Abstract
Background
Allergic and febrile non‐haemolytic transfusion reactions (NHTRs) are the two most common forms of transfusion reaction. Pretransfusion medication with anti‐inflammatory drugs is used in NHTR prevention, however its efficacy and safety remains unclear.
Objectives
To assess the clinical effects and safety of pharmacological interventions for preventing NHTR in patients with and without a history of transfusion reactions.
Search methods
The search strategy included The Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library (Issue 4, 2008), Cochrane Injuries Group's Specialised Register (December 17, 2008), MEDLINE (1950 to November (week 3) 2008), EMBASE (1988 to November (week 3) 2008), LILACS (1982 to January 12, 2009), CINAHL (1982 to December 2008), ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED): 1970 to December 2008). There was no language restriction.
Selection criteria
Randomised controlled trials (RCTs) assessing the effectiveness of interventions for the prevention of NHTR.
Data collection and analysis
Authors independently selected studies, assessed the risks of bias and extracted data. Relative risks (RR) were estimated in RCTs with parallel design (PD). Odds ratio (OR) was estimated for one RCT with crossover design (CD). No meta‐analysis was attempted due to differences in the pharmacotherapy of pre‐transfusion medication and methodology between the studies; a per‐protocol analysis was used.
Main results
This review includes three RCTs (two PD and one CD). The PD‐RCTs employed disparate units of randomisation (UofR); patient or transfusion, while the CD‐RCT applied the patient as the UofR. The PD‐RCTs administered leukodepleted blood products. Both PD‐RCTs compared acetaminophen plus diphenhydramine (ApD) at different regimens with placebo, while the CD‐RCT contrasted hydrocortisone pharmacotherapy with diphenhydramine. Both PD‐RCTs found no statistically significant difference in allergic reactions (RR 0.13, 95% confidence interval (CI) 0.01 to 2.39, RR 1.46, 95% CI 0.78 to 2.73) and febrile reactions (RR 0.52, 95% CI 0.22 to 1.26). The CD‐RCT found a statistically significant difference in the odds of febrile reactions (OR 2.38, 95% CI 1.07 to 5.27). The trials did not report anaphylactic reactions, deaths related to transfusion reactions or other adverse events.
Authors' conclusions
None of the three studies found that medication prior to transfusion reduces NHTR. This applied regardless of the patient's history of NHTR and the use of leukodepleted blood products in the transfusion. However, this conclusion is based on three trials of moderate to low quality. A better‐powered RCT is necessary to evaluate the role of pretransfusion medication in the prevention of NHTR. Inclusion criteria should be restricted to patients at high risk of developing NHTR, with no restriction by age, history of transfusion reactions and type of blood products (leukodepleted or not).
Keywords: Humans; Premedication; Transfusion Reaction; Acetaminophen; Acetaminophen/administration & dosage; Anti‐Inflammatory Agents, Non‐Steroidal; Anti‐Inflammatory Agents, Non‐Steroidal/administration & dosage; Diphenhydramine; Diphenhydramine/administration & dosage; Fever; Fever/etiology; Fever/prevention & control; Histamine H1 Antagonists; Histamine H1 Antagonists/administration & dosage; Hydrocortisone; Hydrocortisone/administration & dosage; Hypersensitivity; Hypersensitivity/etiology; Hypersensitivity/prevention & control; Randomized Controlled Trials as Topic
Pre‐transfusion drugs for preventing side effects from blood transfusions
Febrile non‐haemolytic transfusion reactions (FNHTRs) and allergic reactions are the most common adverse reactions to blood transfusion. These reactions are often related to other dangerous side effects from transfusion such as sepsis due to contaminated blood products and intravascular red cell haemolysis.
In an effort to prevent these reactions, patients are given drugs prior to transfusion. Three kinds of drugs are commonly used for this pre‐transfusion medication, either alone or in combination. However, this practice is not standardised and there is controversy about its effectiveness.
This review found that current evidence from three trials in which 462 patients were analysed indicates pre‐transfusion medication in any regimen does not reduce the risk of allergic and febrile non‐haemolytic transfusion reactions.
Summary of findings
Summary of findings for the main comparison.
Acetaminophen plus Diphenhydramine versus placebo for preventing allergic and febrile non‐haemolytic transfusion reactions (Unit of randomisation: Patient).
| Acetaminophen plus Diphenhydramine versus placebo for preventing allergic and febrile non‐haemolytic transfusion reactions (Unit of randomisation: Patient). | ||||||
| Patient or population: patients with requirement of prophylaxis to avoid allergic and febrile non‐haemolytic transfusion reactions Settings: Hospital Intervention: Acetaminophen plus Diphenhydramine versus placebo (Unit of randomisation: Patient) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Acetaminophen plus Diphenhydramine versus placebo (Unit of randomisation: Patient) | |||||
| Febrile reactions (fever with or without chills, chills with or without rigors) ‐ Intention‐to treat analysis | Study population | RR 0.52 (0.21 to 1.25) | 334 (1 study) | ⊕⊕⊝⊝ low1,2,3 | ||
| 82 per 1000 | 43 per 1000 (17 to 102) | |||||
| Medium risk population | ||||||
| 82 per 1000 | 43 per 1000 (17 to 102) | |||||
| Anaphylatic reactions ‐ not reported | Study population | RR 0 (0 to 0) | 0 (0) | See comment | ||
| See comment | See comment | |||||
| Medium risk population | ||||||
| Death ‐ not reported | Study population | RR 0 (0 to 0) | 0 (0) | See comment | ||
| See comment | See comment | |||||
| Medium risk population | ||||||
| Adverse events | Study population | RR 0 (0 to 0) | 0 (0) | See comment | ||
| See comment | See comment | |||||
| Medium risk population | ||||||
| Allergic reactions (urticaria with or without pruritus) ‐ Intention‐to treat analysis | Study population | RR 1.45 (0.78 to 2.72) | 334 (1 study) | ⊕⊝⊝⊝ very low1,2,4 | ||
| 88 per 1000 | 128 per 1000 (69 to 239) | |||||
| Medium risk population | ||||||
| 88 per 1000 | 128 per 1000 (69 to 239) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Data on randomisation (sequence generation, allocation concealment) unavailable. 2 Not applicable (1 trial included) 3 Low event rate (21 reported) 4 Low event rate (36 reported)
Summary of findings 2.
Acetaminophen plus Diphenhydrammine versus placebo (Unit of randomisation: transfusion) for requirement of prophylaxis to avoid allergic and febrile non‐haemolytic transfusion reactions
| Acetaminophen plus Diphenhydrammine versus placebo (Unit of randomisation: transfusion) for requirement of prophylaxis to avoid allergic and febrile non‐haemolytic transfusion reactions | ||||||
| Patient or population: patients with requirement of prophylaxis to avoid allergic and febrile non‐haemolytic transfusion reactions Settings: Hospital Intervention: Acetaminophen plus Diphenhydrammine versus placebo (Unit of randomisation: transfusion) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Acetaminophen plus Diphenhydrammine versus placebo (Unit of randomisation: transfusion) | |||||
| Fever ‐ Per protocol analysis | Study population | RR 1.77 (0.57 to 5.49) | 98 (1 study) | ⊕⊕⊝⊝ low1,2,3 | ||
| 87 per 1000 | 154 per 1000 (50 to 478) | |||||
| Medium risk population | ||||||
| 87 per 1000 | 154 per 1000 (50 to 478) | |||||
| Anaphylatic reactions ‐ not reported | Study population | RR 0 (0 to 0) | 0 (0) | See comment | ||
| See comment | See comment | |||||
| Medium risk population | ||||||
| Hives ‐ Per protocol analysis | Study population | RR 0.13 (0.01 to 2.39) | 98 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 65 per 1000 | 8 per 1000 (1 to 155) | |||||
| Medium risk population | ||||||
| 65 per 1000 | 8 per 1000 (1 to 155) | |||||
| Death ‐ not reported | Study population | RR 0 (0 to 0) | 0 (0) | See comment | ||
| See comment | See comment | |||||
| Medium risk population | ||||||
| Adverse events | Study population | RR 0 (0 to 0) | 0 (0) | See comment | ||
| See comment | See comment | |||||
| Medium risk population | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Data on randomisation (sequence generation, allocation concealment) unavailable. 2 Not applicable (1 trial included) 3 Low event rate (36 reported)
Background
Description of the condition
Blood transfusion is employed in the treatment of many illnesses including infections, immune injury and cancer. Febrile non‐haemolytic transfusion reactions (FNHTRs) where antibodies act against donor leukocytes and allergic reactions, both considered immunological adverse events, are the most common transfusion reactions (Geiger 2007).
FNHTRs are defined as a temperature rise of at least 1ºC post‐transfusion (Brecher 2005). Allergic reactions can be classified as mild or severe. Mild allergic reactions appear as urticaria or hives (erythematous circumscribed lesions, with or without pruritus), while severe allergic reactions are known as an anaphylaxis (systemic symptoms of dyspnoea, wheezing, hypotension, tachycardia, loss of consciousness, shock, and in rare cases death) (Tobian 2007).
Little is know about the exact risk for developing FNHTRs or allergic reactions after transfusion (Geiger 2007) and there are discrepancies in the reported data. During the non‐leukocyte reduced era, FNHTRs were observed in up to 30% of transfusions, but this incidence has now been reduced to between 0.03% and 2.18%. This reduction is explained by two combined approaches: leukoreduction of blood products, and the use of single‐donor apheresis platelet units (Paglino 2004; Pruss 2004). The frequency of allergic reactions post‐transfusion ranges from less than 1% up to 17% (Domen 2003; Paglino 2004; Pruss 2004). The frequency of severe allergic reactions (anaphylaxis, anaphylactoid signs and symptoms, hypotension) is 7.7% of allergic reactions, or 1.3% of all transfusion reactions (Domen 2003).
The pathophysiology of FNHTRs and allergic reactions post‐transfusion has been widely reviewed (Geiger 2007; Heddle 1999).
Although these adverse transfusion events are usually not associated with serious clinical sequelae (Perrotta 2001) or a reduction in the effectiveness of the transfusion (Sarkodee‐Adoo 1998), the high rate of reactions in patients transfused with pooled platelet concentrate or non‐leukoreduced blood components has led to the practice of pre‐transfusion medication in an attempt to reduce reaction rates. This pre‐transfusion medication can involve acetaminophen (paracetamol), alone or in combination with diphenhydramine, or hydrocortisone (glucocorticoid).
Description of the intervention
Acetaminophen (paracetamol) is a non‐steroidal anti‐inflammatory drug with potent antipyretic and analgesic properties but with very weak anti‐inflammatory properties (Botting 2000). The main toxicity of acetaminophen is liver damage. The common dose of this drug is 650 mg for adults and 10 mg/kg for children (Geiger 2007). The lethal dose of acetaminophen is 15 times this dose (10g) (Geiger 2007). However, this drug should be used cautiously in patients with liver disease.
Diphenhydramine is a first‐generation antihistamine drug used for treating acute allergic reactions (Banerji 2007). Contrary to acetaminophen, diphenhydramine crosses the blood‐brain barrier (Hawkins 2008), penetrating the central nervous system (CNS), causing drowsiness, affecting alertness, and impairing cognitive performance (Banerji 2007; Geiger 2007). Diphenhydramine, as with other first‐generation antihistamine drugs, can cause cardiotoxicity and arrhythmias (Ramachandran 2008).
Hydrocortisone is the pharmacological name for cortisol, the main glucocorticoid agent generated by the adrenal cortex. Its anti‐inflammatory and immunosuppressive properties make hydrocortisone pharmacologically useful for preventing and treating severe allergic reactions such as anaphylaxis and allergy mediated angiooedema. The mechanism of action involves the inhibition of leukocyte (white blood cell) functions (Brunton 2008).
Due to their pharmacological actions mentioned above, acetaminophen, diphenhydramine and hydrocortisone are used for the prevention of allergic and febrile transfusion reactions, the pathophysiology of which is explained by Geiger 2007.
Why it is important to do this review
There is controversy about the use of pre‐transfusion medication. Febrile and allergic transfusion reactions are rare in children and patients transfused with leukoreduced, irradiated blood products whether or not they have received pre‐transfusion medication (Sanders 2005). In adults, one study suggested that pre‐transfusion medication provides significant advantages for patients but that its use does not yield significant cost benefits for the healthcare provider (Ezidiegwu 2004). One randomised controlled trial did not find clinical effectiveness in preventing non‐haemolytic reactions (Wang 2002). Two papers (Geiger 2007 and Tobian 2007) found no evidence to support pre‐transfusion medication as a clinical approach in the prevention of transfusion reactions.
Therefore, the aim of this Cochrane review is to answer the following clinical question: 'What is the efficacy and safety of pre‐transfusion medications for preventing allergic and febrile non‐haemolytic transfusion reactions?'.
Objectives
To determine, for patients with and without a history of transfusion reactions, whether pharmacological interventions:
are effective in preventing allergic and febrile non‐haemolytic transfusion reactions, and death;
are safe; and
differ in their efficacy or safety.
Methods
Criteria for considering studies for this review
Types of studies
Both published and unpublished RCTs were included.
Types of participants
Any patient requiring a blood transfusion. Participants could be of any age or sex, could be treated in any setting and could have cancer, haematologic malignancies, non‐haematologic malignancies and require a chronic transfusion regimen.
Types of interventions
Only pharmacological interventions were considered. Trials could compare different pre‐transfusion approaches or different doses and routes of administration for the same pre‐transfusion. Transfusions with whole blood or blood components were considered.
Types of outcome measures
Primary outcomes
Febrile reactions (fever with or without chills, chills with or without rigors).
Allergic reactions (urticaria with or without pruritus).
Anaphylactic reactions (dyspnoea, wheezing, hypotension, tachycardia, loss of consciousness, shock).
Death related to transfusion reactions.
Other adverse events.
Search methods for identification of studies
We did not restrict the searches by date, language or publication status.
Electronic searches
We searched the following electronic databases:
Cochrane Injuries Group's Specialised Register (December 17, 2008),
CENTRAL (The Cochrane Library; Issue 4, 2008),
MEDLINE (1950 to November (week 3) 2008),
EMBASE (1950 to November (week 3) 2008),
LILACS (1982 to January 12, 2009),
CINAHL (1982 to December 2008),
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED): 1970 to December 2008,
Proceedings Citation Index‐ Science (CPCI‐S): 1990 to December 2008,
PubMed (last 6 months; searched December 18 2008)
Searching other resources
In addition, we searched:
US National Library of Medicine ‐ Clinical Trials Registry (http://www.clinicaltrials.gov ) (January 12, 2009),
controlled trials; metaRegister (http://www.controlled‐trials.com/mrct/) (January 12, 2009),
http://www.excelenciaclinica.net/ (January 12, 2009),
http://www.transfusionguidelines.org.uk/Index.aspx?Publication=SRI&Section=24&pageid=1406 (March 31, 2009),
International Network of Agencies for Health Technology Assessment (http://www.inahta.org) (March 31, 2009),
Supplementary searches of the Web using the Internet search engines Google (www.google.co.uk/) and Google scholar (www.scholar.google.co.uk/) were used to identify grey literature and authors in this field. Bibliographies of relevant trials were searched for additional material and to identify additional authors. Authors were contacted directly to identify information on completed and ongoing trials.
Data collection and analysis
Selection of studies
Each reference identified by the searches was independently checked by two review authors (Arturo Martí‐Carvajal (AMC) and Luís E. González (LEG)) against the agreed inclusion criteria. Disagreements were resolved through discussion and Ivan Solà (IS) was consulted on any discrepancies.
Data extraction and management
Data extraction was carried out by two review authors (AMC and LEG) using a pre‐designed data extraction form containing publication type, details such as patient population, randomisation, allocation concealment, details of blinding measures, description of interventions and results (Zavala 2006). Discrepancies were resolved through discussion or by consulting a third author (IS). Data were entered into Review Manager software (RevMan 2008) and IS checked for accuracy.
Assessment of risk of bias in included studies
This section describes the recommended approach for assessing risk of bias in trials included in Cochrane reviews (Higgins 2009).
Three authors (AMC, IS, LEG) independently assessed each included trial for risk of bias in six domains:
Methods used to generate the allocation sequence.
Concealment of allocation.
Blinding (clinician, participant, outcome assessor).
Incomplete outcome data.
Selective outcome reporting.
Other sources of bias.
A judgement was reached relating to the risk of bias by answering a pre‐specified question about the adequacy of the study in relation to each of these domains. A judgement of ‘Yes’ indicated low risk of bias, ‘No’ indicated high risk of bias, and ‘Unclear’ indicated unclear or unknown risk of bias (Higgins 2009). The detailed risk of bias assessment is included in the Characteristics of included studies table.
Studies were grouped in to two categories: low risk of bias (when the allocation concealment was adequate and double blind) and high risk of bias (for all other scenarios).
Measures of treatment effect
For dichotomous data, we presented results as summary risk ratios with 95% confidence intervals. Because one of the included studies used a cross‐over design (Wang 1992), we used the Becker‐Balagtas marginal estimated odds ratio (OR) to summarise the transfusion reactions outcomes (Elbourne 2002).
Unit of analysis issues
Two trials included in this review (Kennedy 2008 and Wang 2002) used simple, parallel group design. Kennedy 2008 used patients recruited as unit of analysis, while Wang 2002 used transfusion as unit of analysis. Wang 1992 used a cross‐over design and the patient as its unit of analysis. We used statistical methodology guidelines suggested by Elbourne 2002 to analyse data from this trial.
Dealing with missing data
For all outcome analyses that were carried out on an intention‐to‐treat (ITT) basis, we attempted to include all participants randomised to each group. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing. We did contact the main author of each RCT in an attempt to obtain these missing data. We did ITT analysis by using the imputation method (worst‐case scenario versus best‐case scenario).
Assessment of heterogeneity
This review did not include meta‐analysis. For future updates, we will use the I2 statistic to measure heterogeneity among the trials in each analysis. I2 describes the percentage of total variation across studies due to heterogeneity rather than sampling error (Higgins 2003). If substantial heterogeneity (I² > 50%) is identified we will explore it by prespecified subgroup analysis.
Assessment of reporting biases
Where reporting bias was suspected, study authors were contacted and asked to provide absent outcome data. Where this was not possible, and the missing data thought to introduce serious bias, the impact of including such studies in the overall assessment of results was explored by sensitivity analysis.
For future updates, we will also attempt to assess whether the review is subject to publication bias by using a funnel plot.
Data synthesis
Statistical analysis was carried out using Review Manager software (RevMan 2008) and Comprehensive Meta‐analysis® version 2. Despite this Cochrane review containing three randomised controlled trials (Kennedy 2008; Wang 1992; Wang 2002), data was not pooled due to heterogeneity of pharmacotherapy and methodology. In subsequent updates, we will use random‐effects model meta‐analysis for combining data where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar.
Subgroup analysis and investigation of heterogeneity
In subsequent updates of this review, when sufficient data are available, we plan to carry out the following subgroup analyses:
Leukoreduced blood products versus non‐leukoreduced blood products.
Type of blood products (platelet concentrate, packed red cell, platelet rich plasma).
Patients with history of NHTR versus patients without history of NHTR.
Type or types of drug used for preventing allergic and febrile non‐haemolytic reactions.
Type of disorder (non‐haematological, haematological malignancies, and non‐haematological malignancies).
The following outcomes will be used in subgroup analysis:
Febrile reactions (fever with or without chills, chills with or without rigors).
Mild allergic reactions (urticaria with or without pruritus).
Anaphylatic reactions (dyspnoea, wheezing, hypotension, tachycardia, loss of consciousness, or shock).
Death related to transfusion reactions.
Other adverse events.
For random‐effects meta‐analyses we will assess differences between subgroups by inspection of their confidence intervals; where non‐overlapping confidence intervals indicate a statistically significant difference in treatment effect between the subgroups.
Sensitivity analysis
In subsequent updates we also plan to conduct a sensitivity analysis comparing the results using all studies and using only those of high methodological quality.
Results
Description of studies
We identified 148 references from our search strategy, of which 134 were excluded as irrelevant after examining the title and abstract.
Full text copies of the remaining 14 references were obtained for more detailed examination.
Included studies
Three randomised controlled trials (RCTs) published between 1992 and 2008 met inclusion criteria, giving a total of 462 participants for analysis (Wang 1992; Wang 2002; Kennedy 2008). A detailed description of the included trials is provided in the Characteristics of included studies table. The trials varied in the following characteristics: pre‐transfusion randomisation, participant population, study design, pre‐transfusion medication composition, study country, blood product transfusion, history of transfusion reaction, measures of treatment effect, sample size and duplicate publication.
All three RCTs were at moderate risk of bias.
1. Pre‐transfusion medication randomisation Wang 1992 and Kennedy 2008 randomised pre‐transfusion by patient. Wang 2002 randomised pre‐transfusion by transfusion to be delivered.
2. Participants population Kennedy 2008 and Wang 2002 only included patients with cancer and haematologic malignancies. Wang 1992 included patients gathered from haematology and gastroenterology wards.
3. Study design Two studies were conducted using parallel study design (Kennedy 2008; Wang 2002), and one used a crossover design (Wang 1992).
4. Pre‐transfusion medication composition Two RCTs used acetaminophen (500 or 650 mg per oral) and diphenhydramine (25 mg IV) as pre‐transfusion for preventing transfusion reactions (Kennedy 2008; Wang 2002). Wang 1992 used small doses of hydrocortisone (50 mg IV) plus diphenhydramine (40 mg IV) as pharmacotherapy preventing transfusion reaction.
5. Study country Two studies were conducted in the United States of America (Kennedy 2008; Wang 2002), and one in the People's Republic of China (Wang 1992).
6. Blood product transfusion
Two RCTs transfused leukodepleted or leukoreduced blood products (Kennedy 2008; Wang 2002). Wang 1992 did not describe blood product characteristics. Wang 2002 used irradiated and pre‐storage leukocyte reduced single‐donor apheresis units platelet transfusions. Kennedy 2008 administered red blood cell and platelet transfusions. Patients undergoing bone marrow transplantation received irradiated products. Single‐donor apheresis units platelet transfusions were used.
7. History of previous reactions Kennedy 2008 excluded patients with history of FNHTR or allergic transfusion reactions, while Wang 2002 included patients with history of both allergic and FNHTRs, and Wang 1992 included only patients with history of FNHTR.
8. Measures of treatment effect Kennedy 2008 and Wang 2002 both used risk ratio to quantify risk reduction and found pre‐transfusion reduced the chance of bad outcome (allergic and febrile NHTR). Wang 1992 used odds ratio to compare two treatments against each other and found a benefit increase with diphenhydramine compared to hydrocortisone.
9. Sample size Two studies were performed with less than 100 participants (Wang 1992; Wang 2002), and one was conducted with less than 400 patients (Kennedy 2008). Three studies reported sample size calculation (Kennedy 2008; Wang 1992; Wang 2002). One used 90% power to detect between 40% reduction in end points (Kennedy 2008).
10. Duplicate publications The search strategy identified one duplicate publication with preliminary results (Kennedy 2008). Febrile and urticarial reaction definitions are described in Table 6.
Table 1.
Definitions of transfusion reactions
| Study‐Year | Febrile reactions | Urticarial | Non‐haemolytic transfusion reactions |
| Kennedy 2008 | Temperature greater than 100.5°F or an increase of 1°F of an already existing fever. | It was classified as " hives with or without itching". | ‐‐‐‐ |
| Wang 1992 | Elevation of body temperature of 1ºC or more during a blood transfusion or within two hours of its completion. | ‐‐‐‐ | ‐‐‐‐ |
| Wang 2002 | New temperature >38°C, or an increase in temperature >1°C above baseline. | ‐‐‐‐ | Fever (new temperature >38°C, or an increase in temperature >1°C above baseline), subjective chills with or without rigors, urticaria or rash, in the absence of haemolysis. |
Excluded studies
Eleven studies did not fulfil the inclusion criteria and were excluded (Ezidiegwu 2004; Geiger 2007; Goodnough 1993; Karpushyn 1965; Patterson 2000; Reverberi 1989; Sanders 2005; Tan 1993; Tobian 2007; Westphal 1982; Zatseva 1984). The majority of these excluded studies were retrospective. See Characteristics of excluded studies table for a detailed account of the reasons for exclusion.
Studies ongoing and pending publication The search did not identify any ongoing studies.
Studies awaiting classification The search found one study (Ricevuti 1984) which is awaiting classification. Attempts were made to contact its main author but no reply was received. See Studies awaiting classification.
Risk of bias in included studies
The risk of bias of Wang 1992; Wang 2002; and Kennedy 2008 is summarised in Figure 1 and Figure 2.
Figure 1.
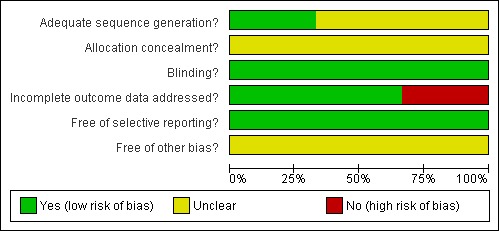
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies. Three studies are included in this review.
Figure 2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Only Wang 1992 developed an adequate randomisation sequence by means of a random table. The other two RCTs did not report detailed information about this domain (Kennedy 2008; Wang 2002). The trials did not provide information about allocation concealment.
Kennedy 2008, Wang 1992 and Wang 2002 were double blind RCTs.
Kennedy 2008 and Wang 2002 were analysed by per‐protocol analysis. Wang 1992 was analysed by intention‐to treat (Table 3).
See the Characteristics of included studies table for details.
Effects of interventions
Main analysis
Results are based on three RCTs, and summarised in Table 1 and Table 2.
Meta‐analysis was not performable because pre‐transfusion randomisation was done by patient in Kennedy 2008 while Wang 2002 randomised pre‐transfusion by transfusion. Wang 1992 compared two active drugs (hydrocortisone versus diphenhydramine).
Primary outcomes
(1) Febrile reactions (fever with or without chills, chills with or without rigors).
Acetaminophen plus diphenhydramine versus placebo (Kennedy 2008, Wang 2002)
The trials included in the review showed no difference between febrile reaction rates when comparing acetaminophen plus diphenhydramine with placebo.
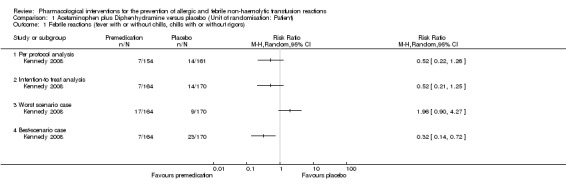
Kennedy 2008 (315 participants; 21 events) showed a non‐significant risk ratio for febrile reactions (RR 0.52, 95% CI 0.22 to 1.26 ‐ see Analysis 1.1). Similarly, Wang 2002 (98 transfusions; 12 events) showed that the risk ratio for febrile reactions was statistically non‐significant when comparing acetaminophen plus diphenhydramine with placebo (RR 1.77, 95% CI 0.57 to 5.49 ‐ see Analysis 2.1).
Analysis 1.1.
Comparison 1 Acetaminophen plus Diphenhydramine versus placebo (Unit of randomisation: Patient), Outcome 1 Febrile reactions (fever with or without chills, chills with or without rigors).
Analysis 2.1.

Comparison 2 Acetaminophen plus Diphenhydrammine versus placebo (Unit of randomisation: transfusion), Outcome 1 Fever.
Diphenhydramine versus hydrocortisone
Wang 1992 (73 randomised participants, 146 participants for paired analysis, 116 beneficial events) showed that patients treated with hydrocortisone had a lower rate of febrile reactions compared to those treated with diphenhydramine (OR 2.38, 95% CI 1.07 to 5.26). Table 7 shows raw data from Wang 1992.
Table 2.
Raw data from Wang 1992
| Prevention of transfusion febrile reaction on both treatments. | Prevention of transfusion febrile reaction diphenhydramine only. | Prevention of transfusion febrile reaction on hydrocortisone only. | Prevention of transfusion febrile reaction on neither treatments. |
| 47 | 6 | 16 | 4 |
These data was supplied by Dr. Wang.
(2) Allergic reactions (urticaria with or without pruritus).
The trials comparing acetaminophen plus diphenhydramine with placebo (Kennedy 2008, Wang 2002) showed no difference in the rate of allergic reactions.
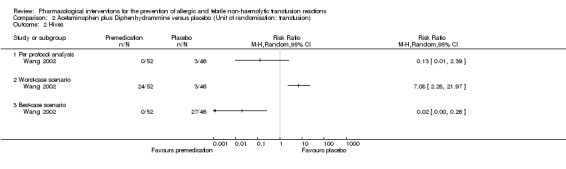
Kennedy 2008 (315 participants; 36 events) showed that the risk ratio for allergic reactions was statistically non‐significant when comparing acetaminophen plus diphenhydramine with placebo (RR 0.52, 95% CI 0.22 to 1.26 ‐ see Analysis 1.2). Wang 2002 (98 transfusions; 3 events) showed that the risk ratio for allergic reactions was statistically non‐significant when comparing acetaminophen plus diphenhydramine with placebo (RR 0.13, 95% CI 0.01 to 2.39 ‐ see Analysis 2.2).
Analysis 1.2.

Comparison 1 Acetaminophen plus Diphenhydramine versus placebo (Unit of randomisation: Patient), Outcome 2 Allergic reactions (urticaria with or without pruritus).
Analysis 2.2.
Comparison 2 Acetaminophen plus Diphenhydrammine versus placebo (Unit of randomisation: transfusion), Outcome 2 Hives.
The trials included did not assess the following outcomes of interest in this review: anaphylactic reactions (dyspnoea, wheezing, hypotension, tachycardia, loss of consciousness, or shock), death related to transfusion reactions and other adverse events (Kennedy 2008; Wang 1992; Wang 2002).
Intention‐to Treat Analysis and Sensitivity Analysis:
An intention‐to‐treat analysis was performed including the 19 patients who were excluded by Kennedy 2008.
For Kennedy 2008 (334 participants; 21 events), there was no significant difference between the intervention and control group for febrile reactions (RR 0.52, 95% CI 0.21 to 1.25 ‐ see Analysis 1.1). There was no significant difference between the intervention and control group for allergic reactions (334 participants; 36 events) (RR 1.46, 95% CI 0.78 to 2.73 ‐ see Analysis 1.2).
Sensitivity analyses of febrile reactions to account for the 19 excluded patients showed discrepancy on this outcome. According to Kennedy 2008 et al, the RRs for the worst‐case scenario (all participants with missing outcomes in the intervention group had 'febrile reactions', and all those with missing outcomes in the placebo group had 'normal' outcomes) and the best‐case scenario (all participants with missing outcomes in the intervention group had 'normal' outcomes, and all those with missing outcomes in the placebo group had 'febrile reactions') were: RRworst scenario 1.96 (95% CI 0.90 to 4.27) for febrile reactions and RRbest scenario 0.32 (95% CI 0.74 to 0.72) for febrile reactions ‐ see Analysis 1.1.
Sensitivity analyses of allergic reactions to account for the 19 excluded patients showed discrepancy on this outcome. According to Kennedy 2008 et al, the RRs for the worst‐case scenario (all participants with missing outcomes in the intervention group had 'allergic reactions', and all those with missing outcomes in the placebo group had 'normal' outcomes) and the best‐case scenario (all participants with missing outcomes in the intervention group had 'normal' outcomes, and all those with missing outcomes in the placebo group had 'allergic reactions') were: RRworst scenario 1.34 (95% CI 0.82 to 2.18) for allergic reactions and RRbest scenario 1.90 (95% CI 0.72 to 5.02) allergic reactions ‐ see Analysis 1.2.
The 24 transfusions excluded by this study could not be included in an ITT analysis because we were unable to determine their transfusion allocation group. The study author was contacted for this information but we received no reply.
Sensitivity analyses of non‐haemolytic transfusion reactions (fever) to account for the 24 excluded transfusions showed discrepancy on this outcome. According to Wang 2002, the RRs for the worst‐case scenario (all participants with missing outcomes in the intervention group had 'febrile reactions', and all those with missing outcomes in the placebo group had 'normal' outcomes) and the best‐case scenario (all participants with missing outcomes in the intervention group had 'normal' outcomes, and all those with missing outcomes in the placebo group had 'febrile reactions') were: RRworst scenario 7.08 (95% CI 2.71 to 18.50) for febrile reactions and RRbest scenario 0.25 (95% CI 0.13 to 0.50) febrile reactions ‐ see Analysis 2.1. Sensitivity analyses of allergic reaction (hives) to account for the 24 excluded transfusions showed discrepancy on this outcome. According to Wang 2002, the RRs for the worst‐case scenario (all participants with missing outcomes in the intervention group had 'allergic reactions', and all those with missing outcomes in the placebo group had 'normal' outcomes) and the best‐case scenario (all participants with missing outcomes in the intervention group had 'normal' outcomes, and all those with missing outcomes in the placebo group had 'allergic reactions') were: RRworst scenario 7.08 (95% CI 2.28 to 21.97) for allergic reactions and RRbest scenario 0.02 (95% CI 0.00 to 0.26) allergic reactions ‐ see Analysis 2.2.
Discussion
Summary of main results
This review of pharmacological interventions for preventing allergic and febrile non‐haemolytic transfusion reactions included three randomised controlled trials whose critical assessment does not support the clinical use of these interventions. We did not find significant differences on incidence of febrile reactions (fever with or without chills, chills with or without rigors), mild allergic reactions (urticaria with or without pruritus). The data from the included studies (462 participants) is inconclusive. Randomised controlled trials differed somewhat in the following characteristics: pre‐transfusion randomisation, participant population, study design, pre‐transfusion medication composition, study country, blood product transfusion, history of transfusion reaction, measures of treatment effect, sample size and duplicate publication.
Overall completeness and applicability of evidence
The main limitation of this Cochrane review was the impossibility of pooling the results into a meta‐analysis. However, this is explained by several clinical and methodological differences in the included RCTs (Wang 1992; Wang 2002; Kennedy 2008) that prevented a pooled analysis. Although the incidence of transfusion reactions from these RCTs was low, these studies included patients with or without history of transfusion reactions, leading to incomplete data and no definitive conclusion. All included RCTs were conducted using less than 500 patients.
Quality of the evidence
The review authors' assessment of the risk of bias of the included studies has been described previously and a summary can be found in Figure 1 and Figure 2. The studies were at moderate risk of bias.
Potential biases in the review process
Publication bias represents a major threat to the validity of systematic reviews. We aimed to minimise bias by completing an exhaustive search which included many clinical trial registries.
Agreements and disagreements with other studies or reviews
Despite differences in methodology, this review drew the same conclusions as Sanders 2005 and Tobian 2007, namely that there was no evidence of effect from pre‐transfusion medications on the risk of NHTRs. Sanders 2005 reported a very low incidence of transfusion reactions (1.3 percent of the 518 transfusions) including patients with prior reactions (≥2) and a low incidence of febrile reactions (0.53 percent of 3792 transfusions) without, and 0.95 percent of 4108 transfusion, with acetaminophen pre‐transfusion medication, concluding that "no clinical trials have demonstrated their efficacy for this purpose" (Sanders 2005). Tobian 2007 meanwhile concluded that "in the absence of definitive evidence‐based studies, pre‐transfusions should not be encouraged". These studies were not systematic reviews.
Ezidiegwu 2004 did contradict the results of this Cochrane review, however it was a retrospective review without a control group. The review included 120,000 units of transfused blood products, 80% of which were preceded by pre‐transfusion. The main target of this study was determining the cost implications of pre‐transfusion medications, and while it found pre‐transfusion reduced reaction rates, this did not provide a significant cost benefit to the healthcare provider.
Authors' conclusions
We found no evidence that pre‐transfusion medication prevents NHTR. This applies regardless of the patient's history of NHTR and whether or not they were transfused with leukodepleted blood products. This conclusion is based on three trials with moderate risk of bias. Practically, this implies the prescription of pre‐transfusion medication is not justified, unless new evidence from a large high quality trial modifies this conclusion.
A powerful RCT is necessary to effectively evaluate the role of pre‐transfusion medication. Inclusion criteria should only involve patients with high risk of developing febrile, allergic or anaphylactic reactions. There should be no restriction by history of transfusion reactions, age, type of blood products used (leukodepleted or not) and pre‐transfusion transfusion safety. Trials should be structured and reported according to the 'Consort Statement' checklist to improve the quality of findings with the aim to standardise patient management.
Acknowledgements
We want to express our gratitude to:
1. Dr Vassily Vlassov who translated Karpushyn 1965 and Zatseva 1984 from Russian and Ukranian respectively. 2. Prof David L. Sackett who supplied the thesis of Wang 1992. 3. Mrs Marta Roquè for her help in preparing the protocol for this review and statistical support relating to Wang 1992. 4. Dr Pablo Perel for his statistical advice.
Appendices
Appendix 1. Search strategy
Cochrane Injuries Group’s Specialised Register (searched Dec 17 2008): 5 records (Febrile and non‐hemolytic) or (Allerg* and non‐hemolytic) or FNHTR* or (post‐transfusion* and reaction*) or (anaphylactic* and transfusion*) or ((allerg* or febrile) and (transfusion* and reaction*)) or (plasma and tranfus* and allerg*) CENTRAL (The Cochrane Library 2008, issue 4): 10 records #1MeSH descriptor Blood Transfusion explode all trees with qualifiers: AE,CO #2MeSH descriptor Erythrocyte Transfusion explode all trees with qualifiers: AE,CO #3MeSH descriptor Platelet Transfusion explode all trees with qualifiers: AE,CO #4MeSH descriptor Blood Component Transfusion explode all trees with qualifiers: AE,CO #5((temperature) near3 (high or rise or raise*)) and (transfusion*):ab,ti #6(Febrile near3 non‐hemolytic):ab,ti #7(Allerg* near3 non‐hemolytic):ab,ti #8(transfusion*) near3 (reaction*):ab,ti #9FNHTR*:ab,ti #10 (post‐transfusion* near5 reaction*):ab,ti #11 (anaphylactic* near transfusion*):ab,ti #12 (allerg* or febrile) and (transfusion*) and (reaction*):ab,ti #13 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12) #14 MeSH descriptor Plasma explode all trees #15 (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12) #16 (#14 AND #15) #17 (#13 OR #16) #18 MeSH descriptor pretransfusion explode all trees #19 pre‐medication or pretransfusion:ab,ti #20 MeSH descriptor Acetaminophen explode all trees #21 MeSH descriptor Diphenhydramine explode all trees #22MeSH descriptor Analgesics, Non‐Narcotic explode all trees #23Paracetamol or Acetaminophen or Diphenhydramine:ab,ti #24MeSH descriptor Anti‐Inflammatory Agents, Non‐Steroidal explode all trees #25MeSH descriptor Ethylamines explode all trees #26MeSH descriptor Hydrocortisone explode all trees #27(hydrocortisone or cortisol or cortifair or cortril):ab,ti #28(#18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27) #29(#17 AND #28) MEDLINE 1950 to November (Week 3) 2008: 82 records 1.exp Blood Transfusion/ae, co [Adverse Effects, Complications] 2.exp Erythrocyte Transfusion/ae [Adverse Effects] 3.exp Platelet Transfusion/ae [Adverse Effects] 4.exp Blood Component Transfusion/ae [Adverse Effects] 5.(temperature adj3 (high or rise or raise*) adj5 transfusion*).ab,ti. 6.(Febrile adj3 non?hemolytic).ab,ti. 7.(Allerg* adj3 non?hemolytic).ab,ti. 8.(transfusion* adj3 reaction*).ab,ti. 9.FNHTR*.ab,ti. 10.(post?transfusion* adj5 reaction*).ab,ti. 11.(anaphylactic* adj5 transfusion*).ab,ti. 12.((allerg* or febrile) adj5 transfusion* adj5 reaction*).ab,ti. 13.or/1‐12 14.exp Plasma/ 15.or/5‐12 16.14 and 15 17.13 or 16 18.exp pretransfusion/ 19.pre?medication*.ab,ti. 20.exp Acetaminophen/ 21.exp Diphenhydramine/ 22.Analgesics, Non‐Narcotic/tu [Therapeutic Use] 23.(Paracetamol or Acetaminophen or Diphenhydramine).ab,ti. 24.exp Anti‐Inflammatory Agents, Non‐Steroidal/tu [Therapeutic Use] 25.exp Ethylamines/tu [Therapeutic Use] 26.exp Hydrocortisone/ 27.(hydrocortisone or cortisol or cortifair or cortril).ab,ti. 28.or/18‐27 29.17 and 28 EMBASE 1980 to Dec 2008 (Week 50): 70 records retrieved 1.exp Blood Transfusion/ 2.exp Erythrocyte Transfusion/ 3.exp Thrombocyte Transfusion/ 4.exp Blood Component Therapy/ 5.exp Plasma Transfusion/ 6.1 or 2 or 3 or 4 or 5 7.exp Allergic Reaction/ 8.(temperature adj3 (high or rise or raise*) adj5 transfusion*).ab,ti. 9.(Febrile adj3 non?hemolytic).ab,ti. 10.(Allerg* adj3 non?hemolytic).ab,ti. 11.(transfusion* adj3 reaction*).ab,ti. 12.FNHTR*.ab,ti. 13.(post?transfusion* adj5 reaction*).ab,ti. 14.(anaphylactic* adj5 transfusion*).ab,ti. 15.((allerg* or febrile) adj5 transfusion* adj5 reaction*).ab,ti. 16.or/7‐15 17.6 and 16 18.exp pretransfusion/ 19.pre?medication*.ab,ti. 20.exp Paracetamol/ 21.exp Diphenhydramine/ 22.exp Analgesic Agent/ 23.(Paracetamol or Acetaminophen or Diphenhydramine).ab,ti. 24.exp Nonsteroid Antiinflammatory Agent/ 25.exp Ethylamine/ 26.exp Hydrocortisone/ 27.(hydrocortisone or cortisol or cortifair or cortril).ab,ti. 28.or/18‐27 29.28 and 17 CINAHL 1982 to Dec 2008: 4 records S17 S16 and S9 S16 (S15 or S14 or S13 or S12 or S11 or S10) S15 (pre‐medication or pretransfusion ) or ( hydrocortisone or cortisol or cortifair or cortril or ethylamine*) S14 (MH "Hydrocortisone") S13 (MH "Antiinflammatory Agents, Non‐Steroidal+") or (MH "Analgesics, Nonnarcotic+") S12 (MH "Diphenhydramine") S11 (MH "Acetaminophen") S10 (MH "pretransfusion") S9 S8 or S1 S8 S7 and S4 S7 S6 or S5 S6 (post‐transfusion* N3 reaction*) or (anaphylactic* N3 transfusion*): or (allerg* N3 reaction*) or (febrile N3 reaction*) or FNHTR S5 (MH "Allergic Reaction Control (Saba CCC)") or (MH "Antigen‐Antibody Reactions+") S4 S3 or S2 S3 (MH "Platelet Transfusion") S2 (MH "Blood Transfusion+") or (MH "Blood Component Transfusion+") S1 (MH "Blood Transfusion Reaction") or (MH "Blood Transfusion Reaction Control (Iowa NOC)") ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) 1970 to Dec 2008, Conference Proceedings Citation Index‐ Science (CPCI‐S) 1990 to Dec 2008: 32 records Topic=(blood transfusion* or erythrocyte transfusion* or platelet transfusion* or blood component transfusion* or plasma transfusion*) AND Topic=(reaction* or febrile or allerg* or anaphylactic* or FNHTR) AND Topic=(pretransfusion* or pre‐medication* or Acetaminophen or Diphenhydramine or Paracetamol or Acetaminophen or Diphenhydramine or analgesic* or Anti‐Inflammatory Agent* or Ethylamines or hydrocortisone or cortisol or cortifair or cortril) PubMed (last 6 months; searched Dec 18 2008): 0 records Search (blood transfusion* or erythrocyte transfusion* or platelet transfusion* or blood component transfusion* or plasma transfusion*) AND (reaction* or febrile or allergy or allergies or allergic or anaphylactic* or FNHTR) AND (pretransfusion* or pre‐medication* or Acetaminophen or Diphenhydramine or Paracetamol or Acetaminophen or Diphenhydramine or analgesic* or Anti‐Inflammatory Agent* or Ethylamines or hydrocortisone or cortisol or cortifair or cortril)
LILACS (1982 to January, 2009): 0 records
((Pt ENSAYO CONTROLADO ALEATORIO OR Pt ENSAYO CLINICO CONTROLADO OR Mh ENSAYOS CONTROLADOS ALEATORIOS OR Mh DISTRIBUCIÓN ALEATORIA OR Mh METODO DOBLE CIEGO OR Mh METODO SIMPLECIEGO OR Pt ESTUDIO MULTICÉNTRICO) or ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT ((Ct ANIMALES OR Mh ANIMALES OR Ct CONEJOS OR Ct RATÓN OR MH Ratas OR MH Primates OR MH Perros OR MH Conejos OR MH Porcinos) AND NOT (Ct HUMANO AND Ct ANIMALES)) [Palavras] and transfusion [Palavras] and pretransfusion [Palavras]
Data and analyses
Comparison 1.
Acetaminophen plus Diphenhydramine versus placebo (Unit of randomisation: Patient)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Febrile reactions (fever with or without chills, chills with or without rigors) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Per protocol analysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Intention‐to treat analysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Worst scenario case | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Best‐scenario case | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Allergic reactions (urticaria with or without pruritus) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Per protocol analysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Intention‐to treat analysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Worst‐case scenario | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Best‐case scenario | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2.
Acetaminophen plus Diphenhydrammine versus placebo (Unit of randomisation: transfusion)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fever | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Per protocol analysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Worst‐scenario case | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Best‐scenario case | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Hives | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Per protocol analysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Worst‐case scenario | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Best‐case scenario | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3.
Diphenhidramine versus Hidrocortisone
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Febrile reactions | 1 | Odds Ratio (Random, 95% CI) | Totals not selected |
Analysis 3.1.

Comparison 3 Diphenhidramine versus Hidrocortisone, Outcome 1 Febrile reactions.
What's new
| Date | Event | Description |
|---|---|---|
| 28 May 2010 | Amended | The 'Sources of support' section has been amended. |
Differences between protocol and review
Methodology for summarising data from Wang 1992 due to its cross‐over design.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, double blind, placebo‐controlled trial. Study type: Single centre study. Location: USA (North Carolina). Study phase: III Study design: Parallel. Randomisation: Block randomisation. Allocation concealment: Not described. Blinding: Caregivers and members of the study team. Follow‐up period: 3.5 years. Analysis: per protocol (315/315). |
|
| Participants | Randomised: 334 (Intervention group: 164, Control group: 170) Excluded (post randomisation):19 (5.7%) (Intervention group: 10 (6%), Control group: 9 (5.3%)) Active participants: 315 (94.3%) (Intervention group: 154 (94%), Control group: 161 (94.7%)) Reason for exclusion:
Gender (Women): 185 (Intervention group: 93 (60%), Control group: 92 (57%)) Age (years): (Median Interquartile Range ‐ IR): Intervention group: 46 (19‐64), Control group: 46 (18‐65) Inclusion criteria: 1. Age: 18 to 65 years. 2. In hospital at leukaemia or bone marrow transplant services. Exclusion criteria: 1. Allergy to either acetaminophen or diphenhydramine. 2. History of febrile or allergic transfusion reaction. |
|
| Interventions |
Intervention group: Oral administration of acetaminophen (500 mg) and diphenhydramine (25 mg) 30 min before the first blood or platelet transfusion. Control group: Oral administration of a placebo thirty minutes prior the first transfusion. Characteristics of placebo not described. Quote: "30 minutes before the first transfusion and no other acetaminophen or diphenhydramine was given 4 hours before the administration of the study medications or 4 hours after the transfusion. If sequential RBC or PLT transfusions were administered after pre‐transfusion medication, then all transfusions occurring 4 hours after pre‐transfusion medication were included in the data" (Page 2286). Co‐interventions: Leukofilter was used for all transfusions. Irradiated blood products were transfused in patients receiving bone marrow transplantation. Single‐donor apheresis platelets were transfused. |
|
| Outcomes |
Transfusion reactions:
|
|
| Notes | Start date: October 1993. Site: Comprehensive Cancer Center of Wake Forest University, North Carolina, USA. Sample size expected: 320 patients (Power: 90%, Hazard ratio: risk of a transfusion reaction of 0.4, (in the treatment group relative to placebo), level of significance: 10% percent one‐sided, expected frequency of reactions: 10%). Information on patient exclusion received from Dr. Kennedy on May 2, 2009. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Patients were assigned to the placebo or the active treatment with equal probability using blocked randomisation" (Page 2286). |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | Low risk | Quote: "All other caregivers and members of the study team were blinded throughout the study duration and data collection until the study was complete" (Page 2286). |
| Incomplete outcome data addressed? All outcomes | Low risk | ‐ |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes. |
| Free of other bias? | Unclear risk | Table 1 exclusively shows characteristics of the patients post exclusion. |
| Methods | Randomised, double blind, controlled trial. Study type: Single centre study. Location: People's Republic of China (Sichuan). Study phase: III Study design: Crossover. Randomisation: Randomisation table. Allocation concealment: Not described. Blinding: Patients and healthcare workers. Follow‐up period: No information provided. |
|
| Participants | Enrolled: 73 participants.
1. History of febrile transfusion reaction. 2. Age: 15 to 70 years. 3. No febrile disease within the previous seven days.
1. Pre‐transfusion temperature above 37.5 ºC. 2. Age: < 15 or > 70 years old. 3. Abnormal mental condition or patients who are uncooperative. 4. Physical condition too poor to justify the burden of hourly temperature readings. 5. History of allergic reaction (urticaria, asthma or allergic transfusion reactions). 6. Blood transfusion for emergency care. |
|
| Interventions |
Group 1: Low dose of hydrocortisone (50 mg) disposed in saline solution infusion (100 ml). Group 2: Diphenhydramine (50 mg) disposed in saline solution infusion (100 ml). Infusion started 30 minutes prior to the blood transfusion. Both drug ampoules were colourless. |
|
| Outcomes | Febrile transfusion reaction. | |
| Notes | Site: Haematology and gastroenterology wards. Recruitment: May 1983 to May 1985. Sample size: 81 patients (β: 80%, α: 0.05 (one tail test: 1.96)). Expected frequency of reactions without treatment: 50%. Expected frequency of reaction reduced by hydrocortisone: 30%. Expected frequency of reaction reduced by diphenhydramine: 10%. Information described above comes from Dr. Wang's doctoral thesis, supplied by Dr. David L. Sackett. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation table. Data obtained from the study protocol. |
| Allocation concealment? | Unclear risk | Insufficient information provided in the study protocol. |
| Blinding? All outcomes | Low risk | Patients and healthcare workers. |
| Incomplete outcome data addressed? All outcomes | Low risk | No missing outcome data. |
| Free of selective reporting? | Low risk | The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
| Free of other bias? | Unclear risk | ‐ |
| Methods | Randomised, Double blind, placebo‐controlled trial. Study type: Single centre study. Location: USA (California). Study phase: III Study design: Parallel. Randomisation: Not described. Allocation concealment: Not described. Blinding: Patients and healthcare workers. Investigational pharmacist was not blinded. Follow‐up period: Assumed to be 3.5 years based on study report. |
|
| Participants | Enrolled: 55 patients (122 transfusions): 100% Assessable transfusions: 98 (51 patients): 80.3% Withdrawals: 19.7% Assessments not performed: 24 Reasons for not performing assessment:
1. Age: ≥18 years of age. 2. Informed consent and complete study questionnaire.
1. Fever on two occasions in the prior 24 hrs. 2. Fever at the onset of transfusion. 3. History of haemolytic transfusion reaction. 4. Concurrent corticosteroid therapy. 5. Acetaminophen or diphenhydramine administered within the past 6 hrs. |
|
| Interventions |
Intervention group: Acetaminophen (650 mg) unlabeled capsule and diphenhydramine (25 mg) IV. Control group: Dextrose (650 mg) unlabeled capsule and 100 ml of normal saline IV. Information on the timing of the intervention was not reported. |
|
| Outcomes | Non‐haemolytic transfusion reactions. | |
| Notes | Start date: March 1998 to March 2000. Site: Hematology Oncology Ward and Infusion room at the University of California—Davis Medical Center (USA). Sample size calculation: not described in detail. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: 'Each transfusion in enrolled patients was randomized to pre‐transfusion or placebo prior to platelet transfusion' (Page 192). |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | Low risk | All patients and healthcare workers were blinded, except the investigational pharmacist. |
| Incomplete outcome data addressed? All outcomes | High risk | Comments: 1. 19.3% of transfusions were not assessed. 2. There is inconsistency between 'patients characteristics' and 'transfusion reaction' tables. 3. Author noted "There were not significant differences between each group of patients (Table I)" (Page 192). The randomisation unit was the platelet transfusion. Therefore, the table number 1 should show transfusion characteristics from the 122 transfusions. 4. Reason for missing outcome data likely to be related to imbalance in numbers or reasons for missing data across intervention groups. 5. Authors did not report reason by intervention groups. |
| Free of selective reporting? | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes. |
| Free of other bias? | Unclear risk | ‐ |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ezidiegwu 2004 | Retrospective review. |
| Geiger 2007 | Narrative review. |
| Goodnough 1993 | Retrospective review of non‐pharmacological intervention. |
| Karpushyn 1965 | Retrospective analysis of clinical data. |
| Patterson 2000 | Non randomised controlled trial. |
| Reverberi 1989 | Non‐pharmacological intervention. |
| Sanders 2005 | Retrospective study. |
| Szelei‐Stevens 2006 | Not RCT (Retrospective). |
| Tan 1993 | Retrospective review of non‐pharmacological intervention. |
| Tobian 2007 | Narrative review. |
| Westphal 1982 | Non‐pharmacological intervention. |
| Zatseva 1984 | Analysis of different aspects of transfusion reactions from two hospital departments. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Design: not described. |
| Participants | Insufficient information was supplied. Sample size: 25. |
| Interventions | Hydrocortisone: 100 mg. Phenytoin: 10 mg/kg. |
| Outcomes | Fever (temperature > 38º C). |
| Notes | Blood product: granulocyte transfusion. Source: Letter to editor. |
Contributions of authors
Conceiving the review (guarantor): Arturo Martí‐Carvajal (AMC)
Undertaking searches: Karen Blackhall, Cochrane Injuries Group Trials Search Co‐ordinator
Screening search results: AMC, Luís E. González (LEG)
Screening retrieved papers against inclusion criteria: AMC, LEG
Appraising quality of papers: AMC, LEG, IS
Extracting data from papers: AMC, LEG
Data management for the review: AMC
Entering data into Review Manager (RevMan 5.0): AMC, IS
Double entry of data: AMC, LEG
Interpretation of data: AMC, LEG, IS, Nelcy Rodríguez (NR), and Graciela León (GL)
Statistical analysis: NR, AMC
Writing the review: AMC
Comment and editing of review drafts: AMC, LEG, IS, NR, GL
Responsible for reading and checking review before submission: AMC, IS
Sources of support
Internal sources
CIBER de Epidemiología y Salud Pública (CIBERESP), Spain.
External sources
Iberoamerican Cochrane Center, Spain.
Agencia de Calidad y Consumo del SNS, Ministry of Health, Spain for Agencia de Calidad del Sistema Nacional de Salud, Ministry of Health, Spain.
Declarations of interest
In 2004 Arturo Martí‐Carvajal was employed by Eli Lilly to run a four hour workshop on 'how to critically appraise clinical trials on osteoporosis and how to teach this'. This activity was not related to his work with The Cochrane Collaboration or any Cochrane review. In 2007 Arturo Martí‐Carvajal was employed by Merck to run a four hour workshop 'how to critically appraise clinical trials and how to teach this'. This activity was not related to his work with The Cochrane Collaboration or any Cochrane review.
Other authors: none.
Edited (no change to conclusions)
References
References to studies included in this review
- Kennedy LD, Case LD, Hurd DD, Cruz JM, Pomper GJ. A prospective, randomized, double‐blind controlled trial of acetaminophen and diphenhydramine pretransfusion medication versus placebo for the prevention of transfusion reactions. Transfusion 2008;48:2285‐91. [DOI] [PubMed] [Google Scholar]; Kennedy LD, Cruz JM, Restino MS. Comparison of acetaminophen and diphenhydramine versus placebo for the prevention of febrile or allergic transfusion associated reactions. Blood 1999;94(10 Suppl 1):375a. [Google Scholar]
- Wang JS, Sackett DJ, Yuan YM. Randomized clinical controlled cross‐over trial (RCT) in the prevention of blood transfusion febrile reactions with small dose hydrocortisone versus anti‐histamines. Zhonghua Nei Ke Za Zhi [Chinese Journal of Internal Medicine] 1992;31:536‐6. [PubMed] [Google Scholar]
- Wang SE, Lara PN, Lee‐Ow A, et al. Acetaminophen and diphenhydramine as premedication for platelet transfusions: a prospective randomized double‐blind placebo‐controlled trial. American Journal of Hematology 2002;70:191‐4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Ezidiegwu CN, Lauenstein KJ, Rosales LG, Kelly KC, Henry JB. Febrile nonhemolytic transfusion reactions. Management by premedication and cost implications in adult patients. Archives of Pathology and Laboratory Medicine 2004;128:991‐5. [DOI] [PubMed] [Google Scholar]
- Geiger TL, Howard SC. Acetaminophen and diphenhydramine premedication for allergic and febrile nonhemolytic transfusion reactions: good prophylaxis or bad practice?. Transfusion Medicine Reviews 2007;21:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnough LT, Riddell J 4th, Lazarus H, Chafel TL, Prince G, Hendrix D, et al. Prevalence of platelet transfusion reactions before and after implementation of leukocyte‐depleted platelet concentrates by filtration. Vox Sanguinis 1993;65:103‐7. [DOI] [PubMed] [Google Scholar]
- Karpushyn VP. The use of cortisone and hydrocortisone in the prevention of transfusion reactions in pregnancy. Pediatriia Akusherstvo i Ginekologiia 1965;4:55‐7. [PubMed] [Google Scholar]
- Patterson BJ, Freedman J, Blanchette V, Sher G, Pinkerton P, Hannach B, et al. Effect of premedication guidelines and leukoreduction on the rate of febrile nonhaemolytic platelet transfusion reactions. Transfusion Medicine 2000;10:199‐206. [DOI] [PubMed] [Google Scholar]
- Reverberi R, Ferrari L, Gennari M, Menini C. Prevention of non‐hemolytic transfusion reactions with leucocyte‐poor blood: a prospective study. Haematologica 1989;74:283‐8. [PubMed] [Google Scholar]
- Sanders RP, Maddirala SD, Geiger TL, Pounds S, Sandlund JT, Ribeiro RC, et al. Premedication with acetaminophen or diphenhydramine for transfusion with leucoreduced blood products in children. British Journal of Haematology 2005;130:781‐7. [DOI] [PubMed] [Google Scholar]
- Szelei‐Stevens KA, Narvios A, Al‐Sammak M. Transfusion reactions: to premedicate or not to premedicate?. Transfusion 2006;46 (Suppl 9):95A. 16398736 [Google Scholar]
- Tan KK, Lee WS, Liaw LC, Oh A. A prospective study on the use of leucocyte‐filters in reducing blood transfusion reactions in multi‐transfused thalassemic children. Singapore Medical Journal 1993;34:109‐11. [PubMed] [Google Scholar]
- Tobaian AAR, King KE, Ness PM. Transfusion premedications: a growing practice not based on evidence. Transfusion 2007;47:1089‐96. [DOI] [PubMed] [Google Scholar]
- Westphal R, Goldfinger D. Washed RBC to prevent transfusion reactions. Transfusion 1982;22:82. [DOI] [PubMed] [Google Scholar]
- Zatseva GA, Zheleznova AI, Moiseeva VG, Aribzhanova IM, Vozgegova NP. Prevention of the nonhemolytic type of reactions during the transfusion of blood and its components. Gematologiia i transfuziologiia 1984;29:24‐7. [PubMed] [Google Scholar]
References to studies awaiting assessment
- Ricevuti G, Mazzone A, Danesino M, Toscano M, Rizzo SC. Phenytoin to prevent or control granulocyte transfusion reactions. Lancet 1984;2(8393):37. [DOI] [PubMed] [Google Scholar]
Additional references
- Banerji A, Long AA, Camargo CA Jr. Diphenhydramine versus nonsedating antihistamines for acute allergic reactions: a literature review. Allergy and Asthma Proceedings 2007;28:418‐26. [DOI] [PubMed] [Google Scholar]
- Botting RM. Mechanism of action of acetaminophen: is there a cyclooxygenase 3?. Clinical Infectious Diseases 2000;31 Suppl 5:202‐10. [DOI] [PubMed] [Google Scholar]
- Brecher ME, editor. Technical manual. 15th Edition. Bethesda: American Association of Blood Banks, 2005. [Google Scholar]
- Brunton LL, Parker KL, Blumenthal D, Buxton I (editors). Manual of Pharmacology and Therapeutics. 11th Edition. New York: McGraw Hill, 2008. [DOI: 10.1036/0071443436] [DOI] [Google Scholar]
- Domen RE, Hoeltge GA. Allergic transfusion reactions: an evaluation of 273 consecutive reactions. Archives of Pathology & Laboratory Medicine 2003;127:316‐20. [DOI] [PubMed] [Google Scholar]
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [DOI] [PubMed] [Google Scholar]
- Ezidiegwu CN, Lauenstein KJ, Rosales LG, Kelly KC, Henry JB. Febrile nonhemolytic transfusion reactions. Management by premedication and cost implications in adult patients. Archives of Pathology & Laboratory Medicine 2004;128:991‐5. [DOI] [PubMed] [Google Scholar]
- Geiger TL, Howard SC. Acetaminophen and diphenhydramine premedication for allergic and febrile nonhemolytic transfusion reactions: good prophylaxis or bad practice?. Transfusion Medicine Reviews 2007;21:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Egleton RD. Pathophysiology of the blood‐brain barrier: animal models and methods. Current Topics in Developmental Biology 2008;80:277‐309. [DOI] [PubMed] [Google Scholar]
- Heddle NM. Pathophysiology of febrile nonhemolytic transfusion reactions. Current Opinion in Hematology 1999;6:420‐6. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. Available from www.cochrane‐handbook.org.2009.
- Paglino JC, Pomper GJ, Fisch GS, Champion MH, Snyder EL. Reduction of febrile but not allergic reactions to RBCs and platelets after conversion to universal prestorage leukoreduction. Transfusion 2004;44:16‐24. [DOI] [PubMed] [Google Scholar]
- Perrotta PL, Snyder EL. Non‐infectious complications of transfusion therapy. Blood Reviews 2001;15:69‐83. [DOI] [PubMed] [Google Scholar]
- Pruss A, Kalus U, Radtke H, Koscielny J, Baumann‐Baretti B, Balzer D, et al. Universal leukodepletion of blood components results in a significant reduction of febrile non‐hemolytic but not allergic transfusion reactions. Transfusion and Apheresis Science 2004;30:41‐6. [DOI] [PubMed] [Google Scholar]
- Ramachandran K, Sirop P. Rare complications of diphenhydramine toxicity. Connecticut Medicine 2008;72:79‐82. [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
- Sanders RP, Maddirala SD, Geiger TL, Pounds S, Sandlund JT, Ribeiro RC, et al. Premedication with acetaminophen or diphenhydramine for transfusion with leucoreduced blood products in children. British Journal of Haematology 2005;130:781‐7. [DOI] [PubMed] [Google Scholar]
- Sarkodee‐Adoo CB, Kendall JM, Sridhara R, Lee EJ, Schiffer CA. The relationship between the duration of platelet storage and the development of transfusion reactions. Transfusion 1998;38:229‐35. [DOI] [PubMed] [Google Scholar]
- Tobian AA, King KE, Ness PM. Transfusion premedications: a growing practice not based on evidence. Transfusion 2007;47:1089‐96. [DOI] [PubMed] [Google Scholar]
- Wang SE, Lara PN Jr, Lee‐Ow A, Reed J, Wang LR, Palmer P, et al. Acetaminophen and diphenhydramine as premedication for platelet transfusions: a prospective randomized double‐blind placebo‐controlled trial. American Journal of Hematology 2002;70:191‐4. [DOI] [PubMed] [Google Scholar]
- Zavala D, Martí A, Peña‐Martí G, Comunián G. Sheet to enter data for performing a Cochrane review. Valencia, Venezuela: Universidad de Carabobo, 2006.


