Abstract
Background
Uterine fibroids occur in up to 40% of women aged over 35 years. Some are asymptomatic, but up to 50% cause symptoms that warrant therapy. Symptoms include anaemia caused by heavy menstrual bleeding, pelvic pain, dysmenorrhoea, infertility and low quality of life. Surgery is the first choice of treatment. In recent years, medical therapies have been used before surgery to improve intraoperative and postoperative outcomes. However, such therapies tend to be expensive.
Fibroid growth is stimulated by oestrogen. Gonadotropin‐hormone releasing analogues (GnRHa) induce a state of hypo‐oestrogenism that shrinks fibroids , but has unacceptable side effects if used long‐term. Other potential hormonal treatments, include progestins and selective progesterone‐receptor modulators (SPRMs).
This is an update of a Cochrane Review published in 2000 and 2001; the scope has been broadened to include all preoperative medical treatments.
Objectives
To assess the effectiveness and safety of medical treatments prior to surgery for uterine fibroids.
Search methods
We searched the Cochrane Gynaecology and Fertility Group specialised register, CENTRAL, MEDLINE, Embase, PsycINFO and CINAHL in June 2017. We also searched trials registers (ClinicalTrials.com; WHO ICTRP), theses and dissertations and the grey literature, handsearched reference lists of retrieved articles and contacted pharmaceutical companies for additional trials.
Selection criteria
We included randomised comparisons of medical therapy versus placebo, no treatment, or other medical therapy before surgery, myomectomy, hysterectomy or endometrial resection, for uterine fibroids.
Data collection and analysis
We used standard methodological procedures expected by The Cochrane Collaboration.
Main results
We included a total of 38 RCTs (3623 women); 19 studies compared GnRHa to no pretreatment (n = 19), placebo (n = 8), other medical pretreatments (progestin, SPRMs, selective oestrogen receptor modulators (SERMs), dopamine agonists, oestrogen receptor antagonists) (n = 7), and four compared SPRMs with placebo. Most results provided low‐quality evidence due to limitations in study design (poor reporting of randomisation procedures, lack of blinding), imprecision and inconsistency.
GnRHa versus no treatment or placebo
GnRHa treatments were associated with reductions in both uterine (MD ‐175 mL, 95% CI ‐219.0 to ‐131.7; 13 studies; 858 participants; I² = 67%; low‐quality evidence) and fibroid volume (heterogeneous studies, MD 5.7 mL to 155.4 mL), and increased preoperative haemoglobin (MD 0.88 g/dL, 95% CI 0.7 to 1.1; 10 studies; 834 participants; I² = 0%; moderate‐quality evidence), at the expense of a greater likelihood of adverse events, particularly hot flushes (OR 7.68, 95% CI 4.6 to 13.0; 6 studies; 877 participants; I² = 46%; moderate‐quality evidence).
Duration of hysterectomy surgery was reduced among women who received GnRHa treatment (‐9.59 minutes, 95% CI 15.9 to ‐3.28; 6 studies; 617 participants; I² = 57%; low‐quality evidence) and there was less blood loss (heterogeneous studies, MD 25 mL to 148 mL), fewer blood transfusions (OR 0.54, 95% CI 0.3 to 1.0; 6 studies; 601 participants; I² = 0%; moderate‐quality evidence), and fewer postoperative complications (OR 0.54, 95% CI 0.3 to 0.9; 7 studies; 772 participants; I² = 28%; low‐quality evidence).
GnRHa appeared to reduce intraoperative blood loss during myomectomy (MD 22 mL to 157 mL). There was no clear evidence of a difference among groups for other primary outcomes after myomectomy: duration of surgery (studies too heterogeneous for pooling), blood transfusions (OR 0.85, 95% CI 0.3 to 2.8; 4 studies; 121 participants; I² = 0%; low‐quality evidence) or postoperative complications (OR 1.07, 95% CI 0.43 to 2.64; I² = 0%; 5 studies; 190 participants; low‐quality evidence). No suitable data were available for analysis of preoperative bleeding.
GnRHa versus other medical therapies
GnRHa was associated with a greater reduction in uterine volume (‐47% with GnRHa compared to ‐20% and ‐22% with 5 mg and 10 mg ulipristal acetate) but was more likely to cause hot flushes (OR 12.3, 95% CI 4.04 to 37.48; 5 studies; 183 participants; I² = 61%; low‐quality evidence) compared with ulipristal acetate. There was no clear evidence of a difference in bleeding reduction (ulipristal acetate 5 mg: OR 0.71, 95% CI 0.3 to 1.7; 1 study; 199 participants; moderate‐quality evidence; ulipristal acetate 10 mg: OR 0.39, 95% CI 0.1 to 1.1; 1 study; 203 participants; moderate‐quality evidence) or haemoglobin levels (MD ‐0.2, 95% CI ‐0.6 to 0.2; 188 participants; moderate‐quality evidence).
There was no clear evidence of a difference in fibroid volume between GnRHa and cabergoline (MD 12.71 mL, 95% CI ‐5.9 to 31.3; 2 studies; 110 participants; I² = 0%; low‐quality evidence).
The included studies did not report usable data for any other primary outcomes.
SPRMs versus placebo
SPRMs (mifepristone, CDB‐2914, ulipristal acetate and asoprisnil) were associated with greater reductions in uterine or fibroid volume than placebo (studies too heterogeneous to pool) and increased preoperative haemoglobin levels (MD 0.93 g/dL, 0.5 to 1.4; 2 studies; 173 participants; I² = 0%; high‐quality evidence). Ulipristal acetate and asoprisnil were also associated with greater reductions in bleeding before surgery (ulipristal acetate 5 mg: OR 41.41, 95% CI 15.3 to 112.4; 1 study; 143 participants; low‐quality evidence; ulipristal acetate 10 mg: OR 78.83, 95% CI 24.0 to 258.7; 1 study; 146 participants; low‐quality evidence; asoprisnil: MD ‐166.9 mL; 95% CI ‐277.6 to ‐56.2; 1 study; 22 participants; low‐quality evidence). There was no evidence of differences in preoperative complications. No other primary outcomes were measured.
Authors' conclusions
A rationale for the use of preoperative medical therapy before surgery for fibroids is to make surgery easier. There is clear evidence that preoperative GnRHa reduces uterine and fibroid volume, and increases preoperative haemoglobin levels, although GnRHa increases the incidence of hot flushes. During hysterectomy, blood loss, operation time and complication rates were also reduced. Evidence suggests that ulipristal acetate may offer similar advantages (reduced fibroid volume and fibroid‐related bleeding and increased haemoglobin levels) although replication of these studies is advised before firm conclusions can be made. Future research should focus on cost‐effectiveness and distinguish between groups of women with fibroids who would most benefit.
Plain language summary
Preoperative medical therapy before surgery for uterine fibroids
Review question
We investigated if giving drugs before surgery for uterine fibroids improves outcomes.
Background
Uterine fibroids are smooth muscle tumours of the uterus (womb) that can cause fertility problems, heavy menstrual bleeding, repeated pregnancy loss and pelvic pain. Fibroids are usually treated by surgery. Some drugs, particularly gonadotropin‐releasing hormone analogues (GnRHa), have been used to temporarily control bleeding and reduce fibroid and uterine size before surgery. They are unsuitable for long‐term use because they may cause bone loss. Other drugs, including progestins, dopamine agonists, selective progesterone receptor modulators (SPRMs), oestrogen receptor antagonists and selective oestrogen receptor modulators (SERMs), may also provide benefits used short‐term. However, such therapies tend to be expensive.
Search date
We searched for evidence to June 2017.
Study characteristics
We included 38 studies that involved 3623 women with fibroids that caused symptoms and who were scheduled for surgery to remove the fibroids. Surgeries were either hysterectomy (uterus removal) or myomectomy or resection (removal of fibroids from the uterus wall). Many women were anaemic (had low red blood cell or haemoglobin levels).
The studies compared GnRHa with no treatment or sham treatment, GnRHa with other medical treatments, and SPRMs with sham treatment.
Study funding sources
Fourteen studies were either wholly or partially funded by pharmaceutical companies; three were funded by institutions or hospitals; the source of funding was unclear for 21 trials. It was not possible to determine whether funding source influenced results.
Key results
GnRHa increased haemoglobin levels before surgery and decreased uterine and fibroid size, compared with no treatment or placebo. Blood loss, need for blood transfusion, operation time during hysterectomy and postoperative complications were reduced. However, women were more likely to experience hot flushes during treatment. An SPRM drug (ulipristal acetate) had similar benefits, particularly reduced bleeding. Future research should focus on cost‐effectiveness and distinguish between groups of women with fibroids who would most benefit.
Quality of the evidence
The overall quality of evidence for most outcomes was low or very low, meaning there is substantial uncertainty about findings. Quality limitations included lack of reporting of randomisation methods and allocation concealment, lack of blinding (which means that knowledge of treatment could have influenced the findings) and variation in findings among studies. Some findings were imprecise because they were based on only one study.
Summary of findings
Summary of findings for the main comparison. GnRHa treatment versus placebo or no pretreatment (preoperative outcomes) for uterine fibroids.
| Gonadotropin‐hormone releasing analogue (GnRHa) treatment versus placebo or no pretreatment (preoperative outcomes) for uterine fibroids | ||||||
| Patient or population: women with uterine fibroids Settings: hospitals or outpatient clinics (only preoperative outcomes) Intervention: GnRHa treatment versus placebo or no pretreatment (preoperative outcomes) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control placebo or no treatment | GnRHa pretreatment | |||||
| Uterine volume (mL) (preoperative) | Mean uterine volume in control group ranged from 255 mL to 920 mL | Mean uterine volume (mL) (preoperative) in the intervention groups was 175.34 mL lower (219.04 mL to 131.65 mL lower) | ‐ | 858 (13 studies) | ⊕⊕⊝⊝ low1,2 | This overall estimate assessed effects from studies with two types of control group, either no treatment or placebo |
| Fibroid volume (mL) (preoperative) | See comment | Not estimable | 427 (5 studies) | ⊕⊕⊝⊝ low3,4 | Estimates were too heterogeneous for pooling. Reduction in fibroid volume ranged from 5 mL to 155 mL in the GnRHa group compared to control | |
| Haemoglobin (g/dL) (preoperative) | Mean haemoglobin ranged from 10.9 g/dL to 13.4 g/dL | Mean haemoglobin (g/dL) (preoperative) in the intervention groups was 0.88 mL higher (0.68 mL to 1.08L higher) | ‐ | 834 (10 studies) | ⊕⊕⊝⊝ low5 | This overall estimate assessed effects from studies with two types of control group, either no treatment or placebo |
| Preoperative bleeding | See comment | Not estimable | ‐ | ‐ | This outcome was not measured by validated scales | |
| Adverse events | Study population | OR 2.78 (1.77 to 4.36) | 755 (4 studies) | ⊕⊕⊕⊝ moderate6 | ||
| 579 per 1000 | 793 per 1000 (709 to 857) | |||||
| Moderate | ||||||
| 608 per 1000 | 812 per 1000 (733 to 871) | |||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Evidence quality was downgraded 1 level for serious limitations in study design (few studies had adequate sequence generation, allocation concealment baseline comparability and blinding although lack of blinding was not expected to influence findings. 7 studies had low risk of attrition and reporting bias). 2 Evidence quality was downgraded one level for inconsistency (there was wide variability in the estimates). 3 Evidence quality was downgraded one level for serious limitations in study design (only 1 study had low risk of selection, reporting, performance and detection bias and 2 of 5 had low risk of attrition bias). 4 Evidence quality downgraded one level for substantial heterogeneity. 5 Level of evidence downgraded 1 level for serious limitations in study design (sequence generation and allocation concealment were unclear or inadequate in 7 of 10 studies, selective reporting and completeness of data were unclear or inadequate in 5 of 10 studies, blinding was only assured in 6 studies (participants/investigators) and 2 studies (assessors) and other bias was possible in 6 studies).
6 Evidence quality downgraded one level because of serious limitations in study design (most trials had low risk of selection, reporting and performance biases, but risk of detection and attrition bias was unclear or high).
Summary of findings 2. GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative outcomes for uterine fibroids).
| Gonadotropin‐hormone releasing analogues (GnRHa) treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative outcome for uterine fibroids) | ||||||
| Patient or population: women with uterine fibroids Settings: hospitals or outpatient clinics (only perioperative or postoperative outcomes) Intervention: GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative outcomes) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control placebo or no pretreatment | GnRHa pretreatment | |||||
| Duration of surgery (minutes) | Mean duration of surgery in the control group ranged from 53 minutes to 115 minutes | Mean duration of surgery (minutes) in the intervention groups was 9.59 minutes shorter (15.9 to 3.28 shorter) | ‐ | 617 (6 studies) | ⊕⊕⊝⊝ low1,2 | An additional 3 studies had findings presented in data tables (2 reported no difference between groups and 1 reported a difference of 21 minutes between groups) |
| Intraoperative blood loss (mL) | See comment | Not estimable | 258 (4 studies) | ⊕⊝⊝⊝ very low3,4,5 | Substantial heterogeneity so estimates could not be pooled. Differences between blood loss (mL) between GnRHa and control group participants ranged from 25 mL to 148 mL | |
| Blood transfusions | Study population | OR 0.54 (0.29 to 1.01) | 601 (6 studies) | ⊕⊕⊝⊝ moderate3,5 | Fixed‐effects model: OR 0.54 (95% CI 0.3 to 0.95) | |
| 104 per 1000 | 59 per 1000 (33 to 105) | |||||
| Moderate | ||||||
| 115 per 1000 | 66 per 1000 (36 to 116) | |||||
| Postoperative morbidity | Study population | OR 0.54 (0.32 to 0.91) | 772 (7 studies) | ⊕⊕⊝⊝ low5,6 | ||
| 195 per 1000 | 116 per 1000 (72 to 181) | |||||
| Moderate | ||||||
| 239 per 1000 | 145 per 1000 (91 to 222) | |||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Evidence quality downgraded one level because of serious limitations in study design (approximately half of the included studies had unclear or high risk of selection, performance, detection, attrition and reporting bias). 2 Evidence quality downgraded one level because of serious (moderate) inconsistency. 3 Evidence quality downgraded one level because of serious limitations in study design (half of the studies had unclear selection, reporting and attrition bias. Lack of blinding in the studies was unlikely to affect the results). 4 Evidence quality downgraded one level because of serious inconsistency. 5 Evidence quality downgraded one level because of serious imprecision (wide confidence intervals). 6 Evidence quality downgraded one level because of serious limitations in study design (approximately half of the studies had unclear risk of selection, performance, and attrition bias and risk of detection bias was unclear in all studies).
Summary of findings 3. GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative outcomes for uterine fibroids).
| Gonadotropin‐hormone releasing analogue (GnRHa) treatment versus no pretreatment or placebo before myomectomy (operative and postoperative outcomes) for uterine fibroids | ||||||
| Patient or population: women with uterine fibroids Settings: hospitals or outpatient clinics (only perioperative or postoperative outcomes) Intervention: GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative outcomes) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control placebo or no pretreatment | GnRHa pretreatment | |||||
| Duration of surgery (minutes) | See comment | Not estimable | 443 (6 studies) | ⊕⊝⊝⊝ very low1,2 | Substantial heterogeneity so estimates could not be pooled. Trial where laparoscopic myomectomy was undertaken indicated that GnRHa was associated with greater duration of surgery than control but no other factors were identified to explain the variation and no estimates could be shown. | |
| Intraoperative blood loss (mL) | See comment | Not estimable | 549 (10 studies) | ⊕⊝⊝⊝ very low2,3 | Substantial heterogeneity so estimates could not be pooled. All trials, except 1, found a difference in intraoperative blood loss between GnRHa and control ranging from 21 mL to 157 mL. A single trial where laparoscopic myomectomy was compared with control found that GnRHa pretreatment was associated with 82 mL greater blood loss than control. | |
| Blood transfusions | Study population | OR 0.85 (0.26 to 2.75) | 121 (4 studies) | ⊕⊕⊝⊝ low4,5 | ||
| 143 per 1000 | 124 per 1000 (42 to 314) | |||||
| Moderate | ||||||
| 194 per 1000 | 170 per 1000 (59 to 398) | |||||
| Postoperative morbidity | Study population | OR 1.07 (0.43 to 2.64) | 190 (5 studies) | ⊕⊕⊝⊝ low5,6 | ||
| 146 per 1000 | 154 per 1000 (68 to 311) | |||||
| Moderate | ||||||
| 188 per 1000 | 199 per 1000 (91 to 379) | |||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Evidence quality downgraded one level because of serious limitations in study design (only 1 study had allocation concealment and blinding of participants or investigators. Half of the studies had low risk of selection and detection bias but most had low risk of reporting and attrition bias). 2 Evidence quality downgraded 2 levels because of substantial heterogeneity 3 Evidence quality downgraded 1 level because of serious limitations in study design (risk of attrition and reporting bias was generally low but only 1 study had allocation concealment, risk of selection and performance bias was mostly unclear and detection bias was unclear in about half of the studies). 4 Evidence quality downgraded 1 level for serious limitations in study design (only 1 study had low risk of selection, performance, detection and reporting bias). 5 Evidence quality downgraded 1 level for imprecision (very small trials with wide confidence intervals). 6 Evidence quality downgraded one level for serious limitations in study design (low risk of selection bias (from adequate allocation concealment) and performance bias (from blinding) in only 1 study).
Summary of findings 4. GnRHa treatment versus no pretreatment or placebo before resection for uterine fibroids.
| Gonadotropin‐hormone releasing analogue (GnRHa) treatment versus no pretreatment or placebo before resection for uterine fibroids | ||||||
| Patient or population: women with uterine fibroids Settings: hospitals or outpatient clinics (only perioperative or postoperative outcomes) Intervention: GnRHa treatment versus no pretreatment or placebo before resection | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control placebo or no pretreatment | GnRHa pretreatment | |||||
| Duration of surgery (minutes) | Mean duration of surgery in the control group was 21 minutes | Mean operating time (minutes) in the intervention groups was 5.4 shorter (7.65 to 3.15 shorter) | ‐ | 39 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| Intraoperative blood loss (mL) | ‐ | No studies measured this outcome | Not estimable | ‐ | ‐ | |
| Blood transfusions | ‐ | No studies measured this outcome | Not estimable | ‐ | ‐ | |
| Postoperative morbidity | ‐ | No studies measured this outcome | Not estimable | ‐ | ‐ | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Evidence quality downgraded 1 level for serious limitations in study design (lack of blinding and unclear reporting bias). 2 Evidence quality downgraded 1 level for imprecision (small trial).
Summary of findings 5. GnRHa treatment versus other medical therapies before any surgery for uterine fibroids.
| Gonadotropin‐hormone releasing analogue (GnRHa) treatment versus other medical therapies before any surgery for uterine fibroids | ||||||
| Patient or population: women with uterine fibroids Settings: hospitals or outpatient clinics (only preoperative outcomes) Intervention: GnRHa treatment versus other medical therapies before any surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control (other medical therapies) | GnRHa pretreatment | |||||
| Uterine volume (cm³) | See comment | Not estimable | ‐ | ‐ | Studies too heterogeneous for pooling. One study comparing a GnRHa with a SERM and another study comparing GnRHA with mifepristone found no difference between groups. One trial comparing GnRHa with ulipristal acetate found a greater reduction with GnRHa (‐47%) compared to 5 mg (‐20%) and 10 mg (‐22%) ulipristal acetate | |
| Fibroid volume (cm³) | Fibroid volume in the other treatment group (cabergoline) ranged from 86 cm³ to 278 cm³ | Mean fibroid volume in the intervention groups was 12.71 greater (5.92 lower to 31.34 higher) | ‐ | 110 (2 studies) | ⊕⊕⊝⊝ low1,2 | 2 additional studies with skewed data not suitable for pooling reported no differences between groups (GnRHa vs. raloxifene, GnRHa vs. ulipristal acetate) One additional study found a greater reduction with GnRHa when compared to multiple doses of fulvestrant |
| Preoperative haemoglobin (g/dL) | Mean haemoglobin at end of preoperative treatment in ulipristal acetate group was 12.9 g/dL | Mean haemoglobin at end of preoperative treatment in the intervention groups was 0.2 lower (0.6 lower to 0.2 higher) | ‐ | 188 (1 study) | ⊕⊕⊕⊝ moderate3 | |
| Preoperative bleeding: Reduction in bleeding to PBAC < 75 ulipristal acetate 5 mg | Study population | OR 0.71 (0.3 to 1.68) | 199 (1 study) | ⊕⊕⊕⊝ moderate3 | ||
| 898 per 1000 | 862 per 1000 (725 to 937) | |||||
| Moderate | ||||||
| 898 per 1000 | 862 per 1000 (725 to 937) | |||||
|
Preoperative bleeding: Reduction in bleeding to PBAC < 75 ulipristal acetate 10 mg |
Study population | OR 0.39 (0.14 to 1.06) | 203 (1 study) | ⊕⊕⊕⊝ moderate3 | ||
| 941 per 1000 | 862 per 1000 (691 to 944) | |||||
| Moderate | ||||||
| 941 per 1000 | 861 per 1000 (691 to 944) | |||||
| Adverse events (hot flushes) | 213 per 1000 | 691 per 1000 | OR 12.30 (4.04 to 37.48) | 453 (5 studies) | ⊕⊕⊝⊝ low4 |
These findings were for hot flushes (GnRHa compared to raloxifene, ulipristal acetate, mifepristone, cabergoline and lynestrenol). Headache (with comparators raloxifene, ulipristal acetate, cabergoline and lynestrenol), sleep disorder (vs. lynestrenol) and bone sensitivity (vs. cabergoline) were also increased with GnRHa compared to other medical treatments but fewer studies contributed data. There were no other significant differences. No studies compared total numbers of adverse events |
| The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Evidence quality downgraded 1 level because of limitations in study design (unclear risk of selection and attrition bias and lack of blinding). 2 Evidence quality downgraded 1 level because of imprecision (two small trials with wide confidence intervals). 3 Evidence quality downgraded 1 level (study had pharmaceutical support and it was not possible to determine whether this had influenced the findings). 4 Evidence level downgraded one level for serious limitations in study design (the majority of the studies had significant risk of bias and downgraded one level because of inconsistency (variation between estimates in the studies).
Summary of findings 6. SPRM compared to placebo for uterine fibroids.
| Selective progesterone‐receptor modulators (SPRM) compared to placebo for uterine fibroids | ||||||
| Patient or population: women with uterine fibroids Settings: hospitals or outpatient clinics (only preoperative outcomes) Intervention: selective progesterone‐receptor modulators (SPRM) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | SPRM | |||||
| Uterine volume (cm³) | See comment | Not estimable | ‐ | ‐ | Two studies could not be pooled. One study found a greater proportion of women taking ulipristal acetate had a reduction of uterine volume > 25% than placebo (34% (ulipristal acetate 5 mg) and 28% (ulipristal acetate 10 mg) vs. placebo 6%). The other study found no difference in this outcome with asoprisnil compared to placebo | |
| Fibroid volume (cm³) | See comment | Not estimable | ‐ | ‐ | Four studies could not be pooled. All studies found a significantly greater reduction with SPRMs (regardless of type) compared to placebo (except for the lower dose of asoprisnil (10 mg)). Reductions with ulipristal acetate, mifepristone, CDB‐2914 and asoprisnil 25 mg ranged from 12% to 29% compared to a range of 3% to 6% with placebo | |
| Preoperative haemoglobin (g/dL) | Mean haemoglobin ranged from 12.2 to 12.6 g/dL | Mean haemoglobin (g/dL) in the intervention groups was 0.93 higher (0.52 to 1.35 higher) | ‐ | 173 (2 studies) | ⊕⊕⊕⊕ high | Although one study reported receiving pharmaceutical company funding, results were very similar so funding was unlikely to have influenced the results |
| Preoperative bleeding: (PBAC < 75) ulipristal acetate 5 mg | Study population | OR 41.41 (15.26 to 112.38) | 143 (1 study) | ⊕⊕⊝⊝ low1, 2 | Study was funded by the pharmaceutical company that supplied the intervention | |
| 188 per 1000 | 905 per 1000 (779 to 963) | |||||
| Moderate | ||||||
| 188 per 1000 | 906 per 1000 (779 to 963) | |||||
| Preoperative bleeding: Reduction in menstrual bleeding (PBAC < 75) ulipristal acetate 10 mg | Study population | OR 78.83 (24.02 to 258.74) | 146 (1 study) | ⊕⊕⊝⊝ low1, 2 | Study was funded by the pharmaceutical company that supplied the intervention | |
| 83 per 1000 | 878 per 1000 (686 to 959) | |||||
| Moderate | ||||||
| 83 per 1000 | 877 per 1000 (685 to 959) | |||||
|
Preoperative bleeding: Change in menstrual blood loss from baseline to end of treatment |
Mean menstrual blood loss change score (menstrual pictogram) increased from baseline of 12.6 (menstrual bleeding score) | Mean change in menstrual blood loss from baseline to end of treatment in the intervention groups was 166.9 lower (277.6 to 56.2 lower) | ‐ | 22 (1 study) | ⊕⊕⊝⊝ low3 | |
| Adverse events |
42 per 1000 0 per 1000 63 per 1000 |
0 per 1000 429 per 1000 500 per 1000 |
OR 0.05 (0.0 to 1.0)
OR 25.24 (1.3 to 503.4) OR 15.0 (1.5 to 146.5) |
241 (1 study) (dysmenorrhoea) 30 (1 study) (hot flushes) 30 (1 study) (change in mood) |
⊕⊕⊝⊝ low3 |
No evidence of a difference in serious adverse events. For specific less serious adverse events, results were very imprecise. There was no evidence of significant differences for the other individual adverse events. |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Evidence quality downgraded one level because of potential influence from pharmaceutical company funding.
2 Evidence quality downgraded one level for imprecision (wide confidence intervals). 3 Evidence quality downgraded two levels because of imprecision (very small trial with wide confidence intervals).
Background
Description of the condition
Uterine fibroids (also known as myomas or leiomyomas) are the most common benign tumour of the female reproductive tract which are thought to affect approximately 20% to 40% of women of reproductive age (Jacoby 2010; Wallach 1992), although it is possible prevalence may be even higher (70% to 80%) (Baird 2003). Fibroids are classified according to their anatomic location as subserosal, intramural and submucosal types (Yang 2011). Many fibroids are asymptomatic but a proportion of women have heavy menstrual bleeding (30%), anaemia, dysmenorrhoea, pelvic pain and pressure symptoms (34%), reduced quality of life and reduced fertility (27%) (Buttram 1981). The standard treatment for symptomatic uterine fibroids are surgical and radiological interventions. Fibroids are the most common indication for hysterectomy (Merrill 2008); less invasive procedures include myomectomy (in women wishing to preserve their fertility), hysteroscopic removal, uterine artery embolisation and other radiological interventions (Patel 2014). Fibroids represent one of the most frequent indications for major surgery in premenopausal women (Carls 2008) and as such they constitute a major public health cost.
Description of the intervention
Some medical therapies are currently being investigated as stand‐alone treatments for fibroids, but the role of this review was to investigate medical therapies before surgery. These preoperative medical treatments include gonadotropin‐releasing hormone analogues (GnRHa), progestins, selective oestrogen receptor modulators, dopamine agonists, prostaglandin analogues and selective progesterone receptor modulators.
Since the 1980s, GnRHa treatments, which induce a state of hypo‐oestrogenism by suppressing pituitary ovarian function, have been investigated for women with fibroids. The main effects of this treatment are the temporary control of bleeding and reduction of fibroid and uterine size, but side effects include menopausal symptoms and bone loss with long‐term use. After therapy is stopped, there is re‐growth of both the tumours and the uterus to almost their pretreatment size, and in most women, a recurrence of symptoms (Matta 1989). Thus, they have been approved only for short‐term use, as a preoperative adjunct to surgery.
Other potential hormonal therapies have also been investigated as preoperative treatment. Progestins have been used to reduce heavy menstrual bleeding induced by fibroids but have thromboembolic and metabolic risks (Jourdain 1996). Selective oestrogen receptor modulators (SERMs) are approved for the prevention and treatment of osteoporosis but preclinical studies suggest they may inhibit the proliferation of fibroid cells, consequently limiting their growth (Jirecek 2004). Selective progesterone receptor modulators (SPRMs), such as asoprisnil, mifepristone and ulipristal acetate, have more recently been investigated, and in 2012, ulipristal acetate was licensed by the European Medicines Agency for the treatment of symptomatic fibroids over a maximum of three months for preoperative management (Pérez Lopez 2014).
How the intervention might work
Although the pathogenesis of fibroids is not well established, it has been recognised that fibroid growth and maintenance are stimulated by oestrogen and affected by hormonal cyclic changes (Friedman 1990). Oestradiol and progesterone receptors have been identified in myomatous tissue (Tamaya 1985; Wilson 1980). Because of this dependence of fibroids on steroid hormones, it follows that medications to reduce the levels of gonadal steroids might be options for the treatment of uterine fibroids. If a state of reduced oestrogen secretion could be induced, this would result in the reduction in growth of fibroids and even their regression. Additionally, as progesterone is known to promote the growth of fibroids, modulating the progesterone pathway by acting on progesterone receptors in myometrial tissue may control heavy menstrual bleeding and reduce fibroid bulk (Donnez 2012a).
Pretreatment with medical therapy before hysterectomy is considered particularly useful for women with severe anaemia and to reduce blood loss during surgery. Other indications have included large fibroids or other factors that make surgery technically difficult (West 1992). Pretreatment with medical therapy may also enable greater use of vaginal hysterectomy (Stovall 1991) compared to abdominal hysterectomy or even more conservative surgical options such as laparoscopic or hysteroscopic removal.
Conservative surgery, or myomectomy, has generally been used for women who wish to preserve or enhance their fertility but is often regarded as a more difficult procedure than hysterectomy, with a high risk of postoperative pyrexia (fever), pelvic haematoma formation and postoperative adhesions. Moreover, intraoperative haemorrhage can necessitate emergency blood transfusion or hysterectomy. Myomectomy may be performed via laparotomy, laparoscopy or hysteroscopy and the method must be distinguished in the evaluation of pretreatment with medical agents. Potential benefits of preoperative medical treatments are reduction in blood loss during the operation, ease of operability, better anatomical reconstruction and the possibility of using a transverse (Pfannenstiel‐type) rather than vertical midline incision at laparotomy. However, concern has been expressed that the fibroid capsule would become less evident and may be missed, tumours will not 'shell out' cleanly and the excision may be more difficult (Friedman 1989; Stovall 1989).
A less invasive surgical option, hysteroscopic resection, is often used in women with submucous fibroids. This option offers advantages over myomectomy such as reduced trauma, shorter hospitalisation and recovery times and decreased risk of adhesion formation. GnRH analogues have been used preoperatively before this surgery for some time, but robust evidence to support this practice is weak (Parazzini 1998). Controlled non‐randomised studies have been undertaken but have reported conflicting results (Campo 2005; Perino 1993).
Why it is important to do this review
Fibroids represent one of the most frequent indications for major surgery in premenopausal women. GnRH analogues, and more latterly other types of medical therapy, have been investigated before surgery for uterine fibroids to improve intraoperative and postoperative outcomes. It is important to determine precisely the specific advantages and disadvantages of this practice compared to no presurgical therapies and to compare the effectiveness of individual presurgical therapies.
Objectives
To assess the effectiveness and safety of medical treatments prior to surgery for uterine fibroids.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled comparisons of medical therapies versus placebo or no treatment when administered before any surgery for uterine fibroids.
All randomised controlled comparisons of individual medical therapies versus other individual medical therapies when administered before surgery for uterine fibroids.
Trials of medical therapies used as sole treatment for uterine fibroids, without the expectation of subsequent surgery, were not included.
Types of participants
Premenopausal women, without any other underlying uterine pathology, intending to undergo any surgery for uterine fibroids: either hysterectomy (abdominal, vaginal or laparoscopic), myomectomy (laparotomy or laparoscopy) or resection for uterine fibroids.
Types of interventions
Versions of this review published before 2017 focused on gonadotropin‐hormone releasing analogue (GnRHa) treatment versus no treatment, placebo or other medical therapy before surgery for uterine fibroids.
In this 2017 update, the scope of the review was expanded to include any other types of treatment used before fibroid surgery. The following interventions were also included and compared either with placebo, no treatment or with each other:
progestins;
selective progesterone receptor modulators (SPRMs);
selective oestrogen receptor modulators (SERMs);
dopamine agonists; and
oestrogen receptor antagonists.
Misoprostol, another therapy that has been used particularly before myomectomy, was not included; its effectiveness was considered (along with other interventions for the prevention of haemorrhage specifically in myomectomy) in another Cochrane Review (Kongnyuy 2014).
We made the following comparisons:
GnRHa versus no pretreatment or placebo;
GnRHa versus other pretreatment (progestin, SPRM, SERM, dopamine agonist, oestrogen receptor antagonist); and
SPRMs versus placebo.
The GnRHa comparison was further structured according to the types of outcomes measured. Where outcomes were preoperative, all relevant trials were included; where the outcomes were measured during or after surgery, the comparisons were structured by type of surgery: hysterectomy, myomectomy or resection.
Types of outcome measures
Each of the following outcomes was analysed where data were available. The outcomes were stratified into different groups, according to whether they were measured before, during or after surgery. Trials that measured only surrogate outcomes were excluded from the review.
Primary outcomes
1. Preoperative assessment
Reduction in uterine volume or fibroid volume or both (as reported in the primary study).
Preoperative haemoglobin.
Preoperative bleeding (only if measured by a validated scale).
2. Operative difficulties and postoperative assessment
Duration of surgery.
Intraoperative blood loss.
Frequency of blood transfusions.
Postoperative morbidity (complications such as pyrexia, haematoma formation and incidence of postoperative adhesions).
Secondary outcomes
1. Preoperative assessment
Adverse events (related to the preoperative treatment).
Quality of life (related to the preoperative assessment, assessed subjectively by the participant on a validated scale).
2. Operative difficulties and postoperative assessment
Difficulty of surgery (assessed subjectively by surgeon).
Proportion of women undergoing vaginal hysterectomy (in women undergoing hysterectomy).
Type of abdominal incision (Pfannenstiel transverse versus vertical).
Duration of hospital stay (days).
Intraoperative hysterectomy (for women undergoing myomectomy).
Frequency of postoperative recurrence of myomas.
Postoperative haemoglobin.
Search methods for identification of studies
We searched for all published and unpublished randomised controlled trials (RCTs) of preoperative treatment with either GnRHa, selective progesterone receptor modulators (SPRMs), selective oestrogen receptor modulators (SERMs), oestrogen receptor antagonists, progestins or dopamine antagonists before surgery in women with fibroids. The searches were conducted without language or date restriction and in consultation with the Cochrane Gynaecology and Fertility Group Information Specialist.
Electronic searches
We searched the following electronic databases:
Cochrane Gynaecology and Fertility Specialised Register (inception to 13 June 2017) (Appendix 1);
Cochrane Central Register of Controlled Studies (searched 13 June 2017) (Appendix 2);
MEDLINE (1946 to 13 June 2017) (Appendix 3);
Embase (1980 to 13 June 2017) (Appendix 4);
PsycINFO (1806 to 13 June 2017) (Appendix 5); and
CINAHL (1961 to 13 June 2017) (Appendix 6).
We also searched other electronic sources of trials (trials registers and websites) (13 June 2017):
trials registers for ongoing and registered trials (www.clinicaltrials.gov and the WHO ICTRP www.who.int/trialsearch/Default.aspx);
the Cochrane Library for the Database of Abstracts of Reviews of Effects (DARE);
ProQuest Dissertations and Theses;
Web of Science conference abstracts and other trials;
OpenGrey for unpublished literature from Europe;
PubMed; and
Google Scholar.
Searching other resources
We handsearched the reference lists of included studies and relevant reviews retrieved by the search for additional trials. We also contacted the pharmaceutical company that supplies ulipristal acetate, HRA Pharma, for any clinical trials that may have been undertaken and not published. No reply has been received to date.
Data collection and analysis
Selection of studies
For previous versions, two review authors (a methodologist (AL) and a clinical expert (BV)) selected studies for the review. For the 2017 update, two review authors (a methodologist (AL) and a topic area specialist (LP)) independently selected potentially relevant trials from the search results according to the review eligibility criteria. Where studies appeared eligible, they were retrieved in full text format for further duplicate investigation for eligibility. Disagreements over selection were resolved by consensus. The selection process is documented in a PRISMA flow chart (Figure 1).
1.
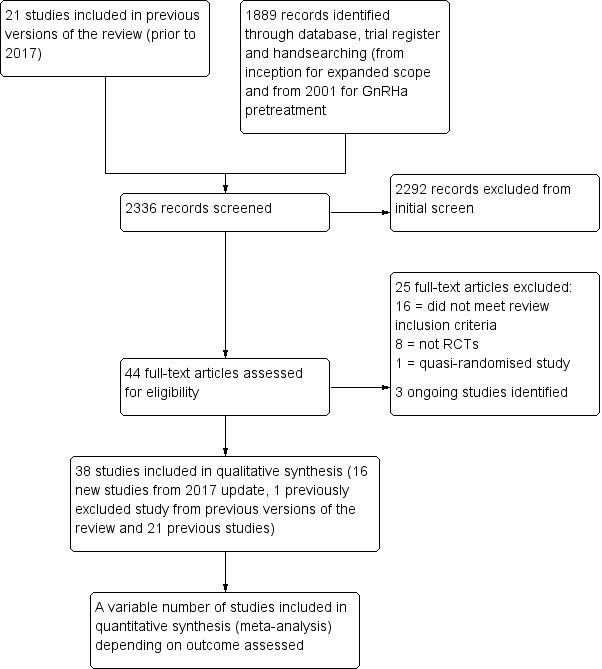
Study flow diagram
Data extraction and management
For previous versions of the review, two review authors independently extracted and managed data. For the 2017 update, two review authors (AL, LP) independently extracted data from the eligible studies using a data extraction form designed and pilot tested by AL. Disagreements were resolved by discussion. The extracted data included relevant study characteristics and effect estimates.
Where there were multiple intervention groups (e.g. different doses of GnRHa), the data were combined, where possible. If combined data could not be calculated:
for binary outcomes with a common placebo group, the dosage group data were entered into the meta‐analysis separately and the placebo numbers were divided as equally as possible between the arms of the intervention; and
for continuous outcomes, the data from the intervention with the lowest dosage were extracted.
Where there were multiple groups of participants (e.g. women with different uterine size: 14 to 18 and > 18 gestational weeks), data from the group with the smaller uterine size were used in the meta‐analysis.
Where studies had multiple publications, the main trial report was used as the reference and additional details were derived from secondary papers, if necessary.
Where data were not clearly reported, we corresponded with the principal author of the study to obtain clarification.
Assessment of risk of bias in included studies
For previous review versions, two review authors (AL, BV) independently assessed the studies for risk of bias in descriptive format.
In the 2017 review update, two review authors (AL, LP) independently assessed the included studies for risk of bias using the Cochrane risk of bias assessment tool (Higgins 2011). The following domains were assessed and scored according to whether they indicated low, unclear or high risk of bias:
generation of allocation sequence;
allocation concealment;
blinding of participants, study personnel and assessors;
incomplete outcome data;
selective reporting; and
other bias (baseline comparability, early stopping of trial etc.).
Disagreements were resolved by consensus. The judgments behind each score were fully recorded in the 'Risk of bias' tables and assessments presented for each study in Figure 2 and in combined format in Figure 3.
2.
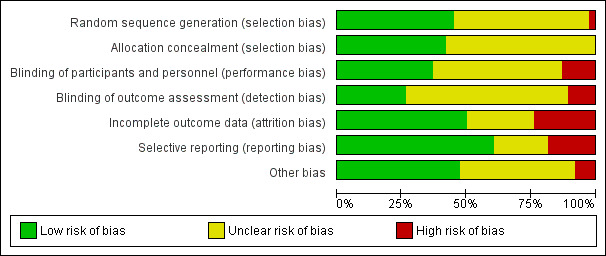
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
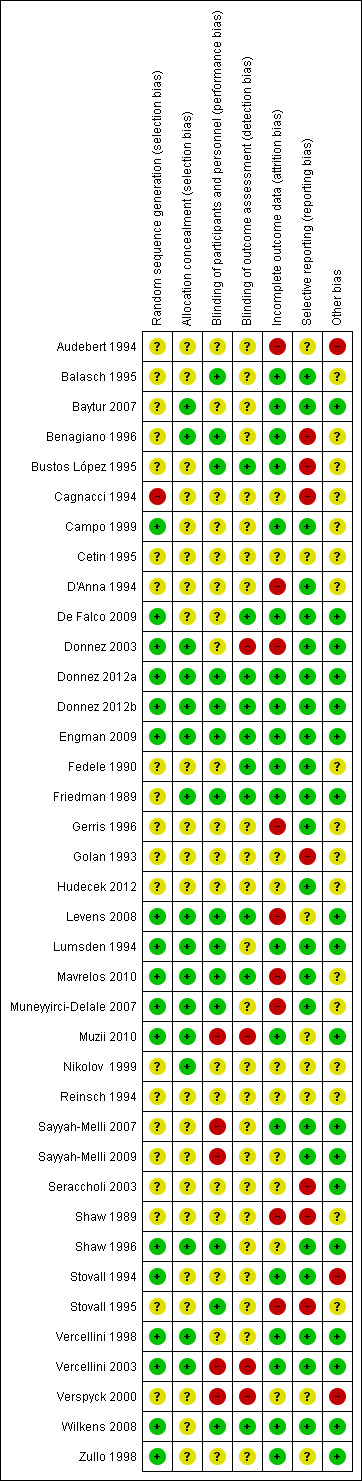
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Measures of treatment effect
For dichotomous data (e.g. incidence of adverse events), we used the number of events in the control (or other treatment) and intervention groups of each study to calculate Mantel‐Haenszel odds ratios (OR). For continuous data, (e.g. uterine volume) we calculated the mean difference (MD) between treatment groups. We reversed the direction of effect of different studies, when required, to ensure consistency across trials. We presented 95% confidence intervals (CIs) for all outcomes.
We compared the magnitude and direction of effect reported by studies with how they were presented in the review, taking account of legitimate differences.
Unit of analysis issues
The unit of analysis is per woman randomised.
Dealing with missing data
The data were analysed on an intention‐to‐treat (ITT) basis, as far as possible, and attempts were made to obtain missing data from the original trialists.
Where data to calculate ORs or MDs were not available, we used the most detailed numerical data available that facilitated similar analyses of the included studies (e.g. test statistics, P values, standard error of the mean). Where this was not possible (e.g. missing measure of variation), we imputed values for the missing data by entering the largest comparable measure used by the other pooled studies. Any imputation was subjected to sensitivity analysis. Otherwise, if imputation was not feasible or realistic, only the available data were analysed.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. Where the decision was made to pool studies, we assessed statistical heterogeneity by inspection of the Chi² test results and the I² statistic.
A rough guide to interpretation of I² values is as follows (Higgins 2011):
0% to 40% might not be important;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity; and
75% to 90% represents considerable heterogeneity.
These overlapping categories were considered, together with the unique characteristics of the outcomes, in the assessment of heterogeneity.
Assessment of reporting biases
The review authors attempted to minimise the potential impact of reporting bias by ensuring a comprehensive search for eligible studies and by being alert to the duplication of data. We planned to use funnel plots, if sufficient studies were identified, to further investigate potential publication bias or small study effects.
Data synthesis
Since many of the outcomes assessed were likely to be influenced by other factors such as different hospital policies in different countries (e.g. hospital stay, duration of surgery) or differences in participants' characteristics (size of fibroids, haemoglobin levels), we combined data using random‐effects models to compare intervention with control (or other treatment).
Outcomes with continuous data were assessed for the likelihood of skew. Where the authors of individual studies reported a median and range, or where the methods used to analyse the data were non parametric, it was considered that skew was likely. For other outcomes, where a mean and SD were reported, a rough check was made, where possible, by calculating the observed mean minus the lowest possible value (or the highest possible value minus the observed mean) and dividing this by the standard deviation. Where this ratio was less than 1, it was considered that skew was likely.
Where skew was considered likely, the outcome data were not pooled in a meta‐analysis but displayed in other data tables. The findings of each of these studies were included in the interpretation of overall results for each outcome.
Subgroup analysis and investigation of heterogeneity
As assessment of some outcomes could be influenced by participant knowledge of whether they were receiving pretreatment or not, we conducted subgroup analyses (where possible) to determine the separate evidence according to whether control group women with fibroids went on to immediate surgery or had no pretreatment, or whether there was placebo control. No other subgroup analysis was undertaken.
In most cases, a pooled effect estimate was calculated to combine the results of both subgroups but where there were markedly different estimates, a summary effect measure was not calculated. The findings within each subgroup informed the interpretation of the results.
Where moderate heterogeneity was detected (I² > 50%), we explored possible explanations by checking the data and by examining clinical and methodological differences among studies to determine whether there was any plausible explanation. We took statistical heterogeneity into account when interpreting the results, particularly when there was variation in the direction of effect.
Where considerable statistical heterogeneity was detected (I² > 75%), we did not pool the studies but displayed individual study results on a forest plot, without calculating a summary effect estimate.
Sensitivity analysis
We conducted sensitivity analyses, where possible, for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding eligibility and analysis. These analyses included consideration of whether the review conclusions would have differed if:
eligibility was restricted to studies without high risk of bias; or
a fixed‐effect model had been adopted.
We also undertook sensitivity analysis for comparison 5: GnRHa versus other medical treatments. 'Other medical treatments' constituted SPRMs, SERMs, dopamine agonists, progestins and oestrogen receptor antagonists. Data from the included studies for the trials assessing these other treatments were scarce and so these treatments were combined until further data becomes available to enable separate sensible comparisons. It was recognised that the different treatments might have different effects on the outcomes and sensitivity analyses were undertaken, where necessary, to assess whether these effects could be distinguished.
Overall quality of the body of evidence
One review author (AL) generated 'Summary of findings' (SoF) tables using GRADEpro software (GRADEpro GDT 2015). Another review author (LP) checked the SoF tables for errors but no disagreements between authors were identified. The SoF tables displayed findings for all the primary outcomes (those considered most critical), as well as adverse events (which was a secondary outcome). The primary outcomes for all stages of assessment were: uterine or fibroid volume, preoperative haemoglobin, reduction of fibroid‐related bleeding, duration of surgery, intraoperative blood loss, requirement for blood transfusion, and complications.
The SoF tables evaluated the overall quality of the body of evidence for the primary review outcomes, using GRADE criteria (study limitations (risk of bias), consistency of effect, imprecision, indirectness and publication bias). Judgments about overall evidence quality (very low, low, moderate or high) were documented alongside the overall results for each of the primary outcomes, enabling judgments to be made with respect to the confidence in these results (see Table 1; Table 2; Table 3; Table 4; Table 5; Table 6).
Results
Description of studies
Results of the search
Searches up to 2017
The review was first published in 2000 with a total of 19 included studies (Lethaby 2000). An additional two randomised controlled trials (RCTs) were included in an updated version in February 2001 (Lethaby 2001). Full details on the potentially eligible studies retrieved during these earlier searches are not available.
Searches for the 2017 update
The 2017 review update included an expanded scope, with inclusion of other medical interventions in addition to gonadotropin‐releasing hormone analogue (GnRHa) agents before surgery for women with fibroids.
A search of electronic databases, trials registers and handsearching in June 2017 identified 44 potentially eligible studies and one previously excluded trial (Reinsch 1994) was considered eligible for inclusion. After further assessment, 25 studies were excluded (see Excluded studies) and three other studies were assessed as ongoing, without full results (Bigatti 2014; NCT01873378; NCT02288130).
We included 16 new studies (plus 1 study that was previously excluded) in the update (Baytur 2007; De Falco 2009; Donnez 2003; Donnez 2012a; Donnez 2012b; Engman 2009; Hudecek 2012; Levens 2008; Mavrelos 2010; Muneyyirci‐Delale 2007; Muzii 2010; Reinsch 1994; Sayyah‐Melli 2007; Sayyah‐Melli 2009; Seraccholi 2003; Vercellini 2003; Wilkens 2008). These 17 new studies were added to the 21 studies previously included in earlier versions of the review. Studies of pretreatment in women with fibroids where surgery was not reported were not considered in this review. Full details of the search results are included in Figure 1.
Included studies
We included 38 RCTs, including 3623 women, for this 2017 update, with broadened scope. See Characteristics of included studies table for full details.
Study design and funding source
All trials were parallel group RCTs. Sixteen reported they were multicentre trials (Audebert 1994; Benagiano 1996; Donnez 2003; Donnez 2012a; Donnez 2012b; Gerris 1996; Lumsden 1994; Muneyyirci‐Delale 2007; Muzii 2010; Seraccholi 2003; Shaw 1996; Stovall 1995; Vercellini 1998; Verspyck 2000; Wilkens 2008; Zullo 1998); the remainder were single centre trials (Balasch 1995; Baytur 2007; Bustos López 1995; Cagnacci 1994; Campo 1999; Cetin 1995; D'Anna 1994; De Falco 2009; Engman 2009; Fedele 1990; Friedman 1989; Golan 1993; Hudecek 2012; Levens 2008; Mavrelos 2010; Nikolov 1999; Reinsch 1994; Sayyah‐Melli 2007; Sayyah‐Melli 2009; Shaw 1989; Stovall 1994; Vercellini 2003).
Fourteen studies were either wholly or partially funded by pharmaceutical companies (Benagiano 1996; Bustos López 1995; Donnez 2003; Donnez 2012a; Donnez 2012b; Friedman 1989; Gerris 1996; Levens 2008; Muneyyirci‐Delale 2007; Shaw 1996; Stovall 1994; Stovall 1995; Vercellini 1998; Wilkens 2008), and three were funded by institutions or hospitals (Engman 2009; Mavrelos 2010; Sayyah‐Melli 2009). The source of funding was unclear for the remaining 21 trials (Audebert 1994; Balasch 1995; Baytur 2007; Cagnacci 1994; Campo 1999; Cetin 1995; D'Anna 1994; De Falco 2009; Fedele 1990; Golan 1993; Hudecek 2012; Lumsden 1994; Muzii 2010; Nikolov 1999; Reinsch 1994; Sayyah‐Melli 2007; Seraccholi 2003; Shaw 1989; Vercellini 2003; Verspyck 2000; Zullo 1998).
Participants
Participants in all studies had symptomatic fibroids, mostly diagnosed by ultrasound, and were scheduled for surgery. About half of the studies did not specify any details regarding size or type of fibroid. The remaining trials had various requirements; some excluded submucous or subserous fibroids, and others only included these types of fibroids; where size of the uterus in gestational weeks was a requirement, this was specified as greater than 8, 12, 14 and 16 gestational weeks with two trials assessing women with large uteri (over 18 gestational weeks in Stovall 1994 or ≥ 600 cm³ in Friedman 1989). Where size of fibroids was a requirement, this was usually specified as larger than 2 cm or larger than 3 cm. Six studies required that women had evidence of anaemia (2 required haemoglobin < 12 g/dL; 2 required haemoglobin < 10 g/dL; and 2 required diagnosis of iron deficiency anaemia). Two other studies required women to have haemoglobin values over 10 g/dL. Two studies enrolled women with fibroids and infertility (Campo 1999; Zullo 1998).
Type of surgery varied among the studies. Surgery was either unspecified or was described as either myomectomy or hysterectomy in 12 studies. Participants had hysterectomy (unspecified or abdominal) in 12 studies and in one study participants received laparoscopic hysterectomy (Seraccholi 2003). Myomectomy (unspecified) was performed in seven studies, two had laparoscopic myomectomy (Campo 1999; Zullo 1998) and one had both laparotomic and laparoscopic myomectomy (Hudecek 2012). Two studies offered women fibroid resection (Mavrelos 2010; Muzii 2010) but only data from Muzii 2010 could be included in analyses; only a proportion of women in Mavrelos 2010 went on to have surgery.
Interventions
Prior to the 2017 update, the review was restricted to gonadotropin‐releasing hormone analogues (GnRHa) as pretreatment. In 2017, the scope of the review was expanded to include other types of pretreatment for fibroid surgery: progestins, selective progesterone receptor modulators (SPRMs), selective oestrogen receptor modulators (SERMs), dopamine agonists, and oestrogen receptor antagonists.
GnRHa
We included 19 studies that compared GnRHa to no pretreatment (Audebert 1994; Balasch 1995; Bustos López 1995; Cagnacci 1994; Campo 1999; Cetin 1995; De Falco 2009; Fedele 1990; Gerris 1996; Golan 1993; Hudecek 2012; Muzii 2010; Nikolov 1999; Seraccholi 2003; Shaw 1989; Stovall 1994; Vercellini 1998; Vercellini 2003; Zullo 1998) and eight studies that compared GnRHa to placebo (Benagiano 1996; D'Anna 1994; Friedman 1989; Lumsden 1994; Mavrelos 2010; Muneyyirci‐Delale 2007; Shaw 1996; Stovall 1995).
A number of different GnRHa preparations were administered via different routes and regimens. Leuprolide acetate, goserelin and triptorelin were given either by intramuscular depot injection or subcutaneous depot implant every four weeks before surgery; in three studies nafarelin or buserelin were given daily by nasal spray (Bustos López 1995; Cetin 1995; Fedele 1990). Duration of treatment ranged from two to three months, and in one study, participants were treated for four months (Shaw 1989). In those trials with no preoperative treatment arm, control group participants had surgery either immediately or as soon as practicable, but in two studies (Nikolov 1999; Cagnacci 1994) there was a three month observation period before surgery equivalent to the duration of treatment in the GnRHa group.
Dosages for depot formulations were 3.6 mg (goserelin), 3.75 mg (leuprolide acetate or triptorelin) or 3.2 mg (triptorelin). However, in two studies a larger dose was administered to cover the three month pretreatment period (Muneyyirci‐Delale 2007; Seraccholi 2003). In Seraccholi 2003, triptorelin was administered as one injection of 11.25 mg and goserelin was administered as one injection of 10.8 mg in Muneyyirci‐Delale 2007. Two doses of leuprolide acetate (3.75 mg and 7.5 mg) were compared with placebo in Stovall 1995, and sensitivity analysis was undertaken to assess whether dosage influenced results. Two of the placebo trials included iron in both treatment arms since participants were anaemic (Benagiano 1996; Stovall 1995). Benagiano 1996 also included a GnRHa + placebo iron arm which was not considered in this review. Sensitivity analysis was also performed with and without the inclusion of the studies with iron treatment in the meta‐analysis to determine if results varied.
Progestins
One trial compared a dose of 10 mg (2 tablets of 5 mg given orally per day) of lynestrenol during days 5 to 26 of the menstrual cycle with four injections of leuprorelin monthly for four months (Verspyck 2000). No other trials used progestin pretreatment.
Selective progesterone receptor modulators (SPRMs)
The SPRMs assessed as pretreatment included ulipristal acetate (5 mg and 10 mg daily), mifepristone (50 mg every other day or 25 mg daily), asoprisnil (10 mg or 25 mg daily) or CDB‐2914 (10 mg or 20 mg daily).
Two trials compared SPRMs with GnRHa pretreatment: Donnez 2012b compared 5 mg and 10 mg of ulipristal acetate with once monthly leuprolide acetate injections 3.75 mg for three months and Reinsch 1994 compared 25 mg of mifepristone daily with once monthly leuprolide acetate for three months.
Four trials compared SPRMs with placebo. Donnez 2012a assessed 5 mg or 10 mg of ulipristal acetate, Engman 2009 compared 50 mg of mifepristone every other day, Wilkens 2008 compared asoprisnil 10 mg or 25 mg and Levens 2008 compared CDB‐2914 10 mg or 20 mg daily.
Selective oestrogen receptor modulators (SERMs)
Baytur 2007 compared 60 mg daily of raloxifene with three cycles of monthly goserelin 3.6 mg.
Dopamine agonists
Two studies from Iran compared the dopamine agonist cabergoline (0.5 mg once per week for 6 weeks) with triptorelin (administered once monthly for 4 weeks) (Sayyah‐Melli 2007; Sayyah‐Melli 2009) in women with fibroids scheduled for surgery to examine the impact on fibroid regression and side effects.
Oestrogen receptor antagonists
Donnez 2003 compared different doses of fulvestrant (50 mg, 125 mg or 250 mg given as an intramuscular injection once per month for 4 months) with goserelin (3.6 mg subcutaneous injection once per month for 4 months) and placebo in women with fibroids awaiting hysterectomy.
Outcomes
Outcomes from the included studies were characterised within the comparisons as preoperative, intraoperative or postoperative. Since intraoperative or postoperative outcomes were influenced by type of surgery, it was necessary to divide the timing of the outcomes in the comparisons, so that these outcomes were measured in separate comparisons according to type of surgery performed. There were sufficient studies to distinguish the comparisons in this way when GnRHa was compared with no treatment or placebo, but not when other types of presurgical treatments were compared.
Preoperative outcomes
Primary review outcomes:
Preoperative uterine or fibroid or both uterine and fibroid volume was calculated either by the prolate ellipsoid method and the formula V = 0.5233 (D1 X D2 X D3), where D1, D2 and D3 are the longitudinal, transverse and cross‐sectional diameters of the uterus or fibroid, respectively (Geirsson 1993), the water displacement method, or magnetic resonance imaging in 24 studies. Other preoperative outcomes included haemoglobin levels (after pretreatment and before surgery commenced (19 studies)) and bleeding prior to surgery; two trials evaluating an SPRM (ulipristal acetate) (Donnez 2012a; Donnez 2012b) measured the influence of interventions on menstrual bleeding before surgery using the pictorial blood assessment chart (PBAC) score and Wilkens 2008 used a similar assessment for menstrual bleeding (a menstrual pictogram).
Secondary review outcomes
Studies reported adverse events (from pretreatment (20 studies)) and withdrawal because of adverse events (8 studies). However, data on adverse events in some trials were either too poorly reported or not given for the control group; data could not be extracted from these trials. Quality of life was measured in four trials (two piloted a Measurement of Discomfort due to Fibroids questionnaire, one study used the SF‐36 and Uterine Fibroid Symptom and Health‐Related Quality of Life Questionnaire (UFS‐QoL), and another used only the UFS‐QoL (but data for these latter two studies were insufficient for extraction)).
Operative difficulties
Primary review outcomes
Duration of surgery was measured by 20 studies (but data could only be extracted from 19 studies). Because of numerous confounding factors likely to influence these outcomes, the studies were not pooled but individual estimates for each trial were shown on forest plots.
Intraoperative blood loss, reported in 22 studies, was estimated by measuring the weight of swabs and the volume of blood collected into receptacles such as aspiration bottles. Sixteen studies also reported whether participants required blood transfusions during surgery.
Secondary review outcomes
Other intraoperative outcomes included: degree of difficulty of surgery (estimated by surgeons) (6 studies), rate of performance of vaginal hysterectomy (3 studies) (in hysterectomy participants), and rate of vertical incisions (5 studies). Duration of hospital stay was measured by 10 studies; however (as with duration of surgery), because of numerous confounding factors likely to influence these outcomes, the studies were not pooled but individual estimates for each trial were shown on forest plots. No studies measured the rate of intraoperative hysterectomy in participants undergoing myomectomy.
Postoperative assessment
Primary review outcomes
Intraoperative and postoperative complications were measured in 12 trials.
Secondary review outcomes
After surgery, postoperative haemoglobin was measured in seven trials and recurrence of fibroids in two studies (at 6 months and from 27 to 38 months after surgery, respectively).
Excluded studies
Nineteen studies were excluded either because the interventions were not preoperative, included add‐back (this is covered by another Cochrane Review: Moroni 2015), investigated misoprostol (also covered by another Cochrane Review: Kongnyuy 2014), outcomes were surrogate measures, there was no control group, the trial was not a true RCT or contained mixed populations with data on women with fibroids not available (see Characteristics of excluded studies table).
Studies awaiting assessment
Gambardella 1995 is waiting assessment (translation from Italian).
Ongoing studies
Three studies are ongoing and will be assessed for inclusion in future updates (Bigatti 2014; NCT01873378; NCT02288130).
Risk of bias in included studies
Summaries of the risk of bias assessments are given in Figure 2 and Figure 3.
Allocation
Almost half of the studies (n = 17) specified an appropriate method for sequence generation (randomisation method), using computer‐generated or other types of randomisation methods; these studies were considered to be at low risk of bias (Campo 1999; De Falco 2009; Donnez 2003; Donnez 2012a; Donnez 2012b; Engman 2009; Levens 2008; Lumsden 1994; Mavrelos 2010; Muneyyirci‐Delale 2007; Muzii 2010; Shaw 1996; Stovall 1994; Vercellini 1998; Vercellini 2003; Wilkens 2008; Zullo 1998). One study (Cagnacci 1994) was considered to be at high risk of bias because it created a subgroup of randomised participants within a much larger study where the remaining participants all received the intervention. The remaining studies were assessed as being at unclear risk of bias; it was reported that participants were randomised but did not specify which method was used for sequence generation.
Fewer than half of the studies (n = 16) indicated that allocation to randomised groups was concealed, either because there was centralised control of the allocation, sealed envelopes were used for allocation or a web integrated interactive voice system was used (Baytur 2007; Benagiano 1996; Donnez 2003; Donnez 2012a; Donnez 2012b; Engman 2009; Friedman 1989; Levens 2008; Lumsden 1994; Mavrelos 2010; Muneyyirci‐Delale 2007; Muzii 2010; Nikolov 1999; Shaw 1996; Vercellini 1998; Vercellini 2003); these studies were considered to be at low risk of bias. The remaining studies were considered as unclear risk of bias because the authors did not report methods used to conceal allocation.
Blinding
Assessments were made with respect to blinding of participants, investigators and assessors, although for some outcomes, participants were the assessors (pictorial blood assessment chart (PBAC) scores) and for others the investigators also undertook assessment: duration of surgery and intraoperative blood loss. The risk of bias assessments in Characteristics of included studies attempted to clarify this for each study.
Blinding of participants and investigators
Fewer than half the studies (n = 14) reported double blinding or provided clear evidence that both participants and investigators were blinded to treatment (Balasch 1995; Benagiano 1996; Bustos López 1995; Donnez 2012a; Donnez 2012b; Engman 2009; Friedman 1989; Levens 2008; Lumsden 1994; Mavrelos 2010; Muneyyirci‐Delale 2007; Shaw 1996; Stovall 1995; Wilkens 2008). One study blinded surgeons but participants knew their allocation because treatments were administered differently (De Falco 2009). Five studies were at high risk of bias for blinding of participants and investigators because they were clearly reported as open studies with different types of treatment administration (Muzii 2010; Sayyah‐Melli 2007; Sayyah‐Melli 2009; Vercellini 2003; Verspyck 2000). The remaining studies were at unclear risk of bias; the authors did not report whether blinding was undertaken.
Blinding of assessors
Only 10 studies provided clear evidence that assessors were blinded; these studies were considered at low risk of bias (Bustos López 1995; De Falco 2009; Donnez 2012a; Donnez 2012b; Engman 2009; Fedele 1990; Friedman 1989; Levens 2008; Mavrelos 2010; Wilkens 2008). Four studies were at high risk of bias (Donnez 2003; Muzii 2010; Vercellini 2003; Verspyck 2000) and the remainder at unclear risk of bias.
Incomplete outcome data
Half of the included studies (n = 19) were at low risk of attrition bias (Balasch 1995; Baytur 2007; Benagiano 1996; Bustos López 1995; Campo 1999; De Falco 2009; Donnez 2012a; Donnez 2012b; Engman 2009; Fedele 1990; Friedman 1989; Lumsden 1994; Muzii 2010; Sayyah‐Melli 2007; Stovall 1994; Vercellini 1998; Vercellini 2003; Wilkens 2008; Zullo 1998). These 19 studies either included all participants in the analysis, or had minimal withdrawals that were balanced between groups or used methods to account for missing data. A further nine studies were assessed at high risk of bias (Audebert 1994; D'Anna 1994; Donnez 2003; Gerris 1996; Levens 2008; Mavrelos 2010; Muneyyirci‐Delale 2007; Shaw 1989; Stovall 1995), mostly because withdrawals were substantial or were unbalanced between randomised groups. The remaining studies were assessed at unclear risk of attrition bias.
Selective reporting
Over half of the studies (n = 23) were at low risk of reporting bias due to selective outcome reporting (Balasch 1995; Baytur 2007; Campo 1999; D'Anna 1994; De Falco 2009; Donnez 2003; Donnez 2012a; Donnez 2012b; Engman 2009; Fedele 1990; Friedman 1989; Gerris 1996; Hudecek 2012; Lumsden 1994; Mavrelos 2010; Muneyyirci‐Delale 2007; Sayyah‐Melli 2007; Sayyah‐Melli 2009; Shaw 1996; Stovall 1994; Vercellini 2003; Verspyck 2000; Wilkens 2008). In these studies, all prespecified outcomes were reported fully or published protocols indicated there was no evidence of selective outcome reporting. A further seven studies were considered at high risk of reporting bias (Benagiano 1996; Bustos López 1995; Cagnacci 1994; Golan 1993; Seraccholi 2003; Shaw 1989; Stovall 1995). In these studies, outcome data were only reported for the intervention group and not for the control group, so a valid comparison could not be made. For the remaining studies, the likelihood of reporting bias due to selective outcome reporting was unclear because some prespecified outcomes were not fully reported.
Other potential sources of bias
Almost half of the studies (n = 18) had low risk of other sources of bias (Baytur 2007; De Falco 2009; Donnez 2003; Donnez 2012a; Donnez 2012b; Engman 2009; Friedman 1989; Levens 2008; Lumsden 1994; Muzii 2010; Sayyah‐Melli 2007; Sayyah‐Melli 2009; Seraccholi 2003; Shaw 1996; Vercellini 1998; Vercellini 2003; Wilkens 2008; Zullo 1998), mostly because participant groups were comparable at baseline and no other potential bias was detected. Three studies had imbalanced groups at baseline and were considered at high risk of other bias (Audebert 1994; Stovall 1994; Verspyck 2000), mainly because the imbalances were likely to influence the findings of the study. For the remaining studies, risk of other bias was unclear; there were some discrepancies in the comparability of the groups at baseline but it was unclear whether this would bias the results.
There were insufficient studies included in the individual comparisons to construct funnel plots to check for potential reporting biases.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Comparisons were divided into:
Comparison 1: GnRHa versus no treatment or placebo (preoperative outcomes, regardless of type of subsequent surgery).
Comparison 2: GnRHa versus no treatment or placebo before hysterectomy (intraoperative or postoperative outcomes).
Comparison 3: GnRHa versus no treatment or placebo before myomectomy (intraoperative or postoperative outcomes).
Comparison 4: GnRHa versus no treatment or placebo before resection (intraoperative or postoperative outcomes).
Comparison 5: GnRHa versus other medical treatments (preoperative outcomes only. Data were insufficient to distinguish between types of surgery, which could influence intra‐ or postoperative outcomes).
Comparison 6: SPRMs versus placebo (preoperative outcomes only. Data were insufficient to distinguish between types of surgery, which could influence intra‐ or postoperative outcomes).
The structure of the comparisons is presented in Table 7.
1. Structure of comparisons according to measured outcomes and type of surgery.
| Comparison | Outcomes | |||
| Preoperative | Intra/postoperative + hysterectomy | Intra/postoperative + myomectomy | Intra/postoperative + resection | |
| GnRHa versus no treatment or placebo | Comparison 1 | Comparison 2 | Comparison 3 | Comparison 4 |
| Primary outcomes .Reduction in uterine volume · Reduction in fibroid volume · Preoperative Hb · Preoperative bleeding Secondary outcomes · Adverse events · QoL |
Primary outcomes · Duration of operation · Intraoperative blood loss · Frequency of blood transfusions Secondary outcomes · Difficulty of surgery · Proportion of women undergoing vaginal hysterectomy · Type of abdominal incision · Duration of hospital stay · Postoperative morbidity · Postoperative recurrence · Postoperative Hb |
Primary outcomes · Duration of operation · Intraoperative blood loss · Frequency of blood transfusions Secondary outcomes · Difficulty of surgery ·Intraoperative hysterectomy · Type of abdominal incision · Duration of hospital stay · Postoperative morbidity · Postoperative recurrence · Postoperative Hb |
Primary outcomes · Duration of operation · Intraoperative blood loss · Frequency of blood transfusions Secondary outcomes · Difficulty of surgery · Type of abdominal incision · Duration of hospital stay · Postoperative morbidity · Postoperative recurrence · Postoperative Hb |
|
| GnRHa versus other medical treatments | Comparison 5 | |||
| Primary outcomes · Reduction in uterine volume · Reduction in fibroid volume · Preoperative Hb · Preoperative bleeding Secondary outcomes · Adverse events · QoL |
not applicable | not applicable | not applicable | |
| SPRMs versus placebo | Comparison 6 | |||
| Primary outcomes · Reduction in uterine volume · Reduction in fibroid volume · Preoperative Hb · Preoperative bleeding Secondary outcomes · Adverse events · QoL |
not applicable | ‐ | ‐ | |
GnRHa: gonadotropin‐releasing hormone analogues
Hb: haemoglobin
Hb: Haemoglobin
QoL: quality of life
SPRM: selective progesterone receptor modulator
GnRHa pretreatment versus no treatment or placebo
Comparison 1: GnRHa versus no treatment or placebo. Preoperative outcomes (regardless of type of subsequent surgery)
See Table 1.
Primary outcomes
Reduction in uterine volume
See Analysis 1.1; Figure 4; Analysis 1.2.
4.
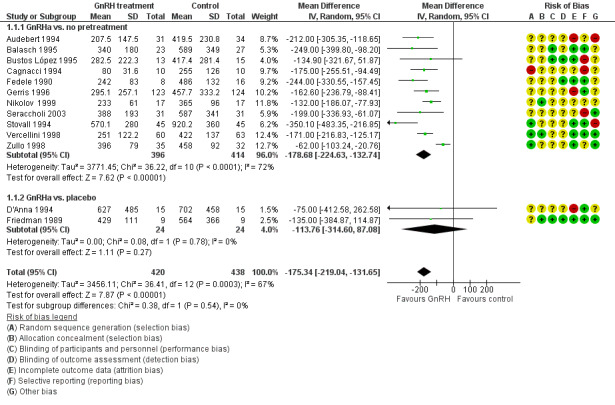
Forest plot of comparison: 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), outcome: 1.1 Uterine volume (mL) (preoperative).
Seventeen studies evaluated this outcome (12 had a no pretreatment arm and 5 had a placebo arm). Four of these studies either had skewed data or the data were in a form that precluded pooling. The individual findings for these studies are reported in tabular format (Analysis 1.2). Given substantial heterogeneity in the subgroup where GnRHa was compared to a no treatment control group, we investigated the characteristics of the studies to determine potential causes of the variation in effects. The elimination of the data from one study (Zullo 1998) markedly reduced the heterogeneity. This study focused on participants where the main fibroids were intramural and GnRHa treatment was given for only two months (with the majority of included studies having three or four months of active treatment). In addition, variation in the findings could be expected given the variability in participants with respect to initial uterus size and number and type of fibroids. Although there was substantial heterogeneity in the body of evidence for this analysis, GnRHa fairly consistently reduced uterine volume when compared to control, with uterus volume ranging from a value of 80 cc to 570.1 cc in the GnRHa groups compared to a range of 255 cc to 920 cc in the control groups.
1.2. Analysis.
Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 2 Uterine volume (preop in data table).
| Uterine volume (preop in data table) | ||||
|---|---|---|---|---|
| Study | Number in study | Comparison | Results | Comment |
| GnRHa vs. no pretreatment | ||||
| Golan 1993 | 53 | GnRHa (D‐Trp LHRH) vs. no pre‐treatment | Results reported as "average" with range (likely to be median plus range prior to surgery: D‐Trp: median 380 mL (300 to 400) No pre‐treatment: median 496 mL (370 mL to 600 mL) |
Authors did not report whether these the reduction in uterine volume was significantly different between groups |
| GnRHa vs. placebo | ||||
| Lumsden 1994 | 69 | GnRHa (goserelin) vs. placebo | Difference between goserelin and placebo at end of treatment (%): 27.7% (95% CI 10.3 to 45.2), P = 0.002 |
Statistical test reported as "calculation of limits of agreement" |
| Muneyyirci‐Delale 2007 | 110 | GnRHa (goserelin) + iron vs. iron + placebo | Goserelin/iron vs iron + placebo: Change in uterine volume: median (IQR): ‐233.1 (IQR NR) vs. +18.9 (IQR NR) cm³ (NS) |
Authors reported that there were no significant differences between groups |
| Stovall 1995 | 179 | GnRHa (leuprolide acetate depot) + iron (7.5 mg and 3.75 mg) vs. placebo + iron | Median change from baseline to presurgery: Leuprolide/iron 7.5 mg: ‐31% (no range reported) Leuprolide/iron 3.75 mg: ‐39% (no range reported) Placebo/iron: +10% (no range reported) |
Authors reported that the change from baseline in both leuprolide groups was significantly different from placebo (P < 0.01) |
When the overall estimates were combined regardless of type of control group (no pretreatment or placebo), GnRHa pretreatment was associated with a greater reduction in uterine volume compared to control (MD 175.3 mL, 95% CI ‐219.0 to ‐131.7; 13 studies; 858 participants; I² = 67%; low‐quality evidence).
Reduction in fibroid volume
See Analysis 1.3; Analysis 1.4.
Seven trials with 675 participants evaluated this outcome, five of which were pooled (Analysis 1.3) with a no pretreatment arm and two other studies with placebo arms (and skewed data) (Analysis 1.4). GnRHa pretreatment was associated with a reduction in fibroid size ranging from 5.7 mL to 155.4 mL (data were too heterogeneous to calculate a summary effect measure). In two other trials with placebo arms, one reported a significant difference from placebo and the other reported no significant difference.
1.3. Analysis.
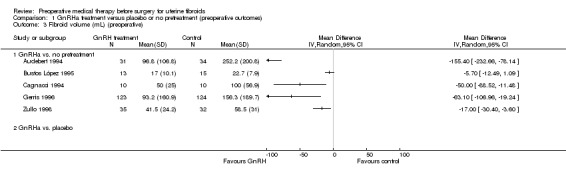
Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 3 Fibroid volume (mL) (preoperative).
1.4. Analysis.
Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 4 Fibroid volume (preop in data table).
| Fibroid volume (preop in data table) | ||||
|---|---|---|---|---|
| Study | Number in study | Comparison | Results | Comment |
| GnRHa vs. placebo | ||||
| Muneyyirci‐Delale 2007 | 110 | (GnRHa (goserelin) + iron vs sham injection + iron | Median (range): GnRHa vs placebo: ‐35.4 cm3 (no range reported) vs +3.9 cm3 (no range reported) |
Auhors reported that there were no significant between group differences |
| Stovall 1995 | 138 | GnRHa (leuprolide acetate depot 7.5mg and 3.75mg) + iron vs placebo + iron | Median % change from baseline: LA/iron 7.5mg: ‐23% (no range reported) LA/iron 3.75mg: ‐27% (no range reported) Placebo/iron: +8% (no range reported) |
Authors reported that both LA groups had significantly greater changes from baseline compared to placebo (p<0.01) |
Preoperative haemoglobin
See Analysis 1.5; Analysis 1.6.
Eleven studies assessed preoperative haemoglobin, six compared to no pretreatment and five compared to placebo (Stovall 1995 included two comparisons reflecting differences in dosage of GnRHa, 3.75 mg or 7.5 mg). One of the placebo‐controlled trials could not be pooled and the findings were reported in table format (Muneyyirci‐Delale 2007). Haemoglobin was consistently increased in women who received GnRHa compared to control, regardless of whether placebo was used (MD 0.88 g/dL, 95% CI 0.7 to 1.1; 10 studies; 834 participants; I² = 0%; moderate‐quality evidence).
Preoperative bleeding (measured by a validated scale)
No included studies measured preoperative bleeding in a validated format.
Secondary outcomes
Adverse events (related to the preoperative treatment)
Adverse events (any) were more common overall in women who received GnRHa pretreatment when compared to placebo (OR 2.8, 95% CI 1.8 to 4.4; 4 studies; 755 participants; I² = 28%; moderate‐quality evidence; Analysis 1.7.2). When specific adverse events were considered, hot flushes (OR 7.7, 95% CI 4.6 to 13.0; 6 studies; 877 participants; I² = 46%; low‐quality evidence; Analysis 1.8.2), headache (OR 1.7, 95% CI 1.0 to 3.0; 6 studies; 877 participants; I² = 49%; low‐quality evidence; Analysis 1.8.3), dizziness (OR 2.1, 95% CI 1.1 to 5.1; 2 studies; 505 participants; I² = 0%; moderate‐quality evidence; Analysis 1.8.6), vaginitis (OR 4.2, 95% CI 1.6 to 11.1; 5 studies; 751 participants; I² = 28%; low‐quality evidence; Analysis 1.8.10), change in breast size (OR 10.9, 95% CI 1.9 to 62.2; 2 studies; 261 participants; I² = 0%; low‐quality evidence; Analysis 1.8.15) and sweating (OR 14.3, 95% CI 6.2 to 33.3; 4 studies; 497 participants; I² = 0%; low‐quality evidence; Analysis 1.8.16) were all more common with GnRHa pretreatment compared to no treatment or placebo. See Analysis 1.7; Analysis 1.8.
1.7. Analysis.
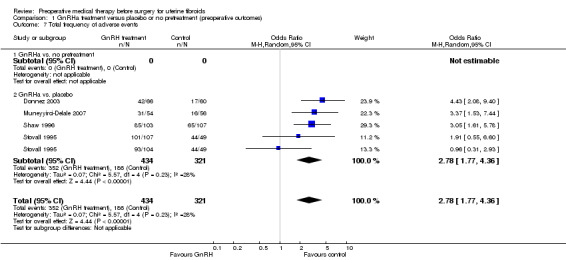
Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 7 Total frequency of adverse events.
1.8. Analysis.
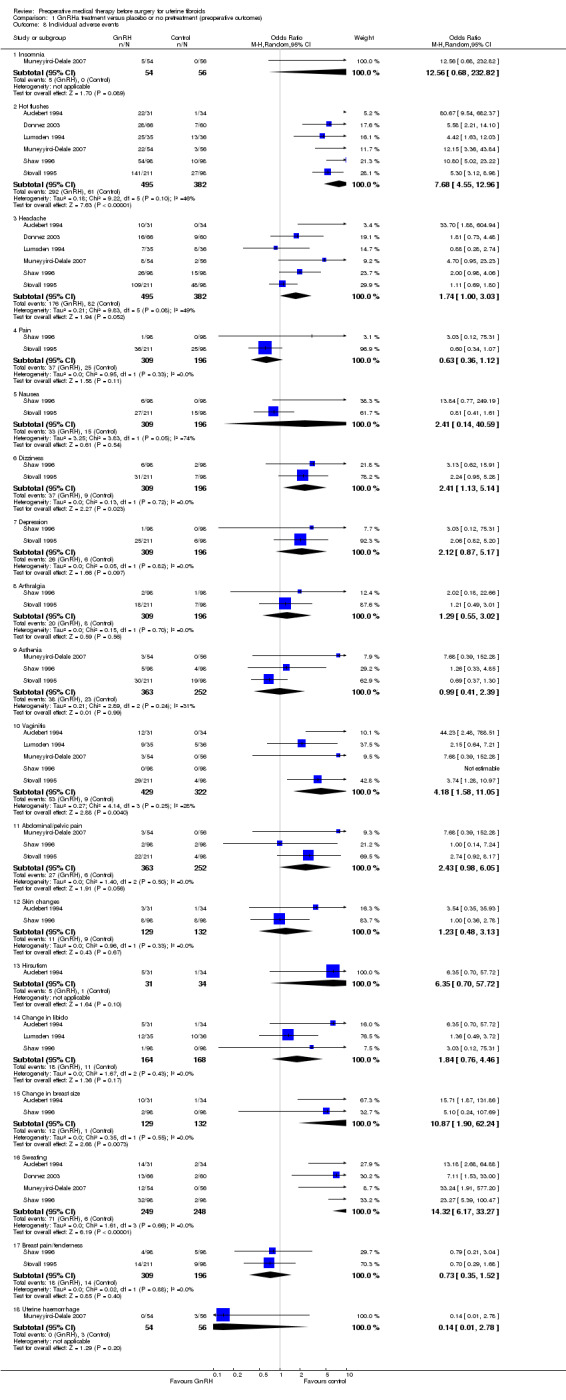
Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 8 Individual adverse events.
Quality of life (related to the preoperative assessment, assessed subjectively by the participant on a validated scale)
No included studies assessed quality of life.
Comparison 2: Intraoperative or postoperative outcomes before hysterectomy
See Table 2.
Primary outcomes
Duration of surgery
Six studies comparing GnRHa with no pretreatment and three studies comparing GnRHa with placebo assessed duration of surgery (2 no pretreatment and 1 placebo‐controlled trials were reported in tabular format; Analysis 2.1; Figure 5). There was moderate evidence of heterogeneity in the body of evidence. Heterogeneity was expected, given differences in the expertise of the surgeons, variable methods of measuring the length of the surgery and, in particular, the type of hysterectomy performed. One trial undertook laparoscopic hysterectomy and two studies performed vaginal as well as abdominal hysterectomy, where possible.
5.
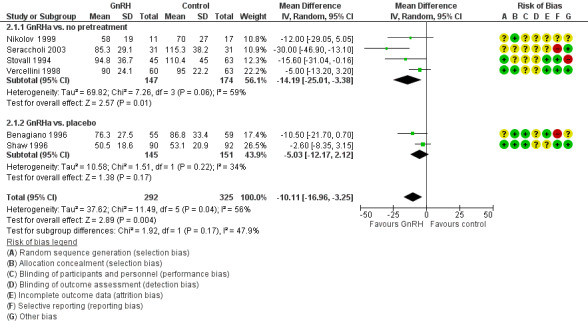
Forest plot of comparison: 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), outcome: 2.1 Duration of surgery (minutes).
Overall, when studies were combined (control group either no pretreatment or placebo), GnRHa reduced duration of surgery by 9.6 minutes (MD 9.6 min, 95% CI ‐15.9 to ‐3.3; 6 studies; 617 participants; I² = 57%; low‐quality evidence; Analysis 2.2).
Intraoperative blood loss
See Analysis 2.3; Analysis 2.4.
Six studies (N = 359 participants) compared blood loss between groups who received GnRHa pretreatment and control. All studies found less blood loss with GnRHa pretreatment, ranging from 25 mL to 148 mL reductions compared to control (summary effect measures could not be calculated because of substantial heterogeneity). Overall, the studies provided very low‐quality evidence.
Frequency of intraoperative blood transfusions
See Analysis 2.5.
Six studies assessed the likelihood of blood transfusions during surgery. Overall, blood transfusions were less likely with GnRHa pretreatment compared to control (no pretreatment and placebo combined) (OR 0.54, 95% CI 0.3 to 1.0; 6 trials; 601 participants; I² = 0%; low‐quality evidence).
Postoperative morbidity
See Analysis 2.6; Analysis 2.7.
Seven trials assessed postoperative complications (any) (5 compared GnRHa with no pretreatment and two compared GnRHa with placebo). Overall, the odds of complications with GnRHa was reduced compared to control (OR 0.54, 95% CI 0.3 to 0.9; 7 studies; 772 participants; I² = 28%; low‐quality evidence).
One trial assessed individual complications in 212 participants (Hudecek 2012). There was no evidence of a difference in the rates of hypermenorrhoea (OR 0.36, 95% CI 0.1 to 1.2; low‐quality evidence, Analysis 2.7.1), dysmenorrhoea (OR 3.9, 95% CI 0.2 to 82.3; very low‐quality evidence, Analysis 2.7.2), pelvic pain (OR 0.43, 95% CI 0.2 to 1.2; low‐quality evidence, Analysis 2.7.3), difficult defecation (OR 0.76, 95% CI 0.1 to 5.5; low‐quality evidence, Analysis 2.7.4), difficult urination (OR 0.15, 95% CI 0.0 to 3.2; very low‐quality evidence, Analysis 2.7.5), or dyspareunia (OR 3.9, 95% CI 0.2 to 82.3; very low‐quality evidence, Analysis 2.7.6) among groups. See Analysis 2.7.
2.7. Analysis.
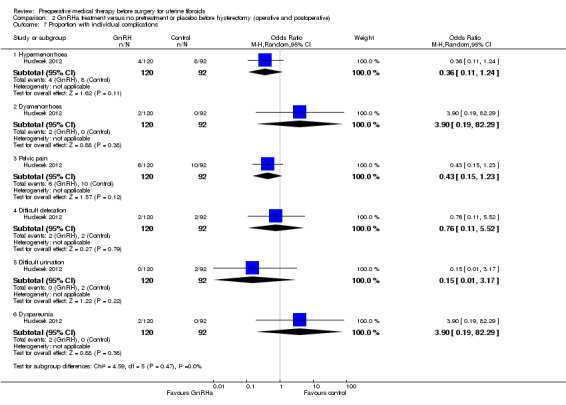
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 7 Proportion with individual complications.
Secondary outcomes
Difficulty of surgery (assessed subjectively by surgeon)
Five trials assessed this outcome (2 with a no pretreatment control group and 3 with a placebo‐control group). The overall summary effect estimate (regardless of control group) indicated the odds of difficult surgery were lowered with GnRHa (OR 0.72, 95% CI 0.5 to 1.0; 95% CI 0.5 to 1.0; 5 studies, 712 participants; 1² = 0%; low‐quality evidence; Analysis 2.8).
Proportion of women undergoing vaginal hysterectomy
Three studies (395 participants) compared GnRHa with control (2 studies had no pretreatment and 1 used a placebo‐control group). Studies were too heterogeneous to pool. In two studies with no pretreatment control, both found an increase in the odds of undertaking a vaginal procedure with GnRHa, but there were limitations in study design, with substantial heterogeneity. One placebo‐controlled study, with moderate‐quality evidence, did not report any differences between randomised groups. See Analysis 2.9.
Type of abdominal incision
Four studies (2 compared GnRHa with no pretreatment and 2 compared GnRHa with placebo) assessed this outcome. The odds of vertical incision during hysterectomy was reduced with GnRHa pretreatment (overall OR 0.34, 95% CI 0.2 to 0.5; 4 studies; 529 participants; I² = 0%; moderate‐quality evidence; Analysis 2.10).
Duration of hospital stay (days)
Findings were mixed (and too heterogeneous to pool) in five trials (344 participants) assessing this outcome. Two trials reported that GnRHa pretreatment was associated with less time in hospital when compared with no pretreatment (1 day to 2.6 days less) but three other trials reported no evidence of a difference in hospital stay between randomised groups. See Analysis 2.11; Analysis 2.12.
Postoperative recurrence of myomas
No included studies assessed recurrence.
Postoperative haemoglobin
See Analysis 2.13.
Three studies (2 studies with no pretreatment as control and 1 placebo‐controlled trials) assessed this outcome. The overall summary effect estimate (regardless of type of control group) suggested that GnRHa improved postoperative haemoglobin levels (MD 0.85, 95% CI 0.3 to 1.4, 3 studies; 240 participants; I² = 41%; low‐quality evidence).
Comparison 3: Intraoperative or postoperative outcomes with myomectomy
See Table 3
Primary outcomes
Duration of operation
See Analysis 3.1; Figure 6; Analysis 3.2.
6.
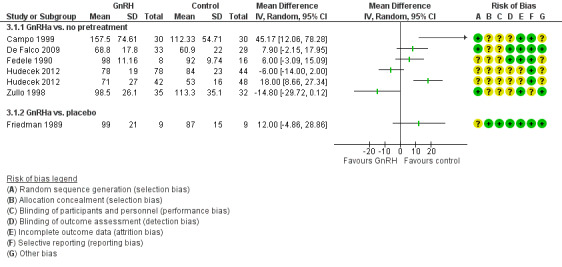
Forest plot of comparison: 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), outcome: 3.1 Duration of surgery (minutes).
Eight trials, including 494 participants, assessed this outcome (7 comparing GnRHa to no pretreatment and 1 comparing GnRHa to placebo).
Trials could not be pooled due to substantial heterogeneity. There was no evidence that duration of surgery was influenced by whether participants received GnRHa pretreatment or not. Individual studies did not report significant differences between randomised groups, except for two studies comparing GnRHa pretreatment to no pretreatment before laparoscopic myomectomy was undertaken. In these two trials (Campo 1999; Hudecek 2012), GnRHa was associated with a significant increase in the time taken to undertake laparoscopic myomectomy compared to no pretreatment (ranging from an increase of 18 minutes to 45 minutes).
Intraoperative blood loss
See Analysis 3.3.
We included 10 studies (549 participants) that assessed this outcome (9 compared GnRHa to no pretreatment and 1 compared GnRHa to placebo).
Trials could not be pooled, due to substantial heterogeneity and varied findings. Most trials reported that GnRHa reduced blood loss, ranging from a reduction of 22 mL to 157 mL, although findings were mostly outside the level of significance and the quality of the evidence was very low. Most trials reported that surgery was myomectomy, either unspecified or open. Three trials where laparoscopic myomectomy was performed had mixed results; two trials reported that GnRHa pretreatment reduced blood loss by either 37 mL or 60 mL and the other trial reported a greater intraoperative blood loss with GnRHa compared to control (82 mL) (Campo 1999; Hudecek 2012; Zullo 1996).
Frequency of intraoperative blood transfusions
See Analysis 3.4.
In four trials assessing this outcome, there was no evidence of a difference in the odds of intraoperative blood transfusion between randomised groups (OR 0.85, 95% CI 0.3 to 2.8; 4 studies; 121 participants; I² = 0%; low‐quality evidence).
Postoperative morbidity
The odds of any postoperative complications was assessed in five trials. There was no evidence of a significant difference between groups (overall OR 1.07, 95% CI 0.4 to 2.6; 5 trials; 190 participants; I² = 0%; low‐quality evidence; Analysis 3.5).
Secondary outcomes
Difficulty of surgery
No included studies assessed this outcome.
Type of abdominal incision
In one small trial with 28 participants, there was no evidence of a significant difference between GnRHa pretreatment or no presurgical treatment (OR 0.07, 95% CI 0.0 to 1.4; low‐quality evidence; Analysis 3.6) (Bustos Lopez 1995).
Duration of hospital stay (days)
Three studies including 290 participants assessed this outcome but heterogeneity was substantial and data could not be pooled. There was no evidence that GnRHa pretreatment influenced duration of hospital stay when compared with no pretreatment or placebo. See Analysis 3.7.
Intraoperative hysterectomy
No included studies assessed this outcome.
Postoperative recurrence of myomas
See Analysis 3.8.
FIndings were inconsistent in two very small trials assessing this outcome. There was no evidence of a difference in recurrence between randomised groups in the overall estimate (with placebo and no presurgical treatments combined) (OR 4.16, 95% CI 0.6 to 29.1; 2 studies; 42 participants; I² = 48%; very low‐quality evidence).
Postoperative haemoglobin
In one trial, GnRHa pretreatment increased postoperative haemoglobin when compared to no pretreatment (MD 0.8 g/dL, 95% CI 0.2 to 1.4; 67 participants; low‐quality evidence; Zullo 1996; Analysis 3.9).
Comparison 4: Intraoperative and postoperative outcomes with resection
See Table 4
Two studies assessed intraoperative and postoperative outcomes during resection of fibroids (Mavrelos 2010; Muzii 2010), but in Mavrelos 2010, only a proportion of women randomised to groups went on to have surgery, so outcomes for this trial were not extracted.
Primary outcomes
Duration of surgery
In one small study (Muzii 2010), duration of surgery was reduced with GnRHa pretreatment when compared to no pretreatment (MD 5.4 minutes, 95% CI ‐3.2 to ‐7.7, 1 study; N = 39; low‐quality evidence; Analysis 4.1).
Intraoperative blood loss
The included studies did not assess this outcome.
Frequency of blood transfusions
The included studies did not assess this outcome.
Postoperative morbidity
The included studies did not assess this outcome.
Secondary outcomes
Difficulty of surgery (assessed subjectively by surgeon)
See Analysis 4.2.
In one small trial with 39 participants (Muzii 2010), there was no evidence of a difference in a visual analogue scale (VAS) (with categories of perceived difficulty) between women who received GnRHa pretreatment and those who did not receive pretreatment (MD ‐1.4, 95% CI ‐3.1 to 0.3; low‐quality evidence). Surgeons were not blinded, so it is not possible to exclude the possibility that knowledge of treatment influenced the findings.
Type of abdominal incision (Pfannenstiel transverse versus vertical)
The included studies did not assess this outcome.
Duration of hospital stay (days)
The included studies did not assess this outcome.
Postoperative recurrence of myomas
In one small trial with 39 participants (Muzii 2010), there were no incidences of fibroid recurrence over a mean of nine months after surgery in any participants (Analysis 4.3).
Postoperative haemoglobin
The included studies did not assess this outcome.
Comparison 5: GnRHa pretreatment compared to other medical therapy pretreatment before surgery
See Table 5
GnRHa pretreatment was compared with a combined group of 'other medical treatment' because there were few data on these other treatments. Given the established effectiveness of GnRHa as a pretreatment, at least some of the trials comparing GnRHa with other treatment were non‐inferiority trials, designed in a way to establish whether they were as effective as GnRHa, but without the associated adverse effects. Where necessary, sensitivity analyses were undertaken to assess the differential effects of the other treatments. The structure of the comparisons is provided in Table 7.
Seven studies were included (Baytur 2007; Donnez 2003; Donnez 2012b; Reinsch 1994; Sayyah Melli 2007; Sayyah‐Melli 2009; Verspyck 2000) in this analysis. One study (Verspyck 2000) assessed the effects of lynestrenol (a progestin), another (Donnez 2012b) assessed the effects of two different doses of an SPRM (ulipristal acetate 5 mg and 10 mg), one (Reinsch 1994) assessed another type of SPRM (mifepristone), another (Baytur 2007) assessed a SERM (raloxifene), two assessed a dopamine agonist (cabergoline (Sayyah‐Melli 2007; Sayyah‐Melli 2009) and another (Donnez 2003) assessed multiple doses of an oestrogen receptor agonist (fulvestrant 50 mg, 125 mg and 250 mg). Donnez 2012b was the only trial that was placebo‐controlled with participants and investigators blinded to allocation.
Only preoperative outcomes were measured, because data were insufficient to distinguish between types of surgery, which could influence intra or postoperative outcomes.
Primary outcomes
Reduction in uterine volume
Three trials including 353 participants assessed uterine volume but could not be pooled because of skewed data. There was no evidence of a significant difference between GnRHa pretreatment compared to raloxifene or GnRHa pretreatment compared to mifepristone (Baytur 2007) or when GnRHa pretreatment was compared to mifepristone (Reinsch 1994). A large placebo‐controlled trial (Donnez 2012b) reported that leuprolide acetate pretreatment had greater reduction in uterine volume (‐47%) compared to either 5 mg of ulipristal acetate (‐20%) or 10 mg of ulipristal acetate (‐22%). See Analysis 5.1.
Reduction in fibroid volume
Five trials assessed fibroid volume but only two could be pooled (Sayyah Melli 2007; Sayyah‐Melli 2009); both trials compared GnRHa with cabergoline (a dopamine agonist). For these studies, there was no evidence of a difference in fibroid volume between groups (MD 12.71, 95% CI ‐5.9 to 31.3; 2 studies; 110 participants; I² = 0%; low‐quality evidence; Analysis 5.2). The three trials that could not be pooled (646 participants) compared GnRHa with raloxifene (a SERM) (Baytur 2007), GnRHa with ulipristal acetate (a SPRM) in two doses (Donnez 2012b) and GnRHa with fulvestrant (an oestrogen receptor antagonist) in multiple doses (Donnez 2003). There was no evidence of a significant difference between treatments for any of the studies except the latter, where GnRHa was associated with a greater fibroid reduction than any dose of fulvestrant (see Analysis 5.3).
5.2. Analysis.
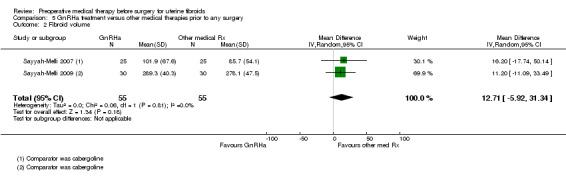
Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 2 Fibroid volume.
5.3. Analysis.
Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 3 Fibroid volume (descriptive table).
| Fibroid volume (descriptive table) | ||||
|---|---|---|---|---|
| Study | Number in study | Comparison | Results | Comment |
| Baytur 2007 | 32 | GnRHa (goserelin 3.6mg monthly for 3 months) vs SERM (raloxifene 60mg daily orally) | Difference from baseline to after treatment (median (range)): GnRHa: 30cc (20 to 200) Raloxifene: 18 (12 to 65) |
No significant difference between groups |
| Donnez 2003 | 313 | GnRHa (goserelin 3.6mg monthly for 3 months) vs fluvestrant (50mg, 125mg and 250mg for 3 months) ‐ this comparison not blinded | Ratio of the generalised least squares means (fulvestrant or goserelin): Fulvestrant 50mg vs goserelin: 1.88 (95% CI 1.3 to 2.8), p=0.001, n=82 Fulvestrant 125mg vs goserelin: 1.82 (95% CI 1.2 to 2.7), p=0.0002, n=80 Fulvestrant 250mg vs goserelin: 1.56 (95% CI 1.1 to 2.3), p=0.023, n=83 |
Authors reported that goserelin was associated with a significantly greater reduction in fibroid volume than any dose of fulvestrant. Note: this analysis was per protocol with significant attrition ‐ the ITT analyses were not reported. |
| Donnez 2012b | 307 | GnRHa (leuprolide acetate 3.75mg) vs ulipristal acetate (5mg and 10mg) | Per protocol results: Percentage change from baseline in 3 largest fibroids (IQ range) Ulipristal acetate 5mg: ‐36% (‐58 to ‐11) Ulipristal acetate 10mg: ‐42% (‐69 to ‐41) Leuprolide acetate 3.75mg: ‐53% (‐69 to ‐36) Difference in % points: UA 5mg vs LA 3.75mg: 1.23 (0.99 to 1.52) UA 10mg vs LA 3.75mg: 1.12 (0.91 to 1.38) |
Authors reported that all 3 treatments reduced the volume of the 3 largest fibroids |
Preoperative haemoglobin
One large trial with 188 participants reported that there was no evidence of a difference between the levels of preoperative haemoglobin after pretreatment with GnRHa compared to ulipristal acetate 10 mg (MD ‐0.2, 95% CI ‐0.6 to 0.2; moderate‐quality evidence; Analysis 5.4) (Donnez 2012b).
Reduction in preoperative bleeding
One non‐inferiority trial, with 307 participants, comparing GnRHa to ulipristal acetate assessed the proportion of women whose bleeding reduced to < 75 units by the PBAC as a result of presurgical treatment (Donnez 2012b). There was no evidence of a difference in bleeding rates between the 2 groups leading the authors to conclude that ulipristal acetate was non inferior to GnRHa in controlling uterine bleeding (ulipristal acetate 5 mg: OR 0.71, 95% CI 0.3 to 1.7; moderate‐quality evidence; ulipristal acetate 10 mg: OR 0.39, 95% CI 0.1 to 1.1; moderate‐quality evidence; Analysis 5.5).
Secondary outcomes
Adverse events
Many individual adverse events were measured by only a few of the relevant trials and definitions may have varied influencing the reliability of the results. As GnRHa has been compared to other medical treatments before surgery, overall totals where there are more than one trial need to be broken down. There was clear evidence that hot flushes were more likely with GnRHa pretreatment compared to other medical pretreatments (which included raloxifene, ulipristal acetate, mifepristone, cabergoline and lynestrenol) (OR 12.3, 95% CI 4.0 to 37.5; 5 studies; 183 participants; I² = 61%; low‐quality evidence). The odds of headache (OR 4.51, 95% CI 1.1 to 18.6; 4 studies; 102 participants; I² = 67%; low‐quality evidence; Analysis 5.6.2), sleep problems (OR 20.71, 95% CI 2.5 to 172; 1 study; 56 participants; very low‐quality evidence; Analysis 5.6.6) and bone sensitivity (OR 125.8, 95% CI 6.8 to 2343.3; 1 study; 50 participants; very low‐quality evidence; Analysis 5.6.20) were also increased with GnRHa. There was no evidence of a difference between groups in other specific adverse events. See Analysis 5.6.
5.6. Analysis.
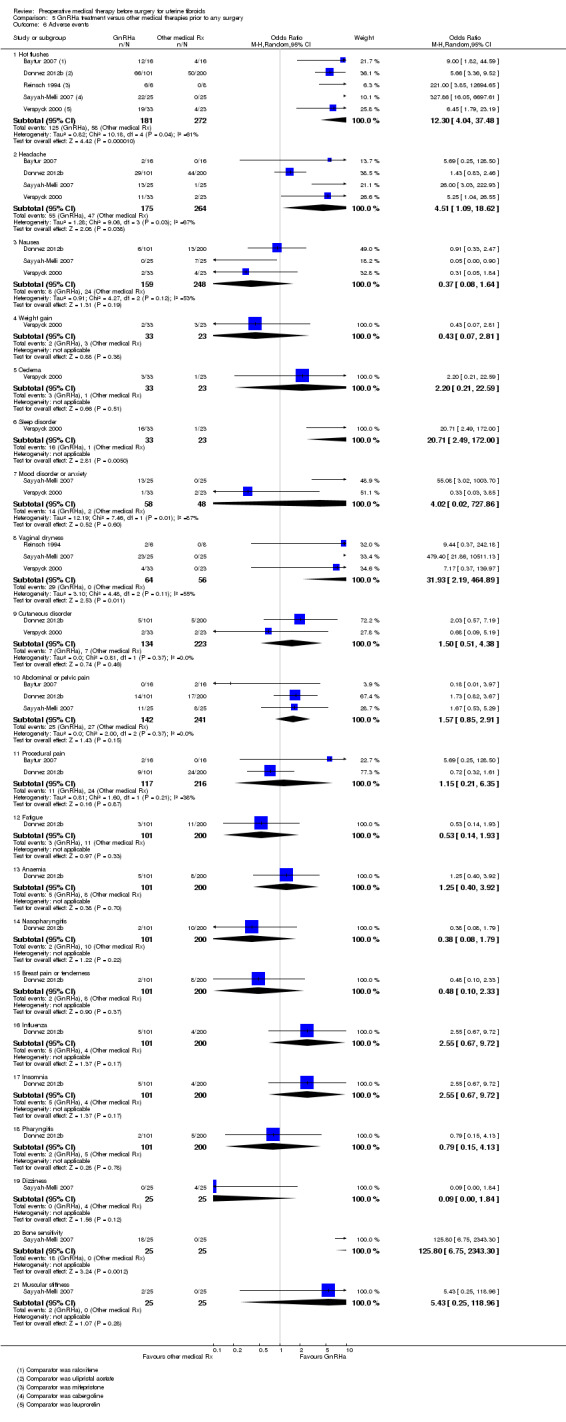
Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 6 Adverse events.
Where there was more than one study in the comparison, sensitivity analyses were undertaken to establish the differential effects of the other medical therapies (which were combined as control in the comparison). For hot flushes, GnRHa was associated with a greater odds of hot flushes when compared with other medical treatments independently, with odds ratios varying from 5.7 (ulipristal acetate), 6.45 (lynestrenol), 9.0 (raloxifene), 221.0 (mifepristone) and 327.9 (cabergoline). Two of the four studies measuring headache also reported independently greater odds with GnRHa compared to cabergoline (OR 26.0) and lynestrenol (OR 5.3), but there was no evidence of differences with raloxifene (Baytur 2007) or ulipristal acetate (Donnez 2012b).
Quality of life
There was no evidence of a difference between GnRHa and ulipristal acetate (either dose) with respect to quality of life (measured in a specific fibroid symptom questionnaire) (Donnez 2012b). The difference in the percentage change from baseline compared to GnRHa was 2.5% with ulipristal acetate 5 mg and 5.6% with ulipristal acetate 10 mg. See Analysis 5.7.
Comparison 6: SPRMs versus placebo
See Table 6.
Only preoperative outcomes were measured, as data were not sufficient to distinguish between types of surgery, which could influence intra or postoperative outcomes.
The structure of the comparisons according to outcomes measured is provided in Table 7.
Primary outcomes
Reduction in uterine volume
Two studies with 275 participants compared either ulipristal acetate (5 mg and 10 mg) with placebo (Donnez 2012a) or asoprisnil (10 mg or 25 mg) with placebo (Wilkens 2008). The studies could not be pooled because of potentially skewed data. Ulipristal acetate was associated with a greater reduction in uterine volume than placebo (Donnez 2012a) but there was no evidence of a significant difference between asoprisnil and placebo (Wilkens 2008). See Analysis 6.1.
Reduction in fibroid volume
Four studies including 327 participants compared various SPRMs with placebo: either ulipristal acetate (5 mg or 10 mg) (Donnez 2012a), mifepristone (50 mg every other day) (Engman 2009), CDB‐2914 (10 mg or 20 mg) (Levens 2008) or asoprisnil (10 mg or 25 mg) (Wilkens 2008). The outcomes measured were mostly median change from baseline which was compared between randomised groups, and so studies were not pooled. All studies reported that SPRMs were associated with greater reductions in uterine fibroids, except for the lower dose of asoprisnil (10 mg). Donnez 2012a reported a 3% increase with placebo compared to a 21% and 12.3% decrease with ulipristal acetate 5 mg and 10 mg, respectively; Engman 2009 reported a 6% increase with placebo compared to 28% decrease with mifepristone; Levens 2008 found a 6% increase with placebo compared to a 29% decrease with CDB‐2914; and Wilkens 2008 reported a 5% increase with placebo compared to a 26% decrease with asoprisnil. See Analysis 6.2.
Preoperative haemoglobin
Two studies compared haemoglobin levels before surgery after ulipristal acetate pretreatment (5 mg or 10 mg) (Donnez 2012a) or mifepristone pretreatment (Engman 2009) when compared to placebo. Both treatments were associated with,an increased mean of almost 1 g/dL haemoglobin (MD 0.93, 95% CI 0.5 to 1.4; 2 studies; 173 participants; I² = 0%; high‐quality evidence; Analysis 6.3).
Reduction in preoperative bleeding
One trial comparing ulipristal acetate with placebo assessed the proportion of women who achieved a reduction in bleeding to < 75 units by PBAC after presurgical intervention (Donnez 2012a). The odds of bleeding reduction was higher in women receiving both doses of ulipristal acetate compared to placebo (ulipristal acetate 5 mg: OR 41.41, 95% CI 15.3 to 112.4; 1 study; 143 participants; low‐quality evidence; Analysis 6.4.1; ulipristal acetate 10 mg: OR 78.83, 95% CI 24.0 to 258.7; 1 study; 146 participants; low‐quality evidence; Analysis 6.4.2). Another small study (Wilkens 2008) compared change in menstrual blood loss from baseline to the end of treatment (asoprisnil) before surgery; asoprisnil was associated with a significant reduction in blood loss when compared to placebo (MD 166.9, 95% CI ‐56.2 to ‐277.6; 1 study; 22 participants; low‐quality evidence; Analysis 6.5).
Secondary outcomes
Adverse events
Serious events: There was no evidence of a significant difference in the rates of breast cancer, uterine or ovarian haemorrhage, fibroid protrusion, menometrorrhagia or hyperplasia between groups in three studies (Donnez 2012a; Levens 2008; Wilkens 2008) where the SPRMs included ulipristal acetate (5 mg or 10 mg), asoprisnil (10 mg or 25 mg) or CDB‐2914 (10 mg or 20 mg), although many of these specific adverse events were measured by only one trial (Donnez 2012a) (Analysis 6.6).
Other specific adverse events: Three trials measured other less serious adverse events (Donnez 2012a; Engman 2009; Wilkens 2008) although most of these events included data from only one trial (Donnez 2012a). The odds of hot flushes and change of mood was increased with mifepristone in one small trial (hot flushes: OR 25.24, 95% CI 1.3 to 503.4; Engman 2009; 30 participants; low‐quality evidence; mood change Analysis 6.7.18: OR 15.0, 95% CI 1.5 to 146.5; Donnez 2012a; 30 participants; low‐quality evidence; Analysis 6.7.22). Dysmenorrhoea was less likely with ulipristal acetate in another larger trial (OR 0.05, 95% CI 0.0 to 1.0; 1 study; 241 participants; low‐quality evidence; Analysis 6.7.12). The findings from these two studies should be treated with considerable caution, as the findings were very imprecise, with very wide confidence intervals. There was no evidence of significant differences in any of the other adverse events.
6.7. Analysis.
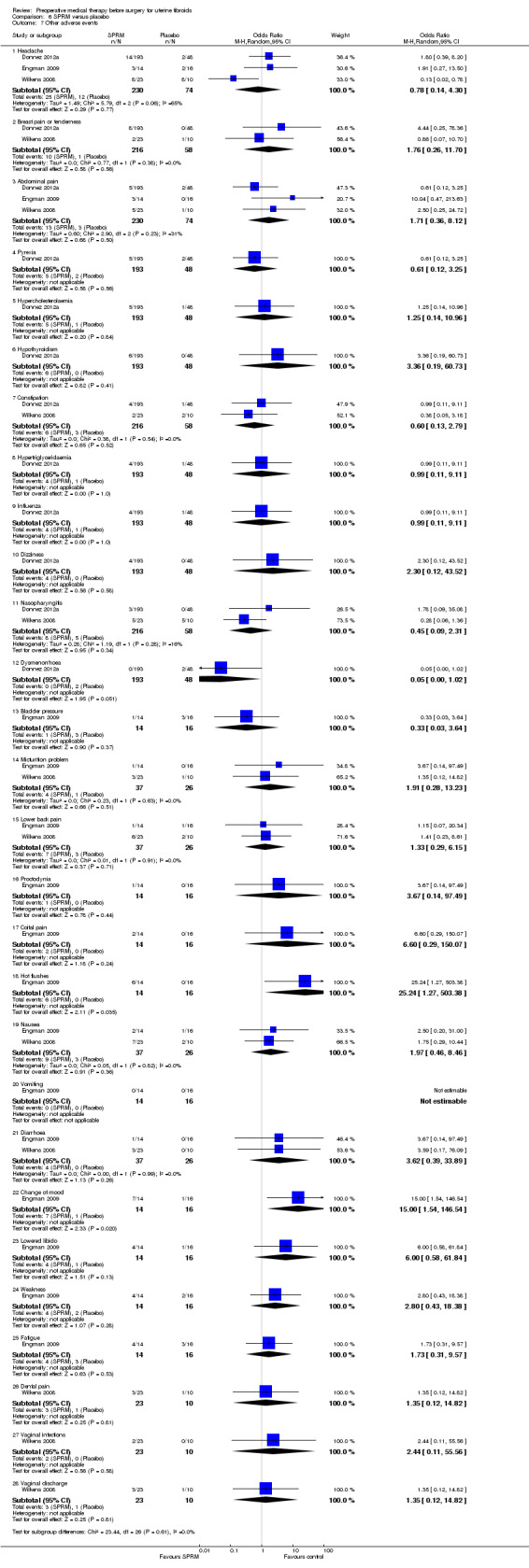
Comparison 6 SPRM versus placebo, Outcome 7 Other adverse events.
Quality of life
One trial with 239 participants found that either ulipristal acetate 5 mg or 10 mg increased quality of life (by a median reduction of 4 points) (measured by a uterine fibroid symptoms and quality of life questionnaire with a total range of 28 points) when compared to placebo (Donnez 2012a). See Analysis 6.8.
Discussion
Summary of main results
We assessed the effect of preoperative medical therapy on a number of important preoperative, intraoperative and postoperative outcomes for resection, myomectomy and hysterectomy in the surgical treatment of women with uterine fibroids. A rationale for the use of preoperative medical therapy is to reduce the difficulty of any surgical procedure and thereby improve associated outcomes. The included studies did not contribute data to every outcome and, for some outcomes, findings were stratified according to whether the control group was no pretreatment or placebo. Summaries of overall results for the main outcomes, with overall quality assessments, are presented in Table 1, Table 2, Table 3Table 4, Table 5 and Table 6.
Gonadotropin‐hormone releasing analogues (GnRHa) versus no pretreatment or placebo
Preoperative outcomes (versus no treatment or placebo)
The combined results of both placebo‐controlled and GnRHa versus no treatment trials strongly suggest that, regardless of subsequent surgery, the use of GnRHa is associated with an increase in preoperative haemoglobin, and a reduction in both uterine and fibroid volume, although this came at the expense of a greater likelihood (odds) of adverse events, in particular hot flushes, headache, dizziness, vaginitis, change in breast size and sweating.
Outcomes during and after hysterectomy
Duration of surgery was reduced by up to 14 minutes with GnRHa pretreatment in most studies, although some studies with skewed data did not find a benefit of pretreatment. Other benefits of GnRHa pretreatment included: a significant reduction in blood loss and the need for blood transfusions, smaller odds of requiring vertical incision, lower likelihood (odds) of difficult surgery (only in placebo trials) and improved postoperative haemoglobin levels. Evidence was inconsistent with respect to duration of hospital stay and odds of performance of vaginal rather than abdominal surgery. The odds of complications were also reduced with GnRHa pretreatment.
Outcomes during and after myomectomy
In women undergoing myomectomy, most trials found that GnRHa reduced intraoperative blood loss, although substantial heterogeneity was found and no overall pooled estimate could be calculated. There was no evidence that pretreatment influenced duration of surgery, odds of intraoperative blood transfusions, duration of hospital stay, and recurrence of fibroids or postoperative haemoglobin levels. One small trial found that in women undergoing abdominal myomectomy, the odds of vertical incision were reduced and another trial found that pretreatment was associated with greater postoperative haemoglobin levels. However, because of small numbers, these findings are very uncertain.
Outcomes during and after endometrial resection
One small study found that duration of surgery was reduced by a mean of 5.4 minutes with GnRHa pretreatment when compared to no pretreatment, although this is not likely to be clinically important. There was no evidence of a difference in the perception of difficult surgery or recurrence of fibroids with GnRHa pretreatment.
GnRHa versus other medical therapies (lynestrenol, selective progesterone‐receptor modulators (SPRMs), selective oestrogen receptor modulators (SERMs), dopamine agonists, oestrogen receptor agonists)
For most comparisons and outcomes, only one trial contributed data, because control groups were stratified according to type of medical therapy being compared to GnRHa. Two trials compared GnRHa with multiple doses of the control medical therapy (of ulipristal acetate and fulvestrant). Only the trial comparing GnRHa with ulipristal acetate was placebo‐controlled with double blinding. Because of few data, the other medical therapies were combined in one control group, and sensitivity analyses undertaken to determine the differential effects of the treatments.
With regard to uterine volume, there was no evidence of a difference between GnRHa and raloxifene or mifepristone, but GnRHa pretreatment was associated with a greater reduction in volume than ulipristal acetate, regardless of dosage of ulipristal acetate. With respect to fibroid volume, there was no evidence of significant differences between groups (in comparisons of control groups with dopamine agonist, raloxifene and ulipristal acetate); however, GnRHa was associated with a greater reduction in volume than either dose of fulvestrant. There was no evidence of differences between groups for other outcomes: preoperative haemoglobin, reduction in bleeding, blood transfusion rates and quality of life. However, GnRHa was more likely to be associated with hot flushes than other medical therapies.
SPRMs versus placebo
SPRMs were associated with greater reductions in uterine volume (ulipristal acetate and asoprisnil) and fibroid volume (ulipristal acetate, mifepristone, CDB2914 and asoprisnil) than placebo pretreatment, and with increased preoperative haemoglobin levels (ulipristal acetate and asoprisnil). Ulipristal acetate and asoprisnil were also associated with a greater reduction in bleeding before surgery than placebo and quality of life was greater with ulipristal acetate pretreatment. There was insufficient evidence to determine rates of adverse events, because these were mostly measured by only one trial.
Overall completeness and applicability of evidence
For this 2017 update, the review was expanded from assessment of the role of GnRHa treatment before surgery for uterine fibroids to include all potential medical pretreatments (except for misoprostol which is covered by another Cochrane Review (Kongnyuy 2014)). We included other medical treatment options such as SPRMs (asoprisnil, ulipristal acetate, mifepristone or CDB‐2914), progestins (lynestrenol), SERMs (raloxifene), dopamine agonists (cabergoline), and oestrogen receptor antagonists (fulvestrant). This update included 38 randomised controlled trials (RCTs), with 3560 participants but there were multiple comparisons with different types of interventions, which mean that some comparisons were underpowered. Hence, results based on small numbers of participants should be treated with caution. Only 12 of the 38 studies had 100 participants or more.
Participants in the studies all had symptomatic fibroids, with the expectation of surgery, but there was substantial variation among studies in types of fibroids included, size of uterus, degree of anaemia and type of subsequent surgery, limiting the generalisability of the results. Evidence on GnRHa pretreatment was based on a reasonable number of participants but results from other medical pretreatments was based on much smaller numbers of women.
Adverse events were sometimes only anecdotally reported in text format in the included studies but the larger studies mostly provided full tables of individual symptoms associated with pretreatment. Although hyperplasia rates did not differ, the authors of two large trials comparing ulipristal acetate with placebo or GnRHa (Williams 2012) noted that a spectrum of morphological endometrial changes were associated with three months of treatment with ulipristal acetate (that had been described previously in women receiving SPRM treatment), but these disappeared two months after the end of therapy.
Quality of the evidence
The quality of the included studies was determined in two ways; risk of bias was assessed for each individual study and an overall quality grading for the body of the evidence was also assessed for each outcome, based on the GRADE criteria: limitations in study design, consistency, indirectness, imprecision and likelihood of publication bias.
Only three of the included studies had low risk of bias for all domains. When summarised as a body of evidence (Figure 2), less than half the included studies had low risk of bias for allocation, or blinding of participants and investigators or other potential bias, such as baseline comparability. About 30% had low risk of bias for outcome assessment, and about 50% had low risk of bias for incomplete outcome data and selective reporting. The remainder of the included trials had either unclear or high risk of bias for each these domains. A number of the included studies reported receiving pharmaceutical company funding or support; for these studies, it was not possible to determine whether the conflict of interest had influenced the findings.
With respect to overall quality assessment, heterogeneity was substantial for many of the outcomes (particularly those influenced by different hospital policies, participants' uterine size, fibroid type, experience of surgeons etc.). Thus, for many outcomes, pooled estimates could not be calculated and the forest plots display individual estimates for studies, because combining these was not sensible. Imprecision, with findings based on either small trials, low number of events, or with very wide confidence intervals, was also a characteristic of the findings of some outcomes, leading to uncertainties about benefits or harms.
As a result, for most of the primary outcomes reported in the 'Summary of findings' tables, the overall quality of the evidence (using GRADE criteria) was low, with some exceptions. This suggests there are some uncertainties associated with many of the findings in this review.
For summaries of the primary outcome results together with GRADE overall quality assessments, see Table 1; Table 2; Table 3; Table 4; Table 5; Table 6.
Potential biases in the review process
Efforts were made to retrieve all eligible studies by thorough searching of electronic databases and trials registers. However, the possibility remains that some unpublished studies were not retrieved. Rigorous processes were followed for selection of studies, data extraction and data entry, with efforts undertaken to access missing or unclear data from the publications to ensure accuracy of all data.
Agreements and disagreements with other studies or reviews
Three systematic reviews have evaluated the role of GnRHa preoperatively: before any surgery (Zhang 2014), before laparoscopic myomectomy (Chen 2011) and before hysteroscopic resection (for submucous fibroids) (Kamath 2014). One systematic review and network meta‐analysis (Gurusamy 2016) provided a more general analysis of the role of medical therapies for fibroids, both before surgery and when used alone. Two analyses have been undertaken to assess the economic effects of ulipristal acetate; one was a cost‐effectiveness analysis (Nagy 2014) and the other a cost minimisation and budget impact analysis (Zakiyah 2017).
The findings of this review broadly reflect the findings of the Zhang 2014 review which included 26 studies: these were that preoperative GnRHa reduces fibroid volume, increases haemoglobin levels, reduces the chance of a vertical incision, increases the chance of a vaginal procedure, without any increase in postoperative complications. In addition, our review also found a reduction in uterine volume before surgery, a reduction in blood loss (during either hysterectomy or myomectomy), a reduced chance of blood transfusions and postoperative complications (where surgery was hysterectomy). However, GnRHa was associated with an increased risk of adverse events before surgery (in particular, hot flushes). Reduction in intraoperative blood loss when surgery was restricted to laparoscopic myomectomy was also reported in the systematic review by Chen 2011.
The systematic review by Kamath 2014 included both trials from this review where women underwent hysteroscopic resection for submucous fibroids. Kamath 2014 concluded there was insufficient evidence of benefit to support the routine use of GnRHa before resection for this particular indication; this finding mirrors our conclusion.
The network meta‐analysis by Gurusamy 2016 ranked all potential medical treatments before any surgery, according to different outcomes. Gurusamy 2016 found that trials were at high risk of bias and overall quality of evidence was low. Gurusamy 2016 concluded that no medical treatment could currently be recommended before surgery for fibroids without consideration of the relative importance that women ascribe to adverse events and without consideration of cost‐effectiveness analyses.
A group of authors from Hungary (Nagy 2014) undertook a cost‐effectiveness analysis of ulipristal acetate using a Markov model and based estimates on the findings from Pearl I (Donnez 2012a) (a study included in the current review), together with a multicentre cohort study and estimates from an expert panel. Nagy 2014 found that adding three months of preoperative ulipristal acetate before surgery rather than immediate hysterectomy resulted in an incremental cost effectiveness ratio of EUR 3575 per quality‐adjusted life year in women with moderate to severe bleeding as a result of their fibroids. This finding was limited to effects on symptoms. Nagy 2014 did not assess the cost‐effectiveness of any other preoperative treatment. A cost minimisation analysis was undertaken to compare ulipristal acetate to leuprolide, which was considered the standard of care in the Netherlands (Zakiyah 2017). Zakiyah 2017 concluded that ulipristal acetate was a cost‐saving option for preoperative treatment of moderate and severe symptoms of fibroids compared to leuprolide in the short term, with the potential to provide savings on the healthcare budget in the Netherlands.
Authors' conclusions
Implications for practice.
One of the most frequently asked questions in daily practice is whether preoperative treatment actually makes fibroid surgery easier. There is clear evidence from randomised controlled trials (RCTs) that preoperative gonadotropin‐hormone releasing analogues (GnRHa) can reduce both uterine and fibroid volume and improve haemoglobin levels, although at the expense of increased adverse effects such as hot flushes, before surgery. Rates of vertical incision and blood loss are also reduced (in women undergoing hysterectomy or myomectomy) and women are more likely to have a vaginal procedure and less likely to have postoperative complications when undergoing hysterectomy. However, there is inadequate evidence to support the use of GnRHa for all women with fibroids undergoing hysterectomy or myomectomy. GnRHa could be considered for preoperative use in women with greatly enlarged uteri, preoperative anaemia or where a midline rather than transverse incision was planned. In addition, some women undergoing hysterectomy would benefit from a less invasive vaginal rather than an abdominal procedure. There was insufficient evidence of benefit for other patient or surgical outcomes, such as duration of surgery or hospital stay.
Recent RCTs suggest that ulipristal acetate, an SPRM, may offer another alternative pretreatment to enhance outcomes during and after surgery in women with fibroid‐related anaemia, although to date the evidence is based on only two RCTs (both which received pharmaceutical company funding). When compared to GnRHa, ulipristal acetate was not as effective at reducing uterine volume but both pretreatments appeared to have similar effects on other outcomes. These findings will need to be replicated before routine use can be confirmed, with clarification about which women will benefit.
Implications for research.
Although duration of surgery, total blood loss, postoperative haemoglobin and postoperative complications can be used as surrogates for surgical difficulty, few blinded placebo‐controlled data have conclusively measured this outcome and future trials should be both blinded and consider evaluating operative difficulty in a reproducible way.
Cost‐effectiveness data are lacking in all published trials, and given the very significant cost of preoperative agents, some attempt should be made to generate such data in future trials.
The question of whether the chances of fibroid recurrence and women's quality of life is increased after the use of preoperative medical therapy should also be evaluated further in future randomised trials.
What's new
| Date | Event | Description |
|---|---|---|
| 13 June 2017 | New search has been performed | Updated in 2017 ‐ 17 additional studies added (Baytur 2007; De Falco 2009; Donnez 2003; Donnez 2012a; Donnez 2012b; Engman 2009; Hudecek 2012; Levens 2008; Mavrelos 2010; Muneyyirci‐Delale 2007; Muzii 2010; Reinsch 1994; Sayyah‐Melli 2007; Sayyah‐Melli 2009; Seraccholi 2003; Vercellini 2003; Wilkens 2008). |
| 13 June 2017 | New citation required and conclusions have changed | Review scope changed. From inception, the review assessed only GnRHa as preoperative treatment. In 2017, the scope was broadened to include all preoperative treatment. |
History
Protocol first published: Issue 4, 1997 Review first published: Issue 4, 1998
| Date | Event | Description |
|---|---|---|
| 17 June 2008 | Amended | Converted to new review format. |
| 10 January 2001 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors acknowledge the helpful comments of those who refereed previous versions of this review. The authors are also very grateful for the translations of papers provided by Ms Christine Aguilar, San Antonio Cochrane Centre, Petr Tomek of Auckland University and Ms Kirsten Duckitt, Royal College of Obstetrics and Gynaecology, London, and to all trialists who provided additional material for this review: Dr A Cagnacci, Dr AJ Friedman, Kristof Chwalisz and Dr A Nikolov. Special thanks are due to Ms Ruth Jepson, Ms Sarah Hetrick and Ms Helen Nagels, Managing Editors, for their professionalism and help with the inevitable problems that arise, to Mrs Sue Furness and Mrs Marian Showell, Trials Search Coordinators, for their assistance with identifying trials and to Mrs Sue Hall, Secretary of the Review Group, for her secretarial help (Lethaby 2000; Lethaby 2001).
We thank Drs Amita Ray, Sujoy Ray, Aneesh George, Dr Martin Sowter, and Antonia Steed for their contributions to an early draft of the 2017 update of this review.
For the 2017 update, Marian Showell (Information Specialist) assisted with additional searches.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group specialised register search strategy
PROCITE platform
Searched from inception to 13 June 2017
Keywords CONTAINS "myomectomy" or "Hysterectomy" or "Hysterectomy,abdominal" or "hysterectomy, laparoscopically assisted vaginal" or "myoma" or "myomas" or "myomata" or "myomatous uterus" or "fibroids" or "Leiomyoma" or "leiomyomata" or "abdominal hysterectomy" or "abdominal myomectomy" or "laparoscopic" or "laparoscopic hysterectomy" or "laparoscopic myomectomy" or "uterine fibroids" or "uterine leiomyomas" or Title CONTAINS "myomectomy" or "Hysterectomy" or "Hysterectomy,abdominal" or "hysterectomy, laparoscopically assisted vaginal" or "myoma" or "myomas" or "myomata" or "myomatous uterus" or "fibroids" or "Leiomyoma" or "leiomyomata" or "abdominal hysterectomy" or "abdominal myomectomy" or "laparoscopic" or "laparoscopic hysterectomy" or "laparoscopic myomectomy" or "uterine fibroids" or" uterine leiomyomas"
AND
Keywords CONTAINS "Gonadorelin" or "GnRH analogue" or "GnRH analog" or "GnRh" or "GnRHa" or "GnRHa‐gonadotropin" or "gonadotrophin" or "gonadotropin" or "gonadotropin‐releasing hormone" or "Goserelin" or "Gosereline " or "Lh recombinant" or "LHRH" or "luteinizing hormone" or "Fsh" or "leuprolide " or "leuprorelin" or "leuprolin" or "Zoladex" or "Lupron" or "decapeptyl" or "goserelin pretreatment" or "Pretreatment" or Title CONTAINS "Gonadorelin" or "GnRH analogue" or "GnRH analog" or "GnRh" or "GnRHa" or "GnRHa‐gonadotropin" or "gonadotrophin" or "gonadotropin" or "gonadotropin‐releasing hormone" or "Goserelin" or "Gosereline " or "Lh recombinant" or "LHRH" or "luteinizing hormone" or "Fsh" or "leuprolide " or "leuprorelin" or "leuprolin" or "Zoladex" or "Lupron" or "decapeptyl" or "goserelin pretreatment" or "Pretreatment"
OR
Keywords CONTAINS "mifepristone" or "RU486" or "selective progesterone receptor modulator" or "CDB‐2914" or "asoprisnil" or "Ulipristal" or Title CONTAINS "mifepristone" or "RU486" or "selective progesterone receptor modulator" or "CDB‐2914" or "asoprisnil" or "Ulipristal"
OR
Keywords CONTAINS "progestagen" or "progesteron" or "Progesterone" or "progesterone, micronized" or "progestin" or "progestins" or "progestogen" or "progestogens" or "*Medrogestone" or "medroxyprogesterone" or "Medroxyprogesterone Acetate*"or "Depoprovera" or "depot medroxyprogesterone" or "depot medroxyprogesterone acetate" or "levonorgestrel intrauterine system" or "levonorgestrel‐releasing intrauterine device" or "levonorgestrel‐releasing intrauterine system" or "Levonorgestrel‐Therapeutic‐Use" or "LNG‐IUS" or "Mirena" or "norethindrone" or "noresthisterone" or "Norethisterone" or "Norgestimate" or "Norgestrel" or "desogestrel" or "desogestral" or "Gestagen" or "gestodene" or "Levonorgestrel" or Title CONTAINS "progestagen" or "progesteron" or "Progesterone" or "progesterone, micronized" or "progestin" or "progestins" or "progestogen" or "progestogens" or "*Medrogestone" or "medroxyprogesterone" or "Medroxyprogesterone Acetate*"or "Depoprovera" or "depot medroxyprogesterone"
OR
Keywords CONTAINS "androgen antagonists" or "androgens" or "danazol" or "gestrinone" or Title CONTAINS "androgen antagonists" or "androgens" or "danazol" or "gestrinone"
OR
Keywords CONTAINS "aromatase inhibition" or "aromatase inhibitor" or "letrozole" or "anastrozole" or "arimidex" or Keywords CONTAINS "aromatase inhibition" or "aromatase inhibitor" or "letrozole" or "anastrozole" or "arimidex"
(65 hits)
Appendix 2. Cochrane Register of Studies (CRS Online)
Web platform
Searched 13 June 2017
#1 MESH DESCRIPTOR Gonadotropin‐Releasing Hormone EXPLODE ALL TREES 2040
#2 (GnRH* or lhrh or gn‐rh or lfrh or lh‐rh or lhfshrh):TI,AB,KY 2933
#3 (Gonadotropin‐Releasing Hormone*):TI,AB,KY 1749
#4 (Gonadotrophin‐Releasing Hormone*):TI,AB,KY 362
#5 (luteini?ing hormone releasing):TI,AB,KY 365
#6 (fsh releasing hormone*):TI,AB,KY 1
#7 (gonadorelin or luliberin or luteinizing hormone‐releasing hormone or cystorelin):TI,AB,KY 1174
#8 (dirigestran or factrel or gonadoliberin):TI,AB,KY 5
#9 (buserelin or goserelin or leuprolide or nafarelin or triptorelin):TI,AB,KY 2324
#10 (luprorelin or Zoladex):TI,AB,KY 232
#11 (suprecur or suprefact):TI,AB,KY 9
#12 (lupron or prostap):TI,AB,KY 42
#13 (enantone or lucrin):TI,AB,KY 21
#14 (trenantone or synarel):TI,AB,KY 4
#15 (synarella or decapeptyl or gonapeptyl):TI,AB,KY 63
#16 Elagolix:TI,AB,KY 16
#17 (Pretreatment or pre treatment):TI,AB,KY 14757
#18 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 19202
#19 MESH DESCRIPTOR Receptors, Progesterone EXPLODE ALL TREES 398
#20 (selective progesterone receptor modulator*):TI,AB,KY 26
#21 SPRM*:TI,AB,KY 18
#22 MESH DESCRIPTOR Mifepristone EXPLODE ALL TREES 388
#23 Mifepristone:TI,AB,KY 746
#24 mifegyne:TI,AB,KY 0
#25 mifeprex:TI,AB,KY 0
#26 (r38486 or ru‐38486 or ru38486 or ru‐486 or ru486):TI,AB,KY 149
#27 Asoprisnil:TI,AB,KY 10
#28 J867:TI,AB,KY 1
#29 (Telapristone or Progenta):TI,AB,KY 2
#30 (Ulipristal or Ella):TI,AB,KY 62
#31 (Proellex or esmya or CDB‐4124):TI,AB,KY 4
#32 antiprogest*:TI,AB,KY 84
#33 (progesterone receptor antagonist*):TI,AB,KY 5
#34 CDB‐2914:TI,AB,KY 9
#35 #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 1229
#36 MESH DESCRIPTOR progesterone EXPLODE ALL TREES 2430
#37 MESH DESCRIPTOR Medroxyprogesterone Acetate EXPLODE ALL TREES 844
#38 (progesterone or medroxyprogesterone):TI,AB,KY 6203
#39 (progest?gen* or progestin*):TI,AB,KY 2047
#40 DPMA:TI,AB,KY 0
#41 (LNG‐IUS or IUS or mirena):TI,AB,KY 218
#42 (hormone‐releasing intrauterine system*):TI,AB,KY 0
#43 (Norethisterone or norethindrone or Utovlan*):TI,AB,KY 1083
#44 (ethynyltestosterone or progestational):TI,AB,KY 93
#45 (megestrol or Megace):TI,AB,KY 475
#46 Lynestrenol:TI,AB,KY 75
#47 (desogestrel or gestodene or norgestimate or dienogest):TI,AB,KY 862
#48 (drospirenone or levonorgestrel):TI,AB,KY 1454
#49 #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 9728
#50 (androgen* or danazol):TI,AB,KY 4702
#51 gestrinone:TI,AB,KY 63
#52 #50 OR #51 4738
#53 MESH DESCRIPTOR Aromatase Inhibitors EXPLODE ALL TREES 520
#54 (Aromatase Inhibitor*):TI,AB,KY 1194
#55 letrozole:TI,AB,KY 958
#56 (Anastrozole or Arimidex):TI,AB,KY 772
#57 #53 OR #54 OR #55 OR #56 2172
#58 #18 OR #35 OR #49 OR #52 OR #57 33021
#59 MESH DESCRIPTOR Leiomyoma EXPLODE ALL TREES 431
#60 MESH DESCRIPTOR Myoma EXPLODE ALL TREES 21
#61 MESH DESCRIPTOR Uterine Myomectomy EXPLODE ALL TREES 31
#62 (uter* adj2 fibroma*):TI,AB,KY 14
#63 (leiomyom* or Myomectom*):TI,AB,KY 854
#64 (myoma* or hysteromyom*):TI,AB,KY 769
#65 fibromyom*:TI,AB,KY 12
#66 fibroid*:TI,AB,KY 504
#67 #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 1471
#68 #58 AND #67 472
Appendix 3. MEDLINE (Ovid) search strategy
Ovid platform
From 1946 to 13 June 2017
1 exp Gonadotropin‐Releasing Hormone/ (31036) 2 (GnRH$ or lhrh or gn‐rh or lfrh or lh‐rh or lhfshrh).tw. (30505) 3 Gonadotropin‐Releasing Hormone$.tw. (12767) 4 Gonadotrophin‐Releasing Hormone$.tw. (2797) 5 luteini?ing hormone releasing.tw. (6086) 6 fsh releasing hormone$.tw. (56) 7 (gonadorelin or luliberin or luteinizing hormone‐releasing hormone or cystorelin).tw. (5775) 8 (dirigestran or factrel or gonadoliberin).tw. (166) 9 (buserelin or goserelin or leuprolide or nafarelin or triptorelin).tw. (4654) 10 (luprorelin or Zoladex).tw. (390) 11 (suprecur or suprefact).tw. (31) 12 (lupron or prostap).tw. (173) 13 (enantone or lucrin).tw. (35) 14 (trenantone or synarel).tw. (16) 15 (synarella or decapeptyl or gonapeptyl).tw. (221) 16 Elagolix.tw. (16) 17 (Pretreatment or pre treatment).tw. (186609) 18 or/1‐17 (229012) 19 exp Receptors, Progesterone/ (17898) 20 selective progesterone receptor modulator$.tw. (194) 21 SPRM$.tw. (134) 22 exp Mifepristone/ (5723) 23 Mifepristone.tw. (3214) 24 mifegyne.tw. (14) 25 mifeprex.tw. (13) 26 r38486.tw. (1) 27 ru‐38486.tw. (440) 28 ru38486.tw. (373) 29 ru‐486.tw. (1688) 30 ru486.tw. (2153) 31 Asoprisnil.tw. (48) 32 J867.tw. (13) 33 Telapristone.tw. (12) 34 Progenta.tw. (1) 35 Ulipristal.tw. (273) 36 Ella.tw. (273) 37 Proellex.tw. (8) 38 CDB‐4124.tw. (19) 39 esmya.tw. (8) 40 antiprogest$.tw. (1454) 41 progesterone receptor antagonist$.tw. (314) 42 CDB‐2914.tw. (45) 43 or/19‐42 (25818) 44 exp progesterone/ or exp medroxyprogesterone acetate/ (67942) 45 progesterone.tw. (77003) 46 medroxyprogesterone.tw. (5938) 47 progest?gen$.tw. (7276) 48 progestin$.tw. (11065) 49 DPMA.tw. (103) 50 LNG‐IUS.tw. (607) 51 hormone‐releasing intrauterine system$.tw. (12) 52 IUS.tw. (977) 53 mirena.tw. (259) 54 (Norethisterone or norethindrone or Utovlan$).tw. (3196) 55 ethynyltestosterone.tw. (16) 56 progestational.tw. (1725) 57 (megestrol or Megace).tw. (1415) 58 Lynestrenol.tw. (397) 59 (desogestrel or gestodene or norgestimate or dienogest).tw. (2006) 60 drospirenone.tw. (668) 61 levonorgestrel.tw. (4218) 62 or/44‐61 (123508) 63 androgen$.tw. (71781) 64 danazol.tw. (2418) 65 gestrinone.tw. (184) 66 or/63‐65 (73988) 67 exp Aromatase Inhibitors/ (6919) 68 Aromatase Inhibitor$.tw. (6479) 69 letrozole.tw. (2386) 70 (Anastrozole or Arimidex).tw. (1703) 71 or/67‐70 (10651) 72 fibroid$.tw. (5519) 73 exp Leiomyoma/ or exp Uterine Myomectomy/ (19974) 74 (uter$ adj2 fibroma$).tw. (325) 75 exp Uterine Neoplasms/ and fibroid$.tw. (2683) 76 (leiomyom$ or Myomectom$).tw. (15100) 77 exp Myoma/ (2714) 78 myoma$.tw. (5539) 79 hysteromyom$.tw. (73) 80 fibromyom$.tw. (720) 81 or/72‐80 (30096) 82 18 or 43 or 62 or 66 or 71 (421080) 83 81 and 82 (2617) 84 randomized controlled trial.pt. (465934) 85 controlled clinical trial.pt. (94208) 86 randomized.ab. (408090) 87 randomised.ab. (79890) 88 placebo.tw. (195471) 89 clinical trials as topic.sh. (186853) 90 randomly.ab. (283087) 91 trial.ti. (182982) 92 (crossover or cross‐over or cross over).tw. (75700) 93 or/84‐92 (1199535) 94 exp animals/ not humans.sh. (4417040) 95 93 not 94 (1106420) 96 83 and 95 (360)
Appendix 4. Embase (Ovid) search strategy
Ovid platform
From 1980 to 13 June 2017
1 exp gonadorelin derivative/ or gonadorelin/ or gonadorelin agonist/ (61481) 2 (GnRH or lhrh or gn‐rh or lfrh or lh‐rh or lhfshrh).tw. (34042) 3 Gonadotropin‐Releasing.tw. (13875) 4 (gonadorelin or luliberin or luteinizing hormone‐releasing hormone or cystorelin).tw. (5383) 5 (dirigestran or factrel or gonadoliberin).tw. (272) 6 (buserelin or goserelin or leuprolide or nafarelin or triptorelin).tw. (6225) 7 fsh releasing hormone$.tw. (32) 8 Gonadotrophin‐Releasing Hormone$.tw. (2940) 9 (luprorelin or Zoladex).tw. (2061) 10 (suprecur or suprefact).tw. (1248) 11 (lupron or prostap).tw. (1825) 12 (enantone or lucrin).tw. (676) 13 (trenantone or synarel).tw. (375) 14 (synarella or decapeptyl or gonapeptyl).tw. (1834) 15 Elagolix.tw. (39) 16 (Pretreatment or pre treatment).tw. (219650) 17 or/1‐16 (286994) 18 exp progesterone receptor modulator/ (489) 19 selective progesterone receptor modulator$.tw. (303) 20 SPRM$.tw. (203) 21 exp mifepristone/ (11582) 22 Mifepristone.tw. (3995) 23 mifegyne.tw. (186) 24 mifeprex.tw. (109) 25 r38486.tw. (1) 26 ru38486.tw. (407) 27 ru‐38486.tw. (912) 28 ru‐486.tw. (4162) 29 ru486.tw. (2533) 30 Asoprisnil.tw. (65) 31 J867.tw. (14) 32 Telapristone.tw. (18) 33 Progenta.tw. (4) 34 Ulipristal.tw. (528) 35 Ella.tw. (409) 36 Proellex.tw. (24) 37 CDB‐4124.tw. (51) 38 esmya.tw. (59) 39 antiprogest$.tw. (1524) 40 progesterone receptor antagonist$.tw. (356) 41 CDB‐2914.tw. (108) 42 or/18‐41 (13961) 43 exp progeria/ or exp progesterone/ or exp gestagen/ (150242) 44 medroxyprogesterone acetate.m_titl. (1912) 45 exp medroxyprogesterone acetate/ or exp injectable contraceptive agent/ (16457) 46 progesterone.tw. (82490) 47 medroxyprogesterone.tw. (6375) 48 progest?gen$.tw. (7481) 49 progestin$.tw. (11861) 50 DPMA.tw. (103) 51 LNG‐IUS.tw. (902) 52 hormone‐releasing intrauterine system$.tw. (17) 53 IUS.tw. (1621) 54 mirena.tw. (1384) 55 exp levonorgestrel/ (10556) 56 (Norethisterone or norethindrone or Utovlan$).tw. (2908) 57 ethynyltestosterone.tw. (10) 58 progestational.tw. (1315) 59 (megestrol or Megace).tw. (1979) 60 Lynestrenol.tw. (222) 61 (desogestrel or gestodene or norgestimate or dienogest).tw. (2453) 62 drospirenone.tw. (1003) 63 levonorgestrel.tw. (5052) 64 or/43‐63 (184895) 65 androgen$.tw. (84806) 66 danazol.tw. (2937) 67 gestrinone.tw. (206) 68 or/65‐67 (87486) 69 exp aromatase inhibitor/ (26304) 70 Aromatase Inhibitor$.tw. (9432) 71 letrozole.tw. (3883) 72 (Anastrozole or Arimidex).tw. (3615) 73 or/69‐72 (27258) 74 17 or 42 or 64 or 68 or 73 (547758) 75 fibroid$.tw. (8679) 76 exp leiomyoma/ or exp myomectomy/ (21166) 77 (uter$ adj2 fibroma$).tw. (306) 78 (leiomyom$ or Myomectom$).tw. (18913) 79 exp uterus myoma/ (12963) 80 myoma$.tw. (7019) 81 hysteromyom$.tw. (147) 82 or/75‐81 (38057) 83 74 and 82 (4742) 84 Clinical Trial/ (923008) 85 Randomized Controlled Trial/ (451676) 86 exp randomization/ (74101) 87 Single Blind Procedure/ (27406) 88 Double Blind Procedure/ (136615) 89 Crossover Procedure/ (51583) 90 Placebo/ (293634) 91 Randomi?ed controlled trial$.tw. (159829) 92 Rct.tw. (24361) 93 random allocation.tw. (1640) 94 randomly.tw. (348476) 95 randomly allocated.tw. (27408) 96 allocated randomly.tw. (2234) 97 (allocated adj2 random).tw. (779) 98 Single blind$.tw. (19161) 99 Double blind$.tw. (171699) 100 ((treble or triple) adj blind$).tw. (683) 101 placebo$.tw. (249662) 102 prospective study/ (382418) 103 or/84‐102 (1940548) 104 case study/ (47724) 105 case report.tw. (329915) 106 abstract report/ or letter/ (997401) 107 or/104‐106 (1367149) 108 103 not 107 (1895249) 109 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5743778) 110 108 not 109 (1765108) 111 83 and 110 (1030)
Appendix 5. PsycINFO (Ovid) search strategy
Ovid platform
From 1806 to 13 June 2017
1 exp Gonadotropic Hormones/ (3880) 2 (GnRH$ or lhrh or gn‐rh or lfrh or lh‐rh or lhfshrh).tw. (995) 3 Gonadotropin‐Releasing Hormone$.tw. (603) 4 Gonadotrophin‐Releasing Hormone$.tw. (200) 5 luteini?ing hormone releasing.tw. (207) 6 fsh releasing hormone$.tw. (1) 7 (gonadorelin or luliberin or luteinizing hormone‐releasing hormone or cystorelin).tw. (197) 8 (dirigestran or factrel or gonadoliberin).tw. (1) 9 (buserelin or goserelin or leuprolide or nafarelin or triptorelin).tw. (122) 10 (luprorelin or Zoladex).tw. (4) 11 (suprecur or suprefact).tw. (0) 12 (lupron or prostap).tw. (16) 13 (enantone or lucrin).tw. (2) 14 (trenantone or synarel).tw. (0) 15 (synarella or decapeptyl or gonapeptyl).tw. (2) 16 Elagolix.tw. (0) 17 (Pretreatment or pre treatment).tw. (15577) 18 or/1‐17 (19869) 19 selective progesterone receptor modulator$.tw. (1) 20 SPRM$.tw. (3) 21 Mifepristone.tw. (203) 22 Mifegyne.tw. (0) 23 mifeprex.tw. (1) 24 r38486.tw. (0) 25 ru‐38486.tw. (41) 26 ru‐486.tw. (83) 27 Ulipristal.tw. (3) 28 CDB‐4124.tw. (2) 29 or/19‐28 (308) 30 exp progesterone/ (1931) 31 medroxyprogesterone.tw. (263) 32 progesterone.tw. (3621) 33 progest?gen$.tw. (185) 34 progestin$.tw. (553) 35 levonorgestrel‐releasing intrauterine.tw. (15) 36 DPMA.tw. (6) 37 LNG‐IUS.tw. (16) 38 hormone‐releasing intrauterine system$.tw. (0) 39 IUS.tw. (96) 40 mirena.tw. (9) 41 or/30‐40 (4366) 42 18 or 29 or 41 (24055) 43 fibroid$.tw. (50) 44 Leiomyoma$.tw. (14) 45 exp Gynecological Disorders/ and fibroid$.tw. (11) 46 (uter$ adj2 fibroma$).tw. (4) 47 myoma$.tw. (23) 48 hysteromyom$.tw. (2) 49 fibromyom$.tw. (1) 50 or/43‐49 (89) 51 42 and 50 (4)
Appendix 6. CINAHL (EBSCO) search strategy
EBSCO platform
From 1961 to 13 June 2017
| # | Query | Results |
| S75 | S62 AND S74 | 75 |
| S74 | S63 OR S64 OR S65 OR S66 OR S67 OR S68 OR S69 OR S70 OR S71 OR S72 OR S73 | 1,139,541 |
| S73 | TX allocat* random* | 6,922 |
| S72 | (MH "Quantitative Studies") | 15,879 |
| S71 | (MH "Placebos") | 10,179 |
| S70 | TX placebo* | 46,226 |
| S69 | TX random* allocat* | 6,922 |
| S68 | (MH "Random Assignment") | 43,219 |
| S67 | TX randomi* control* trial* | 126,136 |
| S66 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 892,196 |
| S65 | TX clinic* n1 trial* | 206,835 |
| S64 | PT Clinical trial | 80,034 |
| S63 | (MH "Clinical Trials+") | 215,251 |
| S62 | S54 AND S61 | 279 |
| S61 | S55 OR S56 OR S57 OR S58 OR S59 OR S60 | 3,506 |
| S60 | TX hysteromyom* | 5 |
| S59 | TX myoma* | 498 |
| S58 | TX fibroid* | 1,291 |
| S57 | TX (uter* N2 fibroma*) | 8 |
| S56 | TX Leiomyoma* | 2,862 |
| S55 | (MM "Leiomyoma") | 1,969 |
| S54 | S12 OR S26 OR S44 OR S48 OR S53 | 26,298 |
| S53 | S49 OR S50 OR S51 OR S52 | 2,396 |
| S52 | TX (Anastrozole or Arimidex) | 557 |
| S51 | TX letrozole | 539 |
| S50 | TX Aromatase Inhibitor* | 1,976 |
| S49 | (MH "Aromatase Inhibitors+") | 1,487 |
| S48 | S45 OR S46 OR S47 | 203 |
| S47 | TX gestrinone | 18 |
| S46 | TX danazol | 193 |
| S45 | (MM "Danazol") | 48 |
| S44 | S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 | 7,852 |
| S43 | TX levonorgestrel | 1,441 |
| S42 | TX drospirenone | 213 |
| S41 | TX(desogestrel or gestodene or norgestimate or dienogest) | 206 |
| S40 | TX Lynestrenol | 4 |
| S39 | TX (megestrol or Megace) | 278 |
| S38 | TX progestational | 1,908 |
| S37 | TX (Norethisterone or norethindrone or Utovlan*) | 202 |
| S36 | TX mirena | 100 |
| S35 | TX hormone‐releasing intrauterine | 8 |
| S34 | TX LNG‐IUS | 130 |
| S33 | TX DPMA | 5 |
| S32 | TX progestin* | 1,253 |
| S31 | TX progest?gen* | 609 |
| S30 | TX medroxyprogesterone | 1,396 |
| S29 | (MM "Medroxyprogesterone Acetate") | 316 |
| S28 | (MH "Progestational Hormones, Synthetic+") | 1,626 |
| S27 | (MH "Progestational Hormones+") OR (MM "Megestrol") | 3,967 |
| S26 | S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 | 1,067 |
| S25 | TX CDB‐4124 | 2 |
| S24 | TX Ulipristal | 117 |
| S23 | TX Telapristone | 3 |
| S22 | TX J867 | 1 |
| S21 | TX Asoprisnil | 5 |
| S20 | TX ru‐486 | 172 |
| S19 | TX ru‐38486 | 10 |
| S18 | TX ru38486 | 10 |
| S17 | TX mifeprex | 21 |
| S16 | TX Mifepristone | 889 |
| S15 | (MM "Mifepristone") | 411 |
| S14 | TX SPRM* | 12 |
| S13 | TX selective progesterone receptor modulator* | 29 |
| S12 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 | 15,556 |
| S11 | TX (Pretreatment or pre treatment) | 13,636 |
| S10 | TX (suprecur or suprefact) | 2 |
| S9 | TX (luprorelin or Zoladex) | 23 |
| S8 | TX(buserelin or goserelin or leuprolide or nafarelin or triptorelin) | 639 |
| S7 | TX(gonadorelin or luliberin or cystorelin) | 957 |
| S6 | TX fsh releasing hormone* | 2 |
| S5 | TX luteini?ing hormone releasing | 186 |
| S4 | TX(GnRH* or lhrh or gn‐rh or lfrh or lh‐rh or lhfshrh) | 681 |
| S3 | TX gonadotrophin releasing hormone* | 96 |
| S2 | TX gonadotropin‐releasing hormone* | 527 |
| S1 | (MH "Gonadorelin+") | 1,328 |
Data and analyses
Comparison 1. GnRHa treatment versus placebo or no pretreatment (preoperative outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine volume (mL) (preoperative) | 13 | 858 | Mean Difference (IV, Random, 95% CI) | ‐175.34 [‐219.04, ‐131.65] |
| 1.1 GnRHa vs. no pretreatment | 11 | 810 | Mean Difference (IV, Random, 95% CI) | ‐178.68 [‐224.63, ‐132.74] |
| 1.2 GnRHa vs. placebo | 2 | 48 | Mean Difference (IV, Random, 95% CI) | ‐113.76 [‐314.60, 87.08] |
| 2 Uterine volume (preop in data table) | Other data | No numeric data | ||
| 2.1 GnRHa vs. no pretreatment | Other data | No numeric data | ||
| 2.2 GnRHa vs. placebo | Other data | No numeric data | ||
| 3 Fibroid volume (mL) (preoperative) | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 GnRHa vs. no pretreatment | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 GnRHa vs. placebo | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Fibroid volume (preop in data table) | Other data | No numeric data | ||
| 4.1 GnRHa vs. placebo | Other data | No numeric data | ||
| 5 Haemoglobin (preoperative) | 10 | 834 | Mean Difference (IV, Random, 95% CI) | 0.88 [0.68, 1.08] |
| 5.1 GnRHa vs. no pretreatment | 6 | 308 | Mean Difference (IV, Random, 95% CI) | 0.91 [0.52, 1.30] |
| 5.2 GnRHa vs. placebo | 4 | 526 | Mean Difference (IV, Random, 95% CI) | 0.87 [0.62, 1.13] |
| 6 Haemoglobin (preop in data table) | Other data | No numeric data | ||
| 6.1 GnRHa vs. placebo | Other data | No numeric data | ||
| 7 Total frequency of adverse events | 4 | 755 | Odds Ratio (M‐H, Random, 95% CI) | 2.78 [1.77, 4.36] |
| 7.1 GnRHa vs. no pretreatment | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 GnRHa vs. placebo | 4 | 755 | Odds Ratio (M‐H, Random, 95% CI) | 2.78 [1.77, 4.36] |
| 8 Individual adverse events | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Insomnia | 1 | 110 | Odds Ratio (M‐H, Random, 95% CI) | 12.56 [0.68, 232.82] |
| 8.2 Hot flushes | 6 | 877 | Odds Ratio (M‐H, Random, 95% CI) | 7.68 [4.55, 12.96] |
| 8.3 Headache | 6 | 877 | Odds Ratio (M‐H, Random, 95% CI) | 1.74 [1.00, 3.03] |
| 8.4 Pain | 2 | 505 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.36, 1.12] |
| 8.5 Nausea | 2 | 505 | Odds Ratio (M‐H, Random, 95% CI) | 2.41 [0.14, 40.59] |
| 8.6 Dizziness | 2 | 505 | Odds Ratio (M‐H, Random, 95% CI) | 2.41 [1.13, 5.14] |
| 8.7 Depression | 2 | 505 | Odds Ratio (M‐H, Random, 95% CI) | 2.12 [0.87, 5.17] |
| 8.8 Arthralgia | 2 | 505 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [0.55, 3.02] |
| 8.9 Asthenia | 3 | 615 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.41, 2.39] |
| 8.10 Vaginitis | 5 | 751 | Odds Ratio (M‐H, Random, 95% CI) | 4.18 [1.58, 11.05] |
| 8.11 Abdominal/pelvic pain | 3 | 615 | Odds Ratio (M‐H, Random, 95% CI) | 2.43 [0.98, 6.05] |
| 8.12 Skin changes | 2 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.48, 3.13] |
| 8.13 Hirsutism | 1 | 65 | Odds Ratio (M‐H, Random, 95% CI) | 6.35 [0.70, 57.72] |
| 8.14 Change in libido | 3 | 332 | Odds Ratio (M‐H, Random, 95% CI) | 1.84 [0.76, 4.46] |
| 8.15 Change in breast size | 2 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 10.87 [1.90, 62.24] |
| 8.16 Sweating | 4 | 497 | Odds Ratio (M‐H, Random, 95% CI) | 14.32 [6.17, 33.27] |
| 8.17 Breast pain/tenderness | 2 | 505 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.35, 1.52] |
| 8.18 Uterine haemorrhage | 1 | 110 | Odds Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.78] |
1.1. Analysis.
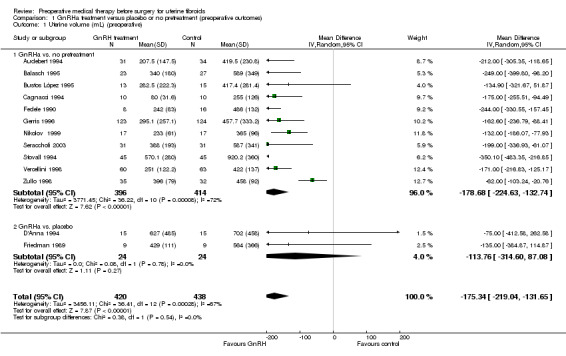
Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 1 Uterine volume (mL) (preoperative).
1.5. Analysis.
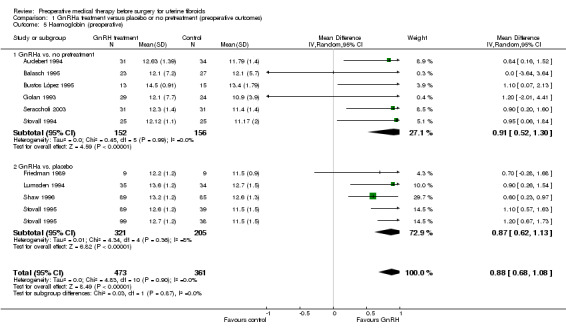
Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 5 Haemoglobin (preoperative).
1.6. Analysis.
Comparison 1 GnRHa treatment versus placebo or no pretreatment (preoperative outcomes), Outcome 6 Haemoglobin (preop in data table).
| Haemoglobin (preop in data table) | ||||
|---|---|---|---|---|
| Study | Number in study | Comparison | Results | Comment |
| GnRHa vs. placebo | ||||
| Muneyyirci‐Delale 2007 | 110 | GnRHa (goserelin) + iron vs sham injection + iron | Difference of least squares mean: 1.17 g/dL (95% CI 0.7 to 1.7), p<0.001 |
Significantly higher in goserelin group |
Comparison 2. GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of surgery (minutes) | 6 | 617 | Mean Difference (IV, Random, 95% CI) | ‐10.11 [‐16.96, ‐3.25] |
| 1.1 GnRHa vs. no pretreatment | 4 | 321 | Mean Difference (IV, Random, 95% CI) | ‐14.19 [‐25.01, ‐3.38] |
| 1.2 GnRHa vs. placebo | 2 | 296 | Mean Difference (IV, Random, 95% CI) | ‐5.03 [‐12.17, 2.12] |
| 2 Duration of surgery (data table) | Other data | No numeric data | ||
| 2.1 GnRHa vs. no pretreatment | Other data | No numeric data | ||
| 2.2 GnRHa vs. placebo | Other data | No numeric data | ||
| 3 Intraoperative blood loss (mL) | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 GnRHa vs. no pretreatment | 4 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Intraoperative blood loss (data table) | Other data | No numeric data | ||
| 4.1 GnRHa vs. no pretreatment | Other data | No numeric data | ||
| 4.2 GnRHa vs. placebo | Other data | No numeric data | ||
| 5 Proportion with blood transfusions | 6 | 601 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.29, 1.01] |
| 5.1 GnRHa vs. no pretreatment | 5 | 487 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.22, 1.08] |
| 5.2 GnRHa vs. placebo | 1 | 114 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.23, 1.78] |
| 6 Proportion with postoperative complications | 7 | 772 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.32, 0.91] |
| 6.1 GnRHa vs. no treatment | 5 | 507 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.29, 1.23] |
| 6.2 GnRHa vs. placebo | 2 | 265 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.19, 1.11] |
| 7 Proportion with individual complications | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Hypermenorrhoea | 1 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.11, 1.24] |
| 7.2 Dysmenorrhoea | 1 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 3.90 [0.19, 82.29] |
| 7.3 Pelvic pain | 1 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.15, 1.23] |
| 7.4 Difficult defecation | 1 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.11, 5.52] |
| 7.5 Difficult urination | 1 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 0.15 [0.01, 3.17] |
| 7.6 Dyspareunia | 1 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 3.90 [0.19, 82.29] |
| 8 Proportion with difficult surgery | 5 | 712 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.51, 1.00] |
| 8.1 GnRH vs. no pretreatment | 2 | 347 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.54, 1.44] |
| 8.2 GnRH vs. placebo | 3 | 365 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.38, 0.94] |
| 9 Proportion undergoing vaginal rather than abdominal procedure | 3 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 GnRHa vs. no treatment | 2 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 GnRHa vs. placebo | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Proportion with vertical incision | 4 | 529 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.21, 0.54] |
| 10.1 GnRHa vs. no pretreatment | 2 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.18, 0.59] |
| 10.2 GnRHa vs. placebo | 2 | 228 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.17, 0.75] |
| 11 Duration of hospital stay (days) | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.1 GnRHa vs. no pretreatment | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 GnRHa vs. placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Duration of hospital stay (data table) | Other data | No numeric data | ||
| 12.1 GnRHa vs. no pretreatment | Other data | No numeric data | ||
| 13 Postoperative haemoglobin | 3 | 240 | Mean Difference (IV, Random, 95% CI) | 0.85 [0.31, 1.38] |
| 13.1 GnRHa vs. no pretreatment | 2 | 173 | Mean Difference (IV, Random, 95% CI) | 1.05 [0.39, 1.71] |
| 13.2 GnRHa vs. placebo | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.35, 1.15] |
2.1. Analysis.
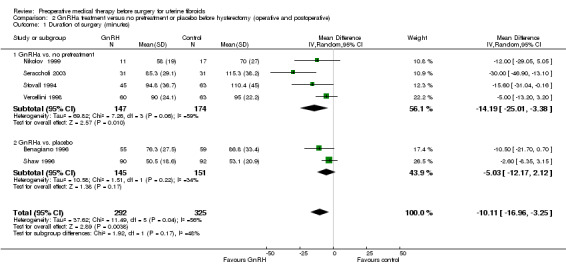
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 1 Duration of surgery (minutes).
2.2. Analysis.
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 2 Duration of surgery (data table).
| Duration of surgery (data table) | ||||
|---|---|---|---|---|
| Study | Number in study | Comparison | Results | Comment |
| GnRHa vs. no pretreatment | ||||
| Balasch 1995 | 50 | GnRHa (triptorelin) vs no preRx | GnRHa vs no preRx: mean (SD) 110 mins (81.5) vs 107 mins (166.3) (NS) |
Authors reported that there was no evidence of a significant difference between groups Data reported in table format as it appears skewed |
| Golan 1993 | 32 | GnRHa (triptorelin) vs no preRx | GnRHa vs no preRx: mean (SD): 49 (37.1) vs 70 (131.7) (p<0.05) |
Authors reported a significant difference between groups Data reported in table format as it appears skewed |
| GnRHa vs. placebo | ||||
| Lumsden 1994 | 71 | GnRHa (goserelin) vs placebo | GnRHa vs placebo: mean (SD): 61 mins (16) vs 68 mins (208) (NS) |
The authors reported that there was no evidence of a significant difference between groups Data reported in table format as it appears skewed |
2.3. Analysis.
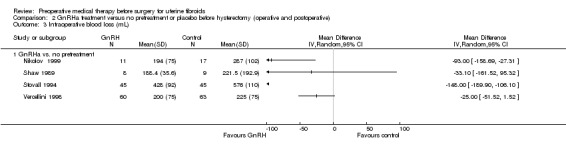
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 3 Intraoperative blood loss (mL).
2.4. Analysis.
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 4 Intraoperative blood loss (data table).
| Intraoperative blood loss (data table) | ||||
|---|---|---|---|---|
| Study | Number in study | Comparison | Results | Comment |
| GnRHa vs. no pretreatment | ||||
| Golan 1993 | 32 | GnRHa (triptorelin) vs no preRx | GnRHa vs no preRx: mean (SD) 208 (263.9) vs 309 (313.7) p<0.05 |
Authors reported a significant difference between groups Data reported in table format as it appears skewed |
| GnRHa vs. placebo | ||||
| Lumsden 1994 | 69 | GnRHa (goserelin) vs placebo | GnRHa vs placebo: median (range): 187 mls (60 to 600) vs 307.5 (118 to 1000) p<0.05 |
Authors reported a significant difference between groups Data reported in table format as it appears skewed |
2.5. Analysis.
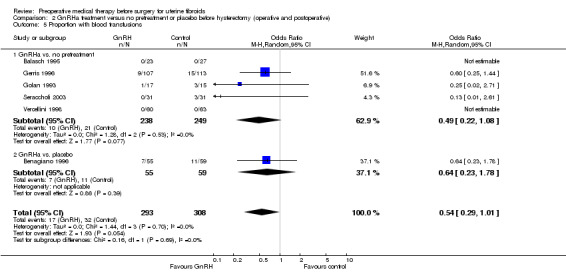
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 5 Proportion with blood transfusions.
2.6. Analysis.
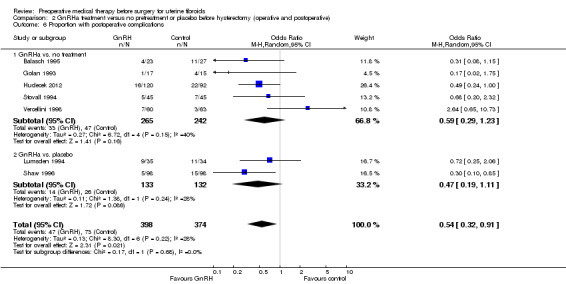
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 6 Proportion with postoperative complications.
2.8. Analysis.
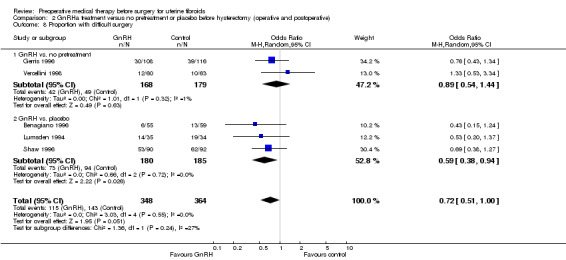
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 8 Proportion with difficult surgery.
2.9. Analysis.
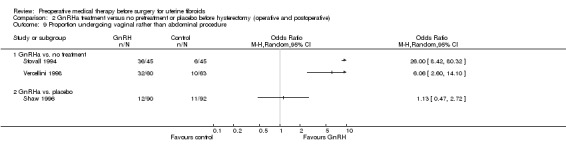
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 9 Proportion undergoing vaginal rather than abdominal procedure.
2.10. Analysis.
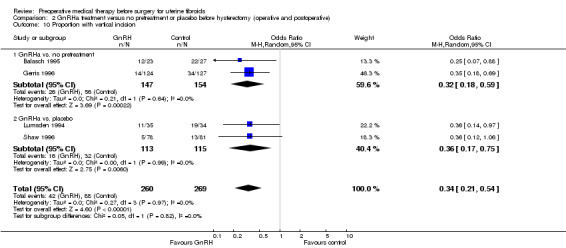
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 10 Proportion with vertical incision.
2.11. Analysis.
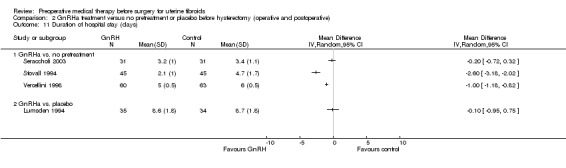
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 11 Duration of hospital stay (days).
2.12. Analysis.
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 12 Duration of hospital stay (data table).
| Duration of hospital stay (data table) | ||||
|---|---|---|---|---|
| Study | Number in study | Comparison | Results | Comment |
| GnRHa vs. no pretreatment | ||||
| Balasch 1995 | 50 | GnRHa (triptorelin) vs no preRx | GnRHa vs no preRx: mean (SD): 7.2 (2.9) vs 8.6 (18.7) (NS) |
Authors reported that there was no evidence of a significant difference between groups Data reported in table format as it appears skewed |
2.13. Analysis.
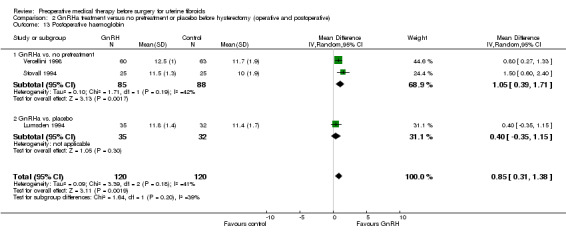
Comparison 2 GnRHa treatment versus no pretreatment or placebo before hysterectomy (operative and postoperative), Outcome 13 Postoperative haemoglobin.
Comparison 3. GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of surgery (minutes) | 6 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 GnRHa vs. no pretreatment | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 GnRHa vs. placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Duration of surgery (descriptive data) | Other data | No numeric data | ||
| 2.1 GnRHa vs. no pretreatment | Other data | No numeric data | ||
| 3 Intraoperative blood loss (mL) | 10 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 GnRHa vs. no pretreatment | 9 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 GnRHa vs. placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Proportion with blood transfusions | 4 | 121 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.26, 2.75] |
| 4.1 GnRHa vs. no pretreatment | 3 | 103 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.20, 3.19] |
| 4.2 GnRHa vs. placebo | 1 | 18 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.11, 9.23] |
| 5 Proportion with postoperative complications | 5 | 190 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.43, 2.64] |
| 5.1 GnRHa vs. no pretreatment | 4 | 172 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.44, 3.54] |
| 5.2 GnRHa vs. placebo | 1 | 18 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.10, 4.11] |
| 6 Proportion with vertical incision | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.43] |
| 6.1 GnRHa vs. no pretreatment | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.43] |
| 6.2 GnRHa vs. placebo | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Duration of hospital stay (days) | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 GnRHa vs. no pretreatment | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 GnRHa vs. placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Proportion with postoperative recurrence of myomas | 2 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 4.16 [0.59, 29.09] |
| 8.1 GnRHa vs. no pretreatment | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 11.67 [1.49, 91.54] |
| 8.2 GnRHa vs. placebo | 1 | 18 | Odds Ratio (M‐H, Random, 95% CI) | 1.6 [0.24, 10.81] |
| 9 Postoperative haemoglobin | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.80 [0.22, 1.38] |
| 9.1 GnRHa vs. no pretreatment | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.80 [0.22, 1.38] |
| 9.2 GnRHa vs. placebo | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
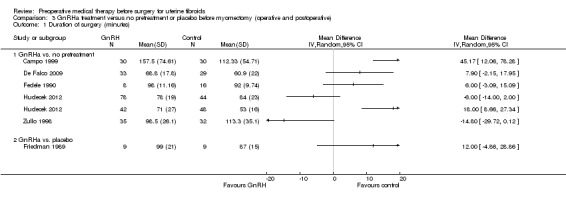
Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 1 Duration of surgery (minutes).
3.2. Analysis.
Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 2 Duration of surgery (descriptive data).
| Duration of surgery (descriptive data) | ||||
|---|---|---|---|---|
| Study | Number of participants | Comparison | Results | Comment |
| GnRHa vs. no pretreatment | ||||
| Cetin 1995 | 30 | GnRHa (buserelin 900 ugr/day for 3 months) vs no pretreatment (immediate surgery) | GnRHa (mean (SD)): 87 mins (174.3) Control (mean (SD)): 102 mins (135.6) |
Authors reported that the difference was not statistically significant, p>0.05 |
| Golan 1993 | 21 | GnRHa (decapeptyl 3.2 mg for 3 months) vs no pretreatment | GnRHa (mean (SD)): 80 mins (145.5 Control (mean (SD)): 96 mins (138.0) |
Authors reported that the difference was not statistically significant |
3.3. Analysis.
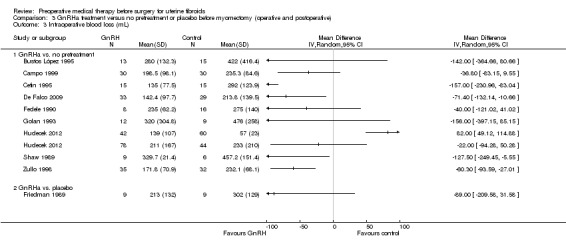
Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 3 Intraoperative blood loss (mL).
3.4. Analysis.
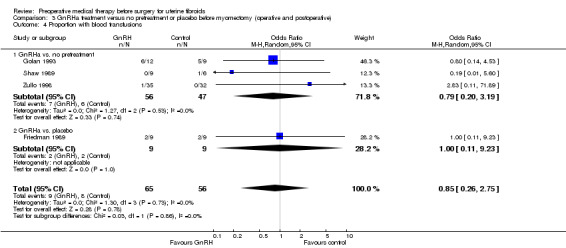
Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 4 Proportion with blood transfusions.
3.5. Analysis.
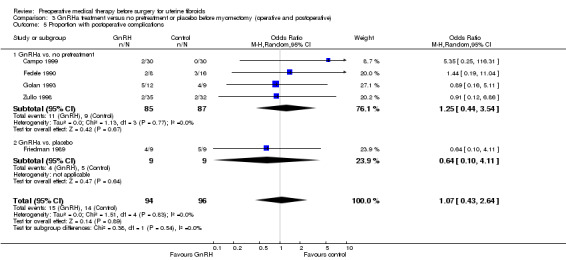
Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 5 Proportion with postoperative complications.
3.6. Analysis.
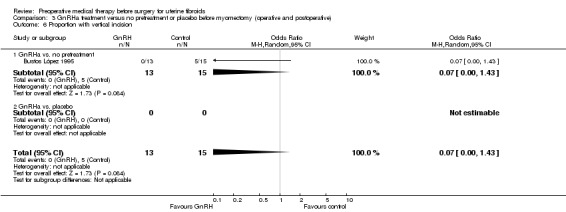
Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 6 Proportion with vertical incision.
3.7. Analysis.
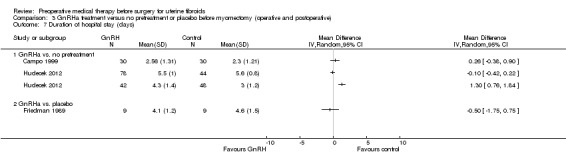
Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 7 Duration of hospital stay (days).
3.8. Analysis.
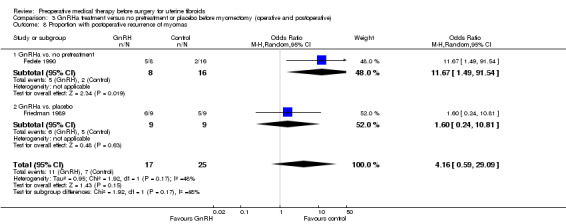
Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 8 Proportion with postoperative recurrence of myomas.
3.9. Analysis.
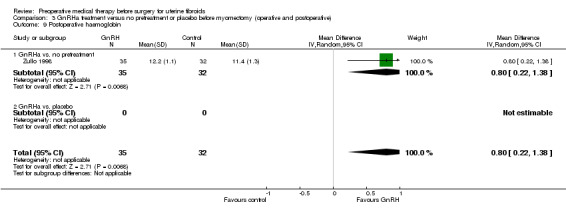
Comparison 3 GnRHa treatment versus no pretreatment or placebo before myomectomy (operative and postoperative), Outcome 9 Postoperative haemoglobin.
Comparison 4. GnRHa treatment versus no pretreatment or placebo prior to resection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of surgery (minutes) | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐5.4 [‐7.65, ‐3.15] |
| 2 Difficulty of surgery (VAS) | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐3.05, 0.25] |
| 3 Fibroid recurrence | 1 | 39 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.
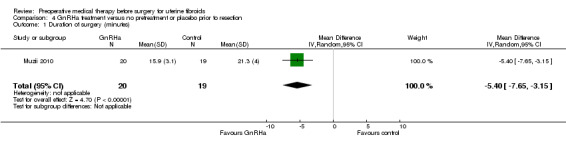
Comparison 4 GnRHa treatment versus no pretreatment or placebo prior to resection, Outcome 1 Duration of surgery (minutes).
4.2. Analysis.
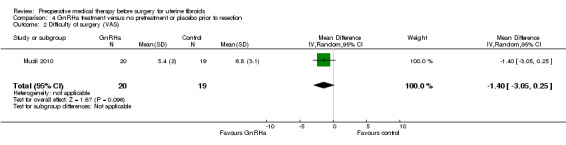
Comparison 4 GnRHa treatment versus no pretreatment or placebo prior to resection, Outcome 2 Difficulty of surgery (VAS).
4.3. Analysis.
Comparison 4 GnRHa treatment versus no pretreatment or placebo prior to resection, Outcome 3 Fibroid recurrence.
Comparison 5. GnRHa treatment versus other medical therapies prior to any surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine volume (descriptive table) | Other data | No numeric data | ||
| 2 Fibroid volume | 2 | 110 | Mean Difference (IV, Random, 95% CI) | 12.71 [‐5.92, 31.34] |
| 3 Fibroid volume (descriptive table) | Other data | No numeric data | ||
| 4 Haemoglobin at end of preoperative treatment | 1 | 188 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.60, 0.20] |
| 5 Reduction in bleeding to PBAC < 75 | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Ulipristal acetate 5 mg | 1 | 199 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.30, 1.68] |
| 5.2 Ulipristal acetate 10 mg | 1 | 203 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.14, 1.06] |
| 6 Adverse events | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Hot flushes | 5 | 453 | Odds Ratio (M‐H, Random, 95% CI) | 12.30 [4.04, 37.48] |
| 6.2 Headache | 4 | 439 | Odds Ratio (M‐H, Random, 95% CI) | 4.51 [1.09, 18.62] |
| 6.3 Nausea | 3 | 407 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.08, 1.64] |
| 6.4 Weight gain | 1 | 56 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.07, 2.81] |
| 6.5 Oedema | 1 | 56 | Odds Ratio (M‐H, Random, 95% CI) | 2.2 [0.21, 22.59] |
| 6.6 Sleep disorder | 1 | 56 | Odds Ratio (M‐H, Random, 95% CI) | 20.71 [2.49, 172.00] |
| 6.7 Mood disorder or anxiety | 2 | 106 | Odds Ratio (M‐H, Random, 95% CI) | 4.02 [0.02, 727.86] |
| 6.8 Vaginal dryness | 3 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 31.93 [2.19, 464.89] |
| 6.9 Cutaneous disorder | 2 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 1.50 [0.51, 4.38] |
| 6.10 Abdominal or pelvic pain | 3 | 383 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.85, 2.91] |
| 6.11 Procedural pain | 2 | 333 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.21, 6.35] |
| 6.12 Fatigue | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.14, 1.93] |
| 6.13 Anaemia | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.40, 3.92] |
| 6.14 Nasopharyngitis | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.08, 1.79] |
| 6.15 Breast pain or tenderness | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.10, 2.33] |
| 6.16 Influenza | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 2.55 [0.67, 9.72] |
| 6.17 Insomnia | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 2.55 [0.67, 9.72] |
| 6.18 Pharyngitis | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.15, 4.13] |
| 6.19 Dizziness | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.00, 1.84] |
| 6.20 Bone sensitivity | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 125.80 [6.75, 2343.30] |
| 6.21 Muscular stiffness | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 5.43 [0.25, 118.96] |
| 7 Quality of life (Uterine Fibroid Symptom and QoL questionnaire) | Other data | No numeric data |
5.1. Analysis.
Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 1 Uterine volume (descriptive table).
| Uterine volume (descriptive table) | ||||
|---|---|---|---|---|
| Study | Number in study | Comparison | Results | Comment |
| Baytur 2007 | 32 | GnRHa (goserelin 3.6mg monthly for 3 months) vs SERM (raloxifene 60mg daily orally) | Difference from baseline to after treatment (median (range)): GnRHa: 95cc (37 to 452) Raloxifene: 62.5 (10 to 118) |
Not significantly different between groups |
| Donnez 2012b | 307 | GnRHa (leuprolide acetate 3.75mg) vs ulipristal acetate (5mg and 10mg) | Per protocol results: Median percent change from baseline in uterine volume (IQ range): Ulipristal acetate 5mg: ‐20% (‐40 to ‐3) Ulipristal acetate 10mg: ‐22% (‐45 to 0) Leuprolide acetate 3.75mg: ‐47% (‐57 to ‐35) Difference in % points: UA 5mg vs LA 3.75mg: 1.48 (1.25 to 1.74) UA 10mg vs LA 3.75mg: 1.41 (1.19 to 1.66) |
Authors reported that LA was associated with a significantly greater reduction in uterine volume than either UA group |
| Reinsch 1994 | 14 | GnRHa (leuprolide acetate 3.75mg) vs mifepristone (RU 486 25mg) | Results reported in the text (median percent reduction and range) Leuprolide acetate 3.75mg: 54% (22 to 84) RU 486 25mg: 32% (1 to 65) |
Authors reported that there was no significant change in volume reduction between the 2 groups. |
5.4. Analysis.
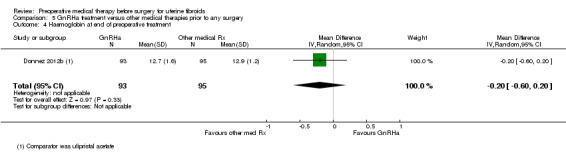
Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 4 Haemoglobin at end of preoperative treatment.
5.5. Analysis.
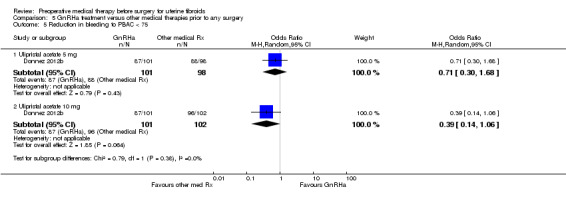
Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 5 Reduction in bleeding to PBAC < 75.
5.7. Analysis.
Comparison 5 GnRHa treatment versus other medical therapies prior to any surgery, Outcome 7 Quality of life (Uterine Fibroid Symptom and QoL questionnaire).
| Quality of life (Uterine Fibroid Symptom and QoL questionnaire) | ||||
|---|---|---|---|---|
| Study | No of participants | Comparison | Results | Comment |
| Donnez 2012b | 281 | UA 5mg or UA 10mg versus LA 3.75mg | UA 5mg vs LA (change from baseline): 2.5% (‐7.3 to 12.3) UA 10mg vs LA (change from baseline: 5.6% (‐3.9 to 15.1) |
No significant difference between groups |
Comparison 6. SPRM versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction in uterine volume | Other data | No numeric data | ||
| 2 Reduction in fibroid volume | Other data | No numeric data | ||
| 3 Haemoglobin (g/dL) | 2 | 173 | Mean Difference (IV, Random, 95% CI) | 0.93 [0.52, 1.35] |
| 4 Reduction in menstrual bleeding (PBAC < 75) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Ulipristal acetate 5 mg | 1 | 143 | Odds Ratio (M‐H, Random, 95% CI) | 41.41 [15.26, 112.38] |
| 4.2 Ulipristal acetate 10 mg | 1 | 146 | Odds Ratio (M‐H, Random, 95% CI) | 78.83 [24.02, 258.74] |
| 5 Change in menstrual blood loss from baseline to treatment end | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐166.9 [‐277.60, ‐56.20] |
| 6 Serious adverse events | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Breast cancer | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 2.04] |
| 6.2 Uterine haemorrhage | 2 | 274 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.00, 12.70] |
| 6.3 Ovarian haemorrhage | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.03, 18.84] |
| 6.4 Fibroid protruding through cervix | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 2.04] |
| 6.5 Menometrorrhagia | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 2.04] |
| 6.6 Hyperplasia | 2 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.02, 8.38] |
| 7 Other adverse events | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Headache | 3 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.14, 4.30] |
| 7.2 Breast pain or tenderness | 2 | 274 | Odds Ratio (M‐H, Random, 95% CI) | 1.76 [0.26, 11.70] |
| 7.3 Abdominal pain | 3 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 1.71 [0.36, 8.12] |
| 7.4 Pyrexia | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.12, 3.25] |
| 7.5 Hypercholesterolaemia | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.14, 10.96] |
| 7.6 Hypothyroidism | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 3.36 [0.19, 60.73] |
| 7.7 Constipation | 2 | 274 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.13, 2.79] |
| 7.8 Hypertriglyceridaemia | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.11, 9.11] |
| 7.9 Influenza | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.11, 9.11] |
| 7.10 Dizziness | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 2.30 [0.12, 43.52] |
| 7.11 Nasopharyngitis | 2 | 274 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.09, 2.31] |
| 7.12 Dysmenorrhoea | 1 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 1.02] |
| 7.13 Bladder pressure | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.03, 3.64] |
| 7.14 Micturition problem | 2 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 1.91 [0.28, 13.23] |
| 7.15 Lower back pain | 2 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.29, 6.15] |
| 7.16 Proctodynia | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 3.67 [0.14, 97.49] |
| 7.17 Coital pain | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 6.6 [0.29, 150.07] |
| 7.18 Hot flushes | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 25.24 [1.27, 503.38] |
| 7.19 Nausea | 2 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 1.97 [0.46, 8.46] |
| 7.20 Vomiting | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.21 Diarrhoea | 2 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 3.62 [0.39, 33.89] |
| 7.22 Change of mood | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 15.00 [1.54, 146.54] |
| 7.23 Lowered libido | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 6.0 [0.58, 61.84] |
| 7.24 Weakness | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 2.8 [0.43, 18.38] |
| 7.25 Fatigue | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 1.73 [0.31, 9.57] |
| 7.26 Dental pain | 1 | 33 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.12, 14.82] |
| 7.27 Vaginal infections | 1 | 33 | Odds Ratio (M‐H, Random, 95% CI) | 2.44 [0.11, 55.56] |
| 7.28 Vaginal discharge | 1 | 33 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.12, 14.82] |
| 8 Quality of life (Uterine Fibroid Symptoms and QoL questionnaire) | Other data | No numeric data |
6.1. Analysis.
Comparison 6 SPRM versus placebo, Outcome 1 Reduction in uterine volume.
| Reduction in uterine volume | ||||
|---|---|---|---|---|
| Study | Number in study | Comparison | Results | Comment |
| Donnez 2012a | 242 | Ulipristal acetate 5 mg or 10 mg daily vs. placebo | Reduction in uterine volume prior to surgery of 25% or greater: Ulipristal acetate 5 mg: 30/88 (34%) Ulipristal acetate 10 mg: 24/85 (28%) Placebo: 3/47 (6%) Difference UA 5 mg vs. placebo: 28 (11 to 40) Difference UA 10 mg vs. placebo: 22 (6 to 35) |
Authors reported that both UA groups had a significantly greater reduction in uterine volume of 25% or greater than the placebo group |
| Wilkens 2008 | 33 | Asoprisnil 10 mg and 25 mg vs. placebo | Median percentage change (from baseline): Asoprisnil 10 mg vs. placebo: 7.9% vs. ‐2.1% (NS) Asoprisnil 25 mg vs placebo: ‐5.1% vs ‐2.1% (NS) |
Authors reported no significant difference between groups |
6.2. Analysis.
Comparison 6 SPRM versus placebo, Outcome 2 Reduction in fibroid volume.
| Reduction in fibroid volume | ||||
|---|---|---|---|---|
| Study | Number in study | Comparison | Study findings | Comment |
| Donnez 2012a | 242 | Ulipristal acetate 5mg/day and 10mg/day vs placebo | Median change: UA 5mg vs placebo: ‐18.9% vs +1.9% (p=0.002) Difference UA 5mg vs placebo: ‐19.6% (‐31.2 to ‐6.5) UA 10mg vs placebo: ‐6.2% vs +1.9% (p=0.006) Difference UA 10mg vs placebo: ‐14.2% (‐25.9 to ‐2.4) |
Clinically and statistically significant differences between UA and placebo in both per protocol and modified ITT analyses |
| Engman 2009 | 30 | Mifepristone 50mg/every other day vs placebo | Mean % change from baseline (CI): MP vs placebo: ‐28% (‐48 to ‐8) vs +6% (‐13 to 25 (p=0.02) |
Authors reported that decrease with MP was significantly lower than placebo |
| Levens 2008 | 22 | CDB‐2914 10mg/day and 20mg/day vs placebo | Change: CDB‐2914 (combined dosages) vs placebo: +6% vs ‐29% (p=0.01) |
Not clear if mean or median change ‐ no variation measure reported |
| Wilkens 2008 | 33 | Asoprisnil 10mg/day and 25mg/day vs placebo | Median percent change in largest fibroid volume: Asoprisnil 10mg vs placebo: ‐0.4% vs 4.9% (NS) Asoprisnil 25mg vs placebo: ‐25.8% vs 4.9% (0.04) |
Authors reported that only the higher dose asoprisnil group reduction was significantly different from placebo (lower dose NS) |
6.3. Analysis.
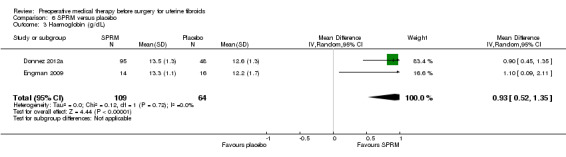
Comparison 6 SPRM versus placebo, Outcome 3 Haemoglobin (g/dL).
6.4. Analysis.
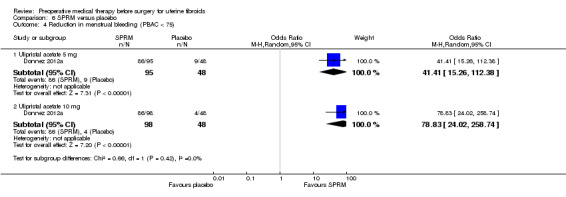
Comparison 6 SPRM versus placebo, Outcome 4 Reduction in menstrual bleeding (PBAC < 75).
6.5. Analysis.
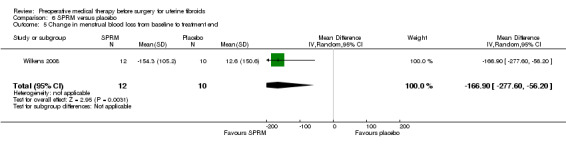
Comparison 6 SPRM versus placebo, Outcome 5 Change in menstrual blood loss from baseline to treatment end.
6.6. Analysis.
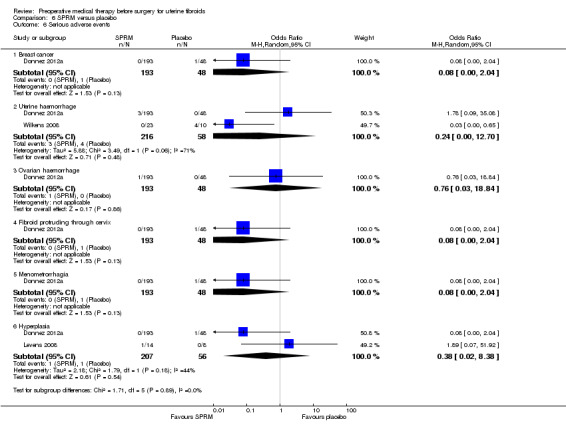
Comparison 6 SPRM versus placebo, Outcome 6 Serious adverse events.
6.8. Analysis.
Comparison 6 SPRM versus placebo, Outcome 8 Quality of life (Uterine Fibroid Symptoms and QoL questionnaire).
| Quality of life (Uterine Fibroid Symptoms and QoL questionnaire) | ||||
|---|---|---|---|---|
| Study | No of participants | Comparison | Results | Comment |
| Donnez 2012a | 239 | UA 5 mg or UA 10 mg versus placebo | Questionnaire assessing discomfort from fibroids (ranging from 0 to 28 points): UA 5 mg vs. placebo (change from baseline): ‐4.0 (‐6.0 to ‐1.0), P = 0.001 UA 10 mg vs. placebo (change from baseline): ‐4.0 (‐7.0 to ‐2.0), P < 0.001 |
Differences from placebo group significant |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Audebert 1994.
| Methods | Randomisation method not given. Multicentre study with no blinding. Number of women randomised: N = 71. Number of withdrawals: N = 24 (mainly for administrative reasons); 6 withdrew before treatment, 9 before surgery, 1 immediately after surgery and 8 during follow up. No power calculation made and no intention‐to‐treat analysis. No source of funding given. | |
| Participants | Premenopausal women aged over 25 years diagnosed with uterine fibroids confirmed by ultrasound scan and awaiting hysterectomy or myomectomy were recruited from a number of different hospitals in France. Exclusion criteria were pregnancy, receiving hormone treatment and serious concomitant illnesses. | |
| Interventions | Rx: Subcutaneous goserelin 3.6 mg once every month for 3 months followed by hysterectomy (N = 15) or myomectomy (N = 10). Control: Immediate surgery (hysterectomy: N = 23, myomectomy: N = 8) Duration: 3 months (treatment group only). | |
| Outcomes | Preoperative haemoglobin Preoperative uterine volume (mL) Preoperative fibroid volume (mL) Pelvic symptom score Adverse events Intraoperative blood loss (mL) Difficulty of surgery Duration of surgery (minutes) Duration of hospital stay (days) Frequency of blood transfusions Postoperative haemoglobin (g/dL) | |
| Notes | Groups not comparable at baseline (preoperative uterine and fibroid size greater in the immediate surgery group). Hysterectomy and myomectomy were both performed as surgical procedures and intraoperative and postoperative outcomes not reported separately for each type of surgery so only preoperative outcomes were considered in the review. The author was contacted for additional data but no reply received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were randomized either to immediate surgery or to treatment” – randomisation method not reported. |
| Allocation concealment (selection bias) | Unclear risk | No details of allocation concealment reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded due to the control group having immediate surgery. It is not stated whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether personnel were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 24 of 71 participants withdrew before completion of study. “The results of the study must be viewed in light of these withdrawals”. “The immediate surgery group assessment did not include 7 patients who required intraoperative blood transfusion.” Intention‐to‐treat analysis was not used. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Results did not specify numbers included in the results, so the results could not be included in a meta‐analysis. |
| Other bias | High risk | Groups not comparable at baseline for pre‐operative uterine and fibroid size. “Although the patients not receiving goserelin were scheduled to immediate surgery, the operation took place at a mean of 76 days after randomization” |
Balasch 1995.
| Methods | Method of randomisation not stated. Single centre study with ultrasonographer and surgeon blinded. Number of women randomised: N = 50. No withdrawals reported. No power calculation made. No source of funding reported. | |
| Participants | Women aged 37 to 52 years with uterine fibroids and menorrhagia, pelvic pain or pressure recruited from Provincial Hospital in Barcelona, Spain. Inclusion criteria: fibroids ≥ 12 weeks gestational size, no suspicion of uterine or ovarian malignancy, endometriosis or pelvic inflammatory disease from clinical or ultrasound examination, stable general condition. | |
| Interventions | Rx: Intramuscular decapeptyl 3.75 mg every 4 weeks for 2 injections before hysterectomy, N = 23 Control: Abdominal hysterectomy within 4 weeks of randomisation, N = 27 Duration: 8 weeks (treatment group) | |
| Outcomes | Preoperative haemoglobin (g/dL) Preoperative haematocrit (%) Preoperative uterine volume (mL) Duration of surgery Type of incision Frequency of blood transfusions Duration of hospital stay (days) Postoperative complications | |
| Notes | Groups not comparable at baseline (measurements of uterine volume and pretreatment haemoglobin and haematocrit lower in the treatment than in the control group). Author contacted for additional data but no reply received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The patients were randomised to pre‐operative gonadotropin releasing hormone agonist treatment or to immediate hysterectomy”. Did not state allocation method. |
| Allocation concealment (selection bias) | Unclear risk | No details were reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “All ultrasonic measurements of fibroid dimensions were performed by the same blinded ultrasonographer”. “The operations were performed by a staff specialist and a senior gynaecology resident who were blinded as to the treatment groups.” No information was provided regarding participants being blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details relating to outcome assessment were available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data, no participants withdrew from the study. |
| Selective reporting (reporting bias) | Low risk | All prespecified and expected outcomes of interest were reported. |
| Other bias | Unclear risk | Pretreatment uterine volume and haemoglobin/haematocrit between comparison groups were not comparable at baseline. |
Baytur 2007.
| Methods | Randomisation method not reported. Single centre, parallel group study with no apparent blinding. Number of women randomised: N = 32. Number of women analysed (primary outcomes): N = 32 (6 and 5 women in each group did not proceed to surgery but outcomes measured preoperatively). Power calculation for sample size calculated; 16 subjects per group to detect a difference of 25 cm³ in 3 months fibroid volume chance between groups. Source of funding not reported. |
|
| Participants | Inclusion criteria: healthy premenopausal women with regular cycles (ranging from 25 to 35 days); mild fibroid symptoms such as anaemia, pain or pressure symptoms. Exclusion criteria: women requiring emergency surgery due to severe fibroid symptoms and disorders such as anaemia (Hb < 10 mg/dL), pain, dysmenorrhoea, menstrual bleeding and osteoporosis, severe vasomotor symptoms, blood coagulation diseases, history or family history of vascular thrombosis, suspicion of systemic neoplastic and infectious disease, suspicion of uterine malignancies, endometrial abnormalities detected by Pipelle endometrial biopsy and transvaginal ultrasound. Recruited from clinic in Manisa, Turkey. Mean ages: 46.6 years and 45.2 years. |
|
| Interventions |
Interventions were compared with a control group (age matched but not randomised) but this group has not been included in comparisons in this review. |
|
| Outcomes | Primary: change in fibroid volume between randomised groups. Other outcomes: adverse effects. The other outcomes measured were not included in this review. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported. |
| Allocation concealment (selection bias) | Low risk | Closed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts before surgery ‐ outcomes measured before surgery (substantial attrition from both groups for surgery). |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
Benagiano 1996.
| Methods | Randomisation scheme controlled by Zeneca and women allocated sequentially as they entered the study. Multinational (Denmark, the Netherlands, Spain, Finland, Norway, Scotland, Portugal, Northern Ireland, Italy), multicentre, double‐blind study. Number of women randomised: N = 185. Withdrawals: N = 17 (2 refused treatment, 10 during treatment and 5 at the end of the study). Power calculation made for sample size and analysis by intention‐to‐treat. Source of funding: Zeneca Pharmaceuticals (UK). Zeneca Pharmaceuticals produced the randomisation scheme (pharmaceutical company producing Zoladex) | |
| Participants | Premenopausal women aged over 25 years with menorrhagia or metrorrhagia and anaemia associated with uterine fibroids and awaiting hysterectomy recruited from 30 centres in 10 countries. Inclusion criteria: fibroids confirmed from manual exam, haemoglobin < 12 g/dL, negative cervical smear within previous 12 months, informed consent. Exclusion criteria: serious renal, hepatic, haemopoietic or endocrine disorders other than anaemia due to fibroids, history of drug and/or alcohol abuse within the previous year, gynaecological malignancy, sex hormone therapy within the past month or GnRHa treatment within the past 6 months, blood transfusions within the previous 3 months or other therapy affecting menstrual loss, sensitivity to GnRHa or iron replacement, any medical condition which would render the woman unsuitable. | |
| Interventions | Rx 1: Goserelin acetate depot 3.6 mg once monthly + iron 600 mg/day before hysterectomy, N = 55 (ITT not performed) Rx 2: Goserelin acetate depot 3.6 mg once monthly + placebo iron, N = 54 Control: Sham injection once monthly + iron 600 mg/day before hysterectomy, N = 59 Duration: 3 months | |
| Outcomes | Preoperative haemoglobin (g/dL) Preoperative haematocrit (%) Preoperative uterine volume (mL) Preoperative fibroid volume (mL) Pelvic symptoms Adverse events Duration of surgery (minutes) Intraoperative blood loss (mL) Frequency of blood transfusions Difficulty of surgery | |
| Notes | Groups comparable at baseline for age, weight and height but differences in fibroid volumes, uterine volumes and haemoglobin concentrations. Author contacted for additional information but no reply received. The outcomes with suitable data considered in the review were duration and difficulty of surgery, transfusion rate and withdrawal because of adverse effects. For all other outcomes, data were not suitable. The second treatment group was not considered in the review because the control group was not comparable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “A separate randomization scheme was produced for each centre by the Biometrics group, Zeneca Pharmaceuticals and patients were randomised in a ratio of 1:1:1…strictly sequentially as patients entered the study”. Unclear as to whether quasi‐random. |
| Allocation concealment (selection bias) | Low risk | Central control of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The study was a… double‐blind comparison”. Sham injection given to control group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported on blinding of assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “All analyses were performed on an intention‐to‐treat basis”. 185 participants were recruited, 17 withdrew with reasons given. |
| Selective reporting (reporting bias) | High risk | The outcomes with suitable data considered in the review were duration and difficulty of surgery, transfusion rate and withdrawal because of adverse effects. For all other outcomes the results were reported incompletely only as P values. |
| Other bias | Unclear risk | Difference in baseline values for fibroid and uterine volumes between groups. |
Bustos López 1995.
| Methods | Randomisation method not stated. Single centre, parallel group, double blinding (investigator and assessor, not participants) Number of women randomised: N = 28 No reported withdrawals No power calculation performed Source of funding: Syntex | |
| Participants | Women aged up to 40 years with diagnosis of uterine fibroids confirmed by clinical examination, ultrasonography and/or laparoscopy and with a desire to preserve their fertility, recruited in Mexico City. Other inclusion criteria: informed consent, normal endometrial biopsy and cervical cytology. Exclusion criteria: women with intolerable side effects, desire to not continue with the study and suspicion of malignancy. | |
| Interventions | Rx: Nafarelin intranasal spray 200 µg twice daily before myomectomy, N = 13. Control: No preoperative treatment before myomectomy, N = 15. Duration: 3 months. | |
| Outcomes | Preoperative uterine volume (cc) Preoperative myoma volume (cc) Preoperative haemoglobin (g/dL) Type of incision Intraoperative blood loss (mL) | |
| Notes | Authors contacted but no reply received. Paper translated by Christine Aguilar. Some of the calculations with the raw data did not match the means reported in the tables. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised to 2 groups ‐ method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators were blinded but participants were not blinded ‐ however the outcomes could not be influenced by the participants' knowledge of group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There did not appear to be any dropouts from the study. |
| Selective reporting (reporting bias) | High risk | The outcomes were measured in the intervention group at baseline, 30, 60 and 90 days but were only measured in the control group at baseline so a true comparison between groups could not be made. Postsurgical complication rates were not clearly reported. |
| Other bias | Unclear risk | There appear to be differences between groups at baseline. |
Cagnacci 1994.
| Methods | Randomisation by alternation and no blinding. Number of women randomised: N = 20. No withdrawals. No power calculation reported. No source of funding reported. | |
| Participants | Women aged 30 to 49 years in good health and with ultrasound evidence of uterine fibroids were recruited from a centre in Italy. Inclusion criteria: pre menopause, requirement for surgery and voluntary informed consent. Exclusion criteria: abnormal Pap test, uterine cancer, alterations of coagulation, glucose or lipid metabolism and liver or kidney disease. | |
| Interventions | Rx: Goserelin depot 3.6 mg every 28 days before surgery, N = 10. Control: No treatment before surgery (type not specified by authors), N = 10. Duration: 3 months | |
| Outcomes | Uterine volume (cc) Fibroid volume (cc) Preoperative haematocrit (%) Blood loss (mL) (data for this outcome not entered in review) | |
| Notes | Authors contacted for additional information and reply received. Groups not comparable at baseline (uterine and fibroid volume higher in controls). Type of surgery not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “Administered for 6 months to 22 subjects, and for 12 months to 8 subjects. The other 20 subjects… were randomly allocated for 3 months to no treatment or goserelin depot administration.” 20 women were randomised, the other 30 women were in groups of 22 and 8, and it is unclear whether they were randomised. The method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded. Unclear whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | None reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals from the study were mentioned, but final numbers were not reported in results. |
| Selective reporting (reporting bias) | High risk | Data were not reported for the 20 women randomised who required surgery. Adverse events were not reported. |
| Other bias | Unclear risk | Baseline variables between groups not reported. |
Campo 1999.
| Methods | Randomisation according to a computer generated sequence but no description of attempts to conceal allocation and no blinding. Number of women randomised: 60 Number of women analysed: 60 No power calculation reported. No source of funding reported. | |
| Participants | Women aged 25 to 42 years selected for laparoscopic myomectomy between June 1993 and December 1996 at a clinic in Italy. Inclusion criteria: presence of symptomatic subserosal or intramural myomas; presence of uterine myomas as the only plausible explanation for a history of recurrent abortion or infertility. Diagnosis by transvaginal sonography indicated by fibroid symptoms. Exclusion criteria: submucous myomas; myomas > 10 cm in diameter; women with more than 3 myomas > 4 cm in diameter. | |
| Interventions | Rx: Decapeptyl 3.75 mg intramuscularly every 28 days for 3 months before surgery, N = 30. Control: No preoperative treatment before surgery, N = 30. Duration 3 months. | |
| Outcomes | Duration of surgery (minutes) Postoperative complications Blood transfusion rate Duration of hospital stay (days) Fertility rate (number of pregnancies) Blood loss (mL) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients included in the present series were randomized according to a computer‐generated sequence”. |
| Allocation concealment (selection bias) | Unclear risk | No details regarding allocation concealment were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded, as they either received immediate surgery or treatment and delayed surgery. It is not stated whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details were provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women were followed‐up for a minimum 6 months. No missing data. |
| Selective reporting (reporting bias) | Low risk | Outcomes of interest were reported. |
| Other bias | Unclear risk | No details provided of baseline variables between groups. |
Cetin 1995.
| Methods | Randomisation method not specified and no blinding. Number of women randomised: N = 30. Number of women analysed: N = 30. No power calculation reported. No source of funding reported. | |
| Participants | Women with symptomatic fibroids attending the obstetrics and gynaecology department at a hospital in Turkey. Inclusion criteria: symptomatic fibroids; no other pathology. Diagnosis confirmed by pelvic, abdominal and ultrasonographic examinations. Exclusion criteria: none stated. Main symptoms were infertility in 14 women and menorrhagia in 6 women. | |
| Interventions | Rx: Buserelin intranasally 900 μg/day in 3 doses for 3 months, = 15. Control: No preoperative treatment, N = 15. Duration: 3 months. | |
| Outcomes | Volume of myomas (cm³) Pre‐operative haemoglobin (g/dL) Duration of surgery (minutes) Blood loss (mL) Side effects | |
| Notes | The principal review author noted an error in the published paper which was confirmed by the principal study author. The authors were contacted for additional information on side effects rates but a reply has not yet been received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Subjects were randomly divided into two groups”. “Prospective, randomised, controlled study”. Method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding of participants. Most likely no blinding of personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | “Ultrasonographic examinations were performed by the same sonographers in all cases”. “All of the myomectomies were performed by the same surgeons”. Did not state whether these personnel were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers included in analysis not stated in results. It appears there were no withdrawals from the study but this is unclear. |
| Selective reporting (reporting bias) | Unclear risk | Mistake was acknowledged in paper by author via past contact. Assuming is related to lack of referenced tables in text. |
| Other bias | Unclear risk | Unclear if baseline variables comparable between groups. |
D'Anna 1994.
| Methods | Randomisation method not stated and blinding not clear. Number of women randomised: N = 30. No withdrawals reported. No power calculation made. No source of funding reported. | |
| Participants | Premenopausal women aged 36 to 50 years awaiting hysterectomy for uterine fibroids recruited from a clinic in Messina, Italy. Inclusion criteria: uterine fibroids with an average diameter of 3 cm. No exclusion criteria reported. | |
| Interventions | Rx: Leuprolide acetate depot 3.75 mg monthly before hysterectomy, N = 15. Control: Placebo monthly before hysterectomy, N = 15. Duration: 2 months. | |
| Outcomes | Uterine volume (cc) Adverse events (no control data provided so this outcome was not considered in the review) | |
| Notes | Paper translated by Kirsten Duckitt. Groups not comparable at baseline (uterine volume higher in treatment group compared to control group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “In a randomised manner”. Randomisation method not stated. |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | A placebo was used to blind participants, no detail provided as to what the placebo was. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details were reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 dropouts. Adverse events reported with treatment were not compared to adverse events reported with placebo. |
| Selective reporting (reporting bias) | Low risk | No protocol viewed but all outcomes reported in the methods section were reported in the results section. |
| Other bias | Unclear risk | It is not reported whether the difference between the groups of uterine volume pretreatment is statistically significant or not. |
De Falco 2009.
| Methods | Single‐centre (Italy), randomised controlled trial. Sequential numerical allocation to a randomisation list. Number of women randomised: N = 62 Number of withdrawals: none Intention‐to‐treat not mentioned but all participants included in analysis. Power calculation and source of funding not mentioned. |
|
| Participants | Inclusion criteria: Premenopausal women with single intramural symptomatic uterine leiomyoma, referred between 2005 and 2007 to the outpatient clinic of the department of Obstetrical‐Gynaecological and Urological Science and Reproductive Medicine of a University. Exclusion criteria: taking hormonal therapy, delivered within 12 months of the study, or had malignant neoplasm. Previous pelvic surgery, uterine malformations, present or past pelvic inflammatory disease, coagulation disorders and unstable general conditions. |
|
| Interventions | Treatment: 22 women received 3.75 mg triptorelin subcutaneous depot injection, once a month for 3 months. Surgery carried out at the latest 3 weeks after third injection. Control: 29 women underwent immediate surgery during follicular phase of the menstrual cycle. Duration: 3 months for treatment group. |
|
| Outcomes | Fibroid diameter. Total operating time. Intraoperative blood loss. Clear identification of cleavage plane. PCNA expression. CD34 expression. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...patients were randomised using a sequential numerical allocation to a randomisation list prepared before commencing the study" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Control group participants did not receive a placebo injection, and thus participants were aware of study allocation. “Surgeons were blinded to the pre‐surgical medical treatment”. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Surgeons were blinded to the pre‐surgical medical treatment". “Sections were examined and immunostaining was graded without previous knowledge of the clinical data of the patients.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of ITT analysis. Authors did not state whether all participants included in the analyses but it appears there were no dropouts because of the percentages quoted for dichotomous outcomes. |
| Selective reporting (reporting bias) | Low risk | Protocol not viewed. All outcomes described in methods were reported in results section. |
| Other bias | Low risk | In addition to participants enrolled in the study, 20 samples obtained retrospectively were randomly selected from the Pathology Unit database. It was not specified how these samples were randomly selected, or whether these samples were demographically similar to the women enrolled in the study; however, the outcome evaluated was not relevant to this review. Randomised groups appeared similar at baseline. |
Donnez 2003.
| Methods | Women were randomised to one of five groups on a 1:1:1:1:1 basis with 50 participants per group. Placebo group was sub‐randomised to each of the four placebo arms. overall randomisation 4:4:4:4:1:1:1:1:1 Multicentre (Belgium, Spain, Czech Republic, France) trial. Due to nature of injections both medical personnel and participant could not be blinded to the two different (GnRHa and fulvestrant) injections but they were blinded to whether it was placebo or active. Total no randomised N = 313. Total withdrawals N = 12. 4 from fulvestrant 50 mg, 3 from fulvestrant 125 mg,1 from 250 mg and 4 from goserelin group. Intention‐to‐treat analysis was done but not presented. The per protocol analyses had substantial withdrawals for most outcomes. Power calculation done. Supported by Astra Zeneca. |
|
| Participants | Inclusion criteria: Premenopausal women with measurable fibroids that required hysterectomy; not involved in night shift work; prepared to use barrier contraception for the study period and could provide signed informed consent. Exclusion criteria: Used GnRHa in the past for > 3 months or had finished the same treatment within 3 months of study entry; used sex hormone therapy, oral contraceptives or danazol within 4 weeks of study entry; had disease effecting bone or steroid metabolism; had changes in menstrual frequency or any changes reflecting the onset of menopause. |
|
| Interventions | Rx: Fulvestrant 50 mg IM injection once every 4 weeks for 3 injections, N = 59 Fulvestrant 125 mg IM injection once every 4 weeks for 3 injections, N = 66 Fulvestrant 250 mg IM injection once every 4 weeks for 3 injections, N = 62 Goserelin 3.6 mg SC every 4 weeks for 3 injections, N = 66 Each of the groups had a placebo group which received fulvestrant matched placebo or sham goserelin, N = 60 |
|
| Outcomes | Preoperative Primary
Secondary
|
|
| Notes | Intraoperative outcomes not assessed. Haematocrit values assessed at base line only and not as outcome. No blinding as regards to fulvestrant and GnRHa but that should not be considered significant as all outcomes except vaginal blood loss were objective. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The treatment received by individual patients was determined centrally, with separate schemes produced for each center" |
| Allocation concealment (selection bias) | Low risk | Central allocation to treatment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and medical personnel were aware of allocation to fulvestrant or goserelin arm of the trial. However could not distinguish between active drug and placebo. “Because of the differences in the nature of injections, both patients and medical personnel could distinguish between the two medications and, because of the differences in volumes, between the doses of fulvestrant being given. However, it was impossible to distinguish between active agent and placebo (sham) for any of the treatments”. However, most outcomes were objective and unlikely to be influenced by knowledge of treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Medical personnel were aware of drug assignment (fulvestrant or goserelin) but were not aware of placebo vs. active drug. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Methods section states that both ITT analysis and per‐protocol analysis carried out, however ITT data not provided in study and the data from per protocol analyses suggested significant attrition. |
| Selective reporting (reporting bias) | Low risk | Protocol not viewed. All outcomes from methods section of paper reported in the results section. |
| Other bias | Low risk | Baseline variables similar between groups. |
Donnez 2012a.
| Methods | Multinational (Belgium, Ukraine, France, Romania, Hungary, Czech Republic, Switzerland, UK), multicentre parallel group RCT with double blinding. Number of women randomised: 242 Number of women analysed: 241 (modified ITT) but per protocol analyses also undertaken. Number of withdrawals: 1 (in the 5 mg ulipristal acetate group who was withdrawn before she received the study drug). Power calculation performed for sample size: based on the endpoint of change in fibroid volume. Source of funding: PregLem (data handled by independent data management organisation). |
|
| Participants | Women aged 18 to 50 years were recruited between October 2008 and August 2010 from 38 academic centres in 6 countries. Inclusion criteria: PBAC > 100 during days 1 to 8 of menstruation, fibroid related anaemia (Hb ≤ 10.2 g/dL without macrocytosis, fibroid uterus with a size equivalent to a uterus of 16 weeks or less of gestation, at least 1 fibroid ≥ 3.cm in diameter but with no fibroid measuring more than 10.cm in diameter (US), BMI 18 to 40 kg/m². Exclusion criteria: history of uterine surgery, endometrial ablation or uterine artery embolisation, history of current gynaecological cancer, current endometrial hyperplasia, Hb ≤ 6 g/dL or any condition requiring immediate blood transfusion, known haemoglobinopathy, known severe coagulation disorder, large uterine polyp (> 2 cm), one or more ovarian cysts ≥ 4 cm in diameter (U/S), previous or current treatment for fibroids with an SPRM or a GnRHa, treatment with agents known to affect hepatic cytochrome CYP3A4, progestins, acetylsalicylic acid, mefenamic acid, anticoagulants, antifibrinolytic drugs or systemic glucocorticoid treatments. |
|
| Interventions | Rx: Ulipristal acetate 5.mg or 10.mg orally once per day, N = 96 and N = 98). Control: placebo (identical pill) orally once per day. Duration: 13 weeks of treatment (before surgery) with follow up at weeks 17, 26, and 38, N = 48. |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Protocol and supplementary data were available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated list". |
| Allocation concealment (selection bias) | Low risk | "Web integrated interactive voice system" under central control. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical treatments, placebo‐controlled study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were not aware of participant allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Modified ITT analysis (1 participant excluded, withdrawn before taking medication). |
| Selective reporting (reporting bias) | Low risk | Protocol viewed and all predetermined outcomes reported in full. |
| Other bias | Low risk | Participant groups comparable at baseline. |
Donnez 2012b.
| Methods | Multinational (Belgium, Poland, Spain, France, Austria, Italy, UK), multicentre parallel group RCT with double dummy design. Number of women randomised: N = 307. Number of women analysed: N = 301 (for safety), N = 297 for modified ITT analysis and N = 281 for per protocol analyses. Number of withdrawals: N = 1 in ulipristal acetate 5 mg group (did not receive study drug), N = 2 in ulipristal acetate10 mg group (did not receive study drug, missing efficacy data), N = 2 in LA group (missing efficacy data). Power calculation for sample size (based on non inferiority of LA with ulipristal acetate). Source of funding: PregLem (supplied ulipristal acetate). |
|
| Participants | Inclusion criteria: premenopausal women aged 18 to 50 years, BMI between 18 and 40, heavy uterine bleeding caused by fibroids, at least one fibroid measuring 3 cm or more in diameter (no fibroid measuring > 10 cm), uterine size equivalent to a pregnancy of no more than 18 weeks in gestation; eligible for surgery. Exclusion criteria: history of uterine surgery, endometrial ablation or uterine artery embolisation, history of current gynaecological cancer, current endometrial hyperplasia, Hb ≤ 6 g/dL or any condition requiring immediate blood transfusion, known haemoglobinopathy, known severe coagulation disorder, large uterine polyp (> 2 cm), one or more ovarian cysts ≥ 4 cm in diameter (U/S), previous or current treatment for fibroids with an SPRM or a GnRHa, treatment with agents known to affect hepatic cytochrome CYP3A4, progestins, acetylsalicylic acid, mefenamic acid, anticoagulants, antifibrinolytic drugs or systemic glucocorticoid treatments. |
|
| Interventions | Rx 1: ulipristal acetate (SPRM) 5 mg or 10 mg oral tablet daily + intramuscular saline injection once monthly, N = 98 and N = 104. Rx 2: daily oral placebo + intramuscular injection of 3.75 mg leuprolide acetate (GnRHa) once monthly, N = 101. Duration: 13 weeks (before surgery) with follow up at weeks 17, 26 and 38. Iron supplementation could be used at the discretion of the physician. |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Non inferiority trial ‐ PEARL II | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Low risk | Web integrated voice system under central control. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, double dummy trial with uterine volume assessed by ultrasound at each centre. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biopsy samples were assessed by 3 independent pathologists who were unaware of the study group assignments, the visit sequence and each others assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Modified ITT ‐ did not include 5 participants (2 participants (one in each ulipristal acetate group) who never received the study drug and were not followed and 3 participants (1 who was assigned to receive ulipristal acetate10 mg and 2 in the LA group) with missing efficacy data after baseline. Per protocol analysis also performed (modified ITT population with the exclusion of women with major protocol deviations and a compliance rate of < 80%). |
| Selective reporting (reporting bias) | Low risk | Protocol published and viewed ‐ all outcomes reported. |
| Other bias | Low risk | Groups comparable at baseline. |
Engman 2009.
| Methods | Single centre, parallel group RCT. Number of women randomised: N = 30. Number of women analysed: N = 28. Number of withdrawals: N = 2 (both from placebo group, with reasons). Power calculation for sample size (at least 10% in % fibroid volume change between groups). Source of funding: Swedish Research Council, Karolinska Institute and Stockholm city. |
|
| Participants | Inclusion criteria: healthy non pregnant women referred for evaluation to outpatient clinic due to fibroid related problems indicating surgical intervention. Exclusion criteria: steroid hormonal therapy for a minimum of 3 months before recruitment, any history of breast cancer or other malignancy, uncontrollable bleeding requiring urgent surgical treatment, abnormal mammogram or breast biopsy at baseline, adnexal abnormality or suspicion of leiomyosarcoma upon TVUS, abnormal FSH and LH levels or any other hormonal dysfunction of clinical significance, lab findings that would give suspicion of blood, liver or renal dysfunction, abnormal Pap smear at screening, any other contraindication to mifepristone. Mean age: 41 years Recruited from outpatient clinic at Karolinska University Hospital, Stockholm, Sweden |
|
| Interventions |
Duration of treatment 3 months (ended the day before surgery). |
|
| Outcomes | Primary: reduction in uterine fibroid size. Other: number of bleeding days, endometrial assessment (from biopsy), symptom scores. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation method. |
| Allocation concealment (selection bias) | Low risk | Central control from pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assumed that assessors were the same as study staff. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition 12% from placebo group (reasons unrelated to intervention). |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | Groups were generally comparable at baseline. |
Fedele 1990.
| Methods | Randomisation list on a 1:2 ratio with no blinding. Number of women randomised: N = 24. No withdrawals reported. No power calculation made. No source of funding reported. | |
| Participants | Women aged 24 to 38 years (mean 33.6 years) with symptomatic multiple uterine fibroids recruited from a clinic in Milan, Italy. Prevalent symptoms were infertility in 18 and menorrhagia in 6 women. No exclusion criteria reported. | |
| Interventions | Rx: Intranasal buserelin 1200 µg/day before myomectomy, N = 8. Control: Immediate myomectomy surgery, N = 16. Duration: 3 months. | |
| Outcomes | Preoperative uterine volume (mL) Duration of surgery (minutes) Intra‐operative blood loss (mL). Adverse events (specific information not available from author). Postoperative febrile complications. Recurrence of myomas at 6 months. | |
| Notes | Author contacted for additional information on adverse events but no reply received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Using a randomisation list the patients were allocated in a 1:2 ratio”. List unclear whether this was sequential or random. |
| Allocation concealment (selection bias) | Unclear risk | No information pertaining to allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information pertaining to blinding was provided but unlikely as control participants had immediate surgery ‐ however recurrence is an objective outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Measurements were performed… in all patients by a physician unaware of the patient’s group allocation”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no withdrawals from the study. Data for all 24 participants were reported. |
| Selective reporting (reporting bias) | Low risk | No previous protocol information was available but all outcomes from methods section were reported in the results section. |
| Other bias | Unclear risk | Insufficient information to determine if groups comparable at baseline |
Friedman 1989.
| Methods | Randomisation by permuted blocks controlled by pharmacy and stratified into 2 groups: moderate (< 600 cm³) or large (≥ 600 cm³). Single centre and double blind. Number of women randomised: N = 20. Exclusions post randomisation: N = 2 in 1992 study (because myomectomy technique different). No power calculation made and not intention‐to‐treat. Source of funding: Takeda‐Abbott Research and Development and General Clinical Research Centre, Brigham and Womens' Hospital. | |
| Participants | Premenopausal women aged 29 to 41 years recruited from Brigham and Womens' Hospital, Massachusetts, USA. Inclusion criteria: Aged < 42 years, premenopausal (FSH < 30 mU/mL), not pregnant or lactating, prepared to avoid pregnancy (either sterilised or using contraception), presence of at least 1 fibroid > 3 cm in diameter or at least 50 cm³ with multiple fibroids on ultrasound, absence of uterine calcification on ultrasound, moderate to severe symptoms from fibroids, no suspicion of ovarian or uterine malignancy from physical examination or ultrasound, absence of hyperplasia on endometrial sample in women with menorrhagia. | |
| Interventions | Rx: Intramuscular leuprolide acetate depot 3.75 mg monthly for 4 injections before myomectomy, N = 9. Control: Intramuscular placebo monthly for 4 injections before myomectomy, N = 9. Duration: 12 treatment weeks before myomectomy (follow up 27 to 38 months after surgery). | |
| Outcomes | Preoperative uterine volume (cc). Preoperative haemoglobin (g/dL). Preoperative haematocrit (%). Duration of surgery (minutes). Intraoperative blood loss (mL). Postoperative morbidity. Frequency of blood transfusion. Duration of hospital stay (days). Recurrence of myomas. Change in quality of life. | |
| Notes | Author contacted for additional information and reply received. Study population stratified into 2 groups after pretreatment ultrasound: uterine volume < 600 cc and ≥ 600 cc and sensitivity analysis performed in different strata. Outcomes from 2 separate publications but same study population. Same surgical technique performed on participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...patients were randomised by permuted blocks” The size of the blocks was not stated. Treatment allocation can be predicted at the end of each block. |
| Allocation concealment (selection bias) | Low risk | Central control of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “…to receive either LA depot 3.75mg or placebo intramuscularly every 4 weeks for four injections” “All patients and examiners were blinded with respect to treatment group throughout the study”. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assumed that examiners were also the assessors of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “All patients enrolled completed the study protocol and were included in data analysis”. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all outcomes appear to have been reported in full. |
| Other bias | Low risk | Groups appear comparable at baseline. |
Gerris 1996.
| Methods | Randomisation method not given. Multinational (Belgium, the Netherlands, Portugal, Sweden, UK) multicentre study. Number of women randomised: N = 254. Withdrawals post randomisation and before treatment: N = 7. Withdrawals after treatment and before surgery: N = 32 (20 from treatment group: 7 due to side effects, 11 unable/unwilling to continue, 1 ovarian cyst, 1 lost to follow up; 12 from surgery only group: 5 unwilling/unable to continue, 3 lost to follow up, 1 menopausal symptoms, 1 started norethisterone, 2 operation could not be performed). No power calculation made but analysis by intention‐to‐treat. Source of funding: Zeneca Pharmaceuticals. | |
| Participants | Women aged over 25 years recruited from 6 clinics or hospitals in 5 countries. Inclusion criteria: premenopausal, diagnosis of benign uterine fibroids from ultrasound, awaiting hysterectomy, and either symptomatic, haemoglobin level < 12 g/dL or pelvic mass > 12 weeks in gestational size. Exclusion criteria: pregnant or breastfeeding, concomitant illness that would warrant exclusion, sex hormone therapy within 2 months of entry into the study. | |
| Interventions | Rx: Subcutaneous goserelin 3.6 mg monthly before hysterectomy, N = 127. Control: No treatment before hysterectomy, N = 127. Duration: 3 months. | |
| Outcomes | Preoperative uterine volume (cc). Preoperative fibroid volume (cc). Preoperative haemoglobin (g/dL). Preoperative haematocrit (%). Pelvic symptoms (score). Withdrawal due to adverse events. Postoperative haemoglobin (g/dL). Postoperative haematocrit (%). Intraoperative blood loss (mL). Duration of surgery (minutes). Difficulty of surgery. Frequency of blood transfusions. Duration of hospital stay (days). Type of operative incision. | |
| Notes | Author contacted for additional information and request forwarded to Zeneca but no reply received. 2 women randomised to Zoladex had surgery alone and 3 women randomised to surgery alone had Zoladex. Outcomes considered in this review were uterine and fibroid volume, pelvic symptom score, transfusion rate, difficulty of surgery, type of incision and withdrawal due to adverse events. Data were unsuitable for all other outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...patients were randomised.” Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment is not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “Patients were randomized to surgery alone…. Or to Zoladex treatment 3.6mg every month subcutaneously for 3 months prior to surgery” Participants were not blinded. No mention of personnel being blinded to study allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding is not reported and unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition unbalanced between groups ‐ higher in treatment than control group. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all outcomes in methods section were reported in full in the results section. |
| Other bias | Unclear risk | The authors acknowledged that the mean uterine volume for the Zoladex group was approximately 50 cm³ bigger than the surgery alone group mainly due to larger fibroids. Also women in surgery alone group had higher mean haemoglobin than the Zoladex group at entry. |
Golan 1993.
| Methods | Randomisation method not stated and no blinding. Number of women randomised: N = 53. No withdrawals reported. No power calculation made. No source of funding reported. | |
| Participants | Women with symptomatology related to uterine fibroids recruited from medical centre in Israel. No other specific inclusion and exclusion criteria specified although all uteri were at least the size of 12 weeks gestation. | |
| Interventions | Rx: Intramuscular D‐Trp LHRH 3.2 mg micro capsules (Decapeptyl) monthly before surgery (hysterectomy, N = 17; myomectomy, N = 12). Control: No preoperative treatment (hysterectomy, N = 15; myomectomy, N = 9). Duration: 2 months | |
| Outcomes | Preoperative uterine volume (mL) all participants. All other outcomes given separately for hysterectomy and myomectomy participants. Preoperative haemoglobin (g/dL). Duration of surgery (minutes). Intraoperative blood loss (mL). Frequency of blood transfusions. Duration of hospital stay (days). Postoperative complications. | |
| Notes | Author contacted for additional information but unable to supply this information. Each treatment group had a combination of hysterectomy and myomectomy surgery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The patients were randomly allocated”. Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided regarding allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not reported and unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details regarding blinding of outcome assessment were recorded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is not reported whether any participants dropped out during the study |
| Selective reporting (reporting bias) | High risk | No protocol available but all outcomes from methods section were reported in the results section. |
| Other bias | Unclear risk | “Intraoperative blood loss estimated by the senior surgeon based of the volume of aspirated blood by the suction apparatus and the count of soaked abdominal pads”. Not convinced this is an accurate way of measuring blood loss when this was considered to be a primary outcome. This was acknowledged in the discussion. It is also not clear whether the groups were comparable at baseline. |
Hudecek 2012.
| Methods | Parallel group single centre RCT. Number of women randomised: 212. Number of women analysed: not clear, assumed it was 212. Number of withdrawals: not reported. Power calculation for sample size not reported. Source of funding: not reported. |
|
| Participants | Participants recruited from Gynecological and Obstetric Clinic of Medical Facility of Masaryk University and the University Hospital Brno, Czech Republic. Inclusion criteria: reproductive aged females with uterine symptomatic myomatosis. Exclusion criteria: not reported. |
|
| Interventions | Rx: Goserelin acetate 3.6 mg SC 3 times once every 4 weeks, N = 120. Control: No pretreatment before surgery, N = 92. 42.5% of participants had laparoscopic myomectomy and 57.5% of participants had open laparotomic myomectomy. |
|
| Outcomes | Perioperative blood loss. Duration of surgery. Length of hospital stay. Perioperative and postoperative complications. |
|
| Notes | Translated from Czech by Petr Tomek, Auckland University. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported but stated as ITT. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. SLL outcomes not reported as these were not measured in this review. |
| Other bias | Unclear risk | Czech and English abstracts of the article report different numbers of women treated with open myomectomy (78 vs. 44 respectively). |
Levens 2008.
| Methods | Parallel group single centre RCT. Number of women randomised: N = 22. Number of women analysed: N = 18. Number of withdrawals: N = 4 (but secondary analysis performed to evaluate whether significant differences existed between dropouts and completers). No power calculation for sample size reported. Source of funding: (in part) Reproductive Biology and Medicine branch (NIH, Bethseda Maryland) and HRA Pharma France. |
|
| Participants | Inclusion criteria: healthy non pregnant women aged 33 to 50 years with regular menses (cycles every 24 to 35 days) and one or more leiomyomata > 2 cm in diameter; desiring hysterectomy; haemoglobin > 10 g/dL, current use of non hormonal contraception, BMI < 33 kg/m². Exclusion criteria: inability to complete study requirements, prior uterine artery embolisation, menopausal status (FSH > 20 mU/mL), cervical dysplasia, adnexal mass, genetic cause of rapid growth of leiomyomata, unexplained vaginal bleeding, use of glucocorticoids, progestins or agents that alter ovarian or hepatic function. Mean age: 45, 43 and 44 years Recruitment not clear ‐ study location USA. |
|
| Interventions |
Duration: 3 cycles or 90 to 102 days if no menses occurred |
|
| Outcomes | Primary: Fibroid volume (determined by MRI). Other: Proportion of amenorrhoea, change in haemoglobin and haematocrit, ovulation inhibition, quality of life. |
|
| Notes | Target enrolment was 36 participants but recruitment was terminated after 22 participants were enrolled because of slow recruitment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated blocks of 6. |
| Allocation concealment (selection bias) | Low risk | Authors stated that allocation concealment was "assured". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "...both patients and health care providers" were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assumption that assessors were also blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Very small study with 18% withdrawals overall (25% withdrawal from 2 of the 3 randomised groups). Quality of life assessments performed in only 50% of original participants. |
| Selective reporting (reporting bias) | Unclear risk | Quality of life assessments not reported in full. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
Lumsden 1994.
| Methods | Randomisation by third party who opened the code‐break. Multicentre study with double blinding. Number of women randomised: N = 71. Number of withdrawals: N = 6 (3 from treatment group due to adverse events and 3 from the placebo group: 1 due to pregnancy, 1 due to inclusion criteria not met, the other not specified). Previous pilot study conducted, power calculation for sample size performed and analysis by intention‐to‐treat. Source of funding not reported. | |
| Participants | Premenopausal women with mean age 43 years awaiting total abdominal hysterectomy for uterine fibroids recruited from hospitals in Edinburgh, Glasgow and Newcastle, UK. No other specific inclusion or exclusion criteria reported although all women had regular menstrual cycles, fibroids were confirmed by ultrasound, there was no recent history of dilatation and curettage and none were pregnant. | |
| Interventions | Rx: Subcutaneous goserelin 3.6 mg monthly before hysterectomy, N = 35. Control: Subcutaneous placebo monthly before hysterectomy, N = 6. Duration: 3 months. | |
| Outcomes | Preoperative uterine volume (cc). Preoperative haemoglobin (g/dL). Pelvic symptoms (score). Adverse events. Intraoperative blood loss (mL). Duration of surgery (minutes). Difficulty of surgery. Type of operative incision. Duration of hospital stay (days). Postoperative haemoglobin (g/dL). | |
| Notes | Author contacted for additional data who forwarded the request to Zeneca but reply not received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomised by a third party who opened the code‐break bearing the next consecutive patient number which contained the randomisation to either goserelin or placebo treatment”. |
| Allocation concealment (selection bias) | Low risk | “Randomisation was performed by the research nurses involved so that the medical staff did not know into which group the patients fell”. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded. “Randomisation was performed by the research nurses involved so that the medical staff did not know into which group the patients fell”. “All applicators (goserelin and sham) were provided with transparent windows covered with a previously coded label so that they look identical, although the sham applicator was actually empty”. “Surgeons were requested not to ask the date of the last menstrual period at the time of the pre‐operative ward round as this would un‐blind the study”. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | “Randomisation was performed by the research nurses involved so that the medical staff did not know into which group the patients fell”. No further information on whether these medical staff performed outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis, “all randomised patients recruited into the study for whom data were available were included in the efficacy analysis”. Three women in each group withdrew, with details reported on each and it appears they were included in the analyses. |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes of interest were reported. Previous pilot study reported same outcomes as full study. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
Mavrelos 2010.
| Methods | Single‐centre (UK), prospective, randomised, placebo‐controlled trial. Randomisation carried out by a computer‐generated simple randomisation sequence with opaque sealed envelopes and staff nurses who were not part of the trial. Double‐blind study. Number of women randomised: N = 47, 24 to treatment group and 23 to placebo. Number of withdrawals: 7 women did not undergo planned operation, 3 in treatment group, 4 in placebo. Reasons: allergic reaction in one, two women opted for abdominal myomectomy, four did not attend for operation. Primary outcome was analysed with Intention‐to‐treat. Power calculation carried out. Study supported by Kings College Hospital NHS Foundation Trust. |
|
| Participants | Inclusion criteria: history of heavy or irregular menstrual periods. Diagnosis of Type I or Type II submucous fibroid on ultrasound. Type 1 = fibroids with < 50% contained within the myometrium, Type II = ≥ 50% contained within myometrium. No specific exclusion criteria stated. |
|
| Interventions | Treatment: 24 women received goserelin 3.6 mg (Zoladex, AstraZeneca) three injections given at 4 weekly intervals. Surgery took place 4 weeks after the last injection. Control: subcutaneous injections of placebo (5 mL 1% lignocaine), three injections given at 4 weekly intervals. Surgery took place 4 weeks after the last injection. Duration: three months with 6 weeks post‐operative follow up. |
|
| Outcomes | Completion of fibroid resection. Volume of fluid infusion. Fluid deficit > 1500 mL. Duration of surgery. Complications. Recurrence of myomas. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation carried out by a computer‐generated simple randomisation sequence with staff nurses who were not part of the trial. |
| Allocation concealment (selection bias) | Low risk | "...consecutively numbered, opaque, sealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "...both patients and clinicians were blinded to the group allocation". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Patients underwent hysteroscopic transcervical resection of myoma by a single experienced operator.” "clinicians blinded to group allocation". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial attrition. Only postoperative complications were assessed in all participants. |
| Selective reporting (reporting bias) | Low risk | Protocol viewed, all outcomes stated in method and protocol were reported. |
| Other bias | Unclear risk | Participants in treatment group were younger than those in control group. |
Muneyyirci‐Delale 2007.
| Methods | Randomisation schedule prepared by Biometrics Group, AstraZeneca Pharmaceuticals. Study personnel had to contact the randomisation desk for allocation of treatment. Phase III, multicentre (sites in North America), double blind controlled trial. Number of women randomised: N = 110, 54 to treatment and 56 to control Number of withdrawals: 38 participants dropped out of the study, 20 from treatment group and 18 from control. Reasons include: loss to follow up; adverse event or intercurrent illness; protocol non‐compliance, or withdrawal of informed consent. Power calculation performed, intention‐to‐treat analysis carried out on primary outcome. Study funded by AstraZeneca. |
|
| Participants | Premenopausal women aged over 18 years, with a history of excessive menstrual bleeding causing iron‐deficiency anaemia (IDA) who were candidates for hysterectomy or myomectomy. Participants underwent screening to demonstrate uterus ≥ 8 weeks gestation in size and the presence of ≥ 1 non‐calcified leiomyoma of ≥ 3 cm diameter. Participants were required to have a negative cervical smear test within 6 months of trial entry and a negative endometrial biopsy within the 45 day period before randomisation. Exclusion criteria: women with any blood disorder other than IDA (thalassaemia, sickle cell anaemia, folic‐acid deficiency, coagulopathy). Women with renal or hepatic impairment, gynaecological malignancy or pre malignancy, adrenal, pancreatic, ovarian or pituitary tumours, osteoporosis, osteopenia or metabolic bone disease. Women with any other medical condition which might confound the haematologic parameters. Blood transfusion within 8 weeks of randomisation or blood donation within two weeks was not permitted. Women who had received treatment with an LHRH analogue within previous 6 months, or who had a known hypersensitivity to LHRH, LHRH agonists or analogues, or any of the components of the study medication. Pregnant women were excluded. |
|
| Interventions | Intervention: Injection of goserelin acetate 10.8 mg depot 12 weeks before planned surgery. Supplied as a pre‐filled sterile delivery device, and dispersed in a cylindrical rod of D,L, lactide glycolide polymer. Control: Sham depot injection containing copolymer only, supplied in a sterile syringe applicator identical to goserelin device, 12 weeks before planned surgery. Every study participant received 325 mg ferrous sulphate taken three times daily, for 12 weeks until surgery. |
|
| Outcomes | Haemoglobin concentration at time of surgery (g/dL). Percentage of women achieving an increase in Hb ≥ 2 g/dL. Percentage of women achieving haematologic recovery where Hb ≥ 12 g/dL. Symptoms associated with uterine leiomyomas. Requirement for blood transfusion at pre‐, peri‐, and postoperative visits. Ability to donate blood for autologous transfusion. Fibroid and total uterine volume measured by ultrasound (cm³) |
|
| Notes | Author contacted for additional data on 21.11.11 and awaiting reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatment group was determined by a randomisation schedule prepared by the Biometrics Group, AstraZeneca Pharmaceuticals." |
| Allocation concealment (selection bias) | Low risk | "Investigators were instructed to contact the randomisation desk for a subject number and allocation". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "sham injection in a double‐blind manner". “The sham depot was… identical to the goserelin device.” Treatment administrators were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details regarding blinding of surgeons or ultrasonographers. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis only carried out for Hb levels and adverse events. “Of the 110 subjects treated, 72 completed the trial”. 34.5% of patients withdrew from the study. “Reasons for withdrawal were similar in the 2 groups” although more participants were lost to follow up in the goserelin group than the sham group and more were lost due to protocol noncompliance in the sham group compared to the goserelin group. |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods section were reported in the results section. Adverse events reported in full. |
| Other bias | Unclear risk | Unclear if groups comparable at baseline. |
Muzii 2010.
| Methods | Multicentre parallel group RCT. Number of women randomised: N = 39. Number of women analysed: N = 39. No withdrawals. Power calculation for sample size (reduction of 50% in operating time with GnRHa). Source of funding: not reported. |
|
| Participants | Inclusion criteria: premenopausal women with submucous fibroids (diagnosed by TVUS) with diameter between 10 mm and 35 mm, grade GO or G1 (fibroids either completely intracavity or with an intramural portion of < 50%), BMI between 18 and 30 kg/m². Exclusion criteria: present or past history of cancer, a preoperative clinical suspicion of associated multiple or large polyps, planned associated non hysteroscopic surgical procedures or > 2 fibroids requiring hysteroscopic resection. Mean age: 42 years. Recruited from 3 tertiary care hospitals in Rome, Italy. |
|
| Interventions |
|
|
| Outcomes | Operating times, fluid absorption, difficulty of the operation, surgeon satisfaction with the procedure, intraoperative and postoperative complications, postoperative pain, patient satisfaction. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer generated sequence". |
| Allocation concealment (selection bias) | Low risk | "...sealed opaque envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes not reported in full. |
| Other bias | Low risk | Groups comparable at baseline. |
Nikolov 1999.
| Methods | Randomisation by sealed opaque sequentially numbered identical envelopes. Single centre, parallel group with no blinding. Number of women randomised: N = 34. No withdrawals or loss to follow up. No power calculation performed. Source of funding not reported. | |
| Participants | Premenopausal women aged 25 to 50 years awaiting surgery for uterine fibroids were recruited from the State Maternity Hospital in Sofia, Bulgaria. Other inclusion criteria: aged over 25 years, benign uterine fibroids confirmed by ultrasound, anaemia (Hb < 10 g/dL or 7 nmol/L) or with pelvic mass < 12 gestational weeks. Exclusion criteria: pregnant or breastfeeding, sex hormone therapy for last 12 months, severe illness interfering with the aims of the study. | |
| Interventions | Rx: Subcutaneous goserelin 3.6 mg monthly before myomectomy (N = 6) or hysterectomy (N = 11). Control: No treatment before hysterectomy surgery (N = 17) but observation period for 3 months. Duration: 3 months. | |
| Outcomes | Preoperative uterine volume (mL) Preoperative fibroid volume (mL) Preoperative haemoglobin levels (g/dL) Intraoperative blood loss (mL) Duration of surgery (days) | |
| Notes | Data given separately for intraoperative outcomes according to whether myomectomy or hysterectomy performed but data entered only for hysterectomy because this was the only surgery performed in the control group. Author contacted for additional information and reply received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of the method of random sequence generation. "...the women were subdivided into two groups by randomisation principle". |
| Allocation concealment (selection bias) | Low risk | Sealed opaque sequentially numbered identical envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It seems there were no losses to follow up. In the treatment group 6 women had myomectomy and 11 had hysterectomy, while in the control group all 17 had hysterectomy. |
| Selective reporting (reporting bias) | Unclear risk | For all continuous variables (as outcome measures), the authors did not present the numbers experiencing the event. |
| Other bias | Unclear risk | Unclear if groups similar at baseline. |
Reinsch 1994.
| Methods | Single centre, parallel group RCT. Number of women randomised: N = 14. No apparent withdrawals. Power calculation for sample size not reported. Source of funding: not reported. |
|
| Participants | Inclusion criteria: not clearly specified ‐ all women had uterine fibroids. Exclusion criteria: not reported. Mean age of participants: not reported. Recruitment source not reported. |
|
| Interventions |
|
|
| Outcomes | Uterine artery blood flow, uterine volume, adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned" but method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported but unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported but unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study authors did not report whether there were any withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes not fully reported. |
| Other bias | Unclear risk | Study authors stated that groups were comparable at baseline but no values were reported. |
Sayyah‐Melli 2007.
| Methods | Single centre (Iran), parallel group RCT. Number of women randomised: N = 50. No withdrawals. No power calculation for sample size reported. Source of funding: not reported. |
|
| Participants | Inclusion criteria: women with uterine myoma nodules > 5 cm in diameter with irregular menstrual cycle and candidates for myomectomy. Exclusion criteria: > 40 years of age, abnormal uterine pathology, infection, hypersensitivity to any ergot alkaloids, hepatic and renal disorders, history of toxemia of pregnancy, cardiovascular disease, peptic ulcer, taking antipsychotic medications. Mean age: 30 and 32 years. Recruited from Alzahra University Hospital, Tabriz, Iran. |
|
| Interventions |
|
|
| Outcomes | Reduction in fibroid volume, symptoms, adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...assigned randomly" but no method reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not reported and highly unlikely because of different administration of intervention regimens (injection and tablet). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not reported and unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported withdrawals. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes fully reported. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
Sayyah‐Melli 2009.
| Methods | Single centre (Iran) parallel group RCT. Number of women randomised: N = 60. It appears that there are no withdrawals so presumably all participants were analysed. Power calculation for sample size not reported. Source of funding: grant from Tabriz University of Medical Sciences, no funding from drug company. |
|
| Participants | Women with uterine fibroids recruited from Iranian hospital between September 2007 and November 2008. Inclusion criteria: women of reproductive age who had abnormal bleeding or infertility with uterine intramural fibroids. Exclusion criteria: submucous or subserous fibroids, abnormal uterine pathology and infection, aged ≥ 43 years. |
|
| Interventions | Rx 1: Dipheredine 3.75 mg (GnRHa) 4 times every 28 days, N = 30. Rx 2: Dostinex (Cabergoline) 0.5 mg once per week for 6 weeks, N = 30. Only a proportion of women went on to have surgery. Duration of Rx: 6 weeks to 4 months. |
|
| Outcomes | Fibroid volume. Adverse effects. |
|
| Notes | Data on adverse effects were inconsistent between table and text so were not extracted. Intraoperative outcomes could not be used in the review as only a small proportion of women went on to have surgery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were obviously not blinded because of different treatment administration schedules. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported if any participants withdrew. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
Seraccholi 2003.
| Methods | Randomised controlled single centre (Italy) trial. Number of women randomised: N = 62. Number of withdrawals: not clear. Power calculation not reported and unclear if intention to treat analysis. Source of funding not reported. |
|
| Participants | Inclusion criteria: women with symptomatic fibroid, with mobile uterus and vaginal accessibility with uterus size between 16 to 20 weeks clinically and volume between 380 mL and 680 mL ultrasonographically. Exclusion criteria: women with pelvic pathology as prolapse, pelvic floor relaxation, SI, adnexal mass; women with medical conditions requiring monitoring as diabetes, IHD; women who had therapy with GnRHa, danazol or progestational agents in last 6 months; women who had undergone surgery requiring longitudinal laparotomy; women with any contraindication to operative laparoscopy. |
|
| Interventions | Treatment group: triptorelin depot 11.25 mg starting in mid luteal phase 3 months before surgery, N = 31. Control group: no therapy, N = 31. |
|
| Outcomes |
Preoperative Pretreatment: uterine volume and weight, haemoglobin, uterine bleeding, pelvic pain, urinary urgency. Operative Time of operation from skin incision and pneumoperitoneum to closure. Postoperative
|
|
| Notes | Age: Treatment group 47.6 ± 3.5 years Control group 48.4 ± 4.6 years |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were assigned at a ratio 1:1 by random selection” – method of randomisation not specified. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Women were randomised to injection or no treatment. No details on personnel blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details regarding outcome assessment were provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether all participants were included in analysis. |
| Selective reporting (reporting bias) | High risk | The study authors only reported adverse events and changes in uterine volume/weight in the intervention group before surgery ‐ no comparison was made with control so this outcome was not relevant to the review. Introduction mentions evaluating “operating time, surgical complications, conversion to laparotomy, blood loss, hospital stay, and costs”. Results report all of these except costs. |
| Other bias | Low risk | Groups appear comparable at baseline. |
Shaw 1989.
| Methods | Method of randomisation not stated. Single centre (UK), parallel group design with no blinding. Number of women analysed: N = 32. No information given about actual numbers randomised or withdrawals. No power calculation for sample size or intention to treat analysis reported. Source of funding not stated. | |
| Participants | Women with large fibroid uteri, from 14 to 30 weeks in gestational size were recruited. No specific inclusion or exclusion criteria reported. | |
| Interventions | Rx: Goserelin depot 3.6 mg before surgery (myomectomy and hysterectomy). Control: No treatment before surgery (myomectomy and hysterectomy). Duration in treated group: 4 months. | |
| Outcomes | Uterine volume before surgery (mL) (data not given). Intraoperative blood loss (mL) (reported separately for myomectomy and hysterectomy). Blood transfusion rate (given for myomectomy only). | |
| Notes | Data not provided for uterine volume before surgery. Author contacted for additional information but no reply received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “They were randomised to receive Zoladex depot for 4 months or to act as controls with no treatment”. Method of randomisation not specified. |
| Allocation concealment (selection bias) | Unclear risk | No details on allocation concealment reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants or personnel not reported and unlikely because of the differing treatment regimens. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not specified who assessed the outcomes or whether the assessor/s was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 32 women were included in the analysis. It is not stated how many were recruited or randomised, or if there were any withdrawals. |
| Selective reporting (reporting bias) | High risk | Uterine volume before surgery was not reported. Intraoperative blood loss was reported, without the numbers of the groups provided. Blood transfusion rate only provided for one group. No adverse effects information was reported. |
| Other bias | Unclear risk | No characteristics of the two assigned groups were provided so we could not confirm if they were comparable at baseline. |
Shaw 1996.
| Methods | Randomisation on a 1:1 basis according to a randomisation schedule controlled by Hoechst and stratified according to uterine size to minimise bias. Multicentre study (23 centres: UK (21), Israel (2)) with double blinding. Number of women randomised: 210 Number of women analysed: 196 (intention‐to‐treat); 164 (per protocol). Intention to treat analysis and power calculation performed for sample size. Source of funding: Hoechst UK. | |
| Participants | Women aged 29 to 52 years were recruited from 21 medical centres in the UK and 2 in Israel. Inclusion criteria: Aged ≥ 20 years; willing and able to participate; informed consent; fibroids clinically diagnosed at gynaecological examination and confirmed by US; uterus with size at least 8 weeks of pregnancy; menorrhagia and/or other symptoms of sufficient intensity to require hysterectomy. Exclusion criteria: persistent symptoms characteristic of menopause; FSH levels suggestive of ovarian failure; rapidly increasing uterine size; irregular vaginal bleeding of unknown origin; requirement for immediate hysterectomy; pregnancy or breastfeeding; history of hypersensitivity to the study medication or similar drugs; likelihood of requiring treatment during the study with drugs not permitted by study protocol; treatment with any other investigational drug in the last 3 months; terminal disease; history of drug or alcohol abuse; any serious endocrine disorder other than stable diabetes; impaired renal or hepatic function; impaired mental condition; history of major depression within last 3 years; treatment with LHRH analogue in previous 6 months; evidence of uncooperative attitude; treatment with oral contraceptives or progestogens; previous entry to the study. | |
| Interventions | Rx: Buserelin 3.6 mg monthly (intramuscular), N = 98. Control: Placebo monthly, N = 98. Duration: 3 months. | |
| Outcomes | Primary: menstrual blood loss during treatment; blood loss during surgery. Secondary: fibroid/uterine volume; haemoglobin levels, ease of surgery; type of incision; type of hysterectomy; duration of surgery; change in symptoms, adverse events. | |
| Notes | Unpublished study released by Hoechst. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Subjects were randomised to the trial of whom 98 received buserelin and 98 placebo”. Randomisation on a 1:1 basis according to a randomisation schedule controlled by Hoechst and stratified according to uterine size to minimise bias. |
| Allocation concealment (selection bias) | Low risk | Central control. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “This study was a… double blind comparison”. Participants received either buserelin 3.6 mg monthly or placebo monthly for 3 months. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not specified who assessed the outcomes or whether the assessment was performed by a blinded party. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 210 women randomised, 196 women intention‐to‐treat analysis, 164 subjects per‐protocol analysis. Both analyses presented and reasons given for withdrawals. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | Groups comparable at baseline. |
Stovall 1994.
| Methods | Randomisation by computer generated random number table with no blinding. Number of women randomised: N = 150 (in 1994 study). No withdrawals reported. No power calculation made. Source of funding: TAP Pharmaceticals Inc (in part). | |
| Participants | Premenopausal women aged 29 to 51 years with symptomatic uterine fibroids scheduled to undergo hysterectomy were recruited in Tennessee, USA. Inclusion criteria: Inclusion criteria: FSH < 30 mIU/mL, negative urine pregnancy test, presence of fibroids ≥ 14 gestational weeks on pelvic examination, absence of uterine calcification on ultrasound examination, symptoms of increased vaginal bleeding, pain or pressure, no evidence of ovarian or uterine malignancy from pelvic examination or ultrasonography, benign endometrial histologic features where sampling was indicated and normal cervical cytologic characteristics. No specific exclusion criteria were reported. | |
| Interventions | Rx 1: Either subcutaneous leuprolide acetate 0.5 mg daily or intramuscular depot leuprolide acetate 3.75 mg monthly before hysterectomy, N = 45.
Control: no preoperative treatment before hysterectomy, N = 45. Data were only analysed from the subgroup who had gestational size of 14 to 18 weeks. Duration: 2 months (treatment group only). |
|
| Outcomes | Preoperative uterine size (gestational weeks). Preoperative uterine volume (mL) measured by ultrasound. Duration of surgery (minutes). Intraoperative blood loss (mL). Postoperative complications. Frequency of blood transfusions. Duration of hospital stay (days). Proportion undergoing vaginal rather than abdominal hysterectomy. Preoperative and post‐operative haemoglobin and haematocrit (from smaller number of participants in 1991 study). | |
| Notes | Author contacted for additional information but no reply received. Subgroup analysis performed in 2 separate treatment and control groups: women with uterine size 14 to 18 gestational weeks and women with uterine size > 18 gestational weeks. Vaginal hysterectomy attempted if uterus mobile and ≤ 14 weeks in gestational size. Participants from earlier study in 1991 a subset of later study in 1994 and haematological parameters provided only for the this subset of women. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomised by a computer‐generated random number table”. |
| Allocation concealment (selection bias) | Unclear risk | “Patients were randomised by a computer‐generated random number table”. No other details were given with respect to concealing allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Immediate and delayed surgery so participants were unable to be blinded; not clear if personnel blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not specified who assessed the outcomes or whether the assessment was performed by a blinded party. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appears there were no withdrawals from the study by checking the percentages recorded for dichotomous outcomes. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | High risk | The first 10 participants in group IIB were given leuprolide acetate 0.5 mg subcutaneously daily for 8 weeks, the remaining women received two intramuscular injections of depot leuprolide acetate, 3.75 mg 4 weeks apart ‐ not clear whether this could cause bias. In addition, there was a short‐stay protocol for vaginal hysterectomy resulting in a reduction in hospital stay unrelated to the treatment with GnRHa therapy. |
Stovall 1995.
| Methods | Randomisation method not stated. Multicentre, parallel group study with double blinding. Number of women randomised: N = 309. Exclusions post randomisation: N = 44 (due to insufficient washout period after hormone therapy or failure to meet stated haematologic criteria). Number of additional withdrawals: N = 47 (women who decided not to have surgery). Power calculation for sample size performed and analysis by intention‐to‐treat. Source of funding: TAP Pharmaceuticals Inc. | |
| Participants | Women aged 23 to 52 years (mean age 39 years) recruited from 50 centres in the USA. Inclusion criteria: not pregnant or lactating, aged > 18 years, free of gynaecological malignancy, history of prolonged or excessive bleeding for 3 months, pelvic masses consistent with fibroids established by history and pelvic exam and confirmed by ultrasound and MRI, consent to surgical management, hematocrit ≤ 30% and/or haemoglobin ≤ 10.2, no evidence of concomitant disease with the potential for producing bleeding that would result in iron‐deficiency anaemia. No additional exclusion criteria reported. | |
| Interventions | Rx 1: Intramuscular leuprolide acetate depot 7.5 mg + iron monthly (results for this treatment group not included in the review), N = 107. Rx 2: Intramuscular leuprolide acetate depot 3.75 mg + iron monthly, N = 104. Control: Placebo + iron monthly, N = 98. Duration: 3 months. | |
| Outcomes | Preoperative haemoglobin (g/dL). Preoperative haematocrit (%). Preoperative uterine size (gestational weeks). Preoperative uterine volume. Preoperative fibroid volume. Preoperative pelvic symptoms. Frequency of adverse events (listed). Frequency of blood transfusions (not recorded in the review because different types of surgery performed and this data not given separately). | |
| Notes | Study author contacted for additional information but no reply received. Different types of surgery performed (hysterectomy in 137 women (63%), myomectomy in 80 women (37%) and endometrial ablation in 1 woman). The only outcomes considered in this review were preoperative haemoglobin and hematocrit (no SD given), pelvic symptoms and adverse events. For all of the other outcomes, the data were not in a suitable form for meta‐analysis. Results analysed in 2 strata: A: baseline hematocrit ≤ 28%; B: baseline haematocrit > 28%. Data from the first treatment group with the higher dosage of 7.5 mg leuprolide acetate not considered in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants stratified “arbitrarily” into one of two strata based on their pre‐study haematocrit level. “Within each stratum, patients were randomised to one of three treatment arms”. Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | No details on allocation concealment reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “A blinded central reader was used for all bone mineral densitometry scans”. “Each patient received an intramuscular injection of study drug or placebo”. Study stated to be “double‐blinded”. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 309 women were enrolled and treated, of these only 265 women (86%) were evaluated for efficacy. 47/265 women “decided not to have surgery” so surgical outcomes were not reported. There was also substantial attrition for some other outcomes. All women were included in the adverse event analysis. |
| Selective reporting (reporting bias) | High risk | Data for main outcomes not fully reported. |
| Other bias | Unclear risk | The study authors reported that there were no significant differences between randomised groups but did not clearly report the individual values. |
Vercellini 1998.
| Methods | Method of randomisation by computer generated randomisation sequences stratified per centre with consecutively numbered opaque sealed envelopes. Multicentre (4 centres in northern Italy) study with single blinding. Number of women randomised: N = 127. Number of withdrawals: N = 4 (2 in each group; one who refused treatment, one for personal non‐medical reasons, one due to menopause and one who decided to have surgery in another hospital). Power calculation for sample size performed and analysis by intention‐to‐treat. Source of funding not reported. | |
| Participants | Premenopausal women with median age 46 years (range 43 to 48 years) were recruited from 4 Italian centres specialising in vaginal surgery. Inclusion criteria: premenopausal (FSH < 30 mIU/mL), symptomatic fibroids requiring hysterectomy, uterine volume of 12 to 16 gestational weeks, mobile uterus with volume 380 mL to 680 mL on ultrasound, regular vaginal accessibility, no adnexal tumours at clinical and ultrasound examination. Exclusion criteria: uncertainty about future childbearing, use of GnRHa in the past 6 months, previous pelvic interventions with the exception of caesarian section, pelvic inflammatory disease or endometriosis, urinary stress incontinence, moderate or severe genital prolapse, clotting disorders, unstable general conditions. | |
| Interventions | Rx: Intramuscular triptorelin depot injections 3.75 mg (Decapeptyl) monthly before hysterectomy, N = 62. Control: Immediate surgery (hysterectomy), N = 65. Duration: 3 months. | |
| Outcomes | Preoperative uterine volume (mL). Duration of surgery (minutes). Intraoperative blood loss (mL). Difficulty of surgery. Frequency of blood transfusions. Proportion undergoing vaginal rather than abdominal hysterectomy. Postoperative complications. Postoperative haemoglobin (g/dL). Postoperative haematocrit (%). Patient satisfaction (not included in the review). | |
| Notes | Hysterectomy was by both the vaginal and abdominal route but data not provided separately for these groups so separate analysis not possible. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Performed in a separate setting in accordance with computer‐generated randomisation sequences stratified per centre”. |
| Allocation concealment (selection bias) | Low risk | “Using consecutively numbered opaque, sealed envelopes.” “The evaluator was blinded with regard to treatment allocation”. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Surgeon was not allowed to interview participants, and participants were requested to avoid mention of their last menstrual period. However, participants were not blinded, as they either received immediate surgery or treatment and delayed surgery. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome was vaginal vs. abdominal hysterectomy, no women who were recommended vaginal hysterectomy required conversion to abdominal hysterectomy. The surgeon who evaluated which surgery the woman would have was blinded to the treatment allocation (same surgeon as above), however “it is possible that the evaluator could have recalled examining the same woman three months before” as “only the patients allocated to pre‐operative medical treatment were examined twice.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four women withdrew after randomisation and before surgery, two from each arm. These 4 participants were also included in the efficacy analysis. “The inclusion of the four withdrawn patients in the analysis did not modify the appreciably the above estimates”. All women operated on attended the follow‐up evaluation. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported clearly. |
| Other bias | Low risk | Randomised groups appeared comparable at baseline. |
Vercellini 2003.
| Methods | Method of randomisation in a proportion of 1:1 by a computer generated randomisation sequence using serially numbered sealed opaque envelopes. Single centre study. Open labelled study (single blind). Number randomised N = 100. Number of withdrawals N = 3, 2 in immediate surgery group (one became pregnant, and one opted for surgery at different hospital,1 in the GnRHa group had to undergo hysterectomy. Power calculation for sample size performed and analysis by intention to treat. Source of funding not reported. Triptorelin depot injections provided by IPSEN Biotech Pharmaceuticals, Milan, Italy. |
|
| Participants | Premenopausal women aged 18 to 40 years with symptomatic intramural or subserous fibroid > 3 cm were included. Exclusion criteria: If predominantly intracavitary fibroids, previous pelvic surgery for leiomyomas or other genital abnormalities,uterine malformations, present or past pelvic inflammatory diseases, use of GnRHa up to 6 months prior, ultrasonography showing signs of uterine calcifications, coagulation disorders and unstable general conditions. |
|
| Interventions | Rx: Intramuscular triptorelin depot injections 3.75 mg (Decapeptyl) on 2 occasions 28 days apart starting during mid luteal phase, N = 50. Control: Immediate surgery (abdominal myomectomy), N = 50. Duration: 2 months. | |
| Outcomes | No preoperative evaluation. Operative Duration of surgery (minutes) Intraoperative blood loss (mL) Difficulty of surgery Frequency of blood transfusions Post operative Duration of hospital stay. Postoperative complications. Postoperative haemoglobin (g/dL). Postoperative haematocrit (%). Patient satisfaction (not included in the review). |
|
| Notes | No preoperative assessment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatment allocation was performed with a computer‐generated randomization sequence". |
| Allocation concealment (selection bias) | Low risk | “...using serially numbered, opaque, sealed envelopes”. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could not be blinded as they either received immediate surgery or treatment and delayed surgery. Study reported as "open label". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were low in number and were evenly distributed, and all available data appeared to be reported. After randomisation and before surgery, 3 women withdrew from the study, 2 from control group and 1 in triptorelin group and were not included in the analysis (reasons given). |
| Selective reporting (reporting bias) | Low risk | It appears that all obvious outcomes were reported. |
| Other bias | Low risk | Groups appear comparable at baseline. |
Verspyck 2000.
| Methods | Balanced randomisation list predefined for each centre and balanced after every 4 women. Multicentre study (10 centres) with no blinding. Number of women randomised: 56. Number of women analysed: 46. Exploratory intention to treat analysis but 10 women withdrew before the completion of the study (5 from each group), 2 for inefficacy, 6 for adverse events, 1 for protocol deviation and 1 lost to follow‐up. No power calculation for sample size. Source of funding not stated. | |
| Participants | Women with symptomatic fibroids indicating surgery (mean age 41.3 years) were recruited from 10 medical centres in France. Inclusion criteria: baseline pelvic ultrasound showing evidence of ≥ 1 fibroid ≥ 5 cm in diameter or submucous fibroids. Exclusion criteria: amenorrhoea; progestin or GnRHa treatment in previous 6 months; administration of another hormone therapy (except insulin); calcified fibroids; fibroids causing acute compressive complications. | |
| Interventions | Rx: Leuprorelin 3.75 mg monthly (subcutaneous), N = 33. Control: Lynestrenol 5 mg twice daily from day 5 to 25 of the cycle, N = 23. Duration: 4 months. | |
| Outcomes | Ultrasound reduction of myoma diameter. Percentage decrease in myoma diameter. Intensity of pelvic pain. Proportion with change in pelvic pain. Proportion with change in other symptoms. Preoperative haemoglobin. Postoperative haemoglobin. Blood transfusion rate. Adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The treatments were allocated according to a randomisation list predefined for each centre as balanced after every four patients”. It is not clear how the balancing worked or whether it had the potential for bias. |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment was reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “An open‐label study design was used because of the different administration conditions for the two products i.e. leuprorelin is injected subcutaneously and lynestrenol is administered orally. A double‐blind design would have been ideal, but the injection of a placebo for leuprorelin raises ethical problems”. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Exploratory intent‐to‐treat analysis, Withdrawals described, but substantial attrition for some outcomes. Details on surgical outcomes were only found for 25/33 participants for the leuprorelin group, and 17/23 in the lynestrenol group. |
| Selective reporting (reporting bias) | Unclear risk | Some outcome details not clearly reported. |
| Other bias | High risk | Significant differences in characteristics between the two groups. Statistically significant difference in distribution by category, more participants with multiple myomas in the leuprorelin group. “Larger number of myomas observed in patients in the leuprorelin group compared to those in the lynestrenol group. Consequently, the group treated with leuprorelin would rather have been put a disadvantage”. In addition, there was poor recruitment, “Three centres had only enrolled the first patient list (leuprorelin group each time).” |
Wilkens 2008.
| Methods | Multicentre (4 centres in the UK), parallel group, RCT (phase 2 trial). Number of women randomised: 33. Number of women analysed: 33 (no withdrawals). Power calculation for sample size: 95% power to detect a 0.08 difference between asoprisnil and placebo in RI (resistance index). Source of funding: TAP Pharmaceutical Products Inc (2 authors appear to have major conflicts of interest). |
|
| Participants | Premenopausal women scheduled for hysterectomy due to symptomatic fibroids were recruited from 4 UK centres. Inclusion criteria: general good health, menstrual cycle between 17 and 42 days, symptoms related to fibroid size, pressure and/or heavy menstrual bleeding, at least one intramural non‐pedunculated, submucosal or subserosal fibroid with a diameter of at least 2 cm or multiple small fibroids with uterine volume 200 cm³ on ultrasonography, age over 18 years; negative pregnancy test; a washout period of 2 to 12 months for hormonal therapies; serum FSH 30 mIU/mL 21 at commencement; agreement to use double barrier method of contraception (condom/diaphragm/sponge plus spermicide) throughout the study until hysterectomy, unless surgically sterile by bilateral tubal ligation or vasectomy of partner and normal Papanicolaou test. Exclusion criteria: abnormal endometrial biopsy report based on an adequate specimen taken within 3 months of commencement. |
|
| Interventions | Rx: Asoprisnil 10 mg or 25 mg orally once daily for 12 weeks, N = 12 and N = 11. Control: placebo, N = 10. All women then proceeded to hysterectomy. |
|
| Outcomes | Volume of the largest fibroid and the uterus. Menstrual blood loss (measured by menstrual pictogram). Adverse events. Quality of life (measured by Uterine Fibroid Symptom and Health‐Related Quality of Life Questionnaire (UFS‐QOL). |
|
| Notes | The primary outcomes in this study were not measured in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were sequentially assigned to subject numbers in ascending numerical order that encoded the assignment of the woman via a randomisation schedule into one of three arms of the study. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and all study personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and all study personnel were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | Groups comparable at baseline. |
Zullo 1998.
| Methods | Computer generated random assignment to 2 centres in the study, with no blinding. Two centres (Italy), parallel group design. Number of women randomised: N = 74. Number of exclusions post randomisation: N = 7 (2 from Rx group and 5 from control group, either because fibroid pedunculated or < 4 cc in volume or because of severe adhesions or endometriosis). Power calculation performed and intention‐to‐treat analysis. Source of funding not stated. | |
| Participants | Women, aged 24 to 45 years (mean 37.2 years), with symptomatic fibroids, recruited from a university department and a private centre for surgery in Naples, Italy. Inclusion criteria: history of infertility > 3 years or recurrent abortions, symptoms of increased vaginal bleeding, pelvic pain or pressure, lack of pedunculation of the main myoma with size 4 cc to 500 cc from ultrasound, presence of ≤ 4 myomas per woman, absence of submucosal fibroids from hysteroscope, absence of calcification in main myoma from ultrasound, absence of atypical hyperplasia from endometrial biopsy, absence of abnormal pap smear, negative urine pregnancy test result. | |
| Interventions | Rx: Intramuscular leuprolide acetate depot 3.75 mg followed by laparoscopic myomectomy, N = 35. Control: No preoperative treatment before laparoscopic myomectomy, N = 32. Duration of GnRHa treatment: 2 months. | |
| Outcomes |
Main outcomes
Secondary outcomes
|
|
| Notes | Attempt made to contact author for additional data but no reply received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The enrolled patients were allocated to one of the two groups according to the same computer‐generated random assignment for both centres”. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants would not be blinded to immediate or delayed surgery. There were no details on whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “All randomized patients recruited for the study for whom data were available were included in the efficacy analysis”. No loss to follow‐up: “All 67 patients have a clinical follow‐up of at least 6 months”. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not clearly reported. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
BMI: body mass index cc: cubic centimetres CD34: hematopoietic progenitor cell antigen CDB‐2914: a type of selective progesterone receptor modulator cm³: cubic centimetres CYP384: a type of oxidising enzyme D‐Trp: D‐Tryptophan, an amino acid FSH: follicle stimulating hormone g/dL: grams per decilitre G1: fibroids with intramural portion of < 50% GnRHa: gonadotropin‐releasing hormone analogues GO: fibroids completely intracavity Hb: haemoglobin IDA: iron deficiency anaemia IM: intramuscular kg/m²: kilograms per square metre LA: leucocyte antigen LH: luteinizing hormone LHRH: luteinizing hormone releasing hormone mg: milligram mL: millilitre MRI: magnetic resonance imaging NIH: National Institute of Health nmol/L: nanomoles per litre PBAC: pictorial blood assessment chart PCNA: anti proliferating cell nuclear antigen RCT: randomised controlled trial Ru 486: mifepristone Rx: treatment SC: subcutaneous SHBG: sex hormone binding globulin SLL: second look laparoscopy SPRM: selective progesterone receptor modulator TVUS: transvaginal ultrasound UFS‐QOL: Uterine Fibroid Symptom and Health‐Related Quality of Life Questionnaire
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bassaw 2014 | Randomisation was alternate which is subject to bias. |
| Benagiano 1992 | The relevant outcome in the publication, post‐operative complications, was given as a score rather than a proportion as contained in the table of comparisons. Contact was attempted with the principal author for extra information but no reply was received. |
| Bizzari 2015 | Prospective study but not randomised. |
| Bondi 2016 | Retrospective analysis of a prospectively collected database. |
| Coddington 2009 | Objectives of trial to measure other outcomes not included in the review. |
| De Falco 2006 | Intervention was not relevant to this review. |
| Di Lieto 2005 | Trial of add‐back therapy which is covered in another Cochrane Review (Moroni 2015). |
| Donnez 2014 | Intervention was given long term and women participants were trying to avoid surgery. |
| Elzaher 2013 | Participants had either fibroids or adenomyosis and no data provided for only women with fibroids. |
| Ferrero 2016a | Retrospective analysis of a prospectively collected database. |
| Ferrero 2016b | Prospective study but not randomised. |
| Hasan 2014 | Intervention was misoprostol which is covered in another Cochrane Review (Kongnyuy 2014). |
| Hutsikava 2016 | No indication whether the study was randomised ‐ unequal numbers in the two groups. |
| Leone Roberti Maggiore 2014 | Not an RCT. |
| Mizutani 2005 | The outcome of this study was not relevant to this review. |
| Nakano 1998 | Appeared to be a dose finding study of different types of GnRHa without a control arm. |
| Palomba 2001 | Trial of add‐back therapy which is covered in another Cochrane Review (Moroni 2015). |
| Parsanezhad 2010 | Wrong participants ‐ no mention of surgery after interventions. |
| Russo 1998 | Not RCT ‐ participants could chose their treatment. |
| Rutgers 1995 | The outcomes measured in the trial were not relevant to the review. |
| Simon 2016 | RCT, but treatment was not given preoperatively ‐ no indication whether the participants went on to have surgery. |
| Tabatabai 2015 | Intervention was misoprostol which is covered in another Cochrane Review (Kongnyuy 2014). |
| Triolo 2006 | Participants had either endometrial polyps, submucous myoma or septate uterus and results were reported for the whole group. |
| Weeks 2000 | The women in this study did not have fibroids. |
| Ylikorkala 1995 | The study author was contacted for extra information not contained in the publication but no reply was received. The study population consisted of women with fibroids and women with menometrorrhagia and pelvic pain and data were not provided separately for the women with fibroids. |
GnRHa: gonadotropin‐hormone releasing analogues; RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Gambardella 1995.
| Methods | Parallel group RCT. |
| Participants | N = 58, aged 25 to 51 years, with symptomatic fibroids. |
| Interventions | Goserelin depot 3.6 mg/month for 6 months followed by surgery (unspecified) versus immediate surgery without preoperative medical therapy. |
| Outcomes | Uterine volume, intraoperative blood loss and adverse events. |
| Notes | Study published in Italian, translation pending. |
Characteristics of ongoing studies [ordered by study ID]
Bigatti 2014.
| Trial name or title | Not reported |
| Methods | Randomised into 5 groups. No other details reported |
| Participants | Women with submucosal fibroids < 3 cm |
| Interventions | 5 separate interventions:
3 month observation period before surgery. Surgery was with intrauterine Bigatti shaver (IBS) |
| Outcomes | Fluid balance Operation time Complications Conversion to bipolar resectoscopy. |
| Starting date | September 2013 ‐ one year later 7 participants had been recruited into the study. |
| Contact information | Not reported. |
| Notes | Abstract presented at European Society of Gynaecological Endoscopy meeting in Belgium 2014. |
NCT01873378.
| Trial name or title | NCT01873378 |
| Methods | Randomised parallel group study. |
| Participants | Premenopausal women aged 18 years to 55 years with submucous fibroids (diagnosed by vaginal ultrasonography and confirmed by diagnostic hysteroscopy). Women with present or past history of cancer, pregnancy, presence of multiple polyps, presence of more than 2 fibroids and with associated non‐hysteroscopic surgical procedures were excluded. |
| Interventions | GnRHa: triptorelin 3.75 mg, intramuscularly, monthly, for 3 months Control: no pharmacological treatment |
| Outcomes | Duration of surgery, fluid absorption during the procedure. |
| Starting date | January 2013, completed August 2015 |
| Contact information | sandro.gerli@unipg.it |
| Notes | Principal investigator contacted by email; trial completed and manuscript submitted for publication to Obstetrics and Gynecology journal. Awaiting data. |
NCT02288130.
| Trial name or title | NCT02288130 |
| Methods | Double‐blind parallel group randomised controlled trial. Multicenter (9 in Netherlands) with computer generated randomisation sequences stratified per centre. |
| Participants | Premenopausal women with symptomatic fibroids and eligible for laparoscopic myomectomy. Women were excluded if they were pregnant, had a suspicion of malignancy, used hormonal agents and not willing to discontinue use, used anticoagulants, used NSAIDs impacting bleeding before surgery, had contraindication to laparoscopy, had allergy to leuprolide acetate or ulipristal acetate, had coagulopathy, had any type 0 to 2 fibroids smaller than 5 cm, had more than 2 type 3 to 6 fibroids > 5 cm that needed to be removed (except type 7 fibroids of any size). |
| Interventions | GnRHa and placebo tablets: intramuscular leuprorelin acetate depot 11.25 mg once and placebo tablets for 12 weeks. Ulipristal acetate, 5 mg once daily for 12 weeks plus single saline injection (placebo) at the onset of pretreatment. Control: No pretreatment before laparoscopic myomectomy. |
| Outcomes | Primary: intraoperative blood loss. Secondary: reduction of fibroid volume, haemoglobin levels pre and postoperatively, conversion rate to laparotomy, complication rate, re‐intervention rate, duration of surgery, surgical ease, quality of life during preoperative treatment and postoperatively up until 6 months. |
| Starting date | December 2014 |
| Contact information | i.demi@vumc.nl |
| Notes | Contacted the contact author and details of the trial from the register were confirmed. |
Differences between protocol and review
In the original version of the review, GnRHa alone was assessed as treatment before fibroid surgery. All methods have been updated to comply with current Cochrane standards for the 2017 review update. In addition, the review authors and the Co‐ordinating Editor of the Cochrane Gynaecology and Fertility Group decided to expand the scope of the review. Eligible interventions were expanded to include all preoperative medical agents, where surgery was subsequently expected.
We clarified in the 2017 version of the review that trials that measured only surrogate outcomes were not eligible for inclusion.
Title changed in 2017 (was previously Pre‐operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids).
Contributions of authors
Beverley Vollenhoven selected trials for inclusion in the first review published in 1998, assessed the included trials for quality and performed data extraction, reviewed both the protocol and final draft of the review and wrote the conclusions section in the abstract.
Martin Sowter wrote the Discussion and Conclusions sections of the review and commented on the final draft.
Anne Lethaby registered the title, prepared the protocol and incorporated suggested changes, performed searches, selected trials for inclusion in the review, assessed the included trials for quality and performed data extraction, entered data, prepared the final draft of the review and incorporated suggested changes from the peer review.
The review was updated in October 2000 (Lethaby 2000). Additional searches were performed by Anne Lethaby, Sue Furness and information specialists employed by BMJ Clinical Evidence. Anne Lethaby and Beverley Vollenhoven selected additional trials for inclusion and extracted data from the included trials. Anne Lethaby entered the data and made modifications to the text of the review.
For the 2017 update, Anne Lethaby conducted additional searches. Anne Lethaby and Lucian Puscasiu selected additional trials for inclusion and extracted data in duplicate. Anne Lethaby entered data and these were checked by Lucian Puscasiu. Both authors made modifications to the text of the review. Beverley Vollenhoven made some suggestions for the revised background and approved the remaining review.
Sources of support
Internal sources
Department of Obstetrics and Gynaecology, University of Auckland, New Zealand.
External sources
Health Research Council, Auckland, New Zealand.
Declarations of interest
Anne Lethaby: None known.
Lucian Puscasiu (LP) is a co‐author of an included trial in this review (Donnez 2012a). LP has received publication and speaking fees as well as travel expenses and fees in connection with ESMYA launch symposium in March 2012 in Barcelona from the company Gedeon‐Richter. LP also received investigator's fees from the company ICON during the PEARL I study.
Beverley Vollenhoven: None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Audebert 1994 {published data only}
- Audebert AJM, Madenelat P, Querleu D, Pontonnier G, Racinet C, Renaud R, et al. Deferred versus immediate surgery for uterine fibroids: clinical trial results. British Journal of Obstetrics and Gynaecology 1994;101(Suppl):29‐32. [DOI] [PubMed] [Google Scholar]
Balasch 1995 {published data only}
- Balasch J, Manau D, Mimó J, Duran M, Puerto B, Vanrell JA. Trial of routine gonadotropin releasing hormone agonist treatment before abdominal hysterectomy for leiomyoma. Acta Obstetricia et Gynecologica Scandinavica 1995;74(7):562‐5. [DOI] [PubMed] [Google Scholar]
Baytur 2007 {published data only}
- Baytur YB, Ozbilgin K, Cilaker S, Lacin S, Kurtul O, Oruc S, et al. A comparative study of the effect of raloxifene and gosereline on uterine leiomyoma volume changes and estrogen receptor, progesterone receptor, bcl‐2 and p53 expression immunohistochemically in premenopausal women. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2007;135(1):94‐103. [DOI] [PubMed] [Google Scholar]
Benagiano 1996 {published data only}
- Benagiano G, Kivinen ST, Fadini R, Cronje H, Klintorp S, Spuy Z. Zoladex (goserelin acetate) and the anemic patient: results of a multicenter fibroid study. Fertility and Sterility 1996;66(2):223‐9. [PubMed] [Google Scholar]
Bustos López 1995 {published data only}
- Bustos López HH, Miranda Rodríguez JA, Kably Ambe A, Serviere Zaragoza C, Espinoza de los Monteros A, Alvarado Durán A. Pre‐operative medical treatment of uterine leiomyomatosis with hypophysiary gonadotropin releasing hormone analogues [Tratamiento médico preoperatorio de leiomiomatosis uterina con análogos de hormona liberadora de gonadotropinas hipofisiarias]. Ginecología y Obstetricia de México 1995;63(8):356‐64. [PubMed] [Google Scholar]
Cagnacci 1994 {published data only}
- Cagnacci A, Paoletti AM, Soldani R, Angiolucci M, Arangino S, Falqui A, et al. Role of goserelin‐depot in the clinical management of uterine fibroids. Clinical and Experimental Obstetrics & Gynecology 1994;21(4):263‐5. [PubMed] [Google Scholar]
Campo 1999 {published data only}
- Campo S, Garcea N. Laparoscopic myomectomy in premenopausal women with and without preoperative treatment using gonadotropin‐releasing hormone analogues. Human Reproduction (Oxford, England) 1999;14(1):44‐8. [DOI] [PubMed] [Google Scholar]
Cetin 1995 {published data only}
- Cetin MT, Vardar MA, Demir SC. Kibar M. Administration of preoperative gonadotropin releasing hormone agonist (buserelin) for uterine leiomyomas. Annals of Medical and Health Sciences Research 1995;4:102‐8. [Google Scholar]
D'Anna 1994 {published data only}
- D'Anna R, Palmara V, Lo Re C, Scilipoti A, Leonardi I. Short treatment with leuprolide acetate depot before hysterectomy for uterine leiomyomata [Breve trattamento con leuprolide acetato depot prima di intervento chirurgico per leiomiomatosi uterina]. Minerva Ginecologica 1994;46(6):343‐6. [PubMed] [Google Scholar]
De Falco 2009 {published data only}
- Falco M, Staibano S, Mascolo M, Mignogna C, Improda L, Ciociola F, et al. Leiomyoma psuedocapsule after presurgical treatment with gonadotropin‐releasing hormone agonists: relationship between clinical features and immunohistochemical changes. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2009;144(1):44‐7. [DOI] [PubMed] [Google Scholar]
Donnez 2003 {published data only}
- Donnez J, Vivancos B, Kudela M, Audebert A, Jadoul P. A randomized, placebo‐controlled, dose‐ranging trial comparing fulvestrant with goserelin in premenopausal patients with uterine fibroids awaiting hysterectomy. Fertility and Sterility 2003;79(6):1380‐9. [DOI] [PubMed] [Google Scholar]
Donnez 2012a {published data only}
- Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, et al. PEARL I Study Group. Ulipristal acetate versus placebo for fibroid treatment before surgery. New England Journal of Medicine 2012;366(5):409‐20. [DOI] [PubMed] [Google Scholar]
Donnez 2012b {published data only}
- Donnez J, Tomaszewski J, Vázquez F, Bouchard P, Lemieszczuk B, Baró F, et al. PEARL II Study Group. Ulipristal acetate versus leuprolide acetate for uterine fibroids. New England Journal of Medicine 2012;366(5):421‐32. [DOI] [PubMed] [Google Scholar]
Engman 2009 {published data only}
- Engman M, Granberg S, Williams AR, Meng CX, Lalitkumar PGL, Gemzell‐Danielsson K. Mifepristone for treatment of uterine leiomyoma. A prospective randomised placebo controlled trial. Human Reproduction (Oxford, England) 2009;24(8):1870‐9. [DOI] [PubMed] [Google Scholar]
Fedele 1990 {published data only}
- Fedele L, Vercellini P, Bianchi S, Brioschi D, Dorta M. Treatment with GnRH agonists before myomectomy and the risk of short‐term myoma recurrence. British Journal of Obstetrics and Gynaecology 1990;97(5):393‐6. [DOI] [PubMed] [Google Scholar]
Friedman 1989 {published data only}
- Friedman AJ, Daly M, Juneau‐Norcross M, Fine C, Rein MS. Recurrence of myomas after myomectomy in women pretreated with leuprolide acetate depot or placebo. Fertility and Sterility 1992;58(1):205‐8. [DOI] [PubMed] [Google Scholar]
- Friedman AJ, Rein MS, Harrison‐Atlas D, Garfield JM, Doubilet PM. A randomized, placebo‐controlled, double‐blind study evaluating leuprolide acetate depot treatment before myomectomy. Fertility and Sterility 1989;52(5):728‐33. [PubMed] [Google Scholar]
Gerris 1996 {published data only}
- Gerris J, Degueldre M, Peters AAW, Romao F, Stjernquist M, al‐Taher H. The place of Zoladex in deferred surgery for uterine fibroids. Zoladex Myoma Study Group. Hormone Research 1996;45(6):279‐84. [DOI] [PubMed] [Google Scholar]
Golan 1993 {published data only}
- Golan A, Bukovsky I, Pansky M, Schneider D, Weinraub Z, Caspi E. Pre‐operative gonadotropin‐releasing hormone agonist treatment in surgery for uterine leiomyomata. Human Reproduction (Oxford, England) 1993;8(3):450‐2. [DOI] [PubMed] [Google Scholar]
- Golan A, Bukovsky I, Schneider D, Pansky M, Caspi E. Preoperative GnRH‐analog treatment in surgery for uterine myomas. 3rd International Sympoisum on Gynaecological Endocrinology, Switzerland, 25‐28 Feb 1993. 1993:15.
Hudecek 2012 {published data only}
- Hudecek R, Ivanová Z, Smerdová M, Pánková S, Krajcovicová R. Effect of GnRH analogues pre‐treatment on myomectomy outcomes in reproductive age women. Ceska Gynekologie 2012;77(2):109‐17. [PubMed] [Google Scholar]
Levens 2008 {published data only}
- Levens ED, Potlog‐Nahari C, Armstrong AY, Wesley R, Premkumar A, Blithe DL, et al. CDB‐2914 for uterine leiomyomata treatment. Obstetrics and Gynecology 2008;111(5):1129‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lumsden 1994 {published data only}
- Lumsden MA, West CP, Thomas E, Coutts J, Hillier H, Thomas N, et al. Treatment with the gonadotropin releasing hormone‐agonist goserelin before hysterectomy for uterine fibroids. British Journal of Obstetrics and Gynaecology 1994;101(5):438‐42. [DOI] [PubMed] [Google Scholar]
Mavrelos 2010 {published data only}
- Mavrelos D, Ben‐Nagi J, Davies A, Lee C, Salim R, Jurkovic D. The value of preoperative treatment with GnRH analogues in women with submucous fibroids: a double blind placebo controlled randomised trial. Human Reproduction (Oxford, England) 2010;25(9):2264‐9. [DOI] [PubMed] [Google Scholar]
Muneyyirci‐Delale 2007 {published data only}
- Muneyyirci‐Delale O, Richard‐Davis G, Morris T, Armstrong J. Goserelin acetate 10.8 mg plus iron monotherapy prior to surgery in premenopausal women with iron deficiency anemia due to uterine leiomyomas: results from a phase III, randomized, multicenter, double blind, controlled trial. Clinical Therapeutics 2007;29(8):1682‐91. [DOI] [PubMed] [Google Scholar]
Muzii 2010 {published data only}
- Muzii L, Boni T, Bellati F, Marana R, Ruggiero A, Zullo MA, et al. GnRH analogue treatment before hysteroscopic resection of submucous myomas: a prospective, randomised, multicenter study. Fertility and Sterility 2010;94(4):1496‐9. [DOI] [PubMed] [Google Scholar]
Nikolov 1999 {published data only}
- Nikolov A, Karag'osov I. Comparative study on the efficacy of preoperative use of GnRH agonists in patients with uterine fibromyomas. Akuserstvo i Ginekologija 1999;38(4):38‐42. [PubMed] [Google Scholar]
- Nikolov N, Karag'osov I. Preoperative use of Zoladex for treatment of uterine fibromyomas (abstract). Acta Obstetricia et Gynecologica Scandinavica 1997;76(Suppl):31. [Google Scholar]
Reinsch 1994 {published data only}
- Reinsch RC, Murphy AA, Morales AJ, Yen SSC. The effects of RU 486 and leuprolide acetate on uterine artery blood flow in the fibroid uterus: a prospective randomised study. American Journal of Obstetrics and Gynecology 1994;170(6):1623‐8. [PubMed] [Google Scholar]
Sayyah‐Melli 2007 {published data only}
- Sayyah‐Melli M, Farzadi L, Madarek EO. Comparison of the effect of gonadotropin‐releasing hormone analog (Diphereline) and Cabergoline (Dostinex) treatment on uterine myoma regression. Saudi Medical Journal 2007;28(3):445‐50. [PubMed] [Google Scholar]
Sayyah‐Melli 2009 {published data only}
- Sayyah‐Melli M, Tehrani‐Gadim S, Dastranj‐Tabrizi A, Gatrehsamani F, Morteza G, Ouladesahebmadarek E, et al. Comparison of the effect of gonadotropin‐releasing hormone agonist and dopamine receptor agonist on uterine myoma growth. Saudi Medical Journal 2009;30(8):1024‐33. [PubMed] [Google Scholar]
Seraccholi 2003 {published data only}
- Seracchioli R, Venturoli S, Colombo F, Bagnoli A, Vianello F, Govoni F, et al. GnRH agonist treatment before total laparoscopic hysterectomy for large Uteri. Journal of the American Association of Gynecologic Laparoscopists 2003;10(3):316‐9. [DOI] [PubMed] [Google Scholar]
Shaw 1989 {published data only}
- Shaw RW. Mechanism of LHRH analogue action in uterine fibroids. Hormone Research 1989;32(Suppl 1):150‐3. [DOI] [PubMed] [Google Scholar]
Shaw 1996 {unpublished data only}
- Shaw RW. Placebo controlled comparison of the effectiveness of the microparticles depot formulation of buserelin in the pre‐operative management of patients with uterine fibroids. Clinical/biometric report, Hoechst Marion Roussel, UK Ltd 1996.
Stovall 1994 {published data only}
- Stovall TG, Ling FW, Henry LC, Woodruff MR. A randomised trial evaluating leuprolide acetate before hysterectomy as treatment for leiomyomas. American Journal of Obstetrics and Gynecology 1991;164(6 Pt 1):1420‐5. [DOI] [PubMed] [Google Scholar]
- Stovall TG, Summitt Jr RL, Washburn SA, Ling FW. Gonadotropin‐releasing hormone agonist use before hysterectomy. American Journal of Obstetrics and Gynecology 1994;170(6):1744‐51. [PubMed] [Google Scholar]
Stovall 1995 {published data only}
- Stovall TG, Muneyyirci‐Delale O, Summitt Jr RL, Scialli AR. GnRH agonist and iron versus placebo and iron in the anemic patient before surgery for leiomyomas: a randomized controlled trial. Leuprolide Acetate Study Group. Obstetrics and Gynecology 1995;86(1):65‐71. [DOI] [PubMed] [Google Scholar]
Vercellini 1998 {published data only}
- Vercellini P, Crosignani PG, Mangioni C, Imparato E, Ferrari A, Giorgi O. Treatment with a gonadotrophin releasing hormone agonist before hysterectomy for leiomyomas: results of a multicentre, randomised controlled trial. British Journal of Obstetrics and Gynaecology 1998;105(11):1148‐54. [DOI] [PubMed] [Google Scholar]
Vercellini 2003 {published data only}
- Vercellini P, Trespidi L, Zaina B, Vicentini S, Stellato G, Crosignani PG. Gonadotropin‐releasing hormone agonist treatment before abdominal myomectomy: a controlled trial. Fertility and Sterility 2003;79(6):1390‐5. [DOI] [PubMed] [Google Scholar]
Verspyck 2000 {published data only}
- Verspyck E, Marpeau L, Lucas C. Leuporelin depot 3.75 mg versus lynestrenol in the preoperative treatment of symptomatic uterine myomas: a multicentre randomised trial. European Journal of Obstetrics, Gynecology, and Reproductive BIology 2000;89(1):7‐13. [DOI] [PubMed] [Google Scholar]
Wilkens 2008 {published data only}
- Wilkens J, Chwalisz K, Han C, Walker J, Cameron IT, Ingamells S, et al. Effects of the selective progesterone receptor modulator asoprisnil on uterine artery blood flow, ovarian activity and clinical symptoms in patients with uterine leiomyomata scheduled for hysterectomy. Journal of Clinical Endocrinology and Metabolism 2008;93(12):4664‐71. [DOI] [PubMed] [Google Scholar]
Zullo 1998 {published data only}
- Zullo F, Pellicano M, Stefano R, Zupi E, Mastrantonio P. A prospective randomised study to evaluate leuprolide acetate treatment before laparascopic myomectomy: efficacy and ultrasonographic predictors. American Journal of Obstetetrics and Gynecology 1998;178(1 Pt 1):108‐12. [DOI] [PubMed] [Google Scholar]
- Zullo F, Pellicano M, Carlo C, Stefano R, Mastrantonio P, Nappi C. GnRH‐a pretreatment and laparoscopic intramural myomectomy: efficacy and ultrasonographic correlations (abstract). American Society for Reproductive Medicine. 1996:S134.
References to studies excluded from this review
Bassaw 2014 {published data only}
- Bassaw B, Mohammed N, Jaggat A, SIngh‐Bhola M, Ramkissoon A, Singh P, et al. Experience with a gonadotropin‐releasing hormone agonist prior to myomectomy ‐ comparison of twice‐ vs thrice‐monthly doses and a control group. Journal of Obstetrics and Gynaecology 2014;34(5):415‐9. [DOI] [PubMed] [Google Scholar]
Benagiano 1992 {published data only}
- Benagiano G, Morini A, Primiero FM. Fibroids: overview of current and future treatment options. Journal of Obstetrics and Gynaecology 1992;99(Suppl 7):18‐22. [DOI] [PubMed] [Google Scholar]
Bizzari 2015 {published data only}
- Bizzarri N, Ghirardi V, Remorgida V, Venturini PL, Ferrero S. Three‐month treatment with triptorelin, letrozole and ulipristal acetate before hysteroscopic resection of uterine myomas: prospective comparative pilot study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2015;192:22‐6. [DOI] [PubMed] [Google Scholar]
Bondi 2016 {published data only}
- Bondi C, Maggiore LRU, Alessandri F, Venturini PL, Vellone VG, Ferrero S. Three‐month treatment with ulipristal acetate prior to laparoscopic myomectomy of large uterine myomas. Human Reproduction 2016;31(Suppl 1):i467. [DOI] [PubMed] [Google Scholar]
Coddington 2009 {published data only}
- Coddington CC, Grow D, Ahmed MS, Toner JP, Cook E, Diamond MP. Gonadotropin‐releasing hormone agonist pretreatment did not decrease postoperative adhesion formation after abdominal myomectomy in a randomized control trial. Fertility and Sterility 2009;91(5):1909‐13. [DOI] [PubMed] [Google Scholar]
De Falco 2006 {published data only}
- Falco M, Staibano S, D'Armiento FP, Mascolo M, Salvatore G, Busiello A. Preoperative treatment of uterine leiomyomas: clinical findings and expression of transforming growth factor‐beta3 and connective tissue growth factor. Journal of the Society for Gynecologic Investigation 2006;13(4):297‐300. [DOI] [PubMed] [Google Scholar]
Di Lieto 2005 {published data only}
- Lieto A, Falco M, Mansueto G, Rosa G, Pollio F, Staibano S. Preoperative administration of GnRH‐a plus tibolone to premenopausal women with uterine fibroids: evaluation of the clinical response, the immunohistochemical expression of PDGF, bFGF and VEGF and the vascular pattern. Steroids 2005;70(2):95‐102. [DOI] [PubMed] [Google Scholar]
Donnez 2014 {published data only}
- Donnez J, Vazquez F, Tomaszewski J, Nouri K, Bouchard P, Fauser BCJM, et al. the PEARL III and PEARL III extension study group. Long term treatment of uterine fibroids with ulipristal acetate. Fertility and Sterility 2014;10(6):1565‐73. [DOI] [PubMed] [Google Scholar]
Elzaher 2013 {published data only}
- Elzaher MA, Moawad A, Madkour WA, Ali M, Salah Eldin Abdel Hamid AM, Zaheer H. Does medical debulking with gonadotropin‐releasing hormone agonist facilitate vaginal hysterectomy with a moderate enlarged uterus? A randomised controlled study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2013;169(2):326‐30. [DOI] [PubMed] [Google Scholar]
Ferrero 2016a {published data only}
- Ferrero S, Racca A, Tafi E, Alessandri F, Venturini PL, Maggiore LRU, et al. Ulipristal acetate before high complexity hysteroscopic myomectomy: a retrospective comparative study. Journal of Minimally Invasive Gynecology 2016;23(3):390‐5. [DOI] [PubMed] [Google Scholar]
Ferrero 2016b {published data only}
- Ferrero S, Racca A, Tafi E, Scala C, Alessandri F, Venturini PL, et al. Ulipristal acetate versus triptorelin prior to laparoscopic myomectomy: prospective study. Journal of Minimally Invasive Gynecology 2016;23(7 Suppl 1):S199. [Google Scholar]
Hasan 2014 {published data only}
- Hasan M, Nasir A, Saba E. Color Doppler misoprostol response study (CDMRS): an evaluation tool for patients awaiting myomectomy. Journal of Medical Ultrasound 2014;22(2):78‐82. [Google Scholar]
Hutsikava 2016 {published data only}
- Hutsikava L. The preoperative treatment of women with myoma of the uterus using ulipristal acetate. Gynecological Endocrinology 2016;32(Suppl 1):50. [Google Scholar]
Leone Roberti Maggiore 2014 {published data only}
- Leone Roberti Maggiore U, Scala C, Venturini PL, Ferrero S. Preoperative treatment with letrozole in patients undergoing laparoscopic myomectomy of large uterine myomas: a prospective non randomised study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2014;181:157‐62. [DOI] [PubMed] [Google Scholar]
Mizutani 2005 {published data only}
- Mizutani T, Sugihara A, Honma H, Komura H, Nakamuro K, Terada N. Effect of steroid add‐back therapy on the proliferative activity of uterine leiomyoma cells under gonadotropin‐releasing hormone agonist therapy. Gynecological Endocrinology 2005;20(2):80‐3. [DOI] [PubMed] [Google Scholar]
Nakano 1998 {published data only}
- Nakano H, Kawasima M, Okada S, Igarashi T, Maejima M, Ogino M. The study of the effect of presurgical GnRH agonist treatment for women with uterine leiomyoma ‐ effect on the myoma volume and the operation. Japanese Journal of Fertility and Sterility 1998;43:289‐93. [Google Scholar]
Palomba 2001 {published data only}
- Palomba S, Massimiliano P, Affinito P, Carlo C, Zullo F, Nappi C. Effectiveness of short term administration of tibolone plus gonadotropin‐releasing hormone analogue on the surgical outcome of laparoscopic myomectomy. Fertility and Sterility 2001;75(2):429‐33. [DOI] [PubMed] [Google Scholar]
- Palomba S, Morelli M, Nola R, Santagata M, Oliverio A, Sena T, et al. Short‐term administration of tibolone plus GnRH analog before laparoscopic myomectomy. Journal of the American Association of Gynecologic Laparoscopists 2002;9(2):170‐4. [DOI] [PubMed] [Google Scholar]
Parsanezhad 2010 {published data only}
- Parsanezhad ME, Azmoon M, Alborzi S, Rajaeefard A, Zarei A, Kazerooni T, et al. A randomised controlled clinical trial comparing the effects of aromatase inhibitor (letrazole) and gonadotropin‐releasing hormone agonist (triptorelin) on uterine leiomyoma volume and hormonal status. Fertility and Sterility 2010;93(1):192‐8. [DOI] [PubMed] [Google Scholar]
Russo 1998 {published data only}
- Russo P, Ciolli P, Atlante M, Carico EE, Mancini R, Russo R, et al. Clinical use of leuprolide acetate depot in a group of gynecologic patients in the preoperative period. Minerva Ginecologica 1998;50(11):499‐502. [PubMed] [Google Scholar]
Rutgers 1995 {published data only}
- Rutgers J, Spong C, Sinow R, Heiner J. Leuprolide acetate treatment and myoma arterial size. Obstetrics and Gynecology 1995;86(3):386‐8. [DOI] [PubMed] [Google Scholar]
Simon 2016 {published data only}
- Simon J, Catherino WH, Segars J, Blakesley R, Chan A, Sniukiene V, et al. First US‐based phase 3 study of ulipristal acetate (UPA) for symptomatic uterine fibroids (UF): results of VENUS‐1. Fertility and Sterility 2016;106(3):e376. [Google Scholar]
Tabatabai 2015 {published data only}
- Tabatabai A, Karimi‐Zarchi M, Meibodi B, Vaghefi M, Yazdian P, Zeidabadi M, et al. Effects of a single rectal dose of Misoprostol prior to abdominal hysterectomy in women with symptomatic leiomyoma: a randomised double blind clinical trial. Electronic Physician 2015;7(6):1372‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Triolo 2006 {published data only}
- Triolo O, Vivo A, Benedetto V, Falcone S, Antico F. Gestrinone versus danazol as preoperative treatment for hysteroscopic surgery: a prospective randomised evaluation. Fertility and Sterility 2006;85(4):1027‐31. [DOI] [PubMed] [Google Scholar]
Weeks 2000 {published data only}
- Weeks A, Duffy S, Walker J. A double‐blind randomised trial of leuprorelin acetate prior to hysterectomy for dysfunctional uterine bleeding. British Journal of Obstetrics and Gynaecology 2000;107(3):323‐8. [DOI] [PubMed] [Google Scholar]
- Weeks AD, Duffy SRG, Walker JJ. Uterine ultrasonographic changes with gondatrophin‐releasing hormone agonists. American Journal of Obstetrics and Gynecology 1999;180(1):8‐13. [DOI] [PubMed] [Google Scholar]
Ylikorkala 1995 {published data only}
- Ylikorkala O, Tiitinen A, Hulkko S, Kivinen S, Nummi S. Decrease in symptoms, blood loss and uterine size with nafarelin acetate before abdominal hysterectomy: a placebo‐controlled, double‐blind study. Human Reproduction 1995;10(6):1470‐4. [PubMed] [Google Scholar]
References to studies awaiting assessment
Gambardella 1995 {published data only}
- Gambardella F, Capicotto V, Cuneo P, Daminani A, Proto M. Using analogous preoperative Gn‐RH in the treatment of symptomatic uterine leiomyomatosis [Uso preoperatorio degli analoghi del Gn‐RH nel trattamento della leiomiomatosi uterina sintomatica]. Giornarle Italiano di Ostetrica e Ginecologica 1995;11:658‐62. [Google Scholar]
References to ongoing studies
Bigatti 2014 {published data only}
- Bigatti G, Iemmello R, Desgro M, Pollini S, Santirocco M. Progesterone preoperative treatment before myomectomy with the Intrauterine Bigatti Shaver (IBS). Gynecological Surgery 2014;11(1 Suppl 1):119‐20. [Google Scholar]
NCT01873378 {unpublished data only}
- NCT01873378. GnRH agonist pretreatment in hysteroscopic myomectomy. clinicaltrials.gov/ct2/show/NCT01873378 (first received 1 June 2013).
NCT02288130 {unpublished data only}
- NCT02288130. Ulipristal acetate vs GnRHa prior to laparoscopic myomectomy (MYOMEX). clinicaltrials.gov/ct2/show/NCT02288130 (first received 6 November 2014).
Additional references
Baird 2003
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. American Journal of Obstetrics and Gynecology 2003;188(1):100‐7. [DOI] [PubMed] [Google Scholar]
Buttram 1981
- Buttram VC Jr, Reiter RC. Uterine leiomyomas: etiology, symptomatology, and management. Fertility and Sterility 1981;36(4):433‐45. [DOI] [PubMed] [Google Scholar]
Campo 2005
- Campo S, Campo V, Gambadauro P. Short term and long term results of resectoscopic myomectomy with and without pretreatment with GnRH analogs in premenopausal women. Acta Obstetricia et Gynecologica Scandinavica 2005;84(8):756‐60. [DOI] [PubMed] [Google Scholar]
Carls 2008
- Carls GS, Lee DW, Ozminkowski RJ, Wang S, Gibson TB, Stewart E. What are the total costs of surgical treatment for uterine fibroids?. Journal of Women's Health 2008;17(7):1119‐32. [DOI] [PubMed] [Google Scholar]
Chen 2011
- Chen I, Motan T, Kiddoo D. Gonadotropin‐releasing hormone agonist in laparoscopic myomectomy: systematic review and meta‐analysis of randomised controlled trials. Journal of Minimally Invasive Gynecology 2011;18(3):303‐9. [DOI] [PubMed] [Google Scholar]
Friedman 1990
- Friedman AJ, Lobel SM, Rein MS, Barbieri RL. Efficacy and safety considerations in women with uterine leiomyomas treated with gonadotrophin‐releasing hormone agonists: the estrogen window hypothesis. American Journal of Obstetrics and Gynecology 1990;163(4 Part 1):1114‐9. [DOI] [PubMed] [Google Scholar]
Geirsson 1993
- Geirsson RT. Intrauterine volume measurement techniques and uteroplacental growth. In: Chervenak FA, Isaacson GC, Campbell S editor(s). Ultrasound in Obstetrics and Gynecology. Boston: Little, Brown and Company, 1993:405‐12. [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to 18 July 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gurusamy 2016
- Gurusamy KS, Vaughan J, Fraser IS, Best LMJ, Richards T. Medical therapies for uterine fibroids ‐ a systematic review and network meta‐analysis of randomised controlled trials. PloS One 2016;11(2):e0149631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jacoby 2010
- Jacoby VL, Fujimoto VY, Guidice LC, Kupperman M, Washington AE. Racial and ethnic disparities in benign gynecologic conditions and associated surgeries. American Journal of Obstetrics and Gynecology 2010;202(6):514‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jirecek 2004
- Jirecek S, Lee A, Pavo I, Crans G, Eppel W, Wenzl R. Raloxifene prevents the growth of uterine leiomyomas in premenopausal women. Fertility and Sterility 2004;81(1):132‐6. [DOI] [PubMed] [Google Scholar]
Jourdain 1996
- Jourdain O, Descamps P, Abusada N, Ventrillon E, Dallay D, Lansac J, et al. Treatment of fibromas. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1996;66(2):99‐107. [DOI] [PubMed] [Google Scholar]
Kamath 2014
- Kamath MS, Kalampokas EE, Kalampokas TE. Use of GnRH analogues preoperatively for hysteroscopic resection of submucous fibroids: a systematic review and metaanalysis. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2014;177:11‐8. [DOI] [PubMed] [Google Scholar]
Kongnyuy 2014
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD005355.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matta 1989
- Matta WHM, Shaw RW, Nye M. Long‐term follow‐up of patients with uterine fibroids after treatment with the LHRH agonist buserelin. British Journal of Obstetrics and Gynaecology 1989;96(2):200‐6. [DOI] [PubMed] [Google Scholar]
Merrill 2008
- Merrill RM. Hysterectomy surveillance in the United States, 1997 to 2005. Medical Science Monitor 2008;14(1):CR24‐31. [PubMed] [Google Scholar]
Moroni 2015
- Moroni RM, Martins WP, Ferriani RA, Vieria CS, Nastri CO, Candido Dos Reis FJ, et al. Add‐back therapy with GnRH analogues for uterine fibroids. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD010854.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nagy 2014
- Nagy B, Timar G, Józwiak‐Hagymásy J, Kovács G, Merész G, Vámossy I, et al. The cost effectiveness of ulipristal acetate tablets in treating patients with moderate to severe symptoms of uterine fibroids. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2014;175:75‐81. [DOI] [PubMed] [Google Scholar]
Parazzini 1998
- Parazzini F, Vercellini P, Giorgi O, Pesole A, Ricci E, Crosignani PG. Efficacy of preoperative medical treatment in facilitating hysteroscopic endometrial resection, myomectomy and metroplasty: literature review. Human Reproduction (Oxford, England) 1998;13(9):2592‐7. [DOI] [PubMed] [Google Scholar]
Patel 2014
- Patel A, Malik M, Britten J, Cox J, Catherino WH. Alternative therapies in management of leiomyomas. Fertility and Sterility 2014;102(3):649‐55. [DOI] [PubMed] [Google Scholar]
Perino 1993
- Perino A, Chianchiano N, Petronio M, Cittadini E. Role of leuprolide acetate depot in hysteroscopic surgery: a controlled study. Fertility and Sterility 1993;59:507‐10. [DOI] [PubMed] [Google Scholar]
Pérez Lopez 2014
- Pérez‐López FR, Ornat L, Ceausu I, Depypere H, Erel CT, Lambrinoudaki I, et al. EMAS position statement: management of uterine fibroids. Maturitas 2014;79(1):106‐16. [DOI] [PubMed] [Google Scholar]
Stovall 1989
- Stovall TG. Rationale for the short‐term use of luteinising hormone‐releasing hormone analogues in the treatment of uterine myomata. Hormone Research 1989;32(Suppl 1):134‐6. [DOI] [PubMed] [Google Scholar]
Stovall 1991
- Stovall TG, Ling FW, Henry LC, Woodruff MR. A randomized trial evaluating leuprolide acetate before hysterectomy as a treatment for leiomyomas. American Journal of Obstetrics and Gynecology 1991;164(6 Pt 1):1420‐5. [DOI] [PubMed] [Google Scholar]
Tamaya 1985
- Tamaya T, Fujimoto J, Okada H. Comparison of cellular levels of steroid receptors in uterine leiomyomas and myometrium. Acta Obstetricia et Gynaecologica Scandinavica 1985;64(4):307‐9. [DOI] [PubMed] [Google Scholar]
Wallach 1992
- Wallach EE. Myomectomy. In: Thompson JD, Rock JA editor(s). Te Linde's Operative Gynaecology. 7th Edition. Philadelphia: Lippincott, 1992:647‐62. [Google Scholar]
West 1992
- West CP, Lumsden MA, Baird DT. Goserelin (Zoladex) in the treatment of fibroids. British Journal of Obstetrics and Gynaecology 1992;99(Suppl 7):27‐30. [DOI] [PubMed] [Google Scholar]
Williams 2012
- Williams ARW, Bergeron C, Barlow DH, Ferenczy A. Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. International Journal of Gynecological Pathology 2012;31(6):556‐69. [DOI] [PubMed] [Google Scholar]
Wilson 1980
- Wilson EA, Yang F, Rees ED. Estradiol and progesterone binding in uterine leiomyomata and in normal uterine tissue. Obstetrics and Gynecology 1980;55(1):20‐4. [PubMed] [Google Scholar]
Yang 2011
- Yang JH, Chen MJ, Chen DC, Chen CL, Ho HN, Yang YS. Impact of submucous fibroid on the severity of anemia. Fertility and Sterility 2011;95(5):1769‐72. [DOI] [PubMed] [Google Scholar]
Zakiyah 2017
- Zakiyah N, Asselt ADI, Postma MJ. Ulipristal acetate for pre‐operative treatment of moderate‐to‐severe uterine fibroids in women of reproductive age in the Netherlands: cost minimisation analysis and budget impact analysis. Journal of Medical Economics 2017;20(3):280‐7. [DOI] [PubMed] [Google Scholar]
Zhang 2014
- Zhang Y, Sun L, Guo Y, Cheng J, Wang Y, Fan S, et al. The impact of preoperative gonadotropin‐releasing hormone agonist treatment on women with uterine fibroids: a metaanalysis. Obstetrical & Gynecological Survey 2014;69(2):100‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lethaby 2000
- Lethaby A, Vollenhoven B, Sowter M. Pre‐operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD000547] [DOI] [PubMed] [Google Scholar]
Lethaby 2001
- Lethaby A, Vollenhoven B, Sowter M. Pre‐operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD000547] [DOI] [PubMed] [Google Scholar]


