Abstract
Background
Traumatic brain injury (TBI) is a major public health problem and a fundamental cause of morbidity and mortality worldwide. The burden of TBI disproportionately affects low‐ and middle‐income countries. Intracranial hypertension is the most frequent cause of death and disability in brain‐injured people. Special interventions in the intensive care unit are required to minimise factors contributing to secondary brain injury after trauma. Therapeutic positioning of the head (different degrees of head‐of‐bed elevation (HBE)) has been proposed as a low cost and simple way of preventing secondary brain injury in these people. The aim of this review is to evaluate the evidence related to the clinical effects of different backrest positions of the head on important clinical outcomes or, if unavailable, relevant surrogate outcomes.
Objectives
To assess the clinical and physiological effects of HBE during intensive care management in people with severe TBI.
Search methods
We searched the following electronic databases from their inception up to March 2017: Cochrane Injuries' Specialised Register, CENTRAL, MEDLINE, Embase, three other databases and two clinical trials registers. The Cochrane Injuries' Information Specialist ran the searches.
Selection criteria
We selected all randomised controlled trials (RCTs) involving people with TBI who underwent different HBE or backrest positions. Studies may have had a parallel or cross‐over design. We included adults and children over two years of age with severe TBI (Glasgow Coma Scale (GCS) less than 9). We excluded studies performed in children of less than two years of age because of their unfused skulls. We included any therapeutic HBE including supine (flat) or different degrees of head elevation with or without knee gatch or reverse Trendelenburg applied during the acute management of the TBI.
Data collection and analysis
Two review authors independently checked all titles and abstracts, excluding references that clearly didn't meet all selection criteria, and extracted data from selected studies on to a data extraction form specifically designed for this review. There were no cases of multiple reporting. Each review author independently evaluated risk of bias through assessing sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias.
Main results
We included three small studies with a cross‐over design, involving a total of 20 participants (11 adults and 9 children), in this review. Our primary outcome was mortality, and there was one death by the time of follow‐up 28 days after hospital admission. The trials did not measure the clinical secondary outcomes of quality of life, GCS, and disability. The included studies provided information only for the secondary outcomes intracranial pressure (ICP), cerebral perfusion pressure (CPP), and adverse effects.
We were unable to pool the results as the data were either presented in different formats or no numerical data were provided. We included narrative interpretations of the available data.
The overall risk of bias of the studies was unclear due to poor reporting of the methods. There was marked inconsistency across studies for the outcome of ICP and small sample sizes or wide confidence intervals for all outcomes. We therefore rated the quality of the evidence as very low for all outcomes and have not included the results of individual studies here. We do not have enough evidence to draw conclusions about the effect of HBE during intensive care management of people with TBI.
Authors' conclusions
The lack of consistency among studies, scarcity of data and the absence of evidence to show a correlation between physiological measurements such as ICP, CCP and clinical outcomes, mean that we are uncertain about the effects of HBE during intensive care management in people with severe TBI.
Well‐designed and larger trials that measure long‐term clinical outcomes are needed to understand how and when different backrest positions can affect the management of severe TBI.
Plain language summary
Elevation of the head during intensive care management in people with severe traumatic brain injury
Review question
How does the position of the backrest of the bed (and therefore the position of the head) affect people who have had an injury to the head that caused serious brain damage?
Background
Raised pressure within the skull (intracranial hypertension) because of swelling is the most common cause of death and disability in brain‑injured people. How well someone with intracranial hypertension recovers often depends on how they are treated. Some people think that some positions of the backrest of the bed (called the 'head‐of‐bed elevation' or HBE) might affect this pressure and improve the person's recovery. The position of the backrest of the bed is a simple and cheap intervention. This is important as most brain injury happens in low‐ and middle‐income countries with relatively undeveloped health systems and few resources to deal with brain injury.
Search date
In March 2017 the review authors searched for randomised studies.
Study characteristics
We found three small studies, with a total of 20 people (11 adults and 9 children). The studies had a cross‐over design (participants received the study interventions in a random order, and served as their own control) and looked at the effect of different head positions. Researchers measured the pressure inside the skull (intracranial pressure (ICP)) and the pressure gradient causing blood flow to the brain (cerebral perfusion pressure (CPP)). Two studies were funded by research grants from the national Department of Health, and one study received no funding.
Key results
At the time of follow‐up 28 days following hospital admission, one child had died. None of the studies assessed quality of life, Glasgow Coma Scale (a measurement of how conscious someone is), or disability. The studies gave varied results and our certainty in the results is very low, so we do not consider the body of evidence to be reliable. None of the studies found any evidence of a change in CPP due to different backrest positions. The results for ICP were more mixed but there is still no convincing evidence that HBE changes ICP. There is insufficient evidence to say whether the intervention is safe. One child experienced an increase in ICP in response to the intervention, which resolved when the height of the bed was returned to the normal position. We are uncertain about the effects of different backrest positions in people with serious brain injury.
Quality of the evidence
The body of evidence for this research question is very low due to variability in physiological response in the study participants, unclear risk of bias in the study methods, and the small number of people enrolled in each study.
Conclusions
We are uncertain about the effects of different backrest positions in people with serious brain injury. Well‐designed and larger trials are needed. Trials also need to measure the right patient outcomes over a longer period of time in order to understand how and when different backrest positions can affect people with brain injury.
Summary of findings
Summary of findings for the main comparison. Altered backrest position for severe traumatic brain injury.
| Altered backrest position for severe traumatic brain injury | ||||
|
Patient or population: people with severe traumatic brain injury (adults and children) Settings: intensive care Intervention: altered backrest position (head‐of‐bed elevation (HBE)) Comparison: any other backrest position (the studies used a flat position or 30 degree elevation as the baseline) | ||||
| Outcomes | Results | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Mortality | Out of a total of 20 participants who served as their own control, there was one death at final follow‐up 28 days after hospital admission | 20 (3 studies) | ⊕⊝⊝⊝ Very low1,2 | We are uncertain about the effects of altered backrest position in participants with severe TBI |
| Intracranial pressure (ICP) Follow‐up: ≤ 1 h | The findings across these three small studies were inconsistent, with two showing no evidence of an effect of HBE on ICP, and one showing a negative linear association with high intra‐study heterogeneity | 20 (3 studies) | ⊕⊝⊝⊝ Very low1,2,3 | Due to the unclear risk of bias, inconsistency and the very small number of participants, we are uncertain about the effect of altered backrest position on ICP in participants with severe TBI |
| Cerebral perfusion pressure (CPP) Follow‐up: ≤ 1 h | These three small studies showed no effect of HBE on CCP | 20 (3 studies) | ⊕⊝⊝⊝ Very low1,2 | Due to the unclear risk of bias, and in particular the very small number of participants, we are uncertain about the effect of altered backrest position on CPP in participants with severe TBI |
|
Quality of life; Glasgow Coma Score; Disability |
No studies measured quality of life, Glasgow Coma Score or disability | |||
| Adverse events | All three studies reported adverse events, and none occurred. One participant experienced an increase in ICP in response to the intervention, which resolved when the height of the bed was returned to the baseline position. | 20 (3 studies) |
⊕⊝⊝⊝ Very low1,2 | We are uncertain about the adverse effects of altered backrest position in participants with severe TBI |
| CPP: cerebral perfusion pressure; HBE: head‐of‐bed elevation; ICP: intracranial pressure; TBI: traumatic brain injury | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
1Downgraded one level due to limitations in design and execution leading to risk of bias: there are methodological aspects that are unclear (allocation concealment, blinding of participants and personnel, and outcome assessment). 2Downgraded two levels due to a high imprecision: the number of participants is very low and the confidence intervals very wide. 3Downgraded one level due to inconsistency: the three studies that measured this outcome had inconsistent results.
Background
Description of the condition
Traumatic brain injury (TBI) is a major public health problem and a fundamental cause of morbidity and mortality worldwide. Improving patient outcomes following TBI requires the implementation of effective, evidence‐based interventions (March 1990; Meixensberger 1997; Ng 2004; Puvanachandra 2009). The global incidence of TBI is approximately 200 per 100,000 population, with mortality of 20 per 100,000 population. The incidence in different countries ranges from 91 per 100,000 population to 430 per 100,000 hospital admissions. Some studies show regional variations, with higher mortality for people injured in rural and low‐income settings compared to urban and high‐income areas (Reilly 2007). It has been estimated that TBI affects over 10 million people annually and that TBI will surpass many diseases as the leading cause of mortality and morbidity by the year 2020 (Zitnay 2005). It is also the most common disabling injury accounting for 26% of all deaths due to trauma in the USA, where it is also the leading cause of death for children (Bergen 2008). Children are over‐represented in the 1.4 million cases of TBI and the 50,000 deaths each year estimated by the Centers for Disease Control and Prevention (Langlois 2005).
The burden of TBI injury disproportionately affects low‐ and middle‐income countries (LMICs), which not only face a higher prevalence of risk factors for TBI, but also have less developed health systems than high‐income countries and fewer resources to deal with the associated health outcomes. Thus, both the incidence and case‐fatality of this type of injury in LMICs can be devastating (Puvanachandra 2008). Simple, cost‐effective interventions that improve outcomes following TBI would be of particular importance to physicians in LMICs.
Intracranial hypertension is a common pathophysiology in TBI that contributes to worsened outcomes. The prognosis of people with intracranial hypertension often depends on the medical, surgical and critical care management resources available to treat it. Intracranial hypertension may result from one or more secondary injury mechanisms in the injured brain, including cerebral oedema and intracranial mass lesions.
Intracranial hypertension is the most frequent cause of death and disability in brain‐injured people. Early medical treatment for hypoxia and hypotension, in the pre‐hospital phase and in the emergency department, are essential. However, special interventions in the intensive care unit (ICU) are also required to minimise factors contributing to secondary brain injury, cerebral oedema, and raised intracranial pressure. Some of these interventions include head‐of‐the‐bed elevation (HBE) (also known as backrest elevation), sedation, use of paralytic agents, cerebrospinal fluid drainage, osmotic therapy including mannitol and hypertonic saline, barbiturates, hyperventilation and hypothermia (Meyer 2010a; Meyer 2010b). There has been increasing clinical and basic research to understand the pathophysiologic responses associated with TBI, including differences between paediatric brain responses that are distinct from the traumatised adult brain. In addition, there appear to be age‐dependent responses following trauma (Adelson 1997; Aldrich 1992; Huh 2009).
Description of the intervention
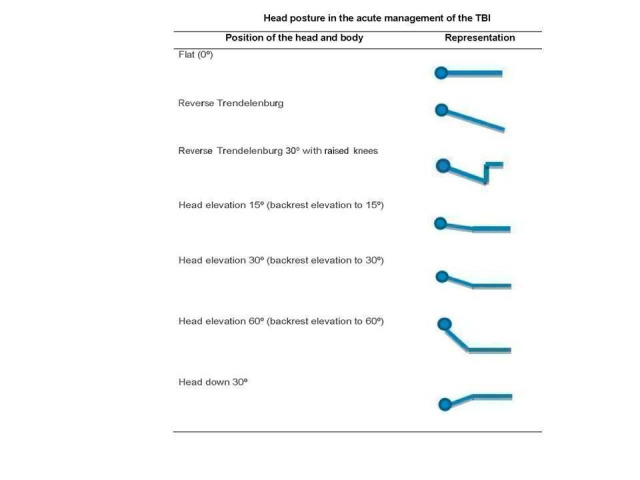
The aim of this review is to evaluate the evidence related to the clinical and physiological effects of different backrest positions of the head including flat, 30° elevated, 30° with knee gatch elevation and reverse Trendelenburg, and more than 30° of elevation, in people with severe TBI (Glasgow Coma Score less than 9) during the acute management of TBI. These different backrest positions are illustrated in Figure 1.
1.
How the intervention might work
Management of people with TBI in the ICU focuses on prevention of secondary brain injury through resuscitation, multi‐system stabilisation, and the treatment of intracranial hypertension. Therapeutic head positioning has known effects on multiple organ systems, including beneficial effects on brain physiology in severe TBI (Sullivan 2000).
Prevention of secondary injury resulting from increased intracranial pressure (ICP) and the promotion of adequate cerebral blood flow (CBF) are major concerns of healthcare professionals taking care of people with TBI. Some researchers argue that people with intracranial hypertension should be placed in a horizontal position. The rationale behind this is that this position will increase the cerebral perfusion pressure (CPP) and thereby improve CBF. However, ICP is generally substantially higher when the person is in the horizontal position (Meixensberger 1997). Since the 1990s, studies have indicated that raising the head of the bed lowers the ICP. However, when perfusion pressure has been measured along with ICP, results have been conflicting: some investigators found that CPP decreased with HBE, while others found no change (March 1990). Brain oedema and the resulting increase in ICP may be reduced using HBE as a conventional procedure for people with TBI with intracranial hypertension. It is performed with the intention of reducing ICP by means of a non‐invasive physical intervention. Venous return improvement and cerebrospinal fluid (CSF) distribution to the subarachnoid spinal space are, in theory, some of the benefits of raising the head above the heart level (Fan 2004; Magnaes 1978). Major routes for cerebral venous drainage include the deep and superficial sinus system and the internal and external jugular veins (Kenning 1981). These venous systems are valveless channels that allow cerebral venous return without interruption after HBE. Postural impact on the systemic haemodynamics causes sudden displacement of approximately 30% of the blood volume from the upper body into the peripheral veins. In addition to the intravascular pressure in the heart, gravity contributes to more pressure to the vessels below the heart, which is why standing up causes the compliant veins to distend (Fan 2004). This is known as a venous pooling, an effect of hydrostatic pressure. Together, the redistribution of CSF and venous pooling decrease the venous return to the heart (Fan 2004).
It is a common practice to position people with TBI in bed with the head elevated above the level of the heart in order to reduce ICP. The theoretical basis is that the head is above the level of the heart on the vertical axis and, as a result, CSF is redistributed from the cranial to the spinal subarachnoid space (Kenning 1981), facilitating cerebral venous return (Magnaes 1976; Magnaes 1978; Marmarou 1975; Potts 1973). However, it has been reported that the supine position may improve CPP (Ng 2004). In summary, the effect of 0°, 30º and more than 30° HBE on ICP, CPP, CBF, mean carotid pressure, and other cerebral and systemic physiological parameters has been studied in people with head‐injuries (Meixensberger 1997) with different results, and to date there is no strong evidence to suggest a beneficial influence from any HBE strategy. Some data suggest that an individual approach to HBE at different degrees is preferred over a flat position. HBE can produce a dilemma for healthcare providers because, in certain circumstances, elevating the head of the bed may decrease ICP, while HBE may risk increasing ICP and cerebral ischaemia in people with impaired cerebral auto‐regulation or unstable arterial blood pressure (Ledwith 2010; Rosner 1986; Simmons 1997). Aditionally, to date, paediatric TBI case series rarely include a description of the degree of HBE (Tasker 2012). It is currently unknown what the optimal or 'age‐appropriate' CPP for paediatric TBI is, and there is no evidence that targeting a specific CPP for a specific age of the paediatric patient improves outcome (Chambers 2001; Huh 2009). In a recent study, CPP values of 53 mmHg for two to six year‐olds, 63 mmHg for seven to 10 year‐olds, and 66 mmHg for 11‐16 year‐olds were suggested to represent minimum values for a favourable outcome (Chambers 2005).
Why it is important to do this review
Several studies have reached divergent conclusions regarding the impact of HBE (Durward 1983; March 1990; Meyer 2010b; Winkelman 2000), and to date there is no strong evidence that suggests a beneficial effect from any one specific HBE strategy. Paediatric TBI case series rarely include a description of degree of HBE and while no paediatric studies are known, the same degree of HBE in adults with midline position to promote venous drainage is currently recommended in the paediatric guidelines (Adelson 2005; Figaji 2009; Huh 2009; Prabhakaran 2004; Tasker 2012). For these reasons, a systematic analysis of the available literature is needed.
In addition, many of the interventions used in the acute management of TBI in intensive or critical care are not available in low‐income countries where the majority of the TBI population is present (Stelfox 2008). Therefore, assessing the utility of this simple intervention is of great interest.
Objectives
To assess the clinical and physiological effects of HBE during intensive care management in people with severe TBI.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCT) involving patients with TBI who underwent HBE or backrest elevation. Studies may have had a parallel or crossover design.
Types of participants
Adults and children over two years of age with severe TBI (Glasgow Coma Scale Score less than 9).
Studies performed in participants less than two years old were ineligible, because small children have unfused skulls (the ICP pathophysiology seems to be the same in children as in adults, but there is still a lot of discussion regarding the differences between ICP physiology in fused and unfused skulls).
Types of interventions
Any therapeutic HBE including supine (flat) or different degrees with or without knee gatch or reverse Trendelenburg (Figure 1), that is applied during the acute management of the TBI.
Types of outcome measures
Primary outcomes
Mortality at the end of study follow‐up.
Secondary outcomes
Quality of life (Short Form‐36 or other validated scale) at 90 days or at the end of study follow‐up (if it lasted less time)
Glasgow Coma Score at the end of study follow‐up
Disability (Glasgow Outcome Scale, Glasgow Outcome Scale‐Extended) at 90 days or at the end of study follow‐up (if it lasted less time)
Intracranial Pressure (ICP) after the intervention
Cerebral Perfusion Pressure (CPP) after the intervention
Adverse effects over the course of the study
Information size calculation
We require outcome data from at least 6,084 people in order to have certainty in the effect of the treatment on mortality, assuming 25% mortality in the control group and a 10% benefit with the intervention, at 90% power at the 5% statistical significant level. We are aware that this estimate may be under‐ or over‐estimated with the current lack of prior data. In a future update of this review, if there are additional studies to consider we may revise this estimate accordingly.
Search methods for identification of studies
In order to reduce publication and retrieval bias we did not restrict our search by date, language or publication status.
Electronic searches
The Cochrane Injuries Group's Information Specialist ran searches on the following electronic databases:
Cochrane Injuries' Specialised Register (SR‐INJ) (2 March 2017);
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 2) in the Cochrane Library;
MEDLINE (Ovid SP) (1950 to 2 March 2017);
Embase (Ovid SP) (1980 to 2 March 2017);
CINAHL (EBSCO) (1982 to 2 March 2017);
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to 2 March 2017);
ISI Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) (1990 to 2 March 2017);
International trial registers (ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
The search strategies are reported in Appendix 1.
Searching other resources
We reviewed the bibliographies of all identified RCTs and other review articles on management of TBI.
In March 2017, the Cochrane Injuries Group's Information Specialist ran a cited reference search on the Web of Science for research citing the three included studies to date (Agbeko 2012; March 1990; Winkelman 2000). We were also in contact with the authors of studies selected for inclusion in the review.
Data collection and analysis
Selection of studies
The Cochrane Injuries Group's Information Specialist ran the search for studies, collated the search results, removed duplicates and sent the list of individual records to the review authors for screening. Two review authors (JDA, AR) checked all titles and abstracts independently and excluded references that did not clearly meet all selection criteria. For those that were unclear or potentially relevant, we obtained the full text for independent assessment. These same two review authors decided which trials met the inclusion criteria. There were no cases of multiple reporting. There were no disagreements in the decisions made. A third author (GU) verified the study selection.
Data extraction and management
Two review authors (JDA, AR) extracted data from selected studies on to a data extraction form specifically designed for this review and a third author (GU) verified the data extraction.
Assessment of risk of bias in included studies
Two review authors (JDA, AR) independently assessed the studies for methodological quality using the Cochrane 'Risk of bias' tool, which examines bias in studies (Higgins 2011), and a third author (GU) verified the judgements.
We examined the adequacy of the methods used to generate the allocation sequence, the methods of allocation concealment and the level of blinding (clinician, participant or outcome assessor). We further examined the presence of incomplete outcome data, selective reporting, and other potential sources of bias (see 'Risk of bias in included studies').
We classified each domain as 'high', 'unclear', or 'low' risk of bias. We described the reason for each judgment from details provided in the trial reports. We considered a trial to be at 'low' risk of bias when it concealed allocation and blinded participants and outcome assessors, if it reported complete outcome data, and where we did not suspect selective outcome reporting (we assessed prespecification of outcomes from methods sections of trial publications). If one or more of these key domains were not met, we considered the trial to be at 'high' risk of bias. If one or more of these key domains were unclear, we considered the trial as 'unclear' with respect to risk of bias (see table 8.7.a of the Cochrane Handbook for Systematic Reviews of Interventions) (Higgins 2011).
Where there was disagreement in judgements of bias, we discussed this and reached a consensus. Where information was unavailable to make a judgement, we contacted the study authors and sought further information.
Measures of treatment effect
We had planned to calculate the pooled risk ratio (RR) for dichotomous outcomes, and the mean difference (MD) and its variance for continuous outcomes, and the corresponding 95% confidence intervals (CI) using study data based on an intention‐to‐treat (ITT) analysis. However, we have only presented a narrative description of results, as we thought pooling of results across studies was not appropriate, and therefore we did not calculate any measure of global effect. We have presented and discussed results in the review as they were reported in the study reports. Data were available for continuous outcomes (ICP and CPP), and results were expressed as a mean difference in one study, in a graphical form in another and as an average change per 10 cm of head elevation in another. We considered a P value < 0.05 as statistically significant.
Unit of analysis issues
The unit of analysis was the participant. All RCTs included in the review were cross‐over trials, where participants received all interventions being tested across the study in a random order and served as their own control. We analysed these studies according to Elbourne 2002.
Dealing with missing data
For each outcome variable we had planned to evaluate the number of losses, their distribution in each study group, and analyse data on an intention‐to‐treat basis. If there were a greater number of losses in one branch, we planned to conduct a sensitivity analysis, with missing values considered as therapeutic failure or no response. However, due to a lack of data, we were unable to carry out a sensitivity analysis.
In the Winkelman 2000 study, four out of 12 recrutied patients dropped out before randomisation. Data from the eight study participants are incuded in the review. In the Agbeko 2012 study, one out of 10 recruited patients dropped out before randomisation. One participant dropped out following randomisation and one head of bed change. As there are no comparison data to analyse for this one participant, the study reported the usable data for eight participants. Data were analysed as presented in the trials, without performing any type of statistical manipulation such as imputation.
Assessment of heterogeneity
We would have assessed heterogeneity through inspection of a graphical display of the estimated treatment effects from the included trials. We had planned to use the Chi2 statistic, with statistical significance set at P value < 0.10 (Deeks 2011). In addition, we had planned to investigate the degree of heterogeneity by calculating the I2 statistic (Higgins 2003).
However, because we could not combine results in a meta‐analysis, we did not formally assess heterogeneity. Moreover, due to the limited data available and the lack of statistical power in all the RCTs, we have only considered the consistency of the results (trends).
Assessment of reporting biases
We planned to assess publication bias by funnel plots, however, there were fewer than 10 included studies and so the analysis would not have been meaningful (Sterne 2011). We planned to assess reporting bias by comparing the protocol for each study with the final study report.
Data synthesis
We had planned to use a fixed‐effect model if I2 was less than 40% and a random‐effects model if there was more statistical heterogeneity between studies in the meta‐analysis.
For cross‐over studies, we calculated the RR or the MD for differences of paired outcomes, along with the standard errors and 95% CIs following the indications of Elbourne 2002 and we assumed a within‐participant correlation coefficient of 0.05.
Subgroup analysis and investigation of heterogeneity
It was not possible to perform a subgroup analysis according to the age (less than 55 years or 55 years and over) of the participants and severity (Acute Injury Score: 3 or less or more than 3) of the injuries, as we stated in the protocol, due to lack of data.
Sensitivity analysis
We had planned to carry out sensitivity analyses to explore the effect of potential bias associated with inadequate allocation concealment and the effect of published versus unpublished studies on the outcomes of the review. However, given the very limited data available, we were unable to perform a sensitivity analysis.
'Summary of findings' tables
We constructed a 'Summary of findings' table using the GRADEpro Guideline Development Tool (GRADEpro GDT 2015). We included all outcomes in the 'Summary of findings' table, although the included studies only measured mortality, ICP, CCP, and provided limited information on adverse effects. Since we could not pool the results, we summarised them narratively. We used the principles of the GRADE system to assess the quality of the body of evidence associated with these outcomes. The GRADE method appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Evaluation of the quality of a body of evidence considers within‐study risk of bias, the directness of the evidence, heterogeneity (inconsistency) in the data, precision of effect estimates, and risk of publication bias (Schünemann 2011).
Results
Description of studies
Results of the search
The electronic searches retrieved a total of 686 individual references after removing duplicates (Figure 2). We excluded 533 after reading the abstracts, as they did not fulfil the inclusion criteria. We excluded 153 studies after reviewing the full text and selected 17 studies potentially relevant for this review. We excluded 14 of the 17 studies because all except one were not truly randomised trials (they were controlled studies where all participants were placed in different positions in a sequential order). In addition, many of them studied a mixed population of critical patients with intracranial hypertension including but not limited to traumatic brain injury (TBI). Only one randomised controlled trial (RCT) with a parallel design that was performed in children with severe TBI was excluded (Prabhakaran 2004). We excluded it because it compared two different strategies for TBI management. Head‐of‐bed elevation (HBE) was included in one strategy along with various other interventions. It was not possible to examine the effect of HBE separately from the other interventions. Additionally, we excluded one case‐series study (Ropper 1982). Ultimately, three studies that met all the selection criteria were included (Agbeko 2012; March 1990; Winkelman 2000).
2.

Flow diagram of study selection.
Included studies
Studies in adults
March 1990 is a randomised cross‐over trial conducted in a single centre in Seattle (USA), that included three adults (age range 19 to 30 years) with a diagnosis of severe TBI (Glasgow Coma Scale 8 or less). There was no information given regarding the method of recruitment or the recruitment period. All of the participants were included in the analysis. The main outcomes were: intracranial pressure (ICP), measured by a Spectramed TDX transducer; cerebral perfusion pressure (CPP), calculated by the formula mean atrial pressure (MAP) minus ICP; arterial blood pressure (ABP) and cerebral blood flow (CBF), measured with transcranial Doppler (TCD) at five and 12 minutes after each change in backrest position. This study examined the effect of four different positions of the head: supine position (flat); head elevated to 30°; head up to 30° with high strapped knees; and head elevated at 30° in reverse Trendelenburg. Each participant took part in the study on one day. Participants were initially placed in the flat position for 15 minutes, followed by 15 minutes in each of the other three positions of supine with the head elevated at 30º in a random order. The evaluation of the results was unblinded.
Winkelman 2000 is a randomised cross‐over trial conducted in Cleveland (USA) from 1996 to 1997. Each participant took part in the study on one day, for two to four hours total. The study recruited 12 participants between 18 and 45 years old with closed severe head injury (Glasgow Coma Scale 8 or less). The participants had been injured within the preceeding 12 to 36 hours. Four participants became ineligible to participate in the study due to instability in the period between obtaining permission to enrol from surrogtaes and randomisation. Eight people participated in, and completed, the study. The study compared the horizontal supine (flat) position and 30° head elevation with reverse Trendelenburg position; all positions for a period of 60 minutes, with a period of inactivity of 15 minutes between them. At baseline, participants had been previously placed in the opposite position to their randomly assigned position for 65 to 75 minutes until they had reached haemodynamic balance. During this period there were no measurements. The main outcomes (ICP and CPP) were measured by the Physiologic Data Acquisition System ICP Camino® at five, 15, 30 and 60 minutes after the change of position was performed. No other interventions were implemented during this period of 60 minutes.
Studies in children
Agbeko 2012 is a randomised cross‐over trial carried out in Portland (USA) and London (UK). Participants were recruited from two reference centres of neurotrauma in the period 2004 to 2006. Both centres applied the same management protocol for TBI in children (aged under 18 years) with a diagnosis of severe TBI (Glasgow Coma Scale 8 or less), intubated and ventilated, and with the presence of a device for ICP monitoring (Physiologic Data Acquisition System ICP Camino®). The number of participants recruited was 10, but one child was not randomised due to consciousness, and one child dropped out shortly after randomisation having undergone a single change in elevation of the head of the bed. Eight children completed the study, with an age range from two to 16 years (mean: 10 years). The study compared various head positions between 0° and 40°, with successive increments (increases in angle) or decrements (decreases in angle) of 10°, from a basal position of 30° elevation. The order of the positions was determined randomly, and participants remained in each position for ten minutes. The intervention was performed for a maximum daily total of six HOB changes (including baseline and recovery recordings) during which time the device for ICP monitoring was in place. Children took part in the study for one to four days. This resulted in a total of 18 experimental sessions, where 66 position changes were evaluated. The outcomes measured were ICP and CPP (calculated by MAP minus ICP), five minutes before and two minutes after each change of position.
Further details of these studies are reported in the table 'Characteristics of included studies'.
Excluded studies
The only RCT that we excluded was a parallel‐design trial conducted in children with severe trauma (Prabhakaran 2004). We excluded it because it was not possible to separate HBE from the other interventions. Additionally, we excluded one case‐series study (Ropper 1982) and 12 non‐randomised controlled studies because they compared different elevation positions of the head in a non‐randomised but sequential order (pre‐post test). All but two were conducted in adults (Durward 1983; Feldman 1992; Ledwith 2010; Mahfoud 2010; Meixensberger 1997; Moraine 2000; Ng 2004; Parsons 1984; Ropper 1982; Rosner 1986; Schneider 1993; Schulz‐Stubner 2006). Many of them studied a mixed population of critical care patients with intracranial hypertension including but not limited to TBI (Durward 1983; Ledwith 2010; Mahfoud 2010; Moraine 2000; Ropper 1982; Rosner 1986; Schneider 1993; Schulz‐Stubner 2006).
We have described excluded studies, along with the reasons for exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies
The three studies included were RCTs with a cross‐over design, and described the methods used for randomisation. None of the studies reported how they had concealed allocation, so the risk of bias was unclear. They had an open design, given the impossibility of masking the evaluated interventions. None reported performing a blinded evaluation of outcomes. They all measured only surrogate physiological variables (especially ICP and CPP) in the short term, with a low risk of bias due to the nature of variables that were measured (automated measures).
All of the studies were very small in sample size (3 to 9 participants), and with a cross‐over design (each participant was subjected to different positions, and served as their own control). In this context, this was not a major cause of bias, because the main variables analysed by the studies were mortality, ICP and CPP, which appear to be stable shortly after performing the change of position.
Overall, we assessed these studies as being of unclear risk of bias. Given the specific context (management of people with severe TBI in intensive care), it is difficult to perform high‐quality methodological studies that also analyse clinical outcomes in the longer term.
Figure 3 and Figure 4 show a visual representation of the risk of bias assessments.
3.
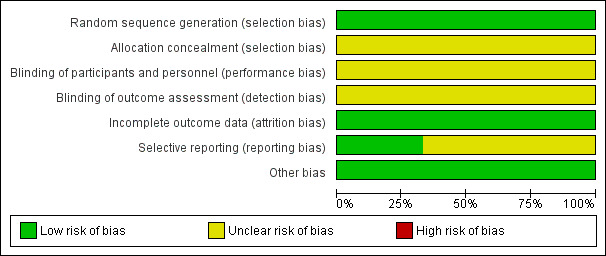
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Three studies are included in this review.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The risk of bias for Agbeko 2012 is unclear. Although the use of envelopes is stated as a method for assigning the order of interventions, it is uncertain whether the envelopes were opaque and were closed and sequentially numbered. March 1990 did not describe the method used for randomisation of the head positions and concealing the allocation sequence, thus we judged the risk of bias to be unclear. Through correspondence with Winkelman 2000, we learned that randomisation was by coin toss and there was no concealment of the randomisation sequence.
Blinding
None of the studies reported that they performed a blinded assessment of outcomes. We assumed that the study design was completely open for practical reasons. However, due to the objective nature of the physiological outcome variables (ICP and CPP with automated measurements), it is reasonable to think that there was no significant risk of bias because of this open design. We judged the risk of bias for this domain to be low.
Incomplete outcome data
Agbeko 2012 analysed eight of the nine randomised participants. The reason for exclusion seemed justified; the one participant received only a single change of HBE. In addition, participants were subjected to a variable number of sessions of position changes (from the data it appears to be between two to three, with a total of 66 changes analysed in a total of 18 sessions for eight participants). The reasons for not completing all sessions was not clear. We analysed all the changes for which data were available. Given the number of analysed changes we judged the risk of bias to be low.
March 1990 reported the outcomes for all three participants, and Winkelman 2000 reported the outcomes for all eight participants.
Selective reporting
The review authors did not have access to the study protocols, so we assessed the risk of bias to be unclear.
Other bias
We did not identify any other sources of bias.
Effects of interventions
See: Table 1
Primary outcomes
Mortality
There were no deaths in the March 1990 and Winkelman 2000 studies.
One out of ten participants in the Agbeko 2012 study had died at the time of final follow‐up at 28 days.
Secondary outcomes
Quality of life
The Agbeko 2012 and March 1990 studies did not report measuring quality of life. The Winkelman 2000 study did not measure quality of life.
Glasgow Coma Score
The Agbeko 2012 and March 1990 studies did not report measuring GCS at the end of the study. The Winkelman 2000 study did not measure GCS at the end of the study.
Disability (GOS, GOS‐E)
The Agbeko 2012 and March 1990 studies did not report measuring disability at the end of the study.
The Winkelman 2000 study did not measure GOS at the end of the study. APACHE and MEDICUS scores were obtained from participants' records at the time of enrollment, but as the scores were not recorded at the end of the study a comparison cannot be made.
Intracranial pressure (ICP)
We assessed the body of evidence for this outcome to be very low quality (see Quality of the evidence) and we are therefore uncertain about the effect of HBE on ICP. The body of evidence was downgraded due to inconsistency, imprecision and risk of bias.
Agbeko 2012 showed a negative and linear association between ICP and an increase in head elevation with marked inter‐individual variability (mainly attributable to the participant's height and thus the vertical height at the level of foramen of Monro) (eight participants, very low‐quality evidence). According to a linear regression analysis, each increase of 10 cm in the elevation of the head was associated with a decline of the ICP of ‐3.9 mmHg (SD ± 3.2 mmHg, P value < 0.001) on average. Participants showed a wide variability in the observed change of the ICP (range: ‐8.4 to 1.9 mmHg/10 cm). At higher elevations of the backrest, greater reductions in the ICP were generally observed, although in 21% of cases we observed the opposite response. This was partially attributed to the height of the participant.
March 1990 did not provide numerical data, they presented the results graphically, which prevents a more precise analysis. However, the study authors presented no evidence of an increase of ICP with a HBE at 30º compared to a flat position (three participants, very low‐quality evidence).
In the Winkelman 2000 study there was a statistically significant improvement in ICP (decrease) with the HBE at 30º compared to a flat position (very low‐quality evidence) (an average change of ‐4.0 mmHg that was judged as clinically important by the study authors). However, we performed a re‐analysis of the data taking into account the cross‐over design, and found no evidence of a reduction of ICP (MD ‐5.05, 95% CI ‐73.02 to 62.92) (Table 1).
Cerebral perfusion pressure (CPP)
We assessed the body of evidence for this outcome to be of very low quality (see Quality of the evidence) and we are therefore uncertain about the effect of HBE on CPP.
In Agbeko 2012 there was no evidence of a change in CPP after position changes at 10 minutes (eight participants, very low‐quality evidence). The regression model showed a 0° slope (P value < 0.957) with a marked variability among participants.
March 1990, showed no evidence of a decrease in CPP with HBE at 30º after 15 minutes compared to a flat position (three participants, very low‐quality evidence). There was marked variability among participants.
In the Winkelman 2000 study, taking into account the cross‐over design, we calculated the effect size of CCP comparing HBE at 30º with a flat position. There was no difference between groups at 60 minutes: MD 4.12 (95% CI ‐100.10 to 108.33) (eight participants, very low‐quality evidence) (Table 1).
Adverse effects
One study reported adverse events specifically (Agbeko 2012). No adverse events were identified during the 66 HBE changes. However, for one participant, ICP increased by 20 mmHg after the HBE was reduced from 10° to 0°. ICP returned to baseline level as soon as the head position was increased to 30º (baseline). This increase in ICP was not classified as an adverse event because the intervention protocol was stopped within the 5‐minute cut‐off point.
The March 1990 study authors did not report adverse effects, and it is unclear if any data were collected on adverse effects.
There were no adverse events in the Winkelman 2000 study.
We assessed the body of evidence for this outcome to be very low quality and we are therefore uncertain about the adverse effects of HBE.
Discussion
Summary of main results
Three small cross‐over studies including a total of 20 randomised participants are incuded in this review. The participants served as their own control through the cross‐over design. The scarcity of available data, inconsistency across studies, heterogeneity of individual responses to the intervention, unclear risk of bias and low power of the included studies mean that we are uncertain about the effect of HBE on severe TBI from this body of evidence.
At the time of follow‐up 28 days following hospital admission, one participant had died. None of the studies assessed the clinical outcomes quality of life, disability or Glasgow Coma Score. None of the participants experienced a lasting adverse event during the study.
Very low quality evidence from two RCTs conducted in adults with severe TBI showed no evidence of a benefit of HBE at 30º on ICP or CPP (March 1990; Winkelman 2000).
In children, a single study of nine participants (Agbeko 2012) indicated that HBE (at least 30°) decreases ICP without compromising CPP. However, there was high heterogeneity among participants, which seems to relate to participant height, and there were some paradoxical responses. We have very little confidence in this result due to the very low quality of the evidence.
Two studies used the same technique for ICP measurement (Agbeko 2012; Winkelman 2000). All three studies used the same approach to measure CPP (Agbeko 2012; March 1990; Winkelman 2000).
Overall completeness and applicability of evidence
In general, the objective of this review was not completed because none of the included studies evaluated the clinical outcomes of interest such as quality of life and disability. Consequently, no conclusions can be drawn about the effects of HBE on these outcomes.
The applicability of the evidence is low, because the RCTs included in this review are too small and limited in their design to be able to determine whether there were clinical benefits or risks associated with the use of HBE. Newer, high‐quality studies are necessary, preferably RCTs, with larger samples, of both adults and children. These studies ideally need to measure clinical outcomes or, alternatively, they need to show the relationship between ICP, CPP and mortality, disability or time to recovery. Many of the interventions used in the acute management of TBI in intensive or critical care are not available in low‐income countries where the majority of the TBI population is present (Stelfox 2008). Therefore, assessing the usefulness of this simple intervention is of great interest for those settings.
Quality of the evidence
The quality of the overall body of evidence for the intervention of HBE for severe TBI is very low due to the scarcity of available data, inconsistency across studies, heterogeneity within studies, unclear risk of bias and low power of the included studies. This means that we have very little confidence in these results and we are very limited in the conclusions we are able to draw about this intervention.
Potential biases in the review process
We did not detect any potential biases in the review process.
Agreements and disagreements with other studies or reviews
There are two other systematic reviews on the same topic. Fan 2004 included 11 studies (the two RCTs in adults included in this review and nine other non‐RCTs conducted in people with severe brain‐injury, not necessarily caused by trauma) and concluded that HBE of 30° is a therapeutic intervention to be considered for preventing ICP in brain‐injured people. Due to the same general limitations with the quality of the evidence that we found in our review, as well as the inter‐individual variability observed in most of the studies, the authors concluded that "in clinical practice, intensive care unit staff members need to cautiously perform head elevation with a thorough understanding of its physiologic effect and potential hazard."
The authors also commented on the need for new evidence for best practices of positioning neurosurgical patients in the ICU, although we foresee major practical difficulties in conducting RCTs in ICUs due to the unpredictable and debilitating conditions of critically ill individuals. We also agree that there is a necessity for new studies, ideally RCTs, to confirm these suggested beneficial effects. Similar studies of head positioning in different conditions (i.e. stroke) indicate that such studies are also feasible in this context (Anderson 2017).
The second review is a systematic review of non‐pharmacological interventions in acquired brain injury (Meyer 2010a). One of the included interventions was head position, and the authors analysed data from two RCTs in adults and five additional non‐randomised controlled studies. This review has been subsequently updated and published as an extensive report (Module 16: Acute Interventions for Acquired Brain Injury) and is available at the website of Evidence‐Based Review of Moderate To Severe Acquired Brain Injury (ERABI), a "joint project to develop an evidence‐based review of the literature for rehabilitation or rehabilitation‐related interventions for acquired brain injury" (www.abiebr.com). The review included the three RCTs already included in this review plus seven other quasi‐experimental studies. Taking into account this body of evidence, the authors concluded: 1. "there is Level 2 evidence suggesting a 30° head elevation reduces intracranial pressure with concomitant increments in CPP", and 2. "there is Level 2 evidence to suggest head elevation does reduce ICP in children post TBI; however, it was not found to have a significant impact of CPP." The levels of evidence used to summarise the findings in this review were based on the modified Sackett criteria (Sackett 1989), where Level 2 means, "Randomized controlled trial (PEDro score < 6); Non‐RCTs and Cohort studies (using at least 2 similar groups with one exposed to a particular condition)."
Authors' conclusions
Implications for practice.
The lack of consistency among studies, the scarcity of data, the variability in responses observed among participants, and the absence of evidence to show an appropriate correlation between this effect and the clinical outcomes, prevent us from drawing conclusions about practice.
According to these results it seems reasonable to suggest that the optimum angle of the head‐of‐bed elevation (HBE) needs to be decided individually after an analysis of the response of intracranial pressure (ICP), cerebral perfusion pressure (CPP) and cerebral blood flow (CBF) in each backrest position. This relationship may need to be analysed on a daily basis, keeping in mind what clinical goal is desirable (decrease of ICP or maintenance/increase in CPP) for each particular person.
Implications for research.
There is very little evidence in the existing literature to determine effects of HBE for severe traumatic brain injury (TBI). More, well‐designed clinical trials are needed, that include children and adults and have larger sample sizes to increase the power to detect potential effects. Ideally these trials should measure long‐term clinically relevant outcomes, and not just physiological outcomes such as ICP and CPP, as the relationship between ICP and CPP, and survival, disability or time to recovery is not clear.
Similar studies that are currently ongoing support the idea that such studies are feasible (Muñoz‐Venturelli 2015). These studies will probably have a cross‐over design, with participants as their own control in order to control for between participant variability. These studies should fully describe the experimental and control groups, and should state how long the participants stayed in each position before the measurements were taken so that it is clear whether the values reflect an immediate response or an equilibrated response. Multiple time point measurements would capture whether the effects were transient or sustained over time.
In addition, the safety of HBE for severe TBI should be assessed more carefully in future trials, and adverse effects recorded systematically.
As most people with TBI have combined multisystem injuries or underlying diseases, or both, a more comprehensive approach is needed with respect to selecting a variety of outcomes that may reflect the impact of the intervention on the relevant systems. In addition, these studies should evaluate the effect of other backrest and body positions (e.g. lateral side‐lying positions) for brain‐injured people who have multisystem involvement.
Notes
In the future when this review is updated:
we may conduct a network meta‐analysis of the various head‐of‐bed elevations;
we will group data for all outcomes and analyse them in clinically similar periods.
Acknowledgements
José Domingo Alarcón is a doctoral candidate at the Paediatrics, Obstetrics and Gynecology, and Preventive Medicine Department, Universitat Autònoma de Barcelona, Barcelona (Spain).
We thank Cochrane Injuries for their support, and particularly Helen Wakeford, Managing Editor.
We thank Dr. Chris Winkelman for providing unpublished data.
This project was supported by the UK National Institute for Health Research, through Cochrane Infrastructure funding to Cochrane Injuries. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Search strategies
The date of the latest search was 2 March 2017 and the databases searched are listed below.
1. Cochrane Injuries Specialised Register (SR‐INJ) (posture OR "patient* position" OR (head NEAR/3 elevat*) OR backrest* OR "back rest" OR recumbent OR HOBOE OR "head of bed") AND ("intracrani* pressure" OR “intra‐crani* pressure” OR head OR crani* or brain* or cerebr* or swell* or oedema or edema) [All Fields In Register]
2. Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 2) in the Cochrane Library #1 MeSH descriptor Patient Positioning explode all trees #2 MeSH descriptor Posture explode all trees #3 (patient* near/3 position*):ti,ab,kw #4 ((upper* near/3 body near/5 elevat*) or ((backrest* or back‐rest*) near3 position*)):ti,ab,kw #5 ((head near/3 elevat*) or "head of bed"):ti,ab,kw #6 (head near/3 bed* near/5 (rais* or low*)):ti,ab,kw #7 ((backrest* or "back rest*") near/3 position*) #8 ("intracranial pressure" and bed*):ti,ab,kw #9 (bed* and "30 degrees" or "45 degrees"):ti,ab,kw #10 (semirecumbent or semi‐recumbent):ti,ab,kw #11 MeSH descriptor: [Beds] this term only #12 MeSH descriptor: [Bed Rest] this term only #13 MeSH descriptor: [Head] this term only #14 ((#11 or #12) and #13) #15 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #14) #16 ((head or crani* or cerebr* or brain* or intra‐cran* or intracran* or intra‐cereb* or intracereb*) near/3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or hernia* or bleed* or pressure or hypertension or oedema or edema or swell*)) #17 (((traumatic or severe) near/3 (brain or head) adj3 injur*) or TBI or sTBI) #18 (#16 OR #17) #19 (#15 AND #18)
3. MEDLINE (Ovid) 1950 to 2 March 2017 1. exp Patient Positioning/ 2. exp Posture/ 3. HOBOE.ab,ti. 4. (patient* adj3 position*).ab,ti. 5. (upper* adj3 body adj5 elevat*).ab,ti. 6. ((backrest* or back rest*) adj3 position*).ab,ti. 7. (head adj3 elevat*).ti,ab. 8. head of bed.ti,ab. 9. (head adj3 bed* adj5 (elevat* or rais* or low*)).ab,ti. 10. (bed* adj5 "intracranial pressure").ab,ti. 11. (bed* adj5 ("30 degrees" or "45 degrees")).ab,ti. 12. (semirecumbent or semi recumbent).ab,ti. 13. ((Beds/ or Bed Rest/) and Head/) 14. or/1‐13 15. Intracranial Pressure/ 16. ((head or crani* or cerebr* or brain* or intra‐cran* or intracran* or intra‐cereb* or intracereb*) adj3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or hernia* or bleed* or pressure or hypertension or oedema or edema or swell*)).ti,ab. 17. (((traumatic or severe) adj3 (brain or head) adj3 injur*) or TBI or sTBI).ti,ab. 18. or/15‐17 19. randomi#ed.ab,ti. 20. randomised controlled trial.pt. 21. controlled clinical trial.pt. 22. placebo.ab. 23. clinical trials as topic.sh. 24. randomly.ab. 25. trial.ti. 26. or/19‐25 27. (animals not (humans and animals)).sh. 28. 26 not 27 29. (14 and 18 and 28)
4. Embase (Ovid SP) 1980 to 2 March 2017 1. exp patient positioning/ 2. HOBOE.ti,ab. 3. (patient* adj3 position*).ti,ab. 4. (upper* adj3 body adj5 elevat*).ti,ab. 5. ((backrest* or back rest*) adj3 position*).ti,ab. 6. (head adj3 elevat*).ti,ab. 7. (head adj3 bed* adj5 (elevat* or rais* or low*)).ti,ab. 8. (bed* adj5 "intracranial pressure").ti,ab. 9. (bed* adj5 ("30 degrees" or "45 degrees")).ti,ab. 10. (semirecumbent or semi recumbent).ti,ab. 11. ((Beds/ or Bed Rest/) and Head/) 12. or/1‐11 13. Intracranial Pressure/ 14. Traumatic Brain Injury/ 15. *Head Injury/ 16. ((head or crani* or cerebr* or brain* or intra‐cran* or intracran* or intra‐cereb* or intracereb*) adj3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or hernia* or bleed* or pressure or hypertension or oedema or edema or swell*)).ti,ab. 17. (((traumatic or severe) adj3 (brain or head) adj3 injur*) or TBI or sTBI).ti,ab. 18. or/13‐17 19. exp Randomized Controlled Trial/ 20. exp Controlled Clinical Trial/ 21. exp Controlled Study/ 22. randomi#ed.ab,ti. 23. placebo.ab. 24. *Clinical Trial/ 25. exp Major Clinical Study/ 26. randomly.ab. 27. (trial or study).ti. 28. Cross‐over Procedure/ 29. or/19‐28 30. exp animal/ not (exp human/ and exp animal/) 31. 29 not 30 32. (12 and 18 and 31) 33. limit 32 to exclude medline journals 34. Conference Abstract.pt. 35. 32 or (32 and 34)
5. CINAHL (EBSCO) to 2 March 2017 1 (MH "Patient Positioning") 2 (MH "Posture+") 3 TX hoboe 4 TX patient* n3 position* 5 TX upper* n3 body n5 elevat* 6 TX ((backrest* or back‐rest*) n3 position*) 7 TX (head n3 bed* n5 (elevat* or rais* or low*)) 8 TX (bed* n5 "intracranial pressure") 9 TX (bed* N5 ("30 degrees" or "45 degrees")) 10 TX semirecumbent or semi‐recumbent 11 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 12 (MM "Intracranial Pressure") 13 TX ((head or crani* or cerebr* or brain* or intracran* or intercran* or intra‐cran* or inter‐cran*) N3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressure)) 14 TX ((brain or cerebral or intracranial or intra‐cran*) N3 (oedema or edema or swell*)) 15 S12 or S13 or S14 16 (MH "Clinical Trials") 17 PT clinical trial* 18 TX clinical N3 trial* 19 TI ( (singl* N3 blind*) or (doubl* N3 blind*) or (trebl* N3 blind*) or (tripl* N3 blind*) ) or TI ( (singl* N3 mask*) or (doubl* N3 mask*) or (trebl* N3 mask*) or (tripl* N3 mask*) ) or AB ( (singl* N3 blind*) or (doubl* N3 blind*) or (trebl* N3 blind*) ) or AB ( (singl* N3 mask*) or (doubl* N3 mask*) or (trebl* N3 mask*) or (tripl* N3 mask*) ) 20 TX randomi?ed N3 control* N3 trial* 21 (MH "Placebos") 22 TX placebo* 23 (MH "Random Assignment") 24 TX random* N3 allocat* 25 MH quantitative studies 26 S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 27 S11 and S15 and S26 (Limiters ‐ Exclude MEDLINE records)
6 & 7. ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) ISI Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) Search 1 (to March 2016): 1. TS=((brain or cerebral or intracranial or intra?cran*) near/3 (oedema or edema or swell*)) OR ((head or crani* or cerebr* or brain* or intracran* or intercran* or intra?cran* or inter?cran*) near/3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressure)) 2. TS=(Semirecumbent or semi?recumbent) OR (bed* and "intracranial pressure") OR (backrest* position*) OR (upper body elevat*) OR (patient* position*) OR (posture OR position*) OR HOBOE 3.TS=((singl* OR doubl* OR trebl* OR tripl*) SAME (blind* OR mask*)) OR TS=(controlled clinical trial OR controlled trial OR clinical trial OR placebo) OR TS=(randomised OR randomised OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random OR randomised controlled trial) AND TS=(human*) 4. 1 and 2 and 3
Search 2 (2 March 2017): TS=((semirecumbent OR semi‐recumbent OR “head of bed” OR (bedrest SAME "intracranial pressure*") OR ((backrest* or “back rest”) SAME (position* or elevat*)) OR “patient* position*” OR posture OR ((head OR “upper body”) SAME elevat*)) AND (((trauma* or severe) SAME (brain or head) SAME injur*) OR ((crani* OR intracran* OR intra‐cran*) SAME (oedema or edema or swell* OR haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressure* or hypertens*))) AND (((singl* OR doubl* OR trebl* OR tripl*) SAME (blind* OR mask*)) OR “controlled clinical trial” OR placebo OR randomised OR randomised OR (random* SAME (order or allocat* or sequence)) OR “at random” or crossover or cross‐over)) NOT TI=(rat OR rats OR rodent* OR mice OR mouse OR murine OR horse* OR equine OR pig OR pigs OR piglet* OR swine) Indexes=SCI‐EXPANDED, CPCI‐S, CPCI‐SSH, ESCI Timespan=2016‐2017
A citation search was also conducted in March 2017 for research citing the three included studies.
8. International trials registers ClinicalTrials.gov basic search 1: (intracranial AND pressure) AND (backrest OR head elevation OR head of bed or HOB or HOBE) ClinicalTrials.gov basic search 2: (brain injury) AND (backrest OR head elevation OR head of bed or HOB or HOBE) Records were screened for relevant studies.
ICTRP advanced search 1: Intervention=head elevation or head of bed or HOB or HOBE and Title=brain injury or intracranial pressure ICTRP advanced search 2: Intervention=head elevation or head of bed or HOB or HOBE and Condition=brain injury or intracranial pressure
The following synonyms were automatically applied to the ICTRP search 2: ‐ PRESSURE, INTRACRANIAL, PRESSURE, SUBARACHNOID, PRESSURES, INTRACRANIAL, PRESSURES, SUBARACHNOID, SUBARACHNOID PRESSURE, SUBARACHNOID PRESSURES, intracranial pressure ‐ BRAIN DAMAGE, BRAIN INJ, BRAIN INJURIES, BRAIN LESION (FROM INJURY), BRAIN TRAUMA, CONCUSSION, INJ BRAIN, INJURIES, BRAIN, INJURY, BRAIN, brain injury ‐ head elevation ‐ head of bed ‐ hob
Data and analyses
Comparison 1. Altered backrest position compared with flat position for severe traumatic brain injury.
1.1. Analysis.
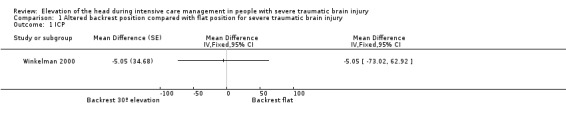
Comparison 1 Altered backrest position compared with flat position for severe traumatic brain injury, Outcome 1 ICP.
1.2. Analysis.
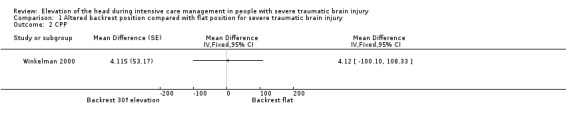
Comparison 1 Altered backrest position compared with flat position for severe traumatic brain injury, Outcome 2 CPP.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agbeko 2012.
| Methods |
Design: RCT (cross‐over design) Unit of randomisation: participant Unit of analysis: the participant, while engaged in a study position Follow‐up: 28 days |
|
| Participants |
Number of centres: 2 Setting: Paediatric ICU Country: UK and USA Number of participants: 9 Age (years; mean and SD): 10 (± 5) years; range 2–16 years for the 8 participants who completed the study (the age was not provided for the one participant who withdrew from the study) Gender: 7 male and 1 female (the gender was not provided for the one participant who withdrew from the study) Inclusion criteria: age < 18 years, with a diagnosis of severe TBI (GCS ≤ 8) necessitating ICP monitoring Method of recruitment: not reported Recruitment dates: 2004‐2006 |
|
| Interventions |
Intervention: the physiological challenge protocol was identical for both study sites. The degree of HBE was routinely maintained at 30°. From this baseline position, the degree of HBE was varied between 0° and 40° in 10° increments/decrements in a random order, with the axis of rotation at the foot of the bed. The angle of elevation was measured with a protractor. Midline position of the head relative to the neck and thorax was maintained. Including baseline and recovery recordings, a maximum daily total of 6 physiologic signal recording episodes during HBE changes were obtained, each of 10‐min duration. If an intraventricular ICP‐monitoring device was in place, then this remained closed during the HBE challenges. A set of physiological challenges, including 4 random changes in HBE were administered in random order each day to each participant as long as there was an ICP‐monitoring device in place and the participant was deemed clinically stable by the treating physician. Routine neurocritical care included supine position with the HBE at 30° (hips extended); intubation and ventilation to normocapnia; sedation/analgesia with morphine, fentanyl, or midazolam, or a combination of these; and norepinephrine or phenylephrine to increase mean arterial pressure to maintain CPP at 40–70 mmHg dependent on age. In addition, if treatment goals were not met, then barbiturate coma to burst suppression on electroencephalogram monitoring, muscle paralysis with a short‐acting paralytic, and drainage of cerebrospinal fluid via an intraventricular drain were used at the discretion of the attending physician. |
|
| Outcomes |
ICP, both parenchymal and ventricular, was measured with the Physiologic Data Acquisition System ICP Camino® (Camino Laboratories, San Diego, CA) CPP was calculated: MAP minus ICP ABP was measured with the Physiologic Data Acquisition System ICP Camino® |
|
| Notes | Quote: The change in orthostatic pressure (ICP) attributable to a change in HBE angle is proportional to the change in vertical height between the subject’s jugular venous bulb and right atrium. This relationship was confounded by subject height in this paediatric cohort. Therefore, the relationship between ICP change and change in HBE position was normalised to the vertical distance change between the subject’s jugular bulb and right atrium. This was estimated by measuring the distance between the left tragus and ipsilateral nipple. A linear model was used based on the premise that hydrostatic mechanisms would account for most of the change in ICP changes. Ethical approval for the study was obtained from the "Great Ormond Street Hospital for Children National Health Serice Trust/Institute of Child Health Research Ethics Committee and Oregon Health and Science University Institutional Review Board. Parental or legal guardian informed consent was obtained at both sites according to the Declaration of Helsinki guidelines." p. e40 Funding source: "This work was undertaken at Great Ormond Street Hospital/UCL Institute of Child Health, which received a proportion of funding from the Department of Health's NIHR Biomedical Research Centre's funding scheme." p. e39 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The order of the interventions was determined by pulling folded pieces of paper stating the angle degrees from an envelope by a member of the clinical staff not involved in the study. |
| Allocation concealment (selection bias) | Unclear risk | The paper reported the use of envelopes for the assignment of interventions, but it is uncertain whether the envelopes were opaque or not and if they were closed or sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although the study seems to have had an open design (no blinding was mentioned in the paper), the short duration of the HBE challenges protocol (10 min) and the prespecified criteria (clinical status deterioration) to stop the protocol make a performance bias unlikely to have occurred. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although the study seems to have had an open design (no blinding was mentioned in the paper), the nature of the outcomes with an automated measure makes it unclear to what extent a detection bias may have occurred. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 participants were analysed from a total of 10 that had been initially recruited. Reasons for exclusion seem justified (1 participant was conscious at the beginning of the protocol and was not randomised into the study, and another participant was randomised but only received one change of HBE instead of 3). In addition, participants received a variable number of sessions of change in position (HBE "challenges"). Usable data were obtained from 8 participants during 18 sessions, resulting in a total of 66 HBE challenges. Number of sessions per participant ranged between 1 and 4. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. From the information published in the articles, all outcomes seemed to be reported. However, we cannot exclude a risk of selective reporting. |
| Other bias | Low risk | No other sources of bias were identified. |
March 1990.
| Methods |
Design: RCT (cross‐over design) Unit of randomisation: participant Unit of analysis: the participant, while engaged in a study position Follow‐up: not specified |
|
| Participants |
Number of centres: 1 Setting: Harborview Medical Center (Seattle) Country: USA Number of participants: 3 Age (range): 19‐30 years Gender: 1 male and 2 female Method of recruitment: not reported Inclusion criteria: not reported. Participants consisted of 3 comatose head‐injured people ranging from 19‐30 years old with a GCS score ranging from 4‐7 Recruitment dates: not reported |
|
| Interventions |
Interventions: each participant underwent backrest manipulation 3 times:
The order of positions was randomly assigned. Procedure: each participant was placed in the flat position for 15 min followed by 15 min in 1 of the 3 randomly assigned elevated backrest positions. CBF, as measured with a transcranial Doppler, was obtained at the 10th min in the flat position and at 2 and 15 min after backrest elevation. ICP and systemic ABP were monitored continuously but digital data were used from the 10th min prior to position change and the 5th and 10th min after each position change. This process was repeated at hourly intervals for each of the study positions with the participants placed in their non‐study positions between study positions. |
|
| Outcomes |
ICP was measured by a ICP subarachnoidal bolt connected to a Spectramed TDX transducer CPP was calculated: MAP minus ICP Systemic ABP was measured by a Spectramed TDX transducer connected to a radial catheter anchored at the wrist and aligned with a reference point at heart level. An extension was added to enable the measurement of systemic ABP at head level (foramen of Monro) CBF was measured with a transcranial Doppler |
|
| Notes | Quote: "Consent was obtained from next of kin prior to data collection on all subjects." p. 376 Funding source: "The study was partially supported by a training grant, Number NU 00218 from the Division of Nursing, Bureau of Health Manpower, Health Resources Administration, U.S. Public Health Service." p. 375 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No details were provided regarding the randomisation procedure. Quote: "the order of positions was randomly assigned". |
| Allocation concealment (selection bias) | Unclear risk | The paper did not describe the method used to conceal the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although the study seems to have had an open design (no blinding was mentioned in the paper), the short duration of the HBE challenges (15 min each position) make a performance bias unlikely to occur. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although the study seems to have had an open design (no blinding was mentioned in the paper), the nature of the outcomes with an automated measure makes unclear to what extent a detection bias may have occurred. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The outcomes were reported for all participants. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. From the information published in the articles, all outcomes seemed to have been reported. However, we cannot exclude a risk of selective reporting. |
| Other bias | Low risk | No other sources of bias were identified. |
Winkelman 2000.
| Methods |
Design: RCT (cross‐over design) Unit of randomisation: participant Unit of analysis: the participant, while engaged in a study position Follow‐up: not specified |
|
| Participants |
Number of centres: 1 Setting: Level I trauma centre emergency department (Metromedical Center, Cleveland, Ohio) Country: USA Number of participants: 8 Age (years; mean and SD): 28 (± 7.2) years Gender: 6 male and 2 female Method of recruitment: "In this crossover experimental study, a homogeneous sample of adult patients admitted to an intensive care unit with brain injury was selected from daily admissions to a large, urban teaching hospital." Inclusion criteria: people with a diagnosis of a closed head injury who had an ICP monitor in place. The convenience sample consisted of adults 18‐45 years old with closed, nonvascular head injuries who had a GCS of ≤ 8 at the time of arrival. All participants had either a subarachnoid or an intravascular device for monitoring ICP and an intra‐arterial catheter for monitoring blood pressure in place. Participants with a sustained ICP > 25 mmHg or a CPP < 60 mmHg were excluded. Recruitment dates: May 1996 through November 1997 |
|
| Interventions |
Interventions: the purpose of the study was to determine the effect of 2 backrest positions on both ICP and CPP during the first 36 h after severe TBI:
Backrest position was randomly assigned to each participant. Each participant was studied in both positions, between 12 and 36 h after injury. All participants had a 15 min rest before the study began so that the effects of interventions known to affect ICP in people with brain injury (e.g. medications, suctioning, family visits) would not be a factor. Procedure: after randomisation to the initial study position, the participant was placed in the position alternative to the study position, with attention to alignment of the neck and trunk. The participant remained in the alternative position for 65‐75 minutes to achieve haemodynamic equilibrium; no interventions were implemented during the final 15 min. Each participant was then placed in the initial study position. Measurements of ICP and systemic ABP were recorded 5, 15, 30, and 60 min after the change in position. Then, after a 15‐min period with no interventions, the participant was placed in the second (alternative) study position, and measurements of ICP and systemic ABP were recorded 5, 15, 30, and 60 min after the change in position. Equilibrium was defined as the mean of the values obtained at 15, 30, and 60 min. An interval for care as needed could occur before the participant was placed in the second study position. No interventions occurred during the 60‐min observation period. |
|
| Outcomes |
ICP was measured by the Physiologic Data Acquisition System ICP Camino® (not specified if parenchymal or ventricular; Camino Laboratories, San Diego, CA) CPP was calculated: MAP minus ICP |
|
| Notes | Quote: "Approval from the appropriate institutional review board was obtained before the study began. Before a subject was enrolled in the study, informed consent was obtained from the person responsible for making medical decisions for the subject." p. 375‐6 Source of funding: The study was unfunded. The study was part of a PhD project. (Confirmed through correspondence with the author.) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study reported: "Backrest position was randomly assigned to each patient, and each patient was studied in both positions." Correspondence with the author confirmed: "A coin toss immediately prior to placing the patient in a resting position. Heads = Up first. Tails = Flat first." |
| Allocation concealment (selection bias) | Unclear risk | Correspondence with the author confirmed: "I did not know the allocation until I completed the coin toss. But I did toss the coin and I did know the allocation immediately following the coin toss." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although the study seems to have had an open design (no blinding was mentioned in the paper), the short duration of the HBE challenges (60 min each position) make a performance bias unlikely to have occurred. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although the study seems to have had an open design (no blinding was mentioned in the paper), the nature of the outcomes with an automated measure makes it unclear to what extent detection bias may have occurred. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 participants were recruited to the study. 4 participants experienced changes in vital signs or ICP before randomisation and never took part in the study. Complete data for the eight participants who took part in the study are presented. |
| Selective reporting (reporting bias) | Low risk | 12 participants were recruited to the study. 4 participants experienced changes in vital signs or ICP before randomisation and never took part in the study. Complete data for the eight participants who took part in the study are presented. |
| Other bias | Low risk | No other sources of bias were identified. |
A BP: arterial blood pressure; CBF: cerebral blood flow; CPP: cerebral perfusion pressure; GCS: Glasgow Coma Score; HBE: head‐of‐bed elevation; ICP: intracranial pressure; ICU: intensive care unit; MAP: mean arterial pressure; RCT: randomised controlled trial; TBI: traumatic brain injury
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Durward 1983 | Quasi‐experimental study (pre‐post test). Mixed population |
| Feldman 1992 | Quasi‐experimental study (pre‐post test) |
| Huh 2008 | Study was not randomised. |
| Ledwith 2010 | Quasi‐experimental study (pre‐post test). Mixed population |
| Mahfoud 2010 | Quasi‐experimental study (pre‐post test). Mixed population |
| Meixensberger 1997 | Quasi‐experimental study (pre‐post test) |
| Moraine 2000 | Quasi‐experimental study (pre‐post test). Mixed population |
| Ng 2004 | Quasi‐experimental study (pre‐post test) |
| Parsons 1984 | Quasi‐experimental study (pre‐post test) |
| Prabhakaran 2004 | RCT did not compare different backrest positions isolated from other interventions |
| Ropper 1982 | Case series. Mixed population |
| Rosner 1986 | Quasi‐experimental study (pre‐post test). Mixed population |
| Schneider 1993 | Quasi‐experimental study (pre‐post test). Mixed population |
| Schulz‐Stubner 2006 | Quasi‐experimental study (pre‐post test). Mixed population |
Differences between protocol and review
We have expanded the focus of the review to include children, but analysed separately studies performed in adults and children. Accordingly, we have renamed the title of the review, eliminating the term 'adult'. We have also modified the following sections accordingly: Background (Description of condition and How the intervention might work), Objectives, Criteria for considering studies for this review (Types of participants), and Measures of treatment effect.
Changes were made with respect to the initial protocol with regard to the methods of analysis. Given the way results are expressed in the original papers, we could not calculate any pooled‐effect measure nor display them in a plot. Nor was it possible to perform sensitivity analyses and subgroup analyses as planned, given the very limited data available. In conclusion, we have presented results only in a descriptive or narrative manner. We included the secondary outcome of adverse effects in the review as we considered that looking at potential harms was important.
We added information on the time to follow‐up and scale for each outcome, although this was not specified in the protocol. This was to improve the quality of the review and comply with MECIR reporting standards. We also added a 'Summary of findings' table to improve the reporting of results.
Contributions of authors
| Development of and running the search strategy | We had the assistance of Ivan Solà (Information Specialist at the Iberoamerican Cochrane Center) and Deirdre Beecher (Information Specialist with Cochrane Injuries) |
| Obtaining copies of studies | Alarcón JD, Rubiano AM |
| Selection of studies to include | Alarcón JD, Rubiano AM, Urrútia G |
| Extraction of data from studies | Alarcón JD, Rubiano AM, Urrútia G |
| RevMan analysis | Martínez‐Zapata MJ |
| Interpretation of the analysis | Alarcón JD, Rubiano AM, Okonkwo DO, Alarcón J, Martínez‐Zapata MJ, Urrútia G, Bonfill X |
| Drafting of the final review | Alarcón JD, Rubiano AM, Okonkwo DO, Alarcón J, Martínez‐Zapata MJ, Urrútia G, Bonfill X |
Sources of support
Internal sources
Iberoamerican Cochrane Center. IIB‐Sant Pau, Barcelona, Spain, Colombia.
Surcolombian University, Neiva, Colombia.
External sources
-
Instituto de Salud Carlos III, Spain.
Dr. Mª José Martinez Zapata is funded by a Miguel Servet research contract from the Instituto de Salud Carlos III (CP15/00116).
Declarations of interest
Jose D Alarcon: none known
Andres M Rubiano: none known
David O Okonkwo: none known
Jairo Alarcón: none known
Maria José Martinez‐Zapata: none known
Gerard Urrútia: none known
Xavier Bonfill Cosp: none known
New
References
References to studies included in this review
Agbeko 2012 {published data only}
- Agbeko RS, Pearson S, Peters MJ, McNames J, Goldstein B. Intracranial pressure and cerebral perfusion pressure responses to head elevation changes in pediatric traumatic brain injury. Pediatric Critical Care Medicine 2012;13:e39 – e47. [DOI] [PubMed] [Google Scholar]
March 1990 {published data only}
- March K, Mitchell P, Grady S, Winn R. Effect of backrest position on intracranial and cerebral perfusion pressures. Journal of Neuroscience Nursing 1990;22(6):375‐81. [PUBMED: 2148767] [DOI] [PubMed] [Google Scholar]
Winkelman 2000 {published and unpublished data}
- Winkelman C. Effect of backrest position on intracranial and cerebral perfusion pressures in traumatically brain‐injured adults. American Journal of Critical Care 2000;9(6):373‐80; quiz 381‐2. [PUBMED: 11072552] [PubMed] [Google Scholar]
- Winkelman C. Question about your study ‐ head elevation [personal communication]. Email to: Cochrane Injuries 4 December 2017.
References to studies excluded from this review
Durward 1983 {published data only}
- Durward QJ, Amacher AL, Maestro RF, Sibbald WJ. Cerebral and cardiovascular responses to changes in head elevation in patients with intracranial hypertension. Journal of Neurosurgery 1983;59(6):938‐44. [PUBMED: 6631516] [DOI] [PubMed] [Google Scholar]
Feldman 1992 {published data only}
- Feldman Z, Kanter MJ, Robertson CS, Contant CF, Hayes C, Sheinberg MA, et al. Effect of head elevation on intracranial pressure, cerebral perfusion pressure, and cerebral blood flow in head‐injured patients. Journal of Neurosurgery 1992;76:207‐11. [DOI] [PubMed] [Google Scholar]
Huh 2008 {published data only}
- NCT00636376. Effects of head elevation on intracranial pressure in children. clinicaltrials.gov/ct2/show/NCT00636376 Accessed 15 March 2012.
Ledwith 2010 {published data only}
- Ledwith MB, Bloom S, Maloney‐Wilensky E, Coyle B, Polomano RC, Roux PD. Effect of body position on cerebral oxygenation and physiologic parameters in patients with acute neurological conditions. Journal of Neuroscience Nursing 2010;42:280‐7. [DOI] [PubMed] [Google Scholar]
Mahfoud 2010 {published data only}
- Mahfoud F, Beck J, Raabe A. Intracranial pressure pulse amplitude during changes in head elevation: a new parameter for determining optimum cerebral perfusion pressure?. Acta Neurochirurgica 2010;152:443‐50. [DOI] [PubMed] [Google Scholar]
Meixensberger 1997 {published data only}
- Meixensberger J, Baunach S, Amschler J, Dings J, Roosen K. Influence of body position on tissue‐pO2, cerebral perfusion pressure and intracranial pressure in patients with acute brain injury. Neurological Research 1997;19(3):249‐53. [PUBMED: 9192375] [DOI] [PubMed] [Google Scholar]
Moraine 2000 {published data only}
- Moraine JJ, Berre J, Melot C. Is cerebral perfusion pressure a major determinant of cerebral blood flow during head elevation in comatose patients with severe intracranial lesions?. Journal of Neurosurgery 2000;92(4):606‐14. [PUBMED: 10761649] [DOI] [PubMed] [Google Scholar]
Ng 2004 {published data only}
- Ng I, Lim J, Wong HB. Effects of head posture on cerebral hemodynamics: its influences on intracranial pressure, cerebral perfusion pressure, and cerebral oxygenation. Neurosurgery 2004;54(3):593‐7; discussion 598. [PUBMED: 15028132] [DOI] [PubMed] [Google Scholar]
Parsons 1984 {published data only}
- Parsons LC, Wilson MM. Cerebrovascular status of severe closed head injured patients following passive position changes. Nursing Research 1984;33(2):68‐75. [PUBMED: 6560425] [PubMed] [Google Scholar]
Prabhakaran 2004 {published data only}
- Prabhakaran P, Reddy AT, Oakes WJ, King WD, Winkler MK, Givens TG. A pilot trial comparing cerebral perfusion pressure‐targeted therapy to intracranial pressure‐targeted therapy in children with severe traumatic brain injury. Journal of Neurosurgery 2004;100(5 Suppl Pediatrics):454‐9. [PUBMED: 15287454] [DOI] [PubMed] [Google Scholar]
Ropper 1982 {published data only}
- Ropper AH, O'Rourke D, Kennedy SK. Head position, intracranial pressure, and compliance. Neurology 1982;32:1288‐91. [DOI] [PubMed] [Google Scholar]
Rosner 1986 {published data only}
- Rosner MJ, Coley IB. Cerebral perfusion pressure, intracranial pressure, and head elevation. Journal of Neurosurgery 1986;65(5):636‐41. [PUBMED: 3772451] [DOI] [PubMed] [Google Scholar]
Schneider 1993 {published data only}
- Schneider GH, Helden GH, Franke R, Lanksch WR, Unterberg A. Influence of body position on jugular venous oxygen saturation, intracranial pressure and cerebral perfusion pressure. Acta Neurochirurgica. Supplement 1993;59:107‐12. [DOI] [PubMed] [Google Scholar]
Schulz‐Stubner 2006 {published data only}
- Schulz‐Stubner S, Thiex R. Raising the head‐of‐bed by 30 degrees reduces ICP and improves CPP without compromising cardiac output in euvolemic patients with traumatic brain injury and subarachnoid haemorrhage: a practice audit. European Journal of Anaesthesiology 2006;23:177‐80. [DOI] [PubMed] [Google Scholar]
Additional references
Adelson 1997
- Adelson PD, Clyde B, Kochanek PM, Wisniewski SR, Marion DW, Yonas H. Cerebrovascular response in infants and young children following severe traumatic brain injury: a preliminary report. Pediatric Neurosurgery 1997;26(4):200‐7. [PUBMED: 9436831] [DOI] [PubMed] [Google Scholar]
Adelson 2005
- Adelson PD, Ragheb J, Kanev P, Brockmeyer D, Beers SR, Brown SD, et al. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery 2005;56(4):740‐54; discussion 740‐54. [PUBMED: 15792513] [DOI] [PubMed] [Google Scholar]
Aldrich 1992
- Aldrich EF, Eisenberg HM, Saydjari C, Luerssen TG, Foulkes MA, Jane JA, et al. Diffuse brain swelling in severely head‐injured children. A report from the NIH Traumatic Coma Data Bank. Journal of Neurosurgery 1992;76(3):450‐4. [PUBMED: 1738026] [DOI] [PubMed] [Google Scholar]
Anderson 2017
- Anderson CS, Arima H, Lavados P, Billot L, Hackett ML, Olavarría VV, et al. Cluster‐randomized, crossover trial of head positioning in acute stroke. New England Journal of Medicine 2017;376(25):2437‐47. [DOI] [PubMed] [Google Scholar]
Bergen 2008
- Bergen G, Frattaroli S, Ballesteros MF, Ta VM, Beach C, Gielen AC. The implementation and utility of fire incident reporting systems: the Delaware experience. Journal of Community Health 2008;33(2):103‐9. [PUBMED: 18074209] [DOI] [PubMed] [Google Scholar]
Chambers 2001
- Chambers IR, Treadwell L, Mendelow AD. Determination of threshold levels of cerebral perfusion pressure and intracranial pressure in severe head injury by using receiver‐operating characteristic curves: an observational study in 291 patients. Journal of Neurosurgery 2001;94:412‐6. [DOI] [PubMed] [Google Scholar]
Chambers 2005
- Chambers IR, Jones PA, Minns RA, Stobbart L, Mendelow AD, Tasker RC, et al. Which paediatric head injured patients might benefit from decompression? Thresholds of ICP and CPP in the first six hours. Acta Neurochirurgica. Supplement 2005;95:21‐3. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving crossover trials: methodological issues. International Journal of Epidemiology 2002;31(1):140–9. [DOI] [PubMed] [Google Scholar]
Fan 2004
- Fan JY. Effect of backrest position on intracranial pressure and cerebral perfusion pressure in individuals with brain injury: a systematic review. The Journal of Neuroscience Nursing 2004;36(5):278‐88. [DOI] [PubMed] [Google Scholar]
Figaji 2009
- Figaji AA, Zwane E, Thompson C, Fieggen AG, Argent AC, Roux PD, et al. Brain tissue oxygen tension monitoring in pediatric severe traumatic brain injury. Part 1: Relationship with outcome. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery 2009;25(10):1325‐33. [PUBMED: 19214532] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Huh 2009
- Huh JW, Raghupathi R. New concepts in treatment of pediatric traumatic brain injury. Anesthesiology Clinics 2009;27(2):213‐40. [PUBMED: 19703674] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kenning 1981
- Kenning JA, Toutant SM, Saunders RL. Upright patient positioning in the management of intracranial hypertension. Surgical Neurology 1981;15(2):148‐52. [PUBMED: 7245008] [DOI] [PubMed] [Google Scholar]
Langlois 2005
- Langlois JA, Rutland‐Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. Journal of Head Trauma Rehabilitation 2005;20(3):229‐38. [PUBMED: 15908823] [DOI] [PubMed] [Google Scholar]
Magnaes 1976
- Magnaes B. Body position and cerebrospinal fluid pressure. Part 2: clinical studies on orthostatic pressure and the hydrostatic indifferent point. Journal of Neurosurgery 1976;44(6):698‐705. [PUBMED: 1271090] [DOI] [PubMed] [Google Scholar]
Magnaes 1978
- Magnaes B. Movement of cerebrospinal fluid within the craniospinal space when sitting up and lying down. Surgical Neurology 1978;10(1):45‐9. [PUBMED: 684606] [PubMed] [Google Scholar]
Marmarou 1975
- Marmarou A, Shulman K, LaMorgese J. Compartmental analysis of compliance and outflow resistance of the cerebrospinal fluid system. Journal of Neurosurgery 1975;43(5):523‐34. [PUBMED: 1181384] [DOI] [PubMed] [Google Scholar]
Meyer 2010a
- Meyer MJ, Megyesi J, Meythaler J, Murie‐Fernandez M, Aubut JA, Foley N, et al. Acute management of acquired brain injury part II: an evidence‐based review of pharmacological interventions. Brain Injury 2010;24(5):706‐21. [PUBMED: 20376996] [DOI] [PubMed] [Google Scholar]
Meyer 2010b
- Meyer MJ, Megyesi J, Meythaler J, Murie‐Fernandez M, Aubut JA, Foley N, et al. Acute management of acquired brain injury part I: an evidence‐based review of non‐pharmacological interventions. Brain Injury 2010;24(5):694‐705. [PUBMED: 20353284] [DOI] [PubMed] [Google Scholar]
Muñoz‐Venturelli 2015
- Muñoz‐Venturelli P, Arima H, Lavados P, Brunser A, Peng B, Cui L, et al. Head Position in Stroke Trial (HeadPoST)‐‐sitting‐up vs lying‐flat positioning of patients with acute stroke: study protocol for a cluster randomised controlled trial. Trials 2015;16:256. [ClinicalTrials.gov identifier: NCT02162017] [DOI] [PMC free article] [PubMed] [Google Scholar]
Potts 1973
- Potts DG, Deonarine V. Effect of positional changes and jugular vein compression on the pressure gradient across the arachnoid villi and granulations of the dog. Journal of Neurosurgery 1973;38(6):722‐8. [PUBMED: 4710651] [DOI] [PubMed] [Google Scholar]
Puvanachandra 2008
Puvanachandra 2009
- Puvanachandra P, Hyder AA. The burden of traumatic brain injury in Asia: a call for research. Pakistan Journal of Neurological Sciences 2009;4(1):27‐32. [Google Scholar]
Reilly 2007
- Reilly P. The impact of neurotrauma on society: an international perspective. Progress in Brain Research 2007;161:3‐9. [PUBMED: 17618966] [DOI] [PubMed] [Google Scholar]
Sackett 1989
- Sackett DL. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest 1989;95:2S‐4S. [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Simmons 1997
- Simmons BJ. Management of intracranial hemodynamics in the adult: a research analysis of head positioning and recommendations for clinical practice and future research. Journal of Neuroscience Nursing 1997;29(1):44‐9. [PUBMED: 9067853] [DOI] [PubMed] [Google Scholar]
Stelfox 2008
- Stelfox HT, Goverman J. The number, content, and quality of randomized controlled trials in the prevention and care of injuries. The Journal of Trauma 2008;65(6):1488‐93. [PUBMED: 19077647] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Version 5.1.0 (updated March 2011). The Cochrane Collaboration.
Sullivan 2000
- Sullivan J. Positioning of patients with severe traumatic brain injury: research‐based practice. Journal of Neuroscience Nursing 2000;32(4):204‐9. [PUBMED: 10994533] [DOI] [PubMed] [Google Scholar]
Tasker 2012
Zitnay 2005
- Zitnay GA. Lessons from national and international TBI societies and funds like NBIRTT. Acta Neurochirurgica. Supplement 2005;93:131‐3. [PUBMED: 15986742] [DOI] [PubMed] [Google Scholar]


