Abstract
Background
Laparoscopic appendectomy is amongst the most common general surgical procedures performed in the developed world. Arguably, the most critical part of this procedure is effective closure of the appendix stump to prevent catastrophic intra‐abdominal complications from a faecal leak into the abdominal cavity. A variety of methods to close the appendix stump are used worldwide; these can be broadly divided into traditional ligatures (such as intracorporeal or extracorporeal ligatures or Roeder loops) and mechanical devices (such as stapling devices, clips, or electrothermal devices). However, the optimal method remains unclear.
Objectives
To compare all surgical techniques now used for appendix stump closure during laparoscopic appendectomy.
Search methods
In June 2017, we searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 6) in the Cochrane Library, MEDLINE Ovid (1946 to 14 June 2017), Embase Ovid (1974 to 14 June 2017), Science Citation Index ‐ Expanded (14 June 2017), China Biological Medicine Database (CBM), the World Health Organization International Trials Registry Platform search portal, ClinicalTrials.gov, Current Controlled Trials, the Chinese Clinical Trials Register, and the EU Clinical Trials Register (all in June 2017). We searched the reference lists of relevant publications as well as meeting abstracts and Conference Proceedings Citation Index to look for additional relevant clinical trials.
Selection criteria
We included all randomised controlled trials (RCTs) that compared mechanical appendix stump closure (stapler, clips, or electrothermal devices) versus ligation (Endoloop, Roeder loop, or intracorporeal knot techniques) for uncomplicated appendicitis.
Data collection and analysis
Two review authors identified trials for inclusion, collected data, and assessed risk of bias independently. We performed the meta‐analysis using Review Manager 5. We calculated the odds ratio (OR) for dichotomous outcomes and the mean difference (MD) for continuous outcomes, with 95% confidence intervals (CIs).
Main results
We included eight randomised studies encompassing 850 participants. Five studies compared titanium clips versus ligature, two studies compared an endoscopic stapler device versus ligature, and one study compared an endoscopic stapler device, titanium clips, and ligature. In our analyses of primary outcomes, we found no differences in total complications (OR 0.97, 95% CI 0.27 to 3.50, 8 RCTs, very low‐quality evidence), intraoperative complications (OR 0.93, 95% CI 0.34 to 2.55, 8 RCTs, very low‐quality evidence), or postoperative complications (OR 0.80, 95% CI 0.21 to 3.13, 8 RCTs, very low‐quality evidence) between ligature and all types of mechanical devices. However, our analyses of secondary outcomes revealed that use of mechanical devices saved approximately nine minutes of total operating time when compared with use of a ligature (mean difference (MD) ‐9.04 minutes, 95% CI ‐12.97 to ‐5.11 minutes, 8 RCTs, very low‐quality evidence). However, this finding did not translate into a clinically or statistically significant reduction in inpatient hospital stay (MD 0.02 days, 95% CI ‐0.12 to 0.17 days, 8 RCTs, very low‐quality evidence). Available information was insufficient for reliable comparison of total hospital costs and postoperative pain/quality of life between the two approaches. Overall, evidence across all analyses was of very low quality, with substantial potential for confounding factors. Given the limitations of all studies in terms of bias and the low quality of available evidence, a clear conclusion regarding superiority of any one particular type of mechanical device over another is not possible.
Authors' conclusions
Evidence is insufficient at present to advocate omission of conventional ligature‐based appendix stump closure in favour of any single type of mechanical device over another in uncomplicated appendicitis.
Plain language summary
Determining the optimal method of securely closing the base of the appendix during keyhole surgery after removal of the inflamed appendix
Background
Appendicitis is an Inflammation of the appendix. The conventional treatment for this condition involves an operation to remove the appendix, called an appendectomy. In recent years, this operation has been increasingly performed as keyhole surgery ‐ laparoscopic appendectomy. For removal of the appendix during laparoscopic appendectomy, the best method of closing the remaining appendix stump to avoid leakage of bowel contents is unclear. Traditional approaches have involved ligatures and knots. However, in recent years, some surgeons have elected to use automated mechanical devices rather than ligatures, and it is unclear whether these devices reduce complications during laparoscopic appendectomy when compared with ligatures.
Study characteristics
We searched for all relevant randomised controlled trials up to 14 June 2017. This systematic review included eight randomised controlled trials involving a total of 850 participants. All trials compared mechanical devices versus ligatures for appendix stump closure. Five of the eight trials compared use of clips versus ligature, two trials compared an automated stapler versus ligature, and one trial compared all three methods.
Key results
Use of mechanical devices to close the appendix stump during laparoscopic appendectomy did not make a significant difference in the rate of overall complications when compared with use of a ligature, or in the rate of complications that happened during or after the appendectomy procedure. However, mechanical devices did make the operation nine minutes quicker when compared with ligatures. Mechanical devices did not make a substantial difference in overall hospital stay. We did not have enough information to reliably evaluate hospital costs, pain, or quality of life for either of these comparisons. As a result, we have not found enough evidence at present that would lead us to strongly recommend any particular method over another. More research should be undertaken to better compare available newer methods.
Quality of the evidence
The evidence used to derive our conclusions was generally of low quality. The studies we included for each analysis were vulnerable to different types of bias and contained inconsistencies and imprecision in their results due to small numbers of participants and events in each included study arm. It is likely that future research will substantially change our conclusions; further studies in this field are needed.
Summary of findings
Summary of findings for the main comparison. Mechanical devices versus ligature for appendix stump closure during laparoscopic appendectomy.
| Mechanical devices vs ligatures for appendix stump closure during laparoscopic appendectomy | |||||
| Patient or population: patients undergoing appendix stump closure during laparoscopic appendectomy Setting: hospital Intervention: mechanical devices (endoscopic stapler/clips) Comparison: ligature (intra/extracorporeal knot/Endoloop) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with ligatures | Risk with mechanical devices | ||||
| Total complications | 205 per 1000 | 169 per 1000 (119 to 225) | OR 0.97 (0.27 to 3.50) |
850 (8 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
| Intraoperative complications | 76 per 1000 | 63 per 1000 (36 to 108) | OR 0.93 (0.34 to 2.55) |
850 (8 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
| Postoperative complications | 129 per 1000 | 109 per 1000 (71 to 154) | OR 0.80 (0.21 to 3.13) |
850 (8 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
| Postoperative superficial infections | 26 per 1000 | 13 per 1000 (5 to 33) | OR 0.58 (0.18 to 1.93) | 850 (8 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
| Postoperative ileus | 41 per 1000 | 20 per 1000 (8 to 46) | OR 0.47 (0.19 to 1.18) | 850 (8 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
| Postoperative deep infections | 14 per 1000 | 12 per 1000 (4 to 34) | OR 0.79 (0.24 to 2.53) | 850 (8 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
| Operative time (minutes) | Mean operative time was 40.6 minutes. |
Mean operative time (minutes) in the intervention group was 9.04 minutes shorter (12.97 minutes shorter to 5.11 minutes shorter). |
‐ | 850 (8 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
| Hospital stay (days) | Mean hospital stay was 1.4 days. |
Mean hospital stay in the intervention group was 0.02 days longer (0.12 days shorter to 0.17 days longer). |
‐ | 850 (8 RCTs) | ⊕⊝⊝⊝ Very low1,2,3 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial. | |||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level for inconsistency (substantial heterogeneity).
bDowngraded one level for high risk of bias.
cDowngraded one level for imprecision (all included studies had few participants and events and thus wide confidence intervals, limiting the precision of estimates).
Summary of findings 2. Endoscopic stapler versus ligature for appendix stump closure during laparoscopic appendectomy.
| Endoscopic stapler vs ligature for appendix stump closure during laparoscopic appendectomy | |||||
|
Patient or population: patients undergoing appendix stump closure during laparoscopic appendectomy Settings: hospital Intervention: endoscopic stapler Comparison: ligature | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with ligature | Risk with endoscopic stapler | ||||
| Total complications | 421 per 1000 | 198 per 1000 (35 to 637) | OR 0.34 (0.05 to 2.41) | 327 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
| Intraoperative complications | 182 per 1000 | 191 per 1000 (37 to 599) | OR 1.06 (0.17 to 6.70) | 327 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
| Postoperative complications | 239 per 1000 | 250 per 1000 (51 to 678) | OR 0.20 (0.09 to 0.44) | 327 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
| Postoperative superficial infections | 44 per 1000 | 47 per 1000 (8 to 236) | OR 0.10 (0.01 to 0.84) | 327 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
| Postoperative ileus | 88 per 1000 | 93 per 1000 (16 to 393) | OR 0.37 (0.13 to 1.07) | 327 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
| Postoperative deep infections | 31 per 1000 | 33 per 1000 (5 to 179) | OR 0.45 (0.10 to 2.08) | 327 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
| Operative time (minutes) | Mean operative time was 40.6 minutes. | Mean operative time in the intervention group was 8.52 minutes lower (15.64 minutes shorter to 1.39 minutes shorter). |
327 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | |
| Hospital stay (days) | Mean hospital stay was 1.9 days. |
Mean hospital stay in the intervention group was 0.02 days longer (0.38 days shorter to 0.34 days longer). |
327 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded one level for inconsistency (substantial heterogeneity).
bDowngraded one level for high risk of bias.
cDowngraded one level for imprecision (all included studies had few participants and events and thus wide confidence intervals, limiting the precision of estimates).
Summary of findings 3. Clips versus ligature for appendix stump closure during laparoscopic appendectomy.
| Clips vs ligatures for appendix stump closure during laparoscopic appendectomy | |||||
|
Patient or population: patients undergoing appendix stump closure during laparoscopic appendectomy Settings: hospital Intervention: clips Comparison: ligature | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with ligature | Risk with clips | ||||
| Total complications | 17 per 1000 | 18 per 1000 (3 to 105) | OR 2.03 (0.71 to 5.84) |
553 (6 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
| Intraoperative complications | 21 per 1000 | 22 per 1000 (4 to 124) | OR 1.74 (0.33 to 9.04) |
553 (6 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
| Postoperative complications | 17 per 1000 | 18 per 1000 (3 to 105) | OR 1.88 (0.63 to 5.64) |
553 (6 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
| Postoperative superficial infections | 14 per 1000 | 15 per 1000 (2 to 86) | OR 1.25 (0.32 to 4.90) |
553 (6 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
| Postoperative ileus | 10 per 1000 | 11 per 1000 (2 to 65) | OR 0.92 (0.15 to 5.64) |
553 (6 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
| Postoperative deep infections | 3 per 1000 | 4 per 1000 (1 to 23) | OR 1.75 (0.28 to 10.93) |
553 (6 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
| Operative time (minutes) | Mean operative time was 40.0 minutes. |
Mean operative time in the intervention group was 8.14 minutes shorter (11.73 minutes shorter to 4.55 minutes shorter). |
553 (6 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | |
| Hospital stay (days) | Mean hospital stay was 1.5 days. |
Mean hospital stay in the intervention group was 0.03 days shorter (0.16 days shorter to 0.11 days longer). |
553 (6 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded one level for inconsistency (substantial heterogeneity).
bDowngraded one level for high risk of bias.
cDowngraded one level for imprecision (all included studies had few participants and events and thus wide confidence intervals, limiting the precision of estimates).
Summary of findings 4. Endoscopic stapler versus clips for appendix stump closure during laparoscopic appendectomy.
| Endoscopic stapler vs clips for appendix stump closure during laparoscopic appendectomy | |||||
|
Patient or population: patients undergoing appendix stump closure during laparoscopic appendectomy Settings: hospital Intervention: endoscopic stapler Comparison: clips | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with clips | Risk with endoscopic stapler | ||||
| Total complications | 67 per 1000 | 70 per 1000 (12 to 324) | OR 1.00 (0.13 to 7.60) |
60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c |
| Intraoperative complications | 67 per 1000 | 70 per 1000 (12 to 324) | OR 1.00 (0.13 to 7.60) |
[60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c |
| Postoperative complications | 0 events in both treatment arms | NE | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | |
| Postoperative superficial infections | 0 events in both treatment arms | NE | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | |
| Postoperative ileus | 0 events in both treatment arms | NE | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | |
| Postoperative deep infections | 0 events in both treatment arms | NE | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | |
| Operative time (minutes) | Mean operative time was 39.4 minutes. |
Mean operative time in the intervention group was 3.46 minutes shorter (6.94 minutes shorter to 0.02 minutes longer). |
60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | |
| Hospital stay (days) | Mean hospital stay was 2.0 days. |
Mean hospital stay in the intervention group was 0.04 days shorter (0.28 days shorter to 0.20 days longer). |
60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NE: not estimable; OR: odds ratio; RCT: randomised controlled trial. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded one level for single study with limited sample size.
bDowngraded one level for high risk of bias.
cDowngraded one level for imprecision (the sole included studies had few participants and therefore few events, resulting in wide confidence intervals, which limited the precision of estimates).
Background
Appendicitis refers to inflammation of the appendix. Appendectomy (surgical removal of the appendix) is performed as an emergency procedure for treatment of individuals with acute appendicitis (Andersen 2005).
Description of the condition
Acute appendicitis, first described by Fitz in 1886, is the most common cause of acute abdominal pain (Andersen 2005; Rehman 2011; Wilms 2011). The overall incidence of acute appendicitis varies between 76 and 227 cases per 100,000 population per year (Addiss 1990;Andreu‐Ballester 2009;Buckius 2011; Lee 2010; Pieper 1982). The overall lifetime risk for acute appendicitis has been reported to be between 6% and 16% (Addiss 1990;Lee 2010). This condition affects all age groups, with the highest incidence in the second decade (Addiss 1990; Wilms 2011).
The cause of acute appendicitis is an issue of considerable debate (Andersen 2005). Acute appendicitis might be associated with obstruction of the appendix lumen (the inside space of an appendix), which could result in increased intraluminal pressure with transmural tissue necrosis (Andersen 2005). Tissue necrosis is followed by bacterial invasion, leading to inflammation of the appendix (Andersen 2005).
Description of the intervention
Patients with acute appendicitis usually need an appendectomy (irrespective of open or laparoscopic approaches) to relieve symptoms and avoid complications. Laparoscopic appendectomy was first described in 1983 (Schier 1998). Since then, the procedure has undergone some modifications (from four ports to three, then to two). In 1992, Pelosi reported the single‐incision laparoscopic operation, which resulted in less superficial trauma whilst providing a safe operative approach (Pelosi 1992). Both laparoscopic appendectomy and open appendectomy are well accepted by surgeons, and clinical data have shown distinct relative advantages of laparoscopic appendectomy, albeit small in absolute terms. One of the possible drawbacks of the laparoscopic technique is the slightly higher intra‐abdominal abscess rate (Sauerland 2010). In this context, it has been suggested that appendix stump closure techniques play a key role in preventing infectious complications after appendectomy (Krisher 2001).
How the intervention might work
The traditional technique for securing the appendix stump during open appendectomy involved transfixing the appendix base, then applying a purse suture circumferentially around the appendix base to invert it into the caecum. However, this suture is difficult to apply during laparoscopic appendectomy (Houben 1998). Therefore, two other techniques have been introduced for laparoscopic appendectomy. The first technique involves the Roeder loop ‐ a pre‐tied sliding knot that was developed by Roeder (a German ear, nose, and throat (ENT) surgeon) for tonsillectomy (Röder 1918). After one or more of these loops is applied to the base of the appendix, the appendix can be excised (Beldi 2006; Shimi 1994). The second technique utilises a mechanical device such as a gastrointestinal anastomosis (GIA) stapler (Daniell 1991; Klaiber 1994), titanium clips (Akbiyik 2011; Ates 2012; Delibegovic 2012; Gonenc 2012), or an electrothermal bipolar tissue sealing device (Sucullu 2009).
The GIA stapler applies two rows of small staples to hold tissue edges together, so that automatic dissection can be done between the two rows (Beldi 2006). This device can be loaded with different cartridges of staples, thus allowing its application to different types of tissue, such as the appendix base and the mesoappendix with its artery. Use of different types of titanium clips for laparoscopic appendectomy has been described more recently (Hanssen 2007; Partecke 2010; Delibegovic 2009) and offers the advantages of easy application and low costs. The LigaSure Vessel Sealing System (Valleylab, Boulder, Colorado, USA) (Yang 2015) avoids placement of prosthetic clips via an electrothermal bipolar tissue sealing system.
Why it is important to do this review
Traditional ligatures (such as intracorporeal knots or Roeder loops) and mechanical devices (such as GIA stapling devices, clips, or electrothermal devices) are widely used during laparoscopic appendectomies worldwide. It is currently believed that the main difference between the two approaches represents a trade‐off between cost and safety. However, this concept is not evidence‐based; although mechanical devices are more expensive to use, it remains unclear whether they truly provide safer closure of the appendix stump than their cheaper ligature counterparts. Certainly, the degree of local inflammation and the expertise of the operating surgeon play a decisive role in the selection of surgical technique. However, to date, no Cochrane review has determined the preferred technique for securing the appendix stump in laparoscopic appendectomy.
Objectives
To compare all surgical techniques now used for appendix stump closure during laparoscopic appendectomy.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) regardless of publication status and language, including cluster‐randomised studies. We excluded quasi‐randomised trials (in which allocation was done on the basis of a pseudo‐random sequence, e.g. odd/even hospital number, date of birth, alternation) and non‐randomised studies (Higgins 2011a). We included studies reported solely in abstract form if full study data were available.
Types of participants
We included patients (irrespective of age, sex, or race) who were to undergo laparoscopic appendectomy.
Types of interventions
We examined the following comparisons.
Mechanical appendix stump closure (with stapler, clips, or LigaSure device) versus ligation (with Endoloop, Roeder loop, or intracorporeal knot).
Stapler versus ligation (with Endoloop, Roeder loop, or intracorporeal knot).
Clips versus ligation (with Endoloop, Roeder loop, or intracorporeal knot).
Stapler versus clips.
One versus two ligatures (with Endoloop, Roeder loop, or intracorporeal knot).
LigaSure sealing device versus other mechanical devices (with stapler or clips) or versus ligation (with Endoloop, Roeder loop, or intracorporeal knot).
Types of outcome measures
Primary outcome measures focused on complications between different interventions, whereas secondary outcome measures examined health and health economic implications of the different interventions assessed.
Primary outcomes
Total complications (defined as all complications, i.e. sum of intraoperative and postoperative complications)
-
Intraoperative complications:
Intraoperative bleeding
Intraoperative rupture of appendix
Intraoperative organ injury/faecal soiling
Access‐related visceral injury
-
Postoperative complications:
Surgical site infection (superficial)
Deep infection
Postoperative bleeding
Paralytic ileus
Purulent peritonitis
Secondary outcomes
Operative time (minutes)
Hospital stay (days)
Hospital costs (operation, direct and indirect)
Pain/quality of life, measured by a validated instrument (i.e. the visual analogue scale (VAS) scale)
Search methods for identification of studies
Marija Barbateskovic (Information Specialist at the Cochrane Colorectal Cancer Group) helped to design the search strategy, and Nia Roberts (Outreach Librarian at the Bodleian Library, University of Oxford) conducted the search. Sys Johnsen (Information Specialist at the Cochrane Colorectal Cancer Group) subsequently updated the search.
Electronic searches
We searched the following electronic databases with no language or date of publication restrictions.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 6) in the Cochrane Library (Appendix 1).
MEDLINE Ovid (1950 to 14 June 2017) (Appendix 2).
Embase Ovid (1974 to 14 June 2017) (Appendix 3).
Science Citation Index ‐ Expanded (1900 to 14 June 2017) (Appendix 4).
China Biological Medicine Database (CBM) (14 June 2017).
We searched the following databases, including ongoing trials, on 14 June 2017.
World Health Organization International Trials Registry Platform search portal (http://apps.who.int/trialsearch/) (from 2007).
ClinicalTrials.gov (http://www.clinicaltrials.gov/) (from 2000).
Current Controlled Trials (http://www.controlled‐trials.com/) (from 2000).
Chinese Clinical Trial Register (http://www.chictr.org/) (from 2005).
EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/) (from 2004).
Searching other resources
We also searched reference lists of relevant publications and meeting abstracts (via http://www.eaes‐eur.org/, http://www.sages.org/ and Conference Proceedings Citation Index) to explore further relevant clinical trials. These searches were last done on 14 June 2017. We planned to contact the authors of RCTs included in the review to ask for more information, if necessary.
Data collection and analysis
We conducted the systematic review according to the recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
After completing all searches, we merged search results using the software package Endnote X5 (reference management software) and removed duplicate records of the same report. Two independent review authors (EC and FKL) scanned the title and abstract of every record identified by the search for inclusion. We retrieved full‐text versions for further assessment if inclusion criteria were unclear from the abstract. We detected duplicate publication by identifying common authors, centres, details of interventions, numbers of participants, and baseline data (Higgins 2011b). If necessary, we contacted the authors of RCTs to confirm whether trial results had been duplicated. We excluded papers not meeting the inclusion criteria and listed the reasons for exclusion under Characteristics of excluded studies. A third review author (GSM) resolved disagreements between the two authors through discussion and, if required, by consultation with authors of the paper.
Data extraction and management
Two review authors (EC and FKL) independently extracted and entered data onto an electronic data collection form (Figure 1). Two other review authors (MS and GSM) independently checked the data for accuracy and entered these data into Review Manager 5.3 (RevMan 2014). An independent review author (JHBS) compared data from the collection forms versus data entered into RevMan to prevent translational errors. From each study, we collected information on setting, intervention type, number of participants within each intervention arm, intraoperative findings, total number of complications, numbers of participants with specific types of complications, duration of surgery, length of hospital stay, number of participants re‐admitted, number of reoperations, pain and quality of life definitions and scores, and total cost per procedure.
1.
Data collection form (Microsoft Word).
Assessment of risk of bias in included studies
Two review authors (MS and GSM) independently assessed and presented 'Risk of bias' tables. For each trial, we judged each domain as having low, high, or unclear risk of bias according to the Cochrane 'Risk of bias' tool (Appendix 5) (Higgins 2011c). We resolved disagreements at this stage by discussion and by referral to a third review author (JHBS) for adjudication. We defined overall low risk of bias as low risk of bias in randomisation sequence generation, allocation concealment, blinding of outcome assessment, and attrition bias with no high‐risk elements, in accordance with guidance set out by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). We presented results on risk of bias in two figures (a 'Risk of bias graph' figure and a 'Risk of bias summary' figure) generated via Review Manager 5.3 (RevMan 2014). We evaluated the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Selective outcome reporting.
Incomplete outcome data.
Other bias.
Measures of treatment effect
We performed meta‐analyses using the software package Review Manager 5 (RevMan 2014). For dichotomous outcomes, we calculated the Peto odds ratio (Peto OR) with 95% confidence intervals (CIs) (Deeks 2011). For continuous outcomes, we calculated mean differences (MDs) with 95% CIs (Deeks 2011). For continuous outcomes based on different measurement scales in different randomised clinical trials, we calculated standardised mean differences (SMDs) with 95% CIs (Deeks 2011) for comparison. When means were used, we included their standard deviations. When standard deviations were not reported, we imputed them from the means of other studies in the same analysis, as described in Section 16.1.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used weighted means when multiple averages needed to be combined.
Unit of analysis issues
The unit of analysis was each individual participant. We identified no cluster‐randomised trials, but, should we do so in future updates, we will analyse data using the generic inverse variance method, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). For trials with multiple intervention groups, we combined groups to create a single pair‐wise comparison (Higgins 2011a).
Dealing with missing data
We contacted the original investigators to request further information in the case of missing data. If we received no reply, we performed analyses on an intention‐to‐treat (ITT) basis, if applicable (Newell 1992). Otherwise, we used only available published data for the analysis.
Assessment of heterogeneity
We used a three‐step approach to assess heterogeneity. First, we decided whether the included studies were too heterogeneous clinically for inclusion in a meta‐analysis, in which case we planned to write a narrative review. We assessed clinical heterogeneity according to participant characteristics and interventions. Second, assuming clinical homogeneity, we used the I2 statistic to measure the quantity of statistical heterogeneity and followed the recommendations for interpretation set out in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011): 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% may represent considerable heterogeneity. Third, if substantial heterogeneity was present (I2 > 50%), we interpreted results cautiously and further investigated reasons for heterogeneity.
Assessment of reporting biases
We planned to use visual asymmetry from funnel plots combined with Egger's regression method (Egger 1997) to assess the presence of reporting biases, if we identified more than 10 studies (Sterne 2011). This review included only eight studies, thus we did not perform and present funnel plots to assess possible reporting bias.
Data synthesis
We performed meta‐analyses using Review Manager 5 software provided by Cochrane (RevMan 2014). Following evaluation of the characteristics of eligible studies, we assumed that the true effect size might differ from study to study owing to intrinsic differences between trial populations and settings in which the included studies were conducted. As a result, the random‐effects model best met our assumption, and we have reported results using this model throughout our review. We used the Mantel‐Haenszel method for dichotomous outcomes and the inverse variance model for continuous outcomes.
Subgroup analysis and investigation of heterogeneity
If substantial heterogeneity (I2 > 50%) was present, we first checked that data had been entered correctly into Review Manager,then planned to perform the following subgroup analyses. However, owing to insufficient available data from included studies, we did not perform these analyses.
Trials with low risk of bias versus trials with high risk of bias.
Adults versus children.
Complicated (gangrenous or perforated) versus uncomplicated appendicitis.
Single incision versus non‐single incision.
Male versus female.
Sensitivity analysis
We performed the following sensitivity analyses to investigate whether conclusions were robust to decisions made during the review process.
Changing statistics among risk ratios (RRs), risk differences (RDs), and odds ratios (ORs) for dichotomous outcomes.
Changing statistics between mean differences (MDs) and standardised mean differences (SMDs) for continuous outcomes.
Excluding trials at high risk of bias.
Evaluating the impact of using a fixed‐effects model.
If the results did not change, we considered them to have low sensitivity. If the results changed, we considered them to have high sensitivity.
'Summary of findings'
We assessed the quality of evidence of each outcome for all comparisons and for any subgroup analysis and sensitivity analysis by using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach in 'Summary of findings' tables (Schünemann 2011).
The GRADE system classifies the quality of evidence according to one of four grades.
High: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate: Further research is likely to have an impact on our confidence in the estimate of effect and may change the estimate.
Low: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: Any estimate of effect is very uncertain.
We could downgrade the quality of evidence by one (serious concern) or two (very serious concerns) levels for the following reasons: study limitations (risk of bias), inconsistency of results (heterogeneity), indirectness of evidence (indirect population, intervention, control), imprecision (wide confidence intervals), and publication bias.
Results
Description of studies
We have presented search results and a flow chart of studies in Figure 2.
2.
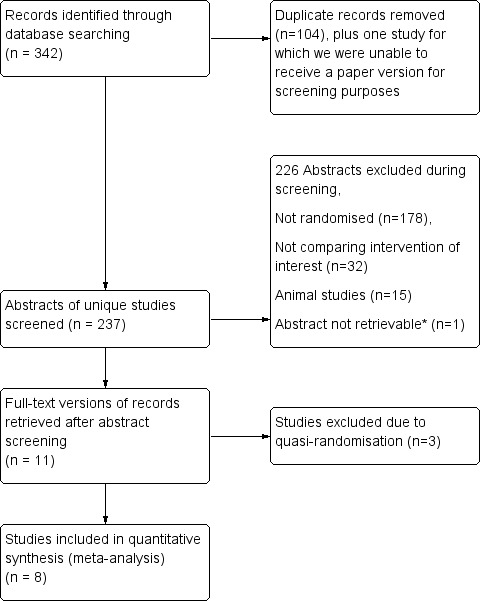
*Lange 1993 was not retrievable following a worldwide search because the journal was published and is going out of print (see Results section).
Results of the search
We identified 342 studies from a search of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE Ovid, Embase Ovid, and Science Citation Index ‐ Expanded. Removal of duplicates yielded 238 studies. One of these studies was not available for screening, as the journal it was published in no longer existed and an online archive could not be located (Lange 1993). A worldwide search commissioned by the Bodleian Library, University of Oxford, could not locate a printed version of this paper for our screening process. After excluding studies from the remaining 237 that did not meet our inclusion criteria, we short‐listed 11 studies for full‐text review and data extraction (Akbiyik 2011; Ates 2012; Beldi 2004; Colak 2013; Delibegovic 2012; Gonenc 2012; Nadeem 2015; Ortega 1995; Shalaby 2001; Sucullu 2009; Yang 2014). Of these, we subsequently excluded three studies from the quantitative meta‐analysis following risk of bias assessment owing to quasi‐randomisation that resulted in an unacceptably high risk of randomisation bias (Ates 2012; Beldi 2004; Sucullu 2009). This resulted in inclusion of eight studies in the final meta‐analysis. All studies were published in the English language, except for Yang 2014, which was published in Chinese and translated by review authors.
Upon re‐running the searches in June 2017, we identified two abstracts (Lv 2016; Sadat‐Safavi 2016). These two abstracts are too recent to have been classified by the publication date of this meta‐analysis, thus we have listed them under Studies awaiting classification and will consider them for inclusion in a future update of this review.
Included studies
Our review included eight randomised controlled trials, with a total of 850 participants (Akbiyik 2011; Colak 2013; Delibegovic 2012; Gonenc 2012; Nadeem 2015; Ortega 1995; Shalaby 2001; Yang 2014). These studies span two decades from Ortega 1995 to Nadeem 2015. One study was reported from the USA (Ortega 1995), three from Turkey (Akbiyik 2011; Colak 2013; Gonenc 2012;), one from Bosnia and Herzegovina (Delibegovic 2012), one from China (Yang 2014), one from Pakistan (Nadeem 2015), and one from Egypt and Saudi Arabia (Shalaby 2001). Six studies compared clips versus a ligatures (Akbiyik 2011; Colak 2013; Delibegovic 2012; Gonenc 2012; Nadeem 2015; Yang 2014), two compared stapler versus ligature (Ortega 1995; Shalaby 2001), and one compared stapler versus clip use (Delibegovic 2012). We have summarised these studies in the Characteristics of included studies tables. No studies were eligible for inclusion in comparisons that examined the question of one ligature versus two ligatures, or LigaSure sealing device versus other mechanical devices (with stapler or clips) or versus ligation (with Endoloop, Roeder loop, or intracorporeal knot).
Excluded studies
We excluded three trials from the quantitative meta‐analysis following risk of bias assessment, as they used quasi‐randomisation, resulting in an unacceptably high risk of randomisation bias (Ates 2012; Beldi 2004; Sucullu 2009). Of these three quasi‐randomised trials, one study compared titanium clips versus a ligature (Endoloop/intracorporeal knot) (Ates 2012), one compared the LigaSure sealing device versus titanium clips (Sucullu 2009), and one compared one ligature (with Endoloop) versus two ligatures (with Endoloops) (Beldi 2004).
Risk of bias in included studies
We have presented results of our risk of bias assessment in Figure 3 and Figure 4. We judged the overall risk of bias for all trials across domains as high.
3.
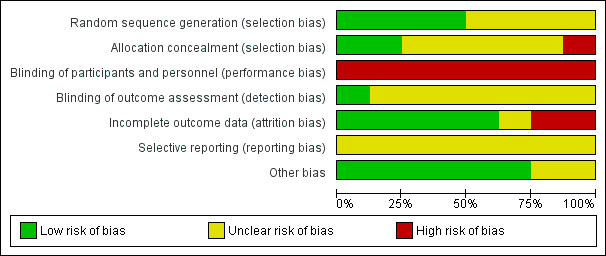
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
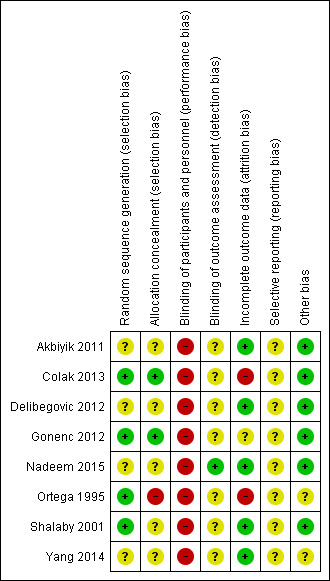
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Details of random sequence generation were unavailable for four of the included trials (Akbiyik 2011; Delibegovic 2012; Nadeem 2015; Yang 2014), in which the only indication of randomisation was seen in variations of the statement, “patients randomly allocated". On this basis, we classified risk of selection bias in these trials as unclear. Trials for which details were available achieved randomisation by using a “computer‐generated randomisation schedule” (Colak 2013), “by the lottery method” (Gonenc 2012), through a “computer‐generated random numbers table” (Ortega 1995), or by using a “table of random numbers” (Shalaby 2001). Colak 2013 recruited a total of 60 participants and excluded four participants postoperatively owing to conversion to open appendectomy, along with three participants owing to loss of follow‐up. Moreover, Ortega 1995 stated that "endoscopic staplers were temporarily unavailable at one point during the study and five patients randomised to endoscopic linear stapler underwent appendectomies with pre‐tied loops" and were subsequently re‐allocated to corresponding groups. We interpreted this as high risk for attrition bias as well as high risk for allocation concealment bias, as it was likely to influence effect estimates.
Blinding
Blinding of performing surgeons to the technique is impossible with this type of intervention, and personnel are likely to be aware of study group allocation from intraoperative and postoperative records. This lack of personnel blinding is an inherent drawback of such surgical intervention trials. We classified all eight trials (Akbiyik 2011; Colak 2013; Delibegovic 2012; Gonenc 2012; Nadeem 2015; Ortega 1995; Shalaby 2001; Yang 2014) as showing high risk of performance bias. Ortega 1995 stated that “data collection was performed in a prospective fashion using two standardised data sheets", and it was unclear whether these data sheets were intended for different arms of the study, or whether they were trial arm‐specific; if so, this would imply complete lack of postoperative blinding of the healthcare team (even those not directly involved in the operation). The remaining studies made no mention of this (Akbiyik 2011; Colak 2013; Delibegovic 2012; Gonenc 2012; Shalaby 2001; Yang 2014).
Nadeem 2015 stated that this was a single‐blinded trial and made efforts to minimise detection bias by ensuring that investigators who collected data were "at the same time blinded for the type of procedure done". However, it was unclear to what extent the operating team could influence the postoperative course outside the remit of data collection. In all studies except Nadeem 2015, it was also unclear whether participants were aware of the method used because no specific reference was made to methods of participant blinding. Studies that described procedures performed by residents had the potential for performance bias (Gonenc 2012; Ortega 1995). Ortega 1995 (n = 253) and Gonenc 2012 (n = 107) contributed some of the largest participant populations to our analysis and were conducted entirely by residents, with attending surgeons experienced in laparoscopic and open surgical techniques present (Ortega 1995). Attendings presumably were holding the camera during these laparoscopic procedures. Trial authors did not refer to the variation in seniority amongst operating residents (although Gonenc 2012 stated that investigators were at least within their second year of residency). The assumption is that all residents were equally skilled and fluent in both randomised methods of appendix stump closure; however, because trial authors did not explicitly state that all residents were trained to equal proficiency in both approaches, we recorded the potential for performance bias as 'unclear'.
Incomplete outcome data
We classified five trials as having low risk of attrition bias, as they were free of postrandomisation exclusions (Akbiyik 2011; Delibegovic 2012; Nadeem 2015; Shalaby 2001; Yang 2014). We classified two trials as having high risk of attrition bias (Colak 2013; Ortega 1995). Colak 2013 recruited a total of 60 participants and excluded four participants postoperatively owing to conversion to open appendectomy and three participants because of loss to follow‐up. Moreover, Ortega 1995 stated that "endoscopic staplers were temporarily unavailable at one point during the study and five patients randomised to endoscopic linear stapler underwent appendectomies with pre‐tied loops" and were subsequently re‐allocated to corresponding groups. We interpreted this as high risk for attrition bias as well as high risk for allocation concealment bias and believed it was likely to influence effect estimates. We classified one trial as having unclear risk of attrition bias (Gonenc 2012). Gonenc 2012 excluded participants with an intraoperative diagnosis of complicated appendicitis and those who had undergone an open appendectomy. However, trial authors provided no information on the number of participants initially recruited to the study before randomisation and how many of these were subsequently excluded, if any. As a result, the level of attrition bias in this study was not clear.
Selective reporting
Similar to the ubiquitous problem of blinding amongst our included studies, we could not identify an a priori publication of intended outcomes from either a published trial protocol or trial registration for any of the studies included in this review. As a result, we considered all studies as having 'unknown' risk of selective reporting bias. In addition, Akbiyik 2011 had a follow‐up period that varied between one week and one year, and no uniform long‐term outcome data were made available for comparison between groups, as this study published only limited data from four‐month follow‐up.
Other potential sources of bias
Postoperative pain constituted one of the primary outcomes in one of our included studies (Ortega 1995), which suffered a combination of attrition and reporting biases because amongst 253 participants randomised at 10 different centres, the comparison of postoperative pain between study arms was undertaken only in a subpopulation of 134 participants from a single centre. It is not clear to what degree participant characteristics at this single centre were similar to or different from those noted in the rest of the study population. We therefore classified Ortega 1995 as having unclear risk of other bias. We classified Yang 2014 as having unclear risk of other potential sources of bias, as only limited methodological information was provided in its published manuscript.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
We present the following results for our a priori primary and secondary outcomes for outlined comparisons.
1. Mechanical appendix stump closure (with stapler, clips, or LigaSure device) versus ligation (with Endoloop, Roeder loop, or intracorporeal knot)
1.1 Primary outcomes
1.1.1 Total complications
The composite comparison of 850 participants from eight randomised studies (Akbiyik 2011; Colak 2013; Delibegovic 2012; Gonenc 2012; Nadeem 2015; Ortega 1995; Shalaby 2001; Yang 2014) of all types of mechanical devices versus ligature (or Endoloop, Roeder loop, or intracorporeal knot) for appendix stump closure during laparoscopic appendectomy showed no significant differences in overall complications (odds ratio (OR) 0.97, 95% confidence interval (CI) 0.27 to 3.50) (Analysis 1.1). However, it should be noted that the wide 95% confidence intervals in this analysis might actually represent imprecision of the estimate, rather than no true difference. This analysis was subject to a high degree of heterogeneity (I2 = 84%); therefore GRADE should be downgraded further by one level to very low quality (i.e. owing to inconsistency), largely because of the addition of the two most recent trials (Nadeem 2015; Yang 2014).
1.1. Analysis.
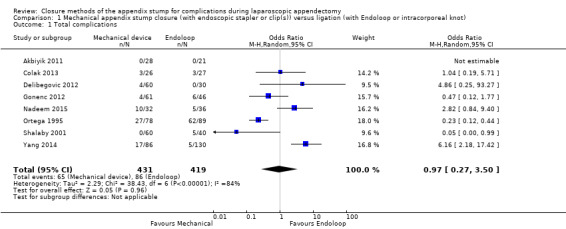
Comparison 1 Mechanical appendix stump closure (with endoscopic stapler or clip(s)) versus ligation (with Endoloop or intracorporeal knot), Outcome 1 Total complications.
1.1.2 Intraoperative complications
Data show no differences in intraoperative complications from the use of any mechanical device when compared with ligature (OR 0.93, 95% CI 0.34 to 2.55; I2 = 25%) (Analysis 1.2).
1.2. Analysis.
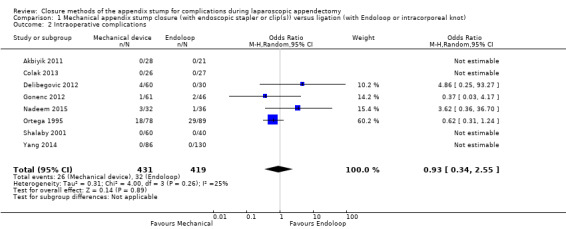
Comparison 1 Mechanical appendix stump closure (with endoscopic stapler or clip(s)) versus ligation (with Endoloop or intracorporeal knot), Outcome 2 Intraoperative complications.
1.1.3 Postoperative complications
Similar to our analysis of intraoperative complications, we found no substantial differences in postoperative complications between the use of any mechanical device versus any ligature‐based appendix stump closure technique (OR 0.80, 95% CI 0.21 to 3.13) (Analysis 1.3). This analysis was subject to substantial heterogeneity (I2 = 83%). More detailed examination by type of postoperative complication helped to reduce heterogeneity but still showed no significant differences between mechanical devices and ligature. Data show no differences in postoperative superficial infection rates (OR 0.58, 95% CI 0.18 to 1.93; I2 = 8%) (Analysis 1.6), deep infection rates (OR 0.79, 95% CI 0.24 to 2.53; I2 = 0%) (Analysis 1.7), and postoperative ileus rates (OR 0.47, 95% CI 0.19 to 1.18; I2 = 0%) (Analysis 1.8), when any mechanical device was compared with ligature.
1.3. Analysis.
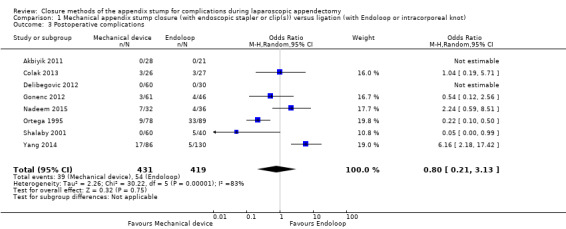
Comparison 1 Mechanical appendix stump closure (with endoscopic stapler or clip(s)) versus ligation (with Endoloop or intracorporeal knot), Outcome 3 Postoperative complications.
1.6. Analysis.
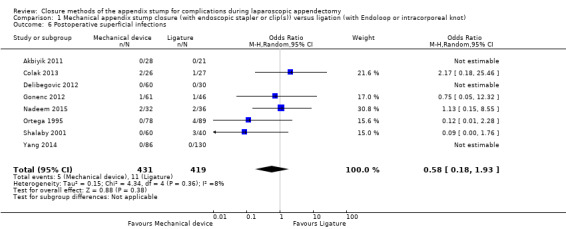
Comparison 1 Mechanical appendix stump closure (with endoscopic stapler or clip(s)) versus ligation (with Endoloop or intracorporeal knot), Outcome 6 Postoperative superficial infections.
1.7. Analysis.
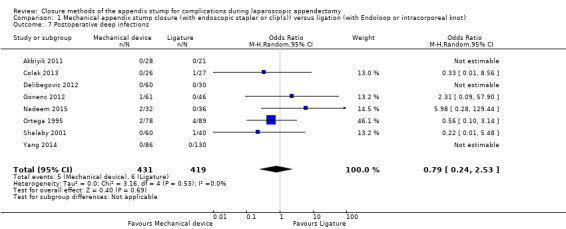
Comparison 1 Mechanical appendix stump closure (with endoscopic stapler or clip(s)) versus ligation (with Endoloop or intracorporeal knot), Outcome 7 Postoperative deep infections.
1.8. Analysis.
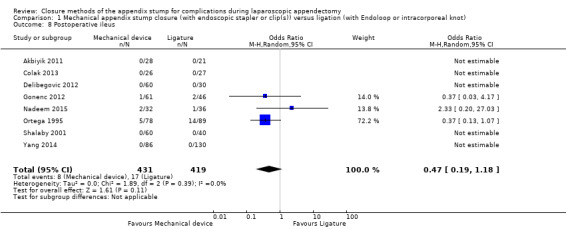
Comparison 1 Mechanical appendix stump closure (with endoscopic stapler or clip(s)) versus ligation (with Endoloop or intracorporeal knot), Outcome 8 Postoperative ileus.
Quality of the evidence
We judged the overall quality of evidence for the primary outcomes for this comparison as very low owing to high risk of bias, imprecision, small sample sizes, lack of long‐term follow‐up, and heterogeneity amongst included studies (Table 1).
1.2 Secondary outcomes
The evidence upon which our secondary outcome analyses were based had a GRADE rating of very low quality for three main reasons: (1) methodological limitations amongst the included studies listed above, (2) the more general subjective nature of using hospital stay as an outcome measure, which can be confounded by a number of factors unaccounted for in the included studies, and (3) the paucity of pain and quality of life‐related outcome measures amongst included studies.
1.2.1 Operative time
Results show a significant reduction in operative time with mechanical devices compared with ligature‐based techniques, with saving of approximately nine minutes on average across all studies (mean difference (MD) ‐9.04 minutes, 95% CI ‐12.97 to ‐5.11 minutes; I2 = 87%) (Analysis 1.4).
1.4. Analysis.
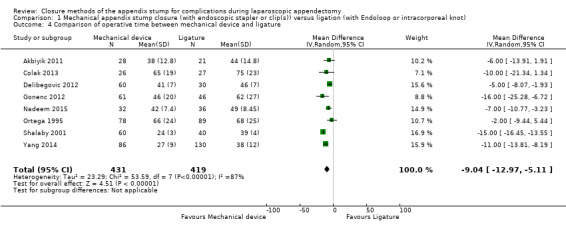
Comparison 1 Mechanical appendix stump closure (with endoscopic stapler or clip(s)) versus ligation (with Endoloop or intracorporeal knot), Outcome 4 Comparison of operative time between mechanical device and ligature.
1.2.2 Duration of hospital stay
We noted no significant differences in reduction in hospital stay with mechanical devices compared with ligature‐based techniques (MD 0.02 days, 95% CI ‐0.12 to 0.17 days; I2 = 30%) (Analysis 1.5).
1.5. Analysis.
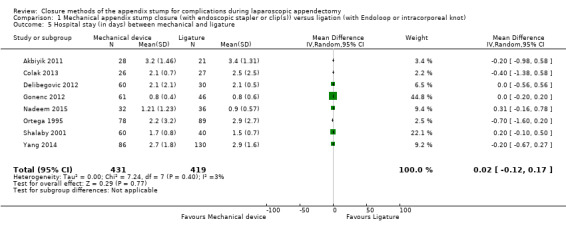
Comparison 1 Mechanical appendix stump closure (with endoscopic stapler or clip(s)) versus ligation (with Endoloop or intracorporeal knot), Outcome 5 Hospital stay (in days) between mechanical and ligature.
1.2.3 Hospital cost
Only four of the included trials commented on the consumable cost of the method used in each comparison arm, with mechanical devices costing at least three‐fold more than ligature‐based methods (Akbiyik 2011; Delibegovic 2012; Nadeem 2015; Shalaby 2001). None of the included studies evaluated total health economic costs such as whether any additional costs in staff time were required for device setup, maintenance, and disposal; or whether the observed reduction in operating time translated into additional emergency operations per day.
1.2.4 Pain/Quality of life
Only Ortega 1995 evaluated postoperative pain and reported showed no significant differences between use of the endoscopic stapler and ligature use. However, the published description suggests that the method used to ascertain this might have been subject to methodological confounding (see section Other potential sources of bias). No other studies evaluated quality of life postoperatively.
Quality of the evidence
We judged the overall quality of evidence for secondary outcomes for this comparison to be very low owing to high risk of bias, imprecision, small sample sizes, lack of long‐term follow‐up, and heterogeneity amongst included studies (Table 1).
2. Stapler versus ligation (with Endoloop, Roeder loop, or intracorporeal knot)
2.1 Primary outcomes
2.1.1 Total complications
Analysis of the comparison of endoscopic stapler device versus ligature amongst 327 participants randomised in three studies showed that the endoscopic stapler device resulted in no substantial differences in overall complications compared with the ligature (Delibegovic 2012; Ortega 1995; Shalaby 2001) (OR 0.34, 95% CI 0.05 to 2.41; I2 = 60%) (Analysis 2.1).
2.1. Analysis.
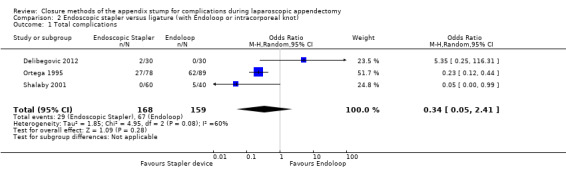
Comparison 2 Endoscopic stapler versus ligature (with Endoloop or intracorporeal knot), Outcome 1 Total complications.
2.1.2 Intraoperative complications
Data show no differences in intraoperative complications in our comparison of endoscopic stapler device versus ligature technique for appendix stump closure (OR 1.06, 95% CI 0.17 to 6.70; I2 = 45%) (Analysis 2.2).
2.2. Analysis.
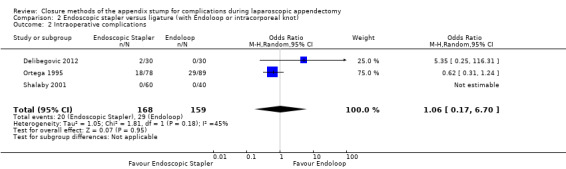
Comparison 2 Endoscopic stapler versus ligature (with Endoloop or intracorporeal knot), Outcome 2 Intraoperative complications.
2.1.3 Postoperative complications
Results show a significant reduction in postoperative complications with use of the stapler device compared with ligature (OR 0.20, 95% CI 0.09 to 0.44; I2 = 0%) (Analysis 2.3); this was masked in the analysis of overall complications by no differences amongst intraoperative complications in this comparison (OR 1.06, 95% CI 0.17 to 6.70; I2 = 45%) (Analysis 2.2). Exploration of this reduction in postoperative complications revealed that it was chiefly driven by a reduction in postoperative superficial wound infections in the endoscopic stapler arm when compared with the ligature arm (OR 0.10, 95% CI 0.01 to 0.84; I2 = 0%) (Analysis 2.6). We noted no significant differences in postoperative deep infection (OR 0.45, 95% CI 0.10 to 2.08; I2 = 0%) (Analysis 2.7) or postoperative ileus (OR 0.37, 95% CI 0.13 to 1.07; I2 = 0%) (Analysis 2.8) between the two groups. No studies reported postoperative bleeding, appendix stump rupture, or purulent peritonitis in either comparison group.
2.3. Analysis.
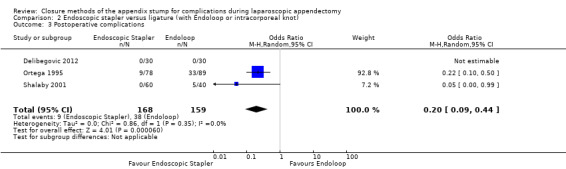
Comparison 2 Endoscopic stapler versus ligature (with Endoloop or intracorporeal knot), Outcome 3 Postoperative complications.
2.6. Analysis.
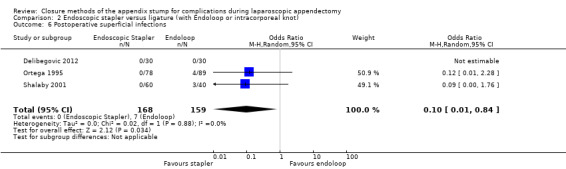
Comparison 2 Endoscopic stapler versus ligature (with Endoloop or intracorporeal knot), Outcome 6 Postoperative superficial infections.
2.7. Analysis.
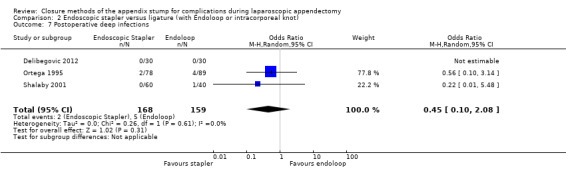
Comparison 2 Endoscopic stapler versus ligature (with Endoloop or intracorporeal knot), Outcome 7 Postoperative deep infections.
2.8. Analysis.
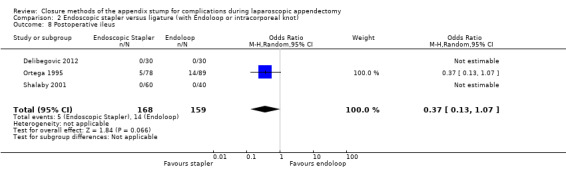
Comparison 2 Endoscopic stapler versus ligature (with Endoloop or intracorporeal knot), Outcome 8 Postoperative ileus.
Quality of the evidence
We judged overall quality of evidence for the primary outcomes for this comparison to be very low owing to high risk of bias, imprecision, and substantial heterogeneity amongst included studies (Table 2).
2.2 Secondary outcomes
The evidence upon which our secondary outcome analyses were based also had a very low GRADE quality rating for the same three main reasons as for the primary outcome analyses, with the addition of subjective reporting of hospital stay as an outcome measure, which can be confounded by several factors unaccounted for in the included studies, and the paucity of pain and quality of life‐related outcomes measures amongst included studies.
2.2.1 Operative time
Data show a significant reduction in operative time with use of the endoscopic stapler device versus the ligature‐based technique (MD ‐8.52 minutes, 95% CI ‐15.64 to ‐1.39 minutes; I2 = 91%) (Analysis 2.4).
2.4. Analysis.
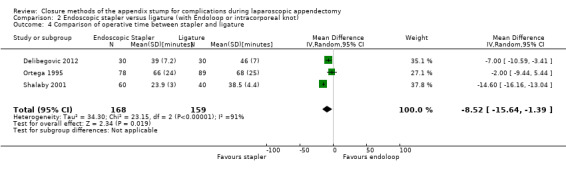
Comparison 2 Endoscopic stapler versus ligature (with Endoloop or intracorporeal knot), Outcome 4 Comparison of operative time between stapler and ligature.
2.2.2 Duration of hospital stay
We noted no significant reduction in differences in hospital stay with use of the endoscopic stapler compared with ligature‐based techniques (MD ‐0.02 days, 95% CI ‐0.38 to 0.34 days; I2 = 66%) (Analysis 2.5).
2.5. Analysis.
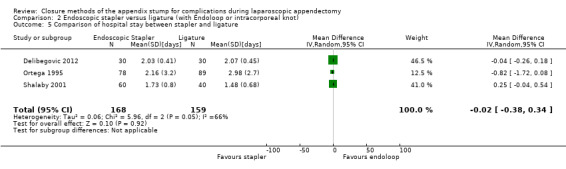
Comparison 2 Endoscopic stapler versus ligature (with Endoloop or intracorporeal knot), Outcome 5 Comparison of hospital stay between stapler and ligature.
2.2.3 Hospital cost
Two of the three studies included in this subanalysis commented on the consumable cost of the method used in each comparison arm (Delibegovic 2012; Shalaby 2001). Delibegovic 2012 commented that the cost per 45‐mm stapler used was EUR 230.7, whereas the cost per ligature (Endoloop) was EUR 28.85 (with two loops generally used). Shalaby 2001 commented that the cost per Endo GIA stapler (Ethicon Endo‐Surgery, Cincinnati, Ohio, USA) was USD 100 (EUR 86.00), whereas the cost per ligature (Endoloop) was USD 30 (EUR 25.80). No studies commented on indirect costs.
2.2.4 Pain/Quality of life
As described in Section 1.2.4, only one study evaluated postoperative pain (Ortega 1995), showing no significant differences between use of the endoscopic stapler and ligature use. However, the published description suggests that the method used to ascertain might have been subject to methodological confounding (see section Other potential sources of bias). No other studies evaluated quality of life postoperatively.
Quality of the evidence
We judged the overall quality of evidence for secondary outcomes for this comparison to be very low owing to high risk of bias, imprecision, and substantial heterogeneity amongst included studies (Table 2).
3. Clips versus ligation (with Endoloop, Roeder loop, or intracorporeal knot)
3.1 Primary outcomes
3.1.1 Total complications
Similarly, data show no significant differences in overall complications between use of clips versus ligature placement (OR 2.03, 95% CI 0.71 to 5.84; I2 = 61%) (Analysis 3.1) amongst 553 participants from six studies (Akbiyik 2011; Colak 2013; Delibegovic 2012; Gonenc 2012; Nadeem 2015; Yang 2014). This analysis was subject to high heterogeneity (I2 = 61%), and, similar to the composite analyses in Analysis 1.1, much of this heterogeneity was contributed by inclusion of a more recent study (Yang 2014).
3.1. Analysis.
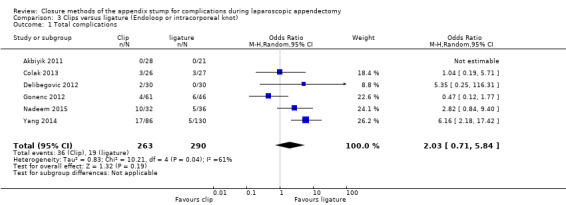
Comparison 3 Clips versus ligature (Endoloop or intracorporeal knot), Outcome 1 Total complications.
3.1.2 Intraoperative complications
We noted no differences in intraoperative complications in our comparison of endoscopic clip placement versus ligature (OR 1.74, 95% CI 0.33 to 9.04; I2 = 19%) (Analysis 3.2).
3.2. Analysis.
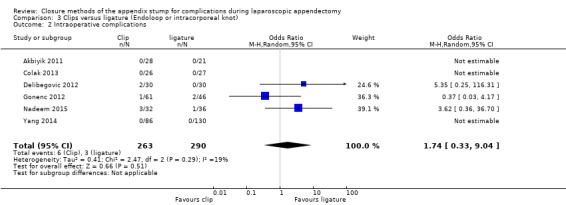
Comparison 3 Clips versus ligature (Endoloop or intracorporeal knot), Outcome 2 Intraoperative complications.
3.1.3 Postoperative complications
Results show no substantial differences in postoperative complications between endoscopic clip placement and ligature placement for appendix stump closure (OR 1.74, 95% CI 0.33 to 9.04; I2 = 19%) (Analysis 3.3).
3.3. Analysis.
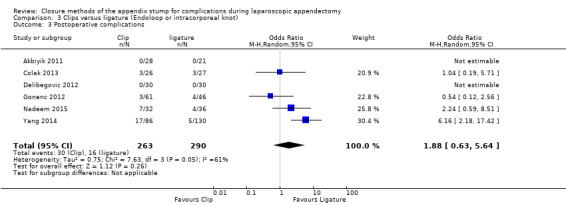
Comparison 3 Clips versus ligature (Endoloop or intracorporeal knot), Outcome 3 Postoperative complications.
Quality of the evidence
We judged the overall quality of evidence for the primary outcomes for this comparison as very low owing to high risk of bias, imprecision, and heterogeneity amongst included studies (Table 3).
3.2 Secondary outcomes
The evidence upon which our secondary outcome analyses were based also had a very low GRADE quality rating for the same three main reasons as for the primary outcome analyses.
3.2.1 Operative time
Data show a significant reduction in operative time with use of endoscopic clips versus a ligature‐based technique (MD ‐8.14 minutes, 95% CI ‐11.73 to ‐4.55 minutes; I2 = 66%) (Analysis 3.4).
3.4. Analysis.
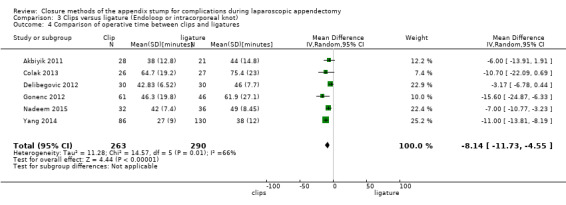
Comparison 3 Clips versus ligature (Endoloop or intracorporeal knot), Outcome 4 Comparison of operative time between clips and ligatures.
3.2.2 Duration of hospital stay
We noted no significant difference in reduction in hospital stay with endoscopic clip use compared with ligature‐based techniques (MD ‐0.03 days, 95% CI ‐0.16 to 0.11 days; I2 = 0%) (Analysis 3.5).
3.5. Analysis.
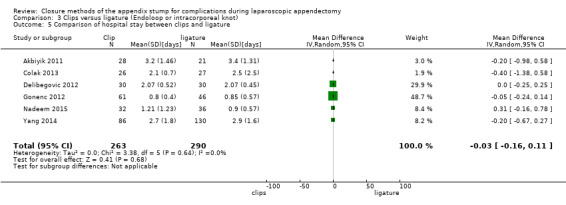
Comparison 3 Clips versus ligature (Endoloop or intracorporeal knot), Outcome 5 Comparison of hospital stay between clips and ligature.
3.2.3 Hospital cost
Three of the six included studies commented on the consumable cost of the method used in each comparison arm (Akbiyik 2011; Delibegovic 2012; Nadeem 2015). Akbiyik 2011 commented that the cost per Endo GIA (Ethicon Endo‐Surgery, Cincinnati, Ohio, USA) stapler was USD 100, whereas the cost per ligature (Endoloop) was USD 60.75 (with two loops generally used). Delibegovic 2012 commented that the cost per one Hem‐o‐lok clip (non‐absorbable polymeric clips) was EUR 4.75 (USD 5.52) but did not comment on the cost of the endoscopic clipping device. Delibegovic 2012 commented that the cost per ligature (Endoloop) was EUR 28.85 (USD 33.55)(with two loops generally used). Nadeem 2015 commented that total cost for the metallic endoclip arm was USD 800, whereas the cost per loop in the ligature arm was USD 200. However, Nadeem 2015 did not provide a justification for these costs. None of the included studies specifically listed indirect costs associated with each comparison arm.
3.2.4 Pain/Quality of life
None of the included studies evaluated pain or quality of life postoperatively.
Quality of the evidence
We judged the overall quality of evidence for secondary outcomes for this comparison to be very low owing to high risk of bias, imprecision, and heterogeneity amongst included studies (Table 3).
4. Stapler versus clips
4.1 Primary outcomes
Only one study with 60 participants directly compared endoscopic staplers versus endoscopic clips (Delibegovic 2012).
4.1.1 Total complications
Only one study reported complications (Delibegovic 2012), noting no significant differences in overall complications (OR 1.00, 95% CI 0.13 to 7.60) (Analysis 4.1).
4.1. Analysis.
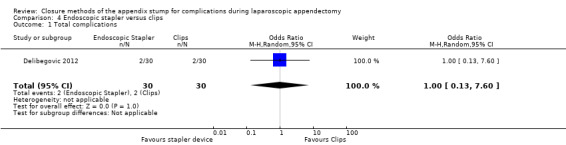
Comparison 4 Endoscopic stapler versus clips, Outcome 1 Total complications.
4.1.2 Intraoperative complications
Data show no differences in intraoperative complications in our comparison of endoscopic stapler versus clips (OR 1.00, 95% CI 0.13 to 7.60).
4.1.3 Postoperative complications
We noted no postoperative complications in either comparison arm in this study.
Quality of the evidence
We graded the quality of evidence for the primary outcomes of this comparison as very low owing to high risk of bias, limited sample size, and lack of longer‐term follow‐up (Table 4).
4.2 Secondary outcomes
The evidence upon which our secondary outcome analyses were based also had a very low GRADE quality rating for the same three main reasons as for the primary outcome analysis, and because investigators did not examine the paucity of pain and quality of life‐related outcomes measures in sufficient detail.
4.2.1 Operative time
Data show no significant differences in reduction in operative time with endoscopic stapler use compared with use of endoscopic clips (MD ‐3.46 minutes, 95% CI ‐6.94 to 0.02) (Analysis 4.4).
4.4. Analysis.
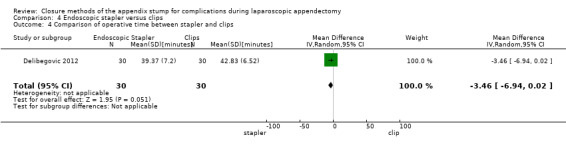
Comparison 4 Endoscopic stapler versus clips, Outcome 4 Comparison of operative time between stapler and clips.
4.2.2 Duration of hospital stay
We noted no significant differences in reduction in hospital stay with endoscopic stapler use compared with use of endoscopic clips (MD ‐0.04 days, 95% CI ‐0.28 to 0.20 days) (Analysis 4.5).
4.5. Analysis.
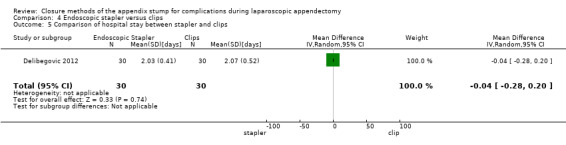
Comparison 4 Endoscopic stapler versus clips, Outcome 5 Comparison of hospital stay between stapler and clips.
4.2.3 Hospital cost
Delibegovic 2012 commented that the cost per 45‐mm stapler was EUR 230.7, whereas the cost per ligature (Endoloop) was EUR 28.85 (with two loops generally used), and that the cost per one Hem‐o‐lok clip (non‐absorbable polymeric clips) was EUR 4.75; however, investigators did not comment on the cost of the endoscopic clipping device and did not describe indirect costs associated with each comparison arm.
4.2.4 Pain/Quality of life
Data show no evaluation of postoperative pain or quality of life.
Quality of the evidence
We graded the quality of evidence for secondary outcomes of this comparison as very low owing to high risk of bias, limited sample size, and lack of longer‐term follow‐up (Table 4).
5. One versus two ligatures (with Endoloop, Roeder loop, or intracorporeal knot)
We found no eligible randomised trials comparing one ligature versus two ligatures for appendix stump closure. Beldi 2004 evaluated this question bycomparing one versus two Endoloops, but we excluded this study from meta‐analysis on the basis of a quasi‐randomised approach to allocating participants to each study arm based on the date of surgery. On odd days, investigators performed the operation using one and on even days two Endoloops to the appendix stump. In total, 208 participants received one Endoloop (n = 109) and 99 participants received two Endoloops to the appendix base. This study found no significant differences in postoperative complications between use of one Endoloop and use of two Endoloops, with each arm reporting five postoperative complications. However, this study was underpowered to demonstrate equivalence between the two arms; therefore the evidence upon which the question of whether one or two Endoloops are appropriate is of very low quality overall.
6. LigaSure sealing device versus other mechanical devices (with stapler or clips) or versus ligation (with Endoloop, Roeder loop, or intracorporeal knot)
We found no eligible randomised trials comparing the LigaSure sealing device versus other mechanical devices (with stapler or clips) or versus ligation (with Endoloop, Roeder loop, or intracorporeal knot).
7. Sensitivity analyses
We have provided in Table 5 a detailed description of all complications seen amongst included studies, and in Table 6 a summary of our sensitivity analysis of primary and secondary outcomes across all comparisons. Our presented results did not vary substantially by any of the a priori defined factors listed in the Methods section. We could not perform our planned sensitivity analysis with exclusion of trials at high risk of bias because all trials were at high risk of overall bias.
1. Primary outcomes in included studies.
| Study ID | Intervention arms | Total no. with complications | Total no. without complications | Intraoperative | Postoperative | |||||||||||
| Bleeding | Intraoperative rupture of appendix | Intraoperative organ injury/ faecal soiling | Access‐related visceral injury | Other | Total | Surgical site infection (superficial) | Deep infection | Bleeding | Paralytic ileus | Purulent peritonitis | Other | Total | ||||
| Ortega 1995 | Endoscopic linear stapler (LAS) | 27 | 51 | 11 | 2 | 5 | 0 | 0 | 18 | 0 | 2 | 0 | 5 | 0 | 2a | 9 |
| 2× catgut ligatures (Endoloops) (LAL) | 62 | 27 | 14 | 4 | 11 | 0 | 0 | 29 | 4 | 4 | 0 | 14 | 0 | 11b | 33 | |
| Open appendectomy (OA) | 44 | 42 | 20 | 5 | 1 | 0 | 0 | 26 | 11 | 0 | 0 | 6 | 0 | 1c | 18 | |
| Akbiyik 2011 | Hem‐o‐lok clip (non‐absorbable polymeric clips) | 0 | 28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ligaure (Endoloop) | 0 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Delibegovic 2012 | 45‐mm stapler | 2 | 28 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 ligature (Endoloop) | 0 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1 Hem‐o‐lok clip (non‐absorbable polymeric clips) | 2 | 28 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Beldi 2004 | 1 ligature (Endoloop) only at appendix base (1 other at 6 to 12 mm distally) | 5 | 104 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 2d | 5 |
| 2 ligatures (Endoloops) at base of appendix (1 other at 6 to 12 mm distally) | 5 | 94 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 1e | 5 | |
| Sucullu 2009 | Endodissector and endoclip | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| LigaSure 5 to 10 mm | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Shalaby 2001 | Endo GIA (Ethicon Endo‐Surgery, Cincinnati, Ohio, USA) stapler | 0 | 60 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ligature (Endoloop) | 5 | 35 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 1f | 5 | |
| Extracorporeal laparoscopically assisted appendectomy | 6 | 44 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 4 | |
| Colak 2013 | Hem‐o‐lok (non‐absorbable polymeric clips) | 3 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1g | 3 |
| Ligature (Endoloop) | 3 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1g | 3 | |
| Gonenc 2012 | Titanium endoclip | 4 | 57 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 3 |
| Intracorporeal knotting | 6 | 40 | 0 | 0 | 1 | 1 | 0 | 2 | 1 | 0 | 0 | 2 | 1 | 0 | 4 | |
| Ates 2012 | Titanium endoclip | 8 | 22 | NS | NS | NS | NS | 1h | 7 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Intracorporeal knotting | 7 | 24 | NS | NS | NS | NS | 0 | 4 | 1 | 0 | 0 | 0 | 0 | 2i | 3 | |
| Yang 2014 | Intracorporeal knotting | 5 | 125 | 0 | NS | NS | NS | NS | 0 | NS | 0 | NS | NS | NS | 5j | 5 |
| Titanium hem‐o‐lok | 17 | 69 | 0 | NS | NS | NS | NS | 0 | NS | 0 | NS | NS | NS | 17k | 17 | |
| Nadeem 2015 | Extracorporeal knotting | 5 | 31 | 1 | NS | 0 | NS | NS | 1 | 2 | 0 | NS | 1 | NS | 1 | 4 |
| Metallic endoclip | 10 | 22 | 2 | NS | 1 | NS | NS | 3 | 2 | 2 | NS | 2 | NS | 1l | 7 | |
NS: non‐significant. aTwo cases of vomiting. bEleven cases of vomiting. cone case of vomiting. dOne case of pulmonary embolism (PE) and one case of persistent port site pain. eOne case of prolonged percutaneous drainage. fOne case of intestinal obstruction. gOne non‐surgical complication. ihTwo open endoclips dropped during procedure and discovered by abdominal X‐ray postoperatively. iOne case of abdominal pain and one case unknown. jThree cases of lower abdominal discomfort, one case of abdominal pain, and two cases of fever. kEight cases of lower abdominal discomfort, three cases of abdominal pain, five cases of fever, and one reoperation. lOne re‐admission occurred in each arm: The re‐admitted participant in the metallic endoclip arm required peritoneal lavage and drain placement.
2. Sensitivity analyses.
| Mechanical appendix stump closure (with endoscopic stapler or clip(s)) vs ligation (with Endoloop or intra/extracorporeal knot) | |||||||||
| Odds ratio (95% CI) | Risk ratio (95% CI) | Risk difference (95% CI) | Mean difference (95% CI) | ||||||
| Outcome | Fixed effect | Random effects | Fixed effect | Random effects | Fixed effect | Random effects | Fixed effect | Random effects | |
| Total complications | 0.77 (0.53 to 1.13) | 0.97 (0.27 to 3.50) | 0.83 (0.64 to 1.08) | 1.09 (0.41 to 2.88) | ‐0.03 (‐0.08 to 0.01) | ‐0.02 (‐0.12 to 0.09) | ‐ | ‐ | |
| Intraoperative complications | 0.81 (0.45 to 1.46) | 0.93 (0.34 to 2.55) | 0.85 (0.53 to 1.35) | 0.93 (0.40 to 2.18) | ‐0.01 (‐0.04 to 0.02) | 0.00 (‐0.02 to 0.02) | ‐ | ‐ | |
| Postoperative complications | 0.80 (0.52 to 1.24) | 0.80 (0.21 to 3.13) | 0.83 (0.57 to 1.19) | 0.86 (0.27 to 2.74) | ‐0.02 (‐0.06 to 0.02) | ‐0.02 (‐0.10 to 0.06) | ‐ | ‐ | |
| Operative time (minutes) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐11.94 (‐13.04 to ‐10.84) | ‐9.04 (‐12.97 to ‐5.11) | |
| Hospital stay (days) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 0.02 (‐0.12 to 0.16) | 0.02 (‐0.12 to 0.17) | |
| Postoperative superficial infections | 0.47 (0.17 to 1.26) | 0.58 (0.18 to 1.93) | 0.48 (0.19 to 1.24) | 0.61 (0.19 to 1.93) | ‐0.02 (‐0.04 to 0.01) | ‐0.01 (‐0.02 to 0.01) | ‐ | ‐ | |
| Postoperative ileus | 0.48 (0.20 to 1.15) | 0.47 (0.19 to 1.18) | 0.51 (0.23 to 1.14) | 0.50 (0.22 to 1.17) | ‐0.02 (‐0.04 to 0.01) | ‐0.01 (‐0.03 to 0.02) | ‐ | ‐ | |
| Postoperative deep infections | 0.86 (0.31 to 2.41) | 0.79 (0.24 to 2.53) | 0.87 (0.32 to 2.35) | 0.79 (0.25 to 2.47) | ‐0.00 (‐0.02 to 0.02) | ‐0.00 (‐0.02 to 0.01) | ‐ | ‐ | |
| Endoscopic stapler vs ligature | |||||||||
| Total complications | 0.26 (0.14 to 0.46) | 0.34 (0.05 to 2.41) | 0.49 (0.35 to 0.68) | 0.51 (0.09 to 2.84) | ‐0.21 (‐0.29 to ‐0.12) | ‐0.13 (‐0.40 to 0.14) | ‐ | ‐ | |
| Intraoperative complications | 0.72 (0.38 to 1.39) | 1.06 (0.17 to 6.70) | 0.79 (0.48 to 1.28) | 1.07 (0.22 to 5.19) | ‐0.04 (‐0.11 to 0.04) | ‐0.00 (‐0.11 to 0.10) | ‐ | ‐ | |
| Postoperative complications | 0.19 (0.09 to 0.41) | 0.20 (0.09 to 0.44) | 0.27 (0.14 to 0.51) | 0.25 (0.08 to 0.75) | ‐0.17 (‐0.24 to ‐0.10) | ‐0.12 (‐0.34 to 0.09) | ‐ | ‐ | |
| Operative time (minutes) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐12.94 (‐14.35 to ‐11.53) | ‐8.36 (‐15.68 to ‐1.03) | |
| Hospital stay (days) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 0.03 (‐0.14 to 0.20) | ‐0.02 (‐0.38 to 0.34) | |
| Postoperative superficial infections | 0.10 (0.01 to 0.86) | 0.10 (0.01 to 0.84) | 0.11 (0.01 to 0.88) | 0.11 (0.01 to 0.87) | ‐0.05 (‐0.08 to ‐0.01) | ‐0.04 (‐0.08 to 0.00) | ‐ | ‐ | |
| Postoperative ileus | 0.37 (0.13 to 1.07) | 0.37 (0.13 to 1.07) | 0.41 (0.15 to 1.08) | 0.41 (0.15 to 1.08) | ‐0.05 (‐0.10 to 0.00) | ‐0.02 ( ‐0.10 to 0.05) | ‐ | ‐ | |
| Postoperative deep infections | 0.45 (0.10 to 2.02) | 0.45 (0.10 to 2.08) | 0.46 (0.11 to 1.95) | 0.47 (0.11 to 2.04) | ‐0.02 (‐0.05 to 0.02) | ‐0.02 (‐0.05 to 0.02) | ‐ | ‐ | |
| Endoscopic stapler vs clips | |||||||||
| Total complications | 1.00 (0.13 to 7.60) | 1.00 (0.13 to 7.60) | 1.00 (0.15 to 6.64) | 1.00 (0.15 to 6.64) | 0.00 (‐0.13 to 0.13) | 0.00 (‐0.13 to 0.13) | ‐ | ‐ | |
| Intraoperative complications | 1.00 (0.13 to 7.60) | 1.00 (0.13 to 7.60) | 1.00 (0.15 to 6.64) | 1.00 (0.15 to 6.64) | 0.00 (‐0.13 to 0.13) | 0.00 (‐0.13 to 0.13) | ‐ | ‐ | |
| Postoperative complications | NE | NE | NE | NE | 0.00 (‐0.06 to 0.06) | 0.00 (‐0.06 to 0.06) | ‐ | ‐ | |
| Operative time (minutes) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐3.46 (‐6.94 to 0.02) | ‐3.46 (‐6.94 to 0.02) | |
| Hospital stay (days) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐0.04 (‐0.28 to 0.20) | ‐0.04 [‐0.28, 0.20] | |
| Postoperative superficial infections | NE | NE | NE | NE | 0.00 (‐0.06 to 0.06) | 0.00 (‐0.06 to 0.06) | ‐ | ‐ | |
| Postoperative ileus | NE | NE | NE | NE | 0.00 (‐0.06 to 0.06) | 0.00 (‐0.06 to 0.06) | ‐ | ‐ | |
| Postoperative deep infections | NE | NE | NE | NE | 0.00 (‐0.06 to 0.06) | 0.00 (‐0.06 to 0.06) | ‐ | ‐ | |
| Clips vs ligature (Endoloop and intra/extracorporeal knot) | |||||||||
| Total complications | 2.33 (1.31 to 4.13) | 2.03 (0.71 to 5.84) | 2.11 (1.29 to 3.47) | 1.84 (0.73 to 4.62) | 0.08 (0.03 to 0.13) | 0.05 (‐0.03 to 0.13) | ‐ | ‐ | |
| Intraoperative complications | 1.79 (0.49 to 6.56) | 1.74 (0.33 to 9.04) | 1.76 (0.51 to 6.01) | 1.69 (0.35 to 8.19) | 0.01 (‐0.02 to 0.04) | 0.00 (‐0.02 to 0.02) | ‐ | ‐ | |
| Postoperative complications | 2.40 (1.28 to 4.48) | 1.88 (0.63 to 5.64) | 2.20 (1.27 to 3.82) | 1.75 (0.66 to 4.61) | 0.07 (0.02 to 0.12) | 0.03 (‐0.04 to 0.11) | ‐ | ‐ | |
| Operative time (minutes) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐8.06 (‐9.85 to ‐6.26) | ‐8.14 (‐11.73 to ‐4.55) | |
| Hospital stay (days) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐0.03 (‐0.16 to 0.11) | ‐0.03 (‐0.16 to 0.11) | |
| Postoperative superficial infections | 1.27 (0.33 to 4.86) | 1.25 (0.32 to 4.90) | 1.25 (0.35 to 4.49) | 1.24 (0.34 to 4.56) | 0.00 (‐0.02 to 0.03) | 0.00 (‐0.02 to 0.02) | ‐ | ‐ | |
| Postoperative ileus | 0.92 (0.19 to 4.56) | 0.92 (0.15 to 5.64) | 0.92 (0.20 to 4.21) | 0.93 (0.16 to 5.33) | ‐0.00 (‐0.02 to 0.02) | ‐0.00 (‐0.02 to 0.02) | ‐ | ‐ | |
| Postoperative deep infections | 1.79 (0.37 to 8.58) | 1.75 (0.28 to 10.93) | 1.77 (0.38 to 8.16) | 1.71 (0.28 to 10.28) | 0.01 (‐0.02 to 0.03) | 0.00 (‐0.01 to 0.02) | ‐ | ‐ | |
CI: confidence interval; NE: not estimable; "‐": not applicable.
Discussion
Summary of main results
Review authors found no significant differences in our primary outcomes of total complications, intraoperative complications, and postoperative complications between the use of any mechanical device versus a ligature technique for closure of the appendix stump during laparoscopic appendectomy. One exception to this was that the stapler device resulted in reduced likelihood of postoperative superficial wound infection when compared with ligature (odds ratio (OR) 0.10, 95% confidence interval (CI) 0.01 to 0.84) (Analysis 2.6). However, this review cannot unequivocally recommend the routine use of any single mechanical device over another for appendix stump closure because reductions in postoperative superficial infection and in our secondary outcome of operative time failed to translate into a clinically or statistically significant reduction in overall complications or in in‐patient hospital stay when compared with ligature use. For indirect comparisons of mechanical devices, analyses of total complications associated with use of an endoscopic stapler compared with a ligature (OR 0.34, 95% CI 0.05 to 2.41) (Analysis 2.1), and with use of endoscopic clips compared with a ligature (OR 2.03, 95% CI 0.71 to 5.84) (Analysis 3.1), were subject to moderate heterogeneity; the only study that directly compared the two devices found no substantial differences in total complications (OR 1.00, 95% CI 0.13 to 7.60) (Analysis 4.1) (Delibegovic 2012). All included studies had limitations in terms of biases; therefore a clear conclusion is not possible in light of the quality of current evidence.
Although our findings suggest that use of the endoscopic stapler results in reduced operative time and decreased rates of postoperative superficial infection compared with ligature (or Endoloop) placement, the reason for the reduction in postoperative superficial infection is not entirely clear. One consideration is that endoscopic staplers require a 10‐ to 12‐mm port, whereas ligatures can be introduced through a 5‐mm port, and wound infection rates may be related to length of the surgical incision. A second explanation may be based on the technique required for use of the stapler device versus ligature placement. The endoscopic stapler requires that care be taken in ensuring that a viable appendix stump is placed between endoscopic stapler arms to allow clear margins of healthy tissue when the device is ‘fired’. Once successfully positioned, stump closure results in little contamination of surrounding viscera and little device displacement on firing and retrieval. The result may be less ambient faecal contamination of surrounding tissues, as both proximal and distal lumens of the appendix are simultaneously closed. In contrast, endoscopic Roeder loops or intracorporeal knot tying requires greater skill in ensuring sufficient economy of motion to secure the knot and excise the appendix without inadvertent faecal contamination of the ends of instruments or of the surrounding viscera (which might thereby seed and spread infection). Potential additional risks of faecal contamination and intra‐abdominal instrumentation when a ligature is secured may explain the excessive postoperative ileus superficial infection rates and operative times reported when mechanical devices are compared. Of interest, we did not see a difference in postoperative ileus or deep infection rates, as might be expected to follow this explanation. In theory, this explanation should be equally applicable to endoscopic clip use and endoscopic stapler use. However, a reduction in postoperative superficial infection is not seen with endoscopic clips in the same way as with the endoscopic stapler. This may have more to do with outcome assessment limitations amongst included studies, particularly for subjective outcomes such as postoperative ileus (see Quality of the evidence), and may not be truly representative of the technique used.
A surgeon must consider two key points when deciding how to close the appendix stump, namely, patient safety and health economic costs. Patient safety may be expanded to include the detrimental effects of prolonged anaesthesia, seen as delays in operative time, potential collateral damage or iatrogenic injury from use of the intervention, and the implications of failure for the intended outcome of the intervention. Economic costs extend beyond hardware costs per use of the intervention and also include the fiscal repercussions of time‐consuming procedures (resulting in reduced time for other operations), prolonged hospital stay, and costs of reoperations or follow‐up.
Any reduction in costs resulting from fewer postoperative complications must be reconciled with the cost of the device, particularly because a stapler device on average is at least four times as expensive as a ligature (in the form of Endoloops). Our included studies did not provide sufficient data to allow a detailed cost‐benefit analysis. Similarly, available information is insufficient for a quantitative morbidity comparison between devices. However, results from our qualitative review show no substantial differences in pain or quality of life associated with any individual intervention.
Overall completeness and applicability of evidence
This review included randomised controlled trials (RCTs) of participants undergoing laparoscopic appendectomy. Two of our included studies excluded participants who were converted to open appendectomy (Colak 2013; Gonenc 2012). Thus, the findings of this review are applicable to patients with diagnosed uncomplicated acute appendicitis who are fit for a laparoscopic procedure.
Quality of the evidence
For this review, meta‐analysis included 850 participants from eight randomised studies. We downgraded the quality of evidence for the primary outcome 'Total complications' for all comparisons by one level for high risk of bias, one level for inconsistency due to substantial heterogeneity, and one level for imprecision. We graded the quality of evidence for remaining outcomes for all comparisons as very low owing to high risk of bias and imprecision (wide confidence intervals).
All studies reported details of intraoperative and postoperative complications. However, no studies provided information on blinding of personnel or participants. Blinding of personnel in randomised studies comparing different surgical procedures is difficult, which is a common drawback of many surgical trials. Blinding can affect the perception of secondary outcomes such as pain and quality of life. It also can influence detection of outcomes such as postoperative ileus, which, although our results show is reduced with use of mechanical devices, can be difficult to objectively quantify even by blinded personnel; none of our included studies clearly specify whether this diagnosis was made by blinded personnel. Every effort should be taken to blind outcome assessment, as blinding can significantly contribute to reduction of detection bias.
Two trials were at high risk of attrition bias or selection bias, as they excluded participants postoperatively (Colak 2013) or changed the allocation of several participants from the group initially randomised to Ortega 1995. In addition, two trials were at unclear risk of attrition bias, as they excluded participants postoperatively but did not provide enough detail on the exclusion process to permit judgement (Gonenc 2012; Nadeem 2015). Both selection bias and attrition bias could have been reduced by appropriate a priori trial protocol publication and registration, as well as by diligent reporting of prespecified outcomes among all randomised participants according to the group to which they were randomly allocated (intention‐to‐treat analysis).
The quality of evidence may also be compromised as the result of heterogeneity of performing surgeons. Ortega 1995 contributed the greatest population of participants to our analysis, with all procedures performed by residents with a reportedly high total number of complications. This can be a significant source of bias in assessing the primary outcomes, especially with no significant differences in complication rates reported by other studies. To ensure homogenous assessment, experienced surgeons with predefined levels of competency should be in charge of performing the procedures. Data available from the included studies were insufficient to allow us to undertake our intended detailed subgroup analyses (see Subgroup analysis and investigation of heterogeneity). In addition, no published studies eligible for inclusion allowed us to undertake our intended analyses of one versus two ligatures and of the LigaSure sealing device versus other methods; this provides scope for future research (see Implications for research).
Potential biases in the review process
We followed guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We applied no language, publication status, or sample size restrictions. We minimised bias in trial selection and included only RCTs. As we applied no restriction on publication date, we did include trials run before the imposition of mandatory trial registration. Therefore, the possibility exists that some trials might not have been reported owing to the direction of results (publication bias). Moreover, excluding trials that did not meet our selection criteria rendered only eight studies for inclusion in quantitative meta‐analysis ‐ a number that was inadequate to generate funnel plots for assessment of reporting bias (Sterne 2011).
Agreements and disagreements with other studies or reviews
Over recent years, different authors have reviewed this topic (Kazemier 2006; Sajid 2009; Shaikh 2015). Similar to our review, all three previous reviews used a random‐effects model to meta‐analyse included studies. However, eligibility criteria for each of these three reviews differed, meaning that different studies were included or excluded in each review. Both Kazemier 2006 and Sajid 2009 included only two of the eight RCTs used in our review (Ortega 1995; Shalaby 2001), but they also included several studies that we excluded for not providing the minimal methodological robustness defined by our eligibility criteria; Kazemier 2006 included Beldi 2006, Klima 1998, and Lange 1993), and Sajid 2009 included Klima 1996, Klima 1998, and Lange 1993. Shaikh 2015 included four of the eight RCTs that we used in our review (Akbiyik 2011; Colak 2013; Delibegovic 2012; Gonenc 2012) but included three additional studies that did not meet our inclusion criteria (Ates 2012; Delibegovic 2009; Hue 2012). These differences may explain why the conclusions drawn by respective review authors have differed so dramatically. Kazemier 2006 concluded that routine use of endoscopic staplers was favourable, and Shaikh 2015 concluded that use of the Endoclip was simple, efficacious, safe, and a cost‐effective alternative whereas Sajid 2009 concluded that although use of the Endoloop took longer than use of the Endo‐GIA, length of hospital stay, perioperative complication rates, and incidence of intra‐abdominal abscess appeared equal. To the best of our knowledge, we have undertaken the most extensive systematic review in this field to date, and our results show that current evidence is insufficient to strongly support the routine use of any single stump closure method over another during laparoscopic appendectomy.
Authors' conclusions
Implications for practice.
Results of this review show no differences between overall complications associated with mechanical devices and ligature methods during appendix stump closure. In light of this, we cannot unequivocally recommend routine use of mechanical devices in appendix stump closure because reduction in operating time has not translated into any clinically significant reductions in in‐patient hospital stay (mean difference 0.02, 95% confidence interval ‐0.12 to 0.17; I2 = 0%) (Analysis 1.5). Similarly, information on the fiscal costs of different mechanical devices is insufficient to show whether additional costs of these devices compared with the costs of ligatures are outweighed by reduced operating time, allowing the possibility of including additional procedures in an operating list. Until such time when these devices show more definitive comparative evidence of efficacy in comparison with each other and with ligatures (as outlined below under Implications for research), it is not possible to advocate omission of conventional ligature‐based appendix stump closure in favour of any single mechanical device over another.
Implications for research.
For our comparison of types of mechanical devices, we were limited to a single study that met our inclusion criteria. The only studies comparing the efficacy of the Medtronic LigaSure vessel sealing system (Valleylab, Boulder, Colorado, USA), or comparing one versus two ligatures in terms of complication rates, used a quasi‐randomisation method and did not meet our inclusion criteria (Sucullu 2009 and Beldi 2004, respectively); therefore we were unable to undertake our planned analyses of these comparisons. Similarly, no robust randomised trials have examined laparoscopic appendectomy using the Ethicon ENSEAL device or the Harmonic scalpel device, and none have compared these against Weck Hem‐o‐lok Polymer Locking Ligation System (Weck Closure Systems, Research Triangle Park, North Carolina, USA), titanium clip devices, or the Ethicon Endo GIA stapler (Ethicon Endo‐Surgery, Inc., Cincinnati, Ohio, USA). Although a plethora of cases series and observational studies from single‐centre experiences have used various types of mechanical devices, they have contributed little conclusive evidence of efficacy because of confounding factors inherent in these types of study design.
None of our included studies have reported postoperative bleeding, appendix stump rupture, or purulent peritonitis in either comparison group, and included studies have poorly reported other outcomes such as hospital costs (operation, direct and indirect) and pain/quality of life. Well‐designed randomised clinical trials are needed to compare contemporary mechanical sealing devices versus each other and versus conventional ligature‐based methods, with particular emphasis on health economic implications and clinically relevant complication rates (such as postoperative peritonitis and appendix stump rupture); they should be designed in a manner that will allow investigators to address the biases identified in existing studies on this topic (see Quality of the evidence). It would be ethically feasible for a double‐blinded trial to ensure that (1) the consenting participant is blinded to the method of appendix stump closure used for the duration of postoperative recovery until study completion, unless a complication precludes this; (2) a senior operating surgeon is blinded to identifiable participant details and is not directly involved in the decision to operate or in providing postoperative care; and (3) the participant's responsible healthcare team is blinded to the operative details, unless clinically relevant reasons preclude this. In such trials, blinded investigators may evaluate outcomes. With this approach, a double‐blinded surgical randomised trial would be feasible and robust enough to avoid confounding factors such as those evident in the studies included in this review.
What's new
| Date | Event | Description |
|---|---|---|
| 10 October 2017 | Amended | Revised version to incorporate feedback from Cochrane Editorial Unit |
History
Protocol first published: Issue 2, 2007 Review first published: Issue 11, 2017
| Date | Event | Description |
|---|---|---|
| 30 July 2017 | Amended | Incorporated feedback from Editorial Assistant |
| 13 July 2017 | Amended | Incorporated feedback from Contact Editor |
| 3 April 2017 | Feedback has been incorporated | Incorporated feedback and comments from Reviewers |
| 24 March 2017 | Feedback has been incorporated | I ncorporated feedback and com ments incorporated from Reviewers |
| 3 January 2017 | Feedback has been incorporated | Included full‐text article (Yang et al) in place of the previously included Abstract only |
| 2 November 2016 | Feedback has been incorporated | Incorporated feedback from Section Editor |
| 24 June 2016 | New search has been performed | Implemented Review protocol and systematic review/Undertook meta ‐analysis /Prepared corresponding manuscript |
| 13 November 2012 | Amended | Updated the protocol published in 2007 |
| 13 August 2012 | New citation required and minor changes | Updated original protocol from 2007 with up‐to‐date references; updated protocol evaluated by the CCCG Editorial Board |
Notes
None.
Acknowledgements
We would like to thank the Cochrane Colorectal Cancer Group, the CCCG Editorial Office, and Dr. Henning Keinke Andersen for assisting in development of the protocol, the review, and careful copy editing; Anne Sofie Christensen, who assisted in development of the protocol; Marija Barbateskovic, who developed the search strategies and ran the initial literature searches; and CCCG editors and peer referees Keith Chapple and Brett Doleman. We also would like to thank Nia Roberts, Outreach Librarian at the Bodliean Library, University of Oxford; and Sys Johnsen, Information Specialist at the Cochrane Colorectal Cancer Group; for updating the systematic search. We would like to acknowledge Stefan Sauerland and Geert Kazemier, who had the idea for the review; and Su Peng, Yao Chen, Jin Zhou, Yalin Zhang, Nansheng Cheng, Yun Liao, and Zong‐Guang Zhou, who drafted the review protocol. And finally Dolores Matheews from the Copy Edit Support team.
Appendices
Appendix 1. Search strategy for the Cochrane Library
#1 MeSH descriptor: [Appendix] explode all trees
#2 MeSH descriptor: [Appendicitis] explode all trees
#3 MeSH descriptor: [Appendectomy] explode all trees
#4 append*:ti,ab,kw
#5 (#1 or #2 or #3 or #4)
#6 MeSH descriptor: [Laparoscopy] explode all trees
#7 (laparoscop* or minimal* invasiv*):ti,ab,kw
#8 (#6 or #7)
#9 MeSH descriptor: [Suture Techniques] explode all trees
#10 MeSH descriptor: [Surgical Staplers] explode all trees
#11 (stump or loop* or ligation or polymer* or stapl* or Roeder or Roder or clips* or sutur* or closure*):ti,ab,kw
#12 (#9 or #10 or #11)
#13 (#5 and #8 and #12)
Appendix 2. Search strategy for MEDLINE (Ovid)
1. exp Appendix/
2. exp Appendicitis/
3. exp Appendectomy/
4. append*.mp.
5. 1 or 2 or 3 or 4
6. exp Laparoscopy/
7. (laparoscop* or minimal* invasiv*).mp.
8. 6 or 7
9. exp Suture Techniques/
10. exp Surgical Staplers/
11. (stump or loop* or ligation or polymer* or stapl* or Roeder or Roder or clips* or sutur* or closure*).mp.
12. 9 or 10 or 11
13. 5 and 8 and 12
14. randomized controlled trial.pt.
15. controlled clinical trial.pt.
16. randomized.ab.
17. placebo.ab.
18. clinical trials as topic.sh.
19. randomly.ab.
20. trial.ti.
21. 14 or 15 or 16 or 17 or 18 or 19 or 20
22. Exp animals/ not humans.sh.
23. 21 not 22
24. 13 and 23
Appendix 3. Search strategy for Embase (Ovid)
1. exp appendix/
2. exp appendix disease/
3. exp appendectomy/
4. append*.mp.
5. 1 or 2 or 3 or 4
6. exp laparoscopy/
7. (laparoscop* or minimal* invasiv*).mp. 8. 6 or 7
9. exp suturing method/
10. exp suture/
11. (stump or loop* or ligation or polymer* or stapl* or Roeder or Roder or clips* or sutur* or closure*).mp.
12. 9 or 10 or 11
13. 5 and 8 and 12
14. CROSSOVER PROCEDURE.sh.
15. DOUBLE‐BLIND PROCEDURE.sh.
16. SINGLE‐BLIND PROCEDURE.sh.
17. (crossover* or cross over*).ti,ab.
18. placebo*.ti,ab.
19. (doubl* adj blind*).ti,ab.
20. allocat*.ti,ab.
21. trial.ti.
22. RANDOMIZED CONTROLLED TRIAL.sh.
23. random*.ti,ab.
24. 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23
25. (exp animal/ or exp invertebrate/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans or man or men or wom?n).ti.)
26. 24 not 25
27. 13 and 26
Appendix 4. Search strategy for Science Citation Index ‐ Expanded
#1 Topic=(append*)
#2 Topic=(laparoscop*)
#3 Topic=(stump or loop* or ligation or polymer* or stapl* or Roeder or Roder or clips* or sutur* or closure*)
#4 Topic=(random* OR controlled OR RCT OR placebo OR trial OR group* OR trial*)
#5 (#1 and #2 and #3 and #4)
Appendix 5. Criteria for risk of bias assessment in the Cochrane 'Risk of bias' tool
|
RANDOM SEQUENCE GENERATION Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | |
| Criteria for a judgement of ‘low risk’ of bias | Investigators describe a random component in the sequence generation process such as: · referring to a random number table; · using a computer random number generator; · tossing a coin; · shuffling cards or envelopes; · throwing dice; · drawing lots; or · minimising*. *Minimisation may be implemented without a random element, and this is considered equivalent to being random. |
| Criteria for the judgement of ‘high risk’ of bias | Investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: · sequence generated by odd or even date of birth; · sequence generated by some rule based on date (or day) of admission; · sequence generated by some rule based on hospital or clinic record number. Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorisation of participants, for example: · allocation by judgement of the clinician; · allocation by preference of the participant; · allocation based on results of a laboratory test or series of tests; or · allocation by availability of the intervention. |
| Criteria for the judgement of ‘unclear risk’ of bias | Insufficient information about the sequence generation process to permit judgement of ‘low risk’ or ‘high risk’ |
|
ALLOCATION CONCEALMENT Selection bias (biased allocation to interventions) due to inadequate concealment of allocations before assignment | |
| Criteria for a judgement of ‘low risk’ of bias | Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation. · Central allocation (including telephone, web‐based, and pharmacy‐controlled randomisation); · Sequentially numbered drug containers of identical appearance; or · Sequentially numbered, opaque, sealed envelopes. |
| Criteria for the judgement of ‘high risk’ of bias | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: · using an open random allocation schedule (e.g. a list of random numbers); · using assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or were not sequentially numbered); · alternation or rotation; · date of birth; · case record number; or · any other explicitly unconcealed procedure. |
| Criteria for the judgement of ‘unclear risk’ of bias | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. This is usually the case if the method of concealment is not described or is not described in sufficient detail to allow a definitive judgement – for example, if use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed. |
|
BLINDING OF PARTICIPANTS AND PERSONNEL Performance bias due to knowledge of allocated interventions by participants and personnel during the study | |
| Criteria for a judgement of ‘low risk’ of bias | Any one of the following. · No blinding or incomplete blinding, but review authors judge that the outcome is not likely to be influenced by lack of blinding. · Blinding of participants and key study personnel ensured, and unlikely that blinding could have been broken. |
| Criteria for the judgement of ‘high risk’ of bias | Any one of the following. · No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding. · Blinding of key study participants and personnel attempted, but likely that blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. |
| Criteria for the judgement of ‘unclear risk’ of bias | Any one of the following. · Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. · The study did not address this outcome. |
|
BLINDING OF OUTCOME ASSESSMENT Detection bias due to knowledge of allocated interventions by outcome assessors | |
| Criteria for a judgement of ‘low risk’ of bias. | Any one of the following. · No blinding of outcome assessment, but review authors judge that the outcome measurement is not likely to be influenced by lack of blinding. · Blinding of outcome assessment ensured, and unlikely that blinding could have been broken. |
| Criteria for the judgement of ‘high risk’ of bias | Any one of the following. · No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding. · Blinding of outcome assessment, but likely that blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. |
| Criteria for the judgement of ‘unclear risk’ of bias | Any one of the following. · Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. · The study did not address this outcome. |
|
INCOMPLETE OUTCOME DATA Attrition bias due to quantity, nature, or handling of incomplete outcome data | |
| Criteria for a judgement of ‘low risk’ of bias | Any one of the following. · No missing outcome data. · Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias). · Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. · For dichotomous outcome data, proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate. · For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size. · Missing data imputed using appropriate methods. |
| Criteria for the judgement of ‘high risk’ of bias | Any one of the following. · Reasons for missing outcome data likely to be related to true outcome, with imbalance in numbers or reasons for missing data across intervention groups. · For dichotomous outcome data, proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate. · For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size. · ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation. · Potentially inappropriate application of simple imputation. |
| Criteria for the judgement of ‘unclear risk’ of bias | Any one of the following. · Insufficient reporting of attrition/exclusions to permit judgement of ‘low risk’ or ‘high risk’ (e.g. number randomised not stated, no reasons for missing data provided). · The study did not address this outcome. |
|
SELECTIVE REPORTING Reporting bias due to selective outcome reporting | |
| Criteria for a judgement of ‘low risk’ of bias | Any of the following. · The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. · The study protocol is not available but it is clear that published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon). |
| Criteria for the judgement of ‘high risk’ of bias | Any one of the following. · Not all of the study’s prespecified primary outcomes have been reported. · One or more primary outcomes are reported using measurements, analysis methods, or subsets of data (e.g. subscales) that were not prespecified. · One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect). · One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis. · The study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
| Criteria for the judgement of ‘unclear risk’ of bias | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. It is likely that most studies will fall into this category. |
|
OTHER BIAS Bias due to problems not covered elsewhere in the table | |
| Criteria for a judgement of ‘low risk’ of bias | The study appears to be free of other sources of bias. |
| Criteria for the judgement of ‘high risk’ of bias | There is at least one important risk of bias. For example, the study: · had a potential source of bias related to the specific study design used; · has been claimed to have been fraudulent; or · had some other problem. |
| Criteria for the judgement of ‘unclear risk’ of bias | There may be a risk of bias, but there is either: · insufficient information to assess whether an important risk of bias exists; or · insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Mechanical appendix stump closure (with endoscopic stapler or clip(s)) versus ligation (with Endoloop or intracorporeal knot).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total complications | 8 | 850 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.27, 3.50] |
| 2 Intraoperative complications | 8 | 850 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.34, 2.55] |
| 3 Postoperative complications | 8 | 850 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.21, 3.13] |
| 4 Comparison of operative time between mechanical device and ligature | 8 | 850 | Mean Difference (IV, Random, 95% CI) | ‐9.04 [‐12.97, ‐5.11] |
| 5 Hospital stay (in days) between mechanical and ligature | 8 | 850 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.12, 0.17] |
| 6 Postoperative superficial infections | 8 | 850 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.18, 1.93] |
| 7 Postoperative deep infections | 8 | 850 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.24, 2.53] |
| 8 Postoperative ileus | 8 | 850 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.19, 1.18] |
Comparison 2. Endoscopic stapler versus ligature (with Endoloop or intracorporeal knot).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total complications | 3 | 327 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.05, 2.41] |
| 2 Intraoperative complications | 3 | 327 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.17, 6.70] |
| 3 Postoperative complications | 3 | 327 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.09, 0.44] |
| 4 Comparison of operative time between stapler and ligature | 3 | 327 | Mean Difference (IV, Random, 95% CI) | ‐8.52 [‐15.64, ‐1.39] |
| 5 Comparison of hospital stay between stapler and ligature | 3 | 327 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.38, 0.34] |
| 6 Postoperative superficial infections | 3 | 327 | Odds Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 0.84] |
| 7 Postoperative deep infections | 3 | 327 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.10, 2.08] |
| 8 Postoperative ileus | 3 | 327 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.13, 1.07] |
Comparison 3. Clips versus ligature (Endoloop or intracorporeal knot).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total complications | 6 | 553 | Odds Ratio (M‐H, Random, 95% CI) | 2.03 [0.71, 5.84] |
| 2 Intraoperative complications | 6 | 553 | Odds Ratio (M‐H, Random, 95% CI) | 1.74 [0.33, 9.04] |
| 3 Postoperative complications | 6 | 553 | Odds Ratio (M‐H, Random, 95% CI) | 1.88 [0.63, 5.64] |
| 4 Comparison of operative time between clips and ligatures | 6 | 553 | Mean Difference (IV, Random, 95% CI) | ‐8.14 [‐11.73, ‐4.55] |
| 5 Comparison of hospital stay between clips and ligature | 6 | 553 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.16, 0.11] |
| 6 Postoperative superficial infections | 6 | 553 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.32, 4.90] |
| 7 Postoperative deep infections | 6 | 553 | Odds Ratio (M‐H, Random, 95% CI) | 1.75 [0.28, 10.93] |
| 8 Postoperative ileus | 6 | 553 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.15, 5.64] |
3.6. Analysis.
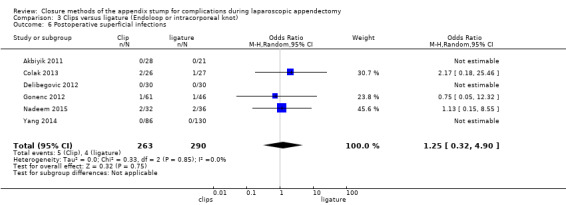
Comparison 3 Clips versus ligature (Endoloop or intracorporeal knot), Outcome 6 Postoperative superficial infections.
3.7. Analysis.
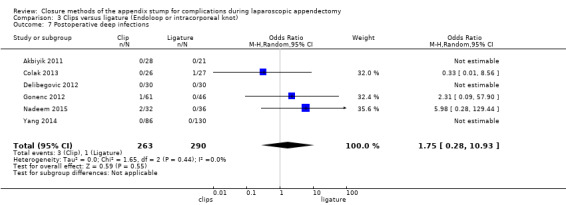
Comparison 3 Clips versus ligature (Endoloop or intracorporeal knot), Outcome 7 Postoperative deep infections.
3.8. Analysis.
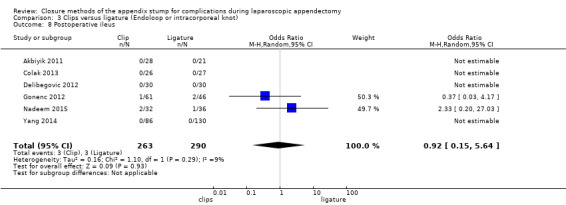
Comparison 3 Clips versus ligature (Endoloop or intracorporeal knot), Outcome 8 Postoperative ileus.
Comparison 4. Endoscopic stapler versus clips.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total complications | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.13, 7.60] |
| 2 Intraoperative complications | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.13, 7.60] |
| 3 Postoperative complications | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Comparison of operative time between stapler and clips | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐3.46 [‐6.94, 0.02] |
| 5 Comparison of hospital stay between stapler and clips | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.28, 0.20] |
| 6 Postoperative superficial infections | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Postoperative deep infections | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Postoperative ileus | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
4.2. Analysis.
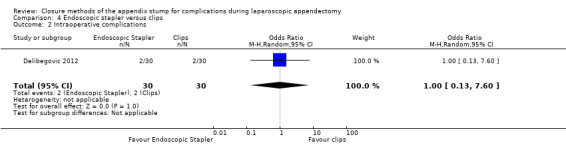
Comparison 4 Endoscopic stapler versus clips, Outcome 2 Intraoperative complications.
4.3. Analysis.
Comparison 4 Endoscopic stapler versus clips, Outcome 3 Postoperative complications.
4.6. Analysis.
Comparison 4 Endoscopic stapler versus clips, Outcome 6 Postoperative superficial infections.
4.7. Analysis.
Comparison 4 Endoscopic stapler versus clips, Outcome 7 Postoperative deep infections.
4.8. Analysis.
Comparison 4 Endoscopic stapler versus clips, Outcome 8 Postoperative ileus.
Comparison 5. Sensitivity analysis: mechanical appendix stump closure (with endoscopic stapler or clip(s)) versus ligation (with Endoloop or intracorporeal knot) using fixed effect model.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total complications | 8 | 850 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.53, 1.13] |
| 2 Intraoperative complications | 8 | 850 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.45, 1.46] |
| 3 Postoperative complications | 8 | 850 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.52, 1.24] |
| 4 Comparison of operative time between mechanical device and ligature | 8 | 850 | Mean Difference (IV, Fixed, 95% CI) | ‐11.94 [‐13.04, ‐10.84] |
| 5 Hospital stay (in days) between mechanical device and ligature | 8 | 850 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.12, 0.16] |
| 6 Postoperative superficial infections | 8 | 850 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.17, 1.26] |
| 7 Postoperative deep infections | 8 | 850 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.31, 2.41] |
| 8 Postoperative ileus | 8 | 850 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.20, 1.15] |
5.1. Analysis.
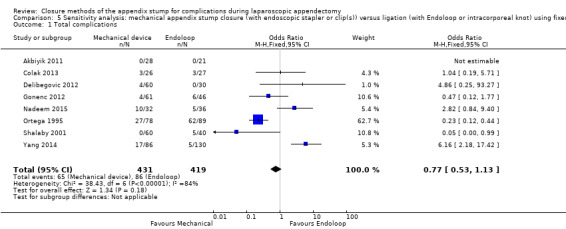
Comparison 5 Sensitivity analysis: mechanical appendix stump closure (with endoscopic stapler or clip(s)) versus ligation (with Endoloop or intracorporeal knot) using fixed effect model, Outcome 1 Total complications.
5.2. Analysis.

Comparison 5 Sensitivity analysis: mechanical appendix stump closure (with endoscopic stapler or clip(s)) versus ligation (with Endoloop or intracorporeal knot) using fixed effect model, Outcome 2 Intraoperative complications.
5.3. Analysis.
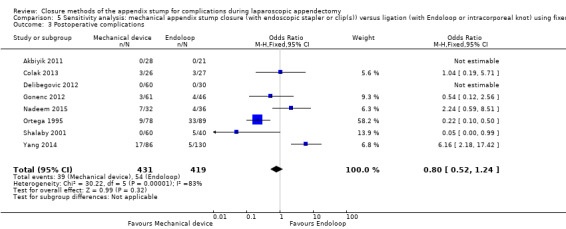
Comparison 5 Sensitivity analysis: mechanical appendix stump closure (with endoscopic stapler or clip(s)) versus ligation (with Endoloop or intracorporeal knot) using fixed effect model, Outcome 3 Postoperative complications.
5.4. Analysis.
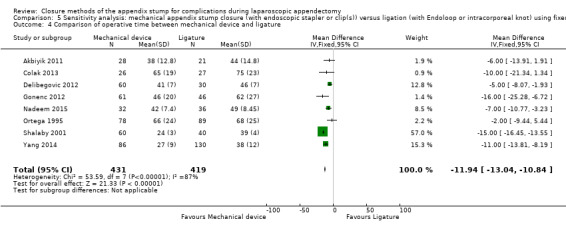
Comparison 5 Sensitivity analysis: mechanical appendix stump closure (with endoscopic stapler or clip(s)) versus ligation (with Endoloop or intracorporeal knot) using fixed effect model, Outcome 4 Comparison of operative time between mechanical device and ligature.
5.5. Analysis.
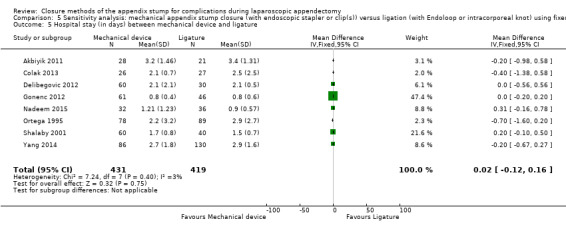
Comparison 5 Sensitivity analysis: mechanical appendix stump closure (with endoscopic stapler or clip(s)) versus ligation (with Endoloop or intracorporeal knot) using fixed effect model, Outcome 5 Hospital stay (in days) between mechanical device and ligature.
5.6. Analysis.
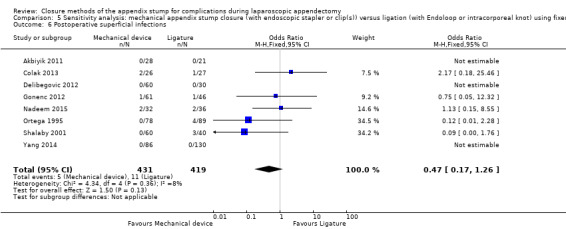
Comparison 5 Sensitivity analysis: mechanical appendix stump closure (with endoscopic stapler or clip(s)) versus ligation (with Endoloop or intracorporeal knot) using fixed effect model, Outcome 6 Postoperative superficial infections.
5.7. Analysis.
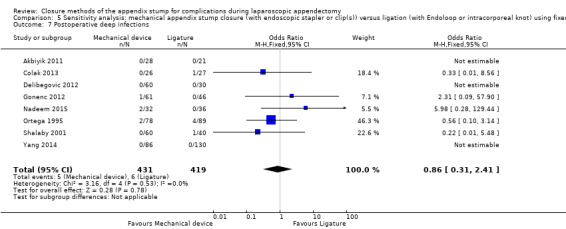
Comparison 5 Sensitivity analysis: mechanical appendix stump closure (with endoscopic stapler or clip(s)) versus ligation (with Endoloop or intracorporeal knot) using fixed effect model, Outcome 7 Postoperative deep infections.
5.8. Analysis.
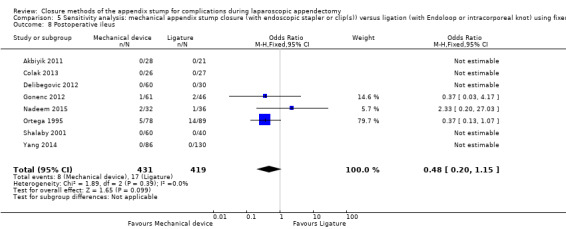
Comparison 5 Sensitivity analysis: mechanical appendix stump closure (with endoscopic stapler or clip(s)) versus ligation (with Endoloop or intracorporeal knot) using fixed effect model, Outcome 8 Postoperative ileus.
Comparison 6. Sensitivity analysis: endoscopic stapler versus ligature (with Endoloop or intracorporeal knot) using fixed effect model.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total complications | 3 | 327 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.14, 0.46] |
| 2 Intraoperative complications | 3 | 327 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.38, 1.39] |
| 3 Postoperative complications | 3 | 327 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.09, 0.41] |
| 4 Comparison of operative time between stapler and ligature | 3 | 327 | Mean Difference (IV, Fixed, 95% CI) | ‐12.99 [‐14.39, ‐11.58] |
| 5 Comparison of hospital stay between stapler and ligature | 3 | 327 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.14, 0.20] |
| 6 Postoperative superficial infections | 3 | 327 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.86] |
| 7 Postoperative deep infections | 3 | 327 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.10, 2.02] |
| 8 Postoperative ileus | 3 | 327 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.13, 1.07] |
6.1. Analysis.
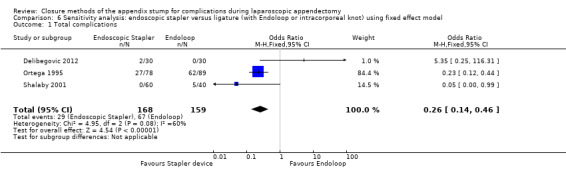
Comparison 6 Sensitivity analysis: endoscopic stapler versus ligature (with Endoloop or intracorporeal knot) using fixed effect model, Outcome 1 Total complications.
6.2. Analysis.
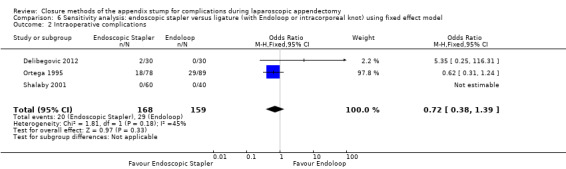
Comparison 6 Sensitivity analysis: endoscopic stapler versus ligature (with Endoloop or intracorporeal knot) using fixed effect model, Outcome 2 Intraoperative complications.
6.3. Analysis.
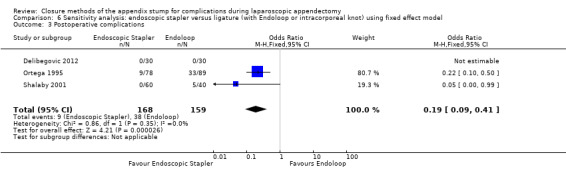
Comparison 6 Sensitivity analysis: endoscopic stapler versus ligature (with Endoloop or intracorporeal knot) using fixed effect model, Outcome 3 Postoperative complications.
6.4. Analysis.

Comparison 6 Sensitivity analysis: endoscopic stapler versus ligature (with Endoloop or intracorporeal knot) using fixed effect model, Outcome 4 Comparison of operative time between stapler and ligature.
6.5. Analysis.
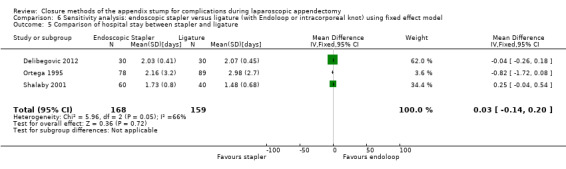
Comparison 6 Sensitivity analysis: endoscopic stapler versus ligature (with Endoloop or intracorporeal knot) using fixed effect model, Outcome 5 Comparison of hospital stay between stapler and ligature.
6.6. Analysis.
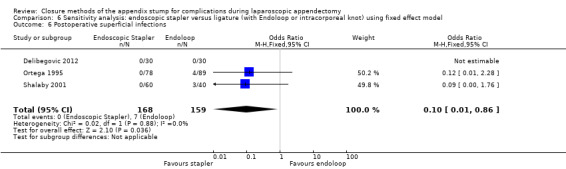
Comparison 6 Sensitivity analysis: endoscopic stapler versus ligature (with Endoloop or intracorporeal knot) using fixed effect model, Outcome 6 Postoperative superficial infections.
6.7. Analysis.
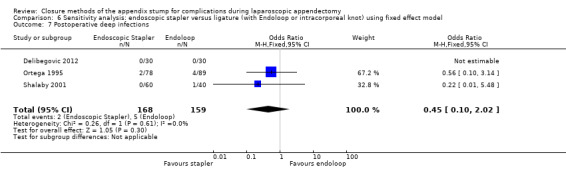
Comparison 6 Sensitivity analysis: endoscopic stapler versus ligature (with Endoloop or intracorporeal knot) using fixed effect model, Outcome 7 Postoperative deep infections.
6.8. Analysis.
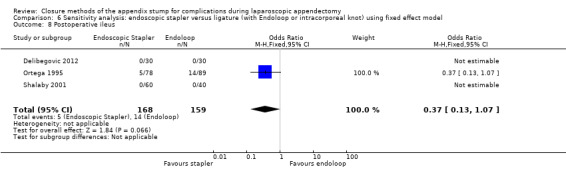
Comparison 6 Sensitivity analysis: endoscopic stapler versus ligature (with Endoloop or intracorporeal knot) using fixed effect model, Outcome 8 Postoperative ileus.
Comparison 7. Sensitivity analysis: clips versus ligature (Endoloop or intracorporeal knot) using fixed effect model.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total complications | 6 | 553 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.31, 4.13] |
| 2 Intraoperative complications | 6 | 553 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.49, 6.56] |
| 3 Postoperative complications | 6 | 553 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.40 [1.28, 4.48] |
| 4 Comparison of operative time between clips and ligature | 6 | 553 | Mean Difference (IV, Fixed, 95% CI) | ‐8.06 [‐9.85, ‐6.26] |
| 5 Comparison of hospital stay between clips and ligature | 6 | 553 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.16, 0.11] |
| 6 Postoperative superficial infections | 6 | 553 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.33, 4.86] |
| 7 Postoperative deep infections | 6 | 553 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.37, 8.58] |
| 8 Postoperative ileus | 6 | 553 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.19, 4.56] |
7.1. Analysis.
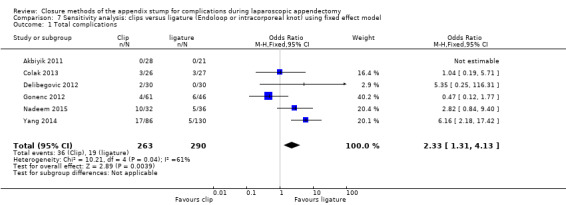
Comparison 7 Sensitivity analysis: clips versus ligature (Endoloop or intracorporeal knot) using fixed effect model, Outcome 1 Total complications.
7.2. Analysis.
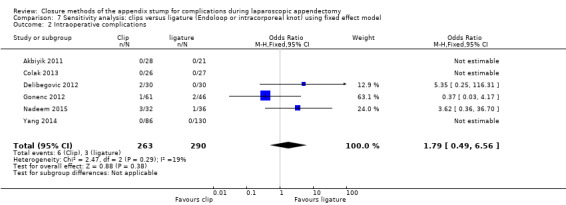
Comparison 7 Sensitivity analysis: clips versus ligature (Endoloop or intracorporeal knot) using fixed effect model, Outcome 2 Intraoperative complications.
7.3. Analysis.
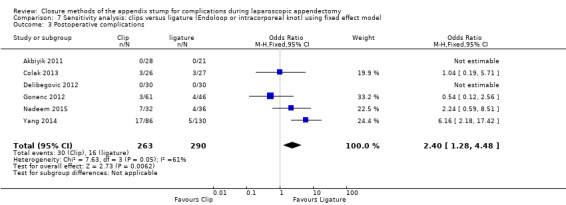
Comparison 7 Sensitivity analysis: clips versus ligature (Endoloop or intracorporeal knot) using fixed effect model, Outcome 3 Postoperative complications.
7.4. Analysis.
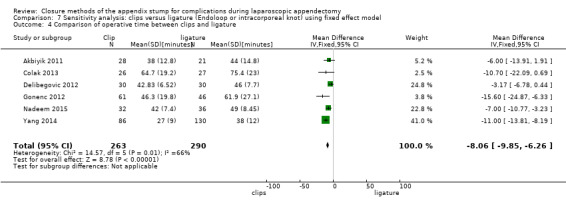
Comparison 7 Sensitivity analysis: clips versus ligature (Endoloop or intracorporeal knot) using fixed effect model, Outcome 4 Comparison of operative time between clips and ligature.
7.5. Analysis.
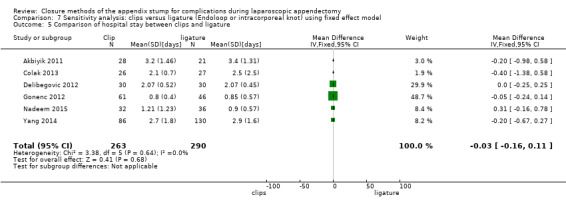
Comparison 7 Sensitivity analysis: clips versus ligature (Endoloop or intracorporeal knot) using fixed effect model, Outcome 5 Comparison of hospital stay between clips and ligature.
7.6. Analysis.
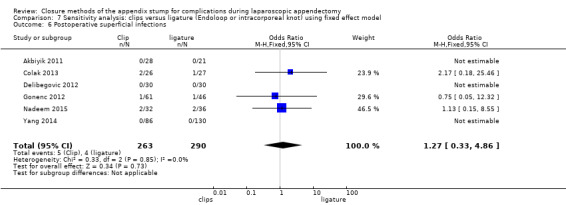
Comparison 7 Sensitivity analysis: clips versus ligature (Endoloop or intracorporeal knot) using fixed effect model, Outcome 6 Postoperative superficial infections.
7.7. Analysis.
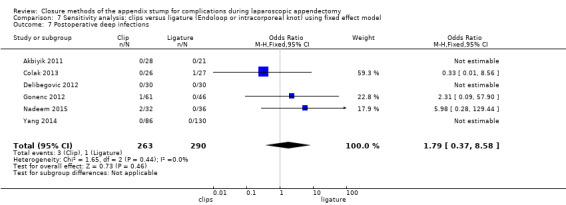
Comparison 7 Sensitivity analysis: clips versus ligature (Endoloop or intracorporeal knot) using fixed effect model, Outcome 7 Postoperative deep infections.
7.8. Analysis.

Comparison 7 Sensitivity analysis: clips versus ligature (Endoloop or intracorporeal knot) using fixed effect model, Outcome 8 Postoperative ileus.
Comparison 8. Sensitivity analysis: endoscopic stapler versus clips using fixed effect model.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total complications | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.60] |
| 2 Intraoperative complications | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.60] |
| 3 Postoperative complications | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Comparison of operative time between stapler and clips | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐3.46 [‐6.94, 0.02] |
| 5 Comparison of hospital stay between stapler and clips | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.28, 0.20] |
| 6 Postoperative superficial infections | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Postoperative deep infections | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Postoperative ileus | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
8.1. Analysis.
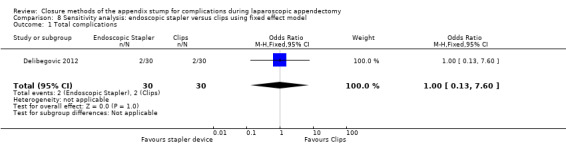
Comparison 8 Sensitivity analysis: endoscopic stapler versus clips using fixed effect model, Outcome 1 Total complications.
8.2. Analysis.
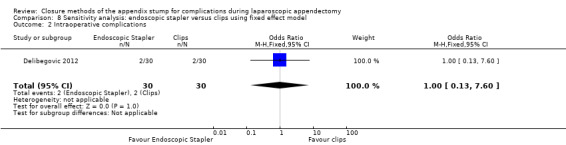
Comparison 8 Sensitivity analysis: endoscopic stapler versus clips using fixed effect model, Outcome 2 Intraoperative complications.
8.3. Analysis.
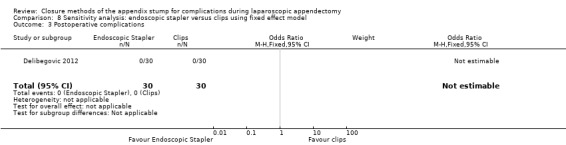
Comparison 8 Sensitivity analysis: endoscopic stapler versus clips using fixed effect model, Outcome 3 Postoperative complications.
8.4. Analysis.
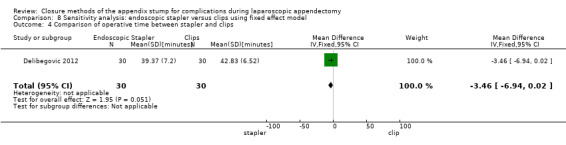
Comparison 8 Sensitivity analysis: endoscopic stapler versus clips using fixed effect model, Outcome 4 Comparison of operative time between stapler and clips.
8.5. Analysis.
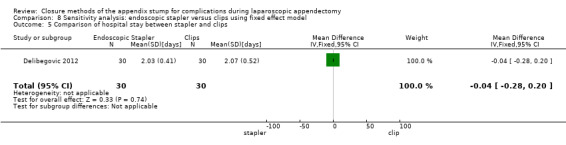
Comparison 8 Sensitivity analysis: endoscopic stapler versus clips using fixed effect model, Outcome 5 Comparison of hospital stay between stapler and clips.
8.6. Analysis.
Comparison 8 Sensitivity analysis: endoscopic stapler versus clips using fixed effect model, Outcome 6 Postoperative superficial infections.
8.7. Analysis.
Comparison 8 Sensitivity analysis: endoscopic stapler versus clips using fixed effect model, Outcome 7 Postoperative deep infections.
8.8. Analysis.
Comparison 8 Sensitivity analysis: endoscopic stapler versus clips using fixed effect model, Outcome 8 Postoperative ileus.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akbiyik 2011.
| Methods | Randomised controlled trial | |
| Participants | Number of participants: 49 Number of centres: 1 Mean age: not specified (age ranged from 1 to 15 years) Number of males: 32 Number of females: 17 Inclusion criteria: diagnosis of acute or perforated appendicitis between May 2008 and May 2009 Exclusion criteria: NS |
|
| Interventions | Intervention arm: hem‐o‐lok clip (non‐absorbable polymeric clips) Control arm: ligature (Endoloop) Antibiotic use: not specified |
|
| Outcomes | Primary outcome measures: intraoperative complications, postoperative complications, and postoperative radiological appearance Secondary outcome measures: cost, operative time, and hospital stay |
|
| Notes | Level of seniority of operating surgeon: all participants operated on by a single surgeon | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "compared prospectively" Comment: information about the sequence generation process insufficient to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is unclear whether participants were aware of the method used. Personnel would likely be aware from operative records. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Personnel would likely be aware which study group participants had been assigned to on the basis of postoperative imaging findings and operative records. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Quote: "Patients were followed up for a period of 1 week to 1 year". Comment: Follow‐up period varied from 1 week to 1 year. No uniform longer‐term outcome data were available for comparison between arms. Additionally, no a priori publication of intended outcomes was identified from either a published trial protocol or trial registration. |
| Other bias | Low risk | This study appears to be free of other sources of bias. |
Colak 2013.
| Methods | Randomised controlled trial | |
| Participants | Number of participants: 53 Number of centres: 1 Mean age: 29 years Number of males: 28 Number of females: 25 Inclusion criteria:diagnosis of acute appendicitis and admission to General Surgery Department of Samsun Education and Research Hospital between September 2010 and July 2011 Exclusion criteria: < 16 years of age, previous major abdominal operations, pregnancy, refusal to consent to participation in the study, and conversion to open appendectomy |
|
| Interventions | Intervention arm: hem‐o‐lok (non‐absorbable polymeric clips) Control arm: ligature (Endoloop) Antibiotic use: prophylactic dose of third‐generation cephalosporin given intravenously after GA induction |
|
| Outcomes | Primary outcome measures: intraoperative complications Secondary outcome measures: operative time and surgical findings |
|
| Notes | Level of seniority of operating surgeon: The same surgical team (level of seniority not specified) performed all operations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients randomly allocated" Comment: computer randomisation method used |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was conducted by using a computer‐generated randomisation schedule". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is unclear whether participants were aware of the method used. Personnel would likely be aware from operative records. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficent to allow judgement, but personnel would likely be aware from operative records. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Four participants excluded postoperatively owing to conversion to open appendectomy, and 3 participants owing to loss of follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No a priori publication of intended outcomes was identified from either a published trial protocol or trial registration. |
| Other bias | Low risk | This study appears to be free of other sources of bias. |
Delibegovic 2012.
| Methods | Randomised controlled trial | |
| Participants | Number of participants: 90 Number of centres: 1 Mean age: 27 years Number of males: 48 Number of female: 42 Inclusion criteria: diagnosis of acute appendicitis and admission to General Surgery Department of University Clinic Center Tuzla, between January 2010 and May 2011 Exclusion criteria: NS |
|
| Interventions | Intervention arm 1: 45‐mm stapler Intervention arm 2: 1 hem‐o‐lok clip (non‐absorbable polymeric clips) Control arm: 1 ligature (Endoloop) Antibiotic use: NS |
|
| Outcomes | Primary outcome measures: intraoperative complications and postoperative complications Secondary outcome measures: cost, operative time, and hospital stay |
|
| Notes | Level of seniority of operating surgeon not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided" Comment: method of randomisation not explicitly specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified, but investigator likely to be aware of allocation pattern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is unclear whether participants were aware of the method used. Personnel would likely be aware from operative records. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to allow judgement, but personnel would likely be aware from operative records. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | No a priori publication of intended outcomes was identified from either a published trial protocol or trial registration. |
| Other bias | Low risk | This study appears to be free of other sources of bias. |
Gonenc 2012.
| Methods | Randomised controlled trial | |
| Participants | Number of participants: 107 Number of centres: 1 Mean age: 27 years Number of males: 61 Number of females: 56 Inclusion criteria: all those given diagnosis of acute appendicitis between December 2010 and May 2011 Exclusion criteria: unwillingness to participate, inability to give informed consent (mental disabilities), age < 15 years, pregnancy, preference for the open procedure, severe sepsis or septic shock on admission, medical or technical contraindication for laparoscopy, American Society of Anesthesiologists class III and IV, intraoperative diagnosis of complicated appendicitis, conversion to an open procedure, and normal appendix at histopathological examination |
|
| Interventions | Intervention arm: titanium endoclip Control arm: intracorporeal knotting Antibiotic use: single dose of cefuroxime axetil (1500 mg, intravenously) during GA induction |
|
| Outcomes | Primary outcome measures: postoperative complications, including re‐admissions, rehospitalisations, and reoperations Secondary outcome measures: operative time, intraoperative complications, and length of hospital stay |
|
| Notes | Level of seniority of operating surgeon: All operations were performed by the residents, who were at least within their second year, under the supervision of the chief resident or the attending surgeon. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done by the lottery method". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "One of the residents who had no idea about the preoperative data and who would not join the operation was chosen as the card picker". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is unclear whether participants were aware of the method used. Personnel would likely be aware from operative records. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to allow judgement, but personnel would likely be aware from operative records. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Patients on intraoperative diagnosis of complicated appendicitis or open appendectomy were excluded from the study". Comment: information on exclusion process insufficient to allow judgement |
| Selective reporting (reporting bias) | Unclear risk | No a priori publication of intended outcomes was identified from either a published trial protocol or trial registration. |
| Other bias | Low risk | This study appears to be free of other sources of bias. |
Nadeem 2015.
| Methods | Randomised controlled trial | |
| Participants | Number of participants: 68 Number of centres: 3 Mean age: 24 years Number of males: 37 Number of females: 31 Inclusion criteria: undergoing laparoscopic appendectomy in 3 tertiary care hospitals in Peshawar from 1 June 2013 to 1 June 2014 Exclusion criteria: perforation of appendix, local and diffuse peritonitis, friable appendix base, evidence of pelvic inflammatory disease, conversion to open procedure, and possible other diagnoses |
|
| Interventions | Intervention arm: metallic endoclip Control arm: extracorporeal ligature tie Antibiotic use: oral cefixime for 5 to 7 days |
|
| Outcomes | Primary outcome measures: bleeding, organ injury, postoperative ileus, intra‐abdominal infection, surgical site infection, re‐admission, and reoperation Secondary outcome measures: cost, operative time, and hospital stay |
|
| Notes | Level of seniority of operating surgeon: All participants underwent minimal access surgery performed by certified surgeons with more than 10 years' experience in laparoscopic procedures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised controlled trial" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "were divided randomly into two groups" Comment: no information on allocation method available to allow judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "single‐blinded" Comment: This is a drawback of these types of trials, as it is impossible to blind surgeons to the procedure; however, single‐blinded suggests that participants were not aware of the method used. Personnel would likely be aware from operative records. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The residents/intern present at the time of procedure would collect the data on data sheets with no blinded investigators who could collect data and at the same time be blinded for the type of procedure done". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No a priori publication of intended outcomes was identified from either a published trial protocol or trial registration. |
| Other bias | Low risk | This study appears to be free of other sources of bias. |
Ortega 1995.
| Methods | Randomised controlled trial | |
| Participants | Number of participants: 253 Number of centres: 10 Mean age: 25 years Number of males: 180 Number of females: 73 Inclusion criteria: clinical diagnosis of appendicitis or lower quadrant pain of uncertain etiology and suitable candidates for laparoscopy and laparotomy Exclusion criteria: pregnancy, minors, prisoners, or incapable of providing informed consent |
|
| Interventions | Intervention arm 1: endoscopic linear stapler (LAS) Intervention arm 2: open appendectomy (OA) Control arm: 2× catgut ligatures (Endoloops) (LAL) Antibiotic use: NS |
|
| Outcomes | Primary outcome measures: intraoperative blood loss, fragmentation of appendix, faecal soilage of abdomen, postoperative abscess, vomiting, ileus, wound infection, and re‐admissions Secondary outcome measures: operative time, pain, length of stay, and resumption of activity |
|
| Notes | Level of seniority of operating surgeon: All participants were operated on by residents with attending surgeons experienced in laparoscopic and open surgical techniques. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was executed". Comment: computer randomisation method used |
| Allocation concealment (selection bias) | High risk | Quote: "computer‐generated random numbers table administered centrally via a toll‐free telephone connection" Comment: probably done Quote: "endoscopic staplers were temporarily unavailable (...), 5 patients with LAS underwent appendectomies with pre‐tied loops" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is unclear whether participants were aware of the method used. Personnel would likely be aware from operative records. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Data collection was performed in a prospective fashion using two standardized data sheets". Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Endoscopic staplers were temporarily unavailable at one point during the study, 5 patients randomised to LAS underwent appendectomies with pre‐tied loops". |
| Selective reporting (reporting bias) | Unclear risk | No a priori publication of intended outcomes was identified from either a published trial protocol or trial registration. |
| Other bias | Unclear risk | Quote: "A subgroup of 134 patients at one institution were evaluated using a visual analogue pain scale"
Quote: "Endoscopic staplers were temporarily unavailable at one point during the study, 5 patients randomised to LAS underwent appendectomies with pre‐tied loops". Comment: insufficient rationale that an identified problem will introduce bias |
Shalaby 2001.
| Methods | Randomised controlled trial | |
| Participants | Number of participants: 150 Number of centres: 2 Mean age: 10 years Number of males: 67 Number of females: 83 Inclusion criteria: diagnosis of acute appendicitis from October 1997 to October 1999 Exclusion criteria: NS |
|
| Interventions | Intervention arm 1: Endo GIA (Ethicon Endo‐Surgery, Cincinnati, Ohio, USA) stapler Intervention arm 2: extracorporeal laparoscopically assisted appendectomy Control arm: ligature (Endoloop) Antibiotic use: 50 mg/kg ceftriaxone preoperatively, then 1 or 2 doses postoperatively. Metronidazole 25 mg/kg to those with suppurative and gangrenous appendicitis |
|
| Outcomes | Primary outcome measures: residual abscess, wound infection, bleeding, and intestinal obstruction Secondary outcome measures: cost, operative time, and hospital stay |
|
| Notes | Level of seniority of operating surgeon not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomly assigned to one of the groups using a table of random numbers. The randomisation procedure was not restricted". |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to allow judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is unclear whether participants were aware of the method used. Personnel would likely be aware from operative records. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to allow judgement, but personnel would likely be aware from operative records. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | No a priori publication of intended outcomes was identified from either a published trial protocol or trial registration. |
| Other bias | Low risk | This study appears to be free of other sources of bias. |
Yang 2014.
| Methods | Randomised controlled trial | |
| Participants | Number of participants: 216 Number of centres: 1 Mean age: NS Number of males: NS Number of females: NS Inclusion criteria: undergoing laparoscopic appendectomy from July 2004 to June 2013 Exclusion criteria: NS |
|
| Interventions | Intervention arm: titanium hem‐o‐lok Control arm: extracorporeal knotting Antibiotic use: NS |
|
| Outcomes | Primary outcome measures: operation time, amount of bleeding, intestinal function recovery time, and hospital stay after operation and complications Secondary outcome measures: NS |
|
| Notes | Published paper translated from Chinese Level of seniority of operating surgeon not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided" |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to allow judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding unlikely to have been in place appropriately |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to allow judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | No a priori publication of intended outcomes was identified from either a published trial protocol or trial registration. |
| Other bias | Unclear risk | Information insufficient to allow judgement |
GA: gestational age. NS: not specified.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ates 2012 | Quasi‐randomised trial |
| Beldi 2004 | Quasi‐randomised trial |
| Sucullu 2009 | Quasi‐randomised trial |
Characteristics of studies awaiting assessment [ordered by study ID]
Lv 2016.
| Methods | Randomised controlled trial |
| Participants | Number of participants: 1100 Number of centres: 1 Mean age: 37 years Number of males: 505 Number of females: 595 Inclusion criteria: undergoing laparoscopic appendectomy from April 2012 to February 2015 with appendicular base < 12 mm in diameter, and acute appendicitis (except in cases of perforation or a gangrenous base). Patients with malignant appendicular diseases including carcinoid tumours, adenocarcinoma, and mucinous adenocarcinoma (confirmed by pathology) were excluded, and a randomised label given to the next patient. Exclusion criteria: conversion to open surgery or malignant appendicular disease |
| Interventions | Intervention arm: absorbable polymeric surgical clips (Lapro‐Clips) Control arm: non‐absorbable polymeric clips (hem‐o‐lok clips) Antibiotic use: not specified |
| Outcomes | Primary outcome measures 1. Postoperative complications including intra‐abdominal abscess, superficial wound infection, appendicular stump leakage, and postoperative abdominal pain (defined as abdominal complaints after surgery requiring prolonged clinical observation or additional biochemical or radiological tests) 2. Re‐interventions including percutaneous and/or transrectal drainage, reoperation (laparoscopy/laparotomy), and prolonged use of intravenous antibiotics (> 3 to 5 days) 3. Duration of the operation (time from skin incision to skin closure), duration of hospital stay, and re‐admission (duration of a re‐admission was included in the hospital stay calculation) |
| Notes | Level of seniority of operating surgeon not specified, but states, "All surgeons participating in this study could perform appendicular closure with Lapro‐Clips or Hemo‐ lok clips proficiently". |
Sadat‐Safavi 2016.
| Methods | Randomised controlled trial |
| Participants | Number of participants: 76 Number of centres: 1 Mean age: 37 years Number of males: 34 Number of females: 42 Inclusion criteria: undergoing laparoscopic appendectomy between 1 March 2013 and 25 May 2015, after receiving clinical diagnosis of acute appendicitis Exclusion criteria: conversion to open surgery or malignant appendicular disease, pain longer than 4 days, mass in the right lower quadrant area identified during examination, phlegmon in images or peritonitis symptoms, underwent surgery that turned into open laparoscopic owing to adhesion and improper anatomical conditions |
| Interventions | Intervention arm: absorbable polymeric surgical clips (Lapro‐Clips) Control arm: non‐absorbable polymeric clips (Hem‐o‐lok clips) Antibiotic use: not specified |
| Outcomes | Primary outcome measures: operative time (minutes), hospital stay (days), wound infection, surgical site pain, technical complications, stump leak, reoperations |
| Notes | Level of seniority of operating surgeon not specified, but states, "all operations were performed by single surgeon" |
Differences between protocol and review
We appropriately refined primary comparisons compared with those in the published protocol (Peng 2012) and followed results of the updated systematic search for 'mechanical devices compared to ligature devices' due to paucity of published research on different subtypes of mechanical devices, as discussed in Quality of the evidence and Implications for research. Furthermore, whereas the protocol specified the inclusion of all studies irrespective of length of publication, we decided that in cases when studies were reported solely in abstract form, we would include them in our quantitative synthesis only if full study data were made available to us. In preparation of this review, these refined inclusion criteria did not result in subsequent exclusion of any studies. In light of the type of outcome measures reported amongst included studies, we deemed Peto odds ratio (Peto OR) with 95% confidence intervals to be more appropriate for dichotomous outcomes when compared with relative risk estimates. However, we have presented the results of both in our sensitivity analysis.
Contributions of authors
Gurdeep S. Mannu coordinated all aspects of the review team and prepared the final manuscript.
Maria Sudul extracted data from published papers, entered data into Review Manager 5, helped carry out the analysis, and helped prepare the final review.
Joao H. Bettencourt‐Silva entered data into Review Manager 5 and helped carry out the analysis.
Elspeth Cumber and Fangfang Kate Li selected which trials were included/excluded and extracted data from included trials.
Allan B Clark provided statistical expertise.
Yoon K Loke helped interpret the analysis within the review and provided methodological expertise.
All review authors agreed on the final version of the manuscript for publication.
Sources of support
Internal sources
West China Hospital, Sichuan University, China.
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies included in this review
Akbiyik 2011 {published data only}
- Akbiyik F, Senel E, Bayram‐Kabacam G, Demirkan H, Atayurt H, Tiryaki T. A comparison of polymer clips and Endoloop applications for securing the appendiceal stump during laparoscopic surgery in children. Surgical Laparoscopy Endoscopy & Percutaneous Techniques 2011;21(5):349‐52. [DOI] [PubMed] [Google Scholar]
Colak 2013 {published data only}
- Colak E, Kement M, Ozlem N, Mutlu T, Yildirim K, Gurer A, et al. A comparison of nonabsorbable polymeric clips and Endoloop ligatures for the closure of the appendicular stump in laparoscopic appendectomy: a prospective, randomized study. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2013;23(3):255‐8. [DOI] [PubMed] [Google Scholar]
Delibegovic 2012 {published data only}
- Delibegovic S. The use of a single Hem‐o‐lok clip in securing the base of the appendix during laparoscopic appendectomy. Journal of Laparoendoscopic & Advanced Surgical Techniques. Part A 2012;22(1):85‐7. [DOI] [PubMed] [Google Scholar]
Gonenc 2012 {published data only}
- Gonenc M, Gemici E, Kalayci MU, Karabulut M, Turhan AN, Alis H. Intracorporeal knotting versus metal endoclip application for the closure of the appendiceal stump during laparoscopic appendectomy in uncomplicated appendicitis. Journal of Laparoendoscopic & Advanced Surgical Techniques. Part A 2012;22(3):231‐5. [DOI] [PubMed] [Google Scholar]
Nadeem 2015 {published data only}
- Nadeem M, Khan SM, Ali S, Shafiq M, Elahi MW, Abdullah F, et al. Comparison of extracorporeal knot‐tying suture and endoclips in laparoscopic appendiceal stump closure in uncomplicated acute appendicitis. Surgical Endoscopy and Other Interventional Techniques 2015;2(29):S528. [Google Scholar]
Ortega 1995 {published data only}
- Ortega AE, Hunter JG, Peters JH, Swanstrom LL, Schirmer B, Laparoscopic Appendectomy Study Group. A prospective, randomized comparison of laparoscopic appendectomy with open appendectomy. American Journal of Surgery 1995;169:208‐13. [DOI] [PubMed] [Google Scholar]
Shalaby 2001 {published data only}
- Shalaby R, Arnos A, Desoky A, Samaha AH. Laparoscopic appendectomy in children: evaluation of different techniques. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2001;11:22‐7. [PubMed] [Google Scholar]
Yang 2014 {published data only}
- Yang X, Si R, Qu K. Comparison study of two approaches for treatment of the appendicular stump during laparoscopic appendectomy. Journal of Laparoscopic Surgery 2014;19(8):610‐2. [Google Scholar]
References to studies excluded from this review
Ates 2012 {published data only}
- Ates M, Dirican A, Ince V, Ara C, Isik B, Yilmaz S. Comparison of intracorporeal knot‐tying suture (polyglactin) and titanium endoclips in laparoscopic appendiceal stump closure: a prospective randomized study. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2012;22(3):226‐31. [DOI] [PubMed] [Google Scholar]
Beldi 2004 {published data only}
- Beldi G, Muggli K, Helbling C, Schlumpf R. Laparoscopic appendectomy using Endoloops: a prospective, randomized clinical trial. Surgical Endoscopy 2004;18:749‐50. [DOI] [PubMed] [Google Scholar]
Sucullu 2009 {published data only}
- Sucullu I, Filiz AI, Kurt Y, Yilmaz I, Yildiz M. The effects of LigaSure on the laparoscopic management of acute appendicitis: "LigaSure assisted laparoscopic appendectomy". Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2009;19(4):333‐5. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Lv 2016 {published data only}
- Lv B, Zhang X, Li J, Leng S, Li S, Zeng Y, et al. Absorbable polymeric surgical clips for appendicular stump closure: a randomized control trial of laparoscopic appendectomy with Lapro‐Clips. Oncotarget 2016;7(27):41265‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sadat‐Safavi 2016 {published data only}
- Sadat‐Safavi SA, Nasiri S, Shojaiefard A, Jafari M, Abdehgah AG, Notash AY, et al. Comparison of the effect of stump closure by endoclips versus Endoloop on the duration of surgery and complications in patients under laparoscopic appendectomy: a randomized clinical trial. Journal of Research in Medical Sciences 2016;21:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Addiss 1990
- Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. American Journal of Epidemiology 1990;132(5):910‐25. [DOI] [PubMed] [Google Scholar]
Andersen 2005
- Andersen BR, Kallehave FL, Andersen HK. Antibiotics versus placebo for prevention of postoperative infection after appendicectomy. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD001439.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreu‐Ballester 2009
- Andreu‐Ballester JC, González‐Sánchez A, Ballester F, Almela‐Quilis A, Cano‐Cano MJ, Millan‐Scheiding M, et al. Epidemiology of appendectomy and appendicitis in the Valencian community (Spain), 1998‐2007. Digestive Surgery 2009;26(5):406‐12. [DOI] [PubMed] [Google Scholar]
Beldi 2006
- Beldi G, Vorburger SA, Bruegger LE, Kocher T, Inderbitzin D, Candinas D. Analysis of stapling versus Endoloops in appendiceal stump closure. British Journal of Surgery 2006;93:1390‐3. [DOI] [PubMed] [Google Scholar]
Buckius 2011
- Buckius MT, McGrath B, Monk J, Grim R, Bell T, Ahuja V. Changing epidemiology of acute appendicitis in the United States: study period 1993–2008. Journal of Surgical Research 2011;7(17):1‐6. [DOI] [PubMed] [Google Scholar]
Daniell 1991
- Daniell JF, Gurley LD, Kurtz BR, Chambers JF. The use of an automatic stapling device for laparoscopic appendectomy. Obstetrics and Gynecology 1991;78:721‐3. [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, editors. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org..
Delibegovic 2009
- Delibegovic S, Matovic E. Hem‐o‐lok plastic clips in securing of the base of the appendix during laparoscopic appendectomy. Surgical Endoscopy 2009;23:2851–4. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanssen 2007
- Hanssen A, Plotnikov S, Dubois R. Laparoscopic appendectomy using a polymeric clip to close the appendicular stump. Journal of the Society of Laparoendoscopic Surgeons 2007;11:59–62. [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. [Google Scholar]
Higgins 2011a
- Higgins JP, Deeks JJ, Altman DG, editors. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org..
Higgins 2011b
- Higgins JP, Deeks JJ, editors. Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org..
Higgins 2011c
- Higgins JP, Altman DG, Sterne JAC, editors. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org..
Houben 1998
- Houben F, Willmen HR. Simplified appendectomy without stump embedding. Experiences of 20 years conventional and 5 years laparoscopic application [Vereinfachte Appendektomie ohne Stumpfversenkung. Erfahrungen aus 20‐jähriger konventioneller und 5‐jähriger laparoskopischer Anwendung.]. Chirurg 1998;69:66‐71. [DOI] [PubMed] [Google Scholar]
Hue 2012
- Hue CS, Kim JS, Kim KH, Nam SH, Kim KW. The usefulness and safety of Hem‐o‐lok clips for the closure of appendicular stump during laparoscopic appendectomy. Journal of Korean Surgical Society 2013;84:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kazemier 2006
- Kazemier G, Hof KH, Saad S, Bonjer HJ, Sauerland S. Securing the appendiceal stump in laparoscopic appendectomy: evidence for routine stapling?. Surgical Endoscopy 2006;20:1473‐6. [DOI] [PubMed] [Google Scholar]
Klaiber 1994
- Klaiber C, Wagner M, Metzger A. Various stapling techniques in laparoscopic appendectomy: 40 consecutive cases . Surgical Laparoscopy & Endoscopy 1994;4:205‐9. [PubMed] [Google Scholar]
Klima 1996
- Klima S, Schyra B. Technique and significance of stump management for outcome of laparoscopic appendectomy. Langenbecks Archieves für Chirugie Supplement Kongressband 1996;113:556‐8. [PubMed] [Google Scholar]
Klima 1998
- Klima S. Appendix stump closure in laparoscopic appendectomy [Bedeutung der Appendixstumpfversorgung bei derlaparoskopischen Appendektomie]. Zentralblatt für Chirurgie 1998;123(Suppl 4):90–3. [PubMed] [Google Scholar]
Krisher 2001
- Krisher SL, Browne A, Dibbins A, Tkacz N, Curci M. Intra‐abdominal abscess after laparoscopic appendectomy for perforated appendicitis. Archives of Surgery 2001;136:438‐41. [DOI] [PubMed] [Google Scholar]
Lange 1993
- Lange J, Zünd MR, Nägeli J. Prospective randomised study: Roeder knot vs Endo‐GIA for laparoscopic appendectomy [Prospektiv randomisierte Studie: Roederschlinge versus Endo‐GIA bei der laparoskopischen Appendektomie [abstract]]. Minerva Invasive Chirugie 1993;2(Suppl 1):8. [Google Scholar]
Lee 2010
- Lee JH, Park YS, Choi JS. The epidemiology of appendicitis and appendectomy in South Korea: national registry data. Journal of Epidemiology 2010;20(2):97‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
Partecke 2010
- Partecke LI, Kessler W, Bernstorff W, Diedrich S, Heidecke CD, Patrzyk M. Laparoscopic appendectomy using a single polymeric clip to close the appendicular stump. Langenbecks Archives für Surgery 2010;395(8):1077–82. [DOI] [PubMed] [Google Scholar]
Pelosi 1992
- Pelosi MA, Pelosi MA 3rd. Laparoscopic appendectomy using a single umbilical puncture (minilaparoscopy). Journal of Reproductive Medicine 1992;37(7):588. [PubMed] [Google Scholar]
Peng 2012
- Peng S, Cheng Y, Zhang Y, Zhou J, Liao Y, Cheng N, Kazemier G, Sauerland S, Zhou ZG. Appendix stump closure during laparoscopic appendectomy. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD006437] [DOI] [Google Scholar]
Pieper 1982
- Pieper R, Kager L. The incidence of acute appendicitis and appendectomy. An epidemiological study of 971 cases. Acta Chirurgica Scandinavica 1982;148(1):45‐9. [PubMed] [Google Scholar]
Rehman 2011
- Rehman H, Rao AM, Ahmed I. Single incision versus conventional multi‐incision appendicectomy for suspected appendicitis. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD009022.pub2] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Röder 1918
- Röder H. On the technique for healing tonsillar disease [Die Technik der Mandelgesundungsbestrebungen]. Aerztl Rundschau 1918;57:169‐71. [Google Scholar]
Sajid 2009
- Sajid MS, Rimple J, Cheek E, Baig MK. Use of endo‐GIA versus endo‐loop for securing the appendicular stump in laparoscopic appendicectomy: a systematic review. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2009;19(1):11‐5. [DOI] [PubMed] [Google Scholar]
Sauerland 2010
- Sauerland S, Jaschinski T, Neugebauer EA. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database of Systematic Reviews 2010, Issue (10). [DOI: 10.1002/14651858.CD001546.pub2] [DOI] [PubMed] [Google Scholar]
Schier 1998
- Schier F. Laparoscopic appendectomy with 1.7‐mm instruments. Pediatric Surgery International 1998;14(1):142–3. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org..
Shaikh 2015
- Shaikh FM, Bajwa R, McDonnell CO. Management of appendiceal stump in laparoscopic appendectomy ‐ clips or ligature: a systematic review and meta‐analysis. Journal of Laparoendoscopic & Advanced Surgical Technigues. Part A 2015;25(1):21‐7. [DOI] [PubMed] [Google Scholar]
Shimi 1994
- Shimi SM, Lirici M, Vander Velpen G, Cuschieri A. Comparative study of the holding strength of slipknots using absorbable and nonabsorbable ligature materials. Surgical Endoscopy 1994;8:1285‐91. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D, editors. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org..
Wilms 2011
- Wilms IMHA, Hoog DENM, Visser DC, Janzing HMJ. Appendectomy versus antibiotic treatment for acute appendicitis. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD008359.pub2] [DOI] [PubMed] [Google Scholar]
Yang 2015
- Yang XJ, Si RH, Ma BQ, Qu KP, Gao P. Comparison study of two approaches for treatment of the appendicular stump during laparoscopic appendectomy. European Surgery ‐ Acta Chirurgica Austriaca 2015;47:S64‐S65. [Google Scholar]


