Abstract
Background
The aim of diabetes management is to normalise blood glucose levels, since improved blood glucose control is associated with reduction in development, and progression, of complications. Nutritional factors affect blood glucose levels, however there is currently no universal approach to the optimal dietary treatment for diabetes. There is controversy about how useful the glycaemic index (GI) is in diabetic meal planning. Improved glycaemic control through diet could minimise medications, lessen risk of diabetic complications, improve quality of life and increase life expectancy.
Objectives
To assess the effects of low glycaemic index, or low glycaemic load, diets on glycaemic control in people with diabetes.
Search methods
We performed electronic searches of The Cochrane Library, MEDLINE, EMBASE and CINAHL with no language restriction.
Selection criteria
We assessed randomised controlled trials of four weeks or longer that compared a low glycaemic index, or low glycaemic load, diet with a higher glycaemic index, or load, or other diet for people with either type 1 or 2 diabetes mellitus, whose diabetes was not already optimally controlled.
Data collection and analysis
Two reviewers independently extracted data on study population, intervention and outcomes for each included study, using standardised data extraction forms.
Main results
Eleven relevant randomised controlled trials involving 402 participants were identified. There was a significant decrease in the glycated haemoglobin A1c (HbA1c) parallel group of trials, the weighted mean difference (WMD) was ‐0.5% with a 95% confidence interval (CI) of ‐ 0.9 to ‐0.1, P = 0.02; and in the cross‐over group of trials the WMD was ‐0.5% with a 95% CI of ‐1.0 to ‐0.1, P = 0.03. Episodes of hypoglycaemia were significantly fewer with low compared to high GI diet in one trial (difference of ‐0.8 episodes per patient per month, P < 0.01), and proportion of participants reporting more than 15 hyperglycaemic episodes per month was lower for low‐GI diet compared to measured carbohydrate exchange diet in another study (35% versus 66%, P = 0.006). No study reported on mortality, morbidity or costs.
Authors' conclusions
A low‐GI diet can improve glycaemic control in diabetes without compromising hypoglycaemic events.
Plain language summary
Low glycaemic index, or low glycaemic load, diets for diabetes mellitus
Nutritional factors affect blood glucose levels, however there is currently no universal approach to the optimal dietary strategy for diabetes. Different carbohydrate foods have different effects on blood glucose and can be ranked by the overall effect on the blood glucose levels using the so‐called glycaemic index. By contributing a gradual supply of glucose to the bloodstream and hence stimulating lower insulin release, low glycaemic index foods, such as lentils, beans and oats, may contribute to improved glycaemic control, compared to high glycaemic index foods, such as white bread. The so‐called glycaemic load represents the overall glycaemic effect of the diet and is calculated by multiplying the glycaemic index by the grammes of carbohydrates.
We identified eleven relevant randomised controlled trials, lasting 1 to 12 months, involving 402 participants. Metabolic control (measured by glycated haemoglobin A1c (HbA1c), a long‐term measure of blood glucose levels) decreased by 0.5% HbA1c with low glycaemic index diet, which is both statistically and clinically significant. Hypoglycaemic episodes significantly decreased with low glycaemic index diet compared to high glycaemic index diet. No study reported on mortality, morbidity or costs.
Background
Description of the condition
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. A consequence of this is chronic hyperglycaemia (that is elevated levels of plasma glucose) with disturbances of carbohydrate, fat and protein metabolism. Long‐term complications of poorly controlled diabetes mellitus include retinopathy, nephropathy and neuropathy, and increased risk of cardiovascular disease. The prevalence of type 2 diabetes is increasing and is being diagnosed at increasingly younger ages (Silink 2002). For a detailed overview of diabetes mellitus, please see under 'Additional information' in the Metabolic and Endocrine Disorders Group section in The Cochrane Library (see 'About', 'Cochrane Review Groups (CRGs)'). For an explanation of methodological terms, see the main glossary in The Cochrane Library.
Description of the intervention
The aim of diabetes management is to normalise blood glucose levels, since improved blood glucose control is associated with a reduction in the development of, and progression of, complications (Stratton 2000). Nutritional factors affect blood glucose levels, however there is currently no universal approach to the optimal dietary strategy for diabetes (ADA 2008). Improvement in glycaemic control achieved through dietary interventions would lessen the risk of diabetic complications, improve quality of life for people with diabetes, increase their life expectancy, and minimise, or even avoid, the necessity for expensive medications and diabetic health care. Different carbohydrate foods have different effects on blood glucose and can be ranked by the overall effect on the blood glucose levels using the glycaemic index (Jenkins 1981). By contributing a gradual supply of glucose to the bloodstream and hence stimulating lower insulin release, low glycaemic index foods, such as lentils, beans and oats, may contribute to improved glycaemic control, compared to high glycaemic index foods, such as white bread (Jenkins 1981). Low glycaemic index diets may increase insulin sensitivity by minimising fluctuations in blood glucose levels and reducing the secretion of insulin over the day (Crapo 1977). The glycaemic load represents the overall glycaemic effect of the diet and is calculated by multiplying the glycaemic index by the grammes of carbohydrates (Salmeron 1997).
How the intervention might work
There is controversy over how useful the glycaemic index or glycaemic load is in meal planning for people with diabetes. The authors of a recent Cochrane systematic review concluded that there were no high‐quality data on the efficacy of diet alone for the treatment of type 2 diabetes (Nield 2008), but low glycaemic index diets were not one of the diets considered in that review. However, in another review, it was concluded that low glycaemic index diets exert a small, but clinically useful effect on medium‐term glycaemic control in diabetes (Brand‐Miller 2003).
The American Diabetes Association recommends that "with regard to the glycaemic effects of carbohydrates, the total amount of carbohydrate in meals or snacks is more important than the source or type" (ADA 2004). This recommendation was based on five studies (Franz 2004) in all of which glycated haemoglobin levels did not change. However, all these studies had interventions of six weeks or less and percentage glycated haemoglobin reflects glycaemic control over two to three months (ADA 2004). Short trials, however, may show differences in the degree of glycation of serum proteins (mostly albumin), measured by fructosamine or glycated serum albumin (GSA) assays, since these tests reflect glycaemic control during the preceding one to four weeks (ADA 2004; Goldstein 1995; Winocour 1988). Four of the five studies (Franz 2004) reported either fructosamine or GSA results, which decreased after the low glycaemic index diet, indicating a positive effect on glycaemic control.
The most recent position statement from the American Diabetes Association (ADA) maintains that glycaemic control is best attained through monitoring total carbohydrate via carbohydrate counting, exchange or by experienced‐based estimation (ADA 2008). This ADA position statement considers that use of glycaemic index or load may provide possibly only a modest secondary benefit above consideration of total carbohydrate alone (ADA 2008), even though questions on the glycaemic index were considered in an ADA statement issued in 2004 (Sheard 2004).
Why it is important to do this review
Our Cochrane systematic review may clarify issues surrounding the role of low glycaemic index, or low glycaemic load, diets in the management of diabetes mellitus. Our review will include all relevant studies with diet interventions lasting four weeks or longer. If alterations in the glycaemic index or glycaemic load of the diet can alone improve glycaemic control in diabetes, the use of low glycaemic index, or low glycaemic load, diets could have significant health and cost benefits for people with diabetes and the community as a whole.
Objectives
To assess the effects of low glycaemic index, or low glycaemic load, diets on glycaemic control in people with diabetes.
Methods
Criteria for considering studies for this review
Types of studies
Inclusion criteria
Trial Design
We considered all randomised controlled trials that compared a low glycaemic index, or low glycaemic load, diet with a higher glycaemic index diet for people with diabetes.
Trial Duration
We included trials with dietary interventions lasting four weeks or longer. Efficacy was assessed as short term (if follow‐up was less than six months), intermediate (six months to less than 12 months) and long‐term (12 months and over).
Exclusion criteria
We excluded studies in which the intervention was only a generalised recommendation to increase the proportion of low glycaemic index foods in the diet, or to reduce the glycaemic load, without provision of explicit detail; studies in which the intervention was either not directly supervised or well‐documented, for example, through the use of food diaries or the provision of food; studies in which there was a co‐intervention in the experimental group that was not also applied to the control group.
Types of participants
Participants were males and females of any age who were classified as having diabetes mellitus using validated and specified criteria. To be consistent with changes in classification and diagnostic criteria of diabetes mellitus through the years, the diagnosis should have been established using the standard criteria valid at the time of the beginning of the trial. Ideally, diagnostic criteria should have been described. If necessary, the authors' definition of type 2 diabetes mellitus was used.
Types of interventions
We included studies that compared a low glycaemic index, or low glycaemic load, diet with a higher glycaemic index diet or other diets.
Types of outcome measures
Glycated haemoglobin is the best measure of long‐term glycaemic control, since it represents the average blood glucose levels over several months (ADA 2004; UKPDS 38 1998; UKPDS33 1998). Hence, for the review, glycated haemoglobin was defined as the main outcome measure of glycaemic control for studies where the intervention lasted over six weeks. Fructosamine or glycated serum albumin (GSA) levels were used, when provided, as the measure of glycaemic control for studies where the intervention lasted six weeks or less, since in these cases, fructosamine or GSA levels are more reliable indicators of glycaemic control than the degree of glycation of haemoglobin (ADA 2004; Winocour 1988). The turnover of human serum albumin is much shorter (half‐life of 14 to 20 days) than that of haemoglobin (erythrocyte life span 120 days), so the degree of glycation of serum proteins (mostly albumin), indicated by fructosamine or GSA, shows the level of glycaemia better over shorter time periods than does glycation of haemoglobin (ADA 2004). "Measurements of total glycated serum protein and GSA correlate well with one another and with measurements of glycated haemoglobin" (ADA 2004; Goldstein 1995).
Primary outcomes
glycaemic control as measured by glycated haemoglobin, fructosamine, glycated serum albumin or other test measuring glycated proteins;
adverse effects.
Secondary outcomes
insulin action (fasting plasma insulin, insulin sensitivity, insulin area under the curve, total insulin released per day, insulin‐to‐glucose ratio);
morbidity (for example diabetes and cardiovascular related morbidity, like angina pectoris, myocardial infarction, stroke, peripheral vascular disease, neuropathy, retinopathy, nephropathy, erectile dysfunction, amputation);
quality of life (using a validated instrument);
costs;
mortality.
Timing of outcome assessment (length of intervention)
Studies were classified as short term (less than six months), medium term (six to less than twelve months), or long‐term (12 months and over), according to the timing of the final outcome assessments after the intervention.
Search methods for identification of studies
Electronic searches
We searched the following sources for the identification of trials:
The Cochrane Library (Issue 2, 2008);
MEDLINE (up June 2008);
EMBASE (up to June 2008);
CINAHL (up to June 2008).
The included search strategy (see detailed search strategy under Appendix 1) was used for MEDLINE. This was slightly modified for searches of EMBASE, The Cochrane Library and CINAHL. We placed no language restrictions on either the search or the included trials.
Searching other resources
We hand searched the reference lists of review articles and included studies for other potentially eligible studies.
Data collection and analysis
Selection of studies
Two reviewers independently reviewed the titles, abstract sections and keywords of every record retrieved from the literature searches to identify potentially eligible studies. Articles that clearly do not meet the inclusion criteria were rejected at this initial review. We obtained the full text of the remaining articles for further examination. We assessed each study for eligibility for inclusion against the defined selection criteria and eliminated any trial that did not fulfil this criteria, for example was not a randomised controlled trial, did not involve people who had diabetes, had no comparator, included a co‐intervention, or in which the trial period was less than four weeks. The decision to eliminate a trial was based on agreement by both reviewers. We had planned to calculate inter‐rater agreement for study selection using Cohen's kappa statistic (Cohen 1960; Fleiss 1981), and resolve any differences through discussion. However, the authors identified the same abstracts for further investigation and later for inclusion, so this was not performed. An adapted QUOROM (quality of reporting of meta‐analyses) flow‐chart of study selection is attached (Moher 1999) (Figure 1).
1.
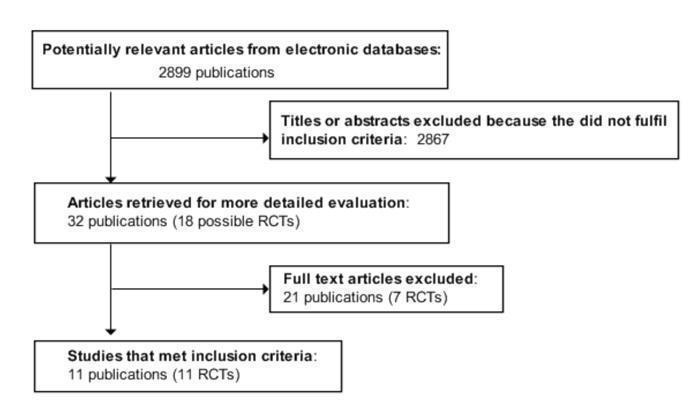
Adapted QUOROM (quality of reporting of meta‐analyses) flow‐chart of study selection
Data extraction and management
Two reviewers independently extracted the data on the study population, intervention and outcomes for each included study, using a standardised data extraction form. We extracted the following data:
general information: published or unpublished, title, authors, study setting, source, contact address, country, language, year of publication, duplicate publication, funding source;
trial characteristics: design, randomisation (and method), allocation concealment (and method), blinding of outcome assessors, withdrawals, losses to follow‐up.
intervention and comparator; duration;
participants: Inclusion criteria, exclusion criteria, number in intervention and comparison groups, sex, age, health status, medication status, type of diabetes, diagnostic criteria, similarity of groups at baseline;
outcomes: outcomes specified in the methods, other outcomes assessed in the study;
results: For continuous variables, we extracted the number of participants, and the baseline and post‐intervention means with standard deviations (SD) or standard error of the mean (SEM) or 95% confidence interval (95% CI) for the intervention and control groups. We transformed SEM or 95% CI into SD, if appropriate. Dichotomous outcomes were also recorded.
Any variations in data extraction were resolved by consensus, referring back to the original data.
Assessment of risk of bias in included studies
Two reviewers independently assessed the methodological quality of each included trial, based on quality criteria specified by Schulz and Jadad (Jadad 1996; Schulz 1995): (1) Minimisation of selection bias ‐ a) was the randomisation procedure adequate? b) was the allocation concealment adequate? (2) Minimisation of attrition bias ‐ a) were withdrawals and dropouts completely described? b) was the analysis by intention‐to treat? (3) Minimisation of detection bias ‐ were the outcome assessors blind to the intervention? Blinding of either the participant or the administrator of the intervention is generally not possible in dietary intervention studies, and it is often not feasible to have an assessor who has had no part in the trial, hence blinding was not assessed as a quality criterion. However, blinding of outcome assessors was recorded.
As there were insufficient trials, sensitivity analyses on the quality of the included trials (A ‐ low risk of bias: all quality criteria met; B ‐ moderate risk of bias: one or more of the quality criteria only partially met; C ‐ high risk of bias: one or more quality criteria not met) were not performed (Cochrane Handbook for Systematic Reviews of Interventions) (Higgins 2005).
We had planned to calculate the level of inter‐rater agreement on quality assessment using the kappa statistic (Cohen 1960; Fleiss 1981), however, as there was no variation, this was not performed.
Assessment of heterogeneity
We tested for heterogeneity between trial results using the standard χ2 test to examine whether any variation in study results could be due to the variation expected by chance alone, with significance set at α = 0.1. Quantification of the effect of heterogeneity was assessed by means of I2, ranging from 0% to 100% including its 95% confidence interval (Higgins 2002). I2 demonstrates the percentage of total variation across studies due to heterogeneity and was used to judge the consistency of evidence. I2 values of 50% and more indicate a substantial level of heterogeneity (Higgins 2003). If heterogeneity had been found, we planned to explore it using subgroup and sensitivity analyses.
Where summarising the results seemed appropriate (sufficiently similar studies of similar quality), we used a random‐effects model which assumes the effect size varies across studies. We used intention‐to‐treat analysis where possible.
Assessment of reporting biases
Publication bias was explored through assessment of funnel plot asymmetry (Cooper 1994; Tang 2000).
Data synthesis
All data were initially analysed with a fixed effect model. We summarized the data statistically, including meta‐analysis of trial results where appropriate, that is if the data were available and results were sufficiently homogeneous and of sufficient quality. For dichotomous outcomes, we planned to express the effect size in terms of relative risk with 95% confidence interval (CI), but no dichotomous outcomes were included in this review.
For continuous outcomes, we calculated weighted mean differences. We extracted the baseline and post‐intervention means with standard deviations (SD) (or standard error of the mean (SEM) or 95% confidence interval (CI)) for the intervention and control groups, transforming any SEM or 95% CI into SD where appropriate. For absolute changes in outcome between baseline and post‐intervention for the control and intervention groups, mean difference was calculated, if required, by subtracting the control absolute change from the intervention absolute change. The estimate of variance for each of these changes equals Vpre + Vpost ‐ 2r(SEpre x SEpost), where Vpre and SEpre are the variance and standard error of the mean baseline value; Vpost and SEpost are the variance and standard error of the mean post‐intervention value; and r is the correlation between baseline and post‐intervention values. The variance of the total change is then the sum of the variance of the change in the intervention group and the variance of the change in the control group. If the value of r was not given, we assumed that r equalled 0.5.
When post‐intervention measures of dispersion were not given (for example if the results were presented as percentage change from baseline), the baseline measures of dispersion were also used as the post‐intervention values. This is a conservative approach, since variation at baseline should be larger than that at post‐intervention, but this approach was only taken when pre‐ and post‐ measures of dispersion for the same outcome were similar to each other in other trials. If the results were given on different scales, we used standardised mean differences. When data were only presented graphically, an estimate of the mean and SD was obtained from the graph.
Drop‐outs, losses to follow‐up and withdrawn study participants were investigated. Where there were two papers reporting on the same study, we maximised the yield of information by simultaneous evaluation of all available data, with the original publication given priority.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was planned where a primary outcome parameter was statistically significantly different between dietary groups. The following subgroup analyses were planned:
age, less than or equal to 18 years, 19 to 40 years, 41 to 65 years, more than 65 years;
duration of trial intervention: short term (less than or equal to three months), medium term (three to six months), long term (more than six months);
difference in the glycaemic index, or load, between the intervention and comparator diets; diabetes diagnosis (type 1 or type 2);
duration of diabetes; follow‐up timing: less than or equal to six months, 6 to 12 months, more than 12 months.
Sensitivity analysis
We had planned to perform sensitivity analyses to explore the influence of the following factors on effect size, by repeating the analysis:
excluding unpublished studies;
taking study quality, as specified above, into account;
excluding any long or large studies to determine their influence on the results;
excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country.
However, there were insufficient studies to perform these analyses.
Results
Description of studies
Results of the search
From the initial search, 2899 records were identified. From the abstracts of these, we identified 32 papers for examination of the full text. The other papers were excluded on the basis of their abstract because they did not fit the criteria for the review. Main reasons for exclusion were: papers were reviews, not relevant, duplicates, some or all of the participants did not have diagnosed diabetes, study had no control group or no randomisation, studies did not compare similar groups, there was a co‐intervention or the intervention was less than four weeks. Eleven studies met the inclusion criteria (Brand 1991; Collier 1988; Fontvieille 1992; Frost 1994; Giacco 2000; Gilbertson 2001; Jimenez‐Cruz 2003; Komindr 2001; Luscombe 1999; Rizkalla 2004; Wolever 1992). Six reported percentage glycated haemoglobin (Brand 1991; Giacco 2000; Gilbertson 2001; Komindr 2001; Jimenez‐Cruz 2003; Rizkalla 2004), four reported fructosamine (Fontvieille 1992; Frost 1994; Luscombe 1999; Wolever 1992) and one reported glycosylated albumin (Collier 1988). One study reported both percentage glycated haemoglobin and fructosamine, but as the intervention was less than six weeks, only the fructosamine results were included (Fontvieille 1992). For an adapted QUOROM (quality of reporting of meta‐analyses) flow‐chart of study selection see Figure 1.
Missing data
No authors were successfully contacted for further information or clarification.
Assessment of inter‐rater agreement
Both authors reviewed the studies, and were in agreement on those to be fully assessed. From these, studies eligible for inclusion in the review were identified. Both authors agreed on the final papers chosen for assessment and on the quality assessment of the studies.
Included studies
Details of the characteristics of the included studies are given in the table Characteristics of included studies. The following gives a brief overview:
Study types
All eleven studies identified for the review were randomised controlled trials (Brand 1991; Collier 1988; Fontvieille 1992; Frost 1994; Giacco 2000; Gilbertson 2001; Jimenez‐Cruz 2003; Luscombe 1999; Komindr 2001; Rizkalla 2004; Wolever 1992). They were conducted in Australia (Brand 1991; Gilbertson 2001; Luscombe 1999), Canada (Wolever 1992), France (Fontvieille 1992; Rizkalla 2004), Italy (Giacco 2000) Mexico (Jimenez‐Cruz 2003) and UK (Frost 1994). The duration ranged from four weeks (Komindr 2001; Luscombe 1999; Rizkalla 2004 ) to 52 weeks (Gilbertson 2001). The maximum length of follow up was 12 months from the start of the intervention (Gilbertson 2001).
Participants
The included studies involved a total of 402 participants. The number of participants ranged from 104 participants in a parallel trial (Gilbertson 2001) to six participants in a crossover trial (Wolever 1992). The mean age ranged from 10 (SD 2) years (Gilbertson 2001) to 63 (SD 4) years (Wolever 1992) and more males than females participated. There was a total of 247 participants in the six studies reporting percentage glycated haemoglobin included in the percentage glycated haemoglobin meta‐analysis (Brand 1991; Giacco 2000; Gilbertson 2001; Jimenez‐Cruz 2003; Komindr 2001; Rizkalla 2004) and a total of 141 participants in the four studies reporting fructosamine included in the fructosamine meta‐analysis (Fontvieille 1992; Frost 1994; Luscombe 1999; Wolever 1992). There were 14 participants in the trial reporting results as glycosylated albumin (Collier 1988). 209 of these participants received the low glycaemic index or load diet intervention. Two studies involved children (Collier 1988; Gilbertson 2001).
Interventions
Ten studies compared a low glycaemic index diet with a higher glycaemic index diet (Brand 1991; Collier 1988; Fontvieille 1992; Frost 1994; Giacco 2000; Jimenez‐Cruz 2003; Komindr 2001; Luscombe 1999; Rizkalla 2004; Wolever 1992). One study compared the low‐GI diet to a diet using measured carbohydrate exchanges (Gilbertson 2001).
Duration of studies
The low glycaemic index dietary interventions ranged from four weeks duration (Komindr 2001; Luscombe 1999; Rizkalla 2004) to 12 months (Gilbertson 2001).
Outcomes
Original data can be found in Appendix 3 and Appendix 4.
Primary outcomes
Glycaemic control
Glycated haemoglobin: Seven of the included trials reported percentage glycated haemoglobin ( Brand 1991; Fontvieille 1992; Giacco 2000; Gilbertson 2001; Jimenez‐Cruz 2003; Komindr 2001; Rizkalla 2004). One of these trials reported both percentage glycated haemoglobin and fructosamine results, hence as this trial had a duration of only five weeks, as per the protocol only the value for fructosamine was included in the meta‐analysis (Fontvieille 1992).
Fructosamine: Four trials (n=141) reported fructosamine (Fontvieille 1992; Frost 1994; Luscombe 1999; Wolever 1992).
Adverse events
Two trials reported adverse events or included the incidence of hypo‐ and hyperglycaemia in the outcomes (Giacco 2000; Gilbertson 2001).
Secondary outcomes
Insulin action (fasting plasma insulin, insulin sensitivity, insulin area under the curve, total insulin released/day, insulin‐to‐glucose ratio)
Five studies included parameters related to insulin action as an outcome (Fontvieille 1992; Giacco 2000; Gilbertson 2001; Luscombe 1999; Rizkalla 2004).
Morbidity (diabetes and cardiovascular related morbidity, like angina pectoris, myocardial infarction, stroke, peripheral vascular disease, neuropathy, retinopathy, nephropathy, erectile dysfunction, amputation)
No trial included morbidity as outcome.
Quality of life (using a validated instrument)
One trial included quality of life as an outcome (Gilbertson 2001).
Costs
No trial included costs as an outcome
Mortality
No trial included mortality as an outcome.
Excluded studies
Studies which were excluded not at the time of the literature search, but after the entire papers had been perused and the reasons for their exclusion are given in the table Characteristics of excluded studies.
Risk of bias in included studies
For details see Appendix 2.
Allocation
While all included trials were described as randomised, only one included trial reported the method of randomisation, through the use of random number tables ( Frost 1994).
Incomplete outcome data
Most studies described losses to follow‐up or dropouts, if any occurred in the study. There were no dropouts in six trials (Brand 1991; Collier 1988; Fontvieille 1992; Komindr 2001; Rizkalla 2004; Wolever 1992). Dropouts were encountered in the other five trials (Frost 1994; Giacco 2000; Gilbertson 2001; Jimenez‐Cruz 2003; Luscombe 1999) and reasons for the dropouts were described in four of these trials (Frost 1994; Gilbertson 2001; Jimenez‐Cruz 2003; Luscombe 1999). One study, with nine dropouts, did not give reasons (Giacco 2000).
Other potential sources of bias
No trial included in the review reported any significant differences between groups in the main characteristics of participants at baseline.
Effects of interventions
Glycaemic index of the intervention and control group diets
The glycaemic index (GI) of the low‐GI diet in the Brand 1991 study was 15% lower than that of the high‐GI diet (77 ± 3 SE versus 91 ± 3, P < 0.01). The mean GI for the low‐GI diet was 12 units lower than the control high‐GI diet in the Collier 1988 study (68 ± 3 SE versus 82 ± 1 units P < 0.005), and this change was achieved by exchanging approximately 50% of the carbohydrates coming from high‐GI foods for low‐GI foods. In the Fontvieille 1992 study, 38 ± 5% SD was the GI for the low‐GI diet, while the GI for the high‐GI diet was 64 ± 2%, P < 0.001. The GI of the low‐GI diet in the Frost 1994 study was 77 ± 1% SEM, while the GI of the high‐GI diet was 82 ± 1%, P < 0.01. The average GI of the two diets was also different in the Giacco 2000 study, where the intervention diet had a GI of 70% and the control diet had a GI of 90%. In the Gilbertson 2001 study, in which the participants were all children with type 1 diabetes (n = 104) the low‐GI diet, which was designed to be flexible, was compared to a measured carbohydrate exchange diet. Both the GI and the glycaemic load (GL) of the low‐GI diet were significantly lower the the high‐GI diet for the Jimenez‐Cruz 2003 study (P = 0.0001). In the Komindr 2001 study, the GI of the low‐GI diet versus the high‐GI diet was approximately 70 versus 100 GI units. The GI of the low‐GI diet was 20 units lower than that of the high‐GI diet in the Luscombe 1999 study (43 GI units versus 63 GI units). The low‐GI diet had a GI of 58 compared with 86 for the high‐GI diet in the Wolever 1992 study.
Primary outcomes
Glycaemic control
Pooled data from the six studies reporting glycated haemoglobin A1c (HbA1c) with participants whose glycated haemoglobin was not yet optimised (Brand 1991; Giacco 2000; Gilbertson 2001; Jimenez‐Cruz 2003; Komindr 2001; Rizkalla 2004) showed that there was a significant decrease in HbA1c levels, indicating improved glycaemic control (WMD ‐0.5 % HbA1c, 95% CI ‐0.8 to ‐0.2, P = 0.001). When parallel and cross‐over trials were analysed separately, pooled effect estimates remained stable: In the HbA1c parallel group of trials (Giacco 2000 and Gilbertson 2001) the WMD was ‐0.5% with a 95% CI of ‐ 0.9 to ‐0.1, P = 0.02; and in the cross‐over group of trials the WMD was ‐0.5% with a 95%CI of ‐1.0 to ‐0.1, P = 0.03
Considering the studies individually, in the study comparing the low glycaemic index diet with the carbohydrate exchange diet, by 12 months the mean difference in HbA1c levels between the groups was not significant (P = 0.05), however twice as many participants in the low GI group (45%) attained acceptable HbA1c levels compared with only 22% of participants in the carbohydrate exchange group (P = 0.02 after adjustment for baseline values) (Gilbertson 2001). Percentage HbA1c was also significantly lower after the low‐GI diet compared to after the high‐GI diet in the Jimenez‐Cruz 2003 study (P < 0.02), in the Giacco 2000 study (P < 0.05), in the Brand 1991 study (P < 0.05) and in the Rizkalla 2004 study (P < 0.05). In the Rizkalla 2004 study, the reduction in the change in HbA1c after the low‐GI diet was also significantly more than after the high‐GI diet (P < 0.01).
One study which had a 12 week intervention reported the main outcome as fructosamine (Frost 1994). The three studies with an intervention duration of 4 to 6 weeks also reported results as fructosamine (Fontvieille 1992; Luscombe 1999; Wolever 1992), WMD ‐0.20 mmol/L (95% CI ‐0.46 to 0.07, P = 0.14).
Considering these studies individually, in the Fontvieille 1992 study, the reduction in fructosamine with the low‐GI diet was significant compared to the high‐GI diet (3.9 ± 0.9 versus 3.4 ± 0.4, mmol/L, P < 0.05). In the parallel Frost 1994 study, the within‐group change in fructosamine levels, which decreased in the low‐GI diet group but not in the high‐GI diet group, was also significant (P < 0.05). The Wolever 1992 study reported that serum fructosamine fell significantly after the low‐GI diet, with no change after the high‐GI diet (P < 0.05). Glycosylated albumin levels decreased significantly in the low glycaemic index intervention, but not in the high glycaemic intervention in the one study reporting this outcome (low glycaemic index diet 13.2 ± 1.5 % to 10.7 ± 2.2 %, P < 0.05; high glycaemic index diet 13.1 ± 2.3 % to 14.6 ± 1.9 %, not significant) (Collier 1988).
Adverse effects
Two trials reported on hypo‐ or hyperglycaemic events, but did not give further infomation on whether they were mild/moderate or severe (Giacco 2000; Gilbertson 2001). In the meta‐analysis for episodes of hypoglycaemia, there was heterogeneity (I2 = 50.8%) and so the results for the two studies are reported separately: In one study, where the control diet was a higher GI diet, episodes of hypoglycaemia were significantly fewer with the low‐GI diet compared to the control diet (mean difference ‐0.8 episodes per patient per month, P < 0.01) (Giacco 2000). In the second study, where the control diet was a measured carbohydrate exchange diet in children with type 1 diabetes, the proportion of participants reporting more than 15 episodes of hyperglycaemia per month was significantly lower for the low‐GI diet group compared to the measured carbohydrate exchange group, (35% versus 66%, P = 0.006 after adjustment for baseline values) at 12 months (Gilbertson 2001).
Secondary outcomes
Insulin action
Five included studies reported on parameters related to insulin action (Fontvieille 1992; Giacco 2000; Gilbertson 2001; Luscombe 1999; Rizkalla 2004).
Whole body peripheral insulin sensitivity, measured by euglycaemic‐hyperinsulinaemic clamp, was significantly higher after the low‐GI diet than after the high‐GI diet (glucose disposal: 7.0 ± 1.3 versus 4.8 ± 0.9 mg glucose/kg/min, P < 0.001)(Rizkalla 2004).
Another study reported that no significant differences were found in insulin or drug requirements, or in insulin binding to erythrocytes (Fontvieille 1992). In the two studies reporting insulin dose, both found no significant differences (Giacco 2000; Gilbertson 2001). There were also no significant differences reported in plasma insulin levels between groups (Luscombe 1999).
Morbidity
No study reported on morbidity.
Quality of life (using a validated instrument)
One trial, in children, reported on quality of life and found that it was significantly influenced by the type of diet (Gilbertson 2001), although validation measures for the questionnaire were not reported. In this trial, twice as many parents in the low‐GI group stated that their child had no difficulties in selecting their own meals at the 12‐month time point (51% versus 24%, P = 0.01). Almost twice as many parents from the low‐GI group also reported that diabetes never limited the type of family activities pursued (53% versus 27%, P = 0.02).
Costs
No study reported on costs.
Mortality
No study reported on mortality.
Heterogeneity
There was heterogeneity in the adverse events results for episodes of hypoglycaemia. In the meta‐analysis for episodes of hypoglycaemia, as there was heterogeneity (I2 = 50.8%), the results for the two studies have been reported separately. In one study, where the control diet was a higher GI diet, episodes of hypoglycaemia were significantly fewer with the low‐GI diet compared to the control diet (WMD ‐0.8 episodes, 95% CI ‐1.3 to ‐0.3, P < 0.01) (Giacco 2000). In the second study, the control diet was a measured carbohydrate exchange diet in children with type 1 diabetes and there was no difference reported in hypoglycaemic episodes (Gilbertson 2001).
Subgroup analysis
Not performed due to the small number of included studies.
Sensitivity analysis
The results were substantially unaffected by omitting individual studies from the analysis.
Assessment of publication bias
There were too few studies for detailed analysis of the funnel plot.
Follow‐up
One study reported results at 12 month follow‐up from the commencement of the study, reporting that rates of excessive hyperglycaemia (>15 episodes per month) were significantly lower in the low‐GI group (35 versus 66%, P = 0.006) (Gilbertson 2001).
Discussion
Summary of main results
This review indicates that glycaemic control in people with diabetes improved significantly with a low glycaemic index diet, compared to those on higher glycaemic index diets or measured carbohydrate exchange diets. The decrease of 0.5% glycated haemoglobin A1c (HbA1c) is clinically significant and is similar to decreases achieved through medications for newly diagnosed type 2 diabetes ( Holman 1999; UK PDSG 1995). Improvements of this size have been associated with a significant reduction in the risk of microvascular complications (Stratton 2000). The UK Prospective Diabetes Study Group (UKPDSG) found that each 1% reduction in glycated haemoglobin was associated with a reduction in risk of 21% (95% confidence interval (CI) 17% to 24%, P < 0.0001) for any end point related to diabetes and a reduction in risk of 37% (95% CI 33% to 41%, P < 0.0001) for microvascular complications and that any reduction in glycated haemoglobin is likely to reduce the risk of complications (Stratton 2000).
Of additional clinical significance, improved glycaemic control was also associated with a decrease in adverse outcomes, namely hypoglycaemic episodes. In the two trials that reported this outcome, improved glycated haemoglobin was associated with a reduction in hypoglycaemic events with the low‐GI diet compared to a high‐GI diet in one trial (Giacco 2000), and in the other trial, the proportion of participants reporting more than 15 hyperglycaemic episodes per month was lower for the low‐GI diet compared to a measured carbohydrate exchange diet (Gilbertson 2001).
Considering each of the studies individually, the improvement in glycaemic control in people on low‐GI diets versus other diets reached statistical significance in nine of the individual included studies: five which were reporting HbA1c (Brand 1991; Giacco 2000; Gilbertson 2001; Jimenez‐Cruz 2003; Rizkalla 2004), three reporting fructosamine (Fontvieille 1992; Frost 1994; Wolever 1992) and one reporting per cent glycosylated albumin (Collier 1988). The meta‐analysis confirmed these results. Of the eleven studies included in this review, three studies had participants with type 1 diabetes (Collier 1988, Giacco 2000; Gilbertson 2001), seven with type 2 diabetes (Brand 1991; Frost 1994; Jimenez‐Cruz 2003; Komindr 2001; Rizkalla 2004; Wolever 1992) and one study had participants with either type 1 or type 2 (Fontvieille 1992). Two studies involved children, all of whom had type 1 diabetes (Collier 1988; Gilbertson 2001).
One excluded study reported that there was a greater reduction in the change in percentage glycated haemoglobin after the low‐GI diet compared to that after the high‐GI diet (‐0.3% HbA1c) (Heilbronn 2002). This study was excluded from this review because participants were of unknown diabetic status at the start of the intervention (diabetic or improved diabetic), only having been diagnosed as having type 2 diabetes during some time in the previous ten years.
In the Wolever 2008 study, participants commenced the study with already optimised HbA1c levels (6.2 ± 1% HbA1c) and hence this study was also excluded, since the effect of the diets on HbA1c was our primary outcome. This study found, however, that there were sustained reductions in both postprandial glucose and C‐reactive protein on the low‐GI diet, also indicating that it could be beneficial in the ongoing management of type 2 diabetes.
In another excluded study, in which medications were adjusted as necessary, significantly less diabetic medication was required in people on the low glycaemic index diet, compared to the ADA diet, to achieve equivalent control of HbA1c levels (Ma 2008).
Insulin sensitivity was affected by the glycaemic index of the diet, with a significant increase in the whole body peripheral insulin sensitivity, measured via euglycaemic‐hyperinsulinaemic clamp after the low‐GI diet compared to the high‐GI diet (Rizkalla 2004).
All studies in the review compared the low glycaemic index diet with a high glycaemic index diet, except for one study in which the comparison diet was a measured carbohydrate exchange diet (Gilbertson 2001). In this study comparing the low‐GI diet to a restricted carbohydrate exchange diet, involving children with type 1 diabetes (Gilbertson 2001), twice as many participants in the low‐GI group reached acceptable HbA1c levels at 12 months without any increase in the rate of hypoglycaemic occurrences, compared to the carbohydrate exchange group. Hence, even when compared to the restricted carbohydrate exchange diet, the low glycaemic index diet showed greater improvement in glycaemic control.
Overall completeness and applicability of evidence
This review suggests that a low‐GI diet is beneficial for improving glycaemic control in people with diabetes, and that a low‐GI diet is associated with a decrease in the number of hypoglycaemic episodes. Participants in the included trials were both adults and children with diabetes, suggesting that the results would be relevant to a broad spectrum of age groups in other similar communities. Inclusion criteria for studies were either type 1 or type 2 diabetes, or both, and hence the results of the review have relevance to both types of diabetes. None of the trials occurred in developing countries.
Potential biases in the review process
Eleven relevant studies were identified, all of which were randomised controlled trials. Some methodological limitations were present such as failure to conceal allocation and lack of reporting on blinding of outcome assessors.
Authors' conclusions
Implications for practice.
The studies included in this systematic review were all randomised controlled trials, and all had interventions of at least four weeks or longer. The longest trial was 12 months. This review provides data that low glycaemic index diets can significantly improve diabetic control in less than optimally controlled people with diabetes by lowering percentage glycated haemoglobin A1c (HbA1c) levels by 0.5%. This is clinically significant and comparable to decreases achieved through medications for newly diagnosed type 2 diabetes (Holman 1999; UK PDSG 1995). The UK Prospective Diabetes Study Group found that 1% reduction in glycated haemoglobin was associated with reductions in risk of 21% for any end point related to diabetes and a reduction in risk of 37% for microvascular complications and that any reduction in glycated haemoglobin is likely to reduce the risk of complications (Stratton 2000). Importantly, reduction in glycated haemoglobin with the low‐GI diet was associated with decreased risk of hypoglycaemic episodes. When compared with a high‐GI diet, the low‐GI diet reduced hypoglycaemic events significantly (Giacco 2000). Similarly, the proportion of participants reporting more than 15 hyperglycaemic episodes per month was significantly lower for the low‐GI diet compared to the measured carbohydrate exchange diet (Gilbertson 2001).
Whole body peripheral insulin sensitivity, measured via euglycaemic‐hyperinsulinaemic clamp, was also significantly affected by the glycaemic index of the diet, significantly increasing in the low‐GI group compared to the high‐GI group (Rizkalla 2004). The improvement may benefit the patient with diabetes by lessening or even avoiding the requirement for medication.
Hence, lowering the glycaemic index of foods in the diet appears to be an effective method of improving glycaemic control in diabetes without compromising the number of hypoglycaemic episodes.
Implications for research.
While one study provided follow‐up data at 12 months after the start of the intervention, it would be useful if further long‐range studies could be performed, including quality of life outcomes with validated instruments to determine the acceptability of incorporating a low‐GI diet in a person's lifestyle, as well as measurement of long‐term glycaemic control. There are indications that the improvement can be maintained, as an excluded study reported that at 12 months, there was no significant change in HbA1c levels which had already been optimised before the commencement of the study (Wolever 2008). There were two studies in children, all of whom had type 1 diabetes, and further longer range studies with children would be useful to confirm the impact of low glycaemic index diets on long‐term glycaemic control, adverse events and quality of life.
Feedback
Description of the intervention, 20 March 2009
Summary
The intervention isn't clearly described in a way that would allow it to be reproduced (or at least I couldn't find this ‐ it isn't in the "Description of the intervention"). As it doesn't define what constitutes low GI or low glycaemic load, a reader with diabetes might not know what the implications of this review's findings are for improving his or her diet.
Reply
As the glycaemic index (GI) represents the degree that a carbohydrate can raise the blood glucose, it is necessary to test a food in people to determine its GI, rather than predict the GI through its structure. The GI of a food can be affected by several factors such as the cooking method used for preparation, the form of the food, the type of starch it contains, how much fibre it contains and also how much and what type of sugar is present in the food. Processing increases the gelatinised starch content and so can raise the GI of the food. Grains and legumes that have fibrous husks, as well as foods containing soluble fibre, such as oatmeal and apples tend to have a lower GI. The low GI diet is a diet generally high in carbohydrate foods, but with legumes, whole grains (eg oats, barley, rye), and low GI versions of bread, rice, pasta, fruit and vegetables forming the basis. A low GI diet can be achieved by substituting the high GI foods in the diet with lower GI alternatives. Hence, consuming semolina, muesli or porridge instead of processed cereals; basmati rice, instead of short grain rice; sweet potato instead of white potato; whole grain, rye or sourdough bread instead of white bread can significantly lower the GI of the diet. Pasta is also a low GI option. If the lower GI alternative food is eaten for these carbohydrate rich foods, then a low GI diet can include whatever types of vegetables and meat that are required to provide a balanced healthy diet, as these contain, by comparison, little carbohydrate. These changes from higher to lower GI foods can be gradually incorporated into the eating pattern, so that they become part of the normal diet and the GI of the overall diet significantly lowers.
Contributors
Comments made by Amanda, occupation doctor ( ajburls@yahoo.co.uk).
Diana Thomas replied to the comments on behalf of the review authors for the review.
What's new
| Date | Event | Description |
|---|---|---|
| 12 May 2009 | Feedback has been incorporated | Clarification about the description of the intervention |
Acknowledgements
Professor Chris Cowell, The University of Sydney, Head of Endocrinology at The Children's Hospital at Westmead, for expert advice on clinical aspects; Samantha Clarke, Acting Head Diabetes Dietitian, The Children's Hospital at Westmead, for assistance in trial searching and Sunita Chauhan for assistance in search strategy development.
Appendices
Appendix 1. Search strategy
| Search terms |
| Unless otherwise stated, search terms are free text terms; MeSH = Medical subject heading (Medline medical index term); exp = exploded MeSH; the dollar sign ($) stands for any character(s); the question mark (?) = to substitute for one or no characters; tw = text word; pt = publication type; sh = MeSH; adj = adjacent. MEDLINE: A) Diabetes mellitus, general 1 exp diabetes mellitus/ 2 diabet$.tw. 3 IDDM.tw. 4 NIDDM.tw. 5 MODY.tw. 6 insulin$ secret$ dysfunc$.tw. 7 impaired glucose toleran$.tw. 8 exp glucose intolerance/ 9 glucose intoleran$.tw. 10 exp insulin resistance/ 11 insulin$ resist$.tw. 12 (non insulin$ depend$ or noninsulin$ depend$ or non insulin?depend$ or noninsulin?depend$).tw. 13 (insulin? depend$ or insulin?depend$).tw 14 metabolic$ syndrom$.tw. 15 (pluri metabolic$ syndrom$ or plurimetabolic$ syndrom$).tw. 16 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 17 exp diabetes insipidus/ 18 diabet$ insipidus.tw. 19 17 or 18 20 16 not 19 B) Controlled trials* 21 randomized‐controlled trial.pt. 22 controlled‐clinical trial.pt. 23 randomized‐controlled‐trials.sh. 24 random allocation.sh. 25 double‐blind method.sh. 26 single‐blind method.sh. 27 21 or 22 or 23 or 24 or 25 or 26 28 animal.sh. 29 human.sh. 30 28 not 29 31 27 not 30 C) Glycaemic index or glycaemic load 32 (diet adj5 glyc?emic$).tw. 33 Glycemic Index/ or glyc?emic index.tw. 34 (all bran or wholegrain or pasta or oat$ or apple$ or appricot$ or bean$ or lentil$ or wheat bran or barley porridge or raw oats or basmati rice).ti, ab. 35 (gi adj10 (diet or food or carbohydrate$)).tw. 36 (food adj5 glyc?emic$).tw. 37 (carbohydrate$ adj5 (blood glucose or blood sugar) adj5 (low or less$ or high$)).tw. 38 (puls$ adj10 (diet or food)).tw. 39 lentil$.tw. 40 or/32‐39 41 dietary carbohydrates/ 42 blood glucose.sh,rn,rw. 43 41 and 42 44 40 or 43 D) Systematic reviews, meta‐analyses, health‐technology assessment reports 45 exp meta‐analysis/ 46 exp Review Literature/ 47 meta‐analysis.pt. 48 systematic review$.tw. 49 search$.tw. 50 medline.tw. 51 cochrane database of systematic reviews.jn. 52 45 or 46 or 47 or 48 or 49 or 50 or 51 53 letter.pt. 54 comment.pt. 55 editorial.pt. 56 historical‐article.pt. 57 53 or 54 or 55 or 56 58 52 not 57 59 exp Technology Assessment, Biomedical/ 60 HTA.tw. 61 (health technology adj6 assessment$).tw. 62 (biomedical adj6 technology assessment$).tw. 63 60 or 61 or 62 64 58 or 63 65 31 or 64 66 20 and 44 67 65 and 66 * Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. International Journal of Epidemiology 2002;31:150‐3. |
Appendix 2. Risk of bias
| Study | At baseline | Randomisation | Allocation concealed | Intention‐to ‐treat | Assessor blinding | Losses accounted for |
| Brand 1991 | similar | yes | not reported | yes | not reported | no losses |
| Collier 1988 | similar | yes | not reported | yes | not reported | no losses |
| Fontvieille 1992 | similar | yes | not reported | yes | not reported | no losses |
| Frost 1994 | similar | yes | not reported | no | not reported | failed to complete study |
| Giacco 2000 | similar | yes | not reported | no | not reported | reasons not given |
| Gilbertson 2001 | similar | yes | not reported | yes, but not at 12 months | yes | yes |
| Jimenez‐Cruz 2003 | similar | yes | not reported | no | not reported | yes |
| Komindr 2001 | similar | yes | not reported | yes | not reported | no losses |
| Luscombe 1999 | similar | yes | not reported | no | not reported | yes |
| Rizkalla 2004 | similar | yes | not reported | yes | not reported | no losses |
| Wolever 1992 | similar | yes | not reported | yes | not reported | 2 participants sampled 1 week early for final analysis for both diets |
Appendix 3. Original study data
| Glycaemic index or low glycaemic load diet versus other diet Glycated haemoglobin (%HbA1c) Study ID: Low glycaemic N Low glycaemic Mean Low glycaemic SD Control N Control Mean Control SD Brand 1991: 16 7.00 0.60 16 7.90 2.00 Giacco 2000: 29 8.80 1.00 25 9.10 1.30 Gilbertson 2001: 51 8.00 1.00 38 8.60 1.40 Komindr 2001: 10 10.97 1.55 10 11.15 2.02 Jiminez‐Cruz 2003: 14 8.10 0.90 14 8.60 0.90 Rizkalla 2004: 12 7.17 1.35 12 7.57 1.21 Fructosamine Study ID: Treatment N Treatment Mean Treatment SD Control N Control Mean Control SD Fontvieille 1992: 18 3.41 0.95 18 3.88 0.42 Frost 1994: 25 3.20 1.00 26 3.60 1.00 Luscombe 1999: 21 3.22 5.04 21 3.28 5.50 Wolever 1992: 6 4.56 1.30 6 5.12 1.40 Episodes of hypoglycemia Study ID: Treatment N Treatment Mean Treatment SD Control N Control Mean Control SD Giacco 2000: 29 0.70 0.70 25 1.50 1.20 Gilbertson 2001: 51 6.90 6.80 38 5.80 5.50 |
Appendix 4. Baseline percentage glycated haemoglobin values reported in studies
| Significantly different from endpoint | 1.4 SEM | 8.3 (baseline not significantly different from endpoint) | 1.5 SEM | Giacco 2000 |
| 8.8 | 1.4 SD | 8.8 | 1.4 SD | Gilbertson 2001 |
| 8.3 | 1.3 SD | 8.6 (carbohydrate exchange) | 1.4 SD | Jimenez‐Cruz 2003 |
| 8.5 | 0.3 SEM | 8.6 | 0.3 SEM | Komindr 2001 |
| 13.8 | 1.1 SEM | 13.8 | 1.1 SEM | Rizkalla 2004 |
| 7.6 | 0.4 SE | 7.5 | 0.4 SE | Studies with HbA1c levels already less than 7.0% at baseline (excluded) |
| Heilbronn 2002 (mixed diabetic/ improved diabetic subjects. Diabetic status unknown at baseline) | ||||
| 6.6 | 0.3 SEM | 6.3 | 0.3 SEM | Wolever 2008 (Participants already had optimal mean HbA1c at baseline) |
| 6.2 | 0.8 SEM | 6.2 | 1.0 SEM |
Data and analyses
Comparison 1. Low glycaemic index or low glycaemic load diet versus other diet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Glycated haemoglobin (%HbA1c) | 6 | 247 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐0.81, ‐0.20] |
| 2 Fructosamine (mMol/L) | 4 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.47, 0.00] |
1.1. Analysis.
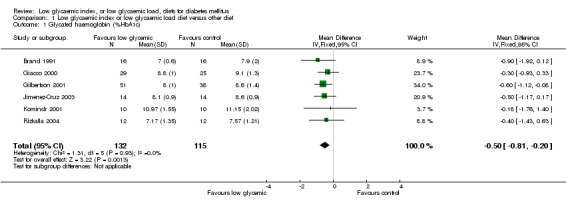
Comparison 1 Low glycaemic index or low glycaemic load diet versus other diet, Outcome 1 Glycated haemoglobin (%HbA1c).
1.2. Analysis.
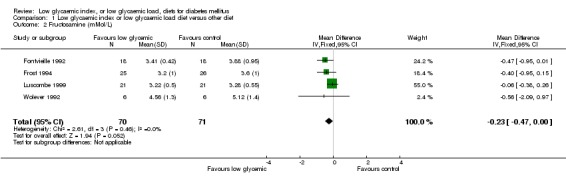
Comparison 1 Low glycaemic index or low glycaemic load diet versus other diet, Outcome 2 Fructosamine (mMol/L).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brand 1991.
| Methods | Trial Design: RCT Randomisation procedure: randomly assigned crossover design Allocation concealment: not reported Blinding of outcome assessors: Not reported Intention to treat analysis: Yes | |
| Participants | Country: Australia Setting: Community Number: 16 in crossover design Age: 62 ± 9 yr Sex: 6 female, 10 male Inclusion criteria: well‐controlled NIDDM (defined by the National Diabetes Data Group), no history of ketosis or brittle diabetes, insidious onset of diabetes with minimal symptoms Other characteristics: mean duration of diabetes 5 yr (range 1‐22 yr), body mass 75.9 ± 14.1 kg. Body Mass Index 25 ± 5 kg/m2. All subjects in good healthy except for diabetes. Ten participants on sulfonylureas plus diet therapy, and six on diet therapy alone. Medication was not altered during study. | |
| Interventions | Trial intervention: low glycaemic index diet comparison intervention: high glycaemic index diet Duration: 12 weeks | |
| Outcomes | Main outcome measures: %HbA1c, weight, fasting plasma glucose, urinary glucose, plama cholesterol, plasma triglycerides, low‐ and high‐ density lipoprotein cholesterol | |
| Notes | Source of funding: Sydney University Nutrition Research Foundation, CSL‐Novo Pty Ltd, Apex‐Australian Diabetes Foundation Drop‐outs: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Collier 1988.
| Methods | Trial Design: RCT Randomisation procedure: randomly assigned crossover design. Allocation concealment: not reported Blinding of outcome assessors: not reported Intention to treat analysis: yes | |
| Participants | Country: Canada Setting: community Number: 7 Age: 12 ± 2 years Sex: 6 male, 1 female Inclusion criteria: Children with diabetes Other characteristics: 102 ± 3% normal body weight, average insulin dose = 41.7 Units per day | |
| Interventions | Trial intervention: low glycaemic index starchy diet comparison intervention: high glycaemic index diet Duration: 6 weeks per intervention with a 4 week washout period | |
| Outcomes | Main outcome measures: lipids, glucose, %HbA1c, glycosylated albumin Other outcomes: C‐peptide, insulin dose, growth | |
| Notes | Source of funding: Natural Sciences and Engineering Research Council of Canada and the Hospital for Sick Children Foundation Drop‐outs: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Fontvieille 1992.
| Methods | Trial Design: RCT Randomisation procedure: not reported, crossover design Allocation concealment: not reported Blinding of outcome assessors: Unclear Intention to treat analysis: yes | |
| Participants | Country: France Setting: Community Number: 18 Age: 47 ± 12 years Sex: 12 male, 6 female Inclusion criteria: well‐controlled diabetes Exclusion criteria: not stated Other characteristics: type 1 diabetes n=12 (insulin controlled), type 2 diabetes n=6 (oral antidiabetic drugs), diabetes duration 12 ± 6 years, BMI 25 ± 3 kg /m2 | |
| Interventions | Trial intervention: 5 weeks on low glycaemic index diet GI=38 ± 5 SD comparison intervention: 5 weeks on high glycaemic index diet GI= 64 ± 2 SD | |
| Outcomes | Main outcome measures: fructosamine Other outcomes: self‐measured fasting blood glucose, postprandial blood glucose, daily blood glucose, body weight, HbA1c, insulin requirements, serum lipid levels | |
| Notes | Source of funding: BSN, General Biscuit France, Pierre and Marie Curie University, Paris Drop‐outs: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Frost 1994.
| Methods | Trial Design: RCT Randomisation procedure: random number tables Allocation concealment: not reported Blinding of outcome assessors: not reported Intention to treat analysis: no | |
| Participants | Country: UK Setting: Community Number: 60 Age: intervention group 54 ± 2 years; comparison group 56 ± 3 years Sex: low‐GI diet group 16 males, 9 females, control group 20 males, 6 females Inclusion criteria: newly diagnosed type 2 diabetes Exclusion criteria: pregnant or lactating women, subjects aged more than 70 years, patients with other endocrine or lipid disorders, had received dietary advice previously, requiring oral hypoglycaemic or insulin therapy, patients where language barrier made instruction difficult Other characteristics: BMI: low‐GI group 30.1± 0.0 kg/m2, control group 29.1 ± 1.3 kg/m2 | |
| Interventions | Trial intervention: 12 weeks low glycaemic index diet GI= 77 ± 1 comparison intervention: 12 weeks standard dietary advice GI= 82 ± 1 Duration: 12 weeks | |
| Outcomes | Main outcome measure: fructosamine Other outcomes: fasting blood glucose, cholesterol, triglycerides, body weight | |
| Notes | Source of funding: British Diabetic Association Drop‐outs: 9, as they failed to complete the study as they did not attend the final appointment (5 in intervention group, 4 in comparison group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Giacco 2000.
| Methods | Trial Design: RCT Randomisation procedure: not stated, randomised with 2 parallel groups Allocation concealment: not reported Blinding: not reported Intention to treat analysis: stated intention to treat, but all participants originally randomised not included (9 excluded) | |
| Participants | Country: Italy Setting: community Number: 63 Age: low‐GI group 29 ± 11 years, high‐GI group 26 ± 8 years Sex: low‐GI group 12 males,17 females, high‐GI group 9 males, 16 females Inclusion criteria: type 1 diabetic patients Exclusion criteria: renal failure, liver disease or symptomatic cardiovascular disease Other characteristics: C‐peptide negative, BMI 23.9 ± 0.6 kg/m2, duration of diabetes 10.3 ± 6.3 years, treated with insulin, HbA1c levels between 7 and 10% | |
| Interventions | Trial intervention: low glycaemic index diet (GI= 70%, 50g/day fibre, comparison intervention: high gycemic index diet (GI=90%, 15 g/day fibre) Duration: 24 weeks | |
| Outcomes | Main outcome measure: % glycated haemoglobin, mean daily plasma glucose, lipids, hypoglycaemic events, body weight, insulin dose | |
| Notes | Source of funding: Italian National Research Council and Bayer Italy Drop‐outs: 9 drop outs, reasons not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gilbertson 2001.
| Methods | Trial Design: RCT Randomisation procedure: not reported, parallel study Allocation concealment: not reported Blinding of outcome assessors: yes Intention to treat analysis: yes, but not at 12 months Power calculation: yes | |
| Participants | Country: Australia Setting: community Number: 104 children, low‐GI group n=55, CHOx group n=49 Age: children low‐GI group 10.7 ± 1.6 years, CHOx group 10.2 ± 1.6 years Sex: low‐GI group 49% male, CHOx group 51% male Inclusion criteria: children with type 1 diabetes | |
| Interventions | Trial intervention: low glycaemic index diet, comparison intervention: measured carbohydrate exchange (CHOx) Duration: 12 months | |
| Outcomes | Main outcome measures: % glycated haemoglobin, insulin dose, weight, height, dietary intake, incidence of hypo‐ and hyper‐glycemia, quality of life | |
| Notes | Source of funding: Diabetes Australia Research Trust Drop‐outs: accounted for | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Jimenez‐Cruz 2003.
| Methods | Trial Design: RCT Randomisation procedure: not reported, randomly allocated crossover design Allocation concealment: not reported Blinding of outcome assessors: not reported Intention to treat analysis: no | |
| Participants | Country: Mexico Setting: community Number: 36 (14 completed study) Age: 53 ± 9 years Sex: of 14 who completed study 6 male, 8 female Inclusion criteria: type 2 diabetes with BMI >25 kg/m2 Other characteristics: mean diabetes duration 8 ± 7 years, BMI 30 ± 6 kg/m2, mean fasting glucose 9.5 mmol/L, mean A1c 8.4% | |
| Interventions | Trial intervention: low glycaemic index diet comparison intervention: high glycaemic index diet Duration: crossover design 2 x 6 week periods with 6 week washout period in between | |
| Outcomes | Main outcome measures: % glycated haemoglobin, weight, fasting serum glucose, BMI, body mass, serum lipids | |
| Notes | Source of funding: Omnilife‐Conacyt Drop‐outs: accounted for, 4 dropped out during the low‐GI diet, 8 during the high‐GI diet and 10 did not complete records | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Komindr 2001.
| Methods | Trial Design: RCT Randomisation procedure: not reported Allocation concealment: not reported Blinding of outcome assessors: not reported Intention to treat analysis: yes | |
| Participants | Country: Thailand Setting: community Number: 10 Age: 32‐60 years Sex: 10 female Inclusion criteria: type 2 diabetes, absence of diabetic complications, co‐operative, ability to consume the different test‐diets, keep food records and be followed up for at least 4 months. Other characteristics: non‐insulin dependent treated with diet alone, or diet and oral hypoglycaemic agents, for 2 to 7 years, fasting plasma glucose levels 140 to 280 mg/dL | |
| Interventions | Trial intervention: low glycaemic index diet comparison intervention: high glycaemic index diet Duration: 4 weeks | |
| Outcomes | Main outcome measures: HbA1c, plasma glucose and insulin, urinary glucose secretion, body weight | |
| Notes | Source of funding: Mahidol University, Sithinan Co Ltd Drop‐outs: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Luscombe 1999.
| Methods | Trial Design: RCT Randomisation procedure: not stated, randomly assigned crossover design Allocation concealment: not reported Blinding: not reported Intention to treat analysis: no | |
| Participants | Country: Australia Setting: community Number: 28 (7 dropouts), 21 analysed Age: 57.4 ± 2.9 years Sex: 14 males, 7 females Body mass: 87 ± 3 kg Inclusion criteria: NIDDM but no history of renal disease, retinopathy or vascular problems Other characteristics: 16 subjects treated with oral hypoglycaemic agents (sulphonylureas and metformin) and 5 by diet alone. Drug dosage was not altered during study. | |
| Interventions | Trial intervention: low‐GI diet (GI=63 GI units using glucose =100) comparison intervention: high‐GI diet (GI=43 GI units) Duration of each intervention: 4 weeks with no washout period between interventions | |
| Outcomes | Main outcome measures: fructosamine, glycated plasma protein (%) Other outcomes: plasma insulin, urinary glucose, urinary C‐peptide, plasma lipids, plasma glucose, body weight | |
| Notes | Source of funding: CSIRO Human Nutrition Drop‐outs: 7 (due to work commitments and illness unrelated to diabetes) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rizkalla 2004.
| Methods | Trial Design: RCT Randomisation procedure: not stated, randomly allocated crossover design Allocation concealment: not reported Blinding: not reported Intention to treat analysis: yes | |
| Participants | Country: France Setting: community Number: 12 Age: 54 ± 2 years Sex: 12 males Body mass: 93 ± 3 kg, Inclusion criteria: type 2 diabetes Other characteristics: BMI 31 ± 1 kg/m2, fasting glycaemia 8.7 ± 0.7 mmol/L, 11 men on antidiabetic agents and 1 on dietary regime alone. Exclusion criteria: abnormal renal, hepatic and thyroid functions | |
| Interventions | Trial intervention: low‐GI diet comparison intervention: high‐GI diet (GI= GI units) Duration of each intervention: 4 weeks with 4 week washout period between interventions | |
| Outcomes | Main outcome measures: % glycated haemoglobin Other outcomes: plasma glucose, plasma insulin, plasma lipids, body weight | |
| Notes | Source of funding: INSERM Drop‐outs: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wolever 1992.
| Methods | Trial Design: RCT Randomisation procedure: not reported, crossover design Allocation concealment: not reported Blinding of outcome assessors: not reported Intention to treat analysis: yes | |
| Participants | Country: Canada Setting: community Number: 6 in crossover study Age: 63 ± 4 years Sex: 3 male, 3 female Inclusion criteria: NIDDM plus obese/overweight Other characteristics: BMI 32.1 ± 2.4kg/m2 | |
| Interventions | Trial intervention: low glycaemic index diet GI= 58, comparison intervention: high glycaemic index diet GI=86 Duration of study: 6 weeks. 4 to 6 week washout period in between diets. | |
| Outcomes | Main outcome measures: fructosamine, body weight, lipids | |
| Notes | Source of funding: Canadian Diabetes Association, Bristol Myers Company, NY Drop‐outs: 2 subjects were sampled one week early for the final analysis for both diets. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
GI = glycaemic index RCT= randomised controlled trial BMI = Body Mass Index NIDDM = non‐insulin dependent diabetes mellitus CHOx = Measured carbohydrate exchange %HbA1c = percentage glycated haemoglobin
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Heilbronn 2002 | Participants were of unknown diabetic status at the start of the intervention (diabetic or improved diabetic), although all had previously been diagnosed as having type 2 diabetes in the last ten years |
| Jarvi 1999 | Duration of intervention less than 4 weeks (24 days) |
| Jenkins 1988 | Duration of intervention less than 4 weeks (2 weeks) |
| Lafrance 1998 | Duration of intervention less than 4 weeks (12 days) |
| Ma 2008 | Co‐intervention of alteration of the medications when diagnosed as necessary according to %HbA1c levels |
| Wolever 1992 b | Duration of intervention less than 4 weeks (2 weeks) |
| Wolever 2008 | Participants commenced the study with already optimised HbA1c levels (6.2 ± 1% HbA1c) and hence this study was excluded, since the effect of diet on HbA1c was our primary outcome |
Contributions of authors
DIANA THOMAS: conceived the idea for the review, designed and co‐ordinated the review, developed the protocol, searched for trials, screened search results, assessed trial quality, extracted and entered data, analysed and interpreted data, developed the review.
ELIZABETH ELLIOTT: screened search results, assessed trials for quality, analysed and interpreted data, provided clinical perspective, developed the review.
Sources of support
Internal sources
The Children's Hospital at Westmead, NSW, Australia.
University of Sydney, Australia.
External sources
Elizabeth Elliott is supported by a National Health and Medical Research Fellowship (457084), Australia.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Brand 1991 {published data only}
- Brand JC, Colagiuri S, Crossman S, Allen A, Roberts DC, Truswell AS. Low‐glycemic index foods improve long‐term glycemic control in NIDDM. Diabetes Care 1991;14(2):95‐101. [DOI] [PubMed] [Google Scholar]
Collier 1988 {published data only}
- Collier GR, Giudici S, Kalmusky J, Wolever TM, Helman G, Wesson V, Ehrlich RM Jenkins DJ. Low glycemic index starchy foods improve glucose control and lower serum cholesterol in diabetic children. Diabetes Nutrition and Metabolism 1988;1:11‐19. [Google Scholar]
Fontvieille 1992 {published data only}
- Fontvieille AM, Rizkalla SW, Penfornis A, Acosta M, Bornet FR, Slama G. The use of low glycaemic index foods improves metabolic control of diabetic patients over five weeks. Diabetic Medicine 1992;9:444‐50. [DOI] [PubMed] [Google Scholar]
Frost 1994 {published data only}
- Frost G, Wilding J, Beecham J. Dietary advice based on the glycaemic index improves dietary profile and metabolic control in type 2 diabetic patients. Diabetic Medicine 1994;11:397‐40. [DOI] [PubMed] [Google Scholar]
Giacco 2000 {published data only}
- Giacco R, Parillo M, Rivellese A, Lasorella G, Giacco A, D'Episcopo L, Riccardi G. Long‐term dietary treatment with increased amounts of fiber‐rich low‐glycemic index natural foods improves blood glucose control and reduces the number of hypoglycemic events in type 1 diabetic patients. Diabetes Care 2000;23:1461‐6. [DOI] [PubMed] [Google Scholar]
Gilbertson 2001 {published data only}
- Gilbertson H, Brand‐Miller J, Thorburn A, Evans S, Chondros P, Werther G. The effect of flexible low glycemic index dietary advice versus measured carbohydrate exchange diets on glycemic control in children with type 1 diabetes. Diabetes Care 2001;24:1137‐43. [DOI] [PubMed] [Google Scholar]
Jimenez‐Cruz 2003 {published data only}
- Jimenez‐Cruz A, Bacardi‐Gascon M, Turnbull WH, Rosales‐Garay P, Severino‐Lugo I. A Flexible, Low‐Glycemic Index Mexican‐Style Diet in Overweight and Obese Subjects With Type 2 Diabetes Improves Metabolic Parameters During a 6‐Week Treatment Period. Diabetes Care 2003;26:1967‐70. [DOI] [PubMed] [Google Scholar]
Komindr 2001 {published data only}
- Komindr S, Ingsriswang S, Lerdvuthisopon N, Boontawee A. Effect of long‐term intake of Asian food with different glycemic indices on diabetic control and protein conservation in type 2 diabetic patients. Journal of the Medical Association of Thailand 2001;84:85‐97. [PubMed] [Google Scholar]
Luscombe 1999 {published data only}
- Luscombe ND, Noakes M, Clifton PM. Diets high and low in glycemic index versus high monounsaturated fat diets: effects on glucose and lipid metabolism in NIDDM. European Journal of Clinical Nutrition 1999;53:473‐8. [DOI] [PubMed] [Google Scholar]
Rizkalla 2004 {published data only}
- Rizkalla SW, Taghrid L, Laromiguiere M, Huet D, Boillot J, Rigoir A, Elgrably F, Slama. Improved Plasma Glucose Control, Whole‐Body Glucose Utilization, and Lipid Profile on a Low‐Glycemic Index Diet in Type 2 Diabetic Men: A randomized controlled trial. Diabetes Care 2004;27:1866‐72. [DOI] [PubMed] [Google Scholar]
Wolever 1992 {published data only}
- Wolever TM, Jenkins DJ, Vuksan V, Jenkins AL, Wong GS, Josse RG. Beneficial effect of low‐glycemic index diet in overweight NIDDM subjects. Diabetes Care 1992;15:562‐4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Heilbronn 2002 {published data only}
- Heilbronn LK, Noakes M, Clifton PM. The Effect of High‐ and Low‐Glycemic Index Energy Restricted Diets on Plasma Lipid and Glucose Profiles in Type 2 Diabetic Subjects with Varying Glycemic Control. Journal of the American College of Nutrition 2002;21:120‐7. [DOI] [PubMed] [Google Scholar]
Jarvi 1999 {published data only}
- Jarvi A, Karlstrom B, Granfeldt Y, Bjorck I, Asp N, Vessby B 1999. Improved glycemic control and lipid profile and normalized fibrinolytic activity on a low‐glycemic index diet in type 2 diabetic patients. Diabetes Care 1999;22:10‐8. [DOI] [PubMed] [Google Scholar]
Jenkins 1988 {published data only}
- Jenkins DJ, Wolever TM, Buckley G, Lam KY, Giudici S, Kalmusky J, Jenkins AL, Patten RL, Bird J, Wong GS. Low‐glycemic‐index starchy foods in the diabetic diet. American Journal of Clinical Nutrition 1988;48:248‐54. [DOI] [PubMed] [Google Scholar]
Lafrance 1998 {published data only}
- Lafrance L, Rabasa‐Lhoret R, Poisson D, Ducros F, Chiasson JL. Effects of different glycaemic index foods and dietary fibre intake on glycaemic control in type 1 diabetic patients on intensive insulin therapy. Diabetic Medicine 1998;15(11):972‐8. [DOI] [PubMed] [Google Scholar]
Ma 2008 {published data only}
- Ma Y, Olendzki BC, Merriam PA, Chiriboga DE, Culver AL, et al. A randomized clinical trial comparing low‐glycemic index versus ADA dietary education among individuals with type 2 diabetes. Nutrition 2007;24:45‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolever 1992 b {published data only}
- Wolever TMS, Jenkins DJA, Vuskan V, Jenkins AL, Buckley GC, Wong GS, Josse RG. Beneficial effect of a low glycaemic index diet in type 2 diabetes. Diabetic Medicine 1992;9:451‐8. [DOI] [PubMed] [Google Scholar]
Wolever 2008 {published data only}
- Wolever TMS, Gibbs AL, Mehling C, Chiasson JL, Connelly PW, Josse RG, et al. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1‐y controlled trial of low‐glycemic‐index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C‐reactive protein. American Journal of Clinical Nutrition 2008;87:114‐25. [DOI] [PubMed] [Google Scholar]
Additional references
ADA 2004
- Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan DM, Peterson CM. Tests of Glycemia in Diabetes. Diabetes Care 2004;27(1 Suppl):S91‐3. [DOI] [PubMed] [Google Scholar]
ADA 2008
- American Diabetes Association. Nutrition recommendations and interventions for diabetes. Diabetes Care 2008;31:S61‐78. [DOI] [PubMed] [Google Scholar]
Brand‐Miller 2003
- Brand‐Miller J, Hayne S, Petocz P, Colagiuri S. Low‐Glycemic Index Diets in the Management of Diabetes. Diabetes Care 2003;26(8):2261‐7. [DOI] [PubMed] [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 37‐46, 1960. [Google Scholar]
Cooper 1994
- Cooper H, Hedges LV. The handbook of research synthesis. NewYork: Russell Safe Foundation, 1994. [Google Scholar]
Crapo 1977
- Crapo PA, Reaven G, Olefsky J Crapo PA, Reaven G, Olefsky J. Postprandial plasma‐glucose and ‐insulin responses to different complex carbohydrates. Diabetes 1977;26:1178‐83. [DOI] [PubMed] [Google Scholar]
Fleiss 1981
- Fleiss. Statistical Methods for Rates and Proportions. 2nd Edition. Wiley, New York, 1981. [Google Scholar]
Franz 2004
- Bantle JP, Beebe C, Brunzell JD, Chiasson JL, Garg A, Franz MJ, et al. Nutrition principles and recommendations in diabetes. Diabetes Care 2004;27(1 Suppl):S36‐46. [DOI] [PubMed] [Google Scholar]
Goldstein 1995
- Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan DM, Peterson CM. Tests of glycemia in diabetes (Technical Review). Diabetes Care 1995;18:896‐909. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. In: The Cochrane Library, Issue 3, 2005. Chichester, UK: John Wiley & Sons, Ltd.
Holman 1999
- Holman RR, Cull CA, Turner RC. A Randomized Double‐Blind Trial of Acarbose in Type 2 Diabetes Shows Improved Glycemic Control Over 3 Years (U.K. Prospective Diabetes Study 44). Diabetes Care 1999;22:960‐4. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Jenkins 1981
- Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. American Journal of Clinical Nutrition 1981;34(3):362‐6. [DOI] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;354(9193):1896‐900. [DOI] [PubMed] [Google Scholar]
Nield 2008
- Nield L, Moore HJ, Hooper L, Cruickshank JK, Vyas A, Whittaker V, Summerbell CD. Dietary advice for treatment of type 2 diabetes mellitus in adults (Review). Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004097.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salmeron 1997
- Salmeron J, Jenkins DJ, Ascerio A, Stampfer MJ, Rimm EB, et al. Dietary fiber, glycemic load and risk of NIDDM in men. Diabetes Care 1997;20:545‐50. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Sheard 2004
- Sheard NF, Clark NG, Brand‐Miller JC, Franz MJ, Pi‐Sunyer FX, Mayer‐Davis E, Kulkarni K, Geil P. Dietary Carbohydrate (Amount and Type) in the Prevention and Management of Diabetes: A statement by the American Diabetes Association. Diabetes Care 2004;27:2266‐71. [DOI] [PubMed] [Google Scholar]
Silink 2002
- Silink M. Childhood diabetes: a global perspective. Hormone Research 2002;57 Suppl 1:1‐5. [DOI] [PubMed] [Google Scholar]
Stratton 2000
- Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. British Medical Journal 2000;321:405‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2000
- Tang JL, Liu JL. Misleading funnel plot for detection of bias in meta‐analysis. Journal of clinical epidemiology 2000;53(5):477‐84. [DOI] [PubMed] [Google Scholar]
UK PDSG 1995
- United Kingdom Prospective Diabetes Study Group. UKPDS 13: relative efficacy of randomly allocated diet, sulphonylurea, insulin or metformin in patients with newly diagnosed non‐insulin dependent diabetes followed for three years. British Medical Journal 1995;310:83‐8. [PMC free article] [PubMed] [Google Scholar]
UKPDS 38 1998
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: (UKPDS 38). BMJ 1998;317:703‐13. [PMC free article] [PubMed] [Google Scholar]
UKPDS33 1998
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837‐53. [PubMed] [Google Scholar]
Winocour 1988
- Winocour P, Bhatnagar D, Kalsi P, Hillier V, Anderson D. Relative clinical usefulness of glycosylated serum albumin and fructosamine during short‐term changes in glycemic control in IDDM. Diabetes Care 1988;12:665‐72. [DOI] [PubMed] [Google Scholar]


