Abstract
Background
Peripheral nerve block (infiltration of local anaesthetic around a nerve) is used for anaesthesia or analgesia. A limitation to its use for postoperative analgesia is that the analgesic effect lasts only a few hours, after which moderate to severe pain at the surgical site may result in the need for alternative analgesic therapy. Several adjuvants have been used to prolong the analgesic duration of peripheral nerve block, including perineural or intravenous dexamethasone.
Objectives
To evaluate the comparative efficacy and safety of perineural dexamethasone versus placebo, intravenous dexamethasone versus placebo, and perineural dexamethasone versus intravenous dexamethasone when added to peripheral nerve block for postoperative pain control in people undergoing surgery.
Search methods
We searched the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, DARE, Web of Science and Scopus from inception to 25 April 2017. We also searched trial registry databases, Google Scholar and meeting abstracts from the American Society of Anesthesiologists, the Canadian Anesthesiologists' Society, the American Society of Regional Anesthesia, and the European Society of Regional Anaesthesia.
Selection criteria
We included all randomized controlled trials (RCTs) comparing perineural dexamethasone with placebo, intravenous dexamethasone with placebo, or perineural dexamethasone with intravenous dexamethasone in participants receiving peripheral nerve block for upper or lower limb surgery.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 35 trials of 2702 participants aged 15 to 78 years; 33 studies enrolled participants undergoing upper limb surgery and two undergoing lower limb surgery. Risk of bias was low in 13 studies and high/unclear in 22.
Perineural dexamethasone versus placebo
Duration of sensory block was significantly longer in the perineural dexamethasone group compared with placebo (mean difference (MD) 6.70 hours, 95% confidence interval (CI) 5.54 to 7.85; participants1625; studies 27). Postoperative pain intensity at 12 and 24 hours was significantly lower in the perineural dexamethasone group compared with control (MD ‐2.08, 95% CI ‐2.63 to ‐1.53; participants 257; studies 5) and (MD ‐1.63, 95% CI ‐2.34 to ‐0.93; participants 469; studies 9), respectively. There was no significant difference at 48 hours (MD ‐0.61, 95% CI ‐1.24 to 0.03; participants 296; studies 4). The quality of evidence is very low for postoperative pain intensity at 12 hours and low for the remaining outcomes. Cumulative 24‐hour postoperative opioid consumption was significantly lower in the perineural dexamethasone group compared with placebo (MD 19.25 mg, 95% CI 5.99 to 32.51; participants 380; studies 6).
Intravenous dexamethasone versus placebo
Duration of sensory block was significantly longer in the intravenous dexamethasone group compared with placebo (MD 6.21, 95% CI 3.53 to 8.88; participants 499; studies 8). Postoperative pain intensity at 12 and 24 hours was significantly lower in the intravenous dexamethasone group compared with placebo (MD ‐1.24, 95% CI ‐2.44 to ‐0.04; participants 162; studies 3) and (MD ‐1.26, 95% CI ‐2.23 to ‐0.29; participants 257; studies 5), respectively. There was no significant difference at 48 hours (MD ‐0.21, 95% CI ‐0.83 to 0.41; participants 172; studies 3). The quality of evidence is moderate for duration of sensory block and postoperative pain intensity at 24 hours, and low for the remaining outcomes. Cumulative 24‐hour postoperative opioid consumption was significantly lower in the intravenous dexamethasone group compared with placebo (MD ‐6.58 mg, 95% CI ‐10.56 to ‐2.60; participants 287; studies 5).
Perinerual versus intravenous dexamethasone
Duration of sensory block was significantly longer in the perineural dexamethasone group compared with intravenous by three hours (MD 3.14 hours, 95% CI 1.68 to 4.59; participants 720; studies 9). We found that postoperative pain intensity at 12 hours and 24 hours was significantly lower in the perineural dexamethasone group compared with intravenous, however, the MD did not surpass our pre‐determined minimally important difference of 1.2 on the Visual Analgue Scale/Numerical Rating Scale, therefore the results are not clinically significant (MD ‐1.01, 95% CI ‐1.51 to ‐0.50; participants 217; studies 3) and (MD ‐0.77, 95% CI ‐1.47 to ‐0.08; participants 309; studies 5), respectively. There was no significant difference in severity of postoperative pain at 48 hours (MD 0.13, 95% CI ‐0.35 to 0.61; participants 227; studies 3). The quality of evidence is moderate for duration of sensory block and postoperative pain intensity at 24 hours, and low for the remaining outcomes. There was no difference in cumulative postoperative 24‐hour opioid consumption (MD ‐3.87 mg, 95% CI ‐9.93 to 2.19; participants 242; studies 4).
Incidence of severe adverse events
Five serious adverse events were reported. One block‐related event (pneumothorax) occurred in one participant in a trial comparing perineural dexamethasone and placebo; however group allocation was not reported. Four non‐block‐related events occurred in two trials comparing perineural dexamethasone, intravenous dexamethasone and placebo. Two participants in the placebo group required hospitalization within one week of surgery; one for a fall and one for a bowel infection. One participant in the placebo group developed Complex Regional Pain Syndrome Type I and one in the intravenous dexamethasone group developed pneumonia. The quality of evidence is very low due to the sparse number of events.
Authors' conclusions
Low‐ to moderate‐quality evidence suggests that when used as an adjuvant to peripheral nerve block in upper limb surgery, both perineural and intravenous dexamethasone may prolong duration of sensory block and are effective in reducing postoperative pain intensity and opioid consumption. There is not enough evidence to determine the effectiveness of dexamethasone as an adjuvant to peripheral nerve block in lower limb surgeries and there is no evidence in children. The results of our review may not apply to participants at risk of dexamethasone‐related adverse events for whom clinical trials would probably be unsafe.
There is not enough evidence to determine the effectiveness of dexamethasone as an adjuvant to peripheral nerve block in lower limb surgeries and there is no evidence in children. The results of our review may not be apply to participants who at risk of dexamethasone‐related adverse events for whom clinical trials would probably be unsafe. The nine ongoing trials registered at ClinicalTrials.gov may change the results of this review.
Plain language summary
Dexamethasone and peripheral nerve block
What is a peripheral nerve block?
A nerve block prevents or relieves pain by interrupting pain signals that travel along a nerve to the brain. It involves an injection of local anaesthetic (a numbing agent) around a nerve either during or immediately after surgery. Pain relief from nerve block may last only a few hours after surgery, after which people may experience moderate to severe pain.
What is dexamethasone?
Dexamethasone is a steroid that may reduce pain and the inflammatory response to tissue damage after surgery (heat, pain, redness and swelling). In people receiving nerve block, dexamethasone may be given with the local anaesthetic around the nerve (perineural) or into a vein (intravenous) to prolong the pain relief from the peripheral nerve block.
What did the researchers investigate?
We looked for randomized controlled trials that investigated whether perineural or intravenous dexamethasone prolongs the length of time people experience pain relief from the peripheral nerve block when undergoing upper and lower limb surgery and reduces the intensity of pain after surgery. We also investigated whether perineural or intravenous dexamethasone cause any side effects or harms. We searched the medical literature for articles that included either adults or children undergoing upper or lower limb surgery with peripheral nerve block published up until 25 April 2017. We also assessed the quality of evidence for each outcome.
What did the researchers find?
We included 35 studies involving 2702 aged 15 to 78 years.
When compared with placebo, the duration of sensory block was prolonged in the perineural dexamethasone group by 6 and a half hours (27 studies, 1625 participants, low‐quality evidence) and in the intravenous dexamethasone group by six hours (8 studies, 499 participants, moderate‐quality evidence). When perineural and intravenous dexamethasone were compared, the duration of sensory block was longer in the perineural dexamethasone group by three hours (9 studies, 720 participants, moderate‐quality evidence).
Postoperative pain intensity at 12 hours after surgery was lower in the perineural dexamethasone group compared with placebo (5 studies, 257 participants, very low‐quality evidence) and at 24 hours after surgery (9 studies, 469 participants, low‐quality evidence). When we compared intravenous dexamethasone with placebo, postoperative pain intensity was also lower in the intravenous dexamethasone group than in the placebo group at 12 hours (3 studies, 162 participants, low‐quality evidence) and 24 hours (5 studies, 257 participants, low‐quality evidence). The amount of opioid pain medication required was also lower in participants receiving perineural and intravenous dexamethasone. There was no difference in postoperative pain intensity or the amount of opioid pain medication required when perineural and intravenous dexamethasone were compared. We concluded that one way of administering dexamethasone does not provide better pain relief over the other.
Five serious adverse events were reported in three studies. One block‐related adverse event (pneumothorax or collapsed lung) occurred in one participant in a trial comparing perineural dexamethasone and placebo; however group allocation was not reported. The remaining events were non‐block‐related and occurred in two trials comparing perineural dexamethasone, intravenous dexamethasone and placebo. Two participants in the control group required hospitalization within one week of surgery; one for a fall and one for a bowel infection. One participant in the placebo group developed a chronic pain syndrome called Complex Regional Pain Sydrome, and one participant in the intravenous dexamethasone group developed pneumonia. The quality of evidence for safety issues was very low.
Summary of findings
Background
Description of the condition
Peripheral nerve block is a technique whereby local anaesthetic solution is infiltrated perineurally to provide anaesthesia, or analgesia, or both. Peripheral nerve block for intraoperative and postoperative pain management is associated with improved analgesia, fewer opioid‐related adverse events, earlier ambulation, and shorter hospital stay when compared with intravenous opioid analgesia alone (Barreveld 2013; Charlton 2010; Lin 2013). A limitation to the use of peripheral nerve blocks is that the analgesic effect of the block lasts only a few hours, resulting in early, moderate to severe pain, and thus the need for adjuvant therapies (Choi 2014; Cummings 2011). Peripheral nerve catheters that provide continuous infusion of local anaesthetic have been used to prolong the effects of local anaesthesia; however, continuous catheters require greater time and skill to insert than single‐shot peripheral block, may dislodge while in use, may be difficult to remove, and may add additional costs to health care (Adhikary 2012; Bowens 2011; Choi 2014). Several adjuvants have been used to attempt to prolong the analgesia provided by peripheral nerve block, including perineural and intravenous dexamethasone (Brummett 2012; Choi 2014; Popping 2009).
Description of the intervention
Dexamethasone is a corticosteroid drug that has been used as an adjuvant to reduce postoperative pain. Two systematic reviews have shown that study participants who received a single dose of intravenous dexamethasone perioperatively had lower pain scores and decreased opioid consumption after surgery compared with those given placebo (De Oliveira 2011; Waldron 2013). De Olivera 2013 studied three different intravenous doses: low‐dose (< 0.10 mg/kg), intermediate‐dose (0.11 to 0.20 mg/kg) and high‐dose (≥ 0.21 mg/kg). Low‐dose dexamethasone was not effective in reducing pain and opioid consumption; however, intermediate and high doses were effective (De Olivera 2013). Waldron 2013 performed a subgroup analysis of two doses of dexamethasone: 4 mg to 5 mg; and 8 mg to 10 mg, and did not find a dose‐response relationship.
Several randomized control trials (RCTs) have studied the use of perineural dexamethasone (i.e. dexamethasone added to the local anaesthesia solution) as an adjuvant to peripheral nerve block to improve analgesia provided by local anaesthetic alone (Bias 2014; Biradar 2013; Cummings 2011; Dar 2013; Golwala 2009; Movafegh 2006; Parrington 2010; Shaikh 2013; Tandoc 2011; Viera 2010; Yadov 2008). Perineural dexamethasone, as an adjuvant to peripheral nerve block, has been associated with faster onset of anaesthesia (Golwala 2009; Shrestha 2003; Talukdar 2013; Yadov 2008), longer duration of anaesthesia/analgesia (Biradar 2013; Cummings 2011; Dar 2013; Golwala 2009Movafegh 2006; Parrington 2010; Shaikh 2013; Talukdar 2013; Tandoc 2011; Viera 2010; Vishnu 2014), decreased postoperative pain intensity (Cummings 2011; Dar 2013; Tandoc 2011; Yadov 2008), and decreased postoperative analgesia requirements compared with local anaesthetic alone (Shaikh 2013; Talukdar 2013; Tandoc 2011; Vishnu 2014; Yadov 2008).
Five systematic reviews have evaluated the efficacy of perineural dexamethasone versus placebo in participants undergoing surgery with peripheral nerve block. The number of trials and participants in each trial are as follows: Albrecht 2015 ‐ 29 trials, 1695 participants; Choi 2014 ‐ nine trials, 809 participants; De Oliveira 2014 ‐ nine trials, 760 participants; Huynh 2015 ‐ 12 trials, 512 participants; and Knezivic 2015 ‐ 14 trials, 1022 participants.
In all five reviews, the use of perineural dexamethasone was associated with longer duration of sensory block compared with placebo (Albrecht 2015; Choi 2014; De Oliveira 2014; Huynh 2015, Knezivic 2015). Neither the De Oliveira 2014 review nor the Huynh 2015 review found a difference in intensity of postoperative pain among participants who received perineural dexamethasone compared with placebo. The Knezivic 2015 review found intensity of pain at 24 and 48 hours after surgery was lower with dexamethasone compared with block alone. The remaining reviews did not evaluate intensity of postoperative pain (Albrecht 2015; Choi 2014). Opioid consumption was evaluated in three of five reviews. The De Oliveira 2014 and Knezivic 2015 reviews found a reduction in opioid consumption among participants who received perineural dexamethasone but the Choi 2014 review did not. Similarly, only two reviews evaluated postoperative nausea and vomiting, both reporting a reduction in the incidence of postoperative nausea and vomiting among participants who received perineural dexamethasone (Albrecht 2015; Huynh 2015). None of the reviews compared perineural dexamethasone with systemic dexamethasone, or systemic dexamethasone with placebo.
How the intervention might work
The exact mechanism by which dexamethasone reduces pain is not known. The decrease in pain intensity and the prolonged analgesia attained with the use of perineural dexamethasone may be the result of a local, or systemic action, or both (Fredrickson 2013). Dexamethasone may act locally on glucocorticosteroid receptors to induce vasoconstriction, thereby decreasing systemic absorption of local anaesthetics (Shishido 2002; Wang 2011). Other potential mechanisms of action include suppression of C‐fibre transmission of pain signals and direct action on the nerve cell to reduce neural discharge (Johansson 1990). Dexamethasone may act systemically by reducing the inflammatory response caused by surgical tissue injury (Christiansson 2009).
Why it is important to do this review
It is important to treat postoperative pain effectively. People who experience severe pain in the early postoperative period are at risk for development of chronic pain (Kehlet 2006; Vandenkerkoff 2012), which can dramatically affect their quality of life (Galvez 2007; Lame 2005; Smith 2007), and increase healthcare costs (Blyth 2003). In an attempt to augment postoperative pain management, people are often treated with opioids, which are associated with adverse events such as respiratory depression, nausea, vomiting, constipation and pruritus. Adequate treatment of people with pain through the use of peripheral nerve block may result in reduced opioid use and fewer opioid‐related harms (Avidan 2003; Hadzic 2005).
Use of perineural dexamethasone as an adjuvant to peripheral nerve block for postoperative pain is controversial. Animal studies have suggested that perineural dexamethasone is neurotoxic to peripheral nerves and has the potential to cause peripheral nerve damage; however, data in humans are limited (Ma 2010). Although no symptoms of peripheral nerve damage were reported in four RCTs examining perineural versus intravenous dexamethasone (Abdallah 2015; Desmet 2013; Kawanishi 2014; Rahangdale 2014), these studies may have been underpowered to detect differences in potential neurotoxic events (Williams 2014). Furthermore, in most studies, participants were followed for short periods (24 to 48 hours). Thus, adverse events such as persistent nerve palsy caused by peripheral nerve damage may not have been detected.
Intravenous dexamethasone may be used as an alternative to perineural dexamethasone and as an adjuvant to peripheral nerve block. In four RCTs, the effects of perineural and intravenous dexamethasone in participants receiving peripheral nerve block were studied (Abdallah 2015; Desmet 2013; Kawanishi 2014; Rahangdale 2014). In three of these studies, both perineural and intravenous dexamethasone were associated with prolonged sensory block when compared with placebo (Abdallah 2015; Desmet 2013; Rahangdale 2014). In one study, perineural but not intravenous dexamethasone was associated with prolonged sensory block when compared with placebo (Kawanishi 2014). In all four studies, no difference was observed in the duration of sensory block when perineural and intravenous dexamethasone were compared with each other.
Single‐dose intravenous dexamethasone is associated with complications such as hyperglycaemia, perineal irritation, postoperative infection, and delayed wound healing (Bartlett 2013; Crandell 2004; Pasternak 2004; Percival 2010; Perron 2003; Yared 2000). Rare adverse events include tumour lysis syndrome and psychosis after a single dose and avascular necrosis of bone after short‐term use (Fast 1984; Lerza 2002; Mc Donnell 2008; McKee 2001).
Although four systematic reviews have compared the efficacy of perineural dexamethasone versus placebo (Albrecht 2015; Choi 2014; De Oliveira 2014; Huynh 2015), to date, no comprehensive review has compared each method of dexamethasone delivery versus placebo, or perineural versus intravenous dexamethasone.
Objectives
To evaluate the comparative efficacy and safety of perineural dexamethasone versus placebo, intravenous dexamethasone versus placebo, and perineural dexamethasone versus intravenous dexamethasone when added to peripheral nerve block for postoperative pain control in people undergoing surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) that evaluated the effectiveness of dexamethasone as an adjuvant to peripheral nerve block, irrespective of blinding and other design features (parallel or factorial). We did not exclude any study on the basis of language of publication or publication status. We excluded observational studies, quasi‐randomized trials and cluster‐randomized trials.
Types of participants
We included children (aged 1 month to 18 years) and adults (aged 19 years and older) undergoing upper and lower limb surgery who received a peripheral nerve block or a peripheral nerve block with the addition of dexamethasone. We excluded neonates.
Types of interventions
Our intervention groups included the following.
Participants who received peripheral nerve block and perineural dexamethasone (dexamethasone mixed with the local anaesthetic solution) versus those receiving peripheral nerve block and a perineural placebo or a non‐active comparator.
Participants who received peripheral nerve block and intravenous dexamethasone versus those receiving peripheral nerve block and intravenous placebo or a non‐active comparator.
Participants who received peripheral nerve block and perineural dexamethasone versus those receiving peripheral nerve block and intravenous dexamethasone.
We excluded participants who received local anaesthetic, or dexamethasone, or both, via more than one route (e.g. perineurally and subcutaneously).
Types of outcome measures
Primary outcomes
Duration of sensory block. We included all studies describing duration of sensory block regardless of how it was described.
Incidence of serious adverse events. We used the National Institutes of Health (NIH) definition of adverse events. A serious event includes death, a life‐threatening event that requires hospitalization or prolonged hospitalization, disability or congenital anomaly (NIH 2013).
Secondary outcomes
Duration of motor block. We included all studies describing duration of motor block, regardless of how it was described.
Incidence of mild to moderate adverse events such as nausea/vomiting, pruritus, somnolence, oxygen desaturation, urinary retention, numbness/tingling.
Postoperative pain intensity (pain scores) at 12, 24 and 48 hours.
Postoperative opioid consumption at 12, 24 and 48 hours. We converted all opioids to oral morphine equivalents.
Participant satisfaction with pain control. Participant satisfaction is typically measured on a numerical rating scale (NRS).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (inception to 25 April 2017), (Appendix 1) MEDLINE via Ovid (1966 to 25 April 2017) (Appendix 2), Embase via Ovid (1947 to 25 April 2017) (,Appendix 3) the Database of Abstracts of Reviews of Effectiveness (DARE) (inception to 25 April 2017), and Web of Science (Appendix 4) and Scopus (inception to 25 April 2017) . An experienced librarian assisted with the search strategy. The MEDLINE search strategy presented in Appendix 2 was adopted for searching the DARE and Scopus databases. We did not impose any language restrictions.
Searching other resources
We reviewed the reference lists of all included trials for additional studies that met our criteria, as well as trial registry databases (ClinicalTrials.gov (clinicaltrials.gov), EU Cinical Trials Register (clinicaltrialsregister.eu), and Current Controlled Trials (isrctn.com), Google Scholar and meeting abstracts from the American Society of Anesthesiologists, the Canadian Anesthesiologists' Society, the American Society of Regional Anesthesia, and the European Society of Regional Anaesthesia (2010 to April 2017).
Data collection and analysis
Selection of studies
Using the results of all searches, two review authors (AK and CP) independently screened titles and abstracts for eligibility according to the following criteria:.
The study described was an RCT.
Participants received a peripheral nerve block.
Dexamethasone was given perineurally (mixed with the local anaesthetic) or intravenously.
In cases of disagreement on eligibility, we consulted a third review author (BJ) to determine eligibility. If additional information was required, we contacted the first author of the trial.
Data extraction and management
Two review authors (AP and CP) independently extracted data and assessed the quality of each trial using a standardized, pre‐piloted form (Appendix 5). We resolved disagreements through discussion with a senior review author (BJ).
Assessment of risk of bias in included studies
Using the Cochrane 'Risk of bias' instrument, we assessed the risk of bias of each study using the following domains (Higgins 2011).
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Selective reporting.
Missing participant data.
For each study, we classified risk of bias of each domain as low, high or unclear. If risk of bias was low for all domains or was low for five out of the six domains then we classified the risk of bias as low for that study. If two or more domains were classified as high or unclear risk of bias, we determined the study to be at high or unclear risk of bias, respectively.
Measures of treatment effect
We analysed continuous outcomes including pain scores, analgesic consumption, duration of sensory and motor block. and participant satisfaction by calculating the mean difference (MD) with corresponding 95% confidence interval (CI). We pooled dichotomous outcomes to calculate the risk ratio (RR) and the risk difference (RD) with corresponding 95% CI. We assumed a normal distribution of pain scores on the Visual Analogue Scale (VAS) or the numerical rating scale (NRS) among intervention and placebo groups, and we considered a 1.2 cm change/1.2 point change on the VAS/NRS as representative of a minimally important difference (MID) in acute pain (Johnston 2013; Kelly 2001).
Unit of analysis issues
We avoided unit of analysis errors as follows: if included studies had more than two study arms, we combined relevant groups to create a single pair‐wise comparison, or, if not possible, we selected one pair of interventions and excluded the others.
Dealing with missing data
We assessed the completeness of outcome data and determined whether missing outcome data may put continuous and dichotomous outcomes at risk of bias. If primary analyses for our critical outcome of interest suggested significant benefit (or harm), we conducted a sensitivity meta‐analysis to address missing participant data (Akl 2012; Ebrahim 2013).
To determine whether missing participant data represented risk of bias for continuous outcomes (pain at 12, 24 and 48 hours), we used the method described by Ebrahim 2013. For each outcome with missing data, we used four progressively stringent data input strategies based on observed outcomes for those individuals in the intervention and placebo arms for whom data were not missing.
If we found a significant difference in serious adverse events, to determine whether missing participant data represented risk of bias for dichotomous outcomes (serious adverse events), we conducted a sensitivity meta‐analysis using the worst‐case scenario assumption described by Akl 2013. If results were robust to the worst‐case scenario (all missing participants in the treatment experienced the outcome of interest, and none of the participants in the placebo group did), we concluded that the missing data did not represent a source of bias. If results of the sensitivity meta‐analysis were not robust to the worst‐case scenario, we tested more plausible assumptions. For participants missing from the intervention group, we assumed a range of ratios of event rates for those with missing data compared with those successfully followed of 2:1, 3:1 and 5:1 (Akl 2012).
Assessment of heterogeneity
We used the Cochran Q and I2 tests to assess the potential for statistical heterogeneity between trials. For the Cochran Q, the null hypothesis is that the underlying effect is the same in each of the included studies. A P value less than 0.10 means that random error provides an unlikely explanation for observed differences in study results between trials. The I2 statistic shows the percentage of variability due to differences between studies such that I2 > 75% indicates considerable heterogeneity (Higgins 2011; Riley 2011).
Assessment of reporting biases
For outcomes reported in 10 or more trials, we assessed publication biases using a funnel plot (Higgins 2011).
Data synthesis
We entered data into Review Manager statistical software and analysed data using the DerSimonian‐Laird random‐effects model (Review Manager 2014).
If data for some outcomes were not amenable to meta‐analysis, we planned to use tables to describe the characteristics of each trial that contributed to our a priori outcomes. We planned to describe all trial populations, interventions, comparator(s), outcome(s) and follow‐up time points for outcomes not amenable to meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We analysed the following subgroups.
Long‐acting local anaesthetics (e.g. ropivacaine, bupivacaine, levobupivacaine) versus medium‐acting local anaesthetics (e.g. lidocaine, mepivacaine).
Additives to local anaesthetics (e.g. clonidine, epinephrine) versus no additives to local anaesthetics.
Low‐dose dexamethasone (4 mg to 5 mg) versus high‐dose dexamethasone (8 mg to 10 mg).
Adult versus paediatric participants. (See Differences between protocol and review)
Studies at high/unclear risk of bias versus studies at low risk of bias.
Following are our a priori hypotheses for explaining heterogeneity between trials.
-
For the outcome, duration of sensory block, we anticipated that participants receiving dexamethasone along with long‐acting local anaesthetics (e.g. ropivacaine, bupivacaine, levobupivacaine) would show larger effects than those receiving dexamethasone with medium‐acting local anaesthetics (e.g. lidocaine, mepivacaine).
Choi et al in a systematic review and meta‐analysis compared the effects of short‐acting local anaesthetics and medium‐acting anaesthetics and found that the duration of analgesia in participants receiving long‐acting anaesthetics was longer than those receiving medium‐acting local anaesthetics (Choi 2014).
-
For the outcome, duration of sensory block, we anticipated that participants who receive additives to local anaesthesia (e.g. clonidine, epinephrine) would show a larger effect than those who do not.
In a systematic review of 20 RCTs of 573 participants, Popping and colleagues found that the duration of intermediate and long‐acting local anaesthetics was longer in participants who received clonidine (Popping 2009).
-
Although we conducted a subgroup analysis on dose of dexamethasone for the outcomes: pain intensity, duration of analgesia, and serious adverse events, we did not expect that participants who receive high‐dose dexamethasone would show any difference in effect when compared with those receiving low‐dose dexamethasone.
Albrecht and colleagues, in a systematic review of 29 RCTs of 1695 participants did not find a difference in duration of analgesia in participants who received 4 mg of dexamethasone compared with those who received 8 mg (Albrecht 2015). Differences in other outcomes, including intensity of pain and adverse events were not reported.
-
For the outcome of adult versus paediatric participants, we did not anticipate a difference in duration of analgesia or intensity of pain.
Currently, no evidence supports that the pharmacokinetics of dexamethasone is different in children when compared with adults.
-
For the outcomes of intensity of pain and duration of sensory block, we expected that trials with high risk of bias would show a larger effect than those with low risk of bias.
Our subgroup on risk of bias is based on previous literature suggesting that studies at high risk of bias are more likely to overestimate treatment effects (Nuesch 2009; Wood 2008).
Sensitivity analysis
We conducted a sensitivity analysis to assess the completeness of outcome data and to determine whether missing outcome data put continuous and dichotomous outcomes at risk of bias, using the methods described in Dealing with missing data.
'Summary of findings' table and GRADE
Two review authors (AP and CP) independently prepared a 'Summary of findings' table using GRADEprofiler software to assess the confidence of estimates of effect (GRADEpro GDT 2015), for the following outcomes of interest.
Duration of sensory block.
Incidence of serious adverse events
Postoperative pain intensity at 12 hours.
Postoperative pain intensity at 24 hours.
Postoperative pain intensity at 48 hours.
We used GRADE principles as described by Guyatt 2008, to independently assess the confidence in our pooled estimates of effect (i.e. overall quality of evidence) using the following criteria.
Risk of bias.
Consistency.
Directness.
Precision.
Reporting bias.
For RCTs, we initially assigned high confidence ratings, but rated confidence as moderate, low or very low if we detected issues with risk of bias, consistency or other GRADE criteria. In particular, we categorized the quality of each pooled estimate as high (we are very confident that the true effect lies close to that of the estimate of the effect), moderate (we are moderately confident in the effect estimate ‐ the true effect is likely to be close to the estimate of the effect, but may be substantially different), low (our confidence in the effect estimate is limited ‐ the true effect may be substantially different from the estimate of the effect) or very low (we have very little confidence in the effect estimate ‐ the true effect is likely to be substantially different from the estimate of effect) (Guyatt 2008).
We referred discrepancies in assessment of the quality of evidence to a third review author (BJ) for a final decision.
Results
Description of studies
Results of the search
Please see the PRISMA flowchart for the selection process of the included studies (Figure 1):
1.
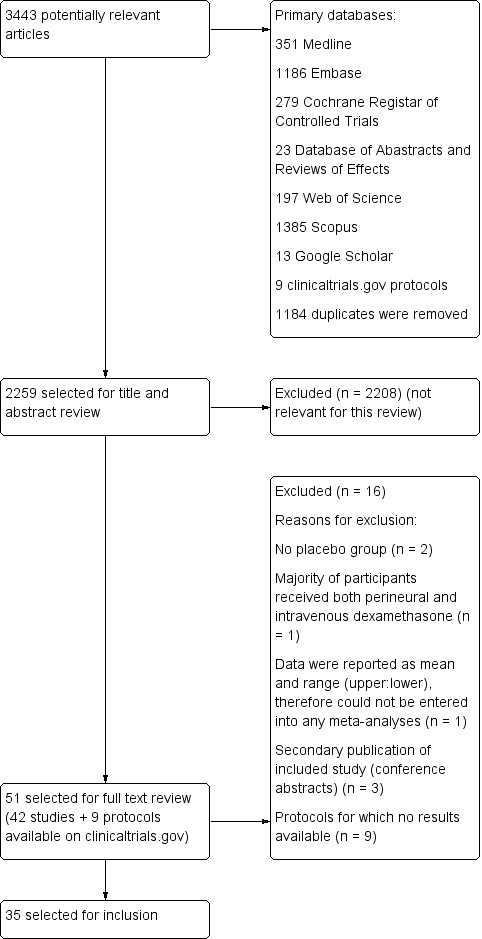
Flow diagram of included studies.
We identified 3443 unique records in our literature search. Of these, 51 were potentially eligible. Nine were protocols found on ClinicalTrials.gov for which no results were available (NCT01277159; NCT01495624; NCT01586806; NCT01971645; NCT02178449; NCT02322242; NCT02436694; NCT02462148; NCT02506660). We excluded seven studies: two because there was no placebo group (Fredrickson 2013; Shethra 2007); one because participants received both perineural and intravenous dexamethasone (Lui 2015): one because the authors reported only a means without any variances, therefore we could not enter the data into a meta‐analysis (Percec 2014); and three were secondary publications of included studies (Arora 2010; Desmet 2012; Kim 2010), leaving 35 for inclusion in the review.
Included studies
Participants
The 35 included trials involved 2702 participants. All studies were in Americal Anesthesiology Society (ASA) I to III adolescent and adult participants aged 15 to 78 years. We did not find any studies in children aged less than 15 years. Length of follow‐up ranged from one day to six months after surgery. Surgical procedures included the forearm and hand (not including the elbow) (Abdallah 2015; Aliste 2017; Alarasan 2017; Lee 2016; Leurcharusmee 2016; Movafegh 2006; Parrington 2010; Saritas 2014; Shah 2015; Yadov 2008), forearm and hand (including the elbow) (Biradar 2013; Shah 2015; Shaikh 2013), arthroscopic shoulder (Chalifoux 2017; Chun 2016; Desmet 2013; Jadon 2015; Kawanishi 2014; Kim 2012; Sakae 2017; Tandoc 2011; Viera 2010; Woo 2015), both arthroscopic and open shoulder (Cummings 2011; Nallam 2014; Rosenfeld 2016), upper limb (Bias 2014; Dar 2013; Ganvit 2014; Golwala 2009; Kumar 2014; Talukdar 2013; Vishnu 2014), rotator cuff repair or subacromial decompression (Desmet 2015), and foot and ankle (Dawson 2016; Rahangdale 2014).
Type of block included interscalene brachial plexus (Chun 2016; Chalifoux 2017; Cummings 2011; Desmet 2013; Desmet 2015; Ganvit 2014; Jadon 2015; Kawanishi 2014; Kim 2012; Nallam 2014; Tandoc 2011; Viera 2010; Woo 2015), supraclavicular brachial plexus (Abdallah 2015; Alarasan 2017; Bias 2014; Biradar 2013; Dar 2013; Golwala 2009; Kumar 2014; Parrington 2010; Shaikh 2013; Talukdar 2013; Vishnu 2014; Yadov 2008), axillary brachial plexus (Aliste 2017; Movafegh 2006; Rosenfeld 2016; Saritas 2014), infraclavicular brachial plexus (Leurcharusmee 2016; Sakae 2017; Shah 2015), sciatic nerve (Rahangdale 2014), and ankle block (Dawson 2016).
Exclusion criteria
Exclusion criteria were: pregnancy (Abdallah 2015; Biradar 2013; Chalifoux 2017; Cummings 2011; Desmet 2013; Desmet 2015; Ganvit 2014; Golwala 2009; Kawanishi 2014; Kim 2012; Kumar 2014; Movafegh 2006; Rahangdale 2014; Sakae 2017; Saritas 2014; Talukdar 2013; Viera 2010; Yadov 2008), neurological deficit or neuropathy (Aliste 2017; Biradar 2013; Chalifoux 2017; Chun 2016; Cummings 2011; Desmet 2013; Desmet 2015; Ganvit 2014; Kawanishi 2014; Kim 2012; Kumar 2014; Leurcharusmee 2016; Parrington 2010; Rahangdale 2014; Sakae 2017; Shah 2015; Talukdar 2013; Tandoc 2011; Vishnu 2014), peptic ulcer (Biradar 2013; Ganvit 2014; Golwala 2009; Kawanishi 2014; Kumar 2014; Movafegh 2006; Parrington 2010; Shah 2015; Shaikh 2013; Talukdar 2013; Woo 2015; Yadov 2008), diabetes mellitus (Abdallah 2015; Biradar 2013; Chun 2016; Desmet 2013; Desmet 2015; Ganvit 2014; Golwala 2009; Kawanishi 2014; Kim 2012; Lee 2016; Parrington 2010; Rahangdale 2014; Rosenfeld 2016; Shaikh 2013; Talukdar 2013; Vishnu 2014; Woo 2015), hypertension (Biradar 2013; Ganvit 2014; Tandoc 2011; Yadov 2008), endocrine disorder (Biradar 2013; Kumar 2014; Sakae 2017; Saritas 2014; Shah 2015), cardiac disease (Biradar 2013; Kumar 2014; Saritas 2014; Sakae 2017; Shah 2015; Yadov 2008), circulatory instability (Golwala 2009), hepatic or renal disease (Aliste 2017; Biradar 2013; Ganvit 2014; Golwala 2009; Kawanishi 2014; Kumar 2014; Lee 2016; Leurcharusmee 2016; Movafegh 2006; Sakae 2017; Saritas 2014: Shaikh 2013; Talukdar 2013), lung disease (Desmet 2013 , Desmet 2015; Kim 2012; Kumar 2014; Shah 2015; Tandoc 2011; Rosenfeld 2016; Woo 2015), respiratory disorder (Chun 2016; Yadov 2008), psychiatric history (Abdallah 2015, Kumar 2014; Shah 2015; Yadov 2008), clavicular fracture (Abdallah 2015), electrolyte imbalance, (Saritas 2014), head injury (Kumar 2014; Sakae 2017; Shah 2015), neuromuscular disease (Shaikh 2013; Yadov 2008), drug/alcohol dependency (Kawanishi 2014; Kim 2012; Kumar 2014; Talukdar 2013; Yadov 2008), pre‐existing chronic pain (Abdallah 2015; Chalifoux 2017; Kim 2012), preoperative opioid use (Biradar 2013; Chun 2016; Dawson 2016; Kawanishi 2014; Movafegh 2006; Sakae 2017; Rahangdale 2014; Rosenfeld 2016; Sakae 2017; Shah 2015; Woo 2015), preoperative corticosteroid use (Chalifoux 2017; Chun 2016; Cummings 2011; Dawson 2016; Desmet 2013; Desmet 2015; Golwala 2009; Kumar 2014; Rahangdale 2014; Sakae 2017; Talukdar 2013; Vishnu 2014; Woo 2015), contraindication to peripheral nerve block (skin infection, coagulopathy, bleeding diathesis, deformities in the operative site (Abdallah 2015; Aliste 2017; Bias 2014; Chalifoux 2017; Chun 2016; Cummings 2011; Dawson 2016; Jadon 2015; Kawanishi 2014; Kim 2012; Kumar 2014; Lee 2016; Leurcharusmee 2016; Sakae 2017; Shah 2015; Talukdar 2013; Tandoc 2011; Vishnu 2014; Woo 2015), allergy/hypersensitivity to any of the study drugs (Abdallah 2015; Bias 2014; Biradar 2013; Dawson 2016; Desmet 2013; Desmet 2015; Ganvit 2014; Golwala 2009; Jadon 2015; Kim 2012; Kumar 2014; Parrington 2010; Rahangdale 2014; Rosenfeld 2016; Shaikh 2013; Shah 2015; Shaikh 2013; Tandoc 2011; Viera 2010; Vishnu 2014; Woo 2015).
Settings
All trials took place between 2006 and 2017 in hospital settings in Australia (Dawson 2016), Bangledesh (Talukdar 2013), Belguim (Desmet 2013; Desmet 2015), Brazil (Sakae 2017), Canada (Abdallah 2015; Aliste 2017; Chalifoux 2017; Leurcharusmee 2016; Parrington 2010), India (Alarasan 2017; Bias 2014; Dar 2013; Ganvit 2014; Golwala 2009; Jadon 2015; Kumar 2014; Nallam 2014; Shah 2015; Shaikh 2013; Vishnu 2014), Iran (Movafegh 2006), Japan (Kawanishi 2014), Korea (Chun 2016; Kim 2012; Lee 2016; Woo 2015), Nepal (Yadov 2008), Thailand (Aliste 2017; Leurcharusmee 2016), Turkey (Saritas 2014), and USA (Cummings 2011; Rahangdale 2014; Rosenfeld 2016; Tandoc 2011; Viera 2010).
Interventions
Twenty‐three studies (1488 participants) compared perineural dexamethasone and placebo (Alarasan 2017; Bias 2014; Biradar 2013; Cummings 2011; Dar 2013; Ganvit 2014; Golwala 2009; Jadon 2015; Kim 2012; Kumar 2014; Lee 2016; Movafegh 2006; Nallam 2014; Parrington 2010; Saritas 2014; Shah 2015; Shaikh 2013; Talukdar 2013; Tandoc 2011; Viera 2010; Vishnu 2014; Woo 2015; Yadov 2008), two (n = 195) compared intravenous dexamethasone and control (Chalifoux 2017; Desmet 2015), four (n = 460) compared perineural and intravenous dexamethasone (Alarasan 2017; Chun 2016; Leurcharusmee 2016; Sakae 2017), and six (n = 564) compared perineural dexamethasone, intravenous dexamethasone and placebo (Abdallah 2015; Dawson 2016; Desmet 2013; Kawanishi 2014; Rahangdale 2014; Rosenfeld 2016).
Techniques used for block placement included nerve stimulation (Biradar 2013; Cummings 2011; Desmet 2013; Ganvit 2014; Jadon 2015; Kumar 2014; Movafegh 2006; Nallam 2014; Saritas 2014; Shah 2015; Shaikh 2013; Tandoc 2011; Viera 2010; Vishnu 2014; Yadov 2008), ultrasound guidance (Abdallah 2015; Alarasan 2017; Aliste 2017; Dawson 2016; Kawanishi 2014; Kim 2012; Leurcharusmee 2016; Parrington 2010; Rahangdale 2014; Rosenfeld 2016; Viera 2010; Woo 2015), both nerve stimulation and ultrasound guidance (Chalifoux 2017; Chun 2016; Desmet 2015; Lee 2016; Sakae 2017), landmark method (Bias 2014; Dar 2013; Golwala 2009), and paraesthesia technique (Talukdar 2013).
Local anaesthetics included ropivacaine 0.5% (Bias 2014; Chalifoux 2017; Chun 2016; Dar 2013; Dawson 2016; Desmet 2013; Desmet 2015; Jadon 2015; Kawanishi 2014; Kumar 2014; Lee 2016; Rosenfeld 2016; Sakae 2017; Woo 2015), bupivacaine 0.5% (Abdallah 2015; Alarasan 2017; Cummings 2011; Rahangdale 2014; Shaikh 2013; Talukdar 2013; Tandoc 2011; Viera 2010; Vishnu 2014), lidocaine 1.5% (Biradar 2013; Movafegh 2006; Shah 2015; Yadov 2008), levobupivacaine 0.5 % (Kim 2012; Nallam 2014), bupivacaine 0.5% and lidocaine 1.5% mixture (Aliste 2017; Ganvit 2014; Golwala 2009; Leurcharusmee 2016), mepivacaine (Parrington 2010), and prilocaine 2% (Saritas 2014).
Additives to local anaesthetic agent included epinephrine (Alarasan 2017; Biradar 2013; Ganvit 2014; Golwala 2009; Leurcharusmee 2016; Rahangdale 2014; Shaikh 2013; Tandoc 2011; Viera 2010; Yadov 2008), and clonidine (Viera 2010). No additives were used in the remaining studies.
Dexamethasone dose included 4 mg (Kawanishi 2014; Sakae 2017; Yadov 2008), 5 mg (Alarasan 2017; Chun 2016; Kim 2012), 7.5 mg (Woo 2015), 8 mg (Abdallah 2015; Aliste 2017; Bias 2014; Biradar 2013; Cummings 2011; Dar 2013; Dawson 2016; Ganvit 2014; Golwala 2009; Jadon 2015; Kumar 2014; Leurcharusmee 2016; Movafegh 2006; Nallam 2014; Parrington 2010; Rahangdale 2014; Rosenfeld 2016; Saritas 2014; Shah 2015; Shaikh 2013; Talukdar 2013; Tandoc 2011; Viera 2010; Vishnu 2014), and 10 mg (Chalifoux 2017; Desmet 2013; Desmet 2015; Lee 2016).
Comparators
In all included studies, participants received a peripheral nerve block with local anaesthesia. In studies comparing perineural dexamethasone and placebo, participants received either perineural dexamethasone or an equal volume of perineural saline. In studies comparing intravenous dexamethasone and placebo, participants received either intravenous dexamethasone or an equal volume of intravenous saline. In studies comparing perineural and intravenous dexamethasone, participants in the perineural dexamethasone group received dexamethasone perineurally and intravenous saline. Those in the intravenous dexamethasone group received dexamethasone intravenously and perineural saline.
Funding sources
Funding sources included: Merit Award form the Department of Anesthesia, Univerity of Toronto (Abdallah 2015), departmental sources (Alarasan 2017; Chalifoux 2017; Cummings 2011), Belgian Association for Regional Anesthesia (Desmet 2015), Department of Anesthesiology, Northwestern University (Rahangdale 2014), Buffalo Anesthesiology Associates (Tandoc 2011), and Department of Anesthesiology, Baystate Medical Center, Springfield, Massachutes (Viera 2010) (see Characteristics of included studies).
Contact with authors
We attempted to contact 15 authors for additional information (Abdallah 2015; Cummings 2011; Dar 2013; Desmet 2013; Desmet 2015; Jadon 2015; Kawanishi 2014; Kumar 2014; Parrington 2010; Rahangdale 2014; Rosenfeld 2016; Sakae 2017; Shah 2015; Viera 2010; Woo 2015), and were successful in obtaining data from seven (Abdallah 2015; Cummings 2011; Rahangdale 2014; Rosenfeld 2016; Sakae 2017; Shah 2015; Viera 2010).
Excluded studies
We excluded four studies from our review. Two lacked a placebo group (Fredrickson 2013; Shethra 2007), one reported data as median and range (minimum to maximum), therefore we could not enter the results in a meta‐analysis (Percec 2014), and in another, participants received both perineural and intravenous dexamethasone (Lui 2015) (see Characteristics of excluded studies).
Ongoing studies
We found nine ongoing trials at ClinicalTrials.gov (NCT01277159; NCT01495624; NCT01586806; NCT01971645; NCT02178449; NCT02322242; NCT02436694; NCT02462148; NCT02506660) (see Characteristics of ongoing studies).
Studies awaiting classification
There are no studies awaiting classification.
Risk of bias in included studies
The overall risk of bias was low in 13 studies (Abdallah 2015; Aliste 2017; Chalifoux 2017; Cummings 2011; Dawson 2016; Desmet 2013; Desmet 2015; Kumar 2014; Leurcharusmee 2016; Parrington 2010; Rahangdale 2014; Rosenfeld 2016; Woo 2015) and high/unclear in the remaining 22. Figure 2 shows authors' judgements about each risk of bias item presented as percentages across all included studies and Figure 3 shows review authors' judgements about each risk of bias item for each included study.
2.
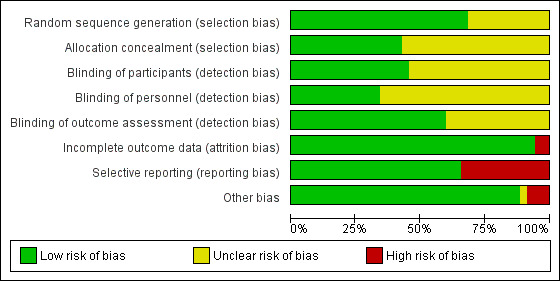
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
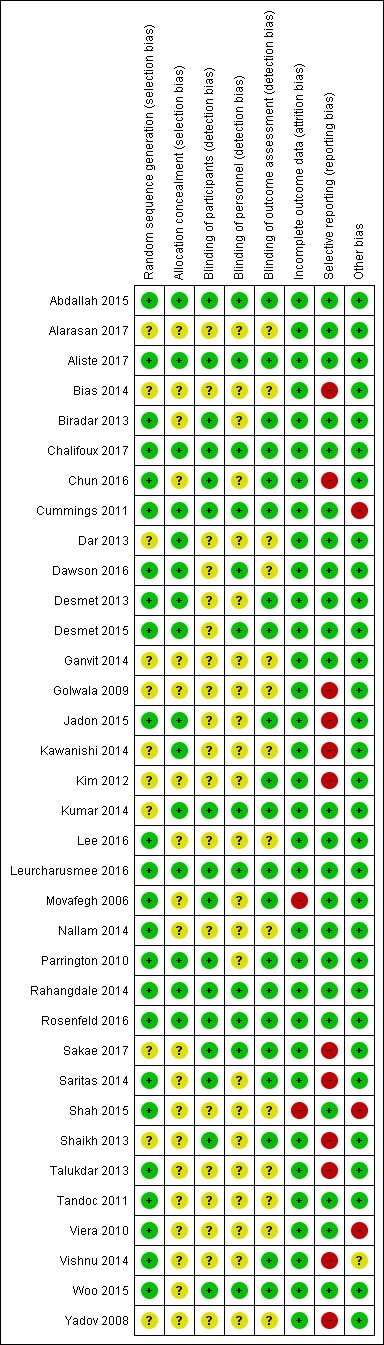
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In 24 studies the method of random sequence generation was adequately described, and we judged the risk of bias to be low (Abdallah 2015; Aliste 2017; Biradar 2013; Chalifoux 2017; Chun 2016; Cummings 2011; Dawson 2016; Desmet 2013; Desmet 2015; Jadon 2015; Lee 2016; Leurcharusmee 2016; Movafegh 2006; Nallam 2014; Parrington 2010; Rahangdale 2014; Rosenfeld 2016; Saritas 2014; Shah 2015; Talukdar 2013; Tandoc 2011; Viera 2010; Vishnu 2014; Woo 2015). In the 11 remaining, we judged the risk of bias to be unclear because the random sequence was not described.
In 15 studies the method of allocation concealment was adequately described, and we judged the risk of bias to be low (Abdallah 2015; Aliste 2017; Chalifoux 2017; Cummings 2011; Dar 2013; Dawson 2016; Desmet 2013; Desmet 2015; Jadon 2015; Kawanishi 2014; Kumar 2014; Leurcharusmee 2016; Parrington 2010; Rahangdale 2014; Rosenfeld 2016). In the remaining 20 we judged the risk of bias to be unclear because the method of allocation concealment was not described.
Blinding
Blinding of participants was adequately described in 16 studies (Abdallah 2015; Aliste 2017; Biradar 2013; Chalifoux 2017; Chun 2016; Cummings 2011; Kumar 2014; Leurcharusmee 2016; Movafegh 2006; Parrington 2010; Rahangdale 2014; Rosenfeld 2016; Sakae 2017; Saritas 2014; Shaikh 2013; Vishnu 2014).
Blinding of personnel was adequately described in 12 studies (Abdallah 2015; Aliste 2017; Chalifoux 2017; Cummings 2011; Dawson 2016; Desmet 2015; Kumar 2014; Leurcharusmee 2016; Rahangdale 2014; Rosenfeld 2016; Sakae 2017; Woo 2015).
Bliding of outcome assessors was adequately described in 21 studies(Abdallah 2015; Aliste 2017; Biradar 2013; Chalifoux 2017; Chun 2016; Cummings 2011; Desmet 2013; Desmet 2015; Jadon 2015; Kim 2012; Kumar 2014; Leurcharusmee 2016; Movafegh 2006; Parrington 2010; Rahangdale 2014; Rosenfeld 2016; Sakae 2017; Saritas 2014; Shaikh 2013; Vishnu 2014; Woo 2015).
Incomplete outcome data
We judged the risk for attrition bias to be low in 33 studies. There were no missing outcome data in 16 (Abdallah 2015; Alarasan 2017; Bias 2014; Cummings 2011; Dar 2013; Dawson 2016; Kim 2012; Kumar 2014; Lee 2016; Sakae 2017; Saritas 2014; Talukdar 2013; Viera 2010; Vishnu 2014; Woo 2015; Yadov 2008), and in 17, the number of participants with missing outcome data was balanced between groups (Aliste 2017; Biradar 2013; Chalifoux 2017; Chun 2016; Desmet 2013; Desmet 2015; Ganvit 2014; Golwala 2009; Jadon 2015; Kawanishi 2014; Leurcharusmee 2016; Nallam 2014; Parrington 2010; Rahangdale 2014; Rosenfeld 2016; Shaikh 2013; Tandoc 2011). We judged two studies to be at high risk of attrition bias. In one, over 30% of participants in each group were excluded from the study (Movafegh 2006), and in the other, only 41 of 53 participants enrolled were included in the analysis (Shah 2015).
Selective reporting
We judged 23 studies to be at low risk of reporting bias. Protocols were available for eight and all prespecified outcomes were reported (Abdallah 2015; Aliste 2017; Cummings 2011; Leurcharusmee 2016; Parrington 2010; Rahangdale 2014; Rosenfeld 2016; Woo 2015). In the remaining 15, protocols were not available, but all outcomes prespecified in the methods section were reported (Alarasan 2017; Biradar 2013; Chalifoux 2017; Dar 2013; Dawson 2016; Desmet 2013; Desmet 2015; Ganvit 2014; Kumar 2014; Lee 2016; Movafegh 2006; Nallam 2014; Shah 2015; Tandoc 2011; Viera 2010). Twelve studies were at high risk of selective outcome bias. In two, protocols were available but not all outcomes were reported as per protocol (Chun 2016; Sakae 2017), and in 10, not all outcomes were reported as described in the methods section (Bias 2014; Golwala 2009; Jadon 2015; Kawanishi 2014; Kim 2012; Saritas 2014; Shaikh 2013; Talukdar 2013; Vishnu 2014; Yadov 2008).
Other potential sources of bias
There were other potential sources of bias in two studies. Both were stopped early for benefit (Cummings 2011; Shah 2015), which may be a source of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Perineural dexamethasone versus placebo.
|
Patient or population: participants undergoing surgery with peripheral nerve block
Setting: participants undergoing upper and lower limb surgery in hospitals in Australia, Bangledesh, Belgium, Brazil, India, Iran, Japan, Korea, Nepal, Turkey and USA Intervention: perineural dexamethasone Comparison: placebo | ||||
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with placebo | Risk with perineural dexamethasone | |||
| Duration of sensory block (we included all studies describing duration of sensory block, regardless of how it was described) |
The mean duration of sensory block was 10.2 hours | The mean duration of sensory block in the perineural dexamethasone group was 6.70 hours longer (5.54 longer to 7.85 longer) | 1625 (27 RCTs) | ⨁⨁◯◯ LOW a |
| Incidence of serious adverse events (we used the NIH definition of adverse events. A serious event includes death, a life‐threatening event that requires hospitalization or prolonged hospitalization, disability or congenital anomaly) |
In seven studies, authors reported that they assessed for serious adverse events. Five serious adverse events were reported in three studies: one block‐related adverse event (pneumothorax) occurred in one participant in a trial comparing perineural dexamethasone and placebo; however, group allocation was not reported. The remaining non‐block‐related events occurred in two trials comparing perineural dexamethasone, intravenous dexamethasone and placebo. Two participants in the placebo group required hospitalization within one week of surgery; one for a fall, and one for a bowel infection. One participant in the placebo group developed Complex Regional Pain Syndrome Type I and one in the intravenous dexamethasone group developed pneumonia. | 620 (7 RCTs) |
⨁◯◯◯
VERY LOWb |
|
| Postoperative pain intensity at 12 hours (assessed by pain scores on an 11‐point VAS) |
The mean postoperative pain intensity at 12 hours was 3.0 | The mean postoperative pain score at 12 hours in the perineural dexamethasone group was 2.08 points lower (1.52 lower to 2.63 lower) | 257 (5 RCTs) | ⨁◯◯◯
VERY LOWc |
| Postoperative pain intensity at 24 hours. (assessed by pain scores on an 11‐point VAS) |
The mean postoperative pain intensity at 24 hours was 3.9 | The mean postoperative pain score at 24 hours in the perineural dexamethasone group was 1.63 points lower (0.93 lower to 2.34 lower) | 469 (9 RCTs) | ⨁⨁◯◯ LOW d |
| Postoperative pain intensity at 48 hours (assessed by pain scores on an 11‐point VAS) |
The mean postoperative pain intensity at 48 hours was 3.3 | The mean postoperative pain score at 48 hours in the perineural dexamethasone group was 0.61 points lower (1.24 lower to 0.03 higher) | 296 (3 RCTs) | ⨁⨁◯◯ LOW e |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; NIH: National Institute of Health; RCT: randomized controlled trial; VAS: Visual Analogue Scale. | ||||
| GRADE Working Group grades of evidence High‐quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low‐quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
aDowngraded by one level for risk of bias as 19 out of 27 studies are at unclear risk of bias. Reasons include lack of reporting on random sequence generation, concealment allocation, blinding, and attrition bias. Downgraded by one level for inconsistency (I2 = 99%, P value for heterogeneity is < 0.00001) and heterogeneity is not explained by subgroup analyses; point estimates vary widely among studies, confidence intervals show minimal overlap).
bDowngraded by one level for risk of bias as four out of the seven studies are at unclear risk of bias. Reasons include lack of reporting on random sequence generation, concealment allocation, blinding, and evidence of selective reporting bias. Downgraded by two levels for imprecision due to very low number of events.
c Downgraded by one level for risk of bias. Three out of five studies are at unclear risk of bias. Reasons include lack of
reporting on random sequence generation and allocation concealment and evidence of attrition bias, selective reporting bias, and stopping early for benefit. Downgraded by one level for inconsistency (I2 = 61%, P value for heterogeneity is 0.03) and heterogeneity is not explained by subgroup analyses; point estimates vary widely among studies, confidence intervals show minimal overlap
dDowngraded by one level for inconsistency (I2 = 80%, P value for heterogeneity is < 0.00001) and heterogeneity is not explained by subgroup analyses; point estimates vary widely across studies. Downgraded by one level for imprecision (95% confidence interval includes no clinical effect and a clinical effect). By no clinical effect we mean the lower bound of the CI did not surpass our chosen MID threshold of 1.2 on VAS.
e Downgraded by two levels for imprecision because of a sparse number of participants (n=296) and a very wide confidence interval demonstrating that the treatment effect is not statistically significant and of questionable clinical significance.
Summary of findings 2. Intravenous dexamethasone versus placebo.
|
Patient or population: participants undergoing surgery with peripheral nerve block
Setting: participants undergoing upper and lower limb surgery in hospitals in Australia, Belgium, Brazil, Canada, Japan, Korea, Thailand and USA Intervention: intravenous dexamethasone Comparison: placebo | ||||
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with placebo | Risk with intravenous dexamethasone | |||
| Duration of sensory block (we included all studies describing duration of sensory block regardless of how it was described) |
The mean duration of sensory block was 16.1 hours | The mean duration of sensory block in the intravenous dexamethasone group was 6.21 hours longer (3.53 longer to 8.88 longer) | 499 (8 RCTs) | ⨁⨁⨁◯ MODERATE a |
| Incidence of serious adverse events (we used the NIH definition of adverse events. A serious event includes death, a life‐threatening event that requires hospitalization or prolonged hospitalization, disability or congenital anomaly) |
Please see incidence of serious adverse events in the perineural dexamethasone versus placebo 'Summary of findings' table. | |||
| Postoperative pain intensity at 12 hours (measured using pain scores on an 11‐point VAS) |
The mean postoperative pain score at 12 hours was 2.6 | The mean postoperative pain score at 12 hours in the intravenous dexamethasone group was 1.24 points lower (2.44 lower to 0.04 lower) | 162 (3 RCTs) | ⨁⨁◯◯ LOW b |
| Postoperative pain intensity at 24 hours (measured using pain scores on an 11‐point VAS) | The mean postoperative pain score at 24 hours was 4.4 | The mean postoperative pain score at 24 hours in the intravenous dexamethasone group was 1.26 points lower (2.23 lower to 0.29 lower) | 257 (5 RCTs) | ⨁⨁◯◯ LOW c |
| Postoperative pain intensity at 48 hours (measured using pain scores on an 11‐point VAS) |
The mean postoperative pain score at 48 hours was 3.7 | The mean postoperative pain score at 48 hours in the intravenous dexamethasone group was 0.21 points lower (0.83 lower to 0.41 higher) | 172 (3 RCTs) | ⨁⨁◯◯ LOW d |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; NIH: National Institute of Health; RCT: randomized controlled trial; VAS: Visual Analogue Scale. | ||||
| GRADE Working Group grades of evidence High‐quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low‐quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
aDowngraded by one level for inconsistency (considerable heterogeneity (I2 = 88% and P value for heterogeneity <0.0001) and subgroup analyses did not explain observed heterogeneity. Downgraded by one level for imprecision because of a sparse number of participants (n=162).
bDowngraded by one level for inconsistency (I2 = 61% and P value for heterogeneity 0.08) and subgroup analyses did not explain observed heterogeneity. Downgraded by one level for imprecision. Confidence interval includes both no clinical effect (minimally important difference 1.2 on VAS) and clinical effect (minimally important difference greater than 1.2 on VAS).
cDowngraded by one level for inconsistency(I2 = 65% and P value for heterogeneity 0.02) and subgroup analyses did not explain observed heterogeneity. Point estimates vary widely across studies. Downgraded by one level for imprecision (95% confidence interval includes no clinical effect and a clinical effect). By no clinical effect we mean the lower bound of the CI did not surpass our chosen MID threshold of 1.2 on VAS.
dDowngraded by two levels for precision (small sample size (n=172) and confidence interval crosses the line of null effect)..
Summary of findings 3. Perineural versus intravenous dexamethasone.
|
Patient or population: peripheral nerve block Setting: people undergoing upper or lower limb surgery with peripheral nerve block in hospitals in Australia, Belgium, Brazil, Canada and USA Intervention: perineural dexamethasone Comparison: intravenous dexamethasone | ||||
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with intravenous dexamethasone | Risk with perineural dexamethasone | |||
| Duration of sensory block (we included all studies describing duration of sensory block regardless of how it was described) |
The mean duration of sensory block was 20.6 hours | The mean duration of sensory block in the perineural dexamethasone group was 3.13 hours longer (1.68 longer to 4.58 longer) | 720 (9 RCTs) | ⨁⨁⨁◯ MODERATE a |
| Incidence of serious adverse events (we used the NIH definition of adverse events. A serious event includes death, a life‐threatening event that requires hospitalization or prolonged hospitalization, disability or congenital anomaly) |
Please see incidence of serious adverse events in the perineural dexamethasone versus placebo 'Summary of findings' table. | |||
| Postoperative pain intensity at 12 hours (measured using pain scores on an 11‐point VAS) |
The mean postoperative pain score at 12 hours was 2.3 | The mean postoperative pain score at 12 hours in the perineural dexamethasone group was 1.01 points lower (0.50 lower to 1.51 lower) | 217 (3 RCTs) | ⨁⨁◯◯ LOW b |
| Postoperative pain intensity at 24 hours (measured using pain scores on an 11‐point VAS) |
The mean postoperative pain score at 24 hours was 2.9 | The mean postoperative pain score at 24 hours in the perineural dexamethasone group was 0.77 points lower (0.08 lower to 1.47 lower) | 309 (5 RCTs) | ⨁⨁⨁◯ MODERATE c |
| Postoperative pain intensity at 48 hours (measured using pain scores on an 11‐point VAS) |
The mean postoperative pain score at 48 hours was 2.8 | The mean postoperative pain score at 48 hours in the perineural dexamethasone group was 0.13 points higher (0.35 lower to 0.61 higher) | 227 (3 RCTs) | ⨁⨁◯◯ LOW d |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; NIH: National Institute of Health; RCT: randomized controlled trial; VAS: Visual Analogue Scale. | ||||
| GRADE Working Group grades of evidence High‐quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low‐quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
aDowngraded by one level for inconsistency (I2 = 67% and P value for heterogeneity is 0.001).
bDowngraded by one level for risk of bias. Two out of the three studies are at unclear risk of bias. Reasons include unclear random sequence generation, unclear allocation concealment, and selective outcome reporting. Downgraded by one level for imprecision because of a sparse number of participants (n=217) and because the 95% confidence interval includes no clinical effect and a clinical effect. By no clinical effect we mean the lower bound of the CI did not surpass our chosen MID threshold of 1.2 on VAS.
cDowngraded by one level for imprecision (95% confidence interval includes no clinical effect and a clinical effect). By no clinical effect we mean the lower bound of the CI did not surpass our chosen MID threshold of 1.2 on VAS.
dDowngraded by one level for risk of bias. The one study that is at unclear risk of bias contributes half the data for this outcome. Downgraded by one level for imprecision because of a small sample size (n=227).
See: Table 1, Table 2, Table 3
Perineural dexamethasone verus placebo
Primary outcomes
1. Duration of sensory block
Duration of sensory block was defined inconsistently across studies. Definitions included the following.
The interval between administration of block and:
first report of pain (Abdallah 2015; Ganvit 2014; Movafegh 2006; Rahangdale 2014; Yadov 2008);
participant detected complete resolution of block (Dar 2013; Lee 2016; Sakae 2017; Saritas 2014; Viera 2010);
Visual Analogue Scale (VAS) greater than three (Alarasan 2017);
VAS greater than four (Vishnu 2014);
VAS three to six (Kumar 2014);
VAS eight to ten (Talukdar 2013);
first analgesia request or administration (Desmet 2013; Kawanishi 2014).
The interval between onset of sensory block and:
first administration of analgesia after discharge from the recovery room (Cummings 2011);
first report of pain (Bias 2014; Shah 2015; Shaikh 2013).
Duration of sensory block also included the interval between completion of surgery and numerical rating scale (NRS) greater than three (Nallam 2014), and the interval between hospital discharge until VAS greater than three (Tandoc 2011).
The duration of sensory block was significantly longer in the perineural dexamethasone group compared with placebo (MD 6.70 hours, 95% CI 5.54 to 7.85) (Abdallah 2015; Alarasan 2017; Bias 2014; Biradar 2013; Cummings 2011; Dar 2013; Desmet 2013; Ganvit 2014; Jadon 2015; Kawanishi 2014; Kumar 2014; Lee 2016; Movafegh 2006; Nallam 2014; Parrington 2010; Rahangdale 2014; Rosenfeld 2016; Sakae 2017; Saritas 2014; Shah 2015; Shaikh 2013; Talukdar 2013; Tandoc 2011; Viera 2010; Vishnu 2014; Woo 2015; Yadov 2008); (Figure 4), (Analysis 1.1).
4.
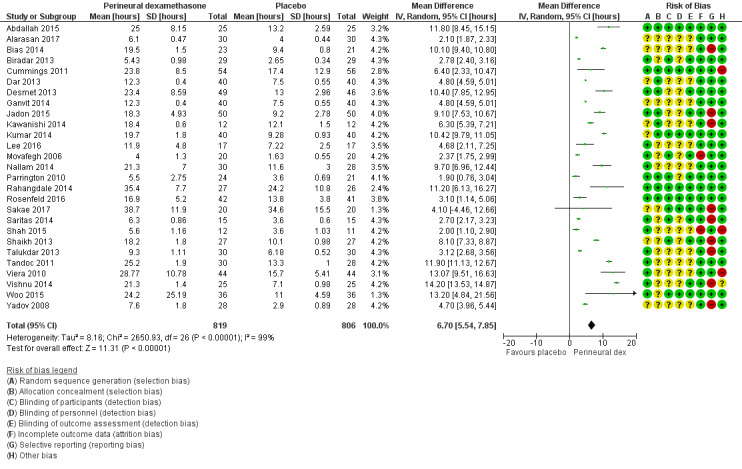
Forest plot of comparison: 1 Duration of sensory block: perineural dexamethasone versus placebo, outcome: 1.1 Duration of sensory block [hours].
1.1. Analysis.
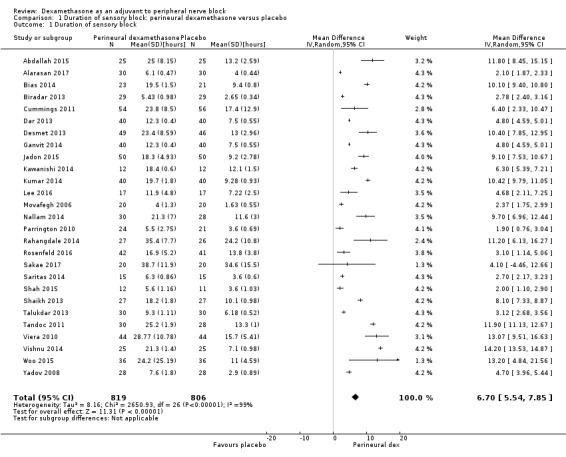
Comparison 1 Duration of sensory block: perineural dexamethasone versus placebo, Outcome 1 Duration of sensory block.
Subgroup analysis
The duration of sensory block was significantly longer in the long‐ versus short‐acting local anaesthetic subgroup (P = 0.00) (Analysis 1.2), and in the high‐ versus low‐dose dexamethasone subgroup (P = 0.06) (Analysis 1.4). There was no significant difference between the remaining subgroups: additives versus no additives (P = 0.72) (Analysis 1.3); or high/unclear versus low risk of bias (P = 0.33) (Analysis 1.5).
1.2. Analysis.
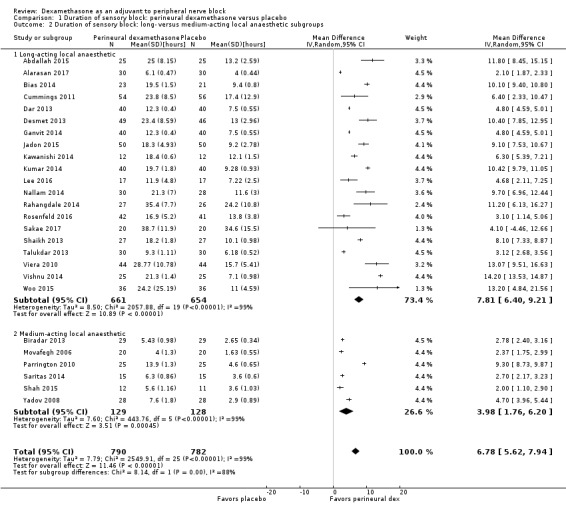
Comparison 1 Duration of sensory block: perineural dexamethasone versus placebo, Outcome 2 Duration of sensory block: long‐ versus medium‐acting local anaesthetic subgroups.
1.4. Analysis.
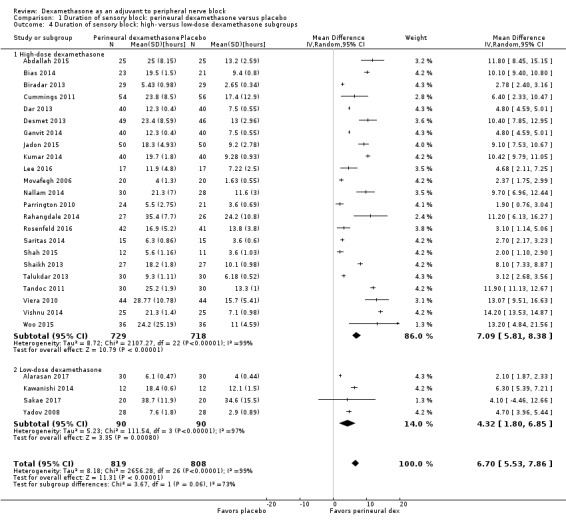
Comparison 1 Duration of sensory block: perineural dexamethasone versus placebo, Outcome 4 Duration of sensory block: high‐ versus low‐dose dexamethasone subgroups.
1.3. Analysis.
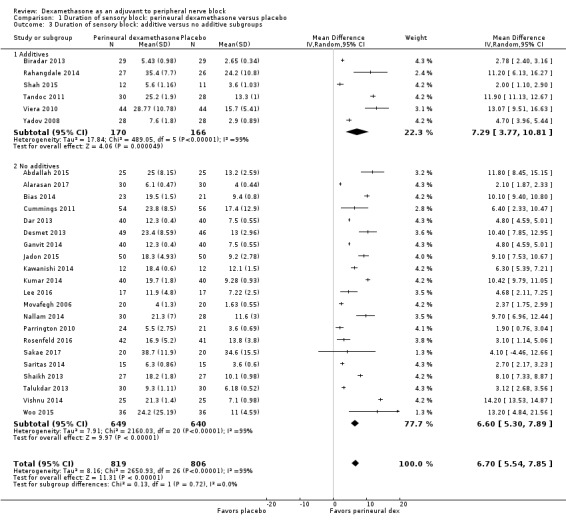
Comparison 1 Duration of sensory block: perineural dexamethasone versus placebo, Outcome 3 Duration of sensory block: additive versus no additive subgroups.
1.5. Analysis.
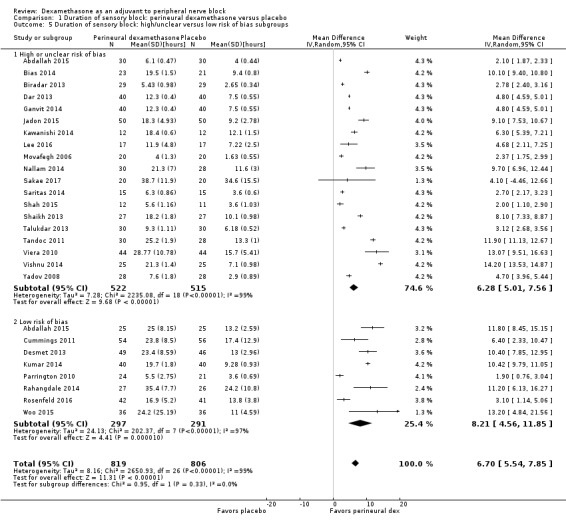
Comparison 1 Duration of sensory block: perineural dexamethasone versus placebo, Outcome 5 Duration of sensory block: high/unclear versus low risk of bias subgroups.
Quality of evidence
We assessed the quality of evidence as low. We downgraded by one level for risk of bias because the majority of studies are at unclear risk of bias and by one level for inconsistency because of considerable heterogeneity (I2 = 99%, P < 0.00001); point estimates vary widely across studies and confidence intervals show minimal overlap. Our subgroup analyses did not explain the observed heterogeneity.
2. Incidence of serious adverse events
We used the National Institutes of Health (NIH) definition of adverse events. A serious event includes death, a life‐threatening event that requires hospitalization or prolonged hospitalization, or disability (NIH 2013). Seven studies reported that they assessed for serious adverse events (Desmet 2015; Jadon 2015; Kim 2012; Kumar 2014; Rosenfeld 2016; Shaikh 2013; Tandoc 2011). Five serious adverse events were reported among three trials (Desmet 2015; Rosenfeld 2016; Tandoc 2011). One block‐related event (pneumothorax) was reported in a study comparing perineural dexamethasone and placebo; however, the group allocation was not reported (Tandoc 2011). The four remaining events were non‐block‐related. In a study comparing intravenous dexamethasone and placebo, one participant in the placebo group developed Chronic Regional Pain syndrome Type I (Desmet 2015). In a study comparing perineural dexamethasone, intravenous dexamethasone and placebo, one participant in the placebo group developed pneumonia and two participants in the placebo group required hospitalization within one week of surgery; one for a bowel infection and one for a fall (Rosenfeld 2016).
Quality of evidence
We assessed the quality of evidence as very low. We downgraded by one level for risk of bias because over half the studies reporting serious adverse events are at unclear risk of bias, and by two levels for imprecision because of an extremely small number of events.
Secondary outcomes
1. Duration of motor block
Duration of motor block was defined inconsistently across studies. Definitions included the following.
The interval between completion of block and:
modified Brommage score of 0 (Vishnu 2014);
return to baseline motor strength in the operative limb (Abdallah 2015; Alarasan 2017; Viera 2010);
complete recovery of motor functions in all distributions (Biradar 2013; Dar 2013; Ganvit 2014; Movafegh 2006; Saritas 2014);
participant was able to lift operated limb (Kumar 2014; Nallam 2014; Tandoc 2011);
participant was able to move great toe (Rahangdale 2014).
The interval between onset of motor block and:
time finger movement was regained (Bias 2014);
complete recovery of motor functions in all distributions (Shah 2015; Shaikh 2013).
Duration of motor block also included the interval between successful block and recovery of all movements in the arm (Sakae 2017).
The duration of motor block was significantly longer in the perineural dexamethasone compared with control (MD 5.87 hours, 95% CI 4.44 to 7.30; participants = 912; studies = 16; I2 = 99) (Abdallah 2015; Bias 2014; Biradar 2013; Dar 2013; Ganvit 2014; Kumar 2014; Movafegh 2006; Nallam 2014; Rahangdale 2014; Sakae 2017; Saritas 2014; Shah 2015; Talukdar 2013; Tandoc 2011; Viera 2010; Vishnu 2014); (Analysis 2.1).
2.1. Analysis.
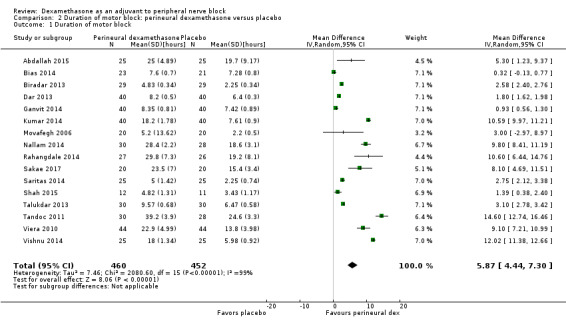
Comparison 2 Duration of motor block: perineural dexamethasone versus placebo, Outcome 1 Duration of motor block.
Subgroup analysis
The duration of motor block was significantly longer in the long‐acting local anaesthetic versus medium‐acting local anaesthesia subgroup (P = 0.00) (Analysis 2.2); however, there was no statistically significant difference between the remaining subgroups: additive versus no additive (P = 0.33) (Analysis 2.3), high‐ versus low‐dose dexamethasone and P = 0.22) (Analysis 2.4), and high/unclear versus low risk of bias (P = 0.41) (Analysis 2.5).
2.2. Analysis.
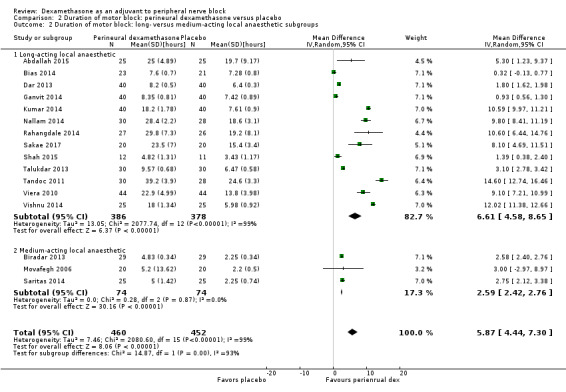
Comparison 2 Duration of motor block: perineural dexamethasone versus placebo, Outcome 2 Duration of motor block: long‐ versus medium‐acting local anaesthetic subgroups.
2.3. Analysis.
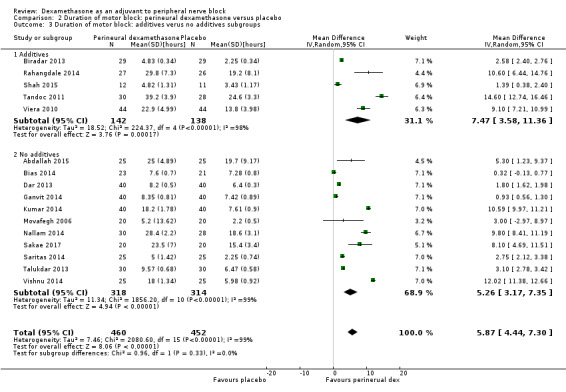
Comparison 2 Duration of motor block: perineural dexamethasone versus placebo, Outcome 3 Duration of motor block: additives verus no additives subgroups.
2.4. Analysis.
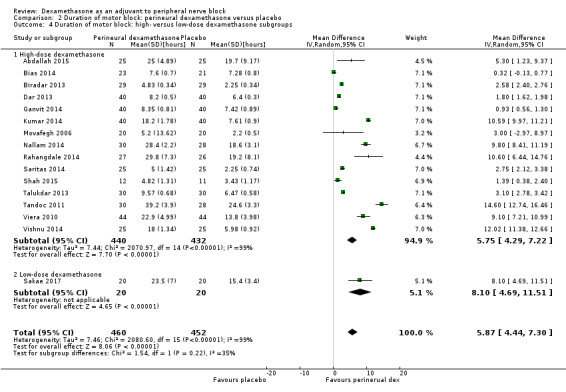
Comparison 2 Duration of motor block: perineural dexamethasone versus placebo, Outcome 4 Duration of motor block: high‐ versus low‐dose dexamethasone subgroups.
2.5. Analysis.
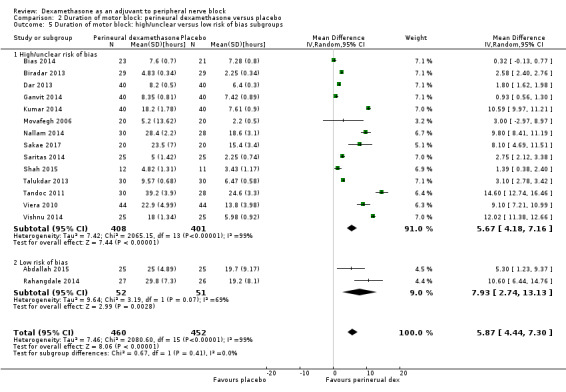
Comparison 2 Duration of motor block: perineural dexamethasone versus placebo, Outcome 5 Duration of motor block: high/unclear versus low risk of bias subgroups.
2. Incidence of mild to moderate adverse events such as nausea/vomiting, pruritus, somnolence, oxygen desaturation, urinary retention, numbness/tingling
Bock‐related adverse events
Ten studies reported that they assessed for block‐related adverse events (Abdallah 2015; Cummings 2011; Desmet 2013; Jadon 2015; Kawanishi 2014; Parrington 2010; Rahangdale 2014; Rosenfeld 2016; Shah 2015; Woo 2015). In one study, the authors reported that numbness/tingling had resolved in all participants at eight weeks after surgery (Rahangdale 2014). None of the other studies described whether block‐related complications had resolved. There was no significant difference between perineural dexamethasone and control in the incidence of overall adverse or each individual adverse event. Overall block‐related adverse events occurred in 97 out of 340 participants in the perineural dexamethasone group versus 81 out of 337 in the control group (risk ratio (RR) 1.17, 95% CI 0.99 to 1.39; participants = 677; studies = 10; I2 = 0%) (Analysis 3.1). The incidence of each event is as follows.
3.1. Analysis.
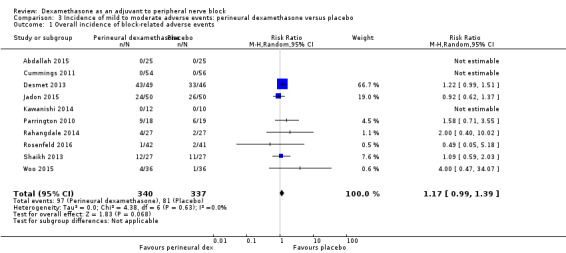
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 1 Overall incidence of block‐related adverse events.
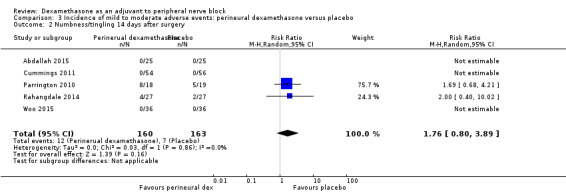
Numbness/tingling 14 days after surgery: 12 out of 160 in the perineural dexamethasone group versus seven out of 163 in the placebo group (RR 1.76, 95% CI 0.80 to 3.89; participants = 323; studies = 5; I2 = 0%); (Abdallah 2015; Cummings 2011; Parrington 2010; Rahangdale 2014; Woo 2015); (Analysis 3.2).
Residual motor block/muscle weakness 24 hours after surgery: five out of 130 in the perineural dexamethasone group versus one out of 129 in the placebo group (RR 4.69, 95% CI 0.57 to 38.68; participants = 259; studies = 3; I2 = 0%) (Cummings 2011; Desmet 2013; Rahangdale 2014); (Analysis 3.3).
Horner syndrome: 47 out of 162 in the perineural dexamethasone group versus 47 out of 159 in the placebo group (RR 0.99, 95% CI 0.73 to 1.36; participants = 321; studies = 4; I2 = 0%) (Desmet 2013; Jadon 2015; Shaikh 2013; Woo 2015); (Analysis 3.4).
Hoarseness: 16 out of 177 in the perineural dexamethasone versus 13 out of 176 in the placebo group (RR 1.23, 95% CI 0.65 to 2.34; participants = 353; studies = 4; I2 = 0%) (Desmet 2013; Jadon 2015; Shaikh 2013; Woo 2015); (Analysis 3.5).
Diaphragmatic paresis: 14 out of 86 in the perineural versus 9 out of 86 in the placebo group (RR 1.46, 95% CI 0.66 to 3.23; participants = 172; studies = 2; I2 = 1%) (Jadon 2015; Woo 2015); (Analysis 3.6).
Dyspnoea: zero out of 138 in the perineural dexamethasone group versus one out of 136 in the placebo group (RR 0.34, 95% CI 0.01 to 8.14; participants = 274; studies = 4; I2 = 100%) (Desmet 2013; Kawanishi 2014; Rosenfeld 2016; Woo 2015); (Analysis 3.7).
Vascular injury: two out of 50 in the perineural dexamethasone group versus one out of 50 in the placebo group (RR 2.00, 95% CI 0.19 to 21.36; participants = 100; studies = 1) (Jadon 2015); (Analysis 3.8).
Cranial nerve 12 palsy: zero out of 42 in the perineural dexamethasone group versus 1 out of 41 in the placebo group (RR 0.33, 95% CI 0.01 to 7.77; participants = 83; studies = 1) (Rosenfeld 2016); (Analysis 3.9)
Bruising at the injection site: one out of 18 in the perineural dexamethasone group versus one out of 19 in the placebo group (RR 1.06, 95% CI 0.07 to 15.64; participants = 37; studies = 1) (Parrington 2010); (Analysis 3.10).
3.2. Analysis.
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 2 Numbness/tingling 14 days after surgery.
3.3. Analysis.
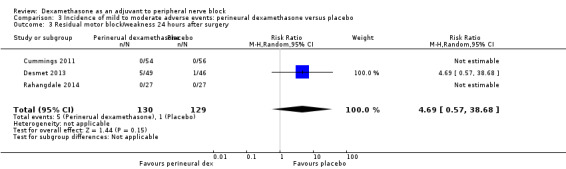
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 3 Residual motor block/weakness 24 hours after surgery.
3.4. Analysis.
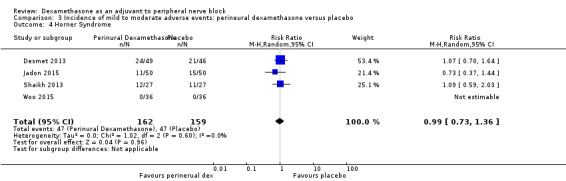
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 4 Horner Syndrome.
3.5. Analysis.
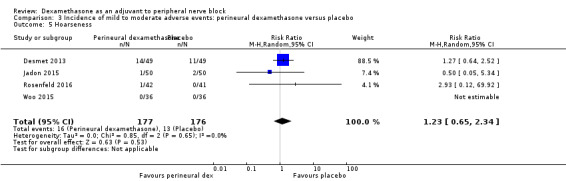
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 5 Hoarseness.
3.6. Analysis.
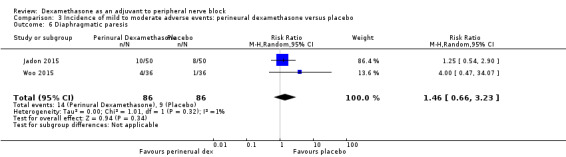
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 6 Diaphragmatic paresis.
3.7. Analysis.
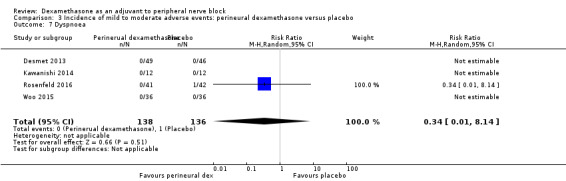
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 7 Dyspnoea.
3.8. Analysis.
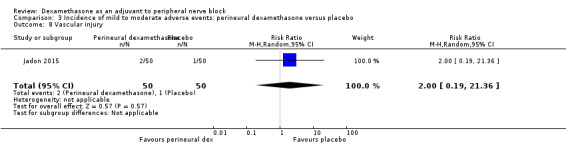
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 8 Vascular injury.
3.9. Analysis.
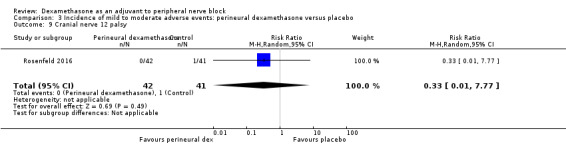
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 9 Cranial nerve 12 palsy.
3.10. Analysis.
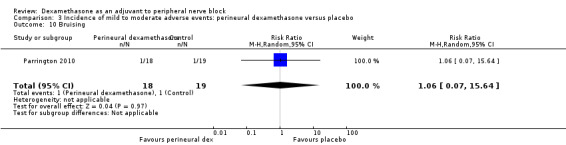
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 10 Bruising.
Non‐block‐related adverse events
In 10 studies, non‐block‐related adverse events were assessed (Abdallah 2015; Dar 2013; Dawson 2016; Golwala 2009; Kawanishi 2014; Parrington 2010; Rosenfeld 2016; Talukdar 2013; Vishnu 2014; Woo 2015). There was no significant difference between perineural dexamethasone and placebo in the incidence overall or individual non‐block‐related events (Analysis 3.1). The overall incidence was 33 out of 313 in the perineural dexamethasone group versus 38 out of 312 in the placebo group (RR 0.76, 95% CI 0.35 to 1.68; participants = 625; studies = 10; I2 = 49%). The incidence of individual events is as follows:
Postoperative nausea and vomiting: 13 out of 293 in the perineural dexamethasone versus 26 out of 292 in the placebo group ((RR 0.55, 95% CI 0.26 to 1.14; participants = 585; studies = 10; I2 = 10%) (Abdallah 2015; Dar 2013; Dawson 2016; Golwala 2009; Kawanishi 2014; Kim 2012; Parrington 2010; Rosenfeld 2016; Vishnu 2014); (Analysis 3.12).
Deep sedation: three out of 30 in the perineural dexamethasone group versus zero out of 30 in the placebo group (RR 7.00, 95% CI 0.38 to 129.93; participants = 60; studies = 1) (Talukdar 2013); (Analysis 3.13).
Dermatological symptoms (pruritus/rash): three out of 42 in the perineural dexamethasone group versus one out of 41 in the placebo group (RR 2.93, 95% CI 0.32 to 27.02; participants = 83; studies = 1) (Rosenfeld 2016); (Analysis 3.14).
Syncope/fainting: two out of 42 in the perineural dexamethasone group versus one out of 41 in the placebo group (RR 1.95, 95% CI 0.18 to 20.71; participants = 83; studies = 1) (Rosenfeld 2016); (Analysis 3.15).
Bradycardia: two out of 30 in the perineural dexamethasone group versus three out of 30 in the placebo group; (RR 0.67, 95% CI 0.12 to 3.71; participants = 60; studies = 1; I2 = 0%); (Talukdar 2013); (Analysis 3.16).
Hypotension: four out of 70 in the perineural dexamethasone group versus six out of 70 in the control group; (RR 0.67, 95% CI 0.21 to 2.13; participants = 140; studies = 2; I2 = 0%); (Dar 2013; Talukdar 2013); Analysis 3.17
Each of the following outcomes occurred in one out of 42 in the perineural dexamethasone group versus zero out of 41 in the placebo group (RR 2.93, 95% CI 0.12 to 69.92; participants = 83; studies = 1): headache, 10‐pound fluid gain over 24 hours, diarrhoea, frequent urination, and muscle soreness (Rosenfeld 2016); (Analysis 3.18).
3.12. Analysis.
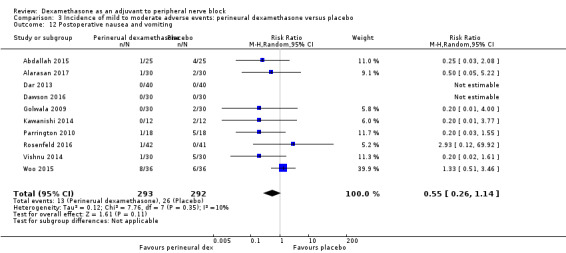
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 12 Postoperative nausea and vomiting.
3.13. Analysis.
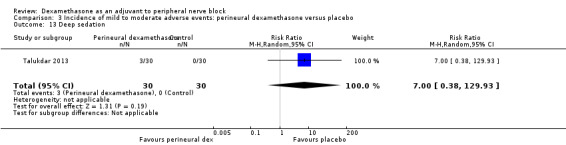
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 13 Deep sedation.
3.14. Analysis.
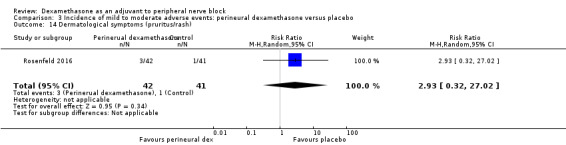
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 14 Dermatological symptoms (pruritus/rash).
3.15. Analysis.
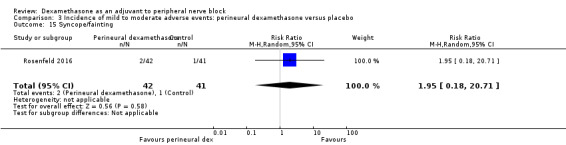
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 15 Syncope/fainting.
3.16. Analysis.
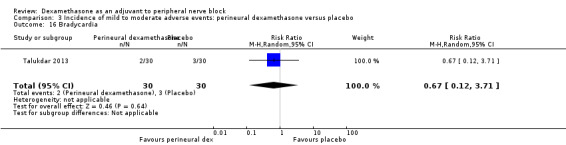
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 16 Bradycardia.
3.17. Analysis.
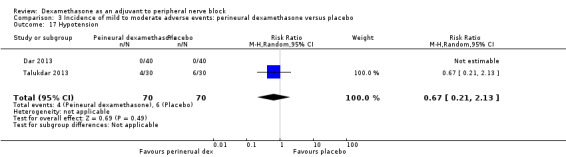
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 17 Hypotension.
3.18. Analysis.
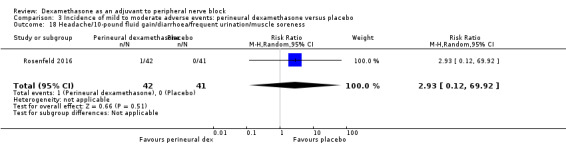
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 18 Headache/10‐pound fluid gain/diarrhoea/frequent urination/muscle soreness.
3a Postoperative pain intensity at 12 hours
Postoperative pain scores at 12 hours were significantly lower in the dexamethasone group compared with placebo (MD ‐2.08, 95% CI ‐2.63 to ‐1.52; participants = 257; studies = 5; I2 = 62%) (Kim 2012; Rosenfeld 2016; Sakae 2017; Shah 2015; Woo 2015); (Figure 5), (Analysis 4.1).
5.
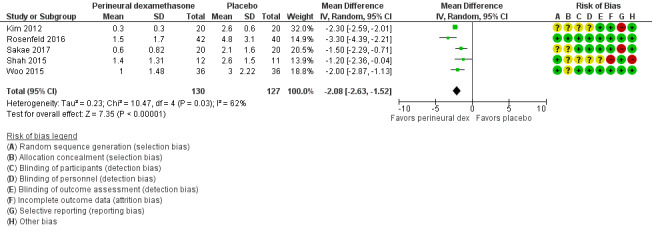
Forest plot of comparison: 4 Postoperative pain intensity at 12 hours: perineural dexamethasone versus placebo, outcome: 4.1 Postoperative pain intensity at12 hours.
4.1. Analysis.
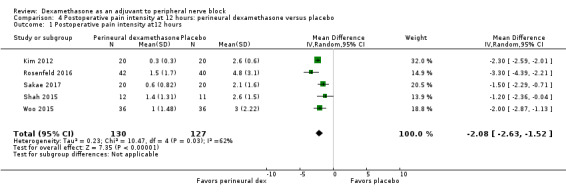
Comparison 4 Postoperative pain intensity at 12 hours: perineural dexamethasone versus placebo, Outcome 1 Postoperative pain intensity at12 hours.
Subgroup analysis
There was no significant difference in effect size between any of the subgroups: long‐ versus medium‐acting local anaesthetic (P = 0.13) (Analysis 4.2); additive versus no additive (P = 0.12) (Analysis 4.3); high‐ versus low‐dose dexamethasone (P = 0.79) (Analysis 4.4); or high/unclear versus low risk of bias (P = 0.28) (Analysis 4.5).
4.2. Analysis.
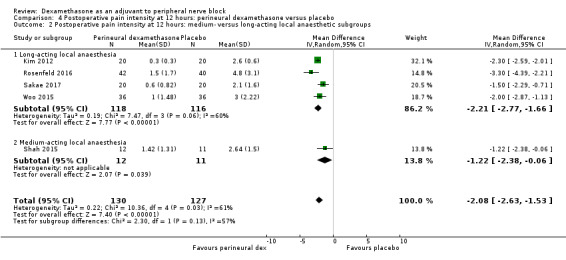
Comparison 4 Postoperative pain intensity at 12 hours: perineural dexamethasone versus placebo, Outcome 2 Postoperative pain intensity at 12 hours: medium‐ versus long‐acting local anaesthetic subgroups.
4.3. Analysis.
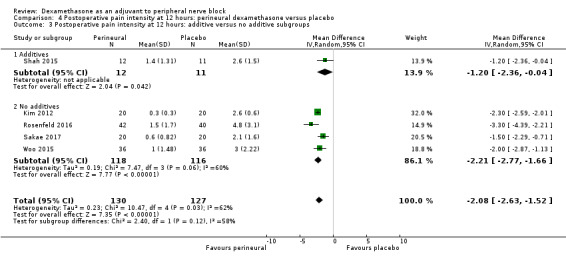
Comparison 4 Postoperative pain intensity at 12 hours: perineural dexamethasone versus placebo, Outcome 3 Postoperative pain intensity at 12 hours: additive versus no additive subgroups.
4.4. Analysis.
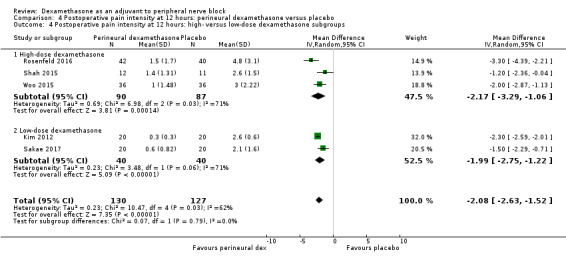
Comparison 4 Postoperative pain intensity at 12 hours: perineural dexamethasone versus placebo, Outcome 4 Postoperative pain intensity at 12 hours: high‐ versus low‐dose dexamethasone subgroups.
4.5. Analysis.
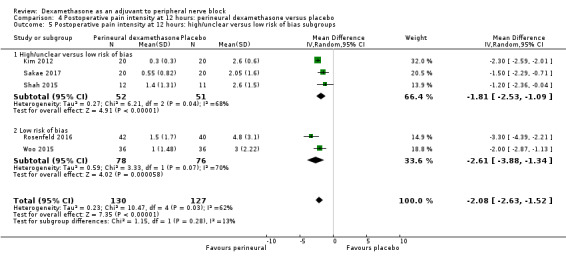
Comparison 4 Postoperative pain intensity at 12 hours: perineural dexamethasone versus placebo, Outcome 5 Postoperative pain intensity at 12 hours: high/unclear versus low risk of bias subgroups.
Quality of evidence
We assessed the quality of evidence as very low. We downgraded by one level for risk of bias because three out of five of the studies are at unclear risk of bias; we downgraded by one level for inconsistency because of substantial heterogeneity (I2 = 62%, P = 0.03). Our subgroup analyses did not explain observed heterogeneity. We also downgraded by one level for imprecision. For continuous outcomes, Cochrane guidelines suggest downgrading if fewer than 400 participants.
3b Postoperative pain intensity at 24 hours
Postoperative pain scores at 24 hours were significantly lower in the dexamethasone group compared with placebo (MD ‐1.63, 95% CI ‐2.34 to ‐0.93; participants = 469; studies = 9; I2 = 79%) (Abdallah 2015; Dawson 2016; Kim 2012; Parrington 2010; Rahangdale 2014; Rosenfeld 2016; Sakae 2017; Viera 2010; Woo 2015); (Figure 6), (Analysis 5.1).
6.
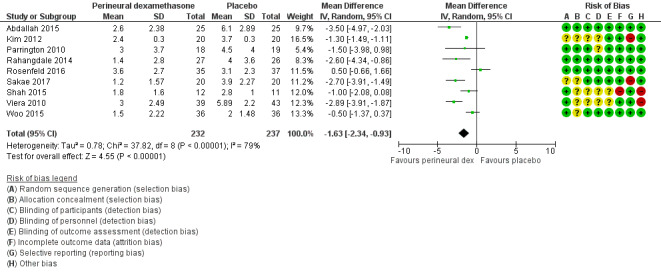
Forest plot of comparison: 5 Postoperative pain intensity at 24 hours: perineural dexamethasone versus placebo, outcome: 5.1 Postoperative pain intensity at 24 hours.
5.1. Analysis.
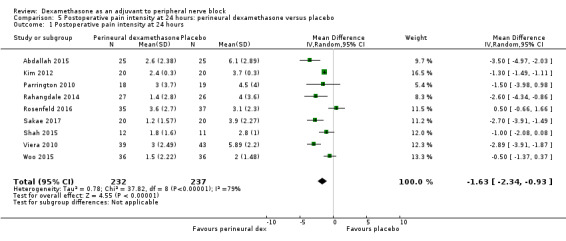
Comparison 5 Postoperative pain intensity at 24 hours: perineural dexamethasone versus placebo, Outcome 1 Postoperative pain intensity at 24 hours.
Subgroup analysis
Three was no significant difference in effect size between any of the subgroups: long‐ versus medium‐acting local anaesthetic (P = 0.31) (Analysis 5.2); additive versus no additive (P = 0.37) (Analysis 5.3); high‐ versus low‐dose dexamethasone (P = 0.76) (Analysis 5.4); and high/unclear versus low risk of bias (P = 0.60) (Analysis 5.5).
5.2. Analysis.
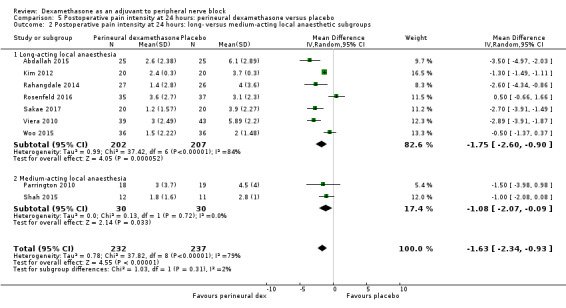
Comparison 5 Postoperative pain intensity at 24 hours: perineural dexamethasone versus placebo, Outcome 2 Postoperative pain intensity at 24 hours: long‐ versus medium‐acting local anaesthetic subgroups.
5.3. Analysis.
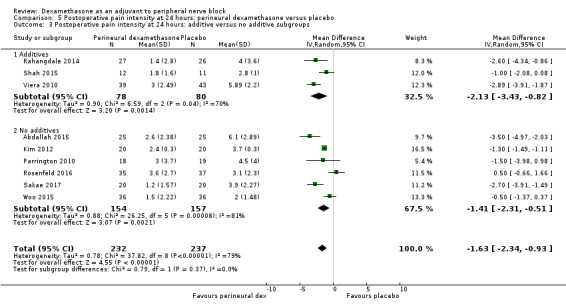
Comparison 5 Postoperative pain intensity at 24 hours: perineural dexamethasone versus placebo, Outcome 3 Postoperative pain intensity at 24 hours: additive versus no additive subgroups.
5.4. Analysis.
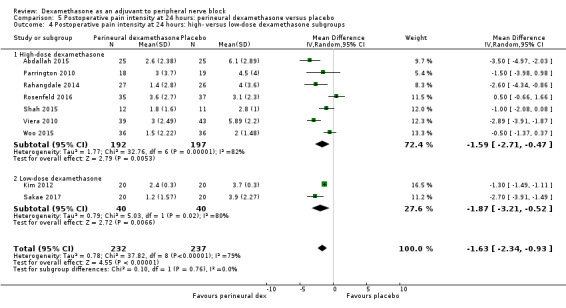
Comparison 5 Postoperative pain intensity at 24 hours: perineural dexamethasone versus placebo, Outcome 4 Postoperative pain intensity at 24 hours: high‐ versus low‐dose dexamethasone subgroups.
5.5. Analysis.
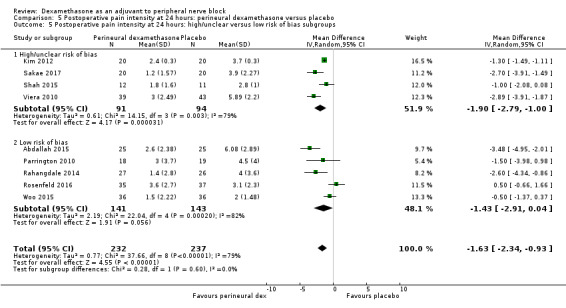
Comparison 5 Postoperative pain intensity at 24 hours: perineural dexamethasone versus placebo, Outcome 5 Postoperative pain intensity at 24 hours: high/unclear versus low risk of bias subgroups.
Quality of evidence
We assessed the quality of evidence to be low. We downgraded by one level for inconsistency because of considerable heterogeneity (I2 = 80% and P < 0.00001) not explained by subgroup analyses and by one level for imprecision because the confidence interval includes both no clinical effect (minimally important difference (MID) less than 1.2) and clinical effect (MID greater than 1.2).
3c Postoperative pain intensity at 48 hours
There was no significant difference in postoperative pain scores at 48 hours between perineural dexamethasone and placebo (MD ‐0.61, 95% CI ‐1.24 to 0.03; participants = 296; studies = 4; I2 = 41%) (Rahangdale 2014; Rosenfeld 2016; Viera 2010; Woo 2015); (Analysis 6.1).
6.1. Analysis.
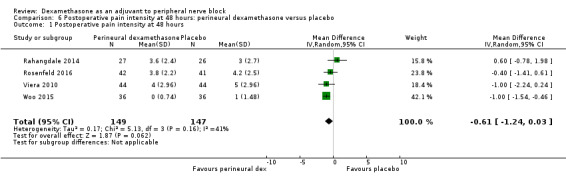
Comparison 6 Postoperative pain intensity at 48 hours: perineural dexamethasone versus placebo, Outcome 1 Postoperative pain intensity at 48 hours.
Subgroup analysis
There was no statistically significant difference in effect size between the additive and no additive subgroups (P = 0.45) (Analysis 6.2); and the high/unclear risk of bias subgroups (P = 0.47) (Analysis 6.3). In all four studies, long‐acting local anaesthetic and high‐dose dexamethasone were used.
6.2. Analysis.
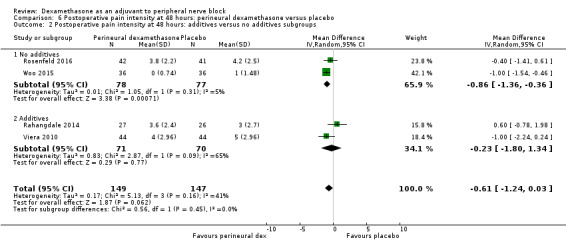
Comparison 6 Postoperative pain intensity at 48 hours: perineural dexamethasone versus placebo, Outcome 2 Postoperative pain intensity at 48 hours: additives versus no additives subgroups.
6.3. Analysis.
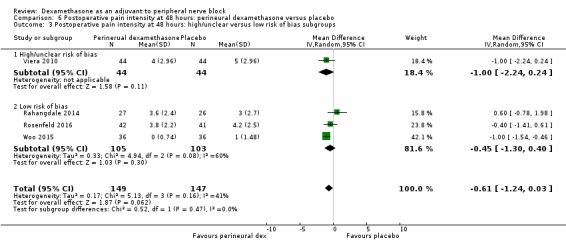
Comparison 6 Postoperative pain intensity at 48 hours: perineural dexamethasone versus placebo, Outcome 3 Postoperative pain intensity at 48 hours: high/unclear versus low risk of bias subgroups.
Quality of evidence
We assessed the quality of evidence to be low. We downgraded by two levels for imprecision because the confidence interval includes both no clinical effect (MID less than 1.2 on VAS) and clinical effect (MID greater than 1.2 on VAS).
4a Postoperative opioid consumption at 12 hours
No studies evaluated postoperative opioid consumption at 12 hours.
4b Postoperative opioid consumption at 24 hour
Cummulative opioid administration at 24 hours was reported in six studies. Reasons for opioid administration varied across studies and included VAS greater than four (Abdallah 2015), and "as needed" (Dawson 2016; Rahangdale 2014; Rosenfeld 2016). No criteria for opioid administration was provided in the remaining two studies (Parrington 2010; Viera 2010). Postoperative opioid consumption (oral morphine equivalents) at 24 hours in the perineural dexamethasone group was significantly lower compared with placebo (MD 19.25 mg, 95% CI 5.99 to 32.51; participants = 380; studies = 6; I2 = 88%) (Analysis 7.1).
7.1. Analysis.
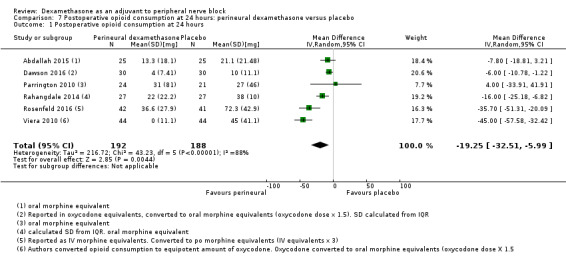
Comparison 7 Postoperative opioid consumption at 24 hours: perineural dexamethasone versus placebo, Outcome 1 Postoperative opioid consumption at 24 hours.
Subgroup analysis
There was no significant difference in effect size between the long‐ versus medium‐acting local anaesthetic subgroups (P = 0.22) or the additive versus no additive subgroups (P = 0.28). Opioid consumption was significantly higher in the high/unclear risk of bias subgroup (P = 0.00001) (Analysis 7.2; Analysis 7.3; Analysis 7.4). In all six studies, high‐dose dexamethasone was used.
7.2. Analysis.
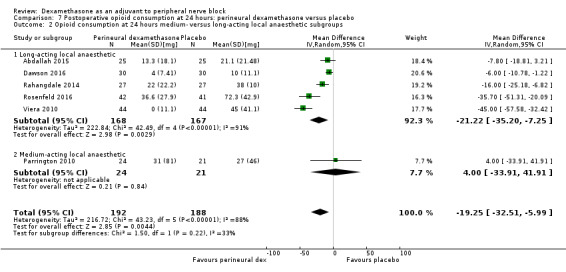
Comparison 7 Postoperative opioid consumption at 24 hours: perineural dexamethasone versus placebo, Outcome 2 Opioid consumption at 24 hours medium‐ versus long‐acting local anaesthetic subgroups.
7.3. Analysis.
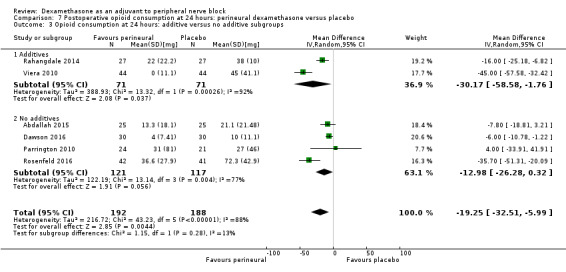
Comparison 7 Postoperative opioid consumption at 24 hours: perineural dexamethasone versus placebo, Outcome 3 Opioid consumption at 24 hours: additive versus no additive subgroups.
7.4. Analysis.
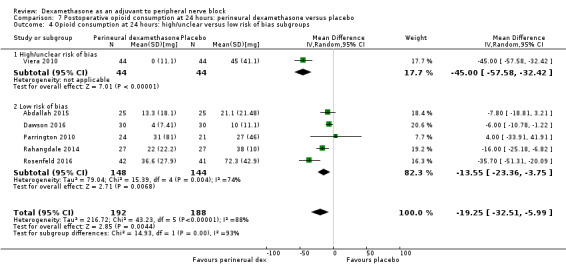
Comparison 7 Postoperative opioid consumption at 24 hours: perineural dexamethasone versus placebo, Outcome 4 Opioid consumption at 24 hours: high/unclear versus low risk of bias subgroups.
4c Postoperative opioid consumption at 48 hours
No studies reported cumulative opioid consumption at 48 hours.
5 Participant satisfaction with pain control
There was no statistically significant difference in satisfaction scores on an 11‐point VAS between perineural dexamethasone and placebo (MD 0.83, 95% CI ‐0.05 to 1.71; participants = 224; studies = 4; I2 = 0%) (Analysis 8.1)
8.1. Analysis.
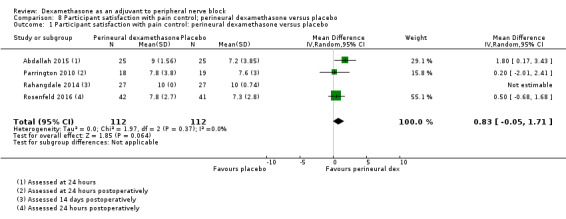
Comparison 8 Participant satisfaction with pain control; perineural dexamethasone versus placebo, Outcome 1 Participant satisfaction with pain control: perineural dexamethasone versus placebo.
Intravenous dexamethasone versus placebo
Primary outcomes
1. Duration of sensory block
Duration of sensory block was defined inconsistently across six studies. Definitions included the following.
The interval between administration of block and:
first report of pain (Abdallah 2015; Rahangdale 2014);
participant detected complete resolution of block (Rosenfeld 2016);
first analgesia request or administration (Desmet 2013; Desmet 2015; Kawanishi 2014).
Duration of sensory block also included the interval between onset of sensory block and first analgesic request (Chalifoux 2017), and the time interval between successful block and complete recovery of all senses in the operative limb (Sakae 2017).
Duration of sensory block was significantly longer in the intravenous group compared with placebo (MD 6.21, 95% CI 3.53 to 8.88; participants = 499; studies = 8; I2 = 88%); (Figure 7), (Analysis 9.1).
7.
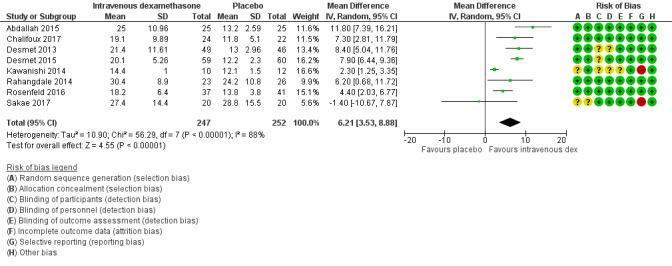
Forest plot of comparison: 9 Duration of sensory block: intravenous dexamethasone versus placebo , outcome: 9.1 Duration of sensory block.
9.1. Analysis.
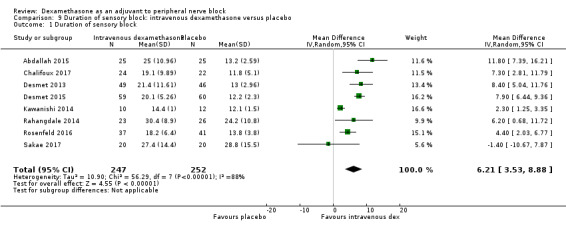
Comparison 9 Duration of sensory block: intravenous dexamethasone versus placebo, Outcome 1 Duration of sensory block.
Subgroup analysis
The duration of sensory block was significantly longer in the high‐dose versus low‐dose dexamethasone subgroup (P = 0.00) Analysis 9.3), and the low risk of bias versus high risk of bias subgroup (P = 0.00); Analysis 9.4). There was no statistically significant difference in the duration of sensory block between the additive and no additive subgroups (P = 1.0) (Analysis 9.2). In all studies, long‐acting local anaesthetic was used.
9.3. Analysis.
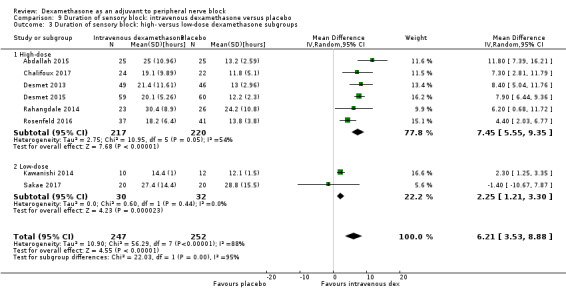
Comparison 9 Duration of sensory block: intravenous dexamethasone versus placebo, Outcome 3 Duration of sensory block: high‐ versus low‐dose dexamethasone subgroups.
9.4. Analysis.
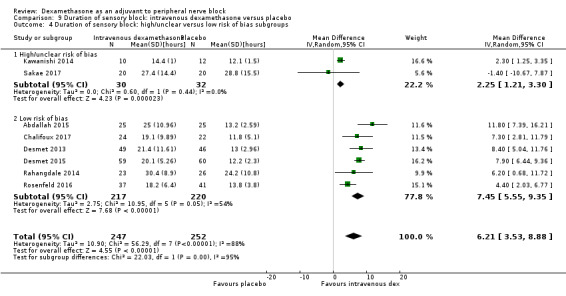
Comparison 9 Duration of sensory block: intravenous dexamethasone versus placebo, Outcome 4 Duration of sensory block: high/unclear versus low risk of bias subgroups.
9.2. Analysis.
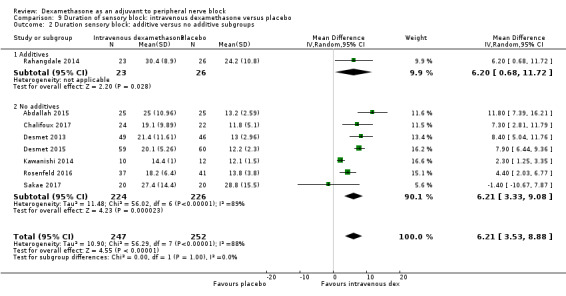
Comparison 9 Duration of sensory block: intravenous dexamethasone versus placebo, Outcome 2 Duration sensory block: additive versus no additive subgroups.
Quality of evidence
We assessed the quality of evidence as moderate. We downgraded by one level for inconsistency because of considerable heterogeneity (I2 = 88%, P < 0.00001). Our subgroup analyses did not explain the observed heterogeneity.
2. Incidence of severe adverse events
See incidence of severe events in the perineural dexamethasone versus placebo section.
Secondary outcomes
1. Duration of motor block
Duration of motor block was defined as the interval between completion of block until return to baseline motor strength in the operative limb (Abdallah 2015), the time interval between successful block and complete recovery of all movements of the arm (Sakae 2017), or when the participant was able to move the great toe (Rosenfeld 2016). Duration of motor block was significantly longer in the intravenous dexamethasone group compared with placebo (MD 5.04 hours, 95% CI 3.07 to 7.00; participants = 139; studies = 3; I2 = 27%) (Analysis 10.1).
10.1. Analysis.
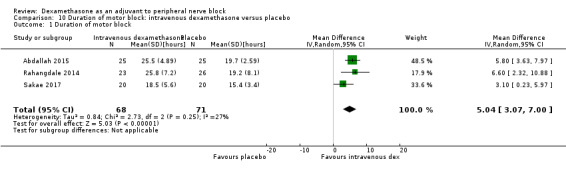
Comparison 10 Duration of motor block: intravenous dexamethasone versus placebo, Outcome 1 Duration of motor block.
Subgroup analysis
There was no significant difference in the duration of motor block between the additive and no additive subgroup (P = 0.46) (Analysis 10.2); in the high‐ versus low‐dose subgroups (P = 0.11) (Analysis 10.3); or in the high versus low risk of bias subgroups (P = 0.11) (Analysis 10.4). In all three studies, long‐acting local anaesthetic was used.
10.2. Analysis.
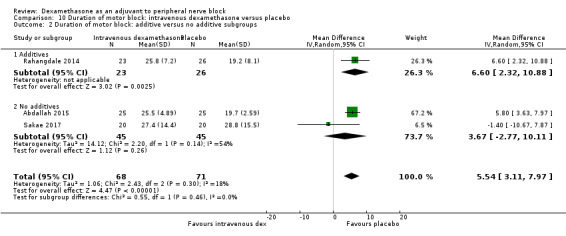
Comparison 10 Duration of motor block: intravenous dexamethasone versus placebo, Outcome 2 Duration of motor block: additive versus no additive subgroups.
10.3. Analysis.
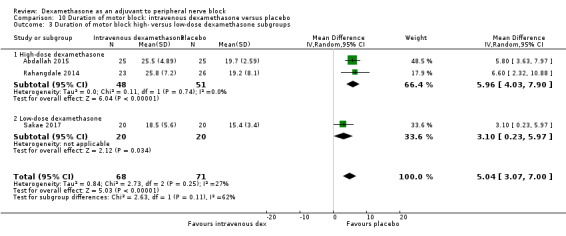
Comparison 10 Duration of motor block: intravenous dexamethasone versus placebo, Outcome 3 Duration of motor block high‐ versus low‐dose dexamethasone subgroups.
10.4. Analysis.
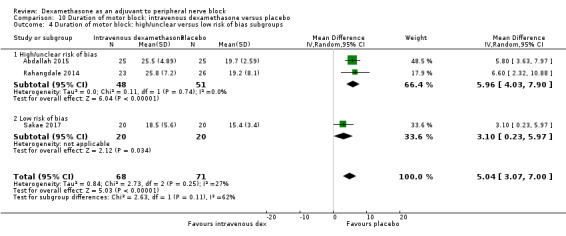
Comparison 10 Duration of motor block: intravenous dexamethasone versus placebo, Outcome 4 Duration of motor block: high/unclear versus low risk of bias subgroups.
2. Incidence of mild to moderate adverse events such as nausea/vomiting, pruritus, somnolence, oxygen desaturation, urinary retention, numbness/tingling
Block‐related adverse events
Five studies reported that they assessed for block‐related adverse events. There was no significant difference between intravenous dexamethasone and control in the overall incidence of events or each individual event. The incidence of overall block‐related events was 75 out of 195 in the intravenous dexamethasone group versus 70 out of 198 in the control group (RR 1.09, 95% CI 0.69 to 1.70; I2 = 59%).
The incidence of each adverse event is as follows.
Numbness/tingling 14 days after surgery: three out of 49 in the intravenous group versus two out of 52 in the placebo group (RR 1.69, 95% CI 0.31 to 9.26; participants = 101; studies = 2; I2 = 0%) (Abdallah 2015; Rahangdale 2014); (Analysis 11.2).
Residual motor block/muscle weakness 24 hours after surgery: nine out of 133 in the intravenous dexamethasone group versus three out of 132 in the placebo group (RR 2.68, 95% CI 0.80 to 8.90; studies = 3; I2 = 0%) (Desmet 2013; Desmet 2015; Rahangdale 2014); (Analysis 11.3).
Horner syndrome: 38 out of 109 in the intravenous dexamethasone group versus 41 out of 105 in the placebo group (RR 0.89, 95% CI 0.63 to 1.26; participants = 214; studies = 2) (Desmet 2013; Desmet 2015); (Analysis 11.4).
Hoarseness: 16 out of 109 in the intravenous versus 17 out of 106 in the placebo group (RR 0.88, 95% CI 0.45 to 1.71; participants = 215; studies = 2; I2 = 8%) (Desmet 2013; Desmet 2015); (Analysis 11.5).
Dyspnoea: one out of 107 in the intravenous dexamethasone group versus three out of 112 in the placebo group (RR 0.63, 95% CI 0.11 to 3.74; participants = 219; studies = 3; I2 = 0%) (Desmet 2015; Kawanishi 2014; Rosenfeld 2016); (Analysis 11.6).
Cranial nerve 12 palsy: zero out of 37 in the intravenous group versus one out of 41 in the placebo group (RR 0.37, 95% CI 0.02 to 8.77; participants = 78; studies = 1; I2 = 0%) (Rosenfeld 2016); (Analysis 11.7).
11.2. Analysis.
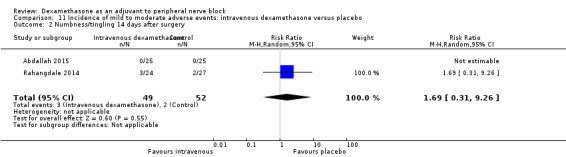
Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 2 Numbness/tingling 14 days after surgery.
11.3. Analysis.
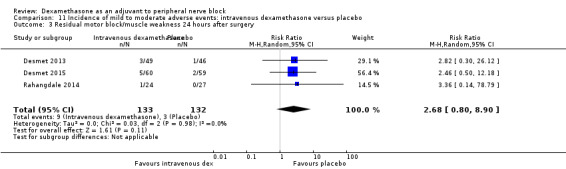
Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 3 Residual motor block/muscle weakness 24 hours after surgery.
11.4. Analysis.
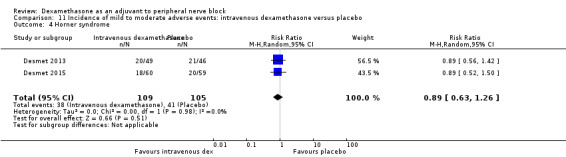
Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 4 Horner syndrome.
11.5. Analysis.
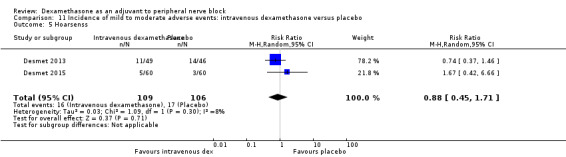
Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 5 Hoarsenss.
11.6. Analysis.
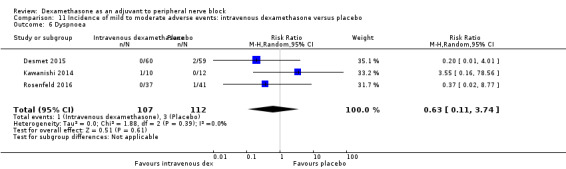
Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 6 Dyspnoea.
11.7. Analysis.
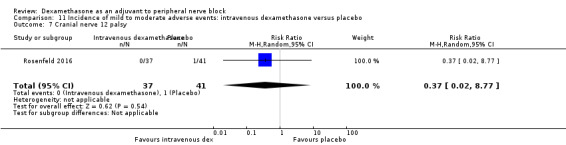
Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 7 Cranial nerve 12 palsy.
Non block‐related adverse events
Five studies reported that they assessed for non‐block‐related adverse events (Abdallah 2015; Chalifoux 2017; Dawson 2016; Kawanishi 2014; Rosenfeld 2016); (Analysis 11.8). There was no significant difference between intravenous dexamethasone and placebo (8 out of 128 in the intravenous group versus 5 out of 122 in the placebo group (RR 1.23, 95% CI 0.38 to 3.97; participants = 258; studies = 5; I2 = 0%).
11.8. Analysis.
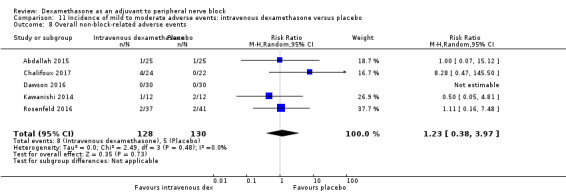
Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 8 Overall non‐block‐related adverse events.
Postoperative nausea and vomiting: two out of 67 in the intravenous group versus three out of 67 in the placebo group (RR 0.66, 95% CI 0.12 to 3.78; participants = 134; studies = 3; I2 = 0%) (Abdallah 2015; Dawson 2016; Kawanishi 2014); (Analysis 11.9).
Dermatological symptoms (pruritus/rash): four out of 61 in the intravenous dexamethasone group versus one out of 63 in the placebo group (RR 1.88, 95% CI 0.09 to 40.62; participants = 124; studies = 2; I2 = 52%) (Chalifoux 2017; Rosenfeld 2016); (Analysis 11.10).
Each of the following adverse events occurred in one out of 37 in the intravenous dexamethasone group versus zero out of 41 in the placebo group: dizziness, wrist, hand or finger pain, constipation (RR 0.37, 95% CI 0.02 to 8.77; participants = 78; studies = 1) (Rosenfeld 2016); (Analysis 11.11).
11.9. Analysis.
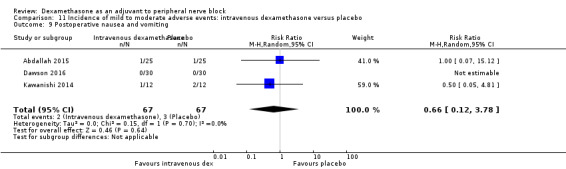
Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 9 Postoperative nausea and vomiting.
11.10. Analysis.
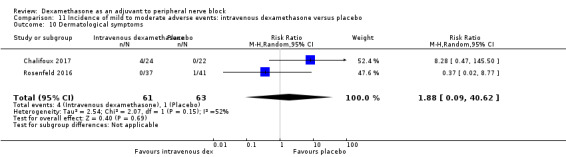
Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 10 Dermatological symptoms.
11.11. Analysis.
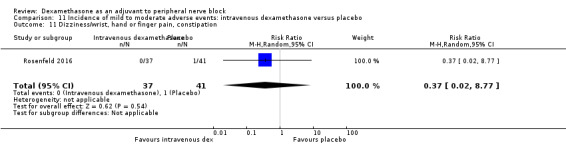
Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 11 Dizziness/wrist, hand or finger pain, constipation.
3a. Postoperative pain intensity at 12 hours
Pain scores were significantly lower in the intravenous dexamethasone group compared with placebo (MD ‐1.24, 95% CI ‐2.44 to ‐0.04; participants = 162; studies = 3; I2 = 61%) (Chalifoux 2017; Rosenfeld 2016; Sakae 2017); (Figure 8), (Analysis 12.1).
8.
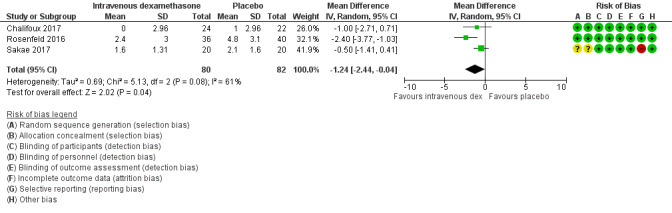
Forest plot of comparison: 12 Postoperative pain intensity at 12 hours: intravenous dexamethasone versus placebo, outcome: 12.1 Postoperative pain intensity at 12 hours.
12.1. Analysis.
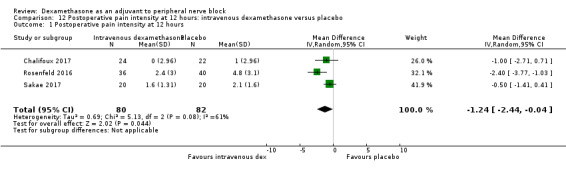
Comparison 12 Postoperative pain intensity at 12 hours: intravenous dexamethasone versus placebo, Outcome 1 Postoperative pain intensity at 12 hours.
Subgroup analysis
There was no difference in effect size between the low‐ and high‐dose dexamethasone subgroups (P = 0.12) (Analysis 21.2); or between the high/unclear versus low risk of bias subgroups (P = 0.12) (Analysis 22.3). In all three studies, long‐acting local anaesthetic was used, and none used additives.
21.2. Analysis.
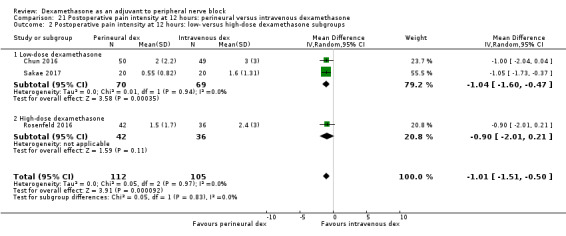
Comparison 21 Postoperative pain intensity at 12 hours: perineural versus intravenous dexamethasone, Outcome 2 Postoperative pain intensity at 12 hours: low‐ versus high‐dose dexamethasone subgroups.
22.3. Analysis.
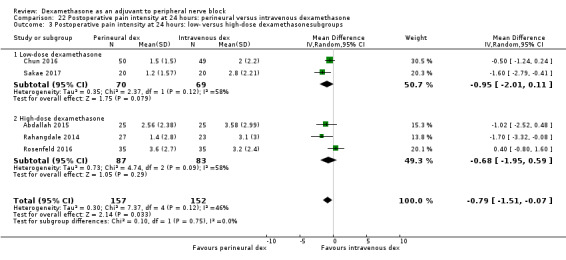
Comparison 22 Postoperative pain intensity at 24 hours: perineural versus intravenous dexamethasone, Outcome 3 Postoperative pain intensity at 24 hours: low‐ versus high‐dose dexamethasonesubgroups.
Quality of evidence
We assessed the quality of evidence to be low. We downgraded by one level due to moderate heterogeneity (I2= 61%, P = 0.08) not explained by subgroup analyses, and by one level for imprecision because the CI includes both no clinical effect (MID less than 1.2 on VAS) and clinical effect (MID greater than 1.2 on VAS).
3b. Postoperative pain intensity at 24 hours
Pain scores were significantly lower in the intravenous dexamethasone group compared with placebo (MD ‐1.26, 95% CI ‐2.23 to ‐0.29; participants = 257; studies = 5; I2 = 65%) (Abdallah 2015; Chalifoux 2017; Rahangdale 2014; Rosenfeld 2016; Sakae 2017); (Figure 9), (Analysis 13.1).
9.
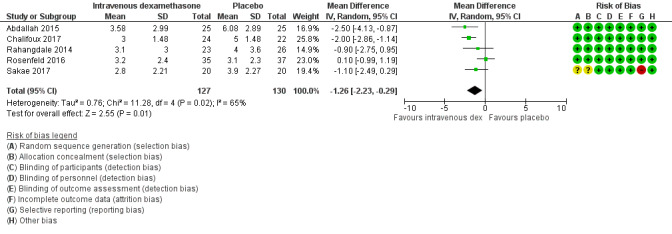
Forest plot of comparison: 13 Postoperative pain intensity at 24 hours: intravenous dexamethasone versus placebo, outcome: 13.1 Postoperative pain intensity at 24 hours.
13.1. Analysis.
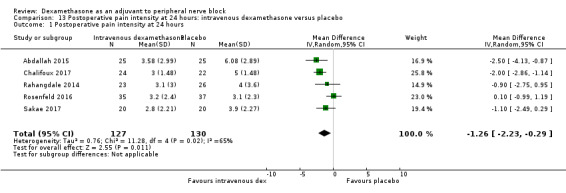
Comparison 13 Postoperative pain intensity at 24 hours: intravenous dexamethasone versus placebo, Outcome 1 Postoperative pain intensity at 24 hours.
Subgroup analysis
There was no significant difference in effect size between the additive or no additive subgroups (P = 0.70) (Analysis 13.2); the high‐ versus low‐dose dexamethasone subgroups (P = 0.83) (Analysis 13.3); or the high/unclear versus low risk of bias subgroups (P = 0.83) (Analysis 13.4). In all studies, long‐acting local anaesthetic was used.
13.2. Analysis.
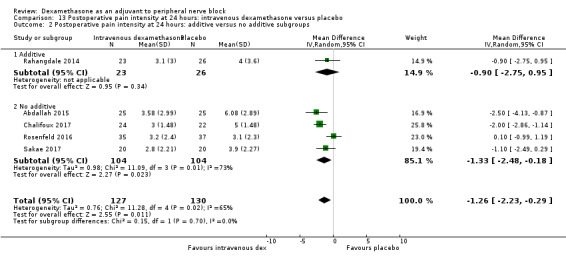
Comparison 13 Postoperative pain intensity at 24 hours: intravenous dexamethasone versus placebo, Outcome 2 Postoperative pain intensity at 24 hours: additive versus no additive subgroups.
13.3. Analysis.
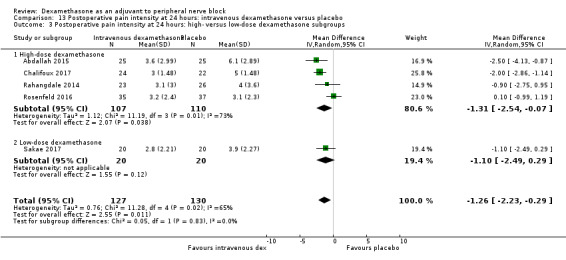
Comparison 13 Postoperative pain intensity at 24 hours: intravenous dexamethasone versus placebo, Outcome 3 Postoperative pain intensity at 24 hours: high‐ versus low‐dose dexamethasone subgroups.
13.4. Analysis.
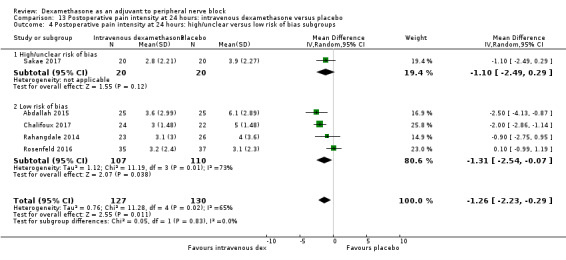
Comparison 13 Postoperative pain intensity at 24 hours: intravenous dexamethasone versus placebo, Outcome 4 Postoperative pain intensity at 24 hours: high/unclear versus low risk of bias subgroups.
Quality of evidence
We assessed the quality of evidence to be low. We downgraded by one level for inconsistency because of considerable heterogeneity (I2 = 65 %, P = 0.02) not explained by subgroup analyses, and by one level for imprecision because the CI includes both no clinical effect (MID less than 1.2 on VAS) and clinical effect (MID greater than 1.2 on VAS).
3c. Postoperative pain intensity at 48 hours
There was no significant difference in postoperative pain intensity at 48 hours between intravenous dexamethasone and placebo (MD ‐0.18, 95% CI ‐0.80 to 0.44; participants = 172; studies = 3; I2 = 0%) (Chalifoux 2017; Rahangdale 2014; Rosenfeld 2016); (Analysis 14.1).
14.1. Analysis.
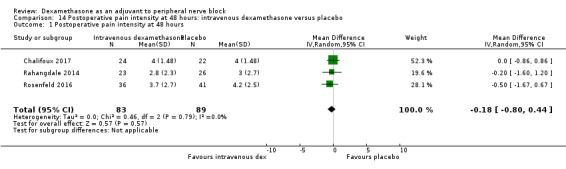
Comparison 14 Postoperative pain intensity at 48 hours: intravenous dexamethasone versus placebo, Outcome 1 Postoperative pain intensity at 48 hours.
Subgroup analysis
There was no significant difference in effect size between the additive and no additive subgroups (P = 0.97) (Analysis 14.2). In all studies, long‐acting local anaesthetic and high‐dose dexamethasone were used, and all were at low risk of bias.
14.2. Analysis.
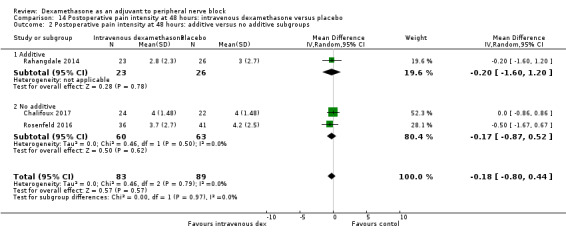
Comparison 14 Postoperative pain intensity at 48 hours: intravenous dexamethasone versus placebo, Outcome 2 Postoperative pain intensity at 48 hours: additive versus no additive subgroups.
Quality of evidence
We assessed the quality of evidence to be low. We downgraded by two levels for imprecision because of small sample size and the CI crosses the line of null effect.
4a Postoperative opioid consumption at 12 hours
One study in 46 participants reported the cumulative opioid consumption at 12 hours. Median and interquartile range of opioid consumption was zero in both the intravenous dexamethasone and control groups (Chalifoux 2017).
4b Postoperative opioid consumption at 24 hours
Cummulative opioid consumption at 24 hours was reported in five studies. Postoperative opioids were administered for VAS greater than four (Abdallah 2015; Chalifoux 2017), or as needed (Dawson 2016; Rahangdale 2014; Rosenfeld 2016). Twenty‐four hour opioid consumption was significantly lower in the intravenous dexamethasone group compared with control (MD ‐6.58 mg, 95% CI ‐10.56 to ‐2.60; participants = 287; studies = 5; I2 = 60%) (Analysis 15.1).
15.1. Analysis.
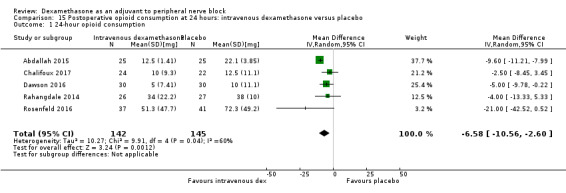
Comparison 15 Postoperative opioid consumption at 24 hours: intravenous dexamethasone versus placebo, Outcome 1 24‐hour opioid consumption.
Subgroup analysis
There was no significant difference in effect size between the additive and no additive subgroups (P = 0.58) (Analysis 15.2). In all three studies, long‐acting local anaesthesia and high‐dose dexamethasone were used, and all were at low risk of bias.
15.2. Analysis.
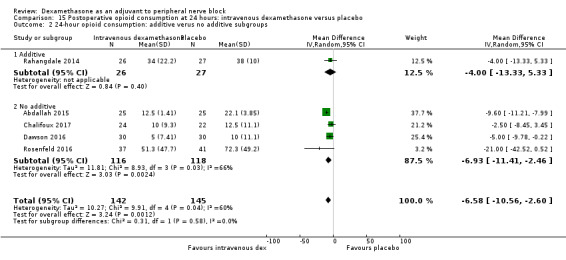
Comparison 15 Postoperative opioid consumption at 24 hours: intravenous dexamethasone versus placebo, Outcome 2 24‐hour opioid consumption: additive verus no additive subgroups.
4c Postoperative opioid consumption at 48 hours
In one study (46 participants), postoperative opioid consumption was significantly lower in the intravenous dexamethasone group versus placebo (MD 22.50 mg, 95% CI 5.15 to 39.85) (Chalifoux 2017); (Analysis 16.1).
16.1. Analysis.
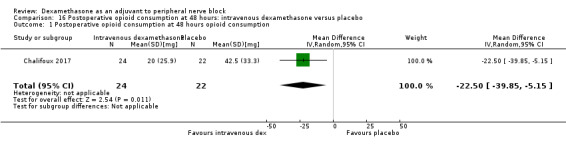
Comparison 16 Postoperative opioid consumption at 48 hours: intravenous dexamethasone versus placebo, Outcome 1 Postoperative opioid consumption at 48 hours opioid consumption.
5 Participant satisfaction with pain control
There was no statistically significant difference between intravenous dexamethasone and placebo in participant satisfaction with pain control (MD 1.07, 95% CI ‐0.08 to 2.22; participants = 181; studies = 3; I2 = 27%) (Analysis 17.1).
17.1. Analysis.
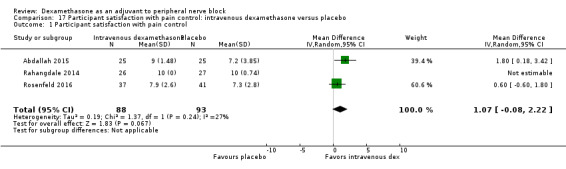
Comparison 17 Participant satisfaction with pain control: intravenous dexamethasone versus placebo, Outcome 1 Participant satisfaction with pain control.
Perineural versus intravenous dexamethasone
1. Duration of sensory block
We identified nine trials that compared perineural versus intravenous dexamethasone. The duration of sensory block was defined inconsistently across studies. Definitions included the following.
The interval between administration of block and:
first report of pain (Abdallah 2015; Aliste 2017; Leurcharusmee 2016; Rahangdale 2014);
participant detected complete resolution of block (Rosenfeld 2016);
first analgesia request or administration (Desmet 2013; Kawanishi 2014).
Time of completion of surgery to first analgesic request (Chun 2016)
Time of successful block to recovery of sensation (Sakae 2017).
The duration of sensory block was significantly longer in the perineural dexamethasone group compared with intravenous dexamethasone (MD 3.13 hours, 95% CI 1.68 to 4.58; participants = 720; studies = 9; I2 = 63%) (Analysis 18.1).
18.1. Analysis.
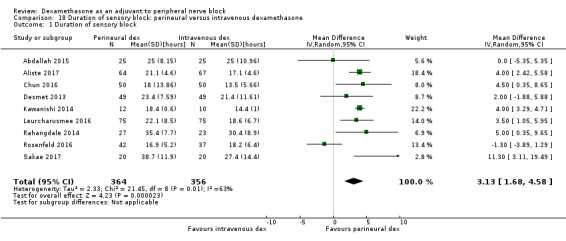
Comparison 18 Duration of sensory block: perineural versus intravenous dexamethasone, Outcome 1 Duration of sensory block.
Subgroup analysis
There was no significant difference in the duration of sensory block between the additive and no additive subgroups (P = 0.40) (Analysis 18.2); between the high‐ versus low‐dose dexamethasone subgroups (P = 0.22) (Analysis 18.3); or between the high/unclear risk of bias subgroups (P = 0.14). In all studies, long‐acting local anaesthesia was used.
18.2. Analysis.
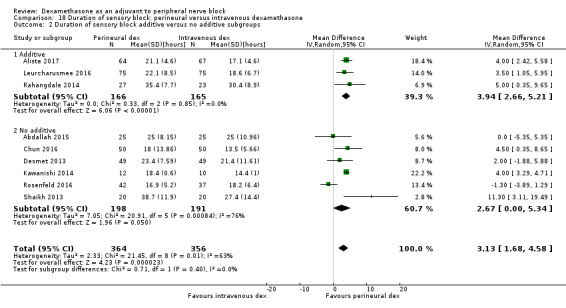
Comparison 18 Duration of sensory block: perineural versus intravenous dexamethasone, Outcome 2 Duration of sensory block additive versus no additive subgroups.
18.3. Analysis.
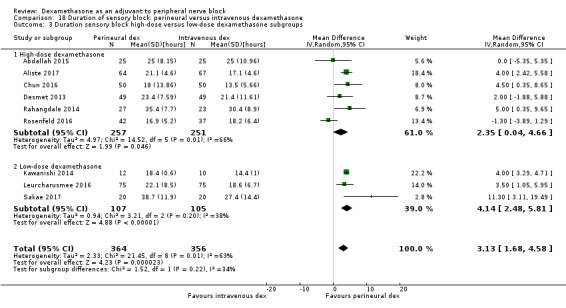
Comparison 18 Duration of sensory block: perineural versus intravenous dexamethasone, Outcome 3 Duration sensory block high‐dose versus low‐dose dexamethasone subgroups.
Quality of evidence
We assessed the quality of evidence as moderate. We downgraded by one level for inconsistency because of considerable heterogeneity (I2 = 63%, P = 0.006) and point estimates vary widely. Subgroup analyses did not explain observed heterogeneity.
2. Incidence of serious adverse events
See incidence of serious adverse events in perineural versus control section.
Secondary outcomes
1. Duration of motor block
Duration of motor block was defined as the interval between administration of block until return to baseline motor strength in the operative limb (Abdallah 2015), or the participant was able to move the great toe (Rahangdale 2014). The duration of motor block was significantly longer in the perineural dexamethasone group compared with the intravenous dexamethasone group (MD 3.13 hours, 95% CI 0.99 to 5.27; participants = 421; studies = 5; I2 = 71%) (Analysis 19.1).
19.1. Analysis.
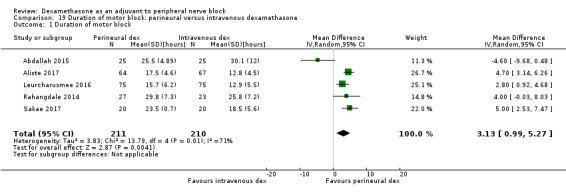
Comparison 19 Duration of motor block: perineural versus intravenous dexamethasone, Outcome 1 Duration of motor block.
Subgroup analysis
There was no significant difference in motor block between the additive versus no additive subgroups (P = 0.53) (Analysis 19.2); between the high‐ versus low‐dose dexamethasone subgroups (P = 0.18) (Analysis 19.3); or between the high/unclear versus low risk of bias subgroups (P = 0.18) (Analysis 19.4). In all studies, long‐acting local anaesthesia was used.
19.2. Analysis.
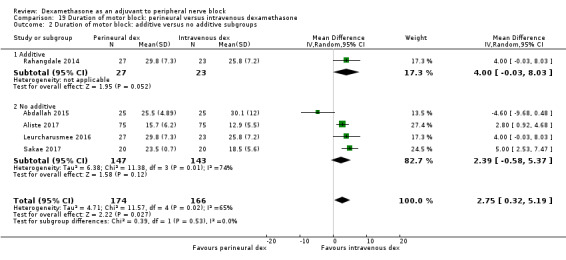
Comparison 19 Duration of motor block: perineural versus intravenous dexamethasone, Outcome 2 Duration of motor block: additive versus no additive subgroups.
19.3. Analysis.
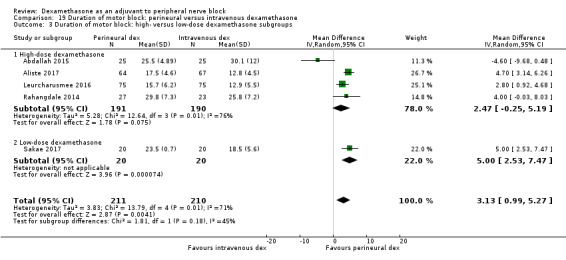
Comparison 19 Duration of motor block: perineural versus intravenous dexamethasone, Outcome 3 Duration of motor block: high‐ versus low‐dose dexamethasone subgroups.
19.4. Analysis.
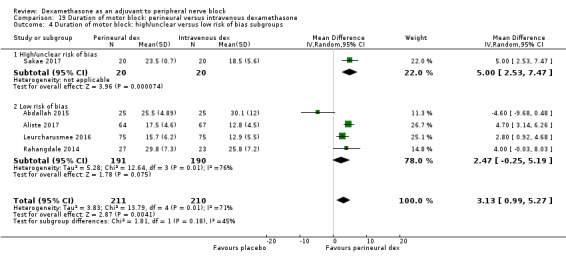
Comparison 19 Duration of motor block: perineural versus intravenous dexamethasone, Outcome 4 Duration of motor block: high/unclear versus low risk of bias subgroups.
2. Incidence of mild to moderate adverse events such as nausea/vomiting, pruritus, somnolence, oxygen desaturation, urinary retention, numbness/tingling
Block‐related adverse events
Five studies reported that they assessed for block‐related adverse events (Abdallah 2015; Aliste 2017; Dawson 2016; Kawanishi 2014; Rosenfeld 2016). There was no statistically significant difference between perineural and intravenous dexamethasone in the overall or individual incidence of block‐related adverse events (42 out of 207 in the perineural dexamethasone group versus 36 out of 199 in the intravenous dexamethasone group (RR 1.20, 95% CI 0.93 to 1.55; participants = 406; studies = 5; I2 = 0%) (Analysis 20.1). Individual events are as follows.
20.1. Analysis.
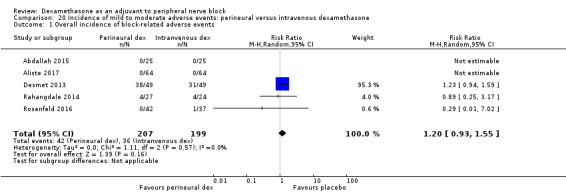
Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 1 Overall incidence of block‐related adverse events.
Numbness/tingling 14 days after surgery: four out of 116 in the perineural group versus four out of 116 in the intravenous group (RR 0.97, 95% CI 0.27 to 3.49; participants = 232; studies = 3; I2 = 0%) (Abdallah 2015; Aliste 2017; Rahangdale 2014); (Analysis 20.2).
Residual motor block/muscle weakness at 24 hours: 16 out of 126 in the perineural dexamethasone group versus 13 out of 122 in the intravenous dexamethasone group (RR 1.22, 95% CI 0.62 to 2.37; participants = 248; studies = 3; I2 = 0%) (Chun 2016; Desmet 2013; Rahangdale 2014); (Analysis 20.3).
Horner syndrome: 24 out of 99 in the perineural versus 20 out of 98 in the intravenous dexamethasone group (RR 1.20, 95% CI 0.77 to 1.87; participants = 197; studies = 2; I2 = 0%) (Chun 2016; Desmet 2013); (Analysis 20.4).
Hoarseness: 11 out of 99 in the perineural versus 11 out of 98 in the intravenous dexamethasone group (RR 1.00, 95% CI 0.48 to 2.09; participants = 197; studies = 2; I2 = 0%)(RR 1.00, 95% CI 0.48 to 2.09; participants = 98; studies = 1; I2 = 0%) (Chun 2016; Desmet 2013); (Analysis 20.5).
Cranial nerve 12 palsy: zero out of 42 in the perineural dexamethasone group versus one out of 41 in the intravenous dexamethasone group (RR 0.31, 95% CI 0.01 to 7.39; participants = 81; studies = 1; I2 = 0%) (Rosenfeld 2016); (Analysis 20.6).
20.2. Analysis.
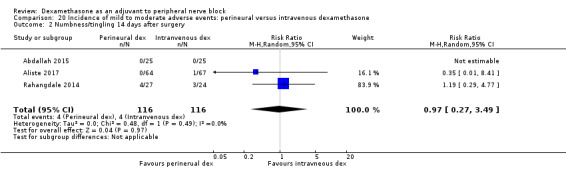
Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 2 Numbness/tingling 14 days after surgery.
20.3. Analysis.
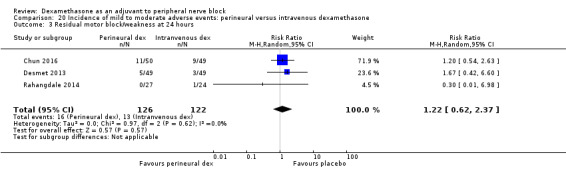
Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 3 Residual motor block/weakness at 24 hours.
20.4. Analysis.
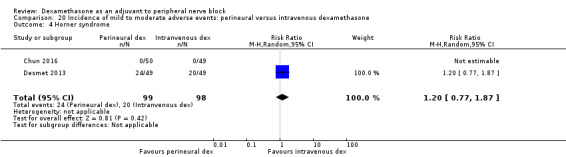
Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 4 Horner syndrome.
20.5. Analysis.
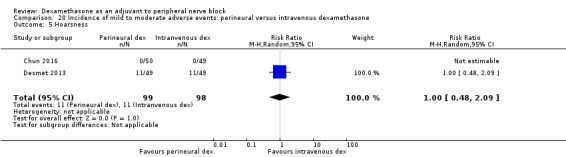
Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 5 Hoarsness.
20.6. Analysis.
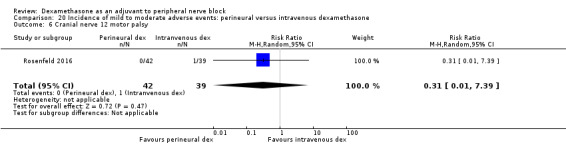
Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 6 Cranial nerve 12 motor palsy.
Non‐block‐related adverse events
Five studies reported that they assessed for non‐block‐related adverse events (Abdallah 2015; Chun 2016; Dawson 2016; Kawanishi 2014; Rosenfeld 2016). There was no statistically significant difference between perineural and intravenous dexamethasone (26 out of 159 in the perineural dexamethasone group versus 21 out of 157 in the intravenous dexamethasone group ((RR 1.34, 95% CI 0.37 to 4.78; participants = 316; studies = 5; I2 = 63%) (Analysis 20.7). The incidence for each event is as follows.
20.7. Analysis.
Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 7 Overall incidence of non block‐related adverse events.
Postoperative nausea and vomiting: five out of 159 in the perineural dexamethasone group versus eight out of 153 in the intravenous dexamethasone group (RR 0.63, 95% CI 0.22 to 1.80; participants = 312; studies = 5; I2 = 0%) (Abdallah 2015; Chun 2016; Dawson 2016; Kawanishi 2014; Rosenfeld 2016); (Analysis 20.8).
Dermatological symptoms (pruritus/rash): two out of 42 in the perineural dexamethasone group versus zero out of 37 in the intravenous dexamethasone group (RR 4.42, 95% CI 0.22 to 89.18; participants = 79; studies = 1) (Rosenfeld 2016); (Analysis 20.9).
Syncope/fainting: two out of 42 in the perineural dexamethasone group versus zero out of 37 in the intravenous dexamethasone group (RR 4.42, 95% CI 0.22 to 89.18; participants = 79; studies = 1; I2 = 0%) (Rosenfeld 2016); (Analysis 20.10).
Dizziness: one out of 92 in the perineural dexamethasone group versus three out of 86 in the intravenous dexamethasone group (RR 0.41, 95% CI 0.06 to 2.72; participants = 178; studies = 2; I2 = 0%) (Rosenfeld 2016); (Analysis 20.11).
Wrist, hand or finger pain: zero out of 42 in the perineural dexamethasone group versus one out of 37 in the intravenous dexamethasone group (RR 0.29, 95% CI 0.01 to 7.02; participants = 79; studies = 1) (Rosenfeld 2016); (Analysis 20.12).
Each of the following outcomes occurred in one out of 42 in the perineural dexamethasone group versus zero out of 37 in the intravenous dexamethasone group: 10‐lb weight gain in 24 hours, headache, diarrhoea, frequent urination and muscle soreness (RR 2.65, 95% CI 0.11 to 63.16; participants = 79; studies = 1) (Rosenfeld 2016); (Analysis 20.13).
20.8. Analysis.
Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 8 Postoperative nausea and vomiting.
20.9. Analysis.
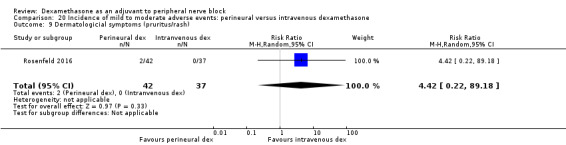
Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 9 Dermatologicial symptoms (pruritus/rash).
20.10. Analysis.
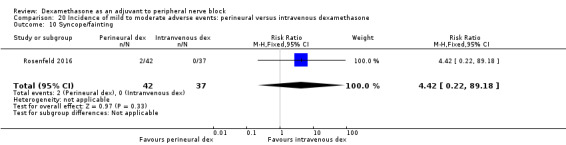
Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 10 Syncope/fainting.
20.11. Analysis.
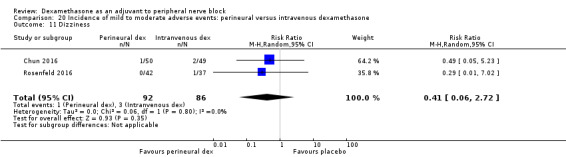
Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 11 Dizziness.
20.12. Analysis.
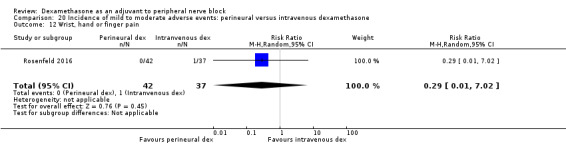
Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 12 Wrist, hand or finger pain.
20.13. Analysis.
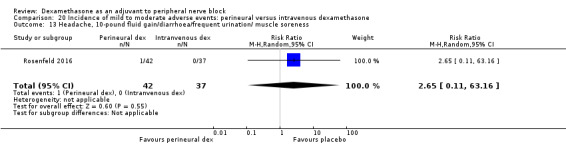
Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 13 Headache, 10‐pound fluid gain/diarrhoea/frequent urination/ muscle soreness.
3a. Postoperative pain intensity at 12 hours
Pain scores were significantly lower in the perineural dexamethasone group compared with intravenous dexamethasone. The MD did not surpass the MID of 1.2, therefore the difference in effect size is not clinically significant (MD ‐1.01, 95% CI ‐1.51 to ‐0.50; participants = 217; studies = 3; I2 = 0%) (Chun 2016; Rosenfeld 2016; Sakae 2017); (Analysis 21.1).
21.1. Analysis.
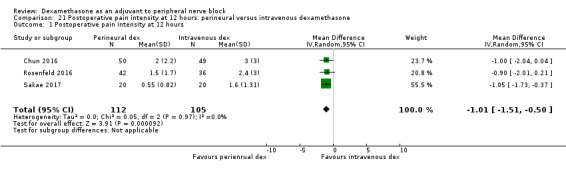
Comparison 21 Postoperative pain intensity at 12 hours: perineural versus intravenous dexamethasone, Outcome 1 Postoperative pain intensity at 12 hours.
Subgroup analysis
There was no significant difference in effect size between the high‐and low‐dose dexamethasone subgroups (P = 0.83) (Analysis 21.2 or between the high/unclear and low risk of bias subgroups (P = 0.83) Analysis 21.3. In all three studies, long‐acting local anaesthetic was used and no additives were used.
21.3. Analysis.
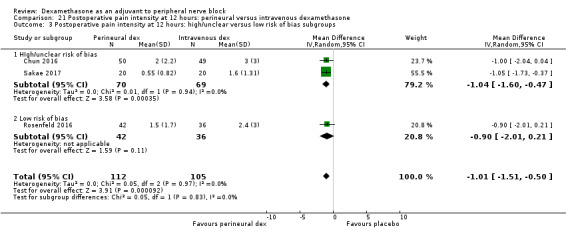
Comparison 21 Postoperative pain intensity at 12 hours: perineural versus intravenous dexamethasone, Outcome 3 Postoperative pain intensity at 12 hours: high/unclear versus low risk of bias subgroups.
Quality of evidence
We assessed the quality of evidence to be low. We downgraded by one level for risk of bias because two of the three studies are at unclear risk of bias, and by one level for imprecision because the CI includes both no clinical effect (MID less than 1.2 on VAS) and clinical effect (MID greater than 1.2 on VAS).
3b. Postoperative pain intensity at 24 hours
Pain scores were significantly lower in the perineural dexamethasone group compared with intravenous dexamethasone. The MD did not surpass the MID of 1.2 on the VAS, therefore the difference in effect size is not clinically significant (MD ‐0.79, 95% CI ‐1.51 to ‐0.07; participants = 309; studies = 5; I2 = 46%) (Abdallah 2015; Chun 2016; Rahangdale 2014; Rosenfeld 2016; Sakae 2017); (Analysis 22.1).
22.1. Analysis.
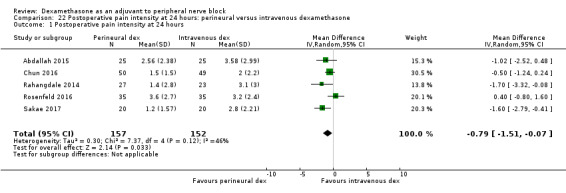
Comparison 22 Postoperative pain intensity at 24 hours: perineural versus intravenous dexamethasone, Outcome 1 Postoperative pain intensity at 24 hours.
Subgroup analysis
There was no significant difference in effect size between the additive and no additive subgroups (P = 0.24) (Analysis 22.2), the low‐versus high‐dose dexamethasone subgroups (P = 0.75) (Analysis 22.3) or the high/unclear versus low risk of bias subgroups (P = 0.75) (Analysis 22.4). In all five studies, long‐acting local anaesthetic was used.
22.2. Analysis.
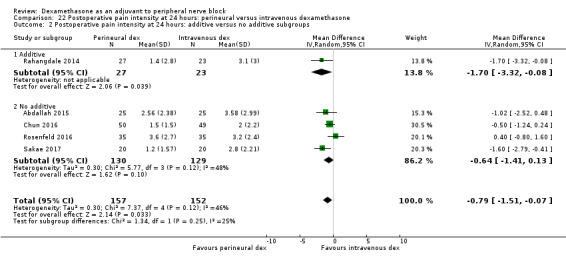
Comparison 22 Postoperative pain intensity at 24 hours: perineural versus intravenous dexamethasone, Outcome 2 Postoperative pain intensity at 24 hours: additive versus no additive subgroups.
22.4. Analysis.
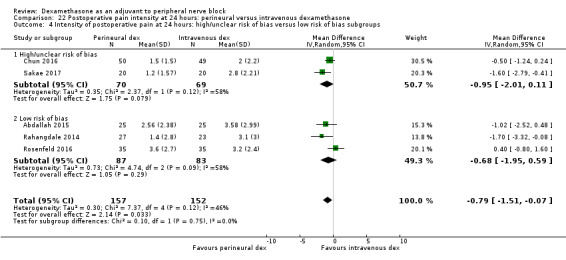
Comparison 22 Postoperative pain intensity at 24 hours: perineural versus intravenous dexamethasone, Outcome 4 Intensity of postoperative pain at 24 hours: high/unclear risk of bias versus low risk of bias subgroups.
Quality of evidence
We assessed the quality of evidence to be moderate. We downgraded by one level for imprecision because the CI includes both no clinical effect (MID less than 1.2 on VAS) and clinical effect (MID greater than 1.2 on VAS).
3c. Postoperative pain intensity at 48 hours
There was no significant difference in pain scores at 48 hours between perineural and intravenous dexamethasone (MD 0.13, 95% CI ‐0.35 to 0.61; participants = 227; studies = 3; I2 = 0%) (Chun 2016; Rahangdale 2014; Rosenfeld 2016); (Analysis 23.1).
23.1. Analysis.
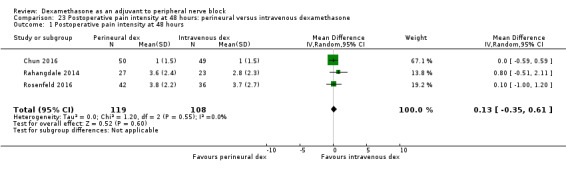
Comparison 23 Postoperative pain intensity at 48 hours: perineural versus intravenous dexamethasone, Outcome 1 Postoperative pain intensity at 48 hours.
Subgroup analysis
There was no significant difference in effect size between the additive and the no additive subgroups (P = 0.28) (Analysis 23.2), the low‐versus high‐dose dexamethasone subgroups (P = 0.46) (Analysis 23.3) and the high/unclear versus low risk of bias subgroups (P = 0.46) (Analysis 23.4). In all three studies, long‐acting local anaesthetic was used.
23.2. Analysis.
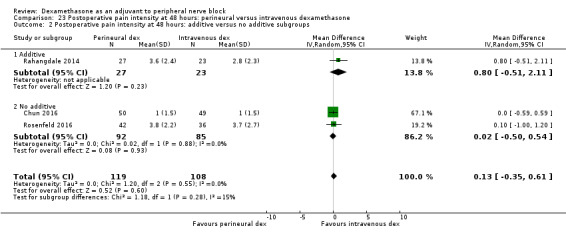
Comparison 23 Postoperative pain intensity at 48 hours: perineural versus intravenous dexamethasone, Outcome 2 Postoperative pain intensity at 48 hours: additive versus no additive subgroups.
23.3. Analysis.
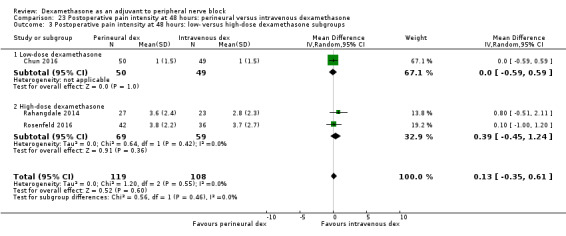
Comparison 23 Postoperative pain intensity at 48 hours: perineural versus intravenous dexamethasone, Outcome 3 Postoperative pain intensity at 48 hours: low‐ versus high‐dose dexamethasone subgroups.
23.4. Analysis.
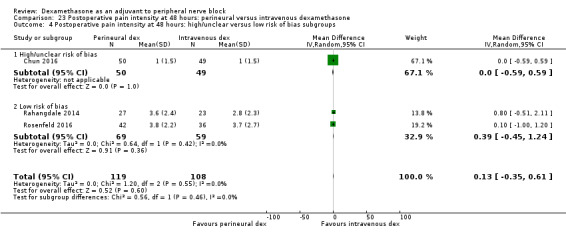
Comparison 23 Postoperative pain intensity at 48 hours: perineural versus intravenous dexamethasone, Outcome 4 Postoperative pain intensity at 48 hours: high/unclear versus low risk of bias subgroups.
Quality of evidence
We assessed the quality of evidence to be low. We downgraded by one level for risk of bias because the one study that is at unclear risk of bias contributes half the data for this outcome, and by one level for imprecision because of small sample size.
4a. Postoperative opioid consumption at 12 hours
No studies evaluated postoperative opioid consumption at 12 hours.
4b. Postoperative opioid consumption at 24 hours
Cummulative postoperative consumption at 24 hours was reported in four studies. Postoperative opioids were administered for VAS greater than four (Abdallah 2015), or as needed (Dawson 2016; Rahangdale 2014; Rosenfeld 2016). There was no significant difference in the 24‐hour opioid consumption between perineural and intravenous dexamethasone (MD ‐3.87 mg, 95% CI ‐9.93 to 2.19; participants = 242; studies = 4; I2 = 44%) (Analysis 24.1).
24.1. Analysis.
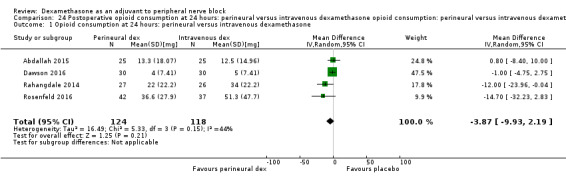
Comparison 24 Postoperative opioid consumption at 24 hours: perineural versus intravenous dexamethasone opioid consumption: perineural versus intravenous dexamethasone subgroups, Outcome 1 Opioid consumption at 24 hours: perineural versus intravenous dexamethasone.
Subgroup analysis
There was no significant difference in effect size between the additive or no additive subgroups (P = 0.11) (Analysis 24.2). In all four studies, long‐acting local anaesthetic and high‐dose dexamethasone were used, and all four studies were at low risk of bias.
24.2. Analysis.
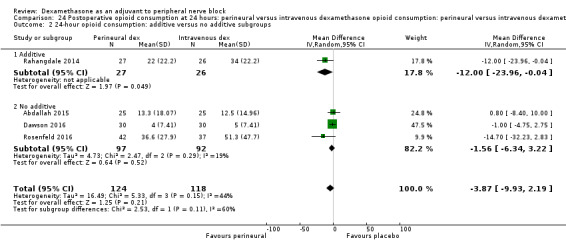
Comparison 24 Postoperative opioid consumption at 24 hours: perineural versus intravenous dexamethasone opioid consumption: perineural versus intravenous dexamethasone subgroups, Outcome 2 24‐hour opioid consumption: additive versus no additive subgroups.
4c. Postoperative opioid consumption at 48 hours
No studies reported the cumulative opioid consumption at 48 hours.
5. Participant satisfaction with pain control
There was no significant difference in participant satisfaction between perineural and intravenous dexamethasone (MD 0.19, 95% CI ‐0.33 to 0.70; participants = 181; studies = 3; I2 = 0%) (Analysis 25.1). The SD was zero in both the perineural and intravenous dexamethasone groups in one of the two studies, therefore the 95% CI was not estimable and the analysis was based on one study in 50 participants.
25.1. Analysis.
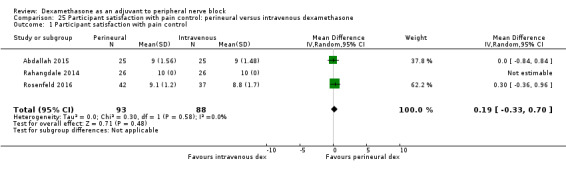
Comparison 25 Participant satisfaction with pain control: perineural versus intravenous dexamethasone, Outcome 1 Participant satisfaction with pain control.
Discussion
Summary of main results and quality of evidence
The objective of this review was to evaluate the comparative efficacy and safety of perineural dexamethasone and intravenous dexamethasone as adjuvants to peripheral nerve block for postoperative pain control in people undergoing upper or lower limb surgery. Our primary outcomes were duration of sensory block and incidence of severe adverse events. We conducted a comprehensive search for trials evaluating our study objectives. We assessed the quality of evidence for outcomes important for clinical decision‐making, including duration of sensory block, intensity of postoperative pain at 12, 24 and 48 hours, and incidence of severe adverse events. In total, we found 35 eligible trials involving 2707 participants. We describe our findings and provide a summary of the quality of evidence for each comparison below.
Perineural dexamethasone verus placebo
Among 27 trials (1625 participants) the duration of sensory block was longer in the perineural dexamethasone group by approximately six and a half hours. The quality of evidence is low. We downgraded by one level for risk of bias because the majority of studies are at unclear risk of bias and by one level for inconsistency because of considerable heterogeneity not explained by subgroup analyses; point estimates varied widely among studies and confidence intervals showed minimal overlap. Motor block was also longer in the perineural dexamethasone group compared with control by approximately six hours (16 studies, 912 participants).
Among five studies (257 participants), postoperative pain intensity at 12 hours in the perineural dexamethasone group was 2.1 points lower on an 11‐point numeric rating scale. The quality of evidence is very low; we downgraded by one level for risk of bias because half of the studies were at high/unclear risk of bias, by one level for inconsistency due to considerable heterogeneity not explained by subgroup analyses, and by one level for imprecision due to small sample size. At 24 hours, perineural dexamethasone reduced postoperative pain intensity by 1.6 points (9 studies, 469 participants). The quality of evidence is low; we downgraded by one level for inconsistency because of considerable heterogeneity not explained by subgroup analysis, and by one level for imprecision because the confidence interval includes both no clinical effect and clinical effect. For postoperative pain intensity at 12 and 24 hours, the minimally important difference (MID) of 1.2 points was surpassed. There was no difference in postoperative pain intensity between perineural dexamethasone and placebo at 48 hours (3 studies, 296 participants). The quality of evidence is low; we downgraded by one level for inconsistency due to moderate heterogeneity not explained by subgroup analyses, and by one level for imprecision because the confidence interval includes both no clinical effect and clinical effect. Cumulative opioid consumption 24 hours postoperatively was lower in the perineural dexamethasone group compared with placebo by 19 mg oral morphine equivalents.
Based on our a priori hypotheses, the duration of sensory block was significantly longer in long‐ versus medium‐acting local anaesthetic subgroup and the high‐ versus low‐dose dexamethasone subgroup. There was no significant difference in effect size between the high‐ and low‐dose dexamethasone subgroups in postoperative pain intensity at 12‐ and 24‐hour outcomes; therefore, the longer duration of sensory block in the long‐acting local anaesthetic and high‐dose dexamethasone subgroups are likely not clinically significant.
Intravenous dexamethasone versus placebo
Among eight trials (499 participants), the duration of sensory block was longer in the intravenous dexamethasone group by approximately six hours. The quality of evidence is moderate; we downgraded by one level for inconsistency because of considerable heterogeneity not explained by subgroup analyses. The duration of motor block was also longer in the intravenous dexamethasone group compared with control by approximately five hours.
Among three studies (162 participants), postoperative pain intensity at 12 hours was lower in the intravenous dexamethasone group compared with placebo by 1.2 points on an 11‐point numeric rating scale. The quality of evidence is low; we downgraded by one level for inconsistency because of considerable heterogeneity not explained by subgroup analyses, and by one level for imprecision because the confidence interval included both no clinical effect and clinical effect. At 24 hours (5 studies, 257 participants), postoperative pain intensity was lower in the intravenous dexamethasone group by 1.3 points. The quality of evidence is low; we downgraded by one level for inconsistency for considerable heterogeneity not explained by subgroup analyses, and by one level for imprecision because the confidence interval includes both no clinical benefit and clinical benefit. The MID of 1.2 points was surpassed in postoperative pain intensity at 12‐ and 24‐hour outcomes. Among three trials (172 participants) there was no difference in postoperative pain intensity at 48 hours. The quality of evidence is low; we downgraded by two levels for imprecision because the confidence interval crosses the line of null effect, and because of the small sample size. Opioid consumption 24 hours postoperatively was lower in the intravenous dexamethasone group.
Based on our a priori hypotheses, the duration of sensory block was significantly longer in the high‐ versus low‐dose dexamethasone subgroup. There was no significant difference in effect size between the high‐ and low‐dose dexamethasone subgroups in the intensity of postoperative pain at 12‐ and 24‐hour outcomes, therefore the longer duration of sensory block in the high‐dose dexamethasone is likely not clinically significant.
Perineural versus intravenous dexamethasone
Among nine studies (720 participants) the duration of sensory block was longer in the perineural dexamethasone group compared with intravenous dexamethasone by approximately three hours. The quality of evidence is moderate; we downgraded by one level for considerable heterogeneity not explained by subgroup analysis. Duration of motor block was also longer in the perineural dexamethasone group by approximately three hours (3 studies, 139 participants).
Postoperative pain intensity at 12 hours was lower in the perineural dexamethasone group compared with intravenous dexamethasone (3 studies, 217 participants). The MID of 1.2 was not surpassed; therefore the lower intensity of pain is not clinically significant. The quality of evidence is very low; we downgraded by one level for risk of bias because two out of the three included studies are at unclear risk of bias, and by one level for imprecision because the confidence interval includes both no clinical effect and clinical effect. At 24 hours, although the postoperative pain intensity was significantly higher in the perineural dexamethasone group compared with intravenous dexamethasone, the MID of 1.2 was not surpassed; therefore the lower intensity of pain is not clinically significant (5 studies, 309 participants). The quality of evidence is moderate; we downgraded by one level for imprecision because the confidence interval includes both clinical effect and no clinical effect. At 48 hours postoperatively, there was no difference in postoperative pain intensity between perineural and intravenous dexamethasone. The quality of evidence is low; we downgraded by one level for risk of bias because half the data comes from one study at unclear risk of bias and by one level for imprecision because of the small sample size. There was no difference between perineural and intravenous dexamethasone in 24‐hour postoperative opioid consumption. We did not find any difference in effect size between any of our subgroups.
Incidence of severe adverse events
Authors reported that they assessed for serious adverse events in seven studies. Five serious adverse events were reported in three studies including pneumothorax, pneumonia, development of Chronic Regional Pain Syndrome Type I, and two unexpected hospitalizations within one week of surgery; one for a fall, the other for a bowel infection. The quality of evidence is very low, downgraded by one level for risk of bias because the majority of studies were at high/unclear risk of bias, and by two levels for imprecision due to the small sample size.
Mild to moderate adverse events
We categorized mild to moderate adverse events into block‐related and non‐block‐related adverse events. Block‐related adverse events included numbness/tingling, residual motor block and muscle weakness, Horner's syndrome, hoarseness, diaphragmatic paresis, dyspnoea, cranial nerve 12 motor palsy, vascular injury, and bruising at the injection site. Non‐block‐related adverse events included bradycardia/hypotension, postoperative nausea and vomiting, pruritus/rash, syncope, dizziness, headache, fluid gain, diarrhoea, frequent urination, muscle soreness, wrist, hand or finger pain, and constipation.
We found no difference between the incidence of block‐related or non‐block‐related adverse events in any of the three comparisons. Because the incidence of severe and block‐related adverse events associated with the use of peripheral nerve block is rare, our review may not have included enough participants to detect a difference in any of the comparisons, therefore our confidence in the estimate is low (sparse number of participants and events). In only two studies did the authors report that block‐related symptoms had resolved, therefore it is not possible to determine whether participants reporting block‐related adverse events in other studies were later diagnosed with nerve injury.
Overall completeness and applicability of evidence
The majority of studies included in our review were conducted in upper limb surgery; as only two studies were conducted in lower limb surgery, we cannot draw any meaningful conclusions about the effectiveness of dexamethasone as an adjuvant to lower limb blocks. More studies for lower limb surgery are needed in order to determine whether our results are applicable in this population. The nine ongoing trials on ClinicalTrials.gov may change the results of this review.
The results of our review may not be applicable to participants who are at risk for dexamethasone‐related adverse events in whom clinical trials would likely to be unsafe. People with diabetes mellitus, peptic ulcer and psychiatric disorders were excluded from many of the trials. Additionally, our results may also not be applicable to those at risk for postoperative infection and delayed wound healing, including people with immunodeficiency disorders, those undergoing radiation therapy, people with circulatory disorder, obesity, poor nutritional status, or the elderly. Other populations excluded from some of the trials include those with renal, liver, cardiac or lung disease, head injury, hypertension, drug/alcohol dependence, pregnant women, and those who had used steroids or opioids preoperatively. Finally, there were no studies in infants and children under the age of 15 years, and so our results are not directly applicable to this population.
We found that the duration of sensory block was longer in the high‐ versus low‐dose dexamethasone subgroups in the perineural versus control and in the intravenous versus control comparisons, but the longer duration in the high‐dose dexamethasone subgroups was not associated with lower postoperative pain intensity. There were fewer studies using low‐dose than high‐dose dexamethasone. It is possible that the sample size was too small to detect a difference between high‐ and low‐dose dexamethasone. Dose‐finding studies would be beneficial to determine the ideal perineural and intravenous doses.
For the duration of sensory block outcome, we did not determine a priori the minimally important difference (MID) that would be clinically significant. In the perineural dexamethasone versus control and the intravenous dexamethasone versus control comparisons, the longer duration of sensory block in the dexamethasone groups was also associated with lower postoperative pain intensity and opioid consumption. In the perineural versus intravenous dexamethasone comparison, the longer duration of sensory block in the perineural dexamethasone was not associated with a reduction in postoperative pain intensity or opioid consumption, and we concluded that the longer duration of sensory block was unlikely to be clinically significant. In 10 of the included studies, duration of sensory block was reported without also reporting pain outcomes; therefore it is not known whether the longer duration of sensory block in the dexamethasone groups was effective in reducing postoperative pain and opioid consumption. In all future studies, duration of sensory block should be reported in conjunction with other pain outcomes to determine the efficacy of dexamethasone in reducing postoperative pain.
Potential biases in the review process
In order to reduce potential bias in the review process, two review authors independently assessed each trial for eligibility, extracted the data, assessed risk of bias, and assessed the quality of evidence. Furthermore, we did not impose any language restrictions. With the assistance of an experienced librarian, we did an extensive literature review of six databases and we searched Google Scholar and found additional studies we had not found through scientific databases. There were no marginal decisions around the inclusion or exclusion of studies or use and analysis of data. We made minor changes to the protocol, however, it is unlikely that any changes would have been a source of bias.
We conducted subgroup analyses to explore heterogeneity for all outcomes regardless of the observed heterogeneity (I2). In particular, we explored whether the type of local anaesthetic (long‐acting versus medium‐acting), the dose of dexamethasone (high‐ versus low‐dose), whether additives to local anaesthetics were used, and whether risk of bias (high/unclear versus low) could explain the observed heterogeneity. Our subgroup hypotheses were determined as possible factors that may influence the results based on the literature. There may be other reasons for heterogeneity that we did not explore.
Among our 35 eligible trials, 14 had incomplete reports (e.g. missing variance data, unclear presentation of data on figures). We attempted to obtain unpublished data for our meta‐analyses, but we were only able to obtain data from six of the 15 study authors we contacted. The missing information may have introduced a source of bias. With respect to publication bias, only two of the outcomes in the perineural versus placebo comparison included 10 or more trials (duration of sensory block and duration of motor block). Because our remaining outcomes in all three comparisons included fewer than 10 trials we were not able to adequately assess publication bias. Published protocols were available for 10 of the studies. For the remaining 25, because we relied on the information provided in the methods section to assess risk of selection bias, we could not ascertain whether all outcomes were reported as planned, so our assessment of selection bias is limited.
Agreements and disagreements with other studies or reviews
We found five reviews evaluating the effectiveness of perineural dexamethasone on postoperative outcomes that are in agreement with our findings. Two reviews were in participants undergoing upper limb surgery with brachial plexus block (Choi 2014; Knezivic 2015), and the remaining three were in participants undergoing surgery with a variety of nerve blocks, including peribulbar, transversus abdominis, axillary, supraclavicular, sciatic, and interscalene (Albrecht 2015; De Oliveira 2014; Huynh 2015). In all five reviews, the duration of sensory and motor block was longer after perineural dexamethasone compared with placebo. Albrecht 2015 and De Oliveira 2014 found that 24‐hour postoperative opioid consumption was lower after perineural dexamethasone compared with placebo.
We found two systematic reviews that evaluated the effectiveness of intravenous dexamethasone for postoperative pain (De Oliveira 2011; Waldron 2013). Neither of these reviews included studies in participants undergoing peripheral nerve block. Postoperative pain intensity at 24 hours, opioid consumption, and the incidence of postoperative nausea and vomiting was lower in the intravenous dexamethasone group compared with placebo (De Oliveira 2011; Waldron 2013). We did not find any difference between intravenous dexamethasone and placebo in postoperative pain intensity at 24 hours or the incidence of postoperative nausea and vomiting; however, our review included fewer participants than the previous reviews.
Authors' conclusions
Implications for practice.
Low‐ to moderate‐quality evidence suggests that when used as an adjuvant to peripheral nerve block in upper limb surgery, both perineural and intravenous dexamethasone may prolong the duration of sensory block and are effective in reducing postoperative pain intensity and opioid consumption. Perineural dexamethasone is not likely to be more effective than intravenous dexamethasone. There is not enough evidence to determine the effectiveness of dexamethasone as an adjuvant to peripheral nerve block in lower limb surgeries and there is no evidence in children. The results of our review may not apply to participants who are at risk of dexamethasone‐related adverse events in whom clinical trials would likely to be unsafe.The nine ongoing trials on ClinicalTrials.gov may change the results of this review.
Implications for research.
Future trials would benefit from long‐term follow‐up to determine the safety of dexamethasone as an adjuvant to peripheral nerve block. Dose‐finding studies to determine the optimum intravenous and perineural dose of dexamethasone are needed. In addition, additional research should include the paediatric population. Future studies evaluating the duration of sensory block should also evaluate outcomes, such as, postoperative pain intensity and postoperative opioid consumption.
Acknowledgements
We would like to thank Rodrigo Cavallazzi (content editor), Cathal Walsh (statistical editor), and Vaughan L Thomas and Faraj W Abdallah (peer reviewers) for help and editorial advice provided during preparation of the protocol for this Cochrane Review (Pehora 2015).
We would like to thank Rodrigo Cavallazzi (content editor), Vibeke E Horstmann (statistical editor), Andrew Moore, Stephen Choi, Brett Doleman, Faraj W Abdallah (peer reviewers), and Janet Wale (consumer editor) for help and editorial advice during the preparation of this Cochrane Review.
Appendices
Appendix 1. CENTRAL (the Cochrane Library) search strategy
#1 MeSH descriptor: [Glucocorticoids] explode all trees #2 glucocorticoid* or etiprednol dicloacetate or fluocinolone acetonide or icometasone enbutate or locicortolone dicibate or melengestrol acetate or mometasone furoate or ulobetasol propionate or alclometasone or algestone or amcinonide or amelometasone or baycuten or beclomet?asone or budesonide or butixocort or celestamine or chloroprednisone or ciclesonide or ciprocinonide or clobetaso* or clocortolone or cloprednol or cortisone or cortivazol or daktacort or deflazacort or desonide or desoximet?asone or dexatopic or diflorasone or diflucortolone or difluprednate or domoprednate or drocinonide or dutimelan or epihydrocortisone or fluclorolone or fludrocortisone or fludroxycortide or flumet?asone or flumoxonide or flunisolide or fluocinolone or fluocinonide or fluocortin or fluocortolone or fluorometholone or fluprednidene or fluprednisolone or flurandrenolone or fluticasone or formocortal or halcinonide or halometasone or halopredone or hydrallostane or hydrocortamate or hydrocortisone or isoflupredone or itrocinonide or lorinden or loteprednol or mazipredone or medrysone or meprednisone or methylprednisolone or mycolog or nicocortonide or nivacortol or oropivalone or paramethasone or prednicarbate or prednisolone or prednisone or prednival acetate or prednylidene or pregnenolone or procinonide or promestriene or resocortol or rimexolone or rofleponide or sofradex or terracortril or tetrahydrocortiso* or ticabesone or timobesone or tipredane or tixocortol or triamcinolone or trophigil or uniderm or zoticasone #3 #1 or #2 #4 MeSH descriptor: [Anesthesia, Conduction] explode all trees #5 MeSH descriptor: [Anesthesia, Epidural] explode all trees #6 MeSH descriptor: [Anesthesia, Local] explode all trees #7 MeSH descriptor: [Anesthesia, Spinal] explode all trees #8 MeSH descriptor: [Nerve Block] explode all trees #9 MeSH descriptor: [Anesthetics, Local] explode all trees #10 ((an?eth* or analg*) near (wipe or local or block* or topical or caudal or conduct* or epidural or extradural or peridural or infiltration or regional* or sacral or spinal or retrobulbar or subarachnoid or lumbar)) or (block* near (nerv* or ganglion* or brachial or paracervical or autonomic or pterygopalatine or sympathetic or sphenopalatine or caud* or dural or epidural or extradural or intercostal or neurogenic or subarachnoid or transversus or abdominis)) or (chemical neurolys?s or chemodenervation* or gangliopleg* or (huneke near neural therapy) or rachian?esth*) or benzyl alcohol or carcainium chloride or pseudotropine benzoate or amydricaine or amylocaine or articaine or aslavital or benzocaine or benzofurocaine or bucricaine or bumecaine or bupivacaine or butacaine or butanilicaine or butethamine or butoxycaine or butylcaine or carbisocaine or carticaine or centbucridine or cetacaine or chloroprocaine or cinchocaine or cocaine or cyclomethycaine or dibucaine or dimethocaine or diperodon or diphenhydramine or dyclonine or emla or ethyl chloride or etidocaine or eugenol or euprocin or fluress or fomocaine or guafecainol or heptacaine or hexathricin or hexylcaine or instillagel or ipravacaine or isobutamben or ketocaine or levobupivacaine or lidamidine or lidocaine or mepivacaine or meprylcaine or metabutethamine or myrtecaine or oxetacaine or oxybuprocaine or pentacaine or phenacaine or phenol or piperocaine or polidocanol or pramocaine or prilocaine or procaine or propanocaine or propoxycaine or propylcaine or proxymetacaine or pseudococaine or pyrrocaine or quinisocaine or ropivacaine or tanax or tetracaine or tetrodotoxin or tolycaine or tricaine or trimecaine or xyloproct or zolamine #11 #4 or #5 or #6 or #7 or #8 or #9 or #10 #12 MeSH descriptor: [Intraoperative Period] explode all trees #13 MeSH descriptor: [Postoperative Period] explode all trees #14 MeSH descriptor: [Anesthesia Recovery Period] explode all trees #15 (intra?operat* or peroperat* or postoperat* or (an?esthesia near recover*)) #16 #12 or #13 or #14 or #15 #17 #3 and #11 and #16
Appendix 2. MEDLINE (Ovid SP) search strategy
1. exp Glucocorticoids/ or glucocorticoid*.mp. or ("etiprednol dicloacetate" or "fluocinolone acetonide" or "icometasone enbutate" or "locicortolone dicibate" or "melengestrol acetate" or "mometasone furoate" or "ulobetasol propionate" or alclometasone or algestone or amcinonide or amelometasone or baycuten or beclomet?asone or budesonide or butixocort or celestamine or chloroprednisone or ciclesonide or ciprocinonide or clobetaso* or clocortolone or cloprednol or cortisone or cortivazol or daktacort or deflazacort or desonide or desoximet?asone or dexatopic or diflorasone or diflucortolone or difluprednate or domoprednate or drocinonide or dutimelan or epihydrocortisone or fluclorolone or fludrocortisone or fludroxycortide or flumet?asone or flumoxonide or flunisolide or fluocinolone or fluocinonide or fluocortin or fluocortolone or fluorometholone or fluprednidene or fluprednisolone or flurandrenolone or fluticasone or formocortal or halcinonide or halometasone or halopredone or hydrallostane or hydrocortamate or hydrocortisone or isoflupredone or itrocinonide or lorinden or loteprednol or mazipredone or medrysone or meprednisone or methylprednisolone or mycolog or nicocortonide or nivacortol or oropivalone or paramethasone or prednicarbate or prednisolone or prednisone or "prednival acetate" or prednylidene or pregnenolone or procinonide or promestriene or resocortol or rimexolone or rofleponide or sofradex or terracortril or tetrahydrocortiso* or ticabesone or timobesone or tipredane or tixocortol or triamcinolone or trophigil or uniderm or zoticasone).mp. 2. exp Anesthesia, Conduction/ or exp Anesthesia, Epidural/ or exp Anesthesia, Local/ or exp Anesthesia, Spinal/ or exp Nerve Block/ or exp Anesthetics, Local/ or ((an?eth* or analg*) adj3 (wipe or local or block* or topical or caudal or conduct* or epidural or extradural or peridural or infiltration or regional* or sacral or spinal or retrobulbar or subarachnoid or lumbar)).mp. or (block* adj3 (nerv* or ganglion* or brachial or paracervical or autonomic or pterygopalatine or sympathetic or sphenopalatine or caud* or dural or epidural or extradural or intercostal or neurogenic or subarachnoid or transversus or abdominis)).mp. or ("chemical neurolys?s" or "chemodenervation*" or gangliopleg* or (huneke adj2 neural therapy) or rachian?esth*).mp. or ("benzyl alcohol" or "carcainium chloride" or "pseudotropine benzoate" or amydricaine or amylocaine or articaine or aslavital or benzocaine or benzofurocaine or bucricaine or bumecaine or bupivacaine or butacaine or butanilicaine or butethamine or butoxycaine or butylcaine or carbisocaine or carticaine or centbucridine or cetacaine or chloroprocaine or cinchocaine or cocaine or cyclomethycaine or dibucaine or dimethocaine or diperodon or diphenhydramine or dyclonine or emla or ethyl chloride or etidocaine or eugenol or euprocin or fluress or fomocaine or guafecainol or heptacaine or hexathricin or hexylcaine or instillagel or ipravacaine or isobutamben or ketocaine or levobupivacaine or lidamidine or lidocaine or mepivacaine or meprylcaine or metabutethamine or myrtecaine or oxetacaine or oxybuprocaine or pentacaine or phenacaine or phenol or piperocaine or polidocanol or pramocaine or prilocaine or procaine or propanocaine or propoxycaine or propylcaine or or pseudococaine or pyrrocaine or quinisocaine or ropivacaine or tanax or tetracaine or tetrodotoxin or tolycaine or tricaine or trimecaine or xyloproct or zolamine).mp. 3. exp Intraoperative Period/ or exp Postoperative Period/ or exp Anesthesia Recovery Period/ or (intra?operat* or peroperat* or postoperat* or (an?esthesia adj3 recover*)).mp. 4. 1 and 2 and 3 5. ((randomized placeboled trial or placeboled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 6, 4 and 5
Appendix 3. Embase (Ovid SP) search strategy
1 exp glucocorticoid/ or glucocorticoid*.mp. or ("etiprednol dicloacetate" or "fluocinolone acetonide" or "icometasone enbutate" or "locicortolone dicibate" or "melengestrol acetate" or "mometasone furoate" or "ulobetasol propionate" or alclometasone or algestone or amcinonide or amelometasone or baycuten or beclomet?asone or budesonide or butixocort or celestamine or chloroprednisone or ciclesonide or ciprocinonide or clobetaso* or clocortolone or cloprednol or cortisone or cortivazol or daktacort or deflazacort or desonide or desoximet?asone or dexatopic or diflorasone or diflucortolone or difluprednate or domoprednate or drocinonide or dutimelan or epihydrocortisone or fluclorolone or fludrocortisone or fludroxycortide or flumet?asone or flumoxonide or flunisolide or fluocinolone or fluocinonide or fluocortin or fluocortolone or fluorometholone or fluprednidene or fluprednisolone or flurandrenolone or fluticasone or formocortal or halcinonide or halometasone or halopredone or hydrallostane or hydrocortamate or hydrocortisone or isoflupredone or itrocinonide or lorinden or loteprednol or mazipredone or medrysone or meprednisone or methylprednisolone or mycolog or nicocortonide or nivacortol or oropivalone or paramethasone or prednicarbate or prednisolone or prednisone or "prednival acetate" or prednylidene or pregnenolone or procinonide or promestriene or resocortol or rimexolone or rofleponide or sofradex or terracortril or tetrahydrocortiso* or ticabesone or timobesone or tipredane or tixocortol or triamcinolone or trophigil or uniderm or zoticasone).mp. 2 exp regional anesthesia/ or exp epidural anesthesia/ or exp local anesthesia/ or exp spinal anesthesia/ or exp nerve block/ or exp local anesthetic agent/ or ((an?eth* or analg*) adj3 (wipe or local or block* or topical or caudal or conduct* or epidural or extradural or peridural or infiltration or regional* or sacral or spinal or retrobulbar or subarachnoid or lumbar)).mp. or (block* adj3 (nerv* or ganglion* or brachial or paracervical or autonomic or pterygopalatine or sympathetic or sphenopalatine or caud* or dural or epidural or extradural or intercostal or neurogenic or subarachnoid or transversus or abdominis)).mp. or ("chemical neurolys?s" or "chemodenervation*" or gangliopleg* or (huneke adj2 neural therapy) or rachian?esth*).mp. or ("benzyl alcohol" or "carcainium chloride" or "pseudotropine benzoate" or amydricaine or amylocaine or articaine or aslavital or benzocaine or benzofurocaine or bucricaine or bumecaine or bupivacaine or butacaine or butanilicaine or butethamine or butoxycaine or butylcaine or carbisocaine or carticaine or centbucridine or cetacaine or chloroprocaine or cinchocaine or cocaine or cyclomethycaine or dibucaine or dimethocaine or diperodon or diphenhydramine or dyclonine or emla or ethyl chloride or etidocaine or eugenol or euprocin or fluress or fomocaine or guafecainol or heptacaine or hexathricin or hexylcaine or instillagel or ipravacaine or isobutamben or ketocaine or levobupivacaine or lidamidine or lidocaine or mepivacaine or meprylcaine or metabutethamine or myrtecaine or oxetacaine or oxybuprocaine or pentacaine or phenacaine or phenol or piperocaine or polidocanol or pramocaine or prilocaine or procaine or propanocaine or propoxycaine or propylcaine or proxymetacaine or pseudococaine or pyrrocaine or quinisocaine or ropivacaine or tanax or tetracaine or tetrodotoxin or tolycaine or tricaine or trimecaine or xyloproct or zolamine).mp. 3 exp intraoperative period/ or exp postoperative period/ or exp anesthetic recovery/ or (intra?operat* or peroperat* or postoperat* or (an?esthesia adj3 recover*)).mp. 4 1 and 2 and 3 5 (randomized‐placeboled‐trial/ or randomization/ or placeboled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 6 4 and 5
Appendix 4. ISI Web of Science search strategy
#1 TS=(glucocorticoid* or etiprednol dicloacetate or fluocinolone acetonide or icometasone enbutate or locicortolone dicibate or melengestrol acetate or mometasone furoate or ulobetasol propionate or alclometasone or algestone or amcinonide or amelometasone or baycuten or beclomet?asone or budesonide or butixocort or celestamine or chloroprednisone or ciclesonide or ciprocinonide or clobetaso* or clocortolone or cloprednol or cortisone or cortivazol or daktacort or deflazacort or desonide or desoximet?asone or dexatopic or diflorasone or diflucortolone or difluprednate or domoprednate or drocinonide or dutimelan or epihydrocortisone or fluclorolone or fludrocortisone or fludroxycortide or flumet?asone or flumoxonide or flunisolide or fluocinolone or fluocinonide or fluocortin or fluocortolone or fluorometholone or fluprednidene or fluprednisolone or flurandrenolone or fluticasone or formocortal or halcinonide or halometasone or halopredone or hydrallostane or hydrocortamate or hydrocortisone or isoflupredone or itrocinonide or lorinden or loteprednol or mazipredone or medrysone or meprednisone or methylprednisolone or mycolog or nicocortonide or nivacortol or oropivalone or paramethasone or prednicarbate or prednisolone or prednisone or prednival acetate or prednylidene or pregnenolone or procinonide or promestriene or resocortol or rimexolone or rofleponide or sofradex or terracortril or tetrahydrocortiso* or ticabesone or timobesone or tipredane or tixocortol or triamcinolone or trophigil or uniderm or zoticasone) #2 TS=((an?eth* or analg*) SAME (wipe or local or block* or topical or caudal or conduct* or epidural or extradural or peridural or infiltration or regional* or sacral or spinal or retrobulbar or subarachnoid or lumbar)) or TS=(block* SAME (nerv* or ganglion* or brachial or paracervical or autonomic or pterygopalatine or sympathetic or sphenopalatine or caud* or dural or epidural or extradural or intercostal or neurogenic or subarachnoid or transversus or abdominis)) or TS=(chemical neurolys?s or chemodenervation* or gangliopleg* or (huneke SAME neural therapy) or rachian?esth*) or TS=(benzyl alcohol or carcainium chloride or pseudotropine benzoate or amydricaine or amylocaine or articaine or aslavital or benzocaine or benzofurocaine or bucricaine or bumecaine or bupivacaine or butacaine or butanilicaine or butethamine or butoxycaine or butylcaine or carbisocaine or carticaine or centbucridine or cetacaine or chloroprocaine or cinchocaine or cocaine or cyclomethycaine or dibucaine or dimethocaine or diperodon or diphenhydramine or dyclonine or emla or ethyl chloride or etidocaine or eugenol or euprocin or fluress or fomocaine or guafecainol or heptacaine or hexathricin or hexylcaine or instillagel or ipravacaine or isobutamben or ketocaine or levobupivacaine or lidamidine or lidocaine or mepivacaine or meprylcaine or metabutethamine or myrtecaine or oxetacaine or oxybuprocaine or pentacaine or phenacaine or phenol or piperocaine or polidocanol or pramocaine or prilocaine or procaine or propanocaine or propoxycaine or propylcaine or proxymetacaine or pseudococaine or pyrrocaine or quinisocaine or ropivacaine or tanax or tetracaine or tetrodotoxin or tolycaine or tricaine or trimecaine or xyloproct or zolamine) #3 TS=(intra?operat* or peroperat* or postoperat* or (an?esthesia SAME recover*)) #4 #3 AND #2 AND #1
Appendix 5. Data Collection Tool
CARG
Data collection form
Intervention review – RCTs only
Notes on using a data extraction form:
· Be consistent in the order and style you use to describe the information for each report.
· Record any missing information as unclear or not described, to make it clear that the information was not found in the study report(s), not that you forgot to extract it.
· Include any instructions and decision rules on the data collection form, or in an accompanying document. It is important to practice using the form and to give training to any other authors using the form.
| Review title or ID |
| Study ID(surname of first author and year first full report of study was published, e.g. Smith 2001) |
| Report IDs of other reports of this study(e.g. duplicate publications, follow‐up studies) |
| Notes: |
1. General information
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data | |
|
Report title (title of paper/abstract/report from which data are extracted) |
|
|
Report ID (ID for this paper/abstract/report) |
|
| Reference details | |
| Report author contact details | |
|
Publication type (e.g. full report, abstract, letter) |
|
|
Study funding sources (including role of funders) |
|
|
Possible conflicts of interest (for study authors) |
|
| Notes: | |
2. Study eligibility
| Study characteristics |
Eligibility criteria (insert eligibility criteria for each characteristic as defined in the Protocol) |
Yes | No | Unclear |
Location in text (pg & ¶/fig/table) |
|
| Type of study | Randomized control trial | |||||
| Participants | ||||||
| Types of interventions | ||||||
| Types of outcome measures | ||||||
| INCLUDE | EXCLUDE | |||||
| Reason for exclusion | ||||||
| Notes: | ||||||
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW
3. Population and setting
|
Description (include comparative information for each group (i.e. intervention and placebos) if available) |
Location in text (pg & ¶/fig/table) |
||
|
Population description (from which study participants are drawn) |
|||
| Inclusion criteria | |||
| Exclusion criteria | |||
| Method/s of recruitment of participants | |||
| Informed consent obtained | Yes/No/Unclear | ||
| Notes: | |||
4. Methods
| Descriptions as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Aim of study | |||
| Design(e.g. parallel, cross‐over, cluster) | |||
|
Unit of allocation (by individuals, clusters/groups or body parts) |
|||
| Start date | |||
| End date | |||
| Total study duration | |||
| Ethical approval needed/obtained for study | Yes/No/Unclear | ||
| Notes: | |||
5. Risk of bias assessment
| Domain | Risk of bias | Support for judgement |
Location in text (pg & ¶/fig/table) |
||
| Low risk | High risk | Unclear risk | |||
|
Random sequence generation (selection bias) |
|||||
|
Allocation concealment (selection bias) |
|||||
|
Blinding of participants and personnel (performance bias) |
Outcome group: All/ | ||||
| (if required) | Outcome group: | ||||
|
Blinding of outcome assessment (detection bias) |
Outcome group: All/ | ||||
| (if required) | Outcome group: | ||||
|
Incomplete outcome data (attrition bias) |
|||||
|
Selective outcome reporting? (reporting bias) |
|||||
| Other bias | |||||
| Notes: | |||||
6. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Total no. randomly assigned (or total population at start of study for NRCTs) |
||
|
Withdrawals and exclusions (if not provided below by outcome) |
||
| Age | ||
| Sex | ||
| American Society of Anesthesiologists (ASA) classification | ||
| Subgroups measured | ||
| Subgroups reported | ||
| Notes: | ||
7. Intervention groups
Control
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Group name | ||
|
No. randomly assigned to group (specify whether no. people or clusters) |
||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| Duration of treatment period | ||
| Timing(e.g. frequency, duration of each episode) | ||
| Notes: | ||
Perineural dexamethasone
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Group name | ||
|
No. randomly assigned to group (specify whether no. people or clusters) |
||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| Duration of treatment period | ||
| Timing(e.g. frequency, duration of each episode) | ||
| Notes: | ||
Intravenous dexamethasone
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Group name | ||
|
No. randomly assigned to group (specify whether no. people or clusters) |
||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| Duration of treatment period | ||
| Timing(e.g. frequency, duration of each episode) | ||
| Notes: | ||
8. Outcomes
Severity of pain at 12 hours
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes/No/Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
Severity of pain at 24 hours
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes/No/Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
Severity of pain at 48 hours
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes/No/Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
Serious adverse event 1
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes/No/Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
Serious adverse event 2
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes/No/Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
Serious adverse event 3
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes/No/Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
Mild to moderate adverse event 1
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes/No/Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
Mild to moderate adverse event 2
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes/No/Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
Mild to moderate adverse event 3
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes/No/Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
Participant satisfaction
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes/No/Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
Duration of sensory block
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Outcome name | ||
| Outcome definition(with diagnostic criteria if relevant) | ||
| Person measuring/reporting | ||
|
Unit of measurement (if relevant) |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | ||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
||
| Power | ||
| Notes: | ||
Duration of motor block
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Outcome name | ||
| Outcome definition(with diagnostic criteria if relevant) | ||
| Person measuring/reporting | ||
|
Unit of measurement (if relevant) |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | ||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
||
| Power | ||
| Notes: | ||
Postoperative opioid requirement 12 hours
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Outcome name | ||
| Time points measured | ||
| Time points reported | ||
| Outcome definition(with diagnostic criteria if relevant) | ||
| Person measuring/reporting | ||
|
Unit of measurement (if relevant) |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | ||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
||
| Power | ||
| Notes: | ||
Postoperative opioid requirement 24 hours
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Outcome name | ||
| Time points measured | ||
| Time points reported | ||
| Outcome definition(with diagnostic criteria if relevant) | ||
| Person measuring/reporting | ||
|
Unit of measurement (if relevant) |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | ||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
||
| Power | ||
| Notes: | ||
Postoperative opioid requirement 48 hours
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Outcome name | ||
| Time points measured | ||
| Time points reported | ||
| Outcome definition(with diagnostic criteria if relevant) | ||
| Person measuring/reporting | ||
|
Unit of measurement (if relevant) |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | ||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
||
| Power | ||
| Notes: | ||
9. Results
Severity of pain at 12 hours
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post‐intervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
| Other results reported | ||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes/No/Unclear | |||||||||
| Reanalysis possible? | Yes/No/Unclear | |||||||||
| Reanalysed results | ||||||||||
| Notes: | ||||||||||
Severity of pain at 24 hours
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post‐intervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
| Other results reported | ||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes/No/Unclear | |||||||||
| Reanalysis possible? | Yes/No/Unclear | |||||||||
| Reanalysed results | ||||||||||
| Notes: | ||||||||||
Severity of pain at 48 hours
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post‐intervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
| Other results reported | ||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes/No/Unclear | |||||||||
| Reanalysis possible? | Yes/No/Unclear | |||||||||
| Reanalysed results | ||||||||||
| Notes: | ||||||||||
Serious adverse event 1
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||||
| Comparison | |||||
| Outcome | |||||
| Subgroup | |||||
| Time point (specify whether from start or end of intervention) | |||||
| Results | Intervention | Comparison | |||
| No. events | No. participants | No. events | No. participants | ||
| No. missing participants and reasons | |||||
| No. participants moved from other group and reasons | |||||
| Other results reported | |||||
| Unit of analysis(by individuals, clusters/groups or body parts) | |||||
Serious adverse event 2
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||||
| Comparison | |||||
| Outcome | |||||
| Subgroup | |||||
| Time point (specify whether from start or end of intervention) | |||||
| Results | Intervention | Comparison | |||
| No. events | No. participants | No. events | No. participants | ||
| No. missing participants and reasons | |||||
| No. participants moved from other group and reasons | |||||
| Other results reported | |||||
| Unit of analysis(by individuals, clusters/groups or body parts) | |||||
Serious adverse event 3
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||||
| Comparison | |||||
| Outcome | |||||
| Subgroup | |||||
| Time point (specify whether from start or end of intervention) | |||||
| Results | Intervention | Comparison | |||
| No. events | No. participants | No. events | No. participants | ||
| No. missing participants and reasons | |||||
| No. participants moved from other group and reasons | |||||
| Other results reported | |||||
| Unit of analysis(by individuals, clusters/groups or body parts) | |||||
Mild to moderate adverse event 1
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||||
| Comparison | |||||
| Outcome | |||||
| Subgroup | |||||
| Time point (specify whether from start or end of intervention) | |||||
| Results | Intervention | Comparison | |||
| No. events | No. participants | No. events | No. participants | ||
| No. missing participants and reasons | |||||
| No. participants moved from other group and reasons | |||||
| Other results reported | |||||
| Unit of analysis(by individuals, clusters/groups or body parts) | |||||
Mild to moderate adverse event 2
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||||
| Comparison | |||||
| Outcome | |||||
| Subgroup | |||||
| Time point (specify whether from start or end of intervention) | |||||
| Results | Intervention | Comparison | |||
| No. events | No. participants | No. events | No. participants | ||
| No. missing participants and reasons | |||||
| No. participants moved from other group and reasons | |||||
| Other results reported | |||||
| Unit of analysis(by individuals, clusters/groups or body parts) | |||||
Mild to moderate adverse event 3
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||||
| Comparison | |||||
| Outcome | |||||
| Subgroup | |||||
| Time point (specify whether from start or end of intervention) | |||||
| Results | Intervention | Comparison | |||
| No. events | No. participants | No. events | No. participants | ||
| No. missing participants and reasons | |||||
| No. participants moved from other group and reasons | |||||
| Other results reported | |||||
| Unit of analysis(by individuals, clusters/groups or body parts) | |||||
Participant satisfaction
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post‐intervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
| Other results reported | ||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes/No/Unclear | |||||||||
| Reanalysis possible? | Yes/No/Unclear | |||||||||
| Reanalysed results | ||||||||||
| Notes: | ||||||||||
Duration of motor block
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post‐intervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
| Other results reported | ||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes/No/Unclear | |||||||||
| Reanalysis possible? | Yes/No/Unclear | |||||||||
| Reanalysed results | ||||||||||
| Notes: | ||||||||||
Duration of sensory block
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post‐intervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
| Other results reported | ||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes/No/Unclear | |||||||||
| Reanalysis possible? | Yes/No/Unclear | |||||||||
| Reanalysed results | ||||||||||
| Notes: | ||||||||||
Postoperative opioid requirement 12 hours
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post‐intervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
| Other results reported | ||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes/No/Unclear | |||||||||
| Reanalysis possible? | Yes/No/Unclear | |||||||||
| Reanalysed results | ||||||||||
| Notes: | ||||||||||
Postoperative opioid requirement 24 hours
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post‐intervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
| Other results reported | ||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes/No/Unclear | |||||||||
| Reanalysis possible? | Yes/No/Unclear | |||||||||
| Reanalysed results | ||||||||||
| Notes: | ||||||||||
Postoperative opioid requirement 48 hours
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post‐intervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
| Other results reported | ||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes/No/Unclear | |||||||||
| Reanalysis possible? | Yes/No/Unclear | |||||||||
| Reanalysed results | ||||||||||
| Notes: | ||||||||||
10. Applicability
|
Does the study directly address the review question? (issues of partial or indirect applicability) |
Yes/No/Unclear | |
| Notes: | ||
11. Other information
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Key conclusions of study authors | ||
| References to other relevant studies | ||
| Correspondence required for further study information(from whom, what and when) | ||
| Notes: | ||
Data and analyses
Comparison 1. Duration of sensory block: perineural dexamethasone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of sensory block | 27 | 1625 | Mean Difference (IV, Random, 95% CI) | 6.70 [5.54, 7.85] |
| 2 Duration of sensory block: long‐ versus medium‐acting local anaesthetic subgroups | 26 | 1572 | Mean Difference (IV, Random, 95% CI) | 6.78 [5.62, 7.94] |
| 2.1 Long‐acting local anaesthetic | 20 | 1315 | Mean Difference (IV, Random, 95% CI) | 7.81 [6.40, 9.21] |
| 2.2 Medium‐acting local anaesthetic | 6 | 257 | Mean Difference (IV, Random, 95% CI) | 3.98 [1.76, 6.20] |
| 3 Duration of sensory block: additive versus no additive subgroups | 27 | 1625 | Mean Difference (IV, Random, 95% CI) | 6.70 [5.54, 7.85] |
| 3.1 Additives | 6 | 336 | Mean Difference (IV, Random, 95% CI) | 7.29 [3.77, 10.81] |
| 3.2 No additives | 21 | 1289 | Mean Difference (IV, Random, 95% CI) | 6.60 [5.30, 7.89] |
| 4 Duration of sensory block: high‐ versus low‐dose dexamethasone subgroups | 27 | 1627 | Mean Difference (IV, Random, 95% CI) | 6.70 [5.53, 7.86] |
| 4.1 High‐dose dexamethasone | 23 | 1447 | Mean Difference (IV, Random, 95% CI) | 7.09 [5.81, 8.38] |
| 4.2 Low‐dose dexamethasone | 4 | 180 | Mean Difference (IV, Random, 95% CI) | 4.32 [1.80, 6.85] |
| 5 Duration of sensory block: high/unclear versus low risk of bias subgroups | 26 | 1625 | Mean Difference (IV, Random, 95% CI) | 6.70 [5.54, 7.85] |
| 5.1 High or unclear risk of bias | 19 | 1037 | Mean Difference (IV, Random, 95% CI) | 6.28 [5.01, 7.56] |
| 5.2 Low risk of bias | 8 | 588 | Mean Difference (IV, Random, 95% CI) | 8.21 [4.56, 11.85] |
Comparison 2. Duration of motor block: perineural dexamethasone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of motor block | 16 | 912 | Mean Difference (IV, Random, 95% CI) | 5.87 [4.44, 7.30] |
| 2 Duration of motor block: long‐ versus medium‐acting local anaesthetic subgroups | 16 | 912 | Mean Difference (IV, Random, 95% CI) | 5.87 [4.44, 7.30] |
| 2.1 Long‐acting local anaesthetic | 13 | 764 | Mean Difference (IV, Random, 95% CI) | 6.61 [4.58, 8.65] |
| 2.2 Medium‐acting local anaesthetic | 3 | 148 | Mean Difference (IV, Random, 95% CI) | 2.59 [2.42, 2.76] |
| 3 Duration of motor block: additives verus no additives subgroups | 16 | 912 | Mean Difference (IV, Random, 95% CI) | 5.87 [4.44, 7.30] |
| 3.1 Additives | 5 | 280 | Mean Difference (IV, Random, 95% CI) | 7.47 [3.58, 11.36] |
| 3.2 No additives | 11 | 632 | Mean Difference (IV, Random, 95% CI) | 5.26 [3.17, 7.35] |
| 4 Duration of motor block: high‐ versus low‐dose dexamethasone subgroups | 16 | 912 | Mean Difference (IV, Random, 95% CI) | 5.87 [4.44, 7.30] |
| 4.1 High‐dose dexamethasone | 15 | 872 | Mean Difference (IV, Random, 95% CI) | 5.75 [4.29, 7.22] |
| 4.2 Low‐dose dexamethasone | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 8.1 [4.69, 11.51] |
| 5 Duration of motor block: high/unclear versus low risk of bias subgroups | 16 | 912 | Mean Difference (IV, Random, 95% CI) | 5.87 [4.44, 7.30] |
| 5.1 High/unclear risk of bias | 14 | 809 | Mean Difference (IV, Random, 95% CI) | 5.67 [4.18, 7.16] |
| 5.2 Low risk of bias | 2 | 103 | Mean Difference (IV, Random, 95% CI) | 7.93 [2.74, 13.13] |
Comparison 3. Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall incidence of block‐related adverse events | 10 | 677 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.99, 1.39] |
| 2 Numbness/tingling 14 days after surgery | 5 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [0.80, 3.89] |
| 3 Residual motor block/weakness 24 hours after surgery | 3 | 259 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [0.57, 38.68] |
| 4 Horner Syndrome | 4 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.73, 1.36] |
| 5 Hoarseness | 4 | 353 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.65, 2.34] |
| 6 Diaphragmatic paresis | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.66, 3.23] |
| 7 Dyspnoea | 4 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.14] |
| 8 Vascular injury | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.19, 21.36] |
| 9 Cranial nerve 12 palsy | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.77] |
| 10 Bruising | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.07, 15.64] |
| 11 Overall non‐block‐related adverse events | 10 | 625 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.68] |
| 12 Postoperative nausea and vomiting | 10 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.26, 1.14] |
| 13 Deep sedation | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.38, 129.93] |
| 14 Dermatological symptoms (pruritus/rash) | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 2.93 [0.32, 27.02] |
| 15 Syncope/fainting | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.18, 20.71] |
| 16 Bradycardia | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.12, 3.71] |
| 17 Hypotension | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.21, 2.13] |
| 18 Headache/10‐pound fluid gain/diarrhoea/frequent urination/muscle soreness | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 2.93 [0.12, 69.92] |
3.11. Analysis.
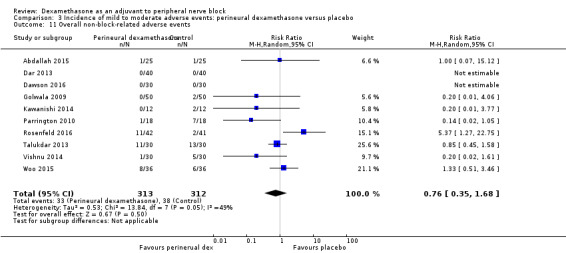
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 11 Overall non‐block‐related adverse events.
Comparison 4. Postoperative pain intensity at 12 hours: perineural dexamethasone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative pain intensity at12 hours | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐2.63, ‐1.52] |
| 2 Postoperative pain intensity at 12 hours: medium‐ versus long‐acting local anaesthetic subgroups | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐2.63, ‐1.53] |
| 2.1 Long‐acting local anaesthesia | 4 | 234 | Mean Difference (IV, Random, 95% CI) | ‐2.21 [‐2.77, ‐1.66] |
| 2.2 Medium‐acting local anaesthesia | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.38, ‐0.06] |
| 3 Postoperative pain intensity at 12 hours: additive versus no additive subgroups | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐2.63, ‐1.52] |
| 3.1 Additives | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.36, ‐0.04] |
| 3.2 No additives | 4 | 234 | Mean Difference (IV, Random, 95% CI) | ‐2.21 [‐2.77, ‐1.66] |
| 4 Postoperative pain intensity at 12 hours: high‐ versus low‐dose dexamethasone subgroups | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐2.63, ‐1.52] |
| 4.1 High‐dose dexamethasone | 3 | 177 | Mean Difference (IV, Random, 95% CI) | ‐2.17 [‐3.29, ‐1.06] |
| 4.2 Low‐dose dexamethasone | 2 | 80 | Mean Difference (IV, Random, 95% CI) | ‐1.99 [‐2.75, ‐1.22] |
| 5 Postoperative pain intensity at 12 hours: high/unclear versus low risk of bias subgroups | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐2.63, ‐1.52] |
| 5.1 High/unclear versus low risk of bias | 3 | 103 | Mean Difference (IV, Random, 95% CI) | ‐1.81 [‐2.53, ‐1.09] |
| 5.2 Low risk of bias | 2 | 154 | Mean Difference (IV, Random, 95% CI) | ‐2.61 [‐3.88, ‐1.34] |
Comparison 5. Postoperative pain intensity at 24 hours: perineural dexamethasone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative pain intensity at 24 hours | 9 | 469 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.34, ‐0.93] |
| 2 Postoperative pain intensity at 24 hours: long‐ versus medium‐acting local anaesthetic subgroups | 9 | 469 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.34, ‐0.93] |
| 2.1 Long‐acting local anaesthesia | 7 | 409 | Mean Difference (IV, Random, 95% CI) | ‐1.75 [‐2.60, ‐0.90] |
| 2.2 Medium‐acting local anaesthesia | 2 | 60 | Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐2.07, ‐0.09] |
| 3 Postoperative pain intensity at 24 hours: additive versus no additive subgroups | 9 | 469 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.34, ‐0.93] |
| 3.1 Additives | 3 | 158 | Mean Difference (IV, Random, 95% CI) | ‐2.13 [‐3.43, ‐0.82] |
| 3.2 No additives | 6 | 311 | Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐2.31, ‐0.51] |
| 4 Postoperative pain intensity at 24 hours: high‐ versus low‐dose dexamethasone subgroups | 9 | 469 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.34, ‐0.93] |
| 4.1 High‐dose dexamethasone | 7 | 389 | Mean Difference (IV, Random, 95% CI) | ‐1.59 [‐2.71, ‐0.47] |
| 4.2 Low‐dose dexamethasone | 2 | 80 | Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐3.21, ‐0.52] |
| 5 Postoperative pain intensity at 24 hours: high/unclear versus low risk of bias subgroups | 9 | 469 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.34, ‐0.93] |
| 5.1 High/unclear risk of bias | 4 | 185 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.79, ‐1.00] |
| 5.2 Low risk of bias | 5 | 284 | Mean Difference (IV, Random, 95% CI) | ‐1.43 [‐2.91, 0.04] |
Comparison 6. Postoperative pain intensity at 48 hours: perineural dexamethasone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative pain intensity at 48 hours | 4 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.24, 0.03] |
| 2 Postoperative pain intensity at 48 hours: additives versus no additives subgroups | 4 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.24, 0.03] |
| 2.1 No additives | 2 | 155 | Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.36, ‐0.36] |
| 2.2 Additives | 2 | 141 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐1.80, 1.34] |
| 3 Postoperative pain intensity at 48 hours: high/unclear versus low risk of bias subgroups | 4 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.24, 0.03] |
| 3.1 High/unclear risk of bias | 1 | 88 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐2.24, 0.24] |
| 3.2 Low risk of bias | 3 | 208 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.30, 0.40] |
Comparison 7. Postoperative opioid consumption at 24 hours: perineural dexamethasone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative opioid consumption at 24 hours | 6 | 380 | Mean Difference (IV, Random, 95% CI) | ‐19.25 [‐32.51, ‐5.99] |
| 2 Opioid consumption at 24 hours medium‐ versus long‐acting local anaesthetic subgroups | 6 | 380 | Mean Difference (IV, Random, 95% CI) | ‐19.25 [‐32.51, ‐5.99] |
| 2.1 Long‐acting local anaesthetic | 5 | 335 | Mean Difference (IV, Random, 95% CI) | ‐21.22 [‐35.20, ‐7.25] |
| 2.2 Medium‐acting local anaesthetic | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐33.91, 41.91] |
| 3 Opioid consumption at 24 hours: additive versus no additive subgroups | 6 | 380 | Mean Difference (IV, Random, 95% CI) | ‐19.25 [‐32.51, ‐5.99] |
| 3.1 Additives | 2 | 142 | Mean Difference (IV, Random, 95% CI) | ‐30.17 [‐58.58, ‐1.76] |
| 3.2 No additives | 4 | 238 | Mean Difference (IV, Random, 95% CI) | ‐12.98 [‐26.28, 0.32] |
| 4 Opioid consumption at 24 hours: high/unclear versus low risk of bias subgroups | 6 | 380 | Mean Difference (IV, Random, 95% CI) | ‐19.25 [‐32.51, ‐5.99] |
| 4.1 High/unclear risk of bias | 1 | 88 | Mean Difference (IV, Random, 95% CI) | ‐45.0 [‐57.58, ‐32.42] |
| 4.2 Low risk of bias | 5 | 292 | Mean Difference (IV, Random, 95% CI) | ‐13.55 [‐23.36, ‐3.75] |
Comparison 8. Participant satisfaction with pain control; perineural dexamethasone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant satisfaction with pain control: perineural dexamethasone versus placebo | 4 | 224 | Mean Difference (IV, Random, 95% CI) | 0.83 [‐0.05, 1.71] |
Comparison 9. Duration of sensory block: intravenous dexamethasone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of sensory block | 8 | 499 | Mean Difference (IV, Random, 95% CI) | 6.21 [3.53, 8.88] |
| 2 Duration sensory block: additive versus no additive subgroups | 8 | 499 | Mean Difference (IV, Random, 95% CI) | 6.21 [3.53, 8.88] |
| 2.1 Additives | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 6.20 [0.68, 11.72] |
| 2.2 No additives | 7 | 450 | Mean Difference (IV, Random, 95% CI) | 6.21 [3.33, 9.08] |
| 3 Duration of sensory block: high‐ versus low‐dose dexamethasone subgroups | 8 | 499 | Mean Difference (IV, Random, 95% CI) | 6.21 [3.53, 8.88] |
| 3.1 High‐dose | 6 | 437 | Mean Difference (IV, Random, 95% CI) | 7.45 [5.55, 9.35] |
| 3.2 Low‐dose | 2 | 62 | Mean Difference (IV, Random, 95% CI) | 2.25 [1.21, 3.30] |
| 4 Duration of sensory block: high/unclear versus low risk of bias subgroups | 8 | 499 | Mean Difference (IV, Random, 95% CI) | 6.21 [3.53, 8.88] |
| 4.1 High/unclear risk of bias | 2 | 62 | Mean Difference (IV, Random, 95% CI) | 2.25 [1.21, 3.30] |
| 4.2 Low risk of bias | 6 | 437 | Mean Difference (IV, Random, 95% CI) | 7.45 [5.55, 9.35] |
Comparison 10. Duration of motor block: intravenous dexamethasone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of motor block | 3 | 139 | Mean Difference (IV, Random, 95% CI) | 5.04 [3.07, 7.00] |
| 2 Duration of motor block: additive versus no additive subgroups | 3 | 139 | Mean Difference (IV, Random, 95% CI) | 5.54 [3.11, 7.97] |
| 2.1 Additives | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 6.60 [2.32, 10.88] |
| 2.2 No additives | 2 | 90 | Mean Difference (IV, Random, 95% CI) | 3.67 [‐2.77, 10.11] |
| 3 Duration of motor block high‐ versus low‐dose dexamethasone subgroups | 3 | 139 | Mean Difference (IV, Random, 95% CI) | 5.04 [3.07, 7.00] |
| 3.1 High‐dose dexamethasone | 2 | 99 | Mean Difference (IV, Random, 95% CI) | 5.96 [4.03, 7.90] |
| 3.2 Low‐dose dexamethasone | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 3.10 [0.23, 5.97] |
| 4 Duration of motor block: high/unclear versus low risk of bias subgroups | 3 | 139 | Mean Difference (IV, Random, 95% CI) | 5.04 [3.07, 7.00] |
| 4.1 High/unclear risk of bias | 2 | 99 | Mean Difference (IV, Random, 95% CI) | 5.96 [4.03, 7.90] |
| 4.2 Low risk of bias | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 3.10 [0.23, 5.97] |
Comparison 11. Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall incidence of block‐related adverse events | 5 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.69, 1.70] |
| 2 Numbness/tingling 14 days after surgery | 2 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.31, 9.26] |
| 3 Residual motor block/muscle weakness 24 hours after surgery | 3 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 2.68 [0.80, 8.90] |
| 4 Horner syndrome | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.63, 1.26] |
| 5 Hoarsenss | 2 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.45, 1.71] |
| 6 Dyspnoea | 3 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.11, 3.74] |
| 7 Cranial nerve 12 palsy | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 8.77] |
| 8 Overall non‐block‐related adverse events | 5 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.38, 3.97] |
| 9 Postoperative nausea and vomiting | 3 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.12, 3.78] |
| 10 Dermatological symptoms | 2 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [0.09, 40.62] |
| 11 Dizziness/wrist, hand or finger pain, constipation | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 8.77] |
11.1. Analysis.
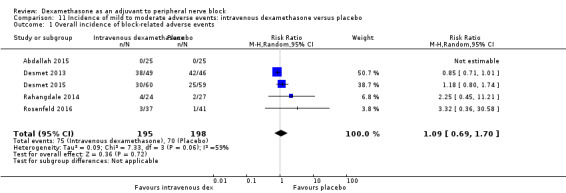
Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 1 Overall incidence of block‐related adverse events.
Comparison 12. Postoperative pain intensity at 12 hours: intravenous dexamethasone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative pain intensity at 12 hours | 3 | 162 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐2.44, ‐0.04] |
| 2 Postoperative pain intensity at 12 hours: high‐ versus low‐dose dexamethasone subgroups | 3 | 162 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐2.44, ‐0.04] |
| 2.1 High‐dose dexamethasone | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐3.16, ‐0.44] |
| 2.2 Low‐dose dexamethasone | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.41, 0.41] |
| 3 Postoperative pain intensity at 12 hours: high/unclear versus low risk of bias subgroups | 3 | 162 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐2.44, ‐0.04] |
| 3.1 High/unclear risk of bias | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.41, 0.41] |
| 3.2 Low risk of bias | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐3.16, ‐0.44] |
12.2. Analysis.
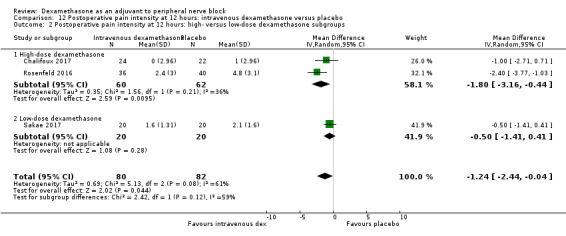
Comparison 12 Postoperative pain intensity at 12 hours: intravenous dexamethasone versus placebo, Outcome 2 Postoperative pain intensity at 12 hours: high‐ versus low‐dose dexamethasone subgroups.
12.3. Analysis.
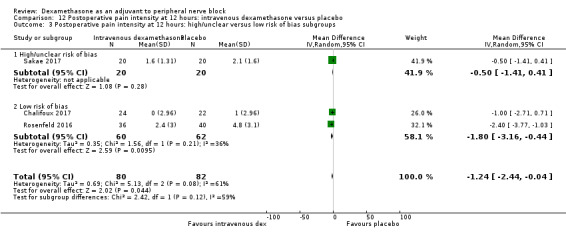
Comparison 12 Postoperative pain intensity at 12 hours: intravenous dexamethasone versus placebo, Outcome 3 Postoperative pain intensity at 12 hours: high/unclear versus low risk of bias subgroups.
Comparison 13. Postoperative pain intensity at 24 hours: intravenous dexamethasone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative pain intensity at 24 hours | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐2.23, ‐0.29] |
| 2 Postoperative pain intensity at 24 hours: additive versus no additive subgroups | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐2.23, ‐0.29] |
| 2.1 Additive | 1 | 49 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.75, 0.95] |
| 2.2 No additive | 4 | 208 | Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐2.48, ‐0.18] |
| 3 Postoperative pain intensity at 24 hours: high‐ versus low‐dose dexamethasone subgroups | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐2.23, ‐0.29] |
| 3.1 High‐dose dexamethasone | 4 | 217 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐2.54, ‐0.07] |
| 3.2 Low‐dose dexamethasone | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.1 [‐2.49, 0.29] |
| 4 Postoperative pain intensity at 24 hours: high/unclear versus low risk of bias subgroups | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐2.23, ‐0.29] |
| 4.1 High/unclear risk of bias | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.1 [‐2.49, 0.29] |
| 4.2 Low risk of bias | 4 | 217 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐2.54, ‐0.07] |
Comparison 14. Postoperative pain intensity at 48 hours: intravenous dexamethasone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative pain intensity at 48 hours | 3 | 172 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.80, 0.44] |
| 2 Postoperative pain intensity at 48 hours: additive versus no additive subgroups | 3 | 172 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.80, 0.44] |
| 2.1 Additive | 1 | 49 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.60, 1.20] |
| 2.2 No additive | 2 | 123 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.87, 0.52] |
Comparison 15. Postoperative opioid consumption at 24 hours: intravenous dexamethasone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 24‐hour opioid consumption | 5 | 287 | Mean Difference (IV, Random, 95% CI) | ‐6.58 [‐10.56, ‐2.60] |
| 2 24‐hour opioid consumption: additive verus no additive subgroups | 5 | 287 | Mean Difference (IV, Random, 95% CI) | ‐6.58 [‐10.56, ‐2.60] |
| 2.1 Additive | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐13.33, 5.33] |
| 2.2 No additive | 4 | 234 | Mean Difference (IV, Random, 95% CI) | ‐6.93 [‐11.41, ‐2.46] |
Comparison 16. Postoperative opioid consumption at 48 hours: intravenous dexamethasone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative opioid consumption at 48 hours opioid consumption | 1 | 46 | Mean Difference (IV, Random, 95% CI) | ‐22.5 [‐39.85, ‐5.15] |
Comparison 17. Participant satisfaction with pain control: intravenous dexamethasone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant satisfaction with pain control | 3 | 181 | Mean Difference (IV, Random, 95% CI) | 1.07 [‐0.08, 2.22] |
Comparison 18. Duration of sensory block: perineural versus intravenous dexamethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of sensory block | 9 | 720 | Mean Difference (IV, Random, 95% CI) | 3.13 [1.68, 4.58] |
| 2 Duration of sensory block additive versus no additive subgroups | 9 | 720 | Mean Difference (IV, Random, 95% CI) | 3.13 [1.68, 4.58] |
| 2.1 Additive | 3 | 331 | Mean Difference (IV, Random, 95% CI) | 3.94 [2.66, 5.21] |
| 2.2 No additive | 6 | 389 | Mean Difference (IV, Random, 95% CI) | 2.67 [0.00, 5.34] |
| 3 Duration sensory block high‐dose versus low‐dose dexamethasone subgroups | 9 | 720 | Mean Difference (IV, Random, 95% CI) | 3.13 [1.68, 4.58] |
| 3.1 High‐dose dexamethasone | 6 | 508 | Mean Difference (IV, Random, 95% CI) | 2.35 [0.04, 4.66] |
| 3.2 Low‐dose dexamethasone | 3 | 212 | Mean Difference (IV, Random, 95% CI) | 4.14 [2.48, 5.81] |
| 4 Duration sensory block high/unclear versus low risk of bias subgroups | 9 | 720 | Mean Difference (IV, Random, 95% CI) | 3.13 [1.68, 4.58] |
| 4.1 High/unclear risk of bias | 3 | 162 | Mean Difference (IV, Random, 95% CI) | 4.67 [2.29, 7.04] |
| 4.2 Low risk of bias | 6 | 558 | Mean Difference (IV, Random, 95% CI) | 2.30 [0.23, 4.37] |
18.4. Analysis.
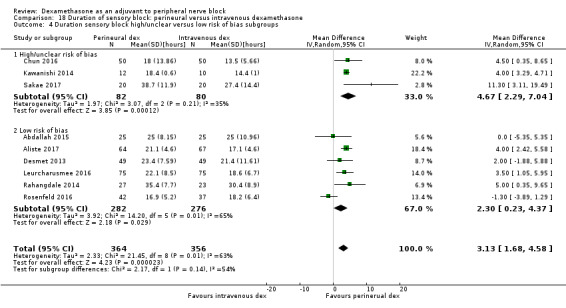
Comparison 18 Duration of sensory block: perineural versus intravenous dexamethasone, Outcome 4 Duration sensory block high/unclear versus low risk of bias subgroups.
Comparison 19. Duration of motor block: perineural versus intravenous dexamethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of motor block | 5 | 421 | Mean Difference (IV, Random, 95% CI) | 3.13 [0.99, 5.27] |
| 2 Duration of motor block: additive versus no additive subgroups | 5 | 340 | Mean Difference (IV, Random, 95% CI) | 2.75 [0.32, 5.19] |
| 2.1 Additive | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐0.03, 8.03] |
| 2.2 No additive | 4 | 290 | Mean Difference (IV, Random, 95% CI) | 2.39 [‐0.58, 5.37] |
| 3 Duration of motor block: high‐ versus low‐dose dexamethasone subgroups | 5 | 421 | Mean Difference (IV, Random, 95% CI) | 3.13 [0.99, 5.27] |
| 3.1 High‐dose dexamethasone | 4 | 381 | Mean Difference (IV, Random, 95% CI) | 2.47 [‐0.25, 5.19] |
| 3.2 Low‐dose dexamethasone | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 5.0 [2.53, 7.47] |
| 4 Duration of motor block: high/unclear versus low risk of bias subgroups | 5 | 421 | Mean Difference (IV, Random, 95% CI) | 3.13 [0.99, 5.27] |
| 4.1 HIgh/unclear risk of bias | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 5.0 [2.53, 7.47] |
| 4.2 Low risk of bias | 4 | 381 | Mean Difference (IV, Random, 95% CI) | 2.47 [‐0.25, 5.19] |
Comparison 20. Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall incidence of block‐related adverse events | 5 | 406 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.93, 1.55] |
| 2 Numbness/tingling 14 days after surgery | 3 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.27, 3.49] |
| 3 Residual motor block/weakness at 24 hours | 3 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.62, 2.37] |
| 4 Horner syndrome | 2 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.2 [0.77, 1.87] |
| 5 Hoarsness | 2 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.48, 2.09] |
| 6 Cranial nerve 12 motor palsy | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.39] |
| 7 Overall incidence of non block‐related adverse events | 5 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.37, 4.78] |
| 8 Postoperative nausea and vomiting | 5 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.22, 1.80] |
| 9 Dermatologicial symptoms (pruritus/rash) | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 4.42 [0.22, 89.18] |
| 10 Syncope/fainting | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.42 [0.22, 89.18] |
| 11 Dizziness | 2 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.06, 2.72] |
| 12 Wrist, hand or finger pain | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 7.02] |
| 13 Headache, 10‐pound fluid gain/diarrhoea/frequent urination/ muscle soreness | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 2.65 [0.11, 63.16] |
Comparison 21. Postoperative pain intensity at 12 hours: perineural versus intravenous dexamethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative pain intensity at 12 hours | 3 | 217 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.51, ‐0.50] |
| 2 Postoperative pain intensity at 12 hours: low‐ versus high‐dose dexamethasone subgroups | 3 | 217 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.51, ‐0.50] |
| 2.1 Low‐dose dexamethasone | 2 | 139 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.60, ‐0.47] |
| 2.2 High‐dose dexamethasone | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.01, 0.21] |
| 3 Postoperative pain intensity at 12 hours: high/unclear versus low risk of bias subgroups | 3 | 217 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.51, ‐0.50] |
| 3.1 HIgh/unclear risk of bias | 2 | 139 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.60, ‐0.47] |
| 3.2 Low risk of bias | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.01, 0.21] |
Comparison 22. Postoperative pain intensity at 24 hours: perineural versus intravenous dexamethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative pain intensity at 24 hours | 5 | 309 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.51, ‐0.07] |
| 2 Postoperative pain intensity at 24 hours: additive versus no additive subgroups | 5 | 309 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.51, ‐0.07] |
| 2.1 Additive | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐3.32, ‐0.08] |
| 2.2 No additive | 4 | 259 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.41, 0.13] |
| 3 Postoperative pain intensity at 24 hours: low‐ versus high‐dose dexamethasonesubgroups | 5 | 309 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.51, ‐0.07] |
| 3.1 Low‐dose dexamethasone | 2 | 139 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐2.01, 0.11] |
| 3.2 High‐dose dexamethasone | 3 | 170 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.95, 0.59] |
| 4 Intensity of postoperative pain at 24 hours: high/unclear risk of bias versus low risk of bias subgroups | 5 | 309 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.51, ‐0.07] |
| 4.1 High/unclear risk of bias | 2 | 139 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐2.01, 0.11] |
| 4.2 Low risk of bias | 3 | 170 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.95, 0.59] |
Comparison 23. Postoperative pain intensity at 48 hours: perineural versus intravenous dexamethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative pain intensity at 48 hours | 3 | 227 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.35, 0.61] |
| 2 Postoperative pain intensity at 48 hours: additive versus no additive subgroups | 3 | 227 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.35, 0.61] |
| 2.1 Additive | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.51, 2.11] |
| 2.2 No additive | 2 | 177 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.50, 0.54] |
| 3 Postoperative pain intensity at 48 hours: low‐ versus high‐dose dexamethasone subgroups | 3 | 227 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.35, 0.61] |
| 3.1 Low‐dose dexamethasone | 1 | 99 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.59, 0.59] |
| 3.2 High‐dose dexamethasone | 2 | 128 | Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.45, 1.24] |
| 4 Postoperative pain intensity at 48 hours: high/unclear versus low risk of bias subgroups | 3 | 227 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.35, 0.61] |
| 4.1 High/unclear risk of bias | 1 | 99 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.59, 0.59] |
| 4.2 Low risk of bias | 2 | 128 | Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.45, 1.24] |
Comparison 24. Postoperative opioid consumption at 24 hours: perineural versus intravenous dexamethasone opioid consumption: perineural versus intravenous dexamethasone subgroups.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Opioid consumption at 24 hours: perineural versus intravenous dexamethasone | 4 | 242 | Mean Difference (IV, Random, 95% CI) | ‐3.87 [‐9.93, 2.19] |
| 2 24‐hour opioid consumption: additive versus no additive subgroups | 4 | 242 | Mean Difference (IV, Random, 95% CI) | ‐3.87 [‐9.93, 2.19] |
| 2.1 Additive | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐10.00 [‐23.96, ‐0.04] |
| 2.2 No additive | 3 | 189 | Mean Difference (IV, Random, 95% CI) | ‐1.56 [‐6.34, 3.22] |
Comparison 25. Participant satisfaction with pain control: perineural versus intravenous dexamethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant satisfaction with pain control | 3 | 181 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.33, 0.70] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdallah 2015.
| Methods | Parallel group RCT. | |
| Participants | In Canada, 75 ASA class I‐III participants aged 18‐80 years with BMI less than 35 m2 undergoing elective forearm or hand surgery with supraclavicular brachial plexus block were included. Those with cognitive or psychiatric history, pregnancy, diabetes mellitus, clavicular fracture, surgical procedure 180 minutes or longer, severe respiratory disease, chest or shoulder deformities on the operative side, preexisting chronic pain, preexisting neurological deficit or neuropathy in the upper extremities, allergy to any drugs used in the study or contraindication to peripheral nerve block such as local skin infection, coagulopathy or bleeding diathesis were excluded. | |
| Interventions |
Block All participants underwent ultrasound‐guided supraclavicular block with bupivacaine 0.5 % 30 ml. Dexamethasone/placebo Perineural dexamethasone group: dexamethasone 8 mg perineurally and normal saline 2 ml intravenously. Intravenous dexamethasone group: dexamethasone 8 mg intravenously, normal saline 2 ml perineurally. Placebo group: normal saline 2 ml perineurally and normal saline 2 ml intravenously. Intraperative anaesthesia/analgesia Intraoperative sedation with midazolam (1‐3 mg), fentanyl (1‐2 micrograms/kg) and/or propofol (25‐75 micrograms/kg/min) titrated to participant comfort. Postoperative anaesthesia/analgesia Fentanyl was administered every 5 minutes as needed up to 200 micrograms/hour to participants reporting moderate to severe pain (VAS 4 or greater) or at participant request. Participants requiring additional analgesics received acetaminophen 1g followed by oxycodone 5 mg as needed. |
|
| Outcomes |
Outcomes of interest for the review Duration of analgesia defined as time in hours to the first report of postoperative pain. Duration of motor block defined as time in hours to return to normal (or baseline) motor strength in the operative limb. Postoperative pain intensity (VAS) at 24 hours. Cummulative intraoperative opioid consumption converted to intravenous morphine equivalent. Cummulative postoperative opioid consumption converted to oral morphine equivalent at 24 hours. Incidence of postoperative nausea and vomiting at 24 hours after surgery. Participant satisfaction with pain relief (expressed as VAS) at 24 hours after surgery. Occurrence of any block‐related complications including new paraesthesia (numbness or tingling) or weakness in the operative limb at 2 weeks after surgery. Other outcomes Postoperative pain intensity at eight hours, and at seven days and 14 days. |
|
| Notes | Funding: Drs. Faraj Abdallah and Richard Brull are supported by the Merit Award Program, Department of Anesthesia, University of Toronto. Conflicts of interest: Vincient Chan received equipment support from BK Medical, Philips Medical Systems, SonoSite and Ultrasonix. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Allocation sequence was concealed by sealed, opaque envelopes. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | The anaesthesiologist performing the block, the intraoperative anaesthesiologists, surgeons and nurses were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as states in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
Alarasan 2017.
| Methods | Parallel group RCT. | |
| Participants | In India, 60 ASA class I or II participants aged 20‐69 years undergoing elective upper limb surgery (expected duration 60‐120 minutes) with ultrasound‐guided supraclavicular brachial plexus block. Participants with communication difficulties, hypersensitivity to local anaesthetics and dexamethasone, those on sedative medications and perioperative intravenous steroids were excluded from the study. | |
| Interventions |
Block All participants received supraclavicular brachial plexus block with bupivacaine 0.5% 20 ml. Dexamethasone/placebo Perineural dexamethasone group: dexamethasone 8 mg perineurally Placebo group: normal saline 2 ml intravenously. Intraoperative anaesthesia/analgesia Diazepam 0.15 mg orally the night before and on the morning of surgery. Postoperative anaesthesia/analgesia Diclofenac 1.5 mg intravenously for VAS > 30. |
|
| Outcomes |
Outcomes of interest for the review Duration of sensory block defined as the onset of block and appearance of pain requiring analgesia. Duration of motor block defined as the time interval between complete motor paralysis to the compete return of motor power. Adverse events including nausea, vomiting, bradycardia, hypotension, convulsions, haematoma. Other outcomes Onset of block defined as the interval between injection of study drug to complete loss of cold perception and complete paralysis. Severity of pain at 90, 150, 210, 270, 330, 390 and 450 minutes after surgery. |
|
| Notes | Funding: Gajira Raja Medical College, Gwalior, Madhya Pradesh, India. Conflicts of interest: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | Anesthesiologist performing the block was blinded, however, no indication whether other personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes were reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
Aliste 2017.
| Methods | Parallel group RCT. | |
| Participants | In Canada and Thailand, 150 ASA class I‐II participants aged 18‐80 years undergoing forearm, wrist or hand surgery with ultrasound‐guided axillary block. Participants with sepsis, coagulopathy, allergy to local anaesthesia, hepatic or renal failure, pre‐existing upper limb neuropathy and who had prior surgery to the axilla were excluded. | |
| Interventions |
Block All participants received ultrasound‐guided axillary nerve block with equal parts of lidocaine 2% and bupivacaine 0.5% with epinephrine 5 micrograms/ml 25 ml. Dexamethasone/placebo Perineural dexamethasone group: dexamethasone 8 mg (0.8 mg) perineurally and normal saline 0.8 ml intravenously. Intravenous dexamethasone group: dexamethasone 8 mg (0.8 ml) intravenously and normal saline 0.8 ml perineurally. Intraperative anaesthesia/analgesia Intraoperative sedation with midazolam 0.015‐0.03 mg/kg and fentanyl 0.6 micrograms/kg intravenously. In the case of anxiety (as reported by the participant or determined by the blinded treating anaesthesiologist), propofol (25‐80 micrograms/kg/min) was administered. Postoperative anaesthesia/analgesia None reported. |
|
| Outcomes |
Outcomes of interest for the review Duration of motor block defined as time between block administration and time when participant regained movement of fingers. Duration of sensory block defined as time between block administration and time participant regained sensation of fingers. Duration of analgesia defined as time between block administration and time participant experienced pain in the operative site. Incidence of adverse events such as numbness, paraesthesia and motor deficit. Other outcomes Block performance time. Block onset time. Number of passes required to complete block. Block‐related pain as measured on 0‐10 pain scale. Incidence of vascular puncture. |
|
| Notes | Funding: none. Conflict of interest: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Group allocation was concealed in sealed envelopes. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | Personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants with missing data was balanced between groups (8 in the IV dexamethasone group and 11 in the perineural dexamethasone group). |
| Selective reporting (reporting bias) | Low risk | Trial registered on clinicaltrials.gov NCT02629835. All outcomes reported as stated in the protocol. |
| Other bias | Low risk | Appears to be free of any other bias. |
Bias 2014.
| Methods | Parallel group RCT. | |
| Participants | In India, 50 ASA class I‐II participants aged 15 to 54 years undergoing upper limb surgeries with supraclavicular block. Participants classified as ASA III to IV and those with infection at the block site, with comorbidities, coagulopathies and hypersensitivity to any of the study drugs were excluded. | |
| Interventions |
Block All participants underwent supraclavicular block with ropivacaine 0.5% 30 ml using landmarks. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Midazolam IV 1 mg was administered to all participants. Postoperative analgesia Diclofenac IM was administered when the participant reported pain. |
|
| Outcomes |
Outcomes of interest for the review Duration of sensory block defined as time interval between onset of sensory block to the time when participant first complains of pain at the site of surgery. Duration of motor block defined as interval between the time of loss of finger movements to the time the participant first regains finger movements. Intensity of pain assessed on a 5‐point VAS. Other outcomes Onset of sensory block defined as the time interval between administration of local anaesthetic to complete analgesia of forearm in relation to the distrubution of each major nerve as tested by pinprick over the forearm between elbow and wrist. Onset of motor block defined as time interval between administration of local anaesthetic to the time when finger movements are lost completely. |
|
| Notes | Funding: no information provided. Conflicts of interest: no information provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | Haemodynamic variables which could potentially be an indicator of adverse events were not reported as stated. |
| Other bias | Low risk | Appears to be free of any other bias. |
Biradar 2013.
| Methods | Parallel group RCT. | |
| Participants | In India, 60 ASA class I‐II participants aged 20‐70 years undergoing elective surgery of the hand, forearm or elbow with supraclavicular brachial plexus block were included. Participants with uncontrolled diabetes mellitus, hypertension, peripheral neuropathy, hepatic or renal disease, pregnancy, acid peptic disease or hypersensitivity to local anaesthetics were excluded from the study. | |
| Interventions |
Block All participants underwent nerve stimulator‐guided supraclavicular brachial plexus block with lidocaine 1.5% with adrenaline 1:200,000 (7 mg/kg) using nerve stimulator for guidance. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia None described. Postoperative analgesia Diclofenac IM 1.5 mg/kg was administered when the participant first complained of pain. Morphine IV 2 mg was administered every 10 minutes until VAS was less than 30. |
|
| Outcomes |
Outcomes of interest for the review Duration of sensory block defined as the time interval between brachial injection of local anaesthetic and the first postoperative pain. Duration of motor block defined as the time interval between brachial injection of local anaesthetic and complete recovery of motor function of all nerve distributions. Other outcomes Onset of sensory block defined as the time between the last brachial injection of local anaesthetic to the total abolition of pinprick response in all nerve distributions. Onset of motor block defined as the time between the last brachial injection of local anaesthetic to complete paralysis in all nerve distributions. |
|
| Notes | Funding: none. Conflicts of interest: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence table was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | Anaesthesiologist who performed the block was blinded but no indication that other personnel (surgeon, nurses) were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one participant from each group was excluded. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
Chalifoux 2017.
| Methods | Parallel group RCT. | |
| Participants | In Canada, 75 participants undergoing arthroscopic shoulder surgery with interscalene brachial plexus block. Exclusion criteria included participants with contraindications to interscalene block (coagulopathies, severe bronchopulmonary disease, contralateral diaphragmatic paralysis, prior contralateral pneumonectomy, preexisting neuropathy involving the surgical limb), preference for general anaesthesia, allergy or intolerance to one or more medications of the study protocol (dexamethasone, acetaminophen, morphine, or hydromorphone), chronic pain syndrome, chronic opioid use, chronic systemic corticosteroid use, weight\50 kg and pregnancy. | |
| Interventions |
Block All participants received ultrasound and nerve stimulator‐guide interscalene brachial plexus block with ropivacaine 0.5% 20 ml. Dexamethasone/placebo Participants in the dexamethasone group received dexamethasone 10 mg diluted to a final volume with normal saline of 20 ml intravenously. Participants in the placebo group received 20 ml normal saline intravenously. Intraoperative anaesthesia/analgesia All participants received midazolam 1‐2 mg and/or fentanyl 25‐50 ug before block administration. Participants could receive an additional midazolam 1‐2 mg intravenously every 30 minutes and/or propofol 25‐100 ug/kg/min. Postoperative analgesia Acetaminopen 650 mg orally every six hours. Hydormorphone 1‐2 mg orally or morphine 5‐10 mg orally every 4 hours for pain score greater than or equal to 4. |
|
| Outcomes |
Outcomes of interest for the review Duration of sensory block defined as the time from the onset of block to the first analgesic request. Intensity of postoperative pain at 12, 24 and 48 hours. Cummulative opioid consumption at 12, 24 and 48 hours. Participant satisfaction. Adverse events including pruritus on administration of study drug and residual motor block at 24 and 48 hours postoperatively. Other outcomes Intensity of postoperative pain at 36 hours. Cummulative opioid consumption at 36 hours. Differences in variation of blood glucose concentration. |
|
| Notes | This was a three‐arm study comparing dexamethasone 4 mg, dexamethasone 10 mg and placebo. In order to avoid unit of analysis issues we decided to include the dexamethasone 10 mg group and exclude the 4 mg group because high‐dose dexamethasone is used most often in clinical practice. Funding: funded by the Department of Anesthesiology, Hôpital Mainsonneve, Montréal, Quebec. Conflicts of interest: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sealed envelopes. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | Personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant from the dexamethasone group and three participants from the placebo group were excluded. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as described in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
Chun 2016.
| Methods | Parallel group RCT. | |
| Participants | In Korea, 100 ASA class I‐II participants aged 20‐80 years undergoing elective arthroscopic shoulder surgery with interscalene brachial plexus block. Exclusion criteria included any neuropathy, coagulopathy, respiratory diseases, systemic steroid use or chronic opioid use, and uncontrolled diabetes mellitus. | |
| Interventions |
Block All participants received ultrasound‐guided interscalene brachial plexus block with ropivacaine 0.75%, 60 mg. Dexamethasone/placebo Participants in the perineural dexamethasone group, participants received dexamethasone 5 mg perineurally + 3 ml 0.9% saline intravenously. Participants in the intravenous dexamethasone group received dexamethasone 5 mg intravenously + 4 ml 0.9% saline. Intraoperative anaesthesia/analgesia In all participants anaesthesia was induced with thiopental sodium 4 mg/kg, fentanyl 1‐2 ug/kg and rocuronium 0.6 mg/kg and maintained with sevoflurane. Postoperative analgesia Tramadol 50 mg intravenously for pain scores three or higher. Ketorolac 30 mg intravenously was given if tramadol was insufficient. |
|
| Outcomes |
Outcomes of interest for the review Duration of sensory block defined as the time from the completion of surgery to the first analgesic request. Severity of postoperative pain at 12, 24 and 48 hours. The incidence of adverse events including motor block, numbness and any other side effects in the first two days after surgery. Other outcomes Severity of postoperative pain at 6 hours. Number of participants requiring analgesic after surgery. |
|
| Notes | Funding: no information provided. Conflicts of interest: no information provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how treatment allocation was concealed. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one participant in the intravenous group was excluded from the study. |
| Selective reporting (reporting bias) | High risk | Protocol available from the Clinical Trials Registry of Korea. In the protocol, the primary outcome was stated as time to first analgesic request. In the study, the authors state the primary outcome was median analgesic time defined as the time to first analgesic request in > 50% of participants. |
| Other bias | Low risk | Appears to be free from any other bias. |
Cummings 2011.
| Methods | Parallel group RCT. | |
| Participants | In USA, 110 participants undergoing moderately to severely painful shoulder surgery with interscalene block were included. Participants with contraindication to interscalene block (severe lung disease, contralateral diaphragmatic paralysis and coagulopathy), pregnancy, pre‐existing neuropathy in the surgical limb, use of corticosteroids for two weeks or longer within six months of surgery or chronic opioid use were excluded from the study. | |
| Interventions |
Block All participants underwent interscalene block with bupivacaine 0.5%, 30 ml, using nerve stimulator for guidance. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia General anaesthesia. No other details were provided. Postoperative analgesia Morphine IV 2 mg every 5 minutes for pain score > 2 was given in PACU. Acetominophen 325‐650 mg and oxycodone 5‐10 mg orally every 4 hours as needed for VAS > 4 after discharge from PACU. Morphine IV for pain unrelieved by oral analgesics (VRS persistently > 4). |
|
| Outcomes |
Outcomes of interest for the review Duration of sensory block. Postoperative pain intensity at rest and movement on postoperative day 1 and 2. Incidence of adverse events including numbness, paraesthesia, weakness in the operative limb, persistent hoarseness, respiratory difficulty, injection site infection or haematoma. Other outcomes Postoperative pain intensity on postoperative day 7. |
|
| Notes | The effect of ropivacaine 0.5% was also examined; however to avoid unit of analysis errors, we chose to include only the bupivacaine arms since bupivacaine is more commonly used in clinical practice. Funding: support was solely from departmental sources. Conflicts of interest: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Group allocation was concealed by sealed, sequentially numbered opaque envelopes. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | Personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Protocol available on cllinicaltrials.gov. All outcomes reported as stated in the protocol. |
| Other bias | High risk | Study was stopped early for benefit according to predetermined stopping rule. |
Dar 2013.
| Methods | Parallel group RCT. | |
| Participants | In India, 80 ASA class I‐II participants aged 20‐50 years undergoing upper limb surgery with supraclavicular brachial plexus block were included. No exclusion criteria were stated. | |
| Interventions |
Block All participants received supraclavicular brachial plexus block with ropivacaine 0.5% 30 ml using landmarks. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia None described. Postoperative analgesia Diclofenac IM 75 mg was administered when the VAS was greater than 4. |
|
| Outcomes |
Outcomes of interest for the review Duration of sensory block defined as the time interval between the end of local anaesthetic administration and complete resolution of sensory block (normal sensation). Duration of motor block defined as the time interval between the end of local anaesthetic administration and the recovery of full power in the relevant muscle group. Incidence of adverse events including hypotension (a 20% decrease in relation to baseline), bradycardia (heart rate less than 50 beats per minute), hypoxaemia (SpO2 < 90%) and nausea and vomiting. Other outcomes Onset of sensory block defined as the time interval between the end of local anaesthetic administration and complete sensory block. Onset of motor block defined as the time interval between the end of local anaesthetic administration and the time of no movement in the relevant group. Quality of intraoperative analgesia judged by the investigator on a 4‐point scale. |
|
| Notes | Funding: none. Conflicts of interest: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used to conceal treatment allocation. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as described in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
Dawson 2016.
| Methods | Parallel group RCT. | |
| Participants | In Australia, 90 ASA class I‐III participants undergoing metatarsal osteotomy with ankle block were included. Participants classified as ASA greater than III, those less than 18 years old, those with coagulopathy, sepsis or infection at the operative site, allergy to ropivacaine, those taking regular opioids or glucocorticoids were excluded from the study. | |
| Interventions |
Block All participants received ankle block with ropivacaine 0.75% 20 ml with ultrasound guidance. Dexamethasone/placebo Perineural dexamethasone group: dexamethasone 8 mg perineurally and normal saline 2 ml intravenously. Intravenous dexamethasone group: dexamethasone 8 mg intravenously and normal saline 2 ml mixed with the block solution. Placebo group: normal saline 2 ml mixed with the block solution and 2 ml intravenously. Intraperative anaesthesia/analgesia None reported. Postoperative analgesia Paracetamol 665 mg. Oxycodone 5 mg. Tamadol 50 mg. |
|
| Outcomes |
Outcomes of interest for the review Postoperative opioid consumption. Incidence of PONV. Other outcomes Pain score when block wore off, at seven days after surgery and maximum pain score during study period. Duration of block defined as the time when sensation and movement returned to normal. |
|
| Notes | Funding: none. Conflicts of interest: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was concealed in sealed envelopes. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | Assume personnel were blinded since study drugs were prepared by a nurse not involved in the study and all study drugs were similar in appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all outcomes reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
Desmet 2013.
| Methods | Parallel group RCT. | |
| Participants | In Belguim, 150 participants greater than 18 years undergoing arthroscopic shoulder surgery with interscalene block were included. Participants less than 18 years old, those with diabetes, brachial plexus neuropathies, severe bronchopulmonary disease, systemic glucocorticoid use, pregnancy, routine use of opioids or sensitivity to any of the study drugs were excluded. | |
| Interventions |
Block All participants underwent nerve stimulator‐guided interscalene block with ropivacaine 0.5% 30 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 10 mg perineurally and normal saline 2 ml intravenously. Intravenous dexamethasone group: normal saline 2 ml perineurally and dexamethasone 10 mg intravenously. Placebo group: normal saline 2 ml perineurally and normal saline 2 ml intravenously. Intraoperative anaesthesia/analgesia General anaesthesia was induced with target‐controlled propofol infusion 3‐5 micrograms/ml, remifentanil (loading dose 1 microgram/kg, continuous infusion 0.05‐0.3 microgram/kg/min) and cisatracurium 0.5 mg/kg. Postoperative analgesia Paracetamol was administered for VRS more than 2 on a 5‐point VRS. Diclofenac IV 50 mg was administered for inadequate analgesia with paracetamol. Piritramide IM 15‐20 mg was administered as needed. |
|
| Outcomes |
Outcomes of interest for the review Duration of sensory block defined as the time between performance of the block and the time to first analgesic request. Participant satisfaction measured on a 2‐point scale. Other outcomes Number of participants experiencing moderate to severe pain. Mean postoperative paracetamol consumption. Postoperative blood glucose concentrations. |
|
| Notes | Funding: no information provided. Conflicts of interest: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sealed, opaque envelopes. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | Assume operating room personnel were blinded since study drugs prepared by staff member not involved int he study and delivered in unidentifiable syringes, however, no indication whether other personnel were blinded (surgeon, recovery room and ward nurses) was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only four participants were excluded from the placebo group. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
Desmet 2015.
| Methods | Parallel group RCT. | |
| Participants | In Belgim, 120 participants aged 18 years and older undergoing shoulder rotator cuff repair or subacromial decompression with interscalene brachial plexus block were included. Participants less than 18 years old, those with diabetes, brachial plexus neuropathies, severe bronchopulmonary disease, systemic glucocorticoid use, or pregnancy were excluded. | |
| Interventions |
Block All participants received nerve‐stimulator/ultrasound‐guided interscalene block with 0.5% ropivacaine. Dexamethasone/placebo Dexamethasone group: dexamethasone 10 mg intravenously. Placebo group: normal saline intravenously. Intraoperative anaesthesia/analgesia Oral lorazepam 2.5 mg 1 hour before surgery + intravenous midazolam 2 mg and sufentanil 2‐5 micrograms before block placement. General anaesthesia was induced with target‐controlled propofol infusion 3‐5 micrograms/ml, remifentanil (loading dose 1 microgram/kg, continuous infusion 0.05‐0.3 microgram/kg/min) and cisatracurium 0.5 mg/kg. Postoperative analgesia Paracetamol for moderate or severe pain or on participant request to a maximum 4 grams/24 hours supplemented with diclofenac to a maximum of 100 mg/24 hours and intramuscular piritramide 15‐20 mg. |
|
| Outcomes |
Outcomes of interest for the review Duration of sensory block defined by the interval between the time block was done and the time to first analgesia request. Arm weakness at 24 hours. Incidence of sleep disturbance, postoperative nausea and vomiting. Satisfaction. Other outcomes Number of participants with no/mild pain at 24 and 48 hours. |
|
| Notes | This was a four‐arm study which included a placebo arm and three doses of dexamethasone: 1.25 mg. 2.5. mg and 10 mg. In order to avoid unit of analysis issues, we chose to include the dexamethasone 10 mg arm and placebo and exclude the other arms. Funding: supported through a grant from the Belgian Association for Regional Anesthesia. Conflicts of interest: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sealed, opaque envelopes. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | Assume operating room personnel were blinded since study drugs prepared by staff member not involved the study and delivered in unidentifiable syringes, however, no indication whether other personnel were blinded (surgeon, recovery room and ward nurses) was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one participant was lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
Ganvit 2014.
| Methods | Parallel group RCT. | |
| Participants | In India, 60 ASA class I‐II participants aged 18‐60 years undergoing elective and emergency upper limb surgery with supraclavicular block were included. Participants with uncontrolled diabetes or hypertension, peripheral neuropathy, hepatic or renal disease, pregnancy, acid peptic diease or allergy or hypersensitivity to local anaesthetics were excluded. | |
| Interventions |
Block All participants underwent nerve stimulator‐guided supraclavicular block with bupivacaine 0.5% 15 ml + lidocaine 2% 15 ml + 5 micrograms 1:200,000 adrenaline. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Oral diazepam 0.15 mg/kg was administered the morning of surgery. Postoperative analgesia Diclofenac IM 1.5 mg/kg was administered when participant first complained of pain. |
|
| Outcomes |
Outcomes of interest for the review Duration of sensory block as defined as the time from injection of local anaesthetic to the time rescue analgesia was given. Other outcomes Onset of sensory block as defined by the time from injection of local anaesthesia to patient report of dull sensation along any of the nerve distributions. Onset of motor block as defined by the time from injection of local anaesthesia to time patient felt heaviness on abduction of arm at shoulder. |
|
| Notes | Funding: no information provided. Conflicts of interest: no information provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No indication that outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only two participants were excluded from the study. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
Golwala 2009.
| Methods | Parallel group RCT. | |
| Participants | In India, 60 ASA class I‐II participants undergoing elective or emergency upper limb surgery with supraclavicular brachial plexus block were included. Participants with a history or uncontrolled diabetes, renal or liver disease, circulatory instability, pregnancy, peptic ulcer disease, allergy to local anaesthetics or receiving long‐term steroid therapy were excluded. | |
| Interventions |
Block All participants underwent landmark‐guided supraclavicular block with lidocaine 2 % 15 ml + bupivacaine 0.5% 15 ml + adrenaline 1:200,000 Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Midazolam IV 1 mg was administered after the block. Postoperative analgesia Diclofenac IM 1.5 mg/kg was administered when VAS was 5 or greater. |
|
| Outcomes |
Outcomes of interest for the review Intensity of postoperative pain measured on an 11‐point VAS every 3 hours after surgery. Duration of sensory block defined as the time from drug injection in brachial plexus to VAS = 5. Incidence of side effects in the intraoperative and postoperative period. Other outcomes Onset of sensory block defined as dull sensation along any nerve distrubution. Onset of motor block defined as the time when the patient felt heaviness on abduction of arm at the shoulder. |
|
| Notes | Duration of block was reported as a range without any measure of central tendency. Pain scores were reported up to six hours in the placebo group and 15 hours in the dexamethasone group, therefore the data for this study could not be included in the meta‐analysis. Incidence of side effects was the only outcome that could be included in the analysis. Funding: no information provided. Conflicts of interest: no information provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how treatment allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | No protocol available. Pain scores were reported up to six hours in the placebo group and up to 15 hours in the dexamethasone group. |
| Other bias | Low risk | Appears to be free of any other bias. |
Jadon 2015.
| Methods | Parallel group RCT. | |
| Participants | In India, 112 ASA class I‐II participants aged 18‐70 years undergoing arthroscopic shoulder surgery with interscalene block were included. Participants with known hypersensitivity to study drugs or a contraindication to interscalene block were excluded. | |
| Interventions |
Block All participants underwent nerve stimulator‐guided interscalene block with ropivacaine 0.5% 30 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Alprazolam (by mouth) 0.5 mg was administered 2 hours before surgery. Midazolam IV 0.05 mg/kg was administered before block. Postoperative analgesia Diclofenac IM 1 mg/kg was administered when the VAS was greater than 3 or on participant request. Tramadol IV 1 mg/kg was administered if VAS was 3 or greater 45 minutes after diclofenac administration. |
|
| Outcomes |
Outcomes of interest for the review Duration of analgesia. Intensity of postoperative pain measured on an 11‐point VAS at 12 and 24 hours. Analgesic consumption at 24 hours. Incidence of block‐related complications. Other outcomes Intensity of postoperative pain at 1, 2, 3, 8, 16 and 20 hours. Onset of sensory block. Onset of motor block. |
|
| Notes | Funding: none. Conflicts of interest: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was concealed in sealed, opaque envelopes. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | Assume anaesthesiologist performing the block and operating room personnel were blinded since medication were prepared by an anaesthesiologist not involved in the study and delivered in similar syringes, however, no indication whether other personnel were blinded (surgeon, nurses). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Twelve participants were excluded: six from the dexamethasone group and six from the placebo group. |
| Selective reporting (reporting bias) | High risk | No protocol available. All outcomes were reported as stated in the methods section, however, the SD was not reported for pain scores but was reported for other outcomes. |
| Other bias | Low risk | Appears to be free of any other bias. |
Kawanishi 2014.
| Methods | Parallel group RCT. | |
| Participants | In Japan, 39 participants aged 20 and 75 years undergoing arthroscopic shoulder surgery with interscalene block. Participants with coagulation disorder, skin infection at site of surgery, preexisting neuropathy involving upper limb, drug dependency, systemic opioid use within the previous six months, peptic ulcer disease, diabetes mellitus, renal or hepatic disease or pregnancy were excluded. | |
| Interventions |
Block All participants underwent ultrasound‐guided interscalene block with ropivacaine 0.5% 20 ml after the surgical procedure. Dexamethasone/placebo Dexamethasone group: dexamethasone 4 mg perineurally. Placebo group: dexamethasone 4 mg intravenously. Intraoperative anaesthesia/analgesia Anaesthesia was induced and maintained by propofol 1mg/kg, remifentanil infusion 0.1 ‐0.3 microgram/kg/min, rocuronium 0.7 mg/kg and sevoflurane 1.0‐1.5 minimum alveolar concentration. Morphine 5 mg was administered after induction of anaesthesia. Postoperative analgesia Flurbiprofen IV was administered in the recovery room. Loxoprofen (by mouth) was administered after discharge from recovery room. Participants were instructed to request analgesia as soon as pain developed. |
|
| Outcomes |
Outcomes of interest for the review Intensity of postoperative pain measured on an 11‐point NRS the morning after surgery. Duration of sensory block defined as the interval between the time the block was performed and the first analgesic administration. Incidence of sleep disturbances measured on a 2‐point scale. Participant satisfaction measured on a 5‐point scale. Incidence of adverse events including nausea and vomiting, interscalene site infection, redness or neuropathy. |
|
| Notes | Funding: no information provided. Conflicts of interest: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was concealed by closed envelopes. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants were excluded in the intravenous group, one in the placebo group and one in the perineural dexamethasone group. |
| Selective reporting (reporting bias) | High risk | There was an outlier in the intravenous dexamethasone group that was not included in the analysis. |
| Other bias | Low risk | Appears to be free of any other bias. |
Kim 2012.
| Methods | Parallel group RTC. | |
| Participants | In Korea, 40 ASA I‐II participants undergoing arthroscopic shoulder surgery with interscalene brachial plexus block were included. Participants with diabetes, pregnancy, coagulation disorders, sensitivity to local anaesthetic, severe chronic pulmonary disease, neurological deficiencies, neuropathy, infection at the surgical site, drug or alcohol dependency or history or chronic pain were excluded. | |
| Interventions |
Block All participants received ultrasound‐guided interscalene block with levobupivacaine 0.5% 10 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 5 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Midazolam IV 1‐3 mg and fentanyl IV 25‐50 micrograms was administered before block was performed. After block was performed, glycopyrrolate IV 0.2 mg, pentothal sodium IV 4 mg/kg, fentanyl 1‐2 micrograms/kg and rocuronium IV 0.6 mg/kg was administered. Postoperative analgesia Ketorolac IV or opioid IM was administered when the participant reported VAS more than 4 or on participant request. |
|
| Outcomes | Intensity of postoperative pain measured on a 11‐point VAS assessed 12, 24 and 48 hours. Incidence of adverse events including nausea, vomiting, respiratory difficulties and neurological disabilities. |
|
| Notes | This was a three‐arm study of 60 participants. In group III epinephrine 1:400 000 was given perineurally, however, the 20 participants of this arm are not included in this review as this is not an intervention of interest. Funding: no information provided. Conflicts of interest: no information provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how the random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how the treatment allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication of whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication of whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | The authors state analgesic consumption was not significantly different, however the results are not presented. In the abstract, the authors state they would assess sleep quality and satisfaction, however the results are not reported. |
| Other bias | Low risk | Appears to be free of any other bias. |
Kumar 2014.
| Methods | Parallel group RCT. | |
| Participants | In India, 80 ASA I‐II participants aged 16‐60 years undergoing elective upper limb surgery with supraclavicular brachial plexus block were included. Those with infection at the surgical site, local site anatomical abnormality, allergy to study drugs, use of corticosteroid for two weeks or longer, drug abuse, peripheral neuropathy, head injury, psychiatric disorder, severe pulmonary, cardiac, renal or endocrine disorder, peptic ulcer disease or pregnancy were excluded. | |
| Interventions |
Block All participants underwent nerve‐stimulator‐guided supraclavicular block with ropivacaine 0.5% 30 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: sterile water 2 ml perineurally. Intraoperative anaesthesia/analgesia None reported. Postoperative analgesia Diclofenac (by mouth) 50 mg was administered when participant reported VAS 3‐6. Diclofenac injection 75 mg was administered if participant reported VAS greater than 6. |
|
| Outcomes |
Outcomes of interest for the review Duration of analgesia as defined by the interval between the onset of sensory block and the initial use of rescue analgesia for surgical site pain. Duration of block. Intensity of postoperative pain measured on VAS. Postoperative analgesic consumption. Incidence of adverse events including nausea, vomiting, dysrhythmias, hypotension, convulsions, pneumothorax, pruritus, jerking movements and hypersensitivity reaction for the study drug. Other outcomes Onset of block. Peak effect of block. |
|
| Notes | Authors did not specify the type of VAS. It was not possible to obtain pain scores from the figure in the manuscript. Authors were contacted to provide raw data, however it was unavailable; therefore not included in the analysis. Funding: no information provided. Conflicts of interest: no information provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Low risk | Group allocation was concealed in opaque, sealed envelopes. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | Personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
Lee 2016.
| Methods | Parallel group RCT. | |
| Participants | In Korea, 34 ASA class I‐II participants aged 18 years and older undergoing elective forearm and hand surgery with ultrasound and nerve stimulator‐guided axillary brachial plexus block. Participants with hypertension, cardiac or hepatic disease, diabetes mellitus or coagulopathy were excluded from the study. | |
| Interventions |
Block All participants received ultrasound and nerve stimulator‐guided axillary brachial plexus block with ropivacaine 0.5% 20 ml. Dexamethasone/placebo Perienural dexamethasone group: dexamethasone 10 mg perineurally Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Fentanyl 50 micrograms intravenously for pain score more than 4 on VAS. An additional 50 micrograms was given if pain persisted five minutes after first administration. Postoperative analgesia Not described. |
|
| Outcomes |
Outcomes of interest for the review Duration of sensory block as defined as time between successful block and complete restoration of all the senses controlled by the radial, ulnar, median and musculocutaneous nerves. Incidence of adverse events including hypotension, bradycardia, hypoxaemia and nausea and vomiting. Other outcomes Onset of sensory block defined as time between the end of local anaesthetic injection and the loss of pinprick sensation. Quality of anaesthesia determined by the need for supplemental opioids during surgery. |
|
| Notes | Funding: none reported. Conflicts of interest: none reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication of whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication of whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | A blinded observer recorded the onset of sensory block but unclear of whether the person who observed the primary outcome (duration of sensory block) was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all outcomes reported as described in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
Leurcharusmee 2016.
| Methods | Parallel group RCT. | |
| Participants | In Canada and Thailand, 150 ASA class I‐III participants aged 18‐80 years undergoing forearm, wrist or hand surgery with ultrasound‐guided infraclavicular block. Participants with sepsis, coagulopathy, allergy to local anaesthesia, hepatic or renal failure, pre‐existing upper limb neuropathy and who had prior surgery in the infraclavicular fossa were excluded. | |
| Interventions |
Block All participants received ultrasound‐guided Infraclavicular nerve block with equal parts of lidocaine 2% and bupivacaine 0.5% with epinephrine 5 micrograms/ml 35 ml. Dexamethasone/placebo Perineural dexamethasone group: dexamethasone 5 mg (0.5 ml) perineurally and normal saline 0.5 ml intravenously. Intravenous dexamethasone group: dexamethasone 5 mg (0.5 ml) intravenously and normal saline 0.5 ml perineurally. Intraperative anaesthesia/analgesia Intraoperative sedation with midazolam 0.015‐0.03 mg/kg and fentanyl 0.6 micrograms/kg intravenously was administered as necessary. Postoperative anaesthesia/analgesia Not described. |
|
| Outcomes |
Outcomes of interest for the review Duration of motor block defined as time between block administration and time when participant regained movement of fingers. Duration of sensory block defined as time between block administration and time participant regained sensation of fingers. Duration of analgesia defined as time between block administration and time participant experienced pain in the operative site. Incidence of adverse events such as numbness, paraesthesia and motor deficit. Other outcomes Block performance time. Block onset time. Number of passes required to complete block. Block‐related pain as measured on 0‐10 pain scale. Incidence of vascular puncture. |
|
| Notes | Funding: no information provided. Conflicts of interest: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Group allocation was concealed in sealed envelopes. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | Personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of participants and reasons for exclusion were balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Protocol published on clinicaltrials.in.th TCTR20150624001. All outcomes reported as per protocol. |
| Other bias | Low risk | Appears to be free of any other bias. |
Movafegh 2006.
| Methods | Parallel group RCT. | |
| Participants | In Iran, 60 ASA I‐II participants aged 20‐50 years undergoing elective hand and forearm surgery with axillary brachial plexus block were included. Participants with a history of peptic ulcer disease, diabetes, hepatic or renal failure, pregnancy and those receiving premedications including opioids, benzodiazepines and clonidine were excluded. | |
| Interventions |
Block All participants received nerve stimulator‐guided axillary brachial plexus block with lidocaine 1.5% 34 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Not described. Postoperative analgesia Not described. |
|
| Outcomes |
Outcomes of interest for the review Duration of sensory block defined as the time interval between administration of local anaesthetic and the first postoperative pain. Duration of motor block defined as the time interval between administration of local anaesthetic and complete recovery of motor functions. Other outcomes Onset of sensory block defined as the time between the last injection and complete abolition of the pinprick response. Onset of motor block defined as the time between the last injection and complete paralysis in all nerve distributions. |
|
| Notes | Funding: no information provided. Conflicts of interest: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The anaesthesiologists who evaluated the sensory and motor block were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Thirty participants were randomized to each group. Ten participants in the placebo group were excluded for failed block leaving 20 for analysis (33% excluded). In the dexamethasone group, six participants were excluded for failed block. The total of the remaining participants is reported to be 20. There are four participants that are not accounted for. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes were reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
Nallam 2014.
| Methods | Parallel group RCT. | |
| Participants | In India, 90 ASA class I‐II participants aged 18‐65 years undergoing shoulder surgery with interscalene brachial plexus block were included. No exclusion criteria were stated. | |
| Interventions |
Block All participants underwent nerve stimulator‐guided brachial plexus block with levobupivacaine 0.5% 35 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Midazolam 1 mg and fentanyl 30mg was administered before the block. Postoperative anaesthesia/analgesia Acetaminophen 325 mg. Participants were advised to take one or two tablets if the pain exceeded 3 on an 11‐point VAS. Ibuprophen 400 mg was administered if the pain persisted. |
|
| Outcomes |
Outcomes of interest for the review Duration of analgesia defined as the time in hours from the time of completion of surgery to the time participant felt pain from the incision at an intensity > 3 on numerical rating scale. Duration of motor block defined as the time of completion of nerve block to the time when patient was able to abduct the arm at least 2 inches away from the body. Other outcomes Total analgesic consumption defined as the number of analgesic used within the first 72 hours after surgery. |
|
| Notes | This was a three‐arm study comparing dexamethasone 8 mg, dexamethasone 4 mg and placebo. In order to avoid unit of analysis errors, we chose to include the dexamethasone 8 mg arm and exclude the dexamethasone 4 mg arm since 8 mg is the dose most commonly used in clinical practice. Funding: no information provided. Conflicts of interest: no information provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Groups were allocated using a randomization table. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication of whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | The block was performed by a blinded anaesthesiologist, however there is no indication whether other personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No indication whether outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants were excluded from the placebo group. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
Parrington 2010.
| Methods | Parallel group RCT. | |
| Participants | In Canada, 45 ASA I‐III participants undergoing elective hand or forearm surgery with brachial plexus block were included. Participants scheduled for surgery less than 30 minutes or more than 120 minutes, hypersensitivity to local anaesthetics or dexamethasone, peripheral neuropathy, peptic ulcer, diabetes mellitus, coagulopathy or contraindication to supraclavicular brachial plexus block were excluded. | |
| Interventions |
Block All participants underwent ultrasound‐guided brachial plexus block with mepivacaine 1.5% 30 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Midazolam IV 0.03‐0.04 mg was administered before the block. Postoperative analgesia Fentanyl was administered in 25 microgram increments to participants with a pain score of 4 or greater on the VAS. Once oral intake was initiated, acetaminophen 300 mg/codeine 30 mg or acetaminophen 325 mg/oxycodone 5 mg was administered. |
|
| Outcomes |
Outcomes of interest for the review Intensity of postoperative pain measured on a 0‐100 mm VAS at 1 day, 7 days. Duration of sensory block defined as interval between the end of local anaesthetic injection and the patient's first report of postoperative pain at the surgical site. Postoperative analgesic consumption at 8 hours, 1 day, after surgery. Other outcomes Onset of sensory block defined as the time interval between the end of local anaesthetic injection and the loss of sensation to pinprick. Onset of motor block defined as the time interval between the end of local anaesthetic injection and paresis in the distributions of all 4 peripheral nerves. Intensity of pain measured on a 0‐100 mm VAS at 8 hours, 7 days and 14 days after surgery. Postoperative analgesic consumption at 0 hours, 8 hours, 7 days and 14 days after surgery. |
|
| Notes | Funding: none reported. Conflicts of interest: Dr. Richard Brull is a consultant for B. Braun. Dr. Vincient Chan receives equipment support and honoraria from Philips Medcial Systems, SonoSite and GE Medical. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Group allocation was concealed in opaque, sealed envelopes. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | Anaesthesiologist performing the block and the anaesthesiologist providing intraoperative care was blinded, however, there was no indication whether other personnel (surgeon, nurses) was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of participants with missing data balanced between groups (six in the dexamethasone group and seven in the placebo group). |
| Selective reporting (reporting bias) | Low risk | Protocol available on clinicaltrials.gov. All outcomes reported as stated. |
| Other bias | Low risk | Appears to be free of any other bias. |
Rahangdale 2014.
| Methods | Parallel group RCT. | |
| Participants | In the USA, 80 participants aged 18 to 70 years undergoing elective ankle and foot surgery with sciatic nerve block were included. Participants with contraindication to regional anaesthesia, history of allergy to amide local anaesthetics, neurological deficit, coagulopathy, infection, type 1 or 2 diabetes mellitus, systemic use of corticosteroids within six months of surgery, chronic use of opioids, pregnancy and those undergoing midfoot and forefoot surgery were excluded. | |
| Interventions |
Block All participants underwent ultrasound‐guided sciatic nerve block with bupivacaine 0.5% with epinephrine 1:300,000 (0.45 mg/kg) Dexamethasone/placebo Perineural dexamethasone group: dexamethasone 8 mg perineurally and normal saline 2 ml intravenously. Intravenous dexamethasone group: normal saline 2 ml perineurally and dexamethasone 8 mg intravenously. Placebo group: normal saline 2 ml perineurally and normal saline 2 ml intravenously. Intraoperative anaesthesia/analgesia Midazolam IV 2‐5 mg was administered to all participants and fentanyl IV 25‐50 micrograms was administered incrementally if necessary before the block. Propofol 25‐75 micrograms/kg/min was administered to provide sedation while maintaining responsiveness to tactile or verbal stimulation after the block. Postoperative analgesia Hydrocodone 10 mg + acetaminophen 325 mg every 4 hours as needed. |
|
| Outcomes |
Outcomes of interest for the review Intensity of postoperative pain measured on an 11‐point NRS on postoperative day one and day two. Postoperative opioid consumption on postoperative day one and two. Duration of sensory block defined as time to first pain not in saphenous distribution. Duration of motor block defined as time to first toe movement. Incidence of postoperative neurological sequale. Participant satisfaction measured on an 11‐point VAS. Other outcomes Quality of recovery measured by Quality of Recovery‐40 scale. Intensity of pain measured on an 11‐point NRS two weeks after surgery. Postoperative opioid consumption two weeks after surgery. |
|
| Notes | Funding: Department of Anesthesiology, Northwestern University. Conflicts of interest: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Group allocation was concealed in opaque, sequentially numbered, sealed envelopes. |
| Blinding of participants (detection bias) | Low risk | No indication that participants were blinded in the paper, however the clincialtrials.gov document states that participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | No indication that participants were blinded in the paper, however the clincialtrials.gov document states that caregivers were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of participants with missing data balanced between groups; three in the intravenous dexamethasone group, one from the placebo group, and none from the perineural dexamethasone group. |
| Selective reporting (reporting bias) | Low risk | Protocol available on clinicaltrials.gov. All outcomes were reported as stated in the protocol. |
| Other bias | Low risk | Appears to be free of any other bias. |
Rosenfeld 2016.
| Methods | Parallel group RCT. | |
| Participants | In USA, 130 participants ASA I‐III participants undergoing shoulder surgery (arthroplasty, open and arthroscopic rotator cuff repair and acromisoplasty) with ultrasound‐guided brachial plexus block were included. Participants taking more than 60 mg oral morphine equivalents per day, those with diabetes mellitus, allergy to local anaesthetic or dexamethasone, coagulopathy, local infection or severe lung disease were excluded. | |
| Interventions |
Block All participants received brachial plexus block with ropivacaine 0.5% 28 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally and normal saline 5 ml intravenously. Intravenous dexamethasone group: dexamethasone, 8 mg intravenously and normal saline 5 ml mixed with the block solution. Placebo group: normal saline 5 ml both intravenously and mixed with the block solution. Intraoperative anaesthesia/analgesia All participants received fentanyl IV up to 100 micrograms and midazolam up to 4 mg for sedation for block placement. All participants underwent general anaesthesia with propofol, fentanyl, rocuronium and/or succinylcholine, sevoflurane in air‐oxygen and ondansetron. Intraoperative fentanyl was limited to 250 micrograms and no long‐acting opioids were used. Neuromusclular block was reversed with neostigmine/glycopyrrolate. Postoperative analgesia For participants not discharged the day of surgery, ketorolac IV was given every six hours for the first 24 hours and intravenous morphine or hydromorphone and oral hydrocodone or oxycodone as needed. Participants who were discharged the day of surgery were prescribed ibuprofen 800 mg every eight hours and hydrocodone, oxycodone and non‐steroidal anti‐inflammatory medications as needed. |
|
| Outcomes |
Outcomes of interest for the review Duration of sensory block defined as the time until the patient detected complete resolution of sensory block in the shoulder region. 24‐hour postoperative opioid consumption. Pain scores at rest measured on 11‐point VAS 12 , 24 and 48 hours after surgery. Satisfaction with pain placebo. Incidence of adverse events. Other outcomes Pain scores at rest measured on 11‐point VAS 8 hours, 20 hours, and 1 week after surgery. |
|
| Notes | Funding: none. Conflicts of interest: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was generated by a statistician. |
| Allocation concealment (selection bias) | Low risk | Treatment allocation schedule was stored by the pharmacy and randomization occurred after informed consent was obtained and before any study drugs were prepared. |
| Blinding of participants (detection bias) | Low risk | The clinicaltrials.gov protocol states that participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | The clinicaltrials.gov document states personnel was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The clinicaltrial.gov document states outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only two participants were excluded form the perineural dexamethasone group, five from the intravenous group and three from the placebo group. |
| Selective reporting (reporting bias) | Low risk | Protocol was registered on clinicaltrial.gov. All outcomes were reported as stated in the protocol. |
| Other bias | Low risk | Appears to be free of any other bias. |
Sakae 2017.
| Methods | Parallel group RCT. | |
| Participants | In Brazil, 60 ASA class I‐II participants aged 18 years and older undergoing arthroscopic shoulder surgery with interscalene brachial plexus block. Exclusion criteria were: infection at the site, history of allergy to any of the study drugs, systemic use of corticosteroid for two weeks or longer, drug abuse, peripheral neuropathy, head injury, psychiatric disorder, coagulation disorder, severe pulmonary, cardiac, renal or endocrine disorder and pregnancy. | |
| Interventions |
Block All participants received ultrasound and nerve stimulator‐guided interscalene brachial plexus block with ropivacaine 0.5% 20 ml. Dexamethasone/placebo Participants in the perineural dexamethasone group received dexamethasone 4 ml perineurally. Participants in the intravenous dexamethasone group received dexamethasone 4 mg intravenously + 1 ml normal saline perineurally. Participants in the control group received 1 ml of normal saline perineurally. Interoperative anaesthesia/analgesia All participants received fentanyl 50 micrograms intervenously. General anaesthesia was given by Total Anaesthesia Target Control Infusion induced with propofol 1% and remifentanil 50 micrograms then titrated to effect. Rocuronium 0.5 mg/kg was administered. Postoperative analgesia Parecoxib 40 mg was administered as soon as participant reported pain. Morphine 0.1 mg/kg was used as rescue medication. |
|
| Outcomes |
Outcomes of interest for the review Duration of sensory block defined as time between successful block and complete recovery of arm sensation. Duration of motor block defined as time interval between successful block and complete recovery of all movements in the arm. Severity of postoperative pain at 12 hours. Severity of postoperative pain at 24 hours. Postoperative opioid requirement. Incidence of postoperative nausea and vomiting. |
|
| Notes | Funding: no information provided. Conflicts of interest: no information provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how allocation was concealed. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | Personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | Protocol was published on www.ensaiosclinicos.gov.br. Adverse events not reported as per published protocol. |
| Other bias | Low risk | Appears to be free of any other bias. |
Saritas 2014.
| Methods | Parallel group RCT. | |
| Participants | In Turkey, 30 ASA I‐II participants aged 18‐60 years undergoing elective hand and forearm surgery with brachial plexus block were included. Participants with severe hepatic, renal or cardiovascular disorders, electrolyte imbalance or pregnancy were excluded. | |
| Interventions |
Block All participants underwent nerve stimulator‐guided brachial plexus block with prilocaine 2% 5 mg/kg. Dexamethasone/placebo Dexamethsone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Not described. Postoperative analgesia Diclofenac 1 mg/kg was administered to participants when they first complained of pain. |
|
| Outcomes |
Outcomes of interest for the review Duration of sensory block defined as the first postoperative pain. Incidence of any side effects (nausea, vomiting, methaemoglobinaemia, cardiovascular issues). Other outcomes Onset of sensory block defined as the time between completion of local anaesthetic injection and no response to pinprick. Onset of motor block defined as the time between completion of local anaesthetic injection and paralysis. |
|
| Notes | This was a three‐arm study in 75 participants. In group II, 15 participants received brachial plexus block with levobupivacaine however, this arm was not included in the review because there was no equivalent placebo or non‐active comparator group. Funding: no information provided. Conflicts of interest: no information provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | The anaesthesiologists who performed the block were blinded, however, there is no indication whether other personnel (surgeon, nurses) were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | Pain scores, analgesic consumption, incidence of adverse events and vital signs not reported as stated. |
| Other bias | Low risk | Appears to be free of any other bias. |
Shah 2015.
| Methods | Parallel group RCT. | |
| Participants | In India, 53 ASA I‐II participants aged 18‐60 years undergoing upper limb surgery below mid‐humerus with infraclavicular brachial plexus block were included. Participants with head injury, psychiatric disorders, infection at surgical site, severe pulmonary, cardiac, renal, endocrine disorders, peptic ulcer disease, peripheral neuropathies, allergy to any of the study drugs were excluded. | |
| Interventions |
Block All participants received nerve stimulator‐guided infraclavicular brachial plexus block with lignocaine 1.5% 0.6 ml/kg. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Midazolam IV was administered in incremental doses of 1 mg to a maximum of 3 mg and fentanyl was administered in 25 microgram incremental boluses to a maximum of 2 micrograms/kg before the block. Postoperative analgesia Patient controlled analgesia with morphine 1 mg/ml solution bolus 1 ml, lockout 5 min, 4 hour limit of 10 mg without background infusion. |
|
| Outcomes |
Outcomes of interest for the review Intensity of postoperative pain assessed by 11‐point NRS at 12 and 24 hours. Duration of sensory block defined as the time interval between the onset of sensory block and the first postoperative pain. Duration of motor block as defined as the time interval between the onset of motor block and complete recovery of motor functions. Patient satisfaction measured on a 4‐point scale. Other outcomes NRS assessed every 30 minutes until 6 hours and then at 6 hour intervals until 24 hours after surgery. |
|
| Notes | This is a three‐arm study. In group C (19 participants), clonidine was given perineurally, however this was not an intervention of interest for our study therefore not included in the analysis. Funding: no information provided. Conflicts of interest: no information provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Authors state 53 participants were included in the study, however only 41 were included in the analysis. It is not clear how many participants were randomized to each group. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all outcomes reported as described in the methods section. |
| Other bias | High risk | No sample size was done a priori. An interim analysis showed significant difference with 13 participants in the placebo group and the study was stopped for benefit. |
Shaikh 2013.
| Methods | Parallel group RCT. | |
| Participants | In India, 60 ASA I‐II participants undergoing elective elbow, forearm and hand surgery with supraclavicular brachial plexus block were included. Participants classified as ASA III or more, those with history or peptic ulcer, diabetes mellitus, hepatic or renal failure, history of neurological, psychiatric, neuromuscular disease or hypersensitivity to any of the study drugs were excluded. | |
| Interventions |
Block All participants underwent nerve stimulator‐guided supraclavicular brachial plexus block with bupivacaine 0.5% 38 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Midazolam IV 0.03‐0.04 was administered before the block. Postoperative analgesia Diclofenac IM 75 mg was administered when participant reported VAS 30 or greater on a 100‐mm VAS. |
|
| Outcomes |
Outcomes of interest for the review Duration of sensory block defined as the time interval between the onset of sensory block and the first postoperative pain. Duration of motor block defined as the time interval between the onset of motor block and complete recovery of motor functions. Other outcomes Onset of sensory block defined as the time interval between the end of local anaesthetic injection and loss of sensation to pinprick in all nerve distrubution. Onset of motor block defined as the time interval between the end of local anaesthetic and paresis in all nerve distributions. Number of diclofenac injections required in the first 24 hours after surgery. |
|
| Notes | Funding: no information provided. Conflicts of interest: no information provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants per group were excluded. |
| Selective reporting (reporting bias) | High risk | Pain scores were not reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
Talukdar 2013.
| Methods | Parallel group RCT. | |
| Participants | In Bangledesh, 60 ASA I‐II participants ages 18 to 60 years undergoing elective upper limb surgery with supraclavicular brachial plexus block were included. Participants with coagulation disorder, skin infection at surgical site, pre‐existing upper limb neuropathy, drug dependency, systemic use of steroid within the past six months, peptic ulcer disease, diabetes mellitus, renal or hepatic disease or pregnancy were excluded. | |
| Interventions |
Block All participants received a supraclavicular block with bupivacaine 0.5% 38 ml using paraesthesia technique. Dexamethasone/placebo Dexamethsaone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Not described. Postoperative analgesia Not described. |
|
| Outcomes |
Outcomes of interest for the review Duration of sensory block. Intensity of postoperative pain measured on an 11‐point VAS at 12 and 24 hours. Incidence of sedation, nausea, vomiting, hypotension, arrhythmia and shivering. Other outcomes Onset of sensory block. Onset of motor block. Intensity of postoperative pain measured on an 11‐point VAS at 0.5 and 1 hour. |
|
| Notes | Funding: none. Conflicts of interest: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was generated by card sampling method. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | Pain scores were assessed only up to 16 hours instead of up to 24 hours as stated in the methods section. The incidence of arrhythmias not reported as stated in the methods section. P values were reported for only statistically significant results. |
| Other bias | Low risk | Appears to be free of any other bias. |
Tandoc 2011.
| Methods | Parallel group RCT. | |
| Participants | In the USA, 78 participants aged 18 to 78 years undergoing elective arthroscopic shoulder surgery were included. Participants with coagulopathy, allergy to local anaesthetics, hypertension, peripheral neuropathy or chronic obstructive pulmonary disease were excluded. | |
| Interventions |
Block All participants underwent nerve stimulator‐guided brachial plexus block with bupivacaine 0.5% with epinephrine 1:200,000 40 ml. Dexamethasone/placebo Dexamethsone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Midazolam 1‐2 mg and/or fentanyl 50‐100 micrograms was administered before the block. Anaesthesia was induced with propofol 2 mg/kg and maintained with sevoflurane 1.0‐1.5 MAC. Postoperative analgesia Acetaminophen 325 mg + hydrocodone 7.5 mg 1‐2 tablets was administered if pain score was greater than 3. If pain persisted, ibuprofen 400 mg was administered. |
|
| Outcomes |
Otcomes of interest for review Duration of sensory block defined as time of discharge to the time the patient experienced pain at or greater than 3. Duration of motor block defined as the time from discharge to the time when the patient was able to abduct the arm at least 2 inches away from the body. Participant satisfaction measured on a 5‐point scale. Other outcomes Number of acetaminophen 325 mg + hydrocodone 7.5 mg tablets taken in the first 72 hours after surgery. Incidence of adverse events. |
|
| Notes | This was a three‐arm study comparing dexamethasone 8 mg, dexamethasone 4 mg and placebo. In order to avoid unit of analysis errors, we chose to include the dexamethasone 8 mg arm and exclude the dexamethasone 4 mg arm since 8 mg is the dose most commonly used in clinical practice. Funding: provided by Buffalo Anesthesiology Associates. Conflicts of interest: no information provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized using a randomization table. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Nurse who assessed the outcomes after discharge was blinded. Unclear whether the anaesthesiologist assessing the incidence of postoperative adverse events was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were excluded from the dexamethasone group and two were excluded from the placebo group. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
Viera 2010.
| Methods | Parallel group RCT. | |
| Participants | In the USA, 120 ASA I‐III participants 18 years or older undergoing elective shoulder arthroscopy with interscalene brachial plexus block were included. Participants with a contraindication to bupivacaine, epinephrine, clonidine or dexamethasone as well as pregnant participants were excluded. | |
| Interventions |
Block All participants underwent ultrasound‐guided supraclavicular block with bupivacaine 5 mg/ml + epinephrine 5 microgram/ml and clonidine 75 microgram/ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Anaesthesia was induced with propofol and maintained with sevoflurane, desflurane or propofol with nitrous oxide after the block. Postoperative anaesthesia/analgesia Participants were prescribed hydrocodone, oxycodone or hydromorphone. |
|
| Outcomes |
Outcomes of interest for the review Intensity of pain measured on an 11‐point VAS at 24 and 48 hours after surgery. Duration of sensory and motor block. Participants were given a diary to record the time at which they felt the sensory and motor block had resolved based on increase in pain, sensation and strength in the arm. Participant satisfaction with pain placebo measured on an 11‐point VAS Other outcomes Intensity of pain on admission to PACU, 1 and 2 hours after surgery and on discharge from PACU. |
|
| Notes | Funding: departmental funding from the Department of Anesthesiology, Baystate Medical Center, Springfied, Massachuttes. Conflicts of interest: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as stated in the methods section. |
| Other bias | High risk | Sample size was 88; however, 120 participants were enrolled in order to obtain reliable data from 88 participants. |
Vishnu 2014.
| Methods | Parallel group RCT. | |
| Participants | In India, 50 participants ASA I‐II aged 20‐45 years undergoing upper extremity surgery with supraclavicular block were included. Participants classified as ASA III to IV, those with allergy to local anaesthetic or dexamethasone, coagulopathy, diabetes mellitus, local infection at block site, pre‐existing neuropathy of the surgical limb and systemic use of corticosteroids within six months of surgery were excluded. | |
| Interventions |
Block All participants underwent nerve stimulator‐guided supraclavicular brachial plexus block with bupivacaine 0.5% 28 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Midazolam IM 0.05 mg/kg was administered one hour before surgery. Postoperative analgesia Diclofenac IM 75 mg was administered as rescue analgesia. |
|
| Outcomes |
Outcomes of interest for the review Duration of sensory block defined as the time interval between the end for local anaesthetic administration to the time when the patient had VAS 4 or greater Incidence of nausea. Incidence of tingling/numbness. Other outcomes Onset of sensory block defined as the time interval between the end of local anaesthetic administration and loss of sensation to pin prick. Onset of motor block defined as the time between the end of local anaesthetic administration and the inability to move fingers. |
|
| Notes | Funding: no information provided. Conflicts of interest: no information provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was achieved by simple random sampling. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | VAS scores not reported. |
| Other bias | Unclear risk | Appears to be free of any other bias. |
Woo 2015.
| Methods | Parallel group RCT. | |
| Participants | In Korea 36 ASA 1 to 2 participants aged 20 to 70 years undergoing arthroscopic shoulder surgery with interscalene brachial plexus block were included. Participants with coagulopathy, infection at block site, neurological deficit in the surgical limb, severe lung disease, contralateral diaphragmatic paralysis, systemic glucocorticoid use, chronic opioid use, peptic ulcer disease, uncontrolled diabetes mellitus or allergy to ropivacaine were excluded. | |
| Interventions |
Block All participants underwent interscalene brachial plexus block with ropivacaine 0.75% using nerve stimulator guidance. Dexamethasone/placebo Dexamethsaone group: dexamethasone 7.5 mg perineurally. Placebo group: normal saline perineurally. Intraoperative anaesthesia/analgesia Thiopentone 4 mg/kg. Fentanyl one to two micrograms/kg. Rocuronium 0.6 mg/kg. Sevoflurane in 50% air/oxygen mixture 1.0 to 1.5 minimum alveolar concentration. Postoperative analgesia Tramadol 100 mg up to 3 times a day when pain was at least three on Numerical Rating Scale or patient request. Ketorolac 30 mg up to 90 mg a day for insufficient analgesia. |
|
| Outcomes |
Outcomes of interest for the review Duration of sensory block defined as the time the block was performed to the time of first analgesic request. Incidence of arm weakness and adverse events for the first 48 hours after surgery. Pain scores at 12, 24 and 48 hours after surgery. Other outcomes Number of participants not requiring analgesia. Analgesia consumption. Pain scores at 6 hours after surgery. |
|
| Notes | This was a three‐arm study comparing three doses of dexamethasone (2.5 mg, 5 mg and 7.5 mg) and placebo. In order to avoid unit of analysis issues we chose to include the dexamethasone 7.5 mg arm since this is the dose used most often in practice and to exclude the other arms. Funding: none. Conflicts of interest: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication how group allocation was concealed. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | Personnel was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | OUtcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Protocol was registered with the Clinical Trial Registry of Korea. All outcomes reported as stated in the protocol. |
| Other bias | Low risk | Appears to be free of any other bias. |
Yadov 2008.
| Methods | Parallel group RCT. | |
| Participants | In Nepal, 60 ASA I‐II participants undergoing forearm or hand surgery with brachial plexus block were included. Participants with uncontrolled hypertension, Ischaemic heart disease, acid peptic disease, neurological, psychiatric neuromuscular or respiratory disorder, drug addiction, pregnant or lactating women were excluded. | |
| Interventions |
Block All participants underwent nerve stimulator‐guided brachial plexus block with lignocaine (1.5%) and adrenaline (1:200,000) 24 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 4 mg perineurally. Placebo group: nerve block only. Intraoperative anaesthesia/analgesia None described. Postoperative analgesia Diclofenac (by mouth) 50 mg was administered if VAS was 3‐5. Diclofenac IV 75 mg was administered if VAS was 6 or greater. |
|
| Outcomes |
Outcomes of interest for the review Intensity of postoperative pain on 11‐point VAS and 12 hours after surgery. Duration of analgesia defined as the time between onset of analgesia to first pain perception by the patient. Postoperative nausea and vomiting. Other outcomes Intensity of pain measured on VAS at 1 min, 15 minutes, 30 minutes, 1 hour, 2 hours, 3 hours, 6 hours after surgery. Postoperative analgesic consumption (non‐opioid). Surgeons satisfaction score measured on an 11‐point VAS. |
|
| Notes | This is a three‐arm study of 90 participants. In group B (30 participants) neostigmine 0.5 mg was added to the block solution, however this is not an outcome of interest for this review and this group was not included in any of the analyses. Funding: no information provided. Conflicts of interest: no information provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | Assume anaesthesiologist performing the block was blinded since study drugs were prepared by an anaesthesiologist not involved in the study, however no indication whether other personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | No protocol available. The authors report mean and SD for all outcomes except pain scores. |
| Other bias | Low risk | Appears to be free of any other bias. |
ASA = Americal Anesthesiology Society; BMI = body mass index; IM = intramuscularly; IV = intravenously; kg = kilograms; MAC = maximum alveolar concentration; mg = milligrams; ml = millilitres; mm = millimetres; NRS = Numerical Rating Scale; PACU = Postanaesthesia care unit; PONV = postoperative nausea and vomiting; RCT = randomized controlled trial; SD = standard deviation; VAS = Visual Analalogue Scale; VRS = Verbal Rating Scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Fredrickson 2013 | All participants received both perineural and intravenous dexamethasone. |
| Lui 2015 | The majority of participants (81/89) received both perineural and intravenous dexamethasone. |
| Percec 2014 | Data for all outcomes were reported as median and range (upper/lower), therefore could not be used in any meta‐analyses. |
| Shethra 2007 | No non‐active comparator. |
Characteristics of ongoing studies [ordered by study ID]
NCT01277159.
| Trial name or title | Duration of analgesia after popliteal fossa nerve blockade: Effects of dexamethasone and buprenorphine |
| Methods | RCT |
| Participants | In the United States of America (New York, New York), participants undergoing ankle surgery. |
| Interventions | Drug: A. Control nerve block. IV dexamethasone (4 mg). Drug: B. Nerve block with dexamethasone (4 mg). IV saline. Drug: C. Control nerve block. IV dexamethasone (4 mg). IV buprenorphine (0.3 mg). Drug: D. Nerve block with buprenorphine (0.3 mg). IV dexamethasone (4 mg). Drug: E. Nerve block with dexamethasone (4 mg)/block buprenorphine (0.3 mg). |
| Outcomes | Time it takes for nerve block to wear off. |
| Starting date | October 2012. |
| Contact information | Hospital for special surgery, New York. |
| Notes | Completed study. Published results not available. |
NCT01495624.
| Trial name or title | The effect of systemic or perineural dexamethasone on the duration of interscalene nerve blocks with ropivacaine |
| Methods | RCT |
| Participants | In the United States of America (Clevland, Ohio) participants age 18 to 75 years undergoing shoulder surgery with interscalene brachial plexus block. |
| Interventions | Placebo comparator: ropivacaine with perineural dexamethasone
30 ml 0.5% ropivacaine plus dexamethasone 8 mg (2 ml) mixed with the local anaesthetic with 2 ml normal saline given intravenously (systemic placebo). Active comparator: ropivacaine with systemic steroid 30 ml 0.5% ropivacaine for interscalene block mixed with 2 ml normal saline (perineural placebo) plus dexamethasone 8 mg (2 ml) administered systemically. Other: ropivacaine plus dexamethasone anaesthetic. Subjects will receive interscalene block comprised of 30 ml 0.5% ropivacaine plus dexamethasone 8 mg (2 ml) mixed with the local anaesthetic with 2 ml normal saline given intravenously (systemic placebo). Other: ropivacaine plus saline plus dexamethasone anaesthetic. Subjects will receive interscalene block with 30 ml 0.5% ropivacaine for interscalene block mixed with 2 ml normal saline (perineural placebo) plus dexamethasone 8 mg (2 ml) administered systemically. |
| Outcomes | The clinical duration of the interscalene nerve block, which will be measured by time from onset of sensory block until first administration of analgesic medication or requirement for initiation of the perineural catheter infusion. Maximum Verbal Response Score with rest (time frame: upon admission to PACU through postoperative day 2, postoperative day 14). Verbal VRS with movement (time frame: upon admission daily through postoperative day 2, postoperative day 14). Total opioid consumption (time frame: daily through postoperative day 2). |
| Starting date | September 2011. |
| Contact information | Principal Investigator: Kenneth Cummings, MD, The Cleveland Clinic. |
| Notes | Study terminated (accrual insufficient to complete study in a feasible time frame). No results available. |
NCT01586806.
| Trial name or title | Postoperative analgesia comparing subsartorial saphenous nerve block with and without dexamethasone in ACL reconstruction |
| Methods | RCT |
| Participants | In the United States of America (New York, New York), ASA I‐III participants age 16‐65 undergoing anterior cruciate ligament reconstruction. |
| Interventions | Bupivacaine 0.5% 13 ml + 1 mg dexamethasone perineurally. Bupivacaine 0.5% 13 ml + 4 mg dexamethasone perineurally. Bupivacaine 0.5% 13 ml perineurally. |
| Outcomes | Patient perceived duration of analgesia. Intensity of pain measured on NRS. Patient satisfaction measured on an 11‐point NRS. Postoperative morphine consumption. Incidence of opioid‐related side effects. |
| Starting date | July 2012. |
| Contact information | Hospital for Special Surgery, New York. |
| Notes | Completed study. No published results. |
NCT01971645.
| Trial name or title | Dexamethasone as an adjuvant to ropivacaine for femoral nerve blocks in children undergoing knee surgery |
| Methods | RCT |
| Participants | In the United States of America (Columbus, Ohio), ASA I‐II participants age 10‐19 years undergoing arthroscopic surgery of the knee. |
| Interventions | Ropivacaine 0.5% 2 mg/kg + 0.1 mg/kg dexamethasone given perineurally + normal saline intramuscularly. Ropivacaine 0.5% 2 mg/kg + 0.1 mg/kg dexamethasone given perineurally + dexamethasone 0.1 mg/kg intramuscularly. Ropivacaine 0.5% 2 mg/kg + normal saline given perineurally + normal saline intramuscularly. |
| Outcomes | Post‐PACU opioid consumption. |
| Starting date | July 2014. |
| Contact information | Giorgio Veneziano, Nationwide Children's Hospital. |
| Notes | Currently recruiting participants. |
NCT02178449.
| Trial name or title | Prolongation of pain free time by the use of dexamethasone in peripheral nerve blockade |
| Methods | RCT |
| Participants | In Austria, ASA I to II participants aged 18 years and older undergoing shoulder arthroscopy with interscalene block. |
| Interventions | Experimental: Ropivacaine and dexamethasone perineurally Active comparator: Ropivacaine + saline placebo. |
| Outcomes | Pain free time measured by the duration between block and the point to first analgesic request. VAS on movement and rest 10 hours after surgery. |
| Starting date | March 2014 |
| Contact information | Christoph Homann. |
| Notes | Recruitment status of this study is unknown because the information has not been verified recently. |
NCT02322242.
| Trial name or title | The effects of dexamethasone on low‐dose interscalene brachial plexus block |
| Methods | RCT |
| Participants | In Canada (Toronto, Ontario), ASA class I to II participants aged 18 to 80 years undergoing arthroscopic shoulder surgery with interscalene block. |
| Interventions | Active comparator: ropivacaine 0.5% + dexamethasone 4 mg given perineurally. Systemic dexamethasone: ropivacaine 0.5% given perineurally + dexamethasone 4 mg given intravenously. |
| Outcomes | Duration of sensory block defined as the time from completion of block to NRS > 0. Time to first opioid consumption. Duration of motor block. Postoperative oxygen saturation on room air. Pulse oximetry one hour after arrival in postoperative recovery room. Opioid consummation 12, 24 and 7 days after surgery. Incidence of nerve damage defined as persistent paraesthesia and sensory/motor block at 7 days. Postoperative nausea and vomiting assessed at 12 hours, 24 hours and 7 days after surgery. |
| Starting date | January 2015. |
| Contact information | Stephen Choi, Sunnybrook Health Science Centre. |
| Notes | Ongoing study July 2015. |
NCT02436694.
| Trial name or title | The effect of systemic or perineural dexamethasone on the duration of interscalene nerve blocks with ropivacaine |
| Methods | RCT |
| Participants | In the Unitied States of America (Cleveland, Ohio), participants undergoing shoulder surgery with interscalene block. |
| Interventions | Placebo comparator: ropivacaine 0.5% 30 ml + dexamethasone 8 mg given perineurally + normal saline 2 ml given intravenously. Active comparator: ropivacaine 0.5% 30 ml + normal saline 2 ml given perineurally + dexamethasone 8 mg given intravenously. |
| Outcomes | Duration of sensory block measured by the time of onset of sensory block until the first administration of analgesic or requirement for perineural catheter infusion. Maximum VAS at rest. Maximum VAS on movement. Postoperative opioid consumption. |
| Starting date | December 2011. |
| Contact information | Kenneth Cummings, The Cleveland Clinic. |
| Notes | This study is currently recruiting participants. |
NCT02462148.
| Trial name or title | Perineural steroids for peripheral nerve blocks |
| Methods | RCT |
| Participants | In the United States of America (Winston‐Salem, North Carolina), participants aged 18 to 90 years undergoing surgery with saphenous nerve block. |
| Interventions | Experimental: bupivacaine 0.25% 20 ml with epinephrine 1:400,000 + dexamethasone 4 mg. Placebo comparator: bupivacaine 0.25% 20 mg with epinephrine 1:400,000. |
| Outcomes | Duration of sensory block. Pain scores 24 and 36 hours after surgery. Incidence of postoperative nausea and vomiting. Incidence of neurologic complications. Opioid consumption 24‐36 hours after surgery. Time to first analgesic request. |
| Starting date | July 2015. |
| Contact information | Daryl Steven, Wake Forest Baptist Health. |
| Notes | This study is currently recruiting participants. |
NCT02506660.
| Trial name or title | Interscalene block with low‐dose IV vs. perineural dexamethasone for shoulder arthroscopy |
| Methods | RCT |
| Participants | In the United States of America (New York, New York), participants aged 18‐70 years undergoing arthroscopic shoulder surgery with ultrasound‐guided interscalene block. |
| Interventions | Active comparator: bupivacaine 0.5% 15 cc perineurally + dexamethasone 1 mg intravenously. Experinmental: bupivacaine 0.5% 15 cc perineurally + dexamethasone 1 mg perineurally. |
| Outcomes | Duration of sensory block. Pain scores for duration of stay in recovery room after surgery, postoperative day 2 and postoperative day 3. Opioid consumption on postoperative day 2 and 3. Adverse events. Opioid‐related symptom distress scale. Satisfaction with block. |
| Starting date | August 2015. |
| Contact information | Jennifer Cheng, Hosptial for Special Surgery, New York. |
| Notes | This study is currently recruiting participants. |
ASA = Amercian Society of Anesthesiologists; IV = intravenous; kg = kilograms; mg = milligrams; ml = millilitres; NRS = Numeric Rating Score; PACU = postanaesthesia care unit; RCT = randomized controlled trial; VAS = Visual Analogue Scale; VRS = Verbal Rating Scale.
Differences between protocol and review
There are several differences between our protocol and review (Pehora 2015).
We stated that we would perform a sensitivity analysis to determine whether missing outcome data put continuous and dichotomous outcomes at risk of bias. We found that the missing outcome data was less than 10% in all outcomes, therefore we did not perform a sensitivity analysis.
We stated that we would perform a subgroup analysis to determine if there was any difference between adult and paediatric participants. Since we did not find any studies in children under the age of 15 we could not perform this subgroup analysis.
One of our secondary outcomes was the incidence of mild to moderate adverse events. We chose to classify mild to moderate adverse events into two categories: block‐related and non‐block‐related. A concern with the use of perineural dexamethasone is that it may be neurotoxic and may cause neuropathy. By separating the block‐related adverse events from those that are not block‐related we could evaluate the number of participants who experienced signs and symptoms that could potentially indicate neuropathy.
The incidence of numbness and tingling was reported at 24 hours, 48 hours, seven days, and 14 days after surgery. In our protocol we did not specify any a priori time points. We chose to analyse the 14‐day time point because it would be more indicative of potential nerve injury than that of the 24‐hour and 7‐day time points.
Our protocol states that we would convert pain data to risk difference (RD), number needed to treat for an additional beneficial outcome (NNTB), and ratio of means (RoM) with corresponding 95% CIs. When we wrote our protocol we were anticipating that some of the studies would report pain on a scale other than a 0‐10 scale. All the studies in our analyses reported pain on a 0‐10 scale, therefore our pain outcomes are reported in their natural units, which are easily understood by clinicians.
Contributions of authors
Carolyne Pehora (CP), Annabel ME Pearson (AP), Alka Kaushal (AK), Mark Crawford (MC), Bradley Johnston (BJ).
Conceiving the review: CP, AP, MWC, BJ.
Co‐ordinating the review: CP, BJ.
Undertaking manual searches: CP.
Screening search results: CP, AK.
Organizing retrieval of papers: CP.
Screening retrieved papers against inclusion criteria: CP, AK.
Appraising quality of papers: CP, AP, BJ.
Abstracting data from papers: CP, AP.
Writing to authors of papers for additional information: CP, AK.
Providing additional data about papers: CP, AK.
Obtaining and screening data on unpublished studies: CP.
Managing data for the review: CP, BJ.
Entering data into Review Manager 5 (Review Manager 2014): CP, BJ.
Interpreting data: CP, AK, AP, MC, BJ.
Making statistical inferences: BJ.
Securing funding for the review: none.
Serving as guarantor for the review (one author): CP.
Taking responsibility for reading and checking the review before submission: BJ.
Sources of support
Internal sources
No sources of support supplied
External sources
None, Other.
Declarations of interest
Carolyne Pehora: none known.
Annabel Pearson: none known.
Alka Kaushal: none known.
Mark Crawford: none known.
Bradley Johnston: none known.
New
References
References to studies included in this review
Abdallah 2015 {published and unpublished data}
- Abadallah FW, Johnson J, Chan V, Murgatroyd H, Ghafari M, Ami N, et al. Intravenous dexamethasone and perineural dexamethasone similarly prolong the duration of analgesia after supraclavicular brachial plexus block. Regional Anesthesia and Pain Medicine 2015;40:125‐32. [PUBMED: 25629321 ] [DOI] [PubMed] [Google Scholar]
Alarasan 2017 {published data only}
- Alarassan AK, Agrawal J, Choudhary B, Melhortra A, Uike S, Mukherji A, et al. Effect of dexamethasone in low volume supraclavicular brachial plexus block: a double‐blinded randomized clinical study. Journal of Anesthesiology, Clinical Pharmacology 2017;32:234‐9. [PUBMED: PMC4874081 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aliste 2017 {published data only}
- Aliste J, Leurcharusmee P, Engsusophon P, Gordon A, Michelagnoli G, Sriparkdee C, et al. A randomized comparison between intravenous and perineural dexamethasone for ultrasound‐guided axillary block. Canadian Journal of Anesthesia 2017;64:29‐36. [PUBMED: 27663451] [DOI] [PubMed] [Google Scholar]
Bias 2014 {published data only}
- Bais D, Geetha C, Kawale A. Effectiveness of addition of dexamethasone to 0.5% ropivacaine in providing perioperative analgesia for supraclavicular brachial plexus block. Journal of Evolution of Medical and Dental Sciences 2014;3(10):2456‐64. [DOI: 10.14260/jemds/2014/2158] [DOI] [Google Scholar]
Biradar 2013 {published data only}
- Biradar PA, Kaimar P, Gopalakrishna K. Effect of dexamethasone added to lidocaine in supraclavicular brachial plexus block: a prospective randomized, double blind study. Indian Journal of Anaesthesia 2013;57(2):180‐4. [PUBMED: 23825819] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chalifoux 2017 {published data only}
- Chalifoux F, Colin F, St‐Pierre P, Godin N, Brulotte V. Low dose intravenous dexamethasone (4 mg and 10 mg) significantly prolongs the analgesic duration of single‐shot interscalene block after arthroscopic shoulder surgery: a prospective randomized placebo‐controlled study. Canadian Journal of Anesthesia 2017;64:280‐9. [PUBMED: 28050801] [DOI] [PubMed] [Google Scholar]
Chun 2016 {published data only}
- Chun EH, Kim YJ, Woo JH. Which is your choice for prolonging the analgesic duration of single‐shot interscalene brachial plexus block for arthroscopic shoulder surgery? intravenous dexamethasone 5 mg vs. perineural dexamethasone 5 mg randomized controlled, clinical trial. Medicine 2006;95(23):e3828. [PUBMED: 27281084] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cummings 2011 {published and unpublished data}
- Cummings KC, Napierkowski DE, Parra‐Sanchez I, Kurz A, Dalton JE, Brems JJ, et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. British Journal of Anaesthesia 2011;107(3):446‐53. [PUBMED: 21676892] [DOI] [PubMed] [Google Scholar]
Dar 2013 {published data only}
- Dar FA, Najar MR, Jan N. Effect of addition of dexamethasone to ropivacaine in supraclavicular brachial plexus block. Indian Journal of Pain 2013;27(3):165‐9. [DOI: 10.4103/0970-5333.124602] [DOI] [Google Scholar]
Dawson 2016 {published data only}
- Dawson RL, McLeod DH, Koerber JP, Plummer JL, Dracopoulos GC. A randomized controlled trial of perineural vs intravenous dexamethasone for foot surgery. Anaesthesia 2016;71(3):285‐90. [PUBMED: 26682721] [DOI] [PubMed] [Google Scholar]
Desmet 2013 {published data only}
- Desmet C, Braems H, Reynvoet M, Plasschaert S, Cauwelaert J, Pottel H, et al. I.V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single‐shot interscalene block with ropivacaine for shoulder surgery: a prospective, randomized, placebo‐controlled study. British Journal of Anaesthesia 2013;111(3):445‐52. [PUBMED: 23587875] [DOI] [PubMed] [Google Scholar]
Desmet 2015 {published data only}
- Desmet M, Vanneste B, Reynovoet M, Cauwelaert J, Verhelst L, Pottell H, et al. A randomized controlled trial of intravenous dexamethasone combined with interscalene brachial plexus block for shoulder surgery. Anaesthesia 2015;70(10):1180‐5. [PUBMED: 26082203] [DOI] [PubMed] [Google Scholar]
Ganvit 2014 {published data only}
- Ganvit KS, Akshay HM, Singhal I, Upadhyay MR. The efficacy of dexamethasone added as an adjuvant to ropivacaine (0.5%) for brachial plexus block. International Journal of Research in Medicine 2014;3(1):71‐4. [ISSN: 2320‐2734] [Google Scholar]
Golwala 2009 {published data only}
- Golwala MP, Swadia VN, Dhimar AA, Sridhar NV. Pain relief by dexamethasone as an adjuvant to local anaesthetics in supraclavicular brachial plexus blocks. Journal of Anaesthesiology and Clinical Pharmacology 2009;25(3):285‐8. [Google Scholar]
Jadon 2015 {published data only}
- Jadon A, Dixit S, Kedia SK, Chakraborty S, Agrawal A, Sinha N. Interscalene brachial plexus block for shoulder surgery: Prospective randomized controlled study of effects of 0.5% ropivacaine and 0.5% ropivacaine with dexamethasone. Indian Journal of Anaesthesia 2015;59(3):171‐6. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kawanishi 2014 {published and unpublished data}
- Kawanishi R, Yamamoto K, Tobetto Y, Nomura K, Kato M, Go R, et al. Perineural but not systemic low‐dose dexamethasone prolongs the duration of interscalene block with ropivacaine: a prospective randomized trial. Local and Regional Anesthesia 2014;7:5‐9. [PUBMED: 24817819] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2012 {published data only}
- Kim YJ, Lee GY, Kim DY, Kim CH, Baik HJ, Heo S. Dexamethasone added to levobupivacaine improves postoperative analgesia in ultrasound guided interscalene brachial plexus blockade for arthroscopic shoulder surgery. Korean Journal of Anesthesiology 2012;62(2):130‐4. [PUBMED: 22379567] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 2014 {published data only}
- Kumar S, Palaria U, Sinha AK, Punera DC, Pandey V. Comparative evaluation of ropivacaine and ropivacaine with dexamethasone in supraclavicular brachial plexus block for postoperative analgesia. Anesthesia: Essays and Researches 2014;8(2):202‐8. [PUBMED: 25886227] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2016 {published data only}
- Lee MJ, Koo DJ, Chou YS, Lee KC, Kim HY. Dexamethasone or dexmedetomidine as local anesthetic agents for ultrasound‐guided axillary brachial plexus blocks with nerve stimulation. Korean Jounal of Pain 2016;29:29‐33. [PUBMED: 26839668 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leurcharusmee 2016 {published data only}
- Leurcharusmee P, Aliste J, Zundert CRV, Engsusophon P, Arnuntasupakul V, Tiyaprasertkul W, et al. A multicenter randomized comparison between intravenous and perineural dexamethasone for ultrasound‐guided infraclavicular block. Regional Anesthesia and Pain Medicine 2016;41:328‐33. [PUBMED: 27015546] [DOI] [PubMed] [Google Scholar]
Movafegh 2006 {published data only}
- Movafegh A, Razazian M, Hajimaohamadi F, Meysamie A. Dexamethasone added to lidocaine prolongs axillary brachial plexus blockade. Anesthesia and Analgesia 2006;102(1):263‐7. [PUBMED: 16368840] [DOI] [PubMed] [Google Scholar]
Nallam 2014 {published data only}
- Nallam SR. Interscalene brachial plexus block: Comparison of efficacy of varying doses of dexamethasone combined with levobupivacaine: A double‐blind randomized trial. Journal of Evolution of Medical and Dental Sciences 2014;3(25):6900‐8. [Google Scholar]
Parrington 2010 {published data only}
- Parrington SJ, O'Donnell D, Chan VW, Brown‐Shreves D, Subramanyam R, Qu M, et al. Dexamethasone added to mepivacaine prolongs the duration of analgesia after supraclavicular brachial plexus block. Regional Anesthesia and Pain Medicine 2010;35(5):422‐6. [PUBMED: 20814282 ] [DOI] [PubMed] [Google Scholar]
Rahangdale 2014 {published and unpublished data}
- Rahangdale R, Kendall MC, McCarthy RJ, Tureanu L, Doty R, Weingart A, et al. The effects of perineural versus intravenous dexamethasone on sciatic nerve blockade outcomes: a randomized, double‐blind, placebo controlled study. Anesthesia and Analgesia 2014;118(5):1113‐9. [PUBMED: 24686045] [DOI] [PubMed] [Google Scholar]
Rosenfeld 2016 {published and unpublished data}
- Rosenfeld DM, Ivancic MG, Hattrup SJ, Renfree KJ, Watkins AR, Hentz JG, et al. Perineural versus intravenous dexamethasone as adjuncts to local anaesthetic brachial plexus block for shoulder surgery. Anaesthesia 2016;71(4):380‐8. [PUBMED: 26899862] [DOI] [PubMed] [Google Scholar]
Sakae 2017 {published data only}
- Sakae TM, Marchioro P, Schuelter‐Trevisol F, Trevison DJ. Dexamethasone as a ropivacaine adjuvant for ultrasound‐guided interscalene brachial plexus block: A randomized, double‐blinded clinical trial. Journal of Clinical Anesthesia 2017;38:113‐6. [DOI] [PubMed] [Google Scholar]
Saritas 2014 {published data only}
- Saritas A, Sabuncu C. Comparison of clinical effects of prilocaine, dexamethasone added to prilocaine and levobupivacaine on brachial plexus block. Journal of the Pakistani Medical Association 2014;64(4):433‐6. [PUBMED: 24864639] [PubMed] [Google Scholar]
Shah 2015 {published and unpublished data}
- Shah DM, Arora M, Trikha A, Prasad G, Sunder R, Kotwal P, et al. Comparison of dexamethasone and clonidine as an adjuvant to 1.5% lignocaine with adrenaline in infraclavicular brachial plexus block for upper limb surgeries. Journal of Anaesthesiology Clinical Pharmacology 2015;13(3):354‐9. [PUBMED: 26330715 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaikh 2013 {published data only}
- Shaikh MR, Majumdar S, Das A, Saha TK, Bandyopadhyay SN, Mikherjee D, et al. Role of dexamethasone in supraclavicular brachial plexus block. IOSR Journal of Dental and Medical Sciences 2013;12(1):1‐7. [Google Scholar]
Talukdar 2013 {published data only}
- Talukdar M, Begum H, Shoman MM, Khatun UHS. Anaesthetic and analgesic effects of adding dexamethasone to bupivacaine in supraclavicular brachial plexus block ‐ a comparative study. Journal of Bangladesh College of Physicans and Surgeons 2013;31(1):11‐7. [Google Scholar]
Tandoc 2011 {published data only}
- Tandoc MN, Fan L, Kolesnikov S, Kruglov A, Nader ND. Adjuvant dexamethasone with bupivacaine prolongs the duration of interscalene block: a prospective randomized trial. Journal of Anesthesia 2011;25(5):704‐9. [PUBMED: 21681533] [DOI] [PubMed] [Google Scholar]
Viera 2010 {published and unpublished data}
- Viera PA, Pulai I, Tsao GC, Manikantan P, Keller B, Connelly NR. Dexamethasone with bupivacaine increases duration of analgesia in ultrasound‐guided interscalene brachial plexus block. European Journal of Anaesthesiology 2010;27(3):285‐8. [PUBMED: 20009936] [DOI] [PubMed] [Google Scholar]
Vishnu 2014 {published data only}
- Vishnu VA, Vishnu MB, Sai NL. Effect of dexamethasone with bupivacaine on duration of supra clavicular block compared to bupivacaine with normal saline: A prospective, randomized and double blind study. Journal of Evolution of Medical and Dental Sciences 2014;3(28):7861‐9. [DOI: 10.14260/jemds/2014/2984] [DOI] [Google Scholar]
Woo 2015 {published data only}
- Woo JH, Kim YJ, KIm DY, Cho S. Dose‐dependency of dexamethasone on th analgesic effect of interscalene block for arthroscopic should surgery using ropivacaine 0.5%. European Journal of Anaesthesiology 2015;32(9):650‐5. [PUBMED: 25603389] [DOI] [PubMed] [Google Scholar]
Yadov 2008 {published data only}
- Yadov RK, Sah BP, Kumar P, Singh SN. Effectiveness of addition of neostigmine or dexamethasone to local anaesthetic in providing perioperative analgesia for brachial plexus block: a prospective, randomized, double blinded, controlled study. Kathmandu University Medical Journal 2008;6(23):302‐9. [PUBMED: 20071811] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Fredrickson 2013 {published data only}
- Fredrickson Franzca MJ, Danesh‐Clough TK, White R. Adjuvant dexamethasone for bupivacaine sciatic and ankle blocks. Results from 2 randomized‐controlled trials. Regional Anesthesia and Pain Medicine 2013;38(4):300‐7. [PUBMED: 23698496] [DOI] [PubMed] [Google Scholar]
Lui 2015 {published data only}
- Lui J, Richman KA, Grodfsky SR, Bhatt S, Huffman GR, Kelly JD, et al. Is there a dose relationship of dexamethasone as adjuvant for supraclavicular brachial plexus block? A prospective randomized double‐blinded clinical study. Journal of Clinical Anesthesia 2015;27(3):237‐42. [PUBMED: 25637938] [DOI] [PubMed] [Google Scholar]
Percec 2014 {published data only}
- Persec J, Persec Z, Kopljar M, Zupcic M, Sakic L, Zrinjscak IK, et al. Low‐dose dexamethasone with levobupivacaine improves analgesia after supraclavicular brachial plexus blockade. International Orthopedics 2014;38(1):101‐5. [PUBMED: 24173676] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shethra 2007 {published data only}
- Shesthra BR, Maharjan SK, Shrestha S, Gautam B, Thapa C, Thapa PB, et al. Comparative study between tramadol and dexamethasone as an admixture to bupivacaine in supraclavicular brachial plexus block. Journal of the Napali Medical Association 2007;46(168):158‐64. [PUBMED: 18340366] [PubMed] [Google Scholar]
References to ongoing studies
NCT01277159 {published data only}
- NCT01277159. Duration of analgesia after popliteal fossa nerve blockade: effects of dexamethasone and buprenophine. clinicaltrials.gov/show/NCT01277159 (first received 13 January 2011).
NCT01495624 {published data only}
- NCT01495624. The effect of systemic or perineural dexamethasone in the duration of interscalene nerve blocks with ropivacaine. clinicaltrials.gov/show/NCT1495624 (first received 12 December 2011).
NCT01586806 {published data only}
- NCT01586806. Comparing subsartorial saphenous nerve blocks with and without dexamethasone from anterior cruciate ligament reconstruction. clinicaltrials.gov/show/NCT01586806 (first received 25 April 2012).
NCT01971645 {published data only}
- NCT01971645. Decadon as adjuvant in femoral nerve blocks in knee arthroscopy. clinicaltrials.gov/show/NCT01971645 (first received 22 October 2013).
NCT02178449 {published data only}
- NCT02178449. Prolongation of pain free time by the use of dexamethasone in peripheral nerve blocks. clinicaltrials.gov/show/NCT02178449 (first received 26 May 2014).
NCT02322242 {published data only}
- NCT02322242. The effects of dexamethasone on low dose interscalene brachial plexus block (ISB‐Dex). clinicaltrials.gov/show/NCT02322242 (first received 16 December 2014).
NCT02436694 {published data only}
- NCT02436694. Nerve blocks with dexamethasone and local anaesthetic to improve postoperative analgesia. clinicaltrials.gov/show/NCT02436694 (first received 1 April 2015).
NCT02462148 {published data only}
- NCT02462148. Perineural steroids for peripheral nerve block. clinicaltrials.gov/show/NCT02462148 (first received 1 June 2015).
NCT02506660 {published data only}
- NCT02506660. Interscalene block with low‐dose IV vs. perineural dexamethasone for shoulder arthroscopy (Dex ISB). clinicaltrials.gov/show/NCT02506660 (first received 2 July 2015).
Additional references
Adhikary 2012
- Adhikary SD, Armstrong K, Chin KJ. Perineural entrapment of an interscalene stimulating catheter. Anaesthesia and Intensive Care 2012;40(3):527‐30. [PUBMED: 22577921] [DOI] [PubMed] [Google Scholar]
Akl 2012
- Akl EA, Briel M, You JJ, Sun X, Johnston BC, Busse JW, et al. Potential impact on estimated treatment effects of information lost to follow‐up in randomised controlled trials (LOST‐IT): systematic review. BMJ 2012;18(344):e2809. [PUBMED: 22611167] [DOI] [PubMed] [Google Scholar]
Akl 2013
- Akl EA, Johnston BC, Alonso‐Coello P, Neumann I, Ebrahim S, Briel M, et al. Addressing dichotomous data for participants excluded from trial analysis: a guide for systematic reviewers. PLoS One 2013;8(2):e0057132. [PUBMED: 23451162] [DOI] [PMC free article] [PubMed] [Google Scholar]
Albrecht 2015
- Albrecht E, Kern C, Kirkham KR. A systematic review and meta‐analysis of perineural dexamethasone for peripheral nerve blocks. Anaesthesia 2015;70:71‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arora 2010
- Arora M, Shah D. Evaluation of dexamethasone versus clonidine as adjuvants to 1.5% lignocaine with adrenaline in infraclavicular brachial plexus block for upper limb surgeries. Journal of Pain 2010;11:S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Avidan 2003
- Avidan A, Drenger B, Ginosar Y. Peripheral nerve block for ambulatory surgery and postoperative analgesia. Current Opinion in Anesthesiology 2003;16:567‐73. [PUBMED: 17021512] [DOI] [PubMed] [Google Scholar]
Barreveld 2013
- Barreveld A, Witte J, Chahal K, Durieux ME, Strichartz G. Preventative analgesia by local anesthetics: the reduction of postoperative pain by peripheral nerve blocks and intravenous drugs. Anesthesia and Analgesia 2013;116(5):1141‐61. [PUBMED: 23408672] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bartlett 2013
- Bartlett R, Hartle AJ. Routine use of dexamethasone for postoperative nausea and vomiting: the case against. Anaesthesia 2013;68(9):892‐6. [PUBMED: 23848377] [DOI] [PubMed] [Google Scholar]
Blyth 2003
- Blyth FM, March LM, Cousins MJ. Chronic pain‐related disability and use of analgesia and health services in a Sydney community. Medical Journal of Australia 2003;179(2):84‐7. [PUBMED: 12864718] [DOI] [PubMed] [Google Scholar]
Bowens 2011
- Bowens C, Briggs ER, Malchow RJ. Brachial plexus entrapment of interscalene nerve catheter after uncomplicated ultrasound guided placement. Pain Medicine 2011;12(7):1117‐20. [PUBMED: 21699651 ] [DOI] [PubMed] [Google Scholar]
Brummett 2012
- Brummett CM, Williams BA. Additives to local anesthetics for peripheral nerve blockade. International Anesthesiology Clinics 2012;49(4):104‐16. [PUBMED: 21956081] [DOI] [PMC free article] [PubMed] [Google Scholar]
Charlton 2010
- Charlton S, Cyna AM, Middleton P, Griffiths JD. Perioperative transversus abdominis plane (TAP) blocks for analgesia after abdominal surgery. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD007705.pub2; PUBMED: 21154380 ] [DOI] [PubMed] [Google Scholar]
Choi 2014
- Choi S, Rodseth R, McCartney CJ. Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: a systematic review and meta‐analysis of randomized trials. British Journal of Anaesthesia 2014;112(3):427‐39. [PUBMED: 24413428] [DOI] [PubMed] [Google Scholar]
Christiansson 2009
- Christiansson. Update on adjuvants in regional anaesthesia. Periodicum Biologorum 2009;3:161‐70. [ISSN: 00315362] [Google Scholar]
Crandell 2004
- Crandell JT. Perineal pruritus after the administration of iv dexamethasone. Canadian Journal of Anesthesia 2004;51(4):398. [PUBMED: 15064273 ] [DOI] [PubMed] [Google Scholar]
De Olivera 2013
- Oliveria GS, Castro‐Alves LJ, Ahmad S, Kendall MC, McCarthy RJ. Dexamethasone to prevent postoperative nausea and vomiting. An updated meta‐analysis of randomized control trials. Anesthesia and Analgesia 2013;116(1):58‐74. [PUBMED: 23223115] [DOI] [PubMed] [Google Scholar]
De Oliveira 2011
- Oliveira GS, Almeida M, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain. A meta‐analysis of randomized controlled trials. Anesthesiology 2011;115(3):575‐88. [PUBMED: 21799397 ] [DOI] [PubMed] [Google Scholar]
De Oliveira 2014
- Oliveira GS, Castro‐Alves LJ, Nader A, Kendall MC, Rahangdale R, McCarthy RJ. Perineural dexamethasone to improve postoperative analgesia with peripheral nerve blocks: a meta‐analysis of randomized control trials. Pain Research and Treatment 2014;2014:179029. [PUBMED: 25485150] [DOI] [PMC free article] [PubMed] [Google Scholar]
Desmet 2012
- Desmet M, Pottel H, Missant C, Velde M. The effect of dexamethasone on the duration and quality of single shot interscalene block with ropivacaine for shoulder surgery: A prospective randomized control trial. Reginal Anesthesia and Pain Medicine 2012;37(7):E188‐9. [Google Scholar]
Ebrahim 2013
- Ebrahim S, Alk EA, Mustafa RA, Sun X, Walter SD, Heels‐Ansdell D, et al. Addressing continuous data for participants excluded from trial analysis. Journal of Clinical Epidemiology 2013;66(9):1014‐21. [PUBMED: 23774111] [DOI] [PubMed] [Google Scholar]
Fast 1984
- Fast A, Alon M, Weiss S, Zer‐Aviv FR. Avascular necrosis of bone following short‐term dexamethasone therapy for brain edema. Journal of Neurosurgery 1984;61(5):983‐5. [PUBMED: 6491744] [DOI] [PubMed] [Google Scholar]
Galvez 2007
- Galvez R, Marsal C, Vidal J, Ruiz M, Rejas J. Cross‐sectional evaluation of patients functioning and health‐related quality of life in patients with neuropathic pain under standard care conditions. European Journal of Pain 2007;11(3):244‐55. [PUBMED: 16563819] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version Date accessed: April 25, 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ, et al. What is “quality of evidence” and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hadzic 2005
- Hadzic A, Karaca PE, Hobeika P, Unis G, Dermksian J, Yufa M, et al. Peripheral nerve block results in superior recovery profile compared with general anesthesia in outpatient knee arthroscopy. Anesthesia and Analgesia 2005;100(4):976‐81. [PUBMED: 15781509] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration. [Available from: www.cochrane‐handbook.org.]
Huynh 2015
- Huynh TM, Marret E, Bonnet F. Combination of dexamethasone and local anesthetic solution in peripheral nerve blocks. A meta‐analysis of randomised placebo trials. European Journal of Anaesthesiology 2015;32(11):751‐8. [PUBMED: 25774458] [DOI] [PubMed] [Google Scholar]
Johansson 1990
- Johansson A, Hao J, Sjölund B. Local corticosteroid application blocks transmission in normal nociceptive C‐fibres. Acta Anaesthesiologica Scandinavia 1990;34(5):335‐8. [PUBMED: 2167604] [DOI] [PubMed] [Google Scholar]
Johnston 2013
- Johnston BC, Patrick DL, Thorlund K, Busse JW, Costa BR, Schünemann HJ, et al. Patient‐reported outcomes in meta‐analyses. Part 2: methods for improving interpretability for decision‐makers. Health and Quality of Life Outcomes 2013;21(11):211. [PUBMED: 24359184] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kehlet 2006
- Kehlet H, Jensen TS, Woolf C. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367(9522):1618‐25. [PUBMED: 16698416] [DOI] [PubMed] [Google Scholar]
Kelly 2001
- Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emergency Medicine Journal 2001;18(3):205‐7. [PUBMED: 11354213] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2010
- Kim YJ, Han JI, Baik HJ, Lee GY. 5 mg dexamathasone added to levobupivacaine improves postoperative analgesia in ultrasound guided interscalene brachial plexus blockade for arthroscopic shoulder surgery. Regional Anesthesia and Pain Medicine 2010;35(5):464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knezivic 2015
- Knezevic NN, Anantamongkol U, Candido KD. Perinerual dexamethasone added to local anesthesia for brachial plexus block improves pain but delays block onset and motor blockade recovery. Pain Physcian 2015;18(1):1‐14. [PUBMED: 25675053] [PubMed] [Google Scholar]
Lame 2005
- Lame IE, Peters ML, Vlaeyen JW, Kleef M, Patijn J. Quality of life in chronic pain is more associated with beliefs about pain, than with pain intensity. European Journal of Pain 2005;9(1):15‐24. [PUBMED: 15629870] [DOI] [PubMed] [Google Scholar]
Lerza 2002
- Lerza R, Botta M, Barsotto B, Schenone E, Mencoboni M, Bogliolo G, et al. Dexamethazone‐induced acute tumor lysis syndrome in a T‐cell malignant lymphoma. Leukemia & Lymphoma 2002;43(5):1129‐32. [PUBMED: 12148896 ] [DOI] [PubMed] [Google Scholar]
Lin 2013
- Lin E, Choi J, Hadzic A. Peripheral nerve blocks for outpatient surgery: evidence based indications. Current Opinion in Anesthesiology 2013;26(4):467‐74. [PUBMED: 23820103] [DOI] [PubMed] [Google Scholar]
Ma 2010
- Ma R, Wang X, Lu C, Li C, Cheng Y, Ding G, et al. Dexamethasone attenuated bupivacaine‐induced neuron injury in vitro through a threonine‐serine protein kinase B‐dependent mechanism. Neuroscience 2010;167(2):329‐42. [PUBMED: 20038443] [DOI] [PubMed] [Google Scholar]
Mc Donnell 2008
- McDonnell C, Barlow R, Campisi P, Grant R, Malkin D. Fatal peri‐operative acute tumour lysis syndrome precipitated by dexamethasone. Anaesthesia 2008;63(6):652‐5. [PUBMED: 18477278] [DOI] [PubMed] [Google Scholar]
McKee 2001
- McKee MD, Waddell JP, Kudo PA, Schemitsch EH, Richards RR. Osteonecrosis of the femoral head in men following short‐course corticosteroid therapy: a report of 15 cases. Canadian Medical Association Journal 2001;164(2):205‐6. [PUBMED: 11332313 ] [PMC free article] [PubMed] [Google Scholar]
NIH 2013
- National Institute of Health. Adverse events. https://www.nhlbi.nih.gov/research/funding/research‐support/crg/glossary.htm (accessed 26 September 2014).
Nuesch 2009
- Nuesch E, Reichenback S, Trelle S, Rutjes AWS, Liewald K, Sterchi R, et al. The importance of allocation concealment and patient blinding in osteoarthritis trials: a meta‐epidemiologic study. Arthritis and Rheumatism 2009;61(12):1633‐41. [PUBMED: 19950329] [DOI] [PubMed] [Google Scholar]
Pasternak 2004
- Pasternak JJ, McGregor DG, Lanier WL. Effect of single‐dose dexamethasone on blood glucose concentration in patients undergoing craniotomy. Journal of Neursurgical Anesthesiology 2004;16(2):122‐5. [PUBMED: 15021280] [DOI] [PubMed] [Google Scholar]
Percival 2010
- Percival VG, Riddell J, Corcoran TB. Single dose dexamethasone for postoperative nausea and vomiting‐a matched case‐placebo study of postoperative infection risk. Anaesthesia and Intensive Care 2010;38(4):661‐6. [PUBMED: 20715728] [DOI] [PubMed] [Google Scholar]
Perron 2003
- Perron G, Dolbec P, Germain J, Bechard P. Perineal pruritis after i.v. dexamethasone administration. Canadian Journal of Anesthesia 2003;50(7):749‐50. [PUBMED: 12944454 ] [DOI] [PubMed] [Google Scholar]
Popping 2009
- Popping DM, Ellia N, Marre E, Wenk M, Tramer MR. Clonidine as an adjuvant to local anesthetic for peripheral nerve block. A meta‐analysis of randomized trials. Anesthesiology 2009;111(2):406‐15. [PUBMED: 19602964] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2011
- Riley RD, Higgins J, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:964‐7. [PUBMED: 21310794] [DOI] [PubMed] [Google Scholar]
Shishido 2002
- Shishido H, Kikuchi S, Heckman H, Myers RR. Dexamethasone decreases blood flow in normal nerves and dorsal root ganglia. Spine 2002;27(6):581‐6. [PUBMED: 11884905] [DOI] [PubMed] [Google Scholar]
Shrestha 2003
- Shrestha BR, Maharjan SK, Tabedar S. Supraclavicular and brachial plexus block with and without dexamethasone ‐ a comparative study. Kathmandu University Medical Journal 2003;1(3):158‐60. [PUBMED: 16388222] [PubMed] [Google Scholar]
Smith 2007
- Smith BH, Torrance N, Bennett MI, Lee AJ. Health and quality of life associated with chronic pain of predominantly neuropathic origin in the community. Clinical Journal of Pain 2007;23(2):143‐9. [PUBMED: 17237663] [DOI] [PubMed] [Google Scholar]
Vandenkerkoff 2012
- Vandenkerkoff EG, Hopman WM, Goldstein DH, Wilson RA, Towheed TE, Lam M, et al. Impact of perioperative pain intensity, pain qualities and chronic pain after surgery. A prospective cohort study. Regional Anesthesia and Pain Medicine 2012;37(1):19‐27. [PUBMED: 22157741] [DOI] [PubMed] [Google Scholar]
Waldron 2013
- Waldron NH, Jones CA, Gan TJ, Allen TK, Habib AS. Impact of perioperative dexamethasone on postoperative analgesia and side‐effects: systematic review and meta‐analysis. British Journal of Anaesthesia 2013;110(2):191‐200. [PUBMED: 23220857] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2011
- Wang PH, Tsai CL, Lee JS, Wu KC, Cheng KI, Jou IM. Effects of topical corticosteroids on the sciatic nerve: an experimental study to adduce the safety in treating carpel tunnel syndrome. Journal of Hand Surgery: European Volume 2011;36(3):236‐43. [PUBMED: 21282223] [DOI] [PubMed] [Google Scholar]
Williams 2014
- Williams BA, Schott NJ, Mangione MP, Ibinson JW. Perineural dexamethasone and multimodal perineural analgesia: how much is too much?. Anesthesia and Analgesia 2014;118(5):912‐4. [PUBMED: 24781562] [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [PUBMED: 18316340] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yared 2000
- Yared JP, Starr NJ, Torres FK, Bashour CA, Bourdakos G, Piedmonte M, et al. Effects of single dose, post induction dexamethasone on recovery after cardiac surgery. Annals of Thoracic Surgery 2000;69(5):1420‐4. [PUBMED: 10881816] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Pehora 2015
- Pehora C, Pearson AME, Kaushal A, Crawford M, Johnston BC. Dexamethasone as an adjuvant to peripheral nerve block. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011770] [DOI] [PMC free article] [PubMed] [Google Scholar]


