Abstract
Background
Heparin is an anticoagulant medication that is usually injected subcutaneously. Subcutaneous administration of heparin may result in complications such as bruising, haematoma, and pain at the injection site. One of the factors that may affect pain, haematoma, and bruising is injection speed. For patients and healthcare providers, strategies that can reduce pain and bruising are considered important. Reducing patients' discomfort and concerns whenever and wherever possible is an important aim of nursing. Several studies have been carried out to see if speed of injection affects the amount of pain and bruising where the injection is given, but results of these studies have differed and study authors have not reached a clear final conclusion. This is the first update of the review first published in 2014.
Objectives
To assess the effects of duration (speed) of subcutaneous heparin injection on pain, haematoma, and bruising at the injection site in people admitted to hospitals or clinics who require treatment with unfractionated heparin (UFH) or low molecular weight heparin (LMWH).
Search methods
For this update, the Cochrane Vascular Information Specialist (CIS) searched the Specialised Register (last searched March 2017) and the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 2). The CIS also searched trials registries for details of ongoing or unpublished studies. Review authors searched two Persian databases ‐ Iranmedex and Scientific Information Database (SID) ‐ as well as Google Scholar.
Selection criteria
We sought randomised controlled trials (RCTs) comparing the effects of different durations of subcutaneous injection of heparin on pain, bruising, and haematoma at the injection site.
Data collection and analysis
Two review authors (MM, LJ), working independently, extracted data onto a structured form and assessed study quality. We used the criteria recommended by Cochrane to assess the risk of bias of included studies. For the outcomes, we calculated the mean difference (MD) or the standardised MD (SMD) with corresponding 95% confidence intervals (CIs). We pooled data using fixed‐effect and random‐effects models. We used GRADE to assess the overall quality of evidence supporting outcomes assessed in this review.
Main results
For this update, we identified three new studies and therefore included in the Review four studies with a total of 459 participants who received subcutaneous injections of LMWH into the abdomen. Only one trial reported the injected drug volume (0.4 mL). Owing to the nature of the intervention, it was not possible to blind participants and care givers (personnel) in any included study. Two studies described blinding of outcome assessors; therefore overall, the methodological quality of included studies was moderate. The duration of the fast injection was 10 seconds and the duration of the slow injection was 30 seconds in all included studies.
Three studies reported site pain intensity after each injection at different time points. Two studies assessed site pain intensity immediately after each injection, and meta‐analysis on 140 participants showed no clear difference in site pain intensity immediately post slow injection when compared to fast injection (low‐quality evidence; P = 0.15). In contrast, meta‐analysis of two studies with 59 participants showed that 48 hours after the heparin injection, slow injection was associated with less pain intensity compared to fast injection (low‐quality evidence; P = 0.007). One study (40 participants) reported pain intensity at 60 and 72 hours after injection. This study described no clear difference in site pain intensity at 60 and 72 hours post slow injection compared to fast injection.
All four included studies assessed bruise size at 48 hours after each injection. Meta‐analysis on 459 participants showed no difference in bruise size after slow injection compared to fast injection (low‐quality evidence; P = 0.07). None of the included studies measured the incidence of haematoma as an outcome.
Authors' conclusions
We found four RCTs that evaluated the effect of subcutaneous heparin injection duration on pain intensity and bruise size. Owing to the small numbers of participants, we found insufficient evidence to determine any effect on pain intensity immediately after injection or at 60 and 72 hours post injection. However, slow injection may reduce site pain intensity 48 hours after injection (low‐quality evidence). We observed no clear difference in bruise size after slow injection compared to fast injection (low‐quality evidence). We judged this evidence to be of low quality owing to imprecision and inconsistency.
Keywords: Humans; Middle Aged; Anticoagulants; Anticoagulants/administration & dosage; Anticoagulants/adverse effects; Contusions; Contusions/chemically induced; Contusions/prevention & control; Heparin; Heparin/administration & dosage; Heparin/adverse effects; Injections, Subcutaneous; Injections, Subcutaneous/adverse effects; Injections, Subcutaneous/methods; Pain; Pain/etiology; Pain/prevention & control; Randomized Controlled Trials as Topic; Time Factors
Does the speed of injection make a difference in the amount of pain and bruising in people receiving heparin injections?
Background
Heparin is a drug that is used to help stop blood from clotting. It comes in two forms ‐ unfractionated heparin (UFH) and low molecular weight heparin (LMWH). These are usually given by injection just underneath the skin. Injected heparin goes into the layer of fat under the skin so that it is released slowly into the body. This type of injection can sometimes cause bruising and pain at the site where the needle goes in. It can sometimes result in a swelling that contains blood, called a haematoma. For patients and healthcare providers, strategies that can reduce pain and bruising are considered important. Reducing patients' discomfort and concerns whenever and wherever possible is an important aim of nursing. Several studies have been carried out to see if the speed of injection affects the amount of pain and bruising at the site where the injection is given, but their results differed and study authors did not reach a clear final conclusion.
Study characteristics and key results
We searched for studies that investigated the effects of speed of injection on the amount of pain and bruising where the injection is given (current to March 2017) and found four studies that fitted our review criteria. These studies took place in Turkey, Italy, and China. They enrolled a total of 459 people including 287 female and 172 male participants. All patients received LMWH, and none of the studies used UFH. Participants were treated in hospital, in neurology, orthopaedic, and cardiology units.
Investigators injected heparin slow or fast into the abdomen (stomach) of participants. Participants could watch the injection being given and knew whether it was fast (10 seconds long) or slow (30 seconds long).
Participants given injections said that pain was less with the slow injection after 48 hours. Owing to the small numbers of participants, we found insufficient evidence to determine any effect on pain intensity immediately after injection or at 60 hours and 72 hours after injection. The bruise was not smaller with the slow injection. None of the included studies reported if participants had a swelling with blood inside (haematoma).
Quality of the evidence
We graded the quality of evidence as low because we found only a small number of published studies that reported on this question. These studies were small and had contradictory results. The fact that participants knew whether they received a fast or a slow injection may have affected the results.
Summary of findings
Summary of findings for the main comparison.
Slow versus fast subcutaneous heparin injection for prevention of bruising and site pain intensity
| Slow vs fast subcutaneous heparin injection for prevention of bruising and site pain intensity | |||||
|
Patient or population: patients treated with subcutaneous heparin injections Settings: hospital outpatient and inpatient units Intervention: slow injection (injection speed of 20 or more seconds) Comparison: fast injection (injection speed of less than 20 seconds) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk with fast injection | Corresponding risk with slow injection | ||||
|
Intensity of injection pain immediately after injection (VAS 0 to 10 cm) 0 (no pain) to 10 (worst possible pain) |
Mean pain intensity reported by the 2 studies ranged across fast injection groups from 2 to 5. | Mean pain intensity in the slow injection group was 1.52 points less than in the fast group (3.56 lower to 0.53 higher; P = 0.15). | 140 (2 RCTs) | ⊕⊕⊝⊝a low | |
|
Intensity of injection pain 48 hours after injection (VAS 0 to 10 cm) 0 (no pain) to 10 (worst possible pain) |
Mean pain intensity ranged across fast injection groups from 2.1 to 2.8. | Mean pain intensity in the slow injection group was 1.68 points less than in the fast group (2.91 lower to 0.45 lower; P = 0.007). | 59 (2 RCTs) | ⊕⊕⊝⊝b low | |
|
Bruise size 48 hours after injection (mm/mm2) |
See comment. | Mean bruising size in the slow injection group was 0.6 SD lower than in the fast injection group (1.24 lower to 0.04 higher; P = 0.07). | 459 (4 RCTs) | ⊕⊕⊝⊝c low | Bruise size was measured on different scales; therefore we used the SMD to pool data. |
| Haematoma at injection site | See comment. | No studies measured this outcome. | |||
| *The basis for the assumed risk (e.g. median control group risk across studies) for pain intensity was the range of mean pain score reported following fast injection by the 2 studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the mean difference of the intervention (and its 95% CI). CI: confidence interval; cm: centimetre; mm: millimetre; RCTs: randomised controlled trials; SD: standard deviation; SMD: standardised mean difference; VAS: visual analogue scale. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aWe downgraded the quality of evidence by two steps owing to study limitations, as we identified few studies with small numbers of participants (imprecision) and high heterogeneity (I2 = 73%) (inconsistency). bWe downgraded the quality of evidence by two steps owing to study limitations, as we identified few studies with small numbers of participants (imprecision) and high heterogeneity (I2 = 72%) (inconsistency). cWe downgraded the quality of evidence by two steps owing to study limitations, as we identified few studies with small numbers of participants (imprecision) and high heterogeneity (I2 = 85%) across studies (inconsistency).
Background
Description of the condition
Heparin is an anticoagulant medication that is used to prevent further development of an existing thrombus or new clot formation. Heparin is prescribed for treatment or prevention of thromboembolic disorders. Different forms of heparin are available. Unfractionated heparin (UFH) may be administered by subcutaneous (SC) or intravenous (IV) injection, but low molecular weight heparin (LMWH) is administered only subcutaneously (Hodgson 2007).
Description of the intervention
Subcutaneous administration of heparin is frequently carried out as a nursing intervention (Wooldridge 1988). Subcutaneous injection is normally chosen when slow and continuous absorption of a drug (e.g. insulin, heparin) is needed. The drug is injected into fat and connective tissue underlying the dermis, where less blood flow results in a slower absorption rate. Suitable sites for subcutaneous injections are often the umbilical region of the abdomen and the lateral sides of the arms and thighs (Hunter 2008). Administration and injection techniques that are used for subcutaneous heparin injection may cause adverse outcomes such as bruising, haematoma, and pain at the injection site (Chan 2001; Kuzu 2001). The incidence of bruising at the injection site when 3‐mL and 1‐mL syringes are used has been reported as 69% and 79%, respectively (Hadley 1996).
How the intervention might work
Adverse drug reactions are a relatively common problem that might cause harm to patients. Nurses should apply techniques that minimise the side effects of subcutaneous injections, including site pain, haematoma, and bruising. It can be argued that slow versus fast injection of heparin might significantly reduce pain, haematoma, and bruising at the injection site. However, a systematic review has not been conducted to explore this theory. Slow administration of heparin allows time for subcutaneous tissue to accommodate the injected volume, resulting in reduced pressure, capillary bleeding, and site pain, and minimising the likelihood of other damage (Chan 2001).
Why it is important to do this review
Some patients receive subcutaneous heparin for a long time from the time of hospital admission until after hospital discharge (Delate 2012). With daily heparin injections for several weeks, the risk of extensive bruising in addition to pain at the injection site is high. Therefore, for patients and healthcare providers, strategies that can reduce pain and bruising are considered important. Reducing patients' discomfort and concerns whenever and wherever possible is an important aim of nursing. Several studies have explored the effects of factors such as temperature, syringe size, needle gauge, injection volume, and air bubble in the syringe on the incidence of bruising, haematoma, and pain at the injection site (Chan 2001; Kuzu 2001; Rahmani 2016; Sendir 2015). Other studies have explored the effect of duration of the injection on the incidence of bruising and pain (Dadaeen 2015; Dehghani 2012; Zaybak 2008). Some investigators have recommended that subcutaneous heparin injections must be given slowly to reduce bruising and pain, but others have reported no significant differences between the two techniques in terms of bruising and pain (Balci Akpinar 2008; Chenicek 2004; Pourghaznein 2014; Rahmani 1999). Several studies have compared slow versus fast subcutaneous injection of heparin, but researchers have reported variable results and study authors were not able to reach a clear final conclusion about the exact speed of heparin injection (Chenicek 2004; Sendir 2015; Zaybak 2008). This controversy highlights the importance of conducting a systematic review to explore the effect of different durations of heparin injection on complications at the injection site.
Objectives
To assess the effects of duration (speed) of subcutaneous heparin injection on pain, haematoma, and bruising at the injection site in people admitted to hospitals or clinics who require treatment with unfractionated heparin (UFH) or low molecular weight heparin (LMWH).
Methods
Criteria for considering studies for this review
Types of studies
We searched for randomised controlled trials (RCTs) that we could include in this review. We excluded clinical controlled trials (CCTs), quasi‐randomised controlled trials (QRCTs), and quasi‐experimental studies.
Types of participants
We included studies in which participants were males and females 18 years of age or older who were admitted to hospitals or clinics and treated with subcutaneous injections of heparin including LMWH and UFH. We excluded trials involving people treated with other anticoagulant drugs.
Types of interventions
We included studies in which the intervention consisted of slow subcutaneous administration of heparin (LMWH or UFH) compared to fast subcutaneous administration of heparin. We considered an injection speed of 20 or more seconds as a slow injection, and an injection speed of less than 20 seconds as a fast injection.
Types of outcome measures
Primary outcomes
Pain intensity (measured at injection site by any scale, including visual analogue scale (VAS), numerical rating, McGill scales, and descriptive or other pain scales)
Size of bruise at the injection site
Secondary outcomes
Incidence of haematoma at the injection site
Search methods for identification of studies
We did not restrict language of publication.
Electronic searches
For this update, the Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials.
Cochrane Vascular Specialised Register (20 March 2017).
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 2) via the Cochrane Register of Studies Online.
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and the Allied and Complementary Medicine Database (AMED), and through handsearching of relevant journals. The full list of databases, journals, and conference proceedings that have been searched, as well as the search strategies used, is presented in the Specialised Register section of the Cochrane Vascular Module in the Cochrane Library (Specialised Register; www.cochranelibrary.com).
The CIS also searched the following trials registries for details of ongoing and unpublished studies.
ClinicalTrials.gov (www.clinicaltrials.gov).
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch).
International Standard Registered Clinical/Social Study Number (ISRCTN) Register (www.isrctn.com/).
See Appendix 2 for details of trial registries searches.
In addition, review authors searched Google Scholar (November 2016) and updated the search of two Persian databases ‐ Iranmedex (November 2016) and the Scientific Information Database (SID) (November 2016) ‐ using the search strategies provided in Appendix 3 and Appendix 4 (http://health.barakatkns.com/irmedex/index.asp; http://www.sid.ir/En/index.asp).
Searching other resources
For this update, we reviewed the reference lists of included studies to identify other studies for inclusion. We also tried to contact relevant trial authors to identify additional studies.
Data collection and analysis
Selection of studies
Two review authors (MM, LJ) independently assessed titles and abstracts of the studies retrieved by searching to determine whether each study met the inclusion criteria. If it was not possible to include or exclude a study on the basis of the title or abstract, review authors obtained the full version of the article. The same two review authors (MM, LJ) then assessed the full papers independently to explore if they met the inclusion criteria. We resolved disagreements by discussion and, if necessary, by consultation with the third review author (AAS).
Data extraction and management
Two review authors (MM, LJ) independently extracted data from the included studies using a structured data extraction form. We resolved disagreements by discussion and, if necessary, by consultation with the third review author (AAS). We collected the following data from the included studies: study design; method of randomisation; method of concealment of allocation; blinding, details of participants, details of interventions, and duration of interventions; main inclusion and exclusion criteria; outcomes; methods of measuring pain, bruising, and haematoma; and methods of performing statistical analysis and reporting results.
Assessment of risk of bias in included studies
Two review authors (MM, LJ) independently assessed the risk of bias of included studies. We resolved disagreements by discussion and, if necessary, by consultation with the third review author (AAS). We used the Cochrane 'Risk of bias' tool as described by Higgins when assessing risk of bias (Higgins 2011).
Measures of treatment effect
Bruise size and pain intensity were continuous outcome measures. Therefore, we calculated the mean difference (MD) and 95% confidence intervals (95% CIs) for these outcomes. As investigators measured bruise size using different scales, we calculated the standardised mean difference (SMD) and 95% CIs to pool data.
Unit of analysis issues
Cross‐over trials were eligible for inclusion in this review because heparin has a temporary effect and the half‐life of heparin is 4.5 to 7 hours after administration. Therefore, the first administration of heparin has no effect on formation of bruises at the second injection. We did not expect to find cluster‐randomised trials, and we identified none for inclusion in this review. The individual participant was the unit of analysis.
Dealing with missing data
We contacted trialists to ask them to provide missing information when needed.
Assessment of heterogeneity
We visually inspected forest plots and determined the I2 and Chi2 statistics to evaluate heterogeneity among the included studies.
Assessment of reporting biases
It is recommended that the test for funnel plot asymmetry to detect publication bias should be used when at least 10 studies are included in the review (Higgins 2011). We included only four studies and determined that it was not appropriate to prepare a funnel plot.
Data synthesis
We summarised study outcomes using narrative and quantitative methods. We applied a fixed‐effect model for meta‐analyses unless heterogeneity was high (I2 > 40%), in which case we used a random‐effects model for data synthesis. We used MD or SMD to pool continuous outcomes.
Subgroup analysis and investigation of heterogeneity
For this update, we subgrouped outcomes according to different measures used by trialists to recording bruise size (mm or mm2) and different time points of assessment.
If sufficient studies are included, and if appropriate in future updates, we will perform subgroup analyses according to participants' sex, age, etc.
Sensitivity analysis
We planned to perform a sensitivity analysis to assess possible influences of the following factors on effect size, if sufficient studies met the inclusion criteria of this review.
Exclusion of unpublished studies.
Consideration of the quality of studies.
Exclusion of studies conducted in different countries.
'Summary of findings'
We created a 'Summary of findings' table using GRADEpro software to summarise the evidence derived when investigators compared fast subcutaneous heparin injections versus slow injections in patients requiring heparin (GRADEproGDT 2015) (Table 1). We included outcomes of pain intensity, bruise size, and extent of haematoma, as described under Types of outcome measures. We calculated assumed control intervention risks by using mean measurements in the control groups of selected studies for each outcome. We used the GRADE system to grade the quality of evidence as high, moderate, low, and very low, upon assessing within‐study risk of bias, inconsistency, directness of evidence, imprecision, and publication bias (Atkins 2004).
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
See Figure 1.
Figure 1.
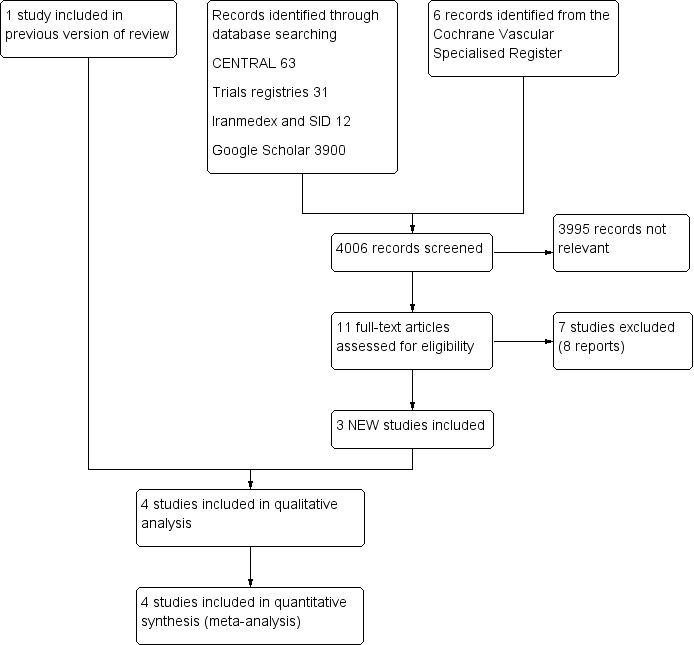
Study flow diagram.
Included studies
For this update, we identified three new studies (Palese 2013; Sendir 2015; Zhao 2016). This review includes four studies with a total of 459 participants that were conducted in Italy (Palese), Turkey (Sendir and Zaybak), and China (Zhao) (Palese 2013; Sendir 2015; Zaybak 2008; Zhao 2016). Three studies were published in English (Palese, Sendir, and Zaybak) and one in Chinese (Zhao) (Palese 2013; Sendir 2015; Zaybak 2008; Zhao 2016). Three studies were small, including from 19 to 100 participants. The largest study involved 300 participants (Palese 2013). Two trials included more females (Palese and Sendir), one included more males (Zhao), and one included equal numbers of males and females (Zaybak) (Palese 2013; Sendir 2015; Zaybak 2008; Zhao 2016).
All studies included participants who received subcutaneous injections of LMWH, and no studies included participants given UFH. All studies performed subcutaneous injections into the abdomen. Only one trial reported the injected drug volume (0.4 mL) (Palese 2013).
The included studies enrolled participants who were hospitalised in orthopaedics units (Palese and Sendir); cardiology units (Zhao); and neurology, orthopaedics, and cardiology units (Zaybak) (Palese 2013; Sendir 2015; Zaybak 2008; Zhao 2016).
Sendir randomised participants to three groups: group A (injection duration 10 seconds), group B (injection duration 30 seconds and 5‐minute dry cold local application), and group C (injection duration 30 seconds) (Sendir 2015). We did not consider results from the group with injection duration of 30 seconds that applied 5‐minute dry cold locally, as this was not an objective of the review (Sendir 2015).
Zhao divided participants into six groups. Four groups investigated the effects of different injection durations and different pressing time on the injection site (Zhao 2016). We did not consider results from these four groups, as these were not the objectives of our review. We included as the control group the group that injected heparin for 10 seconds without pressing, and as the intervention group the group that injected heparin for 30 seconds without pressing (Zhao 2016).
Two studies used an applied self‐controlled design whereby every participant received the slow injection into one side of the abdomen (as intervention) and the fast injection into the other side of the abdomen (as control) (Palese 2013; Zaybak 2008).
Two other studies applied parallel design whereby investigators randomly allocated participants to different groups (Sendir 2015; Zhao 2016).
Only one trial reported the source of funding (Zaybak 2008).
Details of all included studies are given in the Characteristics of included studies table.
Excluded studies
For this update, we excluded seven additional studies (Avsar 2013; Dadaeen 2015; Dehghani 2012; Deng 2009; Fathi 2014; Rahmani 2013; Uzun 2016). In total, we excluded 21 studies. We excluded eight studies because they used a quasi‐randomised design (Babaie Asl 2008; Balci Akpinar 2008; Chan 2001; Dadaeen 2015; Dehghani 2012; Gholam Nezhad 2004; Sanagoo 2011; Tehrani Neshat 2005). We excluded three studies because they used a non‐randomised design (Deng 2009; Nair 2008; Uzun 2016). We excluded two studies because investigators used other anticoagulant drugs during the study (Chenicek 2004; Rahmani 1999). We excluded one study because researchers compared 10‐second heparin injection versus 30‐second heparin injection plus air lock (Fathi 2014). We excluded another study because study authors compared 10‐second heparin injection versus 10‐second injection and waiting for 10 seconds before withdrawing the needle (Rahmani 2013). We excluded one study because heparin injection duration was less than 20 seconds in all comparison groups (Pourghaznein 2014).
The remaining excluded studies compared various techniques of heparin injection but did not explore the effects of injection duration on study outcomes. Two of these studies compared two techniques (McGowan 1990; Wooldridge 1988). One study compared three different techniques (Vanbree 1984). Two studies compared four techniques of heparin injection (Avsar 2013; Jesús Gómez 2005).
Details of the excluded studies are given in the Characteristics of excluded studies table.
Risk of bias in included studies
For a summary of 'Risk of bias', see the Characteristics of included studies table and Figure 2.
Figure 2.
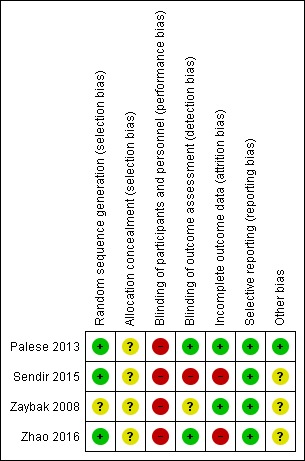
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two studies adequately described methods of random sequence generation (Sendir 2015; Zhao 2016). One of these studies used a computerised randomisation programme to generate random sequence (Sendir 2015). The other study used a table of random numbers (Zhao 2016). However, methods of allocation concealment were unclear in these studies (Sendir 2015; Zhao 2016).
Two studies gave every participant a slow injection into one side of the abdomen and a fast injection into the other side of the abdomen (Palese 2013; Zaybak 2008). Therefore, it was not possible to randomise participants into two separate groups. However, participants could instead be randomised into intervention or control groups according to treatment order. Each participant was given one of the two injection techniques (injection duration of 10 or 30 seconds) for the first injection and the second technique 12 hours later. For each individual, the technique to be used first (injection duration of 10 or 30 seconds) was identified randomly using a randomised sequence. Two studies did not describe the methods of sequence generation and allocation concealment used (Palese 2013; Zaybak 2008). The CIS contacted the authors of the Palese study to request information about the randomisation method used, and they stated that the random sequence was generated by computer (Palese 2013).
Blinding
Owing to the nature of the intervention, it was not possible to blind participants and care givers in all included studies. Two studies described blinding of outcome assessors (Palese 2013; Zhao 2016). The Zaybak study was at unclear risk of detection bias because, although study authors mentioned assessor blinding, they provided no description of assessor blinding for bruising (Zaybak 2008). Sendir investigators reported that they used unblinded methods, leading to high risk of performance bias (Sendir 2015).
Incomplete outcome data
All included studies provided an adequate description of individual study withdrawals and dropouts. Sendir and Zhao reported 6.25% and 10% dropouts in one group, respectively, with no described intention‐to‐treat analysis, so we assigned high risk of bias (Sendir 2015; Zhao 2016).
Selective reporting
Although we did not have access to individual study protocols, study reports suggest that investigators reported all expected outcomes.
Other potential sources of bias
Three studies did not provide clear descriptions about some aspects of heparin injection (e.g. heparin temperature, syringe size, injection volume, air bubble in the syringe) (Sendir 2015; Zaybak 2008; Zhao 2016). These factors may have affected study outcomes, and so we judged these studies as being at unclear risk of other bias.
Effects of interventions
See: Table 1
Pain intensity
Three studies evaluated site pain intensity at different time points after each injection. One study reported pain intensity immediately after injection and at 48, 60, and 72 hours after each injection (Sendir 2015). Another reported pain intensity immediately after injection (Zaybak 2008). The third reported pain intensity at 48 hours after each injection (Zhao 2016). The fourth study did not report injection pain intensity (Palese 2013).
Heterogeneity was high for this outcome, so we pooled results of different studies using a random‐effects method. In addition, we pooled data according to different time points reported in included studies, so numbers of studies and of participants at each time point were small.
Meta‐analysis of data from two trials showed no clear differences in site pain intensity immediately after slow injection compared to fast injection (mean difference (MD) ‐1.52, 95% confidence interval (CI) ‐3.56 to 0.53; participants = 140; I2 = 73%; P = 0.15; low‐quality evidence) (Sendir 2015; Zaybak 2008). See Analysis 1.1.
Analysis 1.1.
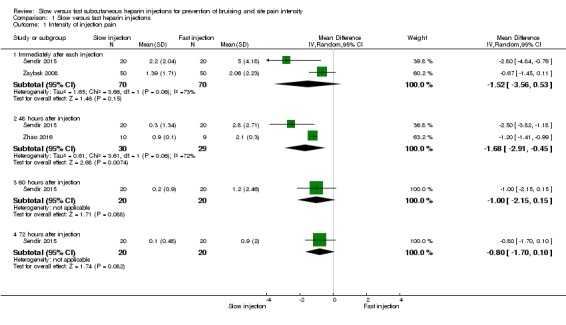
Comparison 1 Slow versus fast heparin injections, Outcome 1 Intensity of injection pain.
Meta‐analysis of data from two RCTs showed reduced site pain intensity 48 hours after slow injection compared to fast injection (MD ‐1.68, 95% CI ‐2.91 to ‐0.45; participants = 59; I2 = 72%; P = 0.007; low‐quality evidence) (Sendir 2015; Zhao 2016). See Analysis 1.1.
Only one study reported pain intensity at 60 and 72 hours after injection (Sendir 2015). This study described no clear differences in site pain intensity at 60 hours post slow injection compared to fast injection (MD ‐1.00, 95% CI ‐2.15 to 0.15; participants = 40; I2 = 0%; P = 0.09); or at 72 hours post injection (MD ‐0.80, 95% CI ‐1.70 to 0.10; participants = 40; I2 = 0%; P = 0.08; low‐quality evidence). See Analysis 1.1.
Size of bruise
Two studies used millimetric measuring papers to measure the area of the bruise and recorded it as square millimetres (mm2) (Sendir 2015; Zaybak 2008). Two studies measured the largest diameter of the bruise and recorded it as millimetres (mm) (Palese 2013; Zhao 2016).
We used the standardised mean difference (SMD) to pool data from these different outcome measures.
All four studies reported bruise size 48 hours after injection. Heterogeneity was high for this outcome (I2= 85%), so we pooled results of different studies using the random‐effects method. We pooled data according to different time points reported in included studies, so the numbers of studies and of participants at each time point were small.
Meta‐analysis revealed no difference in bruise size after slow injection compared to fast injection after 48 hours (SMD ‐0.60, 95% CI ‐1.24 to 0.04; participants = 459; studies = 4; P = 0.07) and detected no subgroup differences (P = 0.43). See Analysis 1.2.
Analysis 1.2.
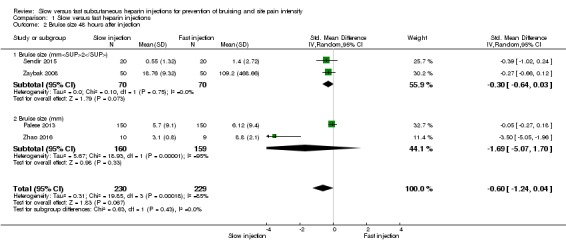
Comparison 1 Slow versus fast heparin injections, Outcome 2 Bruise size 48 hours after injection.
Only one study assessed bruise size after 60 hours, showing no clear differences between intervention and control groups when these data were pooled (MD ‐3.85, 95% CI ‐8.99 to 1.29; participants = 40; P = 0.14; low‐quality evidence) (Sendir 2015). See Analysis 1.3.
Analysis 1.3.
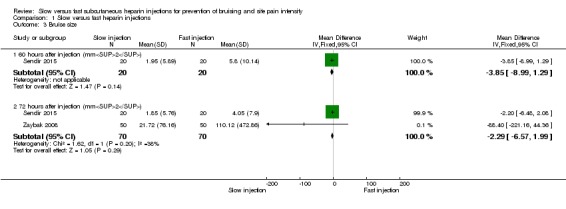
Comparison 1 Slow versus fast heparin injections, Outcome 3 Bruise size.
Two studies assessed bruise size after 72 hours (Sendir 2015; Zaybak 2008). Heterogeneity was low for this outcome (I2 = 38%), therefore we pooled results of these studies using a fixed‐effect method and found no clear difference between slow and fast injections of heparin (MD ‐2.29, 95% CI ‐6.57 to 1.99; participants = 140; P = 0.29). See Analysis 1.3.
Incidence of haematoma
None of the included studies measured the incidence of haematoma as an outcome.
Discussion
Summary of main results
This systematic review incorporated data from four trials enrolling 459 participants to assess the effects of duration of subcutaneous heparin injection on pain and bruise size at the injection site. Results of this review show that slow injection may reduce pain intensity after 48 hours compared to fast injection, but that this reduction may not be detected immediately, nor at 60 or 72 hours after injection (low‐quality evidence). Review results also show that the slow injection technique had no clear effect on bruise size compared to the fast injection technique (low‐quality evidence). None of the included studies measured haematoma incidence after injection.
Overall completeness and applicability of evidence
All studies included in this update injected heparin subcutaneously into the abdomen, but two excluded studies injected heparin into the arm (Babaie Asl 2008; Balci Akpinar 2008). Therefore, it should be considered that pain intensity and bruise size at the injection site could be different depending on whether heparin is injected into the arm, thigh, or abdomen.
Similarily, all included studies used low molecular weight heparin (LMWH), and it is possible that outcomes could have been different had they used unfractionated heparin (UFH). To minimise any confounding effect, we excluded studies in which investigators also treated participants with antiplatelet or anticoagulant drugs because of the possible effects of these agents on bruising and haematoma size. Included studies provided no clear description of how analgesic medications were used. In addition, studies used different injection protocols such as needle gauge, syringe size, heparin volume injected, injection technique used, experience of the injector, etc, and did not clearly describe these. Therefore, it should be considered that these factors, along with injection speed, may also affect pain intensity and bruise size at the injection site.
Quality of the evidence
See Table 1.
All studies included in this review were randomised controlled trials (RCTs), and we assessed the overall methodological quality of these studies as moderate, as the nature of the intervention made a double‐blind study design impossible. Two trials blinded outcome assessors (Palese 2013; Zhao 2016).
We assessed the overall quality of evidence presented using the GRADE approach. We judged the body of evidence related to pain intensity and size of the bruise to be of low quality. We downgraded the quality of the evidence owing to study limitations, as we identified only a few studies with small numbers of participants. This may have affected the precision of results.
Pooling of data from all included studies in the meta‐analyses was impossible, as some studies reported outcomes at different time points or used different measurements, thus reducing the numbers of available studies and participants for each outcome. One study addressed only bruise size (Palese 2013). The other studies addressed all primary outcomes. We detected heterogeneity across studies.
Potential biases in the review process
None of the review authors was involved in any of the included or excluded trials. Furthermore, none of the review authors had any conflicts of interest. To minimise the possibility of bias, we tried to locate eligible RCTs by conducting a broad and comprehensive search of several national and international databases. However, it is still possible that we did not recognise all eligible studies. In addition, two trained review authors independently performed study selection, data collection, and quality assessment of included studies. We followed Cochrane processes as described by Higgins when assessing risk of bias (Higgins 2011).
Agreements and disagreements with other studies or reviews
A recent published systematic review evaluated the effects of heparin injection duration on injection pain intensity and bruising (Yi 2016). Some of the findings of the previous review were not in line with the findings of our review. Yi reported that slow heparin injection reduced pain intensity and bruise size at 48 and 60 hours after each injection (Yi 2016). The Yi review included three RCTs and five quasi‐experimental studies (Yi 2016). Our review did not include quasi‐experimental studies because of potential risk of bias due to inadequate allocation concealment, as this could lead to overestimation of treatment efficacy by 30% to 40% compared to trials with adequate allocation concealment (Schulz 1995). Inclusion of quasi‐experimental studies could account for the difference between results of the two reviews.
In addition, Yi and colleagues limited their search strategies to studies published only in Chinese and English, and our search strategies did not restrict the language of publication (Yi 2016). Although we identified only papers published in Chinese and English in the current version of our review, this may not be the case in future searches, and we believe our search strategy is more comprehensive.
Authors' conclusions
Some patients receive subcutaneous heparin for a long time from hospital admission to after hospital discharge. With daily heparin injections for several weeks, the risk of extensive bruising in addition to pain at the injection site is high. Therefore, patients and healthcare providers consider strategies that can reduce pain and bruising to be important. For this reason, we sought to evaluate the effectiveness of subcutaneous heparin injection duration on pain intensity and bruise size and found four randomised controlled trials (RCTs). Owing to the small numbers of participants, evidence was insufficient for review authors to confirm an effect on pain intensity immediately after injection (low‐quality evidence) or at 60 and 72 hours post injection. However, slow injection may reduce site pain intensity 48 hours after injection (low‐quality evidence). We observed no clear differences in bruise size after slow injection compared to fast injection (low‐quality evidence).
Results of this review suggest that slow injection may be associated with lower pain intensity 48 hours post injection compared to fast injection. However, new trials with a more robust design and focus on different injection techniques and broader outcomes including haematoma might be useful in strengthening our certainty around these results. In addition, trials are needed to evaluate other aspects that might impact adverse outcomes such as bruising, haematoma, and pain at the injection site, for example, different types of heparin given (unfractionated (UFH) or low molecular weight heparin (LMWH)), needle gauge, drug volume injected, cold applied to the injection site, injection site and injection technique used, and injector role and experience.
Acknowledgements
We would like to thank Cochrane Vascular for extensive and kind support provided, and Dr Saharnaz Nedjat for input into the previous version of this review.
Appendices
Appendix 1. CENTRAL search strategy
| Search run on Mon Mar 20 2017 | ||
| #1 | MESH DESCRIPTOR Heparin EXPLODE ALL TREES | 3883 |
| #2 | *heparin*:TI,AB,KY | 9079 |
| #3 | LMWH:TI,AB,KY | 847 |
| #4 | (nadroparin* or fraxiparin* or enoxaparin* or Clexane or klexane or lovenox or dalteparin* ):TI,AB,KY | 2110 |
| #5 | (ardeparin or normiflo or tinzaparin or logiparin or Innohep or certoparin or sandoparin or reviparin or clivarin* or danaproid or danaparoid ):TI,AB,KY | 371 |
| #6 | (Fragmin or Kabi 2165 or Kabi2165 or FR 860 or FR860):TI,AB,KY | 209 |
| #7 | (antixarin or ardeparin* or bemiparin* or Zibor or cy 222 or cy222 or embolex or monoembolex or parnaparin* or rd 11885 or tedelparin or Kabi 2165 or Kabi2165 ):TI,AB,KY | 154 |
| #8 | (emt 966 or emt966 or emt 967 or emt967 or pk 10169 or pk10169):TI,AB,KY | 8 |
| #9 | (cy 216 or cy216 or seleparin* or tedegliparin or seleparin* or tedegliparin* or tedelparin or calciparin*):TI,AB,KY | 70 |
| #10 | (kb 101 or kb101 ):TI,AB,KY | 3 |
| #11 | (lomoparan or orgaran):TI,AB,KY | 28 |
| #12 | (parnaparin or fluxum or lohepa or lowhepa or parvoparin ):TI,AB,KY | 34 |
| #13 | (op 2123 or op2123 ):TI,AB,KY | 1 |
| #14 | (AVE5026 or AVE 5026):TI,AB,KY | 2 |
| #15 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 | 10033 |
| #16 | MESH DESCRIPTOR Injections, Subcutaneous EXPLODE ALL TREES | 3847 |
| #17 | subcutan*:TI,AB,KY | 16583 |
| #18 | (sc or s.c):TI,AB,KY | 7296 |
| #19 | #16 OR #17 OR #18 | 19485 |
| #20 | MESH DESCRIPTOR Contusions | 93 |
| #21 | bruis*:TI,AB,KY | 486 |
| #22 | contus*:TI,AB,KY | 537 |
| #23 | indurat*:TI,AB,KY | 858 |
| #24 | MESH DESCRIPTOR Hematoma | 402 |
| #25 | haematoma*:TI,AB,KY | 525 |
| #26 | hematoma*:TI,AB,KY | 2882 |
| #27 | MESH DESCRIPTOR Pain EXPLODE ALL TREES | 34252 |
| #28 | pain*:TI,AB,KY | 94480 |
| #29 | #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 | 102440 |
| #30 | #15 AND #19 AND #29 | 303 |
| #31 | * NOT SR‐PVD:CC AND 31/08/2013 TO 28/02/2017:DL | 79662 |
| #32 | #30 AND #31 | 63 |
Appendix 2. Trials registries searches
ClinicalTrials.gov
12 studies found for: heparin AND subcutaneous AND pain
4 studies found for: heparin AND subcutaneous AND bruising
World Health Organization International Clinical Trials Registry Platform
5 records for 5 trials found for: heparin AND subcutaneous AND bruising (2 NEW and not NCT)
6 records for 6 trials found for: heparin AND subcutaneous AND pain (2 new not in NCT)
ISRCTN Register
3 results subcutaneous AND heparin AND pain (0 new)
1 results subcutaneous AND heparin AND bruising (0 new)
Appendix 3. Authors' Iranian search strategy
| 1 | Heparin AND Subcutaneous AND Injection (title or abstract) | 12 |
Appendix 4. Google Scholar search strategy
| 1 | ((heparin OR enoxaparin OR LMWH) AND subcutaneous AND injection AND (duration OR speed) AND (bruising OR pain) AND trial)) ABSTRACT | 3900 |
Data and analyses
Comparison 1.
Slow versus fast heparin injections
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intensity of injection pain | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Immediately after each injection | 2 | 140 | Mean Difference (IV, Random, 95% CI) | ‐1.52 [‐3.56, 0.53] |
| 1.2 48 hours after injection | 2 | 59 | Mean Difference (IV, Random, 95% CI) | ‐1.68 [‐2.91, ‐0.45] |
| 1.3 60 hours after injection | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐2.15, 0.15] |
| 1.4 72 hours after injection | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.8 [‐1.70, 0.10] |
| 2 Bruise size 48 hours after injection | 4 | 459 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.24, 0.04] |
| 2.1 Bruise size (mm2) | 2 | 140 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.64, 0.03] |
| 2.2 Bruise size (mm) | 2 | 319 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐5.07, 1.70] |
| 3 Bruise size | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 60 hours after injection (mm2) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐3.85 [‐8.99, 1.29] |
| 3.2 72 hours after injection (mm2) | 2 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐2.29 [‐6.57, 1.99] |
What's new
Last assessed as up‐to‐date: 20 March 2017.
| Date | Event | Description |
|---|---|---|
| 2 October 2017 | Amended | Error in contact details of contact author corrected |
| 2 October 2017 | New citation required but conclusions have not changed | Error in contact details of contact author corrected |
History
Protocol first published: Issue 4, 2009 Review first published: Issue 7, 2014
| Date | Event | Description |
|---|---|---|
| 19 September 2017 | New search has been performed | New search run. Three new studies included and 7 new studies excluded |
| 19 September 2017 | New citation required and conclusions have changed | New search run. Three new studies included and 7 new studies excluded. Text amended to reflect current Cochrane standards. 'Summary of findings' table added. Conclusions changed |
| 1 June 2015 | Amended | Error in plain language summary regarding speed of intervention amended |
Differences between protocol and review
The protocol stated that this review would include randomised controlled trials, clinical controlled trials, and quasi‐experimental studies. However, on the basis of discussions with the Vascular Group editorial base, review authors agreed to exclude clinical controlled trials and quasi‐experimental studies to reduce risk of bias.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: randomised and self‐controlled trial | |
| Participants | Baseline: 150 participants ‐ 102 female and 48 male; mean age of participants was 74.8 (SD 12.37) years Setting: patients who were hospitalised in the 2 orthopaedic units of a teaching hospital in northern Italy Country: Italy Injection site: left or right side of lower abdomen Injection protocol: needle gauge 27.5, 5/8 inch, syringe volume 0.4 mL, enoxaparin 4000 IU Inclusion criteria: received SC injection of LMWH, were monitored for at least 3 days after first injection Exclusion criteria: already received SC heparin injection; had haematological, cardiologic, or liver disease; were pregnant; were taking oral anticoagulant or antiaggregate drugs; had altered integrity of abdominal skin |
|
| Interventions | Slow injection (30 seconds) vs fast injection of heparin (10 seconds) Time between 2 injections was 24 hr. |
|
| Outcomes | Extent of injection site bruising was evaluated at 48 hr after each injection with a plastic ruler to measure maximum horizontal diameter of bruise recorded as mm | |
| Notes | Funding sources: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was reported but how sequence was generated was not described. Quote: "the order of the treatment (A or B) was randomly selected." The CIS contacted trial authors to ask for information about randomisation method; trial authors stated that random sequence was generated by computer. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel blinding was impossible in this trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The decision (treatment A, 10 seconds left hypochondrium; treatment B, 30 seconds right hypochondrium) was written in a paper and placed in an envelope and kept in a locked safe." Quote: "Nurses evaluating the bruising did not know which treatment had been performed on the left and right side of the abdomen. The data analysis was also performed in a blinded fashion. The envelope containing this information was opened after analysis was complete." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Trial authors reported no losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Low risk | No potential other bias was identified. |
| Methods | Randomised parallel controlled trial | |
| Participants | Baseline: 60 participants were divided into 3 groups. We reported the results of 20 participants in the 10‐second heparin injection group and 20 participants in the 30‐second heparin injection group ‐ 13 male and 27 female; mean age in the intervention group was 62.7 (SD 8.83) years and in the control group 57.9 (SD 12.5) years. Setting: patients who were hospitalised in the orthopedic wards of a university hospital in Turkey Country: Turkey Injection site: LMWH was injected SC into the tissue of the lower abdominal wall (umbilical region) Injection protocol: insertion angle 90°, grasping of tissue at injection site, injection without drug aspiration Inclusion criteria: 18 years of age or older, received SC injections of LMWH once a day, had normal platelet values, were conscious, did not have any complications in the perioperative days, did not have acute painful disease Exclusion criteria: were pregnant, had abnormalities of coagulation or haematologic and allergic diseases, received any other injections at the abdominal site during the days of the research, had any incision or scar tissue at the abdominal site |
|
| Interventions | Slow injection (30 seconds) vs fast injection of heparin (10 seconds) | |
| Outcomes | Site pain intensity was assessed by VAS (0 to 100 mm) immediately, and at 48, 60, and 72 hr after injection. Injection site bruising was evaluated at 48, 60, and 72 hr after each injection with a transparent mm ruler to measure the surface area of the bruise and record it as mm2. |
|
| Notes | Study authors were contacted about any use of anticoagulant drugs; they reported that participants were excluded if participants took any anticoagulant drugs before the start of the study. Funding sources: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computerized randomisation program was used to allocate the patients." |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "non‐blinded study design was used." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "non‐blinded study design was used." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6.25% dropout after randomisation in the intervention group, as 4 participants were hospitalised in intensive care unit after randomisation. Use of intention‐to‐treat analysis was not reported. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Unclear risk | Other aspects of heparin injection (e.g. heparin temperature, syringe size, injection volume, air bubble in the syringe) were not clearly described. This may have affected study outcomes. |
| Methods | Design: randomised self‐controlled trial | |
| Participants | Baseline: 50 participants ‐ 25 male and 25 female; mean age of participants was 55.25 (SD 12.37) years Setting: patients who were hospitalised in the neurology, orthopaedics, and cardiology units of a university hospital in Izmir, Turkey Country: Turkey Injection site: right or left side of abdomen Injection protocol: insertion angle 90°, grasping the tissue of the injection site, injection without drug aspiration Inclusion criteria: received SC injection of LMWH, were conscious, platelet values were within normal limits before the trial started Exclusion criteria: were pregnant; had haematological disease, abnormal coagulation, or any allergic disease; received any other injections at the abdominal site during the trial, had any incision or scar tissue at the abdominal site |
|
| Interventions | Slow injection (30 seconds) versus fast injection of heparin (10 seconds) Time between 2 injections was 12 hr. |
|
| Outcomes | Site pain intensity was assessed by VAS (0 to 100 mm) immediately after injection. Injection site bruising was evaluated at 48 and 72 hr after each injection with millimetric measuring paper to measure area of the bruise recorded as mm2. |
|
| Notes | Study authors were contacted, but no response was received. Funding sources: Research Foundation of Ege University, Izmir, Turkey |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was reported but how sequence was generated was not described. Quote: "Each of participant randomised into intervention or control group according to treatment order." |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was impossible in this trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Another rater who was blind to the research operated the stop‐watch to determine the pain period." Assessor blinding for measurement of bruise was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts or losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Unclear risk | Other aspects of heparin injection (e.g. heparin temperature, syringe size, needle gauge, injection volume, air bubble in the syringe) were not clearly described. This may have affected outcomes. |
| Methods | Design: RCT, factorial design | |
| Participants | Baseline: 60 participants were divided into 6 groups. We reported the results of 9 participants in the 10‐second heparin injection group and 10 participants in the 30‐second heparin injection group ‐ 13 male and 6 female; mean age in the intervention group was 61.5 (SD 11.5) years and in the control group 61.6 (SD 6.5) years. Setting: patients who were hospitalised in the cardiology unit of a university hospital in China Country: China Injection site: about 5 cm up and down the navel Injection protocol: injection without drug aspiration Inclusion criteria: received SC injection of enoxaparin sodium 4100 IU; were conscious; platelet values were within the limits of 100,000 to 300,000/dL; activated partial thromboplastin time (APTT) in the reference range 25 to 37.5 seconds; no large abdominal skin bruising, induration, or skin disease; not taking antiplatelet drugs such as aspirin or clopidogrel before the start of the study Exclusion criteria: liver and kidney dysfunction, significant weight loss, body mass index less than 18.5 kg/m2, previously injected with LMWH |
|
| Interventions | Slow injection (30‐second injection) as intervention vs fast injection of heparin (10‐second injection) as control | |
| Outcomes | Site pain intensity was assessed by VAS (0 to 100 mm) immediately after each injection. Extent of injection site bruising was evaluated at 48 hr after injection with ruler to measure the maximum diameter of bruise recorded as mm. |
|
| Notes | Funding sources: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was impossible in this trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researcher who evaluated outcomes was blinded to the group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | One participant in the control group (10%) was lost to follow‐up. Study did not report intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported. |
| Other bias | Unclear risk | Other aspects of heparin injection (e.g. heparin temperature, syringe size, needle gauge, injection volume, air bubble in the syringe) were not clearly described. This may have affected outcomes. |
APTT: activated partial thromboplastin time. CIS: Cochrane Vascular Information Specialist. cm: centimetres. hr: hours. IU: international units. LMWH: low molecular weight heparin. mm: millimetres. RCT: randomised controlled trial. SC: subcutaneous. SD: standard deviation. VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Avsar 2013 | Intervention: comparing 4 different techniques of heparin injection |
| Babaie Asl 2008 | Quasi‐experimental design |
| Balci Akpinar 2008 | Quasi‐experimental design |
| Chan 2001 | Quasi‐experimental design |
| Chenicek 2004 | Participants used anticoagulant drugs during the study |
| Dadaeen 2015 | Quasi‐randomised design |
| Dehghani 2012 | Quasi‐randomised design |
| Deng 2009 | Non‐random design |
| Fathi 2014 | Intervention: comparing 10‐second heparin injection vs 30‐second heparin injection plus air lock |
| Gholam Nezhad 2004 | Quasi‐experimental design |
| Jesús Gómez 2005 | Intervention: comparing 4 administration techniques of heparin injection |
| McGowan 1990 | Intervention: comparing 2 administration techniques of heparin injection |
| Nair 2008 | Non‐random design |
| Pourghaznein 2014 | Intervention: comparing 4 administration techniques of heparin injection with speeds of less than 20 seconds |
| Rahmani 1999 | Participants used anticoagulant drugs during the study |
| Rahmani 2013 | Intervention: comparing 10‐second injection and waiting for 10 seconds before withdrawal of the needle vs 10‐second injection |
| Sanagoo 2011 | Quasi‐experimental design |
| Tehrani Neshat 2005 | Quasi‐experimental design |
| Uzun 2016 | Non‐random design |
| Vanbree 1984 | Intervention: comparing 3 different techniques of heparin injection |
| Wooldridge 1988 | Intervention: comparing 2 administration techniques of heparin injection |
Contributions of authors
MM: design of the review; literature search and identification of trials for inclusion; evaluation of methodological quality of included trials; data extraction; contact with trial authors; interpretation of data; writing of the draft review; and assuming responsibility for writing of future updates. LJ: design of the review; literature search and identification of trials for inclusion; evaluation of methodological quality of included trials; data extraction; interpretation of data; and writing the review. AAS: design of the review; methodological advice; writing of the review; overall supervision.
Sources of support
Internal sources
Tehran University of Medical Sciences, Tehran, Iran.
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Vascular Group editorial base is supported by the Chief Scientist Office.
Declarations of interest
MM: none known. LJ: none known. AAS: none known.
Edited (no change to conclusions)
References
References to studies included in this review
- Palese A, Aidone E, Dante A, Pea F. Occurrence and extent of bruising according to duration of administration of subcutaneous low‐molecular‐weight heparin: a quasi‐experimental case‐crossover study. Journal of Cardiovascular Nursing 2013;28:473‐82. [DOI] [PubMed] [Google Scholar]
- Sendir M, Buyukyilmaz F, Celik Z, Taskopru I. Comparison of 3 methods to prevent pain and bruising after subcutaneous heparin administration. Clinical Nurse Specialist CNS 2015;29(3):174‐80. [DOI] [PubMed] [Google Scholar]
- Zaybak A, Khorshid L. A study on the effect of the duration of subcutaneous heparin injection on bruising and pain. Journal of Clinical Nursing 2008;17(3):378‐85. [DOI] [PubMed] [Google Scholar]
- Zhao B, Yi L, Wang L, Liu J. Effect of injection duration and pressing duration on adverse reactions caused by subcutaneous injection of low molecular weight heparin [低分子肝素皮下注射时间和按压时间对不良反应的影响研究]. Chinese General Practice 2016;19(9):1037‐41. [Google Scholar]
References to studies excluded from this review
- Avsar G, Kasikci M. Assessment of four different methods in subcutaneous heparin applications with regard to causing bruise and pain. International Journal of Nursing Practice 2013;19(4):402‐8. [DOI] [PubMed] [Google Scholar]
- Babaie Asl F. Effect of injection duration on bruise size associated with subcutaneous heparin. Journal of Babol University of Medical Sciences 2008;10(4):49‐55. [Google Scholar]; Babaie Asl F, Kheradmand M, Jafarian R. Effect of duration of subcutaneous heparin injection on its subsequent pain. Feyz 2008;12(2):34‐8. [Google Scholar]
- Balci Akpinar R, Celebioglu A. Effect of injection duration on bruising associated with subcutaneous heparin: a quasi‐experimental within‐subject design. International Journal of Nursing Studies 2008;45(6):812‐7. [DOI] [PubMed] [Google Scholar]
- Chan H. Effects of injection duration on site‐pain intensity and bruising associated with subcutaneous heparin. Journal of Advanced Nursing 2001;35(6):882‐92. [DOI] [PubMed] [Google Scholar]
- Chenicek TE. Effects of Injection Duration on Site‐Pain Intensity and Bruising Associated With Subcutaneous Administration of Lovenox (Enoxaparin Sodium) [Masters thesis]. Tallahassee, Florida (USA): Florida State University School of Nursing, 2004.
- Dadaeen A, Bahrein M, Bazi P, Ostovar A, Raeisi A, Dobaradaran S. The effect of duration of subcutaneous injection on the extent of bruising and pain intensity at injection sites among patients receiving enoxaparin sodium: a randomized self‐controlled clinical trial. International Cardiovascular Research Journal 2015;9:77‐82. [Google Scholar]
- Dehghani KH, Dehghani H, Najari Z. Effect of subcutaneous enoxaparin injection duration on site‐pain intensity in acute coronary syndrome patients hospitalized in CCU Afshar Hospital, Yazd, 2011. Journal of Shahid Sadoughi University of Medical Sciences 2012;20(4):517‐23. [Google Scholar]; Dehghani KH, Najari Z, Dehghani H. Effect of subcutaneous enoxaparin injection duration on bruising size in acute coronary syndrome patients. Iranian Journal of Nursing and Midwifery Research 2014;19(6):564‐8. [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Li R, Li X. The effect of the duration of subcutaneous heparin injection on bruising and pain [低分子肝素皮下注射持续时间对皮下出血和疼痛的影响]. West China Medical Journal 2009;24(2):300‐3. [Google Scholar]
- Fathi R, Imanipour M, Pasheypoor SH, Nikbakht Nasrabadi AR. Effect of simultaneous use of air lock and injection duration on ecchymosis extension and pain intensity associated with subcutaneous heparin injection. Nursing Research. IRANIAN JOURNAL OF NURSING RESEARCH, 2014; Vol. 9, issue 3:62‐7.
- Gholam Nezhad H, Jafari S, Boloorchi Fard F, Allavi Majd H. Effects of Speed of Subcutaneous Heparin Injection on Pain Intensity and Bruising on Orthopedic Patients Hospitalised in Akhtar Hospital at Tehran [Masters thesis]. Tehran, Iran: Faculty of Nursing and Midwifery of Shahid Beheshti University of Medical Sciences and Health Services, 2004. Tehran: Shaheed Beheshti university of medical sciences. ; Jafari S, Gholam Nezhad H, Boloorchi Fard F, Allavi Majd H. Effects of subcutaneous heparin injection duration on pain intensity. Journal of Faculty of Nursing and Midwifery of Shahid Beheshti University of Medical Sciences and Health Services 2004;45(14):13‐8. [Google Scholar]
- Jesús Gómez M, Antonia Martínez M, García I. What is the ideal technique to reduce local complications secondary to subcutaneous enoxaparin administration? A randomized clinical trial [Spanish]. Enfermeria Clinica 2005;15(6):329‐34. [Google Scholar]
- McGowan S, Wood A. Administering heparin subcutaneously: an evaluation of techniques used and bruising at the injection site. Australian Journal of Advanced Nursing 1990;7(2):30‐9. [PubMed] [Google Scholar]
- Nair P, Kaur S, Sharma YP. Effect of time taken in injecting subcutaneous heparin injection with reference to site pain and bruising among patients receiving heparin therapy. Nursing and Midwifery Research 2008;4(1):7‐15. [Google Scholar]
- Pourghaznein T, Vahedian Azimi A, Jafarabadi MA. The effect of injection duration and injection site on pain and bruising of subcutaneous injection of heparin. Journal of Clinical Nursing 2014;23:1105–13. [Article first published online: 12 JUL 2013] [DOI] [PubMed] [Google Scholar]
- Rahmani R. The effect of some physical condition of subcutaneous heparin injection on bruising in patients admitted to cardiac care unit of Baghiatalh Alazam Ag Hospital in 1997. Kowsar Medical Journal 1999;4(Part 2):10‐4. [Google Scholar]
- Rahmani Anaraki H, Farhange Ranjbar M, Abdolahi AA, Behnampour N. The effect of injection duration of subcutaneous enoxaparin sodium on pain intensity and bruising of injection site. Journal of Research Development in Nursing & Midwifery 2013;10(1):10‐6. [Google Scholar]
- Sanagoo A, Kor A, Jouybari L, Shirafkan A, Batyar MM, Nasiri A, et al. A study on the effect of the duration of subcutaneous heparin injection on bruising and pain of Panje Azar Hospital in Gorgan, 2008. Journal of Gorgan Bouyeh Faculty of Nursing & Midwifery 2011;8(1):11‐9. [Google Scholar]
- Tehrani Neshat B, Azizzadeh Forouzi M, Mohammad Alizadeh S. Effects of duration of heparin injection on site‐pain intensity. Scientific Journal of Hamadan University of Medical Sciences and Health Services 2005;11(4):55‐9. [Google Scholar]; Tehrani Neshat B, Azizzadeh Forouzi M, Mohammad Alizadeh S. Study of the relation between duration of injection of subcutaneous heparin and extent of local skin discoloration at the Fatima and Shahid Beheshti Cardiac Hospitals, Shiraz, 2002. Journal of Shahid Sadoughi University of Medical Sciences and Health Services 2005;12(4):86‐94. [Google Scholar]
- Uzun S, Aciksoz S, Arslan F, Yildiz C, Akyol M. The effect of administration protocol of subcutaneous enoxaparin injection on formation of ecchymosis. Orthopaedic Nursing 2016;35(2):120‐5. [DOI] [PubMed] [Google Scholar]
- Vanbree NS, Hollerbach AD, Brooks GP. Clinical evaluation of three techniques for administering low‐dose heparin. Nursing Research 1984;33(1):15‐9. [PubMed] [Google Scholar]
- Wooldridge JB, Jackson JG. Evaluation of bruises and areas of induration after two techniques of subcutaneous heparin injection. Heart and Lung 1988;17(5):476‐82. [PubMed] [Google Scholar]
Additional references
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delate T, Witt DM, Ritzwoller D, Weeks JC, Kushi L, Hornbrook MC, et al. Outpatient use of low molecular weight heparin monotherapy for first‐line treatment of venous thromboembolism in advanced cancer. The Oncologist 2012;17(3):419‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton (ON): McMaster University (developed by Evidence Prime). GRADEproGDT. Version accessed March 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
- Hadley SA, Chang M, Rogers K. Effect of syringe size on bruising following subcutaneous heparin injection. International Journal of Trauma Nursing 1996;2(4):119‐20. [PubMed] [Google Scholar]
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Chichester: John Wiley & Sons.
- Hodgson BB, Kizior RJ. Saunders Nursing Drug Handbook. Philadelphia, PA (USA): Saunders Elsevier, 2007. [Google Scholar]
- Hunter J. Subcutaneous injection technique. Nursing Standard 2008;22(21):41‐4. [DOI] [PubMed] [Google Scholar]
- Kuzu N, Ucar H. The effect of cold on the occurrence of bruising, haematoma and pain at the injection site in subcutaneous low molecular weight heparin. International Journal of Nursing Studies 2001;38(1):51‐9. [DOI] [PubMed] [Google Scholar]
- Rahmani AH, Farhange Ranjbar M, Kavosi A, Nasiri H, Shariati AR. Effect of local cold on pain and bruising at the injection site of subcutaneous enoxaparin sodium. Journal of Research Development in Nursing and Midwifery 2016;11(2):15‐21. [Google Scholar]
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
- Yi LJ, Shuai T, Tian X, Zeng Z, Ma L, Song G. The effect of subcutaneous injection duration on patients receiving low‐molecular‐weight heparin: evidence from a systematic review. International Journal of Nursing Sciences 2016;3(1):79‐88. [Google Scholar]
References to other published versions of this review
- Akbari Sari A, Janani L, Mohammady M, Nedjat S. Slow versus fast subcutaneous heparin injections for prevention of bruising and site‐pain intensity. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD008077.pub3] [DOI] [PubMed] [Google Scholar]
- Mohammady M, Janani L, Akbari Sari A, Nedjat S, Ebrahimi SM. Slow versus fast subcutaneous heparin injections for prevention of bruising and site‐pain intensity. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008077] [DOI] [PubMed] [Google Scholar]
- Mohammady M, Janani L, Akbari Sari A, Nedjat S. Slow versus fast subcutaneous heparin injections for prevention of bruising and site‐pain intensity. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD008077.pub2] [DOI] [PubMed] [Google Scholar]


