Abstract
Background
Patellofemoral pain syndrome, now generally referred to as patellofemoral pain (PFP), is one of the most common orthopaedic disorders, characterised by pain in the anterior or retropatellar knee region. Neuromuscular electrical stimulation (NMES) has been proposed generally as a complementary treatment, associated with other interventions such as exercise, or as a single treatment to increase muscle force, reduce knee pain, and improve function.
Objectives
To assess the effects (benefits and harms) of neuromuscular electrical stimulation for people with patellofemoral pain.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, PEDro, CINAHL, SPORTDiscus, AMED, LILACS, trial registers, conference abstracts, and reference lists. We carried out the search in May 2017.
Selection criteria
We included randomised controlled clinical trials that evaluated the use of NMES for people with PFP.
Data collection and analysis
Two review authors independently performed the process of study selection, data extraction, and 'Risk of bias' assessment in duplicate. The primary outcomes were knee pain, knee function, and adverse events. The timing of outcome measurements was up to three months (short term), three to 12 months (medium term), and 12 months and above from trial entry (long term). We calculated risk ratios for dichotomous data and mean differences or standardised mean differences for continuous data. Where appropriate, we pooled data using the fixed‐effect model.
Main results
We included eight randomised clinical trials, reporting results for 345 participants with PFP. The mean ages of trial populations ranged from 25 to 43 years, and the majority (53% to 100%) of participants were female. There was a wide duration of symptoms, with the minimum duration of symptoms for trial inclusion ranging from one to six months. In addition to the study inclusion criteria, studies varied widely in the characteristics of the NMES and its application, and associated co‐interventions. We assessed all trials as at high risk of bias in at least one domain, particularly blinding and incomplete outcome data. The results of a laboratory‐based trial reporting knee pain immediately after a single 15‐minute session of NMES are not reported here as these are of questionable clinical relevance. The seven remaining trials provided evidence for three comparisons. We assessed the overall quality of the evidence, using GRADE, for all primary outcomes for all comparisons as very low, thus we are very unsure of the findings.
Four studies compared NMES plus exercise versus exercise alone. Patellar taping was applied as well as exercise to all participants of one study, and patellar taping and ice were also applied in another study. Each trial tested a different multiple‐session NMES programme. Pooled data from three studies (118 participants) provided very low‐quality evidence that NMES is associated with reduced pain at the end of treatment (ranging from 3 to 12 weeks): mean difference ‐1.63, 95% confidence interval (CI) ‐2.23 to ‐1.02; visual analogue scale (VAS) 0 to 10; higher scores = worse pain. However, this result may not be clinically relevant since the minimal clinically important difference for VAS during activities (1.5 to 2.0, out of 10 points) lies within the 95% CI. We found very low‐quality evidence from pooled data from two trials of little effect of NMES on knee function, as measured by two knee function rating systems. We found inconclusive and very low‐quality evidence from one trial (29 participants) of little effect of NMES on pain and function at one‐year follow‐up. None of the four trials reported on adverse effects of treatment.
One study (94 participants) compared NMES, applied four hours per day on a daily basis for four weeks, with two types of exercises (isometric and isokinetic). The study did not report on knee pain or adverse events. The study provided very low‐quality evidence of no important difference between the two groups in knee function at the end of the four‐week treatment. Of note is the potentially onerous NMES schedule in this study, which does not correspond to that typically used in clinical practice.
Two studies compared different types of NMES. Simultaneously delivered high‐low frequencies NMES was compared with sequentially delivered high‐low frequencies NMES in one trial (14 participants) and with fixed frequency NMES in the second trial (64 participants). The studies provided very low‐quality evidence of no important differences at the end of the six‐week treatment programme between the simultaneous frequencies NMES and the two other NMES programmes in overall knee pain, knee function, or in quadriceps fatigue (an adverse event).
Authors' conclusions
This review found insufficient and inconclusive evidence from randomised controlled trials to inform on the role of NMES for treating people with PFP in current clinical practice. The very low‐quality evidence available means that we are uncertain whether or not a multiple‐session programme of NMES combined with exercise over several weeks versus exercise alone results in clinically important differences in knee pain and function at the end of the treatment period or at one year. There were no data on adverse effects such as muscle fatigue and discomfort. High‐quality randomised clinical trials are needed to inform on the use of NMES for people with PFP. However, professional and stakeholder consensus is required on prioritisation of the research questions for interventions for treating people with PFP, including on the NMES treatment protocol for trials testing NMES.
Plain language summary
Muscle stimulation for people with anterior knee pain
Background
Patellofemoral pain syndrome, commonly known as anterior knee pain, is characterised by short‐ or long‐term pain in the front part of the knee or behind the kneecap. Muscle stimulation has been proposed as a treatment for this condition. This involves the use of a device that produces a muscle contraction by placing electrodes on the skin of the leg. Muscle stimulation is often used together with exercises and other treatments but can also be used on its own.
Results of the search
We searched the medical literature up to May 2017 and found eight studies reporting results for a total of 345 participants who had anterior knee pain for at least one month, and sometimes over several years. Most of the participants were female. The average age of participants in the studies ranged from 25 to 43 years. All the studies were small and had flaws that meant they were at risk of bias. There was very little evidence on longer‐term outcome. The results of one study that reported on immediate pain after a single 15‐minute session of muscle stimulation are not reported here as these are of questionable clinical relevance.
Key results
Each of the seven remaining studies tested one of three comparisons.
Four studies compared a multiple‐session muscle stimulation programme combined with exercise over several weeks with exercise on its own for the same period. All participants in two studies had adhesive tape applied across their knee cap, with ice also being applied in one study. We found very low‐quality evidence that muscle stimulation with exercise may slightly reduce knee pain at the end of a treatment period of between 3 and 12 weeks better than exercise alone. However, very low‐quality evidence did not show an effect on knee function. None of the studies reported on harms such as muscle fatigue and discomfort. There was very little useful information on longer‐term effects.
One study compared muscle stimulation lasting four hours each day for four weeks with exercise. Very low‐quality evidence showed no important difference between the two groups in knee function at the end of the four‐week treatment. Of note is that the duration of muscle stimulation is much longer than used nowadays.
Two studies compared different types of muscle stimulation. Very low‐quality evidence showed no important differences at the end of the six‐week treatment programme between the different types of muscle stimulation.
Quality of the evidence
The overall quality of the evidence for all reported outcomes was very low. This means that we are very uncertain about the findings of these studies.
Conclusions
We found insufficient evidence to inform on the role of neuromuscular electrical stimulation for treating people with anterior knee pain. Further research is needed.
Summary of findings
Summary of findings for the main comparison. Neuromuscular electrical stimulation + other intervention (e.g. exercise) versus same other intervention only for patellofemoral pain syndrome.
| Neuromuscular electrical stimulation (NMES) plus other intervention (e.g. exercise) versus no NMES plus same other intervention for patellofemoral pain syndrome | ||||||
|
Patient or population: people with patellofemoral pain syndrome1
Settings: outpatient rehabilitation and home‐based therapy
Intervention: NMES2 plus other active intervention (e.g. exercise)3 Comparison: no NMES control plus same other active intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No NMES plus same other intervention | NMES plus other intervention (e.g. exercise) | |||||
| Knee pain (short term) VAS scale: 0 to 10; higher score = worse pain. Follow‐up: 3 to 12 weeks (all were at the end of the treatment programme2) | The mean knee pain ranged across control groups from 2.36 to 2.70 points. | The mean knee pain in the intervention groups was 1.63 lower (2.23 to 1.02 lower). | MD ‐1.63 (‐2.23 to ‐1.02) | 118 (3 studies) | ⊕⊝⊝⊝ very low4 | This difference may not be clinically important since the MCID for VAS (1.5 to 2.0, out of 10 points)5 lies within the 95% CI. |
| Knee pain (long term) VAS scale: 0 to 10; higher score = worse pain. Follow‐up: 1 year | The median pain score in the study control group was 0.4 points (IQR 0.2 to 3.4). | The median pain score in the NMES group was 1.8 points (IQR 0.1 to 3.6). | See comment | 29 (1 study) | ⊕⊝⊝⊝ very low6 | The difference was reported as not statistically significant. |
| Knee function (short term) 2 tools used: Cincinnati Knee Rating System (6 to 100; higher scores = better function) and Lower Extremity Functional Scale (LEFS) scale (0 to 80; higher scores = better function). Follow‐up: 3 to 6 weeks (at the end of the treatment programme) | The mean knee function in the study control groups was 72.4 (LEFS scale) and 83.3 (Cincinnati score). | The mean difference in knee function in the intervention groups was 0.37 SDs higher (0.11 lower to 0.84 higher). | SMD 0.37 (‐0.11 to 0.84) | 70 (2 studies) | ⊕⊝⊝⊝ very low7 | 0.2 SD represents a small difference, 0.5 SD a moderate difference, and 0.8 SD a large difference. However, the mean differences in the 2 trials were small and unlikely to be clinically important (LEFS scale: MD 0.73; Cincinnati score: MD 4.65). |
|
Knee function (long term) Kujala Patellofemoral Score (KPS) (0 to 100; higher score = better function). Follow‐up: 1 year |
The median KPS in the study control group was 95 (IQR 85 to 96). | The median KPS in the NMES group was 94 (IQR 88 to 96). | See comment | 29 (1 study) | ⊕⊝⊝⊝ very low6 | The very small difference was reported as not statistically significant. |
| Adverse events ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IQR: interquartile range; MCID: minimal clinically important difference; MD: mean difference; SD: standard deviation; SMD: standardised mean difference; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Where reported, there was a higher percentage of females (63% to 100%). The mean ages of the participants in the four trials ranged from 25 to 39 years. There was a wide duration of symptoms, with the minimum duration of symptoms for trial inclusion ranging from one to six months. 2The format of the NMES varied among the four trials. Sessions of NMES lasted between 10 to 20 minutes, applied 2 to 5 times a week, for between 3 and 12 weeks. 3The co‐intervention in all four trials was exercise. Patellar taping was also applied to all participants in two trials, and ice was applied in one trial. 4We downgraded the evidence two levels due to very serious risk of bias (performance bias), one level for imprecision reflecting small sample size, and one level for indirectness (time point of pain assessment was very far from the end of the intervention). 5The minimal clinically important difference for visual analogue scale usual pain was set at 1.5 to 2.0 (out of 10) points (Crossley et al. Archives of Physical Medicine and Rehabilitation 2004;85(5):815‐22). 6We downgraded the evidence two levels due to very serious risk of bias (performance, detection, and attrition biases) and two levels for imprecision reflecting single‐trial data and small sample size. 7We downgraded the evidence two levels due to very serious risk of bias (performance bias) and one level for imprecision reflecting small sample size.
Summary of findings 2. Neuromuscular electrical stimulation versus exercise for patellofemoral pain syndrome.
| Neuromuscular electrical stimulation (NMES) versus exercise for patellofemoral pain syndrome | ||||||
|
Patient or population: people with patellofemoral pain syndrome
Settings: at home
Intervention: NMES (2‐hour session, twice a day, every day for 4 weeks)1 Comparison: exercise (either isokinetic or isometric; data combined in the analyses) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Exercise | NMES | |||||
| Knee pain (short term: < 3 months) | See comment | See comment | Not estimable | ‐ | See comment | Not estimable |
| Knee pain (longer term: > 3 months) | See comment | See comment | Not estimable | ‐ | See comment | Not estimable |
| Knee function (short term) Arpège function scale (0 to 18; higher score = better function). Follow‐up: 4 weeks (at end of treatment) | The mean knee function in the study control group was 15.34 points. | The mean knee function in the intervention groups was 0.94 lower (2.1 lower to 0.22 higher). | MD ‐0.94 (‐2.10 to 0.22) | 94 (1 study) | ⊕⊝⊝⊝ very low2 | |
| Knee function (longer term: > 3 months) | See comment | See comment | Not estimable | ‐ | See comment | Not estimable |
| Adverse events | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1This very demanding schedule is unlikely to be found in clinical practice. 2We downgraded the evidence two levels for very serious risk of bias (including high risk of performance bias and attrition bias), one level for imprecision (low numbers and wide 95% confidence interval), and one level for indirectness (the scheme used for NMES (during 4 hours/day) does not correspond to that used in clinical practice).
Background
Description of the condition
Patellofemoral pain syndrome (also known as patella malalignment syndrome, chondromalacia patellae, and anterior knee pain syndrome) is characterised by pain in the anterior or retropatellar (behind the kneecap) knee region that is exacerbated during activities that overload the patellofemoral joint such as running, prolonged sitting, stair climbing, kneeling, or squatting. Additional symptoms of the disorder include reduced muscle strength and knee function (Collins 2013; Petersen 2013; Rathleff 2013). In line with the International Patellofemoral Pain consensus statements published in 2014, we now use the term 'patellofemoral pain' (PFP) in this review (Witvrouw 2014). Patellofemoral pain is one of the most common orthopaedic disorders, accounting for 25% to 40% of all sports‐related knee problems; it predominantly affects adolescents and young adults, especially physically active women (Fagan 2008; Frye 2012). The prevalence of PFP is higher in active populations, including athletes and military recruits, than in the general population. However, the prevalence of this disorder in unselected populations is unclear (Lankhorst 2012; Petersen 2013; Roush 2012).
The aetiology of PFP is multifactorial. Common causes include mechanical and structural changes in the patellofemoral joint, malalignment of the patella (also known as patella maltracking), weakness of the knee extensor muscles (quadriceps musculature), poor muscle flexibility, and altered lower extremity kinematics (Bolgla 2011; Lankhorst 2012; Powers 2003). There are no specific diagnostic tests for PFP, and the diagnosis is usually based on clinical symptoms and physical examination (Crossley 2002; Roush 2012).
It is widely believed that patellofemoral malalignment is the primary mechanism responsible for anterior knee pain. Since the patella acts as a lever for the knee extensor muscles, any changes in the angle of the knee will also affect the peak force of the quadriceps (Bolgla 2011; Powers 2012). Recent studies have shown that the patella of people with PFP tends to deviate laterally within the femoral trochlear groove to a greater extent than normal during knee movement, leading to both a decrease in the patellofemoral contact area and an increase in the pressure on the articular surface, thus resulting in peripatellar tissue inflammation and pain (Kaya 2011; Powers 2012; Salsich 2001).
Several factors, including large quadriceps angle, abnormalities of the patellofemoral joint, excessive foot pronation, and tightness of hamstrings, calf, and lateral retinaculum can lead to abnormal patellar gliding (Barton 2009; Davies 2000; Fagan 2008; Pal 2012). However, according to biomechanical studies, hip muscle weakness and decreased quadriceps torque (especially the imbalance between activation of the vastus lateralis (VL) and vastus medialis oblique (VMO) muscles) are the main causes of patellar maltracking and patellofemoral pain (Chiu 2012; Cowan 2009; Pal 2012; Petersen 2013).
The response of people with PFP to conservative (non‐surgical) interventions such as physical therapy (including patellar taping, joint mobilisation, quadriceps strengthening, and foot orthoses, among others) and analgesic medications is variable. Long‐term results are not always satisfactory, and therefore the disorder can become chronic (Pattyn 2012; Rathleff 2012). A recent study showed that approximately half of treated patients reported persistent symptoms after 12 months (Collins 2013). Factors that may be associated with poor prognosis include higher age, female gender, overweight, reduced muscle strength, sports participation (overuse of the patellofemoral joint), and duration of symptoms over two months (Collins 2013; Pattyn 2012; Rathleff 2012). Those prognostic factors indicate that early diagnosis and treatment may lead to better long‐term results (Rathleff 2012).
Description of the intervention
Exercise therapy has been highly regarded as an essential tool in the treatment of people with PFP (Rathleff 2012). According to several clinical trials, muscle‐strengthening exercises help to correct abnormal patellar alignment and reduce clinical symptoms such as pain and impaired function (Bolgla 2011; Chiu 2012; Crossley 2002; Frye 2012). However, in some people, exercise can exacerbate pain and inflammation and reduce voluntary muscle activation (Dye 2005). Neuromuscular electrical stimulation (NMES) has been proposed as a complementary treatment, associated with other interventions such as voluntary exercise, or as a single treatment to increase muscle force by activating motor units and promoting muscle contraction (Lake 2011; Monaghan 2010; Taradaj 2013).
Electrical stimulation is produced by a device that delivers intermittent electrical impulses to muscle fibres through electrodes placed on the skin. These impulses induce action potentials, which stimulate motor nerves, thus producing contractions (Doucet 2012). These parameters include: frequency (usually set between 50 to 70 Hz); pulse (monophasic or biphasic, in waveform geometrical patterns such as rectangular); pulse duration (between 1 to 1000 µs, most often between 100 to 400 µs); duty cycle/duration of muscle contraction (intermittent pulse stimulation, i.e. pulses on and off, usually set as 10 seconds on and 50 seconds off); and intensity (adjusted according to individual tolerance) (Doucet 2012; Maffiuletti 2010; Sillen 2013). When comparing an NMES group with a control or a placebo group (e.g. NMES parameters configured not to produce muscle contraction), there is a significant increase in quadriceps muscle strength in people who receive active NMES (Maddocks 2013).
Although there is still no consensus on the standardisation of optimal NMES parameters, some studies suggest that electrical stimulation should be able to produce 50% of maximal voluntary contraction and recommend the use of biphasic rectangular pulses of 100 to 400 microseconds delivered at 50 to 100 Hz, at the highest tolerated intensity. The usual treatment for quadriceps muscles involves two or more sessions per week, 10 to 30 minutes each, for a period of four to five months (Sillen 2013; Vanderthommen 2007).
The main adverse effects of NMES are muscular discomfort caused by the electrical stimuli and excessive neuromuscular fatigue, which can be reduced by adjusting the NMES parameters (Maffiuletti 2014). Furthermore, the use of NMES over the thoracic region can cause changes in cardiac rhythm, therefore this intervention is not recommended for people with hypertension or those using pacemakers (Doucet 2012; Maffiuletti 2010; Monaghan 2010).
How the intervention might work
Researchers report the use of NMES in people with PFP to strengthen the quadriceps muscles in general and also to selectively strengthen the VMO muscle in cases where this muscle is hypotrophic and contracting after the VL muscle (Maffiuletti 2010; Werner 1993). Because NMES promotes simultaneous VMO and VL muscle contractions, it apparently leads to a significant increase in quadriceps muscles' force (Vengust 2001; Werner 1993).
Good results also depend on using electrodes of appropriate size and their adequate positioning (Doucet 2012; Maffiuletti 2010). Since the electric current produced by NMES is superficial, stimulation delivered is not sufficient to recruit a large number of motor units and produce an effective muscle contraction, therefore the characteristics and the spatial position of surface electrodes can influence the effectiveness of the muscle contraction. Despite the lack of a standard procedure, electrodes are often used in pairs of the same size, placed side by side over the muscle (Maffiuletti 2010; Sillen 2013; Vanderthommen 2007). Some authors have shown that the use of multiple electrodes, of large size, produces better results in muscle strengthening and reduction of discomfort, presumably due to the wide distribution and greater intensity of the electrical current. Additionally, gradual increase of the NMES intensity is recommended in order to activate deep muscle fibres and optimise muscle contraction (Maffiuletti 2010; Maffiuletti 2014).
Why it is important to do this review
Neuromuscular electrical stimulation is currently widely used by physical therapists to treat people with several types of knee problems and in postoperative rehabilitation; however, the effectiveness of NMES for PFP remains uncertain. Through a critical summary of the evidence for NMES for treating PFP, this review aims to help health professionals and patients make informed clinical decisions about treatment choices for PFP and identify research gaps in this area in relation to NMES.
Objectives
To assess the effects (benefits and harms) of neuromuscular electrical stimulation for people with patellofemoral pain.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled clinical trials evaluating the use of NMES for people with PFP.
Types of participants
We included studies involving adolescents and adults diagnosed with PFP. We excluded studies of participants with patellar luxation, patellar fracture, osteoarthritis, or other intra‐articular knee pathology. We excluded studies that included a mixed population where a percentage of the participants had some other knee pathology or were children, unless the results of people with PFP were presented separately or the proportion of those with other knee pathology or children was small (less than 5%) and preferably balanced between groups.
Types of interventions
We included trials comparing any kind of NMES with:
placebo or sham intervention (e.g. stimulation parameters below the level required to promote muscle contraction or no stimulator output);
no treatment;
exercise.
We also included trials comparing different programmes of NMES (e.g. different frequencies, intensity, and duration). For these comparisons, we designated the control group to be the one with a lower frequency, less intensive, or shorter duration programme.
We included studies that tested NMES as an isolated procedure or in combination with other conservative interventions (e.g. exercise programme), as long as the same intervention was also offered to people in the control group.
Types of outcome measures
Primary outcomes
Knee pain (measured by validated pain scores, such as visual analogue scales (VAS)) (Revill 1976).
Knee function measured by validated knee questionnaires (such as Kujala Patellofemoral Score and Lysholm score) (Kujala 1993; Lysholm 1982).
Adverse events (e.g. skin injuries, excessive discomfort, excessive fatigue, bradycardia or other cardiac arrhythmia, or substantially increased pain as a direct effect of treatment).
Secondary outcomes
Objectively measured performance tests (such as hop test, timed up‐and‐go, stair climbing).
Health‐related quality of life (measured by validated assessment tools such as Medical Outcomes Study 36‐item short‐form health survey (SF‐36) for general measures) (Ware 1992).
Participant satisfaction (preferably measured by validated assessment tools).
Muscle strength (directly measured, e.g. by isokinetic dynamometer).
We did not consider trials where muscle strength or outcome measures such as EMG (electromyogram) data and gait analysis were studied without evaluation of pain or knee function.
Timing of outcome measurement
We adjusted the previous criteria subsequent to external referee's feedback (see Differences between protocol and review). This included starting the follow‐up at trial entry.
Up to three months (short term); this coincided with the end of the treatment programme in all trials
Three to up to 12 months (medium term)
12 months or over (long term)
Search methods for identification of studies
Electronic searches
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (9 May 2017), the Cochrane Central Register of Controlled Trials (CENTRAL) (2017; Issue 5), Ovid MEDLINE (including MEDLINE In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and Epub Ahead of Print) (1946 to 5 May 2017), Embase (Ovid Online) (1974 to 2017 Week 18), the Physiotherapy Evidence Database (PEDro) (1999 to 15 July 2016), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO) (1982 to 5 May 2017), SPORTDiscus (EBSCO) (1985 to 5 May 2017), Allied and Complementary Medicine Database (AMED) (Ovid Online) (1985 to 5 May 2017), and Latin American and Caribbean Health Sciences (LILACS) (BIREME) (1982 to 5 May 2017). We also searched the WHO International Clinical Trials Registry Platform (apps.who.int/trialsearch/), ClinicalTrials.gov (www.clinicaltrials.gov/), and the ISRCTN registry (www.isrctn.com/) for ongoing trials (5 May 2017). We did not apply any restrictions based on publication status or language.
In MEDLINE (Ovid Online), the subject specific strategy was combined with the sensitivity‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011). The search strategies for all databases are reported in Appendix 1.
Searching other resources
We searched the reference lists of relevant studies and conference abstracts of the International Patellofemoral Pain Research Retreat (IPRR) (ipfrn.org/conference/) (from 2009 to 2013) and the International Patellofemoral Pain Clinical Symposium (ipfrn.org/clinicalsymposium/) (2013). We also contacted experts in the field to identify published, unpublished, or ongoing trials.
Data collection and analysis
We carried out data collection and analysis according to the methods stated in the published protocol (Martimbianco 2014).
Selection of studies
Two review authors (ALCM and GP) independently screened the titles and abstracts retrieved through the search strategy. The full texts of all studies considered potentially eligible for inclusion were retrieved and read independently by the two review authors for final selection. Disagreements were settled by a third author (RR).
Data extraction and management
Two review authors (ALCM and GP) independently extracted the data from all reports of the included studies using a piloted extraction form that included information on participant characteristics, methodological aspects, interventions, comparisons, outcomes, follow‐up, and results. Unresolved disagreements between the two review authors were settled by a third review author (RR). We attempted to contact trial authors for clarification and additional information when necessary.
Assessment of risk of bias in included studies
Two review authors (ALCM and BNGA) independently assessed the risk of bias in the included studies using the Cochrane 'Risk of bias' tool (Higgins 2011), which assesses seven domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other sources of bias. Regarding this last domain we looked for major baseline imbalance as a potential cause of bias. We judged each of the seven domains as being at high, low, or unclear risk of bias. Disagreements between the two review authors were settled by a third review author (RR).
Measures of treatment effect
We calculated risk ratios (RR) with 95% confidence intervals (95% CI) for dichotomous data and mean differences (MD) with 95% CIs for continuous data. In cases where different scales were used to measure the same outcome, we planned to use standardised mean differences (SMD) with 95% CIs.
Unit of analysis issues
As we expected, the unit of randomisation in the included trials was the individual participant. In future updates of this review, if we find studies in which the unit of analysis is the knee rather than the individual participant, and where corrections have not been made, we will consider presenting the data for the trials where the disparity between the units of analysis and randomisation is small. Had we found studies with cross‐over designs, we would have considered the first stage as indicated in our protocol. We avoided unit of analysis issues related to repeated observations of the same outcome. For example, where trial results were presented for several periods of follow‐up, we presented data for these separately according to the different follow‐up periods defined in Types of outcome measures.
Dealing with missing data
We contacted trial authors to obtain any missing data and information. Where we were successful, we recorded the date of communication and the information obtained. We planned to conduct intention‐to‐treat analyses whenever possible. If necessary and possible, for continuous data we planned to calculate missing standard deviations (SDs) from exact P values, 95% CIs, or standard errors.
Assessment of heterogeneity
We assessed heterogeneity by visual inspection of forest plots and by using the I2 statistic, as recommended by Higgins 2011: 0% to 40% indicated no significant heterogeneity; 30% to 60% indicated moderate heterogeneity; 50% to 90% could represent substantial heterogeneity; and 75% to 100% was indicative of very substantial heterogeneity. We also used the Chi2 test: statistical significance (P < 0.1) was interpreted as significant heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are at least 10 studies in a meta‐analysis of any primary outcome, we intend to perform a funnel plot to explore potential publication bias.
Data synthesis
When considered appropriate and where data were available, we pooled the results of comparable groups of trials using the fixed‐effect or random‐effects analysis models. Our choice of the fixed‐effect model for reporting purposes was guided by a careful consideration of the extent of heterogeneity and whether it could be explained, in addition to other factors such as the number and size of the included studies. We planned not to pool data where there was considerable heterogeneity (I2 > 75%) that could not be explained by differences in methodological or clinical features among the trials. Where it was not appropriate to pool data, we presented trial data in the analyses for illustrative purposes and reported these in the text.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses: according to control group intervention (placebo versus no NMES control); when NMES was the main intervention or a complementary or auxiliary intervention; age of participants (18 years or more versus less than 18 years); gender; physical activity level (athletic, i.e. regular sports participation, versus non‐athletic); and duration of PFP symptoms (acute, i.e. less than three months, versus chronic). However, subgroup analysis was not possible because data were insufficient. In future updates, if sufficient data are available, we will perform subgroup analysis to investigate whether the results of subgroups were significantly different by inspecting the overlap of CIs and by performing the test for subgroup differences available in Review Manager 5 (RevMan 2014).
Sensitivity analysis
In future updates of this review, if sufficient trials are available, we plan to perform sensitivity analyses examining various aspects of trial and review methodology, including: the effects of missing data; of excluding trials at high or unclear risk of bias (specifically, selection bias from lack of allocation concealment, and detection bias from lack of outcome assessor blinding); trials only reported in abstracts; trials with unit of analysis problems related to the inclusion of participants with bilateral PFP; and the selection of the statistical model (fixed‐effect versus random‐effects). We also plan to examine the effect of including trials that provide suboptimal dosing: less than two sessions per week, lasting under 15 minutes each, for less than two weeks (Doucet 2012; Maffiuletti 2010).
Assessing the quality of the evidence and presenting 'Summary of findings' tables
We assessed the quality of the evidence for the primary outcomes (pain, function, and adverse events) according to the GRADE approach per Section 12.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We prepared two 'Summary of findings' tables for the following comparisons: NMES plus other intervention (e.g. exercise) versus no NMES plus same other intervention; and NMES versus exercise. We presented knee pain and knee function at end of treatment (short term) and in the longer term (three months or above) and adverse events.
Results
Description of studies
Results of the search
We retrieved a total of 340 records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (5); CENTRAL (21), MEDLINE (47), Embase (123), PEDro (19), CINAHL (29), SPORTDiscus (14), AMED (16), LILACS (9), the WHO International Clinical Trials Registry Platform (11), ClinicalTrials.gov (44), and the ISRCTN registry (2). After excluding 87 duplicates, we screened 253 citations and excluded 241 that were not relevant. We judged 12 reports to be potentially eligible, which we selected for full‐text reading. Eight studies (nine reports) met our selection criteria and were included in the review (Akarcali 2002; Bily 2008; Callaghan 2001; Callaghan 2004; Glaviano 2016 (two reports); Gobelet 1992; Kaya 2013; Tunay 2003). We excluded two studies (Dursun 2001; Kuru 2012), and identified one ongoing study from our search of ClinicalTrials.gov (NCT02441712). There are no trials awaiting classification. A flow diagram summarising the study selection process is shown in Figure 1.
1.
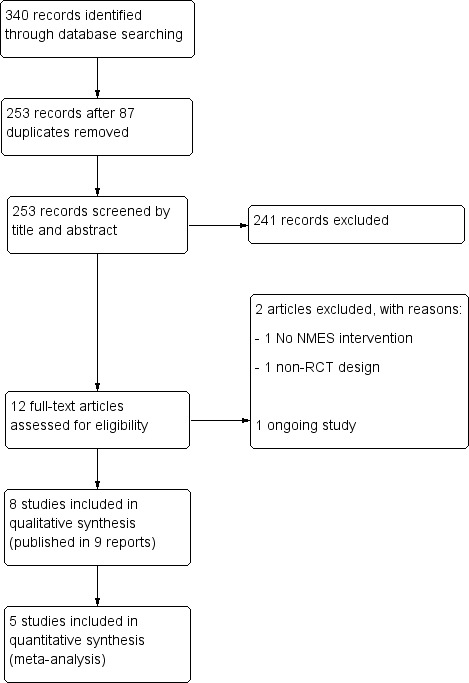
Study flow diagram.
Included studies
We have provided full details on the individual studies in the Characteristics of included studies table. We contacted the authors of all studies for clarification and additional information and received responses from authors of five studies (Akarcali 2002; Bily 2008; Callaghan 2001; Callaghan 2004; Tunay 2003). One study, Glaviano 2016, was reported in two different articles, one of which reported the findings for the 15 female participants of the 22 participants in the full report.
Design
All eight included studies were randomised, controlled, parallel‐group clinical trials. Two of them were pilot studies (Bily 2008; Callaghan 2001). Glaviano 2016 was a sports laboratory‐based study that assessed the immediate effects of a single session of NMES. Gobelet 1992 had three treatment groups; we considered the two groups testing different types of exercise (isokinetic versus isometric) together in this review. Kaya 2013 had three treatment groups, of which one was excluded as it was not relevant for this review, and Tunay 2003 had four groups, two of which were excluded as they were not relevant.
Sample size
Overall, 390 participants were randomised and 345 were available for analysis. Sample sizes in the eight studies ranged from 16, in Callaghan 2001, to 120, in Gobelet 1992. The number of participants in the intervention group ranged from 7, in Callaghan 2001, to 37, in Callaghan 2004.
Setting
The trials were conducted in five different countries: one in Switzerland (Gobelet 1992), two in the United Kingdom (Callaghan 2001; Callaghan 2004), one in Austria (Bily 2008), three in Turkey (Akarcali 2002; Kaya 2013; Tunay 2003), and one in the United States (Glaviano 2016). The publication dates ranged from 1992, in Gobelet 1992, to 2016, in Glaviano 2016.
Participants
All studies included participants with PFP diagnosed through clinical examination of the knee, including pain provoking clinical tests (prolonged sitting, kneeling, squatting, ascending or descending stairs), in Bily 2008, Callaghan 2001, Callaghan 2004, and Glaviano 2016, and positive patellar compression tests (Akarcali 2002; Glaviano 2016). Two studies also used magnetic resonance imaging for diagnosis (Callaghan 2001; Kaya 2013). Three trials included X‐rays to investigate anatomical bone changes (Akarcali 2002; Bily 2008; Gobelet 1992). Four studies included only participants with unilateral complaints (Callaghan 2001; Callaghan 2004; Kaya 2013; Tunay 2003). Akarcali 2002 and Glaviano 2016 included people with bilateral PFP, but the authors treated only the most symptomatic knee in these participants; Bily 2008 included only people with bilateral PFP, and both knees were treated and evaluated; and Gobelet 1992 provided no information on the side(s) affected. Seven studies reported the minimum duration of symptoms (Akarcali 2002; Bily 2008; Callaghan 2001; Callaghan 2004; Glaviano 2016; Kaya 2013; Tunay 2003), which ranged from one month, in Tunay 2003, to six months (Bily 2008; Callaghan 2001; Callaghan 2004; Kaya 2013). The mean age of participants ranged from 25.4 years, in Bily 2008, to 42.7 years, in Kaya 2013. Tunay 2003 did not report the gender of the participants. All of the other seven trials recruited a higher percentage of females, ranging from 53% in Gobelet 1992 to 100% in Kaya 2013, which recruited females only. None of the included studies described the level of activity of their participants.
Interventions and controls
All studies gave the participants in the intervention group an NMES portable device for use at home (Bily 2008; Callaghan 2001; Callaghan 2004; Gobelet 1992), during an outpatient rehabilitation programme (Akarcali 2002; Kaya 2013; Tunay 2003), or in a research laboratory (Glaviano 2016). The participants using the portable device at home received instructions about the stimulation programme and electrode placement. In four studies, self adhesive electrodes were to be placed on the quadriceps muscles (Bily 2008; Callaghan 2001; Callaghan 2004; Kaya 2013). In two studies (Akarcali 2002; Gobelet 1992), the investigators aimed to specifically stimulate the vastus medialis oblique (VMO) muscle. In one study (Glaviano 2016), the electrodes were positioned over the VMO and gluteus medius muscles (agonist muscles), and over the adductor and the hamstrings muscle groups (antagonist muscles). Tunay 2003 did not describe the position of the electrodes.
Glaviano 2016 used a novel form of NMES (patterned electrical neuromuscular stimulation (PENS) with a frequency of 50 Hz) that mimics voluntary movement patterns of muscular contractions (stimulation of the agonist muscles, followed by antagonist muscles), and compared it with a sham intervention ("sub sensory" treatment).
Four studies compared NMES plus another intervention versus no NMES plus the same other intervention (Akarcali 2002; Bily 2008; Kaya 2013; Tunay 2003). The other intervention was an exercise programme in Akarcali 2002 and Bily 2008; an exercise programme and patellar taping in Kaya 2013; and ice, patellar taping, and exercises in Tunay 2003. Akarcali 2002 and Kaya 2013 used a high‐voltage pulsed galvanic stimulation (HVPGS) with a frequency of 60 Hz, and Bily 2008 and Tunay 2003 used an NMES device with a frequency of 40 Hz and 30 Hz, respectively.
Gobelet 1992 compared NMES (with sequentially combined periods of high and low frequencies (50 Hz and 10 Hz)) alone versus an exercise programme (isokinetic and isometric exercises).
The last two studies compared different NMES programmes (Callaghan 2001; Callaghan 2004). Callaghan 2001 used an NMES device that simultaneously combined high and low frequencies superimposed, comparing it with an NMES device that sequentially combined high and low frequencies (control group). Callaghan 2004 used a new type of NMES with simultaneous delivery of mixed frequencies, comparing it with a fixed‐frequency NMES device (control group).
All studies reported that the intensity of stimulation was adjusted to the highest possible level that was tolerated by the participants. The duration of the stimulation session ranged from 10 minutes, in Akarcali 2002 and Tunay 2003, to four hours per day (Gobelet 1992). NMES session frequency ranged from a single session, in Glaviano 2016, to seven times per week, in Callaghan 2001. The treatment duration ranged from 15 minutes, in Glaviano 2016, to 12 weeks, in Bily 2008.
Outcomes
All studies evaluated outcomes in the short term, which was timed after the single session in Glaviano 2016, and at end of the treatment programme (3 weeks in Tunay 2003 to 12 weeks in Bily 2008). Bily 2008 also assessed participants at 12 months after the end of treatment (long term). Seven trials used a visual analogue scale (VAS) to assess knee pain (Akarcali 2002; Bily 2008; Callaghan 2001; Callaghan 2004; Glaviano 2016; Kaya 2013; Tunay 2003). Three studies used the Kujala Patellofemoral Score to assess knee function (Bily 2008; Callaghan 2001; Callaghan 2004); one used the Cincinnati Knee Rating System (Tunay 2003); one used the Lower Extremity Functional Scale (LEFS) (Kaya 2013); and one used the Arpège function scale (Gobelet 1992). Only two studies evaluated adverse events, such as muscle fatigue using bipolar electrode surface electromyography (EMG) (Callaghan 2001; Callaghan 2004). Four studies assessed muscle flexion strength: Callaghan 2001 and Callaghan 2004 used an isokinetic dynamometer; Bily 2008 used a specifically designed chair with a circuit configuration that measured maximal isometric contractions; and Akarcali 2002 used Lovett’s manual muscle test (grades from 0 to 5; higher indicates better muscle strength). Two studies assessed lower limb muscle function (Callaghan 2001; Callaghan 2004), measured through performance tests such as step up and down and squat flexion. None of the included studies reported quality of life or participant satisfaction.
Excluded studies
We excluded two potentially eligible studies for reasons detailed in the Characteristics of excluded studies table (Dursun 2001; Kuru 2012). Briefly, Dursun 2001 investigated the effects of electromyographic biofeedback (EMG) on PFP, and Kuru 2012 was not a randomised controlled trial.
Ongoing studies
We found one ongoing study, which aimed to recruit 32 participants to test the results at four weeks of the same comparison as in Glaviano 2016. Further details are given in Characteristics of ongoing studies.
Risk of bias in included studies
We assessed studies for risk of bias ('low', 'high', or 'unclear') relating to seven domains. The results for individual studies are summarised in Figure 2, with details given in the Characteristics of included studies table. An overall summary of the ratings for each domain is shown in Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence allocation was adequate in four studies: two studies used a computer‐generated randomisation list (Callaghan 2001; Callaghan 2004); one used a sample of shuffled sealed envelopes, and the participant took one out of the batch (Bily 2008, in reply to our email); and one study used the coin‐tossing technique (Akarcali 2002, in reply to our email). The other four trials provided insufficient information about the randomisation process and were therefore judged as at unclear risk of bias for this domain (Gobelet 1992; Glaviano 2016; Kaya 2013; Tunay 2003). We judged all eight studies as at unclear risk of bias relating to allocation concealment. Six studies provided no details about the method used for allocation concealment (Akarcali 2002; Callaghan 2001; Callaghan 2004; Gobelet 1992; Kaya 2013; Tunay 2003). Bily 2008 and Glaviano 2016 used sealed envelopes but did not mention if they were opaque.
Blinding
Seven studies provided no information on participant blinding and were classified as being at high or unclear risk of performance bias depending on whether blinding would have been feasible: we judged five studies that compared different interventions between groups as at high risk of bias (Akarcali 2002; Bily 2008; Gobelet 1992; Kaya 2013; Tunay 2003), and two studies that compared the same intervention (different NMES parameters) as at unclear risk of bias for this domain (Callaghan 2001; Callaghan 2004). Glaviano 2016 compared NMES with a sham control but, since the authors did not exclude people who had received previous NMES therapy, it is difficult to judge whether the blinding was effective; we therefore also classified this study as at unclear risk of bias for this domain. Regarding detection bias, four trials reported blinding of outcome assessors and were judged to be at low risk of bias (Akarcali 2002; Callaghan 2004; Gobelet 1992; Kaya 2013). Bily 2008 reported by email that no measures were used to ensure blinding of the outcome assessors, and was therefore judged as at high risk of bias. Callaghan 2001, Glaviano 2016, and Tunay 2003 did not clearly report if the outcome assessors were blinded; we judged the first two studies as being at unclear risk of bias for this domain, and Tunay 2003 as at high risk of bias given the clear differences in the interventions.
Incomplete outcome data
We categorised only two studies as at low risk of bias for this domain because all participants completed the study (Glaviano 2016; Kaya 2013). Tunay 2003 provided insufficient information to make a judgement regarding losses and was assessed as being at unclear risk of bias for this domain. We judged the remaining five trials as at high risk of bias because they had at least one of the following issues: a high dropout rate (> 20%), an imbalance between groups due to loss of participants, no reporting of intention‐to‐treat analysis and uncertainty of the effects of postrandomisation exclusions due to adverse events (Akarcali 2002; Bily 2008; Callaghan 2001; Callaghan 2004; Gobelet 1992).
Selective reporting
None of the trials had a published study protocol or trial registration. It was unclear in six studies if the results included all expected outcomes (Akarcali 2002; Bily 2008; Glaviano 2016; Gobelet 1992; Kaya 2013; Tunay 2003); furthermore, these studies did not consider adverse event as an outcome, and were therefore judged as at unclear risk of bias for this domain. We assessed two studies as at further high risk of bias: Glaviano 2016 because of the very short follow‐up and data inconsistencies between the two reports of the trial, and Kaya 2013 because of contradictions between the text and tables for pain results. We categorised Callaghan 2001 and Callaghan 2004 as being at low risk of bias because although they considered only one adverse event (muscle fatigue), this is one of the most important adverse events in clinical practice.
Other potential sources of bias
Three studies were apparently free of other sources of bias (Akarcali 2002; Callaghan 2004; Tunay 2003), including major between‐group imbalances in key baseline characteristics, and were rated as at low risk of bias for this domain. We judged four studies as at unclear risk of bias: Bily 2008 because of some imbalances in participant characteristics, particularly in pre‐training muscle strength; Callaghan 2001 due to lack of information on participant characteristics at baseline; Glaviano 2016 because of some doubts as to the reliability of the presented data; and Gobelet 1992 because the authors did not provide information on the side(s) affected, and it was unclear which knee was treated. We judged Kaya 2013 as at high risk of other bias due to there being a major difference between the two groups in the initial pain during activities data, given that pain is the top primary outcome of this review.
Effects of interventions
Comparison 1: NMES versus placebo
One laboratory study compared NMES versus sham NMES in a single 15‐minute session (Glaviano 2016). Glaviano 2016 assessed knee pain immediately at the end of the treatment in 22 participants. This study did not report on all other outcomes pertinent to our review, such as function or adverse events. We rated the quality of the evidence as very low, downgrading one level for serious risk of bias, one level for indirectness (the single session of NMES does not correspond to that used in clinical practice; the results immediately post‐treatment may not be representative), and one level for serious imprecision, reflecting the low numbers available.
Knee pain (VAS: 0 to 10; higher scores = worse pain)
Glaviano 2016 found NMES was associated with reduced pain during a single leg squat (mean difference (MD) ‐1.90, 95% confidence interval (CI) ‐3.10 to ‐0.70) and during a lateral step‐down (MD ‐2.20, 95% CI ‐3.47 to ‐0.93) (Analysis 1.1). However, the 95% CI also included the probability that this benefit may not be clinically relevant because the 95% CI overlap the minimal clinically important difference (MCID) for VAS during activities (1.3 out of 10 points; Crossley 2004).
1.1. Analysis.

Comparison 1 NMES versus placebo (sham device), Outcome 1 Knee pain during activities (end of the treatment, single 15‐minute NMES session): VAS scale: 0 to 10; higher score = worse pain.
Comparison 2: NMES plus other active intervention (e.g. exercise) versus no NMES plus same other active intervention
Four studies compared NMES versus no NMES, where all participants received another intervention, typically exercise. Two studies compared NMES associated with exercise versus exercise alone (Akarcali 2002; Bily 2008). In Kaya 2013, the common co‐intervention was an exercise programme and patellar taping, and in Tunay 2003, all participants received exercises, patellar taping, and ice. All studies reported results at the end of the treatment (short term): three weeks in Tunay 2003, six weeks in Akarcali 2002 and Kaya 2013, and 12 weeks in Bily 2008. Bily 2008 also reported results at one year after treatment (long term). None of the four trials reported on adverse events, performance tests, quality of life or participant satisfaction. We rated the quality of the evidence for all reported outcomes as very low, downgrading one or two levels for serious or very serious risk of bias, and one or two levels for serious or very serious imprecision, reflecting the low numbers available and often broad confidence intervals.
Knee pain (VAS: 0 to 10; higher scores = worse pain)
Pooled results from three studies showed NMES was associated with lower pain scores in the short term (ranging from 3 to 12 weeks): MD ‐1.63, 95% CI ‐2.23 to ‐1.02; 118 participants; 3 studies; I2 = 2%; Analysis 2.1 (Akarcali 2002; Bily 2008; Tunay 2003). However, this benefit may not be clinically relevant since the absolute value (1.02) for the upper 95% CI was less than the MCID for VAS during activities (1.5 to 2.0, out of 10 points; Crossley 2004).
2.1. Analysis.

Comparison 2 NMES (+ other intervention) versus no NMES (+ same other intervention), Outcome 1 Knee pain (end of the treatment, 3 to 12 weeks): VAS scale: 0 to 10; higher score = worse pain.
Conversely, Kaya 2013 (30 participants), which evaluated knee pain during three activities after six weeks of treatment, reported clinically important higher pain scores in the NMES group during step‐down (MD 3.32, 95% CI 2.38 to 4.26), and step‐up (MD 3.15, 95% CI 2.10 to 4.20); Analysis 2.2. This did not apply for pain during squatting (MD 0.58, 95% CI ‐0.91 to 2.07). However, these results must be considered in the context of the imbalances in baseline pain scores, which were statistically significantly higher in the NMES group: step‐down: MD 2.12, 95% CI 0.2 to 4.04; step‐up: MD 1.90, 95% CI 0.01 to 3.79; and during squat: MD 1.77, 95% CI 0.22 to 3.32. Additionally, these findings disfavouring NMES were contradicted in the abstract conclusions for this trial, which stated that "Additional HVPGS [NMES] application in PFP rehabilitation may decrease pain levels during activities including step up and down".
2.2. Analysis.

Comparison 2 NMES (+ other intervention) versus no NMES (+ same other intervention), Outcome 2 Knee pain during activities (end of the treatment, 6 weeks): VAS scale: 0 to 10; higher score = worse pain.
Bily 2008 reported that the NMES group had a median pain score of 1.5 (interquartile range (IQR) 0.3 to 2.8; 18 participants) at 12 weeks, while the control group had a median score of 1.3 (IQR 0.4 to 3.3; 18 participants) (reported P = 0.64). At one‐year follow‐up, the NMES group had a higher median score of 1.8 (IQR 0.1 to 3.6; 16 participants), while the control group had a median score of 0.4 (IQR 0.2 to 3.4; 13 participants) (P value not available). However, the study reported that the change score for pain at one‐year follow‐up "remained constant" to that at 12 weeks (Analysis 2.1).
Knee function
Kaya 2013 measured knee function using the Lower Extremity Functional Scale (LEFS) scale (0 to 80; higher scores indicate better function) after six weeks of treatment. Tunay 2003 assessed knee function using the Cincinnati Knee Rating System (6 to 100; higher scores indicating better function) after three weeks of treatment. Pooled results from these two studies did not confirm a difference between the groups (standardised mean difference 0.37 favouring NMES, 95% CI ‐0.11 to 0.84; 70 participants; I2 = 7%; Analysis 2.3). The between‐group differences in the two scores in the individual trials were also small and unlikely to be clinically important (LEFS scale: MD 0.73; Cincinnati score: MD 4.65).
2.3. Analysis.

Comparison 2 NMES (+ other intervention) versus no NMES (+ same other intervention), Outcome 3 Knee function (end of the treatment, 3 and 6 weeks): Cincinnati Knee Rating System and LEFS; higher score = better function.
Bily 2008 evaluated knee function using the Kujala Patellofemoral Score (KPS) (0 to 100; higher scores indicating better function) and found no statistically significant difference between groups after the end of the 12‐week treatment and at one year. After 12 weeks, the NMES group had a median score of 89 (IQR 82 to 96; 18 participants), while the control group had a median score of 90 (IQR 85 to 95; 18 participants) (reported P = 0.29). At one‐year follow‐up, the NMES group had a median of 94 (IQR 88 to 96; 16 participants), while the control group had a median of 95 (IQR 85 to 96; 13 participants) (P value not available). There was no clinically important difference in change score for KPS at 12 weeks (MD 3.70, 95% CI ‐2.90 to 10.30; 36 participants; Analysis 2.4). The study reported that the change score for KPS at one‐year follow‐up remained constant.
2.4. Analysis.

Comparison 2 NMES (+ other intervention) versus no NMES (+ same other intervention), Outcome 4 Change score for KPS (end of treatment, 12 weeks) (0 to 100; higher score = better function).
Muscle strength
Akarcali 2002 assessed muscle strength using Lovett’s manual muscle test (graded between 0 and 5; higher scores indicating better muscle strength). There was very little difference between the two groups with good or normal muscle strength (grades 4 and 5) at the end of the six‐week treatment: 20/20 versus 21/22; risk ratio 1.04, 95% CI 0.92 to 1.19; very low‐quality evidence; Analysis 2.5.
2.5. Analysis.

Comparison 2 NMES (+ other intervention) versus no NMES (+ same other intervention), Outcome 5 Good or normal muscle strength (end of the treatment, 6 weeks): grades 4 to 5 Lovett’s manual muscle scale.
Bily 2008 (36 participants) evaluated isometric knee extensor strength (measured in newtons (N)) through a specifically designed chair (see Characteristics of included studies). Neuromuscular electrical stimulation was associated with greater muscle strength at 12 weeks (30° knee flexion: MD 38.30 N, 95% CI 13.71 to 62.89; and 60° knee flexion: MD 50.00 N, 95% CI 11.30 to 88.70; Analysis 2.6). However, we are not confident about the clinical relevance of these differences, especially since the muscle strengths before training were greater in the NMES group at both knee flexion settings (MD 14 N and 36 N).
2.6. Analysis.

Comparison 2 NMES (+ other intervention) versus no NMES (+ same other intervention), Outcome 6 Muscle strength (end of the treatment, 12 weeks): isokinetic dynamometer (N).
Comparison 3: NMES versus exercise
One trial compared NMES versus exercises (isometric and isokinetic exercises), reporting results at the end of the four‐week treatment (short term) for 94 participants (Gobelet 1992). In our analyses, we pooled data from the two exercise groups. Since Gobelet 1992 did not report which knee was treated and assessed, we considered the participant as the unit of analysis. Gobelet 1992 did not report on knee pain, adverse events, performance tests, quality of life or participant satisfaction. For both reported outcomes, we rated the quality of the evidence as very low, downgrading one level for serious risk of bias, one level for indirectness given the protracted application of NMES (four hours per day), and one level for serious imprecision, reflecting the low numbers available.
Knee function
Gobelet 1992, which used the Arpège function scale (0 to 18; higher scores indicating better function) to assess knee function, found no important difference in knee function at four weeks between the two groups (MD ‐0.94, 95% CI ‐2.10 to 0.22; 94 participants; Analysis 3.1).
3.1. Analysis.

Comparison 3 NMES versus exercise, Outcome 1 Knee function (end of the treatment, 4 weeks): Arpège function scale: 0 to 18; higher score = better function.
Muscle strength
Gobelet 1992 assessed muscle strength using an isokinetic dynamometer (measured in Nm) at speeds of 30°/s and 300°/s. There were no important differences in strength at four weeks between the two groups at either speed (at 30°/s: MD 0.06 Nm, 95% CI ‐29.67 to 29.79; 94 participants; at 300°/s: MD 1.04 Nm, 95% CI ‐14.00 to 16.08; 94 participants; Analysis 3.2).
3.2. Analysis.

Comparison 3 NMES versus exercise, Outcome 2 Muscle strength (end of the treatment, 4 weeks): isokinetic dynamometer (Nm).
Comparison 4: NMES (simultaneous frequencies) versus control NMES (sequential or fixed frequencies)
Two studies assessed this comparison (Callaghan 2001; Callaghan 2004). Callaghan 2001 compared simultaneously delivered high‐low frequencies versus sequentially delivered high‐low frequencies (control group). Callaghan 2004 compared simultaneously delivered high‐low frequencies versus a fixed frequency (control group). Both studies assessed outcomes in the short term, at the end of the six‐week treatment programme. Neither study reported on quality of life or participant satisfaction. We rated the quality of the evidence as very low for all reported outcomes, downgrading one level for serious risk of bias, one level for indirectness given the experimental nature of the intervention, and one level for serious imprecision, reflecting the low numbers available.
Knee pain (VAS: 0 to 10; higher scores = worse pain)
Both studies assessed this outcome and provided very low‐quality evidence of no important difference in knee pain between the two groups at six weeks (MD 0.40, 95% CI ‐1.76 to 2.56; 14 participants; 1 study; Analysis 4.1). In Callaghan 2004 (74 participants), participants in the NMES group with simultaneous frequencies had a median score of 2 points (IQR 0 to 4; 37 participants), and those in the NMES group with fixed frequencies had a median score of 2 points (IQR 1 to 4; 37 participants) (reported P = 0.249).
4.1. Analysis.

Comparison 4 NMES (simultaneous frequencies) versus control NMES (sequential or fixed frequencies), Outcome 1 Knee pain (end of the treatment, 6 weeks): VAS scale: 0 to 10; higher score = worse pain.
Knee function (KPS: 0 to 100; higher scores = better function)
We found very low‐quality evidence of no important difference between the groups in knee function at six weeks (MD ‐1.16, 95% CI ‐6.79 to 4.47; 88 participants; 2 studies; I2 = 15%; Analysis 4.2).
4.2. Analysis.

Comparison 4 NMES (simultaneous frequencies) versus control NMES (sequential or fixed frequencies), Outcome 2 Knee function (end of the treatment, 6 weeks): KPS: 0 to 100 scale; higher score = better function.
Adverse events
Both studies assessed muscle fatigue only as an adverse event (quadriceps fatigue rates through bipolar electrode surface EMG, at 60% of maximum contraction for 60 seconds). Both studies reported no statistically significant difference between groups (Callaghan 2001: reported P > 0.05; Callaghan 2004: reported P = 0.724). There were no reports of dropouts due to adverse events.
Functional performance tests
Both studies assessed this outcome by combining the step‐up and step‐down tests in a single measure ('steps up and down') and presented the total number of steps achieved until the onset of patellar pain. Neither study found evidence showing a difference between the two groups at six weeks (MD 4.50 steps, 95% CI ‐8.21 to 17.21; 14 participants; 1 study; Analysis 4.3). In Callaghan 2004, the participants of the NMES group with simultaneous frequencies had a median of 27 steps (IQR 16 to 46; 37 participants), and those in the NMES fixed‐frequency group had a median of 28 steps (IQR 11 to 60; 37 participants) (reported P = 0.562).
4.3. Analysis.

Comparison 4 NMES (simultaneous frequencies) versus control NMES (sequential or fixed frequencies), Outcome 3 Functional performance (end of the treatment, 6 weeks): number of steps up and down until pain.
Muscle strength
Both studies assessed quadriceps isometric and isokinetic muscle strength using an isokinetic dynamometer (measured in Nm), with angular velocity at 90°/s. Pooled results showed no evidence of important differences between the two groups at six weeks for either isometric muscle strength (MD ‐1.15 Nm, 95% CI ‐16.24 to 13.94; 88 participants; 2 studies; I2 = 0%; Analysis 4.4) or isokinetic muscle strength (MD ‐7.28 Nm, 95% CI ‐24.45 to 9.89; 88 participants; 2 studies; I2 = 0%; Analysis 4.5).
4.4. Analysis.
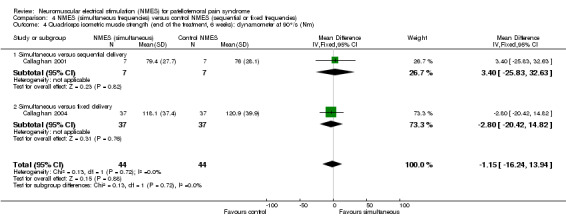
Comparison 4 NMES (simultaneous frequencies) versus control NMES (sequential or fixed frequencies), Outcome 4 Quadriceps isometric muscle strength (end of the treatment, 6 weeks): dynamometer at 90°/s (Nm).
4.5. Analysis.

Comparison 4 NMES (simultaneous frequencies) versus control NMES (sequential or fixed frequencies), Outcome 5 Quadriceps isokinetic muscle strength (end of the treatment, 6 weeks): dynamometer at 90°/s (Nm).
Subgroup and sensitivity analyses
There were insufficient data to perform our planned subgroup and sensitivity analyses.
Discussion
Summary of main results
This review evaluated the effects (benefits and harms) of NMES for people with a diagnosis of PFP. We included eight heterogeneous randomised clinical trials reporting results for a total of 345 participants. These studies contributed data to one of the four comparisons summarised below.
NMES alone versus placebo (sham NMES)
One laboratory‐based study compared a single 15‐minute session of NMES versus sham (placebo control) in 22 participants. This study reported only on pain during two functional activities assessed immediately after treatment (short term). We found very low‐quality evidence (downgraded one level for serious risk of bias, one level for indirectness given that the single 15‐minute session of NMES does not correspond to clinical practice, and one level for imprecision) that NMES is associated with reduced pain during both functional activities. However, the 95% CI results also include the probability that the difference was not clinically important. There were no data on function, adverse events, or longer‐term outcomes. Overall, the evidence from this study is limited and of uncertain clinical relevance.
NMES plus another intervention (e.g. exercise) versus no NMES control plus the same other intervention
The evidence for this comparison, which is summarised in Table 1, was from four trials, each testing a different multiple‐session NMES programme. Exercise was the common intervention in all four trials in this comparison; in one trial patellar taping was also used, and in another trial patellar taping and ice were also used.
We found very low‐quality evidence from three trials (118 participants) that NMES is associated with reduced pain in the short term (at the end of the 3 and 12 weeks' treatment programmes). However, the 95% CI included the possibility that the difference is not clinically important. We found very low‐quality evidence from pooled data from two trials of little effect of NMES on knee function at the end of treatment (three and six weeks). There was inconclusive and very low‐quality evidence from one trial (29 participants) of little effect of NMES on pain and function at one‐year follow‐up. None of the four trials reported on adverse effects of treatment.
NMES versus exercise
The evidence for this comparison, which is summarised in Table 2, was from one trial (94 participants) that compared NMES applied four hours per day on a daily basis for four weeks, with two types of exercises (isometric and isokinetic). The trial did not report on knee pain or adverse events. There was very low‐quality evidence of no important difference between the two groups in knee function at the end of the four‐week treatment (short term).
Different types of NMES
Two studies reporting data for 88 participants compared simultaneously delivered high‐low frequencies NMES versus control NMES, which was either sequentially delivered high‐low frequencies (14 participants) or fixed frequency (74 participants). We found very low‐quality evidence of no important differences at the end of the six‐week treatment programme between the simultaneous frequencies NMES and the control NMES in overall knee pain, knee function, or quadriceps fatigue (adverse event).
Overall completeness and applicability of evidence
Overall, outcome data from the eight included trials were available for 345 participants, thus 88.5% of the 390 randomised participants. The majority (26 participants; 22% of 120) were lost from the largest and oldest trial (Gobelet 1992). The eight trials contributed data to one of four comparisons, thus reducing again the quantity of data available to address individual questions. Clinical and methodological heterogeneity among studies in the same comparison precluded the pooling of data for several outcomes. The maximum number of participants in any analysis was 118 participants from three studies (Analysis 2.1). There were very few data for longer‐term outcomes.
Except Glaviano 2016, which was carried out in a sports laboratory, the trials were conducted in typical settings, with NMES applied in outpatients or at home.
Where details were provided, the study populations were representative of those treated for PFP. In three studies, the minimum duration of the complaints was from one to three months, which could present a better prognosis than the other four studies, which only included participants who had symptoms for more than six months. Three studies stipulated a maximum duration of symptoms of three and five years, and a further study recorded a maximum duration of symptoms of five years (Tunay 2003). On duration of symptoms alone, there was clearly a large variation within each study. None of the included studies reported the level of physical activity of their participants, which can also influence treatment effect. Finally, it was not possible to assess the influence of age and gender in the effectiveness of the intervention, but these factors were in accordance with the incidence of the disease. The variation in the inclusion and diagnostic criteria and the actual population characteristics could thus limit the applicability of the evidence.
The included studies used different NMES devices and stimulation parameters (e.g. frequency, pulse waveform, pulse duration, duty cycle, intensity, and treatment duration), as well as different types of co‐interventions (exercises, taping, ice). Only one study compared NMES with placebo, and although this comparison is considered clinically relevant (since it evaluates the specific active effect of electrostimulation), this study evaluated only a single NMES session, which could be insufficient to produce a true clinical benefit. In contrast, in the study that compared NMES versus exercise, participants were prescribed NMES for four hours a day, which seems to be very demanding and impracticable. Moreover, the length of treatment also varied between studies, ranging from a single session to 12 weeks.
Although appropriate outcome measures were generally used, most studies did not record or report on adverse events, and none reported on quality of life and participant satisfaction, both of which are important patient‐related outcomes. Moreover, most studies measured the outcome immediately at the end of treatment (in the short term), and there were very few data on the longer‐term effects of NMES.
All these factors contribute to the poor quality and applicability of the available evidence. For example, it is not possible to identify the best NMES programme and whether it is applicable to all people with PFP.
Quality of the evidence
All eight trials included in this review were at high risk of bias for at least one of the seven assessed domains, and at unclear risk of bias for several other domains, notably for allocation concealment, where no trial was judged as meeting the criteria for low risk of selection bias. We considered the overall quality of the evidence, based on the GRADE approach, as very low for all primary outcomes for each comparison, which means that we are very uncertain about the estimates of treatment effect. We downgraded evidence levels due methodological limitations that resulted in serious risk of bias, imprecision (small sample size and usually wide confidence intervals), and indirectness (the NMES scheme did not correspond to what is used in clinical practice, and the time point of pain assessment was very far from the end of the intervention). We did not assess publication bias due to the small number of studies available for pooling. While there was clinical heterogeneity as illustrated above, statistical heterogeneity was low in all pooled analyses, and thus we did not downgrade for inconsistency.
Potential biases in the review process
To minimise the probability of bias, we followed the recommendations on searching, trial selection, data extraction and analysis in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The search strategy was broad and sensitive, and we anticipate that we have identified all fully published randomised clinical trials that met the inclusion criteria. However, we cannot rule out that we have missed some trials, especially unpublished trials that are available, for example, only in conference proceedings. We contacted all trial authors via email for additional information. Although adhering to our protocol, our decision to pool data from clearly heterogeneous trials may be questioned (comparison 2). However, we have been cautious in our interpretation of the results and made clear the limitations in the applicability of the evidence.
Agreements and disagreements with other studies or reviews
Although we did not find another published systematic review on this topic, we located two Cochrane Reviews on related topics. One Cochrane Review assessed the effects of NMES for quadriceps strengthening before and after total knee replacement (Monaghan 2010). Based on two studies with a high risk of bias, Monaghan 2010 concluded that the evidence for the use of NMES for this clinical situation was unclear. A more recent Cochrane Review that included 31 heterogeneous trials evaluated exercises for treating PFP (van der Heijden 2015). van der Heijden 2015 concluded that there is very low‐quality but consistent evidence that exercise may improve clinical outcomes such as pain, function, and long‐term recovery.
Authors' conclusions
Implications for practice.
This review found insufficient and inconclusive evidence from randomised trials to inform on the role of neuromuscular electrical stimulation (NMES) for treating people with patellofemoral pain (PFP) in current clinical practice. The very low‐quality evidence available means that we are uncertain whether or not a multiple‐session programme of NMES combined with exercise over several weeks versus exercise alone results in a clinically important difference in knee pain and function at the end of the treatment period or at one year. There were no data on medium‐term outcome (between 3 and up to 12 months) and adverse effects such as muscle fatigue and discomfort.
Implications for research.
In addition to insufficient evidence for the use of NMES in people with PFP, we note the absence of a) formal diagnostic criteria for PFP, b) standardisation regarding NMES parameters, and c) definition of priority outcomes. Achieving professional and stakeholder consensus relating to these three aspects, thus on the patient population, the NMES protocol, and outcome assessment, is a key precursor to setting up high‐quality, multicentric randomised clinical trials to remedy the current lack of evidence. Agreement is also required in terms of prioritisation of research on NMES in the context of the known deficiencies in the evidence relating to other interventions for PFP, for example on exercise (van der Heijden 2015), and knee orthoses (Smith 2015). In addition to conforming to best‐quality methodological and reporting standards (CONSORT 2010), future trials on this topic should assess adverse events related to the intervention, quality of life, and patient satisfaction, and also include both short‐ and long‐term assessment of outcomes, crucially several months after completion of the treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 20 December 2017 | Amended | Amended contact details for Maria Regina Torloni |
Acknowledgements
We thank Lindsey Elstub and Laura MacDonald for their assistance in the preparation of this review, Joanne Elliott for her advice on developing the search strategies, and the Brazilian Cochrane Centre team for their methodological support. We also thank Helen Handoll, Zipporah Iheozor‐ejiofor, Catherine Sherrington, Toby Smith, and Martin Underwood for their feedback and suggestions on the protocol and review.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. Search strategies
CENTRAL (Cochrane Register of Studies Online)
#1 MESH DESCRIPTOR Patellofemoral Pain Syndrome (83) #2 MESH DESCRIPTOR Patellofemoral Joint (17) #3 MESH DESCRIPTOR Patella (246) #4 MESH DESCRIPTOR Knee (660) #5 #2 OR #3 OR #4 (914) #6 MESH DESCRIPTOR Arthralgia (568) #7 MESH DESCRIPTOR pain EXPLODE ALL TREES (34418) #8 #6 OR #7 (34418) #9 #5 AND #8 (222) #10 (anterior knee pain):TI,AB,KY (192) #11 (((patell* or femoropatell* or femoro‐patell* or retropatell*) adj2 (pain or syndrome or dysfunction))):TI,AB,KY (304) #12 (((lateral compression or lateral facet or lateral pressure or odd facet) adj syndrome)):TI,AB,KY (1) #13 (((chondromalac* or chondropath*) adj2 (knee*1 or patell* or femoropatell* or femoro‐patell* or retropatell*))):TI,AB,KY (27) #14 #10 OR #11 OR #12 OR #13 (479) #15 #1 OR #9 OR #14 (651) #16 MESH DESCRIPTOR Electric Stimulation Therapy EXPLODE ALL TREES (5281) #17 (((neuro* or musc* or electr* or nerve) adj3 stim*)):TI,AB,KY (9513) #18 (electrotherap* or myostim* or electrostim* or electroneurostim* or neurostim* or EMS or NMES):TI,AB,KY (2458) #19 #16 OR #17 OR #18 (13201) #20 #15 AND #19 (21)
MEDLINE (Ovid Online)
1 Patellofemoral Pain Syndrome/ (658) 2 Patella/ or Knee Joint/ or Knee/ (63784) 3 Arthralgia/ or Pain/ (131605) 4 2 and 3 (3930) 5 anterior knee pain.tw. (1429) 6 ((patell* or femoropatell* or femoro‐patell* or retropatell*) adj2 (pain or syndrome or dysfunction)).tw. (2305) 7 ((lateral compression or lateral facet or lateral pressure or odd facet) adj syndrome).tw. (23) 8 ((chondromalac* or chondropath*) adj2 (knee*1 or patell* or femoropatell* or femoro‐patell* or retropatell*)).tw. (548) 9 Chondromalacia Patellae/ (79) 10 or/1,4‐9 (7209) 11 exp Electric Stimulation Therapy/ (70205) 12 ((neuro* or musc* or electr* or nerve) adj3 stim*).tw. (118525) 13 electrotherap*.tw. (1222) 14 myostim*.tw. (92) 15 electrostim*.tw. (3230) 16 electroneurostim*.tw. (42) 17 neurostim*.tw. (2230) 18 (EMS or NMES).tw. (10788) 19 or/11‐18 (186863) 20 10 and 19 (96) 21 Randomized controlled trial.pt. (462116) 22 Controlled clinical trial.pt. (94040) 23 randomized.ab. (403322) 24 placebo.ab. (188773) 25 Drug therapy.fs. (1991827) 26 randomly.ab. (280166) 27 trial.ab. (422340) 28 groups.ab. (1725939) 29 or/21‐28 (4099404) 30 exp Animals/ not Humans/ (4396757) 31 29 not 30 (3544787) 32 20 and 31 (47)
Embase (Ovid Online)
1 Patellofemoral Pain Syndrome/ (1016) 2 Patellofemoral Joint/ or Patella/ or Knee/ (64001) 3 Arthralgia/ or Pain/ (306136) 4 3 and 2 (8189) 5 Knee Pain/ (11759) 6 anterior knee pain.tw. (1578) 7 ((patell* or femoropatell* or femoro‐patell* or retropatell*) adj2 (pain or syndrome or dysfunction)).tw. (2630) 8 ((lateral compression or lateral facet or lateral pressure or odd facet) adj syndrome).tw. (26) 9 ((chondromalac* or chondropath*) adj2 (knee*1 or patell* or femoropatell* or femoro‐patell* or retropatell*)).tw. (715) 10 Patella Chondromalacia/ (738) 11 or/1,4‐10 (22056) 12 Neuromuscular Electrical Stimulation/ (1165) 13 electrostimulation therapy/ or nerve stimulation/ (40993) 14 electrostimulation/ (75363) 15 ((neuro* or musc* or electr* or nerve) adj3 stim*).tw. (139859) 16 electrotherap*.tw. (1722) 17 myostim*.tw. (112) 18 electrostim*.tw. (3841) 19 electroneurostim*.tw. (55) 20 neurostim*.tw. (3599) 21 (EMS or NMES).tw. (14372) 22 or/12‐21 (211498) 23 11 and 22 (378) 24 Randomized controlled trial/ (442254) 25 Clinical trial/ (918094) 26 Controlled clinical trial/ (428691) 27 Randomization/ (73053) 28 Single blind procedure/ (26265) 29 Double blind procedure/ (136826) 30 Crossover procedure/ (50503) 31 Placebo/ (303176) 32 Prospective study/ (366226) 33 ((clinical or controlled or comparative or placebo or prospective* or randomi#ed) adj3 (trial or study)).tw. (1000672) 34 (random* adj7 (allocat* or allot* or assign* or basis* or divid* or order*)).tw. (244729) 35 ((singl* or doubl* or trebl* or tripl*) adj7 (blind* or mask*)).tw. (201706) 36 (cross?over* or (cross adj1 over*)).tw. (87005) 37 ((allocat* or allot* or assign* or divid*) adj3 (condition* or experiment* or intervention* or treatment* or therap* or control* or group*)).tw. (331952) 38 RCT.tw. (23420) 39 or/24‐38 (2408554) 40 Case Study/ or Abstract Report/ or Letter/ (1055033) 41 39 not 40 (2363242) 42 23 and 41 (123)
PEDro
Advanced search option
Abstract & Title: *Patellofemoral Therapy: electrotherapies, heat, cold Problem: no selection Body part: lower leg or knee Subdiscipline: no selection Method: clinical trial Match all search terms (AND) (16)
Abstract & Title: *Chondromalacia Therapy: electrotherapies, heat, cold Problem: no selection Body part: lower leg or knee Subdiscipline: no selection Method: clinical trial Match all search terms (AND) (3)
CINAHL (Ebsco)
S1 (MH "Patellofemoral Pain Syndrome") (1,105) S2 TX anterior knee pain (691) S3 TX (patell* or femoropatell* or femoro‐patell* or retropatell*) n2 (pain or syndrome or dysfunction) (1,646) S4 TX ((lateral compression or lateral facet or lateral pressure or odd facet) n2 syndrome) (11) S5 TX (chondromalac* or chondropath*) n2 (knee*1 or patell* or femoropatell* or femoro‐patell* or retropatell*) (113) S6 (MH "Chondromalacia Patella") (67) S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6 (2,188) S8 (MH "Electric Stimulation") OR (MH "Electrical Stimulation, Neuromuscular") (9,097) S9 (neuro* or musc* or electr* or nerve) n3 stim* (17,142) S10 TX electrotherap* or myostim* or electrostim* or electroneurostim* or neurostim* or EMS or NMES (16,328) S11 S8 OR S9 OR S10 (31,931) S12 S7 AND S11 (43) S13 (MH "Clinical Trials+") (213,292) S14 (MH "Evaluation Research+") (53,474) S15 (MH "Comparative Studies") (116,883) S16 (MH "Crossover Design") (14,538) S17 PT Clinical Trial (80,011) S18 (MH "Random Assignment") (42,824) S19 S13 or S14 or S15 or S16 or S17 or S18 (350,510) S20 TX ((clinical or controlled or comparative or placebo or prospective or randomi?ed) and (trial or study)) (1,043,596) S21 TX (random* and (allocat* or allot* or assign* or basis* or divid* or order*)) (96,806) S22 TX ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)) (912,205) S23 TX ( crossover* or 'cross over' ) or TX cross n1 over (20,051) S24 TX ((allocat* or allot* or assign* or divid*) and (condition* or experiment* or intervention* or treatment* or therap* or control* or group*)) (131,291) S25 S20 or S21 or S22 or S23 or S24 (1,784,630) S26 S19 or S25 (1,794,552) S27 S12 AND S26 (29)
SPORTDiscus (Ebsco)
S1 DE "PLICA syndrome" (400) S2 TX anterior knee pain (707) S3 TX (patell* or femoropatell* or femoro‐patell* or retropatell*) n2 (pain or syndrome or dysfunction) (1,487) S4 TX ((lateral compression or lateral facet or lateral pressure or odd facet) n2 syndrome) (11) S5 TX (chondromalac* or chondropath*) n2 (knee*1 or patell* or femoropatell* or femoro‐patell* or retropatell*) (247) S6 DE "CHONDROMALACIA patellae" (176) S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6 (2,115) S8 DE "ELECTRIC stimulation" (3,435) S9 TX ((neuro* or musc* or electr* or nerve) n3 stim*) (7,526) S10 TX electrotherap* or myostim* or electrostim* or electroneurostim* or neurostim* or EMS or NMES (1,955) S11 S8 OR S9 OR S10 (8,570) S12 S7 AND S11 (40) S13 TX ( (clinic* N3 trial) or (controlled N3 trial) or (comparative N3 trial) or (placebo N3 trial) or (prospective N3 trial) or (randomi?ed N3 trial) ) or TX ( (clinic* N3 study) or (controlled N3 study) or (comparative N3 study) or (placebo N3 study) or (prospective N3 study) or (randomi?ed N3 study) ) (74,084) S14 (random* N7 allot*) or (random* N7 assign*) or (random* N7 basis*) or (random* N7 divid*) or (random* N7 order*) (10,220) S15 TX ( (singl* N7 blind*) or (doubl* N7 blind*) or (trebl* N7 blind*) or (tripl* N7 blind*) ) or TX ( (singl* N7 mask*) or (doubl* N7 mask*) or (trebl* N7 mask*) or (tripl* N7 mask*) ) (6,348) S16 TX (cross#over*) or TX (cross N1 over*) (5,067) S17 TX randomi?ed control* trial* (12,639) S18 TX ( (allocat* N3 condition*) or (allocat* N3 experiment*) or (allocat* N3 intervention*) or (allocat* N3 treatment*) or (allocat* N3 therap*) or (allocat* N3 control*) or (allocat* N3 group*) ) or TX ( (allot* N3 condition*) or (allot* N3 experiment*) or (allot* N3 intervention*) or (allot* N3 treatment*) or (allot* N3 therap*) or (allot* N3 control*) or (allot* N3 group*) ) or TX ( (assign* N3 condition*) or (assign* N3 experiment*) or (assign* N3 intervention*) or (assign* N3 treatment*) or (assign* N3 therap*) or (assign* N3 control*) or (assign* N3 group*) ) or TX ( (divid* N3 condition*) or (divid* N3 experiment*) or (divid* N3 intervention*) or (divid* N3 treatment*) or (divid* N3 therap*) or (divid* N3 control*) or (divid* N3 group*) ) (11,324) S19 TX placebo* (9,165) S20 S13 or S14 or S15 or S16 or S17 or S18 or S19 (91,979) S21 S12 AND S20 (14)
AMED (Ovid Online)
1 patellofemoral pain syndrome/ (120) 2 anterior knee pain.tw. (141) 3 ((patell* or femoropatell* or femoro‐patell* or retropatell*) adj2 (pain or syndrome or dysfunction)).tw. (526) 4 ((lateral compression or lateral facet or lateral pressure or odd facet) adj syndrome).tw. (1) 5 ((chondromalac* or chondropath*) adj2 (knee*1 or patell* or femoropatell* or femoro‐patell* or retropatell*)).tw. (27) 6 or/1‐5 (647) 7 electric stimulation/ (1221) 8 ((neuro* or musc* or electr* or nerve) adj3 stim*).tw. (4141) 9 electrotherap*.tw. (953) 10 myostim*.tw. (4) 11 electrostim*.tw. (107) 12 electroneurostim*.tw. (1) 13 neurostim*.tw. (22) 14 (EMS or NMES).tw. (192) 15 or/7‐14 (4840) 16 6 and 15 (16)
LILACS (BIREME)
MH Patellofemoral Pain Syndrome or MH Chondromalacia Patellae OR (TW rótula or TW patela or TW patell$ or TW femoropatell$ or TW femoro‐patell$ or TW retropatell$) AND (TW pain or TW dolor or TW dor or TW syndrome or TW síndrome or TW dysfunc$ or TW disfunç$)) OR ((TW chondromalac$ or TW chondropath$) AND (TW knee$ or TW rodilla or TW joelho or TW patell$ or TW femoropatell$ or TW femoro‐patell$ or TW retropatell$)) [Words] AND MH Electric Stimulation Therapy OR ((TW neuro$ or TW musc$ or TW electr$ or TW elétric$ or TW eléctrico or TW nerv$) AND TW stim$) OR TW electrotherapy$ or TW myostim$ or TW electrostim$ or TW electroneurostim$ or TW neurostim$ or TW EMS or TW NMES [Words] (9)
WHO International Clinical Trials Registry Platform (ICTRP)
patellofemoral AND electric* OR patellofemoral AND neuromuscular OR patellofemoral AND stimulat* OR patellofemoral AND NMES OR chondromalacia AND electric* OR chondromalacia AND neuromuscular OR chondromalacia AND stimulat* OR chondromalacia AND NMES OR anterior knee pain AND electric* OR anterior knee pain AND neuromuscular OR anterior knee pain AND stimulat* OR anterior knee pain AND NMES (11)
ClinicalTrials.gov
(patellofemoral OR Patellofemoral Pain Syndrome OR Chondromalacia OR Anterior knee pain) AND (electrical OR electric OR neuromuscular OR stimulation OR NMES) (44)
ISRCTN Registry
(patellofemoral OR patellofemoral pain syndrome OR chondromalacia OR anterior knee pain) AND (Electrical Stimulation) [All Registers] (2)
Data and analyses
Comparison 1. NMES versus placebo (sham device).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Knee pain during activities (end of the treatment, single 15‐minute NMES session): VAS scale: 0 to 10; higher score = worse pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain during a single‐leg squat | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain during a lateral step‐down | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. NMES (+ other intervention) versus no NMES (+ same other intervention).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Knee pain (end of the treatment, 3 to 12 weeks): VAS scale: 0 to 10; higher score = worse pain | 3 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐1.63 [‐2.23, ‐1.02] |
| 2 Knee pain during activities (end of the treatment, 6 weeks): VAS scale: 0 to 10; higher score = worse pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Pain during step‐down | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Pain during step‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Pain during squat | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Knee function (end of the treatment, 3 and 6 weeks): Cincinnati Knee Rating System and LEFS; higher score = better function | 2 | 70 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.37 [‐0.11, 0.84] |
| 4 Change score for KPS (end of treatment, 12 weeks) (0 to 100; higher score = better function) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Good or normal muscle strength (end of the treatment, 6 weeks): grades 4 to 5 Lovett’s manual muscle scale | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Muscle strength (end of the treatment, 12 weeks): isokinetic dynamometer (N) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Isometric strength with 30° knee flexion | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Isometric strength with 60° knee flexion | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. NMES versus exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Knee function (end of the treatment, 4 weeks): Arpège function scale: 0 to 18; higher score = better function | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Muscle strength (end of the treatment, 4 weeks): isokinetic dynamometer (Nm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Isokinetic dynamometer at 30°/s | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Isokinetic dynamometer at 300°/s | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. NMES (simultaneous frequencies) versus control NMES (sequential or fixed frequencies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Knee pain (end of the treatment, 6 weeks): VAS scale: 0 to 10; higher score = worse pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Simultaneous versus sequential delivery | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Knee function (end of the treatment, 6 weeks): KPS: 0 to 100 scale; higher score = better function | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐6.79, 4.47] |
| 2.1 Simultaneous versus sequential delivery | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐5.90 [‐16.14, 4.34] |
| 2.2 Simultaneous versus fixed delivery | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐5.84, 7.64] |
| 3 Functional performance (end of the treatment, 6 weeks): number of steps up and down until pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Simultaneous versus sequential delivery | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Quadriceps isometric muscle strength (end of the treatment, 6 weeks): dynamometer at 90°/s (Nm) | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐1.15 [‐16.24, 13.94] |
| 4.1 Simultaneous versus sequential delivery | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 3.40 [‐25.83, 32.63] |
| 4.2 Simultaneous versus fixed delivery | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐20.42, 14.82] |
| 5 Quadriceps isokinetic muscle strength (end of the treatment, 6 weeks): dynamometer at 90°/s (Nm) | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐7.28 [‐24.45, 9.89] |
| 5.1 Simultaneous versus sequential delivery | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 6.10 [‐30.70, 42.90] |
| 5.2 Simultaneous versus fixed delivery | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐11.0 [‐30.41, 8.41] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akarcali 2002.
| Methods | Randomised controlled trial Trial protocol registration: not reported |
|
| Participants |
Country: Turkey Setting: Hacettepe University, School of Physiotherapy and Rehabilitation, and Department of Orthopaedics and Traumatology, Ankara Data collection period: not reported Inclusion criteria: anterior knee pain (longer than 2 months), positive patellar compression test, age between 15 and 45 years, negative findings in the clinical examination of knee ligaments, bursae, menisci, synovial plicae, hamstring, quadriceps, and patellar tendons Exclusion criteria: history or clinical evidence of patellofemoral dislocation, subluxation, or severe osteoarthritis, X‐rays showing lateral displacement of the patella Mean duration of symptoms: 15.74 ± 9.31 months 62.5% of bilateral complaints, but only the most symptomatic knee was treated Study participants: 44 people with patellofemoral pain assigned and 42 assessed
Mean age (SD): 39.0 (9.6) years Gender (number of women/men): 31/13 |
|
| Interventions |
Comparison: NMES + other intervention (exercise) versus no NMES + same other intervention Treatment duration: 6 weeks Treatment setting: outpatient rehabilitation programme Details of interventions:
|
|
| Outcomes |
Outcomes analysed in the study and used in this review:
Follow‐up assessments: at 6 weeks (at end of treatment) |
|
| Notes | Description of condition: patellofemoral pain syndrome The trial authors provided additional information on random sequence generation, allocation concealment, and blinding (participants, personnel, and assessors) via email (10 March 2015). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The subjects were randomly introduced into either HVPGS and exercise, or only exercise (control group)" Authors' reply: "At the time of study, it was feasible for us to use coin tossing technique to assign patients to either HVPGS and exercise group, or only exercise (control group) in consecutively." |
| Allocation concealment (selection bias) | Unclear risk | Available information did not permit judgement. Authors' reply: "Assigning patients to intervention groups was done by the first author with coin tossing technique to prevent second and third author/researcher from influencing concealing the allocation sequence in the study." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Available information did not permit judgement. Authors' reply: "Patients were also blinded to their intervention groups. Exercise programs were applied by physiotherapist (third researcher/author)" However, since no sham/placebo was used, it is unlikely that the blinding was kept. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Available information did not permit judgement. Authors reply: "Pre and post treatment muscle strength and VAS measurements were evaluated by the same physiotherapist (second researcher) who is blind from the patient’s group" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “Two patients of the HVPGS group did not complete the study and their data results were excluded” (4.5% dropout) No reasons were provided. We are uncertain of the potential effect of these missing data. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. It is not clear if the results included all expected outcomes (e.g. retropatellar pain during activities: steps up and down and squatting). This study did not consider adverse event as an outcome. |
| Other bias | Low risk | The study appears to be free of other sources of bias. Baseline characteristics were balanced between groups. |
Bily 2008.
| Methods | Randomised controlled trial Trial protocol registration: not reported |
|
| Participants |
Country: Austria Setting: Department of Physical Medicine and Rehabilitation, Wilhelminenspital Vienna and Core Unit for Medical Statistics and Informatics, Section of Clinical Biometrics, Medical University of Vienna Data collection period: between June 2003 and August 2005 Inclusion criteria: bilateral anterior knee pain for 6 to 120 months and at least 3 of the following 4 clinical criteria: pain associated with prolonged sitting with bended knees, descending stairs, kneeling and squatting, or sports activities Exclusion criteria: clinical evidence of patellar dislocation or subluxation, periarticular bursitis or tendonitis, ligamentous instability, or intra‐articular pathology. Before beginning therapy, all participants were thoroughly clinically examined. Those who did not reveal any obvious reason for a systemic disorder like patellar or lower‐extremity alignment problems or benign joint hypermobility syndrome were not excluded. To rule out osteoarthritic changes or hypoplastic femoral trochlea, radiographs were performed. Pregnancy, a history of knee surgery, or oral or intra‐articular administration of drugs within the last 3 months Mean duration of symptoms: 14 months (6 to 24) Only bilateral complaints (but did not clarify which knee was treated and assessed) Study participants: 38 people with patellofemoral pain assigned and 29 assessed
Mean age (SD): 25.4 (6.7) years Gender (number of women/men): 24/14 |
|
| Interventions |
Comparison: NMES + other intervention (exercise) versus no NMES + same other intervention Treatment duration: 12 weeks Treatment setting: at home Details of interventions:
|
|
| Outcomes |
Outcomes analysed in the study and used in this review:
Follow‐up assessments: at 12 weeks (at end of treatment) and 1 year |
|
| Notes | Description of condition: patellofemoral pain syndrome The trial authors provided additional information on random sequence generation, allocation concealment and blinding (participants, personnel, and assessors) via email (16 March 2015). Additionally, the trial authors provided information regarding which knee was treated and assessed (9 August 2016). Authors' reply: "EMS was applied on both knees" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors' reply: "Random allocation of the patients to the 2 treatment groups was performed by using shuffled sealed envelopes” |
| Allocation concealment (selection bias) | Unclear risk |
Authors' reply: "With sealed envelopes" The authors did not mention if the envelopes were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: probably not done because the interventions were different between groups.
Personnel: probably not done due to the nature of the intervention. Authors' reply: No measures were used to ensure blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Available information did not permit judgement. Authors' reply: No measures were used to ensure blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Some participants did not complete the study (5.2% dropout at the end of the treatment and 19% dropout in the long term), and it is unclear how the authors dealt with these missing data. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. It is not clear if the results included all expected outcomes. This study did not consider adverse event as an outcome. |
| Other bias | Unclear risk | The study appears to be free of other sources of bias. However, although baseline characteristics were balanced between groups, muscle strength before training was greater in the NMES group. |
Callaghan 2001.
| Methods | Randomised controlled trial Trial protocol registration: not reported |
|
| Participants |
Country: United Kingdom Setting: Centre for Rehabilitation Science, Manchester Royal Infirmary, Manchester Data collection period: not reported Inclusion criteria: atraumatic peripatellar pain (greater than 6 months and not longer than 3 years); patellofemoral pain was provoked by 1 of the following alone or in combination: prolonged sitting, deep squatting, kneeling, ascending or descending stairs; quadriceps cross‐sectional area differences between affected and unaffected limb greater than 4% Exclusion criteria: epilepsy, cancer, cardiac pacemaker, suspected heart problem, recent surgery (not including arthroscopy). In order to exclude abnormal foot and ankle pronation as the cause of patellofemoral pain, the participants were screened by kinetic gait analysis to detect abnormal values of mediolateral force. Pain from the lumbar spine and hip joint, severe leg length discrepancy, knee ligament, quadriceps tendon, and meniscal pathologies, Hoffa’s syndrome, medial plica syndrome, femoral anteversion and tibial torsion Mean duration of symptoms: not reported All unilateral complaints Study participants: 16 people with patellofemoral pain assigned and 14 assessed
Mean age (SD): 29.6 (5.9) years Gender (number of women/men): 12/2 |
|
| Interventions |
Comparison: NMES (simultaneous mixed frequencies) versus control NMES (sequential mixed frequencies) Treatment duration: 6 weeks Treatment setting: at home Details of interventions:
|
|
| Outcomes |
Outcomes analysed in the study and used in this review:
Follow‐up assessments: 6 weeks (at end of treatment) |
|
| Notes | Description of condition: patellofemoral pain syndrome The trial authors provided additional information on NMES parameters via email (9 March 2015). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated by computer program to either the experimental stimulation or standard stimulation treatment regimes" |
| Allocation concealment (selection bias) | Unclear risk | Available information did not permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants: Available information did not permit judgement. Personnel: Available information did not permit judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Available information did not permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 participants (12% dropout) did not complete the study and were excluded from the analysis. |
| Selective reporting (reporting bias) | Low risk | No study protocol available. The authors considered only 1 adverse event (muscle fatigue); however, this is 1 of the most important adverse events in clinical practice. |
| Other bias | Unclear risk | Unable to judge because it is unclear if the groups were similar regarding relevant characteristics at baseline (age, gender, level of pain) |
Callaghan 2004.
| Methods | Randomised controlled trial Trial protocol registration: not reported |
|
| Participants |
Country: United Kingdom Setting: Centre for Rehabilitation Science, Manchester Royal Infirmary, Manchester Data collection period: not reported Inclusion criteria: atraumatic peripatellar pain (greater than 6 months and not longer than 3 years), patellofemoral pain was provoked by 1 of the following alone or in combination: prolonged sitting, deep squatting, kneeling, ascending or descending stairs Exclusion criteria: epilepsy, cancer, cardiac pacemaker, suspected heart problem, recent surgery (not including arthroscopy). In order to exclude abnormal foot and ankle pronation as the cause of patellofemoral pain, the participants were screened by kinetic gait analysis to detect abnormal values of mediolateral force. Presence of other lower extremity dysfunction that could account for the knee symptoms Mean duration of symptoms: not reported All unilateral complaints Study participants: 80 people with patellofemoral pain assigned and 74 assessed
Mean age (SD): 35 (11.4) years Gender (number of women/men): 43/31 |
|
| Interventions |
Comparison: NMES (simultaneous mixed frequencies) versus control NMES (fixed frequency) Treatment duration: 6 weeks Treatment setting: at home Details of interventions:
Stimulation intensity for both groups was the highest comfortably tolerable for all the participants. |
|
| Outcomes |
Outcomes analysed in the study and used in this review:
Follow‐up assessments: 6 weeks (at end of treatment) |
|
| Notes | Description of condition: patellofemoral pain syndrome
3 participants were excluded due to device failure. The trial authors provided additional information on NMES parameters via email (9 March 2015). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by consulting 4 computer‐generated randomization lists, 1 for each of the 4 stratified groups." |
| Allocation concealment (selection bias) | Unclear risk | Available information did not permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants: Available information did not permit judgement. Personnel: Quote: "To preserve the blinding for the study, both devices were fully explained to and demonstrated on the patients by an independent investigator" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The lead investigator who examined and measured the patients was not part of the randomisation process, thus ensuring blindness to the stimulator allocation" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Some participants did not complete the study (12% dropout), and their data were excluded from the analysis. Losses not balanced between groups. |
| Selective reporting (reporting bias) | Low risk | No study protocol available. The authors considered only 1 adverse event (muscle fatigue); however, this is 1 of the most important adverse events in clinical practice. |
| Other bias | Low risk | The study appears to be free of other sources of bias. Baseline characteristics were balanced between groups. |
Glaviano 2016.
| Methods | Double‐blinded, randomised sham‐controlled trial Trial protocol registration: not reported |
|
| Participants |
Country: United States Setting: laboratory (single NMES session) Data collection period: not described Inclusion criteria: age between 15 and 65, atraumatic knee pain (greater than 3 months), pain with more than 2 of the following activities: jumping, kneeling, prolonged sitting, quadriceps contraction, running, squatting, or stair climbing or when pressure was placed on the patella. Participants were required to score less than 85 of 100 on the Anterior Knee Pain Scale. Exclusion criteria: previous knee surgery, ligamentous instability, meniscal injury, or other sources of anterior knee pain, such as patellar tendinitis, bursitis, or patella subluxation. Contraindications to electrical stimulation: implanted biomedical devices, history of neuropathy, muscular abnormality, hypersensitivity to electrical stimulation, or active infection where the electrodes would be placed. Mean duration of symptoms: not reported People who presented bilateral complaints were included, but only the most symptomatic knee was treated. Study participants: 22 people with patellofemoral pain assigned and assessed
Mean age (SD): 26.0 (7.9) years Gender (number of women/men): 15/7 |
|
| Interventions |
Comparison: NMES versus placebo Treatment duration: single session (15‐minute treatment) Treatment setting: laboratory Details of interventions:
At the end of the intervention, the PENS electrodes were removed, and the participants were instructed to perform 2 functional movements. Outcome data were collected for both tasks.
|
|
| Outcomes |
Outcomes analysed in the study and used in this review:
Follow‐up assessments: immediately at the end of the single‐session treatment (after completing the single‐leg squat and lateral step‐down) |
|
| Notes | Description of condition: patellofemoral pain The trial authors provided additional information on random sequence generation, allocation concealment, and blinding (participants, personnel, and assessors) via email (12 August 2016). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Available information did not permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "One researcher concealed treatment interventions in envelopes, which were randomly allocated to participants before enrolment." The authors did not mention if the envelopes were sealed and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "sham controlled laboratory study" Since the authors did not exclude people who had received previous NMES therapy, it is difficult to affirm that the blinding was effective. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "At the conclusion of the 15‐minute treatment, electrodes were removed, the blinded researcher left the laboratory, and the primary researcher returned to the laboratory to conduct post‐intervention assessments" It is not clear if the outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. |
| Selective reporting (reporting bias) | High risk | No study protocol available. It is unclear if the results included all expected outcomes. This study did not consider adverse event as an outcome. Another report of this study that included a subgroup of 15 females presented the same baseline characteristics (age, height, mass, anterior knee pain score, and VAS pain knee) as those for the 22 participants in the study. |
| Other bias | Unclear risk | Although the baseline characteristics were balanced between groups, we are uncertain whether these are correct. The pain scores (1.9 in both groups) were below the threshold ("more than 2") for inclusion in the trial. |
Gobelet 1992.
| Methods | Randomised controlled trial Trial protocol registration: not reported |
|
| Participants |
Country: Switzerland Setting: Physical Medicine and Rehabilitation, Sion Hospital, Switzerland and Orthopaedic Hospital of Lausanne Data collection period: not reported Inclusion criteria: non‐traumatic retropatellar painful chondropathy, without radiological lesion, with or without Wiberg patellar dysplasia type I or II Exclusion criteria: Wiberg dysplasia type III Mean duration of symptoms: not reported % of bilateral complaints not reported. Study participants: 120 people with patellofemoral pain assigned and 94 assessed
Mean age (SD): 26.4 (11.2) years Gender (number of women/men): 50/44 |
|
| Interventions |
Comparison: NMES versus exercise (isokinetic) versus exercise (isometric) Treatment duration: 4 weeks Treatment setting: at home Details of co‐interventions:
|
|
| Outcomes |
Outcomes analysed in the study and used in this review:
Follow‐up assessments: 4 weeks (at end of treatment) |
|
| Notes | Description of condition: retropatellar chondropathy It was not possible to obtain additional data on random sequence generation, allocation concealment, and blinding (participants, personnel, and assessors) after attempt to contact authors by email (9 March 2015). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Available information did not permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Available information did not permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: probably not done because the interventions were different between groups. Personnel: probably not done due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The evaluation of the isokinetic strength and Arpège criteria was performed by a neutral observer. This investigator does not belong to the rehabilitation team" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Some participants did not complete the study (22% dropout) and it is unclear how the authors dealt with these missing data. Losses imbalanced between the groups. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. It is unclear if the results included all expected outcomes. This study did not consider adverse event as an outcome. |
| Other bias | Unclear risk | This study provided no information on the side(s) affected, and it is unclear which knee was treated. |
Kaya 2013.
| Methods | Randomised controlled trial Trial protocol registration: not reported |
|
| Participants |
Country: Turkey Setting: Faculty of Medicine, Department of Sports Medicine, Hacettepe University, Ankara Data collection period: not reported Inclusion criteria: pain longer than 6 months, presence of retropatellar pain, crepitation and pain in patellar grinding, age between 18 to 40 years, no abnormalities on magnetic resonance imaging Exclusion criteria: history or clinical evidence of patellofemoral dislocation, subluxation, or osteoarthritis, presence in the clinical examination of injury or dysfunction to the knee ligaments, bursae, menisci, and synovial plicae, history of lower extremity surgery, radiographic evidence of osteoarthritis in any compartments of the knee joint. Mean duration of symptoms: not reported All unilateral complaints Study participants: 30 participants with patellofemoral pain assigned and assessed
Mean age (SD): 42.7 (10.0) years Gender (number of women/men): only women were included. |
|
| Interventions |
Comparison: NMES + other intervention (exercise + taping) versus no NMES + same other intervention Treatment duration: 6 weeks Treatment setting: outpatient rehabilitation programme Details of interventions:
|
|
| Outcomes |
Outcomes analysed in the study and used in this review:
Follow‐up assessments: short term (6 weeks) |
|
| Notes | Description of condition: patellofemoral pain syndrome This trial had 3 treatment arms. Data from 1 group (NMES alone) were not included in this review. It was not possible to obtain additional data on random sequence generation, allocation concealment, and blinding (participants, personnel, and assessors) after attempt to contact the authors by email (10 March 2015). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The patients were randomly allocated into three groups by the second author who was blinded in measurements and assessments” The sentence above is unclear about the method used. |
| Allocation concealment (selection bias) | Unclear risk | Available information did not permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: probably not done because the interventions were different between groups. Personnel: probably not done due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The patients were randomly allocated into three groups by the second author who was blinded in measurements and assessments” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients completed the rehabilitation program and all assessment procedures" |
| Selective reporting (reporting bias) | High risk | No study protocol available. It is unclear if the results included all expected outcomes. This study did not consider adverse event as an outcome. The conclusions for pain were contradicted by the data, raising doubts regarding their reliability. |
| Other bias | High risk | Baseline characteristics were balanced between groups, except for pain, which was significantly lower in the control group for all 3 functional activities. |
Tunay 2003.
| Methods | Randomised controlled trial Trial protocol registration: not reported |
|
| Participants |
Country: Turkey Setting: Hacettepe University, School of Physiotherapy and Rehabilitation, Sports Physiotherapy and Gulhane Military Medical Academy, Department of Orthopaedics and Traumatology, Ankara Data collection period: not reported Inclusion criteria: unilateral patellofemoral pain lasting more than 1 month Exclusion criteria: history or clinical findings of patellar dislocation, meniscal or ligamentous injury, synovial plicae, knee surgery or trauma Mean duration of symptoms: 1.8 years (range 1 month to 5 years) All unilateral complaints Study participants: 40 people with patellofemoral pain assigned and assessed
Mean age (SD): 32.9 (7.3) years Gender (number of women/men): no information |
|
| Interventions |
Comparison: NMES + other intervention (exercise, taping, and ice) versus no NMES + same other intervention Treatment duration: 3 weeks (total of 15 sessions) Treatment setting: outpatient rehabilitation programme Details of interventions:
|
|
| Outcomes |
Outcomes analysed in the study and used in this review:
Follow‐up assessments: short term (3 weeks) |
|
| Notes | Description of condition: patellofemoral pain syndrome This trial had 4 treatment arms. Data from 2 groups (NMES, ice, medial patellar glide and exercises; ice and home exercises) were not included in this review. The trial authors provided additional information on NMES parameters and exercise programme via email, confirming that "same exercises were given to the all groups" (12 March 2015). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Available information did not permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Available information did not permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: probably not done because the interventions were different between groups. Personnel: probably not done due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Available information did not permit judgement, but seems unlikely given the nature of the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is not clear if there were losses. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. It is unclear if the results included all expected outcomes. This study did not consider adverse event as an outcome. |
| Other bias | Low risk | The study appears to be free of other sources of bias. Baseline characteristics were balanced between groups. |
EMG: electromyography HVPGS: high‐voltage pulsed galvanic simulation mA: milliamp NMES: neuromuscular electrical stimulation PENS: patterned electrical neuromuscular stimulation SD: standard deviation VAS: visual analogue scale VMO: vastus medialis oblique
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dursun 2001 | The purpose of this randomised controlled trial was to investigate the effects of electromyographic biofeedback treatment in people with patellofemoral pain (biofeedback group versus control group). No neuromuscular electrical stimulation intervention. |
| Kuru 2012 | This was not a randomised or quasi‐randomised controlled trial. |
Characteristics of ongoing studies [ordered by study ID]
NCT02441712.
| Trial name or title | Rehabilitation with patterned electrical neuromuscular stimulation for patients with patellofemoral pain (PENS for PFP) |
| Methods | Randomised controlled trial |
| Participants |
Country: United States Setting: University of Virginia, Charlottesville Data collection period: this study was recruiting participants (verified May 2015) Inclusion criteria: insidious onset of symptoms, presence of peripatellar or retropatellar knee pain during at least 2 of the following functional activities: stair ascent or descent, running, kneeling, squatting, prolonged sitting, jumping; pain for more than 3 months (> 3/10 on VAS); 85 or less on the Anterior Knee Pain Scale Exclusion criteria: previous knee surgery; internal derangement; ligamentous instability, other sources of anterior knee pain (patella tendonitis, Osgood Schlatter, knee plica, etc.), neurological involvement, any biomedical device; muscular abnormalities; currently pregnant; hypersensitivity to electrical stimulation; active infection over the site of the electrode placement Study participants: people with patellofemoral pain, ages between 15 and 40 years, both genders Estimated sample: 32 participants |
| Interventions |
Comparison: NMES versus placebo Treatment duration: single session (15‐minute treatment) Treatment setting: outpatient rehabilitation programme Details of interventions:
|
| Outcomes | Data collection was planned for 4 weeks.
|
| Starting date | 4 May 2015 |
| Contact information | Neal Glaviano, MEd, ATC University of Virginia, Charlottesville, Virginia, United States 22902, 434‐924‐6184; email: ng2w@virginia.edu |
| Notes | A related laboratory study testing the same intervention from the same team is available (Glaviano 2016). A check on the status of this trial on 1 May 2017 found that "The recruitment status of the study is unknown. The completion data has passed and the status has not been verified in more than two years." |
NMES: neuromuscular electrical stimulation PENS: patterned electrical neuromuscular stimulation VAS: visual analogue scale
Differences between protocol and review
Consistent with the International Patellofemoral Pain consensus statements published in 2014, we replaced the terminology 'patellofemoral pain syndrome' with 'patellofemoral pain' in the written text.
As noted by the external referee, PFP is a multifactorial condition, and people with PFP can take several months of treatment to gain sustained improvement in outcomes. We therefore adjusted the 'Timing of outcome measurement' section from: at the end of the treatment, up to three months after treatment (short term), and over three months after treatment (long term) to: up to three months (short term), three to up to 12 months (medium term), and 12 months or above (long term). We adjusted the start point for follow‐up to the start of treatment (postrandomisation) and note that the timing of the short‐term follow‐up usually coincided with the end of treatment.
Contributions of authors
Ana Luiza C Martimbianco: drafted the protocol and review, methodological and content issues Maria R Torloni: drafted the protocol and review, content issues, language (English) issues Brenda NG Andriolo: drafted the protocol and review, methodological and content issues Gustavo Porfirio: drafted the protocol and review, content issues Rachel Riera: drafted the protocol and review, methodological issues
Sources of support
Internal sources
Brazilian Cochrane Centre, Brazil.
External sources
No sources of support supplied, Other.
Declarations of interest
Ana Luiza C Martimbianco: none known Maria R Torloni: none known Brenda NG Andriolo: none known Gustavo Porfirio: none known Rachel Riera: none known
Edited (no change to conclusions)
References
References to studies included in this review
Akarcali 2002 {published data only}
- Akarcali I. Additional information on random sequence generation, allocation concealment and blinding [personal communication] Email to: AL Martimbianco 10 March 2015.
- Akarcali I, Tugay N, Kaya D, Atay A, Doral MN. The role of high voltage electrical stimulation in the rehabilitation of patellofemoral pain. The Pain Clinic 2002;14(3):207–12. [Google Scholar]
Bily 2008 {published data only}
- Bily W. Additional information on random sequence generation, allocation concealment and blinding (participants, personnel and assessors) [personal communication]. Email to: AL Martimbianco 16 March 2015.
- Bily W. Additional information regarding which knee was treated and assessed [personal communication]. Email to: AL Martimbianco 9 August 2016.
- Bily W, Trimmel L, Mödlin M, Kaider A, Kern H. Training program and additional electric muscle stimulation for patellofemoral pain syndrome: a pilot study. Archives of Physical Medicine and Rehabilitation 2008;89:1230‐6. [DOI] [PubMed] [Google Scholar]
Callaghan 2001 {published data only}
- Callaghan MJ. Additional information on NMES parameters [personal communication]. Email to: AL Martimbianco 9 March 2015.
- Callaghan MJ, Oldham JA, Winstanley J. A comparison of two types of electrical stimulation of the quadriceps in the treatment of patellofemoral pain syndrome. A pilot study. Clinical Rehabilitation 2001;15:637–46. [DOI] [PubMed] [Google Scholar]
Callaghan 2004 {published data only}
- Callaghan M. Additional information on NMES parameters [personal communication]. Email to: AL Martimbianco 9 March 2015.
- Callaghan MJ, Oldham JA. Electric muscle stimulation of the quadriceps in the treatment of patellofemoral pain. Archives of Physical Medicine and Rehabilitation 2004;85:956‐62. [DOI] [PubMed] [Google Scholar]
Glaviano 2016 {published data only}
- Glaviano N. Additional information on random sequence generation, allocation concealment and blinding (participants, personnel and assessors) [personal communication]. Email to: AL Martimbianco 12 August 2016.
- Glaviano NR, Huntsman S, Dembeck A, Hart JM, Saliba S. Improvements in kinematics, muscle activity and pain during functional tasks in females with patellofemoral pain following a single patterned electrical stimulation treatment. Clinical Biomechanics 2016;32:20‐7. [DOI] [PubMed] [Google Scholar]
- Glaviano NR, Saliba SA. Immediate effect of patterned neuromuscular electrical stimulation on pain and muscle activation in individuals with patellofemoral pain. Journal of Athletic Training 2016;51(2):118‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gobelet 1992 {published data only}
- Gobelet C, Frey M, Bonard A. Muscle training techniques and retropatellar chondropathy [Techniques de musculation et chondropathie rétro‐patellaire]. Revue du Rhumatismeet des Maladies Osteo‐Articulaires 1992;59(1):23‐7. [PubMed] [Google Scholar]
Kaya 2013 {published data only}
- Kaya D, Yüksel Ý, Callaghan MJ, Güney H, Atay ÖA, Çitaker S, et al. High voltage pulsed galvanic stimulation adjunct to rehabilitation program for patellofemoral pain syndrome: a prospective randomized controlled trial. Fizyoterapi Rehabilitasyon 2013;24(1):1‐8. [Google Scholar]
Tunay 2003 {published data only}
- Tunay VB. Additional information on NMES parameters and exercise programme [personal communication]. Email to: AL Martimbianco 12 March 2015.
- Tunay VB, Baltaci G, Tunay S, Ergun N. A comparison of different treatment approaches to patellofemoral pain syndrome. The Pain Clinic 2003;15(2):179‐84. [Google Scholar]
References to studies excluded from this review
Dursun 2001 {published data only}
- Dursun N, Dursun E, Kiliç Z. Electromyographic biofeedback–controlled exercise versus conservative care for patellofemoral pain syndrome. Archives of Physical Medicine and Rehabilitation 2001;82:1692‐5. [DOI] [PubMed] [Google Scholar]
Kuru 2012 {published data only}
- Kuru T, Yaliman A, Dereli EE. Comparison of efficiency of Kinesio taping and electrical stimulation in patients with patellofemoral pain syndrome. Acta Orthopaedica et Traumatologica Turcica 2012;46(5):385‐92. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02441712 {published data only}
- NCT02441712. Rehabilitation with patterned electrical neuromuscular stimulation for patients with patellofemoral pain (PENS for PFP). clinicaltrials.gov/ct2/show/NCT02441712 (first received 12 May 2015).
Additional references
Barton 2009
- Barton CJ, Levinger P, Menz HB, Webster KE. Kinematic gait characteristics associated with patellofemoral pain syndrome: a systematic review. Gait & Posture 2009;30(4):405‐16. [DOI] [PubMed] [Google Scholar]
Bolgla 2011
- Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. International Journal of Sports Physical Therapy 2011;6(2):112‐25. [PMC free article] [PubMed] [Google Scholar]
Chiu 2012
- Chiu JK, Wong YM, Yung PS, Ng GY. The effects of quadriceps strengthening on pain, function, and patellofemoral joint contact area in persons with patellofemoral pain. American Journal of Physical Medicine & Rehabilitation 2012;91(2):98‐106. [DOI] [PubMed] [Google Scholar]
Collins 2013
- Collins NJ, Bierma‐Zeinstra SM, Crossley KM, Linschoten RL, Vicenzino B, Middelkoop M. Prognostic factors for patellofemoral pain: a multicentre observational analysis. British Journal of Sports Medicine 2013;47(4):227‐33. [DOI] [PubMed] [Google Scholar]
CONSORT 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. Journal of Clinical Epidemiology 2010;63(8):834–40. [DOI] [PubMed] [Google Scholar]
Cowan 2009
- Cowan SM, Crossley KM, Bennell KL. Altered hip and trunk muscle function in individuals with patellofemoral pain. British Journal of Sports Medicine 2009;43(8):584‐8. [DOI] [PubMed] [Google Scholar]
Crossley 2002
- Crossley K, Bennell K, Green S, Cowan S, McConnell J. Physical therapy for patellofemoral pain: a randomized, double‐blinded, placebo‐controlled trial. American Journal of Sports Medicine 2002;30(6):857‐65. [DOI] [PubMed] [Google Scholar]
Crossley 2004
- Crossley KM, Bennell KL, Cowan SM, Green S. Analysis of outcome measures for persons with patellofemoral pain: which are reliable and valid?. Archives of Physical Medicine and Rehabilitation 2004;85(5):815‐22. [DOI] [PubMed] [Google Scholar]
Davies 2000
- Davies AP, Costa ML, Shepstone L, Glasgow MM, Donell S. The sulcus angle and malalignment of the extensor mechanism of the knee. Journal of Bone and Joint Surgery ‐ British Volume 2000;82(B):1162‐6. [DOI] [PubMed] [Google Scholar]
Doucet 2012
- Doucet BM, Lam A, Griffin L. Neuromuscular electrical stimulation for skeletal muscle function. Yale Journal of Biology and Medicine 2012;85(2):201‐15. [PMC free article] [PubMed] [Google Scholar]
Dye 2005
- Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clinical Orthopaedics and Related Research 2005;436:100‐10. [DOI] [PubMed] [Google Scholar]
Fagan 2008
- Fagan V, Delahunt E. Patellofemoral pain syndrome: a review on the associated neuromuscular deficits and current treatment options. British Journal of Sports Medicine 2008;42(10):489‐95. [DOI] [PubMed] [Google Scholar]
Frye 2012
- Frye JL, Ramey LN, Hart JM. The effects of exercise on decreasing pain and increasing function in patients with patellofemoral pain syndrome: a systematic review. Sports Health 2012;4(3):205‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kaya 2011
- Kaya D, Citaker S, Kerimoglu U, Atay OA, Nyland J, Callaghan M, et al. Women with patellofemoral pain syndrome have quadriceps femoris volume and strength deficiency. Knee Surgery, Sports Traumatology, Arthroscopy 2011;19(2):242‐7. [DOI] [PubMed] [Google Scholar]
Kendall 2005
- Kendall FP, McCreary EK, Provance PG, Rodgers MM, Romani WA. Muscles: Testing and Function, With Posture and Pain. 5th Edition. Baltimore: Lippincott Williams & Wilkins, 2005. [Google Scholar]
Kujala 1993
- Kujala UM, Jaakkola LH, Koskinen SK, Taimela S, Hurme M, Nelimarkka O. Scoring of patellofemoral disorders. Arthroscopy 1993;9(2):159‐63. [DOI] [PubMed] [Google Scholar]
Lake 2011
- Lake DA, Wofford NH. Effect of therapeutic modalities on patients with patellofemoral pain syndrome: a systematic review. Sports Health 2011;3(2):182‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lankhorst 2012
- Lankhorst NE, Bierma‐Zeinstra SM, Middelkoop M. Risk factors for patellofemoral pain syndrome: a systematic review. Journal of Orthopaedic and Sports Physical Therapy 2012;42(2):81‐94. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lysholm 1982
- Lysholm J, Gillquist J. Evaluation of the knee ligament surgery results with special emphasis on use of a scoring scale. American Journal of Sports Medicine 1982;10(3):150‐4. [DOI] [PubMed] [Google Scholar]
Maddocks 2013
- Maddocks M, Gao W, Higginson IJ, Wilcock A. Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD009419.pub2] [DOI] [PubMed] [Google Scholar]
Maffiuletti 2010
- Maffiuletti NA. Physiological and methodological considerations for the use of neuromuscular electrical stimulation. European Journal of Applied Physiology 2010;110(2):223‐34. [DOI] [PubMed] [Google Scholar]
Maffiuletti 2014
- Maffiuletti NA, Vivodtzev I, Minetto MA, Place N. A new paradigm of neuromuscular electrical stimulation for the quadriceps femoris muscle. European Journal of Applied Physiology 2014;114(6):1197‐205. [DOI: 10.1007/s00421-014-2849-2] [DOI] [PubMed] [Google Scholar]
Monaghan 2010
- Monaghan B, Caulfield B, O’Mathúna DP. Surface neuromuscular electrical stimulation for quadriceps strengthening pre and post total knee replacement. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007177.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pal 2012
- Pal S, Besier TF, Draper CE, Fredericson M, Gold GE, Beaupre GS, et al. Patellar tilt correlates with vastus lateralis: vastus medialis activation ratio in maltracking patellofemoral pain patients. Journal of Orthopaedic Research 2012;30(6):927‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pattyn 2012
- Pattyn E, Mahieu N, Selfe J, Verdonk P, Steyaert A, Witvrouw E. What predicts functional outcome after treatment for patellofemoral pain?. Medicine and Science in Sports Exercise 2012;44(10):1827‐33. [DOI] [PubMed] [Google Scholar]
Petersen 2013
- Petersen W, Ellermann A, Gösele‐Koppenburg A, Best R, Rembitzki IV, Brüggemann GP, et al. Patellofemoral pain syndrome. Knee Surgery, Sports Traumatology, Arthroscopy 2013 Nov 13 [Epub ahead of print]. [DOI: 10.1007/s00167-013-2759-6] [DOI] [PMC free article] [PubMed]
Powers 2003
- Powers CM. The influence of altered lower‐extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. Journal of Orthopaedic and Sports Physical Therapy 2003;33(11):639‐46. [DOI] [PubMed] [Google Scholar]
Powers 2012
- Powers CM, Bolgla LA, Callaghan MJ, Collins N, Sheehan FT. Patellofemoral pain: proximal, distal, and local factors, 2nd International Research Retreat. Journal of Orthopaedic and Sports Physical Therapy 2012;42(6):A1‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rathleff 2012
- Rathleff MS, Roos EM, Olesen JL, Rasmussen S. Early intervention for adolescents with patellofemoral pain syndrome ‐ a pragmatic cluster randomised controlled trial. BMC Musculoskeletal Disorders 2012;27(13):1‐9. [DOI: 10.1186/1471-2474-13-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rathleff 2013
- Rathleff MS, Roos EM, Olesen JL, Rasmussen S, Arendt‐Nielsen L. Lower mechanical pressure pain thresholds in female adolescents with patellofemoral pain syndrome. Journal of Orthopaedic and Sports Physical Therapy 2013;43(6):414‐21. [DOI] [PubMed] [Google Scholar]
Revill 1976
- Revill SI, Robinson JO, Rosen M, Hogg MI. The reliability of a linear analogue for evaluating pain. Anaesthesia 1976;31(9):1191‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roush 2012
- Roush JR, Curtis Bay R. Prevalence of anterior knee pain in 18‐35 year‐old females. International Journal of Sports Physical Therapy 2012;7(4):396‐401. [PMC free article] [PubMed] [Google Scholar]
Salsich 2001
- Salsich GB, Brechter JH, Powers CM. Lower extremity kinetics during stair ambulation. Clinical Biomechanics 2001;16:906‐12. [DOI] [PubMed] [Google Scholar]
Sillen 2013
- Sillen MJ, Franssen FM, Gosker HR, Wouters EF, Spruit MA. Metabolic and structural changes in lower‐limb skeletal muscle following neuromuscular electrical stimulation: a systematic review. PLoS ONE 2013;8(9):e69391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2015
- Smith TO, Drew BT, Meek TH, Clark AB. Knee orthoses for treating patellofemoral pain syndrome. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD010513.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taradaj 2013
- Taradaj J, Halski T, Kucharzewski M, Walewicz K, Smykla A, Ozon M, et al. The effect of neuromuscular electrical stimulation on quadriceps strength and knee function in professional soccer players: return to sport after ACL reconstruction. Biomed Research International 2013 Dec 5 [Epub ahead of print]. [DOI: 10.1155/2013/802534] [DOI] [PMC free article] [PubMed]
van der Heijden 2015
- Heijden RA, Lankhorst NE, Linschoten R, Bierma‐Zeinstra SMA, Middelkoop M. Exercise for treating patellofemoral pain syndrome. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD010387.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vanderthommen 2007
- Vanderthommen M, Duchateau J. Electrical stimulation as a modality to improve performance of the neuromuscular system. Exercise and Sport Sciences Review 2007;35(4):180‐5. [DOI] [PubMed] [Google Scholar]
Vengust 2001
- Vengust R, Strojnik V, Pavlovcic V, Antolic V, Zupanc O. The effect of electrostimulation and high load exercises in patients with patellofemoral joint dysfunction. A preliminary report. European Journal of Physiology 2001;442(6):153‐4. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36) I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
Werner 1993
- Werner S, Arvidsson H, Arvidsson I, Eriksson E. Electrical stimulation of vastus medialis and stretching of lateral thigh muscles in patients with patello‐femoral symptoms. Knee Surgery, Sports Traumatology, Arthroscopy 1993;1(2):85‐92. [DOI] [PubMed] [Google Scholar]
Witvrouw 2014
- Witvrouw E, Callaghan MJ, Stefanik JJ, Noehren B, Bazett‐Jones DM, Willson JD, et al. Patellofemoral pain: consensus statement from the 3rd International Patellofemoral Pain Research Retreat held in Vancouver, September 2013. British Journal of Sports Medicine 2014;48(6):411‐4. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Martimbianco 2014
- Martimbianco ALC, Torloni MR, Andriolo BNG, Porfirio G, Riera R. Neuromuscular electrical stimulation (NMES) for patellofemoral pain syndrome. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD011289] [DOI] [PMC free article] [PubMed] [Google Scholar]


