Abstract
Background
Non‐small cell lung cancer (NSCLC) is the most common lung cancer, accounting for approximately 80% to 85% of all cases. For patients with localised NSCLC (stages I to III), it has been speculated that immunotherapy may be helpful for reducing postoperative recurrence rates, or improving the clinical outcomes of current treatment for unresectable tumours. While several new agents have now entered phase III clinical trials, we felt a systematic review was needed to address the question of the effectiveness and safety of immunotherapy in patients with stages I to III NSCLC.
Objectives
To evaluate the effectiveness and safety of immunotherapy (excluding checkpoint inhibitors) in patients with localised NSCLC (stages I to III) who received surgery or radiotherapy with curative intent.
Search methods
We searched the following databases (from inception to 20 January 2017): CENTRAL, MEDLINE, Embase, and CINAHL, and five trial registers. We also manually checked abstracts or reports from relevant conference proceedings and the reference lists of included trials.
Selection criteria
We searched for randomised controlled trials (RCTs) in adults (≥ 18 years) with histologically‐confirmed early‐stage (stages I to III) NSCLC after surgical resection, and those with unresectable locally advanced stage III NSCLC who had received radiotherapy with curative intent. For patients who had received primary surgical treatment, postoperative radiotherapy or chemoradiotherapy was allowed if it was used for both experimental and control groups.
Data collection and analysis
Two review authors independently selected eligible trials, assessed risk of bias, and extracted data. We used survival analysis to pool time‐to‐event data, expressing the intervention effect as a hazard ratio (HR). We calculated risk ratios (RR) for dichotomous data, and mean differences for continuous data, with 95% confidence intervals (CI). Due to clinical heterogeneity (immunotherapeutic agents with different underlying mechanisms), we used random‐effects models for our meta‐analyses.
Main results
We identified nine eligible trials that randomised 4940 participants, who had received surgical resection or curative radiotherapy, to either an immunotherapy group or a control group. Included immunological interventions were active immunotherapy (i.e. Bacillus Calmette‐Guérin (BCG)), adoptive cell transfer (i.e. transfer factor (TF), tumour‐infiltrating lymphocytes (TIL), dendritic cell‐cytokine induced killer (DC‐CIK), and antigen‐specific cancer vaccines (melanoma‐associated antigen 3 (MAGE‐A3) and L‐BLP25). Except for one small trial, which provided insufficient information for risk assessment, we assessed five studies at high risk of bias for at least one of the seven biases studied; we considered the risk of bias in the other three trials to be low. We included data from seven of the nine trials in the meta‐analyses (4695 participants). We pooled data from 3693 participants from the three high quality RCTs to evaluate overall survival (OS) and progression‐free survival (PFS). We found a small, but not statistically significant, improvement in OS (HR 0.94, 95% CI 0.83 to 1.06; P = 0.35), and PFS (HR 0.93, 95% CI 0.81 to 1.07; P = 0.19; high‐quality evidence). The addition of immunotherapy resulted in a small, but not statistically significant, increased risk of having any adverse event (RR 1.15, 95% CI 0.97 to 1.37; P = 0.11, three trials, 3955 evaluated participants, moderate‐quality evidence), or severe adverse events (RR 1.10, 95% CI 0.88 to 1.39; four trials, 4362 evaluated participants; low‐quality evidence).
We analysed data from six studies for one‐, two‐, and three‐year survival rates (4265 participants), and from six studies for five‐year survival rates (4234 participants). We observed no clear between‐group differences (low‐quality evidence for one‐ and two‐year survival rates, and moderate‐quality evidence for three‐ and five‐year survival rate).
No trial reported the overall response rates; only one trial provided health‐related quality of life results.
Authors' conclusions
The current literature does not provide evidence that suggests a survival benefit from adding immunotherapy (excluding checkpoint inhibitors) to conventional curative surgery or radiotherapy, for patients with localised NSCLC (stages I to III). The addition of vaccine‐based immunotherapy might increase the risk of adverse events. Several ongoing trials with immune checkpoints inhibitors (PD‐1/PD‐L1) might bring new insights for role of immunotherapy for patients with stages I to III NSCLC.
Keywords: Adult; Humans; Antigens, Neoplasm; Antigens, Neoplasm/immunology; Cancer Vaccines; Cancer Vaccines/therapeutic use; Carcinoma, Non‐Small‐Cell Lung; Carcinoma, Non‐Small‐Cell Lung/pathology; Carcinoma, Non‐Small‐Cell Lung/radiotherapy; Carcinoma, Non‐Small‐Cell Lung/surgery; Carcinoma, Non‐Small‐Cell Lung/therapy; Combined Modality Therapy; Combined Modality Therapy/methods; Disease‐Free Survival; Immunotherapy; Immunotherapy/adverse effects; Immunotherapy/methods; Immunotherapy, Adoptive; Immunotherapy, Adoptive/methods; Lung Neoplasms; Lung Neoplasms/pathology; Lung Neoplasms/radiotherapy; Lung Neoplasms/surgery; Lung Neoplasms/therapy; Neoplasm Proteins; Neoplasm Proteins/immunology; Neoplasm Staging; Survival Rate
Effect of immunotherapy on the prognosis for stages I to III non‐small cell lung cancer treated with surgery or radiotherapy with curative intent
Review question
Do treatments that help the body’s immune system fight cancer cells (immunotherapy) make patients with non‐small cell lung cancer (NSCLC) who have had surgery or radiotherapy aimed at a cure, live longer?
Background
Many people with NSCLC, who have had surgery or radiotherapy to cure their cancer, eventually die because the cancer comes back, either in the chest, or somewhere else in the body. There have been a number of clinical trials over the years that have looked at whether immunotherapy helps patients live longer. Some seemed to show a benefit, others did not.
Study characteristics
We searched four computerised databases and five trial registers to 20 January 2017. We looked for all trials that randomly allocated participants to one treatment or another (randomised controlled trials (RCTs)), and included adults (aged 18 years or older) with early non‐small cell lung cancer (stages I to III), confirmed by laboratory testing of a sample of the tumour. We found nine RCTs, which included nearly 5000 participants who had received surgery or curative radiotherapy, and were randomly allocated to receive either immunotherapy or no further treatment.
Key results
We found that giving immunotherapy, mainly vaccine‐based (aiming to activate the host immune system to induce human immune response to tumour‐specific antigens), after surgery or radiotherapy did not, on average, make people live longer. We did not find any results that could tell us whether the addition of immunotherapy improved the quality of life, but it seemed that those who were given vaccine‐based immunotherapy may have experienced, on average, more side effects. At the moment, there is no evidence to support or refute giving immunotherapy (mainly vaccine‐based) to people with localized NSCLC (stages I to III). RCTs are in progress that are testing new, more promising immunotherapy drugs (e.g. checkpoint inhibitors).
Quality of the evidence
The evidence we found about overall survival and progression‐free survival was high quality. When we looked for evidence about how many patients lived to one, two, three, or five years, it was only moderate or low quality, because the RCTs were not very well done, and their results did not agree with each other.
Summary of findings
Summary of findings for the main comparison.
Immunotherapy for surgically‐treated patients with NSCLC, with or without radiotherapy with curative intent
| Immunotherapy for surgically‐treated NSCLC patients, with or without radiotherapy | ||||||
| Patient or population: stages I to III non‐small cell lung cancer (NSCLC) treated with surgery or radiotherapy with curative intent Setting: hospital Intervention: Immunotherapy plus surgery (adjuvant chemotherapy or chemoradiotherapy was allowed, provided it was applied for both experimental and control groups) Comparison: surgical treatment with placebo, best supportive care, no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk with surgical treatment only (control group) | Corresponding risk with immunotherapy plus surgery (experimental group) | |||||
| Overall survival Duration of follow‐up: varied between studies (the median follow‐up time ranged from 37.7 months to 70 months) |
The median overall survival time ranged across control groups from 22.3 to 60.2 months | The median overall survival time ranged across experimental groups from 25.6 to 62.0 months | HR 0.94 (0.83 to 1.06) | 3693 (3 RCTs) |
⊕⊕⊕⊕ HIGH | |
| Progression‐free survival | The median progression‐free survival time ranged across control groups from 11.4 to 57.9 months | The median progression‐free survival time ranged across experimental groups from 14.2 to 60.5 months | HR 0.93 (0.81 to 1.07) | 3693 (3 RCTs) | ⊕⊕⊕⊕ HIGH | |
| Overall survival, 1‐year survival rate | Study population | RR 1.01 (0.95 to 1.08) | 4265 (6 RCTs) | ⊕⊕⊝⊝ LOW1 2 |
The presence of heterogeneity could be partly explained by the inclusion of data from studies with high risk of bias. | |
| 824 per 1000 | 832 per 1000 (782 to 873) | |||||
| Overall survival, 3‐year survival rate | Study population | RR 1.00 (0.89 to 1.12) | 4265 (6 RCTs) | ⊕⊕⊕⊝ MODERATE2 |
||
| 394 per 1000 | 399 per 1000 (348 to 453) | |||||
| Overall survival, 5‐year survival rate | Study population | RR 0.98 (0.86 to 1.12) | 4234 (6 RCTs) | ⊕⊕⊕⊝ MODERATE2 | ||
| 126 per 1000 | 124 per 1000 (97 to 157) | |||||
| Adverse event (any) Duration of follow‐up: varied between studies (the median follow‐up time ranged from 37.7 months to 70 months) |
Study population | RR 1.15 (0.97 to 1.37) | 3955 (3 RCTs) | ⊕⊕⊕⊝ MODERATE1 | The presence of heterogeneity could be explained by the different agents applied in different trials. By restricting to MEAG‐A3 trials, we observed statistically significant elevation in general adverse event risk (RR 1.23, 95% CI 1.18 to 1.29; I² = 0%). | |
| 800 per 1,000 | 922 per 1,000 (817 to 969) | |||||
| Adverse event (severe, grade > 2) Duration of follow‐up: varied between studies (the median follow‐up time ranged from 37.7 months to 70 months) |
Study population | RR 1.10 (0.88 to 1.39) | 4362 (4 RCTs) | ⊕⊕⊝⊝ LOW1 2 | The presence of heterogeneity could be explained by the involvement of low quality trials | |
| 240 per 1000 | 266 per 1000 (209 to 334) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; HR: hazard ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgrade one level due to the inconsistency: significant heterogeneity was detected during analysis
2 Downgrade one level due to methodological limitations: inclusion of data from low‐quality trials
Background
Description of the condition
Lung cancer is the most common cancer in men, and the leading cause of cancer‐related death worldwide (GLOBOCAN 2012). In 2012, there were an estimated 1.8 million new lung cancer cases (accounting for 12.9% of all new cancer cases), 58% of which occurred in the less economically‐developed regions of the world (GLOBOCAN 2012). Clinically, there are two main types of lung cancer: non‐small cell lung cancer (NSCLC), the most common type, accounting for approximately 80% to 85% of all lung cancer cases, and small cell lung cancer (SCLC; (Roy 2008)). This classification is important for deciding treatment and predicting prognosis (Roy 2008). Another key issue for clinical management is lung cancer staging ‐ the assessment of the extent of spread of cancer from its original source (Detterbeck 2009). For NSCLC, the best outcomes are achieved with complete surgical resection of a stage IA tumour, with up to a 70% five‐year survival rate (Mountain 1997). However, the corresponding rates for later stages drop sharply to less than 20% for stage IIIA; the worst survival is seen in stage IV patients, with 2% living longer than five years from diagnosis.
Despite the recent advances in the treatment of NSCLC, there has been little improvement in overall survival (National Cancer Institute 2017). The current standard treatment is surgical resection (lobectomy and adequate mediastinal lymph node evaluation), with or without adjuvant chemotherapy, for those with early stage disease (Howington 2013). For patients with unresectable, locally advanced tumours (some stage IIIA and all stage IIIB patients), curative radiotherapy combined with chemotherapy is usually offered (Ramnath 2013). Although there is a chance of being cured, overall outcomes for patients with stages I to III are not very good. Even after curative treatment, many patients develop a local or distant recurrence (Lardinois 2005). We need more effective, and better tolerated therapeutic options, to prevent relapse and improve the rate of cure. Cancer immunotherapy is one possible approach.
Description of the intervention
'Cancer immunotherapy' generally refers to the use of a number of agents that activate or potentiate immune responses and increase anticancer immunity (Mellman 2011). Immunotherapy can be either active or passive. Active immunotherapy is treatment that stimulates the innate immune system to attack cancer cells (Hirschowitz 2006). Antigen‐specific cancer immunotherapeutics (ASCIs) use exogenous antigens, ideally as tumour‐specific as possible, to induce the immune system to produce an effective T‐cell response against cancer cells (Tyagi 2009). A strong adjuvant component to stimulate the immune response and a proper delivery system for promoting antigen presentation are needed for ASCIs to be effective (Mellman 2011). Passive immunotherapy provides immune effector molecules or effector cells made outside of the human body to modulate immunity. These include monoclonal antibodies and adoptive cell transfer of autologous T‐cells genetically engineered to attack tumour cells.
Currently, immunotherapy has been used successfully in patients with malignant melanoma and renal cell carcinoma, because of their high immunogenicity (Drake 2014). Although there are suggestions that lung cancer is a highly immunogenic tumour, initial attempts to administer immunotherapy to patients with NSCLC have failed to show clinical benefit (Dasanu 2012). However, significant improvements may come from newer agents, by identifying more relevant, targeted antigens, and developing better adjuvants and delivery systems (Finn 2008; Forde 2014). Trial reports have recorded significant objective response rates using novel agents that block immune checkpoint molecules (Topalian 2012). Also, with promising results from phase II studies, several new approaches have recently been tested in randomised phase III clinical trials, targeting different stages of NSCLC (Vansteenkiste 2013).
How the intervention might work
The idea of cancer immunotherapy originated with the better understanding of immune surveillance, a process by which the immune system can recognise malignant cells as foreign, and then induce immune responses to eliminate them (Finn 2008). Physiologically, a normal cellular immune response starts with the uptake of tumour antigens by antigen‐presenting cells, such as dendritic cells or macrophages. Antigen‐presenting cells then process the antigens to T‐cells, by presenting them on their surfaces via major histocompatibility complex classes I and II. With assistance from co‐stimulatory signals, different downstream immune effectors (e.g. plasma cells, nature killer cells, and cytotoxic T‐cells) may be activated, consequently causing the apoptosis (death) of tumour cells (Figure 1). However, immune surveillance may be limited by other factors. If there is an immunosuppressive microenvironment, even malignant tumour cells that carry unusual antigens can escape an immune‐mediated attack (Drake 2006). One such resistance mechanism involves a series of immune checkpoint molecules presenting on the cell surface, including programmed death‐ligand 1 (PD‐L1) and other ligands to inhibitory T‐cell receptors, which can substantially suppress T‐cell proliferation and its killing capacity (Keir 2008).
Figure 1.
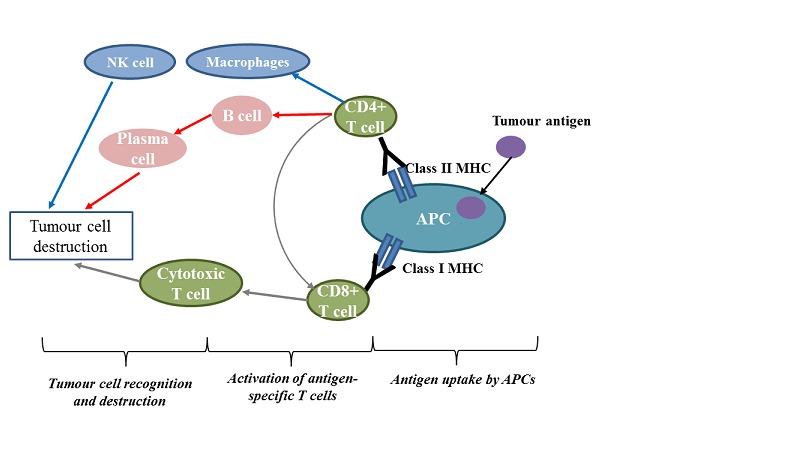
Schematic diagram of the immune components and events involved in cancer immunotherapy
Abbreviations: APCs: antigen‐presenting cells; MHC: major histocompatibility complex; NK cell: natural killer cell
Therapeutic cancer vaccination and monoclonal antibodies that block immune checkpoints are the most widely used immunotherapies for NSCLC treatment, especially for stages I to III. Our main focus for the review was the effect of therapeutic cancer vaccines, which can be summarised by the type of antigens described below.
Cell‐based vaccines
Autologous cell vaccines, generated from lysate or whole cells from the tumours of individual patients, have the advantage of stimulating an immune response to a large variety of tumour‐specific antigens expressed by the patient's cancer cells. In contrast, allogeneic cell vaccines use mixtures of different cancer cell lines. For instance, the belagenpumatucel‐L vaccine (Lucanix®, NovaRx, San Diego, CA) is composed of several lung cancer cell lines (two adenocarcinomas, one squamous cell carcinoma, and one large cell carcinoma), and two adjuvants, which form a major histocompatibility complex and antisense molecule, targeting transforming growth factor ß2 (TGF‐ß2; (Giaccone 2013)). Theoretically, expression of the TGF‐ß2 antisense molecule can undermine the TGF‐ß‐related immunosuppressive effect and potentiate dendritic cell activation, resulting in increased immunogenicity of gene‐modified cancer cells (Nemunaitis 2006).
Compound‐directed vaccines
Peptide vaccines are based on amino acid sequences. However, since they can only target a few epitopes, their major shortcoming is poor immunogenicity (Kochenderfer 2007). This defect can be circumvented by incorporating an efficient delivery system or immunoadjuvants, such as the L‐BLP25 vaccine (known as Stimuvax® or Tecemotide). The L‐BLP25 vaccine consists of a 25‐amino acid sequence from the glycoprotein mucin‐1 (MUC‐1) protein, along with an immunoadjuvant (monophosphoryl lipid A), and a liposomal delivery system (Sangha 2007). MUC‐1 is a highly glycosylated transmembrane protein found on the epithelial cell surface. In cancer cells, MUC‐1 was reported to be frequently overexpressed, with an abnormally glycosylated status (Bafna 2010). Cancer‐associated MUC‐1 can induce abnormal interactions between receptor tyrosine kinases and other cell surface receptors, which in turn lead to inappropriate activation of intracellular signalling pathways. These events then facilitate the growth, proliferation, and survival of cancer cells (Acres 2005; Bafna 2010). In preclinical studies, the L‐BLP25 vaccine induced a cellular immune response, characterised by T‐cell proliferation in response to MUC‐1.
Protein‐based vaccines can elicit an immune response targeting multiple epitopes, but efficient implementation requires that they are combined with immunoadjuvants. Melanoma‐associated antigen 3 (MAGE‐A3) is considered to be a highly exclusive tumour‐specific antigen, since normally it is only expressed in the testes and placenta, where it remains inaccessible to T‐cells because of the lack of major histocompatibility complex molecules to present the antigens (Simpson 2005). Therefore, the MAGE‐A3 vaccine is expected to be a well‐tolerated therapy with minimal side effects. In several types of cancer cells, MAGE‐A3 expression increases with tumour stage (Van den Eynde 1997). MAGE‐A3 is detected in about 35% to 50% of NSCLC tumours (Tyagi 2009).
Viral‐based vaccines
Finally, another way to produce cancer vaccines is to incorporate the target antigen into a viral backbone. One such vaccine, consisting of a modified Ankara virus, known as TG4010, has been developed to target MUC‐1 (Kochenderfer 2007).
Why it is important to do this review
Although immunotherapy for NSCLC showed disappointing results in earlier trials, more promising evidence of its efficacy have emerged in the last decade. For patients with localised NSCLC (stages I to III), immunotherapy has been used to reduce the postoperative recurrence rate or negative clinical outcomes of current chemoradiotherapy for unresectable tumours. While several agents have now entered phase III clinical trials, there is a need for a systematic review to address the question of the effectiveness and safety of immunotherapy in such patients. It is also unclear what the most effective type of immunotherapeutic agents is, and which group of patients could benefit most from this treatment. Therefore, our subgroup analysis might offer supportive evidence to optimise its further development and application in the clinic.
Objectives
To evaluate the effectiveness and safety of immunotherapy (excluding checkpoint inhibitors) in patients with localised NSCLC (stages I to III), who received surgery or radiotherapy with curative intent.
Methods
Criteria for considering studies for this review
Types of studies
We only included randomised controlled trials (RCTs).
Types of participants
We included all adults (18 years or older) with histologically‐confirmed early‐stage NSCLC (stages I to III) after surgical resection (with or without chemotherapy), and those with unresectable locally advanced stage III NSCLC who had received radiotherapy, or radiotherapy combined with chemotherapy with curative intent.
Types of interventions
Surgical treatment + immunotherapy agents versus surgical treatment with placebo, best supportive care, or no intervention.
Radical radiotherapy (with or without chemotherapy) + immunotherapy agents versus radical radiotherapy (with or without chemotherapy) with placebo, best supportive care, or no intervention.
Types of outcome measures
Primary outcomes
Overall survival (OS): defined as the interval between the date of randomisation and the date of death from any cause.
Progression‐free survival (PFS): defined as the time from randomisation to either death or disease progression, whichever occurred first. Disease progression was defined according to RECIST, as at least a 20% increase in the sum of the longest diameter of target lesions, taking as reference the smallest sum of the longest diameter recorded since the treatment starts, or the appearance of one or more new lesions (Therasse 2000).
Secondary outcomes
Overall survival rates: the percentage of participants in a study who were still alive for a certain period of time.
Adverse events or side effects: graded severity with the National Cancer Institute‐Common Terminology Criteria for Adverse Events (NCI‐CTCAE), including the percentage of treatment‐related deaths.
Overall response: response assessed according to RECIST guidelines (Therasse 2000), or immune‐related response criteria (Wolchok 2009).
Health‐related quality of life: measured by a validated scale.
We included all primary outcomes, as well as parts of secondary outcomes in a ’Summary of findings’ table (Table 1).
Search methods for identification of studies
Electronic searches
We conducted a literature search to identify all published or unpublished RCTs. The literature search identified potential studies in all languages.
We searched the following electronic databases for potential studies:
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1) in the Cochrane Library, searched 20 January 2017 (Appendix 1);
MEDLINE PubMed (1966 to 20 January 2017; Appendix 2);
Embase (1988 to 20 January 2017; Appendix 3);
CINAHL (1982 to 20 January 2017).
The search string for MEDLINE was developed according to the Cochrane Highly Sensitive Search Strategy, sensitivity‐maximising version, as referenced in Chapter 6.4.11.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We adapted the terms and the search strategies for CENTRAL, MEDLINE, and Embase to search CINAHL.
Searching other resources
We searched on Clinical Trial Registers: www.clinicaltrials.gov, www.controlled‐trials.com, databases of the Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the International Clinical Trials Registry Platform (ITCRP), to identify information about ongoing studies. We searched all databases from their inception to 20 January 2017.
We checked reference lists of all included studies and related reviews for additional references. We asked experts in the field and manufacturers of relevant drugs to provide details of outstanding clinical trials and any relevant unpublished material. We also contacted authors of identified trials and asked them to identify other published and unpublished studies.
We manually checked for potential trials in abstracts or reports from the following relevant conference proceedings (from 1990 to present): American Society of Clinical Oncology (ASCO), European Society of Medical Oncology (ESMO), European Cancer Conference Organisation (ECCO), and International Association for the Study of Lung Cancer (IASLC) World Lung Cancer Conference.
We searched for errata or retractions from eligible trials on www.ncbi.nlm.nih.gov/pubmed on 20 January, 2017.
Data collection and analysis
Selection of studies
Two review authors (OT, ET) independently screened titles and abstracts of all studies that we identified as a result of the search, and labelled them as 'retrieve' (eligible, potentially eligible, or unclear) or 'do not retrieve'. For the ones coded as 'retrieve', we then referred to their full‐text study reports or publication. Two review authors (HS, RL) independently screened the full‐text reports. The procedure of study identification for inclusion and exclusion was well documented by using standard screening forms. We resolved any disagreement through discussion, or consulted a third person (CS).
We identified and excluded duplicates and collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Liberati 2009), and 'Characteristics of excluded studies' table.
Data extraction and management
We used a data collection form for study characteristics and outcome data, which we tested on one study in the review. One review author (RL) extracted study characteristics from included studies. We extracted the following study characteristics.
Methods: study design (for example, parallel or cross‐over design), number of study centres and location (country), total duration (for example, date of study, follow‐up period, early stopping of trial), method of randomisation (including imbalanced randomisation ratio), methods of allocation concealment, blinding;
Participants: N, age, gender, Eastern Co‐operative Oncology Group (ECOG) performance status, medical history, the severity of condition (stage), diagnostic criteria, inclusion criteria, exclusion criteria;
Interventions: intervention, comparison, concomitant medications, excluded medications;
Outcomes: primary and secondary outcomes specified and collected, time points reported;
Notes: funding for trial, or any notable conflicts of interest of trial authors.
Two review authors (HS, RL) independently extracted outcome data from the included studies. We noted in the 'Characteristics of included studies' table if outcome data were reported in an unusable way. We resolved disagreements by consensus, or by involving a third person (CS). One review author (RL) copied the data from the data collection form into the Review Manager file (RevMan 2014). We double‐checked that the data were entered correctly by comparing the study reports with the data in the systematic review. A second review author (JZ) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (HS, RL) independently assessed the risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were resolved by discussion, or by involving a third assessor (JZ). We assessed the risk of bias according to the following domains.
Random sequence generation;
Allocation concealment;
Blinding of participants and personnel;
Blinding of outcome assessment;
Incomplete outcome data;
Selective outcome reporting;
Other potential bias.
We graded each potential source of bias as high, low, or unclear, and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the 'risk of bias' judgements across different studies for each of the domains listed. For overall risk of bias, we considered studies that had adequate random sequence generation, adequate allocation concealment, adequate blinding, adequate handling of incomplete outcome data, no selective outcome reporting, and were without other bias risks, as being at overall low risk of bias. We considered studies that were assessed as being at high or unclear risk of bias in the majority of domains as being at overall high risk of bias; and the remaining studies to be at moderate risk of bias. We considered blinding separately for different key outcomes where necessary e.g. for unblinded outcome assessment, the risk of bias for all‐cause mortality may be very different than for a participant‐reported pain scale. Where information on the risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Measures of treatment effect
We analysed the primary outcomes based on intention‐to‐treat (ITT) analyses, where available. We measured effect estimates by hazard ratios (HRs) for time‐to‐event variables, and risk ratios (RRs) for dichotomous variables. For continuous variables, we calculated mean differences (MDs) with their corresponding 95% confidence intervals (CIs) if studies used the same measurement, and standardised mean differences (SMDs) and 95% CIs when studies use different scales. We contacted the corresponding authors for missing information about standard deviations or standard errors. For reports without available data for pooling, we tried to measure rates from figures, and calculated effect estimates from P values, t statistics, ANOVA tables, or other statistics as appropriate.
Unit of analysis issues
We did not find any trials with non‐standard design (such as cluster‐randomised trials for potential ’unit‐of‐analysis error’) for this review. But if such eligible trials emerge in future literature searches, we will carefully assess these studies (in terms of recruitment bias, baseline imbalance, loss of clusters, and comparability with individually randomised trials). Furthermore, we will apply proper statistical methods (such as multilevel models and generalised estimating equations) for analysis, according to theHandbook for Systematic Review of Interventions (Higgins 2011). The individual participant was the unit of analysis for this review.
If there had been data from a cross‐over trial with eligible intervention performances, we would have used the data from the first phase only, i.e. from randomisation to the point of cross‐over.
Had multiple trial arms been reported in a single trial, we would only have included the relevant arms. In future, if we enter two comparisons (e.g. drug A versus placebo and drug B versus placebo) from the same trial into the same meta‐analysis, we will halve the control group to avoid double counting.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and obtain missing outcome data where applicable (e.g. when a study was identified as an abstract only). For full‐text reports with missing details relevant to our analysis, we also contacted the authors of the studies by email. In the case of non‐response after repeated attempts, we dropped these incomplete data from the analysis, stating this clearly in the Results section, and discussed it further under the Potential biases in the review process section of the Discussion.
Assessment of heterogeneity
We carried out tests for heterogeneity using the Chi² test, to assesses whether observed differences in results were compatible with chance alone. We used the I² statistic to quantify inconsistency across studies. The presence of heterogeneity was defined by P < 0.05 from the Chi² test, and I² > 50% (Higgins 2011). If moderate or higher heterogeneity (50% to 100%) was detected, we applied a thorough exploration of possible sources of heterogeneity, by means of subgroup and sensitivity analyses (as stated below). Given the limitations of the methods, the P value from the Chi² test and the value of I² were only referred to as a guide, and we exercised caution when interpreting the results.
Assessment of reporting biases
We attempted to contact study authors, asking them to provide missing outcome data. When this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by conducting a sensitivity analysis.
We did not include enough studies for each outcome in the current review to create a funnel plot. But if we are able to pool more than 10 trials in future updates, we will do so to explore possible publication biases (intervention effect estimate versus standard error of intervention effect estimate). If we find funnel plot asymmetry, we will further investigate clinical diversity of studies as a possible explanation. If there are enough studies (> 10), we also will use the 'contour‐enhanced' funnel plot to differentiate asymmetry due to other factors (Peters 2008). If the supposed missing studies are in areas of higher statistical significance, the cause of the asymmetry is highly suggestive of being due to factors other than publication bias.
Data synthesis
We used Review Manager 5 (RevMan 2014) for pooling data and for statistical analysis. We used random‐effects models for primary analyses since the agents of interest had different mechanisms of action. In the future (since currently no subgroup analysis was successfully conducted), provided that the studies in some subgroups are found to be homogeneous (in terms of age, diagnostic subtype, intervention type, intervention duration), we will use both the fixed‐effect and the random‐effects models, and compare the results. In the absence of heterogeneity and significant reporting bias, these two models should yield the same results. In this case, we will report the results from the fixed‐effect model only. If the results are different, indicating significant heterogeneity, we will report the results from the random‐effects model only.
We created a 'Summary of findings' table presenting all our primary and secondary outcomes except overall response and health‐related quality of life, using the GRADEpro software, version 3.2 (GRADEpro GDT 2015). We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence as it related to the studies that contributed data to the meta‐analyses for the pre‐specified outcomes. We justified decisions to downgrade or upgrade the quality of the evidence using footnotes, and made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We had planned to perform the following exploratory subgroup analyses on the primary outcomes, using Review Manager 5 (RevMan 2014).
Participants receiving immunotherapy who present with a different stage of NSCLC (stages I, II, or III);
Participants receiving different types of immunotherapy;
Participants with a specific biomarker: e.g. participants with a gene‐signature profile (MAGE‐A3‐positive).
In the current review, we did not include enough studies for each population (subgroup) of interest to conduct subgroup analyses. In future updates, we will perform a subgroup analysis for primary outcomes when we have at least three studies for a subgroup.
Considering the differences between subgroups, we will first examine them by visual inspection of their CIs; non‐overlapping CIs indicate a statistically significant difference in treatment effect between subgroups. Also, we will use the approach of Borenstein 2008 to formally investigate differences between two or more subgroups.
Sensitivity analysis
We performed sensitivity analyses, defined a priori, to assess the robustness of our conclusions. This was achieved by repeating the analyses to explore the influence of the following factors on effect size.
Exclusion of unpublished studies (in the current review, we did not include unpublished data, but will conduct this analysis if unpublished data are included in future updates);
Exclusion of lower quality studies (those at high or unclear risk of bias).
Results
Description of studies
Results of the search
Figure 2 shows details of the search results. We identified 3612 citations (631 from CENTRAL, 1347 from MEDLINE, and 1634 from Embase), excluding duplicates. In addition, we found five ongoing studies in ClinicalTrials.gov. After screening their abstracts, we excluded 3564 records, leaving 53 records, which we considered to be highly relevant to our review, for further detailed assessment. We were unable to access the full data and relevant study information for the ongoing studies, since they were still recruiting participants (Antonia 2017; Canadian Cancer Trials Group 2014; Roche 2015; Sharp 2015), or had not yet published a report (Merck KGaA 2015). Macchiarini 1989 and Schlieben 1984 were only available as abstracts; repeated attempts to contact the authors were fruitless. In the end, we found 46 full‐text reports, nine of which met the inclusion criteria of our protocol (Butts 2014; Giovanni 1996; Vansteenkiste 2013; Vansteenkiste 2016; Stanley 1986; Macchiarini 1991; Matthay 1986; Fujisawa 1996; Zhao 2014).
Figure 2.
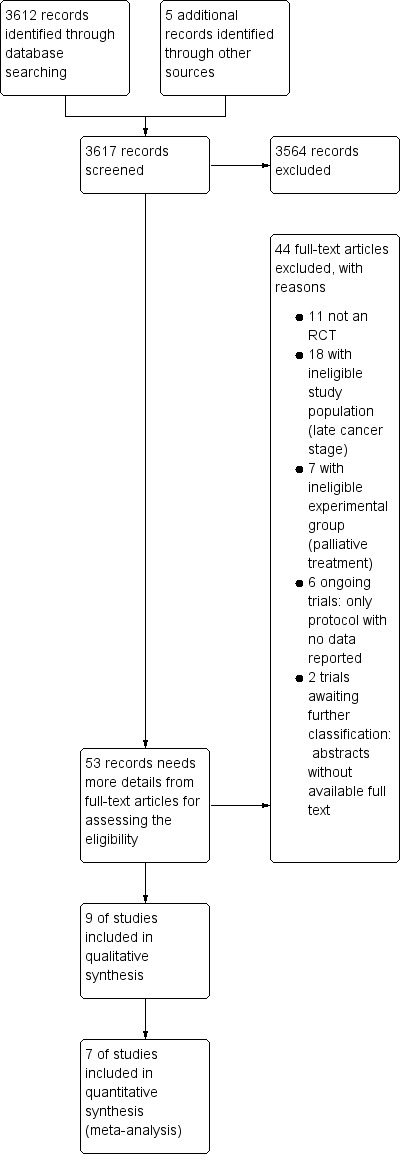
Study flow diagram.
Wu 2011 was an ongoing study with a published protocol that was potentially eligible for our review. However, according to the latest information (16 September 2016 at clinicaltrials.gov), it was terminated prematurely as the sponsor decided to discontinue its program of tecemotide in NSCLC.
We did not find any relevant abstracts or trials from conference proceedings by handsearching.
Included studies
Overall, we included nine reports after carefully evaluating potentially eligible articles, corresponding to nine individual RCTs, with a total of 4940 participants (Butts 2014; Giovanni 1996; Vansteenkiste 2013; Vansteenkiste 2016; Stanley 1986; Macchiarini 1991; Matthay 1986; Fujisawa 1996; Zhao 2014). We summarised the characteristics of the included studies in the 'Characteristics of included studies' tables.
Unfortunately, we did not manage to extract any relevant results from Matthay 1986, a small trial containing 48 Bacillus Calmette‐Guérin (BCG)‐treated and 40 control subjects.This trial reported survival time by tumour stage (stage I, stage II, and stage III), without providing any detailed data on overall survival, or adverse events, which were described in a very general way, where only fever and transient malaise were mentioned, without grading the severity. Also, because of the poor study quality of Zhao 2014, especially the conflicting data reported in the paper (survival rates in abstract, full text, and figures were different from each other), we decided not to include results of this study in our analysis.
Study design
Three studies were double‐blind RCTs (Butts 2014; Vansteenkiste 2013; Vansteenkiste 2016). With the exception of Stanley 1986, where the details of blinding were unclear, the other five had an open‐label design (Macchiarini 1991; Matthay 1986; Fujisawa 1996;Giovanni 1996; Zhao 2014). We included four multicenter international trials (Butts 2014; Vansteenkiste 2013; Vansteenkiste 2016; Stanley 1986), enrolling participants from the USA, Europe, and Asia. Other studies recruited participants from China (Zhao 2014), Italy (Giovanni 1996; Macchiarini 1991), Japan (Fujisawa 1996), or the USA (Matthay 1986).
Participants
All included trials enrolled participants with histologically‐confirmed NSCLC. Two studies enrolled participants with stages I to III NSCLC (Matthay 1986; Vansteenkiste 2016). Three RCTs focused on stage I or II completely resected NSCLC (Fujisawa 1996; Stanley 1986; Vansteenkiste 2013); two studies were conducted on participants with stage II or III NSCLC (Giovanni 1996; Macchiarini 1991); and the remaining two only included participants with locally advanced NSCLC (stage III; (Butts 2014; Zhao 2014)). Vansteenkiste 2013 and Vansteenkiste 2016 were separate phase II and phase III clinical trials on MAGE‐A3 immunotherapy, and they only included participants with MAGE‐A3‐positive NSCLC. As stated above, nine eligible trials enrolled a total of 4940 participants; after we excluded the two trials without usable information on outcomes of interest (Matthay 1986; Zhao 2014), the total number of participants that contributed to the analyses was 4695. The mean age of analysed participants was 61 years, with a range of 19 to 89 years; 74.8% of them were men (Giovanni 1996 did not report the number of men and women).
Interventions
Only one trial included participants with unresectable NSCLC who were receiving chemoradiotherapy, and were randomly assigned to receive immunotherapy (L‐BLP25) or placebo (Butts 2014). All other included RCTs included participants with surgically‐treated NSCLC. Four RCTs compared the immunotherapy group to a control group of either placebo, best supportive care, or no intervention (Fujisawa 1996; Matthay 1986; Stanley 1986; Vansteenkiste 2013); the other four trials allowed adjuvant chemotherapy (Vansteenkiste 2016; Zhao 2014), or chemoradiotherapy (Giovanni 1996; Macchiarini 1991), for both experimental and control groups. It is noteworthy that the immunotherapy agents used in these trials differed over the years. Earlier trials mainly studied active immunotherapy, such as BCG injected into the intrapleural space (Macchiarini 1991; Stanley 1986), or into the tumour (Matthay 1986). The focus of research then gradually moved to passive immunotherapy, with adoptive cell transfer (transfer factor (TF; (Fujisawa 1996)), tumour‐infiltrating lymphocytes (TIL; (Giovanni 1996)), and dendritic cell‐cytokine induced killer (DC‐CIK; (Zhao 2014)). Most recently, antigen‐specific cancer vaccines (MAGE‐A3 (Vansteenkiste 2013; Vansteenkiste 2016)) and L‐BLP25 (Butts 2014) were widely introduced and evaluated.
Outcome measures
All trials reported overall survival time, although measured in different ways. Matthay 1986 reported survival time by tumour stage (stage I, stage II, and stage III); but they only provided survival probability curves for stage I and stage III. Therefore, we could not extract any survival outcome data from this study. Only the three recent trials measured the difference in survival between experimental and control group by time‐to‐event analysis and hazard ratio (HR; (Butts 2014; Vansteenkiste 2013; Vansteenkiste 2016)). Fujisawa 1996 reported 5‐ and 10‐year survival rates, and all other RCTs reported survival by median or mean survival with ranges or standard deviation (SD). To enable the use of these data for meta‐analysis, we extracted data for one‐, two‐, three‐, and five‐year survival rates from all included studies (where possible), from either text statements or survival curves, as secondary outcomes. We extracted progression‐free survival (PFS) data, as HRs, from three RCTs (Butts 2014; Vansteenkiste 2013; Vansteenkiste 2016).
No trial reported overall response. Adverse events were mentioned in six trials, but we only included data from four of them in the analysis of adverse events (Butts 2014; Stanley 1986; Vansteenkiste 2013; Vansteenkiste 2016), since Matthay 1986 and Giovanni 1996 reported adverse events in general, without grading the severity. Quality of life was reported in only one RCT (Vansteenkiste 2016).
Because of the inconsistencies in the duration of follow‐up in the different studies, these point estimates should be interpreted with caution.
Excluded studies
Please see Characteristics of excluded studies.
Risk of bias in included studies
In Figure 3 and Figure 4, we summarised the risk of bias in the included studies. Overall, three recently conducted trials were considered to be well‐designed and well‐conducted, and therefore assessed as having low risk of bias (Butts 2014; Vansteenkiste 2013; Vansteenkiste 2016). However, for all of them, the involvement of the sponsors during study design, analysis, and results interpretation was mentioned in their reports. One trial had unclear risk of bias owing to limited study information for risk assessment reported in their published paper (Stanley 1986). The other five trials were at high risk of bias, mainly because of non‐blinding design (Fujisawa 1996; Giovanni 1996; Macchiarini 1991; Matthay 1986; Zhao 2014).
Figure 3.
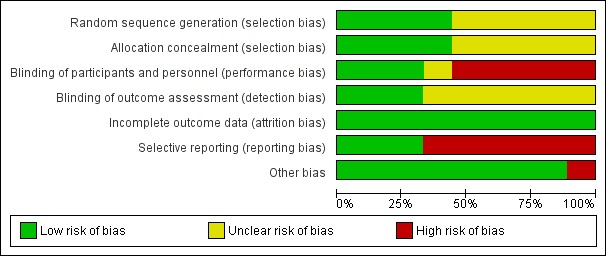
Risk of bias graph: review authors' judgements about each risk of bias domain, presented as percentages across all included studies.
Figure 4.
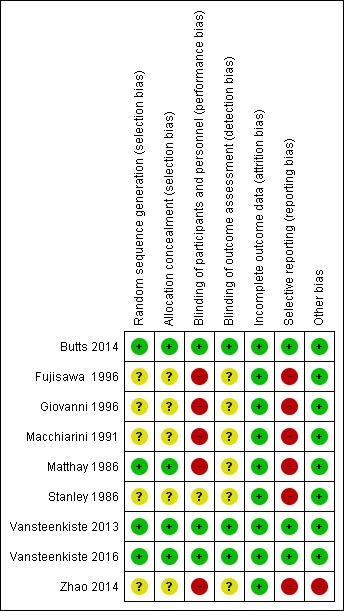
Risk of bias summary: review authors' judgements about each risk of bias domain, for each included study.
Allocation
Four trials described how they carried out the randomisation procedure in detail (Butts 2014; Matthay 1986; Vansteenkiste 2013; Vansteenkiste 2016). The other studies did not provide details on how they randomised the participants into different treatment arms, so we considered them to be at unclear risk of selection bias (Fujisawa 1996; Giovanni 1996; Macchiarini 1991; Stanley 1986; Zhao 2014). For Butts 2014, Vansteenkiste 2013, and Vansteenkiste 2016, randomisation was done centrally, via Internet, minimising the risk of lack of allocation concealment. Matthay 1986 randomised with a printed table of random numbers, which was concealed from investigators with a sealed envelope.
Blinding
We judged the three trials with a double‐blind, placebo‐controlled design to be at low risk of bias for blinding of participants and researchers, as well as blinding of outcome assessors (Butts 2014; Vansteenkiste 2013; Vansteenkiste 2016). Stanley 1986 had a placebo comparator, but did not provide a detailed explanation of blinding of participants, so it was evaluated to be an unclear risk. We considered five studies to be at high risk of performance bias because they had no placebo comparator (open‐label design; (Fujisawa 1996; Giovanni 1996; Macchiarini 1991; Matthay 1986; Zhao 2014)).
None of the other studies provided details on blinding of outcome assessors in their reports (classified as unclear risk). To assess the influence of detection bias, we considered the concept of each outcome: OS, yearly survival rates, and severe adverse events were classified as objective effects of treatment, and so unlikely to be affected by the non‐blinding of outcome assessment. But PFS, response, quality of life, and less severe adverse events are subjective outcomes, and could be affected by the outcome assessors’ knowledge of treatment and the participants’ awareness of the assignment status. We assessed all studies to be at low risk of detection bias for objective outcomes. For subjective outcomes (i.e., where the judgements were made by assessors, not natural/obvious events), since all data included in the meta‐analysis were extracted from double‐blind trials with masking for assessors, the risk of detection bias was low (PFS and any adverse event in Butts 2014, Vansteenkiste 2013, and Vansteenkiste 2016, and quality life in Vansteenkiste 2016). Overall response was not documented in any of the included trials.
Incomplete outcome data
We considered all the included studies to be at low risk of bias, either because the number of participants missing from follow‐up was very low (dropout rates below 5%), or because the primary survival analysis was done on the intention‐to‐treat population of all participants randomly allocated to treatment.
Selective reporting
The protocols of three trials were available, where all their prespecified (primary and secondary) outcomes were described in detail (Butts 2014; Vansteenkiste 2013; Vansteenkiste 2016). Since the pre‐published protocols were consistent with their reports, we judged these three studies to be at low risk of selective outcome reporting. We considered other trials had a high risk for selective reporting bias, because their study protocol was unavailable and they just reported part of the primary outcomes (Fujisawa 1996; Giovanni 1996; Macchiarini 1991; Matthay 1986; Stanley 1986; Zhao 2014). Only Butts 2014, Vansteenkiste 2013, and Vansteenkiste 2016 reported OS, together with PFS. All others reported OS, but in different formats (Fujisawa 1996; Giovanni 1996; Macchiarini 1991; Matthay 1986; Stanley 1986; Zhao 2014).
Other potential sources of bias
Zhao 2014 reported conflicting data in their paper (survival rates in abstract, full text, and figures were different from each other), and so we considered this study had high risk of other potential bias. For the remaining trials, since there was no obvious potential source of bias, we classified them to be at low risk of other potential biases. However, the three high quality trials were funded by pharmaceutical companies, and the sponsors played critical roles in study design, data collection, management and statistical analysis (Butts 2014; Vansteenkiste 2013; Vansteenkiste 2016). The other six RCTs had no connection with these companies and confirmed their independence in the study implementation.
Effects of interventions
See: Table 1
Primary outcomes
Effect of immunotherapy on overall survival for patients with stages I to III NSCLC
To enable the maximum use of eligible data for the meta‐analysis of survival outcomes, we used two approaches to pool data, the first examined overall survival, and the second examined overall survival rates during different time slots. We have provided details of the second analyses under Secondary outcomes.
We extracted hazard ratios (HRs) from three trials (Butts 2014; Vansteenkiste 2013; Vansteenkiste 2016). In total, 4007 participants were involved in these studies, and 3693 of them were evaluated for OS (92% of all randomised participants). Using a random‐effects model, the pooled results illustrated that the study groups did not have a statistically significantly reduced risk of death compared to the control groups (HR 0.94, 95% confidence interval (CI) 0.83 to 1.06; Analysis 1.1; Figure 5); we detected low heterogeneity across the trials (I² = 5%; P = 0.35). Since all three studies were considered to be at low risk of bias, with consistent results, we considered that the quality of the evidence was high (Table 1). However, it is notable that only three out of nine studies contributed data for this meta‐analysis.
Analysis 1.1.
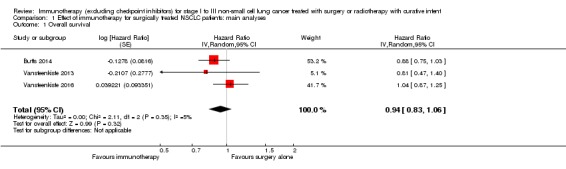
Comparison 1 Effect of immunotherapy for surgically treated NSCLC patients: main analyses, Outcome 1 Overall survival.
Figure 5.
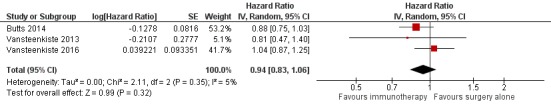
Forest plot of comparison: Effect of immunotherapy for surgically‐treated NSCLC patients: main analyses, outcome: Overall survival.
Effect of immunotherapy on progression‐free survival (PFS) for patients with stages I to III NSCLC
Three trials reported PFS (Butts 2014; Vansteenkiste 2013; Vansteenkiste 2016). Although different agents were used in these studies, we found no statistically significant heterogeneity between the results of these three trials (I² = 39%; P = 0.19); using a random‐effects model, we found that immunotherapy plus surgery showed no statistically significant advantage compared to surgery alone (HR 0.93, 95% CI 0.81 to 1.07; Analysis 1.2; Figure 6). The quality of evidence was high (Table 1).
Analysis 1.2.
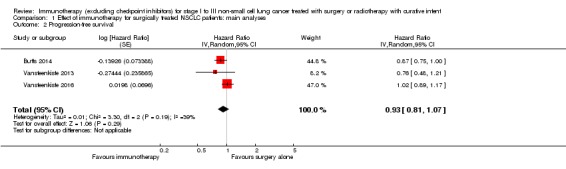
Comparison 1 Effect of immunotherapy for surgically treated NSCLC patients: main analyses, Outcome 2 Progression‐free survival.
Figure 6.
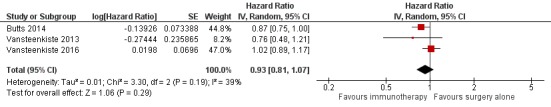
Forest plot of comparison: Effect of immunotherapy for surgically‐treated NSCLC patients: main analyses, outcome: Progression‐free survival.
Secondary outcomes
Overall survival rates
We added this outcome after assessing the data, in order to capture as much information as we could on participant survival. We extracted one‐, two‐, and three‐year survival rates from six trials with 4265 participants (Butts 2014; Giovanni 1996; Macchiarini 1991; Stanley 1986; Vansteenkiste 2013; Vansteenkiste 2016). The results from a random‐effects model showed no clear difference in one‐year survival probabilities for participants treated with immunotherapy compared to participants assigned to control groups (risk ratio (RR) 1.01, 95% CI 0.95 to 1.08; Analysis 1.3). The RRs were similar for two‐year (1.01, 95% CI 0.91 to 1.11; Analysis 1.4) and three‐year survival probability (1.00, 95% CI 0.89 to 1.12; Analysis 1.5). There was moderate heterogeneity for one‐ (I² = 61%; P = 0.02) and two‐year (I² = 53%; P = 0.06) survival rates; while three‐year survival rates showed low between‐trial heterogeneity (I² = 35%; P = 0.18). Five‐year survival rates were available from six trials that included 2731 participants from the experimental and 1503 participants from the control groups (Butts 2014; Fujisawa 1996; Macchiarini 1991; Stanley 1986; Vansteenkiste 2013; Vansteenkiste 2016). Analysis showed there was no clear benefit for five‐year survival by adding immunotherapy (RR 0.98, 95% CI 0.86 to 1.12; Analysis 1.6), and there was no heterogeneity (I² = 0%; P = 0.74). We concluded that the quality of evidence for one‐ and two‐year survival rates was low, primarily due to the inconsistency of effect across the studies and the involvement of trials with unclear or high risks of bias; the quality of evidence for three‐ and five‐year survival rates was moderate, because of the involvement of trials with high or unclear risk of selection bias.
Analysis 1.3.
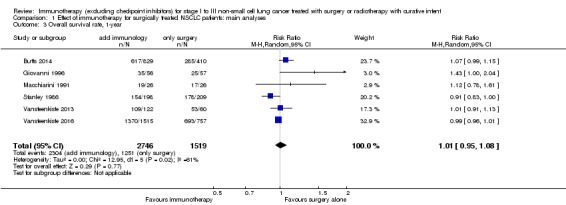
Comparison 1 Effect of immunotherapy for surgically treated NSCLC patients: main analyses, Outcome 3 Overall survival rate, 1‐year.
Analysis 1.4.
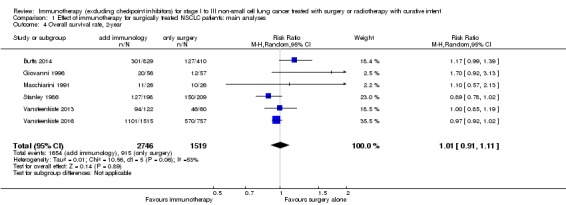
Comparison 1 Effect of immunotherapy for surgically treated NSCLC patients: main analyses, Outcome 4 Overall survival rate, 2‐year.
Analysis 1.5.
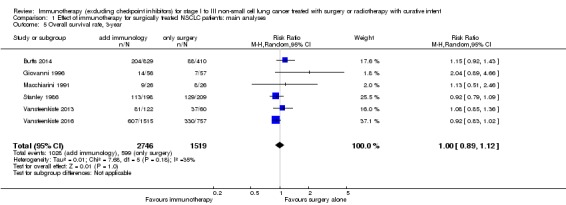
Comparison 1 Effect of immunotherapy for surgically treated NSCLC patients: main analyses, Outcome 5 Overall survival rate, 3‐year.
Analysis 1.6.
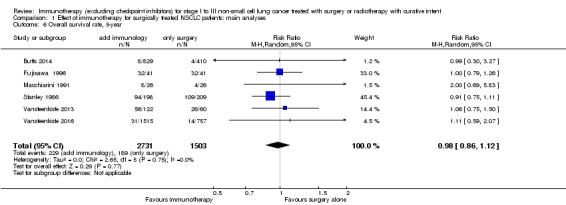
Comparison 1 Effect of immunotherapy for surgically treated NSCLC patients: main analyses, Outcome 6 Overall survival rate, 5‐year.
Because of significant heterogeneity for one‐ and two‐year survival rates, we repeated the analyses, restricting it to trials at low risk of bias. This sensitivity analyses suggested that the heterogeneity for one‐year survival rates could be partly explained by the differences in study quality. For two‐year survival rates, the inconsistency of effect size remained after the exclusion of low‐quality trials.
Effect of immunotherapy on adverse events for patients with stages I to III NSCLC
Three studies, with a total of 3955 evaluated participants, provided data on the proportion of participants with any adverse event, graded for severity (Butts 2014; Vansteenkiste 2013; Vansteenkiste 2016). Using a random‐effects model, our analysis showed that the addition of immunotherapy to surgery or curative radiotherapy might lead to a small and not statistically significant increase in the risk of experiencing any adverse events among these participants (RR 1.15, 95% CI 0.97 to 1.37; P = 0.11; Analysis 1.7). The effect size varied substantially between studies (suggesting a drug‐specific effect on this outcome), which corresponded to a high level of heterogeneity between individual outcomes (I² = 97%; P < 0.00001). We also observed a statistically significant increase in the general adverse event risk (RR 1.23, 95% CI 1.18 to 1.29; I² = 0%; P < 0.00001) by restricting the analysis to MAGE‐A3 trials (Vansteenkiste 2013; Vansteenkiste 2016).
Analysis 1.7.
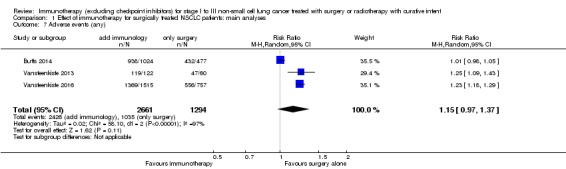
Comparison 1 Effect of immunotherapy for surgically treated NSCLC patients: main analyses, Outcome 7 Adverse events (any).
Four trials, with 2859 evaluated participants in the experimental and 1503 evaluated participants in the control groups, showed that participants receiving immunotherapy did not have a clearly higher risk of severe adverse events (severity grade > 2) than their controls (RR 1.10, 95% CI 0.88 to 1.39; Analysis 1.8; Butts 2014; Stanley 1986; Vansteenkiste 2013; Vansteenkiste 2016). We also found high heterogeneity among the results of these trials (I² = 70%; P = 0.02). Sensitivity analysis that excluded the low quality study implied that this inconsistency might originate from the inclusion of this single high risk trial (RR 0.96, 95% CI 0.86 to1.08; I² = 0%; P = 0.69; Stanley 1986).
Analysis 1.8.
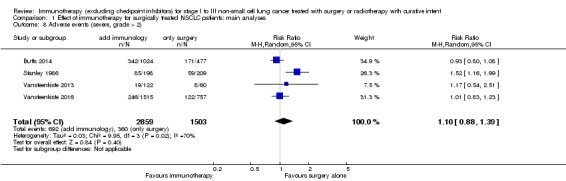
Comparison 1 Effect of immunotherapy for surgically treated NSCLC patients: main analyses, Outcome 8 Adverse events (severe, grade > 2).
In summary, we considered there was moderate‐quality evidence for any adverse event (because of the significant heterogeneity), and low‐quality evidence for severe adverse events (due to the involvement of a low quality study and significant heterogeneity).
Effect of immunotherapy on response rate for patients with stages I to III NSCLC
None of the included studies reported response rate.
Effect of immunotherapy on quality of life for for patients with stages I to III NSCLC
Only Vansteenkiste 2016 assessed the quality of life of their participants (1515 experimental and 757 control), using both European Quality of Life‐5 Dimensions (EQ‐5D) utility scores and visual analogue scores (VAS). They did not find any evidence that the application of melanoma‐associated antigen 3 (MAGE‐A3) improved quality of life. Instead, the immunotherapy group reported significantly lower scores (indicating poorer life quality) on quality‐of‐life assessments on the day after the first and the third MAGE‐A3 administrations, compared to the control group.
Subgroup analysis
As described in our protocol, we had planned to perform subgroup analysis for primary outcomes according to participants at different tumour stages (I, II, or III), type of immunotherapy, and specific prognostic biomarker that were used for participant selection. However, since we only found three trials with data for primary outcome analyses, we were not able to conduct any subgroup analyses at this stage.
Discussion
Summary of main results
This review, which pooled survival data from trials that focused mainly on vaccine‐based immunotherapy, showed that there was no clear evidence of additional survival benefit from immunotherapy (excluding checkpoint inhibitors) for participants with stages I to III NSCLC, who had undergone surgery or received radiotherapy with curative intent. Pooled data from three (3693 participants analysed) of the nine included studies suggested a small, but not statistically significant, decrease in death (overall survival (OS) hazard ratio (HR) 0.94, 95% confidence interval (CI) 0.83 to 1.06) or risk of progression(progression‐free survival (PFS) HR 0.93, 95% CI 0.81 to 1.07) for those receiving immunotherapy, compared to their controls. Similarly, survival rate analyses of data from six studies (4265 participants) showed no improvement of one‐, two‐, three‐, or five‐year survival associated with the addition of immunotherapy agents. Sensitivity analysis showed that the exclusion of low quality data could not explain the observed variation in study results. Due to the small number of trials, we could not perform subgroup analyses to detect the possible differences caused by tumour stage, type of immunotherapy, or the use of a specific prognostic biomarker.
Importantly, we observed a small and not statistically significant increase in the risk of experiencing any adverse event (15% for MAGE‐A3 and L‐BLP25 together (P = 0.11), and 23% specifically for MAGE‐A3 (P < 0.00001)) for participants who were assigned to immunotherapy, compared to the control groups. The risk of having severe adverse events showed no clear increase (RR 1.10, 95% CI 0.88 to 1.39).
The immunotherapy group in one study reported significantly lower scores (indicating poorer life quality) on quality‐of‐life assessments on the day after the first and the third MAGE‐A3 administrations, compared to the control group.
Due to lack of data, we were unable to assess overall response.
Overall completeness and applicability of evidence
In order to reduce the publication bias, our literature search sought unpublished or ongoing trials, without any limitation on publication language. However, the identified studies only partially addressed the objectives of the review, mainly because data about the effect of immunotherapy for participants with stages I to III NSCLC were insufficiently described. Survival outcomes were reported in various ways. Only three recent, high quality studies, (33% of included studies) provided reliable and adequate data for OS and PFS (Butts 2014; Vansteenkiste 2013; Vansteenkiste 2016). Due to the absence of a uniform approach for group comparison, data extraction and analyses from earlier trials was difficult. In addition, because of clinical (different agents used in each trial, and participants with different tumour stages) and statistical heterogeneity, the legitimacy of pooling results may be debatable. The limited number of included trials prevented us from detailed comparisons within more homogeneous subgroups. Also, some important outcomes, such as response rates and health‐related quality of life, could not be examined in this review; response rates were not measured, and quality of life was only measured in one trial.
Before the publication of our review, we found a newly published phase I/II trial (the same as Merck KGaA 2015, which was classified as an ongoing trial at the time of our literature search) on the safety and effectiveness of maintenance tecemotide (i.e. L‐BLP25) versus placebo in Japanese patients with stage III unresectable NSCLC (Katakami 2017). Similar to our meta‐analysis, this trial did not find an increase in OS and PFS resulting from L‐BLP25. We were aware of updated reports for the included START trial, with up to 20 months of additional median follow‐up time compared with the primary analysis (Mitchell 2015; Shepherd 2013). However, since only OS data were updated, with results that were consistent with the primary analysis, we did not change our meta‐analysis accordingly. These trial reports will be fully assessed and included when the review is updated.
At the time of our literature search, results from ongoing trials applying new immunotherapy agents, i.e. checkpoint inhibitors (PD‐1/PD‐L1), were not available. However, the most recently published data for an interim analysis of the ongoing PACIFIC trial showed a significant PFS benefit from durvalumab (Antonia 2017). Therefore, further reviews that focus on the effectiveness of checkpoint inhibitors for patients with stage I to III NSCLC will be needed when results from several trials are available.
Quality of the evidence
We identified nine eligible RCTs. We could not extract useful data (for our outcomes of interest) from Matthay 1986, and we discarded results from Zhao 2014 because of poor study quality and contradictory reports of the primary outcomes, leaving only seven trials for further analysis. Data from studies performed before 2000 generally were of poor quality, because of the open‐label design and the lack of information provided about randomisation methods (particularly allocation concealment). The three newer double‐blinded RCTs were considered to be of high quality (Butts 2014; Vansteenkiste 2013; Vansteenkiste 2016). However, all of their industry sponsors were highly involved in the study management, which might have an uncertain influence on their reported outcomes. Therefore, we carefully compared final reports with their previously published protocols. The consistency between these two documents partly mitigated this concern, by indicating that in spite of the sponsors’ involvement, these studies were conducted as planned, and with full supervision by independent administrators.
It should be noted that the available data for the meta‐analyses of primary outcomes was incomplete. All participants included in the final analysis were from only three trials, and evaluated two different drugs, although we did not downgrade the quality of the evidence because of this. Clinical and statistical heterogeneity lead to a problem of imprecision for most of our outcomes of interest. We found no suggestion of publication bias, and therefore, no serious limitation was assumed. In addition, we could not explain the inconsistency between individual trial results by our predefined subgroups.
Potential biases in the review process
For the primary analyses, we combined all intervention groups’ data, regardless of the type of immunotherapeutic agents administered, and the specific biomarker used for participant selection. Consequently, our analyses were subject to high potential risk of between‐study heterogeneity, due to the clinical diversity. Further, we observed significant statistical heterogeneity in the analysis of one‐ and two‐year survival rates. But, because only a limited number of eligible trials was included in these analyses, we were not able to fully explore the reasons for the variation by subgroup or sensitivity analysis. The unexplained inconsistency between individual trial results may undermine the reliability of our effectiveness assessment.
Another issue relevant to the potential bias of our pooled results was the small proportion of studies (33%) included in the meta‐analyses of OS and PFS, our primary outcomes (i.e., a large proportion of included trials were at high risk of selective reporting bias). Although these three trials contained more than 80% of all participants (4007/4940) from all eligible trials, such analyses were deemed to be incomplete, with over‐representation of the effects of most recent agents (Butts 2014; Vansteenkiste 2013; Vansteenkiste 2016). This naturally limited our analysis to antigen‐specific vaccines (MAGE‐A3 and L‐BLP25). On the other hand, since other trials (with smaller numbers of participants) produced mixed results, we were unable to assess the efficacy of other types of immunotherapeutic agents in the current review.
Two review authors independently carried out study selection, assessment of risks of bias, and data collection, without blinding. Regarding missing data and missing information related to study quality assessment, we tried to contact authors by email to obtain these details. However, even after repeated attempts, we did not receive any response from these authors (since these trials were conducted a long time ago, the non‐response rates were expected to be high). In the end, we were unable to get enough information to assess the eligibility of two studies (Macchiarini 1989; Schlieben 1984). For the others, we deemed that there were unclear risks of certain study bias (see Figure 4), and we extracted and used available data for the meta‐analysis for each outcome. Again, this could influence the accuracy and reliability of our results because of incomplete study assessment and data pooling.
Agreements and disagreements with other studies or reviews
The survival benefit of immunotherapy for patients with localised NSCLC has been discussed for decades. For early (stage I and II) or locally advanced (IIIA) NSCLC, the first attempts to use immunotherapy (mainly cellular‐immunity based therapeutic strategies) on these patients started in the 1980s, using active immunotherapy agents (e.g. BCG) or adoptive cell transfer (e.g. TF). Striking improvement in survival was observed in some clinical trials, including both RCTs (Fujisawa 1996; Macchiarini 1991), and non‐randomised clinical studies; while null results were reported by others (Matthay 1986).These attempts were finally thought to fail since the largest RCT with 441 participants found no significant difference between experimental and control groups (Stanley 1986). New efforts were launched after 2000, mainly applying vaccine‐based immunotherapy agents. Promising outcomes from small studies inspired further explorations (Vansteenkiste 2013). However, disappointing results were reported from later phase III RCTs (Butts 2014; Vansteenkiste 2016). Similar conclusions were drawn from other published reviews (Carrizosa 2015; Reckamp 2015). Nevertheless, new trials in this area have been started, with a focus on novel agents, especially immune checkpoint inhibitors, for which superior PFS has been reported in the planned interim analysis of one phase III RCT trial (Antonia 2017).
Authors' conclusions
So far, there is no evidence showing better survival outcomes associated with the addition of immunotherapy (excluding checkpoint inhibitors) for patients with stages I to III NSCLC. However, the probability of experiencing adverse events may be greater for patients receiving immunotherapy, especially the MAGE‐A3 vaccine. A major concern about the evidence on overall survival (OS) and progression‐free survival (PFS) was the small proportion of included trials (33%) that contributed usable data for group comparisons. For yearly survival rate analyses, for which more data were available, we found no clear differences between the treatment groups for one‐, two‐, three‐, or five‐year survival rates, with significant heterogeneity across the trials for one‐ and two‐ year rates, which could not be fully explained by either variation in study quality or the clinical differences in the trials.
Based on these results, we consider that at present, there is insufficient evidence to support or negate the use of adjuvant immunotherapy (excluding checkpoint inhibitors) for patients with stages I to III NSCLC. Several planned or ongoing trials that aim to treat patients with stages IB to IIIA NSCLC with adjuvant checkpoint inhibitors (PD‐1/PD‐L1) may provide new evidence in favour of immunotherapy.
We found no ongoing clinical trials on patients with stages I to III NSCLC that could provide additional evidence on the effectiveness of vaccine‐based immunotherapy. Several planned or ongoing trials were identified with a focus on adjuvant checkpoint inhibitors (PD‐1/PD‐L1). The interim analysis for one of these trials showed superior PFS for treated participants with stage III NSCLC. Future efforts should be put into the development of novel, effective immunotherapy, and the detection of more useful prognostic biomarker to guide the use of these immunotherapeutic drugs.
Acknowledgements
We thank Corynne Marchal, Managing Editor, Cochrane Lung Cancer Group for providing administrative and logistical support for the conduct of the current review. We thank Ludovic Trinquart, Noelle O’Rourke, and Sophie Paget‐Bailly for their comments on the protocol. We thank François Calais for helping us revise the search strategy and also for running the search for us. We thank Noelle O’Rourke, Fergus Macbeth, Frederic Fiteni, Bertrand Mennecier, Virginie Westeel, and Lars Lidgard for their helpful advice for review revision
Appendices
Appendix 1. CENTRAL search strategy
1. MeSH descriptor: [Lung Neoplasms] explode all trees 2. MeSH descriptor: [Carcinoma, Non‐Small‐Cell Lung] explode all trees 3. lung carcinom* 4. lung neoplasm* 5. lung cancer* 6. nsclc 7. non small cell lung 8. #1 or #2 or #3 or #4 or #5 or #6 or #7 9. MeSH descriptor: [Immunotherapy] explode all trees 10. immunother* 11. MeSH descriptor: [Cancer Vaccines] explode all trees 12. vaccin* 13. immunisation 14. immunization 15. #9 or #10 or #11 or #12 or #13 or #14 16. #8 and #15
Appendix 2. MEDLINE PubMed search strategy
1. Carcinoma, Non‐Small‐Cell Lung[Mesh]
2. nsclc[Title/Abstract]
3. lung cancer*[Title/Abstract]
4. lung carcinoma*[Title/Abstract]
5. lung neoplasm*[Title/Abstract]
6. lung tumor*[Title/Abstract]
7. lung tumour*[Title/Abstract]
8. non‐small cell*[Title/Abstract]
9. nonsmall cell*[Title/Abstract]
10. #3 OR #4 OR #5 OR #6 OR #7
11. #8 OR #9
12. #10 AND #11
13. #1 OR #2 OR #12
14. immunotherapy[MeSH Terms]
15. immunother*[Title/Abstract]
16. Cancer vaccines[MeSH Terms]
17. vaccin*[Title/Abstract]
18. immunisation[Title/Abstract]
19. immunization[Title/Abstract]
20. #14 OR #15 OR #16 OR #17 OR #18 OR #19
21. #13 AND #20
Appendix 3. Embase search strategy
1. 'non small cell lung cancer'/exp
2. 'lung tumor'/exp
3. 'nsclc':ab,ti
4. 'lung carcinom*':ab,ti
5. 'lung cancer*':ab,ti
6. 'lung neoplasm*':ab,ti
7. 'lung tumor*':ab,ti
8. 'lung tumour*':ab,ti
9. 'nonsmall cell*':ab,ti
10. 'non‐small cell*':ab,ti
11. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
12. 'immunotherapy'/exp
13. 'immunother*':ab,ti
14. 'cancer vaccine'/exp
15. 'vaccin*':ab,ti
16. 'immunisation':ab,ti
17. 'immunization':ab,ti
18. #12 OR #13 OR #14 OR #15 OR #16 OR #17
19. 'crossover procedure'/exp OR 'double‐blind procedure'/exp OR 'randomized controlled trial'/exp OR 'single‐blind procedure'/exp OR random* OR factorial* OR crossover* OR cross NEXT/1 over* OR placebo* OR doubl* NEAR/1 blind* OR singl* NEAR/1 blind* OR assign* OR allocat* OR volunteer*
20. #11 AND #18 AND #19
21. #11 AND #18 AND #19 NOT [20‐1‐2017]/sd
Data and analyses
Comparison 1.
Effect of immunotherapy for surgically treated NSCLC patients: main analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 3 | Hazard Ratio (Random, 95% CI) | 0.94 [0.83, 1.06] | |
| 2 Progression‐free survival | 3 | Hazard Ratio (Random, 95% CI) | 0.93 [0.81, 1.07] | |
| 3 Overall survival rate, 1‐year | 6 | 4265 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.95, 1.08] |
| 4 Overall survival rate, 2‐year | 6 | 4265 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.11] |
| 5 Overall survival rate, 3‐year | 6 | 4265 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.89, 1.12] |
| 6 Overall survival rate, 5‐year | 6 | 4234 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.86, 1.12] |
| 7 Adverse events (any) | 3 | 3955 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.97, 1.37] |
| 8 Adverse events (severe, grade > 2) | 4 | 4362 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.88, 1.39] |
Differences between protocol and review
The title of the published protocol was 'Immunotherapy for stage I‐III non‐small cell lung cancer treated with surgery or radiotherapy with curative intent'. However, since the available data for new immunotherapy agent was very limited, and the meta‐analysis performed in the review only pooled data for limited types of immunotherapy (mainly BCG, MAGE‐A3, L‐BLP25), we decided to narrow the title to 'immunotherapy (excluding checkpoint inhibitors)'. In the future, in order to answer a specific question about the effectiveness of specific new immunotherapy agent, such as checkpoint inhibitors, additional reviews should be planned.
Because we were not able to extract adequate data to calculate time‐to‐death hazard ratios for most of the included studies, we added a secondary outcome of ‘Overall survival rates: the percentage of participants in a study who were still alive for a certain period of time’. We compared overall survival in terms of yearly overall survival rates for one, two, three, and five years.
We updated the search strategies.
Four new authors (Rui Li, Raheleh Roudi, Olivia Teghararian, and Eva Tiselius) were involved during the review stage. OT and ET completed the abstract screenings and data extraction, together with RL. RL also helped with full‐text checking, data extraction, assessment of risk of bias, and manuscript drafting. RR helped the data analysis, and manuscript drafting and revision.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | International RCT (264 centres, 33 countries), Phase III | |
| Participants |
Number of participants: 1513 (Feb 22, 2007 to Nov 15, 2011) Number randomised: Experimental group (cyclophosphamide and tecemotide): 1006 Control group (saline and placebo): 507 Number evaluated: Experimental group: 829, age (median and range): 61.0 (19.0 to 89.0) years Control group: 410, age (median and range): 61.5 (24,0 to 83.0) years Diagnosis: patients with unresectable stage III non‐small‐cell lung cancer Inclusion: eligible patients should be: aged 18 years or older with histologically or cytologically unresectable stage III non‐small‐cell lung cancer and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Stage was confirmed and documented by CT, MRI, or PET. All histological subtypes of non‐small‐cell lung cancer were included. |
|
| Interventions |
Experimental group: 3 days before administration of study drug, one dose of intravenous cyclophosphamide (300 mg/m², maximum dose 600 mg) was administered to participants, initial therapy consisted of eight consecutive weekly subcutaneous injections of tecemotide (806 μg lipopeptide). Control group: 3 days before administration of study drug, a corresponding intravenous saline infusion was given to participants, initial therapy consisted of eight consecutive weekly subcutaneous injections of placebo. In the absence of progressive cancer or toxicity, maintenance tecemotide or placebo every 6 weeks was continued until disease progression. |
|
| Outcomes | Duration of follow‐up (median): experimental group: 39.9 months (IQR 21.2 to 48.7), control group: 37.7 months (IQR 19.9 to 49.7) overall response, adverse events, time‐to‐disease progression, time‐to‐disease progression, safety. | |
| Notes | Merck KGaA, the study sponsor, designed the trial in collaboration with the investigators, developed the protocol and statistical analysis plan, provided the study drug, co‐ordinated the management of study sites and the clinical data management, did statistical analyses, and participated in the interpretation of data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | a computerized interactive voice response system was used for randomisation and allocation |
| Allocation concealment (selection bias) | Low risk | a computerized interactive voice response system was used for randomisation and allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "interactive voice randomisation system staff assigned patients and were not involved in the rest of the trial. To maintain blinding, tecemotide and placebo for the primary and maintenance treatment phases were packaged in identical containers" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'With the exception of a designated unblinded statistician on the data monitoring board, interactive voice randomisation system staff, a designated pharmacist, and a study monitor for cyclophosphamide drug accountability records, the randomisation code was masked from the sponsor and other individuals monitoring the trial.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1239 randomised participants were included in analysis (81.9%) |
| Selective reporting (reporting bias) | Low risk | Previous protocol was available |
| Other bias | Low risk | No obvious potential source of bias |
| Methods | Japanese RCT | |
| Participants |
Number of participants: 82 (Between 1986 and 1990) Number randomised: Experimental group (surgery + transfer factor (TF) + nocardia rubra‐cell wall skeleton (N‐CWS)): 41 Control group (surgery only): 41 Number evaluated: Experimental group: 41 Control group: 41 Diagnosis: Patients with non‐small cell lung carcinoma who had undergone a complete resection and mediastinal lymph node dissection. Inclusion: The eligibility criteria was as follows: non‐small cell carcinoma; pathologic Stage T1N0M0 or T2N0M0 disease; compIete resection; age younger than 75 years; Karnofsky performance status of 90% or greater; no other active malignancy; no hepatorenal dysfunction; no severe complications. |
|
| Interventions |
Experimental group: Transfer factor (TF) and nocardia rubra‐cell wall skeleton (N‐CWS) One vial of TF, equivalent to 5 x 10⁸ peripheral lymphocytes, was administered subcutaneously (sc) every 4 weeks, and N‐CWS (200 mg) was administered sc every 2 weeks, beginning 1 month after resections, and continuing for 1 year. Control group: participants received surgery alone without any particular treatment until recurrence. |
|
| Outcomes | Duration of follow‐up (median): experimental group: 99 months, control group: 83 months. Overall survival, disease‐specific survival, recurrence‐free survival |
|
| Notes | Supported in Part by Grants‐in‐Aid for Scientific Research 05453481 from the Ministry of Education and Culture of Japan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | The author reported "Patients were randomised into two groups, TF + N‐CWS or control, by closed envelope", without detailed information about how the envelope was generated and kept. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 82 randomised participants were included in analysis (100%) |
| Selective reporting (reporting bias) | High risk | Prior protocol was unavailable, and no pre‐specified primary outcome was reported. |
| Other bias | Low risk | No obvious potential source of bias |
| Methods | Italy; RCT | |
| Participants |
Number of participants: 113 Number randomised: Experimental group (adoptive immunotherapy ± radiotherapy): 56 Control group (chemoradiotherapy): 57 Number evaluated: Experimental group: 56, age (year ± SD): 61± 7 years Control group: 57, age (year ± SD): 62 ± 8 years Diagnosis: patients with (completely or incompletely) surgically removed stage II, IIIa, or IIIb NSCLC. Inclusion: eligible patients should have: (1) histologically confirmed NSCLC; (2) preoperative assessment suggesting potentially resectable disease; (3) cardiopulmonary function adequate for planned surgery; (4) performance status (PS) from 0 to 1; (5) normal hematologic, renal, and hepatic function; (6) no prior therapy with biologic response modifiers (such as interferon (IF) or Interleukin (IL)); (7) negative serologic testing for HN and hepatitis B virus antibodies; (8) no previous treatment with antineoplastic therapy; and (9) no steroid therapy. |
|
| Interventions |
Experimental group: Stage II participants underwent AI (tumour‐infiltrating lymphocytes and recombinant interleukin‐2); participants at Stage III received AI plus radiotherapy. Six to 8 weeks after surgery, TIL were infused i.v. (Day 0). A number of TIL ranging from 4 to 70 x 10⁹ cells was infused. rIL‐2 was administered subcutaneously (s.c.), starting from the day of TIL infusion and escalating from 2 to 16X10⁶ IU/m²/day, from Day 0 to Day 14. Each dose increment was 2 to 16X10⁶ IU/m²/ two days. Radiotherapy started 60 to 90 days from TIL infusion. Control group: Stage II participants received no treatment; participants at Stage III received chemotherapy plus radiotherapy. Chemotherapy consisted of vinblastine (5 mg/m² of body‐surface area given in an i.v. bolus weekly) and cisplatin (80 mg/m², given i.v. over 30 to 60 minutes on Day 1 and Day 22). The same radiation therapy regimen was used in both treatment arms. The radiation dose was 50Gy/25f over a 5‐week period for completely resected participants. |
|
| Outcomes |
Duration of follow‐up: Range from 6 to 40 months OS (survival), adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised by the method of random number (how the random number was generated was not specified in the report) |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 113 randomised participants were included in analysis (100%) |
| Selective reporting (reporting bias) | High risk | Prior protocol was unavailable, and no pre‐specified primary outcome was reported. |
| Other bias | Low risk | No obvious potential source of bias |
| Methods | Italy; RCT | |
| Participants |
Number of participants: 52 (Between January 1979 and December 1980) Number randomised: Experimental group (adjuvant chemo immunotherapy CAV + BCG ): 26 Control group (adjuvant chemotherapy CAV): 26 Number evaluated: Experimental group: 26, age: 53.8% < Median (57.5 yrs), 46.2% > Median (57.5 yrs) Control group: 26, age: 46.2% < Median (57.5 yrs), 53.8% > Median (57.5 yrs) Diagnosis: Patients stages II and III NSCLC following complete surgical resection Inclusion: eligible patients should: Patients aged < 76 years with no prior history of malignancy except for non‐melanoma skin cancer, or in situ cervical cancer, or both; no previous treatment by chemotherapy, immunotherapy, or thoracic radiation; Karnofsky performance status > 69; histologically confirmed NSCLC classified postsurgically as stage II or III disease; adequate renal, liver, bone marrow, and cardiopulmonary functions. |
|
| Interventions |
Experimental group: Chemotherapy including cyclophosphamide (1 g/m² iv, day 1), doxorubicin (45 mg/m² iv, day 1), and vincristine (1.2 mg/m² iv, day 1) was started within 4 weeks after operation and repeated every 3 weeks. Intrapleural BCG consisted of 5.5 per 10⁸ colony‐forming units of BCG Pasteur administered into the pleural space as a single dose between postoperative days 4 and 14. 14 days after BCG administration, Isionazide (INH), 300 mg/day, was administered orally for 12 weeks. Control group: Chemotherapy including cyclophosphamide (1 g/m² iv, day 1), doxorubicin (45 mg/m² iv, day 1), and vincristine (1.2 mg/m² iv, day 1) was started within 4 weeks after operation and repeated every 3 weeks. |
|
| Outcomes |
Duration of follow‐up (median): 111 months OS (survival time), disease‐free survival |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 62 randomised participants were included in analysis (100%) |
| Selective reporting (reporting bias) | High risk | previous protocol was unavailable, and no pre‐specified primary outcome was reported. |
| Other bias | Low risk | No obvious potential source of bias |
| Methods | An USA prospective randomised trial | |
| Participants | Number of participants: 88 Number randomised: Experimental group (BCG‐treated + surgery): 48 Control group (Control subjects + surgery): 40 Number evaluated: Experimental group: 48, age (Mean ± SD): 61 ± 9 years Control group: 37, age (Mean ± SD): 61 ± 10 years Diagnosis: Patients with potentially resectable non‐small cell carcinoma of the lung Inclusion: Patients suspected of having lung cancer were excluded if a definite diagnosis had not been established before thoracotomy. | |
| Interventions |
Experimental group : Injecting BCG 10⁷ viable organisms for PPD skin test‐negative participants and 5x10⁶ for PPD‐positive participants into the tumour mass. Then underwent thoracotomy 2 to 3 weeks after intratumoural injection of BCG. Control group: All control participants underwent thoracotomy immediately following randomisation. A 12‐week course of 300 mg INH daily was given to all participants. |
|
| Outcomes |
Duration of follow‐up (median ± SD): experimental group: 986 ± 412 days; control group: 1062 ± 390 days OS, Median time to recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomisation method."Patients were divided into 2 groups according to a table of random numbers and sealed‐envelope technique in strict numerical order of entry" |
| Allocation concealment (selection bias) | Low risk | "Patients were divided into 2 groups according to a table of random numbers and sealed‐envelope technique in strict numerical order of entry" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 85 randomised participants were included in analysis (96.6%) |
| Selective reporting (reporting bias) | High risk | No prior protocol was available, and no pre‐specified primary outcome was reported. |
| Other bias | Low risk | No obvious potential source of bias |
| Methods | International randomised multicenter trial | |
| Participants |
Number of participants: 441 (February 1979 to October 1981) Number randomised: Experimental group (BCG + Isoniazid): Not specified in the report Control group (Saline): Not specified in the report. Number evaluated: Experimental group: 198, age: < 70 years Control group: 209, age: < 70 years Diagnosis: Patients with a completely resected stage I and stage II non‐small cell bronchogenic carcinoma. Inclusions: eligible patients should: This trial included only those patients with a resected non‐small cell carcinoma pT1N0M0, pT2N0M0, pT1N1M0, or pT2N1M0 (Stages I and 11). Patients < 70 years of age, and those without history of previous malignancy, radiotherapy, chemotherapy, or immunosuppressive treatment. |
|
| Interventions |
Experimental group : A single dose of 1 X 10⁷ units of BCG Tice were given into the pleural space between day 6 and day 12 postoperatively. Isoniazid (INH) 300 mg/day by mouth, starting on the 14th post‐injection day and continuing for 12 weeks. Control group: 35 cc. of saline injected through the pleural catheter and no INH was given. |
|
| Outcomes |
Duration of follow‐up (median): 4.7 years Disease‐free interval, overall survival, adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Had placebo comparator without detailed explanation of blinding of participants or researchers |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 407 randomised participants were included in analysis (90.8%) |
| Selective reporting (reporting bias) | High risk | No protocol was available, and no pre‐specified primary outcome was reported. |
| Other bias | Low risk | No obvious potential source of bias |
| Methods | International RCT (41 investigation sites in 12 European countries), Phase II | |
| Participants |
Number of participants: 182 (September 2005 to June 2010) Number randomised: Experimental group (MAGE‐A3 protein):122 Control group (Placebo): 60 Number evaluated: Experimental group: 122, age (mean and range): 63 (46 to 78) years Control group: 60, age (mean and range): 62.5 (45 to 81) years Diagnosis: Patients with completely resected MAGE‐A3–positive TNM Stage IB or II NSCLC Inclusion: eligible patients should: Be at least 18 years of age at the time of resection. Be completely resected and pathologically confirmed MAGE‐A3–positive stage IB or II NSCLC. Resection had to be anatomic and had to include at least a lobectomy. Patients were to have recovered (performance status 0 to 1) from surgery that had taken place no more than 8 weeks earlier and to have adequate bone marrow reserve and hepatic and renal functions. |
|
| Interventions |
Experimental group: MAGE‐A3 protein + GlaxoSmithKline’s proprietary immunostimulant AS02B was administered intramuscularly in 0.5 mL doses. First administration was 4 to 8 weeks after resection, five doses at 3‐week intervals, followed by eight doses at 3‐month intervals. Control group: Placebo was sucrose in the tocopherol oil‐in‐water emulsion‐based adjuvant AS03A, was administered intramuscularly in 0.5 mL doses. First administration was 4 to 8 weeks after resection, five doses at 3‐week intervals followed by eight doses at 3‐month intervals. |
|
| Outcomes | Duration of follow‐up (median): participants were followed for 7.3 years, with a median of 70 months after resection. Disease‐free interval (DFI), OS, disease‐free survival (DFS), safety | |
| Notes | Sponsor: Vincent G. Brichard The sponsor was involved in the study design, data collection, and analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A supply randomisation list was generated at GSK Biologicals, Rixensart, using a standard SAS program and used to identify uniquely each individual vaccine dose (= treatment box). A randomisation blocking scheme (2:1 ratio) was used to ensure that balance between treatments was maintained. |
| Allocation concealment (selection bias) | Low risk | The person in charge of the vaccination accessed the randomisation system via the Internet. The randomisation system determined the treatment randomised and provided the treatment box number to be used for the first vaccination of this patient. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "scans were evaluated by the investigator or a radiologist blinded to the treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 182 participants included in the analysis (100%) |
| Selective reporting (reporting bias) | Low risk | Prior protocol was available. |
| Other bias | Low risk | No obvious potential source of bias |
| Methods | International multicenter RCT (590 centres in 34 countries), Phase III | |
| Participants |
Number of participants: 2312 (Oct 18, 2007, and July 17, 2012) Number randomised: Experimental group (recMAGE‐A3 with AS15 immunostimulant): 1541 Control group (placebo): 771 Number evaluated: Experimental group: 1515, age (median and range): 63 (57 to 70) years Control group: 757, age (median and range): 63 (57 to 70) years Diagnosis: patients with completely resected stage IB, II, and IIIA MAGE‐A3‐positive NSCLC who did or did not receive adjuvant chemotherapy. Inclusion: eligible patients should be: > 18 years; Male or female patient with completely resected, pathologically proven stage IB, II or IIIA NSCLC; tumour shows expression of MAGE‐A3 gene; the surgical technique for resection of the patient's tumour is anatomical, involving at least a lobectomy or a sleeve lobectomy; ECOG performance status of 0, 1 or 2 at the time of randomisation; adequate bone‐marrow reserve, adequate renal function, and adequate hepatic function as assessed by standard laboratory criteria, and defined as: absolute neutrophil count ≥ 1.0 x 10E9/L Platelet count ≥ 75 x 10E9/L, serum creatinine ≤ 1.5 times, the Upper Limit of Normal (ULN) ≤ 3.0 times, the ULN if due to platinum adjuvant chemotherapy, total bilirubin ≤ 1.5 times the ULN Alanine transaminase (ALAT) ≤ 2.5 times the ULN. |
|
| Interventions |
Experimental group : participants received GSK1572932A Antigen‐Specific up to 13 intramuscular injections during 27 months. Five doses were given every 3 weeks, followed by eight doses every 12 weeks. Control group : participants received placebo up to 13 intramuscular injections during 27 months. Five doses were given every 3 weeks, followed by eight doses every 12 weeks. Treatment started at the time of randomisation. |
|
| Outcomes |
Duration of follow‐up (median): Experimental group: 38.1 months (IQR 27.9 to 48.4), control group:39.5 months (IQR 27.9 to 50·4) disease‐free survival, overall survival, quality of life, adverse event. |
|
| Notes | This study was designed, funded, and interpreted by GlaxoSmithKline in cooperation with an international steering committee. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation at the investigator site was done centrally via Internet with stratification for chemotherapy versus no chemotherapy. Within each chemotherapy stratum, a minimisation algorithm was used to allocate study group, accounting for the number of chemotherapy cycles received (1 to 2 or 3 to 4), disease stage (IB, II, or IIIA), lymph node sampling procedure (mediastinal lymph node dissection or sampling), PS score (0 to 1 or 2), and lifetime smoking status (never, past, or current)" |
| Allocation concealment (selection bias) | Low risk | The randomisation was done centrally, so that the researchers would have no way of knowing in advance the allocation of the next participant. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Individual treatment assignment was masked at all levels, masking was managed by the central randomisation system with access limited to a single sponsor database administrator who was independent from the trail |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Individual treatment assignment was masked at all levels, masking was managed by the central randomisation system with access limited to a single sponsor database administrator who was independent from the trail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in analysis (100%) |
| Selective reporting (reporting bias) | Low risk | Prior protocol was available |
| Other bias | Low risk | No obvious potential source of bias |
| Methods | Chinese RCT | |
| Participants |
Number of participants: 157 (June 2010 to June 2013) Number randomised: Experimental group (GP regimen + DC‑CIK cell immunotherapy): 79 Control group (GP regimen therapy): 78 Number evaluated: Experimental group: 79, average age: 59.6 ± 10.7 years Control group: 78, average age: 58.2 ± 11.2 years Diagnosis: stage Ⅲ NSCLC that had received complete surgery resection Inclusion: eligible patients: i) Patients were pathologically diagnosed with adenocarcinoma, squamous cell carcinoma or adenosquamous‑mixed NSCLC; ii) patients were at stage ⅢA according to the International Union Against Cancer NSCLC criteria; iii) patients were aged 30 to 78 years; iv) patients exhibited heart symptoms; v) Karnofsky performance status score of the patients was > 60 points; and vi) patients provided a signed informed consent sheet. |
|
| Interventions | Experimental group: GP chemotherapy and DC‑CIK cell immunotherapy Control group: Gemcitabine (1,000 mg/m²) intravenously infused at Day 1 and Day 8 + 30 mg/m² cisplatin intravenously injected at Days 1 to 3. The treatment cycle was 21 days and each participant underwent four cycles of treatment | |
| Outcomes |
Duration of follow‐up:The two groups both were followed up for 36 months postoperative cellular immune function, disease‑free survival time, cumulative recurrence rate and cumulative survival rate were analysed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 157 randomised participants were included in analysis (100%) |
| Selective reporting (reporting bias) | High risk | Prior protocol was unavailable, and no pre‐specified primary outcome was reported. |
| Other bias | High risk | Suvival rates in abstract, full text, and figures were different from each other |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Belani 2013 | Included advanced cancer stage patients (treatments were not implemented for curative intent) |
| García 2008 | Included advanced cancer stage patients (treatments were not implemented for curative intent) |
| Kimura 1997 | Included advanced cancer stage patients (treatments were not implemented for curative intent) |
| Kimura 2015 | Included advanced cancer stage patients (treatments were not implemented for curative intent) |
| Kotsakis 2014 | Included advanced cancer stage patients (treatments were not implemented for curative intent) |
| Lissoni 1994 | Included advanced cancer stage patients (treatments were not implemented for curative intent) |
| Nemunaitis 2004 | Included advanced cancer stage patients (treatments were implemented not for curative intent) |
| Neninger 2009 | Included advanced cancer stage patients (treatments were not implemented for curative intent) |
| Pujol 2015 | The definition of experiment group did not meet criterion |
| Ramalingam 2014 | The definition of experiment group did not meet criteria |
| Ruckdeschel 1981 | Included advanced cancer stage patients (treatments were not implemented for curative intent) |
| Skachkova 2013 | An experimental study focusing on immunologic biomarker after immunotherapy, not a clinical trial. |
| Woodruff 1983 | Included advanced cancer stage patients (treatments were not implemented for curative intent) |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT |
| Participants | BCG‐treated patients (N = 17), control patients (N = 17) |
| Interventions | Experiment group: bacillus Calmette‐Guérin (BCG) + surgery Control group: surgery |
| Outcomes | 5‐year and median survival |
| Notes | Full text is unobtainable and full details of trial results were unavailable from the authors |
| Methods | RCT |
| Participants | Not mentioned in the abstract |
| Interventions | Not mentioned in the abstract |
| Outcomes | Not mentioned in the abstract |
| Notes | Full text is unobtainable and full details of trial results were unavailable from the authors |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A global study to assess the effects of MEDI4736 following concurrent chemoradiation in patients with stage III unresectable non‐small cell lung cancer (PACIFIC) |
| Methods | Interventional RCT, Phrase III |
| Participants |
Number of participants: 713 (May 2014 to Februray 2025 ) Number randomised: Experimental group (MEDI47361 immunotherapy): 476 Control group (Placebo): 237 Number evaluated: Experimental group: 473 Control group: 236 Diagnosis: patients with locally advanced unresectable stage III NSCLC Inclusion: The eligibility criteria are as follows:
|
| Interventions |
Experimental group: MEDI4736 by intravenous infusion. Treatment from Day 1 for a maximum of 12 months or study drug withdrawal if this occurs earlier . Control group: PLACEBO by intravenous infusion. Treatment from Day 1 for a maximum of 12 months or study drug withdrawal if this occurs earlier. |
| Outcomes | Disease Free Survival, overall survival, adverse events |
| Starting date | May 7 2014 |
| Contact information | scott.antonia@moffitt.org |
| Notes | This study is ongoing, but not recruiting participants |
| Trial name or title | A phase III prospective double blind placebo controlled randomized study of adjuvant MEDI4736 in completely resected non‐small cell lung cancer |
| Methods | Interventional RCT, Phrase III |
| Participants |
Number of participants: 1100 (October 2014 to January 2025 ) Number randomised: Experimental group (MEDI47361 immunotherapy): not specified Control group (Placebo): not specified Number evaluated: Experimental group: not specified Control group: not specified Diagnosis: patients with locally advanced unresectable stage III NSCLC Inclusion: The eligibility criteria are as follows: ≥ 18 years of age; Histologically confirmed diagnosis of primary non‐small cell carcinoma of the lung. Patients must be classified post‐operatively as Stage IB (≥ 4 cm in the longest diameter), II or IIIA on the basis of pathologic criteria. Complete surgical resection of the primary NSCLC is also mandatory. All gross disease must have been removed at the end of surgery. All surgical margins of resection must be negative for tumour. Resection may be accomplished by open or VATS techniques |
| Interventions |
Experimental group: MEDI4736 by intravenous infusion. Treatment from Day 1 for a maximum of 12 months or study drug withdrawal if this occurs earlier. Control group: PLACEBO by intravenous infusion. Treatment from Day 1 for a maximum of 12 months or study drug withdrawal if this occurs earlier. |
| Outcomes | This trial is currently recruiting participants. Disease Free Survival, overall survival, adverse events |
| Starting date | October 22, 2014 |
| Contact information | Correspondence for published protocol: cocallaghan@ctg.queensu.ca |
| Notes | Patient recruiting stage |
| Trial name or title | Study of tecemotide (l‐blp25) in participants with stage III unresectable non‐small cell lung cancer (NSCLC) following primary chemoradiotherapy |
| Methods | Japanese RCT, Phrase I/II |
| Participants |
Number of participants: 172 (December 2008 to June 2015) Number randomised: Experimental group (Tecemotide (L‐BLP25) + cyclophosphamide):114 Control group (placebo + saline control): 58 Number evaluated: Experimental group: not specified Control group: not specified Diagnosis: patients with locally advanced unresectable stage III NSCLC Inclusion: The eligibility criteria are as follows: ≥ 20 years of age; receipt of concomitant or sequential chemoradiotherapy, consisting of a minimum of two cycles of platinum‐based chemotherapy and a minimum radiation dose of greater than or equal to (≥) 50 Gray (Gy); histologically or cytologically documented unresectable stage III NSCLC. Cancer stage must be confirmed and documented by Computed Tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET) scan; documented stable disease or objective response, according to RECIST, after primary chemoradiotherapy for unresectable stage III disease, within four weeks prior to randomisation; receipt of concomitant or sequential chemoradiotherapy, consisting of a minimum of two cycles of platinum‐based chemotherapy and a minimum radiation dose of ≥ 50 Gy; Eastern cooperative Oncology Group (ECOG) performance status of 0 or 1; adequate bone marrow function |
| Interventions |
Experimental group: Tecemotide (L‐BLP25) + Cyclophosphamide Control group: Placebo + Saline |
| Outcomes | Overall survival |
| Starting date | December 2008 |
| Contact information | |
| Notes | This study has been completed, but no publication was found at the time of literature searching |
| Trial name or title | A phase III, open‐label, randomized study to investigate the efficacy and safety of MPDL3280a (anti‐PD‐L1 antibody) compared with best supportive care following adjuvant cisplatin‐based chemotherapy in PD‐L1‐selected patients with completely resected stage IB‐IIIA non‐small cell lung cancer |
| Methods | Interventional RCT, Phrase III |
| Participants |
Number of participants: 1127 (October 2015 to February 2026) Number randomised: Experimental group (Atezolizumab): not specified Control group (Placebo): not specified Number evaluated: Experimental group: not specifid Control group: not specified Diagnosis: patients were stage IB to IIIA NSCLC Inclusion: The eligibility criteria are as follows: ≥ 18 years of age, ECOG performance status of 0 or 1, histological or cytological diagnosis of stage IB (tumours ≥ 4 cm) to IIIA (T2‐3 N0, T1‐3 N1, T1‐3 N2) NSCLC, Patients must have had complete resection of NSCLC 6‐12 weeks (≥ 42 days and ≤ 84 days) prior to enrolment and must be adequately recovered from surgery. Complete mediastinal lymph node dissection (MLND) is required. If mediastinoscopy was not performed preoperatively, mediastinal lymph node systematic sampling will have occurred. Systematic sampling is defined as removal of at least one representative lymph node at specified levels. MLND entails resection of all lymph nodes at those same levels. For a right thoracotomy, sampling or MLND is required at levels 4 and 7 and for a left thoracotomy, levels 5, 6 (or both) and 7. If there is clear documentation in the operative report or in a separately submitted addendum by the surgeon of exploration of the required lymph node areas, the patient will be considered eligible if no lymph nodes are found in those areas. If patients have documented N2 disease in one level, not all levels need to be sampled. Eligibility to receive a cisplatin‐based chemotherapy regimen. Adequate hematologic and end‐organ function, defined by the following laboratory results obtained within 14 days prior to enrolment. Women who are not postmenopausal (≥ 12 months of non‐therapy‐induced amenorrhoea) or surgically sterile must have a negative serum pregnancy test result within 14 days prior to initiation of cisplatin‐based chemotherapy. |
| Interventions |
Experimental group: Atezolizumab (MPDL3280A) 1200 mg will be administered intravenously (IV) every 3 weeks for 16 cycles Control group: Best supportive care (BSC) |
| Outcomes | Disease‐free survival, overall survival, adverse events, immunogenicity, pharmacokinetics. |
| Starting date | October 2015 |
| Contact information | Correspondence for published protocol: global.rochegenentechtrials@roche.com |
| Notes | Patient recruiting stage |
| Trial name or title | A randomized, phase 3 trial with anti‐PD‐1 monoclonal antibody pembrolizumab (MK‐3475) versus placebo for patients with early stage NSCLC after resection and completion of standard adjuvant therapy (PEARLS) |
| Methods | Interventional RCT, Phrase III |
| Participants |
Number of participants: 1380 (November 2015 to August 2021) Number randomised: 1380 Experimental group (pembrolizumab immunotherapy): not specified Control group (Placebo): not specified Number evaluated: Experimental group: not specified Control group: not specified Diagnosis: participants with Stage IB or II to IIIA non‐small cell lung cancer (NSCLC) who have undergone surgical resection (lobectomy or pneumonectomy) with or without adjuvant chemotherapy will be treated with pembrolizumab or placebo. Inclusion: Pathological diagnosis of NSCLC confirmed at surgery, any histology. UICC v7 stage IB with T ≥ 4 cm, II to IIIA NSCLC at complete surgical resection with no residual disease (R0) after complete surgical resection. Tumor sample obtained at surgical resection for PD‐L1 Immunohistochemistry (IHC) expression assessment. ECOG Performance Status 0 to 1. Adequate organ function performed within 10 days of treatment initiation. Female participants must have a negative urine or serum pregnancy test at screening. The serum pregnancy test must be negative for the participant to be eligible. Female participants of childbearing potential should be willing to use 2 methods of birth control or be surgically sterile, or abstain from heterosexual activity for the course of the study through 120 days after the last dose of study treatment. Female patients who are breast feeding should discontinue nursing prior to the first dose of study medication and until 44 months after the last study treatment. Male participants should agree to use an adequate method of contraception starting with the first dose of study treatment through 120 days after the last dose of study treatment. Absence of severe comorbidities that in the opinion of the Investigator might hamper the participation to the study and/or the treatment administration. |
| Interventions |
Experimental group: Participants receive pembrolizumab 200 mg intravenously, every 3 weeks, for one year (expected maximum 18 doses). Control group: participants receive placebo intravenously, every 3 weeks, for one year (expected maximum 18 doses). |
| Outcomes | Overall survival, disease‐free survival |
| Starting date | November 2015 |
| Contact information | Correspondence for published protocol: 1‐888‐577‐8839 |
| Notes | participant recruiting stage |
| Trial name or title | Cancer vaccine study for stage III, unresectable, non‐small cell lung cancer (NSCLC) in the Asian population (INSPIRE) |
| Methods | International RCT (45 investigation sites in 5 countries ), Phase III |
| Participants |
Number of participants: 285 (03 Dec 2009 to 10 Sep 2014) Number randomised: Experimental group (Tecemotide (L‐BLP25) + Cyclophosphamide + Best Supportive Care): 191 Control group (Saline + Placebo + BSC): 94 Number evaluated: Experimental group: 191, age (mean): 56.5 years (8.93) Control group: 94, age (mean): 59.3 years (9.08) Diagnosis: patients were locally advanced unresectable stage III NSCLC Inclusion: The eligibility criteria were as follows: ≥ 18 years of age; Histologically documented unresectable stage III NSCLC, with stage confirmed by imaging (CT, MRI, or PET, or a combination); Completion of chemoradiotherapy (concomitant or sequential) ≥ 4 weeks and ≤ 12 weeks prior to randomisation, consisting of ≥ 2 cycles of platinum‐based chemotherapy and a radiation dose of ≥ 50 Gy; Stable disease or objective response after primary chemoradiotherapy according to RECIST documented ≤ 4 weeks prior to randomisation ECOG performance status of 0 or 1; Platelet count ≥ 140 × 109/L; WBC ≥ 2.5 × 109/L; haemoglobin ≥ 90 g/L. |
| Interventions |
Experimental group: A single IV infusion of 300 mg/m² (to a maximum 600 mg) of low dose cyclophosphamide was given 3 days prior to first tecemotide (L‐BLP25) vaccination. After receiving single low dose cyclophosphamide, subjects received 8 consecutive weekly (Week 1, 2, 3, 4, 5, 6, 7, and 8 primary treatment phase) subcutaneous tecemotide (L‐BLP25) vaccinations at a dose of 918 mcg and then at 6‐week intervals, beginning at Week 14 (maintenance phase) until PD is documented or the subject discontinued for any other reason. The BSC was provided as per the investigator’s discretion and was not limited to palliative radiation, psychosocial support, analgesics, and nutritional support. Control group: A single IV infusion of 0.9% sodium chloride (saline) was administered 3 days prior to first placebo vaccination. After receiving saline solution, subjects received 8 consecutive weekly subcutaneous vaccinations with placebo at Week 1, 2, 3, 4, 5, 6, 7 and 8 followed by maintenance treatment at 6‐week intervals, beginning at Week 14, until PD is documented or the subject discontinued for any other reason. The BSC was provided as per the investigator’s discretion and was not limited to palliative radiation, psychosocial support, analgesics and nutritional support. |
| Outcomes | Data cut‐off date: June 2015 OS, PFS,time‐to‐symptom progression (TTSP), time‐to‐progression (TTP), adverse events |
| Starting date | December 2009 |
| Contact information | Correspondence for published protocol: tony@clo.cuhk.edu.hk |
| Notes | Supported by Merck KGaA, Darmstadt, Germany This study was terminated prematurely as the sponsor decided to discontinue program with Tecemotide in NSCLC |
Contributions of authors
Conceiving the review: HS
Designing the review: HS, JZ, CS
Co‐ordinating the review: HS, JZ
Designing search strategies: HS, Cochrane Lung Cancer Group
Study selection, data extraction, data analysis, risk assessment: HS, JZ, CS, RL, ET, OT, RR
Writing the review: JZ, HS, RL, RR, JW
Providing general advice on the review: CS
Securing funding for the protocol: none
Performing previous work that was the foundation of the current study: HS, JZ, SC
Sources of support
Internal sources
Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Sweden.
External sources
No sources of support supplied
Declarations of interest
HS declares no conflict of interest.
JZ declares no conflict of interest.
RL declares no conflict of interest.
ET declares no conflict of interest.
RR declares no conflict of interest.
CS declares no conflict of interest.
OT declares no conflict of interest.
New
References
References to studies included in this review
- Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L, Krzakowski M, et al. Tecemotide (L‐BLP25) versus placebo after chemoradiotherapy for stage III non‐small‐cell lung cancer(START): a randomised, double‐blind, phase 3 trial. Lancet Oncolology 2014;15(1):59‐68. [DOI] [PubMed] [Google Scholar]
- Fujisawa T, Yamaguchi Y. Postoperative immunostimulation after complete resection improves survival of patients with stage I non‐small cell lung carcinoma. Cancer 1996;78(9):1892‐8. [PubMed] [Google Scholar]
- Giovanni BR, Paolo Z, Sandro M, Paolo M, Riccardo A, Giovanni F, et al. A randomized trial of adoptive immunotherapy with tumor‐infiltrating lymphocytes and interleukin‐2 versus standard therapy in the postoperative treatment of resected non‐small cell lung carcinoma. Cancer 1996;78(2):244‐51. [DOI] [PubMed] [Google Scholar]
- Macchiarini P, Hardin M, Angeletti CA. Long‐term evaluation of intrapleural Bacillus Calmette‐Guérin with or without adjuvant chemotherapy in completely resected stages II and III non‐small‐cell lung cancer. American Journal of Clinical Oncology 1991;14(4):291‐7. [DOI] [PubMed] [Google Scholar]
- Matthay RA, Mahler DA, Beck GJ, Loke J, Baue AE, Carter DC, et al. Intratumoral Bacillus Calmette‐Guérin immunotherapy prior to surgery for carcinoma of the lung:results of a prospective randomized trial. Cancer Research 1986;46(11):5963‐8. [PubMed] [Google Scholar]
- Stanley K, The Ludwig Lung Cancer Study Group (LLCSG). Immunostimulation with intrapleural BCG as adjuvant therapy in resected non‐small cell lung cancer. Cancer 1986;58(11):2411‐6. [DOI] [PubMed] [Google Scholar]
- Vansteenkiste J, Zielinski M, Linder A, Dahabreh J, Gonzalez EE, Malinowski W, et al. Adjuvant MAGE‐A3 immunotherapy in resected non‐small‐cell lung cancer: phase II randomized study results. Journal of Clinical Oncology 2013;31(19):2396‐403. [DOI] [PubMed] [Google Scholar]
- Vansteenkiste JF, Cho BC, Vanakesa T, Pas T, Zielinski M, Kim MS, et al. Efficacy of the MAGE‐A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE‐A3‐positive non‐small‐cell lung cancer (MAGRIT): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncology 2016;17(6):822‐35. [DOI] [PubMed] [Google Scholar]
- Zhao M, Li H, Li L, Zhang Y. Effects of a gemcitabine plus platinum regimen combined with a dendritic cell‐cytokine induced killer immunotherapy on recurrence and survival rate of non‐small cell lung cancer patients. Experimental and Therapeutic Medicine 2014;7(5):1403‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Belani CP, Nemunaitis JJ, Chachoua A, Eisenberg PD, Raez LE, Cuevas JD, et al. Phase 2 trial of erlotinib with or without PF‐3512676 (CPG 7909, a Toll‐like receptor 9 agonist) inpatients with advanced recurrent EGFR‐positive non‐small cell lung cancer. Cancer Biology & Therapy 2013 Jul;14(7):557‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García B, Neninger E, Torre A, Leonard I, Martínez R, Viada C, et al. Effective inhibition of the epidermal growth factor/epidermal growth factor receptor binding by anti‐epidermal growth factor antibodies is related to better survival in advanced non‐small‐cell lung cancer patients treated with the epidermal growth factor cancer vaccine. Clinical Cancer Research 2008;14(3):840‐6. [DOI] [PubMed] [Google Scholar]
- Kimura H, Yamaguchi, Y. A phase III randomized study of interleukin‐2 lymphokine‐activated killer cell immunotherapy combined with chemotherapy or radiotherapy after curative or noncurative resection of primary lung carcinoma. Cancer 1997;80(1):42‐9. [PubMed] [Google Scholar]
- Kimura H, Matsui Y, Ishikawa A, Nakajima T, Yoshino M, Sakairi Y. Randomized controlled phase III trial of adjuvant chemo‐immunotherapy with activated killer T cells and dendritic cells in patients with resected primary lung cancer. Cancer Immunology, Immunotherapy 2015;64(1):51‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsakis A. Papadimitraki E, Vetsika EK, Aggouraki D, Dermitzaki EK, Hatzidaki D, et al. A phase II trial evaluating the clinical and immunologic response of HLA‐A2(+) non‐small cell lung cancer patients vaccinated with an hTERT cryptic peptide. Lung Cancer 2014;86(1):59‐66. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Meregalli S, Fossati V, Paolorossi F, Barni S, Tancini G, et al. A randomized study of immunotherapy with low‐dose subcutaneous interleukin‐2 plus melatonin vs chemotherapy with cisplatin andetoposide as first‐line therapy for advanced non‐small cell lung cancer. Tumori 1994;80(6):464‐7. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J, Sterman D, Jablons D, Smith JW, Fox B, Maples P, et al. Granulocyte‐macrophage colony‐stimulating factor gene‐modified autologous tumor vaccines in non‐small‐cell lung cancer. Journal of the National Cancer Institute 2004;96(4):326‐31. [DOI] [PubMed] [Google Scholar]
- Neninger E, Verdecia BG, Crombet T, Viada C, Pereda S, Leonard I, et al. Combining an EGF‐based cancer vaccine with chemotherapy in advanced non‐small cell lung cancer. Journal of Immunotherapy 2009;32(1):92‐9. [DOI] [PubMed] [Google Scholar]
- Pujol JL, Vansteenkiste JF, Pas TM, Atanackovic D, Reck M, Thomeer M, et al. Safety and immunogenicity of MAGE‐A3 cancer immunotherapeutic with or without adjuvant chemotherapy in patients with resected stage IB to III MAGE‐A3‐positive non‐small‐cell lung cancer. Journal of Thoracic Oncology 2015;10(10):1458‐67. [DOI] [PubMed] [Google Scholar]
- Ramalingam SS, Mitchell P, Vansteenkiste JF, Debus J, Curran WJ, Socinski MA, et al. START2: tecemotide in unresectable stage III NSCLC after first‐line concurrent chemoradiotherapy. Journal of Clinical Oncology 2014 [Epub ahead of print];32(15 Suppl 1):TPS7608. [DOI: 10.1200/jco.2014.32.15_suppl.tps7608] [DOI] [Google Scholar]
- Ruckdeschel JC, McKneally MF, Baxter DH, DeVote C, Kellar S, Killam D, et al. Regional immunotherapy has a detrimental effect on the response to combined irradiation and chemotherapy in locally advanced non‐small cell bronchogenic carcinoma. Cancer Immunology, Immunotherapy 1981;11(4):277‐82. [Google Scholar]
- Skachkova OV, Khranovska NM, Gorbach OI, Svergun NM, Sydor RI, Nikulina VV. Immunological markers of anti‐tumor dendritic cells vaccine efficiency in patients with non‐small cell lung cancer. Experimental Oncology 2013;35(2):109‐13. [PubMed] [Google Scholar]
- Woodruff M, Walbaum P. A phase‐II trial of corynebacterium parvum as adjuvant to surgery in the treatment of operable lung cancer. Cancer Immunology, Immunotherapy 1983;16(2):114‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Macchiarini P, Mussi A, Angeletti CA. Intrapleural BCG in postsurgical stage I non‐small cell lung cancer. Anticancer Research 1989;9(2):391‐3. [PubMed] [Google Scholar]
- Schlieben I, Calavrezos A, Koschel G, Seysen U. Adjuvant BCG therapy in radical resection of lung cancer. Praxis und Klinik der Pneumologie 1984;38(3):102‐3. [PubMed] [Google Scholar]
References to ongoing studies
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small‐Cell Lung Cancer. The New England Journal of Medicine. 2017 Sep 8;[Epub ahead of print]:doi: 10.1056/NEJMoa1709937. [Google Scholar]; Haiyan Jiang. A Global Study to Assess the Effects of MEDI4736 Following Concurrent Chemoradiation in Patients With Stage III Unresectable Non‐Small Cell Lung Cancer (PACIFIC). ClinicalTrials.gov.
- Canadian Cancer Trials Group. A Phase III Prospective Double Blind Placebo Controlled Randomized Study of Adjuvant MEDI4736 In Completely Resected Non‐Small Cell Lung Cancer. ClinicalTrials.gov.
- Merck KGaA. Study of Tecemotide (L‐BLP25) in Participants With Stage III Unresectable Non‐small Cell Lung Cancer (NSCLC) Following Primary Chemoradiotherapy. ClinicalTrials.gov.
- Hoffmann‐La Roche. Study to Assess Safety and Efficacy of Atezolizumab (MPDL3280A) Compared to Best Supportive Care Following Chemotherapy in Patients With Lung Cancer. ClinicalTrials.gov.
- Sharp M, Corp D. Study of Pembrolizumab (MK‐3475) vs Placebo for Participants With Non‐small Cell Lung Cancer After Resection With or Without Standard Adjuvant Therapy (MK‐3475‐091/KEYNOTE‐091) (PEARLS). ClinicalTrials.gov.
- Wu YL, Park K, Soo RA, Sun Y, Tyroller K, Wages D, Ely G, et al. INSPIRE: A phase III study of the BLP25 liposome vaccine (L‐BLP25) in Asian patients with unresectable stage III non‐small cell lung cancer. BMC Cancer 2011 Oct 7;11:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
- Acres B, Limacher JM. MUC1 as a target antigen for cancer immunotherapy. Expert Review of Vaccines 2005;4(4):493‐502. [DOI] [PubMed] [Google Scholar]
- Bafna S, Kaur S, Batra SK. Membrane‐bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene 2010;29(20):2893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta‐analysis (Statistics in Practice). Chichester: John Wiley & Sons, 2008. [Google Scholar]
- Carrizosa DR, Gold KA. New strategies in immunotherapy for non‐small cell lung cancer. Translational Lung Cancer Research 2015;4(5):553‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasanu CA, Sethi N, Ahmed N. Immune alterations and emerging immunotherapeutic approaches in lung cancer. Expert Opinion on Biological Therapy 2012;12(7):923‐37. [DOI] [PubMed] [Google Scholar]
- Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009;136(1):260‐71. [DOI] [PubMed] [Google Scholar]
- Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Advances in Immunology 2006;90:51‐81. [DOI] [PubMed] [Google Scholar]
- Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nature Reviews. Clinical Oncology 2014;11(1):24‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn OJ. Cancer immunology. New England Journal of Medicine 2008;358(25):2704‐15. [DOI] [PubMed] [Google Scholar]
- Forde PM, Kelly RJ, Brahmer JR. New strategies in lung cancer: translating immunotherapy into clinical practice. Clinical Cancer Research 2014;20(5):1067‐73. [DOI] [PubMed] [Google Scholar]
- Giaccone G, Bazhenova L, Nemunaitis J, Juhasz E, Ramlau R, Heuval MM, et al. A phase III study of belagenpumatucel‐L therapeutic tumor cell vaccine for non‐small cell lung cancer (NSCLC). European Cancer Congress 2013. Amsterdam, 2013:Abstract n2. [Google Scholar]
- International Agency for Research on Cancer (IARC) CancerBase. Cancer Incidence and Mortality Worldwide. www.globocaniarcfr 2012 (accessed 11 June 2014).
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime, Inc.), 2015.
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Hirschowitz EA, Hiestand DM, Yannelli JR. Vaccines for lung cancer. Jpurnal of Thoracic Oncology 2006;1(1):93‐104. [PubMed] [Google Scholar]
- Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non‐small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2013 May;143(5 Suppl):e278S‐e313S. [DOI] [PubMed] [Google Scholar]
- Katakami N, Hida T, Nokihara H, Imamura F, Sakai H, Atagi S et, al. Phase I/II study of tecemotide as immunotherapy in Japanese patients with unresectable stage III non‐small cell lung cancer. Lung Cancer 2017 Mar;105:23‐30. [DOI] [PubMed] [Google Scholar]
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD‐1 and its ligands in tolerance and immunity. Annual Review of Immunology 2008;26:677‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer JN, Gress RE. A comparison and critical analysis of preclinical anticancer vaccination strategies. Experimental Biology and Medicine 2007;232(9):1130‐41. [DOI] [PubMed] [Google Scholar]
- Lardinois D, Suter H, Hakki H, Rousson V, Betticher D, Ris HB. Morbidity, survival, and site of recurrence after mediastinal lymph‐node dissection versus systematic sampling after complete resection for non‐small cell lung cancer. Annals of Thoracic Surgery 2005;80(1):268‐74. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480(7378):480‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P, Thatcher N, Socinski MA, Wasilewska‐Tesluk E, Horwood K, Szczesna A, et al. Tecemotide in unresectable stage III non‐small‐cell lung cancer in the phase III START study: updated overall survival and biomarker analyses. Annals of Oncolology 2015 Jun;26(6):1134‐42. [DOI] [PubMed] [Google Scholar]
- Mountain CF. Revisions in the international system for staging lung cancer. Chest 1997;111:1710–17. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Cancer Stat Facts: Lung and Bronchus Cancer. Available at: https://seer.cancer.gov/statfacts/html/lungb.html Vol. Accessed November 14, 2017.
- Nemunaitis J, Dillman RO, Schwarzenberger PO, Senzer N, Cunningham C, Cutler J, et al. Phase II study of belagenpumatucel‐L, a transforming growth factor beta‐2 antisense gene‐modified allogeneic tumor cell vaccine in non‐small‐cell lung cancer. Journal of Clinical Oncology 2006;24(29):4721‐30. [DOI] [PubMed] [Google Scholar]
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour‐enhanced meta‐analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991–6. [DOI] [PubMed] [Google Scholar]
- Ramnath N, Dilling TJ, Harris LJ, Kim AW, Michaud GC, Balekian AA, et al. Treatment of stage III non‐small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2013 May;143(5 Suppl):e314S‐e340S. [DOI] [PubMed] [Google Scholar]
- Reckamp KL. Advances in immunotherapy for non‐small cell lung cancer. Clinical Advances in Hematology & Oncology 2015 Dec;13(12):847‐53. [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. The New England Journal of Medicine 2008;359(13):1367‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha R, Butts C. L‐BLP25: a peptide vaccine strategy in non small cell lung cancer. Clinical Cancer Research 2007;13(15 Pt 2):s4652‐4. [DOI] [PubMed] [Google Scholar]
- Shepherd F, Socinski M, Mitchell P, Thatcher N, Havel L, Krzakowski M, et al. Updated analysis and secondary endpoints with L‐BLP25 in unresectable stage III non‐small cell lung cancer in the phase III START study. Poster discussion session at the European Cancer Congress; 2013 Sept 29. 2013:Abstract No: 3419. [Google Scholar]
- Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nature Reviews 2005;5(8):615‐25. [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of Canada, National Cancer Institute of the United States. Journal of the National Cancer Institute 2000;92(3):205‐16. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. New England Journal of Medicine 2012;366(26):2443‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi P, Mirakhur B. MAGRIT: the largest‐ever phase III lung cancer trial aims to establish a novel tumor‐specific approach to therapy. Clinical Lung Cancer 2009;10(5):371‐4. [DOI] [PubMed] [Google Scholar]
- Eynde BJ, Bruggen P. T cell defined tumor antigens. Current Opinion in Immunology 1997;9(5):684‐93. [DOI] [PubMed] [Google Scholar]
- Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune‐relatedresponse criteria. Clinical Cancer Research 2009;15(23):7412‐20. [DOI] [PubMed] [Google Scholar]


