Abstract
Background
Diabetes mellitus is a chronic metabolic disorder that is associated with an increased risk of cardiovascular disease, retinopathy, nephropathy, neuropathy, sexual dysfunction and periodontal disease. Improvements in glycaemic control may help to reduce the risk of these complications. Several animal studies show that cinnamon may be effective in improving glycaemic control. While these effects have been explored in humans also, findings from these studies have not yet been systematically reviewed.
Objectives
To evaluate the effects of cinnamon in patients with diabetes mellitus.
Search methods
Pertinent randomised controlled trials were identified through AARP Ageline, AMED, AMI, BioMed Central gateway, CAM on PubMed, CINAHL, Dissertations Abstracts International, EMBASE, Health Source Nursing/Academic edition, International Pharmaceutical Abstracts, MEDLINE, Natural medicines comprehensive database, The Cochrane Library and TRIP database. Clinical trial registers and the reference lists of included trials were searched also (all up to January 2012). Content experts and manufacturers of cinnamon extracts were also contacted.
Selection criteria
All randomised controlled trials comparing the effects of orally administered monopreparations of cinnamon (Cinnamomum spp.) to placebo, active medication or no treatment in persons with either type 1 or type 2 diabetes mellitus.
Data collection and analysis
Two review authors independently selected trials, assessed risk of bias and trial quality, and extracted data. We contacted study authors for missing information.
Main results
Ten prospective, parallel‐group design, randomised controlled trials, involving a total of 577 participants with type 1 and type 2 diabetes mellitus, were identified. Risk of bias was high or unclear in all but two trials, which were assessed as having moderate risk of bias. Risk of bias in some domains was high in 50% of trials. Oral monopreparations of cinnamon (predominantly Cinnamomum cassia) were administered at a mean dose of 2 g daily, for a period ranging from 4 to 16 weeks. The effect of cinnamon on fasting blood glucose level was inconclusive. No statistically significant difference in glycosylated haemoglobin A1c (HbA1c), serum insulin or postprandial glucose was found between cinnamon and control groups. There were insufficient data to pool results for insulin sensitivity. No trials reported health‐related quality of life, morbidity, mortality or costs. Adverse reactions to oral cinnamon were infrequent and generally mild in nature.
Authors' conclusions
There is insufficient evidence to support the use of cinnamon for type 1 or type 2 diabetes mellitus. Further trials, which address the issues of allocation concealment and blinding, are now required. The inclusion of other important endpoints, such as health‐related quality of life, diabetes complications and costs, is also needed.
Plain language summary
Cinnamon for diabetes mellitus
Diabetes mellitus is a chronic metabolic disorder. People with diabetes are known to be at greater risk of cardiovascular disease (including heart attack, stroke, and peripheral vascular disease such as acute or chronic ischaemia of a leg resulting in severe pain when walking short distances). There is also an increased risk of eye disease, kidney failure, nerve damage and sexual dysfunction when compared to the general population. Improvements in the regulation of blood sugar levels may help to reduce the risk of these complications.
Cinnamon bark has been shown in a number of animal studies to improve blood sugar levels, though its effect in humans is not too clear. Hence, the review authors set out to determine the effect of oral cinnamon extract on blood sugar and other outcomes. The authors identified 10 randomised controlled trials, which involved 577 participants with diabetes mellitus. Cinnamon was administered in tablet or capsule form, at a mean dose of 2 g daily, for four to 16 weeks. Generally, studies were not well conducted and lacked in quality.
The review authors found cinnamon to be no more effective than placebo, another active medication or no treatment in reducing glucose levels and glycosylated haemoglobin A1c (HbA1c), a long‐term measurement of glucose control. None of the trials looked at health‐related quality of life, morbidity, death from any cause or costs. Adverse reactions to cinnamon treatment were generally mild and infrequent.
Further trials investigating long‐term benefits and risks of the use of cinnamon for diabetes mellitus are required. Rigorous study design, quality reporting of study methods, and consideration of important outcomes such as health‐related quality of life and diabetes complications, are key areas in need of attention.
Summary of findings
for the main comparison.
| Cinnamon compared with placebo, no treatment, or active medication for diabetes mellitus | ||||||
|
Patient or population: patients with diabetes mellitus Settings: predominantly university outpatient clinics Intervention: oral monopreparations of cinnamon Comparison: placebo, no treatment, or active medication (such as insulin, oral hypoglycaemic agents, or other herbal / nutritional preparations) | ||||||
| Outcomes | Assumed risk | Corresponding risk | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
|
Death from any cause (follow‐up: 30 days to 16 weeks) |
Not estimable | See comment | See comment | Not investigated | ||
|
Morbidity (follow‐up: 30 days to 16 weeks) |
Not estimable | See comment | See comment | Not investigated | ||
| Health‐related quality of life (follow‐up: 30 days to 16 weeks) | Not estimable | See comment | See comment | Not investigated | ||
|
Adverse events (follow‐up: 30 days to 16 weeks) |
0.82 (0.21 to 3.23) | 264 (4) | ⊕⊕⊕⊝ moderate 1 | Adverse reactions to oral cinnamon were infrequent and generally mild in nature | ||
|
Costs (follow‐up: 30 days to 16 weeks) |
Not estimable | See comment | See comment | Not investigated | ||
|
HbA1c (follow‐up: 3 to 4 months) |
The mean HbA1c ranged across control groups from 6.8% to 8.8% | The mean HbA1c in the intervention groups was 0.3% lower to 0.2% higher |
MD ‐0.1% (‐0.3% to 0.2%) | 405 (6) | ⊕⊕⊕⊝ moderate 2 | |
1 Only four out of 10 studies reported adverse events; short follow‐up; unclear or high risk of bias in several domains.
2 Short follow‐up; imprecision of results; unclear or high risk of bias in several domains.
Background
Description of the condition
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. A consequence of this is chronic hyperglycaemia with disturbances of carbohydrate, fat and protein metabolism. Long‐term complications of diabetes mellitus include retinopathy, nephropathy, neuropathy, periodontal disease, and sexual dysfunction. The risk of cardiovascular disease is also increased. For a detailed overview of diabetes mellitus, please see 'Additional information' in the information on the Metabolic and Endocrine Disorders Group in The Cochrane Library (see 'About the Cochrane collaboration', 'Collaborative Review Groups (CRGs)'). For an explanation of methodological terms, see the main glossary in The Cochrane Library.
Description of the intervention
True cinnamon (Cinnamomum zeylanicum), Chinese cinnamon (Cinnamomum cassia) and Indonesian cinnamon (Cinnamomum burmanii) are among 300 species of Cinnamomum that belong to the Lauraceae family. The aromatic bark of the cinnamon tree is used worldwide for culinary purposes, but is also used in Ayurvedic and traditional Chinese medicine for its hypoglycaemic, digestive, antispasmodic and antiseptic properties (Battaglia 1995; Ody 1993).
Adverse effects of the intervention
Isolated case reports of cinnamon‐induced stomatitis venenata (inflammation of the mucous lining of any of the structures in the mouth) secondary to contact allergy have been reported with consumption of the herb as a flavouring agent (De Rossi 1998). However, there have been no documented adverse effects associated with the oral administration of cinnamon extract in clinical studies to date.
How the intervention might work
Animal studies have demonstrated that cinnamon, and its active constituent cinnamaldehyde, dose‐dependently improved glycaemic control and hyperlipidaemia in normal and streptozocin‐induced diabetic rats (Kannappan 2006; Kim 2006; Subash 2007). The mode of action for this hypoglycaemic action is unclear, but may be attributed to an increase in serum insulin levels, hepatic glycogen storage (Subash 2007), improved insulin‐receptor signalling (Qin 2004), an insulinomimetic effect (Roffey 2006), or a reduction in intestinal alpha‐glucosidase activity (Kim 2006). In clinical terms, these actions could lead to improvements in glycaemic control and insulin sensitivity, and a possible reduction in diabetic complications.
Why it is important to do this review
While there are a number of over‐the‐counter products that contain cinnamon, which make claim of a glucose‐regulating effect, the evidence of effectiveness for cinnamon in diabetes mellitus remains limited, and is still in its infancy. Therefore, there is a need to grow this evidence base with high‐quality research evidence in order to provide healthcare stakeholders, such as consumers, health professionals and funders, access to best evidence on the use of cinnamon for diabetes. By doing so, healthcare policies and practices can be informed by current best evidence.
Objectives
To evaluate the effects of cinnamon in patients with diabetes mellitus.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), without restriction on language.
Types of participants
Participants were limited to people with either type 1 or type 2 diabetes mellitus. To be consistent with changes in classification and diagnostic criteria of type 2 diabetes mellitus through the years, the diagnosis should have been established using the standard criteria valid at the time of the beginning of the trial (e.g. ADA 1997; ADA 1999; ADA 2003; WHO 1980; WHO 1985; WHO 1999). Ideally, diagnostic criteria should have been described. If necessary, an authors' definition of diabetes mellitus was used.
Participants with normal fasting blood glucose levels (FBGL) or postprandial glucose (PPG) levels were excluded.
Types of interventions
Intervention
Any orally administered monopreparation of cinnamon (Cinnamomum spp.) of any dose and form.
Combination preparations of cinnamon were excluded, although the simultaneous administration of cinnamon with insulin, oral hypoglycaemic agents or both was included.
Control
Placebo.
No treatment.
Active medication, such as insulin, oral hypoglycaemic agents, or other herbal/nutritional preparations.
Types of outcome measures
Primary outcomes
FBGL.
PPG levels.
Adverse events.
Secondary outcomes
Glycosylated haemoglobin A1c (HbA1c).
Serum insulin.
Insulin sensitivity (homeostasis model assessment of insulin resistance (HOMA‐IR)).
Health‐related quality of life (HRQoL).
Morbidity (all‐cause morbidity as well as diabetes and cardiovascular related morbidity).
Costs.
Covariates, effect modifiers and confounders
Compliance with treatment.
Co‐medication (insulin, oral hypoglycaemic agents).
Timing of outcome measurement
Data for all primary and secondary outcomes were collected from studies of any duration, except for HbA1c, where a period of at least three months was required to accurately measure changes in HbA1c.
Search methods for identification of studies
Electronic searches
The authors used the following sources from inception to specified time for the identification of trials.
The Cochrane Library (issue 12, 2011).
MEDLINE (until January 2012).
EMBASE (until January 2012).
CINAHL (until January 2012).
AARP Ageline (until January 2012).
BioMed Central gateway (until January 2012).
CAM on PubMed (until January 2012).
Health Source Nursing/Academic edition (until January 2012).
Natural medicines comprehensive database (until January 2012).
Dissertations Abstracts International (until January 2012).
AMI (until December 2009).
AMED (until January 2012).
International Pharmaceutical Abstracts (until January 2012).
Turning Research Into Practice (TRIP) database (until January 2012).
The authors also searched databases of ongoing trials (www.controlled‐trials.com/ [with links to several databases] and www.clinicaltrialsregister.eu/). Authors provided information (including trial identifier) about recognised studies in the table 'Characteristics of ongoing studies'.
For detailed search strategies see Appendix 1.
Searching other resources
The authors searched the reference lists of included trials, as well as pertinent reviews and textbooks, to identify additional studies. Content experts and manufacturers of cinnamon extracts were also contacted in order to obtain additional references, as well as details of unpublished trials and ongoing trials. The grey literature was also searched for unpublished studies using 'Dissertations Abstracts International' and 'Proceedings First'.
Data collection and analysis
Selection of studies
Two review authors (ML, SK) independently scanned the title and abstract of every record retrieved. All articles that appeared to meet the selection criteria, as well as those that could not be adequately assessed from the information given, were retrieved and investigated as full text.
Data extraction and management
For studies that fulfilled the inclusion criteria, two review authors (ML, SK) independently abstracted relevant population and intervention characteristics using standard data extraction templates (see Characteristics of included studies; Table 2; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6) with any disagreements resolved by discussion. Where possible, any relevant missing information on the trial was sought from the original author(s) of the article. An adapted PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow‐chart of study selection is attached (Figure 1) (Liberati 2009).
1. Overview of study populations.
|
Characteristic Study ID |
Intervention(s) and control(s) | [n] Screened/eligible | [n] Randomised | [n] Safety | [n] ITT | [n] Finishing study | Percentage of randomised participants finishing study |
| Akilen 2010 | I1: cinnamon C1: placebo |
I1: ‐ C1: ‐ T: 68 |
I1: 30 C1: 28 T: 58 |
‐ | I1: 30 C1: 28 T: 58 |
I1: 29 C1: 26 T: 55 |
I1: 97 C1: 93 T: 95 |
| Altschuler 2007 | I1: cinnamon C1: placebo |
I1: ‐ C1: ‐ T: 132 |
I1: 36 C1: 36 T: 72 |
‐ | I1: 28 C1: 29 T: 57 |
I1: 28 C1: 29 T: 57 |
I1: 78 C1: 81 T: 79 |
| Blevins 2007 | I1: cinnamon C1: placebo |
I1: ‐ C1: ‐ T: 77 |
I1: 30 C1: 30 T: 60 |
‐ | I1: 29 C1: 28 T: 57 |
I1: 21 C1: 22 T: 43 |
I1: 70 C1: 73 T: 72 |
| Crawford 2009 | I1: cinnamon C1: usual care |
I1: ‐ C1: ‐ T: 190 |
I1: 55 C1: 54 T: 109 |
‐ | I1: 55 C1: 54 T: 109 |
I1: 46 C1: 43 T: 89 |
I1: 84 C1: 80 T: 82 |
| Khan 2003 | I1: cinnamon 1 g I2: cinnamon 3 g I3: cinnamon 6 g C1: placebo 2 cap C2: placebo 6 cap C3: placebo 12 cap |
‐ | I1: 10 I2: 10 I3: 10 C1: 10 C2: 10 C3: 10 T: 60 |
‐ | I1: 10 I2: 10 I3: 10 C1: 10 C2: 10 C3: 10 T: 60 |
I1: 10 I2: 10 I3: 10 C1: 10 C2: 10 C3: 10 T: 60 |
I1: 100 I2: 100 I3: 100 C1: 100 C2: 100 C3: 100 T: 100 |
| Khan 2010 | I1: cinnamon C1: placebo |
‐ | I1: 7 C1: 7 T: 14 |
‐ | ‐ | I1: 7 C1: 7 T: 14 |
I1: 100 C1: 100 T:100 |
| Mang 2006 | I1: cinnamon C1: placebo |
‐ | I1: ‐ C1: ‐ T: 79 |
‐ | I1: 33 C1: 32 T: 65 |
I1: 33 C1: 32 T: 65 |
T: 82 |
| Rosado 2010 | I1: cinnamon C1: placebo |
‐ | I1: 20 C1: 20 T: 40 |
‐ | I1: 20 C1: 20 T: 40 |
I1: 20 C1: 20 T: 40 |
I1: 100 C1: 100 T: 100 |
| Suppapitiporn 2006 | I1: cinnamon C1: placebo |
‐ | I1: 20 C1: 40 T: 60 |
‐ | I1: 20 C1: 40 T: 60 |
I1: 20 C1: 40 T: 60 |
I1: 100 C1: 100 T: 100 |
| Vanschoonbeek 2006 | I1: cinnamon C1: placebo |
‐ | I1: 12 C1: 13 T: 25 |
‐ | I1: 12 C1: 13 T: 25 |
I1: 12 C1: 13 T: 25 |
I1: 100 C1: 100 T: 100 |
| Total | All interventions | 240 1 | |||||
| All controls | 258 1 | ||||||
| All interventions and controls | 577 | ||||||
"‐" denotes not reported
1data not available for all included studies
C: control; cap: capsules; I: intervention; ITT: intention to treat; T: total.
1.
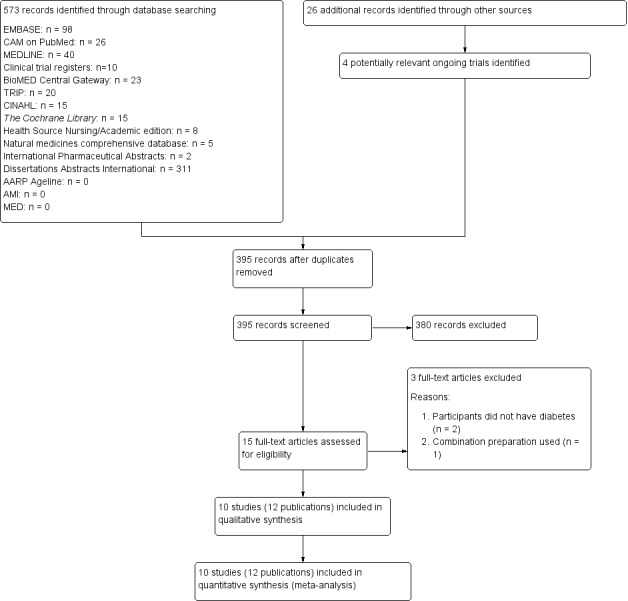
Study flow diagram.
Assessment of risk of bias in included studies
Two review authors (ML, SK) assessed risk of bias of each trial, independently, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). Any disagreement was resolved by consensus. A 'Risk of bias' table was completed for each included study (Characteristics of included studies). The results were also summarised graphically (Figure 2; Figure 3).
2.
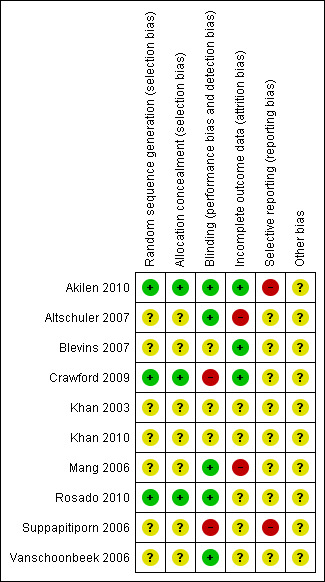
Risk of bias summary: review authors' judgements about each 'Risk of bias' item for each included study.
3.
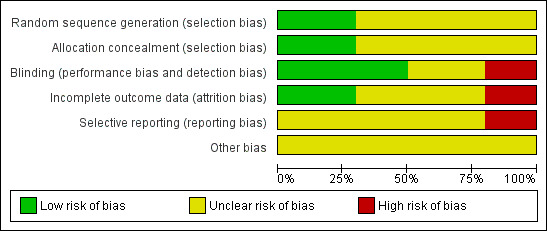
Risk of bias graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
Measures of treatment effect
Dichotomous data
Dichotomous outcomes were expressed as odds ratios (OR) with 95% confidence intervals (CI).
Continuous data
Continuous outcomes were expressed as mean differences (MD) with 95% CI.
Dealing with missing data
We obtained relevant missing data from authors, where possible. Evaluation of important numerical data, such as screened, eligible and randomised patients, as well as intention‐to‐treat (ITT) and per‐protocol (PP) population, is presented in Table 2. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals, were investigated. Issues of missing data were critically appraised.
Dealing with duplicate publications
In the case of duplicate publications and companion papers of a primary study, the authors maximised yield of information by simultaneous evaluation of all available data. In cases of doubt, the original publication (usually the oldest version) was given priority.
Assessment of heterogeneity
We identified heterogeneity by visual inspection of the forest plots and by using a standard Chi2 test with a significance level of α = 0.1, in view of the low power of this test. We specifically examined heterogeneity employing the I2 statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003), where an I2 statistic of 75% and more indicates a considerable level of inconsistency (Higgins 2008).
When we found heterogeneity, we attempted to determine potential reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
Funnel plots were planned in an exploratory data analysis to assess for the potential existence of small study bias if there were 10 studies or more for a given outcome. There are a number of explanations for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to study size, poor methodological design of small studies and publication bias (Sterne 2001). Thus, this exploratory data instrument may be misleading, so review authors did not place undue emphasis on this tool (Lau 2006).
Data synthesis
Data were summarised statistically if available, sufficiently similar and of sufficient quality, using Review Manager (RevMan) 5 software (RevMan 2011) and a random‐effects model. Statistical analysis was performed according to the statistical guidelines referenced in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were performed if one of the primary outcome parameters demonstrated statistically significant differences between intervention groups. In any other case, subgroup analyses were clearly marked as a hypothesis generating exercise.
The following subgroup analyses were planned.
Effect of different cinnamon species (e.g. C. zeylanicum, C. cassia, C. burmanii) on primary outcome measures.
Effect of cinnamon dosage (e.g. ≤ 1 g, 1.5 to 2 g, 3 g) on primary outcome measures.
Effect of treatment duration (e.g. < 12 weeks, 12 weeks or more) on primary outcome measures.
Effect of diabetes type (e.g. type 1 diabetes mellitus, type 2 diabetes mellitus) on primary outcome measures.
Sensitivity analysis
The review authors performed sensitivity analyses in order to explore the influence of the following factors on effect size.
Repeating the analysis excluding unpublished studies.
Repeating the analysis excluding low quality/high risk of bias studies (studies were defined as low quality/high risk of bias if any of the first three domains of the 'Risk of bias' table (i.e. random sequence generation, treatment concealment or blinding) were rated as unclear or high risk; studies were defined as moderate quality/moderate risk of bias if each of the first three domains of the 'Risk of bias' table were rated as low‐risk; studies were defined as high quality/low risk of bias if all domains of the 'Risk of bias' table were rated as low‐risk).
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results.
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country.
Results
Description of studies
For a detailed description of studies, see Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies.
Results of the search
The initial search identified 599 records; from these, 15 full‐text papers were identified for further examination. The other studies were excluded on the basis of their abstracts because they did not meet the inclusion criteria, were not relevant to the question under study or were a duplicate report (see Figure 1 for the amended PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow chart). After screening the full text of the selected papers, 10 studies (12 papers) met the inclusion criteria. All studies were published in English. Additional data and clarification of methodological issues were sought from the authors of all studies. Two review authors responded to these requests (Akilen 2010; Blevins 2007).
Included studies
A detailed description of the characteristics of included studies is presented elsewhere (see Characteristics of included studies). The following is a brief overview.
Study design
All studies were RCTs, with the exception of Vanschoonbeek 2006, of which randomisation was uncertain. All 10 trials adopted a parallel group design, and all but one study (Crawford 2009) used a placebo control. Two trials were multicentred (Akilen 2010; Altschuler 2007), with the number of centres being two (Altschuler 2007) or three (Akilen 2010). In terms of blinding, six studies were double‐blinded (Akilen 2010; Altschuler 2007; Blevins 2007; Mang 2006; Rosado 2010; Vanschoonbeek 2006), two single‐blinded (Crawford 2009; Suppapitiporn 2006), and in two studies, blinding was not defined (Khan 2003; Khan 2010). The duration of studies ranged from 4.3 to 16 weeks, with a mean study period of 10.8 weeks. No studies had a run‐in period, and two studies had a follow‐up period of 20 days (Khan 2003; Rosado 2010).
Participants
A total of 577 participants were included in the 10 trials. The individual sample size ranged from 14 to 109. Participants' gender was approximately distributed evenly, except for the trials by Akilen 2010 and Vanschoonbeek 2006. The trial by Akilen 2010 had more females in the intervention group compared to the control group. The trial by Vanschoonbeek 2006 was exclusively focused on a group of postmenopausal women. Participant gender was not reported by Khan 2010. The mean age of participants in the trials ranged from 52 to 63 years. One trial involved adolescents with a mean age of 15 years (Altschuler 2007). The mean body mass index (BMI) at baseline ranged from 24.8 to 33.4 kg/m2, with most study participants classified as obese (BMI 30 kg/m2 or more). Most trials included participants from economically developed countries, except three trials, which recruited participants from Pakistan (Khan 2003, Khan 2010) and Thailand (Suppapitiporn 2006). The duration of diabetes was reported in eight trials (except Crawford 2009 and Khan 2010), with the mean duration of diabetes in adolescents being six to seven years (Altschuler 2007) and in adults four to seven years. Only two trials reported co‐morbidities of participants (Akilen 2010; Rosado 2010). Criteria for entry into the individual studies are outlined in the Characteristics of included studies.
Diagnosis
Participants were diagnosed with type 2 diabetes mellitus in all but one study (Altschuler 2007), for which participants had type 1 diabetes mellitus. Four studies confirmed the diagnosis of type 2 diabetes against standard diagnostic criteria; two against WHO 1999 criteria (Akilen 2010; Vanschoonbeek 2006), and two against ADA 2003 criteria (Blevins 2007; Rosado 2010). The remaining five studies did not refer to standard diagnostic criteria; but instead, relied on third party diagnosis of diabetes prior to study enrolment.
Interventions
All studies used oral monopreparations of cinnamon in tablet or capsule form. The species of cinnamon used in seven out of 10 studies was Cinnamomum cassia or Chinese cinnamon. One study used Cinnamomum burmanii (Rosado 2010), and two did not define the type of cinnamon used (Altschuler 2007; Khan 2010). The daily dosage of cinnamon varied: 0.5 g (Rosado 2010), 1 g (Altschuler 2007; Blevins 2007; Crawford 2009; Khan 2003), 1.5 g (Khan 2010; Suppapitiporn 2006; Vanschoonbeek 2006), 2 g (Akilen 2010), 3 g (Khan 2003; Mang 2006) and 6 g (Khan 2003); with an average daily dosage of 1.9 g. All but one study (Crawford 2009) used a matching placebo as the control intervention. The ingredients in the control tablets were varied and included wheat flour (Blevins 2007; Khan 2003; Vanschoonbeek 2006), starch (Akilen 2010), microcrystalline cellulose (Mang 2006), lactose (Altschuler 2007), maize flour (Khan 2010) and bran cereal (Rosado 2010).The duration of treatment ranged from 4.3 to 16 weeks, with a mean treatment duration of 10.3 weeks.
In terms of concomitant treatments, the use of other diabetes medication (i.e. insulin, oral hypoglycaemic agents, or both) was similar between groups in five trials (Akilen 2010; Altschuler 2007; Crawford 2009; Khan 2003; Rosado 2010). In one study (Blevins 2007), use of diabetes medication was much higher in the placebo group relative to the cinnamon group (91% vs. 77%, respectively). Four trials (Khan 2010; Mang 2006; Suppapitiporn 2006; Vanschoonbeek 2006) did not provide sufficient data to make between‐group comparisons of diabetes medication use. Without this information, it is difficult to determine whether the findings of these studies are affected by additional risk of bias.
Outcomes
FBGL was measured in eight studies (Akilen 2010; Blevins 2007; Khan 2003; Khan 2010; Mang 2006; Rosado 2010; Suppapitiporn 2006; Vanschoonbeek 2006). All but one study (Khan 2003) reported HbA1c. Two studies assessed serum insulin (Blevins 2007; Vanschoonbeek 2006) and insulin sensitivity (Altschuler 2007; Vanschoonbeek 2006), five reported on adverse events (Akilen 2010; Altschuler 2007; Crawford 2009; Mang 2006; Suppapitiporn 2006) and one reported PPG (Rosado 2010). No studies measured HRQoL, morbidity or cost of treatment. For a summary of all endpoints assessed in each study, see Appendix 2.
Settings
Four of the nine studies were conducted in the US (Altschuler 2007; Blevins 2007; Crawford 2009; Rosado 2010). The other studies were completed in the UK (Akilen 2010), Pakistan (Khan 2003; Khan 2010), Germany (Mang 2006), Thailand (Suppapitiporn 2006) and the Netherlands (Vanschoonbeek 2006). For further details, see Characteristics of included studies.
Excluded studies
Three studies had to be excluded after careful evaluation of the full publication (Graham 2005; Wainstein 2011; Ziegenfuss 2006). Main reasons for exclusion were, failure to meet the criteria for diagnosis of type 1 or type 2 diabetes mellitus (Graham 2005; Ziegenfuss 2006), and use of a combination preparation (Wainstein 2011). For further details, see Characteristics of excluded studies.
Risk of bias in included studies
The 10 RCTs could be classified by their quality into two with moderate risk of bias (Akilen 2010; Rosado 2010) and eight with unclear or high risk of bias (Altschuler 2007; Blevins 2007; Crawford 2009; Khan 2003; Khan 2010; Mang 2006; Suppapitiporn 2006; Vanschoonbeek 2006). The results of the 'Risk of bias' assessments were summarised graphically (Figure 2, Figure 3).
Allocation
All selected trials were described as randomised, except for Vanschoonbeek 2006, where randomisation was uncertain. Only three studies reported the method of randomisation (Akilen 2010; Crawford 2009; Rosado 2010). Allocation concealment was reported in two studies (Crawford 2009; Rosado 2010).
Blinding
Five studies explicitly stated that blinding of the participants and investigator was undertaken (Akilen 2010; Altschuler 2007; Mang 2006; Rosado 2010; Vanschoonbeek 2006). Two studies reported that single blinding was undertaken, though it was unclear as to who and how this was achieved (Crawford 2009; Suppapitiporn 2006). Three studies did not provide sufficient information about blinding procedures (Blevins 2007; Khan 2003; Khan 2010).
Incomplete outcome data
Numbers of study withdrawals were described in six trials that had losses to follow‐up (Akilen 2010; Altschuler 2007; Blevins 2007; Crawford 2009; Mang 2006; Rosado 2010). Analysis was reported to be by ITT in Akilen 2010, Blevins 2007 and Crawford 2009. No ITT analysis was undertaken in the trials by Altschuler 2007 and Mang 2006. No loss to follow‐up was reported by Khan 2003, Khan 2010, Suppapitiporn 2006 and Vanschoonbeek 2006. Detailed descriptions of participants' withdrawals and reasons underpinning them were not provided in studies by Akilen 2010, Altschuler 2007, Blevins 2007Crawford 2009 and Mang 2006.
Selective reporting
While 8 of the 10 trials (Altschuler 2007; Blevins 2007; Crawford 2009; Khan 2003; Khan 2010; Mang 2006; Rosado 2010; Vanschoonbeek 2006) reported all primary and secondary outcomes, none of them published or lodged the trial protocol. Two trials (Akilen 2010; Suppapitiporn 2006) failed to report all primary and secondary outcomes.
Other potential sources of bias
Information on enrolments, exclusions, withdrawals or baseline characteristics was either limited or missing in studies by Khan 2003, Khan 2010, Rosado 2010, Suppapitiporn 2006 and Vanschoonbeek 2006.
Effects of interventions
See: Table 1
Baseline characteristics
For details of baseline characteristics, see Appendix 3.
Primary outcomes
Fasting blood glucose level
Eight trials reported data on FBGL for 338 participants. There was no statistically significant difference in FBGL between cinnamon and placebo (MD ‐0.83 mmol/L; 95% CI ‐1.67 to 0.02; P = 0.06; n = 388; 8 trials) (Analysis 1.1). A considerable level of heterogeneity (I2 = 82%) was present. Subgroup analysis based on study duration (Analysis 2.2), and sensitivity analysis restricted to trials with moderate risk of bias (MD ‐0.08 mmol/L; 95% CI ‐0.39 to 0.22; P = 0.59; n = 98, 2 trials) (Analysis 3.1) could not explain the heterogeneity; subgroup analysis for dosage was not suitable owing to repeated observations. Visual inspection of the funnel plot identified Khan 2003 and Khan 2010 as extreme outliers, which reported markedly different intervention effect estimates. A possible reason for this is the questionable quality of the Khan 2003 and Khan 2010 studies owing to inadequate methodological reporting; with insufficient details reported for all items in the 'Risk of bias' table. When Khan 2003 and Khan 2010 were removed from the analysis, the I2 statistic dropped to 0%. The analysis of six studies found no statistically significant difference in FBGL between cinnamon and placebo groups (MD ‐0.08 mmol/L; 95% CI ‐0.34 to 0.18; P = 0.55; n = 304; 6 trials, Analysis 1.2) (Figure 4).
1.1. Analysis.
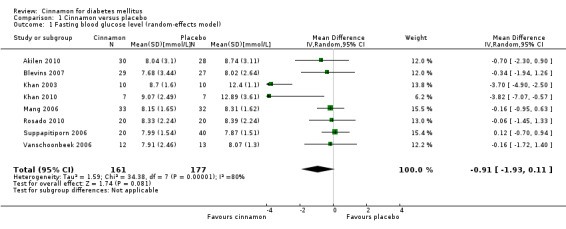
Comparison 1 Cinnamon versus placebo, Outcome 1 Fasting blood glucose level (random‐effects model).
2.2. Analysis.
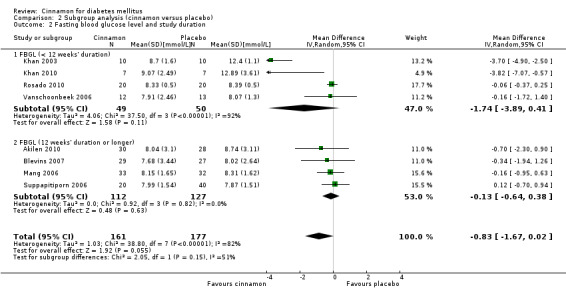
Comparison 2 Subgroup analysis (cinnamon versus placebo), Outcome 2 Fasting blood glucose level and study duration.
3.1. Analysis.
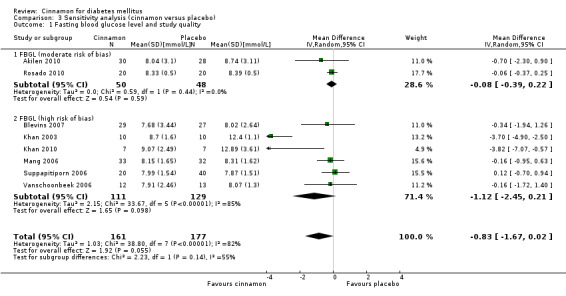
Comparison 3 Sensitivity analysis (cinnamon versus placebo), Outcome 1 Fasting blood glucose level and study quality.
1.2. Analysis.
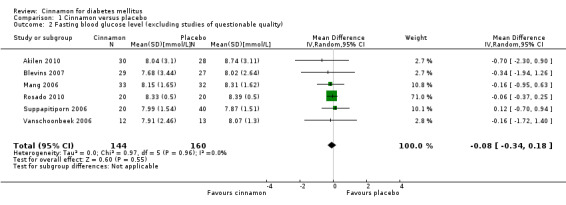
Comparison 1 Cinnamon versus placebo, Outcome 2 Fasting blood glucose level (excluding studies of questionable quality).
4.
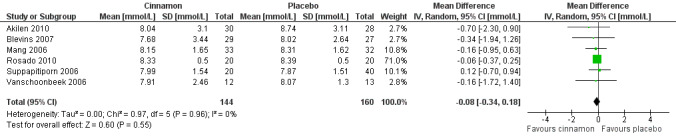
Forest plot of comparison: Cinnamon versus placebo; Outcome ‐ fasting blood glucose level (mmol/L; excludes studies of questionable quality).
Postprandial blood glucose level
One trial reported data on PPG for 40 participants. There was no statistically significant difference in PPG between cinnamon and placebo groups (MD ‐0.39 mmol/L; 95% CI ‐0.83 to 0.05; P = 0.08; n = 40; 1 trial) (Analysis 1.3).
1.3. Analysis.
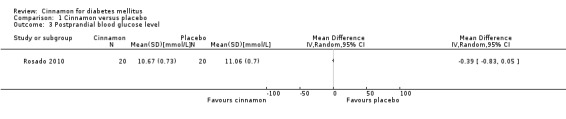
Comparison 1 Cinnamon versus placebo, Outcome 3 Postprandial blood glucose level.
Adverse events
Four trials reported data on adverse events for 264 participants; including three events in 133 participants receiving cinnamon, and four events in 131 participants receiving control. Crawford 2009 observed that one participant in the treatment group developed a rash after discontinuing cinnamon. Rosado 2010 identified one case of nausea in the control group. Altschuler 2007 reported that one participant in the treatment group developed hives, while another had a hypoglycaemic seizure. In the same trial, two participants from the control group reported adverse events; one reported stomach aches and the other frequent illness. All four participants withdrew from the study. Akilen 2010 stated that one participant from the placebo group developed mild gastric pain for two days. There was no statistically significant difference in the rate of adverse events between cinnamon and placebo groups (OR 0.83; 95% CI 0.22 to 3.07; P = 0.77; n = 264; 4 trials) (Analysis 1.4, Figure 5). There also was no significant difference in the OR of any adverse event between treatment groups in the subgroup analyses for dosage (Analysis 2.3) and study duration (Analysis 2.4), or the sensitivity analysis restricted to trials with moderate risk of bias (OR 0.31; 95% CI 0.03 to 3.07; P = 0.32; n = 98; 2 trials) (Analysis 3.2).
1.4. Analysis.
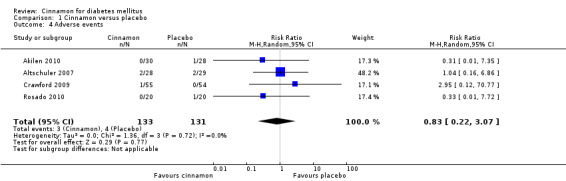
Comparison 1 Cinnamon versus placebo, Outcome 4 Adverse events.
5.
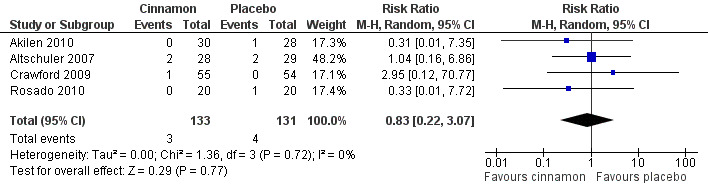
Forest plot of comparison: Cinnamon versus placebo; Outcome ‐ total number of adverse events (n).
2.3. Analysis.
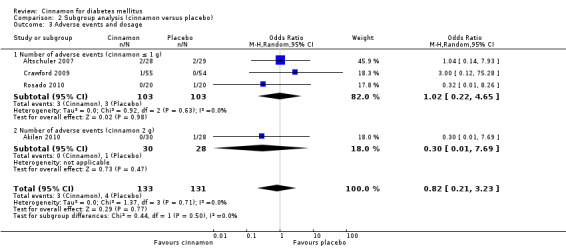
Comparison 2 Subgroup analysis (cinnamon versus placebo), Outcome 3 Adverse events and dosage.
2.4. Analysis.
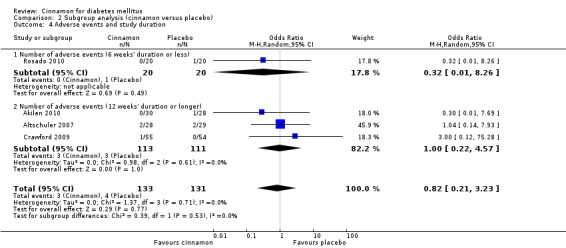
Comparison 2 Subgroup analysis (cinnamon versus placebo), Outcome 4 Adverse events and study duration.
3.2. Analysis.
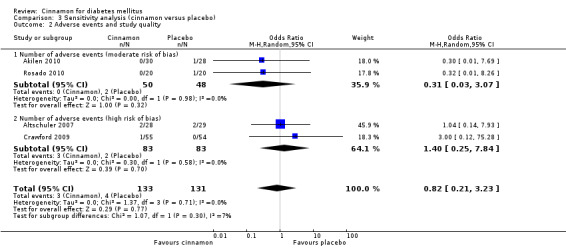
Comparison 3 Sensitivity analysis (cinnamon versus placebo), Outcome 2 Adverse events and study quality.
Secondary outcomes
Glycosylated haemoglobin A1c
Six trials (of at least three months' duration) reported data on HbA1c for 405 participants. There was no statistically significant difference in HbA1c between cinnamon and control groups (MD ‐0.06%; 95% CI ‐0.29 to 0.18; P = 0.63; n = 405; 6 trials) (Analysis 1.5, Figure 6). There also was no clear difference in HbA1c between treatment groups in the subgroup analyses for dosage (Analysis 2.5) and diabetes type (Analysis 2.7). Subgroup analysis for study duration and all planned sensitivity analyses were not suitable owing to insufficient data.
1.5. Analysis.
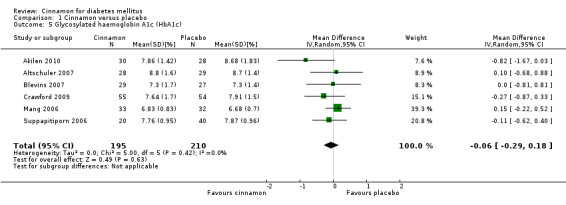
Comparison 1 Cinnamon versus placebo, Outcome 5 Glycosylated haemoglobin A1c (HbA1c).
6.
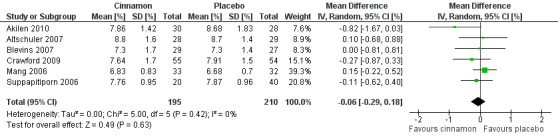
Forest plot of comparison: Cinnamon versus placebo; Outcome ‐ glycosylated haemoglobin A1c (HbA1c, %).
2.5. Analysis.
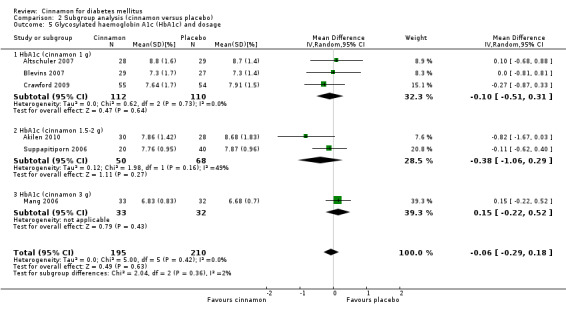
Comparison 2 Subgroup analysis (cinnamon versus placebo), Outcome 5 Glycosylated haemoglobin A1c (HbA1c) and dosage.
2.7. Analysis.
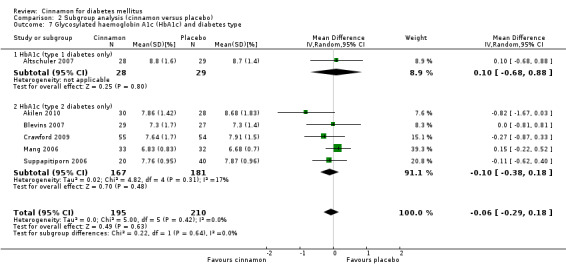
Comparison 2 Subgroup analysis (cinnamon versus placebo), Outcome 7 Glycosylated haemoglobin A1c (HbA1c) and diabetes type.
Serum insulin
Two trials reported data on serum insulin for 81 participants. There was no statistically significant difference in serum insulin between cinnamon and placebo groups (MD ‐6.77 pmol/L; 95% CI ‐37.0 to 23.46; P = 0.66: n = 81; 2 trials (Analysis 1.6). There also was no clear difference in serum insulin between treatment groups in subgroup analyses for dosage (Analysis 2.8) and study duration (Analysis 2.9). Subgroup analysis for diabetes type and all planned sensitivity analyses were not suitable owing to insufficient data.
1.6. Analysis.
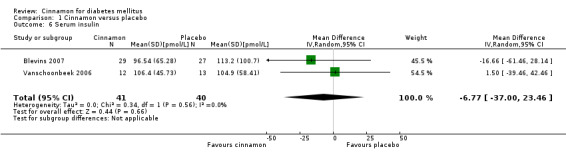
Comparison 1 Cinnamon versus placebo, Outcome 6 Serum insulin.
2.8. Analysis.
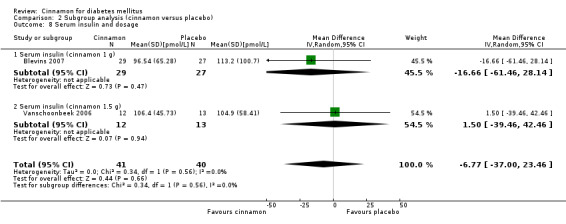
Comparison 2 Subgroup analysis (cinnamon versus placebo), Outcome 8 Serum insulin and dosage.
2.9. Analysis.
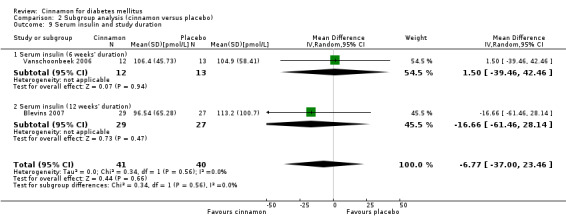
Comparison 2 Subgroup analysis (cinnamon versus placebo), Outcome 9 Serum insulin and study duration.
Insulin sensitivity
Two trials reported data on insulin sensitivity for 82 participants. Altschuler 2007 reported the ratio of carbohydrates to insulin (CHO/unit insulin) to demonstrate insulin sensitivity. Their findings indicated that there was no statistically significant difference in insulin sensitivity between cinnamon and placebo groups (MD 0; 95% CI ‐1.56 to 1.56; P = 1.00; n = 48; 1 trial) (Analysis 1.7). Vanschoonbeek 2006 measured insulin sensitivity as HOMA‐IR. Their findings indicate that there was no statistically significant difference in insulin sensitivity between treatment groups (MD 0.22; 95% CI ‐0.70 to 1.14; P = 0.64; n = 25; 1 trial) (Analysis 1.8). Data were not suitable for subgroup or sensitivity analysis.
1.7. Analysis.
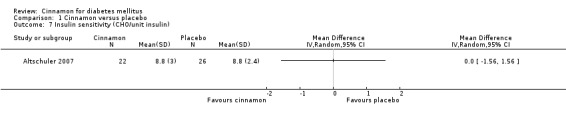
Comparison 1 Cinnamon versus placebo, Outcome 7 Insulin sensitivity (CHO/unit insulin).
1.8. Analysis.
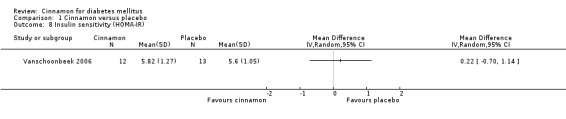
Comparison 1 Cinnamon versus placebo, Outcome 8 Insulin sensitivity (HOMA‐IR).
Health‐related quality of life, morbidity and costs
No trial explored HRQoL, morbidity or costs as endpoints.
Discussion
Summary of main results
This systematic review of cinnamon for diabetes mellitus pooled 10 prospective, parallel‐group design, RCTs, studying a total of 577 adolescents and adults with type 1 and type 2 diabetes. All studies administered oral monopreparations of cinnamon (primarily Cinnamomum cassia) in tablet or capsule form, at an average daily dose of 2 g, for a mean period of 11 weeks. In all but one study (which compared cinnamon to usual care), placebo was used as the control intervention.
In the meta‐analysis of trials assessing glycaemic control, no conclusions could be made regarding the efficacy of cinnamon in reducing FBGL. Meta‐analyses of secondary outcomes found no statistically significant difference in HbA1c or serum insulin between cinnamon and control groups. Study results could not be combined for insulin sensitivity owing to the different outcome measures used; even so, both trials found no significant difference in insulin sensitivity between groups. Similarly, there were too few studies to combine data for PPG levels; the one study reporting this outcome found no significant difference in PPG between the two groups. In general, cinnamon was well tolerated, with less than 2.7% of participants reporting adverse events, most of which were mild in nature. No trials examined HRQoL, morbidity or costs as endpoints.
These findings add to the body of emerging evidence on the effectiveness of cinnamon for diabetes (Baker 2008; Pham 2007). While the best available evidence does not support the use of orally administered cinnamon for diabetes mellitus, there is adequate justification for conducting further studies in this area. For instance, no studies have investigated the effects of cinnamon in young children with diabetes mellitus. It is also unclear whether different species of cinnamon (e.g. Cinnamomum zeylanicum), routes of administration (e.g. subcutaneous), methods of extraction (e.g. ethanolic extraction) or types of preparation (e.g. liquid extract) exhibit different effects in people with diabetes mellitus. Given the findings of our subgroup analyses, it is unlikely that differences in cinnamon dosage, frequency of administration or treatment duration would yield more favourable results. The high or unclear risk of bias of included studies also suggests that more rigorous trials of cinnamon for diabetes are warranted.
Overall completeness and applicability of evidence
The objective of this review was to evaluate the effects of cinnamon in patients with diabetes mellitus. Commonly reported outcomes include FBGL, HbA1c, serum insulin, insulin sensitivity and adverse events. Only a few trials reported all of these outcomes measures. Equally important measures such as HRQoL, morbidity and costs were not measured by any of the included studies, and PPG was measured in only one trial. Notwithstanding, several of these outcomes (i.e. HRQoL and PPG) are reportedly being measured in ongoing trials (Ridout 2007; Stoecker 2010).
The variety of dosages and wide range of intervention periods (i.e. four to 16 weeks) made comparisons difficult. Further, there was little information regarding long‐term follow‐up and therefore, it is unclear what, if any, long‐term benefits are likely to occur as a result of this intervention. Unfortunately, ongoing trials do not appear to address this issue. Overall, this review has good external validity as the participants in the trials resemble patients in clinical practice, and further, the intervention is generally safe and feasible to carry out in clinical practice.
Quality of the evidence
Two out of 10 trials were assessed as having moderate risk of bias (i.e. each of the first three domains of the 'Risk of bias' table were rated as low risk); five trials showed high risk of bias in one of the investigated domains. Selection bias may have played a role in some of the included trials as important information about sample characteristics and sampling was missing in several studies. While all the included trials were labelled as RCTs, only three studies explicitly reported the randomisation method, with only two studies reporting concealed allocation. This highlights the inherent risk of allocation bias. Half of the included trials either did not provide adequate information or had high risk of bias regarding blinding processes, which raises the possibility of performance bias. When explored further, we were unable to determine how many trials had blinded outcome assessment. While loss to follow‐up was reported in 6 out of 10 trials, ITT analysis was only explicitly undertaken in three of these trials. Furthermore, reasons for drop‐outs were inconsistently reported. Therefore, attrition bias may play a role here. While eight of the 10 trials reported on all primary and secondary outcomes, none of these trials published or lodged the trial protocol. Therefore, it is unclear if all the trial processes were adhered to or what, if any, variations to the processes did occur. As two trials did not report on all primary and secondary outcomes, reporting bias may play a role here. It is also unclear if there were significant differences between groups in concomitant diabetes medication use in the trials not reporting these data, and whether this constituted an unfair comparison of groups, and thereby an additional risk of bias. Taking into account these threats to internal validity, the quality of evidence underpinning this review needs to be carefully considered.
Potential biases in the review process
The review was not without limitations. For instance; whilst the search strategy was comprehensive, and no limits were placed on language of publication, it is possible that pertinent unpublished reports or studies published in languages other than English could have been missed, unintentionally. Thus, language and publication bias cannot be excluded entirely. The degree of rigour with which the studies were conducted is not clear also; because, even though the overall risk of bias of most included studies was rated high or unclear, much of this risk was attributed to inadequate reporting, including the lack of detailed information on blinding procedures, participant withdrawals and methods of randomisation. This was in spite of attempts to contact study authors for further information.
Agreements and disagreements with other studies or reviews
This review agrees with a previous review on the findings that cinnamon does not appear to improve a number of clinical parameters (such as HbA1c and FBGL) in patients with diabetes (Baker 2008). The meta‐analysis undertaken by Baker and colleagues also highlighted the significant limitations to the current evidence in terms of the limited evidence base, high proportion of underpowered studies, and range of methodological issues. The results from two systematic reviews (Akilen 2012; Davis 2011) present conflicting findings. Akilen and colleagues concluded that while the majority of studies showed no potential therapeutic benefits, cinnamon may be a viable addition to a range of conventional diabetes management options for patients with poorly controlled type 2 diabetes mellitus with a HbA1c greater than 7% (Akilen 2012). The meta‐analysis by Davis and Yokoyama identified that cinnamon, administered either whole or as an extract, resulted in the lowering of FBGL in people with type 2 diabetes mellitus or pre‐diabetes (Davis 2011). The findings of Akilen 2012 and Davis 2011 may have differed from the results of our review owing to differences in the study inclusion criteria (such as the inclusion of the pre‐diabetic population by Davis 2011). While there are differences in the findings, these reviews agree that the current evidence base is small (hence potentially underpowered) with important methodological limitations.
Authors' conclusions
Implications for practice.
This systematic review has shown that in people with type 1 or type 2 diabetes mellitus, orally administered cinnamon (Cinnamomum cassia) in tablet or capsule form, at a dose of 0.5 to 6 g daily for a period of four to 16 weeks, is no more effective than placebo or control intervention at improving glycosylated haemoglobin A1c (HbA1c) or serum insulin levels. The effect of cinnamon on fasting and postprandial blood glucose levels is inconclusive. The review is unable to draw any conclusions regarding the efficacy of other species, routes of administration or types of preparation of cinnamon for diabetes mellitus.
Implications for research.
Many of the included trials were of poor methodological quality (leading to high or unclear risk of bias) and hence, there is a need for rigorous, higher‐quality RCTs. A common finding among the included trials was the poor reporting standards. There are several reporting standards for clinical trials that could be used as a useful framework for future publications. Future research should include adequate samples, with clear justification and evidence of power calculations, with a comprehensive suite of outcome measures that capture short‐ and long‐term outcomes. There currently persists a research gap in the literature that investigates the effect of cinnamon in young children. With diabetes becoming more prevalent, research in this important area should be undertaken. Particular to cinnamon, future research should explore other species of cinnamon and different parameters of administration, extraction and preparation. Outcomes are likely to be different for each of these groups.
Feedback
New feedback, 29 September 2013
Summary
Some analysis seemed inappropriate here: (1) The reviewers chose wrong variables for meta‐analysis. For example, the difference of the fasting blood glucose levels in the cinnamon and placebo groups at the end of the clinical trial (Figure 4, the numbers were post‐intervention levels) could not reflect the effect of treatment because each individual started at a different level. Many cited studies (except for Khan 2003, Crawford 2009, Khan 2010) did paired comparison and showed changes between pre‐ and post‐ intervention levels and calculated the SD accordingly; the failure with regard to the treated paired samples and the corresponding analysis methods resulted in loss of information and lower contribution from these trials to the final synthesis. The meta‐analysis should compare the differences of changes between the cinnamon and placebo groups. The presented analysis is not interpretable. Both figures 4 and 6 should be re‐analyzed. Many of the corresponding analyses (e.g. analysis 1.1‐1.3 and 1.5) should also be repeated using the correct variables. (2) The SD of baseline fasting glucose levels in the Rosado thesis was by far the lowest in the listed trials. The text in her thesis showed (page 65) the SD at baseline was nearly 37.67 mg/dL (2.09 mM). The SD listed in figures 1 and 2 were all near 8.77 mg/dL (0.49 mM) (page 67). Clearly, she misrepresented the standard error of the mean (SD divided by the square root of the sample size in that group, which varied from 16 to 20) as the SD. (3) The authors thought Khan 2003 article had a high probability of bias. This inference was supported and could be quantified by the data. The control group (3) had baseline fasting glucose levels of 16.7 (1.4) and the rest had means between 11.4 to 13.0, SD between 1.0 to 1.7. This randomization in the initial allocation (F=20.5 df=5,54, p=0.00000000002) was unusually poor.
Reply
We would like to thank Yeh for the commentary on our Cochrane review of cinnamon for diabetes. Yeh stated that the choice to use post‐intervention data in our meta‐analyses was incorrect. At the beginning of this review, there was much deliberation as to whether we should use change from baseline data or post‐intervention data for the meta‐analyses. Under the advice of the Cochrane Collaboration and the Cochrane Handbook, we made the decision to go with post‐intervention data. There were several good reasons for this. Firstly, not all studies reported change from baseline data; by mixing both types of data in the meta‐analysis (which is not necessarily a problem in the eyes of the Cochrane Collaboration), it is possible to introduce bias through the selection of data that may exaggerate results (either intentionally or unintentionally). It is also important to be consistent with the approach taken. Second, because few studies explicitly reported the use of intention‐to‐treat analysis, we were not confident that the number of participants in the baseline and post‐intervention groups in each study were the same; because of this, it was not appropriate to use change from baseline data.
We thank Yeh for also bringing to our attention the possibility that the standard deviations reported in Rosado’s thesis could be standard errors. We were unable to confirm this, but can state that the impact of this on the meta‐analysis of FBGL (fasting blood glucose levels) data was negligible in terms of effect size (changing from ‐0.83 to ‐0.91), heterogeneity [I2] (changing from 82% to 80%) and level of significance [P] (changing from 0.06 to 0.08).
Contributors
Jih‐I Yeh. Department of Family Medicine, Tzu‐Chi General Hospital and Tzu‐Chi University, Hualien, Taiwan. Email: jihiyeh@gms.tcu.edu.tw.
For the authors: Matthew Leach.
What's new
| Date | Event | Description |
|---|---|---|
| 12 December 2013 | Feedback has been incorporated | New feedback incorporated |
History
Protocol first published: Issue 2, 2008 Review first published: Issue 9, 2012
| Date | Event | Description |
|---|---|---|
| 20 February 2008 | New citation required and major changes | Substantive amendment |
Acknowledgements
The review authors wish to acknowledge the support provided by the International Centre of Allied Health Evidence (iCAHE) during the development of the protocol.
Appendices
Appendix 1. Search strategies
| Search terms and databases |
| Unless otherwise stated, search terms are free text terms. Abbreviations: '$': stands for any character; '?': substitutes one or no character; adj: adjacent (i.e. number of words within range of search term); exp: exploded MeSH; MeSH: medical subject heading (MEDLINE medical index term); pt: publication type; sh: MeSH; tw: text word. |
| The Cochrane Library |
| 1. cinnamon.tw 2. cinnamomum.tw 3. Lauraceae.tw 4. 1 or 2 or 3 5. diabetes.tw 6. diabetes mellitus.tw 7. insulin dependent diabetes mellitus.tw 8. non insulin dependent diabetes mellitus.tw 9. maturity onset diabetes mellitus.tw 10. glucose intolerance.tw 11. insulin resistance.tw 12. IDDM.tw 13. NIDDM.tw 14. MODY.tw 15. T1DM.tw 16. T2DM.tw 17. 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18. 4 and 17 |
| MEDLINE |
| 1. exp Cinnamomum/ 2. exp Cinnamomum zeylanicum/ 3. exp lauraceae/ 4. (cinnamomum or cinnamon).tw 5. or/1‐4 6. exp prospective studies/ 7. exp clinical trial/ 8. randomized controlled trial.pt 9. controlled clinical trial.pt. 10. clinical trial, Phase III.pt 11. clinical trial, Phase III.pt 12. randomized controlled trial.sh 13. random allocation.sh. 14. double‐blind method.sh 15. single‐blind method.sh 16. ((singl$ or doubl$ or tripl$ or trebl$) adj6 (mask$ or blind$)).tw 17. (random$ adj25 (trial$ or stud$ or investigat$ or cross over or crossover)).tw 18. or/6‐17 19. exp meta‐analysis/ 20. exp Review Literature/ 21. meta‐analysis.pt. 22. systematic review$.tw 23. search$.tw 24. medline.tw 25. cochrane database of systematic reviews.jn 26. or/19‐25 27. letter.pt 28. comment.pt 29. editorial.pt 30. historical‐article.pt. 31. or/27‐30 32. 26 not 31 33. exp Technology Assessment, Biomedical/ 34. HTA.tw 35. (health technology adj6 assessment$).tw 36. (biomedical adj6 technology assessment$).tw 37. or/33‐36 38. exp diabetes mellitus/ 39. diabet$.tw 40. IDDM.tw 41. NIDDM.tw 42. MODY.tw 43. exp glucose intolerance/ 44. (late onset adj diabet$).tw 45. (maturity onset adj diabet$).tw 46. (non insulin$ depend$ or noninsulin$ depend$ or non insulin?depend$ or noninsulin?depend$).tw 47. ((typ$ 1 or typ$ 2) adj6 diabet$).tw 48. ((typ$ I or typ$ II) adj6 diabet$).tw 49. (insulin$ depend$ or insulin?depend$).tw 50. exp insulin resistance/ 51. (T1DM or T2DM).tw 52. or/38‐51 53. 5 and 18 and 52 54. 5 and 32 and 52 55. 5 and 37 and 52 56. 53 or 54 or 55 |
| EMBASE |
| 1. exp Cinnamomum/ 2. exp Cinnamomum cassia/ 3. exp Cinnamomum cassia extract/ 4. exp Cinnamomum zeylanicum/ 5. exp cinnamon/ 6. exp cinnamon extract/ 7. lauraceae.tw 8. (cinnamomum or cinnamon).tw. 9. or/1‐8 10. exp prospective study/ 11. exp clinical study/ 12. exp controlled clinical trial/ 13. exp.phase 3 clinical trial/ 14. exp placebo/ 15. ((singl$ or doubl$ or tripl$ or trebl$) and (mask$ or blind$)).tw 16. random$ and (trial$ or stud$ or investigat$ or cross over or crossover).tw 18. or/10‐16 19. animal studies / animals. 20. 18 not 19 21. exp diabetes mellitus/ 22. exp insulin dependent diabetes mellitus/ 23. exp non insulin dependent diabetes mellitus/ 24. exp maturity onset diabetes mellitus/ 25. diabet$.tw 26. IDDM.tw 27. NIDDM.tw 28. MODY.tw 29. exp glucose intolerance/ 30. exp insulin resistance/ 31. (T1DM or T2DM).tw 32. (late onset adj diabet$).tw 33. or/21‐32 34. 9 and 20 and 33 |
| CINAHL |
| 1. cinnamon.tw 2. cinnamomum.tw 3. Lauraceae.tw 4. or/1‐3 5. diabetes.tw 6. diabetes mellitus.tw 7. insulin dependent diabetes mellitus.tw 8. non insulin dependent diabetes mellitus.tw 9. maturity onset diabetes mellitus.tw 10. glucose intolerance.tw 11. insulin resistance.tw 12. IDDM.tw 13. NIDDM.tw 14. MODY.tw 15. T1DM.tw 16. T2DM.tw 17. or/5‐16 18. prospective study.tw 19. clinical trial.tw 20. randomized controlled trial.tw 21. randomized clinical trial.tw 22. controlled clinical trial.tw 23. double‐blind.tw 24. single‐blind.tw 25. or/18‐24 26. 4 and 17 and 25 |
| AMED |
| 1. exp Cinnamomum/ 2. lauraceae.tw 3. (cinnamomum or cinnamon).tw 4. or/1‐3 5. exp clinical trial/ 6. exp randomized controlled trials/ 7. randomized controlled trial.pt 9. controlled clinical trial.pt. 10. clinical trial, phase III.pt 11. clinical trial.pt 12. ((singl$ or doubl$ or tripl$ or trebl$) and (mask$ or blind$)).tw 13. random$ and (trial$ or stud$ or investigat$ or cross over or crossover).tw 14. double‐blind method.sh 15. single‐blind method.sh 16. or/5‐15 17. exp diabetes mellitus/ 18. diabet$.tw 19. IDDM.tw 20. NIDDM.tw 21. MODY.tw 22. glucose intolerance.tw 23. insulin resistance.tw 24. (late onset adj diabet$).tw 25. (maturity onset adj diabet$).tw 26. (non insulin$ depend$ or noninsulin$ depend$ or non insulin?depend$ or noninsulin?depend$).tw 27. ((typ$ 1 or typ$ 2) adj6 diabet$).tw 28. ((typ$ I or typ$ II) adj6 diabet$).tw 29. (insulin$ depend$ or insulin?depend$).tw 30. (T1DM or T2DM).tw 31. or/17‐30 32. 4 and 16 and 31 |
| BIOMED CENTRAL GATEWAY |
| 1. cinnamon.tw
2. cinnamomum.tw
3. Lauraceae.tw
4. or/1‐3
5. diabetes.tw
6. diabetes mellitus.tw
7. insulin dependent diabetes mellitus.tw
8. non insulin dependent diabetes mellitus.tw
9. maturity onset diabetes mellitus.tw
10. glucose intolerance.tw
11. insulin resistance.tw
12. IDDM.tw
13. NIDDM.tw
14. MODY.tw
15. T1DM.tw
16. T2DM.tw
17. or/5‐16
18. prospective study.tw
19. clinical trial.tw
20. randomized controlled trial.tw
21. randomized clinical trial.tw
22. controlled clinical trial.tw
23. double‐blind.tw
24. single‐blind.tw
25. or/18‐24 26. 4 and 17 and 25 |
| CAM ON PUBMED |
| 1. cinnamon.tw 2. cinnamomum.tw 3. Lauraceae.tw 4. 1 or 2 or 3 5. diabetes.tw 6. diabetes mellitus.tw 7. insulin dependent diabetes mellitus.tw 8. non insulin dependent diabetes mellitus.tw 9. maturity onset diabetes mellitus.tw 10. glucose intolerance.tw 11. insulin resistance.tw 12. IDDM.tw 13. NIDDM.tw 14. MODY.tw 15. T1DM.tw 16. T2DM.tw 17. 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18. prospective study.tw 19. clinical trial.tw 20. randomized controlled trial.tw 21. randomized clinical trial.tw 22. controlled clinical trial.tw 23. double‐blind.tw 24. single‐blind.tw 25. 18 or 19 or 20 or 21 or 22 or 23 or 24 |
| HEALTH SOURCE NURSING / ACADEMIC EDITION |
| 1. cinnamon.tw 2. cinnamomum.tw 3. Lauraceae.tw 4. 1 or 2 or 3 5. diabetes.tw 6. diabetes mellitus.tw 7. insulin dependent diabetes mellitus.tw 8. non insulin dependent diabetes mellitus.tw 9. maturity onset diabetes mellitus.tw 10. glucose intolerance.tw 11. insulin resistance.tw 12. IDDM.tw 13. NIDDM.tw 14. MODY.tw 15. T1DM.tw 16. T2DM.tw 17. 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18. prospective study.tw 19. clinical trial.tw 20. randomized controlled trial.tw 21. randomized clinical trial.tw 22. controlled clinical trial.tw 23. double‐blind.tw 24. single‐blind.tw 25. 18 or 19 or 20 or 21 or 22 or 23 or 24 |
| INTERNATIONAL PHARMACEUTICAL ABSTRACTS |
| 1. cinnamon.tw 2. cinnamomum.tw 3. Lauraceae.tw 4. 1 or 2 or 3 5. diabetes.tw 6. diabetes mellitus.tw 7. insulin dependent diabetes mellitus.tw 8. non insulin dependent diabetes mellitus.tw 9. maturity onset diabetes mellitus.tw 10. glucose intolerance.tw 11. insulin resistance.tw 12. IDDM.tw 13. NIDDM.tw 14. MODY.tw 15. T1DM.tw 16. T2DM.tw 17. 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18. prospective study.tw 19. clinical trial.tw 20. randomized controlled trial.tw 21. randomized clinical trial.tw 22. controlled clinical trial.tw 23. double‐blind.tw 24. single‐blind.tw 25. 18 or 19 or 20 or 21 or 22 or 23 or 24 |
| NATURAL MEDICINES COMPREHENSIVE DATABASE |
| 1. Cinnamon (subject heading) |
| TURNING RESEARCH INTO PRACTICE |
| 1. cinnamon.tw 2. cinnamomum.tw 3. Lauraceae.tw 4. 1 or 2 or 3 5. diabetes.tw 6. diabetes mellitus.tw 7. insulin dependent diabetes mellitus.tw 8. non insulin dependent diabetes mellitus.tw 9. maturity onset diabetes mellitus.tw 10. glucose intolerance.tw 11. insulin resistance.tw 12. IDDM.tw 13. NIDDM.tw 14. MODY.tw 15. T1DM.tw 16. T2DM.tw 17. 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18. prospective study.tw 19. clinical trial.tw 20. randomized controlled trial.tw 21. randomized clinical trial.tw 22. controlled clinical trial.tw 23. double‐blind.tw 24. single‐blind.tw 25. 18 or 19 or 20 or 21 or 22 or 23 or 24 |
| DISSERTATIONS ABSTRACTS INTERNATIONAL |
| 1. cinnamon.tw 2. cinnamomum.tw 3. Lauraceae.tw 4. 1 or 2 or 3 5. diabetes.tw 6. diabetes mellitus.tw 7. insulin dependent diabetes mellitus.tw 8. non insulin dependent diabetes mellitus.tw 9. maturity onset diabetes mellitus.tw 10. glucose intolerance.tw 11. insulin resistance.tw 12. IDDM.tw 13. NIDDM.tw 14. MODY.tw 15. T1DM.tw 16. T2DM.tw 17. 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18. prospective study.tw 19. clinical trial.tw 20. randomized controlled trial.tw 21. randomized clinical trial.tw 22. controlled clinical trial.tw 23. double‐blind.tw 24. single‐blind.tw 25. 18 or 19 or 20 or 21 or 22 or 23 or 24 26. Limit to dissertations and theses |
| AARP |
| 1. cinnamon.tw 2. cinnamomum.tw 3. Lauraceae.tw 4. 1 or 2 or 3 5. diabetes.tw 6. diabetes mellitus.tw 7. insulin dependent diabetes mellitus.tw 8. non insulin dependent diabetes mellitus.tw 9. maturity onset diabetes mellitus.tw 10. glucose intolerance.tw 11. insulin resistance.tw 12. IDDM.tw 13. NIDDM.tw 14. MODY.tw 15. T1DM.tw 16. T2DM.tw 17. 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18. prospective study.tw 19. clinical trial.tw 20. randomized controlled trial.tw 21. randomized clinical trial.tw 22. controlled clinical trial.tw 23. double‐blind.tw 24. single‐blind.tw 25. 18 or 19 or 20 or 21 or 22 or 23 or 24 |
| AMI |
| 1. cinnamon.tw 2. cinnamomum.tw 3. Lauraceae.tw 4. 1 or 2 or 3 5. diabetes.tw 6. diabetes mellitus.tw 7. insulin dependent diabetes mellitus.tw 8. non insulin dependent diabetes mellitus.tw 9. maturity onset diabetes mellitus.tw 10. glucose intolerance.tw 11. insulin resistance.tw 12. IDDM.tw 13. NIDDM.tw 14. MODY.tw 15. T1DM.tw 16. T2DM.tw 17. 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18. prospective study.tw 19. clinical trial.tw 20. randomized controlled trial.tw 21. randomized clinical trial.tw 22. controlled clinical trial.tw 23. double‐blind.tw 24. single‐blind.tw 25. 18 or 19 or 20 or 21 or 22 or 23 or 24 |
Appendix 2. Matrix of study endpoints
|
Characteristic Study ID |
Primarya endpoint(s) | Secondaryb endpoint(s) | Otherc endpoint(s) | Time points for outcome measurement |
| Akilen 2010 | ‐ | ‐ | HbA1c, diastolic and systolic blood pressure, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, fasting plasma glucose, total energy intake, body mass index | 12 weeks |
| Altschuler 2007 | HbA1c | ‐ | Daily insulin intake, adverse events, insulin sensitivity | 12 weeks |
| Blevins 2007 | ‐ | ‐ | HbA1c, fasting glucose, total cholesterol, LDL cholesterol, HDL cholesterol, triglyceride, serum insulin, body mass index | 4 weeks, 8 weeks, 12 weeks |
| Crawford 2009 | HbA1c | ‐ | ‐ | 12 weeks |
| Khan 2003 | ‐ | ‐ | Fasting serum glucose, fasting serum triglyceride, fasting serum cholesterol, fasting serum HDL level, fasting serum LDL level | 20 days, 40 days, 60 days |
| Khan 2010 | ‐ | ‐ | Fasting serum glucose, fasting serum triglycerides, fasting serum cholesterol, fasting serum HDL cholesterol, fasting serum LDL cholesterol | 30 days |
| Mang 2006 | ‐ | ‐ | HbA1c, fasting plasma glucose, total cholesterol, LDL, HDL, triacylglycerol | 16 weeks |
| Rosado 2010 | ‐ | ‐ | HbA1c, fasting blood glucose, postprandial glucose, total cholesterol, triglycerides, LDL cholesterol, HDL cholesterol | 20 days, 40 days, 60 days |
| Suppapitiporn 2006 | ‐ | ‐ | HbA1c, fasting plasma glucose, total cholesterol, triglyceride, HDL, creatinine, SGOT, SGPT, BUN, body weight, blood pressure | 12 weeks |
| Vanschoonbeek 2006 | ‐ | ‐ | HbA1c, plasma glucose, plasma insulin, OGIS; ISIcomp, HOMA‐IR, total cholesterol, LDL cholesterol, HDL cholesterol, triacylglycerol | 2 weeks, 6 weeks |
|
Footnotes: "‐" denotes not reported a,b verbatim statement in the publication or (registered) trial document; c not explicitly stated as primary or secondary endpoint(s) in the publication or (registered) trial document BUN: blood urea nitrogen; HbA1c: glycosylated haemoglobin A1c; HDL: high‐density lipoprotein; HOMA‐IR: homeostasis model assessment of insulin resistance; ISIcomp: index of composite whole‐body insulin sensitivity; LDL: low‐density lipoprotein; OGIS: oral glucose insulin sensitivity; SGOT: serum glutamic oxaloacetic transaminase; SGPT: serum glutamic pyruvic transaminase. | ||||
Appendix 3. Baseline characteristics (I)
|
Characteristic Study ID |
Intervention(s) and control(s) | Duration of intervention | Participating population | Pharmaco‐naive patients [%] | Country | Setting | Sex [female, %] | Age [mean years (SD)] | Ethnic groups [%] |
| Akilen 2010 | I1: cinnamon C1: placebo |
12 weeks | Adults with type 2 diabetes | 0 | UK | Community diabetes clinics | I1: 63 C1: 46 |
I1: 54.9 (10.1) C1: 54.4 (12.5) |
I1: White 20, Asian 57, Black 23 C1: White 14, Asian 57, Black 29 |
| Altschuler 2007 | I1: cinnamon C1: placebo |
12 weeks | Adolescents with type 1 diabetes | 0 | USA | Medical centre outpatient clinic | I1: 54 C1: 55 |
I1: 14.7 (1.4) C1: 15.2 (1.7) |
‐ |
| Blevins 2007 | I1: cinnamon C1: placebo |
12 weeks | Patients with type 2 diabetes | I1: 23 C1: 9 |
USA | University research centre | T: 51 | I1: 63.6 (9.3) C1: 58.0 (10.9) T: 56 |
White 68 Native American 16 African American 7 Hispanic 4 Asian 2 Unknown 3 |
| Crawford 2009 | I1: cinnamon C1: usual care |
90 days | Adults with type 2 diabetes | ‐ | US | Military base primary care clinics | I1: 42 C1: 41 |
I1: 60.5 (10.7) C1: 59.9 (9.2) |
I1: White 76, Black 16, Latino 2, Asian 5 C1: White 76, Black 13, Latino 5, Asian 5 |
| Khan 2003 | I1: cinnamon 1 g I2: cinnamon 3 g I3: cinnamon 6 g C1: placebo 2 cap C2: placebo 6 cap C3: placebo 12 cap |
40 days | Adults > 40 years of age with type 2 diabetes | 0 | Pakistan | University | I1‐3: 50 C1‐3: 50 |
I1‐3: 52.0 (5.9) C1‐3: 52.0 (6.9) |
‐ |
| Khan 2010 | I1: cinnamon C1: placebo |
30 days | Adults ≥ 40 years of age with type 2 diabetes | ‐ | Pakistan | University | ‐ | ‐ | ‐ |
| Mang 2006 | I1: cinnamon C1: placebo |
16 weeks | Patients with type 2 diabetes | T: 23 | Germany | University research centre | I1: 36 C1: 28 |
I1: 62.8 (8.4) C1: 63.7 (7.2) |
‐ |
| Rosado 2010 | I1: cinnamon C1: placebo |
40 days | Adults 30 to 70 years of age with type 2 diabetes | ‐ | US | Medical centre outpatient clinics | I1: 50 C1: 55 |
I1: 53.9 (9.2) C1: 54.9 (10.8) |
I1: White 35, Pacific Islander 35,
Asian 20, African American 5, Hispanic 5 C1: White 40, Pacific Islander 15, Asian 35, African American 10, Hispanic 0 |
| Suppapitiporn 2006 | I1: cinnamon C1: placebo |
16 weeks | Adults 30 to 70 years of age with type 2 diabetes | 0 | Thailand | Hospital outpatient clinic | I1: 60 C1: 50 |
I1: 59.9 (8.7) C1: 58.5 (8.6) |
‐ |
| Vanschoonbeek 2006 | I1: cinnamon C1: placebo |
6 weeks | Postmenopausal women with type 2 diabetes | 0 | The Netherlands | University research laboratory | I1: 100 C1: 100 |
I1: 62 (7.2) C1: 64 (6.9) |
‐ |
|
Footnotes: "‐" denotes not reported C: control; cap: capsules; I: intervention; T: total. | |||||||||
Appendix 4. Baseline characteristics (II)
|
Characteristic Study ID |
Intervention(s) and control(s) | Duration of disease [mean years (SD]) | BMI [mean kg/m2 (SD)] | HbA1c [mean % (SD)] | Co‐morbidities | Co‐medications | Fasting plasma glucose [mean mmol/L (SD)] | Postprandial blood glucose [mean mmol/L (SD)] | Serum insulin [mean pmol/L (SD)] | Insulin sensitivity [mean (SD) variable ] |
| Akilen 2010 | I1: cinnamon C1: placebo |
I1: 5.6 (4.2) C1: 6.0 (5.0) |
I1: 33.4 (4.2) C1: 32.1 (8.3) |
I1: 8.2 (1.2) C1: 8.6 (1.8) |
Hypertension (29%), dyslipidaemia (15%), hypertension and dyslipidaemia (24%) | Oral hypoglycaemic agents | I1: 8.82 (3.45) C1: 8.77 (2.59) |
‐ | ‐ | ‐ |
| Altschuler 2007 | I1: cinnamon C1: placebo |
I1: 7.1 (4.6) C1: 6.1 (5.6) |
Z‐score: I1: 0.8 (0.7) C1: 0.8 (0.6) |
I1: 8.4 (1.3) C1: 8.7 (1.3) |
‐ | Insulin pump or injections | ‐ | ‐ | ‐ | I1: 9.0 (3.2) (g CHO/unit insulin) C1: 9.7 (3.3) (g CHO/unit insulin) |
| Blevins 2007 | I1: cinnamon C1: placebo |
I1: 7.8 (8.1) C1: 8.4 (7.4) |
I1: 32.5 (8.8) C1: 32.0 (7.5) |
I1: 7.2 (1.4) C1: 7.2 (1.3) |
‐ | Oral hypoglycaemic agents HMG‐CoA reductase inhibitors | I1: 7.38 (2.79) C1: 8.04 (3.02) |
‐ | I1: 89.59 (50.00) C1: 81.95 (56.26) |
‐ |
| Crawford 2009 | I1: cinnamon C1: usual care |
‐ | I1: 31.9 (6.4) C1: 32.9 (6.4) |
I1: 8.5 (1.8) C1: 8.3 (1.3) |
‐ | Insulin, oral hypoglycaemic agents | ‐ | ‐ | ‐ | ‐ |
| Khan 2003 | I1: cinnamon 1 g I2: cinnamon 3 g I3: cinnamon 6 g C1: placebo 2 cap C2: placebo 6 cap C3: placebo 12 cap |
I1‐3: 7.1 (3.3) C1‐3: 6.7 (2.3) |
‐ | ‐ | ‐ | Sulphonylurea drugs |
Serum glucose I1: 11.6 (1.7) I2: 11.4 (1.2) I3: 13.0 (1.4) C1: 12.2 (1.0) C2: 12.4 (1.0) C3: 16.7 (1.4) |
‐ | ‐ | ‐ |
| Khan 2010 | I1: cinnamon C1: placebo |
‐ | ‐ | ‐ | ‐ | ‐ | I1: 12.02 (2.93) C1: 11.34 (2.48) |
‐ ‐ |
‐ | ‐ |
| Mang 2006 | I1: cinnamon C1: placebo |
I1: 7.1 (5.2) C1: 6.8 (4.7) |
I1: 29.6 (4.6) C1: 30.1 (5.2) |
I1: 6.9 (1.0) C1: 6.7 (0.7) |
‐ | Oral hypoglycaemic agents | I1: 9.26 (2.26) C1: 8.66 (1.47) |
‐ ‐ |
‐ | ‐ |
| Rosado 2010 | I1: cinnamon C1: placebo |
I1: 5.4 (5.9) C1: 4.9 (4.8) |
I1: 31.5 (2.9) C1: 31.2 (3.7) |
I1: 7.8 (0.3) C1: 7.8 (0.2) |
Hyperlipidaemia (70%) | Metformin, hypolipidaemic agents, and any other prescribed medications | I1: 9.02 (0.34) C1: 9.12 (0.49) |
I1: 10.94 (0.69) C1: 11.44 (0.69) |
‐ | ‐ |
| Suppapitiporn 2006 | I1: cinnamon C1: placebo |
I1: 4.7 (2.3) C1: 4.4 (2.2) |
I1: 24.8 (1.7) C1: 24.9 (1.2) |
I1: 8.1 (1.1) C1: 8.1 (1.1) |
‐ | Oral hypoglycaemic agents | I1: 8.58 (1.37) C1: 8.01 (1.56) |
‐ | ‐ | ‐ |
| Vanschoonbeek 2006 | I1: cinnamon C1: placebo |
I1: 7.6 (4.9) C1: 7.1 (5.8) |
I1: 30.7 (3.8) C1: 30.1 (5.1) |
I1: 7.4 (1.0) C1: 7.1 (0.7) |
‐ | Oral hypoglycaemic agents | I1: 8.37 (2.04) C1: 8.28 (1.19) |
‐ | I1: 110.1 (45.03) C1: 111.0 (55.89) |
I1: 6.21 (3.88) (HOMA‐IR) C1: 6.01 (3.71) (HOMA‐IR) |
|
Footnotes: "‐" denotes not reported BMI: body mass index; C: control; CHO: carbohydrate; HMG‐CoA: 3‐hydroxy‐3‐methyl‐glutaryl coenzyme A; HOMA‐IR: homeostasis model assessment of insulin resistance; I: intervention; Z‐score: The WHO Global Database on Child Growth and Malnutrition uses a Z‐score cut‐off point of more than +2 standard deviations for classification of 'high weight‐for‐height' as overweight in children. | ||||||||||
Appendix 5. Adverse events (I)
|
Characteristic Study ID |
Intervention(s) and control(s) | Deaths [n] | Adverse events [n (%)] | Serious adverse events [n (%)] | Left study owing to adverse events [n (%)] | Hospitalisation [n (%)] | Outpatient treatment [n (%)] |
| Akilen 2010 | I1: cinnamon C1: placebo |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 1 T: 1 |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
| Altschuler 2007 | I1: cinnamon C1: placebo |
I1: 0 C1: 0 T: 0 |
I1: 2 (3) C1: 2 (3) T: 4 (6) |
I1: 1 (1) C1: 0 T: 1 (1) |
I1: 2 (3) C1: 2 (3) T: 4 (6) |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
| Blevins 2007 | I1: cinnamon C1: placebo |
I1: 0 C1: 0 T: 0 |
‐ | ‐ | ‐ | ‐ | ‐ |
| Crawford 2009 | I1: cinnamon C1: usual care |
I1: 0 C1: 0 T: 0 |
I1: 1 (2) C1: 0 T: 1 (1) |
I1: 0 C1: 0 T: 0 |
I1: 1 (2) C1: 0 T: 1 (1) |
‐ | ‐ |
| Khan 2003 | I1‐3: cinnamon 1/3/6 g C1‐3: placebo 2/6/12 cap |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Khan 2010 | I1: cinnamon C1: placebo |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mang 2006 | I1: cinnamon C1: placebo |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
‐ | ‐ |
| Rosado 2010 | I1: cinnamon C1: placebo |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 1 T: 1 |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 1 T: 1 |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
| Suppapitiporn 2006 | I1: cinnamon C1: placebo |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
| Vanschoonbeek 2006 | I1: cinnamon C1: placebo |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Footnotes:
"‐" denotes not reported C: control; cap: capsules; I: intervention; T: total. | |||||||
Appendix 6. Adverse events (II)
|
Characteristic Study ID |
Intervention(s) and control(s) | Hypoglycaemic episodes [n (%)] | Severe hypoglycaemic episodes [n (%)] | Definition of severe hypoglycaemic episodes | Nocturnal hypoglycaemic episodes [n (%)] | Symptoms [n (%)] | Notes |
| Akilen 2010 | I1: cinnamon C1: placebo |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
‐ | I1: 0 C1: 0 T: 0 |
I1: 0 C1: mild gastric pain 1 (4) T: 1 (2) |
3 Drop‐outs (5%) |
| Altschuler 2007 | I1: cinnamon C1: placebo |
‐ | I1: 1 (1) C1: 0 T: 1 |
Hypoglycaemic seizure | ‐ | I1: hives 1 (1),
hypoglycaemic seizure (1) C1: stomach aches (1), frequent illness (1) T: 4 (6) |
15 Drop‐outs (21%) |
| Blevins 2007 | I1: cinnamon C1: placebo |
‐ | ‐ | ‐ | ‐ | ‐ | 17 Drop‐outs (28%) |
| Crawford 2009 | I1: cinnamon C1: usual care |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
‐ | I1: 0 C1: 0 T: 0 |
I1: rash 1 (2) C1: 0 T: 1 (1) |
20 Drop‐outs (18%) |
| Khan 2003 | I1‐3: cinnamon 1/3/6 g C1‐3: placebo 2/6/12 cap |
‐ | ‐ | ‐ | ‐ | ‐ | 0 Drop‐outs (0 %) |
| Khan 2010 | I1: cinnamon C1: placebo |
‐ | ‐ | ‐ | ‐ | ‐ | 0 Drop‐outs (0%) |
| Mang 2006 | I1: cinnamon C1: placebo |
‐ | ‐ | ‐ | ‐ | I1: 0 C1: 0 T: 0 |
14 Withdrawals (18%) |
| Rosado 2010 | I1: cinnamon C1: placebo |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
‐ | ‐ | I1: 0 C1: nausea (5) T: 1 (3) |
3 Drop‐outs (8%) |
| Suppapitiporn 2006 | I1: cinnamon C1: placebo |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
‐ | I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
0 Drop‐outs (0%) |
| Vanschoonbeek 2006 | I1: cinnamon C1: placebo |
‐ | ‐ | ‐ | ‐ | ‐ | Drop‐out rate could not be determined |
|
Footnotes:
"‐" denotes not reported C: control; cap: capsules; I: intervention; T: total. | |||||||
Data and analyses
Comparison 1. Cinnamon versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fasting blood glucose level (random‐effects model) | 8 | 338 | Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.93, 0.11] |
| 2 Fasting blood glucose level (excluding studies of questionable quality) | 6 | 304 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.34, 0.18] |
| 3 Postprandial blood glucose level | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Adverse events | 4 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.22, 3.07] |
| 5 Glycosylated haemoglobin A1c (HbA1c) | 6 | 405 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.29, 0.18] |
| 6 Serum insulin | 2 | 81 | Mean Difference (IV, Random, 95% CI) | ‐6.77 [‐35.00, 23.46] |
| 7 Insulin sensitivity (CHO/unit insulin) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Insulin sensitivity (HOMA‐IR) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Subgroup analysis (cinnamon versus placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fasting blood glucose level and dosage | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 FBGL (cinnamon ≤ 1 g) | 3 | 116 | Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐3.71, 1.01] |
| 1.2 FBGL (cinnamon 1.5‐2 g) | 4 | 157 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.57, 0.56] |
| 1.3 FBGL (cinnamon 3 g) | 2 | 85 | Mean Difference (IV, Random, 95% CI) | ‐1.72 [‐4.80, 1.36] |
| 2 Fasting blood glucose level and study duration | 8 | 338 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.67, 0.02] |
| 2.1 FBGL (< 12 weeks' duration) | 4 | 99 | Mean Difference (IV, Random, 95% CI) | ‐1.74 [‐3.89, 0.41] |
| 2.2 FBGL (12 weeks' duration or longer) | 4 | 239 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.64, 0.38] |
| 3 Adverse events and dosage | 4 | 264 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.21, 3.23] |
| 3.1 Number of adverse events (cinnamon ≤ 1 g) | 3 | 206 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.22, 4.65] |
| 3.2 Number of adverse events (cinnamon 2 g) | 1 | 58 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 7.69] |
| 4 Adverse events and study duration | 4 | 264 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.21, 3.23] |
| 4.1 Number of adverse events (6 weeks' duration or less) | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.26] |
| 4.2 Number of adverse events (12 weeks' duration or longer) | 3 | 224 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.22, 4.57] |
| 5 Glycosylated haemoglobin A1c (HbA1c) and dosage | 6 | 405 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.29, 0.18] |
| 5.1 HbA1c (cinnamon 1 g) | 3 | 222 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.51, 0.31] |
| 5.2 HbA1c (cinnamon 1.5‐2 g) | 2 | 118 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.06, 0.29] |
| 5.3 HbA1c (cinnamon 3 g) | 1 | 65 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.22, 0.52] |
| 6 Glycosylated haemoglobin A1c (HbA1c) and study duration | 6 | 405 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.29, 0.18] |
| 6.1 HbA1c (12 weeks' duration or longer) | 6 | 405 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.29, 0.18] |
| 7 Glycosylated haemoglobin A1c (HbA1c) and diabetes type | 6 | 405 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.29, 0.18] |
| 7.1 HbA1c (type 1 diabetes only) | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.68, 0.88] |
| 7.2 HbA1c (type 2 diabetes only) | 5 | 348 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.38, 0.18] |
| 8 Serum insulin and dosage | 2 | 81 | Mean Difference (IV, Random, 95% CI) | ‐6.77 [‐35.00, 23.46] |
| 8.1 Serum insulin (cinnamon 1 g) | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐16.66 [‐61.46, 28.14] |
| 8.2 Serum insulin (cinnamon 1.5 g) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 1.5 [‐39.46, 42.46] |
| 9 Serum insulin and study duration | 2 | 81 | Mean Difference (IV, Random, 95% CI) | ‐6.77 [‐35.00, 23.46] |
| 9.1 Serum insulin (6 weeks' duration) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 1.5 [‐39.46, 42.46] |
| 9.2 Serum insulin (12 weeks' duration) | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐16.66 [‐61.46, 28.14] |
2.1. Analysis.
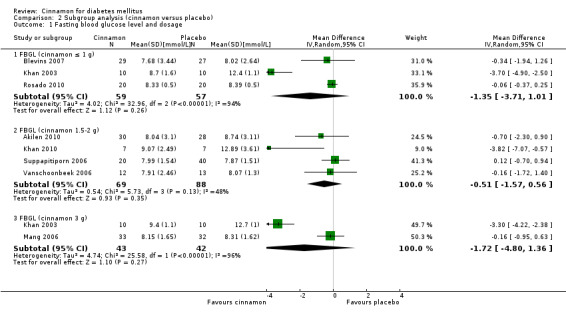
Comparison 2 Subgroup analysis (cinnamon versus placebo), Outcome 1 Fasting blood glucose level and dosage.
2.6. Analysis.
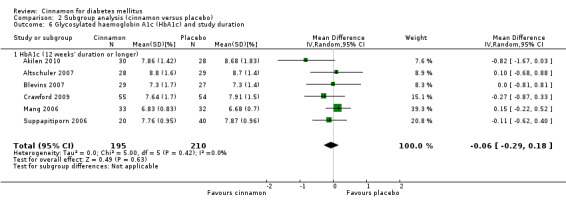
Comparison 2 Subgroup analysis (cinnamon versus placebo), Outcome 6 Glycosylated haemoglobin A1c (HbA1c) and study duration.
Comparison 3. Sensitivity analysis (cinnamon versus placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fasting blood glucose level and study quality | 8 | 338 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.67, 0.02] |
| 1.1 FBGL (moderate risk of bias) | 2 | 98 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.39, 0.22] |
| 1.2 FBGL (high risk of bias) | 6 | 240 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐2.45, 0.21] |
| 2 Adverse events and study quality | 4 | 264 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.21, 3.23] |
| 2.1 Number of adverse events (moderate risk of bias) | 2 | 98 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.03, 3.07] |
| 2.2 Number of adverse events (high risk of bias) | 2 | 166 | Odds Ratio (M‐H, Random, 95% CI) | 1.40 [0.25, 7.84] |
| 3 Serum insulin and study quality | 2 | 81 | Mean Difference (IV, Random, 95% CI) | ‐6.77 [‐35.00, 23.46] |
| 3.1 Serum insulin (high risk of bias) | 2 | 81 | Mean Difference (IV, Random, 95% CI) | ‐6.77 [‐35.00, 23.46] |
3.3. Analysis.
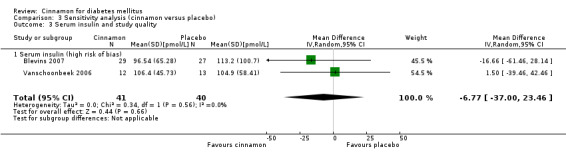
Comparison 3 Sensitivity analysis (cinnamon versus placebo), Outcome 3 Serum insulin and study quality.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akilen 2010.
| Methods | Design: randomised, double‐blind, placebo‐controlled, parallel group, multicentre clinical trial Randomisation ratio: not stated |
|
| Participants | Participants: 58 adults randomised, 58 analysed (cinnamon = 30, placebo = 28). Mean age (cinnamon = 54.9 ± 10.1 years, placebo = 54.4 ± 12.5 years). Sex (male/female) (cinnamon = 11/19, placebo = 15/13). Duration of diabetes (cinnamon = 5.6 ± 4.2 years, placebo = 6.0 ± 5.0 years) Inclusion criteria: type 2 diabetes mellitus; aged ≥ 18 years; treated with oral hypoglycaemic agents Exclusion criteria: insulin use; pregnant, lactating, or both; cinnamon supplementation; supplementation with other antidiabetic herbs; acute health disorders; unable to read/understand English Diagnostic criteria: source of criteria not stated ‐ 2 consecutive fasting glucose measurements of ≥ 7 mmol/L, and HbA1c ≥ 7.0% Co‐morbidities: hypertension (29%), dyslipidaemia (15%), hypertension and dyslipidaemia (24%) Co‐medications: oral hypoglycaemic agents (metformin, sulphonylureas) | |
| Interventions | Number of study centres: 3 Country/location: Brent, Greater London, UK Setting: community diabetes clinics Intervention (route, total dose/day, frequency): oral, cinnamon (C. cassia) capsule, 500 mg (1 x 500 mg) with breakfast, 1000 mg (2 x 500 mg) with lunch and 500 mg (1 x 500 mg) with dinner Control (route, total dose/day, frequency): oral, starch capsule, 500 mg (1 x 500 mg) with breakfast, 1000 mg (2 x 500 mg) with lunch and 500 mg (1 x 500 mg) with dinner Treatment before study: not stated Titration period: not applicable | |
| Outcomes | Primary outcome(s) (as stated in the publication): not stated Secondary outcomes (as stated in the publication): not stated Additional/other outcomes: HbA1c; diastolic and systolic blood pressure; total cholesterol; low‐density lipoprotein cholesterol; high‐density lipoprotein cholesterol; triglycerides; FBGL; total energy intake; BMI | |
| Study details | Duration of intervention: 12 weeks Duration of follow‐up: not applicable Run‐in period: not applicable |
|
| Publication details | Language of publication: English No (non‐)/commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim of study | "To determine the therapeutic effect of cinnamon on glycated hemoglobin [sic] (HbA1c), blood pressure and lipid profiles in people with type 2 diabetes" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization...was...by use of a computer generated randomized list" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "the capsules were sealed independently in serially numbered containers of equal appearance and weight (allocation concealment). The investigator and clinicians involved in the clinical trial at different sites received sealed bottles of capsules (A and B) for distribution and were unaware which were active and placebo" Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blinding of this trial was ensured by use of matching colour, size and smell of placebo and cinnamon"; "The investigator...was unaware which were active and which were placebo until the end of the trial" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT "For those patients who withdrew (n=3), their remaining data were included in the analysis using last observation carried forward method" |
| Selective reporting (reporting bias) | High risk | No study protocol was published or lodged. Nonetheless, not all outcomes listed were reported (e.g. week 12 anthropometrics) |
| Other bias | Unclear risk | 3 participants withdrew from the study ‐ the number and reasons for withdrawal differed between groups. Baseline differences in sex were evident |
Altschuler 2007.
| Methods | Design: randomised, double‐blind, placebo‐controlled, parallel group, multicentre clinical trial Randomisation ratio: not stated |
|
| Participants | Participants: 72 adolescents randomised, 57 analysed (cinnamon = 28, placebo = 29). Mean age (cinnamon = 14.7 ± 1.4 years, placebo = 15.2 ± 1.7 years). Sex (male/female) (cinnamon = 13/14, placebo = 13/15). Duration of diabetes (cinnamon = 7.1 ± 4.6 years, placebo = 6.1 ± 5.6 years)
Inclusion criteria: type 1 diabetes mellitus > 18 months duration; aged 13 to 18 years; presentation to medical centre endocrinology clinic for routine care; ability to be accessed by telephone
Exclusion criteria: pregnant; history of hospitalisation for medical or psychiatric reasons in the last 12 months Diagnostic criteria: not stated Co‐morbidities: not stated Co‐medications: insulin pump or injections |
|
| Interventions | Number of study centres: 2 Country/location: Lebanon and Manchester, New Hampshire, US Setting: medical centre outpatient clinic Intervention (route, total dose/day, frequency): oral, cinnamon 1000 mg tablet, daily Control (route, total dose/day, frequency): oral, lactose tablet, daily Treatment before study: not stated Titration period: not applicable | |
| Outcomes | Primary outcome(s) (as stated in the publication): HbA1c Secondary outcomes (as stated in the publication): not stated Additional/other outcomes: daily insulin intake, adverse events, insulin sensitivity | |
| Study details | Duration of intervention: 3 months (12 weeks) Duration of follow‐up: not applicable Run‐in period: not applicable |
|
| Publication details | Language of publication: English Non‐commercial finding Publication status: peer‐reviewed journal |
|
| Stated aim of study | To determine the effect of cinnamon on glycaemic control in adolescents with type 1 diabetes mellitus | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each pill bottle was assigned a randomly determined study number before being distributed to subjects" (method not described) Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described Comment: probably not done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind"; "...cinnamon and placebo pills appeared identical" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "intention‐to‐treat" was quoted, but the analysis consisted only of patients who completed the 90 days of treatment |
| Selective reporting (reporting bias) | Unclear risk | All primary and secondary outcomes listed were reported, though no study protocol was published or lodged |
| Other bias | Unclear risk | 15 participants withdrew from the study and were excluded from the analysis; the number and reasons for withdrawal were similar between groups |
Blevins 2007.
| Methods | Design: randomised, double‐blind, placebo‐controlled, parallel group, single‐centre clinical trial Randomisation ratio: not stated |
|
| Participants | Participants: 60 participants randomised, 57 analysed (cinnamon = 29, placebo = 28). Mean age (cinnamon = 63.6 ± 9.3 years, placebo = 58.0 ± 10.9 years). Sex (male/female) (49%/51%). Duration of diabetes (cinnamon = 7.8 ± 8.1 years, placebo = 8.4 ± 7.4 years) Inclusion criteria: type 2 diabetes mellitus; no age limit Exclusion criteria: insulin use; cinnamon supplementation; HbA1c < 6.0%; acute illness Diagnostic criteria: American Diabetes Association (2003) criteria Co‐morbidities: not stated Co‐medications: oral hypoglycaemic agents (metformin; thiazolidinediones); HMG‐CoA reductase inhibitors | |
| Interventions | Number of study centres: 1 Country/location: Oklahoma city, Oklahoma, US Setting: university research centre Intervention (route, total dose/day, frequency): oral, cinnamon (C. cassia) 500 mg capsule, twice a day Control (route, total dose/day, frequency): oral, wheat flour capsule, twice a day Treatment before study: not stated Titration period: not applicable | |
| Outcomes | Primary outcome(s) (as stated in the publication): not stated Secondary outcomes (as stated in the publication): not stated Additional/other outcomes: HbA1c; FBGL; total cholesterol; low‐density lipoprotein cholesterol; high‐density lipoprotein cholesterol; triglyceride; serum insulin; BMI | |
| Study details | Duration of intervention: 3 months (12 weeks) Duration of follow‐up: not applicable Run‐in period: not applicable | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim of study | To examine the effect of cinnamon on glucose and lipid levels in persons with type 2 diabetes mellitus | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Enrolled subjects were stratified by sex and randomised to receive either cinnamon...or placebo" (method not described) Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Investigators and subjects were blinded to group assignment" (method not described) Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Investigators and subjects were blinded to...capsule content"; though there was no assurance how this was achieved Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Intention‐to‐treat analysis" |
| Selective reporting (reporting bias) | Unclear risk | All primary and secondary outcomes listed were reported, though no study protocol was published or lodged |
| Other bias | Unclear risk | A similar proportion of patients withdrew from each group, though the reasons for withdrawal were not given for each group separately |
Crawford 2009.
| Methods | Design: randomised, controlled, parallel group, single‐centre clinical trial Randomisation ratio: 1:1 |
|
| Participants | Participants: 109 participants analysed (cinnamon = 55, placebo = 54). Mean age (cinnamon = 60.5 ± 10.7 years, placebo = 59.9 ± 9.2 years). Sex (male/female) (cinnamon = 32/23, placebo = 32/22). Duration of diabetes not stated Inclusion criteria: type 2 diabetes mellitus; HbA1c ≥ 7.0% in the last 6 months; listed in the population health database as a patient with diabetes Exclusion criteria: pregnancy, age < 18 years, allergy to cinnamon Diagnostic criteria: not stated Co‐morbidities: not stated Co‐medications: insulin, oral hypoglycaemic agents | |
| Interventions | Number of study centres: 3
Country/location: Florida, US Setting: military base primary care clinics Intervention (route, total dose/day, frequency): oral, 1000 mg (2 x 500 mg) cinnamon (C. cassia) capsules, daily Control (route, total dose/day, frequency): usual care Treatment before study: not stated Titration period: not applicable |
|
| Outcomes | Primary outcome(s) (as stated in the publication): HbA1c Secondary outcomes (as stated in the publication): not applicable Additional/other outcomes: not applicable | |
| Study details | Duration of intervention: 90 days (12.9 weeks) Duration of follow‐up: not applicable Run‐in period: not applicable | |
| Publication details | Language of publication: English (Non‐)/commercial finding: not stated Publication status: peer‐reviewed journal |
|
| Stated aim of study | To determine whether cinnamon lowers HbA1c in patients with type 2 diabetes | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We randomised patients by blocking...in groups of 10" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "[treatment] allocation was concealed until that time [of consent]" Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Neither the participants nor investigators were blinded, but the laboratory... was blinded to group allocation" Comment: not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Intention‐to‐treat analysis...using the carry‐forward method" |
| Selective reporting (reporting bias) | Unclear risk | The only outcome listed (HbA1c) was reported, though no study protocol was published or lodged |
| Other bias | Unclear risk | Number and reasons for withdrawal differed between groups; intervention was not standardised or tested for quality; study was underpowered |
Khan 2003.
| Methods | Design: randomised, placebo‐controlled, parallel group, single‐centre clinical trial Randomisation ratio: not stated |
|
| Participants | Participants: 60 participants analysed (cinnamon (C. cassia) 1 g/day = 10, cinnamon 3 g/day = 10, cinnamon 6 g/day = 10, placebo (wheat flour) 1 tablet/day = 10, placebo 3 tablets/day = 10, placebo 6 tablets/day = 10). Mean age (cinnamon groups = 52.0 ± 6.87 years, placebo groups = 52.0 ± 5.85 years). Sex (male/female) (cinnamon groups = 15/15, placebo groups = 15/15). Duration of diabetes (cinnamon groups = 7.1 ± 3.3 years, placebo groups = 6.7 ± 2.3 years) Inclusion criteria: type 2 diabetes mellitus; > 40 years of age; FBGL 7.8 to 22.2 mmol/L Exclusion criteria: insulin therapy; non‐diabetic medication Diagnostic criteria: not stated Co‐morbidities: not stated Co‐medications: sulphonylurea drugs | |
| Interventions | Number of study centres: 1 Country/location: Peshawar, Pakistan Setting: university Intervention (route, total dose/day, frequency): oral, 1 g (2 x 500 mg), 3 g (6 x 500 mg) or 6 g (12 x 500 mg) cinnamon (C. cassia) capsules, daily (3 groups) Control (route, total dose/day, frequency): oral, 2, 6 or 12 wheat flour capsules, daily (3 groups) Treatment before study: not stated Titration period: not applicable | |
| Outcomes | Primary outcome(s) (as stated in the publication): not stated Secondary outcomes (as stated in the publication): not stated Additional/other outcomes: FBGL; fasting serum triglyceride; fasting serum cholesterol; fasting serum high‐density lipoprotein cholesterol; fasting serum low‐density lipoprotein cholesterol | |
| Study details | Duration of intervention: 40 days (5.7 weeks) Duration of follow‐up: 20 days Run‐in period: not applicable | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim of study | To determine whether cinnamon has a dose‐dependent effect on clinical variables associated with diabetes and cardiovascular disease in people with type 2 diabetes | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "individuals...[were] divided randomly into six equal groups" (method not described) Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described Comment: probably not done |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Method of blinding not described Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not mentioned, though all randomised participants appeared to be included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | All primary outcomes listed were reported, though no study protocol was published or lodged |
| Other bias | Unclear risk | Information on enrolments, exclusions, withdrawals and baseline characteristics was either limited or missing |
Khan 2010.
| Methods | Design: randomised, placebo‐controlled, parallel group, single‐centre clinical trial Randomisation ratio: not stated |
|
| Participants | Participants: 14 participants analysed (cinnamon = 7, placebo = 7) Inclusion criteria: type 2 diabetes mellitus; ≥ 40 years of age; FBGL ≥ 125 mg/dL (6.9 mmol/L) Exclusion criteria: not stated Diagnostic criteria: not stated Co‐morbidities: not stated Co‐medications: not stated | |
| Interventions | Number of study centres: 1 Country/location: Peshawar, Pakistan Setting: university Intervention (route, total dose/day, frequency): oral, 1.5 g (3 x 500 mg) cinnamon capsules, daily Control (route, total dose/day, frequency): oral, 1.5 g (3 x 500 mg) maize flour capsules, daily Treatment before study: not stated Titration period: not applicable | |
| Outcomes | Primary outcome(s) (as stated in the publication): not stated Secondary outcomes (as stated in the publication): not stated Additional/other outcomes: FBGL; fasting serum triglycerides; fasting serum cholesterol; fasting serum high‐density lipoprotein cholesterol; fasting serum low‐density lipoprotein cholesterol | |
| Study details | Duration of intervention: 30 days (4.3 weeks) Duration of follow‐up: not applicable Run‐in period: not applicable | |
| Publication details | Language of publication: English (Non)/commercial funding: not stated Publication status: peer‐review journal |
|
| Stated aim of study | To confirm the previous findings that cinnamon intake reduces glucose, triglycerides and cholesterol in type 2 diabetic individuals | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The registered patients were randomly divided into two groups" (method not described) Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described Comment: probably not done |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Method of blinding not described Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not mentioned, though all randomised participants appeared to be included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | All primary outcomes listed were reported, though no study protocol was published or lodged |
| Other bias | Unclear risk | Information on enrolments, exclusions, withdrawals and baseline characteristics was absent |
Mang 2006.
| Methods | Design: randomised, double‐blind, placebo‐controlled, parallel group, single‐centre clinical trial Randomisation ratio: not stated |
|
| Participants | Participants: 79 participants recruited, 65 analysed (cinnamon = 33, placebo = 32). Mean age (cinnamon = 62.8 ± 8.37 years, placebo = 63.7 ± 7.17 years). Sex (male/female) (cinnamon = 21/12, placebo = 23/9). Duration of diabetes (cinnamon = 7.1 ± 6.2 years, placebo = 6.8 ± 4.7 years) Inclusion criteria: type 2 diabetes mellitus Exclusion criteria: not stated Diagnostic criteria: not stated Co‐morbidities: not stated Co‐medications: oral hypoglycaemic agents (metformin, sulphonylureas, glinides, glitazones, or combination therapy) | |
| Interventions | Number of study centres: 1 Country/location: Hannover, Germany Setting: university research centre Intervention (route, total dose/day, frequency): oral, cinnamon (aqueous extract of C. cassia) 1000 mg capsule, 3 times a day Control (route, total dose/day, frequency): oral, 1 microcrystalline cellulose (placebo) capsule, 3 times a day Treatment before study: not stated Titration period: not applicable | |
| Outcomes | Primary outcome(s) (as stated in the publication): not stated Secondary outcomes (as stated in the publication): not stated Additional/other outcomes: HbA1c; FBGL; total cholesterol; low‐density lipoprotein cholesterol; high‐density lipoprotein cholesterol; triacylglycerol | |
| Study details | Duration of intervention: 4 months (16 weeks) Duration of follow‐up: not applicable Run‐in period: not applicable | |
| Publication details | Language of publication: English Commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim of study | To investigate the effects of aqueous cinnamon extract on HbA1c, fasting plasma glucose and serum lipids in type 2 diabetes | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised"; "patients...[were] randomly assigned to take either cinnamon...or...placebo" (method not described) Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described Comment: probably not done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind"; "placebo capsules looked identical [to cinnamon capsules]" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT not mentioned; withdrawn participants were excluded from the analysis |
| Selective reporting (reporting bias) | Unclear risk | All primary outcomes listed were reported, though no study protocol was published or lodged |
| Other bias | Unclear risk | The number and reasons for withdrawals were not given for each group separately |
Rosado 2010.
| Methods | Design: randomised, double‐blind, placebo‐controlled, parallel group, single‐centre clinical trial Randomisation ratio: not stated |
|
| Participants | Participants: 40 participants recruited, 40 analysed (cinnamon = 20, placebo = 20). Mean age (cinnamon = 53.9 ± 9.2 years, placebo = 54.9 ± 10.8 years). Sex (male/female) (cinnamon = 10/10, placebo = 9/11). Duration of diabetes (cinnamon = 5.4 ± 5.9 years, placebo = 4.9 ± 4.8 years) Inclusion criteria: type 2 diabetes mellitus; 30 to 70 years of age; taking metformin for glucose control for at least 3 months (at a daily dose of at least 1000 mg); and FBGL 7.0 to 16.7 mmol/L or HbA1c > 7% Exclusion criteria: pregnancy; known allergy to cinnamon; history of peptic ulceration; BMI > 35 kg/m2; receiving tetracycline therapy; receiving insulin therapy Diagnostic criteria: American Diabetes Association (2003) criteria Co‐morbidities: hyperlipidaemia (70%) Co‐medications: metformin, hypolipidaemic agents, and any other prescribed medications (other than excluded medications) | |
| Interventions | Number of study centres: 1
Country/location: Honolulu, Hawaii, US
Setting: medical centre outpatient clinics
Intervention (route, total dose/day, frequency): oral, cinnamon 250 mg (water‐soluble extract of C. burmanii; Cinnulin PF®) capsule, twice a day Control (route, total dose/day, frequency): oral, 250 mg bran cereal (control) capsule, twice a day Treatment before study: not stated Titration period: not applicable |
|
| Outcomes | Primary outcome(s) (as stated in the publication): not stated Secondary outcomes (as stated in the publication): not stated Additional/other outcomes: HbA1c; FBGL, PPG, total cholesterol; triglycerides; low‐density lipoprotein cholesterol; high‐density lipoprotein cholesterol |
|
| Study details | Duration of intervention: 40 days (5.7 weeks) Duration of follow‐up: 20 days Run‐in period: not applicable | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: dissertation |
|
| Stated aim of study | To determine whether cinnamon improves blood glucose, triglyceride, total cholesterol, and low‐density lipoprotein cholesterol levels in persons with type‐2 diabetes | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized"; "subjects were assigned a sequential number..(and)...a sequential number...was assigned to each capsule container based on the computer‐generated (allocation) table...pharmacy personnel randomized the study capsule containers to treatment or control" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "The investigative team did not know the capsule allocation table results"; "The computer‐generated allocation was maintained by...Pharmacy personnel in a sealed envelope" Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind"; "(capsulated) cinnamon...for the treatment group and identical capsules...for the control group" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not mentioned, though it appeared that all randomised participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | All primary outcomes listed were reported, though no study protocol was published or lodged |
| Other bias | Unclear risk | 3 participants withdrew from the study ‐ the reasons for withdrawal differed between groups. Information on enrolments and exclusions was missing |
Suppapitiporn 2006.
| Methods | Design: randomised, single‐blind, placebo‐controlled, parallel group, single‐centre clinical trial Randomisation ratio: not stated |
|
| Participants | Participants: 60 participants recruited, 60 analysed (cinnamon = 20, placebo = 40). Mean age (cinnamon = 59.9 ± 8.7 years, placebo = 58.5 ± 8.7 years). Sex (male/female) (cinnamon = 8/12, placebo = 20/20). Duration of diabetes (cinnamon = 4.7 ± 2.3 years, placebo = 4.4 ± 2.2 years) Inclusion criteria: type 2 diabetes mellitus; maintained a fixed dose of hypoglycaemic medication over the past 3 months; aged 30 to 70 years; FBGL 120 to 180 mg/dL (6.67 to 10.0 mmol/L); HbA1c > 7% Exclusion criteria: type 1 diabetes mellitus; type 2 diabetes mellitus treated with insulin; diabetes secondary to chronic pancreatitis; genetic defects of beta‐cell function; genetic defects in insulin action; haemochromatosis; endocrinopathies; poorly controlled diabetes secondary to intercurrent illness, infection, surgery, or liver/renal disease Diagnostic criteria: not stated Co‐morbidities: not stated Co‐medications: oral hypoglycaemic agents (metformin, sulphonylureas) | |
| Interventions | Number of study centres: 1 Country/location: Bangkok, Thailand Setting: hospital outpatient clinic Intervention (route, total dose/day, frequency): oral, cinnamon (C. cassia) 1500 mg capsule, 3 times a day Control (route, total dose/day, frequency):oral, 1 placebo capsule, 3 times a day Treatment before study: not applicable Titration period: not applicable | |
| Outcomes | Primary outcome(s) (as stated in the publication): not stated Secondary outcomes (as stated in the publication): not stated Additional/other outcomes: HbA1c; FBGL; total cholesterol; triglyceride; high‐density lipoprotein cholesterol; creatinine; serum glutamic oxaloacetic transaminase; serum glutamic pyruvic transaminase; blood urea nitrogen; body weight; blood pressure | |
| Study details | Duration of intervention: 4 months (16 weeks) Duration of follow‐up: not applicable Run‐in period: not applicable | |
| Publication details | Language of publication: English (Non‐)/commercial funding: not stated Publication status: peer‐reviewed journal Publication status: journal supplement |
|
| Stated aim of study | To investigate the effects of aqueous cinnamon extract on HbA1c, FBGL and serum lipids in type 2 diabetes | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised"; "...randomly assigned...patients" (method not described) Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described Comment: probably not done |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "single blind" (method not described) Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not mentioned, though it appeared that all randomised participants were included in the analysis |
| Selective reporting (reporting bias) | High risk | Not all outcomes listed were reported (e.g. low‐density lipoprotein cholesterol, blood pressure); no study protocol was published or lodged |
| Other bias | Unclear risk | Information on enrolments, exclusions and withdrawals was missing |
Vanschoonbeek 2006.
| Methods | Design: double‐blind, placebo‐controlled, parallel group, single‐centre clinical trial Randomisation ratio: not stated |
|
| Participants | Participants: 25 postmenopausal woman recruited, 25 analysed (cinnamon = 12, placebo = 13). Mean age (cinnamon = 62 ± 2 years, placebo = 64 ± 2 years). Duration of diabetes (cinnamon = 7.6 ± 1.4 years, placebo = 7.1 ± 1.6 years) Inclusion criteria: type 2 diabetes mellitus Exclusion criteria: impaired liver or renal function; cardiovascular disease; exogenous insulin therapy Diagnostic criteria: WHO (1999) criteria Co‐morbidities: not stated Co‐medications: oral hypoglycaemic agents (metformin, sulphonylureas, thiazolidinediones) | |
| Interventions | Number of study centres: 1 Country/location: Maastricht, Netherlands Setting: university research laboratory Intervention (route, total dose/day, frequency): oral, cinnamon 500 mg (C. cassia) capsule, 3 times a day Control (route, total dose/day, frequency): oral, 1 wheat flour capsule, 3 times a day Treatment before study: not applicable Titration period: not applicable | |
| Outcomes | Primary outcome(s) (as stated in the publication): not stated Secondary outcomes (as stated in the publication): not stated Additional/other outcomes: HbA1c; FBGL; fasting plasma insulin; OGIS; ISIcomp; HOMA‐IR; total cholesterol; low‐density lipoprotein cholesterol; high‐density lipoprotein cholesterol; triacylglycerol | |
| Study details | Duration of intervention: 6 weeks Duration of follow‐up: not applicable Run‐in period: not applicable | |
| Publication details | Language of publication: English (Non‐)/commercial funding: not stated Publication status: peer‐reviewed journal |
|
| Stated aim of study | To determine the effects of cinnamon supplementation on FBGL, insulin, HbA1c, whole‐body insulin sensitivity, and serum lipids | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of treatment allocation not mentioned Comment: probably not done |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described Comment: probably not done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind"; "capsules...could not be distinguished by color, scent, or taste" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not mentioned; though it appeared that all randomised participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | All primary outcomes listed were reported, though no study protocol was published or lodged |
| Other bias | Unclear risk | Information on enrolments, exclusions and withdrawals was missing |
BMI: body mass index; FBGL: fasting blood glucose level; HbA1c: glycosylated haemoglobin; HMG‐CoA: 3‐hydroxy‐3‐methyl‐glutaryl‐CoA; HOMA‐IR: homeostasis model assessment of insulin resistance; ISIcomp: index of composite whole‐body insulin sensitivity; ITT: intention to treat; OGIS: oral glucose insulin sensitivity; PPG: postprandial glucose.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Graham 2005 | Participants in this study had gestational diabetes |
| Wainstein 2011 | The study used a combination preparation (i.e. cinnamon, zinc gluconate and tricalcium phosphate) |
| Ziegenfuss 2006 | In accordance with ADA and WHO criteria, participants in this study did not have diabetes mellitus (FBGL < 7 mmol/L) |
ADA: American Diabetes Association; FBGL: fasting blood glucose level; WHO: World Health Organization.
Characteristics of ongoing studies [ordered by study ID]
Crawford 2011.
| Trial name or title | Cinnamon bark, water‐soluble cinnamon extract, and metformin as initial treatment for type 2 diabetes mellitus: a randomized, controlled trial |
| Methods | Randomised, active‐controlled trial |
| Participants | Adults (18 years or older); newly diagnosed type 2 diabetes mellitus within the last month |
| Interventions | Metformin 1000 mg extended‐release daily, cinnamon bark 1000 mg daily or 500 mg Cinnulin PF daily for 90 days |
| Outcomes | Primary outcomes: HbA1c; low‐density lipoprotein cholesterol; waist circumference |
| Starting date | October 2011 |
| Contact information | Dr P Crawford. Email: paul.crawford@nellis.af.mil |
| Notes | Participant recruitment had not commenced as at December 2011. Trial ID: NCT01302743 |
DeVries 2006.
| Trial name or title | Metabolic effects of Diabecinn (oral cinnamon extract) in diabetes type 2, a placebo‐controlled randomised clinical trial |
| Methods | Randomised placebo‐controlled trial |
| Participants | Adults (35‐70 years); type 2 diabetes; HbA1c between 7% and 12% inclusive |
| Interventions | Diabeccin (oral cinnamon extract) or placebo, orally, 3 times a day, for unknown duration |
| Outcomes | Primary outcome: HbA1c Secondary outcome: lipid profile; 6‐point glucose profile; hypoglycaemia; body weight; free fatty acids; C‐reactive protein |
| Starting date | May 2006 |
| Contact information | Dr J DeVries. Email: j.h.devries@amc.uva.nl |
| Notes | The study has been stopped owing to insufficient enrolments. Trial ID: ISRCTN36704940 |
Ridout 2007.
| Trial name or title | The antidiabetic and cholesterol‐lowering effects of cinnamon and cassia bark |
| Methods | Randomised placebo‐controlled trial |
| Participants | Adults > 30 years; type 2 diabetes; not taking hypoglycaemic or hypolipidaemic medication, OR on a stable drug regimen for the past 3 months; FBGL 8 to 15 mmol/L |
| Interventions | 280 mg Cinnamonforce (C. aromaticum and C. verum blend) or placebo, orally, twice a day, for 12 weeks |
| Outcomes | Primary outcomes: FBGL; insulin; HbA1c Secondary outcomes: total cholesterol; triglycerides; low‐density lipoprotein cholesterol; high‐density lipoprotein cholesterol; blood pressure; BMI; waist‐hip ratio; insulin resistance; liver function; renal function; quality of life |
| Starting date | July 2007 |
| Contact information | Dr Rowena Ridout. Email: rowena.ridout@uhn.on.ca |
| Notes | The study had not reached completion as at June 2011. Trial ID: NCT00479973 |
Stoecker 2010.
| Trial name or title | Cinnamon extract lowers blood glucose in hyperglycemic subjects [abstract title] |
| Methods | Double‐blind, placebo‐controlled trial |
| Participants | Adults with hyperglycaemia |
| Interventions | 250 mg CinSulin (dried water‐extract of cinnamon) or placebo, orally, twice a day, for 2 months |
| Outcomes | Insulin resistance; fasting glucose; PPG; insulin; triglycerides; high‐density lipoprotein cholesterol; fructosamine; BMI; blood pressure |
| Starting date | Unknown |
| Contact information | Dr Barbara Stoecker. Email: barbara.stoecker@okstate.edu |
| Notes | The study has reached completion but results of the study have yet to be published in full |
BMI: body mass index; FBGL: fasting blood glucose level; HbA1c: glycosylated haemoglobin.
Differences between protocol and review
There are no differences between the protocol and review.
Contributions of authors
Matthew Leach: protocol draft, search strategy development, acquirement of trial copies, trial selection, data extraction, data analysis, data interpretation, review draft and update draft.
Saravana Kumar: protocol draft, search strategy development, trial selection, data extraction, data interpretation and review draft.
Declarations of interest
None known.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Akilen 2010 {published and unpublished data}
- Akilen R, Tsiami A, Devendra D, Robinson N. Glycated haemoglobin and blood pressure‐lowering effect of cinnamon in multi‐ethnic type 2 diabetic patients in the UK: a randomized, placebo‐controlled, double‐blind clinical trial. Diabetic Medicine 2010;27(10):1159‐67. [DOI] [PubMed] [Google Scholar]
Altschuler 2007 {published data only}
- Altschuler JA, Casella SJ, MacKenzie TA, Curtis KM. The effect of cinnamon on A1c among adolescents with type 1 diabetes. Diabetes Care 2007;30(4):813‐6. [DOI] [PubMed] [Google Scholar]
Blevins 2007 {published data only}
- Blevins SM, Leyva MJ, Brown J, Wright J, Scofield RH, Aston CE. Effect of cinnamon on glucose and lipid levels in non‐insulin‐dependent type 2 diabetes. Diabetes Care 2007;30(9):2236‐7. [DOI] [PubMed] [Google Scholar]
Crawford 2009 {published data only}
- Crawford P. Effectiveness of cinnamon for lowering hemglobin A1c in patients with type 2 diabetes: a randomised, controlled trial. Journal of the American Board of Family Medicine 2009;22(5):507‐12. [DOI] [PubMed] [Google Scholar]
Khan 2003 {published data only}
- Khan A, Safdar M, Khan MMA, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 2003;26(12):3215‐8. [DOI] [PubMed] [Google Scholar]
- Safdar M, Khan A, Khattak MMAK, Siddique M. Effect of various doses of cinnamon on blood glucose in diabetic individuals. Pakistan Journal of Nutrition 2004;3(5):268‐72. [Google Scholar]
Khan 2010 {published data only}
- Khan R, Khan Z, Shah SH. Cinnamon may reduce glucose, lipid and cholesterol level in type 2 diabetic individuals. Pakistan Journal of Nutrition 2010;9(5):430‐3. [Google Scholar]
Mang 2006 {published data only}
- Mang B, Wolters M, Schmitt B, Kelb K, Lichtinghagen R, Stichtenoth DO, et al. Effects of a cinnamon extract on plasma glucose, HbA1c, and serum lipids in diabetes mellitus type 2. European Journal of Clinical Investigation 2006;36(5):340‐4. [DOI] [PubMed] [Google Scholar]
Rosado 2010 {published data only}
- Rosado J. A Study to Determine the Effects of Cinnamon on Blood Glucose and Lipid Levels in Persons with type‐2 Diabetes [dissertation]. Honolulu: University of Hawaii at Manoa, Honolulu, 2010. [Google Scholar]
Suppapitiporn 2006 {published data only}
- Suppapitiporn S, Kanpaksi N, Suppapitiporn S. The effect of cinnamon cassia powder in type 2 diabetes mellitus. Journal of the Medical Association of Thailand 2006;89(Suppl. 3):S200‐5. [PubMed] [Google Scholar]
Vanschoonbeek 2006 {published data only}
- Vanschoonbeek K, Thomassen JW, Senden JM, Wodzig WKWH, Loon LJC. Cinnamon supplementation does not improve glycemic control in postmenopausal type 2 diabetes patients. The Journal of Nutrition 2006;136:977‐80. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Graham 2005 {published data only}
- Graham G, Johnson B, Johnson A, Anderson R, Devine P. Cinnamon for glycemic control in gestational diabetes: a randomised double‐blind placebo controlled pilot study. The American Journal of Obstetrics & Gynecology 2005;193(6, Suppl):S91. [Google Scholar]
Wainstein 2011 {published data only}
- Wainstein J, Stern N, Heller S, Boaz M. Dietary cinnamon supplementation and changes in systolic blood pressure in subjects with type 2 diabetes. Journal of Medicinal Food 2011;14(12):1505‐10. [DOI] [PubMed] [Google Scholar]
Ziegenfuss 2006 {published data only}
- Ziegenfuss TN, Hofheins JE, Mendel RW, Landis J, Anderson RA. Effects of a water‐soluble cinnamon extract on body composition and features of the metabolic syndrome in pre‐diabetic men and woman. Journal of the International Society of Sports Nutrition 2006;3(2):45‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Crawford 2011 {published data only}
- Cinnamon bark, water‐soluble cinnamon extract, and metformin as initial treatment for type 2 diabetes mellitus: a randomized, controlled trial. Ongoing study October 2011.
DeVries 2006 {unpublished data only}
- Metabolic effects of Diabecinn (oral cinnamon extract) in diabetes type 2, a placebo‐controlled randomised clinical trial. Ongoing study May 2006.
Ridout 2007 {unpublished data only}
- The antidiabetic and cholesterol‐lowering effects of cinnamon and cassia bark. Ongoing study July 2007.
Stoecker 2010 {published data only (unpublished sought but not used)}
- Stoecker BJ, Zhan Z, Luo R, Mu X, Guo X, Liu Y, et al. Cinnamon extract lowers blood glucose in hyperglycemic subjects. FASEB Journal 2010;24:722.1. [Google Scholar]
Additional references
ADA 1997
- American Diabetes Association. Report on the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20(Suppl. 1):S5‐20. [DOI] [PubMed] [Google Scholar]
ADA 1999
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1999;22(Suppl. 1):S5‐19. [DOI] [PubMed] [Google Scholar]
ADA 2003
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26(Suppl. 1):S5‐20. [DOI] [PubMed] [Google Scholar]
Akilen 2012
- Akilen R, Tsiami A, Devendra D, Robinson N. Cinnamon in glycaemic control: systematic review and meta analysis. Clinical Nutrition 2012 Jul 19 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Baker 2008
- Baker WL, Gutierrez‐Williams G, White CM, Kluger J, Coleman CI. Effect of cinnamon on glucose control and lipid parameters. Diabetes Care 2008;31(1):41‐3. [DOI] [PubMed] [Google Scholar]
Battaglia 1995
- Battaglia S. The Complete Guide to Aromatherapy. Virginia, Queensland: The Perfect Potion, 1995. [Google Scholar]
Davis 2011
- Davis PA, Yokoyama W. Cinnamon intake lowers fasting blood glucose: meta‐analysis. Journal of Medicinal Food 2011;14:1‐6. [DOI] [PubMed] [Google Scholar]
De Rossi 1998
- Rossi SS, Greenberg MS. Intraoral contact allergy: a literature review and case reports. Journal of the American Dental Association 1998;129:1435‐41. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT. Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kannappan 2006
- Kannappan S, Jayaraman T, Rajasekar P, Ravichandran MK, Anuradha CV. Cinnamon bark extract improves glucose metabolism and lipid profile in the fructose‐fed rat. Singapore Medical Journal 2006;47(10):858‐63. [PubMed] [Google Scholar]
Kim 2006
- Kim SH, Hyun SH, Choung SY. Anti‐diabetic effect of cinnamon extract on blood glucose in db/db mice. Journal of Ethnopharmacology 2006;104(1‐2):119‐23. [DOI] [PubMed] [Google Scholar]
Lau 2006
- Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006;333:597‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Med 1999;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ody 1993
- Ody P. The Complete Medicinal Herbal. Ringwood: Viking Books, 1993. [Google Scholar]
Pham 2007
- Pham A, Kourlas H, Pham DQ. Cinnamon supplementation in patients with type 2 diabetes mellitus. Pharmacotherapy 2007;27(4):595‐9. [DOI] [PubMed] [Google Scholar]
Qin 2004
- Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Cinnamon extract prevents the insulin resistance induced by a high‐fructose diet. Hormone & Metabolic Research 2004;36(2):119‐25. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Roffey 2006
- Roffey B, Atwal A, Kubow S. Cinnamon water extracts increase glucose uptake but inhibit adiponectin secretion in 3T3‐L1 adipose cells. Molecular Nutrition & Food Research 2006;50(8):739‐45. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JAC, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care; Meta‐analysis in Context. London: BMJ Publishing Group, 2001:189‐208. [Google Scholar]
Subash 2007
- Subash Babu P, Prabuseenivasan S, Ignacimuthu S. Cinnamaldehyde ‐ a potential antidiabetic agent. Phytomedicine 2007;14(1):15‐22. [DOI] [PubMed] [Google Scholar]
WHO 1980
- WHO Expert Committee on Diabetes Mellitus. Second Report. Technical Report Series 646. Geneva: World Health Organization, 1980. [PubMed] [Google Scholar]
WHO 1985
- WHO Expert Committee on Diabetes Mellitus. Technical Report Series 727. Geneva: World Health Organization, 1985. [PubMed] [Google Scholar]
WHO 1999
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organization, 1999. [Google Scholar]


