Abstract
Background
Asthma exacerbations can be frequent and range in severity from mild to life‐threatening. The use of magnesium sulfate (MgSO₄) is one of numerous treatment options available during acute exacerbations. While the efficacy of intravenous MgSO₄ has been demonstrated, the role of inhaled MgSO₄ is less clear.
Objectives
To determine the efficacy and safety of inhaled MgSO₄ administered in acute asthma.
Specific aims: to quantify the effects of inhaled MgSO₄ I) in addition to combination treatment with inhaled β₂‐agonist and ipratropium bromide; ii) in addition to inhaled β₂‐agonist; and iii) in comparison to inhaled β₂‐agonist.
Search methods
We identified randomised controlled trials (RCTs) from the Cochrane Airways Group register of trials and online trials registries in September 2017. We supplemented these with searches of the reference lists of published studies and by contact with trialists.
Selection criteria
RCTs including adults or children with acute asthma were eligible for inclusion in the review. We included studies if patients were treated with nebulised MgSO₄ alone or in combination with β₂‐agonist or ipratropium bromide or both, and were compared with the same co‐intervention alone or inactive control.
Data collection and analysis
Two review authors independently assessed trial selection, data extraction and risk of bias. We made efforts to collect missing data from authors. We present results, with their 95% confidence intervals (CIs), as mean differences (MDs) or standardised mean differences (SMDs) for pulmonary function, clinical severity scores and vital signs; and risk ratios (RRs) for hospital admission. We used risk differences (RDs) to analyse adverse events because events were rare.
Main results
Twenty‐five trials (43 references) of varying methodological quality were eligible; they included 2907 randomised patients (2777 patients completed). Nine of the 25 included studies involved adults; four included adult and paediatric patients; eight studies enrolled paediatric patients; and in the remaining four studies the age of participants was not stated. The design, definitions, intervention and outcomes were different in all 25 studies; this heterogeneity made direct comparisons difficult. The quality of the evidence presented ranged from high to very low, with most outcomes graded as low or very low. This was largely due to concerns about the methodological quality of the included studies and imprecision in the pooled effect estimates.
Inhaled magnesium sulfate in addition to inhaled β₂‐agonist and ipratropium
We included seven studies in this comparison. Although some individual studies reported improvement in lung function indices favouring the intervention group, results were inconsistent overall and the largest study reporting this outcome found no between‐group difference at 60 minutes (MD −0.3 % predicted peak expiratory flow rate (PEFR), 95% CI −2.71% to 2.11%). Admissions to hospital at initial presentation may be reduced by the addition of inhaled magnesium sulfate (RR 0.95, 95% CI 0.91 to 1.00; participants = 1308; studies = 4; I² = 52%) but no difference was detected for re‐admissions or escalation of care to ITU/HDU. Serious adverse events during admission were rare. There was no difference between groups for all adverse events during admission (RD 0.01, 95% CI −0.03 to 0.05; participants = 1197; studies = 2).
Inhaled magnesium sulfate in addition to inhaled β₂‐agonist
We included 13 studies in this comparison. Although some individual studies reported improvement in lung function indices favouring the intervention group, none of the pooled results showed a conclusive benefit as measured by FEV1 or PEFR. Pooled results for hospital admission showed a point estimate that favoured the combination of MgSO₄ and β₂‐agonist, but the confidence interval includes the possibility of admissions increasing in the intervention group (RR 0.78, 95% CI 0.52 to 1.15; participants = 375; studies = 6; I² = 0%). There were no serious adverse events reported by any of the included studies and no between‐group difference for all adverse events (RD −0.01, 95% CI −0.05 to 0.03; participants = 694; studies = 5).
Inhaled magnesium sulfate versus inhaled β₂‐agonist
We included four studies in this comparison. The evidence for the efficacy of β₂‐agonists in acute asthma is well‐established and therefore this could be considered a historical comparison. Two studies reported a benefit of β₂‐agonist over MgSO₄ alone for PEFR and two studies reported no difference; we did not pool these results. Admissions to hospital were only reported by one small study and events were rare, leading to an uncertain result. No serious adverse events were reported in any of the studies in this comparison; one small study reported mild to moderate adverse events but the result is imprecise.
Authors' conclusions
Treatment with nebulised MgSO₄ may result in modest additional benefits for lung function and hospital admission when added to inhaled β₂‐agonists and ipratropium bromide, but our confidence in the evidence is low and there remains substantial uncertainty. The recent large, well‐designed trials have generally not demonstrated clinically important benefits. Nebulised MgSO₄ does not appear to be associated with an increase in serious adverse events. Individual studies suggest that those with more severe attacks and attacks of shorter duration may experience a greater benefit but further research into subgroups is warranted.
Despite including 24 trials in this review update we were unable to pool data for all outcomes of interest and this has limited the strength of the conclusions reached. A core outcomes set for studies in acute asthma is needed. This is particularly important in paediatric studies where measuring lung function at the time of an exacerbation may not be possible. Placebo‐controlled trials in patients not responding to standard maximal treatment, including inhaled β₂‐agonists and ipratropium bromide and systemic steroids, may help establish if nebulised MgSO₄ has a role in acute asthma. However, the accumulating evidence suggests that a substantial benefit may be unlikely.
Plain language summary
Is inhaled magnesium sulfate a safe and effective treatment for people with asthma attacks?
Background
Asthma attacks are common in adults and children. People having an attack may need to be treated in a hospital emergency department (A&E). Even with the best treatment, some people need to be admitted to hospital or even into the intensive care unit. Some guidelines suggest that giving magnesium sulfate, either by injection or inhaled straight into the lungs, may be beneficial. In this review we focused on inhaled (or 'nebulised') magnesium sulfate. We were particularly interested in finding out the effects of magnesium sulfate on lung function (breathing tests), severity scores and hospital admissions. We also wanted to know if it was safe.
Study characteristics
We looked for studies in adults and children attending the emergency department with an asthma attack. We included studies which compared giving inhaled magnesium sulfate, plus standard treatment, with standard treatment alone. We also included studies that compared inhaled magnesium sulfate directly with standard treatment. We included studies carried out anywhere in the world, at any time and written in any language.
Key results
We found 25 studies in total, which included nearly 3000 people with asthma attacks. This latest update of the review includes several large trials that were carried out to a very high standard. We found that adding inhaled magnesium sulfate to standard treatments may result in small benefits in terms of lung function, hospital admission and severity scores, but we are uncertain about these findings. This is because many of the studies were carried out in different ways and measured different outcomes at different times so it was quite hard to combine the results from individual studies. Inhaled magnesium sulfate did not seem to cause any serious side effects in the studies we found. We did not find evidence that using inhaled magnesium sulfate instead of standard treatment is beneficial.
Quality of the evidence
We used a scoring system to rate how confident we are in the findings presented. Our scores ranged from high confidence to very low confidence, but most outcomes we rated as low or very low. This is because we had concerns about the way in which some of the studies were carried out: for example, it was perhaps not clear how people were chosen for the two different treatment groups in the study; or it was unclear whether the patients or people running the trial knew who was getting which treatment. Another factor that reduced our confidence was uncertainty about the combined results: for example in some cases we could not tell whether magnesium sulfate was better, worse or the same.
Key message
There is some limited evidence that inhaled magnesium sulfate may have a small benefit for people having asthma attacks when added to standard treatment. However, the most recent, high‐quality trials did not generally show important benefits. Also, we cannot be sure if some groups may benefit more than other, for example those having more severe attacks.
Summary of findings
Background
Description of the condition
Asthma is a chronic respiratory disease characterised by reversible airflow obstruction, with periods of relative control and episodes of deterioration referred to as exacerbations. Exacerbations range in severity from mild to life‐threatening and can result in visits to healthcare providers and emergency departments, at times necessitating hospital admission. While rare, admissions to the intensive care setting, mechanical ventilation and deaths from severe acute asthma exacerbations do still occur (NRAD 2014): thus the prevention and treatment of exacerbations are important considerations for everyone with asthma. Due to its chronicity, variability, risk of mortality, and cost to the healthcare system, asthma remains the cause of significant personal and social burden.
Description of the intervention
Asthma exacerbations are characterised by acute episodes of bronchoconstriction and airway inflammation. These episodes generally result in increased requirements for inhaled beta₂‐agonist (β₂‐agonist) therapy (Cates 2004). Unfortunately, in acute asthmatic episodes, β₂‐agonists may not be enough to relieve bronchospasm and reduce dyspnoea. The evidence‐based guideline for the management of asthma developed by the British Thoracic Society (BTS) and the Scottish Intercollegiate Guideline Network (SIGN) offers comprehensive guidance on the acute and chronic management of asthma in children and adults (BTS/SIGN 2016). Although the management of children and adults is broadly similar, differences remain between the management of acute exacerbations of asthma in children (less than 16 years old) and adults (16 years and older) (BTS/SIGN 2016).
For children and adults seen in an emergency department (ED or A&E) with an asthma exacerbation, the BTS/SIGN guideline recommends inhaled or nebulised β₂‐agonists, systemic corticosteroids, and oxygen if needed. International guidelines also recommend the use of inhaled ipratropium for all adults, and children over the age of 5 with severe exacerbations (GINA 2017). For poorly responsive children the next step is nebulised ipratropium (if not already given), and consideration of nebulised magnesium sulfate (MgSO₄) if life‐threatening features are identified. Intravenous (IV) MgSO₄ (Shan 2013), salbutamol and aminophylline are considered if response remains poor. For poorly responsive adults, or those with a life‐threatening exacerbation, the addition of nebulised ipratropium (if not already given) is recommended, with consideration of IV MgSO₄ (Kew 2014). Nebulised magnesium sulfate is not recommended for the treatment of adults with acute asthma (BTS/SIGN 2016).
How the intervention might work
Magnesium sulfate has been proposed as a possible additive treatment in acute asthma, and has been shown to be effective in severe acute asthma when delivered intravenously (Shan 2013; Kew 2014). It may be effective in acute asthma through one or more of a variety of mechanisms. There is evidence that magnesium sulfate may augment the beta receptor response to salbutamol (Turner 2017). Magnesium sulfate has been shown to relax smooth muscle by inhibiting calcium ion influx (Gourgoulianis 2001); it inhibits acetylcholine and histamine release from cholinergic motor nerve terminals and mast cells respectively (Del Castillo 1954; Bois 1962), and promotes synthesis of nitric oxide (Ashutosh 2000) and prostacyclin (Nadler 1987), which stimulate broncho‐ and vasodilation. Finally, magnesium ions may have an anti‐inflammatory role, attenuating neutrophil activation in adults with asthma (Cairns 1996).
Why it is important to do this review
The potential clinical benefits of nebulised MgSO₄ have been studied and research publications have produced conflicting results. Subgroup analysis from one large multi‐centre RCT suggests a possible role for MgSO₄ in the treatment of children with acute severe asthma (Powell 2013), and has led to current guidance to consider nebulised MgSO₄ for children presenting with a life‐threatening acute asthma attack (BTS/SIGN 2016). However, nebulised MgSO₄ has not yet been used widely in the acute care setting.
In the previous version of this Cochrane Review (Powell 2012), sixteen trials involving 896 patients were included. Seven studies compared nebulised MgSO₄ with β₂‐agonist to β₂‐agonist alone, three studies compared nebulised MgSO₄ to β₂‐agonist alone, and two studies compared nebulised MgSO₄ with β₂‐agonist and ipratropium to β₂‐agonist and ipratropium alone. The review concluded that there was no good evidence that inhaled MgSO₄ could be used as a substitute for inhaled β₂‐agonists; and when used in addition to standard inhaled treatments there was no clear evidence of improved pulmonary function or reduced hospital admissions. However, individual study results from three trials suggest possible improved pulmonary function in those with severe asthma exacerbations. The review called for further studies focusing on inhaled MgSO₄ in addition to the current guideline treatment for acute asthma and including those with more severe exacerbations.
A 2013 systematic review including nine trials of nebulised magnesium sulfate (some of which were excluded from the 2012 Cochrane Review) identified benefits in pulmonary function for adults treated with nebulised magnesium sulfate compared to placebo (Shan 2013).
Thus, MgSO₄ administration in combination with β₂‐agonists may be of benefit with respect to pulmonary function in patients presenting to the emergency department with severe acute exacerbations of asthma, and there may be evidence that MgSO₄ administered in combination with β₂‐agonists reduces hospitalisations. Due to significant heterogeneity among studies, both in terms of treatments and outcome measures, there remains a need for further trials before recommendations can be made regarding the use of nebulised magnesium sulfate for acute asthma exacerbations. The rationale for completing this updated systematic review was to examine the influence any further studies would make on these conclusions.
Objectives
To determine the efficacy and safety of inhaled MgSO₄ administered in acute asthma.
Specific aims: to quantify the effects of inhaled MgSO₄ I) in addition to combination treatment with inhaled β₂‐agonist and ipratropium bromide, ii) in addition to inhaled β₂‐agonist and iii) in comparison to inhaled β₂‐agonist.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised (or quasi‐randomised) controlled trials. We included only parallel study designs; cross‐over trials were excluded.
Types of participants
We included studies restricting enrolment to patients with acute asthma; patients with chronic or 'stable' asthma were excluded from the review. We included studies involving all ages; however, we sub‐grouped data into adults and children where possible. We accepted any reasonable diagnosis of asthma, namely clinical and guideline‐based criteria.
Types of interventions
We included studies where participants were randomised to receive inhaled MgSO₄ compared with a control inhaled treatment. That is, studies comparing the efficacy of:
inhaled MgSO₄ and β₂‐agonist and ipratropium versus β₂‐agonist and ipratropium and placebo;
inhaled MgSO₄ and β₂‐agonist versus β₂‐agonist and placebo;
inhaled MgSO₄ versus β₂‐agonist.
We allowed co‐interventions, and recorded information we received about them.
Types of outcome measures
Primary outcomes
Change in pulmonary function from baseline using the following indices.
Forced expiratory volume in one second (FEV1) and percentage predicted FEV1;
Peak expiratory flow (PEF) and percentage predicted PEF.
Secondary outcomes
Clinical severity scores.
Proportion of patients requiring admission to hospital.
Duration of symptoms.
Vital signs (pulse and respiratory rates; systolic and diastolic blood pressure).
Adverse events (tremor, nausea, etc).
For the 2017 update, we chose to extract and present outcomes including lung function, vital signs and severity scores at — or as close as possible to — 60 minutes post‐baseline. The rationale for this decision is given in the Potential biases in the review process section.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Trials Register, which is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org).
Weekly searches of MEDLINE Ovid SP 1946 to date.
Weekly searches of Embase Ovid SP 1974 to date.
Monthly searches of PsycINFO Ovid SP 1967 to date.
Monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature) 1937 to date.
Monthly searches of AMED EBSCO (Allied and Complementary Medicine).
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings are in Appendix 1. See Appendix 2 for the search terms we used to identify studies for this review.
We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) trials portal (apps.who.int/trialsearch) (Appendix 2). We searched all sources from their inception to the present and we placed no restriction on the language of publication. Search methods used in the previous version of this review are detailed in Appendix 3. The previously published version included searches up to September 2012. The search period for this update is September 2012 to 6 September 2017.
Searching other resources
We examined the reference lists of all selected articles, primary studies and review articles for relevant studies. We contacted primary authors of studies to request information on additional trials (published and unpublished). We contacted clinicians, colleagues, collaborators and trialists to identify potentially relevant studies. Since MgSO₄ is not currently commercially delivered, we did not contact any industry sponsor.
Data collection and analysis
Selection of studies
The selection of studies involved two steps. First, to retrieve studies two independent investigators screened by title, abstract, MeSH headings and keywords the initial search of all databases and reference lists to identify all citations of randomised controlled trials (RCTs) or possible RCTs with potential relevance. We obtained the full texts of those selected articles for 'formal inclusion' review. Second, another review author independently decided on trial inclusion using pre‐determined eligibility criteria.
Data extraction and management
We extracted data independently using a standardised data collection form. We extracted the following information, if available: characteristics of the study (design, methods of randomisation, withdrawals/dropouts); participants (age, gender); intervention (type, dose, route of administration, timing and duration of therapy, co‐interventions); control (agent and dose); outcomes (types of outcome measures measured and reported, timing of outcomes, adverse events); and results. We requested unpublished data from the primary authors when necessary. For this update, two review authors (RK and RN) entered data into Review Manager 2014.
Assessment of risk of bias in included studies
We applied the Cochrane 'Risk of bias' tool in this 2017 update (Higgins 2011). Two review authors (RK and RN) independently assessed the risk of bias for all new included studies for the following six items: random sequence generation; allocation concealment; blinding; incomplete outcome data; selective outcome reporting; and other types of bias. We recorded the judgement as high, low or unclear risk of bias and added a description from the trial reports. We discussed any disagreements and resolved them by consensus.
Measures of treatment effect
For dichotomous variables, we expressed data as risk ratio (RR) with 95% confidence intervals (CIs) and reported adverse events as risk difference (RD) together with 95% CIs. For the continuous variables 'pulmonary function' and 'clinical severity score', we reported data as mean differences (MD) or standardised mean differences (SMD) with 95% CIs.
Unit of analysis issues
The unit of analysis was the patient.
Dealing with missing data
If baseline or outcome data or information on trial design were missing, we attempted to contact trial authors.
Assessment of heterogeneity
We assessed heterogeneity by visual inspection of the forest plots. We also used the Chi² test (where a P value < 0.10 indicates substantial heterogeneity); however, we exercised caution in interpretation due to the low power associated with this test. I² was calculated and a guide to interpretation is:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
We planned to test for publication bias using a funnel plot if there was a sufficient number of trials included in a single forest plot (more than 10). It should be noted that an asymmetrical funnel plot can be caused by heterogeneity, outcome reporting bias and small‐study effects as well as publication bias.
Data synthesis
We combined data using a fixed‐effect model except in cases where we identified substantial heterogeneity, as defined above, where we employed a random‐effects model as a sensitivity analysis.
Subgroup analysis and investigation of heterogeneity
A priori subgroup analyses were planned to examine the effect of:
age (two to 16 years old (paediatric) and > 16 years old (adult));*
severity of asthma as measured by pre‐administration spirometric deviation from predicted (baseline FEV1 or PEF < 50% predicted).
*For the 2017 update, if the age range of participants was unclear, we classified the study according to average age of participants.
Sensitivity analysis
We planned to conduct sensitivity analyses to assess the effect of the overall risk of bias of included trials, but there were insufficient trials of varied methodological quality in the meta‐analysis for a sensitivity analysis (e.g. either all the studies were of similar methodological quality, or removing a trial in which we had concerns about risk of bias made no difference to the pooled result).
For the 2017 update we performed a post‐hoc sensitivity analysis using a random‐effects model when we encountered a study with unusually small standard deviations, which was therefore dominating the meta‐analysis. Results of such random‐effects model meta‐analyses should be interpreted with caution as the model is based on the assumption of a normal distribution of the true effect from each study; this is problematic in analyses with few studies.
We also employed a random‐effects model as a sensitivity analysis if substantial heterogeneity was detected, as previously described.
'Summary of findings' table
For this update we included a 'Summary of findings' table for each main comparison and assessed the quality of the evidence using the five GRADE domains (study limitations, imprecision, inconsistency, indirectness and publication bias). We decided a priori to include lung function, clinical severity scores, hospital admissions and adverse events. We used GRADEPro software (GRADEpro GDT) to create the 'Summary of findings' tables.
Results
Description of studies
Results of the search
The previous version of the review included 16 trials. For this update, the database search yielded 45 records and we identified six records from additional sources. Fourteen records were excluded on the basis of the title or abstract and we assessed 37 full texts for eligibility. We excluded a further 10 full texts, with reasons, and identified three ongoing studies. We included nine new studies (22 records) in the review, bringing the total number of included studies to 25 (43 records). See Figure 1, Characteristics of excluded studies and Characteristics of ongoing studies for further details. In addition, we moved two studies which had previously been excluded to Studies awaiting classification (Abd 1997; Bustamante 2000); and added one additional study to Studies awaiting classification (ISRCTN61336225)
1.
Study flow diagram: review update
Included studies
We incorporated 25 trials (43 references) including 2907 randomised participants (2777 of whom completed) into the review (see Characteristics of included studies). All of the studies included in this manuscript were published since 1995. There is no particular geographic preference, with Argentina, Egypt, India, Iran, Mexico, New Zealand, Tenerife, Turkey, the UK and the USA represented.
We requested lung function data from the primary authors for two included studies (Meral 1996; Drobina 2006); and further information on trials design and baseline data from four authors (Neki 2006; Badawy 2014; Hossein 2016; Sarhan 2016). We also requested clarification on adverse event data from one author (Powell 2013). With the exception of clarification from the authors of Badawy 2014 and Powell 2013 we did not receive a reply before this review was published. Should information subsequently become available, we will include it in a future update.
Populations
Nine of the 25 included studies involved adults exclusively (Nannini 2000; Abreu‐Gonzalez 2002; Bessmertny 2002; Hughes 2003; Kokturk 2005; Gaur 2008; Gallegos‐Solórzano 2010; Goodacre 2013; Hossein 2016); and four included adults and children (Mangat 1998; Aggarwal 2006; Neki 2006; Sarhan 2016). Eight studies enrolled children (Meral 1996; Mahajan 2004; Ashtekar 2008; Khashabi 2008; Powell 2013; Mohammedzadeh 2014; Alansari 2015; Turker 2017); and in the remaining four studies the age of participants was not stated (Dadhich 2005; Drobina 2006; Ahmed 2013; Badawy 2014).
The severity of disease varied between studies (Table 4). Fourteen studies enrolled patients based on specific lung function criteria (Meral 1996; Mangat 1998; Nannini 2000; Bessmertny 2002; Hughes 2003; Mahajan 2004; Dadhich 2005; Neki 2006; Gaur 2008; Gallegos‐Solórzano 2010; Goodacre 2013; Powell 2013; Alansari 2015; Hossein 2016), while the remaining studies enrolled patients previously diagnosed with asthma using accepted clinical standards, or did not specify how asthma was diagnosed. Based on the baseline demographic data, 15 studies were considered to enrol severe acute exacerbations of asthma (FEV1 or PEF < 50% predicted at baseline or symptom criteria defined by BTS/SIGN guideline) (Meral 1996; Mangat 1998; Nannini 2000; Bessmertny 2002; Hughes 2003; Mahajan 2004; Dadhich 2005; Kokturk 2005; Aggarwal 2006; Neki 2006; Ashtekar 2008; Gaur 2008; Gallegos‐Solórzano 2010; Goodacre 2013; Powell 2013).
1. Summary of Severity.
| Study | Severity of asthma exacerbation | Diagnosis based on | Population (adult/mixed/paediatric) |
| MgSO₄ and SABA and Ipratropium bromide versus SABA and Ipratropium | |||
| Ashtekar 2008 | Severe | BTS definition clinical features | Paediatric (2 to 16) |
| Drobina 2006 | Unclear | PEF and clinical signs | Adults |
| Gallegos‐Solórzano 2010 | Moderate to severe | FEV1 < 60% | Adults >18 |
| Gaur 2008 | Severe | FEV1 < 30% | Adults (18 to 60) |
| Goodacre 2013 | Severe | BTS definition | Adult (≥ 16) |
| Hossein 2016 | Moderate to severe | PEF < 70% and clinical signs | Adult (> 16) |
| Powell 2013 | Severe after conventional treatment | BTS definition | Paediatric (2 to 16) |
| MgSO4 and SABA versus SABA | |||
| Abreu‐Gonzalez 2002 | Moderate | FEV1 and PEF at baseline | Adults |
| Aggarwal 2006 | Severe and life threatening | BTS definition clinical features and PEF | Mixed (13 to 60) |
| Ahmed 2013 | Severe | PEF | Not documented |
| Alansari 2015 | Moderate to severe | Clinical score | Paediatric (2 to 14) |
| Badawy 2014 | Unclear | N/A | Adult |
| Bessmertny 2002 | Moderate to severe | PEF between 40% to 80% | Adults (18 to 65) |
| Dadhich 2005 | Severe | PEF < 50% | Adults |
| Hughes 2003 | Severe | FEV1 < 50% | Adults (16 to 65) |
| Khashabi 2008 | Unclear | Clinically defined as respiratory distress | Paediatric (mean age 3.55 years) |
| Kokturk 2005 | Moderate to severe | Clinical scores and PEF | Adults (18 to 60) |
| Mahajan 2004 | Moderate to severe | FEV1 between 45% and 75% | Paediatric (5 to 17) |
| Mohammedzadeh 2014 | Moderate to severe | GINA definition | Paediatric (5 to 14) |
| Nannini 2000 | Severe | PEF < 50% | Adult (> 18) |
| Sarhan 2016 | Unclear | PEF < 300L/min | Mixed (11 to 70) |
| Turker 2017 | Moderate | Not described | Children (3 to 15) |
| MgSO₄ versus SABA | |||
| Dadhich 2005 | Severe | PEF < 50% | Adults |
| Mangat 1998 | Moderate to severe | PEF < 300 L/Min | Mixed (12 to 60) |
| Meral 1996 | Moderate to severe | PEF < 75% | Paediatric |
| Neki 2006 | Severe | FEV1 < 40% or PEF < 300 L/Min | Adult (15 to 60) |
| Sarhan 2016 | Unclear | PEF < 300L/min | Mixed (11 to 70) |
BTS: British Thoracic Society
GINA: Global Initiative for Asthma FEV1: Forced expiratory volume in one second PEF: Peak Expiratory Flow Rate
Sixteen studies recruited participants from emergency departments; two from outpatient or emergency departments (Badawy 2014; Sarhan 2016); and one in a children's assessment unit after general practitioner referral (Ashtekar 2008). Department of presentation was unclear in the remaining six studies (see Table 5).
2. Summary of Characteristics of the studies – where patients were recruited from, additional treatment, exclusion criteria and side effects.
| Study | Presentation to which department? | Origin | Primary outcome(s) | Total n randomised | Side effects (patients in study) | Pharmaceutical exclusions | Other Interventions |
| MgSO₄ and SABA and Ipratropium bromide versus SABA and Ipratropium | |||||||
| Ashtekar 2008 | Children’s Assessment Unit after GP referral | Cardiff, Wales | ASS (Yung) | 17 | 1 tingling in fingers and 1 transient hypotension | None stated | All management followed the BTS/SIGN guidelines; all children received 2 mg/kg prednisolone |
| Drobina 2006 | ED | USA | PEF, admissions | 110 | No comment on side effects in paper | Not stated | All subjects received 50 mg of oral prednisone at the onset of the treatment |
| Gallegos‐Solórzano 2010 | ED | Mexico City, Mexico | % change FEV1, O₂ post treatment, admission rates |
112 | Dry and bitter mouth (MgSO₄ group 1), dizziness (MgSO₄ 1; placebo 1) | Use of steroids prior to presentation | All participants received one IV dose of 125 mg methylprednisolone at admission and 1 mg/kg/day for 10 days prednisolone,on discharge. Other treatments were administered according to the treating physician |
| Gaur 2008 | ED | Delhi, India | FEV1 | 60 | None reported | None stated | All participants received IV hydrocortisone on arrival |
| Goodacre 2013 | ED | UK | Admission within 7d, visual analogue scale for breathlessness at 2 h | 703 | AEs (41 MgSO₄/salbutamol; 36 placebo/salbutamol) | MgSO₄ in the past 24 h | All participants were managed according to BTS/SIGN guidelines (consisting of oxygen, nebulised salbutamol (5 mg), nebulised ipratropium (500 μg), and oral prednisolone administered during recruitment, followed by up to 5 mg salbutamol added to each trial nebuliser. Other treatments were provided at the discretion of the clinician |
| Hossein 2016 | ED | Tehran, Iran | PEFR improvement, admission rate | 50 | No serious side effects reported | None stated | All participants received 50 mg oral prednisolone |
| Powell 2013 | ED and children's assessment units | UK | Yung asthma severity score | 508 | 47 in MgSO₄ group and 59 in control group | None | Hospital‐defined conventional treatment |
| MgSO4 and SABA versus SABA | |||||||
| Abreu‐Gonzalez 2002 | ‐ | Tenerife Spain | FEV1, PEF | 24 | None reported | None stated | Not stated |
| Aggarwal 2006 | ED | New Delhi India | PEF | 100 | Palpitations (MgSO₄/salbutamol 13; salbutamol/placebo 11) and tremors (7; 7). | None stated | Clinicians free to administer steroids, salbutamol, IV hydrocortisone if judged to be required |
| Ahmed 2013 | ‐ | Mymensingh, Bangladesh | PEF | 120 | None reported | None stated | Not stated |
| Alansari 2015 | Paediatric emergency centre | Doha, Qatar | Time to readiness for discharge | 400 | Chest tightness and facial rash (MgSO₄/salbutamol 191), excessive cough (placebo/salbutamol 174) | None stated | All participants received methylprednisolone 1 mg/kg IV every 12h and additional nebulised albuterol at clinicians' discretion |
| Badawy 2014 | Outpatient department and ED | Sohag, Egypt | Exacerbations post intervention, delivery outcome, post‐partum health status | 60 | None reported | None stated | All participants received 100 mg hydrocortisone IV, 500 mg aminophylline IV |
| Bessmertny 2002 | ED | Brooklyn, USA | FEV1 (% pred) | 74 | No SAEs reported | No theophylline or anticholinergics 2 h prior to presentation | Intravenous hydrocortisone, 2 mg/kg every 6 h, was administered to patients who failed to show an adequate improvement of pulmonary function after 3 initial doses of albuterol |
| Dadhich 2005 | ED | Ajmer India | PEF | 71 | "Side effects were self limiting" | Not stated | Not stated |
| Hughes 2003 | ED | Wellington New Zealand | FEV1 | 52 | None reported | None | All participants received 100 mg hydrocortisone IV |
| Khashabi 2008 | ‐ | Urmia, Iran | Reduced mean duration of O₂ therapy in MgSO₄ group, no change in Respiratory Distress Score) |
40 | No side effects | Not stated | Not stated |
| Kokturk 2005 | ED | Gazi, Turkey | PEF difference | 26 | Transient hypotension (1 MgSO₄), palpitation (1 salbutamol) | None | All participants received 1 mg/kg prednisolone. Theophylline, anticholinergics and salbutamol given at clinicians discretion |
| Mahajan 2004 | ED | Detroit, USA | % change in FEV1 | 62 | No side effects | Steroids, ipratropium or theophylline in the last 3 days. | All participants received 2 mg/kg of prednisone |
| Mohammedzadeh 2014 | ‐ | Babol, Iran | Pulmonary index, PEFR, adjusted PEFR | 80 | ‐ | Corticosteroids; steroids, theophylline or ipratropium use within last 72 h | Not stated |
| Nannini 2000 | ED | 4 hospitals in Argentina | PEF, admissions | 35 | None reported | Oral or parenteral steroids in the last 7 days | No other medications were permitted during the study except supplemental oxygen; if the patient’s condition worsened, a 2.5 mg dose of nebulized salbutamol was administered at the discretion of the treating physician |
| Sarhan 2016 | Chest and ED | Minia, Egypt | Clinical improvement, PEFR | 30 | None severe enough to warrant withdrawal | Bronchodilators in last 6 h, steroids in last 12 h | Nebulised salbutamol, IV hydrocortisone, IV aminophylline at clinicians' discretion |
| Turker 2017 | ED | Turkey | Modified pulmonary index score | 100 | "No side effect caused by magnesium was observed in any of the patients in the study" | Not stated | Nebulised salbutamol (0.15 mg/kg), methylprednisolone 1 mg/kg IV; Oxygen was given to patients with SaO2≤ 95% |
| MgSO₄ versus SABA | |||||||
| Dadhich 2005 | ED | Ajmer India | PEF | 71 | "Side effects were self limiting" | Not stated | Not stated |
| Mangat 1998 | ED | St John’s College, India | PEF, Fischl index score, admissions | 33 | Transient self limiting hypotension (1) palpitation (1) tremors (2) all in control group and only 1 transient hypotension in MgSO₄ group (33) | Oral parenteral bronchodilators (6 h) steroids (last 12 h) | All participants received 100 mg hydrocortisone IV |
| Meral 1996 | ‐ | Izmir, Turkey | % change in PEF ASS (Davies Leffert, Dabbous score) |
40 | No side effects | Beta2‐agonists or theophylline in the last 12 h | No other medication given |
| Neki 2006 | ‐ | Amritsar Punjab | PEF, RR, Fischl index | 40 | ‐ | Oral, inhaled or parenteral steroids in last 12 h | All participants received 100 mg hydrocortisone IV |
| Sarhan 2016 | Chest and ED | Minia, Egypt | Clinical improvement, PEFR | 30 | None severe enough to warrant withdrawal | Bronchodilators in last 6 h, steroids in last 12 h | Nebulised salbutamol, IV hydrocortisone, IV aminophylline at clinicians' discretion |
ASS: Asthma Severity Score; BP: blood pressure; ED: emergency department; FEV1: Forced expiratory volume in 1 second; h: hour(s) HR: heart rate; IV: intravenous; MgSO₄: magnesium sulfate; PEF: Peak Expiratory Flow Rate; SAEs: serious adverse events
Badawy 2014 recruited exclusively pregnant women. Due to concerns about baseline imbalance in this study, and the narrow population recruited, we did not include this study in our meta‐analyses and instead present the results narratively. The study has been included in another Cochrane Review that addresses asthma treatment options in pregnant women (Bain 2014).
Participants were excluded for a number of reasons including pre‐existing lung conditions and features of infection on examination. There was great variation in pharmaceutical exclusion due to drugs taken before recruitment (see Table 5).
Interventions
All studies used nebulised MgSO₄ in the intervention group but the comparison and placebo nebulised solutions varied (Table 6). Three studies compared MgSO₄ with β₂‐agonist directly with no placebo (Meral 1996; Mangat 1998; Neki 2006). Twelve studies compared β₂‐agonist with MgSO₄ to β₂‐agonist with placebo (normal saline) (Nannini 2000; Abreu‐Gonzalez 2002; Bessmertny 2002; Hughes 2003; Mahajan 2004; Kokturk 2005; Aggarwal 2006; Khashabi 2008; Ahmed 2013; Badawy 2014; Mohammedzadeh 2014; Turker 2017) . Five studies compared β₂‐agonist and ipratropium with MgSO₄ to β₂‐agonist and ipratropium with placebo (Ashtekar 2008; Gaur 2008; Gallegos‐Solórzano 2010; Powell 2013; Hossein 2016), and Drobina 2006 compared β₂‐agonist and ipratropium with MgSO₄ to β₂‐agonist and ipratropium only (i.e. no placebo). Alansari 2015 compared β₂‐agonist with MgSO₄ to β₂‐agonist with placebo (normal saline) after both groups had received one hour of therapy with combined β₂‐agonist and ipratropium, and thus is included in comparison one. Two studies had three groups and investigated MgSO₄ versus β₂‐agonist versus MgSO₄ plus β₂‐agonist (Dadhich 2005 and Sarhan 2016) and thus appear in comparisons 2 and 3. Goodacre 2013 studied one group with nebulised MgSO₄, β₂‐agonist, ipratropium and IV placebo, one with IV MgSO₄, β₂‐agonist, ipratropium and nebulised placebo, and a third group with β₂‐agonist, ipratropium and both nebulised and IV placebo. The comparison involving IV MgSO₄ has been covered in other reviews (Kew 2014; Griffiths 2016).
3. Summary of Interventions.
| Study (N) | Magnesium sulfate | Control | ||||
| Dose | N | Co‐interventions | Dose | N | Co‐interventions | |
| MgSO₄ and SABA and Ipratropium bromide versus SABA and Ipratropium | ||||||
| Ashtekar 2008 | 2.5 mL isotonic MgSO₄ (151 mg /dose) | 7 | 500 mcg Ipratropium bromide 2.5 mg salbutamol or 5 mg salbutamol (depending on age) 3 times per h |
2.5 mL of isotonic saline) | 10 | Same as for MgSO₄ group |
| Drobina 2006 | 150 mg MgSO₄ (0.3 mL of 50% MgSO₄ heptahydrate) | 60 | Albuterol sulfate (0.5%) 5 mg/mL) and 0.5 mg ipratropium bromide (0.02% inhalation solution) (frequency*) | No placebo so volume will be less: i.e. blinding may be an issue) | 50 | Same as for MgSO₄ group |
| Gallegos‐Solórzano 2010 | 3 mL (333 mg) of 10% isotonic MgSO₄ (1 g/10 mL) | 60 (30 withdrawals) | 2.5 mg albuterol and 500 mcg ipratropium 3 doses per hour | 3 mL isotonic saline | 52 (22 withdrawals) | Same as for MgSO₄ group |
| Gaur 2008 | 3 mL (3.2 g%) isotonic MgSO₄ |
30 | Salbutamol and ipratropium (dose*, frequency*) | Saline | 30 | Same as for MgSO₄ group |
| Goodacre 2013 | 2 mmol MgSO₄ | 339 (7 withdrawal) | 7.5 mL 0.9% NaCl nebulised, 3 doses; 100 mL 0.9% NaCl IV once, BTS/SIGN standard treatments plus others at clinicians' discretion | 7.5 mL 0.9% saline nebulised, 3 doses, 100 mL 0.9% NaCl IV once | 364 (7 withdrawal) | BTS/SIGN standard treatments plus others at clinicians' discretion |
| Hossein 2016 | 3 mL (260 mmol/L) MgSO4 | 25 | 2.5 mg salbutamol, 0.5 mg ipratropium nebulised every 20 to 60 minutes, 50 mg oral prednisolone (once*) | 3 mL 0.9% NaCl | 25 | Same as for MgSO₄ group |
| Powell 2013 | 2.5 mL 250 mmol/L MgSO₄ | 252 (13 withdrawals) | 3 doses every 20 min. Hospital‐defined conventional treatment | 2.5 mL isotonic saline | 256 (10 withdrawals) | Same as for MgSO₄ group |
| MgSO4 and SABA versus SABA | ||||||
| Abreu‐Gonzalez 2002 | 2 mL MgSO₄ (isotonic) | 13 | 400 mcg salbutamol (once*) |
2 mL of a physiological serum of an inhaled form 11 patients |
11 | 400 mcg salbutamol |
| Aggarwal 2006 | 1 mL of 500 mg/mL MgSO₄ | 50 | 1 mL salbutamol (dose*, 8 mL distilled water, (295 mOsml/kg) 3 times per h ultrasonic nebuliser |
7.5 mL normal saline | 50 | 1 mL salbutamol (dose*), 1.5 mL distilled water (287 mOsml/kg) 3 times per h |
| Ahmed 2013 | MgSO₄ (dose* frequency*) | 60 | Not recorded | Normal saline (dose* frequency*) | 60 | Not recorded |
| Alansari 2015 | 800 mg (15 mL) MgSO₄ | 208 (17 withdrawals) | 5 mg albuterol, divided into 3 doses over 1 h. Methylprednisolone 1 mg/kg IV every 12 h. 3 doses nebulized 1 mL albuterol (5 mg/mL), 250 mcg ipratropium, 2 mL normal saline before trial doses started | 15 mL 0.9% NaCl | 192 (18 withdrawals) | Same as for MgSO₄ group |
| Badawy 2014 | 500 mg (1mL) MgSO₄ | 30 | 1 mL salbutamol solution (dose*), 8 mL 0.9% NaCl, max 3 doses with 20 mins apart. 100 mg hydrocortisone IV, 500 mg aminophylline IV (once*) | 1 mL 0.9% NaCl | 30 | Same as for MgSO₄ group |
| Bessmertny 2002 | MgSO₄ (384 mg) | 37 (3 withdrawals) | Followed by ( i.e. not mixed) albuterol 2.5 mg/mL 3 times per h | Normal saline (no volume documented) | 37 (3 withdrawals) | Same as for MgSO₄ group |
| Dadhich 2005 | MgSO₄ | 26 | No doses in any group or co‐interventions described | Not stated | 24 | No doses in any group or co‐interventions described |
| Hughes 2003 | 2.5 mL isotonic MgSO₄ (250 mmol/L 151 mg) 28 patients |
28 | 2.5 mg salbutamol 3 times per 30 minutes |
2.5 mL normal saline | 24 | Same as for MgSO₄ group |
| Khashabi 2008 | Isotonic MgSO₄ (dose*, frequency*) |
* | Salbutamol (dose*) | 2.5 mL normal saline (frequency*) | * | Same as for MgSO₄ group |
| Kokturk 2005 | Isotonic MgSO₄ (2.5 mL) | 14 | Salbutamol (dose*) 3 times per h then 1 per h for 3 h |
2.5 mL normal saline | 12 | Same as for MgSO₄ group |
| Mohammedzadeh 2014 | 3 mL 7.5% MgSO₄ | 40 | 0.15 mg/kg salbutamol 3 doses, every 20 min | 3 mL normal saline | 40 | Same as for MgSO₄ group |
| Mahajan 2004 | 2.5 mL Isotonic (6.3%) MgSO₄ solution | 31 | Albuterol 2.5 mg 1 dose | 2.5 mL normal saline | 31 | Same as for MgSO₄ group |
| Nannini 2000 | 3 mL isotonic MgSO₄ (286 mOsml, 7.5%, 225 mg) |
19 | 0.5 mL 2.5 mg salbutamol 1 dose* |
3 mL normal saline | 16 | Same as for MgSO₄ group |
| Sarhan 2016 | 2.5 mL MgSO4 (100 mg), 0.5 mL salbutamol (2.5 mg) | 10 | 4 doses at 20 min intervals. If needed: additional nebulised salbutamol, IV hydrocortisone, IV aminophylline | 2.5 mL isotonic saline | 10 | Same as for MgSO4 group |
| Turker 2017 | 1 mL magnesium sulfate (15%) + 1.5 mL isotonic saline | 50 | 3 doses at 20 min intervals. Also nebulised salbutamol (0.15 mg/kg), methylprednisolone 1 mg/kg IV; Oxygen was given to patients with SaO2 ≤ 95% | 1.5 mL isotonic saline | 50 | Same as for MgSO₄ group |
| MgSO₄ versus SABA | ||||||
| Dadhich 2005 | MgSO₄ | 21 | No doses in any group or co‐interventions described | Not stated | 24 | No doses in any group or co‐interventions described |
| Mangat 1998 | 3.2% solution MgSO₄ = 95 mg) | 16 | 4 doses every 20 minutes | 3 mL (2.5 mg) salbutamol | 17 | Four doses every 20 minutes |
| Meral 1996 | 2 mL MgSO₄ (280 mmol/L) | 20 | 1* dose given over 10 to 15 minutes | Salbutamol 2.5 mg in 2.5 mL | 20 | 1 dose* given over 10 to 15 minutes |
| Neki 2006 | 20 patients 3.2 G % MgSO₄ |
20 | 4 doses every 20 min | 3 mL of 25 mg* salbutamol (likely decimal point missing) | 20 | Same as for MgSO4 group |
| Sarhan 2016 | 3 mL (100 mg) MgSO4 | 10 | 4 doses at 20 min intervals. If needed: additional nebulised salbutamol, IV hydrocortisone, IV aminophylline | 0.5 mL salbutamol (2.5 mg) | 10 | Same as for MgSO₄ group |
| TOTAL: 2907 randomised to comparisons of interest. 130 withdrawn, 2777 completed | TOTAL: 1476 randomised, 70 withdrawn = 1406 completed intervention | TOTAL: 1431 randomised, 60 withdrawn = 1371 completed control | ||||
* denotes uncertainty
Most studies used 0.9% normal saline as placebo; Aggarwal 2006 used distilled water (as well as normal saline) for placebo and Abreu‐Gonzalez 2002 used ‘physiological serum’ as placebo.
We identified the following comparisons which have been used throughout the review to lend structure.
MgSO₄ and β₂‐agonist and ipratropium versus placebo (saline) and β₂‐agonist and ipratropium (seven studies: Drobina 2006; Ashtekar 2008; Gaur 2008; Gallegos‐Solórzano 2010; Goodacre 2013; Powell 2013; Hossein 2016).
MgSO₄ with β₂‐agonist versus placebo (saline) and β₂‐agonist (15 studies: Nannini 2000; Abreu‐Gonzalez 2002; Bessmertny 2002; Hughes 2003; Mahajan 2004; Dadhich 2005; Kokturk 2005; Aggarwal 2006; Khashabi 2008; Ahmed 2013; Badawy 2014; Mohammedzadeh 2014; Alansari 2015; Sarhan 2016; Turker 2017).
MgSO₄ versus β₂‐agonist alone (five studies: Meral 1996; Mangat 1998; Dadhich 2005; Neki 2006; Sarhan 2016).
Dose, formulation and dose frequency of MgSO₄ differed, meaning that the overall dose of MgSO₄ given differed between studies (Table 6). Not all studies reported the concentration of MgSO₄ nebulised, but when the information was available most included studies used MgSO₄ of similar concentration and osmolality. However, dose per nebulisation and the number of nebulisations performed varied.
Ten studies nebulised three doses of MgSO₄ at 20 minutes intervals (Bessmertny 2002; Hughes 2003; Aggarwal 2006; Ashtekar 2008; Gallegos‐Solórzano 2010; Goodacre 2013; Powell 2013; Mohammedzadeh 2014; Alansari 2015; Turker 2017). Three studies nebulised four doses at 20 minute intervals (Mangat 1998; Neki 2006; Sarhan 2016). Kokturk 2005 nebulised hourly up to four hours after the initial treatment of three doses in one hour. Five studies nebulised only one treatment (Meral 1996; Nannini 2000; Abreu‐Gonzalez 2002; Mahajan 2004; Ahmed 2013). Khashabi 2008 gave two doses of treatment but the timing was unclear. Three studies were unclear how frequently the doses were given but probably only one dose was given (Dadhich 2005; Drobina 2006; Gaur 2008). One study gave up to three doses at 20 minute intervals (Badawy 2014). Hossein 2016 gave treatments every 20 to 60 minutes but the total number of doses given was unclear.
All control or placebo interventions were similar in appearance to the treatment drug. The most frequent placebo was saline. One study collected data on participants' ability to distinguish between the treatment and control, and noted no ability to discern (Hughes 2003). Even when not expressly stated, it can reasonably be assumed that the control (placebo) would be similar in appearance to the treatment drug (especially if given in a β₂‐agonist vehicle).
Co‐interventions
Co‐interventions used added complexity and heterogeneity to the review (Table 5). In 11 studies, systemic corticosteroids were administered to all participants, although the timing (before/after nebulised treatment) varied (Mangat 1998; Hughes 2003; Mahajan 2004; Kokturk 2005; Neki 2006; Ashtekar 2008; Gaur 2008; Badawy 2014; Alansari 2015; Sarhan 2016; Turker 2017). In one study, systemic corticosteroids were administered if there was no improvement after the three doses of study treatment (Bessmertny 2002). Overall, 15 studies routinely administered corticosteroids, but in different doses, routes and frequency. In three studies, corticosteroids were administered according to local standard/conventional treatment, or at the clinicians’ discretion (Aggarwal 2006; Goodacre 2013; Powell 2013). Meral 1996 gave no further medication as a co‐intervention. Six studies made no comments on co‐interventions (Nannini 2000; Abreu‐Gonzalez 2002; Dadhich 2005; Khashabi 2008; Ahmed 2013; Mohammedzadeh 2014).
Outcomes
A summary of the outcomes relevant to this review reported in the included studies is given in Table 7.
4. Outcomes.
| Study ID (author, date of publication) | Review primary outcomes | Review secondary outcomes | |||||
| FEV1 | PEF | Clinical severity scores | Hospital admissions | Duration of symptoms | Vital signs | Adverse effects | |
| MgSO₄ and SABA and Ipratropium bromide versus SABA and Ipratropium | |||||||
| Ashtekar 2008 | N | N | Y | N | N | N | Y |
| Drobina 2006 | N | P | N | N | N | N | P |
| Gallegos‐Solórzano 2010 | Y | N | N | N | N | N | Y |
| Gaur 2008 | Y | N | N | N | N | N | N |
| Goodacre 2013 | N | Y | N | Y | N | Y | Y |
| Hossein 2016 | N | Y | Y | Y | N | Y | N |
| Powell 2013 | N | N | Y | P | N | N | Y |
| MgSO4 and SABA versus SABA | |||||||
| Abreu‐Gonzalez 2002 | Y | Y | N | N | N | N | N |
| Aggarwal 2006 | N | Y | N | Y | N | Y | Y |
| Ahmed 2013 | N | P | N | N | N | N | N |
| Alansari 2015 | N | N | Y | P | N | N | Y |
| Badawy 2014 | Y | Y | N | N | N | Y | N |
| Bessmertny 2002 | P | N | N | N | N | N | Y |
| Dadhich 2005 | P | P | N | N | N | N | Y |
| Hughes 2003 | Y | N | N | Y | N | N | Y |
| Khashabi 2008 | N | N | N | N | N | N | N |
| Kokturk 2005 | N | Y | P | Y | N | N | Y |
| Mahajan 2004 | Y | N | N | Y | N | N | Y |
| Mohammedzadeh 2014 | N | Y | Y | N | N | N | N |
| Nannini 2000 | N | Y | N | Y | N | N | Y |
| Sarhan 2016 | N | Y | Y | N | N | Y | N |
| Turker 2017 | N | N | Y | Y | N | N | Y |
| MgSO₄ versus SABA | |||||||
| Dadhich 2005 | P | P | N | N | N | N | Y |
| Mangat 1998 | N | Y | N | Y | N | N | Y |
| Meral 1996 | N | Y | N | N | N | N | Y |
| Neki 2006 | N | Y | N | N | N | Y | N |
| Sarhan 2016 | N | Y | Y | N | N | Y | N |
N ‒ the study did not report the outcome but it is not clear whether the outcome was measured or not
Y ‒ full reporting
P ‒ partial reporting
Ongoing trials and unpublished data
We have identified three ongoing studies relevant to this review (Motamed 2015; Saucedo 2015; Schuh 2016a).
Excluded studies
During the history of this review, 65 studies have been excluded for the following reasons: 19 not acute asthma, 12 reviews articles, 11 not randomised controlled trials, eight investigated intravenous magnesium sulfate, seven investigated oral supplements, two investigated intravenous versus inhaled magnesium sulfate, two letters, one study unobtainable, one editorial, one study comparing nebulised magnesium sulfate to ipratropium/fenoterol, and one study in bronchiolitis (see Characteristics of excluded studies).
Risk of bias in included studies
See Figure 2 for summary of the risk of bias judgements.
2.
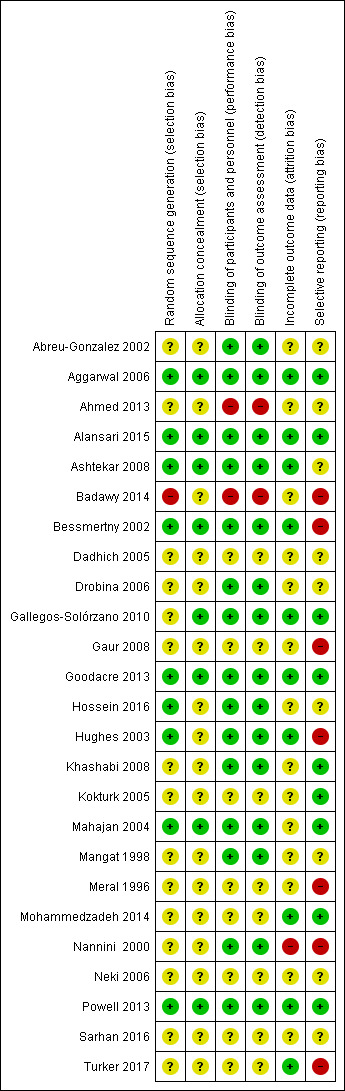
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Fourteen studies were described as 'randomised' but the method of sequence generation was not described; these studies were therefore at an unclear risk of bias (Abreu‐Gonzalez 2002; Ahmed 2013; Dadhich 2005; Drobina 2006; Gallegos‐Solórzano 2010; Gaur 2008; Khashabi 2008; Kokturk 2005; Mangat 1998; Meral 1996; Mohammedzadeh 2014; Nannini 2000; Sarhan 2016; Turker 2017). One further study was at an unclear risk of bias as it is not clear if the study was randomised; we contacted the author for clarification but received no response (Neki 2006). One study was described as ‘randomised’ but no indication was given of random sequence generation and we were unable to confirm that the groups were balanced with regard to baseline clinical asthma criteria; this study was at high risk of bias (Badawy 2014).
Nine studies were at low risk of bias (Figure 2): the randomisation lists were computer‐generated for four studies (Bessmertny 2002; Powell 2013; Alansari 2015; Hossein 2016); produced by the pharmacy for two studies (Hughes 2003; Ashtekar 2008); produced by random number tables for two studies (Mahajan 2004; Aggarwal 2006); and produced by a web‐based randomisation system for one study (Goodacre 2013).
No details, or minimal details, were provided on allocation concealment in 17 studies; they were therefore assessed as at unclear risk of bias (Meral 1996; Mangat 1998; Nannini 2000; Abreu‐Gonzalez 2002; Hughes 2003; Dadhich 2005; Kokturk 2005; Drobina 2006; Neki 2006; Gaur 2008; Khashabi 2008; Ahmed 2013; Badawy 2014; Mohammedzadeh 2014; Hossein 2016; Sarhan 2016; Turker 2017). A description of allocation concealment was provided in eight studies and they were assessed as at low risk of bias (Bessmertny 2002; Mahajan 2004; Aggarwal 2006; Ashtekar 2008; Gallegos‐Solórzano 2010; Goodacre 2013; Powell 2013; Alansari 2015).
Blinding
Fifteen studies gave details as to their double blinding and were therefore at low risk of bias (Mangat 1998; Nannini 2000; Abreu‐Gonzalez 2002; Bessmertny 2002; Hughes 2003; Mahajan 2004; Aggarwal 2006; Drobina 2006; Ashtekar 2008; Khashabi 2008; Gallegos‐Solórzano 2010; Goodacre 2013; Powell 2013; Alansari 2015; Hossein 2016). Two studies were single blind and therefore at unclear risk of performance and assessment bias (Kokturk 2005; Gaur 2008). One study was described as an open trial and was at high risk of bias (Ahmed 2013). One study gave no baseline clinical asthma data and no details about blinding and was therefore at high risk of detection bias (Badawy 2014). No details were provided of blinding procedure or who was blinded for eight studies so we deemed them to have an unclear risk of bias (Meral 1996; Dadhich 2005; Kokturk 2005; Neki 2006; Gaur 2008; Mohammedzadeh 2014; Sarhan 2016; Turker 2017).
Incomplete outcome data
Fourteen studies were at unclear risk of attrition bias for the following reasons. Six studies were reported as conference abstracts only, with no details provided regarding dropouts (Abreu‐Gonzalez 2002; Dadhich 2005; Drobina 2006; Gaur 2008; Khashabi 2008; Ahmed 2013); and no dropout data were given in six studies (Meral 1996; Mangat 1998; Mahajan 2004; Neki 2006; Hossein 2016; Sarhan 2016). In Kokturk 2005 it appears as though there were no dropouts but the published report states that a participant was later excluded because the final diagnosis was COPD and the treatment group is not stated. On further correspondence, Badawy 2014 gave appropriate reasons for exclusions, but details regarding the groups from which participants were excluded were not given.
There was a high risk of bias in one study as three participants were enrolled more than once; only the initial visit was used in the analysis but the treatment group was not stated (Nannini 2000).
There was a low risk of bias in ten studies, with all randomised participants completing in four studies (Aggarwal 2006; Ashtekar 2008; Mohammedzadeh 2014; Turker 2017); and reasons fully described for dropouts in six studies (Bessmertny 2002; Hughes 2003; Gallegos‐Solórzano 2010; Goodacre 2013; Powell 2013; Alansari 2015).
Selective reporting
Ten studies were at unclear risk of reporting bias. Six studies were only reported in conference abstracts and therefore the risk of selective reporting bias is unclear (Abreu‐Gonzalez 2002; Dadhich 2005; Drobina 2006; Neki 2006; Ashtekar 2008; Ahmed 2013). Hossein 2016 was also at unclear risk of bias as not all primary outcome data were reported, adverse events were recorded only as “no treatment‐related complications” and there were clear mistakes in the reporting of vital signs. Sarhan 2016 did not distinguish between primary or secondary outcomes, while Mangat 1998 mentioned but did not report two outcomes.
Seven studies were judged to be at high risk of bias. One study was considered at high risk of bias as outcomes were partially reported and not statistically significant (Gaur 2008). Badawy 2014 was at high risk of bias as no primary outcome was stated. On further correspondence, adverse event data but no clinical asthma baseline characteristics were given. Bessmertny 2002 did not present data for outcomes which were described as not statistically significant, and only means were presented for FEV1. We did not identify a prospective trial registration for Turker 2017, adverse events were reported as “no side effect caused by magnesium was observed in any of the patients in the study” and the modified pulmonary index score was reported numerically at 120 minutes only. Hughes 2003, Meral 1996 and Nannini 2000 were also at high risk of bias as the trial report stated there was no difference in blood pressure and heart rate between the groups and no data were reported.
Eight studies were judged to be at low risk of bias. Four studies were at low risk of bias as all outcomes stated in the methods were reported, although no protocols were available (Mahajan 2004; Kokturk 2005; Aggarwal 2006; Gallegos‐Solórzano 2010). Pre‐registered protocols were available for four studies, in which all planned outcomes were reported (Goodacre 2013; Powell 2013; Mohammedzadeh 2014; Alansari 2015).
Other potential sources of bias
No other risks of bias were identified.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. MgSO4 + SABA + ipratropium compared to SABA + ipratropium in the treatment of acute asthma.
| MgSO₄+ SABA + ipratropium compared to SABA + ipratropium in the treatment of acute asthma | ||||||
| Patient or population: adults and children with acute exacerbation of asthma Setting: emergency department/inpatient Intervention: MgSO₄ + SABA + ipratropium Comparison: SABA + ipratropium | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with SABA + ipratropium | Risk with MgSO4 + SABA + ipratropium | |||||
| Pulmonary function (% predicted FEV1) (90 to 120 minutes) |
The mean pulmonary function (% predicted FEV1) was 65% | % predicted FEV1 was 3.28% higher (1.06 higher to 5.49 higher) | ‐ | 120 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | Outcome measured at 90 mins in 1 study and 120 mins in the other. 1 study (Gaur 2008) has reported much smaller standard deviations and contributes almost 90% of analysis weight |
| Pulmonary function % predicted PEF (60 minutes) |
The mean pulmonary function % predicted PEF was 50.45% | % predicted PEF was 0.05 higher (2.33 lower to 2.42 higher) | ‐ | 636 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 2 4 5 | Both studies in adults Mean control group % predicted PEF was 36% in 1 study and 64.9% in the other |
| Clinical severity scores (60 minutes) |
The mean dyspnoea VAS was 31.8; the mean Yung ASS was 4.95 | SMD 0.01 higher (0.11 lower to 0.12 higher) | ‐ | 1130 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 6 | 1 study reported Yung ASS and the other change in dyspnoea VAS |
| Admission at first presentation | 819 per 1000 | 778 per 1000 (745 to 819) | RR 0.95 (0.91 to 1.00) | 1308 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 7 8 9 | Adults vs children test for subgroup difference: P = 0.72, I² = 0% |
| Readmission (7 to 30 days) |
26 per 1000 | 46 per 1000 (22 to 100) | RR 1.80 (0.84 to 3.87) | 750 (2 RCTs) | ⊕⊕⊝⊝ LOW 10 | Outcome measured at 7 days in 1 study and 30 days in the other. |
| Serious adverse events (during admission) | 43 per 1000 | Not estimable. See comment. | ‐ | 557 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 11 | Risk difference: −0.03 (95% CI −0.06 to 0.00) Adults vs children test for subgroup difference: P = 0.39, I² = 0% Goodacre 2013 also reported participants with 1 or more SAE within 30 days: 35/332 in the MgSO₄ group and 28/358 in the placebo group (RD: 0.03; 95% CI −0.02 to 0.07) |
| Any adverse event (during admission) | 144 per 1000 | Not estimable. See comment. | ‐ | 1197 (2 RCTs) | ⊕⊕⊕⊕ HIGH | Risk Difference: 0.01 (95% CI −0.03 to 0.05) Adults vs children test for subgroup difference: P = 0.34, I² = 0% Goodacre 2013 also reported participants with 1 or more adverse event within 30 days: 52/332 in the MgSO₄ group and 36/358 in the placebo group (OR 1.66, 95% CI 1.05 to 2.62) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ASS: asthma severity score; CI: Confidence interval; RD: risk difference; RR: Risk ratio; OR: Odds ratio; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 One study contributing most of weight at unclear risk of bias in multiple domains (−1 study limitations)
2 I² > 50% (−1 inconsistency)
3 Studies equal size but one study contributes almost 90% of weight to analysis due to much smaller standard deviations. Result no longer significant if random‐effects model applied (−1 imprecision)
4 Although one study at unclear risk of bias in several domains, the larger study, which contributes vast majority of weight to analysis, if of high methodological quality (no downgrade)
5 Although confidence interval includes no difference, they are sufficiently tight to effectively rule out an important between‐group difference (no downgrade)
6 Confidence intervals include both harm and benefit of intervention (−1 imprecision)
7 Although two of the studies at unclear risk of bias in several domains the two large studies contributing nearly 95% of weight in analysis are both of high methodological quality (no downgrade)
8 Although the I² = 52%, the two large studies contributing to this analysis show consistent results (no downgrade)
9 Confidence intervals include no difference (−1 imprecision)
10 Confidence intervals include no difference and appreciable harm or benefit of the intervention (−2 imprecision)
11 Events rare and confidence intervals include no difference (−1 imprecision)
Summary of findings 2. MgSO4 + SABA compared to SABA in the treatment of acute asthma.
| MgSO₄+ SABA compared to SABA in the treatment of acute asthma | ||||||
| Patient or population: adults and children with acute exacerbation of asthma Setting: emergency department/inpatient Intervention: MgSO₄ + SABA Comparison: SABA | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with SABA | Risk with MgSO4 + SABA | |||||
| Pulmonary function % predicted FEV1 (20 minutes to 2 to 3 h) |
The mean pulmonary function % predicted FEV1 was 56.55% | % predicted FEV1 was 3.34% higher (1.58 lower to 8.26 higher) | ‐ | 208 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Adults vs children test for subgroup difference: P = 0.35, I² = 0% Severe vs moderate asthma exacerbation test for subgroup difference: P = 0.15, I² = 51.8% (favouring a greater effect in the more severe subgroup) |
| Pulmonary function PEF L/min ‐ Adults (20 minutes to 2 to 3 h) |
The mean pulmonary function PEF was 233 L/min | PEF was 11.91 L/min higher (4.12 lower to 27.95 higher) | ‐ | 155 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | |
| Pulmonary function PEF L/min ‐ Children (60 minutes) |
The mean pulmonary function PEF was 143.5 | PEF was 11.9 L/min higher (6.86 lower to 30.66 higher) | ‐ | 80 (1 RCT) | ⊕⊕⊝⊝ LOW 2 3 | |
| Admission to hospital at initial presentation | 202 per 1000 | 158 per 1000 (105 to 233) | RR 0.78, (0.52 to 1.15) | 375 (6 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Adults vs children test for subgroup difference: P = 0.35, I² = 0% |
| Serious adverse events (During ED/hospital admission) |
Not estimable | Not estimable. See comment | ‐ | 243 (5 RCTs) | ⊕⊕⊝⊝ LOW 1 4 | Risk difference: 0.00 (95% CI −0.04 to 0.04) No events reported |
| Any adverse events (During ED/hospital admission) |
107 per 1000 | Not estimable. See comment | ‐ | 694 (5 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Risk difference: −0.01 (95% CI −0.05 to 0.03) Adults vs children test for subgroup difference: P = 0.77, I² = 0% |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; ED: emergency department; FEV1: forced expiratory volume in 1 second; OR: Odds ratio; PEF: peak expiratory flow; RD: risk difference; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Several studies were at unclear or high risk of bias in one or more domain (−1 study limitations)
2 Confidence intervals include both possible harm and benefit of the intervention (−1 imprecision)
3 Study at unclear risk of bias in several domains (−1 study limitations)
4 No events reported but less than 250 participants in total. Risk difference confidence intervals include a possible important harm or benefit of the intervention (−1 imprecision)
Summary of findings 3. MgSO4 compared to SABA in the treatment of acute asthma.
| MgSO₄compared to SABA in the treatment of acute asthma | ||||||
| Patient or population: adults and children with acute exacerbation of asthma Setting: emergency department/inpatient Intervention: MgSO₄ Comparison: SABA | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with SABA | Risk with MgSO4 | |||||
| Lung function | Reported narratively in text | |||||
| Clinical severity score ‐ Fischl index (120 minutes) |
The Fischl index score was 2.1 | Fischl index score 0.13 lower (0.62 lower to 0.36 higher) | ‐ | 93 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | Time point 120 minutes in 2 studies and unclear in the third study Wide range of control group scores (0.3, 0.76 and 4.81). Scale out of 7 with higher score indicating more severe symptoms. 4.81 reported in study with unclear time point. |
| Admission to hospital at initial presentation | 118 per 1000 | 62 per 1000 (6 to 625) | RR 0.53 (0.05 to 5.31) | 33 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 4 5 | |
| Serious adverse events (During ED/hospital admission) |
Not estimable | Not estimable. See comment | 53 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 6 | Risk difference: 0.00 (95% CI −0.10 to 0.10) No events reported |
|
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Several studies at unclear or high risk of bias in one or more domains (−1 study limitations)
2 Confidence intervals include both possible harm and benefit of the intervention (−1 imprecision)
3 Time‐point for measurement unclear in one study (−1 indirectness)
4 Study at unclear risk of bias in several domains (−1 study limitations)
5 One small study. Confidence intervals include appreciable harm or benefit of the intervention (−2 imprecision)
6 Two small studies. No events reported. Risk difference confidence intervals include appreciable harm or benefit of the intervention (−1 for imprecision)
As detailed in the Methods section, we have presented effects of interventions within the following comparisons.
MgSO₄ and β₂‐agonist and ipratropium versus placebo (saline) and β₂‐agonist and ipratropium (comparison 1).
MgSO₄ with β₂‐agonist versus placebo (saline) and β₂‐agonist (comparison 2).
MgSO₄ versus β₂‐agonist alone (comparison 3).
MgSO₄ and β₂‐agonist and ipratropium versus placebo (saline) and β₂‐agonist and ipratropium (comparison 1)
Pulmonary function
Four studies including 1279 participants reported on FEV1 or PEF (Gaur 2008; Gallegos‐Solórzano 2010; Goodacre 2013; Hossein 2016).
Gallegos‐Solórzano 2010 and Gaur 2008, both adult studies, reported a greater %FEV1 in the MgSO₄ and β₂‐agonist and ipratropium group at 90 minutes (MD: 8.57, 95% CI 1.99 to 15.15; participants = 60) and 120 minutes (MD: 2.60, 95% CI 0.25 to 4.95; participants = 60) respectively with the pooled fixed‐effect result favouring the MgSO₄ intervention (MD 3.28, 95% CI 1.06 to 5.49; participants = 120; studies = 2; I² = 64%; Analysis 1.1).
1.1. Analysis.
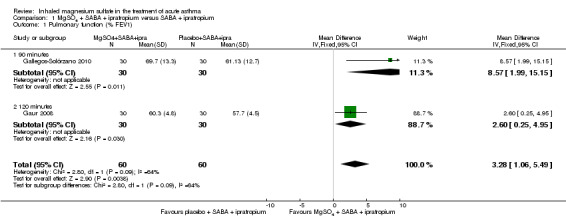
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 1 Pulmonary function (% FEV1).
Despite being similar‐sized studies, Gaur 2008 contributed nearly 90% of the weight to the pooled analysis due to reporting much smaller standard deviations. Sensitivity analysis with a random‐effects model results in reduced weighting for that study, and increased the size of the CI such that the lower confidence interval included no difference (MD 4.76, 95% CI −0.86 to 10.39; participants = 120; studies = 2).
Hossein 2016 reported a significantly greater per cent predicted PEF in the MgSO₄ and β₂‐agonist and ipratropium group at 20 minutes (MD 6.90, 95% CI 1.63 to 12.17) but an important between‐group difference was not found when 60 minute data were combined with 60 minute data from the large Goodacre 2013 trial (MD 0.05, 95% CI −2.33 to 2.42; participants = 636; studies = 2; I² = 67%). A random‐effects model substantially increases imprecision, and the effect estimate remains inconclusive. The Goodacre results taken individually did not demonstrate an important between‐group difference (MD −0.30%, 95% CI −2.71% to 2.11%).
Alansari 2015, Ashtekar 2008 and Powell 2013 did not report this outcome. Drobina 2006 reported that "peak flow measurements improved over time in both groups (p < 0.001). The addition of aerosolized magnesium sulfate did not result in a statistically significant increase in either the maximum or the average peak flow over time (p = 0.279 and p = 0.399, respectively)." As this research is only available in abstract form, it is unclear how many participants were in each group and no data were reported to include in the meta‐analysis.
Clinical severity scores
Powell 2013 reported a lower (therefore better) Yung asthma severity score (ASS) in children receiving MgSO₄ and β₂‐agonist and ipratropium compared to placebo (saline) and β₂‐agonist and ipratropium at 60 minutes (MD −0.23, 95% CI −0.48 to 0.02; participants = 472). The minimal important difference on this nine point scale is not known, but in Powell 2013 it was regarded as 0.5 by the trial steering group. Goodacre 2013 reported no significant difference in the change in dyspnoea visual analogue scale between the groups at 60 (MD 3.10, 95% CI −0.53 to 6.73; participants = 658) or 120 minutes (MD 3.12, 95% CI −1.35 to 7.59; participants = 619). The minimal important difference on the 100 mm scale used in Goodacre 2013 is thought to be 22 mm. When the '60 minute' data from each study are combined using an SMD analysis there is no between‐group difference (SMD 0.01, 95% CI −0.11 to 0.12; participants = 1130; studies = 2) but a high level of heterogeneity (I² = 83%). As the studies were of a similar size and weight in this analysis, a random‐effects model has little impact on the effect estimates, although the confidence interval is widened.
Ashtekar 2008 reported that there was no significant difference between the median area under the curve of ASS of the MgSO₄ compared with the placebo‐treated group (1530 versus 1355).
Of note: Powell 2013 performed subgroup analysis for asthma severity score, investigating whether participants with a more severe asthma exacerbation or an exacerbation of shorter duration derived more benefit. Although not powered to detect a difference, the study results support the hypothesis that children with more severe exacerbations and children with shorter duration of symptoms prior to presentation benefit more from inhaled MgSO₄ as measured using the Yung asthma severity score.
Admission to hospital
Gallegos‐Solórzano 2010 reported admissions to the emergency department and the general ward; Goodacre 2013 reported admissions to hospital, HDU and ICU; and Powell 2013 reported admissions to PICU/HDU or intubation.
Pooled results for adults and children for admissions from the emergency department at initial presentation suggests that admissions are decreased in those receiving MgSO₄, β₂‐agonist and ipratropium compared to placebo (saline), β₂‐agonist and ipratropium (RR 0.95, 95% CI 0.91 to 1.00; participants = 1308; studies = 4; I² = 52%) but the upper confidence interval reaches no difference (Analysis 1.4). The overall risk of admission was 82% on placebo which translates into a 78% risk of admission (95% CI 75% to 82%) with nebulised magnesium (Figure 3, Table 1). The RR is the same if a random‐effects model is used, but the result is less precise (RR 0.95, 95% CI 0.87 to 1.05).
1.4. Analysis.
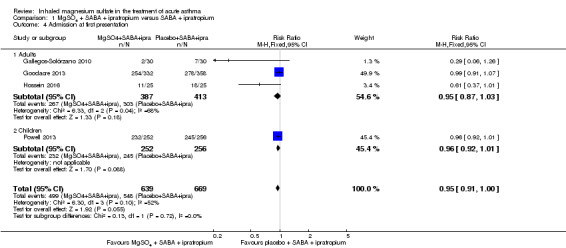
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 4 Admission at first presentation.
3.
In the control group 82 people out of 100 had hospital admission , compared to 78 (95% CI 75 to 82) out of 100 for the active treatment group.
Gallegos‐Solórzano 2010, an adult study, also reported admission to the emergency department (RR 0.38, 95% CI 0.16 to 0.94; participants = 60).
Goodacre 2013 and Gallegos‐Solórzano 2010, both adult studies, reported on readmission after initial attendance (up to 7 days in Goodacre 2013; time point unclear in Gallegos‐Solórzano 2010). There was no significant difference in admission rates between the two groups, but the result is imprecise (RR 1.80, 95% CI 0.84 to 3.87; participants = 750; studies = 2; I² = 37%, Analysis 1.6).
1.6. Analysis.
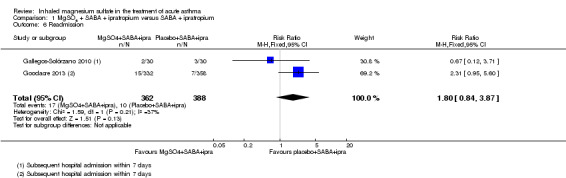
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 6 Readmission.
Goodacre 2013 did not detect a between‐group difference for either HDU admission (RR 1.19, 95% CI 0.66 to 2.13; participants = 690) or ICU (RR 1.94, 95% CI 0.66 to 5.73; participants = 690) but events were infrequent and both results inconclusive (Analysis 1.5). Similarly Powell 2013, a study in children, reported HDU or ICU admissions/intubations and did not detect a between‐group difference (RR 1.48, 95% CI 0.79 to 2.79; participants = 505).
1.5. Analysis.
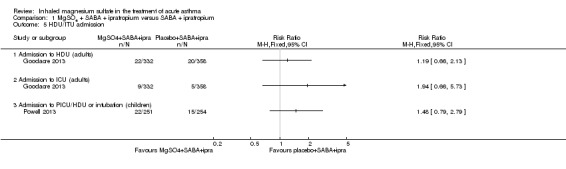
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 5 HDU/ITU admission.
Duration of symptoms
Not reported.
Vital signs
Hossein 2016 and Goodacre 2013 reported on vital signs at various time points. For this update, we chose to present the 60‐minute time point in the meta‐analysis. There are concerns that administration of MgSO₄ may lead to an unwanted drop in blood pressure. Goodacre 2013 reported a significantly higher (therefore better in this context) diastolic blood pressure in participants receiving MgSO₄ and β₂‐agonist and ipratropium compared to placebo and β₂‐agonist and ipratropium at 60 minutes (MD 2.40, 95% CI 0.29 to 4.51; participants = 674), but this difference is unlikely to be clinically meaningful (Analysis 1.10). There was no significant difference in respiratory rate, heart rate or systolic and diastolic blood pressure at any other time point. Similarly there was no significant difference in the change in vital signs between groups at 60 or 120 minutes (Goodacre 2013; data not shown).
1.10. Analysis.
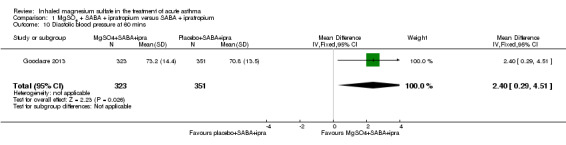
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 10 Diastolic blood pressure at 60 mins.
Drobina 2006 stated that vital signs were measured in the conference abstract but data were not reported.
Adverse events
Four studies including 2067 participants reported adverse events (Goodacre 2013; Powell 2013; Alansari 2015; Hossein 2016). During admission, more serious adverse events were reported in the placebo group compared to the MgSO₄ group, but only one of the two studies reporting this outcome contributed events and the confidence interval includes no difference (RD −0.03, 95% CI −0.06 to −0.00; participants = 557; studies = 2; I² = 0%; Analysis 1.11). There was no significant difference in adverse events between groups for any adverse event (RD 0.01, 95% CI −0.03 to 0.05; participants = 1197; studies = 2; I² = 0%; Analysis 1.12).
1.11. Analysis.
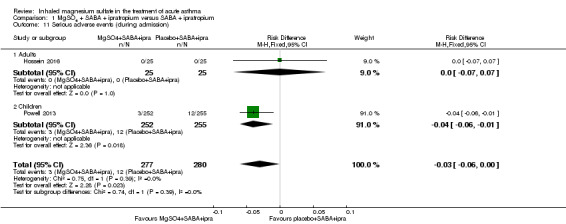
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 11 Serious adverse events (during admission).
1.12. Analysis.
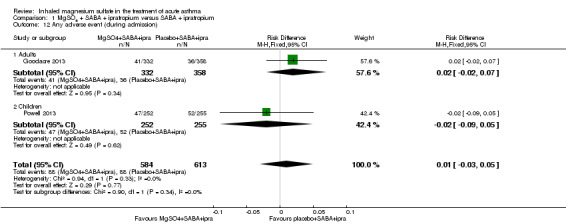
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 12 Any adverse event (during admission).
Goodacre 2013 reported a significantly higher 'all adverse event' rate within 30 days of primary attendance for those participants receiving MgSO₄ and β₂‐agonist and ipratropium compared to placebo (saline) and β₂‐agonist and ipratropium (RD 0.06, 95% CI 0.01 to 0.11; participants = 690; Analysis 1.14) but there was no difference in serious adverse events (Analysis 1.13). Hossein 2016 reported no serious adverse events in either group in the first 60 minutes of treatment.
1.14. Analysis.
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 14 Any adverse event (within 30 days).
1.13. Analysis.
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 13 Serious adverse events (within 30 days).
Both Goodacre 2013 and Powell 2013 reported hypotension and flushing but did not detect a significant between‐group difference for either outcome (Analysis 1.15; Analysis 1.16).
1.15. Analysis.
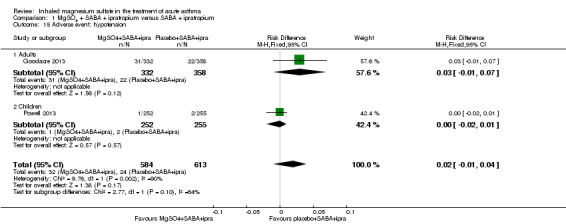
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 15 Adverse event: hypotension.
1.16. Analysis.
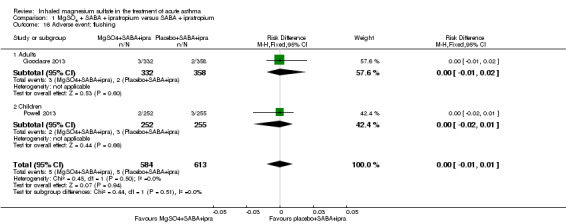
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 16 Adverse event: flushing.
Ashtekar 2008 reported that one child had a transiently low blood pressure and another had tingling of the fingers; both received nebulised MgSO₄. Drobina 2006 reported that there were no significant side effects noted in either treatment group, but did not report data. Gaur 2008 did not report adverse effects.
Gallegos‐Solórzano 2010 reported that the most common adverse reaction associated with MgSO₄ was a dry and bitter mouth, but no other side effect was associated with treatment. Electrocardiography (ECG) was abnormal in some participants (43% versus 36%): most commonly, sinus tachycardia (40% versus 36%). One person in the MgSO₄ group developed supraventricular extrasystole that did not require additional management. One from each group reported dizziness.
MgSO₄ with β₂‐agonist versus placebo (saline) and β₂‐agonist (comparison 2)
Pulmonary function
Eleven studies involving 589 participants reported at least one measure of lung function (Nannini 2000; Abreu‐Gonzalez 2002; Bessmertny 2002; Hughes 2003; Mahajan 2004; Kokturk 2005; Aggarwal 2006; Ahmed 2013; Badawy 2014; Mohammedzadeh 2014; Sarhan 2016).
Five studies reported per cent predicted FEV1; two at 120 minutes (Badawy 2014; Sarhan 2016), two at 60 minutes (Bessmertny 2002; Hughes 2003) and one at 20 minutes (Mahajan 2004). Due to our concerns about potential baseline imbalance and the population recruited in Badawy 2014 (all pregnant women) we chose not to include this study in the analysis.
Pulmonary function based on per cent predicted FEV1 was improved in those who received MgSO₄ and a β₂‐agonist compared to β₂‐agonist and placebo, but the confidence interval includes no difference (MD 3.34, 95% CI −1.58 to 8.26; participants = 208; studies = 4), with moderate between‐study heterogeneity identified (I² = 43%). We did not detect a difference between adults and children, but only one study involving children contributed to the analysis. We were only able to present subgrouping based on severity at presentation with three studies as we were unable to characterise severity in Sarhan 2016. Results suggest the combination of MgSO₄ and salbutamol may be more beneficial in people with a more severe exacerbation (FEV1 < 50% predicted) but the test for subgroup difference was negative (I² = 51.8%, P = 0.15). Badawy 2014 reports significantly higher post‐treatment per cent predicted FEV1 in pregnant women receiving MgSO₄ in addition to salbutamol compared to salbutamol alone; end‐point scores were 56.31% (SD 8.25) in the intervention group compared to 32.68% (SD 7.15) (P < 0.001, 30 participants), but baseline FEV1 in each group was not measured.
Six studies reported peak expiratory flow rate (PEF): one at 20 minutes (Nannini 2000), two at 60 minutes (Aggarwal 2006; Mohammedzadeh 2014), two at 120 minutes (Badawy 2014; Sarhan 2016), one at discharge (Kokturk 2005). Where studies reported at more than one time point we have extracted and presented the closest time point to 60 minutes. We excluded Badawy 2014 due to the reasons given above and Kokturk 2005 due to the incompatible time point. We have not pooled adults and children in this analysis. In adults, there was small improvement in PEF compared to the control group, but the confidence interval includes no difference (MD 11.91 L/min, 95% CI −4.12 to 27.95, participants 155, studies 3, I² = 13%, Analysis 2.3). A similar small and imprecise effect was seen in Mohammedzadeh 2014, the study involving children: MD 11.90 L/min, 95% CI −6.86 to 30.66, participants 80. Mohammedzadeh 2014 also reports a change from baseline "adjusted % PEF", which favours the combination of MgSO₄ and salbutamol compared to salbutamol alone (MD 6.70, 95% CI 3.80 to 9.60; data not displayed). Kokturk 2005 reported "both groups displayed comparable improvement in PEF (%) and clinical scores over 120 min" and displayed results graphically. Discharge mean (SD) per cent predicted PEF was similar in both groups (71.18 (11.55) and 70.50 (12.34)).
2.3. Analysis.
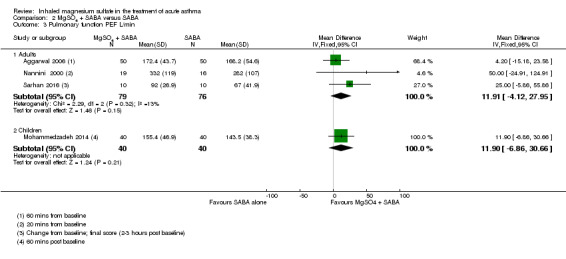
Comparison 2 MgSO4 + SABA versus SABA, Outcome 3 Pulmonary function PEF L/min.
One study (Ahmed 2013; an abstract) reported "the percentage increase in peak flow" at 10 and 20 minutes, but the lack of clarity about whether this was a per cent change in the absolute values or a change in the per cent predicted meant we could not include data in the analyses. The study reported a greater improvement in the intervention group compared to the control group at both 10 and 20 minutes (20 (SD 4) vs 13 (3) at 10 minutes; and 35 (7) vs 24 (6) at 20 minutes).
Abreu‐Gonzalez 2002 (n = 24), also a conference abstract, selectively reports improvement in PEF at 30 minutes for people in the β₂‐agonist and MgSO₄ group compared to the placebo plus β₂‐agonist group and improvement in FEV1 at 45 minutes for those in the β₂‐agonist alone with placebo group compared to the β₂‐agonist and MgSO₄ group.
Clinical severity score
Four studies involving 575 participants reported clinical severity scores with enough detail for data extraction (Mohammedzadeh 2014; Alansari 2015; Sarhan 2016; Turker 2017), but we did not perform meta‐analysis. Pulmonary index score was significantly lower (therefore better) for those receiving MgSO₄ and β₂‐agonist compared to β₂‐agonist and placebo at 90 minutes in Mohammedzadeh 2014 (MD −0.90, 95% CI −1.43 to −0.37, participants = 80; ) but not at other time points. Sarhan 2016 reported no significant difference in change in Fischl index at two hours between the two groups (MD −0.10, 95% CI −1.22 to 1.02; participants = 20; studies = 1). Alansari 2015 reported PRAM asthma severity score at seven time points (from four to 48 hours). PRAM was significantly lower (therefore better) for those receiving placebo (saline) and β₂‐agonist and ipratropium compared to MgSO₄ and β₂‐agonist and ipratropium at the earliest time point (4 hours; MD −0.4, 95% CI −0.7 to −0.01; P = 0.05; participants = 365;) but not at later time points. Turker 2017 measured the modified pulmonary index score at 20, 40 and 120 minutes post baseline but it was only possible to extract data at 120 minutes; no between‐group difference was identified (MD 0.38, 95% CI −0.25 to 1.01; participants = 100; studies = 1).
Kokturk 2005 reported that both groups displayed comparable improvement in clinical scores over 120 minutes. Khashabi 2008 reported a non‐significant difference in respiratory distress scores, but it is not clear which score was used and the number of participants in each group is not clear. The author was contacted for further information before the 2012 update but no response was received.
Admission to hospital
Six studies involving 375 participants reported admissions for MgSO₄ and β₂‐agonist compared to β₂‐agonist and placebo (Nannini 2000; Hughes 2003; Mahajan 2004; Kokturk 2005; Aggarwal 2006; Turker 2017). Although there was a reduction in the risk of admission for people receiving the MgSO₄ intervention, the confidence interval includes no difference (risk ratio 0.78, 95% CI 0.52 to 1.15; participants = 375; studies = 6; I² = 0%). It should be noted that this analysis is dominated by evidence from adult studies. As we did not detect any statistical heterogeneity in the analysis we did not perform our prespecified subgroup analyses. One study in children reported that one child from each group required admission/re‐admission in the two weeks after discharge (Alansari 2015).
Vital signs
Three studies including 190 participants reported heart rate at 120 minutes (Aggarwal 2006; Badawy 2014; Sarhan 2016). As previously, we have not included Badawy 2014 in the analysis and combining the remaining two studies resulted in such substantial heterogeneity (I² = 82%) that we did not perform meta‐analysis (Analysis 2.5). Participants receiving MgSO₄ and β₂‐agonist had a slightly lower (therefore better) heart rate at 120 minutes compared to those receiving β₂‐agonist and placebo in Aggarwal 2006 (MD −2.70, 95% CI −6.15 to 0.75; participants = 100), but the opposite effect was seen in Sarhan 2016 (MD 22.60, 95% CI 1.61 to 43.59; participants = 20). Badawy 2014 reported a MD of −25.46 (95% CI −28.38 to −22.54) favouring the MgSO₄ group.
2.5. Analysis.
Comparison 2 MgSO4 + SABA versus SABA, Outcome 5 Heart rate at 120 mins.
There was no significant difference between the two groups in systolic or diastolic blood pressure or respiratory rate in the studies reporting these outcomes.
Adverse events
Serious adverse events were reported by five studies including 243 participants (Nannini 2000; Bessmertny 2002; Hughes 2003; Mahajan 2004; Sarhan 2016). No serious events occurred in any of the studies (Analysis 2.9).
2.9. Analysis.
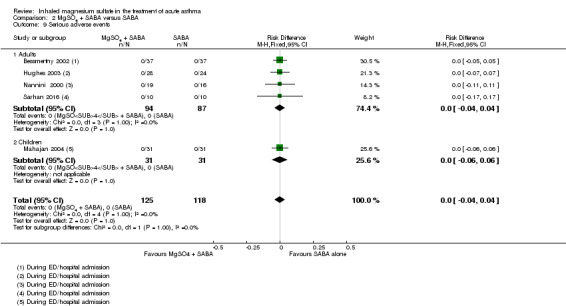
Comparison 2 MgSO4 + SABA versus SABA, Outcome 9 Serious adverse events.
Three studies reported on mild‐moderate adverse events (Nannini 2000; Bessmertny 2002; Aggarwal 2006); and two reported all adverse events (Ahmed 2013; Alansari 2015). There was no significant difference in those experiencing one or more events between the two groups (RD −0.01, 95% CI −0.05 to 0.03; participants = 694; studies = 5; I² = 0%; Analysis 2.10); and no difference detected between adults and children.
2.10. Analysis.
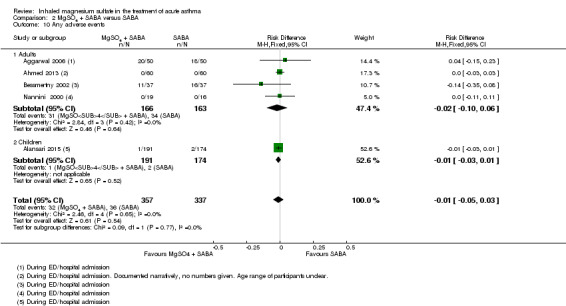
Comparison 2 MgSO4 + SABA versus SABA, Outcome 10 Any adverse events.
Turker 2017 reports narratively that "no side effect caused by magnesium was observed in any of the patients in the study"; this data has not been included in the forest plots.
Aggarwal 2006 reported that tremor was the same in both groups. Khashabi 2008 reported no side effects but it is not clear how many participants were in each group, so their data were not included in this analysis. Kokturk 2005 reported that two participants in the MgSO₄ group and four in the placebo group required additional therapy. Two participants developed transient hypotension after receiving MgSO₄ and β₂‐agonist. No one needed nebulisation to be withheld. One participant in the placebo group suffered palpitations after the second salbutamol nebulisation. No other side effects were reported. Alansari 2015 reported that no participants experienced hypotension; one participant in the placebo group had excessive cough after the first nebulisation and was withdrawn by his parents; one participant in the MgSO₄ group experienced chest tightness and facial rash after the third nebulisation (both resolved after 30 minutes); and one placebo group participant required paediatric ICU admission for refractory status asthmaticus.
MgSO₄ versus β₂‐agonist alone (comparison 3)
Pulmonary function
Four studies involving 133 participants reported PEF, but due to the range of time points and ways of reporting the outcome we have not presented the results on a forest plot. Mangat 1998 found no significant difference in % predicted PEF for MgSO₄ alone compared with β₂‐agonist alone at 60 minutes (MD 4.20, 95% CI −12.29 to 20.69; participants = 33). Neki 2006 shows a significant advantage for β₂‐agonist alone but the time point reported is unclear (MD −50 L/min, 95% CI −67.83 to −32.17; participants = 4). Meral 1996 reported the mean improvement per group in PEFR (%) at five minutes, favouring the β₂‐agonist group (MD −36.77, 95% CI −61.26 to −12.28; participants = 40). Sarhan 2016 reported change in per cent predicted PEF and found no significant difference between groups (MD −1.70, 95% CI −8.45 to 5.05; participants = 20).
Clinical severity score
The Fischl index (a composite of vital signs, PEF and clinical features ranging from 0 to 7 with scores > 4 indicating acute severe asthma) was reported in three studies (Mangat 1998; Neki 2006; Sarhan 2016); and the Davies, Leffert, Drabous score (a composite measure of retraction, nasal flaring, cyanosis and wheeze) in one study (Meral 1996). We pooled the results for studies reporting the Fischl Index, which suggests no significant between‐group difference (MD −0.13, 95% CI −0.62 to 0.36; participants = 93; studies = 3; I² = 0%).
Meral 1996 reported the maximum clinical severity score in the first hour (MD −3.20; 95% CI −17.62 to 11.22; participants = 40).
Admission to hospital
There was no significant difference in risk of admission between those receiving MgSO₄ alone compared with β₂‐agonist alone (RR 0.53, 95% CI 0.05 to 5.31; participants = 33, studies = 1; Analysis 3.2); however, the wide confidence interval indicates, due to there being few events in a small trial, that equivalence cannot be claimed. With a single trial contributing data (Mangat 1998), no additional analyses were possible. Three studies did not appear to measure or report this outcome (Meral 1996; Neki 2006; Sarhan 2016).
3.2. Analysis.
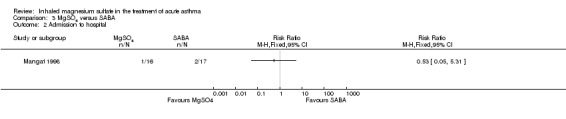
Comparison 3 MgSO4 versus SABA, Outcome 2 Admission to hospital.
Vital signs
Sarhan 2016 reported a significantly lower (therefore better) mean heart rate in people receiving β₂‐agonist alone compared to MgSO₄ alone (MD 21.20, 95% CI 0.17 to 42.23; participants = 20). Respiratory rate was reported as significantly lower (therefore better) in those receiving MgSO₄ alone compared to β₂‐agonist alone (MD −2.40, 95% CI −3.91 to −0.89; participants = 60; studies = 2; I² = 0%)(Neki 2006; Sarhan 2016). There was no significant difference in systolic or diastolic blood pressure between those receiving MgSO₄ alone compared with β₂‐agonist alone in the one study which reported this (Analysis 3.5; Analysis 3.6).Two studies did not appear to measure or report these outcomes (Meral 1996; Mangat 1998).
3.5. Analysis.
Comparison 3 MgSO4 versus SABA, Outcome 5 Systolic pressure (120 mins).
3.6. Analysis.
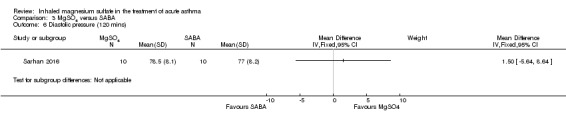
Comparison 3 MgSO4 versus SABA, Outcome 6 Diastolic pressure (120 mins).
Adverse events
Two studies reported that there were no serious adverse events in either arm (participants = 53; studies = 2) (Mangat 1998; Sarhan 2016). One study reported mild to moderate adverse events and no difference was detected between groups (RD −0.17, 95% CI −0.41 to 0.06; participants = 33; Analysis 3.8). Meral 1996 also reported that there were no adverse events in either group. One study did not report adverse events (Neki 2006).
3.8. Analysis.
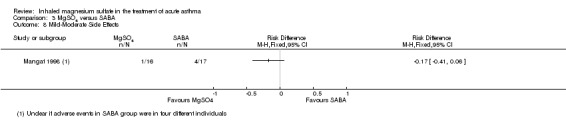
Comparison 3 MgSO4 versus SABA, Outcome 8 Mild‐Moderate Side Effects.
Reporting biases
Too few studies were included in any meta‐analysis to produce a funnel plot. However, the impact of publication bias was likely limited through a thorough search strategy.
Discussion
Summary of main results
This systematic review summarises evidence from twenty‐four trials including 2807 randomised participants with acute exacerbations of asthma. The most recent update added three new large trials (Goodacre 2013; Powell 2013; Alansari 2015), as well as several small trials. Overall, the results are conflicting with some, generally small, benefits seen of adding MgSO₄ to standard therapy for an exacerbation of asthma. The larger and more recent studies typically show a smaller effect than some of the older/smaller studies. Serious adverse events were rare across all four comparisons, suggesting the treatment is generally well tolerated in people experiencing moderate to severe exacerbations of asthma. Differences between studies in the populations, outcomes reported and time points limited the number of meta‐analyses performed.
Comparison 1: MgSO₄ and β₂‐agonist and ipratropium versus placebo and β₂‐agonist and ipratropium
We included seven studies in this comparison and were able to extract data on lung function, clinical severity scores, admissions to hospital, vital signs and adverse events. Although some individual studies reported improvement in lung function indices favouring the intervention group, results were inconsistent and may not be clinically relevant overall. The largest study reporting this outcome reported no between‐group difference at 60 minutes (Goodacre 2013).
Similarly, clinical severity scores typically did not demonstrate a benefit of the intervention compared to control. Powell 2013, a large study in children, reported a benefit at 60 minutes using the Yung asthma severity score, but the mean difference is unlikely to be clinically important. Admissions to hospital at initial presentation may be reduced by the addition of inhaled magnesium sulfate, but the upper end of the confidence interval includes no difference (RR 0.95, 95% CI 0.91 to 1.00) and it is hard to compare the studies as in Powell 2013 and Goodacre 2013 almost everyone was admitted to hospital, while in the smaller studies a smaller proportion of participants were admitted.
No difference was detected for re‐admissions or escalation of care to ITU/HDU, but events were generally rare. Administration of magnesium sulfate has been associated with hypotension and Goodacre 2013 reported a slightly higher diastolic blood pressure in the control group, but the effect size was small (MD 2.40 mmHg, 95% CI 0.29 to 4.51). No other between‐group differences were detected in the studies reporting vital signs. Finally, serious adverse events during admission were rare and only one study contributed to this analysis. There was no difference between groups for all adverse events during admission (RD 0.01, 95% CI −0.03 to 0.05). Goodacre 2013 reported a lower risk of all adverse events in the 30 days after admission in the placebo group, but no difference in serious adverse events (around 10% of people experience a serious adverse event and 15% an adverse event).
Comparison 2: MgSO₄ with β₂‐agonist versus β₂‐agonist
We included 15 studies in this comparison and were able to extract data on lung function, clinical severity scores, admissions to hospital, vital signs and adverse events. While some individual studies reported improvement in lung function indices favouring the intervention group, none of the pooled results showed a clinically meaningful benefit as measured by FEV1 or PEFR. Clinical severity scores were reported using a number of different scales and at different time points, but no consistent difference between the two groups was identified. Pooled results for hospital admission favoured the combination of MgSO₄ and β₂‐agonist, but the confidence interval includes the possibility of admissions increasing in the intervention group (RR 0.78, 95% CI 0.52 to 1.15). Few studies reported vital signs and of those that did, none detected a significant between‐group difference, with results often imprecise. There were no serious adverse events reported by any of the included studies and no between‐group difference for all adverse events (RD −0.01, 95% CI −0.05 to 0.03).
Comparison 3: MgSO₄ versus β₂‐agonist alone
We included five studies in this comparison and were able to extract data on lung function, clinical severity scores, admissions to hospital, vital signs and adverse events. As the evidence for use of β₂‐agonists in acute asthma is well‐established this could be considered a historical comparison, no longer in keeping with current clinical practice. Two studies reported a benefit in lung function of β₂‐agonist over MgSO₄ alone and two studies reported no difference; we did not pool these results. No between‐group difference was detected for clinical severity scores (Fischl index) but results are based on only a few small studies, which measured at different time points (MD −0.13, 95% CI −0.62 to 0.36; participants = 93). Admissions to hospital were only reported by one small study and events were rare, leading to an uncertain result. Effects on vital signs were inconsistent; one small study reported lower heart rate in the β₂‐agonist group, while pooled results from two studies found a lower respiratory rate in the MgSO₄ group. No serious adverse events were reported in any of the studies in this comparison; one small study reported mild to moderate adverse events but the result is imprecise.
Overall completeness and applicability of evidence
Comparisons 1 and 2, in which MgSO₄ was combined with other bronchodilators, are the most consistent with current clinical guidance, and therefore most applicable to practice (BTS/SIGN 2016; GINA 2017). Comparison 3, in which MgSO₄ was the only inhaled bronchodilator given during the intervention period in one arm, could be considered a more historical comparison. Given the strong evidence of the efficacy of beta‐agonists in acute asthma, it is unlikely that studies with this design would receive ethical approval in many settings.
Within comparisons 1 and 2, populations, interventions, outcomes and time points were heterogeneous, which limited the number of meta‐analyses performed and therefore the conclusions reached. However three large, well‐powered studies of high methodological quality were added to this 2017 update and overall did not demonstrate a substantial benefit of the addition of inhaled MgSO₄ to standard therapy (Goodacre 2013; Powell 2013; Alansari 2015), although modest benefits were seen in some individual studies and meta‐analyses. However, we were not able to fully implement our planned subgroup analysis for severity and thus there remains uncertainty about whether those with more severe exacerbations derive greater benefit.
The included studies used a variety of different dosing regimens for MgSO₄, including different numbers and frequency of doses and different MgSO₄ formulations. The precise regimen was not described in all included studies (Table 6). A limitation of this review is that we did not attempt to subgroup studies according to dosing regimen used. It is possible that this is an important effect modifier which may require further investigation. Also, we have not attempted to explore the cost‐effectiveness of using inhaled MgSO₄; any clinical benefits need to be considered in the context of possible increased costs associated with delivering MgSO₄ and monitoring patients during treatment.
We chose lung function (FEV1 and PEFR) as the primary outcome in this review. Lung function may be considered a surrogate outcome in asthma and does not always correlate well with a patient's symptoms, quality of life and asthma control (Carranza Rosenzweig 2004; Aburuz 2005). In an acute setting, however, lung function — particularly peak flow — is frequently used to guide management and assess response to therapy; and validated patient‐reported outcomes such as the Asthma Control Questionnaire and Asthma Quality of Life Questionnaire have limited applicability during acute exacerbations.
Other uncertainties in the findings are introduced by the lack of consistency between settings in deciding whether, and if so when, to admit patients from the emergency department into the hospital. There were marked differences between studies in terms of the proportion of patients admitted; this may reflect different practices in different hospitals, varied exacerbation severity in those recruited and differences between practices in paediatric and adult populations. Although these variations should be accounted for in the randomisation process within each trial, it introduces clinical heterogeneity into our meta‐analyses and further complicates the interpretation of the evidence.
Finally, adverse events were not consistently reported by all studies. However, we were able to extract data extracted from the more recent larger studies and the combined evidence suggest that inhaled MgSO₄ is unlikely to be associated with an important increase in serious adverse events in the populations studied — it should be noted that events were rare.
Quality of the evidence
Our confidence in the results presented in this review ranges from high to very low, but overall most of the evidence was rated as being of low or very low quality. This means the true effect may be substantially different from the estimate of the effect presented. Please see Table 1; Table 2; Table 3 for full details of our GRADE assessments. Our confidence was reduced by a number of different considerations.
Most studies were at unclear or high risk of bias in multiple domains; only four studies were considered to be at low risk of bias in all domains (Aggarwal 2006; Goodacre 2013; Powell 2013; Alansari 2015). Many studies did not clearly report their methods of randomisation or allocation. In some studies it was not clear who was masked to treatment group assignment and we had concerns about attrition bias and selective reporting in multiple studies. We downgraded for study limitations when we were judged that a study about which we had important methodological concerns contributed sufficient weight to analysis to potentially affect the overall estimate.
Imprecision was also a problem in many of the meta‐analyses with sample sizes too small and events too few to rule out potential important harm or benefit of the intervention. This was a problem in the lung function, hospitalisation and adverse event analyses. Unexplained statistical heterogeneity was encountered less frequently but this may be a result of us being unable to perform meta‐analyses with substantial numbers of studies contributing data. Indirectness was not thought to be a problem in any of the outcomes to which we applied GRADE and we did not have sufficient studies in any one analysis to formally assess publication bias using a funnel plot.
Potential biases in the review process
Publication bias may have influenced the result of this meta‐analysis. For example, by missing unpublished negative trials we may be over‐estimating the effect of MgSO₄ treatment. In order to reduce bias, however, a comprehensive and systematic search of the published and unpublished literature for potentially relevant studies was conducted and we have recently updated it. This was followed by our attempts to contact corresponding and first authors. However, we recognise that unpublished data may exist.
We conducted our review in accordance with Cochrane methods and have detailed changes between the protocol and the review in Differences between protocol and review section. One such difference, which may have introduced bias, is the selection of the '60 minute' time point for reporting outcomes. Many studies reported outcome at multiple time points and for consistency we selected the outcome closest to 60 minutes from baseline for meta‐analysis. We chose this time point by consensus to maximise the homogeneity of pooled results and because this was identified as a clinically relevant time point for decision making about treatment escalation (i.e. there would be an expectation that most patients are showing a response to treatment with an hour). It is conceivable that choice of a different time point might have led to different conclusions, especially for the lung function and clinical severity score indices, which may be more susceptible to fluctuations over short periods of time.
Agreements and disagreements with other studies or reviews
The conclusions in this updated Cochrane Review are broadly consistent with the previous version of the review (Powell 2012): that there may be some modest benefits associated with addition of magnesium sulfate (MgSO₄) to standard therapy for acute asthma. However, the addition of several large and well‐conducted studies suggests that there is unlikely to be a substantial additional benefit associated with the addition of inhaled MgSO₄. Although we now have more data in children and the effect appears to be similar to that seen in adults, formal subgrouping by age was not always possible due to lack of sufficient data and between‐trial heterogeneity.
To our knowledge, no other systematic review has included as many primary studies as the present review and therefore conclusions are not entirely comparable and findings are somewhat conflicting. Overall, systematic reviews published to date have failed to demonstrate a conclusive benefit of inhaled magnesium sulfate in acute asthma and have suggested the role of the intervention remains unclear. For example, a systematic review published in 2006 included six studies, all of which are included in the present review. Despite some evidence of benefit, the authors were unable to reach conclusions due to lack of evidence and stated that the role of inhaled magnesium sulfate remains unclear. Similarly, a 2005 review also included six studies and concluded that nebulised MgSO₄ may confer some benefits in terms of pulmonary function and hospital admission, but called for more research (Blitz 2005). These findings are also consistent with the systematic review by Mohammed 2007.
More recently, a 2016 systematic review summarised the literature for both inhaled and intravenous magnesium sulfate in children with acute asthma (Su 2017). The review included four studies of nebulised magnesium sulfate in children, three of which we included and one which we excluded as it was a study of methacholine challenge test in stable asthma. The review concluded there was no impact on hospital admission or lung function. Similarly, a 2012 systematic review in adults included six studies of nebulised MgSO₄ (all of which are included in the present review) and concluded that there was insufficient evidence to recommend the use of this intervention (Song 2012). A 2016 review identified 10 relevant trials and concluded "adding nebulized MgSO₄ neither improved pulmonary function nor reduced the number of hospital admissions in adult patients with acute asthma" (Ling 2016). Finally, a 2013 review of both intravenous and inhaled magnesium sulfate suggested that there is a benefit for adults in terms of hospital admissions and lung function, but no benefit seen in children (Shan 2013). However, this study included only nine trials of inhaled magnesium sulfate, all of which are included in the present review with the exception of one, because we did not consider it to be an RCT.
Finally, two recent Cochrane Reviews have examined the efficacy and safety of intravenous (IV) MgSO₄ in adults and children with acute asthma (Griffiths 2016; Kew 2014). The review in adults included 14 studies, randomising over 2000 participants, and concluded that "a single infusion of 1.2 g or 2 g IV MgSO₄ over 15 to 30 minutes reduces hospital admissions and improves lung function in adults with acute asthma who have not responded sufficiently to oxygen, nebulised short‐acting beta₂‐agonists and IV corticosteroids". Limited evidence was found for other measures of benefit and safety (Kew 2014). The evidence in children is more limited with only five studies, involving 182 children, included in the review (Griffiths 2016). The authors conclude that IV MgSO₄ may reduce the need for hospital admission in children with moderate to severe asthma exacerbations, but emphasise the small number of trials and participants. Clinicians managing acute asthma and considering the use of a magnesium preparation will need to choose between the inhaled and IV route; at this time, the evidence for IV magnesium may seem more persuasive, in keeping with guidance (BTS/SIGN 2016).
Authors' conclusions
Implications for practice.
Treatment with nebulised MgSO₄ may result in modest additional benefits when added to inhaled β₂‐agonists and ipratropium bromide, but our confidence in the evidence is low and there remains substantial uncertainty. The recent large, well‐designed trials have generally not demonstrated clinically important benefits. Nebulised MgSO₄ does not appear to be associated with an increase in serious adverse events or all adverse events, but serious events were rare and results lacked precision. Those with a more severe exacerbation may experience a greater benefit but as we were unable to implement our planned subgroup analysis for severity this remains an area of uncertainty. Evidence regarding the use of nebulised MgSO₄ as an alternative to beta‐agonists is sparse and inconclusive. Given the wealth of evidence about the use of beta‐agonists in acute asthma it seems unlikely that future trials will address this question directly.
Implications for research.
Despite including 25 trials in this review update we were unable to pool data for all outcomes of interest and this has limited the strength of the conclusions reached. An agreement on the core outcomes for studies in acute asthma is needed so that any acute asthma study has the same outcomes measured — physiological, cost and those relevant to patients. This is particularly important in paediatric studies where lung function measurement may be more challenging.
Placebo‐controlled trials in patients not responding to standard maximal treatment, including inhaled β₂‐agonists and ipratropium bromide and systemic steroids, may help establish if nebulised MgSO₄ has a role in acute asthma, although the accumulating evidence suggests that a substantial benefit may be unlikely. Trials comparing the safety and efficacy of inhaled MgSO₄ to intravenous MgSO₄, including an economic evaluation, are also of interest.
What's new
| Date | Event | Description |
|---|---|---|
| 6 September 2017 | New citation required and conclusions have changed | Nine new trials with 2051 participants were added to the 896 (16 trials) in the previous version of the review. We re‐ordered the comparisons to reflect current asthma management. The evidence has been strengthened by the addition of several large well‐conducted trials. We are more confident that the treatment is likely to be well tolerated; however, there remains uncertainty about modest benefits for lung function and hospital admission when added to standard therapies. |
| 6 September 2017 | New search has been performed | New literature search run and incorporated. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 28 September 2012 | New citation required and conclusions have changed | Ten new trials with 600 participants added to the 296 in the previous version of the review. We added a new comparison of inhaled magnesium sulfate in addition to inhaled β2 ‐agonist and ipratropium bromide. The evidence remains inconclusive, but whilst there is no good evidence that inhaled magnesium sulfate can be used as a substitute for inhaled beta2‐agonists, there is a suggestion of benefit in pulmonary function when used in addition to inhaled beta2‐agonists (with or without ipratropium) in severe asthma exacerbations. |
| 28 September 2012 | New search has been performed | New literature search run. |
| 28 July 2008 | Amended | Converted to new review format. |
| 22 August 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The original version of this review in 2000 included the following acknowledgment: "The authors would like to acknowledge the assistance of Toby Lasserson and Liz Arnold of the Cochrane Airways Review Group. We would also like to acknowledge Dr. P Mahajan for providing the data from his in revision paper for inclusion into the review. Finally, the assistance of Dr. Chris Cates (Cochrane Airways Review Group Co‐ordinating Editor) was greatly appreciated."
The 2012 update of the review included the following acknowledgement: "The authors responsible for the update of this review would particularly like to acknowledge the excellent support and assistance from Emma Welsh, Liz Stovold, and Emma Jackson of the Cochrane Airways Review group, together with the greatly appreciated guidance from Chris Cates (Cochrane Airways Review Group Co‐ordinating Editor). We are grateful to Taixiang Wu, Alieksei Seniukovich and Sharon Kramer for kindly providing a translation of studies of potential relevance to the review. The support provided by librarians Judith Scammel, Jane Appleton and Hilary Garrett at St Georges University London is also greatly appreciated."
The 2017 update authors would like to thank Kerry Dwan and Richard Beasley for their contributions to the previous versions of the review. We would also like to acknowledge Chris Cates, Emma Dennett, Emma Jackson and Liz Stovold (members of the Cochrane Airways editorial team) for their support and advice. We thank Mohamed Badawy for providing additional details about Badawy 2014 and Sepideh Banava and Narges Khanjani for their excellent translations of Mohammedzadeh 2014.
Chris Cates was the Editor for this review and commented critically on the review.
The Background and Methods section of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| CENTRAL (The Cochrane Library) | Monthly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Appendix 2. Search strategies to identify relevant studies
Cochrane Airways Trials Register
#1 AST:MISC1 #2 MeSH DESCRIPTOR Asthma Explode All #3 asthma*:ti,ab #4 #1 or #2 or #3 #5 magnesium* #6 MgSO4 #7 #5 or #6 #8 #4 and #7
[In search line #1, MISC1 denotes the field in the record where the reference has been coded for condition, in this case, asthma]
ClinicalTrials.gov & WHO ICTRP
Search terms: magnesium and asthma
Study type: Interventional Studies
Appendix 3. Search methods for previous version of review (2005‐2012)
Electronic searches
The Cochrane Airways Groups "Asthma and Wheez* RCT" register was searched for the following terms: magnesium OR MgSO4 OR Mg OR MS OR magnesium sulfate or magnesium sulphate. The results of this search were screened to omit studies that clearly involved only intravenous or parenteral administration of magnesium.
In addition, searches were also conducted on the following computerized bibliographic databases: MEDLINE (1966‐2005), EMBASE (1988 to 2005), LILACS, Cochrane Clinical Trials Registry, Web of Science and Dissertation Abstracts.
Data and analyses
Comparison 1. MgSO4 + SABA + ipratropium versus SABA + ipratropium.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pulmonary function (% FEV1) | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | 3.28 [1.06, 5.49] |
| 1.1 90 minutes | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 8.57 [1.99, 15.15] |
| 1.2 120 minutes | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [0.25, 4.95] |
| 2 Pulmonary function % predicted PEF | 2 | 636 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐2.33, 2.42] |
| 3 Clinical severity scores (closest to 60 mins) | 2 | 1130 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.11, 0.12] |
| 3.1 Yung ASS at 60 minutes | 1 | 472 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.35, 0.02] |
| 3.2 Change in dyspnoea VAS at 60 minutes | 1 | 658 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.02, 0.28] |
| 4 Admission at first presentation | 4 | 1308 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.91, 1.00] |
| 4.1 Adults | 3 | 800 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.87, 1.03] |
| 4.2 Children | 1 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.92, 1.01] |
| 5 HDU/ITU admission | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Admission to HDU (adults) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Admission to ICU (adults) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Admission to PICU/HDU or intubation (children) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Readmission | 2 | 750 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.84, 3.87] |
| 7 Respiratory rate at 60 mins | 2 | 723 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.14, 1.53] |
| 8 Heart rate at 60 mins | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9 Systolic blood pressure at 60 mins | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10 Diastolic blood pressure at 60 mins | 1 | 674 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [0.29, 4.51] |
| 11 Serious adverse events (during admission) | 2 | 557 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.06, ‐0.00] |
| 11.1 Adults | 1 | 50 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.07, 0.07] |
| 11.2 Children | 1 | 507 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.06, ‐0.01] |
| 12 Any adverse event (during admission) | 2 | 1197 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 12.1 Adults | 1 | 690 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.02, 0.07] |
| 12.2 Children | 1 | 507 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.09, 0.05] |
| 13 Serious adverse events (within 30 days) | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14 Any adverse event (within 30 days) | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15 Adverse event: hypotension | 2 | 1197 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.01, 0.04] |
| 15.1 Adults | 1 | 690 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.01, 0.07] |
| 15.2 Children | 1 | 507 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 16 Adverse event: flushing | 2 | 1197 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.01, 0.01] |
| 16.1 Adults | 1 | 690 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.01, 0.02] |
| 16.2 Children | 1 | 507 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.01] |
1.2. Analysis.
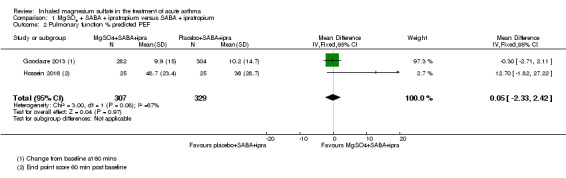
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 2 Pulmonary function % predicted PEF.
1.3. Analysis.
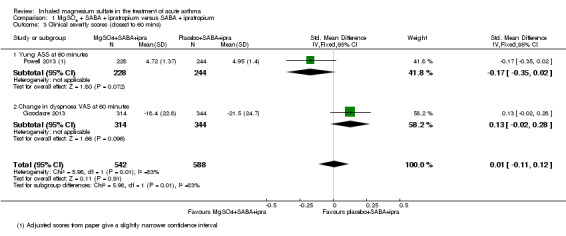
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 3 Clinical severity scores (closest to 60 mins).
1.7. Analysis.
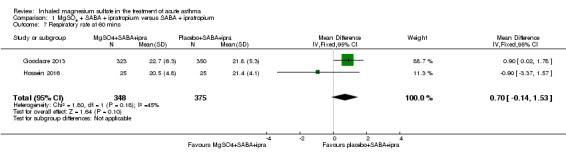
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 7 Respiratory rate at 60 mins.
1.8. Analysis.
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 8 Heart rate at 60 mins.
1.9. Analysis.
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 9 Systolic blood pressure at 60 mins.
Comparison 2. MgSO4 + SABA versus SABA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pulmonary function % predicted FEV1 | 4 | 208 | Mean Difference (IV, Fixed, 95% CI) | 3.34 [‐1.58, 8.26] |
| 1.1 Adults | 3 | 146 | Mean Difference (IV, Fixed, 95% CI) | 2.18 [‐3.30, 7.67] |
| 1.2 Children | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 8.10 [‐3.03, 19.23] |
| 2 % predicted FEV1: subgroup: severity | 3 | 188 | Mean Difference (IV, Fixed, 95% CI) | 4.12 [‐1.81, 10.06] |
| 2.1 Severe (FEV1 <50% predicted) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 9.90 [0.05, 19.75] |
| 2.2 Moderate | 2 | 136 | Mean Difference (IV, Fixed, 95% CI) | 0.84 [‐6.59, 8.27] |
| 3 Pulmonary function PEF L/min | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Adults | 3 | 155 | Mean Difference (IV, Fixed, 95% CI) | 11.91 [‐4.12, 27.95] |
| 3.2 Children | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 11.90 [‐6.86, 30.66] |
| 4 Admission to hospital | 6 | 375 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.52, 1.15] |
| 4.1 Adults | 4 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.45, 1.07] |
| 4.2 Children | 2 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.44, 2.98] |
| 5 Heart rate at 120 mins | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Respiratory rate at 120 mins | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 Diastolic blood pressure at 120 mins | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.72 [‐1.35, 2.80] |
| 8 Systolic blood pressure at 120 mins | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.89 [‐2.69, 4.48] |
| 9 Serious adverse events | 5 | 243 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.04, 0.04] |
| 9.1 Adults | 4 | 181 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.04, 0.04] |
| 9.2 Children | 1 | 62 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.06, 0.06] |
| 10 Any adverse events | 5 | 694 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 10.1 Adults | 4 | 329 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.10, 0.06] |
| 10.2 Children | 1 | 365 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.01] |
2.1. Analysis.
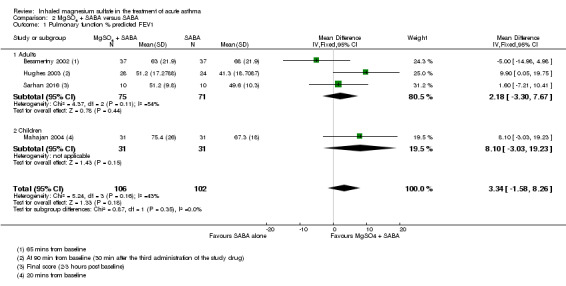
Comparison 2 MgSO4 + SABA versus SABA, Outcome 1 Pulmonary function % predicted FEV1.
2.2. Analysis.
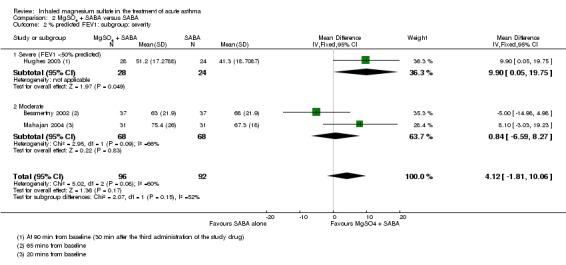
Comparison 2 MgSO4 + SABA versus SABA, Outcome 2 % predicted FEV1: subgroup: severity.
2.4. Analysis.
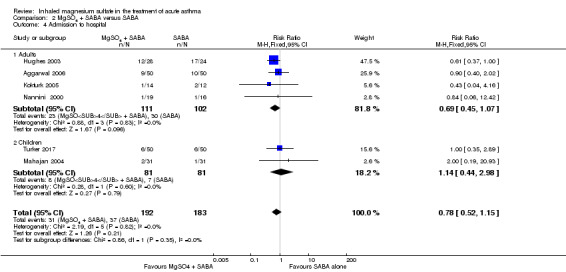
Comparison 2 MgSO4 + SABA versus SABA, Outcome 4 Admission to hospital.
2.6. Analysis.
Comparison 2 MgSO4 + SABA versus SABA, Outcome 6 Respiratory rate at 120 mins.
2.7. Analysis.
Comparison 2 MgSO4 + SABA versus SABA, Outcome 7 Diastolic blood pressure at 120 mins.
2.8. Analysis.
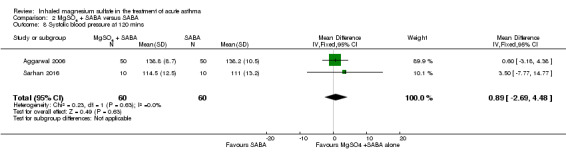
Comparison 2 MgSO4 + SABA versus SABA, Outcome 8 Systolic blood pressure at 120 mins.
Comparison 3. MgSO4 versus SABA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical severity score | 3 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.62, 0.36] |
| 1.1 Fischl index final score (120 mins) | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐1.07, 0.41] |
| 1.2 Fischl index score (time point unclear) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.11, 0.71] |
| 1.3 Change in Fischl index at 120 mins | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.67, 1.27] |
| 2 Admission to hospital | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Heart rate (120 mins) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 21.20 [0.17, 42.23] |
| 4 Respiratory rate | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐3.91, ‐0.89] |
| 5 Systolic pressure (120 mins) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Diastolic pressure (120 mins) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 Serious adverse events | 2 | 53 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.10, 0.10] |
| 8 Mild‐Moderate Side Effects | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.
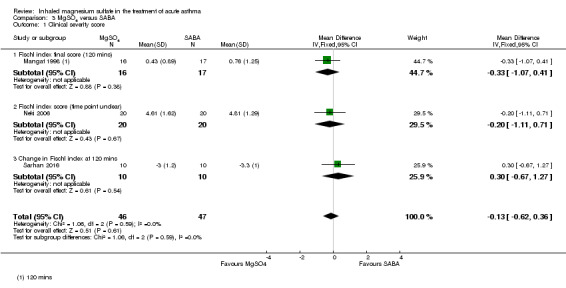
Comparison 3 MgSO4 versus SABA, Outcome 1 Clinical severity score.
3.3. Analysis.
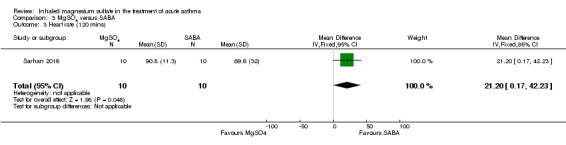
Comparison 3 MgSO4 versus SABA, Outcome 3 Heart rate (120 mins).
3.4. Analysis.
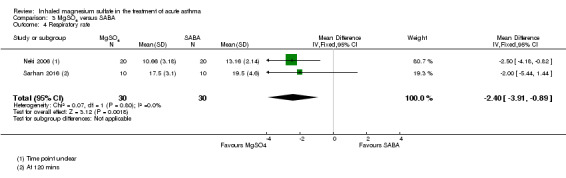
Comparison 3 MgSO4 versus SABA, Outcome 4 Respiratory rate.
3.7. Analysis.
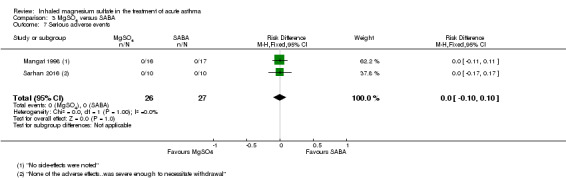
Comparison 3 MgSO4 versus SABA, Outcome 7 Serious adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abreu‐Gonzalez 2002.
| Methods | Randomised, controlled, double blind study, 2 groups. 1 centre in Tenerife. |
|
| Participants | 24 patients (Intervention 13, Control 11), adults, acute asthma, moderate obstruction. | |
| Interventions | Intervention: 2 mL of MgSO₄ (isotonic) dose and 400 mcg of salbutamol (delivery probably by MDI). Control: 2 mL of a physiological serum of an inhaled form, 400 mcg of salbutamol (delivery probably by MDI). Nebuliser: no details. |
|
| Outcomes | FEV1 and PEF at 0, 15, 30 45 minutes. | |
| Notes | Funding: Gobierno Autonomo Canarias. Abstract only. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details but stated as "randomised". |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only and not all time points reported. |
Aggarwal 2006.
| Methods | Double blind, randomised controlled trial, parallel. 1 emergency department in India. |
|
| Participants | Inclusion criteria: participants aged 13 to 60, BTS definition acute asthma (PEF and clinical features). Exclusion criteria: first episode of wheeze, chronic bronchitis or emphysema, heart failure, angina, renal failure, temperature > 38 ºC, ET tube required, no consent, pregnancy, failure to do peak flow. Intervention: 50 randomised. Mean age (years): 46.26 (13.96). Men:women: 27:23. Acute severe: 29. Acute life threatening: 21. Smokers: 9. Baseline PEF: 118.6 (41.3). Duration of attack; days (SD) 4.16 (1.69). Control: 50 randomised. Mean age (years): 41.00 (16.66). Men: women: 33:17. Acute severe: 30. Severe life threatening: 20. Smokers: 5. Baseline PEF: 111.6 (43.3). Duration of attack; days (SD) 4.28 (1.99). |
|
| Interventions | Intervention: MgSO₄ (1 mL of 500 mg/mL MgSO₄) and salbutamol (1 mL of salbutamol) 8 mL distilled water – 295 mOsmol/kg ×3 in an hour. Control: salbutamol 1 mL, 1.5 mL distilled water, 7.5 mL normal saline – 287 mOsmol/kg ×3 in an hour. Treatment over 1 h; 3 nebulisers 20 minutes apart. Follow‐up for 20 minutes. Ultrasonic nebuliser. |
|
| Outcomes | PEF, heart rate, systolic pressure, diastolic pressure, time in ED, blood gases (O₂ and CO₂— 0 and 120 minutes), magnesium levels (0 and 120 minutes). Time points 0, 15, 60, 75, 120 minutes. |
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Low risk | Separate envelopes to ensure concealment until inclusion (where they were kept and whether tamper proof — not mentioned). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The 2 researchers were blinded to the treatments so measurements (normal clinical outcomes) remained blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 50 participants both sides at beginning and 50 participants both sides completed the study with full outcome data. |
| Selective reporting (reporting bias) | Low risk | Follow‐up data and longer‐term outcome data not collected. No apparent indication of selective reporting. |
Ahmed 2013.
| Methods | Randomised open controlled trial. 1 hospital in Bangladesh. |
|
| Participants | Inclusion criteria: severe acute asthma. Exclusion criteria: none stated. 120 randomised. Intervention: 60 randomised. Control: 60 randomised. |
|
| Interventions | Intervention: salbutamol with MgSO₄. Control: salbutamol with normal saline. |
|
| Outcomes | PEF, respiratory rate, pulse rate, systolic, diastolic blood pressure, adverse effects. | |
| Notes | Funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details but states randomized. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear how many participants completed the trial or if any were excluded from analysis. |
| Selective reporting (reporting bias) | Unclear risk | Conference abstract. no prospective trial registration identified, no outcome measures pre‐specified. |
Alansari 2015.
| Methods | Double‐blind, randomised controlled trial. 1 Paediatric emergency centre, Qatar. |
|
| Participants | Inclusion criteria: moderate/severe asthma exacerbation, age 2‐14 years, previous diagnosis of asthma. Exclusion criteria: prematurity, critical illness needing ICU admission for IV bronchodilator, NIV or invasive ventilation, transfer to other institution, history of hypersensitivity to MgSO₄, history of neuromuscular/cardiac/renal disease, underlying structural lung disease, received systemic steroid/theophylline/ipratropium in prior 72 h, consolidation on chest XR, received IV MgSO₄ before randomisation, prior participation in the study, haemodynamic instability. Number randomised: 400. Intervention: 208 randomised. Mean age (years): 5.6 (3.1). Male:female: 133:75. Moderate:severe: 168:40. Mean baseline asthma severity score: 7.6 (1.3). Control: 192 randomised. Mean age (years): 5.8 (3.1). Male:female: 115:77. Moderate:severe: 163:29. Mean baseline asthma severity score: 7.5 (1.3). |
|
| Interventions | Intervention: 800 mg MgSO₄ (15 mL). Control: 15 mL 0.9% NaCl. Medication divided into 3 doses over 1 h. Jet nebuliser. |
|
| Outcomes | Time to medical readiness for discharge, mean asthma severity score (4, 8, 12, 24, 36, 48 h), mean asthma severity score at discharge, need for revisit or readmission (2 weeks). Adverse events: chest tightness and facial rash (1; intervention group). Excessive cough (1; control group). ICU admission (1; control group). |
|
| Notes | Funding: Hamad Medical Corporation; Number: 12095/12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation without blocks. |
| Allocation concealment (selection bias) | Low risk | Randomisation list provided to pharmacy resulted in preparation of identical‐appearing sealed numbered vials. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All study personnel were blinded to treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All study personnel were blinded to treatment. The paper was not explicit re. outcome assessors ‒ they were assumed to also have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Over 90% completed the trial in both arms. Balanced number were excluded from each arm, with reasons given. |
| Selective reporting (reporting bias) | Low risk | Prospective trial registration. All listed outcomes are reported. |
Ashtekar 2008.
| Methods | Parallel 1 Children’s Assessment Unit, 1 hospital (UHW). |
|
| Participants | Inclusion criteria: age range 2 to 16 years, acute severe asthma. Exclusion criteria: chronic lung disease, congenital heart disease, unable to understand English. 17 randomised (8 boys). Intervention: 7 completed. Control: 10 completed. |
|
| Interventions | Intervention: 2.5 mL isotonic MgSO₄ (3 occasions at 20‐minute intervals), salbutamol and ipratropium bromide. Control: 2.5 mL isotonic saline (3 occasions at 20‐minute intervals), salbutamol and ipratropium bromide. 3 dosages over 1 h: follow‐up for 240 minutes. |
|
| Outcomes | Asthma severity scores (ASS), the sum of wheeze, accessory muscle use and heart rate, were computed on 6 occasions over 4 h. The primary endpoint was the area under the curve of the ASS at the 6 time points for each child. | |
| Notes | Funding: local R and D pilot funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation by pharmacy at source ‒ in ED as sequential vials (code in pharmacy). |
| Allocation concealment (selection bias) | Low risk | As above – absolute concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial described as double blind: as above. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trial described as double blind: as above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data collected for the 17 patients. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. Outcomes partially reported. |
Badawy 2014.
| Methods | Randomised controlled trial. Outpatient department and Emergency department from 1 hospital, Egypt. |
|
| Participants | Inclusion criteria: pregnancy, acute exacerbation of asthma partially or not completely controlled on routine acute asthma therapy. Exclusion criteria: congestive heart failure, history of angina, renal problems, history suggestive of pulmonary oedema, very severe asthma (altered consciousness, respiratory acidosis, needing intubation, arrest), any associated medical illness e.g. diabetes/hypertension, fever > 38°C, inability to perform PEF. Number randomised: 60. All participants female. Intervention: 30 randomised. Mean age (years): 25.7 (3.8). Control: 30 randomised. Mean age (years): 25.9 (4.0). |
|
| Interventions | Intervention: 500 mg (1 mL) MgSO₄ with 1 mL salbutamol solution and 8 mL 0.9% NaCl. Control: 1 mL salbutamol solution with 9 mL 0.9% NaCl. Treatments given over 8 minutes; max 3 sets of nebulisation 20 minutes apart. |
|
| Outcomes | PEF, FEV1, FVC, FEV1/FVC ratio, FEF 25‐75%, arterial blood pCO2, pO2 and pH, oxygen saturations, serum potassium. Recorded at end of therapy ‒ assumed to be 2 h from baseline. | |
| Notes | Funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were randomly classified into groups comparable in socio‐demographic criteria, but no indication is given of random sequence generation. Baseline clinical characteristics are given, and there is no indication that the groups were balanced with regard to clinical criteria. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomized into 2 groups through sealed opaque envelopes, but no indication is given whether participants or research personnel were aware of group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were randomized into 2 groups through sealed opaque envelopes, but no mention of procedures to blind personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of procedures to blind personnel. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | On further correspondence, appropriate exclusion criteria were applied but no indication given whether excluded participants were balanced across groups, and no dropout data were given. |
| Selective reporting (reporting bias) | High risk | No prospective trial registration identified. Primary outcome on which this trial was powered is not stated. On further correspondence, adverse event data were given but no clinical baseline characteristics are given. |
Bessmertny 2002.
| Methods | Design: parallel randomised controlled trial. Method of randomisation: computer‐generated random numbers. Concealment of allocation: yes. Blinding: double‐blinded, placebo‐controlled. Withdrawals/dropouts: 6 (4 unable to complete spirometry, 2 inappropriate randomisation). | |
| Participants | Location: 1 university hospital in Brooklyn, NY. Participants: 74 patients, presenting to the emergency department with acute asthma exacerbation, PEF between 40% and 80% predicted. Exclusions: smoking history > 10 pack years, known hypersensitivity to albuterol or MgSO₄, known chronic obstructive pulmonary disease, known history of renal impairment, known history of cardiac dysrhythmias, congestive heart failure or angina, fever more than 38 °C, receipt of theophylline or anti‐cholinergic within 2 h of arrival to ED. | |
| Interventions | Treatment: albuterol 2.5 mg/3 mL nebule followed by 384 mg isotonic MgSO₄ every 20 min × 3. Control: albuterol 2.5 mg/3 mL nebule followed by normal saline every 20 min × 3. | |
| Outcomes | Measured FEV1 every 20 minutes for 2 h. Adverse events: no serious adverse events noted. | |
| Notes | Funding: supported by an unrestricted educational grant from Astra Pharmaceutical Company; no Astra Pharmaceutical Company products were used in the study. Mouthpieces for the spirometer were supplied at no charge from Mallinkrodt Nellcor Puritan Bennett. Circulaire nebulizers were supplied by Westmed Inc. at a reduced rate. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An assigned third party randomised participants by means of a computer‐generated random table (1:1 randomisation) to either the treatment or control group. |
| Allocation concealment (selection bias) | Low risk | An assigned third party randomised participants by means of a computer‐generated random table (1:1 randomisation) to either the treatment or control group. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded, placebo‐controlled. A log of the identification number and specific treatment of each participant was kept and remained closed to the investigators until the completion of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded, placebo‐controlled. A log of the identification number and specific treatment of each participant was kept and remained closed to the investigators until the completion of the study. Outcomes were assessed every 20 minutes for 2 h. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts: 3 in each group. Albuterol plus normal saline solution (3 unable to complete spirometry); and albuterol plus magnesium (2 inappropriate randomisation, 1 unable to perform spirometry). |
| Selective reporting (reporting bias) | High risk | Mean values only given for FEV1, no SDs and the text reports that there were no statistically significant differences in FEV1 between the groups. The text also states "The analysis of continuous safety variables (BP, pulse rate, respiratory rate, oxygen saturation, and serum magnesium concentrations) did not demonstrate any clinically or statistically significant differences between the 2 groups at any point during the study." |
Dadhich 2005.
| Methods | Random allocation into 3 groups parallel study. | |
| Participants | Location: 1 emergency department teaching hospital in India. Acute severe asthma , PEF < 50%. Group A = 24 Group B = 26 Group C = 21 |
|
| Interventions | Group A: salbutamol; Group B; salbutamol and MgSO₄; Group C MgSO₄ alone; no details on dose or frequency. | |
| Outcomes | FEV1, FVC, FEV1/FVC, PEF, "Vital parameters" |
|
| Notes | 2 abstracts only (the same). Funding: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only and no data reported except there was a significant improvement in groups B and C compared to group A. |
Drobina 2006.
| Methods | Parallel. | |
| Participants | A total of 110 participants. | |
| Interventions | Intervention: received the control treatment with the addition of 150 mg of MgSO₄ (0.3 mL of 50% MgSO₄ heptahydrate) to each nebulised dose of medication. Control: received nebulised treatments of albuterol sulfate 0.5% (5 mg/mL) combined with 0.5 mg of ipratropium bromide 0.02% inhalation solution (Atrovent). |
|
| Outcomes | Vital signs and peak flow measurements were also assessed at the end of each treatment (a maximum of 3 treatments) and just prior to discharge. A 24‐hour follow‐up call was made to each participant, during which peak flow measurements were again obtained. |
|
| Notes | Abstract only. Funding: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no detail. |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised but no detail. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double blind but no detail. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as double blind but no detail. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Very limited information ‒ impossible to judge. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. Vital signs are mentioned as being recorded but are not reported. |
Gallegos‐Solórzano 2010.
| Methods | RCT, parallel | |
| Participants | Inclusion criteria: adults, >18 years in the emergency dept with asthmatic crisis, FEV1 < 60% predicted. Exclusion criteria: smokers, those with ambulatory use of systemic steroids, with associated co‐morbidities (neuropathy, nephropathy, heart disease, liver disease), fever at admission, use of dietary supplements with MgSO₄, irreversible airway obstruction (persistent abnormal spirometry), near‐fatal asthma, requirement of endotracheal intubation at admission, anatomic abnormalities of the bronchial tree (bronchiectasis, tuberculosis), history of pulmonary or thoracic surgery, hypersensitivity to MgSO₄, and pregnancy or breastfeeding. Location: National Institute of Respiratory Diseases, a tertiary care teaching hospital and national referral centre in Mexico City. Date of study: June 2008 to March 2009. Intervention: 60 randomised, 30 completed. Mean age (years): 34.3 (12.4). Men:women: 9:21. Control: 52 randomised, 30 completed. Mean age (years): 40.3 (11.6). Men:women: 9:21. |
|
| Interventions | Each nebulisation lasted 20 mins. Intervention: standard nebulisation but diluted with 3 mL (333 mg) of 10% isotonic MgSO₄ (Magnefusin PISA, Guadalajara, Mexico; 1 g/10 mL). Also received 125 mg of IV methylprednisolone. Control: 1 IV dose of 125 mg methylprednisolone and nebulisation with 7.5 mg of albuterol and 1.5 mg of ipratropium bromide in 3 divided doses. Standard nebulisation diluted in 3 mL of isotonic saline solution (SS) as placebo. |
|
| Outcomes | FEV1 post‐BD (absolute in litres and as percentage of predicted), clinical improvement, oxygen saturation, admission to the ED, admission to the asthma ward, hospital readmissions. At 30‐min post‐nebulisation, patients were clinically and functionally re‐evaluated. Also evaluated at 30 days. |
|
| Notes | Funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. |
| Allocation concealment (selection bias) | Low risk | After randomisation, diluents were prepared by a physician outside the study who was not responsible for the participants’ care and only had control of the pre‐filled syringes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind. Both diluents are odourless, tasteless and colourless to the eye and did not differ when transparency was measured. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The physician responsible for the participants’ care along with the nurse and respiratory therapist were blinded to the type of treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons given for dropouts in both groups in the CONSORT diagram. It seems as though there are a high percentage of dropouts but the majority are post‐randomisation exclusions based on exclusion criteria. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the Methods section are reported. Best judgement with no access to trial protocol. |
Gaur 2008.
| Methods | Parallel RCT. | |
| Participants | Age: 18 to 60 years. Location: emergency department of a tertiary referral centre in India. Acute asthma and FEV1 < 30% predicted. Intervention: 30. Control: 30. |
|
| Interventions | Intervention: nebulised similarly using isotonic MgSO₄ (3 mL of 3.2 g%) as a vehicle ‒ unsure if this is “Nebulized salbutamol and ipratropium”. Control: nebulised salbutamol and ipratropium using isotonic saline as a vehicle thrice at 20‐min intervals. |
|
| Outcomes | FEV% predicted at 120 minutes, pooled discharge rate proportion of groups attaining PEF > 60% predicted and relief in dyspnoea at 30, 60, 90, 120 min). | |
| Notes | Abstract only. Funding: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single blind – no further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Single blind – no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | High risk | 1 outcome partially reported and not significant. Abstract only. |
Goodacre 2013.
| Methods | Double blind, randomised controlled trial. 34 emergency departments, UK. |
|
| Participants | Inclusion criteria: severe (BTS/SIGN quantified) asthma attack, age ≥16 years. Exclusion criteria: life‐threatening features, contraindication to MgSO₄, participant unable to give verbal/written consent, previous participation in the study; criteria amended to exclude those who had received MgSO₄ in the past 24 h. 1109 randomised. Intervention 1 (nebulised MgSO₄): 339 randomised. Mean age (years): 36.5 (14.8). Men:women: 107:232. Smokers: 98. Mean predicted PEF (L/min): 430 (118.8). Intervention 2 (intravenous MgSO₄): 406 randomised. Mean age (years): 35.6 (13.1). Men:women: 130:279. Smokers: 138. Mean predicted PEF (L/min): 431.8 (116.9). Control: 364 randomised. Mean age (years): 36.4 (14.1). Men:women: 112:252. Smokers: 127. Mean predicted PEF (L/min): 435.0 (110.8). |
|
| Interventions | Intervention 1: 100 mL 0.9% NaCl IV and 2 mmol MgSO₄ in 7.5 mL 0.9% NaCl nebulised. Intervention 2: 8 mmol MgSO₄ in 100 mL 0.9% NaCl IV and 7.5 mL 0.9% NaCl nebulised. Control: 100 mL 0.9% NaCl IV and 7.5 mL 0.9% NaCl nebulised. IV infusion given once over 20 mins, nebulisers given 3 times, each over 20 minutes. |
|
| Outcomes | Admission (4 h, 7 days); change in participant's assessment of breathlessness via visual analogue scale, change in PEF, heart rate, respiratory rate, BP, oxygen saturations (1, 2 h); adverse events (2 h); mortality, length of hospital stay, admission to HDU or ICU. Adverse events: treatment group 41 adverse events; control group 36 adverse events. |
|
| Notes | Funding: UK National Institute for Health Research Health Technology Assessment Programme. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple and blocked randomisation sequences used to allocate participants to numbered treatment packs. |
| Allocation concealment (selection bias) | Low risk | Allocated treatment pack numbers were only revealed after participant details recorded and the participant irreversibly entered into the trial. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, hospital staff, and research staff were masked to allocated treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, hospital staff, and research staff were masked to allocated treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Over 95% of randomised participants in each arm were included in primary analysis. All participants clearly accounted for in flow diagram. There was an inevitable 'drop off' in participants available at each time point for many of the secondary outcomes; it is unclear what impact this may have had on the results. |
| Selective reporting (reporting bias) | Low risk | Prospective trial registration identified. All primary and secondary outcomes listed in the trial register were reported. |
Hossein 2016.
| Methods | Double blind, randomised controlled trial. 2 emergency departments, Iran. |
|
| Participants | Inclusion criteria: moderate/severe asthma exacerbation defined by PEFR < 40% to 69% predicted or limiting speech/normal activity, age > 16 years. Exclusion criteria: need for immediate intubation, significant impairment of heart function, kidney or liver disease, fever > 38.3 °C, chronic lung disease, pregnancy, lactation, pneumonia. 50 randomised. Intervention: 25 randomised. Mean age (years): 52.4 (16.9). Men:women: 11:14. Acute moderate: 3. Acute severe: 22. Mean predicted PEF (%): 15.1 (4.7). Control: 25 randomised. Mean age (years): 53.9 (16.2). Men:women: 14:11. Acute moderate: 3. Acute severe: 21 Mean predicted PEF (%): 14.7 (6.4). |
|
| Interventions | Intervention: 3 mL MgSO₄ solution (260 mmol/L) nebulised. Control: 3 mL 0.9% NaCl nebulised. Nebulised medication given every 20 to 60 minutes. |
|
| Outcomes | Predicted PEFR (%), oxygen saturations, respiratory rate, dyspnoea severity index (20, 60 minutes); need for admission, serious side‐effect rate (60 minutes). Adverse effects: no "serious side‐effects" reported. |
|
| Notes | Funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation software used. |
| Allocation concealment (selection bias) | Unclear risk | Data not given. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both patients and investigators were blinded to the content of identical treatment vials. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Both patients and investigators were blinded to the content of identical treatment vials. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The report does not state that all 25 randomised participants in each arm completed the trial, but as the trial finished at 60 mins is it likely that they did. |
| Selective reporting (reporting bias) | Unclear risk | Trial registered while recruiting. Pre‐specified primary and secondary outcomes are reported; note omission of PEFR/dyspnoea scale reporting at 40 mins and no data given to support report of "no treatment‐related complications". Clear mistakes in reporting of vital signs. |
Hughes 2003.
| Methods | Design: parallel randomised controlled trial. Method of randomisation: unknown. Concealment of allocation: yes. Blinding: double‐blinded, placebo‐controlled. Withdrawals/dropouts: 6 (4 COPD, 2 pneumonia). | |
| Participants | Location: 2 university hospitals in New Zealand. Participants: 52 patients, presenting to the emergency department with acute asthma exacerbation, FEV1 < 50% predicted. Exclusions: known irreversible lung disease, pneumonia, pregnancy, significant renal/cardiac impairment, hypotension (sBP < 100 mmHg), required intubation. | |
| Interventions | Standard of care: salbutamol 2.5 mg nebulised ×1 or more, hydrocortisone 100 mg IV at presentation. Treatment: salbutamol 2.5 mg nebule with 2.5 mL isotonic MgSO₄ (250 mmol/L) every 30 min ×3. Control: salbutamol 2.5 mg nebule with 2.5 mL normal saline every 30 min ×3. Participants were unable to distinguish solutions. | |
| Outcomes | Measured at baseline and after each treatment (every 30 min ×3): FEV1, % predicted FEV1, BP, heart rate, O₂ saturation. Requirement for admission at 90 minutes. Adverse events: no serious adverse events noted. | |
| Notes | Funding: the study was funded by a research grant from the University of Otago. The study sponsor had no role in study design, data collection, data analysis, data interpretation, or writing of the report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to their treatment groups in accordance with the allocation sequence determined by the hospital pharmacy. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded, placebo‐controlled. Participants and investigators were unaware of treatment allocation through provision by the hospital pharmacy of pre‐prepared identical unmarked syringes containing the study drug. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded, placebo‐controlled. Participants and investigators were unaware of treatment allocation through provision by the hospital pharmacy of pre‐prepared identical unmarked syringes containing the study drug. Outcomes assessed every 30 minutes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 in total. MgSO₄ (1 COPD, 1 pneumonia). Saline (3 COPD, 1 pneumonia). |
| Selective reporting (reporting bias) | High risk | The primary outcome, FEV1, was fully reported but other outcomes were not. "The change in blood pressure and heart rate did not differ between the two groups. No clinically significant adverse events were reported." |
Khashabi 2008.
| Methods | Parallel RCT. | |
| Participants | Location: authors based in Iran. Participants: 40 asthmatic children in total between 2 groups. Mean age: 3.55 years. |
|
| Interventions | Intervention: nebulised salbutamol, as a vehicle isotonic MgSO₄ mixed with salbutamol. Control: nebulised salbutamol, as a vehicle 2.5 mL of normal saline. |
|
| Outcomes | Days of hospital stay, hours of need for oxygen, respiratory distress. Measured 1 h before and 1 h after the second course of treatment. |
|
| Notes | Abstract only. Funding: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly enrolled. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | Low risk | Outcomes stated as measured, reported. Abstract only. |
Kokturk 2005.
| Methods | Parallel RCT | |
| Participants | Inclusion criteria: moderate to severe asthma attacks, 18 to 60 years. Exclusion criteria: patients with febrile disease, diabetes, congestive heart failure, atherosclerotic heart disease, intractable hypertension, chronic obstructive lung disease, renal and hepatic failure and arrhythmia were excluded from the study. Pregnant and breast‐feeding women, patients who had already taken theophylline, antihistaminics, and systemic steroids in the previous 24 h, who had acute or chronic respiratory failure, who had been on long‐term oxygen therapy, and a history of allergy to salbutamol and MgSO₄ have been excluded as well. Location: emergency department, Turkey. Intervention: 14. Mean age: 46.43 (years) (3.31) range 18 to 3. Men:women: 4:10. Control: 12. Mean age: 37.83 (years) (9.26) range 20 to 52. Men:women: 3:9. |
|
| Interventions | Every 20 mins for first hour and every hour for the rest of 4 h. Intervention: isotonic MgSO₄ (2.5mL) + salbutamol (2.5 mL). Control: salbutamol (2.5 mL) + saline (2.5 mL). |
|
| Outcomes | PEF, clinical scores, discharge rates, admission rates. 20th, 60th, 120th, 180th, 240th minute (180 and 240 not compared as most patients completed study in 2 h). |
|
| Notes | Funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised ‒ details of sequence generation not included in trial report. |
| Allocation concealment (selection bias) | Unclear risk | Information not available in trial report. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single blind – no further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Single blind – no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information provided in trial report on discharges from both groups up to 240 minutes. |
| Selective reporting (reporting bias) | Low risk | No apparent indication of selective reporting. |
Mahajan 2004.
| Methods | Design: parallel randomised controlled trial. Method of randomisation: table of random numbers. Concealment of allocation: not stated. Blinding: double‐blinded, placebo‐controlled. Withdrawals/dropouts: none described. | |
| Participants | Location: 1 paediatric emergency department in Detroit, Michigan. Participants: 62 patients age 5 to 17, presenting to the emergency department with acute asthma exacerbation, FEV1 between 45% and 75% predicted. Exclusions: Fever (> 39 °C), chronic disease (bronchopulmonary dysplasia, cystic fibrosis), known allergy to albuterol or magnesium, received any of steroids, theophylline or ipratropium bromide in the prior 3 days. | |
| Interventions | Treatment: albuterol 2.5 mg nebule with 2.5 mL isotonic MgSO₄ (6.3% solution); 1 dose. Control: albuterol 2.5 mg nebule with 2.5 mL normal saline; 1 dose. Both groups received corticosteroids (2 mg/kg) after inhaled treatment. | |
| Outcomes | Lung function (FEV1 and % predicted FEV1) at baseline, then at 10 and 20 minutes after treatment. Also report vital signs and hospital admission rates. State that none of the patients showed any side effects. | |
| Notes | Funding: this work was funded by an unrestricted grant from the Division of Pediatric Emergency Medicine, Children’s Hospital of Michigan, Detroit, Michigan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A table of random numbers was used to provide randomisation and this was performed by a senior research pharmacist at the institution. |
| Allocation concealment (selection bias) | Low risk | A table of random numbers was used to provide randomisation and this was performed by a senior research pharmacist at the institution. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded, placebo controlled. The study medications were provided in identical syringes and both the pharmacy and the investigator were blinded to their contents. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded, placebo controlled. The study medications were provided in identical syringes and both the pharmacy and the investigator were blinded to their contents. Outcomes assessed at 10 and 20 minutes after treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None described. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section are reported. |
Mangat 1998.
| Methods | Design: parallel randomised controlled trial. Method of randomisation: unknown. Concealment of allocation: yes. Blinding: double‐blind, placebo‐controlled. Withdrawals/dropouts: 0. | |
| Participants | Location: emergency department, St John's Medical College Hospital, India. Screened: 63. Participants: 33, 12 to 60 years of age, known or newly diagnosed asthmatics with PEF < 300 L/min. Exclusions: patient enrolled at prior presentation, febrile, lower respiratory tract infection, history or evidence of cardiac/renal/hepatic dysfunction, pregnancy, requirement for ventilatory care, oral/parenteral bronchodilators within previous 6 h, steroids within previous 12 h. | |
| Interventions | Standard of care: hydrocortisone 100 mg IV. Treatment: MgSO₄ 3 mL (3.2% solution = 95 mg) nebulised every 20 min ×4. Control: salbutamol 3 mL (2.5 mg) nebulised every 20 min ×4. | |
| Outcomes | Clinical score: Fischl Index, clinical examination. Pulmonary function: PEF. Vitals: respiratory rate, heart rate, BP, pulsus paradoxus. Admission rates, vital signs. Adverse events/side effects:
|
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled. Outcomes assessed at 20 minute intervals. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None described. |
| Selective reporting (reporting bias) | Unclear risk | Pulsus paradoxus and BP are mentioned but not reported, but pulsus paradoxus is included as part of the Fischl index. |
Meral 1996.
| Methods | Design: randomised controlled trial. Method of randomisation: unknown. Concealment of allocation: unknown. Blinding: unknown. Withdrawals/dropouts: 0. | |
| Participants | Location: Department of Paediatric Asthma of Ege University Hospital, Turkey. Participants: 40 randomly selected and divided into 2 groups of 20. Mean ages 10.6 and 11 years of age. Previously diagnosed as asthmatic using ATS definitions; PEF decreased by ≥ 25%. Exclusions: medication within 12 h of study, cardiac/renal dysfunction. | |
| Interventions | Treatment: MgSO₄ 2 mL (280 mmol/L, 258 mOsm, pH 6.7). Control: salbutamol 2.5 mg in 2.5 mL. Administration: nebulised, inhaled over 10 to 15 minutes. | |
| Outcomes | Evaluations at: 5, 15, 30, 60, 180,240 and 360 minutes. Clinical score: Davis‐Leffert‐Dabbous respiratory distress score pulmonary function: PEF. Adverse reactions/side effects: none observed. | |
| Notes | Funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly selected for the study and divided into 2 groups. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None described. |
| Selective reporting (reporting bias) | High risk | No statistical differences were found between the groups for respiratory rate, heart rate and BP. It is also unclear as to the time point reported as although 5 minutes was prespecified, there were also several other time points specified and only the maximum values were presented. |
Mohammedzadeh 2014.
| Methods | Randomised controlled trial. 1 hospital, Iran. |
|
| Participants | Inclusion criteria: moderate to severe asthma (GINA‐defined) with acute attack. Exclusion criteria: corticosteroid therapy, steroid/theophylline/ipratropium in past 72 h, chronic lung disease e.g. bronchopulmonary dysplasia/CF, allergy to MgSO₄ or salbutamol, not co‐operative. 80 randomised. Intervention 1 (nebulised MgSO₄): 40 randomised. Mean age (years): 9 (2.2). Male:female: 10:30. Control: 40 randomised. Mean age (years): 8.5 (2.4) Male:female: 17:23. |
|
| Interventions | Intervention: 3 mL 7.5% MgSO₄, 0.15 mg/kg salbutamol. Control: 3 mL normal saline, 0.15 mg/kg salbutamol. 3 doses at 20 minute intervals. |
|
| Outcomes | Pulmonary index, PEFR, adjusted PEFR at 30, 60 and 90 minutes | |
| Notes | Funding: Babol University of Medical Sciences ‒ Research and Technology Institute. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "divided into two groups randomly" but no details given. |
| Allocation concealment (selection bias) | Unclear risk | Data not given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as "double‐blind" in prospective trial registration but no details given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as "double‐blind" in prospective trial registration but no details given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered; planned outcomes were fully reported. |
Nannini 2000.
| Methods | Design: randomised controlled trial. Method of randomisation: unknown. Concealment of allocation: yes. Blinding: double‐blind, placebo‐controlled. Solutions were pre‐packaged in identical appearing vials. Withdrawals/dropouts: 3 participants were enrolled more than once, only the initial visit was used in the analysis. | |
| Participants | Location: emergency departments in 4 Argentinian hospitals. Participants: 35 patients at least 18 years of age presenting to the emergency department with an acute asthma exacerbation who were able to have PEF measured were enrolled. (% predicted PEF: 38 + 18 in treatment group, 38 + 12 in control group). Exclusions: current smokers of ≥ 5 pack years, concurrent medical illness, pregnant, breast feeding, oral or parenteral steroids within the previous 7 days. | |
| Interventions | Standard of care: all patients received supplemental oxygen. If patient condition worsened patient may receive salbutamol 2.5 mg nebulised at discretion of physician. Treatment: 0.5 mL salbutamol (2.5 mg) diluted in 3 mL isotonic MgSO₄ (286 mOsm, 7.5% = 225 mg). Control: 0.5 mL salbutamol (2.5 mg) diluted in 3 mL normal saline. Administration: jet nebulised using oxygen at 10 L/min via mouthpiece until dry. | |
| Outcomes | Measurements made at baseline, 10 minutes after treatment and 20 minutes after treatment. Pulmonary functions: primary endpoint : % increase in peak flow = ((change/baseline) × 100). Other: peak flow (best of 3 attempts). Vital signs: respiratory rate, pulse rate, BP. Duration of emergency room care. No adverse events reported in either the experimental or control group. | |
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 patients were enrolled more than once, only the initial visit was used in the analysis but treatment group not stated. |
| Selective reporting (reporting bias) | High risk | There were no significant differences between the groups in changes in BP, heart rate, or respiratory rate at either 10 minutes or 20 minutes. |
Neki 2006.
| Methods | Parallel. | |
| Participants | Inclusion criteria: patients in age group of 15 to 60 years with severe bronchial asthma, as judged by Fischl index having PEF < 300 L/min or FEV in 1st second less than 40% of the predicted value were included in the study. Exclusion criteria: all patients who had received oral inhaler or parenteral bronchodilators in the past 6 h or steroid in the previous 12 h were excluded from the study. Adults and children with severe asthma (15 to 60 years) ‒ 40 participants. 30 female and 10 male but unclear how divided between groups. Intervention: 20 completed. Control: 20 completed. |
|
| Interventions | Intervention: given 4 doses of nebulised solution of "3.2G%" MgSO₄, 20 minutes apart. Control: received 4 doses of nebulised salbutamol (each dose of 3 mL containing 25 mg), 20 minutes apart. |
|
| Outcomes | PEF (L/min), respiratory rate, Fischl index and SaO₂. |
|
| Notes | Abstract only. Funding: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of random sequence generation not included in trial report. There is no reference to randomisation in trial report and trial not reported as randomised – seeking clarification from author. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not included in trial report. There is no reference to randomisation in trial report and trial not reported as randomised – seeking clarification from author. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | If the trial was not blinded, there is a strong likelihood that outcome assessment was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. No apparent indication of selective reporting. |
Powell 2013.
| Methods | Double blind, randomised controlled trial. 30 emergency departments or children's assessment units, UK. |
|
| Participants | Inclusion criteria: severe (BTS/SIGN quantified) asthma exacerbation after conventional treatment, age 2 to 16 years. Exclusion criteria: coexisting respiratory disease, severe renal disease, severe liver disease, known pregnancy, known previous reaction to magnesium, inability to give informed consent, previous randomisation into the trial, life‐threatening symptoms, current or previous (in the 3 months preceding screening) involvement with a trial of a medicinal product. 508 randomised. Intervention: 252 randomised. Median age (years): 4 (3 to 7). Male:female: 143:109. Control: 256 randomised. Median age (years): 4 (3 to 7). Male:female: 150:106. |
|
| Interventions | Intervention: 2.5 mL MgSO₄ (250 mmol/L) nebulised. Control: 2.5 mL isotonic saline nebulised. 3 doses given at roughly 20 minute intervals. |
|
| Outcomes | Mean Yung asthma severity score, treatment step‐down (60 minutes); length of stay, need for additional intravenous bronchodilator, admission to PICU/HDU or intubation, adverse events (until discharge). Adverse events: treatment group 47, control group 59 |
|
| Notes | Funding: National Institute of Health Research Health Technology Assessment Programme. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated blocked randomisation sequence stratified by centre was generated by an independent statistician who had no further involvement in the study. |
| Allocation concealment (selection bias) | Low risk | Treatment packs were identical in appearance and numbered sequentially for each centre. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants (patients, clinicians, research team, and statisticians) were masked to the treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The statistical analyses were completed with masked data, with treatment groups revealed only after final analyses had been completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Over 90% randomised participants in each arm were included in the adjusted primary analysis. All participants who withdrew or were excluded are clearly accounted for in the flow diagram. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered trial. All listed outcomes are reported. |
Sarhan 2016.
| Methods | Double blind, randomised controlled trial. Chest and emergency departments at 1 hospital, Egypt. |
|
| Participants | Inclusion criteria: diagnosis of asthma. Exclusion criteria: fever, lower respiratory tract infection, cardiac/renal/hepatic dysfunction, needed NIV/intubation, near‐fatal asthma, pregnancy, lactation, failed to use PEF meter, inhaled/oral/intravenous bronchodilator use within past 6 h or steroid use within past 12 h. 30 randomised. Intervention 1 (magnesium): 10 randomised. Mean age (years): 33.5 (17.8). Men:women: 4:6. Mean % of predicted PEF at presentation: 33.9 (9.8). Intervention 2 (salbutamol and placebo) : 10 randomised. Mean age (years): 48.6 (9.9). Men:women: 3:7. Mean % of predicted PEF at presentation: 36.4 (10.5). Intervention 3 (salbutamol and magnesium): 10 randomised. Mean age (years): 51.3 (15.8). Men:women: 7:3. Mean % of predicted PEF at presentation: 34.1 (9.4). |
|
| Interventions | Intervention 1: 3 mL MgSO₄ (3.3% solution) nebulised. Intervention 2: 0.5 mL salbutamol (0.5% solution) in 2.5 mL isotonic saline nebulised. Intervention 3: 0.5 mL salbutamol (0.5% solution) in 2.5 mL MgSO₄ (4% solution) nebulised. 4 doses given at 20 minute intervals. Ultrasonic nebuliser. |
|
| Outcomes | PEF improvement, respiratory rate, heart rate, blood pressure, oxygen saturations, improvement in Fischl index of clinical severity, adverse event rate (all at "final" time point, assumed to be 2 h). Adverse events: no events "severe enough to warrant withdrawal" reported. |
|
| Notes | Funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reports patients were randomised into 3 groups but no details given. |
| Allocation concealment (selection bias) | Unclear risk | Data not given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as "double blind" but no details given about who was blinded or how. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as "double blind" but no details given about who was blinded or how. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The report does not specify how many randomised participants completed the trial. |
| Selective reporting (reporting bias) | Unclear risk | No prospective trial registration identified. Primary and secondary outcomes not defined. No power calculation reported. |
Turker 2017.
| Methods | Double blind, randomised controlled trial. 1 emergency department, Turkey |
|
| Participants | Inclusion criteria: children aged 3 to 15 years with asthma admitted to the emergency department due to a moderate asthma exacerbation. Exclusion criteria: any associated chronic diseases such as cystic fibrosi and bronchiectasis. 100 randomised. Intervention: 50 randomised. Mean age, months (SD): 76.06 (27.33). Male:female: 25:25. Median (IQR) modified pulmonary index score at presentation 8 (7‐8). Control: 50 randomised. Mean age, months (SD): 74.96 (33.65). Male:female: 29/21. Median (IQR) modified pulmonary index score at presentation 7 (7 to 9). |
|
| Interventions | Intervention: nebulised salbutamol (0.15 mg/kg) + 1 mL magnesium sulfate (15%) + 1.5 mL isotonic saline. Control: nebulised salbutamol (0.15 mg/kg) + 1.5 mL isotonic saline. 3 doses given at 20 min intervals. |
|
| Outcomes | Primary outcome: Modified Pulmonary Index Score (MPIS); secondary outcomes: hospitalisation rates, symptoms of magnesium imbalance such as nausea, vomiting, abdominal pain, chest pain, headache, fatigue, hypotension and fever. | |
| Notes | Funding: "this research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were assigned consecutively to the control or intervention group based on a stratified randomisation procedure" but no further detail about how the randomisation sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Data not given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as "double‐blind" but no details of who was blinded and the blinding procedure. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as "double‐blind" but no details of who was blinded and the blinding procedure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All patients enrolled in the study completed it". |
| Selective reporting (reporting bias) | High risk | No trial registration or prospective protocol identified. Adverse events reported as: "no side effect caused by magnesium was observed in any of the patients in the study". Modified pulmonary index score reported numerically at 120 minutes only; other time points presented graphically with no measure of variance |
ASS: Asthma Severity Score (ASS) ATS: American Thoracic Society BP: blood pressure BTS: British Thoracic Society COPD: Chronic obstructive pulmonary disease ED: emergency department FEV1: Forced expiratory volume in 1 second FVC: Forced vital capacity h: hour(s) IV: intravenous MDI: metered dose inhaler MgSO₄: magnesium sulfate PEF: Peak Expiratory Flow Rate R&D: research and development sBP: systolic blood pressure SD: standard deviation SIGN: Scottish Intercollegiate Guidelines Network
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Balter 1989 | Review |
| Bede 2003 | Oral supplementation in chronic asthma |
| Bede 2004 | Oral supplementation in chronic asthma |
| Bede 2008 | Oral supplementation in chronic asthma |
| Bernstein 1995 | Study does not assess people with acute asthma |
| Cairns 1996 | Study does not assess people with acute asthma |
| Castillo Rueda 1991 | Letter to the Editor |
| Chande 1992 | Study of stable asthma and methacholine challenge tests |
| Corbridge 1995 | Review |
| DiGregorio 1999 | Not a randomised controlled study |
| Emelyanov 1997 | Study not a randomised trial and in mild‐to‐moderate persistent asthma rather than acute asthma |
| Emelyanov 1990 | Not a randomised controlled trial |
| Emelyanov 1996 | Exercise induced bronchospasm and challenge test. Not a randomised controlled trial. |
| Fathi 2014 | Oral supplementation in chronic asthma |
| Fedoseev 1991 | Study does not assess people with acute asthma and is not a randomised controlled trial |
| Gandia 2012 | Study of stable asthma and methacholine challenge tests |
| Gurkan 1999 | Randomised controlled trial of intravenous MgSO4 |
| Harari 1998 | Review |
| Hardin 2001 | Review |
| Harmanci 1996 | Stable asthma histamine‐induced bronchospasm adults |
| Hill 1995 | Study does not assess people with acute asthma. Dose response study in 20 normal individuals and 19 with chronic asthma. |
| Hill 1997a | Study does not assess people with acute asthma. Stable asthma histamine challenge tests. |
| Hill 1997b | Stable adult asthma with histamine challenges |
| Irazuzta 2014 | Randomised controlled trial of intravenous MgSO₄ |
| Irazuzta 2016 | Randomised controlled trial of intravenous MgSO₄ |
| Kenyon 2001 | Review |
| Kreutzer 2001 | Review |
| Manzke 1990 | Paediatric exercise‐induced bronchospasm. Not a randomised controlled trial. |
| McFadden 1995 | Review |
| Nannini 1997 | Study does not assess people with acute asthma |
| Nunez‐Torres 1995 | Not a randomised controlled trial |
| Pelton 1998 | Study does not assess people with acute asthma |
| Pelton 1999 | Review |
| Petrov 2014 | Oral supplementation in uncontrolled and partly controlled atopic asthma |
| Puente‐Maestu 1999 | Review |
| Qureshi 1999 | Review |
| Rodger 2003 | Oral supplementation on people with unstable asthma |
| Rodrigo 2000 | Systematic review, includes intravenous MgSO₄ |
| Rolla 1987a | Study does not assess people with acute asthma |
| Rolla 1987b | Study does not assess people with acute asthma |
| Rolla 1988a | Study does not assess people with acute asthma |
| Rolla 1988b | Letter to the editor |
| Scarfone 1998 | Randomised controlled trial of intravenous MgSO₄ |
| Scarfone 2000 | Intravenous MgSO₄ |
| Shishimorov 2015 | Oral supplementation in children with uncontrolled asthma |
| Singh 2008a | Intravenous MgSO₄ |
| Singh 2008b | Comparison between inhaled versus intravenous MgSO₄ |
| Singhi 2014 | Randomised controlled trial of intravenous MgSO₄ |
| Sinitsina 1991 | Not a randomised controlled trial |
| Skobeloff 1982 | Editorial |
| Sun 2014 | Study of stable asthma and methacholine challenge tests |
| Talukdar 2005 | Not a randomised controlled trial |
| Teeter 1999 | Review |
| Telia 2005 | Study does not assess people with acute asthma |
| Tereshchenko 2006 | Looking at ipratropium bromide mixed with either MgSO₄ or saline for bronchiolitis (up to age 11.5 months) |
| Tetikkurt 1992 | Study does not assess people with acute asthma |
| Tetikkurt 1993 | Study does not assess people with acute asthma |
| Torres 2012 | Randomised controlled trial of intravenous MgSO₄ |
| Watanatham 2015 | Randomised controlled trial of intravenous versus nebulised MgSO₄ |
| Wijetunge 2002 | No response to attempts made to contact first author from 2002 to 2012. First author sadly died in 2014. |
| Wongwaree 2017 | Randomised controlled trial of nebulized magnesium sulfate versus ipratropium bromide/fenoterol in children with severe asthma exacerbation |
| Xu 2002 | Not a randomised controlled trial |
| Yemelyanov 1997 | Study does not assess people with acute asthma |
| Zandsteeg 2009 | Study does not assess people with acute asthma (stable chronic asthma) and is not a randomised controlled trial |
| Zhu 2003 | Intravenous MgSO₄ and not a randomised controlled trial |
MgSO₄: magnesium sulfate
Characteristics of studies awaiting assessment [ordered by study ID]
Abd 1997.
| Methods | "Ventilatory, cardiovascular and metabolic responses to salbutamol, ipratropium bromide and magnesium sulfate in bronchial asthma: comparative study" |
| Participants | No details |
| Interventions | No details |
| Outcomes | No details |
| Notes | Full‐text unobtainable |
Bustamante 2000.
| Methods | "Inhaled magnesium sulfate as adjunct therapy for moderate to severe asthma exacerbations, a randomized control clinical trial" |
| Participants | No details |
| Interventions | No details |
| Outcomes | No details |
| Notes | Full‐text unobtainable |
ISRCTN61336225.
| Methods | Prospective double‐blind placebo controlled trial |
| Participants | Children diagnosed as asthmatic according to The Global Initiative for Asthma (GINA) guidelines, aged 5 to 14 years old, capable of measuring PEFR, presenting with moderate to severe acute exacerbation according to paediatric asthma severity score and PEFR |
| Interventions | Group A: participants receive inhaled salbutamol solution (0.15 mL/kg) plus isotonic magnesium sulfate (2 mL) in a nebulizer chamber; Group B: participants receive inhaled salbutamol solution (0.15 mL/kg), diluted with placebo (normal saline 2 mL) in a nebulizer chamber. |
| Outcomes | 1. Asthma severity measured using the Pediatric Asthma Severity Score (PASS) at baseline, 20, 40 and 60 minutes post‐nebulisation 2. Oxygen saturation measured using pulse oximetry at baseline, 20, 40 and 60 minutes post‐nebulisation 3. Lung function assessed through measuring peak expiratory flow rate (PEFR) at baseline, 20, 40 and 60 minutes post‐nebulisation |
| Notes | Trial stated as complete February 2016 but no associated publication identified. Contact person emailed on 7 September 2017 to enquire about status of results/publication. No response received at time of review publication. |
Characteristics of ongoing studies [ordered by study ID]
Motamed 2015.
| Trial name or title | Comparison of clinical and spirometric response between nebulized salbutamol, MgSO₄ and nebulized salbutamol alone in acute asthma attack |
| Methods | Randomized, double‐blind controlled trial 1 hospital, Iran |
| Participants | Inclusion criteria: having a history of asthma, a minimum 18 years and maximum 65 years Exclusion criteria: COPD; kidney disease; CHF; pneumonitis; underlying respiratory disease 146 randomised |
| Interventions | Intervention: nebulized MgSO₄ 1/5 mL (20 g / 100 mL) with salbutamol 2/5 mL Control: normal saline with nebulized salbutamol 2/5 mL |
| Outcomes | Clinical state, FEV1, PEFR |
| Starting date | 22 March 2014 |
| Contact information | Hasan_motamed@yahoo.com |
| Notes |
Saucedo 2015.
| Trial name or title | Nebulized Magnesium Sulfate as an Adjunct to Standard Therapy in Asthma Exacerbation |
| Methods | Randomized, double‐blind controlled trial 1 paediatric emergency department, Mexico |
| Participants | Inclusion criteria: clinical history of asthma, clinical diagnosis of moderate or severe asthma exacerbations, age 2 to 15 years Exclusion criteria: coexistence of lung disease, severe kidney or liver disease, pregnancy, previous reaction to magnesium, no parental consent, prior inclusion in this study, presence of life‐threatening co‐morbidities, need for advanced airway management, life‐threatening symptoms. Estimated enrolment: 152 |
| Interventions | Intervention: nebulized salbutamol 2.5 mg (2 to 5 years) or 5 mg (≥ 6 years) and ipratropium bromide 250 mcg mixed with 2.5 mL of isotonic MgSO₄ (150 mg) per dose every 20 minutes during the first hour, continued with nebulized standard treatment every hour for 4 h, plus IV methylprednisolone or PO prednisolone 2 mg/kg/day for each treatment. Control: nebulized salbutamol 2.5 mg (2‐5 years) or 5 mg (≥ 6 years) and ipratropium bromide 250 mcg mixed with 2.5 mL of isotonic saline per dose every 20 minutes during the first hour, continued with nebulized standard treatment every hour for 4 h, plus IV methylprednisolone or PO prednisolone 2 mg/kg/day for each treatment. |
| Outcomes | Primary outcome measure: change from Baseline Preschool Respiratory Assessment Measure (PRAM) at 20, 40, 60, 120, 180 and 240 minutes after beginning treatment. Secondary outcome measures: rate of hospitalisation at 4 h, change from baseline heart rate, respiratory rate and blood pressure at 20, 40, 60, 120, 180 and 240 minutes after beginning treatment. |
| Starting date | September 2015 |
| Contact information | abisaipec@msn.com |
| Notes | Estimated study completion date: January 2018 |
Schuh 2016a.
| Trial name or title | Magnesium nebulization utilization in management of paediatric asthma (MagNUM PA) trial: study protocol for a randomized controlled trial |
| Methods | Randomized double‐blind controlled trial in 7 Canadian paediatric emergency departments |
| Participants | The trial will include 816 otherwise healthy children who are 2 to 17 years old, having had at least 1 previous wheezing episode, have received systemic corticosteroids, and have a Pediatric Respiratory Assessment Measure (PRAM) ≥ 5 points after 3 salbutamol and ipratropium treatments for a current acute asthma exacerbation. |
| Interventions | 3 doses nebulized salbutamol with either 600 mg MgSO₄ or placebo 20 min apart. |
| Outcomes | Primary outcome: hospitalisation within 24 h of the start of the experimental therapy for persistent respiratory distress or supplemental oxygen. Secondary outcomes include all‐cause hospitalisation within 24 h, PRAM, vital signs, number of bronchodilator treatments by 240 min, association between the difference in the primary outcome between the groups, age, gender, baseline PRAM, atopy, and “viral induced wheeze” phenotype. |
| Starting date | November 2014 |
| Contact information | Suzanne Schuh: Suzanne.schuh@sickkids.ca Division of Paediatric Emergency Medicine, The Hospital for Sick Children, Child Health Evaluative Sciences, SickKids Research Institute, University of Toronto, 555 University Avenue, Toronto, ON M5G 1X8, Canada |
| Notes | Estimated completed: December 2017 |
RCT: randomised controlled trial
Differences between protocol and review
For the 2012 update the 'Risk of bias' tool has been updated to that advised in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Three new review authors were added and the sensitivity analyses have been amended to investigating risk of bias rather than methodological quality.
In 2017 the following changes were made.
Background, Results and Discussion substantially re‐drafted.
Comparisons re‐ordered to reflect current clinical practice.
We chose to present outcomes at, or as close to as possible, 60 minutes from baseline. This time point was decided by consensus.
We performed a post‐hoc sensitivity analysis excluding trials with unusually small standard deviations.
We chose to exclude one study from the meta‐analyses due to concerns about baseline imbalance and the narrow population recruited (pregnant women only) (Badawy 2014).
We added a 'Summary of findings' table.
Two review authors stepped down (Richard Beasley and Kerry Dwan) and two new authors were added (Rachel Knightly and Rebecca Normansell).
Contributions of authors
For the 2017 update, RK and RN identified the included studies, extracted data, assessed risk of bias and analysed the data. RN and SM performed the GRADE assessments. RK and RN drafted the manuscript CP provided advice. All authors read and approved the final version for publication.
Sources of support
Internal sources
Department of Emergency Medicine, University of Alberta, Edmonton, AB, Canada.
National Institute of Health Research (SJM), UK.
External sources
Alberta Cancer Board, Canada.
Canadian Institutes of Health Research (CIHR), Ottawa (BHR), Canada.
Canadian Institutes of Health Research (CIHR), Ottawa, ON (BH Rowe),, Canada.
Declarations of interest
Drs. Hughes and Beasley were involved as Primary and Co‐investigator on one of the trials included in this review (Hughes 2003). Dr Powell was a co‐author of the pilot work completed in Ashtekar 2008 and was the chief investigator of the MAGNETIC study in children (Powell 2013). Dr Powell was not involved in the selection of studies for inclusion, data extraction, risk of bias assessment or GRADE assessments.
None of the other review authors has any known conflict of interest.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abreu‐Gonzalez 2002 {published data only}
- Abreu‐Gonzalez J, Rodríguez‐Díaz CY. Magnesium and bronchodilator effect of beta‐adrenergic. American Journal of Respiratory and Critical Care Medicine 2002;165:A185. [Google Scholar]
Aggarwal 2006 {published data only}
- Aggarwal P, Dwivedi S, Handa R. Nebulized magnesium sulfate and salbutamol combination compared to salbutamol alone in the treatment of acute bronchial asthma: a randomized study. Annals of Emergency Medicine 2004;44(4 Suppl):S38. [Google Scholar]
- Aggarwal P, Sharad S, Handa R, Dwiwedi SN, Irshad M. Comparison of nebulised magnesium sulphate and salbutamol combined with salbutamol alone in the treatment of acute bronchial asthma: a randomised study. Emergency Medicine Journal 2006;23(5):358‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahmed 2013 {published data only}
- Ahmed S, Sutradhar S, Miah A, Bari M, Hasan M, Alam M, et al. Comparison of salbutamol with normal saline and salbutamol with magnesium sulphate in the treatment of severe acute asthma. Mymensingh Medical Journal : MMJ 2013;22(1):1‐7. [PubMed] [Google Scholar]
Alansari 2015 {published data only}
- Alansari K, Ahmed W, Davidson B, Alamri M, Zakaria I. Nebulized magnesium for moderate and severe pediatric asthma: a randomized trial. American Journal of Respiratory and Critical Care Medicine 2015;191:A2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alansari K, Ahmed W, Davidson B, Alamri M, Zakaria I, Alrifaai M. Nebulized magnesium for moderate and severe pediatric asthma: a randomized trial. Pediatric Pulmonology 2015;50(12):1191‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed H, Al‐Ansari K. Inhaled magnesium for moderate and severe paediatric asthma. Archives of Disease in Childhood 2014;99(Suppl 2):A101‐2. [Google Scholar]
Ashtekar 2008 {published data only}
- Ashtekar CS, Powell C, Hood K, Doull I. Magnesium nebuliser trial (magnet): a randomised double‐blind placebo controlled pilot study in severe acute asthma. Archives of Disease in Childhood 2008;93:A100‐6. [Google Scholar]
Badawy 2014 {published data only}
- Badawy M, Hasannen I. The value of magnesium sulfate nebulization in treatment of acute bronchial asthma during pregnancy. Egyptian Journal of Chest Diseases and Tuberculosis 2014;63(2):285‐9. [Google Scholar]
- Badawy M, Hasannen I. The value of magnesium sulfate nebulization in treatment of acute bronchial asthma during pregnancy. European Respiratory Journal 2012;40(Suppl 56):1789. [Google Scholar]
Bessmertny 2002 {published data only}
- Bessmertny O, DiGregorio RV, Cohen H, Becker E, Looney D, Golden J, et al. A randomized clinical trial of nebulized magnesium sulfate in addition to albuterol in the treatment of acute mild‐to‐moderate asthma exacerbations in adults. Annals of Emergency Medicine 2002;39(6):585‐91. [DOI] [PubMed] [Google Scholar]
Dadhich 2005 {published data only}
- Dadhich P, Tailor M, Gupta ML, Gupta R. Magnesium sulphate nebulisation in acute severe asthma [Abstract]. Indian Journal of Allergy, Asthma and Immunology 2005;19:117. [Google Scholar]
- Dadhich P, Vats M, Lokendra D, Gupta RC, Gupta ML, Gupta N. Magnesium sulphate nebulization in acute severe asthma. Chest 2003;124(4):107S. [Google Scholar]
Drobina 2006 {published data only}
- Drobina BJ, Kostic MA, Roos JA. Nebulized magnesium has no benefit in the treatment of acute asthma in the emergency department. Academic Emergency Medicine 2006; Vol. 13, issue 5 Suppl. 1:S26.
Gallegos‐Solórzano 2010 {published data only}
- Gallegos‐Solórzano MC, Pérez‐Padilla R, Hernández‐Zenteno RJ. Usefulness of inhaled magnesium sulfate in the coadjuvant management of severe asthma crisis in an emergency department. Pulmonary Pharmacology and Therapeutics 2010;23(5):432‐7. [DOI] [PubMed] [Google Scholar]
Gaur 2008 {published data only}
- Gaur SN, Singh A, Kumar R. Evaluating role of inhaled magnesium sulphate as an adjunct to salbutamol and ipratropium in severe acute asthma [Abstract]. Chest 2008;134(4_MeetingAbstracts):91003. [DOI: 10.1378/chest.134.4_MeetingAbstracts.p91003] [DOI] [Google Scholar]
Goodacre 2013 {published data only}
- Goodacre S. The 3Mg Study: A randomised trial of intravenous or nebulised magnesium sulphate versus placebo for acute severe asthma. ISCRTN Register 2007. [DOI] [PMC free article] [PubMed]
- Goodacre S, Bradburn M, Cohen J, Gray A, Benger J, Coats T. Prediction of unsuccessful treatment in patients with severe acute asthma. Emergency Medicine Journal 2014;31(e1):e40‐5. [DOI: 10.1136/emermed-2013-203046] [DOI] [PubMed] [Google Scholar]
- Goodacre S, Cohen J, Bradburn M, Gray A, Benger J, Coats T, 3Mg Research Team. Intravenous or nebulised magnesium sulphate versus standard therapy for severe acute asthma (3Mg trial): a double‐blind, randomised controlled trial. Lancet: Respiratory Medicine 2013;1(4):293‐300. [DOI: 10.1016/S2213-2600(13)70070-5] [DOI] [PubMed] [Google Scholar]
- Goodacre S, Cohen J, Bradburn M, Stevens J, Gray A, Benger J, et al. The 3Mg trial: a randomised controlled trial of intravenous or nebulised magnesium sulphate versus placebo in adults with acute severe asthma. Health Technology Assessment 2014;18(22):1‐168. [DOI: 10.3310/hta18220] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN04417063. Magnesium sulphate for treatment of severe acute asthma. isrctn.com/ISRCTN04417063 (first received 26 February 2007). [DOI: 10.1186/ISRCTN04417063] [DOI]
Hossein 2016 {published data only}
- Hossein S, Pegah A, Davood F, Said A, Babak M, Mani M, et al. The effect of nebulized magnesium sulfate in the treatment of moderate to severe asthma attacks: a randomized clinical trial. American Journal of Emergency Medicine 2016;34(5):883‐6. [DOI] [PubMed] [Google Scholar]
Hughes 2003 {published and unpublished data}
- Hughes RJ, Goldkorn AL, Masoli M, Weatherall M, Brugess C, Beasley CRW. The use of nebulised isotonic magnesium sulphate as an adjuvant to salbutamol in the treatment of asthma in adults. Thorax 2002;57:iii10. [DOI] [PubMed] [Google Scholar]
- Hughes RJ, Goldkorn AL, Masoli M, Weatherall M, Burgess C, Beasley CR. Use of isotonic nebulized magnesium as an adjuvant to salbutamol in treatment of severe asthma in adults: randomised placebo‐controlled trial. Lancet 2003;361(9375):2114‐7. [DOI] [PubMed] [Google Scholar]
- Hughes RJ, Goldkorn AL, Masoli M, Weatherall M, Burgess C, Beasley R. The use of isotonic nebulised magnesium as an adjuvant to salbutamol in the treatment of severe asthma. American Journal of Respiratory and Critical Care Medicine 2002; Vol. 165:A186.
Khashabi 2008 {published data only}
- Khashabi J, Asadolahi S, Karamiyar M, Salari Lak S. Comparison of magnesium sulfate to normal saline as a vehicle for nebulized salbutamol in children with acute asthma: a clinical trial [Abstract]. European Respiratory Society 18th Annual Congress; 2008 Oct 3‐7; Berlin. 2008:[4597].
Kokturk 2005 {published data only}
- Kokturk N, Turktas H, Kara P, Mullaoglu S, Yilmaz F, Karamercan A. A randomized clinical trial of magnesium sulphate as a vehicle for nebulized salbutamol in the treatment of moderate to severe asthma attacks. Pulmonary Pharmacology and Therapeutics 2005;18(6):416‐21. [DOI] [PubMed] [Google Scholar]
- Turktas H, Kokturk N, Kara P, Mullaoglu SB, Yilmaz F, Karamercan A. A randomized clinical trial of magnesium sulphate as a vehicle for nebulized salbutamol in the treatment of moderate to severe asthma attacks [Abstract]. European Respiratory Journal 2004; Vol. 24:343s. [DOI] [PubMed]
Mahajan 2004 {published and unpublished data}
- Mahajan P, Haritos D, Rosenberg N, Thomas R. Comparison of nebulized magnesium plus albuterol to nebulized albuterol plus saline in children with mild to moderate asthma. Journal of Emergency Medicine 2004;27(1):21‐5. [DOI] [PubMed] [Google Scholar]
Mangat 1998 {published data only}
- Mangat HS, D'Souza GA, Jacob MS. Nebulized magnesium sulphate versus nebulized salbutamol in acute bronchial asthma: a clinical trial. European Respiratory Journal 1998;12(2):341‐4. [DOI] [PubMed] [Google Scholar]
Meral 1996 {published data only}
- Meral A, Coker M, Tanac R. Inhalation therapy with magnesium sulfate and salbutamol in bronchial asthma. Turkish Journal of Pediatrics 1996;38(2):169‐75. [PubMed] [Google Scholar]
Mohammedzadeh 2014 {published data only}
- Mohammedzadeh I, Mohammadi M, Khodabakhsh E. Effect of salbutamol and magnesium sulfate nebulizer compared with salbutamol and normal saline nebulizer in the treatment of acute asthma attack. Journal of Babol University of Medical Sciences 2014;16(3):7‐12. [Google Scholar]
Nannini 2000 {published data only}
- Nannini LJ Jr, Pendino JC, Corna RA, Mannarino S, Quispe R. Magnesium sulfate as a vehicle for nebulized salbutamol in acute asthma. American Journal of Medicine 2000;108(3):193‐7. [DOI] [PubMed] [Google Scholar]
Neki 2006 {published data only}
- Neki NS. A comparative clinical efficacy and safety profile of inhaled magnesium sulphate and salbutamol nebulisations in severe asthma [Abstract]. Indian Journal of Allergy, Asthma and Immunology 2006;20:131. [Google Scholar]
Powell 2013 {published data only}
- Kolamunnage‐Dona R, Powell C, Williamson P. Modelling variable dropout in randomised controlled trials with longitudinal outcomes: application to the MAGNETIC study. Trials 2016;17(1):222. [DOI: 10.1186/s13063-016-1342-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrou S, Boland A, Khan K, Powell C, Kolamunnage‐Dona R, Lowe J, et al. Economic evaluation of nebulized magnesium sulphate in acute severe asthma in children. International Journal of Technology Assessment in Health Care 2014;30(4):354‐60. [DOI: 10.1017/S0266462314000440] [DOI] [PubMed] [Google Scholar]
- Powell C. MAGNETIC; a randomised, double blind, placebo controlled study of nebulised magnesium sulphate in acute asthma in children [Abstract]. European Respiratory Journal. 2011; Vol. 38, issue Suppl 55:1406. []
- Powell C, Kolamunnage‐Dona R, Lowe J, Boland A, Petrou S, Doull I, et al. MAGNEsium Trial In Children (MAGNETIC): a randomised, placebo‐controlled trial and economic evaluation of nebulised magnesium sulphate in acute severe asthma in children. Health Technology Assessment 2013;17(45):1‐216. [DOI: 10.3310/hta17450] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell C, Kolamunnage‐Dona R, Lowe J, Boland A, Petrou S, Doull I, et al. MAGNEsium trial in children (MAGNETIC): a randomised, placebo controlled trial and economic evaluation of nebulised magnesium sulphate in acute severe asthma in children. Health Technology Assessment 2013;17(45):1‐216. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell C, Kolamunnage‐Dona R, Lowe J, Boland A, Petrou S, Doull I, et al. Magnesium sulphate in acute severe asthma in children (MAGNETIC): a randomised, placebo‐controlled trial. Lancet Respiratory Medicine 2013;1(4):301‐8. [DOI] [PubMed] [Google Scholar]
- Powell CVE. A randomised double, blind , placebo controlled study of nebulised magnesium sulphate in acute asthma in children –The MAGNETIC study. Archives of Disease in Children 2012;97:A2‐3. [DOI: 10.1136/archdischild-2012-301885.6] [DOI] [Google Scholar]
Sarhan 2016 {published data only}
- Sarhan H, El‐Garhy O, Ali M, Youssef N. The efficacy of nebulized magnesium sulfate alone and in combination with salbutamol in acute asthma. Drug Design, Development and Therapy 2016;10:1927‐33. [DOI: 10.2147/DDDT.S103147] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turker 2017 {published data only}
- Turker S, Dogru M, Yildiz F, Yilmaz SB. The effect of nebulised magnesium sulphate in the management of childhood moderate asthma exacerbations as adjuvant treatment. Allergologia et immunopathologia 2017;45(2):115‐20. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Balter 1989 {published data only}
- Balter MS, Rebuck AS. Treatment of the recalcitrant asthmatic. Annals of Allergy 1989;63:297‐300. [PubMed] [Google Scholar]
Bede 2003 {published data only}
- Bede O, Suranyi A, Pinter K, Szlavik M, Gyurkovits K. Urinary magnesium excretion in asthmatic children receiving magnesium supplementation: a randomized, placebo‐controlled, double‐blind study. Magnesium Research 2003;16:262‐70. [PubMed] [Google Scholar]
Bede 2004 {published data only}
- Bede O, Suranyi A, Pinter K, Szlavik M, Gyurkovits K. Efficacy of long‐lasting magnesium supplementation in asthmatic children [Abstract]. European Respiratory Journal 2004;24:212s. [Google Scholar]
Bede 2008 {published data only}
- Bede O, Nagy D, Suranyi A, Horvath I, Szlavik M, Gyurkovits K. Effects of magnesium supplementation on the glutathione redox system in atopic asthmatic children. Inflammation Research 2008;57:279‐86. [DOI] [PubMed] [Google Scholar]
Bernstein 1995 {published data only}
- Bernstein WK, Khasgir T, Khastgir A, Hernandex E, Miller J, Schonfeld SA, et al. Lack of effectiveness of magnesium in chronic stable asthma. Archives of Internal Medicine 1995;155:271‐6. [DOI] [PubMed] [Google Scholar]
Cairns 1996 {published data only}
- Cairns CB, Kraft M. Magnesium attenuates the neutrophil respiratory burst in adult asthmatic patients. Academic Emergency Medicine 1996;3(12):1093‐7. [DOI] [PubMed] [Google Scholar]
Castillo Rueda 1991 {published data only}
- Castillo Rueda A, Recarte Garcia‐Andrade C, Torres Segovia FJ. Magnesium, the 4th drug in asthma treatment?. Revista Clinica Espanola 1991;189(5):250. [PubMed] [Google Scholar]
Chande 1992 {published data only}
- Chande VT, Skoner DP. A trial of nebulized magnesium sulfate to reverse bronchospasm in asthmatic patients. Annals of Emergency Medicine 1992;21(9):1111‐5. [DOI] [PubMed] [Google Scholar]
Corbridge 1995 {published data only}
- Corbridge TC, Hall JB. The assessment and management of adults with status‐asthmaticus. American Journal of Respiratory and Critical Care Medicine 1995;151(5):1296‐316. [DOI] [PubMed] [Google Scholar]
DiGregorio 1999 {published data only}
- DiGregorio RV, Bessmertny O, Pringle G, Cohen H. Effect of nebulized magnesium sulfate on serum magnesium levels in asthmatic patients. ASHP Midyear Clinical Meeting 1999; Vol. 34:[P‐383E].
Emelyanov 1990 {published data only}
- Emelyanov AV, Fedoseev GB, Emanuel VL, Sinitsina TM, Krunchak LV. Effect of magnesium‐sulfate aerosol on external respiration in bronchial‐asthma patients. Klinicheskaya Meditsina 1990;68(11):31‐4. [PubMed] [Google Scholar]
Emelyanov 1996 {published data only}
- Emelyanova AV, Goncharova VA, Sinitsina TM. Magnesium sulfate in management of bronchial asthma. Klinicheskaya Meditsina (Mosk) 1996;74(8):55‐8. [PubMed] [Google Scholar]
Emelyanov 1997 {published data only}
- Emelyanov AV, Goncharova VA, Sinitsyna TM. Magnesium sulphate aerosol efficacy in bronchial asthma. Terapevticheskii Arkhiv 1997;69(3):35‐9. [PubMed] [Google Scholar]
Fathi 2014 {published data only}
- Fathi N, Hosseini SA, Tavakkol H, Khodadady A, Tabesh H. Effect of oral magnesium citrate supplement on lung function and magnesium level in patients with asthma. [Persian]. Journal of Mazandaran University of Medical Sciences 2014;24(111):43‐51. [Google Scholar]
Fedoseev 1991 {published data only}
- Fedoseev GB, Emelyanov AV, Malakauskas KK, Goncharova V, Sinitsina TM, Didur MD, et al. Therapeutic potentialities of magnesium‐sulfate in bronchial‐asthma. Terapevticheskii Arkhiv 1991;63(12):27‐9. [PubMed] [Google Scholar]
Gandia 2012 {published data only}
- Gandia F, Guenard H, Sriha B, Tabka Z, Rouatbi S. Inhaled magnesium sulphate in the treatment of bronchial hyperresponsiveness. Magnesium Research 2012;25(4):168‐76. [DOI] [PubMed] [Google Scholar]
Gurkan 1999 {published data only}
- Gurkan F, Hospolat K, Bosnak M, Dikici B, Derman O, Ece A. Intravenous magnesium sulphate in the management of moderate to severe acute asthmatic children nonresponding to conventional therapy. European Journal of Emergency Medicine 1999;6(3):201‐5. [DOI] [PubMed] [Google Scholar]
Harari 1998 {published data only}
- Harari M, Barzillai R, Shani J. Magnesium in the management of asthma: critical review of acute and chronic treatments, and Deutsches Medizinisches Zentrum's (DMZ's) clinical experience at the Dead Sea. Journal of Asthma 1998;35(7):525‐36. [DOI] [PubMed] [Google Scholar]
Hardin 2001 {published data only}
- Hardin KA, Kallas HJ, McDonald RJ. Pharmacologic management of the hospitalized pediatric asthma patient. Clinical Reviews in Allergy and Immunology 2001;20(3):293‐326. [DOI] [PubMed] [Google Scholar]
Harmanci 1996 {published data only}
- Harmanci E, Ekici M, Erginel S, Ozdemir N, Ozkan G. Comparison of effects of nebulized to intravenous magnesium sulfate on bronchial hyperreactivity and expiratory flow rate in asthmatic patients. European Respiratory Journal 1996;9 Suppl 23:34s. [Google Scholar]
Hill 1995 {published data only}
- Hill J, Britton J. Dose‐response relationship and time‐course of the effect of inhaled magnesium sulphate on airflow in normal and asthmatic subjects. British Journal of Clinical Pharmacology 1995;40:539‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hill 1997a {published data only}
- Hill J, Lewis S, Britton J. Studies of the effects of inhaled magnesium on airway reactivity to histamine and adenosine monophosphate in asthmatic subjects. Clinical and Experimental Allergy 1997;27(5):546‐51. [PubMed] [Google Scholar]
Hill 1997b {published data only}
- Hill J, Lewis S, Britton J. Studies of the effects of inhaled magnesium on airway reactivity to histamine and adenosine monophosphate in asthmatic subjects. Clinical and Experimental Allergy 1997; Vol. 27:546‐51. [PubMed]
Irazuzta 2014 {published data only}
- Irazuzta J, Pavlovich V, Paredes F. High dose magnesium sulfate infusions in children with status asthmaticus in the emergency department, efficacy study. Pediatric Critical Care Medicine 2014;15(4):99. [Google Scholar]
Irazuzta 2016 {published data only}
- Irazuzta JE, Paredes F, Pavlicich V, Dominguez SL. High‐dose magnesium sulfate infusion for severe asthma in the emergency department: efficacy study. Pediatric Critical Care Medicine 2016;17(2):e29‐e33. [DOI] [PubMed] [Google Scholar]
Kenyon 2001 {published data only}
- Kenyon N, Albertson TE. Status asthmaticus: From the emergency department to the intensive care unit. Clinical Reviews in Allergy and Immunology 2001;20(3):271‐92. [DOI] [PubMed] [Google Scholar]
Kreutzer 2001 {published data only}
- Kreutzer ML, Louie S. Pharmacologic treatment of the adult hospitalized asthma patient. Clinical Reviews in Allergy and Immunology 2001;20(3):357‐83. [DOI] [PubMed] [Google Scholar]
Manzke 1990 {published data only}
- Manzke H, Thiemeier M, Elster P, Lemke J. Magnesium sulfate as adjuvant in beta‐2‐sympathicomimetic inhalation therapy of bronchial asthma. Pneumologie 1990;44(10):1190‐2. [PubMed] [Google Scholar]
McFadden 1995 {published data only}
- McFadden ER. Asthma. Lancet 1995;345(8959):1215‐20. [DOI] [PubMed] [Google Scholar]
Nannini 1997 {published data only}
- Nannini Jr LJ, Hofer D. Effect of inhaled magnesium sulfate on sodium metabisulfite‐induced bronchoconstriction in asthma. Chest 1997;111:858‐61. [DOI] [PubMed] [Google Scholar]
Nunez‐Torres 1995 {published data only}
- Nunez‐Torres C, Abreu Glez J, Glez Martin I, Martinez Riera A, Glez Reimers E, Santolaria FF. The magnesium sulphate does not have bronchodilator effect in the acute bronchial asthma when it is used by inhaled way [abstract]. European Respiratory Society Annual Congress; 1995 Oct 9‐13; Barcelona 1995; Vol. 8:98S.
Pelton 1998 {published data only}
- Pelton R. Nutrients can reduce asthma severity. American Druggist 1998;215(5):34, 36. [Google Scholar]
Pelton 1999 {published data only}
- Pelton R. Don't forget magnesium. American Druggist 1999;December:48‐9. [Google Scholar]
Petrov 2014 {published data only}
- Petrov VI, Shishimorov IN, Perminov AA, Nefedov IV. [Influence of magnesium deficiency correction on the effectiveness of bronchial asthma pharmacotherapy in children] [Russian]. Eksperimental'naia i klinicheskaia farmakologiia 2014;77(8):23‐7. [PubMed] [Google Scholar]
Puente‐Maestu 1999 {published data only}
- Puente‐Maestu L, Abad YM. Treatment of asthmatic crisis. Revista Clinica Espanola 1999;199(7):473‐7. [PubMed] [Google Scholar]
Qureshi 1999 {published data only}
- Qureshi F. Management of children with acute asthma in the emergency department. Pediatric Emergency Care 1999;15(3):206‐14. [DOI] [PubMed] [Google Scholar]
Rodger 2003 {published data only}
- Rodger KA, McLachlan J. The effects of oral magnesium in asthma [abstract]. American Thoracic Society 99th International Conference; 2003 May 16‐21; Seattle. 2003:C104 Poster D42.
Rodrigo 2000 {published data only}
- Rodrigo G, Rodrigo C, Burschtin O. Efficacy of magnesium sulfate in acute adult asthma: a meta‐analysis of randomized trials. American Journal of Emergency Medicine 2000;18(2):216‐21. [DOI] [PubMed] [Google Scholar]
Rolla 1987a {published data only}
- Rolla G, Bucca C, Bugiani M, Arossa W, Spinaci S. Reduction of histamine‐induced bronchoconstriction by magnesium in asthmatic subjects. Allergy 1987;42:186‐8. [DOI] [PubMed] [Google Scholar]
Rolla 1987b {published data only}
- Rolla G, Bucca C, Arossa W, Bugiani M. Magnesium attenuates methacholine‐induced bronchoconstriction in asthmatics. Magnesium 1987;6(4):201‐4. [PubMed] [Google Scholar]
Rolla 1988a {published data only}
- Rolla G, Bucca C, Caria E. Dose‐related effect of inhaled magnesium‐sulfate on histamine bronchial challenge in asthmatics. Drugs under Experimental and Clinical Research 1988;14(9):609‐12. [PubMed] [Google Scholar]
Rolla 1988b {published data only}
- Rolla G, Bucca C. Magnesium, beta‐agonists and asthma. Lancet 1988;April 30:989. [DOI] [PubMed] [Google Scholar]
Scarfone 1998 {published data only}
- Scarfone RJ, Loiselle JM, Joffe MD, Mull C, Stiller S, Thompson, et al. Magnesium sulfate in the emergency department treatment of acute asthma in children. Pediatrics 1998;102:711. [Google Scholar]
Scarfone 2000 {published data only}
- Scarfone RJ, Lioselle JM, Joffe MD, Mull CC, Stiller S, Thompson K, et al. A randomized trial of magnesium in the emergency department treatment of children with asthma. Annals of Emergency Medicine 2000;36(6):572‐8. [DOI] [PubMed] [Google Scholar]
Shishimorov 2015 {published data only}
- Shishimorov I, Petrov V, Magnitskaya O, Perminov A, Nefedov I. Magnesium deficiency correction for improvement of children bronchial asthma treatment results. European Respiratory Journal 2015;46(0):PA1286. [Google Scholar]
Singh 2008a {published data only}
- Singh AK, Gaur S, Kumar R. A randomized controlled trial of intravenous magnesium sulphate as an adjunct to standard therapy in acute severe asthma. Iranian Journal of Allergy, Asthma and Immunology 2008; Vol. 7:221‐9. [PubMed]
Singh 2008b {published data only}
- Singh AK, Gaur SN, Kumar R. Comparison of efficacy of intravenous and inhaled magnesium sulphate as an adjunct to standard therapy in acute severe asthma [Abstract]. European Respiratory Society 18th Annual Congress; 2008 Oct 3‐7; Berlin. 2008:[P3620]. [PubMed]
Singhi 2014 {published data only}
- Singhi S, Grover S, Bansal A, Chopra K. Randomised comparison of intravenous magnesium sulphate, terbutaline and aminophylline for children with acute severe asthma. Acta Paediatrica 2014;103(12):1301‐6. [DOI] [PubMed] [Google Scholar]
Sinitsina 1991 {published data only}
- Sinitsina TM, Shchemelinina TI, Didur MD, Evsyukova EV, Emelyanov AV, Nazarova VA. The follow‐up of bronchial hyperreactivity in risk group subjects and bronchial‐asthma patients ‐ approaches to its correction. Terapevticheskii Arkhiv 1991;63(8):21‐5. [PubMed] [Google Scholar]
Skobeloff 1982 {published data only}
- Skobeloff EM. An ion for the lungs. Academic Emergency Medicine 1982;3(12):1082‐4. [DOI] [PubMed] [Google Scholar]
Sun 2014 {published data only}
- Sun YX, Gong CH, Liu S, Yuan XP, Yin LJ, Yan L. Effect of inhaled MgSO4 on FEV1 and PEF in children with asthma induced by acetylcholine: a randomized controlled clinical trial of 330 cases. Journal of Tropical Pediatrics 2014;60(2):1268‐73. [DOI] [PubMed] [Google Scholar]
Talukdar 2005 {published data only}
- Talukdar T, Singhal P, Jain A, Kumar R, Gaur SN. Inhaled magnesium sulfate in the treatment of severe asthma. Indian Journal of Allergy Asthma and Immunology 2005; Vol. 19:29‐35.
Teeter 1999 {published data only}
- Teeter JG. Bronchodilator therapy in status asthmaticus. Chest 1999;115(4):911‐2. [DOI] [PubMed] [Google Scholar]
Telia 2005 {published data only}
- Telia A, Tutashvili M, Donguzashvili S, Pirtskhalava N. Effect of magnesium and furosemide on bronchial asthma. Georgian Medical News 2005, (128):55‐9. [PubMed] [Google Scholar]
Tereshchenko 2006 {published data only}
- Tereshchenko S, Turgenkova N, Gorbacheva N, Procoptzeva N, Goncharov B, Vlasova M. Administration of isotonic nebulised magnesium sulphate as an adjuvant to ipratropium bromide in treatment of viral wheezing in children [Abstract]. European Respiratory Journal 2006;28:265s [P1537]. [Google Scholar]
Tetikkurt 1992 {published data only}
- Tetikkurt C, Kocyigit E, Disci R. Bronchodilating effect of inhaled and intravenous magnesium‐sulfate (compared with aeresol terbutaline). Magnesium Bulletin 1992;14(2):49‐52. [Google Scholar]
Tetikkurt 1993 {published data only}
- Tetikkurt C, Kocyigit E. The bronchodilating effect of inhaled magnesium. Magnesium Bulletin 1993;15(1):1‐2. [Google Scholar]
Torres 2012 {published data only}
- Torres S, Sticco N, Bosch JJ, Lolster T, Siaba A, Rivarola M, et al. Effectiveness of magnesium sulfate as initial treatment of acute severe asthma in children, conducted in a tertiary‐level university hospital: a randomized, controlled trial. Archivos Argentinos de Pediatria 2012;110(4):291‐6. [DOI] [PubMed] [Google Scholar]
Watanatham 2015 {published data only}
- Watanatham S, Pongsamart G, Vangveeravong M, Daengsuwan T. Comparison efficacy and safety of inhaled magnesium sulfate to intravenous magnesium sulfate in childhood severe asthma exacerbation. Journal of Allergy and Clinical Immunology 2015;135(2):AB241. [Google Scholar]
Wijetunge 2002 {unpublished data only}
- Wijietunge DB. A trial of nebulised magnesium sulphate versus placebo in addition to conventional bronchodilator treatment in acute asthma of moderate severity. webarchive.nationalarchives.gov.uk/20130506081956/http://www.hta.ac.uk/protocols/200600010002.pdf (accessed prior to 31 July 2017).
Wongwaree 2017 {published data only}
- Wongwaree S. Comparison efficacy of randomized nebulized magnesium sulfate and ipratropium bromide/fenoterol in children with severe asthma exacerbation. Journal of allergy and clinical immunology 2017;139(2 Suppl 1):AB94. [DOI] [PubMed] [Google Scholar]
Xu 2002 {published data only}
- Xu CQ, Yang J, Meng XK. Clinical study of salbutamol combined with magnesium sulfate by nebulization in the treatment of paroxysmal asthma. Chinese Journal of Clinical Pharmacology and Therapeutics 2002;7:446‐8. [Google Scholar]
Yemelyanov 1997 {published data only}
- Yemelyanov A, Fedoseev G, Gonocharova V, Sinitcina T, Lintsov A. The effects of magnesium sulfate aerosol on pulmonary function tests in asthmatic patients [abstract]. European Respiratory Journal 1997;10:472S. [Google Scholar]
Zandsteeg 2009 {published data only}
- Zandsteeg AM, Hirmann P, Pasma HR, Yska JP, Brinke A. Effect of MgSO4 on fev1 in stable severe asthma patients with chronic airflow limitation. Magnesium Research 2009;22:256‐61. [DOI] [PubMed] [Google Scholar]
Zhu 2003 {published data only}
- Zhu BF, Tao YJ. 1,6‐Fructose diphosphate and magnesium sulfate therapy for 34 patients with severe asthma. Chinese Journal of Medical Writing 2003;10:888‐90. [Google Scholar]
References to studies awaiting assessment
Abd 1997 {published data only}
- Abd El Kader F. Ventilatory, cardiovascular and metabolic responses to salbutamol, ipratropium bromide and magnesium sulfate in bronchial asthma: comparative study. Alexandria Medical Journal 1997;39(1):43‐64. [Google Scholar]
Bustamante 2000 {published data only}
- Bustamante APL. Inhaled magnesium sulfate as adjunct therapy for moderate to severe asthma exacerbations, a randomized control clinical trial. Philippine Journal of Pediatrics 2000;49:237‐9. [Google Scholar]
ISRCTN61336225 {published data only}
- Dawood M, Mahmoud LMZ. Magnesium sulfate in the treatment of acute asthma. ISRCTN registry 2016. [http://www.isrctn.com/ISRCTN61336225]
References to ongoing studies
Motamed 2015 {published data only}
- IRCT2015111015446N8. Comparison of clinical and Spirometric response between nebulized salbutamol _ magnesium sulfate and nebulized salbutamol alone in acute asthma attack. en.search.irct.ir/view/26734 (first received 27 November 2015).
Saucedo 2015 {published data only}
- NCT02584738. Nebulized magnesium sulfate as an adjunct to standard therapy in asthma exacerbation. clinicaltrials.gov/ct2/show/NCT02584738 (first received 14 October 2015).
Schuh 2016a {published data only}
- Schuh S, Sweeney J, Freedman S, Coates A, Johnson D, Thompson G, et al. Magnesium nebulization utilization in management of pediatric asthma (MagNUM PA) trial: study protocol for a randomized controlled trial. Trials 2016;17:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aburuz 2005
- Aburuz S, McElnay J, Gamble J, Millership J, Heaney L. Relationship between lung function and asthma symptoms in patients with difficult to control asthma. Journal of Asthma 2005;42(10):859‐64. [DOI] [PubMed] [Google Scholar]
Ashutosh 2000
- Ashutosh K. Nitric oxide and asthma: a review. Current Opinion in Pulmonary Medicine 2000;6(1):21‐5. [DOI] [PubMed] [Google Scholar]
Bain 2014
- Bain E, Pierides KL, Clifton VL, Hodyl NA, Stark MJ, Crowther CA, et al. Interventions for managing asthma in pregnancy. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD010660.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blitz 2005
- Blitz M, Blitz S, Hughes R, Diner B, Beasley R, Knopp J, et al. Aerosolized magnesium sulfate for acute asthma: a systematic review. Chest 2005;128(1):337‐44. [DOI] [PubMed] [Google Scholar]
Bois 1962
- Bois P. Effect of magnesium deficiency on mast cells and urinary histamine in rats. British Journal of Experimental Pathology 1963;44:151‐5. [PMC free article] [PubMed] [Google Scholar]
BTS/SIGN 2016
- SIGN 2016. Sign 153. British guideline on the management of asthma. A national clinical guideline. September 2016. brit‐thoracic.org.uk/document‐library/clinical‐information/asthma/btssign‐asthma‐guideline‐2016/ (accessed prior to 31 July 2017). [ISBN 9781909103474]
Carranza Rosenzweig 2004
- Carranza Rosenzweig JR, Edwards L, Lincourt W, Dorinsky P, ZuWallack RL. The relationship between health‐related quality of life, lung function and daily symptoms in patients with persistent asthma. Respiratory Medicine 2004;98(12):1157‐65. [DOI] [PubMed] [Google Scholar]
Cates 2004
- Cates CCJ, Bara A, Crilly JA, Rowe BH. Holding chambers versus nebulisers for beta‐agonist treatment of acute asthma. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD000052] [DOI] [PubMed] [Google Scholar]
Del Castillo 1954
- Castillo J, Engbaek L. The nature of the neuromuscular block produced by magnesium. Journal of Physiology 1954;124:370‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
GINA 2017
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention 2017. ginasthma.org (accessed prior to 31 July 2017).
Gourgoulianis 2001
- Gourgoulianis KI, Chatziparasidis G, Chatziefthimiou A, Molyvdas PA. Magnesium as a relaxing factor of airway smooth muscles. Journal of Aerosol Medicine 2001;14(3):301‐7. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 8 May 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Griffiths 2016
- Griffiths B, Kew KM. Intravenous magnesium sulfate for treating children with acute asthma in the emergency department. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011050.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kew 2014
- Kew K, Kirtchuk L, Michell C. Intravenous magnesium sulfate for treating adults with acute asthma in the emergency department. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD010909.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ling 2016
- Ling ZG, Wu YB, Kong JL, Tang ZM, Liu W, Chen YQ. Lack of efficacy of nebulized magnesium sulfate in treating adult asthma: A meta‐analysis of randomized controlled trials. Pulmonary Pharmacology and Therapeutics 2016;41:40‐7. [DOI] [PubMed] [Google Scholar]
Mohammed 2007
- Mohammed S, Goodacre S. Intravenous and nebulised magnesium sulphate for acute asthma: systematic review and meta‐analysis. Emergency Medicine Journal 2007;24(12):823‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nadler 1987
- Nadler J, Goodson S, Rude R. Evidence that prostacyclin mediates the vascular action of magnesium in humans. Hypertension 1987;9(4):379‐83. [DOI] [PubMed] [Google Scholar]
NRAD 2014
- Royal College of Physicians. Why asthma still kills: the national review of asthma deaths (NRAD) confidential enquiry report. rcplondon.ac.uk/projects/outputs/why‐asthma‐still‐kills. [ISBN 978‐1‐86016‐531‐3]
Powell 2012
- Powell C, Dwan K, Milan S, Beasley R, Hughes R, Knopp‐Sihota J, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD003898.pub5] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shan 2013
- Shan Z, Rong Y, Yang W, Wang D, Yao P, Xie J, et al. Intravenous and nebulized magnesium sulfate for treating acute asthma in adults and children: A systematic review and meta‐analysis. Respiratory Medicine 2013;107(3):321‐30. [DOI: 10.1016/j.rmed.2012.12.001] [DOI] [PubMed] [Google Scholar]
Song 2012
- Song WJ, Chang YS. Magnesium sulfate for acute asthma in adults: a systematic literature review. Asia Pacific Allergy 2012;2(1):76‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Su 2017
- Su Z, Li R, Gai Z. Intravenous and nebulized magnesium sulfate for treating acute asthma in children: a systematic review and meta‐analysis. journals.lww.com/pec‐online/Abstract/publishahead/Intravenous_and_Nebulized_Magnesium_Sulfate_for.98881.aspx 2016 Oct 4 [Epub ahead of print].
Turner 2017
- Turner DL, Ford WR, Kidd EJ, Broadley KJ, Powell C. Effects of nebulised magnesium sulphate on inflammation and function of the guinea‐pig airway. European Journal of Pharmacology 2017;801:79‐85. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Blitz 2004
- Blitz M, Blitz S, Beasely R, Diner BM, Hughes R, Knopp JA, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD003898.pub2] [DOI] [Google Scholar]
Blitz 2005a
- Blitz M, Blitz S, Beasely R, Diner BM, Hughes R, Knopp JA, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003898.pub3] [DOI] [PubMed] [Google Scholar]
Blitz 2005b
- Blitz M, Blitz S, Beasely R, Diner B, Hughes R, Knopp JA, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003898.pub4] [DOI] [PubMed] [Google Scholar]


