Abstract
Background
Functional dyspepsia (FD or non‐ulcer dyspepsia) is defined as continuous or frequently recurring epigastric pain or discomfort for which no organic cause can be found. Acid suppressive therapy, including proton pump inhibitors (PPIs), has been proposed as a therapeutic option in FD, but its efficacy remains controversial. While PPIs are generally considered safe and well tolerated, they have been associated with adverse events, especially in the long term. For this reason, decisions on whether to initiate or continue PPI therapy should be made based on an appropriate clinical indication. Therefore, we conducted a systematic review to evaluate whether PPI therapy provides symptomatic relief in FD.
Objectives
To determine the efficacy of proton pump inhibitors in the improvement of global symptoms of dyspepsia and quality of life compared to placebo, H2 receptor antagonists or prokinetics, in people with functional dyspepsia.
Search methods
We searched in the following electronic databases: the Cochrane Library (to May 2017), MEDLINE (OvidSP; to May 2017), Embase (OvidSP; to May 2017), and SIGLE grey literature (up to May 2017) and clinical trial registries; we handsearched abstracts from conferences up to May 2017. We screened non‐systematic reviews, systematic reviews and guidelines to identify any additional trials. We contacted trialists to obtain missing information.
Selection criteria
All randomized controlled trials (RCTs) comparing any PPI with placebo, H2 receptor antagonists (H2RAs) or prokinetics for the treatment of FD of at least two weeks' duration. Participants were adults (aged 16 years or greater) with an adequate diagnosis of FD (any validated criteria such as Rome I, II, III or Lancet Working Group).
Data collection and analysis
Two review authors independently assessed eligibility and trial quality, and extracted data. We collected data on dyspeptic symptoms, quality of life and number of overall adverse events. Specific adverse events were beyond the scope of this review.
Main results
We identified 25 RCTs from 27 papers (with 8453 participants) studying the effect of PPIs versus placebo, H2RAs or prokinetics for improvement of global symptoms of dyspepsia and quality of life in people with FD. Low‐dose PPIs had similar efficacy as standard‐dose PPIs, therefore we combined these subgroups for the analysis. PPI was more effective than placebo at relieving overall dyspepsia symptoms in people with FD (risk ratio (RR) 0.88, 95% confidence interval (CI) 0.82 to 0.94; participants = 6172; studies = 18; number needed to treat for an additional beneficial outcome (NNTB) 11; moderate quality evidence). PPIs may have little or no effect compared with H2RAs (RR 0.88, 95% CI 0.74 to 1.04; participants = 740; studies = 2; low quality evidence), and may be slightly more effective than prokinetics (RR 0.89, 95% CI 0.81 to 0.99; participants = 1033; studies = 5; NNTB 16; low quality evidence) at relieving overall dyspepsia symptoms in people with FD. PPIs plus prokinetics have probably little or no effect compared with PPIs alone at relieving overall dyspepsia symptoms (RR 0.85, 95% CI 0.68 to 1.08; participants = 407; studies = 2; moderate quality evidence).
There was no difference when subgrouped by Helicobacter pylori status, country of origin, or presence of reflux or Rome III subtypes. There were no differences in the number of adverse events observed between PPIs and any of the other treatments. There were fewer adverse events in the combination of PPI plus prokinetics compared to prokinetics alone (RR 0.60, 95% CI 0.39 to 0.93; participants = 407; studies = 2; moderate quality evidence).
Authors' conclusions
There is evidence that PPIs are effective for the treatment of FD, independent of the dose and duration of treatment compared with placebo. PPIs may be slightly more effective than prokinetics for the treatment of FD; however, the evidence is scarce. The trials evaluating PPIs versus prokinetics are difficult to interpret as they are at risk of bias. Although the effect of these drugs seems to be small, the drugs are well tolerated.
Plain language summary
Proton pump inhibitors for functional dyspepsia
Review question
How effective are medicines that suppress stomach acid for the treatment of indigestion in adults with no other major disease?
Background
Acid suppression is a possible treatment for functional dyspepsia (indigestion), which is recurring pain over the stomach, bloating, burping or the feeling of being full. Several medicines are used to treat functional dyspepsia; proton pump inhibitors (PPIs) and H2 receptor antagonists (H2RAs) reduce stomach acid, and prokinetics accelerate stomach emptying. There is no clear evidence that one medicine is more effective than another. Although these are considered safe, a few people have side effects. The most common side effects are headache, tummy (abdominal) pain, bloating, diarrhoea and feeling sick (nausea). Long‐term use of PPIs has been associated with infectious diarrhoea (inflammation of the stomach and small intestine), bone fracture and bacterial overgrowth. Therefore, we need to know whether these medications are effective and safe for people with indigestion.
Search date
We searched medical databases for clinical trials in which treatment was allocated by chance (called randomized controlled trials) in adults with functional dyspepsia up to May 2017. We included results from 25 studies from 27 publications. We found two studies awaiting further details and no other ongoing studies.
Study characteristics
We included 25 studies (with 8453 participants). There were six studies (2304 participants) comparing low‐dose PPIs versus standard‐dose PPIs (the dose used in clinical practice); 18 studies (6172 participants) comparing PPIs with placebo (pretend treatment); two studies (740 participants) comparing PPIs with H2RAs; five studies (1033 participants) comparing PPIs with prokinetics and two studies (407 participants) comparing PPIs plus prokinetics versus prokinetics alone.
The duration of the treatment lasted at least two weeks. Seven studies reported treatment for two weeks, 12 studies reported treatment for four weeks and five studies reported more than six weeks of treatment. The treatment period was unclear in one study.
Study funding sources
Seventeen of the 25 studies were sponsored or funded by a pharmaceutical company and two by an institution grant. There was no information on funding in eight studies.
Key results
Our review showed that PPIs are more effective than placebo, and are probably slightly more effective than prokinetics for the treatment of functional dyspepsia. Low‐dose and standard‐dose PPIs were similarly effective on the relief of indigestion, so we combined the results of the two doses of PPI. PPI was more effective than placebo, with 31% of the PPI group reporting no or minimal symptoms compared with 26% of the placebo group. The effect of PPI was probably slightly more effective than H2RAs; however, the two studies involved in the analysis were so different that it may have influenced the results. There was no difference in the number of reported side effects when comparing PPIs, H2RAs and prokinetics.
Quality of the evidence
The studies evaluating the effect of PPIs compared to placebo or PPIs combined with prokinetics versus prokinetics were in general of good quality. However, the studies that compared PPIs versus H2RAs and prokinetics had serious quality issues.
Summary of findings
Background
Description of the condition
See 'Glossary of medical terms' in Appendix 1.
Since the mid‐to‐late 1990s, the definition of functional dyspepsia (FD, or non‐ulcer dyspepsia) has undergone major changes since the initial working party definition in 1988, to the consecutive Rome I to III (Colin‐Jones 1988; Drossman 1999; Drossman 2006), and most recently the Rome IV definitions (Stanghellini 2016). This process has been in line with a change in our understanding of the pathophysiological basis of FD and its categorization (Talley 2016).
FD is defined as continuous or frequently recurring epigastric pain or discomfort for which no organic cause can be found (Drossman 2006). Other symptoms, such as upper abdominal bloating, excessive burping and early satiety could also be present; a normal upper endoscopy is usually required to rule out any underlying organic disease (Abraham 2004). FD is a highly prevalent disorder, affecting 10% to 15% of the general population (Lacy 2013), and it accounts for 3% to 5% of all primary care clinic visits in North America. About 50% of European and North American people with dyspepsia receive pharmacological treatment, and more than 30% report missing work or school hours because of burdensome symptoms (Overland 2014). Therefore, FD incurs direct and indirect costs (total yearly cost estimated at USD1595 per person), with additional costs associated with their impaired work productivity (Lacy 2013).
The clinical management of FD is problematic, reflecting the unknown cause and poorly understood pathophysiology (Talley 1991; Talley 1995). Different treatments have been proposed for the condition, including H2 receptor antagonists (H2RA), prokinetic agents (Bekhti 1979; Holtmann 2002; Talley 1998 (BOND); Talley 1998 (OPERA); Van Outryve 1993), proton pump inhibitors (PPIs) (McColl 1998; Wong 2002), Helicobacter pylori eradication therapy (Blum 1998; Froehlich 2001; Hamilton 2000; Talley 1999a; Talley 1999b), and even antidepressants or psychological interventions (Bolling‐Sternevald 2003; Calvert 2002). It has been previously shown that omeprazole (a PPI) produces higher intragastric pH values in H pylori‐positive compared to H pylori‐negative people (Verdú 1995). The apparently increased effect of omeprazole during H pylori infection may be a result of the production of acid‐neutralizing compounds by the H pylori (Bercik 2000).
Drugs that reduce gastric acid secretion are commonly prescribed for people with dyspepsia, but the efficacy of acid suppression in treating the condition is still controversial. Gastric acid secretion is a complex process regulated by at least three types of receptors (histamine, gastrin and acetylcholine) on the parietal cell. In contrast to H2RAs or anticholinergic agents, which only partially inhibit histamine‐, gastrin‐ or acetylcholine‐stimulated acid secretion, PPIs inhibit acid secretion in response to all stimulatory agents (Robinson 2004). Although gastric acid secretion is normal in people with FD (Chen 2000), a subset of these people benefit from strong acid suppression with a PPI (Wong 2002). Acid secretion inhibitory drugs are therefore widely prescribed to people with FD worldwide, but the underlying mechanisms of their effect are unknown (Suzuki 2011). It has been shown that about one‐third of people with FD have a normal 24‐hour pH profile (Chen 2000; Moayyedi 2011), and a clear relationship between acid exposure and severity of symptoms is far from evident in these people (Moayyedi 2003; Moayyedi 2011). The effect of H2RAs seems to be overestimated (Talley 1998 (BOND); Talley 1998 (OPERA)), and studies on the efficacy of PPIs have had variable results, depending on the protocol and inclusion criteria used (Bolling‐Sternevald 2003; Hansen 1998; Suzuki 2011). While PPIs are generally considered safe and well tolerated, there have been reports of associated Clostridium difficile infection, pneumonia, risk of fractures and acute interstitial nephritis in the long term (Wilhelm 2013). This has been a controversial topic that has been studied in detail (Abramowitz 2016; Scarpignato 2016). For this reason, decisions on whether to initiate or continue PPI therapy should be made based on an appropriate clinical indication (Yang 2010).
Description of the intervention
PPIs are the most widely used agents for the suppression of gastric acid. Following on from their demonstrated success in the treatment of gastroesophageal reflux disease and peptic ulcers, PPIs have been widely employed in the treatment of dyspeptic symptoms and in people with FD (Camillieri 2013; Lacy 2012). They have been proposed as the first step in the treatment of people with FD after H pylori eradication (in people who are positive for H pylori). However, the real effect of PPIs has been controversial. Evidence from randomized controlled trials (RCTs) suggests that the efficacy of PPIs in FD may be confined to those people who have coexisting reflux symptoms (Lacy 2012).
How the intervention might work
PPIs may be beneficial in a subset of people with FD. One meta‐analysis of placebo‐controlled RCTs of PPIs in FD included 3725 participants across seven studies (Wang 2007). Overall, the meta‐analysis concluded that PPI treatment was superior to placebo with a number needed to treat for an additional beneficial outcome (NNTB) of 15. In subgroup analyses, they found that the benefit of PPI over placebo was confined to people with 'ulcer‐like' and 'reflux‐like' dyspepsia; they found no advantage of PPI treatment among people with 'dysmotility‐like' or unspecified dyspepsia (Drossman 1999).
PPIs may also have advantages compared to prokinetics. Prokinetic agents are conceptually appealing: they have the potential to improve gastric emptying and are commonly used worldwide; however, the effect in FD is not clearly supported by the evidence (Lacy 2012).
PPIs may also benefit in comparison with H2RAs (Barbera 1995).
Why it is important to do this review
From 2000 to 2007, several systematic reviews and meta‐analyses were published which considered different treatments for FD, including PPIs (Hansen 1998; Moayyedi 2011; Suzuki 2011). Since then, newer RCTs addressing this issue have been added to the medical literature. However, no new systematic reviews have evaluated these studies and a former Cochrane Review has been withdrawn (Moayyedi 2011).
Acid secretion inhibitory drugs are widely prescribed to people with FD worldwide, but the underlying mechanisms of their effect are unknown. PPIs have been considered to be 'safe' drugs; however, some adverse effects were reported (Johnson 2013). Evidence of the real effect of PPIs in FD will therefore help us to understand better the need for PPIs in this specific population and to avoid the indiscriminate use of these drugs.
Due to the importance of the topic, we have conducted a systematic review of RCTs evaluating PPI therapy in FD using Cochrane methodology.
Objectives
To determine the efficacy of proton pump inhibitors in the improvement of global symptoms of dyspepsia and quality of life compared to placebo, H2 receptor antagonists or prokinetics, in people with functional dyspepsia.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs comparing the efficacy of different PPIs in people with an adequate diagnosis of FD (any validated criteria such as Rome I, II, III, IV or Lancet Working Group) (Colin‐Jones 1988; Drossman 1999; Drossman 2006; Stanghellini 2016). We included cross‐over studies only if the results were available before the cross‐over, so that the study could be evaluated as a parallel‐group study. We excluded cluster‐randomized trials.
Types of participants
We considered studies involving people aged over 16 years, of both genders, with a diagnosis of FD according to any well‐defined criteria (such as Rome I, II, III, IV or Lancet Working Group; Table 5), with a normal upper gastrointestinal endoscopy and with upper gastrointestinal symptoms including epigastric pain/discomfort. We excluded studies involving participants with other gastrointestinal conditions, such as peptic ulcer, organic dyspepsia and reflux disease. If a study included populations with different conditions, we only considered people with FD.
1. Definitions of functional dyspepsia.
| Functional dyspepsia | Rome I (1991) | Rome II (1999) |
Rome III (2006) Rome IV (2016) |
AGA Working Group | Lancet Working Group (1998) |
| Main criteria | Pain or discomfort centred in the upper abdomen with no evidence of organic disease. | Persistent or recurrent symptoms (pain or discomfort centred in the upper abdomen). AND No evidence that dyspepsia is exclusively relieved by defecation or associated with the onset of a change in stool frequency or stool form (exclude irritable bowel syndrome and exclude reflux). |
≥ 1 symptoms need to be present:
|
Chronic or recurrent pain or discomfort centred in the upper abdomen. | Chronic or recurrent pain or discomfort centred in the upper abdomen or retrosternal pain, discomfort, heartburn, nausea, vomiting or other symptoms of the gastrointestinal tract. |
| Normal upper endoscopy | Required. | Required. | Required. | Required. | Required. |
| Symptoms present for the last... | ‐ | 12 weeks, which need not be consecutive. | Criteria fulfilled for the last 3 months. | ≥ 3 months. | ≥ 4 weeks. |
| Onset of symptoms | ‐ | 12 months. | 6 months. | ‐ | ‐ |
| Subtypes |
|
|
|
‐ | Reflux‐like dyspepsia. |
AGA: American Gastroenterological Association.
Types of interventions
We included trials comparing oral administration of any dose of any PPI available (omeprazole, esomeprazole, pantoprazole, lansoprazole, dex‐lansoprazole or rabeprazole; for doses see Table 6) with placebo, H2RAs or prokinetics. We considered a combination of treatments in either intervention or control groups only if the combination of treatment was present in both groups. We considered therapy of at least two weeks' duration. We recorded and compared the time of the intervention and follow‐up.
2. Proton pump inhibitor equivalent doses.
| Proton pump inhibitor | Daily standard dose |
| Dex‐lansoprazole | 30 mg |
| Esomeprazole | 20 mg to 40 mg |
| Lansoprazole | 30 mg |
| Omeprazole | 20 mg |
| Pantoprazole | 40 mg |
| Rabeprazole | 20 mg |
Types of outcome measures
We measured outcomes as continuous (mean score pre‐ and post‐treatment) and dichotomous (improved or not improved). We measured the number of events in each group for adverse events.
Primary outcomes
Global symptoms of dyspepsia (using the most stringent definition of not symptom‐free) or epigastric pain/discomfort if global symptoms were not reported.
Secondary outcomes
Quality of life (QoL).
Adverse events.
Search methods for identification of studies
Electronic searches
We conducted a literature search to identify all published and unpublished RCTs. We considered studies regardless of language and publication status to avoid biases. We translated the non‐English language papers and fully assessed them for potential inclusion in the review as necessary. We only included data from abstracts if we were able to obtain further details from the investigators. We searched the following electronic databases for potential studies:
the Cochrane Library (to May 2017) (Appendix 2);
MEDLINE (OvidSP) (1946 to May 2017) (Appendix 3);
Embase (OvidSP) (1974 to May 2017) (Appendix 4).
The Cochrane Library databases include Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Methodology Register (CMR), Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment Database (HTA) and NHS Economic Evaluation Database (EED). The search strategies were constructed by using a combination of subject headings and text words relating to dyspepsia and PPIs.
Searching other resources
We performed handsearching of healthcare journals and conference proceedings (i.e. Digestive Diseases Week, United European Gastroenterology Week). We checked the reference lists of all primary studies and review articles for additional references. We contacted the authors of identified trials and ask them to identify other published and unpublished studies. We contacted manufacturers and experts in the field. We searched for errata or retractions from eligible trials in PubMed (www.ncbi.nlm.nih.gov/pubmed) and reported the date this was done within the review. We also conducted a search of ClinicalTrials.gov for clinical trials. We made efforts to identify unpublished studies. We searched the grey literature (e.g. conference reports, technical reports and dissertations) using SIGLE (Appendix 5)). EAGLE (the European Association for Grey Literature Exploitation) has closed the SIGLE (System for Information on Grey Literature; www.opengrey.eu/) database, which was one of the most widely used databases of grey literature.
Data collection and analysis
Selection of studies
We used Review Manager 5 to collect and manage citations (Reference Manager 2014). We identified and excluded duplicates and collate multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1).
1.
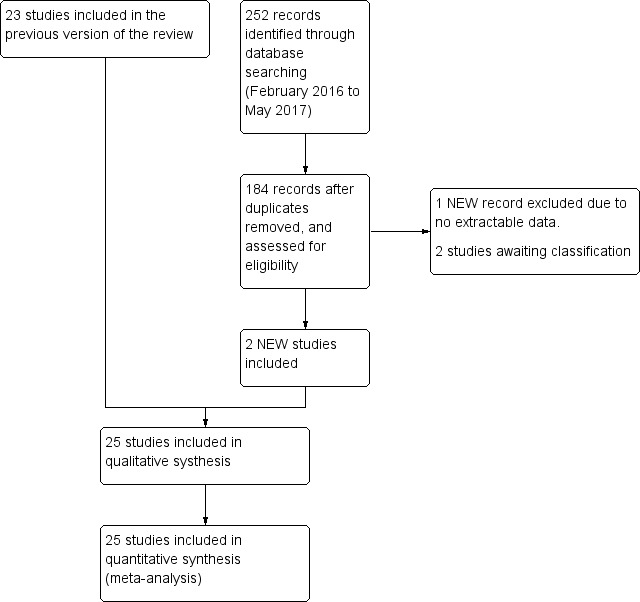
Study flow diagram.
To ensure that we identified all eligible studies, two review authors (MIP and YY) independently screened the abstracts and selected trials according to the inclusion and exclusion criteria. We documented study selection or exclusion and created a list of studies to be included in the analysis (see Characteristics of included studies table). We resolved any disagreement through discussion or consultation with a third review author (PM). At this initial stage, we included studies where there was disagreement or where it was difficult to decide whether a study should be included.
We identified studies in which participants with an adequate diagnosis of FD (any valid criteria for FD with a normal upper gastrointestinal endoscopy) were randomized to receive any type of PPI versus another prespecified treatment or placebo. We considered and recorded the general characteristics and outcomes of each study using a screening form. We piloted the form on the first five studies included in the list and made changes if necessary. The screening form recorded the title, author, date, study design (only RCTs were included), population characteristics, intervention and control treatment duration, and outcomes according to the PICO question (population (P), intervention (I), comparison (C) and outcome(s) (O)). We provided a section for general comments, for any review author considerations and future discussion. We identified and removed duplicate studies at this initial stage.
We combined the results of the title and abstract screening performed by the review authors and document and discussed decisions about inclusion in the final full‐text screening list. To ensure that inclusion and exclusion criteria were properly interpreted and selection bias was minimized, three different review authors (AH, MIP and YY) performed the screening of the full texts. For papers in languages other than English, we requested translation by a translator from Cochrane with experience in systematic reviews and medicine. If the paper met the inclusion criteria, we asked the translator to extract data on the predefined data extraction form. The two review authors received the full‐text journal articles and translations to perform the screening. We collected the full‐text screening data in an Excel sheet and compared the results. We calculated the level of agreement after each step: title and abstract screening, full‐text screening and data extraction using Kappa statistics for categorical data (GraphPad), and raw agreement for continuous data. We reported raw agreement as a percentage and Kappa as fair agreement (К = 0.4 to 0.59), good agreement (0.6 to 0.74) or excellent agreement (0.75 or greater).
Data extraction and management
We used a standard data collection form for study characteristics and outcome data, which was piloted on five studies. Three review authors (AH, MIP and YY) extracted the following study characteristics from included studies.
Methods: study design, total duration of study and run‐in, number of study centres and location, study setting, withdrawals, date of study.
Participants: number, mean age, age range, gender, severity of condition, diagnostic criteria, inclusion criteria, exclusion criteria.
Interventions: intervention, comparison, concomitant medications, excluded medications.
Outcomes: primary and secondary outcomes specified and collected, time points reported.
Notes: funding for trial, notable conflicts of interest of trial authors.
Two review authors (MIP and AH) independently extracted outcome data from the included studies. We noted in the Characteristics of included studies table if outcome data were reported in an unusable way. We resolved disagreements by consensus or by involving a third review author (PM). One review author (MIP) copied across the data from the data collection form into Review Manager 5 (RevMan 2014). Two review authors (YY and PM) double‐checked that the data were entered correctly by comparing the study reports with how the data were presented in the systematic review.
We collected blinding information by individually identifying the person blinded. If this information was not reported, we recorded the study as 'single‐blind' (implying that probably only the study participants were blinded), 'double‐blind' (implying that the study participants, healthcare providers, data collectors and assessors were blinded but not the data analysts) or 'triple‐blind' (implying that the data analysts were also blinded).
If any information was missing at the end of data extraction process, we contacted the authors of the trials to recover the specific information. We included information on the following outcomes on the form: global symptoms, QoL and adverse events. We detailed common adverse events (such as such as diarrhoea, intolerance, nausea, headaches). We recorded participant demographics, treatment outcomes and adverse events as mean (standard deviation (SD)), n/N or % when applicable. We also collected information to assess possible risk of bias (randomization, concealment, blinding of participants and outcome assessors, incomplete outcome data, selective reporting and other biases).
Assessment of risk of bias in included studies
Three review authors (AH, MIP and YY) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We assessed the risk of bias according to the following domains: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective outcome reporting and other bias. We graded each potential source of bias as high, low or unclear and provide a quote from the study report together with a justification for our judgement in the 'Risk of bias' table for each study.
We summarized the 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary, for example, for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different from a participant‐reported pain scale). Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias of the studies that contributed to that outcome. We entered the data related to risk of bias into Review Manager 5 (RevMan 2014) and construct the 'Risk of bias' tables. We generated two figures with the Review Manager 5 software: a 'Risk of bias' summary, which represents all the judgements in a cross‐tabulation of study by entry and a 'Risk of bias' graph, which illustrates the proportion of studies complying with each of the judgements (low, high and unclear risk of bias).
GRADE and 'Summary of findings' tables
We used the GRADE system for the assessment of the quality of the evidence, and developed 'Summary of findings' tables using the GRADEpro software (GRADEpro). One review author (MIPS) performed GRADE assessment and another checked the consistency of the information. We graded the quality of evidence as high, moderate, low or very low, depending on study limitations, consistency, directness, precision and publication bias of each outcome.
Measures of treatment effect
We analyzed dichotomous data as risk ratio (RR) and continuous data as mean difference (MD) or standardized mean difference (SMD). We reported information regarding the study population follow‐up (participants enrolled and randomized) as the data collected from discontinued participants over the total number of participants for each arm (n/N). We reported the total number of participants with symptoms related to dyspepsia in each arm at each time point (before and after treatment) as a number over the total sample population (n/N) in each arm.
We reported the comparison of binary data as an RR with an associated 95% confidence interval (CI), and the NNTB. The number needed to treat was calculated according to the following formula: NNTB = 100/ARR and ARR = 100 × ACR × (1 ‐ RR), where ARR was the absolute risk reduction and ACR was the assumed control risk (Higgins 2011). Standardized MD (SMD) was used to pool post‐treatment quality scores from different studies, since different scales were used to assess QoL. However,post‐treatment scores and change from baseline scores from different quality scales were not pooled as final values and change scores should not be combined as standardized mean differences (Deeks 2011).
We collected continuous outcome data in three different ways:
unit of measurement or, if unit of measurement could not be reported (i.e. visual analogue scale), we considered the data to be unit‐less;
measure of central tendency: mean, median, mode;
measure of variance, such as SD, standard error (SE), interquartile range or 95% CI.
If we were not provided with the raw data, we collected the reported analysis.
We collected change scores (the difference between scores before and after intervention) for comparison. We compared the final values of post‐treatment scores when change scores were not available. We undertook meta‐analyses only where this was meaningful (i.e. if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense). A common way in which trialists indicate that they have skewed data is by reporting medians and interquartile ranges. When we encounter this, we noted that the data were skewed and considered the implication of this. Where multiple trial arms were reported in a single trial, we included only the relevant arms. If two comparisons (e.g. drug A versus placebo and drug B versus placebo) needed to be entered into the same meta‐analysis, we halved the control group to avoid double‐counting.
Unit of analysis issues
The unit of analysis was the individual participant included in the studies. We analyzed cross‐over studies as parallel‐group study only if the results were available before the cross‐over.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as an abstract only). We contacted study investigators whenever possible to request missing data. If this was not possible, or data were not provided, we considered that participants with missing data did not have the outcome of interest. We performed sensitivity analyses to assess robustness of the results relative to reasonable changes in the assumptions that were made.
We addressed the potential impact of missing data on the findings of the meta‐analysis in the Discussion section.
Assessment of heterogeneity
Heterogeneity in systematic reviews can occur because of artefactual or real differences in treatment effects across the different studies included in the review (Tett 2013), and the reasons behind it should be carefully investigated. We considered all EPICOT components (evidence, population, intervention, comparison, outcome and time stamp), as well as internal validity issues (such as compliance, cointervention and randomization) in the analysis.
We preidentified potential sources of heterogeneity that could be related to the criteria considered for the FD definition and differences in the demographics of the included population: time, duration and dose of PPI; undetected cointervention and differences in outcomes measurements. To address the most important possible sources of heterogeneity, we performed subgroup analysis.
We assessed statistical heterogeneity with both the I2 statistic and the Chi2 test. An I2 value of 0% indicates no observed heterogeneity and larger values denote heterogeneity. We considered that heterogeneity might be not important when the I2 statistic was between 0% and 40%; moderate heterogeneity when between 30% and 60%; substantial heterogeneity when between 50% and 90% and considerable heterogeneity when greater than 75%; or there was a P value of less than 0.1 for the Chi2 test (Higgins 2011).
Assessment of reporting biases
We attempted to contact study authors and asked them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
If we could pool more than 10 trials, we created and examined a funnel plot to explore possible publication biases. In the graph, the effect estimates were shown on the horizontal scale and the measure of study size on the vertical axis. Asymmetric funnel plots suggested small‐study effects, publication bias, delayed publication (time lag), selective reporting outcome or even differences in methodological quality. Egger's test was used for detecting significant funnel plot asymmetry (RevMan 2014).
Data synthesis
To be able to combine the results, we considered some possible differences before performing the meta‐analysis. We created a forest plot of the meta‐analysis for quantitative synthesis. We addressed differences in the research question, population, intervention, comparators, outcomes and methodology. We included different comparators (placebo or other active comparators such as H2RAs or prokinetics) in the analysis. However, we separated studies with different comparators into different subgroups for their analysis. For quantitative analysis, we performed a meta‐analysis using Review Manager 5 (RevMan 2014). We calculated a summary statistic for each study to describe the observed intervention effect. In the case of dichotomous outcomes, we calculated an RR and for continuous data we calculated an MD. When outcomes were assessed by different instruments, we used the SMD when possible. We calculated a summary (pooled) intervention effect estimate as a weighted mean of the intervention effects estimated in the individual studies. We chose the weights to reflect the amount of information that each study contained. For the combination of intervention effect estimates across studies, we assumed that the studies were not all estimating the same intervention effect, but estimating intervention effects that followed a distribution across studies. We therefore considered a random‐effects model meta‐analysis to be adequate (Kwok 2013). However, since the correct selection of the model is controversial, we also performed a fixed‐effect model analysis and compared the results of both. If these models had similar results, we considered that the chances of heterogeneity being present across the studies were low. Otherwise, if the results were different, we considered the random‐effects model as the most appropriate for the reasons previously described. To communicate the strength of evidence against the null hypothesis of no intervention effect, we used measures of dispersion (such as SE) to derive a CI and a P value.
Subgroup analysis and investigation of heterogeneity
We carried out the following subgroup analyses to reveal any effect that might explain any heterogeneity:
treatment duration (less than four weeks versus four weeks and versus greater than four weeks);
dose (standard‐dose versus low‐dose of PPI);
PPI subtype;
H pylori status (H pylori‐negative versus H pylori‐positive);
presence of reflux, defined as abnormal 24‐hour pH study (pH less than 4 for more than 4% of the 24‐hour recording versus pH less than 4 for less than 4% of the 24‐hour recording);
risk of bias (low versus unclear versus high risk of bias);
geographical location (e.g. western versus eastern studies);
trial funding sources (industry‐sponsored versus non‐industry‐sponsored studies).
We assessed differences between subgroups with the I2 statistic to test for subgroup interactions.
Sensitivity analysis
We performed sensitivity analyses using different summary statistics (RR versus odds ratio (OR)) and meta‐analytic models (fixed‐effect versus random‐effects), to assess the robustness of our results.
Results
Description of studies
Results of the search
The search up to May 2017 identified 1876 records. After removal of duplicates, we initially screened 1521 citations resulting from the electronic searches. Based on consideration of their titles and abstracts, we excluded 1453 citations while 67 papers were targeted for full‐article review, either because they were potentially relevant, or because not enough information was reported in the title and abstract to make a final decision. We included 25 studies from 27 papers (Characteristics of included studies table), excluded 38 studies (Characteristics of excluded studies table), two studies were classified as awaiting classification (Characteristics of studies awaiting classification table). (Figure 1). There was very good inter‐reviewer agreement at the full‐text stage (k = 0.94, SE of kappa = 0.046; 95% CI 0.84 to 1.0).
Included studies
We included 25 studies from 27 publications (Characteristics of included studies table). Of the 25 included studies, 22 were published in English and three in Chinese (Jiang 2011; Li 2003; Yang 2014). Four studies identified during the search were published only as abstracts (Catapani 2015; Dillon 2004; Hengels 1998; Tominaga 2010). We contacted authors from these studies and requested further information. Only two authors supplied additional information (Catapani 2015; Tominaga 2010).
Country of origin
Four of 25 studies were from Japan (Iwakiri 2013; Kamiya 2017; Suzuki 2013 (ELF); Tominaga 2010); three from the US (Gerson 2005; Majewski 2016; Peura 2004); three from China (Jiang 2011; Li 2003; Yang 2014); two from the UK (Dillon 2004; Fletcher 2011); two from Germany (Blum 2000; Hengels 1998); one from Canada (Van Zanten 2006); one from Hong Kong (Wong 2002); one from Korea (Jung 2016); one from Norway (Farup 1999); one from Brazil (Catapani 2015), and one from Taiwan (Hsu 2011). Five studies were from multiple countries (Bolling‐Sternevald 2002; Talley 1998 (BOND); Talley 1998 (OPERA); Talley 2007; Van Rensburg 2008).
Interventions
Seventeen studies included two treatment arms (Bolling‐Sternevald 2002; Catapani 2015; Dillon 2004; Fletcher 2011; Gerson 2005; Hengels 1998; Hsu 2011; Jiang 2011; Kamiya 2017; Li 2003; Majewski 2016; Suzuki 2013 (ELF); Talley 2007; Tominaga 2010; Van Rensburg 2008; Van Zanten 2006; Yang 2014); six studies included three arms (Farup 1999; Jung 2016; Peura 2004; Talley 1998 (BOND); Talley 1998 (OPERA); Wong 2002); and two studies included four arms (Blum 2000; Iwakiri 2013).
From the studies with two arms, 12 compared standard doses of PPIs either with placebo (Bolling‐Sternevald 2002; Fletcher 2011; Gerson 2005; Majewski 2016; Talley 2007; Van Rensburg 2008; Van Zanten 2006), prokinetics (Hsu 2011; Jiang 2011; Kamiya 2017; Yang 2014), or H2RA (ranitidine) (Dillon 2004); four compared low‐dose PPIs either with placebo (Hengels 1998; Suzuki 2013 (ELF); Tominaga 2010), or prokinetics (Li 2003). The dose of PPI was not reported in Catapani 2015.
From the studies with three arms, five compared low and standard doses of PPIs with placebo (Farup 1999; Peura 2004; Talley 1998 (BOND); Talley 1998 (OPERA); Wong 2002), and one compared standard‐dose PPI, prokinetic and PPI plus prokinetic (Jung 2016).
From the two studies with four arms, one compared low‐dose PPI with standard‐dose PPI, ranitidine and placebo (Blum 2000), and one compared low‐dose, standard‐dose and high‐dose PPIs and placebo (Iwakiri 2013).
Duration of intervention
The duration of intervention ranged from two to eight weeks. Seven studies reported treatment for two weeks (Blum 2000; Bolling‐Sternevald 2002; Fletcher 2011; Hengels 1998; Hsu 2011; Jiang 2011; Li 2003); 12 studies reported treatment for four weeks (Farup 1999; Gerson 2005; Jung 2016; Kamiya 2017; Majewski 2016; Suzuki 2013 (ELF); Talley 1998 (BOND); Talley 1998 (OPERA); Tominaga 2010; Van Rensburg 2008; Wong 2002; Yang 2014); five studies reported eight weeks of treatment (Dillon 2004; Iwakiri 2013; Peura 2004; Talley 2007; Van Zanten 2006); and one study had unclear duration although participants were followed up for six months (Catapani 2015).
Definition of functional dyspepsia
The definition for FD differed according to the study and the year of publication. Six studies defined FD as "persistent or recurrent epigastric pain and/or discomfort in participants with normal findings at upper gastrointestinal endoscopy and with symptoms at least one month's duration" (Blum 2000; Bolling‐Sternevald 2002; Farup 1999; Li 2003; Talley 1998 (BOND); Talley 1998 (OPERA)). The same studies considered the presence of symptoms during the seven‐day run‐in period. Seven studies defined FD based on the Rome II criteria (Catapani 2015; Dillon 2004; Gerson 2005; Majewski 2016; Peura 2004; Van Zanten 2006; Wong 2002); eight studies on Rome III criteria (Hsu 2011; Iwakiri 2013; Jiang 2011; Jung 2016; Kamiya 2017; Suzuki 2013 (ELF); Tominaga 2010; Yang 2014); and three using the American Gastroenterological Association (AGA) Working Group definition (Fletcher 2011; Talley 2007; Van Rensburg 2008). One study included participants with dyspeptic symptoms present for at least one week (Hengels 1998).
Excluded studies
After full‐text review, we excluded 38 studies (Almazar 2015; Bolling‐Sternvald 2003; Burkov 2009; Bytzer 2000; Cheung 2013; Chuang 2001; Delaney 2008; Fan 2012; Fransen 2012; Goves 1998; Guo 2011; Ivanova 2002; Jones 1997; Jones 1999; Kamada 2013; Leung 2007; Lewin van den Broek 2001; Madsen 2004; Madsen 2008; Mazure 1996; Meineche‐Schmidt 1999; Meineche‐Schmidt 2000; Meineche‐Schmidt 2004; Miwa 2015; Mönnikes 2009; Mönnikes 2012; Nagahara 2015; Pilichiewicz 2011; Rabeneck 2001 (SODA); Reimer 2010; Rui 2015; Sakaguchi 2012; Sakurai 2012 (J‐FOCUS); Schwartz 2001; Theodoropoulos 2009; van Zanten 2007; Veldhuyzen van Zanten 2005; Zeng 2007). The reasons for exclusion were detailed in Characteristics of excluded studies table.
Studies awaiting classification
Two studies, one published only as an abstract, did not reported extractable data (Puttapitakpong 2016; Yamawaki 2016). We contacted the authors requesting further information but received no response.
Ongoing studies
The search found no ongoing studies.
Risk of bias in included studies
Sequence generation (selection bias)
We categorized 18 trials at low risk of selection bias (Bolling‐Sternevald 2002; Catapani 2015; Fletcher 2011; Hsu 2011; Iwakiri 2013; Jiang 2011; Jung 2016; Kamiya 2017; Li 2003; Majewski 2016; Peura 2004; Suzuki 2013 (ELF); Talley 1998 (BOND); Talley 1998 (OPERA); Talley 2007; Van Rensburg 2008; Van Zanten 2006; Wong 2002), and the rest at unclear risk of selection bias. A graphical representation of the 'risk of bias' assessment is shown in Figure 2 (a summary of the risk of bias) and Figure 3 (which shows the risk of bias for individual studies).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
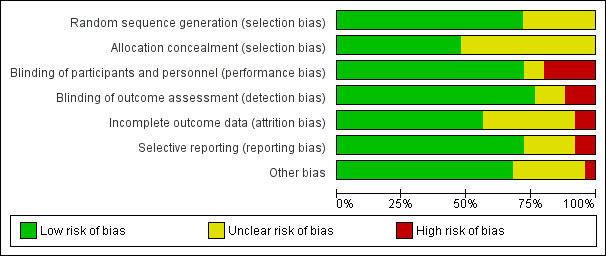
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Allocation concealment was adequate and therefore at low risk of bias in 12 trials (Fletcher 2011; Gerson 2005; Hsu 2011; Iwakiri 2013; Jung 2016; Suzuki 2013 (ELF); Talley 1998 (BOND); Talley 1998 (OPERA); Talley 2007; Van Rensburg 2008; Van Zanten 2006; Wong 2002). The rest of the studies were at unclear risk of bias.
Blinding
Eighteen studies were designed as double‐blind, and therefore at low risk of bias (Blum 2000; Bolling‐Sternevald 2002; Farup 1999; Fletcher 2011; Gerson 2005; Hengels 1998; Iwakiri 2013; Jung 2016; Majewski 2016; Peura 2004Suzuki 2013 (ELF); Talley 1998 (BOND); Talley 1998 (OPERA); Talley 2007; Tominaga 2010; Van Rensburg 2008; Van Zanten 2006; Wong 2002). Five studies were at high risk for performance bias (one single‐blind study (Dillon 2004), four open‐label studies (Hsu 2011; Jiang 2011; Kamiya 2017; Li 2003). Blinding of personnel was performed and was unclear for participants in one study (Catapani 2015). Blinding of participants and personnel was unclear in one study (Yang 2014).
Nineteen studies were at low risk of detection bias (Blum 2000; Bolling‐Sternevald 2002; Catapani 2015; Fletcher 2011; Gerson 2005; Hengels 1998; Iwakiri 2013; Jung 2016; Li 2003; Majewski 2016; Peura 2004; Suzuki 2013 (ELF); Talley 1998 (BOND); Talley 1998 (OPERA); Talley 2007; Tominaga 2010; Van Rensburg 2008; Van Zanten 2006; Wong 2002) and three studies were at high risk of detection bias (Hsu 2011; Jiang 2011; Kamiya 2017). Blinding of outcomes assessors was unclear in three studies (Dillon 2004; Farup 1999; Yang 2014).
Incomplete outcome data
Fourteen studies reported all planned outcomes, which also provided data for intention‐to‐treat (ITT) analysis (Blum 2000; Bolling‐Sternevald 2002; Fletcher 2011; Gerson 2005; Hsu 2011; Iwakiri 2013; Jiang 2011; Jung 2016; Li 2003; Majewski 2016; Suzuki 2013 (ELF); Talley 1998 (BOND); Talley 1998 (OPERA); Yang 2014). Discontinued participants were imbalanced between arms in two studies, and therefore identified at high risk for attrition bias (Catapani 2015; Talley 2007). Information on lost to follow‐up or discontinuation of treatment was unclear or the potential impact of missing data on effect estimates was unclear in nine studies (Dillon 2004; Farup 1999; Hengels 1998; Kamiya 2017; Peura 2004; Tominaga 2010; Van Rensburg 2008; Van Zanten 2006; Wong 2002). Most trials did not report data from participants who dropped out.
Selective reporting
We attempted to identify protocols to check that studies reported prespecified outcomes. Eighteen studies reported all intended outcomes, and therefore were at low risk of bias (Blum 2000; Bolling‐Sternevald 2002; Fletcher 2011; Gerson 2005; Iwakiri 2013; Jiang 2011; Jung 2016; Kamiya 2017; Li 2003; Peura 2004; Suzuki 2013 (ELF); Talley 1998 (BOND); Talley 1998 (OPERA); Talley 2007: Van Rensburg 2008; Van Zanten 2006; Wong 2002; Yang 2014). One study reported that symptomatic improvement was initially planned for low‐dose omeprazole versus high‐dose (Farup 1999). Because of similar effect and low numbers of participants, the results were grouped; however, the individual data were not shown. This study, and another that terminated early (Hsu 2011), were identified at high risk of reporting bias. There was insufficient information on planned outcomes in other five trials and therefore risk for reporting bias was unclear for these studies (Catapani 2015; Dillon 2004; Hengels 1998; Majewski 2016; Tominaga 2010).
Other potential sources of bias
Seventeen trials were at low risk of other bias (Blum 2000; Fletcher 2011; Hsu 2011; Iwakiri 2013; Jiang 2011; Jung 2016; Kamiya 2017; Li 2003; Majewski 2016; Peura 2004; Talley 1998 (BOND); Talley 1998 (OPERA); Talley 2007; Van Rensburg 2008; Van Zanten 2006; Wong 2002; Yang 2014). Seven trials were at unclear risk of other bias (Bolling‐Sternevald 2002; Catapani 2015; Dillon 2004; Farup 1999; Hengels 1998; Suzuki 2013 (ELF); Tominaga 2010). We identified one study in which the groups had imbalanced symptoms scores at baseline, with a potential bias on the treatment effect estimate (Gerson 2005).
Reporting bias
A funnel plot was performed including studies comparing PPI versus placebo. Visually the funnel plot appeared to be symmetrical (Figure 4); decreasing the chances of publication bias. No significant funnel plot asymmetry was seen using Egger's test (P = 0.47).
4.
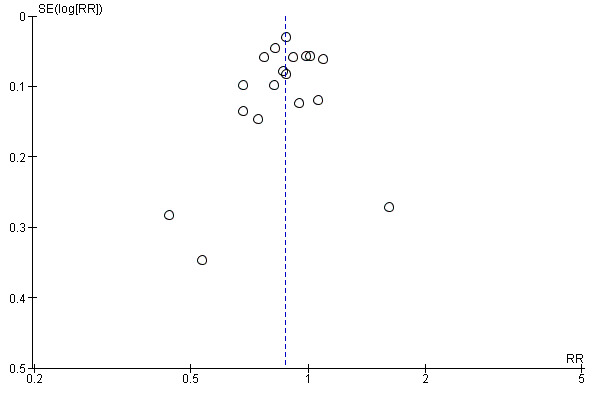
Funnel plot of comparison: 3 Proton pump inhibitors versus placebo, outcome: 3.1 Global symptoms (two to eight weeks).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Proton pump inhibitors (PPI) compared to placebo for functional dyspepsia.
| PPI versus placebo for functional dyspepsia | ||||||
| Patient or population: functional dyspepsia Setting: secondary and tertiary centres Intervention: PPI Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Proton pump inhibitors (PPI) | |||||
| Global symptoms of dyspepsia (> 2 weeks) | Study population | RR 0.88 (0.82 to 0.94) | 6172 (18 RCTs) | ⊕⊕⊕⊝ Moderate1 | Measurement of no improvement. | |
| 714 per 1000 | 629 per 1000 (586 to 671) | |||||
| Quality of life Psychological General Well‐Being Index (Scale from: 22 to 132) and SF‐36 (Scale from: 0 to 100) combined | The mean post‐treatment PGWB score was 99.84, the mean post‐treatment SF‐36 score was 66.2 | SMD 0.01 higher (0.09 lower to 0.11 higher) | 1630 (3 RCTs) | ⊕⊕⊕⊝ Moderate2 | Higher scores means better quality of life. | |
| Adverse events | Study population | RR 0.99 (0.73 to 1.33) | 2693 (6 RCTs) | ⊕⊕⊕⊝ Moderate1 | Number of adverse events. | |
| 191 per 1000 | 189 per 1000 (140 to 254) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; PPI: proton pump inhibitor; RCT: randomized controlled trial; RR: risk ratio; SF‐36: 36‐item Short Form. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level due to serious inconsistency between studies.
2Downgraded one level due to imprecision.
Summary of findings 2. Proton pump inhibitors (PPI) compared to H2 receptor antagonists (H2RA) for functional dyspepsia.
| PPI versus H2RA for functional dyspepsia | ||||||
| Patient or population: adults with functional dyspepsia Setting: secondary centres Intervention: PPI Comparison: H2RA | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with H2RA | Risk with PPI | |||||
|
Global symptoms of dyspepsia Follow‐up: range 2‐8 weeks |
Study population | RR 0.88 (0.74 to 1.04) | 740 (2 RCTs) | ⊕⊕⊝⊝ Low1 | Measurement of no improvement. | |
| 739 per 1000 | 650 per 1000 (547 to 769) | |||||
|
Quality of life Follow‐up: range 2‐8 weeks |
‐ | ‐ | Not estimable | (0 studies) | ‐ | No data available. |
|
Adverse events Follow‐up range 2‐8 weeks |
Study population | RR 0.97 (0.64 to 1.46) | 589 (1 RCT) | ⊕⊕⊕⊝ Moderate2 | Number of adverse events. | |
| 144 per 1000 | 137 per 1000 (89 to 209) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; H2RA: H2 receptor antagonist; PPI: proton pump inhibitor; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded two levels due to imprecision, substantial heterogeneity (I2 = 51%) and high risk of bias in one of the two studies.
2Downgraded one level due to serious imprecision (95% CI included appreciable benefit and harm and low number of events).
Summary of findings 3. Proton pump inhibitors (PPI) compared to prokinetics for functional dyspepsia.
| PPI versus prokinetics for functional dyspepsia | ||||||
| Patient or population: adults with functional dyspepsia Setting: secondary and tertiary centres Intervention: PPI Comparison: prokinetic | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with prokinetic | Risk with PPI | |||||
|
Global symptoms of dyspepsia Follow‐up: range 2‐4 weeks |
Study population | RR 0.89 (0.81 to 0.99) | 1033 (5 RCTs) | ⊕⊕⊝⊝ Low1,2 | Measurement of no improvement. | |
| 495 per 1000 | 441 per 1000 (401 to 490) | |||||
|
Quality of life
Korean version of the dyspepsia related Nepean Dyspepsia Index (NDI) from: 0 to 99 Follow‐up: range 2‐4 weeks |
The mean NDI score change from baseline was 20.4 | MD 0.5 lower (4.42 lower to 3.42 higher) | 262 (1 RCT) | ⊕⊕⊕⊝ Moderate1 | Higher scores denote better outcome. | |
|
Adverse events Follow‐up: 4 weeks |
Study population | RR 1.09 (0.79 to 1.49) | 1033 (5 RCTs) | ⊕⊕⊕⊝ Moderate3 | Number of adverse events. | |
| 113 per 1000 | 123 per 1000 (89 to 168) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PPI: proton pump inhibitor; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level due to serious imprecision.
2Downgraded one level due to risk of bias in four of the five studies.
3We did not consider the impact of risk of bias and inconsistency on the results to be serious enough to justify fully downgrading two levels so we have downgraded one level in respect of both considerations.
Summary of findings 4. Proton pump inhibitors plus prokinetics compared to prokinetics alone for functional dyspepsia.
| PPI + prokinetics versus prokinetics alone for functional dyspepsia | ||||||
| Patient or population: adults with functional dyspepsia Setting: secondary and tertiary centres Intervention: PPI + prokinetic Comparison: prokinetic alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with prokinetic alone | Risk with PPI + prokinetic | |||||
|
Global symptoms of dyspepsia Follow‐up range: 2 to 4 weeks |
Study population | RR 0.85 (0.68 to 1.08) | 407 (2 RCTs) | ⊕⊕⊕⊝ Moderate1 | Measurement of no improvement. | |
| 444 per 1000 | 377 per 1000 (302 to 479) | |||||
|
Quality of life
Korean version of the dyspepsia related Nepean Dyspepsia Index (0 to 99) and Functional Digestive Disorders Quality of Life questionnaire (FDDQL) scale Follow‐up: 4 weeks |
NDI | ‐ | 258 (1 RCT) | ⊕⊕⊕⊝ Moderate1 | Higher scores denote better outcome. | |
| The mean NDI score change from baseline was 20.4 | MD for NDI score change from baseline 1.10 lower (5.22 lower to 3.02 higher) | |||||
| FDDQL | ‐ | 149 (1 RCT) | ⊕⊕⊕⊝ Moderate1 | Higher scores denote better outcome. | ||
| The mean post‐treatment FDDQL score was 70.56 | MD for FDDQL score 18.96 higher (17.01 lower to 20.91 higher) | |||||
|
Adverse events Follow‐up: 4 weeks |
Study population | RR 0.60 (0.39 to 0.93) | 407 (2 RCTs) | ⊕⊕⊕⊝ Moderate1 | Number of adverse events. | |
| 220 per 1000 | 132 per 1000 (86 to 204) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; PPI: proton pump inhibitor; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level due to serious imprecision .
Some trials contributed more than one comparison as some evaluated more than two interventions.
Standard‐dose versus low‐dose proton pump inhibitor
Global symptoms of dyspepsia
Six studies with 2304 participants provided data to compare low‐ and standard‐dose PPI therapy for a reduction of global symptoms of dyspepsia (Blum 2000; Iwakiri 2013; Peura 2004; Talley 1998 (BOND); Talley 1998 (OPERA); Wong 2002). There was no difference between the two doses of PPI therapy (RR of remaining dyspeptic on standard‐dose PPI 0.97, 95% CI 0.92 to 1.02; P = 0.21) with no heterogeneity between subgroups (I2 = 0%; Chi2 = 2.54; degrees of freedom (df) = 5; P = 0.77) (Analysis 1.1). Therefore, we combined results of all doses of PPI therapy in the following analyses.
1.1. Analysis.

Comparison 1 Standard‐dose versus low‐dose proton pump inhibitors (PPI), Outcome 1 Global symptoms of dyspepsia.
Proton pump inhibitor versus placebo
Global symptoms of dyspepsia
Eighteen eligible studies with 6172 participants were included in the global assessment of dyspepsia analysis (Blum 2000; Bolling‐Sternevald 2002; Catapani 2015; Farup 1999; Fletcher 2011; Gerson 2005; Hengels 1998; Iwakiri 2013; Majewski 2016; Peura 2004; Suzuki 2013 (ELF); Talley 1998 (BOND); Talley 1998 (OPERA); Talley 2007; Tominaga 2010; Van Rensburg 2008; Van Zanten 2006; Wong 2002).
PPI therapy was more effective than placebo, with 31.1% of the PPI group reporting no or minimal symptoms compared with 25.8% of the placebo group (RR of remaining dyspeptic 0.88, 95% CI 0.82 to 0.94; P < 0.001, random‐effects model) with an NNTB of 11. There was considerable heterogeneity between trials (I2 = 71%; Chi2 = 57.74; df = 17; P < 0.001) (Analysis 2.1) with no significant funnel plot asymmetry Egger's bias (‐0.63, 95% CI ‐2.49 to 1.21; P = 0.47). In a sensitivity analysis, the effect remained significant with OR (OR 0.63, 95% CI 0.49 to 0.81) and with fixed‐effect model (RR 0.88, 95% CI 0.85 to 0.91).
2.1. Analysis.
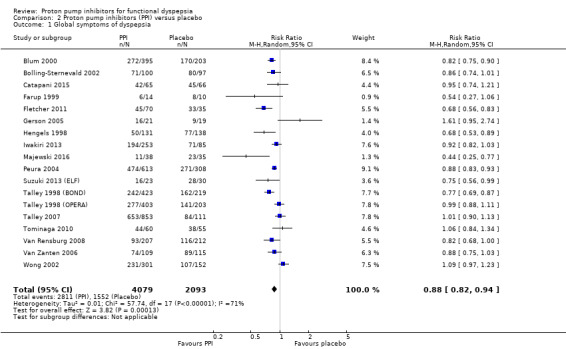
Comparison 2 Proton pump inhibitors (PPI) versus placebo, Outcome 1 Global symptoms of dyspepsia.
Quality of life
Six studies investigated the effect of PPIs versus placebo on QoL (Blum 2000; Bolling‐Sternevald 2002; Talley 1998 (BOND); Talley 1998 (OPERA); Van Zanten 2006; Wong 2002).
Blum 2000 assessed QoL using a validated questionnaire translated into German. The authors reported an improvement on QoL with standard doses of omeprazole compared to placebo in H pylori‐positive participants, but the effect was not so pronounced in H pylori‐negative participants (data were not shown). In a similar direction, Van Zanten 2006 found differences in improvement of five domains of the Quality of Life in Reflux and Dyspepsia (QoLRAD) questionnaire at four weeks but not at eight weeks for esomeprazole versus placebo. In contrast, four trials found no differences in QoL assessed by the Psychological General Well‐Being Index (PGWBI) (Bolling‐Sternevald 2002; Talley 1998 (BOND); Talley 1998 (OPERA)), or by the 36‐item Short Form (SF‐36) (Wong 2002). Data were not shown in the study by Bolling‐Sternevald 2002.
We found data for quantitative analysis for three studies from two papers (Talley 1998 (BOND); Talley 1998 (OPERA); Wong 2002). There were no differences in QoL between PPIs and placebo assessed by the PGWBI (Talley 1998 (BOND); Talley 1998 (OPERA)), and by SF‐36 (Wong 2002) (SMD 0.01, 95% CI ‐0.09 to 0.11) (Analysis 2.10).
2.10. Analysis.

Comparison 2 Proton pump inhibitors (PPI) versus placebo, Outcome 10 Quality of life.
Adverse events
Fourteen studies comparing PPIs versus placebo reported information on adverse events (Blum 2000; Bolling‐Sternevald 2002; Farup 1999; Fletcher 2011; Hengels 1998; Iwakiri 2013; Peura 2004; Suzuki 2013 (ELF); Talley 1998 (BOND); Talley 1998 (OPERA); Talley 2007; Van Rensburg 2008; Van Zanten 2006; Wong 2002). We excluded data from eight studies from the meta‐analysis (Bolling‐Sternevald 2002; Farup 1999; Peura 2004; Suzuki 2013 (ELF); Talley 1998 (BOND); Talley 1998 (OPERA); Van Zanten 2006; Wong 2002). Peura 2004 and Van Zanten 2006 reported individual adverse events rather than overall adverse events. Farup 1999 found no clinically significant adverse events; however, information on adverse events was not shown. Suzuki 2013 (ELF) reported only mild symptoms during the test period; however, it was unclear to which group the adverse events were attributed. The other four trials reported participants that dropped out of the study due to adverse events rather than overall adverse events (Bolling‐Sternevald 2002; Talley 1998 (BOND); Talley 1998 (OPERA); Wong 2002).
There were no differences on adverse events in PPIs compared to placebo (RR 0.99, 95% CI 0.73 to 1.33). However, there was significant heterogeneity between studies (I2 = 55%, P = 0.06) (Analysis 2.11).
2.11. Analysis.
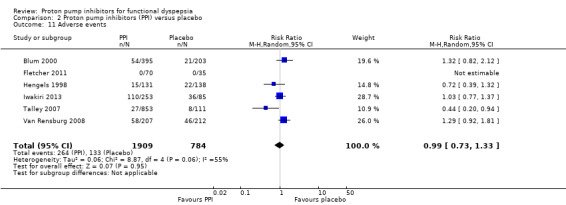
Comparison 2 Proton pump inhibitors (PPI) versus placebo, Outcome 11 Adverse events.
Proton pump inhibitor versus H2 receptor antagonist
Global symptoms of dyspepsia
We identified two trials with 740 participants, comparing the effect of PPIs versus H2RA on global assessment of dyspepsia (Blum 2000; Dillon 2004). One study compared omeprazole 20 mg and 10 mg with ranitidine 150 mg over two weeks (Blum 2000). The other study was a completed trial that was only published in abstract form, evaluating the effect of lansoprazole 30 mg versus ranitidine 150 mg twice daily over eight weeks (Dillon 2004). There was no difference between PPI and H2RA therapy (RR of remaining dyspeptic on PPI therapy 0.88, 95% CI 0.74 to 1.04; P = 0.14). There was substantial heterogeneity between the trials (I2 = 51%; Chi2 = 2.05; df = 1) (Analysis 3.1). In a sensitivity analysis, the effect remained non‐significant with a random‐effects model (OR 0.66, 95% CI 0.40 to 1.07) or with a fixed‐effect model (RR 0.90, 95% CI 0.81 to 0.99).
3.1. Analysis.

Comparison 3 Proton pump inhibitors (PPI) versus H2 receptor antagonists (H2RA), Outcome 1 Global symptoms of dyspepsia.
Quality of life
No studies compared the effect of PPIs versus H2RAs for QoL.
Adverse events
One study comparing PPIs versus H2RA reported adverse events (Blum 2000). There were no differences on adverse events in PPIs compared to H2RA therapy (RR 0.97, 95% CI 0.64 to 1.46) (Analysis 3.2).
3.2. Analysis.
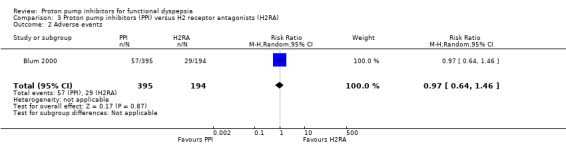
Comparison 3 Proton pump inhibitors (PPI) versus H2 receptor antagonists (H2RA), Outcome 2 Adverse events.
Proton pump inhibitor versus prokinetic
Global symptoms of dyspepsia
We identified five studies with 1033 participants comparing the effect of PPI versus different prokinetics (Hsu 2011; Jiang 2011; Jung 2016; Kamiya 2017; Li 2003). The length of treatment for all studies was two to four weeks. Two of the studies compared mosapride 5 mg three times a day versus lansoprazole (Hsu 2011) or pantoprazole (Jiang 2011) for two weeks. One study compared itopride versus rabeprazole (Kamiya 2017), one study compared cisapride versus omeprazole (Li 2003), and one study investigated the effect of a new prokinetic, DA 9701 with pantoprazole 40 mg (Jung 2016).
Pooled data revealed a small difference in favour of PPI compared to prokinetics (RR 0.89, 95% CI 0.81 to 0.99; P = 0.03) with an NNTB of 16. There was no heterogeneity between the studies (I2 = 0%; Chi2 = 0.92; df = 4; P = 0.92) (Analysis 4.1). In a sensitivity analysis, the effect was in a similar direction with an OR (OR 0.78, 95% CI 0.60 to 1.00) and with a fixed‐effect model (RR 0.90, 95% CI 0.81 to 1.00). Whilst an NNT of 16 may (or may not) be clinically significant, the 95%CI do include a clinically significant effect.
4.1. Analysis.

Comparison 4 Proton pump inhibitors (PPI) versus prokinetics, Outcome 1 Global symptoms of dyspepsia (2‐4 weeks).
Quality of life
One study evaluated the effect of pantoprazole versus prokinetics using the Korean version of the Nepean Dyspepsia Index (Jung 2016). There was no difference on change of QoL scores from baseline after four weeks of treatment (MD ‐0.50, 95% CI ‐4.42 to 3.42) (Analysis 4.2).
4.2. Analysis.
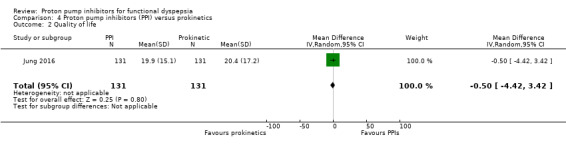
Comparison 4 Proton pump inhibitors (PPI) versus prokinetics, Outcome 2 Quality of life.
Adverse events
All studies comparing PPIs versus prokinetics reported adverse events. There were no differences on adverse events in PPIs compared to prokinetics therapy (RR 1.09, 95% CI 0.79 to 1.49) (Analysis 4.3). No significant heterogeneity was observed between studies (I2 = 0%, P = 0.71).
4.3. Analysis.
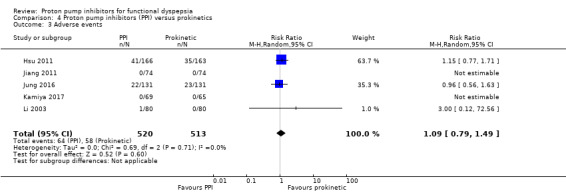
Comparison 4 Proton pump inhibitors (PPI) versus prokinetics, Outcome 3 Adverse events.
Proton pump inhibitor plus prokinetic versus prokinetic alone
Global symptoms of dyspepsia
Two studies evaluated the effect of PPIs combined with prokinetics compared to prokinetics alone on global assessment of dyspepsia (Jung 2016; Yang 2014). One study evaluated the effect of pantoprazole plus mosapride versus mosapride three times a day for one, three and six months (Yang 2014). One study evaluated the effect of pantoprazole plus DA 9701 compared to pantoprazole alone or DA 9701 alone for four weeks (Jung 2016).
There was no difference between PPI plus prokinetics compared to prokinetics alone (RR 0.85, 95% CI 0.68 to 1.08; P = 0.18). There was no heterogeneity between the studies (I2 = 0%; Chi2 = 0.45; df = 1; P = 0.5) (Analysis 5.1). In a sensitivity analysis, the effect remained non‐significant with a random‐effects model (OR 0.77, 95% CI 0.52 to 1.14) and with a fixed‐effect model (RR 0.86, 95% CI 0 to 68 to 1.08).
5.1. Analysis.

Comparison 5 Proton pump inhibitors (PPI) plus prokinetics versus prokinetics alone, Outcome 1 Global symptoms of dyspepsia (2‐4 weeks).
Quality of life
Two studies evaluated QoL in people with FD treated with PPIs plus prokinetics versus prokinetics alone (Jung 2016; Yang 2014). One study compared pantoprazole plus DA 9701 versus DA 9701 alone using the Korean version of the Nepean Dyspepsia Index and reported the change of QoL scores from baseline (MD ‐1.10, 95% CI ‐5.22 to 3.02); the second study reported post‐treatment QoL scores assessed by the Functional Digestive Disorders Quality of Life questionnaire (FDDQL) (MD 18.96, 95% CI 17.01 to 20.91). There were no differences in QoL between PPIs+ prokinetics vs prokinetics alone. Data were not pooled as final values and change scores should not be combined as standardized mean differences (Deeks 2011). (Analysis 5.2).
5.2. Analysis.

Comparison 5 Proton pump inhibitors (PPI) plus prokinetics versus prokinetics alone, Outcome 2 Quality of life.
Adverse events
Two studies comparing PPIs combined with prokinetics versus prokinetics alone reported adverse events (Jung 2016; Yang 2014). There were slightly fewer adverse events with PPIs plus prokinetics compared to prokinetics alone (RR 0.60, 95% CI 0.39 to 0.93) (Analysis 5.3).
5.3. Analysis.
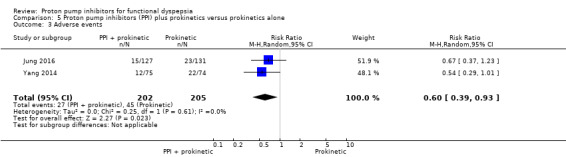
Comparison 5 Proton pump inhibitors (PPI) plus prokinetics versus prokinetics alone, Outcome 3 Adverse events.
Subgroup analyses
Duration of treatment
We performed a subgroup analysis to assess the efficacy of PPI versus placebo according to the duration of treatment (Analysis 2.2). One of the 18 studies did not provide information on the duration of treatment (Catapani 2015). From the 18 studies, four studies involving 1169 participants evaluated the effect of PPI versus placebo after two weeks of treatment (RR of remaining dyspeptic after two weeks 0.78, 95% CI 0.70 to 0.87) (Blum 2000; Bolling‐Sternevald 2002; Fletcher 2011; Hengels 1998). Nine studies from eight papers involving 2425 participants evaluated the effect of PPI versus placebo after four weeks of treatment (Farup 1999; Gerson 2005; Majewski 2016; Suzuki 2013 (ELF); Talley 1998 (BOND); Talley 1998 (OPERA); Tominaga 2010; Van Rensburg 2008; Wong 2002) (RR of remaining dyspeptic after four weeks 0.89, 95% CI 0.76 to 1.03). Four studies involving 2447 participants evaluated the effect of PPI versus placebo after eight weeks of treatment (RR of remaining dyspeptic after eight weeks 0.92, 95% CI 0.86 to 0.98) (Analysis 2.2) (Iwakiri 2013; Peura 2004; Talley 2007; Van Zanten 2006). There was significant difference between subgroups (I2 = 66.6%; Chi2 = 5.99; df = 2; P = 0.05).
2.2. Analysis.
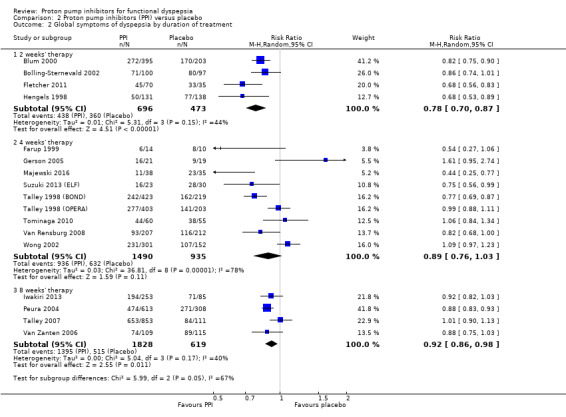
Comparison 2 Proton pump inhibitors (PPI) versus placebo, Outcome 2 Global symptoms of dyspepsia by duration of treatment.
Dose
As mentioned at the beginning of the Effects of interventions section, there was no difference between the two doses of PPI therapy (RR of remaining dyspeptic on standard‐dose versus low‐dose PPI (RR 0.97, 95% CI 0.92 to 1.02; P = 0.21) with no heterogeneity between studies (I2 = 0%; Chi2 = 2.54; df = 5; P = 0.77) (Analysis 1.1). All doses were combined for the analyses, therefore subgroup analyses on dose was not possible.
Geographical location
We performed a subgroup analysis to assess the efficacy of PPI versus placebo according to the origin of the study (Analysis 2.3). For the multicentre studies, we allocated them to the Western countries subgroup, as most of the sites were in the Western area. Fourteen studies, involving 5213 participants evaluating the effect of PPI versus placebo originated in western countries (RR of remaining dyspeptic 0.85, 95% CI 0.79 to 0.92) (Blum 2000; Bolling‐Sternevald 2002; Catapani 2015; Farup 1999; Fletcher 2011; Gerson 2005; Hengels 1998; Majewski 2016; Peura 2004; Talley 1998 (BOND); Talley 1998 (OPERA); Talley 2007; Van Rensburg 2008; Van Zanten 2006). Four studies involving 959 participants evaluating the effect of PPI versus placebo originated in Eastern countries (RR of remaining dyspeptic 0.97, 95% CI 0.84 to 1.11) (Analysis 2.3) (Iwakiri 2013; Suzuki 2013 (ELF); Tominaga 2010; Wong 2002). There was no difference between subgroups (Chi2 = 2.46; df = 1; P = 0.12).
2.3. Analysis.
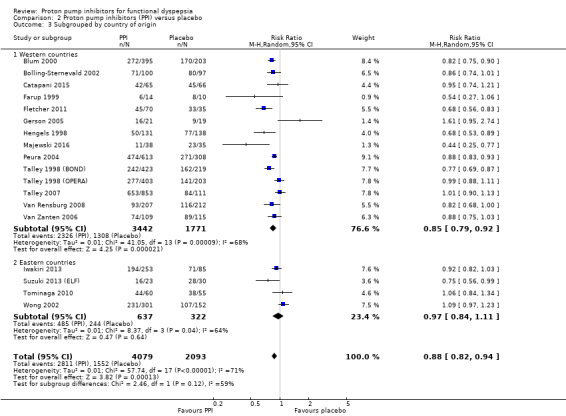
Comparison 2 Proton pump inhibitors (PPI) versus placebo, Outcome 3 Subgrouped by country of origin.
Helicobacter pylori status
Nine studies comparing PPIs versus placebo provided information of the impact of H pylori status on dyspepsia (Blum 2000; Bolling‐Sternevald 2002; Hengels 1998; Iwakiri 2013; Talley 1998 (BOND); Talley 1998 (OPERA); Talley 2007; Van Zanten 2006; Wong 2002). Data from individual studies Talley 1998 (BOND) and Talley 1998 (OPERA) were not provided in the paper, therefore they were combined for the analysis. There was insufficient information on H pylori status in eligible comparison in the study by Talley 2007, for the previous reason this study was excluded from the analysis. In addition, one of the studies reported that H pylori status had no effect on treatment response; however, data were not reported in the paper (Van Zanten 2006). Subgroup analysis in studies evaluating PPI versus placebo in the relief of dyspeptic symptoms in H pylori‐positive participants (RR 0.84, 95% CI 0.76 to 0.93) and H pylori‐negative participants (RR 0.94, 95% CI 0.83 to 1.06) showed no difference between subgroups (I2 = 44.2%; Chi2 = 1.79; df = 1; P = 0.18) (Analysis 2.4).
2.4. Analysis.
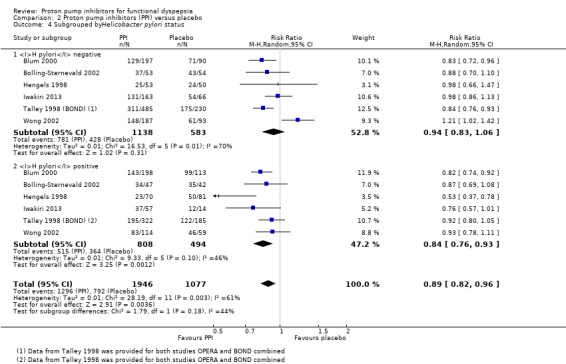
Comparison 2 Proton pump inhibitors (PPI) versus placebo, Outcome 4 Subgrouped byHelicobacter pylori status.
Proton pump inhibitor subtype
We performed a subgroup analysis to assess the efficacy of PPI versus placebo according to the PPI subtype (Analysis 2.5). Seven studies including 2238 participants compared omeprazole (doses were combined) versus placebo (RR of dyspeptic symptoms on omeprazole 0.88, 95% CI 0.79 to 0.98) (Blum 2000; Bolling‐Sternevald 2002; Catapani 2015; Farup 1999; Gerson 2005; Talley 1998 (BOND); Talley 1998 (OPERA)). Five studies compared lansoprazole versus placebo; three used standard‐dose (30 mg/day) (Fletcher 2011; Peura 2004; Wong 2002), and four use low‐dose (15 mg/day) (Hengels 1998; Peura 2004; Suzuki 2013 (ELF); Wong 2002). The RR of dyspeptic symptoms on lansoprazole was 0.82 (95% CI 0.70 to 0.97 (combined doses)). Three studies compared esomeprazole 40 mg/day (RR of dyspeptic symptoms on esomeprazole 0.84, 95% CI 0.65 to 1.09) (Majewski 2016; Talley 2007; Van Zanten 2006). One study compared pantoprazole 20 mg versus placebo (RR of dyspeptic symptoms on pantoprazole 0.82, 95% CI 0.68 to 1.00) (Van Rensburg 2008). One study compared different doses of rabeprazole (10 mg/day, 20 mg/day and 40 mg/day) with placebo (Iwakiri 2013) and one study compared rabeprazole 10 mg/day with placebo (Tominaga 2010). The RR of dyspeptic symptoms with rabeprazole was 0.95 (95% CI 0.84 to 1.08 (doses combined)). There was no difference between subgroups (I2 = 0%; Chi2 = 2.71; df = 4; P = 0.61) (Analysis 2.5).
2.5. Analysis.
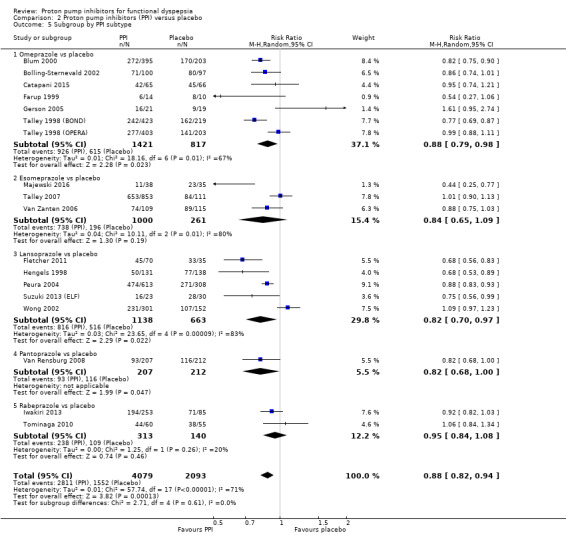
Comparison 2 Proton pump inhibitors (PPI) versus placebo, Outcome 5 Subgroup by PPI subtype.
Presence of reflux (abnormal 24‐hour pH study)
Two studies provided data on the efficacy of PPI therapy versus placebo in participants subgrouped according to abnormal 24‐hour pH study (greater than 4% pH less than 4: RR 0.91, 95% CI 0.70 to 1.19) or normal 24‐hour pH study (less than 4% pH less than 4: RR 1.27, 95% CI 0.49 to 3.29) (Bolling‐Sternevald 2002; Gerson 2005). There was no difference between subgroups (I2 = 0%; Chi2 = 0.45; df = 1; P = 0.50) (Analysis 2.6).
2.6. Analysis.
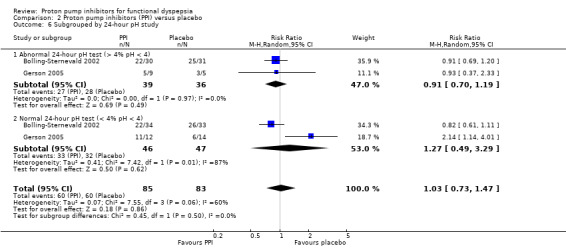
Comparison 2 Proton pump inhibitors (PPI) versus placebo, Outcome 6 Subgrouped by 24‐hour pH study.
Rome III dyspepsia subtypes
Two studies provided data on the efficacy of PPI therapy versus placebo in participants subgrouped according to Rome III epigastric pain syndrome (RR of remaining dyspeptic 0.99, 95% CI 0.76 to 1.28) and postprandial distress subtypes (RR of remaining dyspeptic 0.89, 95% CI 0.77 to 1.03) (Iwakiri 2013; Suzuki 2013 (ELF)). There was no difference between subgroups (I2 = 0%; Chi2 = 0.48; df = 1; P = 0.49) (Analysis 2.7).
2.7. Analysis.
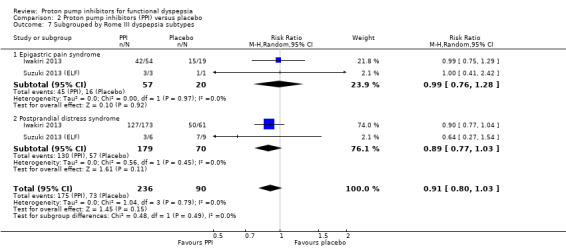
Comparison 2 Proton pump inhibitors (PPI) versus placebo, Outcome 7 Subgrouped by Rome III dyspepsia subtypes.
Risk of bias
Four studies comparing PPIs versus placebo had high risk of bias (Catapani 2015; Farup 1999; Gerson 2005; Talley 2007) (RR 1.00, 95% CI 0.80 to 1.24). The other studies were at low risk of bias (4 studies, RR 0.84, 95% CI 0.73 to 0.98) or unclear risk of bias (10 studies, RR 0.86, 95% CI 0.79 to 0.94). There was no difference between subgroups (I2 = 0%; Chi2 = 1.61; df = 2; P = 0.45) (Analysis 2.8).
2.8. Analysis.
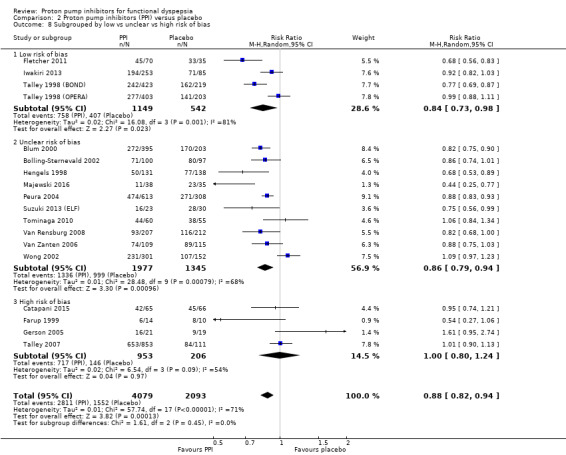
Comparison 2 Proton pump inhibitors (PPI) versus placebo, Outcome 8 Subgrouped by low vs unclear vs high risk of bias.
Funding
Source of funding was unclear in eight studies (Catapani 2015; Dillon 2004; Hengels 1998; Hsu 2011; Jiang 2011; Kamiya 2017; Li 2003; Yang 2014), and 17 studies were funded by a pharmaceutical company (Blum 2000; Bolling‐Sternevald 2002; Farup 1999; Fletcher 2011; Gerson 2005; Iwakiri 2013; Jung 2016; Majewski 2016; Peura 2004; Suzuki 2013 (ELF); Talley 1998 (BOND); Talley 1998 (OPERA); Talley 2007; Tominaga 2010; Van Rensburg 2008; Van Zanten 2006; Wong 2002). Considering that pharmaceutical companies sponsored all the studies with clear funding source, there was no need to perform the intended subgroup analysis to explore whether trial funding sources might have influenced the results.
Data from abstracts
Three included studies were published solely in abstract form (RR 0.89, 95% CI 0.68 to 1.15) (Catapani 2015; Hengels 1998; Hsu 2011). The other studies were published as full text (14 studies, RR 0.87, 95% CI 0.81 to 0.94). There was no difference between subgroups (I2 = 0%; Chi2 = 0.01; df = 1; P = 0.90) (Analysis 2.9).
2.9. Analysis.

Comparison 2 Proton pump inhibitors (PPI) versus placebo, Outcome 9 Subgrouped by publication type.
Discussion
Summary of main results
Our updated review demonstrated that PPIs were more effective than placebo for the treatment of FD, independent of the dose or duration of treatment. The symmetry found in the forest plot suggested that reporting bias (publication bias) was not a risk. We performed both effect estimates models and found results were similar. However, we found substantial heterogeneity between the studies. In concordance with our previous review, subgroup analysis suggested that the variation in results between studies may not be explained by differences on country of origin, H pylori status or subtype of PPI.
The effect of PPIs over placebo was not influenced by the presence of reflux symptoms or different subtypes of FD, suggesting that differentiation of these subtypes may not be needed.
PPIs may be slightly more effective than H2RAs for the treatment of FD. However, no study has been published since our last review, therefore the evidence remains from the previous two RCTs including 740 participants and the effect size was small. Therefore, we consider there is insufficient data to be confident on the real effect estimate.
PPIs may be slightly more effective than prokinetics at relieving overall dyspepsia symptoms in people with FD. However, the studies involved in the analysis had methodological problems, which are likely to influence the results.
PPIs were no more effective than placebo in improving QoL. However, we found no new data in our update. Future studies are needed to confirm these results.
Finally, similar to our previous analysis, there were no differences in the rate of adverse events between PPIs and any of the comparisons including placebo, indicating that PPIs are safe for the treatment of FD.
Overall completeness and applicability of evidence
We identified two new studies comparing PPIs versus placebo (Catapani 2015; Majewski 2016). In the previous version of the review, we listed Catapani 2015 as an ongoing study as it was only available as an abstract. For this update, the authors of the study provided us with additional information, and therefore, we were able to include this study in our analysis. Kamiya 2017 was included in the previous version of this review listed under authors' latest publication in 2011. Since then, the authors have published two further reports.
Considering the 18 studies comparing PPIs versus placebo included in this review, we can conclude that these results are applicable to all people with FD independently of the criteria for definition. The fact that the results from our review were similar to the findings of previous systematic reviews (Moayyedi 2006; Wang 2007) support the beneficial effect of PPIs in FD (see Agreements and disagreements with other studies or reviews). All the five studies included in the meta‐analysis of PPIs versus prokinetics originated from eastern countries, which may limit the results to this specific population (Hsu 2011; Jiang 2011; Jung 2016; Kamiya 2017; Li 2003).
Quality of the evidence
Proton pump inhibitor versus placebo
Overall, the quality of evidence was moderate. We downgraded the quality of evidence due to serious inconsistency. Even though some of the trials had an unclear risk of bias, the effect was evident and the majority of the studies reported a beneficial effect of PPI versus placebo. We were moderately confident that the true effect was likely to be close to that of the estimate of the effect.
Proton pump inhibitor versus H2 receptor antagonist
The quality of evidence was low. We downgraded the quality of evidence two levels due to imprecision, substantial heterogeneity (I2 = 51%) and high a risk bias in one of the two included studies. Our confidence in the effect estimate was limited: the true effect may be substantially different from the estimate of the effect.
Proton pump inhibitor versus prokinetic
All studies except one were open‐label; therefore, there was a high risk of performance and detection bias attributed to these studies. We downgraded the quality of evidence to low meaning that our confidence in the effect estimate was limited: the true effect may be substantially different from the estimate of the effect.
Proton pump inhibitors plus prokinetics versus prokinetics alone
The quality of evidence was moderate. We downgraded the quality of evidence one level due to serious imprecision. We were moderately confident that the true effect was likely to be close to that of the estimate of the effect.
Potential biases in the review process
We based our methods and reporting on recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We consider that our methods and meta‐analyses were rigorous while searching for studies, selecting studies, performing data extraction and analysing data.
We aimed to be as transparent as possible and published the protocol in advance. However, as seen in all meta‐analyses, there may be some potential limitations related to our study.
One potential limitation could be related to the lack of information obtained from authors in one study (Pilichiewicz 2011), which we excluded from the review due to a lack of data. Although the lack of this information is likely to decrease our confidence in the results, this was outside of the control of the review authors.
Agreements and disagreements with other studies or reviews
There are multiple narrative reviews (Camillieri 2013; Savarino 2017; Stanghellini 2016; Suzuki 2011), but only two systematic reviews and meta‐analyses investigating the effects of PPIs in FD (Moayyedi 2006; Wang 2007). The scope of the reviews differed considerably. Moayyedi 2006 was a comprehensive review evaluating different strategies for the treatment of FD including PPIs, while Wang 2007 limited the analysis to the effect of PPIs compared to placebo for the treatment of FD. The current review aimed to determine the efficacy of PPIs for FD compared with placebo and to other therapeutic options. In our review, two to eight weeks of treatment with PPIs were more effective than placebo in overall symptom improvement in people with FD. Although our review included eight more studies evaluating the effect of PPIs versus other treatments, compared to the Moayyedi 2006 review, the results were consistent with the previous analyses. Similar to Moayyedi 2006 and Wang 2007, we found that PPIs were more effective than placebo in treating people with FD. We anticipated potential differences between studies that may have influenced the results, and prespecified analyses to identify sources of heterogeneity. In concordance with previous analyses, we found no differences in the effect between duration of treatment and dose of PPIs, or by the presence of Rome III dyspepsia subtype. There is a discrepancy with our findings on the dyspepsia subtype compared to the analysis from Wang 2007, and this could be related to the inclusion of studies with different definitions of FD. While Wang 2007 considered reflux‐like or dysmotility‐like dyspepsia according to Rome II criteria, our analysis also included investigated epigastric pain syndrome versus postprandial distress syndrome according to Rome III criteria. The definition of FD using the Rome III criteria is stricter than previous definitions of FD in excluding participants with reflux symptoms, and, therefore, this could lead to differences on the results.
We have confirmed the findings from Moayyedi 2006 on the lack of a positive effect of PPIs over placebo on improving QoL.
We performed a subgroup analysis to investigate whether the effect of PPIs compared with placebo changed according to country of origin and PPI subtype. Although this was suggested by Wang 2007 as a potential source of heterogeneity, this was not the case in our analysis as we found no differences between subgroups. We also found no difference between PPI subtype. Our meta‐analysis is the first to perform subgroup analysis on PPI subtype and these results should be confirmed in future analyses.
Our analysis found that PPIs were slightly more effective than H2RAs. As previously reported by Moayyedi 2006, we found substantial heterogeneity and methodological differences between the two studies that may explain the inconsistent results. Wang 2007 did not assess the effect of PPIs versus H2RAs. Therefore, there is a need of studies comparing the effect of PPIs versus H2RAs in the treatment of FD, as they may be a more cost‐effective alternative.
Our results found that PPIs were slightly more effective than prokinetics at relieving overall dyspepsia symptoms in people with FD. This goes in a similar direction to the review by Moayyedi 2006. However, we consider that these results should be taken with caution, due to serious methodological concerns related to the majority of studies comparing PPIs versus prokinetics.
Finally, our review demonstrated that PPIs were relatively safe drugs with a similar number of adverse events compared to placebo, H2RAs and prokinetics. The safety of PPIs was in concordance with the results by Moayyedi 2006, but not explored in Wang 2007.
Authors' conclusions
Implications for practice.
Our updated review confirmed that proton pump inhibitors (PPIs) are effective at reducing overall symptoms of functional dyspepsia (FD). The effect is independent of the dose and duration of treatment. The studies included in our analysis were generally of high methodological quality and a funnel plot found no evidence of publication bias. There was substantial heterogeneity between the studies that could not be explained by predefined criteria. There was no evidence of a difference between PPIs and placebo on quality of life; however, the results were reported by a minority of the studies and should be interpreted with caution. PPIs have a similar effectiveness to H2 receptor antagonists (H2RAs) for the treatment of FD. However, the evidence is scarce and of low quality.
We should be careful in the interpretation of the trials evaluating prokinetic therapy, as they have serious methodological concerns. Further research comparing PPIs with prokinetics and H2RAs is required before any firm conclusions can be reached with respect to their efficacy in the treatment of FD. The effect of antisecretory drugs and prokinetics is likely to be small and many participants may take them on a long‐term basis, so ideally these therapies should be well tolerated. Although the assessment of individual adverse events was out of the scope of our review, we showed that PPIs, H2RAs and prokinetics are overall well‐tolerated drugs.
Implications for research.
This updated review confirmed that PPIs are effective to treat people with FD. There is sufficient evidence that treatment with more than two weeks of PPIs is more effective than placebo. It should be noted that there was considerable heterogeneity between studies and that the effect in the long term was not investigated. It is important to use well‐defined criteria for FD, and a clear definition of treatment response. Further research may be justified to identify the specific subgroup of people who will benefit from the treatment. Further large double‐blind randomized controlled trials are needed to address the remaining uncertainty about the relative effects of PPIs compared to other drugs such as H2RAs and prokinetics. Trials should measure compliance, report all intended outcomes (including quality of life) and analyze the results using an intention‐to‐treat method. There is also a need for trials to assess the longer‐term (at least six to 12 months) benefits and harms of PPIs in FD.
What's new
| Date | Event | Description |
|---|---|---|
| 20 February 2018 | Amended | Correction made to reporting of Quality of Life outcome. |
History
Protocol first published: Issue 7, 2014 Review first published: Issue 2, 2017
| Date | Event | Description |
|---|---|---|
| 30 October 2017 | New citation required but conclusions have not changed | Two new studies incorporated. Conclusions unchanged. |
| 17 May 2017 | New search has been performed | Searches rerun. |
Acknowledgements
We thank the Cochrane Upper Gastrointestinal and Pancreatic Diseases (UGPD) Group for providing administrative and logistical support for preparation of this review, and for developing and executing the search strategies.
We would like to thank Dr Kamiya (Kamiya 2017) and Dr Tominaga (Tominaga 2010) for kindly providing with information related to their studies.
We thank the peer referees, copy editors and the Cochrane Editorial Unit for their comments.
Appendices
Appendix 1. Glossary of medical terms
Clostridium difficile: (also known as C difficile or C diff) a bacterium that can cause symptoms ranging from diarrhoea or life‐threatening inflammation of the colon (part of the large intestine).
Dysmotility: alteration in the movements of the gut.
Endoscopy: a procedure in which a hollow tube with a light and camera on the end is passed through a hollow organ or tube in the body to allow visual inspection or the passage of small surgical instruments.
Epigastric: over the stomach.
Eradication: elimination.
Gastric: relating to the stomach.
Gastro‐oesophageal reflux disease: (GORD) a digestive disorder in which the stomach acid flows backwards into the oesophagus causing heartburn or acid indigestion.
Helicobacter pylori: a form of bacteria associated with stomach and duodenal (involving the first section of the small intestine) ulcers.
Interstitial nephritis: a condition of the kidneys causing swelling of the kidney tubules which affects the ability to clear waste from the blood.
Parietal cell: any of the microscopic structures of the stomach mucous lining that secretes acid.
Pathophysiological: relating to the functional changes that accompany a disease.
Peptic: relating to digestion.
pH profile: measurement of the acid in the stomach.
Pharmacological: drug‐related.
Pneumonia: a disease of the lungs normally caused by infection which leads to inflammation of lung tissue.
Postprandial: after meal.
Reflux: the backward flow of stomach acid.
Satiety: the sensation of being full or having eaten too much.
Appendix 2. The Cochrane Library databases search strategy
#1 MeSH descriptor: [Dyspepsia] explode all trees
#2 Dyspepsia or dyspeptic or NUD or FD:ti,ab,kw (Word variations have been searched)
#3 indigestion or indigestive:ti,ab,kw (Word variations have been searched)
#4 #1 or #2 or #3
#5 MeSH descriptor: [Proton Pump Inhibitors] explode all trees
#6 (proton near/2 pump near/2 inhibitor*) or PPI or PPIs:ti,ab,kw (Word variations have been searched)
#7 MeSH descriptor: [Esomeprazole] explode all trees
#8 Esomeprazole or Nexium or Esotrex or Alenia or Escz or Esofag or Nexiam:ti,ab,kw (Word variations have been searched)
#9 MeSH descriptor: [Omeprazole] explode all trees
#10 omeprazole or losec or nexium or prilosec or rapinex or zegerid or ocid or Lomac or Omepral or Omez:ti,ab,kw (Word variations have been searched)
#11 pantoprazole or protium or protonix or Pantotab or Pantopan or Pantozol or Pantor or Pantoloc or Astropan or Controloc or Pantecta or Inipomp or Somac or Pantodac or Zurcal or Zentro:ti,ab,kw (Word variations have been searched)
#12 rabeprazole or aciphex or dexrabeprazole or pariet or Zechin or Rabecid or Nzole‐D or Rabeloc:ti,ab,kw (Word variations have been searched)
#13 Dexlansoprazole or Kapidex or Dexilant:ti,ab,kw (Word variations have been searched)
#14 lansoprazole or lanzoprazole or agopton or bamalite or Inhibitol or Levant or Lupizole or lanzor or monolitum or ogast or ogastro or opiren or prevacid or prezal or pro ulco or promeco or takepron or ulpax or zoton:ti,ab,kw (Word variations have been searched)
#15 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14
#16 #4 and #15
Appendix 3. MEDLINE (OvidSP) search strategy
1. exp dyspepsia/
2. (Dyspepsia or dyspeptic or NUD or FD).mp.
3. (indigestion or indigestive).tw.
4. or/1‐3
5. exp Proton Pump Inhibitors/
6. ((proton adj2 pump adj2 inhibitor$) or PPI or PPIs).tw.
7. Esomeprazole Sodium/
8. (Esomeprazole or Nexium or Esotrex or Alenia or Escz or Esofag or Nexiam).tw.
9. Omeprazole/
10. (omeprazole or losec or nexium or prilosec or rapinex or zegerid or ocid or Lomac or Omepral or Omez).tw.
11. (pantoprazole or protium or protonix or Pantotab or Pantopan or Pantozol or Pantor or Pantoloc or Astropan or Controloc or Pantecta or Inipomp or Somac or Pantodac or Zurcal or Zentro).tw.
12. (rabeprazole or aciphex or dexrabeprazole or pariet or Zechin or Rabecid or Nzole‐D or Rabeloc).tw.
13. (Dexlansoprazole or Kapidex or Dexilant).tw.
14. (lansoprazole or lanzoprazole or agopton or bamalite or Inhibitol or Levant or Lupizole or lanzor or monolitum or ogast or ogastro or opiren or prevacid or prezal or pro ulco or promeco or takepron or ulpax or zoton).tw.
15. or/5‐14
16. 4 and 15
17. randomized controlled trial.pt.
18. controlled clinical trial.pt.
19. randomized.ab.
20. placebo.ab.
21. drug therapy.fs.
22. randomly.ab.
23. trial.ab.
24. groups.ab.
25. or/17‐24
26. 16 and 25
27. exp animals/ not humans.sh.
28. 26 not 27
Appendix 4. Embase (OvidSP) search strategy
1. exp dyspepsia/
2. (Dyspepsia or dyspeptic or NUD or FD).mp.
3. (indigestion or indigestive).tw.
4. or/1‐3
5. exp proton pump inhibitor/
6. ((proton adj2 pump adj2 inhibitor$) or PPI or PPIs).tw.
7. esomeprazole/
8. (Esomeprazole or Nexium or Esotrex or Alenia or Escz or Esofag or Nexiam).tw.
9. omeprazole/
10. (omeprazole or losec or nexium or prilosec or rapinex or zegerid or lomac or ocid or Lomac or Omepral or Omez).tw.
11. pantoprazole/
12. (pantoprazole or protium or protonix or Pantotab or Pantopan or Pantozol or Pantor or Pantoloc or Astropan or Controloc or Pantecta or Inipomp or Somac or Pantodac or Zurcal or Zentro).tw.
13. rabeprazole/
14. (rabeprazole or aciphex or dexrabeprazole or pariet or Zechin or Rabecid or Nzole‐D or Rabeloc).tw.
15. lansoprazole/
16. (lansoprazole or lanzoprazole or agopton or bamalite or Inhibitol or Levant or Lupizole or lanzor or monolitum or ogast or ogastro or opiren or prevacid or prezal or pro ulco or promeco or takepron or ulpax or zoton).tw.
17. (Dexlansoprazole or Kapidex or Dexilant).tw.
18. or/5‐17
19. 4 and 18
20. random:.tw. or placebo:.mp. or double‐blind:.tw.
21. 19 and 20
Appendix 5. SIGLE search strategy
(dyspep* OR belch* OR indig*) AND (PPI* OR proton pump inhibitor* OR Omeprazole OR Lansoprazole OR Pantoprazole or Esomeprazole OR Rabeprazole OR Dexlansoprazole)
Data and analyses
Comparison 1. Standard‐dose versus low‐dose proton pump inhibitors (PPI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global symptoms of dyspepsia | 6 | 2304 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.92, 1.02] |
Comparison 2. Proton pump inhibitors (PPI) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global symptoms of dyspepsia | 18 | 6172 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.82, 0.94] |
| 2 Global symptoms of dyspepsia by duration of treatment | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 2 weeks' therapy | 4 | 1169 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.70, 0.87] |
| 2.2 4 weeks' therapy | 9 | 2425 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.76, 1.03] |
| 2.3 8 weeks' therapy | 4 | 2447 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.86, 0.98] |
| 3 Subgrouped by country of origin | 18 | 6172 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.82, 0.94] |
| 3.1 Western countries | 14 | 5213 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.79, 0.92] |
| 3.2 Eastern countries | 4 | 959 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.84, 1.11] |
| 4 Subgrouped byHelicobacter pylori status | 6 | 3023 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.82, 0.96] |
| 4.1 H pylori negative | 6 | 1721 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.83, 1.06] |
| 4.2 H pylori positive | 6 | 1302 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.76, 0.93] |
| 5 Subgroup by PPI subtype | 18 | 6172 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.82, 0.94] |
| 5.1 Omeprazole vs placebo | 7 | 2238 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.79, 0.98] |
| 5.2 Esomeprazole vs placebo | 3 | 1261 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.65, 1.09] |
| 5.3 Lansoprazole vs placebo | 5 | 1801 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.97] |
| 5.4 Pantoprazole vs placebo | 1 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.68, 1.00] |
| 5.5 Rabeprazole vs placebo | 2 | 453 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.08] |
| 6 Subgrouped by 24‐hour pH study | 2 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.73, 1.47] |
| 6.1 Abnormal 24‐hour pH test (> 4% pH < 4) | 2 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.70, 1.19] |
| 6.2 Normal 24‐hour pH test (< 4% pH < 4) | 2 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.49, 3.29] |
| 7 Subgrouped by Rome III dyspepsia subtypes | 2 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.80, 1.03] |
| 7.1 Epigastric pain syndrome | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.76, 1.28] |
| 7.2 Postprandial distress syndrome | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.77, 1.03] |
| 8 Subgrouped by low vs unclear vs high risk of bias | 18 | 6172 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.82, 0.94] |
| 8.1 Low risk of bias | 4 | 1691 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.73, 0.98] |
| 8.2 Unclear risk of bias | 10 | 3322 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.79, 0.94] |
| 8.3 High risk of bias | 4 | 1159 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.80, 1.24] |
| 9 Subgrouped by publication type | 18 | 6172 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.82, 0.94] |
| 9.1 Full text | 15 | 5657 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.81, 0.94] |
| 9.2 Abstract | 3 | 515 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.68, 1.15] |
| 10 Quality of life | 3 | 1630 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.09, 0.11] |
| 10.1 Psychological General Well‐Being Index | 2 | 1177 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.09, 0.15] |
| 10.2 36‐item Short Form | 1 | 453 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.25, 0.14] |
| 11 Adverse events | 6 | 2693 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.73, 1.33] |
Comparison 3. Proton pump inhibitors (PPI) versus H2 receptor antagonists (H2RA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global symptoms of dyspepsia | 2 | 740 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.74, 1.04] |
| 2 Adverse events | 1 | 589 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.64, 1.46] |
Comparison 4. Proton pump inhibitors (PPI) versus prokinetics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global symptoms of dyspepsia (2‐4 weeks) | 5 | 1033 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.81, 0.99] |
| 2 Quality of life | 1 | 262 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐4.42, 3.42] |
| 3 Adverse events | 5 | 1033 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.79, 1.49] |
Comparison 5. Proton pump inhibitors (PPI) plus prokinetics versus prokinetics alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global symptoms of dyspepsia (2‐4 weeks) | 2 | 407 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.68, 1.08] |
| 2 Quality of life | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Nepean Dyspepsia Index | 1 | 258 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐5.22, 3.02] |
| 2.2 FDDQL scale | 1 | 149 | Mean Difference (IV, Random, 95% CI) | 18.96 [17.01, 20.91] |
| 3 Adverse events | 2 | 407 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.39, 0.93] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blum 2000.
| Methods | Setting: multicentre study in Germany. Design: double‐blind, placebo‐controlled RCT. |
|
| Participants | 974 participants (792 randomized) with epigastric symptoms in the absence of organic disease. Dyspeptic symptoms present for at least 4 weeks and were severe on at least 3 days of the 7‐day run‐in screening period. | |
| Interventions | PPI: omeprazole 10 mg/day. PPI: omeprazole 20 mg/day. H2RA: ranitidine 150 mg/day. Placebo. Duration: 2 weeks. |
|
| Outcomes | Disappearance of dyspeptic symptoms requiring further treatment. Severity of symptom was assessed and graded according to a 4‐point scale. Improvement in QoL. Decrease in time spent off work. |
|
| Notes | Funding by Swiss Science Research Foundation and Astra‐Hässle. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind double‐dummy, placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Analyst blinded until allocation in ITT analysis performed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported data in ITT sample, early termination, < 20% and balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Reported prespecified outcomes. |
| Other bias | Low risk | No other risk of bias identified. |
Bolling‐Sternevald 2002.
| Methods | Setting: multicentre study in Denmark and Sweden. Design: double‐blind, placebo‐controlled RCT. |
|
| Participants | 265 participants (197 randomized) with recurrent symptoms (epigastric pain/discomfort) at least 1 month and normal OGD; HP+ were not excluded. Exclusions: people with predominant reflux or IBS symptoms. |
|
| Interventions | PPI: omeprazole 20 mg twice daily. Placebo. Duration: 2 weeks. |
|
| Outcomes | No epigastric pain on the last day of assessment. No dyspepsia symptoms on the last 2 days of assessment. |
|
| Notes | Funding by AstraZeneca. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list in blocks of 4 for each centre. |
| Allocation concealment (selection bias) | Unclear risk | No detailed information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study, personnel and participants blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study, physicians and participants blinded throughout the study. It is likely that the outcome assessors (physicians) were also blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 participants in each group did not have complete outcome data; however, the number of participants and the reasons for incomplete outcome were balanced between the groups (PPI vs placebo: 0 vs 1 withdrawn, 2 vs 1 lost to follow‐up, 2 vs 3 discontinued treatment due to worsening symptoms, 2 vs 1 stopped treatment due to adverse events). It is unlikely to have impacted on the treatment effect estimates. |
| Selective reporting (reporting bias) | Low risk | Reported planned outcome data; however, no SD reported for quality scores, although mean scores and P values reported. |
| Other bias | Unclear risk | 9 participants who did not fulfil the inclusion criteria were randomized by mistake, it is unclear whether these participants impacted on the treatment effect estimates. |
Catapani 2015.
| Methods | Setting: single centre in Brazil. Design: parallel RCT. |
|
| Participants | 131 participants with FD (enrolled and randomized) who met Rome II criteria. | |
| Interventions | Group A1: traditional medical therapy + omeprazole (dose unknown). Group A2: traditional medical therapy + placebo. Group B1: therapeutic encounter + omeprazole. Group B2: therapeutic encounter + placebo. Data from A1 + B1 were combined as PPI arm, data from A2 + B2 were combined as control arm in this systematic review. |
|
| Outcomes | ≥ 50% reduction from the initial score of the symptoms questionnaire. Participants followed for 6 months. |
|
| Notes | Some information provided by authors. Duration and dose of omeprazole is unknown. We contacted the authors for further information but received no reply before the review submission. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each participant was allocated using a numbered card taken in a blind manner from a ballot box by a third person (not 1 of the investigators). |
| Allocation concealment (selection bias) | Unclear risk | Data not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double blind not stated; mentioned that investigators were blinded but did not mention participants; unlikely that participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigator responsible for collecting symptom questionnaire was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In total, only 74 (56%) participants completed treatment, per‐protocol analysis only included 65% PPI users and 33% placebo users. Unclear how many participants dropped out and for what reasons. |
| Selective reporting (reporting bias) | Unclear risk | Conference abstract; unclear if other outcomes were planned and reported, especially for adverse events. |
| Other bias | Unclear risk | Conference proceedings only, no other information. |
Dillon 2004.
| Methods | Setting: 41 centres in UK. Design: double‐blind, placebo‐controlled RCT. |
|
| Participants | 480 participants enrolled (152 randomized) with dyspepsia (Rome II). | |
| Interventions | PPI: lansoprazole 30 mg/day. H2RA: ranitidine 150 mg 2 times/day. Duration: 8 weeks. |
|
| Outcomes | Severity of daytime epigastric pain at 2, 4 and 8 weeks. | |
| Notes | Funding by AstraZeneca. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization was described, but details provided. |
| Allocation concealment (selection bias) | Unclear risk | Single‐blind study. Information for allocation concealment is not known. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described, but likely to have been unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract conference. |
| Selective reporting (reporting bias) | Unclear risk | Abstract conference. |
| Other bias | Unclear risk | Abstract conference. |
Farup 1999.
| Methods | Setting: secondary centre in Norway. Design: double‐blind, placebo‐controlled RCT. |
|
| Participants | 24 participants (enrolled and randomized) with epigastric pain/discomfort for at least 1 month, 7 days from study period and least 3 days during the last week before enrolment and normal OGD. Exclusions: people with predominant alarm symptoms; HP+ and NSAIDS users. |
|
| Interventions | PPI: omeprazole 10 mg/day. PPI: omeprazole 20 mg/day. Placebo. Duration: 4 weeks. |
|
| Outcomes | Sufficient relief of dyspepsia symptoms (binary). Differences in reflux episodes in responders to PPI vs non‐responders. |
|
| Notes | Funding by Astra Norge AS. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported randomization but unclear sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessor not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Primary and secondary outcomes not properly described. Missing data not described. Adverse event table not shown (there were no clinically significant adverse events). |
| Selective reporting (reporting bias) | High risk | Symptomatic improvement was initially planned report as low‐dose vs high‐dose; however, because of similar effect and low number of participants, the groups were combined. Data not shown. Dropouts not reported. Demographics from treatment groups not reported, therefore, bias due to characteristics of population not evaluable. |
| Other bias | Unclear risk | Initial sample size not provided; however, there were low numbers of participants in each group and unbalanced. Possibly stopped early, but not stated. |
Fletcher 2011.
| Methods | Setting: tertiary centre in UK. Design: double‐blind, placebo‐controlled RCT. |
|
| Participants | 154 participants enrolled (105 randomized) with symptoms (epigastric pain/discomfort) for at least 3 months and normal OGD. Exclusions: people with predominant alarm symptoms, HP+ and NSAIDS users. |
|
| Interventions | PPI: lansoprazole 30 mg/day. Placebo. Duration: 2 weeks. |
|
| Outcomes | Reduction in symptom severity score (GDSS) during days 7‐14 of treatment. Value of symptoms and 24‐hour pH metry and oesophageal manometry in predicting the response to PPI in this group of participants. |
|
| Notes | Funding by Wyeth Pharmaceuticals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were allocated to either active or placebo medications through block randomisation conducted by hospital pharmacy using a computer random number generator." |
| Allocation concealment (selection bias) | Low risk | "Randomisation was conducted by hospital pharmacy using a computer random number generator." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medication was blinded to both participants and investigators. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study, medication was blinded to both participants and investigators. Likely that outcome assessors were also blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | PPI vs placebo: 2 vs 1 participants withdrew from the study, it is unlikely to have impacted on treatment effect estimates. |
| Selective reporting (reporting bias) | Low risk | Reported all planned outcomes. |
| Other bias | Low risk | No other risk of bias identified. |
Gerson 2005.
| Methods | Setting: tertiary centre in the US. Design: double‐blind, placebo‐controlled RCT. |
|
| Participants | 40 participants (enrolled and randomized) with FD diagnosis (RomeII), HP‐ and normal OGD. Exclusions: people with GORD predominant symptoms, NSAID users and H2RA blocker users. |
|
| Interventions | PPI: omeprazole 20 mg/day. Placebo. Duration: 4 weeks. |
|
| Outcomes | Abnormal 24‐hour pH ambulatory oesophageal monitoring. Symptoms: GSRS. |
|
| Notes | Funding by AstraZeneca and ADHF grant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomized by a pharmacist in a sequential fashion; however, it is unclear whether a randomization list was generated by the true randomization method then the participants were allocated to treatment arms according to the list in sequence, or the treatment allocation was simply based on the participants' admission sequence. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by a pharmacist. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study, it is likely that participants and physicians were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study, it is likely that outcome assessors were also blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the follow‐up. |
| Selective reporting (reporting bias) | Low risk | Reported all planned outcomes. |
| Other bias | High risk | The 2 groups had imbalanced GSRS scores at baseline, the treatment effect estimates are unlikely to be associated with the treatment. |
Hengels 1998.
| Methods | Setting: multicentre study in Germany. Design: RCT. |
|
| Participants | 269 participants (enrolled and randomized) with FD defined as: dyspeptic symptoms present for at least 1 week, nocturnal pain waking the person, nausea with or without vomiting, postprandial pain, pain reduction by minor food intake or antacids, referred pain in extra‐abdominal regions; 0 to 100 VAS score > 60. | |
| Interventions | PPI: lansoprazole 15 mg/day. Placebo. Duration: 2 weeks. |
|
| Outcomes | Cure rate (cure = absence of pain in epigastrium and retrosternal region VAS < 20) during the last 5 days of treatment and absence of regurgitation, heartburn, nausea or vomiting. | |
| Notes | Funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but details not provided. |
| Allocation concealment (selection bias) | Unclear risk | No details on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as double‐blind, likely outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Conference proceedings, no information available. |
| Selective reporting (reporting bias) | Unclear risk | Conference proceedings, no information available. |
| Other bias | Unclear risk | Conference proceedings, no information available. |
Hsu 2011.
| Methods | Setting: secondary care centre in Taiwan. Design: open‐label, 2‐arm, parallel RCT. |
|
| Participants | 329 participants (enrolled and randomized) with FD diagnosis (Rome III) with normal OGD. Exclusions: NSAID users. HP not routinely tested. |
|
| Interventions | PPI: lansoprazole 30 mg/day. Prokinetic: mosapride 5 mg 3 times/day. Duration: 2 weeks. |
|
| Outcomes | Resolution of dyspeptic symptoms defined as a dyspepsia score of ≤ 16 points on Hong Kong Index Questionnaire. Absolute change in dyspepsia score. |
|
| Notes | Compliance > 80%. Funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Participants and treating physicians unaware of allocation sequence and the code not revealed until participants assigned to treatment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study, "Without specific blinding measures." Likely outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all planned outcomes. PPI vs prokinetic: 8.4% vs 6.7% of participants lost to follow‐up. However, it is unclear whether it had an impact on the treatment effect estimate. 3.0% vs 14% had poor compliance, but these participants were included in our ITT analysis. |
| Selective reporting (reporting bias) | High risk | Study was terminated early because the interim analysis (12 months after the study) determined that the results would not have changed even if the enrolment of 364 participants had been completed. |
| Other bias | Low risk | No other risk detected. |
Iwakiri 2013.
| Methods | Setting: 66 centres from Japan. Design: 4‐arm, parallel RCT. |
|
| Participants | 392 participants enrolled (338 randomized) with FD diagnosis (Rome III) with normal OGD who did not respond to 1 week of single‐blind placebo treatment in a run‐in period. | |
| Interventions | PPI: rabeprazole 10 mg/day. PPI: rabeprazole 20 mg/day. PPI: rabeprazole 40 mg/day. Placebo. Duration: 8 weeks. |
|
| Outcomes | Complete relief of symptoms according to the Dyspepsia Symptom Questionnaire (defined as scores of 1 for all 4 major dyspeptic symptoms at week 8) and a symptom diary (defined as the absence of all 4 symptoms during the 7 days before week 8). Satisfactory relief of symptoms according to the Dyspepsia Symptom Questionnaire (defined as scores of ≤ 2 for all 4 major symptoms at week 8) and a symptom diary (defined as a frequency of ≤ 1 day for all 4 major symptoms during the 7 days before week 8). |
|
| Notes | SAMURAI Study, ClinicalTrials.gov NCT01089543. Funding by Eisai Co. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Third‐party organization (Bellsystem24, Inc., Tokyo, Japan) randomly created key code of study drug (1:1:1:1), assigned the participants to the 4 treatment arms and kept the code until the public key to maintain blinding. |
| Allocation concealment (selection bias) | Low risk | Allocation performed by third party. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy method. Investigators and participants blinded to the assigned treatment during the treatment period. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐dummy method. Investigators and participants blinded to the assigned treatment during the treatment period. It is likely that outcome assessors were also blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data available for 303 participants (PPI 10 mg/day vs 20 mg/day vs 40 mg/day vs placebo: 79 vs 76 vs 75 vs 74 participants). That is, < 20% of participants had no outcome data and were considered to have treatment failure in our analysis (338 ‐ 303)/338 = 10.4%. The proportion of missing participants balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Reported all planned outcomes. |
| Other bias | Low risk | No other risk of bias identified. |
Jiang 2011.
| Methods | Setting: single centre in China. Design: open‐label, 2‐arm, parallel RCT. |
|
| Participants | 148 participants (enrolled and randomized) with FD diagnosis (Rome III) with normal OGD. Exclusions: NSAID users. HP not routinely tested. |
|
| Interventions | PPI: pantoprazole 40 mg/day. Prokinetic: mosapride 5 mg 3 times/day. Duration: 2 weeks. |
|
| Outcomes | Decrease overall symptoms scores of FD. Absolute difference in overall symptoms score decrease. |
|
| Notes | Chinese language. Funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label, participants and personnel not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label, outcome assessors not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Reported planned outcome data. |
| Other bias | Low risk | No other risk of bias identified. |
Jung 2016.
| Methods | Setting: 20 centres from Korea. Design: parallel RCT. |
|
| Participants | 433 participants enrolled (389 randomized) with FD (Rome III). HP tested. | |
| Interventions | PPI: pantoprazole 40 mg/day. Prokinetic: DA 9701 30 mg 3 times/day. PPI + prokinetic: pantoprazole + DA 9701. Duration: 4 weeks. |
|
| Outcomes | Improvement in global symptoms defined as a response of "yes" on the binary outcome survey or "symptom free, or markedly improved symptoms or moderately improved" on the 5‐point Likert scale. > 50% reduction in the total score. Difference in dyspepsia‐specific QoL outcomes. |
|
| Notes | Funding by Donga‐A ST. ClinicalTrials.gov NCT01817465. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization generated using the PLAN procedure (random number generator) contained in SAS. |
| Allocation concealment (selection bias) | Low risk | Concealed allocation was assured using an encrypted code by Clinical Research Organization. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐dummy method. Investigators and participants blinded to the assigned treatment during the treatment period. It is likely that outcome assessors were also blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 20% of participants dropped out and numbers balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Reported all prespecified outcomes. |
| Other bias | Low risk | No other risk of bias identified. |
Kamiya 2017.
| Methods | Setting: 4 centres from Japan. | |
| Participants | 155 participants enrolled. 134 randomized to 2 groups, 69 in the PPI group and 65 in the prokinetic group. | |
| Interventions | PPI: rabeprazole 10 mg/day. Prokinetic: itopride. |
|
| Outcomes | Rate of change in FD in 4 weeks of treatment in as per‐protocol analysis. Rate of change in the EPS score and in the PDS between pretreatment and each time point. Subanalysis of EPS and PDS based on the Rome III criteria. |
|
| Notes | Contacted author who provided more information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Unclear risk | Information not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel not performed. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study. Outcome assessors were likely not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 58/69 participants finished study in PPI group and 58/65 participants in prokinetics group, but enrolment was not balanced. 21 participants withdrew before randomization. |
| Selective reporting (reporting bias) | Low risk | Reported all prespecified outcomes. |
| Other bias | Low risk | No other risk of bias identified. |
Li 2003.
| Methods | Setting: multicentre study in China. Design: open‐label, 2‐arm, parallel RCT. |
|
| Participants | 160 participants (enrolled and randomized) with FD with normal endoscopy. | |
| Interventions | PPI: omeprazole 10 mg/day. Prokinetic: cisapride 5 mg 3 times/day. Duration: 2 weeks. |
|
| Outcomes | Overall dyspepsia score decrease. Individual symptoms score decrease. Proportion of efficiency. |
|
| Notes | Language Chinese. Funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Evaluated by staff blinded to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/160 (4%) eligible participants did not complete the treatment, lost to follow‐up balance between groups (PPI vs prokinetic: 1 vs 0 for adverse events, 3 vs 3 for lost to follow‐up), its impact on treatment effect estimate was likely to be small. |
| Selective reporting (reporting bias) | Low risk | Reported planned outcome data. |
| Other bias | Low risk | No other risk of bias identified. |
Majewski 2016.
| Methods | Setting: multicentre study in US and Poland. Design: double‐blind, 2‐arm, parallel RCT. |
|
| Participants | 73 adults (enrolled and randomized) with FD (Rome II). | |
| Interventions | Esomeprazole 40 mg/day. Placebo. Duration: 4 weeks. |
|
| Outcomes | Satisfactory relief of dyspeptic symptoms. | |
| Notes | Supported by AstraZeneca. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomization codes were assigned sequentially to enrolled participants. |
| Allocation concealment (selection bias) | Unclear risk | Unclear how allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double blind" stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double blind". Likely outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Protocol adherence was excellent" stated, reported outcome data for all participants. |
| Selective reporting (reporting bias) | Unclear risk | "At each visit, patients were asked to rate any adverse effect that occurred as mild, moderate or severe." However, data for adverse events were not reported. |
| Other bias | Low risk | No other risk of bias identified. |
Peura 2004.
| Methods | Setting: multicentre, primary and secondary care, 71 investigators in US. Design: 2 × 3‐arm, parallel RCTs merged. |
|
| Participants | 921 participants enrolled (921 randomized) with FD diagnosis (Rome II) with normal OGD. Exclusions: people with IBS and NSAID users. HP+ not excluded. |
|
| Interventions | PPI: lansoprazole 15 mg/day. PPI: lansoprazole 30 mg/day. Placebo. Duration: 8 weeks. |
|
| Outcomes | Change in percentage of days with upper abdominal discomfort and mean daily severity score. Proportions of participants with symptom resolution. Percentage of days that antacids (Gelusil) were used. Proportions of participants with no symptoms. |
|
| Notes | Funding by TAP Pharmaceutical. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No information provided for allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study, personnel and participants blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study, personnel and participants blinded, it is likely that the outcome assessors (physicians) were also blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Primary study did not provide information on lost to follow‐up to discontinuation treatment due to adverse events. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | No other risk of bias identified. |
Suzuki 2013 (ELF).
| Methods | Setting: 11 centres from Japan. Design: parallel RCT. |
|
| Participants | 54 participants with FD (Rome III). HP tested. | |
| Interventions | PPI: lansoprazole 15 mg/day. Placebo. Duration: 4 weeks. |
|
| Outcomes | Overall dyspeptic symptom relief rates at 4 weeks of treatment. Subgroup analysis according to dyspepsia subtypes. Individual scores GSRS and Likert scales. |
|
| Notes | UMIN Clinical Trials Registry number: UMIN000001759; Partially funded by Takeda Pharmaceutical Company. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random assignment program. |
| Allocation concealment (selection bias) | Low risk | Concealment assured by enciphered code kept by the Site Management Organizations for clinical trials (CMIC CMO, Shizuoka, Japan). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical tablets. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Code opened after the completion of all data collection to the data management department. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 (13%) participants had no data in the placebo group based on participants' request. |
| Selective reporting (reporting bias) | Low risk | Reported all prespecified outcomes. |
| Other bias | Unclear risk | Stopped early due to slow recruitment. 1 participant was excluded after randomization due to ineligibility. |
Talley 1998 (BOND).
| Methods | Setting: multicentre study from Greece, the UK, Belgium, Finland, Portugal, Canada, Norway, Denmark, France, Germany, Holland, Hungary and Poland. Design: parallel RCT. |
|
| Participants | 642 participants (enrolled and randomized) with FD (persistent or recurrent epigastric pain or discomfort, or both, in participants with normal findings at upper gastrointestinal endoscopy. Symptoms at least 1 month of duration, 25% of days during month and least 3 days during the last week before enrolment), normal endoscopy. | |
| Interventions | PPI: omeprazole 10 mg. PPI: omeprazole 20 mg. Placebo. Duration: 4 weeks. |
|
| Outcomes | Overall dyspeptic symptom relief rates at 4 weeks of treatment (Likert scale). Improvement in gastrointestinal symptoms (GSRS). Improvement in psychological well‐being (PGWBI). |
|
| Notes | Funding by Astra‐Hässle. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, omeprazole and placebo capsules identical in appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, investigators and study centres maintained strict blinding throughout the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants without outcome data balanced between groups were < 20% in total (omeprazole 10 mg vs omeprazole 20 mg vs placebo: 3% vs 3% vs 4%). |
| Selective reporting (reporting bias) | Low risk | Reported all prespecified outcomes. |
| Other bias | Low risk | No other risk of bias identified. |
Talley 1998 (OPERA).
| Methods | Setting: multicentre study from Greece, the UK, Belgium, Finland, Portugal, Canada, Norway, Denmark, France, Germany, Holland, Hungary and Poland. Design: parallel RCT. |
|
| Participants | 606 participants (enrolled and randomized) with FD (persistent or recurrent epigastric pain or discomfort, or both, in participants with normal findings at upper gastrointestinal endoscopy. Symptoms at least 1 month of duration, 25% of days during month and least 3 days during the last week before enrolment), normal endoscopy. | |
| Interventions | PPI: omeprazole 10 mg. PPI: omeprazole 20 mg. Placebo. Duration: 4 weeks. |
|
| Outcomes | Overall dyspeptic symptom relief rates at 4 weeks of treatment (Likert scale). Improvement in gastrointestinal symptoms (GSRS). Improvement in psychological well‐being (PGWBI). |
|
| Notes | Funding by Astra‐Hässle. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, omeprazole and placebo capsules identical in appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, investigators and study centres maintained strict blinding throughout the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants without outcome data balanced between groups were < 20% in total (omeprazole 10 mg vs omeprazole 20 mg vs placebo: 3% vs 3% vs 4%). |
| Selective reporting (reporting bias) | Low risk | Reported all prespecified outcomes. |
| Other bias | Low risk | No other risk of bias identified. |
Talley 2007.
| Methods | Setting: 17 countries. Design: placebo‐controlled, parallel group RCT. |
|
| Participants | 2868 participants enrolled (1589 randomized) with intermittent or continuous epigastric pain or burning for at least 3 months and normal OGD irrespective of HP status. Exclusions: people with predominant GORD symptoms, HP eradication and NSAIDS users. |
|
| Interventions | PPI: esomeprazole 40 mg/day. Placebo. Duration: 1 week. Then: PPI: esomeprazole 40 mg/day. Placebo. Duration: 7 weeks. Total duration: 8 weeks. |
|
| Outcomes | Proportion of treatment responders. Trial‐week response was defined as symptom score sum ≤ 1 on last 3 days of therapy; response at 4 weeks and 8 weeks was symptom score sum ≤ 1 over preceding 7 days. | |
| Notes | Funding not stated. Clinicatrials.gov NCT00251914. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated to a treatment sequence in blocks, computer‐generated randomization list (sponsor). |
| Allocation concealment (selection bias) | Low risk | Randomization list concealed to participants, investigators and study personnel. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical tablets for esomeprazole and placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Identical placebo tablets were used to maintain blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Discontinued participants were imbalanced between groups. |
| Selective reporting (reporting bias) | Low risk | Reported all prespecified outcome data. |
| Other bias | Low risk | No other risk of bias identified. |
Tominaga 2010.
| Methods | Setting: multicentre study in Japan. Design: placebo‐controlled, double‐blind RCT. |
|
| Participants | 115 participants (enrolled and randomized) with FD (Rome III) and normal endoscopy. | |
| Interventions | PPI: rabeprazole 10 mg/day. Placebo. Duration: 4 weeks. |
|
| Outcomes | Improvement rate ≥ 50% of FD, EPS and PDS scores after 4 weeks' administration. Decrease of 50% of severity symptoms (GSRS). |
|
| Notes | Abstract DDW2010. Full text not yet published. Authors provided us with poster. Funding by Eisai Co, Ltd. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available. |
| Allocation concealment (selection bias) | Unclear risk | Conference proceedings, no information available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study. Likely outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11 (18%) PPI vs 6 (11%) placebo participants had missing data. It was unclear whether the imbalanced lost to follow‐up had an impact on treatment effect estimates. |
| Selective reporting (reporting bias) | Unclear risk | Data for responder reported in EPS subtype only. Information for adverse events was not reported in the provided poster. |
| Other bias | Unclear risk | Conference proceedings, no information available. |
Van Rensburg 2008.
| Methods | Setting: 57 centres from Belgium, Canada, France, the UK, Italy, Portugal, Spain and South Africa. Design: parallel RCT. |
|
| Participants | 419 participants (enrolled and randomized) with FD defined as intermittent episodes of epigastric pain for at least the 3 months prior to screening. Primary inclusion criterion was predominant epigastric pain centred in the upper abdomen of at least 'moderately to severe' intensity (predefined using a 7‐point Likert scale, ranging from 'none' to 'very severe') for at least 2 days prior to the start of the study. Normal OGD within 3 days prior to ingestion of the first dose of therapy and normal abdominal ultrasound. | |
| Interventions | PPI: pantoprazole 20 mg/day. Placebo. Duration: 4 weeks. |
|
| Outcomes | Complete relief (i.e. absence) from epigastric pain reported for the 2 days prior to the 4‐week visit. Secondary endpoints (per protocol): complete relief of epigastric pain at 7 and 14 days of treatment. Complete relief of epigastric pain at 28 days. Complete relief from other gastrointestinal symptoms (heartburn, acid regurgitation, vomiting, nausea, early satiety, postprandial fullness). |
|
| Notes | Funded by Nycomed Pharmaceutical Company. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list. |
| Allocation concealment (selection bias) | Low risk | Individual treatment codes indicating randomization assignment were kept in individual sealed emergency cards in a locked file and were available to the study investigators in the case of emergency. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo and active drug were identical in appearance, colour and consistency to ensure blinding was maintained. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study, outcome assessors were blinded, the assignment codes were not broken unless in the case of emergency. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rates were balanced between groups but nearly 20% (PPI vs placebo: 19.6% to 20.3% vs 18.9%) did not completed the trial, either due to protocol violation or dropped out. |
| Selective reporting (reporting bias) | Low risk | Reported all prespecified outcomes. |
| Other bias | Low risk | No other risk of bias identified. |
Van Zanten 2006.
| Methods | Setting: 49 centres from Canada. Design: double‐blind, placebo‐controlled, parallel RCT. |
|
| Participants | 502 HP‐ participants enrolled (224 randomized) with FD (Rome II) with normal endoscopy. Exclusions: people with IBS or GORD predominant symptoms. |
|
| Interventions | PPI: esomeprazole 40 mg/day. Placebo. Duration: 8 weeks. |
|
| Outcomes | Proportion of participants with symptom relief at 8 weeks defined as having symptoms of no more than minimal severity (GOS score ≤ 2) during the previous 2 days. Proportions of participants who achieved symptom relief (GOS ≤ 2), symptom resolution (GOS = 1) and symptom improvement (change of GOS ≥ 2) after 4 and 8 weeks of treatment. Changes in individual dyspepsia symptoms and in the most bothersome symptom after 4 and 8 weeks of treatment. Change in QoL. |
|
| Notes | CADET‐HN Study; registration number: DC‐QBE‐0018. Funding by AstraZeneca. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomized in a strictly sequential manner, with computer‐generated randomization numbers being generated by the Biostatistics Department in AstraZeneca. |
| Allocation concealment (selection bias) | Low risk | Concealed to all study personnel and participants. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo‐controlled study, identical‐looking placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study, personnel and physicians were blinded, it is likely that outcome assessors were also blinded. All study personnel, investigators and participants remained blinded to the results until the study was completed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | PPI vs placebo: 6% vs 10% participants discontinued treatment, of them 0% vs 4% lost to follow‐up. It is unclear whether the imbalanced lost to follow‐up had an impact on treatment effect estimates. |
| Selective reporting (reporting bias) | Low risk | Reported all prespecified outcomes. |
| Other bias | Low risk | No other risk of bias identified. |
Wong 2002.
| Methods | Setting: 3 centres from Hong Kong. Design: double‐blind, placebo‐controlled RCT. |
|
| Participants | 456 participants enrolled (453 randomized) with FD (Rome II), normal OGD with predominant epigastric pain/discomfort. | |
| Interventions | PPI: lansoprazole 15 mg once daily. PPI: lansoprazole 30 mg once daily. Placebo. Duration: 4 weeks. |
|
| Outcomes | Proportion of participants with complete relief of epigastric pain/discomfort during the last 3 days of the 4 weeks' treatment. Change in mean dyspepsia score and SF‐36 scores from baseline to the 4‐week visit. Improvement in QoL (SF‐36). |
|
| Notes | Funding by Takeda Pharmaceutical Company. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both the investigators and patients were blinded to the assigned treatment throughout the study. The lansoprazole and placebo capsules were identical in appearance." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study, personnel and physicians blinded, "Both the investigators and patients were blinded to the assigned treatment throughout the study." It is likely that outcome assessors were also blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up or low compliance was imbalanced between the 3 arms (lansoprazole 30 mg once daily vs 15 mg once daily vs placebo: 19% vs 15% vs 13%). Although the data were analyzed based on an ITT sample, it was unclear whether the lost to follow‐up had an impact on treatment effect estimates. |
| Selective reporting (reporting bias) | Low risk | Reported all prespecified outcomes. |
| Other bias | Low risk | No other risk of bias identified. |
Yang 2014.
| Methods | Setting: single centre in China. Design: parallel RCT. |
|
| Participants | 149 participants (enrolled and randomized) with FD (Rome III). HP not tested. | |
| Interventions | PPI + prokinetic: pantoprazole 40 mg/day + mosapride 3 times/day. Prokinetic alone: mosapride 3 times/day. Duration: 4, 12 and 24 weeks. |
|
| Outcomes | Improvement of overall symptoms. Improvement of QoL through Functional Digestive Disorder Quality of Life questionnaire. |
|
| Notes | Published in Chinese. Funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization mentioned but method unclear. |
| Allocation concealment (selection bias) | Unclear risk | Information not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detailed information. Unlikely to be blinded as the drug regimens were different and there was no placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported for all participants. |
| Selective reporting (reporting bias) | Low risk | Reported all prespecified outcomes. |
| Other bias | Low risk | No other risk of bias detected. |
ADHF: American Digestive Health Foundation; EPS: epigastric pain syndrome; FD: functional dyspepsia; GDSS: Glasgow Dyspepsia Severity Score; GORD: gastro‐oesophageal reflux disease; GOS: Global Overall Symptom; GSRS: Gastrointestinal Symptoms Rating Scale; H2RA: H2 receptor antagonist; HP: Helicobacter pylori; HP‐: Helicobacter pylori negative; HP+: Helicobacter pylori positive; IBS: irritable bowel syndrome; ITT: intention to treat; NSAID: non‐steroidal anti‐inflammatory drug; OGD: oesophagogastroduodenoscopy; PDS: postprandial distress syndrome; PGWBI: Psychological General Well‐Being Index; PPI: proton pump inhibitor; QoL: quality of life; RCT: randomized controlled trial; SD: standard deviation; SF‐36: 36‐item Short Form; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Almazar 2015 | Not intended comparison. |
| Bolling‐Sternvald 2003 | Post hoc analysis of Bolling‐Sternevald 2002. Not intended outcome. |
| Burkov 2009 | Not an RCT. |
| Bytzer 2000 | Short time intervention. |
| Cheung 2013 | Not intended outcome. |
| Chuang 2001 | Not intended comparison. |
| Delaney 2008 | Not intended comparison. |
| Fan 2012 | Not intended comparison. |
| Fransen 2012 | Not intended outcome. |
| Goves 1998 | Uninvestigated dyspepsia. |
| Guo 2011 | Not intended comparison. |
| Ivanova 2002 | Short time intervention. |
| Jones 1997 | Not intended comparison. |
| Jones 1999 | Uninvestigated dyspepsia. |
| Kamada 2013 | Not intended comparison. |
| Leung 2007 | Uninvestigated dyspepsia. |
| Lewin van den Broek 2001 | Uninvestigated dyspepsia. |
| Madsen 2004 | Uninvestigated dyspepsia. |
| Madsen 2008 | Uninvestigated dyspepsia. |
| Mazure 1996 | Not intended comparison. |
| Meineche‐Schmidt 1999 | Uninvestigated dyspepsia. |
| Meineche‐Schmidt 2000 | Uninvestigated dyspepsia. |
| Meineche‐Schmidt 2004 | Uninvestigated dyspepsia. |
| Miwa 2015 | Not original study. |
| Mönnikes 2009 | Uninvestigated dyspepsia. |
| Mönnikes 2012 | Uninvestigated dyspepsia. |
| Nagahara 2015 | Not an RCT. |
| Pilichiewicz 2011 | No extractable information, no additional data provided by authors. |
| Rabeneck 2001 (SODA) | Uninvestigated dyspepsia. |
| Reimer 2010 | Not an RCT. |
| Rui 2015 | Not intended comparison. |
| Sakaguchi 2012 | Uninvestigated dyspepsia. |
| Sakurai 2012 (J‐FOCUS) | Uninvestigated dyspepsia. |
| Schwartz 2001 | Not intended outcome. |
| Theodoropoulos 2009 | Not intended comparison. |
| van Zanten 2007 | Uninvestigated dyspepsia. |
| Veldhuyzen van Zanten 2005 | Uninvestigated dyspepsia. |
| Zeng 2007 | Not intended population. |
RCT: randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Puttapitakpong 2016.
| Methods | Setting: Thailand. Design: double‐blind, 2‐arm, parallel RCT. |
| Participants | 34 people with functional dyspepsia (unclear definition). |
| Interventions | Omeprazole (10 participants). Placebo (9 participants). Turmeric (12 participants). |
| Outcomes | Reduction in dyspeptic symptoms. Reduction in pain scores. Improvement of satisfaction. |
| Notes | Conference proceedings. No full text available. We contacted authors for extractable data. |
Yamawaki 2016.
| Methods | Setting: single‐centre in Japan. Design: double‐blind, placebo‐controlled RCT. |
| Participants | 98 adults with functional dyspepsia (Rome III). |
| Interventions | Acotiamide 100 mg 3 times daily (35 participants). Acotiamide + rabeprazole (32 participants). Rabeprazole 10 mg once per day (31 participants). Duration: 4 weeks. |
| Outcomes | Improvement of clinical symptoms. Gastric emptying. Satisfaction with treatment. |
| Notes | Supported in part by grant (No.24590928) from the Ministry of Education, Culture, and Science and from the Ministry of Health, Japan. No extractable data for meta‐analysis |
RCT: randomized controlled trial.
Differences between protocol and review
A new author (Ahmed Hassan) was added to the review team as he assisted with the development of the updated review.
The search strategy was modified after the publication of the protocol to improve sensitivity and precision.
The primary outcome was listed as "Reduction in global dyspeptic symptom score" in the protocol; however, we used "Global symptoms of dyspepsia " in the review as it is considered a more stringent definition of 'not symptom‐free.'
For the subgroup analyses, we found that the dichotomization of studies less than four weeks' duration versus greater than four weeks' duration was impractical at the time to allocate the studies in which the treatment was performed at week four. Therefore, we considered a categorization of two, four and eight weeks a more rational approach. We identified that a previous systematic review defined FD according to Rome II criteria (Wang 2007), and the definition of FD using the Rome III criteria is stricter than previous definitions of FD in excluding participants with reflux symptoms. Therefore, we added Rome III subgroup analysis post hoc.
We planned improvement of individual symptoms such as pain/discomfort and nausea as secondary outcome measures in the protocol; however, due to the length of this review we did not perform the analysis of individual symptoms.
Contributions of authors
Conceiving the review: MIPS, PM.
Designing the review: MIPS, PM.
Co‐ordinating the review: PM.
Designing the search strategies: YY, MIPS, PM.
Writing the review: MIPS, YY, AH.
Performing the analysis: MIPS, YY, AH.
Providing general advice on the review: PB, PM.
Performing previous work that was the foundation of the current review: PB, PM, YY.
Sources of support
Internal sources
McMaster University, Canada.
External sources
No sources of support supplied
Declarations of interest
MIPS: none known.
YY: none known.
AH: none known.
PB: none known.
PM has acted as a consultant and/or received speaker fees from Allergan, Shire and Lupin none of which directly or indirectly profit from PPI therapy. He also has received a research grant from Takeda to evaluate the diet and microbiome in IBS and IBD as part of the IMAGINE network but this company does sell a PPI.
Edited (no change to conclusions)
References
References to studies included in this review
Blum 2000 {published data only}
- Blum AL, Arnold R, Stolte M, Fischer M, Koelz HR. Short course acid suppressive treatment for patients with functional dyspepsia: results depend on Helicobacter pylori status. The Frosch Study Group. Gut 2000;47(4):473‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolling‐Sternevald 2002 {published data only}
- Bolling‐Sternevald E, Lauritsen K, Aalykke C, Havelund T, Knudsen T, Unge P, et al. Effect of profound acid suppression in functional dyspepsia: a double‐blind, randomized, placebo‐controlled trial. Scandinavian Journal of Gastroenterology 2002;37(12):1395‐402. [DOI] [PubMed] [Google Scholar]
Catapani 2015 {published and unpublished data}
- Catapani W, Guedes FS. A clinical trial assessing the efficacy of a therapeutic encounter versus traditional consultation in the management of functional dyspepsia. American Journal of Gastroenterology 2015;110(Suppl 1s):S751‐2. [Google Scholar]
Dillon 2004 {published data only}
- Dillon JF, Finch PJ, Baxter G. A comparison of lansoprazole vs ranitidine in the treatment of functional ulcer‐like dyspepsia as defined by the Rome II criteria. Gut 2004;53(Suppl VI):A285. [Google Scholar]
Farup 1999 {published data only}
- Farup PG, Hovde O, Torp R, Wetterhus S. Patients with functional dyspepsia responding to omeprazole have a characteristic gastro‐oesophageal reflux pattern. Scandinavian Journal of Gastroenterology 1999;34(6):575‐9. [DOI] [PubMed] [Google Scholar]
Fletcher 2011 {published data only}
- Fletcher J, Derakhshan MH, Jones GR, Wirz AA, McColl KEL. BMI is superior to symptoms in predicting response to proton pump inhibitor: randomised trial in patients with upper gastrointestinal symptoms and normal endoscopy. Gut 2011;60:442‐8. [DOI] [PubMed] [Google Scholar]
Gerson 2005 {published data only}
- Gerson LB, Triadafilopoulos G. A prospective study of oesophageal 24‐h ambulatory pH monitoring in patients with functional dyspepsia. Digestive and Liver Disease 2005;37(2):87‐91. [DOI] [PubMed] [Google Scholar]
Hengels 1998 {published data only}
- Hengels KJ. Therapeutic efficacy of 15mg lansoprazole mane in 269 patients suffering from non‐ulcer dyspepsia (NUD): a multicentre, randomised, double‐blind study. Gut 1998;43 (Suppl 2):A89. [Google Scholar]
Hsu 2011 {published data only}
- Hsu YC, Liou JM, Yang TH, Hsu WL, Lin HJ, Wu HT, et al. Proton pump inhibitor versus prokinetic therapy in patients with functional dyspepsia: is therapeutic response predicted by Rome III subgroups?. Journal of Gastroenterology 2011;46:183‐90. [DOI] [PubMed] [Google Scholar]
Iwakiri 2013 {published data only}
- Iwakiri R, Tominaga K, Furuta K, Inamori M, Furuta T, Masuyama H, et al. Randomised clinical trial: rabeprazole improves symptoms in patients with functional dyspepsia in Japan. Alimentary Pharmacology & Therapeutics 2013;16(9):547‐50. [DOI] [PubMed] [Google Scholar]
Jiang 2011 {published data only}
- Jiang Q, Ding X, Zhang S, Wang H, Yu X, Xie S. Comparison of mosapride and pantoprazole in treating functional dyspepsia. Chinese Journal of Gastroenterology 2011;16(9):254‐63. [Google Scholar]
Jung 2016 {published data only}
- Jung HK, Lee KJ, Choi MG, Park H, Lee JS, Rhee PL, et al. Efficacy of DA‐9701 (Motilitone) in functional dyspepsia compared to pantoprazole: a multicenter, randomized, double‐blind, non‐inferiority study. Journal of Neurogastroenterology and Motility 2016;22(2):254‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamiya 2017 {published data only}
- Kamiya T, Shikano M, Hirata Y, Mizushima T, Murakami K, Shimura, et al. A multicenter open‐label randomized trial comparing rabeprazole versus itopride in Japanese functional dyspepsia: the NAGOYA Study. Gastroenterology 2011;140(5):S189. [Google Scholar]
- Kamiya T, Shikano M, Kubota E, Mizoshita T, Wada T, Tanida S, et al. A multicenter randomized trial comparing rabeprazole and Itopride in patients with functional dyspepsia in Japan: the NAGOYA study. Journal of Clinical Biochemistry and Nutrition 2017;60(2):130‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya T, Shikano M, Mizushima T, Hirata Y, Murakami K, Shimura T, et al. T1095 comparison of rabeprazole and itopride in the treatment of functional dyspepsia sub‐analysis: Nagoya multicentre randomised comparative trial. Gastroenterology 2010;138(5):S486‐7. [Google Scholar]
- Kamiya T, Shikano M, Ozeki K, Hirata Y, Mizoshita T, Mori Y, et al. Are there any differences among predictive response factors for a proton pump inhibitor and prokinetics? Sub‐analysis of a multicenter open‐label randomized trial comparing rabeprazole versus itopride in Japanese functional dyspepsia: the NAGOYA study. Gastroenterology 2012;142(5 Suppl 1):S‐468. [Google Scholar]
Li 2003 {published data only}
- Li Z, Guomin X, Yiqi D, Wenzhong L, Xiao SD. Low‐dose omeprazole treatment of functional dyspepsia. Chinese Journal of Gastroenterology 2003;6:337‐9. [Google Scholar]
Majewski 2016 {published data only}
- Majewski M, Sarosiek I, Cooper CJ, Wallner G, McCallum RW, Edlavitch SA, et al. Gastric pH and therapeutic responses to exsomeprazole in patients with functional dyspepsia: potential clinical implications. American Journal of the Medical Sciences 2016;352(6):582‐92. [DOI] [PubMed] [Google Scholar]
Peura 2004 {published data only}
- Peura DA, Kovacs TO, Metz DC, Siepman N, Pilmer BL, Talley NJ. Lansoprazole in the treatment of functional dyspepsia: two double‐blind, randomized, placebo‐controlled trials. American Journal of Medicine 2004;116(11):740‐8. [DOI] [PubMed] [Google Scholar]
Suzuki 2013 (ELF) {published data only}
- Suzuki H, Kusunoki H, Kamiya T, Futagami S, Yamaguchi Y, Nishizawa T, et al. Effect of lansoprazole on the epigastric symptoms of functional dyspepsia (ELF study): a multicentre, prospective, randomized, double‐blind, placebo‐controlled clinical trial. United European Gastroenterology Journal 2013;1(6):445‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Talley 1998 (BOND) {published data only}
- Talley NJ, Meineche‐Schmidt V, Pare P, Duckworth M, Raisanen P, Pap A, et al. Efficacy of omeprazole in functional dyspepsia: double‐blind, randomized, placebo‐controlled trials (the BOND and OPERA studies). Alimentary Pharmacology & Therapeutics 1998;12:1055‐65. [DOI] [PubMed] [Google Scholar]
Talley 1998 (OPERA) {published data only}
- Talley NJ, Meineche‐Schmidt V, Pare P, Duckworth M, Raisanen P, Pap A, et al. Efficacy of omeprazole in functional dyspepsia: double‐blind, randomized, placebo‐controlled trials (the BOND and OPERA studies). Alimentary Pharmacology & Therapeutics 1998;12:1055‐65. [DOI] [PubMed] [Google Scholar]
Talley 2007 {published data only}
- Talley NJ, Vakil N, Lauritsen K, Zanten VS, Flook N, Bolling‐Sternevald E, et al. Randomized‐controlled trial of esomeprazole in functional dyspepsia patients with epigastric pain or burning: does a 1‐week trial of acid suppression predict symptom response?. Alimentary Pharmacology & Therapeutics 2007;26(5):673‐82. [DOI] [PubMed] [Google Scholar]
Tominaga 2010 {published data only}
- Tominaga K, Suzuki H, Umegaki E, Kusunoki H, Tomotsugu N, Higuchi K, et al. Rabeprazole improves the symptoms of functional dyspepsia ‐ a double blind randomized placebo‐controlled multi‐center trial in Japan: the CAESAR Study. Gastroenterology 2010;138(5 Suppl 1):S55. [Google Scholar]
Van Rensburg 2008 {published data only}
- Rensburg C, Berghofer P, Enns R, Dattani ID, Maritz JF, Gonzalez Carro P, et al. Efficacy and safety of pantoprazole 20 mg once daily treatment in patients with ulcer‐like functional dyspepsia. Current Medical Research and Opinion 2008;24(7):2009‐18. [DOI] [PubMed] [Google Scholar]
Van Zanten 2006 {published data only}
- Zanten SV, Armstrong D, Chiba N, Flook N, White RJ, Chakraborty B, et al. Esomeprazole 40 mg once a day in patients with functional dyspepsia: the randomized, placebo‐controlled "ENTER" trial. American Journal of Gastroenterology 2006;101:2096‐106. [DOI] [PubMed] [Google Scholar]
Wong 2002 {published data only}
- Wong WM, Wong BCY, Hung WK, Yee YK, Yip AWC, Szeto ML, et al. Double blind, randomised, placebo controlled study of four weeks of lansoprazole for the treatment of functional dyspepsia in Chinese patients. Gut 2002;51:502‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2014 {published data only}
- Yang ZY. Efficacy and safety of pantoprazole sodium enteric coated tablets combined with mosapride in treatment of functional dyspepsia. World Chinese Journal of Digestology 2014;22(29):4514‐18. [Google Scholar]
References to studies excluded from this review
Almazar 2015 {published data only}
- Almazar AE, Saito YA, Locke G, Camillieru M, Burton DD, Bouras EP, et al. Is proton pump inhibitor use associated with delayed gastric emptying in functional dyspepsia subjects?. Gastroenterology 2015;148(4):S888. [Google Scholar]
Bolling‐Sternvald 2003 {published data only}
- Bolling‐Sternevald E, Lauritsen K, Talley NJ, Junghard O, Glise H. Is it possible to predict treatment response to a proton pump inhibitor in functional dyspepsia?. Alimentary Pharmacology & Therapeutics 2003;18(1):117‐24. [DOI] [PubMed] [Google Scholar]
Burkov 2009 {published data only}
- Burkov SG, Arutiunov AT, Okoemov MN, Anokhina LN, Egorova NV, Makukh EA, et al. Effectiveness of Ultop 10 mg using at treatment of functional (nonulcer) dyspepsia syndrome. Eksperimental'naia i Klinicheskaia Gastroenterologiia [Experimental & Clinical Gastroenterology] 2009;6:122‐6. [PubMed] [Google Scholar]
Bytzer 2000 {published data only}
- Bytzer P, Hansen JM, Rune S, Bonnevie O, Breinstrup H, Funch‐Jensen P, et al. Identifying responders to acid suppression in dyspepsia using a random starting day trial. Alimentary Pharmacology & Therapeutics 2000;14(11):1485‐94. [DOI] [PubMed] [Google Scholar]
Cheung 2013 {published data only}
- Cheung CK, Lee Y, Chan Y, Chan A, Law WT, Lan LL, et al. The effect of proton pump inhibitor (PPI) on plasma 5‐hydroxytryptamine (5‐HT) levels in patients with functional dyspepsia (FD): a double‐blind randomized control trial. Gastroenterology 2013;144(5):S679. [Google Scholar]
Chuang 2001 {published data only}
- Chuang CH, Sheu BS, Yang HB, Wu JJ, Lin XZ. Ranitidine bismuth citrate or omeprazole‐based triple therapy for Helicobacter pylori eradication in Helicobacter pylori‐infected non‐ulcer dyspepsia. Digestive and Liver Disease 2001;33(2):125‐30. [DOI] [PubMed] [Google Scholar]
Delaney 2008 {published data only}
- Delaney BC, Qume M, Moayyedi P, Logan RF, Ford AC, Elliott C, et al. Helicobacter pylori test and treat versus proton pump inhibitor in initial management of dyspepsia in primary care: multicentre randomised controlled trial (MRC‐CUBE trial). BMJ 2008;336:651‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fan 2012 {published data only}
- Fan YH, Cai LJ, Xu GP. Treatment of functional dyspepsia by Chinese medical syndrome typing: a randomized control research. Zhongguo Zhong Xi Yi Jie He za Zhi [Chinese Journal of Integrated Traditional and Western Medicine] 2012;32(12):1592‐7. [PubMed] [Google Scholar]
Fransen 2012 {published data only}
- Fransen GA, Mesters I, Muris JW, Marrewijk CJ, Mujakovic S, Laheij RJ, et al. Patient adherence to prescribed medication instructions for dyspepsia: the DIAMOND study. European Journal of General Practice 2012;18(2):79‐85. [DOI] [PubMed] [Google Scholar]
Goves 1998 {published data only}
- Goves J, Oldring JK, Kerr D, Dallara RG, Roffe EJ, Powell JA, et al. First line treatment with omeprazole provides an effective and superior alternative strategy in the management of dyspepsia compared to antacid/alginate liquid: a multicentre study in general practice. Alimentary Pharmacology & Therapeutics 1998;12(2):147‐57. [DOI] [PubMed] [Google Scholar]
Guo 2011 {published data only}
- Guo LK, Zhang CX, Guo XF. Long‐term efficacy and safety research on functional dyspepsia treated with electroacupuncture and Zhizhu Kuanzhong capsule. Zhongguo Zhen Jiu [Chinese Acupuncture & Moxibustion] 2011;31(12):1071‐7. [PubMed] [Google Scholar]
Ivanova 2002 {published data only}
- Ivanova NG. Inhibitors of proton pump in the treatment of non‐ulcer functional dyspepsia of the reflux‐like type. Eksperimental'naia i Klinicheskaia Gastroenterologiia [Experimental & Clinical Gastroenterology] 2002;101(2):35‐6. [PubMed] [Google Scholar]
Jones 1997 {published data only}
- Jones RH, Baxter G. Lansoprazole 30 mg daily versus ranitidine 150 mg b.d. in the treatment of acid‐related dyspepsia in general practice. Alimentary Pharmacology & Therapeutics 1997;11(3):541‐6. [DOI] [PubMed] [Google Scholar]
Jones 1999 {published data only}
- Jones R, Crouch SL. Low‐dose lansoprazole provides greater relief of heartburn and epigastric pain than low‐dose omeprazole in patients with acid‐related dyspepsia. Alimentary Pharmacology & Therapeutics 1999;13(3):413‐9. [DOI] [PubMed] [Google Scholar]
Kamada 2013 {published data only}
- Kamada T, Fujimura Y, Gotoh K, Imamura H, Manabe N, Kusunoki H, et al. A study on the efficacy of proton pump inhibitors in Helicobacter pylori‐negative primary care patients with dyspepsia in Japan. Gut and Liver 2013;7(1):16‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leung 2007 {published data only}
- Leung WK, Wu JC, Chan FK, Fung SS, Wong VW, Hui AJ, et al. Initial treatment with lansoprazole in young dyspeptic patients with negative urea breath test result: a randomized controlled trial with 12‐month follow‐up. American Journal of Gastroenterology 2007;102(7):1483‐8. [DOI] [PubMed] [Google Scholar]
Lewin van den Broek 2001 {published data only}
- Lewin van den Broek NT, Numans ME, Buskens E, Verheij TJ, Wit NJ, Smout AJ. A randomised controlled trial of four management strategies for dyspepsia: relationships between symptom subgroups and strategy outcome. British Journal of General Practice 2001;51(469):619‐24. [PMC free article] [PubMed] [Google Scholar]
Madsen 2004 {published data only}
- Madsen LG, Bytzer P. Reproducibility of a symptom response to omeprazole therapy in functional dyspepsia evaluated by a random‐starting‐day trial design. Alimentary Pharmacology & Therapeutics 2004;20(3):365‐72. [DOI] [PubMed] [Google Scholar]
Madsen 2008 {published data only}
- Madsen LG, Bytzer P. Symptomatic response to blocked and unblocked pentagastrin stimulation in functional dyspepsia. Comparison of responders and non‐responders to omeprazole identified in a single‐subject trial model. Digestive Diseases (Basel, Switzerland) 2008;26(3):237‐42. [DOI] [PubMed] [Google Scholar]
Mazure 1996 {published data only}
- Mazure PA. Gastric acid secretion inhibitors: H2 receptor antagonists (H2RA), proton pump inhibitors (PPI) or acid pump antagonists (APA). Acta Gastroenterologica Latinoamericana 1996;26(4):263‐5. [PubMed] [Google Scholar]
Meineche‐Schmidt 1999 {published data only}
- Meineche‐Schmidt V, Talley NJ, Pap A, Kordecki H, Schmid V, Ohlsson L, et al. Impact of functional dyspepsia on quality of life and health care consumption after cessation of antisecretory treatment. A multicentre 3‐month follow‐up study. Scandinavian Journal of Gastroenterology 1999;34(6):566‐74. [DOI] [PubMed] [Google Scholar]
Meineche‐Schmidt 2000 {published data only}
- Meineche‐Schmidt V, Christensen E. Which dyspepsia patients will benefit from omeprazole treatment? Analysis of a Danish multicenter trial. American Journal of Gastroenterology 2000;95(10):2777‐83. [DOI] [PubMed] [Google Scholar]
Meineche‐Schmidt 2004 {published data only}
- Meineche‐Schmidt V. Classification of dyspepsia and response to treatment with proton‐pump inhibitors. Alimentary Pharmacology & Therapeutics 2004;20(10):1171‐9. [DOI] [PubMed] [Google Scholar]
Miwa 2015 {published data only}
- Miwa H, Haruma K, Sakamoto S, Sanada K, Hiroi S, Kinoshita Y. Demography and treatment response in patients with predominant non‐erosive reflux disease or functional dyspepsia. Journal of Gastroenterology and Hepatology 2015;30(5):834‐41. [DOI] [PubMed] [Google Scholar]
Mönnikes 2009 {published data only}
- Mönnikes H, Schmidtmann M. Irritable bowel disease with flatulence: is the breath test for carbohydrate malabsorption obligatory?. Deutsche Medizinische Wochenschrift (1946) 2009;134(37):1828. [DOI] [PubMed] [Google Scholar]
Mönnikes 2012 {published data only}
- Mönnikes H, Schwan T, Rensburg C, Straszak A, Theek C, Sander P, et al. Randomised clinical trial: sustained response to PPI treatment of symptoms resembling functional dyspepsia and irritable bowel syndrome in patients suffering from an overlap with erosive gastro‐oesophageal reflux disease. Alimentary Pharmacology & Therapeutics 2012;35(11):1279‐89. [DOI] [PubMed] [Google Scholar]
Nagahara 2015 {published data only}
- Nagahara A, Miwa H, Asaoka D, Shimada Y, Sasaki H, Matsumoto K, et al. Pretreatment prediction of symptom response to proton pump inhibitor therapy. Gastroenterology & Hepatology 2015;Suppl 1:25‐30. [DOI] [PubMed] [Google Scholar]
Pilichiewicz 2011 {published data only}
- Pilichiewicz AN, Andrews JM, Goess C, McMahon J, Fraser RJ, Beilby J, et al. A preliminary analysis of the effect Iberogast, Nexium, dual Iberogast + Nexium or dual placebo on symptoms in patients with functional dyspepsia. Journal of Gastroenterology and Hepatology 2011;26(s4):85. [DOI: 10.1111/j.1440-1746.2011.06826.x] [DOI] [Google Scholar]
Rabeneck 2001 (SODA) {published data only}
- Rabeneck L, Cook KF, Wristers K, Souchek J, Menke T, Wray NP. SODA (severity of dyspepsia assessment): a new effective outcome measure for dyspepsia‐related health. Journal of Clinical Epidemiology 2001;54(8):755‐65. [DOI] [PubMed] [Google Scholar]
Reimer 2010 {published data only}
- Reimer C, Bytzer P. Discontinuation of long‐term proton pump inhibitor therapy in primary care patients: a randomized placebo‐controlled trial in patients with symptom relapse. European Journal of Gastroenterology & Hepatology 2010;22(10):1182‐8. [DOI] [PubMed] [Google Scholar]
Rui 2015 {published data only}
- Rui J, Kang J, Xiu‐Li Z, Yanqing L. Randomized clinical study on functional dyspepsia treatment with herbal preparation puyuanhewei and therapeutic response evaluation by endomicroscopy. Gastroenterology 2015;148(4 Suppl 1):S891. [Google Scholar]
Sakaguchi 2012 {published data only}
- Sakaguchi M, Takao M, Ohyama Y, Oka H, Yamashita H, Fukuchi T, et al. Comparison of PPIs and H2‐receptor antagonists plus prokinetics for dysmotility‐like dyspepsia. World Journal of Gastroenterology 2012;18(13):1517‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sakurai 2012 (J‐FOCUS) {published data only}
- Sakurai K, Nagahara A, Inoue K, Akiyama J, Mabe K, Suzuki J, et al. Efficacy of omeprazole, famotidine, mosapride and teprenone in patients with upper gastrointestinal symptoms: an omeprazole‐controlled randomized study (J‐FOCUS). BMC Gastroenterology 2012;12:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwartz 2001 {published data only}
- Schwartz MP, Samsom M, Berge Henegouwen GP, Smout AJ. Effect of inhibition of gastric acid secretion on antropyloroduodenal motor activity and duodenal acid hypersensitivity in functional dyspepsia. Alimentary Pharmacology & Therapeutics 2001;15(12):1921‐8. [DOI] [PubMed] [Google Scholar]
Theodoropoulos 2009 {published data only}
- Theodoropoulos I, Karakoidas C, Papamichael K, Smyrnidis A, Archavlis EJ, Mantzaris GJ. A lyophilized form of S. boulardii enhances the effect of omeprazole‐triple therapy in patients with organic or functional dyspepsia. Gastroenterology 2009;Suppl 1:A343. [Google Scholar]
van Zanten 2007 {published data only}
- Zanten SV, Armstrong D, Barkun A, Junghard O, White RJ, Wiklund IK. Symptom overlap in patients with upper gastrointestinal complaints in the Canadian Confirmatory Acid Suppression Test (CAST) study: further psychometric validation of the reflux disease questionnaire. Alimentary Pharmacology & Therapeutics 2007;25(9):1087‐97. [DOI] [PubMed] [Google Scholar]
Veldhuyzen van Zanten 2005 {published data only}
- Veldhuyzen van Zanten SJ, Chiba N, Armstrong D, Barkun A, Thomson A, Smyth S, et al. A randomized trial comparing omeprazole, ranitidine, cisapride, or placebo in Helicobacter pylori negative, primary care patients with dyspepsia: the CADET‐HN Study. American Journal of Gastroenterology 2005;100(7):1477‐88. [DOI] [PubMed] [Google Scholar]
Zeng 2007 {published data only}
- Zeng J, Zuo XL, Li YQ, Wei W, Lv GP. Tegaserod for dyspepsia and reflux symptoms in patients with chronic constipation: an exploratory open‐label study. European Journal of Clinical Pharmacology 2007;63(6):529‐36. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Puttapitakpong 2016 {published data only}
- Puttapitakpong C, Jearjesdakul J. Effectiveness of curcuma longa linn compared with omeprazole on treatment of functional dyspepsia. Gastroenterology 2016;150(4 (Suppl 1)):S 43. [Google Scholar]
Yamawaki 2016 {published data only}
- Yamawaki H, Futagami S, Kawagoe T, Maruki Y, Hashimoto S, Nagoya H, et al. Improvement of meal‐related symptoms and epigastric pain in patients with functional dyspepsia treated with acotiamide was associated with acylated ghrelin levels in Japan. Neurogastroenterology and Motility 2016;28(7):1037‐47. [DOI] [PubMed] [Google Scholar]
Additional references
Abraham 2004
- Abraham NS, Moayyedi P, Daniels B, Veldhuyzen Van Zanten SJ. Systematic review: the methodological quality of trials affects estimates of treatment efficacy in functional (non‐ulcer) dyspepsia. Alimentary Pharmacology and Therapeutics 2004;19(6):631‐41. [DOI] [PubMed] [Google Scholar]
Abramowitz 2016
- Abramowitz J, Thakkar P, Isa A, Truong A, Park C, Rosenfeld RM. Adverse event reporting for proton pump inhibitor therapy: an overview of systematic reviews. Otolaryngology and Head and Neck Surgery 2016;155(4):547‐54. [DOI] [PubMed] [Google Scholar]
Barbera 1995
- Barbera R, Feinle C, Read NW. Nutrient specific modulation of gastric mechanosensitivity in patients with functional dyspepsia. Digestive Diseases and Sciences 1995;40(8):1636‐41. [DOI] [PubMed] [Google Scholar]
Bekhti 1979
- Bekhti A, Rutgeerts L. Domperidone in the treatment of functional dyspepsia in patients with delayed gastric emptying. Postgraduate Medical Journal 1979;55(Suppl 1):30‐2. [PubMed] [Google Scholar]
Bercik 2000
- Bercík P, Verdú EF, Armstrong D, Idström JP, Cederberg C, Markert M, et al. The effect of ammonia on omeprazole‐induced reduction of gastric acidity in subjects with Helicobacter pylori infection. American Journal of Gastroenterology 2000;95(4):947‐55. [DOI] [PubMed] [Google Scholar]
Blum 1998
- Blum AL, Talley NJ, O'Moráin C, van Zanten SV, Labenz J, Stolte M, et al. Lack of effect of treating Helicobacter pylori infection in patients with nonulcer dyspepsia. Omeprazole plus clarithromycin and amoxicillin effect one year after treatment (OCAY) study group. New England Journal of Medicine 1998;339(26):1875‐81. [DOI] [PubMed] [Google Scholar]
Bolling‐Sternevald 2003
- Bolling‐Sternevald E, Lauritsen K, Talley NJ, Junghard O, Glise H. Is it possible to predict treatment response to a proton pump inhibitor in functional dyspepsia?. Alimentary Pharmacology and Therapeutics 2003;18(1):117‐24. [DOI] [PubMed] [Google Scholar]
Calvert 2002
- Calvert EL, Houghton LA, Cooper P, Morris J, Whorwell PJ. Long‐term improvement in functional dyspepsia using hypnotherapy. Gastroenterology 2002;123(6):1778‐85. [DOI] [PubMed] [Google Scholar]
Camillieri 2013
- Camillieri M, Stanghellini V. Current management strategies and emerging treatments for functional dyspepsia. Nature Reviews. Gastroenterology & Hepatology 2013;10(3):187‐94. [DOI] [PubMed] [Google Scholar]
Chen 2000
- Chen JD, Ke MY, Lin XM, Wang Z, Zhang M. Cisapride provides symptomatic relief in functional dyspepsia associated with gastric myoelectrical abnormality. Alimentary Pharmacology and Therapeutics 2000;14(8):1041‐7. [DOI] [PubMed] [Google Scholar]
Colin‐Jones 1988
- Colin‐Jones DG, Bloom B, Bodemar G, Crean G, Freston J, Gugler R, et al. Management of dyspepsia: report of a working party. Lancet 1988;331(8585):576‐9. [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Alman DG, Cochrane Statistics Methods Group. Chapter 9.4.5.2: Meta‐analysis of change scores. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Drossman 1999
- Drossman DA, Corazziari E, Talley NJ, Thompson WG, Whitehead WE. Rome II: a multinational consensus document on functional gastrointestinal disorders ‐ functional bowel disorders and functional abdominal pain. Gut 1999;45(2):ii‐43. [Google Scholar]
Drossman 2006
- Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 2006;130:1377‐90. [DOI] [PubMed] [Google Scholar]
Froehlich 2001
- Froehlich F, Gonvers JJ, Wietlisbach V, Burnand B, Hildebrand P, Schneider C, et al. Helicobacter pylori eradication treatment does not benefit patients with nonulcer dyspepsia. American Journal of Gastroenterology 2001;96(8):2329‐36. [DOI] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. Brozek J, Oxman A, Schünemann H, 2008.
GraphPad [Computer program]
- GraphPad Software. GraphPad Software. Version 5.04. La Jolla (CA): GraphPad Software, 2010.
Hamilton 2000
- Hamilton J, Guthrie E, Creed F, Thompson D, Tomenson B, Bennett R, et al. A randomized controlled trial of psychotherapy in patients with chronic functional dyspepsia. Gastroenterology 2000;119(3):661‐9. [DOI] [PubMed] [Google Scholar]
Hansen 1998
- Hansen JM, Bytzer P, Schaffalitzky de Muckadell OB. Placebo‐controlled trial of cisapride and nizatidine in unselected patients with functional dyspepsia. American Journal of Gastroenterology 1998;93(3):368‐74. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holtmann 2002
- Holtmann G, Gschossmann J, Mayr P, Talley NJ. A randomized placebo‐controlled trial of simethicone and cisapride for the treatment of patients with functional dyspepsia. Alimentary Pharmacology and Therapeutics 2002;16(9):1641‐8. [DOI] [PubMed] [Google Scholar]
Johnson 2013
- Johnson DA, Oldfield EC. Reported side effects and complications of long‐term proton pump inhibitor use. Clinical Gastroenterology and Hepatology 2013;11(5):458‐64. [DOI] [PubMed] [Google Scholar]
Kwok 2013
- Kwok CS, Jeevanantham V, Dawn B, Loke YK. No consistent evidence of differential cardiovascular risk amongst proton‐pump inhibitors when used with clopidogrel: meta‐analysis. International Journal of Cardiology 2013;167(3):965‐74. [PUBMED: 22464478] [DOI] [PubMed] [Google Scholar]
Lacy 2012
- Lacy BE, Talley NJ, Locke GR 3rd, Bouras EP, DiBaise JK, El‐Serag HB. Review article: current treatment options and management of functional dyspepsia. Alimentary Pharmacology and Therapeutics 2012;36(1):3‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lacy 2013
- Lacy BE, Weiser KT, Kennedy MD, Crowell MD, Talley NJ. Functional dyspepsia: the economic impact to patients. Alimentary Pharmacology & Therapeutics 2013;38(2):170‐7. [DOI] [PubMed] [Google Scholar]
McColl 1998
- McColl K, Murray L, El‐Omar E, Dickson A, El‐Nujumi A, Wirz A, et al. Symptomatic benefit from eradicating Helicobacter pylori infection in patients with nonulcer dyspepsia. New England Journal of Medicine 1998;339(26):1869‐74. [DOI] [PubMed] [Google Scholar]
Moayyedi 2003
- Moayyedi P, Soo S, Deeks JJ, Delaney B, Innes M, Forman D. Pharmacological interventions for non‐ulcer dyspepsia. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD001960] [DOI] [PubMed] [Google Scholar]
Moayyedi 2006
- Moayyedi P, Soo S, Deeks JJ, Delaney B, Innes M, Forman D. Pharmacological interventions for non‐ulcer dyspepsia. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD001960.pub3] [DOI] [PubMed] [Google Scholar]
Moayyedi 2011
- Moayyedi P, Shelly S, Deeks JJ, Delaney B, Innes M, Forman D. Pharmacological interventions for non‐ulcer dyspepsia. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD001960.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Overland 2014
- Overland MK. Dyspepsia. Medical Clinics of North America 2014;98:549‐64. [DOI] [PubMed] [Google Scholar]
Reference Manager 2014 [Computer program]
- Thomson ISI ResearchSoft. Reference Manager. Version 12.0.3. Copenhagen: Thomson ISI ResearchSoft, 2014.
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 2004
- Robinson M. Review article: the pharmacodynamics and pharmacokinetics of proton pump inhibitors ‐ overview and clinical implications. Alimentary Pharmacology & Therapeutics 2004;20(Suppl 6):1‐10. [DOI] [PubMed] [Google Scholar]
Savarino 2017
- Savarino V, Dulbecco P, Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. European Journal of Internal Medicine 2017;37:19‐24. [DOI] [PubMed] [Google Scholar]
Scarpignato 2016
- Scarpignato C, Gatta L, Zullo A, Blandizzi C, SIF‐AIGO‐FIMMG Group, Italian Society of Pharmacology, the Italian Association of Hospital Gastroenterologists, the Italian Federation of General Practitioners. Effective and safe proton pump inhibitor therapy in acid‐related diseases ‐ a position paper addressing benefits and potential harms of acid suppression. BMC Medicine 2016;14(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanghellini 2016
- Stanghellini V, Chan FK, Hasler WL, Malagelada JR, Suzuki H, Tack J, et al. Gastroduodenal disorders. Gastroenterology 2016;150:1380‐92. [DOI] [PubMed] [Google Scholar]
Suzuki 2011
- Suzuki H, Okada S, Hibi T. Proton pump inhibitors for the treatment of functional dyspepsia. Therapeutic Advances in Gastroenterology 2011;4(4):219‐26. [DOI: 10.1177/1756283X11398735] [DOI] [PMC free article] [PubMed] [Google Scholar]
Talley 1991
- Talley NJ. Drug treatment of functional dyspepsia. Scandinavian Journal of Gastroenterology 1991;182:47‐60. [PubMed] [Google Scholar]
Talley 1995
- Talley NJ. Functional dyspepsia ‐ should treatment be targeted on disturbed physiology?. Alimentary Pharmacology and Therapeutics 1995;9(2):107‐15. [DOI] [PubMed] [Google Scholar]
Talley 1999a
- Talley NJ, Janssens J, Lauritsen K, Rácz I, Bolling‐Sternevald E. Eradication of Helicobacter pylori in functional dyspepsia: randomised double blind placebo controlled trial with 12 months' follow up. The Optimal Regimen Cures Helicobacter Induced Dyspepsia (ORCHID) Study Group. BMJ 1999;318(7187):833‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Talley 1999b
- Talley NJ, Vakil N, Ballard ED 2nd, Fennerty MB. Absence of benefit of eradicating Helicobacter pylori in patients with non‐ulcer dyspepsia. New England Journal of Medicine 1999;341(15):1106‐11. [DOI] [PubMed] [Google Scholar]
Talley 2016
- Talley NJ, Walker M, Holtmann G. Functional dyspepsia. Current Opinions in Gastroenterology 2016;32(6):467‐73. [DOI] [PubMed] [Google Scholar]
Tett 2013
Van Outryve 1993
- Outryve M, Nutte N, Eeghem P. Efficacy of cisapride in functional dyspepsia resistant to domperidone or metoclopramide: a double‐blind, placebo‐controlled study. Scandinavian Journal of Gastroenterology 1993;195:47‐52. [DOI] [PubMed] [Google Scholar]
Verdú 1995
- Verdú EF, Armstrong D, Idström JP, Labenz J, Stolte M, Dorta G, et al. Effect of curing Helicobacter pylori infection on intragastric pH during treatment with omeprazole. Gut 1995;37(6):743‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2007
- Wang WH, Huang JQ, Zheng GF. Effects of proton pump inhibitors on functional dyspepsia: a meta‐analysis of randomized placebo‐controlled trials. Clinical Gastroenterology and Hepatology 2007;5(2):178‐85. [DOI] [PubMed] [Google Scholar]
Wilhelm 2013
- Wilhelm S, Rjater R, Kale‐Pradhan PB. Perils and pitfalls of long‐term effects of proton pump inhibitors. Expert Review of Clinical Pharmacology 2013;6(4):443‐51. [DOI] [PubMed] [Google Scholar]
Yang 2010
- Yang YX, Metz DC. Safety of proton pump inhibitor exposure. Gastroenterology 2010;139(4):115‐27. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Pinto‐Sanchez 2017
- Pinto‐Sanchez MI, Yuan Y, Bercik P, Moayyedi P. Proton pump inhibitors for functional dyspepsia. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD011194.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


