Abstract
Background
Chronically elevated blood glucose levels are associated with significant morbidity and mortality. Many diabetes patients will eventually require insulin treatment to maintain good glycaemic control. There are still uncertainties about the optimal insulin treatment regimens for type 2 diabetes, but the long‐acting insulin analogues seem beneficial. Several reviews have compared either insulin detemir or insulin glargine to NPH insulin, but research directly comparing both insulin analogues is limited.
Objectives
To assess the effects of insulin detemir and insulin glargine compared with each other in the treatment of type 2 diabetes mellitus.
Search methods
We searched MEDLINE, EMBASE, The Cochrane Library, online registries of ongoing trials and abstract books. Date of last search was January 2011.
Selection criteria
All randomised controlled trials comparing insulin detemir with insulin glargine with a duration of 12 weeks or longer were included.
Data collection and analysis
Two authors independently selected the studies and extracted the data. Pooling of studies by means of random‐effects meta‐analysis was performed.
Main results
This review examined four trials lasting 24 to 52 weeks involving 2250 people randomised to either insulin detemir or glargine. Overall, risk of bias of the evaluated studies was high. Insulin glargine was dosed once‐daily in the evening. Insulin detemir was initiated once‐daily in the evening with the option of an additional dose in the morning in three studies and initiated twice‐daily in one study. Of randomised patients 13.6% to 57.2% were injecting insulin detemir twice‐daily at the end of trial.
Glycaemic control, measured by glycosylated haemoglobin A1c (HbA1c) and HbA1c equal to or less than 7% with or without hypoglycaemia, did not differ statistically significantly between treatment groups.
The results showed no significant differences in overall, nocturnal and severe hypoglycaemia between treatment groups.
Insulin detemir was associated with less weight gain. Treatment with insulin glargine resulted in a lower daily basal insulin dose and a lower number of injection site reactions.
There was no significant difference in the variability of FPG or glucose values in 24‐hour profiles between treatment groups. It was not possible to draw conclusions on quality of life, costs or mortality. Only one trial reported results on health‐related quality of life and showed no significant differences between treatment groups.
Authors' conclusions
Our analyses suggest that there is no clinically relevant difference in efficacy or safety between insulin detemir and insulin glargine for targeting hyperglycaemia. However, to achieve the same glycaemic control insulin detemir was often injected twice‐daily in a higher dose but with less weight gain, while insulin glargine was injected once‐daily, with somewhat fewer injection site reactions.
Keywords: Humans; Diabetes Mellitus, Type 2; Diabetes Mellitus, Type 2/blood; Diabetes Mellitus, Type 2/drug therapy; Glycated Hemoglobin A; Glycated Hemoglobin A/metabolism; Hypoglycemic Agents; Hypoglycemic Agents/therapeutic use; Insulin; Insulin/analogs & derivatives; Insulin/therapeutic use; Insulin Detemir; Insulin Glargine; Insulin, Long‐Acting; Randomized Controlled Trials as Topic
Insulin detemir versus insulin glargine for type 2 diabetes mellitus
The two long‐acting insulin analogues (artificial insulins), insulin detemir or insulin glargine differ in their mechanism of attaining protracted action, leading to possible differences in glycaemic control and safety outcomes. Several studies have compared either insulin detemir or insulin glargine to NPH (Neutral Protamin Hagedorn) insulin. Research directly comparing both long‐acting insulin analogues is limited.
Our aim was to systematically review the efficacy and safety of insulin detemir and insulin glargine in head‐to‐head studies in the treatment of type 2 diabetes mellitus.
Four studies investigated a total of 2250 people. Trials lasted between 24 and 52 weeks. Overall, risk of bias of the evaluated studies was high. Our analysis of these intermediate term trials comparing insulin detemir with insulin glargine showed that these two insulins were equally effective in achieving and maintaining glycaemic control (glycosylated haemoglobin A1c (HbA1c)). There were no differences in overall, nocturnal and severe hypoglycaemia when comparing insulin detemir to insulin glargine. Insulin detemir was associated with significantly less weight gain (one study showing a difference of 0.9 kg). Treatment with insulin glargine resulted in a lower daily basal insulin dose and a lower number of injection site reactions (1.8% of patients treated with insulin detemir compared to 0.4% of patients treated with insulin glargine had injection side reactions).
There was no difference in the variability of fasting glucose levels or the variability of glucose values of 24‐hour profiles between the two treatment groups.
From the retrieved trials it was not possible to draw conclusions on the effects of these two insulins on quality of life, their costs or on the number of fatalities. Only one trial reported results on health‐related quality of life and showed no significant differences between treatment groups.
Our analyses suggest that there is no clinically relevant difference in the efficacy or the safety between the use of insulin detemir and insulin glargine for treating type 2 diabetes mellitus. However, to achieve the same glycaemic control insulin detemir was often injected twice‐daily in a higher dose but with less weight gain, while insulin glargine was only injected once‐daily, with somewhat fewer injection site reactions.
Summary of findings
Summary of findings for the main comparison.
Insulin detemir (intervention) vs. Insulin glargin (control) for type 2 diabetes mellitus
| Insulin detemir (intervention) vs. Insulin glargin (control) for type 2 diabetes mellitus | ||||||
| Patient or population: patients with type 2 diabetes mellitus Settings: Intervention: Insulin detemir (intervention) vs. Insulin glargin (control) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Insulin detemir (intervention) vs. Insulin glargin (control) | |||||
| Mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not investigated |
| Quality of life and treatment satisfaction ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Insufficient information as only one included study reported on health‐related quality of life. |
| Change in HbA1c | The mean change in HbA1c ranged across control groups from ‐1.25 to ‐1.68 % | The mean change in HbA1c in the intervention groups was 0.07 higher (0.14 lower to 0.24 higher) | 2250 (4 studies) | ⊕⊕⊝⊝ low1,2 | ||
| Percentage of participants having at least one severe hypoglycaemic event | 33 per 1000 | 27 per 1000 (17 to 43) | RR 0.82 (0.51 to 1.32) | 2252 (4 studies) | ⊕⊕⊝⊝ low3,4 | |
| Percentage of participants having at least one hypoglycaemic event | 544 per 1000 | 533 per 1000 (501 to 571) | RR 0.98 (0.92 to 1.05) | 2252 (4 studies) | ⊕⊕⊝⊝ low3 | |
| Weight gain | The mean weight gain ranged across control groups from 1.4 to 3.8 kg | The mean weight gain in the intervention groups was 0.91 lower (1.21 to 0.61 lower) | 2250 (4 studies) | ⊕⊕⊕⊕ high | ||
| Percentage of participants having at least one injection site reaction | 4 per 1000 | 13 per 1000 (4 to 39) | RR 3.31 (1.13 to 9.73) | 2252 (4 studies) | ⊕⊕⊝⊝ low1,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Low number of events 2 HbA1c is only a weak surrogate for mortality and diabetes‐associated morbidity 3 Due to (i) lack of blinding and (ii) different frequency of injection and different injection devices across treatments 4 Wide confidence interval and low number of total events
Background
Description of the condition
Type 2 diabetes mellitus is a heterogeneous and progressive disorder caused by a combination of insulin resistance and impaired insulin secretion. Initially, the reduced sensitivity to insulin of tissues, particularly the liver and skeletal muscle, is compensated for by an increased insulin production by the pancreatic ß‐cells. The resulting hyperinsulinaemia maintains the blood glucose within the normal range. Eventually, however, relative insulin deficiency, and thus hyperglycaemia, will develop. Chronic hyperglycaemia is associated with microvascular complications like retinopathy, nephropathy and neuropathy, and an increased risk of cardiovascular disease. Lowering blood glucose levels by means of intensive therapy has been shown to reduce these vascular complications in type 2 diabetes patients (Holman 2008; UKPDS 33 1998; UKPDS 34 1998).
Description of the intervention
According to the American Diabetes Association (ADA) / European Association for the Study of Diabetes (EASD) consensus algorithm for the management of type 2 diabetes “an HbA1c level of ≥ 7% should serve as a call to action to initiate or change therapy with the goal of achieving an HbA1c level of < 7%”. The authors also advise that initial therapy should consist of lifestyle intervention and the oral glucose‐lowering drug metformin. As the disease progresses treatment is usually intensified by the addition of one or more oral agents. However, when lifestyle interventions and oral therapy no longer achieve the currently recommended glycaemic goal of an HbA1c level of less than 7%, the introduction of a basal insulin preparation is advocated (Nathan 2006; Nathan 2009). Traditionally, the intermediate‐acting Neutral Protamine Hagedorn (NPH) insulin has been used, but this agent has pharmacodynamic limitations (Heinemann 2000; Plank 2005), predisposing to both hypoglycaemia and hyperglycaemia (Holleman 2007). In order to reach glycaemic targets more effectively and safely, insulin analogues with a modified structure compared to the human insulin molecule were developed. Two long‐acting insulin analogues are currently available: insulin detemir and insulin glargine.
How the intervention might work
The two long‐acting insulin analogues differ in molecular structure and method of protraction. Insulin detemir is produced by removal of the amino acid threonine from position B30 and acylation of lysine at position B29 with myristic acid. These modifications result in an increased self‐association and reversible binding to albumin in the interstitial fluid and plasma, which are responsible for the preparation’s prolonged duration of action (Havelund 2004). In the insulin glargine molecule, glycine has been substituted for asparagine at position A21 and two arginine molecules have been added at position B30, leading to a shift of the isoelectric point. As a result, following injection at physiological pH, insulin glargine forms microprecipitates, which slowly dissolve from the subcutaneous space (Bolli 1999). Euglycaemic clamp studies demonstrated that insulin detemir has a dose‐dependent duration of action (Plank 2005) and a mean time‐action profile somewhat intermediate between the profiles of NPH insulin and insulin glargine (Heise 2004). The time‐action profile of insulin glargine lacks a pronounced peak in effect and its activity may persist up to 24 hours (DeVries 2005; Swinnen 2009).
In clinical trials in type 2 diabetes mellitus, both insulin detemir and insulin glargine have been found to reduce the risks of overall and nocturnal hypoglycaemia compared with NPH insulin (Hermansen 2006; Horvath 2007; Riddle 2003). Additionally, insulin detemir was associated with significantly less weight gain (Hermansen 2006). These advantages of the long‐acting insulin analogues over NPH insulin may improve patient treatment satisfaction and health‐related quality of life.
Adverse effects of the intervention
Hypoglycaemia is an intrinsic adverse effect of insulin treatment and thus insulin detemir and insulin glargine carry the risk of causing hypoglycaemic events. Additionally, insulin therapy is associated with weight gain and injection site reactions. Concern has been raised regarding potential mitogenic effects of insulin analogues, but distinct evidence is lacking. As relatively short term clinical studies will not reliably show a difference in mitogenic potency between insulin detemir and insulin glargine, we will not review these potential adverse effects of the two long‐acting insulin analogues.
Why it is important to do this review
The prevalence of diabetes is increasing rapidly worldwide. A few years ago the total number of people with diabetes was projected to rise from 171 million in 2000 to 366 million in 2030 (Wild 2004). Chronically elevated blood glucose levels are associated with significant morbidity and mortality and many patients will eventually require insulin treatment to maintain good glycaemic control. There are still many uncertainties about the optimal insulin treatment regimens for type 2 diabetes, but the long‐acting insulin analogues seem promising. Several reviews have analysed studies which compared either insulin detemir or insulin glargine to NPH insulin (Chapman 2004; Horvath 2007; Mullins 2007; Rosenstock 2005), but research directly comparing both long‐acting insulin analogues is limited.
Our aim is to systematically review the efficacy and safety of the two currently available long‐acting insulin analogues, insulin detemir and insulin glargine, in the treatment of type 2 diabetes mellitus.
Objectives
To assess the effects of insulin detemir and insulin glargine compared with each other in the treatment of patients with type 2 diabetes mellitus.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) comparing insulin detemir with insulin glargine with a duration of 12 weeks or longer. Trials were included irrespective of blinding, number of patients randomised, and language of the publication.
Types of participants
People with type 2 diabetes mellitus. Ideally, diagnostic criteria should have been described, but if this was not the case we used the authors’ definition of type 2 diabetes mellitus.
Types of interventions
Treatment with insulin detemir versus treatment with insulin glargine. Trials investigating combination therapy, that is insulin treatment combined with oral glucose‐lowering agents or basal‐bolus insulin therapy, were only included if the additional glucose‐lowering intervention was the same in both treatment arms.
Types of outcome measures
Primary outcomes
glycaemic control as measured by: the level of glycosylated haemoglobin A1c (HbA1c) at study endpoint, change in HbA1c level from baseline to study endpoint, fasting glucose level at study endpoint, change in fasting glucose level from baseline to study endpoint, the percentage of participants achieving good glycaemic control (defined as HbA1c equal to or less than 7%) at study endpoint, the percentage of participants achieving good glycaemic control (defined as HbA1c equal to or less than 7%) at study endpoint without hypoglycaemic episodes during the study;
incidence and rate of overall, daytime, nocturnal and severe hypoglycaemia. Hypoglycaemia was defined as a symptomatic or asymptomatic event with plasma glucose less than 3.1 mmol/L and was classified as ‘severe’ if assistance from another person was required.
Secondary outcomes
adverse effects, such as weight gain and injection site reactions;
insulin dose;
variability of fasting glucose levels (calculated as coefficient of variation: SD/mean);
variability of the glucose values included in eight‐ or nine‐point 24‐hour glucose profiles (calculated as coefficient of variation: SD/mean);
health‐related quality of life and treatment satisfaction measured with validated instruments;·
costs;
mortality.
Covariates, confounders, effect modifiers
comparability of the concomitant glucose‐lowering interventions;
compliance;
co‐morbidities.
Timing of outcome assessment
We planned to assess short term (12 to 25 weeks of treatment), intermediate term (26 to 52 weeks of treatment) and long term (more than 52 weeks of treatment) outcome measurements.
Search methods for identification of studies
Electronic searches
We used electronic search strategies to identify relevant RCTs, reviews and meta‐analyses. There were no language or publication year restrictions. We searched the following sources:
The Cochrane Library (issue 10, 2010);
MEDLINE (until January 2011);
EMBASE (until January 2011).
In addition, we searched online registries of ongoing trials:
www.clinicalstudyresults.org
www.clinicaltrials.gov
www.controlled‐trials.com
For detailed search strategies please see under Appendix 1
Searching other resources
We searched the reference lists of included RCTs and relevant reviews and meta‐analyses and we hand searched the abstract books of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) annual meetings from 2000 to 2009.
Data collection and analysis
Selection of studies
Two authors (S.S. and A.S.) independently screened the title, abstract or both of every record retrieved. All potentially relevant publications were investigated as full text. Disagreements were resolved by discussion. An adapted PRISMA (preferred reporting items for systematic reviews and meta‐analyses) flow‐chart of study selection is attached (Liberati 2009).
Data extraction and management
For studies that fulfilled the inclusion criteria, two authors (S.S. and A.S.) independently extracted relevant population and intervention characteristics using standard data extraction templates (for details see 'Characteristics of included studies, Table 3 and Appendix 2). Disagreements were resolved by discussion or, if necessary, by a third party. Any relevant missing information on the trial was sought from the original authors of the publication.
Table 1.
Overview of study populations
| study ID | intervention | [n] screened | [n] randomised | [n] safety | [n] ITT | [n]/% finishing study | comments |
| Hollander 2008 | Intervention (I): Insulin detemir once‐ or twice‐daily + insulin aspart at mealtime Control (C): Insulin glargine once‐daily + insulin aspart at mealtime |
I: ‐ C: ‐ Total: 460 |
I: 216 C: 107 Total: 323 |
I: 214 C: 105 Total: 319 |
I: 214 C: 105 Total: 319 |
I: 173 (80.1%) C: 84 (78.5%) Total: 257 |
Insulin detemir: 92 (43.0%) patients used once‐daily and 122 (57.0%) twice‐daily at study completion. Novo Nordisk A/S web site: Trial report synopsis: "Safety analyses were only performed on the ITT analysis. ITT = all randomised subjects exposed to at least one dose of trial product". |
| Raskin 2009 | Intervention (I): Insulin detemir once‐ or twice‐daily + insulin aspart at mealtime Control (C): Insulin glargine once‐daily + insulin aspart at mealtime |
I: ‐ C: ‐ Total: ‐ |
I: 256 C: 131 Total: 387 |
I: 256 C: 131 Total: 387 |
I: 254 C: 131 Total: 385 |
I: 210 (82.0% of n=256) C: 113 (86.3%) Total: 323 |
Insulin detemir: 222 (87.4%) patients used once‐daily and 32 (12.6%) twice‐daily at study completion. Total: 385 = ITT = randomised and exposed to at least one dose of trial product. |
| Rosenstock 2008 | Intervention (I): Insulin detemir once‐ or twice‐daily Control (C): Insulin glargine once‐daily |
I: ‐ C: ‐ Total: 892 |
I: 291 C: 291 Total: 582 |
I: 291 C: 291 Total: 582 |
I: 291 C: 291 Total: 582 |
I: 231 (79%) C: 252 (87%) Total: 483 |
Insulin detemir: 104 (45.0%) patients used once‐daily and 127 (55.0%) twice‐daily at study completion. Total: 582 = ITT = randomised = exposed. |
| Swinnen 2010 | Intervention (I):Insulin detemir twice‐daily Control (C): Insulin glargine once‐daily |
I: ‐ C: ‐ Total: 1230 |
I: 487 C: 486 Total: 973 |
I: 486 C: 478 Total: 964 |
I: 472 C: 473 Total: 945 |
I: 437 (89.7% of n=487) C: 456 (93.8% of n=486) Total: 893 |
Safety population = all randomised and treated subjects. mITT for primary outcome = randomised and treated subjects with a post‐baseline HbA1c measurement and information on hypoglycaemia, or with either a last HbA1c > 7% or symptomatic hypoglycaemia ≤ 3.1 mmol/L. |
‐ denotes 'not reported'
HbA1c = glycosylated haemoglobin A1c; ITT = intention‐to‐treat; mITT = modified intention‐to‐treat.
Dealing with duplicate publications
In the case of duplicate publications of a primary study, we assessed those articles together to maximise data collection. In case of conflicting information the primary publication had priority.
Assessment of risk of bias in included studies
Risk of bias was assessed using the Cochrane Collaboration’s risk of bias tool (Higgins 2008).Two authors (S.S. and A.S.) independently assessed each included trial. Disagreements were resolved by discussion or, if necessary, by consultation of a third party.
Measures of treatment effect
Dichotomous data
We summarised dichotomous outcome data as risk ratios with 95% confidence intervals (CI).
Continuous data
We summarised continuous outcomes as mean differences with 95% CI.
Dealing with missing data
We obtained relevant missing data from the original authors. We carefully evaluated important numerical data such as the number of screened and randomised patients as well as the intention‐to‐treat (ITT) and per‐protocol (PP) populations. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and techniques to handle these (for example, last‐observation‐carried‐forward (LOCF)) were critically appraised.
Assessment of heterogeneity
In the event of substantial clinical, methodological or statistical heterogeneity, study results were not combined in meta‐analysis. Heterogeneity was examined by visual inspection of the forest plots and by using a standard χ2‐test with a significance level of α = 0.1. Heterogeneity was also quantified with I2 (Higgins 2002), where I2 values of 50% and more indicate a substantial level of heterogeneity (Higgins 2003). When heterogeneity was found, we attempted to determine potential reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
Funnel plots were used to assess for the potential existence of small study bias. There are a number of explanations for asymmetry of a funnel plot (Sterne 2001). Thus, results were carefully interpreted (Lau 2006).
Data synthesis
Data were summarised statistically if they were available, sufficiently similar and of sufficient quality. Statistical analysis was performed according to the statistical guidelines referenced in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were to be performed if one of the primary outcome parameters demonstrated statistically significant differences between intervention groups. We planned the following subgroup analyses:
gender;
age.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size:
repeating the analysis excluding unpublished studies;
repeating the analysis taking account of risk of bias;
repeating the analysis excluding any very long or large studies to establish how much they dominate the results;
repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country.
The robustness of the results was also tested by repeating the analysis using different measures of effects size (relative risk, odds ratio, etc.) and different statistical models (fixed‐effect model and random‐effects model).
Results
Description of studies
Please see Characteristics of included studies and Characteristics of ongoing studies.
Results of the search
Our initial search of EMBASE, MEDLINE and The Cochrane Library yielded 1615 citations, and an updated search yielded an additional 595 citations. Of these two searches, seven studies comparing treatment with insulin detemir versus treatment with insulin glargine in people with type 2 diabetes mellitus were examined as full text. Common reasons for exclusion of citations were non‐randomised controlled trial (mostly review articles) and investigation of a non‐relevant question. Hand searching of the American Diabetes Association's and European Association for the Study of Diabetes' abstract books yielded eight potentially relevant publications, seven (including duplicates) of which we had already identified by our electronic search, resulting in one additional potentially relevant article. We identified five more relevant studies by searching online registries of ongoing trials. Of the thirteen potentially relevant trials, five were excluded because their duration was shorter than 12 weeks (Characteristics of excluded studies). Four were excluded because they were ongoing or unpublished (Characteristics of ongoing studies). In conclusion, we included four studies in our review (Figure 1).
Figure 1.
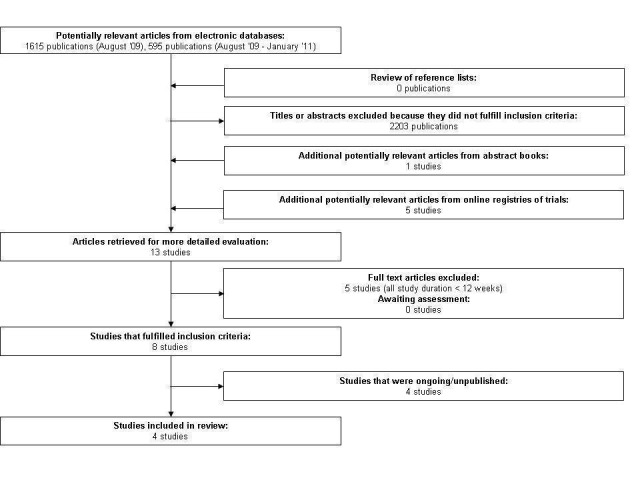
Included studies
All four included studies had a multi‐national, multi‐centre, open‐label, parallel‐group design. Two trials were performed in Europe and the United States (Hollander 2008; Rosenstock 2008), one in Canada and the United States (Raskin 2009) and one in Australia, Brazil, Canada, Europe, India, Korea, Russia, Taiwan and Turkey (Swinnen 2010a). The number of participating study centres ranged from 56 (Hollander 2008) to 122 (Swinnen 2010a). The one study that reported on its setting stated that participants were recruited by endocrinologists and diabetologists (Swinnen 2010a).
Participants
Altogether 2250 people were randomised and exposed to trial drugs in the included studies.
All four studies recruited people who had type 2 diabetes mellitus for at least one year. No study specified diagnostic criteria. Three studies included patients from the age of 18 and one study included people aged 40 to 75 years (Swinnen 2010a). Mean duration of diabetes ranged from 9 to 14 years and mean age from 56 to 58 years. Around 80% of participants were Caucasian. The studies’ inclusion ranges for glycaemic control were a glycosylated haemoglobin A1c (HbA1c) level of ≥ 7.0% or ≥ 7.5% to ≤ 10.0% or ≤ 11.0%. Mean HbA1c level at baseline ranged from 8.4% to 8.7%.
Two studies included insulin‐naive people only, that is patients who had not been treated with insulin prior to study participation (Rosenstock 2008; Swinnen 2010a). The other two trials included both patients who had previously used oral glucose‐lowering drugs and those who had used insulin with or without oral glucose‐lowering drugs (Hollander 2008; Raskin 2009). In the first two studies almost all patients used metformin and most (approx. 75%) used a combination of metformin and an insulin secretagogue. The types of oral glucose‐lowering drugs used at baseline were not reported in the latter two studies but around 20% of participants were treated with oral agents only, one third with insulin only and about 45% with insulin and oral agents.
All studies listed proliferative diabetic retinopathy as an exclusion criterion. Three studies excluded patients with known hypoglycaemia unawareness or with recurrent major hypoglycaemic episodes (Hollander 2008; Raskin 2009; Rosenstock 2008). Rosenstock 2008 also excluded patients treated with thiazolidinediones and Swinnen 2010a excluded those using glucagon‐like peptide‐1 analogues or dipeptidyl peptidase‐4 inhibitors.
Interventions
The two studies that solely included insulin‐naive people investigated basal insulin‐only therapy (Rosenstock 2008; Swinnen 2010a), the other two compared the long‐acting insulin analogues when used in a basal‐bolus insulin regimen. In both studies insulin aspart was used as the bolus or mealtime insulin (Hollander 2008; Raskin 2009).
All four trials had a so‐called treat‐to‐target design. This means that the basal insulin dose, in this case the dose of insulin detemir or insulin glargine, was systematically titrated according to pre‐defined plasma glucose criteria. Insulin glargine was dosed once‐daily in the evening and systematically titrated to achieve a certain pre‐defined fasting plasma glucose target in all studies. Insulin detemir was initiated once‐daily in the evening in three trials, but the titration algorithm allowed an additional dose in the morning if the pre‐dinner plasma glucose value was above target while the fasting plasma glucose target had been achieved. The percentages of patients using insulin detemir twice‐daily at the end of the trial were 57.2% (Hollander 2008), 13.6% (Raskin 2009) and 55% (Rosenstock 2008). In the fourth study insulin detemir was initiated twice‐daily (Swinnen 2010a).
In one of the studies investigating basal insulin‐only therapy in insulin‐naive patients all previous oral glucose‐lowering drugs were continued unchanged (Rosenstock 2008). In the other basal insulin‐only trial thiazolidinediones were stopped at randomisation, insulin secretagogues were stopped at randomisation or maintained at stable dose throughout the study and all other oral agents were continued unchanged (Swinnen 2010a). In the two trials investigating basal‐bolus therapy, insulin secretagogues and α‐glucosidase inhibitors were discontinued prior to the initiation of trial drug and other oral glucose‐lowering agents were continued (Hollander 2008; Raskin 2009).
Treatment duration ranged from 24 (Swinnen 2010a) to 52 weeks (Hollander 2008; Rosenstock 2008). The frequency of contact between the participants and the study team (clinical and telephone contacts combined and standardised to the number or contacts per year) ranged from 25 (Rosenstock 2008) to 44 (Raskin 2009).
Outcomes
Three studies used HbA1c levels at study endpoint as the primary outcome measure. The primary outcome of the fourth study was the percentage of participants achieving an HbA1c less than 7% at end of study without symptomatic hypoglycaemia confirmed by a plasma glucose measurement equal to or less than 3.1 mmol/L during the study (Swinnen 2010a). Endpoint HbA1c level was a secondary outcome of this study.
All four studies reported the following secondary outcomes: the percentage of participants achieving target HbA1c, fasting plasma glucose, 24‐hour plasma glucose profiles, hypoglycaemia, body weight, insulin dose and adverse events.
Excluded studies
The main reason for exclusion of five trials was a study duration of less than 12 weeks. Please see Characteristics of excluded studies.
Risk of bias in included studies
The risk of bias of the included trials was considered as high (see Figure 2 and Figure 3). Although three of the four studies had adequate allocation sequence generation and adequate allocation concealment, all studies were open‐label. Neither participants nor study personnel were blinded to the trial drug received.
Figure 2.
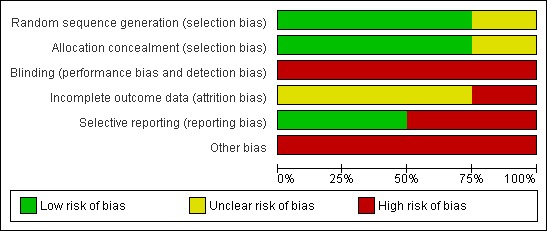
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
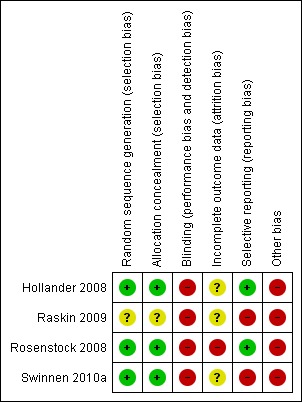
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Allocation concealment was adequate in three studies as they used an automatic telephone system. The fourth study did not describe its randomisation method (Raskin 2009).
Blinding
The lack of blinding of study participants and study personnel in all four trials may have affected outcome measurements and participants' behaviour.
Incomplete outcome data
In two studies the number of missing data for the primary outcome was balanced across the two treatment groups (Rosenstock 2008; Swinnen 2010a). In one of these trials there was an imbalance between intervention groups in numbers of and reasons for missing data for the secondary outcome weight gain (Rosenstock 2008). In all other instances the number of missing data for the analysis of outcomes was not reported.
Selective reporting
The study protocol of one trial was available and not all pre‐specified secondary outcomes are reported in the publication (Swinnen 2010a). For the other three studies we found pre‐defined outcomes on their sponsor’s web site and online trial registries. Most of the pre‐specified outcomes have been reported.
Other potential sources of bias
In three trials an additional daily insulin detemir injection could be added by the study personnel and in the fourth study all participants randomised to treatment with insulin detemir used it twice‐daily. In contrast, insulin glargine was dosed once‐daily in all trials. This once‐ versus twice‐daily dosing also affected the number of home‐glucose measurements that study participants had to perform. People treated with insulin detemir twice‐daily were instructed to not only measure their glucose before breakfast but also before dinner. Finally, part of the patients treated with insulin glargine (namely those in Canada and the United States) used syringes and vials while all detemir‐treated patients used a pen‐injector. The latter is probably more patient‐friendly. All these differences between the insulin detemir and insulin glargine treatment groups may have affected outcome measurements and participants behaviour and compliance.
Effects of interventions
See: Table 1
Primary outcomes
Glycaemic control
Glycosylated haemoglobin A1c (HbA1c)
The mean difference in endpoint HbA1c level between insulin glargine and insulin detemir was not statistically significant: 0.08% (95% CI ‐0.10 to 0.27) (Analysis 1.1). There was substantial statistical heterogeneity between studies (P = 0.01, I2 = 73%). Similarly, meta‐analysis of change in HbA1c level resulted in a not statistically significant estimated mean difference of 0.07% (95% CI ‐0.10 to 0.24) (Analysis 1.2). There was substantial statistical heterogeneity between studies (P = 0.04, I2 = 64%).
Analysis 1.1.
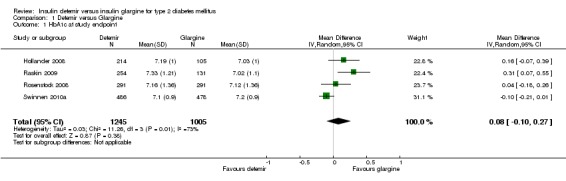
Comparison 1 Detemir versus Glargine, Outcome 1 HbA1c at study endpoint.
Analysis 1.2.
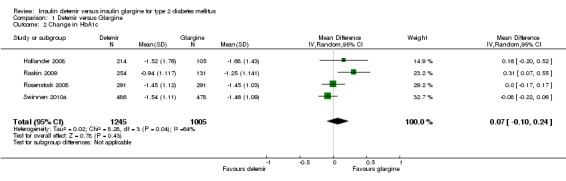
Comparison 1 Detemir versus Glargine, Outcome 2 Change in HbA1c.
Fasting glucose
Insulin glargine was associated with statistical significantly lower fasting glucose at study endpoint than insulin detemir (mean difference of 0.34 mmol/L [95% CI 0.01 to 0.67], but with some statistical heterogeneity [P = 0.11, I2 = 50%] ‐ Analysis 1.5). Insulin glargine also resulted in a not statistical significant 0.18 mmol/L greater lowering of change in fasting glucose from baseline to study endpoint than insulin detemir (95% CI ‐0.10 to 0.47), without evidence for statistical heterogeneity (P = 0.57, I2 = 0% ‐ Analysis 1.6). Data on change in fasting glucose level from baseline to study endpoint were available for all but one study (Rosenstock 2008).
Analysis 1.5.
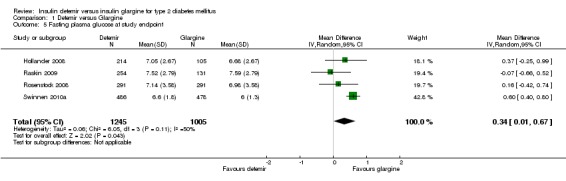
Comparison 1 Detemir versus Glargine, Outcome 5 Fasting plasma glucose at study endpoint.
Analysis 1.6.
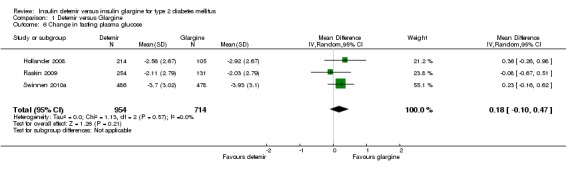
Comparison 1 Detemir versus Glargine, Outcome 6 Change in fasting plasma glucose.
Good glycaemic control with and without hypoglycaemia
The percentage of patients achieving good glycaemic control at study endpoint was similar between the two insulins, with a risk ratio of 0.96 (95% CI 0.81 to 1.14). There was substantial statistical heterogeneity (P = 0.03, I2 = 66%) (Analysis 1.3). When defining good glycaemic control as achieving HbA1c equal to or less than 7% without experiencing hypoglycaemia during the study (Analysis 1.4), there was a not statistically significant difference in favour of insulin glargine, with a risk ratio of 0.87 (95% CI 0.76 to 1.00), without evidence for statistical heterogeneity (P = 0.33, I2 = 13%). Of note, two studies (Hollander 2008; Rosenstock 2008) only included the hypoglycaemic episodes occurring during the last three months of the study.
Analysis 1.3.
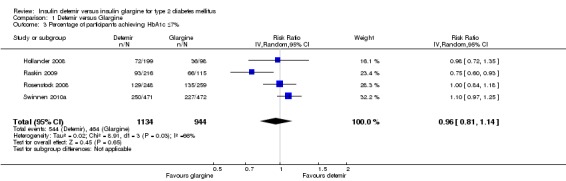
Comparison 1 Detemir versus Glargine, Outcome 3 Percentage of participants achieving HbA1c ≤7%.
Analysis 1.4.
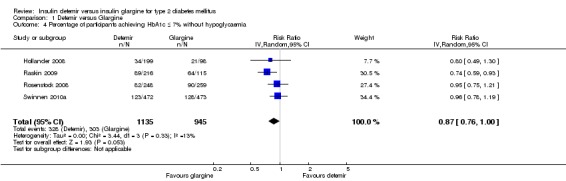
Comparison 1 Detemir versus Glargine, Outcome 4 Percentage of participants achieving HbA1c ≤ 7% without hypoglycaemia.
Hypoglycaemia
After correspondence with the manufacturers of the two insulins we had sufficient data to meta‐analyse the incidence (i.e., the percentage of participants experiencing hypoglycaemia, calculated as risk ratios or relative risks) and the event rate (calculated as rate ratios) of overall, nocturnal and severe hypoglycaemia. For the Raskin 2009 study the standard error of the log rate ratio was imputed as the average of the standard errors of the log rate ratios of the other three studies. As data on daytime hypoglycaemia were available for only two studies (Raskin 2009; Swinnen 2010a) we did not meta‐analyse this outcome.
Overall hypoglycaemia
There was no difference between the two insulins in the relative risk of having at least one hypoglycaemic event (Analysis 1.7): risk ratio of 0.98 (95% CI 0.92 to 1.05), without evidence for statistical heterogeneity (P = 0.42, I2=0%). Similarly, there was no statistically significant difference in the event rate for overall hypoglycaemia (Analysis 1.8): rate ratio of 1.00 (95% CI 0.90 to 1.11), with substantial statistical heterogeneity (P = 0.0006, I2=83%).
Analysis 1.7.
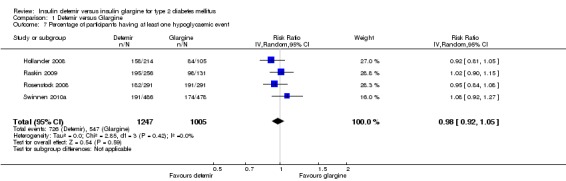
Comparison 1 Detemir versus Glargine, Outcome 7 Percentage of participants having at least one hypoglycaemic event.
Analysis 1.8.
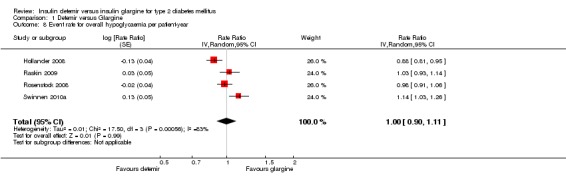
Comparison 1 Detemir versus Glargine, Outcome 8 Event rate for overall hypoglycaemia per patient‐year.
Nocturnal hypoglycaemia
The incidence of nocturnal hypoglycaemia was similar between the two insulins, with a relative risk of having at least one nocturnal hypoglycaemic event (Analysis 1.9) of 1.02 (95% CI 0.90 to 1.16). The test for statistical heterogeneity gave a P value of 0.49 and I2 of 0%. Similarly, there was no statistically significant difference between the two insulins in nocturnal hypoglycaemia event rate (rate ratio of 1.00 [95% CI 0.93 to 1.09] ‐ Analysis 1.10). The test for statistical heterogeneity gave a P value of 0.44 and I2 of 0%.
Analysis 1.9.
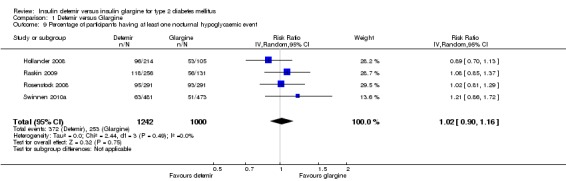
Comparison 1 Detemir versus Glargine, Outcome 9 Percentage of participants having at least one nocturnal hypoglycaemic event.
Analysis 1.10.
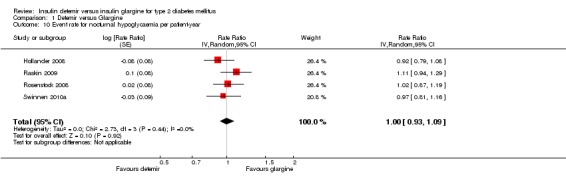
Comparison 1 Detemir versus Glargine, Outcome 10 Event rate for nocturnal hypoglycaemia per patient‐year.
Severe hypoglycaemia
Both relative risk and rate ratio of severe hypoglycaemia were not statistically significantly lower for insulin detemir than for insulin glargine: 0.82 (95% CI 0.51 to 1.32 ‐ Analysis 1.11), the test for statistical heterogeneity gave a P value of 0.94 and I2 of 0% and 0.88 (95% CI 0.59 to 1.30 ‐ Analysis 1.12), the test for statistical heterogeneity gave a P value of 0.84 and I2 of 0%.
Analysis 1.11.
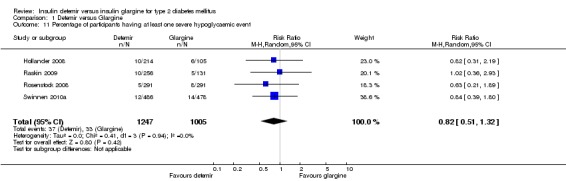
Comparison 1 Detemir versus Glargine, Outcome 11 Percentage of participants having at least one severe hypoglycaemic event.
Analysis 1.12.
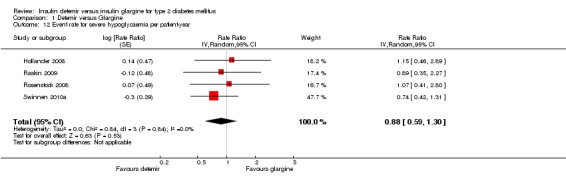
Comparison 1 Detemir versus Glargine, Outcome 12 Event rate for severe hypoglycaemia per patient‐year.
Secondary outcomes
Weight gain
For the Rosenstock 2008 study, the variance in weight gain was calculated using the P value. Based on meta‐analysis of all four studies, insulin detemir was associated with statistically significantly less weight gain than insulin glargine; the mean difference was ‐0.91 kg (95% CI ‐1.21 to ‐0.61), without evidence for statistical heterogeneity (P = 0.50, I2 = 0% ‐ Analysis 1.13).
Analysis 1.13.
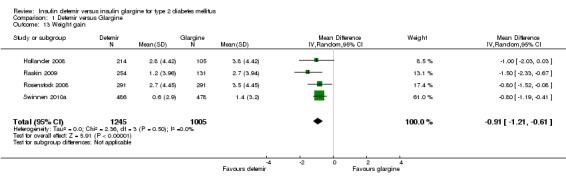
Comparison 1 Detemir versus Glargine, Outcome 13 Weight gain.
Injection site reactions
Statistically significantly more people treated with insulin detemir had injection site reactions as compared with insulin glargine; the risk ratio was estimated to be 3.31 (95% CI 1.13 to 9.73 ‐ Analysis 1.14), with little statistical heterogeneity (P = 0.36, I2 = 6%). Out of the 1247 study participants randomised to insulin detemir 23 patients had an injection site reaction (1.8%), whereas out of the 1005 study participants randomised to insulin glargine 4 patients had an injection site reaction (0.4%).
Analysis 1.14.
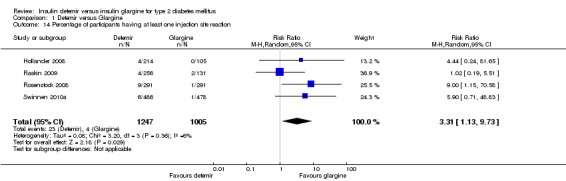
Comparison 1 Detemir versus Glargine, Outcome 14 Percentage of participants having at least one injection site reaction.
Insulin dose
For the Hollander 2008 and Rosenstock 2008 studies the standard errors of the mean insulin doses were imputed as the average of the standard errors of the remaining two studies. The mean difference in daily basal insulin dose was a significant 0.26 U/kg (95% CI 0.11 to 0.41), in favour of insulin glargine (Analysis 1.15). However, there was substantial statistical heterogeneity between studies: P < 0.00001 and I2 = 94%.
Analysis 1.15.
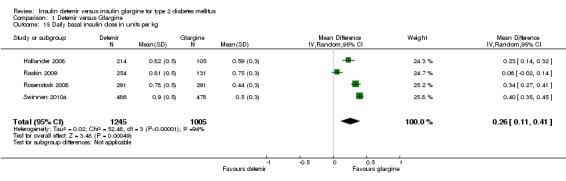
Comparison 1 Detemir versus Glargine, Outcome 15 Daily basal insulin dose in units per kg.
Variability of fasting glucose levels
The variability in fasting plasma glucose profiles was similar for both long‐acting insulin analogues, with a mean difference of ‐0.00 ([95% CI ‐0.03 to 0.02].There was substantial statistical heterogeneity, P = 0.02, I2 = 70% ‐ Analysis 1.16).
Analysis 1.16.
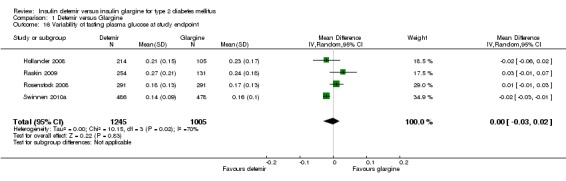
Comparison 1 Detemir versus Glargine, Outcome 16 Variability of fasting plasma glucose at study endpoint.
Variability of glucose values of 24‐hour profiles
There was no difference in variability of the 24‐hour glucose profiles between insulin detemir and insulin glargine with substantial statistical heterogeneity between studies (mean difference of 0.01 [95% CI ‐0.03 to 0.06], P = 0.09, I2 = 59% ‐ Analysis 1.17).
Analysis 1.17.
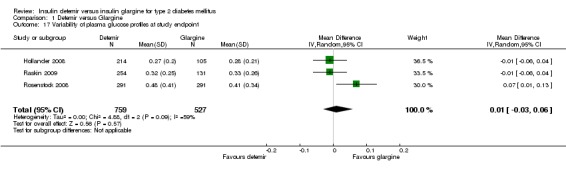
Comparison 1 Detemir versus Glargine, Outcome 17 Variability of plasma glucose profiles at study endpoint.
Health‐related quality of life
Only one trial reported results on health related quality of life (Swinnen 2010a). Swinnen 2010 showed no differences in health related quality of life between treatment groups.
Costs
No study investigated the costs of treatment with a long‐acting insulin analogue.
Mortality
No study was designed or adequately powered to investigate mortality.
Covariates, confounders and effect modifiers
Due to lack of data, we could not assess the influence of the predetermined covariates comparability of the concomitant glucose‐lowering interventions, compliance and co‐morbidity, on the main outcome parameters.
Timing of outcome assessment
The included studies only reported outcomes measured at study endpoint. Thus, the influence of timing of outcome assessment could not be evaluated.
Subgroup analysis and investigation of heterogeneity
We could not perform the two planned subgroup analyses due to lack of data. The robustness of the results was also tested by repeating the analysis using different measures of effects size (relative risk, odds ratio, etc.) and different statistical models (fixed‐effect model and random‐effects model).
The substantial statistical heterogeneity in the pooled effect of endpoint HbA1c, fasting glucose at study endpoint and variability of fasting plasma glucose at study endpoint were caused by the Swinnen 2010a study.
The substantial statistical heterogeneity in the pooled effect of the percentage of participants achieving good glycaemic control without hypoglycaemia was caused by the Raskin 2009 study.
The substantial statistical heterogeneity in the pooled effect of event rate of overall hypoglycaemia was caused by two outlying studies (Hollander 2008; Swinnen 2010a).
There is no explanation for the substantial level of statistical heterogeneity in the pooled effect of variability in daily basal insulin dose in units per kg.
After exclusion of the Swinnen 2010a study, the level of statistical heterogeneity in the pooled effect of endpoint HbA1c level decreased from I2 = 73% to I2 = 24%. The mean difference in endpoint HbA1c level between the two insulin analogues changed from a not statistically significant 0.08% (95% CI ‐0.10 to 0.27) including the Swinnen 2010a study to a statistically significant 0.16% (95% CI 0.01 to 0.32) in favour of insulin glargine, when excluding this study.
Similarly, after exclusion of the Swinnen 2010a study the level of statistical heterogeneity in the pooled effect of fasting glucose at study endpoint decreased from I2 = 50% to I2 = 0%. When excluding the Swinnen 2010 study, the statistically significant difference in endpoint fasting glucose of 0.34 mmol/L (95% CI 0.01 to 0.67) in favour of insulin glargine, was no longer significant (mean difference of 0.15 mmol/L [95% CI ‐0.20 to 0.49]).
Exclusion of the Swinnen 2010a study also decreased the level of statistical heterogeneity in the pooled effect of variability of plasma glucose profiles at study endpoint from I2 = 70% to I2 = 38%. However, excluding the Swinnen 2010a study did not affect the outcome of the pooled effect.
After exclusion of the Raskin 2009 study, the level of statistical heterogeneity in the percentage of participants achieving good glycaemic control without hypoglycaemia, decreased from I2 = 66% to I2 = 0%. However excluding the Raskin 2009 study did not affect the outcome of the pooled effect.
After exclusion of two studies (Hollander 2008; Swinnen 2010a) the level of statistical heterogeneity in the pooled effect of event rate of overall hypoglycaemia decreased from I2 = 83% to I2 = 0% However, we can not report a meta‐analytically pooled effect estimate based on two remaining studies (Raskin 2009;Rosenstock 2008) .
As there is no explanation for the substantial level of statistical heterogeneity in the pooled effect of variability in daily basal insulin dose in units per kg, we cannot report a meta‐analytically pooled effect estimate without statistical heterogeneity.
In conclusion, for two outcome measures, namely HbA1c at study endpoint and fasting plasma glucose at study endpoint, decreasing the level of statistical heterogeneity by excluding studies created a significant difference in the outcome (HbA1c at study endpoint) or eliminated the significant difference in the outcome (fasting plasma glucose at study endpoint) between the two insulin analogues. However, for both outcome measures the significance was of borderline magnitude. In addition, the pooled effect of two related outcome measures, namely change in HbA1c and change in fasting plasma glucose, resulted in a not statistically significant mean difference between the two insulins without statistical heterogeneity. Overall there seems to be no difference in glycaemic control between the two insulins.
For the event rate for overall hypoglycaemia per patient‐year we cannot report a meta‐analytically pooled effect estimate. However, the pooled effect of a related outcome measure, namely the percentage of participants having at least one hypoglycaemic event, showed a non statistically significant difference between the two insulins. In conclusion, there seems to be no statistically significant difference in overall hypoglycaemia when comparing insulin detemir to insulin glargine.
Similarly, for the daily basal insulin dose in units per kg, the meta‐analytically pooled effect estimate is hampered by substantial statistical heterogeneity. However, when taking into account the subtotals, the mean differences of three out of four studies (Hollander 2008; Rosenstock 2008; Swinnen 2010a) are statistically significantly in favour of insulin glargine. Also, there is a pharmacological explanation for a larger insulin need with insulin detemir (Swinnen 2010b).
Sensitivity analysis
We found no unpublished studies, the risk of bias of the included studies was comparable among trials, no study used explicit diagnostic criteria to define diabetes mellitus type 2, and all were industry‐sponsored and published in English‐language journals. Therefore, we could only perform the pre‐specified sensitivity analysis excluding a very large study (Swinnen 2010a). Exclusion of this study resulted in changes of the following outcome measures: endpoint HbA1c level, endpoint fasting glucose level, the percentage of participants achieving good glycaemic control without hypoglycaemia, and the occurrence of injection site reactions. We already addressed the change in mean difference in endpoint HbA1c and fasting glucose at study endpoint between the two insulin analogues after excluding the Swinnen 2010a study (Subgroup analysis and investigation of heterogeneity).
In addition the non statistically significant difference in favour of insulin glargine in the percentage of participants achieving good glycaemic control without hypoglycaemia becomes statistically significant when excluding the Swinnen 2010a study, with a risk ratio of 0.83 (95% CI 0.70 to 0.98), (P = 0.33, I2 = 9%). However, the significance is of borderline magnitude. Finally, after exclusion of the Swinnen 2010a study the difference in the percentage of participants having at least one injection site reaction was no longer statistically significant (estimated risk ratio of 2.94 [95% CI 0.68 to 12.71], P = 0.24, I2 = 30%).
A possible explanation for the change in results after exclusion of the Swinnen 2010a is the distinctive study design of this study in which insulin detemir was initiated twice daily.
Repeating the analyses using different measures of effects size and different statistical models did not change the results materially.
Discussion
Summary of main results
This systematic review and meta‐analysis included four studies comparing the effects of insulin detemir to insulin glargine in patients with type 2 diabetes mellitus. Overall, pooling all four studies resulted in a not statistically significant difference in HbA1c between the two treatment groups, although glargine was associated with a slightly lower fasting plasma glucose.
The results show a not statistically significant difference in overall, nocturnal and severe hypoglycaemia when comparing insulin detemir to insulin glargine.
Insulin detemir was associated with statistically significant relatively small reduction of weight gain. Treatment with insulin glargine resulted in a lower daily basal insulin dose and a lower number of injection site reactions.
There was no difference in the variability of fasting glucose levels and glucose values of 24‐hour profiles between the two treatment groups.
No evidence for differences in patient‐oriented outcomes like health‐related quality of life, costs or mortality could be obtained. It may however well be that the difference in required insulin dose translates in different costs for the two insulins.
Insulin glargine was dosed once‐daily in the evening. Insulin detemir was initiated once‐daily in the evening with the option of an additional dose in the morning in three studies and initiated twice‐daily in the fourth study.
In the three studies, patients randomised to receive insulin detemir could be switched to a second dose given in the morning based on failure to achieve a prespecified pre dinner plasma glucose target. In all probability, a sufficient number of patients randomised to insulin glargine would also have failed to achieve predinner plasma glucose goals and thus would have met the plasma glucose criterion for a switch from once‐ to twice‐daily dosing. This was not done as this would have been off‐label use, although there are reports in literature supporting twice‐daily dosing of glargine (Albright 2004; Ashwell 2006).
The differences between the two basal insulin analogues should be defined on the basis of a similar insulin administration regimen; from a patient perspective this should preferably be once‐daily for both insulins.
Overall completeness and applicability of evidence
Although this meta‐analysis included only four RCTs, almost all outcomes could be investigated using additional information provided by the authors.
The studies were conducted in 25 different countries. Participants included both male and female adults with a mean age of 55 years, hence the external validity of the results seems high.
Quality of the evidence
All four RCTs used appropriate randomisation methods and were adequately powered. In total this review is based on the data of 2250 study participants. Attrition rates were acceptable and most of the pre‐specified outcomes have been reported. However, the risk of bias of the included studies was considered as high. The main limitations were lack of blinding. This source of bias should be considered when interpreting the results of the various meta‐analyses in this review.
Potential biases in the review process
We minimised the risk of publication bias by performing an extensive search of both electronic sources and grey literature. In addition, all 1615 citations identified by our electronic search strategies were assessed independently by two authors.
Agreements and disagreements with other studies or reviews
There are no other systematic reviews comparing insulin detemir with insulin glargine in type 2 diabetes patients.
Authors' conclusions
Our analyses suggest that there is no clinically relevant difference in the efficacy or the safety between insulin detemir and insulin glargine for targeting hyperglycaemia. However, to achieve the same glycaemic control insulin detemir was often injected twice‐daily in a higher dose but with less weight gain, while insulin glargine was only injected once‐daily, with somewhat fewer injection site reactions.
RCTs comparing insulin detemir and insulin glargine using the treat‐to‐target approach and an identical, preferably once‐daily, dosing regimen are needed. Equal dosing frequencies also abolish the need for an open‐label study design. In addition, outcome measures should include health‐related quality of life, treatment satisfaction and costs. Finally, studies with a duration longer than one year are desirable.
Acknowledgements
We would like to thank Christoph Koenen from Novo Nordisk and Marie‐Paule Dain from Sanofi‐Aventis for providing additional information on the trials sponsored by these companies. We thank Karla Bergerhoff, Trials Search Coordinator of the Cochrane Metabolic and Endocrine Disorders Group, for developing and conducting the electronic search strategies. We thank Koos Zwinderman, head of the department of clinical epidemiology and biostatistics in the Academical Medical Centre, for providing statistical assistance.
Appendices
Appendix 1. Search strategies
| Search terms |
| Unless otherwise stated, search terms are free text terms; ab = abstract; adj = adjacent; exp = exploded MeSH; MeSH = medical subject heading (Medline medical index term); ot = original title; pt = publication type; sh = MeSH; tw = text word; ti = title; the dollar sign ($) stands for any character(s); the question mark (?) = to substitute for one or no characters. EMBASE: 1 exp long acting insulin/ 2 ((long‐acting or longacting) adj6 insulin*).tw,ot. 3 exp insulin detemir/ 4 (detemir or levemir).tw,ot. 5 exp insulin glargine/ 6 (glargin* or lantus).tw,ot. 7 or/1‐6 8 exp Diabetes Mellitus, Type 2/ 9 exp Diabetes Complications/ 10 (MODY or NIDDM or TDM2).ab,ti,ot. 11 (non insulin$ depend$ or noninsulin depend$ or noninsulin?depend$ or noninsulin?depend$).ab,ti,ot. 12 ((typ$ 2 or typ$ II) adj3 diabet$).ab,ti,ot. 13 ((keto?resist$ or non?keto$) adj6 diabet$).ab,ti,ot. 14 (((late or adult$ or matur$ or slow or stabl$) adj3 onset) and diabet$).ab,ti. 15 or/8‐14 16 exp Diabetes Insipidus/ 17 diabet$ insipidus.ab,ti,ot. 18 16 or 17 19 15 not 18 20 7 and 19 21 exp Randomized Controlled Trial/ 22 exp Controlled Clinical Trial/ 23 exp Clinical Trial/ 24 exp Comparative Study/ 25 exp Drug comparison/ 26 exp Randomization/ 27 exp Crossover procedure/ 28 exp Double blind procedure/ 29 exp Single blind procedure/ 30 exp Placebo/ 31 exp Prospective Study/ 32 ((clinical or control$ or comparativ$ or placebo$ or prospectiv$ or randomi?ed) adj3 (trial$ or stud$)).ab,ti. 33 (random$ adj6 (allocat$ or assign$ or basis or order$)).ab,ti. 34 ((singl$ or doubl$ or trebl$ or tripl$) adj6 (blind$ or mask$)).ab,ti. 35 (cross over or crossover).ab,ti. 36 or/21‐35 37 exp meta analysis/ 38 (metaanaly$ or meta analy$ or meta?analy$).ab,ti,ot. 39 ((review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinhal or psychinfo or psychlit or healthstar or biosis or current content$ or systematic$)).ab,ti,ot. 40 exp Literature/ 41 exp Biomedical Technology Assessment/ 42 hta.tw,ot. 43 (health technology adj6 assessment$).tw,ot. 44 or/37‐43 45 (comment or editorial or historical‐article).pt. 46 44 not 45 47 36 or 46 48 20 and 47 49 48 not 45 Medline: 1 exp Insulin, Long‐Acting/ 2 ((longacting or long‐acting) adj6 insulin*).tw,ot. 3 (detemir or levemir).tw,ot. 4 (glargin* or lantus).tw,ot. 5 or/1‐4 6 exp Diabetes Mellitus, Type 2/ 7 exp Diabetes Complications/ 8 (MODY or NIDDM or T2DM).tw,ot. 9 (non insulin$ depend$ or noninsulin$ depend$ or noninsulin?depend$ or noninsulin?depend).tw,ot. 10 ((typ$ 2 or typ$ II) adj3 diabet$).tw,ot. 11 ((keto?resist$ or non?keto$) adj6 diabet$).tw,ot. 12 (((late or adult$ or matur$ or slow or stabl$) adj3 onset) and diabet$).ab,ti. 13 or/6‐12 14 exp Diabetes Insipidus/ 15 diabet$ insipidus.tw,ot. 16 14 or 15 17 13 not 16 18 randomized controlled trial.pt. 19 controlled clinical trial.pt. 20 randomi?ed.ab,ti. 21 placebo$.ab,ti. 22 drug therapy.fs. 23 randomly.ab,ti. 24 trial$.ab,ti. 25 group$.ab,ti. 26 or/18‐25 27 Meta‐analysis.pt. 28 exp Review/ 29 exp Meta‐analysis/ 30 systematic review$.tw. 31 search$.tw. 32 medline.tw. 33 cochrane database of systematic reviews.jn. 34 or/27‐33 35 letter.pt. 36 comment.pt.37 editorial.pt. 38 historical‐article.pt. 39 or/35‐38 40 34 not 39 41 exp Technology Assessment, Biomedical/ 42 HTA.tw. 43 (health technology adj6 assessment$).tw. 44 (biomedical adj6 technology assessment$).tw. 45 or/41‐44 46 26 or 34 or 45 47 46 not 39 48 5 and 17 and 47 The Cochrane Library: #1 MeSH descriptor Insulin, Long‐Acting explode all trees #2 ((long‐acting or longacting) near6 insulin*) #3 (detemir or levemir) #4 (glargin* or lantus) #5 (#1 OR #2 OR #3 OR #4) #6 MeSH descriptor Diabetes Mellitus, Type 2 explode all trees #7 MeSH descriptor Diabetes Complications explode all trees #8 (MODY or NIDDM or T2DM) #9 (non insulin* depend* or noninsulin* depend* or non insulin depend* or noninsulindepend*) #10 ((ketoresist* or keto resist* or nonketo* or non keto*) near6 diabet*) #11 ((typ* 2 or typ* II) adj3 diabet*) #12 (((late or adult* or matur* or slow or stabl*) near3 onset) and diabet*) #13 (#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12) #14 #5 and #13 |
Appendix 2. Adverse events
| Characteristic | study ID1 | study ID2 | study ID3 | study ID4 |
| Insulin detemir = Intervention (I) Insulin glargine = Control (C) |
Hollander 2008 | Raskin 2009 | Rosenstock 2008 | Swinnen 2010 |
| Participants who died [n] | I: 1 C: 1 Total: 2 |
I: ‐ C: ‐ Total: ‐ |
I: 1 C: 1 Total: 2 |
I: 0 C: 0 Total: 0 |
| Adverse events [n] | I: 185 patients (743 events) C: 88 patients (377 events) Total: 273 patients (1120 events) |
I: 169 patients (605 events) C: 93 patients (273 events) Total: 262 patients (878 events) |
I: ‐ C: ‐ Total: ‐ |
I: 256 patients (number of events not reported) C: 264 patients (number of events not reported) Total: 520 patients (number of events not reported) |
| Adverse events [%] | I: 86.4% of n=214 C: 83.8% of n=105 Total: 85.6% of n=319 |
I: 66.0% of n=256 C: 71.0% of n=131 Total: 67.7% of n=387 |
I: ‐ C: ‐ Total: ‐ |
I: 52.7% of n=486 C: 55.2% of n=478 Total: 53.9% of n=964 |
| Serious adverse events [n] | I: 31 patients (31 events) C:14 patients (number of events not reported) Total: 45 patients (number of events not reported) |
I: 23 patients (27 events) C: 5 patients (8 events) Total: 28 patients (35 events) |
I: 42 patients (47 events) C: 53 patients (73 events) Total: 95 patients (120 events) |
I: 18 patients (19 events) C: 21 patients (number of events not reported) Total: 39 patients (number of events not reported) |
| Serious adverse events [%] | I: 14.5% of n=214 C: 13.3% of n=105 Total: 14.1% of n=319 |
I: 9.0% of n=256 C: 3.8% of n=131 Total: 7.2% of n=387 |
I: 14.4% of n= 291 C: 18.2% of n= 291 Total: 16.3% of n=582 |
I: 3.7% of n=486 C: 4.4% of n=478 Total: 4.0% of n=964 |
| Drop‐outs due to adverse events [n] | I: 12 patients (13 events) C: 3 patients (4 events) Total: 15 patients (17 events) |
I:10 patients (number of events not reported) C: 3 patients (number of events not reported) Total: 13 patients (number of events not reported) |
I: 23 patients (27 events) C: 11 patients (14 events) Total: 34 patients (41 events) |
I: 22 patients (23 events) C: 7 patients (8 events) Total: 29 patients (31 events) |
| Drop‐outs due to adverse events [%] | I: 5.6% of n=214 C: 2.9% of n=105 Total: 4.7% of n=319 |
I: 3.9% of n=256 C: 2.3% of n=131 Total: 3.4% of n=387 |
I: 7.9% of n=291 C: 3.8% of n= 291 Total: 5.8% of n=582 |
I: 4.5% of n=486 C: 1.5% of n=478 Total: 3.0% of n=964 |
| Hospitalisation [n] | I1: ‐ C1: ‐ Total: ‐ |
I1: ‐ C1: ‐ Total: ‐ |
I1: ‐ C1: ‐ Total: ‐ |
I1: ‐ C1: ‐ Total: ‐ |
| Hospitalisation [%] | I: ‐ C: ‐ Total: ‐ |
I: ‐ C: ‐ Total: ‐ |
I: ‐ C: ‐ Total: ‐ |
I: ‐ C: ‐ Total: ‐ |
| Out‐patient treatment [n] | I1: ‐ C1: ‐ Total: ‐ |
I1: ‐ C1: ‐ Total: ‐ |
I1: ‐ C1: ‐ Total: ‐ |
I1: ‐ C1: ‐ Total: ‐ |
| Out‐patient treatment [%] | I: ‐ C: ‐ Total: ‐ |
I: ‐ C: ‐ Total: ‐ |
I: ‐ C: ‐ Total: ‐ |
I: ‐ C: ‐ Total: ‐ |
| Hypoglycaemic episodes [n] | I: 119 patients (822 episodes) C: 68 patients (502 episodes) Total: 187 (1324 episodes) |
I: ‐ C: ‐ Total: ‐ |
I: 135 patients (737 episodes) C: 151 patients (786 episodes) Total: 286 patients (1523 episodes) |
I: ‐ C: ‐ Total: ‐ |
| Hypoglycaemic episodes [%] | I1: 55.6% of n=214 C1: 64.8% of n=105 Total: 58.6% of n=319 |
I1: ‐ C1: ‐ Total: ‐ |
I1: 46.4% of n=291 C1: 51.9% of n= 291 Total: 49.1% of n=582 |
I1: ‐ C1: ‐ Total: ‐ |
| Severe hypoglycaemic episodes [n] | I: 10 patients (17 episodes) C: 6 patients (6 episodes) Total: 16 patients (23 episodes) |
I: 10 patients (number of episodes not reported) C: 5 patients (number of episodes not reported) Total: 15 patients (number of episodes not reported) |
I: 5 patients (9 episodes) C: 8 patients (8 episodes) Total: 13 patients (17 episodes) |
I: 12 patients (number of episodes not reported) C: 14 patients (number of episodes not reported) Total: 26 patients (number of episodes not reported) |
| Severe hypoglycaemic episodes [%] | I: 4.7% of n=214 C: 5.7% of n=105 Total: 5.0% of n=319 |
I: 3.9% of n=256 C: 3.8% of n=131 Total: 3.9% of n=387 |
I: 1.7% of n=291 C: 2.7% of n=291 Total: 2.2% of n=582 |
I: 2.5% of n=486 C: 2.9% of n=478 Total: 2.7% of n=964 |
| Definition of severe hypoglycaemia | "When patients were not able to treat the episode themselves" | ‐ | "If assistance from another person was required" | "An event with symptoms consistent with hypoglycaemia, requiring the assistance of another person, and associated with measured PG < 2.0 mmol/L or recovery after carbohydrate or glucagon administration" |
| Nocturnal hypoglycaemic episodes [n] | I: 66 patients (229 episodes) C: 40 patients (124 episodes) Total: 106 patients (353 episodes) |
I: ‐ C: ‐ Total: ‐ |
I: 73 patients (212 episodes) C: 71 patients (192 episodes) Total: 144 patients (404 episodes) |
I: ‐ C: ‐ Total: ‐ |
| Nocturnal hypoglycaemic episodes [%] | I: 30.8% of n=214 C: 38.1% of n=105 Total: 33.2% of n=319 |
I: ‐ C: ‐ Total: ‐ |
I1: 25.1% of n=291 C1: 24.4% of n=291 Total: 24.7% of n=582 |
I1: ‐ C1: ‐ Total: ‐ |
| Symptoms [n] | I1: 130 patients (888 episodes) C1: 68 patients (639 episodes) Total: 198 |
I1: ‐ C1: ‐ Total: ‐ |
I: 137 patients (760 episodes) C: 133 patients (866 episodes Total: 270 patients (1626 episodes) |
I: ‐ C: ‐ Total: ‐ |
| Symptoms [%] | I: 60.7% of n=214 C: 64.8% of n=105 Total: 62.1% of n=319 |
I: ‐ C: ‐ Total: ‐ |
I: 47.1% of n=291 C: 45.7% of n=291 Total: 46.4% of n=582 |
I: ‐ C: ‐ Total: ‐ |
|
Footnotes ‐ denotes 'not reported' | ||||
Data and analyses
Comparison 1.
Detemir versus Glargine
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HbA1c at study endpoint | 4 | 2250 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.10, 0.27] |
| 2 Change in HbA1c | 4 | 2250 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.10, 0.24] |
| 3 Percentage of participants achieving HbA1c ≤7% | 4 | 2078 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.81, 1.14] |
| 4 Percentage of participants achieving HbA1c ≤ 7% without hypoglycaemia | 4 | 2080 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.76, 1.00] |
| 5 Fasting plasma glucose at study endpoint | 4 | 2250 | Mean Difference (IV, Random, 95% CI) | 0.34 [0.01, 0.67] |
| 6 Change in fasting plasma glucose | 3 | 1668 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.10, 0.47] |
| 7 Percentage of participants having at least one hypoglycaemic event | 4 | 2252 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 8 Event rate for overall hypoglycaemia per patient‐year | 4 | Rate Ratio (Random, 95% CI) | 1.00 [0.90, 1.11] | |
| 9 Percentage of participants having at least one nocturnal hypoglycaemic event | 4 | 2242 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.90, 1.16] |
| 10 Event rate for nocturnal hypoglycaemia per patient‐year | 4 | Rate Ratio (Random, 95% CI) | 1.00 [0.93, 1.09] | |
| 11 Percentage of participants having at least one severe hypoglycaemic event | 4 | 2252 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.51, 1.32] |
| 12 Event rate for severe hypoglycaemia per patient‐year | 4 | Rate Ratio (Random, 95% CI) | 0.88 [0.59, 1.30] | |
| 13 Weight gain | 4 | 2250 | Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.21, ‐0.61] |
| 14 Percentage of participants having at least one injection site reaction | 4 | 2252 | Risk Ratio (M‐H, Random, 95% CI) | 3.31 [1.13, 9.73] |
| 15 Daily basal insulin dose in units per kg | 4 | 2250 | Mean Difference (IV, Random, 95% CI) | 0.26 [0.11, 0.41] |
| 16 Variability of fasting plasma glucose at study endpoint | 4 | 2250 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.03, 0.02] |
| 17 Variability of plasma glucose profiles at study endpoint | 3 | 1286 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.03, 0.06] |
Differences between protocol and review
Airin Simon replaced Sanne Swinnen as a contact person.
Airin Simon was added as an author.
We did not assess long‐term outcome measurements as none of the included RCTs had a duration of more than 52 weeks. Since the two 52‐week studies did not report interim data we selected the longest follow‐up from each trial (24, 26 and 52 weeks) and used study endpoint data only.
We did not quantify interrater agreement for risk of bias assessments by means of the kappa statistic, as this is no longer routinely done in Cochrane reviews. Also, the two authors who independently assessed the risk of bias of the included RCTs differed in opinion about only one item of one study.
Dichotomous data were expressed as risk ratios rather than odds ratios because risks and risk ratios are less difficult to interpret than odds and odds ratios. Continuous data are expressed as mean differences as the historically used term weighted mean difference (WMD) is no longer used in the Cochrane Database of Systematic Reviews.
We did not perform the planned subgroup analyses because the studies did not report data on separate patient groups (such as males and females) and we did not have individual patient data.
The number of pre‐specified sensitivity analyses that we could perform was very limited as we did not identify any unpublished relevant study, the risk of bias of the included studies was comparable, no study used diagnostic criteria, all were sponsored by a pharmaceutical company and all reports were in English.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RANDOMISED CONTROLLED CLINICAL TRIAL (RCT): Yes. RANDOMISATION RATIO: 2:1. NON‐INFERIORITY DESIGN: Yes. EQUIVALENCE DESIGN: No. PARALLEL / CROSSOVER / FACTORIAL RCT: Parallel. CONTROLLED CLINICAL TRIAL (CCT): Yes. |
|
| Participants | WHO PARTCIPATED: SEX (female% / male%): 34 (42.0%) / 185 (58.0%). AGE (mean years (SD)): Insulin detemir: 59 (11.0); Insulin glargine: 58 (11.0). Novo Nordisk A/S web site: All: 58 (11.0). ETHNIC GROUPS (%): 250 (78.4%) white, 38 (11.9%) black, 26 (8.2%) Hispanic, 5 (1.5%) other. DURATION OF DISEASE (mean years (SD)): Insulin detemir: 13.6 (8.1); Insulin glargine: 13.4 (7.8). Novo Nordisk A/S web site: All: 13.6 (8.0). INCLUSION CRITERIA: Men and women aged ≥ 18 years who had a diagnosis of type 2 diabetes for ≥ 12 months, BMI ≤ 40.0 kg/m2, HbA1c of ≥ 7.0% to ≤ 11.0% at screening and had been receiving any OGLD regimen or insulin with or without OGLDs for > 4 months. EXCLUSION CRITERIA: Not reported in Clinical Therapeutics paper. Novo Nordisk A/S web site: Proliferative retinopathy or maculopathy, recurrent major hypoglycaemia, impaired hepatic or renal function, cardiac problems or uncontrolled hypertension. DIAGNOSTIC CRITERIA: Not specified. CO‐MORBIDITIES: Not reported. CO‐MEDICATIONS: Insulin detemir: 42 (19.6%) OGLDs only, 79 (36.9%) insulin only, 93 (43.5%) insulin + OGLDs; Insulin glargine: 17 (16.2%) OGLDs only, 34 (32.4%) insulin only, 54 (51.4%) insulin + OGLDs; All: 59 (18.5%) OGLDs only, 113 (35.4%) insulin only, 147 (46.1%) insulin + OGLDs. |
|
| Interventions | NUMBER OF STUDY CENTRES: 56. COUNTRY/ LOCATION: Europe (Finland, France, Norway, Sweden) and United States. SETTING: Not reported. INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Insulin detemir: Initiated once‐daily in the evening, titrated according to structured algorithm to FPG ≤ 6.0 mmol/L and (if needed) to pre‐dinner PG ≤ 6.0 mmol/L without hypoglycaemia, additional morning insulin dose if titration of evening dose did not result in pre‐dinner PG ≤ 6.0 mmol/L, but only if FPG ≤ 6.0 mmol/L. Pen‐injector (Flexpen), between 1 hour before dinner and bedtime. Insulin aspart injected immediately before each main meal (Flexpen), initiated and adjusted according to local practice to achieve 2‐hour post‐prandial PG ≤ 9.0 mmol/L, no algorithm for bolus insulin. CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Insulin glargine: Initiated once‐daily in the evening, titrated according to structured algorithm to FPG ≤ 6.0 mmol/L without hypoglycaemia. Pen‐injector (OptiPen Pro 1 in Europa and syringes and vials in United States), between 1 hour before dinner and bedtime. Insulin aspart injected immediately before each main meal (Flexpen), initiated and adjusted according to local practice to achieve 2‐hour post‐prandial PG ≤ 9.0 mmol/L, no algorithm for bolus insulin. TREATMENT BEFORE STUDY: Insulin secretagogues and α‐glucosidase inhibitors were stopped at study entry, thiazolidinediones were also stopped in Europe, other existing OGLD regimens were continued. TITRATION PERIOD: 52 weeks, 13 scheduled visits, occurring biweekly initially, then every 4 weeks, and eventually every 8 weeks, and 16 scheduled telephone contacts. Patients were instructed to monitor FPG and pre‐dinner PG on the 3 days before each of 13 visits. Titration algorithm is given. |
|
| Outcomes | PRIMARY OUTCOME(S) (as stated in the publication): HbA1c at 52 weeks. SECONDARY OUTCOMES (as stated in the publication): Change in weight, proportion of patients achieving HbA1c ≤ 7.0% at 52 weeks, proportion of patients achieving HbA1c ≤ 7.0% with or without symptomatic hypoglycaemia confirmed by PG < 4.0 mmol/L or any PG < 3.1 mmol/L in the last 3 months of treatment, glycaemic control at 52 weeks based on FPG and 10‐point PG profiles, insulin doses, and within‐subject variation in self‐measured FPG and pre‐dinner PG. Major hypoglycaemia = if patients were not able to treat the episode themselves; Minor hypoglycaemia = confirmed by PG < 3.1 mmol/L; Symptoms only = if PG ≥ 3.1 mmol/L or no measurement was made; All = all episodes occurring over 24 hours; Nocturnal = between 11 p.m. and 6 a.m. ADDITIONAL OUTCOMES: Novo Nordisk A/S web site: Adverse events, body weight, hypoglycaemia, PG, basal and bolus insulin doses, insulin treatment satisfaction (ITSQ), laboratory safety parameters, physical examination, vital signs, funduscopy, proportion of patients on once‐daily basal insulin in insulin detemir group, time to change from once‐ to twice‐daily in insulin detemir group, correlation between endogenous insulin and C‐peptide and insulin requirements in insulin‐naive subjects and serum adiponectin levels. |
|
| Study details | DURATION OF INTERVENTION: 52 weeks. DURATION OF FOLLOW‐UP: 52 weeks. RUN‐IN PERIOD: Not specified, but Novo Nordisk A/S web site: Randomisation visit maximum 2 weeks after screening visit. |
|
| Publication details | LANGUAGE OF PUBLICATION: English. COMMERCIAL FUNDING: Yes: "This study was supported by Novo Nordisk A/S, Bagsvaerd, Denmark". NON‐COMMERCIAL FUNDING: No. PUBLICATION STATUS (PEER REVIEW JOURNAL): Yes. PUBLICATION STATUS (JOURNAL SUPPLEMENT): No. PUBLICATION STATUS (ABSTRACT): No. |
|
| Stated aim of study | Quote: "This trial compared the efficacy and safety profiles of detemir and glargine as the basal insulin component of a basal‐bolus regimen in patients with type 2 diabetes who were being treated with OGLDs or insulin, with or without OGLDs". | |
| Notes | Trialnumbers: NN304‐1431 and NCT00097084. Contact information: Priscilla Hollander (PriscilH@baylorhealth.edu). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A telephone randomisation system prepared by Clinical Trial Supplies, Novo Nordisk A/S, Bagsvaerd, Denmark, was used to randomise patients". Comment: Probably done, as the "telephone randomisation system" probably used a computer random number generator. |
| Allocation concealment (selection bias) | Low risk | Quote: "A telephone randomisation system prepared by Clinical Trial Supplies, Novo Nordisk A/S, Bagsvaerd, Denmark, was used to randomise patients". Comment: Done, as central allocation (by means of a telephone system) was used. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "It is possible that the open‐label design of this trial, although necessary given the different dosing schemes for the two insulins, could have introduced some bias". Comment: Neither study participants nor study personnel were blinded and this may have influenced outcome measurements. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of missing data or number of patients used for the calculation of outcomes are not reported. |
| Selective reporting (reporting bias) | Low risk | Probably, as all of the study's pre‐specified outcomes (as listed on www.ClinicalTrials.gov) are reported (except for insulin treatment satisfaction, which was a pre‐specified secondary outcome). |
| Other bias | High risk | Quote: "Patients who had been randomised to receive detemir were to be transferred to twice‐daily dosing if..." and "Irrespective of pre‐dinner PG levels, patients randomised to the glargine group continued to receive a once‐daily evening dose, according to the approved labelling". Also, all patients randomised to insulin detemir used a pen‐injector, whereas United States patients randomised to insulin glargine used syringes and vials. Comment: The study has a potential source of bias related to the specific study design used, i.e. different frequency of injection and different injection devices across treatments. |
| Methods | RANDOMISED CONTROLLED CLINICAL TRIAL (RCT): Yes. RANDOMISATION RATIO: 2:1. NON‐INFERIORITY DESIGN: Yes. EQUIVALENCE DESIGN: No. PARALLEL / CROSSOVER / FACTORIAL RCT: Parallel. CONTROLLED CLINICAL TRIAL (CCT): Yes. |
|
| Participants | WHO PARTCIPATED: SEX (female% / male%): 175 (45.5%) / 210 (54.5%). AGE (mean years (SD)): Insulin det: 55.8 (10.0); Insulin glargine: 55.9 (11.0); All: 55.8 (10.3). ETHNIC GROUPS (%): Insulin detemir: 193 (76.0%) Caucasian, 37 (14.6%) African‐American, 7 (2.8%) Asian, 4 (1.6%) American Indian or Alaskan Native, 13 (5.1%) other; Insulin glargine: 108 (82.4%) Caucasian, 14 (10.7%) African‐American, 3 (2.3%) Asian, 0 (0%) American Indian or Alaskan Native, 6 (4.6%) other; All: 301 (78.2%) Caucasian, 51 (13.2%) African‐American, 10 (2.6%) Asian, 4 (1.0%) American Indian or Alaskan Native, 19 (4.9%) other. DURATION OF DISEASE (mean years (SD)): Insulin detemir: 12.5 (6.8); Insulin glargine: 11.9 (7.4); All: 12.3 (7.0). INCLUSION CRITERIA: Patients with type 2 diabetes, who were at least 18 years old, with a BMI of ≤ 40 kg/m2, an HbA1c ranging from 7% to 11% and who had previously received any OGLD, insulin, or insulin + OGLD treatment regimens. Novo Nordisk A/S web site: Type 2 diabetes ≥ 12 months, currently on any OGLD therapy or on any insulin in any regimen with or without OGLDs ≥ 4 months, age ≥ 18 years, BMI ≤ 40 kg/m2 and HbA1c ≥ 7.0% and ≤ 11.0%. EXCLUSION CRITERIA: Proliferative retinopathy or maculopathy that required acute treatment 6 months before the start of the study, recurrent major hypoglycaemia, anticipated change in medication known to interfere with glucose metabolism, impaired hepatic or renal function believed to interfere with study participation, cardiac problems or uncontrolled hypertension. DIAGNOSTIC CRITERIA: Not specified. CO‐MORBIDITIES: Not reported. CO‐MEDICATIONS: Insulin detemir: 9 (3.5%) OGLD monotherapy, 42 (16.5%) OGLD combination therapy, 83 (32.7%) insulin without OGLDs, 120 (47.2%) insulin with OGLDs; Insulin glargine: 6 (4.6%) OGLD monotherapy, 20 (15.3%) OGLD combination therapy, 41 (31.3%) insulin without OGLDs, 64 (48.9%) insulin with OGLDs; All: 15 (3.9%) OGLD monotherapy, 62 (16.1%) OGLD combination therapy, 124 (32.2%) insulin without OGLDs, 184 (47.8%) insulin with OGLDs. |
|
| Interventions | NUMBER OF STUDY CENTRES: 68. COUNTRY/ LOCATION: Canada (8 sites) and United States (60 sites). SETTING: Not reported. INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Insulin detemir: Initiated once‐daily in the evening at the same time each day, titrated according to structured algorithm to FPG ≤ 6.0 mmol/L and (if needed) to pre‐dinner PG ≤ 6.0 mmol/L without significant hypoglycaemia, additional morning insulin dose if titration of evening dose did not result in pre‐dinner PG ≤ 6.0 mmol/L, but only if FPG ≤ 6.0 mmol/L and after optimisation of bolus doses. Pen‐injector (Flexpen), between 1 hour before dinner and bedtime. Insulin aspart injected in abdomen (Flexpen). CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Insulin glargine: Initiated once‐daily in the evening at the same time each day, titrated according to structured algorithm to FPG ≤ 6.0 mmol/L without significant hypoglycaemia. Syringes and vials, between 1 hour before dinner and bedtime. Insulin aspart injected in abdomen (Flexpen). TREATMENT BEFORE STUDY: Insulin secretagogues and α‐glucosidase inhibitors were discontinued prior to initiating trial drug, thiazolidinediones and metformin were continued unchanged. TITRATION PERIOD: 26 weeks, 9 visits and 13 telephone contacts. "Basal insulin was titrated at the instruction of the investigator according to titration guidelines". Titration algorithm is given but the titration frequency is not reported. |
|
| Outcomes | PRIMARY OUTCOME(S) (as stated in the publication): HbA1c after 26 weeks. SECONDARY OUTCOMES (as stated in the publication): FPG during the trial and body weight. Safety endpoints were the incidence of hypoglycaemic events and adverse events. Nocturnal hypoglycaemia = between 23:00 and 06:00 hour; Daytime hypoglycaemia = between 06:00 and 23:00 hour. ADDITIONAL OUTCOMES: Novo Nordisk A/S web site: Proportion of subjects achieving HbA1c ≤ 7% after 26 weeks, proportion of subjects with HbA1c ≤ 7% without symptomatic hypoglycaemia confirmed by PG < 4.0 mmol/L or any single PG < 3.1 mmol/L in the last 3 months of the treatment, FPG, within‐subject variation of self‐measured FPG and pre‐dinner PG, 9‐point PG profiles, body weight, difference in weight change, basal and bolus insulin doses, time to start detemir twice‐daily, proportion of detemir‐treated subjects on once‐daily basal insulin, insulin treatment satisfaction (ITSQ), incidence of hypoglycaemia (all, minor, major, symptoms only, nocturnal and during the day), adverse events, laboratory assessments, physical examination, fundoscopy, vital signs and ECG. |
|
| Study details | DURATION OF INTERVENTION: 26 weeks. DURATION OF FOLLOW‐UP: 26 weeks. RUN‐IN PERIOD: Not specified. |
|
| Publication details | LANGUAGE OF PUBLICATION: English. COMMERCIAL FUNDING: Yes: "This trial was funded and monitored by Novo Nordisk". NON‐COMMERCIAL FUNDING: No. PUBLICATION STATUS (PEER REVIEW JOURNAL): Yes. PUBLICATION STATUS (JOURNAL SUPPLEMENT): No. PUBLICATION STATUS (ABSTRACT):No. |
|
| Stated aim of study | Quote: "The current study compared the efficacy and safety of the basal insulin insulin detemir and insulin glargine used in combination with insulin aspart as bolus insulin in patients with type 2 diabetes". | |
| Notes | Trialnumbers: NN304‐2175 and NCT00106366. Contact information: Philip Raskin (Philip.Raskin@UTSouthwestern.edu). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomised 2:1 to insulin detemir and insulin glargine treatment groups". Comment: Besides this quote the paper gives no information about the randomisation process. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "were randomised 2:1 to insulin detemir and insulin glargine treatment groups". Comment: Besides this quote the paper gives no information about the randomisation process. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "open‐label". Comment: Neither study participants nor study personnel were blinded and this may have influenced outcome measurements. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of missing data or number of patients used for the calculation of outcomes are not reported. |
| Selective reporting (reporting bias) | High risk | Not all of the study's pre‐specified secondary outcomes as listed on the Novo Nordisk A/S web site are reported (time to start insulin detemir twice‐daily, insulin treatment satisfaction, and hypoglycaemia with symptoms only are not reported). |
| Other bias | High risk | Quote: "For patients randomised to insulin detemir, an optional second daily morning dose could be added at the discretion of the investigator". Also, patients randomised to insulin detemir used a pen‐injector, whereas patients randomised to insulin glargine used syringes and vials. Comment: The study has a potential source of bias related to the specific study design used, i.e. different frequency of injection and different injection devices across treatments. |
| Methods | RANDOMISED CONTROLLED CLINICAL TRIAL (RCT): Yes. RANDOMISATION RATIO: 1:1. NON‐INFERIORITY DESIGN: Yes. EQUIVALENCE DESIGN: No. PARALLEL / CROSSOVER / FACTORIAL RCT: Parallel. CONTROLLED CLINICAL TRIAL (CCT): Yes. |
|
| Participants | WHO PARTCIPATED: SEX (female% / male%): 245 (42.1%) / 337 (57.9%). AGE (mean years (SD)): Insulin detemir: 58.4 (10.2); Insulin glargine: 59.4 (9.6). ETHNIC GROUPS (%): Insulin detemir: 22 (7.6%) black, 250 (85.9%) white, 7 (2.4%) Asian‐Pacific islander, 12 (4.1%) other; Insulin glargine: 12 (4.1%) black, 263 (90.4%) white, 7 (2.4%) Asian‐Pacific islander, 9 (3.1%) other. DURATION OF DISEASE (mean years (SD)): Insulin detemir: 9.1 (6.1); Insulin glargine: 9.1 (6.4). INCLUSION CRITERIA: Insulin‐naive men and women with type 2 diabetes, ≥ 18 years old, ≥ 12 months disease duration, BMI ≤ 40.0 kg/m2, HbA1c between 7.5% and 10.0% and 1 or 2 OGLDs (metformin, insulin secretagogues, α‐glucosidase inhibitors) for ≥ 4 months on at least half the maximum recommended dose. EXCLUSION CRITERIA: Treatment with thiazolidinediones, use of > 2 OGLDs within 6 months, hypoglycaemic unawareness or other medical conditions likely to interfere with trial conduct. Novo Nordisk A/S web site: Current or previous treatment with thiazolidinediones within last 6 months, OGLD treatment with combination of ≥ 3 OGLDs within last 6 months, proliferative retinopathy or maculopathy or known hypoglycaemia unawareness. DIAGNOSTIC CRITERIA: Not specified. CO‐MORBIDITIES: Not reported. CO‐MEDICATIONS: Insulin detemir: 32 (11.0%) metformin monotherapy, 41 (14.1%) insulin secretagogue monotherapy, 212 (72.9%) metformin + insulin secretagogue, 3 (1.0%) metformin + α‐glucosidase inhibitor, 3 (1.0%) insulin secretagogue + α‐glucosidase inhibitor; Insulin glargine: 33 (11.3%) metformin monotherapy, 37 (12.7%) insulin secretagogue monotherapy, 215 (73.9%) metformin + insulin secretagogue, 1 metformin + α‐glucosidase inhibitor (0.3%), 4 insulin secretagogue + α‐glucosidase inhibitor (1.4%), 1 (0.3%) insulin secretagogue + insulin secretagogue. |
|
| Interventions | NUMBER OF STUDY CENTRES: 80. COUNTRY/ LOCATION: Europe (Austria, Belgium, France, Germany, Ireland, UK) and United States. SETTING: Not reported. INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Insulin detemir: Initiated once‐daily in the evening at a dose of 12 units/day, titrated according to structured algorithm to FPG ≤ 6.0 mmol/L in the absence of hypoglycaemia and (if needed) to pre‐dinner PG ≤ 6.0 mmol/L, additional morning insulin dose if pre‐dinner PG > 7.0 mmol/L, but only if FPG < 7.0 mmol/L or nocturnal hypoglycaemia precluded achievement of the FPG target. Pen‐injector (Flexpen), 1 hour before to 1 hour after dinner and (if needed) within 30 minutes of breakfast. CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Insulin glargine: Initiated once‐daily in the evening at a dose of 12 units/day, titrated according to a structured algorithm to FPG ≤ 6.0 mmol/L in the absence of hypoglycaemia. Pen‐injector in Europe (OptiPen Pro 1) and syringes and vials in United States, at bedtime. TREATMENT BEFORE STUDY: Insulin‐naive, 1 or 2 OGLDs (metformin, insulin secretagogues, α‐glucosidase inhibitors) ≥ 4 months on at least half the maximum recommended dose, according to local guidelines. Insulin added to OGLDs, OGLDs were recommended to remain stable during the study. TITRATION PERIOD: 52 weeks, 16 clinical visits and 9 telephone contacts. Dose adjustments were to be based on the average of 3 self‐measurements, during the first 12 weeks weekly investigator contact. Titration algorithm is given. Titration committee. |
|
| Outcomes | PRIMARY OUTCOME(S) (as stated in the publication): Baseline‐adjusted HbA1c at end of treatment. SECONDARY OUTCOMES (as stated in the publication): Clinic FPG, within‐subject variation in PG, 10‐point self‐measured PG profiles, proportion of subjects achieving HbA1c ≤ 7.0% with and without hypoglycaemia, change in body weight, incidence of hypoglycaemia, adverse events and standard safety parameters. Major hypoglycaemia = if assistance of another person was required; Minor hypoglycaemia = confirmed by PG < 3.1 mmol/L; Symptoms only = if PG ≥ 3.1 mmol/L or no measurement was made. ADDITIONAL OUTCOMES: Novo Nordisk A/S web site: Primary outcome: HbA1c after 52 weeks; Secondary outcomes: Insulin antibodies, incidence of hypoglycaemic episodes and adverse events, plasma glucose profiles and change in body weight. |
|
| Study details | DURATION OF INTERVENTION: 52 weeks. DURATION OF FOLLOW‐UP: 52 weeks. RUN‐IN PERIOD: Not specified. |
|
| Publication details | LANGUAGE OF PUBLICATION: English. COMMERCIAL FUNDING: Yes: "The study was funded and monitored by Novo Nordisk". NON‐COMMERCIAL FUNDING: No. PUBLICATION STATUS (PEER REVIEW JOURNAL): Yes. PUBLICATION STATUS (JOURNAL SUPPLEMENT): Yes: American Diabetes Association Scientific Sessions 2004 + American Diabetes Association Scientific Sessions 2006. PUBLICATION STATUS (ABSTRACT): No. |
|
| Stated aim of study | Quote: "The objective of the current study was to compare treatment with insulin detemir and insulin glargine in line with their licensed indications as add‐on therapy to oral glucose‐lowering agents in insulin‐naive patients with type 2 diabetes". | |
| Notes | Trialnumbers: NN304‐1373 and NCT00283751. Contact information: Julio Rosenstock (juliorosenstock@dallasdiabetes.com). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Concealed randomisation was carried out by an automatic telephone system and was stratified according to OGLD monotherapy or combination therapy at study entry". Comment: Probably done, as the "automatic telephone system" probably used a computer random number generator. |
| Allocation concealment (selection bias) | Low risk | Quote: "Concealed randomisation was carried out by an automatic telephone system and was stratified according to OGLD monotherapy or combination therapy at study entry". Comment: Done, as central allocation (by means of telephone) was used. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "An open‐label design was required to allow twice daily‐daily administration of insulin detemir if needed" and "To reduce potential bias, HbA1c results were only disclosed to investigators at randomisation and at trial end". Comment: Neither study participants nor study personnel were blinded and this may have influenced outcome measurements. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Safety analysis: no missing data in either intervention group (291/291 analysed). Primary analysis: 23/291 (=7.9%) excluded from intervention group as no post‐baseline information and 16/291 (=5.5%) excluded from control group as no post‐baseline information. Thus, no imbalance in number of missing data. Primary weight analysis: 60/291 (=20.6%) excluded from intervention group and 39/291 (=13.4%) excluded from control group. Imbalance in numbers and reasons for missing data across intervention groups. |
| Selective reporting (reporting bias) | Low risk | Probably, as all of the study's pre‐specified outcomes (as listed on www.ClinicalTrials.gov) are reported (except for insulin antibodies, which was a pre‐specified secondary outcome). |
| Other bias | High risk | Quote: "In line with its license, people allocated to insulin detemir were allowed to receive an additional morning insulin dose if" and "Glargine was given once‐daily". Also, all patients randomised to insulin detemir used a pen‐injector, whereas United States patients randomised to insulin glargine used syringes and vials. Comment: The study has a potential source of bias related to the specific study design used, i.e. different frequency of injection and different injection devices across treatments. |
| Methods | RANDOMISED CONTROLLED CLINICAL TRIAL (RCT): Yes. RANDOMISATION RATIO: 1:1. NON‐INFERIORITY DESIGN: Yes. EQUIVALENCE DESIGN: No. PARALLEL / CROSSOVER / FACTORIAL RCT: Parallel. CONTROLLED CLINICAL TRIAL (CCT): Yes. |
|
| Participants | WHO PARTCIPATED: SEX (female% / male%): 437 (45.3%) / 527 (54.7%). AGE (mean years (SD)): Insulin detemir: 58.0 (8.0); Insulin glargine: 58.7 (8.5); All: 58.4 (8.3). ETHNIC GROUPS (%): Insulin detemir: 382 (78.6%) white, 80 (16.5%) asian/oriental, 6 (1.2%) black, 9 (1.9%) multiracial, 9 (1.9%) other; Insulin glargine: 368 (77.0%) white, 77 (16.1%) asian/oriental, 12 (2.5%) black, 7 (1.5%) multiracial, 14 (2.9%) other; All: 750 (77.8%) white, 157 (16.3%) asian/oriental, 18 (1.9%) black, 16 (1.7%) multiracial, 23 (2.4%) other. DURATION OF DISEASE (mean years (SD)): Insulin detemir: 9.7 (5.6); Insulin glargine: 10.1 (5.9); All: 9.9 (5.8). INCLUSION CRITERIA: Insulin‐naive people, aged 40 to 75 years, with type 2 diabetes for ≥ 1 year and treated for ≥ 3 months prior to study start with stable doses of OGLDs (including at least metformin ≥ 1 g/day), BMI < 40.0 kg/m2, HbA1c between 7.0% and 10.5% and ability and willingness to perform home glucose monitoring and to use a patient diary. EXCLUSION CRITERIA: Exclusion criteria included previous use of insulin, treatment with GLP‐1 analogues or DPP‐4 inhibitors, active proliferative retinopathy, pregnancy or lactation, treatment with systemic corticosteroids in the preceding 3 months, treatment with investigational products in the preceding 2 months, impaired hepatic or renal function or any major somatic or mental condition that might preclude implementation of the study protocol. Study protocol: Type 1 diabetes, history of hypersensitivity to the study drugs or to drugs with a similar chemical structure, likelihood of requiring treatment during the study period with drugs not permitted by the clinical trial protocol, clinically relevant cardiovascular, hepatic, neurological, endocrine, or other major disease making implementation of the protocol or interpretation of the study results difficult, history of drug or alcohol abuse in the last year or mental condition rendering the patient unable to understand the nature, scope and possible consequences of the study. DIAGNOSTIC CRITERIA: Not used. CO‐MORBIDITIES: Insulin detemir: 79 (16.3%) macroangiopathy, 48 (9.9%) nephropathy, 123 (25.3%) neuropathy, 92 (18.9%) retinopathy; Insulin glargine: 84 (17.6%) macroangiopathy, 71 (14.9%) nephropathy, 134 (28.0%) neuropathy, 105 (22.0%) retinopathy; All: 163 (16.9%) macroangiopathy, 119 (12.3%) nephropathy, 257 (26.7%) neuropathy, 197 (20.4%) retinopathy. CO‐MEDICATIONS: Insulin detemir: 486 (100%) metformin, 417 (85.8%) sulfonylureas, 89 (18.3%) thiazolidinediones, 28 (5.8%) α‐glucosidase inhibitors, 22 (4.5%) glinides, 1 (0.2%) other; Insulin glargine: 478 (100%) metformin, 410 (85.8%) sulfonylureas, 74 (15.5%) thiazolidinediones, 32 (6.7%) α‐glucosidase inhibitors, 27 (5.6%) glinides, 0 (0%) other; All: 964 (100%) metformin, 827 (85.8%) sulfonylureas, 163 (16.9%) thiazolidinediones, 60 (6.2%) α‐glucosidase inhibitors, 49 (5.1%) glinides, 1 (0.1%) other. |
|
| Interventions | NUMBER OF STUDY CENTRES: 122. COUNTRY/ LOCATION: 20 countries (Australia, Brazil, Canada, Europe, India, Korea, Russia, Serbia, Taiwan, Turkey). SETTING: Hospitals and diabetes centres (endocrinologists and diabetologists). INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Insulin detemir: Initiated twice‐daily, at breakfast and dinner, using NovoPen3 injector pen, starting dose 0.2 units/kg/day. Titrated to FPG and pre‐dinner PG < 5.6 mmol/L, increase both doses by 1 unit every 2 days until fasting and pre‐dinner PG ≤ 6.9 mmol/L, subsequently increase evening dose by 2 units every 2 days until FPG < 5.6 mmol/L, finally increase breakfast dose by 2 units every 2 days until pre‐dinner PG < 5.6 mmol/L. CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Insulin glargine: Initiated once‐daily, at dinner or bedtime, but at the same time every day throughout the study, using OptiClik injector pen, starting dose 0.2 units/kg/day. Titrated to FPG < 5.6 mmol/L, increase dose by 2 units every 2 days until FPG < 5.6 mmol/L. TREATMENT BEFORE STUDY: Insulin‐naive, treated for ≥ 3 months prior to study start with stable doses of OGLDs (including at least metformin ≥ 1 g/day), GLP‐1 analogues and DPP‐4 inhibitors were exclusion criteria. Metformin continued at stable dose throughout study, thiazolidinediones stopped at randomisation, insulin secretagogues stopped at randomisation or continued at stable dose throughout (decision made by site‐investigator, considering local guidelines). TITRATION PERIOD: 24 weeks, 8 clinical visits and 8 telephone contacts. Insulin detemir: Increase both doses by 1 unit every 2 days until FPG and pre‐dinner PG ≤ 6.9 mmol/L, subsequently increase evening dose by 2 units every 2 days until FPG < 5.6 mmol/L, finally increase breakfast dose by 2 units every 2 days until pre‐dinner PG < 5.6 mmol/L; Insulin glargine: Increase insulin dose by 2 units every 2 days until FPG < 5.6 mmol/L. |
|
| Outcomes | PRIMARY OUTCOME(S) (as stated in the publication): Percentage of patients reaching HbA1c < 7% at the end of the treatment period without symptomatic hypoglycaemia confirmed by PG ≤ 3.1 mmol/L during these 24 weeks. SECONDARY OUTCOMES (as stated in the publication): Secondary outcomes included proportions of patients achieving HbA1c < 7% and < 6.5%, changes in HbA1c, FPG, 8‐point PG profiles and weight, hypoglycaemia, insulin dose, adverse events and quality of life and treatment satisfaction. Incidence and rates for all symptomatic, symptomatic daytime, symptomatic nocturnal (both with PG ≤3.1 mmol/L and ≤3.9 mmol/L), asymptomatic ≤3.1 mmol/L, and severe (an event with symptoms consistent with hypoglycaemia, requiring the assistance of another person, and associated with measured PG <2.0 mmol/L or recovery after carbohydrate or glucagon administration) hypoglycaemia were assessed. ADDITIONAL OUTCOMES: Statistical analysis plan: Day‐to‐day FPG variability, change in waist circumference, change in hip circumference, change in BMI, change in waist‐to‐hip ratio, HbA1c < 7% without symptomatic hypo ≤ 3.1 mmol/L in last 12 weeks, and mean and SD of 24‐hour profiles. |
|
| Study details | DURATION OF INTERVENTION: 24 weeks. DURATION OF FOLLOW‐UP: Study protocol: 25 weeks (1 telephone contact to be made within the week after the completion visit). RUN‐IN PERIOD: No (1‐ to 4‐week screening phase). |
|
| Publication details | LANGUAGE OF PUBLICATION: English. COMMERCIAL FUNDING: Yes: "This study was sponsored by sanofi‐aventis, Paris, France". NON‐COMMERCIAL FUNDING: No. PUBLICATION STATUS (PEER REVIEW JOURNAL): No. PUBLICATION STATUS (JOURNAL SUPPLEMENT): Yes, Diabetologia 2009 Supplement 1. PUBLICATION STATUS (ABSTRACT): Yes, abstract only. |
|
| Stated aim of study | Quote: "Our primary objective was to demonstrate the non‐inferiority of glargine once‐daily compared to detemir twice‐daily with respect to the percentage of patients reaching HbA1c < 7% without symptomatic hypoglycaemia confirmed by PG ≤ 3.1 mmol/L". | |
| Notes | Trialnumbers: LANTU‐C‐00579 and NCT00405418. Contact information: Sanne Swinnen (s.g.swinnen@amc.uva.nl). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was stratified by centre and treatment allocation was concealed by using an interactive voice‐response system". Comment: Probably done, as the "interactive voice‐response system" probably used a computer random number generator. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was stratified by centre and treatment allocation was concealed by using an interactive voice‐response system". Comment: Done, as central allocation (by means of interactive voice‐response system) was used. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "A limitation of our study was its open‐label design. This design was necessary, however, as detemir was dosed twice‐daily with a separate titration target before dinner". Comment: Neither study participants nor study personnel were blinded and this may have influenced outcome measurements. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Safety analysis: 8/486 (=1.6%) excluded from intervention group and 1/487 (=0.2%) excluded from control group. Imbalance in reasons for missing data across intervention groups. However, plausible effect size among missing outcomes not enough to have a clinically relevant impact on observed effect size. Primary analysis: 13/486 (=2.7%) excluded from intervention group and 15/487 (=3.1%) excluded from control group. Reasons for missing data not reported. However, (virtually) no imbalance in number of missing data and plausible effect size among missing outcomes not enough to have a clinically relevant impact on observed effect size. Quality of life analysis: Number of missing data or number of patients used for the calculation of outcomes are not reported. |
| Selective reporting (reporting bias) | High risk | Not all of the study's pre‐specified secondary outcomes as listed in the study protocol are reported (change in BMI, waist circumference, hip circumference, waist‐to‐hip ratio, and mean and SD of 24‐hour PG profiles and the proportions of patients achieving HbA1c < 7% without symptomatic hypoglycaemia with PG ≤ 3.1 mmol/L in the last 12 weeks of the study are not reported). |
| Other bias | High risk | Quote: "Insulin glargine was administered at dinner or bedtime" and "Insulin detemir was administered at breakfast and dinner" and "Patients randomised to insulin glargine were instructed to perform daily fasting home glucose measurements and to increase their insulin dose by 2 units every 2 days until they reached the titration target of fasting PG < 5.6 mmol/L. Patients randomised to insulin detemir were instructed to measure their glucose before breakfast and before dinner. The goal was to obtain both fasting and pre‐dinner PG < 5.6 mmol/L. The systematic titration of insulin detemir followed three steps. First, both doses were increased by 1 unit every 2 days until FPG and pre‐dinner PG ≤ 6.9 mmol/L. Subsequently, patients were instructed to achieve fasting PG < 5.6 mmol/L by increasing their dinner dose by 2 units every 2 days. Finally, the breakfast dose was increased by 2 units every 2 days until pre‐dinner PG < 5.6 mmol/". Comment: The study has a potential source of bias related to the specific study design used, i.e. insulin detemir was dosed twice‐daily (and insulin glargine once‐daily), patients in the insulin detemir group measured their glucose level twice‐daily (insulin glargine‐treated patients once‐daily) and the titration scheme for insulin detemir may have been more complicated than that for insulin glargine. |
Anti‐GAD = anti‐Glutamic Acid Decarboxylase; BMI = body mass index; DPP‐4 = dipeptidyl peptidase‐4; FPG = fasting plasma glucose; GLP‐1 = glucagon‐like peptide‐1; HbA1c = glycosylated haemoglobin A1c; OGLD = oral glucose‐lowering drug; PG = plasma glucose; SD = standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| King 2009 | Study duration <12 weeks. |
| Klein 2007 | Study duration <12 weeks. |
| Lucidi 2010 | Study duration <12 weeks. |
| Matsuura K 2009 | Study duration <12 weeks. |
| NCT00566124 | Study duration <12 weeks. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Weight gain, eating patterns and development of body composition during initiation of basal insulin therapy in patients with type 2 diabetes: a comparison of insulin detemir and insulin glargine. |
| Methods | Intervention study, supportive care, randomised, open‐label, active‐control, parallel assignment. |
| Participants | Inclusion criteria: Age > 18 years and < 80 years, type 2 diabetes, BMI between 20.0 and 38.0 kg/m2, anti‐GAD antibody negative, FBG > 126 mg/dl, HbA1c between 7.0% and 11.0% and need for insulin therapy. Exclusion criteria: Previous therapy with insulin within the last 3 months prior to inclusion into the study, previous therapy with glitazones within the last 6 months prior to inclusion into the study, change in therapy with lipid‐lowering or anti‐hypertensive agent within one month prior to inclusion into the study, concomitant participation in other clinical trials, type 1 diabetes, cardiac and macrovascular disease, malignancy including leukaemia and lymphoma within the last 5 years, liver disease (cirrhosis or chronic active hepatitis, except fat liver), significant renal dysfunction, other endocrine disease, significant laboratory abnormalities, history of active substance abuse (including an average alcohol consume of > 40g/day and drugs) within the past 2 years, pregnancy or childbearing potential without adequate contraception, present therapy with systemic steroids, presence of psychiatric disorder or intake of anti‐depressive or anti‐psychotic agents with the exception of benzodiazepines and SSRIs/SNRIs, use of anti‐obesity drugs 3 months prior or during the trial, subjects judged by the investigator to be unsuitable for the study, contraindications for MRI scanning (cardiac pacemaker, implants out of metal or claustrophobia) or known hypersensitivity to insulin detemir, insulin glargine or to any of the other components. |
| Interventions | Insulin detemir: the participant will receive an insulin dose of insulin detemir at dinner subcutaneously according to a dosing algorithm. Insulin glargine: the participant will receive an insulin dose of the insulin glargine at dinner subcutaneously according to a dosing algorithm. |
| Outcomes | Changes in hepatic fat content, weight, body fat, visceral adipose tissue, waist circumference, hip circumference, eating behaviour, food selection, well‐being and disease perception. Also, daily insulin dose, fasting BG, hypoglycaemia and safety. |
| Starting date | November 2007. |
| Contact information | Prof. dr. Andreas Fritsche (andreas.fritsche@med.uni‐tuebingen.de). |
| Notes | Estimated completion date: October 2010.. |
| Trial name or title | Effects of adding basal insulin on endothelial progenitor cell levels in poorly‐controlled type 2 diabetic patients with cardiovascular disease. A randomised cross‐over study comparing insulin detemir and glargine. |
| Methods | Intervention study, randomised, open‐label, active‐control, cross‐over assignment, pharmacodynamics study. |
| Participants | Inclusion criteria: Type 2 diabetes, 35 to 80 years, macroangiopathy (coronary, peripheral or cerebrovascular), on oral antidiabetic therapy, and HbA1c > 7.0%. Exclusion criteria: Type 1 diabetes, acute diabetic decompensation, use of glitazones, cancer, acute disease or infection, chronic renal failure (serum creatinine > 2.0 mg/dl), advanced liver disease (Child B‐C), immune disease, organ transplantation, immunosuppression, recent surgery (within 3 months), recent cardiovascular events (within 3 months), inability to provide informed consent, pregnancy or lactation. |
| Interventions | Insulin detemir: Daily bedtime subcutaneous insulin detemir in individualized doses. Insulin glargine: Daily bedtime subcutaneous insulin glargine in individualized doses. |
| Outcomes | Changes in endothelial progenitor cell count and in markers of endothelial damage. |
| Starting date | May 2008. |
| Contact information | Angelo Avogaro (angelo.avogaro@unipd.it) or Gian Paolo Fadini (gianpaolo.fadini@unipd.it). |
| Notes | Estimated completion date: March 2010. |
| Trial name or title | Effects of basal insulin analogue detemir on body composition, epicardial fat and energy metabolism. |
| Methods | Intervention study, randomised, open‐label, parallel assignment, pharmacodynamics study. |
| Participants | Inclusion criteria: Type 2 diabetic patients who require basal (long‐acting) insulin for the control of hyperglycaemia according to the opinion of the investigator, age 18 to 80 years, BMI from 27 to 40 kg/m2, HbA1c ≥ 8.0% to 12.0%, stable body weight for previous 3 months (± 5 kg), structured exercise < 4 hours per week, and metformin ≥ 1.5 g/day. Exclusion criteria: Any medical, social or geographic condition, which, in the opinion of the investigator would not allow safe or reliable completion of the protocol, type 1 diabetes, previous treatment with insulin (< 6 months prior inclusion), secondary diabetes (i.e. steroid induced, Cushing syndrome, chronic pancreatitis, cystic fibrosis, etc.), maturity‐onset diabetes of the young, proliferative retinopathy/maculopathy requiring treatment, hypoglycaemia unawareness or recurrent major hypoglycaemia, pregnancy or breast‐feeding, unstable coronary artery disease, heart failure as defined by class IV according to NYHA classification, recent (< 6 months) history of myocardial infarction, stroke, TIA, ventricular arrhythmias, or unstable supra‐ventricular arrhythmias, renal insufficiency (creatinine clearance < 40 ml/min), acute (< 2 months) or chronic infectious diseases requiring either hospitalisation or antibiotic treatment for > 2 weeks, recent (< 1 year) diagnosis of systemic malignancies except thyroid and skin cancer, major psychiatric diseases, history of drug addiction, previous bariatric surgery or medication that affects weight. |
| Interventions | Insulin detemir: Initial dose of 10 units of insulin at bedtime. The insulin dose will be increased by 1 unit per day until FPG levels are 5.0 mmol/L. Insulin glargine: Initial dose of 10 units of insulin at bedtime. The insulin dose will be increased by 1 unit per day until FPG levels are 5.0 mmol/L. |
| Outcomes | Changes in total fat mass, and epicardial fat, trunk fat, total fat free mass, weight and waist circumference, HbA1c, fasting glucose, RMR, TEF, PAEE and TEE and energy intake. |
| Starting date | March 2009. |
| Contact information | Sylvie Blaquiere (sylvie.blaquiere@ircm.qc.ca) or Stephanie Potvin (stephanie.potvin@ircm.qc.ca). |
| Notes | Estimated completion date: December 2011. |
| Trial name or title | A 26‐week randomised, multinational, open‐labelled, 2‐armed, parallel group, treat‐to‐target once‐daily treatment trial with insulin detemir versus insulin glargine, both in combination with metformin in subjects with type 2 diabetes. |
| Methods | Intervention study, randomised, open‐label, active‐control, parallel assignment, safety/efficacy study. |
| Participants | Inclusion criteria: diagnosed with type 2 diabetes for at least 6 months, age 18 years and older, stable treatment with a total daily dose of at least 1500 mg metformin or maximum tolerated dose (minimum 1000 mg) with or without one other OGLD (sulphonylureas, meglitinides, thiazolidinediones or DPP‐4 inhibitors) for at least 3 months, insulin‐naive (short‐term insulin treatment of up to 14 days is allowed), HbA1c between 7.0% and 9.0 % (both inclusive), and BMI ≤ 35.0 kg/m2. Exclusion criteria: Any contraindication to insulin detemir or insulin glargine according to the local labelling, receipt of any investigational product within 4 weeks, anticipated change of dose of any systemic treatment with products, which in the investigator's opinion could interfere with glucose metabolism (e.g. systemic corticosteroids), clinically significant diseases which, in the Investigator's opinion may confound the results of the trial or pose additional risk in administering trial product or any other condition that the Investigator feels would interfere with trial participation or evaluation of results. |
| Interventions | Insulin detemir: Treat‐to‐target titration according to titration algorithm. Subcutaneous injection once‐daily. Insulin glargine: Treat‐to‐target titration according to titration algorithm. Subcutaneous injection once‐daily. |
| Outcomes | Change in HbA1C from baseline, proportion of subjects achieving HbA1c ≤ 7.0%, proportion of subjects achieving HbA1c ≤ 6.5%, FPG, within‐subject variation of self‐measured PG, 9‐point PG profile, incidence of hypoglycaemic episodes during the trial (nocturnal and over 24 hours) and change in body weight. |
| Starting date | May 2009. |
| Contact information | Novo Nordisk clinical trial call centre (866‐867‐7178). |
| Notes | Estimated completion date: June 2010. |
Anti‐GAD = anti‐Glutamic Acid Decarboxylase; BMI = body mass index; DPP‐4 = dipeptidyl peptidase‐4; FBG = fasting blood glucose, FPG = fasting plasma glucose; HbA1c = glycosylated haemoglobin A1c; MRI = magnetic resonance imaging; NYHA = New York Heart Association; OGLD = oral glucose‐lowering drug; PAEE = physical activity energy expenditure; PG = plasma glucose; RMR = resting metabolic rate; SNRIs = serotonin‐norepinephrine reuptake inhibitors; SSRIs = selective serotonin reuptake inhibitors; TEE = total energy expenditure; TEF = thermic effects of food; TIA = transient ischaemic attack.
Contributions of authors
SANNE SWINNEN: development of protocol, undertaking of searches, selection of studies, data extraction, quality assessment of studies, data analysis, development of final review, contact reviewer.
AIRIN SIMON: selection of studies, data extraction, quality assessment of studies, data analysis, development of final review.
FRITS HOLLEMAN: development of protocol, development of final review.
HANS DEVRIES: development of protocol, development of final review.
JOOST HOEKSTRA: development of protocol, development of final review.
Declarations of interest
Our research group co‐developed the protocol for, conducted and reported one of the included randomised controlled trials. This study was sponsored by Sanofi‐Aventis. We have performed various studies on insulin therapy and other glucose‐lowering interventions in cooperation with Novo Nordisk.
New
References
References to studies included in this review
- Hollander P, Cooper J, Bregnhøi J, Pedersen CB. A 52‐week, multinational, open‐label, parallel‐group, noninferiority, treat‐to‐target trial comparing insulin detemir with insulin glargine in a basal‐bolus regimen with mealtime insulin aspart in patients with type 2 diabetes. Clinical Therapeutics 2008;30:1976‐87. [DOI] [PubMed] [Google Scholar]
- Raskin P, Gylvin T, Weng W, Chaykin L. Comparison of insulin detemir and insulin glargine using a basal‐bolus regimen in a randomized, controlled clinical study in patients with type 2 diabetes. Diabetes Metabolism Research and Reviews 2009;25:542‐8. [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52‐week, treat‐to‐target trial comparing insulin detemir with insulin glargine when administered as add‐on to glucose‐lowering drugs in insulin‐naive people with type 2 diabetes. Diabetologia 2008;51:408‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen SG, Dain MP, Aronson R, Davies M, Gerstein HC, Pfeiffer AF, et al. A 24‐week, randomized, treat‐to‐target trial comparing initiation of insulin glargine once‐daily with insulin detemir twice‐daily in patients with type 2 diabetes inadequately controlled on oral glucose‐lowering drugs. Diabetes Care 2010;33(6):1176‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- King AB. Once‐daily insulin detemir is comparable to once‐daily insulin glargine in providing glycaemic control over 24 h in patients with type 2 diabetes: A double‐blind, randomized, crossover study. Diabetes, Obesity and Metabolism 2009;11:69‐71. [DOI] [PubMed] [Google Scholar]
- Klein O, Lynge J, Endahl L, Damholt B, Nosek L, Heise T. Albumin‐bound basal insulin analogues (insulin detemir and NN344): comparable time‐action profiles but less variability than insulin glargine in type 2 diabetes. Diabetes, Obesity and Metabolism 2007;9:290‐9. [DOI] [PubMed] [Google Scholar]
- P. Lucidi, P. Rosetti, F. Porcellati, P. Candeloro, P. Cioli, S. Marzotti, et al. Basal insulin NPH, glargine and detemir in type 2 diabetes: hepatospecificity, effects on glucose and lipid metabolism, and pancreatic islet alpha and beta cell rest: a PK‐PD study. Diabetologia. 2010; Vol. 53:S391. [Google Scholar]
- Matsuura K, Mori Y, Itoh Y, Yokoyama J, Tajima N. Effect of miglitol on 24‐hour glucose fluctuation in type 2 diabetic patients treated with long‐acting insulin glargine or detemir as single agents as assessed by using continuous glucose monitoring. Diabetes. 2009; Vol. 58:A131. [Google Scholar]
- Basal Insulins ‐ Pharmacodynamics. ClinicalTrials.gov2007.
References to ongoing studies
- Weight gain, eating patterns and development of body composition during initiation of basal insulin therapy in patients with type 2 diabetes: a comparison of insulin detemir and insulin glargine. ClinicalTrials.gov2008.
- Effects of adding basal insulin on endothelial progenitor cell levels in poorly‐controlled type 2 diabetic patients with cardiovascular disease. A randomised cross‐over study comparing insulin detemir and glargine. ClinicalTrials.gov2008.
- Effects of basal insulin analogue detemir on body composition, epicardial fat and energy metabolism. ClinicalTrials.gov2009.
- A 26‐week randomised, multinational, open‐labelled, 2‐armed, parallel group, treat‐to‐target once‐daily treatment trial with insulin detemir versus insulin glargine, both in combination with metformin in subjects with type 2 diabetes. ClinicalTrials.gov2009.
Additional references
- Albright ES, Desmond R, Bell DS. Efficacy of conversion from bedtime NPH insulin injection to once‐ or twice‐daily injections of insulin glargine in type 1 diabetic patients using basal/bolus therapy.. Diabetes Care 2004;27(2):632‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ashwell SG, Gebbie J, Home PD. Twice‐daily compared with once‐daily insulin glargine in people with Type 1 diabetes using meal‐time insulin aspart. Diabetic Medicine 2006;23(8):879‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bolli GB, Marchi RD, Park GD, Pramming S, Koivisto VA. Insulin analogues and their potential in the management of diabetes mellitus. Diabetologia 1999;42:1151‐67. [DOI] [PubMed] [Google Scholar]
- Chapman TM, Perry CM. Insulin detemir: A review of its use in the management of type 1 and 2 diabetes mellitus. Drugs 2004;64:2577‐95. [DOI] [PubMed] [Google Scholar]
- DeVries JH. To: Scholtz HE, Pretorius SG, Wessels DH, Becker RHA (2005) Pharmacokinetic and glucodynamic variability: assessment of insulin glargine, NPH insulin and insulin ultralente in healthy volunteers using a euglycaemic clamp technique. Diabetologia 2005;48(10):1988‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Havelund S, Plum A, Ribel U, Jonassen I, Volund A, Markussen J, et al. The mechanism of protraction of insulin detemir, a long‐acting, acylated analog of human insulin. Pharmaceutical research 2004;21:1498‐504. [DOI] [PubMed] [Google Scholar]
- Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time‐action profile of the long‐acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care 2000;23(5):644‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Heise T, Nosek L, Ronn BB, Endahl L, Heinemann L, Kapitza C, et al. Lower within‐subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes 2004;53:1614‐20. [DOI] [PubMed] [Google Scholar]
- Hermansen K, Davies M, Derezinski T, Martinez G, Clauson P, Home P. A 26‐week, randomized, parallel, treat‐to‐target trial comparing insulin detemir with NPH insulin as add‐on therapy to oral glucose‐lowering drugs in insulin‐naïve people with type 2 diabetes. Diabetes Care 2006;29:1269‐74. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
- Holleman F, Gale EA. Nice insulins, pity about the evidence. Diabetologia 2007;51(4):689‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. The New England Journal of Medicine 2008;359(15):1577‐89. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Horvath K, Jeitler K, Berghold A, Plank J, Pieber TR, Siebenhofer A. Long acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005613.pub3] [DOI] [PubMed] [Google Scholar]
- Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006;333:597‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA Statement for Reporting Systematic and Meta‐Analyses of Studies That Evaluate Interventions: Explanation and Elaboration. PLoS Med 1999;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins P, Sharplin P, Yki‐Jarvinen H, Riddle MC, Haring HU. Negative binomial meta‐regression analysis of combined glycosylated hemoglobin and hypoglycemia outcomes across eleven Phase III and IV studies of insulin glargine compared with neutral protamine Hagedorn insulin in type 1 and type 2 diabetes mellitus. Clinical Therapeutics 2007;29(8):1607‐19. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, et al. Management of hyperglycaemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetologia 2006;49:1711‐21. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care 2009;32(1):193‐203. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plank J, Bodenlenz M, Sinner F, Magnes C, Görzer E, Regittnig W, et al. A double‐blind, randomized, dose‐response study investigating the pharmacodynamic and pharmacokinetic properties of the long‐acting insulin analog detemir. Diabetes Care 2005;28:1107‐12. [DOI] [PubMed] [Google Scholar]
- Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators. The treat‐to‐target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26(11):3080‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Dailey G, Massi‐Benedetti M, Fritsche A, Lin Z, Salzman A. Reduced hypoglycemia risk with insulin glargine: a meta‐analysis comparing insulin glargine with human NPH insulin in type 2 diabetes. Diabetes Care 2005;28:950‐5. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care; Meta‐analysis in Context. London: BMJ Publishing Group, 2001:189‐208. [Google Scholar]
- Swinnen SG, Hoekstra JB, DeVries JH. Insulin therapy for type 2 diabetes. Diabetes Care 2009;32 Suppl 2:S253‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen SG, Dain MP, Mauricio D, DeVries JH, Hoekstra JB, Holleman F. Continuation versus discontinuation of insulin secretagogues when initiating insulin in type 2 diabetes. Diabetes, obesity & metabolism 2010;12(10):923‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837‐53. [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group. Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854‐65. [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047‐53. [DOI] [PubMed] [Google Scholar]


