Abstract
Background
Most tobacco control programmes for adolescents are based around prevention of uptake, but teenage smoking is still common. It is unclear if interventions that are effective for adults can also help adolescents to quit. This is the update of a Cochrane Review first published in 2006.
Objectives
To evaluate the effectiveness of strategies that help young people to stop smoking tobacco.
Search methods
We searched the Cochrane Tobacco Addiction Group's Specialized Register in June 2017. This includes reports for trials identified in CENTRAL, MEDLINE, Embase and PsyclNFO.
Selection criteria
We included individually and cluster‐randomized controlled trials recruiting young people, aged under 20 years, who were regular tobacco smokers. We included any interventions for smoking cessation; these could include pharmacotherapy, psycho‐social interventions and complex programmes targeting families, schools or communities. We excluded programmes primarily aimed at prevention of uptake. The primary outcome was smoking status after at least six months' follow‐up among those who smoked at baseline.
Data collection and analysis
Two review authors independently assessed the eligibility of candidate trials and extracted data. We evaluated included studies for risk of bias using standard Cochrane methodology and grouped them by intervention type and by the theoretical basis of the intervention. Where meta‐analysis was appropriate, we estimated pooled risk ratios using a Mantel‐Haenszel fixed‐effect method, based on the quit rates at six months' follow‐up.
Main results
Forty‐one trials involving more than 13,000 young people met our inclusion criteria (26 individually randomized controlled trials and 15 cluster‐randomized trials). We judged the majority of studies to be at high or unclear risk of bias in at least one domain. Interventions were varied, with the majority adopting forms of individual or group counselling, with or without additional self‐help materials to form complex interventions. Eight studies used primarily computer or messaging interventions, and four small studies used pharmacological interventions (nicotine patch or gum, or bupropion). There was evidence of an intervention effect for group counselling (9 studies, risk ratio (RR) 1.35, 95% confidence interval (CI) 1.03 to 1.77), but not for individual counselling (7 studies, RR 1.07, 95% CI 0.83 to 1.39), mixed delivery methods (8 studies, RR 1.26, 95% CI 0.95 to 1.66) or the computer or messaging interventions (pooled RRs between 0.79 and 1.18, 9 studies in total). There was no clear evidence for the effectiveness of pharmacological interventions, although confidence intervals were wide (nicotine replacement therapy 3 studies, RR 1.11, 95% CI 0.48 to 2.58; bupropion 1 study RR 1.49, 95% CI 0.55 to 4.02). No subgroup precluded the possibility of a clinically important effect. Studies of pharmacotherapies reported some adverse events considered related to study treatment, though most were mild, whereas no adverse events were reported in studies of behavioural interventions. Our certainty in the findings for all comparisons is low or very low, mainly because of the clinical heterogeneity of the interventions, imprecision in the effect size estimates, and issues with risk of bias.
Authors' conclusions
There is limited evidence that either behavioural support or smoking cessation medication increases the proportion of young people that stop smoking in the long‐term. Findings are most promising for group‐based behavioural interventions, but evidence remains limited for all intervention types. There continues to be a need for well‐designed, adequately powered, randomized controlled trials of interventions for this population of smokers.
Plain language summary
Are there any smoking cessation programmes that can help adolescents to stop smoking?
Background
Worldwide, between 80,000 and 100,000 young people start smoking every day. Many adolescent tobacco programmes focus on preventing teenagers from starting to smoke, but some programmes have been aimed at helping those teenagers who are already smoking to quit. We set out to investigate whether these programmes can help young people quit smoking for six months or longer. Searches are up to date as of June 2017.
Study characteristics
We identified 41 studies (around 13,000 participants) that researched ways of helping teenagers to quit smoking. These studies were of mixed quality and looked at various methods for stopping smoking, including one‐to‐one counselling, counselling as part of a group, methods using computers or text messaging, or a combination of these. Four studies used drug treatments such as nicotine patches. Most studies recruited participants from schools, and 29 of the studies were carried out in North America.
Key results
Although some programmes showed promise, especially those that used group counselling and those that combined a variety of approaches, there was no strong evidence that any particular method was effective in helping young people to stop smoking. Trials differed in how they measured whether a person had quit smoking, and many trials did not have enough participants for us to be confident about wider application of the results. Medications such as nicotine replacement and bupropion were not shown to be successful with adolescents, and some adverse events were reported, although these events were generally mild and findings were based on studies with small numbers of participants. Based on these findings we cannot currently identify a programme for helping adolescents to stop smoking that is more successful than trying to stop unaided.
Quality of the evidence
The quality of evidence was low or very low for all of the outcomes in this review. This is because of issues with the quality of some of the studies, the small number of studies and participants for some outcomes, and the differences between the studies.
Summary of findings
Background
In much of the developed world, the prevalence of smoking amongst young people has been falling over the last 20 years. Recent figures from the UK show that for children under the age of 16 years, 18% have tried smoking at least once and 3% are regular smokers, but that regular cigarette smoking has fallen from a peak of around 12% of children aged 11 to 15 years in the mid‐1990s (CRUK 2017). A similar decline has been noted in the USA; in 2016, 13.8% of high school students and 4.3% of middle school students reported current use of combustible tobacco (MMWR 2017), compared with a prevalence of more than 30% 20 years earlier (USDHHS 2012). In developing economies the picture is less clear cut, with wide variation and often higher rates of smoking in young people, including rates of up to 50% in some countries (Eriksen 2015). The incidence of the initiation of smoking first becomes measurable in the 10‐ to 12‐year age range (ONS 2000), and smoking in teenage years is strongly predictive of adult smoking (HSCIC 2012).
Although the major burden of disease caused by smoking falls on the adult population, there are several reasons why smoking cessation interventions that are effective in younger smokers are particularly valuable. Firstly, many adult smokers started smoking in childhood (in the UK, 40% of regular smokers began smoking before the age of 16 years (CRUK 2017)). However, many of the adverse health effects associated with smoking are preventable with cessation at a young age (USDHHS 2004), and there is little loss in life expectancy provided cessation occurs early enough (Jha 2014). There are therefore substantial cumulative potential health benefits to be gained from successful interventions in this age group, as well as the prospect of reducing the demand for cessation services among adult smokers who have been smoking since childhood.
Secondly, there is evidence that those who start earliest and continue to smoke may be more susceptible to disease in adulthood than smokers who start later in life, facing increased risks of lung damage, bowel cancer, and cervical pre‐cancerous lesions (CRUK 2017). There is also evidence that, although levels of dependence may be lower in young smokers than in the adult population (Rubinstein 2007), addiction to nicotine can develop very rapidly in young smokers, making unassisted quitting difficult even among those without a long smoking history (DiFranza 2008a; DiFranza 2008b).
Thirdly, there is evidence that within a short time of commencing, many teenage smokers want to quit (Burt 1998; Hu 1998; MMWR 2009; Stanton 2001; Sussman 1998). Frequent quit attempts are reported in this population (MMWR 2009; Stanton 2001), with many studies reporting more than 50% of teenage smokers making a quit attempt within six months, although many of these attempts are unsuccessful (Bancej 2007; Mermelstein 2003).
Fourthly, smoking may be a particular problem in young people with mental health or behavioural problems. In the UK, smoking rates among 11‐ to 15‐year‐olds were 30% in those with conduct disorder, 19% in those with emotional disorder, and 15% in those with attention deficit hyperactivity disorder (ADHD) compared to 5% in those without such disorders (Green 2004; Thakur 2012).There is now strong evidence that the relationship is causal with respect to depression (Boden 2010), whilst for ADHD molecular genetics would seem to play a role.
There is now a large literature on smoking cessation services for adults. This is reflected in a number of Cochrane Reviews examining several aspects of the subject in detail. Many countries have developed appropriate services for adults. However, whilst some have suggested that similar services, suitably modified, should be considered for young people (Raw 1998), this assertion is open to challenge in view of the difference in smoking pattern, lifestyle and attitudes to services in this age group (TAG 2000). Previous reviews of adolescent smoking cessation have been published, comprising randomized controlled (experimental) trials and non‐randomized, 'quasi‐experimental' or observational studies (McDonald 2003; Patnode 2013; Sussman 1999; Sussman 2002; Sussman 2006). This update, restricted to evidence from randomized controlled trials, is the third version of a Cochrane Review to focus on smoking cessation in young people under 20 years. A further systematic review has looked at strategies for smoking cessation for university‐age smokers (Villanti 2010). The paucity of high‐quality research evidence to answer important clinical questions is a recurrent theme of reviews in this area.
Other Cochrane Reviews of interventions relevant to tobacco addiction amongst young people have mainly focused on primary prevention. These include a review of school‐based prevention programmes (Thomas 2012), and reviews of mass media interventions (Carson 2017), community interventions (Carson 2011), interventions for reducing access by preventing illegal sale of tobacco (Stead 2005), prevention in indigenous youth (Carson 2012), and school smoking policies (Coppo 2012). This review looks at strategies for smoking cessation in young people and, more specifically, at the context in which the interventions are offered, and how young people are enrolled into quit attempts.
Objectives
To evaluate the effectiveness of strategies that help young people to stop smoking tobacco.
Methods
Criteria for considering studies for this review
Types of studies
Eligible study designs are randomized controlled trials, including:
individually randomized controlled trials, that is, trials in which individuals were randomized to either the intervention or the control arm of the experiment, or randomized to receive different interventions;
cluster‐randomized controlled trials, that is, trials that have as the unit of randomization a school, group or organization level, or where clusters of professionals or groups of professionals are implementing interventions.
Types of participants
Participants were young people, aged under 20 years, who were regular, current tobacco smokers. As there is evidence that some young people have an irregular pattern of smoking (Grimshaw 2003; O'Loughlin 2003), we have defined a regular smoker in this review as a young person who smokes an average of at least one cigarette a week, and has done so for at least six months. Trials did not always specify smoking status to this level of detail, but we excluded trials known to target young people who smoked less than this or known to include as 'smokers' people who did not currently smoke, but had smoked in the past.
If a study included participants beyond their 20th birthday (for example, 16‐ to 21‐year‐olds), we have included the study if the majority of participants were aged under 20, and if the design of the programme specifically considered the needs of young people.
Exclusions
We have excluded from this review interventions specifically targeting young women in pregnancy, since this topic is covered by the Pregnancy and Childbirth Group (Chamberlain 2017; Coleman 2015). We have also excluded any programme aimed primarily at the adult population, and have contacted investigators where there was a lack of clarity on this issue.
Types of interventions
Interventions could be specifically designed to meet the needs of young people aged under 20 years, or could also be applicable to adults. Interventions could range from simple ones such as pharmacotherapy, targeting individual young people, through strategic programmes targeting people or organizations associated with young people (for example, their families or schools), to complex programmes targeting the community in which young people study or live, provided the study reported outcomes related to the individual smoker.
To be included, all interventions had to be aimed at helping young people to stop smoking tobacco. We included cessation programmes and strategies that also targeted relapse. We included programmes or strategies that targeted psycho‐social determinants (for example, enhancing self‐efficacy for refusing tobacco), or that focused on developing life skills in order to stay abstinent, if the study design was appropriate. We did not place any restrictions on the setting in which the intervention was offered, for example, school, hospital, doctor's surgery, or dentist.
We excluded smoking prevention programmes, even if they reported cessation data, as they have been the subject of previous reviews (Carson 2011; Carson 2017; Thomas 2012). Within large‐scale, community primary prevention interventions, health‐education programmes/curricula or mass media campaigns that targeted young people, we only considered for inclusion the cessation component of those programmes, where the following three criteria were met: that part of the intervention had been specifically designed to target cessation; that the interventions could be separately assessed; and that the interventions explicitly met the criteria of this review for study design and recruitment.
Control conditions
Interventions in the control arm of the study could be one of the following:
no intervention;
delayed intervention beyond the last date of data acquisition including follow‐up;
information on stopping smoking either delivered to individuals in control groups or as literature (indicated in Characteristics of included studies as 'brief Intervention');
general tobacco education given to all participants in trial.
We also included studies that compared two different cessation interventions or combinations of interventions.
We have not included primary prevention strategies or programmes aimed solely at relapse prevention.
Types of outcome measures
Measures of quitting
The primary outcome of interest was change in smoking behaviour (being a smoker at baseline and becoming an ex‐smoker at follow‐up) at six months' follow‐up or longer. We excluded trials with follow‐up of less than six months. In trials that reported data at multiple follow‐up times, we chose for the primary analysis the shortest follow‐up of at least six months that used the most rigorous available definition of abstinence. We have not included relapse rates in the review.
We have reported the definition of cessation used in each trial, for example abstinence during a particular period, such as in the past seven or 30 days (point prevalence), abstinence from the start of the programme (continuous abstinence), or abstinence following occasional relapse in the two‐week, post‐treatment grace period (prolonged abstinence) (Hughes 2003). If studies reported cessation using more than one definition of abstinence we used the most rigorous outcome. Biochemical confirmation of self‐reported non‐smoking is generally taken to be the gold standard for reporting of quit rates (West 2005). This tests for the presence of smoking‐related substances in exhaled breath, saliva, urine or blood, and is the preferred verification method for reported outcomes where this is available. It should be noted that biochemical validation may not be a very sensitive measure of change in smoking status for irregular smokers; it is possible that some studies may have recruited participants on the basis of self‐reported smoking status who would not have been identified as smokers at baseline if biochemical validation had been used.
Adverse events
We extracted data on adverse events where reported.
Search methods for identification of studies
We used the Cochrane Tobacco Addiction Group search strategies to identify randomized controlled trials, cluster‐randomized controlled trials, and controlled trials of smoking cessation and prevention interventions. Trials relevant to the review were identified using the free text and keywords 'Child' or 'adolescent*' or 'adolescence'. We searched the Cochrane Tobacco Addiction Group Specialized Register on 8 June 2017. At the time of the search the Register included the results of searches of the Cochrane Central Register of Controlled trials (CENTRAL; 2016, issue 11); MEDLINE (via OVID) to update 20170526; Embase (via OVID) to week 201724; PsycINFO (via OVID) to update 20170529. See the Tobacco Addiction Group Module in the Cochrane Library for full search strategies and a list of other resources searched. We have also searched the 'grey literature' (unpublished resources and conference proceedings) and the reference lists of identified studies.
Where necessary, we have contacted the authors of existing trials and other experts for ongoing trials, and for unpublished results pertaining to completed trials, subject to the availability of peer review.
For previous updates, we also contacted smoking cessation e‐networks with a list of the references to extracted studies, to request verification and any additional information, and contacted manufacturers of smoking cessation products.
Data collection and analysis
Selection of studies
We drew up a prospective list of eligibility criteria with two levels of priority: essential and desirable. For the initial review, two original authors (GG and AS; see Acknowledgements) assessed the retrieved abstracts against this list for possible inclusion, to measure the feasibility of each criterion.
After piloting, we applied the agreed criteria to the abstracts of all studies extracted from the databases. We then categorized studies into three groups:
both authors agree on inclusion based on the abstract;
one author suggests inclusion based on the abstract;
both authors agree on exclusion based on the abstract.
We retrieved full‐text articles for groups 1 and 2. We used the processes outlined here and later for all updates.
Two authors independently assessed each full article, using the agreed inclusion criteria. For studies where there was disagreement, the editorial base or a third author was consulted to reach a consensus. Where there was ambiguity in trial reporting or lack of data, we contacted investigators for clarification where possible. If we could not retrieve missing data, a study may have been excluded on that basis.
Data extraction and management
We extracted and reported the following information, where it was available, concerning each study.
Country and study setting
Theoretical framework (including a brief description of the intervention)
Focus of the intervention
Type of intervention, its duration, intensity, delivery format, gatekeeper
Length of follow‐up
Size of eligible population
Recruitment rate
Number of participants or number of clusters and participants
Definition of the study population
Age range, gender, and ethnicity of participants
Definition of smoking status used at baseline
Definition of abstinence
Biochemical validation
Adverse effects of intervention
We have reported any threats to validity or other limitations described by the studies, and where we have contacted study authors for discrete data in the 'notes' section (see Characteristics of included studies).
A selection of potentially relevant studies, which were ultimately excluded, are listed in the Characteristics of excluded studies table.
Assessment of risk of bias in included studies
We rated each included study as being at low, unclear, or high risk of bias in five domains.
Random sequence generation
Concealment of allocation. For cluster‐randomized controlled trials, which recruited after allocation to intervention or control status, we took account of whether individuals may have been selectively recruited or may have differentially refused to participate in the light of the known allocation, where this could be ascertained (Campbell 2004a; Campbell 2004b; Hahn 2005).
Performance bias (blinding of participants and personnel, if applicable)
Detection bias (blinding of outcome assessment, biochemical validation)
Attrition
We also recorded any other risks of bias that did not fit in the above categories (Higgins 2011).
Measures of treatment effect
We summarized the effect size for each individual study as a risk ratio (RR) with 95% confidence interval (CI). The RR is calculated as (number quit in intervention group/number randomized to intervention)/(number quit in control group/number randomized to control), with participants randomized but lost to follow‐up regarded as non‐abstinent.
Unit of analysis issues
Outcomes of all cluster randomized trials were given at the participant level, that is, the unit of analysis was different from that used for randomization. We checked whether the study authors' analysis used a method to account for clustering effects, such as multi‐level modelling. If the analysis clearly stated that they had made an allowance for clustering, or had examined the clustering effect but found it to be negligible, we extracted the effect size and standard error (or 95% CI) reported in the paper, converting odds ratios to approximate RRs if necessary. Otherwise, if a trial was cluster‐randomized but the study authors had not allowed for this in the published analysis, we extracted the log‐RR and its standard error and the average cluster size, and adjusted it using an assumed inter‐cluster correlation (ICC) of 0.03, a similar value to that estimated in several of the included studies.
Dealing with missing data
It is a frequent feature of analysis of smoking cessation studies that cases lost to follow‐up are assumed to be still smoking. Several authors discussed this issue and made adjustments in the analysis (e.g. Haug 2013; Hollis 2005; Joffe 2009) and/or analysed their data on an intention‐to‐treat basis, that is, including all participants in the groups to which they were originally randomized, and classifying those lost to follow‐up as continuing smokers. In this review we also counted cases lost to follow‐up as current smokers, even if the primary studies had not explicitly done this. One other reason for data being unavailable for the review was a tendency for study authors to report results as percentages, sometimes without any particular clarity as to the denominator. Some of the results of our analysis have therefore been imputed from percentages, assuming the denominator to be the number of participants randomized. Where possible, we have contacted study authors to ask for verification of the calculations (Brown 2003; Colby 2005; Killen 2004; Lipkus 2004; Project EX‐1 2001).
Data synthesis
We pooled groups of studies that we consider to be sufficiently similar in their interventions, comparison groups, setting, and participants, provided that there was no evidence of substantial statistical heterogeneity as assessed by the I² statistic (Higgins 2003). Specifically, we have presented results for groups of studies characterized by the mode of delivery of the intervention, the theoretical basis underpinning the intervention, and according to the pharmacotherapy used (where applicable). We estimated a pooled RR using the Mantel‐Haenszel fixed‐effect model, based on the quit rates at follow‐up. Where meta‐analysis was not appropriate, we have presented summary and descriptive statistics. For studies for which we were unable to obtain reliable numerical data, we have reported the main results narratively.
Sensitivity analysis
In sensitivity analyses we assumed values for the ICC of 0 and 0.05 (in addition to the value of 0.03 assumed for the main analysis) in making the adjustment to the standard errors for clustering.
Results
Description of studies
Results of the search
For this update, we identified 247 references, from which 15 new trials (Bailey 2013; Colby 2012; Dalum 2012; Gungormus 2012; Guo 2014; Haug 2013; Mason 2016; Pbert 2011; Pérez‐Milena 2012; Prochaska 2015; Project EX Spain 2015a; Project EX Spain 2015b; Redding 2015; Scherphof 2014; Skov‐Ettrup 2014) were added to the list of included studies. A reassessment of previously excluded studies based on the new inclusion criteria identified four further studies (Abroms 2008; Harris 2010; O'Neill 2000; Project EX‐4 2007) (see Differences between protocol and review). The previous update of this review contained 28 studies. The new inclusion criteria resulted in six of these studies (Chan 1988; Myers 2005; NoT AL 2008; NoT FL 2001; NoT NC 2005; NoT WV 2004) being excluded as they are not randomized trials. This update therefore contains 41 studies (26 individually randomized and 15 cluster‐randomized), which included a total of 13,292 participants. Figure 1 displays the numbers of records screened and studies included in previous versions of the review, as well as the study flow for this update. Trials excluded at the full‐text screening stage are listed in the Characteristics of excluded studies table with reasons for their exclusion, and the characteristics of six ongoing studies can be found in the Characteristics of ongoing studies table.
1.
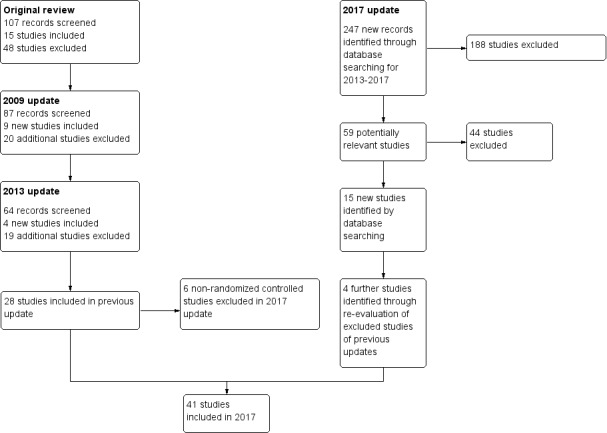
Study flow diagram
Included studies
We have given full details of the included studies in the Characteristics of included studies table, where new trials are identified in the notes as "New for 2017 update". Trials are identified by the first author and the publication year of the main report, except for a group of studies reporting the Not on Tobacco (NoT) programme and the Project EX programme, which are identified by programme type, trial location and publication year of the main report.
Delivery method
We classified studies that used person‐to‐person counselling interventions as those that used individual counselling (eight studies: Bailey 2013; Colby 2005; Colby 2012; Harris 2010; Pbert 2011; Pérez‐Milena 2012; Robinson 2003; Sherbot 2005) and those that used group counselling (10 studies: Greenberg 1978; Hoffman 2008; Joffe 2009; NoT MD 2009; NoT WV 2011; Project EX‐1 2001; Project EX‐4 2007; Project EX Russia 2013; Project EX Spain 2015a; Project EX Spain 2015b). The individual counselling sessions were delivered by trained interventionists, therapists, health educators, general practitioners, or nurses (see Characteristics of included studies). Nine studies used computer‐based or messaging interventions, possibly also including a face‐to‐face counselling component (Aveyard 2001; Haug 2013; Hollis 2005; Mason 2016; O'Neill 2000; Patten 2006; Redding 2015; Skov‐Ettrup 2014; Woodruff 2007). Eight studies, whose interventions explicitly comprised multiple delivery methods, including the provision of self‐help materials, form a separate subcategory (Abroms 2008; Dalum 2012; Gungormus 2012; Guo 2014; Horn 2007; Kelly 2006; Lipkus 2004; Peterson 2009). Of the remaining six studies, four used a purely pharmacological intervention (Killen 2004; Moolchan 2005; Muramoto 2007; Scherphof 2014), described separately below, and two used a combination of counselling and a pharmacological intervention (Bailey 2013; Prochaska 2015).
Theoretical basis of intervention
It was difficult to stratify many of the studies into categories corresponding to a single theoretical model that formed the basis of the intervention, and for some studies no relevant information was available. One intervention, conducted in 1978, used the health promotion strategies of that period (Greenberg 1978). However, many interventions were complex and used combinations of psycho‐social theories (see Sheppard 2009 for discussion of management of reviews of complex interventions). Constructs relating to motivational enhancement and strategies for resisting cultural and social pressures were the most common. Studies of this type included those using motivational interviewing (Brown 2003; Colby 2005; Colby 2012; Harris 2010; Horn 2007; Kelly 2006; Lipkus 2004; Mason 2016; Pérez‐Milena 2012; Sherbot 2005) sometimes combined with some form of relapse prevention advice and ongoing support. Other studies tested interventions based on the Transtheoretical Model of Stages of Change for adolescents (Prochaska 2000; Redding 2015), either alone (Aveyard 2001; Dalum 2012; Gungormus 2012; Haug 2013; O'Neill 2000; Redding 2015) or in combination with other modalities, including brief advice and motivational enhancement (Guo 2014; Hoffman 2008; Hollis 2005; Peterson 2009; Robinson 2003; Woodruff 2007) or, in the case of the Project EX suite of studies, a more eclectic mix, including yoga and meditation (Project EX‐1 2001; Project EX‐4 2007; Project EX Russia 2013; Project EX Spain 2015a; Project EX Spain 2015b). Six studies based their intervention design on social cognitive theory (SCT) (Abroms 2008; NoT MD 2009; NoT WV 2011; Patten 2006; Pbert 2011; Skov‐Ettrup 2014).
Pharmacological interventions
Of the four studies of pharmacological interventions, one compared a nicotine patch with placebo after all participants received a short behavioural intervention (Scherphof 2014). One randomized participants to receive either a nicotine patch with placebo gum, nicotine gum with a placebo patch, or a placebo patch and placebo gum, with all participants receiving a short group cognitive behavioural therapy (CBT) session and self‐help materials (Moolchan 2005). One used bupropion versus a placebo tablet, with all participants receiving brief counselling (Muramoto 2007), and one compared a combination treatment of nicotine patch plus bupropion to nicotine patch plus a placebo tablet, with all participants receiving group skills training (Killen 2004).
Recruitment and settings
The majority of trials were based in North America ‐ one in Canada (Sherbot 2005) and 28 in the USA. Of the remainder, one took place in the UK (Aveyard 2001), two in Denmark (Dalum 2012; Skov‐Ettrup 2014), one in Switzerland (Haug 2013), one in the Netherlands (Scherphof 2014), three in Spain (Pérez‐Milena 2012; Project EX Spain 2015a; Project EX Spain 2015b), one in Russia (Project EX Russia 2013), one in Turkey (Gungormus 2012), one in Australia (Kelly 2006) and one in Taiwan (Guo 2014).
As can be expected from a cohort where most participants were in formal education, recruitment for studies mainly occurred within schools (Aveyard 2001; Bailey 2013; Colby 2012; Dalum 2012; Greenberg 1978; Gungormus 2012; Guo 2014; Haug 2013; Hoffman 2008; Joffe 2009; Kelly 2006; Killen 2004; Mason 2016; Moolchan 2005; NoT MD 2009; NoT WV 2011; Pbert 2011; Pérez‐Milena 2012; Peterson 2009; Project EX‐1 2001; Project EX‐4 2007; Project EX Spain 2015a; Project EX Spain 2015b; Robinson 2003; Scherphof 2014; Woodruff 2007), universities (Abroms 2008; Harris 2010; O'Neill 2000) or summer camps (Project EX Russia 2013). Educational settings have the advantage of easier recruitment and minimization of contamination. Nine studies recruited wholly or partially from the healthcare environment (Brown 2003; Colby 2005; Colby 2012; Hollis 2005; Horn 2007; Mason 2016; Prochaska 2015; Redding 2015; Sherbot 2005) and one further study via a website aimed at smoking cessation (Skov‐Ettrup 2014). Three studies (Lipkus 2004; Muramoto 2007; Patten 2006) recruited directly from the community. Where a school or college was the base, trials were often cluster‐randomized, with the intervention delivered to all students in one school, with matched schools used for control (e.g. Aveyard 2001; Guo 2014; Woodruff 2007), although there were also examples of individually randomized trials in educational settings (e.g. Bailey 2013; Joffe 2009).
The rate of recruitment was commented on by several trialists. Where schools were recruited and matched or randomized, and attendance in the programme was not compulsory, typically fewer than half of the students who smoked showed interest in enrolling (Greenberg 1978; NoT studies; Project EX‐1 2001; Project EX Russia 2013). Some trials that recruited from healthcare settings reported recruitment rates higher than 50% of eligible participants (e.g. Colby 2012; Horn 2007), substantially so in the case of one trial based in a mental‐health setting (Prochaska 2015). Recent trials of text messaging interventions have achieved participation rates of above 70% when recruitment took place in a school‐based, cluster‐randomized trial (Haug 2013) or when the intervention was supplemented with peer support (Mason 2016), but lower recruitment rates when participants were recruited online (Skov‐Ettrup 2014).
For many studies with lower recruitment rates, parental permission was a requirement. Inducements to enrol or remain in the study (money, gift cards or class credits) were also a feature of many trials (Abroms 2008; Bailey 2013; Brown 2003; Colby 2005; Colby 2012; Greenberg 1978; Guo 2014; Haug 2013; Joffe 2009; Killen 2004; Lipkus 2004; Mason 2016; Moolchan 2005; NoT MD 2009; Patten 2006; Peterson 2009; Prochaska 2015; Project EX‐1 2001; Redding 2015; Robinson 2003; Scherphof 2014; Sherbot 2005; Woodruff 2007). In some trials an element of compulsion was present, either with attendance as a consequence of a smoking policy violation (Robinson 2003) or as a controlled regimen in a hospital setting (Brown 2003). Intention to quit smoking was a pre‐requisite of many trials but was not required for inclusion in this review.
Definition of smoking
One of the crucial issues for smoking cessation research for young people is how smoking is defined, and how cessation is defined and verified. The cessation issues are described in the subsection following, in the Risk of bias in included studies section and in the Discussion.
There was variation among the included studies concerning the definition of smoking status, with most relying on self‐reported smoking status at recruitment (see Characteristics of included studies). In general, at least one cigarette per week (cpw) was used as a definition of being a smoker. Studies used many different definitions (e.g. one cigarette per day at recruitment, or ten cigarettes in the previous 30 days) and it is likely that some studies with less stringent inclusion criteria recruited some participants who smoked less frequently than one cpw at the time of recruitment. Where there was doubt, we assured compatibility with our criteria through discussion with study authors, where possible. Hollis 2005 differentiated between smokers and 'experimenters', but no studies explicitly took account of the episodic nature of adolescent smoking (Corby 2000; Grimshaw 2003). Many studies estimated nicotine dependence using some form of scale, most commonly the modified Fagerström Questionnaire (e.g. Killen 2004; Mason 2016), alongside self‐reported measures. Other studies used cotinine or exhaled carbon monoxide in the baseline smoking status assessment or as part of the inclusion criteria (e.g. Muramoto 2007; see Characteristics of included studies for details).
Measurement of outcomes
The primary outcome was individual‐level smoking cessation. Just as a wide variety of definitions of smoking was used, so there were several definitions of cessation.
The gold standard outcome of continuous abstinence (West 2005) was used by three studies (O'Neill 2000; Pérez‐Milena 2012; Peterson 2009). Other continuous measures included "prolonged abstinence", defined as continuous abstinence following an initial two‐week grace period (Moolchan 2005), and "sustained cessation", defined as two sequential reports of seven‐day point prevalence abstinence at four months and eight months from the start of the intervention (Lipkus 2004). One study used a self‐reported measure based solely on the participant's categorization in the Stages of Change model (Redding 2015).
Point prevalence measures were in the majority and these ranged from cessation for one day (Hoffman 2008) to 30‐day cessation (Aveyard 2001; Dalum 2012; Guo 2014; Harris 2010; Haug 2013; Hollis 2005; Joffe 2009; Kelly 2006; Mason 2016; NoT MD 2009; Patten 2006; Pbert 2011; Project EX‐1 2001; Project EX‐4 2007; Project EX Russia 2013; Project EX Spain 2015a; Project EX Spain 2015b; Scherphof 2014; Skov‐Ettrup 2014). Another common outcome measure was seven‐day point prevalence abstinence (Abroms 2008; Aveyard 2001; Bailey 2013; Brown 2003; Colby 2005; Colby 2012; Haug 2013; Killen 2004; Lipkus 2004; Moolchan 2005; Muramoto 2007; NoT WV 2011; Prochaska 2015; Robinson 2003; Woodruff 2007).
Verification of smoking status
Of the 41 studies that satisfied the inclusion criteria for this review, 23 used or attempted some form of biochemical verification of self‐reports of smoking status for the whole cohort or for the full duration of follow‐up. Seven trials used more than one method of biochemical verification (Brown 2003; Colby 2005; Colby 2012; Killen 2004; Moolchan 2005; Prochaska 2015; Robinson 2003). Carbon monoxide levels were measured for verification in 1415 listed trials (Bailey 2013; Brown 2003; Colby 2005; Colby 2012; Killen 2004; Moolchan 2005; Muramoto 2007; NoT WV 2011; Patten 2006; Pérez‐Milena 2012; Prochaska 2015; Project EX‐1 2001; Project EX‐4 2007; Project EX Spain 2015b; Robinson 2003), salivary cotinine in 15 trials (Abroms 2008; Brown 2003; Colby 2005; Colby 2012; Harris 2010; Hoffman 2008; Joffe 2009; Killen 2004; Lipkus 2004; Moolchan 2005; NoT MD 2009; Pbert 2011; Prochaska 2015; Robinson 2003; Scherphof 2014) and urinary cotinine in one trial (Guo 2014). Peterson 2009 used internal verification within questionnaires. Two studies reported using a form of "bogus pipeline" alongside biochemical validation in an attempt to improve the assessment of smoking status (Harris 2010; Robinson 2003).
Risk of bias in included studies
Figure 2 summarizes the review authors' judgements across each risk of bias domain and Figure 3 shows a breakdown for each domain by study. We judged the majority of studies to be at unclear or high risk of bias in at least one domain.
2.
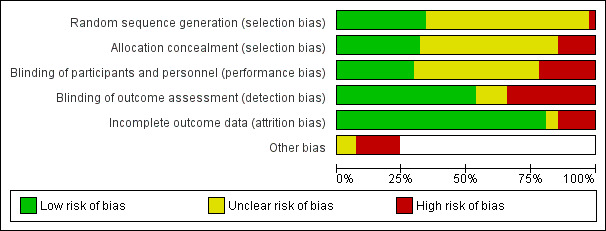
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
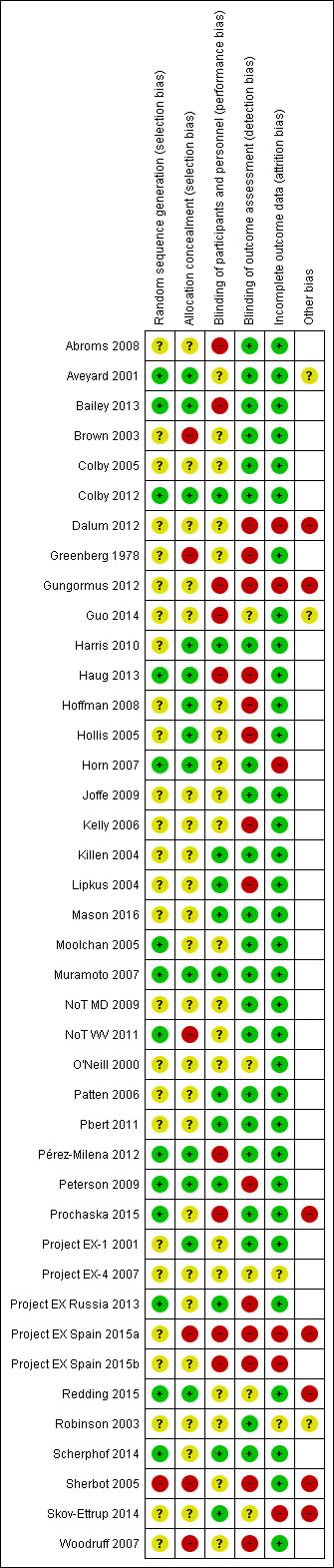
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Of the 41 included studies, 15 were cluster‐randomized (Aveyard 2001; Dalum 2012; Guo 2014; Harris 2010; Haug 2013; Hoffman 2008; NoT WV 2011; Pbert 2011; Peterson 2009; Project EX‐1 2001; Project EX‐4 2007; Project EX Russia 2013; Project EX Spain 2015a; Project EX Spain 2015b; Woodruff 2007) and the remainder allocated individuals. Of the cluster‐randomized trials, we judged three to be at high risk of selection bias because of the way in which institutions or students within clusters were recruited (NoT WV 2011; Project EX Spain 2015a; Woodruff 2007). Three of the individually randomized studies were rated at high risk of selection bias because of the method of allocation or concealment (Brown 2003; Greenberg 1978; Sherbot 2005). Twenty‐five studies did not provide sufficient detail on either randomization or allocation, and hence we judged them to be at unclear risk of selection bias.
Blinding
We rated nine studies as having high risk of performance bias (Abroms 2008; Bailey 2013; Gungormus 2012; Guo 2014; Haug 2013; Pérez‐Milena 2012; Prochaska 2015; Project EX Spain 2015a; Project EX Spain 2015b). These were generally behavioural intervention trials in which there was a considerable difference in the extent of intervention given according to group allocation. We judged 19 studies to be at unclear risk of performance bias, as it was not clear if blinding had taken place or, in the case of behavioural interventions, was not clear whether participants in control groups were aware of the programme the intervention arms were receiving.
We judged 14 studies that involved face‐to‐face contact in the intervention group to be at high risk of detection bias, as they employed inadequate or no biochemical validation and were liable to possible differential misreport (Dalum 2012; Greenberg 1978; Gungormus 2012; Haug 2013; Hoffman 2008; Hollis 2005; Kelly 2006; Lipkus 2004; Peterson 2009; Project EX Russia 2013; Project EX Spain 2015a; Project EX Spain 2015b; Sherbot 2005; Woodruff 2007).
Incomplete outcome data
The percentage of participants lost to follow‐up was less than 10% in some studies, but was often high, and sometimes above 50% (Dalum 2012; Horn 2007; Lipkus 2004; Moolchan 2005; Project EX Spain 2015a; Skov‐Ettrup 2014). We judged six studies to be at high risk of attrition bias, as having particularly high or unexplained dropout, especially if this occurred differentially between groups (Dalum 2012; Gungormus 2012; Horn 2007; Project EX Spain 2015a; Project EX Spain 2015b; Skov‐Ettrup 2014). We judged two further studies to be at unclear risk, as they did not report attrition rates in sufficient detail to make a judgement (Robinson 2003; Project EX‐4 2007). We judged all other studies to be at low risk of attrition bias.
Other potential sources of bias
We also evaluated studies for any other potential sources of bias. We judged four studies to be at unclear or high risk of other bias owing to possible or confirmed issues with treatment fidelity and contamination (Aveyard 2001; Dalum 2012; Robinson 2003; Skov‐Ettrup 2014). We judged one study to be at high risk of other bias due to significant between‐group differences at baseline (Sherbot 2005), and three at high or unclear risk because of inadequate or inconsistent reporting of data by group (Guo 2014; Prochaska 2015; Project EX Spain 2015a). We classified one study as having high risk of other bias because the definition of the cessation outcome measure appeared not to be consistent with the maintenance stage of the Stages of Change model used (Gungormus 2012), and one because of doubts about the extent to which the smoking cessation intervention was delivered (Redding 2015).
Effects of interventions
Summary of findings for the main comparison. Behavioural interventions compared to minimal control for smoking cessation in young people.
| Behavioural interventions compared to minimal control for smoking cessation in young people | ||||||
| Patient or population: young people Setting: community, school and healthcare settings Intervention: behavioural interventions Comparison: minimal control | ||||||
| Comparisons and outcomes1 | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with minimal control | Risk with behavioural interventions | |||||
| Individual counselling (in‐person) vs control Smoking cessation assessed with: biochemical validation and self‐report Follow‐up: range 6 months to 12 months | Study population | RR 1.07 (0.83 to 1.39) | 2088 (7 RCTs) | ⊕⊕⊝⊝ Low2,3 | Control risk based on rates in included studies | |
| 90 per 1000 | 97 per 1000 (75 to 126) | |||||
| Group counselling vs control Smoking cessation assessed with: biochemical validation and self‐report Follow‐up: range 6 months to 12 months | Study population | RR 1.35 (1.03 to 1.77) | 1910 (9 RCTs) | ⊕⊕⊝⊝ Low3,4 | Control risk based on rates in included studies | |
| 142 per 1000 | 191 per 1000 (146 to 251) | |||||
| Computer‐based interventions vs control Smoking cessation assessed with: biochemical validation and self‐report Follow‐up: range 6 months to 12 months | Study population | RR 0.79 (0.50 to 1.24) | 340 (3 RCTs) | ⊕⊕⊝⊝ Low4,5 | Control risk based on rates in included studies | |
| 191 per 1000 | 151 per 1000 (96 to 237) | |||||
| Text messaging‐based interventions vs control Smoking cessation assessed with: self‐report Follow up: range 6 months to 12 months | Study population | RR 1.18 (0.90 to 1.56) | 2985 (3 RCTs) | ⊕⊕⊝⊝ Low4,5 | Some interventions also included access to intervention website. Control risk based on rates in included studies | |
| 57 per 1000 | 67 per 1000 (51 to 89) | |||||
|
Interventions with multiple delivery methods vs control Smoking cessation assessed with: biochemical validation and self‐report Follow‐up: 6 months to 14 months |
Study population | RR 1.26 (0.95 to 1.66) | 2755 (8 RCTs) | ⊕⊝⊝⊝ Very low3,4,5 | This represents a diverse set of delivery modes; all interventions included self‐help materials alongside other, more intensive delivery modes. Control risk based on rates in included studies | |
| 59 per 1000 | 74 per 1000 (56 to 98) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Adverse events not included as not assessed for behavioural interventions. 2Downgraded one level due to risk of bias: all but one study at high or unclear risk of bias. 3Downgraded one level due to inconsistency: interventions were clinically heterogeneous. 4Downgraded one level due to risk of bias: all studies at high or unclear risk of bias. 5Downgraded one level due to imprecision: confidence intervals are consistent with no effect and clinically significant effect.
Summary of findings 2. Pharmacological interventions compared to placebo for smoking cessation in young people.
| Pharmacological interventions compared to placebo for smoking cessation in young people | ||||||
| Patient or population: young people Setting: schools, community Intervention: pharmacological interventions Comparison: placebo | ||||||
| Comparisons and outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with pharmacological interventions | |||||
| NRT vs placebo Smoking cessation assessed with: biochemical verification Follow‐up: range 6 months to 12 months | Study population | RR 1.11 (0.48 to 2.58) | 385 (2 RCTs) | ⊕⊝⊝⊝ Very low1,2 | Both studies included single forms of NRT (patch or gum). No evidence of significant subgroup differences based on NRT type. Control risk based on rates in included studies | |
| 59 per 1000 | 66 per 1000 (28 to 153) | |||||
|
NRT vs placebo
Adverse events assessed with: participant report Follow‐up: range 6 months to 12 months |
No serious adverse events reported. NRT associated with increase in some mild adverse events: sore throat; hiccups; erythema; pruritus; shoulder/arm pain; headache; cough; abnormal dreams; and muscle pain. In the patch studies, successful quitters in NRT group reported a lower level of insomnia than those in the control group. | 385 (2 RCTs) | ⊕⊝⊝⊝ Very low1,2 | Both studies included single forms of NRT (patch or gum) | ||
| Bupropion vs placebo Smoking cessation assessed with: biochemical validation Follow‐up: 26 weeks | Study population | RR 1.49 (0.55 to 4.02) | 207 (1 RCT) | ⊕⊝⊝⊝ Very low1,3 | Control risk based on rates in included studies | |
| 58 per 1000 | 87 per 1000 (32 to 234) | |||||
| Bupropion vs placebo Adverse events assessed with: participant report Follow‐up: 26 weeks | 2 serious adverse events resulting in hospitalization among intervention participants: anticholinergic crisis after ingesting Datura innoxia; intentional overdose on study medication and other substances. High level of mild adverse events reported in both groups (headache, cough, throat symptoms, sleep disturbance and nausea each reported by more than 10% of participants). 8 participants discontinued bupropion because of adverse events. | 207 (1 RCT) | ⊕⊝⊝⊝ Very low1,3 | |||
| Nicotine patch + bupropion vs nicotine patch + placebo Smoking cessation assessed with: biochemical validation Follow‐up: 6 months | Study population | RR 1.05 (0.41 to 2.69) | 211 (1 RCT) | ⊕⊝⊝⊝ Very low1,3 | Control risk based on rates in included studies | |
| 74 per 1000 | 78 per 1000 (30 to 199) | |||||
| Nicotine patch + bupropion vs nicotine patch + placebo Adverse events assessed with: participant report Follow‐up: 6 months | No serious adverse events reported. Nausea most commonly reported adverse event. | 211 (1 RCT) | ⊕⊝⊝⊝ Very low1,3 | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NRT: nicotine replacement therapy; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded two levels due to serious imprecision: small number of events (< 300 overall), confidence intervals are consistent with no effect and a clinically significant effect. 2Downgraded one level due to risk of bias: both studies at unclear risk of bias in at least one domain. 3Downgraded one level due to risk of bias: study at unclear risk of bias.
Smoking cessation
Details of individual study outcomes are given in the Data and analyses section, split by subgroup. Analysis 1 displays results of studies comparing behavioural interventions with control, grouped by type of behavioural intervention. Analysis 2 also displays results of studies comparing behavioural interventions with control, but interventions are grouped by the theoretical basis of the intervention. Analysis 3 contains studies of pharmacological interventions. Analysis 4 contains the results from the Project EX trials. Four studies do not appear in these analyses. For one, we were unable to establish the denominator and the study report was published before follow‐up was complete (Robinson 2003). One study did not report sufficient data for numerical extraction (Project EX Russia 2013). Two studies used a combination of counselling and pharmacological interventions (Bailey 2013; Prochaska 2015) and so did not did not fit into the categorization adopted for this review; one of these (Prochaska 2015) also did not provide sufficient data with which a summary statistic could be calculated.
Behavioural interventions versus control, grouped by delivery mode
Among studies that primarily offered individual counselling as the intervention (Analysis 1.1), the pooled risk ratio (RR) for smoking cessation was 1.07 (95% confidence interval (CI) 0.83 to 1.39, I2 = 1%, seven studies, n = 2088). This estimate was heavily influenced by the results of Harris 2010, which showed a small, negative (albeit not statistically significant) effect of the intervention on 30‐day point prevalence abstinence at six months. This study however claimed a beneficial effect of the intervention on quit attempts and an increased rate of cessation among heavier baseline smokers. Harris 2010 and Pérez‐Milena 2012 also demonstrated notably higher quit rates in both intervention and control groups than the much larger study of Pbert 2011 (crude intervention group quit rate 20% for Harris 2010, 30% for Pérez‐Milena 2012, 5% for Pbert 2011).
1.1. Analysis.
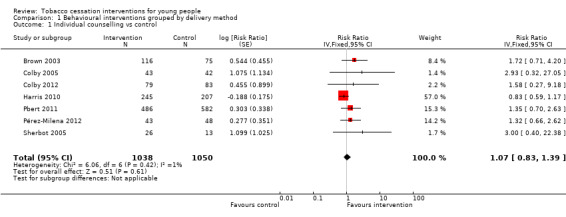
Comparison 1 Behavioural interventions grouped by delivery method, Outcome 1 Individual counselling vs control.
Studies that used group counselling (Analysis 1.2) demonstrated a larger intervention effect (RR 1.35, 95% CI 1.03 to 1.77, I2 = 0%, nine studies, n = 1910). None of the nine contributing studies showed a statistically significant effect of the intervention individually, although in eight of these studies the point estimate of the RR was above one and many had small sample sizes (less than 100 participants per group). Two studies yielded very large point estimates because of very low (Greenberg 1978) or zero (Project EX Spain 2015a) quit rates in the control group.
1.2. Analysis.
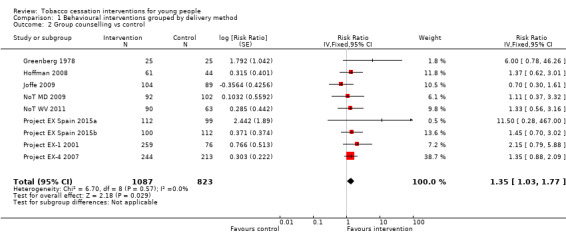
Comparison 1 Behavioural interventions grouped by delivery method, Outcome 2 Group counselling vs control.
Among interventions that used information or communication technology (Analysis 1.3), there were no statistically significant intervention effects for either purely computer‐based interventions (RR 0.79, 95% CI 0.50 to 1.24, I2 = 0%, three studies, n = 340) or messaging interventions (RR 1.18, 95% CI 0.90 to 1.56, I2 = 0%, three studies, n = 2985). When we pooled results from the three studies that used a combination of a computer‐based intervention and face‐to‐face counselling, statistical heterogeneity was high (I2 = 62%), with substantially different results between the two largest trials, and hence we have not presented pooled results for this comparison. Aveyard 2001 (based on the results after one year of follow‐up reported in Aveyard 1999) found no effect (RR 1.03, 95% CI 0.80 to 1.33, n = 1089), whereas Hollis 2005 found a strong, positive intervention effect (RR 1.79, 1.19 to 2.71, n = 448). The latter's authors attribute the difference to greater provision of adjunct counselling alongside the computer‐based materials, as in Aveyard 2001 the face‐to‐face component of the intervention was delivered to participants in a group in a school classroom setting. The effect sizes at one year and two years for Hollis 2005 were very similar, even though the percentage of participants in the intervention group achieving cessation dropped from 28% to 24% between these two time points, while two‐year follow‐up results provided by Aveyard 2001 continued to show a non‐statistically significant intervention effect. The third study in this subgroup found no intervention effect, but this was based on a small subgroup of the total sample who were smokers at baseline (Redding 2015).
1.3. Analysis.
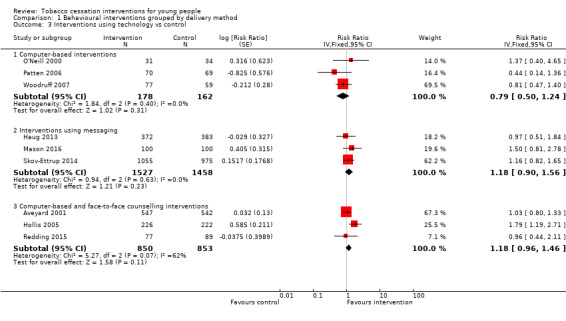
Comparison 1 Behavioural interventions grouped by delivery method, Outcome 3 Interventions using technology vs control.
The diverse category of trials whose interventions included multiple delivery methods (all included self‐help materials alongside other more intensive delivery modes) (Analysis 1.4) showed a pooled RR of 1.26 (95% CI 0.95 to 1.66, I2 = 0%, eight studies, n = 2755). In this analysis there were also two small trials with a very high RR estimate because of a low cessation rate in the control group (Gungormus 2012; Guo 2014); these studies were rated as having high risk of bias but because of their size were relatively uninfluential on the pooled estimate. The three largest trials in this meta‐analysis (Dalum 2012; Lipkus 2004; Peterson 2009) all showed positive, non‐statistically significant findings, with RR estimates ranging between 1.10 and 1.29. All three of these trials used a multifactorial intervention that included elements of counselling alongside self‐help materials, with some level of tailoring to individual requirements.
1.4. Analysis.
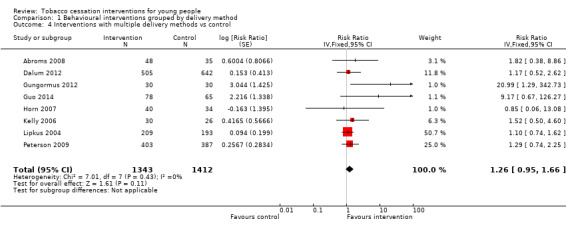
Comparison 1 Behavioural interventions grouped by delivery method, Outcome 4 Interventions with multiple delivery methods vs control.
Behavioural interventions versus control, grouped by theoretical basis
The theoretical basis of behavioural interventions was not always easy to classify, as the studies did not always provide detailed information and some studies used interventions that combined multiple theoretical approaches.
In the three subgroups that could be defined by the theoretical basis of the intervention, corresponding to Stage of Change models (Analysis 2.1), motivational interviewing (Analysis 2.2) and social cognitive theory (SCT) (Analysis 2.3) respectively, all showed small effect sizes (stage of change versus control RR 1.06, 95% CI 0.85 to 1.31, I2 = 0%, six studies, n = 3282; motivational interviewing versus control RR 1.11, 95% CI 0.90 to 1.36, I2 = 0%, ten studies, n = 1752; SCT versus control RR 1.16, 95% CI 0.88 to 1.51, I2 = 0%, six studies, n = 3667).
2.1. Analysis.
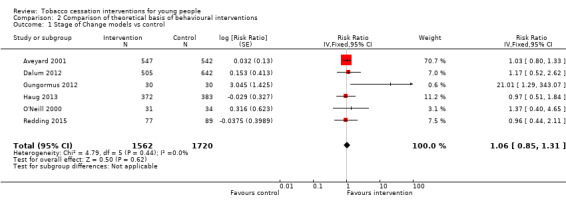
Comparison 2 Comparison of theoretical basis of behavioural interventions, Outcome 1 Stage of Change models vs control.
2.2. Analysis.
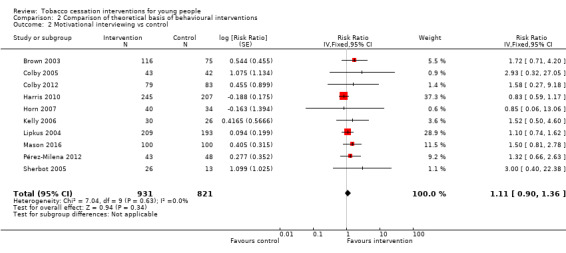
Comparison 2 Comparison of theoretical basis of behavioural interventions, Outcome 2 Motivational interviewing vs control.
2.3. Analysis.
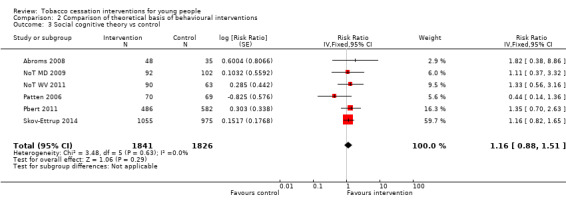
Comparison 2 Comparison of theoretical basis of behavioural interventions, Outcome 3 Social cognitive theory vs control.
In the group of studies that were classified as using a complex theoretical model (e.g. drawing on multiple theories) (Analysis 2.4), there was evidence of a positive intervention effect (RR 1.40, 95% CI 1.14 to 1.74, I2 = 14%, nine studies, n = 2827). One study was quite influential on the pooled estimate, and was the only one of the contributing trials that reported a statistically significant intervention effect (Hollis 2005). Of the two studies that could not be included in the meta‐analysis but also fell into this category, the study of Project EX Russia 2013 in summer recreation camps claimed a higher smoking cessation rate for smoking in the intervention group (in the context of a near‐zero cessation rate among participants in the control group) and Robinson 2003, using a combination of CBT and motivational techniques delivered over four sessions with telephone follow‐up, did not detect any effect on cessation.
2.4. Analysis.
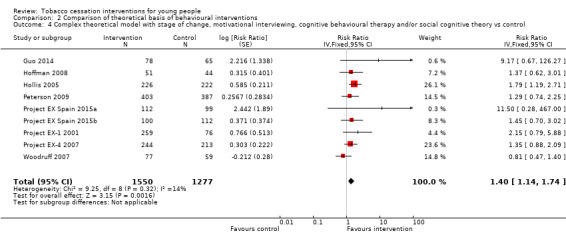
Comparison 2 Comparison of theoretical basis of behavioural interventions, Outcome 4 Complex theoretical model with stage of change, motivational interviewing, cognitive behavioural therapy and/or social cognitive theory vs control.
Pharmacological interventions
This review contains four studies that used pharmacological interventions. Effect sizes are displayed in Analysis 3.1, Analysis 3.2 and Analysis 3.3. All studies were relatively small and abstinence rates were low, and so confidence intervals are wide. The only studies that used directly comparable interventions were two trials that used nicotine replacement therapy (NRT) (Moolchan 2005; Scherphof 2014). These yielded a pooled RR of 1.11 (95% CI 0.48 to 2.58, I2 = 20%, n = 385). Pooled results from nicotine patch yielded an RR of 1.02 (95% CI 0.41 to 2.56, I2 = 57%, n = 319). Moolchan 2005 also used a nicotine gum treatment arm: the RR of 1.74 (95% CI 0.21 to 14.60, n = 66) for gum versus placebo at six months had a very wide confidence interval. Muramoto 2007 did not detect evidence for a benefit of standard dose bupropion (RR 1.49, 95% CI 0.55 to 4.02, n = 207), and Killen 2004 also failed to detect an effect for bupropion used as an adjunct to NRT patches versus patches alone (RR 1.05, 95% CI 0.41 to 2.69, n = 211). Given the small number of individuals in both the intervention or control groups who achieved smoking cessation at any point during follow‐up, these studies appear to be severely underpowered.
3.1. Analysis.
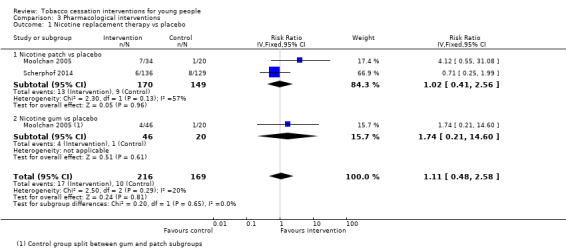
Comparison 3 Pharmacological interventions, Outcome 1 Nicotine replacement therapy vs placebo.
3.2. Analysis.
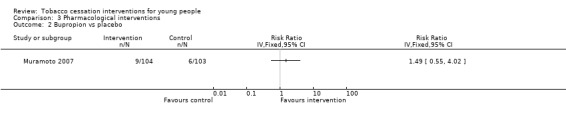
Comparison 3 Pharmacological interventions, Outcome 2 Bupropion vs placebo.
3.3. Analysis.
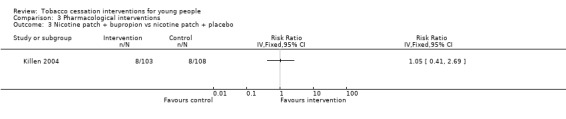
Comparison 3 Pharmacological interventions, Outcome 3 Nicotine patch + bupropion vs nicotine patch + placebo.
Two trials, not included in forest plots, used interventions that combined a pharmacological and a behavioural component. Both the intervention and the control group participants of Bailey 2013 received 10 weeks of group‐based CBT and skills training, followed by nine weeks of therapy using a nicotine patch; the intervention group additionally received nine additional subsequent group sessions ("extended treatment"). The trial resulted in a large increase in smoking cessation in the intervention group compared to control (seven‐day point prevalence abstinence at 6 weeks: 15/72 versus 5/71, RR 2.96, 95% CI 1.14 to 7.71, n = 143 (analysis not shown)). Prochaska 2015 used a complex intervention that consisted of several components, including a Transtheoretical Model (Stages of Change) (TTM)‐based computer intervention, six sessions of CBT and the option of 12 weeks using a nicotine patch for heavier smokers. This study reported 15% seven‐day point prevalence abstinence after 12 months for all trial participants, but no evidence of a difference between the study arms (full results data were not available).
Project EX interventions
Five eligible trials used a version of Project EX, originally developed as a clinic‐based smoking cessation programme (Sussman 2004): an initial evaluation in the USA (Project EX‐1 2001), and four more recent studies in the USA (Project EX‐4 2007), Spain (Project EX Spain 2015a; Project EX Spain 2015b) and Russia (Project EX Russia 2013). The first of these studies (Project EX‐1 2001) contained a third arm in which the Project EX intervention was enhanced with a 'school‐as‐community' component. This was combined with the standard Project EX arm for the purpose of data analysis and the enhanced intervention has not been used in subsequent trials. Among the four studies with data suitable for pooling, the estimated effect RR was 1.48 (95% CI 1.05 to 2.10, I2 = 0%, four studies, n = 1215, Analysis 4.1). Project EX Russia 2013 also stated a beneficial effect on smoking cessation, but did not provide sufficient data to be included in numerical analysis. This result should be taken in the context that the two Spanish studies, which were conducted in similar school settings, were judged at particularly high risk of bias, with concerns relating to both institutional and participant‐level dropout. Additionally, there was a marked variation in absolute quit rates between the Project EX trials, with reported six‐month quit rates in control group participants ranging from zero (Project EX Spain 2015a) to 25% of baseline smokers (Project EX‐4 2007).
4.1. Analysis.
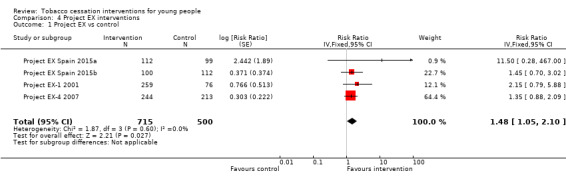
Comparison 4 Project EX interventions, Outcome 1 Project EX vs control.
Sensitivity analysis
Varying the assumed ICC had relatively little effect on the magnitude of pooled RRs as many studies were either individually randomized or had already allowed for clustering in their published analyses, and estimates from other studies already tended to have wide confidence intervals. In the different sensitivity analysis scenarios, point estimates for the pooled RR ranged from 1.34 to 1.38 for Analysis 1.2, from 1.25 to 1.27 for Analysis 1.4, from 1.15 to 1.16 for Analysis 2.3, from 1.39 to 1.43 in Analysis 2.4 and from 1.46 to 1.53 in Analysis 4.1.
Adverse effects
None of the psychosocial trials reported whether any adverse events had occurred. In the trial of nicotine patch or gum versus placebo of Moolchan 2005, one or both of the active medications were associated with an increase compared to placebo in five symptom categories ‐ sore throat, hiccups, erythema, pruritus and shoulder/arm pain. Bailey 2013 reported the occurrence of 73 unspecified adverse events during the open‐label nicotine patch treatment phase, but that none of these was "medically serious". Scherphof 2014 stated that participants using the nicotine patch reported more episodes of headache, cough, abnormal dreams, muscle pain, and "patch‐related adverse events" than those in the control group, but that successful quitters in the nicotine patch group reported a lower level of insomnia than those in the control group, which the authors attribute to withdrawal effects. The authors state these self‐reported side effects to be generally mild.
In the trial of bupropion as an adjunct to nicotine patch (Killen 2004), although young people reported a total of 47 self‐rated "severe" complaints, with nausea the most common, none of these was judged to be severe by the lead study physician. In the trial of bupropion alone (Muramoto 2007), a large number of participants in all study groups, including the control group, reported adverse effects (for example, around half of all participants reported headache; cough, throat symptoms, sleep disturbance and nausea were also each reported by more than 10% of participants). Eight subjects discontinued bupropion treatment because of different adverse events, and two further serious adverse events resulting in hospitalization occurred among participants in the bupropion group: one participant was admitted for anticholinergic crisis after ingesting Datura innoxia and one participant intentionally overdosed on study medication and other substances.
Discussion
Summary of main results
This is an update of a review first published in 2006. The most recent (2017) update includes 19 additional studies. However, our certainty in the findings remains low or very low for all comparisons. For behavioural therapies (Table 1), when we grouped interventions by delivery mode, no interventions showed effects apart from group counselling, but certainty here is limited by inconsistency and risk of bias, and imprecision is an additional issue for the other interventions tested. When we grouped studies by theoretical basis, studies employing complex theoretical models showed the most promise, but again these studies were clinically heterogeneous. There is very limited evidence on pharmacotherapies in this population, with two small studies of nicotine replacement therapy and two small studies of bupropion failing to demonstrate an effect (Table 2). Evidence here is again limited by issues with imprecision and risk of bias. Pooled results from the four studies evaluating Project EX showed a pooled result whose confidence intervals only narrowly exceeded 1. As with previous versions of this review, this update demonstrates that more research is still needed in this field. Our specific recommendations for future research are detailed below (see Implications for research).
Completeness, applicability and quality of the evidence
As detailed in Table 1 and Table 2 the quality of the evidence in this review is limited by issues relating to individual study quality (risk of bias), imprecision due to a small number of included studies and some studies appearing underpowered, and inconsistency due to clinical heterogeneity between studies. This hampers our ability to draw any firm conclusions about the interventions evaluated in this review. Some further issues with the data are discussed in more detail below.
The first of these is that most of the included studies were conducted in high‐income countries. As previously explained (see Background), adolescent smoking rates are, for the most part, declining in high‐income countries. However, they remain high, and in some cases continue to rise, in lower‐ and middle‐income countries (LMICs). Therefore, the majority of the evidence from this review has not been generated in the setting where the interventions are most needed. This is not to say that the interventions tested in high‐income countries are not relevant to LMICs, nor that high income‐countries are not also in need of effective stop‐smoking programmes for adolescents, but LMICs may face particular challenges with implementation that have yet to be adequately explored by the research in this field.
With regards to applicability, it should be noted that where recruitment was by inclusion from self‐reports it is likely that those volunteering, and in some trials obtaining parental consent, could be perceived as a subset of all smokers ‐ those who were both willing to quit and willing to participate in a research study. Some study authors comment on this aspect of recruitment (Kealey 2009b).
A further weakness in the evidence base springs from the definitions of quitting used in different studies. These vary from self‐reported quitting for longer than one day through to seven‐day or 30‐day point prevalence abstinence at the point of ascertainment, to longer or continuous periods (see Characteristics of included studies). With respect to the shorter point prevalence abstinences, a negative result is useful in demonstrating evidence of a lack of effect where the study size is adequate but care should be taken with the shorter quit lengths such as 24 hours. The irregularity and instability of the smoking habit in its early stages (for example, weekend smoking is commonly reported) and the low number of cigarettes smoked at baseline by some participants, calls into question the prognostic value of short‐term point prevalence abstinence measurements of less than 30 days. Several trials recognize this pattern of smoking and use a 30‐day measure of abstinence but continuous abstinence remains the recommended outcome (West 2005). It is tempting to conclude that encouraging an increased number of what are effectively short‐lived (e.g. seven‐day) quit attempts allows young people to 'practice' quitting, and therefore may help to achieve prolonged cessation in the long run. Prolonged quit attempts might also have a health benefit on their own, or interrupt the progression to more regular or heavy smoking. However, we have no data for young people against which we can test these assumptions.
In addition, several studies clearly demonstrate the importance of biochemical verification (Robinson 2003; Killen 2004; Colby 2005) as substantial numbers of participants have given false information regarding quit attempts. This raises possible doubts about the validity of those studies that showed positive results but did not use verification, for example, Hollis 2005. In Project EX‐1 2001, verification was incomplete and a weighting factor was added to results. For NoT WV 2011, verification was added to the intervention but only done at three months. There is a continued need for further studies where smoking status has been verified, but the experience of some studies (e.g. Hoffman 2008) underlines the challenges that face researchers in this area. Muramoto warns that exhaled CO has a short half‐life and may be an insensitive measure given the episodic nature of teen smoking. She reports cotinine confirmed rates 50% to 65% lower than CO rates (Muramoto 2007).
Potential biases in the review process
For the purpose of this review, we have taken a clinical focus on young smokers. In public health terms, the line between young smokers, experimenters and 'potential' smokers is blurred. Some interventions are therefore aimed at the population level, attempting to combine prevention and cessation. Individual clinicians, however, face a different problem: what advice should they give and what works for the young person who has started smoking and expresses a wish to stop? For this review, therefore, we drew what might otherwise be seen as an arbitrary line and developed a protocol that would include those prevention studies that had a cessation intervention component and discrete results for smokers.
Ideally, we would wish to know outcomes in terms of true smoking cessation, that is, quitting smoking and never smoking again, although an absolute measure of cessation in these terms is in practice impossible, as it would require life‐long follow‐up of participants. It is necessary therefore to consider just how well what are effectively proxy measures correspond to the desired outcome. Clearly, longer periods of follow‐up would be of greater value. We therefore limited our review to studies with six months' follow‐up, as recommended elsewhere (Mermelstein 2002; West 2005). There is clear evidence in some of the included studies that performed repeated measures, of a waning effect over this period (e.g. Brown 2003). Early relapse is an obvious danger, especially for young people who have been shown to make many quit attempts (MMWR 2009). In order to standardize comparisons, we took the six‐month period as beginning from baseline measurement. It should be noted however, that studies may not set a quit date until some weeks into the programme (e.g. Project EX) and this may be a source of bias when comparing outcomes.
For our results, we used an intention‐to‐treat analysis, that is, all those randomized were included in their original groups, whether or not they received the full intervention. We counted all those with missing data as continuing smokers. We requested information from authors where necessary to facilitate these calculations. Although this is standard practice in adult cessation studies, the reasons for young people dropping out from follow‐up are diverse, and by no means always related to risk of continued smoking. We accept, therefore, that this assumption leads to a conservative analysis, and that it may bias our results towards the null.
Many studies in this area are cluster‐randomized. Where authors had not allowed for clustering effects in their statistical analysis, we imputed a plausible value of the ICC, and varied this value in a sensitivity analysis. This did not have large influence on estimates of the pooled effect sizes, and the uncertainty due to this analysis appears small compared to the uncertainty in the effect estimate itself, as reflected in generally wide confidence intervals that do not rule out the possibility of clinically important effects.
Agreements and disagreements with other studies or reviews
The results of this review are for the most part consistent with other reviews of smoking cessation interventions in young people, though other reviews are very different from ours (Sussman 1998; Sussman 2002; McDonald 2003; Sussman 2006; Gervais 2007). Some of these reviews had a much wider focus and included non‐experimental studies. For example, Sussman 2006 (also discussed by USDHHS 2012) found some evidence of a modest improvement in quit rate both overall and stratified by the theoretical basis of the intervention but included many non‐randomized studies and did not restrict by the length of follow‐up. Our review update has aimed instead to evaluate the experimental evidence for effectiveness. Our results are also consistent with Riemsma 2003, whose review found results similar to Aveyard 2001. A recent review of nicotine replacement therapy (NRT) in adolescents, which included a broader range of study types than our review, also did not detect evidence of an effect (King 2016).
There is, however, one review of randomized controlled trials of which we are aware that concluded that behavioural interventions for smoking cessation in adolescents were effective (Peirson 2016). This was an update of a 2013 review (Patnode 2013) focusing on primary‐care relevant interventions. Though the 2013 review did not find any evidence of effectiveness, in the 2016 review, inclusion criteria were amended to include only studies with control groups that received no content specifically designed or intended to prevent or treat tobacco smoking. Three studies are therefore included in Peirson 2016's meta‐analysis of behavioural interventions for smoking cessation; of these, one was excluded from our review as it did not meet our inclusion criteria (Pbert 2008). The study driving the observed effect in Peirson 2016 is Hollis 2005. In our review, Hollis 2005 is classed as an intervention using both computer‐based and face‐to‐face counselling interventions; when pooled, the result for this comparison was not statistically significant but we did not present pooled results due to substantial statistical heterogeneity (see Analysis 1.3). In our analysis by theoretical basis, Hollis 2005 is pooled with other interventions using a complex theoretical model (Analysis 2.4); here an effect was detected. However, this group of interventions was clinically heterogeneous and we judged Hollis 2005 to be at high risk of bias as it did not use biochemical verification of smoking status. As this study was the most influential on the effect estimate in this subgroup, having a large sample size, we believe these concerns warrant caution when interpreting results.
Our results contrast with those of systematic reviews that have investigated the effectiveness of similar interventions in adult populations. For some of the intervention methods considered there is not enough evidence from trials of young people to make a definitive comparison with results from trials in adults. However, the pooled effect size estimates for individual and group therapies are lower than those in previous Cochrane Reviews for adults. For individual counselling in young people, the RR estimate of 1.07, with an upper 95% CI limit of 1.39, compares to an estimate of 1.57, with lower 95% CI limit of 1.40 in adults (Lancaster 2017). For group counselling versus control, the effect size estimate for young people (1.35) is also lower than the effect sizes seen for some group‐based interventions in adults (RR 1.88 when compared to self‐help interventions, Stead 2017). The estimated RR for mobile phone‐based interventions is much lower in our review than in the corresponding review in adults (1.67, Whittaker 2016). The lack of evidence regarding the effectiveness of pharmacological interventions in young people is particularly striking. The existing evidence base gives us no reason to believe that the neuropharmacological efficacy, effectiveness and safety of pharmacotherapies for smoking cessation should be different for adolescents than for any other group of smokers, but the context and meaning of smoking in adolescence is very different from that for adult smokers (Amos 2006). While there is strong evidence of the effectiveness of NRT (from 150 trials, Stead 2012) and bupropion (from 65 trials, Hughes 2014) for cessation, our review contains just four relatively small studies that used either of these treatments, and hence their effectiveness in young people remains uncertain. Taken together, these comparisons demonstrate that adult interventions whose effectiveness is well established cannot be assumed to be equally successful in younger age groups.
Future directions
Twenty‐seven of the 41 included studies have been published within the last ten years, with 19 new studies contributing to this update, suggesting an increase in both activity and in some instances in quality. However, this update has not resulted in stronger conclusions regarding the effectiveness of interventions in this area. Due to continuing limitations in the evidence base, we are unable to recommend widespread implementation of any one model. We are aware that there is a growing interest in this topic and we intend to continue regular updates of this review. Over the period that we have been extracting data, teenage prevalence figures have shown some improvement in those countries using global public health campaigns (USDHHS 2012), such as bans on smoking in public places (Frazer 2016), suggesting global measures may have had an impact on smoking initiation. However, we must not lose sight of the fact that a substantial segment of young people still smoke and a high proportion of quit attempts fail (Bancej 2007). In some lower and middle income countries, the prevalence of smoking in young people is rising (Eriksen 2015), and in high income countries, the burden disproportionately falls on people with mental health conditions and on those of lower socioeconomic status. Further research into effective ways to help young people stop smoking continues to be needed.
Authors' conclusions
Implications for practice.
Evidence on the effectiveness of behavioural interventions in this age group is limited by issues with imprecision, heterogeneity, and risk of bias. Group counselling interventions and behavioural interventions designed using complex theoretical models appear to show the most promise.
There remains little evidence on effectiveness of pharmacotherapies in this age group and we judge effect estimates very likely to change should further research become available.
Consequently, there is not sufficient evidence to recommend widespread implementation of any one model or to recommend provision of a particular service to support young people to stop smoking.
Implications for research.
Research is developing and increasingly studies are measuring verified, sustained quitting. This trend is to be encouraged for all new trials for teen smoking. However, our confidence for all findings in this review is limited by issues with imprecision, risk of bias, and inconsistency. More studies are urgently needed evaluating both pharmacological and behavioural interventions for smoking cessation in young people. Studies should minimize risk of bias as much as possible and be adequately powered for cessation. The evidence is developing for complex psychosocial interventions but needs to be replicated and tested in different settings. The theoretical basis of all interventions should be explicit, and reporting using CONSORT standards (Schulz 2010) should be the norm (e.g. Hollis 2005). Trials of brief interventions or self‐help materials would be useful, particularly as these are often used as control conditions for more complex interventions. In addition, the majority of studies in this review were conducted in high‐income countries, despite adolescent smoking rates being substantially higher in lower‐ and middle‐income countries (LMICs). Therefore, further studies conducted in LMICs would be particularly useful.
Likely losses to follow‐up for this age group must also be considered in the research design and the assumption that losses to follow‐up are non‐quitters (whilst representing the current 'gold standard') needs testing. Every effort should be made to keep the latter as small as possible, so that intention‐to‐treat analysis with missing participants treated as continuing smokers can be carried out without excessive bias towards the null. Brown 2003 and Peterson 2009 demonstrate good practice in this respect. Subsidiary analysis of data with other imputed data is acceptable but should not represent the main result.
Biochemical verification remains the gold standard (West 2005) and there is the potential for substantial misclassification of smoking status in adolescents if relying on self‐report alone (Jarvis 2008). If it is used, note should be made on the limitations of exhaled CO, given the episodic nature of smoking in this population. However, although it is recognized that self‐reports in this cohort are not necessarily reliable, voluntary use of verification can affect recruitment and retention, especially if parental consent is required before it is used in adolescents, and a pragmatic decision needs to be taken in study design that balances these factors (SRNT 2002).
Our review did not find any eligible studies that used electronic cigarettes as an intervention for tobacco smoking cessation in adolescents. A recent systematic review of electronic cessation interventions (Hartmann‐Boyce 2016) also found no randomized trials in this age group, and only a single, non‐randomized, study that investigated their use in a young adult population, aged 18 to 24 years (Choi 2014). Given the sharply increasing popularity of electronic cigarettes (Farsalinos 2016), it appears reasonable to expect future evaluations in relation to tobacco smoking cessation in young people as the scope of the research literature in this area continues to grow.
Few of our studies complied with the Russell Standard (West 2005). Six months' follow‐up should be a minimum requirement, and research should use outcomes based on sustained, continuous quitting in line with the Russell Standard. As a complementary measure, long‐term prospective studies of the natural smoking history of those making quit attempts in adolescence are needed. Finally, as the field matures, direct comparisons of effective treatments should become possible and should support full economic analyses.
What's new
| Date | Event | Description |
|---|---|---|
| 21 July 2017 | New search has been performed | Search updated June 2017. 19 new included studies added; inclusion criteria altered and 6 previously included studies removed. Structure of analyses altered from previous version. |
| 21 July 2017 | New citation required but conclusions have not changed | Change in authorship. No major changes to conclusions though review substantially restructured |
History
Protocol first published: Issue 3, 2005 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 24 June 2013 | New search has been performed | Searches updated in February 2013. 4 new studies included and risk of bias tables expanded. |
| 6 June 2013 | New citation required but conclusions have not changed | Change in author order. No major changes to conclusions. |
| 6 November 2009 | New search has been performed | Updated for issue 1, 2010. Eight new studies included, no major change to conclusions |
| 30 October 2009 | New search has been performed | Converted to new review format. |
Acknowledgements
Alan Stanton and Gill Grimshaw originally conceived this review and were authors on this review until this current update; their contribution has been substantial and still shapes much of the review today.
The original authors would like to thank Paul Aveyard and Cathy Backinger for reading and commenting on drafts of the initial review. Their gratitude goes to Review Group Co‐ordinators and staff, and to all the trialists who supplied additional data or information for this review. They would particularly like to thank Steve Sussman for sharing the bibliography of his systematic reviews.
With regards to the most recent update we would like to thank Jong‐Wook Ban (University of Oxford) for his help in interpreting a Korean language paper; Rafael Perera‐Salazar and Carmen Piernas‐Sanchez (both University of Oxford) for their help interpreting a Spanish language paper; Dr Michael Mason (Virginia Commonwealth University) for providing further information and abstinence data for Mason 2016; and Dr Jaimee Heffner (Fred Hutchinson Cancer Research Center) for providing denominator data relating to Peterson 2009.
Data and analyses
Comparison 1. Behavioural interventions grouped by delivery method.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Individual counselling vs control | 7 | 2088 | Risk Ratio (Fixed, 95% CI) | 1.07 [0.83, 1.39] |
| 2 Group counselling vs control | 9 | 1910 | Risk Ratio (Fixed, 95% CI) | 1.35 [1.03, 1.77] |
| 3 Interventions using technology vs control | 9 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 Computer‐based interventions | 3 | 340 | Risk Ratio (Fixed, 95% CI) | 0.79 [0.50, 1.24] |
| 3.2 Interventions using messaging | 3 | 2985 | Risk Ratio (Fixed, 95% CI) | 1.18 [0.90, 1.56] |
| 3.3 Computer‐based and face‐to‐face counselling interventions | 3 | 1703 | Risk Ratio (Fixed, 95% CI) | 1.18 [0.96, 1.46] |
| 4 Interventions with multiple delivery methods vs control | 8 | 2755 | Risk Ratio (Fixed, 95% CI) | 1.26 [0.95, 1.66] |
Comparison 2. Comparison of theoretical basis of behavioural interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stage of Change models vs control | 6 | 3282 | Risk Ratio (Fixed, 95% CI) | 1.06 [0.85, 1.31] |
| 2 Motivational interviewing vs control | 10 | 1752 | Risk Ratio (Fixed, 95% CI) | 1.11 [0.90, 1.36] |
| 3 Social cognitive theory vs control | 6 | 3667 | Risk Ratio (Fixed, 95% CI) | 1.16 [0.88, 1.51] |
| 4 Complex theoretical model with stage of change, motivational interviewing, cognitive behavioural therapy and/or social cognitive theory vs control | 9 | 2827 | Risk Ratio (Fixed, 95% CI) | 1.40 [1.14, 1.74] |
Comparison 3. Pharmacological interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nicotine replacement therapy vs placebo | 2 | 385 | Risk Ratio (IV, Fixed, 95% CI) | 1.11 [0.48, 2.58] |
| 1.1 Nicotine patch vs placebo | 2 | 319 | Risk Ratio (IV, Fixed, 95% CI) | 1.02 [0.41, 2.56] |
| 1.2 Nicotine gum vs placebo | 1 | 66 | Risk Ratio (IV, Fixed, 95% CI) | 1.74 [0.21, 14.60] |
| 2 Bupropion vs placebo | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Nicotine patch + bupropion vs nicotine patch + placebo | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. Project EX interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Project EX vs control | 4 | 1215 | Risk Ratio (Fixed, 95% CI) | 1.48 [1.05, 2.10] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abroms 2008.
| Methods | Country: USA Setting: University in Washington DC Study design: RCT |
|
| Participants | Participants: 83 (I = 48, C = 35), 45.8% female, ethnicity: white = 77.1%, Asian = 3.6%, black = 2.4%, Hispanic = 1.2%, other = 15.7% Age range: 18‐23 years, mean (SD) = 19.8 (1.3) Criteria for inclusion: full‐time or part‐time student, ≥ 1 cpd for last 7 d, aged 18‐24 years, interest in quitting smoking in next 6 months Follow‐up method: over telephone Inducements to enter study: USD 25 for completing follow‐up assessments, additional USD 10 for submitting saliva sample for cotinine analysis if reported abstinence Baseline characteristic equivalence: no significant differences Pre‐test smoking status assessment: self‐report Post‐test smoking status assessment: biochemically validated self‐report |
|
| Interventions | Intervention: X‐Pack programme, tailored to young adults. 15‐min in‐person counselling session during which the participant was encouraged to set a quit date in the following month and key information was reviewed; self‐help kit (The X‐Pack) containing a guidebook, motivational materials and cigarette alternatives (gum, toothpicks, putty); and 10‐12 individually tailored emails from counsellors over the 6 months following the quit date, to which participants were encouraged to respond. Emails were weekly in first month post quit date, and monthly for following 5 months Theoretical basis for intervention: SCT Control: Clear The Air programme, a counselling and self‐help intervention aimed at general adult audience |
|
| Outcomes | Measurement: 7‐day PPA Relevant follow‐up periods: 6 months Verification: salivary cotinine ≤ 10 ng/mL Loss to follow‐up: 31.3% were lost to follow‐up |
|
| Notes | Previously excluded, now included in 2017 update. This is on account of the redefinition of our inclusion criteria for age of participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Counselors were assigned a list of identification numbers for enrolled participants, each of which was randomly assigned to a participant’s condition”. No details on generation of randomization sequence itself |
| Allocation concealment (selection bias) | Unclear risk | Information not sufficient to make judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Behavioural intervention so blinding is impossible, and different amounts of contact time with counsellors between groups causes a high risk of differential misreport. “After the in‐person counselling session, the participant [CTA group] was not provided with additional assistance in quitting.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "We found no significant differences in follow‐up rates between groups at either time point or for the cotinine sample." |
Aveyard 2001.
| Methods | Country: UK Setting: schools in West Midlands Study design: cluster‐RCT. Schools sampled with probability in proportion of size of year group. Combined prevention/cessation trial | |
| Participants | Participants: 1089 adolescent smokers (defined as ≥ 1 cpw) (I = 547; C = 542) Age range: 13‐14 years Criteria for inclusion: inclusion was at level of school; 89 schools approached, 53 agreed to participate. Data extracted for this cessation review based on all pupils in year 9 who smoked ≥ 1 cpw Follow‐up method: questionnaire to all students Inducements to enter study: none Pre‐study smoking status assessment: self‐reported Post‐study smoking status assessment: self‐reported Significant demographic differences between arms of trial: none apparent in published data | |
| Interventions | Intervention: computer 'expert system' designed to diagnose stage of change and deliver material tailored to individual. 6 sessions, 2 per term, 1 class‐based (tutor training mandatory) and 1 computer‐based delivered over period of school year (3 school terms per year in UK) Theoretical basis of intervention: psycho‐social intervention based on Transtheoretical Model of Stages of Change Control: control schools received health education as delivered locally at that time; in addition teachers received 3 lesson plans plus handouts but no specialist training or record of what was delivered. Theoretical basis of control: normal local practice | |
| Outcomes | Measurement: 7‐day and 30‐day PPA (supplied by study author); follow‐up periods > 3 months, 12 months (mean length of follow‐up 359 (I) to 347 (C) days) and 24 months from start of study, equivalent to 4 months and 16 months after end of intervention Verification: none Losses to follow‐up: 11% (I) and 10.7% (C) at 12 months; 14% (I) and 16.9% (C) at 24 months (additional data from study authors) | |
| Notes | This review uses 12‐month follow‐up for the group of baseline regular smokers, treating those lost to follow‐up as continuing smokers, as reported in Aveyard 1999. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomization, balanced by class size |
| Allocation concealment (selection bias) | Low risk | Computerized and anonymous |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No biochemical validation, but follow‐up surveys anonymized (identified only by ID number) and delivered by trained personnel in 'examination' setting, differential misreport judged to be unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analyses tested all models of losses to follow‐up |
| Other bias | Unclear risk | Fidelity of implementation for controls unclear |
Bailey 2013.
| Methods | Country: USA Setting: 10 continuation high schools, San Francisco Bay Area Study design: RCT |
|
| Participants | Participants: 143 smokers (I = 72, C = 71). 38% female Age: mean = 16.9 years, SD = 0.80 Criteria for inclusion: 14‐18 years old, attended a participating school, smoked ≥ 10 cpd, expressed interest in quitting smoking. Excluded if currently receiving treatment for major depression, panic disorder, social anxiety or agoraphobia; taking antidepressants, antipsychotics, benzodiazepines or theophylline; current heavy alcohol or substance abuse; diagnosed heart problems or high blood pressure; current use of nicotine replacement therapy; allergy to adhesive tape; currently pregnant or planning on becoming pregnant. Follow‐up method: self‐report through questionnaire Inducements to enter study: gift cards, values not reported No differences in baseline participant characteristics between trial arms Pre‐test smoking status assessment: cpd mean = 13.9, SD = 5.53, dependence measured with mFTQ mean = 17.5, SD = 4.5 Post‐test smoking status assessment: self‐reported biochemically validated abstinence |
|
| Interventions | Intervention: extended treatment of 24 weeks of group‐based CBT and skills training, concurrent with 9 weeks of nicotine patch therapy. Extended treatment focuses on relapse prevention skills and effective coping plans. Theoretical basis for intervention: CBT (for the non‐pharmacological component of the intervention) Control: 10 weeks of group‐based CBT and skills training, concurrent with 9 weeks of nicotine patch therapy |
|
| Outcomes | Measurement: 7‐day PPA Relevant follow‐up periods: 10 weeks and 26 weeks Verification: expired‐air CO < 10 ppm, using a Bedfont Smokerlyzer Loss to follow‐up: at 26 weeks both intervention and control groups lost 18% of participants Adverse events: specific details not given |
|
| Notes | New for 2017 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated randomisation to extended treatment was conducted by the study statistician" |
| Allocation concealment (selection bias) | Low risk | "Intervention staff and participants remained blind to treatment group assignments until the end of open label treatment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Behavioural intervention makes blinding difficult, and intervention group received extended treatment in comparison to the control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was 18% in both intervention and control groups, sufficiently low and similar to be judged low risk of attrition bias. |
Brown 2003.
| Methods | Country: USA Setting: psychiatric hospital, Providence RI Study design: RCT | |
| Participants | Participants: 191 patients (I = 116 ; C = 75), 62.3% female, ethnicity 94.8% white Age range: 13‐17 year olds, mean 15.4 years Criteria for inclusion: ≥ 1 cpw for previous 4 weeks, 64% daily smokers, on average smoking for 3.6 years (additional data from study authors) Follow‐up method: telephone questionnaire Inducements to enter study: gift certificates to local mall, escalating in value, on completion of each phase No significant demographic differences between arms of trial Other: participants were prohibited from smoking during hospital stay (mean length 9 days) | |
| Interventions | Intervention: MI given in 2 sessions of 45 min, delivered by a study therapist, plus relapse prevention manual and self‐help pamphlet Control: brief advice session plus self‐help pamphlet | |
| Outcomes | Measurement: 7‐day PPA; follow‐up period/s > 3 months, 6 months, 12 months Pre‐study smoking status assessment: Modified Fagerstrom, mean 4.9 (± 1.82) Post‐study smoking status assessment Verification: salivary cotinine and CO Losses to follow‐up: at 6 months 8%; at 12 months 9% | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The assignment of cohorts to treatment condition was determined randomly before the initiation of the study," method of sequence generation not specified |
| Allocation concealment (selection bias) | High risk | Allocation based on time of admission. "Between cohorts, no recruitment occurred until study participants from the previous cohort had been discharged from the hospital." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified, not clear if other hospital personnel blind to treatment assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used at 1‐month, 6‐month and 12‐month follow‐up visits |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 91% followed up at 12 months, "rates of missing data were not significantly different across motivational intervention and brief advice conditions." |
Colby 2005.
| Methods | Country: USA Setting: hospital outpatient or emergency departments in Rhode Island Study design: RCT | |
| Participants | Participants: 85 adolescents (43 = I; 42 = C) Age range: 14‐9 years Criteria for inclusion: reported daily smoking for previous 30 d Follow‐up method: Timeline Follow Back to inform structured interview Inducements to enter study: USD 10 gift voucher for completion Pre‐study smoking status assessment: self‐reported cpd in last 30 days Post ‐tudy smoking status assessment: verified self‐reported smoking pattern in last 90 days Significant demographic differences between arms of trial: not reported | |
| Interventions | Intervention: 35‐min personal MI with a trained interventionist, with 1 week follow‐up phone call of 15‐20 min Theoretical basis of intervention: motivational enhancement Control: 5‐min advice interview plus pamphlet and brief phone call 1 week after visit Theoretical basis of control: brief Intervention | |
| Outcomes | Measurement: 7‐day PPA; follow‐up periods: > 3 months, 6 months Verification: CO and cotinine Losses to follow‐up: 20% at 6 months | |
| Notes | Author of study considers little confounding amongst extensive array of variables High withdrawal and non‐recruitment rate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned," method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The research assistants in this study were blind to treatment condition," unclear if participants in control group knew of intervention being provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants lost to follow‐up counted as smokers, 80% of participants followed up at 6 months, no significant difference in loss to follow‐up between treatment groups |
Colby 2012.
| Methods | Country: USA Setting: 5 high schools, an emergency department, a hospital‐based adolescent outpatient clinic, and a paediatrician's office. Study design: RCT |
|
| Participants | Participants: 162 (I = 79, C = 83), 48% female, ethnicity: 72% non‐Hispanic white, 7% black/African American, 6% Hispanic/Latino, 15% other race/ more than one race Mean age (SD): I = 16.2 (1.3), C = 16.2 (1.2) Criteria for inclusion: aged 14‐18 years, spoke English, smoked ≥ 1 cpw for the past month. Excluded for suicidal ideation or, in medical settings, recent traumatic injury Follow‐up method: self‐report in person or over telephone Inducements to enter study: USD 30 in gift certificates and the opportunity to earn up to an additional USD 80 cash for completion of follow‐up assessments Baseline characteristic equivalence: groups broadly similar save for baseline expired CO measurement, which was significantly higher in the intervention group (mean 11.1 ppm) than control (mean 7.8 ppm) Pre‐test smoking status assessment: mean (SD) cpd in last 30 days: I = 11.3 (8.5) , C = 9.2 (7.0). Stanford Dependence Index, mean (SD): I = 14.1 (4.0), C = 13.5 (4.0). Expired CO given above Post‐test smoking status assessment: self‐report |
|
| Interventions | Intervention: 45‐min personal interview with a trained interventionist, 1 week follow‐up 15‐20‐min telephone call. 15‐20‐min discussion with parents to help them support participants' quit attempt Theoretical basis for intervention: MI Control: Brief advice consisting of a 5‐min meeting where a pamphlet was provided, a telephone booster 1 week after, and a pamphlet delivered to the parents by post |
|
| Outcomes | Measurement: 7‐day PPA Relevant follow‐up periods: 6 months Verification: expired CO < 9 ppm measured with Bedfont Smokerlyzer, salivary cotinine < 14 ng/mL analysed with gas chromatography Loss to follow‐up: I = 23%, C = 14% |
|
| Notes | New for 2017 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A computer‐generated random number sequence allocated participants to treatment groups prior to enrolment” |
| Allocation concealment (selection bias) | Low risk | “Assignments were sealed in envelopes which were filed in a series of sequentially numbered folders” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Behavioural intervention so not possible to blind, but intervention and control groups both received a similar number of contacts during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “All interviewers were blind to condition assignment during assessments”; primary outcome was biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was 23% in intervention and 14% in control group; study authors performed sensitivity analysis treating participants who dropped out as continued smokers, which “yielded lower abstinence rates and were also not significant” |
Dalum 2012.
| Methods | Country: Denmark Setting: 22 continuation high schools Study design: 2‐arm cluster‐RCT |
|
| Participants | Participants: 1147 daily smokers (I = 505, C = 642), 70% female, 86% Danish Age: mean = 17.7, SD = 1.2 Criteria for inclusion: aged 15‐21 years, daily smokers attending participating schools Follow‐up method: written questionnaire completed during school Inducements to enter study: none mentioned Baseline characteristic equivalence: baseline data not presented Pre‐test smoking status assessment: self‐report questionnaire, cpd mean = 11.9, SD = 5.6 Post‐test smoking status assessment: self‐report questionnaire |
|
| Interventions | Intervention: school‐wide interactive sessions weekly for 4 weeks. These included an expired CO measurement, personal short counselling based on TTM, paper self‐help materials, referrals to cessation programmes through text, the internet, or over telephone Theoretical basis for intervention: TTM, self‐regulation theory Control: waiting list control |
|
| Outcomes | Measurement: 30‐day PPA Relevant follow‐up periods: 14 months Verification: none Loss to follow‐up: 68.8% |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study authors state that “randomisation was done by flipping a coin” but then discuss blocked randomization by both county and school type that could not be done simply a flipping a coin. The exact method of randomization is not adequately explained |
| Allocation concealment (selection bias) | Unclear risk | It is not clear at what point the study investigators, the participating school co‐ordinators or the participating individuals became aware of group allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding to the intervention is not possible. It is not stated whether participants were aware of the allocation given to the other group (although the schools were told this in advance) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐report only, and although the control group was ‘waiting list controls’ and received the intervention in the second year, at the time the primary outcome was obtained the control group had received no intervention beyond simple measurement of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | For the follow‐up relevant to this review, three schools were missing some or all of their data. 1147 individuals were identified as current smokers at baseline. Only 369 could be analysed at the longest follow‐up (32.2%). There was a differential in some group characteristics (such as educational level) between those who were successfully followed up and those who were not. |
| Other bias | High risk | Baseline characteristics were not reported and so there may have been imbalance of groups at baseline. Even though the study was cluster‐randomized, school‐level information was not reported and school could not be allowed for in the analysis. There is evidence for very inconsistent delivery of the intervention between schools. The nature of loss of some data via recording errors makes bias likely. |
Greenberg 1978.
| Methods | Country: USA Setting: high schools Study design: RCT | |
| Participants | Participants: open recruitment, first 100 recruited Age range: 14‐16 years (grades 9‐11) Criteria for inclusion: all participants smoked ≥ 5 cpd Inducements to enter study: half a unit credit for experimental groups Pre‐study smoking status assessment: self‐report Post‐study smoking status assessment: self‐report | |
| Interventions | Intervention: Group A (n = 25) received 'scare' education; Group B (n = 25) 'fact'‐based education, Group C (n = 25) 'attitude' approach using affective strategies. All classes took place in weekly sessions over 7 weeks Theoretical basis of intervention: affective teaching strategies consistent with theoretical development at time of trial Control: control group (n = 25) spent time in study hall without any active intervention | |
| Outcomes | Measurement: PPA ("no longer smoked"); follow‐up period/s > 3 months, 5 months after end of intervention. Intervention lasted 7 weeks, so endpoint 6‐7 months post‐baseline No biochemical verification Losses to follow‐up: 22% at final follow‐up Results: All ORs calculated. Quitters: Group A, 3 students; Group B, 0 students; Group C, 6 students and control, 1 student Overall OR for aggregated quitting = 3.27 (0.39 ‐ 27.21) Group A vs control OR = 3.27 (0.32‐33.84) Group B vs control OR = 1(0) Group C vs control OR = 7.58 (0.84 ‐ 68.46) (displayed in analyses | |
| Notes | No power calculations evident from paper Lack of information regarding allocation and potential confounding in this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified, not clear if randomization used |
| Allocation concealment (selection bias) | High risk | "The subjects were divided into four equal groups... designated to meet during four different daily class periods," suggests allocation was not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "An attempt was made to validate the self‐report data by asking about smoking behaviour in two different parts of the questionnaire by two differently‐worded questions." Self‐reported smoking status used, differential misreport possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 78% followed up at 5 months, rates similar in each group |
Gungormus 2012.
| Methods | Country: Turkey Setting: 1st and 2nd years of a single high school in Erzurum, Turkey Study design: 2‐arm RCT |
|
| Participants | Participants: 60 male smokers (I = 30, C = 30) Mean age (SD): I = 17.1 (1.5), C = 17.9 (1.1) Criteria for inclusion: current smoker in 1st or 2nd year of a high school in Erzurum, Turkey Follow‐up method: written questionnaire Inducements to enter study: none reported Baseline characteristic equivalence: groups were equivalent at baseline Pre‐test smoking status assessment: self‐report Post‐test smoking status assessment: self‐report |
|
| Interventions | Intervention: "Transtheoretical model‐based education" delivered in 4 sessions at the 1st, 3rd, 6th and 12th months. "The content of the sessions consisted of training, distribution of training booklets, and application of the [TTM] scales." Theoretical basis for intervention: TTM Control: no intervention, control measurements were made in 1st and 12th months |
|
| Outcomes | Measurement: definition of abstinence is unclear Relevant follow‐up periods: 11 months Verification: none Loss to follow‐up: 10% in both groups |
|
| Notes | New for 2017 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Simple random sampling" |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Behavioural intervention; potential for large difference to be caused by lack of blinding as the control group received no intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐report only and no blinding to intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The original study sample was reduced from 75 to 60 participants, and it is not clearly reported whether this occurred before or after randomisation |
| Other bias | High risk | Definition of abstinence itself is unclear from paper, but is suggested from Table 2 of the paper to be separate from the Maintenance stage of change |
Guo 2014.
| Methods | Country: Taiwan Setting: 6 vocational high schools in New Taipei City, Taiwan I = 3, C = 3) Study design: cluster‐RCT |
|
| Participants | Participants: 143 adolescent smokers (I = 78, C = 65), 16% female Age: mean = 16.06, SD = 0.81 Criteria for inclusion: attending 1/6 vocational high schools in New Taipei City, Taiwan, regular smokers, thinking of quitting smoking, willing to comply with verbal instructions. Exluded if pregnant or suffering from a major chronic disease Follow‐up method: Inducements to enter study: TWD 200 for passing 1st test, TWD 100 for passing 3‐month and 6‐month cotinine tests Baseline characteristic equivalence: groups similar at baseline except for slightly longer mean duration of smoking in intervention than control group (2.31 vs 1.70 years) Pre‐test smoking status assessment: self‐report Post‐test smoking status assessment: biochemically validated self‐report |
|
| Interventions | Intervention: 12‐week programme consisting of 6 courses of two 45‐min classroom‐based smoking cessation sessions, self‐study manual for smoking cessation, a film teaching Chinese acupressure, telephone calls from research assistants at least once a fortnight to provide counselling if required, 10 text messages containing smoking‐cessation cues and support Theoretical basis for intervention: literature cited for each of the three main strands of the intervention (“Strength and skill building”, “New modes of communication for difficulties”, “Credits for the efforts to change”) Control: educational fliers |
|
| Outcomes | Measurement: 30‐day PPA Relevant follow‐up periods: 7 months after baseline Verification: urinary cotinine < 200 ng/mL Loss to follow‐up: 32% from intervention group, 25% from control |
|
| Notes | New for 2017 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be cluster‐randomized trial in figure 1; method of allocation unclear |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants or personnel; very large difference between groups in the amount of intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Difference in dropout between groups at end of trials quite small (32% vs 25%) although larger difference at earlier time points; no sensitivity analysis for dropouts |
| Other bias | Unclear risk | It is implied that some participants refused to take the urine cotinine test at some points during follow‐up, but the numbers were not recorded, and the denominator in the results of the objective cessation assessment suggest that all participants not lost to follow‐up did take the urine test |
Harris 2010.
| Methods | Country: USA Setting: Midwestern University Study design: cluster‐RCT (30 university fraternities/sororities, 15 in intervention and 15 in control) |
|
| Participants | Participants: 452 (I = 245, C = 207) students; 45.6% female; Ethnicity: non‐white (%) I = 4.1, C = 6.3 Age range: 18‐22, mean (SD): I = 19.4 (1.1), C = 19.5 (1.01) Criteria for inclusion: student member of university fraternity/sorority, smoking cigarettes ≥ 1 of the past 30 days, ≥ 18 years old, expected to be enrolled in college for the academic year, interested in participating in a health study, excluded if used medication to help quit smoking in past 30 days or if 30 from the fraternity/sorority were already enrolled. Follow‐up method: computer‐administered survey Inducements to enter study: none Baseline characteristic equivalence: equivalent except for gender, where control group had significantly high proportion of females Pre‐test smoking status assessment: self‐report, mean cpd; 1.9 (calculated), HONC (Hooked on Nicotine Checklist) dependence: 2.4 Post‐test smoking status assessment: biochemically validated self‐report |
|
| Interventions | Intervention: ≤ 4 one‐on‐one sessions of MI with a trained counsellor ‐ first 3 occurred approximately every other week following baseline and the 4th approx 4 weeks after session 3. Sessions were typically 20‐30 min. Participants received MI focused on motivating and assisting participants to quit cigarette smoking. Participants received a self‐help guide tailored for college students that discussed the benefits and methods of quitting at their first session. Heavy smokers were also encouraged to use pharmacotherapy obtainable through the university Theoretical basis for intervention: MI Control: as intervention, but focused on increasing consumption of fruit and vegetables to ≥ 5 servings a day |
|
| Outcomes | Measurement: 30‐day PPA Relevant follow‐up periods: 6 months Verification: salivary cotinine ≤ 15 ng/mL, with additional "bogus pipeline" at follow‐up Loss to follow‐up: 10.2% in intervention, 11.1% in control |
|
| Notes | Previously excluded, now included in 2017 update. This is on account of the redefinition of our inclusion criteria for age of participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Clusters were randomized after participants had been recruited and undergone a baseline assessment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study was unblinded as interventions were behavioural, however the interventions were of the same intensity (matched) and outcome at 6 months was biologically validated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed by a computer and biologically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention group lost 10.2% to follow‐up, control lost 11.1% These are sufficiently low and similar to be judged low risk. |
Haug 2013.
| Methods | Country: Switzerland Setting: vocational schools in German‐speaking regions of Switzerland Study design: cluster‐RCT |
|
| Participants | Participants: 755 (I = 372, C = 383) adolescent smokers, 51.9% female, 20.4% with 1 parent born outside Switzerland, 26.4% with both parents born outside Switzerland Age: mean = 18.2, SD = 2.3 Criteria for inclusion: daily or occasional smoking (≥ 1 cpw for the last month) Follow‐up method: computer‐assisted telephone interviews Inducements to enter study: EUR 8 for completing 6‐month follow‐up, EUR 0.80 for response to text assessments Baseline characteristic equivalence: study authors report possibility of baseline differences in gender, hazardous drinking, smoking status (occasional/daily), cpd and age at onset of smoking (with the control group having higher cpd (mean 11.6 vs 9.6) and more daily smokers (82.0% vs 70.7%) than the intervention group) Pre‐test smoking status assessment: self‐report, cpd: mean = 10.6, SD = 7.6 Post‐test smoking status assessment: self‐report |
|
| Interventions | Intervention: 1) an online assessment of individual smoking behaviour and attitudes toward smoking cessation (2) a weekly SMS text message assessment of smoking‐related target behaviours (3) 2 weekly text messages tailored to the data of the online and the SMS text message assessments (4) an integrated quit day preparation and relapse‐prevention program Theoretical basis for intervention: Health Action Process Approach Control: no intervention |
|
| Outcomes | Measurement: 7‐day PPA, 4 week PPA Relevant follow‐up periods: 6 months Verification: none Loss to follow‐up: I = 23% loss, C = 28% |
|
| Notes | New for 2017 update. Odds ratios for the authors’ ITT analysis using multiple imputation are available: for 7‐day abstinence (intervention vs control), OR = 1.03 (95% CI 0.59 to 1.79); for 4‐week abstinence, OR = 0.97 (95% CI 0.50 to 1.90) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We used block randomisation with computer‐generated, randomly permuted blocks of 4 cases." |
| Allocation concealment (selection bias) | Low risk | "The study assistants who conducted the baseline assessment in the vocational schools were blinded concerning group allocation for each of the school classes. Additionally, group allocation was not released to study participants until they provided informed consent, username, mobile phone number, and baseline data for the smoking‐related variables" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind participants to group allocation and control participants received no intervention and were aware of the intervention that they were not receiving (“Control group participants were informed that they were assigned to the control group and could not participate in the SMS text message program.”) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Impossible to blind participants to group allocation and primary outcome measure is by self‐report only, so high risk of differential misreport |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up at 6 months was 23% in intervention group, 28% in control group; ITT analysis was done using multiple imputation and gave similar conclusion to complete‐case analysis (even though “attrition analysis” gave some evidence that individuals lost to follow‐up were likely to be heavier smokers than those not lost to follow‐up) |
Hoffman 2008.
| Methods | Country: USA Setting: 7 public high schools in Montgomery County, Maryland Study design: cluster‐RCT, randomised at level of school | |
| Participants | Participants: 105 adolescent smokers Age range: 14‐18 years Criteria for inclusion of school: not currently participating in any other smoking cessation interventions Criteria for inclusion of students: those who had smoked ≥ 1 cpd for 30 days and were willing to attend 6 sessions plus follow‐up at 1 year Follow‐up method: project team interviews face‐to‐ face and by telephone Inducements to enter study: none Pre‐study smoking status assessment: self‐reported, 30‐day smoking status No significant demographic differences in participants in arms of study | |
| Interventions | Intervention: ASCENT programme included "cognitive behavioural therapy" tailored to stage of change (TTM), a student workbook, role play, discussion and games and video all delivered over 6 sessions of 1 h/week over 6 weeks. However, as intervention was delivered to a group, TTM component not strictly applied Theoretical basis of intervention: TTM and CBT Control: normal teaching and information giving within school | |
| Outcomes | Measurement: quitting defined as no smoking in 24 h prior to interview Follow‐up periods: 3 months, 1 year Verification: saliva cotinine attempted but either kits failed or students didn't provide sample Losses to follow‐up: 16% at 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Schools were randomized," method of sequence generation not specified |
| Allocation concealment (selection bias) | Low risk | Schools randomized, not participants. Students recruited prior to status of school being known |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Cotinine collected but not used, previous studies have found high misreport in adolescents even when aware biochemical validation would be used, hence misreport cannot be ruled out for this study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16% loss to follow‐up at 12 months, "A series of attrition analyses examining both the 30‐day and 12‐month follow‐up data indicated no differential loss of youth by condition, sex, racial group or having plans to quit in the next 30 days." |
Hollis 2005.
| Methods | Country: USA Setting: 7 pediatrics and family practice departments in Health Maintenance Organization medical centres in Oregon and Washington State Study design: RCT (prevention and cessation). Blocked randomization method, using sealed envelopes | |
| Participants | Participants: 448 adolescent smokers selected from 2524 recruits attending clinic appointments. Age range: 14‐17 years Criteria for inclusion: those who were willing to stay after consultation at clinic and had no intention of leaving geographical area within 1 year Follow‐up method: mailed questionnaires and telephone interviews Inducements to enter study: none Pre‐study smoking status assessment: self‐reported 30‐day smoking status Non‐significant demographic differences between arms of trial at level of P < 0.05 except for small difference in positive at depression screen (P < 0.01) | |
| Interventions | Intervention: 3 sequential interventions plus maximum of 2 boosters: (1) clinical message encouraging quitting or not starting, (2) 10‐12 min individual, multi‐media interactive computer‐delivered expert system tailored to stage of change of individual (3) 3‐5 min of motivational counselling by trained health counsellors. Boosters were delivered at clinic attendance (computer programme and motivation counselling) or by telephone (motivational counselling only). Repeated attempts were made to deliver boosters. Theoretical basis of intervention: prompts to clinicians to give brief advice, TTM and MI Control: dietary advice (5‐a‐day fruit and vegetables); theoretical basis of intervention: brief advice ‐ 3‐5 min motivational counselling | |
| Outcomes | Measurement: 30‐day PPA; follow‐up periods: > 3 months, 1 year and 2 years No verification Losses to follow‐up: 6% at 12 months and 12% at 24 months | |
| Notes | This systematic review uses definition of smoking of 1 cpw for ≥ 6 months to define a regular smoker. Hollis et al confirm that their definition of 'smokers' most closely fits this criterion. We have only used the data for smokers, although the trial included separate smoking uptake prevention results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "blocked over time and stratified according to medical centre and 30‐day cigarette smoking status," method of sequence generation not specified |
| Allocation concealment (selection bias) | Low risk | "Study staff members not involved in recruitment or randomization printed the stratified allocation assignments on index cards and concealed the cards in envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessor blinded, but no biochemical validation used. Differential misreport possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up at 2 years higher in treatment group (14.3%) than in control group (10.1%). 6 types of analyses to model missing data, including ITT analysis, in which participants lost to follow‐up counted as smokers. "Conclusions were largely consistent among the various missing‐data procedures." |
Horn 2007.
| Methods | Country: USA Setting: suburban Emergency Department Study design: RCT | |
| Participants | Participants: presenting for care at an ED (excluding those not competent or in police custody) 40/75 in Intervention and 35/75 in control arm Age range: 14‐17 years Criteria for inclusion: reported smoking within 30 days, willing to participate and providing written consent Follow‐up method: phone calls Inducements to enter study: none Pre‐study smoking status assessment: mFTQ and CO | |
| Interventions | Intervention: 5‐stage MI (1) screening (2) tailored interview of 15‐30 min (3) stage‐sensitive homework book (4) handwritten postcard within 3 days (5) motivational phone calls at 1/12, 3/12 and 6/12 Theoretical basis of intervention: MI Control: brief intervention including screening, generic advice‐giving (2 min) referral to information line Theoretical basis of control: normal care | |
| Outcomes | Measurement: self‐report at 6 months. 1 person quit in both intervention and control Verification. none | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized: "sequentially numbered...as sorted by the SAS random number function" |
| Allocation concealment (selection bias) | Low risk | Allocation concealed in manila study envelope, single pile, sequentially numbered. "Each randomized manila folder contained either the MTI or the BA protocol set of equal size and weight." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Each provider was blinded during the initial screening." No further blinding reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No biochemical validation used, different levels of intensity between groups, differential misreport possible, however, only 1 participant in each group reported abstinence so outcome unlikely to have been affected by detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 60% intervention and 65% control lost to follow‐up at 6 months. Study authors state: "follow‐up found low retention rates, presenting potential biases in our data" though "no significant differences between absent and present teenagers at 6‐month follow‐up were observed." |
Joffe 2009.
| Methods | Country: Maryland, USA Setting: 4 high schools (I = 2; C = 2) Study design: RCT with individuals randomized within schools, schools allocated in balanced blocks | |
| Participants | Participants: 193 students (I = 104; C = 104)
Age range: 14‐18 years, mean 15.9
Criteria for inclusion: self‐report of smoking AND expressed willingness to quit
Follow‐up method: self‐reports and salivary cotinine verification of smoking status Inducements to enter study: sessions conducted over lunch, which was provided, plus "modest incentives" Verification of smoking status: none Pre‐study smoking status assessment: self‐reports, age first smoked and "nicotine dependence" Significant demographic differences between arms of the trial: slight imbalance in ethnicity, age, nicotine dependence and quit attempts Post‐study smoking status assessment: self‐report and salivary cotinine |
|
| Interventions | Intervention: "Kickin' Butts": 15 lunch time sessions of 25/30 min (compared to 8 x 50‐min sessions of original intervention) Theoretical basis of intervention: programme used that of Adelman 2001 (see Excluded studies for references). Programme design "guided by information gathered in preliminary focus groups, directed interviews, and current teen and adult smoking cessation programs." Control: brief Intervention of 1 session with pamphlets | |
| Outcomes | Measurement: 30‐day PPA Follow‐up periods: 6 months and 12 months Verification: self‐reporting verified by salivary cotinine Losses to follow‐up: 69% followed up at 6 months and 62% at 12 months | |
| Notes | Same study also evaluated a NoT intervention, see NoT MD 2009 Used most conservative data presented in paper (Table 4) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomization, no information given on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding of personnel, not clear if participants knew what intervention other group was receiving |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No significant difference between groups in terms of percentage lost to follow‐up. Study authors conducted two ITT analyses, one treating those lost to follow‐up as smokers |
Kelly 2006.
| Methods | Country: Australia Setting: 3 state high schools in Brisbane Study design: RCT. Students referred into trial as a result of violation of smoking policy | |
| Participants | Participants: 56 students (34% female) Age range: 14‐16 years with parental consent Criterion for Inclusion: violation of school smoking policy Pre‐study status assessment: Modified Fagerstrom 3.6 ± 1.4, consumption ∼50 cpw follow‐up method: 1‐, 3‐ and 6‐month self‐reported tobacco use Inducements: not stated Pre‐study smoking status assessment: self‐reporting | |
| Interventions | Intervention: MI with trained interviewer of 1 h duration with information targeted directly at reported experiences of smoking, additional reading following interview Control: standard care interview of 1 h duration and within‐interview use of a "quit kit" plus review of general literature on effects of smoking within interview time | |
| Outcomes | Measurement: 30‐day PPA, no verification follow‐up periods 3/12 and 6/12 Losses to follow‐up: 25% at 6 months assumed relapsed | |
| Notes | Moderate differences in intervention and control groups but not regarded as significant to outcomes of study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned," no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified, not clear if participants knew what interventions the other group was receiving |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemical validation used, interventions delivered by author, differential misreport possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar loss to follow‐up in both groups (6/30 intervention, 8/26 control), participants lost to follow‐up counted as smokers in ITT analysis. "To test for attrition bias, differences between attritors and nonattritors were tested using one‐way ANOVAs... There were no significant differences on any variables except mother's occupational status." |
Killen 2004.
| Methods | Country: USA Setting: 9 continuation high schools in San Francisco, CA Study design: RCT. Quality of allocation concealment confirmed by study author | |
| Participants | Participants: 211 smokers Age range: 15‐18 years Criteria for inclusion: currently smoked ≥ 10 cpd, for ≥ 6 months, with > 1 quit attempt and a score of ≥ 10 on modified FNTQ Inducements to enter study: USD 50 at end of treatment and USD 50 for completing 6‐month assessment Pre‐study smoking status assessment: mean cpd 15 and mean FTQ score 16.6 No significant demographic differences between arms of trial Health screening was conducted; those screened positive for depression (clinical diagnosis) were excluded | |
| Interventions | Intervention: 8 weeks of tailored NRT patch therapy plus 150 mg SR bupropion tablet (for 8 weeks from quit date) and relapse prevention Theoretical basis of intervention: pharmacological plus group work (theoretical basis not given) Control: 8 weeks of tailored NRT patch therapy plus placebo tablet (for 8 weeks from quit date) | |
| Outcomes | Measurement: 7‐day PPA; follow‐up periods: > 3 months, 6 months
Verification: CO monitoring (below 9 ppm) and saliva cotinine (below 20 ng/mL) at 6 months; adherence to bupropion measured at 5 weeks
Losses to follow‐up: 36% at 6 months Adverse events: 47 self‐rated "severe" but none judged severe by the study physician |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | "Assignment to treatment condition was double blind," no further information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double blind," no further information provided, but placebo used and treatment effect not found, performance bias judged to be unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 38% bupropion & 35% placebo lost at 6 months, included in analysis |
Lipkus 2004.
| Methods | Country: USA Setting: 11 shopping malls and an amusement park in North Carolina, South Carolina, Georgia and Tennessee Study design: RCT | |
| Participants | Participants: 402 adolescents (I = 209; C = 193) Age range: 15‐18 years old Criteria for inclusion: ≥ 1 cigarette within preceding 7 days (mean years smoked 3 ± 2, and 10 ± 8 cpd) Follow‐up: telephone survey Inducements to enter study: a movie pass Pre‐study smoking status assessment: nicotine dependence measured using mFTQ No significant demographic differences between arms of trial | |
| Interventions | Intervention: telephone counselling, self‐help materials and a video Theoretical basis of intervention: eclectic but pre‐tested with age‐appropriate group and contained elements of CBT and TTM. Telephone counselling used MI Control: self‐help materials and a video Theoretical basis of control: eclectic, see above | |
| Outcomes | Measurement: 7‐day PPA and sustained abstinence (defined as not smoking at both 4‐month and 8‐month assessment points); follow‐up periods > 3 months, 8 months Verification: saliva cotinine at level of > 10 ng/mL at 4 months; self‐report only at 8 months Losses to follow‐up: 36% at 8 months Results: 7‐day quitting: 21% (calculated as 44 smokers) in intervention and 19% (calculated as 37) in control. Sustained quitting 9% (calculated as 19) in intervention arm and 7% (calculated as 14) in control. ITT for sustained quitting OR = 1.279 (0.622 ‐ 2.627) ITT for 7 day point prevalence OR = 1.124 (0.690 ‐ 1.833) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described, stratified by stage of readiness to quit |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Due to minimal contact nature of intervention, performance bias unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Biochemical validation done but final outcome figures based on self‐report only. High failure to confirm and low response rate. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | I = 46% and C = 51% reached at both follow‐ups. Losses included as smokers |
Mason 2016.
| Methods | Country: USA Setting: participants were recruited from the community (community adolescent substance abuse facility (66 %), public health clinics (21 %), university medical centre paediatric clinics (10 %), and dorms and high schools (3 %). The intervention was mobile‐phone based Study design: RCT |
|
| Participants | Participants: 200 (I = 100, C = 100), 52.5% female, 90.5% black or African American, 6.5% white, 3% unknown or other Age range: 14‐18 years, mean (SD) = 16.2 (1.39) Criteria for inclusion: aged 14‐18 years, scored > 1 in mFTQ assessment of dependence Follow‐up method: text messaging questionnaires Inducements to enter study: enrolled participants could recruit up to 3 peers and earn USD 5 per enrolment. 53% were recruited this way Baseline characteristic equivalence: no significant differences Pre‐test smoking status assessment: self‐report Post‐test smoking status assessment: self‐report |
|
| Interventions | Intervention: provided mobile phone. Received 30 text messages over 5 days, with boosters available if required. Consisted of rapport building, presenting tobacco use feedback, introducing social network information and presenting feedback, and summary and plans. Based off 20‐min intervention shown to be effective. Theoretical basis for intervention: MI Control: attention control. 30 health‐based (diet, exercise, study habits) text messages matched on length and frequency. Booster messages were not available |
|
| Outcomes | Measurement: 30‐day PPA Relevant follow‐up periods: 6 months Verification: none Loss to follow‐up: I = 13%, C = 15% |
|
| Notes | New for 2017 update. Abstinence data were not reported in paper, so were obtained from Dr Mason directly | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomized adolescents into either the intervention or the control group, using a blocked design creating equal numbers allocated to intervention and control groups”; however no details of how sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No details of blinding given; however as the intervention was carried out remotely via mobile phone there was minimal contact with researchers or other participants and therefore a lack of blinding is unlikely to have had an effect |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data was submitted by participants electronically, attention control matched, so despite lack of biochemical validation, risk of detection bias is low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13/100 allocated to intervention and 15/100 allocated to control were lost to 6‐month follow‐up. Therefore between‐arm drop out was comparable |
Moolchan 2005.
| Methods | Country: USA Setting: Baltimore, MD, by invitation through media advertisements, schools, churches Study design: RCT | |
| Participants | Participants: 120 Smokers (I = 80, C = 40) Age range: 13‐17 years Criteria for inclusion: smoking ≥ 10 cpd for ≥ 6 months and motivation to quit > 5 on 10‐point integer scale. Only those who were happy to inform parents of smoking status were included. Follow‐up method: interim and final questionnaires and final visit for verification of smoking status Inducements to enter study: USD 90 for baseline and USD 135 after final visit/completion Pre‐study status assessment: mean 18.8 cpd, 'youth appropriate' FTQ mean 7.04 No significant demographic differences between arms of the trial | |
| Interventions | Intervention: nicotine patch and gum, and self‐help written materials. 2 active groups (a) active patch with placebo gum (n = 34) (b) active gum with placebo patch (n = 46). NRT for both groups was tailored to weight and smoking level. Participants received 11 visits over 12 weeks to receive NRT, and attended 45‐min group CBT session at the end of each visit, + self‐help materials. Theoretical basis of intervention: pharmacological Control: placebo patch and gum (n = 40), same course of CBT sessions as intervention group | |
| Outcomes | Measurement: 7‐day PPA, and "prolonged abstinence", i.e. continuous abstinence after a 2‐week grace period from end of intervention; follow‐up periods: > 3 months, 6 months Verification: CO, salivary cotinine and thiocyanate Losses to follow‐up: 54% | |
| Notes | Timeline for trial was verified with study authors Adverse event "profile consistent with that reported for adults" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomized ... according to an algorithm held by the National Institute on Drug Abuse Pharmacy, with true replacement of the non‐completers" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as "double‐blind, double‐dummy", but no further information |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were included as failures for cessation. Losses fully reported |
Muramoto 2007.
| Methods | Country: Arizona, USA Setting: community recruitment Study design: double‐blind RCT with 2 treatment arms | |
| Participants | Participants: 312 smokers (I = 209; C = 103) Age range: 14‐17 years Criteria for inclusion: smoking ≥ 6 cpd & exhaled CO ≥ 10 ppm & ≥ 2 prior quit attempts & no major psychiatric diagnosis Follow‐up method: telephone visit at 12 weeks and 26 weeks post‐target quit date Inducements to enter study: none Pre‐study smoking status assessment: self‐report previous 90 days, mFTQ and CO verification | |
| Interventions | Intervention 1: bupropion SR 300 mg/d in blister cards Intervention 2: bupropion SR 150 mg/d in blister cards Theoretical basis of intervention: pharmacological phase III trial including "standardised brief individualised counselling" at each visit Control: 0 mg/d placebo tablet identical to active tablets and blister packed Theoretical basis of control: pharmaceutical | |
| Outcomes | Measurement: self‐reports of 7‐day PPA (30‐day PPA stated as an outcome in paper but figures not given, not obtainable from study author) at 26 weeks
Verification: exhaled CO at 26‐week visit Adverse events: headache, cough, throat symptom, sleep disturbance, nausea reported. 8 participants in treatment group discontinued treatment for various adverse events. 2 "serious" and 1 "medically important" adverse events occurred |
|
| Notes | 300 mg vs placebo displayed in analyses. 150 mg had fewer quitters than control (2/105, vs 6/103, RR 0.33 95% CI 0.07 to 1.58). Losses to follow‐up: 19/104 in 300 mg, 31/105 in 150 mg, 19/105 in control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Active study medication and identical‐appearing placebo were prepackaged into 3 sets of identical‐appearing blister cards in accordance with a computer‐generated randomization list." |
| Allocation concealment (selection bias) | Low risk | "... a research assistant assigned the subject the next treatment number (and associated blister cards) in sequence." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Study subjects and researchers remained blind to treatment group assignment throughout the study," identical appearing placebo used (see above) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded and biochemically validated abstinence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Slightly higher loss to follow‐up/declined further participation in placebo group (30%) than active arms (18%). ITT analysis |
NoT MD 2009.
| Methods | Country: Maryland, USA Setting: 4 high schools (I = 2 and C = 2) Study design: RCT, with individuals randomized within schools, schools allocated in balanced blocks | |
| Participants | Participants: 194 students (I = 92; C = 102)
Age range: 14‐18 years, mean 15.9
Criteria for inclusion: self‐report of smoking AND expressed willingness to quit
Follow‐up method: self‐report & salivary cotinine verification Inducements to enter study: sessions conducted over lunch, which was provided plus "modest incentives" Verification of smoking status: none Pre‐study smoking status assessment: self‐reports, age first smoked and "nicotine dependence" Significant demographic differences between arms of the trial: slight imbalance in ethnicity, age, nicotine dependence and quit attempts Post‐study smoking status assessment: self‐report and salivary cotinine |
|
| Interventions | Intervention: modified NoT intervention: 20 lunch time sessions of 25/30 min (compared to 5 x 50 min sessions of other NoT trials) Theoretical basis of intervention: SCT Control: brief Intervention of 1 session with pamphlets | |
| Outcomes | Measurement: 30‐day PPA Follow‐up periods: 6 months and 12 months Verification: self‐reporting verified by salivary cotinine Losses to follow‐up: at 6 months and at 12 months | |
| Notes | Clarification of data and details of incentives sought from study authors but not received Same study also evaluated an alternative intervention, see Joffe 2009 Modified NoT: entered as NoT since basis of intervention same but timescale of delivery modified Used most conservative data presented in paper (Table 4) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | see Joffe 2009 |
| Allocation concealment (selection bias) | Unclear risk | see Joffe 2009 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | see Joffe 2009 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | see Joffe 2009 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | see Joffe 2009 |
NoT WV 2011.
| Methods | Country: USA Setting: 99 public high schools in West Virginia Study design: cluster‐RCT. Intervention schools were allocated to either NoT alone or NoT plus a physical activity programme. This enabled comparison of NoT with NoT plus FIT. | |
| Participants | Participants: 233 participants (I = 170, C = 63). NoT alone had 90 in intervention group and NoT plus FIT had 80 in intervention group Age range: 14‐19 years Criteria for inclusion: ≥ 1 day of smoking in last 30 days Inducements to enter study: none Pre‐study smoking status assessment: self‐reports Post‐study smoking status assessment: self‐reports plus breath CO at 3 months but self‐reported only at 6 months | |
| Interventions | Intervention: NoT intervention: 1 x 50‐min session once/week for 10 weeks, same‐gender small groups (≤ 10 in the group) led by same‐gender facilitators. Covered motivation, smoking history, nicotine dependence, social, psychological and health consequences of smoking, preparation for quitting, urges and cravings, relapse prevention, stress management, family/peer pressure, healthy lifestyle, nutrition. 4 booster sessions offered post‐programme at 2 and 4 weeks Theoretical basis of intervention: SCT Control: brief intervention NoT plus FIT participants were given a pedometer and encouraged to keep a log of steps taken |
|
| Outcomes | Measurement: 7‐day PPA at 3 months, self‐reported quitting at 6 months
Biochemical verification: breath CO at 3 months Losses to follow‐up: > 60% of participants retained |
|
| Notes | Although results analysed as clusters, 21 (out of 40) schools dropped out after randomization but before study onset due to recruitment and logistics. We note that the abstract gives impression that 7‐day PPA was measured at 6 months. However outcome at 6 months was self‐report only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomization |
| Allocation concealment (selection bias) | High risk | 21 out of 40 schools dropped out after randomization but prior to study start, aware of assignments |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information specified, unclear if arms knew what other arms were receiving |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | > 60% followed up, participants lost to follow‐up counted as smokers |
O'Neill 2000.
| Methods | Country: USA Setting: Midwestern University Study design: RCT |
|
| Participants | Participants: 65 (I = 31, C = 34) daily smokers, 63% female Age range: 18‐25 years, mean 19.7 Criteria for inclusion: university undergraduates studying lower‐level psychology, daily cigarette smokers, consented to taking part in study of "computer‐based health education" Follow‐up method: telephone Inducements to enter study: not reported Baseline characteristic equivalence: not reported Pre‐test smoking status assessment: self‐report Post‐test smoking status assessment: self‐report |
|
| Interventions | Intervention: 4 individual computer sessions over 6 weeks. Modules were completed in sessions 1‐3 with a post‐test in session 4. Intervention software was adapted from the Smoke Mall program (unable to find any extra information online about this). The programme was made up of 6 modules, each utilising specific processes of change. Modules were selected by the computer to match the participants’ current stage of change. Theoretical basis for intervention: TTM stages of change theory Control: 4 individual computer sessions over 6 weeks. Modules were completed in sessions 1‐3 with a post‐test in session 4, as in intervention condition. 3 computer modules dealing with health‐related topics other than smoking (dietary assessment, hypertension risk, stress management). Control modules were equivalent to intervention modules in length and general format. |
|
| Outcomes | Measurement: PPA and 6‐month continuous abstinence Relevant follow‐up periods: 7 months Verification: none Loss to follow‐up: 13% in intervention group, 15% control group |
|
| Notes | Previously excluded, now included in 2017 update. This is on account of the redefinition of our inclusion criteria for age of participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although attention matched, Not specified for participants whether they were informed of the content of the other intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not biochemically validated, unclear on levels of participant blinding to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was 13% in intervention group and 15% in control group, sufficiently low and similar to be considered low risk |
Patten 2006.
| Methods | Country: USA
Setting: community‐based in 3 locations; Minnesota, Winsconsin and Conneticut Study Design: RCT Recruitment: community‐based recruitment by television commercials, radio, newspaper announcements and flyers in schools and clinics |
|
| Participants | Participants: 139 smokers: (I = 70; C = 69)
Age range: 11‐18 years, median 16 years
Criteria for inclusion: smoked > 10 cigarettes in previous 30 days, primarily used tobacco, parental consent given
Follow‐up method: clinic visits at 4, 8, 12, 24 and 36 weeks
Pre‐study status assessment: mFTQ = 4.1 ± 1.9, mean cpd 10.1 ± 6 Inducements to enter study: USD 10 per visit for weeks 4‐24 for completed visits, USD 20 at week 36 Post‐study smoking status assessment: self‐reports validated with CO measurement Significant demographic differences between arms of the trial: none |
|
| Interventions | Intervention: 'Stomp out Smokes' (SOS) delivered by home‐based internet and using as theoretical base Social (cognitive) Learning theory, health communication and decision‐making theories. Access to SOS was available for 24 weeks after enrolment. No clinician contact except during assessment clinic visits Control: brief Intervention (office based) developed by American Medical Association and delivered by counsellor at 4 individual weekly sessions No participants required to set quit dates and pharmacotherapy not provided |
|
| Outcomes | Measurement: 30‐day PPA at 24 weeks and 36 weeks Verification: self reports plus CO validation Losses to follow‐up: 33% at week 24 and 43% at week 36 | |
| Notes | As intervention was available up to 24 weeks point outcomes taken from 36 weeks as more realistically demonstrating persistence of intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned," method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Due to nature of intervention any contact likely to be part of intervention, so performance bias unlikely. "Except for the assessment visits, study staff did not have any personal contact with participants." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage attending assessment visit in the intervention and control conditions, respectively, was 42% and 53% at 9 months. All randomized participants included in ITT analysis which produced more conservative outcome. |
Pbert 2011.
| Methods | Country: USA Setting: 35 high schools (16 intervention, 19 control) Study design: Cluster‐RCT |
|
| Participants | Participants: 1068 (I = 486, C = 582) adolescent smokers, 46.7% female, 92.6% white, 10.3% Hispanic Age: mean 16.8 (I = 16.8 (SD 1.2), C = 16.9 (SD 1.9)) Criteria for inclusion: grade 9‐12, smoked within past 30 days, reported interest in quitting smoking Follow‐up method: confidential, self‐administered questionnaire and cotinine assessment Inducements to enter study: none reported Baseline characteristic equivalence: the 2 groups were similar in sociodemographic and smoking characteristics. Approximately 66% of intervention group students planned to quit within the next 12 months compared with 57% of control students. The intervention group had slightly higher depression and anxiety scores Pre‐test smoking status assessment: self‐report. Mean cpd = 6.7 Post‐test smoking status assessment: biochemically validated self‐report |
|
| Interventions | Intervention: Calling It Quits counselling intervention. One 30‐min session/week with the school nurse for 2 weeks before quit date, one 15‐min session/week for 2 weeks after quit date. Sessions based on 5 A's model, adapted for adolescents Theoretical basis for intervention: SCT Control: 4 weekly visits with the school nurse, where informational pamphlets were delivered |
|
| Outcomes | Measurement: 30‐day PPA Relevant follow‐up periods: 12 months Verification: validated with salivary cotinine < 11.4 ng/mL Loss to follow‐up: at 12 months loss to follow‐up was 11% in intervention group and 12% in control group |
|
| Notes | New for 2017 update. Flow diagram says that all participants were included in analysis; therefore where Ns have been calculated from percentages it is assumed that the analysis was intention to treat and N randomized has been used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Thirty‐five schools were recruited, pair‐matched on demographics (percentage white, black, and Hispanic), school size, and percentage of students that are low‐income, and were randomly assigned to either the counseling intervention (16 schools, n _ 486 subjects) or attention control condition (19 schools, n _ 582 subjects). Randomization was conducted after completion of baseline data collection in each school. A random number was generated using Excel for each matched pair of schools.” Unsure how 35th school was accounted for, and how the resulting randomization had a difference of 3 schools between groups, if it truly was pair matched |
| Allocation concealment (selection bias) | Unclear risk | Randomization was conducted after completion of baseline data collection in each school (therefore after participant recruitment). However it is not clear how the randomization was carried out and by who |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The intervention was behavioural so blinding was not possible; however the control was attention matched, so is judged to be low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants completed a confidential, self‐administered questionnaire at baseline and 3 and 12 months after enrolment to assess smoking status. Cotinine was analysed externally by a laboratory. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11% of intervention and 12% of control groups lost to follow‐up at 1 year, sufficiently small and similar to be judged low risk |
Peterson 2009.
| Methods | Country: USA Setting: high schools in Washington State Study Design: matched pair, cluster‐RCT, randomized at school level Recruitment: following smoking status baseline survey in all schools smokers were invited to participate in intervention. Non‐smokers also invited to preserve confidentiality of students | |
| Participants | Participants: 790 smokers: (I = 403; C = 387) Age range: high school smokers, almost all aged 16‐18 years Criteria for inclusion: see Notes as restricted subset. Parental consent sought for those aged under 18 years. Follow‐up method: questionnaire at 12 months from intervention Pre‐study status assessment: baseline survey to identify monthly and "regular" smokers (defined as those reporting smoking on ≥ 20 of the last 30 days) Inducements to enter study: USD 10 per completed post‐study questionnaire (USD 20 if survey returned at second or third prompt). Post‐study smoking status assessment: self‐report Significant demographic differences between arms of the trial: random assignment but experimental group contained higher proportion of daily smokers (statistically corrected in analysis) | |
| Interventions | Intervention: complex intervention including quit kit, tailored telephone counselling, supportive website (TTM based) and school‐wide cessation health promotion campaign. Specific attributes of teen smoking addressed, e.g. need for privacy, confidentiality and sense of being in control, state of motivation, importance of peer support Theoretical basis of intervention: TTM, MI, CBT and SCT‐based counselling Control: normal school‐based activity | |
| Outcomes | Measurement: self‐reported, 6‐month continuous abstinence, measured at 12 months Verification: self‐report. No biochemical validation but internal within‐questionnaire validity checks on reports of smoking status Losses to follow‐up: 11% at week 52 after intervention | |
| Notes | The 2017 review update uses data reported by Heffner 2016, who report on the subgroup of "regular smokers" (smoking on ≥ 20 of the last 30 days) at baseline. Although this is more restrictive than our own criteria, it is this group that is recognized, in the literature, as the most likely to be addicted. Data on the number of participants randomized were provided by the lead study author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Matched‐pair randomization for individual schools, "schools were randomly ordered within each matched pair, and then, one school in each pair was randomly assigned to the experimental or control condition by a computerized coin flip." |
| Allocation concealment (selection bias) | Low risk | Computerized coin flip "performed openly, witnessed, and recorded" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The tracking and data collection staff were blind to experimental vs. control status at outcome data collection and entry." As control was normal, school‐based activity, performance bias unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemical validation used, intervention higher intensity than control, differential misreport possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 92% control and 86% intervention participants completed follow‐up survey, ITT analysis conducted |
Prochaska 2015.
| Methods | Country: USA Setting: mental health settings Study design: RCT |
|
| Participants | Participants: 60 adolescent and young adult smokers, 52% female, 41.6% white, 25.0% Hispanic/Latino, 15.0% multi‐racial, 6.6% African American, 5.0% Asian, 6.4% other, 1.6% American Indian/Alaska Native Age range: 13‐25 years, mean 19.5 (1.2) Criteria for inclusion: adolescents and young adults aged 13‐25 years, receiving mental health treatment at 1 of the recruitment sites, reported smoking ≥ 1 cigarette in the past month and > 100 cigarettes in their lifetime, speak English, not currently receiving smoking cessation treatment Follow‐up method: Inducements to enter study: up to USD 120 could be earned in gift cards, and USD 40 dollars reimbursed for travel costs Baseline characteristic equivalence: details of randomization by trial arm not available Pre‐test smoking status assessment: self‐report. Mean 8.0 (SD = 6.6), dependence measured with mFTQ mean (SD): 4.8 (1.6) Post‐test smoking status assessment: self‐report |
|
| Interventions | Intervention: 2‐staged approach. Stage one was tailored, computer‐assisted, brief counselling and assessment of TTM constructs at baseline, 3 months and 6 months, with feedback compared to others at the same stage and to previous responses. Stage 2 could be initiated in the first 9 months of treatment, and consisted of 6 individual sessions of CBT over 12 weeks, along with 12 weeks of NRT (patch) Theoretical basis for intervention: TTM and CBT Control: usual care, consisting of brief advice and a self‐help brochure |
|
| Outcomes | Measurement: 7‐day PPA Relevant follow‐up periods: 6 and 12 months Verification: exhaled CO < 10 ppm at 6 months, salivary cotinine < 15 ng/mL at 12 months Loss to follow‐up: at 12 months, 14% of intervention and 10% of control were lost |
|
| Notes | New for 2017 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “computer‐generated randomization scheme that blocked on tobacco use (daily vs. nondaily) and stage of change (precontemplation, contemplation, or preparation)” |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded as large part of intervention was behavioural. Interventions were not matched for intensity of support or attention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated, loss to follow‐up was similar |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12‐month follow‐up rates were 86% for treatment group and 90% for control, sufficiently high and similar to be considered low risk |
| Other bias | High risk | Limited reporting of data by trial arm. Investigating the difference in efficacy according to treatment provided was a primary study aim |
Project EX Russia 2013.
| Methods | Country: Russia Setting: summer recreational camps Study design: cluster‐RCT | |
| Participants | Participants: 164 smokers (I = 76, C = 88) Age range: ≤ 19 years old Criteria for inclusion: ≥ 1 cpw for ≥ 6 months prior to enrolment Follow‐up method: at 6 months through telephone calls and emails Pre‐study status assessment: self‐reported Inducements to enter study: none Post‐study smoking status assessment: self‐reported | |
| Interventions | Intervention: standard Project EX (see Project EX‐1 2001) Theoretical basis of intervention: complex intervention including CBT and motivational enhancement Control: standard care on tobacco use (officially tobacco use not allowed during camp) | |
| Outcomes | Measurement: self‐reported 30‐day PPA Biochemical verification. none Losses to follow‐up: 34 out of 164 (I = 16, C = 18) | |
| Notes | We were unable to determine suitable numerical information for including in meta‐analysis. For example, the reported quit rate of 0.1% among control group participants was inconsistent with the follow‐up sample size of 70. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "experimental pilot trial that involved different youth that rotated through camps. Conditions were nested within camps. Two rotations of unique subject groups of smokers (program and standard care control) through each of five camps provided the means of controlling for campsite by condition" Allocation decided by coin toss |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Youth in a given rotation were informed that they would be offered assistance in quitting smoking. However, they were kept blinded to study condition, which was easy considering that totally different cohorts of youth attended the different camp rotations." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Intervention involved face‐to‐face contact, no biochemical validation of smoking status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low loss to follow‐up in both conditions |
Project EX Spain 2015a.
| Methods | Country: Spain Setting: 9 schools in Alicante and Murcia Study design: cluster‐RCT |
|
| Participants | Participants: 211 (I = 112, C = 99) adolescent smokers, 53.3% female, 91% Spanish, 9% other nationality Age range: 14‐19 years, mean (SD) = 16.4 (1.38) Criteria for inclusion: aged 13‐19 years, smoked a cigarette in last 30 days before baseline, willing to attend school‐based clinic programme and joined clinic in first 2 weeks Follow‐up method: questionnaire Inducements to enter study: none Baseline characteristic equivalence: no information reported Pre‐test smoking status assessment: self‐report, mean (SD) cpd = 7.1 (6.3), mFTQ used to measure dependence but no baseline data reported Post‐test smoking status assessment: self‐report |
|
| Interventions | Intervention: Spanish translation of Project EX programme (see Project EX‐1 2001) Theoretical basis for intervention: complex intervention including CBT and motivational enhancement Control: waiting list control |
|
| Outcomes | Measurement: 30‐day PPA Relevant follow‐up periods: 6 months Verification: none Loss to follow‐up: I = 68%, C = 34% |
|
| Notes | New for 2017 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" but no details given |
| Allocation concealment (selection bias) | High risk | 2 schools that were randomized to the intervention dropped out before any students could be recruited. One of these did so “due to a concern to meet academic priorities and overall lack of interest in the program” suggesting that in this school the potential for participant recruitment was heavily dependent on the randomized group allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible and control participants received no intervention until after study follow‐up had been completed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐report only and control participants received no intervention until after study follow‐up had been completed, so high risk of differential misreport |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High level of dropout overall and more dropout by 6 months in the intervention group (68%) than in the control group (34%) |
| Other bias | High risk | The number of quitters post‐test in the programme group was reported inconsistently as 5 smokers and 3.9% (5/112 = 4.5%) and also reported inconsistently at 6 months as 6 smokers and 4.9% (6/112 = 5.4%), which cannot be accounted for by loss to follow‐up. In the abstract the percentage of quitters at 6 months given for the programme group is different again, at 14.28%, which does not match up with a complete case analysis (as 6/35 = 17.1%) |
Project EX Spain 2015b.
| Methods | Country: Spain Setting: schools in the province of Alicante Study design: cluster‐RCT |
|
| Participants | Participants: 212 (I = 100, C = 112) adolescent smokers (1546 participants included in study but 212 reported being current smokers and were analysed separately). 46.4% female, 90.7% Spanish, 9.3% other nationality Age (mean): 15.3 years Criteria for inclusion: not reported Follow‐up method: questionnaire Inducements to enter study: not reported Baseline characteristic equivalence: not reported Pre‐test smoking status assessment: self‐report, but measures not reported Post‐test smoking status assessment: self‐report |
|
| Interventions | Intervention: Spanish translation of Project EX programme (see Project EX‐1 2001) Theoretical basis for intervention: complex intervention including CBT and motivational enhancement Control: waiting list control |
|
| Outcomes | Measurement: 30‐day PPA Relevant follow‐up periods: 12 months Verification: expired CO, measured with Belfont Micro+ Smokerlyzer Loss to follow‐up: 38% of participants from sample of 1546 dropped out |
|
| Notes | New for 2017 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" but no details given; also "schools were carefully matched into pairs prior to assignment" but unclear how this was done |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible with behavioural intervention, and study arms received significantly different levels of contact. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐report only, and control group received no contact until after final assessment, so high risk of differential misreport |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 38% of participants from the sample of 1546 dropped out; not clear how many of these were current smokers at baseline and therefore suitable for inclusion in the review, but there were large differences in the characteristics of individuals lost to follow‐up and those who completed the study on several variables, which makes bias due to differential drop‐out likely |
Project EX‐1 2001.
| Methods | Country: USA Setting: 18 continuation high schools in Southern California Study design: cluster‐RCT (assigned by block randomization) | |
| Participants | Participants: 335 smokers, recruited by advertising and flyers within each school. 139 in 6 Project EX schools, 120 in 6 Project EX plus 'school as community' (SAC) schools, 76 in 6 control schools. Age range: 14‐19 years. Mean age was 16.8 (± 0.8) years Criterion for inclusion: used tobacco in last 30 days Follow‐up method: questionnaires and telephone for those who had left school Inducements to enter study: class credits and class release time Pre‐study smoking status assessment: questionnaire. Mean smoking 8.8 cpd ( ± 9.3) mFTQ scores 30% in range 0‐6, 53% in range 7‐13 and 17% in range 14‐21 Post‐study smoking status assessment: questionnaires No significant demographic differences between arms of trial | |
| Interventions | Intervention: initially schools split into 3 arms: (1) Project EX sessions alone (clinic‐only schools). (2) Project EX plus school community development 'school‐as‐community' (SAC schools). (3) Control: standard care 1. Project Ex was 8 sessions or 'clinics' over a 6‐week period delivered to groups and developed in trials. 4 sessions were preparation for quitting over 2 weeks, and next 4 were weekly during the first month post‐quit Theoretical basis of intervention: complex theoretical constructs including MI etc, and including games for groups, education and anger management, yoga, weight control, meditation, assertiveness training, role play and relapse prevention 2. SAC intervention: modelled on Toward No Drug Abuse programme. Student body organized service, recreational and job training functions, and produced a Project newsletter, to enable expression of anti‐tobacco attitudes. | |
| Outcomes | Measurement: 30‐day PPA; Follow‐up periods: > 3 months, 6 months from start of study
Verification: CO (for 62 students and results adjusted by false quit reporting factor of this group)
Losses to follow‐up: 51% in intervention group ‐ 40% of intervention group dropped out during clinics ‐ 42% in control group lost to follow‐up. Results: no difference in outcomes between two intervention arms of trial so study authors pooled data and compared, as a single arm with control arm Calculated OR based on 17% in intervention = 44 people and 8% in control being 6 people Calculated OR = 2.388 (0.976 to 5.841) |
|
| Notes | Recruitment in intervention arm was voluntary; 90% of participants said they had volunteered because they wanted help with quitting | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized block design procedure," method not specified |
| Allocation concealment (selection bias) | Low risk | Students recruited after schools randomized |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used for 62 students and results adjusted by false quit reporting factor of this group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Per‐protocol analysis and ITT analysis yield similar outcomes, "evidence that the study findings are robust despite the relatively high clinic drop‐out rate." |
Project EX‐4 2007.
| Methods | Country: USA (Southern California) Setting: 12 continuation high schools Study design: cluster‐RCT |
|
| Participants | Participants: 1097 participants attending continuation high school Age: range 13‐19 years, mean 16.5 years, SD 1.0 years Inclusion/exclusion criteria: participants included both smokers and non‐smokers at baseline, no inclusion/exclusion criteria stated Follow‐up method: questionnaires for 6‐month and 12‐month outcome measures, supplemented by a "pipeline assessment protocol" using CO verification. These methods were also used to define baseline smoking status. Inducements to enter study: not reported Baseline comparison by group: not reported for those who were smokers at baseline |
|
| Interventions | Based on the Project EX clinic program similar to the Project EX‐1 2001 intervention. 8 sessions were delivered over a 6‐week period Participants randomized to the comparison group received standard tobacco prevention and cessation activities (if any) that were routinely provided by their school |
|
| Outcomes | Measurement: 30‐day PPA Relevant follow‐up periods: 6 and 12 months Verification: "pipeline assessment protocol" using CO verification for all participants who consented Results for baseline smokers were reported in a corrigendum published in 2010, as an ITT analysis. Level of dropout among baseline smokers not reported |
|
| Notes | Results for baseline smokers were taken from the corrigendum Sussman et al. (2010) rather than the main trial paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Schools were matched and randomly assigned but method of randomization not stated |
| Allocation concealment (selection bias) | Unclear risk | Extent of awareness of the matching not clear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Cluster‐randomized by school – whether participants were aware of group allocation or allocation to other schools is not clear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Paper reports a “pipeline assessment protocol” but unclear whether this was used for all participants. Self‐report was also used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on possible differential drop‐out by group |
Pérez‐Milena 2012.
| Methods | Country: Spain Setting: 5 high schools Study design: RCT |
|
| Participants | Participants: 91 (I = 43, C = 48), 49% female Age: mean 15.4 Criteria for inclusion: ≤ 20 years, attending participating high schools, smoking ≥ 1 cpw over last 6 months. Excluded for mental/psychiatric illness/disability, pregnancy, using any smoking cessation pharmacology, student or parents or tutors not wishing student to participate Follow‐up method: in person Inducements to enter study: none Baseline characteristic equivalence: Pre‐test smoking status assessment: self‐report, I = 84%, C = 81% were daily smokers, cpd 8.3 during the week but 15.9 at weekends, FTND 3.1, 62% were low dependence (0‐3) Post‐test smoking status assessment: biochemically validated self‐report |
|
| Interventions | Intervention: 4 x 15‐min weekly sessions with GPs, focus on initial reduction, signing a declaration to quit at 3rd visit and 4th visit for reinforcement Theoretical basis for intervention: MI Control: a single 15‐min session with brief advice and a leaflet. All participants sent a text message on quit date, the day before and a week after, and monthly emails for a year |
|
| Outcomes | Measurement: 12‐month continuous abstinence Relevant follow‐up periods: 12 months Verification: expired CO ≤ 6 ppm (Smoke Check) Loss to follow‐up: 3 of 91 |
|
| Notes | New for 2017 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomization stratified by school, using Epidat 3.1 |
| Allocation concealment (selection bias) | Low risk | "Blinded allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel would have been aware of group assignment, as it was a behavioural intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 losses, counted as smokers for meta‐analysis |
Redding 2015.
| Methods | Country: USA Setting: 4 family planning clinics (2 in teaching hospitals, 2 in community health centres) in metropolitan areas, Philadelphia Study design: RCT | |
| Participants | Girls, aged 14‐17 years, 84% African‐American ethnicity, registering at family planning clinics Exclusion criteria: pregnant at time of recruitment 828 participants randomized, including 166 "baseline smokers" Baseline smoking status measured by self‐report, with those classified as being in any of the first 3 stages of the Stage of Change model for smoking cessation classified as "baseline smokers" No inducements to recruitment but could receive small non‐monetary gift incentives (e.g. pencil case and teddy bear) at clinic visits to minimize attrition and USD 10 gift vouchers at 12‐ and 18‐month telephone follow‐up |
|
| Interventions | Intervention: computer‐based information and feedback, plus counselling from BA‐/MA‐level counsellors with family planning counselling experience and training on smoking Participants could attend ≤ 4 sessions which included group‐specific, computer‐delivered feedback and in‐person counselling Intervention period: 9 months Theoretical basis: TTM Usual care: generic, non‐tailored computerized information and advice + standard “contraceptive educational counseling” |
|
| Outcomes | Self‐reported: computer‐assisted surveys at baseline, 3, 6, 9 months; telephone phone follow‐up surveys at 12, 18 months (phone survey staff were blind to group allocation) Cessation measure assessed using stages of change – e.g. if reported moving to action or maintenance phase of smoking cessation No biochemical verification |
|
| Notes | New for 2017 update Study was primarily aimed at increasing condom use but smoking cessation formed part of the intervention and results were reported separately for those classified by the authors as “baseline smokers” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The computer randomized participants to either the TTM or SC group (1:1 ratio) within each recruitment site stratified by baseline stage of condom use.” |
| Allocation concealment (selection bias) | Low risk | “The computer randomized participants to either the TTM or SC group (1:1 ratio) within each recruitment site stratified by baseline stage of condom use.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified if family planning personnel were blinded, or if participants in control arm were aware what participants in the intervention arm were given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study personnel making follow‐up telephone calls were blinded to group allocation but cessation was measured by participant self‐report and participant knowledge of group allocation was unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up < 40% (intervention group) at 48% (control group) at 12 months. Study authors report results unaffected by multiple imputation and sensitivity analysis |
| Other bias | High risk | Smoking cessation component of the intervention may not have been delivered successfully: “Subsequent review of these reports for fidelity revealed that counselors were much more ready to discuss condom use than they were ready to discuss smoking related topics in sessions” |
Robinson 2003.
| Methods | Country: USA Setting: 18 schools in Memphis, Tennesee Study design: RCT | |
| Participants | Participants: 316 smokers referred to study by school administrators or parents after violation of school no‐smoking policy, 261 students (I = 169; C = 92) followed up to (2003 Age range: 13‐19 year olds; 64% male Follow‐up method: telephone assessment, self‐reporting Inducements to enter study: fast food coupons, discounts at music stores and money on completion Pre‐study smoking status assessment: mFTQ Significant demographic differences between arms of trial: more cases in intervention than control arms because of school wish to have offenders treated | |
| Interventions | Intervention: 4 x 50‐min sessions behavioural programme, based on STS (Start To Stop) model, delivered by trained health educators, MI at start of programme and monthly phone calls for 1 year to assess smoking status and give brief support, based on stage of change. Theoretical basis of intervention: aocial influence theory, motivational enhancement, CBT and TTM Control: qritten material at start of study, and monthly phone calls to assess smoking status | |
| Outcomes | Measurement: 7‐day PPA; follow‐up periods: > 3 months, 12 months Verification: attempted for all quitters. Salivary cotinine samples obtained for 18/41 cases, CO initially as a "bogus pipeline" for some students | |
| Notes | Paper based on incomplete follow‐up and denominators unclear so data not shown in comparisons. No evidence of effect detected. We were unable to obtain clarification from study authors. Stratified data available on baseline characteristics. Referral to study for violation of school no‐smoking policy raises issues of consent. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization at individual level, method not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used (indicating that 50% of those who had reported quitting had falsified smoking status) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 92% retention but rates in each group not clear |
| Other bias | Unclear risk | Possible contamination as unit of allocation was student, so that controls and interventions mixed in same schools, and there was no concealment of allocation. |
Scherphof 2014.
| Methods | Country: the Netherlands Setting: baseline assessment carried out in schools, outcome data submitted via internet Study design: RCT |
|
| Participants | Participants: 265 (I = 136, C = 129), 52.9% female Age mean (SD): I = 16.56 (1.11), C = 16.70 (1.16) Criteria for inclusion: 12‐18 years old, no major health problems, smoking ≥ 7 cpd, parents of participants were aware of their smoking, participants were motivated to quit Participants excluded if currently using NRT, were pregnant or lactating, or were allergic to patches Follow‐up method: online questionnaires Inducements to enter study: up to EUR 90 for completing all online follow‐up assessments Baseline characteristic equivalence: Only gender was significantly different between groups (I = 59.3%, C = 45.9%) Pre‐test smoking status assessment: self‐report, banded cpd, ≤ 10 (cpd): I = 24.8%, C = 23.1%; 11–20: I = 63.9%, C = 65.3%; > 20: I = 11.3%, C = 11.6%. Using the 265 included in analysis these percentages equal 64 participants smoking ≤ 10 cpd, 171 participants smoking 11‐20 cpd, and 30 participants smoking > 20 cpd Post‐test smoking status assessment: biochemically validated self‐report |
|
| Interventions | Intervention: short behavioural intervention, followed by 6 or 9 weeks of 24 h NRT with patch, depending on smoking level at baseline Theoretical basis for intervention: pharmacotherapy Control: placebo patch control, otherwise identical to intervention |
|
| Outcomes | Measurement: 30‐day PPA Relevant follow‐up periods: 6 months and 12 months Verification: salivary cotinine measured using a NicAlert saliva strip (Nymox) Loss to follow‐up: 7.4% at 6 months, 10.1% at 12 months Adverse events including tiredness, cough, insomnia, itchiness and headache |
|
| Notes | New for 2017 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomized according to a computer‐generated randomization list by the pharmacy of the University Medical Centre to either (1) active study medication (nicotine patch) or (2) an identically appearing placebo (placebo patch)." |
| Allocation concealment (selection bias) | Unclear risk | "participants and research assistants were blind to treatment allocation" however does not specify how this occurred |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial was placebo controlled ‐ "Novartis provided the study medication (Nicotinell and placebo, 21 mg, 14 mg, and 7 mg, identical in appearance)". "participants and research assistants were blind to treatment allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Occurred via on online questionnaire, biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Excluded from analyses prior to follow‐up: n = 8 (1 in intervention, 7 in control), due to inconsistent answers; quitted participation; filled out < 2 questionnaires Participants who did not complete the 7th (n = 19, 7.4%) or 8th (n = 26, 10.1%) online questionnaires were not spread significantly differently across treatment groups |
Sherbot 2005.
| Methods | Country: Canada, Nova Scotia Setting: intervention introduced into a wider programme set up for young people who had been identified as having substance abuse problems (including drugs, alcohol and gambling but not tobacco). Study design: RCT | |
| Participants | Participants: 39 young people, 13 in each study group referred onto programme from both urban and rural settings Age range: 13‐19 years Criterion for Inclusion: enrolled on 'Choices Adolescent Treatment Program' and not taking any psychotropic drugs Inducements: wait list received 2 x CAD 25 each and intervention groups 4 x CAD 25 each. All completing participants at 7/12 received CAD 25 Follow‐up method: participants contacted by phone or mail Pre‐study smoking status assessment: FTND Post‐study smoking status assessment: self‐reported quitting & FTND | |
| Interventions | Intervention: Group A ‐ motivational enhancement therapy delivered by trained therapists over a period of 4 weeks at 1 individual session/week Intervention: Group B ‐ Completion of 'Quit 4 life' booklet over a period of 4 weeks at 2 sessions in the 1st week, 2 sessions in the 2nd week, 2 sessions in the 3rd week, and 3 sessions in the 4th week Theoretical basis of intervention: MI Control: on waiting list | |
| Outcomes | Self‐reported quitting at 6 months; Group A 5; Group B 1; control: 2 Losses to follow‐up: overall 10.3%, Group A 2.6%, Group B 2.1%, control 2.7% | |
| Notes | All referrals to both programme and this study were voluntary. 100% of those studied also used marijuana. Quitting data not verified and large differences between intervention groups in baseline smoking reports, possibly explained by outliers | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Participants had the opportunity to draw either an “A,” “B,” or “C” to determine which group they were to be in" |
| Allocation concealment (selection bias) | High risk | No possibility of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified but due to nature of intervention, performance bias unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding reported, no biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/39 lost at 6 months |
| Other bias | High risk | Large differences between intervention groups in baseline smoking reports, possibly explained by outliers. |
Skov‐Ettrup 2014.
| Methods | Country: Denmark Setting: online, participants were members of Xhale.dk Study design: RCT |
|
| Participants | Participants: 2030 (I = 1055, C = 975) daily smokers, 59.3% female Age range: 15‐25 years, mean (SD): I = 19.4 (3.1), C = 19.5 (3.2) Criteria for inclusion: daily smoker, aged 15‐25 years, valid email address or mobile phone number, self‐chosen quit date between 14 February 2007 and 1 August 2009 Follow‐up method: contacted via email to complete internet‐based questionnaire, email/text reminders sent after 4 days and after 11 days. If there was still no response after 18 days up to 4 attempts were made to contact participants over telephone Inducements to enter study: none Baseline characteristic equivalence: “At baseline there were no statistically significant differences between groups” Pre‐test smoking status assessment: self‐report, mean (SD) cpd: I = 15.4 (7.0), C = 15.6 (6.8) Post‐test smoking status assessment: self‐report |
|
| Interventions | Intervention: access to programme website, which included smoking facts, tests, exercises, videos and a chat forum. In addition there was the option of receiving tailored text messages. This entailed a weekly message up to 4 weeks before their quit date, and a daily message 1–3 days before the quit date. Then they received 2 tailored text messages/d during a period of 4 weeks. For the following 4 weeks, the frequency of text messages declined to 4‐5 text messages/week. The system generated 3 types of tailored messages based on 3 different tailoring parameters: self‐efficacy, beliefs about smoking and themes chosen by the user. Theoretical basis for intervention: Stage of Change theory and theory of planned behaviour Control: also had access to website and the option to activate text messages. These messages were less frequent and untailored. Messages were sent once daily for 5 weeks beginning 5 days before the chosen quit date. Weekly messages were sent for the following 3 weeks. |
|
| Outcomes | Measurement: 30‐day PPA Relevant follow‐up periods: 12 months Verification: none Loss to follow‐up: I = 73.7%, C = 71.9% |
|
| Notes | New for 2017 update. This review used all randomized participants, whether or not they chose to activate text messages. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract states participants were "consecutively randomized", the meaning of which is unclear. No further details of the randomization process were present. |
| Allocation concealment (selection bias) | Unclear risk | Same problems as random sequence generation (above) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were unaware of the random allocation. Personnel did not deliver the intervention, as it was through text messaging and email. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The majority of follow‐up took place online (although if participants did not respond to online prompts, interviews took place via telephone ‐ unclear whether assessors were blinded) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rates of dropout as would be expected from online intervention however rates above 50%, 73.7% in intervention group and 71.9% in control group |
| Other bias | High risk | Participants were given the option to receive text messages so not all participants benefited from differential treatment between study arms. |
Woodruff 2007.
| Methods | Country: USA, San Diego County Setting: 14 schools Study design: cluster‐RCT | |
| Participants | Participants: 136 young people volunteering, (I = 77 ; C = 59) Age range: 14‐19 years Criterion for Inclusion: volunteering and consented (parents and teenagers) and smoking ≥ 1 cigarette within the last 30 days Inducements: participants were asked to complete an online survey and paid (sum in brackets) on completion of survey at baseline(USD 5), immediate post intervention (USD 10), 3 months post completion (USD 15) and 12 months post completion (USD 20) Follow‐up method: completion of online survey with reminders Pre‐study smoking status assessment: self‐reported Post‐study smoking status assessment: self‐reported quitting | |
| Interventions | Intervention: web‐based virtual reality world based on sky mall with students as avatars and counsellor present as avatar. Information represented as "shops" and galleries and chat possible as more than one student can be "present". Chat texted based at foot of screen. Students also offered 1‐to‐1 counselling sessions with Smoking Cessation professional Theoretical basis of intervention: MI and responses in virtual world based on SCT Control: asked to complete online surveys with inducements | |
| Outcomes | Self‐reported quitting (7‐day PPA) at 1 year; I = 19, C = 18 Losses to follow‐up: overall 27.2%, I = 32.5%, C = 20.3% | |
| Notes | "Effects of clustering were small" so analysis at individual level | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomized by school, method not described |
| Allocation concealment (selection bias) | High risk | Students recruited after schools randomized, with different recruitment methods. The 2 conditions did not differ significantly on demographic data, although a significantly greater proportion of intervention subjects were alternative/continuation high school students. The groups differed significantly on several baseline smoking variables |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified but due to nature of intervention, performance bias unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding reported, no biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was 25% post intervention, 21% for the 3‐month follow‐up survey, and 27% at 12 months. Survey non‐response was higher among intervention participants then among controls (33% vs 15%). All randomized participants included in ITT analysis |
C: control group CBT: cognitive behavioural therapy CO: carbon monoxide cpd: cigarettes per day cpw: cigarettes per week ED: Emergency Department FTND: Fagerstrom Test for Nicotine Dependence h: hour(s) I: intervention group ITT: intention‐to‐treat MI: Motivational Interview/ing NoT: Not on Tobacco NRT: nicotine replacement therapy (m)FTQ: (modified) Fagerstrom tolerance questionnaire OR: odds ratio PPA: point prevalence abstinence RCT: randomized controlled trial SCT: social‐cognitive theory SD: standard deviation SR: sustained release TTM: Transtheoretical model (stages of change)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abelin 1989 | NRT double‐blind randomized trial for 112 young people. Reported follow‐up was for 3 months only. |
| Adelman 2001 | RCT of a psycho‐social intervention targeted at young people. Although measurements made at 6 months' follow‐up, the control group were given the intervention 3 months after the intervention group, therefore only 3 months' effectiveness data were available |
| Adelman 2009 | NCT of nasal spray for 6 weeks plus counselling vs counselling alone. Unpleasant adverse effects, poor adherence, and consequent lack of efficacy did support the use of nicotine nasal spray as an adjunct to counselling. Outcome reported at 12 weeks therefore not added to review. |
| Ames 2007 | Median age of study subjects was 20 years with range 18‐21 years. This age range is outside scope of this review. |
| An 2007 | Evaluated recruitment strategies, not smoking cessation |
| Arora 2010 | Study reported prevalence‐level information only so it was not possible to identify individual‐level smoking cessation. Majority of sample (around 95%) were non‐smokers at baseline. Although intervention contained a cessation component it was not possible to separate this from the effect of the other components of the intervention. Study was previously listed as an ongoing study, excluded in 2017 update after publication of the results paper (Harrell 2016). |
| Audrain‐McGovern 2011 | Although a cessation trial, the intervention group could choose reduction rather than cessation as an outcome. Not added to data as not a pure cessation trial. |
| Audrey 2008 | Smoking prevention programme, not cessation |
| Bannink 2014 | Not all participants were smokers |
| Bauman 2000 | The authors state that there were "no activities focused explicitly on cessation or reduction " in their intervention. |
| Bloor 1999 | Controlled trial using pupil advocates but only 3‐month follow‐up |
| Bond 2004 | No discrete cessation component or results |
| Bramley 2005 | Study participants outside age range of review |
| Braverman 1994 | Report not found but unlikely to be a trial |
| Brendryen 2008 | Trial of internet‐based support over 12‐month period for > 18‐year‐olds. Self‐reports of abstinence used with no verification. Main outcome repeated reports of abstinence at 1, 3, 6 and 12 months. |
| Brinker 2016 | Participants were not smokers at baseline. |
| Buller 2014a | Adult population (mean age = 25.0 years) |
| Buller 2014b | Adult population (mean age = 24.9 years) |
| Burton 1994 | This is a report of the secondary cessation component/effects of the Project TNT intervention designed as a preventative programme. Follow‐up was 4 months after start of trial. Summary paper published in 2009. |
| Cai 2000 | Intervention over 4 weeks and follow‐up of cases for further 3 months. Excluded as not having 6‐month follow‐up but results from 3 months give no evidence of effectiveness: 1/12 (end of treatment OR = 1.027 (0.57‐1.84) and 4/12 from beginning of study = OR 0.971 (0.53‐1.77) |
| Campbell 2008 | This trial was not designed as a pure cessation intervention. |
| Cavallo 2007 | Preliminary data giving end‐of‐treatment rates of cessation but no long‐term follow‐up |
| Chan 1988 | Non‐randomized controlled trial. Previously included, but excluded in 2017 update because of updated inclusion criteria. |
| Chen 2006 | Follow‐up only 4 weeks so not eligible for this review |
| Colby 1998 | RCT of brief MI in a hospital setting. Follow‐up at three months so not eligible for this review. |
| Curry 2013 | Review |
| Digiusto 1994 | This study, a "quasi‐experiment" with pair matching for analysis, described 2 interventions (same intervention but different time of delivery) and control. Control data on quitting collected at 6 months but data from 1 intervention arm collected at approximately 19 weeks after allocation. |
| Dino 1998 | West Virginia NoT with 3‐ and 4‐month follow‐up data from baseline |
| Egger 1983 | Community intervention, with cessation component and control population, aimed at adults in community > 18 years. Although subset of population this study was not aimed primarily at young people. |
| Ehrsam 1991 | Average age of participants in intervention group 21.9 ± 6.8 years and control 24.1 ± 6.9 years. Small size of overall study groups (56 cases in each arm) would mean it would be difficult to extract meaningful outcomes from sub‐group analysis for age range of this review. |
| Elsasser 2002 | Conference paper: trial of only 17 cases randomized to treatment or control therefore very underpowered. Outcome measured at 3/12. |
| Emmons 2003 | This study was long‐term follow‐up of children who had had cancer. Current age of participants was 31 ± 6.6 years. |
| Erol 2008 | Uncontrolled before and after study |
| Escoffery 2004 | Programme aimed at college students > 18 years of age. Average age of participants was 21 years |
| Faessel 2009 | Clinical trial of safety and tolerability and pharmacokinetics of 14 days of high‐dose varenicline. Study design did not include cessation outcomes |
| Fagan 2003 | This was an RCT designed to control tobacco use amongst young people and based in the workplace. Outcomes were reduction of use and intention to quit measures rather than actual cessation |
| Figa‐Talamanca 1989 | Educational RCT aimed at whole class groups and not specifically smokers |
| Flay 1995 | Primarily a prevention programme and measured outcomes were in terms of knowledge and intention to quit. Cessation component not discrete |
| Gray 2011 | A trial of sustained‐release bupropion combined with contingency management. The primary outcome was 7‐day cotinine‐verified PPA but follow‐up was only for 12 weeks. |
| Gray 2012 | Last follow‐up only at 12 weeks |
| Ha 2015 | Non‐randomized controlled trial |
| Hamilton 2005 | A school‐based cluster‐RCT designed to test a harm minimization approach. Only prevalence data available, no discrete results for smokers |
| Hancock 2001 | Trial of community intervention aimed at teenagers that reported population prevalence of smoking rather than following up individual smokers |
| Hanson 2003 | Trial of NRT (patches) for 13‐19 year olds. Abstinence reported at 10 weeks post quit date |
| Hanson 2006 | A harm reduction study rather than cessation |
| Haug 2009 | Study of SMS intervention for young adults. Mean age = 25 years |
| Heikkinen 2009 | Finnish study of smokers aged 15‐16 years. 2 intervention groups, information and support offered by dentist or school nurse. Only 3‐month follow‐up |
| Hellmann 1988 | Although (quasi) experimental in design there was no formal randomization or attempt to case match and baseline characteristics not been assessed or compared |
| Helstrom 2004 | Potentailly interesting study with positive results but follow‐up only 5 months in initial report |
| Higgs 2000 | This primarily a prevention trial reporting secondary cessation effects |
| Hollis 1994 | Not targeted at regular smokers and discrete quitting data not available |
| Horn 2004 | Report of West Virginia trial with 3‐month follow‐up data only |
| Hort 1995 | Prevention review. No discrete cessation programme |
| Jason 1982 | This was essentially a trial of 2 whole‐class prevention strategies |
| Josendal 1998 | Primarily a prevention study |
| Kang 2005 | Excluded as follow‐up was 4 weeks |
| Kealey 2009 | Telephone counselling intervention (MI and cognitive behavioral skills training) with matched pair design |
| Kelleher 1999 | Smoking cessation was a component of an intervention to reduce cardiovascular risk. No discrete results measured |
| Kentala 1999 | Intervention by dentists to discuss smoking during annual check up. Young people randomized to brief intervention or normal care. Prevalence data only collected. Individual smokers not followed up |
| Keyser 2014 | Review |
| Killen 1988 | This was a cardiovascular health promotion trial with a smoking cessation component but without discrete results for individual smokers. |
| Kim 2004 | No discrete cessation component in report |
| Knishkowy 2008 | Prevention study |
| Kong 2015 | Follow‐up was 3 months only |
| Krishnan‐Sarin 2013 | Follow‐up was 3 months only |
| La Torre 2013 | Participants were not smokers at time of recruitment |
| Lando 2007 | Study experienced some recruitment issues and it is not clear that all participants were active smokers |
| Lotecka 1983 | Cognitive behavioural intervention trialled in 4 schools. No discrete results available and follow‐up 3 months |
| McCambridge 2004 | Follow‐up of smoking component was 3 months only |
| McCuller 2006 | Project EX intervention that reported 3‐month follow‐up |
| Mermelstein 2006 | Follow‐up 3 months only |
| Minary 2013 | Non‐randomized ‐ the study was controlled; however the differences between arms were investigated at baseline and there were significant differences, which were not controlled for in the analysis |
| Mokina 2015 | Aimed at reducing intensity of smoking activity, not cessation |
| Myers 2005 | Non‐randomized controlled trial. Previously included, but excluded in 2016 update because of updated inclusion criteria |
| Myers 2008 | Although a smoking cessation intervention, it was targeted at and outcomes recorded for other substances |
| Niederhofer 2004 | Trial of bupropion versus placebo. Effectiveness measured at 90 days (3 months) |
| Norman 2008 | No discrete quit data available. Confirmed with study author |
| NoT AL 2008 | Non‐randomized controlled trial. Previously included, but excluded in 2017 update because of updated inclusion criteria. |
| NoT FL 2001 | Non‐randomized controlled trial. Previously included, but excluded in 2017 update because of updated inclusion criteria. |
| NoT NC 2005 | Non‐randomized controlled trial. Previously included, but excluded in 2017 update because of updated inclusion criteria. |
| NoT WV 2004 | Non‐randomized controlled trial. Previously included, but excluded in 2017 update because of updated inclusion criteria. |
| Pallonen 1998 | This was a comparison trial between 2 interventions. There was no control group randomized to 'placebo'/no intervention. The study authors state "The inclusion of two different interventions (for smokers) rather than a treatment/control comparison is for process analysis since the sample size was inadequate for a clinical trial." The number of smokers in study was 135. |
| Park 2015 | Review |
| Patten 2014 | Majority of tobacco consumed by participants was smokeless, outcomes not divided by type of tobacco |
| Pbert 2006 | Excluded as follow‐up only 3 months |
| Pbert 2008 | Not specifically targeted at smokers and no discrete results available at this time |
| Peirson 2016 | Review |
| Perry 1980 | This was primarily a prevention study as the stated aim was to influence the incidence of smoking. The results were presented in such a form that overall prevalence was measured for a whole year group and discrete smokers could not be identified. |
| Prokhorov 2010 | Of 1574 participants, only 62 were smokers |
| Quinlan 2000 | Clinical trial using intervention matched to stage of change (TTM). Age range 18‐55 years. Mean age by group of participants was 20.41 years, 21.71 and 23.3 years and therefore this study falls outside the scope of this review. |
| Rabius 2004 | The age range of this study included a cohort of 18‐25 year olds. it is not possible to disaggregate 18 and 19 year olds from report of study but author contacted for primary data. If available these data will be incorporated in future versions of review |
| Ramo 2015 | Adult population (mean age = 20.8 years) |
| Reynolds 2015 | 6‐week follow‐up only |
| Rice 2010 | Study based on Project Towards No Tobacco (Project EX‐4 2007). Non random allocation instead compared cohorts in different years. |
| Roddy 2006 | Although this study mets all other inclusion criteria the outcome was measured at 13 weeks. This review uses Russell Standards, i.e. a minimum of a 6‐month follow‐up. |
| Rubinstein 2008 | 12‐week follow‐up only |
| Schepis 2006 | Excluded as outcome was measured at 4 weeks |
| Severson 1991 | Essentially a prevention study |
| Shi 2013 | 12‐week follow‐up only |
| Simmons 2011 | Test of web‐based intervention in American college students, participants > 18 years |
| Simmons 2013 | Adult population (mean age = 20.54 years) |
| Sims 2013 | Reported outcomes for young adults aged 18‐24 years; average age not reported but > 20 years. Original study intended to recruit adolescents smokers but low recruitment, and results for 52 adolescents not reported |
| Solomon 2009 | Outcomes long‐term prevalence of smoking |
| Stamm‐Balderjahn 2012 | Non‐randomized controlled trial with 40% of participants being non‐smokers. Unknown if smokers were baseline matched |
| Stein‐Seroussi 2009 | Cluster‐RCTincluding biochemical verification of cessation. Outcome reported after 90‐day follow‐up |
| Stephens 2001 | Good‐quality trial of motivational enhancement for young people but follow‐up only 30 days at end of an intervention of 5 weeks' duration. Study author notes a high dropout rate |
| Stoddard 2005 | Prevalance only measured, no discrete cessation data |
| Sussman 1995 | This was a trial of Project Towards No Tobacco (Project EX‐4 2007), an intervention based on cessation intervention clinics. Outcomes were self‐reported at 4 months after start of intervention |
| Sussman 2012 | Not a trial. Reports on progress of translated versions of Project EX |
| Thrul 2015 | Non‐randomized controlled trial, differences in baseline characteristics were present |
| Travis 2009 | Excluded as aimed at college students with participants median age 21 ± 3 years and only 3‐month follow‐up. |
| Tuisku 2016 | Adult population |
| Turner 2006 | A version of NoT with web‐based component added. Only 3/12 follow‐up |
| Wang 2006 | Not a trial of intervention but a correlation analysis |
| Werch 2008 | Trial of brief, image‐ based, multiple behaviour intervention for adolescents and college students. Aimed at range of substance abuse. 3‐month follow‐up |
| Whittaker 2011 | Although recruiting > 16 years, mean age of participants was 27 years +/‐ 8.7 |
| Winkleby 2004 | Programme aims were to reduce smoking and although gives 6/12 follow‐up, discrete results not available for individual smokers as unit of analysis was school |
| Witkiewitz 2014 | Adult population (mean age = 20.5 years) |
| Wongwiwatthananukit 2010 | Trial of pharmacist‐based cessation programme for youth offenders, 1 arm voluntary cessation, 1 arm compulsory cessation. Excluded as non‐randomized allocation as part of criminal justice process |
| Ybarra 2013 | Adult population (mean age = 21.8 years) |
MI: motivational interview/ing NoT: Not on Tobacco NRT: nicotine replacement therapy OR: odds ratio PPA: point prevalence abstinence RCT: randomized controlled trial SMS: short message service (text) TTM: Transtheoretical model (stages of change)
Characteristics of ongoing studies [ordered by study ID]
Gorzkowski 2016.
| Trial name or title | Implementation and impact of the 5As tobacco conseling intervention with adolescents in pediatric practice |
| Methods | 2‐arm cluster‐RCT |
| Participants | 10,967 adolescents aged > 14 years, 936 of whom were smokers at baseline |
| Interventions | 5As tobacco intervention (Ask‐Advise‐Assess‐Assist‐Arrange) |
| Outcomes | Self‐reported smoking cessation at 4‐6 weeks and 6 months, cpd, quit attempts, relapse after quit attempts, intention to quit |
| Starting date | |
| Contact information | Julie A Gorzkowski |
| Notes | New for 2017 update. Published only as a conference abstract with trial paper pending |
Haug 2014b.
| Trial name or title | Efficacy of an internet and SMS‐based integrated smoking cessation and alcohol intervention for smoking cessation in young people |
| Methods | 2‐arm cluster‐RCT |
| Participants | 1350 daily or occasional smokers who are students at vocational schools in Switzerland |
| Interventions | Mobile coach tobacco plus (MCT+), a tailored web‐ and text‐based integrated smoking and alcohol cessation intervention. The control is Mobile Coach Tobacco (MCT), a tobacco cessation programme delivered by text only |
| Outcomes | 7‐day and 30‐day PPA at 6 months, cigarette consumption per day and per month at 6 months, Health Action Process Approach stage of change, quit attempts within 6‐month period, alcohol consumption |
| Starting date | September 2016 |
| Contact information | Dr Severin Haug. Address for correspondence: Konradstrasse 32, Zurich, 8031, Switzerland email: severin.haug@isgf.uzh.ch |
| Notes | New for 2017 update |
NCT01312909.
| Trial name or title | Smoking cessation in healthy adolescent smokers |
| Methods | RCT |
| Participants | Healthy smokers aged 12‐19 |
| Interventions | Varenicline 1 mg twice/d, 0.5 mg twice/d or placebo |
| Outcomes | Reduction or abstinence through to week 52 |
| Starting date | TBC |
| Contact information | Pfizer 1‐800‐718‐1021 |
| Notes | Added 2013 |
NCT01509547.
| Trial name or title | Varenicline for adolescent smoking cessation |
| Methods | RCT |
| Participants | 14‐21‐year‐old daily smokers with a desire to quit |
| Interventions | Pharmaceutical participants > 55 kg will take varenicline/placebo 0.5 mg once daily for 3 days, titrated to 0.5 mg twice daily for 4 days, titrated to 1 mg twice daily for 11 weeks. Participants ≤ 55 kg will take varenicline/placebo 0.5 mg once daily for 7 days, titrated to 0.5 mg twice daily for 11 weeks |
| Outcomes | Smoking abstinence at 26 weeks confirmed with CO breathalyser, self‐reported cpd, change in urinary cotinine measurement, frequency of treatment‐emergent adverse events |
| Starting date | August 2012 |
| Contact information | Lori Ann Ueberroth, USA telephone number: 843‐792‐8220 email: ueberro@musc.edu |
| Notes | New for 2017 update |
NCT02021175.
| Trial name or title | Korean youth smoking cessation study |
| Methods | 2‐arm RCT |
| Participants | 14‐19 year old Korean or Korean‐American smokers living in Los Angeles County who are interested in quitting smoking |
| Interventions | 6 weeks of cognitive‐behavioural motivational enhancement therapy via internet and cell phones, vs 6 weeks of standard of care |
| Outcomes | 7‐day PPA rates at end of treatment and 6‐month follow‐up, verified with urinary cotinine and exhaled CO |
| Starting date | June 2016 |
| Contact information | Steve Shoptaw Ph.D, USA telephone number: (310) 794‐0619 ext 225, email address: sshoptaw@mednet.ucla.edu |
| Notes | New for 2017 update |
NCT02218281.
| Trial name or title | Developing a smartphone app with mindfulness training for teen smoking cessation |
| Methods | 3‐arm cluster‐RCT |
| Participants | English‐speaking 13‐19‐year‐old smokers interested in quitting in the following 3 weeks |
| Interventions | C2Q‐Teen smartphone app incorporating mindfulness training or NCI’s QuitSTART smartphone app without mindfulness, or written smoking cessation materials |
| Outcomes | 7‐day PPA rates validated with salivary cotinine at 3 and 6 months, feasibility of participant recruitment and retention, acceptability of the 3 interventions and usage of C2Q‐Teen as a predictor of smoking abstinence |
| Starting date | September 2014 |
| Contact information | Lori Pbert PhD, Professor of Medicine, University of Massachusetts, Worcester |
| Notes | New for 2017 update |
CO: carbon monoxide cpd: cigarettes per day PPA: point prevalence abstinence RCT: randomized controlled trial TBC: to be confirmed
Differences between protocol and review
Upon re‐evaluation for the update in 2017 we decided to modify the inclusion criteria such that non‐randomized controlled trials are no longer included in this review. This is because of the increased risk of bias they introduce, which can decrease the certainty of our findings. We re‐assessed previously included studies to ensure consistency, and excluded six studies because they were non‐randomized (Chan 1988, Myers 2005, NoT AL 2008, NoT FL 2001, NoT NC 2005, NoT WV 2004). Another reassignment of studies occurred with our evaluation of ages of participants. We decided to include studies in which more than 50% of the participants were under 20 years so long as there was evidence that the intervention was tailored towards young people. In following this, three studies that had been excluded in previous editions have been included in this edition (Abroms 2008; Harris 2010; O'Neill 2000), and a further study has been included because of the publication of a corrigendum to the original trial paper (Project EX‐4 2007). In addition, in the 2017 update we no longer list participation, retention or enrolment as outcomes, focusing solely on smoking cessation and adverse events, in line with other Cochrane Tobacco Addiction Group reviews. We have moved the outcome, Adverse events to be listed as a primary outcome. We have added 'Summary of findings' tables in line with Cochrane guidance.
Contributions of authors
Previous versions of this review were authored by Gill Grimshaw and Alan Stanton. Both authors conceived the review, and both selected and extracted data for the previous versions. GG and AS wrote the previous versions of the review in collaboration.
The author team for this update changed. For this update, NL, WH, TF, and JLB conducted the majority of screening and data extraction, with contributions from JHB. TF led analysis with contributions from WH, PA and JHB. TF, WH, PA and JHB updated the text. All authors reviewed and approved the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
The NIHR funds the Cochrane Tobacco Addiction Group through a Cochrane Infrastructure Award. This award covers salary and associated costs for TF, NL, JLB and JHB's involvement in this review. The views expressed in this research are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Declarations of interest
TF declares no conflicts of interest.
WH declares no conflicts of interest.
NL is a co‐applicant on a completed trial investigating nicotine patch preloading for smoking cessation (not a harm reduction approach). The nicotine patches were provided free of charge by GlaxoSmithKline; however the trial was funded by the NIHR HTA (09/110/01), and the running and the reporting of the trial were carried out independently to the funder and treatment provider.
PA declares: PA is an author of one of the included studies.
JLB declares no conflicts of interest.
JHB declares no conflicts of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abroms 2008 {published data only}
- Abroms LC, Windsor R, Simons‐Morton B. Getting young adults to quit smoking: a formative evaluation of the X‐Pack Program. Nicotine & Tobacco Research 2008;10:27‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abroms LC, Windsor R, Simons‐Morton BA. A formative evaluation of an email‐based program for smoking cessation in young adults (POS1‐115). Society for Research on Nicotine & Tobacco 13th Annual Meeting Febuary 21‐22 Austin, Texas. 2007.
Aveyard 2001 {published and unpublished data}
- Aveyard P, Cheng KK, Almond J, Sherratt E, Lancashire R, Lawrence T, et al. Cluster randomised controlled trial of expert system based on the transtheoretical (''stages of change'') model for smoking prevention and cessation in schools. BMJ 1999;319:948‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveyard P, Sherratt E, Almond J, Lawrence T, Lancashire R, Griffin C, et al. The change‐in‐stage and updated smoking status results from a cluster‐randomized trial of smoking prevention and cessation using the transtheoretical model among British adolescents. Preventive Medicine 2001;33:313‐24. [DOI] [PubMed] [Google Scholar]
Bailey 2013 {published data only}
- Bailey SR, Hagen SA, Jeffery CJ, Harrison CT, Ammerman S, Bryson SW, et al. A randomized clinical trial of the efficacy of extended smoking cessation treatment for adolescent smokers. Nicotine & Tobacco Research 2013;15(10):1655‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2003 {published data only}
- Brown RA, Ramsey SE, Strong DR, Myers MG, Kahler CW, Lejuez CW, et al. Effects of motivational interviewing on smoking cessation in adolescents with psychiatric disorders. Tobacco Control 2003;12(Suppl iv):iv3‐iv10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Strong DR, Abrantes AM, Myers MG, Ramsey SE, Kahler CW. Effects on substance use outcomes in adolescents receiving motivational interviewing for smoking cessation during psychiatric hospitalization. Addictive Behaviours 2009;34:887‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson L, Strong DR, Kahler CW, Abrantes AM, Ramsey SE, Brown RA. Association of post‐treatment smoking change with future smoking and cessation efforts among adolescents with psychiatric comorbidity. Nicotine Tobacco Research 2007;9(12):1297‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson L, Strong DR, Palm KM, Abrantes AM, Brown RA. Post‐intervention lapse and relapse in adolescent smokers with psychiatric comorbidity (POS1‐84). Society for Research on Nicotine and Tobacco 13th Annual Meeting, February 21‐24, Austin, Texas. 2007.
- Tzilos GK, Strong DR, Abrantes AM, Ramsey SE, Brown RA. Quit intention as a predictor of quit attempts over time in adolescents with psychiatric disorders. American Journal on Addictions / American Academy of Psychiatrists in Alcoholism and Addictions 2014;23(1):84‐9. [CENTRAL: 921019; CRS: 9400129000000198; EMBASE: 2013783516] [DOI] [PMC free article] [PubMed] [Google Scholar]
Colby 2005 {published data only}
- Colby SM, Monti PM, O'Leary Tevyaw T, Barnett NP, Spirito A, Rohsenow DJ, et al. Brief motivational intervention for adolescent smokers in medical settings. Addictive Behaviors 2005;30(5):865‐74. [DOI] [PubMed] [Google Scholar]
- Monti P, Colby SM, O'Leary TA, Spirito A, Woolard RH, Lewander WJ, et al. Motivational interviewing for adolescent smokers: preliminary results from a randomized clinical trial. Society for Research on Nicotine and Tobacco 8th Annual Meeting Rapid Communications Posters (RP‐27) Savannah, Georgia; February 20‐23 2002. 2002.
Colby 2012 {published data only}
- Colby SM, Nargiso J, Tevyaw TO, Barnett NP, Metrik J, Lewander W, et al. Enhanced motivational interviewing versus brief advice for adolescent smoking cessation: results from a randomized clinical trial. Addictive Behaviors 2012;37(7):817‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantini R, McGrath AC, Stein LA, Barnett NP, Monti PM, Colby SM. Misreporting in a randomized clinical trial for smoking cessation in adolescents. Addictive Behaviors 2015;45:57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dalum 2012 {published data only}
- Dalum P, Paludan‐Muller G, Engholm G, Kok G. A cluster randomised controlled trial of an adolescent smoking cessation intervention: short and long‐term effects. Scandinavian Journal of Public Health 2012;40(2):167‐76. [DOI] [PubMed] [Google Scholar]
- Dalum P, Schaalma H, Kok G. The development of an adolescent smoking cessation intervention‐‐an Intervention Mapping approach to planning. Health Education Research 2012;27(1):172‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenberg 1978 {published data only}
- Greenberg JS, Deputat Z. Smoking intervention: comparing three methods in a high school setting. Journal of School Health 1978;48:489‐502. [DOI] [PubMed] [Google Scholar]
Gungormus 2012 {published data only}
- Gungormus Z, Erci B. Transtheorethical Model‐based education given for smoking cessation in higher school students. Southeast Asian Journal of Tropical Medicine and Public Health 2012;43(6):1548‐59. [PubMed] [Google Scholar]
Guo 2014 {published data only}
- Guo JL, Liao JY, Chang LC, Wu HL, Huang CM. The effectiveness of an integrated multicomponent program for adolescent smoking cessation in Taiwan. Addictive Behaviors 2014;39(10):1491‐9. [DOI] [PubMed] [Google Scholar]
Harris 2010 {published data only}
- Harris KJ, Catley D, Good GE, Cronk NJ, Harrar S, Williams KB. Motivational interviewing for smoking cessation in college students: a group randomized controlled trial. Preventive Medicine 2010;51(5):387‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JD, Catley D, Lee HS, Harrar SW, Harris KJ. Health risk perceptions predict smoking‐related outcomes in Greek college students. Psychology of Addictive Behaviors 2014;28(3):743‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haug 2013 {published data only}
- Haug S, Meyer C, Dymalski A, Lippke S, John U. Efficacy of a text messaging (SMS) based smoking cessation intervention for adolescents and young adults: study protocol of a cluster randomised controlled trial. BMC Public Health 2012;12:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug S, Schaub MP, Castro RP, Schmid H. Barriers and resources for smoking cessation in vocational school students [Barrieren und ressourcen für einen rauchstopp bei auszubildenden]. Sucht 2014;60(4):225‐34. [Google Scholar]
- Haug S, Schaub MP, Schmid H. Predictors of adolescent smoking cessation and smoking reduction. Patient Education and Counseling 2014;95(3):378‐83. [DOI] [PubMed] [Google Scholar]
- Haug S, Schaub MP, Venzin V, Meyer C, John U. Efficacy of a text message‐based smoking cessation intervention for young people: a cluster randomized controlled trial. Journal of Medical Internet Research 2013;15(8):142‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug S, Schaub MP, Venzin V, Meyer C, John U. Moderators of outcome in a text messaging (SMS)‐based smoking cessation intervention for young people [Differenzielle wirksamkeit eines short message eervice (SMS)‐basierten programms zur förderung des rauchstopps bei jugendlichen]. Psychiatrische Praxis 2013;40(6):339‐46. [DOI] [PubMed] [Google Scholar]
Hoffman 2008 {published data only}
- Hoffman J, Nemes S, Weil J, Zack S. Adolescent smoking cessation escaping nicotine and tobacco (ASCENT): outcomes. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20‐23 March; Prague, Czech Republic. 2005.
- Hoffman J, Nemes S, Weil J, Zack S, Munly K, Hess L. Evaluation of the ASCENT smoking cessation program for adolescents. Journal of Smoking Cessation 2008;3(1):2‐8. [Google Scholar]
- Hoffman J, Nemes S, Weil J, Zack S, Munly K, Levin H. Adolescent smoking cessation escaping nicotine and tobacco (ASCENT): Outcome evaluation preliminary findings. Society for Research on Nicotine and Tobacco 9th Annual Meeting, February 19‐22 New Orleans, Louisiana. 2003.
- Zack SL, Hoffman J, Nemes S, Weil J, Hess J. Participation in a successful and multifaceted teen cessation program. National Conference on Tobacco or Health. Chicago, 2005.
Hollis 2005 {published data only}
- Hollis JF, Polen MR, Whitlock EP, Lichtenstein E, Mullooly JP, Velicer WF, et al. Teen Reach: outcomes from a randomized, controlled trial of a tobacco reduction program for teens seen in primary medical care. Pediatrics 2005;115(4):981‐9. [DOI] [PubMed] [Google Scholar]
Horn 2007 {published data only}
- Horn K, Dino G, Hamilton C, Noerachmanto N. Efficacy of an emergency department‐based motivational teenage smoking intervention. Preventing Chronic Disease 2007;4(1):A08. [PMC free article] [PubMed] [Google Scholar]
Joffe 2009 {published data only}
- Joffe A, McNeely C, Colantuoni E, An M, Wang W, Scharfstein D. Evaluation of school‐based smoking‐cessation interventions for self‐described adolescent smokers. Pediatrics 2009;124:e187‐e194. [DOI] [PubMed] [Google Scholar]
Kelly 2006 {published data only}
- Kelly AB, Lapworth K. The HYP program‐targeted motivational interviewing for adolescent violations of school tobacco policy. Preventive Medicine 2006;43(6):466‐71. [DOI] [PubMed] [Google Scholar]
Killen 2004 {published data only}
- Killen JD, Robinson TN, Ammerman S, Hayward C, Rogers J, Stone C, et al. Randomized clinical trial of the efficacy of bupropion combined with nicotine patch in the treatment of adolescent smokers. Journal of Consulting and Clinical Psychology 2004;72(4):729‐35. [DOI] [PubMed] [Google Scholar]
Lipkus 2004 {published data only}
- Lipkus IM, McBride CM, Pollak KI, Schwartz‐Bloom RD, Bloom PN, Tilson E. A randomized trial comparing the effects of self‐help materials and proactive telephone counseling on teen smoking cessation. Health Psychology 2004;23(4):397‐406. [DOI] [PubMed] [Google Scholar]
Mason 2016 {published data only}
- Mason M, Mennis J, Way T, Campbell LF. Real‐time readiness to quit and peer smoking within a text message intervention for adolescent smokers: modeling mechanisms of change. Journal of Substance Abuse Treatment 2015;59:67‐73. [DOI] [PubMed] [Google Scholar]
- Mason M, Mennis J, Way T, Zaharakis N, Campbell LF, Benotsch EG, et al. Text message delivered peer network counseling for adolescent smokers: a randomized controlled trial. The Journal of Primary Prevention 2016;37(5):403‐20. [DOI] [PubMed] [Google Scholar]
- Mason MJ, Campbell L, Way T, Keyser‐Marcus L, Benotsch E, Mennis J, et al. Development and outcomes of a text messaging tobacco cessation intervention with urban adolescents. Substance Abuse 2015;36(4):500‐6. [DOI] [PubMed] [Google Scholar]
- Mason MJ, Mennis J, Zaharakis NM, Way T. The dynamic role of urban neighborhood effects in a text‐messaging adolescent smoking intervention. Nicotine & Tobacco Research 2016;18(5):1039‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moolchan 2005 {published data only}
- Collins CC, Epstein DH, Parzynski CS, Zimmerman D, Moolchan ET, Heishman SJ. Puffing behavior during the smoking of a single cigarette in tobacco‐dependent adolescents. Nicotine and Tobacco Research 2010;12(2):164‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken FH, Pickworth WB, Epstein DH, Moolchan ET. Smoking rates and topography predict adolescent smoking cessation following treatment with nicotine replacement therapy. Cancer Epidemiology, Biomarkers & Prevention 2006;15(1):154‐7. [DOI] [PubMed] [Google Scholar]
- Mancha B, Cranford D, Snidow N, Radzius A, Conrad S, Cadet JL. Patterns of compliance of teenage smokers in cessation treatment: are the first two weeks determining? (PO3 75). Society for Research on Nicotine and Tobacco 7th Annual Meeting March 23‐23 2001 Seattle Washington. 2001.
- Moolchan ET, Robinson ML, Ernst M, Cadet JL, Pickworth WB, Heishman SJ, et al. Safety and efficacy of the nicotine patch and gum for treatment of adolescent tobacco addiction. Pediatrics 2005;115(4):407‐14. [DOI] [PubMed] [Google Scholar]
- Moolchan ET, Robinson ML, Schroeder JR, Huestis MA, Ernst M. A randomized trial of the efficacy of the nicotine gum and patch for adolescent smokers (PA2‐8). Society for Research on Nicotine and Tobacco 10th Annual Meeting February 18‐21 2004, Phoenix, Arizona. 2004.
- Robinson ML, Schroeder JR, Moolchan ET. Adolescent smokers screened for a nicotine replacement treatment trial: correlates of eligibility and enrollment. Nicotine and Tobacco Research 2006;8:447‐54. [DOI] [PubMed] [Google Scholar]
- Thorner‐Bantug E, Jaszyna‐Gasior M, Schroeder JR, Collins CC, Moolchan ET. Weight gain, related concerns, and treatment outcomes among adolescent smokers enrolled in cessation treatment. Journal of National Medical Association 2009;101(10):1009‐14. [DOI] [PubMed] [Google Scholar]
Muramoto 2007 {published data only}
- Floden L, Taren DL, Muramoto ML, Leischow SJ. BMI changes in adolescents treated with bupropion SR for smoking cessation. Obesity 2016;24(1):26‐9. [CENTRAL: 1128464; CRS: 9400131000004123; EMBASE: 20151051936; PUBMED: 26692579] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leischow SJ, Muramoto ML, Matthews E, Floden LL, Grana RA. Adolescent smoking cessation with bupropion: the role of adherence. Nicotine & Tobacco Research 2016;18(5):1202‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto M, Leischow S, Sherrill DL. A randomized trial of the efficacy of bupropion for adolescent smoking cessation [RP‐064]. Society for Research on Nicotine and Tobacco 11th annual meeting, March 2005; Prague, Czech Republic. Rapid Communications posters. 2005.
- Muramoto ML, Leischow SJ, Enright P, Strayer L, Hadjioannou M, Matthews E, et al. So you want to do a smoking cessation study with teens ... interim process evaluation of 'Lessons Learned' from a dose response study of safety and efficacy of sustained release bupropion for smoking cessation in adolescents (PO3‐67). Society for Research on Nicotine and Tobacco 7th Annual Meeting, Seattle WA. 2001.
- Muramoto ML, Leischow SJ, Sherrill D, Matthews E, Strayer LJ. Randomized, double‐blind, placebo‐controlled trial of 2 dosages of sustained‐release bupropion for adolescent smoking cessation. Archives of Pediatrics & Adolescent Medicine 2007;161:1068‐74. [DOI] [PubMed] [Google Scholar]
NoT MD 2009 {published data only}
- Joffe A, McNeely C, Colantuoni E, An M, Wang W, Scharfstein D. Evaluation of school‐based smoking‐cessation interventions for self‐described adolescent smokers. Pediatrics 2009;124:e187‐e194. [DOI] [PubMed] [Google Scholar]
NoT WV 2011 {published data only}
- Horn K, Dino, G, Branstetter S, Noerachanto N, Zhang J, Jarrett T, et al. Effects of physical activity on teen smoking cessation. Pediatrics 2011;128(4):e801‐e811. [DOI] [PubMed] [Google Scholar]
O'Neill 2000 {published data only}
- O'Neill HK, Gillispie MA, Slobin K. Stages of change and smoking cessation: a computer‐administered intervention program for young adults. American Journal of Health Promotion 2000;15(2):93‐6, iii. [DOI] [PubMed] [Google Scholar]
Patten 2006 {published data only}
- Croghan IT, Campbell HM, Patten CA, Croghan GA, Schroeder DR, Novotny PJ. A contest to create media messages aimed at recruiting adolescents for stop smoking programs. Journal of School Health 2004;74(8):325‐8. [DOI] [PubMed] [Google Scholar]
- Patten CA, Croghan IT, Meis TM, Decker PA, Pingree S, Colligan RC, et al. Randomized clinical trial of an internet‐based versus brief office intervention for adolescent smoking cessation. Patient Education & Counseling 2006;64(1‐3):249‐58. [DOI] [PubMed] [Google Scholar]
Pbert 2011 {published data only}
- Pbert L, Druker S, DiFranza JR, Gorak D, Reed G, Magner R, et al. Effectiveness of a school nurse‐delivered smoking‐cessation intervention for adolescents. Pediatrics 2011;128(5):926‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pérez‐Milena 2012 {published data only}
- Pérez‐Milena A, Navarrete Guillén AB, Mesa‐Gallardo MI, Martínez Pérez R, Leal‐Helmling FJ, Pérez‐Fuentes C. Efficiency of two motivational interventions for adolescent smokers (brief and intensive) conducted in high schools [Eficiencia de dos intervenciones motivacionales para la deshabituación tabáquica en adolescentes (breve e intensiva) realizadas en Institutos de educación secundaria]. Adicciones 2012;24(3):191‐9. [PubMed] [Google Scholar]
Peterson 2009 {published data only}
- Bricker JB, Liu J, Comstock BA, Peterson AV, Kealey KA, Marek PM. Social cognitive mediators of adolescent smoking cessation: results from a large randomized intervention trial. Psychology of Addictive Behaviors 2010;24(3):436‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner JL, Kealey KA, Marek PM, Bricker JB, Ludman EJ, Peterson AV Jr. Proactive telephone counseling for adolescent smokers: comparing regular smokers with infrequent and occasional smokers on treatment receptivity, engagement, and outcomes. Drug and Alcohol Dependence 2016;165:229‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kealey KA, Ludman EJ, Mann SL, Marek PM, Phares MM, Riggs KR, et al. Overcoming barriers to recruitment and retention in adolescent smoking cessation. Nicotine & Tobacco Research 2007;9(2):257‐70. [DOI] [PubMed] [Google Scholar]
- Kealey KA, Ludman EJ, Marek PM, Mann SL, Bricker JB, Peterson AV. Design and implementation of an effective telephone counseling intervention for adolescent smoking cessation. Journal of the National Cancer Institute 2009;101(19):1393‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Peterson AV Jr, Kealey KA, Mann SL, Bricker JB, Marek PM. Addressing challenges in adolescent smoking cessation: design and baseline characteristics of the HS Group‐Randomized trial. Preventive Medicine 2007;45(2‐3):215‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AV Jr, Kealey KA, Mann SL, Marek PM, Ludman EJ, Liu J, et al. Group‐randomized trial of a proactive, personalized telephone counseling intervention for adolescent smoking cessation. Journal of the National Cancer Institute 2009;101:1378‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AV, Marek PM, Kealey KA, Bricker JB, Ludman EJ, Heffner JL. Does effectiveness of adolescent smoking‐cessation intervention endure into young adulthood? 7‐year follow‐up results from a group‐randomized trial. PLoS ONE 2016;11(2):e0146459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prochaska 2015 {published data only (unpublished sought but not used)}
- Grana RA, Ramo DE, Fromont SC, Hall SM, Prochaska JJ. Correlates of tobacco dependence and motivation to quit among young people receiving mental health treatment. Drug and Alcohol Dependence 2012;125:127‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Fromont SC, Ramo DE, Young‐Wolff KC, Delucchi K, Brown RA, et al. Gender differences in a randomized controlled trial treating tobacco use among adolescents and young adults with mental health concerns. Nicotine & Tobacco Research 2015;17(4):479‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Project EX‐1 2001 {published data only}
- Dent CW, Pfingston YM, Schmuker K, Granstra J. Nicotine replacement in school‐based cessation (RP‐33). Society for Research on Nicotine and Tobacco 8th Annual Meeting Rapid Communications Posters February 20‐23 2002 Savannah, Georgia. 2002.
- McCuller WJ, Sussman S, Wapner M, Dent C, Weiss DJ. Motivation to quit as a mediator of tobacco cessation among at‐risk youth. Addictive Behaviors 2006;31:880‐8. [DOI] [PubMed] [Google Scholar]
- Sussman S, Dent CW, Litchman KL. Project EX: outcomes of teen smoking cessation program. Addictive Behaviors 2001;26:425‐38. [DOI] [PubMed] [Google Scholar]
- Sussman S, McCuller WJ, Zheng H, Pfingston YM, Miyano J, Dent CW. Project EX: a program of empirical research on adolescent tobacco use cessation. Tobacco Induced Diseases 2004;2(3):119‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Project EX‐4 2007 {published data only}
- Sussman S, Miyano J, Rohrbach LA, Dent CW, Sun P. Corrigendum to “Six‐month and 1‐year effects of Project EX‐4, a classroom‐based smoking prevention and cessation intervention program”. Addictive Behaviors 2010;35:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman S, Miyano J, Rohrbach LA, Dent CW, Sun P. Six‐month and one‐year effects of Project EX‐4: a classroom‐based smoking prevention and cessation intervention program. Addictive Behaviors 2007;32:3005‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Project EX Russia 2013 {published data only}
- Idrisov B, Sun P, Akhmadeev L, Arpawong TE, Kukhareva P, Sussman S. Immediate and six‐month effects of Project EX Russia: a smoking cessation intervention pilot program. Addictive Behaviors 2013;38(8):2402‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Project EX Spain 2015a {published data only}
- Espada JP, Gonzálvez MT, Orgilés M, Guillén‐Riquelme A, Soto D, Sussman S. Pilot clinic study of Project EX for smoking cessation with Spanish adolescents. Addictive Behaviors 2015;45:226‐31. [DOI] [PubMed] [Google Scholar]
Project EX Spain 2015b {published data only}
- Espada JP, Gonzalvez MT, Orgiles M, Morales A, Sussman S. Preliminary results of Project EX in Spain: a classroom‐based smoking prevention and cessation program. Drug and Alcohol Dependence 2015;146:e272‐3. [Google Scholar]
- Espada JP, Gonzálvez MT, Guillén‐Riquelme A, Sun P, Sussman S. Immediate effects of project EX in Spain: a classroom‐based smoking prevention and cessation intervention program. Journal of Drug Education: Substance Abuse Research and Prevention 2014;44(1‐2):3‐18. [DOI] [PubMed] [Google Scholar]
- Gonzálvez MT, Espada JP, Orgilés M, Soto D, Sussman S. One‐year effects of Project EX in Spain: a classroom‐based smoking prevention and cessation intervention program. PLoS ONE 2015;10(6):e0130595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Redding 2015 {published data only}
- Redding CA, Prochaska JO, Armstrong K, Rossi JS, Hoeppner BB, Sun X, et al. Randomized trial outcomes of a TTM‐tailored condom use and smoking intervention in urban adolescent females. Health Education Research 2015;30(1):162‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robinson 2003 {published data only}
- Robinson LA, Vander Weg MW, Riedel BW, Klesges RC, McLain‐Allen B. "Start to stop": results of a randomised controlled trial of a smoking cessation programme for teens. Tobacco Control 2003;12(Suppl IV):iv26‐iv33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scherphof 2014 {published data only}
- Scherphof CS, Eijnden RJ, Engels RCME, Vollebergh WA. Short‐term efficacy of nicotine replacement therapy for smoking cessation in adolescents: a randomized controlled trial. Journal of Substance Abuse Treatment 2014;46(2):120‐7. [DOI] [PubMed] [Google Scholar]
- Scherphof CS, Eijnden RJ, Lugtig P, Engels RCME, Vollebergh WA. Adolescents' use of nicotine replacement therapy for smoking cessation: predictors of compliance trajectories. Psychopharmacology 2014;231(8):1743‐52. [DOI] [PubMed] [Google Scholar]
- Scherphof CS, Eijnden RJJM, Engels RCME, Vollebergh WAM. Long‐term efficacy of nicotine replacement therapy for smoking cessation in adolescents: a randomized controlled trial. Drug and Alcohol Dependence 2014;140:217‐20. [DOI] [PubMed] [Google Scholar]
Sherbot 2005 {published data only}
- Sherbot NA. The use of motivational enhancement therapy and the quit 4 life program as a means to facilitate adolescent smoking cessation [doctoral dissertation]. Vol. 66, Dissertation Abstracts International: Section B: The Sciences and Engineering, 2005:574. [Google Scholar]
Skov‐Ettrup 2014 {published data only}
- Skov‐Ettrup LS, Ringgaard LW, Dalum P, Flensborg‐Madsen T, Thygesen LC, Tolstrup JS. Comparing tailored and untailored text messages for smoking cessation: a randomized controlled trial among adolescent and young adult smokers. Health Education Research 2014;29(2):195‐205. [DOI] [PubMed] [Google Scholar]
Woodruff 2007 {published data only}
- Woodruff SI, Conway TL, Edwards CC. Sociodemographic and smoking‐related psychosocial predictors of smoking behavior change among high school smokers. Addictive Behaviors 2008;33:354‐8. [DOI] [PubMed] [Google Scholar]
- Woodruff SI, Conway TL, Edwards CC, Elliott SP, Crittenden J. Evaluation of an internet virtual world chat room for adolescent smoking cessation. Addictive Behaviors 2007;32:1769‐86. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abelin 1989 {published data only}
- Abelin T, Ehrsam R, Buhler Reichert A, Imhof PR, Muller P, Thommen A, et al. Effectiveness of a transdermal nicotine system in smoking cessation studies. Methods and Findings in Experimental and Clinical Pharmacology 1989;11:205‐14. [PubMed] [Google Scholar]
Adelman 2001 {published data only}
- Adelman WP, Duggan A, Hauptman P, Joffe A. Effectiveness of a high school smoking cessation program. Journal of Adolescent Health 2000;26(2):83. [DOI] [PubMed] [Google Scholar]
- Adelman WP, Duggan AK, Hauptman P, Joffe A. Effectiveness of a high school smoking cessation program. Pediatrics 2001;107(4):e50‐9. [DOI] [PubMed] [Google Scholar]
Adelman 2009 {published data only}
- Adelman WP. Nicotine nasal spray neither effective nor well‐tolerated by adolescent smokers. Journal of Pediatrics 2009;154(3):462‐3. [DOI] [PubMed] [Google Scholar]
Ames 2007 {published data only}
- Ames S, Patten C, Werch C, Covil E, Craggs J, Olmos J, et al. Expressive writing as a smoking cessation treatment adjunct for young adult smokers (POS2‐28). Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15‐18, Orlando, Florida. 2006.
- Ames SC, Patten CA, Offord KP, Pennebaker JW, Croghan IT, Tri DM, et al. Expressive writing intervention for young adult cigarette smokers. Journal of Clinical Psychology 2005;61:1555‐70. [DOI] [PubMed] [Google Scholar]
- Ames SC, Patten CA, Werch CE, Schroeder DR, Stevens SR, Fredrickson PA, et al. Expressive writing as a smoking cessation treatment adjunct for young adult smokers. Nicotine & Tobacco Research 2007;9(2):185‐94. [DOI] [PubMed] [Google Scholar]
An 2007 {published data only}
- An LC, Hennrikus DJ, Perry CL, Lein EB, Klatt C, Farley DM, et al. Feasibility of internet health screening to recruit college students to an online smoking cessation intervention. Nicotine and Tobacco Research 2007;9(Suppl 1):s11‐8. [DOI] [PubMed] [Google Scholar]
Arora 2010 {published data only}
- Arora M, Stigler M, Gupta V, Bassi S, Dhavan P, Mathur N, et al. Tobacco control among disadvantaged youth living in low‐income communities in India: introducing Project ACTIVITY. Asian Pacific Journal of Cancer Prevention 2010;11(1):45‐52. [PMC free article] [PubMed] [Google Scholar]
- Harrell MN, Arora M, Bassi S, Gupta VK, Perry CL, Reddy KS. Reducing tobacco use among low socio‐economic status youth in Delhi, India: outcomes from project ACTIVITY, a cluster randomized trial. Health Education Research 2016;31(5):624‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Audrain‐McGovern 2011 {published data only}
- Audrain‐McGovern J, Stevens S, Murray PJ, Kinsman S, Zuckoff A, Pletcher J, et al. The efficacy of motivational interviewing versus brief advice for adolescent smoking behavior change. Pediatrics 2011;128(1):e101‐1. [DOI] [PubMed] [Google Scholar]
- Grossberg P. Motivational interviewing results in minimal change in behavior among adolescent smokers. Journal of Pediatrics 2012;160(3):526‐7. [DOI] [PubMed] [Google Scholar]
- Kalkhuis‐Beam S, Stevens SL, Baumritter A, Carlson EC, Pletcher JR, Rodriguez D, et al. Participant‐ and study‐related characteristics predicting treatment completion and study retention in an adolescent smoking cessation trial. Journal of Adolescent Health 2011;49(4):371‐8. [DOI] [PubMed] [Google Scholar]
Audrey 2008 {published data only}
- Audrey S, Holliday J, Campbell R. Commitment and compatibility: teachers' perspectives on the implementation of an effective school‐based, peer‐led smoking intervention. Health Education Journal 2008;67(2):74‐90. [Google Scholar]
Bannink 2014 {published data only}
- Bannink R, Broeren S, Joosten‐van Zwanenburg E, As E, Looij‐Jansen P, Raat H. Effectiveness of a web‐based tailored intervention (E‐health4Uth) and consultation to promote adolescents' health: randomized controlled trial. Journal of Medical Internet Research 2014;16(5):e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bauman 2000 {published data only}
- Bauman KE, Ennett ST, Foshee VA, Pemberton M, King TS, Koch GG. Influence of a family‐directed program on adolescent cigarette and alcohol cessation. Prevention Science 2000;1:227‐37. [DOI] [PubMed] [Google Scholar]
Bloor 1999 {published data only}
- Bloor M, Frankland J, Langdon NP, Robinson M, Allerston S, Catherine A, et al. A controlled evaluation of an intensive, peer‐led, schools‐based, anti‐smoking programme. Health Education Journal 1999;58(1):17‐25. [Google Scholar]
Bond 2004 {published data only}
- Bond L, Patton G, Glover S, Carlin JB, Butler H, Thomas L, et al. The Gatehouse Project: can a multilevel school intervention affect emotional wellbeing and health risk behaviours?. Journal of Epidemiology and Community Health 2004;58(12):997‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bramley 2005 {published data only}
- Bramley D, Riddell T, Whittaker R, Corbett T, Lin RB, Wills M, et al. Smoking cessation using mobile phone text messaging is as effective in Maori as non‐Maori. New Zealand Medical Journal 2005;118(1216):U1494. [PubMed] [Google Scholar]
- Rodgers A, Corbett T, Bramley D, Riddell T, Wills M, Lin RB, et al. Do u smoke after txt? Results of a randomised trial of smoking cessation using mobile phone text messaging. Tobacco Control 2005;14(4):255‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Braverman 1994 {published data only}
- Braverman MT, Moskowitz JM, D'Onofrio CN, Foster V. Project 4‐health develops program to curb youth tobacco use. California Agriculture 1994;48:39‐43. [Google Scholar]
Brendryen 2008 {published data only}
- Brendryen H, Drozd F, Kraft P. A digital smoking cessation program delivered through internet and cell phone without nicotine replacement (happy ending): randomized controlled trial. Journal of Medical Internet Research 2008;10(5):e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brinker 2016 {published data only}
- Brinker TJ, Holzapfel J, Baudson TG, Sies K, Jakob L, Baumert HM, et al. Photoaging smartphone app promoting poster campaign to reduce smoking prevalence in secondary schools: the Smokersface Randomized Trial: design and baseline characteristics. BMJ Open 2016;6(11):e014288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buller 2014a {published data only}
- Buller DB, Halperin A, Severson HH, Borland R, Slater MD, Bettinghaus EP, et al. Effect of nicotine replacement therapy on quitting by young adults in a trial comparing cessation services. Journal of Public Health Management and Practice 2014;20(2):E7‐E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buller 2014b {published data only}
- Buller DB, Borland R, Bettinghaus EP, Shane JH, Zimmerman DE. Randomized trial of a smartphone mobile application compared to text messaging to support smoking cessation. Telemedicine Journal and e‐Health 2014;20(3):206‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burton 1994 {published data only}
- Burton D. Tobacco cessation programs for adolescents. In: Richmond R editor(s). Interventions for Smokers: an International Perspective. Baltimore: Williams and Wilkins, 1994:95‐136. [Google Scholar]
- Burton D, Chakravorty B, Weekes K, Flay BR. Outcome of TNT tobacco‐cessation randomised trial with high school students. Unpublished material 1994. [DOI] [PMC free article] [PubMed]
- Burton D, Chakravorty B, Weeks K, Flay BR, Dent C, Stacy A, et al. Outcome of a tobacco use cessation randomized trial with high‐school students. Substance Use & Misuse 2009;44(7):965‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cai 2000 {published data only}
- Cai YM, Zhao CX, Wong SU, Zhang L, Lim SK. Laser acupuncture for adolescent smokers ‐ a randomized double‐blind controlled trial. American Journal of Chinese Medicine 2000;28(3‐4):443‐9. [DOI] [PubMed] [Google Scholar]
- Yiming C. Laser acupuncture for adolescent smokers ‐ a randomised double blind controlled study. XI World Congress of Psychiatry, Hamburg, August 6‐11, 1999. 1999; Vol. II:105.
Campbell 2008 {published data only}
- Campbell R, Starkey F, Holliday J, Audrey S, Bloor M, Parry‐Langdon N, et al. An informal school‐based peer‐led intervention for smoking prevention in adolescence (ASSIST): a cluster randomised trial. Lancet 2008;371(10):1595‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth W, Cohen D, Hawkins J, Hughes RA, Moore LA, Holliday JC, et al. Reducing smoking in adolescents: cost‐effectiveness results from the cluster randomized ASSIST (A Stop Smoking In Schools Trial). Nicotine & Tobacco Research. United Kingdom: Oxford University Press (Great Clarendon Street, Oxford OX2 6DP, United Kingdom), 2012; Vol. 14, issue 2:161‐8. [] [DOI] [PMC free article] [PubMed]
- Starkey F, Moore L, Campbell R, Sidaway M, Bloor M, ASSIST. Correction: rationale, design and conduct of a comprehensive evaluation of a school‐based peer‐led anti‐smoking intervention in the UK: the ASSIST cluster randomised trial. BMC Public Health 2007;7:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cavallo 2007 {published data only}
- Cavallo DA, Smith AE, Liss TB, McFetridge AK, Babuscio T, Nich C, et al. Contingency management and cognitive behavioral therapy for smoking cessation in adolescent smokers: comparison of two different CBT formats. Society for Research on Nicotine and Tobacco 13th Annual Meeting February 21‐24, Austin, Texas. 2007:54.
Chan 1988 {published data only}
- Chan CW, Witherspoon JM. Health risk appraisal modifies cigarette smoking behavior among college students. Journal of General Internal Medicine 1988;3(6):555‐9. [DOI] [PubMed] [Google Scholar]
Chen 2006 {published data only}
- Chen HH, Yeh ML. Developing and evaluating a smoking cessation program combined with an internet‐assisted instruction program for adolescents with smoking. Patient Education and Counseling 2006;61(3):411‐8. [DOI] [PubMed] [Google Scholar]
- Chen HH, Yeh ML, Chao YH. Comparing effects of auricular acupressure with and without an internet‐assisted program on smoking cessation and self‐efficacy of adolescents. Journal of Alternative & Complementary Medicine 2006;12(2):147‐52. [DOI] [PubMed] [Google Scholar]
Colby 1998 {published data only}
- Colby SM, Monti PM, Barnett NP, Rohsenow DJ, Weissman K, Spirito A, et al. Brief motivational interviewing in a hospital setting for adolescent smoking: a preliminary study. Journal of Consulting and Clinical Psychology 1998;66(3):574‐8. [DOI] [PubMed] [Google Scholar]
- Monti P, Colby SM, O'Leary TA, Spirito A, Woolard R, Rohsenow D, et al. Motivational interviewing plus parental intervention for adolescent smokers: results from a randomized clinical trial (POS4‐31). Society for Research on Nicotine and Tobacco 9th Annual Meeting February 19‐22 2003 New Orleans, Louisiana. 2003.
Curry 2013 {published data only}
- Curry SJ, Mermelstein RJ, Emery SL, Sporer AK, Berbaum ML, Campbell RT, et al. A national evaluation of community‐based youth cessation programs: end of program and twelve‐month outcomes. American Journal of Community Psychology 2013;51(1‐2):15‐29. [DOI] [PubMed] [Google Scholar]
Digiusto 1994 {published data only}
- Digiusto WE. Pros and cons of cessation interventions for adolescent smokers at school. In: Richmond R editor(s). Interventions for Smoking ‐ an International Perspective. Baltimore: Williams and Wilkins, 1994:107‐36. [Google Scholar]
Dino 1998 {published data only}
- Dino G, Horn K, Meit H. A pilot study of Not On Tobacco: a stop smoking programme for adolescents. Health Education 1998;6:230‐41. [Google Scholar]
Egger 1983 {published data only}
- Egger G, Fitzgerald W, Frape G, Monaem A, Rubinstein P, Tyler C, et al. Results of large scale media antismoking campaign in Australia: North Coast 'Quit for Life' programme. British Medical Journal 1983;287:1125‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehrsam 1991 {published data only}
- Ehrsam RE, Buhler A, Muller P, Mauli D, Schumacher PM, Howald H, et al. Weaning of young smokers using a transdermal nicotine patch [Entwöhnung junger Raucher mit Hilfe eines transdermalen Nikotinpflasters]. Schweizerische Rundschau für Medizin Praxis 1991;80(7):145‐50. [PubMed] [Google Scholar]
Elsasser 2002 {published data only}
- Elsasser GN, Guck TP, Destache CJ, Daher PM, Frey DR, Jones J, et al. Sustained release bupropion in the treatment of nicotine addiction among teenage smokers (RP‐32). Society for Research on Nicotine and Tobacco 8th Annual Meeting Rapid Communications Posters February 20‐23 2002 Savannah, Georgia. 2002.
Emmons 2003 {published data only}
- Emmons KM, Butterfield RM, Puleo E, Park ER, Mertens A, Gritz ER, et al. Smoking among participants in the childhood cancer survivors cohort: the Partnership for Health Study. Journal of Clinical Oncology 2003;21(2):189‐96. [DOI] [PubMed] [Google Scholar]
Erol 2008 {published data only}
- Erol S, Erdogan S. Application of a stage based motivational interviewing approach to adolescent smoking cessation: the Transtheoretical Model‐based study. Patient Education and Counseling 2008;72(1):42‐8. [DOI] [PubMed] [Google Scholar]
Escoffery 2004 {published data only}
- Escoffery C, McCormick L, Bateman K. Development and process evaluation of a web‐based smoking cessation program for college smokers: innovative tool for education. Patient Education and Counseling 2004;53(2):217‐25. [DOI] [PubMed] [Google Scholar]
Faessel 2009 {published data only}
- Faessel H, Ravva P, Williams K. Pharmacokinetics, safety, and tolerability of varenicline in healthy adolescent smokers: a multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study. Clinical Therapeutics 2009;31(1):177‐89. [DOI] [PubMed] [Google Scholar]
Fagan 2003 {published data only}
- Fagan P, Stoddard AM, Hunt MK, Frazier L, Girod K, Sorensen G. The feasibility of evaluating a tobacco control intervention for working youth. Tobacco Control 2003;12:34‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Figa‐Talamanca 1989 {published data only}
- Figa‐Talamanca I, Modolo M. Evaluation of an anti‐smoking educational programme among adolescents in Italy. Hygie 1989;8:24‐8. [PubMed] [Google Scholar]
Flay 1995 {published data only}
- Flay BR, Miller TQ, Hedeker D, Siddiqui O, Britton CF, Brannon BR, et al. The television, school, and family smoking prevention and cessation project. VIII student outcomes and mediating variables. Preventive Medicine 1995;24:29‐40. [DOI] [PubMed] [Google Scholar]
Gray 2011 {published data only}
- Carpenter MJ, Baker NL, Gray KM, Upadhyaya HP. Assessment of nicotine dependence among adolescent and young adult smokers: a comparison of measures. Addictive Behaviours 2010;35(11):977‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Carpenter MJ, Baker NL, Hartwell KJ, Lewis AL, Hiott DW, et al. Bupropion SR and contingency management for adolescent smoking cessation. Journal of Substance Abuse Treatment 2011;40(1):77‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gray 2012 {published data only}
- Gray KM, Carpenter MJ, Lewis AL, Klintworth EM, Upadhyaya HP. Varenicline versus bupropion XL for smoking cessation in older adolescents: a randomized, double‐blind pilot trial. Nicotine & Tobacco Research 2012;14(2):235‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ha 2015 {published data only}
- Ha YS, Choi YH. Effectiveness of the self‐determination theory based a motivational interviewing YOU‐TURN Program for smoking cessation among adolescents. Journal of Korean Academy of Nursing 2015;45(3):347‐56. [DOI] [PubMed] [Google Scholar]
Hamilton 2005 {published data only}
- Hamilton G, Cross D, Resnicow K, Hall M. A school‐based harm minimization smoking intervention trial: outcome results. Addiction 2005;100(5):689‐700. [DOI] [PubMed] [Google Scholar]
Hancock 2001 {published data only}
- Hancock L, Sanson‐Fisher R, Perkins J, Girgis A, Howley P, Schofield M. The effect of a community action intervention on adolescent smoking rates in rural Australian towns: the CART project. Cancer Action in Rural Towns. Preventive Medicine 2001;32(4):332‐40. [DOI] [PubMed] [Google Scholar]
Hanson 2003 {published data only}
- Dickmann PJ, Mooney ME, Allen SS, Hanson K, Hatsukami DK. Nicotine withdrawal and craving in adolescents: effects of sex and hormonal contraceptive use. Addictive Behaviors 2009;34(6‐7):620‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K, Allen S, Jensen S, Hatsukami D. Treatment of adolescent smokers with the nicotine patch. Nicotine & Tobacco Research 2003;5(4):515‐26. [DOI] [PubMed] [Google Scholar]
Hanson 2006 {published data only}
- Hanson K, Zylla E, Allen S, Avery G. Harm reduction: an intervention for adolescent smokers (SYM3B). Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15‐18, Orlando, Florida. 2006.
- Hanson K, Zylla E, Allen S, Li Z, Hatsukami DK. Cigarette reduction: an intervention for adolescent smokers. Drug and Alcohol Dependance 2008;95:164‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haug 2009 {published data only}
- Haug S, Meyer C, Schorr G, Bauer S, John U. Continuous individual support of smoking cessation using text messaging: a pilot experimental study. Nicotine & Tobacco Research 2009;11(8):915‐23. [DOI] [PubMed] [Google Scholar]
Heikkinen 2009 {published data only}
- Heikkinen AM, Broms U, Pitkäniemi J, Koskenvuo M, Meurman J. Key factors in smoking cessation intervention among 15‐16‐year‐olds. Behavioral Medicine 2009;35:93‐9. [DOI] [PubMed] [Google Scholar]
Hellmann 1988 {published data only}
- Hellmann R, OShea RM, Kunz ML, Schimpfhauser FT. University health service physician intervention with cigarette smokers. Journal of American College Health 1988;37:91‐3. [DOI] [PubMed] [Google Scholar]
Helstrom 2004 {published data only}
- Helstrom A, Hutchison K, Bryan A. Motivational enhancement therapy for high‐risk adolescent smokers. Addictive Behaviors 2007;32:2404‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helstrom AW. A smoking intervention for high‐risk adolescents [doctoral dissertation]. Vol. 64, Dissertation Abstracts International: Section B: The Sciences and Engineering, 2004:3525. [Google Scholar]
Higgs 2000 {published data only}
- Higgs PE, Edwards D, Harbin RE, Higgs PC. Evaluation of a self‐directed smoking prevention and cessation program. Pediatric Nursing 2000;26(2):150‐5. [PubMed] [Google Scholar]
Hollis 1994 {published data only}
- Hollis JF, Vogt TM, Stevens V, Biglan A, Severson H, Lichtenstein E. The Tobacco Reduction and Cancer Control (TRACC) program: team approaches to counseling in medical and dental settings. In: U.S.Dept of Healh and Human Services, editor(s). Tobacco and the clinician: interventions for medical and dental practice. Vol. I, Washington DC: U.S. Govt printing office, 1994:143. [Google Scholar]
Horn 2004 {published data only}
- Horn K, Dino G, Kalsekar I, Massey CJ, Manzo‐Tennant K, McGloin T. Exploring the relationship between mental health and smoking cessation: a study of rural teens. Prevention Science 2004;5(2):113‐26. [DOI] [PubMed] [Google Scholar]
Hort 1995 {published data only}
- Hort W, Hort H, Willers R. An interventional study against cigarette smoking among Dusseldorf high school students 1992‐94 [Interventionsstudie gegen das Zigarettenrauchen von Düsseldorfer Hauptschülern 1992‐94]. Zeitschrift Fur Kardiologie 1995;84(9):700‐11. [PubMed] [Google Scholar]
Jason 1982 {published data only}
- Jason LA, Mollica M, Ferrone L. Evaluating an early secondary smoking prevention intervention. Preventive Medicine 1982;11(1):96‐102. [DOI] [PubMed] [Google Scholar]
Josendal 1998 {published data only}
- Josendal O, Aaro LE. Evaluation of an intervention programme for smokefree schools. Tidsskrift for den Norske Laegeforening 2002;122(4):403‐7. [PubMed] [Google Scholar]
- Josendal O, Aaro LE, Bergh IH. Effects of a school‐based smoking prevention program among subgroups of adolescents. Health Education Research 1998;13(2):215‐24. [DOI] [PubMed] [Google Scholar]
Kang 2005 {published data only}
- Kang H‐C, Shin K‐K, Kim K‐K, Youn B‐B. The effects of the acupuncture treatment for smoking cessation in high school student smokers. Yonsei Medical Journal 2005;46(2):206‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kealey 2009 {published data only}
- Kealey KA, Ludman EJ, Marek PM, Mann SL, Bricker JB, Peterson AV. Design and implementation of an effective telephone counseling intervention for adolescent smoking cessation. Journal of the National Cancer Institute 2009;101(20):1393‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelleher 1999 {published data only}
- Kelleher CC, Fallon UB, McCarthy E, Dineen BD, ODonnell M, Killian M, et al. Feasibility of a lifestyle cardiovascular health promotion programme for 8‐15‐year olds in Irish general practice: results of the Galway Health Project. Health Promotion International 1999;14:221‐9. [Google Scholar]
Kentala 1999 {published data only}
- Kentala J, Utriainen P, Pahkala K, Mattila K. Can brief intervention through community dental care have an effect on adolescent smoking?. Preventive Medicine 1999;29:107‐11. [DOI] [PubMed] [Google Scholar]
Keyser 2014 {published data only}
- Keyser MM, Goebel LJ. Update on adolescent tobacco cessation. West Virginia Medical Journal 2014;110(4):46‐52. [PubMed] [Google Scholar]
Killen 1988 {published data only}
- Killen JD, Robinson TN, Telch MJ, Saylor KE, Maron DJ, Rich T, et al. The Stanford Adolescent Heart Health Program. Health Education Quarterly 1989;16(2):263‐83. [DOI] [PubMed] [Google Scholar]
- Killen JD, Telch MJ, Robinson TN, Maccoby N, Taylor CB, Farquhar JW. Cardiovascular disease risk reduction for tenth graders. A multiple‐factor school‐based approach. Journal of the American Medical Association 1988;260:1728‐33. [PubMed] [Google Scholar]
Kim 2004 {published data only}
- Kim S, Nam KA, Seo M, Lee HH. Effectiveness of a smoking cessation program for adolescents. Taehan Kanho Hakhoe Chi 2004;34(4):646‐54. [DOI] [PubMed] [Google Scholar]
Knishkowy 2008 {published data only}
- Knishkowy B, Verbov‐Lei G, Amitai Y, Zamir‐Stein C, Rosen L. Results from a religion‐based tobacco control trial among Haredi (Jewish ultra‐orthodox) male adolescents in Jerusalem. Unpublished Work 2008.
Kong 2015 {published data only}
- Kong G, Larsen H, Cavallo DA, Becker D, Cousijn J, Salemink E, et al. Re‐training automatic action tendencies to approach cigarettes among adolescent smokers: a pilot study. American Journal of Drug and Alcohol Abuse 2015;41(5):425‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen H, Kong G, Becker D, Cavallo DA, Cousijn J, Salemink E, et al. Cognitive bias modification combined with cognitive behavioral therapy: a smoking cessation intervention for adolescents. Drug and Alcohol Dependence 2015;146:e168. [Google Scholar]
Krishnan‐Sarin 2013 {published data only}
- Krishnan‐Sarin S, Balodis IM, Kober H, Worhunsky PD, Liss T, Xu J, et al. An exploratory pilot study of the relationship between neural correlates of cognitive control and reduction in cigarette use among treatment‐seeking adolescent smokers. Psychology of Addictive Behaviors 2013;27(2):526‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan‐Sarin S, Cavallo DA, Cooney JL, Schepis TS, Kong G, Liss TB, et al. An exploratory randomized controlled trial of a novel high‐school‐based smoking cessation intervention for adolescent smokers using abstinence‐contingent incentives and cognitive behavioral therapy. Drug and Alcohol Dependence 2013;132(1‐2):346‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Kong G, Camenga DR, Cavallo DA, Carroll KM, Pittman B, et al. Contingency management improves smoking cessation treatment outcomes among highly impulsive adolescent smokers relative to cognitive behavioral therapy. Addictive Behaviors 2015;42:86‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis TS, Cavallo DA, Kong G, Liss T, Liss A, Krishnan‐Sarin S. Predicting initiation of smoking cessation treatment and outcome among adolescents using stressful life events and coping style. Substance Abuse 2015;36(4):478‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P, Connell C, Kong G, Morean ME, Cavallo DA, Camenga D, et al. Self‐efficacy mediates treatment outcome in a smoking cessation program for adolescent smokers. Drug and Alcohol Dependence 2015;146:e100. [Google Scholar]
Lando 2007 {published data only}
- Lando HA, Hennrikus D, Boyle R, Lazovich D, Stafne E, Rindal B. Promoting tobacco abstinence among older adolescents in dental clinics. Journal of Smoking Cessation 2007;2(1):23‐30. [Google Scholar]
La Torre 2013 {published data only}
- Torre G, Rossini G, Saulle R, Monnocci A, Thiene D, Mauro V, et al. Randomised controlled trial on the promotion of healthy lifestyles among adolescents in the orthodontic setting: study protocol [Trial clinico randomizzato sulla promozione di corretti stili di vita tra gli adolescenti in ambito ortodontico: protocollo dello studio]. Clinica Terapeutica 2013;164(Suppl 4):e301‐e304. [DOI] [PubMed] [Google Scholar]
Lotecka 1983 {published data only}
- Lotecka L, MacWhinney M. Enhancing decision behavior in high school 'smokers'. International Journal of the Addictions 1983;18:479‐90. [DOI] [PubMed] [Google Scholar]
McCambridge 2004 {published data only}
- McCambridge J, Strang J. The efficacy of single session motivational interviewing in reducing drug consumption and perceptions of drug related risk and harm among young people: results from a multi‐site cluster randomized trial. Addiction 2004;99(1):39‐52. [DOI] [PubMed] [Google Scholar]
McCuller 2006 {published data only}
- McCuller WJ, Sussman S, Wapner M, Dent C, Weiss DJ. Motivation to quit as a mediator of tobacco cessation among at‐risk youth. Addictive Behaviors 2006;31(5):880‐8. [DOI] [PubMed] [Google Scholar]
Mermelstein 2006 {published data only}
- Mermelstein R, Turner L. Web‐based support as an adjunct to group‐based smoking cessation for adolescents. Nicotine & Tobacco Research 2006;8 Suppl 1:S69‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner L, Mermelstein R. Web‐based support as an adjunct to group‐based smoking cessation for adolescents (POS2‐50). Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15‐18, Orlando, Florida. 2006. [DOI] [PMC free article] [PubMed]
Minary 2013 {published data only}
- Minary L, Cambon L, Martini H, Wirth N, Acouetey DS, Thouvenot F, et al. Efficacy of a smoking cessation program in a population of adolescent smokers in vocational schools: a public health evaluative controlled study. BMC Public Health 2013;13:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minary L, Martini H, Wirth N, Thouvenot F, Acouetey DS, Martinet Y, et al. TABADO: "Evaluation of a smoking cessation program among adolescents in vocational training centers": Study protocol. BMC Public Health 2009;9:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mokina 2015 {published data only}
- Mokina N, Pjatin V, Mokin E. The practical application of clinical guidelines for smoking cessation in adolescents: the final assessment point and the effectiveness of interventions. European Respiratory Journal 2015;46(Suppl 59):PA1219. [Google Scholar]
Myers 2005 {published data only}
- Myers MG, Brown, SA. A controlled study of a cigarette smoking cessation intervention for adolescents in substance abuse treatment. Psychology of Addictive Behaviors 2005;19(2):230–3. [DOI] [PubMed] [Google Scholar]
- Myers MG, Gwaltney CJ, Strong DR, Ramsey SE, Brown RA, Monti PM, et al. Adolescent first lapse following smoking cessation: situation characteristics, precipitants and proximal influences. Addictive Behaviors 2011;36(12):1253‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Myers 2008 {published data only}
- Myers MG, Gwaltney CJ, Strong DR, Ramsey SE, Brown RA, Monti PM, et al. Adolescent first lapse following smoking cessation: situation characteristics, precipitants and proximal influences. Addictive Behaviors 2011;36(12):1253‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Prochaska JJ. Does smoking intervention influence adolescent substance use disorder treatment outcomes?. Substance Abuse 2008;29(2):81‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Niederhofer 2004 {published data only}
- Niederhofer H, Huber M. Bupropion may support psychosocial treatment of nicotine‐dependent adolescents: preliminary results. Pharmacotherapy 2004;24(11):1524‐8. [DOI] [PubMed] [Google Scholar]
Norman 2008 {published data only}
- Norman CD, Maley O, Li X, Skinner HA. Using the internet to assist smoking prevention and cessation in schools: a randomized, controlled trial. Health Psychology 2008;27(6):799‐810. [DOI] [PubMed] [Google Scholar]
- Skinner H, Maley O, Norman C. Website intervention for youth smoking prevention: findings from school‐based randomized controlled trial. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20‐23 March 2005; Prague, Czech Republic. 2005.
- Skinner H, Norman C, Maley O. Web‐based intervention for youth smoking prevention and cessation. 130th Annual Meeting of APHA, 12th November. 2002.
NoT AL 2008 {published and unpublished data}
- Kohler C, Schoenberger YM, Phillips M. Effectiveness evaluation of a school‐based smoking cessation program for adolescents. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20‐23 March 2005; Prague, Czech Republic. 2005.
- Kohler CL, Schoenberger YM, Beasley TM, Phillips MM. Effectiveness evaluation of the N‐O‐T smoking cessation program for adolescents. American Journal of Health Behavior 2008;32(4):368‐79. [DOI] [PubMed] [Google Scholar]
- Vaid IG. Self‐efficacy to resist smoking as a mediator between nicotine dependence and quit attempt in adolescent smokers in Alabama [doctoral dissertation]. Vol. 69, Dissertation Abstracts International: Section B: The Sciences and Engineering, 2008. [Google Scholar]
NoT FL 2001 {published data only}
- Dino G, Goldcamp J, Fernandes A, Kalsekar I, Massey C. A 2‐year efficacy study of Not On Tobacco in Florida: an overview of program successes in changing teen smoking behavior. Preventive Medicine 2001;33(6):600‐5. [DOI] [PubMed] [Google Scholar]
- Mercincavage M, Branstetter SA, Muscat JE, Horn KA. Time to first cigarette predicts cessation outcomes in adolescent smokers. Nicotine & Tobacco Research 2013;15(12):1996‐2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
NoT NC 2005 {published data only}
- Horn K, Dino G, Kalsekar I, Mody R. The impact of Not On Tobacco on teen smoking cessation. Journal of Adolescent Research 2005;20(6):640‐61. [Google Scholar]
- Mercincavage M, Branstetter SA, Muscat JE, Horn KA. Time to first cigarette predicts cessation outcomes in adolescent smokers. Nicotine & Tobacco Research 2013;15(12):1996‐2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
NoT WV 2004 {published data only}
- Horn K, Fernandes A, Dino G, Massey CJ, Kalsekar I. Adolescent nicotine dependence and smoking cessation outcomes. Addictive Behaviors 2003;28:769‐76. [DOI] [PubMed] [Google Scholar]
- Horn KA, Dino GA, Kalsekar ID, Fernandes AW. Appalachian teen smokers: Not On Tobacco 15 months later. American Journal of Public Health 2004;94(2):181‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pallonen 1998 {published data only}
- Pallonen UE, Velicer WF, Prochaska JO, Rossi JS, Bellis JM, Tsoh JY, et al. Computer‐based smoking cessation interventions in adolescents: description, feasibility, and six‐month follow‐up findings. Substance Use and Misuse 1998;33(4):935‐65. [DOI] [PubMed] [Google Scholar]
Park 2015 {published data only}
- Park E, Drake E. Systematic review: internet‐based program for youth smoking prevention and cessation. Journal of Nursing Scholarship 2015;47(1):43‐50. [DOI] [PubMed] [Google Scholar]
Patten 2014 {published data only}
- Patten CA, Fadahunsi O, Hanza MM, Smith CA, Decker PA, Boyer R, et al. Tobacco cessation treatment for Alaska native adolescents: group randomized pilot trial. Nicotine & Tobacco Research 2014;16(6):836‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pbert 2006 {published data only}
- Pbert L, Osganian SK, Gorak D, Druker S, Reed G, O'Neill KM, et al. A school nurse‐delivered adolescent smoking cessation intervention: a randomized controlled trial. Preventive Medicine 2006;43:312‐20. [DOI] [PubMed] [Google Scholar]
Pbert 2008 {published data only}
- Pbert L, Flint AJ, Fletcher KE, Young MH, Druker S, DiFranza JR. Effect of a pediatric practice‐based smoking prevention and cessation intervention for adolescents: a randomized, controlled trial. Pediatrics 2008;121(4):e738‐e747. [DOI] [PubMed] [Google Scholar]
Peirson 2016 {published data only}
- Peirson L, Ali MU, Kenny M, Raina P, Sherifali D. Interventions for prevention and treatment of tobacco smoking in school‐aged children and adolescents: a systematic review and meta‐analysis. Preventive Medicine 2016;85:20‐31. [DOI] [PubMed] [Google Scholar]
Perry 1980 {published data only}
- Perry C, Killen J, Telch M, Slinkard LA, Danaher BG. Modifying smoking behavior of teenagers: a school‐based intervention. American Journal of Public Health 1980;70(7):722‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prokhorov 2010 {published data only}
- Prokhorov AV, Kelder SH, Shegog R, Conroy JL, Murray N, Peters R, et al. Project ASPIRE: an interactive, multimedia smoking prevention and cessation curriculum for culturally diverse high school students. Substance Use & Misuse 2010;45(6):983‐1006. [DOI] [PubMed] [Google Scholar]
Quinlan 2000 {published data only}
- Quinlan KB, McCaul KD. Matched and mismatched interventions with young adult smokers: testing a stage theory. Health Psychology 2000;19(2):165‐71. [DOI] [PubMed] [Google Scholar]
Rabius 2004 {published data only}
- Rabius V, McAlister AL, Geiger A, Huang P, Todd R. Telephone counseling increases cessation rates among young adult smokers. Health Psychology 2004;23(5):539‐41. [DOI] [PubMed] [Google Scholar]
Ramo 2015 {published data only}
- Ramo DE, Thrul J, Chavez K, Delucchi KL, Prochaska JJ. Feasibility and quit rates of the tobacco status project: a Facebook smoking cessation intervention for young adults. Journal of Medical Internet Research 2015;17(12):e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reynolds 2015 {published data only}
- Reynolds B, Harris M, Slone SA, Shelton BJ, Dallery J, Stoops W, et al. A feasibility study of home‐based contingency management with adolescent smokers of rural Appalachia. Experimental and Clinical Psychopharmacology 2015;23(6):486‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rice 2010 {published data only}
- Rice VH, Weglicki LS, Templin T, Jamil H, Hammad A. Intervention effects on tobacco use in Arab and non‐Arab American adolescents. Addictive Behaviors 2010;35(1):46‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roddy 2006 {published data only}
- Roddy E, Romilly N, Challenger A, Lewis S, Britton J. Use of nicotine replacement therapy in socioeconomically deprived young smokers: a community‐based pilot randomised controlled trial. Tobacco Control 2006;15(5):373‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubinstein 2008 {published data only}
- Rubinstein ML, Benowitz NL, Auerback GM, Moscicki AB. A randomized trial of nicotine nasal spray in adolescent smokers. Pediatrics 2008;122(3):e595‐e600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schepis 2006 {published data only}
- Leeman RF, Schepis TS, Cavallo DA, McFetridge AK, Liss TB, Krishnan‐Sarin S. Nicotine dependence severity as a cross‐sectional predictor of alcohol‐related problems in a sample of adolescent smokers. Nicotine and Tobacco Research 2010;12(5):521‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis TS, Warren KA, Rao U. Evaluation of a cognitive‐behavioral smoking cessation treatment for adolescents and young adults (POS2‐53). Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15‐18, Orlando, Florida. 2006.
Severson 1991 {published data only}
- Severson H, Glasgow RE, Wirt R, Brozovsky P, Zoref L, Black C, et al. Preventing the use of smokeless tobacco and cigarettes by teens: results of a classroom intervention. Health Education Research 1991;6:109‐20. [Google Scholar]
Shi 2013 {published data only}
- Shi HJ, Jiang XX, Yu CY, Zhang Y. Use of mobile phone text messaging to deliver an individualized smoking behaviour intervention in Chinese adolescents. Journal of Telemedicine and Telecare 2013;19(5):282‐7. [DOI] [PubMed] [Google Scholar]
Simmons 2011 {published data only}
- Simmons VN, Heckman B, Fink AC, Patel R, Bello L, Brandon TH. Efficacy of an experiential, web‐based smoking intervention for college smokers [PA2‐2]. SRNT 17th Annual Meeting. Toronto, Ontario, 2011:17.
Simmons 2013 {published data only}
- Simmons VN, Heckman BW, Fink AC, Small BJ, Brandon TH. Efficacy of an experiential, dissonance‐based smoking intervention for college students delivered via the internet. Journal of Consulting and Clinical Psychology 2013;81(5):810‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sims 2013 {published data only}
- Sims T, Smith SS, McAfee T, Baker TB, Fiore M, Sheffer M. Randomized clinical trial to evaluate quitline cessation counseling for 18 to 24 year‐old smokers [PA2‐1] . Society for Research on Nicotine & Tobacco 17th Annual Meeting, February 16‐19, Toronto. 2011.
- Sims TH, McAfee T, Fraser DL, Baker TB, Fiore MC, Smith SS. Quitline cessation counseling for young adult smokers: a randomized clinical trial. Nicotine & Tobacco Research 2013;15(5):932‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomon 2009 {published data only}
- Solomon LJ, Bunn JY, Flynn BS, Pirie PL, Worden JK, Ashikaga T. Mass media for smoking cessation in adolescents. Health Education & Behavior 2009;36(4):642‐59. [DOI] [PubMed] [Google Scholar]
Stamm‐Balderjahn 2012 {published data only}
- Stamm‐Balderjahn S, Groneberg DA, Kusma B, Jagota A, Schonfeld N. Smoking prevention in school students: positive effects of a hospital‐based intervention. Deutsches Arzteblatt International 2012;109(44):746‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein‐Seroussi 2009 {published data only}
- Stein‐Seroussi A, Stockton L, Brodish P, Meyer M. Randomized controlled trial of the ACTION smoking cessation curriculum in tobacco‐growing communities. Addictive Behaviors 2009;34(9):737‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stephens 2001 {published data only}
- Stephens SA. The effectiveness of motivational enhancement therapy in adolescent smoking cessation [doctoral dissertation]. Vol. 62, Dissertation Abstracts International, 2001:1101. [Google Scholar]
Stoddard 2005 {published data only}
- Stoddard AM, Fagan P, Sorensen G, Hunt MK, Frazier L, Girod K. Reducing cigarette smoking among working adolescents: results from the SMART study. Cancer Causes & Control 2005;16(10):1159‐64. [DOI] [PubMed] [Google Scholar]
Sussman 1995 {published data only}
- Sussman S, Dent CW, Burton D, Stacey AW, Flay BR. Cessation Clinic Evaluation. Developing school‐based tobacco use prevention and cessation programmes. California: Sage, 1995:204‐10. [Google Scholar]
Sussman 2012 {published data only}
- Sussman S. International translation of Project EX: a teen tobacco use cessation program. Sucht 2012;58(5):317‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thrul 2015 {published data only}
- Thrul J, Stemmler M, Goecke M, Buhler A. Are you in or out? Recruitment of adolescent smokers into a behavioral smoking cessation intervention. Addictive Behaviors 2015;45:150‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Travis 2009 {published data only}
- Travis HE, Lawrance KA. Randomized controlled trial examining the effectiveness of a tailored self‐help smoking‐cessation intervention for postsecondary smokers. Journal of American College Health 2009;57(4):437‐44. [DOI] [PubMed] [Google Scholar]
Tuisku 2016 {published data only}
- Tuisku A, Salmela M, Nieminen P, Toljamo T. Varenicline and nicotine patch therapies in young adults motivated to quit smoking: a randomised, placebo‐controlled prospective study. Basic & Clinical Pharmacology & Toxicology 2016;119(1):78‐84. [DOI] [PubMed] [Google Scholar]
Turner 2006 {published data only}
- Turner L, Mermelstein R. Web‐based support as an adjunct to group‐based smoking cessation for adolescents (POS2‐50). Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15‐18, Orlando, Florida. 2006. [DOI] [PMC free article] [PubMed]
Wang 2006 {published data only}
- Wang K, Lee AH, Hamilton G, Yau KKW. Multilevel logistic regression modelling with correlated random effects: application to the Smoking Cessation for Youth study. Statistics in Medicine 2006;25(22):3864‐76. [DOI] [PubMed] [Google Scholar]
Werch 2008 {published data only}
- Werch CE, Bian H, Carlson JM, Moore MJ, Diclemente CC, Huang IC, et al. Brief integrative multiple behavior intervention effects and mediators for adolescents. Journal of Behavioral Medicine 2011;34(1):3‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werch CE, Bian H, Diclemente CC, Moore MJ, Thombs D, Ames SC, et al. A brief image‐based prevention intervention for adolescents. Psychology of Addictive Behaviors 2010;24(1):170‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werch CE, Moore MJ, Bian H, DiClemente CC, Ames SC, Weiler RM, et al. Efficacy of a brief image‐based multiple‐behavior intervention for college students. Annals of Behavioral Medicine 2008;36(2):149‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whittaker 2011 {published data only}
- Whittaker R, Dorey E, Bramley D, Bullen C, Denny S, Elley CR, et al. A theory‐based video messaging mobile phone intervention for smoking cessation: randomized controlled trial. Journal of Medical Internet Research 2011;13(1):e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winkleby 2004 {published data only}
- Winkleby MA, Feighery E, Dunn M, Kole S, Ahn D, Killen JD. Effects of an advocacy intervention to reduce smoking among teenagers. Archives of Pediatrics & Adolescent Medicine 2004;158:269‐75. [DOI] [PubMed] [Google Scholar]
Witkiewitz 2014 {published data only}
- Witkiewitz K, Desai SA, Bowen S, Leigh BC, Kirouac M, Larimer ME. Development and evaluation of a mobile intervention for heavy drinking and smoking among college students. Psychology of Addictive Behaviors 2014;28(3):639‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wongwiwatthananukit 2010 {published data only}
- Wongwiwatthananukit S, Dumrongpiwat S, Krittiyanunt S, Dhummaupakorn R, Suwanmajo S. Development and evaluation of pharmacist‐based smoking cessation program for youth offenders. Journal of the American Pharmacists Association 2010;50(2):267‐8. [Google Scholar]
Ybarra 2013 {published data only}
- Filion AJ, Darlington G, Chaput JP, Ybarra M, Haines J. Examining the influence of a text message‐based sleep and physical activity intervention among young adult smokers in the United States. BMC Public Health 2015;15:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybarra ML, Holtrop JS, Prescott TL, Rahbar MH, Strong D. Pilot RCT results of Stop My Smoking USA: a text messaging‐based smoking cessation program for young adults. Nicotine & Tobacco Research 2013;15(8):1388‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Gorzkowski 2016 {published data only}
- Gorzkowski JA, Kaseeska KR, Wright M, Harris DL, Shone L, Whitmore RM, et al. Implementation and impact of the 5As tobacco counseling intervention with adolescents in pediatric practice. Journal of Adolescent Health 2016;58(2):S49. [Google Scholar]
Haug 2014b {published data only}
- Haug S, Castro RP, Filler A, Kowatsch T, Fleisch E, Schaub MP. Efficacy of an internet and SMS‐based integrated smoking cessation and alcohol intervention for smoking cessation in young people: study protocol of a two‐arm cluster randomised controlled trial. BMC Public Health 2014;14:1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01312909 {unpublished data only}
- NCT01312909. Smoking Cessation Study In Healthy Adolescent Smokers. clinicaltrials.gov/ct2/show/NCT01312909 (first received 8 June 2017).
NCT01509547 {published data only}
- NCT01509547. A randomized controlled trial of varenicline for adolescent smoking cessation. clinicaltrials.gov/ct2/show/NCT01509547 (first received 8 June 2017).
NCT02021175 {published data only}
- NCT02021175. Adaptation and development of a web and cell phone quit smoking treatment for Korean youth. clinicaltrials.gov/ct2/show/NCT02021175 (first received 8 June 2017).
NCT02218281 {published data only}
- NCT02218281. Developing a smartphone app with mindfulness training for teen smoking cessation. clinicaltrials.gov/ct2/show/NCT02218281 (first received 8 June 2017).
Additional references
Amos 2006
Aveyard 1999
- Aveyard P, Cheng KK, Almond J, Sherratt E, Lancashire R, Lawrence T, et al. Cluster randomised controlled trial of expert system based on the transtheoretical (''stages of change'') model for smoking prevention and cessation in schools. BMJ 1999;319:948‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bancej 2007
- Bancej C, O'Loughlin J, Platt RW, Paradis G, Gervais A. Smoking cessation attempts among adolescent smokers: a systematic review of prevalence studies. Tobacco Control 2007;16:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boden 2010
- Boden JM, Fergusson DM, Horwood J. Cigarette smoking and depression: tests of causal linkages using a longitudinal birth cohort. British Journal of Psychiatry 2010;196:440‐6. [DOI] [PubMed] [Google Scholar]
Burt 1998
- Burt R, Peterson AV. Smoking cessation among high school seniors. Preventive Medicine 1998;27:319‐27. [DOI] [PubMed] [Google Scholar]
Campbell 2004a
- Campbell MK, Elbourne DR, Altman DG, for the CONSORT Group. CONSORT statement: extension to cluster randomised trials. BMJ 2004;328:702‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2004b
- Campbell MJ. Extending CONSORT to include cluster trials: welcome extension will help to understand trials better and reduce bias. BMJ 2004;328:654‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carson 2011
- Carson KV, Brinn MP, Labiszewski NA, Esterman AJ, Chang AB, Smith BJ. Community interventions for preventing smoking in young people. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD001291.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carson 2012
- Carson KV, Brinn MP, Labiszewski NA, Peters M, Chang AB, Veale A, et al. Interventions for tobacco use prevention in Indigenous youth. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD009325.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Carson 2017
- Carson KV, Ameer F, Sayehmiri K, Hnin K, Agteren JEM, Sayehmiri F, et al. Mass media interventions for preventing smoking in young people. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD001006.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chamberlain 2017
- Chamberlain C, O'Mara‐Eves A, Porter J, Coleman T, Perlen SM, Thomas J, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.CD001055.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2014
- Choi K, Forster JL. Beliefs and experimentation with electronic cigarettes: a prospective analysis among young adults. American Journal of Preventive Medicine 2014;46(2):175‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coleman 2015
- Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi‐Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD010078.pub2] [DOI] [PubMed] [Google Scholar]
Coppo 2012
- Coppo A, Galanti MR, Buscemi D, Giordano L, Faggiano F. School policies for preventing smoking among young people. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009990] [DOI] [PMC free article] [PubMed] [Google Scholar]
Corby 2000
- Corby EA, Roll JM, Ledgerwood DM, Schuster CR. Contingency management interventions for treating the substance abuse of adolescents: a feasibility study. Experimental & Clinical Psychopharmacology 2000;8:371‐6. [DOI] [PubMed] [Google Scholar]
CRUK 2017
- Cancer Research UK. Childhood Smoking Statistics. www.cancerresearchuk.org/health‐professional/cancer‐statistics/risk/childhood‐smoking (accessed 6 September 2017).
DiFranza 2008a
- DiFranza JR, Richmond JB. Let the children be heard. Paediatrics 2008;121(3):623‐4. [DOI] [PubMed] [Google Scholar]
DiFranza 2008b
- DiFranza JM. Hooked from the first cigarette. Scientific American 2008;298(5):82‐7. [DOI] [PubMed] [Google Scholar]
Eriksen 2015
- Eriksen M, Mackay J, Shluger N, Gomeshtapeh FI, Drope J. The Tobacco Atlas, 5th edition. Atlanta, Georgia: American Cancer Society, Inc, 2015. [Google Scholar]
Farsalinos 2016
- Farsalinos KE, Poulas K, Voudris V, Houezec J. Electronic cigarette use in the European Union: analysis of a representative sample of 27 460 Europeans from 28 countries. Addiction 2016;111:2032‐40. [DOI] [PubMed] [Google Scholar]
Frazer 2016
- Frazer K, Callinan JE, McHugh J, Baarsel S, Clarke A, Doherty K, et al. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD005992.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gervais 2007
- Gervais A, O'Loughlin J, Dugas E, Eisenberg M J, Wellman RJ, DiFranza JR. A systematic review of randomized controlled trials of youth smoking cessation interventions. Drogués Santé et société 2007;6(1 Suppl 2):ii1‐ii26. [Google Scholar]
Green 2004
- Green H, McGinnity A, Meltzer H, Ford T, Goodman R. Mental health of children and young people in Great Britain, 2004. Office for National Statistics 2004:http://www.esds.ac.uk/doc/5269/mrdoc/pdf/5269technicalreport.pdf. Accessed 31/1/13.
Grimshaw 2003
- Grimshaw G, Stanton A, Blackburn C, Andrews K, Grimshaw C, Podinovskaya Y, et al. Patterns of smoking, quit attempts and services for a cohort of 15 to 19 year olds. Child: Care, Health and Development 2003;29:457‐64. [DOI] [PubMed] [Google Scholar]
Hahn 2005
- Hahn S, Puffer S, Torgerson DJ, Watson J. Methodological bias in cluster randomised trials. BMC Medical Research Methodology 2005;5(10):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann‐Boyce 2016
- Hartmann‐Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD010216.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
HSCIC 2012
- Statistics on Smoking: England, 2012. Health and Social Care Information Centre 2012.
Hu 1998
- Hu SC, Lanese RR. The applicability of the theory of planned behavior to the intention to quit smoking across workplaces in southern Taiwan. Addictive Behaviors 1998;23:225‐37. [DOI] [PubMed] [Google Scholar]
Hughes 2003
- Hughes JR, Keeley JP, Niaura RS, Ossip‐Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine & Tobacco Research 2003;5(1):13‐25. [PubMed] [Google Scholar]
Hughes 2014
- Hughes JR, Stead LF, Hartmann‐Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD000031.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jarvis 2008
- Jarvis MJ, Fidler J, Mindell J, Feyerabend C, West R. Assessing smoking status in children, adolescents and adults: cotinine cut‐points revisited. Addiction 2008;103(9):1553‐61. [DOI] [PubMed] [Google Scholar]
Jha 2014
- Jha P, Peto R. Global Effects of Smoking, of Quitting, and of Taxing Tobacco. N Engl J Med 2014;370:60‐8. [DOI] [PubMed] [Google Scholar]
Kealey 2009b
- Kealey KA, Ludman EJ, Marek PM, Mann SL, Bricker JB, Peterson A. Design and Implementation of an Effective Telephone Counseling Intervention for Adolescent Smoking Cessation. Journal of the National Cancer Institute 2009;101:1393‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2016
- King JL, Pomeranz JL, Merten JW. A systematic review and meta‐evaluation of adolescent smoking cessation interventions that utilized nicotine replacement therapy. Addictive Behaviors 2016;52:39‐45. [DOI] [PubMed] [Google Scholar]
Lancaster 2017
- Lancaster T, Stead LM. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD001292.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2003
- McDonald P, Colwell B, Backinger C, Huston C, Maule C. Better practices for youth tobacco cessation. evidence of review panel. American Journal of Health Behavior 2003;27:S144‐58. [DOI] [PubMed] [Google Scholar]
Mermelstein 2002
- Mermelstein R, Colby SM, Patten C, Prokhorov A, Brown R, Myers M, et al. Methodological issues in measuring treatment outcomes in adolescent smoking cessation studies. Nicotine & Tobacco Research 2002;4(4):395‐403. [DOI] [PubMed] [Google Scholar]
Mermelstein 2003
- Mermelstein R. Teen smoking cessation. Tobacco Control 2003;12 (Suppl I):i25‐i34. [DOI] [PMC free article] [PubMed] [Google Scholar]
MMWR 2009
- Centers for Disease Control and Prevention. High School Students Who Tried to Quit Smoking Cigarettes — United States, 2007. MMWR, Morbidity and Mortality Weekly Report 2009;58(16):428‐31. [PubMed] [Google Scholar]
MMWR 2017
- Centers for Disease Control and Prevention. Current Tobacco Use Among Middle and High School Students—United States, 2011‐2016. Morbidity and Mortality Weekly Report 2017;66(23):597‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Loughlin 2003
- O'Loughlin J, DiFranza J, Tyndale RF, Meshefedjian G, McMillan‐Davey E, Clarke PBS, et al. Nicotine‐dependence symptoms are associated with smoking frequency in adolescents. American Journal of Preventive Medicine 2003;25:219‐25. [DOI] [PubMed] [Google Scholar]
ONS 2000
- Office for National Statistics. Drug use, smoking and drinking among young teenagers in 1999. www.statistics.gov.uk/pdfdir/drugs0500.pdf (accessed 4th March 2005) 2000; Vol. 189.
Patnode 2013
- Patnode CD, O'Connor E, Whitlock EP, Perdue LA, Soh C, Hollis J. Primary care‐relevant interventions for tobacco use prevention and cessation in children and adolescents: a systematic evidence review for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2013;158(4):253‐60. [DOI] [PubMed] [Google Scholar]
Prochaska 2000
- Prochaska JO. Stages of change model for smoking prevention and cessation in schools. BMJ 2000;320:447. [PMC free article] [PubMed] [Google Scholar]
Raw 1998
- Raw M, McNeill A, West R. Smoking cessation guidelines for health professionals. A guide to effective smoking cessation interventions for the health care system. Health Education Authority. Thorax 1998;53(Suppl 5 (1)):S1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Riemsma 2003
- Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al. Systematic review of the effectiveness of stage‐based interventions to promote smoking cessation. BMJ 2003;326(7400):1175‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubinstein 2007
- Rubinstein ML, Thompson PJ, Benowitz NL, Shiffmann S, Moscicki A‐B. Cotinine levels in relation to smoking behavior and addiction in young adolescent smokers. Nicotine & Tobacco Research 2007;9(1):129‐135. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI: 10.1136/bmj.c332] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheppard 2009
- Shepperd S, Lewin S, Straus S, Clarke M, Eccles MP, Fitzpatrick R, et al. Can we systematically review studies that evaluate complex interventions?. PLoS Med 2009;6(8):e1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
SRNT 2002
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research 2002;4:149‐59. [DOI] [PubMed] [Google Scholar]
Stanton 2001
- Stanton A, Grimshaw GM, Andrews K, Grimshaw CM, Robertson W, Blackburn C. Maybe not tomorrow but soon. Warwick: University of Warwick, 2001. [Google Scholar]
Stead 2005
- Stead LF, Lancaster T. Interventions for preventing tobacco sales to minors. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD001497.pub2] [DOI] [PubMed] [Google Scholar]
Stead 2012
- Stead LF, Perera R, Bullen C, Mant D, Hartmann‐Boyce J, Cahill K, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD000146.pub4] [DOI] [PubMed] [Google Scholar]
Stead 2017
- Stead LF, Carroll AJ, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD001007.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sussman 1998
- Sussman S, Dent CW, Nezami E, Stacy AW, Burton D, Flay BR. Reasons for quitting and smoking temptation among adolescent smokers: gender differences. Substance Use & Misuse 1998;33:2703‐20. [DOI] [PubMed] [Google Scholar]
Sussman 1999
- Sussman S, Lichtman K, Ritt A, Pallonen UE. Effects of thirty‐four adolescent tobacco use cessation and prevention trials on regular users of tobacco products. Substance Use and Misuse 1999;34:1469‐503. [DOI] [PubMed] [Google Scholar]
Sussman 2002
- Sussman S. Effects of sixty‐six adolescent tobacco use cessation trials and seventeen prospective studies of self‐initiated quitting. Tobacco Induced Diseases 2002;1(1):35‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sussman 2004
- Sussman S, McCuller WJ, Zheng H, Pfingston YM, Miyano J, Dent CW. Project EX: A program of empirical research on adolescent tobacco use cessation. Tobacco Induced Diseases 2004;2(3):119‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sussman 2006
- Sussman S, Sun P, Dent CW. A meta‐analysis of teen cigarette smoking cessation. Health Psychology 2006;25(2):549‐57. [DOI] [PubMed] [Google Scholar]
TAG 2000
- Tobacco Advisory Group. Nicotine addiction in Britain. London: Royal College of Physicians, 2000. [Google Scholar]
Thakur 2012
- Thakur GA, Sengupta SM, Grizenko N, Choudhry Z, Joober R. Family‐based association study of ADHD and genes increasing the risk for smoking behaviours. Archives of Disease in Childhood 2012;97:1027‐33. [DOI] [PubMed] [Google Scholar]
Thomas 2012
- Thomas RE, McLellan J, Perera R. School‐based programmes for preventing smoking. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD001293.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
USDHHS 2004
- US Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2004. [Google Scholar]
USDHHS 2012
- US Department of Health and Human Services. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2012. [Google Scholar]
Villanti 2010
- Villanti AC, McKay HS, Abrams DB, Holtgrave DR, Bowie JV. Smoking‐cessation interventions for U.S. young adults: a systematic review. American Journal of Preventive Medicine 2010;39(6):564‐74. [DOI] [PubMed] [Google Scholar]
West 2005
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100(3):299‐303. [DOI] [PubMed] [Google Scholar]
Whittaker 2016
- Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y. Mobile phone‐based interventions for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD006611.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Grimshaw 2006
- Grimshaw G, Stanton A. Tobacco cessation interventions for young people. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003289.pub4] [DOI] [PubMed] [Google Scholar]
Stanton 2013
- Stanton A, Grimshaw G. Tobacco cessation interventions for young people. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD003289.pub5] [DOI] [PubMed] [Google Scholar]


