Abstract
Background
Polycystic ovary syndrome (PCOS) is a common endocrine condition, affecting approximately one in 10 women. PCOS is defined by two of three features: oligo‐ or anovulation, clinical or biochemical hyperandrogenism or both, or polycystic ovaries.
Women with PCOS can have a wide range of health problems, including infrequent and irregular periods, unwanted hair growth and acne, and subnormal fertility. Long‐term health concerns include an increased risk of heart disease, diabetes and the development of precancerous disease of the womb.
Objectives
To assess the effectiveness and harms of ovarian surgery as a treatment for symptomatic relief of hirsutism, acne and menstrual irregularity in PCOS.
Search methods
We searched the Cochrane Gynaecology and Fertility Group specialized register, CENTRAL, MEDLINE, Embase and PsycINFO (from inception to 17 October 2016). We handsearched citation lists, registers of ongoing trials and conference proceedings.
Selection criteria
We included randomized controlled trials (RCTs) of women undergoing ovarian drilling in comparison to no treatment, medical treatment, or other forms of surgical treatment for the symptoms of PCOS.
Data collection and analysis
We used standard methodological procedures recommended by Cochrane. The primary outcome measures were improvement in menstrual regularity and androgenic symptoms of PCOS (hirsutism, acne); the secondary outcome measures included harms, change of body mass index (BMI), waist circumference, androgen levels, metabolic measures and quality of life. We assessed the quality of the evidence using GRADE methods.
Main results
We included 22 RCTs (2278 women analyzed) of participants with PCOS and symptoms of acne, hirsutism or irregular menstrual cycles, all of which included laparoscopic ovarian drilling (LOD) as an intervention.
Two studies reported their funding source (Farquhar 2002 ‐ supported in part by the Auckland Medical Research Foundation; Sarouri 2015 ‐ the authors thank the Vice Chancellor for Research of Guilan University of Medical Sciences for funding this project).
The quality of the evidence ranged from very low to moderate quality. The main limitations were imprecision associated with the low number of studies, inconsistency and risk of bias associated with the inability to blind participants. There were too few studies to assess risk of publication bias.
Menstrual Regularity
Two studies compared LOD versus metformin (n=226) but no conclusions could be drawn with regard to menstrual regularity, as their findings were inconsistent and they were unsuitable for pooling. There appeared to be little or no difference in the rate of women reporting improvement in menstrual regularity when LOD was compared with medical treatment including metformin + clomiphene (OR 1.02, 95% CI 0.64 to 1.64, 2 studies, 332 women, I2 = 13%, low‐quality evidence), letrozole (OR 1.08, 95% CI 0.64 to 1.84, 1 study, 260 women, low‐quality evidence), or metformin + letrozole (OR 0.95, 95% CI 0.49 to 1.81, 1 study, 146 women, low‐quality evidence). However, one study reported that LOD was superior to gonadotrophin (OR 19.2, 95% CI 3.17 to 116.45, 1 study, 35 women, very low‐quality evidence).
There appeared to be little or no difference in the rate of women reporting improvement in menstrual regularity when bilateral unipolar LOD was compared to unilateral LOD (OR 1.51, 95% CI 0.62 to 3.71, 2 studies, 104 women, I2 = 0%, moderate‐quality evidence), transvaginal ultrasound‐guided LOD (OR 1.23, 95% CI 0.64 to 2.37, 1 study, 147 women, low‐quality evidence), LOD using adjusted thermal dose in accordance with the ovarian volume (OR 0.42, 95% CI 0.16 to 1.14, 1 study, 115 women, low‐quality evidence) or bipolar LOD (OR 1.00, 95% CI 0.05 to 18.57, 1 study, 18 women, low‐quality evidence).
Four to five punctures per ovary may improve the rate of women reporting menstrual regularity compared with two or fewer (OR 16.04, 95% CI 4.19 to 61.34, 2 studies, 73 women, I2 = 0%, low‐quality evidence).
Androgenic Symptoms
There was probably little or no difference in improvement in androgenic symptoms when LOD was compared to metformin (OR 1.00, 95% CI 0.42 to 2.37, 1 study, 126 women, moderate‐quality evidence) or gonadotrophins; acne (OR 3.20, 95% CI 0.33 to 30.94, 1 study, 25 women, low‐quality evidence), hirsutism (OR 2.31, 95% CI 0.22 to 23.89, 1 study, 25 women, low‐quality evidence).
There appeared to be little or no difference in improvement of androgenic symptoms when LOD was compared to transvaginal ultrasound‐guided LOD, with respect to hirsutism (OR 1.09, 95% CI 0.30 to 3.91, 1 study, 39 women, low‐quality evidence) or acne (OR 0.84, 95% CI 0.20 to 3.50, 1 study, 31 women, low‐quality evidence).
Harms
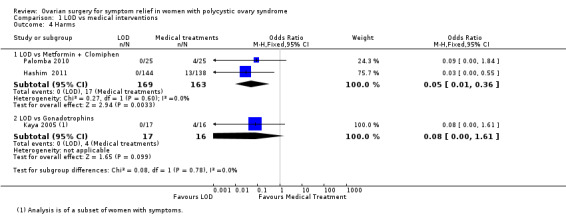
LOD was associated with fewer gastrointestinal side effects than metformin plus clomiphene (OR 0.05, 95% CI 0.01 to 0.36, 2 studies, 332 women, I2 = 0%, moderate‐quality evidence). One study suggested little or no difference in rates of ovarian hyperstimulation syndrome between LOD and gonadotrophins (OR 0.08, 95% CI 0.00 to 1.61, 1 study, 33 women, low‐quality evidence).
There were fewer adhesions with transvaginal hydrolaparoscopy compared to LOD (OR 0.10, 95% CI 0.05 to 0.18, 1 study, 246 women, moderate‐quality evidence). There appeared to be little or no difference in adhesions when variable energy LOD was compared with standard LOD (OR 0.96, 95% CI 0.32 to 2.88, 1 study, 64 women, low‐quality evidence). Another study (44 women) reported that none of the women who returned for surgery following either traditional or unilateral LOD were found to have adhesions.
Authors' conclusions
There was no clear evidence that LOD improves menstrual regularity or the androgenic symptoms of PCOS, compared to most of the medical treatments used in the included studies. LOD was associated with fewer gastrointestinal side effects compared to metformin and clomiphene.
There was also no clear evidence of different effectiveness between types of LOD, except that LOD with four to five punctures per ovary may be more effective than two or fewer punctures. There was little evidence comparing LOD with different types of surgery, although one study concluded that transvaginal hydrolaparoscopy had a lower risk of adhesions than LOD.
There was evidence from one small study of benefit from LOD compared to gonadotrophins for menstrual regulation. However, gonadotrophins are seldom used for this indication.
Plain language summary
Ovarian surgery for symptom relief in women with polycystic ovary syndrome
Review question
Cochrane researchers reviewed the evidence about the effect of ovarian surgery on symptoms of polycystic ovary syndrome (PCOS). We found 22 studies that compared it to surgical and non‐surgical treatments, and variations of surgical technique. The main outcomes measured were improvement in the regularity of periods, and a decrease in unwanted hair growth and acne (androgenic symptoms). We also looked at harms from treatment, change in body weight, change in testosterone levels, changes in metabolic measures and quality of life.
Background
Women with PCOS can have a wide range of health problems, including infrequent and irregular periods, unwanted hair growth and acne, and subnormal fertility. Long‐term health concerns include an increased risk of heart disease, diabetes and the development of precancerous disease of the womb.
Most of the current research has looked at the effect of ovarian surgery in improving fertility in women with PCOS. Our review aims to look at the impact of laparoscopic (keyhole) ovarian surgery (LOD) on the improvement in the other symptoms of PCOS.
Search date
The evidence is current to October 2016.
Study characteristics
We include 22 randomized controlled trials (RCTs), covering 2278 women. A randomized controlled trial is a type of medical experiment where participants are randomly given one or other different treatments in the study. The participants had PCOS and were from different settings around the world.
Ten out of the 22 RCTs compared LOD to medical treatments. These treatments included metformin, clomiphene, gonadotrophins, letrozole and rosiglitazone. Ten out of 22 studies compared traditional LOD to variations in surgical techniques. Two out of 22 RCTs looked at using different energy levels or number of ovarian drill holes during LOD.
Study funding sources
Two studies reported their funding source (Farquhar 2002 ‐ supported in part by the Auckland Medical Research Foundation; Sarouri 2015 ‐ the authors thank the Vice Chancellor for Research of Guilan University of Medical Sciences for funding this project).
Key results
LOD may be better at regulating menstrual cycles than gonadotrophins. However, most doctors would consider other options for first‐line treatment. LOD with four or five drill holes versus two or fewer per ovary may be more effective at menstrual regulation in women with PCOS.
There was not enough evidence to tell whether there is a difference between LOD and other medical treatment or variations in surgical technique in improving the regularity of periods or androgenic symptoms.
LOD was associated with fewer gastrointestinal side effects compared to metformin and clomiphene, but involves surgery and is not standard treatment for menstrual disturbance or unwanted hair growth. There was less scar tissue with transvaginal hydrolaparoscopy compared to LOD.
Overall LOD can be considered to have a low risk of harm, and to be an option in the management of symptoms of PCOS.
Quality of evidence
The quality of the evidence ranged from very low to moderate quality. The main limitations were imprecision associated with the low number of studies, inconsistency and risk of bias associated with the inability to blind participants (conceal the type of treatment from them). There were too few studies to assess risk of publication bias.
Summary of findings
Background
Description of the condition
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders in women of childbearing age. Typically, women will have menstrual disturbances, increased hair growth (hirsutism), acne, infrequent or absent menstrual periods and subfertility. PCOS affects approximately 10% of women worldwide (Adams 1986; Homburg 2008).
A proportion of women with PCOS will present clinically with hirsutism, acne and androgen‐dependent alopecia. Hirsutism in women is defined as an increased growth of terminal hair in a male pattern. The prevalence of clinical signs of hyperandrogenism in women of reproductive age is around 5% to 25% (Azziz 2000). It is characterised biochemically by raised serum concentrations of androgens, particularly testosterone and androstenedione. These features are associated with hypersecretion of luteinising hormone (LH) but with normal or low serum concentrations of follicle stimulating hormone (FSH). In 80% of women with PCOS, the testosterone concentration will exceed the upper limit of 2.4 nmol/l (Taylor 2003).
Women with PCOS also often present with menstrual disorders such as heavy menstrual bleeding and oligomenorrhoea. It is estimated that 30% to 70% of women with PCOS are obese (Vrbikova 2009), and many will develop type 2 diabetes, metabolic disorders and cardiovascular disease (Giallauria 2008; Kiddy 1992).
The Rotterdam meeting in 2003 endorsed a consensus regarding the diagnosis of the syndrome (ESHRE/ASRM PCOS Consensus 2004a; ESHRE/ASRM PCOS Consensus 2004b). This defines PCOS, after the exclusion of related disorders, as a clinical condition exhibiting at least two of the following three features: oligo‐ or anovulation (infrequent or absent menstrual periods); clinical or biochemical, or both, signs of hyperandrogenism (typically, hirsutism or acne); or polycystic ovaries seen on ultrasound.
A further meeting in Greece in 2007 proposed evidence‐based management for the treatment of PCOS‐related subfertility (Thessaloniki ESHRE/ASRM PCOS Consensus 2008). However, very little consensus guidance addresses the management of the non‐fertility‐related symptoms of this condition.
Conservative management with advice on lifestyle changes such as acupuncture (Lim 2016), dieting and weight loss has variable effectiveness. Medical treatment with statins (Raval 2011), hormonal therapy or insulin sensitising agents (Costello 2007) can be associated with significant side effects and is often contraindicated in women wishing to conceive. Laparoscopic ovarian drilling (LOD) is a surgical alternative to medical treatment of anovulation in women with PCOS and subfertility. However, whilst studies have examined the effectiveness of LOD in relation to the improvement of conception, reports on the effect of LOD on non‐fertility‐related PCOS symptoms are contradictory.
Description of the intervention
Ovarian wedge resection was first described in 1935 by Stein and Leventhal (Homburg 2008). This surgery was primarily aimed at reducing ovarian mass by bilateral ovarian wedge resections. With the advent of laparoscopic surgery, a variant of the traditional wedge resection was developed using this technique. During LOD, uni‐ or bipolar electrocautery at various energy levels for a variable duration is used to puncture the ovary. These are typically 2 to 4 mm deep, penetrating into the cortex. The number of puncture holes, the type of energy source and the duration of treatment vary between practitioners. Recent advances in transvaginal hydrolaparoscopy have also allowed ovarian drilling to be performed by the transvaginal route (Gordts 2009), using a hydrolaparoscope or fertiloscope. Ovarian surgery is traditionally performed to induce ovulation in anovulatory women with PCOS. The documented advantage of LOD compared with hormone treatment is the reduction in complication rates associated with ovarian hyperstimulation syndrome (OHSS) and a decreased risk of multiple pregnancy (Farquhar 2007).
How the intervention might work
The mechanism of action of ovarian surgery, wedge resection or more commonly LOD is largely unexplained. Compared to medical treatment, where the treatment effect is dependent on continuous administration of the medication, many LOD‐induced effects appear to be long‐term. It is, however, not known whether LOD exerts its action through a direct effect on the ovary or through a systemic endocrine mechanism. LOD has been shown to reduce long‐term androgen serum levels through a number of mechanisms (Amer 2002). The destruction of androgen‐producing ovarian stroma and the subsequent reduction in substrate for steroid aromatisation may contribute to an overall reduction in androgen production, with improvements in acne and hirsutism. High testosterone levels increase terminal hair growth, and therefore 5α‐reductase inhibitors are implemented in the treatment of hirsutism. A more generalised increased 5α‐reductase activity may be important for increased cortisol metabolism in PCOS (Glintborg 2010). In LOD, it has been hypothesised that the drilling of punctures within the androgen‐producing stroma may depress serum androgen concentrations and offer symptomatic relief. A further possible mechanism is an impact of LOD on insulin resistance. Whilst there is currently very little evidence which documents the effect of LOD on metabolic changes and insulin levels in women with PCOS, small studies have shown that LOD (by electrocautery) can reduce insulin resistance in women with PCOS through decreased IRS‐1 Ser312 phosphorylation (Seow 2007). Other more recent studies have also suggested that LOD can decrease anti‐müllerian hormone (AMH) concentrations and ovarian stroma blood flow in women with PCOS compared with controls (Elmashad 2011).
Why it is important to do this review
The aim of this review is to evaluate the impact of ovarian surgery on symptomatic control of PCOS. Whilst much research has focused on the effect of ovarian surgery in improving fertility in clomiphene‐resistant women with PCOS, the impact of LOD on symptoms of PCOS has not been reviewed.
Objectives
To assess the effectiveness and harms of ovarian surgery as a treatment for symptomatic relief of hirsutism, acne and menstrual disturbances in women with PCOS.
Methods
Criteria for considering studies for this review
Types of studies
We include only randomized controlled trials (RCTs) of surgical interventions for the treatment of symptoms associated with PCOS. Only pre‐crossover data from cross‐over studies was to be included in this study. Other data from cross‐over studies would not be included, even if there was a wash‐out period, as the effect of LOD is long term (for example some women will ovulate regularly after one year).
Types of participants
Women with PCOS (as defined by the Rotterdam Criteria) who are symptomatic with acne, hirsutism or irregular menstrual cycles. Oligomennorhoea is here defined as a menstrual cycle lasting more than 45 days.
Types of interventions
Ovarian surgical interventions for the symptomatic treatment of women with PCOS. Types of comparisons include the following:
1. Surgical (laparotomy or laparoscopy) versus non‐surgical intervention (including placebo or non‐treatment).
2. Comparison between various surgical methods or techniques, e.g.:
Comparing the number of drill holes administered.
Comparison of various energy modalities for the ovarian drilling procedure (e.g. uni‐ versus bipolar electrocautery, or electrocautery versus laser versus harmonic scalpel).
Laparotomy versus laparoscopy.
Types of outcome measures
Primary outcomes
1. Rates of women reporting an improvement in menstrual regularity as defined by the number of cycles per year (or other time frame, e.g. six months) and recorded as regular or irregular.
2. Improvement in androgenic symptoms of PCOS or rates of androgenic symptoms: improvements in hirsutism or acne, as defined by scoring systems such as the Ferriman Gallwey Score (FGS), Global Acne Grading Score (GAGS) or the Leeds revised Acne Grading system (LRAGS).
FGS divides the body into nine areas identified for assessment: upper lip, chin, chest, upper back, lower back, upper abdomen, lower abdomen, upper arms and thighs. Each area is assigned a score between 0 and 4 based on hair growth, giving a potential score of 0 to 36. In white populations a score over seven indicates hyperandrogenism. There is ethnic variation of 'normal'.
GAGS divides the face, chest and back into six areas identified for assessment: forehead, each cheek, nose, chin, chest and back. Each area is assigned a factor of 1, 2 or 3 based on area. Acne lesions are given a value based on severity: no lesions = 0, comedones = 1, papules = 2, pustules = 3 and nodules = 4. Scores of 1 to 18 are considered mild, 19 to 30 are considered moderate, 31 to 38 are severe, and more than 39 very severe.
LRAGS assesses acne on the face, chest and back. A scale of 1 to 10 is used.
Secondary outcomes
1. Harms of surgical and non‐surgical interventions
2. Change in body weight or body mass index
3. Change in waist circumference
4. Testosterone levels, free testosterone or free androgen index
5. Metabolic measures: fasting glucose or insulin levels or haemoglobin A1C (HbA1C)
6. Changes in quality of life
Search methods for identification of studies
We sought all published and unpublished RCTs on surgery for PCOS, using the following search strategy, without language restriction and in consultation with the Gynaecology and Fertility Group (CGF) Information Specialist.
Electronic searches
We searched the following electronic databases, trials registers and websites: Cochrane Gynaecology and Fertility Group (CGF) Specialised Register (from inception to 17 October 2016, Procite platform) (Appendix 1). Central Register of Studies Online (CRSO) (searched 17 October 2016, web platform) (Appendix 2). Ovid MEDLINE® In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE® (from 1946 to 17 October 2016, Ovid platform) (Appendix 3). Ovid Embase (from 1980 to 17 October 2016, Ovid platform) (Appendix 4). Ovid PsycINFO (from 1806 to 17 October 2016, Ovid platform) (Appendix 5).
We searched other electronic sources of trials 17 October 2016, including: Database of Abstracts of Reviews of Effects (DARE) in the Cochrane Library (www.cochrane.org/index.htm); ClinicalTrials.gov (clinicaltrials.gov/ct2/home); World Health Organization International Clinical Trials Registry Platform search portal (apps.who.int/trialsearch/); OpenSigle for grey literature from Europe (opensigle.inist.fr/); China Academic Journal Electronic full text Database in China National Knowledge Infrastructure; Index to Chinese Periodical Literature.
LILACS and other Spanish and Portuguese language databases (LatinAmerican and Caribbean Health Science Information database). This is in the Virtual Health library Regional Portal (VHL) (bvsalud.org/portal/?lang=en).
There were no language or date restrictions in these searches. The search strategies are in the appendices.
Searching other resources
We handsearched the reference lists of articles retrieved by the search and made personal contact with experts in the field. We handsearched ESHRE conference abstracts that were not covered in the CGF Specialised Register, in collaboration with the Information Specialist.
Data collection and analysis
Selection of studies
The four review authors undertook the selection of studies. We used the search strategy described above to obtain titles and, where possible, abstracts of studies that were potentially relevant to the review. We were overly inclusive rather than risk losing relevant studies. All review authors independently assessed whether the studies were eligible for inclusion, with disagreements planned for consultation with a third author, although this was not required. Where papers had insufficient information to enable an accurate assessment of eligibility for inclusion, we sought further information from authors.
The selection process is documented with a PRISMA flow chart in Figure 1.
Data extraction and management
Two review authors independently extracted data and assessed risks of bias using forms designed according to Cochrane guidelines. We resolved disagreements about study eligibility by discussion, without requiring referral to a third review author. Where studies had multiple publications, we used the main trial report as the primary reference and derived additional details from secondary papers. We corresponded with study investigators to clarify further data on methods and results.
Assessment of risk of bias in included studies
Two review authors independently assessed the included studies for risks of bias, using the Cochrane 'Risk of bias' assessment tool (Higgins 2011) to evaluate: selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other potential bias. We assigned judgements as recommended in the Cochrane Handbook for Systematic Reviews of Interventions Section 8.5 (Higgins 2011), resolving disagreements by discussion.
We present our judgements in a 'Risk of bias' table, which we incorporate into the interpretation of review findings by means of sensitivity analyses (see below).
Measures of treatment effect
We performed statistical analysis in accordance with the guidelines and methods developed by Cochrane (Higgins 2011). We expressed dichotomous data results as a Mantel‐Haenszel odds ratio (OR) and continuous data results as a mean difference (MD). We present a 95% confidence interval (CI) for all outcomes.
Unit of analysis issues
The primary analysis was by each woman randomized. Data reported that did not allow valid analysis (e.g. "per cycle" rather than "per woman" where women contributed more than one cycle) were to be briefly summarized in an additional table and would not be meta‐analysed.
Dealing with missing data
In the event that data were missing or not available, we planned to contact the authors to provide further assistance. Where we could obtain no further information, the review authors state this in the review. The review authors determined the individual weight of missing data. We conducted an intention‐to‐treat analysis as far as possible, but otherwise we analyzed only the available data.
Assessment of heterogeneity
We assessed heterogeneity using the I2 statistic, according to the guidelines set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). An I2 value greater than 50% indicates substantial heterogeneity. Where we detected substantial heterogeneity we have explored possible explanations in subgroup analyses (e.g. differing populations) and sensitivity analyses (e.g. differing risks of bias). We have taken heterogeneity in to account in our interpretation of the results.
Assessment of reporting biases
In view of the difficulty in detecting and correcting for publication bias and other reporting bias, we minimised their potential impact by ensuring a comprehensive search for eligible studies and by being aware of duplicated data. In the case where 10 or more studies contributed to our analysis, we planned to use a funnel plot to explore the possibility of a small‐study effect.
Data synthesis
We used a fixed‐effect model inverse variance meta‐analysis for combining data where trials examined the same intervention, and where we judged the trial populations and methods to be sufficiently similar.
An increase in the odds of a particular outcome which was beneficial (for example, return of regular menses) or detrimental (for example, surgical complications) is displayed graphically in the meta‐analyses. The aim was to define analyses that were comprehensive and mutually exclusive so that all eligible study results could be fitted into only one of the strata.
Subgroup analysis and investigation of heterogeneity
We planned, where data allowed, to carry out subgroup analyses to explore possible sources of heterogeneity (for example, differences between participants, interventions and study quality).
Sensitivity analysis
We intended to conduct sensitivity analyses for the primary outcomes to explore the effect of risks of bias assessed by adequate methodology versus poor methodology, where adequate methodology was defined a: adequate randomization method, adequate allocation concealment, analysis by intention‐to‐treat, and losses to follow‐up of less than 20%. However, sensitivity analysis in this respect was not necessary and therefore not performed.
These analyses also included consideration of whether the review conclusions would have differed if:
1. A random‐effects model had been adopted.
2. The summary effect measure had been relative risk rather than odds ratio.
Overall quality of the body of evidence: 'Summary of findings' table
We have prepared a 'Summary of findings' table using GRADEpro and Cochrane methods. This table evaluates the overall quality of the body of evidence for the main review outcomes (improvement in menstrual regularity, improvement in androgenic symptoms of PCOS, harms of medical and surgical intervention) for the main review comparison (LOD versus medical intervention). Table 1.
Summary of findings for the main comparison. LOD compared to medical interventions for symptom relief in women with polycystic ovary syndrome.
| LOD compared to medical interventions for symptom relief in women with polycystic ovary syndrome | ||||||
| Patient or population: Women with symptoms of PCOS Setting: Clinic or hospital Intervention: Laparoscopic ovarian drilling (LOD) Comparison: medical interventions | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality of the evidence (GRADE) | What happens | ||
| Without LOD | With LOD | Difference | ||||
| Menstrual regularity at 6 months LOD vs metformin N of participants: 236 (2 RCTs) LOD vs metformin + clomiphene N of participants: 332 (2 RCTs) LOD vs gonadotropins N of participants: 35 (1 RCT) LOD vs letrozole N of participants: 260 (1 RCT) LOD vs metformin + letrozole N of participants: 146 (1 RCT) |
Findings inconsistent and data unsuitable for pooling | Not calculable | ⊕⊝⊝⊝ VERY LOW 1, 2, 3 | |||
| OR 1.02 (0.64 to 1.64) | 70.6% | 71.0% (60.5 to 79.7) | 0.4% more (10 fewer to 9.2 more) | ⊕⊕⊝⊝ LOW 1, 3 | ||
| OR 19.20 (3.17 to 116.45) | 11.1% | 70.6% (28.4 to 93.6) | 59.5% more (17.3 more to 82.5 more) | ⊕⊕⊝⊝ LOW 1, 3 | ||
| OR 1.08 (0.64 to 1.84) | 68.8% | 70.4% (58.5 to 80.2) | 1.6% more (10.3 fewer to 11.4 more) | ⊕⊕⊝⊝ LOW 1, 3 | ||
| OR 0.95 (0.49 to 1.81) | 52.1% | 50.8% (34.7 to 66.3) | 1.3% fewer (17.3 fewer to 14.2 more) | ⊕⊕⊝⊝ LOW 1, 3 | ||
| Improvement in androgenic symptoms at 6 months (hirsutism/acne) ‐ LOD vs metformin N of participants: 126 (1 RCT) | OR 1.00 (0.42 to 2.37) | 79.4% | 79.4% (61.8 to 90.1) | 0.0% fewer (17.6 fewer to 10.7 more) | ⊕⊕⊝⊝ LOW 1, 3 | |
| Improvement in androgenic symptoms at 6 months (hirsutism/acne) ‐ LOD vs gonadotrophins N of participants: 50 (1 RCT) | Acne: OR 3.20 (0.33 to 30.94) Hirsutism: OR 2.31, (0.22 to 23.89) |
See comments | ⊕⊝⊝⊝VERY LOW 1, 4 | Acne: 4/29 without LOD, 1/21 with LOD Hirsutism: 3/29 without LOD, 1/21 with LOD |
||
| Harms: GI Upset at 6 months ‐ LOD vs metformin + clomiphene
N of participants: 332 (2 RCTs) Harms: OHSS rates at 6 months ‐ LOD vs gonadotrophins N of participants: 33 (1 RCT) |
OR 0.05 (0.01 to 0.36) | 10.4% | 0.6% (0.1 to 4.0) | 9.9% fewer (10.3 fewer to 6.4 fewer) | ⊕⊕⊕⊝ MODERATE 1 | |
| OR 0.08 (0.00 to 1.61) | 25.0% | 2.6% (0.0 to 34.9) | 22.4% fewer (25 fewer to 9.9 more) | ⊕⊕⊝⊝ LOW 1, 3 | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; OHSS: ovarian hyperstimulation syndrome | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded one level for serious risk of bias: Included studies not double‐blinded, and in some cases methods of randomization unclear. 2Downgraded two levels for very serious and unexplained heterogeneity: I2 = 85%, direction of effect inconsistent 3Downgraded one level for serious imprecision. 4Downgraded two levels for very serious imprecision: Broad confidence interval, very few events.
We prepared additional 'Summary of findings' tables for the main review outcomes for other important comparisons (LOD versus other surgical interventions, LOD four to five versus two or fewer punctures, LOD versus variable energy source). Table 2; Table 3; Table 4.
Summary of findings 2. LOD compared to other surgical interventions for symptom relief in women with polycystic ovary syndrome.
| LOD compared to other surgical interventions for symptom relief in women with polycystic ovary syndrome | ||||||
| Patient or population: Women with symptoms of PCOS Setting: Clinic or hospital Intervention: LOD Comparison: other surgical interventions | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality of the evidence (GRADE) | What happens | ||
| Without LOD | With LOD | Difference | ||||
| Menstrual regularity ‐ LOD vs unilateral LOD N of participants: 104 (2 RCTs) | OR 1.51 (0.62 to 3.71) | 71.2% | 78.8% (60.5 to 90.1) | 7.7% more (10.7 fewer to 19 more) | ⊕⊕⊕⊝ MODERATE 1 | 1 study follow‐up at 3 months. 1 study follow‐up at 12 months |
| Menstrual regularity at 6 months ‐ LOD vs ultrasound‐guided transvaginal ovarian drilling N of participants: 147 (1 RCT) | OR 1.23 (0.64 to 2.37) | 54.7% | 59.7% (43.6 to 74.1) | 5.1% more (11.1 fewer to 19.4 more) | ⊕⊕⊝⊝ LOW 1 2 | |
| Menstrual regularity at 12 months Laser LOD vs harmonic scalpel N of participants: 34 (1 RCT) | OR 2.13 (0.17 to 26.03) | 88.2% | 94.1% (56.0 to 99.5) | 5.9% more (32.2 fewer to 11.3 more) | ⊕⊕⊝⊝ LOW 1, 3 | Note control group is NdYAG Laser |
| Improvement in androgenic symptoms at 6 months (Acne) ‐ LOD vs USS guided N of participants:31 (1 RCT) | OR 0.84 (0.20 to 3.5) | 47.1% | 42.7% (15.1 to 75.7) | 4.3% fewer (32 fewer to 28.6 more) | ⊕⊕⊝⊝ LOW 1, 2 | |
| Improvement in androgenic symptoms at 6 months (Hirsutism) ‐ LOD vs USS‐guided N of participants: 39 (1 RCT) | OR 1.09 (0.30 to 3.91) | 40.0% | 42.1% (16.7 to 72.3) | 2.1% more (23.3 fewer to 32.3 more) | ⊕⊕⊝⊝ LOW 1, 2 | |
| Harms: Adhesions at 6 months ‐ LOD vs THL N of participants: 246 (1 RCT) | OR 0.10 (0.05 to 0.18) | 59.3% | 12.7% (6.8 to 20.8) | 46.6% fewer (52.5 fewer to 38.5 fewer) | ⊕⊝⊝⊝ VERY LOW 1, 4 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; THL: transvaginal hydrolaparoscopy | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded one level for serious risk of bias: Included studies not double‐blinded or unclear allocation concealment or unclear randomization method. 2Downgraded one level for serious imprecision. 3Single study, narrow confidence interval. 4Downgraded two levels for very serious imprecision: Broad confidence interval, very few events.
Summary of findings 3. LOD 4‐5 compared to 2 or fewer punctures for symptom relief in women with polycystic ovary syndrome.
| LOD 4 ‐ 5 punctures compared to 2 or fewer punctures for symptom relief in women with polycystic ovary syndrome | ||||||
| Patient or population: Women with symptoms of PCOS Setting: Clinic or hospital Intervention: LOD 4 ‐ 5 punctures Comparison: 2 or fewer punctures | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality of the evidence (GRADE) | What happens | ||
| Without LOD 4‐5 | With LOD 4‐5 | Difference | ||||
| Menstrual regularity at 6 months ‐ LOD 4 ‐ 5 coagulation points compared to 2 or fewer N of participants: 73 (2 RCTs) | OR 16.04 (4.19 to 61.34) | 13.9% | 72.1% (40.3 to 90.8) | 58.2% more (26.4 more to 76.9 more) | ⊕⊕⊝⊝ LOW 1, 2 | |
| Menstrual regularity at 6 months ‐ LOD (4 ‐ 5 laser coagulation points) vs 1 laser coagulation point per ovary N of participants: 40 (1 RCT) | OR 19.00 (2.12 to 170.38) | 5.0% | 50.0% (10.0 to 90.0) | 45.0% more (5 more to 85 more) | ⊕⊕⊝⊝ LOW 1, 2 | |
| Menstrual regularity at 6 months ‐ LOD 4 punctures vs 2 punctures per ovary N of participants: 33 (1 RCT) | OR 14.00 (2.60 to 75.41) | 25.0% | 82.4% (46.4 to 96.2) | 57.4% more (21.4 more to 71.2 more) | ⊕⊕⊝⊝ LOW 1, 2 | |
| Improvement in androgenic symptoms | No data available | |||||
| Harms LOD 4 ‐ 5 versus fewer punctures |
No data available | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded one level for serious risk of bias: Included studies not double‐blinded or methods of randomization unclear. 2Downgraded one level for serious imprecision.
Summary of findings 4. LOD compared to LOD variable energy for symptom relief in women with polycystic ovary syndrome.
| LOD compared to LOD variable energy for symptom relief in women with polycystic ovary syndrome | ||||||
| Patient or population: Women with symptoms of PCOS Setting: Clinic or hospital Intervention: LOD Comparison: LOD variable energy | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality of the evidence (GRADE) | What happens | ||
| Without LOD | With LOD | Difference | ||||
| Menstrual regularity at 6 months ‐ LOD vs adjusted thermal dose N of participants: 115 (1 RCT) | OR 0.42 (0.16 to 1.14) | 87.9% | 75.4% (53.8 to 89.3) | 12.6% fewer (34.1 fewer to 1.3 more) | ⊕⊝⊝⊝ VERY LOW 1, 2 | |
| Menstrual regularity at 3 months ‐ LOD unipolar vs LOD bipolar N of participants: 20 (1 RCT) | OR 1.00 (0.05 to 18.57) | 90.0% | 90.0% (31.0 to 99.4) | 0.0% fewer (59 fewer to 9.4 more) | ⊕⊝⊝⊝ VERY LOW 1, 2 | Groups had different metabolic characteristics at baseline |
| Improvement in androgenic symptoms | No data available | |||||
| Harms: Adhesions at 6 months № of participants: 64 (1 study) | OR 0.96 (0.32 to 2.88) | 28.6% | 27.7% (11.3 to 53.5) | 0.8% fewer (17.2 fewer to 25 more) | ⊕⊝⊝⊝ VERY LOW1, 2 |
Women that remained enrolled for second‐look laparoscopy |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded one level for serious risk of bias: Included studies not double‐blinded or unclear allocation concealment. 2Downgraded two levels for very serious imprecision: Broad confidence interval, very few events.
We assessed the quality of the evidence using GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness and publication bias. Two review authors working independently judged evidence quality (high, moderate, low or very low), with disagreements resolved by discussion. Judgements were justified, documented, and incorporated into the reporting of results for each outcome.
Results
Description of studies
Only pre‐cross‐over data from cross‐over studies were to be included in this review. We do not include other data from cross‐over studies, even if there is a wash‐out period, as the effect of LOD is long‐term (for example, some women will ovulate regularly after one year).
Results of the search
The search retrieved 1115 articles. Thirty‐one studies were potentially eligible and retrieved in full text, with 22 meeting our inclusion criteria and included in the final analysis. See study tables: Characteristics of included studies; Characteristics of excluded studies.
See Figure 1.
1.
Study flow diagram
Included studies
Study Design and setting
22 randomized controlled trials included in this review were set in the high‐, middle‐ and low‐income countries, in private, public and university hospitals. All trials had a parallel design, and all but one were single‐centre studies.
Participants
The studies included 2278 women. All the participants had polycystic ovary syndrome. The characteristics of the participants can be found in the table for each included study. Participants were included regardless of whether their treatment goal was symptom management of PCOS or, more commonly, fertility outcomes.
Interventions
Ten of the 22 studies were designed to assess the impact of LOD on the outcome measures specified earlier, compared to a medical treatment. The comparisons were as follows:
Traditional LOD compared to;
Metformin (Ashrafinia 2009; Hamed 2010)
Metformin + clomiphene (Hashim 2011; Palomba 2010)
Gonadotrophins for ovulation induction (Kaya 2005; Farquhar 2002)
Letrozole (Hashim 2010)
Gonadotrophin analogue + the oral contraceptive pill (Taskin 1996)
Metformin + letrozole (Elgafor 2013)
Rosiglitazone (Roy 2010)
Ten of 22 studies were designed to assess the impact of LOD on the outcome measures specified compared to a different surgical technique/method of ovarian drilling. The comparisons were as follows:
Traditional LOD compared to;
Transvaginal ultrasound‐guided LOD (Badawy 2009)
Minilaparoscopic LOD (Zullo 2000)
Bipolar LOD (as opposed to unipolar) (Sharma 2006)
Unilateral LOD (Abdelhafeez 2013; Roy 2009; Sarouri 2015; Youssef 2007)
Harmonic scalpel LOD (Takeuchi 2002)
Transvaginal hydrolaparoscopy (THL) (Giampaolino 2016)
LOD using adjusted thermal dose in accordance with the ovarian volume (Zakherah 2011)
Two of 22 studies were head‐to‐head comparisons between different dosage/number of punctures of LOD (Selim 2011; Zhu 2010)
No studies compared LOD with no treatment/placebo, or laparotomy. No studies compared LOD to wedge resection.
Outcomes
The following studies reported on the specified outcome measures in this review:
1. Menstrual regularity: Abdelhafeez 2013; Ashrafinia 2009; Badawy 2009; Elgafor 2013; Hamed 2010; Hashim 2010; Hashim 2011; Kaya 2005; Palomba 2010; Roy 2009; Selim 2011; Takeuchi 2002; Zakherah 2011; Zhu 2010
2. Androgenic symptoms: Ashrafinia 2009; Badawy 2009; Farquhar 2002
3. Harms of surgical intervention: short‐ and long‐term surgical complications:
Adverse events relating to surgery: Zullo 2000; Zhu 2010
Ovarian hyperstimulation syndrome (OHSS): Kaya 2005; Roy 2010
Post‐surgical adhesions: Giampaolino 2016; Roy 2009; Zakherah 2011
Gastrointestinal side effects of metformin + clomiphene: Hashim 2011; Palomba 2010
4. Body weight or body mass index (kg/m2): Elgafor 2013; Hamed 2010; Farquhar 2002; Zakherah 2011
5. Change in waist circumference: None of the studies reported on waist circumference.
6. Testosterone levels (nmol/l), free testosterone (pg/ml) or free androgen index: Ashrafinia 2009; Badawy 2009; Elgafor 2013; Hamed 2010; Roy 2009; Roy 2010; Sarouri 2015; Selim 2011; Sharma 2006; Takeuchi 2002; Taskin 1996; Youssef 2007; Zakherah 2011; Zhu 2010; Zullo 2000
7. Metabolic measures: fasting glucose (nmol/L) or insulin levels (pmol/L) or haemoglobin A1C (HbA1C): Elgafor 2013; Hamed 2010; Roy 2010
8. Quality of life: Farquhar 2002 provided a questionnaire on the acceptability and convenience of both procedures.
Excluded studies
We excluded nine studies from the review. Reasons for exclusion include studies not being RCTs (three studies), one study was retracted, one study did not measure testosterone as planned, and the remainder did not meet our inclusion criteria.
Risk of bias in included studies
Risk of bias assessment focused on seven main domains: random sequence generation, allocation concealment, blinding, incomplete data outcome, selective reporting and other potential bias. The findings are summarized in Figure 2 and Figure 3, and details appear in individual 'Risk of bias' included studies tables.
2.
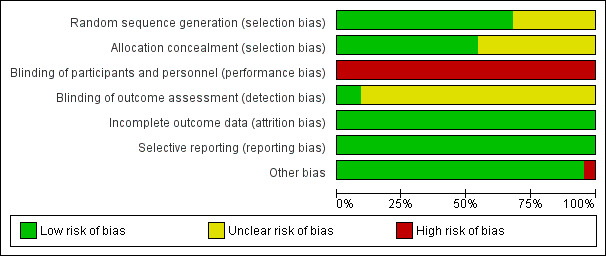
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
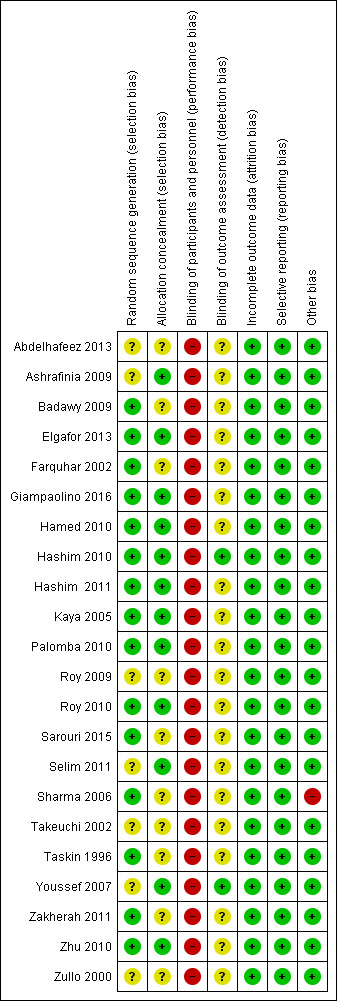
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Eighteen trials were at low risk of selection bias related to sequence generation. Four studies had unclear risk due to a lack of detail about their methods of sequence generation.
Allocation concealment
Twelve trials were at low risk of bias for allocation concealment, with the other 10 trials at unclear risk of bias.
Blinding
Blinding of participants
Due to the nature of the studies, blinding was often not possible. Fourteen trials had no means of blinding due to the surgical study design and so were at high risk of performance and detection bias; the remaining eight trials were deemed to be at high risk as they stated a lack of participant blinding.
Blinding of assessment
Most of the studies (20/22) were at unclear risk of assessment blinding. Two studies were at low risk, as they specified that the assessors were blinded.
Incomplete outcome data
Attrition rates in all studies were low and hence we rated all studies at low risk for this domain. One study (Giampaolino 2016) had attrition rates of 45/246, with 19 lost from one arm and 26 from the other.
Selective reporting
All the studies reported on the outcome specified.
Other potential sources of bias
One study (Sharma 2006) showed a statistically significant difference in baseline levels of glucose between the control and intervention groups.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
We include 22 studies in this review.
As the studies were sufficiently similar, we combined the data using a fixed‐effect model in the following comparisons:
LOD versus medical interventions (stratified by type of medication)
LOD versus other surgical interventions (stratified by type of surgery)
LOD with four to five punctures versus LOD with two or fewer punctures
LOD versus variable energy
1. LOD versus medical treatments
Primary outcomes
1.1 Improvement of menstrual regularity
Seven studies reported this outcome. We did not pool studies comparing different medications, as this resulted in high statistical heterogeneity (I2 = 66%). We interpret the underlying cause of the heterogeneity to be attributable to the differences between the interventions.
1.1.1. LOD versus metformin: Two studies reported this comparison. Findings were inconsistent and the data were unsuitable for pooling due to high statistical heterogeneity (I2=85%). There was no obvious difference between the studies that might explain the heterogeneity. One study (Hamed 2010) suggested a benefit in the LOD arm (OR 2.32, 95% CI 1.02 to 5.28, 110 women) while the other study (Ashrafinia 2009) had the opposite direction of effect but found no conclusive evidence of a difference between the groups (OR 0.55, 95% CI 0.27 to 1.13, 126 women). See Analysis 1.1; Figure 4.
1.1. Analysis.
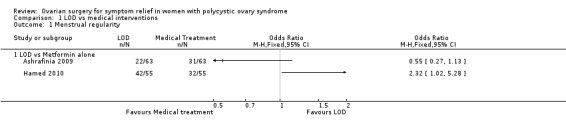
Comparison 1 LOD vs medical interventions, Outcome 1 Menstrual regularity.
4.
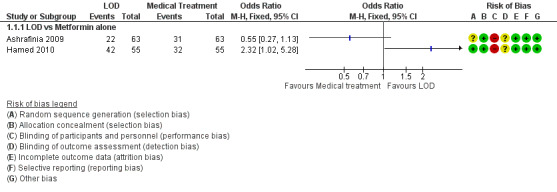
Forest plot of comparison: 1 LOD vs medical interventions, outcome: 1.1 Menstrual regularity.
1.1.2. LOD versus metformin + clomiphene: There was no clear evidence of a difference between the groups at six months (OR 1.02, 95% CI 0.64 to 1.64, 2 RCTs, n = 332, I2 = 13%, low‐quality evidence) (Hashim 2011; Palomba 2010).
1.1.3 LOD versus gonadotrophin: One study showed evidence of benefit of LOD over gonadotrophin at six months (OR 19.20, 95% CI 3.17 to 116.45, 1 RCT, n = 35, low‐quality evidence) (Kaya 2005).
1.1.4. LOD versus letrozole: There was no clear evidence of a difference between the groups at six months (OR 1.08, 95% CI 0.64 to 1.84, 1 RCT, n = 260, moderate‐quality evidence) (Hashim 2010).
1.1.5. LOD versus metformin + letrozole: There was no clear evidence of a difference between the groups at six months (OR 0.95, 95% CI 0.49 to 1.81, 1 RCT, n = 146, moderate‐quality evidence) (Elgafor 2013).
See Analysis 1.2; Figure 5
1.2. Analysis.
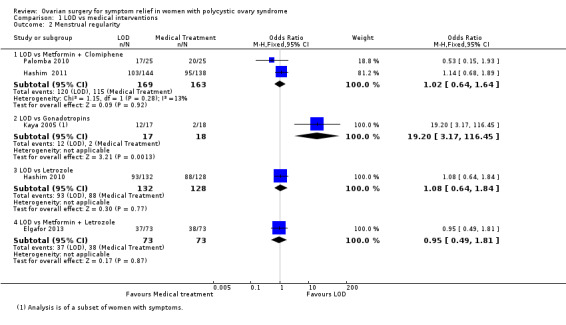
Comparison 1 LOD vs medical interventions, Outcome 2 Menstrual regularity.
5.
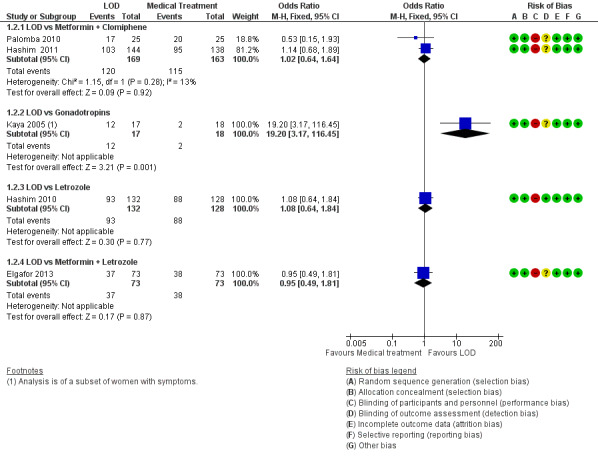
Forest plot of comparison: 1 LOD vs medical interventions, outcome: 1.2 Menstrual regularity.
1.2 Improvement of androgenic symptoms (hirsutism or acne, or both)
1.2.1. LOD versus metformin: There was no clear evidence of a difference between the groups at six months (OR 1.00, 95% CI 0.42 to 2.37, 1 RCT, n = 126, moderate‐quality evidence) (Ashrafinia 2009).
1.2.2. LOD versus gonadotrophins: There was no clear evidence of a difference between the groups in improvement of acne (OR 3.20, 95% CI 0.33 to 30.94, 1 RCT, n = 25, low‐quality evidence) or hirsutism at six months (OR 2.31, 95% CI 0.22 to 23.89, 1 RCT, n = 25, very low‐quality evidence) (Farquhar 2002).
Secondary outcomes
1.3 Harms
1.3.1. LOD versus metformin + clomiphene: There were more gastrointestinal side effects in the metformin + clomiphene group at six months (OR 0.05, 95% CI 0.01 to 0.36, 2 RCTs, n = 332 I2 = 0%, moderate‐quality evidence) (Hashim 2011; Palomba 2010).
1.3.2. LOD versus gonadotrophin: There was no clear evidence of a difference between the groups in OHSS rate at six months (OR 0.08, 95% CI 0.00 to 1.61, 1 study, n = 33, low‐quality evidence) (Kaya 2005).
1.3.3. LOD versus rosiglitazone: There was no occurrence of OHSS in either group at six months (Roy 2010).
1.4 Body weight or body mass index (kg/m2)
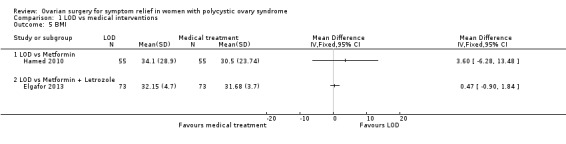
1.4.1. LOD versus metformin: There was a higher body mass index (BMI) at six months follow‐up in the LOD group compared to the metformin group (MD 3.60, 95% CI ‐6.28 to 13.48, 1 RCT, n = 110) (Hamed 2010).
1.4.2. LOD versus metformin + letrozole: There was no clear evidence of a difference in BMI at six months follow‐up between groups (MD 0.47, 95% CI ‐0.90 to 1.84, 1 RCT, n = 146) (Elgafor 2013).
1.5 Waist circumference
None of the studies reported on waist circumference.
1.6 Testosterone levels (nmol/L), free testosterone (pg/ml) or free androgen index
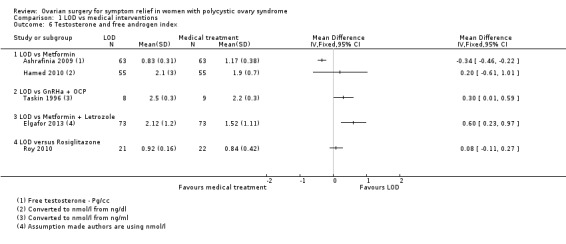
1.6.1. LOD versus metformin: There was no clear evidence of a difference in free testosterone levels post‐surgery at six months follow‐up, (MD ‐0.34, 95% CI ‐0.46 to ‐0.22; n = 126 ; 1 RCT) (Ashrafinia 2009) There was no clear evidence of a difference in mean testosterone levels post‐surgery at six months follow‐up, (MD 0.20, 95% CI ‐0.61 to 1.01, 1 RCTs, n = 110) Hamed 2010).
1.6.2. LOD versus gonadotropin‐releasing hormone antagonist (GnRHa) + oral contraceptive pill (OCP): There was a higher testosterone level one week post‐surgery or at the first menstruation after treatment in the LOD group compared to the GnRHa + OCP group (MD 0.3, 95% CI 0.01 to 0.59, 1 RCT, n = 17) (Taskin 1996).
1.6.3. LOD versus metformin + letrozole: There was a higher testosterone level after LOD compared to metformin + letrozole use (MD 0.60, 95% CI 0.23 to 0.97, 1 RCT, n = 146) (Elgafor 2013).
1.6.4. LOD versus rosiglitazone: There was no clear evidence of a difference between the groups in testosterone levels between rosiglitazone and LOD (MD 0.08, 95% CI ‐0.11 to 0.27, 1 RCT, n = 43) (Roy 2010).
1.7 Metabolic measures: fasting glucose or insulin levels or ratios or haemoglobin A1C (HbA1C)
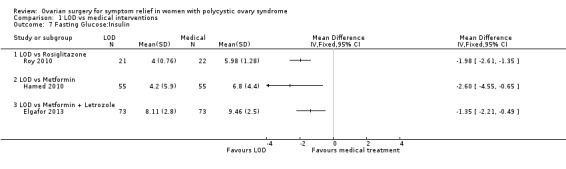
1.7.1. LOD versus rosiglitazone: There was a lower glucose/insulin ratio in the LOD group compared to the rosiglitazone group (MD ‐1.98, 95% CI ‐2.61 to ‐1.35, 1 RCT, n = 43) (Roy 2010).
1.7.2. LOD versus metformin: There was a lower glucose/insulin ratio in the LOD group compared to the metformin group (MD ‐2.60, 95% CI ‐4.55 to ‐0.65, 1 RCT, n = 110) (Hamed 2010).
1.7.3. LOD versus metformin + letrozole: There was a lower glucose/insulin ratio in the LOD group compared to the metformin + letrozole group (MD ‐1.35, 95% CI ‐2.21 to ‐0.49, 1 RCT, n = 146) (Elgafor 2013).
1.8 Quality of life
1.8.1. LOD versus gonadotrophins: Farquhar 2002 provided a questionnaire to the 19 women who underwent both LOD and gonadotrophins regarding the acceptability and convenience of both procedures. Seventeen returned the questionnaire; 15 women preferred LOD and two preferred gonadotrophins. LOD was described as "less traumatic".
2. Bilateral LOD versus other surgical techniques
Primary outcomes
2.1 Improvement of menstrual regularity
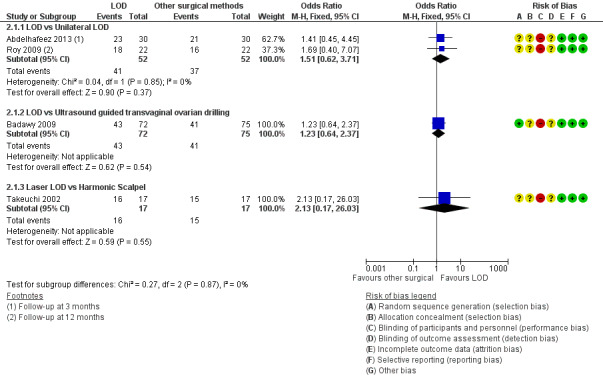
2.1.1. LOD versus unilateral LOD: There was no clear evidence of a difference between the groups (OR 1.51, 95% CI 0.62 to 3.71, 2 RCTs, n = 104, I2 = 0%, moderate‐quality evidence) (Abdelhafeez 2013; Roy 2009). Abdelhafeez 2013 reported at follow‐up of three months (OR 1.41, 95% CI 0.45 to 4.45, 1 RCT, n = 60), while Roy 2009 reported at follow‐up of 12 months (OR 1.69, 95% CI 0.40 to 7.07, 1 RCT, n = 44). We have pooled these data, as they are otherwise similar studies.
2.1.2. LOD versus transvaginal ultrasound‐guided LOD: There was no clear evidence of a difference between the groups at six months (OR 1.23, 95% CI 0.64 to 2.37, 1 RCT, n = 147, low‐quality evidence) (Badawy 2009).
2.1.3. LOD with Hd‐YAG laser versus LOD with harmonic scalpel: There was no clear evidence of a difference between the groups at 12 months (OR 2.13, 95% CI 0.17 to 26.03, 1 RCT, n = 34, low‐quality evidence) (Takeuchi 2002).
See Analysis 2.1; Figure 6.
2.1. Analysis.
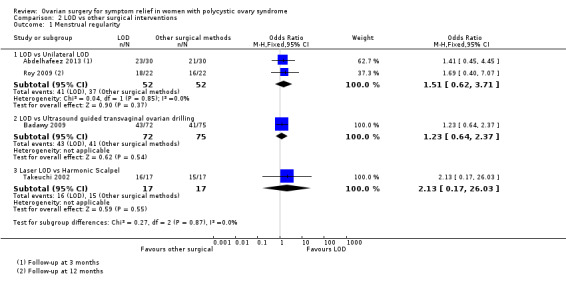
Comparison 2 LOD vs other surgical interventions, Outcome 1 Menstrual regularity.
6.
Forest plot of comparison: 2 LOD vs other surgical interventions, outcome: 2.1 Menstrual regularity.
2.2 Improvement of androgenic symptoms (hirsutism or acne, or both)
LOD versus transvaginal ultrasound‐guided LOD: There was no clear evidence of a difference between the groups in hirsutism (OR 1.09, 95% CI 0.30 to 3.91, 1 RCT, n = 39, low‐quality evidence) or acne at six months (OR 0.84, 95% CI 0.20 to 3.50, 1 RCT, n = 31, low‐quality evidence) (Badawy 2009).
Secondary outcomes
2.3 Harms
2.3.1. LOD versus unilateral LOD: There were no post‐surgical peri‐ovarian adhesions found in a subgroup of women who returned for repeat laparoscopy or caesarean section up to 12 months post‐intervention (Roy 2009).
2.3.2. LOD versus minilaparoscopic LOD under local anaesthetic and conscious sedation: There were no complications from local anaesthetic (Zullo 2000).
2.3.3 LOD versus transvaginal hydrolaparoscopy: There were significantly fewer women with post‐procedure adhesions with THL compared to LOD at six months (OR 0.10, 95% CI 0.05 to 0.18, 1 RCT, n = 246, very low‐quality evidence) (Giampaolino 2016).
2.4 Body weight or body mass index (kg/m2)
None of the studies reported on this outcome.
2.5 Waist circumference
None of the studies reported on this outcome.
2.6 Testosterone levels (nmol/L), free testosterone (pg/ml) or free androgen index
2.4.1. LOD versus transvaginal ultrasound‐guided LOD: The mean testosterone levels were found to be higher in the LOD group compared to the ultrasound‐guided transvaginal LOD group (MD 0.30, 95% CI 0.13 to 0.47, 1 RCT, n = 163) (Badawy 2009).
2.4.2. LOD versus minilaparoscopic LOD under local anaesthetic and conscious sedation: There was no clear evidence of a difference between the groups (MD 0.00, 95% CI ‐0.43 to 0.43, 1 RCT, n = 62) (Zullo 2000).
2.4.3. LOD versus unilateral LOD: There was no clear evidence of a difference in mean testosterone between the groups (MD 0.03, 95% CI ‐0.04 to 0.09, 2 RCTs, n = 112, I2 = 0%) (Roy 2009; Youssef 2007). There was no clear evidence in free testosterone between the groups (MD ‐0.30, 95% CI ‐0.84 to 0.24, 1 RCTs, n = 90) (Sarouri 2015).
2.4.4. LOD with Hd‐YAG Laser versus LOD with harmonic scalpel: There was no clear evidence of difference between the groups (MD 0.0, 95% CI ‐0.28 to 0.28, 1 RCT, n = 34) (Takeuchi 2002).
2.7 Metabolic measures: fasting glucose or insulin levels or ratio or haemoglobin A1C (Hb1AC)
None of the studies reported on this outcome.
2.8 Quality of life
None of the studies reported on this outcome.
3. LOD with different numbers of punctures per ovary
Primary outcomes
3.1 Improvement of menstrual regularity
LOD four to five punctures versus two or fewer punctures per ovary: There is evidence of benefit of applying four or five punctures per ovary compared to two or fewer punctures at six months (OR 16.04, 95% CI 4.19 to 61.34, 2 RCTs, n = 73, I2 = 0%, low‐quality evidence) (Zhu 2010; Selim 2011).
See Analysis 3.1; Figure 7.
3.1. Analysis.
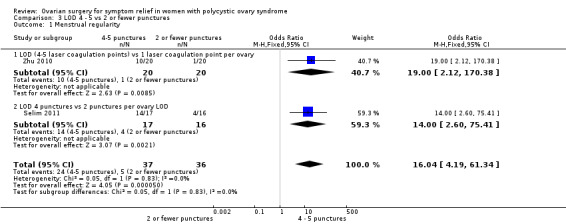
Comparison 3 LOD 4 ‐ 5 vs 2 or fewer punctures, Outcome 1 Menstrual regularity.
7.
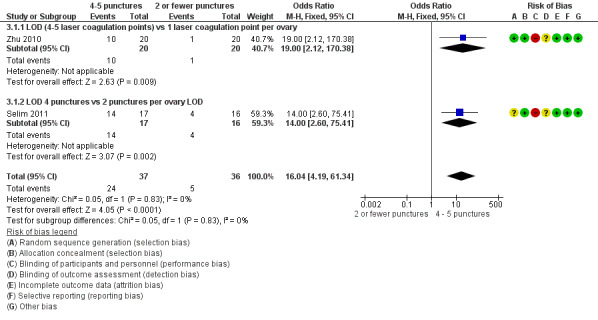
Forest plot of comparison: 3 LOD 4 ‐ 5 vs 2 or fewer punctures, outcome: 3.1 Menstrual regularity.
3.2 Improvement of androgenic symptoms (hirsutism or acne, or both)
None of the studies reported on this outcome.
Secondary outcomes
3.3 Harms
LOD four to five punctures versus two or fewer punctures: There were no surgical adverse events in any group (Zhu 2010).
3.4 Body weight or body mass index (kg/m2)
None of the studies reported on this outcome.
3.5 Waist circumference
None of the studies reported on this outcome.
3.6 Testosterone levels (nmol/L) or free androgen index
Testosterone
3.6.1. LOD four to five punctures versus two or fewer punctures: There is evidence of a difference in applying more punctures per ovary compared to two or fewer punctures (MD ‐0.90, 95% CI ‐1.12 to ‐0.68, 2 RCTs, n = 73, I2 = 0%) (Selim 2011; Zhu 2010) (Analysis 3.2).
3.2. Analysis.
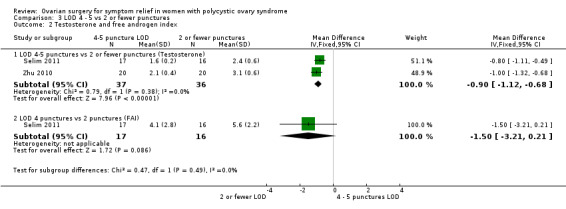
Comparison 3 LOD 4 ‐ 5 vs 2 or fewer punctures, Outcome 2 Testosterone and free androgen index.
Free androgen index (FAI)
3.6.2. LOD four to five punctures versus two or fewer punctures: There was no clear evidence of a difference between the groups (MD ‐1.50, 95% CI ‐3.21 to 0.21, 1 RCT, n = 33) (Selim 2011) (Analysis 3.2).
3.7 Metabolic measures: fasting glucose or insulin levels or haemoglobin A1C (HbA1C)
None of the studies reported on this outcome.
3.8 Quality of life
None of the studies reported on this outcome.
4. LOD with various energy modalities
Primary outcomes
4.1 Improvement of menstrual regularity
4.1.1. LOD versus LOD using adjusted thermal dose in accordance with the ovarian volume: There was no clear evidence of a difference between the groups at six months (OR 0.42, 95% CI 0.16 to 1.14, 1 RCT, n = 115, very low‐quality evidence) (Zakherah 2011).
4.1.2. Unipolar LOD versus bipolar LOD: There was no clear evidence of a difference between the groups at three months (OR 1.00, 95% CI 0.05 to 18.57, 1 RCT, n = 20, very low‐quality evidence) (Sharma 2006).
See Analysis 4.1; Figure 8.
4.1. Analysis.
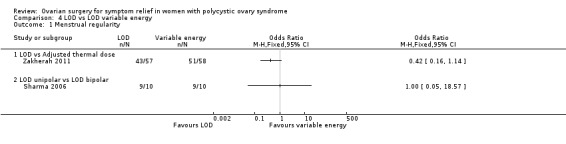
Comparison 4 LOD vs LOD variable energy, Outcome 1 Menstrual regularity.
8.
Forest plot of comparison: 4 LOD vs LOD variable energy, outcome: 4.1 Menstrual regularity.
4.2 Improvement of androgenic symptoms (hirsutism or acne, or both)
None of the studies reported on this outcome.
Secondary outcomes
4.3 Harms
LOD versus LOD using adjusted thermal dose in accordance with ovarian volume: There was no clear evidence of a difference between the groups in the adhesions found at second‐look laparoscopy at six months (OR 0.96, 95% CI 0.32 to 2.88, 1 RCT, n = 64, very low‐quality evidence) (Zakherah 2011).
4.4 Body weight or body mass index (kg/m2)
LOD versus LOD using adjusted thermal dose in accordance with ovarian volume: There was no clear evidence of a difference between the groups (MD 0.20, 95% CI ‐0.71 to 1.11, 1 RCT, n = 115) (Zakherah 2011).
4.5 Waist circumference
None of the studies reported on this outcome.
4.6 Testosterone levels (nmol/L) or free androgen index
4.6.1. LOD versus LOD using adjusted thermal dose in accordance with ovarian volume: Testosterone levels were lower in the thermal dose group (MD 0.70, 95% CI 0.57 to 0.83, 1 RCT, n = 115) (Zakherah 2011) (Analysis 4.4).
4.4. Analysis.
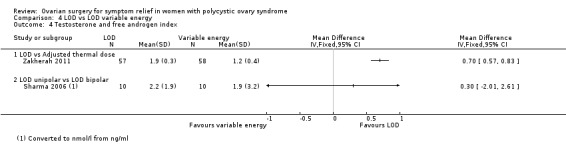
Comparison 4 LOD vs LOD variable energy, Outcome 4 Testosterone and free androgen index.
4.6.2. Unipolar LOD versus bipolar LOD: There was no clear evidence of a difference between the groups in the testosterone levels (MD 0.30, 95% CI ‐2.01 to 2.61, 1 RCT, n = 20) (Sharma 2006) (Analysis 4.4).
4.7 Metabolic measures: fasting glucose or insulin levels or haemoglobin A1C (HbA1C)
4.5.1. Unipolar LOD versus bipolar LOD: There was a significantly lower glucose insulin ratio at three months after surgery in the group who had unipolar LOD compared to those with bipolar LOD (MD 4.10, 95% CI 3.08 to 5.12, 1 RCT, n = 20) (Sharma 2006). However, in this study there was a significant disparity between the baseline characteristics of the glucose insulin ratio between the two groups.
4.8 Quality of life
None of the studies reported on this outcome.
5. LOD versus laparotomy
There were no studies that investigated this.
Other analyses
Upon completing study selection and data extraction we considered no subgroups to be relevant to our analysis.
We conducted sensitivity analyses as planned. The sensitivity analyses resulted in no significant modification of the results or the strength of associations after applying the above considerations to our data. No imputation of data was required. We are unable to address publication bias in this review.
Discussion
Summary of main results
For women with menstrual irregularity, acne or hirsutism due to PCOS there was evidence of benefit of LOD over gonadotrophin, and for high puncture number versus low puncture number in menstrual regularity. These outcomes were based on one and two RCTs respectively. As gonadotrophins are unlikely to be prescribed solely for these indications, it is important to note that these outcomes are secondary.
There was no clear evidence of a difference in the rates of improvement of menstrual regularity or androgenic symptoms when LOD was compared to other medical or other surgical interventions.
Studies reported low adverse effects with LOD, and metformin and clomiphene is associated with greater gastrointestinal side effects compared to LOD. Given the evidence supporting metformin as beneficial in the improvement of menstrual regularity (Tang 2012), LOD may be considered a non‐inferior but invasive alternative in those unable to tolerate metformin.
There were significantly fewer post‐procedure adhesions with transvaginal hydrolaparoscopy when compared with LOD, but this was based on a single RCT.
Overall completeness and applicability of evidence
The studies included addressed the review question, and the participants and outcomes were all relevant. Certain comparisons of surgical technique will not be relevant in centres where the equipment is not available. There were no studies comparing LOD versus no treatment and hence our conclusions can only be drawn from head‐to‐head comparisons of LOD with medical or surgical treatments. Current evidence is largely limited by data obtained from single or small numbers of randomised controlled studies. When confronted with a woman with symptoms of PCOS, this evidence needs to be applied in the wider context of their symptoms and goals, as neither LOD nor the medical interventions are considered first‐line treatments. There may be additional benefit, however, if these interventions are otherwise indicated; they should form part of patient counselling and may guide certain decisions.
Quality of the evidence
The quality of the evidence ranged from very low to moderate quality. The main limitations were imprecision associated with the low number of studies, inconsistency and risk of bias associated with the inability to blind participants (see Table 1; Table 2; Table 3; Table 4). The risk of performance bias in individual studies was high, due to a lack of blinding of outcome assessors, which can be expected with surgical comparisons. There were too few studies to assess risk of publication bias. Other bias was rare, with the exception of Sharma 2006 which we rated at high risk of other bias due to a significant disparity in the baseline characteristics of glucose/insulin ratio.
Potential biases in the review process
We executed an inclusive search, minimising as far as possible incomplete identification of studies and the risk of reporting bias. We made every effort to identify all potentially eligible studies, and sought additional data from study authors as necessary. However, it is possible that there are unpublished studies that we did not retrieve.
Agreements and disagreements with other studies or reviews
We found no other studies or reviews on this topic.
Authors' conclusions
Implications for practice.
There was no clear evidence that LOD improves menstrual regularity or the androgenic symptoms of PCOS, compared to most of the medical treatments used in the included studies. LOD was associated with fewer gastrointestinal side effects compared to metformin and clomiphene.
There was also no clear evidence of different effectiveness between types of LOD, except that LOD with four to five punctures per ovary may be more effective than with two or fewer punctures. There was little evidence comparing LOD with different types of surgery, although one study concluded that transvaginal hydrolaparoscopy had a lower risk of adhesions than LOD.
There was evidence from one small study of benefit from LOD compared to gonadotrophins in terms of menstrual regulation. However, gonadotrophins are seldom used for this indication.
Implications for research.
Studies are required to compare LOD to placebo or to no treatment in order to clarify the impact of LOD alone on the management of symptoms of PCOS. Our conclusions on LOD versus gonadotrophins are based on the results of one small RCT and correlation with further data would be beneficial. Most studies enrolled women for fertility treatment, and primary RCT evidence of surgical treatments of symptoms of PCOS would enhance the body of evidence. Implications of both medical and surgical treatments were generally analyzed in the short and medium term. Long‐term, prospective follow‐up of women with treatments for PCOS would add to our knowledge of the relative effects and harms involved.
Acknowledgements
We thank the Cochrane Gynaecology and Fertility Group for providing us with the search strategy and for proof‐reading the review. We thank Dr Yvonne Obura and Dr Bindiya Jhamb for their contributions to this review.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group (CGF) specialised register search strategy
Procite platform
From inception to 17 October 2016
Keywords CONTAINS "polycystic ovary morphology" or "polycystic ovary syndrome" or "PCOS" or Title CONTAINS "polycystic ovary morphology" or "polycystic ovary syndrome" or "PCOS"
AND
Keywords CONTAINS "laparoscopic" or "laparoscopic ovarian drilling" or "laparoscopic surgery"or "laparoscopic surgical treatment"or "laparoscopic ovarian electro drilling"or"ovarian drilling"or"ovarian resection"or"ovarian surgery"or"ovarian wedge resection"or "resection" or "laparoscopic bipolar coagulation"or"laparoscopic coagulation techniques"or"laparoscopic electrocautery"or"laparoscopic ovarian cautery"or "LaparoSonic coagulation shears"or "laser", "laparotomy"or "laser drilling"or"electrocautery"or "Electrocoagulation"or"Harmonic scalpel"or "hydro laparoscopy"or "diathermy" or Title CONTAINS"laparoscopic" or "laparoscopic ovarian drilling" or "laparoscopic surgery"or "laparoscopic surgical treatment"or "laparoscopic ovarian electro drilling"or"ovarian drilling"or"ovarian resection"or"ovarian surgery"or"ovarian wedge resection"or "resection" or "laparoscopic bipolar coagulation"or"laparoscopic coagulation techniques"or"laparoscopic electrocautery"or"laparoscopic ovarian cautery" (105 hits)
Appendix 2. CENTRAL Register of Studies Online (CRSO) search strategy
Web platform
searched 17 October 2016
#1 MESH DESCRIPTOR Polycystic Ovary Syndrome EXPLODE ALL TREES 874
#2 (Polycystic Ovar*):TI,AB,KY 1674
#3 (PCOD or PCOS):TI,AB,KY 1283
#4 (stein‐leventhal or leventhal):TI,AB,KY 16
#5 (Ovar* Polycystic):TI,AB,KY 513
#6 #1 OR #2 OR #3 OR #4 OR #5 1871
#7 MESH DESCRIPTOR Hand‐Assisted Laparoscopy EXPLODE ALL TREES 7
#8 MESH DESCRIPTOR Laparoscopy EXPLODE ALL TREES 4243
#9 (ovar* adj2 surg*):TI,AB,KY 372
#10 (surg* adj2 ovar*):TI,AB,KY 330
#11 (ovar* adj2 resect*):TI,AB,KY 7
#12 (ovar* adj2 drill*):TI,AB,KY 62
#13 laparoscop*:TI,AB,KY 9365
#14 MESH DESCRIPTOR Laparotomy EXPLODE ALL TREES 622
#15 Laparotom*:TI,AB,KY 1893
#16 electrocauter*:TI,AB,KY 404
#17 MESH DESCRIPTOR Electrocoagulation EXPLODE ALL TREES 625
#18 Electrocoagulation:TI,AB,KY 716
#19 (harmonic scalpel*):TI,AB,KY 173
#20 laser*:TI,AB,KY 10524
#21 Hydrolaparoscop*:TI,AB,KY 8
#22 MESH DESCRIPTOR Diathermy EXPLODE ALL TREES 817
#23 Diathermy:TI,AB,KY 494
#24 microlaparoscop*:TI,AB,KY 26
#25 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 23031
#26 #6 AND #25 144
Appendix 3. MEDLINE search strategy
OVID platform
From 1946 to 17 October 2016
1 exp Polycystic Ovary Syndrome/ (11854) 2 Polycystic Ovar$.tw. (12964) 3 PCOD.tw. (274) 4 PCOS.tw. (8120) 5 (stein‐leventhal or leventhal).tw. (717) 6 (ovar$ adj2 sclerocystic).tw. (99) 7 (ovar$ adj2 degeneration).tw. (126) 8 Ovar$ Polycystic.tw. (35) 9 or/1‐8 (15915) 10 exp Laparoscopy/ (80117) 11 (ovar$ adj2 surg$).tw. (1874) 12 (ovar$ adj2 resect$).tw. (511) 13 (ovar$ adj2 drill$).tw. (237) 14 laparoscop$.tw. (101216) 15 exp Laparotomy/ (17222) 16 Laparotom$.tw. (43177) 17 electrocauter$.tw. (3078) 18 exp Electrocoagulation/ (11204) 19 Electrocoagulation.tw. (2763) 20 harmonic scalpel$.tw. (817) 21 laser$.tw. (219657) 22 Hydrolaparoscop$.tw. (86) 23 exp Diathermy/ (13573) 24 diathermy.tw. (2748) 25 or/10‐24 (399202) 26 9 and 25 (889) 27 randomized controlled trial.pt. (432907) 28 controlled clinical trial.pt. (91818) 29 randomized.ab. (373189) 30 randomised.ab. (76564) 31 placebo.tw. (184972) 32 clinical trials as topic.sh. (180215) 33 randomly.ab. (265223) 34 trial.ti. (163275) 35 (crossover or cross‐over or cross over).tw. (71495) 36 or/27‐35 (1126434) 37 exp animals/ not humans.sh. (4325953) 38 36 not 37 (1038653) 39 26 and 38 (125)
Appendix 4. Embase search strategy
OVID platform
From 1980 to 17 October 2016
1 ovary polycystic disease/ (17673) 2 Polycystic Ovar$.tw. (14205) 3 PCOD.tw. (318) 4 PCOS.tw. (9235) 5 (stein‐leventhal or leventhal).tw. (652) 6 (ovar$ adj2 sclerocystic).tw. (80) 7 (ovar$ adj2 degeneration).tw. (106) 8 Ovar$ Polycystic.tw. (37) 9 or/1‐8 (20299) 10 exp Laparoscopy/ (103721) 11 ovar$ surg$.tw. (428) 12 (ovar$ adj2 resect$).tw. (615) 13 (ovar$ adj2 drill$).tw. (320) 14 exp Laparotomy/ (54228) 15 laparoscop$.tw. (124543) 16 Laparotom$.tw. (47312) 17 electrocauter$.tw. (3533) 18 exp Electrocoagulation/ (9464) 19 Electrocoagulation.tw. (2635) 20 harmonic scalpel$.tw. (1185) 21 laser$.tw. (185050) 22 Hydrolaparoscop$.tw. (118) 23 exp Diathermy/ (4774) 24 diathermy.tw. (2781) 25 or/10‐24 (403611) 26 Clinical Trial/ (837351) 27 Randomized Controlled Trial/ (355791) 28 exp randomization/ (64250) 29 Single Blind Procedure/ (19230) 30 Double Blind Procedure/ (116818) 31 Crossover Procedure/ (40987) 32 Placebo/ (249956) 33 Randomi?ed controlled trial$.tw. (106976) 34 Rct.tw. (15484) 35 random allocation.tw. (1355) 36 randomly allocated.tw. (21244) 37 allocated randomly.tw. (1971) 38 (allocated adj2 random).tw. (720) 39 Single blind$.tw. (15011) 40 Double blind$.tw. (145763) 41 ((treble or triple) adj blind$).tw. (410) 42 placebo$.tw. (205894) 43 prospective study/ (270083) 44 or/26‐43 (1407017) 45 case study/ (29538) 46 case report.tw. (270756) 47 abstract report/ or letter/ (910063) 48 or/45‐47 (1204366) 49 44 not 48 (1368607) 50 9 and 25 and 49 (289)
Appendix 5. PsycINFO search strategy
OVID platform
From 1806 to 17 October 2016
1 exp Endocrine Sexual Disorders/ (1074) 2 Polycystic Ovar$.tw. (333) 3 PCOD.tw. (5) 4 PCOS.tw. (211) 5 (stein‐leventhal or leventhal).tw. (272) 6 (ovar$ adj2 sclerocystic).tw. (1) 7 (ovar$ adj2 degeneration).tw. (0) 8 Ovar$ Polycystic.tw. (0) 9 or/1‐8 (1563) 10 exp Surgery/ (49607) 11 (ovar$ adj2 surg$).tw. (41) 12 (ovar$ adj2 resect$).tw. (4) 13 (ovar$ adj2 drill$).tw. (0) 14 laparoscop$.tw. (393) 15 Laparotom$.tw. (136) 16 electrocauter$.tw. (17) 17 Electrocoagulation.tw. (67) 18 harmonic scalpel$.tw. (0) 19 laser$.tw. (2879) 20 Hydrolaparoscop$.tw. (0) 21 diathermy.tw. (28) 22 or/10‐21 (52666) 23 9 and 22 (41) 24 random.tw. (48055) 25 control.tw. (372760) 26 double‐blind.tw. (20185) 27 clinical trials/ (9924) 28 placebo/ (4697) 29 exp Treatment/ (665894) 30 or/24‐29 (1027614) 31 23 and 30 (38)
Data and analyses
Comparison 1. LOD vs medical interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Menstrual regularity | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 LOD vs Metformin alone | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Menstrual regularity | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 LOD vs Metformin + Clomiphene | 2 | 332 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.64, 1.64] |
| 2.2 LOD vs Gonadotropins | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 19.2 [3.17, 116.45] |
| 2.3 LOD vs Letrozole | 1 | 260 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.64, 1.84] |
| 2.4 LOD vs Metformin + Letrozole | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.49, 1.81] |
| 3 Improvement in androgenic symptoms (hirsutism/acne) | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 LOD vs Metformin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 LOD vs Gonadotrophins | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Harms | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 LOD vs Metformin + Clomiphen | 2 | 332 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.36] |
| 4.2 LOD vs Gonadotrophins | 1 | 33 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.61] |
| 5 BMI | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 LOD vs Metformin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 LOD vs Metformin + Letrozole | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Testosterone and free androgen index | 5 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 LOD vs Metformin | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 LOD vs GnRHa + OCP | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 LOD vs Metformin + Letrozole | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 LOD versus Rosiglitazone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Fasting Glucose:Insulin | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 LOD vs Rosiglitazone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 LOD vs Metformin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 LOD vs Metformin + Letrozole | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.3. Analysis.
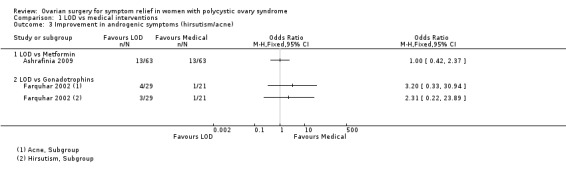
Comparison 1 LOD vs medical interventions, Outcome 3 Improvement in androgenic symptoms (hirsutism/acne).
1.4. Analysis.
Comparison 1 LOD vs medical interventions, Outcome 4 Harms.
1.5. Analysis.
Comparison 1 LOD vs medical interventions, Outcome 5 BMI.
1.6. Analysis.
Comparison 1 LOD vs medical interventions, Outcome 6 Testosterone and free androgen index.
1.7. Analysis.
Comparison 1 LOD vs medical interventions, Outcome 7 Fasting Glucose:Insulin.
Comparison 2. LOD vs other surgical interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Menstrual regularity | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 LOD vs Unilateral LOD | 2 | 104 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.62, 3.71] |
| 1.2 LOD vs Ultrasound guided transvaginal ovarian drilling | 1 | 147 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.64, 2.37] |
| 1.3 Laser LOD vs Harmonic Scalpel | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.13 [0.17, 26.03] |
| 2 Improvement in androgenic symptoms (hirsutism/acne) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Hirsutism | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Acne | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Harms: Adhesions | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Testosterone and free androgen index | 6 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 LOD vs Ultrasound guided transvaginal ovarian drilling | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 LOD versus mini laparoscopy with sedation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 LOD vs unilateral LOD | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Laser LOD vs Harmonic Scalpel | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.2. Analysis.
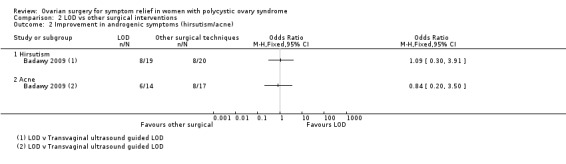
Comparison 2 LOD vs other surgical interventions, Outcome 2 Improvement in androgenic symptoms (hirsutism/acne).
2.3. Analysis.
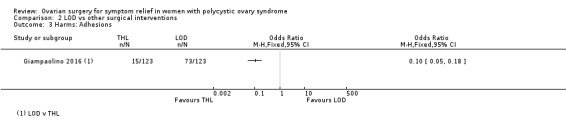
Comparison 2 LOD vs other surgical interventions, Outcome 3 Harms: Adhesions.
2.4. Analysis.
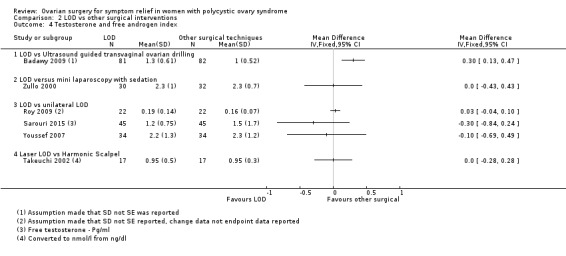
Comparison 2 LOD vs other surgical interventions, Outcome 4 Testosterone and free androgen index.
Comparison 3. LOD 4 ‐ 5 vs 2 or fewer punctures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Menstrual regularity | 2 | 73 | Odds Ratio (M‐H, Fixed, 95% CI) | 16.04 [4.19, 61.34] |
| 1.1 LOD (4‐5 laser coagulation points) vs 1 laser coagulation point per ovary | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 19.0 [2.12, 170.38] |
| 1.2 LOD 4 punctures vs 2 punctures per ovary LOD | 1 | 33 | Odds Ratio (M‐H, Fixed, 95% CI) | 14.0 [2.60, 75.41] |
| 2 Testosterone and free androgen index | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 LOD 4‐5 punctures vs 2 or fewer punctures (Testosterone) | 2 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.12, ‐0.68] |
| 2.2 LOD 4 punctures vs 2 punctures (FAI) | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐3.21, 0.21] |
Comparison 4. LOD vs LOD variable energy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Menstrual regularity | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 LOD vs Adjusted thermal dose | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 LOD unipolar vs LOD bipolar | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Harms | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 BMI | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Testosterone and free androgen index | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 LOD vs Adjusted thermal dose | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 LOD unipolar vs LOD bipolar | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Metabolic measures | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 LOD (unipolar) vs bipolar | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.2. Analysis.
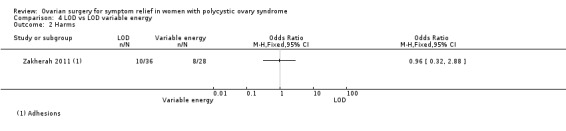
Comparison 4 LOD vs LOD variable energy, Outcome 2 Harms.
4.3. Analysis.
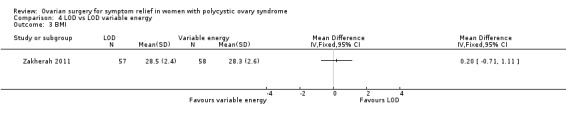
Comparison 4 LOD vs LOD variable energy, Outcome 3 BMI.
4.5. Analysis.
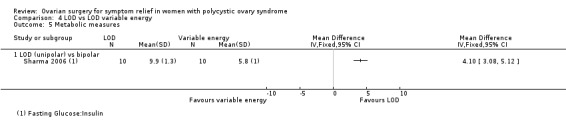
Comparison 4 LOD vs LOD variable energy, Outcome 5 Metabolic measures.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdelhafeez 2013.
| Methods | A randomised controlled trial | |
| Participants | 60 women attending the outpatient infertility clinic with a diagnosis of CC‐resistant PCOS at Ain Shams University Maternity Hospital, Cairo, Egypt between February 2010 and September 2010 | |
| Interventions | 30 women underwent unilateral ovarian drilling and 30 underwent bilateral ovarian drilling | |
| Outcomes | The primary outcome was documented ovulation through a midluteal serum progesterone > 3 ng/ml 3 months after laparoscopy
Secondary outcomes included regularity of menstrual cycles and basal levels of serum FSH and LH within 3 months after laparoscopy No harms reported. |
|
| Notes | Funding source: Not stated. Declarations of interest: Not stated. Email sent requesting further information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible due to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None detected |
Ashrafinia 2009.
| Methods | A randomised controlled trial | |
| Participants | 126 women with PCOS were randomised from March 2006 until February 2008, between the ages of 15 and 45 years, with a history of infertility for at least 1 year and 3 treatment cycles with no response to CC (CC‐resistant), conducted in Tehran, Iran | |
| Interventions | Participants received metformin treatment (n = 63) or underwent LOD (n = 63) | |
| Outcomes | The primary outcome measure was menstrual regularity. The levels of FSH, LH, free testosterone and the level of hirsutism assessed using the Ferriman Gallwey score were included. No harms reported. |
|
| Notes | Funding source: Not stated Declarations of interest: 'None'. Authors contacted requesting further information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | The groups were allocated using serially‐numbered opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible with study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were analyzed |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | None detected |
Badawy 2009.
| Methods | A randomised controlled trial | |
| Participants | The study comprised 163 women (aged 18 – 32 years) with CC‐resistant PCOS among those attending the Outpatient Clinic at the Mansoura University Hospitals, Mansoura, Egypt, and a private practice setting in the period from January 2005 to January 2007 | |
| Interventions | Participants were randomly allocated to either treatment with ultrasound‐guided transvaginal needle ovarian drilling (UTND; n = 82) or laparoscopic electrosurgery ovarian drilling (n = 81) | |
| Outcomes | Normal menstruation, hirsutism and acne, testosterone. No harms reported. |
|
| Notes | Funding source: Not stated Declarations of interest: "All authors have nothing to declare". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random table" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible with study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | None detected |
Elgafor 2013.
| Methods | A randomised controlled trial | |
| Participants | 146 CC‐resistant PCOS women who were attending the infertility clinic were recruited from Zagazig University Hospital, Zagazig, Egypt. Time‐frame not stated | |
| Interventions | Metformin (850 mg (1 tablet daily) + letrozole (n = 73)) versus LOD (n = 73). Metformin dosage was increased after 1 week up to 1700 mg/day (2 tablets daily) and only stopped once pregnancy was confirmed. Letrozole was added from day 3 each month of spontaneous or induced bleeding, and continued for 5 days. LOD was 4 drills to each ovary | |
| Outcomes | Hormonal profile including testosterone, fasting glucose, glu/insulin ratio, regularity of periods, BMI No harms reported. |
|
| Notes | Funding source: Not stated Declarations of interest: "None". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random numeric table" |
| Allocation concealment (selection bias) | Low risk | "concealed in sealed dark envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible with study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | None detected |
Farquhar 2002.
| Methods | A randomised controlled trial | |
| Participants | 50 women were recruited from fertility clinics in New Zealand between mid‐1996 and late 1999 with the following inclusion criteria: 1) 20 ‐ 38 years; 2) infertility > 12 months; 3) Clomiphene resistance; 4) BMI < 33; 5) PCOS | |
| Interventions | Women were randomised into LOD (n = 29) (10 holes per ovary) versus ovulation induction with gonadotrophin (metrodin HP (Serono) or FSH (Puregon)) (n = 21) | |
| Outcomes | Hirsutism, acne. Testosterone pre‐ and post‐LOD, not compared to medical arm. BMI stated as no difference but not quantified Participants were given questionnaires regarding acceptability and convenience of both procedures. No harms reported. |
|
| Notes | Funding source: "Supported in part by Auckland Medical Research Foundation, grant 81310" Declarations of interest: Not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated sequences" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation in "sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible with study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 withdrawals out of 50 |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | None detected |
Giampaolino 2016.
| Methods | A randomised controlled trial | |
| Participants | 286 women with CC‐resistant PCOS attending Department of Obstetrics and Gynecology of the University of Naples ‘‘Federico II’’, Italy. Age 18 ‐ 40 years | |
| Interventions | Women were randomised to either conventional LOD (123 women)or Transvaginal Hydrolaparoscopy (123 women). | |
| Outcomes | Ovarian adhesion formation. | |
| Notes | Study funding: Not reported. Possible conflicts of interest: The authors declare no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Two hundred and forty‐six patients were randomized into two groups in a 1:1 ratio by use of a randomization list generated by a computer with blocks of 4". |
| Allocation concealment (selection bias) | Low risk | The allocation sequence was concealed from the researchers, who enrolled and assessed the participants and attached a sequentially‐numbered, opaque, sealed, and stapled envelope containing the allocated treatment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and surgeon were not blinded to the procedure performed because concealment was not possible due to the differences in the procedures |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal attrition |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | None detected |
Hamed 2010.
| Methods | A randomised controlled trial | |
| Participants | The study was conducted in the Women's Health Center in Cairo, Egypt from May 2007 to September 2008. 200 women were assessed for eligibility, 110 included in the study | |
| Interventions | Participants were randomly allocated to diagnostic laparoscopy plus metformin therapy (group 1, n = 55) or laparoscopic ovarian drilling (group 2, n = 55) | |
| Outcomes | Menstrual cycle regularity, testosterone levels, fasting glucose to insulin ratio, BMI. No harms reported. |
|
| Notes | Funding source: Not stated Declarations of interest: "The authors have no conflicts of interest". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a "computer‐generated random numbers table" |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was done using "serially numbered opaque envelopes. The patient's allocation was not changed after opening the envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible with study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | None detected |
Hashim 2011.
| Methods | A randomised controlled trial | |
| Participants | The study population comprised 282 women with CC‐resistant PCOS attending the Outpatient Clinic in Mansoura University Hospital, Mansoura, Egypt, and a private practice setting from September 2005 to February 2009. Participants in Group A (n = 138) received combined metformin–CC for up to 6 cycles and participants in group B underwent LOD (n = 144) with 6 months follow‐up | |
| Interventions | In group A, all participants received metformin for 5 days starting from day 3 of spontaneous or induced menstruation. In the LOD group (group B), laparoscopy was performed using the 3‐puncture technique. Each ovary was cauterised at 4 points, each for 4 seconds at 40 W for a depth of 4 mm with a mixed current, using a monopolar electrosurgical needle | |
| Outcomes | Resumption of regular menstruation, ovulation rate, mid‐cycle endometrial thickness, pregnancy and miscarriage rates. No intra‐operative or post‐operative complications in either group. |
|
| Notes | Funding source: Not stated Declarations of interest: Not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random sequence was by "computer‐generated random numeric table" |
| Allocation concealment (selection bias) | Low risk | "Opaque envelopes were numbered and sealed containing the allocation information given to a nurse who assigned the patients to study arms of treatment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible with study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | None detected |
Hashim 2010.
| Methods | A randomised controlled trial | |
| Participants | The study comprised 260 women with CC‐resistant PCOS among those attending the Outpatient Clinic in Mansoura University Hospitals, Mansoura, Egypt, and a private practice setting from August 2006 to March 2009 | |
| Interventions | Group A (n = 128) received 2.5 mg letrozole daily for 5 days for up to 6 cycles. Group B (n = 132) underwent LOD with 6 months follow‐up. In letrozole group (group A), treatment continued for up to 6 cycles. In LOD group (group B), laparoscopy was performed using 3‐puncture technique. Each ovary was cauterised at 4 points, each for 4 seconds at 40 W for a depth of 4 mm with a mixed current, using a monopolar electrosurgical needle. Follow‐up continued for 6 months after the procedure | |
| Outcomes | Resumption of regular menstruation, ovulation rate, pregnancy, miscarriage, live birth rates and midcycle endometrial thickness. No operative complications developed, No multiple pregnancies or OHSS in either group. |
|
| Notes | Funding source: Not stated Declarations of interest: "We declare that we have no conflict of interest". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Women were randomized according to a computer‐generated random numeric table prepared by an independent statistician" |
| Allocation concealment (selection bias) | Low risk | "concealment of treatment allocation by use of sealed opaque envelopes that were given to a third party (nurse) who assigned patients to study arms; group A (letrozole) or B (LOD)". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "the treatment was revealed to both the investigator and the patient" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "the radiologist who performed transvaginal ultrasound follow up assessment was blinded to the treatment groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | None detected |
Kaya 2005.
| Methods | A randomised controlled trial | |
| Participants | 35 infertile CC‐resistant women with PCOS were prospectively randomised in 2 groups and evaluated from January 2000 through January 2004. Conducted in Suleyman Demireal University Hospital, Isparta, Turkey | |
| Interventions | 17 women underwent laparoscopic ovarian drilling with a multi‐needle intervention( LOMNI) and 18 women received step‐up ovulation induction treatment with recombinant FSH for ovulation induction in addition to intrauterine insemination | |
| Outcomes | Cycle regularity, pregnancy rate, OHSS and cost | |
| Notes | Funding source: Not stated Declarations of interest: Not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated random sequence" |
| Allocation concealment (selection bias) | Low risk | Allocated using "sealed opaque envelopes" prior to their surgery |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible with study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although 1 woman in the LOMNI group and 2 women in the ovulation induction group were lost to follow‐up, their pregnancy results were available and they all received telephone interviews at the end of the follow‐up period |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | None detected |
Palomba 2010.
| Methods | A randomised controlled trial | |
| Participants | 50 women who were CC‐resistant were recruited between February 2003 and May 2004, conducted in 2 university hospitals in Naples, Italy | |
| Interventions | The participants were randomised to clomiphene + metformin (6 cycles) (n = 25) versus LOD alone (n = 25) and followed up for 6 months. LOD was by 3 ‐ 6 punctures of 3 mm diameter and 4 ‐ 5 mm depth, for 2 ‐ 3 seconds at 40 W) | |
| Outcomes | Amenorrhoea at cycle day 35. No operative complications were identified, no drug‐related adverse effects resulting in treatment discontinuation. |
|
| Notes | Funding source: "Authorship and contribution to the article is limited to the 8 authors indicated. There was no outside funding or technical assistance with the production of this article" Declarations of interest: Not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | "concealed in sealed dark envelopes until intervention was assigned" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible with study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | None detected |
Roy 2009.
| Methods | A randomised controlled trial | |
| Participants | 44 women with PCOS were recruited between June 2005 and June 2007 from an Obstetrics and Gynaecology clinic in New Dehli, India | |
| Interventions | 22 participants underwent unilateral ovarian drilling and 22 underwent bilateral ovarian drilling. The number of drilling sites in each ovary was limited to 5 | |
| Outcomes | The clinical and biochemical response, ovulation and menstrual regularity over a follow‐up period of 1 year were compared. Tubo‐ovarian adhesion rate was compared during caesarean section or during repeat laparoscopy | |
| Notes | Funding source: Not stated Declarations of interest: "None". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Mentioned as randomly allocated but not stated how |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible with study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | None detected |
Roy 2010.
| Methods | A randomised controlled trial | |
| Participants | 43 women were recruited from a gynaecology clinic in New Delhi, India, between January 2006 to 2009, all with CC‐resistant PCOS and subfertility | |
| Interventions | Recruits were randomised into LOD (unilateral, 5 holes) (n = 21) vs rosiglitazone (n = 23) at a dose of 4 mg twice daily and CC at a dose of 100 mg daily from the 3rd day of the period for 5 days | |
| Outcomes | Hormonal profile including testosterone, glucose insulin ratio, ovulation, pregnancy rate. No OHSS in either group. |
|
| Notes | Funding source: Not stated. Declarations of interest: "None". Rosglitazone is no longer available in most countries including the UK, European countries and South Africa, but is still in use in USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | "sealed envelope containing numbers from the computer generated random table" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible with study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | None detected |
Sarouri 2015.
| Methods | A randomised controlled trial | |
| Participants | 121 women with PCOS attending the infertility clinic of Al‐Zahra Hospital in Rasht, Guilan Province, Iran | |
| Interventions | Comparison between bilateral and unilateral ovarian drilling | |
| Outcomes | FSH, LH and free testosterone levels before and after surgery, responses on ovulation and pregnancy rates. Testosterone levels included No harms reported. |
|
| Notes | Study Funding: "The authors thank the Vice Chancellor for Research of Guilan University of Medical Sciences for funding this project" Possible conflicts of interest: Not stated. Study Authors were contacted for further information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomization list was generated using blocked sample randomizations |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible due to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | None detected |
Selim 2011.
| Methods | A randomised controlled trial | |
| Participants | The study was conducted from January 2004 through September 2007 in the infertility unit of Al‐Hammadi Specialized Hospital, Riyadh, Saudi Arabia. 82 women with anovulatory infertility associated with PCOS, who had been CC‐resistant, underwent LOD to assess the optimal number of punctures to be applied to ovarian tissue | |
| Interventions | Women who met inclusion criteria (and had no exclusions) were randomly assigned to 1 of the 5 treatment groups (1:1:1:1:1 ratio). Each randomization number corresponded with 1 of the 5 possible interventions (Gp1: 2 punctures & 300 J; Gp 2: 3 punctures & 450 J; Gp 3: 4 punctures & 600 J; Gp 4: 5 punctures & 750 J; and Gp 5: 6 ‐ 8 punctures & > 900 J). There were 17 cases in groups 3 and 4, and 16 cases in groups 1, 2, and 5 | |
| Outcomes | Menstrual regularity, testosterone, FAI at 6 months follow‐up. No harms reported. |
|
| Notes | Due to the multiple group comparisons, for analysis purposes, the outcomes of those with 2 punctures were compared to those with 4 punctures (considered dose for LOD) ‐ i.e., Gp 3 (LOD with 4 punctures) vs Gp 1 (2 punctures) Funding source: Not stated Declarations of interest: "No competing financial conflicts exist". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Labelling of the randomization number was done by a person not involved with the trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible due to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | None detected |
Sharma 2006.
| Methods | A randomised controlled trial | |
| Participants | 20 women with CC‐resistant PCOS were recruited in the Department of Obstetrics and Gynaecology of the All India Institute of Medical Sciences, New Delhi, India | |
| Interventions | Group I (n = 10) had unipolar ovarian drilling done by unipolar diathermy needle at power settings of 30 ‐ 40 watts. Group II (n = 10) had bipolar ovarian drilling done by bipolar diathermy needle at power settings of 40 ‐ 50 watts | |
| Outcomes | Menstrual regularity at 3 months, hormonal profile including testosterone, Glucose insulin ratio, pregnancy rate, ovulation rate. No harms reported. |
|
| Notes | Funding source: Not stated Declarations of interest: Not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computerised random table" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible due to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | High risk | Baseline values of glucose insulin ratio were significantly different between groups despite randomization |
Takeuchi 2002.
| Methods | A randomised controlled trial | |
| Participants | 34 women diagnosed with PCOS with infertility for more than 2 years in Department of Obstetrics and Gynaecology, Mie University School of Medicine, Mie, Japan. Time frame not stated | |
| Interventions | In Group A (n = 17) laparoscopic ovarian drilling was performed using Harmonic scalpel. In group B (n = 17) it was accomplished using Nd:YAG laser | |
| Outcomes | Hormonal profile including testosterone levels, menstrual regularity. No harms reported. |
|
| Notes | Funding source: Not stated Declarations of interest: "The authors have no connection to any companies or products mentioned in this article". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated". |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible due to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | None detected |
Taskin 1996.
| Methods | A randomised controlled trial | |
| Participants | 17 women with CC‐resistant PCOS were recruited from the Division of Endocrinology and Infertility, Inonu University of Medicine, Malatya, Turkey. Time frame not stated | |
| Interventions | 17 women were randomly assigned to either Group A (ovarian electrocautery; n = 8) or group B (GnRHa + low‐dose OCP (desogestrel 0.15 mg + ethinyl estradiol = 35 mcg); n = 9). 10 ‐ 12 cautery points were applied to each ovary | |
| Outcomes | Hormonal profile including testosterone. No harms from either modality. |
|
| Notes | Low‐dose OCP was given from the beginning of 1st day of induced menstruation and continued for 3 months Funding source: Not stated Declarations of interest: Not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sequential assignment based on table of random numbers". |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible due to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | None detected |
Youssef 2007.
| Methods | A randomised controlled trial | |
| Participants | The study was performed in the Department of Obstetrics and Gynaecology and Fertility care unit, Faculty of Medicine, Mansoura University Hospital, Egypt, from January 2003 to December 2006. 87 women were recruited | |
| Interventions | Participants were allocated to either unilateral (Group A: n = 43) or bilateral (Group B: n = 44) laparoscopic ovarian drilling | |
| Outcomes | Testosterone concentrations, FSH, LH, post‐operative nausea, vomiting and pain; ovulation, pregnancy, and miscarriage rates | |
| Notes | Funding source: Not stated Declarations of interest: Not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated by an independent investigator blinded to treatment groups |
| Allocation concealment (selection bias) | Low risk | "using the closed envelope method" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible due to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An independent assessor blinded to the treatment groups obtained the scores". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None detected |
Zakherah 2011.
| Methods | A randomised controlled trial | |
| Participants | 120 women with polycystic ovary syndrome and clomiphene citrate resistance between January 2007 and December 2009 in the Women's Health Centre and Physiology Department, Assiut University, Egypt. | |
| Interventions | Patients were assigned randomly to 2 groups of 60 women each. Group A received an adjusted thermal dose based on ovarian volume with use of a new model for dose calculation (60 J/cm3 of ovarian tissue), and group B received 600 J per ovary through 4 ovarian holes regardless of size (traditional form of LOD). 1 month afterward, the hormonal profile was re‐evaluated, and second‐look laparoscopy was performed in women who had not conceived by 6 months to evaluate adnexal adhesions | |
| Outcomes | Menstrual cycle regularity, hormonal profile including testosterone, BMI and adhesions | |
| Notes | Funding source: Not stated Declarations of interest: None. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was done using a computer generated random table" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible due to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | None detected |
Zhu 2010.
| Methods | A randomised controlled trial | |
| Participants | 80 women with PCOS and CC–resistant infertility between January 2006 and June 2008 in Shen‐Zen City Materninty and Child Healthcare Hospital, Shen‐Zhen, China. Participants underwent ultrasound‐guided transvaginal ovarian interstitial yttrium aluminium garnet laser treatment. Participants were divided randomly into 4 groups (A, B, C, and D) | |
| Interventions | Group A (n = 20), 1 coagulation point per ovary; group B (n = 20), 2 points; group C (n = 20), 3 points; group D (n = 20), 4 to 5 points | |
| Outcomes | Hormonal profile including testosterone and regularity of menstrual pattern. Follow‐up period was 6 months post‐operation. No complications occurred. |
|
| Notes | Funding source: Not stated Declarations of interest: None. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "80 random numbers generated by computer were divided randomly into 4 groups: A, B, C, and D". |
| Allocation concealment (selection bias) | Low risk | The random allocation sequence was concealed in a closed, dark‐coloured envelope until the surgeries were assigned, and specifically just before entering the operating room. Randomisation occurred after participants agreed to join the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible due to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | None detected |
Zullo 2000.
| Methods | A randomised controlled trial | |
| Participants | 62 infertile women with PCOS from University hospitals and a private day surgery unit in Naples, Italy. Time frame not stated | |
| Interventions | In group A (n = 32), ovarian drilling was performed by mini‐laparoscopy under local anaesthesia plus conscious sedation. In group B (n = 30), the control group underwent ovarian drilling by the traditional laparoscopic approach under general anaesthesia | |
| Outcomes | Hormonal profile including testosterone levels, pain scores post‐surgery (follow‐up period of 1 year) | |
| Notes | Funding source: Not stated Declarations of interest: Not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated". |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but not possible due to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Low risk | None detected |
BMI: body mass index CC: clomiphene citrate FAI: free androgen index FSH: follicle stimulating hormone GnRHa: gonadotrophin‐releasing hormone J: joule(s) LH: luteinising hormone OCP: oral contraceptive pill OHSS: ovarian hyperstimulation syndrome W: watt(s)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amer 2009 | Fertility outcomes only |
| Darwish 2016 | Testosterone outcomes not reported |
| Hashim 2011 | Fertility outcomes only. Compared LOD versus continuing with clomiphene |
| Liu 2015 | Reproductive outcomes only. |
| Malwaki 2003 | Not an RCT |
| Malwaki 2005 | Not an RCT |
| Muenstermann 2000 | Not a true randomised trial as randomization was by 'alternating' treatment protocol |
| Nasr 2013 | Ovarian reserves only |
| Nasr 2015 | Ovarian reserves only |
| Rezk 2016 | Ovarian reserves only |
| Wang 2015 | Study retracted |
Characteristics of ongoing studies [ordered by study ID]
NCT02304536.
| Trial name or title | Comparison between laproscopic ovarian diathermy and urinary purified fsh in women with clomiphene citrate‐resistant polycystic ovarian syndrome: a randomized controlled trial |
| Methods | 210 women with clomiphene‐resistant PCOS will be randomly divided into 3 equal groups using computer‐generated random numbers. Group 1 will receive combined metformin and FSH, group 2 will have LOD and group 3 will act as the control group with no intervention. |
| Participants | 210 women with clomiphene‐resistant PCOS |
| Interventions | Metforimn and FSH, LOD, no intervention |
| Outcomes | Ovulation, pregnancy |
| Starting date | November 2014 |
| Contact information | abdelgany2@gmail.com |
| Notes |
NCT02305693.
| Trial name or title | Comparison between letrozole and laparoscopic ovarian drilling in women with clomiphene‐resistant polycystic ovarian syndrome (PCOS) |
| Methods | 140 women with clomiphene‐resistant PCOS will be randomly divided into 2 equal groups using computer‐generated random numbers. Group 1 will receive letrozole, group 2 will have laparoscopic ovarian drilling (LOD) |
| Participants | 140 women with clomiphene‐resistant PCOS |
| Interventions | Letrozole, LOD |
| Outcomes | Ovulation, pregnancy |
| Starting date | November 2014 |
| Contact information | abdelgany2@gmail.com |
| Notes |
Differences between protocol and review
We have edited the review's Methods to bring them up to current Cochrane standards. These include: addition of a PRISMA flow chart to document our search and addition of 'Summary of findings' tables in accordance with the now mandatory MECIR standards C74 and C75. We have clarified that menstrual regularity is reported as binary data. There has been a change in the author team between protocol and review, so four review authors shared in selecting studies. We have added an I2 percentage for clarification. We planned to use a random‐effects model where data were heterogenous; these analyses using random‐effects model are now sensitivity analyses. The background sections have been updated in the lay and non‐lay sections. We planned to stratify comparisons by some specified characteristics of interventions and planned subgroups by hormone profiles and different surgical techniques; we did not do this because with hindsight the data were not reported in a format applicable to this analysis.
Contributions of authors
SL, JJ and YC performed the analysis and SL was the lead author in the writing of the review. YC wrote the review and contributed to the methodology and content of the review. MM contributed to the content and acted as a moderator.
Sources of support
Internal sources
None, Other.
External sources
-
Support from the Cochrane Gynaecology and Fertility Group, New Zealand.
Help with searches, editorial feedback gratefully acknowledged
Declarations of interest
SL, JJ, MM and YC have no interests to declare.
New
References
References to studies included in this review
Abdelhafeez 2013 {published data only}
- Abdelhafeez MA, Ali MS, Sayed SN. Unilateral versus bilateral laparoscopic ovarian drilling in clomiphene citrate resistant polycystic ovary syndrome. Life Sciences 2013;10(1):3057‐60. [Google Scholar]
Ashrafinia 2009 {published data only}
- Ashrafinia M, Hosseinia R, Moinia A, Eslamia B, Asgar Z. Comparison of metformin treatment and laparoscopic ovarian diathermy in patients with polycystic ovary syndrome. International Journal of Obstetrics and Gynaecology 2009;107:236‐9. [DOI] [PubMed] [Google Scholar]
Badawy 2009 {published data only}
- Badawy A, Khiary M, Ragab A, Hassan M, Sherief L. Ultrasound‐guided transvaginal ovarian needle drilling (UTND) for treatment of polycystic ovary syndrome: a randomized controlled trial. Fertility and Sterility April 2009;4(91):1164‐7. [DOI] [PubMed] [Google Scholar]
Elgafor 2013 {published data only}
- Elgafor A. Efficacy of combined metformin‐letrozole in comparison with bilateral ovarian drilling in clomiphene‐resistant infertile women with polycystic ovarian syndrome. Archives of Gynecology and Obstetrics 2013;288(1):119‐23. [DOI] [PubMed] [Google Scholar]
Farquhar 2002 {published data only}
- Farquhar C, Williamson K, Gudex G, Johnson N, Garland J, Sadler L. A randomised controlled trial of laparoscopic ovarian diathermy versus gonadotrophin therapy for women with clomiphene citrate‐resistant polycystic ovary syndrome. Fertility and Sterility 2002;78(2):404‐11. [DOI] [PubMed] [Google Scholar]
Giampaolino 2016 {published data only}
- Giampaolino P, Morra I, Tommaselli GA, Carlo C, Nappi C, Bifulco G. Post‐operative ovarian adhesion formation after ovarian drilling: a randomized study comparing conventional laparoscopy. Archives of Gynecology and Obstetrics 2016;294(4):791‐6. [DOI] [PubMed] [Google Scholar]
Hamed 2010 {published data only}
- Hamed HO, Hasan AF, Ahmed OG, Ahmed MA. Metformin versus laparoscopic ovarian drilling in clomiphene‐ and insulin‐resistant women with polycystic ovary syndrome. International Journal of Gynecology and Obstetrics 2010;108(2):143‐7. [DOI] [PubMed] [Google Scholar]
Hashim 2010 {published data only}
- Abu Hashim A, Mashaly AM, Badawy A. Letrozole versus laparoscopic ovarian diathermy for ovulation induction in clomiphene‐resistant women with polycystic ovary syndrome: a randomised controlled trial. Archives of Gynecology and Obstetrics 2010;282:567‐71. [DOI] [PubMed] [Google Scholar]
Hashim 2011 {published data only}
- Abu Hashim H, Lakany N, Sherief L. Combined metformin and clomiphene citrate versus laparoscopic ovarian diathermy for ovulation induction in clomiphene‐resistant women with polycystic ovary syndrome: A randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2011;37(3):169‐77. [DOI] [PubMed] [Google Scholar]
Kaya 2005 {published data only}
- Kaya H, Sezik M, Ozkaya O. Evaluation of a new surgical approach for the treatment of clomiphene citrate ‐resistant infertility in polycystic ovary syndrome: laparoscopic ovarian multi‐needle intervention. Journal of Minimally Invasive Gynaecology 2005;12(4):355‐8. [DOI] [PubMed] [Google Scholar]
Palomba 2010 {published data only}
- Palomba S, Falbo A, Battista L, Russo T, Venturella R, Tolino A, et al. Laparoscopic ovarian diathermy vs clomiphene citrate plus metformin as second‐line strategy for infertile anovulatory patients with polycystic ovary syndrome: a randomised controlled trial. American Journal of Obstetrics and Gynecology 2010;202(6):e1‐8. [DOI] [PubMed] [Google Scholar]
Roy 2009 {published data only}
- Roy KK, Baruah J, Moda N, Kumar S. Evaluation of unilateral versus bilateral ovarian drilling in clomiphene citrate resistant cases of polycystic ovarian syndrome. Archives of Gynecology and Obstetrics 2009;280(4):573‐8. [DOI] [PubMed] [Google Scholar]
Roy 2010 {published data only}
- Roy KK, Baruah J, Sharma A, Sharma JB, Kumar S, Kachava, et al. A prospective randomized trial comparing the clinical and endocrinological outcome with rosiglitazone versus laparoscopic ovarian drilling in patients with polycystic ovarian disease resistant to ovulation induction with clomiphene citrate. Archives of Gynecology and Obstetrics 2010;281(5):939‐44. [DOI] [PubMed] [Google Scholar]
Sarouri 2015 {published data only}
- Zahiri Sorouri Z, Sharami SH, Tahersima Z, Salamat F. Comparison between unilateral and bilateral ovarian drilling in clomiphene citrate resistance polycystic ovary syndrome patients: a randomized clinical trial of efficacy. International Journal of Fertility & Sterility 2015;9(1):9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Selim 2011 {published data only}
- Selim MF. The optimal number of ovarian punctures to be applied during laparoscopic ovarian diathermy in women with polycystic ovarian syndrome. Journal of Gynaecologic Surgery 2011;27(4):217‐24. [DOI: 10.1089/gyn.2010.0077] [DOI] [Google Scholar]
Sharma 2006 {published data only}
- Sharma M, Kriplani A, Agarwal N. Laparoscopic bipolar versus unipolar ovarian drilling in infertile women with resistant polycystic ovarian syndrome: a pilot study. Journal of Gynaecologic Surgery 2006;22(3):105‐11. [Google Scholar]
Takeuchi 2002 {published data only}
- Takeuchi S, Futamura N, Takubo S, Noda N, Minoura H, Toyoda N. Polycystic ovarian syndrome treated with laparoscopic ovarian drilling with a harmonic scalpel: a prospective randomized study. Journal of Reproductive Medicine 2002;47(10):816‐20. [PubMed] [Google Scholar]
Taskin 1996 {published data only}
- Taskin O, Yalcinoglu A, Kafkasli A, Burak F, Ozekici U. Comparison of the effects of ovarian cauterization and gonadotropin‐releasing hormone agonist and oral contraceptive therapy combination on endocrine changes in women with polycystic ovary disease. Fertility and Sterility 1996;65(6):1115‐8. [DOI] [PubMed] [Google Scholar]
Youssef 2007 {published data only}
- Youssef H, Atallah MM. Unilateral ovarian drilling in polycystic ovarian syndrome:a prospective randomized study. Reproductive BioMedicine Online 2007;15(4):457‐62. [DOI] [PubMed] [Google Scholar]
Zakherah 2011 {published data only}
- Zakherah MS, Kamal MM, Hamed HO. Laparoscopic ovarian drilling in polycystic ovary syndrome: efficacy of adjusted thermal dose based on ovarian volume. Fertility and Sterility 2011;95(3):1115‐8. [DOI] [PubMed] [Google Scholar]
Zhu 2010 {published data only}
- Zhu W, Fu Z, Chen X, Li X, Tang Z, Zhou Y, et al. Transvaginal ultrasound‐guided ovarian interstitial laser treatment in anovulatory women with polycystic ovary syndrome: a randomized clinical trial on the effect of laser dose used on the outcome. Fertility and Sterility 2010;94(1):268‐75. [DOI] [PubMed] [Google Scholar]
Zullo 2000 {published data only}
- Zullo F, Pellicano M, Zupi E, Guida M, Mastrantonio P, Nappi C. Minilaparoscopic ovarian drilling under local anesthesia in patients with polycystic ovary syndrome. Fertility and Sterility 2000;74(2):376‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amer 2009 {published data only}
- Amer SA, Li TC, Metwally M, Emarh M, Ledger WL. Randomized controlled trial comparing laparoscopic ovarian diathermy with clomiphene citrate as a first‐line method of ovulation induction in women with polycystic ovary syndrome. Human Reproduction 2009;24(1):219‐25. [DOI] [PubMed] [Google Scholar]
Darwish 2016 {published data only}
- Darwish AM, Metwally AB, Shaaban MM, Mohamed S. Monopolar versus bipolar laparoscopic ovarian drilling in clomiphene‐resistant polycystic ovaries (PCO): a preliminary study. Gynecological Surgery 2016;13: 179(3):943‐7. [Google Scholar]
Hashim 2011 {published data only}
- Hashim HA, Foda O, Ghayata E, Elawa A. Laparoscopic ovarian diathermy after clomiphene failure in polycystic ovary syndrome: is it worthwhile? A randomized controlled trial. Archives of Gynecology and Obstetrics 2011;284:1303‐9. [DOI] [PubMed] [Google Scholar]
Liu 2015 {published data only}
- Liu W, Dong S, Li Y, Shi L, Zhou W, Liu Y, Liu J, Ji Y. Randomized controlled trial comparing letrozole with laparoscopic ovarian drilling in women with clomiphene citrate‐resistant polycystic ovary syndrome.. Experimental and Therapeutic Medicine 2015;10(4):1297‐1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malwaki 2003 {published data only}
- Malwaki HY, Qublan HS, Hamaideh H. Medical vs. surgical treatment for clomiphene citrate‐resistant women with polycystic ovary syndrome. Journal of Obstetrics and Gynaecology 2003;23:289‐93. [DOI] [PubMed] [Google Scholar]
Malwaki 2005 {published data only}
- Malwaki HY, Qublan HS, Hamaideh H. Laparoscopic ovarian drilling in the treatment of polycysticovary syndrome: How many punctures per ovary are needed to improve the reproductive outcome?. Journal of Obstetrics and Gynaecology 2005;31:115‐9. [DOI] [PubMed] [Google Scholar]
Muenstermann 2000 {published data only}
- Muenstermann U, Kleinstein J. Long‐term GnRH analogue treatment is equivalent to laparoscopic laser diathermy in polycystic ovarian syndrome patients with severe ovarian dysfunction. Human Reproduction 2000;15(12):2526‐30. [DOI] [PubMed] [Google Scholar]
Nasr 2013 {published data only}
- Nasr A. Impact of unilateral versus bilateral laparoscopic ovarian drilling on ovarian reserve in clomiphene‐resistant PCOS women. Fertility and Sterility 2013;100:114. [Google Scholar]
Nasr 2015 {published data only}
- Nasr A. Impact of ovarian volume‐based adjusted thermal dose versus fixed‐puncture doage in laparoscopic drilling on ovarian reserve in clomiphene citrate‐resistant PCOS women. Fertility and Sterilty 2015;104:124. [Google Scholar]
Rezk 2016 {published data only}
- Rezk A, Sayyed T, Saleh S. Impact of unilateral versus bilateral ovarian drilling on ovarian reserve and pregnancy: A randomized clinical trial. Gynecological Endocrinology 2016;32(5):399‐402. [DOI] [PubMed] [Google Scholar]
Wang 2015 {published data only}
- Wang XH, Wang JQ, Huang LP. Therapeutic effects of metformin and laparoscopic ovarian drilling in treatment of clomiphene and insulin‐resistant plycystic ovary syndrome. Archives of Gynecology and Obstetrics May 2015;291(5):1089‐94. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02304536 {published data only}
- NCT02304536. Comparison between laparoscopic ovarian diathermy and urinary purified FSH in women with clomiphene citrate resistant polycystic ovarian syndrome: a randomised controlled trial. clinicaltrials.gov/ct2/show/NCT02304536 First received 25 November 2014.
NCT02305693 {published data only}
- NCT02305693. Comparison between letrozole and laparoscopic ovarian drilling in women with clomiphene resistant polycystic ovarian syndrome (PCOS). clinicaltrials.gov/ct2/show/NCT02305693 First received 26 November 2014.
Additional references
Adams 1986
- Adams J, Polson DW, Franks S. Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. British Medical Journal (Clinical Research Edition) 1986;293(6543):355‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amer 2002
- Amer SA, Gopalan V, Li TC, Ledger WL, Cooke ID. Long term follow‐up of patients with polycystic ovarian syndrome after laparoscopic ovarian drilling: clinical outcome. Human Reproduction 2002;17(8):2035‐42. [DOI] [PubMed] [Google Scholar]
Azziz 2000
- Azziz R, Carmina E, Sawaya ME. Idiopathic hirsutism. Endocrine Reviews 2000;21(4):347‐62. [DOI] [PubMed] [Google Scholar]
Costello 2007
- Costello M, Shrestha B, Eden J, Sjoblom P, Johnson N. Insulin‐sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005552.pub2] [DOI] [PubMed] [Google Scholar]
Elmashad 2011
- Elmashad A. Impact of laparoscopic ovarian drilling on anti‐Müllerian hormone levels and ovarian stromal blood flow using three‐dimensional power Doppler in women with anovulatory polycystic ovary syndrome. Fertility and Sterility 2011;95(7):2342‐6. [DOI] [PubMed] [Google Scholar]
ESHRE/ASRM PCOS Consensus 2004a
- Rotterdam ESHRE/ASRM‐Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Human Reproduction 2004;19(1):41‐7. [DOI] [PubMed] [Google Scholar]
ESHRE/ASRM PCOS Consensus 2004b
- ESHRE/ASRM PCOS Consensus 2004b. ASRM‐Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome. Fertility and Sterility 2004;81(1):19. [DOI] [PubMed] [Google Scholar]
Farquhar 2007
- Farquhar C, Lilford R, Marjoribanks J, Vanderkerchove P. Laparoscopic drilling by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD001122.pub3] [DOI] [PubMed] [Google Scholar]
Giallauria 2008
- Giallauria F, Orio F, Palomba S, Lombardi G, Colao A, Vigorito C. Cardiovascular risk in women with polycystic ovary syndrome. Journal of Cardiovascular Medicine (Hagerstown) 2008;9(10):987‐92. [DOI] [PubMed] [Google Scholar]
Glintborg 2010
- Glintborg D, Andersen M. An update on the pathogenesis, inflammation, and metabolism in hirsutism and polycystic ovary syndrome. Gynecological Endocrinology 2010;26(4):281‐96. [DOI] [PubMed] [Google Scholar]
Gordts 2009
- Gordts S, Gordts S, Puttemans P, Valkenburg M, Campo R, Brosens I. Transvaginal hydrolaparoscopy in the treatment of polycystic ovary syndrome. Fertility and Sterility 2009;91(6):2520‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Homburg 2008
- Homburg R. Polycystic ovary syndrome. Best practice & research. Clinical Obstetrics and Gynecology 2008;22(2):261‐74. [DOI] [PubMed] [Google Scholar]
Kiddy 1992
- Kiddy DS, Hamilton‐Fairley D, Bush A, Short F, Anyaoku V, Reed MJ, et al. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clinical Endocrinology (Oxford) 1992;36(1):59‐77. [DOI] [PubMed] [Google Scholar]
Lim 2016
- Lim CE, Ng R, Xu K, Cheng NC, Xue CC, Liu JP, et al. Acupuncture for polycystic ovarian syndrome. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD007689.pub3] [DOI] [PubMed] [Google Scholar]
Raval 2011
- Raval AD, Hunter T, Stuckey B, Hart RJ. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD008565.pub2] [DOI] [PubMed] [Google Scholar]
Seow 2007
- Seow KM, Juan CC, Hsu YP, Hwang JL, Huang LW, Ho LT. Amelioration of insulin resistance in women with PCOS via reduced insulin receptor substrate‐1 Ser312 phosphorylation following laparoscopic ovarian electrocautery. Human Reproduction 2007;22(4):1003‐10. [DOI] [PubMed] [Google Scholar]
Tang 2012
- Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin‐sensitising drugs (metformin, rosiglitazone, pioglitazone, D‐chiro‐inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD003053.pub5] [DOI] [PubMed] [Google Scholar]
Taylor 2003
- Taylor A. ABC of subfertility. Making a diagnosis. BMJ 2003;327(7413):494‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thessaloniki ESHRE/ASRM PCOS Consensus 2008
- Thessaloniki ESHRE/ASRM‐Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertility and Sterility 2008;89(3):505‐22. [DOI] [PubMed] [Google Scholar]
Vrbikova 2009
- Vrbikova J, Hainer V. Obesity and polycystic ovary syndrome. Obesity Facts 2009;2(1):26‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Cheong 2011
- Cheong YC, Metwally M, Shreeve N, Sadek K, Farquhar C. Ovarian surgery for symptom relief in women with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858] [DOI] [PMC free article] [PubMed] [Google Scholar]


