Abstract
Background
Children with autistic spectrum disorder (ASD) frequently present with inattention, impulsivity and hyperactivity, which are the cardinal symptoms of attention deficit hyperactivity disorder (ADHD). The effectiveness of methylphenidate, a commonly used ADHD treatment, is therefore of interest in these children.
Objectives
To assess the effects of methylphenidate for symptoms of ADHD (inattention, impulsivity and hyperactivity) and ASD (impairments in social interaction and communication, and repetitive, restricted or stereotypical behaviours) in children and adolescents aged 6 to 18 years with ASD.
Search methods
In November 2016, we searched CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL, 11 other databases and two trials registers. We also checked reference lists and contacted study authors and pharmaceutical companies.
Selection criteria
Randomised controlled trials (RCTs) that investigated the effect of methylphenidate versus placebo on the core symptoms of ASD or ADHD‐like symptoms, or both, in children aged 6 to 18 years who were diagnosed with ASD or pervasive developmental disorder. The primary outcome was clinical efficacy, defined as an improvement in ADHD‐like symptoms (inattention, impulsivity and hyperactivity) and in the core symptoms of ASD (impaired social interaction, impaired communication, and stereotypical behaviours), and overall ASD. Secondary outcomes examined were: rate of adverse events; caregiver well‐being; need for institutionalisation, special schooling or therapy to achieve learning outcomes; and overall quality of life.
Data collection and analysis
We used standard Cochrane methodological procedures. We combined outcome measures that used different psychometric scales, where clinically appropriate. We used a coefficient of 0.6 to calculate standard deviations and adjust for the studies' cross‐over design. We considered a standardised mean difference (SMD) of 0.52 as the minimum clinically relevant inter‐treatment difference. We applied the GRADE rating for strength of evidence for each outcome.
Main results
The studies: we included four cross‐over studies, with a total of 113 children aged 5 to 13 years, most of whom (83%) were boys. We included two studies with five‐year‐old children since we were unable to obtain the disaggregated data for those aged six years and above, and all other participants were in our target age range. All participants resided in the USA. The duration of treatment in the cross‐over phase was one week for each dose of methylphenidate. Studies used a range of outcome scales, rated by parents, teachers or both; clinicians; or programme staff. We report parent‐rated outcomes separately.
Risk of bias: we considered three trials to be at high risk of bias due to selective reporting and all trials to be at unclear risk of bias for blinding of participants and assessors, due to the potential for recognising the side effects of methylphenidate. We judged all trials to be at low or unclear risk of bias for other items.
Primary outcomes: the meta‐analysis suggested that high‐dose methylphenidate (0.43 mg/kg/dose to 0.60 mg/kg/dose) had a significant and clinically relevant benefit on hyperactivity, as rated by teachers (SMD −0.78, 95% confidence interval (CI) −1.13 to −0.43; 4 studies, 73 participants; P < 0.001; low‐quality evidence) and parents (mean difference (MD) −6.61 points, 95% CI −12.19 to −1.03, rated on the hyperactivity subscale of the Aberrant Behviour Checklist, range 0 to 48; 2 studies, 71 participants; P = 0.02; low‐quality evidence). Meta‐analysis also showed a significant but not clinically relevant benefit on teacher‐rated inattention (MD −2.72 points, 95% CI −5.37 to −0.06, rated on the inattention subscale of the Swanson, Nolan and Pelham, Fourth Version questionnaire, range 0 to 27; 2 studies, 51 participants; P = 0.04; low‐quality evidence). There were inadequate data to conduct a meta‐analysis on the symptom of impulsivity. There was no evidence that methylphenidate worsens the core symptoms of ASD or benefits social interaction (SMD −0.51, 95% CI −1.07 to 0.05; 3 studies, 63 participants; P = 0.07; very low‐quality evidence), stereotypical behaviours (SMD −0.34, 95% CI −0.84 to 0.17; 3 studies, 69 participants; P = 0.19; low‐quality evidence), or overall ASD (SMD −0.53, 95% CI −1.26 to 0.19; 2 studies, 36 participants; P = 0.15; low‐quality evidence), as rated by teachers. There were inadequate data to conduct a meta‐analysis on the symptom of impaired communication.
Secondary outcomes: no data were available for the secondary outcomes of caregiver well‐being; need for institutionalisation, special schooling options or therapy to achieve learning outcomes; or overall quality of life. No trials reported serious adverse events. The only adverse effect that was significantly more likely with treatment was reduced appetite as rated by parents (risk ratio 8.28, 95% CI 2.57 to 26.73; 2 studies, 74 participants; P < 0.001; very low‐quality evidence). Subgroup analysis by dose did not identify any significant differences in effect on our primary outcomes between low‐, medium‐ or high‐dose ranges.
Authors' conclusions
We found that short‐term use of methylphenidate might improve symptoms of hyperactivity and possibly inattention in children with ASD who are tolerant of the medication, although the low quality of evidence means that we cannot be certain of the true magnitude of any effect. There was no evidence that methylphenidate has a negative impact on the core symptoms of ASD, or that it improves social interaction, stereotypical behaviours, or overall ASD. The evidence for adverse events is of very low quality because trials were short and excluded children intolerant of methylphenidate in the test‐dose phase. Future RCTs should consider extending the duration of treatment and follow‐up. The minimum clinically important difference also needs to be confirmed in children with ASD using outcome scales validated for this population.
Plain language summary
Effect of methylphenidate for inattentiveness, impulsivity and/or hyperactivity in children aged 6 to 18 years with autistic spectrum disorder
Children with autistic spectrum disorder (ASD) often have trouble paying attention, acting impulsively and sitting still. Methylphenidate, a stimulant drug, is often prescribed to treat children with attention deficit hyperactivity disorder (ADHD) who also have these problems, so it is important to know how well it works for children with ASD.
What is the aim of this review?
The aim of this Cochrane Review was to find out if methylphenidate is helpful for children with ASD. We collected and analysed all relevant studies to answer this question and found four studies.
Key messages
Methylphenidate may improve hyperactivity in children with ASD in the short term, although there was no evidence that methylphenidate improves or worsens ASD symptoms. Some children cannot tolerate the medication's side effects.
What was studied in the review?
We looked for studies that compared children receiving methylphenidate at any dose to placebo (a dummy pill which looks like methylphenidate but has no known effects). We were most interested in investigating the effect of the drug on symptoms of ADHD (inattention, impulsivity and hyperactivity) and ASD (impairments in social interaction and communication, and repetitive, restricted or stereotypical behaviours), but we also looked for information on side effects, caregiver well‐being, the need for special schooling or institutionalisation, and children's overall quality of life.
What are the main results of the review?
We found four studies involving 113 children aged 5 to 13 years and comparing methylphenidate versus placebo. We included two studies with five‐year‐old children because we were unable to separate the data for those aged six years and above, and all other participants were in our target age range. In all of these studies, children took different doses of methylphenidate (low, medium or high) for one week and placebo for another week, and their caregivers (including parents, teachers and clinicians) rated their symptoms at the end of each week. Children who could not tolerate methylphenidate in the test‐dose week (where a dose of medication is given to test the safety and tolerability of the drug) did not participate in the study. All of the studies took place in the USA.
We found that methylphenidate may improve hyperactivity, as assessed by parents and teachers, in the short term. Teachers also tended to report an improvement in children taking methylphenidate in relation to inattention, social interaction, repetitive behaviours, and overall ASD symptoms. However, the studies only lasted for about four weeks, so we do not know if there are any benefits or risks in the long term. There was not enough evidence to say whether methylphenidate has any effect on impulsivity or communication. Teachers and clinicians tended to report more improvement than parents.
We cannot be confident about these findings, mainly because parents and teachers may have recognised which treatment the children were on. The size of the improvement was not very large, except in the case of hyperactivity, where it was probably large enough to really notice the difference. Most of the improvements, except for the improvements in hyperactivity and inattention, could have happened by chance even if methylphenidate is not really effective. We cannot say anything about the likelihood of any harmful effects from methylphenidate, partly because children who had harmful effects prior to the studies, or in the test‐dose phase, are less likely to have participated in the studies.
How up‐to‐date is this review?
The evidence is current to November 2016.
Summary of findings
Summary of findings for the main comparison. High‐dose methylphenidate versus placebo for symptoms of ADHD and ASD as rated by teachers.
| High‐dose methylphenidate versus placebo for symptoms of ADHD and ASD as rated by teachers | ||||||
| Patient or population: children aged 6 to 18 years with ASD Settings: paediatric or psychiatric outpatient or inpatient units, special education units or classes Rater: teachers, clinicians or programme staff Intervention: high‐dose methylphenidate Comparison: placebo Follow‐up: 1 week Measure of effect: if necessary, we transformed results to ensure that lower scores represented fewer symptoms for all comparisons. We standardised results using standardised mean differences (SMD). As such, results are expressed in standardised units, and a negative SMD represents an improvement in symptoms. As a rough guide, an SMD of 0.20 to 0.49 represents a small effect, 0.50 to 0.79 a moderate effect, and ≥ 0.80 a large clinical effect. We used an SMD of 0.52 as the minimum clinically relevant intertreatment difference. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with high‐dose methylphenidate | |||||
| Primary outcome: ADHD‐like symptoms | ||||||
| Inattention Measured using SNAP‐IV inattention subscale (range 0 to 27) |
— | The mean inattention score in the intervention group was 2.72 units lower (5.37 lower to 0.06 lower) | — | 51 teachers (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | — |
| Hyperactivity | — | The mean hyperactivity score in the intervention group was 0.78 standard units lower (1.13 lower to 0.43 lower) | — | 73 teachers (4 RCTs) | ⊕⊕⊝⊝ Lowa,b | — |
| Impulsivity | See comment | — | 36 teachers (1 RCT) |
— | Insufficient data to pool results | |
| Primary outcome: core symptoms of ASD | ||||||
| Impaired social interaction | — | The mean impaired social interaction score in the intervention group was 0.51 standard units lower (1.07 lower to 0.05 higher) | — | 63 teachers (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | — |
| Impaired communication | See comment | — | 24 teachers (1 RCT) |
— | Insufficient data to pool results | |
| Stereotypical behaviours | — | The mean stereotypical behaviours score in the intervention group was 0.34 standard units lower (0.84 lower to 0.17 higher) | — | 69 teachers (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | — |
| Overall ASD | — | The mean overall ASD score in the intervention group was 0.53 standard units lower (1.26 lower to 0.19 higher) | — | 36 teachers (2 RCTs) |
⊕⊕⊝⊝ Lowa,b | — |
| Secondary outcome: rate of adverse effects | ||||||
| Total number of adverse events | See comment | — | 79 teachers (1 RCT) |
— | Insufficient data to pool resultsd | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADHD: attention deficit hyperactivity disorder; ASD: autism spectrum disorders; CI: confidence interval; SNAP‐IV: Swanson, Nolan and Pelham scale, Fourth Revision;RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one point for limitations in design and implementation. bDowngraded one point for imprecision because data came from small studies. cDowngraded one point for indirectness of evidence. dData on individual adverse events are presented in the text.
Summary of findings 2. High‐dose methylphenidate versus placebo for symptoms of ADHD and ASD as rated by parents.
| High‐dose methylphenidate versus placebo for symptoms of ADHD and ASD as rated by parents | ||||||
| Patient or population: Children aged 6 to 18 years with ASD Settings: paediatric or psychiatric outpatient or inpatient units, special education units or classes Rater: parents Intervention: high‐dose methylphenidate Comparison: placebo Follow‐up: 1 week Measure of effect: if necessary, we transformed results to ensure that lower scores represented fewer symptoms for all comparisons. We standardised results using standardised mean differences (SMD). As such, results are expressed in standardised units, and a negative SMD represents an improvement in symptoms. As a rough guide, an SMD of 0.20 to 0.49 represents a small effect, 0.50 to 0.79 a moderate effect, and ≥ 0.80 a large clinical effect. We used an SMD of 0.52 as the minimum clinically relevant intertreatment difference. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with high‐dose methylphenidate | |||||
| Primary outcome: ADHD‐like symptoms | ||||||
| Inattention Measured using SNAP‐IV inattention subscale (range 0 to 27) |
— | The mean inattention score in the intervention group was 3.16 units lower (6.89 lower to 0.57 higher) | — | 71 parents (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | — |
| Impulsivity | See comment | — | 48 parents (1 RCT) |
— | Insufficient data to pool results | |
| Hyperactivity Measured using ABC hyperactivity subscale (range 0 to 48) |
— | The hyperactivity score in the intervention group was 6.61 units lower (12.19 lower to 1.03 lower) | — | 71 parents (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | — |
| Primary outcome: core symptoms of ASD | ||||||
| Impaired social interaction | — | The impaired social interaction score in the intervention group was 0.21 standard units lower (0.60 lower to 0.18 higher) | — | 71 parents (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | — |
| Impaired communication | See comment | — | 48 parents (1 RCT) |
— | Insufficient data to pool results | |
| Stereotypical behaviours | See comment | — | 48 parents (1 RCT) |
— | Insufficient data to pool results | |
| Overall ASD | See comment | — | 48 parents (1 RCT) |
— | Insufficient data to pool results | |
| Secondary outcome: rate of adverse events | ||||||
| Total number of adverse events | See comment | — | 108 parents (1 RCT) |
— | Insufficient data to pool resultsd | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ABC: Aberrant Behavior Checklist; ADHD: attention deficit hyperactivity disorder; ASD: autism spectrum disorders; CI: confidence interval; SNAP‐IV: Swanson, Nolan and Pelham scale, Fourth Revision;RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded one point for limitations in design and implementation. b Downgraded one point for imprecision because data came from small studies. c Downgraded one point for indirectness of evidence. d Data on individual adverse events are presented in the text.
Background
Description of the condition
Autistic spectrum disorder (ASD) is a group of developmental disorders described in theDiagnostic and Statistical Manual of Mental Disorders (DSM), fifth edition (DSM‐5), which includes autistic disorder, Asperger's disorder, childhood disintegrative disorder and pervasive developmental disorder not otherwise specified. ASD encompasses disorders previously referred to as early infantile autism, childhood autism, Kanner's autism, high‐functioning autism and atypical autism. The International Classification of Diseases, 10th revision (ICD‐10) classifies childhood autism as a disorder of psychological development, defined by the presence of abnormal or impaired development that is manifest before three years of age, and the characteristic type of abnormal functioning in all three areas of psychopathology: reciprocal social interaction; communication; and restricted, stereotyped, repetitive behaviour (WHO 2007).
The fourth edition, text revision of the DSM described three characteristic manifestations of ASD (DSM‐IV‐TR): impaired social interaction; impairment of communication; and restricted, repetitive and stereotyped patterns of behaviour (Filipek 1999; Wing 1997). The DSM‐5 uses five diagnostic criteria for the diagnosis of ASD: persistent deficits in social communication and social interaction across multiple contexts; restricted, repetitive patterns of behaviour, interests, or activities; symptoms present in the early developmental period; symptoms causing clinically significant impairment; and disturbances that are not better explained by intellectual disability or global developmental delay. Studies published prior to 2014 used diagnostic criteria from the fourth edition of the DSM (DSM‐IV or DSM‐IV‐TR), as the DSM‐5 was only published in May 2013.
A systematic review of prevalence studies by Williams 2006 estimated an overall prevalence of 7.1 per 10,000 children (95% confidence interval (CI) 1.6 to 30.6) for typical autism and a prevalence of 20.0 per 10,000 children (95% CI 4.9 to 82.1) for ASD (based on the DSM‐IV classification). AABASD 2007 estimated the prevalence of ASD in Australia to be 62.5 per 10,000 for children aged 6 to 12 years. Reported frequencies for ASD across the USA and other countries have approached 1% of the population, with similar estimates in child and adult samples (Brugha 2011). Since DSM‐5 criteria superseded earlier versions of the DSM, reports have shown reduced numbers of children diagnosed with ASD and an increase in the percentage with intellectual impairment (Sturmey 2014).
Early observations of autism by Kanner 1943 led to recognition of the autism spectrum as a heterogenous group of disorders with varying clinical presentations and severities (AAP 2001; NICHD 2014). The onset is typically before three years of age (see DSM‐IV), although diagnosis is often delayed for an additional two or three years (Filipek 2000). Children commonly present with speech delay, poor eye contact, social impairment, unusual or repetitive play, need for routine, difficulty coping with change and obsessions. Approximately 50% or more have intellectual impairment (Sturmey 2014), but others have an intelligence quotient (IQ) in the normal range. Children may also present with abnormal movements, heightened levels of anxiety, phobias, sleeping and eating disturbances, temper tantrums and self‐injurious or aggressive behaviour. Diagnosis of ASD includes history from parents and teachers, clinical observation, psychological and often speech language assessment. Numerous behaviour rating scales have been developed both to aid the assessment of ASD and to monitor response to therapy (AAP 2001; Bertoglio 2009; Filipek 1999; Lord 2000; Santosh 2006; Scahill 2005; Scahill 2006; Wagner 2007; WHO 2007).
Abnormalities of attention (including being overly focused or easily distracted), hyperactivity and impulsivity are common in individuals with ASD (Burack 1997; Lecavalier 2006; Nicolson 2000; Siegel 2012). Studies of children with ASD indicate that 24% to 83% of these children would also meet the criteria for a diagnosis of attention deficit hyperactivity disorder (ADHD) based on their symptoms of inattention, impulsivity and hyperactivity – the cardinal symptoms for ADHD (Frazier 2001; Hyman 2013; Simonoff 2013), although comorbid diagnoses of both ADHD and ASD have only been permitted since the DSM‐5 revision (Hyman 2013). ADHD is a prevalent childhood behavioural disorder, more common in boys, which may persist into adulthood. The prevalence of ADHD in the general population of school‐age children is approximately 3% to 5%, although some reports show even higher incidence (Polanczyk 2007). Children with ADHD appear to have impaired functioning of the prefrontal cortex, a high‐order cortical region that is believed to use representational knowledge of rules and goals, and working memory, to accomplish tasks (Busardò 2016). This region has an important role in inhibiting inappropriate behaviour and sustaining attention. In children with ASD, inattention, impulsivity and hyperactivity increase the risk of poor school performance and academic underachievement, causing further social impairment due to inappropriate and impulsive behaviours, which may also place the child at risk of harm.
The mainstays of treatment for children with ASD are behavioural interventions and pharmacological treatments. The SIGN 2007 guidelines suggest considering behavioural interventions to address a wide range of specific behaviours. There is also some evidence for tailored social communication interventions (such as the use of visual augmentation). Recent Cochrane Reviews of interventions for children with ASD do not support the use of acupuncture (Cheuk 2011), theory of mind skills training (Fletcher‐Watson 2014), omega‐3 fatty acids (James 2011), chelation (James 2015), auditory integration therapy (Sinha 2011), or intravenous secretin (Williams 2012), although there is some evidence that various social competencies may improve with music therapy (Geretsegger 2014), social skills groups (Reichow 2012a), and early intensive behavioural interventions (Reichow 2012b).
Pharmacological treatments have also been widely used in children with ASD to treat both core and co‐morbid symptoms. There is no evidence supporting the use of antidepressants or anticonvulsants to reduce core features of ASD in children (Hurwitz 2012; Williams 2013). There may be a limited role for risperidone, perhaps only in the short term and targeted at specific behaviours (Jesner 2007). Aripiprazole may improve irritability, hyperactivity and repetitive movements in children with ASD in the short term (Ching 2012). Psychostimulants, in particular methylphenidate, have also been prescribed to treat symptoms of hyperactivity, inattention and impulsivity in children with ASD, despite concerns that these medications may worsen ASD symptoms or cause more adverse events than in typically developing children (see Why it is important to do this review).
Description of the intervention
Methylphenidate has been used clinically for over 50 years to treat the symptoms of ADHD in children and is, by far, the most common psychostimulant medication used for this purpose. Over 20 years ago, Aman 1995 identified it as a candidate for treating inattention, impulsivity and hyperactivity in children with ASD. A recent meta‐analysis of the effectiveness of methylphenidate in children with ADHD concluded that methylphenidate may result in improved ADHD symptoms with an estimated effect size of −0.77 standardised mean difference (SMD) (Storebø 2015).
Treatment with immediate‐release formulations of methylphenidate is usually initiated at 5 mg once or twice daily, up to a maximum of 60 mg per day. Treatment with modified‐release formulations of methylphenidate is usually initiated at a dose of 18 mg once daily (in the morning), and increased, if necessary, up to a maximum of 54 mg once daily (NICE 2013). Methylphenidate is also available in some countries in an extended‐release form as a transdermal patch (Mayo Clinic 2014). Short‐release formulations of methylphenidate are absorbed rapidly within 30 minutes, and their effects last up to six hours. Long‐acting or modified‐release forms usually contain both immediate‐ and delayed‐release formulations and are taken once daily (in the morning). They are available in 8‐ and 12‐hour preparations. The advantages of these controlled‐release products include the possible decrease in likelihood and severity of rebound symptoms (Szymansk 2001) and an increase in compliance in children due to reduced dosing frequency and fewer tablets. With short‐acting formulations, the maximum recommended dose is 1.5 mg/kg/day or 60 mg in two or three divided doses (TGL 2012). This can be given as a once‐daily dose in the combination formulations.
The optimal dose of methylphenidate is based on observations of clinical response by the individual, as individual responses are variable, and the optimum dose is not predictable. The dose is initially titrated by increasing the daily dose every week until the effects are observable or until adverse events warrant dose reduction or cessation. In children with ADHD, the ability to attend and focus, particularly in busy environments such as the classroom, indicates clinical effect. Most side effects of methylphenidate are dose dependent (Rossi 2010). Common side effects include headache, loss of appetite, abdominal discomfort, nausea, anxiety and insomnia (Rossi 2010). These effects could lead to intolerance of the drug. Methylphenidate may also increase blood pressure and heart rate, and consultation with a cardiologist is recommended before starting the drug in children with cardiac abnormalities. It also infrequently causes other serious conditions, such as growth restriction, psychosis, liver dysfunction and neuroleptic malignant syndrome, for which monitoring is needed (Medsafe 2010).
How the intervention might work
Although its mechanism of action is uncertain, a number of animal and human studies have investigated the effects of methylphenidate on the way various tasks are performed, and brain imaging and brain chemistry studies have attempted to elucidate how it might work. Methylphenidate is believed to improve symptoms of ADHD by increasing the action of catecholamines, which are a class of neurotransmitters (chemicals that transmit messages in the brain) that include dopamine and noradrenaline (Wilens 2008). Methylphenidate acts in certain areas of the brain, particularly in the prefrontal cortex and striatum. The potential mechanisms by which methylphenidate enhances the action of dopamine include blocking processes in the nerve endings in the brain that remove dopamine from areas where it plays an active role, and extending its duration of action. Methylphenidate is also believed to have a number of complex actions on various dopamine receptors (particularly D2 dopamine autoreceptors and D1 dopamine receptors) in the nervous tissue of the brain. Methylphenidate increases the action of noradrenaline and may have an effect on other neurotransmitters, such as histamine, acetylcholine and serotonin, which, in turn, modify the action of catecholamines (Wilens 2008). The mechanisms by which methylphenidate might work in children with ASD who have inattention, hyperactivity and impulsivity have not yet been established.
Why it is important to do this review
Methylphenidate is commonly believed to be effective for children with ADHD, and a 2015 Cochrane Review of 185 studies of methylphenidate in children with ADHD found that methylphenidate may improve teacher‐rated ADHD symptoms and general behaviour as well as parent‐rated quality of life (Storebø 2015). Up to one third of adolescents with ASD who also meet criteria for a diagnosis of ADHD are currently treated with psychostimulants, most commonly methylphenidate (Frazier 2011). However, a number of studies in the 1970s and 1980s reported that stimulant medication, particularly dextroamphetamine, resulted in increased stereotypical movements in children with ASD (Birmaher 1988; Campbell 1975; Di Martino 2004). Concerns about stereotypy were reinforced by animal research demonstrating stimulant‐induced stereotypy in several animal species (Aman 1982). These concerns about methylphenidate worsening these core symptoms of ASD resulted in methylphenidate being considered contraindicated in children with ASD (Aman 1982; Birmaher 1988). Additional adverse events that may impact negatively on social interaction, including irritability, aggression, self‐mutilating behaviour, emotional lability and dysphoria have also been frequently reported in children with ASD who are treated with psychostimulants (Cortese 2012; Di Martino 2004; Ghuman 2009). However, methylphenidate has also been found to increase positive social interactions and social behaviour and to reduce social anxiety (Patin 2015). Moreover, studies have suggested that methylphenidate improves emotion processing (Conzelmann 2011; Schlochtermeier 2011), also helping children and adolescents with ADHD to recognise faces, facial emotions or both (Demirci 2016;Williams 2008), which may lead to improved social interaction. In addition, methylphenidate has been reported to reduce social interaction deficits in a mouse model of autistic spectrum disorders (Hara 2015), and methylphenidate appeared to improve some social behaviours and self‐regulation in one study of children with ASD (Jahromi 2009 in RUPP 2005). The effect of methylphenidate on impaired social interaction and communication in children with ASD is, however, unknown.
Psychostimulants may be effective in the treatment of ADHD‐like symptoms in children and adolescents with ASD (Cortese 2012). However, the effect of psychostimulants on the core symptoms of ASD (impaired communication, impaired social interaction, and repetitive, restricted or stereotypical behaviours) is uncertain, and their potential to worsen these symptoms remains a concern. It is important to identify whether similar effect sizes and similar risks of adverse events are present in children with ASD and children with ADHD but without ASD.
Objectives
To assess the effects of methylphenidate for symptoms of ADHD (inattention, impulsivity and hyperactivity) and ASD (impairments in social interaction and communication, and repetitive, restricted or stereotypical behaviours) in children and adolescents aged 6 to 18 years with ASD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised (double‐blinded) controlled trials (RCTs).
Types of participants
Children and adolescents aged 6 to 18 years of age, diagnosed with ASD using criteria from DSM III, DSM‐IV or DSM‐IV‐TR, or diagnosed with pervasive developmental disorder according to the ICD‐10 (WHO 2007). We included children with concurrent diagnoses, such as anxiety disorder, intellectual impairment, learning delays and speech impairment, and congenital syndromes such as fragile X syndrome. We included children receiving co‐interventions, including psychotropic medications, dietary modifications and therapist‐based interventions such as speech, occupational and psychological therapy. We excluded children with other established causes of cognitive or behavioural problems such as acquired brain injury.
Types of interventions
We included trials if they used methylphenidate, irrespective of formulation or dose, and compared it to a placebo. We included trials giving methylphenidate to participants in addition to other psychotropic medications if these were provided to both arms. We included trials in which the treatment was administered in any setting, including the home, hospital or residential care.
Types of outcome measures
Primary outcomes
-
Clinical efficacy
An improvement in ADHD‐like symptoms (inattention, impulsivity and hyperactivity), as measured by psychometric instruments or observations of behaviour such as the fourth revision of the Swanson, Nolan and Pelham (SNAP‐IV) questionnaire, Conners' Global Index, and Conners' Parent Rating Scale ‐ Revised (CPRS‐R) (Kollins 2010)
An improvement in the core symptoms of ASD (impaired social interaction, impaired communication, and stereotypical behaviours), and overall ASD, as measured by psychometric instruments or observations of behaviour such as the Aberrant Behaviour Checklist (ABC) and the Child Autism Rating Scale (CARS) (Lord 2014)
Secondary outcomes
Rate of adverse events, for example, common adverse events of nausea and insomnia, and also more serious adverse events such as growth retardation and hypertension
Caregiver well‐being, including levels of parental stress, as assessed using scales such as the Parenting Stress Index (Abidin 1983)
Need for institutionalisation of children or adolescents, special schooling options or therapy to achieve learning outcomes, as measured either dichotomously (by need or no need) for institutionalisation of children or adolescents, special schooling options or therapy, or by duration of institutionalisation, special schooling options or therapy
Overall quality of life of the child or adolescent, as measured by a validated overall quality of life scale such as the Pediatric Quality of Life Inventory (Kollins 2010)
We listed our primary outcomes and rate of adverse events in a 'Summary of findings' table indicating levels of evidence for the findings. For more information see GRADE and 'Summary of findings', beneath Data synthesis.
Search methods for identification of studies
Electronic searches
We searched the following databases in May 2014 and again in November 2016. Appendix 1 reports search strategies for each source, and Appendix 2 provides further details (including exact search dates).
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 4), in the Cochrane Library, which contains the Developmental, Psychosocial and Learning Problems Specialised Register (searched 21 November 2016).
MEDLINE Ovid (1946 to November Week 2 2016).
MEDLINE In‐Process & Other Non‐indexed Citations Ovid (18 November 2016).
MEDLINE Epub Ahead of Print Ovid (18 November 2016).
Embase Ovid (1974 to 18 November 2016).
CINAHLPlus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1936 to 21 November 2016).
PsycINFO Ovid (1806 to November Week 2 2016).
ERIC EBSCOhost (Education Resources Information Center; 1966 to 21 November 2016).
ERIC Proquest (Education Resources Information Center; 1966 to 16 May 2014).
Science Citation Index Web of Science (SCI; 1970 to 22 November 2016).
Social Sciences Citation Index Web of Science (SSCI; 1970 to 22 November 2016).
Conference Proceedings Citation Index ‐ Science Web of Science (CPCI‐S; 1990 to 22 November 2016).
Conference Proceedings Citation Index ‐ Social Sciences & Humanities Web of Science (CPCI‐SS&H; 1990 to 22 November 2016).
Cochrane Database of Systematic Reviews (CDSR; 2016, Issue 11) part of the Cochrane Library (searched 21 November 2016).
Database of Abstracts of Reviews of Effects (DARE; 2015, Issue 2) part of the Cochrane Library (searched 21 November 2016).
AutismData (autism.org.uk/autismdata; searched 22 November 2016).
Proquest Dissertations & Theses (searched 2 December 2016).
ClinicalTrials.gov (clinicaltrials.gov; searched 22 November 2016).
World Health Organization International Clinical Trials Registry Platform (WHO IRCTP; apps.who.int/trialsearch; searched 22 November 2016).
Searching other resources
We contacted drug manufacturers by email, including Mallinckrodt, Novartis, Janssen Pharmaceuticals, Shire and Medice, to obtain unpublished data. We also searched the references of relevant studies and (systematic) reviews to identify additional studies. Finally, we contacted the first author of included RCTs as well as specialists in developmental paediatrics to enquire about other relevant studies.
Data collection and analysis
Selection of studies
Two review authors (NS, MVD) independently read the title and abstracts of all records yielded by the search to determine suitability according to the criteria mentioned above (Criteria for considering studies for this review). They then obtained the full‐text reports of potentially relevant studies, or studies for which more information was needed, and assessed them for eligibility. The two review authors resolved any disagreements by discussion or asked a third review author (LD) to act as arbiter. We listed any excluded studies that initially appeared eligible for inclusion, along with the reasons for exclusion after full‐text review. We created a PRISMA flow diagram illustrating the selection process (Liberati 2009).
Data extraction and management
Two review authors (NS, LD) extracted the following data using the piloted data extraction sheet shown in Appendix 3: type of study, participants, type of intervention (including dose and administration form), measurement scales used, and reported outcomes. A third review author (MVD) checked the extracted data in case of discrepancies that could not be resolved by discussion.
Assessment of risk of bias in included studies
We used a checklist to assess the risk of bias in each included study. Using the Cochrane 'Risk of bias' assessment tool (Higgins 2017), two review authors (NS, MVD) independently assessed each study as being at low, high or unclear risk of bias on each of the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias (see Appendix 4 for a detailed description of the criteria used). In the event of any disagreements, a third review author (LD) evaluated the study and a consensus was reached.
Measures of treatment effect
Continuous outcome data
We use the standardised mean difference (SMD) for all continuous outcomes because this enabled us to combine and compare the results of the different scales used to measure outcomes that are conceptually the same. Where outcomes were assessed using the same scale in separate studies, we used the mean difference (MD). If necessary, we transformed results to ensure that a negative MD or SMD indicated an improvement in functioning for all comparisons.
The SMD expresses the size of the intervention effect in each included study relative to the variability observed in that study (Deeks 2017). This method is based on the assumption that the differences in standard deviations (SD) between trials reflect differences in scales and not real differences in variability between the populations included in the trials. The SMD is 'scale free', that is, since the dependent variable is standardised, the original units are replaced by standardised units. We used Hedges' g formulation to calculate the SMD. It is calculated by dividing the mean differences between groups by the SD. Hedges' g uses a weighting to account for the population sizes in the different studies (Egger 2001).
We included clinician or other trained observer ratings with teacher ratings in a single meta‐analysis where outcomes were measured using psychometric scales rated by a number of different observers (for example, teachers, trained observers and/or clinicians), providing participants were rated in classroom or similarly structured contexts. However, we considered that parent ratings should be considered as a separate outcome because children's behaviour at home may differ from that in more structured clinical or educational settings, and because teachers (or clinicians) have different relationships and contact hours with children compared to parents and may place greater emphasis on academic aspects (Zhang 2005).
We reported effect sizes separately for low, medium and high doses of methylphenidate. We did not combine the data from different doses within included studies because these are repeated measures on the same participants. We reported doses per kg of body weight, whether studies used individualised doses calculated per kg of participant body weight, or used proprietary doses of 2.5 mg, 5 mg, 10 mg and 20 mg of methylphenidate with adjustment for participant weight. We reported data from high‐dose methylphenidate as our primary outcome and performed a subgroup analysis of our results for medium‐ and low‐dose methylphenidate.
Low, medium and high doses are calculated based on the mg/kg/dose ranges. We did not use mg/kg/day because parents or clinicians (or both) may withhold afternoon doses because of adverse events. Low‐dose methylphenidate included doses between 0.11 mg/kg/dose and 0.21 mg/kg/dose, medium‐dose methylphenidate included doses between 0.22 mg/kg/dose and 0.36 mg/kg/dose, and high‐dose methylphenidate included doses between 0.43 mg/kg/dose and 0.6 mg/kg/dose.
Dichotomous outcome data
We reported dichotomous outcomes (such as presence or absence of adverse events and institutionalisation) as risk ratios (RRs), which we calculated as the proportion of patients in the treatment group who experienced the outcome (or event) divided by the proportion of participants in the control group who experience the outcome (or event). Where there were no events in either the treatment group or the control group, we reported these outcomes separately without pooling them. We calculated 95% CIs for all dichotomous outcomes.
Unit of analysis issues
Cross‐over trials
We analysed cross‐over trials, in which each individual participant was allocated to a sequence of interventions (for example, placebo, low dose, medium dose, high dose) in a semi‐randomised order, as follows. If there were no carry‐over or period effects, the appropriate analysis of continuous data for a cross‐over trials is a paired t‐test. However, not all studies clearly reported paired analyses. Therefore, for each study and each comparison (e.g. placebo versus low dose, placebo versus medium dose, placebo versus high dose) we calculated the MD, the SD of the difference and its standard error (SE), the SMD, the pooled SD, and the SE of the SMD in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We used a correlation coefficient of 0.6 in our calculation of the SE (of the MD and the SMD), as this was the within‐subject correlation calculated by RUPP 2005, based on three methylphenidate cross‐over studies involving participants with developmental disabilities.
In the case that studies reported the mean outcome measure for two raters rating the same children using the same scale separately, we combined the data from the two raters by averaging the scores and calculating the combined SD, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
See also Appendix 5 and Redman 2014.
Dealing with missing data
When necessary, we contacted the authors of included studies for information regarding missing data, dropouts and/or data not included in the study report but relevant to the review; for example, we sought outcomes of interest and summary data (such as number of participants and events) if authors had not included them in the published study report. We reported our attempt to obtain additional data and the results of these attempts in the Results section.
See also Appendix 5 and Redman 2014.
Assessment of heterogeneity
We assessed heterogeneity in two steps. First, we assessed clinical heterogeneity by comparing the populations included in the studies, the settings, the treatment modalities, and the outcomes. Clinical heterogeneity was considered sufficient to preclude the pooling of studies if: the participant ages were obviously different (for example, we did not combine data from studies of teenagers aged 16 to 18 years with data from studies of children aged 6 to 8 years); the severity of the ASD was obviously different (for example, we did not combine data from studies of children who require institutional care with data from studies of those with mild symptoms causing little impairment); or the outcome measures were not clinically comparable (for example, we did not combine data from a study that only measures impulsivity with data from a study that only measures hyperactivity).
Second, we assessed statistical heterogeneity by performing a Chi2 test and calculating the I2 value according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017). We interpreted the I2 value as follows (Deeks 2017).
0% to 40%: may not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
We were unable to identify small study effects and publication bias using a funnel plot because of the small number of included studies. See Appendix 5 and Redman 2014.
Data synthesis
We pooled the available data using the generic inverse variance method, and we applied a random‐effects model (Deeks 2017). In the absence of clinical or statistical heterogeneity (see Assessment of heterogeneity), we also applied a fixed‐effect model for pooling, and we compared the effect estimates obtained from each of the two methods in order to assess the robustness of the estimates.
Measures of effect size using SMDs are difficult to interpret in terms of whether they represent a clinically important between‐treatment difference, or a clinically meaningful effect. In this review we used an SMD of 0.52 as a between‐treatment minimum clinically important difference (MCID), based on the Zhang 2005 finding of a MCID of 6.6 on the Attention Deficit Hyperactivity Disorder Rating Scale ‐ Parent Interview (ADHDRS‐PI), which was equivalent to an SMD of 0.52. Storebø 2015 also used this SMD of 0.52 as a clinically meaningful effect size. This aligns with the rule of thumb that an effect size of 0.20 to 0.49 represents a small effect; 0.50 to 0.79, a moderate effect; and 0.80 or above, a large effect, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2017).
Summary of findings table
We created a 'Summary of findings' table using GRADEpro software, GRADEpro GDT 2015, for our prespecified primary outcome of clinical efficacy, as assessed by an improvement in ADHD‐like symptoms (inattention, impulsivity and hyperactivity) and an improvement in symptoms of ASD (including impaired social interaction, impaired communication, and repetitive, restricted or stereotypical behaviour). We also reported one secondary outcome (rates of adverse events) in this table. We reported our findings separately for teacher‐rated and parent‐rated outcomes, and we reported the results for high doses of methylphenidate.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the evidence of studies contributing data to the meta‐analyses for the prespecified outcomes (GRADE 2004). We included the following comparisons: low dose methylphenidate versus placebo, medium dose methylphenidate versus placebo, and high dose methylphenidate versus placebo. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (see Higgins 2011a and Schünemann 2017 respectively) and GRADEpro GDT 2015. We justified all decisions to downgrade the quality of studies (from high to moderate, low or very low) using footnotes, and we provided comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We performed a subgroup analysis on the different doses of methylphenidate (low, medium and high dose).
Sensitivity analysis
We conducted sensitivity analyses to assess:
the effect of the correlation coefficient; one assuming no correlation (correlation coefficient of zero) and one assuming a higher correlation (correlation coefficient of 0.80); and
the influence of the different scales on the same outcome. For example, if trial authors used more than one scale to measure the same outcome in a study, we repeated the meta‐analyses for the different scales in order to assess if this changed the interpretation of our results. We used the SMD to compare the results across the different scales.
See Appendix 5 and Redman 2014.
Results
Description of studies
Results of the search
Our searches retrieved a total of 1102 records, of which 364 were duplicates. We screened the titles and abstracts of the remaining 738 records and excluded 709 irrelevant records. We next obtained and assessed the full texts of the remaining 29 reports for eligibility and excluded 21; most of the excluded studies were either not controlled trials or studied participants who did not meet the criteria for ASD (see Excluded studies; Characteristics of excluded studies). We included four studies in the review (see Included studies; Characteristics of included studies). We contacted the corresponding authors of all included studies for further information, but no authors identified additional studies that met our inclusion criteria (Handen 2000; Pearson 2013; RUPP 2005). See Figure 1.
1.
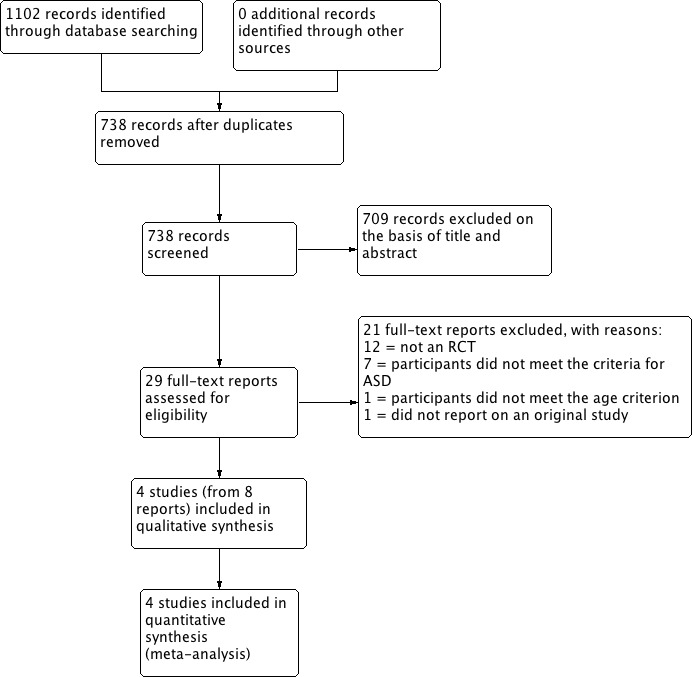
Study flow diagram.
Included studies
We included four studies in this review (Handen 2000; Pearson 2013; Quintana 1995; RUPP 2005). The RUPP 2005 (Research Units on Pediatric Psychopharmacology) study resulted in three journal articles and two conference abstracts, which we treated in the analysis as a single study.
The four included studies all had a randomised, double‐blind, cross‐over design, comparing the effect of placebo and more than one dose of methylphenidate for each study participant. The cross‐over trial design was appropriate for the clinical context, given that ASD is a relatively stable, chronic condition. Furthermore, no period or carry‐over effects would be anticipated for methylphenidate, even in the absence of a washout period, as the elimination half‐life for both the immediate‐ and extended‐release forms is two to three hours (Novartis 2014). The average duration of action of (immediate‐release) methylphenidate is approximately four hours, and the extended‐release form used in Pearson has a duration of action of approximately eight hours (Novartis 2014). Data collection was also focused at the end of each week of intervention, further reducing the risk of any carry‐over effect.
Details for each individual study can be found in the Characteristics of included studies tables.
Study design
All studies were cross‐over studies, completed in four to six weeks, comparing placebo with methylphenidate at either two doses, in Handen 2000 and Quintana 1995, or three, in Pearson 2013 and RUPP 2005.
Location of studies
All studies took place in the USA (Handen 2000; Pearson 2013; Quintana 1995; RUPP 2005).
Participants
Participant diagnoses
In all four included studies participants had ASD and ADHD‐like symptoms.
In Handen 2000, participants had autistic disorder or pervasive developmental disorder ‐ not otherwise specified (PDD‐NOS) (score on the CARS of 30 or more) and symptoms of ADHD (score of 15 or more on the Hyperactivity Index of the Conners' Teacher Rating Scale (CTRS)).
In Pearson 2013, 19 participants had autistic disorder, three had Asperger's disorder and two had PDD‐NOS and symptoms of ADHD (mean ADHD index score from CTRS‐Revised of 67.2 standard deviation (SD) 8.7), with several participants meeting DSM‐IV‐TR criteria for additional disorders, including oppositional defiant disorder, obsessive compulsive disorder and separation anxiety.
In Quintana 1995, participants had autistic disorder (as assessed with the CARS, ranging from 30.0 to 59.5) and a range of baseline behaviours, including temper tantrums and agitation.
In RUPP 2005, participants had autistic disorder, Asperger's disorder or PDD‐NOS and chronic interfering symptoms of hyperactivity or impulsiveness (or both), as assessed with the Clinician Global Impression ‐ Severity (CGI‐S) scale, ranging from moderately to severely ill.
Participant age and sex
Participant ages ranged from 5 to 11 years in Handen 2000; from 7 to 12 years in Pearson 2013; from 7 to 11 years in Quintana 1995; and from 5 to 13 years in RUPP 2005. All studies recruited more boys than girls: Handen 2000 randomised 13 children (10 boys and 3 girls), Pearson 2013 randomised 24 children (19 boys and 5 girls), Quintana 1995 randomised 10 children (6 boys and 4 girls), and RUPP 2005 randomised 66 children (59 boys and 7 girls).
Participant cognitive status
Participant cognitive functioning ranged from severe/profound intellectual impairment to average IQ (Handen 2000), moderate intellectual impairment (IQ 46) to above average IQ (IQ 112) (Pearson 2013), mild intellectual impairment to average IQ (Quintana 1995), and severe intellectual impairment to above average IQ (Slosson IQ 16 to 135) (RUPP 2005).
Participant recruitment
Investigators recruited participants from special education programmes, a psychiatric inpatient unit or an intensive day‐treatment programme (Handen 2000); special education classrooms of a large metropolitan public school district (Pearson 2013); a state psychiatric institute outpatient clinic (Quintana 1995); and five university outpatient centres (RUPP 2005).
Dose of methylphenidate
Handen 2000 used methylphenidate 0.3 mg/kg/dose two or three times daily, and methylphenidate 0.6 mg/kg/dose two or three times daily.
Pearson 2013 adjusted all doses for participant weight, using 10 mg to 20 mg extended‐release methylphenidate in the morning and 2.5 mg to 5 mg immediate‐release in the afternoon in the low‐dose phase, 15 mg to 30 mg extended‐release methylphenidate in the morning and 5 mg to 10 mg immediate‐release in the afternoon in the medium‐dose phase, and 20 mg to 40 mg extended‐release methylphenidate in the morning and 5 mg to 10 mg immediate‐release in the afternoon in the high‐dose phase.
Quintana 1995 used methylphenidate 10 mg and methylphenidate 20 mg, twice a day.
RUPP 2005 adjusted all doses for participant weight, using 2.5 mg to 5 mg methylphenidate two to three times daily, 5 mg to 10 mg methylphenidate two to three times daily, and 10 mg to 20 mg methylphenidate two to three times daily.
Outcomes
The outcomes reported were: overall severity of ADHD symptoms (Handen 2000; Pearson 2013; Quintana 1995; RUPP 2005); hyperactivity (Handen 2000; Pearson 2013; Quintana 1995; RUPP 2005); hyperactivity/impulsivity (RUPP 2005); restlessness‐impulsivity (Pearson 2013); inattention (Pearson 2013; RUPP 2005); overall severity of core features of autism (Handen 2000); lethargy/social withdrawal (Handen 2000; Pearson 2013); social skills (Pearson 2013); social communication with respect to joint attention (RUPP 2005); inappropriate speech (Handen 2000; Pearson 2013); stereotypic behaviour (Handen 2000; Pearson 2013; Quintana 1995); compulsive/repetitive behaviour (RUPP 2005); irritability (Handen 2000; Pearson 2013; Quintana 1995); aggression (Handen 2000); emotional lability (Pearson 2013); oppositional behaviour (Pearson 2013); oppositional defiant disorder (RUPP 2005); self‐regulation (RUPP 2005); compliance (RUPP 2005); regulated affective state (RUPP 2005); abnormal involuntary movements (Quintana 1995); any other atypical behaviours (Pearson 2013) and presence or severity (or both) of common adverse events of methylphenidate (Handen 2000; Pearson 2013; Quintana 1995; RUPP 2005).
Outcome measurement
Included studies used a number of different psychometric instruments or scales to measure our primary outcomes (see Table 3 for symptoms of ADHD and Table 4 for symptoms of ASD). Neither Handen 2000 nor Quintana 1995 nominated a primary outcome measure. Pearson 2013 justified the use of the CTRS‐Revised as their primary outcome measure because it had been shown previously in the literature to be "sensitive to medication treatment response in children with ADHD in the general school‐age population and in children with ASD and symptoms of ADHD". RUPP 2005 did not justify the use of the teacher‐rated hyperactivity subscale of Aberrant Behaviour Checklist as their primary outcome measure.
1. Instruments used to measure ADHD outcomes.
| Instrument | Inattention | Impulsivity | Hyperactivity | |||
| Teachera | Parent | Teachera | Parent | Teachera | Parent | |
| ABC | — | — | — | — | H, Q, R | P, R |
| ACTeRS | P | P | — | — | P | P |
| Conners' Global Index | — | — | P | P | P | P |
| CPRS‐R and CTRS‐R | P | P | — | — | P, Q | P |
| Conners' Abbreviated Parent/Teacher Questionnaire | — | — | — | — | — | — |
| SNAP‐IV | P, R | P, R | — | — | P , R | P, R |
a'Teacher' includes clinician and trained observer raters.
Letters (H, P, Q, R) indicate those studies that used a particular instrument to rate the particular outcome. Letters in bold and underlined font indicate the instrument we used in our meta‐analysis: H: Handen 2000; P: Pearson 2013; Q: Quintana 1995; R: RUPP 2005.
ABC: Aberrant Behavior Checklist; ACTeRS: ADD‐H (Attention deficit disorder ‐ hyperactivity) Comprehensive Teacher Rating Scale; ADHD: attention deficit hyperactivity disorder; CPRS‐R: Conners' Parent Rating Scale ‐ Revised; CTRS‐R: Conners' Teacher Rating Scale ‐ Revised;SNAP‐IV: Swanson, Nolan, and Pelham Questionnaire, Fourth Edition.
2. Instruments used to measure ASD outcomes.
| Instrument | Impaired social interaction | Impaired communication | Stereotypical behaviours | Overall ASD | ||||
| Teacher a | Parent | Teacher a | Parent | Teacher a | Parent | Teacher a | Parent | |
| ABC | H | P | H | P | H, Q | P | — | — |
| ACTeRS | P | P | — | — | — | — | — | — |
| CARS | — | — | — | — | — | — | H | — |
| CYBOCS | — | — | — | — | R | — | — | — |
| CPRS‐R and CTRS‐R | P | P | — | — | — | — | — | — |
| Iowa CTRS | H | — | — | — | — | — | — | — |
| Social communication questionnaire | — | — | — | — | — | — | — | P |
| SNAP‐IV | R | R | — | — | — | — | — | — |
| Clinician Global Impression ‐ Severity | — | — | — | — | — | — | P | — |
a'Teacher' includes clinician and trained observer raters;
Letters (H, P, Q, R) indicate the studies which used a particular instrument to rate the particular outcome. Letters in bolded and underlined font indicate the instrument we used in our meta‐analysis: H: Handen 2000; P: Pearson 2013; Q: Quintana 1995; R: RUPP 2005;
ABC: Aberrant Behavior Checklist; ACTeRS: ADD‐H (Attention deficit disorder ‐ hyperactivity) Comprehensive Teacher Rating Scale; ASD: autism spectrum disorders; CARS: Child Autism Rating Scale; CPRS‐R: Conners' Parent Rating Scale ‐ Revised; CTRS‐R: Conners' Teacher Rating Scale ‐ Revised; CYBOCS: Children's Yale‐Brown Obsessive Compulsive Scales;Iowa CTRS: Iowa Conners' Teacher Rating Scale; SNAP‐IV: Swanson, Nolan, and Pelham Questionnaire, 4th Edition.
Study and author funding
The Fanny Pushkin Rosenberg Research Foundation provided study funding for Handen 2000, and the National Institute of Mental Health Bethesda, the National Institutes of Health Bethesda, and the Korczak Foundation for RUPP 2005. One or more study authors in Pearson 2013 had received funding from or served as a consultant or advisor for: the Forest Research Institute, Curemark LLC, United Biosource Corporation, BioMarin Pharmaceuticals, Bristol‐Myers Squibb, Confluence Pharmaceutica, Hoffman LaRoche, Johnson & Johnson, Supernus Pharmaceutica, Shire, Lilly, Organon, Sigma Tau, Targacept, AstraZeneca, Novartis, Noven, Seaside Therapeutics, Abbotts Laboratories, Pearson Assessments/Psychological Corporation or Ezra Innovations. Quintana 1995 did not report funding sources.
Excluded studies
We excluded 21 studies from this review: 12 because they were not a RCT (Akyol 2015; Aman 1991; Armstrong 2008; Barnard‐Brak 2016; Birmaher 1988; Croteau 2013; Di Martino 2004; Flapper 2008; Gurbuz 2016; Mayes 1994; Scahill 2007; Sinzig 2014); 7 because the participants did not meet the criteria for ASD (Aman 1997; Epstein 2011; Faraone 2001; Simonoff 2013; Steele 2006; Von Morgenstern 2014; Çetín 2015); 1 because it did not report on an original study (Shea 2006), and 1 because the participants in the study did not meet the age criterion (Ghuman 2009).
The excluded RCTs included Aman 1997, which was a double‐blind, placebo‐controlled, cross‐over study of methylphenidate and different doses of fenfluramine in children with mental retardation or borderline IQ and ADHD; participants did not meet criteria for ASD. Birmaher 1988 studied methylphenidate in children with autism and features of ADHD, but this was not a randomised controlled trial. Ghuman 2009 was a randomised, controlled, double‐blind, cross‐over study of methylphenidate for ADHD symptoms in preschoolers with ASD or developmental delay (IQ of less than 70), but participants were aged from three to five years, which is younger than the cutoff for inclusion in our review. Di Martino 2004 studied methylphenidate in children with autism or pervasive developmental disorder and features of ADHD, but it was not a RCT. Simonoff 2013 was a randomised, controlled, double‐blind trial of optimal dose methylphenidate in children and adolescents with severe ADHD and intellectual disability (IQ 30 to 69), but although it measured symptoms of autism using the parent‐reported Social Communication Questionnaire, participants did not meet criteria for ASD. Steele 2006 was an open‐label, randomised trial comparing immediate‐release methylphenidate with extended‐release methylphenidate in children with a diagnosis of ADHD, but although parent stress and social play were measured, participants did not meet criteria for ASD. See Characteristics of excluded studies.
Risk of bias in included studies
We assessed all of the included trials for risk of bias across the seven domains of the Cochrane 'Risk of bias' tool (Higgins 2017). The results of this assessment are shown for each study in the 'Risk of bias' tables, beneath the Characteristics of included studies tables and summarised in 'Risk of bias' graphs (Figure 2; Figure 3).
2.
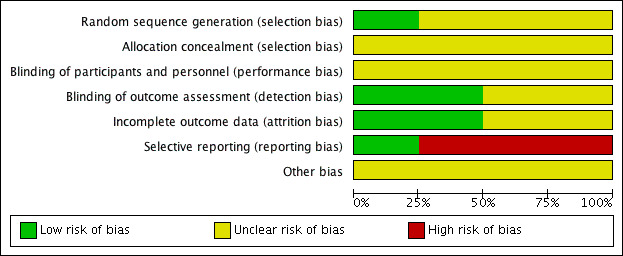
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
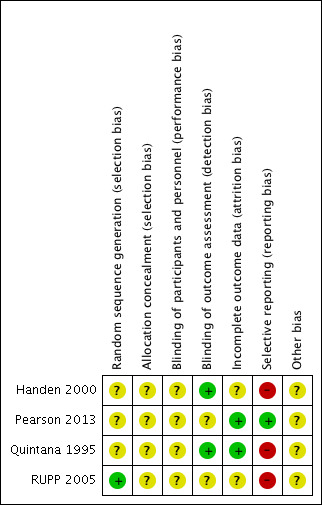
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We assessed three studies as being at unclear risk of selection bias based on inadequate information about the generation of random numbers (Handen 2000; Pearson 2013; Quintana 1995). One study, RUPP 2005, reported an appropriate method for generating a random numbers list, so we considered it to be at low risk of bias.
Allocation concealment
We rated all four studies at unclear risk of selection bias based on inadequate information about the method of allocation concealment (Handen 2000; Pearson 2013; Quintana 1995; RUPP 2005).
Blinding
None of the studies provided enough information to assess to what extent blinding had been successful.
Performance bias
We considered the risk of performance bias to be unclear in all four studies (Handen 2000; Pearson 2013; Quintana 1995; RUPP 2005).
The main issue was inadequate information about whether participants had taken methylphenidate prior to the study, and hence whether some of these children and their parents could have recognised the active medication (by recognising adverse events, for example) (Handen 2000; Pearson 2013). The corresponding author for Handen 2000 was unable to retrieve this information but indicated that from his recall some participants were likely to have had previous exposure to methylphenidate.
Detection bias
We considered the risk of detection bias to be low in Handen 2000 and Quintana 1995 and unclear in Pearson 2013 and RUPP 2005.
The study staff were unblinded during the test‐dose week in Pearson 2013 and RUPP 2005.
Incomplete outcome data
We assessed two studies, Pearson 2013 and Quintana 1995, to be at low risk of attrition bias, because all missing data were accounted for. We assessed Handen 2000 to be at unclear risk of attrition bias because there was insufficient information about the method of imputing missing data, and the corresponding author was not able to retrieve this information. We assessed RUPP 2005 to be at unclear risk of attrition bias because outcome data for the impaired communication outcome was incomplete.
Selective reporting
We considered the risk of reporting bias to be high in Handen 2000, Quintana 1995 and RUPP 2005, and low in Pearson 2013. We assessed Handen 2000 to be at high risk of reporting bias because authors did not provide parent ratings (although they reported that this outcome was incomplete because many participants resided in inpatient clinics or residential halls). Quintana 1995 did not report CARS outcomes, despite listing the CARS score as an outcome. We were unable to contact the corresponding author to clarify this. RUPP 2005 did not report the Clinician Global Impression ‐ Improvement subscale score outcome, although it was used in a composite score to define response. In addition, two subsequent publications (in 2007 and 2009, see RUPP 2005) reported additional outcomes that the original publication did not mention. We rated Pearson 2013 at low risk of reporting bias because the reporting of outcomes was comprehensive.
Other potential sources of bias
We considered all four studies to be at unclear risk of bias due to a lack of information about either study funding (in Handen 2000 and Quintana 1995) or potential conflicts of interest of the investigators/authors (in Pearson 2013 and RUPP 2005). The corresponding author of Handen 2000 could not retrieve this information, and we were unable to contact the corresponding author of Quintana 1995. It is unclear if the affiliations with pharmaceutical companies reported in RUPP 2005 represent a risk of bias, so we judged this study to be at unclear risk of bias. We assessed Pearson 2013 at unclear risk of bias, because the authors reported having received previous financial support from a number of pharmaceutical companies (including manufacturers of pharmaceuticals for behavioural syndromes in children), although the included study was not funded by any pharmaceutical companies.
Effects of interventions
We presented the results for the primary outcome, clinical efficacy (defined as an improvement in ADHD‐like symptoms and an improvement in symptoms of ASD), as rated by teachers for high‐dose methylphenidate in Table 1, and as rated by parents for high‐dose methylphenidate in Table 2.
Below, we report the results separately for the features of ADHD, the core symptoms of ASD and overall ASD. For symptoms of ADHD, we report results on inattention, impulsivity and hyperactivity, as rated by teachers and parents separately. For ASD outcomes, we report results on impaired social interaction, impaired communication, and stereotypical behaviours as well as overall ASD, as rated by teachers and parents separately. No meta‐analysis was possible for the primary outcomes of impulsivity or impaired communication, as only Pearson 2013 measured impulsivity and impaired communication as rated by parents, and only Handen 2000 measured impaired communication as rated by teachers.
Doses differed between studies, with some using doses calculated per kg of participant body weight, and others using proprietary doses of 2.5 mg, 5 mg, 10 mg and 20 mg of methylphenidate with adjustment for participant weight. Doses were three times daily in two studies: Handen 2000, although parents elected to omit the third daily dose for 2/13 children, and RUPP 2005, where the third dose was half of the earlier doses, and no information was available about whether any parents elected to omit the third dose. Quintana 1995 and Pearson 2013 administered doses twice daily, although parents elected to omit the second dose in 5/24 children in the Pearson 2013 study because of "behaviour concerns in the late afternoon/evening".
A number of different psychometric instruments/scales were used both across and within included studies to measure our primary outcomes (see Table 3 for ADHD‐like symptoms and Table 4 for symptoms of ASD). Where an included study used more than one outcome measure, we used the measure in our analysis, which was also used by one or more of our other included studies. This was a pragmatic approach given the absence of a generally accepted gold standard outcome instrument/scale. We therefore report effect sizes as measured on the most commonly used scales across our included studies. The scales used in our meta‐analysis are highlighted in bold and underlined font in Table 3 and Table 4. We used a coefficient of 0.6, which was the within‐subject correlation calculated by RUPP 2005 (based on three methylphenidate cross‐over studies involving participants with developmental disabilities), and we performed sensitivity analyses using a coefficient of 0 and 0.8.
No data were available to assess short‐term (1 to 3 months), medium‐term (3 to 6 months) and long‐term (6 to 12 months) outcomes, as the duration of observation was limited to one week under each experimental condition in all included studies.
We were unable to perform subgroup analyses based on ages 6 to 12 years and 13 to 18 years, because only one study, RUPP 2005, included children aged 13 years and did not report any individual data, and no studies included children aged 14 years or older.
We were unable to perform subgroup analyses based on the formulation of methylphenidate. Only one study, Pearson 2013, used an extended‐release form, so we were unable to compare extended‐release with immediate‐release formulations. Furthermore, Pearson 2013 used both extended‐release methylphenidate (for the morning dose) and immediate‐release methylphenidate (for the afternoon dose, if it was administered). However, parents were asked to focus only on their child's morning behaviour for their ratings, and teachers only saw the children on the extended‐release dose; therefore, we considered this study to have a single treatment arm (extended‐release methylphenidate). No studies used transdermal preparations, so we were unable to compare oral with transdermal methylphenidate.
No data were available on the secondary outcomes of caregiver well‐being; need for institutionalisation, special schooling options or therapy to achieve learning outcomes; or overall quality of life.
Primary outcomes: clinical efficacy
1. Improvement in ADHD‐like symptoms: inattention
Two studies measured inattention as rated by teachers and parents (Pearson 2013; RUPP 2005). Both studies used the inattention subscale of the Swanson, Nolan, and Pelham, Fourth Version (SNAP‐IV) questionnaire, which was used in our primary analysis. This subscale consists of nine items, which are measured on a rating scale ranging from zero to three. Pearson 2013 also used the cognitive‐inattention subscale of the Conners' Parent Rating Scale ‐ Revised (CPRS‐R) short form, and the inattention subscale of the ADD‐H (attention deficit disorder ‐ hyperactivity) Comprehensive Teacher Rating Scale (ACTeRS). See Table 3.
Participants in the Pearson 2013 study differed from those in the RUPP 2005 study in three ways. First, 13/24 children had previous methylphenidate exposure, whereas RUPP 2005 excluded children who had had an adequate trial of methylphenidate in the previous two years. Second, participants in the RUPP 2005 study may have been more unwell than those in the Pearson 2013 study; children in the Pearson 2013 study were rated moderately to severely ill by clinicians and recruited from psychiatric outpatient clinics, while children in the RUPP 2005 study were recruited from special education classrooms. Finally, unlike those in RUPP 2005, participants in the Pearson 2013 study were permitted to remain on other prescribed psychotropic medication, including antidepressant and antipsychotic medication. However, we did not consider that these minor differences were sufficient to preclude their data being combined.
We downgraded the quality of evidence of the pooled effect for both teacher‐rated and parent‐rated inattention to low, due to imprecision (data came from only two small studies), and limitations in study design and implementation in both studies.
1.1 Teacher rated
The pooled difference between treatment and placebo was statistically significant and favoured the treatment group (MD −2.72 points, 95% CI −5.37 to −0.06, rated on SNAP‐IV inattention subscale, range 0 to 27; 2 studies, 51 participants; Analysis 1.1; Table 1).
1.1. Analysis.
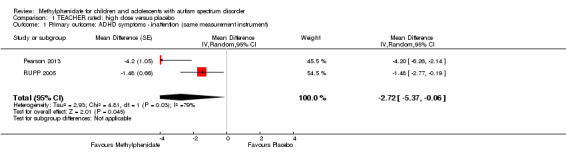
Comparison 1 TEACHER rated: high dose versus placebo, Outcome 1 Primary outcome: ADHD symptoms ‐ inattention (same measurement instrument).
An I2 of 79% indicated considerable heterogeneity.
As a sensitivity analysis, we applied a correlation coefficient of 0 and of 0.8. When we applied a correlation coefficient of 0, the pooled difference between treatment and placebo was no longer statistically significant (MD −2.55 points, 95% CI −5.15 to 0.06, rated on SNAP‐IV inattention subscale, range 0 to 27; 2 studies, 51 participants; Analysis 2.1). When we applied a correlation coefficient of 0.8, the pooled difference between treatment and placebo remained statistically significant and in favour of the treatment group (MD −2.77 points, 95% CI −5.43 to −0.11 rated on SNAP‐IV inattention subscale, range 0 to 27; 2 studies, 51 participants; Analysis 3.1).
2.1. Analysis.
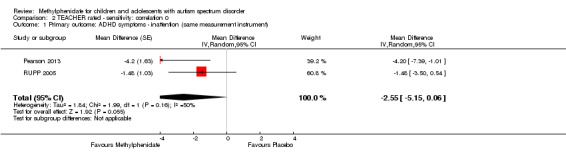
Comparison 2 TEACHER rated ‐ sensitivity: correlation 0, Outcome 1 Primary outcome: ADHD symptoms ‐ inattention (same measurement instrument).
3.1. Analysis.
Comparison 3 TEACHER rated ‐ sensitivity: correlation 0.8, Outcome 1 Primary outcome: ADHD symptoms ‐ inattention (same measurement instrument).
Both studies used the SNAP‐IV measurement scale. Pearson 2013 also used the CPRS‐R and the ACTeRS. Our conclusions did not change when the ACTeRS and the CTRS‐R results were substituted as a sensitivity analysis.
1.2 Parent rated
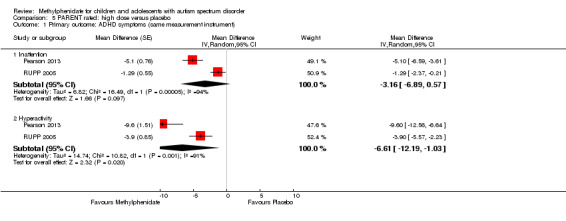
The pooled difference between treatment and placebo was not statistically significant (MD −3.16 points, 95% CI −6.89 to 0.57, rated on SNAP‐IV inattention subscale, range 0 to 27; 2 studies, 71 participants; Analysis 5.1; Table 2).
5.1. Analysis.
Comparison 5 PARENT rated: high dose versus placebo, Outcome 1 Primary outcome: ADHD symptoms (same measurement instrument).
An I2 of 86% indicated considerable heterogeneity.
As a sensitivity analysis, we applied a correlation coefficient of 0 and of 0.8. Using a correlation coefficient of 0 (MD −3.11 points, 95% CI −6.84 to 0.62, rated on SNAP‐IV inattention subscale, range 0 to 27; 2 studies, 71 participants; Analysis 6.1), as well as 0.8 (MD −3.18 points, 95% CI −6.91 to 0.56, rated on SNAP‐IV inattention subscale, range 0 to 27; 2 studies, 71 participants; Analysis 7.1), we could not rule out that the pooled difference between treatment and placebo may have been due to chance.
6.1. Analysis.
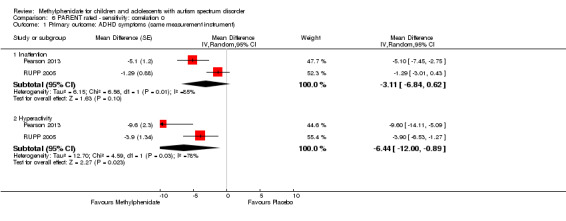
Comparison 6 PARENT rated ‐ sensitivity: correlation 0, Outcome 1 Primary outcome: ADHD symptoms (same measurement instrument).
7.1. Analysis.

Comparison 7 PARENT rated ‐ sensitivity: correlation 0.8, Outcome 1 Primary outcome: ADHD symptoms (same measurement instrument).
Both studies used the SNAP‐IV measurement scale. Pearson 2013 also used the CPRS‐R and the ACTeRS. Our conclusions did not change when the ACTeRS and the CTRS‐R results were substituted as a sensitivity analysis.
2. Improvement in ADHD‐like symptoms: impulsivity
Only Pearson 2013 measured impulsivity (as rated by 24 parents and 18 teachers), so we were unable to pool results. Pearson 2013 used the restless‐impulsivity subscale of the Conners' Global Index to measure impulsivity (see Table 3). This scale was rated by parents as well as teachers.
2.1 Teacher rated
Methylphenidate had a significant, beneficial effect compared to placebo on impulsivity as rated by teachers (MD −14.8 points, 95% CI −18.52 to −11.08, rated on Conner's Global Index restless‐impulsivity subscale, range unknown). We note that Pearson 2013 reported that these results were significant, although they did not provide a P value.
2.2 Parent rated
Methylphenidate had a significant, beneficial effect compared to placebo on impulsivity as rated by parents (MD −11.3 points, 95% CI −14.05 to −8.21, rated on Conner's Global Index restless‐impulsivity subscale, range unknown). We note that Pearson 2013 reported that these results were significant, although without providing a P value.
3. Improvement in ADHD‐like symptoms: hyperactivity
All four studies reported on hyperactivity as rated by parents and teachers (Handen 2000; Pearson 2013; Quintana 1995; RUPP 2005). In three studies both clinicians and teachers rated participants (Handen 2000; Pearson 2013; RUPP 2005), while in Quintana 1995, only the psychiatrist clinicians rated hyperactivity based on a three‐hour observation period. We combined clinician ratings with those of teachers. Studies used five different rating scales: the hyperactivity index of the Conners' Teacher Rating Scale ‐ Revised (CTRS‐R; 10 items rated on a four‐point rating scale) used in Quintana 1995; the hyperactivity subscale of the Aberrant Behavior Checklist (ABC; 16 items, rated on a four‐point rating scale) used in Handen 2000, Pearson 2013, Quintana 1995 and RUPP 2005; the hyperactivity/impulsivity subscale of the SNAP‐IV questionnaire (nine items, rated on a four‐point rating scale) used in Pearson 2013 and RUPP 2005; the hyperactivity subscale of the CPRS‐R, used in Pearson 2013; and the hyperactivity rating scale of the ACTeRS, used in Pearson 2013. See Table 3.
Pearson 2013 reported including children with recent previous methylphenidate exposure (13/24 children), whereas Quintana 1995 excluded children with previous methylphenidate exposure, and RUPP 2005 excluded children who had had an adequate trial of methylphenidate in the previous two years. Handen 2000 did not report on whether or not prior methylphenidate use was an exclusion criterion, and the corresponding author was unable to retrieve this information. The participants in the Pearson 2013 and Quintana 1995 studies may have been less unwell overall than the participants in Handen 2000 (some of whom had severe/profound cognitive impairment) and RUPP 2005 (who were judged moderately to severely unwell). Pearson 2013 recruited from special education classrooms rather than psychiatric outpatient clinics. Unlike in the other studies, participants in Pearson 2013 were permitted to remain on other prescribed psychotropic medication, including antidepressant and antipsychotic medication. The more limited period of observation (three hours) by clinicians in Quintana 1995, compared to other studies, may have led to less developed relationships with the children and less emphasis on academic performance than the teacher ratings in the other studies. However, we did not consider that these differences were sufficient to preclude their data being combined.
We downgraded the quality of evidence of the pooled effect for both teacher‐ and parent‐rated hyperactivity to low, due to imprecision (data came from only two or four small studies) and limitations in design and implementation in all studies.
3.1 Teacher rated
Pooling was possible for all four studies (Handen 2000; Pearson 2013; Quintana 1995; RUPP 2005). The pooled difference between treatment and placebo was statistically significant and favoured the treatment group (SMD −0.78, 95% CI −1.13 to −0.43; 73 participants; Analysis 1.2; Table 1). The SMD of −0.78 corresponded to a moderate clinical effect, which was a clinically important difference.
1.2. Analysis.
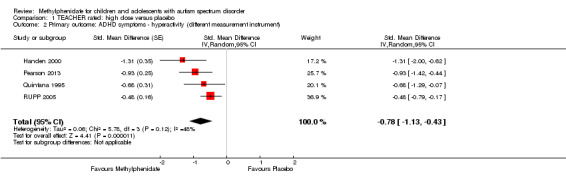
Comparison 1 TEACHER rated: high dose versus placebo, Outcome 2 Primary outcome: ADHD symptoms ‐ hyperactivity (different measurement instrument).
An I2 of 48% indicated moderate heterogeneity.
As a sensitivity analysis, we applied a correlation coefficient of 0 and 0.80. For a correlation coefficient of 0 (SMD −0.70, 95% CI −1.07 to −0.33; 4 studies, 73 participants; Analysis 2.2), as well as 0.8 (SMD −0.81, 95% CI −1.16 to −0.47; 4 studies, 73 participants; Analysis 3.2), the pooled difference between treatment and placebo remained statistically significant.
2.2. Analysis.
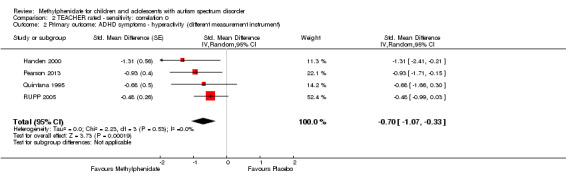
Comparison 2 TEACHER rated ‐ sensitivity: correlation 0, Outcome 2 Primary outcome: ADHD symptoms ‐ hyperactivity (different measurement instrument).
3.2. Analysis.
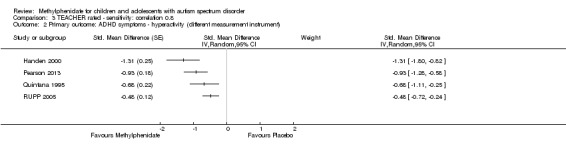
Comparison 3 TEACHER rated ‐ sensitivity: correlation 0.8, Outcome 2 Primary outcome: ADHD symptoms ‐ hyperactivity (different measurement instrument).
In the pooled estimate, three studies used the ABC to measure hyperactivity (Handen 2000; Quintana 1995; RUPP 2005), and one study used the SNAP‐IV (Pearson 2013). As a sensitivity analysis, we also compared hyperactivity as measured by the ABC (Handen 2000; RUPP 2005), the CTRS‐R (Pearson 2013; Quintana 1995), and the SNAP‐IV (Pearson 2013; RUPP 2005). Regardless of which scale was used, the difference between treatment and placebo was statistically significant and favoured the treatment group (see Analysis 4.1).
4.1. Analysis.
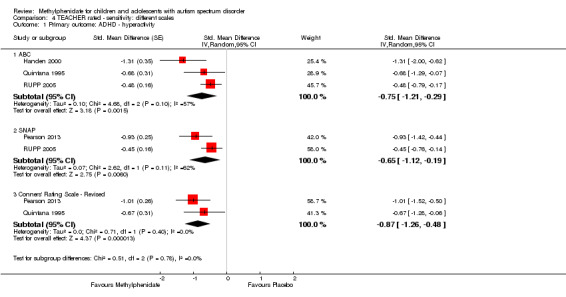
Comparison 4 TEACHER rated ‐ sensitivity: different scales, Outcome 1 Primary outcome: ADHD ‐ hyperactivity.
3.2 Parent rated
Pooling was only possible for two studies (Pearson 2013; RUPP 2005). The pooled difference between treatment and placebo was statistically significant and favoured the treatment group (MD −6.61 points, 95% CI −12.19 to −1.03, rated on ABC hyperactivity subscale, range 0 to 48; 71 participants; Analysis 5.1; Table 2).
An I2 of 69% indicated considerable heterogeneity.
As a sensitivity analysis, we applied a correlation coefficient of 0 and of 0.8. For a correlation coefficient of 0 (SMD −6.44, 95% CI −12.00 to −0.89; 2 studies, 71 participants; Analysis 6.1), as well as 0.8 (SMD −6.67, 95% CI −12.25 to −1.08; 2 studies, 71 participants; Analysis 7.1), the pooled difference between treatment and placebo remained statistically significant.
Both studies used the ABC measurement scale (Pearson 2013; RUPP 2005). As a sensitivity analysis, we also compared hyperactivity as measured by the ABC and the SNAP‐IV (Pearson 2013; RUPP 2005). Irrespective of which scale was used, the difference between treatment and placebo was statistically significant and favoured the treatment group (see Analysis 8.1).
8.1. Analysis.
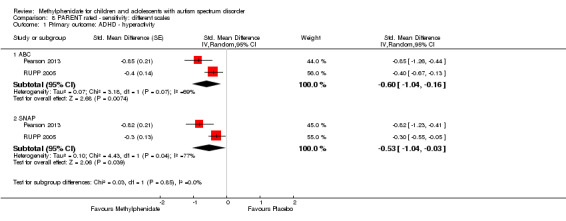
Comparison 8 PARENT rated ‐ sensitivity: different scales, Outcome 1 Primary outcome: ADHD ‐ hyperactivity.
4. Improvement in core symptoms of ASD: impaired social interaction
Three studies reported impaired social interaction (Handen 2000; Pearson 2013; RUPP 2005). RUPP 2005 used the oppositional defiant disorder subscale of the SNAP‐IV (eight items, rated on a four‐point rating scale). Handen 2000 and Pearson 2013 used the ABC lethargy/social withdrawal subscale (16 items, rated on a four‐point rating scale). Handen 2000 also used the aggression subscale of the IOWA Conners' Teacher Rating Scale (five items, rated on a four‐point rating scale). Pearson 2013 also used the oppositional behaviour subscale of the CPRS‐R and the social skills subscale of the ACTeRS. See Table 4.
Pearson 2013 reported including children with previous methylphenidate exposure (13/24 children), and Handen 2000 probably included some participants with previous methylphenidate exposure (based on the corresponding author's recollection), whereas RUPP 2005 excluded children who had had an adequate trial of methylphenidate in the previous two years. Also, the participants in the Pearson 2013 study may have been less unwell overall than the other study participants, who were all recruited from psychiatric outpatient clinics rather than special education classrooms. The participants in Pearson 2013, unlike the other participants, were also permitted to remain on other prescribed psychotropic medication, including antidepressant and antipsychotic medication. However, we did not consider that these differences were sufficient to preclude their data being combined.
We downgraded the quality of evidence of the pooled effect for both teacher‐ and parent‐rated impaired social interaction to very low, due to imprecision (data came from only two or three small studies), limitations in design and implementation in all three studies, and indirectness of evidence in all three studies.
4.1 Teacher rated
Pooling was possible for three studies (Handen 2000; Pearson 2013; RUPP 2005). The pooled difference between treatment and placebo was not statistically significant (SMD −0.51, 95% CI −1.07 to 0.05; 63 participants; Analysis 1.3; Table 1).
1.3. Analysis.
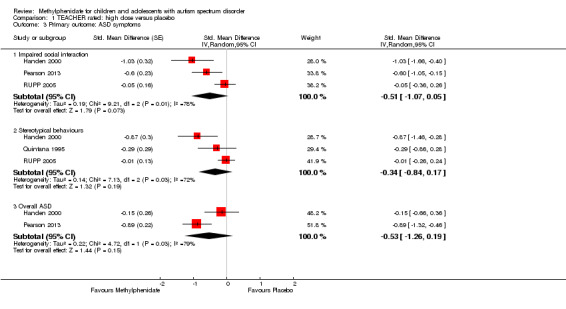
Comparison 1 TEACHER rated: high dose versus placebo, Outcome 3 Primary outcome: ASD symptoms.
An I2 of 78% indicated considerable heterogeneity.
As a sensitivity analysis, we applied a correlation coefficient of 0 and of 0.8. For a correlation coefficient of 0 (SMD −0.44, 95% CI −0.99 to 0.11; 3 studies, 63 participants; Analysis 2.3), as well as 0.8 (SMD −0.53, 95% CI −1.09 to 0.02; 3 studies, 63 participants; Analysis 3.3), we could not rule out that the pooled difference between treatment and placebo may have been due to chance.
2.3. Analysis.
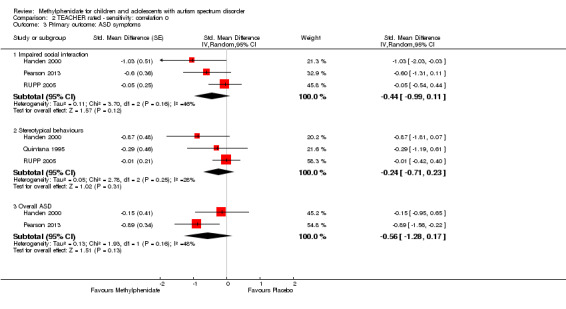
Comparison 2 TEACHER rated ‐ sensitivity: correlation 0, Outcome 3 Primary outcome: ASD symptoms.
3.3. Analysis.
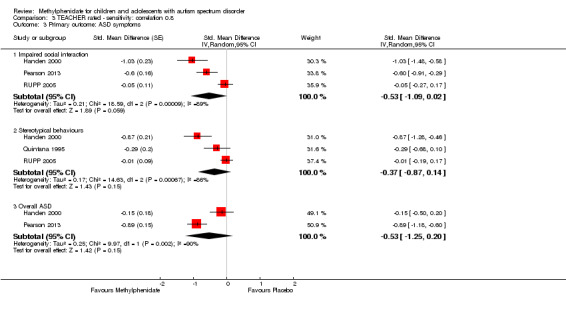
Comparison 3 TEACHER rated ‐ sensitivity: correlation 0.8, Outcome 3 Primary outcome: ASD symptoms.
We used the Iowa Conners' Teachers (Handen 2000), the SNAP‐IV (RUPP 2005), and the CTRS‐R (Pearson 2013) measurement scales for this analysis. Pearson 2013 also used the ACTeRS and Handen 2000 also used the ABC scales. Our conclusions did not change when the ACTeRS and the ABC results were substituted in a sensitivity analysis.
4.2 Parent rated
Pooling was possible for two studies (Pearson 2013; RUPP 2005). The pooled difference between treatment and placebo was not statistically significant (SMD −0.21, 95% CI −0.60 to 0.18; 71 participants; Analysis 5.2; Table 2).
5.2. Analysis.
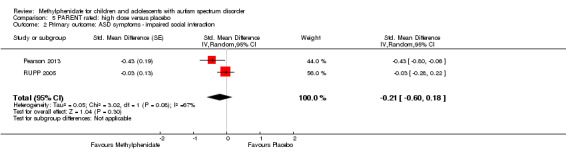
Comparison 5 PARENT rated: high dose versus placebo, Outcome 2 Primary outcome: ASD symptoms ‐ impaired social interaction.
An I2 of 67% indicated considerable heterogeneity.
As a sensitivity analysis, we applied a correlation coefficient of 0 and of 0.8. For a correlation coefficient of 0 (SMD −0.17, 95% CI −0.55 to 0.20; 2 studies, 71 participants; Analysis 6.2) as well as 0.8 (SMD −0.22, 95% CI −0.61 to 0.17; 2 studies, 71 participants; Analysis 7.2), we could not rule out that the pooled difference between treatment and placebo may have been due to chance.
6.2. Analysis.
Comparison 6 PARENT rated ‐ sensitivity: correlation 0, Outcome 2 Primary outcome: ASD symptoms ‐ Impaired social interaction.
7.2. Analysis.
Comparison 7 PARENT rated ‐ sensitivity: correlation 0.8, Outcome 2 Primary outcome: ASD symptoms ‐ Impaired social interaction.
We used the CPRS‐R measurement scale, reported in Pearson 2013, and the SNAP‐IV, reported in RUPP 2005, for this analysis. Pearson 2013 also used the ABC and ACTeRS scales. Our conclusions did not change when the ABC and ACTeRS results were substituted in a sensitivity analysis.
5. Improvement in core symptoms of ASD: impaired communication
Two studies reported impaired communication (Handen 2000; Pearson 2013), and they both used the inappropriate speech subscale of the ABC (four items, rated on a four‐point rating scale). See Table 4. However, we were not able to pool results because teachers rated impaired communication in Handen 2000 and parents in Pearson 2013.
5.1 Teacher rated
Methylphenidate showed a significant beneficial effect compared to placebo on teacher‐rated impaired communication in Handen 2000 (MD −2.25 points, 95% CI −3.41 to −1.09, rated on ABC inappropriate speech subscale, range 0 to 16; 12 participants). We note that Handen 2000 reported that these results were significant, with a P value of less than 0.001.
5.2 Parent rated
Methylphenidate showed a significant beneficial effect compared to placebo on parent‐rated impaired communication in Pearson 2013 (MD −1.30 points, 95% CI −2.08 to −0.52, rated on ABC inappropriate speech subscale, range 0 to 16; 24 participants). We note that Pearson 2013 reported that these results were significant but did not report a P value.
6. Improvement in core symptoms of ASD: stereotypical behaviours
All four studies reported on stereotypical behaviours, as rated by teachers in Handen 2000, Quintana 1995 and RUPP 2005, and as rated by parents in Pearson 2013. Handen 2000, Pearson 2013 and Quintana 1995, used the stereotypic behavior subscale of the ABC (seven items, rated on a four‐point rating scale). RUPP 2005 used the Children's Yale‐Brown Obsessive Compulsive Scales for pervasive developmental disorder (five items, scored from zero to four). See Table 4.
The more limited period of observation by clinicians in Quintana 1995, compared to the other studies, may have led to less developed relationships with the children and less emphasis on academic performance than comparable teacher ratings. However, it is unclear how these differences might have influenced the statistical heterogeneity.
We downgraded the quality of evidence of the pooled effect for teacher‐rated stereotypical behaviours to low, due to imprecision (data came from only four small studies), and limitations in design and implementation in all four studies.
6.1 Teacher rated
Pooling was possible for three studies (Handen 2000; Quintana 1995; RUPP 2005). The pooled difference between treatment and placebo was not significant (SMD −0.34, 95% CI −0.84 to 0.17; 69 participants; Analysis 1.3; Table 1).
An I2 of 72% indicated considerable heterogeneity.
As a sensitivity analysis, we applied a correlation coefficient of 0 and of 0.8. For a correlation coefficient of 0 (SMD −0.24, 95% CI −0.71 to 0.23; 3 studies, 69 participants; Analysis 2.3), as well as 0.8 (SMD −0.37, 95% CI −0.87 to 0.14; 3 studies, 69 participants; Analysis 3.3), the pooled difference between treatment and placebo could have been due to chance.
6.2 Parent rated
Methylphenidate had a statistically significant effect compared to placebo on stereotypical behaviours as rated by parents in Pearson 2013 (MD −1.40 points, 95% CI −2.63 to −0.17, rated on ABC stereotypic behavior subscale, range 0 to 28; 24 participants). We note that Pearson 2013 reported that these results were not significant but did not report a P value.
7. Improvement in overall ASD
Two studies reported on overall ASD (Handen 2000; Pearson 2013). Handen 2000 used the Child Autism Rating Scale (15 items), as rated by teachers. Pearson 2013 used the Social Communication Questionnaire (40 items) in parents and a Clinician Global Impression ‐ Severity score in teachers. See Table 4.
The participants in the Pearson 2013 study may have been less unwell overall than the participants in the Handen 2000 study, as they were recruited from special education classrooms rather than psychiatric outpatient clinics. The participants in the Pearson 2013 study, unlike those in Handen 2000, were also permitted to remain on other prescribed psychotropic medication, including antidepressant and antipsychotic medication. However, we did not consider that these minor differences were sufficient to preclude meta‐analysis.
We downgraded the quality of evidence of the pooled effect for teacher‐rated overall ASD to low, due to imprecision (data came from only two studies) and limitations in design and implementation in both studies.
7.1 Teacher rated
Pooling was possible for two studies (Handen 2000; Pearson 2013). The pooled difference between treatment and placebo was not statistically significant (SMD −0.53, 95% CI −1.26 to 0.19; 36 participants; Analysis 1.3; Table 1).
An I2 of 79% indicated considerable heterogeneity.
As a sensitivity analysis, we applied a correlation coefficient of 0 and of 0.8. For a correlation coefficient of 0 (SMD −0.56, 95% CI −1.28 to 0.17; 2 studies, 36 participants; Analysis 2.3), as well as 0.8 (SMD −0.53, 95% CI −1.25 to 0.20; 2 studies, 36 participants; Analysis 3.3), the pooled difference between treatment and placebo could have been due to chance.
7.2 Parent rated
Methylphenidate had a statistically significant effect compared to placebo on overall ASD as rated by parents in Pearson 2013 (MD −2.10 points, 95% CI −3.65 to −0.55, rated on Social Communication Questionnaire, range 0 to 40; 24 participants). We note that Pearson 2013 reported that these results were not significant but reported no P value.
Secondary outcomes
1. Rate of adverse events: total number
All four studies reported adverse events, as rated by teachers or programme staff in Handen 2000, by teachers in Pearson 2013, and by the paediatrician in Quintana 1995. Parents rated this outcome in Pearson 2013 and RUPP 2005. Only Pearson 2013 reported adverse events data from both parents and teachers, and noted that parents tended to report more adverse events than teachers at higher doses of methylphenidate. Quintana 1995 did not list individual adverse events, instead reporting an overall adverse events checklist score. Handen 2000 reported only moderate or severe adverse events despite also collecting data on mild adverse events. Each study appears to have used a different adverse events checklist. Therefore, the reporting of adverse events varied considerably across all studies, and only one study reported an overall total number of adverse events (RUPP 2005). As a result, we were not able to pool results for the total rate of adverse events.
Only Pearson 2013 used extended‐release methylphenidate, so we were unable to compare rates of adverse events between immediate‐release and extended‐release methylphenidate.
Investigators designed exclusion criteria to minimise serous adverse events. Three studies reported excluding participants with major neurological disorders (Pearson 2013; Quintana 1995; RUPP 2005), two studies excluded participants with major cardiovascular disorders (Quintana 1995; RUPP 2005), and one study excluded previous mood disorders (Pearson 2013). We were unable to adjust for the cross‐over design of our included studies as individual patient data were not available. This conservative approach is likely to result in an underestimation of the risk of adverse events, if there is a within‐person correlation for adverse events.
A number of other factors are likely to have led to an underestimation of adverse events of methylphenidate. First, except for Quintana 1995, the studies included children with prior exposure to methylphenidate. About 50% of the participants in Pearson 2013 and 30% of the participants in RUPP 2005 had taken methylphenidate previously. Children with previous adverse experiences of methylphenidate are not likely to have accepted invitations to participate, and therefore study participants may be more likely to be tolerant of methylphenidate adverse events than the general population of children with ASD and ADHD symptoms. Second, all studies included an unblinded, one‐week period in which the methylphenidate dose was progressively escalated, to test whether participants could tolerate study doses. Investigators did not report the adverse events of participants who withdrew or were excluded during the test‐dose phase prior to randomisation into the cross‐over phase. Third, adverse events from high‐dose methylphenidate are likely to be underestimated because a number of participants were unable to tolerate the high dose due to adverse events in the test‐dose phase and were not randomised to the high dose in the cross‐over phase (16 out of the 66 participants randomised in the RUPP 2005 trial, for example), and therefore are not included in reporting of adverse events for the high‐dose condition. Fourth, several studies reported that some parents did not administer the scheduled afternoon dose of methylphenidate, which is likely to reduce the reporting of evening and overnight adverse events. Therefore, we consider that the evidence for the pooled risk of adverse events is of very low quality.
Please see Analysis 1.4 and Analysis 5.3 and Appendix 6 for results for gastrointestinal effects (abdominal discomfort, reduced appetite and other gastrointestinal effects), general physical effects (dizziness, drowsiness, headache, sleep disturbance, increased activity and other general physical effects), psychological effects (anxiety, depressed mood, irritability, social withdrawal and other psychological effects), repetitive behaviours (general repetitive behaviours, repetitive movements or tics, and other repetitive behaviours) and other adverse events (staring). When we applied a random‐effects model, the only adverse effect that was significantly more likely with treatment was reduced appetite as rated by parents (RR 8.28, 95% CI 2.57 to 26.73; 2 studies, 74 participants; P < 0.01). The number needed to treat for an additional harmful outcome with regard to reduced appetite was 4.7.
1.4. Analysis.
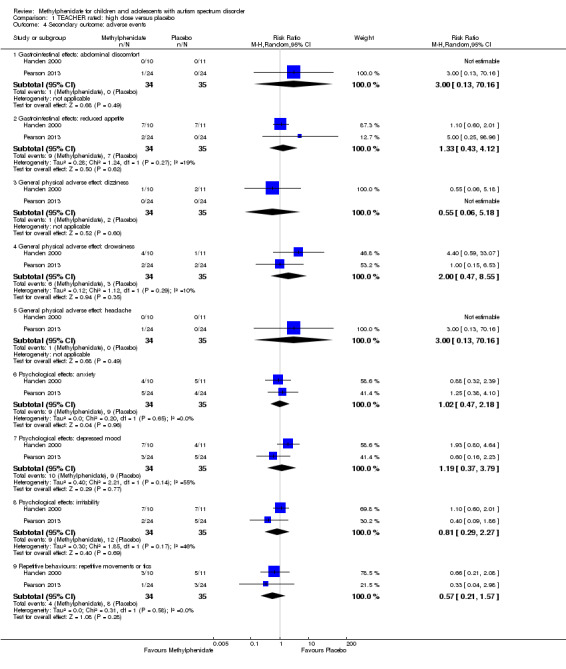
Comparison 1 TEACHER rated: high dose versus placebo, Outcome 4 Secondary outcome: adverse events.
5.3. Analysis.
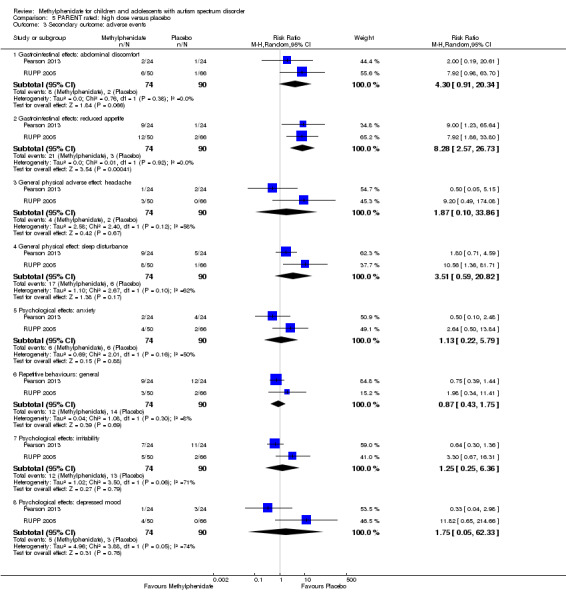
Comparison 5 PARENT rated: high dose versus placebo, Outcome 3 Secondary outcome: adverse events.
When we applied a fixed‐effect model (less conservative) to those adverse events for which statistical heterogeneity was less than 60%, abdominal discomfort (RR 4.74, 95% CI 1.07 to 21.02; 2 studies, 74 participants; P = 0.04) and reduced appetite (RR 8.32, 95% CI 2.57 to 26.91; 2 studies, 74 participants; P < 0.001), as rated by parents, were significantly more likely with treatment (analyses not reported). Results did not differ for adverse events as rated by teachers (analyses not reported).
2. Caregiver well‐being
The included trials did not report on this outcome.
3. Need for institutionalisation, special schooling options or therapy to achieve learning outcomes
The included trials did not report on this outcome.
4. Overall quality of life
The included trials did not report on this outcome.
Subgroup analyses: different doses of methylphenidate
Primary outcomes: clinical efficacy
1. Improvement in ADHD‐like symptoms
1.1 Teacher rated
We found no significant differences between different doses of methylphenidate for the different symptoms of ADHD. However, we were only able to judge this for inattention (P = 0.79; 2 studies; 51 participants; Analysis 9.1) and hyperactivity (P = 0.33; 4 studies; 73 participants; Analysis 9.2). We were not able to assess the effect of different dosages for impulsivity as only one study reported this (Pearson 2013).
9.1. Analysis.
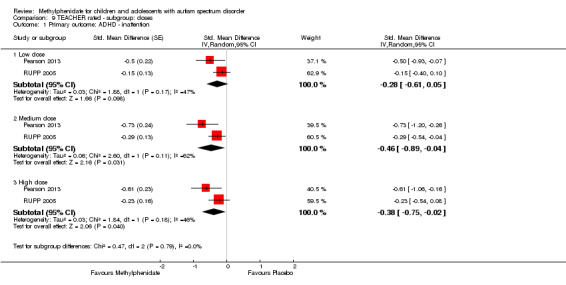
Comparison 9 TEACHER rated ‐ subgroup: doses, Outcome 1 Primary outcome: ADHD ‐ inattention.
9.2. Analysis.
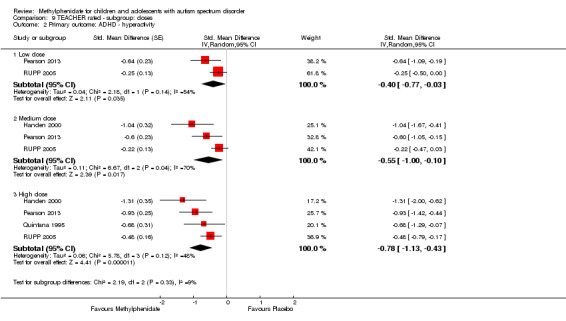
Comparison 9 TEACHER rated ‐ subgroup: doses, Outcome 2 Primary outcome: ADHD ‐ hyperactivity.
1.2 Parent rated
We found no significant differences between different doses of methylphenidate for the different symptoms of ADHD. However, we were only able to judge this for inattention (P = 0.61; 2 studies; 71 participants; Analysis 10.1) and hyperactivity (P = 0.22; 2 studies; 71 participants; Analysis 10.2). We were not able to assess the effect of different dosages for impulsivity as only one study reported this (Pearson 2013).
10.1. Analysis.
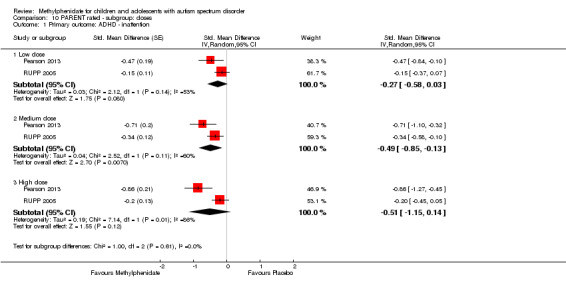
Comparison 10 PARENT rated ‐ subgroup: doses, Outcome 1 Primary outcome: ADHD ‐ inattention.
10.2. Analysis.
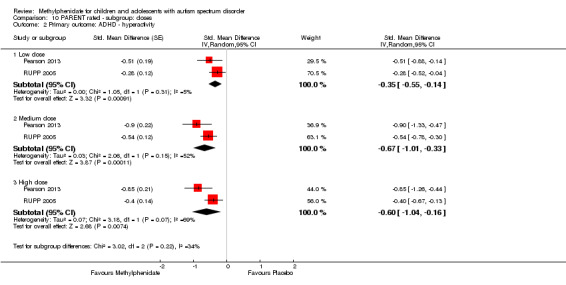
Comparison 10 PARENT rated ‐ subgroup: doses, Outcome 2 Primary outcome: ADHD ‐ hyperactivity.
2. Improvement in core symptoms of ASD
2.1 Teacher rated
We found no significant differences between different doses of methylphenidate for the different symptoms of ASD. However, we were only able to judge this for impaired social interaction (P = 0.77; 3 studies; 63 participants; Analysis 9.3), stereotypical behaviours (P = 0.69; 3 studies; 69 participants; Analysis 9.4), and overall ASD (P = 0.97; 2 studies; 36 participants; Analysis 9.5). We were not able to assess the effect of different dosages for impaired communication as only one study reported this (Handen 2000).
9.3. Analysis.
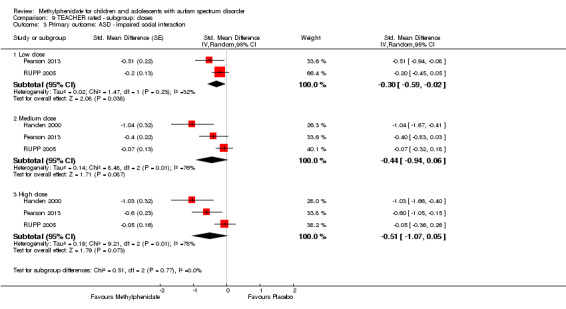
Comparison 9 TEACHER rated ‐ subgroup: doses, Outcome 3 Primary outcome: ASD ‐ impaired social interaction.
9.4. Analysis.
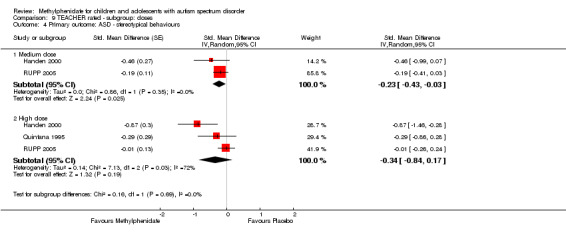
Comparison 9 TEACHER rated ‐ subgroup: doses, Outcome 4 Primary outcome: ASD ‐ stereotypical behaviours.
9.5. Analysis.
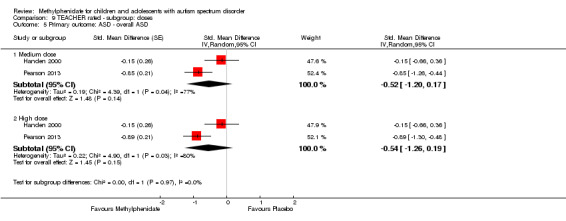
Comparison 9 TEACHER rated ‐ subgroup: doses, Outcome 5 Primary outcome: ASD ‐ overall ASD.
2.2 Parent rated
We found no significant differences between different doses of methylphenidate for the different symptoms of ASD. However, we were only able to judge this for impaired social interaction (P = 0.96; 2 studies; 71 participants; Analysis 10.3). Only one study reported impaired communication, stereotypical behaviours and overall ASD (Pearson 2013).
10.3. Analysis.
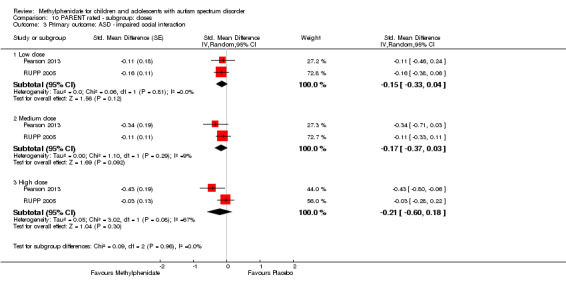
Comparison 10 PARENT rated ‐ subgroup: doses, Outcome 3 Primary outcome: ASD ‐ impaired social interaction.
Secondary outcomes: rate of adverse events
1. Teacher rated
Because the reporting of adverse events varied considerably across all studies, we were not able to pool results for the total rate of adverse events. However, for the specific symptoms (abdominal discomfort, reduced appetite, dizziness, drowsiness, headache, anxiety, depressed mood, irritability, and repetitive movements), we found no significant differences between different doses of methylphenidate (Analysis 9.6; Analysis 9.7; Analysis 9.8; Analysis 9.9; Analysis 9.10; Analysis 9.11; Analysis 9.12; Analysis 9.13; Analysis 9.14).
9.6. Analysis.
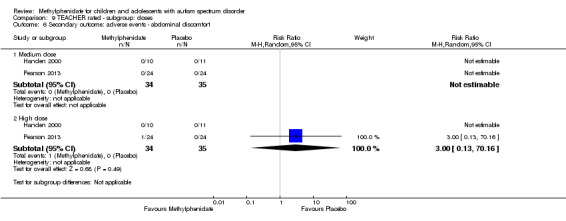
Comparison 9 TEACHER rated ‐ subgroup: doses, Outcome 6 Secondary outcome: adverse events ‐ abdominal discomfort.
9.7. Analysis.
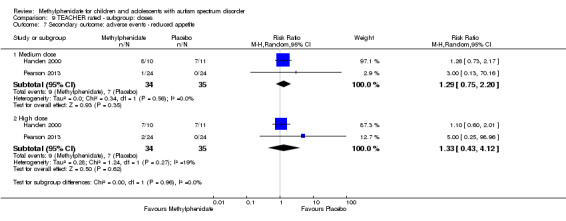
Comparison 9 TEACHER rated ‐ subgroup: doses, Outcome 7 Secondary outcome: adverse events ‐ reduced appetite.
9.8. Analysis.
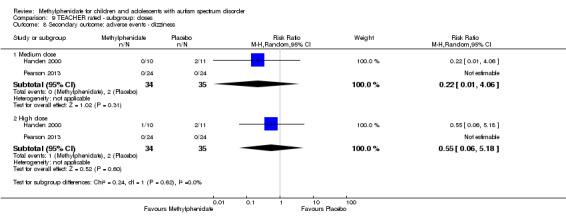
Comparison 9 TEACHER rated ‐ subgroup: doses, Outcome 8 Secondary outcome: adverse events ‐ dizziness.
9.9. Analysis.
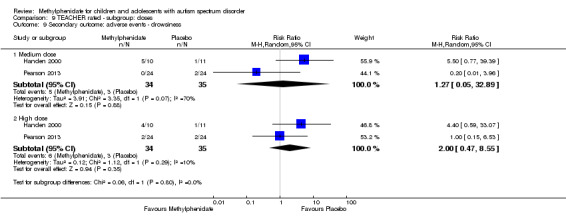
Comparison 9 TEACHER rated ‐ subgroup: doses, Outcome 9 Secondary outcome: adverse events ‐ drowsiness.
9.10. Analysis.
Comparison 9 TEACHER rated ‐ subgroup: doses, Outcome 10 Secondary outcome: adverse events ‐ headache.
9.11. Analysis.
Comparison 9 TEACHER rated ‐ subgroup: doses, Outcome 11 Secondary outcome: adverse events ‐ anxiety.
9.12. Analysis.
Comparison 9 TEACHER rated ‐ subgroup: doses, Outcome 12 Secondary outcome: adverse events ‐ depressed mood.
9.13. Analysis.
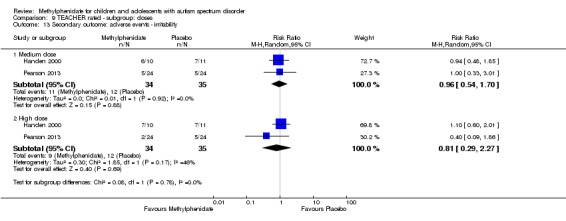
Comparison 9 TEACHER rated ‐ subgroup: doses, Outcome 13 Secondary outcome: adverse events ‐ irritability.
9.14. Analysis.
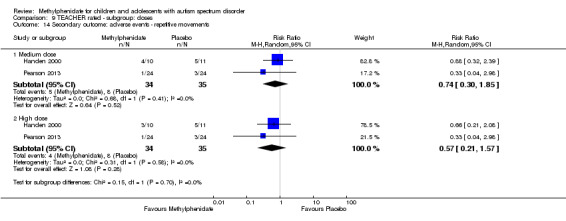
Comparison 9 TEACHER rated ‐ subgroup: doses, Outcome 14 Secondary outcome: adverse events ‐ repetitive movements.
2. Parent rated
Because the reporting of adverse events varied considerably across all studies, we were not able to pool results for the total rate of adverse events. However, for the specific symptoms (abdominal discomfort, reduced appetite, headache, anxiety, depressed mood, irritability, repetitive behaviours, and sleep disturbance), we found no significant differences between different doses of methylphenidate (Analysis 10.4; Analysis 10.5; Analysis 10.6; Analysis 10.7; Analysis 10.8; Analysis 10.9; Analysis 10.10; Analysis 10.11).
10.4. Analysis.
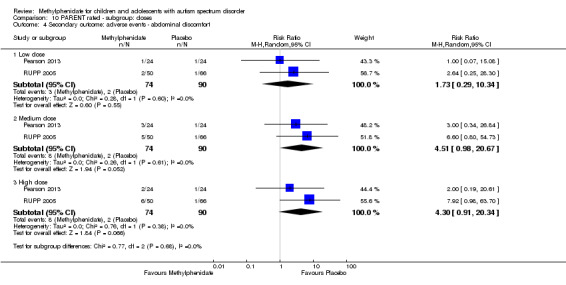
Comparison 10 PARENT rated ‐ subgroup: doses, Outcome 4 Secondary outcome: adverse events ‐ abdominal discomfort.
10.5. Analysis.
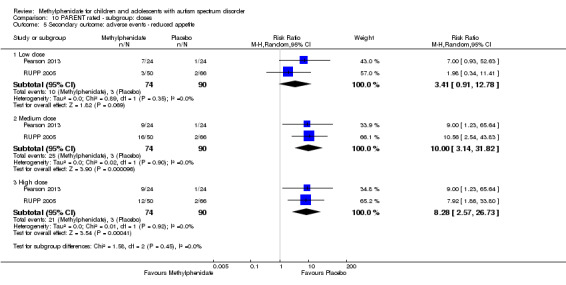
Comparison 10 PARENT rated ‐ subgroup: doses, Outcome 5 Secondary outcome: adverse events ‐ reduced appetite.
10.6. Analysis.
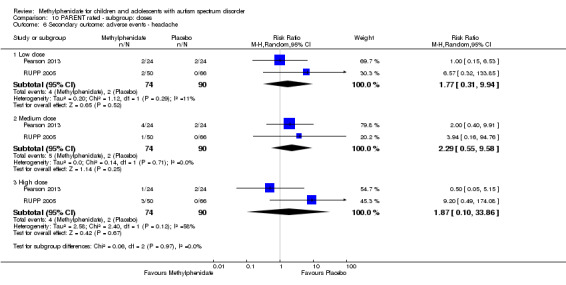
Comparison 10 PARENT rated ‐ subgroup: doses, Outcome 6 Secondary outcome: adverse events ‐ headache.
10.7. Analysis.
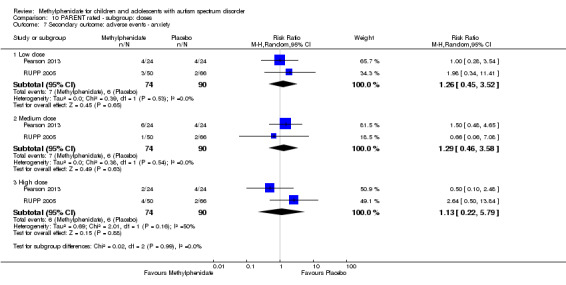
Comparison 10 PARENT rated ‐ subgroup: doses, Outcome 7 Secondary outcome: adverse events ‐ anxiety.
10.8. Analysis.
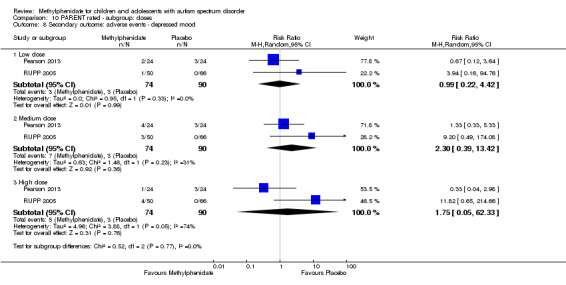
Comparison 10 PARENT rated ‐ subgroup: doses, Outcome 8 Secondary outcome: adverse events ‐ depressed mood.
10.9. Analysis.
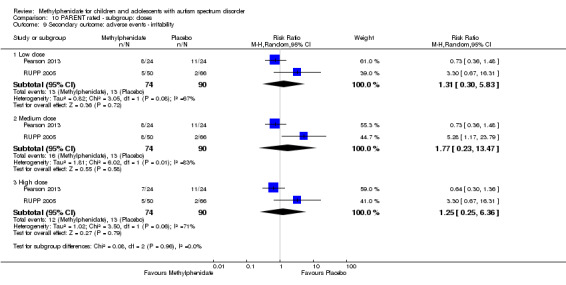
Comparison 10 PARENT rated ‐ subgroup: doses, Outcome 9 Secondary outcome: adverse events ‐ irritability.
10.10. Analysis.
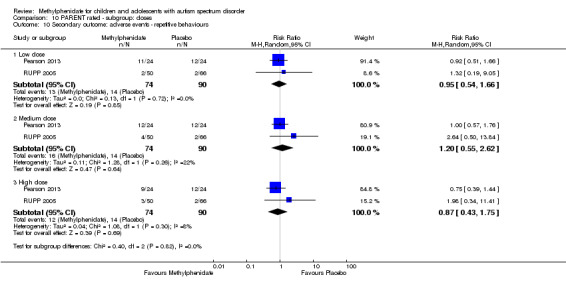
Comparison 10 PARENT rated ‐ subgroup: doses, Outcome 10 Secondary outcome: adverse events ‐ repetitive behaviours.
10.11. Analysis.
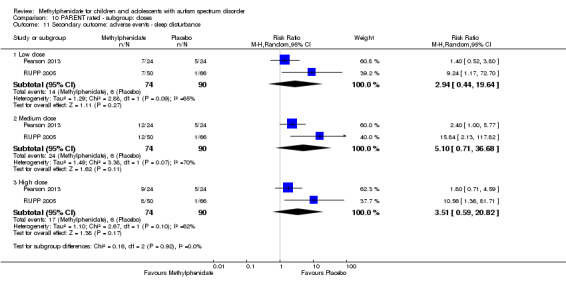
Comparison 10 PARENT rated ‐ subgroup: doses, Outcome 11 Secondary outcome: adverse events ‐ sleep disturbance.
Discussion
Summary of main results
We included four studies in our analysis, with a total of 113 randomised participants (94 (83%) boys; age range 5 to 13 years, all from the USA). All of these were cross‐over studies that compared either two or three different doses of methylphenidate with placebo. The duration of treatment in the cross‐over phase was one week for each dose of methylphenidate. Studies used a range of outcome scales, and several studies used multiple scales to assess one or more outcomes. Parents, teachers (or both), clinicians, and programme staff assessed outcomes. The studies took place between 1995 and 2013.
Primary outcomes: clinical efficacy
Improvement in ADHD‐like symptoms
The meta‐analysis suggested that high‐dose methylphenidate (0.43 mg/kg/dose to 0.60 mg/kg/dose) might have a significant and clinically important benefit on teacher‐rated hyperactivity and parent‐rated hyperactivity. High‐dose methylphenidate may have a significant but not clinically important benefit on teacher‐rated inattention but no benefit on inattention as rated by parents. There were inadequate data to conduct a meta‐analysis on the symptom of impulsivity as rated by either parents or teachers.
Improvement in the core symptoms of ASD and overall ASD
There was no evidence that methylphenidate worsens the core symptoms of ASD or improves social interaction as rated by teachers and parents; stereotypical behaviours as rated by teachers; and overall ASD as rated by teachers. There were inadequate data to conduct a meta‐analysis on: stereotypical behaviours as rated by parents; overall ASD as rated by parents; and impaired communication as rated either by parents or teachers.
Subgroup analysis
A subgroup analysis by dose did not identify any differences in effect on our primary outcomes at low‐, medium‐ or high‐dose ranges.
It was not possible to conduct a subgroup analysis by methylphenidate formulation. Data on duration of effect were not available as duration of follow‐up was only one week.
Secondary outcomes
Rate of adverse events
No studies reported serious adverse events. The only adverse event that was more likely with treatment was reduced appetite, as rated by parents. Other adverse events that were rated by parents or teachers (or both) in more than one included study, but for which the RR did not reach significance, included abdominal discomfort, dizziness, drowsiness, headache, sleep disturbance, anxiety, depressed mood, irritability and involuntary or repetitive movements. It was not possible to calculate the overall RR of one or more non‐serious adverse event(s), as individual participant data were not available. For our analyses of adverse events, we were unable to adjust for the cross‐over design of our included studies as individual patient data were not available. This conservative approach is likely to underestimate the risk of adverse events, if there is a within‐person correlation for adverse events.
No data were available for the secondary outcomes of caregiver well‐being; need for institutionalisation, special schooling options, or therapy to achieve learning outcomes; or overall quality of life.
Overall completeness and applicability of evidence
The evidence was directly applicable and relevant to our review question. Studies, however, were confined to children living in the USA, and our findings may not be generalisable to other countries with different cultural contexts, educational systems and resources. Concomitant psychotropic medication was not an exclusion criterion for this review. Although the concomitant use of such medication by some participants in one, or possibly two, of our included studies may have influenced our findings, it is not possible to estimate in which direction or to what extent such use may have done so. Our data are incomplete in a number of ways. We identified only four small studies, although their cross‐over design increases their power. Importantly, there is no evidence about the use of methylphenidate for longer than one week, and there is no evidence in children older than 13 years. There are also important problems with the completeness of adverse events data, as summarised below (see Quality of the evidence). Furthermore, given that individual participant data were not available, we cannot comment on whether individual participant characteristics (such as severity of ASD or cognitive impairment) predict response to treatment; this information would be useful to clinicians and parents. Data on the total daily dose administered were not available and, in many cases, parents determined whether or not afternoon doses were administered; this information may be relevant if adverse events or benefits of treatment are related to total daily dose. Our review, therefore, has limited external validity.
The included studies are not sufficient to address all of the objectives of the review, in particular the primary outcomes of impulsivity, impaired communication and parent‐rated stereotypical behaviours and overall ASD, and the secondary outcomes of caregiver well‐being; need for institutionalisation, specialised schooling or therapy; and overall quality of life. The trials did not investigate children who were not attending hospital‐based services or special schools, nor did they report medium‐ and long‐term outcomes. Current practice, in terms of prescribing methylphenidate for children with ASD, may vary internationally, but the published literature suggests that methylphenidate is frequently prescribed for these children (Frazier 2011). Our review suggests that there is insufficient evidence to be confident in the effectiveness and safety of this prescribing.
We reported on individual outcomes within the core features of ASD and ADHD‐like symptoms, as well as on overall ASD, allowing these treatment decisions to be personalised to some extent based on the predominant or most troubling symptom(s) a child manifests in particular contexts. This is likely to be helpful for parents and clinicians to determine if and when to administer methylphenidate, given that individual children with ASD and ADHD‐like symptoms manifest a heterogeneous range of different symptoms, which may be more or less troubling depending on the context.
Quality of the evidence
We judged the quality of the evidence to be low overall, as shown in more detail in Table 1 and Table 2. We believe that this is a conservative judgement. We downgraded the quality of the evidence due to imprecision for all outcomes as a result of the small number of included studies and participants. We also downgraded one point for limitations in design and implementation for studies with selective reporting of outcomes and/or unclear risk of bias for allocation and blinding (Handen 2000; Quintana 1995; RUPP 2005). We downgraded one point for indirectness of evidence for the outcome of impaired social interaction, which included measures of aggressive and oppositional defiant behaviour.
We considered three trials to be at high risk of bias due to selective reporting. We assessed all trials as being at unclear risk of bias for blinding of participants and assessors, which was incomplete in all studies, due to the potential for recognising adverse events of methylphenidate. We assessed all trials at low or unclear risk of bias for all other items.
We have low confidence in the adverse events data, because parents of children with previous adverse events from methylphenidate were unlikely to accept invitations to participate in the studies, and participants at high risk of serious adverse events (such as those with cardiological or neurological conditions) were excluded from the studies. Intolerance of methylphenidate in the test‐dose phase also resulted in exclusion of participants from the randomised phase of the study or exclusion from the high‐dose condition. The absence of data about total daily dose administered and the short duration of treatment and follow‐up (one week only) also reduce the quality of this evidence. This short duration may result in an overestimation of the longer‐term risk of some adverse events, such as sleep disturbance, gastrointestinal effects, headaches and emotional lability, which might settle with a longer duration of treatment. On the other hand, the short duration may result in an underestimation of other adverse events that may appear over time such as tolerance, dependence, discontinuation reactions and diversion of doses. Overall, we believe that the very low quality of evidence for adverse events is likely to result in an underestimation of risk. However, it may be safe in the short term to use doses within the dose ranges studied if the child tolerates these, provided that the child has no cardiovascular or neurological conditions and clinicians closely monitor adverse events.
Potential biases in the review process
In order to minimise bias in the review process, two authors independently selected studies for inclusion, assessed risk of bias and extracted data. All authors conducted the analyses and interpreted the data. However, this review has some limitations.
We used a minimum intertreatment clinically important difference (MCID) of 0.52, based on Zhang 2005, who calculated the MCID based on ADHDRS‐PI scores and the Clinical Global Impressions ‐ ADHD ‐ Severity (CGI‐ADHD‐S), a single‐item, clinician rating of the severity of ADHD symptoms. Storebø 2015 also used the MCID of 0.52. Although ADHDRS‐PI scores in Zhang 2005 correlated well with a number of other ADHD scales, it correlated poorly with the Conners' rating scales and was not validated on children with ASD. Consequently, this may be either an underestimate or an overestimate of the true MCID in children with ASD.
It was challenging to locate the exact outcome scales and subscales used in our studies partly because access is restricted by intellectual property and commercial implications, and partly because, in some cases, there are multiple versions that have been edited and modified over time, including multiple versions of the Conners' scales (Sparrow 2010; Westerlund 2009). It was also challenging to identify clinically equivalent outcome scales and subscales, especially as factor analyses from different validation studies have not always produced consistent results. We made decisions about allocating various scales and subscales to our outcomes based on the best information we could access. We did not include James' 'social communication' measure, featured in the 2009 publication of RUPP 2005. This tool measures joint attention and spontaneous attention requests, as a measure of 'impaired social interaction', 'impaired communication' or 'overall ASD' outcomes, but it was unclear which outcomes, if any, this instrument measured. We decided which outcome scales to use in our meta‐analysis based on the most commonly used scales across our studies, and we performed and reported sensitivity analyses using the other scales.
Agreements and disagreements with other studies or reviews
We found a benefit from methylphenidate in children with ASD and ADHD‐like symptoms, which is in general agreement with the literature. We found no evidence of a worsening of ASD symptoms, which had been a theoretical concern in previous literature (Aman 1982). Adverse events also do not appear to be higher than those reported by Storebø 2015, which is reassuring in view of previous concerns in the literature between the 1970s and 1990s that children with ASD were likely to have more adverse events (including an increase in irritability, stereotyped behaviour, dysphoria, agitation and even psychotic symptoms) than children with ADHD. However, our findings on adverse events are tempered by study recruitment factors, test‐dose phase exclusion of participants who were intolerant of methylphenidate, and the short duration of treatment and follow‐up, as previously discussed. Our estimate of decreased appetite (RR 8.28) is actually higher than the RR of 3.66 reported by Storebø 2015. Our finding of no increased risk of sleep disturbance disagrees with the general recognition of sleep disturbance as a common adverse event of methylphenidate; this may be due to parent decisions in our studies to withhold the afternoon dose of methylphenidate for children who experienced this adverse event.
Our finding that teachers tended to report a greater benefit than parents is in agreement with previous studies in children with ADHD (Faries 2001; Hartman 2007), which have suggested that teachers are more sensitive to the beneficial effects of psychostimulants. This may be related to a 'wearing off' of morning methylphenidate by the time children return home from school; children's behaviour at home may also differ from their behaviour in more structured clinical or educational settings, and teachers have different relationships and contact hours with children compared to parents and may place greater emphasis on academic aspects. Some of the symptoms measured by commonly used scales (such as inattention and hyperactivity) may be more important to classroom functioning than functioning at home (whereas improvements in emotional lability and ability to cope with change may be more likely to be noticed by parents).
We do not have adequate data to comment on previous reports that more functionally impaired children have less benefit from methylphenidate (Aman 2003), or that initial benefit from methylphenidate may not be not sustained (Riddle 2013). We cannot comment on optimum doses or dose‐response relationships. As only one of our studies used one of the newer, extended‐release formulations of methylphenidate (Pearson 2013), we are unable to comment on how these compare to the previous immediate‐release formulations.
Authors' conclusions
Implications for practice.
School‐age children up to the age of 13 years with ASD who also have inattention, impulsivity and/or hyperactivity may benefit from a trial of methylphenidate. The results of the meta‐analysis suggest that short‐term use of methylphenidate may improve symptoms of hyperactivity and possibly inattention in children with ASD who are tolerant of the medication. We found no evidence that methylphenidate has a negative impact on the core symptoms of ASD or that it improves social interaction, stereotypical behaviours, and overall ASD. We did not find any effect of methylphenidate dose at the three dose ranges included in our studies. We are unable to comment on whether any benefit from treatment is sustained for longer than one week. The evidence for adverse events is of very low quality because children who were intolerant of methylphenidate were either unlikely to be recruited or were excluded from the randomised, cross‐over phase of the studies. Of note, our studies excluded children with cardiovascular or neurological conditions. The short duration of all studies also reduces the quality of the evidence for adverse events. Although the trials did not identify any serious adverse events, and only reduced appetite was significantly more common in those children receiving methylphenidate compared to those receiving placebo, the quality of the evidence is too low to deliver any implications for practice other than to recommend close, ongoing monitoring for potential adverse events. We are unable to comment on individual factors that might predict the effects of treatment or the optimum dose of methylphenidate.
Implications for research.
Future trials should publish individual participant data as well as means to enable future researchers to identify individual participant characteristics that may increase or reduce sensitivity to intervention effects (including both benefits and adverse events) and predict optimum doses. Single‐case experimental design studies or N‐of‐1 trials should be considered in order to assess the effectiveness of methylphenidate for individual children because these give patient‐specific information in an efficient and methodologically rigorous way. N‐of‐1 trials also provide individual patient data, which can be used in future systematic reviews. In addition, it would be important to develop a core outcome set for future trials to enable meaningful comparisons between studies. As a minimum, studies should also include appendices with a copy of the outcome scale used, as there are a multiplicity of scales and subscales in the literature (Sparrow 2010; Westerlund 2009). The minimum clinically important intertreatment difference also needs to be confirmed for children with ASD using outcome scales validated for this population. Future RCTs should consider extending the duration of treatment and follow‐up in order to assess medium‐ and long‐term effects and to determine whether any initial benefit is sustained or indeed enhanced with ongoing use (which may be the case if some adverse events of methylphenidate diminish over time). Studies should also assess recreational use and abuse of methylphenidate. Furthermore, it would be helpful to report total daily dose administered, as well as morning dose; it was not possible to calculate total daily dose, as the administration of afternoon dose or doses was variable and poorly reported. Additional steps to improve blinding should be considered, possibly including an active placebo, as suggested by Jensen 2017, or nocebo interventions, as suggested by Storebø 2015, although we acknowledge that this presents significant ethical and practical difficulties. Investigators outside the USA should consider further RCTs in children with ASD and ADHD‐like symptoms to assess the generalisability of our findings.
Acknowledgements
We produced this review within the Cochrane Developmental, Psychosocial and Learning Problems review group.
The review authors would like to acknowledge the following people who contributed to the early stages of the development and writing of the protocol for the review: Veena Gullapalli, Raesha Jaffer, Abirami Ratnagopal and Alvin Wong.
The authors would like to acknowledge the following people who contributed to the development and writing of the protocol for this review: Toni Redman, Elly Scheermeyer, Makoto Ogawa, Eddie Sparks, Jeremy Taylor and Vi Tran. Toni Redman generated the idea for this review, provided the content expertise for the protocol and reviewed the final draft of the review, based on her extensive experience in community paediatrics. Elly Scheermeyer contributed to the content and methodology of the protocol. Makoto Ogawa, Eddie Sparks, Jeremy Taylor and Vi Tran reviewed the protocol and contributed responses to editorial and peer reviewers' comments about the protocol. Jeremy Taylor, Makoto Ogawa and Vi Tran also contributed to the initial screening of citations identified by the 2014 search.
The authors would like to acknowledge Mark Jones for his statistical advice.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library
#1MeSH descriptor: [Central Nervous System Stimulants] this term only #2MeSH descriptor: [Methylphenidate] this term only #3methylphenidate* #4(Attenta* or Biphentin* or Centedrin* or Concerta* or Daytrana* or dexmethylphenidat* or Equasym*) #5(Focalin* or Medikinet* or Metadate* or Methylin* or Penid* or Phenidyl* or Ritalin* or Rubifen or tranquilyn* or Tsentedrin*) #6{or #1‐#5} #7[mh "child development disorders, pervasive"] #8[mh "Developmental Disabilities"] #9(pervasive next development* next disorder) #10(pervasive and child*) #11(PDD or PDDs or PDD‐NOS or ASD or ASDs) #12autis* #13asperger* #14kanner* #15"childhood schizophrenia " #16Rett* #17{or #7‐#16} #18 #6 and #17
MEDLINE Ovid
1 Central Nervous System Stimulants/ 2 Methylphenidate/ 3 Methylphenidat$.mp. 4 (Attenta$ or Biphentin$ or Centedrin$ or Concerta$ or Daytrana$ or dexmethylphenidat$ or Equasym$).mp. 5 (Focalin$ or Medikinet$ or Metadate$ or Methylin$ or Methylphenidat$ or Penid$ or Phenidyl$ or Ritalin$ or Rubifen or tranquilyn$ or Tsentedrin$).mp. 6 or/1‐5 7 exp child development disorders, pervasive/ 8 Developmental Disabilities/ 9 pervasive development$ disorder$.tw. 10 (pervasive adj3 child$).tw. 11 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw. 12 autis$.tw. 13 asperger$.tw. 14 kanner$.tw. 15 childhood schizophrenia.tw. 16 Rett$.tw. 17 or/7‐16 18 6 and 17
MEDLINE In‐ Process Ovid
1 Methylphenidat$.mp. 2 (Attenta$ or Biphentin$ or Centedrin$ or Concerta$ or Daytrana$ or dexmethylphenidat$ or Equasym$).mp. 3 (Focalin$ or Medikinet$ or Metadate$ or Methylin$ or Methylphenidat$ or Penid$ or Phenidyl$ or Ritalin$ or Rubifen or tranquilyn$ or Tsentedrin$).mp. 4 pervasive development$ disorder$.tw. 5 (pervasive adj3 child$).tw. 6 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw. 7 autis$.tw. 8 asperger$.tw. 9 kanner$.tw. 10 childhood schizophrenia.tw. 11 Rett$.tw. 12 or/1‐3 13 or/4‐11 14 12 and 13
MEDLINE Epub Ahead of Print Ovid
1 Methylphenidat$.mp. 2 (Attenta$ or Biphentin$ or Centedrin$ or Concerta$ or Daytrana$ or dexmethylphenidat$ or Equasym$).mp. 3 (Focalin$ or Medikinet$ or Metadate$ or Methylin$ or Methylphenidat$ or Penid$ or Phenidyl$ or Ritalin$ or Rubifen or tranquilyn$ or Tsentedrin$).mp. 4 pervasive development$ disorder$.tw. 5 (pervasive adj3 child$).tw. 6 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw. 7 autis$.tw. 8 asperger$.tw. 9 kanner$.tw. 10 childhood schizophrenia.tw. 11 Rett$.tw. 12 or/1‐3 13 or/4‐11 14 12 and 13
Embase Ovid
1 Central nervous system stimulants/ 2 methylphenidate/ 3 Methylphenidat$.mp. 4 (Attenta$ or Biphentin$ or Centedrin$ or Concerta$ or Daytrana$ or dexmethylphenidat$ or Equasym$).mp. 5 (Focalin$ or Medikinet$ or Metadate$ or Methylin$ or Methylphenidat$ or Penid$ or Phenidyl$ or Ritalin$ or Rubifen or tranquilyn$ or Tsentedrin$).mp. 6 or/1‐5 7 exp autism/ 8 pervasive development$ disorder$.tw. 9 (PDD or PDDs or ASD or ASDs).tw. 10 autis$.tw. 11 asperger$.tw. 12 kanner$.tw. 13 childhood schizophreni$.tw. 14 Rett$.tw. (3687) 15 (pervasive adj3 child$).tw. 16 or/7‐15 17 6 and 16 18 Clinical trial/ 19 Randomized controlled trial/ 20 Single blind procedure/ 21 Double blind procedure/ 22 triple blind procedure/ 23 Crossover procedure/ 24 Randomi#ed.tw. 25 (random$ adj3 (allocat$ or assign$)).tw. 26 randomly.ab. 27 groups.ab. 28 trial.ab. 29 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 30 Placebo$.tw. 31 (crossover or cross‐over).tw. 32 or/18‐31 33 (animal/ or nonhuman/ or animal experiment/) and human/ 34 animal/ or nonhuman/ or animal experiment/ 35 34 not 33 36 32 not 35
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
S15 S5 AND S14 S14 S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 S13 Rett* S12 childhood schizophren* S11 kanner* S10 (PDD or PDDs or PDD‐NOS or ASD or ASDs) S9 (pervasive N3 child*) S8 pervasive development* disorder* S7 autis* or asperger* S6 (MH "Child Development Disorders, Pervasive+") S5 S1 OR S2 OR S3 OR S4 S4 (Attenta* or Biphentin* or Centedrin* or Concerta* or Daytrana* or dexmethylphenidat* or Equasym*) S3 (Focalin* or Medikinet* or Metadate* or Methylin* or Penid* or Phenidyl* or Ritalin* or Rubifen or tranquilyn* or Tsentedrin*) S2 methylphenidat* S1 (MH "Methylphenidate")
PsycINFO Ovid
1 CNS stimulating drugs/ 2 methylphenidate/ 3 Methylphenidat$.mp. 4 (Attenta$ or Biphentin$ or Centedrin$ or Concerta$ or Daytrana$ or dexmethylphenidat$ or Equasym$).mp. 5 (Focalin$ or Medikinet$ or Metadate$ or Methylin$ or Methylphenidat$ or Penid$ or Phenidyl$ or Ritalin$ or Rubifen or tranquilyn$ or Tsentedrin$).mp. 6 or/1‐5 7 exp pervasive developmental disorders/ 8 Developmental disabilities/ 9 pervasive development$ disorder$.tw. 10 (pervasive adj3 child$).tw. 11 autis$.tw. 12 asperger$.tw. 13 (autis$ or ASD or ASDs).tw. 14 (ASD or ASDs or PDD or PDDs).tw. 15 Rett$.tw. 16 Kanner$.tw. 17 or/7‐16 18 6 and 17
ERIC (Education Resources Information Center)
ERIC Proquest
Searched up to 2014.
(SU.EXACT.EXPLODE("Pervasive Developmental Disorders") OR autis* OR Asperger* OR kanner* OR "pervasive development* disorder*" OR "childhood schizophrenia" OR pervasive NEAR/3 child* OR pdd OR pdds OR asd OR asds OR pdd‐nos)) AND (Attenta* OR Biphentin* OR Centedrin* OR Concerta* OR Daytrana* OR dexmethylphenidat* OR Equasym* OR Focalin* OR Medikinet* OR Metadate* OR Methylphenidat* OR Methylin* OR Penid* OR Phenidyl* OR Ritalin* OR Rubifen OR tranquilyn* OR Tsentedrin*)
ERIC EBSCOhost
Searched after 2014.
S1DE "Developmental Disabilities" S2DE "Pervasive Developmental Disorders" OR DE "Asperger Syndrome" OR DE "Autism" S3(pervasive development* disorder* or PDD or PDDs) S4(autis* or ASD or ASDs) S5Asperger* S6Rett* S7Kanner* S8childhood schizophren* S9S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 S10methylphenidat* S11(Attenta* or Biphentin* or Centedrin* or Concerta* or Daytrana* or dexmethylphenidat* or Equasym*) S12(Focalin* or Medikinet* or Metadate* or Methylin* or Penid* or Phenidyl* or Ritalin* or Rubifen or tranquilyn* or Tsentedrin*) S13S10 OR S11 OR S12 S14S9 AND S13
Science Citation Index, Social Sciences Citation Index, Conference Proceedings Citation Index ‐ Science, Conference Proceedings Citation Index ‐ Social Sciences & Humanities; all Web of Science
# 7 #6 AND #3 # 6 #5 OR #4 # 5 TS=(Focalin* or Medikinet* or Metadate* or Methylin* or methylphenidat* or Penid* or Phenidyl* or Ritalin* or Rubifen or tranquilyn* or Tsentedrin*) # 4 TS=(Attenta* or Biphentin* or Centedrin* or Concerta* or Daytrana* or dexmethylphenidat* or Equasym*) #3 #2 OR #1 # 2 TS=(PDD or PDDs or PDD‐NOS or ASD or ASDs) # 1 TS=(autis* or asperger* or Rett* or "pervasive developmental " or (pervasive NEAR/3 child) or "childhood schizoprenia")
Cochrane Database of Systematic Reviews (CDSR) part of the Cochrane Library
#1MeSH descriptor: [Central Nervous System Stimulants] this term only #2MeSH descriptor: [Methylphenidate] this term only #3methylphenidate*:ti,ab #4(Attenta* or Biphentin* or Centedrin* or Concerta* or Daytrana* or dexmethylphenidat* or Equasym*):ti,ab #5(Focalin* or Medikinet* or Metadate* or Methylin* or Penid* or Phenidyl* or Ritalin* or Rubifen or tranquilyn* or Tsentedrin*):ti,ab #6{or #1‐#5} #7[mh "child development disorders, pervasive"] #8[mh "Developmental Disabilities"] #9(pervasive next development* next disorder):ti,ab #10(pervasive and child*):ti,ab #11(PDD or PDDs or PDD‐NOS or ASD or ASDs):ti,ab #12autis*:ti,ab #13asperger*:ti,ab #14kanner*:ti,ab #15childhood schizophrenia:ti,ab #16Rett*:ti,ab #17{or #7‐#16}
Database of Abstracts of Reviews of Effects (DARE) part of the Cochrane Library
#1MeSH descriptor: [Central Nervous System Stimulants] this term only #2MeSH descriptor: [Methylphenidate] this term only #3methylphenidate* #4(Attenta* or Biphentin* or Centedrin* or Concerta* or Daytrana* or dexmethylphenidat* or Equasym*) #5(Focalin* or Medikinet* or Metadate* or Methylin* or Penid* or Phenidyl* or Ritalin* or Rubifen or tranquilyn* or Tsentedrin*) #6{or #1‐#5} #7[mh "child development disorders, pervasive"] #8[mh "Developmental Disabilities"] #9(pervasive next development* next disorder) #10(pervasive and child*) #11(PDD or PDDs or PDD‐NOS or ASD or ASDs) #12autis* #13asperger* #14kanner* #15"childhood schizophrenia " #16Rett* #17{or #7‐#16}
AutismData
autism.org.uk/autismdata
methylphenidate and random*
Proquest Dissertations & Theses
ALL(Attenta* or Biphentin* or Centedrin or Concerta* or Daytrana* or dexmethylphenidat* or Equasym* or Focalin* or Medikinet* or Metadate* or Methylin* or Methylphenidat* or Penid* or Phenidyl* or Ritalin* or Rubifen or tranquilyn* or Tsentedrin*) AND ALL(autis* or asperger* or ASD or pervasive)
ClinicalTrials.gov
clinicaltrials.gov
methylphenidate AND autism
World Health Organziation International Clinical Trials Registry Platform (WHO ICTRP)
apps.who.int/trialsearch
Advanced search Condition : autism OR asperger* OR pervasive Intervention: methylphenidate OR ritalin
Synonyms included automatically : AUTISTIC DISORDER, AUTISTIC DISORDERS, AUTISTIC SPECTRUM DISORDER (ASD), DISORDER, AUTISTIC, DISORDERS, AUTISTIC, KANNER SYNDROME, KANNER'S SYNDROME, KANNERS SYNDROME, PERVASIVE DEVELOPMENTAL DISORDER (PDD), SCHIZOPHRENIC REACTION, SYNDROME, KANNER'S, autism ‐ DAYTRANA, RITALIN, methylphenidate
Appendix 2. Summary of searches
| Database | Date of search | Date range or issue | Number of records | Limits applied to searches |
| Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library | 16 May 2014 | 2014 Issue 4 | 24 | No limits |
| 21 November 2016 | 2016 Issue 10 | 18 | 2014‐2016 | |
| MEDLINE Ovid | 15 May 2014 | 1946 to May Week 1 2014 | 181 | No limits |
| 21 November 2016 | 1946 to November Week 2 2016 | 52 | From 2014 | |
| MEDLINE In‐Process Ovid | 15 May 2014 | 14 May 2014 | 17 | No limits |
| 21 November 2016 | 18 November 2016 | 13 | No limits | |
| MEDLINE Epub Ahead of Print Ovid | 21 November 2016 | 18 November 2016 | 1 | No limits |
| Embase Ovid | 16 May 2014 | 1980 to 2014 Week 19 | 284 | No limits |
| 21 November 2016 | 1974 to 18 November 2016 | 66 | From 2014 | |
| CINAHLPlus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature) | 16 May 2014 | 1936 to 16 May 2014 | 46 | No limits |
| 21 November 2016 | 1936 to 21 November 2016 | 6 | 2014‐2016 | |
| PsycINFO Ovid | 16 May 2014 | 1806 to May Week 2 2014 | 114 | No limits |
| 21 November 2016 | 1806 to November Week 2 2016 | 20 | From 2014 | |
| ERIC Proquest (Education Resources Information Center) | 16 May 2014 | 1966 to 16 May 2014 | 17 | No limits |
| ERIC EBSCOhost (Education Resources Information Center) | 22 November 2016 | 1966 to 22 November 2016 | 0 | From 2014 |
| Science Citation Index, Social Sciences Citation Index, Conference Proceedings Citation Index ‐ Science, Conference Proceedings Citation Index ‐ Social Sciences & Humanities; all Web of Science |
16 May 2014 | 1970 to 15 May 2014 | 165 | No limits |
| 22 November 2016 | 1970 to 21 November 2016 | 42 | 2014‐2016 | |
| Cochrane Database of Systematic Reviews part of the Cochrane Library | 16 May 2014 | 2014 Issue 5 | 0 | No limits |
| 21 November 2016 | 2016 Issue 11 | 1 | 2014‐2016 | |
| Database of Abstracts of Reviews of Effects part of the Cochrane Library | 16 May 2014 | 2014 Issue 2 | 0 | No limits |
| 21 November 2016 | 2015 Issue 2 (Final issue) | 0 | 2014‐2016 | |
| AutismData www.autism.org.uk/autismdata | 16 May 2014 | All available years | 6 | No limits |
| 22 November 2016 | All available years | 1 | 2014‐2016 | |
| Proquest Dissertations & Theses | 2 December 2016 | All available years | 11 | No limits |
| ClinicalTrials.gov clinicaltrials.gov | 16 May 2014 | All available years | 6 | No limits |
| 22 November 2016 | All available years | 2 | Registered between 1 May 2014 and 22 November 2016 | |
| WHO ICTRP apps.who.int/trialsearch | 16 May 2014 | All available years | 8 | No limits |
| 22 November 2016 | All available years | 1 | Registered between 1 May 2014 and 22 November 2016 | |
| Total | 1102 | |||
Appendix 3. Data extraction workbook
Primary outcome 1: inattention
| Study reference | Trial data | |||||||||||
| Instrument | ||||||||||||
| Subscale | ||||||||||||
| N items | ||||||||||||
| Item rating | ||||||||||||
| Rater | ||||||||||||
| Participant age | ||||||||||||
| N | ||||||||||||
| Treatment duration | ||||||||||||
| Treatment dose | ||||||||||||
| Short acting or CR | ||||||||||||
| Time point at which outcomes measured | ||||||||||||
| Results |
Primary outcome 2: impulsivity
| Study reference | Trial data |
| Instrument | |
| Subscale | |
| N items | |
| Item rating | |
| Rater | |
| Participant age | |
| N | |
| Treatment duration | |
| Treatment dose | |
| Short acting or CR | |
| Time point at which outcomes measured | |
| Results |
Primary outcome 3: hyperactivity
| Study reference | Trial data |
| Instrument | |
| Subscale | |
| N items | |
| Item rating | |
| Rater | |
| Participant age | |
| N | |
| Treatment duration | |
| Treatment dose | |
| Short acting or CR | |
| Time point at which outcomes measured | |
| Results |
Primary outcome 4: impaired social interaction
| Study reference | Trial data |
| Instrument | |
| Subscale | |
| N items | |
| Item rating | |
| Rater | |
| Participant age | |
| N | |
| Treatment duration | |
| Treatment dose | |
| Short acting or CR | |
| Time point at which outcomes measured | |
| Results |
Primary outcome 5: impaired communication
| Study reference | Trial data |
| Instrument | |
| Subscale | |
| N items | |
| Item rating | |
| Rater | |
| Participant age | |
| N | |
| Treatment duration | |
| Treatment dose | |
| Short acting or CR | |
| Time point at which outcomes measured | |
| Results |
Primary outcome 6: stereotypical behaviours
| Study reference | Trial data |
| Instrument | |
| Subscale | |
| N items | |
| Item rating | |
| Rater | |
| Participant age | |
| N | |
| Treatment duration | |
| Treatment dose | |
| Short acting or CR | |
| Time point at which outcomes measured | |
| Results |
Primary outcome 7: overall ASD
| Study reference | Trial data |
| Instrument | |
| Subscale | |
| N items | |
| Item rating | |
| Rater | |
| Participant age | |
| N | |
| Treatment duration | |
| Treatment dose | |
| Short acting or CR | |
| Time point at which outcomes measured | |
| Results |
Secondary outcome: adverse events
| Study reference | Trial data |
| Instrument | |
| Subscale | |
| N items | |
| Item rating | |
| Rater | |
| Participant age | |
| N | |
| Treatment duration | |
| Treatment dose | |
| Short acting or CR | |
| Time point at which outcomes measured | |
| Results |
Footnotes
ASD: autism spectrum disorder;CR: controlled release ; N: number.
Appendix 4. Criteria for assigning 'Risk of bias' judgements
-
Sequence generation ‐ determines whether generation of the random numbers was adequate. We assessed the risk of bias of sequence generation as low, high or unclear.
Low – computer‐generated random numbers or random number tables.
High – random numbers are generated by sequentially allocating groups.
Unclear – when information about the generation of random numbers is described inadequately or not at all.
-
Allocation concealment ‐ determines whether the method used to conceal allocation was adequate to prevent selection bias during the randomisation process before allocation. We assessed the risk of bias of allocation and concealment as low, high or unclear.
Low – used methods such as central allocation, or sealed opaque envelopes.
High – participants or investigators could possibly foresee the allocated treatment. For example, numbering participants and only including even numbers in the control group.
Unclear – when the method of allocation concealment is described inadequately or not at all.
-
Blinding of participants and personnel. We assessed the risk of bias related to blinding of participants and personnel as low, high or unclear.
Low – participants or investigators are unable to determine the treatment allocated.
High – participants or investigators could possibly determine the treatment allocated.
Unclear – information about blinding is insufficient to make a judgement of low or high risk of bias.
-
Blinding of outcome assessment. We assessed the risk of bias related to blinding of outcome assessment as low, high or unclear.
Low – outcome assessors are unable to determine the treatment allocated.
High – outcome assessors have knowledge or could have knowledge of the allocated treatment, and this could have influenced their assessment (for example, in the case of instruments assessed through interview).
Unclear – information about blinding of outcome assessors is insufficient to make a judgement of low or high risk of bias.
-
Incomplete outcome data – assesses whether missing data were accounted for. We assessed the risk of bias related to incomplete outcome data as low, high or unclear.
Low – no missing outcome data, or reasons for missing outcome data reported and unlikely to be related to true outcome or have a clinically relevant impact on observed effect size.
High – reason for missing outcome data likely to be related to true outcome, or plausible effect size among missing outcomes enough to induce clinically relevant bias in observed effect size, or as‐treated analysis with substantial departure of the intervention received from that assigned at randomisation, or potentially inappropriate application of simple imputation.
Unclear – insufficient reporting of attrition/exclusions or reasons for missing data to permit judgement of risk.
-
Selective reporting – assesses if all planned outcomes are reported. We assessed selective reporting by comparing the reported outcomes with those published in the protocol of the study, if available. If a previously published protocol was not available, we compared the outcomes described in the methods section of the paper with the outcomes reported in the same paper. We assessed risk of bias related to selective reporting as low, high or unclear.
Low – all planned outcomes are reported.
High – not all planned outcomes are reported and no reasons given.
Unclear – insufficient information on planned outcomes available.
-
Other sources of bias – assesses other potential sources of bias not captured by the domains above such as funding of the trial and conflicts of interest of the authors or investigators. We assessed other sources of bias as low, high or unclear.
Low – studies appear to be free of other sources of bias.
High – studies funded by a manufacturer or studies authored by one or more employees of a manufacturer unless there is an explicit and sufficient description of the independence of the funding source and employees in the analysis and reporting of the study results, or potential source of bias related to the specific study design used.
Unclear – insufficient information to assess whether an important risk of bias exists (e.g. conflicts of interest not reported, or authors have previously received funding from relevant pharmaceutical companies which are not directly involved in funding of included studies)
Appendix 5. Unused methods
Unit of analysis issues
The unit of analysis is typically the individual participant. In situations where this is not the case, for example, repeated observations on participants or in cluster‐randomised trials, we planned to undertake the appropriate analysis (see below) that takes these variations into account (Higgins 2011c).
Cluster‐randomised trials
In cluster‐randomised trials, groups rather than individuals are randomised, which requires an adjustment to be made to account for the clustering effect. If trials used cluster randomisation, we would have expected cluster effects to have been appropriately controlled for (robust standard errors or hierarchical linear models). If it was unclear whether appropriate controls for clustering were applied, we planned to contact the investigators for further details. If appropriate controlling was not used, we planned to request and reanalyse individual participant data using appropriate multilevel models. Following this, we planned to analyse effect sizes and standard errors in Review Manager 5 (RevMan 5) (Review Manager 2014) using the generic inverse method (Deeks 2017). If there was insufficient information to control for clustering, we planned to enter outcome data using individuals as the units of analysis, and then conduct a sensitivity analysis to assess the impact of inadequately controlled cluster‐randomised trials on the effect estimate.
The method for adjustment for clustering suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c) is based on reducing the size of effect of each clustered trial to its 'effective sample size', which is the original sample size of the cluster‐randomised trial divided by the 'design effect' (i.e. 1 + (M – 1) *ICC, where M is the average cluster size and ICC is the intracluster correlation coefficient) (Higgins 2011c). We planned to estimate the ICC based upon similar studies (in similar populations, but including other treatments). This would have enabled us to pool non‐clustered and clustered trials to obtain an overall estimate of effect.
Trials with repeated measurements
In trials with repeated measurements for the same patient (for example, measurements at different time points), we planned to attempt to reduce the impact of multiple analysis by analysing the most frequently reported or the most clinically relevant time points (usually the longest duration of follow‐up, which is likely to be the best indication of a clinically sustainable effect), or both (Higgins 2011c).
Trials with multiple treatment arms
We planned to attempt to combine arms to create a single pair‐wise comparison where appropriate (for example, slow‐ or controlled‐release and immediate‐release methylphenidate formulations). If this was not possible, we planned to use all treatment groups but split the comparison (placebo) group evenly across the intervention groups (Higgins 2011c). Criteria for assessing the relevance of the treatment arms for each comparison would have included clinical relevance (is the expected clinical effect likely to be different or not?) and clinical availability or use of the treatment in question (for example, transdermal formulations of methylphenidate are not commonly used. Therefore, if a trial has two active treatment arms with one of them a transdermal patch, we might have chosen to consider only the oral formulation).
Dealing with missing data
We planned to perform an intention‐to‐treat (ITT) analysis to account for missing data. The ITT analysis considers all missing participant data of all randomised patients as a treatment failure. We planned to compare ITT analysis results with the results of 'on‐treatment' or 'complete case analysis' (all participants completing treatment) or per protocol (all participants following protocol or at least one dose of the allocated treatment) results to assess the impact of missing data on the overall estimate of effect.
Assessment of reporting bias
We planned to draw a funnel plot if there were 10 or more studies included in the review. We would have visually examined the graph for asymmetry and, if present, assessed whether the association between estimated intervention effects and study size was greater than what could have been attributed to chance, using tests described in the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2017). We planned to use the 'trim and fill' method, which entails first 'trimming' (removing) the smaller studies causing funnel plot asymmetry, then using the trimmed funnel plot to estimate the true 'centre' of the funnel, and subsequently replacing the omitted studies and their missing 'counterparts' around the centre (filling). As well as providing an estimate of the number of missing studies, we planned to calculate an adjusted intervention effect by performing a meta‐analysis, which would have included the filled studies (Sterne 2017).
Subgroup analysis
We planned to conduct a subgroup analysis based on participant ages (6 to 12 years, and 13 to 18 years), but no studies included participants over the age of 14 years. The one study that included 13‐year‐old participants did not report any individual participant data, so we were unable to extract these results.
We planned to conduct a subgroup analysis based on immediate‐release versus extended‐release formulation, but only study used the extended‐release form (Pearson 2013).
Sensitivity analysis
We planned to perform a sensitivity analysis to assess the impact of risk of bias on the overall result by adding or removing studies with a high risk of bias to the meta‐analysis. We classified studies as being at high risk of bias if one or more of the following items were assessed at high risk: random number generation, allocation concealment, blinding of participants and personnel, and/or blinding outcome assessment.
We planned to explore the impact of heterogeneity on the overall pooled effect estimate by adding or removing studies that were contributing to the heterogeneity. By visually examining the forest plot, studies that are outliers and are potential sources for heterogeneity can be identified. We planned to remove the outliers one by one and assess the impact on the overall outcome.
We planned to perform a sensitivity analysis to explore the impact of missing data on the overall outcome by comparing the analyses with available outcome data with those following the ITT principle (see Dealing with missing data).
Appendix 6. Individual adverse effects
Gastrointestinal events
Abdominal discomfort
Two studies, Handen 2000 and Pearson 2013, included abdominal discomfort in their adverse effects checklist rated by teachers. However, abdominal discomfort was reported for only one child in the treatment group in Pearson 2013, which corresponds to a non‐significant RR of 3.00 (95% CI 0.13 to 70.16). We were not able to pool the results of both studies because abdominal discomfort was not reported in Handen 2000 (Analysis 1.4).
Two studies, Pearson 2013 and RUPP 2005, included abdominal discomfort in their adverse effects checklist rated by parents. The pooled difference in rates between treatment and placebo was not statistically significant (RR 4.30, 95% CI 0.91 to 20.34; 74 participants; Analysis 5.3). There was no clear heterogeneity given the I2 of 0%.
Reduced appetite
Two studies, Handen 2000 and Pearson 2013, both included reduced appetite in their adverse effects checklist rated by teachers. The pooled difference between treatment and placebo was not statistically significant (RR 1.33, 95% CI 0.43 to 4.12; 34 participants; Analysis 1.4).
Only one study, Pearson 2013, reported on weight and found no significant difference in average weight at any dose of methylphenidate. There was no clear heterogeneity given the I2 of 19%. We applied a random‐effects model and this did not change the results.
Two studies, Pearson 2013 and RUPP 2005, both included reduced appetite in their adverse effects checklist rated by parents. The pooled difference between treatment and placebo was statistically significant (RR 8.28, 95% CI 2.57 to 26.73; 74 participants; Analysis 5.3); the risk for reduced appetite was almost eight times higher in the treatment group compared to placebo. This RR is equivalent to a risk difference (RD) of 0.24 (95% CI 0.13 to 0.35), with a number needed to treat for one additional harmful outcome of 4.17 (95% CI 7.69 to 2.86). An I2 of 0% did not indicate heterogeneity.
Other
Other recorded gastrointestinal effects included diarrhoea (RUPP 2005), dry mouth and nausea (Pearson 2013). The difference between treatment and placebo was not statistically significant for any of these symptoms.
General physical adverse events
Dizziness
Two studies, Handen 2000 and Pearson 2013, included symptoms of dizziness in their adverse effects checklist rated by teachers. However, we were not able to pool the results of both studies because dizziness was not reported in the Pearson 2013 study (Analysis 1.4).
Dizziness was not included in any of the studies' adverse effects checklists rated by parents.
Drowsiness
Two studies, Handen 2000 and Pearson 2013, included symptoms of drowsiness in their adverse effects checklist rated by the teachers. The pooled difference between treatment and placebo was not statistically significant (RR 2.00, 95% CI 0.47 to 8.55; 34 participants; Analysis 1.4). An I2 of 10% did not indicate any clear heterogeneity.
Drowsiness was not included in the adverse effects checklists rated by parents in any of the studies.
Headache
Two studies, Handen 2000 and Pearson 2013, included headache in their adverse effects checklists rated by teachers. However, were not able to pool the results of both studies because headache was not reported in the Handen 2000 study (Analysis 1.4).
Two studies, Pearson 2013 and RUPP 2005, included headache in their adverse effects checklists rated by parents. The pooled difference between treatment and placebo was not statistically significant (RR 1.87, 95% CI 0.10 to 33.86; 74 participants; Analysis 5.3). An I2 of 58% indicated moderate heterogeneity. There is no evident clinical explanation for this heterogeneity, although Pearson 2013 used an extended‐release preparation and is likely to have studied less unwell children who may have been more able to communicate the presence of headache.
Sleep disturbance
Sleep disturbance was not included in the adverse effects checklists rated by teachers in any of the studies.
Two studies, Pearson 2013 and RUPP 2005, included sleep disturbance in their adverse effects checklists rated by parents. The pooled difference between treatment and placebo was not statistically significant (RR 3.51, 95% CI 0.59 to 20.82; 74 participants Analysis 5.3). An I2 of 62% indicated moderate heterogeneity. There is no evident clinical explanation for this heterogeneity, although Pearson 2013 used an extended‐release preparation in the morning (and an immediate‐release preparation in the afternoon).
Increased activity
Hyperactivity was one of our primary outcomes and was rated using psychometric scales in all four studies. Results for this outcome are reported above (see Analysis 1.2 for teachers and Analysis 5.2 for parents).
Two studies, Handen 2000 and RUPP 2005, also included increased activity in their adverse effects checklists rated by teachers. Neither reported a significant difference between treatment and placebo. We were unable to pool this data because the effect was rated by teachers in Handen 2000 and by parents in RUPP 2005.
Other
Two studies recorded other general physical effects related to fever and skin rash (Pearson 2013), and fatigue (RUPP 2005). The difference between treatment and placebo was not statistically significant for any of these effects.
Two studies reported blood pressure and pulse (Pearson 2013; RUPP 2005). RUPP 2005 did not report any results and Pearson 2013 reported no significant differences at any dose of methylphenidate as measured by clinicians. Pearson 2013 also included racing heart in their adverse effects checklist rated by parents, and reported no significant difference, while RUPP 2005 included bradycardia in their adverse effects checklist, and reported no significant difference between treatment and placebo (RUPP 2005).
Psychological effects
Anxiety
Two studies, Handen 2000 and Pearson 2013, included anxiety in their adverse effects checklist rated by teachers. The pooled difference between treatment and placebo was not statistically significant (RR 1.02, 95% CI 0.47 to 2.18; 34 participants; Analysis 1.4). There was no clear heterogeneity given the I2 of 0%. We applied a random‐effects model and this did not change the results.
Two studies, Pearson 2013 and RUPP 2005, included anxiety in their adverse effects checklist rated by parents. The pooled difference between treatment and placebo was not statistically significant (RR 1.13, 95% CI 0.22 to 5.79; 74 participants; Analysis 5.3). An I2 of 50% indicated moderate heterogeneity. There is no evident clinical explanation for this heterogeneity, although Pearson 2013 used an extended‐release preparation and is likely to have studied less unwell children who may have been more able to communicate adverse psychological effects.
Depressed mood
Two studies, Handen 2000 and Pearson 2013, included depressed mood in their adverse effects checklists rated by teachers. The pooled difference between treatment and placebo was not statistically significant (RR 1.19, 95% CI 0.37 to 3.79; 34 participants; Analysis 1.4). An I2 of 55% indicated moderate heterogeneity. Pearson 2013 used an extended‐release preparation and is likely to have studied less unwell children who may have been more able to communicate adverse psychological effects.
Two studies, Pearson 2013 and RUPP 2005, included symptoms of depressed mood in their adverse effects checklist rated by parents. The pooled difference between treatment and placebo was not statistically significant (RR 1.75, 95% CI 0.05 to 62.33; 74 participants; Analysis 5.3). An I2 of 74% indicated considerable heterogeneity. Pearson 2013 used an extended‐release preparation and is likely to have studied less unwell children who may have been more able to communicate adverse psychological effects.
Irritability
Two studies, Handen 2000 and Pearson 2013, recorded symptoms of irritability rated by teachers. The pooled difference between treatment and placebo was not statistically significant (RR 0.81, 95% CI 0.29 to 2.27; 34 participants; Analysis 1.4). An I2 of 46% indicated moderate heterogeneity. Pearson 2013 used an extended‐release preparation and is likely to have studied less unwell children who may have been more able to communicate adverse psychological, cognitive and/or affective states.
Two studies, Pearson 2013 and RUPP 2005, both included symptoms of irritability in their adverse effects checklists rated by parents. The pooled difference between treatment and placebo was not statistically significant (RR 1.25, 95% CI 0.25 to 6.36; 74 participants; Analysis 5.3). An I2 of 71% indicated considerable heterogeneity. Pearson 2013 used an extended‐release preparation and is likely to have studied less unwell children who may have been more able to communicate adverse psychological, cognitive and/or affective states.
Social withdrawal
Social withdrawal is one aspect of impaired social interaction and it was one of our primary outcomes. Two studies, Handen 2000 and RUPP 2005, included social withdrawal in their adverse effects checklists. Neither reported a significant difference between methylphenidate and placebo. We were unable to pool this data because the effect was rated by teachers in Handen 2000 and by parents in RUPP 2005.
Impaired social interaction was rated using psychometric scales in three studies (Handen 2000; Pearson 2013; RUPP 2005). Combined results for this outcome are reported above (see Analysis 1.3 for teachers and Analysis 5.2 for parents).
Other psychological effects
Two studies recorded other psychological effects including emotional outbursts and self‐injury in RUPP 2005 and euphoria in Pearson 2013. The difference between treatment and placebo was not statistically significant for self‐injury or euphoria, but RUPP 2005 reported a significant increase in emotional outbursts at medium‐dose methylphenidate.
Repetitive behaviours
We included repetitive, restrictive and stereotypical behaviours as a primary outcome in our review. Four studies assessed this outcome; three by teachers (Handen 2000; Quintana 1995; RUPP 2005), and one by parents (Pearson 2013), using psychometric scales.
Repetitive behaviours, movements and/or tics were also listed on the adverse events checklist used by parents in three studies (Handen 2000; Pearson 2013; RUPP 2005), as reported below.
General repetitive behaviours
Repetitive behaviours were not recorded for teachers.
Two studies, Pearson 2013 and RUPP 2005, included repetitive behaviours in the adverse events checklist rated by parents. The pooled difference between treatment and placebo was not statistically significant (RR 0.87, 95% CI 0.43 to 1.75; 74 participants; Analysis 5.3). An I2 of 8% indicated no clear heterogeneity.
Repetitive movements or tics
Two studies, Handen 2000 and Pearson 2013, included repetitive movements and tics in their adverse events checklists rated by teachers. The pooled difference between treatment and placebo was not statistically significant (RR 0.57, 95% CI 0.21 to 1.57; 34 participants; Analysis 1.4). One study, Quintana 1995, also measured abnormal involuntary movements rated by the paediatrician on a psychometric scale and reported no significant difference between treatment and placebo. An I2 of 0% indicated no heterogeneity.
Repetitive movements were not recorded for parents.
Other repetitive behaviours
One study, Pearson 2013, reported other repetitive behaviours included in their adverse events checklist, namely hair or skin pulling, unusual blinking, and repetitive language. The difference between treatment and placebo was not statistically significant for any of these effects.
Other adverse events
One study, Pearson 2013, also included staring in the adverse events checklist. The difference between treatment and placebo was not statistically significant.
Data and analyses
Comparison 1. TEACHER rated: high dose versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: ADHD symptoms ‐ inattention (same measurement instrument) | 2 | Mean Difference (Random, 95% CI) | ‐2.72 [‐5.37, ‐0.06] | |
| 2 Primary outcome: ADHD symptoms ‐ hyperactivity (different measurement instrument) | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.78 [‐1.13, ‐0.43] | |
| 3 Primary outcome: ASD symptoms | 4 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 Impaired social interaction | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.51 [‐1.07, 0.05] | |
| 3.2 Stereotypical behaviours | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.34 [‐0.84, 0.17] | |
| 3.3 Overall ASD | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.53 [‐1.26, 0.19] | |
| 4 Secondary outcome: adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Gastrointestinal effects: abdominal discomfort | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 70.16] |
| 4.2 Gastrointestinal effects: reduced appetite | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.43, 4.12] |
| 4.3 General physical adverse effect: dizziness | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.06, 5.18] |
| 4.4 General physical adverse effect: drowsiness | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.47, 8.55] |
| 4.5 General physical adverse effect: headache | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 70.16] |
| 4.6 Psychological effects: anxiety | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.47, 2.18] |
| 4.7 Psychological effects: depressed mood | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.37, 3.79] |
| 4.8 Psychological effects: irritability | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.29, 2.27] |
| 4.9 Repetitive behaviours: repetitive movements or tics | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.21, 1.57] |
Comparison 2. TEACHER rated ‐ sensitivity: correlation 0.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: ADHD symptoms ‐ inattention (same measurement instrument) | 2 | Mean Difference (Random, 95% CI) | ‐2.55 [‐5.15, 0.06] | |
| 2 Primary outcome: ADHD symptoms ‐ hyperactivity (different measurement instrument) | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.70 [‐1.07, ‐0.33] | |
| 3 Primary outcome: ASD symptoms | 4 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 Impaired social interaction | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.44 [‐0.99, 0.11] | |
| 3.2 Stereotypical behaviours | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.24 [‐0.71, 0.23] | |
| 3.3 Overall ASD | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.56 [‐1.28, 0.17] |
Comparison 3. TEACHER rated ‐ sensitivity: correlation 0.8.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: ADHD symptoms ‐ inattention (same measurement instrument) | 2 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 2 Primary outcome: ADHD symptoms ‐ hyperactivity (different measurement instrument) | 4 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 3 Primary outcome: ASD symptoms | 4 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 Impaired social interaction | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.53 [‐1.09, 0.02] | |
| 3.2 Stereotypical behaviours | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.87, 0.14] | |
| 3.3 Overall ASD | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.53 [‐1.25, 0.20] |
Comparison 4. TEACHER rated ‐ sensitivity: different scales.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: ADHD ‐ hyperactivity | 4 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 ABC | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.75 [‐1.21, ‐0.29] | |
| 1.2 SNAP | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.65 [‐1.12, ‐0.19] | |
| 1.3 Conners' Rating Scale ‐ Revised | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.87 [‐1.26, ‐0.48] |
Comparison 5. PARENT rated: high dose versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: ADHD symptoms (same measurement instrument) | 2 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 Inattention | 2 | Mean Difference (Random, 95% CI) | ‐3.16 [‐6.89, 0.57] | |
| 1.2 Hyperactivity | 2 | Mean Difference (Random, 95% CI) | ‐6.61 [‐12.19, ‐1.03] | |
| 2 Primary outcome: ASD symptoms ‐ impaired social interaction | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.21 [‐0.60, 0.18] | |
| 3 Secondary outcome: adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Gastrointestinal effects: abdominal discomfort | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 4.30 [0.91, 20.34] |
| 3.2 Gastrointestinal effects: reduced appetite | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 8.28 [2.57, 26.73] |
| 3.3 General physical adverse effect: headache | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.10, 33.86] |
| 3.4 General physical effect: sleep disturbance | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 3.51 [0.59, 20.82] |
| 3.5 Psychological effects: anxiety | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.22, 5.79] |
| 3.6 Repetitive behaviours: general | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.43, 1.75] |
| 3.7 Psychological effects: irritability | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.25, 6.36] |
| 3.8 Psychological effects: depressed mood | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.05, 62.33] |
Comparison 6. PARENT rated ‐ sensitivity: correlation 0.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: ADHD symptoms (same measurement instrument) | 2 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 Inattention | 2 | Mean Difference (Random, 95% CI) | ‐3.11 [‐6.84, 0.62] | |
| 1.2 Hyperactivity | 2 | Mean Difference (Random, 95% CI) | ‐6.44 [‐10.00, ‐0.89] | |
| 2 Primary outcome: ASD symptoms ‐ Impaired social interaction | 2 | Std. Mean Difference (Random, 95% CI) | Subtotals only |
Comparison 7. PARENT rated ‐ sensitivity: correlation 0.8.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: ADHD symptoms (same measurement instrument) | 2 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 Inattention | 2 | Mean Difference (Random, 95% CI) | ‐3.18 [‐6.91, 0.56] | |
| 1.2 Hyperactivity | 2 | Mean Difference (Random, 95% CI) | ‐6.67 [‐12.25, ‐1.08] | |
| 2 Primary outcome: ASD symptoms ‐ Impaired social interaction | 2 | Std. Mean Difference (Random, 95% CI) | Subtotals only |
Comparison 8. PARENT rated ‐ sensitivity: different scales.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: ADHD ‐ hyperactivity | 2 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 ABC | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.60 [‐1.04, ‐0.16] | |
| 1.2 SNAP | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.53 [‐1.04, ‐0.03] |
Comparison 9. TEACHER rated ‐ subgroup: doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: ADHD ‐ inattention | 2 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 Low dose | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.28 [‐0.61, 0.05] | |
| 1.2 Medium dose | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.46 [‐0.89, ‐0.04] | |
| 1.3 High dose | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.38 [‐0.75, ‐0.02] | |
| 2 Primary outcome: ADHD ‐ hyperactivity | 4 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 2.1 Low dose | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.40 [‐0.77, ‐0.03] | |
| 2.2 Medium dose | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.55 [‐1.00, ‐0.10] | |
| 2.3 High dose | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.78 [‐1.13, ‐0.43] | |
| 3 Primary outcome: ASD ‐ impaired social interaction | 3 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 Low dose | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.30 [‐0.59, ‐0.02] | |
| 3.2 Medium dose | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.44 [‐0.94, 0.06] | |
| 3.3 High dose | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.51 [‐1.07, 0.05] | |
| 4 Primary outcome: ASD ‐ stereotypical behaviours | 3 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 4.1 Medium dose | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.43, ‐0.03] | |
| 4.2 High dose | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.34 [‐0.84, 0.17] | |
| 5 Primary outcome: ASD ‐ overall ASD | 2 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 5.1 Medium dose | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.52 [‐1.20, 0.17] | |
| 5.2 High dose | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.54 [‐1.26, 0.19] | |
| 6 Secondary outcome: adverse events ‐ abdominal discomfort | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Medium dose | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 High dose | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 70.16] |
| 7 Secondary outcome: adverse events ‐ reduced appetite | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Medium dose | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.75, 2.20] |
| 7.2 High dose | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.43, 4.12] |
| 8 Secondary outcome: adverse events ‐ dizziness | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Medium dose | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.01, 4.06] |
| 8.2 High dose | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.06, 5.18] |
| 9 Secondary outcome: adverse events ‐ drowsiness | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Medium dose | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.05, 32.89] |
| 9.2 High dose | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.47, 8.55] |
| 10 Secondary outcome: adverse events ‐ headache | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Medium dose | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 High dose | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 70.16] |
| 11 Secondary outcome: adverse events ‐ anxiety | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Medium dose | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.10, 4.46] |
| 11.2 High dose | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.47, 2.18] |
| 12 Secondary outcome: adverse events ‐ depressed mood | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Medium dose | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.46, 2.26] |
| 12.2 High dose | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.37, 3.79] |
| 13 Secondary outcome: adverse events ‐ irritability | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Medium dose | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.54, 1.70] |
| 13.2 High dose | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.29, 2.27] |
| 14 Secondary outcome: adverse events ‐ repetitive movements | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Medium dose | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.30, 1.85] |
| 14.2 High dose | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.21, 1.57] |
Comparison 10. PARENT rated ‐ subgroup: doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: ADHD ‐ inattention | 2 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 Low dose | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.27 [‐0.58, 0.03] | |
| 1.2 Medium dose | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.49 [‐0.85, ‐0.13] | |
| 1.3 High dose | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.51 [‐1.15, 0.14] | |
| 2 Primary outcome: ADHD ‐ hyperactivity | 2 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 2.1 Low dose | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.35 [‐0.55, ‐0.14] | |
| 2.2 Medium dose | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.67 [‐1.01, ‐0.33] | |
| 2.3 High dose | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.60 [‐1.04, ‐0.16] | |
| 3 Primary outcome: ASD ‐ impaired social interaction | 2 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 Low dose | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.15 [‐0.33, 0.04] | |
| 3.2 Medium dose | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.17 [‐0.37, 0.03] | |
| 3.3 High dose | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.21 [‐0.60, 0.18] | |
| 4 Secondary outcome: adverse events ‐ abdominal discomfort | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Low dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.29, 10.34] |
| 4.2 Medium dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 4.51 [0.98, 20.67] |
| 4.3 High dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 4.30 [0.91, 20.34] |
| 5 Secondary outcome: adverse events ‐ reduced appetite | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Low dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 3.41 [0.91, 12.78] |
| 5.2 Medium dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 10.00 [3.14, 31.82] |
| 5.3 High dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 8.28 [2.57, 26.73] |
| 6 Secondary outcome: adverse events ‐ headache | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Low dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.31, 9.94] |
| 6.2 Medium dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 2.29 [0.55, 9.58] |
| 6.3 High dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.10, 33.86] |
| 7 Secondary outcome: adverse events ‐ anxiety | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Low dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.45, 3.52] |
| 7.2 Medium dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.46, 3.58] |
| 7.3 High dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.22, 5.79] |
| 8 Secondary outcome: adverse events ‐ depressed mood | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Low dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.22, 4.42] |
| 8.2 Medium dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [0.39, 13.42] |
| 8.3 High dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.05, 62.33] |
| 9 Secondary outcome: adverse events ‐ irritability | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Low dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.30, 5.83] |
| 9.2 Medium dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.23, 13.47] |
| 9.3 High dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.25, 6.36] |
| 10 Secondary outcome: adverse events ‐ repetitive behaviours | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Low dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.54, 1.66] |
| 10.2 Medium dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.55, 2.62] |
| 10.3 High dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.43, 1.75] |
| 11 Secondary outcome: adverse events ‐ sleep disturbance | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Low dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 2.94 [0.44, 19.64] |
| 11.2 Medium dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 5.10 [0.71, 36.68] |
| 11.3 High dose | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 3.51 [0.59, 20.82] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Handen 2000.
| Methods |
Design: double‐blind, randomised, placebo‐controlled, cross‐over design Duration of study: 3 weeks |
|
| Participants |
Location: USA Setting: recruitment from special education, psychiatric inpatient or psychiatric day treatment programmes Study start date: not specified (prior to 2000) Study end date: not specified Number recruited: not specified Number randomised: 13 children (10 boys, 3 girls) Number completed: 12 Number of dropouts/withdrawals: 1 Mean age: 7.4 years (SD 6.5; range 5.6 to 11.2) Ethnicity: African American (n = 7), white (n = 4), Latino (n = 2) ASD Diagnosis: autistic disorder (n = 9), PDD‐NOS (n = 4) ADHD diagnosis: a score of 15 points or more on the Hyperactivity Index of the Teacher Conners Rating Scale Other diagnosis: oppositional defiant disorder, tuberous sclerosis, mosaic Down's syndrome Cognitive function: ranged from severe/profound disability to average intelligence Stimulant use history: uncertain, likely to have included a "mix of kids with prior experience with stimulants" Handen 2016 (personal communication) and some with no prior experience Concurrent medication: none Inclusion criteria
Exclusion criteria: not specified |
|
| Interventions |
Intervention: 0.3 mg/kg and 0.6 mg/kg doses of MPH Comparison: placebo Administration: each MPH dose and the placebo dose was given 2 to 3 times a day for 7 consecutive days. Doses were given with breakfast and 4 hours later with lunch. 11 participants took a third MPH dose around 4:00 pm. The lower MPH dose always preceded the higher dose. This resulted in three possible drug orders:
|
|
| Outcomes |
Primary outcome measures (teacher rated)
Primary outcome measures (parent rated): parents rated similar questionnaires but data were incomplete and not reported Administration of outcome assessment: outcome measures were completed by the classroom teacher or programme staff at the end of the week for each MPH condition. Information is not available about the administration of questionnaires to parents. |
|
| Aim of study | To determine the efficacy and safety issues of MPH use among children with autism and symptoms of ADHD | |
| Notes |
Comment on design: the cross‐over trial design was appropriate for the clinical context, given that ASD is a relatively stable, chronic condition. No period or carry‐over effects would be anticipated for methylphenidate, even in the absence of a washout period, as the elimination half‐life for both the immediate‐ and extended‐release forms is 2 to 3 hours, and the average duration of action of (immediate‐release) methylphenidate is approximately 4 hours (Novartis 2014). Data collection was also focused at the end of each week of intervention, further reducing the risk of any carry‐over effect. Other comments: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Drug order was randomly assigned. However the lower MPH dose always preceded the higher dose." Comment: method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: all participants and parents were reported to be blinded to doses and/or placebo treatments. It is not reported whether participants had ever taken MPH previously and hence could have recognised the medication. The corresponding author indicated that some participants may have taken MPH previously. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "Both parents and teachers were unaware of the fact that the lower MPH dose would precede the higher dose". Comment: all respondents (parents and teachers) were blinded to doses, placebo treatments or both. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 2 participants had 0.6 mg/kg dose discontinued after only 1 day. A 3rd participant was missing CARS data for placebo condition. For both of these conditions missing data were imputed using a maximum likelihood techniques as outlined in 'Inference and missing data'. A 4th participant was unable to complete the protocol for either MPH dose due to the presence of significant adverse side effects. Data (except for side effects data) for this participant were not included for analysis. |
| Selective reporting (reporting bias) | High risk | Quote: "Only questionnaires from teachers were used" Comment: parent questionnaires were not reported because collection was incomplete (due to many participants residing in inpatient clinics or residential halls). In some cases only subsets of completed scales appear to be reported. |
| Other bias | Unclear risk | Comment: no information available on conflict of interest. The study was supported by a Research Foundation grant. |
Pearson 2013.
| Methods |
Design: double‐blind, placebo‐controlled, cross‐over trial Duration of study: 4 weeks |
|
| Participants |
Location: USA Setting: recruitment from special education classrooms, all children were living at home Study start date: not specified (prior to 2013) Study end date: not specified Number recruited: not specified Number randomised: 24 children (19 boys and 5 girls) Number completed: 24 Number of dropouts/withdrawals: 0 Mean age: 8.8 years (SD 1.7; range 7.1 to 12.7) years Ethnicity: white (n = 13), Hispanic (n = 5), African‐American (n = 4), Asian (n = 1), and multiple races (n = 1) ASD diagnosis: autistic disorder (n = 19), Asperger's disorder (n = 3), PDD‐NOS (n = 2) ADHD diagnosis: combined type (n = 19), predominantly inattentive type (n = 5). Mean Conners' Parent Rating Scale ‐ Revised ADHD Index T score = 76.1 (SD 6.7) and mean Conners' Teacher Rating Scale ‐ Revised ADHD Index T score = 67.2 (SD 8.7) Other diagnosis: oppositional defiant disorder (n = 5), obsessive compulsive disorder (n = 2), separation anxiety (n = 1) Cognitive function: mean full scale IQ 85.0 (SD 16.8) Stimulant‐use history: 13 children had previously taken stimulant medication. This was discontinued 1 week or more (mean = 63 days, range: 7–547 days) prior to entering the trial. Concurrent medication: 7 children continued long‐term medications (at a constant dose) during the trial: risperidone (n = 3), aripiprazole (n = 1), sertraline (n = 1), bupropion (n = 1), and trazodone (n = 1) Inclusion criteria:
Exclusion criteria
|
|
| Interventions |
Intervention: 1 week low‐dose MPH (0.21 mg/kg ER‐MPH morning and 0.14 mg/kg IR‐MPH afternoon), 1 week medium‐dose MPH (0.35 mg/kg ER‐MPH morning and 0.24 mg/kg IR‐MPH afternoon), and 1 week high‐dose MPH (0.48mg/kg ER‐MPH morning and 0.27 mg/kg IR‐MPH afternoon). No child received a dose greater than the equivalent of an IR‐MPH dose of 0.6 mg/kg, and no child's total daily dose exceeded the equivalent of an IR‐MPH twice‐daily dose of 50 mg Comparison: placebo Administration: 1 week of 2 days each of low, medium, and high MPH doses in ascending order to assess tolerability. All 24 children tolerated all 3 doses. 1 week each of the 4 MPH dosing regimens |
|
| Outcomes |
Primary outcome measure: Conners' Teacher Rating Scale ‐ Revised ‐ Short Form (CTRS‐R‐SF; 28 items) Secondary outcome measures
Administration of outcome assessment: Weekly at the end of each week of intervention/placebo |
|
| Aim of study |
Quote: "The purpose of this study was to examine the behavioral effects of four doses of psychostimulant medication, combining extended‐release methylphenidate (MPH) in the morning with immediate‐release MPH in the afternoon." Quote: "Our goals were to determine if: 1) ER‐MPH was associated with improvements in parent and teacher behavioral ratings, and 2) the MPH dose‐response curve was linear (i.e. higher MPH doses were associated with consistent improvements in behavioral functioning), or curvilinear (an initial behavioral improvement with MPH, followed by behavioral declines at higher doses)." |
|
| Notes |
Comment on design: the cross‐over trial design was appropriate for the clinical context, given that ASD is a relatively stable, chronic condition. No period or carry‐over effects would be anticipated for methylphenidate, even in the absence of a washout period, as the elimination half‐life for both the immediate‐ and extended‐release forms is 2‐3 hours (Novartis 2014). The average duration of action of (immediate‐release) methylphenidate is approximately 4 hours, and the extended release form used in Pearson has a duration of action of approximately 8 hours (Novartis 2014). Data collection was also focused at the end of each week of intervention, further reducing the risk of any carry‐over effect. Other comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the order of dosage administration was "counterbalanced across children" using diagram‐balanced Latin squares. This is not a randomising procedure and is more commonly used in larger studies. |
| Allocation concealment (selection bias) | Unclear risk | Comment: it is not clear how dosing sequences were allocated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Quote: "All study personnel with patient contact were blind with respect to dosages given during the drug trial". Comment: it is not stated whether parents were blinded. However, 13/24 participants had previously taken MPH so may have identified whether or not they were taking the active medication based on previous experience. The study physician and the study psychologist were also unblinded during the test‐dose week. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: 13/24 participants had previously taken MPH so parents and teachers are likely to have identified whether or not the children were taking the active medication based on previous experience. 2 blinded clinicians completed the Clinician Global Impression measures, after achieving reliability on training vignettes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all 24 participants completed the trial but 5/24 children discontinued the afternoon IR‐MPH dose because of behavior concerns in late afternoon/evening. All 5 of these children experienced irritability, 2 experienced decreased sleep, and 2 showed increased stereotypical behaviours. Parent ratings were available for all 24 children, whereas teacher ratings were only available for 18 children (6 were assessed in summer when school was in recess). Outcomes are reported for all participants, with the above exceptions. |
| Selective reporting (reporting bias) | Low risk | Comment: comprehensive reporting of outcomes |
| Other bias | Unclear risk | Comment: The authors report having received financial support from a number of pharmaceutical companies (including manufacturers of pharmaceuticals for behavioural syndromes in children). The study was funded by grant MH072263 from the National Institute of Mental Health (NIMH). |
Quintana 1995.
| Methods |
Design: double‐blind, cross‐over study Duration of study: 6 weeks |
|
| Participants |
Location: New York, USA Setting: recruitment from psychiatric outpatient clinic Study start date: not specified, prior to 1995 Study end date: not specified Number recruited: not reported, and the attempt made to contact the corresponding author to clarify this was not successful Number randomised: 10 children (6 boys; 4 girls) Number completed: 10 Number of dropouts/withdrawals: 0 Mean age: 8.5 years (SD 1.3; range 7 to 11) Ethnicity: not specified ASD diagnosis: Baseline Childhood Autism Rating Scale scores between 30.0 and 59.5 ADHD diagnosis: not specified Other diagnosis: not specified, "wide range of baseline behaviours" reported Cognitive function: 7 children met criteria for mild intellectual impairment Stimulant‐use history: nil Concurrent medication: nil (all participants had previously been prescribed neuroleptic medication but this was ceased prior to study) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: MPH treatment appeared to consist of one week of MPH 10 mg twice daily followed by a second week of MPH 20 mg twice daily, although it may have consisted of either 2 weeks of MPH 10 mg twice daily, or two weeks of MPH 20 mg twice daily. Comparison: placebo Administration: cross‐over MPH versus placebo study completed in 6 weeks, with 2 weeks medication‐free baseline, 2 weeks placebo or MPH followed by cross‐over |
|
| Outcomes |
Primary and secondary outcomes were not specified Clinician instruments
Parent Instruments: Conners Abbreviated Parent Questionnaire (CAPQ; 10‐item) Administration of outcome assessment: participants were rated by clinicians in a 3‐hour simulated classroom situation and during free play at the day hospital, at the end of each week. Parent questionnaires were completed weekly based on at‐home behaviour for the week prior to the day hospital assessment |
|
| Aim of study | To evaluate "MPH efficacy and side effects in the treatment of children with autistic disorder" | |
| Notes |
Comment on design: the cross‐over trial design was appropriate for the clinical context, given that ASD is a relatively stable, chronic condition. No period or carry‐over effects would be anticipated for methylphenidate, even in the absence of a washout period, as the elimination half‐life for both the immediate‐ and extended‐release forms is 2‐3 hours and the average duration of action of (immediate‐release) methylphenidate is approximately 4 hours (Novartis 2014). Data collection was also focused at the end of each week of intervention, further reducing the risk of any carry‐over effect. Other comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described but reported as "randomly assigned" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: nursing staff administering morning dose on day of observation may not have been blinded to drug and drug dose, although the "investigators, the children and the parents were blind to drug and drug dose". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Clinicians, children and parents were reported to be "blind to drug and drug dose". None of the children had been on MPH before entry into the study, which minimises the risk of recognition of the effects of MPH. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: unclear if all participants completed study, but table 2 reports n = 10 in the title of the table. |
| Selective reporting (reporting bias) | High risk | Comment: Outcomes for all mentioned instruments are reported in table 2 and in the text except the CARS. This is an autism scale that rates the severity of symptoms based on observation. It was listed as an outcome, and it is unclear why this was omitted in the reporting of the results. |
| Other bias | Unclear risk | Comment: no reporting of conflicts of interest or financial support |
RUPP 2005.
| Methods |
Design: randomised, double‐blind, placebo‐controlled, cross‐over trial, including a 1‐week test‐dose phase to check the tolerability of MPH at each dose; a 4‐week randomised‐order, placebo‐controlled, double‐blind cross‐over phase; and an 8‐week, open‐label continuation phase for responders, at best dose identified in cross‐over phase. Only the results from the 4‐week randomised cross‐over phase are included in our analysis. Duration of study: 4 weeks |
|
| Participants |
Location: USA Setting: 5 centers forming the Research Units on Pediatric Psychopharmacology Autism Network Study start date: 14 November 2001 Study end date: 5 September 2003 Number recruited: 72 (6 of these participants had intolerable adverse events with more than 1 methylphenidate dosage level during the test‐dose phase, and they exited the study prior to randomisation as per protocol) Number randomised: 66 (59 boys, 7 girls) Number completed: 58 Number of dropouts/withdrawals: 8. 1 participant who was randomised withdrew prior to cross‐over phase and 7 children withdrew during cross‐over phase due to intolerable adverse events. Mean age: 7.5 years (SD 2.2; range 5.0 to 13.7 years) Ethnicity: white (n = 48), African‐American (n = 9), Asian (n = 6), Latino (n = 3) ASD diagnosis: autistic disorder, Asperger's disorder, or PDD‐NOS‐ based on DSM‐IV. ADHD diagnosis: based on SNAP‐IV and Clinician Global Impression‐Severity Other diagnosis: not specified Cognitive function: Slosson IQ, mean 62.6 (SD 32.9), range 16‐135 Stimulant‐use history: excluded if adequate trial of MPH within past 2 years Concurrent medication: nil (ceased prior to baseline visit) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Test‐dose phase
Participants were excluded from the cross‐over study if they experienced a severe adverse event, or were rated 'much worse' or 'very much worse' on the CGI, at the low or medium dose. Participants were randomised to a modified cross‐over schedule (omitting high dose) if the adverse event or clinical worsening occurred only on the high dose (15 participants) Study phase Intervention: 3 different doses of MPH (low, medium, high) Comparison: placebo Administration: 4‐week cross‐over phase. Each participant received 1 week placebo and 1 week each of 3 different doses of MPH in random order (except high dose never followed placebo). 16 participants received the modified cross‐over schedule (medium dose administered twice*, no high dose). |
|
| Outcomes |
Primary outcome: teacher‐rated hyperactivity subscale (16 items) of Aberrant Behavior Checklist (ABC) (RUPP 2005) Secondary outcomes
Administration of outcome assessment: ratings were performed at the end of each week of treatment |
|
| Aim of study | To determine the efficacy and safety of MPH in children with PDD and hyperactivity | |
| Notes |
Comment on design: the cross‐over trial design was appropriate for the clinical context, given that ASD is a relatively stable, chronic condition. No period or carry‐over effects would be anticipated for methylphenidate, even in the absence of a washout period, as the elimination half‐life for both the immediate‐ and extended‐release forms is 2‐3 hours, and the average duration of action of (immediate‐release) methylphenidate is approximately 4 hours (Novartis 2014). Data collection was also focused at the end of each week of intervention, further reducing the risk of any carry‐over effect. Other comments: RUPP 2005 only reports means and SDs for teacher and parent rated hyperactivity subscale of ABC; very partial reporting of secondary outcomes (other ABC subscales), with a few effect sizes and P values only. The 2007 article reports means and SDs of a secondary analysis. The 2009 article reports mean and SD for social communication measures in their subset secondary analysis (only measured at some sites of the multicentre trial). *Data from both medium dose weeks were combined. **The Parent and Teacher SNAP‐IV and Clinician CYBOCC‐PDD outcomes were only mentioned and reported in the 2007 secondary analysis article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation was balanced by site to avoid repeating the treatment order within the site. Randomisation lists were generated centrally and were held by an investigational pharmacist at each site. Authors do not describe the exact method of generating the list. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on the role of the pharmacist. Clinicians, the patient, and the caregiver were blind to treatment assignment during cross‐over phase, but not during test‐dose week preceding the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: clinicians, the patient, and the caregiver were blind to treatment assignment during cross‐over phase, but not during test‐dose week preceding the study. No information on the success of blinding is reported. Participants had not had an adequate trial of MPH in past 2 years (exclusion criteria), but they had been exposed to the test dose. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: clinicians, the patient, and the caregiver were blind to treatment assignment during cross‐over phase, but not during test‐dose week preceding the study. No information on the success of blinding is reported. Participants had not had an adequate trial of MPH in past 2 years (exclusion criteria), but they had been exposed to the test dose. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Study dropouts and reasons for drop out are reported. Data for social communication outcomes are incomplete (only 33 out of total of 66 randomised children completed the trial for this outcome). |
| Selective reporting (reporting bias) | High risk | Comment: the main study publication (RUPP 2005) reports all mentioned outcome measures. The overall response outcome was only reported as a combined number, without results for the individual CGI‐I component of this composite outcome measure. The RUPP 2005 study also reports adverse events, but this is not mentioned as an outcome in the methods section of the paper. 2 additional publications (in 2007 and 2009) subsequently reported additional outcomes that were not mentioned in the original publication. Three secondary outcome measures were only mentioned and reported in the 2007 secondary analysis article. 1 outcome measure (social communication) was only mentioned and reported in the 2009 secondary analysis article on a subset of the original patient population. |
| Other bias | Unclear risk | Comment: study supported by funding from National Institutes of Mental Health (NIMH) and universities, USA. Several authors report affiliations with a number of pharmaceutical companies. |
ADHD: attention deficit hyperactivity disorder; ADI‐R: Autism Diagnostic Interview ‐ Revised; ASD: autism spectrum disorders; CARS: Childhood Autism Rating Scale; CGI: Clinical Global Impressions scale; CGI‐I: Clinical Global Impressions ‐ Improvement scale;DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition, Revised;DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition;ER: extended‐release; IQ: intelligence quotient; IR: immediate release; MPH: methylphenidate; PDD: pervasive developmental disorder; PDD‐NOS: pervasive developmental disorder ‐ not otherwise specified; RUPP: Research Units on Pediatric Psychopharmcology Autism Network; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akyol 2015 | Not a RCT |
| Aman 1991 | Not a RCT |
| Aman 1997 | Participants did not meet criteria for ASD |
| Armstrong 2008 | Not a RCT |
| Barnard‐Brak 2016 | Not a RCT |
| Birmaher 1988 | Not a RCT |
| Croteau 2013 | Not a RCT |
| Di Martino 2004 | Not a RCT |
| Epstein 2011 | Participants did not meet criteria for ASD |
| Faraone 2001 | Participants did not meet criteria for ASD |
| Flapper 2008 | Not a RCT |
| Ghuman 2009 | Participants did not meet age criterion for inclusion (too young) |
| Gurbuz 2016 | Not a RCT |
| Mayes 1994 | Not a RCT |
| Scahill 2007 | Not a RCT |
| Shea 2006 | Not an original study |
| Simonoff 2013 | Participants did not meet criteria for ASD |
| Sinzig 2014 | Not a RCT |
| Steele 2006 | Participants did not meet criteria for ASD |
| Von Morgenstern 2014 | Participants did not meet criteria for ASD |
| Çetín 2015 | Participants did not meet criteria for ASD |
ASD: autism spectrum disorder; RCT: randomised controlled trial.
Differences between protocol and review
Authorship: authorship has changed since publication of the Cochrane Protocol: Nancy Sturman assumed the role of lead author; Laura Deckx was added to the team; and Toni Redman, Elly Scheermeyer, Makoto Ogawa, Eddie Sparks, Jeremy Taylor and Vi Tran withdrew from the team (Redman 2014).
Title: the title was changed to 'Methylphenidate for children and adolescents with autistic spectrum disorder', to fit with the character length restriction for proper display on the Cochrane website.
Introduction: the introduction was updated with recent and additional references.
Objectives: we replaced 'concentration, attentiveness and attention' with 'inattention' for consistency with our outcomes. We replaced 'social interaction' with 'impaired social interaction' for consistency with our outcomes. We removed the secondary outcome of 'disturbance of home life' because we were unable to identify relevant measures.
Search methods: in order to ensure our searches were as up‐to date as possible, we searched one additional database (MEDLINE EPub ahead of print), which became available to us after publication of the protocol. We report two ERIC strategies because our ERIC access changed from Proquest to EBSCOhost.
Types of studies: the published protocol did not consider the inclusion of cross‐over trials (Redman 2014), but we included these in the review because they are double‐blind, randomised controlled trials and their single‐case experimental design can be considered analogous to a parallel‐group design, with the additional feature of increasing the power of these studies.
-
Measures of treatment effect
We added information about combining different outcome scales where appropriate. We included two additional tables indicating which scales were used in our meta‐analysis (see Table 3; Table 4).
We reported that we used a generic inverse variance method to analyse the data from cross‐over studies. We presented all continuous data in terms of standardised mean differences (SMD).
We combined teacher, trained staff and clinician ratings and reported these as 'teacher' ratings. We reported teacher and parent ratings separately.
We reported effect sizes separately for low, medium and high dose and performed a subgroup analysis of the effect of the different dose ranges. The protocol did not specify dose ranges for low, medium and high doses (Redman 2014). We determined that low‐dose methylphenidate included doses between 0.11 mg/kg/dose and 0.21 mg/kg/dose; medium‐dose methylphenidate included doses between 0.22 mg/kg/dose and 0.36 mg/kg/dose; and high‐dose methylphenidate included doses between 0.43 mg/kg/dose and 0.6 mg/kg/dose. This determination was based on the dose cut‐offs in our included studies.
We were unable to calculate the risk difference (RD) and the number needed to treat (NNT) as planned had we found a significant effect and the trials were sufficiently homogenous, because all studies assessed improvement on the primary outcomes on a continuous scale. See Redman 2014. We were able to report the number needed to harm in the case of significant adverse events.
-
Unit of analysis issues
We added information about cross‐over studies (as our included studies were cross‐over studies), which was not specified in the protocol (Redman 2014).
We deleted the information about cluster‐randomised trials, trials with repeated measurements and trials with multiple treatment arms as we included no such studies in this review. Appendix 5 provides details of these methods planned for use in future updates of this review.
Dealing with missing data: we were unable to perform an intention‐to‐treat analysis due to inadequate data. Appendix 5 provides details of our planned intention‐to‐treat analysis.
Data synthesis: we added the following paragraph: "Measures of effect size using SMDs are difficult to interpret in terms of whether they represent a clinically important between‐treatment difference, or a clinically meaningful effect. In this review we used an SMD of 0.52 as a between‐treatment minimum clinically important difference (MCID), based on the Zhang 2005 finding of a MCID of 6.6 on the Attention Deficit Hyperactivity Disorder Rating Scale ‐ Parent Interview (ADHDRS‐PI), which was equivalent to an SMD of 0.52. Storebø 2015 also used this SMD of 0.52 as a clinically meaningful effect size. This aligns with the rule of thumb that an effect size of 0.20 to 0.49 represents a small effect; 0.50 to 0.79, a moderate effect; and 0.80 or above, a large effect, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2017)."
Summary of findings table: We included a section on 'Summary of findings table' to the Methods section, which describes how we implemented GRADE in the review, at the request of the editorial base.
-
Subgroup analysis
We were unable to perform subgroup analyses based on participants' ages (ages 6 to 12 years, and 13 to 18 years), as no included studies included participants over the age of 14 years. The one study that included 13‐year‐old participants did not report any individual participant data, so we were unable to extract these results.
We were unable to perform a subgroup analysis based on immediate‐release versus extended‐release formulation, as the only study that used an extended‐release form, Pearson 2013, used both extended‐release methylphenidate (for the morning dose) and immediate‐release methylphenidate (for the afternoon dose, if administered). As investigators asked parents to focus only on their child's morning behaviour for their ratings, and teachers only saw the children on the extended‐release dose, we considered this study to have a single treatment arm (extended‐release methylphenidate).
-
Sensitivity analysis
We performed two sensitivity analyses to assess the effect of the correlation coefficient: one assuming no correlation (correlation coefficient of zero) and one assuming a higher correlation (correlation coefficient of 0.80).
We were unable to perform a sensitivity analysis to assess the impact of risk of bias on the overall result by adding or removing studies with a high risk of bias to/from the meta‐analysis, as we assessed none of our included studies as being at high risk on the assessment items (random number generation, allocation concealment, blinding of participants and personnel, or outcome assessor). See Appendix 5 and Redman 2014.
We were unable to explore the impact of heterogeneity on the overall pooled effect estimate by adding or removing studies that contributed to the heterogeneity, because of the limited number of studies included in this review. See Appendix 5 and Redman 2014.
We were unable to perform a sensitivity analysis to explore the impact of missing data on the overall outcome by comparing the analyses with available outcome data with those following the ITT principle. See Appendix 5 and Redman 2014.
We included an additional sensitivity analysis, testing the influence of the different scales on the same outcome. For example, if a study used more than one scale to measure the same outcome, we repeated the meta‐analyses for the different scales in order to assess if this changed the interpretation of our results. We used the SMD in order to compare the results across the different scales.
Included studies: although our inclusion criteria prespecified children aged 6 to 18 years, we included two studies with children younger than 6 years of age: Handen 2000, which included 13 children aged between 5.6 and 11.2 years (including two children aged 5.6 years, and one child aged 5.9 years) and RUPP 2005, which included 66 children aged between 5.0 and 13.7 years (with a mean age of 7.5 years (SD 2.2 years) and an unspecified number of 5‐year‐old children). We were unable to exclude the results of the five‐year‐old participants because individual participant results were not reported in either study. We included these studies because all other participants were in our target age range. This issue was not foreseen at the protocol stage of our review (Redman 2014), where we intended to target school‐aged children. For future reviews, we suggest including five‐year‐old participant data where this individual data cannot be excluded, and the majority of participants are within the target age range.
Effects of interventions: as no data were available, it was not possible to conduct an analysis of the secondary outcomes of caregiver well‐being; need for institutionalisation, special schooling options or therapy to achieve learning outcomes; or overall quality of life.
Contributions of authors
Nancy Sturman is acting as guarantor for the review. She led the review, contributed to all stages of the process and drafted the review.
Laura Deckx led the data collection and analysis, and reviewed and commented on the draft review.
Mieke L van Driel supervised and coached the team throughout the development and writing of the protocol, advised on the methodology, contributed to the screening and selection of citations identified by the search, led the 'Risk of bias' assessment, and reviewed and commented on the draft review.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Nancy Sturman ‐ none known. Laura Deckx ‐ none known. Mieke L van Driel ‐ none known.
New
References
References to studies included in this review
Handen 2000 {published data only}
- Handen BL. Cochrane Review: Methylphenidate for core and ADHD‐like symptoms in children aged 6 to 18 years with autism spectrum disorders (ASDs) [personal communication]. Email to: N Sturman 12 May 2016.
- Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention‐deficit hyperactivity disorder. Journal of Autism and Developmental Disorders 2000;30(3):245‐55. [PUBMED: 11055460] [DOI] [PubMed] [Google Scholar]
Pearson 2013 {published data only}
- Pearson DA. Cochrane Review: Methylphenidate for core and ADHD‐like symptoms in children aged 6 to 18 years with autism spectrum disorders (ASDs) [personal communication]. Email to: N Sturman 17 May 2016.
- Pearson DA, Santos CW, Aman MG, Arnold LE, Casat CD, Mansour R, et al. Effects of extended release methylphenidate treatment on ratings of attention‐deficit/hyperactivity disorder (ADHD) and associated behavior in children with autism spectrum disorders and ADHD symptoms. Journal of Child and Adolescent Psychopharmacology 2013;23(5):337‐51. [DOI: 10.1089/cap.2012.0096; PMC3689935; PUBMED: 23782128] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quintana 1995 {published data only}
- Quintana H, Birmaher B, Stedge D, Lennon S, Freed J, Bridge J, et al. Use of methylphenidate in the treatment of children with autistic disorder. Journal of Autism and Developmental Disorders 1995;25(3):283‐94. [PUBMED: 7559293] [DOI] [PubMed] [Google Scholar]
RUPP 2005 {published data only}
- Jahromi LB, Kasari CL, McCracken JT, Lee LS, Aman MG, McDougle CJ, et al. Positive effects of methylphenidate on social communication and self‐regulation in children with pervasive developmental disorders and hyperactivity. Journal of Autism and Developmental Disorders 2009;39(3):395‐404. [DOI: 10.1007/s10803-008-0636-9; PMC4374624; PUBMED: 18752063] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey DJ. Cochrane Review: Methylphenidate for core and ADHD‐like symptoms in children aged 6 to 18 years with autism spectrum disorders (ASDs) [personal communication]. Email to: N Sturman 16 May 2015.
- Posey DJ, Aman MG, McCracken JT, Scahill L, Tierney E, Arnold LE, et al. Methylphenidate in pervasive developmental disorders: an analysis of secondary measures. Neuropsychopharmacology 2006;31(Suppl 1):S134. [DOI: 10.1038/sj.npp.1301266] [DOI] [Google Scholar]
- Posey DJ, Aman MG, McCracken JT, Scahill L, Tierney E, Arnold LE, et al. Positive effects of methylphenidate on inattention and hyperactivity in pervasive developmental disorders: an analysis of secondary measures. Biological Psychiatry 2007;61(4):538‐44. [DOI: 10.1016/j.biopsych.2006.09.028; PUBMED: 17276750] [DOI] [PubMed] [Google Scholar]
- Posey DJ, McDougle CJ, Aman MG, Arnold LE, Scahill L, McCracken JT, et al. A randomized, double‐blind, placebo‐controlled, crossover trial of methylphenidate in children with hyperactivity associated with pervasive developmental disorders. Neuropsychopharmacology 2004;29(Suppl S1):S142‐3. [DOI: 10.1038/sj.npp.1300647] [DOI] [Google Scholar]
- Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Archives of General Psychiatry 2005;62(11):1266‐74. [DOI: 10.1001/archpsyc.62.11.1266; PUBMED: 16275814] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akyol 2015 {published data only}
- Ercan E, Akyol Ardic U, Yuce D, Ercan E, Aygunes D, Kosova B. Altered response with methylphenidate to adhd‐like symptoms in pervasive developmental disorder: does CES‐1 enzyme gene polymorphism have a role?. European Child & Adolescent Psychiatry 2015;24(Suppl 1):S233‐4. [DOI: 10.1007/s00787-015-0714-4] [DOI] [PubMed] [Google Scholar]
Aman 1991 {published data only}
- Aman MG, Turbott SH. Prediction of clinical response in children taking methylphenidate. Journal of Autism and Developmental Disorders 1991;21(2):211‐28. [PUBMED: 1864828] [DOI] [PubMed] [Google Scholar]
Aman 1997 {published data only}
- Aman MG, Kern RA, Osborne P, Tumuluru R, Rojahn J, Medico V. Fenfluramine and methylphenidate in children with mental retardation and borderline IQ: clinical effects. American Journal of Mental Retardation 1997;101(5):521‐34. [PUBMED: 9083608] [PubMed] [Google Scholar]
Armstrong 2008 {published data only}
- Armstrong C. Practice guidelines: AAP releases guidelines on management of autism spectrum disorders. American Family Physician 2008;78(12):1399‐404. [Google Scholar]
Barnard‐Brak 2016 {published data only}
- Barnard‐Brak L, Davis TN, Schmidt M, Richman DM. Effects associated with on‐ and off‐label stimulant treatment of core autism and ADHD symptoms exhibited by children with autism spectrum disorder. Developmental Neurorehabilitation 2016; Vol. 19, issue 1:46‐53. [DOI: 10.3109/17518423.2014.904949; PUBMED: 24739141] [DOI] [PubMed]
Birmaher 1988 {published data only}
- Birmaher B, Quintana H, Greenhill LL. Methylphenidate treatment of hyperactive autistic children. Journal of the American Academy of Child and Adolescent Psychiatry 1988;27(2):248‐51. [PUBMED: 3360732] [DOI] [PubMed] [Google Scholar]
- Brugha TS, McManus S, Bankart J, Scott F, Purdon S, Smith J, et al. Epidemiology of autism spectrum disorders in adults in the community in England. Archives of General Psychiatry 2011;68(5):459‐65. [DOI: 10.1001/archgenpsychiatry.2011.38; PUBMED: 21536975] [DOI] [PubMed] [Google Scholar]
Çetín 2015 {published data only}
- Çetín FH, Taş Torun Y, Işik Taner Y. Atomoxetine versus OROS methylphenidate in attention deficit hyperactivity disorder: a six‐month follow up study for efficacy and adverse effects. Turkiye Klinikleri Journal of Medical Sciences 2015;35(2):88‐96. [DOI: 10.5336/medsci.2015-43336] [DOI] [Google Scholar]
Croteau 2013 {published data only}
- Croteau C, Dorais M, Tarride JE, Mottron L, Perreault S. PMH60 ‐ Psychoactive drug use, polypharmacy and co‐morbidities in newly diagnosed patients with pervasive development disorder in the province of Quebec. Value in Health 2013;16(7):A552. [DOI: 10.1016/j.jval.2013.08.1429] [DOI] [Google Scholar]
Di Martino 2004 {published data only}
- Martino A, Melis G, Cianchetti C, Zuddas A. Methylphenidate for pervasive developmental disorders: safety and efficacy of acute single dose test and ongoing therapy: an open‐pilot study. Journal of Child and Adolescent Psychopharmacology 2004;14(2):207‐18. [PUBMED: 15319018] [DOI] [PubMed] [Google Scholar]
Epstein 2011 {published data only}
- Epstein JN, Brinkman WB, Froehlich T, Langberg JM, Narad ME, Antonini TN, et al. Effects of stimulant medication, incentives, and event rate on reaction time variability in children with ADHD. Neuropsychopharmacology 2011;36(5):1060‐72. [DOI: 10.1038/npp.2010.243; PMC3059336; PUBMED: 21248722] [DOI] [PMC free article] [PubMed] [Google Scholar]
Faraone 2001 {published data only}
- Faraone SV, Pliszka SR, Olvera RL, Skolnik R, Biederman J. Efficacy of Adderall and methylphenidate in attention deficit hyperactivity disorder: a reanalysis using drug‐placebo and drug‐drug response curve methodology. Journal of Child and Adolescent Psychopharmacology 2001;11(2):171‐80. [DOI: 10.1089/104454601750284081; PUBMED: 11436957] [DOI] [PubMed] [Google Scholar]
Flapper 2008 {published data only}
- Flapper BC, Schoemaker MM. Effects of methylphenidate on quality of life in children with both developmental coordination disorder and ADHD. Developmental Medicine and Child Neurology 2008;50(4):294‐9. [DOI: 10.1111/j.1469-8749.2008.02039.x; PUBMED: 18352997] [DOI] [PubMed] [Google Scholar]
Ghuman 2009 {published data only}
- Ghuman JK, Aman MG, Lecavalier L, Riddle MA, Gelenberg A, Wright R, et al. Randomized, placebo‐controlled, crossover study of methylphenidate for attention‐deficit/hyperactivity disorder symptoms in preschoolers with developmental disorders. Journal of Child and Adolescent Psychopharmacology 2009;19(4):329‐39. [DOI: 10.1089/cap.2008.0137; PMC2861958; PUBMED: 19702485] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurbuz 2016 {published data only}
- Gurbuz F, Gurbuz BB, Celik GG, Yildirim V, Ucakturk SA, Seydaoglu G, et al. Effects of methylphenidate on appetite and growth in children diagnosed with attention deficit and hyperactivity disorder. Journal of Pediatric Endocrinology & Metabolism 2016; Vol. 29, issue 1:85‐92. [DOI: 10.1515/jpem-2015-0171; PUBMED: 26352086] [DOI] [PubMed]
Mayes 1994 {published data only}
- Mayes SD, Crites DL, Bixler EO, Humphrey FJ 2nd, Mattison RE. Methylphenidate and ADHD: influence of age, IQ and neurodevelopmental status. Developmental Medicine and Child Neurology 1994;36(12):1099‐107. [PUBMED: 7525394] [DOI] [PubMed] [Google Scholar]
Scahill 2007 {published data only}
- Scahill L, Pachler M. Treatment of hyperactivity in children with pervasive developmental disorders. Journal of Child and Adolescent Psychiatric Nursing 2007;20(1):59‐62. [DOI: 10.1111/j.1744-6171.2007.00080.x] [DOI] [PubMed] [Google Scholar]
Shea 2006 {published data only}
- Shea SE. Methylphenidate hydrochloride reduces hyperactivity in children with pervasive development disorders. Evidence‐Based Mental Health 2006;9(2):45. [DOI: 10.1136/ebmh.9.2.45] [DOI] [PubMed] [Google Scholar]
Simonoff 2013 {published data only}
- Simonoff E, Taylor E, Baird G, Bernard S, Chadwick O, Liang H, et al. Randomized controlled double‐blind trial of optimal dose methylphenidate in children and adolescents with severe attention deficit hyperactivity disorder and intellectual disability. Journal of Child Psychology and Psychiatry, and Allied Disciplines 2013;54(5):527‐35. [DOI: 10.1111/j.1469-7610.2012.02569.x; PUBMED: 22676856] [DOI] [PubMed] [Google Scholar]
Sinzig 2014 {published data only}
- Sinzig J, Vinzelberg I, Evers D, Lehmkuhl G. Executive function and attention profiles in preschool and elementary school children with autism spectrum disorders or ADHD. International Journal of Developmental Disabilities 2014; Vol. 60, issue 3:144‐54. [DOI: 10.1179/2047387714y.0000000040] [DOI]
Steele 2006 {published data only}
- Steele M, Weiss M, Swanson J, Wang J, Prinzo RS, Binder CE. A randomized, controlled effectiveness trial of OROS‐methylphenidate compared to usual care with immediate‐release methylphenidate in attention deficit‐hyperactivity disorder. Canadian Journal of Clinical Pharmacology 2006;13(1):e50‐62. [PUBMED: 16456216] [PubMed] [Google Scholar]
Von Morgenstern 2014 {published data only}
Additional references
AABASD 2007
- Australian Advisory Board on Autism Spectrum Disorders. The prevalence of autism in Australia: can it be established from existing data?. www.autismadvisoryboard.org.au/uploads/Autism%20Prevalence%20Study%20FINAL%20Feb%2007.pdf (accessed 12 November 2013).
AAP 2001
- Committee on Children with Disabilities. American Academy of Pediatrics: the pediatrician's role in the diagnosis and management of autistic spectrum disorder in children. Pediatrics 2001;107(5):1221‐6. [PUBMED: 11331713] [DOI] [PubMed] [Google Scholar]
Abidin 1983
- Abidin RR. Parenting Stress Index: Manual, Administration Booklet, [and] Research Update. Charlottesville (VA): Paediatric Psychology Press, 1983. [Google Scholar]
Aman 1982
- Aman MG. Stimulant drug effects in developmental disorders and hyperactivity‐‐toward a resolution of disparate findings. Journal of Autism and Developmental Disorders 1982;12(4):385‐98. [PUBMED: 6131061] [DOI] [PubMed] [Google Scholar]
Aman 1995
- Aman MG, Bourgondien ME, Wolford PL, Sarphare G. Psychotropic and anticonvulsant drugs in subjects with autism: prevalence and patterns of use. Journal of the American Academy of Child and Adolescent Psychiatry 1995;34(12):1672‐81. [PUBMED: 10.1097/00004583‐199512000‐00018; PUBMED: 8543539] [DOI] [PubMed] [Google Scholar]
Aman 2003
- Aman MG, Buican B, Arnold LE. Methylphenidate treatment in children with borderline IQ and mental retardation: analysis of three aggregated studies. Journal of Child and Adolescent Psychopharmacology 2003;13(1):29‐40. [DOI: 10.1089/104454603321666171; PUBMED: 12804124] [DOI] [PubMed] [Google Scholar]
Bertoglio 2009
- Bertoglio K, Hendren RL. New developments in autism. The Psychiatric Clinics of North America 2009;32(1):1‐14. [DOI: 10.1016/j.psc.2008.10.004; PUBMED: 19248913] [DOI] [PubMed] [Google Scholar]
Brugha 2011
- Brugha TS, McManus S, Bankart J, Scott F, Purdon S, Smith J, et al. Epidemiology of autism spectrum disorders in adults in the community in England. Archives of General Psychiatry 2011;68(5):459‐65. [DOI: 10.1001/archgenpsychiatry.2011.38; PUBMED: 21536975] [DOI] [PubMed] [Google Scholar]
Burack 1997
- Burack JA, Ennis JT, Johannes EA. Attention and autism: behavioral and electrophysiological evidence. In: Cohen DJ, Volkmar FR editor(s). Handbook of Autism and Pervasive Developmental Disorder. 2nd Edition. New York (NY): John Wiley & Sons, 1997:226‐47. [Google Scholar]
Busardò 2016
- Busardò FP, Kyriakou C, Cipolloni L, Zaami S, Frati P. From clinical application to cognitive enhancement: the example of methylphenidate. Current Neuropharmacology 2016;14(1):17–27. [PMC4787280; PUBMED: 26813119] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 1975
- Campbell M. Pharmacotherapy in early infantile autism. Biological Psychiatry 1975;10(4):399‐423. [PUBMED: 240449] [PubMed] [Google Scholar]
Cheuk 2011
- Cheuk DK, Wong V, Chen WX. Acupuncture for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD007849.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ching 2012
- Ching H, Pringsheim T. Aripiprazole for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD009043.pub2] [DOI] [PubMed] [Google Scholar]
Conzelmann 2011
- Conzelmann A, Woidich E, Mucha RF, Weyers P, Jacob CP, Lesch KP, et al. Methylphenidate normalizes emotional processing in adult patients with attention‐deficit/hyperactivity disorder: preliminary findings. Brain Research 2011;1381:159‐66. [DOI: 10.1016/j.brainres.2010.12.085; PUBMED: 21215727] [DOI] [PubMed] [Google Scholar]
Cortese 2012
- Cortese S, Castelnau P, Morcillo C, Roux S, Bonnet‐Brilhault F. Psychostimulants for ADHD‐like symptoms in individuals with autism spectrum disorders. Expert Review of Neurotherapeutics 2012;12(4):461‐73. [DOI: 10.1586/ern.12.23; PUBMED: 22449217] [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 9: Analysing data and undertaking metaanalyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Demirci 2016
- Demirci E, Erdogan A. Is emotion recognition the only problem in ADHD? Effects of pharmacotherapy on face and emotion recognition in children with ADHD. Attention Deficit and Hyperactivity Disorders 2016;8(4):197‐204. [DOI: 10.1007/s12402-016-0201-x; PUBMED: 27473346] [DOI] [PubMed] [Google Scholar]
DSM III
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM‐III. 3rd Edition. Washington (DC): American Psychiatric Associate, 1980. [Google Scholar]
DSM‐5
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM‐5. 5th Edition. Washington (DC): American Psychiatric Association, 2013. [Google Scholar]
DSM‐IV
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM‐IV. 4th Edition. Washington (DC): American Psychiatric Association, 1994. [Google Scholar]
DSM‐IV‐TR
- American Psychiatric Association. Diagnostic and Stastical Manual of Mental Disorders: DSM‐IV‐TR. 4th edition, Text Revision. Washington (DC): American Psychiatric Association, 2000. [Google Scholar]
Egger 2001
- Egger M, Davey Smith G, Altman DG, editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London (UK): BMJ Publishing Group, 2001. [Google Scholar]
Faries 2001
- Faries DE, Yalcin I, Harder D, Heiligenstein JH. Validation of the ADHD Rating Scale as a clinician administered and scored instrument. Journal of Attention Disorders 2001;5(2):107‐15. [DOI: 10.1177/108705470100500204] [DOI] [Google Scholar]
Filipek 1999
- Filipek PA, Accardo PJ, Baranek GJ, Cook EH Jr, Dawson G, Gordon B, et al. The screening and diagnosis of autistic spectrum disorders. Journal of Autism and Developmental Disorders 1999;29(6):439‐84. [PUBMED: 10638459] [DOI] [PubMed] [Google Scholar]
Filipek 2000
- Filipek PA, Accardo PJ, Ashwal S, Baranek GT, Cook EH Jr, Dawson G, et al. Practice parameter: screening and diagnosis of autism: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology 2000;55(4):468‐79. [PUBMED: 10953176] [DOI] [PubMed] [Google Scholar]
Fletcher‐Watson 2014
- Fletcher‐Watson S, McConnell F, Manola E, McConachie H. Interventions based on the Theory of Mind cognitive model for autism spectrum disorder (ASD). Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD008785.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frazier 2001
- Frazier JA, Biederman J, Bellordre CA, Garfield SB, Geller DA, Coffey BJ, et al. Should the diagnosis of attention‐deficit/ hyperactivity disorder be considered in children with pervasive developmental disorder?. Journal of Attention Disorders 2001;4(4):203‐11. [DOI: 10.1177/108705470100400402] [DOI] [Google Scholar]
Frazier 2011
- Frazier TW, Shattuck PT, Narendorf SC, Cooper BP, Wagner M, Spitznagel EL. Prevalence and correlates of psychotropic medication use in adolescents with an autism spectrum disorder with and without caregiver‐reported attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2011;21(6):571‐9. [DOI: 10.1089/cap.2011.0057; PMC3279713; PUBMED: 22166171] [DOI] [PMC free article] [PubMed] [Google Scholar]
Geretsegger 2014
- Geretsegger M, Elefant C, Mössler KA, Gold C. Music therapy for people with autism spectrum disorder. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD004381.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004; Vol. 328, issue 7454:1490. [DOI: 10.1136/bmj.328.7454.1490; PMC428525; PUBMED: 15205295] [DOI] [PMC free article] [PubMed]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 17 July 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Hara 2015
- Hara Y, Ago Y, Taruta A, Katashiba K, Haebe S, Takano E, et al. Improvement by methylphenidate and atomoxetine of social interaction deficits and recognition memory impairment in a mouse model of valproic acid‐induced autism. Autism Research 2016;9(9):926‐39. [DOI: 10.1002/aur.1596] [DOI] [PubMed] [Google Scholar]
Hartman 2007
- Hartman CA, Rhee SH, Willcutt EG, Pennington BF. Modeling rater disagreement for ADHD: are parents or teachers biased?. Journal of Abnormal Child Psychology 2007;35(4):536‐42. [DOI: 10.1007/s10802-007-9110-y; PUBMED: 17333362] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, editor(s). Chapter 7: Selecting Studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hurwitz 2012
- Hurwitz R, Blackmore R, Hazell P, Williams K, Woolfenden S. Tricyclic antidepressants for autism spectrum disorders (ASD) in children and adolescents. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD008372.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hyman 2013
- Hyman SL. AAP news: new DSM‐5 includes changes to autism criteria. aapnews.aappublications.org/content/early/2013/06/04/aapnews.20130604‐1 (accessed 24 May 2014).
James 2011
- James S, Montgomery P, Williams K. Omega‐3 fatty acids supplementation for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD007992.pub2] [DOI] [PubMed] [Google Scholar]
James 2015
- James S, Stevenson SW, Silove N, Williams K. Chelation for autism spectrum disorder (ASD). Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD010766.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2017
- Jensen JS, Bielefeldt AØ, Hróbjartsson A. Active placebo control groups of pharmacological interventions were rarely used but merited serious consideration: a methodological overview. Journal of Clinical Epidemiology 2017 July [Epub ahead of print]. [DOI: 10.1016/j.jclinepi.2017.03.001; PUBMED: 28342907] [DOI] [PubMed]
Jesner 2007
- Jesner OS, Aref‐Adib M, Coren E. Risperidone for autism spectrum disorder. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005040.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanner 1943
- Kanner L. Autistic disturbances of affective contact. Nervous Child 1943;2:217‐50. [PubMed] [Google Scholar]
Kollins 2010
- Kollins SH, Sparrow EP. Rating scales for the assessment of ADHD. In: Conners CK editor(s). Guide to Assessment Scales in Attention‐deficit/Hyperactivity Disorder. 2nd Edition. London (UK): Springer Healthcare Ltd, 2010:6‐40. [DOI: 10.1007/978-1-907673-42-9_2] [DOI] [Google Scholar]
Lecavalier 2006
- Lecavalier L. Behavioral and emotional problems in young people with pervasive developmental disorders: relative prevalence, effects of subject characteristics, and empirical classification. Journal of Autism and Developmental Disorders 2006;36(8):1101‐14. [DOI: 10.1007/s10803-006-0147-5; PUBMED: 16897387] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6(7):e1000100. [DOI: 10.1371/journal.pmed.1000100; 19621070; PMC2707010] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lord 2000
- Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule‐generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders 2000;30(3):205‐23. [PUBMED: 11055457] [PubMed] [Google Scholar]
Lord 2014
- Lord C, Corsello C, Grzadzinski R. Diagnostic instruments in autistic spectrum disorders. In: Volkmar FR, Rogers SJ, Paul R, Pelphrey KA editor(s). Handbook of Autism and Pervasive Developmental Disorders. 4th Edition. Vol. 2: assessment, interventions and policy, Hoboken (NJ): John Wiley & Sons, Inc., 2014:609‐60. [DOI: 10.1002/9781118911389.hautc25] [DOI] [Google Scholar]
Mayo Clinic 2014
- Mayo Clinic. Methylphenidate (oral route). www.mayoclinic.com/health/drug‐information/DR601847/DSECTION=proper‐use (accessed 5 April 2014).
Medsafe 2010
- New Zealand Medicines and Medical Devices Safety Authority. Methylphenidate ‐ updated guidance when treating children. www.medsafe.govt.nz/profs/PUArticles/Methylphenidate.htm (accessed 24 May 2014).
NICE 2013
- National Institute for Health and Care Excellence. Autism in under 19s: support and management. www.nice.org.uk/Guidance/CG170 (accessed 1 April 2014).
NICHD 2014
- Eunice Kennedy Shriver National Institute of Child Health and Human Development. Autism spectrum disorder (ASD): overview. www.nichd.nih.gov/health/topics/autism/Pages/default.aspx (accessed 5 April 2014).
Nicolson 2000
- Nicolson R, Castellanos FX. Commentary: considerations on the pharmacotherapy of attention deficits and hyperactivity in children with autism and other pervasive developmental disorders. Journal of Autism and Developmental Disorders 2000;30(5):461‐2. [PUBMED: 11098884] [DOI] [PubMed] [Google Scholar]
Novartis 2014
- Novartis Pharmaceuticals Canada Inc. Product Monograph Ritalin, Ritalin SR. www.novartis.ca/en/our‐products/pharmaceuticals#ui‐id‐1=14 (accessed 15 August 2016).
Patin 2015
- Patin A, Hurlemann R. Social cognition. In: Kantak MN, Wettstein JG editor(s). Handbook of Experimental Pharmacology: Cognitive Enhancement. 2015. Vol. 228, London: Springer, 2015:271‐303. [DOI: 10.1007/978-3-319-16522-6_10; PUBMED: 25977087] [DOI] [PubMed] [Google Scholar]
Polanczyk 2007
- Polanczyk G, Rohde LA. Epidemiology of attention‐deficit/hyperactivity disorder across the lifespan. Current Opinion in Psychiatry 2007;20(4):386–92. [DOI: 10.1097/YCO.0b013e3281568d7a; PUBMED: 17551354] [DOI] [PubMed] [Google Scholar]
Reichow 2012a
- Reichow B, Barton EE, Boyd BA, Hume K. Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD009260.pub2] [DOI] [PubMed] [Google Scholar]
Reichow 2012b
- Reichow B, Steiner AM, Volkmar F. Social skills groups for people aged 6 to 21 with autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD008511.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riddle 2013
- Riddle MA, Yershova K, Lazzaretto D, Paykina N, Yenokyan G, Greenhill L, et al. The Preschool Attention‐Deficit/Hyperactivity Disorder Treatment Study (PATS) 6‐year follow‐up. Journal of the American Academy of Child and Adolescent Psychiatry 2013;52(3):264‐78. [DOI: 10.1016/j.jaac.2012.12.007; PMC3660093; PUBMED: 23452683] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossi 2010
- Rossi S, Hurley E, Sutcliffe A, Abott F, Cardinal T, Curtis J, et al. Australian Medicines Handbook. 11th Edition. Adelaide (SA): Australian Medicines Handbook Pty Ltd, 2010. [Google Scholar]
Santosh 2006
- Santosh PJ, Baird G, Pityaratstian N, Tavare E, Gringras P. Impact of comorbid autism spectrum disorders on stimulant response in children with attention deficit hyperactivity disorder: a retrospective and prospective effectiveness study. Child: Care, Health and Development 2006;32(5):575‐83. [DOI: 10.1111/j.1365-2214.2006.00631.x; PUBMED: 16919137] [DOI] [PubMed] [Google Scholar]
Scahill 2005
- Scahill L. Diagnosis and evaluation of pervasive developmental disorders. Journal of Clinical Psychiatry 2005;66(Suppl 10):19‐25. [PUBMED: 16401146] [PubMed] [Google Scholar]
Scahill 2006
- Scahill L, McDougle CJ, Williams SK, Dimitropoulos A, Aman MG, McCracken JT, et al. Children's Yale‐Brown Obsessive Compulsive Scale modified for pervasive developmental disorders. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(9):1114‐23. [DOI: 10.1097/01.chi.0000220854.79144.e7; PUBMED: 16926619] [DOI] [PubMed] [Google Scholar]
Schlochtermeier 2011
- Schlochtermeier L, Stoy M, Schlagenhauf F, Wrase J, Park SQ, Friedel E, et al. Childhood methylphenidate treatment of ADHD and response to affective stimuli. European Neuropsychopharmacology 2011;21(8):646‐54. [DOI: 10.1016/j.euroneuro.2010.05.001; PUBMED: 20570115] [DOI] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Siegel 2012
- Siegel M, Beaulieu AA. Psychotropic medications in children with autism spectrum disorders: a systematic review and synthesis for evidence‐based practice. Journal of Autism and Developmental Disorders 2012;42(8):1592‐605. [DOI: 10.1007/s10803-011-1399-2; PUBMED: 22068820] [DOI] [PubMed] [Google Scholar]
SIGN 2007
- Scottish Intercollegiate Guidelines Network. Assessment, diagnosis and clinical interventions for children and young people with autism spectrum disorders: a national clinical guideline. www.sign.ac.uk/pdf/sign98.pdf (accessed 11 April 2014).
Sinha 2011
- Sinha Y, Silove N, Hayen A, Williams K. Auditory integration training and other sound therapies for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD003681.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sparrow 2010
- Sparrow EP. Essentials of Conners Behaviour Assessments. Hoboken (NJ): Wiley, 2010. [Google Scholar]
Sterne 2017
- Sterne JAC, Egger M, Moher D, Boutron I, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Storebø 2015
- Storebø OJ, Ramstad E, Krogh HB, Nilausen TD, Skoog M, Holmskov M, et al. Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD009885.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sturmey 2014
- Sturmey P, Dalfern S. The effects of DSM5 autism diagnostic criteria on number of individuals diagnosed with autism spectrum disorders: a systematic review. Review Journal of Autism and Developmental Disorders 2014;1(4):249‐52. [DOI: 10.1007/s40489-014-0016-7] [DOI] [Google Scholar]
Szymansk 2001
- Szymanski ML, Zolotor A. Attention‐deficit/hyperactivity disorder: management. American Family Physician 2001;64(8):1355‐62. [PubMed] [Google Scholar]
TGL 2012
- Therapeutic Guidelines Limited. Management Guidelines: Developmental Disability. 3rd Edition. Melbourne (VIC): Therapeutic Guidelines Limited, 2012. [Google Scholar]
Wagner 2007
- Wagner A, Lecavalier L, Arnold LE, Aman MG, Scahill L, Stigler KA, et al. Developmental Disabilities modification of Children's Global Assessment Scale (DD‐CGAS). Biological Psychiatry 2007;61(4):504‐11. [DOI: 10.1016/j.biopsych.2007.01.001; NIHMS17765; PMC1950959] [DOI] [PMC free article] [PubMed] [Google Scholar]
Westerlund 2009
- Westerlund J, Ek U, Holmberg K, Näswall K, Fernell E. The Conners' 10‐item scale: findings in a total population of Swedish 10‐11‐year‐old children. Acta Paediatrica 2009;98(5):828‐33. [DOI: 10.1111/j.1651-2227.2008.01214.x; PUBMED: 19154524] [DOI] [PubMed] [Google Scholar]
WHO 2007
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. 10th Edition. Geneva (CH): World Health Organization, 2007. [Google Scholar]
Wilens 2008
- Wilens TE. Effects of methylphenidate on the catecholaminergic system in attention‐deficit/hyperactivity disorder. Journal Of Clinical Psychopharmacology 2008;28(3 Suppl 2):S46‐53. [DOI: 10.1097/JCP.0b013e318173312f; PUBMED: 18480677] [DOI] [PubMed] [Google Scholar]
Williams 2006
- Williams JG, Higgins JP, Brayne CE. Systematic review of prevalence studies of autism spectrum disorders. Archives of Disease in Childhood 2006;91(1):8‐15. [DOI: 10.1136/adc.2004.062083; PMC2083083; PUBMED: 15863467] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2008
- Williams LM, Hermens DF, Palmer D, Kohn M, Clarke S, Keage H, et al. Misinterpreting emotional expressions in attention‐deficit/hyperactivity disorder: evidence for a neural marker and stimulant effects. Biological Psychiatry 2008;63(10):917‐26. [DOI: 10.1016/j.biopsych.2007.11.022; PUBMED: 18272140] [DOI] [PubMed] [Google Scholar]
Williams 2012
- Williams K, Wray JA, Wheeler DM. Intravenous secretin for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD003495.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2013
- Williams K, Brignell A, Randall M, Silove N, Hazell P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD004677.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wing 1997
- Wing L. The autistic spectrum. Lancet 1997;350(9093):1761‐6. [DOI: 10.1016/S0140-6736(97)09218-0; PUBMED: 9413479] [DOI] [PubMed] [Google Scholar]
Zhang 2005
- Zhang S, Faries DE, Vowles M, Michelson D. ADHD Rating Scale IV: psychometric properties from a multinational study as a clinician‐administered instrument. International Journal of Methods in Psychiatric Research 2005; Vol. 14, issue 4:186‐201. [PUBMED: 16395872] [DOI] [PMC free article] [PubMed]
References to other published versions of this review
Redman 2014
- Redman T, Scheermeyer E, Ogawa EC, Sparks EC, Taylor JC, Tran VT, et al. Methylphenidate for core and ADHD‐like symptoms in children aged 6 to 18 years with autism spectrum disorders (ASDs). Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD011144] [DOI] [Google Scholar]


