Abstract
Background
Pregnancies with pre‐existing diabetes are high risk, with increased risk of poorer fetal, neonatal, and maternal outcomes. Identifying interventions to improving health outcomes for women with diabetes and their infants is a priority, as rates of diabetes continue to increase.
Exercise has been shown to have benefits for non‐pregnant individuals with pre‐existing type 2 diabetes, such as improving glycaemic control, and reducing visceral adipose tissue and plasma triglycerides. For pregnant women with pre‐existing diabetes, the effects of exercise interventions on the mother and her baby are unknown.
An earlier Cochrane review on 'Exercise for pregnant women with diabetes' considered both pre‐existing diabetes and gestational diabetes. That Cochrane review has now been split into two new reviews (following new protocols) ‐ one on gestational diabetes and one on pre‐existing diabetes (this review).
Objectives
To evaluate the effects of exercise interventions for improving maternal and fetal outcomes in women with pre‐existing diabetes.
Search methods
We searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform (ICTRP) on 27 June 2017, and reference lists of retrieved studies.
Selection criteria
We had planned to include published or unpublished randomised controlled trials (RCT) or cluster‐randomised trials, in full text or abstract format that compared any type of exercise programme, added to standard care, targeted at women with known pre‐gestational diabetes (type 1 or type 2 diabetes), at any stage of pregnancy, compared with 1) standard care alone or 2) standard care plus another exercise intervention. Quasi‐randomised and cross‐over trials were excluded. Conference abstracts were handled in the same way as full‐text publications.
Women with gestational diabetes mellitus were excluded, as they were covered in a separate Cochrane review.
Data collection and analysis
We had planned that two review authors would independently assess all the potential studies we identified as a result of the search strategy. For eligible studies, two review authors would have independently extracted the data using an agreed form. We had planned to resolve discrepancies through discussion, or by consulting a third person. We also had planned to assess the evidence using the GRADE approach.
Main results
We did not identify any randomised controlled trials.
Authors' conclusions
There was no evidence from RCTs that evaluated the effects of exercise interventions for improving maternal and fetal outcomes in women with pre‐existing diabetes.
Good quality, large randomised controlled trials are urgently needed to identify exercise interventions that are safe, and improve health outcomes for women with pre‐existing diabetes and their babies. Future studies in this area could utilise the standardised outcomes in this review, in order to improve consistency between trials in this area, and aid future meta‐analysis.
Plain language summary
Exercise for improving outcomes for women with pre‐existing diabetes and their babies
What is the issue?
Diabetes mellitus can be caused by autoimmune destruction of the cells producing insulin, so that levels are reduced (type 1 diabetes), or the body tissues becoming resistant to insulin (type 2 diabetes). The end result is increased blood glucose levels. Insulin is used to regulate glucose levels for pregnant women with type 1 diabetes. For women with type 2 diabetes, lifestyle changes, including diet and exercise, are an important part of treatment. An oral anti‐diabetic drug (medication that aims to reduce blood sugar levels) or insulin may be added to lower blood glucose levels. We set out to evaluate the effects of exercise interventions, for pregnant women with pre‐existing type 1 or 2 diabetes, on birth outcomes for the mother and her baby. An earlier review on the effects of exercise on diabetes during pregnancy has been split into two reviews ‐ one for women with gestational diabetes, and this review, on women with pre‐existing diabetes.
Why is this important?
Women with diabetes, who become pregnant, are at increased risk of pregnancy loss, or having a baby that is large‐for‐gestational age (baby is larger than would be expected for the number of weeks of pregnancy), is born preterm, who dies around the time of birth, or is born with birth defects. The newborn baby may also have blood sugar levels that are lower than normal, low calcium levels, and excess bilirubin in the blood. Long‐term follow‐up of the infants of diabetic mothers suggests that they are at increased risk of obesity and type 2 diabetes when older.
The number of women who already have diabetes when they become pregnant is increasing, and identifying ways to improve health outcomes for women with diabetes and their babies is a priority. We already know that exercise may be of benefit for non‐pregnant women with type 2 diabetes, as it improves their blood glucose levels and reduces triglyceride fats in the blood. We are unclear if exercise benefits, and is safe for, pregnant women with pre‐existing diabetes and their babies. Physical activity could help to increase fitness and prevent stress urinary incontinence, lower back pain, or depression, and control weight gain during pregnancy.
What evidence did we find?
We searched for evidence on 27 June 2017. We did not identify any randomised controlled trials (RCT) that compared any type of exercise programme (plus standard care) for pregnant women with pre‐existing diabetes with 1) standard care alone, or 2) standard care plus another exercise programme.
What does this mean?
There is no evidence from RCTs to evaluate the effects of exercise interventions for improving mother and baby outcomes in women with pre‐existing diabetes.
Good quality, large studies are urgently needed to find out if exercise interventions are safe, and if they improve health outcomes for pregnant women with diabetes and their babies. Future studies in this area could utilise the outcomes listed in this review, to improve consistency between trials in this area, and aid future analyses.
Background
The original review, Exercise for diabetic pregnant women (Ceysens 2006), has now been split into two new reviews (following new protocols), to reflect the role of exercise for pregnant women with gestational diabetes and for pregnant women with pre‐existing diabetes.
Exercise for pregnant women with gestational diabetes for improving maternal and fetal outcomes (Ceysens 2016)
Exercise for pregnant women with pre‐existing diabetes for improving maternal and fetal outcomes (this review)
There will be similarities in the background, methods and outcomes between these two systematic reviews. Portions of the methods section of this protocol are based on a standard template used by the Cochrane Pregnancy and Childbirth Review Group.
Description of the condition
Diabetes and pregnancy
It is estimated that 2% to 5% of pregnant women have pre‐existing or gestational diabetes (CEMACH 2007). Up to 0.4% of women in the UK and 0.9% of pregnant women in the USA have pre‐existing diabetes (type 1 or type 2; CEMACH 2007; Correa 2015). The prevalence of type 1 and type 2 diabetes is increasing. The number of pregnant women with pre‐existing type 2 diabetes more than quadrupled in the USA between 1994 and 2004, overtaking the rates of pre‐existing type 1 diabetes (0.42% versus 0.33%; Albrecht 2010). This increase in type 2 diabetes in pregnant women has been partly attributed to increasing obesity and older mothers (ACOG 2005; Zhu 2016). Type 2 diabetes has particularly increased in certain minority ethnic groups (including people of African, black Caribbean, South Asian, Middle Eastern, and Chinese family origin; CEMACH 2007). An association between type 2 diabetes and socioeconomic status has also been noted (Correa 2015; Murphy 2009).
Type 1 diabetes tends to begin in childhood, while type 2 diabetes mellitus usually occurs later in adulthood. In type 1 diabetes, sudden onset of insulin deficiency is believed to be caused by an autoimmune attack of the insulin‐producing pancreatic beta‐cells (cells that store and release insulin; Galerneau 2004; Griffith 2004). In type 2 diabetes, tissues become resistant to the action of insulin and insulin secretion is disrupted, leading to increasing levels of glucose in the blood (Dunne 2005).
Gestational diabetes is characterised by glucose intolerance that begins, or is first detected, during pregnancy. The incidence of gestational diabetes is also increasing as a result of higher rates of obesity in the general population, and more pregnancies in older women. After a pregnancy with gestational diabetes, women have an increased risk of progression to ‘pre‐existing’ diabetes (i.e. type 2 diabetes) in the next pregnancy (Khambalia 2013).
Adverse outcomes for women and infants associated with pre‐existing diabetes
Pregnancies with pre‐existing diabetes are high risk, with increased risk of poorer fetal, neonatal, and maternal outcomes (Owens 2015). Women with type 1 diabetes have an elevated risk of pregnancy loss, perinatal mortality, fetal macrosomia (a fetus that is large‐for‐gestational age), and congenital malformations (NICE 2015; Platt 2002). This is also the case for women with pre‐existing type 2 diabetes (CEMACH 2007; Inkster 2006), although neonatal outcomes may be poorer when the mother has type 1 diabetes (Owens 2015). However, a systematic review found perinatal mortality to be higher for women with type 2 compared with type 1 diabetes (odds ratio 1.50, 95% confidence interval 1.15 to 1.96; Balsells 2009).
Organogenesis (development of organs) in early pregnancy can be affected by metabolic disruptions when there are high concentrations of maternal blood glucose. Cardiovascular malformations are the most common birth defects in infants born to diabetic mothers (Inkster 2006). Apart from macrosomia (high birthweight, often defined as more than 4000 g), other adverse outcomes for infants may include large‐for‐gestational age, shoulder dystocia (difficulty in delivering shoulders of baby), neonatal hypoglycaemia (blood sugar that is lower than normal), preterm birth, hyperbilirubinaemia (excess bilirubin), hypocalcaemia (lower than normal calcium), and neonatal intensive care admission (Jensen 2004; Macintosh 2006; Ray 2001; Walkinshaw 2005; Weintrob 1996). Long‐term follow‐up of the infants of diabetic mothers suggests that they also may have an increased risk of obesity and type 2 diabetes when older (Dabelea 2000).
In pregnant women with type 1 (insulin‐dependent) diabetes, insulin is used to control fluctuations in blood glucose concentrations throughout the day (Galerneau 2004). In type 2 diabetes, lifestyle changes (including diet and exercise) are the first line of treatment, with the option of using oral hypoglycaemic agents or insulin to lower blood glucose, if necessary. Therefore, management of diabetes in pregnancy aims for control of glucose concentrations, using careful combinations of diet, exercise, and insulin or other anti‐diabetogenic drugs (medication that aims to reduce blood sugar levels), if required (ACOG 2005; NICE 2015).
Description of the intervention
The American College of Sports Medicine defines physical activity as any bodily movement that is produced as a result of the contraction of skeletal muscle, and defines exercise as physical activity comprising planned, structured, and repetitive body movements, which are undertaken to improve one or more components of physical fitness (ACSM 2014).
Physical activity in non‐diabetic pregnant women has been shown to be beneficial. It has not been shown to be harmful to the fetus, and can potentially lead to long‐term health benefits for the mother. Observed benefits include cardio‐respiratory fitness, prevention of stress urinary incontinence, prevention of lumbar pain, decreased depression, and control of weight gain during pregnancy (Nascimento 2012).
In women with type 2 diabetes who were not pregnant, physical activity, combined with diet and hypoglycaemic medication, has been shown to be effective in maintaining glycaemic control (Tuomilehto 2001).
This evidence may not be generalisable to pregnant women with pre‐existing diabetes, but it does suggest that mild exercise during pregnancy may have the potential to reduce the risk of complications associated with pre‐existing diabetes.
The American College of Obstetricians and Gynecologists notes that physical activity during pregnancy appears to have benefits for most women and has few risks associated with it, although some adaptation may be required, due to anatomical and physiological changes in pregnancy (ACOG 2015). They recommend that pregnant women have a clinical evaluation prior to starting an exercise programme, to ensure that there are no medical contraindications, and that women be encouraged to participate in aerobic and strength‐conditioning exercises before, during, and after uncomplicated pregnancies.
ACOG 2015 recommends that aerobic exercise during pregnancy is contraindicated in a number of medical conditions, including:
cardiac disease;
restrictive lung disease;
incompetent cervix or cerclage;
multiple gestation at risk of preterm birth;
persistent second or third trimester bleeding;
placenta praevia after 26 weeks' gestation;
preterm labour (current pregnancy);
ruptured membranes;
pre‐eclampsia or pregnancy‐induced hypertension;
severe anaemia.
ACOG 2015 considers these activities safe to continue with, or initiate during an uncomplicated pregnancy, following medical advice:
walking;
swimming;
stationary cycling;
low‐impact aerobics;
modified yoga (avoiding positions that result in decreased venous return);
modified Pilates;
racquet sports;
running or jogging;
strength training.
However, running, jogging, or strength training should be undertaken only after consultation with an obstetrical care provider (ACOG 2015). During pregnancy, the duration, frequency, and intensity of physical activity may have to be modified (Nascimento 2012).
ACOG 2015 recommends avoiding these activities during pregnancy:
contact sports (e.g. ice hockey, soccer, boxing);
activities with a high risk of falling (e.g. skiing, surfing, off‐road cycling);
scuba diving;
sky diving;
'hot yoga' or 'hot Pilates'.
How the intervention might work
Physical activity may improve glycaemic control in those with types 1 and 2 diabetes because of the interaction between insulin sensitivity and the uptake of glucose by skeletal muscles (Asano 2014). Skeletal muscle takes glucose from the blood using a membrane transporter; improved insulin sensitivity following regular physical activity can increase the efficiency of this transport mechanism (Chibalin 2000; Dela 1993; Hjeltnes 1998).
Why it is important to do this review
Pre‐existing diabetes during pregnancy is associated with short‐ and long‐term adverse effects for the woman and her infant. Identifying interventions to improving health outcomes for women with diabetes and their infants is a priority, as rates of diabetes continue to increase. Exercise has been shown to have benefits for non‐pregnant individuals with pre‐existing type 2 diabetes, such as improving glycaemic control, and reducing visceral adipose tissue (fat tissue located deep in the abdomen and around internal organs) and plasma triglycerides (fatty molecules found in the blood; Thomas 2006). The benefits and safety for a woman during pregnancy and for her baby remains unclear.
Objectives
To evaluate the effects of exercise interventions for improving maternal and fetal outcomes in women with pre‐existing diabetes.
Methods
Criteria for considering studies for this review
Types of studies
We had planned to include published or unpublished randomised controlled trials or cluster‐randomised trials in full‐text or abstract format. Quasi‐randomised and cross‐over trials were not eligible for inclusion; conference abstracts were handled in the same way as full‐text publications.
Types of participants
Pregnant women diagnosed with pre‐gestational diabetes (type 1 or type 2 diabetes), as defined by the trialists.
Women with gestational diabetes mellitus (GDM) were excluded, as they will be covered in a separate Cochrane review (Ceysens 2016).
Types of interventions
We had planned to include any type of exercise programme, added to standard care, targeted at women with known pre‐gestational diabetes (type 1 or type 2 diabetes), at any stage of pregnancy, compared with 1) standard care alone or 2) standard care plus another exercise intervention.
Types of outcome measures
Primary outcomes
Mother
hypertensive disorders of pregnancy (as reported by the trialists, and including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia);
caesarean section.
Neonate and infant
large‐for‐gestational age (more than 4 kg);
perinatal mortality (stillbirth and neonatal mortality);
mortality or morbidity composite (variously defined by trials, e.g. perinatal or infant death, shoulder dystocia, bone fracture, or nerve palsy);
neurosensory disability (defined as any of the following: legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, developmental delay or impairment (defined as developmental quotient less than two standard deviations (SDs) below the mean).
Secondary outcomes
Short‐term maternal outcomes
induction of labour;
perineal trauma;
placental abruption;
postpartum haemorrhage (more than 500 mL blood loss, or otherwise defined by trialists);
postpartum infection (as defined by trialists);
weight gain during pregnancy;
adherence to the intervention;
behaviour changes associated with the intervention;
relevant biomarker changes associated with the intervention (e.g. adiponectin, free fatty acids, triglycerides, high density lipoproteins, low density lipoproteins, insulin);
sense of well‐being and quality of life (as defined by trialists);
views of the intervention;
breastfeeding (e.g. at discharge, six weeks postpartum);
use of additional pharmacotherapy;
glycaemic control during and at the end of treatment (as defined by trialists);
maternal hypoglycaemia;
maternal mortality.
Long‐term maternal outcomes
postnatal depression (as defined by trialists);
postnatal weight retention or return to pre‐pregnancy weight;
body mass index (BMI);
cardiovascular health (as defined by trialists, including blood pressure (BP), hypertension, cardiovascular disease, metabolic syndrome).
Neonatal and infant outcomes
stillbirth;
neonatal mortality;
gestational age at birth;
preterm birth (less than 37 weeks' gestation, and less than 32 weeks' gestation);
Apgar score (less than seven at five minutes);
macrosomia (higher than 90th percentile);
small‐for‐gestational age (lower than 10th percentile);
birthweight and z‐score;
head circumference and z‐score;
length and z‐score;
Ponderal index;
adiposity (including skin fold thickness, neonatal fat mass);
shoulder dystocia;
bone fracture;
nerve palsy;
respiratory distress syndrome;
hypoglycaemia requiring treatment (as defined by trialists);
hyperbilirubinaemia (as defined by trialists);
neonatal hypocalcaemia (as defined by trialists);
polycythaemia (as defined by trialists);
relevant biomarker changes associated with the intervention (e.g. cord C‐peptide, cord insulin).
Later infant and childhood outcomes
weight and z‐scores;
height and z‐scores;
head circumference and z‐scores;
adiposity (e.g. as measured by BMI, skinfold thickness);
BP;
type 1 diabetes;
type 2 diabetes;
impaired glucose tolerance;
dyslipidaemia or metabolic syndrome;
educational achievement.
Child and adult outcomes
weight;
height;
adiposity (e.g. as measured by BMI, skinfold thickness);
cardiovascular health (as defined by trialists, including BP, hypertension, cardiovascular disease, metabolic syndrome);
type 1 diabetes;
type 2 diabetes;
impaired glucose tolerance (as defined by trialists);
dyslipidaemia or metabolic syndrome (as defined by trialists);
employment, education, social status and achievement.
Health service use
number of hospital or health professional visits (e.g. midwife, obstetrician, physician, dietitian, diabetic nurse);
number of antenatal visits or admissions;
length of antenatal stay;
neonatal intensive care unit admission;
length of postnatal stay (mother);
length of postnatal stay (baby);
costs to families associated with the management provided;
costs associated with the intervention;
cost of maternal care;
cost of offspring care.
Search methods for identification of studies
The following methods sections of this review are based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (27 June 2017).
The Register is a database containing over 23,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register, including the detailed search strategies for CENTRAL, MEDLINE, Embase, and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about Cochrane Pregnancy and Childbirth in the Cochrane Library and select the Specialized Register section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE Ovid;
weekly searches of Embase Ovid;
monthly searches of CINAHL EBSCO;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that would have been fully accounted for in the relevant review section (Included, Excluded, Awaiting Classification or Ongoing).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports. We have provided the search terms in Appendix 1.
Searching other resources
We had planned to search the reference lists of retrieved studies. We did not apply any language or date restrictions.
Data collection and analysis
We did not identify any studies to include in this review. If we identify studies for inclusion in the next update, we plan to use the methods described in Appendix 2.
Results
Description of studies
We did not identify any randomised controlled trials that met the inclusion criteria for this review.
Results of the search
We searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform (ICTRP) on 27 June 2017, and did not identify any randomised controlled trials that compared any type of exercise programme, targeted at women with known pre‐gestational diabetes (type 1 or type 2 diabetes).
See: Figure 1
1.
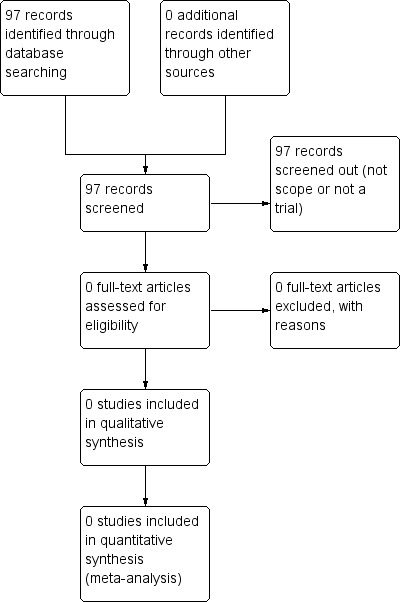
Study flow diagram
Included studies
We did not identify any randomised controlled trials.
Excluded studies
We did not identify any randomised controlled trials.
Risk of bias in included studies
We did not identify any randomised controlled trials.
Effects of interventions
We did not identify any randomised controlled trials.
Discussion
Summary of main results
We did not identify any RCTs for inclusion in this review. Therefore, it remains unclear whether exercise in pregnancy improves maternal and fetal outcomes for women with pre‐existing diabetes.
Overall completeness and applicability of evidence
There is a lack of evidence on exercise interventions for women with pre‐existing diabetes for improving maternal and fetal outcomes.
Quality of the evidence
There were no included studies.
Potential biases in the review process
We made every effort to minimise biases in the review process. We conducted a systematic search of the literature for randomised controlled trial evidence, and we did not use any restrictions for language or publication date.
We adhered to Cochrane methodology for searching, and in our plans for data extraction and analysis.
Agreements and disagreements with other studies or reviews
There were no RCTs on this review topic. Similarly, a brief search of the literature did not identify any observational studies on this topic to inform this discussion.
A physically active pregnancy is generally encouraged, with certain activities dependent on individual medical advice (ACOG 2015). NICE 2015 recommends maintaining a healthy body weight and establishing good glycaemic control preconception. However, they found no evidence to recommend or discourage exercise in pregnancy for women with type 1 or 2 diabetes. Though exercise is beneficial for non‐diabetic pregnant women and is thought to cause no harm to the fetus (Nascimento 2012), evidence regarding the safety of exercise in pregnancy for diabetic women is lacking.
Authors' conclusions
Implications for practice.
There is no evidence from RCTs to evaluate the effects of exercise interventions for improving maternal and fetal outcomes in women with pre‐existing diabetes.
Implications for research.
We identified no RCTs for inclusion in this review. Pre‐existing diabetes during pregnancy is associated with short‐ and long‐term adverse effects for the woman and her infant. Good quality, large randomised controlled trials are urgently needed to identify exercise interventions that are safe, and improve health outcomes for women with diabetes and their babies. Future studies in this area could utilise the standardised outcomes in this review in order to improve consistency between trials in this area, and aid evidence synthesis.
Acknowledgements
We acknowledge the contribution of Dr Rouiller, one of the authors of the original review, who has since died.
We acknowledge the support from the Cochrane Pregnancy and Childbirth editorial teams in Liverpool and the Australian and New Zealand Satellite, and the Liggins Institute, University of Auckland, New Zealand.
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure, and Cochrane Programme Grant funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
As part of the pre‐publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team), members of Cochrane Pregnancy and Childbirth's international panel of consumers, and the Group's Statistical Adviser.
Appendices
Appendix 1. Search terms for ICTRP and ClinicalTrials.gov
exercise AND diabetes AND pregnancy
Appendix 2. Methods of data collection and analysis for use in future updates of this review
Data collection and analysis
Selection of studies
Two review authors will independently assess for inclusion, all the potential studies we identify as a result of the search strategy. We will resolve any disagreement through discussion, or if required, we will consult a third person.
We will create a study flow diagram to map out the number of records identified, included, and excluded.
Data extraction and management
We will design a form to extract data. For eligible studies, two review authors will independently extract the data using the agreed form. We will resolve discrepancies through discussion, or if required, we will consult a third person. We will enter data into Review Manager 5 software and check for accuracy (RevMan 2014). When information regarding any of the above is unclear, we will attempt to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors will independently assess risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will resolve any disagreement by discussion, or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
For each included study, we will describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We will assess the method as:
low risk of bias (any truly random process, e.g. random number table, computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study, we will describe the method used to conceal allocation to interventions prior to assignment, and will assess whether intervention allocation could have been foreseen in advance of, during recruitment, or changed after assignment.
We will assess the methods as:
low risk of bias (e.g. telephone or central randomisation, consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study, we will describe the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We will consider that studies are at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess the methods as:
low, high, or unclear risk of bias for participants;
low, high, or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study, we will describe the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess methods used to blind outcome assessment as:
low, high, or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we will describe the completeness of data, including attrition and exclusions from the analysis. We will state whether attrition and exclusions were reported, and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or can be supplied by the trial authors, we will re‐include missing data in the analyses that we undertake.
We will assess methods as:
low risk of bias (e.g. no missing outcome data, missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups, ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study, we will describe how we investigated the possibility of selective outcome reporting bias and what we found.
We will assess the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported, one or more reported primary outcomes were not pre‐specified, outcomes of interest are reported incompletely and so cannot be used, study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For each included study, we will describe any important concerns we have about other possible sources of bias.
We will assess whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We will make explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we will assess the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We will explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
We will assess the quality of the evidence for outcomes relating to the mother, and the infant, child, or adult for the main comparisons, using the GRADE approach, outlined in the GRADE Handbook and Chapters 11 and 12 of the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011).
Maternal
hypertensive disorders of pregnancy (as reported by trialists, including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia);
caesarean section;
perineal trauma;
postnatal weight retention or return to pre‐pregnancy weight;
postnatal depression (as defined by trialists);
induction of labour.
Child (as a fetus, neonate, child, adult)
large‐for‐gestational age (more than 4 kg);
perinatal mortality (stillbirth and neonatal mortality);
mortality and morbidity composite (variously defined by trials, e.g. perinatal or infant death, shoulder dystocia, bone fracture or nerve palsy);
hypoglycaemia requiring treatment (as defined by trialists);
adiposity (including skin fold thickness, neonatal fat mass)*;
diabetes (type 1, type 2)*;
neurosensory disability (defined as any of the following: legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, developmental delay or impairment (defined as developmental quotient less than two standard deviations (SDs) below the mean).
We will use GRADEpro GDT software to import data from Review Manager 5.3 and create ’Summary of findings’ tables (GRADEpro GDT; RevMan 2014). We will produce a summary of the intervention effect and a measure of quality for each of the above outcomes, using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious, or by two levels for very serious limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias.
*These outcomes will be reported for each stage of life where data are reported.
Measures of treatment effect
Dichotomous data
For dichotomous data, we will present results as a summary risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
For continuous data, we will use the mean difference (MD) with 95% CI if outcomes are measured in the same way between trials. We will use the standardised mean difference (SMD) with 95% CI to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We will include cluster‐randomised trials in the analyses along with individually‐randomised trials. If any included studies are cluster‐randomised trials, we will make adjustments, using the methods described in the Cochrane Handbook of Systematic Reviews for Interventions (Section 16.3.4 or 16.3.6), using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population (Higgins 2011). If we use ICCs from other sources, we will report this, and conduct sensitivity analyses to investigate the effect of variation in the ICC. We will consider it reasonable to combine the results from both cluster‐randomised trials and individually‐randomised trials if there is little heterogeneity between the study designs, and is unlikely there will be an interaction between the effect of intervention and the choice of randomisation unit. If cluster‐randomised trials are included, we will seek statistical advice on the appropriate analysis to enable us to include the data in the meta‐analyses.
Other unit of analysis issues
Multiple pregnancy
There may be unit of analysis issues that arise when the women randomised have a multiple pregnancy. We will present maternal data as per woman randomised, and neonatal data per infant.
Multiple‐arm studies
Where a trial has multiple intervention arms, we will avoid 'double counting' participants by combining groups to create a single pair‐wise comparison, if possible. Where this is not possible, we will split the 'shared' group into two or more groups with smaller sample sizes, and include two or more (reasonably independent) comparisons.
Dealing with missing data
For included studies, we will note levels of attrition. We will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect, by performing a sensitivity analysis.
For all outcomes, we will carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we will attempt to include all participants randomised to each group in the analyses, and all participants will be analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We will assess statistical heterogeneity in each meta‐analysis using the Tau², I², and Chi² statistics. We will regard heterogeneity as substantial, if I² is greater than 30%, and either Tau² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We will carry out statistical analysis using the Review Manager 5 software (RevMan 2014). We will use fixed‐effect meta‐analysis for combining data, where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. We will treat the random‐effects summary as the average of the range of possible treatment effects, and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identify substantial heterogeneity, we will investigate it using subgroup and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses.
Group exercise versus individual exercise;
Low‐intensity exercise (cumulative duration of exercise at 50% VO₂ max (maximal oxygen consumption) for shorter than 180 minutes) versus high‐intensity exercise (cumulative duration of exercise at 50% VO₂ max) for longer than 180 minutes.
We will restrict subgroup analysis to the review's primary outcomes.
We will assess subgroup differences with the interaction tests available within Review Manager 5 (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the I² value.
Sensitivity analysis
If there is evidence of substantial heterogeneity, we will explore this by assessing the impact of the risks of bias of the included trials for the primary outcomes.
We will compare trials that have low risk of bias for allocation concealment with those judged to be of unclear or high risk of bias; we will exclude conference abstracts from the meta‐analysis.
We will also investigate the effect of the randomisation unit (i.e. where we include cluster‐randomised trials along with individually‐randomised trials).
Differences between protocol and review
There are no differences between the published protocol for this review (Brown 2017), and the full review.
Contributions of authors
All of the review authors contributed to the preparation of the full review.
Sources of support
Internal sources
No sources of support supplied
External sources
NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines, UK.
Declarations of interest
Dr Gilles Ceysens ‐ none known.
Dr Julie Brown ‐ none known.
Dr Michel Boulvain received research funding from Centre de Recherche Clinique (Advanced researcher grant scheme (2007‐2010)) to study exercise in pregnancy. One of the studies was a randomised controlled trial evaluating the effects of exercise in women with gestational diabetes. The study will not be eligible for inclusion in this review, but may be eligible for inclusion in the review on exercise for pregnant women with gestational diabetes ‐ Michel Boulvain will not be involved in any decisions relating to the inclusion of his own study in this review. All tasks relating to that study (assessment for inclusion, risk of bias, data extraction) will be carried out by the other members of the review team who were not directly involved in the trial. In 2012, he was invited to speak at the DIP 2012 Congress on gestational diabetes and was reimbursed for travel and accommodation.
New
References
Additional references
ACOG 2005
- ACOG Committee on Practice Bulletins. Pregestational diabetes mellitus: ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician‐Gynecologists. Number 60. Obstetrics and Gynecology 2005;105(3):675‐85. [DOI] [PubMed] [Google Scholar]
ACOG 2015
- American College of Obstetrians and Gynecologists. ACOG Committee Opinion No. 650: physical activity and exercise during pregnancy and the postpartum period. Obstetrics and Gynecology 2015;126(6):1326‐7. [DOI] [PubMed] [Google Scholar]
ACSM 2014
- American College of Sport Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 9th Edition. Philadephia (PA): Wolters Kluwer/Lippincott Williams and Wilkins, 2014. [Google Scholar]
Albrecht 2010
- Albrecht SS, Kuklina EV, Bansil P, Jamieson DJ, Whiteman MK, Kourtis AP, et al. Diabetes trends among delivery hospitalizations in the U.S., 1994‐2004. Diabetes Care 2010;33(4):768‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Asano 2014
- Asano RY, Sales MM, Browne RA, Vila Nova Moraes JF, Coelho HJ Jnr, Moraes MR, et al. Acute effects of physical exercise in type 2 diabetes: a review. World Journal of Diabetes 2014;5(5):659‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balsells 2009
- Balsells M, Garcia‐Patterson A, Gich I, Corcoy R. Maternal and fetal outcome in women with type 2 versus type 1 diabetes: a systematic review and meta‐analysis. Journal of Clinical Endocrinology and Metabolism 2009;94(11):4284‐91. [DOI] [PubMed] [Google Scholar]
CEMACH 2007
- Modder J, Fleming K, Acolet D. Confidential enquiry into maternal and child health. Diabetes in Pregnancy: are we providing the best care? Findings of a National Enquiry. England, Wales and Northern Ireland. CEMACH: London; 2007. www.publichealth.hscni.net/sites/default/files/Diabetes%20in%20Pregnancy‐%20are%20we%20providing%20the%20best%20care.pdf (accessed 1 June 2017).
Ceysens 2016
- Ceysens G, Brown J, Boulvain M. Exercise for pregnant women with gestational diabetes for improving maternal and fetal outcomes. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD012202] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chibalin 2000
- Chibalin AV, Yu M, Ryder JW, Song XM, Galuska D, Krook A, et al. Exercise‐induced changes in expression and activity of proteins involved in insulin signal transduction in skeletal muscle: differential effects on insulin receptor substrates 1 and 2. Proceedings of the National Academy of Sciences of the United States of America 2000;97:38‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Correa 2015
- Correa A, Bardenheier B, Elixhauser A, Geiss LS, Gregg E. Trends in prevalence of diabetes among delivery hospitalizations, United States, 1993–2009. Maternal and Child Health Journal 2015;19:635‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dabelea 2000
- Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow‐up research in the Pima Indians. Journal of Maternal‐Fetal Medicine 2000;9(1):83‐8. [DOI] [PubMed] [Google Scholar]
Dela 1993
- Dela F, Handberg A, Mikines KJ, Vinten J, Galbo H. GLUT4 and insulin receptor binding and kinase activity in trained human muscle. Journal of Physiology 1993;469:615‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunne 2005
- Dunne F. Type 2 diabetes and pregnancy. Seminars in Fetal and Neonatal Medicine 2005;10(4):333‐9. [DOI] [PubMed] [Google Scholar]
Galerneau 2004
- Galerneau F, Inzucchi SE. Diabetes mellitus in pregnancy. Obstetrics and Gynecology Clinics of North America 2004;31(4):907‐33. [DOI] [PubMed] [Google Scholar]
GRADE Handbook
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version (accessed 27 June 2017). Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Griffith 2004
- Griffith J, Conway DL. Care of diabetes in pregnancy. Obstetrics and Gynecology Clinics of North America 2004;31(2):243‐56. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hjeltnes 1998
- Hjeltnes N, Galuska D, Bjornholm M, Aksnes AK, Lannem A, Zierath JR, et al. Exercise‐induced over expression of key regulatory proteins involved in glucose uptake and metabolism in tetraplegic persons: molecular mechanism for improved glucose homeostasis. FASEB Journal 1998;12:1701‐12. [DOI] [PubMed] [Google Scholar]
Inkster 2006
- Inkster ME, Fahey TP, Donnan PT, Leese GP, Mires GJ, Murphy DJ. Poor glycated haemoglobin control and adverse pregnancy outcomes in type 1 and type 2 diabetes mellitus: systematic review of observational studies. BMC Pregnancy and Childbirth 2006;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2004
- Jensen DM, Dam P, Moelsted‐Pedersen L, Ovesen P, Westergaard JG, Moeller M, et al. Outcomes in type 1 diabetic pregnancies. Diabetes Care 2004;27(12):2819‐23. [DOI] [PubMed] [Google Scholar]
Khambalia 2013
- Khambalia AZ, Ford JB, Nassar N, Shand AW, McElduff A, Roberts CL. Occurrence and recurrence of diabetes in pregnancy. Diabetic Medicine 2013;30:452‐6. [DOI] [PubMed] [Google Scholar]
Macintosh 2006
- Macintosh MC, Fleming KM, Bailey JA, Doyle P, Modder J, Acolet D, et al. Perinatal mortality and congenital anomalies in babies of women with type 1 and type 2 diabetes in England, Wales and Northern Ireland: population based study. BMJ 2006;333(7560):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2009
- Murphy HR. Integrating educational and technological interventions to improve pregnancy outcomes in women with diabetes. Diabetes, Obesity and Metabolism 2009;12(2):97‐104. [DOI] [PubMed] [Google Scholar]
Nascimento 2012
- Nascimento SL, Surita FG, Cecatti JG. Physical exercise during pregnancy: a systematic review. Current Opinion in Obstetrics and Gynecology 2012;24(6):387‐94. [DOI] [PubMed] [Google Scholar]
NICE 2015
- National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. NICE guideline (NG3; updated August 2015). www.nice.org.uk/guidance/ng3 (accessed 1 June 2017). [PubMed]
Owens 2015
- Owens LA, Sedar J, Carmody L, Dunne F. Comparing type 1 and type 2 diabetes in pregnancy ‐ similar conditions or is a separate approach required?. BMC Pregnancy and Childbirth 2015;15:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Platt 2002
- Platt MJ, Stanisstreet M, Casson IF, Howard CV, Walkinshaw S, Pennycook S, et al. St Vincent's Declaration 10 years on: outcomes of diabetic pregnancies. Diabetic Medicine 2002;19(3):216‐20. [DOI] [PubMed] [Google Scholar]
Ray 2001
- Ray JG, Vermeulen MJ, Shapiro JL, Kenshole AB. Maternal and neonatal outcomes in pregestational and gestational diabetes mellitus, and the influence of maternal obesity and weight gain: the DEPOSIT study. Diabetes Endocrine Pregnancy Outcome Study in Toronto. QJM: Monthly Journal of the Association of Physicians 2001;94(7):347‐56. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Thomas 2006
- Thomas D, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD002968.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tuomilehto 2001
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne‐Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine 2001;344(18):1343‐50. [DOI] [PubMed] [Google Scholar]
Walkinshaw 2005
- Walkinshaw SA. Pregnancy in women with pre‐existing diabetes: management issues. Seminars in Fetal & Neonatal Medicine 2005;10(4):307‐15. [DOI] [PubMed] [Google Scholar]
Weintrob 1996
- Weintrob N, Karp M, Hod M. Short‐ and long‐range complications in offspring of diabetic mothers. Journal of Diabetes and its Complications 1996;10(5):294‐301. [DOI] [PubMed] [Google Scholar]
Zhu 2016
- Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Current Diabetes Reports 2016;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Brown 2017
- Brown J, Ceysens G, Boulvain M, West HM. Exercise for pregnant women with pre‐existing diabetes for improving maternal and fetal outcomes. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD012696] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ceysens 2006
- Ceysens G, Roullier D, Boulvain M. Exercise for diabetic pregnant women. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004225.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


