Abstract
Background
Multiple sclerosis (MS) is an autoimmune, T‐cell‐dependent, inflammatory, demyelinating disease of the central nervous system, with an unpredictable course. Current MS therapies focus on treating exacerbations, preventing new exacerbations and avoiding the progression of disability. However, at present there is no effective treatment that is capable of safely and effectively reaching these objectives. This has led to the development and investigation of new drugs. Recent clinical trials suggest that alemtuzumab, a humanised monoclonal antibody against cell surface CD52, could be a promising option for MS.
Objectives
To assess the safety and effectiveness of alemtuzumab used alone or associated with other treatments to decrease disease activity in patients with any form of MS.
Search methods
We searched the Trials Register of the Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group (30 April 2015), which contains trials from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, LILACS and the trial registry databases ClinicalTrials.gov and WHO International Clinical Trials Registry Platform. There was no restriction on the source, publication date or language.
Selection criteria
All randomised clinical trials (RCTs) involving adults diagnosed with any form of MS according to the McDonald criteria, comparing alemtuzumab alone or associated with other medications, at any dose and for any duration, versus placebo or any other active drug therapy or alemtuzumab in other dose, regimen or duration. The co‐primary outcomes were relapse‐free survival, sustained disease progression and number of participants with at least one of any adverse events, including serious adverse events.
Data collection and analysis
Two independent review authors performed study selection, data extraction and 'Risk of bias' assessment. A third review author checked the process for accuracy. We used the Cochrane 'Risk of bias' tool to assess the risk of bias of the studies included in the review. We used the GRADE system to assess the quality of the body of evidence. To measure the treatment effect on dichotomous outcomes we used the risk ratio (RR); for the treatment effect on continuous outcomes, we used the mean difference (MD) and for time‐to‐event outcomes we used hazard ratio (HR). We calculated 95% confidence intervals (CI) for these measures. When there was no heterogeneity, we used a fixed‐effect model to pool data.
Main results
Three RCTs (1713 participants) fulfilled the selection criteria and we included them in the review. All three trials compared alemtuzumab versus subcutaneous interferon beta‐1a for patients with relapsing–remitting MS. Patients were treatment‐naive in the CARE‐MS and CAMMS223 studies. The CARE‐MS II study included patients with at least one relapse while being treated with interferon beta or glatiramer acetate. Alemtuzumab was given for 12 or 24 months; for some outcomes, the follow‐up period reached 36 months. The regimens were (a) 12 mg or 24 mg per day administered intravenously, once a day for five consecutive days at month 0 and 12 or (b) 24 mg per day, intravenously, once a day for three consecutive days at month 12 and 24. The patients in the other arm of the trials received interferon beta‐1a 44 μg subcutaneously three times weekly after dose titration.
At 24 months, alemtuzumab 12 mg was associated with: (a) higher relapse‐free survival (hazard ratio (HR) 0.50, 95% CI 0.41 to 0.60; 1248 participants, two studies, moderate quality evidence); (b) higher sustained disease progression‐free survival (HR 0.62, 95% CI 0.44 to 0.87; 1191 participants; two studies; moderate quality evidence); (c) a slightly higher number of participants with at least one adverse event (RR 1.04, 95% CI 1.01 to 1.06; 1248 participants; two studies; moderate quality evidence); (d) a lower number of participants with new or enlarging T2‐hyperintense lesions on magnetic resonance imaging (MRI) (RR 0.74, 95% CI 0.59 to 0.91; 1238 participants; two studies; I2 = 80%); and (e) a lower number of dropouts (RR 0.31, 95% CI 0.23 to 0.41; 1248 participants; two studies, I2 = 29%; low quality evidence).
At 36 months, alemtuzumab 24 mg was associated with: (a) higher relapse‐free survival (45 versus 17; HR 0.21, 95% CI 0.11 to 0.40; one study; 221 participants); (b) a higher sustained disease progression‐free survival (HR 0.33, 95% CI 0.16 to 0.69; one study; 221 participants); and (c) no statistical difference in the rate of participants with at least one adverse event. We did not find any study that reported any of the following outcomes: rate of participants free of clinical disease activity, quality of life, fatigue or change in the numbers of MRI T2‐ and T1‐weighted lesions after treatment. It was not possible to perform subgroup analyses according to disease type and disability at baseline due to lack of data.
Authors' conclusions
In patients with relapsing‐remitting MS, alemtuzumab 12 mg was better than subcutaneous interferon beta‐1a for the following outcomes assessed at 24 months: relapse‐free survival, sustained disease progression‐free survival, number of participants with at least one adverse event and number of participants with new or enlarging T2‐hyperintense lesions on MRI. The quality of the evidence for these results was low to moderate. Alemtuzumab 24 mg seemed to be better than subcutaneous interferon beta‐1a for relapse‐free survival and sustained disease progression‐free survival, at 36 months.
More randomised clinical trials are needed to evaluate the effects of alemtuzumab on other forms of MS and compared with other therapeutic options. These new studies should assess additional relevant outcomes such as the rate of participants free of clinical disease activity, quality of life, fatigue and adverse events (individual rates, serious adverse events and long‐term adverse events). Moreover, these new studies should evaluate other doses and durations of alemtuzumab course.
Plain language summary
Alemtuzumab for multiple sclerosis
Background
Multiple sclerosis (MS) is a chronic disease of the nervous system that affects young and middle‐aged adults. Repeated damage to the myelin sheaths (the membranes that cover and protect nerves) and other parts of the nerves can lead to serious disability. MS may be related to problems in the immune system. Alemtuzumab is a biologic drug (a type of antibody), which has already been used for other diseases.
Study characteristics
We found three studies (including 1713 participants) that fulfilled the review selection criteria. All studies compared alemtuzumab versus subcutaneous interferon beta‐1a for people with relapsing–remitting MS. In two of the studies (CARE‐MS and CAMMS223) the participants were being treated for the first time (treatment‐naive). The third study (CARE‐MS II) included participants with at least one relapse while being treated with interferon beta or glatiramer acetate for at least six months.
Key results
The review of these comparative studies found that, compared to subcutaneous interferon beta‐1a, alemtuzumab reduces the risk of relapse, improves function and seems not to increase the overall risk of adverse events. Additionally, alemtuzumab reduces the risk of new or enlarging lesions of MS detected using magnetic resonance imaging (MRI). However, there is a lack of information about the effects of alemtuzumab on several patient‐related outcomes such as (a) quality of life, (b) the rate of each adverse events (separately) and (c) the frequency of long‐term adverse events and serious adverse events.
Quality of the evidence
The overall methodological quality of the included studies was moderate to high. However, because of the small number of included studies and the low rate of events, we judged the overall quality of the evidence for the main outcomes as very low to moderate. This means that new studies are likely to have an important impact on our confidence in the estimate of effect and may change the estimate or that we are very uncertain about the estimate.
Summary of findings
Summary of findings for the main comparison. Alemtuzumab 12 mg compared to interferon beta‐1a for multiple sclerosis.
| Alemtuzumab 12 mg compared to interferon beta‐1a for multiple sclerosis | ||||||
| Patient or population: patients with multiple sclerosis Settings: outpatients Intervention: alemtuzumab 12 mg Comparison: interferon beta‐1a | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Interferon beta‐1a | Alemtuzumab 12 mg | |||||
| Relapse‐free survival Follow‐up: 24 months | Not estimated | Not estimated |
HR 0.50 (0.41 to 0.60) |
1248 (2 studies) | ⊕⊕⊕⊝ moderate5 | — |
| Sustained disease progression‐free survival Follow‐up: 24 months | Not estimated | Not estimated |
HR 0.62 (0.44 to 0.87) |
1191 (2 studies) | ⊕⊕⊕⊝ moderate2 | — |
| Number of participants with at least one adverse event | Study population | RR 1.04 (1.01 to 1.06) | 1248 (2 studies) | ⊕⊕⊕⊝ moderate1 | — | |
| 94 per 100 | 98 per 100 (95 to 100) | |||||
| Moderate | ||||||
| 94 per 100 | 98 per 100 (95 to 99) | |||||
| Change in EDSS score Follow‐up: 24 months | — | The mean change in EDSS score in the intervention groups was 0.2 lower (0.6 lower to 0.2 higher) | — | 1199 (2 studies) | ⊕⊝⊝⊝ very low2,3 | — |
| Number of participants with new or enlarging T2‐hyperintense lesions Follow‐up: 24 months | 69 per 100 | 51 per 100 (41 to 63) | RR 0.74 (0.59 to 0.91) | 1238 (2 studies) | ⊕⊕⊕⊝ moderate4 | — |
| Dropouts Follow‐up: 24 months | Study population | RR 0.31 (0.23 to 0.41) | 1248 (2 studies) | ⊕⊕⊝⊝ low1,5 | — | |
| 24 per 100 | 8 per 100 (6 to 10) | |||||
| Moderate | ||||||
| 24 per 100 | 7 per 100 (5 to 10) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; HR: hazard ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Participants and personnel were not blinded and this outcome could be affected by this fact. 2Participants, personnel and outcome assessors were not blinded and this outcome could be affected by this fact. 3High heterogeneity; I2 = 88%. 4High heterogeneity; I2 = 80%. 5Low number of events (fewer than 300).
Background
Description of the condition
Multiple sclerosis (MS) is an autoimmune, inflammatory, demyelinating disease of the central nervous system (brain and spinal cord), the causes of which remain unknown (Coles 1999a; Gray 2004). It is the most common cause of non‐traumatic neurological disability in young adults (Noseworthy 2000). Almost two million people in the world are affected by this condition, which can substantially impair patients' quality of life and is associated with high costs for patients, their families and society in general (Multiple Sclerosis International Federation 2010).
Four types of MS have been identified: relapsing‐remitting (RR), secondary‐progressive (SP), primary‐progressive (PP) and progressive‐relapsing (PR). The disease course is unpredictable; while some individuals are minimally affected, others show rapid progression of the disease, reaching total physical incapacity (Lublin 1996). In the first form, MS is characterised by relapses and remissions (RR), but given time sequelae from relapses may cause increased disability (Hawkins 1999). In some patients, the disease is progressive from its onset (PP); others experience periods of progression followed by relapses and remissions (SP) (Lublin 1996). In other cases, MS shows progression from onset but with clear relapses (PR).
Description of the intervention
Therapeutic strategies for MS aim to treat exacerbations, prevent new exacerbations and avoid progression of disability (Filippini 2013). Current disease‐modifying treatments decrease the frequency of relapse and modestly reduce the accumulation of disability (Coles 2006; Rieckmann 2009). Consequently, new agents that effectively control the disease are needed.
Alemtuzumab (Lemtrada, previously known as Campath‐1H) is a humanised monoclonal antibody against cell surface CD52, which can be found in a variety of cell populations, including B and T lymphocytes, thymocytes and monocytes but not in haematological precursors or plasma cells (Gilleece 1993). However, the exact function of CD52 is still unknown (Xia 1991).
In 2001, alemtuzumab was approved for fludarabine‐resistant B‐cell chronic lymphocytic leukaemia (FDA 2001; Keating 2002). Since that time, it has been used for several other diseases (licensed or off‐label use), including immune thrombocytopenic purpura, aplastic anaemia, autoimmune haemolytic anaemia, vasculitis, hematopoietic stem cell transplants (as a conditioning regimen) and organ transplants (as an induction agent) (Gomez‐Almaguer 2012; Lockwood 2003; Waldmann 2005; Weissenbacher 2010).
A study published in 1999, including 36 participants with progressive MS, reported that daily intravenous infusions of alemtuzumab (20 mg over four hours for five days) were associated with a reduction in gadolinium‐enhanced magnetic resonance imaging (MRI) lesions and a reduction in relapses, with no clinical improvement in disability (Coles 1999b). Open studies involving participants with relapsing‐remitting MS reported that the drug reduced relapse rates and disability (Coles 2006; Hirst 2008).
Alemtuzumab is already approved for MS in the European Union (EMA 2013). The US Food and Drug Administration (FDA) approved alemtuzumab for the treatment of people with RR MS who have had an inadequate response to two or more drugs indicated for the treatment of MS (FDA 2014).
The guidelines from the Association of British Neurologists identified alemtuzumab as having greatest activity in preventing relapses. However, because of safety concerns, the guidelines recommended this drug as a second‐line treatment, or for patients with the rapidly evolving RR form (Scolding 2015).
Alemtuzumab is available for the treatment of MS in 12 mg/1.2 mL single‐dose vials (10 mg/mL). The proposed initial dosage for MS is 12 mg daily for five consecutive days (intravenous infusion), followed by a second treatment course of 12 mg/daily for three consecutive days. The second treatment course is administered 12 months after the first course. Premedication with corticosteroids is recommended immediately before alemtuzumab and during the first three days of any treatment course (FDA 2014). The overall half‐life of the drug is approximately 21 days. Alemtuzumab is available as a liquid to be made up into a solution for infusion (drip) into a vein. An infusion provides 12 mg and lasts around four hours.
Alemtuzumab can produce serious adverse events including other autoimmune syndromes affecting the thyroid and blood cells (thrombocytopenia, haemolytic anaemia, pancytopenia) and nephropathies, and it can increase the risk of thyroid cancer (FDA 2014). At five‐year follow‐up, the cumulative risk of autoimmune disease is approximately 22%, Graves' disease 12%, immune thrombocythaemia purpura 3% and Goodpasture's disease (severe glomerulopathy) 0.4% (Cossburn 2011).
Recently, the FDA updated a general overview of recommendations (Risk Evaluation and Mitigation Strategy Program) about Lemtrada for patients, pharmacies and healthcare providers (FDA 2015).
How the intervention might work
Previous researches have suggested that alemtuzumab depletes the T‐ and B‐cells that may be responsible for cellular damage, while sparing innate immune cells (Rao 2012). Change in the composition of lymphocytes that accompanies lymphocyte reconstitution has also been reported (Hill‐Cawthorne 2012).
Why it is important to do this review
Results of randomised controlled trials (RCTs) of alemtuzumab for MS are promising and a systematic review of all RCTs was warranted to evaluate its effectiveness and safety for MS.
Objectives
To assess the safety and effectiveness of alemtuzumab used alone or associated with other treatments to decrease disease activity in people with any form of MS.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, double‐blind clinical trials (RCTs). We did not consider cross‐over trials.
Types of participants
We included adults diagnosed with MS according to the McDonald criteria (McDonald 2001; Polman 2011), or Poser criteria (Poser 1983). We considered participants with any form of MS (relapsing‐remitting, primary‐progressive, secondary‐progressive or progressive‐relapsing) for inclusion.
Types of interventions
Experimental intervention: alemtuzumab alone or associated with other medications at any dose and for any course duration.
Comparator: placebo, any other active drug therapy (i.e. corticosteroids, plasmapheresis, beta interferons, glatiramer acetate, fingolimod, natalizumab, mitoxantrone, teriflunomide or dimethyl fumarate).
Types of outcome measures
Primary outcomes
Relapse‐free survival. Relapse was defined as newly developed or recently worsened symptoms of neurological dysfunction, lasting longer than 24 hours and objectively confirmed. However, we considered less stringent criteria and assessed these separately.
Sustained disease progression‐free survival, defined as a ≥ 1.0‐point increase in the Expanded Disability Status Scale (EDSS) score (Kurtzke 1983) for participants with a baseline score ≤ 5.0 or a ≥ 0.5‐point increase for participants with a baseline score ≥ 5.5 points confirmed at six months. We considered a one‐point increase in EDSS score confirmed at three months' follow‐up as a surrogate outcome measure of progression.
Number of participants with at least one adverse event, including serious adverse events.
All primary outcomes were assessed after 12 and 24 months follow‐up and at the end of the follow‐up period.
Secondary outcomes
Number of participants free of clinical disease activity, defined as no relapses and no sustained accumulation of disability. Sustained accumulation disability was defined as an increase of at least 1.5 points on the Expanded Disability Status Scale (EDSS) for patients with a baseline score of 0 and of at least 1.0 point for patients with a baseline score of 1.0 or more.
Quality of life as assessed by the Multiple Sclerosis Quality of Life scale (MSQOL)‐54 (Vickrey 1995) or the Multiple Sclerosis Quality of Life Inventory (MSQLI) (Fischer 1999).
Change in disability as assessed by the EDSS (Kurtzke 1983).
Fatigue as assessed by the Fatigue Severity Scale or the Fatigue Index Scale (Krupp 1989).
Number of participants with new or enlarging T2‐hyperintense lesions on magnetic resonance imaging (Li 1999).
Number of participants who dropped out.
All secondary outcomes would be assessed after 12 and 24 months and at the end of the follow‐up period.
Search methods for identification of studies
We conducted a systematic search without language restrictions to identify all relevant published randomised controlled trials using the optimally sensitive strategy developed by Cochrane for the identification of RCTs.
Electronic searches
The Trials Search Co‐ordinator searched the Trials Register of the Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group (30 April 2015) which, among other sources, includes trials from:
Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 4);
MEDLINE (PubMed) (1966 to 30 April 2015);
EMBASE (Embase.com) (1974 to 30 April 2015);
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO host) (1981 to 30 April 2015);
Latin American and Caribbean Health Science Information Database (LILACS) (Bireme) (1982 to 30 April 2015);
ClinicalTrials.gov (www.clinicaltrials.gov);
World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch).
Information on the Trials Register of the Review Group and details of search strategies used to identify trials can be found in the 'Specialised Register' section within the Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group module.
The keywords used to search for trials for this review are listed in Appendix 1.
Searching other resources
In addition, we used the following methods.
We screened the bibliographic references of identified studies to identify additional studies.
We contacted pharmaceutical companies (Genzyme‐Sanofi and Bayer) for information on any unpublished trials.
We contacted the main authors of studies if data reported in the original articles were incomplete and we asked experts in this field about additional unpublished or ongoing studies.
Data collection and analysis
Selection of studies
Two review authors (RR and GJMP) independently screened the titles and abstracts of all records retrieved by the search in order to identify potentially relevant studies. We retrieved the full‐text reports/publications of those deemed eligible for inclusion and three review authors (RR, GP and MRT) independently read the full texts to identify studies that met the selection criteria. The review authors recorded the reasons for exclusion of rejected studies. Disagreements between two review authors (RR and GJMP) were discussed until a consensus was reached with the consultation of a third review author (MRT) if needed.
Data extraction and management
We used a data collection form to report information on study characteristics and outcome data. Three review authors (RR, GP and MRT) extracted the following information from the primary studies included in the review.
Publication details (i.e. year, country, authors).
Study design and methods: inclusion/exclusion criteria, randomisation method, allocation concealment, blinding.
Setting.
Population data (i.e. age, severity of disease, type of MS).
Details of intervention (i.e. dose, regimen, duration).
Outcome measures (including effectiveness and adverse effects).
Number of dropouts.
Length of follow‐up.
Types of data analyses (e.g. intention‐to‐treat, modified intention‐to‐treat).
Any other potential risk of bias.
We discussed disagreements until consensus was reached, with the involvement of a third review author (MRT) if needed. One review author (RR) inserted data into the Review Manager 5.3 software (RevMan 2015). We double‐checked that data were entered correctly into the form.
Assessment of risk of bias in included studies
Three review authors (RR, GJMP and MRT) independently assessed the methodological quality of included clinical trials using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the risk of bias according to the following domains.
Sequence generation: Was the allocation sequence adequately generated?
Allocation concealment: Was allocation adequately concealed?
Blinding of participants, personnel and outcome assessors: Was knowledge of the allocated interventions adequately prevented during the study?
Incomplete outcome data: Were outcome data adequately assessed and accounted for? (We considered a loss to follow‐up rate greater than 15% as high risk).
Selective outcome reporting: Were the reports of the study free of any suggestion of selective outcome reporting?
Other potential threats to validity: Was the study apparently free from other problems that could put it at risk of bias?
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table.
We considered the overall quality of the studies good if the sequence generation, allocation concealment and blinding (patients and personnel, and assessors) domains were all at low risk of bias. We considered the overall quality of the studies moderate if one of these domains was categorised as being at unclear risk. Finally, we considered the overall quality high if at least one of these domains was categorised as high risk of bias.
Measures of treatment effect
For each outcome, we calculated a summarised estimate of treatment effect (with 95% confidence interval (CI)) for each comparison. We reported dichotomous outcomes as risk ratios (RRs). We used the mean difference (MD) for continuous outcomes and the hazard ratio (HR) for time‐to‐event outcomes.
Unit of analysis issues
The unit of analysis was the individual participant.
Dealing with missing data
In cases where there were missing or unavailable data, we contacted the primary authors for further information. We performed a search for protocols or additional articles related to the included trials (or both). If relevant data were unavailable, we presented and discuss the results in the main text of the review.
Assessment of heterogeneity
We investigated heterogeneity using the Chi2 test and the I2 statistic, which indicates the degree of variation across studies that is due to heterogeneity rather than due to chance. We considered an I2 value greater than 50% as substantial heterogeneity (Higgins 2011). We checked clinical and methodological differences as potential causes of heterogeneity.
Assessment of reporting biases
As it was not possible to pool more than 10 studies, we did not use funnel plots to explore possible publication bias.
Data synthesis
We summarised data using the Review Manager 5 software (RevMan 2015). When significant methodological or clinical heterogeneity existed,we used a random‐effects model; otherwise we used a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses:
Treatment duration (12 or 24 months).
Different doses and regimens of alemtuzumab (12 mg or 24 mg).
Disease type: relapsing‐remitting, primary‐progressive, secondary‐progressive or progressive‐relapsing.
Disability at baseline (EDSS score ≤ 5.0 or ≥ 5.5).
Naive or previously treated participants.
However, we did not carry out subgroup analyses to consider disease type and disability at baseline due to lack of available data.
Sensitivity analysis
We planned to perform sensitivity analysis by excluding trials of low or moderate quality (or both) and comparing the results with the overall findings. However, since we included only three trials in the review, we deemed this analysis inappropriate.
'Summary of findings' table
We created a 'Summary of findings' table (Table 1) by using the following outcomes:
Relapse‐free survival.
Sustained disease progression‐free survival.
Number of participants with at least one adverse event, including serious adverse events.
Change in disability assessed by the EDSS.
Number of participants with new or enlarging T2‐hyperintense lesions.
Number of participants who dropped out.
We used the five GRADE parameters (risk of bias, inconsistency, imprecision, indirectness and publication bias) to assess the quality of the body of evidence as it relates to the studies that contributed data to the meta‐analyses for prespecified outcomes. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using the GRADEpro software (GRADEpro 2008). We justified all decisions to downgrade or upgrade the quality of studies in the footnotes and we made comments to aid readers' understanding of the review when necessary.
Results
Description of studies
Results of the search
The search strategy retrieved 223 references: two in CENTRAL, 128 in MEDLINE, 82 in EMBASE, three in CINAHL, none in PEDro, none in LILACS, four in ClinicalTrials.gov, none in the WHO International Clinical Trials Registry Platform and four from handsearching. We considered a total of 35 references to be potentially eligible. After reading the full text, we included these 35 records. They referred to three RCTs and 32 ancillary reports about these three primary studies. The flow diagram of the process of study identification and selection is presented in Figure 1.
1.
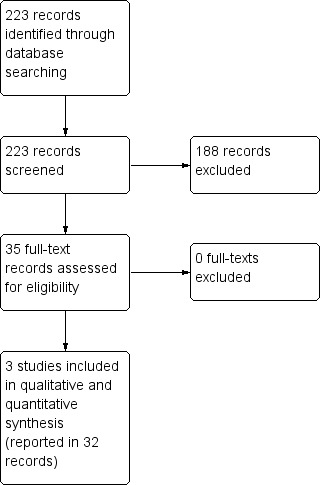
Study flow diagram.
Included studies
The three RCTs included a total of 1713 participants (CAMMS223; CARE‐MS I; CARE‐MS II). All studies were multicentric trials, comparing alemtuzumab versus subcutaneous interferon beta‐1a for patients with relapsing–remitting MS according to the McDonald criteria (McDonald 2001).
Participants were treatment‐naive in the CARE‐MS I and CAMMS223 studies. The CARE‐MS II study included only participants with at least one relapse while being treated with interferon beta or glatiramer for at least six months.
In the CARE‐MS I and CARE‐MS II studies, the interventions were given for 12 months (CARE‐MS I; CARE‐MS II); in the CAMMS223 study, the treatment lasted 24 months (CAMMS223). The following regimens were used in these RCTs:
CAMMS223 study, a phase II trial: alemtuzumab (either 12 mg per day or 24 mg per day) was given by intravenous infusion on five consecutive days during the first month and on three consecutive days at months 12 and 24 (CAMMS223).
CARE‐MS I (or CAMMS323) study, a phase III trial: alemtuzumab (12 mg per day) was given by intravenous infusion on five consecutive days during the first month and on three consecutive days at month 12 (CARE‐MS I).
CARE‐MS II (or CAMMS324) study, a phase III trial: alemtuzumab (either 12 mg per day or 24 mg per day) was given by intravenous infusion on five consecutive days during the first month and on three consecutive days at month 12 (CARE‐MS II).
In all studies, the dose of interferon beta‐1a was 44 μg given subcutaneously three times weekly after dose titration.
Details of these RCTs are available in the table Characteristics of included studies.
Excluded studies
We excluded none of the potentially eligible studies.
Risk of bias in included studies
The risk of bias of each study is detailed in the Characteristics of included studies table. Figure 2 and Figure 3 present the 'Risk of bias' summary along with review authors' judgements about each risk of bias item for each included study. The overall quality of the studies was low since in all of them we categorised at least one of the main domains (generation of allocation sequence, allocation concealment and blinding) as having a high risk of bias.
2.
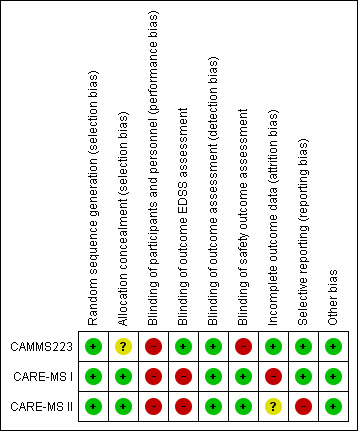
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We classified all studies as low risk of bias for generation of allocation sequence. The methods were reported in the articles and we judged them to be appropriate.
However, for allocation concealment, we classified one study as having an unclear risk of bias because it did not provide enough information to allow judgement (CAMMS223). We classified the other two studies as having a low risk of bias because they provided an adequate method to ensure allocation concealment (CARE‐MS I; CARE‐MS II).
Blinding
We considered all studies as having a high risk of bias of performance bias (participants and personnel) because both drugs (intervention and comparator) had adverse effects that precluded masking.
We judged the following outcomes separately for detection bias (outcome assessment):
EDSS outcome assessment: We classified CARE‐MS I and CARE‐MS II as having a high risk of bias since unmasked raters performed the EDSS assessments. On the other hand, CAMMS223 had a low risk of bias.
Efficacy outcomes assessment (except EDSS): We classified all studies as having a low risk of bias.
Safety outcomes assessment: CARE‐MS I and CARE‐MS II had a low risk of bias, while CAMMS223 had a high risk of bias.
Incomplete outcome data
One study was at low risk of attrition bias (CAMMS223), one was at high risk because it used an inappropriate modified "intention‐to‐treat" analysis (CARE‐MS I), and one study was at unclear risk of attrition bias (CARE‐MS II)
Selective reporting
We classified two studies as having a low risk of selective reporting bias (CAMMS223; CARE‐MS I). We classified one study as having high risk of bias because the results for some previously planned outcomes were not provided (i.e. quality of life) (CARE‐MS II).
Other potential sources of bias
There were no other known potential sources of bias in the three included trials.
Effects of interventions
See: Table 1
Comparison 1: Alemtuzumab 12 mg versus subcutaneous interferon beta‐1a
Primary outcomes
Relapse‐free survival
Alemtuzumab was associated with better relapse‐free survival at 24‐month follow‐up (hazard ratio (HR) 0.50, 95% confidence interval (CI) 0.41 to 0.60; 1248 participants; two studies; moderate quality evidence, I2 = 0%) (Analysis 1.1; Figure 4). This result was consistent when we considered separately naive and previously treated participants.
1.1. Analysis.
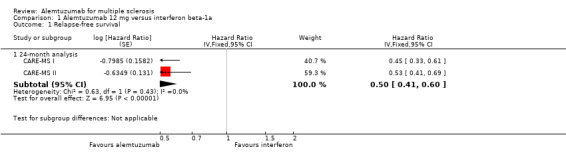
Comparison 1 Alemtuzumab 12 mg versus interferon beta‐1a, Outcome 1 Relapse‐free survival.
4.
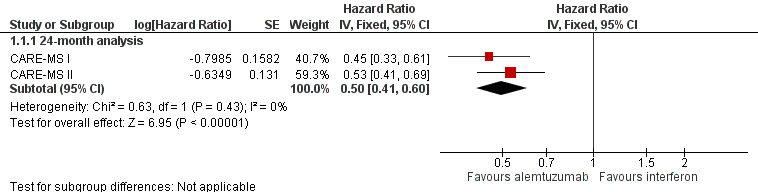
Forest plot of comparison: 1 Alemtuzumab 12 mg versus interferon beta‐1a, outcome: 1.1 Relapse‐free survival.
Only one study assessed this outcome at 36 months (CAMMS223). This study showed a higher number of participants who relapsed with interferon than with alemtuzumab (45 versus 24; HR 0.31, 95% CI 0.18 to 0.52).
None of the included studies provided data for the 12‐month analysis.
Sustained disease progression‐free survival
Alemtuzumab was associated with a lower number of participants with sustained disease progression‐free survival at both 24‐month (HR 0.62, 95% CI 0.44 to 0.87; 1191 participants; two studies; I2 = 0%) and 36‐month follow‐up (HR 0.25, 95% CI 0.11 to 0.57; 223 participants; one study) (Analysis 1.2; Figure 5). This finding was consistent when we considered a subgroup of previously treated participants. However, for naive participants there was no difference between the interventions.
1.2. Analysis.
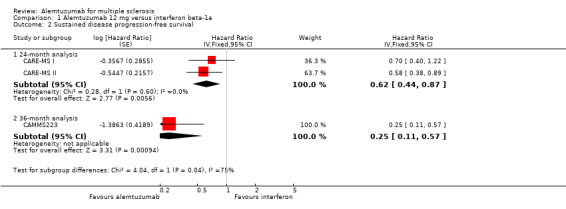
Comparison 1 Alemtuzumab 12 mg versus interferon beta‐1a, Outcome 2 Sustained disease progression‐free survival.
5.
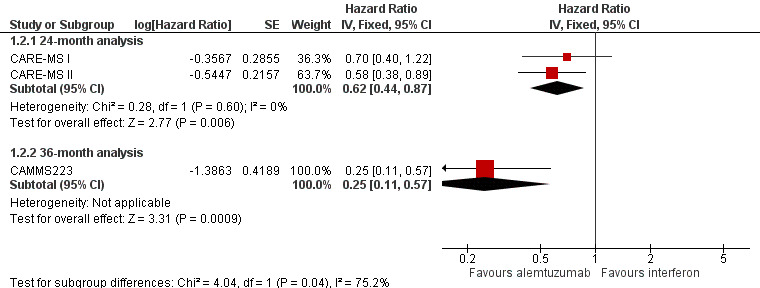
Forest plot of comparison: 1 Alemtuzumab 12 mg versus interferon beta‐1a, outcome: 1.2 Sustained disease progression‐free survival.
None of the included studies provided data for the 12‐month analysis.
Number of participants with at least one adverse event, including serious adverse events
Alemtuzumab was associated with a higher proportion of participants with at least one adverse event after 24 months (risk ratio (RR) 1.04, 95% CI 1.01 to 1.06; 1248 participants; two studies; I2 = 0%; moderate quality evidence), but not at 36 months (RR 1.00, 95% CI 0.98 to 1.02; 224 participants; one study) (Analysis 1.3; Figure 6).
1.3. Analysis.
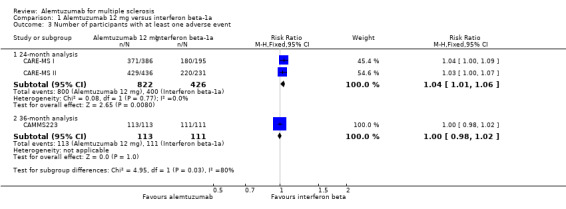
Comparison 1 Alemtuzumab 12 mg versus interferon beta‐1a, Outcome 3 Number of participants with at least one adverse event.
6.
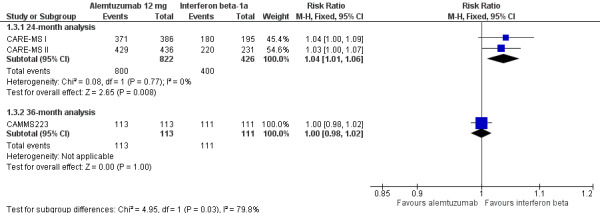
Forest plot of comparison: 1 Alemtuzumab 12 mg versus interferon beta‐1a, outcome: 1.2 Rate of participants with at least one adverse event.
None of the included studies provided data for the 12‐month analysis.
Secondary outcomes
Number of participants free of clinical disease activity
None of the included studies assessed this outcome.
Quality of life
None of the included studies assessed this outcome.
Change in disability as assessed by the Expanded Disability Status Scale (EDSS)
Alemtuzumab was associated with a significant improvement in EDSS scores after 36 months (mean difference (MD) ‐0.70, 95% CI ‐1.04 to ‐0.36; 223 participants; one study) (CAMMS223). At 24 months, considering both treatment‐naive patients and previously treated patients (who failed after interferon beta or glatiramer treatment), there were no differences in EDSS scores (MD ‐0.20, 95% CI ‐0.60 to 0.20; 1199 participants; two studies; I2 = 88%) (Analysis 1.4). However, when only previously treated patients were assessed, alemtuzumab was associated with better results (MD ‐0.41, 95% CI ‐0.62 to ‐0.20; one study; 628 participants) (CARE‐MS II).
1.4. Analysis.
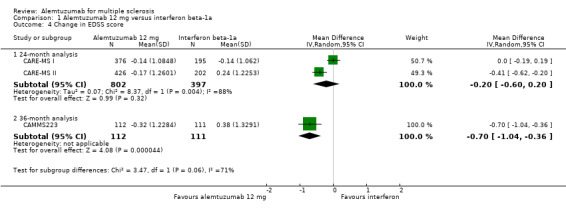
Comparison 1 Alemtuzumab 12 mg versus interferon beta‐1a, Outcome 4 Change in EDSS score.
None of the included studies provided data for the 12‐month analysis.
Fatigue as assessed by the Fatigue Severity Scale or the Fatigue Index Scale
None of the included studies assessed this outcome.
Number of participants with new or enlarging T2‐hyperintense lesions on magnetic resonance imaging
Alemtuzumab was associated with a lower rate of participants presenting with new or enlarging lesions, considering both naive and previously treated participants (RR 0.74, 95% CI 0.59 to 0.91; 1238 participants; two studies; I2 = 80%; random‐effects model) (Analysis 1.5).
1.5. Analysis.

Comparison 1 Alemtuzumab 12 mg versus interferon beta‐1a, Outcome 5 Number of participants with new or enlarging T2‐hyperintense lesions.
Number of participants who dropped out
Alemtuzumab was associated with a lower number of dropouts at 24 months (RR 0.31, 95% CI 0.23 to 0.41; 1248 participants; two studies), but not at 36 months (RR 0.81, 95% CI 0.57 to 1.14; 224 participants; one study) (Analysis 1.6).
1.6. Analysis.
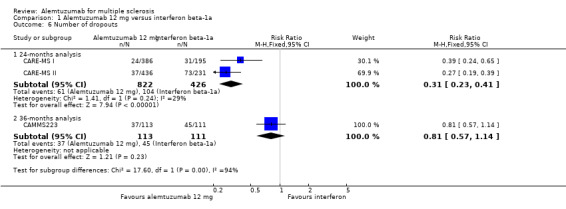
Comparison 1 Alemtuzumab 12 mg versus interferon beta‐1a, Outcome 6 Number of dropouts.
None of the included studies provided data for the 12‐month analysis.
Comparison 2: Alemtuzumab 24 mg versus subcutaneous interferon beta‐1a
Primary outcomes
Relapse‐free survival
Only one study assessed this outcome at 36 months (CAMMS223). This study showed a higher number of relapses in the interferon group than in the alemtuzumab group (45 versus 17; HR 0.21, 95% CI 0.11 to 0.40) (CAMMS223).
None of the included studies provided data for the 12‐ and 24‐month analyses.
Sustained disease progression‐free survival
Alemtuzumab was associated with a lower number of participants with sustained disease progression‐free survival at 36 months (HR 0.33, 95% CI 0.16 to 0.69; 221 participants; one study) (CAMMS223).
None of the included studies provided data for the 12‐ or 24‐month analyses.
Number of participants with at least one adverse event, including serious adverse events
There were no significant differences between alemtuzumab and subcutaneous interferon beta‐1a at 24 months (RR 1.04, 95% CI 1.00 to 1.07; 391 participants; one study) (CARE‐MS II) or at 36 months (RR 0.99, 95% CI 0.97 to 1.02; 220 participants; one study) (CAMMS223).
None of the included studies provided data for the 12‐month analysis.
Secondary outcomes
Number of participants free of clinical disease activity
None of the included studies assessed this outcome.
Quality of life
None of the included studies assessed this outcome.
Change in disability as assessed by the EDSS
Alemtuzumab was associated with a significant improvement in EDSS scores after 36 months (MD ‐0.83, 95% CI ‐1.17 to ‐0.49; 221 participants; one study) (CAMMS223). None of the included studies provided data for the 12‐ and 24‐month analyses.
Fatigue as assessed by the Fatigue Severity Scale or the Fatigue Index Scale
None of the included studies assessed this outcome.
Number of participants with new or enlarging T2‐hyperintense lesions on magnetic resonance imaging
None of the included studies assessed this outcome.
Number of participants who dropped out
Alemtuzumab was associated with a lower number of dropouts at 24 months (RR 0.27, 95% CI 0.16 to 0.46; 404 participants; one study) (CARE‐MS II), but not at 36 months (RR 0.76, 95% CI 0.53 to 1.09; 221 participants; one study) (CAMMS223).
None of the included studies provided data for the 12‐month analysis.
Discussion
Summary of main results
This systematic review aimed to assess the effects (benefits and harms) of alemtuzumab compared with any other drug treatment for any type of multiple sclerosis (MS).
Based on results of three randomised clinical trials (RCTs), compared to subcutaneous interferon beta‐1a, alemtuzumab 12 mg was associated with:
higher relapse‐free survival at 24 months and 36 months;
a lower number of participants with sustained disease progression‐free survival;
a slightly higher number of participants with at least one adverse event after 24 months;
a higher improvement in Expanded Disability Status Scale (EDSS) scores after 36 months;
a higher improvement in EDSS scores after 24 months (for patients previously treated with interferon or glatiramer acetate);
a lower number of participants with new or enlarging T2‐hyperintense lesions on magnetic resonance imaging;
a lower number of dropouts at 24 months, but not at 36 months.
Based on the results of one RCT, compared to subcutaneous interferon beta‐1a, alemtuzumab 24 mg was associated with:
higher relapse‐free survival at 36 months;
a lower number of participants with sustained disease progression‐free survival at 36 months;
no statistical difference in the number of participants with at least one adverse event at 24 and 36 months;
a higher improvement in EDSS scores after 36 months;
a lower number of dropouts at 24 months, but not at 36 months.
The higher number of participants with at least one adverse event was not associated with a higher dropout rate probably because most of these events were mild or moderate. Data for severe adverse events were not provided separately by any of the included studies.
We included change in EDSS scores as a secondary outcome instead of a primary outcome because short‐term changes in EDSS scores may not be a reliable marker of irreversible change in relapsing–remitting MS (Healy 2013).
Overall completeness and applicability of evidence
We included three RCTs that compared alemtuzumab versus subcutaneous interferon beta‐1a in patients with relapsing–remitting MS. Alemtuzumab was given during 12 or 24 months and the participants had a follow‐up of up to 36 months for some outcomes in one of the included studies. The doses were (a) 12 mg or 24 mg per day intravenously, once a day for five consecutive days at month 0 and 12, or (b) 24 mg per day intravenously, once a day for three consecutive days at month 12 and 24. The control groups received interferon beta‐1a, 44 μg subcutaneously three times weekly after dose titration. Therefore the available evidence is limited to these specific interventions and patients.
There is a lack of evidence for the following outcomes:
number of participants free of clinical disease activity;
quality of life;
fatigue (assessed by the Fatigue Severity Scale or the Fatigue Index Scale, for example).
There are two probable reasons for this lack of evidence: (a) the outcomes were initially proposed in the trial protocols but were not available for this review even after contact with the authors of these studies; (b) the outcomes were not originally planned at the protocol stage of the included RCTs.
We must emphasise that the data at 36‐month follow‐up are based on a small number of participants and this can increase the uncertainty of these findings.
Finally, the three studies only included patients with relapsing–remitting MS and we found no evidence for other forms of the disease.
Quality of the evidence
As presented in Table 1, the quality of the body of evidence obtained for each outcome ranged from very low to moderate.
The overall quality of the RCTs was low since in all of them we categorised at least one of the main domains (generation of allocation sequence, allocation concealment and blinding) as having a high risk of bias. In all studies, the participants and personnel were not blinded because the adverse effects related to each intervention preclude the masking. Additionally, in two studies the assessment of the EDSS scores could also be not blinded. Considering these two facts, we judged separately the risk of bias for EDSS and adverse events.
We noted no statistically significant heterogeneity among the studies for the co‐primary outcomes. The quality of the evidence for dropouts was impaired by the low number of events in the trials.
Potential biases in the review process
To avoid the introduction of bias, we strictly followed all of the recommendations on searching, study selection, data collection, and data analysis from the Cochrane Handbook for Systematic Reviews of Interventions in this review (Higgins 2011).
The strengths of this review include a wide literature search and the use of intention‐to‐treat analyses for dichotomous data.
The limitations of this review include: (a) no assessment of publication bias through funnel plot analysis because there were fewer than 10 studies included in the meta‐analysis and (b) the lack of some outcome data in the included RCTs.
Agreements and disagreements with other studies or reviews
During the conduct of this review a non‐Cochrane systematic review assessing all available treatments for MS was published (CADTH 2013). This review evaluated direct and indirect comparisons between several drugs, including alemtuzumab. The findings are similar to those of our review, including the results of meta‐analysis and the risk of bias of the included RCTs.
Authors' conclusions
Implications for practice.
For patients with relapsing‐remitting multiple sclerosis (MS), alemtuzumab 12 mg was better than subcutaneous interferon beta‐1a at 24 months for the outcomes relapse‐free survival, sustained disease progression‐free survival, adverse events, dropouts, Expanded Disability Status Scale (EDSS) scores (this last outcome only for patients previously treated with interferon beta or glatiramer acetate) and number of participants with new or enlarging T2‐hyperintense lesions (very low to moderate quality evidence).
At 36 months, alemtuzumab 24 mg improved the number of relapse‐free patients, reduced the number of patients with sustained disease progression and had no effect on either the number of participants with at least one adverse event or dropouts.
There is a lack of evidence for the number of participants free of clinical disease activity, quality of life, fatigue and changes in lesions on magnetic resonance imaging.
When balancing the potential benefits and harms of this treatment, it is important to consider the risk of other autoimmune syndromes, thyroid disease and malignancies related to alemtuzumab (FDA 2014).
Although the trials assessed two different doses of alemtuzumab, the dose proposed for licensing was 12 mg/day (Genzyme 2013). According to Genzyme, the reasons for this decision were that there was no difference between the 24 mg/day and 12 mg/day dose levels in the pharmacodynamic response and the overall frequency of adverse events was higher in the 24 mg/day group, suggesting better tolerability of the 12 mg/day dose. Additionally, the efficacy on imaging outcomes was reduced with the 12 mg/day regimen, suggesting that larger reductions in dose would probably connote impairment in efficacy (Genzyme 2013).
Implications for research.
Due to a lack of available data, more randomised clinical trials are needed to answer the following questions:
Is alemtuzumab effective for other forms of MS besides relapsing‐remitting MS?
Is alemtuzumab effective for both naive and previously treated MS patients?
Is alemtuzumab more effective than other available treatments (other than interferon beta‐1a)?
Are the observed effects of alemtuzumab sustained after 24 months?
Are other doses and course durations of alemtuzumab effective for MS?
What is the rate of each of the individual adverse events related to alemtuzumab (including autoimmune diseases and thyroiditis)?
Does alemtuzumab improve quality of life, rate of progression‐free participants, rate of participants free of clinical disease activity, fatigue and change in MRI lesions?
Are there long‐term adverse events associated with the use of alemtuzumab in MS patients?
Is alemtuzumab more effective and safe than other therapeutic options for MS?
What's new
| Date | Event | Description |
|---|---|---|
| 30 April 2015 | Amended | The author team has been amended. |
Acknowledgements
The review authors would like to thank:
the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Review Group, especially Liliana Coco (Managing Editor) and Andrea Fittipaldo (Trials Search Co‐ordinator) for their kind help;
the reviewers (content experts and consumer representative), who provided useful comments and suggestions, including Loredana La Mantia and Lorenzo Brait.
the Handbook Study Group from the Brazilian Cochrane Centre for methodological support;
Cristine Migliorini for her effort and dedication in publishing the protocol for this review.
Appendices
Appendix 1. Keywords for searching the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group Trials Register
{alemtuzumab} OR {Campath 1G} OR {Campath‐1G} OR {Campath‐1‐G} OR {Campath 1M} OR {Campath‐1M} OR {MabCampath} OR {Schering brand of alemtuzumab} OR {Campath} OR {Berlex brand of alemtuzumab} OR {Campath 1H} OR {monoclonal antibody Campath‐1H} OR {Campath‐1H} OR {monoclonal antibody*} OR {Antibodies, Monoclonal} OR {lemtrada}
Data and analyses
Comparison 1. Alemtuzumab 12 mg versus interferon beta‐1a.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse‐free survival | 2 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 24‐month analysis | 2 | Hazard Ratio (Fixed, 95% CI) | 0.50 [0.41, 0.60] | |
| 2 Sustained disease progression‐free survival | 3 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 24‐month analysis | 2 | Hazard Ratio (Fixed, 95% CI) | 0.62 [0.44, 0.87] | |
| 2.2 36‐month analysis | 1 | Hazard Ratio (Fixed, 95% CI) | 0.25 [0.11, 0.57] | |
| 3 Number of participants with at least one adverse event | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 24‐month analysis | 2 | 1248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [1.01, 1.06] |
| 3.2 36‐month analysis | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.98, 1.02] |
| 4 Change in EDSS score | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 24‐month analysis | 2 | 1199 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.60, 0.20] |
| 4.2 36‐month analysis | 1 | 223 | Mean Difference (IV, Random, 95% CI) | ‐0.7 [‐1.04, ‐0.36] |
| 5 Number of participants with new or enlarging T2‐hyperintense lesions | 2 | 1238 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.59, 0.91] |
| 6 Number of dropouts | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 24‐months analysis | 2 | 1248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.23, 0.41] |
| 6.2 36‐months analysis | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.57, 1.14] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
CAMMS223.
| Methods |
|
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Main interventions
Comparator
All participants received 1 g of intravenous methylprednisolone for 3 days at baseline and at months 12 and 24, coinciding with infusion cycles as premedication for those receiving alemtuzumab. Some participants also received antihistamines or antipyretics at the investigators' discretion |
|
| Outcomes |
Primary outcome measure
Secondary outcome measures
All adverse events with an onset up to 36 months were reported. In addition, all serious adverse events and autoimmune‐associated disorders occurring before 1 March 2008, were listed. A subsequent adverse event of Burkitt's lymphoma not associated with Epstein–Barr virus (EBV) was also included in this report. |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible participants were randomly assigned in a 1:1:1 with the use of the Pocock and Simon minimisation algorithm. |
| Allocation concealment (selection bias) | Unclear risk | No information available to allow a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Both study drugs have adverse effects that precluded masking of participants and treating clinicians to treatment assignment |
| Blinding of outcome EDSS assessment EDSS | Low risk | "EDSS scores were determined quarterly in a blinded fashion by a neurologist who also adjudicated possible relapses". |
| Blinding of outcome assessment (detection bias) All outcomes, except EDSS | Low risk | "MRI scans were performed annually and interpreted by a neuroradiologist who was unaware of assignments to study groups". |
| Blinding of safety outcome assessment | High risk | "Safety was assessed quarterly by the treating neurologist, who was aware of study‐group assignment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number and reasons of withdrawals were similar in the interventions and control arms. |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Low risk | — |
CARE‐MS I.
| Methods |
|
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Main interventions
After a protocol amendment in January 2009, alemtuzumab participants received oral aciclovir 200 mg twice daily during alemtuzumab infusion and for 28 days thereafter as prophylaxis against herpes infection. Comparator
Participants in both groups received 1 g per day of intravenous methylprednisolone on 3 consecutive days at baseline and at month 12. Concomitant treatment with an antipyretic or antihistamine drug was allowed, at the discretion of the treating neurologist. |
|
| Outcomes |
Primary outcome measures
Secondary outcome measure (24 months)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We randomly allocated patients using an interactive voice response system" |
| Allocation concealment (selection bias) | Low risk | Use of an interactive voice response system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Both study drugs have adverse effects that precluded masking of patients and treating clinicians to treatment assignment, and subcutaneous interferon beta 1a was available only in proprietary prefilled syringes that could not effectively be duplicated for placebo" |
| Blinding of outcome EDSS assessment EDSS | High risk | "In the absence of a masked rater, unmasked raters could submit EDSS assessments" |
| Blinding of outcome assessment (detection bias) All outcomes, except EDSS | Low risk | "Stringent clinical and MRI rater masking, and adjudication of relapses by a committee comprising six independent and masked neurologists" |
| Blinding of safety outcome assessment | Low risk | "Stringent clinical and MRI rater masking, and adjudication of relapses by a committee comprising six independent and masked neurologists" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only participants who received at least one dose of study drugs were included in the efficacy and safety analyses according to treatment assignment (modified ITT analysis) |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Low risk | |
CARE‐MS II.
| Methods |
|
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Main Interventions
Alemtuzumab was administered in 2 annual cycles, once at the beginning of the study and again 1 year later Comparator
|
|
| Outcomes |
Primary outcome measure
Secondary outcome measures
Co‐primary endpoints were relapse rate and time to 6‐month sustained accumulation of disability, comparing alemtuzumab 12 mg and interferon beta‐1a in all participants who received at least one dose of study drug. |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We randomly allocated patients with an interactive voice response system in a 2:2:1 scheme" |
| Allocation concealment (selection bias) | Low risk | Use of an interactive voice response system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Because both study drugs had adverse effects that precluded double‐blinding, and interferon beta 1a proprietary syringes could not effectively be duplicated for placebo" |
| Blinding of outcome EDSS assessment EDSS | High risk | "In the absence of a masked rater, unmasked raters could submit EDSS assessments" |
| Blinding of outcome assessment (detection bias) All outcomes, except EDSS | Low risk | "Raters were masked to treatment‐group assignment" |
| Blinding of safety outcome assessment | Low risk | "Raters were masked to treatment‐group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | — |
| Selective reporting (reporting bias) | High risk | Efficacy outcomes for alemtuzumab 24 mg were not provided. The results from some previously planned outcomes were not provided (i.e. quality of life) |
| Other bias | Low risk | |
EDSS: Expanded Disability Status Scale ITT: intention‐to treat IV: intravenous MRI: magnetic resonance imaging MS: multiple sclerosis SC: subcutaneous VAS: visual analogue scale
Differences between protocol and review
One of the co‐authors of the protocol, for unforeseen reasons, could no longer collaborate on the review and asked to be excluded from authoring.
We planned the following subgroup analyses at the protocol stage but did not perform them due to lack of sufficient data: different doses and regimens, disease type (relapsing‐remitting, primary‐progressive, secondary‐progressive or progressive‐relapsing), disability at baseline (EDSS scores ≤ 5.0 or ≥ 5.5).
We did not plan the subgroup analysis taking into account previous treatment (naive versus pre‐treated patients) at the protocol stage but as it seemed to be clinically relevant, we performed it when there were sufficient data.
In the protocol, we proposed to assess 'number of participants without relapse' and 'number of progression‐free participants' as primary outcomes. However, we found available data only for 'relapse‐free survival' and 'sustained disease progression‐free survival'. We decided to use both of these measures in the final review, since they are the opposite of the same measurement and this change would not influence the effect of the intervention nor its interpretation.
In the protocol, we proposed to assess the 'Changes in the number of MRI T2‐ and T1‐weighted lesions after treatment'. However, we discussed among the review authors and peer reviewers whether 'Patients with new or enlarging T2‐hyperintense lesions' could more relevant for decision‐making, since it comprises the total area of all MS lesions. Moreover, since this is a dichotomous measure, it could be easier for physicians and consumers to interpret. We considered this outcome relevant enough (despite the fact that it is a secondary outcome) to warrant a change in its presentation regardless of the tool/measurement used to assess it. Therefore we decided to change the way it was measured, leading to the presentation of the existing results.
We planned the following outcomes for the 'Summary of findings' table at the protocol stage but we did not include them due to lack of data: one‐point EDSS score increase confirmed at three months' follow‐up and quality of life. We included the outcome dropout rate in the 'Summary of findings' table.
In the protocol we did not pre‐define the use of the hazard ratio to estimate the effect size of time‐to‐event outcomes. We added this information in the final version of the review because we used this measure for the outcomes relapse‐free survival and sustained disease progression.
Contributions of authors
RR was the contact person with the editorial base who co‐ordinated the contributions from co‐authors and was responsible for the final draft of the review.
RR, GJMP and MRT worked on study selection, data extraction, 'Risk of bias' assessment and GRADE.
RR and GJMP performed the results analyses.
RR, GJMP and MRT wrote the discussion and conclusions.
RR responded to the clinical comments of the referees.
RR, GJMP and MRT answered the methodological and statistical questions of the referees.
RR, GJMP and MRT will be in charge of further updating.
Sources of support
Internal sources
Brazilian Cochrane Centre, Brazil.
External sources
No sources of support supplied
Declarations of interest
RR: none
GJMP: none
MRT: none
New
References
References to studies included in this review
CAMMS223 {published data only}
- Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, Margolin DH, et al. Alemtuzumab vs. interferon beta‐1a in early multiple sclerosis. New England Journal of Medicine 2008;359(17):1786‐801. [DOI] [PubMed] [Google Scholar]
- Coles AJ, Fox E, Vladic A, Gazda SK, Brinar V, Selmaj KW, et al. Alemtuzumab more effective than interferon β‐1a at 5‐year follow‐up of CAMMS223 clinical trial. Neurology 2012;78:1069–78. [DOI] [PubMed] [Google Scholar]
- Coles AJ, Fox E, Vladic A, Gazda SK, Brinar V, Selmaj KW, et al. Alemtuzumab versus interferon beta‐1a in early relapsing‐remitting multiple sclerosis: post‐hoc and subset analyses of clinical efficacy outcomes. Lancet Neurology 2011;10(4):338‐48. [DOI] [PubMed] [Google Scholar]
- Daniels GH, Vladic A, Brinar V, Zavalishin I, Valente W, Oyuela P, et al. Alemtuzumab‐related thyroid dysfunction in a phase 2 trial of patients with relapsing‐remitting multiple sclerosis. Journal of Clinical Endocrinology and Metabolism 2014;99(1):80‐9. [DOI] [PubMed] [Google Scholar]
CARE‐MS I {published data only}
- Arnold D, Brinar V, Cohen J, Coles A, Confavreux C, Fisher E, et al. Effect of alemtuzumab vs. RebifTM on brain MRI measurements: results of CARE‐MS I, a phase 3 study. Neurology 2012;78(1):S11.006. [Google Scholar]
- Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, et al. Alemtuzumab versus interferon beta 1a as first‐line treatment for patients with relapsing‐remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012;380(9856):1819‐28. [DOI] [PubMed] [Google Scholar]
- Coles A, Brinar V, Arnold D, Cohen J, Confavreux C, Fox E, et al. Efficacy and safety results from comparison of alemtuzumab and RebifTM efficacy in multiple sclerosis I (CARE‐MS I): a phase 3 study in relapsing‐remitting treatment‐naive patients. Neurology 2012;78(Suppl 1):S01.006. [Google Scholar]
- Fox E, Arnold D, Brinar V, Cohen J, Coles A, Confavreux C, et al. Relapse outcomes with alemtuzumab vs. RebifTM in treatment‐naive relapsing‐remitting multiple sclerosis (CARE‐MS I): secondary and tertiary endpoints. Neurology 2012;78(Suppl 1):PD5.004. [DOI: 10.1212/WNL.78.1_MeetingAbstracts.PD5.004] [DOI] [Google Scholar]
- Giovannoni G, Arnold DL, Cohen J, Coles AJ, Confavreux C, Fox HP, et al. Disease activity‐free status in comparison of alemtuzumab and RebifTM efficacy in multiple sclerosis I (CARE‐MS I) phase 3 study. Journal of Neurology 2012;259(Suppl 1):47. [Google Scholar]
- Habek M, Arnold DL, Cohen J, Coles AJ, Confavreux C, Fox EJ, et al. Thyroid autoimmunity in comparison of Alemtuzumab and RebifTM efficacy in multiple sclerosis studies I and II. Journal of Neurology 2012;259(Suppl 1):66. [Google Scholar]
- Havrdova E, Arnold DL, Cohen J, Coles AJ, Confavreux C, Fox EJ, et al. Infections Phase 3 study: comparison of alemtuzumab and RebifTM efficacy in multiple sclerosis I (CARE‐MS I). Neurology 2012;78(Suppl 1):S41.007. [Google Scholar]
- Lycke J, Arnold DL, Cohen JA, Coles AJ, Confavreux C, Fox EJ, et al. Adverse event profile of alemtuzumab over time in treatment‐naive patients with early, active relapsing‐remitting multiple sclerosis (RRMS; CARE‐MS I study). Journal of the Neurological Sciences 2013;333(Suppl 1):e374‐5. [Google Scholar]
- Lycke J, Arnold DL, Cohen JA, Coles AJ, Fox EJ, Hartung HP, et al. Adverse event profile of alemtuzumab in active relapsing remitting multiple sclerosis patients who participated in the CARE‐MS studies: three‐year follow‐up. 29th Congress or the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), 2‐5 October 2013, Copenhagen, Denmark. 2013.
- Miller T, Arnold D, Cohen J, Coles A, Confavreux C, Fox E, et al. Detection, incidence, and management of thyroid autoimmunity in comparison of alemtuzumab and RebifTM in multiple sclerosis (CARE‐MS) I and II. Neurology 2013;80(1):P01.173. [Google Scholar]
- Selmaj K, Arnold DL, Brinar V, Cohen J, Coles AJ, Confavreux C, et al. Incidence of autoimmunity in a phase 3 trial: comparison of alemtuzumab and RebifTM in multiple sclerosis I (CARE‐MS I). Neurology 2012;78(1):S41.006. [Google Scholar]
- Selmaj K, Arnold DL, Cohen J, Coles AJ, Confavreux C, Fox EJ, et al. Alemtuzumab improves patient‐reported quality of life in relapsing‐remitting multiple sclerosis: CARE‐MS I and II phase 3 trials. Journal of Neurology 2012;259(Suppl 1):S65‐6. [Google Scholar]
CARE‐MS II {published data only}
- Arnold DL, Cohen J, Coles AJ, Confavreux C, Fisher E, Fox EJ, et al. Effect of alemtuzumab vs. Rebif® on brain MRI measurements. Multiple Sclerosis 2012;18(4):397. [Google Scholar]
- Arroyo R, Arnold DL, Cohen JA, Coles AJ, Confavreux C, Fox EJ, et al. Alemtuzumab improves quality of life compared to SC IFNB‐1a in CARE‐MS II. Journal of Neurology 2013;260:S121‐2. [Google Scholar]
- Barkhof F, Fisher E, Palmer J, Margolin DH, Arnold DL. Alemtuzumab demonstrates improvement in MRI outcomes across baseline subgroups versus subcutaneous interferon beta‐1a in relapsing‐remitting multiple sclerosis patients who relapsed on prior therapy. European Journal of Neurology 2014;21:126‐7. [Google Scholar]
- Brinar V, Arnold DL, Cohen J, Coles AJ, Fox EJ, Hartung HP, et al. Alemtuzumab improves expanded disability status scale (EDSS) via effects on functional systems: CARE‐MS II. Multiple Sclerosis. Multiple Sclerosis Journal 2013;19(S1):283‐4. [Google Scholar]
- Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease‐modifying therapy: a randomised controlled phase 3 trial. Lancet 2012;380:1829‐39. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Twyman CL, Arnold D, Cohen J, Coles AJ, Fox EJ, et al. Efficacy of alemtuzumab in relapsing remitting multiple sclerosis (RRMS) patients with highly active disease despite therapy. European Journal of Neurology 2012;19(Suppl 1):458. [Google Scholar]
- Fernandez O, Arnold DL, Cohen JA, Coles AJ, Confavreux C, Fox EJ, et al. Alemtuzumab improves disability by month 6 independent of relapse history in relapsing‐remitting multiple sclerosis patients: CARE‐MS II 20620. Journal of Neurology 2013;260:S14. [Google Scholar]
- Fisher E, Barkhof F, Cohen JA, Fox EJ, Selmaj KW, Margolin DH, et al. Alemtuzumab improves MRI outcomes in relapsing‐remitting multiple sclerosis patients who relapsed on prior therapy: three‐year follow‐up of CARE‐MS II. Multiple Sclerosis Journal 2014;20(Suppl 1):67. [Google Scholar]
- Giovannoni G, Arnold DL, Cohen J, Coles AJ, Confavreux C, Fox E, et al. Disability improvement with alemtuzumab vs. interferon b‐ 1a in relapsing‐remitting multiple sclerosis patients who relapsed on prior therapy (CARE‐MS II). Multiple Sclerosis 2012;18(S1):419. [Google Scholar]
- Giovannoni G, Arnold DL, Cohen J, Coles AJ, Confavreux C, Fox E, et al. Multiple sclerosis: clinical trials outcomes disability improvement with alemtuzumab vs. interferon beta‐1a in relapsing‐remitting multiple sclerosis patients who experienced disease activity while on prior therapy (CARE‐MS II). Neurology 2013;80(Suppl 1):P07.120. [Google Scholar]
- Habek M, Arnold DL, Cohen J, Coles AJ, Confavreux C, Fox EJ, et al. Thyroid autoimmunity in comparison of alemtuzumab and RebifTM efficacy in multiple sclerosis studies I and II. Journal of Neurology 2012;259(Suppl 1):S66. [Google Scholar]
- Hartung H, Vollmer T, Arnold D, Cohen J, Coles A, Confavreux C, et al. Alemtuzumab reduces ms disease activity in active relapsing‐remitting multiple sclerosis patients who had disease activity on prior therapy. Neurology 2013;80(Suppl 1):P07.093. [Google Scholar]
- Hartung HP, Arnold DL, Cohen J, Coles AJ, Confavreux C, Fox EJ, et al. Disability outcomes for alemtuzumab in RRMS patients who relapsed on prior therapy: CARE‐MS II. Journal of Neurology 2012;259(Suppl 1):S47‐8. [Google Scholar]
- Havrdova E, Arnold DL, Cohen J, Coles AJ, Confavreux C, Fox EJ, et al. Safety of alemtuzumab in relapsing‐remitting multiple sclerosis patients who relapsed on prior therapy (CARE‐MS II). Multiple Sclerosis 2012;18(4):235. [Google Scholar]
- Lycke J, Arnold DL, Cohen JA, Coles AJ, Fox EJ, Hartung HP, et al. Adverse event profile of alemtuzumab in active relapsing remitting multiple sclerosis patients who participated in the CARE‐MS studies: three‐year follow‐up. 29th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), 2‐5 October 2013, Copenhagen, Denmark 2013;19(S1):487‐8. [Google Scholar]
- Miller T, Arnold D, Cohen J, Coles A, Confavreux C, Fox E, et al. Detection, incidence, and management of thyroid autoimmunity in comparison of alemtuzumab and RebifTM in multiple sclerosis (CARE‐MS) I and II. Neurology 2013;80(1):P01.173. [Google Scholar]
- Moreau T, Margolin DH, Kasten L, Singer B. Alemtuzumab improves quality of life in relapsing remitting multiple sclerosis patients who relapsed on prior therapy: 3‐year follow‐up of CARE‐MS II. Multiple Sclerosis 2014;20(1):86. [Google Scholar]
- Selmaj K, Arnold DL, Cohen J, Coles AJ, Confavreux C, Fox EJ, et al. Alemtuzumab improves patient‐reported quality of life in relapsing‐remitting multiple sclerosis: CARE‐MS I and II phase 3 trials. Journal of Neurology 2012;259(Suppl 1):S65‐6. [Google Scholar]
- Wray S, Arnold D, Cohen J, Coles A, Confavreux C, Fox E, et al. Comparison of infection risk with alemtuzumab and sc IFNB‐1a in patients with multiple sclerosis who experienced disease activity while on prior therapy (CARE‐MS II). Neurology 2013;80(1):P01.172. [Google Scholar]
Additional references
CADTH 2013
- Canadian Agency for Drugs and Technologies in Health. Management of relapsing‐remitting multiple sclerosis. http://www.ncbi.nlm.nih.gov/books/NBK169748/ (accessed 11 March 2016).
Coles 1999a
- Coles AJ, Wing M, Smith S, Coraddu F, Greer S, Taylor C, et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet 1999;354:1691‐5. [DOI] [PubMed] [Google Scholar]
Coles 1999b
- Coles AJ, Wing MG, Molyneux P, Paolillo A, Davie CM, Hale G, et al. Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Annals of Neurology 1999;46:296‐304. [DOI] [PubMed] [Google Scholar]
Coles 2006
- Coles AJ, Cox A, Page E, Jones J, Trip SA, Deans J, et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. Journal of Neurology 2006;253:98‐108. [DOI] [PubMed] [Google Scholar]
Cossburn 2011
- Cossburn M, Pace AA, Jones J, Ali R, Ingram G, Baker K, et al. Autoimmune disease after alemtuzumab treatment for multiple sclerosis in a multicenter cohort. Neurology 2011;77(6):573‐9. [DOI] [PubMed] [Google Scholar]
EMA 2013
- European Medicines Agency. Lemtrada ‐ Committee for Medicinal Products for Human Use (CHMP) ‐ Assessment report. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/003718/WC500150522.pdf (accessed 11 March 2016).
FDA 2001
- US Food, Drug Administration. Campath (alemtuzumab) Product Approval Information ‐ Application number BLA 103948/0. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/103948_0000_Campath_AprvbleLtr2.pdf (accessed 11 March 2016).
FDA 2014
- US Food, Drug Administration. Alemtuzumab (Lemtrada) Product Approval Information. Licensing Action 2014. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2014/103948Orig1s5139ltr.pdf (accessed 11 March 2016).
FDA 2015
- US Food, Drug Administration. Risk Evaluation and Mitigation Strategies (REMS). Lemtrada 2015. http://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=340 (accessed 11 March 2016).
Filippini 2013
- Filippini G, Giovane C, Vacchi L, D'Amico R, Pietrantonj C, Beecher D, et al. Immunomodulators and immunosuppressants for multiple sclerosis: a network meta‐analysis. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD008933.pub2] [DOI] [PubMed] [Google Scholar]
Fischer 1999
- Fischer JS, LaRocca NG, Miller DM, Ritvo PG, Andrews H, Paty D. Recent developments in the assessment of quality of life in multiple sclerosis (MS). Multiple Sclerosis 1999;5:251‐9. [DOI] [PubMed] [Google Scholar]
Genzyme 2013
- Peripheral and Central Nervous System Drugs Advisory Committee. Alemtuzumab Advisory Committee Briefing Document. BLA 103948. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PeripheralandCentralNervousSystemDrugsAdvisoryCommittee/UCM374188.pdf (accessed 11 March 2016).
Gilleece 1993
- Gilleece M, Dexter T. Effect of campath‐1h antibody on human hematopoietic progenitors in vitro. Blood 1993;82:807–12. [PubMed] [Google Scholar]
Gomez‐Almaguer 2012
- Gomez‐Almaguer D, Jaime‐Perez J, Ruiz‐Arguelles G. Antibodies in the treatment of aplastic anaemia. Archivum Immunologiae et Therapiae Experimentalis 2012;60:99‐106. [DOI] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- GRADE Working Group. GRADEpro. Version 3.2 for Windows. GRADE Working Group, 2008.
Gray 2004
- Gray O, McDonnell GV, Forbes RB. Methotrexate for multiple sclerosis. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD003208.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawkins 1999
- Hawkins SA, McDonnell GV. Benign multiple sclerosis? Clinical course, long term follow up, and assessment of prognostic factors. Journal of Neurology Neurosurgery and Psychiatry 1999;67:148‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Healy 2013
- Healy BC, Engler D, Glanz B, Musallam A, Chitnis T. Assessment of definitions of sustained disease progression in relapsing‐remitting multiple sclerosis. Multiple Sclerosis International 2013;2013:189624. [PUBMED: 23555057] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hill‐Cawthorne 2012
- Hill‐Cawthorne GA, Button T, Tuohy O, Jones JL, May K, Somerfield J, et al. Long term lymphocyte reconstitution after alemtuzumab treatment of multiple sclerosis. Journal of Neurology, Neurosurgery and Psychiatry 2012;83(3):298‐304. [DOI] [PubMed] [Google Scholar]
Hirst 2008
- Hirst CL, Pace A, Pickersgill TP, Jones R, McLean BN, Zajicek JP, et al. Campath1‐H treatment in patients with aggressive relapsing remitting multiple sclerosis. Journal of Neurology 2008;255:231‐8. [DOI] [PubMed] [Google Scholar]
Keating 2002
- Keating M, Flinn I, Jain V, Binet J, Hillmen P, Byrd J, et al. Therapeutic role of alemtuzumab (Campath‐1H) in patients who have failed fludarabine: results of a large international study. Blood 2002;99:3554–61. [DOI] [PubMed] [Google Scholar]
Krupp 1989
- Krupp LB, LaRocca NG, Muir‐Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology 1989;46:1121–3. [DOI] [PubMed] [Google Scholar]
Kurtzke 1983
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale. Neurology 1983;33:1444‐52. [DOI] [PubMed] [Google Scholar]
Li 1999
- Li DK, Paty DW, the UBC MS/MRI Analysis Research Group and the PRISMS Study Group. Magnetic resonance imaging results of the PRISMS trial: a randomised, double‐blind, placebo‐controlled study of interferon‐beta la in relapsing–remitting multiple sclerosis. Annals of Neurology 1999;46:197‐206. [DOI] [PubMed] [Google Scholar]
Lockwood 2003
- Lockwood C, Hale G, Waldmann H, Jayne D. Remission induction in Behcet’s disease following lymphocyte depletion by the anti‐CD52 antibody CAMPATH‐1H. Rheumatology 2003;42:1539‐44. [DOI] [PubMed] [Google Scholar]
Lublin 1996
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. Neurology 1996;46:907‐11. [DOI] [PubMed] [Google Scholar]
McDonald 2001
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Annals of Neurology 2001;50:121‐7. [DOI] [PubMed] [Google Scholar]
Multiple Sclerosis International Federation 2010
- Multiple Sclerosis International Federation (MSIF). Global Economic Impact of Multiple Sclerosis. http://www.msif.org/wp‐content/uploads/2014/09/Global_economic_impact_of_MS.pdf (accessed 11 March 2016).
Noseworthy 2000
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. New England Journal of Medicine 2000;343(13):938‐52. [DOI] [PubMed] [Google Scholar]
Polman 2011
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology 2011;69(2):292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poser 1983
- Poser CM, Paty DW, Scheinberg L, McDonal WI, Davis FA, Ebers GC. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13:227‐31. [DOI] [PubMed] [Google Scholar]
Rao 2012
- Rao SP, Sancho J, Campos‐Rivera J, Boutin PM, Severy PB, Weeden T, et al. Human peripheral blood mononuclear cells exhibit heterogeneous CD52 expression levels and show differential sensitivity to alemtuzumab mediated cytolysis. PLoS One 2012;7(6):e39416. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2015 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2015.
Rieckmann 2009
- Rieckmann P. Concepts of induction and escalation therapy in multiple sclerosis. Journal of Neurosurgical Sciences 2009;277(Suppl 1):42‐5. [DOI] [PubMed] [Google Scholar]
Scolding 2015
- Scolding N, Barnes D, Cader S, Chataway J, Chaudhuri A, Coles A, et al. Association of British Neurologists: revised (2015) guidelines for prescribing disease‐modifying treatments in multiple sclerosis. Practical Neurology 2015;15(4):273‐9. [DOI] [PubMed] [Google Scholar]
Vickrey 1995
- Vickrey BG, Hays RD, Harooni R, Myers LW, Ellison GW. A health‐related quality of life measure for multiple sclerosis. Quality of Life Research 1995;4(3):187‐206. [DOI] [PubMed] [Google Scholar]
Waldmann 2005
- Waldmann H, Hale G. Campath: from concept to clinic. Philosophical transactions of the Royal Society B: Biological Sciences 2005;360:1707–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weissenbacher 2010
- Weissenbacher A, Boesmueller C, Brandacher G, Oellinger R, Pratschke J, Schneeberger S. Alemtuzumab in solid organ transplantation and in composite tissue allotransplantation. Immunotherapy 2010;2:783‐90. [DOI] [PubMed] [Google Scholar]
Xia 1991
- Xia M, Tone M, Packman L, Hale G, Waldmann H. Characterization of the Campath‐1 (CDW2) antigen: biochemical analysis and cDNA cloning reveal an unusually small peptide backbone. European Journal of Immunology 1991;21:1677‐84. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Riera 2014
- Riera R, Porfirio G, Migliorini CR, Torloni MR. Alemtuzumab for multiple sclerosis. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD011203] [DOI] [PMC free article] [PubMed] [Google Scholar]


