Abstract
Background
Self‐monitoring of blood glucose is essential to optimise glycaemic control in type 1 diabetes mellitus. Continuous glucose monitoring (CGM) systems measure interstitial fluid glucose levels to provide semi‐continuous information about glucose levels, which identifies fluctuations that would not have been identified with conventional self‐monitoring. Two types of CGM systems can be defined: retrospective systems and real‐time systems. Real‐time systems continuously provide the actual glucose concentration on a display. Currently, the use of CGM is not common practice and its reimbursement status is a point of debate in many countries.
Objectives
To assess the effects of CGM systems compared to conventional self‐monitoring of blood glucose (SMBG) in patients with diabetes mellitus type 1.
Search methods
We searched The Cochrane Library, MEDLINE, EMBASE and CINAHL for the identification of studies. Last search date was June 8, 2011.
Selection criteria
Randomised controlled trials (RCTs) comparing retrospective or real‐time CGM with conventional self‐monitoring of blood glucose levels or with another type of CGM system in patients with type 1 diabetes mellitus. Primary outcomes were glycaemic control, e.g. level of glycosylated haemoglobin A1c (HbA1c) and health‐related quality of life. Secondary outcomes were adverse events and complications, CGM derived glycaemic control, death and costs.
Data collection and analysis
Two authors independently selected the studies, assessed the risk of bias and performed data‐extraction. Although there was clinical and methodological heterogeneity between studies an exploratory meta‐analysis was performed on those outcomes the authors felt could be pooled without losing clinical merit.
Main results
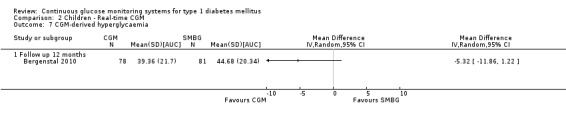
The search identified 1366 references. Twenty‐two RCTs meeting the inclusion criteria of this review were identified. The results of the meta‐analyses (across all age groups) indicate benefit of CGM for patients starting on CGM sensor augmented insulin pump therapy compared to patients using multiple daily injections of insulin (MDI) and standard monitoring blood glucose (SMBG). After six months there was a significant larger decline in HbA1c level for real‐time CGM users starting insulin pump therapy compared to patients using MDI and SMBG (mean difference (MD) in change in HbA1c level ‐0.7%, 95% confidence interval (CI) ‐0.8% to ‐0.5%, 2 RCTs, 562 patients, I2=84%). The risk of hypoglycaemia was increased for CGM users, but CIs were wide and included unity (4/43 versus 1/35; RR 3.26, 95% CI 0.38 to 27.82 and 21/247 versus 17/248; RR 1.24, 95% CI 0.67 to 2.29). One study reported the occurrence of ketoacidosis from baseline to six months; there was however only one event. Both RCTs were in patients with poorly controlled diabetes.
For patients starting with CGM only, the average decline in HbA1c level six months after baseline was also statistically significantly larger for CGM users compared to SMBG users, but much smaller than for patients starting using an insulin pump and CGM at the same time (MD change in HbA1c level ‐0.2%, 95% CI ‐0.4% to ‐0.1%, 6 RCTs, 963 patients, I2=55%). On average, there was no significant difference in risk of severe hypoglycaemia or ketoacidosis between CGM and SMBG users. The confidence interval however, was wide and included a decreased as well as an increased risk for CGM users compared to the control group (severe hypoglycaemia: 36/411 versus 33/407; RR 1.02, 95% CI 0.65 to 1.62, 4 RCTs, I2=0% and ketoacidosis: 8/411 versus 8/407; RR 0.94, 95% CI 0.36 to 2.40, 4 RCTs, I2=0%).
Health‐related quality of life was reported in five of the 22 studies. In none of these studies a significant difference between CGM and SMBG was found. Diabetes complications, death and costs were not measured.
There were no studies in pregnant women with diabetes type 1 and in patients with hypoglycaemia unawareness.
Authors' conclusions
There is limited evidence for the effectiveness of real‐time continuous glucose monitoring (CGM) use in children, adults and patients with poorly controlled diabetes. The largest improvements in glycaemic control were seen for sensor‐augmented insulin pump therapy in patients with poorly controlled diabetes who had not used an insulin pump before. The risk of severe hypoglycaemia or ketoacidosis was not significantly increased for CGM users, but as these events occurred infrequent these results have to be interpreted cautiously.There are indications that higher compliance of wearing the CGM device improves glycosylated haemoglobin A1c level (HbA1c) to a larger extent.
Keywords: Adolescent; Adult; Aged; Aged, 80 and over; Child; Female; Humans; Male; Middle Aged; Young Adult; Blood Glucose Self‐Monitoring; Blood Glucose Self‐Monitoring/methods; Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 1/blood; Glycated Hemoglobin; Glycated Hemoglobin/analysis; Hypoglycemia; Hypoglycemia/diagnosis; Monitoring, Ambulatory; Monitoring, Ambulatory/methods; Quality of Life; Retrospective Studies
Plain language summary
Continuous glucose monitoring systems for type 1 diabetes mellitus
Type 1 diabetes is a disease in which the pancreas has lost its ability to make insulin. A deficit in insulin leads to increases in blood glucose levels, these elevated blood glucose levels can lead to complications which may affect the eyes, kidneys, nerves and the heart and blood vessels. Since there is no cure for type 1 diabetes, patients need to check their blood glucose levels often by fingerprick and use these blood glucose values to decide on their insulin dosages. Fingerpricks are often regarded as cumbersome and uncomfortable by patients. In addition, fingerprick measurements only provide information about a single point in time, so it is difficult to discern trends in decline of rises in blood glucose levels.
Continuous glucose monitoring systems (CGM) measure blood glucose levels semi‐continuously. Most modern CGM systems consist of a small needle which is inserted in the abdominal subcutaneous fat. The tip of the needle houses a small glucose sensor which can measure glucose levels in the fluid which surrounds the fatty tissue. Here we explore whether CGM systems help the patient to increase quality of life and her glycaemic control, which reflects how well the patient's diabetes is treated.
In this review 22 studies were included. These studies randomised 2883 patients with type 1 diabetes to receive a form of CGM or to use self measurement of blood glucose (SMBG) using fingerprick. The duration of follow‐up varied between 3 and 18 months; most studies reported results for six months of CGM use. This review shows that CGM helps in lowering the glycosylated haemoglobin A1c (HbA1c) value (a measure of glycaemic control). In most studies the HbA1c value decreased (denoting improvement of glycaemic control) in both the CGM and the SMBG users, but more in the CGM group. The difference in change in HbA1c levels between the groups was on average 0.7% for patients starting on an insulin pump with integrated CGM and 0.2% for patients starting with CGM alone. The most important adverse events, severe hypoglycaemia and ketoacidosis did not occur frequently in the studies, and absolute numbers were low (9% of the patients, measured over six months). Diabetes complications, death from any cause and costs were not measured. There are no data on pregnant women with diabetes type 1 and patients with diabetes who are not aware of hypoglycaemia.
Summary of findings
Summary of findings for the main comparison. Continuous glucose monitoring (CGM) augmented pump therapy for type 1 diabetes mellitus in insulin pump naive patients.
| CGM augmented pump therapy for type 1 diabetes mellitus in insulin pump naive patients | ||||||
| Patient or population: patients with type 1 diabetes mellitus Intervention: CGM augmented pump therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | CGM augmented pump therapy | |||||
| Diabetic complications | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Severe hypoglycaemia Follow‐up: 6 months | 29 per 1000 | 93 per 1000 (11 to 795) | RR 3.26 (0.38 to 27.82) | 78 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| Ketoacidosis Follow‐up: 6 months | (Moderate risk population) 10 per 1000 | 25 per 1000 (1 to 585) | RR 2.45 (0.1 to 58.45) | 78 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| Quality of life ‐ Various features | 412 (5 studies) | |||||
| Quality of life ‐ Physical health domain SF‐36 Short form Follow‐up: 6 months | The mean quality of life ‐ physical health in the control groups was 91 | The mean quality of life ‐ physical health in the intervention groups was 1.3 higher (4.2 lower to 6.8 higher) | 75 (1 study) | ⊕⊝⊝⊝ very low1,3 | Scale from 0 to 100; higher values indicate better quality of life. | |
| Quality of life ‐ Mental health domain SF‐36 Short form Follow‐up: 6 months | The mean quality of life ‐ mental health in the control groups was 77 | The mean quality of life ‐ mental health in the intervention groups was 2.4 higher (4.4 lower to 9.2 higher) | 75 (1 study) | ⊕⊝⊝⊝ very low1,3 | Scale from 0 to 100; higher values indicate better quality of life. | |
| Change in HbA1c (%) Follow‐up: 6 months | The mean change in Hba1c ranged across control groups from ‐0.1 to ‐0.2 | The mean change in Hba1c in the intervention groups was 0.7 lower (0.8 to 0.5 lower) | 562 (2 studies) | ⊕⊕⊕⊝ moderate4 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Only one study. 2 Substantial imprecision because of very low number of events; 95% CI includes appreciable benefit as well as appreciable harm. 3 Substantial imprecision because of small population size; 95% CI includes improved as well as worsened quality of life.
4 Substantial inconsistency as CIs are hardly overlapping; I2 = 84%. However, results of both studies are clinically and statistically significant.
Summary of findings 2. Continuous real‐time glucose monitoring (CGM) for type 1 diabetes mellitus.
| CGM for type 1 diabetes mellitus | ||||||
| Patient or population: patients with type 1 diabetes mellitus Intervention: Continuous real‐time glucose monitoring | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Continuous Real‐time CGM | |||||
| Diabetic complications | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Severe hypoglycaemia Follow‐up: 6 months | 75 per 1000 | 79 per 1000 (47 to 133) | RR 1.05 (0.63 to 1.77) | 689 (5 studies) | ⊕⊕⊝⊝ low1 | |
| Ketoacidosis Follow‐up: 6 months | 23 per 1000 | 20 per 1000 (7 to 52) | RR 0.85 (0.32 to 2.26) | 689 (5 studies) | ⊕⊕⊝⊝ low1 | |
| Quality of life ‐ Physical health domain SF‐12 Follow‐up: 6 months | The mean quality of life ‐ physical health in the control groups was 54 | The mean quality of life ‐ physical health in the intervention groups was 1.4 higher (0.2 lower to 3 higher) | 226 (1 study) | ⊕⊝⊝⊝ very low2,3 | ||
| Quality of life ‐ Mental health domain SF‐12 Follow‐up: 6 months | The mean quality of life ‐ mental health in the control groups was 75 | The mean quality of life ‐ mental health in the intervention groups was 1.9 higher (1.4 lower to 5.2 higher) | 226 (1 study) | ⊕⊝⊝⊝ very low2,3 | ||
| Quality of life ‐ Parents SF‐12 Follow‐up: 6 months | The mean quality of life ‐ parents in the control groups was 49 | The mean quality of life ‐ parents in the intervention groups was 0.3 lower (2.9 lower to 2.3 higher) | 226 (1 study) | ⊕⊝⊝⊝ very low2,4 | ||
| Change in HbA1c (%) Follow‐up: 6 months | The mean change in Hba1c ranged across control groups from ‐0.6 to 0 | The mean change in Hba1c in the intervention groups was 0.2 lower (0.4 to 0.1 lower) | 963 (6 studies) | ⊕⊕⊕⊝ moderate5 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Substantial imprecision because of very low number of events; 95% CI includes appreciable benefit as well as appreciable harm.
2 Only one study.
3 Substantial imprecision because of small population size; 95% CI includes no effect as well as improved quality of life.
4 Substantial imprecision because of small population size; 95% CI includes improved as well as worsened quality of life.
5 Inconsistency because of heterogeneity (different study designs and patient populations; I2 = 55%).
Background
Description of the condition
Diabetes mellitus (DM) is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. A consequence of this is chronic hyperglycaemia (that is elevated levels of plasma glucose) with disturbances of carbohydrate, fat and protein metabolism. Long‐term complications of DM include retinopathy, nephropathy and neuropathy. The risk of cardiovascular disease is increased. For a detailed overview of DM, please see under 'Additional information' in the information on the Metabolic and Endocrine Disorders Group in The Cochrane Library (see 'About', 'Cochrane Review Groups (CRGs)'). For an explanation of methodological terms, see the main Glossary in The Cochrane Library."
Several types of diabetes are distinguished (WHO 1998). In type 1 DM the body is unable to produce insulin and therefore people with this type are treated with insulin. Type 1 DM accounts for 10% of cases and is typically seen in young adults (less than 30 years), and is often referred to as the insulin dependent diabetes.
Description of the intervention
Self‐monitoring of blood glucose is an essential part of diabetes management and is used to optimise glycaemic control (DCCT 1993; NCCWCH 1994). Good control of blood glucose levels plays an important role in reducing the risk of serious long‐term complications, including microvascular damage (nephropathy, retinopathy) and neuropathy as well as macrovascular damage (cardiovascular disease) (DCCT 1993; Nathan 2005). Regular testing of blood glucose levels is therefore recommended. This allows patients with diabetes to adjust therapy (insulin dosage) appropriately.
Conventional self‐monitoring of blood glucose is achieved by obtaining a finger‐capillary blood sample, where the blood glucose is usually measured employing a small handheld device ‐ a blood glucose meter. This provides a value of the blood glucose at the moment when the blood was sampled. Although this method has been found to provide an accurate estimate of the glucose level, marked fluctuations in blood glucose can be missed, hampering optimal glycaemic control (Boland 2001; Brauker 2009). In addition, blood glucose self‐monitoring requires a number of finger punctures per day to assess the glucose concentration. Many patients find the multiple finger punches that blood glucose self‐monitoring requires uncomfortable and painful (Wentholt 2007).
Continuous glucose monitoring (CGM) systems measure interstitial fluid glucose levels to provide semi‐continuous information about glucose levels, which may identify fluctuations that would not be identified with self‐monitoring alone. Currently, the use of CGM is not common practice (Brauker 2009; Wentholt 2007).
CGM is considered to be particularly useful for children (to reduce the often very high number of finger punctures in this group), for patients with poorly controlled diabetes, for pregnant women in whom tight glucose control is essential with respect to the outcome of pregnancy and for patients with hypoglycaemia unawareness (to prevent dangerous episodes of hypoglycaemia).
Two types of CGM systems can be defined (Wentholt 2007):
systems that measure the glucose concentration during a certain time span: the information is stored in a monitor and can be downloaded later ('retrospective systems');
real‐time systems that continuously provide the actual glucose concentration on a display.
Most systems use a needle sensor, inserted under the skin, but also non‐invasive systems exist that aim to measure the glucose concentration in exudate that is triggered by iontophoresis (Chase 2005). The GlucoWatch is a near‐continuous real‐time CGM device with alarms for high and low glucose values and is shaped like a large watch. The glucose level is measured and displayed every 10 min for up to 13 hours (Thierny 2000). Although this technology seemed attractive as there was no transdermal pricking, drawbacks were the delay between two values, combined with the cumbersome calibration procedure and limited accuracy during hypoglycaemia. Hence, this device has been removed from the market because of the upcoming development of novel more promising diabetes management products (Girardin 2009).
CGM is used continuously or intermittently (e.g. a couple of days per month or in intervals of three days), the latter approach of course being less costly.
Adverse effects of the intervention
Some CGM devices have been associated with skin irritation (Klonoff 2005).
Why it is important to do this review
The advantage of CGM is the continuous provision of information regarding the blood‐glucose concentration, to facilitate the adjustment of the insulin dosage. Disadvantages of CGM are the couple of minutes delay of the measurements which may impede optimal monitoring and some patients may not like the continuous provision of information that confronts them with their illness all the time. However, data on how patients experience CGM systems are sparse (Wentholt 2007). Moreover, the precision of the current CGM systems’ measurements is variable; deviations lower than 20% of the real value are considered to be acceptable (Wentholt 2005; Wentholt 2008). Finally CGM associated costs are higher than conventional self‐monitoring expenditures (each sensor has to be replaced every five days on average).
The future role of CGM might be increasingly important when used in so‐called 'closed loops' in which CGM systems are combined with insulin pumps which adjust their dosage automatically on the basis of the real time blood‐glucose concentration.
The current review has been conducted to enable careful weighting of the benefits and harms of CGM compared to conventional self‐monitoring.
Previous systematic reviews focused only on retrospective devices (Chetty 2008; Golicki 2008) or on specific patient groups, e.g. children (Golicki 2008). The search strategy of the reviews was limited. The current review comprises all types of CGM devices and all patient groups. Recently two meta‐analyses on the effectiveness of CGM have been published, one in adults and children with type 1 or type 2 diabetes mellitus (Pickup 2011) and one in men and non‐pregnant women with type 1 diabetes mellitus (Ghandi 2011). Compared to this review, there were differences in search strategy, eligibility criteria and method of meta‐analysis. The results of these recent meta‐analyses will be discussed in the discussion section.
Objectives
To assess the effects of continuous glucose monitoring systems compared with each other and compared to conventional self‐monitoring of blood glucose in patients with type 1 diabetes mellitus.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials comparing any type of continuous glucose monitoring (CGM) system with conventional self‐monitoring of blood glucose levels or with another type of CGM system in patients with type 1 diabetes mellitus (DM).
Types of participants
Participants were males and females of any age who were classified as having type 1 DM using accepted criteria. To be consistent with changes in classification and diagnostic criteria of diabetes mellitus through the years, the diagnosis should have been established using the standard criteria valid at the time of the beginning of the trial (ADA 1999; WHO 1980; WHO 1985; WHO 1998). Ideally, diagnostic criteria should have been described. If necessary, authors' definition of diabetes mellitus were used. We planned to subject the diagnostic criteria to a sensitivity analysis.
Types of interventions
Intervention
Continuous glucose monitoring systems (invasive retrospective and real‐time systems). Studies on the GlucoWatch were excluded because this device has been removed from the market because of the upcoming development of novel more promising diabetes management products.
Control
conventional self‐monitoring of blood glucose (SMBG), defined as measuring the blood glucose by finger‐capillary blood sample at least once a day. The glucose level is measured using a blood glucose meter;
another type of continuous glucose monitoring system.
Types of outcome measures
Primary outcomes
Glycaemic control
change in glycosylated haemoglobin A1c level (HbA1c);
number of episodes of severe hypoglycaemia (a hypoglycaemic event requiring assistance of another person; documented or undocumented by measured plasma glucose level);
number of episodes with mild hypoglycaemia (symptoms easily controlled by the person);
number of ketoacidotic events.
Quality of life
quality of life: diabetes‐specific, measured with a validated instrument like the 'Diabetes Symptom Checklist' or the 'Diabetes well‐being questionnaire' (Bradley 1994a; Grootenhuis 1994) or generic, measured with a validated instrument like the SF‐36 (McHorney 1993);
patient satisfaction measured with a validated instrument like the 'Diabetes Mellitus Treatment Satisfaction Questionnaire' (Bradley 1994b).
Secondary outcomes
Complications and adverse effects
local adverse effects, e.g. skin irritation and wound infection;
specific diabetes complications (retinopathy, nephropathy, neuropathy, diabetic foot);
among pregnant women: birth weight, macrosomia and congenital malformations of the child, perinatal complications.
CGM derived glycaemic control (with blinded CGM for the control group)
nocturnal hypoglycaemic episodes;
glucose levels less than 3.9 mmol/L (mean area under CGM curve, number of episodes or both);
glucose levels equal or greater than 10 mmol/L (mean area above CGM curve, number of episodes or both).
Death (all causes)
Costs
Covariates, effect modifiers and confounders
patients with hypoglycaemia unawareness (failure to recognize autonomic warning symptoms before the development of neuro‐glycopenia (Cryer 2004));
patients with poorly controlled diabetes (defined as HbA1c greater than 8.0%).
Timing of outcome measurement
Analyses were planned for measurements performed at:
three months follow‐up (short‐term effects);
six months, 1, 2, 5 and 10 years follow‐up (long‐term effects).
Search methods for identification of studies
Electronic searches
We used the following sources for the identification of studies:
The Cochrane Library (issue 6, 2011);
MEDLINE (2003 until June 8, 2011);
EMBASE (2003 until June 8, 2011);
CINAHL (until June 2011).
We also searched prospective trial registers to find ongoing trials:
Dutch Trial Register (NTR);
Australian New Zealand Clinical Trials Registry (ANZCTR);
ISRCTN register (ISRCTN.org);
ClinicalTrials.gov;
Chinese Clinical Trial Register (ChiCTR);
Clinical Trials Registry ‐ India (CTRI);
Sri Lanka Clinical Trials Registry (SLCTR).
For detailed search strategies please see under Appendix 1. Studies published in any language were included.
Searching other resources
Reference lists of included trials and (systematic) reviews, meta‐analyses and health technology assessment reports were checked to identify additional studies. Furthermore, to find relevant but unpublished trials, we contacted experts in the field. We planned to check the abstract books of the major annual European and American diabetes conferences, but as we were confident the currently available evidence was complete, we omitted the abstract books.
Data collection and analysis
Selection of studies
To determine the studies to be assessed further, two authors (ML, LH) independently scanned the title, abstract or both sections of every record retrieved. All potentially relevant articles were investigated as full text. Interrater agreement for study selection was measured using the kappa statistic (Cohen 1960). When there was only an abstract available, we tried to find the final publication of the trial. Studies without a final publication were considered separately. In the case of duplicate publications and accompanying reports of a primary study, we tried to maximise yield of information by simultaneous evaluation of all available data. In cases of doubt, the original publication (usually the oldest version) obtained priority.
The full text articles were examined for compliance with eligibility criteria. We included studies in the review if:
they were based on RCTs;
they included patients with type 1 DM;
the intervention included a CGM system.
We excluded studies if:
the CGM system was not compared with conventional self‐monitoring of blood glucose levels or with another type of CGM system;
none of the above mentioned outcomes were reported;
the results on type 1 DM were not presented separately.
Two researchers (ML, LH) performed study selection independently. Differences in opinion were resolved through discussion. An adapted PRISMA (preferred reporting items for systematic reviews and meta‐analyses) flow‐chart (Figure 1) of study selection is attached (Liberati 2009).
1.
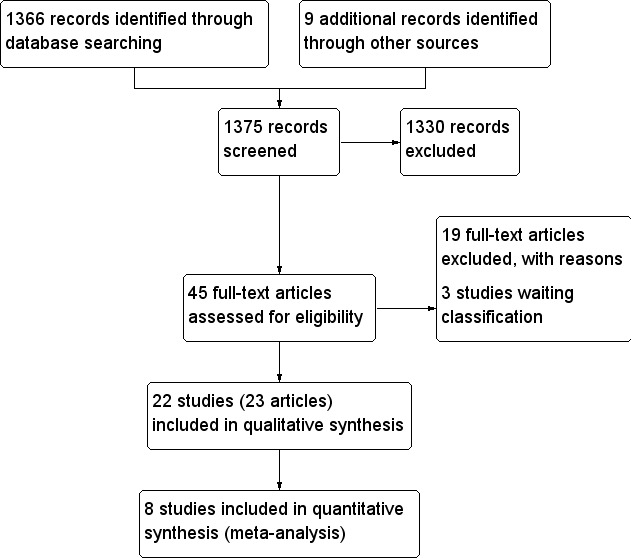
Study flow diagram.
Data extraction and management
For studies that fulfilled the inclusion criteria, two out of three possible authors (ML, YL, RS) independently abstracted relevant population and intervention characteristics using standard data extraction templates (for details see 'Characteristics of included studies', Table 3, Appendix 2, Appendix 3, Appendix 4, Appendix 5, Appendix 6 and Appendix 7). Disagreements were resolved by discussion. Any relevant missing information on the study was sought from the original author(S) of the article, when required.
1. Overview of study populations.
|
Characteristic ► Study ID▼ |
intervention (I) control (C) | [n] screened | [n] randomised | [n] safety | [n] ITT | [n] finishing study | [%] of randomised participants finishing study [%] | comments |
| Battelino 2011 | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
122 | I: 62 C: 58 total: 120 |
‐ | ‐ | I: 53 C: 48 total: 101 |
I: 85 C: 83 |
|
| Bergenstal 2010 | I: Continuous glucose monitoring with CSII C: Conventional glucose self‐monitoring with MDI |
495 | I: 244 C: 241 total: 485 |
32 discontinued or withdrawn (unspecified) | ‐ | total: 443 | total: 89.5 | |
| Chase 2001 | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
12 | I: 6 C: 6 total: 12 |
‐ | ‐ | I: 5 C: 6 total: 11 |
I: 83 C: 100 total: 92 |
|
| Chico 2003 | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
‐ | I: 40 C: 35 total: 75 |
‐ | ‐ | I: 40 C: 35 total: 75 |
I: 100 C: 100 total: 100 |
|
| Cooke 2009 | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
2235 | I: 202 C: 202 total: 404 |
‐ | ‐ | I: 162 C: 168 total: 330 In subanalysis: I: 98 C: 99 total: 197 |
I: 80 C: 83 total: 82 |
Intervention group consisted of 2 subgroups using the Glucowatch (n = 100) or Medtronic device (n = 102). |
| Cosson 2009 | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
56 | I: 25 C: 23 total: 48 |
‐ | ‐ | I: 14 C: 20 total: 34 |
I: 56 C: 87 total: 72 |
Analysed: 9 with diabetes type 1 (3 CGM, 6 control). No further information on randomised number of diabetes type 1 patients. |
| Deiss 2006 | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
30 | I: 15 C: 15 total: 30 |
‐ | ‐ | I: 30 C: 30 total: 30 |
I: 100 C: 100 total: 100 |
Cross‐over design |
| Deiss 2006a | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
193 | I: 108 C: 54 total: 162 |
‐ | ‐ | I: 102 C: 54 total: 156 |
I: 94 C: 100 total: 96 |
Intervention group consisted of 2 subgroups with 54 patients included in both subgroups. |
| Hermanides 2011 | I: Sensor augmented pump therapy c: Conventional glucose self‐monitoring |
93 | I: 44 C: 39 total: 83 |
1 withdrawn (had to undergo surgery for prior health problem) | ‐ | I: 43 C: 35 total: 78 |
I: 98 C: 90 total: 94 |
|
| Hermanns 2009 | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
‐ | I: 25 C: 25 total: 50 |
‐ | ‐ | I: 50 C: 50 total: 50 |
I: 100 C: 100 total: 100 |
Cross‐over design |
| Hirsch 2008 | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
‐ | I: 74 C: 72 total: 146 |
1 withdrawn (pregnancy) | ‐ | I: 72 C: 66 total: 138 |
I: 97 C: 92 total: 95 |
|
| Juvenile 2008 | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
‐ | I: 165 C: 157 total: 322 |
‐ | ‐ | I: 162 C: 155 total: 317 |
I: 98 C: 99 total: 98 |
Intervention groups were subdivided in various age categories |
| Juvenile 2009 | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
‐ | I: 67 C: 62 total: 129 |
‐ | ‐ | I: 67 C: 60 total: 129 |
I: 100 C: 98 total: 100 |
|
| Kordonouri 2010 | I: Continous glucose monitoring C: Conventional glucose monitoring |
357 | I: 80 C: 80 total: 160 |
‐ | ‐ | I: 76 C: 78 total:154 |
I: 95 C:98 total: 96 |
|
| Lagarde 2006 | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
‐ | I: 18 C: 9 total: 27 |
‐ | ‐ | I: 18 C: 9 total: 27 |
I: 100 C: 100 total: 100 |
|
| Logtenberg 2009 | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
20 | I: 6 C: 6 total: 12 |
‐ | ‐ | I: 12 C: 12 total: 24 |
I: 100 C: 100 total: 100 |
Cross‐over design |
| Ludvigsson 2003 | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
‐ | I: 16 C: 16 total: 32 |
1 withdrawn (pregnancy) | ‐ | I: 13 C: 14 total: 27 |
I: 81 C: 88 total: 84 |
Cross‐over design |
| O'Connell 2009 | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
77 | I: 31 C: 31 total: 62 |
‐ | I: 26 C: 29 total: 55 |
I:26 C:29 total:55 |
I: 84 C: 94 total: 89 |
|
| Peyrot 2009 | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
‐ | I: 14 C: 14 total: 28 |
‐ | ‐ | I: 14 C: 13 total: 27 |
I: 100 C: 93 total: 96 |
|
| Raccah 2009 | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
148 | total: 132 | ‐ | I: 55 C: 60 total: 115 |
I: 46 C: 54 total: 109 |
I: 84 C: 90 total: 95 |
|
| Tanenberg 2004 | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
‐ | I: 62 C: 66 total: 128 |
I: 0 C: 1 withdrawn (pregnancy) |
‐ | I: 51 C: 54 total: 105 |
I: 83 C: 82 total: 82 |
|
| Wysocki 2006 | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
‐ | I: 99 C: 101 total: 200 |
‐ | ‐ | I: 97 C: 101 total: 198 |
I: 98 C: 100 total: 99 |
|
| Yates 2006 | I: Continuous glucose monitoring C: Conventional glucose self‐monitoring |
75 | I: 19 C: 17 total: 36 |
‐ | ‐ | I: 19 C: 17 total: 36 |
I: 100 C: 100 total: 100 |
|
| Total | 2883 | 2751 |
"‐" denotes "not reported"
Abbreviations: C: control; CSII: continuous subcutaneuous insulin infusion (insulin pump); I: intervention; ITT: intention‐to‐treat; MDI: multiple daily injections
The following data were extracted:
(1) general information: title, authors, reference/source, year of publication and language of publication, reviewer, date
(2) in‐ and exclusion criteria (confirmation of eligibility, reason for exclusion)
(3) study characteristics:
study design (RCT, parallel or crossover; single or multicenter, country, trial start year, duration of intervention and duration of follow‐up);
patients: number, gender and age distribution, ethnic group distribution, setting, diagnostic criteria for type 1 diabetes mellitus, average duration of disease, baseline HbA1c, body mass index, insulin use (pump or injections), co‐morbidity, co‐medication, treatment before study, percentage pregnant women, percentage children (age less than 18 years), percentage patients with poorly controlled diabetes (HbA1c greater than 8.0%) and percentage patients with hypoglycaemia unawareness;
interventions: type of CGM system, intermittent or continuous use, duration of CGM system use and type of self‐monitoring (times per day);
outcomes: definition, timing and unit of measurement (for scales: upper and lower limits and whether a high or low score is favourable).
(4) results (for each outcome):
dichotomous: number of patients with outcome and total number of patients in the intervention group and in the control group;
continuous: number of patients, mean effect, standard deviation (SD) in the intervention group and in the control group;
number of drop outs in the intervention group and in the control group.
(5) funding source.
Assessment of risk of bias in included studies
Two authors assessed each study independently. Disagreements were resolved by consensus. Risk of bias was assessed using the Cochrane Collaboration’s tool for assessing risk of bias (Higgins 2008). We used the following criteria:
was the allocation sequence adequately generated?
was the allocation adequately concealed?
was knowledge of the allocated interventions adequately prevented during the study (blinding)?
were incomplete outcome data adequately addressed?
were reports of the study free of suggestion of selective outcome reporting?
was inappropriate influence of funding party suspected?
were the reports of the study free of conflicts of interest of the authors?
was the study apparently free of other problems that could put it at risk of bias (baseline imbalance, early stopping)?
Except for blinding and incomplete outcome data, the criteria were addressed per study. For blinding, we assessed the risk of bias for the subjective and objective outcomes separately. The item incomplete outcome data was addressed for short‐term (three months and less) and long‐term (from three months onwards) endpoints.
Measures of treatment effect
Dichotomous outcome data (e.g. severe hypoglycaemia) are expressed as risk ratio (RR) with 95% confidence intervals (CI). In the case of rare events (incidence less than 1%) a Peto odds‐ratio was calculated for each study (Bradburn 2007).
Continuous outcomes are summarized as mean differences with 95% CI and an overall mean difference was calculated in the meta‐analysis. For studies which addressed the same outcome but used different outcome measures, for example different scales measuring quality of life, standardised mean differences (SMD) were used.
Unit of analysis issues
Special issues in the analysis of studies with non‐standard designs, such as cluster‐randomised or cross‐over trials, are described. For cross‐over studies we planned to extract the point estimates of the results and their standard error if these were the result of a correct analysis (analysis of the paired differences). In that case we would have used the generic inverse variance method for combining those study results. If the results in the cross‐over studies were presented as if the trial had been a parallel group trial with standard deviations for each intervention separately, we planned to estimate the standard error of the mean difference using these intervention‐specific standard deviations and impute a correlation coefficient of 0 (Higgins 2008).
Dealing with missing data
Relevant missing data were obtained from authors, if feasible. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat (ITT) and per‐protocol (PP) population was carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated.
Assessment of heterogeneity
A priori the authors evaluated clinical diversity of the included studies. In case of excessive clinical heterogeneity, that is, if the studies were not considered to be sufficiently homogeneous in terms of participants, interventions and outcomes, the results were not pooled in a meta‐analysis.
We assessed statistical heterogeneity by visual inspection of the forest plots, by use of a standard Chi2 test and a significance level of α = 0.10, in view of the low power of such tests. We quantified heterogeneity by the use of the I2 statistic. I2 values of 50% and more indicate a substantial level of heterogeneity (Higgins 2003). When heterogeneity was found, we attempted to determine potential reasons for it by examination of the individual study and subgroup characteristics.
In case of considerable statistical heterogeneity (insufficient overlap of the 95% confidence intervals and an I2 statistic higher than 75%) and availability of at least 10 studies we planned to perform a meta‐regression analysis to identify factors than may explain the heterogeneity. The following study characteristics would have been considered:
country (USA versus Europe versus other countries; because of differences in diabetes care and cultural factors);
baseline HbA1c (disease severity; improvement in HbA1c is not to be expected in people with already low HbA1c values but suffering from high frequencies of hypoglycaemia);
insulin use (pump versus injection; the benefits of CGM may be more readily discerned in those using the most optimal tool for insulin delivery).
Assessment of reporting biases
We planned to use funnel plots to assess the potential existence of small study bias, if there were at least 10 studies available. Possible sources of asymmetry in funnel plots are publication bias, poor methodological quality of smaller studies and true heterogeneity in effect associated with study size (Higgins 2008; Lau 2006; Sterne 2001).
Data synthesis
The following comparisons were included in the analyses:
CGM system versus conventional self‐monitoring;
CGM system versus another type of CGM system.
For each comparison, separate analyses were performed for four different patient groups:
children (0 to 14 years);
adolescents (15 to 23 years);
adults (men and non‐pregnant women) patients;
pregnant women.
We carried out the statistical analysis using the Review Manager software (RevMan 2008). Statistical analysis was performed according to the statistical guidelines referenced in the newest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Studies were grouped per age group (children, adolescents, adults and all ages) and type of device (retrospective or real‐time system).
We performed an exploratory meta‐analysis with all studies (regardless of age group), since all systems provide similar accuracy. We included three types of studies (1a, 1b and 2):
-
studies where real‐time CGM was used continuously for a period of at least six months. We choose to include only studies with a relatively long follow‐up time, because studies with a follow‐up below six months are more prone to over‐report problems with the CGM or other issues such as the occurrence of minor hypoglycaemia during the adjustment period and subsequent learning‐curve the patient follows when provided with a CGM; here we make a distinction between:
studies in insulin‐pump naive patients comparing CGM augmented insulin pump therapy with multiple daily injections of insulin and SMBG;
other studies on real‐time CGM with a follow‐up of at least six months;
studies where CGM was used intermittently (e.g. three days every two weeks).
We choose to include all age‐groups in the meta‐analysis because the CGM devices in children were operated by their adult caregivers who were also the ones who acted on the information the CGM provided. In case of adolescents who operated the CGM system themselves, there was no compelling reason to believe that their decision making process was far inferior to those of young adults. In studies which included patients of all ages and reported outcome measurements per age group, the outcome measures across age‐groups were significantly different in one study (Juvenile 2008) but similar in two studies (Bergenstal 2010; Hirsch 2008). However, it is likely that adults used CGM a greater percentage of time than children or young adults.
Data were combined using a random‐effects model, which assumes that individual studies are estimating a range of treatment effects. The fixed‐effect model is based on the mathematical assumption that a single common effect underlies every study in the meta‐analysis. Although the random‐effects model fits best to our research question, this model is less robust when the number of studies is small. For subgroups with less than five studies we therefore used the fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
The following subgroup analyses were planned:
patients with hypoglycaemia unawareness; in these patients HbA1c is often already relatively low, therefore the number of episodes with severe hypoglycaemia has been used as primary outcome;
patients with poorly controlled diabetes (defined as HbA1c greater than 8.0%).
Sensitivity analysis
We planned to perform sensitivity analyses by repeating the meta‐analyses excluding studies with:
inadequate allocation concealment, inadequate blinding of the outcome assessors, incomplete follow‐up;
suspected reporting bias;
funding by a interested party (e.g. CGM system manufacturer) or possible conflicts of interest of the authors.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The search conducted up to June 8, 2011 for all databases identified 1366 records. From these, 45 full text reports were retrieved for further examination. The other studies were excluded on the basis of their title and/or abstract because they were not relevant to the study question, mainly because the article was not about continuous glucose monitoring (CGM) ‐ 1375 articles)). After screening the full text of the 45 selected studies 22 RCTs, reported in 23 articles, finally met the inclusion criteria. Another 19 studies were excluded for various other reasons (Characteristics of excluded studies) and three studies are awaiting classification (Characteristics of studies awaiting classification). Of these three studies, for one RCT only the methods were published (Conget 2010) and two RCTs were published as a conference abstract with limited data on the results (Lange 2010; Langeland 2010). In total, 22 RCTs were included.
An adapted PRISMA (preferred reporting items for systematic reviews and meta‐analyses) flow‐chart of study selection is attached (Liberati 2009), see Figure 1.
Assessment of inter‐rater agreement
Two authors reviewed the studies, and were in agreement on those to be fully assessed. From these, studies eligible for inclusion in the review were identified. Both authors agreed on the final reports chosen for assessment and on the risk of bias assessment of the studies. The kappa for inter‐rater agreement in the second part of the selection process was 0.86, reflecting excellent agreement.
Included studies
Twenty‐two RCTs meeting the inclusion criteria of this review were identified. Details of all studies are shown in Characteristics of included studies, Table 3 and Table 4.
2. Overview of study characteristics.
|
Characteristic ► Study ID▼ |
Type of CGM | Type of RCT | Duration (months) | Control | HbA1c inclusion criterion |
| Children | |||||
| Chase 2001 | CGMS (Medtronic) | parallel | 3 | SMBG | > 8.0% |
| Ludvigsson 2003 | CGMS (Medtronic) | cross‐over | 2 x 3 | SMBG | ≥ 8.0% |
| Deiss 2006 | CGMS (Medtronic) | cross‐over | 2 x 3 | SMBG | ‐ |
| Lagarde 2006 | CGMS (Medtronic) | parallel | 6 | SMBG | ‐ |
| Yates 2006 | CGMS (Medtronic) | parallel | 3 | SMBG | ≤ 10.0% |
| Juvenile 2008 Juveline 2009 |
Different types | parallel | 6 | SMBG | 7.0% ‐ 10.0% |
| Bergenstal 2010 | Paradigm (Medtronic) | parallel | 12 | MDI + SMBG | 7.4% ‐ 9.5% |
| Kornodouri 2010 | Paradigm (Medtronic) | parallel | 12 | pump + SMBG | onset diabetes |
| Adolescents | |||||
| Juvenile 2008 | Different types | parallel | 6 | SMBG | 7.0% ‐ 10.0% |
| Hirsch 2008 | Paradigm (Medtronic) | parallel | 6 | pump + SMBG | ≥ 7.5% |
| Adults | |||||
| Chico 2003 | CGMS (Medtronic) | parallel | 3 | SMBG | inadequate control |
| Tanenberg 2004 | CGMS (Medtronic) | parallel | 3 | SMBG | > 7.9% |
| Cooke 2009 | CGMS (Medtronic) | parallel | 18 | SMBG | > 7.5% |
| Hermanns 2009 | GlucoDay (Menarini) | cross‐over | 2 periods | retrospective CGM | ‐ |
| Cosson 2009 | GlucoDay (Menarini) | parallel | 3 | blinded CGM | 8.0% ‐ 10.5% |
| Peyrot 2009 | Paradigm (Medtronic) | parallel | 4 | pump + SMBG | suboptimal glucose control |
| Logtenberg 2009 | Paradigm (Medtronic) | cross‐over | 2 x 6 days | blinded retrospective CGM | > 7.5% and/or ≥ 5 hypoglycaemic episodes per week |
| Juvenile 2008 | Different types | parallel | 6 | SMBG | 7.0% ‐ 10.0% |
| Hirsch 2008 | Paradigm (Medtronic) | parallel | 6 | pump + SMBG | ≥ 7.5% |
| Bergenstal 2010 | Paradigm (Metronic) | parallel | 12 | MDI + SMBG | 7.4% ‐ 9.5% |
| Hermanides 2009 | Paradigm (Medtronic) | parallel | 6 | pump + SMBG | > 8.2% |
| All ages | |||||
| Battelino 2011 | Freestyle Navigator | parallel | 6 | SMBG | < 7.5% |
| Deiss 2006a | Guardian (Medtronic) | parallel | 3 | SMBG | > 8.1% |
| Juvenile 2009 | Different types | parallel | 6 | SMBG | < 7.0% |
| Hirsch 2008 | Paradigm (Metronic) | parallel | 6 | pump + SMBG | ≥ 7.5% |
| Raccah 2009 | Paradigm (Metronic) | parallel | 6 | pump + SMBG | ≥ 8.0% |
| O'Connell 2009 | Paradigm (Medtronic) | parallel | 3 | pump + SMBG | ≤ 8.5% |
Abbreviations: CGM: continuous glucose monitoring; HbA1c: glycosylated haemoglobin A1c; MDI: multiple daily injections; SMBG: self‐monitoring blood glucose
The first RCT was published in 2001, followed by three RCTs between 2003 and 2005, six RCTs between 2006 and 2008 and 12 RCTs between 2009 and 2011. The studies are discussed according to the age group of the patients (children, adolescents, adults and all ages) and the type of CGM (retrospective, non‐invasive or real‐time). There were no RCTs addressing pregnant women with diabetes.
Two RCTs were part of a larger cohort of patients (Juvenile 2008; Juvenile 2009). One RCT included patients with HbA1c between 7.0% and 10% (Juvenile 2008) and the other RCT included well‐controlled patients (Juvenile 2009) (HbA1c less than 7.0%). The first RCT consisted in fact of three sub‐RCTs as the authors analysed all results stratified according to age groups: children, adolescents and adults (Juvenile 2008).
In all but one RCT the CGM system was used in an outpatient setting (Hermanns 2009). Most studies (19 out of 22) investigated one type of CGM system. In the Juvenile Diabetes Research Foundation (JDRF) trials three different devices were used, including the real‐time CGMS with insulin pump. The device was assigned on the basis of device features and patients preferences (Juvenile 2008; Juvenile 2009). Cooke et al studied two devices, but we excluded the results of the GlucoWatch system (Cooke 2009).
All studies compared CGM with self‐monitoring of blood glucose (SMBG) or blinded CGM. There were no head‐to‐head comparisons.
The majority of the RCTs used baseline glycosylated haemoglobin A1c (HbA1c) levels as an inclusion criterion. Seven studies were conducted in patients with HbA1c‐defined poorly controlled diabetes (our definition was HbA1c greater than 8.0%, but we accepted greater than 7.9%) (Chase 2001; Cosson 2009; Deiss 2006a; Hermanides 2011; Ludvigsson 2003; Raccah 2009; Tanenberg 2004). Three RCTs focused on well‐controlled patients (i.e., according to the author's definition: less than 7.5%, 7.0% or 8.5%) (Battelino 2011; Juvenile 2009; O'Connell 2009). Six RCTs used an HbA1c range which could include both patients with well‐controlled diabetes and patients with poorly controlled diabetes (greater than 7.0% or 7.5% or less than 10.0%), that is, according to our definition (Bergenstal 2010; Cooke 2009; Hirsch 2008; Juvenile 2008; Logtenberg 2009; Yates 2006). Six RCTs did not use HbA1c as an inclusion criterion; three of these RCTs specified their patients qualitatively ('onset of diabetes', 'sub‐optimal glucose control' and 'inadequate metabolic control') (Chico 2003; Kordonouri 2010; Peyrot 2009).
Children
Ten studies were performed in children. Five RCTs investigated the effects of a retrospective CGMS (Chase 2001; Deiss 2006; Lagarde 2006; Ludvigsson 2003; Yates 2006), one RCT investigated three different types of real‐time systems (Juvenile 2008) and two RCTs investigated the use of CGM augmented insulin pump therapy (Bergenstal 2010; Kordonouri 2010).
Retrospective CGM systems
Five RCTs studied the effects of the retrospective Minimed CGMS (Chase 2001; Deiss 2006; Lagarde 2006; Ludvigsson 2003; Yates 2006). Two studies were cross‐over studies (Deiss 2006; Ludvigsson 2003). The duration of the parallel group trials were three months (Chase 2001), six months (Lagarde 2006) and three months intervention followed by a three month follow‐up period (Yates 2006). The duration of the cross‐over trials was two times three months. In all studies the CGM sensors were used for three days at several time points or intervals. The control group used SMBG alone or blinded CGM with SMBG.
Two studies included only children with poorly controlled diabetes (HbA1c greater than 8.0%) (Chase 2001; Ludvigsson 2003) and one study included children with HbA1c lower than 10% (Yates 2006). The two other studies did not use HbA1c as an eligibility criterion (Deiss 2006; Lagarde 2006). The children in the studies were recruited in paediatric diabetes clinics. The children were treated with insulin pump or multiple daily injections (MDI) of insulin, except for one RCT (Deiss 2006), these children used solely multiple daily injections of insulin.
The sample sizes of the studies were small, ranging from 11 to 36 children.
Real‐time CGM systems
Three RCTs investigated the effects of real‐time CGM systems in children (Bergenstal 2010; Juvenile 2008; Kordonouri 2010). One trial included patients younger than 18 years of age, but reported the results for children/adolescents and adults separately (Bergenstal 2010).
In the JDRF trial the CGM group used three different types of CGM systems: a Dexcom SEVEN, Paradigm or Freestyle Navigator device (Juvenile 2008). The Paradigm system combines an insulin pump with a CGM system. In this trial 114 children (age 8 to 14 years) were included. The duration of the trial was six months and results were also reported for three months after baseline. The baseline HbA1c level was between 7% and 10%.
In the two other studies the Paradigm system was used. One RCT randomised 160 children who were recently diagnosed with type 1 diabetes to insulin pump treatment with CGM or insulin pump treatment with SMBG (Kordonouri 2010). In the other RCT sensor‐augmented pump therapy was compared with a regimen of multiple daily injections of insulin, in 156 children with inadequately controlled type 1 diabetes (HbA1c level between 7.4% and 9.5%) (Bergenstal 2010). Duration of both studies was 12 months.
Adolescents
Two RCTs reported results for adolescents (Juvenile 2008; Hirsch 2008), albeit in one study separate results are reported only for HbA1c (Hirsch 2008).
In one RCT the CGM group used three different CGM devices: a Dexcom SEVEN, Paradigm or Freestyle Navigator device (all real‐time systems) (Juvenile 2008). The Paradigm system combines an insulin pump with a CGM system. The study included 110 adolescents (15 to 24 years of age) and the duration was six months. All patients had a baseline HbA1c levels between 7% and 10%.
In the other study, the use of the Paradigm system was compared with insulin pump use and SMBG (Hirsch 2008). The age of the patients was between 12 and 18 years and their initial HbA1c values were above 7.5%. All were previously treated with an insulin pump for at least six months. The duration of the study was six months.
Adults
For adults, 11 studies were available. Two studies reported on retrospective CGMS (Chico 2003; Tanenberg 2004), two on the real‐time Glucoday CGMS (Cosson 2009; Hermanns 2009), one on retrospective CGMS (Cooke 2009), five on a combined real‐time CGMS and insulin pump device (Paradigm) (Bergenstal 2010; Hermanides 2011; Hirsch 2008; Logtenberg 2009; Peyrot 2009) and one on three different types of real‐time systems (Juvenile 2008).
Retrospective CGM systems
Two RCTs studied the effects of retrospective Minimed CGMS on glycaemic control in adults (Chico 2003; Tanenberg 2004). Both were parallel group trials with a duration of three months in patients with inadequate metabolic control. In one RCT (n = 75) the CGM device was used for one period of three days (Chico 2003), in the other RCT (n = 128) two periods of three days (Tanenberg 2004). The control groups used SMBG. In one study the patients were treated with continuous subcutaneous insulin infusion (insulin pump) or multiple daily injections of insulin (Tanenberg 2004), in the other study there were no pump users at baseline (Chico 2003).
One study had four study arms: retrospective CGM system, GlucoWatch Biographer, standard care and attention control. This study included both type 1 and type 2 diabetic patients and had a duration of 18 months (Cooke 2009). All patients had a HbA1c level of at least 7.5%; the majority of them were on insulin treatment by multiple daily injections. The results of the RCT are not presented separately for type 1 and type 2 diabetes, but the authors have provided us with the results for type 1 diabetes.
Real‐time CGM systems
In adults the effects of real‐time CGM systems were investigated in nine studies (Bergenstal 2010; Cooke 2009; Cosson 2009; Hermanides 2011; Hermanns 2009; Hirsch 2008; Juvenile 2008; Logtenberg 2009; Peyrot 2009). Two of these studies included patients younger than 18 years of age, but reported the results for children/adolescents and adults separately (Bergenstal 2010; Hirsch 2008). Two studies were cross‐over trials in patients with poorly controlled diabetes (Hermanns 2009; Logtenberg 2009).
The first cross‐over trial (n = 12, Paradigm system) included patients from an outpatient clinic who used continuous intraperitoneal insulin infusion (CIPII) (Logtenberg 2009). CIPII (an implanted insulin pump) is mainly used (very rarely) in patients who, despite intensive subcutaneous insulin therapy, do not reach acceptable glycaemic control, or have frequent hypoglycaemic episodes (especially when accompanied by hypoglycaemia unawareness) or have subcutaneous insulin resistance. At the moment CIPII is only available in a few European countries, mostly in France, Sweden, and The Netherlands.The other cross‐over trial (n = 50, GlucoDay system) had an inpatient setting and included both insulin pump users and patients who used multiple daily injections of insulin (Hermanns 2009). Both studies compared open versus blinded use of real‐time CGM. Treatment decisions in the open phase were made based on real‐time CGM values and in the blinded phase on SMBG (Logtenberg 2009) or on retrospective access of the CGM data (Hermanns 2009). Patients used CGM for a short period (six days and approximately two days) and switched over to the other CGM option (open or blinded). HbA1c was not an outcome measure in both studies.
The other eight studies were parallel group RCTs, with durations of 3, 4, 6, 12 and 18 months. Two studies were in patients with poorly controlled diabetes (HbA1c between 8.0% to 10.5%, Cosson 2009 and HbA1c equal to or greater than 8.2%, Hermanides 2011). The other studies were in patients with ‘suboptimal metabolic control’ (Peyrot 2009), HbA1c greater than 7.0% (Juvenile 2008), HbA1c between 7.4% to 9.5% (Bergenstal 2010) and HbA1c greater than 7.5% (Battelino 2011; Cooke 2009; Hirsch 2008).
In one study (n = 9) the Glucoday system was used (Cosson 2009) and in four studies (n = 28, n = 329, n = 83 and n = 98) the Paradigm device. In the first two studies patients had never used an insulin pump (pump naive) (Bergenstal 2010; Peyrot 2009), in the third study patients had not used an insulin pump in the six months before inclusion (Hermanides 2011), and in the last study patients had used an insulin pump at least six months before intake (Hirsch 2008). In one RCT (n = 98, JDRF adults) the CGM group used three different CGM devices: a Dexcom SEVEN, Paradigm or Freestyle Navigator device (Juvenile 2008).
In one RCT all patients used a CGM device for two days, in addition to SMBG. In the intervention group diabetes treatment was managed using the CGM data, in the control group treatment was adjusted using SMBG values (Cosson 2009). In the other RCTs patients used the CGM devices continuously (Bergenstal 2010; Hermanides 2011; Hirsch 2008; Juvenile 2008; Peyrot 2009). In three of these RCTs the use of insulin pump therapy combined with CGM was compared with multiple daily injections and SMBG, in insulin pump therapy naive patients (Bergenstal 2010; Hermanides 2011; Peyrot 2009).
All ages
Six studies had a broad age range and included both children and adults (Battelino 2011; Deiss 2006a; Hirsch 2008; Juvenile 2009; O'Connell 2009; Raccah 2009). These studies investigated real‐time CGMS and real‐time CGMS combined with an insulin pump. There were no subgroup analyses for specific age groups, except for one study on HbA1c results (adolescents and adults, Hirsch 2008).
Three studies studied the Paradigm system (Hirsch 2008; Raccah 2009; O'Connell 2009). In one RCT (n = 62) the patients had an HbA1c level below 8.5% and used an insulin pump for at least three months before randomisation (O'Connell 2009). According to the authors’ definition these patients were considered to have well‐controlled diabetes. The study had a duration of three months and included adolescents and adults, but no children. In the second Paradigm system trial (n = 138), duration six months, the patients had an HbA1c level of at least 7.5% and had used an insulin previously in the six months preceding the study (Hirsch 2008). In the third Paradigm system trial (n = 132), the patients were insulin pump naive and their diabetes was poorly controlled (HbA1c greater than 8.0%) (Raccah 2009). The duration was six months. In this study, the Paradigm system (insulin pump combined with a CGM system) was compared to treatment with an insulin pump and SMBG, in patients naive to both treatment forms.
One RCT (n = 162) investigated the Guardian real‐time CGM system (Deiss 2006a). Patients in this study had poorly controlled diabetes (HbA1c greater than 8.1%), despite intensified insulin treatment (pump or multiple daily injections). The study had three arms: continuous and intermittent use (i.e., bi‐weekly for 3‐day periods) was compared with SMBG. The duration was three months.
One RCT (n = 120) studied the Freestyle Navigator (Battelino 2011). Patients had reasonable metabolic control (HbA1c less than 7.5%). The duration of the trial was six months.
In the last RCT (n = 129, Juvenile 2009) the participants had well‐controlled diabetes (HbA1c less than 7.0%) and the CGM group used three different real‐time CGM systems (including the Paradigm system). The duration of this study was six months.
Excluded studies
Studies were excluded because there was no control group, the device used was not CGM or the study design was not an RCT, see Characteristics of excluded studies.
Risk of bias in included studies
Figure 2 and Figure 3 present a summary of the results for the risk of bias assessment.
2.
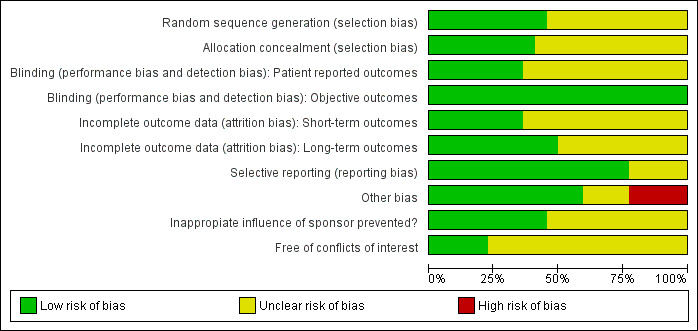
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
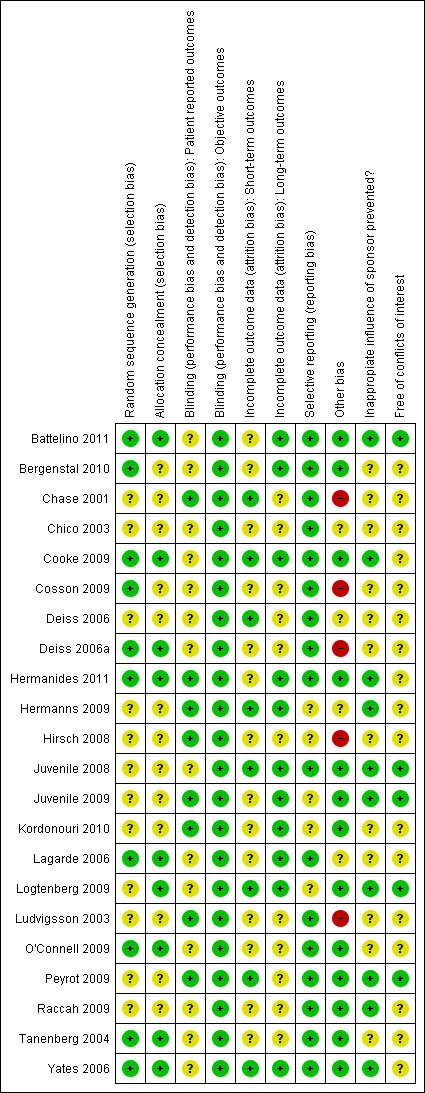
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Of the 22 RCTs, there was insufficient information for 12 RCTs (55%) to judge whether the randomisation sequence generation was adequate; for 13 RCTs (59%) there was insufficient information whether allocation concealment was adequate. For the studies with sufficient information, sequence generation and concealment of allocation was adequate.
Blinding
In the comparison of CGM versus SMBG blinding is not possible. For the glycaemic control outcomes (HbA1c, severe hypoglycaemia, ketoacidosis), lack of blinding is not likely to introduce risk of bias because these outcomes can be measured objectively. However, if use of a new technology is associated with greater expectations by the patient, lack of blinding could introduce risk of bias with regard to subjective outcomes like health‐related quality of life and patient satisfaction. In that case, patients wearing the CGM sensor might report higher quality of life and patient satisfaction scores, compared to patients using SMBG. The differences between the groups are then ‐ at least partly ‐ attributable to wearing the device and not because the improvement in glycaemic control.
Incomplete outcome data
Overall, dropout rates and the risk of selective dropout were relatively low. The ‘unclear’ judgements include no information on withdrawals, discrepancy between text and table on dropouts, dropouts not reported for each study and not mentioning the reasons for dropouts. For four studies there could be a risk of selective dropout as the reasons for dropout were related to the CGM device (Cosson 2009; Hirsch 2008; O'Connell 2009; Tanenberg 2004).
Selective reporting
Most studies were free of selective reporting. In two studies the subgroup analyses were not pre‐specified (Chico 2003; Hirsch 2008), and in another study the variables measuring glycaemic control were not predefined (Hermanns 2009).
Other potential sources of bias
Other sources of bias were imbalance in baseline characteristics (Cosson 2009; Deiss 2006; Lagarde 2006; Ludvigsson 2003), no intention‐to‐treat analysis (Hirsch 2008), possible carry‐over effect (cross‐over design; Deiss 2006), and assessment of the number of hypoglycaemic episodes by SMBG and CGM (CGM has more time points and will consequently end up with a higher number of episodes; Chase 2001). Two RCTs were poorly reported (Chico 2003; Hermanns 2009).
Funding issues and conflicts of interests
All studies were sponsored by the CGM manufacturer, either by a research grant or by providing the CGM devices. In one study the source of funding was not mentioned (Chico 2003). Seven studies had a statement of independency or stated that the research grant was ‘unrestricted’ and three studies used different types of CGM systems. In such cases, we considered that inappropriate influence of funding was prevented (Battelino 2011; Cooke 2009; Hermanides 2011; Hermanns 2009; Juvenile 2008; Juvenile 2009; Logtenberg 2009; Ludvigsson 2003; Peyrot 2009; Raccah 2009; Yates 2006). In all other cases we considered inappropriate influence of funding 'unclear'. One study reported that all data were transferred to the sponsor who provided editorial assistance (Bergenstal 2010).
In nine RCTs there was no declaration of conflicts of interest. When conflicts of interest were reported this meant that one or more authors were employed by the CGM manufacturer or had received consulting, travel or speaking fees from the CGM manufacturer. The influence of these employments and fees on the study results is unclear.
Effects of interventions
The results are presented in four sections: children, adolescents, adults and all age groups.
Children
Eight RCTs were performed in children (Bergenstal 2010; Chase 2001; Deiss 2006; Lagarde 2006; Ludvigsson 2003; Yates 2006; Juvenile 2008; Bergenstal 2010; Kordonouri 2010).
Retrospective CGM systems
Glycaemic control
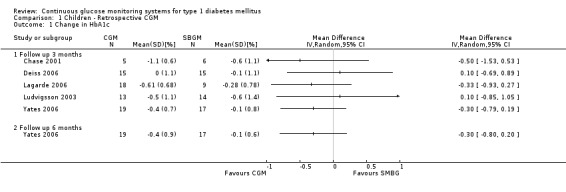
In four out of the five RCTs the HbA1c levels decreased in both the CGM and SMBG group during follow‐up (Chase 2001; Lagarde 2006; Ludvigsson 2003; Yates 2006). In one RCT (Deiss 2006), the HbA1c level did not change in the CGM group but decreased in the SMBG group. The mean difference (MD) between CGM group and SMBG group in change in HbA1c ranged from ‐0.5% to 0.1% (Analysis 1.1). Because of the small sample sizes, the confidence intervals were wide. The mean difference in change in HbA1c level was not statistically significant in any of the five RCTs.
1.1. Analysis.
Comparison 1 Children ‐ Retrospective CGM, Outcome 1 Change in HbA1c.
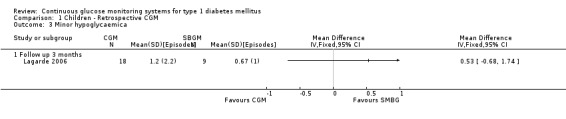
Severe hypoglycaemia (Analysis 1.2) was measured in four studies. The occurrence of events was very low: for two children, one in the CGM and one in the SMBG group a severe hypoglycaemic event was reported in one RCT (Ludvigsson 2003). Minor hypoglycaemia (Analysis 1.3) was measured in one study (Lagarde 2006). Compared to the SMBG group the CGM group had a slightly higher, but non‐significant, number of episodes in three months follow‐up (1.2 versus 0.7, MD 0.5, 95% CI ‐0.7 to 1.7). Ketoacidosis (Analysis 1.5) was measured in one study (Yates 2006), again the number of events was very small (one in the CGM group and null in the SMBG group).
1.2. Analysis.
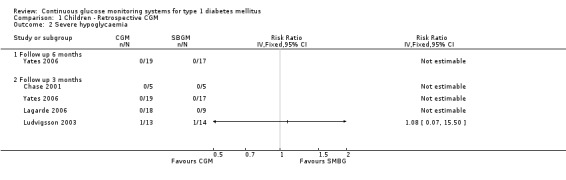
Comparison 1 Children ‐ Retrospective CGM, Outcome 2 Severe hypoglycaemia.
1.3. Analysis.
Comparison 1 Children ‐ Retrospective CGM, Outcome 3 Minor hypoglycaemica.
1.5. Analysis.
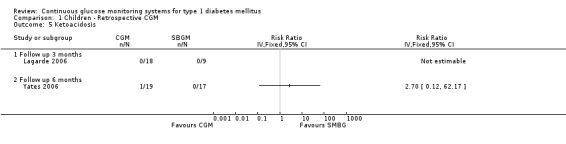
Comparison 1 Children ‐ Retrospective CGM, Outcome 5 Ketoacidosis.
Quality of life
One RCT measured quality of life with the DCCT quality of life questionnaire. The DCCT quality of life questionnaire is based on a 5‐point Likert scale (1 = disease has no impact, 5 = highly impacted by the disease). The authors found no significant differences between CGM and SMBG (data not reported) (Chase 2001).
CGM‐derived hypoglycaemia and hyperglycaemia
CGM derived glycaemic control (Analysis 1.4, Analysis 1.6, Analysis 1.7) was measured in one RCT (Lagarde 2006). None of the outcomes showed significant differences between the study arms.
1.4. Analysis.
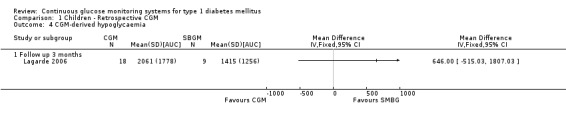
Comparison 1 Children ‐ Retrospective CGM, Outcome 4 CGM‐derived hypoglycaemia.
1.6. Analysis.
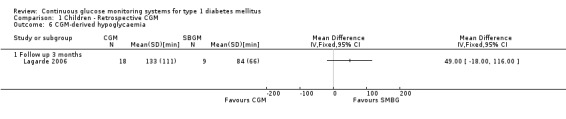
Comparison 1 Children ‐ Retrospective CGM, Outcome 6 CGM‐derived hypoglycaemia.
1.7. Analysis.
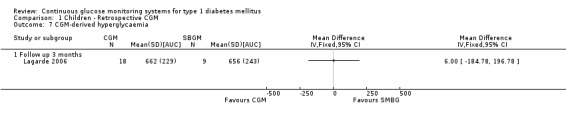
Comparison 1 Children ‐ Retrospective CGM, Outcome 7 CGM‐derived hyperglycaemia.
Real‐time CGM systems
Glycaemic control
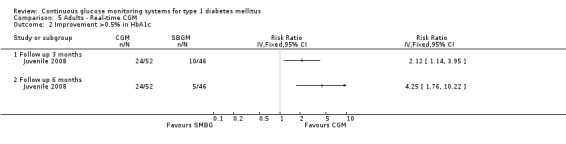
In all three studies the HbA1c levels in both the CGM and SMBG group declined during the study period (Bergenstal 2010; Juvenile 2008; Kordonouri 2010). Three months after baseline the difference in change was statistically significant (change in HbA1c ‐0.5% versus ‐0.2%, MD in change ‐0.2%, 95% CI ‐0.3% to 0.0%, one RCT) (Juvenile 2008). At six months and 12 months follow‐up, however, the difference in change in HbA1c level decreased and was no longer significant (Analysis 2.1). The proportion patients who improved their HbA1c level with at least 0.5% was significantly larger in the CGM group at three months (46% versus 28%, RR 1.68, 95% CI 1.02 to 2.78, one RCT) and at six months after baseline (54% versus 31%, RR 1.73, 95% CI 1.10 to 2.72, one RCT) (Analysis 2.2). One study reported that patients with regular sensor use had lower HbA1c levels, compared to those who had no or low sensor usage (Kordonouri 2010); another study reported that lower HbA1c levels were only seen in those patients who used the sensor more than 60% of the time (Bergenstal 2010).
2.1. Analysis.
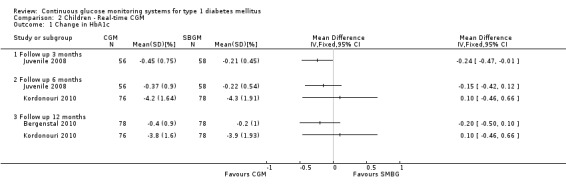
Comparison 2 Children ‐ Real‐time CGM, Outcome 1 Change in HbA1c.
2.2. Analysis.
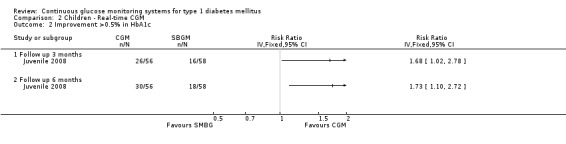
Comparison 2 Children ‐ Real‐time CGM, Outcome 2 Improvement >0.5% in HbA1c.
The occurrence of severe hypoglycaemia after six months of follow‐up was somewhat lower in the CGM study arm, but the difference was not statistically significant (7% [5 events] versus 12% [7 events], RR 0.74, 95% CI 0.25 to 2.19, one RCT) (Analysis 2.3). Two studies measured the occurrence of severe hypoglycaemia at 12 months follow‐up, with inconsistent results and wide confidence intervals (Analysis 2.3). Ketoacidosis events did not occur at six months follow‐up and rarely (three events) after 12 months follow‐up (Analysis 2.4).
2.3. Analysis.
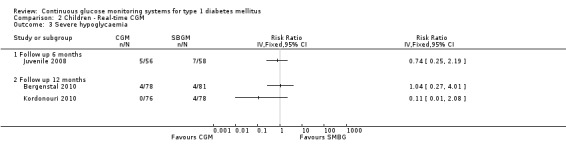
Comparison 2 Children ‐ Real‐time CGM, Outcome 3 Severe hypoglycaemia.
2.4. Analysis.
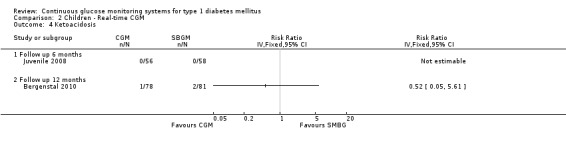
Comparison 2 Children ‐ Real‐time CGM, Outcome 4 Ketoacidosis.
Quality of life
Two studies examined the quality of life of its participants (Juvenile 2009; Kordonouri 2010). In the first study the PedsQL questionnaire (diabetes‐ specific) was used. The scale was 0 to 100, with higher scores denoting higher quality of life (Juvenile 2009). In the other study the WHO‐5 questionnaire was used; in this scale a higher score also indicates a more favourable quality of life. For both studies the differences were small (SMD at six months less than 0.08) and not statistically significant (Analysis 2.5).
2.5. Analysis.
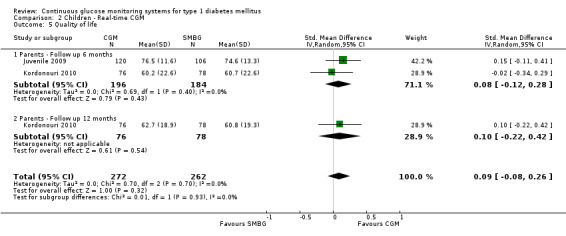
Comparison 2 Children ‐ Real‐time CGM, Outcome 5 Quality of life.
CGM‐derived hypoglycaemia and hyperglycaemia
CGM derived glycaemic control was measured in one RCT (Bergenstal 2010). None of the outcomes showed significant differences between the study arms (Analysis 2.6 and Analysis 2.7).
2.6. Analysis.
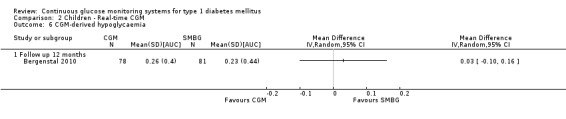
Comparison 2 Children ‐ Real‐time CGM, Outcome 6 CGM‐derived hypoglycaemia.
2.7. Analysis.
Comparison 2 Children ‐ Real‐time CGM, Outcome 7 CGM‐derived hyperglycaemia.
The outcomes patient satisfaction, diabetes complications, death and costs were not measured in any of the studies in children.
Adolescents
Real‐time CGM systems
Glycaemic control
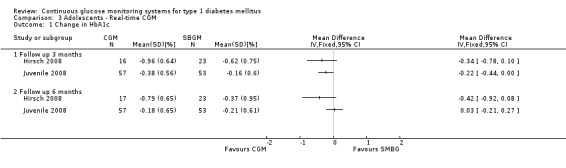
There were two studies among adolescents, both studies used real‐time CGM systems (Hirsch 2008; Juvenile 2008). In both studies the HbA1c levels in the CGM and SMBG group declined during the study period. Three months after baseline the difference in change was ‐0.3% (95% CI ‐0.8% to 0.1%) (Hirsch 2008) and ‐0.2% (95% CI ‐0.4% to 0.0%) (Juvenile 2008), respectively. The differences were not statistically significant. At six months follow‐up, the difference in change in HbA1c level decreased (Analysis 3.1). The proportion of patients that had improved their HbA1c level with at least 0.5% was equal in both groups (36% versus 37%, one RCT) (Analysis 3.2) (Juvenile 2008).
3.1. Analysis.
Comparison 3 Adolescents ‐ Real‐time CGM, Outcome 1 Change in HbA1c.
3.2. Analysis.
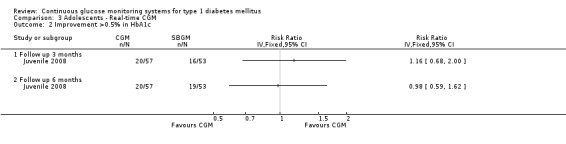
Comparison 3 Adolescents ‐ Real‐time CGM, Outcome 2 Improvement >0.5% in HbA1c.
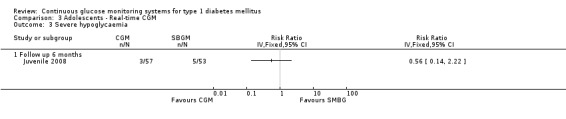
Severe hypoglycaemic and ketoacidotic events were infrequent; there were no significant differences between the groups (severe hypoglycaemia: 5% [3 events] versus 9% [5 events], RR 0.56, 95% CI 0.14 to 2.22, one RCT) (Analysis 3.3, Analysis 3.4) (Juvenile 2008).
3.3. Analysis.
Comparison 3 Adolescents ‐ Real‐time CGM, Outcome 3 Severe hypoglycaemia.
3.4. Analysis.
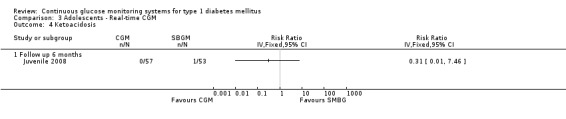
Comparison 3 Adolescents ‐ Real‐time CGM, Outcome 4 Ketoacidosis.
The outcomes quality of life, patient satisfaction, diabetes complications, CGM‐derived glucose control, death and costs were not measured in any of the studies in adolescents.
Adults
Eleven studies with adults were available (Bergenstal 2010; Chico 2003; Cooke 2009; Cosson 2009; Hermanides 2011; Hermanns 2009; Hirsch 2008; Juvenile 2008; Logtenberg 2009; Peyrot 2009; Tanenberg 2004).
Retrospective CGM systems
Glycaemic control
Change in HbA1c level was measured in two RCTs addressing retrospective CGM (Chico 2003; Tanenberg 2004). There was no difference in change between the study arms in one study (‐0.7% change in HbA1c in both the CGM and SMBG group) (Tanenberg 2004) and a non‐significant difference in the other study (MD in change in HbA1c level ‐0.3%, 95% CI ‐0.9% to 0.3%) (Chico 2003) (Analysis 4.1).
4.1. Analysis.
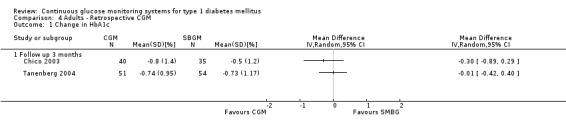
Comparison 4 Adults ‐ Retrospective CGM, Outcome 1 Change in HbA1c.
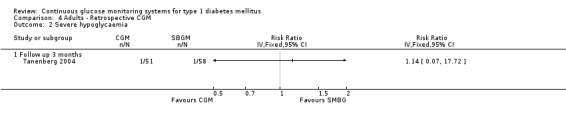
Severe hypoglycaemia was reported in one study, in both the CGM and the SMBG group 2% of the patients (one event in each group) had an episode of hypoglycaemia (Tanenberg 2004) (Analysis 4.2).
4.2. Analysis.
Comparison 4 Adults ‐ Retrospective CGM, Outcome 2 Severe hypoglycaemia.
CGM‐derived hypoglycaemia and hyperglycaemia
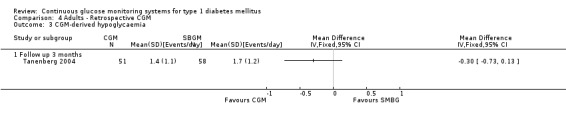
In one RCT the occurrence and duration of CGM‐derived hypoglycaemic and hyperglycaemic events were registered for a three day period at the end of the study (three months) (Tanenberg 2004). The number of events per day did not differ between the study arms, but the mean duration of the hypoglycaemic events was 32 minutes per event shorter in the CGM group (49 versus 80 minutes per event, MD ‐32, 95% CI ‐51 to ‐12, one RCT). The mean duration of the hyperglycaemic periods was 37 minutes longer in the CGM group, but the difference was not statistically significant (209 versus 172 minutes per event, MD 37, 95% CI ‐7 to 81) (Analysis 4.3, Analysis 4.4).
4.3. Analysis.
Comparison 4 Adults ‐ Retrospective CGM, Outcome 3 CGM‐derived hypoglycaemia.
4.4. Analysis.
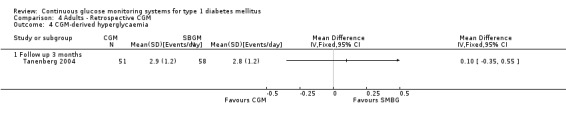
Comparison 4 Adults ‐ Retrospective CGM, Outcome 4 CGM‐derived hyperglycaemia.
Real‐time CGM systems
Glycaemic control
Three months after baseline the change in decrease in HbA1c varied between ‐0.1% and 1.1% (five studies). In three studies the difference was statistically significant. The same pattern was seen six months after baseline: the change in decrease in HbA1c varied between ‐0.1% and 1.1% and the difference was statistically significant in two of the three studies. After 12 months, the change in HbA1c was larger for the CGM group compared to the SMBG group (‐1.0% versus ‐0.4%, MD in change in HbA1c ‐0.6%, 95% CI ‐0.5% to ‐0.4%, one RCT) (Analysis 5.1). In the study of Hirsch et al a sensor usage of more than 60% was associated with HbA1c reduction (P = 0.046) (Hirsch 2008). In the CGM group a larger proportion of patients improved their HbA1c with at least 0.5% (46% versus 11%, RR 4.25%, 95% CI 1.76 to 10.22, one RCT) (Analysis 5.2).
5.1. Analysis.
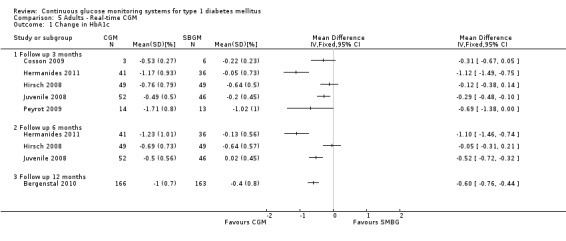
Comparison 5 Adults ‐ Real‐time CGM, Outcome 1 Change in HbA1c.
5.2. Analysis.
Comparison 5 Adults ‐ Real‐time CGM, Outcome 2 Improvement >0.5% in HbA1c.
One study measured HbA1c levels after 18 months follow‐up (Cooke 2009). This study included diabetes type 1 patients as well as diabetes type 2 patients. The authors provided us with additional data for diabetes type 1 patients. The HbA1c levels after 18 months showed a relative percentual decline of ‐2.0 (0.9)% in the CGM‐Glucowatch group and ‐4.1% (1.1)% in the CGMS group at 18 months versus ‐4.6 (1.2)% in the attention control group and ‐5.5 (1.2)% in the standard control group. The overall difference between groups was insignificant (P = 0.458).
Four studies measured the occurrence of severe hypoglycaemia, at three months (Peyrot 2009), six months (Hermanides 2011; Juvenile 2008) and 12 months (Bergenstal 2010). At three months, the number of events was very low (three events). At six and 12 months, the risk of severe hypoglycaemia was increased for CGM users, but the difference was not statistically significant and the confidence intervals were wide (Analysis 5.3). The number of ketoacidosis events was very small (six events in total for all follow‐up periods) (Analysis 5.4).
5.3. Analysis.
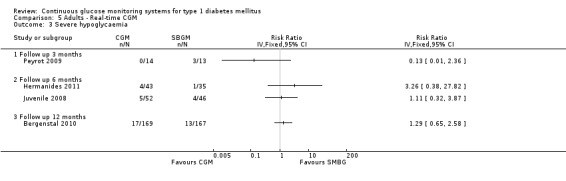
Comparison 5 Adults ‐ Real‐time CGM, Outcome 3 Severe hypoglycaemia.
5.4. Analysis.
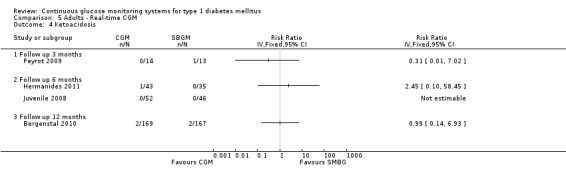
Comparison 5 Adults ‐ Real‐time CGM, Outcome 4 Ketoacidosis.
Quality of life
Two studies measured quality of life after six months (Juvenile 2008; Hermanides 2011). Both studies used the generic SF‐instruments, the SF‐12 (Juvenile 2008) and the SF‐36 Short Form version 2 (Hermanides 2011). The scale of both questionnaires ranges from 0 to 100, with higher scores denoting higher quality of life. The differences between the CGM and SMBG group were small and not statistically significant (Analysis 5.5).
5.5. Analysis.
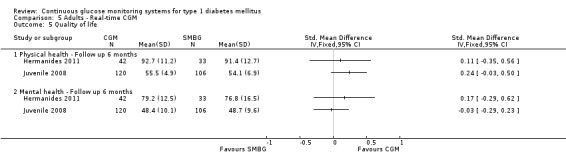
Comparison 5 Adults ‐ Real‐time CGM, Outcome 5 Quality of life.
Patient satisfaction
Two studies on the Paradigm system investigated patient satisfaction, one after three months (Peyrot 2009) and one after six months follow‐up (Hermanides 2011). Both RCTs compared the Paradigm system with insulin administrated by multiple daily injections and SMBG. The studies used different measurement scales for patient satisfaction. Peyrot et al. used the Insulin Delivery System Rating Scale (IDSRS) to measure satisfaction with the Paradigm device and the Blood Glucose Delivery System Rating Scale (BGDSRS) to measure satisfaction with SMBG. The range for both scales was 0 to 100 (Peyrot 2009). Hermanides et al. used the Diabetes Treatment Satisfaction Questionnaire (DTSQ) (Hermanides 2011). The DTSQ comprises six items and is scored on a 0 to 36 scale. Higher scores indicate higher satisfaction in both studies. To compare the results of the different scales, we calculated the SMD. Patients in the CGM group scored significantly higher on overall satisfaction, measured by the IDSRS and BGDSRS (mean score 74 versus 41, SMD 1.10, 95% CI 0.29 to 1.92) (Peyrot 2009) and the DTSQ (mean score 32 versus 24, SMD 1.73, 95% CI 1.20 to 2.26) (Analysis 5.6). As patients in both studies did not use an insulin pump before the start of the study, this difference could be attributed to CGM or the insulin pump or the combination of both.
5.6. Analysis.
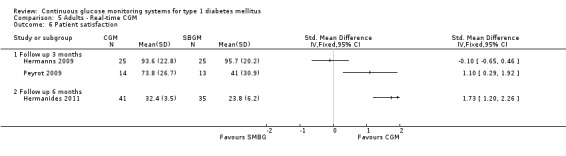
Comparison 5 Adults ‐ Real‐time CGM, Outcome 6 Patient satisfaction.
One study measured patient satisfaction for retrospective versus real‐time access to CGM. After using the CGM device the patient reported benefits were reduced (mean score on the CGM satisfaction questionnaire baseline: 101; after retrospective CGM access: 96; after real‐time CGM access: 94). However, the difference between retrospective and real‐time access was not significant (SMD ‐0.10, 95% CI ‐0.65 to 0.46) (Hermanns 2009).
CGM‐derived hypoglycaemia and hyperglycaemia
In the short duration cross‐over trials (Hermanns 2009; Logtenberg 2009) CGM‐derived hypoglycaemia was measured. In the GlucoDay trial, the time spent in hypoglycaemia while having real‐time access to the CGM values was almost one hour per day longer compared to having retrospective access to the CGM values, the difference showed a tendency towards significance (mean hours per day: 3.3 versus 2.4, MD 0.9, P = 0.08) (Hermanns 2009). In the Paradigm trial there was no difference at all (P = 0.61 for difference between open and blinded CGM in time spent in hypoglycaemia, exact data not reported) (Logtenberg 2009).
Two parallel RCTs measured CGM‐derived hypoglycaemia and hyperglycaemia with (blinded) CGM after six (Hermanides 2011) and 12 months (Bergenstal 2010), using different units of effect measure (percentage time and AUC). CGM‐derived hypoglycaemia, measured as percentage of time, was significantly shorter for the CGM group compared to the SMBG group: 22% versus 38%, MD 17%, 95% ‐25% to ‐8% (Hermanides 2011) (Analysis 5.8). The differences in the other analyses were not statistically significant (Analysis 5.7; Analysis 5.9; Analysis 5.10).
5.8. Analysis.
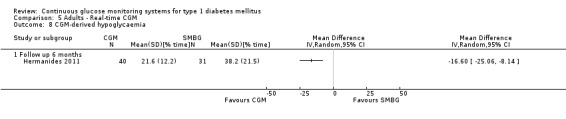
Comparison 5 Adults ‐ Real‐time CGM, Outcome 8 CGM‐derived hypoglycaemia.
5.7. Analysis.
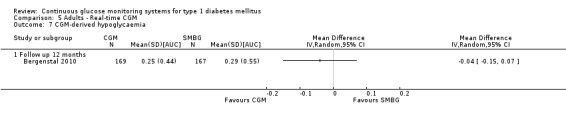
Comparison 5 Adults ‐ Real‐time CGM, Outcome 7 CGM‐derived hypoglycaemia.
5.9. Analysis.
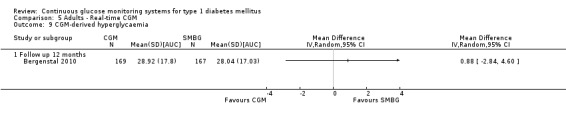
Comparison 5 Adults ‐ Real‐time CGM, Outcome 9 CGM‐derived hyperglycaemia.
5.10. Analysis.
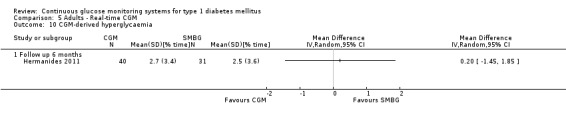
Comparison 5 Adults ‐ Real‐time CGM, Outcome 10 CGM‐derived hyperglycaemia.
The outcomes diabetes complications, death and costs were not measured in any of the studies in adults.
All ages
The six studies differed widely in patient groups and comparisons. Therefore we discuss the studies according to the CGM system used (Analysis 6.1 to Analysis 6.13).
6.1. Analysis.
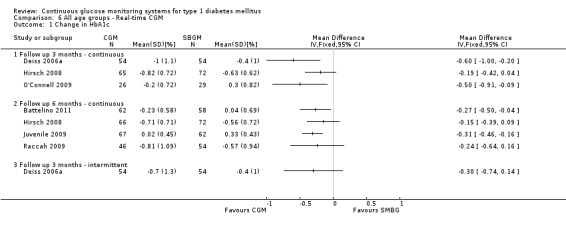
Comparison 6 All age groups ‐ Real‐time CGM, Outcome 1 Change in HbA1c.
6.13. Analysis.
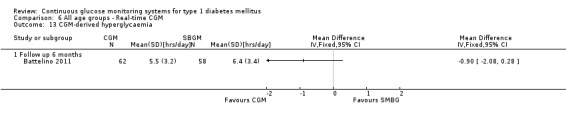
Comparison 6 All age groups ‐ Real‐time CGM, Outcome 13 CGM‐derived hyperglycaemia.
Freestyle Navigator
One study used the Freestyle Navigator as CGM device (Battelino 2011). The patient group had a HbA1c level lower than 7.5%, and consisted of insulin pump users and MDI users. The change in HbA1c six months after baseline was significantly larger in the CGM group compared to the SMBG group (change in HbA1c: ‐0.2% versus 0.0%, MD ‐0.3%, 95% ‐0.5% to 0.0%). Compared to the SMBG group the CGM group spent also significantly less time in hypoglycaemia (hours per day) (0.5 versus 1.0 hours, MD ‐0.5, 95% ‐0.9 to ‐0.1). The time spent in hyperglycaemia was not statistically different between the groups.
Guardian
In the Guardian trial (Deiss 2006a), the improvement in HbA1c between baseline and end of the intervention (three months) was significantly better for the continuous CGM users compared to the SMBG users (‐1.0% versus ‐0.4%, MD in change ‐0.6%, 95% CI ‐1.0 to ‐0.2%). The HbA1c of the intermittent CGM users showed also a better improvement than the SMBG group, but the difference was not statistically significant (‐0.7% versus ‐0.4%, MD in change ‐0.3%, 95% ‐0.7% to 0.1%).
Paradigm
In the study with insulin pump users with well‐controlled diabetes the change in HbA1c from baseline to the end of the study was larger for CGM users compared to SMBG users, but the difference was not statically significant (‐0.2% versus 0.3%, MD ‐0.5%, 95% CI ‐0.9% to 0.1%). Severe hypoglycaemia and ketoacidosis events did not occur. The percentage of time spent in hypo‐ or hyperglycaemic ranges did not differ significantly between the study arms (O'Connell 2009).
In the study with insulin pump users with HbA1c levels of at least 7.5%, the change in HbA1c was not different between the CGM and SMBG users (change in HbA1c after six months: ‐0.7% versus ‐0.6%, MD ‐0.2%, 95% ‐0.4% to 0.1%). The CGM users spent less time in the hypoglycaemic range at the end of the study compared to baseline, for the SMBG users there was no such decline (AUC (area under the curve) less than 3.9 mmol/L [mmol/L per minute], change from baseline ‐0.02 versus 0.01, P < 0.0001). There was no difference for the hyperglycaemic ranges (Hirsch 2008).
Among insulin pump naive patients with poorly controlled diabetes there was no difference between the study arms in change in HbA1c between baseline and end of treatment (six months; ‐0.8% versus ‐0.6%, MD ‐0.2%, 95% CI ‐0.6% to 0.2%). At the end of the study, CGM users had a slightly increased, but non‐significant duration of hypoglycaemic episodes (change in hours per day with glucose levels less than 3.9 mmol/L: 0.3 versus 0.0, MD 0.3, 95% CI 0.0 to 0.6) and a statistically significant decreased duration of hyperglycaemic episodes (change in hours per day with glucose levels greater than 10.6 mmolL: ‐3.5 versus ‐0.7, MD ‐2.8, 95% CI ‐4.5 to ‐1.0). The number of hypo‐ and hyperglycaemic episodes did not change. There was however a significant between group difference in HbA1c levels favouring the sensor augmented pump therapy when only those patients who were protocol compliant were analysed (i.e. those with adequate sensor usage) (Raccah 2009).
Different types of CGM systems
In the study with different CGM systems in well‐controlled diabetes (Juvenile 2009), the HbA1c level of the CGM group did not change after six months, while the HbA1c levels of the control group increased (+0.02% versus +0.3%, MD in change in HbA1c ‐0.3%, 95% CI ‐0.5% to ‐0.2%). The relative better glycaemic control of CGM users compared to SMBG users was consistent for different outcomes.
The outcomes quality of life, patient satisfaction, diabetes complications, death and costs were not measured.
Exploratory meta‐analysis (across age groups)
Continuous use of real‐time CGM augmented insulin pump therapy in insulin pump naive patients
Two studies studied compared CGM augmented insulin pump therapy (Paradigm device) with conventional therapy (MDI and SMBG). Study participants had not used an insulin pump before and had a mean HbA1c higher than 8.0% (Bergenstal 2010; Hermanides 2011).
Glycaemic control
Six months after baseline the estimated decline in HbA1c level was significantly larger for Paradigm users compared to the control group (MD in HbA1c change from baseline ‐0.7%, 95% CI; ‐0.8% to ‐0.5%, 2 RCTs, 562 patients, I2=84%) (Analysis 7.1). After 12 months, the mean difference in HbA1c level change from baseline was ‐0.6% (95% CI ‐0.8% to ‐0.5%, 1 RCT, 485 patients), in favour of the Paradigm users (Analysis 7.1).
7.1. Analysis.
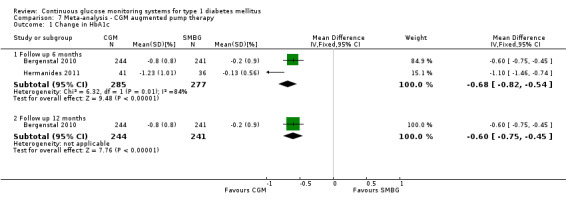
Comparison 7 Meta‐analysis ‐ CGM augmented pump therapy, Outcome 1 Change in HbA1c.
Both studies reported the occurrence of severe hypoglycaemia from baseline to six months (Hermanides 2011) or 12 months of follow‐up (Bergenstal 2010). The risk of hypoglycaemia was increased for the CGM users, but the confidence intervals were (very) wide and included unity (4/43 versus 1/35; RR 3.26, 95% CI 0.38 to 27.82 and 21/247 versus 17/248; RR 1.24, 95% CI 0.67 to 2.29) (Analysis 7.2). One study reported the occurrence of ketoacidosis from baseline to six months (Hermanides 2011). There was however only one event (Analysis 7.3).
7.2. Analysis.
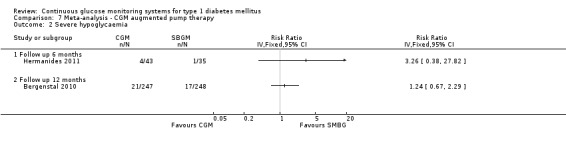
Comparison 7 Meta‐analysis ‐ CGM augmented pump therapy, Outcome 2 Severe hypoglycaemia.
7.3. Analysis.
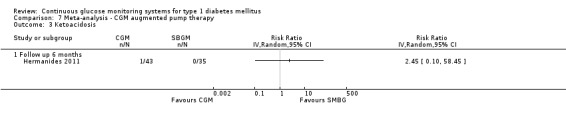
Comparison 7 Meta‐analysis ‐ CGM augmented pump therapy, Outcome 3 Ketoacidosis.
Quality of life
Hermanides et al reported on reported on quality of life in the domains physical and mental health (Hermanides 2011). The differences between the Paradigm and control group were small (MD 1.3, 95% ‐4.2 to 6.8 for physical health and 2.4, 95% CI ‐4.4 to 9.2 for mental health, on a scale from 0 to 100), with wide confidence intervals that included no effect (Analysis 7.4).
7.4. Analysis.
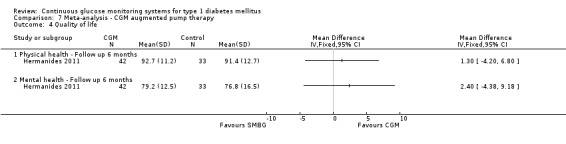
Comparison 7 Meta‐analysis ‐ CGM augmented pump therapy, Outcome 4 Quality of life.
The outcomes patient satisfaction, diabetes complications, death and costs were not measured.
Continuous use of real‐time CGM
Six studies investigated continuous use of real‐time CGM in patients that used either an insulin pump or MDI (Battelino 2011; Hirsch 2008; Juvenile 2008; Juvenile 2009; Kordonouri 2010; Raccah 2009). For the JDRF trial, we used the data of the children, adolescents and adults as subgroups (Juvenile 2008). Only one RCT reported on outcomes on 12 months, therefore we describe only the results for six months of follow‐up (data in the Analysis section).
Glycaemic control
Six months after baseline, the average decline in HbA1c level was statistically significantly larger for CGM users compared to the SMBG users (MD in HbA1c change from baseline ‐0.2%, 95% CI: ‐0.4% to ‐0.1%, 6 RCTs, 963 patients, I2=55%) (Analysis 8.1).
8.1. Analysis.
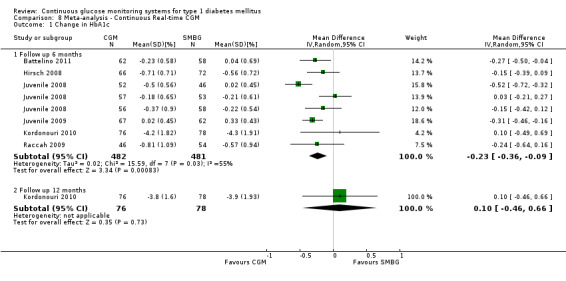
Comparison 8 Meta‐analysis ‐ Continuous Real‐time CGM, Outcome 1 Change in HbA1c.
On average, there was no difference in risk of severe hypoglycaemia or ketoacidosis between CGM and SMBG users. The confidence interval however, was wide and included a decreased as well as an increased risk for CGM users compared to the control group (severe hypoglycaemia: 36/411 versus 33/407; RR 1.02, 95% CI: 0.65 to 1.62, 4 RCTs, I2=0% and ketoacidosis: 8/411 versus 8/407; RR 0.94, 95% CI: 0.36 to 2.40, 4 RCTs, I2=0%) (Analysis 8.2; Analysis 8.3).
8.2. Analysis.
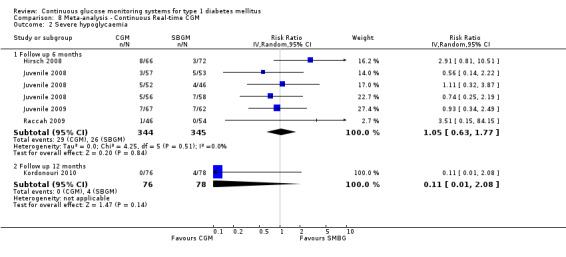
Comparison 8 Meta‐analysis ‐ Continuous Real‐time CGM, Outcome 2 Severe hypoglycaemia.
8.3. Analysis.
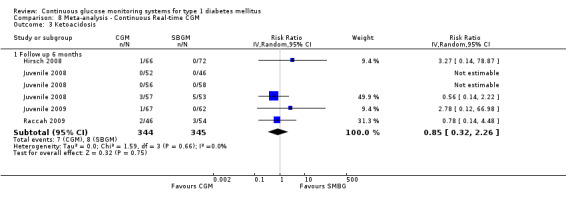
Comparison 8 Meta‐analysis ‐ Continuous Real‐time CGM, Outcome 3 Ketoacidosis.
Quality of life
Quality of life at six months after baseline was measured in one RCT (Juvenile 2008), for adults on the physical and mental domain and in general in parents of patients younger than 18 years of age. The differences between the study arms were small, with SMDs varying between ‐0.03 and 0.24 (small size of effect). The confidence intervals were wide and comprised a higher as well as a lower quality of life for CGM users compared to SMBG users (Analysis 8.4).
8.4. Analysis.
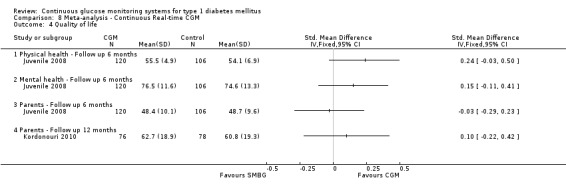
Comparison 8 Meta‐analysis ‐ Continuous Real‐time CGM, Outcome 4 Quality of life.
The outcomes patient satisfaction, diabetes complications, death and costs were not measured.
Intermittent CGM use (retrospective CGM system)
Five studies reported change in HbA1c levels at three months when CGM has been used intermittently to provide data based on which treatment changes were made (Chico 2003; Cosson 2009; Ludvigsson 2003; Tanenberg 2004; Yates 2006).
Glycaemic control
The decrease in HbA1c level three months after baseline was larger for CGM users, but the confidence interval included no effect (MD in change in HbA1c level ‐0.2%, 95% CI ‐0.4% to 0.1%, 5 RCTs, 216 patients, I2=0%) (Analysis 9.1).
9.1. Analysis.
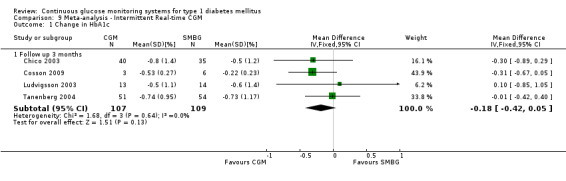
Comparison 9 Meta‐analysis ‐ Intermittent Real‐time CGM, Outcome 1 Change in HbA1c.
Two studies reported occurrence of severe hypoglycaemia (Ludvigsson 2003; Yates 2006) and one study reported on the occurrence of ketoacidosis (Yates 2006). The number of events was very low (two events and one event, respectively) (Analysis 9.2; Analysis 9.3).
9.2. Analysis.
Comparison 9 Meta‐analysis ‐ Intermittent Real‐time CGM, Outcome 2 Severe hypoglycaemia.
9.3. Analysis.
Comparison 9 Meta‐analysis ‐ Intermittent Real‐time CGM, Outcome 3 Ketoacidosis.
The outcomes quality of life, patient satisfaction, diabetes complications, death and costs were not measured.
Pregnant women with diabetes type
We identified one study on pregnant women with diabetes, but the data were not presented for type 1 and type 2 diabetes separately (Murphy 2008).
Subgroup analysis
We planned subgroup analyses for patients with hypoglycaemia unawareness and patients with poorly controlled diabetes. There were no studies that included patients with hypoglycaemia unawareness.
Seven RCTs were performed in patients with poorly controlled diabetes (HbA1c greater than 8.0%): three for retrospective CGM systems (Chase 2001; Ludvigsson 2003; Tanenberg 2004) and four for real‐time CGM (Raccah 2009; Deiss 2006a; Cosson 2009; Hermanides 2011).
For the retrospective CGM systems the evidence for improved glycaemic control is conflicting. Significantly lower (Ludvigsson 2003), as well as significantly higher HbA1c levels (Chase 2001) for the CGM group at the end of the study were found, and a third RCT showed no effect at all (Tanenberg 2004).
For real‐time CGM systems the results of the different RCTs were more in line. There is limited evidence for improved glycaemic control. The change in HbA1c was larger in the CGM group than in the SMBG group in all four RCTs, and a statistically and clinically significant effect in two high quality RCTs (Hermanides 2011; Deiss 2006a).
In one of these RCTs (Raccah 2009) a subgroup of patients who were fully protocol‐compliant (including CGM sensor wear equal to or greater than 70% of the time) was analysed according to a pre‐specified analysis. Fully compliant CGM users showed a larger improvement in HbA1c than CGM‐users in the total group (mean change at six months ‐0.96% [SD 0.93] versus ‐0.81% [SD 1.09%]). In this per‐protocol analysis the difference in improvement between CGM and SMBG group was statistically significant (P = 0.004 for difference between study arms), in contrast to the intention‐to‐treat analysis (Raccah 2009).
Of the eight studies that were included in the meta‐analysis for real‐time CGM, two studies were in patients with clearly defined poorly controlled diabetes (HbA1c equal to or greater than 8.0% as eligibility criterion) (Hermanides 2011; Raccah 2009). The study designs of these studies were too heterogenous to pool. One study compared CGM augmented insulin pump therapy (Paradigm device) with conventional therapy (MDI and SMBG) (Hermanides 2011) while the other study compared the Paradigm device with insulin pump therapy combined with SMBG (Raccah 2009). Both studies were in insulin pump naive patients. CGM use was associated with a larger decrease in HbA1c compared to the control group in both studies, but in the Hermanides study this difference was statistically significant and much larger than in the study by Raccah et al (MD in change in HbA1c level ‐1.1%, 95% CI ‐0.8% to ‐0.5% and MD in change in HbA1c level ‐0.2%, 95% CI ‐0.6% to 0.2%).
Sensitivity analysis
We could not perform our planned sensitivity analysis as there were not many differences in the risk of bias items between the studies.
Discussion
Summary of main results
Children
For children there is limited evidence, based on three RCTs with low risk of bias, for the effectiveness of real‐time continuous glucose monitoring (CGM) systems on glycaemic control. More patients in the CGM group had a decrease of at least 0.5% in glycosylated haemoglobin A1c (HbA1c) compared to the self‐monitoring of blood glucose (SMBG) group. However, the mean change in HbA1c differed significantly after three months but not after six or 12 months. The reduction in HbA1c levels seems to be related to actual CGM use. After 12 months, those patients who used their CGM frequently had a significantly lower HbA1c level compared to patients who showed low or no sensor usage (Kordonouri 2010).
For the retrospective CGM systems the evidence is conflicting. Significantly lower (Lagarde 2006; Ludvigsson 2003), as well as significantly higher HbA1c levels for the CGM group at the end of the study were found (Chase 2001). In one RCT the difference in change after three months was clinically relevant (change of at least 0.4%), but not statistically significant (Deiss 2006). In one RCT the absolute HbA1c level was significantly lower in the CGM group, but the difference in change in HbA1c did not reach significance (Lagarde 2006).
Adolescents
For adolescents no statistically significant differences in any outcome were found, although among experienced insulin pump users the improvement in HbA1c over six months was 0.4% larger in the group that used real‐time CGM compared to the SMBG group (Hirsch 2008).
Adults
For retrospective CGM systems there were no statistically significant differences between CGM and SMBG in the primary glycaemic control outcome measures.
Short‐term (three months) results showed a small, but statistically significant difference on glycaemic control for the real‐time CGM systems. With regard to patient satisfaction, there is very limited evidence (one small RCT, low risk of bias) that Paradigm users are more satisfied with their type of insulin treatment compared to the SMBG group (who injected insulin) (Peyrot 2009). Patient satisfaction did not differ for patients who had six days real‐time access to CGM values compared to patients with retrospective access (one RCT, some limitations in study design) (Hermanns 2009).
Long‐term (at least six months) glycaemic control outcomes for real‐time CGM systems showed inconsistent results: two RCTs (low risk of bias) showed a statistically and clinically significant higher improvement in HbA1c for the CGM group (Hermanides 2011; Juvenile 2008), while in the third trial (some limitations in study design) there was no difference (Hirsch 2008).
In one RCT among adolescents and adults significantly greater improvements in HbA1c were noted in subgroups with better compliance (Hirsch 2008). When sensor use was correlated with HbA1c values in a statistical model, each 10% increase in compliance was associated with a 41% increase in the probability of a 0.5% reduction in HbA1c (P = 0.045). Further details of the model were not reported, nor a comparison of highly compliant CGM‐users with SMBG‐users.
Across age groups
The studies included in this review are subject to heterogeneity which makes meta‐analysis difficult. This heterogeneity surfaces in the duration and kind of intervention, the reported outcome measures and the study populations. The results of individual studies, as discussed previously, often showed a positive effect in favour of CGM without reaching statistically significance. This suggests a statistical power problem with the majority of the included studies for certain endpoints. In these cases a meta‐analysis can be useful and relevant. The question arises whether, despite of certain aspects of heterogeneity between studies, some outcome measures can become subject to meta‐analysis. We are of the opinion that the meta‐analyses hold their merit, in spite of some heterogeneity between studies. For outcome measures that can be objectively measured, meta‐analysis could be performed.
In our exploratory meta‐analysis among patients across all age groups we found a statistically significant larger decline in HbA1c level for real‐time CGM users starting insulin pump therapy compared to patients using multiple daily injections of insulin (MDI) and SMBG (conventional therapy) (MD in HbA1c level change from baseline ‐0.7%, 95% CI; ‐0.8% to ‐0.5%, 2 RCTs, 562 patients, I2=84% (Analysis 7.1). Change in HbA1c was higher in comparison to studies in which patients used different types of real‐time CGM devices, where 80% of patients used pumps in both arms of the studies (Juvenile 2008; Juvenile 2009). This implies an HbA1c‐lowering effect which could be attributed to the combination of pump and CGM over and above the effect of the sensor. Also, the use of the bolus wizard feature on the sensor‐augmented pump, which integrates sensor data in an advice to the patient about an appropriate meal‐time bolus, further illustrates that sensor‐augmented pump therapy should be considered as an distinct treatment platform.
For patients where only the CGM was a new device the average decline in HbA1c level was also statistically significant larger for CGM users compared to the SMBG users. The decline was however much smaller than in the group with the sensor‐augmented insulin pump: the average difference change in HbA1c was 0.2% (MD in HbA1c change from baseline ‐0.2%, 95% CI: ‐0.4% to ‐0.0%, 6 RCTs, 963 patients, I2=55%) (Analysis 8.1). A prediction interval would stretch from ‐0.9% to +0.3%, indicating that although on average the intervention seems effective this will not always be the case in an individual setting. With regard to intermittent (retrospective) sensor use we did not find a difference in change in HbA1c between CGM and SMBG users (MD in HbA1c levels at three months ‐0.2%, 95% CI ‐0.4% to 0.1%, Analysis 7.1). There were no statistically significant differences in the risk of severe hypoglycaemia or ketoacidosis (RR 1.02, 95% CI: 0.65 to 1.62 and RR 0.94, 95% CI 0.37 to 2.40) (Analysis 7.2).
Against intuitive expectations, CGM is not associated with lower incidence of severe hypoglycaemia in type 1 diabetes. Most studies however, were underpowered to detect a difference and studies in patients with hypoglycaemia unawareness are lacking. Moreover, definitions of hypoglycaemia were often not reported. When the data of these heterogeneous studies are subjected to meta‐analysis, a statistically significant and clinically relevant difference in decrease in HbA1c levels in favour of the CGM group was found. This additional decrease in HbA1c levels with the use of CGM could perhaps also explain the trend towards moderately higher occurrence of severe hypoglycaemia. We believe that even though the studies are methodologically and clinically heterogeneous, the results of this meta‐analysis merit the use of CGM in clinical settings to help lower HbA1c levels.
Special patient groups: patients with poorly controlled diabetes, patient with hypoglycaemia unawareness and pregnant women
There is limited evidence for improved glycaemic control of CGM use in patients with poorly controlled diabetes. The change in HbA1c was larger in the CGM group than in the SMBG group in all four RCTs, and a statistically and clinically significant effect in two high quality RCTs (Hermanides 2011; Deiss 2006a).
We did not find any studies in pregnant women and in patients with hypoglycaemia unawareness.
Overall completeness and applicability of evidence
The goal of this systematic review was to investigate the short (less than six months) and long (equal to or greater than six months) term effects of CGM in children, adolescents, adults and pregnant women with diabetes type 1 on glycaemic control ‐ including both HbA1c as a measure of mean glucose and hypoglycaemia ‐ and quality of life. We were specifically interested in the outcomes for children, pregnant women, patients with poorly controlled diabetes and patients with hypoglycaemia unawareness, as these patient groups may benefit the most from CGM. We considered all types of CGM systems, comparisons between CGM and SMBG and head‐to‐head comparisons of different CGM systems for inclusion.
We found 22 RCTs, performed in children, adolescents, adults and patients of all ages, but not in pregnant women with type 1 diabetes mellitus. In some of the RCTs pregnancy was mentioned as an exclusion criterion (Ludvigsson 2003; Logtenberg 2009; Lagarde 2006; Cosson 2009) or reason for dropout (Hirsch 2008). One of the excluded studies was a RCT in pregnant women with type 1 and type 2 diabetes. The study was excluded because the data for type 1 patients were not reported separately (Murphy 2008).
With regard to our specific interests, 11 studies were conducted in children, and eight studies in patients with HbA1c‐defined poorly controlled diabetes. Studies in patients with hypoglycaemia unawareness were lacking and seem highly warranted.
Glycaemic control was an outcome measure in all RCTs and most studies reported change in HbA1c level. A difference of 0.4% HbA1c between CGM and SMBG group is considered clinically significant, according to the US Food and Drug Administration (FDA) and widely accepted in the field of diabetes research (FDA 2009). In power analyses a clinically significant difference of 0.5% HbA1c is used to calculate sample size ( Deiss 2006; Juvenile 2008; Raccah 2009). Two studies reported only CGM‐derived hypo‐ and hyperglycaemia (Hermanns 2009; Logtenberg 2009). Severe hypoglycaemia and ketoacidosis occurred infrequently. Most studies were therefore underpowered to detect differences for this outcome.
Quality of life and patient satisfaction were also under‐evaluated. Only five studies measured quality of life, in one study the data on quality of life were assessed but not reported (Chase 2001). Two studies assessed patient satisfaction (Hermanns 2009; Peyrot 2009). In order to balance the benefits and costs of CGM correctly, more studies with patient‐reported outcomes are needed.
All RCTs compared CGM with SMBG; there were no head‐to‐head comparisons between CGM systems. One trial compared real‐time access of CGM with retrospective access and SMBG (Hermanns 2009). In one trial two different types of CGM were used (Cooke 2009) and in one RCT three different types of CGM were used, but subgroup analyses of the different CGM systems were not reported (Juvenile 2008; Juvenile 2009).
Sensor use compliance
The efficacy of CGM is influenced by the compliance with sensor use. In the JDRF study among patients with HbA1c between 7% and 10% for example, improved HbA1c was found for CGM using adults, but not for children and adolescents. The authors suggest that the observed age effect may be related to a substantially greater use of sensors in the adults than in patients in the younger age groups. Fifty percent of the children used the sensor at least six days per week, compared to 30% of the adolescents and 83% of the adults. In this patient population, frequency of sensor use was an independent predictor of change in HbA1c; increased sensor use was associated with larger improvement in HbA1c. In those using the sensor at least six days per week the mean decrease in HbA1c was 0.6% (SD not reported), irrespective of age group and baseline HbA1c (Juvenile 2008).
Several RCTs in this review investigated the association between sensor use and glycaemic control by performing a subgroup analysis or per protocol analysis (see 'Summary of main results').
From a methodological point of view, an intention‐to‐treat analysis is the recommended analysis of choice for RCTs, because analysing the patients in the group to which they were randomly allocated keeps the randomisation intact. In a per protocol analysis, a selection of the patients is analysed (those completing the protocol). The problem is that the factors behind the selection process are unknown. In other words, the results may be biased (often leading to an overestimation) because the prognostic factors in the intervention and control group are no longer comparable. On the other hand, per protocol analysis reflects the real use of the intervention.
There is growing consensus among diabetes professionals that per protocol analysis has additional value to intention‐to‐treat analysis when it comes to CGM (Hermanides 2011). The reasoning is that whether a sensor is used or not can easily be checked by the health care professional by making a read‐out of the sensor. The difference with e.g. antihypertensive medication is that the health care professional can never be completely sure whether the patients take their antihypertensives or not.
Quality of the evidence
The overall quality of the RCTs was moderate (some limitations in study design) to high.
Potential biases in the review process
The studies in this review addressed different patient groups (e.g. according to age and way of using of insulin), investigated a number of different CGM devices and used different study designs (e.g. continuous or intermittent CGM use, blinded versus unblinded CGM). We choose to describe the results of the studies separately for the different age and CGM device groups, but other choices may be valid as well.
Agreements and disagreements with other studies or reviews
Recently three systematic reviews have been published on the same topic as our review (Ghandi 2011; Hoeks 2010; Pickup 2011). Compared to the review of Hoeks et al, our review is more rigorous and detailed with regard to searching the literature, quality assessment and data‐extraction. Their review included nine RCTs, a subset of the RCTs included in our review. Hoeks et al did not perform a meta‐analysis. The other two reviews differ slightly in objectives (inclusion of type 2 diabetes) and methods (individual patient data analysis), but their results are in line with our findings and conclusions.
Authors' conclusions
Implications for practice.
There is limited evidence for the effectiveness of real‐time continuous glucose monitoring (CGM) use in children, adults and patients with poorly controlled diabetes. The largest improvements in glycaemic control were seen for sensor‐augmented insulin pump therapy in patients with poorly controlled diabetes who had not used an insulin pump before.There are indications that higher compliance of wearing the CGM device improves glycosylated haemoglobin A1c level (HbA1c) to a larger extent.
Implications for research.
Studies that are sufficiently powered to detect a difference in improvement in glycosylated haemoglobin A1c level (HbA1c) and risk of hypoglycaemia are necessary to evaluate this finding, especially studies comparing continuous glucose monitoring (CGM) in pump users to pump users alone, and in specific patient groups: children and adolescents, pregnant women, and patients with hypoglycaemia unawareness. These studies should focus on clinical outcomes as well as health‐related quality of life.
Acknowledgements
We would like to thank Jacqueline Limpens and Faridi van Etten, clinical librarians at the Academic Medical Center in Amsterdam, for developing the search strategy and performing the CINAHL search, and Karla Bergerhoff, trial search coordinator of the Metabolic and Endocrine Disorders Review Group for performing the CENTRAL, MEDLINE and EMBASE search. We thank Dr. Cooke for providing additional data.
Appendices
Appendix 1. Search strategies
| Search terms |
| Unless otherwise stated, search terms are free text terms; MeSH = Medical subject heading (Medline medical index term); exp = exploded MeSH; the dollar sign ($) stands for any character(s); the question mark (?) substitutes one or no characters; tw = text word; pt = publication type; sh = MeSH; adj = adjacent. The Cochrane Library #1 MeSH descriptor Diabetes Mellitus, Type 1 explode all trees #2 MeSH descriptor Diabetic Ketoacidosis explode all trees #3 (IDDM or T1DM or T1D) #4 (insulin* depend* or insulin depend* or insulin‐depend*) #5 ((diabet* or dm) near5 ((typ? near3 (one or '1' or I)) or typ?1 or typ?I)) #6 ((earl* or acidos* or juvenil* or child* or keto* or labil* or britt* or p?ediatric) near6 (diabet* or dm)) #7 ((auto‐immun* or autoimmun* or sudden onset) near6 (diabet* or dm)) #8 (insulin* defic* near6 absolut*) #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) #10 MeSH descriptor Blood Glucose Self‐Monitoring explode all trees #11 (cgm or cgms) #12 (GlucoWatch or (navigator and freestyle) or Medronic or guardian or glucosemeter*) #13 ((glucos* or sugar or HbA*) near6 (sensor* or Monitor*)) #14 (#10 OR #11 OR #12 OR #13) #15 (#9 AND #14) MEDLINE 1. exp Diabetes Mellitus, Type 1/ 2. exp Diabetic Ketoacidosis/ 3. (IDDM or T1DM or T1D).tw,ot. 4. (insulin depend$ or insulindepend$ or insulin‐depend$).tw,ot. 5. ((diabet$ or dm) adj5 ((typ? adj3 (one or '1' or I)) or typ?1 or typ?I)).tw,ot. 6. ((earl$ or acidos$ or juvenil$ or child$ or keto$ or labil$ or britt$ or p?ediatric) adj6 (diabet$ or dm)).tw,ot. 7. ((auto‐immun$ or autoimmun$ or sudden onset) adj6 (diabet$ or dm)).tw,ot. 8. (insulin$ defic$ adj6 absolut$).tw,ot. 9. or/1‐8 10. exp Blood Glucose Self‐Monitoring/ 11. (cgm or cgms).tw,ot. 12. (GlucoWatch or (navigator and freestyle) or Medtronic or guardian or glucosemeter$).tw,ot. 13. ((glucos$ or sugar or HbA$) adj6 (sensor$ or monitor$)).tw,ot. 14. or/10‐13 15. randomised controlled trial.pt. 16. controlled clinical trial.pt. 17. randomi?ed.ab. 18. placebo.ab. 19. clinical trials as topic.sh. 20. randomly.ab. 21. trial.ti. 22. or/15‐21 23. Meta‐analysis.pt. 24. exp Technology Assessment, Biomedical/ 25. hta.tw,ot. 26. (health technology adj6 assessment$).tw,ot. 27. (meta analy$ or metaanaly$ or meta?analy$).tw,ot. 28. ((review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinhal or psychinfo or psychlit or healthstar or biosis or current content$ or systemat$)).tw,ot. 29. or/23‐28 30. (comment or editorial or historical‐article).pt. 31. 29 not 30 32. 9 and 14 and 22 33. 9 and 14 and 31 34. (animals not (humans and animals)).sh. 35. 32 not 34 36. 33 not 34 37. limit 35 to yr="2003‐2009" 38. limit 36 to yr="2003‐2009" EMBASE 1. Insulin Dependent Diabetes Mellitus/ 2. exp Diabetic Ketoacidosis/ 3. ("insulin$ depend$" or insulindepend$ or insulin‐depend$).tw,ot. 4. (IDDM or T1DM or T1D).tw,ot. 5. ((diabet* or dm) adj5 ((typ? adj3 (one or '1' or I)) or typ?1 or typ?I)).tw,ot. 6. ((earl* or acidos* or juvenil* or child* or keto* or labil* or britt* or p?ediatric) adj6 (diabet* or dm)).tw,ot. 7. ((auto‐immun* or autoimmun* or sudden onset) adj6 (diabet* or dm)).tw,ot. 8. (insulin* defic* adj6 absolut*).tw,ot. 9. or/1‐8 10. exp Blood Glucose Monitoring/ 11. (cgm or cgms).tw,ot. 12. (GlucoWatch or (navigator and freestyle) or Medtronic or guardian or glucosemeter*).tw,ot. 13. ((glucos* or sugar or HbA*) adj6 (sensor* or monitor*)).tw,ot. 14. or/10‐13 15. exp Randomized Controlled Trial/ 16. exp Controlled Clinical Trial/ 17. exp Clinical Trial/ 18. exp Comparative Study/ 19. exp Drug comparison/ 20. exp Randomization/ 21. exp Crossover procedure/ 22. exp Double blind procedure/ 23. exp Single blind procedure/ 24. exp Placebo/ 25. exp Prospective Study/ 26. ((clinical or control$ or comparativ$ or placebo$ or prospectiv$ or randomi?ed) adj3 (trial$ or stud$)).ab,ti. 27. (random$ adj6 (allocat$ or assign$ or basis or order$)).ab,ti. 28. ((singl$ or doubl$ or trebl$ or tripl$) adj6 (blind$ or mask$)).ab,ti. 29. (cross over or crossover).ab,ti. 30. or/15‐29 31. exp meta analysis/ 32. (metaanaly$ or meta analy$ or meta?analy$).ab,ti,ot. 33. ((review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinhal or psychinfo or psychlit or healthstar or biosis or current content$ or systematic$)).ab,ti,ot. 34. exp Literature/ 35. exp Biomedical Technology Assessment/ 36. hta.tw,ot. 37. (health technology adj6 assessment$).tw,ot. 38. or/31‐37 39. (comment or editorial or historical‐article).pt. 40. 38 not 39 41. 30 and 14 and 9 42. 14 and 9 and 40 43. limit 42 to yr="2003‐2009" 44. limit 41 to yr="2003‐2009" 45. limit 44 to human 46. limit 43 to human CINAHL S1. (MH "Diabetes Mellitus, Insulin‐Dependent") or (MH "Diabetic Ketoacidosis") S2. ( TX IDDM or T1DM or T1D ) or (insulindepend* or insulin‐depend* ) S3. TX diabet* N5 typ? one or TX diabet* n5 typ? I or TX diabet* n5 typ? 1 or TX diabet* n5 typ?1 or TX diabet* n5 typ?I S4. TX dm N5 typ? one or TX dm n5 typ? I or TX dm n5 typ? 1 or TX dm n5 typ?1 or TX dm n5 typ?I S5. ( TX earl* N6 diabet* or TX acidos* N6 diabet* or TX juvenil* N6 diabet* or TX child* N6 diabet* or TX keto* N6 diabet* or TX labil* N6 diabet* or TX britt* N6 diabet* or TX p?ediatric N6 diabet* ) or ( TX earl* N6 dm or TX acidos* N6 dm or TX juvenil* N6 dm or TX child* N6 dm or TX keto* N6 dm or TX labil* N6 dm or TX britt* N6 dm or TX p?ediatric N6 dm ) or ( TX auto‐immun* N6 diabet* or TX autoimmun* N6 diabet* or TX sudden onset N6 diabet* ) S6. ( TX auto‐immun* N6 dm or TX autoimmun* N6 dm or TX sudden onset N6 dm ) or TX insulin* defic* N6 absolut* or ( TX gestation* N3 diabet* or TX gdm N3 diabet* ) S7. S1 or S2 or S3 or S4 or S5 or S6 S8. MH "Blood Glucose Self‐Monitoring" S9. ( TX cgm or TX cgms ) or ( TX GlucoWatch or TX Medtronic or TX guardian or TX glucosemeter* or (TX navigator and TX freestyle) ) or ( ( TX glucose* N6 sensor* or TX sugar N6 sensor* or TX HbA* N6 sensor* ) or ( TX glucose* N6 monitor* or TX sugar N6 monitor* or TX HbA* N6 monitor* or TX paradigm or TX glucoday ) ) S10. S8 or S9 S11. (MH "Clinical Trials+") or (MH "Comparative Studies") or (MH "Crossover Design") S12. (MH "Double‐Blind Studies") or (MH "Single‐Blind Studies") or (MH "Placebos") S13. (MH "Prospective Studies+") or ( ( TI clinical n3 trial* or AB clinical n3 trial* ) or ( TI control* n3 trial* or AB control* n3 trial* ) or ( TI comparativ* n3 trial* or AB comparativ* n3 trial* ) ) or ( ( TI placebo* n3 trial* or AB placebo* n3 trial* ) or ( TI prospectiv* n3 trial* or AB prospectiv* n3 trial* ) or ( TI randomi?ed n3 trial* or AB randomi?ed n3 trial* ) ) S14. ( ( TI clinical n3 stud* or AB clinical n3 stud* ) or ( TI control* n3 stud* or AB control* n3 stud* ) or ( TI comparativ* n3 stud* or AB comparativ* n3 stud* ) ) or ( ( TI placebo* n3 stud* or AB placebo* n3 stud* ) or ( TI prospectiv* n3 stud* or AB prospectiv* n3 stud* ) or ( TI randomi?ed n3 stud* or AB randomi?ed n3 stud* ) ) or ( ( ( TI singl* n6 blind* or AB singl* n6 blind* ) or ( TI doubl* n6 blind* or AB doubl* n6 blind* ) or ( TI trebl* n6 blind* or AB trebl* n6 blind* ) or ( TI tripl* n6 blind* or AB tripl* n6 blind* ) ) or ( ( TI singl* n6 mask* or AB singl* n6 mask* ) or ( TI doubl* n6 mask* or AB doubl* n6 mask* ) or ( TI trebl* n6 mask* or AB trebl* n6 mask* ) or ( TI tripl* n6 mask* or AB tripl* n6 mask* ) ) ) S15. ( ( TI cross over or AB cross over ) or ( TI crossover or AB crossover ) ) or ( ( TI random* n6 alloc* or AB random* n6 alloc* ) or ( TI random* n6 assign* or AB random* n6 assign* ) or ( TI random* n6 basis or AB random* n6 basis ) or (TI random* n6 order* or AB random* n6 order* ) ) S16. S11 or S12 or S13 or S14 or S15 S17. (MH "Meta Analysis") or ( ( TI metaanaly* or AB metaanaly* ) or ( TI meta analy* or AB meta analy* ) or ( TI meta?analy* or AB meta?analy* ) ) or (MH "Literature+") S18. ( TX hta or TX health technology n6 assessment* ) or ( ( TI search* n10 literature* or AB search* n10 literature* ) or ( TI search* n10 medical database* or AB search* n10 medical database* ) or ( TI search* n10 medline or AB search* n10 medline) or ( TI search* n10 pubmed or AB search* n10 pubmed ) or ( TI search* n10 embase or AB search* n10 embase) or ( TI search* n10 cochrane or AB search* n10 cochrane) or ( TI search* n10 cinahl or AB search* n10 cinahl) or ( TI search* n10 psychinfo or AB search* n10 psychinfo) or ( TI search* n10 psychlit or AB search* n10 psychlit) or ( TI search* n10 healthstar or AB search* n10 healthstar) or ( TI search* n10 biosis or AB search* n10 biosis) or ( TI search* n10 current content* or AB search* n10 current content*) or ( TI search* n10 systematic* or AB search* n10 systematic*) ) or ( ( TI review* n10 literature* or AB review* n10 literature* ) or ( TI review* n10 medical database* or AB review* n10 medical database* ) or ( TI review* n10 medline or AB review* n10 medline) or ( TI review* n10 pubmed or AB review* n10 pubmed ) or ( TI review* n10 embase or AB review* n10 embase) or ( TI review* n10 cochrane or AB review* n10 cochrane) or ( TI review* n10 cinahl or AB review* n10 cinahl) or ( TI review* n10 psychinfo or AB review* n10 psychinfo) or ( TI review* n10 psychlit or AB review* n10 psychlit) or ( TI review* n10 healthstar or AB review* n10 healthstar) or ( TI review* n10 biosis or AB review* n10 biosis) or ( TI review* n10 current content* or AB review* n10 current content*) or ( TI review* n10 systematic* or AB review* n10 systematic*) ) S19. S17 or S18 S20. PT comment or PT editorial or PT historical‐article S21. S19 not S20 S22. S7 and S10 and S16 S23. S7 and S10 and S21 S24. S7 and S10 and S16 S25. S7 and S10 and S21 |
Appendix 2. Description of interventions
|
Characteristic ► Study ID ▼ |
Intervention (CGM type) | Control |
| Battelino 2011 | Freestyle Navigator | SMBG (+ blinded CGM) |
| Bergenstal 2010 | Paradigm | MDI + SMBG |
| Chase 2001 | Minimed CGMS | SMBG |
| Chico 2003 | Minimed CGMS | SMBG |
| Cooke 2009 | Minimed CGMS and Glucowatch | SMBG with standard control SMBG with attention control |
| Cosson 2009 | GlucoDay | SMBG (+ blinded CGM) |
| Deiss 2006 | Minimed CGMS | SMBG (+ blinded CGM) |
| Deiss 2006a | Guardian RT | SMBG |
| Hermanides 2011 | Paradigm | MDI + SMBG |
| Hermanns 2009 | Glucoday | SMBG (+ blinded CGM) |
| Hirsch 2008 | Paradigm | SMBG |
| Juvenile 2008 | Dexcom Seven, Paradigm Freestyle Navigator | SMBG |
| Juvenile 2009 | Dexcom Seven, Paradigm Freestyle Navigator | SMBG |
| Kordonouri 2010 | Paradigm | SMBG |
| Lagarde 2006 | Minimed CGMS | SMBG (+ blinded CGM) |
| Logtenberg 2009 | Paradigm | SMBG (+ blinded CGM) |
| Ludvigsson 2003 | Minimed CGMS | SMBG (+ blinded CGM) |
| O'Connell 2009 | Paradigm | SMBG |
| Peyrot 2009 | Paradigm | MDI + SMBG |
| Raccah 2009 | Paradigm | SMBG |
| Tanenberg 2004 | Minimed CGMS | SMBG |
| Yates 2006 | Minimed CGMS | SMBG |
|
Footnotes ‐ denotes 'not reported' Abbreviations: CGM: continuous glucose monitoring; MDI: multiple daily injections; SMBG: standard monitoring of blood glucose | ||
Appendix 3. Baseline characteristics (I)
|
Characteristic ► Study ID ▼ |
Intervention(s) | Control(s) | Participating population | Country | Setting |
| Battelino 2011 | CGM | SMBG (+ blinded CGM) | adults, children | Slovenia, Israel, Sweden | outpatients |
| Bergenstal 2010 | CGM | MDI + SMBG | adults, children | USA, Canada | outpatients |
| Chase 2001 | CGM | SMBG | children | USA | outpatients |
| Chico 2003 | CGM | SMBG | adults | Spain | outpatients |
| Cooke 2009 | CGM | SMBG with standard control SMBG with attention control | adults | UK | outpatients |
| Cosson 2009 | CGM | SMBG (+ blinded CGM) | adults | France | outpatients |
| Deiss 2006 | CGM | SMBG (+ blinded CGM) | children | Germany | outpatients |
| Deiss 2006a | CGM | SMBG | adults, children | Europe, Israel | outpatients |
| Hermanides 2011 | CGM | MDI + SMBG | adults | Denmark, Belgium, France, Italy, Sweden, Switzerland, The Netherlands, UK | outpatients |
| Hermanns 2009 | CGM | blinded CGM | adults | Germany | inpatients |
| Hirsch 2008 | CGM | SMBG | adolescents, adults | USA | outpatients |
| Juvenile 2008 | CGM | SMBG | adults, children | USA | outpatients |
| Juvenile 2009 | CGM | SMBG | adults, children | USA | outpatients |
| Kordonouri 2010 | CGM | SMBG | adolescents, children | Europe | outpatients |
| Lagarde 2006 | CGM | SMBG (+ blinded CGM) | children | USA | outpatients |
| Logtenberg 2009 | CGM | SMBG (+ blinded CGM) | adults | The Netherlands | outpatients |
| Ludvigsson 2003 | CGM | SMBG (+ blinded CGM) | children | Sweden | outpatients |
| O'Connell 2009 | CGM | SMBG | adolescents, adults | Australia | outpatients |
| Peyrot 2009 | CGM | MDI + SMBG | adults | USA | outpatients |
| Raccah 2009 | CGM | SMBG | adults, children | France | outpatients |
| Tanenberg 2004 | CGM | SMBG | adults | USA | outpatients |
| Yates 2006 | CGM | SMBG | children | Australia | outpatients |
|
Footnotes "‐" denoted not reported Abbreviations: C: control; CGM: continuous glucose monitoring; I: intervention; MDI: multiple daily injections; NA: not acknowledged; SMBG: standard monitoring of blood glucose | |||||
Appendix 4. Baseline characteristics (II)
|
Characteristic ► Study ID ▼ |
Sex [female%] | Age [mean years (SD) or (range)] | Duration of disease [mean years (SD) or (range)] | Ethnic groups [%] |
Duration of intervention [time] |
Duration of follow‐ up [time] |
| Battelino 2011 | I: 42 C: 33 | I: 25.7 (14.1) C: 26.0 (14.6) | I: 11.6 (11.3) C: 11.4 (11.4) | ‐ | 6 months | 6 months |
| Bergenstal 2010 | I: 57 C: 56 | Adults: I: 41.9 (12.3) C: 40.6 (12.0) Children: I: 11.7 (3.0) C: 12.7 (3.1) | Adults: I: 20.2 (12.2) C: 20.2 (11.7) Children: I: 4.7 (3.1) C: 5.4 (3.7) | I: White: 91 Hispanic: 3 Other: 7 C: White: 92 Hispanic: 3 Other: 5 | 12 months | 12 months |
| Chase 2001 | 45.5 | I: 14.8(2.2) C: 12.0(0.6) | I: 10.0 (0.7) C: 9.0 (1.2) | ‐ | 30 days, 18 sensor days | 3 months |
| Chico 2003 | 50.5 | I: 36.5 (12) in the type 1 group, 58 (11) in the type 2 group C: 41 (10) | I: 17 (12) in the type 1 group, 12 (8) in the type 2 group C: 21 (10) | ‐ | 3 days | 3 months |
| Cooke 2009 | 54.7 | 52 (41‐63) (Median (IQR)) | 16 (10‐25) (Median (IQR)) | ‐ | 3 times 72 hours during the first phase of the trial, then an additional 3 times during the second phase for the CGMS. For the glucowatch a minimum of 4 times per month during the first phase of the trial, then ad libitum during the second phase. | 18 months |
| Cosson 2009 | 38.2 | I: 57.2 (4.4) in the type 2 group, 47.3 (7.1) in the type 1 group C: 57.3 (5.9) in the type 2 group, 52.0 (12.7) in the type 1 group | I: 10.5 (8.0) in the type 2 group, 15.0 (2.7) in the type 1 group C: 12.6 (9.9) in the type 2 group, 21.1 (9.9) in the type 1 group | ‐ | 48 hours | 3 months |
| Deiss 2006 | ‐ | total: 11.1 (2.3‐16.3) | total: 2.1 (0.2‐7.1) | ‐ | NA | 6 months |
| Deiss 2006a | ‐ | 14.4 (8.0‐18.9) for children 39.1 (19.5‐59.5) for adults | total: 2.1 (0.2‐7.1) | ‐ | continuous (3 months) or bi‐weekly 3 day periods | 3 months |
| Hermanides 2011 | I: 22 C: 18 | I: 39.3 (11.9) C: 37.3 (10.7) | I: 16.9 (10.7) C: 21.0 (9.4) | ‐ | 6 months | 6 months |
| Hermanns 2009 | ‐ | Total: 41.7 (12.3) | total: 14.75 (11.9) | ‐ | 2 times 48 hours | 3 months |
| Hirsch 2008 | 53.4 | I: 33.2 (16.39) C: 33.0 (14.60) | I: 16.7 (10.49) C: 20.8 (12.41) | Asian: 1.4 Black: 1.4 Latino: 7.2 White: 89.9 | 26 weeks | 26 weeks |
| Juvenile 2008 | 55.9 | I: 41.2 (11.6) in the >25 years group, 18.8 (3.0) in the 15‐24 years group, 11.4 (2.0) in the 8‐14 years group C: 44.6 (12.3) in the >25 years group, 18.2 (2.7) in the 15‐25 years group, 11.6 (2.1) in the 8‐14 years group | I: 23.6 (10.6) in the >25 years group, 9.5 (4.8) in the 15‐24 years group, 6.2 (3.1) in the 8‐14 years group C: 21.8 (10.4) in the >25 years group, 8.8 (4.0) in the 15‐25 years group, 5.3 (2.8) in the 8‐14 years group | White: 91.9 Other: 8.1 | 26 weeks | 26 weeks |
| Juvenile 2009 | 52.7 | I: 29.3 (16.3) C: 32.0 (17.7) | I: 25.6 (16.6) in the >25 years group, 8.7 (5.3) in the 15‐24 years group, 4.9 (2.6) in the 8‐14 years group C: 28.6 (12.7) in the >25 years group, 8.1 (4.5) in the 15‐25 years group, 4.4 (3.2) in the 8‐14 years group | White: 93.8 Other: 6.2 | 26 weeks | 26 weeks |
| Kordonouri 2010 | 51.2 | I: 8.5 (4.6) C: 9.1 (4.2) | study started immediately after diagnosis | ‐ | 52 weeks | 52 weeks |
| Lagarde 2006 | 55.5 | I: 9.94 (3.2) C: 14.22 (2.9) | I: 4.5 (2.5) C: 4.2 (2.1) | White: 96.3 Black: 3.7 | 72‐h period at 0,2 and 4 months | 6 months |
| Logtenberg 2009 | 58.3 | total: 43.8 (12.5) | total: 24.2 (9.7) | ‐ | 6 days | 12 days |
| Ludvigsson 2003 | ‐ | total: 12.5 (3.3) | total: 7.0 (3.9) | ‐ | 3 days every 2 weeks for 3 months | 24 weeks |
| O'Connell 2009 | 50 | I: 23.4 (8.6) C: 23.0 (8.1) | I: 11.1(7.6) C: 9.2(7.2) | ‐ | 3 months | 3 months |
| Peyrot 2009 | ‐ | total: 47.2 (13.2) | total: 25.0 (12.6) | ‐ | 3 months | 3 months |
| Raccah 2009 | I: 45.5 C: 43.3 | I: 28.1 (15.1) C: 12.3 (8.8) | I: 11.2 (9.0) C: 12.3 (8.8) | ‐ | 6 months | 6 months |
| Tanenberg 2004 | 50.8% | I: 44.0 (10.2) C: 44.5 (12.6) | I: 20.4 (10.7) C: 19.5 (11.9) | White: 84.4 Other: 15.6 | 12 weeks | 12 weeks |
| Yates 2006 | 67 | I: 14.7 (13.6‐14.4) C: 14.1 (12.8‐15.3) | ‐ | ‐ | 3 months | 6 months |
|
Footnotes "‐" denoted not reported Abbreviations: C: control; CGM: continuous glucose monitoring; I: intervention; MDI: multiple daily injections; NA: not acknowledged; SMBG: standard monitoring of blood glucose; | ||||||
Appendix 5. Matrix of study endpoints
|
Characteristic ► Study ID ▼ |
Intervention(s) | Control(s) | Primary1 endpoints | Secondary2 endpoint(s) | Other3 endpoint(s) |
| Battelino 2011 | CGM | SMBG and blinded CGM | time spent in hypoglycaemia | HbA1c adverse events | |
| Bergenstal 2010 | CGM | SMBG with MDI | HbA1c | severe hypoglycaemia | NA |
| Chase 2001 | CGM | SMBG | HbA1c | quality of Life (DCCT questionnaire) | change in insulin dose, fear of hypoglycaemia, nocturnal hypoglycaemia (glucose levels <3.25 mmol/L) |
| Chico 2003 | CGM | SMBG | HbA1c | asymptomatic hypoglycaemia (glucose levels <3.3 mmol/L) | distribution of hypoglycaemia, ease of use, confidence in use. |
| Cooke 2009 | CGM | SMBG | HbA1c | HbA1c change at 3, 6 and 12 months, proportion of patients reaching 12.5% reduction in HbA1c levels. Glucose levels <3.5 mmol/L | NA |
| Cosson 2009 | CGM | blinded CGM | HbA1c | glucose control, glucose variability, hypoglycaemia | tolerability and acceptability |
| Deiss 2006 | CGM | blinded CGM | HbA1c | fructosamine, average glucose per 24 hours, number of glucose excursions, duration and area under the curve (AUC) above 10·6 and below 3·9 mmol/L | NA |
| Deiss 2006a | CGM | SMBG | HbA1c | SMBG measurements, insulin dose, severe hypoglycaemia | NA |
| Hermanides 2011 | CGM | SMBG and blinded CGM | HbA1c | CGM derived time spent in hyperglycaemia and hypoglycaemia. Number of hypo‐ and hyperglycaemic events per day. Sensor use. Proportion of patients reaching HbA1c <7%, contact time with study personnel, number of SMBG measurements per 3 weeks, insulin dose. | Health‐related quality of life (36‐item Short Form version 2). The Problem Areas in Diabetes Scale. The Diabetes Treatment Satisfaction Questionnaire. The 13‐item worry subscale of the Hypoglycaemia Fear Survey. |
| Hermanns 2009 | CGM | blinded CGM | CGM satisfaction scale | SAT advantage, SAT disadvantage, mean duration CGM, MARD, correlation coefficient between sensor and reference glucose, mean glucose values, time spent in euglyceamia, time spent in hyperglycaemia, time spent in hypoglycaemia | NA |
| Hirsch 2008 | CGM | SMBG | HbA1c | percentage of subjects achieving 7% HbA1c, hypoglycaemia and hyperglycaemia AUC and incidence. safety | NA |
| Juvenile 2008 | CGM | SMBG | HbA1c | time spent in hypoglycaemia, euglycaemia and hyperglycaemia. glucose variability. hypoglycaemic events | NA |
| Juvenile 2009 | CGM | SMBG | HbA1c | time spent in hypoglycaemia, euglycaemia and hyperglycaemia. glucose variability, hypoglycaemic events. | NA |
| Kordorouni 2010 | CGM | SMBG | HbA1c | fasting C‐peptide, glycaemic variability, sensor usage, adverse events, children's health related quality of life and parent's well being | NA |
| Lagarde 2006 | CGM | blinded CGM | HbA1c | mean daily area under the CGMS curve for glucose <70 mg/dL area under the curve, mean daily time <70 mg/dL, daily area under the CGMS curve for glucose >180 mg/dL. | NA |
| Logtenberg 2009 | CGM | blinded CGM | percentage of time spent in euglycaemia (4.0‐10.0 mmol/L) | percentage of time spent in hypoglycaemia and hyperglycaemia, the incidence of adverse effects, patient satisfaction and agreement of paired SMBG and RT‐CGM measurements | NA |
| Ludvigsson 2003 | CGM | blinded CGM | HbA1c | percentage of time spent in hypoglycaemia, occurrence of hypoglycaemia. | NA |
| O'Connell 2009 | CGM | SMBG | difference in the proportion of time in the target glycaemic range during the 3 month study period (derived from CGM, target range 4‐10 mmol/L) | HbA1c, time in hypoglycaemic (below or equal to 3.9 mmol/L) and hyperglycaemic (above or equal to 10.1 mmol/L) ranges and glycaemic variability | NA |
| Peyrot 2009 | CGM | SMBG | HbA1c | weight, reliability of measures, patient opinion | NA |
| Raccah 2009 | CGM | SMBG | HbA1c | mean glucose change and descriptive parameters for biochemical hyperglycaemia (>70 mg/dL). Daily insulin use. | NA |
| Tanenberg 2004 | CGM | SMBG | HbA1c | sensor performance, hypoglycaemia | NA |
| Yates 2006 | CGM | SMBG | HbA1c | adverse events, AUC, hypoglycaemia | NA |
|
Footnotes 1,2 as stated in the publication; 3 not stated as primary or secondary endpoint(s) in the publication Abbreviations: CGM: continuous glucose monitoring; HbA1c: glycosylated haemoglobin A1c; MDI: multiple daily injections; NA: not acknowledged; SMBG: standard monitoring of blood glucose |
|||||
Appendix 6. Adverse events (I)
|
Characteristic ► Study ID ▼ |
Intervention(s) | Control(s) | Deaths [n] | Adverse events [n (%)] | Serious adverse events [n (%)] | Drop‐outs due to adverse events [n (%)] | Hospitalisation [n (%)] |
| Battelino 2011 | CGM | SMBG and blinded CGM | none | ‐ | total: 4 | none | none |
| Bergenstal 2010 | CGM | SMBG with MDI | none | I: 32 C: 27 | I: 2 C: 1 total: 3 | ‐ | I: 2 |
| Chase 2001 | CGM | SMBG | none | ‐ | none | none | none |
| Chico 2003 | CGM | SMBG | none | I: 8 C: NA total: NA | ‐ | ‐ | ‐ |
| Cooke 2009 | CGM | SMBG | 8 | total: 30 | ‐ | ‐ | 2 patients visited E.R. |
| Cosson 2009 | CGM | blinded CGM | none | I: 4 (5.8) C: NA total: NA | none | none | none |
| Deiss 2006 | CGM | blinded CGM | none | total: 60 | total: 7 | ‐ | ‐ |
| Deiss 2006a | CGM | SMBG | none | ‐ | ‐ | ‐ | ‐ |
| Hermanides 2011 | CGM | SMBG and blinded CGM | none | I: 4 (9) C: 1 (3) | I: 2 C: 5 | C: 1 | incomplete data |
| Hermanns 2009 | CGM | blinded CGM | none | I: 2.4 (5.5) C: 3.3 (2.9) | ‐ | ‐ | ‐ |
| Hirsch 2008 | CGM | SMBG | none | ‐ | total: 17 | ‐ | ‐ |
| Juvenile 2008 | CGM | SMBG | none | I: 4 C: 3 total: 5 (not including hypoglycaemia) | none | none | ‐ |
| Juvenile 2009 | CGM | SMBG | none | ‐ | none | none | ‐ |
| Kordonouri 2010 | CGM | SMBG | none | ‐ | ‐ | ‐ | ‐ |
| Lagarde 2006 | CGM | blinded CGM | none | none | none | none | none |
| Logtenberg 2009 | CGM | blinded CGM | none | total: 4 | none | none | none |
| Ludvigsson 2003 | CGM | blinded CGM | none | ‐ | ‐ | ‐ | ‐ |
| O'Connell 2009 | CGM | SMBG | none | total: 1 | none | none | total: 1 |
| Peyrot 2009 | CGM | SMBG | none | ‐ | I: 0 C: 4 total: 4 | none | ‐ |
| Raccah 2009 | CGM | SMBG | none | ‐ | I: 3 C: 7 total: 10 | ‐ | ‐ |
| Tanenberg 2004 | CGM | SMBG | none | total: 5 | I: 2 C: 1 total: 3 | ‐ | I: 4 C: 2 total: 6 |
| Yates 2006 | CGM | SMBG | none | none | total: 2 | ‐ | C: 1 |
|
Footnotes: ‐ denotes "not reported" Abbreviations: C: control; CGM: continuous glucose monitoring; E.R.: emergency room; I: intervention; MDI: multiple daily injections; SMBG: standard monitoring of blood glucose | |||||||
Appendix 7. Adverse events (II)
|
Characteristic ► Study ID ▼ |
Out‐patient treatment [n (%)] | Hypoglycaemic episodes [n (%)] | Severe hypoglycaemic episodes [n (%)] | Definition of severe hypoglycaemia | Nocturnal hypoglycaemic episodes [n (%)] | Symptoms [n (%)] |
| Battelino 2011 | none | ‐ | none | <63 mg/dL | ‐ | ‐ |
| Bergenstal 2010 | ‐ | ‐ | I: 32 C: 27 | ‐ | ‐ | ‐ |
| Chase 2001 | none | I: 12.8 (1.6) (mean (SEM) per participant) C: 6.7 (1.1) (mean (SEM) per participant) | ‐ | ‐ | I: 20 C: NA total: 40 | I: 3 C: NA total: 4 (10) |
| Chico 2003 | ‐ | I: 81 C: NA total: NA | ‐ | ‐ | ‐ | ‐ |
| Cooke 2009 | ‐ | Glucowatch: (9.4), CGMS: (8.5) Control: (7.8) Attention control: (6.7) | ‐ | ‐ | ‐ | ‐ |
| Cosson 2009 | none | I: 0(0) (mean (SEM)) C: 0.58 (1.08) (mean(SEM)) | ‐ | ‐ | ‐ | ‐ |
| Deiss 2006 | ‐ | I: 0.027 ± 0.041 mmol/L*24h in the continuous group, 0.041 ± 0.086 mmol/L*24h in the biweekly group. C: 0.023 ± 0.042 mmol/L*24h | total: 2 | ‐ | ‐ | ‐ |
| Deiss 2006a | ‐ | ‐ | I: 2 C: 0 total: 2 | ‐ | ‐ | ‐ |
| Hermanides 2011 | incomplete data | I: 0.7 (0.1) (mean(SD)) C: 0.5 (0.5) (mean(SD)) | I: 4 (9) C: 1 (3) | glucose ≤ 2.8 mmol/L resulting in seizure or coma for which i.v. glucose / glucagon or any form of third party assistance is necessary | ‐ | I: 2.4 (1.2) (mean (SD)) C: 2.2 (1.3) (mean (SD)) |
| Hermanns 2009 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Hirsch 2008 | ‐ | I: 0.8828 (0.756) C: 1.1663 (0.744) total: 17 | I: 11 C: 6 total: 17 | ‐ | ‐ | ‐ |
| Juvenile 2008 | ‐ | ‐ | I: 79.2 per 100 person‐yr C: 74.6 per 100 person‐yr | ‐ | ‐ | ‐ |
| Juvenile 2009 | ‐ | ‐ | I: 7(10) C: 7(11) | ‐ | ‐ | ‐ |
| Kordonouri 2010 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Lagarde 2006 | none | I: 1.2 (2.2) C: 0.67 (1.0) | I: 0 C: 0 total: 0 | severe central nervous system symptoms consistent with hypoglycaemia (mental status changes, vision disturbances, speech disturbances, loss of consciousness, and seizures), where the participant was unable to treat him/herself along with one or both of the following (1): capillary BG (CBG) < 50 mg/dL or (2) reversal of symptoms after glucose intake or glucagon administration | ‐ | ‐ |
| Logtenberg 2009 | none | total: (1.9) | ‐ | ‐ | ‐ | ‐ |
| Ludvigsson 2003 | ‐ | total: 0.8 episodes/day | ‐ | ‐ | total: 0.4 episodes/night | ‐ |
| O'Connell 2009 | none | I: 9.2 ± 8.7% of time C: 9.1 ± 6.9% of time | none | an episode of hypoglycaemia resulting in seizure or coma or requiring third‐party assistance or the use of glucagon or intravenous glucose for recovery | ‐ | ‐ |
| Peyrot 2009 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Raccah 2009 | ‐ | I: 0.1 (0.9) (mean (SEM)) episodes/day C: 0.1 (0.7) (mean (SEM)) episodes/day | I: 1 C: 0 | ‐ | ‐ | ‐ |
| Tanenberg 2004 | ‐ | I: 1.9 (1.6) mean (SEM) events/week C: 2.3 (2.3) mean (SEM) events/week | I: 9 C: 13 total: 22 | ‐ | ‐ | ‐ |
| Yates 2006 | ‐ | I: 0.7 events/day C: 0.4 events/day | none | hypoglycaemia causing coma or seizure | total: 18 | total: 6 |
|
Footnotes: ‐ denotes "not reported" Abbreviations: BG: blood glucose; C: control; I: intervention; i.v.: intravenous; SD: standard deviation; SEM: standard error of the mean | ||||||
Data and analyses
Comparison 1. Children ‐ Retrospective CGM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in HbA1c | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Follow up 3 months | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Follow up 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Severe hypoglycaemia | 4 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Follow up 6 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Follow up 3 months | 4 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Minor hypoglycaemica | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Follow up 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 CGM‐derived hypoglycaemia | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Follow up 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Ketoacidosis | 2 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Follow up 3 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Follow up 6 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 CGM‐derived hypoglycaemia | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Follow up 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 CGM‐derived hyperglycaemia | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Follow up 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Children ‐ Real‐time CGM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in HbA1c | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Follow up 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Follow up 6 months | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Follow up 12 months | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Improvement >0.5% in HbA1c | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Follow up 3 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Follow up 6 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Severe hypoglycaemia | 3 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Follow up 6 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Follow up 12 months | 2 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Ketoacidosis | 2 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Follow up 6 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Follow up 12 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Quality of life | 2 | 534 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.08, 0.26] |
| 5.1 Parents ‐ Follow up 6 months | 2 | 380 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.12, 0.28] |
| 5.2 Parents ‐ Follow up 12 months | 1 | 154 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.22, 0.42] |
| 6 CGM‐derived hypoglycaemia | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Follow up 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 CGM‐derived hyperglycaemia | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Follow up 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Adolescents ‐ Real‐time CGM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in HbA1c | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Follow up 3 months | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Follow up 6 months | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Improvement >0.5% in HbA1c | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Follow up 3 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Follow up 6 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Severe hypoglycaemia | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Follow up 6 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Ketoacidosis | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Follow up 6 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Adults ‐ Retrospective CGM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in HbA1c | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Follow up 3 months | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Severe hypoglycaemia | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Follow up 3 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 CGM‐derived hypoglycaemia | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Follow up 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 CGM‐derived hyperglycaemia | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Follow up 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Adults ‐ Real‐time CGM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in HbA1c | 6 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Follow up 3 months | 5 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Follow up 6 months | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Follow up 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Improvement >0.5% in HbA1c | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Follow up 3 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Follow up 6 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Severe hypoglycaemia | 4 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Follow up 3 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Follow up 6 months | 2 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Follow up 12 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Ketoacidosis | 4 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Follow up 3 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Follow up 6 months | 2 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Follow up 12 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Quality of life | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Physical health ‐ Follow up 6 months | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Mental health ‐ Follow up 6 months | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Patient satisfaction | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Follow up 3 months | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Follow up 6 months | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 CGM‐derived hypoglycaemia | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Follow up 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 CGM‐derived hypoglycaemia | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Follow up 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 CGM‐derived hyperglycaemia | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 Follow up 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 CGM‐derived hyperglycaemia | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 Follow up 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. All age groups ‐ Real‐time CGM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in HbA1c | 6 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Follow up 3 months ‐ continuous | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Follow up 6 months ‐ continuous | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Follow up 3 months ‐ intermittent | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Severe hypoglycaemia | 5 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Follow up 3 months ‐ continuous | 2 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Follow up 6 months ‐ continuous | 3 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Follow up 3 months ‐ intermittent | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Ketoacidosis | 5 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Follow up 3 months ‐ continuous | 2 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Follow up 6 months ‐ continuous | 3 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Follow up 3 months ‐ intermittent | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 CGM‐derived hypoglycaemia (change from baseline) | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Follow up 3 months ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Follow up 3 months ‐ intermittent | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Follow up 6 months ‐ continuous | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 CGM‐derived hypoglycaemia (change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Follow up 3 months ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Follow up 3 months ‐ intermittent | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Follow up 6 months ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 CGM‐derived hypoglycaemia (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 CGM‐derived hypoglycaemia (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Follow up 6 months ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 CGM‐derived hypoglycaemia | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Follow up 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 CGM‐derived hyperglycaemia (change from baseline) | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Follow up 3 months ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Follow up 3 months ‐ intermittent | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Follow up 6 months ‐ continuous | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 CGM‐derived hyperglycaemia (change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Follow up 3 months ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Follow up 3 months ‐ intermittent | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Follow up 6 months ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 CGM‐derived hyperglycaemia (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 CGM‐derived hyperglycaemia (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Follow up 6 months ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 CGM‐derived hyperglycaemia | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Follow up 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.2. Analysis.
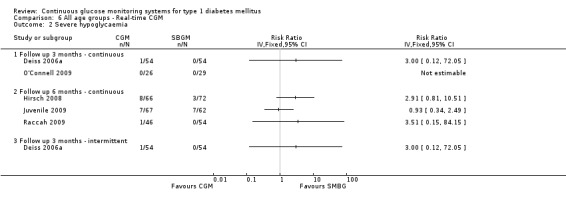
Comparison 6 All age groups ‐ Real‐time CGM, Outcome 2 Severe hypoglycaemia.
6.3. Analysis.
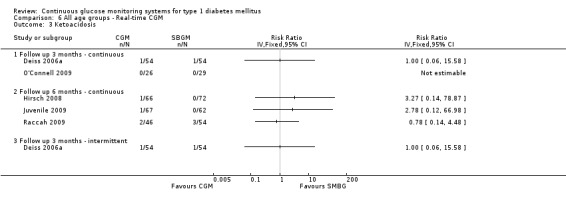
Comparison 6 All age groups ‐ Real‐time CGM, Outcome 3 Ketoacidosis.
6.4. Analysis.
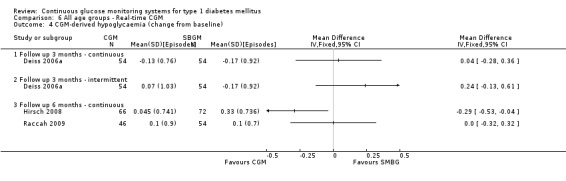
Comparison 6 All age groups ‐ Real‐time CGM, Outcome 4 CGM‐derived hypoglycaemia (change from baseline).
6.5. Analysis.
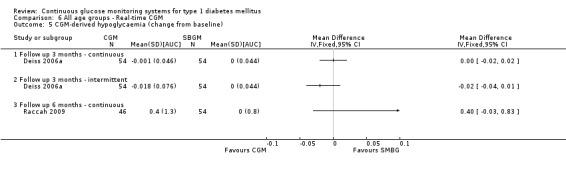
Comparison 6 All age groups ‐ Real‐time CGM, Outcome 5 CGM‐derived hypoglycaemia (change from baseline).
6.6. Analysis.
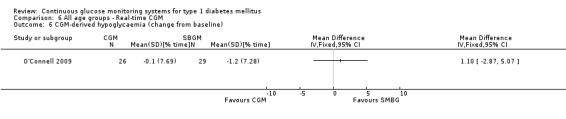
Comparison 6 All age groups ‐ Real‐time CGM, Outcome 6 CGM‐derived hypoglycaemia (change from baseline).
6.7. Analysis.
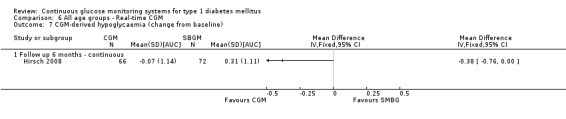
Comparison 6 All age groups ‐ Real‐time CGM, Outcome 7 CGM‐derived hypoglycaemia (change from baseline).
6.8. Analysis.
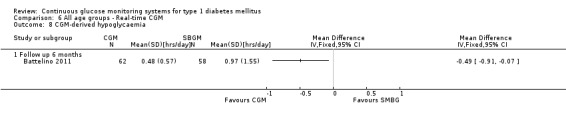
Comparison 6 All age groups ‐ Real‐time CGM, Outcome 8 CGM‐derived hypoglycaemia.
6.9. Analysis.
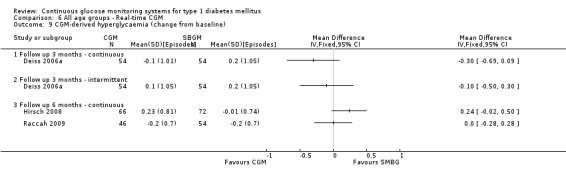
Comparison 6 All age groups ‐ Real‐time CGM, Outcome 9 CGM‐derived hyperglycaemia (change from baseline).
6.10. Analysis.
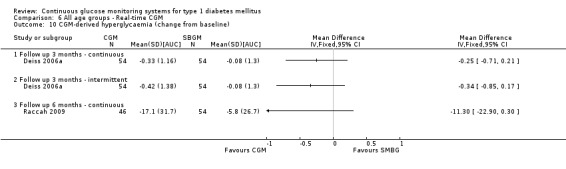
Comparison 6 All age groups ‐ Real‐time CGM, Outcome 10 CGM‐derived hyperglycaemia (change from baseline).
6.11. Analysis.
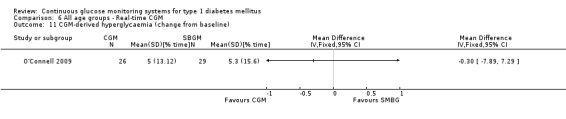
Comparison 6 All age groups ‐ Real‐time CGM, Outcome 11 CGM‐derived hyperglycaemia (change from baseline).
6.12. Analysis.
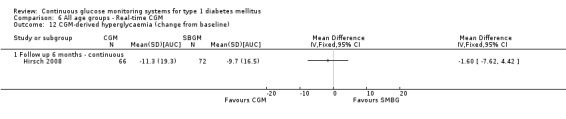
Comparison 6 All age groups ‐ Real‐time CGM, Outcome 12 CGM‐derived hyperglycaemia (change from baseline).
Comparison 7. Meta‐analysis ‐ CGM augmented pump therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in HbA1c | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Follow up 6 months | 2 | 562 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐0.82, ‐0.54] |
| 1.2 Follow up 12 months | 1 | 485 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐0.75, ‐0.45] |
| 2 Severe hypoglycaemia | 2 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Follow up 6 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Follow up 12 months | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Ketoacidosis | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Follow up 6 months | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Physical health ‐ Follow up 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Mental health ‐ Follow up 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Meta‐analysis ‐ Continuous Real‐time CGM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in HbA1c | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Follow up 6 months | 6 | 963 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.36, ‐0.09] |
| 1.2 Follow up 12 months | 1 | 154 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.46, 0.66] |
| 2 Severe hypoglycaemia | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Follow up 6 months | 4 | 689 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.63, 1.77] |
| 2.2 Follow up 12 months | 1 | 154 | Risk Ratio (IV, Random, 95% CI) | 0.11 [0.01, 2.08] |
| 3 Ketoacidosis | 4 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Follow up 6 months | 4 | 689 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.32, 2.26] |
| 4 Quality of life | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Physical health ‐ Follow up 6 months | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Mental health ‐ Follow up 6 months | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Parents ‐ Follow up 6 months | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Parents ‐ Follow up 12 months | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Meta‐analysis ‐ Intermittent Real‐time CGM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in HbA1c | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Follow up 3 months | 4 | 216 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.42, 0.05] |
| 2 Severe hypoglycaemia | 2 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Follow up 3 months | 2 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Ketoacidosis | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Follow up 3 months | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Battelino 2011.
| Methods | PARALLEL RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 120 patients; 62 in the CGM group and 58 in the CGM group SEX: 42% in the CGM group and 33% in the control group AGE (mean age (SD)): adults: 25.7 (14.1) in the CGM group and 26.0 (14.1) in the control group. 44% children in CGM group and 45% children in control group INSULIN PUMP USERS: 76% in CGM group and 59% in control group DURATION OF DISEASE (mean years (SD)): 11.6 (11.3) in the CGM group and 11.4 (11.4) in the control group. INCLUSION CRITERIA: aged between 10 and 65 years, type 1 diabetes diagnosed for more than 1 year, reasonable metabolic control assessing carbohydrate intake and self‐adjusting insulin, MDI or pump, HbA1c between <7.5% and not using a CGM device for at least 4 weeks. EXCLUSION CRITERIA: n.a. DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 6 months DURATION OF FOLLOW‐UP: 6 months |
|
| Interventions |
STUDY CENTRES: 3 COUNTRY: Slovenia, Israel, Sweden SETTING: outpatients CGM SYSTEM: FreeStyle Navigator CONTROL: SMBG and blinded CGM TREATMENT BEFORE STUDY: SMBG with MDI or insulin pump |
|
| Outcomes |
PRIMARY: amount of time per day spent in hypoglycaemia (<63 mg/dL) after 6 months SECONDARY: n.a. ADDITIONAL: HbA1c; amount of time per day spent in hyperglycaemia (>180 mg/dL or 250 mg/dL) or target range (70 to 180 mg/dL or 90 to 180 mg/dL); number of hypoglycaemic excursions (<55 and <63 mg/dL) per day and separately during the night period; adverse events |
|
| Study details |
RUN‐IN PERIOD: 1 month STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING: Abbott Diabetes Care and various other pharmaceutical companies NON‐COMMERCIAL FUNDING: Slovenian National Research Agency Grants PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "We designed a randomized, controlled, multicenter clinical trial to evaluate the effect of continuous glucose monitoring on hypoglycaemia in children and adults with type 1 diabetes." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were allocated to either group by permuted block randomization stratified according to age (10 to 17 years pediatric, 18 to 65 years adult) and study center. The randomization sequence was computer generated, and allocations were concealed using envelopes." |
| Allocation concealment (selection bias) | Low risk | "The randomization sequence was computer generated, and allocations were concealed using envelopes." |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: No blinding, but lack of blinding is not likely to influence outcome. |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Low risk | "The study was completed by 48 patients (83%) and 53 patients (85%), respectively." |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes reported. |
| Other bias | Low risk | |
| Inappropiate influence of sponsor prevented? | Low risk | "Abbott Diabetes Care provided funding, device‐related training, and analytical support. Abbott Diabetes Care was permitted to review themanuscript and suggest changes, but the final decision on content and submission of the manuscript was exclusively retained by the authors,who take responsibility for the accuracy and integrity of the data and analyses. This study was an investigator‐initiated trial. The study and protocol were designed by the investigators. The manuscript was prepared by the investigators." |
| Free of conflicts of interest | Low risk | Comment: Several authors received research grant support, however from various manufacturers. |
Bergenstal 2010.
| Methods | PARALLEL RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 485 patients, 329 adults and 156 children SEX: 274 males and 211 females AGE (mean age (SD)): Adults: 41.9 (12.3) in the CGM group and 40.6 (12.0) in the control group. Children: 11.7 (3.0) in the CGM group and 12.7 (3.1) in the control group. ETHNIC GROUPS: 14 Hispanic, 443 white, 28 other DURATION OF DISEASE (mean years (SD)): Adults: 20.2 (12.2) in the CGM group and 20.2 (11.7) in the control group. Children: 4.7 (3.1) in the CGM group and 5.4 (3.7) in the control group. INCLUSION CRITERIA: aged between 7 and 70 years, MDI for at least 3 months, HbA1c between 7.4 and 9.5%, under care for at least 6 months, access to a computer at home, history of SMBG average 4 times a day or more for the previous 30 days. EXCLUSION CRITERIA: Use of insulin pump therapy within previous 3 years, history of at least two severe hypoglycaemic events in the year before enrolment, use of pharmacologic non‐insulin treatment for diabetes during the previous 3 months, pregnancy or intention to become pregnant. DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 12 months DURATION OF FOLLOW‐UP: 12 months |
|
| Interventions |
STUDY CENTRES: not reported COUNTRY: United States and Canada SETTING: outpatients CGM SYSTEM: Minimed CGMS linked with Paradigm pump CONTROL: SMBG with MDI TREATMENT BEFORE STUDY: SMBG with MDI |
|
| Outcomes |
PRIMARY: HbA1c SECONDARY: severe rates of hypoglycaemia ADDITIONAL: n.a. |
|
| Study details |
RUN‐IN PERIOD: no STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING: Supported by Medtronic PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "In this unmasked, randomized, controlled trial, called Sensor‐Augmented Pump Therapy for A1C Reduction (STAR) 3, we evaluated the use of sensor‐augmented pump therapy and injection therapy at 30 diabetes centers in the United States and Canada for 1 year." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to receive either sensor‐augmented pump therapy (pump therapy) or a regimen of multiple daily injections (injection therapy) with the use of a block design, stratified according to age group: adults (19 to 70 years of age) or children (7 to 18 years of age)." |
| Allocation concealment (selection bias) | Unclear risk | "Patients were randomly assigned to receive either sensor‐augmented pump therapy (pump therapy) or a regimen of multiple daily injections (injection therapy) with the use of a block design, stratified according to age group: adults (19 to 70 years of age) or children (7 to 18 years of age)." Comment: no information on allocation concealment. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: No blinding, but outcome and the outcome measurement are not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Low risk | Comment: 9.3% in the CGM group and 11.6% in the SMBG group did not complete follow‐up. Reasons are provided and it is not likely that bias is introduced. |
| Selective reporting (reporting bias) | Low risk | Comment: Weight was not registered as an outcome in the protocol. No bias expected. |
| Other bias | Low risk | |
| Inappropiate influence of sponsor prevented? | Unclear risk | Comment: All data were transferred to the sponsor, Medtronic. The manuscript was written with editorial assistance from representatives of the sponsor. |
| Free of conflicts of interest | Unclear risk | Comment: Several authors received consulting fees, honoraria and grant support from Medtronic. |
Chase 2001.
| Methods | PARALLEL RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 12 children with type 1 diabetes were included in the study, randomised 1:1 to CGM or SMBG. 1 dropout in CGM group. INSULIN PUMP USERS: 55% SEX: 5 females, 6 males AGE (mean age (SD)): 14.8 (2.2) years in the CGM group and 12.0 (0.6) in the SMBG group. ETHNIC GROUPS: n.a. DURATION OF DISEASE (mean years (SD)): 10.0 (0.7) in the CGM group and 9.0 (1.2) in the SMBG group. INCLUSION CRITERIA: mean HbA1c >8.0% measured in the last 6 months, intensive insulin treatment, informed consent. EXCLUSION CRITERIA: n.a. DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 6 sensors (18 total sensor days) in a 30‐day period DURATION OF FOLLOW‐UP: 3 months |
|
| Interventions |
STUDY CENTRE: 1 COUNTRY: USA SETTING: outpatients CGM SYSTEM: CGMS (Metronic Minimed) CONTROL: SMBG |
|
| Outcomes |
PRIMARY: HbA1c SECONDARY: quality of life (DCCT questionnaire) ADDITIONAL: change in insulin dose, fear of hypoglycaemia, nocturnal hypoglycaemia (glucose levels <3.25 mmol/l), severe hypoglycaemia. |
|
| Study details |
RUN‐IN PERIOD: no STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING: Minimed proved the equipment for CGM NON‐COMMERCIAL FUNDING: Children's Diabetes Foundation, Denver PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "The purpose of this pilot trial was to determine whether continuous subcutaneous glucose monitoring might be helpful in detecting unrecognized nocturnal hypoglycemia and in lowering hemoglobin A1c (HbA1c) values in children with type 1 diabetes and poor glucose control." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The 11 children were randomised to either receive the CGMS or to serve as controls." Comment: no information on randomisation procedure. |
| Allocation concealment (selection bias) | Unclear risk | "The 11 children were randomised to either receive the CGMS or to serve as controls." Comment: no information on allocation concealment. |
| Blinding (performance bias and detection bias) Patient reported outcomes | Low risk | Comment: No blinding, but outcome and the outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: No blinding, but outcome and the outcome measurement are not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Comment: Only one participant did not successfully complete the trial. Reason is provided and it is not likely that bias is introduced. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes are reported. |
| Other bias | High risk | Comment: The difference in documented hypoglycaemic events is at least partially inherent to the study design: in the study group the number of hypoglycaemic events was counted by a continuous registration in contrast to the control group in which this number was based on far less numbers of glucose measurements. |
| Inappropiate influence of sponsor prevented? | Unclear risk | Comment: Research was funded by the Children’s Diabetes Foundation. Medtronic MiniMed Inc provided the equipment for continuous glucose monitoring. |
| Free of conflicts of interest | Unclear risk | Comment: No statement of conflicts of interest. |
Chico 2003.
| Methods | PARALLEL RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 105 patients, 75 with type 1 DM, randomised to CGM (n=40) or SMBG (n=30). All patients completed follow‐up. INSULIN PUMP USERS: 0% SEX: 35 males, 40 females AGE (mean age (SD)): 36.5 (12) in the T1DM CGM group, 41 (10) in the T1DM control group ETHNIC GROUPS: n.a. DURATION OF DISEASE (mean years (SD)): 17 (12) in the T1DM CGM group, 21 (10) in the T1DM control group. INCLUSION CRITERIA: inadequate metabolic control EXCLUSION CRITERIA: n.a. DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 3 days DURATION OF FOLLOW‐UP: 3 months |
|
| Interventions |
STUDY CENTRES: 1 COUNTRY: Spain SETTING: outpatients CGM SYSTEM: CGMS (Metronic Minimed) CONTROL: SMBG |
|
| Outcomes |
PRIMARY: HbA1c SECONDARY: asymptomatic hypoglycaemia (glucose levels <3.3 mmol/L) ADDITIONAL: distribution of hypoglycaemia, ease of use, confidence in use. |
|
| Study details |
RUN‐IN PERIOD: no STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING: not reported NON‐COMMERCIAL FUNDING: not reported PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "The three main objectives of this study are to investigate whether the CGMS is more useful than frequent capillary glucose measurements with a view to modifying treatment and improving metabolic control of type 1 diabetic subjects, to evaluate the incidence of unrecognized hypoglycemias in type 1 and type 2 diabetic patients, and to evaluate the actual incidence of technical problems related to CGMS use." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Type 1 diabetic patients with inadequate metabolic control were randomly assigned to either the group to be monitored with the CGMS (n=40) or the control group (n=35).” Comment: no further information on randomisation process is given. |
| Allocation concealment (selection bias) | Unclear risk | "Type 1 diabetic patients with inadequate metabolic control were randomly assigned to either the group to be monitored with the CGMS (n=40) or the control group (n=35).” Comment: no further information on allocation concealment is given. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: Lack of blinding, but unlikely to influence outcomes. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | Comment: Insufficient information, no flow chart. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes addressed. Non‐specified subgroup analyses, but only with respect to outcomes other than of our interest. |
| Other bias | Unclear risk | Comment: Badly reported study. |
| Inappropiate influence of sponsor prevented? | Unclear risk | Comment: Insufficient information. |
| Free of conflicts of interest | Unclear risk | Comment: Insufficient information. |
Cooke 2009.
| Methods | PARALLEL RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants | All data given from the original publication, data from sub‐analysis provided by author are given where available. WHO PARTICIPATED: 404 patients (201 patients with type 1 diabetes) INSULIN PUMP USERS: 2% SEX: 221 males, 183 females AGE (median age (IQR)): 52 (41‐63) ETHNIC GROUPS: n.a. DURATION OF DISEASE (mean years (IQR)): 16 (10‐25) INCLUSION CRITERIA: 18 years or older, duration of diabetes > 6 months, >2 injections daily or CSII with poor diabetes control EXCLUSION CRITERIA: prior use of studied devices, pregnancy, planned surgery, dialysis treatment, haemoglobinopathies, inability to use study devices. DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 3 times 72 hours during the first phase of the trial, then an additional 3 times during the second phase for the CGMS. For the glucowatch a minimum of 4 times per month during the first phase of the trial, then ad libitum during the second phase. DURATION OF FOLLOW‐UP: 18 months |
|
| Interventions |
STUDY CENTRES: 4 COUNTRY: United Kingdom SETTING: outpatients CGM SYSTEM: CGMS (Metronic Minimed), Glucowatch CONTROL: SMBG both standard and attention control |
|
| Outcomes |
PRIMARY: percentage change HbA1c from baseline to 18 months SECONDARY: HbA1c change at 3, 6 and 12 months, proportion of patients reaching 12.5% reduction in HbA1c levels. Glucose levels <3.5 mmol/L ADDITIONAL: distribution of hypoglycaemia, ease of use, confidence in use. |
|
| Study details |
RUN‐IN PERIOD: no STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English NON‐COMMERCIAL FUNDING: National Institute for Health Research, Health Technology Assessment Programme PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "Commencing in 2002, this trial was designed to address the limitations of previous studies by examining the impact on glycaemic control of two CGM devices that had received regulatory approval at that time [GlucoWatch G2 Biographer (Animas Corporation, West Chester, PA, USA) and the MiniMed continuous glucose monitoring system (CGMS; Medtronic, Northridge, CA, USA)]." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was concealed until the point of allocation and was carried out centrally by telephone. To reduce imbalance between groups, allocation was performed using minimization of three factors: centre, age and type of diabetes. The minimization algorithm contained a random element. Randomization was carried out centrally by telephone." |
| Allocation concealment (selection bias) | Low risk | "Randomization was concealed until the point of allocation and was carried out centrally by telephone. To reduce imbalance between groups, allocation was performed using minimization of three factors: centre, age and type of diabetes. The minimization algorithm contained a random element. Randomization was carried out centrally by telephone." |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: No blinding, but lack of blinding is unlikely to influence outcomes. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Comment: Comparable drop‐out rates in all groups. |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Low risk | Comment: Comparable drop‐out rates in all groups. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes measures reported. |
| Other bias | Low risk | Comment: Groups comparable at baseline. |
| Inappropiate influence of sponsor prevented? | Low risk | Comment: Study funded by the National Institute for Health Research. Two different types of CGM systems. |
| Free of conflicts of interest | Unclear risk | Comment: Various authors have received honoraria from CGM manufacturers. |
Cosson 2009.
| Methods | PARALLEL RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 48 patients with type 1 or 2 diabetes with poor glycaemic control, randomised to CGM (n=25) or control group (blinded CGM, n=23), 34 patients completed the study, of which 9 with type 1 diabetes (3 in CGM group and 6 in control group). INSULIN PUMP USERS: 78% SEX: 4 males, 5 females AGE (mean age (SD)): 47.3 (7.1) in the T1DM CGM group and 52.0 (12.7) in the T1DM control group. ETHNIC GROUPS: n.a. DURATION OF DISEASE (mean years (SD)): 15.0 (5.7) in the T1DM CGM group and 21.1 (9.9) in the T1DM control group. INCLUSION CRITERIA: patients with T1D, aged 18–70 years, treated with continuous insulin infusion or at least three insulin injections per day, and performing SMBG at least three times a day, or patients with T2D, 40–70 years of age, treated with oral antidiabetic agents with or without one insulin injection per day at a stable dosage over the past 3 months, and performing SMBG at least four times a week. HbA1c = 8.0–10.5%. Routine follow‐up (two to four visits) at the study centre for at least 1 year. No previous experience with CGM. EXCLUSION CRITERIA: pregnancy, acute disease with subsequent poor glycaemic control, proliferative retinopathy and renal failure, defined as a creatinine clearance below 30 mL/min. DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 48 hours DURATION OF FOLLOW UP: 3 months |
|
| Interventions |
STUDY CENTRES: 5 COUNTRY: France SETTING: outpatients CGM SYSTEM: GlucoDay (Menarini) CONTROL: blinded CGM |
|
| Outcomes |
PRIMARY: HbA1c SECONDARY: Glucose control, glucose variability, hypoglycaemia. ADDITIONAL: tolerability and acceptability |
|
| Study details |
RUN‐IN PERIOD: no STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING: Menarini Diagnostics PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "The objective of the present study was to evaluate the impact of CGM on glycaemic control using the GlucoDay® system for 48 h in adults with T1D and those with insulin‐requiring or non‐insulin‐requiring T2D." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A randomisation scheme was centrally generated, by computer, for each study hospital and according to diabetes type (1 or 2), using a 1:1 ratio. A randomisation scheme was centrally generated, by computer." |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Commenet: Lack of blinding unlikely to influence outcome |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | Comment: In the CGM group, 7/25 patients did not receive the intervention (evaluation based on CGM) because their sensor data were either inadequate or not sufficiently reliable. This might be related to outcomes. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes reported. |
| Other bias | High risk | Comment: Discrepancies at baseline, see table 1 of article. |
| Inappropiate influence of sponsor prevented? | Unclear risk | Comment: Sponsor: Menarini. |
| Free of conflicts of interest | Unclear risk | Comment: Conflicts of interest not stated. |
Deiss 2006.
| Methods | CROSS‐OVER RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 30 children with type 1 diabetes. 15 were randomised to start with open CGM and 15 patients with blinded CGM (control group). No dropouts. INSULIN PUMP USERS: 0% SEX: 16 boys and 14 girls AGE (median age (range)): 11.1 (2.3‐16.3) years ETHNIC GROUPS: n.a. DURATION OF DISEASE (mean years (range)): 2.1 (0.2‐7.1) INCLUSION CRITERIA: type 1 diabetes EXCLUSION CRITERIA: n.a. DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 2 x 3 months DURATION OF FOLLOW‐UP: 6 months for total period, crossover after 3 months |
|
| Interventions |
STUDY CENTRES: 1 COUNTRY: Germany SETTING: outpatients CGM SYSTEM: CGMS (Metronic Minimed) CONTROL: blinded CGM |
|
| Outcomes |
PRIMARY: HbA1c SECONDARY: n.a. ADDITIONAL: n.a. |
|
| Study details |
RUN‐IN PERIOD: no STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING: Medtronic Minimed PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "We aimed to study whether a single use of the CGMS may positively influence glycaemic control and improve HbA1c in a represententative cohort of young patients with type 1 diabetes." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were stratified according to their pubertal stage and randomly assigned to arm A (open) or arm B (blinded)." Comment: Insufficient data about sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | "Patients were stratified according to their pubertal stage and randomly assigned to arm A (open) or arm B (blinded)." Comment: Insufficient data about sequence generation. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: Crossover study with an open arm and a blinded arm; outcome measurement is not likely to be influenced by lack of blinding of the second arm. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Comment: No dropouts. Complete 24 hour CGMS profiles were available in 23 patients (77%) at the first study point , 27 patients (90%) at the second and 25 (83%) of 30 patients at third study point. No reasons for less than 18 hour measurements reported. |
| Selective reporting (reporting bias) | Low risk | Comment: The study protocol is not available, but the published report includes all the pre‐specified outcomes. |
| Other bias | Unclear risk | Comment: Mean HbA1c was slightly lower in A than in B at baseline (7.8 vs 8.4, p=0.146). Clinically relevant, but no statistically significant difference. |
| Inappropiate influence of sponsor prevented? | Unclear risk | "This study was kindly supported by a research grant Metronic MiniMed Inc., Germany". |
| Free of conflicts of interest | Unclear risk | Comment: Insufficient information. |
Deiss 2006a.
| Methods | PARALLEL RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 81 children and 81 adults with type 1 diabetes, randomised 1:1:1 to two CGM (both n=54) and 1 control group (n=54). 156 patients completed the study. INSULIN PUMP USERS: 48% SEX: CGM groups 54% and 30% males, SMBG 48% males AGE (mean years (SD)): CGM groups 26.2 (13.4) and 25.9 (14.0), SMBG 27.4 (16.5) ETHNIC GROUPS: n.a. DURATION OF DISEASE (mean years (SD)): CGM groups 12.4 (9.7) and 12.6 (8.5), SMBG 13.1 (10.8) INCLUSION CRITERIA: type 1 diabetes, Hba1c >8.1% despite intensive insulin treatment. EXCLUSION CRITERIA: hearing or vision impairment or other chronic illnesses DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: continuous (3 months) or bi‐weekly 3 day periods. DURATION OF FOLLOW‐UP: 3 months |
|
| Interventions |
STUDY CENTRES: 8 COUNTRY: Europe and Israel SETTING: outpatients CGM SYSTEM: Guardian RT (Medtronic Minimed), continuous or biweekly (intermittent) CONTROL: SMBG |
|
| Outcomes |
PRIMARY: HbA1c SECONDARY: SMBG measurements, insulin dose, severe hypoglycaemia. ADDITIONAL: n.a. |
|
| Study details |
RUN‐IN PERIOD: no STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING: Medtronic PUBLICATION STATUS: Peer review journal (published as brief report; complete manuscript from author) |
|
| Stated aim of study | "In contrast to previous studies using retrospective CGM data, the present study evaluates a new real‐time glucose monitor that allows the user to see the glucose readings and to set hypo‐ and hyperglycaemic alarms. The device, Guardian®RT (Medtronic MiniMed Inc., Northridge, CA), also provides trend information on how fast and in which direction glucose values are changing. This is the first large‐scale, open‐label, randomised, controlled multicenter study to assess the impact of continuous real‐time glucose display and alerts on glycaemic control for patients with type 1 diabetes." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed by the study sponsor per center in alternating block sizes of 3 and 6, by means of a computergenerated scheme. Sites were distributed in sealed envelopes containing the treatment assignment, to be opened sequentially as patients were enrolled." |
| Allocation concealment (selection bias) | Low risk | "Randomisation was performed by the study sponsor per center in alternating block sizes of 3 and 6, by means of a computergenerated scheme. Sites were distributed in sealed envelopes containing the treatment assignment, to be opened sequentially as patients were enrolled." |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: Open study, but outcome and the outcome measurement are not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | Comment: In total, 156 patients (out of 162) completed the evaluation. One discontinued before the start of intervention. Four discontinued arm 1, and one discontinued arm 2 due to difficulties with continuous sensor use and/or alarms. No biased outcome assessment to be expected. |
| Selective reporting (reporting bias) | Low risk | Comment: All expected outcomes reported. |
| Other bias | High risk | |
| Inappropiate influence of sponsor prevented? | Unclear risk | Comment: Funding by CGM manufacturer "The sponsor of the study participated in study design, data collection, monitoring and data analysis. The authors had full access to the data. This report was prepared by the authors independently of the funding source, and although the sponsors were allowed to comment on the manuscript they had no right of veto over any of its contents." |
| Free of conflicts of interest | Unclear risk | Comment: (Many authors) received travel grants to participate in scientific meetings, and/or received consultancies and honoraria to contribute to advisory boards and/or educational meetings, or to do other research reimbursed by the medical device industry (Medtronic, Roche, Lifescan, Abbott Diabetes Care). |
Hermanides 2011.
| Methods | PARALLEL RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 83 adults, randomised to 44 in the CGM group and 39 in the control group. 78 patients completed the study INSULIN PUMP USERS: 0% SEX: CGM group 78% males, control 82% males AGE (mean years (SD)): CGM group 39.3 (11.9), control 37.3 (10.7) ETHNIC GROUPS: n.a. DURATION OF DISEASE (mean years (SD)): CGM group 16.9 (10.7), control 21.0 (9.4) INCLUSION CRITERIA: age 18‐65 years, type 1 diabetes at least one year, Hba1c ≥8.2% despite efforts to improve by re‐education, including insulin pump therapy availability. EXCLUSION CRITERIA: hearing or vision impairment or other chronic illnesses, pump treatment in the last 6 months. DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: continuous (6 months) DURATION OF FOLLOW‐UP: 6 months |
|
| Interventions |
STUDY CENTRES: 8 COUNTRY: Denmark, Switzerland, The Netherlands, Sweden, France, United Kingdom, Belgium, Italy. SETTING: outpatients CGM SYSTEM: Paradigm sensor‐augmented pump therapy (Medtronic Minimed) continuous CONTROL: SMBG with 2 times 6 day blinded CGM measurement without subsequent treatment advice. |
|
| Outcomes |
PRIMARY: HbA1c SECONDARY: CGM derived time spent in hyperglycaemia and hypoglycaemia. Number of hypo‐ and hyperglycaemic events per day. Sensor use. Proportion of patients reaching HbA1c <7%, contact time with study personnel, number of SMBG measurements per 3 weeks, insulin dose. ADDITIONAL: Questionnaires: Health‐related quality of life was assessed using the 36‐item Short Form version 2. The Problem Areas in Diabetes Scale is a 20‐item questionnaire that scores diabetes‐related physiological distress. The Diabetes Treatment Satisfaction Questionnaire comprises six items and is scored on a 0–36 scale, with higher scores indicating higher satisfaction.The 13‐item worry subscale of the Hypoglycaemia Fear Survey was administered. The Hypoglycaemia Fear Survey and the Problem Areas in Diabetes Scale could not be administered in all centres, because of lack of validated translations. |
|
| Study details |
RUN‐IN PERIOD: 6 days STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING: Medtronic PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "Therefore, we compared sensor‐augmented pump therapy to intensive multiple daily injection therapy in patients with suboptimally controlled Type 1 diabetes mellitus in a randomized controlled multi‐centre trial." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was stratified per centre in computer‐generated sequences unknown to the investigator. Via a secured Internet database (Oracle Corporation, Redwood City, CA, USA), the investigators performed the randomization. Computer generated sequences were used." |
| Allocation concealment (selection bias) | Low risk | "Randomization was stratified per centre in computer‐generated sequences unknown to the investigator. Via a secured Internet database (Oracle Corporation, Redwood City, CA, USA), the investigators performed the randomization. Computer generated sequences were used." |
| Blinding (performance bias and detection bias) Patient reported outcomes | Low risk | Comment: Unable to blind due to nature of intervention, however lack of blinding is unlikely to influence objective outcomes. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: Unable to blind due to nature of intervention, however lack of blinding is unlikely to influence objective outcomes. |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Low risk | "The trial was completed by 43/44 (98%) patients in the sensor‐augmented insulin pump group and 35/39 (90%) patients in the multiple daily injections group." |
| Selective reporting (reporting bias) | Low risk | Comment: All outcome measures reported. |
| Other bias | Low risk | Comment: Groups comparable at baseline. |
| Inappropiate influence of sponsor prevented? | Low risk | "The funding source had an advising role in trial design details and drafting of the report and was only involved in the collection of the sensor data. The funding source had no role in the conduct of the analyses, interpretation of the data or in the decision to approve publication." |
| Free of conflicts of interest | Unclear risk | Comment: Two authors received fees from Medtronic. |
Hermanns 2009.
| Methods | CROSS‐OVER RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 50 patients with type 1 diabetes, randomised 1:1 to start with open or blinded CGM. No dropouts. INSULIN PUMP USERS: 31% SEX: n.a. AGE (mean age (SD)): 41.7 (12.3) years ETHNIC GROUPS: n.a. DURATION OF DISEASE (mean years (SD)): 14.75 (11.9) INCLUSION CRITERIA: type 1 diabetes; diabetes duration of >6 months; age >18 years. EXCLUSION CRITERIA: current diagnosis of psychiatric disease DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 2 times 48 hours DURATION OF FOLLOW‐UP: 3 months |
|
| Interventions |
STUDY CENTRES: 1 COUNTRY: Germany SETTING: inpatients CGM SYSTEM: GlucoDay CONTROL: blinded CGM |
|
| Outcomes |
PRIMARY: CGM satisfaction scale SECONDARY: SAT advantage, SAT disadvantage, mean duration CGM, MARD, correlation coefficient between sensor and reference glucose, mean glucose values, time spent in euglycaemia, time spent in hyperglycaemia, time spent in hypoglycaemia. ADDITIONAL: n.a. |
|
| Study details |
RUN‐IN PERIOD: no STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING: Menarini Diagnostics PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "The current randomized crossover trial examines the effect of CGM with real‐time access (RTA) to glucose data and alarm functions versus CGM with a retrospective analysis (RA) of glucose data on satisfaction with CGM and other patientreported outcomes." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The order of these two conditions was randomised.” Comment: Method of sequence generation not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Method to conceal allocation not described |
| Blinding (performance bias and detection bias) Patient reported outcomes | Low risk | Comment: Crossover study with open arms; outcome and the outcome measurement are not likely to be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: Crossover study with open arms; outcome and the outcome measurement are not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Comment: Outcome data are complete. |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Low risk | Comment: Outcome data are complete. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Variables measuring glycaemic control (time spent during euglycaemia and in hypoglycaemic range) were not clearly predefined. |
| Other bias | Unclear risk | Comment: Baseline balance could not be checked. |
| Inappropiate influence of sponsor prevented? | Low risk | Comment: Unrestricted grant of Menarini. |
| Free of conflicts of interest | Unclear risk | Comment: Two authors are employees of Menarini Diagnostics, the manufacturer of the GlucoDay device. |
Hirsch 2008.
| Methods | PARALLEL RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 146 patients with type 1 diabetes, treated with insulin by pump, randomised to insulin pump with integrated CGM (n=72) or insulin pump + SMBG (n=74). 8 patients did not complete follow‐up INSULIN PUMP USERS: 100% SEX: 78 females, 60 males AGE (mean age (SD)): 33.2 (16.39) years in the CGM group, 33.0 (14.60) years in the control group. ETHNIC GROUPS: 2 Asian, 2 black, 10 Hispanic, 124 white. DURATION OF DISEASE (mean years (SD)): 16.7 (10.49) in the CGM group, 20.8 (12.41) years in the control group. INCLUSION CRITERIA: between the ages of 12 and 72 years. A1C > 7.5%, and were diagnosed with type 1 diabetes > 1 year prior to entering the study. CSII for at least 6 months. EXCLUSION CRITERIA: none DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 26 weeks DURATION OF FOLLOW‐UP: 26 weeks |
|
| Interventions |
STUDY CENTRES: 7 COUNTRY: USA SETTING: outpatients CGM SYSTEM: Paradigm (Medtronic Minimed) CONTROL: Insulin pump + SMBG |
|
| Outcomes |
PRIMARY: HbA1c SECONDARY: percentage of subjects achieving 7% HbA1c, hypoglycaemia and hyperglycaemia AUC and incidence. Safety ADDITIONAL: n.a. |
|
| Study details |
RUN‐IN PERIOD: no STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING: Medtronic Inc PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "The purpose of this was to assess the safety and clinical efficacy of a sensor‐augmented insulin pump in adolescent and adult subjects." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomised" Comment: Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | "Subjects were randomised" Comment: Insufficient information. |
| Blinding (performance bias and detection bias) Patient reported outcomes | Low risk | Comment: These episodes (severe hypoglycaemia and other serious adverse events) were reported by subjects in their workbook. Lack of blinding is not likely to influence outcomes. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: Laboratory values. No blinding, but lack of blinding is not likely to influence outcomes. |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Unclear risk | Comment: 11% dropout, only in CGM group. |
| Selective reporting (reporting bias) | Unclear risk | Comment: All predefined outcomes are presented. Subgroup analysis (adults and adolescents, for HbA1c) not predefined. |
| Other bias | High risk | Comment: No intention‐to‐treat analysis. |
| Inappropiate influence of sponsor prevented? | Unclear risk | "Supported by a grant from Medtronic Inc.". |
| Free of conflicts of interest | Unclear risk | Comment: Several authors received honoraria and grant support from Medtronic. |
Juvenile 2008.
| Methods | PARALLEL RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 322 patients with type 1 diabetes, in three age groups (n=CGM/ n=SMBG): 8‐14 years (56/58), 15‐25 years (57/53) and >25 years (52/46). 98% completed follow‐up. INSULIN PUMP USERS: 84% / 71% / 84% SEX: female: 49% / 61% / 58% AGE (mean age (SD)): 8‐14 years group: 11.4 (2.0) in the CGM group, 11.6 (2.1) in the control group. 15‐24 years group: 18.8 (3.0) for the CGM group, 18.2 (2.7) for the control group. >25 years group: 41.2 (11.2) for the CGM group, 44.6 (12.3) for the control group. ETHNIC GROUPS: 296 (92%) non‐Hispanic white race DURATION OF DISEASE (mean years (SD)): in the >25 years group: 23.6 (10.6) for the CGM group, 21.8 (10.4) for the control group. In the 15‐24 years group: 9.5 (4.8) for the CGM group, 8.8 (4.0) for the control group. In the 8‐14 years group: 6.2 (3.1) in the CGM group, 5.3 (2.8) in the control group. INCLUSION CRITERIA: 8 years of age or older, type 1 diabetes at least 1 year before randomisation, use an insulin pump or received at least three daily insulin injections, HbA1c level of 7.0 to 10.0%. EXCLUSION CRITERIA: use of CGM at home in the 6 months leading up to the trial. DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 6 months DURATION OF FOLLOW‐UP: 6 months |
|
| Interventions |
STUDY CENTRES: 10 COUNTRY: USA SETTING: outpatients CGM SYSTEM: Dexcom Seven (Dexcom), Paradigm Real‐Time Insulin Pump (Minimed Medtronic) and CGMs, Freestyle Navigator (Abbott) CONTROL: SMBG |
|
| Outcomes |
PRIMARY: HbA1c SECONDARY: time spent in hypoglycaemia, euglycaemia and hyperglycaemia. glucose variability. hypoglycaemic events. ADDITIONAL: n.a. |
|
| Study details |
RUN‐IN PERIOD: yes STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English NON‐COMMERCIAL FUNDING: Juvenile Diabetes Research Foundation PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "In this randomized, multicente, clinical trial, we evaluated the efficacy and safety of continuous glucose monitoring in adults and children with type 1 diabetes." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients meeting these criteria were randomly assigned to receiving CGM or home monitoring with the use of a permuted block design.” Comment: Sequence generation process not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Method to conceal allocation not described. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: Outcomes: lab‐measurements. Study staff not blinded and patients partly blinded (the control group had blinded CGM at 13 and 26 weeks). Lack of blinding is not likely to introduce bias. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Comment: Low drop‐out rates (<5%, Fig 1 supplementary appendix of article). |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Low risk | Comment: Low drop‐out rates (<5%, Fig 1 supplementary appendix of article). |
| Selective reporting (reporting bias) | Low risk | Comment: All predefined outcomes are reported. |
| Other bias | Low risk | |
| Inappropiate influence of sponsor prevented? | Low risk | Comment: Many different manufactures and pharmaceutical companies are listed in the acknowledgements. Supported by grants from the Juvenile Diabetes Research Foundation. |
| Free of conflicts of interest | Low risk | Comment: Several authors received lecture fees and grant support from the CGM manufacturers. Bias is not likely since different manufacturers are involved in this study. |
Juvenile 2009.
| Methods | PARALLEL RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 129 patients with type 1 diabetes, randomised to CGM (n=67) or control group (n=62). 127 patients completed follow‐up INSULIN PUMP USERS: 86% SEX: 68 females, 61 males AGE (mean age (SD)): 29.3 (16.3) in the CGM group, 32.0 (17.7) in the control group. ETHNIC GROUPS: 121 white DURATION OF DISEASE (mean years (SD)): in the >25 years group: 25.6 (16.6) for the CGM group, 28.6 (12.7) for the control group. In the 15‐24 years group: 8.7 (5.3) for the CGM group, 8.1 (4.5) for the control group. In the 8‐14 years group: 4.9 (2.6) in the CGM group, 4.4 (3.2) in the control group. INCLUSION CRITERIA: age > 8 years, type 1 diabetes for at least 1 year, use of either an insulin pump or at least three daily insulin injections, and baseline A1C level <7.0%. EXCLUSION CRITERIA: n.a. DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 26 weeks DURATION OF FOLLOW‐UP: 26 weeks |
|
| Interventions |
STUDY CENTRES: 10 COUNTRY: USA SETTING: outpatienta CGM SYSTEM: Dexcom SEVEN (Dexcom), Paradigm, (Metronic Minimed), Freestyle Navigator (Abbott) CONTROL: SMBG |
|
| Outcomes |
PRIMARY: HbA1c, quality of life SECONDARY: time spent in hypoglycaemia, euglycaemia and hyperglycaemia. glucose variability. hypoglycaemic events, hypoglycaemia fear survey ADDITIONAL: n.a. |
|
| Study details |
RUN‐IN PERIOD: yes STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English NON‐COMMERCIAL FUNDING: Juvenile Diabetes Research Fund PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "To evaluate the efficacy and safety of continuous glucose monitoring in adults and children with type 1 diabetes who had already succesfully achieved AC1 levels <7.0% with intensive insulin therapy." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Sequence generation process not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Method to conceal allocation not described. |
| Blinding (performance bias and detection bias) Patient reported outcomes | Low risk | Comment: Not blinded, but outcome and outcome measurement is not likely to be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: Not blinded, but outcome and outcome measurement is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Low risk | "Adjusting for imbalances between baseline factors and imputing for missing data using Rubin’s method (10) did not alter the results (data not shown)." Comment: Missing baseline data: Home glucose meter readings per day 6/67 intervention group and 4/62 of control group. Drop out rate: 1/67 intervention group vs 4/62 (wk13) and 2/62 (26wk) control group. Balanced and reasons given not likely to influence results. One subject in the CGM group was missing sensor data. Two subjects in the control group dropped out before the 26‐week visit. |
| Selective reporting (reporting bias) | Unclear risk | Comment: The study protocol is available (NCT00406133). Cost‐effectiveness of CGM is not (yet) not reported. |
| Other bias | Low risk | Comment: None of the mentioned reasons for potential threats are likely. |
| Inappropiate influence of sponsor prevented? | Low risk | Comment: Many different manufactures and pharmaceutical companies are listed in the acknowledgements. Also, they had no involvement in the design, conduct, or analysis of the trial or the preparation of this article. |
| Free of conflicts of interest | Low risk | Comment: Many different manufactures and pharmaceutical companies are listed in the acknowledgements. Also, they had no involvement in the design, conduct, or analysis of the trial or the preparation of this article. |
Kordonouri 2010.
| Methods | PARALLEL RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 160 patients with type 1 diabetes SEX: 80 females, 74 males AGE (mean age (SD)): 8.5 (4.6) in the CGM group, 9.1 (4.2) in the control group. ETHNIC GROUPS: n.a. DURATION OF DISEASE (mean years (SD)): study started immediately after diagnosis INCLUSION CRITERIA: diagnosed with type 1 diabetes within 4 weeks of inclusion date, aged 1 through 16 years EXCLUSION CRITERIA: n.a. DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 52 weeks DURATION OF FOLLOW‐UP: 52 weeks |
|
| Interventions |
STUDY CENTRES: 5 centres COUNTRY: Pan‐European SETTING: outpatients CGM SYSTEM: Medtronic Paradigm CONTROL: SMBG with CSII TREATMENT BEFORE STUDY: none |
|
| Outcomes |
PRIMARY: HbA1c after 12 months SECONDARY: fasting C‐peptide, glycaemic variability, sensor usage, adverse events, children's health‐related quality of life and parent's well being. ADDITIONAL: n.a. |
|
| Study details |
RUN‐IN PERIOD: no STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING: Medtronic Inc PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "To assess the acceptance, efficacy and safety of the use of CGM in combination with insulin pump therapy from the diagnosis of type 1 diabetes in children and adolescents. Particularly, we set out to determine whether the use of sensor‐augmented insulin pump therapy leads to better glycaemic control, lower daily insulin requirements, higher residual beta cell function, lower incidence of severe hypoglycaemia and better quality of life after 1 year of treatment compared with the use of a conventional insulin pump combined with conventional self‐monitoring of blood glucose." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were assigned by a central randomisation procedure." |
| Allocation concealment (selection bias) | Unclear risk | "Patients were assigned by a central randomisation procedure." |
| Blinding (performance bias and detection bias) Patient reported outcomes | Low risk | Comment: Patients and staff not blinded. Lack of blinding is not likely to introduce bias. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: Patients and staff not blinded. Lack of blinding is not likely to introduce bias. |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Low risk | Comment: Low drop‐out rates (4/80 in the CGM group and 2/80 in the SMBG group), reasons provided. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Weight and occurrence of hypoglycaemia below 70 mg/dl (3.9 mmol/l) during 24 hour expressed as AUC below 70 mg/dl (3.9 mmol/l) and occurrence of hyperglycaemia above 200 mg/dl (11.1 mmol/l) during 24 hours expressed as AUC were mentioned in the protocol, but not listed or reported in the article. |
| Other bias | Low risk | Comment: No baseline imbalances. |
| Inappropiate influence of sponsor prevented? | Unclear risk | Comment: Funding by Medtronic, no further details. |
| Free of conflicts of interest | Unclear risk | Comment: Investigator‐initiated trial, supported by Medtronic. Several authors received honoraria, consulting fees and travel reimbursement from Medtronic. |
Lagarde 2006.
| Methods | PARALLEL RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 27 children with type 1 diabetes, randomised to CGM (n=18) or SMBG (n=9). No dropouts. INSULIN PUMP USERS: 70% SEX: 15 females, 12 males AGE (mean age (SD)): 9.94 (3.2) in the CGM group, 14.22 (2.9) in the control group. ETHNIC GROUPS: 17 Caucasian and 1 African American in the CGM group, 9 Caucasian in the control group. DURATION OF DISEASE (mean years (SD)): 4.5 (2.5) years in the CGM group, 4.2 (2.1) years in the control group. INCLUSION CRITERIA: age 5‐17 years; a diagnosis of type 1 diabetes treated with insulin for 1 yr or more; availability for all study visits; and willingness to wear a medical device for 72 consecutive hours. EXCLUSION CRITERIA: history of acute metabolic decompensation such as diabetic ketoacidosis within 1 month of study enrolment; use of chronic medications known to affect glucose levels such as systemic corticosteroids; and pregnancy. DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 72‐h periods at 0, 2, and 4 months DURATION OF FOLLOW‐UP: 4 months |
|
| Interventions |
STUDY CENTRES: 1 COUNTRY: USA SETTING: outpatients CGM SYSTEM: CGMS (Metronic Minimed) CONTROL: blinded CGM |
|
| Outcomes |
PRIMARY: HbA1c SECONDARY: mean daily area under the CGMS curve for glucose <70 mg/dL area under the curve (AUC<70), mean daily time <70 (MDT<70), daily area under the CGMS curve for glucose >180 mg/dL (AUC>180). ADDITIONAL: n.a. |
|
| Study details |
RUN‐IN PERIOD: no STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English NON‐COMMERCIAL FUNDING: Agency for Health Care Research and Quality PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "The purpose of this study was to determine if the use of CGMS improves metabolic control in children with T1DM." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomised 2:1 into an intervention group (CGMS data utilized) or control group (CGMS data blinded) using a computer‐ generated randomisation list created by a statistician." |
| Allocation concealment (selection bias) | Low risk | "Allocation was concealed from the investigators, and the participants were blinded to which study group they were assigned." |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: HbA1c probably “Yes”. Other data from the devices “No” because they were used for review in the intervention group. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | Comment: All participants completed the study. |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes mentioned in the methods section, were reported. |
| Other bias | Unclear risk | Comment: Difference in baseline characteristics. Lower age in intervention group favours this group (see JDRF 2008a). |
| Inappropiate influence of sponsor prevented? | Unclear risk | Comment: Provision of study CGM devices by manufacturer. |
| Free of conflicts of interest | Unclear risk | Comment: Insufficient information. |
Logtenberg 2009.
| Methods | CROSS‐OVER RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 12 patients with type 1 diabetes on continuous intraperitoneal insulin infusion (CIPII), randomised 1:1 to start with open or blinded CGM. No dropouts. INSULIN PUMP USERS: 100% SEX: 7 females, 5 males AGE (mean age (SD)): 43.8 (12.5) ETHNIC GROUPS: na DURATION OF DISEASE (mean years (SD)):24.2 (9.7) INCLUSION CRITERIA: People with type 1 diabetes mellitus with less than adequate glycaemic control, defined as haemoglobin A1c (HbA1c) 7.5% and/or five or more incidents of hypoglycaemia (defined as SMBG measurement below 72mg/dL [4.0 mmol/L])per week, over 18 years of age, and treated with CIPII. EXCLUSION CRITERIA: Failure to obtain informed consent, any condition preventing proper handling of the device (for instance, hearing impairment or visual impairment), known allergy to sensor (parts), and currently pregnant or trying to conceive. DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 6 days DURATION OF FOLLOW‐UP: 12 days |
|
| Interventions |
STUDY CENTRES: 1 COUNTRY: Netherlands SETTING: outpatients CGM SYSTEM: Paradigm (Metronic Minimed) CONTROL: blinded CGM |
|
| Outcomes |
PRIMARY: percentage of time spent in euglycaemia (4.0‐10.0 mmol/L) SECONDARY:percentage of time spent in hypoglycaemia and hyperglycaemia, the incidence of adverse effects, patient satisfaction, and agreement of paired SMBG and RT‐CGM measurements. ADDITIONAL: n.a. |
|
| Study details |
RUN‐IN PERIOD: no STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING: no NON‐COMMERCIAL FUNDING: no PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "The objective of this study therefore is to investigate the effectiveness and safety of RT‐CGM in this patient category." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Randomization method not mentioned. |
| Allocation concealment (selection bias) | Low risk | "After informed consent was obtained, randomisation was performed using sealed opaque envelopes, which indicated the assigned mode of RT‐CGM in the first study phase; blinded or open, respectively." |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: No blinding not likely to influence these objective outcomes. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Comment: All patients completed the study. |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Comment: All outcomes mentioned in the methods section, were reported (but see also item 'free of other bias'). |
| Other bias | Low risk | Comment: The following subgroup analyses were not specified: “More narrow glucose ranges are also shown; there are no significant differences in percentage of time in any of these glucose ranges between open and blinded RT‐CGM use”. No major bias to be expected, however |
| Inappropiate influence of sponsor prevented? | Low risk | Comment: Sponsored, but not by CGM manufacturer. |
| Free of conflicts of interest | Low risk | Comment: Declaration of no competing financial interests. |
Ludvigsson 2003.
| Methods | CROSS‐OVER RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 32 patients with type 1 diabetes treated with intensive insulin therapy or insulin infusion pumps, randomised 1:1 to start with open CGM or blinded CGM (control group). 5 patients did not complete follow‐up. INSULIN PUMP USERS: 59% SEX: n.a. AGE (mean age (SD)): 12.5 (3.3) years ETHNIC GROUPS: n.a. DURATION OF DISEASE (mean years (SD)):7.0 (3.9) INCLUSION CRITERIA: type 1 diabetes, HbA1C of 8.0% or above EXCLUSION CRITERIA: pregnancy DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 3 days every 2 weeks for 3 months DURATION OF FOLLOW‐UP: 6 months for total period, crossover after 3 months |
|
| Interventions |
STUDY CENTRES: 1 COUNTRY: Sweden SETTING: outpatients CGM SYSTEM: CGMS (Metronic Minimed) CONTROL: blinded CGM |
|
| Outcomes |
PRIMARY: HbA1c SECONDARY: percentage of time spent in hypoglycaemia, occurrence of hypoglycaemia. ADDITIONAL: n.a. |
|
| Study details |
RUN‐IN PERIOD: no STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING: Minimed Inc PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "The aim of this study was to see if we could further improve metabolic control with the use of CGMS." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Half of the patients were randomised into an open study arm and the remaining patients into the blinded arm.” Comment: Sequence generation process not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Method to conceal allocation not described. |
| Blinding (performance bias and detection bias) Patient reported outcomes | Low risk | Comment: Outcome: severe hypoglycaemia. Patients and staff not blinded. Lack of blinding is not likely to introduce bias |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: Outcomes: lab‐measurements. Patients and staff not blinded. Lack of blinding is not likely to introduce bias |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | Comment: Dropout rate = 15%. Dropouts not reported per study arm. “5 patients did not complete all 12 of the sensor evaluation periods; however, their HbA1c results were included in the statistical analysis on a intention‐to‐treat basis." Comment: Same 5 patients as dropouts? HbA1c measured in all patients at baseline, 3 and 6 months? |
| Selective reporting (reporting bias) | Low risk | Comment: All predefined outcomes are reported. |
| Other bias | High risk | Comment: Crossover after 3‐months: carry over effect not mentioned or investigated. No baseline characteristics per study arm. |
| Inappropiate influence of sponsor prevented? | Unclear risk | “Unrestricted grant from Minimed Inc.” Comment: Medtronic Minimed provided the sensors. |
| Free of conflicts of interest | Unclear risk | Comment: One author had received speakers’ honoraria from Minimed. |
O'Connell 2009.
| Methods | PARALLEL RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 62 patients with type 1 diabetes on CSII, randomised 1:1. 54 patients completed follow‐up INSULIN PUMP USERS: 100% SEX: 29% males in each group AGE (mean age (SD)): 23.4 (8.6) in the CGM group, 23.0 (8.1) in the control group. ETHNIC GROUPS: n.a. DURATION OF DISEASE (mean years (SD)): 11.1 (7.6) in the CGM group, 9.2 (7.2) in the control group. INCLUSION CRITERIA: age 13.0‐40.0 years, type 1 diabetes for >1 year, use of insulin pump therapy including proficiency with use of a bolus‐dose calculator for >3 months, HbA1c under or equal to 8.5%, reliably performing self‐monitoring of blood glucose (SMBG) at least four times daily, and internet access. Willingness to use the subcutaneous sensor component of the system for at least 70% of the total 3 month study period was a further protocol requirement. EXCLUSION CRITERIA: co‐existent medical problems that would interfere with their ability to use the system (e.g. impaired vision), co‐existent illness that otherwise predisposes to hypoglycaemia (e.g. adrenal insufficiency) or a history of severe hypoglycaemia while using insulin pump therapy DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 3 months DURATION OF FOLLOW UP: 3 months |
|
| Interventions |
STUDY CENTRES: 5 COUNTRY: Australia SETTING: outpatients CGM SYSTEM: Paradigm (Metronic Minimed) CONTROL: SMBG |
|
| Outcomes |
PRIMARY: difference in the proportion of time in the target glycaemic range during the 3 month study period (derived from CGM, target range 4?10 mmol/l) SECONDARY:HbA1c, time in hypoglycaemic ( below or equal to 3.9 mmol/l) and hyperglycaemic (above or equal to 10.1 mmol/l) ranges and glycaemic variability. ADDITIONAL: n.a. |
|
| Study details |
RUN‐IN PERIOD: no STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING: Medtronic inc PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "The aim of this study, therefore, was to assess the impact of patientled use of sensor‐guided pump management on indices of glycaemic control in adolescents and young adults with type 1 diabetes and compare the impact with that of standard insulin pump therapy." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Once recruited, a pair of participants was entered, in order of study number, into a computer generated schedule which randomly assigned each of the pair to one of the two study groups. The randomisation schedule was administered centrally; clinicians involved in participant recruitment had no access to the schedule." |
| Allocation concealment (selection bias) | Low risk | "Once recruited, a pair of participants was entered, in order of study number, into a computer generated schedule which randomly assigned each of the pair to one of the two study groups. The randomisation schedule was administered centrally; clinicians involved in participant recruitment had no access to the schedule." |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | "All HbA1c measurements were performed at a central independent DCCT‐accredited laboratory." |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | Comment: Higher drop out rate in intervention group (17% versus 7%), most reasons for dropping out were related to wearing the CGM device. |
| Selective reporting (reporting bias) | Low risk | Comment: All predefined outcomes were reported and addressed. |
| Other bias | Low risk | |
| Inappropiate influence of sponsor prevented? | Unclear risk | Comment: This investigator‐initiated study was supported by Medtronic Australasia. |
| Free of conflicts of interest | Unclear risk | Comment: Several authors have received travel or research support by manufacturer. |
Peyrot 2009.
| Methods | PARALLEL RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 28 patients with type 1 diabetes, CSII‐naive, randomised 1:1 to insulin pump with integrated CGM or insulin pump + SMBG. 27 patients completed follow‐up INSULIN PUMP USERS: 0% SEX: 54% female AGE (mean age (SD)): 47.2 (13.2) ETHNIC GROUPS: 79% white DURATION OF DISEASE (mean years (SD)): 25.0 (12.6) INCLUSION CRITERIA: CSII‐naive adults with type 1 diabetes in suboptimal control (mean HbA1c 8.6%) EXCLUSION CRITERIA: n.a. DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 3 months DURATION OF FOLLOW‐UP: 3 months |
|
| Interventions |
STUDY CENTRES: 2 COUNTRY: USA SETTING: outpatients CGM SYSTEM: Paradigm (Metronic Minimed) CONTROL: insulin pump + SMBG |
|
| Outcomes |
PRIMARY: HbA1c SECONDARY:weight, reliability of measures, patient satisfaction ADDITIONAL: n.a. |
|
| Study details |
RUN‐IN PERIOD: no STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING: Medtronic Inc PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "The study examines the effects of the system on intermediate‐term glucose control and assesses patient‐reported outcomes (PRO) using a validated measure of treatment satisfaction and quality of life in addition to user acceptance measures for the components of the integrated system." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information. |
| Blinding (performance bias and detection bias) Patient reported outcomes | Low risk | Comment: Not blinded, but not likely that the outcomes could be influenced. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: Not blinded, but not likely that the HbA1c results could be influenced. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Comment: Drop out rate: 1/28. One subject who started in the control arm dropped out of the study prior to completion. Not likely to influence results. |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available, but al mentioned outcomes in methods are reported in the results section. |
| Other bias | Low risk | |
| Inappropiate influence of sponsor prevented? | Low risk | "This study was funded by an unrestricted grant from Medtronic MiniMed Corp." |
| Free of conflicts of interest | Low risk | Comment: Competing interests, authors have received honorariums for speaking at research symposiums sponsored by manufacturers and research grant. However, many manufacturers are listed. |
Raccah 2009.
| Methods | PARALLEL RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 132 patients with type 1 diabetes, treated with MDI, randomised to insulin use by pump with integrated CGM (n=55) or pump + SMBG (n=60). 15 patients did not complete follow‐up INSULIN PUMP USERS: 0% SEX: 54.5% males in the CGM group and 56.7% males in the control group AGE (mean age (SD)): 28.1 (15.1) in the CGM group, 28.8 (16.7) in the control group ETHNIC GROUPS: n.a. DURATION OF DISEASE (mean years (SD)): 11.2 (9.0) in the CGM group, 12.3 (8.8) in the control group INCLUSION CRITERIA: age between 2 and 65 years, type 1 diabetes diagnosed for >12 months, follow‐up by the respective investigator for at least 3 months, A1C ≥8%, and treatment with basal/bolus MDI with rapid insulin analogs at mealtimes. EXCLUSION CRITERIA: n.a. DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 6 months DURATION OF FOLLOW‐UP: 6 months |
|
| Interventions |
STUDY CENTRES: 8 COUNTRY: France SETTING: outpatients CGM SYSTEM: Paradigm (Medtronic Minimed) CONTROL: Insulin pump + SMBG |
|
| Outcomes |
PRIMARY: HbA1c SECONDARY: mean glucose change and descriptive parameters for biochemical hyperglycaemia (>190 mg/dl) and hypoglycaemia (<70 mg/dl). Daily insulin use was also compared. ADDITIONAL: n.a. |
|
| Study details |
RUN‐IN PERIOD: no STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING: Medtronic Inc PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "In this trial we randomly initiated pump therapy in patients with insufficient metabolic control despite optimized basal‐bolus injection regimens with either the MiniMed Paradigm REAL‐Time insulin pump (PRT), an insulin pump that can receive and display CGM data from a separate subcutaneous glucose sensor, or conventional CSII, and compared glycemic outcomes after 6 months." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information. |
| Blinding (performance bias and detection bias) Patient reported outcomes | Unclear risk | Comment: No blinding, but unlikely to have influence on outcomes. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: No blinding, but unlikely to have influence on outcomes. |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Unclear risk | “..and 132 (81 adults, 51 children) fulfilling the inclusion criteria were randomised.” Comment: Four adults withdrew before visit 3. “The full analysis (FAS) population (n = 115) excluded an additional 13 patients who did not have HbA1c measured after the baseline visit. The FAS population included 55 patients in the PRT arm (22 children, 33 adults) and the 60 patients in the CSII arm (24 children, 36 adults). Analysis on this population was intention‐to‐treat.” “A total of 20 patients abandoned the study: 14 from the PRT group (6 children and 8 adults) and 6 from the CSII group (6 adults).” So, 55‐14=41 and 60‐6=54 patients should have been analysed; Table 2, however, states 46 and 54 patients, respectively. |
| Selective reporting (reporting bias) | Low risk | Comment: The authors presented some ancillary analyses which did not address the outcomes of this review. |
| Other bias | Low risk | |
| Inappropiate influence of sponsor prevented? | Low risk | Comment: The study was funded by CGM system manufacturer, but: ”The study was designed by investigators and approved by the sponsor. Collection, analysis and interpretation of data were the responsibility of C. Cotton, Statitec France, and her staff. The preparation of the manuscript was the responsibility of the investigators." |
| Free of conflicts of interest | Unclear risk | Comment: Insufficient information. |
Tanenberg 2004.
| Methods | PARALLEL RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 128 patients with insulin treated diabetes, randomised to CGM (n=62) or SMBG (n=66). 19 patients dropped out; 4 had missing HbA1c values. INSULIN PUMP USERS: 46% SEX: 19 males, 32 females in the CGM group, 25 males, 33 females in the control group. AGE (mean age (SD)): 44.0 (10.2) in the CGM group, 44.5 (12.6) in the control group ETHNIC GROUPS: 44 whites, 7 other in the CGM group. 48 white, 10 other in the control group. DURATION OF DISEASE (mean years (SD)): 20.4 (10.7) in the CGM group, 19.5 (11.9) in the control group INCLUSION CRITERIA: insulin treated diabetes, age 17‐76 years, Hba1c >7.9% EXCLUSION CRITERIA: n.a. DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 2 periods of 3 days (week 1 and week 3) DURATION OF FOLLOW‐UP: 3 months |
|
| Interventions |
STUDY CENTRES: 7 COUNTRY: USA SETTING: outpatients CGM SYSTEM: CGMS (Metronic Minimed) CONTROL: SMBG |
|
| Outcomes |
PRIMARY: HbA1c SECONDARY: sensor performance, hypoglycaemia ADDITIONAL: n.a. |
|
| Study details |
RUN‐IN PERIOD: no STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING: Medtronic Inc PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "The purpose of this study was to show improved glycemic control in patients with insulin‐treated diabetes after adjustments to the diabetes management plan based on either continuous glucose monitoring using the CGMS or frequent SMBG using a home blood glucose meter." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A random number list, computer generated by Medtronic Minimed with SAS statistical software was used.” |
| Allocation concealment (selection bias) | Low risk | "Random assignments to the treatment or control group were provided to the study centers in sealed envelopes." |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: Lack of blinding is not likely to introduce bias. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | Comment: Dropout rate 18% (11/62) in CGM versus 12% (8/66) in control group (fig 1). “In each group 14 patients had incomplete end‐of‐study downloads (failure of centre to transmit data).” |
| Selective reporting (reporting bias) | Low risk | Comment: All predefined outcomes were reported, except for adverse reactions. |
| Other bias | Low risk | |
| Inappropiate influence of sponsor prevented? | Unclear risk | “This study is sponsored by Medtronic Minimed.” |
| Free of conflicts of interest | Unclear risk | Comment: Two authors have received funds from Medtronic, of which one is also a member of the medical board. Two authors are employees of Medtronic. |
Yates 2006.
| Methods | PARALLEL RANDOMISED CONTROLLED CLINICAL TRIAL | |
| Participants |
WHO PARTICIPATED: 36 patients with type 1 diabetes, 19 in the CGM group and 17 in the control group. No dropouts. INSULIN PUMP USERS: 47% SEX: 7 (37%) males in the CGM group, 6 (36%) males in the control group. AGE (mean age (range)): 14.7 (13.6‐14.4) years in the CGM group, 14.1 (12.8‐15.3) in the control group. ETHNIC GROUPS: n.a. DURATION OF DISEASE (mean years (SD)): n.a. INCLUSION CRITERIA: age 18 years or less, type 1 diabetes for at least 1 year; use of CSII or an MDI regimen that included glargine for at least 3 months. EXCLUSION CRITERIA: known poor compliance or A1C >10%. DIAGNOSTIC CRITERIA: n.a. CO‐MORBIDITIES: n.a. CO‐MEDICATION: n.a. DURATION OF INTERVENTION: 3 months, 72 hours of CGM use every 3 weeks DURATION OF FOLLOW‐UP: 6 months |
|
| Interventions |
STUDY CENTRES: 1 COUNTRY: Australia SETTING: outpatients CGM SYSTEM: CGMS (Medtronic Minimed) CONTROL: SMBG |
|
| Outcomes |
PRIMARY: HbA1c SECONDARY: adverse events, AUC, hypoglycaemia ADDITIONAL: n.a. |
|
| Study details |
RUN‐IN PERIOD: no STUDY TERMINATED BEFORE REGULAR END: no |
|
| Publication details |
LANGUAGE OF PUBLICATION: English COMMERCIAL FUNDING: Medtronic Inc PUBLICATION STATUS: Peer review journal |
|
| Stated aim of study | "The purpose of this study was to assess the effect on diabetes control of guiding insulin adjustment with four cycles of CGMS over 3 months in children on near physiological insulin replacement regimen." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done by an independent body using biased coin randomisation." |
| Allocation concealment (selection bias) | Low risk | "Group allocation was blinded with opaque sealed envelopes." |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Comment: Not blinded, but lack of blinding is unlikely to influence the results. Patients were not asked to adhere to a special diet or exercise routine but were encouraged to continue their usual behaviour. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Comment: No drop outs (n=19 vs n=17), see Figure 1. |
| Incomplete outcome data (attrition bias) Long‐term outcomes | Low risk | Comment: No drop outs (n=19 vs n=17), see Figure 1. |
| Selective reporting (reporting bias) | Low risk | Comment: The study protocol is not publicly available, but the published report includes all the pre‐specified outcomes. |
| Other bias | Low risk | |
| Inappropiate influence of sponsor prevented? | Low risk | Comment: Statement of ‘unrestricted funding’. |
| Free of conflicts of interest | Unclear risk | Comment: K.Y. and G.A. have received grant/research support from Medtronic MiniMed. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bode 2004 | Comparison of CGM with and without alarm |
| Bode 2004a | No CGM (InDuo) |
| Chase 2003 | GlucoWatch study |
| Chase 2005 | GlucoWatch study |
| Feldman 2003 | Accuracy study |
| Fiallo‐Scharer 2005 | Report of run‐in phase Chase 2005 |
| Garg 2006 | Type 1 and type 2 patients, no subgroup analysis |
| Garg 2008 | Insulin guidance software; no CGM |
| Haupt 2005 | No CGM (InDuo) |
| Jeha 2004 | Only follow‐up of CGM study arm |
| Juvenile 2009a | No control group |
| Murphy 2008 | Type 1 and type 2 patients, no subgroup analysis |
| Rowen 2007 | Qualitative pilot study |
| Tamborlane 2008 | Research methods JDRF 2008a |
| Weinzimer 2008 | No control group |
| Wilhelm 2006 | Observational study |
| Wysocki 2005 | No comparison CGM versus SMBG or other CGM |
| Yogev 2003 | No RCT; patients were their own control |
| Yogev 2003a | No RCT; patients were their own control |
Characteristics of studies awaiting assessment [ordered by study ID]
Conget 2010.
| Methods | Multicenter, randomized, controlled, crossover study |
| Participants | Diagnosed with type 1 diabetes mellitus at least 12 months prior to informed consent form; age between 6 and 70 years of age; HbA1c between 7.5% and 9.5%; treated with CSII at least 6 months prior to informed consent. |
| Interventions | INTERVENTION: Paradigm with sensor switched on CONTROL: Paradigm REAL‐Time with sensor switched off |
| Outcomes | PRIMARY: HbA1c level after 6 months of follow‐up SECONDARY: time spent in different glycemic ranges, percentage of patients with HbA1c <7%, number of hypoglycemic events, glucose variability parameters, safety outcomes, treatment satisfaction, and quality of life. |
| Notes | Recruitment occurred between January 2008 and February 2009. A total of 153 patients were randomized. Study completion is anticipated in July 2010. No published results at the time of the search (June 8, 2011). |
Lange 2010.
| Methods | See Kordonouri 2010. |
| Participants | |
| Interventions | |
| Outcomes | Quality of life and psychological wellbeing |
| Notes | Quality of life results for Kordonouri trial. Conference abstract with limited data on the results. |
Langeland 2010.
| Methods | Randomized controlled crossover trial |
| Participants | 30 patients with diabetes type 1, mean age 34 +/‐ 9 years of age, with moderately good glucose control (HbA1c between 7.0% and 10.0%) |
| Interventions | INTERVENTION: 1 month of CGMS (Medtronic Guardian RT) CONTROL: intensified conventional fingerprick measurements |
| Outcomes | PRIMARY: HbA1c level, hypoglycemic episodes, treatment satisfaction, quality of life |
| Notes | Conference abstract with limited data on the results. |
Differences between protocol and review
Change in authors: I. Wentholt and A. Burt did not participate in performing the full review. Y. Luijf was added to the authors of the full review.
We widened the definition of severe hypoglycaemia from "a hypoglycaemic event with neurological symptoms requiring assistance of another person and/or receiving carbohydrate, glucagon or other resuscitative actions; documented or undocumented by measured plasma glucose level)" to "a hypoglycaemic event requiring assistance of another person; documented or undocumented by measured plasma glucose level".
We planned to check the abstract books of the major annual European and American diabetes conferences, but as we felt the current evidence was complete, we omitted the abstract books.
CGM device: we planned to evaluated the GlucoWatch device, but omitted this as this type of CGM system is no longer on the market.
In the protocol we made a strict distinction between the different age groups, as our aim was to evaluate CGM for different patient groups. For the review however, we decided to present an exploratory meta‐analysis across all age groups. The reason was that because the CGM devices in children were operated by their adult caregivers which were also the ones who acted on the information the CGM provided. In case of adolescents who operated the CGM system themselves, there was no compelling reason to believe that their decision making process was far inferior to those of young adults.
In the meta‐analysis we made a distinction between studies in which the introduction of a insulin pump and a CGM device was investigated in insulin‐pump naive patients and studies that investigated CGM use only (in both insulin pump naive patients and insulin pump users).
We added results on frequency of sensor‐use.
Contributions of authors
All authors have contributed to the production of the protocol and the review. RS, LH, JHDV and AM wrote the protocol. ML and YL led the writing of the review. ML, LH and RS screened records for potential eligibility. ML, LH, RS, AM, YL, and JHDV independently assessed potentially eligible studies, performed quality assessment and abstract data from included studies. ML and YL undertook the analysis. RS acted as an arbitrator for resolving any disagreements that arose during the review process.
Sources of support
Internal sources
No sources of support supplied
External sources
Dutch Health Care Insurance Board, Netherlands.
Declarations of interest
J. Hans DeVries is co‐author of the Hermanides trial (Hermanides 2011).
Other authors: none known.
Authors Langendam and Luijf contributed equally
Authors Langendam and Luijf contributed equally
Edited (no change to conclusions)
References
References to studies included in this review
Battelino 2011 {published data only}
- Battelino T, Nimri R, Philip M, Oskarsson P, Bratina N, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes.. Diabetes Care 2011;34(4):795‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergenstal 2010 {published data only}
- Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. Effectiveness of Sensor‐Augmented Insulin‐Pump Therapyin Type 1 Diabetes. British Medical Journal 2010;363(4):311‐20. [DOI] [PubMed] [Google Scholar]
Chase 2001 {published data only}
- Chase HP, Kim LM, Owen SL, MacKenzie TA, Klingensmith G, Murtfeldt R, et al. Continuous subcutaneous glucose monitoring in children with type 1 diabetes. Pediatrics 2001;107(2):222‐6. [DOI] [PubMed] [Google Scholar]
Chico 2003 {published data only}
- Chico A, Vidal‐Rios P, Subira M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care 2003;4:1153‐7. [DOI] [PubMed] [Google Scholar]
Cooke 2009 {published data only}
- Cooke D, Hurel SJ, Casbard A, Steed L, Walker S, Meredith A, et al. Randomized controlled trial to assess the impact ofcontinuous glucose monitoring on HbA1c in insulintreateddiabetes (MITRE Study). Diabetic Medicine 2009;26:540‐547. [DOI] [PubMed] [Google Scholar]
Cosson 2009 {published data only}
- Cosson E, Hamo‐Tchatchouang E, Dufaitre‐Patouraux L, Attali J‐R, Paris J, Schaepelynck BP. Multicentre, randomised, controlled study of the impact of continuous sub‐cutaneous glucose monitoring (GlucoDay) on glycaemic control in type 1 and type 2 diabetes patients. Diabetes & Metabolism 2009;35:312‐8. [DOI] [PubMed] [Google Scholar]
Deiss 2006 {published data only}
- Deiss D, Hartmann R, Schmidt J, Kordonouri O. Results of a randomised controlled cross‐over trial on the effect of continuous subcutaneous glucose monitoring (CGMS) on glycaemic control in children and adolescents with type 1 diabetes. Experimental and Clinical Endocrinology and Diabetes 2006;2:63‐7. [DOI] [PubMed] [Google Scholar]
Deiss 2006a {published data only}
- Deiss D, Bolinder J, Riveline JP, Battelino T, Bosi E, Tubiana‐Rufi N, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real‐time continuous glucose monitoring. Diabetes Care 2006;29(12):2730‐2. [DOI] [PubMed] [Google Scholar]
Hermanides 2011 {published data only (unpublished sought but not used)}
- Hermanides J, Norgaard K, Bruttomesso D, Mathieu C, Frid A, Dayan CM, Diem P, Vermon C, Wentholt I, DeVries JH. Sensor‐augmented pump therapy lowers HbA1c in suboptimally controlled type 1 diabetes: a randomized controlled trial.. Diabetic Medicine 2011;28(10):1158‐67. [DOI] [PubMed] [Google Scholar]
Hermanns 2009 {published data only}
- Hermanns N, Kulzer B, Gulde C, Eberle H, Pradler E, Patzelt‐Bath A, et al. Short‐term effects on patient satisfaction of continuous glucose monitoring with the glucoday with real‐time and retrospective access to glucose values: A crossover study. Diabetes Technology and Therapeutics 2009;5:275‐81. [DOI] [PubMed] [Google Scholar]
Hirsch 2008 {published data only}
- Hirsch IB, Abelseth J, Bode BW, Fischer JS, Kaufman FR, Mastrototaro J, et al. Sensor‐augmented insulin pump therapy: Results of the first randomized treat‐to‐target study. Diabetes Technology and Therapeutics 2008;5:377‐83. [DOI] [PubMed] [Google Scholar]
Juvenile 2008 {published data only}
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. New England Journal of Medicine 2008;359(14):1464‐76. [DOI] [PubMed] [Google Scholar]
Juvenile 2009 {published data only}
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Group. Quality‐of‐life measures in children and adults with type 1 diabetes: Juvenile Diabetes Research Foundation Continuous Glucose Monitoring randomized trial. Diabetes Care 2010;33:2175‐2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. The effect of continuous glucose monitoring in well‐controlled type 1 diabetes. Diabetes Care 2009;32(8):1378‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kordonouri 2010 {published data only}
- Kordonouri O, Pankowska E, Rami B, Kapellen T, Coutant R, Hartmann R, et al. Sensor‐augmented pump therapy from the diagnosis of childhood type 1 diabetes: results of the Pediatric Onset Study (ONSET) after 12 months of treatment. Diabetologia 2010;53(12):2487‐95. [DOI] [PubMed] [Google Scholar]
Lagarde 2006 {published data only}
- Lagarde WH, Barrows FP, Davenport ML, Kang M, Guess HA, Calikoglu AS. Continuous subcutaneous glucose monitoring in children with type 1 diabetes mellitus: A single‐blind, randomized, controlled trial. Pediatric Diabetes 2006;3:159‐64. [DOI] [PubMed] [Google Scholar]
Logtenberg 2009 {published data only}
- Logtenberg SJJ, Kleefstra N, Groenier KH, Gans ROB, Bilo HJG. Use of short‐term real‐time continuous glucose monitoring in type 1 diabetes patients on continuous intraperitoneal insulin infusion: A feasibility study. Diabetes Technology and Therapeutics 2009;5:293‐9. [DOI] [PubMed] [Google Scholar]
Ludvigsson 2003 {published data only}
- Ludvigsson J, Hanas R. Continuous subcutaneous glucose monitoring improved metabolic control in pediatric patients with type 1 diabetes: A controlled crossover study. Pediatrics 2003;5:933‐8. [DOI] [PubMed] [Google Scholar]
O'Connell 2009 {published data only}
- O'Connell MA, Donath D, O'Neal DN, Colman P, Ambler GR, Jones TW, et al. Glycaemic impact of patient‐led use of sensor‐guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia 2009;52:1250‐7. [DOI] [PubMed] [Google Scholar]
Peyrot 2009 {published data only}
- Peyrot M, Rubin RR. Patient‐reported outcomes for an integrated real‐time continuous glucose monitoring/insulin pump system. Diabetes Technology & Therapeutics 2009;11(1):57‐62. [DOI] [PubMed] [Google Scholar]
Raccah 2009 {published data only}
- Raccah D, Sulmont V, Reznik Y, Guerci B, Renard E, Hanaire‐Broutin H, et al. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: The RealTrend study. Diabetes Care 2009;32:2245‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanenberg 2004 {published data only}
- Tanenberg R, Bode B, Lane W, Levetan C, Mestman J, Harmel AP, et al. Use of the Continuous Glucose Monitoring System to guide therapy in patients with insulin‐treated diabetes: a randomized controlled trial. Mayo Clinic Proceedings 2004;79(12):1521‐6. [DOI] [PubMed] [Google Scholar]
Yates 2006 {published data only}
- Yates K, Milton AH, Dear K, Ambler G. Continuous glucose monitoring‐guided insulin adjustment in children and adolescents on near‐physiological insulin regimens: A randomized controlled trial. Diabetes Care 2006;7:1512‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bode 2004 {published data only}
- Bode B, Gross K, Rikalo N, Schwartz S, Wahl T, Page C, et al. Alarms Based on Real‐Time Sensor Glucose Values Alert Patients to Hypo‐ and Hyperglycemia: The Guardian Continuous Monitoring System. Diabetes Technology and Therapeutics 2004;2:105‐13. [DOI] [PubMed] [Google Scholar]
Bode 2004a {published data only}
- Bode B, Shelmet J, Gooch B, Hassman DR, Liang J, Smedegaard JK, et al. Patient perception and use of an insulin injector/glucose monitor combined device. Diabetes Educator 2004;30(2):301‐9. [DOI] [PubMed] [Google Scholar]
Chase 2003 {published data only}
- Chase HP, Roberts MD, Wightman C, Klingensmith G, Garg SK, Wyhe M, et al. Use of the GlucoWatch biographer in children with type 1 diabetes. Pediatrics 2003;4:790‐4. [DOI] [PubMed] [Google Scholar]
Chase 2005 {published data only}
- Chase HP. A randomized multicenter trial comparing the glucowatch biographer with standard glucose monitoring in children with type 1 diabetes. Diabetes Care 2005;5:1101‐6. [DOI] [PubMed] [Google Scholar]
Feldman 2003 {published data only}
- Feldman B, Brazg R, Schwartz S, Weinstein R. A Continuous Glucose Sensor Based on Wired Enzyme[trademark] Technology ‐ Results from a 3‐Day Trial in Patients with Type 1 Diabetes. Diabetes Technology and Therapeutics 2003;5:769‐79. [DOI] [PubMed] [Google Scholar]
Fiallo‐Scharer 2005 {published data only}
- Fiallo‐Scharer R. Eight‐point glucose testing Versus the continuous glucose monitoring system in evaluation of glycemic control in type 1 diabetes. Journal of Clinical Endocrinology and Metabolism 2005;6:3387‐91. [DOI] [PubMed] [Google Scholar]
Garg 2006 {published data only}
- Garg S, Zisser H, Schwartz S, Bailey T, Kaplan R, Ellis S, et al. Improvement in glycemic excursions with a transcutaneous, real‐time continuous glucose sensor: A randomized controlled trial. Diabetes Care 2006;1:44‐50. [DOI] [PubMed] [Google Scholar]
Garg 2008 {published data only}
- Garg SK, Bookout TR, Mcfann KK, Kelly WC, Beatson C, Ellis SL, et al. Improved glycemic control in intensively treated adult subjects with type 1 diabetes using insulin guidance software. Diabetes Technology & Therapeutics 2008;10(5):369‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haupt 2005 {published data only}
- Haupt A, Berg B, Paschen P, Dreyer M, Haring HU, Smedegaard J, et al. InDuo, a novel combined insulin injection and blood glucose monitoring device ‐ effective and save as other devices, and patient preference. Experimental & Clinical Endocrinology & Diabetes 2005;113(9):541‐4. [DOI] [PubMed] [Google Scholar]
Jeha 2004 {published data only}
- Jeha GS, Karaviti LP, Anderson B, Smith EO'B, Donaldson S, Mcgirk TS, et al. Continuous glucose monitoring and the reality of metabolic control in preschool children with type 1 diabetes. Diabetes Care 2004;12:2881‐6. [DOI] [PubMed] [Google Scholar]
Juvenile 2009a {published data only}
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Sustained benefit of continuous glucose monitoring and HbA1c, glucose profiles, and hypoglycemia in adults with type 1 diabetes. Diabetes Care 2009;32(11):2047‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2008 {published data only}
- Murphy HR, Rayman G, Lewis K, Kelly S, Johal B, Duffield K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: Randomised clinical trial. British Medical Journal 2008;7675:907‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowen 2007 {published data only}
- Rowen M, Schneider DJ, Pratley RE, Sobel BE. On rendering continuous glucose monitoring ready for prime time in the cardiac care unit. Coronary Artery Disease 2007;18(5):405‐9. [DOI] [PubMed] [Google Scholar]
Tamborlane 2008 {published data only}
- Tamborlane WV, Ruedy KJ, Wysocki T, O'Grady M, Kollman C, Block J, et al. JDRF randomized clinical trial to assess the efficacy of real‐time continuous glucose monitoring in the management of type 1 diabetes: Research design and methods. Diabetes Technology and Therapeutics 2008;4:310‐21. [DOI] [PubMed] [Google Scholar]
Weinzimer 2008 {published data only}
- Weinzimer S, Xing D, Tansey M, Fiallo‐Scharer R, Mauras N, Wysocki T, et al. FreeStyle navigator continuous glucose monitoring system use in children with type 1 diabetes using glargine‐based multiple daily dose regimens: Results of a pilot trial diabetes research in children network (DirecNet) study group. Diabetes Care 2008;3:525‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilhelm 2006 {published data only}
- Wilhelm B, Forst S, Weber MM, Larbig M, Pfutzner A, Forst T. Evaluation of CGMS during rapid blood glucose changes in patients with type 1 diabetes. Diabetes Technology and Therapeutics 2006;2:146‐55. [DOI] [PubMed] [Google Scholar]
Wysocki 2005 {published data only}
- Wysocki T. Youth and parent satisfaction with clinical use of the glucoWatch G2 Biographer in the management of pediatric type 1 diabetes. Diabetes Care 2005;8:1929‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yogev 2003 {published data only}
- Yogev Y, Ben‐Haroush A, Chen R, Kaplan B, Phillip M, Hod M. Continuous glucose monitoring for treatment adjustment in diabetic pregnancies ‐ A pilot study. Diabetic Medicine 2003;7:558‐62. [DOI] [PubMed] [Google Scholar]
Yogev 2003a {published data only}
- Yogev Y, Chen R, Ben‐Haroush A, Phillip M, Jovanovic L, Hod M. Continuous glucose monitoring for the evaluation of gravid women with type 1 diabetes mellitus. Obstetrics and Gynecology 2003;4:633‐8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Conget 2010 {published data only}
- Conget I, Battelino T, Gimenez M, Gough H, Castaseda J, Bolinder J. The SWITCH study (Sensing with insulin pump therapy to control HbA1c). Design and methods of a randomized controlled cross‐over trial on sensor‐augmented insulin pump efficacy in type 1 diabetes sub‐optimally controlled with pump therapy. Diabetes Technology and Therapeutics 2011;13(1):49‐54. [DOI] [PubMed] [Google Scholar]
Lange 2010 {published data only}
- Lange K, Coutant R, Danne T, Kapellen T, Pankowska, Rami B, Aschemeier B, Blsig S, Hartmann R, Krug N, Marquardt E, Remus K, Kordonouri O. High quality of life in children and psychological wellbeing in mothers 12 months after diabetes onset: results of the paediatric onset‐trial of sensor‐enhanced CSII. Pediatric Diabetes 2010;11(Suppl s14):101. [Google Scholar]
Langeland 2010 {published data only}
- Langeland LL, Salvesen O, Selle H, Carlsen SM, Fougner KJ. Continuous glucose monitoring: effect on glucose control and treatment satisfaction in diabetes mellitus type 1. Diabetologica 2010;53(Supplement 1):S423. [DOI] [PubMed] [Google Scholar]
Additional references
ADA 1999
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1999;22 Suppl 1:S5‐19. [DOI] [PubMed] [Google Scholar]
Boland 2001
- Boland E, Monsod T, Delucia M, Brandt CA, Fernando S, Tamborlane WV. Limitations of conventional methods of self‐monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care 2001;24:1858‐62. [DOI] [PubMed] [Google Scholar]
Bradburn 2007
- Bradburn MJ, Deeks JJ, Berlin JA, Russell LA. Much ado about nothing: a comparison of the performance of meta‐analytical methods with rare events. Statistics in Medicine 2007;26:53‐77. [DOI] [PubMed] [Google Scholar]
Bradley 1994a
- Bradley C. The Well‐being Questionnaire. In: Bradley C editor(s). Handbook of Psychology and Diabetes: a guide to psychological measurement in diabetes research and practice. Harwood Academic Publishers, 1994:89‐109. [Google Scholar]
Bradley 1994b
- Bradley C. Diabetes Treatment Satisfaction Questionnaire (DTSQ). In: Bradley C editor(s). Handbook of Psychology and Diabetes. Harwood Academic Publishers, 1994:111‐32. [Google Scholar]
Brauker 2009
- Brauker J. Continuous glucose sensing: future technology developments. Diabetes Technology & Therapeutics 2009;11(s1):s25‐s36. [DOI] [PubMed] [Google Scholar]
Chetty 2008
- Chetty VT, Almulla A, Odueyungbo A, Thabane L. The effect of continuous subcutaneous glucose monitoring (CGMS) versus intermittent whole blood finger‐stick glucose monitoring (SBGM) on hemoglobin A1c (HBA1c) levels in Type I diabetic patients: a systematic review. Diabetes Research and Clinical Practice 2008;81:79‐87. [DOI] [PubMed] [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46. [Google Scholar]
Cryer 2004
- Cryer PE. Diverse causes of hypoglycaemia‐associated autonomic failure in diabetes. New England Journal of Medicine 2004;350:2272‐9. [DOI] [PubMed] [Google Scholar]
DCCT 1993
- DCCT Research Group. The diabetes control and complications trial research group. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. New England Journal of Medicine 1993;329(14):977‐86. [DOI] [PubMed] [Google Scholar]
FDA 2009
- US Food, Drug Administration. Guidance for Industry. Diabetes Mellitus: developing drugs and therapeutic biologics for treatment and prevention (Draft guidance). US Food and Drug Administration 2009.
Ghandi 2011
- Ghandi GY, Kovalaske M, Kudva Y, Walsh K, Elamin MB, Beers M, Coyle C, Goalen M, Safwan Murad M, Erwin PJ, Corpus J, Montori VM, Murad MH. Efficacy of continuous glucose monitoring in improving glycaemic control and reducing hypoglycemia: a systematic review and meta‐analysis of randomized trials. Journal of Diabetes Science and Technology 2011;5(4):952‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Girardin 2009
- Girardin CM, Huot C, Gonthier M, Delvin E. Continuous glucose monitoring: a review of biochemical perspectives and clinical use in type 1 diabetes. Clinical Biochemistry 2009;42:136‐142. [DOI] [PubMed] [Google Scholar]
Golicki 2008
- Golicki DT, Golicka D, Groele L, Pankowska E. Continuous Glucose Monitoring System in children with type 1 diabetes mellitus: a systematic review and meta‐analysis. Diabetologica 2008;51:233‐40. [DOI] [PubMed] [Google Scholar]
Grootenhuis 1994
- Grootenhuis PA, Snoek FJ, Heine RJ, Bouter LM. Development of a type 2 diabetes symptom checklist: a measure of symptom severity. Diabetic Medicine 1994;11(3):253‐61. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. British Medical Journal 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Hoeks 2010
- Hoeks LBEA, Greven WL, Valk HW. Review Article: Real‐time continuous glucose monitoring system fortreatment of diabetes: a systematic review. Diabetic Medicine 2010;doi: 10.1111/j.1464‐5491.2010.03177.x:In press. [DOI] [PubMed] [Google Scholar]
Klonoff 2005
- Klonoff DC. Continuous glucose monitoring. Roadmap for 21st century diabetes therapy. Diabetes Care 2005;28:1231‐9. [DOI] [PubMed] [Google Scholar]
Lau 2006
- Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. British Medical Journal 2006;333:597‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA Statement for Reporting Systematic and Meta‐Analyses of Studies That Evaluate Interventions: Explanation and Elaboration. PLoS Med 1999;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
McHorney 1993
- McHorney CA, Ware JE Jr, Raczek AE. The MOS 36‐Item Short‐Form Health Survey (SF‐36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs.. Medical Care 1993;31(3):247‐63. [DOI] [PubMed] [Google Scholar]
Nathan 2005
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. New England Journal of Medicine 2005;353(25):2643‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCCWCH 1994
- National Collaborating Centre for Women's and Children's Health. Type 1 diabetes diagnosis and management of type 1 diabetes in children and young people. London: RCOG Press, 2004. [PubMed] [Google Scholar]
Pickup 2011
- Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta‐analysis of randomised controlled trials using individual patient data. British Medical Journal 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). 5.0 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Sterne 2001
- Sterne JAC, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care; Meta‐analysis in Context. London: BMJ Publishing Group, 2001:189‐208. [Google Scholar]
Thierny 2000
- Thierney MJ, Tamada JA, Potts RO, Eastman RC, Pitzer K, Ackerman NR, Fermi SJ. The GlucoWatch Biographer: a frequent, automatic and noninvasive glucose monitor. Annals in Medicine 2000;32:641. [DOI] [PubMed] [Google Scholar]
Wentholt 2005
- Wentholt IM, Vollebregt MA, Hart AA, Hoekstra JB, Vries JH. Comparison of a needle‐type and a microdialysis continuous glucose monitor in type 1 diabetic patients. Diabetes Care 2005;28(12):2871‐6. [DOI] [PubMed] [Google Scholar]
Wentholt 2007
- Wentholt IME, Hoekstra JBL, Vries JH. Continuous glucose monitors: the long awaited watch dogs?. Diabetes Technology & Therapeutics 2007;9(5):399‐409. [DOI] [PubMed] [Google Scholar]
Wentholt 2008
- Wentholt IM, Hart AA, Hoekstra JB, Vries JH. How to assess and compare the accuracy of continuous glucose monitors?. Diabetes Technol Ther 2008;10(2):57‐68. [DOI] [PubMed] [Google Scholar]
WHO 1980
- WHO Expert Committee on Diabetes Mellitus. Second report. Technical Report Series 646. Geneva. WHO, 1980. [PubMed]
WHO 1985
- WHO Expert Committee on Diabetes Mellitus. World Health Organization, 1985. Technical Report Series 727. [PubMed]
WHO 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its compliactions. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. [DOI] [PubMed] [Google Scholar]


