Abstract
Root extracts made from maize (Zea mays) seedlings submerged for 2 h showed an increased 32P-labeling of a 90-kD polypeptide in a Ca2+-dependent manner. This protein was identified as sucrose synthase (SS) by immunoprecipitation and mutant analysis. Metabolic labeling with 32Pi indicated that the aerobic levels of SS phosphorylation were maintained up to 2 h of anoxia. In contrast, during prolonged anoxia the protein was under-phosphorylated, and by 48 h most of the protein existed in the unphosphorylated form. In seedlings submerged for 2 h or longer, a part of SS became associated with the microsomal fraction and this membrane localization of SS was confined only to the root tip. This redistribution of SS in the root tip preceded callose induction, an indicator of cell death. The sh1 mutants showed sustained SS phosphorylation and lacked the anoxia-induced relocation of SS, indicating that it was the SH1 form of the enzyme that was redistributed during anoxia. The sh1 mutants also showed less callose deposition and greater tolerance to prolonged anoxia than their non-mutant siblings. EGTA accentuated anoxic effects on membrane localization of SS and callose accumulation, whereas Ca2+ addition reversed the EGTA effects. These results indicate that the membrane localization of SS is an important early event in the anoxic root tip, probably associated with the differential anoxic tolerance of the two SS mutants. We propose that beside the transcriptional control of genes encoding SS, the reversible phosphorylation of SS provides a potent regulatory mechanism of sugar metabolism in response to developmental and environmental signals.
We reported earlier that a rise in the cytosolic calcium ([Ca2+]i) transduces the oxygen deprivation stimulus in maize (Zea mays; Subbaiah et al., 1994a, 1994b). Our interest is to unravel the pathways that translate the ionic signal into cellular and whole plant responses. One common mechanism that cells use to rapidly decipher and/or amplify the [Ca2+]i changes is reversible protein phosphorylation. Changes in protein phosphorylation have been implicated in the transduction of many environmental signals in plants (for review, see Stone and Walker, 1995). Addition or removal of phosphate can lead to changes in the activation status, catalytic activity, or cellular localization of effector proteins (e.g. Kim et al., 1994; Huber and Huber, 1996). These changes, in turn, can lead to transient alterations in gene expression and metabolism or even long-lasting modifications in the plant form and function (e.g. Ferreira et al., 1993; Maurel et al., 1995; Stone et al., 1998; Fankhauser et al., 1999).
In this report we show that an anaerobically induced polypeptide, Suc synthase1 (e.g. Springer et al., 1986; Suc synthase, SUS: EC 2.4.1.13; abbreviated as SS in this report), is post-translationally regulated by phosphorylation, and this regulation is among the early responses that culminate in the death of primary root tip in anoxic maize seedlings (Subbaiah et al., 2000). SS is encoded by two genes in maize, sh1 (encoding SH1) and sus1 (encoding SUS1) and is a homo/heterotetramer of the 90-kD gene products in maize (Hannah et al., 1994). This enzyme is unique in its ability to mobilize Suc into diverse pathways that are critical in structural (e.g. cellulose or callose biosynthesis), storage (starch synthesis), and metabolic (e.g. glycolysis) functions of plant cells (e.g. Ruan et al., 1997). The essentiality of SS for anoxic tolerance has recently been demonstrated in maize, using double mutants in the enzyme (Ricard et al., 1998), although no extensive analysis has been done on the anoxia tolerance of single mutants. Ca2+-dependent phosphorylation of SS and its potential implications have been studied earlier (Huber et al., 1996 and refs. therein; Winter et al., 1997, 1998). Here we show that prolonged anoxia induces dephosphorylation of SS, as well as its association with the microsomal fraction. We further show that this redistribution of the enzyme occurs only in the root tip and is associated with the extensive induction of callose, a marker of cell death caused by biotic and abiotic stresses in plants (e.g. Wissemeier et al., 1987; Chen and Howlett, 1996). Furthermore, our genetic analysis suggests that this response is isoform-specific and correlated with the superior anoxia tolerance of sh1 mutants to that of the non-mutant or sus1 mutant.
RESULTS
SS Is a Prominent Phosphoprotein in Anoxic Maize Roots
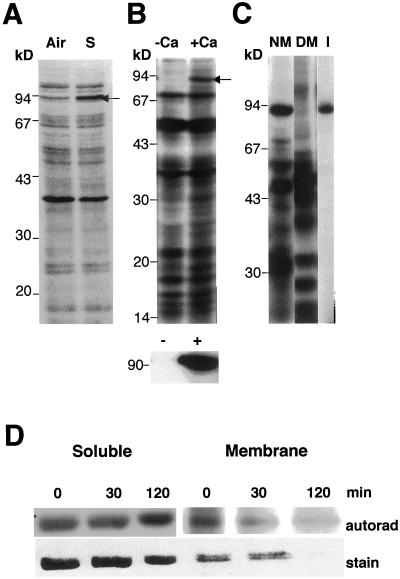
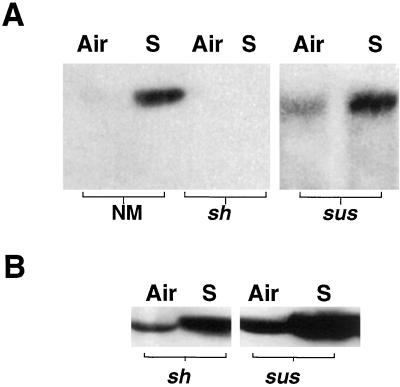
We used detergent-solubilized maize root extracts to study changes in Ca2+-mediated endogenous protein phosphorylation induced by a 2-h submergence. Though most proteins were phospholabeled equally in the aerobic and anoxic extracts, a polypeptide in the 90 kD region was labeled more intensely in the anoxic sample than in the aerobic root extract (Fig. 1A). The labeling of the polypeptide was sensitive to Ca2+ in the assay buffer (Fig. 1B). The band comigrated with a protein recognized by anti-SS in one- and two-dimensional gels, indicating that it could be SS (data not shown). This was confirmed by the precipitation of a protein by anti-SS antisera with similar molecular size and phosphorylation properties (Fig. 1B, bottom). Furthermore, the 90-kD polypeptide was not labeled in mutants that lack both forms of SS (Fig. 1C, lane DM). Phosphoamino acid analysis indicated that in vitro-labeled 90-kD protein and immunoprecipitated SS were phosphorylated on a Ser residue (data not shown). Huber et al. (1996) also reported that in 32P-fed maize seedling shoots, SS was labeled on a Ser residue at the N terminus.
Figure 1.
A, Autoradiograph showing the 32P-labeled products of endogenous protein kinase activities in root extracts from aerobic or submerged seedlings. Preparation of extracts, assay conditions, and electrophoretic analysis were as described in “Materials and Methods.” Arrow indicates a 90-kD polypeptide, which comigrates with SS in immunoblots. Air, Aerobic; S, submerged. B, Ca2+ dependence of phosphotransfer to 90-kD polypeptide. The in vitro phosphorylation assay was carried out in the absence or presence Ca2+. Bottom, The immunoprecipitation of the 90-kD phosphoprotein by SS antisera from aliquots of reactions shown above. −Ca, 0.2 mm EGTA; +Ca, 1 mm CaCl2. C, Double mutant for SS (sh1sus1) lacks the 90-kD phosphoprotein. Root extracts prepared from the non-mutant (Sh1Sus1) and mutant genotypes were tested for endogenous protein phosphorylation activities as described above. The comigration of immunoprecipitated SS with the 90-kD phosphoprotein is also shown. The amount of mutant protein used in the assay was twice that of the non-mutant (for improved detection of the 90-kD band) and therefore, more labeled bands are seen in the mutant lane. NM, Non-mutant; DM, double mutant for SS; I, radiolabeled immunoprecipitate of SS protein. Size markers are indicated on the left side of all panels. D, Immunoprecipitation of SS from in vivo labeled maize seedling roots with 32Pi. The Coomassie-stained gel (stain) was included for a quantitative comparison of labeling (autorad) on a protein basis. Seedlings were labeled for 0, 30, or 120 min of anoxia.
To confirm the above observations, we carried out 32P labeling of seedlings and immunoprecipitation of SS from root tissue. Anoxia up to 2 h maintained or mildly increased the labeling of the enzyme in comparison with the aerobic seedlings (Fig. 1D). The increase occurred in the soluble (30% by 2 h) and membrane fractions (quantitative comparisons are not made, since no protein was detectable in the 2 h lane), relative to Coomassie staining.
SS Phosphorylation Is Altered during Prolonged Anoxia in an Isoform-Specific Manner
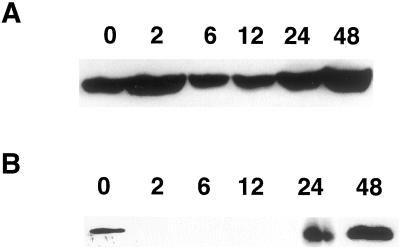
We asked whether prolongation of anoxia leads to a heavier labeling of SS in maize roots. On the contrary, incubation of seedlings even for 4 h under anoxia led to a decreased labeling of SS in the soluble fraction (Fig. 2A) and no labeling in the membrane fraction (data not shown), although 32P uptake did not appreciably decrease under anoxia (data not shown). Of the two genes that encode SS in maize, only sh1 is known to be transcriptionally and translationally induced under anoxia (e.g. McCarty et al., 1986; Springer et al., 1986; Zeng et al., 1998). We examined whether the differential regulation of SS genes under anoxia also extends to the post-translational regulation of the gene products. By making use of sh1 and sus1 mutants (lacking SH1 and SUS1, respectively) we dissected the phosphorylation rates of SS isoforms in anoxic maize roots. The sh1 mutant had very low amounts of immunoprecipitable SS in aerobic and 4-h anoxic primary roots and showed no appreciable difference in SS phospholabeling (data not shown). However, analysis with sus1 mutant indicated that anoxia caused a 75% reduction in the phosphorylation of SH1, compared to a 49% decrease in the phospholabeling of total SS (Fig. 2A).
Figure 2.
Autoradiography of immunoprecipitated SS from maize seedling roots, labeled in vivo with 32Pi under long-term anoxia. A, Labeling was for 4 h of anoxia after an aerobic pre-incubation of non-mutant and sus1 seedlings with 32P for 2 h. B, Labeling was carried out during the final 3 h of a 48-h anoxic treatment of non-mutant and sh1 seedlings. Air, Aerobic incubation; S, anoxia; NM, non-mutant
Under our submergence conditions (in the presence of antibiotics and transitory hypoxia), root tips in intact maize seedlings survive up to 48 h of anoxia (Subbaiah et al., 2000). Our preliminary protein blotting experiments also indicated that the amount of SS continued to increase in the root tissue for at least up to 48 h of anoxia. Therefore, we analyzed the effect of prolonged anoxia on SS phosphorylation in non-mutant and mutant seedlings. Under a 48-h anoxic treatment, 32P incorporation into total root protein was reduced to 40% to 50% of that in air. On the other hand, labeling of SS was more drastically reduced in the non-mutant, as shown in Figure 2B. An identical pattern was observed in the sus1 genotype, as well (data not shown). The 32P incorporation into SS was 35-fold less, although 20- to 23-fold more immunoprecipitable SS (or SH1) protein was recoverable under anoxia. Thus anoxia caused a 300- to 400-fold reduction (even after compensating for the lower 32P incorporation) in the phosphorylation of SS per protein basis. Figure 2B also shows a similar analysis with a sh1 mutant. Forty-eight hours of anoxia caused a 12.9-fold increase in the amount of SUS1 as recovered by immunoprecipitation, but the treatment also led to a 7.7-fold increase in the phospholabeling of the protein. Considering the decreased uptake of 32Pi by anoxic roots, these results indicate that the phosphorylation of SUS1 was maintained to a certain extent even under prolonged anoxia.
Anoxia Induces the Relocation of SS into the Microsomal Fraction
As shown by in vivo labeling studies, in spite of a large increase in SS (in SH1, predominantly) under anoxia, most of the protein existed as the dephosphorylated form (Fig. 2B). The major consequence of SS dephosphorylation is proposed to be its migration to the membrane (Winter et al., 1997). Furthermore, during our in vitro analysis, inclusion of Triton X-100 in the extraction buffer caused a stronger labeling of SS than without the detergent (data not shown), indicating that an additional substrate (or a protein kinase) was solubilized from the membrane. Therefore, we examined the distribution of SS in soluble and microsomal fractions of maize root extracts under continued anoxia. Since SS was shown to localize to the plasma membrane and Golgi apparatus in maize (Carlson and Chourey, 1996; Winter et al., 1997; Buckeridge et al., 1999), total microsomes were used for analysis without further fractionation. As shown in Figure 3A, there was a steady presence of SS in the soluble fraction, with an increase after 24 h of submergence. In contrast, the membrane fraction had very little SS protein until the first 12 h of submergence, but thereafter showed a substantial amount of SS associated with it (Fig. 3B). Thus a decrease in the phospholabeling of SS was followed by an increase its membrane association.
Figure 3.
Immunoblot showing SS distribution in soluble and membrane fractions of root extracts from anoxic maize seedlings. SS was detected by western analysis as detailed in “Materials and Methods.” A and B, Distribution of SS in soluble and membrane fractions respectively. The numbers (0–48) on the top of the lanes indicate hours submerged.
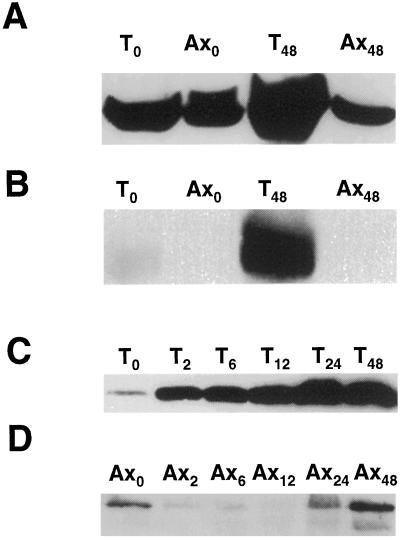
The Anoxic Increase in Membrane-Localized SS Occurs Predominantly in the Root Tip and Coincides with the Kinetics of Its Dephosphorylation
Submergence of maize seedlings for 48 h or longer induces the death of the primary root tip and is preceded by the induction a Cys protease at 12 h (Subbaiah et al., 2000). We determined whether the cellular distribution of SS is also altered in the root tip prior to the initiation of cell death. Soluble and membrane protein extracts, prepared separately from the root tip and axis, were probed with SS antisera. In seedlings submerged for 48 h, all of the anoxic increase in SS was localized to the root tip (Fig. 4, A and B). Using immunocytochemistry, Rowland et al. (1989) also reported that the anoxia-induced increase in SS protein was restricted to the root tip in 24-h submerged maize seedlings. Our analysis further showed that this increase was greater in the membrane fraction of the root tip (Fig. 4B). The axis had very little membrane-bound SS in aerobic or submerged seedlings. Under aerobic incubation even the tip had only a barely detectable amount of SS in its microsomes (Fig. 4B).
Figure 4.
Immunoblot analysis of SS distribution in the tip and axis regions of maize primary root. A and B, Distribution of SS in soluble and membrane fractions, respectively. Roots from aerobic and 48-h submerged seedlings were separated into tip and axis regions before protein extraction. T0 and Ax0, Tip and axis regions from aerobic seedlings; T48 and Ax48, tips and axes from 48-h submerged seedlings. C and D, Time course of SS accumulation in microsomal fractions of root tips and axes, respectively. Maize seedlings were submerged for indicated duration (in hours). Microsomes were prepared from root tips and axes separately and used for immunoblotting. T0 and Ax0 to T48 and A48, Tips and axes from seedlings submerged for 0 to 48 h.
We predicted that the membrane localization of SS in the tip would have started concomitant with its dephosphorylation. As the tip constitutes only to 15% to 20% of the primary root, this was not obvious in our whole root analysis (shown in Fig. 3B) due to a potential dilution of SS. Therefore, we determined the kinetics of SS-membrane association separately in root apices and axes of submerged seedlings. As shown in Figure 4C, the membrane localization of SS increased by as early as 2 h of submergence in the root tip, roughly coinciding with the dephosphorylation kinetics of the protein (Fig. 2A). With prolonged exposures of the blot, weak signals of SS protein were detected in the axis microsomes (Fig. 4D). The time course resembled that of the whole-root membranes (Fig. 3B) in that the amount of membrane-bound SS decreased immediately after anoxia, but was restored or mildly increased only after 24 h. These data also explain the apparent lack of correlation between the steady-state levels of sh1 mRNA and protein accumulation in anoxic maize roots as observed in some studies where only soluble proteins of whole root extracts were analyzed (e.g. McElfresh and Chourey, 1988). However, most of the SH1 induction is confined to the root tip (Fig. 4, A and B; Rowland et al., 1989) and a significant component becomes membrane associated as anoxia is prolonged (Fig. 4C).
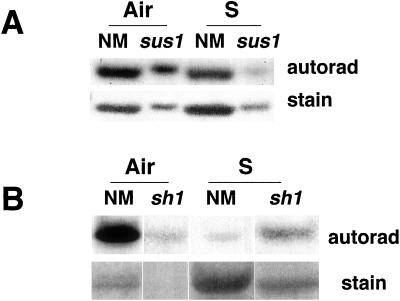
sh1 Mutants Lack the Membrane Localization of SS
If dephosphorylation of SS leads to its membrane localization, then the cellular distribution of the two isoforms under anoxia should parallel their differential (de)phosphorylation rates as revealed by metabolic labeling studies (Fig. 2). We addressed this question using mutants available in the SS isozymes. In Figure 5A, the distribution of SS in the membrane fraction of sh1 and sus1 mutants is compared with that in non-mutant siblings. Anoxia-induced membrane localization of SS was observed only in the non-mutant or sus1 genotypes, but not in the sh1 mutant. This indicated that only the SH1 protein was redistributed in response to anoxia in maize roots. Figure 5B shows the distribution of SS in the soluble fractions of mutants. The SUS1 was substantially induced by prolonged anoxia in the sh1 mutant (Figs. 2B and 5B; Guglielminetti et al., 1996). However, it was only approximately 50% of the amount of the SH1 protein in anoxic sus1 roots, raising the possibility that low levels of SS protein (beyond the limits of detectability by chemiluminescence) could exist on membranes in the sh1 genotype. Nevertheless, its continued phosphorylation and cytosolic presence may be advantageous in maintaining the fermentative metabolism in anoxic cells (Guglielminetti et al., 1996).
Figure 5.
Immunoblot showing the differential intracellular localization of SS in sh1 and sus1 mutants. A, Membrane distribution of SS in the root tissue of non-mutant and mutant maize seedlings. B, The distribution of SS in the root soluble fractions of mutant genotypes. Air, Aerobic; S, 48-h submerged; NM, non-mutant; sh and sus, mutants lacking SH1 and SUS1, respectively (though sus1 is known to be a leaky mutant with aberrant transcripts, no signals were detected with the monoclonal antisera used).
Ca2+ Deprivation Alters the Dynamics of SS in Maize Roots
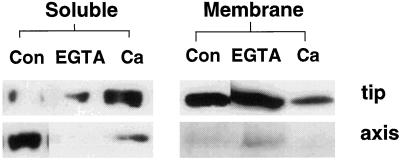
As the phospholabeling of SS is a Ca2+dependent process (Fig. 1B; Huber et al., 1996), we tested whether manipulating the cytosolic Ca2+ levels would change the cellular distribution of SS under anoxia, via alterations in its phosphorylation status. Ruthenium red kills the seedlings within 2 h of anoxia (Subbaiah et al., 1994b) and could not be used in our present studies. Because Ca2+ chelators do not kill anoxic seedlings for at least 2 h (Subbaiah et al., 1994b), we used EGTA to prevent influx of extracellular Ca2+. EGTA addition to the submergence buffer decreased the amount of SS recovered in the soluble fraction of the tip or axis (Fig. 6), while allowing a large membrane relocation of the enzyme in 2 h submerged seedlings (Fig. 6). EGTA also caused a large decrease in the 32P labeling of the enzyme in aerobic and anoxic seedlings (data not shown). Ca2+addition along with EGTA reversed the effect of the chelator (Fig. 6), as expected from the Ca2+-dependence of the cognate kinase.
Figure 6.
Immunoblot analysis of SS localization in maize roots, under Ca2+ deprivation. Effect of external Ca2+ concentration on SS distribution in soluble and membrane fractions of root tips and axes under short-term anoxia. Seedlings were submerged for 2 h with different additions to the submergence buffer. Con, No addition; EGTA, 2 mm EGTA; Ca, 2 mm EGTA + 4 mm CaCl2.
The Cellular Redistribution of SS Is Correlated with the Induction of Callose in the Anoxic Root Tip
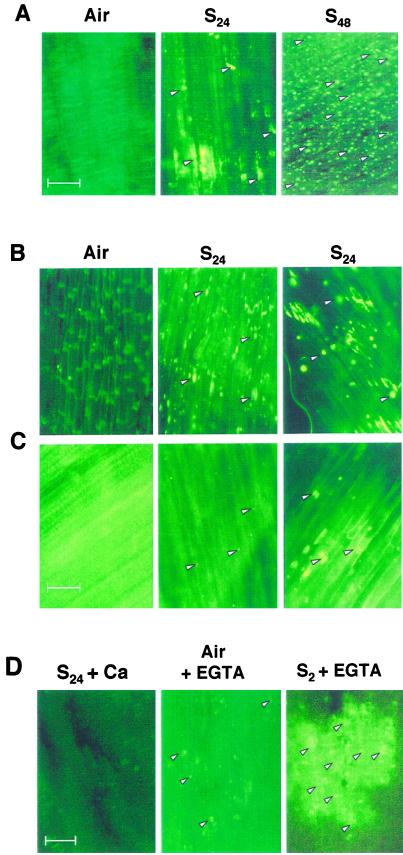
One of the symptoms that is found to precede cell death in the O2-deprived root tip is an induction of callose as early as 2 h (data not shown), which becomes intense by 24 h of submergence (Fig. 7A: S24). The appearance of callose and a progressive increase in its severity with prolonged anoxia (Fig. 7A) correlated with the kinetics of membrane localization of SS (Fig. 4C). The intensity of callose deposition decreased from the extreme tip toward the root axis, rarely being severe above the root hair zone (data not shown). Thus the spatial distribution of callose in the root was correlated with the pattern of membrane localization of SS (Fig. 4, B–D) and tip specific induction of SH1 in anoxic maize roots (Rowland et al., 1989).
Figure 7.
Fluorescence detection of callose within the 15-mm tip of maize roots. A, Non-mutant seedlings were incubated aerobically (Air) or submerged for 24 (S24) or 48 h (S48), fixed, and stained in aniline blue. Bright fluorescence spots (a few indicated by arrowheads) represent callose deposits. B and C, Callose development in the root tip of sus1 and sh1 mutant maize seedlings, respectively. Subpanel designations are as in A. Right and middle, S24 is from proximal (within 0.5 cm of the root apex) and distal regions of the root tip (the region above 0.5 cm of the root apex), respectively. In these genotypes there was a clear distinction in the staining of these two zones. D, Effect of EGTA and Ca2+ supplementation on callose development in non-mutant seedlings. S24+Ca, Seedlings submerged for 24 h in the presence of 2 mm CaCl2; Air + EGTA, seedlings incubated aerobically in 2 mm EGTA; S2+EGTA, seedlings submerged for 2 h in the presence of 2 mm EGTA. The glare in some control panels is due to light intensity adjustment to compensate the dark background caused by a lack of specific aniline blue staining. Scale bars = 50 μm.
As the sh1 mutant lacked membrane-localized SS (Fig. 5), we investigated if the callose accumulation is also affected in this mutant. As shown in Figure 7B, the sus1 mutant showed anoxic induction of dense callose deposits in the root tip (S24 panels). In contrast, the sh1 mutant showed much less callose in the anoxic root tip (Fig. 7C: S24). Furthermore, addition of Ca2+ to the submergence buffer substantially reduced callose in the non-mutant (Fig. 7D: S24 + Ca) or sus1 genotype (data not shown). On the contrary, addition of EGTA led to heavy callose induction in the root tip within 2 h of submergence and a mild induction even under aerobic incubation (Fig. 7D: S2 + EGTA and Air + EGTA). Taken together, treatments that favored the dephosphorylation and membrane localization of SS (e.g. anoxia and/or EGTA) also promoted callose deposition, whereas conditions allowing a cytosolic accumulation of the enzyme (sh1 mutation or Ca2+ supply) decreased callose.
Does Reduced Callose Induction in sh1 Mutants Mean Superior Submergence Tolerance?
If synthesis of callose is a symptom of cell death initiation (e.g. Wissemeier et al., 1987; Chen and Howlett, 1996), a decreased callose deposition in sh1 mutation should indicate reduced cell death and ultimately increased anoxia tolerance. This assumption was tested by measuring the submergence tolerance of non-mutant and mutant siblings in different sh1 alleles of maize differing also in their genetic background. The anoxia tolerance of the sus1 mutant is also provided for comparison (Table I). The sh1 mutations (in all genetic backgrounds) caused a slower shoot elongation rate compared with the non-mutant siblings and imposed a definite disadvantage during early seedling growth. Nevertheless, as shown in Table I, sh1 mutants were more tolerant to prolonged anoxia than their non-mutant siblings (also observed by K. Koch, personal communication) or the sus1 mutant. The roots in sh1 mutant showed increased survival and greater proliferation rate during post-submergence growth. This allowed the mutants to survive after longer periods of submergence (Table I; certain sh1 genotypes survived even after 96 h of submergence; data not shown).
Table I.
Effect of sh1 or sus1 mutation on post-submergence survival and growth of maize seedlings
| Treatment | Survival
|
Grams of Fresh
Wt per Seedling
|
|||||
|---|---|---|---|---|---|---|---|
| NMa | sh1 | sus1 | NMb
|
sh1
|
|||
| Root | Shoot | Root | Shoot | ||||
| % | |||||||
| Aerobic | 100 ± 0 | 95 ± 3 | 94 ± 5 | 0.43 ± 0.05 | 2.09 ± 0.10 | 0.45 ± 0.04 | 1.72 ± 0.05 |
| 24 h Subb | 94 ± 3 | 88 ± 17 | 93 ± 9 | 0.45 ± 0.05 | 1.35 ± 0.25 | 0.51 ± 0.05 | 1.22 ± 0.13 |
| 48 h Sub | 76 ± 8 | 80 ± 9 | 29 ± 19 | 0.22 ± 0.09 | 0.68 ± 0.19 | 0.41 ± 0.06 | 0.89 ± 0.07 |
| 72 h Sub | 15 ± 10 | 72 ± 9 | 1 ± 1 | 0.03 ± 0.01c | 0.42 ± 0.07 | 0.13 ± 0.02 | 0.54 ± 0.04 |
The survival data (means ± se) are 10 seedlings of each genotype (pooled from three different backgrounds) at every time point. Each genotype was tested at least twice. The growth data (means ± se) is shown only for the non-mutant and sh1 genotypes. Under anoxia, no significant difference was observed in the root and shoot growth patterns between non-mutant and sus1 seedlings.
NM, Non-mutant.
Sub, submerged.
The 72-h submerged non-mutant seedlings lost their primary roots during post-submergence growth and had only small seminal roots.
DISCUSSION
Our initial studies showed that Ca2+-dependent phosphorylation of SS is an important early event in anoxic maize seedling roots (Fig. 1). However, the functional significance of this post-translational modification was not immediately obvious until Amor et al. (1995) presented evidence that the plasma membrane association of SS probably complexed with glucan synthases in developing cotton fibers. The authors proposed that this association of SS facilitates the on-site supply of UDP-Glc needed for the synthesis of cellulose and callose. Membrane association of SS in maize tissues has also been observed subsequently (Carlson and Chourey, 1996; Buckeridge et al., 1999). Based on these reports and our finding that anoxia induces callose synthesis in the root apices of anoxic maize roots we hypothesized that the phosphorylation changes may be involved in its cellular distribution and structural changes in anoxic seedlings. More recent studies showing that the phosphorylation status of SS may act as a switch in its partitioning between the cytosol and membranes (Winter et al., 1997) lent support to our hypothesis. In this report we tested the occurrence of this molecular shuttling in the roots of anoxic maize seedlings, as well as its significance at cellular and organismal level.
The results indicate that dephosphorylation and subsequent membrane localization of SS may be part of the early events that culminate in the death of anoxic root tip. Furthermore, this response is isoform-specific (predominantly to SH1), as indicated by our genetic analysis and is related to the differential anoxia tolerance of Sh1 recessive and dominant genotypes. The evidence is as follows: Under prolonged anoxia, there was a localized induction of SS protein (largely SH1) in the root tip. However, phospholabeling of SH1 drastically declined under prolonged anoxia (Fig. 2B). At the same time, a significant component of SS migrated to the membrane fraction, only in the root tip (Fig. 4), which spatially coincided with the pattern of callose deposition in the root. sh1 mutants (lacking SH1) showed only a minor induction of SUS1, mostly in the phospho-form (thus localized to the cytosol) and had less severe callose induction (Figs. 5 and 7, B and C). Ca2+ deprivation by EGTA repressed SS phosphorylation (data not shown), leading to its increased membrane association, as well as callose deposition (Figs. 6 and 7D). The genotype, O2, and Ca2+-dependent effects on SS distribution and callose induction correlated with tissue necrosis and seedling survival (Table I; data not shown). We have initiated ultrastructural and immunocytochemical studies to further elucidate the specific interaction of SS with membranes, which appears to trigger callose biosynthesis. It is not clear if mechanisms other than phosphorylation may also contribute to the cellular distribution of SS, as indicated by phospho-labeling of membrane-bound SS (Fig. 1D). However, short-term labeling experiments may not allow a definitive quantitative comparison between phosphorylation and membrane association.
The differing long- and short-term (as well as tissue-specific) responses of SS to anoxia may reflect the cellular ATP levels, among several other factors. The meristem and the elongation zone (“root tip”) have the greatest demand for O2 (Armstrong et al., 1991) and may become quickly depleted of ATP under an O2 deficit. The increase in the phospho-SS during the initial hours of anoxia (Fig. 1) suggests that a release of the substrate (and/or its kinase) from the membranes might occur under anoxia, predominantly in the root axis. This was evinced by a mild increase in the soluble component of SS in 2-h submerged seedlings with a concomitant decrease in the membrane fraction when root axes or whole roots were used for analysis (Figs. 3 and 4D). An increased sucrolytic activity due to the release of SS into the cytoplasm may favor greater energy supply through the fermentative pathway in O2-deprived tissues. Furthermore, the immediate cessation of growth in anoxic seedlings obviates a role for a membrane-associated component of SS in the synthesis of cell wall components.
On the other hand, the increased membrane association of SS in the root tip soon after submergence may facilitate the death of the root tissues by diverting the limited carbon resources into the synthesis of callose. A rapid elimination of the tip, a metabolically demanding, but soon to be non-functional sink, may prolong the survival of the root axis and the shoot under anoxic conditions. Our recent work shows that removal of the root tip before submergence improves the seedling tolerance to anoxia (Subbaiah et al., 2000; also see Zeng et al., 1999). However, in most maize genotypes we studied, the root tip death is a prolonged process during which the necrosis spreads into the root axis and leads to seedling mortality. The sh1 mutation with its decreased total sucrolytic activity confined only to the cytosol may allow a better survival of the root during anoxia, as manifested by a decreased callose accumulation and superior submergence tolerance of this genotype. Previous analysis using this mutant also suggests that the SH1 is not required for maintaining the fermentative pathway even under prolonged anoxia (Guglielmenetti et al., 1996). Nonetheless, it is intriguing that the inducible form of SS under anoxia (i.e. SH1) confers a definite disadvantage to the post-stress survival of maize seedlings. SS induction may have an adaptive advantage in such genotypes where it leads to a rapid killing of the root tip. In addition, the induction of SH1 may provide an advantage under anoxia in the later stages of maize development.
Our results indicate that in anoxic maize roots, an isoform-specific post-translational regulation of SS directs them to different cellular compartments and consequently to different metabolic pathways (i.e. the synthesis of callose mostly driven by the SH1, whereas the SUS1, being soluble, contributes mainly to the glycolytic pathway). Chourey et al. (1998) recently proposed that a similar functional dichotomy of SS isoforms may exist in the developing endosperm, in that the SH1 plays a dominant role in supplying the substrate to cellulose biosynthesis, whereas the SUS1 provides monomers primarily to starch biosynthesis. As indicated in the present studies, a differential phosphorylation and intracellular distribution may determine the distinctive roles of SS isozymes in the developing endosperm also. Taken together, the post-translational regulation of SS by reversible phosphorylation appears to play a critical role in the responses of maize cells to environmental and developmental cues.
MATERIALS AND METHODS
Maize (Zea mays) Genotypes
The maize inbred line B73Ht was used in all experiments except when mutants were used. sh1-ref mutation was used in three different genetic backgrounds and the deletion mutant, sh1-bz1-x2, was also used for comparison. In all cases, sibling progeny of crosses between sh/sh and sh/Sh segregating 1:1 (mutant:non-mutant) were used. sus1 and SS double mutants were gifts from Dr. Prem Chourey. Segregating sh1 sus1: Sh1 sus1 were generated by crossing a double mutant line with a line homozygous for the sus1 mutant and selfing.
Plant Growth and Stress Treatments
Maize seedlings were grown as described previously (Subbaiah et al., 1994b). Seedlings used in the study had only the primary root (5–8 cm) and two small seminal roots (<10 mm). The terms “tip” and “axis” apply to the apical and distal portions of the primary root (Subbaiah et al., 2000). Anoxia was imposed by submerging the seedlings in “flooding buffer” or by incubating in an anaerobic chamber (Forma Scientific, Marietta, OH) as described in Subbaiah et al. (1994a). Results were similar with either treatment and hence the terms “submergence” and “anoxia” were used interchangeably.
32P Labeling Studies
For in vitro phospholabeling studies maize seedlings were incubated aerobically or submerged for 30 min to 2 h. Roots were excised in the anaerobic chamber and quickly frozen. The tissue was ground in Suc/Triton/EGTA buffer (50 mm Tris-Cl, pH 8.0, 0.3 m Suc, 2% [w/v] Triton X-100, 1 mm EGTA, and protease inhibitors) and fractionated on diethylaminoethyl-cellulose. Endogenous kinase activities were assayed by incubation of fractions in 10 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]-KOH, pH 7.5, 5 mm MgCl2, 1 mm CaCl2, and 0.5 μm γ-32P-ATP (0.01 μCi/assay). The reaction was terminated by boiling in SDS-containing sample buffer and the products were separated by acrylamide gel electrophoresis.
To separate membrane and soluble fractions, the tissue was ground in the STE buffer lacking Triton X-100. After clarifying the extract by centrifugation at 10,000g for 5 min, microsomes were pelleted at 100,000g for 90 min. The supernatant was considered as the soluble fraction. Protein was estimated using the Detergent-Compatible Protein Assay system (Bio-Rad, Hercules, CA).
For in vivo labeling, five to six seedlings per treatment were incubated in 4 mL of sterile deionized water containing 0.5 mCi 32Pi (400–800 mCi mL−1, carrier free), such that part of the root was covered by the solution. After 2 h of aerobic incubation sets of seedlings were transferred to the anaerobic chamber. The aerobic pre-incubation allowed an almost equal 32Pi incorporation among treatments. The duration of labeling was equal for aerobic and anoxic sets. Root tissue was collected at the indicated time points, rinsed briefly in 2 L of 100 mm Na2HPO4, pH 8.0, containing 5 mm EDTA and 1 mm EGTA, and snap-frozen in liquid N2. The frozen tissue was powdered in liquid N2 and extracted in 10 mm HEPES-KOH, pH 7.5, 220 mm Suc, 15 mm EDTA, 2 mm EGTA, 10 nm calyculin A, and protease inhibitors (Complete,2 Roche Molecular Biochemicals, Indianapolis). The clarified homogenate was separated into soluble and microsomal fractions by ultracentrifugation as described above. Trichloroacetic acid-precipitable proteins from an aliquot of each fraction were estimated and counted for radioactivity. Aliquots containing equal amount of protein were processed for immunoprecipitation of SS using monoclonal antisera that cross-react with both forms of SS (HL 253, a gift from Dr. Prem Chourey). Proteins were resuspended in radio-immunoprecipitation analysis buffer (Anderson and Blobel, 1983), precleared using preimmune serum and Pansorbin (Calbiochem, La Jolla, CA), and incubated with anti-SS antisera and Pansorbin. The immunoprecipitate was washed in radio-immunoprecipitation analysis buffer and resolved by SDS-PAGE.
Callose Staining
Roots were rapidly fixed under vacuum in 4% (w/v) formaldehyde buffered at pH 7.5 by 25 mm K-PO4. For callose detection the tissue was incubated in 0.1% (w/v) aniline blue (in 0.1 m K3-PO4, pH 9), washed once in 50% (v/v) ethanol followed by three times in water, squashed, and viewed in a fluorescence microscope (model AH2-BT, Olympus Vanox Photomicroscope, Olympus Optical, Tokyo). The excitation was by 390 nm light from a xenon lamp and fluorescence was collected using a blue filter. The representative sections were photographed by a charge-coupled device video camera system (model VI-470, Optronics Engineering, Goleta, CA) and color video printer (model UP-3000, Mavigraph, Sony, Japan). The authenticity of callose staining was confirmed by immunological detection using callose-specific antibodies (BioSupplies Australia, Victoria, Australia). We could not quantify callose, as none of the published methods gave a complete or uniform extraction. The extractability varied among treatments, probably reflecting a variable extent of cell wall-integrated callose.
Gel Electrophoresis, Western Analysis, and Autoradiography
Protein samples were separated in a 6% to 10% linear gradient acrylamide gel in the presence of SDS. The gels were stained, dried, and autoradiographed or were processed for western analysis after electroblotting onto nitrocellulose. The blotted membrane was blocked and probed with monoclonal anti-SS antisera, followed by peroxidase-conjugated anti-mouse serum. The signal was developed by chemiluminescence (Super Signal Chemiluminescence Substrate, Pierce, Rockford, IL). Protein electrophoresis data (after digitization) was analyzed using an NIH Image program available on the public domain. The band intensities were subtracted from background and integrated with areas. Each figure shown in the manuscript is a representative of three different experiments with less than 10% variability.
Submergence Tolerance Tests
Maize seedlings were submerged and post-submergence survival, as well as growth, was measured as described earlier (Subbaiah et al., 1994b).
ACKNOWLEDGMENTS
We thank Prem S. Chourey (University of Florida, Gainesville) for his generous gift of SS monoclonal antisera and seeds of sus1 and double mutants, Karen E. Koch (University of Florida, Gainesville) for sharing unpublished data, Lane Rayburn (University of Illinois, Urbana) for providing microscopy facilities, and Daniel Bush (University of Illinois, Urbana) for critical reading of the manuscript. We also thank Lizz Funk for her assistance during the initial stages of this project.
Footnotes
This work is supported by the National Research Initiative Competitive Grants Program (grant no. 96–35100–3143 to M.M.S. and C.C.S.) from the U.S. Department of Agriculture and by the Illinois Council of Food and Agricultural Research (grant no. 00I–062–3 to C.C.S.).
Product names are necessary to report factually on available data; however, neither the U.S. Department of Agriculture nor the University of Illinois guarantees or warrants the standard of the product, and the use of the names implies no approval of the product to the exclusion of others that may be suitable.
LITERATURE CITED
- Amor Y, Haigler C, Johnson S, Wainscott M, Delmer DP. A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA. 1995;92:9353–9357. doi: 10.1073/pnas.92.20.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ, Blobel G. Immunoprecipitation of proteins from cell-free translation. Methods Enzymol. 1983;96:111–120. doi: 10.1016/s0076-6879(83)96012-3. [DOI] [PubMed] [Google Scholar]
- Armstrong W, Beckett PM, Justin SHFW, Lythe S. Modeling and other aspects of root aeration by diffusion. In: Jackson MB, Davies DD, editors. Plant Life under Oxygen Deprivation. The Hague, The Netherlands: SPB Academic Publishing; 1991. pp. 267–282. [Google Scholar]
- Buckeridge MS, Vergara CE, Carpita NC. The mechanism of synthesis of a mixed-linkage (1→3), (1→4) β-d-glucan in maize: evidence for multiple sites of glucosyl transfer in the synthase complex. Plant Physiol. 1999;120:1105–1116. doi: 10.1104/pp.120.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SJ, Chourey PS. Evidence for plasma membrane-associated forms of sucrose synthase in maize. Mol Gen Genet. 1996;252:303–310. doi: 10.1007/BF02173776. [DOI] [PubMed] [Google Scholar]
- Chen CY, Howlett BJ. Rapid necrosis of guard cells is associated with the arrest of fungal growth in leaves of Indian mustard (Brassica juncea) inoculated with avirulent isolates of Leptosphaeria maculans. Physiol Mol Plant Pathol. 1996;48:73–81. [Google Scholar]
- Chourey PS, Taliercio EW, Carlson SJ, Ruan Y-L. Genetic evidence that the two isozymes of sucrose synthase present in developing maize endosperm are critical, one for cell wall integrity and the other for starch biosynthesis. Mol Gen Genet. 1998;259:88–96. doi: 10.1007/s004380050792. [DOI] [PubMed] [Google Scholar]
- Fankhauser C, Yeh K-C, Lagarias JC, Zhang H, Elic TD, Chory J. PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science. 1999;284:1539–1541. doi: 10.1126/science.284.5419.1539. [DOI] [PubMed] [Google Scholar]
- Ferreira PC, Hemerly AS, van Montagu M. A protein phosphatase 1 from Arabidopsis thaliana restores temperature sensitivity of a Schizosaccharomyces pombe cdc25ts/wee1− double mutant. Plant J. 1993;4:81–87. doi: 10.1046/j.1365-313x.1993.04010081.x. [DOI] [PubMed] [Google Scholar]
- Guglielminetti L, Alpi A, Perata P. Shrunken-1-encoded sucrose synthase is not required for the sucrose-ethanol transition in maize under anaerobic conditions. Plant Sci. 1996;119:1–10. [Google Scholar]
- Hannah LC, Frommer W, Su J-C, Chourey P, Park W. Sucrose synthases. Plant Mol Biol. 1994;12:72–73. [Google Scholar]
- Huber JLA, Huber SC. Role and regulation of sucrose-phosphate synthase in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:431–444. doi: 10.1146/annurev.arplant.47.1.431. [DOI] [PubMed] [Google Scholar]
- Huber SC, Huber JL, Liao PC, Gage DA, McMichael RW, Chourey PS, Hannah LC, Koch K. Phosphorylation of serine-15 of maize leaf sucrose synthase: occurrence in vivo and possible regulatory significance. Plant Physiol. 1996;112:793–802. doi: 10.1104/pp.112.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Blackshear PJ, Johnson JD, McLaughlin S. Phosphorylation reverses the membrane association of peptides that correspond to the basic domains of MARCKS and neuromodulin. Biophys J. 1994;67:227–237. doi: 10.1016/S0006-3495(94)80473-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Kado RT, Guern J, Chrispeels MJ. Phosphorylation regulates the water channel activity of the seed-specific aquaporin α-tip. EMBO J. 1995;14:3028–3035. doi: 10.1002/j.1460-2075.1995.tb07305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty DR, Shaw JR, Hannah LC. The cloning, genetic mapping, and expression of the constitutive sucrose synthase locus of maize. Proc Natl Acad Sci USA. 1986;83:542–546. doi: 10.1073/pnas.83.23.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElfresh KC, Chourey PS. Anaerobiosis induces transcription but not translation of sucrose synthase in maize. Plant Physiol. 1988;87:542–546. doi: 10.1104/pp.87.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard B, Van Toai T, Chourey P, Saglio P. Evidence for the critical role of sucrose synthase for anoxic tolerance of maize roots using a double mutant. Plant Physiol. 1998;116:1323–1331. doi: 10.1104/pp.116.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LJ, Chen Y-C, Chourey PS. Anaerobic treatment alters the cell specific expression of Adh-1, Sh and Sus genes in roots of maize seedlings. Mol Gen Genet. 1989;218:33–40. [Google Scholar]
- Ruan Y-L, Chourey PS, Delmer DP, Perez-Grau L. The differential expression of sucrose synthase in relation to diverse patterns of carbon partitioning in developing cotton seed. Plant Physiol. 1997;115:375–385. doi: 10.1104/pp.115.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer B, Werr W, Starlinger P, Bennett CD, Zokolica M, Freeling M. The shrunken gene on chromosome 9 of Zea mays L. is expressed in various plant tissues and encodes an anaerobic protein. Mol Gen Genet. 1986;205:461–468. doi: 10.1007/BF00338083. [DOI] [PubMed] [Google Scholar]
- Stone JM, Trotochaud AE, Walker JC, Clark SE. Control of meristem development by CLAVATA1 receptor kinase and kinase-associated protein phosphatase interactions. Plant Physiol. 1998;117:1217–1235. doi: 10.1104/pp.117.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Walker JC. Plant protein kinase families and signal transduction. Plant Physiol. 1995;108:451–457. doi: 10.1104/pp.108.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Bush DS, Sachs MM. Elevation of cytosolic calcium precedes anoxic gene expression in maize suspension cultured cells. Plant Cell. 1994a;6:1747–1762. doi: 10.1105/tpc.6.12.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Kollipara K, Sachs MM. A Ca2+-dependent cysteine protease is associated with anoxia-induced root tip death in maize. J Exp Bot. 2000;51:721–730. [PubMed] [Google Scholar]
- Subbaiah CC, Zhang J, Sachs MM. Involvement of intracellular calcium in anaerobic gene expression and survival of maize seedlings. Plant Physiol. 1994b;105:369–376. doi: 10.1104/pp.105.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Huber J, Huber SC. Membrane association of sucrose synthase: changes during the graviresponse and possible control by protein phosphorylation. FEBS Lett. 1997;420:151–155. doi: 10.1016/s0014-5793(97)01506-8. [DOI] [PubMed] [Google Scholar]
- Winter H, Huber J, Huber SC. Identification of sucrose synthase as an actin-binding protein. FEBS Lett. 1998;430:205–208. doi: 10.1016/s0014-5793(98)00659-0. [DOI] [PubMed] [Google Scholar]
- Wissemeier AH, Klotz F, Horst WJ. Aluminum-induced callose synthesis in roots of soybean (Glycine max L.) J Plant Physiol. 1987;129:487–492. [Google Scholar]
- Zeng Y, Avigne WT, Koch KE. Rapid repression of maize invertase by low oxygen: invertase/sucrose synthase balance, sugar signaling potential, and seedling survival. Plant Physiol. 1999;121:599–608. doi: 10.1104/pp.121.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Wu Y, Avigne WT, Koch KE. Differential regulation of sugar-sensitive sucrose synthases by hypoxia and anoxia indicate complementary transcriptional and post-transcriptional responses. Plant Physiol. 1998;116:1573–1583. doi: 10.1104/pp.116.4.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]