Abstract
Background
Vulvovaginal candidiasis (VVC) is estimated to be the second most common form of infection after bacterial vaginosis. The ability of probiotics in maintaining and recovering the normal vaginal microbiota, and their potential ability to resist Candidas give rise to the concept of using probiotics for the treatment of VVC.
Objectives
To assess the effectiveness and safety of probiotics for the treatment of vulvovaginal candidiasis in non‐pregnant women.
Search methods
We searched the following databases to October 2017: Sexually Transmitted Infections Cochrane Review Group's Specialized Register, CENTRAL, MEDLINE, Embase and eight other databases. We searched in following international resources: World Health Organization International Clinical Trials Registry Platform, ClinicalTrials.gov, Web of Science and OpenGrey. We checked specialty journals, reference lists of published articles and conference proceedings. We collected information from pharmaceutical companies and experts in the field.
Selection criteria
Randomized controlled trials (RCT) using probiotics, alone or as adjuvants to conventional antifungal drugs, to treat VVC in non‐pregnant women. Trials recruiting women with recurrent VVC, coinfection with other vulvovaginal infections, diabetes mellitus, immunosuppressive disorders or taking immunosuppressant medication were ineligible for inclusion. Probiotics were included if they were made from single or multiple species and in any preparation type/dosage/route of administration.
Data collection and analysis
Two review authors independently assessed trials for eligibility and quality and extracted data. We resolved any disagreements through consensus. The quality of the evidence was assessed using the GRADE approach.
Main results
Ten RCTs (1656 participants) met our inclusion criteria, and pharmaceutical industry funded none of these trials. All trials used probiotics as adjuvant therapy to antifungal drugs. Probiotics increased the rate of short‐term clinical cure (risk ratio (RR) 1.14, 95% confidence interval (CI) 1.05 to 1.24, 695 participants, 5 studies, low quality evidence) and mycological cure (RR 1.06, 95% CI 1.02 to 1.10, 969 participants, 7 studies, low quality evidence) and decreased relapse rate at one month (RR 0.34, 95% CI 0.17 to 0.68, 388 participants, 3 studies, very low quality evidence). However, this effect did not translate into a higher frequency of long‐term clinical cure (one month after treatment: RR 1.07, 95% CI 0.86 to 1.33, 172 participants, 1 study, very low quality evidence; three months after treatment: RR 1.30, 95% CI 1.00 to 1.70, 172 participants, one study, very low quality evidence) or mycological cure (one month after treatment: RR 1.26, 95% CI 0.93 to 1.71, 627 participants, 3 studies, very low quality evidence; three months after treatment: RR 1.16, 95% CI 1.00 to 1.35, 172 participants, one study, very low quality evidence). Probiotics use did not increase the frequency of serious (RR 0.80, 95% CI 0.22 to 2.94; 440 participants, 2 studies, low quality evidence). We found no eligible RCTs for outcomes as time to first relapse, need for additional treatment at the end of therapy, patient satisfaction and cost effectiveness.
Authors' conclusions
Low and very low quality evidence shows that, compared with conventional treatment, the use of probiotics as an adjuvant therapy could increases the rate of short‐term clinical and mycological cure and decrease the relapse rate at one month but this did not translate into a higher frequency of long‐term clinical or mycological cure. Probiotics use does not seem to increase the frequency of serious or non‐serious adverse events. There is a need for well‐designed RCTs with standardized methodologies, longer follow‐up and larger sample size.
Plain language summary
Probiotics for vulvovaginal candidiasis in non‐pregnant women
Question
In this Cochrane Review, we assessed the effect and safety of probiotics for the treatment of vulvovaginal candidiasis (VVC) in non‐pregnant women compared with conventional antifungal drugs, or probiotics used to change the effects of conventional antifungal drugs.
Background
The condition of VVC occurs because of an imbalance in the normal vaginal microorganism habitat (microbiota). It is characterized by a decrease of a type of bacteria called lactobacilli and a concomitant overgrowth of a fungus called Candida. Although treatments for VVC by conventional antifungal drugs are quite effective at providing clinical cure (no apparent vaginal symptoms), there is an increasing in resistance to the drugs and recurrence of VVC. Conventional antifungal drugs can also cause many side effects. Probiotics are microorganisms that are believed to provide health benefits when consumed. The ability of probiotics in maintaining and recovering the normal vaginal microbiota, and their potential ability to resist Candidas gives rise to the concept of using probiotics for the treatment of VVC. We wanted to find out whether using probiotics could be useful in treating VVC in non‐pregnant women without high risk or side effects.
Study characteristics
We searched evidence up to October 2017 and included 10 clinical trials with 1656 participants. The trials lasted between three months and five years. All trials used at least one laboratory method for diagnosis. Four trials compared vaginal suppository (solid medicine inserted directly into the vagina) or tablet of clotrimazole (antifungal medicine) plus vaginal capsules of probiotics with vaginal suppository or tablet of clotrimazole alone. Three trials compared vaginal suppository of miconazole (antifungal medicine) plus vaginal capsules of probiotics with vaginal suppository of miconazole alone. Two trials compared oral fluconazole (antifungal medicine) plus oral capsules of probiotics with oral fluconazole plus oral capsules of placebo (pretend treatment). One trial compared oral fluconazole and vaginal fenticonazole (antifungal medicines) with oral fluconazole plus vaginal fenticonazole plus probiotic.
Key results
Compared with conventional antifungal drugs used alone, probiotics as adjuvant therapy could enhance their effect in improving the rate of short‐term (within five to 10 days) clinical cure, short‐term mycological cure (no abnormal laboratory results) and relapse at one month (recurrence of problems), but does not seem to influence the rate of long‐term (within one to three months) clinical cure, long‐term mycological cure, serious and non‐serious side events.
However, because of the low quality of evidence available, there is insufficient evidence for the use of probiotics as adjuvants to conventional antifungal medicines or used alone for the treatment of VVC in non‐pregnant women.
Quality of the evidence
The quality of the evidence was low or very low in this review, so we have very little confidence in the results.
Summary of findings
Summary of findings for the main comparison. Probiotics used as adjuvants to conventional antifungal drugs compared with conventional antifungal drugs for the treatment of vulvovaginal candidiasis in non‐pregnant women.
| Probiotics used as adjuvants to conventional antifungal drugs compared with conventional antifungal drugs for the treatment of vulvovaginal candidiasis in non‐pregnant women | |||||||
|
Patient or population: non‐pregnant women with vulvovaginal candidiasis Settings: outpatient clinics in Brazil, Bulgaria, Iran and China Intervention: probiotics used as adjuvants to conventional antifungal drugs Comparison: conventional antifungal drugs | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | ||
| Assumed risk | Corresponding risk | ||||||
| Conventional antifungal drugs | Probiotics used as adjuvants to conventional antifungal drugs | ||||||
|
Clinical cure rate (short‐term) follow‐up: 5‐10 days |
Medium risk population | RR 1.14 (1.05 to 1.24) | 695 (5) | ⊕⊕⊝⊝ Low1 | ‐ | ||
| 721 per 1000 | 822 per 1000 (757 to 894) | ||||||
| Clinical cure rate (long‐term) |
1 month after treatment follow‐up: 1 month |
Medium risk population | RR 1.07 (0.86 to 1.33) | 172 (1) | ⊕⊝⊝⊝ Very low1,2 | ‐ | |
| 635 per 1000 | 679 per 1000 (546 to 845) | ||||||
|
3 months after treatment follow‐up: 3 months |
Medium risk population | RR 1.30 (1.00 to 1.70) | 172 (1) | ⊕⊝⊝⊝ Very low1,2 | ‐ | ||
| 494 per 1000 | 642 per 1000 (494 to 840) | ||||||
|
Mycological cure rate (short‐term) follow‐up: 5‐10 days |
Medium risk population | RR 1.06 (1.02 to 1.10) | 969 (7) | ⊕⊕⊝⊝ Low1 | See 3 in footnotes. | ||
| 880 per 1000 | 933 per 1000 (898 to 968) | ||||||
| Mycological cure rate (long‐term) |
1 month after treatment follow‐up: 28‐30 days |
Medium risk population | RR 1.26 (0.93 to 1.71) | 627 (3) | ⊕⊝⊝⊝ Very low1,2,4 | See 5 in footnotes. | |
| 706 per 1000 | 890 per 1000 (657 to 1000) | ||||||
|
3 months after treatment follow‐up: 3 months |
Medium risk population | RR 1.16 (1.00 to 1.35) | 172 (1) | ⊕⊝⊝⊝ Very low1,2 | ‐ | ||
| 741 per 1000 | 860 per 1000 (741 to 1000) | ||||||
|
Relapse rate follow‐up: 30‐37 days after treatment |
Medium risk population | RR 0.34 (0.17 to 0.68) | 388 (3) | ⊕⊝⊝⊝ Very low1,6 | ‐ | ||
| 145 per 1000 | 49 per 1000 (25 to 99) | ||||||
|
Rate of serious adverse events follow‐up: 5‐90 days after treatment |
Medium risk population | RR 0.80 (0.22 to 2.94) | 440 (2) | ⊕⊕⊝⊝ Low1,2 | ‐ | ||
| 23 per 1000 | 18 per 1000 (5 to 68) | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||||
1Downgraded two levels due to very serious risk of bias: the included studies had a high or unclear risk of bias in at least one key domain i.e. random sequence generation, allocation concealment or blinding.
2Downgraded one level due to serious imprecision: the 95% CI were wide and included null effects.
3A sensitivity analysis that excluded studies with high risk of bias and included only one study showed that the rate of short‐term mycological cure was changed to no significantly difference between two arms. For the heterogeneity and difference in results, we think the reasons may be associated with the small sample of included study, high risk of bias of studies that were excluded for sensitivity analysis.
4Downgraded one level due to inconsistency: there was a substantial heterogeneity between studies.
5There was heterogeneity that may be attributable to differences in route of the administration of probiotics, we undertook a subgroup analysis, the results showed no statistically significant difference between two arms in either subgroup.
6Downgraded one level due to serious imprecision: small sample size and few events.
Background
Description of the condition
Epidemiology
Vaginitis is one of the most common reasons for women to seek medical assistance from obstetrician and gynecologists (Nyirjesy 2008). Vulvovaginal candidiasis (VVC) is estimated to be the second most common form of vaginal infection after bacterial vaginosis (Sobel 2007; Martinez 2009a). Data about the incidence of VVC are limited and incomplete, since it is not a reportable disease and is often diagnosed without mycological confirmatory (fungi identified) tests and treated with non‐prescription drugs (Sobel 1998; Nyirjesy 2003). Misdiagnosis is common, and it has been shown that about two‐thirds of all non‐prescription drugs for VVC are used without the disease, and that women who overused non‐prescription antifungal drugs, may increase the risk of resistance to antifungal treatments (Sobel 1998; Sobel 2007; Nyirjesy 2008).
Approximately 70% to 75% of women experience at least one episode of VVC in their lives (Sobel 1998; Sobel 2007). Age appears to be an important factor in the overall incidence of VVC because episodes occur mostly during childbearing years and rarely in premenarchal and postmenopausal years (Sobel 1998; Sobel 2007). Approximately 40% to 45% of women will experience two or more episodes of VVC in one year (Sobel 2007; Nyirjesy 2008). One epidemiological study indicated that the frequency of the first diagnosis of VVC increased rapidly after 17 years of age. By the age of 25 years, 54.7% of female college students experienced at least one episode of physician‐diagnosed VVC (Geiger 1995). Recurrent VVC (RVVC) is defined as four or more episodes in one year, and affects 5% to 8% of adult women (Foxman 1998; Sobel 1998; Falagas 2006; Sobel 2007; Nyirjesy 2008). It is reported that the incidence of VVC almost doubled from 1980 to 1990, based on the number of prescriptions written to treat VVC during that period (Sobel 2007). The annual combined cost of health care and lost productivity due to VVC in the US was estimated to be USD1.8 billion, and is projected to reach USD3.1 billion by 2014 (Foxman 2000).
Pathology
The vaginal microenvironment is a complex microecological system, and Lactobacillus is the most commonly dominant flora (the aggregate of bacteria, fungi or other microorganisms) in the vaginal microecological system (Redondo‐Lopez 1990; Kledanoff 1991; Sobel 2007). In normal conditions, vaginal lactobacilli produce lactic acid, thus acidifying the healthy vagina to a low pH level (4.5 or less), which could inhibit the overgrowth of other pathogenic bacteria or Candida (Redondo‐Lopez 1990; Kledanoff 1991). Many species of Lactobacillus also produce other substances, such as hydrogen peroxide and bacteriocin, which further prevent the overgrowth of the pathogenic microbes (Redondo‐Lopez 1990; Kledanoff 1991; Sobel 1998; Jeavons 2003; Nyirjesy 2003; Sobel 2007).
The condition of VVC occurs as a result of an imbalance in the normal vaginal microbiota (the group of microbe) and is characterized by a decrease or depletion of the lactobacilli species and a concomitant overgrowth of Candida species, epidemiological research has shown that Candida organisms can be found in approximate 20% of asymptomatic healthy women (Sobel 2007). Candida organisms gain access to the vaginal lumen and discharge mainly from the adjacent perianal area, effective anti‐Candida defense in the microenvironment of the vagina allows candidal microbes to persist as an avirulent commensal (Bertholf 1983; Beigi 2004; Sobel 2007; Nyirjesy 2008). There are two potential elements during the development of symptomatic VVC: the first is the Candida species' vaginal colonization, adhesion, invasion and growth, and the second is the transformation from the asymptomatic to the symptomatic phase (Sobel 2007; Nyirjesy 2008). Candida enters the vagina through different sources, including local spread from the perineum (the area in front of the anus extending to the fourchette of the vulva) and gastrointestinal tract, digital introduction and unclean sexual activity (Sobel 2007; Nyirjesy 2008). Estrogen is believed to be crucial in the maintenance of colonization (Hillier 1997). The penetrative ability of hyphae enhances the colonization by the adherence to vaginal epithelial cells (Redondo‐Lopez 1990; Ross 1995; Sobel 2007; Nyirjesy 2008). One study indicated that Candida albicans adheres in significantly higher numbers to vaginal epithelial cells than non‐albicans Candida species (Soll 1989). The Candida strains isolated from the vaginas of women with VVC are mainly Candida albicans, while the rest (range 5% to 15%) are non‐albicans Candida species. Candida glabrata is considered the most common of non‐albicans Candida species (Nyirjesy 2008). However, vulvovaginitis induced by non‐albicans Candida cannot be distinguished clinically from that caused by Candida albicans. RVVC is often caused by non‐albicans Candida, which are frequently more resistant to conventional antifungal treatment (Spinillo 1994; Ross 1995; Sobel 2007; Nyirjesy 2008). Some studies have shown that the widespread and long‐term use of antifungal drugs such as azoles, particularly fluconazole, may lead to a pathogen shift and increase the incidence of the non‐albicans Candida such as Candida glabrata, and the extensive use of azoles may effectively suppress Candida albicans but facilitate the overgrowth of non‐albicans Candida (Sanglard 2002; Hettiarachchi 2010; Mahmoudi 2011). Depressed or reduced protective local immunoregulatory mechanisms, cytokine (any of several non‐antibody proteins, released by a cell population on contact with a specific antigen and act as intercellular mediators, as in the generation of an immune response) elaboration and certain genetic polymorphisms may result in increased susceptibility to RVVC (Giraldo 2007).
The overuse of antibiotics; pregnancy; diabetes mellitus; immunosuppression; frequent and unclean sexual activity; use of oral contraceptives, diaphragms, spermicide and intrauterine devices; and vaginal douching are considered important risk factors for the development of VVC and RVVC (Sobel 1998; Nyirjesy 2003; Sobel 2007; Nyirjesy 2008).
VVC can be classified as uncomplicated VVC and complicated VVC (Sobel 2007; Nyirjesy 2008; CDC 2015).
-
Uncomplicated VVC is defined by:
sporadic or infrequent VVC, or
mild‐to‐moderate VVC, or
likely to be Candida albicans infection, or
non‐immunocompromised host.
-
Complicated VVC is defined by:
four or more episodes of candidiasis per year (RVVC), or
severe symptoms or findings (severe VVC), or
non‐albicans Candida infection, or
abnormal host (e.g. uncontrolled diabetes, debilitation or immunosuppression).
Diagnosis
A combination of clinical signs and symptoms, microscopic examination, vaginal culture, or a combination of these is used to diagnose VVC; although acute pruritus and vaginal discharge are the common clinical symptoms of VVC, neither of them is specific (Anderson 2004; Sobel 2007). The typical vaginal discharge has been described as 'cottage‐cheese‐like,' and in practice it could vary from watery to thick (Sobel 2007). Women with VVC may also complain of irritation, soreness, vulvar burning or dyspareunia. Occasionally, VVC can cause external dysuria (difficult or painful urination) by the burning that occurs when urine hits the inflamed vulvar tissues (Eckert 1998). If there is an odor, it is generally insignificant and inoffensive (Sobel 2007). Clinical symptoms may recur or exacerbate in the week before menstruation (Schaaf 1990; Anderson 2004; Sobel 2007). On vulvar examination, women may exhibit erythema (redness of the skin caused by dilation and congestion of the capillaries, often a sign of inflammation or infection), swelling, fissures, or excoriations of the labia and vulva, and vaginal signs of erythema or an adherent curd‐like vaginal discharge may be found (Sobel 1998; Nyirjesy 2003; Sobel 2007; Nyirjesy 2008).
Most women with symptoms of VVC can be easily diagnosed when:
saline and 10% potassium hydroxide microscopy examination or Gram stain (63.2% to 65% sensitivity, 97.2% to 100% specificity (Omar 2001; Ilkit 2011)) of vaginal discharge demonstrates Candida species, hyphae or pseudohyphae; or
a vaginal culture test yields a Candida species (Sobel 1998; Sobel 2007; Nyirjesy 2008; CDC 2015).
Because Candida vaginitis is associated with a normal vaginal pH (less than 4.5), pH testing is not useful for diagnosis, but the finding of a normal pH helps to exclude bacterial vaginosis, trichomoniasis, atrophic vaginitis or some type of mixed infection (Nyirjesy 2008; CDC 2015).
The 10% potassium hydroxide microscopy examination (50% to 85% sensitivity) should be taken for all women with symptoms or signs of VVC, and women with a positive result should receive treatment (Ilkit 2011; Mylonas 2011). The gold standard for diagnosis is still the growth of the infecting organism in fungal culture on Sabouraud dextrose agar (Ilkit 2011). Up to 50% of women with culture‐positive symptomatic vulvovaginal candidiasis will have negative microscopy (Sobel 2007), so, although routine cultures are not necessary if microscopy is positive, vaginal culture should be performed for women with a negative microscopy result and a normal pH and who are symptomatic (Sobel 2007; CDC 2015). Vaginal culture is useful to identify the species of Candida (Bieber 2006). For women with complicated VVC, vaginal culture can guide the choice of therapy regimen, since non‐albicans species tend to be resistant to the azole drugs (Bieber 2006; Nyirjesy 2008). Table 2 lists species of Candida isolated from the lower genital tract in women with VVC. Candida identified by vaginal culture in the absence of symptoms or signs is not an indication for treatment, because many women harbor Candida species in the vagina. If the 10% potassium hydroxide examination is negative and vaginal culture cannot be performed, an empiric treatment may be considered for symptomatic women with any sign of VVC on examination (CDC 2015).
1. Species of Candida isolated from lower genital tract in women with vulvovaginal candidiasis.
| Species | Frequency | Response to azoles |
| Candida albicans | 80‐90% | Sensitive |
| Candida glabrata | 5‐10% | Resistant |
| Candida krusei | < 1% | Tends to be resistant |
| Candida lusitaniae | < 1% | Tends to be resistant |
| Candida parapsilosis | < 1% | Tends to be resistant |
| Candida pseudotropicalis | < 1% | Tends to be resistant |
| Candida tropicalis | < 1% | Tends to be resistant |
Source: Bieber 2006.
Although polymerase chain reaction (PCR) testing for Candida species is available, its usefulness is limited because it depends on obtaining PCR for the full spectrum of organisms that can cause VVC, with the associated costs (Trama 2005).
Description of the intervention
The recommended treatments for uncomplicated VVC involve a short course of antifungal drugs (Nurbhai 2007; Pappas 2009; CDC 2015). Table 3 lists the Centers for Disease Control (CDC)‐recommended treatments for uncomplicated VVC (CDC 2015). The oral and topical preparations have similar effects (Pappas 2009; CDC 2015), and treatment with azole drugs results in relief of symptoms and negative cultures in 80% to 90% of women who complete therapy (CDC 2015). The recommended treatments for complicated VVC involve an intensive, longer course of antifungals. Table 4 lists the CDC‐recommended treatments for complicated VVC (CDC 2015).
2. CDC recommended treatments for uncomplicated vulvovaginal candidiasis.
| Non‐prescription intravaginal agents | Prescription intravaginal agents |
| Butoconazole 2% cream 5 g intravaginally for 3 days OR Clotrimazole 1% cream 5 g intravaginally for 7‐14 days OR Clotrimazole 2% cream 5 g intravaginally for 3 days OR Miconazole 2% cream 5 g intravaginally for 7 days OR Miconazole 4% cream 5 g intravaginally for 3 days OR Miconazole 100 mg vaginal suppository, 1 suppository for 7 days OR Miconazole 200 mg vaginal suppository, 1 suppository for 3 days OR Miconazole 1200 mg vaginal suppository, 1 suppository for 1 day OR Tioconazole 6.5% ointment 5 g intravaginally in a single application |
Butoconazole 2% cream (single dose bioadhesive product), 5 g intravaginally for 1 day OR Nystatin 100,000 unit vaginal tablet, 1 tablet for 14 days OR Terconazole 0.4% cream 5 g intravaginally for 7 days OR Terconazole 0.8% cream 5 g intravaginally for 3 days OR Terconazole 80 mg vaginal suppository, 1 suppository for 3 days |
CDC: Centers for Disease Control and Prevention.
Source: CDC 2015.
3. CDC recommended treatments for complicated vulvovaginal candidiasis.
| Recurrent vulvovaginal candidiasis | |
|
Initial regimen: 7‐14 days of any topical azole drug OR Fluconazole 100 mg, 150 mg or 200 mg orally once daily every 3rd day for a total of 3 doses (days 1, 4 and 7) |
Maintenance regimen: Fluconazole 100 mg, 150 mg or 200 mg orally once weekly for 6 months |
| Severe vulvovaginal candidiasis | |
| Intravaginally once daily for 7 to 14 days of any topical azole drug OR Fluconazole 150 mg orally once daily in 2 doses (second dose 72 hours after initial dose) | |
| Non‐albicans vulvovaginal candidiasis | |
| Non‐fluconazole azole (oral or topical) 7‐14 days OR Boric acid gelatin capsule Intravaginally once daily for 14 days | |
| Abnormal host | |
| More prolonged (i.e. 7‐14 days) conventional antifungal drugs is necessary | |
CDC: Centers for Disease Control and Prevention.
Source: CDC 2015.
Azole antifungals are a group of fungistatic agents with broad‐spectrum activity in treating systemic and topical fungal infections. They are classified into two groups: triazoles and imidazoles (Nurbhai 2007; CDC 2015). One Cochrane systematic review showed that both imidazole and triazole antifungal treatment (by oral or intravaginal route of administration) achieved clinical cure in over 80% of women (Nurbhai 2007). However, they can cause many adverse effects, including vomiting, diarrhea, abdominal pain, urination, pelvic cramps, paresthesia, rhinorrhea, headache, dizziness, fever, chills, vaginal burning, stinging, itching and irritation (Nurbhai 2007; CDC 2015); more systemic adverse effects are likely to be reported with oral compared with intravaginal antifungal administration (Nurbhai 2007). For the half‐life, duration and any known interactions with other drugs of all azole antifungals for VVC, we recommend following this Merck Manual (www.merckmanuals.com), to reference further information.
Probiotics confer a wide range of effects, and have been used for the prevention and treatment of various medical conditions and to support wellness. Some of their effects against diarrheal diseases, Crohn disease, ulcerative colitis, irritable bowel syndrome, bacterial vaginosis, VVC and urinary tract infections have been validated (Reid 2005; Senok 2005; Doron 2006; Santosa 2006; Sanders 2008; Senok 2008). Probiotics are defined as live microorganisms that, when administered in adequate amounts, exert a health benefit on the host by treating and preventing diseases, they are regulated as dietary supplements and foods, consisting of bacteria or yeast, and they are available as capsules, tablets or powders, and may contain a single microorganism or a mixture of several species (Reid 2003a; Falagas 2006; Othman 2007; Sanders 2008). Table 5 lists common microorganisms used as probiotics (Kopp‐Hoolihan 2001; Senok 2005; Doron 2006; Santosa 2006). Products containing bacteria or yeast are not classified as probiotics, unless they have been shown to be viable and stable at the time of use in sufficient quantity to exert a health benefit. The organisms themselves must be speciated using appropriate molecular methods, and given a designation (Reid 2005; Senok 2005; Doron 2006; Santosa 2006; Vanderhoof 2008).
4. Common microorganisms used as probiotics.
|
Lactobacillusspecies: Lactobacillus acidophilus Lactobacillus bulgaricus Lactobacillus casei Lactobacillus crispatus Lactobacillus delbrueckii Lactobacillus fermentum Lactobacillus gasseri Lactobacillus johnsonii Lactobacillus lactis Lactobacillus plantarum Lactobacillus reuteri Lactobacillus rhamnosus GG |
Streptococcusspecies: Streptococcus thermophilus |
|
Yeast: Saccharomyces boulardii | |
|
Other species: Bacillus cereus Enterococcus faecalisa Enterococcus faeciuma Escherichia coli Nissle | |
|
Bifidobacteriumspecies: Bifidobacterium adolescentis Bifidobacterium animalis Bifidobacterium bifidum Bifidobacterium breve Bifidobacterium infantis Bifidobacterium lactis Bifidobacterium longum |
Sources: Senok 2005; Doron 2006; Santosa 2006.
Probiotics used in the prevention and treatment of Candida infections include Lactobacillus fermentum RC‐14, Lactobacillus fermentum B‐54, Lactobacillus rhamnosus GR‐1, Lactobacillus rhamnosus GG and Lactobacillus acidophilus (Reid 2001a; Jeavons 2003; Reid 2005; Falagas 2006; Martinez 2009a). Administration of probiotics can be oral, intravaginal or combined (Reid 2004). Probiotics are safe and effective for urogenital infections, with no severe adverse effects (Reid 2003b). The recommended dose is 109 to 1011 colony‐forming units of bacteria, by any route of administration (Reid 2003b; Andreu 2004). Probiotic preparations should not be taken together with bismuth preparations, tannic acid, activated charcoal or tincture. Because of the sensitivity of probiotics to antibiotics, they should not be taken together, to avoid dilution of their effectiveness (Zhang 2008).
How the intervention might work
The concept of treatment with probiotics stems from a belief that modern humans do not consume or replenish the beneficial microbes in their bodies, and that they can do so by taking probiotics (Reid 2005). The normal vaginal microenvironment is predominantly populated by Lactobacillus species, which tend to suppress growth of other bacterial species (Reid 2004). This dominance of lactobacilli and their potential ability to resist VVC gave rise to the concept of oral or vaginal instillation of probiotic Lactobacillus strains to restore the vaginal microbiotic balance. After the live bacteria in probiotic preparations have colonized the vagina, they grow and reproduce, and their metabolic substances can have toxic effects on Candida species (Reid 2001b; Reid 2001c; Reid 2003b). Evidence suggests that oral intake of probiotics leads to transfer of the organisms from the rectum to the vagina, as well as an overall depletion of coliforms and yeasts in the vagina (Reid 2001b; Reid 2001c; Reid 2003b). Certain probiotic strains, including Lactobacillus fermentum RC‐14 and Lactobacillus rhamnosus GR‐1, are able to remain in the vagina for several months after introduction.
The actual mechanism of action of probiotics in the vagina is probably multifactorial; they may block and prevent the Candida species' colonization, adhesion, invasion and growth by lactic acid, hydrogen peroxide and bacteriocin, which are toxic to Candida species (Reid 2003a; Reid 2003c; Reid 2004; Sobel 2007). In addition, probiotics may take the action of competitive exclusion, which is caused by a stronger affinity of Lactobacillus than pathogens to the receptors of the vaginal epithelial cells (Kaewsrichan 2006). The inflammatory temperance effect, provided by stable colonization with probiotics in the vagina, may help to regulate the vaginal immune environment (Fichorova 2011; Rose 2012). It has also been shown that Lactobacillus rhamnosus GR‐1 and Lactobacillus reuteri RC‐14 may directly influence the response of the vaginal epithelial cells to Candida albicans infections (Reid 2003c; Reid 2004; Martinez 2009b). After eliminating most of the Candidas, Lactobacillus could restore and maintain a normal vaginal microenvironment to prevent recurrence (Reid 2003a; Reid 2003c; Reid 2004; Reid 2005).
Why it is important to do this review
A growing number of women are troubled by the high prevalence and recurrence rates of VVC (Martinez 2009a). Although anticandidal agents are quite effective at providing clinical cure for VVC, resistance to the drugs is increasing. In addition, drugs may reduce the normal protective vaginal flora to increase the risk of recurrent infection, and can also cause many adverse effects (Nurbhai 2007; Martinez 2009a; CDC 2015). The increasing availability of probiotic products makes it important that family physicians understand what to look for when making recommendations (Reid 2005). The use of probiotics in augmenting normal bacterial populations is gradually achieving scientific acceptance (Reid 2003c). Probiotics have already been used for treatment of vulvovaginal inflammations in clinical practice (Reid 2001a; Reid 2003a; Reid 2003c; Othman 2007). Some evaluations have shown that probiotics are effective against VVC, and that their adverse effects are minor (Jeavons 2003; Falagas 2006; Martinez 2009a), while others demonstrated no efficacy in VVC (Pirotta 2004; Falagas 2006). There is no consensus on the use of probiotics for treating VVC (Jeavons 2003; Van Kessel 2003). Therefore, it is necessary to conduct a rigorous systematic review of the available clinical trials, to help determine the effectiveness and safety of probiotics for the treatment of VVC, and to identify strategic areas for future research.
Objectives
To assess the effectiveness and safety of probiotics for the treatment of vulvovaginal candidiasis in non‐pregnant women.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCT). We excluded quasi‐randomized trials because such trials produce effects estimates that indicate more extreme benefits when they are compared with RCTs (Higgins 2011). We excluded cross‐over trials because the carry over effect (Higgins 2011).
Types of participants
Non‐pregnant women diagnosed with VVC, regardless of age and race. Diagnosis of VVC was confirmed by the presence of clinical symptoms and signs, and a positive microscopic examination or positive vaginal culture, or both.
We excluded trials of women with RVVC, diabetes mellitus, immunosuppressive disorders; taking immunosuppressant medication; or trials that recruited women with evidence of coinfection with other vulvovaginal infections.
Types of interventions
Any probiotic: single or multiple strains, any preparation type, dosage regimen or route of administration.
Used alone versus conventional antifungal drugs.
Used as adjuvants to conventional antifungal drugs (before, during or after antifungal treatment) versus conventional antifungal drugs alone.
Conventional antifungal drugs described here refer to the common drugs for VVC treatment, such as azole drugs (triazoles and imidazoles) and nystatin.
Types of outcome measures
Primary outcomes
Clinical cure rate (disappearance of symptoms and signs, and no evidence of fungal infection proved by microscopic examination or vaginal culture), split into 'short‐term clinical cure rate' (zero to 14 days after treatment) and 'long‐term clinical cure rate' (one, three and six months after treatment).
Mycological cure rate (no evidence of fungal infection proved by microscopic examination or vaginal culture), split into 'short‐term mycological cure rate' (zero to 14 days after treatment)' and 'long‐term mycological cure rate' (one, three and six months after treatment).
Relapse rate (symptom recurrence confirmed by microscopic examination or vaginal culture at one, three and six after mycological cure).
Rate of serious adverse events (death, internal organ injury, severe skin and mucosal injury).
Secondary outcomes
Time to first relapse.
Rate of non‐serious adverse events (mild symptoms include vomiting, diarrhea, abdominal pain, abnormal urination, pelvic cramps, paresthesia, rhinorrhea, headache, dizziness, fever, chills, vaginal burning, stinging, itching and irritation).
Need for any additional treatment at the end of therapy.
Patient satisfaction with treatment.
Cost effectiveness.
Search methods for identification of studies
We attempted to identify as many relevant RCTs as possible of "probiotics" for "VVC", regardless of language, publication date or publication status (published, unpublished, in press and in progress). We used both electronic searching in bibliographic databases and handsearching, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Electronic searches
We contacted the Information Specialist of the Cochrane Sexually Transmitted Infections Group to implement a comprehensive search strategy to identify as many relevant RCTs as possible in the electronic databases. The search strategies included a combination of controlled vocabulary (MeSH, EMTREE, DeCS, including exploded terms) and free‐text terms for "probiotics" and "VVC", with field labels (title and abstract), wildcards (truncation), proximity operators (adj) and boolean operators (OR, AND).
We searched the following electronic databases:
Sexually Transmitted Infections Cochrane Review Group's Specialized Register (to October 2017);
Cochrane Central Register of Controlled Trials (CENTRAL), Ovid (1991 to October 2017);
MEDLINE, Ovid (1946 to October 2017);
MEDLINE In‐Process & Other Non‐Indexed Citations, Ovid (1946 to October 2017);
MEDLINE Daily Update, Ovid (1946 to October 2017);
Embase (1947 to October 2017);
LILACS, IAHx interface (1982 to October 2017);
PsycINFO, Ovid (1946 to October 2017);
AMED, Ovid (1946 to October 2017);
CBMdisc and CNKI: inception to October 2017.
The search strategies for the STI Specialized Register, CENTRAL, MEDLINE, Embase, AMED, CBMdisc and CNKI, PsycINFO and LILACS can be found in Appendix 1, Appendix 2, Appendix 3, Appendix 4, Appendix 5, Appendix 6, and Appendix 7.
We placed no language restrictions.
Searching other resources
We attempted to identify other published, unpublished and ongoing relevant RCTs by:
-
searching trials registers:
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) portal (apps.who.int/trialsearch/);
ClinicalTrials.gov (clinicaltrials.gov/);
searching Web of Science (inception to October 2017);
searching grey literature in System for Information on Grey Literature in Europe "OpenGrey" (www.opengrey.eu/) (inception to October 2017);
contacting authors of included RCTs and experts in the field to identify any additional published and unpublished materials;
contacting pharmaceutical companies producing "probiotics" for "VVC";
handsearching the following journals: Anatolian Journal of Obstetrics & Gynecology, Current Medical Literature Gynecology & Obstetrics, Current Obstetrics and Gynecology Reports, ISRN Obstetrics and Gynecology, Journal of South Asian Federation of Obstetrics & Gynecology, Obstetrics and Gynecology International, Obstetrics Gynaecology and Reproductive Medicine and Sexual Science: the newsletter of the Society for the Scientific Study of Sexuality and Sexualities;
-
handsearching the conference proceeding abstracts in the following events:
International Society for Sexually Transmitted Diseases Research (ISSTDR) (www.isstdr.org/): 2007, 2009, 2011, 2013 and 2015;
British Association for Sexual Health and HIV (BASHH) (www.bashh.org/): 2004, 2006, 2007, 2009, 2010, 2011 and 2014;
International Congress on Infectious Diseases (ICID) (www.isid.org/): 2010, 2012 and 2014;
International Union against Sexually Transmitted Infections (IUSTI) (www.iusti.org/): 2011, 2012, 2013 and 2014;
International Society for Infectious Diseases (ISID) (www.isid.org/): 2011;
International Meeting on Emerging Diseases and Surveillance (IMED) (www.isid.org/): 2007, 2009, 2013 and 2014;
Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) (www.icaac.org/): 2011, 2012, 2013, 2014 and 2015;
International Federation of Gynecology and Obstetrics (FIGO) (www.figo.org): 2012 and 2015;
handsearching previous systematic reviews and other relevant publications on the same topic;
handsearching reference lists of all RCTs identified by other methods, including available review articles on the topic, and contacting authors of all RCTs identified by other methods.
Data collection and analysis
Selection of studies
Two authors (DW and HC) independently scanned all titles and abstracts from the initial search, to exclude trials that did not meet the inclusion criteria. Multiple reports of the same study were collected and collated so that each study, rather than each report, was used. Two authors (DW and HC) independently assessed the full text of trials that appeared to meet the inclusion criteria. Two authors (DW and HC) contacted the authors of articles if any important information was missing. We resolved any discrepancies through discussion with a third author (HX).
Data extraction and management
Two authors (LM and XW) independently extracted data using a previously designed form for this review. The data extraction form was pilot tested using one of the included studies. We collected the following information.
-
Study characteristics.
Year and language of publication.
Inclusion and exclusion criteria.
Randomization process.
Allocation concealment.
Blinding.
Number of withdrawals (participants excluded from analysis or lost to follow‐up) and reasons.
Intention‐to‐treat analysis.
Duration of follow‐up.
-
Basic participant information.
Number.
Mean age and age range of the participants.
Type of participants (health status, uncomplicated VVC or complicated VVC).
-
Intervention.
Drug.
Type (single or multiple).
Preparation.
Dosage and route of administration.
-
Outcome.
Clinical cure rate.
Mycological cure rate.
Relapse rate.
Time to first relapse.
-
Adverse events.
Serious adverse events.
Non‐serious adverse events.
Cost effectiveness: if the data were mentioned and available.
-
Others.
Funding sources reported.
Ethical issues: use of signed informed consent and ethics approval.
We discussed any disagreements referring back to the trial report until we reached consensus. If data from the trial reports were insufficient or missing, we contacted the trial authors for additional information.
Two authors (LM, XW) entered data into Review Manager 5 (RevMan 2014) and a third author (HX) checked them to ensure data quality. When information regarding any of the above were unclear, we contacted the authors of the studies to ask for further details. For a single randomized controlled clinical trial report, we extracted data directly onto the data extraction form and in case of multiple reports, we extracted data from each report separately and then combined them across the forms. Studies reported a language other than English or Chinese were translated.
Assessment of risk of bias in included studies
Two authors (DW and HC) assessed the methodological quality of each trial using the Cochrane 'Risk of bias' tool. We resolved any disagreements by consensus or by involving a third author (FF). The authors assessing risk of bias were thematic and methodology experts. We assessed the following information which was used for the development of the 'Risk of bias' tables.
1. Random sequence generation (checking for possible selection bias). We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed the method as: low risk of bias (any truly random process, e.g. random number table, computer random number generator); high risk of bias (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number) or unclear risk of bias.
2. Allocation concealment (checking for possible selection bias). We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomization, consecutively numbered sealed opaque envelopes); high risk of bias (open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth) or unclear risk of bias.
-
3. Blinding
3.1 Blinding of participants and personnel (checking for possible performance bias). We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes. We assessed the methods as: low, high or unclear risk of bias for participants; and low, high or unclear risk of bias for personnel.
3.2 Blinding of outcome assessment (checking for possible detection bias). We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes. We assessed methods used to blind outcome assessment as low, high or unclear risk of bias.
4. Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data). We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We reported whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we reincluded missing data in the analyses that we undertook. We assessed methods as low risk of bias (e.g. no missing outcome data, missing outcome data balanced across groups); high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups, 'as treated' analysis done with substantial departure of intervention received from that assigned at randomization) or unclear risk of bias. We used a cut‐off point of 20% to consider that a study was at low or high risk of bias according to the level of missing data.
5. Selective reporting (checking for reporting bias). We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as: low risk of bias (where it was clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review were reported); high risk of bias (where not all the study's prespecified outcomes were reported, one or more reported primary outcomes were not prespecified, outcomes of interest were reported incompletely and so could not be used, study failed to include results of a key outcome that would have been expected to have been reported or unclear risk of bias).
6. Other bias (checking for bias due to problems not covered under points 1 to 5 above). We described for each included study any important concerns we had about other possible sources of bias (stopped early due to some data‐dependent process or extreme baseline imbalance or was claimed to be fraudulent or have been sponsored by industry). We assessed whether each study was free of other problems that could put it at risk of bias.
7. Overall risk of bias. We made explicit judgments about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to points 1 to 6 above, we assessed the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings.
Two authors (DW and HC) independently applied the 'Risk of bias' tool, and resolved differences by discussion or by discussion with a third author (FF). We presented results in a 'Risk of bias' graph. The risk of bias findings were used to inform any meta‐analyses.
Measures of treatment effect
For dichotomous outcomes, we pooled the results in meta‐analyses and presented results as summary risk ratios (RRs) with 95% confidence intervals (CIs). We expressed continuous data as mean differences (MDs) with 95% CIs.
Unit of analysis issues
The primary unit of analysis in meta‐analysis was the participant. For trials that contributed multiple, correlated comparisons, we planned to combine all relevant experimental intervention groups of the trials into a single group and combined all relevant control intervention groups into a single control group so we could create a single pair‐wise comparison (Higgins 2011) when the objective was to compare the experimental branch with any other control group.
Dealing with missing data
Whenever possible, we contacted the original authors to request missing data. We identified levels of attrition for included trials and we performed analyses for all outcomes, as far as possible, on an intention‐to‐treat basis. We attempted to include all participants randomized to each group in the analyses, and all participants were analyzed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. We conducted sensitivity analyses to explore the impact of studies with missing data, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
If there was obvious heterogeneity (populations, interventions, etc.), we conducted subgroup analyses to investigate potential sources of heterogeneity. We assessed statistical heterogeneity in each meta‐analysis using the T2, I2 and Chi2 statistics. We regarded heterogeneity as substantial if the I² statistic was greater than 40% and either T2 was greater than zero or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
We planned to assess reporting bias by constructing a funnel plot. If 10 or more original trials were identified, then a funnel plot would be produced in an attempt to identify any publication bias. However, for each outcome, we included fewer than 10 trials in the meta‐analysis, so we did not perform these analyses.
Data synthesis
We performed the statistical analyses by using Review Manager 5 (RevMan 2014). If the trials were judged to be similar, and in the absence of statistical heterogeneity, we pooled the data using a fixed‐effect model, and if statistical heterogeneity was present, we used a random‐effect model. If the outcome data could not be combined, we described the outcome separately. If the data were available, we conducted time‐to‐event analyses for time to recurrence by extracting data from published curves (Parmar 1998). We assessed the quality of the body of evidence.
Because the Chi2 test has low power when sample sizes or number of studies are small, it may fail to detect heterogeneity. In the event of few trials or small samples, we proposed to conduct both a random‐effects and a fixed‐effect meta‐analysis, even if there was no heterogeneity detected.
'Summary of findings' table
We summarized the results for the main comparison in a 'Summary of findings' table. We used the GRADE approach to assess the quality of evidence in relation to each outcome included) and used GRADE profiler (GRADEpro) software to import data from Review Manager 5 (RevMan 2014) to create a 'Summary of findings' table (Higgins 2011).
We produced a 'Summary of findings' table for eight critical outcomes in each type of comparison:
clinical cure rate (short‐term);
clinical cure rate (long‐term/one month after treatment);
clinical cure rate (long‐term/three months after treatment);
mycological cure rate (short‐term);
mycological cure rate (long‐term/one month after treatment);
mycological cure rate (long‐term/three months after treatment);
relapse rate;
rate of serious adverse events.
For each outcome, quality was categorized into four ratings: high quality (further research is very unlikely to change our confidence in the estimate of effect), moderate quality (further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate), low quality (further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate) and very low quality (we are very uncertain about the estimate) (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
We explored a subgroup analysis in cases of high heterogeneity and assessed the effect of certain subgroups for all outcomes:
probiotics: single versus multiple species;
route of administration: intravaginal versus oral;
different candidal strains: Candida albicans versus non‐albicans;
age group of participants: aged less than 18 years versus aged 18 years or greater.
Sensitivity analysis
We planned to perform a sensitivity analysis to explore whether the results of the review were robust, depending on study quality, for each outcome variable. We excluded studies with a high risk of bias, comparing findings within the remainder of the included studies with the original meta‐analysis.
Results
Description of studies
Results of the search
We searched the available literature up to October 2017 and retrieved 2525 references, of which we screened 2481 after we removed duplicates. We excluded 2454 clearly irrelevant references. We screened the full‐text articles of the remaining 27 references. Ten published trials met our inclusion criteria (Zhang 2005; Han 2006; Lin 2006; Ma 2007; Mai 2007; Hua 2008; Martinez 2009; Yang 2009; Nouraei 2012; Kovachev 2015). We excluded 16 studies (see Characteristics of excluded studies table) and one trial is awaiting classification (see the Characteristics of studies awaiting classification table). We presented a PRISMA diagram in Figure 1 to illustrate the study selection process.
1.
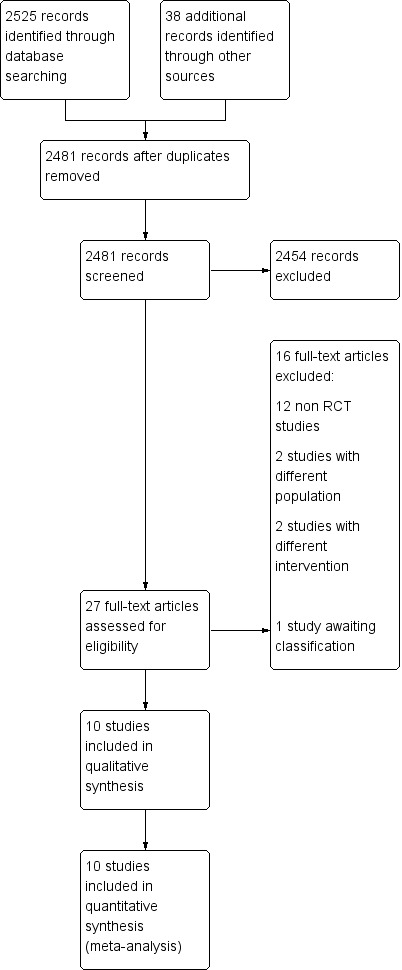
Study flow diagram. RCT: randomized controlled trial.
Included studies
We included 10 trials in this review (Zhang 2005; Han 2006; Lin 2006; Ma 2007; Mai 2007; Hua 2008; Martinez 2009; Yang 2009; Nouraei 2012; Kovachev 2015), and included data from 1656 women, with a sample size ranging from 68 to 436 participants. These trials were from China (Zhang 2005; Han 2006; Lin 2006; Ma 2007; Mai 2007; Hua 2008; Yang 2009), Bulgaria (Kovachev 2015), Brazil (Martinez 2009), and Iran (Nouraei 2012). Only one was multicentric trial (Martinez 2009). Seven trials were published in Chinese (Zhang 2005; Han 2006; Lin 2006; Ma 2007; Mai 2007; Hua 2008; Yang 2009), and three trials were published in English (Martinez 2009; Nouraei 2012; Kovachev 2015).
Population
Study populations were heterogeneous. The included trials recruited participants aged between 16 and 50 years. All trials used at least one laboratory method for diagnosis. All the selected trials recruited participants without RVVC, diabetes mellitus, immunosuppressive disorders, taking immunosuppressant medication or evidence of co‐infection with other vulvovaginal infections. One trial implemented an added exclusion criterion related with human papillomavirus infection (Kovachev 2015). Two trials only recruited married participants (Zhang 2005; Nouraei 2012). Three trials required that participants had no sexual activity during treatment and follow‐up (Zhang 2005; Mai 2007; Kovachev 2015), five trials required participants had no sexual activity during treatment and used a condom during follow‐up (Han 2006; Lin 2006; Ma 2007; Hua 2008; Yang 2009), and two trials did not mention the requirement of sexual activity (Martinez 2009; Nouraei 2012).
Interventions
All trials used probiotics as adjuvant therapy to antifungal drugs. Five trials used probiotics of Lactobacillus delbrueckii subsp. Lactis DM8909 (Zhang 2005; Han 2006; Mai 2007; Hua 2008; Yang 2009), two trials used probiotics of Streptococcus faecalis (Lin 2006; Ma 2007), one trial used probiotics of Lactobacillus rhamnosus GR‐1 and Lactobacillus reuteri RC‐14 (Martinez 2009), one trial used probiotics of Lactobacillus acidophilus, Lactobacillus rhamnosus, Streptococcus thermophilus and Lactobacillus delbrueckii subsp. Bulgaricus (Kovachev 2015), and one trial used probiotics contained seven strains of probiotic bacteria (Lactobacillus casei, Lactobacillus rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, Lactobacillus acidophilus, Bifidobacterium longum and Lactobacillus bulgaricus) (Nouraei 2012).
The most frequently used conventional antifungal drug was clotrimazole (Han 2006; Lin 2006; Mai 2007; Yang 2009), three trials used miconazole (Zhang 2005; Ma 2007; Hua 2008), two trials used fluconazole (Martinez 2009; Nouraei 2012), and one trial used fluconazole plus fenticonazole (Kovachev 2015). Table 6 lists a brief summary of interventions in included studies.
5. Brief summary of interventions in included studies.
| Included study | Intervention groups | Intervention doses and duration | Intervention administration route |
| Han 2006 | Clotrimazole + probiotic group | 1 tablet of clotrimazole 500 mg on day 1 and day 4 + 1 capsule of Lactobacillus delbrueckii Subsp. Lactis DM8909 (each capsule contained 0.25 × 106 colony forming units), QD from day 1 to day 10. | Vaginal |
| Clotrimazole group | 1 tablet of clotrimazole 500 mg on day 1 and day 4. | Vaginal | |
| Hua 2008 | Miconazole + probiotic group | 1 suppository of miconazole nitrate 400 mg, QD from day 1 to day 6, and then 1 capsule of Lactobacillus delbrueckii subsp. Lactis DM8909 (each capsule contained 0.25 × 106 colony forming units), QD from day 7 to day 16. | Vaginal |
| Miconazole group | 1 vaginal suppository of Miconazole nitrate (400 mg), QD from day 1 to day 6 | Vaginal | |
| Kovachev 2015 | Azole + vaginal probiotic group | Fluconazole 150 mg + 1 globule of fenticonazole 600 mg on the same day; however, 10 applications of probiotics (Lactobacillus acidophilus,Lactobacillus rhamnosus,Streptococcus thermophilus and Lactobacillus delbrueckii subsp. Bulgaricus) were also administered beginning the 5th day after azole treatment. | Fluconazole: oral. Fenticonazole: vaginal Probiotics: vaginal |
| Azole group | Fluconazole 150 mg + 1 globule of fenticonazole 600 mg on the same day. | Fluconazole: oral Fenticonazole: vaginal |
|
| Lin 2006 | Clotrimazole + probiotic group | 1 suppository of clotrimazole 150 mg, QD from day 1 to day 7, then, 1 capsule of Streptococcus faecalis (each capsule contained 6 × 107 colony forming units), QD from day 8 to day 14. | Vaginal |
| Clotrimazole group | 1 suppository of clotrimazole 150 mg, QD from day 1 to day 7. | Vaginal | |
| Ma 2007 | Miconazole + probiotic group | 1 suppository of miconazole nitrate 200 mg, QD from day 1 to day 14, and on day 8, 1 capsule of Streptococcus faecalis (each capsule contained 6 × 107 colony forming units), QD from day 8 to day 14. | Vaginal |
| Miconazole group | 1 suppository of miconazole nitrate 200 mg, QD from day 1 to day 14. | Vaginal | |
| Mai 2007 | Clotrimazole + probiotic group | 1 suppository of clotrimazole 150 mg, QN + 1 capsule of Lactobacillus delbrueckii Subsp. Lactis DM8909 (each capsule contained 0.25 × 106 colony forming units), QD from day 1 to day 10. | Vaginal |
| Clotrimazole group | 1 suppository of clotrimazole 150 mg, QN from day 1 to day 10. | Vaginal | |
| Martinez 2009 | Fluconazole + probiotic group | 1 dose of fluconazole 150 mg + 2 capsules of Lactobacillus rhamnosus GR‐1 and Lactobacillus reuteri RC‐14 (each capsule contained 1 × 109 viable cells of both strains) for 28 days | Oral |
| Fluconazole + placebo group | 1 dose of fluconazole 150 mg + 2 oral capsules of placebo once daily (every morning) for 28 days. | Oral | |
| Nouraei 2012 | Fluconazole + oral protexin group | 1 dose of fluconazole 300 mg (2 × 150 mg) + 2 protexin capsules per day (after meals in the morning and evening) for 3 days. | Oral |
| Fluconazole + placebo group | 1 dose of fluconazole 300 mg (2 × 150 mg) + 2 placebo capsules daily (after meals in the morning and evening) for 3 days. | Oral | |
| Yang 2009 | Clotrimazole + probiotic group | 1 tablet of clotrimazole 500 mg on day 1 and day 4 + 1 capsule of Lactobacillus delbrueckii subsp. Lactis DM8909 (each capsule contained 0.25 × 106 colony forming units), QD from day 1 to day 10. | Vaginal |
| Clotrimazole group | 1 tablet of clotrimazole 500 mg on day 1 and day 4. | Vaginal | |
| Zhang 2005 | Miconazole + probiotic group | 1 suppository of miconazole nitrate 200 mg, QD from day 1 to day 7, and then, 1 capsule of Lactobacillus delbrueckii subsp. Lactis DM8909 (each capsule contained 0.25 × 106 colony forming units), QD from day 8 to day 17. | Vaginal |
| Miconazole group | 1 suppository of miconazole nitrate 200 mg, QD from day 1 to day 7. | Vaginal |
QD: every day; QN: every night.
Comparison
Four trials compared vaginal suppository or tablet of clotrimazole plus vaginal capsules of probiotics with vaginal suppository or tablet of clotrimazole alone (Han 2006; Lin 2006; Mai 2007; Yang 2009), three trials compared vaginal suppository of miconazole plus vaginal capsules of probiotics with vaginal suppository of miconazole alone (Zhang 2005; Ma 2007; Hua 2008), two trials compared oral fluconazole plus oral capsules of probiotics with oral fluconazole plus oral capsules of placebo (Martinez 2009; Nouraei 2012), and one trial compared oral fluconazole and vaginal globule of fenticonazole plus oral capsules of probiotics with oral fluconazole and vaginal globule of fenticonazole (Kovachev 2015).
Outcomes
All the included trials reported at least one prespecified primary outcome of this review. Five trials reported short‐term clinical cure rate (Han 2006; Ma 2007; Hua 2008; Yang 2009; Nouraei 2012), and only one trial reported long‐term clinical cure rate (Zhang 2005). Seven trials reported short‐term mycological cure rate (Han 2006; Lin 2006; Ma 2007; Mai 2007; Hua 2008; Yang 2009; Nouraei 2012), and three trials reported short‐term mycological cure rate (Zhang 2005; Martinez 2009; Kovachev 2015). Three trials reported the first relapse after treatment (Han 2006; Hua 2008; Yang 2009). Two trials reported the rate of serious adverse events (Zhang 2005; Hua 2008). Seven trials reported the rate of non‐serious adverse events (Zhang 2005; Han 2006; Ma 2007; Hua 2008; Martinez 2009; Yang 2009; Nouraei 2012). We did not obtain any data on the secondary outcomes: time to first relapse, need for any additional treatment at the end of the therapy, patient satisfaction with treatment and cost effectiveness.
Excluded studies
Sixteen trials were initially identified as potentially eligible for inclusion but were subsequently found to be ineligible. The main reason was that they were not RCTs (Ozkinay 2005; Yang 2005; Zhang 2006; Li 2007; Liu 2008; Patel 2008; Anukam 2009; Fu 2009; Ehrstrom 2010; Wang 2010; Song 2011; Fu 2012; Shi 2012; Nagornaya 2013; Wu 2013; Sigridov 2007). The reasons for exclusions are listed in the Characteristics of excluded studies table.
Risk of bias in included studies
We have summarized the 'Risk of bias' assessment in Figure 2 and Figure 3. In addition, we provided additional details of the included trials in the 'Risk of bias' tables of the Characteristics of included studies' table.
2.
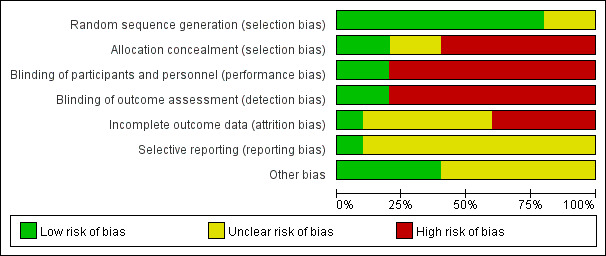
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
3.
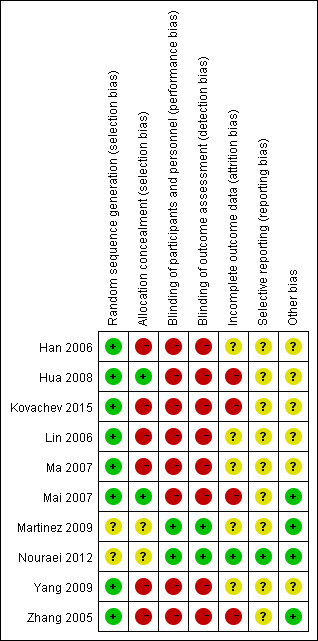
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
Random sequence generation (checking for possible selection bias)
Eight studies adequately reported the random sequence generation method by using a Research Randomizer software or a random draw method (All participants were numbered on an individual piece of paper, then took the paper (relevant to participant) out of a container randomly, without being able to see what was written on it; we judged this as a valid method after contacting the original authors.) making selection bias at entry unlikely (Zhang 2005; Han 2006; Lin 2006; Ma 2007; Mai 2007; Hua 2008; Yang 2009; Kovachev 2015). The remaining two studies did not report the random sequence generation methods, making the risk of selection bias at entry unclear (Martinez 2009; Nouraei 2012).
Allocation concealment (checking for possible selection bias)
Two studies had adequate allocation concealment by using codified opaque‐sealed envelopes, and the risk of selection bias related to allocation concealment was low (Mai 2007; Hua 2008). Six studies had a high selection bias as they did not conceal the allocation (Zhang 2005; Han 2006; Lin 2006; Ma 2007; Yang 2009; Kovachev 2015). Two studies had no description regarding concealment allocation disclosed; they were judged with unclear risk of bias for allocation concealment (Martinez 2009; Nouraei 2012).
Blinding
Performance bias
Two studies used double blinding and a placebo‐controlled method; the participants and investigators were blind to the interventions, making performance bias unlikely (Martinez 2009; Nouraei 2012). Six studies were at high performance bias as no blinding was adopted, as the judgment of symptoms and signs were subjectively evaluated; however, in these studies, both participants and investigators knew the allocated interventions during the study and outcomes may be influenced (Zhang 2005; Han 2006; Lin 2006; Ma 2007; Yang 2009; Kovachev 2015). Two studies used single blinding to the investigators, no blinding to participants as lacked a placebo control, and judgment of symptoms and signs by participants may have been influenced, so we also judged the two studies as having high risk of performance bias (Mai 2007; Hua 2008).
Detection bias
Risk of detection bias was low in two studies as the outcome assessors were blinded adequately (Martinez 2009; Nouraei 2012). Six studies had a high detection bias as no blinding was adopted and the judgment of symptoms and signs outcomes by participants may be influenced (Zhang 2005; Han 2006; Lin 2006; Ma 2007; Yang 2009; Kovachev 2015). In two studies, although the outcome assessors were blinded, they still may have been influenced by participants as there was no blinding to participants, so the two studies were judged at high risk of detection bias (Mai 2007; Hua 2008).
Incomplete outcome data
Five studies reported no withdrawals, but these trial did not have enough information for us to judge "yes" or "no" in intention‐to‐treat analyses and as a consequence, we assessed them as at unclear risk of attrition bias (Han 2006; Lin 2006; Ma 2007; Martinez 2009; Yang 2009). Hua 2008 reported a withdrawal rate of 2.1% (less than 20%) during the treatment, Kovachev 2015 reported a withdrawal rate of 4.6% (less than 20%) after the treatment, Mai 2007 reported a withdrawal rate of 6.1% (less than 20%) after the treatment and Zhang 2005 reported a withdrawal rate of 6.5% during the treatment and 7% after the treatment (less than 20%). The results of the four studies were not analyzed on an intention‐to‐treat basis, so we assessed them as at high risk of attrition bias. As Nouraei 2012 reported no attrition or exclusion, we assessed it at low risk of attrition bias.
Selective reporting
We found only one study reporting all the expected outcomes mentioned in their trial protocol (protocols number IRCT201106206807N3); it was clear that the published reports include all prespecified outcomes, so we judged it at low risk of selective reporting bias (Nouraei 2012).
For the other nine studies, the trial protocols were not available and it was unclear if the published reports included all the expected outcomes, including those that were prespecified. The reports had insufficient information to permit judgment of "yes" or "no," so we rated them at unclear risk of bias.
Other potential sources of bias
One study did not mention available data of the baseline characteristics between the two groups to judge the balance, we judged the study as having unclear risk of bias (Kovachev 2015). Although authors of five included studies mentioned there were no significant differences in baseline characteristics, they provided no detailed data; therefore, we judged these studies at unclear risk of bias (Han 2006; Lin 2006; Ma 2007; Hua 2008; Yang 2009). One study mentioned that one author held some patents associated with lactobacilli; however, his input was in protocol design, logistics, student supervision and assistance with the manuscript not in the accumulation of data. In addition, the author was blinded to the results until after the code was broken and findings acquired. We judged that probably this author had a conflict of interest that did not affect the quality of the evidence and there was low risk of bias in this study (Martinez 2009). The remaining studies appeared to be free of other sources of bias (Zhang 2005; Mai 2007; Nouraei 2012).
Effects of interventions
See: Table 1
1. Probiotics used alone versus conventional antifungal drugs
None of the included trials analyzed probiotics used alone compared with conventional antifungal drugs.
2. Probiotics used as adjuvants to conventional antifungal drugs (before, during or after antifungal treatment) versus conventional antifungal drugs
All 10 included trials compared probiotics used as adjuvants to conventional antifungal drugs (before, during or after antifungal treatment) with conventional antifungal drugs (Zhang 2005; Han 2006; Lin 2006; Ma 2007; Mai 2007; Hua 2008; Martinez 2009; Yang 2009; Nouraei 2012; Kovachev 2015). Data were available on 1656 outpatients with VVC, they were all non‐pregnant and without RVVC, diabetes mellitus, immunosuppressive disorders, taking immunosuppressant medication or evidence of co‐infection with other vulvovaginal infections.
Primary outcomes
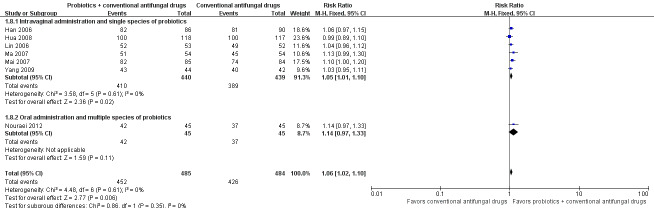
Clinical cure rate: short‐term (zero to 14 days after treatment)
Five trials evaluated 695 participants for short‐term clinical cure rate (Han 2006; Ma 2007; Hua 2008; Yang 2009; Nouraei 2012).
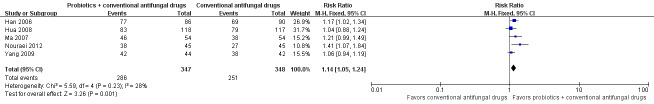
In the probiotics used as adjuvants to conventional antifungal drugs group, clinical cure occurred in 286/347 participants. In the conventional antifungal drugs group, a clinical cure occurred in 251/348 participants. Probiotics as adjuvants therapy was associated with a significantly improved on short‐term clinical cure rate when compared to conventional antifungal drugs alone (combined RR 1.14, 95% CI 1.05 to 1.24; 5 trials, 695 participants, I2 = 28%; Analysis 1.1; Figure 4).
1.1. Analysis.
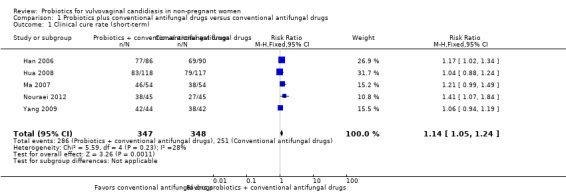
Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 1 Clinical cure rate (short‐term).
4.
Forest plot of comparison: 2 Probiotics + conventional antifungal drugs versus conventional antifungal drugs, outcome: 1.1 Clinical cure rate (short‐term).
A sensitivity analysis excluding the four studies at high risk of bias (Han 2006, Hua 2008, Ma 2007, Yang 2009), only included Nouraei 2012; however, the rate of short‐term clinical cure was still significantly increased in the probiotics used as adjuvants group compared to the conventional antifungal drugs alone group (RR 1.41, 95% CI 1.07 to 1.84; 1 trial, 90 participants; Analysis 1.2).
1.2. Analysis.
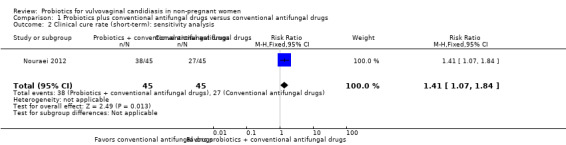
Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 2 Clinical cure rate (short‐term): sensitivity analysis.
The GRADE quality of the evidence for this outcome was low due to the limitations of risk of bias (see Table 1 for details).
Clinical cure rate: long‐term (one month after treatment)
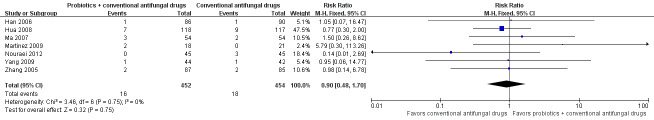
Only one trial reported the long‐term clinical cure rate at one month after treatment (Zhang 2005). There was no significant improvement associated with probiotics as adjuvant therapy on long‐term clinical cure rate at one month after treatment compared to conventional antifungal drugs alone (RR 1.07, 95% CI 0.86 to 1.33; 1 trial, 172 participants; Analysis 1.4).
1.4. Analysis.
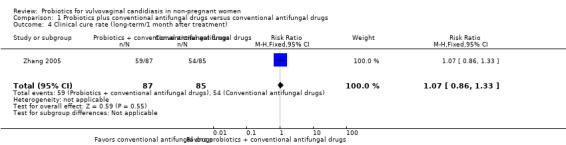
Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 4 Clinical cure rate (long‐term/1 month after treatment).
The quality of the evidence for this outcome was very low due to the limitations of risk of bias and imprecision (see Table 1 for details).
Clinical cure rate: long‐term (three months after treatment)
One trial reported long‐term clinical cure rate at three months after treatment (Zhang 2005). There was no significant improvement associated with probiotics as adjuvant therapy on long‐term clinical cure rate at three months after treatment compared to conventional antifungal drugs alone (RR 1.30, 95% CI 1.00 to 1.70; 1 trial, 172 participants; Analysis 1.5).
1.5. Analysis.
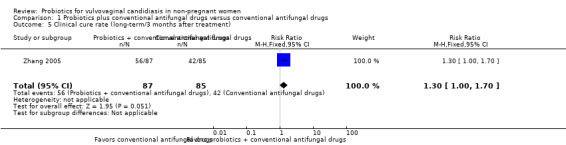
Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 5 Clinical cure rate (long‐term/3 months after treatment).
The quality of the evidence for this outcome was very low due to the limitations of risk of bias and imprecision (see Table 1 for details).
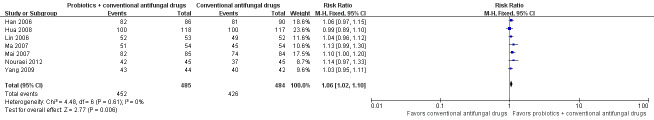
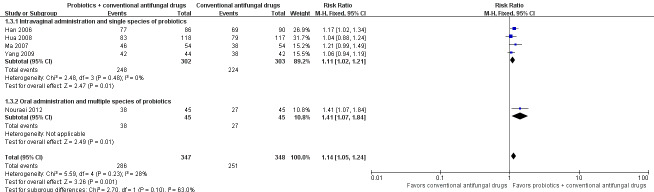
Mycological cure rate: short‐term (zero to 14 days after treatment)
Seven trials with 969 participants evaluated short‐term mycological cure rate (Han 2006; Lin 2006; Ma 2007; Mai 2007; Hua 2008; Yang 2009; Nouraei 2012).
In the probiotics used as adjuvants to conventional antifungal drugs group, mycological cure occurred in 452/485 participants. In the conventional antifungal drugs group, mycological cure occurred in 426/484 participants. Probiotics as adjuvants therapy was associated with a significantly improved short‐term mycological cure rate compared conventional antifungal drugs alone (RR 1.06, 95% CI 1.02 to 1.10; 7 trials, 969 participants, I2 = 0%; Analysis 1.6; Figure 5).
1.6. Analysis.

Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 6 Mycological cure rate (short‐term).
5.
Forest plot of comparison: 2 Probiotics + conventional antifungal drugs versus conventional antifungal drugs, outcome: 1.6 Mycological cure rate (short‐term).
A sensitivity analysis excluding the six trials at high risk of bias (Han 2006; Lin 2006; Ma 2007; Mai 2007; Hua 2008; Yang 2009), only included Nouraei 2012; it found the rate of short‐term mycological cure was no longer significantly difference between the groups (RR 1.14, 95% CI 0.97 to 1.33; 1 trial, 90 participants; Analysis 1.7). The result of this sensitivity analysis must be treated and interpreted carefully because of the small sample in Nouraei 2012.
1.7. Analysis.
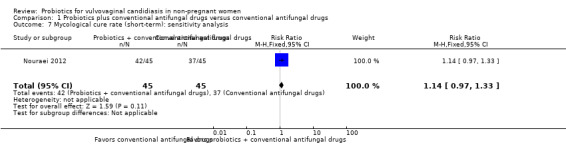
Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 7 Mycological cure rate (short‐term): sensitivity analysis.
The quality of the evidence for this outcome was low due to the limitations of risk of bias (see Table 1 for details).
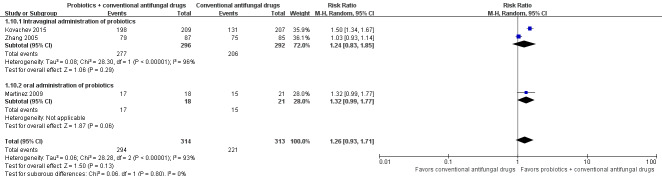
Mycological cure rate: long‐term (one month after treatment)
Three trials with 627 participants evaluated long‐term mycological cure rate at one month after treatment (Zhang 2005; Martinez 2009; Kovachev 2015).
In the probiotics used as adjuvants to conventional antifungal drugs group, mycological cure occurred in 294/314 participants. In the conventional antifungal drugs group, mycological cure occurred in 221/313 participants. Probiotics as adjuvants therapy was associated with no significant improvement on long‐term mycological cure rate at one month after treatment when compared to conventional antifungal drugs alone (RR 1.26, 95% CI 0.93 to 1.71; 3 trials, 627 participants, I2 = 93%; Analysis 1.10; Figure 6).
1.10. Analysis.
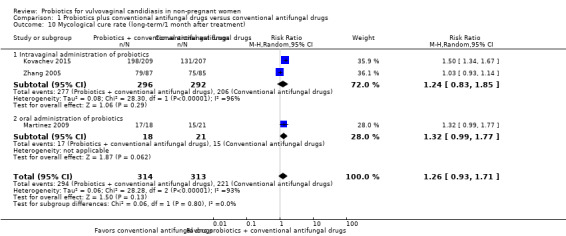
Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 10 Mycological cure rate (long‐term/1 month after treatment).
6.
Forest plot of comparison: 2 Probiotics + conventional antifungal drugs versus conventional antifungal drugs, outcome: 1.10 Mycological cure rate (long‐term/1 month after treatment).
A sensitivity analysis excluding two trials at high risk of bias (Zhang 2005; Kovachev 2015), only included Martinez 2009; however, the rate of long‐term mycological cure rate at one month after treatment was still not significantly difference between probiotics used as adjuvants and conventional antifungal drugs alone (RR 1.32, 95% CI 0.99 to 1.77; 1 trial, 90 participants; Analysis 1.11). This result must be treated and interpreted carefully because of the small sample.
1.11. Analysis.
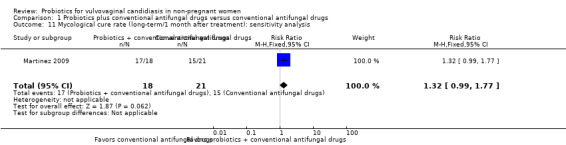
Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 11 Mycological cure rate (long‐term/1 month after treatment): sensitivity analysis.
The quality of the evidence for this outcome was very low due to the limitations of risk of bias, imprecision and inconsistency (see Table 1 for details).
Mycological cure rate: long‐term (three months after treatment)
Only one trial with 172 participants reported long‐term mycological cure rate at three months after treatment (Zhang 2005). There was no statistically significant difference between probiotics used as adjuvants and conventional antifungal drugs alone in the rate of long‐term mycological cure at three months after treatment (RR 1.16, 95% CI 1.00 to 1.35; 1 trial, 172 participants; Analysis 1.12).
1.12. Analysis.
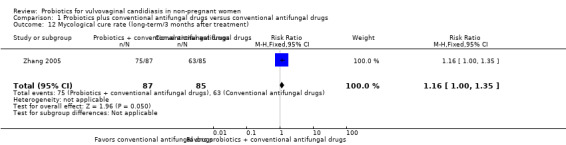
Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 12 Mycological cure rate (long‐term/3 months after treatment).
The quality of the evidence for this outcome was very low due to the limitations of risk of bias and imprecision (see Table 1 for details).
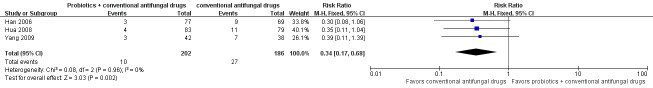
Relapse rate
Three trials with 388 participants evaluated first relapse after treatment (Han 2006; Hua 2008; Yang 2009). In the probiotics used as adjuvants to conventional antifungal drugs group, relapse occurred in 10/202 participants. In the conventional antifungal drugs group, relapse occurred in 27/186 participants. Probiotic use significantly decreased relapse rate at one month (RR 0.34, 95% CI 0.17 to 0.68; 3 trials, 388 participants, I2 = 0%; Analysis 1.13; Figure 7). No sensitivity analysis or subgroup analyses was done from the available trials.
1.13. Analysis.
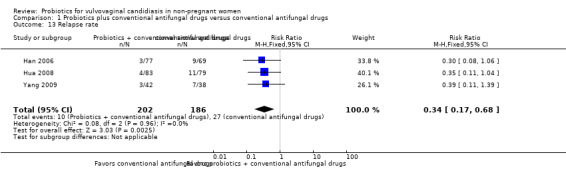
Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 13 Relapse rate.
7.
Forest plot of comparison: 2 Probiotics + conventional antifungal drugs versus conventional antifungal drugs, outcome: 1.13 Relapse rate.
The quality of the evidence for this outcome was very low due to the limitations of risk of bias and imprecision (see Table 1 for details).
Rate of serious adverse events
Two trials with 440 participants evaluated rate of serious adverse events (Zhang 2005; Hua 2008). The reported adverse events included severe vaginal burning, stinging or rash after the first use of miconazole nitrate vaginal suppository. There was no statistically significant difference between probiotics used as adjuvants and conventional antifungal drugs alone in the rate of serious adverse events (RR 0.80, 95% CI 0.22 to 2.94; 2 trials, 440 participants, I2 = 0%; Analysis 1.14). No other serious adverse events were reported.
1.14. Analysis.
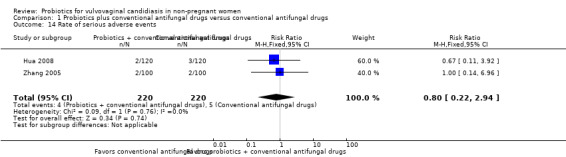
Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 14 Rate of serious adverse events.
The quality of the evidence for this outcome was low due to the limitations of risk of bias and imprecision (see Table 1 for details).
Secondary outcomes
Time to first relapse
We found no eligible RCTs reporting time to first relapse.
Rate of non‐serious adverse events
Seven trials with 906 participants evaluated rate of non‐serious adverse events (Zhang 2005; Han 2006; Ma 2007; Hua 2008; Martinez 2009; Yang 2009; Nouraei 2012).
The reported adverse events included mild vaginal dryness, burning, stinging, headache, loose stool and nausea. There was no statistically significant difference between probiotics used as adjuvants and conventional antifungal drugs alone in the rate of non‐serious adverse events (RR 0.90, 95% CI 0.48 to 1.70; 7 trials, 906 participants, I2 = 0%; Analysis 1.15; Figure 8).
1.15. Analysis.
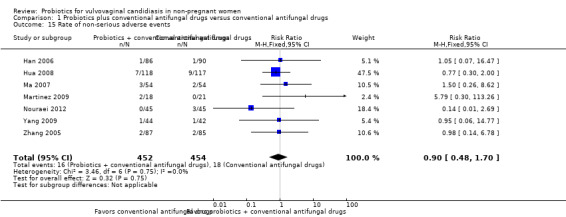
Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 15 Rate of non‐serious adverse events.
8.
Forest plot of comparison: 2 Probiotics + conventional antifungal drugs versus conventional antifungal drugs, outcome: 1.14 Non‐serious adverse events.
The quality of the evidence for this outcome was low due to the limitations of risk of bias and imprecision (CIs were wide and included null effects).
Need for any additional treatment at the end of the therapy
We found no eligible RCTs reporting need for any additional treatment at the end of the therapy.
Patient satisfaction with treatment
We found no eligible RCTs reporting patient satisfaction with treatment.
Cost effectiveness
We found no eligible RCTs reporting cost effectiveness.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses to explore the substantial heterogeneity we found in the analyses "Clinical cure rate: short‐term (zero to 14 days after treatment)." For this analysis, we explored whether differences in species of probiotics and route of administration (intravaginal administration of single species of probiotic or oral administration of multiple species of probiotics). Subgroups did not appear to be different (P = 0.10; Analysis 1.3; Figure 9). The effect of the intervention for this outcome did not change according to these intervention characteristics.
1.3. Analysis.
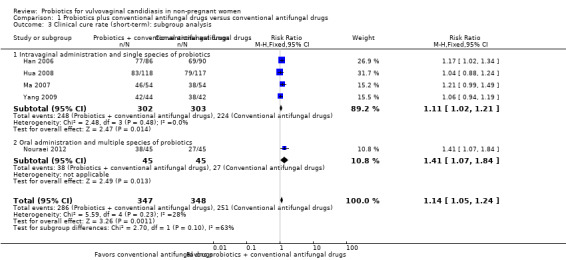
Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 3 Clinical cure rate (short‐term): subgroup analysis.
9.
Forest plot of comparison: 2 Probiotics + conventional antifungal drugs versus conventional antifungal drugs, outcome: 1.3 Clinical cure rate (short‐term): subgroup analysis.
In the analyses for "mycological cure rate: short‐term (zero to 14 days after treatment)," we explored whether differences in species of probiotics and route of administration (intravaginal administration of single species of probiotic or oral administration of multiple species of probiotics), subgroups did not appear to be different (P = 0.35; Analysis 1.8; Figure 10). The effect of the intervention for this outcome did not change according to these intervention characteristics.
1.8. Analysis.
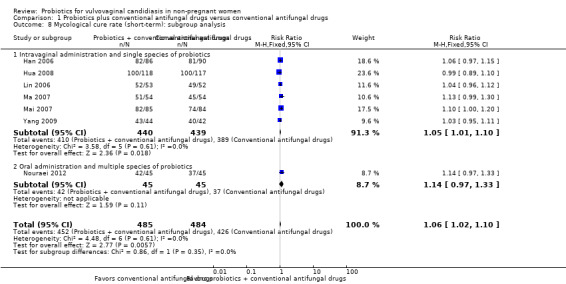
Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 8 Mycological cure rate (short‐term): subgroup analysis.
10.
Forest plot of comparison: 2 Probiotics + conventional antifungal drugs versus conventional antifungal drugs, outcome: 1.8 Mycological cure rate (short‐term): subgroup analysis.
In addition, we explored the differences between different candidal strains (Candida albicans versus non‐albicans), and found that the subgroup estimates for the different candidal strains did not differ significantly in the oral administration of probiotics (P = 0.88; Analysis 1.9).
1.9. Analysis.
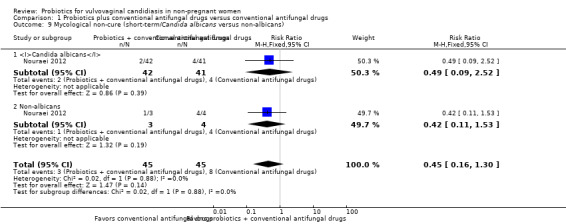
Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 9 Mycological non‐cure (short‐term/Candida albicans versus non‐albicans).
In the analyses "mycological cure rate: long‐term (one month after treatment),", we explored the differences between routes of administration (intravaginal or oral administration), subgroups did not appear to be different (P = 0.80; Analysis 1.10). Subgroup analysis did not appear to explain the variability in the overall summary effect measures for the outcome long‐term mycological cure rate, so these findings should be interpreted with caution.
We did not perform the subgroup analysis "age group of participants" because none of the included trials provided information.
Discussion
Summary of main results
Ten RCTs including 1656 participants fulfilled the inclusion criteria and were included. We judged the risk of bias for the included trials to be high. All the 10 included trials were in the comparison of "probiotics used as adjuvants to conventional antifungal drugs versus conventional antifungal drugs alone," and none of the retrieved studies compared "probiotic alone versus conventional treatment."
We found the following results.
Compared with conventional antifungal drugs alone, the addition of probiotics could enhance their effect in improving short‐term clinical cure rate (RR 1.14, 95% CI 1.05 to 1.24), regardless of the route of administration, and whether multiple species or single species of probiotics were used. The results did not seem to show a significant improvement between the two groups in long‐term clinical cure rate at one month after treatment (RR 1.07, 95% CI 0.86 to 1.33) and at three months after treatment (RR 1.30, 95% CI 1.00 to 1.70).
Compared with conventional antifungal drugs alone, the addition of probiotics could enhance their effect in improving short‐term mycological cure rate (RR 1.06, 95% CI 1.02 to 1.10), but the addition of probiotics did not seem to be different to conventional antifungal drugs used alone in improving the long‐term mycological cure rate at one month after treatment (RR 1.26, 95% CI 0.93 to 1.71) and at three months after treatment (RR 1.16, 95% CI 1.00 to 1.35).
Compared with conventional antifungal drugs alone, the addition of probiotics may reduce the first relapse rate at one month after treatment (RR 0.34, 95% CI 0.17 to 0.68). However, this result should be interpreted with caution because of the limitations related to the low quality of primary studies.
There appears to be no difference between the addition of probiotics to conventional antifungal drugs and conventional antifungal drugs alone in the rate of serious adverse events (RR 0.80, 95% CI 0.22 to 2.94), the reported adverse events included severe vaginal burning, stinging or rash after the first use of miconazole nitrate vaginal suppository.
There was no difference between the addition of probiotics to conventional antifungal drugs and conventional antifungal drugs alone in the rate of non‐serious adverse events (RR 0.90, 95% CI 0.48 to 1.70). The reported adverse events included mild vaginal dryness, burning, stinging, headache, loose stool and nausea.
There was no available evidence for evaluating the time to first relapse, need for any additional treatment at the end of therapy, patient satisfaction with treatment and cost effectiveness.
However, all the results found in this review should be interpreted with caution because of the limitations related to the low and very low quality of primary studies.
Overall completeness and applicability of evidence
Although we employed several methods to try to identify all RCTs and included both published and unpublished data in this review, this systematic review included trials at high risk of bias and consequently with very low to low confidence on the estimate of effect (see Table 1). We conducted sensitivity analyses or subgroup analyses (or both) that did not change the results of short‐term clinical cure rate, long‐term clinical cure rate and long‐term mycological cure rate. It strengthened the confidence that could be placed in these results, considering that most of the outcomes were very low or low quality evidence. This review was unable to report on the results of meta‐analysis of long‐term clinical cure rate (at one month and three months after treatment) and long‐term mycological cure rate (three months after treatment), as only one of the included studies offered the relevant data (Zhang 2005). This review was unable to address the issue of whether an oral administration of probiotics used as adjuvants to conventional antifungal drugs could improve the short‐term mycological cure rate, as only one study included with small sample offered the relevant data (Nouraei 2012). For the same reason, it was also unable to address the issue of whether the different candidal strains (Candida albicans versus non‐albicans) could influence the effectiveness of probiotics. For relapse rate, this review estimated the evidence only from studies that used the intravaginal probiotics. This review was also unable to address the issue of whether the different age group of women with VVC could influence the effectiveness of probiotics, because as of 2017, no RCT had focused on these topics. Likewise, none of the included studies assessed time to first relapse, need for any additional treatment at the end of therapy and patient satisfaction with treatment. Finally, this review could provide no evidence for assessing the effectiveness and safety of probiotics used alone versus conventional antifungal drugs, because none of the RCTs focused on these topics.
This review included 10 studies that enrolled 1656 participants. These studies were conducted in China (seven studies), Brazil (one study), Bulgaria (one study) and Iran (one study), and were representative of a relatively wide range of racial, cultural and social groups. However, there was no study investigating the treatment of VVC in resource‐poor settings and this is a limitation to the general applicability of the results. This review focused on non‐pregnant women with VVC, and from the inclusion criteria, the results from this review could not be applied to pregnant women; women with RVVC, diabetes mellitus or immunosuppressive disorders; or women taking immunosuppressant medication. There was a small number of mild adverse events reported, and there were no other serious adverse events reported except severe vaginal burning, stinging or rash after the first use of miconazole nitrate vaginal suppository. It appears that probiotics could be considered safe in general from current limited data.
Quality of the evidence
We considered the 10 included trials to be at high risk of bias, and the confidence in the estimated effect in this review, assessed with the GRADE tool, ranged from very low to low (see Figure 2, Figure 3, and Table 1). There is little confidence in the effect estimates, and the true effect is likely to be substantially different. The confidence is very low due to trial limitations (on risk of bias domain by sequence generation, allocation concealment and blinding of the included studies), inconsistency (substantial heterogeneity between studies and single study in some results) and imprecise results (outcome events with wide CIs, small sample size and few events). We could not evaluate publication bias, because there were too few included trials into each comparison.
Potential biases in the review process
In our review, reporting bias could not be assessed because of the limited numbers of studies for each outcome. Although we undertook several methods to try to identify all trials and included both published and unpublished data in this review, we could not exclude the possibility that studies with negative findings remain unpublished. There was substantial heterogeneity for some outcomes and our investigation of heterogeneity sources (which was based on a small number of trials for each comparison) could have limited value. In addition, the balance in baseline characteristics was not well explained and as confirmed by both published reports and contact authors, there may be unclear risk of bias for the results and conclusions.
Agreements and disagreements with other studies or reviews
We found one systematic review evaluating the effects of miconazole alone and miconazole plus a living preparation of Lactobacillus for the treatment of the uncomplicated VVC (Cui 2010). The authors included only trials using miconazole as the antifungal drug, and methodology details were unavailable. In contrast to our review, it concluded that currently available evidence showed that the effect in the combined group was better and the recurrence rate was lower. However, it did not report adverse events. Although Nouraei 2012, which used an oral administration of probiotics, showed that probiotics used as adjuvants to conventional antifungal drugs performed better at improving the short‐term clinical cure rate, it showed no difference in short‐term mycological cure rate. Likewise, Martinez 2009, which also used an oral administration of probiotics, found no significant difference in long‐term mycological cure rate, but it showed a statistically significant difference in favor of probiotics used as adjuvants to conventional antifungal drugs in enhancing long‐term clinical improvement, which was determined as cure or persistence of Candida species and absence of symptoms. However, this result should be treated with caution as the small size was sample. Ehrstrom 2010 showed that adjunctive treatment of VVC with probiotics led to fewer recurrences and only a few adverse events (single episodes of headache and nausea), but had no impact on short‐term cure rate. However, the large and imbalanced rate of lost to follow‐up and the small sample could influence the result, and, as pointed out in the publication, probiotics were unavailable for women to use as an adjuvant to urogenital care in Nigeria, and even if probiotics were introduced, many of the population could not afford them.
Authors' conclusions
Implications for practice.
Low and very low quality evidence shows that, compared with conventional treatment, probiotics as adjuvant therapy may increase the rate of short‐term clinical and mycological cure and decrease relapse rate at one month but this did not translate into a higher frequency of long‐term clinical or mycological cure. Probiotic use may not increase the frequency of serious or non‐serious adverse events. The benefits and harms of probiotics for vulvovaginal candidiasis in non‐pregnant women should be regarded with caution due to the risk of bias, imprecision and inconsistency for many of the outcomes we assessed in this Cochrane Review.
Implications for research.
Because the quality of available evidence is low, there is a need for well‐designed randomized controlled trials with adequate allocation concealment and blinding of participants, personnel and outcome assessment; longer follow‐up and larger sample size; and when probiotics are designed to be used as adjuvants to conventional antifungal drugs, placebo should be used in the control group to avoid any possible bias from the judgment of symptoms and signs by participants. The possible influence from different route of administration, probiotics species, candidal strains and age group of participants should be followed with interest. Sustained attention should be paid to the adverse events to determine the safety of probiotics. More range of racial, cultural and social groups are needed. Any future high‐quality trials should include the outcomes evaluated in this review as much as possible. Other types of comparisons are also needed.
What's new
| Date | Event | Description |
|---|---|---|
| 15 November 2017 | Amended | The final adjustment to the comments from the copy editor |
History
Protocol first published: Issue 4, 2013 Review first published: Issue 11, 2017
| Date | Event | Description |
|---|---|---|
| 13 September 2017 | Amended | The final adjustment to the comments from the CoEd and CEU |
| 12 July 2017 | Amended | The final adjustment to the comments from the editorial unit of cochrane |
Acknowledgements
We would like to thank the Cochrane Sexually Transmitted Infections Group, the Cochrane Menstrual Disorders and Subfertility Group and the Cochrane Editorial Unit.
Appendices
Appendix 1. Sexually Transmitted Infections Cochrane Review Group's Specialized Register
Using the terms "probiotic" and "lactobacillus" in title, abstract and keywords.
Appendix 2. MEDLINE and CENTRAL (Ovid)
1 exp Candidiasis, Vulvovaginal/ 2 (candid$ adj5 vulvovagin$).tw. 3 (candid$ adj5 vagin$).tw. 4 (candida adj5 colpitis).tw. 5 colpitis mycotica.tw. 6 (monilias$ adj5 vulvovagin$).tw. 7 (monilial adj5 vaginiti$).tw. 8 (vagin$ adj5 yeast).tw. 9 (vagin$ adj5 fung$).tw. 10 or/1‐9 11 exp Probiotics/ 12 probiotic$.tw. 13 exp Lactobacillus/ 14 lactobac$.tw. 15 exp Lactobacillus acidophilus/ 16 lactic acid bacteria$.tw. 17 exp Bifidobacterium/ 18 bifidobacteri$.tw. 19 or/11‐18 20 randomized controlled trial.pt. 21 controlled clinical trial.pt. 22 randomized.ab. 23 placebo.ab. 24 clinical trials as topic.sh. 25 randomly.ab. 26 trial.ti. 27 or/20‐26 28 exp animals/ not humans.sh. 29 27 not 28 30 10 and 19 and 29 Note: the CENTRAL search strategy doesn't include the terms #20 ‐ #29.
Appendix 3. Embase (embase.com)
1. 'vagina candidiasis'/exp 2. (candid* NEAR/5 vagin*):ab,ti 3. (candid* NEAR/5 vulvovagin*):ab,ti 4. (candida NEAR/5 colpitis):ab,ti 5. colpitis mycotica:ab,ti 6. (monilias* NEAR/5 vulvovagin*):ab,ti 7. (monilial NEAR/5 vaginiti*):ab,ti 8. (vagin* NEAR/5 yeast):ab,ti 9. (vagin* NEAR/5 fung*):ab,ti 10 .#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 11. ‘probiotic agent’/exp 12. probiotic*:ab,ti 13. 'Lactobacillus'/exp 14. lactobac*:ab,ti 15. betabacterium:ab,ti 16. ‘Lactobacillus acidophilus’/exp 17. (acidophilus NEAR/5 bacillus):ab,ti 18. ‘lactic acid bacterium’/exp 19. (lactic NEAR/5 acid NEAR/5 bacteri*):ab,ti 20. ‘Bifidobacterium’/exp 21. bifidobacteri*:ab,ti 22. #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 23. "randomized controlled trial"/de 24. "controlled clinical study"/de 25. random*:ti,ab 26. randomization/de 27. "intermethod comparison"/de 28. placebo:ti,ab 29. (compare OR compared OR comparison):ti 30. ((evaluated OR evaluate OR evaluating OR assessed OR assess) AND (compare OR compared OR comparing OR comparison)):ab 31. (open NEAR/1 label):ti,ab 32. ((double OR single OR doubly OR singly) NEAR/1 (blind OR blinded OR blindly)):ti,ab 33. "double blind procedure"/de 34. (parallel NEXT/1 group*):ti,ab 35. (crossover OR "cross over"):ti,ab 36. ((assign* OR match OR matched OR allocation) NEAR/5 (alternate OR group* OR intervention* OR patient* OR subject* OR participant*)):ti,ab 37. (assigned or allocated):ti,ab 38. (controlled NEAR/7 (study OR design OR trial)):ti,ab 39. (volunteer OR volunteers):ti,ab 40. trial:ti 41. "human experiment"/de 42. #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 43. #10 AND #22 AND #42
Appendix 4. AMED (Ovid)
1 exp candidiasis/ 2 (Candid$ adj2 Vulvovagina$).tw. 3 (Candid$ adj2 Vulv$).tw. 4 (Candid$ adj2 Vagin$).tw. 5 (vagin$ adj2 fung$).tw. 6 vaginosis.tw. 7 vaginitis.tw. 8 vaginoses.tw. 9 colpitis.tw. 10 (yeast$ adj2 vagin$).tw. 11 or/1‐10 12 exp Probiotics/ 13 Lactobacill$.tw. 14 Probiotic$.tw. 15 lactic acid bacter$.tw. 16 acidophil$.tw. 17 bifidobacteri$.tw. 18 or/12‐17 19 11 and 18
Appendix 5. CBMdisc and CNKI
1 exp Candidiasis, Vulvovaginal/ 2 (Candid$ adj2 Vulvovagina$).tw. 3 (Candid$ adj2 Vulv$).tw. 4 (Candid$ adj2 Vagin$).tw. 5 (vagin$ adj2 fung$).tw. 6 vaginosis.tw. 7 vaginitis.tw. 8 vaginoses.tw. 9 colpitis.tw. 10 (yeast$ adj2 vagin$).tw. 11 or/1‐10 12 exp Probiotics/ 13 exp Lactobacillus/ 14 Probiotic$.tw. 15 Lactobacill$.tw. 16 lactic acid bacter$.tw. 17 exp Lactobacillus acidophilus/ 18 acidophil$.tw. 19 bifidobacteri$.tw. 20 or/12‐19 21 11 and 20 22 randomized controlled trial.pt. 23 controlled clinical trial.pt. 24 randomized.ab. 25 placebo.tw. 26 clinical trials as topic.sh. 27 randomly.ab. 28 trial.ti. 29 (crossover or cross‐over or cross over).tw. 30 or/22‐29 31 21 and 30
Appendix 6. PsycINFO (Ovid)
1 exp Gynecological Disorders/ 2 exp Infectious Disorders/ 3 (Candid$ adj2 Vulvovagina$).tw. 4 (Candid$ adj2 Vulv$).tw. 5 (Candid$ adj2 Vagin$).tw. 6 (vagin$ adj2 fung$).tw. 7 vaginosis.tw. 8 vaginitis.tw. 9 vaginoses.tw. 10 colpitis.tw. 11 (yeast$ adj2 vagin$).tw. 12 or/1‐11 13 Probiotic$.tw. 14 Lactobacill$.tw. 15 lactic acid bacter$.tw. 16 acidophil$.tw. 17 bifidobacteri$.tw. 18 or/13‐17 19 12 and 18
Appendix 7. LILACS (iAHx interface)
(mh:(Candidiasis Vulvovaginal)) OR (ti:(candidiasis vulvovaginal)) OR (ab:(candidiasis vulvovaginal)) OR (ti:(moniliasis vulvovaginal)) OR (ab:(moniliasis vulvovaginal)) OR (ti:(vaginitis monilial)) OR (ab:(vaginitis monilial)) AND (mh:(Probióticos)) OR (ti:(probiótico$)) OR (ab:(probiótico$)) OR (mh:(Lactobacillus)) OR (ti:(Lactobacillus)) OR (ab:(Lactobacillus))
RCTs filter:
((PT:"ensayo clinico controlado aleatorio" OR PT:"ensayo clinico controlado" OR PT:"estudio multicéntrico" OR MH:"ensayos clinicos controlados aleatorios como asunto" OR MH:"ensayos clinicos controlados como asunto" OR MH:"estudios multicéntricos como asunto" OR MH:"distribución aleatoria" OR MH:"método doble ciego" OR MH:"metodo simple‐ciego") OR ((ensaio$ OR ensayo$ OR trial$) AND (azar OR acaso OR placebo OR control$ OR aleat$ OR random$ OR enmascarado$ OR simpleciego OR ((simple$ OR single OR duplo$ OR doble$ OR double$) AND (cego OR ciego OR blind OR mask))) AND clinic$)) AND NOT (MH:animales OR MH:conejos OR MH:ratones OR MH:ratas OR MH:primates OR MH:perros OR MH:gatos OR MH:porcinos OR PT:"in vitro")
Data and analyses
Comparison 1. Probiotics plus conventional antifungal drugs versus conventional antifungal drugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure rate (short‐term) | 5 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.05, 1.24] |
| 2 Clinical cure rate (short‐term): sensitivity analysis | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.07, 1.84] |
| 3 Clinical cure rate (short‐term): subgroup analysis | 5 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.05, 1.24] |
| 3.1 Intravaginal administration and single species of probiotics | 4 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.02, 1.21] |
| 3.2 Oral administration and multiple species of probiotics | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.07, 1.84] |
| 4 Clinical cure rate (long‐term/1 month after treatment) | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.86, 1.33] |
| 5 Clinical cure rate (long‐term/3 months after treatment) | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.00, 1.70] |
| 6 Mycological cure rate (short‐term) | 7 | 969 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [1.02, 1.10] |
| 7 Mycological cure rate (short‐term): sensitivity analysis | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.97, 1.33] |
| 8 Mycological cure rate (short‐term): subgroup analysis | 7 | 969 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [1.02, 1.10] |
| 8.1 Intravaginal administration and single species of probiotics | 6 | 879 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [1.01, 1.10] |
| 8.2 Oral administration and multiple species of probiotics | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.97, 1.33] |
| 9 Mycological non‐cure (short‐term/Candida albicans versus non‐albicans) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.16, 1.30] |
| 9.1 Candida albicans | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.52] |
| 9.2 Non‐albicans | 1 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.11, 1.53] |
| 10 Mycological cure rate (long‐term/1 month after treatment) | 3 | 627 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.93, 1.71] |
| 10.1 Intravaginal administration of probiotics | 2 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.83, 1.85] |
| 10.2 oral administration of probiotics | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.99, 1.77] |
| 11 Mycological cure rate (long‐term/1 month after treatment): sensitivity analysis | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.99, 1.77] |
| 12 Mycological cure rate (long‐term/3 months after treatment) | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.00, 1.35] |
| 13 Relapse rate | 3 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.17, 0.68] |
| 14 Rate of serious adverse events | 2 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.22, 2.94] |
| 15 Rate of non‐serious adverse events | 7 | 906 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.48, 1.70] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Han 2006.
| Methods | Randomized controlled study. Setting: Dagang Hospital of Tianjin City, Tianjin, China. Study period: January 2005 to May 2005. |
|
| Participants | Inclusion criteria: first episode of curd‐like vaginal discharge associated with any of the following symptoms and signs: vulvar itching, vulvar burning, vaginal feeling, dyspareunia and vaginal mucosa hyperemia; vaginal samples positive for Candida species, hyphae or pseudohyphae by microscopic examination method. No sexual activity allowed during treatment and used condom during follow‐up. Exclusion criteria: pregnant, lactating, diabetes, allergic responses to azole drugs, positive for trichomoniasis or mycoplasma infection. Number enrolled: 176 women enrolled and randomized. Numbers per group: clotrimazole + probiotic: 86; clotrimazole alone: 90. Age (mean): 37 years (range 19‐48 years). Separate age data per group not available. |
|
| Interventions | Clotrimazole + probiotic group: 1 vaginal tablet of clotrimazole 500 mg on days 1 and 4 + 1 vaginal capsules of Lactobacillus delbrueckii subsp. Lactis DM8909 (each capsule contained 0.25 × 106 colony forming units), QD from day 1 to day 10. Clotrimazole alone group: 1 vaginal tablet of clotrimazole 500 mg on days 1 and 4. |
|
| Outcomes | Follow‐up: at 7‐10 days and 1 month after completion of treatment, clinical and laboratory symptoms were re‐evaluated. Outcomes:
|
|
| Notes | Study obtained written consent from all participants and had ethical clearance from review committee of hospital. No funding source or declaration of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We contacted the original author and found that they used a draw method (all participants were numbered on individual pieces of paper, then took the paper relevant to participant out of a container without seeing what was written on it). |
| Allocation concealment (selection bias) | High risk | No allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported no withdrawals, but there was not enough information for us to judge "yes" or "no" in intention‐to‐treat analysis (per protocol analysis) and consequences. |
| Selective reporting (reporting bias) | Unclear risk | No information available to make a judgment. |
| Other bias | Unclear risk | No available data on baseline characteristics for the 2 groups to judge the balance. (We contacted the original authors and they reported no significant differences in baseline characteristics, but provided no detailed data.) |
Hua 2008.
| Methods | Randomized controlled study. Setting: Second Affiliated Hospital of Wenzhou Medical College, Wenzhou, Zhejiang province, China. Study period: March 2007 to October 2007. |
|
| Participants | Inclusion criteria: first episode of curd‐like vaginal discharge associated with any of the following symptoms and signs: vulvar itching, vulvar burning, vaginal feeling and vaginal mucosa hyperemia, vaginal samples positive for Candida species, hyphae or pseudohyphae by microscopic examination method. No sexual activity allowed during treatment and used condom during follow‐up. Exclusion criteria: pregnant, lactating, diabetes, diseases of the liver and kidney system, allergic responses to azole drugs. Number enrolled: 240 women enrolled and randomized. Numbers per group: miconazole + probiotic: 120; miconazole alone: 120. Age (mean ± SD): 28.5 ± 6.7 years (range 19‐45 years). Separate age per group not available. Withdrawals: miconazole + probiotic group: 2 withdrew due to severe vaginal burning, stinging or rash after the first use of miconazole; miconazole alone group: 3 due to severe vaginal burning, stinging or rash. Original author did not use intention‐to‐treat analyses. |
|
| Interventions | Miconazole + probiotic group: 1 vaginal suppository of miconazole nitrate 400 mg, QD from day 1 to day 6, and then 1 vaginal capsule of Lactobacillus delbrueckii subsp. Lactis DM8909 (each capsule contained 0.25 × 106 colony forming units) QD from day 7 to day 16. Miconazole alone group: 1 vaginal suppository of miconazole nitrate 400 mg, QD from day 1 to day 6. |
|
| Outcomes | Follow‐up: at 5‐7 days and 33‐37 days after completion of treatment, clinical and laboratory symptoms were re‐evaluated. Outcomes:
|
|
| Notes | Study obtained written consent from all participants and had ethical clearance from review committee of hospital. No funding source or declaration of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We contacted the original author and found that they used a draw method (all participants were numbered on individual pieces of paper, then took the paper relevant to participant, out of a container without being able to see what was written on it). |
| Allocation concealment (selection bias) | Low risk | We contacted the original author and found that they used codified, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single blinding for all investigators. We consider the judgment of symptoms and signs outcomes by participants may have been influenced. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessors. But we consider outcome assessors may have been influenced by participants, because there was no blinding to participants. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reported withdrawals rate of 2.1% (less than 20%) during the treatment, but the results were not analyzed with an intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | No information available to make a judgment. |
| Other bias | Unclear risk | No available data on baseline characteristics for the 2 groups to judge the balance. (We contacted the original authors and they reported no significant differences in baseline characteristics, but provided no detailed data.) |
Kovachev 2015.
| Methods | Single‐center, randomized, open‐label study. Setting: Outpatient Group Practice for Specialized Care in Obstetrics and Gynecology "GynArt," Sofia, Bulgaria. Study period: 2008‐2013. |
|
| Participants | Inclusion criteria: aged 17‐50 years with clinically or microbiologically identified predominantly Candida albicans vaginal infections. Exclusion criteria: pregnant, infection with Neisseria gonorrhoeae, herpes simplex virus, human papillomavirus, Chlamydia trachomatis or HIV; using corticosteroids, antibiotics, azoles or probiotics within last month; using vaginal agents within last month; immunocompromised diseases, autoimmune diseases, endocrine diseases, diabetes diseases or malignancies. Number enrolled: 436 women enrolled and randomized. Numbers per group: azole + vaginal probiotic: 217; azole alone: 219. Lost to follow‐up after treatment: azole + vaginal probiotic: 8; azole alone: 12. Original author did not use intention‐to‐treat analyses. |
|
| Interventions | Azole + vaginal probiotic group: oral fluconazole 150 mg + 1 vaginal globule of fenticonazole 600 mg on same day; however, 10 applications of vaginal probiotics (Lactobacillus acidophilus, Lactobacillus rhamnosus, Streptococcus thermophilus and Lactobacillus delbrueckii subsp. Bulgaricus) were also administered beginning on 5th day after azole treatment. Azole group: oral fluconazole 150 mg + 1 vaginal globule of fenticonazole 600 mg on same day. |
|
| Outcomes | Follow‐up: 35‐40 days after completion of treatment, clinical and microbiological data obtained at the follow‐up examination were analyzed, proportion of participants within each treatment group with improvements recorded, number of participants with decreases in specific complaints due to treatment were compared. Outcomes:
|
|
| Notes | Study obtained written consent from all participants and had approval from Local Ethics Committee. No funding source or declaration of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors used Research Randomizer software (version 3.0) for random sequence generation. |
| Allocation concealment (selection bias) | High risk | No allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reported withdrawal rate of 4.6% (less than 20%) after the treatment, but results not analyzed on an intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | No information available to make a judgment. |
| Other bias | Unclear risk | No available data of the baseline characteristics between the 2 groups to judge the balance. |
Lin 2006.
| Methods | Randomized controlled study. Setting: People's Hospital of Meizhou City, Meizhou, Guangdong Province, China. Study period: July 2005 to October 2005. |
|
| Participants | Inclusion criteria: first episode of curd‐like vaginal discharge associated with any of the following symptoms and signs: vulvar or vaginal itching, vulvar burning and vaginal mucosa hyperemia; vaginal samples positive for Candida species, hyphae or pseudohyphae by microscopic examination method. They received no systemic or intravaginal antibiotic during the treatment and follow‐up. Sexual activity during treatment not allowed and they used condom during follow‐up. Exclusion criteria: pregnant, lactating, diabetes, diseases of the liver and kidney system, allergic responses to azole drugs. Number enrolled: 105 women enrolled and randomized. Number per group: clotrimazole + probiotic: 53; clotrimazole alone: 52. Age (mean): 30 years (range 20‐44 years). Separated age data per group not available. |
|
| Interventions | Clotrimazole + probiotic group: 1 vaginal suppository of clotrimazole 150 mg, QD from day 1 to day 7, and then 1 vaginal Lactasin capsules of Streptococcus faecalis (each capsule contained 6 × 107 colony forming units), QD from day 8 to day 14. Clotrimazole alone group: 1 vaginal suppository of clotrimazole 150 mg, QD from day 1 to day 7. |
|
| Outcomes | Follow‐up: 7 days after completion of treatment, clinical and laboratory symptoms were re‐evaluated. Outcomes:
|
|
| Notes | Study obtained written consent from all participants and had ethical clearance from review committee of hospital. No funding source or declaration of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We contacted the original author and found that they used a draw method (all participants were numbered on individual pieces of paper, then took the paper relevant to participant out of a container seeing what was written on it). |
| Allocation concealment (selection bias) | High risk | No allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported no withdrawals, but there was not enough information for us to judge "yes" or "no" in intention‐to‐treat analysis (per protocol analysis) and consequences. |
| Selective reporting (reporting bias) | Unclear risk | No information available to make a judgment. |
| Other bias | Unclear risk | No available data of baseline characteristics for the 2 groups to judge the balance. (We contacted the original authors and they reported no significant differences in baseline characteristics, but provided no detailed data.) |
Ma 2007.
| Methods | Randomized controlled study. Setting: Zhongnan Hospital of Wuhan University, Wuhan, Hubei province, China. Study period: February 2005 to October 2005. |
|
| Participants | Inclusion criteria: first episode of curd‐like vaginal discharge associated with any of the following symptoms and signs: vulvar itching, vulvar burning, vaginal feeling, vaginal mucosa hyperemia and dysuria; vaginal samples positive for Candida species, hyphae or pseudohyphae by microscopic examination method. They received no systemic or intravaginal antibiotic during the treatment. Sexual activity during treatment not allowed and they used condoms during follow‐up. Exclusion criteria: using exogenous hormones or glucocorticoid, pregnant, immunodeficiency disease, diabetes and positive for BV or trichomoniasis. Number enrolled: 108 women enrolled and randomized. Numbers per group: miconazole + probiotic: 54; miconazole alone: 54. Age (mean ± SD): 26.0 ± 3.2 years (range 22‐38 years). Separate age data per group not available. |
|
| Interventions | Miconazole + probiotic group: 1 vaginal suppository of miconazole nitrate 200 mg, QD from day 1 to day 14, and at day 8 used 1 vaginal Lactasin capsules of Streptococcus faecalis (each capsule contained 6 × 107 colony forming units), QD from day 8 to day 14. Miconazole alone group: 1 vaginal suppository of miconazole nitrate 200 mg, QD from day 1 to day 14. |
|
| Outcomes | Follow‐up: 7 days after completion of treatment, clinical and laboratory symptoms were re‐evaluated. Outcomes:
|
|
| Notes | Study obtained written consent from all participants and had ethical clearance from review committee of hospital. No funding source or declaration of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We contacted the original author and found that they used a draw method (all participants were numbered on individual pieces of paper, then took the paper relevant to participant, out of a container without seeing what was written on it). |
| Allocation concealment (selection bias) | High risk | No allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported no withdrawals, but there was not enough information for us to judge "yes" or "no" in intention‐to‐treat analysis (per protocol analysis) and consequences. |
| Selective reporting (reporting bias) | Unclear risk | No information available to make a judgment. |
| Other bias | Unclear risk | No available data on baseline characteristics for the 2 groups to judge the balance. (We contacted the original authors and they reported no significant differences in baseline characteristics, but provided no detailed data.) |
Mai 2007.
| Methods | Randomized controlled study. Setting: People's Hospital of Guangdong province, Guangzhou, Guangdong province, China. Study period: October 2004 to October 2005. |
|
| Participants | Inclusion criteria: first episode of curd‐like vaginal discharge associated with any of the following symptoms and signs: vulvar or vaginal itching, vulvar or vaginal burning and vaginal mucosa hyperemia; vaginal samples positive for Candida species, hyphae or pseudohyphae by microscopic examination method; married; normal menstrual. Sexual activity during treatment and follow‐up not allowed. Exclusion criteria: received antibiotic within past week or vaginal drugs within past 2 weeks of appointment, pregnant, lactating, no menstruation, other disease. Number enrolled: 180 women enrolled and randomized. Number per group: clotrimazole + probiotic: 90; clotrimazole alone: 90. Age (mean ± SD): 30.1 ± 5.4 years (range 20‐47 years). Separate age data per group not available. Lost to follow‐up after treatment: clotrimazole + probiotic: 5; clotrimazole alone: 6. Original author did not use intention‐to‐treat analyses. |
|
| Interventions | Clotrimazole + probiotic group: 1 vaginal suppository of clotrimazole 150 mg, QN + 1 vaginal capsules of Lactobacillus delbrueckii subsp. Lactis DM8909 (each capsule contained 0.25 × 106 colony forming units), QD from day 1 to day 10. Clotrimazole alone group: 1 vaginal suppository of clotrimazole 150 mg, QN from day 1 to day 10. |
|
| Outcomes | Follow‐up: 7 days after completion of treatment, clinical and laboratory symptoms were re‐evaluated. Outcomes:
|
|
| Notes | Study obtained written consent from all participants and had ethical clearance from review committee of hospital. No funding source or declaration of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We contacted the original author and found that they used a draw method (all participants were numbered on individual pieces of paper, then took the paper relevant to participant out of a container without seeing what was written on it). |
| Allocation concealment (selection bias) | Low risk | Author stated they concealed allocation by codified, opaque, sealed envelopes. Comment: probably done. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single blinding to all the investigators. We consider the judgment of symptoms and signs outcomes by participants may have been influenced. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding to outcome assessors. We consider the outcome assessors may have been influenced by participants, because there was no blinding of participants. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reported withdrawals rate of 6.1% (less than 20%) after the treatment, but results were not analyzed on an intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | No information available to make a judgment. |
| Other bias | Low risk | Study appeared free of other sources of bias. |
Martinez 2009.
| Methods | Randomized, double‐blind, placebo‐controlled study. Setting: CSE‐FMRP‐USP and 3 other affiliated sites (Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto (HCFMRP)‐USP, Sistema Integrado de Saúde (SIS)‐USP and Centro de Saúde Vila Lobato (CSVL)‐FMRP‐USP, Brazil). Study period: September 2006 to April 2007. |
|
| Participants | Inclusion criteria: vaginal discharge associated with any of the following symptoms: vaginal itching and burning, dyspareunia and dysuria; whose vaginal samples were positive for Candida species by culture method. Exclusion criteria: pregnant, HIV positive, positive for BV or trichomoniasis; received systemic or intravaginal antibiotic or antifungal agents currently or within 2 weeks prior to appointment, menses during samples collection; allergic responses to fluconazole. Number enrolled: 68 women; 55 randomized. Numbers per group: fluconazole + probiotic: 18 with VVC and 11 with RVVC; fluconazole + placebo: 21 with VVC and 5 with RVVC. Both VVC participants' outcome data and RVVC participants' outcome data were available and provided for each group. Age (mean ± SD): fluconazole + probiotic: 29.1 ± 7.5 years (range 16‐46 years); fluconazole + placebo: 26.9 ± 7.8 years (range 16‐42 years). |
|
| Interventions | Fluconazole + probiotic group: 1 dose of fluconazole 150 mg + 2 oral capsules of Lactobacillus rhamnosus GR‐1 and Lactobacillus reuteri RC‐14 (each capsule contained 1 × 109 viable cells of both strains) for 28 days. Fluconazole + placebo group: 1 dose of fluconazole 150 mg + 2 oral capsules of placebo once daily (every morning) for 28 days. |
|
| Outcomes | Follow‐up: day 28 after beginning of treatment, presence of vaginal discharge was evaluated along with the presence of any symptoms and signs (itching and burning vaginal feeling, dyspareunia and dysuria) and adverse effects related to drug or probiotic, or both. Outcomes:
|
|
| Notes | Study obtained the written consent from all participants. Funded by São Paulo State Foundation. Declarations of interest: G Reid (corresponding author) holds some patents associated with lactobacilli. However, his input was in protocol design, logistics, student supervision and assistance with the manuscript, not in the accumulation of data, and he was blinded to the results until after the code was broken and findings acquired. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects diagnosed with VVC were randomized to treatment with a single dose of fluconazole (150 mg) plus either two oral capsules of L. [Lactobacillus] GR‐1 and L. reuteri RC‐14 or placebo once daily (every morning) for 28 days starting on the day of fluconazole use." Comment: no more information for judgment. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote 1: "The strains L. [Lactobacillus] rhamnosus GR‐1 and L. reuteri RC‐14 were provided by Chr. Hansen, Horsholm, Denmark, in gelatin capsules manufactured under good manufacturing practices. Each capsule contained 1 × 109 viable cells of both strains. Gelatin capsules containing cellulose and magnesium stearate were used as placebo." Quote 2: "Findings were blinded to all the investigators until the analyses had been completed." Comment: probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote 1: "Findings were blinded to all the investigators until the analyses had been completed." Quote 2: "G. Reid holds some patents associated with lactobacilli. However, his input was in protocol design, logistics, student supervision and assistance with the manuscript, not in the accumulation of data, and he was blinded to the results until after the code was broken and findings acquired." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported no withdrawals, but there was not enough information for us to judge "yes" or "no" in intention‐to‐treat analysis (per protocol analysis) and consequences. |
| Selective reporting (reporting bias) | Unclear risk | No information available to make a judgment. |
| Other bias | Low risk | Study appeared free of other sources of bias. |
Nouraei 2012.
| Methods | Randomized, double‐blind, placebo‐controlled, study. Setting: Obstetrics Unit of the clinic affiliated with Shahid Beheshti University of Medical Sciences, Iran. |
|
| Participants | Inclusion criteria: aged 18‐40 years, married, in monogamous relationship. Exclusion criteria: pregnant or lactating; menstruating at time of referral; not receiving clotrimazole; presence of condition improvement; using any vaginal medication, antibiotics, immunosuppressive drugs or exogenous hormones, including oral contraceptives during the 2 weeks before study initiation; intercourse or vaginal douche in previous 24 hours; other trichomonal vaginal infections or bacterial vaginosis; known systemic disease such as diabetes or autoimmune disease; positive potassium hydroxide samples in culture. Number enrolled: 102 women enrolled; 90 randomized. Numbers per group: fluconazole + oral protexin: 45; fluconazole + placebo: 45. No significant differences in mean age, age at marriage, marriage duration, age at first pregnancy, number of pregnancies, cesarean delivery, natural labor, abortion or curettage in the 2 groups according to the results of t‐test and Mann‐Whitney U test. |
|
| Interventions | Fluconazole + oral protexin group: 1 dose of fluconazole 300 mg (2 × 150 mg) + 2 oral protexin capsules per day (after meals in the morning and evening) for 3 days. Fluconazole + placebo group: 1 dose of fluconazole 300 mg (2 × 150 mg) + 2 oral placebo capsules daily (after meals in the morning and evening) for 3 days. |
|
| Outcomes | Follow‐up: referred to medical center 5‐7 days after start of treatment for re‐evaluation of clinical and laboratory symptoms. Outcomes:
|
|
| Notes | Study obtained written consent from all participants and had ethical clearance from review committee of hospital. No funding source or declaration of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "90 of the original 102 participants in this study were randomly classified into two treatment groups." Comment: no more information for judgment. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Subjects in these groups were codified." Comment: no more information for judgment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote 1: "Subjects in these groups were codified and the researcher was blinded to the groupings until the end of the study period." Quote 2: "The same protocol was used in the fluconazole‐oral protexin group, except that instead of placebo 20 protexin capsules were distributed." Comment: probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Subjects in these groups were codified and the researcher was blinded to the groupings until the end of the study period." Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of outcome data, and performed per protocol analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes of interest reported. |
| Other bias | Low risk | Study appeared free of other sources of bias. |
Yang 2009.
| Methods | Randomized controlled study. Setting: People's Hospital of Wuhai City, Wuhai, Inner Mongolia, China. Study period: January 2007 to December 2007. |
|
| Participants | Inclusion criteria: first episode of curd‐like vaginal discharge associated with any of the following symptoms and signs: vulvar itching, vulvar or vaginal burning, dyspareunia and vaginal mucosa hyperemia; vaginal samples positive for Candida species, hyphae or pseudohyphae by microscopic examination method. Sexual activity during treatment not allowed and they used condoms during follow‐up. Exclusion criteria: pregnant, lactating, diabetes, allergic responses to azole drugs, positive for trichomoniasis or mycoplasma infection. Number enrolled: 86 enrolled and randomized. Numbers per group: clotrimazole + probiotic: 44; clotrimazole alone: 42. Age (mean): 36 years (range 25‐48 years). Separate age data per group not available. |
|
| Interventions | Clotrimazole + probiotic group: 1 vaginal tablet of clotrimazole 500 mg on day 1 and day 4 + 1 vaginal capsule of Lactobacillus delbrueckii subsp. Lactis DM8909 (each capsule contained 0.25 × 106 colony forming units), QD from day 1 to day 10. Clotrimazole alone group: 1 vaginal tablet of clotrimazole 500 mg on day 1 and day 4. |
|
| Outcomes | Follow‐up: 7‐10 days and 1 month after completion of treatment, clinical and laboratory symptoms were re‐evaluated. Outcomes:
|
|
| Notes | Study obtained written consent from all participants and had ethical clearance from review committee of hospital. No funding source or declaration of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We contacted the author and found that they used a draw method (all participants were numbered on individual pieces of paper, then took the paper relevant to participant out of a container without seeing what was written on it). |
| Allocation concealment (selection bias) | High risk | No allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported no withdrawals, but there was not enough information for us to judge "yes" or "no" in intention‐to‐treat analysis (per protocol analysis) and consequences. |
| Selective reporting (reporting bias) | Unclear risk | No information available to make a judgment. |
| Other bias | Unclear risk | No available data on baseline characteristics for the 2 groups to judge the balance. (We contacted the original author and they reported no significant differences in baseline characteristics, but provided no detailed data.) |
Zhang 2005.
| Methods | Randomized controlled study. Setting: Rizhao Maternity and Child Healthcare Hospital of Shandong, Rizhao, Shandong province, China. Study period: May 2003 to August 2004. |
|
| Participants | Inclusion criteria: aged 25‐45 years; first episode of curd‐like vaginal discharge associated with any of the following symptoms and signs: vulvar itching, vulvar burning, dyspareunia, dysuria and vaginal mucosa hyperemia; vaginal samples positive for Candida species, hyphae or pseudohyphae by microscopic examination method; married; normal menstrual. Sexual activity during treatment and follow‐up not allowed. Exclusion criteria: received antibiotics within past week, vaginal drugs within 2 weeks prior to appointment, pregnant, lactating, other disease. Number enrolled: 200 women enrolled and randomized. Numbers per group: miconazole + probiotic: 100; miconazole alone: 100. No significant differences in baseline characteristics between groups. Age (mean ± SD): miconazole + probiotic: 33.3 ± 6.8 years; miconazole alone: 32.8 ± 5.7 years. Withdrawals: miconazole + probiotic: 4 due to using β‐lactam antibiotics during treatment (2 participants), severe vaginal burning, stinging or rash after first use of miconazole nitrate vaginal suppository (2 participants); miconazole alone: 5 due to using β‐lactam antibiotics during treatment (3 participants), severe vaginal burning, stinging or rash after first use of miconazole nitrate vaginal suppository (2 participants). Loss to follow‐up: miconazole + probiotic: 3 during treatment, 6 after treatment; miconazole alone: 2 during treatment, 8 after treatment. Original author did not use intention‐to‐treat analyses. |
|
| Interventions | Miconazole + probiotic group: 1 vaginal suppository of miconazole nitrate 200 mg, QD from day 1 to day 7, and 1 vaginal capsule of Lactobacillus delbrueckii subsp. Lactis DM8909 (each capsule contained 0.25 × 106 colony forming units), QD from day 8 to day 17. Miconazole alone group: 1 vaginal suppository of miconazole nitrate 200 mg, QD from day 1 to day 7. |
|
| Outcomes | Follow‐up: 1 and 3 months after completion of treatment, clinical and laboratory symptoms were re‐evaluated. Outcomes:
|
|
| Notes | Study obtained written consent from all participants and had ethical clearance from review committee of hospital. No funding source or declaration of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a draw method (all participants were numbered on individual piece of paper, then took the paper relevant to participant out of a container without seeing what was written on it). |
| Allocation concealment (selection bias) | High risk | Inadequate. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reported withdrawal rate of 6.5% during treatment and 7% after treatment (less than 20%), but the results were not analyzed on an intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | No information available to make a judgment. |
| Other bias | Low risk | Study appeared free of other sources of bias. |
Protexin: A probiotic production contained 7 strains of probiotic bacteria (Lactobacillus casei, Lactobacillus rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, Lactobacillus acidophilus, Bifidobacterium longum and Lactobacillus bulgaricus, made by Science and nature in balance Co, UK.
BV: bacterial vaginosis; QD: every day; QN: every night; SD: standard deviation; VVC: vulvovaginal candidiasis.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anukam 2009 | Participants included VVC mixed with RVVC, separate data not available for VVC. |
| Ehrstrom 2010 | Study focused on vaginal colonization of probiotic strains and prevention of recurrences of VVC. Participants included BV or VVC, or both, but separated outcome data of VVC were unavailable. |
| Fu 2009 | Retrospective control study. |
| Fu 2012 | Unable to contact authors on methodology used. Although full text was available, there was no detail information on methodology used for us to identify whether it was an RCT, and insufficient baseline data provided. |
| Li 2007 | Retrospective control study. |
| Liu 2008 | Not an RCT. |
| Nagornaya 2013 | Not an RCT. |
| Ozkinay 2005 | Compared probiotics in combination with low dose estriol versus placebo. |
| Patel 2008 | Compared probiotics in combination with clotrimazole and tinidazole versus polyherbal (a complementary and alternative medicine). |
| Shi 2012 | Not an RCT. |
| Song 2011 | Unable to contact authors on methodology used. Although full text was available, there was no detailed information on methodology for us to identify whether it was an RCT, and insufficient baseline data provided. |
| Wang 2010 | Not an RCT. |
| Wu 2013 | Unable to contact authors on methodology used. Although full text was available, there was no detail information on methodology for us to identify whether it was an RCT, and insufficient baseline data provided. |
| Yang 2005 | Not an RCT. |
| Zhang 2006 | Retrospective control study. |
| Zhang 2007 | Retrospective control study. |
BV: bacterial vaginosis; RCT: randomized controlled trial; RVVC: recurrent vulvovaginal candidiasis; VVC: vulvovaginal candidiasis.
Characteristics of studies awaiting assessment [ordered by study ID]
Sigridov 2007.
| Methods | Placebo‐controlled study. No mention of randomization. |
| Participants | 32 women aged 18‐32 years (12 with VVC, 20 with BV). |
| Interventions | Lactofem (viable lactobacilli) versus placebo. |
| Outcomes | Long‐term clinical cure rate (1 and 3 months after treatment). Rate of adverse events. |
| Notes | Potentially relevant study for inclusion from the title and abstract, but the original article or data were unavailable, so it cannot be assessed for inclusion until additional data or information are obtained. |
BV: bacterial vaginosis; VVC: vulvovaginal candidiasis.
Differences between protocol and review
We redefined the "Clinical cure rate" in the primary outcome as "disappearance of symptoms and signs, and no evidence of fungal infection proved by microscopic examination or vaginal culture", split into 'short‐term clinical cure rate (zero to 14 days after treatment)' and 'long‐term clinical cure rate (one, three and six months after treatment).'
We redefined the types of interventions, deleted the type of "any probiotic used alone versus placebo or no intervention" and "used as adjuvants to conventional antifungal drugs (before, during or after antifungal treatment) versus placebo or no intervention".
We moved "rate of serious adverse events" from secondary outcomes to primary outcomes.
We redefined "the rate of non‐serious adverse events" in the secondary outcomes as mild symptoms include vomiting, diarrhea, abdominal pain, abnormal urination, pelvic cramps, dysmenorrhea, paresthesia, rhinorrhea, headache, dizziness, fever, chills, vaginal burning, stinging, itching and irritation.
We changed the "Sensitivity analysis" in the methods section as "We planned to perform a sensitivity analysis to explore whether the results of the review were robust, depending on study quality, for each outcome variable. We excluded studies with a high risk of bias, comparing findings within the remainder of the included studies with the original meta‐analysis."
Contributions of authors
HX and DF developed the review and were in charge of searching for studies, quality assessment, data extraction and data analysis.
FF was in charge of data analysis and review development, and offered clinical expertise.
DW participated in searching for studies and quality assessment.
HC participated in searching for studies and quality assessment.
LM participated in data extraction and data analysis.
XW participated in data extraction and data analysis.
Sources of support
Internal sources
West China Second University Hospital, West China Women's and Children's Hospital, Sichuan University, China.
External sources
No sources of support supplied
Declarations of interest
HX: none.
DF: none.
DW: none.
LM: none.
HC: none.
XW: none.
FF: none.
New
References
References to studies included in this review
Han 2006 {published data only}
- Han YX, Zhao SW. Clinical observation of Ding Jun Sheng and clotrimazole vaginal tablet in the treatment of vulvovaginal candidiasis. Anhui Medical Journal 2006;27(6):528‐9. [Google Scholar]
Hua 2008 {published data only}
- Hua Y, Lin M, Wang LD, Xia L. Observation of curative effect of miconazole and lactobacillus on vulvovaginal candidiasis treated. Chinese Journal of Microecology 2008;20(4):386‐7. [Google Scholar]
Kovachev 2015 {published data only}
- Kovachev SM, Vatcheva‐Dobrevska RS. Local probiotic therapy for vaginal Candida albicans infections. Probiotics and Antimicrobial Proteins 2015;7(1):38‐44. [DOI] [PubMed] [Google Scholar]
Lin 2006 {published data only}
- Lin H, Meng XB. Clinical application of Lactasin capsules in vaginitis. Journal of Practical Medicine 2006;22(16):1927‐8. [Google Scholar]
Ma 2007 {published data only}
- Ma L, Li L. Miconazole plus Lactasin capsules to treat vulvovaginal candidiasis (54 cases). Herald of Medicine 2007;26(9):1041‐2. [Google Scholar]
Mai 2007 {published data only}
- Mai XY. Analysis of probiotic lactobacillus's effect on vulvovaginal candidiasis. Chinese Journal of Microecology 2007;19(4):362‐3. [Google Scholar]
Martinez 2009 {published data only}
- Martinez RC, Franceschini SA, Patta MC, Quintana SM, Candido RC, Ferreira JC, et al. Improved treatment of vulvovaginal candidiasis with fluconazole plus probiotic Lactobacillus rhamnosus GR‐1 and Lactobacillus reuteri RC‐14. Letters in Applied Microbiology 2009;48(3):269‐74. [DOI] [PubMed] [Google Scholar]
Nouraei 2012 {published data only}
- Nouraei S, Amir Ali Akbari S, Jorjani M, Alavi Majd H, Afrakhteh M, Ghafoorian, A, et al. Comparison between fluconazole with oral protexin combination and fluconazole in the treatment of vulvovaginal candidiasis. ISRN Obstetrics and Gynecology 2012;2012:375806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2009 {published data only}
- Yang Q, Zhao WF, Zheng JP. Clinical observation of mycobacterium lacticola preparation in treatment of vaginal vini candidiasis using clotrimazole effervescent tablets. Chinese Journal of Hospital Pharmacy 2009;29(16):1377‐9. [Google Scholar]
Zhang 2005 {published data only}
- Zhang CX, Gao YT, Gao HL. Preventive effect of probiotic Lactobacillus on vulvovaginal candidiasis. Maternal and Child Health Care of China 2005;20(17):2239‐40. [Google Scholar]
References to studies excluded from this review
Anukam 2009 {published data only}
- Anukam KC, Duru MU, Eze CC, Egharevba J, Aiyebelehin A, Bruce A, et al. Oral use of probiotics as an adjunctive therapy to fluconazole in the treatment of yeast vaginitis: a study of Nigerian women in an outdoor clinic. Microbial Ecology in Health and Disease 2009;21(2):72‐77. [Google Scholar]
Ehrstrom 2010 {published data only}
- Ehrstrom S, Daroczy K, Rylander E, Samuelsson C, Johannesson U, Anzen B, et al. Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microbes and Infection 2010;12(10):691‐9. [DOI] [PubMed] [Google Scholar]
Fu 2009 {published data only}
- Fu JH. Effect observation of two treatment methods for vulvovaginal candidiasis. Contemporary Medicine 2009;15(32):95. [Google Scholar]
Fu 2012 {published data only}
- Fu YJ. Effect observation of Ding Jun Sheng plus clotrimazole to treat Candida vaginitis. Journal of Henan Medical College for Staff and Workers 2012;24(6):760‐1. [Google Scholar]
Li 2007 {published data only}
- Li M, Hu LQ, Yang JZ. Clinical observation of Daktarin vaginal suppository and (or) Ding Jun Sheng to treat vulvovaginal candidiasis. Journal of Gannan Medical College 2007;27(4):606‐7. [Google Scholar]
Liu 2008 {published data only}
- Liu JF, Zuo ZH, Song L, Zhang JQ, Wang JL. Clinical observation of Lactobacillus preparation for vulvovaginal candidiasis. Maternal and Child Health Care of China 2008;23:4644‐6. [Google Scholar]
Nagornaya 2013 {published data only}
- Nagornaya VF, Baylo NV. Pathogenetic treatment of candidal vulvovaginitis. China Journal of Modern Medicine 2013;23(2):21‐4. [Google Scholar]
Ozkinay 2005 {published data only}
- Ozkinay E, Terek MC, Yayci M, Kaiser R, Grob P, Tuncay G. The effectiveness of live lactobacilli in combination with low dose oestriol (Gynoflor) to restore the vaginal flora after treatment of vaginal infections. BJOG 2005;112(2):234‐40. [DOI] [PubMed] [Google Scholar]
Patel 2008 {published data only}
- Patel Y, Gopalan S, Bagga R, Sharma M, Chopra S, Sethi S. A randomized trial comparing a polyherbal pessary (a complementary and alternative medicine) with Ginlac‐V pessary (containing clotrimazole, tinidazole and lactobacilli) for treatment of women with symptomatic vaginal discharge. Archives of Gynecology and Obstetrics 2008;278(4):341‐7. [DOI] [PubMed] [Google Scholar]
Shi 2012 {published data only}
- Shi HC. The clinical effect of live preparation of Lactobacillus in treatment of vulvovaginal candidiasis and the recurrence rate. Chinese Journal of Microecology 2012;24(3):278‐80. [Google Scholar]
Song 2011 {published data only}
- Song LP. Effect of fluconazole combined Lactobacillus in treatment of vulvovaginal candidiasis. Journal of Modern Clinical Medicine 2011;37(3):181‐2. [Google Scholar]
Wang 2010 {published data only}
- Wang LP. The treatment and prevention of recurrence of vulvovaginal candidiasis. Strait Journal of Preventive Medicine 2010;16(1):89‐90. [Google Scholar]
Wu 2013 {published data only}
- Wu HY, Hu MC, Qu HM, Sun LH. Lactasin capsules for postpartum vaginitis. Chinese Journal of New Drugs 2013;22(7):819‐21. [Google Scholar]
Yang 2005 {published data only}
- Yang LQ. Ding Jun Sheng used in the inhibition of vaginal yeast infection recurrence. Hainan Medical Journal 2005;16(10):102. [Google Scholar]
Zhang 2006 {published data only}
- Zhang J, Ying P. Effect observation of Ding Jun Sheng to treat colpitis mycotica. Modern Journal of Integrated Traditional Chinese and Western Medicine 2006;15(2):144‐5. [Google Scholar]
Zhang 2007 {published data only}
- Zhang DY, Lin L, Zhang HX. Clinical observation of Ding Jun Sheng plus nystatin to treat vulvovaginal candidiasis. Progress in Obstetrics and Gynecology 2007;16(7):555‐7. [Google Scholar]
References to studies awaiting assessment
Sigridov 2007 {published data only}
- Sigridov I, Ivanov S, Batashki I. Preliminary results of Lacofem trial ‐ first impressions. Akusherstvo i Ginekologiia 2007;46 Suppl 2:29‐32. [PubMed] [Google Scholar]
Additional references
Anderson 2004
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA 2004;291:1368. [DOI] [PubMed] [Google Scholar]
Andreu 2004
- Andreu A. Lactobacillus as a probiotic for preventing urogenital infections. Reviews in Medical Microbiology 2004;15:1‐6. [Google Scholar]
Beigi 2004
- Beigi RH, Meyn LA, Moore DM, Krohn MA, Hillier SL. Vaginal yeast colonization in nonpregnant women: a longitudinal study. Obstetrics and Gynecology 2004;104:926‐30. [DOI] [PubMed] [Google Scholar]
Bertholf 1983
- Bertholf ME, Stafford MJ. Colonization of Candida albicans in vagina, rectum, and mouth. Journal of Family Practice 1983;16:919‐24. [PubMed] [Google Scholar]
Bieber 2006
- Bieber EJ, Sanfilippo JS, Horowitz IR. Clinical Gynecology. Philadelphia (PA): Churchill Livingstone, 2006. [Google Scholar]
CDC 2015
- Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. Journal of the Mississippi State Medical Association 2015;56(12):372‐5. [PubMed] [Google Scholar]
Cui 2010
- Cui L, Wang C, Fu J, Xie LX, Hu LN. Miconazole versus miconazole plus living preparation of Lactobacillus for vulvovaginal candidiasis: a systematic review. Chinese Journal of Evidence‐Based Medicine 2010;10(1):89‐93. [Google Scholar]
Doron 2006
- Doron S, Gorbach SL. Probiotics: their role in the treatment and prevention of disease. Expert Review of Anti‐Infective Therapy 2006;4(2):261‐75. [DOI] [PubMed] [Google Scholar]
Eckert 1998
- Eckert LO, Hawes SE, Stevens CE, Koutsky LA, Eschenbach DA, Holmes KK. Vulvovaginal candidiasis: clinical manifestations, risk factors, management algorithm. Obstetrics and Gynecology 1998;92:757‐65. [DOI] [PubMed] [Google Scholar]
Falagas 2006
- Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. Journal of Antimicrobial Chemotherapy 2006;58(2):266‐72. [DOI] [PubMed] [Google Scholar]
Fichorova 2011
- Fichorova RN, Yamamoto HS, Delaney ML, Onderdonk AB, Doncel GF. Novel vaginal microflora colonization model providing new insight into microbicide mechanism of action. MBio 2011;2(6):e00168‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foxman 1998
- Foxman B, Marsh JV, Gillespie B, Sobel JD. Frequency and response to vaginal symptoms among white and African American women: results of a random digit dialing survey. Journal of Womens Health 1998;7:1167‐74. [DOI] [PubMed] [Google Scholar]
Foxman 2000
- Foxman B, Barlow R, D'Arcy H, Gillespie B, Sobel JD. Candida vaginitis: self‐reported incidence and associated costs. Sexually Transmitted Diseases. 2000;27:230‐5. [DOI] [PubMed] [Google Scholar]
Geiger 1995
- Geiger AM, Foxman B, Gillespie BW. The epidemiology of vulvovaginal candidiasis among university students. American Journal of Public Health 1995;85(8 Pt 1):1146‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giraldo 2007
- Giraldo PC, Babula O, Gonçalves AK, Linhares IM, Amaral RL, Ledger WJ, et al. Mannose‐binding lectin gene polymorphism, vulvovaginal candidiasis, and bacterial vaginosis. Obstetrics and Gynecology 2007;109(5):1123‐8. [DOI] [PubMed] [Google Scholar]
Hettiarachchi 2010
- Hettiarachchi N, Ashbee HR, Wilson JD. Prevalence and management of non‐albicans vaginal candidiasis. Sexually Transmitted Infections 2010;86(2):99‐100. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hillier 1997
- Hillier SL, Lau RJ. Vaginal microflora in postmenopausal women who have not received estrogen replacement therapy. Clinical Infectious Diseases 1997;25(Suppl 2):S123‐6. [DOI] [PubMed] [Google Scholar]
Ilkit 2011
- Ilkit M, Guzel AB. The epidemiology, pathogenesis, and diagnosis of vulvovaginal candidosis: a mycological perspective. Critical Reviews in Microbiology 2011;37(3):250‐61. [DOI] [PubMed] [Google Scholar]
Jeavons 2003
- Jeavons HS. Prevention and treatment of vulvovaginal candidiasis using exogenous Lactobacillus. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2003;32(3):287‐96. [DOI] [PubMed] [Google Scholar]
Kaewsrichan 2006
- Kaewsrichan J, Peeyananjarassri K, Kongprasertkit J. Selection and identification of anaerobic lactobacilli producing inhibitory compounds against vaginal pathogens. FEMS Immunology and Medical Microbiology 2006;48(1):75‐83. [DOI] [PubMed] [Google Scholar]
Kledanoff 1991
- Kledanoff SJ, Hillier SL, Eschenbach DA, Waltersdorph AM. Control of the microbial flora of the vagina by H2O2 generating lactobacilli. Journal of Infectious Diseases 1991;164(1):94‐100. [DOI] [PubMed] [Google Scholar]
Kopp‐Hoolihan 2001
- Kopp‐Hoolihan L. Prophylactic and therapeutic uses of probiotics: a review. Journal of the American Dietetic Association 2001;101(2):229‐38. [DOI] [PubMed] [Google Scholar]
Mahmoudi 2011
- Mahmoudi Rad M, Zafarghandi S, Abbasabadi B, Tavallaee M. The epidemiology of Candida species associated with vulvovaginal candidiasis in an Iranian patient population. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2011;155(2):199‐203. [DOI] [PubMed] [Google Scholar]
Martinez 2009a
- Martinez RC, Franceschini SA, Patta MC, Quintana SM, Candido RC, Ferreira JC, et al. Improved treatment of vulvovaginal candidiasis with fluconazole plus probiotic Lactobacillus rhamnosus GR‐1 and Lactobacillus reuteri RC‐14. Letters in Applied Microbiology 2009;48(3):269‐74. [DOI] [PubMed] [Google Scholar]
Martinez 2009b
- Martinez RC, Seney SL, Summers KL, Nomizo A, De Martinis EC, Reid G. Effect of Lactobacillus rhamnosus GR‐1 and Lactobacillus reuteri RC‐14 on the ability of Candida albicans to infect cells and induce inflammation. Microbiology and Immunology 2009;53(9):487‐95. [DOI] [PubMed] [Google Scholar]
Mylonas 2011
- Mylonas I, Bergauer F. Diagnosis of vaginal discharge by wet mount microscopy: a simple and underrated method. Obstetrical & Gynecological Survey 2011;66(6):359‐68. [DOI] [PubMed] [Google Scholar]
Nurbhai 2007
- Nurbhai M, Grimshaw J, Watson M, Bond C, Mollison J, Ludbrook A. Oral versus intra‐vaginal imidazole and triazole anti‐fungal treatment of uncomplicated vulvovaginal candidiasis (thrush). Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD002845.pub2] [DOI] [PubMed] [Google Scholar]
Nyirjesy 2003
- Nyirjesy P, Sobel JD. Candidal vulvovaginitis. Obstetrics and Gynecology Clinics of North America 2003;30(4):671‐84. [DOI] [PubMed] [Google Scholar]
Nyirjesy 2008
- Nyirjesy P. Vulvovaginal candidiasis and bacterial vaginosis. Infectious Disease Clinics of North America 2008;22(4):637‐52. [DOI] [PubMed] [Google Scholar]
Omar 2001
- Omar AA. Gram stain versus culture in the diagnosis of vulvovaginal candidiasis. Eastern Mediterranean Health Journal 2001;7(6):925‐34. [PubMed] [Google Scholar]
Othman 2007
- Othman M, Neilson JP, Alfirevic Z. Probiotics for preventing preterm labour. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005941.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pappas 2009
- Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, Edwards JE Jr, et al. Infectious Diseases Society of America. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clinical Infectious Diseases 2009;48(5):503‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analysis of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Pirotta 2004
- Pirotta M, Gunn J, Chondros P, Grover S, O'Malley P, Hurley S, et al. Effect of Lactobacillus in preventing post‐antibiotic vulvovaginal candidiasis: a randomized controlled trial. BMJ 2004;329:548‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Redondo‐Lopez 1990
- Redondo‐Lopez V, Cook RL, Sobel JD. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Reviews of Infectious Diseases 1990;12(5):856‐72. [DOI] [PubMed] [Google Scholar]
Reid 2001a
- Reid G, Bruce AW. Selection of Lactobacillus strains for urogenital probiotic applications. Journal of Infectious Diseases 2001;183(Suppl 1):77‐80. [DOI] [PubMed] [Google Scholar]
Reid 2001b
- Reid G, Bruce AW, Fraser N, Heinemann C, Owen J, Henning B. Oral probiotics can resolve urogenital infections. FEMS Immunology and Medical Microbiology 2001;30(1):49‐52. [DOI] [PubMed] [Google Scholar]
Reid 2001c
- Reid G, Beuerman D, Heinemann C, Bruce AW. Probiotic Lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunology and Medical Microbiology 2001;32(1):37‐41. [DOI] [PubMed] [Google Scholar]
Reid 2003a
- Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clinical Microbiology Reviews 2003;16(4):658‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reid 2003b
- Reid G, Charbonneau D, Erb J, Kochanowski B, Beuerman D, Poehner R, et al. Oral use of Lactobacillus rhamnosus GR‐1 and L. fermentum RC‐14 significantly alters vaginal flora: randomized, placebo‐controlled trial in 64 healthy women. FEMS Immunology and Medical Microbiology 2003;35(2):131‐4. [DOI] [PubMed] [Google Scholar]
Reid 2003c
- Reid G, Bruce AW. Urogenital infections in women: can probiotics help?. Postgraduate Medical Journal 2003;79(934):428‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reid 2004
- Reid G, Burton J, Devillard E. The rationale for probiotics in female urogenital healthcare. Medscape General Medicine 2004;6(1):49. [PMC free article] [PubMed] [Google Scholar]
Reid 2005
- Reid G, Hammond JA. Probiotics: some evidence of their effectiveness. Canadian Family Physician 2005;51(11):1487‐93. [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rose 2012
- Rose WA 2nd, McGowin CL, Spagnuolo RA, Eaves‐Pyles TD, Popov VL, Pyles RB. Commensal bacteria modulate innate immune responses of vaginal epithelial cell multilayer cultures. PloS One 2012;7(3):e32728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ross 1995
- Ross RA, Lee ML, Onderdonk AB. Effect of Candida albicans infection and clotrimazole treatment on vaginal microflora in vitro. Obstetrics and Gynecology 1995;86(6):925‐30. [DOI] [PubMed] [Google Scholar]
Sanders 2008
- Sanders ME. Probiotics: definition, sources, selection, and uses. Clinical Infectious Diseases 2008;46(Suppl 2):S58‐61. [DOI] [PubMed] [Google Scholar]
Sanglard 2002
- Sanglard D, Odds FC. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infectious Diseases 2002;2(2):73‐85. [DOI] [PubMed] [Google Scholar]
Santosa 2006
- Santosa S, Farnworth E, Jones PJ. Probiotics and their potential health claims. Nutrition Reviews 2006;64(6):265‐74. [DOI] [PubMed] [Google Scholar]
Schaaf 1990
- Schaaf VM, Perez‐Stable EJ, Borchardt K. The limited value of symptoms and signs in the diagnosis of vaginal infections. Archives of Internal Medicine 1990;150:1929. [PubMed] [Google Scholar]
Senok 2005
- Senok AC, Ismaeel AY, Botta GA. Probiotics: facts and myths. Clinical Microbiology and Infection 2005;11(12):958‐66. [DOI] [PubMed] [Google Scholar]
Senok 2008
- Senok A, Verstraelen H, Temmerman M, Botta G. Probiotics for the treatment of bacterial vaginosis. Cochrane Database of Systematic Reviews 2008, Issue 12. [DOI: 10.1002/14651858.CD006289.pub2] [DOI] [PubMed] [Google Scholar]
Sobel 1998
- Sobel JD, Faro S, Force RW, Foxman B, Ledger WJ, Nyirjesy PR, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. American Journal of Obstetrics and Gynecology 1998;178(2):203‐11. [DOI] [PubMed] [Google Scholar]
Sobel 2007
- Sobel JD. Candidal vulvovaginitis. Lancet 2007;369(9577):1961‐71. [DOI] [PubMed] [Google Scholar]
Soll 1989
- Soll DR, Galask R, Isley S, Rao TV, Stone D, Hicks J, et al. Switching of Candida albicans during successive episodes of recurrent vaginitis. Journal of Clinical Microbiology 1989;27(4):681‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spinillo 1994
- Spinillo A, Nicola S, Colonna L, Marangoni E, Cavanna C, Michelone G. Frequency and significance of drug resistance in vulvovaginal candidiasis. Gynecologic and Obstetric Investigation 1994;38(2):130‐3. [DOI] [PubMed] [Google Scholar]
Trama 2005
- Trama JP, Adelson ME, Raphaelli I, Stemmer SM, Mordechai E. Detection of Candida species in vaginal samples in a clinical laboratory setting. Infectious Diseases in Obstetrics and Gynecology 2005;13(2):63‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Kessel 2003
- Kessel K, Assefi N, Marrazzo J, Eckert L. Common complementary and alternative therapies for yeast vaginitis and bacterial vaginosis: a systematic review. Obstetrical & Gynecological Survey 2003;58(5):351‐8. [DOI] [PubMed] [Google Scholar]
Vanderhoof 2008
- Vanderhoof JA, Young R. Probiotics in the United States. Clinical Infectious Diseases 2008;46(Suppl 2):S67‐72. [DOI] [PubMed] [Google Scholar]
Zhang 2008
- Zhang HY, Lv X, Li Y. Safety and clinical use of microecological preparations. Adverse Drug Reactions Journal 2008;10(5):340‐5. [Google Scholar]