it was reported that the neurologist had access to all study results and personal medical records to make the diagnosis (Ong 2015). However, the reference standard(s) were clearly established (McKhann 1984, McKeith 1996Neary 1998; Hauw 1994).
In the flow and timing domain, we judged the study to be at high risk of bias because in our additional signalling question there were potential conflicts of interest due to the financial support for the study and, in addition, three authors were employees of the previous manufacturer of 18F‐florbetaben tracer and the other three authors were employees of the actual manufacturer of 18F‐florbetaben (Ong 2015). However, the four years of interval between the index test and the reference standard was considered an appropriate interval, all participants received the same reference standard(s), and all 45 participants were accounted for in the analysis.
For assessment of applicability, there was no concern that the included patients and setting, and the conduct and interpretation of the index test, did not match the review question (Ong 2015). However, the target condition (as defined by the reference standard) was of high concern due to the fact that diagnosis was made with full access to study results and medical records at four years follow‐up.
Findings
The key characteristics of the study are summarised in Characteristics of included studies. The summary of main results for the only included study is presented in the 'Summary of findings' table (Table 1).
Ong 2015 included data on 45 participants with MCI diagnosed with Petersen criteria (Petersen 2004), and Winblad 2004. The study used two different assessments to evaluate the PET: visual assessment PET positive if increased tracer uptake was visible in any of four different cerebral regions (frontal, parietal, temporal, and posterior cingulate/precuneus cortices) and quantitative assessment with a SUVR > 1.45 for a positive 18F‐florbetaben. At four years follow‐up, the diagnosis of ADD was made using NINCDS‐ADRDA criteria (McKhann 1984). Lewy Body dementia was made using McKeith 1996 criteria, FTD diagnosis was made using Lund criteria (Neary 1998) and PSP diagnosis was made using (Hauw 1994) criteria.
18F‐florbetaben for Alzheimer’s disease dementia (ADD)
Visual Assessment: 18F‐florbetaben PET scan had a sensitivity of 100% (95% CI 84% to 100%) and a specificity of 83% (95% CI 63% to 95%) to predict the progression from MCI to ADD at four years follow‐up. Of 45 participants who were given an initial clinical diagnosis of MCI, 21 were true positive, 4 were false positives, 0 were false negative, and 20 were true negative (Figure 3).
3.

Forest plot of tests: 1 18F‐florbetaben visual assessment and progression to ADD, 2 18F‐florbetaben SUVR and progression to ADD, 3 18F‐florbetaben visual assessment and progression to any other form of non‐ADD, 4 18F‐florbetaben SUVR and progression to any other form of non‐ADD, 5 18F‐florbetaben visual assessment and progression to any form of dementia, 6 18F‐florbetaben SUVR and progression to any form of dementia.
SUVR:18F‐florbetaben PET scan had a sensitivity of 100% (95% CI 84% to 100%) and a specificity of 88% (95% CI 68% to 97%) to predict the progression from MCI to ADD at four years follow‐up. Of 45 participants who were given an initial clinical diagnosis of MCI, 21 were true positive, 3 were false positives, 0 were false negative, and 21 were true negative (Figure 3).
18F‐florbetaben for any other form of dementia (non‐ADD)
Visual Assessment: 18F‐florbetaben PET scan had a sensitivity of 0.0% (95% CI 0.0% to 52%) and a specificity of 38% (95% CI 23% to 54%) to predict the progression from MCI to any other form of dementia (non‐ADD) at four years follow‐up. Of 45 participants who were given an initial clinical diagnosis of MCI, 0 were true positive; 25 were false positives, 5 were false negative (3 FTD, 1 Lewy body dementia, and 1 PSP), and 15 were true negative (Figure 3).
SUVR:18F‐florbetaben PET scan had a sensitivity of 0.0% (95% CI 0.0% to 52%) and a specificity of 40% (95% CI 25% to 57%) to predict the progression from MCI to any other form of dementia (non‐ADD) at four years follow‐up. Of 45 participants who were given an initial clinical diagnosis of MCI, 0 were true positive, 24 were false positives, 5 were false negative (3 FTD, 1 Lewy body dementia and 1 PSP), and 16 were true negative (Figure 3).
18F‐florbetaben for any form of dementia
Visual Assessment: 18F‐florbetaben PET scan had a sensitivity of 81% (95% CI 61% to 93%) and a specificity of 79% (95% CI 54% to 94%) to predict the progression from MCI to any form of dementia at four years follow‐up. Of 45 participants who were given an initial clinical diagnosis of MCI; 21 were true positive, 4 were false positives, 5 were false negative (3 FTD, 1 Lewy body dementia, and 1 PSP) and 15 were true negative (Figure 3).
SUVR:18F‐florbetaben PET scan had a sensitivity of 81% (95% CI 61% to 93%) and a specificity of 84% (95% CI 60% to 97%) to predict the progression from MCI to any form of dementia at four years follow‐up. Of 45 participants who were given an initial clinical diagnosis of MCI, 21 were true positive, 3 were false positives, 5 were false negative (3 FTD, 1 Lewy body dementia, and 1 PSP), and 16 were true negative (Figure 3).
Investigation of heterogeneity
We were able to include only one study, therefore, issues of heterogeneity did not arise.
Sensitivity analyses
There were insufficient data to permit any sensitivity analyses.
Discussion
Summary of main results
The volume and quality of evidence regarding the DTA of 18F‐florbetaben for early diagnosis of ADD and other dementias in people with MCI is very limited. We identified only one study in this systematic review and for that reason we were not able to conduct a meta‐analysis, sensitivity analysis, or heterogeneity analyses (Ong 2015). The results are summarised in a 'Summary of findings' table (Table 1). The study was evaluated as at high risk of bias, mainly due to the potential conflicts of interest (financial support of the study and also because three authors were employees of the previous company who manufactured the 18F‐florbetaben tracer and three authors were employees of the current company that manufactures the 18F‐florbetaben tracer (Characteristics of included studies)).
Regarding our objectives: to determine the DTA of the 18F‐florbetaben PET scan for detecting people with MCI at baseline who will clinically progress to ADD, or to other forms of dementia or any form of dementia at follow‐up, the results were the following:
18F‐florbetaben PET scan for Alzheimer’s disease dementia (ADD)
Progression from MCI to ADD analysed by visual assessment: sensitivity of 100% (95% CI 84% to 100%) and a specificity of 83% (95% CI 63% to 95%).
Progression from MCI to ADD analysed by SUVR > 1.45: sensitivity of 100% (95% CI 84% to 100%) and a specificity of 88% (95% CI 68% to 97%).
18F‐florbetaben has a close to perfect sensitivity and a good specificity for predicting progression to ADD through visual assessment evaluation or SUVR (Ong 2015). However, a positive 18F‐florbetaben PET scan for Aβ, has been found in other neurological conditions clinically diagnosed, and it was positive in vascular dementia, frontotemporal dementia and dementia with Lewy bodies (Villemagne 2011). Nevertheless, in one study with 12 cases with non‐ADD at autopsy, the 18F‐florbetaben PET scan was negative in all of them (Sabbagh 2017). On the other hand, in other amyloid biomarkers like PET PiB, the false positive rate could be explained because it has affinity to amyloid in vessel walls, in particular, to cerebral amyloid angiopathy (CAA) (Zhang 2014). We would think that the pathological diagnosis of some patients with clinically probable ADD may be vascular dementia secondary to CAA and some people with MCI may be have vascular MCI due to CAA.
As other amyloid tracers, 18F‐florbetaben has probed the detection of amyloid plaques that are composed of insoluble Aβ peptides (EMA 2014, FDA 2014), however, the soluble Aβ oligomers have been playing a central role in Alzheimer's pathogenesis in the amyloid hypothesis (Heyden 2013), with the possibility of producing false negatives. In addition, amyloid tracers do not bind to the other histopathologic core of Alzheimer's disease, the neurofibrillary tangles (NFTs). There is evidence that indicates that plaques and tangles independently contribute to cognitive impairment over the clinical course of Alzheimer's disease (Serrano‐Pozo 2013). Moreover, in another cohort study, the NFT formation might be either unrelated to amyloid plaques formation or a temporally distinct process, or both(Royall 2014). The latest could explain why, in 44 participants with ADD and positive Aβ histopathology, one had a negative 18F‐florbetaben PET scan (Sabri 2015).
Another important factor to be considered in predicting the progression to ADD is the duration of follow‐up, because the reported progression rate of MCI to ADD is between 8% and 16% per year (Mitchell 2009). Therefore, a high percentage of people with baseline MCI would progress to Alzheimer’s disease dementia if we could include a longer follow‐up period, which would consequently affect the predictive accuracy of the 18F‐florbetaben PET scan. However the progression rate at two years was 44% (including two participants who at four years follow‐up reverted to MCI or converted to another form of dementia (non‐ADD)) and at four years was 47%. This is more than normal and probably can be explained by the setting of recruitment or demographic or MCI characteristics and maybe other underlying factors that can increase the progression rate. In addition, in one systematic review regarding the progression from MCI to ADD with PiB PETp‐u, a correlation between longer follow‐up and higher specificities was found (Ma 2014). However, due to the lack of data, we were not able to investigate the effect of the follow‐up on the progression rate from MCI to ADD, or any form of dementia.
On the other hand, MCI subtypes have been related to progression to dementia. In a large longitudinal study with 550 MCI participants, evidence were found that the MCI subtype, presence of storage memory impairment, multiple domain condition, and presence of APOE ϵ4 allele increased the risk of progression to dementia. Multivariate survival and Kaplan‐Meier analyses showed that amnestic MCI with storage memory impairment had the most and closest risk of progression to dementia (Espinosa 2013). Specifically, in our systematic review, the study of Ong 2015 (Ong 2015), included amnestic and non‐amnestic MCI, and after adjusting for both 18F‐florbetaben PET scan positive status and hippocampal atrophy, the hazard ratio for the development of ADD from amnestic MCI was not significant. Additionally, some other risk factors like family history of dementia, APOE ϵ4 allele presence, and Aβ and tau protein levels in cerebrospinal fluid may contribute to a faster progression rate to dementia. In conclusion, further updated systematic reviews should include high quality research with more detailed data about the characteristics of MCI that are required to not only explore the underlying mechanisms but also to elucidate the causal pathways that link 18F‐florbetaben PET scan positivity of diverse MCI subtypes and disease progression.
18F‐florbetaben PET scan for any other forms of dementia (non‐Alzheimer’s disease dementia (non‐ADD))
Progression to any other form of non‐ADD analysed by visual assessment: sensitivity of 0.0% (95% CI 0.0% to 52%) and a specificity of 38% (95% CI 23% to 54%).
Progression to any other form of non‐ADD analysed by SUVR > 1.45: sensitivity of 0.0% (95% CI 0.0% to 52%) and a specificity of 40% (95% CI 25% to 57%).
The study reported only 5 people converting to non‐ADD at four years follow‐up: frontotemporal dementia (3), Lewy body dementia (1) and progressive supranuclear palsy (1); all of them were 18F‐florbetaben negative (Ong 2015).
18F‐florbetaben PET scan cortical binding has been observed in non‐ADD; 9% (1/11) of FTLD, 25% (1/4) of VaD, 29% (2/7) of DLB in a study from Australia (Villemagne 2011) and in 11% (3/27) in those with confirmed non‐Alzheimer's disease neurodegenerative pathologies at autopsy (Sabri 2015). However, in this study none of the five converters to non‐ADD at four years follow‐up were 18F‐florbetaben positive; the latest could explain the sensitivity of 0% and specificity of 38% with visual assessment in the included study. Nevertheless, according to histopathological studies, we would expect that studies with more participants, or participants with positive PET scans that progress to non‐ADD, could potentially increase the DTA to predict progression to other forms of dementia and decrease the DTA to predict the progression to ADD.
The latest data suggested the test was insufficient to evaluate the early diagnostic value for progression from MCI to any form of non‐ADD.
18F‐florbetaben PET scan for any form of dementia
Progression to any form of dementia analysed by visual assessment: sensitivity of 81% (95% CI 61% to 93%) and a specificity of 79% (95% CI 54% to 94%).
Progression to any form of dementia analysed by SUVR > 1.45: sensitivity of 81% (95% CI 61% to 93%) and a specificity of 84% (95% CI 60% to 97%).
Ong 2015 reported lower sensitivity and specificity for prediction of any form of dementia other than ADD (Ong 2015). This is explained because the test has a close to perfect sensitivity and good specificity to predict the progression to ADD and if we add the data with those that are PET negative with other types of non‐ADD, the sensitivity decreased to 81% and the specificity to 79%.
According to the aforementioned, in the cases of 18F‐florbetaben PET scans for any other forms of dementia, there are cases that are PET positive with other neurological conditions in the literature, but not in this study; and this paucity of data with this type of participant, could be explained due to the small sample of participants of this study. For that reason, we would expect that in other studies where we would check the progression to any form of dementia, the DTA would be higher because it is probable to find participants with DBL, FTD and others with a PET positive result, and also we would expect an decrease in the DTA to predict the progression to ADD.
Strengths and weaknesses of the review
We conducted an extensive, comprehensive, and sensitive literature search using 11 different electronic databases without any limitation to language or publication status. However, we only identified one study with 45 eligible participants, therefore our DTA estimates are relatively imprecise. This paucity of evidence reflects the very significant challenges inherent in conducting long term prospective studies of well characterised participants, followed up to the point of progression of a clinical dementia. The methodological quality assessment and data syntheses were based on recommended methods. To increase the reliability of our findings, we included only studies that fulfilled delayed verification of progression from MCI to ADD or any other form of dementia (non‐ADD) or any form of dementia at follow‐up.
The included study did have significant methodological limitations that weakened confidence in the findings of the review. The study lacked information about the selection of the participants, the reference standard was made with knowledge of the medical studies and medical records, and the major problem was a potential conflict of interest due to the relationship with the companies who produced and produce the tracer. On the other hand, considerable uncertainty remained concerning the clinical diagnosis of ADD; the histopathological diagnosis would be the better way to probe the diagnosis, however, this option is not realistic for a clinical trial.
Applicability of findings to the review question
Regarding the question of this review:
Could the 18F‐florbetaben PET scan identify those people with MCI who would progress to clinical dementia at follow‐up?. There was no applicability concern that the included patients, the setting, the conduct, and interpretation of the index test in the included study did not match the review question. However, there was a high applicability concern about the target condition (as defined by the reference standard) because the diagnosis at follow‐up was made with access to the study tests and medical records for all participants and, therefore, due to the one study included, it was difficult to extend the findings into clinical practice without a meta‐analysis.
The diagnostic utility of 18F‐florbetaben PET scan for identifying Alzheimer’s disease pathology and identifying those people with MCI who would convert to ADD could be affected by a number of factors that have not been determined so far. The most important was the lack of a large study to evaluate this question, as we included one study that addressed the question with only 45 participants at follow‐up. Conducting a 18F‐florbetaben test is expensive, therefore it is important to clearly demonstrate its accuracy prior to recommending its adoption in clinical practice.
Authors' conclusions
Implications for practice.
Today, the use of 18F‐florbetaben is not indicated in people with MCI (FDA and EMA) except in clinical trials and research studies. However, the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association have proposed the usage of amyloid PET in people with persistent or progressive unexplained MCI (Johnson 2013). The DTA of 18F‐florbetaben PET scans, as determined in this SR, suggests a limited use due to a lack of information based only on one study with 45 participants to predict the progression from MCI to ADD and any form of dementia. Despite this, in the sole study, the sensitivity was 100% and the specificity was 83% for visual assessment analysis to predict the progression to ADD and the sensitivity was 81% and the sensitivity was 79% to predict the progression to any form of dementia. The prediction to other forms of dementia (non‐ADD) was poor, however, this could be explained because the pathology of the other neurodegenerative conditions is not based on the Aβ plaques. Finally, we have to consider the risk of bias due to the access to medical tests and medical records by the neurologist who made the diagnosis at four years follow‐up, because this could overestimate the DTA of 18F‐florbetaben (Lijmer 1999). Due to the aforementioned and the methodological limitations of the included study, it is not possible to recommend the routine use of 18F‐florbetaben in clinical practice. The 18F‐florbetaben biomarker is expensive, therefore, it is important to clearly demonstrate its DTA and to standardise the process for the diagnostic modality prior to it being widely used.
Implications for research.
The FDA and EMA had established the 18F‐florbetaben positivity criteria in order that the use in ADD patients' evaluation and use in people with MCI is accepted in research settings and clinical trials (Albert 2011). On the other hand, it has been proposed for use in clinical practice by the Nuclear Medicine Society and the Alzheimer's Association (Johnson 2013).
The interpretation of the results of the 18F‐florbetaben PET scan studies could be difficult due to the use of different methods to define the result of the test. It is still used in many studies with different SUVR, visual assessment or both, and this promotes different accuracies for the tracer, even in people with ADD when they are compared with healthy people without ADD. Therefore, it is necessary to consider that visual assessment is the most important option to interpret the 18F‐florbetaben PET scan, because this is the approach to the interpretation established by FDA and EMA (FDA 2014, EMA 2014).
Moreover, clinical assessment in people with memory complaints is not always undertaken with only one test, as clinical assessment could use different tests, like volumetric hippocampal MRI, FDG‐PET, SPECT, CSF, and others. This makes sense because neurodegenerative diseases are complex disorders with occasionally multiple and overlapping pathophysiological processes, and multitracer imaging may be helpful in combining metabolic, inflammation, or apoptosis markers with those labelling typical protein aggregations seen in the progression of MCI to Alzheimer’s disease dementia. In future, various PET imaging modalities are needed to evaluate the usefulness of the various PET tracers as predictors of progression to Alzheimer’s disease dementia in MCI studies with clinical follow‐up. There is a hypothesis that amyloid deposition is an early event in Alzheimer’s disease that reaches a relative plateau even at the MCI stage, while downstream biomarkers measure neuronal loss and dysfunction, and cognitive measures are more dynamic at the symptomatic disease stage (Jack 2010). Based on this hypothesis, the combination of structural imaging, functional imaging, and cognitive tests may be better predictors of when an individual will convert. However, in this way, the Ong 2015 study showed that a 18F‐florbetaben PET positive result predicted progression after adjusting for both aMCI status and hippocampal atrophy evaluated with sMRI (Ong 2015). Nevertheless, there is a lack of studies with 18F‐florbetaben combined with other tests.
Additionally, if we consider the hierarchical evidence needed for the level of efficacy of diagnostic imaging tests, we are currently in the second step of six according to Herscovitch (Herscovitch 2015): technical efficacy, diagnostic accuracy efficacy, diagnostic thinking efficacy, therapeutic impact, patient health outcomes, and, finally, societal efficacy. Therefore, we need further research about accuracy before progressing to the other steps with their specific studies before we can incorporate the 18F‐florbetaben PET scan into clinical practice.
Acknowledgements
Gabriel Martínez is a PhD candidate in the Methodology of Biomedical Research and Public Health at the Department of Paediatrics, Obstetrics and Gynaecology and Preventive Medicine, Universitat Autònoma de Barcelona, Barcelona, Spain.
We are grateful to the authors of the included and excluded studies who responded to our requests for additional information.
We thank the strong support of the Cochrane Dementia and Cognitive Improvememnt Group (CDCIG), especially Sue Marcus in finalizing the review.
We thank Anna Noel‐Storr, Information Specialist of the CDCIG, for her assistance with the design of the search strategy.
We thank Gerard Urrútia and Marta Roqué i Figuls for their contribution in the preparation of the protocol for the review (Martínez 2016)
We thank the peer reviewers for their many helpful suggestions.
Appendices
Appendix 1. Glossary
Aetiology: the cause, set of causes, or manner of causation of a disease or condition.
Amyloid beta (Aβ): an amyloid that is derived from a larger precursor protein and is the primary component of plaques characteristic of Alzheimer's disease.
Biomarker: measurable and quantifiable biological parameters (e.g., specific enzyme concentration, specific hormone concentration, presence of biological substances) which serve as indices for health‐ and physiology‐related assessments, such as disease risk, psychiatric disorders, environmental exposure and its effects, disease diagnosis; metabolic processes; etc.
Bolus: a single dose of a drug or other medicinal preparation given all at once.
Cingulate cortex: one of the convolutions on the medial surface of the cerebral hemispheres.
Cortical: the thin layer of grey matter on the surface of the cerebral hemispheres. It reaches its highest development in humans and is responsible for intellectual faculties and higher mental functions.
Epiphenomenon: A secondary effect or by‐product. A secondary symptom or pathology, occurring simultaneously with a disease or condition but not directly related to it.
Frontotemporal: relating to the frontal and the temporal cerebral lobes.
Histopathology: the study of changes in tissues caused by disease.
Hypothyroidism: a syndrome that results from abnormally low secretion of thyroid hormones from the thyroid gland.
Index test: the test under evaluation.
In vivo: (of processes) performed or taking place in a living organism.
Ligand: a molecule that binds to another molecule, used especially to refer to a small molecule that binds specifically to a larger molecule, e.g., an antigen binding to an antibody, a hormone or neurotransmitter binding to a receptor, or a substrate or allosteric effector binding to an enzyme.
Neuritic plaques: accumulations of extracellularly deposited amyloid fibrils within tissues. Is one of the hallmarks of Alzheimer's disease.
Neurofibrillary tangles: abnormal structures located in various parts of the brain and composed of dense arrays of paired helical filaments (neurofilaments and microtubules). Are aggregates of hyperphosphorylated tau protein that are most commonly known as a primary marker of Alzheimer's disease.
Parietal lobe: upper central part of the cerebral hemisphere. It is located anterior to the occipital lobe, and superior to the temporal lobes.
Positron: an extremely small piece of matter with a positive electrical charge, having the same mass as an electron.
Precuneus: is a part of the parietal lobe of the brain, lying on the medial surface of the cerebral hemisphere.
Prodromal: Relating to prodrome; indicating an early stage of a disease.
Radionuclide (sometimes called a radioisotope or isotope): is a chemical which emits a type of radioactivity called gamma rays. The radioactivity can be detected by special scanners.
Reference standard: the best available method for establishing the presence or absence of the target condition.
Sensitivity: a measure of a test’s ability to correctly detect people with the disease. It is the proportion of diseased cases that are correctly identified by the test. It is calculated as follows: Sensitivity = Number with disease who have a positive test/Number with disease.
Specificity: a measure of a test’s ability to correctly identify people who do not have the disease. It is the proportion of people without the target disease who are correctly identified by the test. It is calculated as follows: Specificity = Number without disease who have a negative test/Number without disease.
Stilbene: organic compounds that contain 1,2‐diphenylethylene as a functional group.
Target condition: the disease or condition that the index test is expected to detect.
Temporal lobe: lower lateral part of the cerebral hemisphere responsible for auditory, olfactory, and semantic processing. It is located inferior to the lateral fissure and anterior to the occipital lobe.
Vascular: relating to, affecting, or consisting of a vessel or vessels, especially those which carry blood.
Appendix 2. Search strategy for 18F‐florbetaben PET ligand
| Source | Search strategy |
| MEDLINE In‐process and other non‐indexed citations and Medline® 1946 to May 2017 (Ovid SP) | 1. Florbetaben.ti,ab,nm. 2. (NEURACEQ or neuraceq*).ti,ab,nm. 3. "florbetaben‐fluorine‐18".ti,ab,nm. 4. "18F‐BAY94‐9172".ti,ab,nm. 5. "[18F]Florbetaben".ti,ab,nm. 6. "florbetaben‐PET".ti,ab,nm. 7. or/1‐6 8. Fluorine Radioisotopes/du 9. Aniline Compounds/du 10. Ethylene Glycols/du 11. Stilbenes/du 12. Radioligand Assay/ 13. radioligand*.ti,ab. 14. or/8‐13 15. Alzheimer Disease/ri [Radionuclide Imaging] 16. Plaque, Amyloid/ri [Radionuclide Imaging] 17. or/15‐16 18. 14 and 17 19. 7 or 18 |
| Embase 1974 to May 2017 (Ovid SP) | 1. Florbetaben.ti,ab. 2. (NEURACEQ or neuraceq*).ti,ab. 3. "florbetaben‐fluorine‐18".ti,ab. 4. "18F‐BAY94‐9172".ti,ab. 5. "[18F]Florbetaben".ti,ab. 6. "florbetaben‐PET".ti,ab. 7. exp florbetaben f 18/ 8. or/1‐7 9. exp *radioligand/ 10. Alzheimer disease/ 11. Alzheimer*.ti,ab. 12. amyloid plaque/di [Diagnosis] 13. mild cognitive impairment/ 14. or/10‐13 15. 9 and 14 16. 8 or 15 |
| PsycINFO 1806 to May 2017 (Ovid SP) | 1. Florbetaben.ti,ab. 2. (NEURACEQ or neuraceq*).ti,ab. 3. "florbetaben‐fluorine‐18".ti,ab. 4. "18F‐BAY94‐9172".ti,ab. 5. "[18F]Florbetaben".ti,ab. 6. "florbetaben‐PET".ti,ab. 7. or/1‐6 |
| BIOSIS Citation Index (Thomson Reuters Web of Science) (1922 to May 2017) | Topic=(Florbetaben OR NEURACEQ OR neuraceq* OR "florbetaben‐fluorine‐18" OR "18F‐BAY94‐9172" OR "[18F]Florbetaben" OR "florbetaben‐PET") Timespan=All years. Databases=BCI |
| Web of Science Core Collection, including the Science Citation Index and the Conference Proceedings Citation Index (Thomson Reuters Web of Science) (1946 to May 2017) |
Topic=(Florbetaben OR NEURACEQ OR neuraceq* OR "florbetaben‐fluorine‐18" OR "18F‐BAY94‐9172" OR "[18F]Florbetaben" OR "florbetaben‐PET") Timespan=All years. Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, CCR‐EXPANDED, IC. |
| LILACS (BIREME) | Florbetaben OR NEURACEQ OR neuraceq* OR "florbetaben‐fluorine‐18" OR "18F‐BAY94‐9172" OR "[18F]Florbetaben" OR "florbetaben‐PET" [Words] |
| CINAHL (EBSCOhost) (1980 to May 2017) | S1 TX Florbetaben S2 TX NEURACEQ S3 TX neuraceq* S4 TX "florbetaben‐fluorine‐18" S5 TX "18F‐BAY94‐9172" S6 TX "[18F]Florbetaben" S7 TX "florbetaben‐PET" S8 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 |
| ClinicalTrials.gov (www.clinicaltrials.gov) | Florbetaben OR NEURACEQ OR neuraceq* OR "florbetaben‐fluorine‐18" OR "18F‐BAY94‐9172" OR "[18F]Florbetaben" OR "florbetaben‐PET" |
| World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (http://apps.who.int/trialsearch) | Florbetaben OR NEURACEQ OR neuraceq OR "florbetaben‐fluorine‐18" OR "18F‐BAY94‐9172" OR "[18F]Florbetaben" OR "florbetaben‐PET" |
| ALOIS, the Cochrane Dementia & Cognitive Improvement Group’s specialized register of dementia studies (http://www.medicine.ox.ac.uk/alois/) | Imaging AND PET |
Appendix 3. Tables (2 × 2) cross‐relating index test results of the reference standards
Table 1. Progression from mild cognitive impairment (MCI) to Alzheimer’s disease dementia (ADD)
| Index test information | References standard information | |
| ADD present | ADD absent | |
| Index test‐positive | 18F‐florbetaben PET ligand for Aβ (+) who progress to ADD (TP) | 18F‐florbetaben PET ligand for Aβ (+) who remain MCI (FP) and 18F‐florbetaben PET ligand Aβ (+) who progress to non‐ADD (FP) |
| Index test‐negative | 18F‐florbetaben PET ligand for Aβ (‐) who progress to ADD (FN) | 18F‐florbetaben PET ligand for Aβ (‐) who remain MCI (TN) and 18F‐florbetaben PET ligand for Aβ (‐) who progress to non‐ADD (TN) |
ADD: Alzheimer's disease dementia FN: false negative FP: false positive
MCI: mild cognitive impairment
PET: positron emission tomography TN: true negative TP: true positive
Table 2. Progression from mild cognitive impairment (MCI) to non‐Alzheimer’s disease dementia (non‐ADD)
| Index test information | References standard information | |
| Non‐ADD present | Non‐ADD absent | |
| Index test‐positive | 18F‐florbetaben PET ligand for Aβ (+) who progress to non‐ADD (TP) |
18F‐florbetaben PET ligand for Aβ (+) who remain MCI (FP) and 18F‐florbetaben PET ligand for Aβ (+) who progress to ADD (FP) |
| Index test‐negative | 18F‐florbetaben PET ligand for Aβ (‐) who progress to non‐ADD (FN) |
18F‐florbetaben PET ligand for Aβ (‐) who remain MCI (TN) and 18F‐florbetaben PET ligand for Aβ (‐) who progress to ADD (TN) |
ADD: Alzheimer's disease dementia FN: false negative FP: false positive MCI: mild cognitive impairment
PET: positron emission tomography TN: true negative TP: true positive
Table 3. Progression from mild cognitive impairment (MCI) to any form of dementia
| Index test information | References standard information | |
| Any forms of dementia present | Dementia absent | |
| Index test‐positive | 18F‐florbetaben PET ligand for Aβ (+) who progress to any form of dementia (TP) | 18F‐florbetaben PET ligand for Aβ (+) who remain MCI (FP) |
| Index test‐negative | 18F‐florbetaben PET ligand for Aβ (‐) who progress to any form of dementia (FN) | 18F‐florbetaben PET ligand for Aβ (‐) who remain MCI (TN) |
FN: false negative FP: false positive
MCI: mild cognitive impairment
PET: positron emission tomography TN: true negative TP: true positive
Appendix 4. Assessment of methodological quality table: Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS‐2) tool
| Domain | Patient selection | Index test | Reference standard | Flow and timing |
| Description | Describe methods of patient selection: describe included patients (prior testing, presentation, intended use of index test and setting) | Describe the index test and how it was conducted and interpreted |
Describe the reference standard and how it was conducted and interpreted | Describe any patients who did not receive the index test(s) or reference standard, or both, or who were excluded from the 2 × 2 table (refer to flow diagram): describe the time interval and any interventions between index test(s) and reference standard |
| Signalling questions (yes/no/unclear) | Was a consecutive or random sample of patients enrolled? | Were the index test results interpreted without knowledge of the results of the reference standard? | Is the reference standard likely to correctly classify the target condition? | Was there an appropriate interval between index test(s) and reference standard? |
| Was a case‐control design avoided? | If a threshold was used, was it prespecified? | Were the reference standard results interpreted without knowledge of the results of the index test? | Did all patients receive a reference standard? | |
| Did the study avoid inappropriate exclusions? | Did all patients receive the same reference standard? | |||
| Were all patients included in the analysis? | ||||
| Risk of bias (high/low/unclear) | Could the selection of patients have introduced bias? | Could the conduct or interpretation of the index test have introduced bias? | Could the reference standard, its conduct, or its interpretation have introduced bias? | Could the patient flow have introduced bias? |
| Concerns regarding applicability (high/low/unclear) | Are there concerns that the included patients do not match the review question? | Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Are there concerns that the target condition as defined by the reference standard does not match the review question? |
Appendix 5. Anchoring statements for quality assessment of 18F‐florbetaben PET ligand for Aβ diagnostic studies
Table 4. Review question and inclusion criteria
| Category | Review question | Inclusion criteria |
| Patients | Participants with mild cognitive impairment (MCI), no dementia | Participants that fulfil the criteria for the clinical diagnosis of MCI at baseline |
| Index test | 18F‐florbetaben PET ligand for Aβ biomarker | 18F‐florbetaben PET ligand for Aβ biomarker |
| Target condition | Alzheimer’s disease dementia (ADD) (progression from MCI to ADD) Any other forms of dementia (progression from MCI to any other forms of dementia | ADD (progression from MCI to ADD) Any other forms of dementia (progression from MCI to any other forms of dementia) |
| Reference standard | NINCDS‐ADRDA; DSM; ICD; McKeith criteria; Lund criteria; International Behavioural Variant FTD Criteria Consortium; NINDS‐ARIEN criteria | NINCDS‐ADRDA; DSM; ICD; McKeith criteria; Lund criteria; International Behavioural Variant FTD Criteria Consortium; NINDS‐ARIEN criteria |
| Outcome | N/A | Data to construct a 2 × 2 table |
| Study design | N/A | Longitudinal cohort studies and nested case‐control studies if they incorporate a delayed verification design (case‐control nested in cohort studies) |
ADD: Alzheimer's disease dementia DSM: Diagnostic and Statistical Manual of Mental Disorders FTD: Frontotemporal dementia ICD: International Classification of Diseases MCI: Mild cognitive impairment NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association NINDS‐AIREN: National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l'Enseignement en Neurosciences PET: Positron emission tomography
Anchoring statements for quality assessment 18F‐florbetaben PET ligand for Aβ diagnostic studies
We have provided some core anchoring statements for quality assessment in the diagnostic test accuracy (DTA) review of the 18F‐florbetaben PET ligand for Aβ biomarker in dementia. These statements are designed for use with the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS‐2) tool and are based on the guidance for quality assessment of DTA reviews of Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) in dementia (Quinn 2014). In assessing individual items, the score of unclear should only be given if there is genuine uncertainty. In these situations, we contacted the relevant study teams for additional information. Whenever we scored one question as high risk of bias, we considered the study as having a high risk of bias.
Table 5. Anchoring statements to assist with the 'Risk of bias' assessment
| Question | Response and weighting | Explanation |
| Patient selection | ||
| Was the sampling method appropriate? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | Where sampling is used, the designs least likely to cause bias are consecutive sampling or random sampling. Sampling that is based on volunteers or selecting subjects from a clinic or research resource is prone to bias. |
| Was a case‐control or similar design avoided? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | Designs similar to case‐control that may introduce bias are those designs where the study team deliberately increase or decrease the proportion of subjects with the target condition, which may not be representative. Some case‐control methods may already be excluded if they mix subjects from various settings. |
| Are exclusion criteria described and appropriate? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | We automatically graded the study as unclear if the study authors did not detail exclusions (pending contact with study authors). Where a study details exclusions, we graded the study as 'low risk' if we considered exclusions to be appropriate. Certain exclusions common to many studies of dementia are: medical instability; terminal disease; alcohol/substance misuse; concomitant psychiatric diagnosis; other neurodegenerative conditions. Exclusions are not appropriate if they comprise ‘difficult to diagnose’ patients. We labelled post‐hoc and inappropriate exclusions as at 'high risk' of bias. |
| Index test | ||
| Was the 18F‐florbetaben PET ligand for Aβ biomarker's assessment/interpretation performed without knowledge of clinical dementia diagnosis? |
No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | Terms such as 'blinded' or 'independently and without knowledge of' are sufficient and full details of the blinding procedure are not required. Interpretation of the results of the index test may be influenced by knowledge of the results of the reference standard. If the index test is always interpreted prior to the reference standard, then the person interpreting the index test cannot be aware of the results of the reference standard and so this item could be rated as ‘yes’. For certain index tests, the result is objective and knowledge of the reference standard should not influence the result, e.g. level of protein in cerebrospinal fluid; in this instance, the quality assessment may be 'low risk' even if blinding was not achieved. |
| Was the 18F‐florbetaben PET ligand for Aβ biomarker's threshold prespecified? |
No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | For scales and biomarkers, there is often a reference point (in units or categories) above which subjects are classified as 'test‐positive'; this may be referred to as the threshold, clinical cut‐off, or dichotomisation point. A study is classified at high risk of bias if the study authors define the optimal cut‐off post‐hoc based on their own study data because selecting the threshold to maximise sensitivity and/or specificity may lead to overoptimistic measures of test performance. Certain papers may use an alternative methodology for analysis that does not use thresholds and these papers should be classified as not applicable. |
| Was the 18F‐florbetaben PET ligand for Aβ scan interpretation done by a trained reader physician? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | If a trained reader physician performed the scan interpretation, we scored this item as ’yes’. If no definition of trained reader was done, we scored this item as ’unclear’. If a nontrained reader physician performed the scan interpretation, we scored this item as ’no’. |
| Did the study provide a clear definition of what was considered to be a 18F‐florbetaben PET ligand for Aβ biomarker’s positive result? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | If the study clearly stated the definition of a positive result (e.g. SUV), we scored this item as ’yes’. If the study did not give a definition of what it considered a positive result or the definition of a positive result varied between the participants, we scored this item as ’no’. If the study gave insufficient information to permit judgement, we scored the item as ’unclear’. |
| Reference standard | ||
| Is the assessment used for clinical diagnosis of dementia acceptable? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | Commonly used international criteria to assist with clinical diagnosis of dementia included those detailed in DSM‐IV and ICD‐10. Criteria specific to dementia subtypes included but were not limited to NINCDS‐ADRDA criteria for Alzheimer’s dementia; McKeith criteria for Lewy body dementia; Lund criteria and International Behavioural Variant FTD Criteria Consortium for frontotemporal dementia; and the NINDS‐AIREN criteria for vascular dementia. Where the criteria used for assessment were not familiar to the review authors or the Cochrane Dementia and Cognitive Improvement group (‘unclear’), we classified this item as 'high risk of bias'. |
| Were clinical assessments for dementia performed without knowledge of the 18F‐florbetaben PET ligand for Aβ biomarker? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | Terms such as 'blinded' or 'independently and without knowledge of' were sufficient and full details of the blinding procedure were not required. Interpretation of the results of the reference standard may be influenced by knowledge of the results of the index test. |
| Patient flow | ||
| Was there an appropriate interval between 18F‐florbetaben PET ligand for Aβ biomarker and clinical dementia assessment? |
No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | As we test the accuracy of the 18F‐florbetaben PET ligand for Aβ biomarker for MCI progression to dementia, there will always be a delay between the index test and the reference standard assessments. The time between the reference standard and the index test will influence the accuracy (Geslani 2005; Okello 2007; Visser 2006), and therefore we noted time as a separate variable (both within and between studies) and will test its influence on the diagnostic accuracy. We have set a minimum mean time to follow‐up assessment of 1 year. If more than 16% of subjects have assessment for MCI progression before nine months, this item was scored ‘no’. |
| Did all subjects get the same assessment for dementia regardless 18F‐florbetaben PET ligand for Aβ biomarker? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | There may be scenarios where participants who score 'test‐positive' on the index test have a more detailed assessment. Where dementia assessment differs between participants, this should be classified as high risk of bias. |
| Were all patients who received 18F‐florbetaben PET ligand for Aβ biomarker’s assessment included in the final analysis? |
No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | If the number of patients enrolled differs from the number of patients included in the 2 × 2 table, then there is the potential for bias. If patients lost to dropouts differ systematically from those who remain, then estimates of test performance may differ. If there are dropouts, these should be accounted for; a maximum proportion of dropouts for a study to remain at low risk of bias has been specified as 20%. |
| Were missing 18F‐florbetaben PET ligand for Aβ biomarker's results reported? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | Where missing or uninterpretable results are reported, and if there is substantial attrition (we have set an arbitrary value of 50% missing data), we will score this as ‘no’. If the study did not report these results, we scored this as ‘unclear’ and we contacted the study authors. |
| Was the study with 18F‐florbetaben PET ligand for Aβ biomarker free of commercial funding? | No = high risk of bias Yes = low risk of bias Unclear = unclear risk of bias | If the funding source is clearly stated and is not commercial, this should be scored as ‘no’. If the funding source is clearly stated and is commercial, this should be scored as ’yes ’. If not enough information is given to assess whether the funding source is commercial, the scored is ’unclear’. |
| Anchoring statements to assist with assessment for applicability | ||
| Question | Explanation | |
| Were included patients representative of the general population of interest? | The included patients should match the intended population as described in the review question. The review authors should consider population in terms of symptoms; pretesting; potential disease prevalence; setting. If there is a clear ground for suspecting an unrepresentative spectrum, the item should be rated poor applicability. | |
| Index test | ||
| Were sufficient data on 18F‐florbetaben PET ligand for Aβ biomarker’s application given for the test to be repeated in an independent study? | Variation in technology, test execution, and test interpretation may affect estimate of accuracy. In addition, the background, and training/expertise of the assessor should be reported and taken in consideration. If 18F‐florbetaben PET ligand for Aβ biomarker was not performed consistently, this item should be rated poor applicability. | |
| Reference standard | ||
| Was clinical diagnosis of dementia made in a manner similar to current clinical practice? | For many reviews, inclusion criteria and 'Risk of bias' assessments will already have assessed the dementia diagnosis. For certain reviews, an applicability statement relating to the reference standard may not be applicable. There is the possibility that a form of dementia assessment, although valid, may diagnose a far larger proportion of people with disease than usual clinical practice. In this instance, the item should be rated poor applicability. | |
DSM: Diagnostic and Statistical Manual of Mental Disorders FTD: Frontotemporal dementia ICD: International Classification of Diseases MCI: Mild cognitive impairment NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association NINDS‐AIREN: National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l'Enseignement en Neurosciences PET: Positron emission tomography
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.

18F‐florbetaben visual assessment and progression to ADD.
2. Test.

18F‐florbetaben SUVR and progression to ADD.
3. Test.

18F‐florbetaben visual assessment and progression to any other form of non‐ADD.
4. Test.

18F‐florbetaben SUVR and progression to any other form of non‐ADD.
5. Test.

18F‐florbetaben visual assessment and progression to any form of dementia.
6. Test.

18F‐florbetaben SUVR and progression to any form of dementia.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ong 2015.
| Study characteristics | |||
| Patient sampling | Forty‐five older adults who were referred from local memory clinics in Australia. No further details of patient sampling and recruitment were reported. Participants had to be at least 60 years old and to have had at least 7 years of formal schooling. They were also required to communicate fluently in English. Exclusion criteria included the presence of dementia, a score lower than 23 on the MMSE, the presence of other conditions that may impair their cognition and independence, including other neurological (stroke, multiple sclerosis, epilepsy, moderate‐severe traumatic brain injury), psychiatric (psychotic symptoms, bipolar disorder), or substance use conditions (e.g. drug and alcohol dependence, use of acetylcholinesterase inhibitors and memantine. |
||
| Patient characteristics and setting | 45 MCI participants diagnosed by the Petersen 2004 and Winblad 2004 criteria. Demographic data were reported for 45 MCI participants (with SUVR); demographic data were not available for those classified by visual assessment. 18F‐florbetaben positive: 24, 18F‐florbetaben negative: 21 Gender: not described Age Mean (SD): 18F‐florbetaben positive: 73.5 (6.9), 18F‐florbetaben negative:71.8 (6.1) years APOE ϵ4 carrier: not reported MMSE Mean (SD) 18F‐florbetaben positive: 26.7 (1.9), 18F‐florbetaben negative: 27.9 (1.4) Years of education Mean (SD): 18F‐florbetaben positive: 13.8 (4.2), 18F‐florbetaben negative: 13.5 (3.0) Sources of referral: not reported Setting: secondary care (memory clinic) |
||
| Index tests |
Administration instructions and tracer dosis 18F‐florbetaben was injected intravenously over 38 ± 17 s, with a mean specific activity at the time of injection of 60 ± 29 GBq/μmol. Each participant received on average 286 ± 19 MBq of 18F‐florbetaben. PET imaging was conducted using a 3D GSO Phillips Allegro PET camera. A 2‐min transmission scan using a rotating 137Cs source was done for attenuation correction immediately prior to scanning. Images obtained between 90 to 110 min post injection were analysed. Images were reconstructed using a 3D RAMLA. Image interpretation PET images were processed with a semiautomatic volume of interest (VOI) method. This method used a preset template of narrow cortical VOI that was applied to either the spatially normalized 18F‐florbetaben scan or via placement on the subject’s spatially normalized coregistered MRI by a single operator who was blinded to the subject’s clinical status. Minor manual adjustments on the MRI were made to ensure that overlap with white matter and cerebrospinal fluid was minimized. Spatial normalization and coregistration of the PET and MRI images was performed using SPM8. Mean radioactivity values were obtained from VOI for cortical, subcortical, and cerebellar regions. The cerebellar cortical VOI was placed taking care to avoid cerebellar white matter. No correction for partial volume effect was applied to the PET data.
Baseline 18F‐florbetaben PET images underwent visual assessment by five independent nuclear medicine physicians blinded to clinical data. Readers had limited or no prior experience with amyloid PET visual interpretation and were trained on an electronic training tool. The image assessment was performed on axial slices, in a grey scale. The regional cortical tracer uptake (RCTU) scoring system was used to assess the beta‐amyloid deposition in the following four regions: frontal cortex, posterior cingulate/precuneus, lateral temporal cortex, and parietal cortex. A RCTU value of 1, 2, or 3 was assigned if either no, moderate, or pronounced tracer uptake was observed respectively as described below: 1 No tracer uptake: Tracer uptake (i.e. signal intensity) in grey matter in the region is lower than in white matter 2 Moderate tracer uptake: Smaller area(s) of tracer uptake equal to or higher than that present in white matter; ‐ extending beyond the white matter rim to the outer cortical margin. ‐ involving the majority of the slices within the respective region. 3 Pronounced tracer uptake: A large confluent area of tracer uptake equal to or higher than that present in white matter; ‐ extending beyond the white matter rim to the outer cortical margin. ‐ and involving the entire region including the majority of the slices within the respective region. The RCTU scores for the four brain regions were condensed into a binary brain amyloid plaque load (BAPL) score with positive or negative interpretation as described below: 18F‐florbetaben PET scan negative: scan without β‐amyloid deposition (RCTU score 1 in each of the 4 brain regions 1, 2, 3, and 4). 18F‐florbetaben PET scan positive: scan with moderate β‐amyloid deposition (RCTU score 2 in any or all of the 4 brain regions 1, 2, 3, and 4 and no score 3 in these 4 regions) or scan with pronounced β‐amyloid deposition (RCTU score 3 at least in one of the brain regions 1, 2, 3, and 4). The majority read approach established the final result.
PET images were processed with a semiautomatic volume of interest (VOI) method. This method used a preset template of narrow cortical VOI that was applied to either the spatially normalized 18F‐florbetaben scan or via placement on the subject’s spatially normalised coregistered MRI by a single operator who was blinded to the subject’s clinical status. Minor manual adjustments on the MRI were made to ensure that overlap with white matter and cerebrospinal fluid was minimized. Spatial normalization and coregistration of the PET and MRI images was performed using SPM8. Mean radioactivity values were obtained from VOI for cortical, subcortical, and cerebellar regions. The cerebellar cortical VOI were placed taking care to avoid cerebellar white matter. No correction for partial volume effect was applied to the PET data. The standardised uptake value (SUV), defined as the decay‐corrected brain radioactivity concentration normalised for injected dose and body weight, was calculated for all regions. These were then used to derive the SUV ratio (SUVR), which was referenced to cerebellar cortex. Neocortical Aβ burden was expressed as the average SUVR of the area‐weighted mean for the following cortical ROIs: frontal (consisting of dorsolateral prefrontal, ventrolateral prefrontal, and orbitofrontal regions), superior parietal, lateral temporal, lateral occipital, and anterior and posterior cingulate. The prespecified SUVR used was ≥ 1.45. |
||
| Target condition and reference standard(s) | Target condition as ADD with NINCDS‐ADRDA criteria were established with access to all study results and personal medical records | ||
| Flow and timing | Duration of follow‐up: 4 years Number included in analysis: 45 participants Visual Assessment: 25 18F‐florbetaben (+) and 20 18F‐florbetaben (‐) Progression from MCI to ADD by visual assessment: 18F‐florbetaben (+): 21 MCI to ADD and 4 MCI‐MCI; 18F‐florbetaben (‐): 0 MCI to ADD and 20 MCI‐MCI TP = 21; FP = 4; FN = 0; TN = 20 Progression from MCI to any other form of dementia (non‐ADD) by visual assessment: 18F‐florbetaben (+): 0 MCI to non‐ADD and 25 MCI‐MCI; 18F‐florbetaben (‐): 5 MCI to non‐ADD and 15 MCI‐MCI TP = 0; FP = 25; FN = 5; TN = 15 Progression from MCI to any form of dementia by visual assessment: 18F‐florbetaben (+): 21 MCI to any dementia and 4 MCI‐MCI; 18F‐florbetaben (‐): 5 MCI to any dementia and 15 MCI‐MCI TP = 21; FP = 4; FN = 5; TN = 15 SUVR: 24 18F‐florbetaben (+) and 21 18F‐florbetaben (‐) Progression from MCI to ADD by SUVR: 18F‐florbetaben (+): 21 MCI to ADD and 3 MCI‐MCI; 18F‐florbetaben (‐): 0 MCI to ADD and 21 MCI‐MCI TP = 21; FP = 3; FN= 0; TN = 21 Progression from MCI to any other form of dementia (non‐ADD) by SUVR: 18F‐florbetaben (+): 0 MCI to non‐ADD and 24 MCI‐MCI or ADD; 18F‐florbetaben (‐): 5 MCI to non‐ADD and 16 MCI‐MCI or ADD TP = 0; FP = 24; FN = 5; TN = 16 Progression from MCI to any form of dementia by SUVR: 18F‐florbetaben (+): 21 MCI to any dementia and 3 MCI‐MCI; 18F‐florbetaben (‐): 5 MCI to any dementia and 16 MCI‐MCI TP = 21; FP = 3; FN = 5; TN = 16 |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the PET scan interpretation done by a trained reader physician? | Yes | ||
| Was there a clear definition of a positive result? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the study free of commercial funding? | No | ||
| High | |||
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Kim 2017 | No data for constructing a 2 x 2 table was provided. The study focused on different cortical thickness subgroups and the progression to dementia in aMCI participants whom were evaluated at baseline with PiB PET or 18F‐florbetaben. We emailed the authors to resolve this issue, however, no response from the lead author was received. |
ADD: Alzheimer's disease dementia APOE: Apolipoprotein E BAPL: Brain amyloid plaque load FN: False negative FP: False positive MCI: Mild cognitive impairment MMSE: Mini Mental State Examination MRI: Magnetic resonance imaging NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association PET: Positron emission tomography RCTU: Regional cortical tracer uptake RAMLA: Row action maximum likelihood algorithm ROI: Region of interest SD: Standard deviation SPM8: Statistical parametric mapping 8 SUV: Standardised uptake value SUVR: Standardised uptake value ratio TN: True negative TP: True positive VOI: Volume of interest
Characteristics of ongoing studies [ordered by study ID]
EUCTR2013‐004671‐12‐BE.
| Trial name or title | Predictive value of biomarkers in patients with amnestic mild cognitive impairment |
| Target condition and reference standard(s) | Cognitive decline after two years of follow‐up, reference standard not specified |
| Index and comparator tests | 18F‐florbetaben |
| Starting date | June 2014 |
| Contact information | rik.vandenberghe@uzleuven.be University of Leuven |
| Notes | Dr. Vandenberghe was contacted; he provided requested information regarding the tracer used; email from Dr. Vandenberghe on 23/01/17 |
EUCTR2014‐000562‐21‐NL.
| Trial name or title | Amyloid‐PET as a diagnostic marker in daily practice |
| Target condition and reference standard(s) | Progression to dementia, reference standard not specified |
| Index and comparator tests | 18F‐florbetaben |
| Starting date | January 2015 |
| Contact information | Alzheimer Center, VU University Medical Center Alzheimer Center alzheimercentrum@vumc.nl |
| Notes |
EUCTR2014‐004244‐35‐IT.
| Trial name or title | Amyloid load in prodromal ADD with limbic‐predominant phenotype |
| Target condition and reference standard(s) | Clinical 'indices', reference standard not specified |
| Index and comparator tests | 18F‐florbetaben |
| Starting date | March 2015 |
| Contact information | Medicina Nucleare, IRCCS Ospedale San Raffaele perani.daniela@hsr.it |
| Notes |
NCT01222351.
| Trial name or title | BAY 94‐9172 PET/CT in cognitively normal older adults, older adults with mild cognitive impairment, and older adults with Alzheimer's disease |
| Target condition and reference standard(s) | Alzheimer's disease, reference standard not specified |
| Index and comparator tests | 18F‐florbetaben |
| Starting date | 2010 |
| Contact information | Oksana Taterina, ot2004@columbia.edu Yaakov Stern, ys11@columbia.edu |
| Notes |
NCT02854033.
| Trial name or title | Alzheimer's disease neuroimaging initiative 3 (ADNI3) protocol |
| Target condition and reference standard(s) | Rate of progression to dementia due to ADD, reference standard not specified |
| Index and comparator tests | 18F‐florberaben, 18F‐florbetapir |
| Starting date | October 2016 |
| Contact information | Dr. Paul Aisen, Director, Alzheimer's Therapeutic Research Institute, University of Southern California |
| Notes |
ADD: Alzheimer's disease dementia ADNI3: Alzheimer's disease neuroimaging initiative 3 CT: Computed tomography PET: Positron emission tomography
Differences between protocol and review
We added as the reference standard the definition of progressive supranuclear palsy (PSP) (Hauw 1994).
Contributions of authors
Gabriel Martínez, Robin WM Vernooij, and Paulina Fuentes Padilla: contributed to conception, design, and draft of the protocol; overall responsibility of study selection; data extraction; contacted the authors; draft of discussion and authors’ conclusion sections.
Javier Zamora: reviewed draft protocol, updated statistical methods section, performed statistical analyses and final manuscript.
Leon Flicker: contributed to conception, and designed and reviewed draft protocol and final manuscript.
Xavier Bonfill Cosp: reviewed draft protocol and final manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
This protocol was supported by the NIHR, via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the protocol authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS, or the Department of Health.
Declarations of interest
Gabriel Martínez has no known conflicts of interest. Robin WM Vernooij has no known conflicts of interest. Paulina Fuentes Padilla has no known conflicts of interest. Javier Zamora has no known conflicts of interest.
Leon Flicker has no known conflicts of interest. Xavier Bonfill Cosp has no known conflicts of interest.
New
References
References to studies included in this review
Ong 2015 {published data only}
- Bahar‐Fuchs A, Villemagne V, Ong K, Chetélat G, Lamb F, Reininger CB, et al. Prediction of amyloid‐β pathology in amnestic mild cognitive impairment with neuropsychological tests. Journal of Alzheimer's Disease 2013;33(2):451‐62. [DOI] [PubMed] [Google Scholar]
- Ong D, Villemagne V, Bahar‐Fuchs A, Lamb F, Jones G, Reinlinger C, et al. Conversion from mild cognitive impairment to Alzheimer's disease over 12 months: predictive value of AB imaging with 18F‐Florbetaben. Internal Medicine Journal 2011;41 Suppl S3:39. [Google Scholar]
- Ong K, Villemagne V, Bahar‐Fuchs A, Lamb F, Chetelat G, Holl G, et al. Conversion from mild cognitive impairment to Alzheimer's disease over 12 months: predictive value of AB imaging with 18F‐florbetaben. Alzheimer's & Dementia 2011;7 Suppl(4):S217. [Google Scholar]
- Ong K, Villemagne V, Bahar‐Fuchs A, Lamb F, Reininger C, Putz B, et al. Cognitive change and Ab deposition in MCI subjects studied with serial 18F‐florbetaben PET over 2 years. Alzheimer's & Dementia 2012;8 Suppl(4):P22‐3. [Google Scholar]
- Ong K, Villemagne VL, Bahar‐Fuchs A, Lamb F, Jones G, Reininger C, et al. A two‐year longitudinal assessment of abeta deposition in MCI with 18f‐florbetaben. Internal Medicine Journal 2012;42 Suppl S3:12. [Google Scholar]
- Ong KT, Villemagne VL, Bahar‐Fuchs A, Lamb F, Langdon N, Catafau AM, et al. Aβ imaging with 18F‐florbetaben in prodromal Alzheimer’s disease: a prospective outcome study. Journal of Neurology, Neurosurgery and Psychiatry 2015;86(4):431‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Kim 2017 {published data only}
- Kim HJ, Seo SW, Kim Y, Jang H, Kim KW, Lee JS, et al. Anatomical subtypes of amnestic mild cognitive impairment. Neuro‐degenerative Diseases 2017; Vol. 17, issue Suppl 1:1039.
References to ongoing studies
EUCTR2013‐004671‐12‐BE {unpublished data only}
- EUCTR2013‐004671‐12‐BE. Predictive value of biomarkers in patients with amnestic mild cognitive impairment. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013‐004671‐12‐BE (first received 20 May 2014).
EUCTR2014‐000562‐21‐NL {unpublished data only}
- EUCTR2014‐000562‐21‐NL. Amyloid‐PET as a diagnostic marker in daily practice. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2014‐000562‐21‐NL (first received 5 January 2015).
EUCTR2014‐004244‐35‐IT {unpublished data only}
- EUCTR2014‐004244‐35‐IT. Amyloid load in prodromal AD with limbic‐predominant phenotype [Amyloid load in prodromal AD with limbic‐predominant phenotype principal investigator ‐ Studio del Carico di Amiloide in AD prodromico con fenotipo limbico]. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2014‐004244‐35‐IT (first received 13 November 2014).
NCT01222351 {unpublished data only}
- NCT01222351. Measuring brain amyloid plaque load in older adults using BAY 94‐9172. clinicaltrials.gov/show/NCT01222351 (first received 18 October 2010).
NCT02854033 {unpublished data only}
- NCT02854033. Alzheimer's disease neuroimaging initiative 3 (ADNI3) protocol. clinicaltrials.gov/show/NCT02854033 (first received 3 August 2016).
Additional references
Albert 2011
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia 2011;7(3):270‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alzheimer's Association 2010
- Alzheimer's Association. Changing the trajectory of Alzheimer's disease: a national imperative. http://www.alzheimersreadingroom.com/2010/05/changing‐trajectory‐of‐alzheimers.html (accessed prior to 12 October 2017).
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington, DC: American Psychiatric Association, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
Archer 2015
- Archer HA, Smailagic N, John C, Holmes RB, Takwoingi Y, Coulthard EJ, et al. Regional Cerebral Blood Flow Single Photon Emission Computed Tomography for detection of Frontotemporal dementia in people with suspected dementia. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD010896.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arevalo‐Rodriguez 2015
- Arevalo‐Rodriguez I, Smailagic N, Figuls MRI, Ciapponi A, Sanchez‐Perez E, Giannakou A, et al. Mini‐Mental State Examination (MMSE) for the detection of Alzheimer's disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD010783.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barthel 2011
- Barthel H, Gertz HJ, Dresel S, Peters O, Bartenstein P, Buerger K, et al. Cerebral amyloid‐β PET with florbetaben (18F) in patients with Alzheimer's disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet. Neurology 2011;10(5):424‐35. [DOI] [PubMed] [Google Scholar]
Bossuyt 2008
- Bossuyt PM, Leeflang MM. Chapter 6: Developing criteria for including studies. In: Deeks JJ, Bossuyt PM, Gatsonis C, editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. The Cochrane Collaboration, 2013. Available from srdta.cochrane.org.
Boxer 2005
- Boxer AL, Miller BL. Clinical features of frontotemporal dementia. Alzheimer Disease and Associated Disorders 2005;19(Suppl 1):S3‐6. [DOI] [PubMed] [Google Scholar]
Brun 1994
- Brun A, Englund B, Gustafson L, Passant U, Mann DMA, Neary D, et al. Clinical and neuropathological criteria for frontotemporal dementia. Journal of Neurology, Neurosurgery and Psychiatry 1994;57(4):416‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruscoli 2004
- Bruscoli M, Lovestone S. Is MCI really just early dementia? A systematic review of conversion studies. International Psychogeriatrics 2004;16(2):129‐40. [DOI] [PubMed] [Google Scholar]
Chan 2014
- Chan CCH, Fage BA, Smailagic N, Gill SS, Herrmann N, Nikolaou V, et al. Mini‐Cog for the diagnosis of Alzheimer’s disease dementia and other dementias within a secondary care setting. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD011414] [DOI] [PMC free article] [PubMed] [Google Scholar]
CMS 2013
- The Centers for Medicare & Medicaid Services. Decision memo for beta amyloid positron emission tomography in dementia and neurodegenerative disease (CAG‐00431N). cms.gov/medicare‐coverage‐database/details/nca‐decision‐memo.aspx?NCAId=265 (accessed 08 October 2015).
Cordella 2013
- Cordella CB, Borson S, Boustani M, Chodosh J, Reuben D, Verghese J, et al. Alzheimer's Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimer's & Dementia 2013;9(2):141‐50. [DOI] [PubMed] [Google Scholar]
Creavin 2016
- Creavin S, Wisniewski S, Noel‐Storr A, Trevelyan C, Hampton T, Rayment D, et al. Mini‐Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011145.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 2013
- Davis DHJ, Creavin ST, Noel‐Storr A, Quinn TJ, Smailagic N, Hyde C, et al. Neuropsychological tests for the diagnosis of Alzheimer’s disease dementia and other dementias: a generic protocol for cross‐sectional and delayed‐verification studies. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD010460] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 2015
- Davis DHJ, Creavin ST, Yip JLY, Noel‐Storr AH, Brayne C, Cullum S. Montreal Cognitive Assessment for the diagnosis of Alzheimer’s disease and other dementias. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD010775.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
De la Torre 2004
- Torre JC. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet. Neurology 2004;3(3):184‐90. [DOI] [PubMed] [Google Scholar]
Dubois 2014
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG‐2 criteria. Lancet. Neurology 2014;13(6):614‐29. [DOI] [PubMed] [Google Scholar]
Elias‐Sonnenschein 2014a
- Elias‐Sonnenschein LS, Viechtbauer W, Ramakers I, Ukoumunne O, Verhey FRJ, Visser PJ. APOE‐ϵ4 allele for the diagnosis of Alzheimer's and other dementia disorders in people with mild cognitive impairment in a community setting. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD010948] [DOI] [Google Scholar]
Elias‐Sonnenschein 2014b
- Elias‐Sonnenschein LS, Viechtbauer W, Ramakers I, Ukoumunne O, Verhey FRJ, Visser PJ. APOE‐ϵ4 allele for the diagnosis of Alzheimer's and other dementia disorders in people with mild cognitive impairment in a primary care setting. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD010949] [DOI] [Google Scholar]
Elias‐Sonnenschein 2014c
- Elias‐Sonnenschein LS, Viechtbauer W, Ramakers I, Ukoumunne O, Verhey FRJ, Visser PJ. APOE‐ϵ4 allele for the diagnosis of Alzheimer's and other dementia disorders in people with mild cognitive impairment in a secondary care setting. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD010950] [DOI] [Google Scholar]
EMA 2014
- European Medicines Agency. Neuraceq. Annex 1. Summary of product characteristics. www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/002553/WC500162592.pdf (accessed 8th April 2015).
Espinosa 2013
- Espinosa A, Alegret M, Valero S, Vinyes‐Junque G, Hernandez I, Mauleon A, et al. A longitudinal follow‐up of 550 mild cognitive impairment patients: evidence for large conversion to dementia rates and detection of major risk factors involved. Journal of Alzheimer’s Disease 2013;34(3):769‐80. [DOI] [PubMed] [Google Scholar]
Fage 2015
- Fage BA, Chan CCH, Gill SS, Noel‐Storr AH, Herrmann N, Smailagic N, et al. Mini‐Cog for the diagnosis of Alzheimer's disease dementia and other dementias within a community setting. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD010860.pub2] [DOI] [PubMed] [Google Scholar]
FDA 2014
- Food, Drug Administration. Neuroceq. www.accessdata.fda.gov/drugsatfda_docs/label/2014/204677s000lbl.pdf (accessed 08/04/2015).
Filippini 2012
- Filippini G, Casazza G, Bellatorre AG, Lista C, Duca P, Beecher D, et al. The role of MRI in the early diagnosis of Alzheimer’s disease or other dementias in persons with mild cognitive impairment (MCI). Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009628] [DOI] [Google Scholar]
Garcia‐Alloza 2011
- Garcia‐Alloza M, Gregory J, Kuchibhotla KV, Fine S, Wei Y, Ayata C, et al. Cerebrovascular lesions induce transient β‐amyloid deposition. Brain 2011;134(12):3697‐707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geslani 2005
- Geslani DM, Tierney MC, Herrmann N, Szalai JP. Mild cognitive impairment: an operational definition and its conversion rate to Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders 2005;19(5‐6):383‐9. [DOI] [PubMed] [Google Scholar]
Goedert 2006
- Goedert M, Spillantini MG. A century of Alzheimer's disease. Science 2006;314(5800):777‐81. [DOI] [PubMed] [Google Scholar]
Harrison 2014
- Harrison JK, Fearon P, Noel‐Storr AH, McShane R, Stott DJ, Quinn TJ. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the diagnosis of dementia within a general practice (primary care) setting. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD010771.pub2] [DOI] [PubMed] [Google Scholar]
Harrison 2015
- Harrison JK, Fearon P, Noel‐Storr AH, McShane R, Stott DJ, Quinn TJ. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the diagnosis of dementia within a secondary care setting. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD010772.pub2] [DOI] [PubMed] [Google Scholar]
Hauw 1994
- Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, et al. Preliminary NINDS neuropathologic criteria for Steele‐Richardson‐Olszewski syndrome (progressive supranuclear palsy). Neurology 1994;44(11):2015‐9. [DOI] [PubMed] [Google Scholar]
Hendry 2014
- Hendry K, Lees RA, McShane R, Noel‐Storr AH, Stott DJ, Quinn TJ. AD‐8 for diagnosis of dementia across a variety of healthcare settings. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD011121] [DOI] [PMC free article] [PubMed] [Google Scholar]
Herscovitch 2015
- Herscovitch P. Regulatory approval and insurance reimbursement: the final steps in clinical translation of amyloid brain imaging. Clinical and Translational Imaging 2015;3:75‐7. [Google Scholar]
Heyden 2013
- Hayden EY, Teplow DB. Amyloid β‐protein oligomers and Alzheimer’s disease. Alzheimer's Research & Therapy 2013;5(6):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hyman 2012
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, et al. National Institute on Aging‐Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimer's & Dementia 2012;8(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICD‐10
- World Health Organization. International statistical Classification of Diseases and related health problems (ICD‐10 Version 2010). apps.who.int/classifications/icd10/browse/2010/en (accessed 29 January 2015).
Jack 2010
- Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet. Neurology 2010;9(1):119‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jellinger 2006
- Jellinger K. Clinicopathological analysis of dementia disorders in the elderly ‐ an update. Journal of Alzheimer's Disease 2006;9(Supplement 3):61‐70. [DOI] [PubMed] [Google Scholar]
Johnson 2013
- Johnson KA, Minoshima S, Bohnen NI, Donohoe KJ, Foster NL, Herscovitch P, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer's Association. Journal of Nuclear Medicine 2013;54(3):476‐90. [DOI] [PubMed] [Google Scholar]
Knottnerus 2002
- Knottnerus JA, Weel C, Muris JW. Evaluation of diagnostic procedures. BMJ 2002;324(7335):477‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kokkinou 2014
- Kokkinou M, Smailagic N, Noel‐Storr AH, Hyde C, Ukoumunne O, Worrall RE, et al. Plasma and Cerebrospinal fluid (CSF) Abeta42 for the differential diagnosis of Alzheimer's disease dementia in participants diagnosed with any dementia subtype in a specialist care setting. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD010945] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lees 2014
- Lees RA, Stott DJ, McShane R, Noel‐Storr AH, Quinn TJ. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the early diagnosis of dementia across a variety of healthcare settings. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD011333] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lijmer 1999
- Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, Meulen JH, et al. Empirical evidence of design‐related bias in studies of diagnostic tests. JAMA 1999;282(11):1061‐6. [DOI] [PubMed] [Google Scholar]
Lundh 2017
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2014
- Ma Y, Zhang S, Li J, Zheng DM, Guo Y, Feng J, et al. Predictive accuracy of amyloid imaging for progression from mild cognitive impairment to Alzheimer disease with different lengths of follow‐up: a meta‐analysis. Medicine 2014;93(27):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martínez 2016
- Martínez G, Flicker L, Vernooij RWM, Fuentes Padilla P, Zamora J, Figuls MRI, et al. 18F PET ligands for the early diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD012216] [DOI] [Google Scholar]
Matthews 2008
- Matthews FE, Stephan BC, McKeith IG, Bond J, Brayne C, Medical Research Council Cognitive Function and Ageing Study. Two‐year progression from mild cognitive impairment to dementia: to what extent do different definitions agree?. Journal of the American Geriatrics Society 2008;56(8):1424‐33. [DOI] [PubMed] [Google Scholar]
Mattsson 2009
- Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 2009;302(4):385‐93. [DOI] [PubMed] [Google Scholar]
McCleery 2015
- McCleery J, Morgan S, Bradley KM, Noel‐Storr AH, Ansorge O, Hyde C. Dopamine transporter imaging for the diagnosis of dementia with Lewy bodies. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD010633.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKeith 1996
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996;47(5):1113‐24. [DOI] [PubMed] [Google Scholar]
McKeith 2005
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology 2005;65(12):1863‐72. [DOI] [PubMed] [Google Scholar]
McKhann 1984
- McKhann GM, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34(7):939‐44. [DOI] [PubMed] [Google Scholar]
McKhann 2011
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia 2011;7(3):263‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 2009
- Mitchell AJ, Shiri‐Feshki M. Rate of progression of mild cognitive impairment to dementia ‐ meta‐analysis of 41 robust inception cohort studies. Acta Psychiatrica Scandinavica 2009;119(4):252‐65. [DOI] [PubMed] [Google Scholar]
Morris 1993
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43(11):2412‐4. [DOI] [PubMed] [Google Scholar]
NAO 2007
- National Audit Office. Improving services and support for people with dementia. Report by the Comptroller and Auditor General. HC 604 Session General 2006‐2007. www.nao.org.uk/wp‐content/uploads/2007/07/0607604.pdf (accessed 25th March 2015).
Neary 1998
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51(6):1546‐54. [DOI] [PubMed] [Google Scholar]
NICE 2006
- National Institute for Health Care Excellence. Dementia: supporting people with dementia and their carers in health and social care. NICE guidelines [CG42]. www.nice.org.uk/guidance/cg42 (accessed 17th April 2015).
Noel‐Storr 2013
- Noel‐Storr AH, Flicker L, Ritchie CW, Nguyen GH, Gupta T, Wood P, et al. Systematic review of the body of evidence for the use of biomarkers in the diagnosis of dementia. Alzheimer's & Dementia 2013;9(3):e96‐105. [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2014
- Noel‐Storr AH, McCleery JM, Richard E, Ritchie CW, Flicker L, Cullum SJ, et al. Reporting standards for studies of diagnostic test accuracy in dementia: the STARDdem Initiative. Neurology 2014;83(4):364‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okello 2007
- Okello A, Edison P, Archer H, Hinz R, Fox N, Kennedy AM, et al. Amyloid deposition and cerebral glucose metabolism in mild cognitive impairment: a longitudinal 11C‐PIB and 18F‐FDG PET Study. Journal of Neurology, Neurosurgery and Psychiatry 2007;78(2):219‐20. [Google Scholar]
Petersen 1999
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology 1999;56(3):303‐8. [DOI] [PubMed] [Google Scholar]
Petersen 2004
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine 2004;256(3):183‐94. [DOI] [PubMed] [Google Scholar]
Petersen 2009
- Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, et al. Mild cognitive impairment: ten years later. Archives of Neurology 2009;66(12):1447‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quinn 2014
- Quinn TJ, Fearon P, Noel‐Storr AH, Young C, McShane R, Stott DJ. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the diagnosis of dementia within community dwelling populations. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD010079.pub2] [DOI] [PubMed] [Google Scholar]
Rascovsky 2011
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134(Pt 9):2456‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richard 2012
- Richard E, Schmand B, Eikelenboom P, Westendorp RG, Gool WA. The Alzheimer myth and biomarker research in dementia. Journal of Alzheimer's Disease 2012;31(Suppl 3):S203‐9. [DOI] [PubMed] [Google Scholar]
Ritchie 2013
- Ritchie C, Smailagic N, Ladds EC, Noel‐Storr AH, Ukoumunne O, Martin S. CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010803] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ritchie 2014
- Ritchie C, Smailagic N, Noel‐Storr AH, Takwoingi Y, Flicker L, Mason SE, et al. Plasma and cerebrospinal fluid amyloid beta for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD008782.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Román 1993
- Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology 1993;43(2):250‐60. [DOI] [PubMed] [Google Scholar]
Royall 2014
- Royall DR, Palmer RF. The temporospatial evolution of neuritic plaque‐related and independent tauopathies: implications for dementia staging. Journal of Alzheimer’s Disease 2014;40(3):541‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sabbagh 2017
- Sabbagh MN, Schäuble B, Anand K, Richards D, Murayama S, Akatsu H, et al. Histopathology and florbetaben PET in patients incorrectly diagnosed with Alzheimer's disease.. Journal of Alzheimer's Disease 2017;56(2):441‐46. [DOI] [PubMed] [Google Scholar]
Sabri 2015
- Sabri O, Sabbagh MN, Seibyl J, Barthel H, Akatsu H, Ouchi Y, et al. Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer's disease: phase 3 study. Alzheimer's & Dementia 2015;11(8):964‐74. [DOI] [PubMed] [Google Scholar]
Savva 2009
- Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C, Medical Research Council Cognitive Function and Ageing Study. Age, neuropathology, and dementia. New England Journal of Medicine 2009;360(22):2302‐9. [DOI] [PubMed] [Google Scholar]
Schneider 2007
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community‐dwelling older persons. Neurology 2007;69(24):2197‐204. [DOI] [PubMed] [Google Scholar]
Schneider 2009
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Annals of Neurology 2009;66(2):200‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seitz 2014
- Seitz DP, Fage BA, Chan CCH, Gill SS, Herrmann N, Smailagic N, et al. Mini‐Cog for the diagnosis of Alzheimer’s disease dementia and other dementias within a primary care setting. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD011415] [DOI] [PMC free article] [PubMed] [Google Scholar]
Selkoe 2016
- Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. European Molecular Biology Organization 2016;8(6):595‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Serrano‐Pozo 2013
- Serrano‐Pozo A, Qian J, Monsell SE, Frosch MP, Betensky RA, Hyman BT. Examination of the clinicopathologic continuum of Alzheimer disease in the autopsy cohort of the National Alzheimer Coordinating Center. Journal of Neuropathology and Experimental Neurology 2013;72(12):1182‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smailagic 2015
- Smailagic N, Vacante M, Hyde C, Martin S, Ukoumunne O, Sachpekidis C. 18F‐FDG PET for the early diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD010632.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sperling 2011
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia 2011;7(3):280‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Villemagne 2011
- Villemagne VL, Ong K, Mulligan RS, Holl G, Pejoska S, Jones G, et al. Amyloid imaging with (18)F‐florbetaben in Alzheimer disease and other dementias. Journal of Nuclear Medicine 2011;52(8):1210‐7. [DOI] [PubMed] [Google Scholar]
Visser 2006
- Visser PJ, Kester A, Jolles J, Verhey F. Ten‐year risk of dementia in subjects with mild cognitive impairment. Neurology 2006;67(7):1201‐7. [DOI] [PubMed] [Google Scholar]
White 2009
- White L. Brain lesions at autopsy in older Japanese‐American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu‐Asia aging study. Journal of Alzheimer's Disease 2009;18(3):713‐25. [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AWS, Westwood ME, Mallet S, Deeks JJ, Reitsma JB. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [DOI] [PubMed] [Google Scholar]
WHO 2012
- World Health Organization, Alzheimer's Disease International. Dementia: a public health priority. www.who.int/mental_health/publications/dementia_report_2012/en/ (accessed 23th September 2015).
Winblad 2004
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment ‐ beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine 2004;256(3):240‐6. [DOI] [PubMed] [Google Scholar]
Zhang 2005
- Zhang W, Oya S, Kung MP, Hou C, Maier DL, Kung HF. F‐18 Polyethyleneglycol stilbenes as PET imaging agents targeting Abeta aggregates in the brain. Nuclear Medicine and Biology 2005;32(8):799‐809. [DOI] [PubMed] [Google Scholar]
Zhang 2014
- Zhang S, Smailagic N, Hyde C, Noel‐Storr AH, Takwoingi Y, McShane R, et al. 11C‐PIB‐PET for the early diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD010386.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


