Abstract
Background
Multiple sclerosis (MS) is a chronic disease of the central nervous system, affecting approximately 2.5 million people worldwide. People with MS may experience limitations in muscular strength and endurance – including the respiratory muscles, affecting functional performance and exercise capacity. Respiratory muscle weakness can also lead to diminished performance on coughing, which may result in (aspiration) pneumonia or even acute ventilatory failure, complications that frequently cause death in MS. Training of the respiratory muscles might improve respiratory function and cough efficacy.
Objectives
To assess the effects of respiratory muscle training versus any other type of training or no training for respiratory muscle function, pulmonary function and clinical outcomes in people with MS.
Search methods
We searched the Trials Register of the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group (3 February 2017), which contains trials from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL, LILACS and the trial registry databases ClinicalTrials.gov and WHO International Clinical Trials Registry Platform. Two authors independently screened records yielded by the search, handsearched reference lists of review articles and primary studies, checked trial registers for protocols, and contacted experts in the field to identify further published or unpublished trials.
Selection criteria
We included randomized controlled trials (RCTs) that investigated the efficacy of respiratory muscle training versus any control in people with MS.
Data collection and analysis
One reviewer extracted study characteristics and study data from included RCTs, and two other reviewers independently cross‐checked all extracted data. Two review authors independently assessed risk of bias with the Cochrane 'Risk of bias' assessment tool. When at least two RCTs provided data for the same type of outcome, we performed meta‐analyses. We assessed the certainty of the evidence according to the GRADE approach.
Main results
We included six RCTs, comprising 195 participants with MS. Two RCTs investigated inspiratory muscle training with a threshold device; three RCTs, expiratory muscle training with a threshold device; and one RCT, regular breathing exercises. Eighteen participants (˜ 10%) dropped out; trials reported no serious adverse events.
We pooled and analyzed data of 5 trials (N=137) for both inspiratory and expiratory muscle training, using a fixed‐effect model for all but one outcome. Compared to no active control, meta‐analysis showed that inspiratory muscle training resulted in no significant difference in maximal inspiratory pressure (mean difference (MD) 6.50 cmH2O, 95% confidence interval (CI) −7.39 to 20.38, P = 0.36, I2 = 0%) or maximal expiratory pressure (MD −8.22 cmH2O, 95% CI −26.20 to 9.77, P = 0.37, I2 = 0%), but there was a significant benefit on the predicted maximal inspiratory pressure (MD 20.92 cmH2O, 95% CI 6.03 to 35.81, P = 0.006, I2 = 18%). Meta‐analysis with a random‐effects model failed to show a significant difference in predicted maximal expiratory pressure (MD 5.86 cmH2O, 95% CI −10.63 to 22.35, P = 0.49, I2 = 55%). These studies did not report outcomes for health‐related quality of life.
Three RCTS compared expiratory muscle training versus no active control or sham training. Under a fixed‐effect model, meta‐analysis failed to show a significant difference between groups with regard to maximal expiratory pressure (MD 8.33 cmH2O, 95% CI −0.93 to 17.59, P = 0.18, I2 = 42%) or maximal inspiratory pressure (MD 3.54 cmH2O, 95% CI −5.04 to 12.12, P = 0.42, I2 = 41%). One trial assessed quality of life, finding no differences between groups.
For all predetermined secondary outcomes, such as forced expiratory volume, forced vital capacity and peak flow pooling was not possible. However, two trials on inspiratory muscle training assessed fatigue using the Fatigue Severity Scale (range of scores 0‐56 ), finding no difference between groups (MD, −0.28 points, 95% CI−0.95 to 0.39, P = 0.42, I2 = 0%). Due to the low number of studies included, we could not perform cumulative meta‐analysis or subgroup analyses. It was not possible to perform a meta‐analysis for adverse events, no serious adverse were mentioned in any of the included trials.
The quality of evidence was low for all outcomes because of limitations in design and implementation as well as imprecision of results.
Authors' conclusions
This review provides low‐quality evidence that resistive inspiratory muscle training with a resistive threshold device is moderately effective postintervention for improving predicted maximal inspiratory pressure in people with mild to moderate MS, whereas expiratory muscle training showed no significant effects. The sustainability of the favourable effect of inspiratory muscle training is unclear, as is the impact of the observed effects on quality of life.
Plain language summary
Respiratory muscle training in multiple sclerosis
Background
Multiple sclerosis (MS) is a chronic disease of the central nervous system, affecting approximately 2.5 million people worldwide. Although the exact cause of the disease is unknown, it is generally accepted that MS involves an abnormal immune response within the central nervous system. Depending on the severity of the disease, people with MS may experience varying limitations in, for example muscular strength and endurance, including in the muscles needed to breathe (respiratory muscles). Strength of the respiratory muscles is related to people's ability to function and to exercise, and respiratory muscle weakness can lead to less effective coughing, which may result in aspiration pneumonia (when food, saliva or other liquids are breathed into airways instead of being swallowed) or even acute failure of respiratory function. These pulmonary complications are frequently reported causes of death in people with MS. Training of the respiratory muscles might improve breathing and cough effectiveness.
Study characteristics
We searched electronic databases for randomized controlled trials (where participants are assigned at random to either a treatment or a control arm) published up to 3 February 2017 that investigated respiratory muscle training in people with MS. In addition, we contacted experts in the field to identify additional studies.
Key results
We found six trials involving 195 participants with MS. Training consisted of two or three sets of 10 to 15 repetitions, twice a day for at least three days a week, and interventions lasted for six weeks to three months. Follow‐up ranged from no follow‐up to six months. Two of the included trials investigated inspiratory muscle training with a threshold device (i.e. a portable breathing device that increases airflow resistance while inhaling or exhaling). Three trials investigated expiratory muscle training with a threshold device, and one trial investigated breathing exercises. We found benefits with inspiratory muscle training for improving predicted maximal inspiratory pressure, but not for improving measured maximal inspiratory pressure. We did not find any effects for maximal expiratory pressure. Only one study measured quality of life, but it did not find any effects; two trials measured fatigue and also failed to find a difference between the treatment and control groups. Eighteen participants (˜ 10%) dropped out, and no trials reported any serious adverse events.
Quality of the evidence
The six trials that were eligible for inclusion in this review were small, so statistical power was low, making analyses less precise. In addition, studies were heterogeneous in terms of the type of respiratory muscle training, dosing/intensity, and the severity of MS. In addition, we could not analyze the effects of training on, for example, cough efficacy, pneumonia, and quality of life, as the included trials did not report on these outcomes even though they are important for patients, caregivers and healthcare professionals. Altogether, this review provides low‐quality evidence that resistive inspiratory muscle training improves predicted inspiratory muscle strength in people with MS. We did not find any effects for resistive expiratory muscle training. More high‐quality research in respiratory muscle training in MS is needed.
Summary of findings
Summary of findings for the main comparison. Summary of findings: respiratory muscle training versus sham training or no training for people with multiple sclerosis.
| Respiratory muscle training versus sham training or no training for people with multiple sclerosis | ||||||
|
Patient or population: people with multiple sclerosis Settings: home, outpatient rehabilitation, and outpatient neurology department Intervention: respiratory muscle training (inspiratory or expiratory muscle training) Comparison: sham training or no training | ||||||
| Outcomes | Type of intervention | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Experimental | |||||
|
Maximal inspiratory pressure (cmH2O) Postintervention for insp. training at 10 weeks, for exp. training after 2‐3 months |
Inspiratory muscle training | The mean maximal inspiratory pressure across control groups was 58.5 cmH2O and 71.8 cmH2O | The mean maximal inspiratory pressure in the intervention groups was 6.50 cmH2O higher (7.39 lower to 20.38 higher) | 56 (2 trials) | ⊕⊕⊝⊝ Low | Limitations in design and implementation (‐1) Imprecision of results (‐1) |
| Expiratory muscle training | The mean maximal inspiratory pressure ranged across control groups from 24.42 cmH2O to 82 cmH2O | The mean maximal inspiratory pressure in the intervention groups was 3.54 higher (5.04 lower to 12.12 higher) | 81 (3 trials) | ⊕⊕⊝⊝ Low | Limitations in design and implementation (‐1) Imprecision of results (‐1) |
|
|
Maximal expiratory pressure (cmH2O) Postintervention for insp. training at 10 weeks, for exp. training after 2‐3 months |
Inspiratory muscle training | The mean maximal expiratory pressure across control groups was 61.25 cmH2O and 85.8 cmH2O | The mean maximal expiratory pressure in the intervention groups was 8.22 cmH2O lower (26.20 lower to 9.77 higher) | 56 (2 trials) | ⊕⊕⊝⊝ Low | Limitations in design and implementation (‐1) Imprecision of results (‐1) |
| Expiratory muscle training | The mean maximal expiratory pressure ranged across control groups from 23.04 cmH2O to 100 cmH2O | The mean maximal expiratory pressure in the intervention groups was 8.33 cmH2O higher (0.93 lower to 17.59 higher) | 81 (3 trials) | ⊕⊕⊝⊝ Low | Limitations in design and implementation (‐1) Imprecision of results (‐1) |
|
|
Predicted maximal inspiratory pressure (%) Postintervention after 10 weeks |
Inspiratory muscle training | The mean % of predicted maximal inspiratory pressure across control groups was 58.8% and 77.9% | The mean % of predicted maximal inspiratory pressure in the intervention groups was 20.92 cmH2O higher ( 6.03 to 35.81 higher) | 56 (2 trials) | ⊕⊕⊝⊝ Low | Limitations in design and implementation (‐1) Imprecision of results (‐1) |
|
Predicted maximal expiratory pressure (%) Postintervention after 10 weeks |
Inspiratory muscle training | The mean % of predicted maximal expiratory pressure across control groups was 45.38% and 50.1% | The mean % of predicted maximal expiratory pressure in the intervention groups was 5.86% higher (10.63% lower to 22.35% higher) | 56 (2 trials) | ⊕⊕⊝⊝ Low | Limitations in design and implementation (‐1) Imprecision of results (‐1) |
|
Quality of Life (EQ‐5D VAS, score range: 0‐100) Postintervention after 2 months |
Expiratory muscle training | No separate group data are presented Overall health status was 64.6 (SD 21.5) at baseline and 67.3 (SD 21.6) at follow‐up, | No separate group data are presented Overall health status was 64.6 (SD 21.5) at baseline and 67.3 (SD 21.6) at follow‐up, | 48 (1 trial) |
⊕⊕⊝⊝ Low |
Limitations in design and implementation (‐1) Imprecision of results (‐1) |
|
Fatigue (Fatigue Severity Scale, score range: 0‐56) Post intervention after 10 weeks |
Inspiratory muscle training | The mean fatigue across control groups was 5.3 and 5.6 points on the Fatigue Severity Scale | The mean fatigue in the intervention groups was 0.28 points lower (0.95 lower to 0.39 higher) | 56 (2 trials) | ⊕⊕⊝⊝ Low | Limitations in design and implementation (‐1) Imprecision of results (‐1) |
| *We chose the median control group risk across studies as the basis for the assumed risk. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to determine the quality of the evidence. According to Cochrane Handbook Chapter 12 ‘Interpreting results and drawing conclusions’ (Higgins 2011), the quality level was downgraded based on presence of bias, indirectness of the evidence, heterogeneity or inconsistency of results, imprecision and the probability of publication bias. Upgrading took place in case of a large effect, confounding factors that were taken into account, and the presence of a dose‐response gradient. Reasons for down‐ or upgrading were explained in the Summary of findings table.
Background
Multiple sclerosis (MS) is a chronic disease of the central nervous system. There are approximately 2.5 million people with MS worldwide. Wide demographic variations exist across populations in terms of disease incidence and prevalence. Over the past decades, the prevalence of MS has increased, predominantly due to longer survival (Koch‐Henriksen 2010).
Although the exact aetiology of the disease is unknown, it is generally accepted that MS involves an abnormal immune response within the central nervous system. The variable distribution of demyelinization throughout the central nervous system may lead to disorders of muscular strength and endurance, including in respiratory muscles (Fry 2007; Smeltzer 1988). Respiratory muscle performance is an important aspect of disease management given the association between respiratory muscle weakness and the Expanded Disability Status Scale (EDSS), functional performance (six‐minute walking test; 6MWT), and reduced exercise capacity in MS, all affecting quality of life (Bosnak‐Guclu 2012; Chiara 2006; Foglio 1994; Ray 2013).
Description of the condition
Impairments of the respiratory muscles in people with MS are common. Fry 2007 reported that 64% of people with MS and a low to medium EDSS score (3.96 ± 1.8, range 2.0 to 6.5; score range 0‐10) had respiratory muscle weakness. Impairments in respiratory muscles and pulmonary functions increase in tandem with disease progression (Bosnak‐Guclu 2012; Gosselink 2000). Respiratory muscle weakness can lead to diminished performance on coughing, which may result in aspiration pneumonia or even acute ventilatory failure. These pulmonary complications are frequently reported causes of death in MS (Sumelahti 2010).
Description of the intervention
Respiratory muscle weakness as a consequence of MS is related to neurological impairment, physical inactivity and respiratory complications (Motl 2011a). Diminished motor performance and severe fatigue, as experienced by most people with MS in all stages of the disease (Stuke 2009), influence the level and pattern of physical activity and pulmonary function (Motl 2011b). Therefore, specific exercise training for respiratory muscles may be beneficial.
How the intervention might work
Physical training may play an important role in maintaining or increasing levels of activity to prevent people from muscle disuse, including the respiratory muscles. Strengthening respiratory muscles may improve respiratory function and efficacy of coughing while preventing deterioration of pulmonary function. Possible mechanisms of breathing exercises with positive expiratory or inspiratory pressure include improving respiratory muscle strength and momentarily increasing lung volume, which may facilitate secretion mobilization. Training interventions typically involve threshold devices, that is, portable breathing devices that increase airflow resistance during inspiration or expiration, creating positive inspiratory pressure or positive expiratory pressure. Respiratory musculature must therefore work harder to obtain the required respiratory air exchange (overload principle). Intensity usually ranges from 40% to 60% of maximal inspiratory or expiratory pressure, with or without progressive increase in intensity, during a training period of 8 to 12 weeks.
Why it is important to do this review
No Cochrane Review has formally assessed the effects of respiratory muscle training in people with MS. Previous non‐Cochrane reviews have included quasi‐experimental and pre‐experimental designs. Our systematic review is restricted to randomized clinical trials. Healthcare professionals and people with MS could benefit from having high‐quality evidence from RCTs in order to best inform strategies for managing MS.
Objectives
To assess the effects of respiratory muscle training versus any other type of training or no training for respiratory muscle function, pulmonary function and clinical outcomes in people with MS.
Methods
Criteria for considering studies for this review
Types of studies
We restricted our review to randomized controlled trials (RCTs), in which investigators randomly allocate eligible people into treatment and control groups (Higgins 2011). In line with Cochrane guidelines, we considered cross‐over trials as RCTs, and we used results prior to treatment cross‐over if they came from two or more groups of independent participants.
Types of participants
We considered only studies that included adults with the clinical diagnoses of MS according to the International Classification of Diseases, ninth edition (ICD‐9) (code 340) or to the definitions proposed by McDonald 2001, Poser 1983 or Schumacher 1965.
Types of interventions
Experimental intervention
We included trials of respiratory muscle function training, as defined by our review. We used the description of respiratory muscle training proposed by WHO 2001: "therapeutic exercises specifically aimed to improve strength and endurance of inspiratory and expiratory muscles". The goal of the respiratory muscle training should be associated with one or both of the following codes of the ICF‐classification: b440 respiratory functions and b445 respiratory muscle functions. Therefore, the included interventions pertain to studies that assessed: inspiratory muscle strength training, expiratory muscle strength training, and/or breathing exercises in any intensity, frequency and duration to induce training effects in defined outcomes (see Types of outcome measures). We excluded studies if the goal of the therapy primarily focused on improving physical functions not associated with respiratory muscles, that is, all interventions that did not fit with the review authors' definition of respiratory muscle training (see above).
Control intervention
Control interventions included general rehabilitation or physical therapy (with or without training equipment); exercise training; functional training; home physical training; aquatic therapy; learning to handle products, technology and equipment in daily living; electrotherapy; electrical muscle stimulation (functional, neuromuscular); transcutaneous electrical nerve stimulation or no training.
Types of outcome measures
We assessed the following outcomes at the end of the intervention period, and if possible at follow‐up.
Primary outcomes
Respiratory muscle function parameters measured on the body function level of the International Classification of Functioning, Disability and Health (ICF) (WHO 2001), such as maximal expiratory pressure and maximal inspiratory pressure.
Quality of life, measured with a validated instrument such as the EuroQoL EQ‐5D‐VAS (EuroQol 1990).
Secondary outcomes
Pulmonary function parameters, measured on the body function level of the ICF (WHO 2001): forced expiratory volume, forced vital capacity and peak flow.
Clinical pulmonary parameters, measured on the body function level of the ICF: for instance, cough efficacy and pulmonary complications such as pneumonia as well as acute and chronic ventilatory failure.
Fatigue, measured with the Fatigue Severity Scale (FSS) (Rietberg 2010).
Adverse events of any severity, such as pain, discomfort, relapses or death, whether expected or unexpected; or study‐related, possibly study‐related, or not study‐related.
Search methods for identification of studies
We applied no language restrictions to the search.
Electronic searches
The Information Specialist searched the Trials Register of the Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group, which, among other sources, contains trials from:
Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 2);
MEDLINE (PubMed) (from 1966 to 3 February 2017);
Embase (Embase.com) (from 1974 to 3 February 2017);
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO host) (from 1981 to 3 February 2017);
Latin American and Caribbean Health Science Information Database (LILACS) (Bireme) (from 1982 to 3 February 2017);
ClinicalTrials.gov (www.clinicaltrials.gov);
World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch).
Information on the Cochrane Multiple Sclerosis Group's Trials Register and details of search strategies used to identify trials are available in the 'Specialised Register' section within the Cochrane Multiple Sclerosis Group's module.
The keywords used to search for trials for this review are listed in Appendix 1.
We contacted the authors of studies who reported incomplete data for further information when needed.
Searching other resources
To identify other relevant study data, we also:
screened reference lists of review articles and primary studies;
checked trial registers for protocols (i.e. clinicaltrialsregister.eu, trialregister.nl and isrctn.com); and
contacted experts in the field to identify further published or unpublished trials.
Data collection and analysis
Selection of studies
Following an initial screening based on title and abstract, two reviewers (MBR, JMV) independently selected trials for inclusion in this review using predetermined inclusion criteria. We resolved any disagreements about inclusion of studies by consensus and consultation with a third review author (EvW).
Data extraction and management
For each included study, one reviewer (JMV) documented the following information in the predefined form used by the Review Manager 5 (RevMan 5) table manager (Chapter 7.3 in Higgins 2011; RevMan 2014).
Study design.
Patient characteristics (number, setting, age, type of MS, EDSS score, functional ability, country).
Inclusion and exclusion criteria.
Brief description of the experimental intervention.
Brief description of the control intervention.
Outcomes (including unit of measurement and interpretation of scores i.e. if a high score is good or bad) and time points reported.
Two other reviewers (MBR, EvW) cross‐checked these data. Two reviewers (JMV, MBR) independently extracted study outcome data for quantitative analysis. We contacted principal investigators to request additional relevant (unpublished) information on outcome data (Rietberg 2016a; Rietberg 2016bRietberg 2016c). We imported all the extracted information into both printed data extraction forms and RevMan 2014.
Assessment of risk of bias in included studies
Two reviewers (JMV, EvW) independently assessed the risk of bias for all included studies using the Cochrane 'Risk of bias' assessment tool (Chapter 8.5 in Higgins 2011). We used a standard form that included information on sequence generation, allocation concealment, blinding (participants, personnel and outcome assessor), incomplete outcome data, selective outcome reporting and other sources of bias. We assessed the methodological components of the trials as being at low, high or unclear risk of bias, in line with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed blinding separately for different outcomes, and we assessed incomplete outcome data separately for the same outcome at different time points. When differences arose, we resolved them through discussions with the third review author (MBR).
Measures of treatment effect
As all trials used the same measurement instrument to assess each of the predefined outcomes, we calculated the mean difference (MD) with its corresponding 95% confidence interval (CI).
Unit of analysis issues
We identified only RCTs with a parallel group design and two trial arms. We did not identify eligible studies with a cross‐over design. Therefore, we did not need to combine data from relevant experimental arms and relevant control arms to create single pairwise comparisons in the case of more than two trial arms, nor to determine the time point of analysis in the case of a cross‐over design.
Dealing with missing data
In the meta‐analyses, we used study data as reported in the publication or as provided by the authors.
Assessment of heterogeneity
We examined heterogeneity across studies by inspecting the distribution of point estimates for the effect measure and the overlap in their confidence interval on the forest plot. We used the I2 statistic to check the statistical consistency, defined as the ratio of between‐study variation compared to the overall variation (Chapter 9.5.2 in Higgins 2011). We set the cut‐off for interpretation above which statistical heterogeneity is substantially present at 50%.
Assessment of reporting biases
To estimate the influence of unpublished papers on the overall effects (i.e. publication bias), we would have used funnel plots had more than 10 studies been included in any meta‐analysis.
Data synthesis
We pooled the data from individual studies for each outcome by applying a fixed‐effect model when statistical homogeneity (I2 <50%) was present. In the situation of substantial heterogeneity (I2 ≥ 50%), we used a random‐effects model to adjust for between‐study variance.
Subgroup analysis and investigation of heterogeneity
In case of sufficient power, we planned subgroup analyses to establish the influence of participant‐related characteristics (such as age and MS type) and intervention‐related characteristics (including dose of therapy and type of intervention), on overall effects.
Sensitivity analysis
We planned to assess the influence of factors related to risk of bias (e.g. concealed allocation) on the overall effect through a sensitivity analysis to determine the overall robustness to assumptions made in the meta‐analysis.
Summary of findings table and GRADE
A Summary of Findings table was made for the following outcomes at post intervention and if possible at follow‐up, divided by type of respiratory training:
Primary outcomes:
‐ Respiratory muscle function parameters, such as maximal inspiratory pressure (cmH2O), maximal expiratory pressure (cmH2O), predicted maximal inspiratory pressure (%), predicted maximal expiratory pressure (%)
‐ Quality of Life
Secondary outcomes:
‐ Pulmonary function parameters
‐ Clinical pulmonary parameters
‐ Fatigue (Fatigue Severity Scale);
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to determine the quality of the evidence. According to Cochrane Handbook Chapter 12 ‘Interpreting results and drawing conclusions’ (Higgins 2011), the quality level was downgraded based on presence of bias, indirectness of the evidence, heterogeneity or inconsistency of results, imprecision and the probability of publication bias. Upgrading took place in case of a large effect, confounding factors that were taken into account, and the presence of a dose‐response gradient. Reasons for down‐ or upgrading were explained in the Summary of findings table.
Results
Description of studies
Results of the search
See Figure 1.
1.
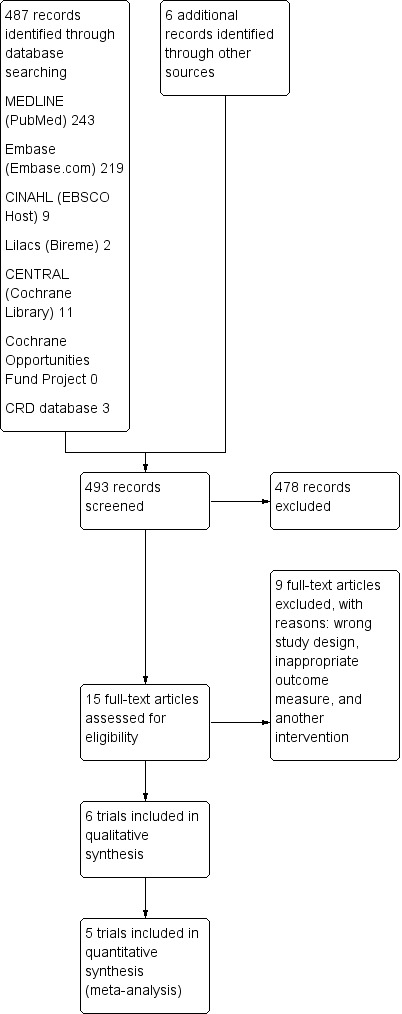
Study flow diagram.
The electronic search yielded 493 unique records; after excluding 478 during initial screening, we assessed the full text of 15 records for eligibility. The main reasons for excluding studies were because they used the wrong study design or an ineligible intervention. We did not identify any further studies through handsearching reference lists or contacting experts in the field of respiratory training and MS. Six RCTs (N = 195) met the inclusion criteria for our systematic review (Fry 2007; Gosselink 2000; Klefbeck 2003; Mutluay 2007; Smeltzer 1996; Westerdahl 2016).
Included studies
See the Characteristics of included studies table.
Two RCTs investigated the effect of inspiratory muscle training in people with MS (Fry 2007; Klefbeck 2003), while three RCTs investigated expiratory muscle training (Gosselink 2000; Smeltzer 1988; Westerdahl 2016). One trial focused on breathing‐enhanced upper extremity exercises, with focus on deep nasal inspiration and forced oral expiration (Mutluay 2007).
There was broad variation in the mobility levels of the included participants. For example, in Fry 2007 and Westerdahl 2016, the participants had to be ambulatory, while in Gosselink 2000, participants had to be bedridden or wheelchair‐bound.
Training with the threshold device consisted of two or three sets of 10 to 15 repetitions, twice a day for at least three days a week, and interventions lasted for six weeks to three months. The total number of repetitions (i.e. breathing motions) ranged from 2100 to 5400. The initially applied resistance was 60% of the participant's maximum expiratory pressure (PEmax) (Gosselink 2000), 40% to 60% of the participant's maximum inspiratory pressure (PImax) (Klefbeck 2003), PEmax (Smeltzer 1996), or an expiratory pressure of 10 cmH2O to 15 cmH2O (Westerdahl 2016). Three trials reported that participant‐reported resistance increased progressively during the programme (Fry 2007; Klefbeck 2003; Smeltzer 1996). Fry 2007 and Mutluay 2007 did not conduct follow‐up assessments at any time points after the postintervention assessment, while Klefbeck 2003 assessed participants at one month postintervention, Westerdahl 2016 at two months and Gosselink 2000 at six months.
Therapist supervision was done by bi‐weekly home visits Klefbeck 2003, weekly home visits Smeltzer 1996 and weekly recall visits to hospital in Mutluay 2007.
Eighteen (10%) of the 195 participants dropped out: 7 in the experimental groups and 8 in the control groups; group allocation for the other 3 dropouts was unclear. The reported reasons for dropout were lack of cooperation, illness unrelated to exacerbation, family discord, stress or competing time commitments, and unwillingness to participate in follow‐up measurements (Fry 2007; Gosselink 2000; Mutluay 2007; Smeltzer 1996; Westerdahl 2016).
Two RCTs provided information about adverse events. In Mutluay 2007, participants did not report any diseases or relapses, and in Westerdahl 2016, four participants reported some study‐related discomfort that could be expected due to the intensity of the intervention protocol.
Three RCTs provided information about adherence to the experimental training protocol. In Fry 2007, adherence to the threshold training protocol averaged 81% (standard deviation (SD) 6.93). Mutluay 2007 reported that 85% of the participants fully complied with the programme, with a 94% (SD 10) scheduled exercise completion rate. Westerdahl 2016 reported that participants performed the programme once or twice a day with 30 (SD 8) breaths.
Excluded studies
See Characteristics of excluded studies; Characteristics of ongoing studies.
We excluded nine studies because they were not RCTs, did not use a respiratory training intervention or did not report respiratory outcomes. We also identified three ongoing studies.
Risk of bias in included studies
Two review authors (JMV, EvW) independently assessed the risk of bias of the six included RCTs. We reached full agreement after a consensus meeting. Figure 2 presents an overview of the risk of bias assessment scores. In general, we assessed most of the items as unclear (21 items, ˜ 58%), while we assessed fewer items as being at low risk of bias (9 items, 25%) or high risk of bias (6 items, ˜ 17%) (Figure 3).
2.
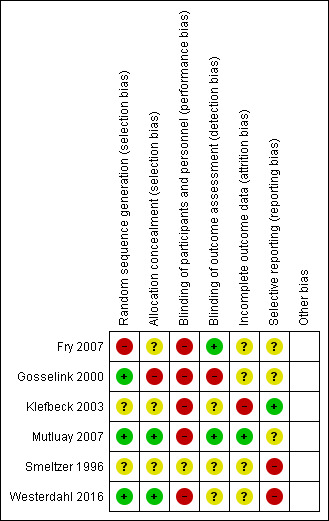
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
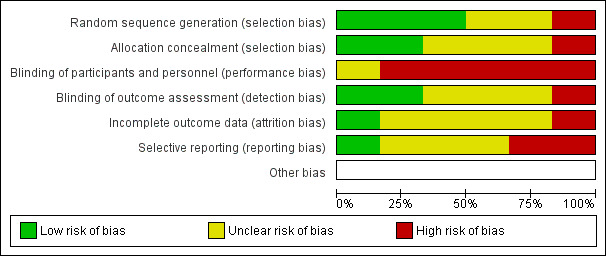
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All trials randomized participants to an experimental and control group. The method of allocation carried a low risk of bias in two RCTs, i.e. the concealment method was properly described (Gosselink 2000; Mutluay 2007), an unclear risk in three RCTs, i.e. the concealment method was incompletely described (Klefbeck 2003;Smeltzer 1996; Westerdahl 2016), and a high risk of bias in one RCT (Fry 2007), i.e. no concealment method was described.(Figure 2)
Blinding
Five RCTs were at high risk of bias for blinding of participants and personnel (performance bias) (Fry 2007; Gosselink 2000; Klefbeck 2003; Mutluay 2007; Westerdahl 2016), an inherent feature of investigations of complex rehabilitation interventions. In one RCT the risk was unclear, as participants in the control group performed sham training using a device without resistance (Smeltzer 1996). Two RCTs were at low risk of bias for blinding of outcome assessment (detection bias) (Fry 2007; Mutluay 2007), while the risk of bias was unclear in four RCTs (Gosselink 2000; Klefbeck 2003; Smeltzer 1996: Westerdahl 2016).
Incomplete outcome data
Eighteen (˜ 10%) of the 195 participants included in this review dropped out, with the number of dropouts per trial ranging from 0 in Mutluay 2007 to 5 each in Fry 2007 and Smeltzer 1996. In general, dropouts were equally distributed over the treatment arms. The reported reasons for dropout were lack of cooperation, illness unrelated to exacerbation, family discord, stress or competing time commitments, and unwillingness to participate in follow‐up measurements (Fry 2007; Gosselink 2000; Mutluay 2007; Smeltzer 1996; Westerdahl 2016). One RCT was at low risk of bias for incomplete outcome data (attrition bias) (Mutluay 2007). The risk of bias was unclear in four trials (Fry 2007; Gosselink 2000; Smeltzer 1996; Westerdahl 2016), while it was high in one (Klefbeck 2003).
Selective reporting
One RCT was at low risk of selective reporting (reporting bias) (Klefbeck 2003), while the risk was unclear in three RCTs (Fry 2007; Gosselink 2000; Mutluay 2007), and it was high in two RCTs (Smeltzer 1996; Westerdahl 2016).
Other potential sources of bias
We did not detect any other potential risks of bias.
Effects of interventions
See: Table 1
Table 1 gives an overview of grading the evidence for inspiratory muscle training and expiratory muscle training found in this systematic review with meta‐analysis.
Primary outcome measures
Inspiratory muscle training
Maximal inspiratory pressure
Based on two RCTs (N = 56) (Fry 2007; Klefbeck 2003), a meta‐analysis comparing inspiratory muscle training versus any other type of physical therapy intervention under a fixed‐effect model failed to show a significant summary effect size on maximal inspiratory pressure (MD 6.50 cmH2O, 95% CI −7.39 to 20.38, P = 0.36, I2 = 0%; Analysis 1.1). For predicted maximal inspiratory pressure, however, results did show a significant benefit in the experimental arm (MD 20.92 cmH2O, 95% CI 6.03 to 35.81, P = 0.006, I2 = 18%; Analysis 1.2).
1.1. Analysis.
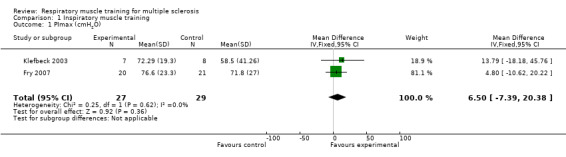
Comparison 1 Inspiratory muscle training, Outcome 1 PImax (cmH2O).
1.2. Analysis.
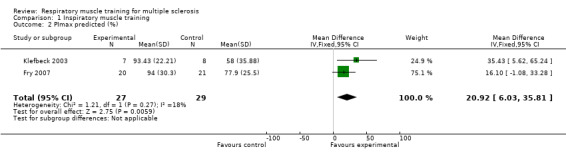
Comparison 1 Inspiratory muscle training, Outcome 2 PImax predicted (%).
Maximal expiratory pressure
After pooling the results of two RCTs (N = 56) (Fry 2007; Klefbeck 2003), we did not find a significant difference between the experimental and control groups for maximal expiratory pressure (MD −8.22 cmH2O, 95% CI −26.20 to 9.77, P = 0.37, I2 = 0%; fixed‐effect; Analysis 1.3) or for predicted maximal expiratory pressure (MD 5.86 cmH2O, 95% CI −10.63 to 22.35, P = 0.49, I2 = 55%, random‐effects; Analysis 1.4 ).
1.3. Analysis.
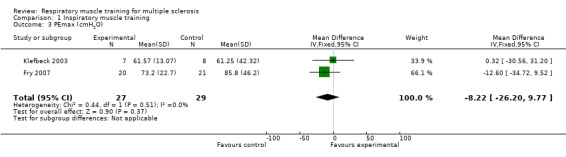
Comparison 1 Inspiratory muscle training, Outcome 3 PEmax (cmH2O).
1.4. Analysis.
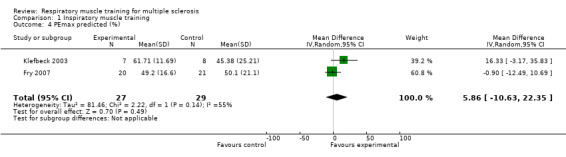
Comparison 1 Inspiratory muscle training, Outcome 4 PEmax predicted (%).
Quality of life
None of the studies on inspiratory muscle training reported the results of a health‐related quality of life outcome measure.
Expiratory muscle training
Maximal expiratory pressure
Based on three RCTs (N = 81) (Gosselink 2000; Smeltzer 1996; Westerdahl 2016), a meta‐analysis comparing expiratory muscle training versus control under a fixed‐effect model failed to show a significant effect on maximal expiratory pressure (MD 8.33 cmH2O, 95% CI −0.93 to 17.59, P = 0.18, I2 = 42%; Analysis 2.1 ).
2.1. Analysis.
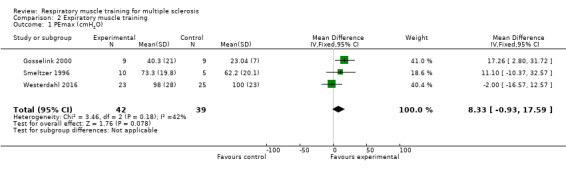
Comparison 2 Expiratory muscle training, Outcome 1 PEmax (cmH2O).
Maximal inspiratory pressure
Based on data from three RCTs (N = 81) (Gosselink 2000; Smeltzer 1996; Westerdahl 2016), meta‐analysis under a fixed‐effect model did not show any significant differences between groups (MD 3.54 cmH2O 95% CI −5.04 to 12.12, P = 0.42, I2 = 41%; Analysis 2.2).
2.2. Analysis.
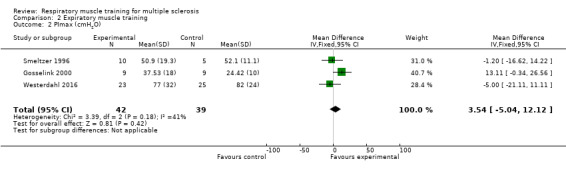
Comparison 2 Expiratory muscle training, Outcome 2 PImax (cmH2O).
Quality of life
One study on expiratory muscle training reported results on a health‐related quality of life outcome measure (EuroQol‐5D‐VAS, range 0‐100) (Westerdahl 2016). Separate group data were not presented, but overall health status was 64.6 (SD 21.5) at baseline and 67.3 (SD 21.6) at follow‐up, with no significant differences between the experimental groups (P < 0.136).
Secondary outcomes
For inspiratory muscle training, expiratory muscle training and breathing exercises, pooling was not possible for pulmonary function parameters or clinical parameters. Two inspiratory muscle training RCTs measured fatigue with the Fatigue Severity Scale (FSS, range 0‐56), showing no significant differences between groups (MD, −0.28 points, 95% CI −0.95 to 0.39, P = 0.42, I2 = 0%; fixed‐effect; Analysis 1.5; Fry 2007; Klefbeck 2003).
1.5. Analysis.
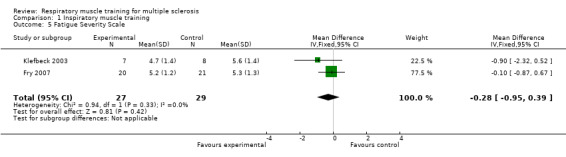
Comparison 1 Inspiratory muscle training, Outcome 5 Fatigue Severity Scale.
It was not possible to perform a meta‐analysis for adverse events. Three studies did not provide any information regarding the presence or absence of adverse events. Two studies explicitly stated that there were no complications such as disease relapses (Klefbeck 2003; Mutluay 2007). Westerdahl 2016 stated that: "discomfort related to the exercises was reported by 4% to a high degree and 13% to some extent. Adverse perceptions were related to dizziness, strenuousness and tediousness". Adverse events were not included in the Summary of Findings table as data was not uniformly presented by included studies and proper quality assessment was not possible.
Subgroup analysis
Due to the low number of included trials, it was not possible to explore if participant‐related characteristics (such as EDSS score, time since diagnosis, age and MS type) or intervention‐related characteristics (dose of therapy, type of intervention) affected overall summary effects.
Sensitivity analysis meta‐analysis
Since we found few studies, we were unable to perform the sensitivity analysis of included studies to investigate the impact of bias on calculated summary effect sizes.
Discussion
Summary of main results
This Cochrane Review, including just six RCTs in 195 participants, reflects the scarcity of research in respiratory muscle training in people with MS. Concerning our primary outcome measures, we found that inspiratory muscle training using a threshold device was beneficial for predicted maximal inspiratory pressure but not for maximal inspiratory pressure. In addition, we found no significant results for postintervention maximal expiratory pressure or fatigue, while pooling was not possible for other secondary outcomes.
For expiratory muscle training with a threshold device, we did not find significant results for maximal expiratory pressure or maximal inspiratory pressure measured right after the intervention period. Only one trial assessed quality of life (Westerdahl 2016), so performing a meta‐analysis was not possible. In this case, Westerdahl 2016 found no significant results for expiratory muscle training on QoL.
Hence, this review provides low‐quality evidence that resistive inspiratory muscle training is moderately effective postintervention for improving inspiratory muscle function in people with mild to moderate MS. Due to large variation in follow‐up periods (ranging from no follow‐up to six months), it is unclear whether effects are sustained over time and whether they are clinically meaningful. What these studies did confirm, is that the baseline values for pulmonary parameters in people with MS are generally below the reference values for healthy people (Neder 1999; Wilson 1984). However, it was not possible to perform a meta‐analysis for adverse events, no serious adverse were mentioned in any of the included trials.
Overall completeness and applicability of evidence
The results of this review are meagre, but there were only a few small phase II trials available with substantial clinical heterogeneity between them. For example, there was large variation in MS types (e.g. relapsing remitting, primary progressive, secondary progressive) and disease severity (EDSS scores ranged from 3 to 8) as well as in the participants' respiratory muscle function and mobility capabilities. We did not assess comparability with other neurological diseases, so generalizability to these patient groups is unclear, although recent reviews suggest that respiratory muscle training is effective in people with spinal cord injury and stroke (Berlowitz 2013; Gomes‐Neto 2016). Also, the applied experimental treatment protocols varied both in terms of focus (inspiratory versus expiratory muscle training) and intensity/dose. Unfortunately, we were unable to statistically analyze outcomes that are highly relevant for patients, like health‐related quality of life. With that, the transfer of effects on the body function level to patient relevant outcomes remains unclear. We could not present any information about long‐term effects or the usefulness of respiratory muscle training to prevent pulmonary complications such as aspiration pneumonia, atelectasis or acute respiratory deficiency, outcomes which future trials should investigate.
The trials reporting information about treatment compliance suggest that resistive respiratory muscle training is feasible in people with MS, even when they mostly perform the exercises unsupervised. In addition, inspiratory muscle training did not negatively influence fatigue.
Quality of the evidence
We assessed the quality of the evidence for both inspiratory muscle training and expiratory muscle training in people with MS to be low. For all performed meta‐analyses, only two or three small phase II trials were available, and with the exception of Mutluay 2007, each of these trials had at least one item with a high risk of bias score. For most items, the risk of bias was unclear.
Potential biases in the review process
Based on the extensive search performed in a large number of databases, it is unlikely that we missed relevant trials. In addition, with two independent review authors (MR, JV) screening the records found in the search, we are confident that we did not overlook eligible trials during the screening. By cross‐checking trial data as well meta‐analysis data, we diminished the chance of introducing bias.
Agreements and disagreements with other studies or reviews
Laciuga 2014 performed a narrative review for functional outcomes associated with expiratory muscle strength training in neurological diseases, but review authors also reported on pulmonary parameters on the impairment level. They concluded that this type of respiratory muscle training is promising in neurological diseases. The only trial they included in people with MS was Smeltzer 1996, also included in our review. With its narrative nature and the inclusion of only one trial on MS, readers should exercise even more caution when interpreting its results than they do with the present review.
Martín‐Valero 2014 performed a systematic review and meta‐analysis for training of respiratory muscles in people with MS. They used a broader definition for interventions than we did, focusing on ventilatory training in general rather than specifically on respiratory muscle training. All of our included trials were also included in their review, except for Westerdahl 2016. Martín‐Valero 2014 found significant effects for both maximum inspiratory and expiratory pressure but did not differentiate between type of respiratory training. In the present review we did differentiate between inspiratory muscle training and expiratory muscle training. To check the findings of Martín‐Valero 2014 we combined both types of training in a supplementary analysis (not shown in this review) and could not replicate their results.
Reyes 2013 reviewed respiratory muscle training in neurodegenerative disorders but did not perform meta‐analysis due to variations in diagnoses and other participant‐ and trial‐related characteristics. They concluded that there was insufficient evidence to support the use of these types of training.
Toomey 2012 reviewed the effects of physical rehabilitation, including respiratory resistance training, in people with MS scoring 7.0 or more on the EDSS. For respiratory muscle training, they included two RCTs that are also included in our review (Gosselink 2000; Klefbeck 2003), concluding that for more impaired patients "the cumulative evidence from two 'low'‐grade RCTs suggests that expiratory or inspiratory muscle training improves respiratory muscle strength, with no changes in respiratory function." These findings are largely consistent with ours regarding PImax. We identified three other studies in which MS patients participated with lower mean baseline EDSS scores (Fry 2007: 3.9; Mutluay 2007: 4.8; Westerdahl 2016: 5.0); however, we could not determine a relationship between EDSS score or time since diagnosis and outcomes due to the low number of studies.
Finally, Ferreira 2016 reviewed RCTs assessing the effects of respiratory training in people with multiple sclerosis and lateral amyotrophic sclerosis, reporting significant summary effect sizes for ventilatory function (forced expiratory volume in one second, or FEV1) and respiratory muscle strength (maximum inspiratory and expiratory pressure). As Ferreira 2016 combined two separate participant groups, their results are not directly comparable with ours. Moreover, they did not perform a separate sensitivity analysis for inspiratory muscle training versus expiratory muscle training in participants with MS. There is also a potential methodological problem in their review, as they double‐counted data from participants from one study that was reported in two separate papers (Fry 2007; Pfalzer 2011). We confirmed this overlap through personal communication with the authors from these separate papers (Fry 2007; Pfalzer 2011).
Authors' conclusions
Implications for practice.
Currently, there is low‐quality evidence for resistive inspiratory muscle training in people with MS. We cannot explain why the percentage of predicted maximum inspiratory pressure showed a significant effect in favour of inspiratory muscle training while measured maximum inspiratory pressure (cmH2O) did not. The results do suggest that people with MS can use more of their maximum predicted inspiratory capacity after inspiratory muscle training. Caution is warranted, as this effect is based on two studies with few participants (N=56) and could change or be strengthened in light of results from future, well‐controlled RCTs. Application of partly unsupervised respiratory muscle training seems to be feasible with acceptable adherence rates (e.g. 81% in Fry 2007. Unfortunately, due to lack of defined minimal clinically important differences for lung function tests in people with MS, the clinical relevance of our results on maximal inspiratory pressure following resistive inspiratory muscle training remains unclear. In addition, it is uncertain whether these effects translate to improved quality of life or a decrease in the number of pulmonary complications frequently seen in people with MS, such as pneumonia or atelectases, or a decrease in prescribed antibiotics.
Implications for research.
Future research investigating respiratory training in people with MS is needed in order to formulate recommendations based on high‐quality evidence regarding its application in clinical practice. For that purpose, numerous RCTs at low risk of bias should investigate the efficacy of various respiratory muscle training intervention types (e.g. inspiratory and expiratory respiratory muscle training or combinations; supervised versus unsupervised; telemedicine) with varying intensities. Specific attention should be given to reducing performance bias by blinding participants and personell to treatment allocations whenever possible and to reducing detection bias by blinding assessors to treatment allocation. It would also be necessary to determine the efficacy of respiratory muscle training according to disease severity. Although mildly affected patients might not have clinical problems with their respiratory system, their respiratory muscle strength and pulmonary functions are below the average of healthy controls, and these indicators are related to functional outcomes. For people with higher EDSS scores, effects on health‐related quality of life are relevant, as is the incidence of pulmonary complications or the use of antibiotics. Investigators should also assess body function level by measuring respiratory muscle strength and pulmonary functions, as these are important for understanding the possible underlying mechanisms of the intervention. Trials should perform these measurements in the long term, not only postintervention. Any sustained effects may be especially relevant for pulmonary complications, which none of the included studies reported and may require longer follow‐up periods than implemented to date.
Acknowledgements
We gratefully thank Andrea Fittipaldo for assisting with the literature search.
Appendices
Appendix 1. Keywords for searching the MS Group Specialised Register
{exercise therapy} OR {respiratory function} OR {respiratory muscle\*} OR {respiration} OR {cough} OR {breathing exercise\*} OR {pneumonia} OR {FVC} OR {FEV} OR {pimax} OR {pressure} OR {inspiration} OR {expiration} OR {respiratory complication\*} OR {pemax} OR {inspiratory muscle training } OR {expiratory muscle strength training}
Data and analyses
Comparison 1. Inspiratory muscle training.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PImax (cmH2O) | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | 6.50 [‐7.39, 20.38] |
| 2 PImax predicted (%) | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | 20.92 [6.03, 35.81] |
| 3 PEmax (cmH2O) | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐8.22 [‐26.20, 9.77] |
| 4 PEmax predicted (%) | 2 | 56 | Mean Difference (IV, Random, 95% CI) | 5.86 [‐10.63, 22.35] |
| 5 Fatigue Severity Scale | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.95, 0.39] |
Comparison 2. Expiratory muscle training.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PEmax (cmH2O) | 3 | 81 | Mean Difference (IV, Fixed, 95% CI) | 8.33 [‐0.93, 17.59] |
| 2 PImax (cmH2O) | 3 | 81 | Mean Difference (IV, Fixed, 95% CI) | 3.54 [‐5.04, 12.12] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fry 2007.
| Methods | Randomized controlled trial | |
| Participants | N = 46 (23/23) randomized, N = 41 (20/21) analyzed Inclusion criteria: aged > 18 years, ambulatory, clinically diagnosed MS Exclusion criteria: acute respiratory infection, oral temperature > 100ºF, unstable physical conditions not related to MS, current smoking history Experimental group (N = 20): EDSS, mean ± SD: 3.96 ± 1.80 Age, mean ± SD: 50 ± 9.1 years Women: 91% MS type (%): RR 52; SP 16; PP 21; PR 11 Time since diagnosis: — Control group (N = 21): EDSS, mean ± SD: 3.36 ± 1.47 Age, mean ± SD: 46.2 ± 9.4 years Women: 74% MS type (%): RR 72; SP 18; PP 5; PR 5 Time since diagnosis: — Country: USA |
|
| Interventions |
Experimental group: Home‐based inspiratory muscle training with a threshold inspiratory muscle trainer device Duration: 3 months Frequency: 2 sessions daily Repetitions and sets: 3 sets of 15 repetitions Resistance and progression: initial resistance (H2O) IMT set on 30% of pretest PImax; weekly progression based on baseline MIP, Borg 6‐20 RPE, and symptoms Control group: No treatment, called at 4, 8 and 10 weeks to control for complications and for changes in level of physical activity. |
|
| Outcomes | PImax, predicted PImax (%), PEmax, predicted PEmax (%), MVV, predicted MVV (%), FSS, FVC, predicted FVC (%), FEV1, predicted FEV1 (%), FEV1/FVC, predicted FEV1/FVC (%), FEF 25‐75%, predicted FEF 25‐75%, VC, predicted VC (%), impaired ventilatory muscle strength inspiratory, impaired ventilatory muscle strength expiratory, pulmonary disorder classification, FSS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote p 163: "participants were randomly placed into a home exercise I group or C group by date of enrolment in the study." |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information Comment: blinding participants and personnel not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote p 163: "The investigator who conducted the pre‐pulmonary and post‐pulmonary function tests was blinded to group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote p 163: "Of the 46 subjects who participated in the pre‐test session, two withdrew due to illness unrelated to a neurological exacerbation and three subjects no longer wished to participate in the study." Comment: unclear which participants withdrew from which group and why. |
| Selective reporting (reporting bias) | Unclear risk | No published study protocol available Comment: relevant outcomes, but not all (e.g., QoL, basic ADLs, cough efficacy), were reported. |
Gosselink 2000.
| Methods | Randomized controlled trial | |
| Participants | N = 21 randomized, N = 18 (9/9) analyzed Inclusion criteria: bedridden or wheelchair‐bound MS patients, clinically stable condition for at least 4 weeks (no recent infection or exacerbation) Exclusion criteria: — Experimental group (N = 9): EDSS, median (range): 8 (7‐9) Age, mean ± SD: 54 ± 13 years Women: 67% MS type: — Time since diagnosis, mean ± SD: 24 ± 15 years Control group (N = 9): EDSS, median (range): 8.5 (8‐9.5) Age, mean ± SD: 59 ± 14 years Women: 33% MS type: — Time since diagnosis, mean ± SD: 31 ± 13 years Country: Belgium |
|
| Interventions |
Experimental group: Expiratory contractions (60% PEmax) with the threshold adapted for expiratory loading Duration: 3 months Frequency: 2 sessions daily Repetitions and sets: 3 sets of 15 expiratory repetitions Resistance and progression: 60% of PEmax Control group: Breathing exercises to enhance maximal inspirations as routine part of physical therapy treatment |
|
| Outcomes | PImax, PEmax, PI (pulmonary index, cough efficacy), FVC, NFF (Neck Flexion Force), EDSS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote p 748: "were randomized by random numbers" |
| Allocation concealment (selection bias) | High risk | Quote p 748: "were randomized by random numbers" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information Comment: blinding participants and personnel not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information Comment: blinding participants and personnel not possible |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote p 748: "three patients dropped out form the study because of lack of cooperation" Comment: unknown how many patient were initially allocated to the experimental and control group |
| Selective reporting (reporting bias) | Unclear risk | No published study protocol available Comment: relevant outcomes, but not all (e.g., QoL, basic ADLs), were reported. |
Klefbeck 2003.
| Methods | Randomized controlled trial | |
| Participants | N = 16 (8/8) randomized, N = 15 (7/8)* analyzed Inclusion criteria: clinically diagnosed MS according to Poser criteria Exclusion criteria: EDSS score between 0.0‐6.0, pulmonary diseases, heart insufficiency, chronic pain, other diagnosis or disorder Experimental group (N = 7): EDSS, median (range): 7.5 (6.5‐8) Age, median (range): 46 years (37‐49) Women: 14% MS type: SP, PP Time since diagnosis, median (range): 12 years (3‐19) Control group (N = 8): EDSS, median (range): 8.0 (6.5‐9.0) Age, median (range): 52.5 years (38‐61) Women: 63% MS type: SP, PP Time since diagnosis, median (range): 20 years (12‐35) Country: Sweden |
|
| Interventions |
Experimental group: Home‐based inspiratory muscle training with a threshold inspiratory muscle trainer device Duration: 10 weeks Frequency: 2, 10 minute sessions, 3‐4 times a week Repetitions and sets: 3 sets of 10 repetitions Resistance and progression: initial resistance (H2O) IMT set on 40%‐60% of pretest PImax, and at the end of the training session was not to be perceived as more than 17 (very hard) on the Borg 6–20 RPE scale Control group: No specific feedback on the study; deep‐breathing exercises were a routine part of their ordinary physical treatment therapy |
|
| Outcomes | PImax, predicted PImax (%), PEmax, predicted PEmax (%), FSS, Borg 6‐20 RPE scale | |
| Notes | *based on additional data as provided by the authors (Rietberg 2016b) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote p 995: "Patients were randomized to a training (N = 8) or a control (N = 8) group." Comment: random component not described |
| Allocation concealment (selection bias) | Unclear risk | No information on method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information Comment: blinding participants and personnel not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote p 995: "one patient in the training group dropped out after 4 weeks, having failed to cooperate, and was excluded from data analysis." Comment: small sample with 1 dropout |
| Selective reporting (reporting bias) | Low risk | Table 2. Author information |
Mutluay 2007.
| Methods | Randomized controlled trial | |
| Participants | N = 40 (20/20) randomized and analyzed Inclusion criteria: clinically diagnosed MS according to Poser criteria Exclusion criteria: pulmonary disease, MS exacerbation, bulbar dysfunction Experimental group (N = 20): EDSS, mean ± SD: 4.85 ± 1.3 Age, mean ± SD: 40.3 ± 6 years Women: 60% MS type (%): RR: 20; RR: 25; SP: 55 Time since diagnosis, mean ± SD: 9.8 ± 5.6 years Control group (N = 20): EDSS, mean ± SD: 4.18 ± 1.7 Age, mean ± SD: 38.1 ± 7 years Women: 60% MS type (%): RR: 40; PP: 15; SP: 45 Time since diagnosis, mean ± SD: 9.0 ± 4.6 years Country: Turkey |
|
| Interventions |
Experimental group: Home‐based breathing‐enhanced upper extremity exercises according to Watchie Duration: 6 weeks Frequency: 30 min daily Repetitions and sets: — Resistance and progression: — Control group: No treatment; waiting list |
|
| Outcomes | FEV1, FEV1/FVC, PImax, PEmax, PDI, 6 MWT, Borg 6‐20 RPE | |
| Notes | Not included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote p 596: "using random number table" |
| Allocation concealment (selection bias) | Low risk | Quote p 596: "sealed in an opaque envelope; … (even: training, odd: control)" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information Comment: blinding participants and personnel not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote p 596: "data collectors who were all blinded to both groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Figure 1, Table 2 |
| Selective reporting (reporting bias) | Unclear risk | No published study protocol available |
Smeltzer 1996.
| Methods | Randomized controlled trial | |
| Participants | N = 20 (10/10) randomized, N = 15 (10/5) analyzed Inclusion criteria: clinically definite MS, PEmax between 45%‐60% of predicted Exclusion criteria: pulmonary diseases Total study group (N = 15) EDSS, range: 6.5‐9.5 Age: — MS type: — Experimental group (N = 10): Women: 91% Time since diagnosis, mean ± SD: 18.3 ± 6.6 years Control group (N = 5): Women: 81 Time since diagnosis, mean ± SD: 14.1 ± 6.6 years Country: USA |
|
| Interventions |
Experimental group: Expiratory training using a threshold training device Duration: 3 months Frequency: 2 sessions daily Repetitions and sets: 3 sets of 15 repetitions with 5 min rest between sets Resistance and progression: initial threshold level was PEmax and increased or decreased on the basis of observations of the ability to perform the training exercise and participant's report of difficulty doing so Control group: Sham training, using the same device without an expiratory training threshold load, configured to train the inspiratory muscles |
|
| Outcomes | PImax, PEmax | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote p 909: "patients who met inclusion criteria were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote p 909: "They were informed about possible blind assignment to a sham group." Comment: probably blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote p 909: "Twenty subjects with a definite diagnosis of MS were recruited for the study and randomly assigned to a training (n = 10) or sham training group (n = 10). However only 15 subjects completed enough of the study to provide data for analysis, 10 in the experimental group and 5 in the control or sham training group." |
| Selective reporting (reporting bias) | High risk | No published study protocol available Comment: only PImax and PEmax reported, which are not assumed to be the only outcomes, and not clinically relevant for patients |
Westerdahl 2016.
| Methods | Randomized controlled trial | |
| Participants | N = 52 (26/26) randomized, N = 48 (23/25) analyzed Inclusion criteria: mild‐moderate MS according to McDonald criteria, relapse‐free for at least 3 months prior to study entry, preserved ability to walk with or without use of walking devices Exclusion criteria: other diseases or conditions impacting functional ability, language or cognitive difficulties that could adversely influence the performance of lung function tests Experimental group (N = 23): EDSS, median: 5.0 Age, mean ± SD: 55 ± 12 years Female (N): 17 MS type (N): RR: 11; PP: 1; SP: 11 Time since diagnosis, mean ± SD: 24 ± 11 years Control group (N = 25): EDSS, median: 4.5 Age, mean ± SD: 56 ± 9 years Female (N): 18 MS type (N): RR: 9; PP: 1; SP: 15 Time since diagnosis, mean ± SD: 23 ± 11 years Country: Sweden |
|
| Interventions |
Experimental group: Expiratory training using a positive expiratory pressure device Duration: 2 months Frequency: 2 sessions daily Repetitions and sets: 3 sets of 10 repetitions with 30‐60 s rest between sets Resistance and progression: expiratory pressure of 10‐15 cmH2O, progression not reported Control group: No breathing exercises |
|
| Outcomes | PImax, predicted PImax (%), PEmax, predicted PEmax (%), FVC, VC, FEV1, FEV1/VC, PEF, SpO2, thoracic excursion, subjective breathing and coughing ability, EQ‐5D VAS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote p 2: "a computer‐generated list was used to randomize eligible patients." |
| Allocation concealment (selection bias) | Low risk | Quote p 2 "This list had been administered by an independent secretary, who printed notes showing group assignment and put them in sequentially numbered, sealed, nontransparent envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information Comment: blinding participants and personnel not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote p 2 "single‐blinded, parallel‐group trial" p 4 "Next, a physiotherapist experienced in neurology and blinded to group allocation assessed ..." For patient reported outcomes: p 4 "… and each patient answered self‐reported questionnaires covering descriptive data, ability to breathe and cough, physical activity, and health‐related quality of life." Comment: participant‐reported outcomes were measured; knowledge of allocation could influence people's perception. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote p 5 "Reasons for withdrawal after randomization in the breathing group (n=3) and the control group (n=1) were other morbidities, failure to cooperate or unwillingness to perform the follow‐up." Unclear whether the reasons for dropout differed between the control and experimental group. Reasons for dropout could be related to the intervention. No long‐term outcome measurement |
| Selective reporting (reporting bias) | High risk | The study protocol is not available. Based on the information in clinicaltrials.gov, it is assumed that more outcomes were measured, but not reported Clinicaltrials.gov, no. NCT01774201: "The hypothesis is that there is a relationship between respiratory function, walking capacity and fatigue and that daily deep breathing exercise during two months will improve respiratory function, walking capacity and fatigue." |
ADL: activities of daily living; EDSS: Expanded Disability Status Scale; FEF: forced expiratory flow; FEV1: forced expiratory volume in 1 second; FSS: Fatigue Severity Scale; FVC: forced vital capacity; IMT: inspiratory muscle training; MIP: maximal inspiratory pressure; MS: multiple sclerosis; MVV: maximum voluntary ventilation; PEF: peak expiratory flow; PEmax: maximum expiratory pressure; PImax: maximum inspiratory pressure; PP: primary progressive; PR: progressive relapsing; QoL: quality of life; RR: relapsing remitting; RPE: rating of perceived exertion;SD: standard deviation; SP: secondary progressive; VC: vital capacity.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chiara 2006 | Not an RCT |
| Chiara 2007 | Not an RCT |
| Feltman 2013 | No respiratory training |
| Hansen 2015 | No respiratory training |
| Natour 2012 | Not an RCT |
| Olgiati 1989 | Not an RCT |
| Pfalzer 2011 | No respiratory outcomes |
| Rampello 2007 | No respiratory training |
| Ray 2013 | Not an RCT |
RCT: randomized controlled trial.
Characteristics of ongoing studies [ordered by study ID]
NCT00856518.
| Trial name or title | Expiratory muscle training for persons with neurodegenerative disease |
| Methods | Allocation: randomized Intervention model: parallel assignment Masking: double‐blind (participant, outcomes assessor) Primary purpose: treatment |
| Participants | Parkinson's disease/multiple sclerosis Inclusion criteria: Multiple sclerosis participants: diagnosis of primary, secondary, or relapsing‐remitting MS by a neurologist; over 85% of the patient populations that come from the study sites demonstrate relapsing‐remitting MS with an average relapse frequency of once every 3 years Parkinson's disease (PD) participants: Hoehn & Yahr, stage II and III as indicated by certified movement disorders neurologist All participants: aged 35‐80 years; non‐smoking for at least the previous 5 years. No history of head and neck cancer, asthma or COPD, untreated hypertension. Sufficient facial muscle strength so as to achieve and maintain adequate lip closure around a circular mouthpiece. Cognition within normal limits as determined by the: Mini Mental Status Exam (MMSE; 1975). No neurological (other than MS or PD) condition that adversely affects respiratory muscle or gas exchange system. Reduced MEPs compared to published normative data for age and sex. Reduced expiratory peak flow rates (6‐8 L/s for young to middle‐aged adults and 3.6 L/s for 65 and older) during voluntary cough production for age and sex (Smith Hammond 2001). Participant report of symptoms related to swallow impairment Exclusion criteria: DBS, COPD, asthma, smoking within preceding 5 years |
| Interventions |
Experimental group: Arm 1: EMST. The experimental group receives 5 weeks of expiratory muscle strength training (EMST) using a positive pressure threshold device. Pressure threshold device (Expiratory Muscle Strength Trainer) targeted at increase muscle force generation of expiratory and submental muscles. Control group: Arm 2: sham group. The sham group undergoes the same 5‐week EMST exercise as the experimental group using the same device but without a spring for minimal pressure load on the target muscle group |
| Outcomes | All outcomes measured at baseline and again after 5‐week EMST exercise.
|
| Starting date | 2009, finished 2014, no peer‐reviewed published data |
| Contact information | Dr Janis Daly, North Florida/South Georgia Veterans Health System phone: (+1) 352‐376‐1611 ext 5223 Email: janis.daly@neurology.ufl.edu |
| Notes | NCT00856518 . No data on group composition provided |
NCT02104492.
| Trial name or title | Clinical research on the effect of a 12‐week respiratory muscles training program in persons with relapsing‐remitting multiple sclerosis |
| Methods | Study type: interventional Study design: allocation: randomized Intervention model: parallel assignment Masking: single blind (investigator) |
| Participants | Relapsing‐remitting multiple sclerosis Inclusion criteria: aged 18 or older with diagnosis of relapsing‐remitting multiple sclerosis according to revised McDonald criteria (Polman 2005). Have mild or moderate impairment of gait according to Hauser ambulatory index. The score 1 and 5 will be included (both values inclusive). Participants who have read, understood, signed and dated the informed consent form. Exclusion criteria: disability caused by other diseases; clinically relevant cognitive or linguistic disorders that impede participants from filling in the questionnaires by themselves; used medication with corticosteroids within the last month (or 30 days) prior to study day 1; recent outbreak (in previous month) not stabilized prior to inclusion in the study |
| Interventions |
Experimental group: A 12‐week respiratory muscles training program (RMTP) with ORYGEN Dual device and peripheric resistive muscle training programme. Every session will last 50 min and be performed under supervision of a physiotherapist. It will comprise 30 min of progressive aerobic training followed by peripheric resistive muscle training, which will be supplemented by a 12‐week Respiratory Muscles Training Program (RMTP) with ORYGEN Dual device for 20 min. Participants will be instructed to maintain adequate inspiration and expiration while using the Origen‐Dual valve at a rate of 15‐20 breaths/minute. Participants will perform 5 sets of 10 repetitions followed by 1‐2 minutes of unloaded recovery breathing off the device, once a day, 2 days per week, for 12 weeks. The respiratory muscles training will start at 30% of MIP achieved at baseline and increased by 5% each week to reach 60% of the baseline assessment MIP. Control group: Peripheric resistive muscle training programme and health education program. Every session will be comprised of 30 min of progressive aerobic training followed by peripheric resistive muscle training and a health education program. |
| Outcomes |
Primary outcome measures (at baseline and 12 weeks):
Secondary outcome measures (at baseline and 12 weeks unless otherwise specified):
|
| Starting date | 2014 |
| Contact information | Rocío Martín Valero, Ph D, University of Malaga University of Malaga Malaga, Spain |
| Notes | NCT00856518 |
NCT02726672.
| Trial name or title | Fatigue and inspiratory muscles training against resistance in patients with multiple sclerosis with severe disabilities |
| Methods | Study type: interventional Study design: allocation: randomized Intervention model: parallel assignment Masking: open label |
| Participants |
Inclusion criteria: remitting or progressive multiple sclerosis defined by McDonald criteria revised in 2005, with an EDSS between 6 and 7.5; aged 18‐65 years; able to maintain the Powerbreathe; no flare‐ups in previous 6 weeks; at least 4 weeks since a corticoid bolus; at least 6 weeks since a botulinum toxin injection; at least 4 weeks since a pulmonary infection; Questionnaire EMIF‐SEP ≥ 55; informed consent; beneficiaries or affiliates of a social security regimen Exclusion criteria: neurologic antecedents other that multiple sclerosis; respiratory disorders other than those induced by multiple sclerosis; orthopaedic, cardiac, and rheumatologic invalidating antecedents; comprehension or cognitive disorders impeding the realization of rehabilitation; pregnant women; under legal guardianship, or safeguard of justice; participating or planning to participate within 3 months in another clinical research project |
| Interventions |
Experimental group: Respiratory rehabilitation respiratory rehabilitation: Powerbreathe training of the inspiratory muscles at home twice a day (2 sessions of 30 inspirations/day) during 10 weeks Control group: No respiratory rehabilitation |
| Outcomes |
Primary outcome measures (at 3 months):
Secondary outcome measures (at 3 months):
|
| Starting date | 2014 |
| Contact information | Amélie Lansiaux, MD, PhD 003320225269 lansiaux.amelie@ghicl.net Hospital Group of the Catholic Institute of Lille Lomme, France, 59462 |
| Notes | NCT02726672 |
COPD: chronic obstructive pulmonary disease; DBS: deep brain stimulation; DYMUS: DYsphagia in MUltiple Sclerosis; EMIF‐SEP: French version of Fatigue Impact Scale; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; MEP: maximal expiratory pressure; MIP: maximal inspiratory pressure; MS: multiple sclerosis.
Differences between protocol and review
The following aspects in the review differ from the protocol.
1. In the Types of interventions section, we further specified the intervention and specifically searched for studies that applied inspiratory muscle training, expiratory muscle training, and/or breathing exercises. Thus, we excluded physical therapy, fitness training and endurance training.
2. In the Secondary outcomes section we added 'fatigue', measured with the Fatigue Severity Scale (FSS), as well as 'adverse events'.
3. In the Measures of treatment effect section, we deleted the text concerning subgroup analyses depending on the type of control group, due to the lack of a large number of studies.
4. In the Measures of treatment effect section, we deleted information concerning statistical methods for pooling dichotomous data because of the continuous nature of the reported study data.
5. In the Measures of treatment effect and Data synthesis sections, we deleted mention of using the standardized mean difference (SMD) because continuous data were measured in the same way. Therefore, we calculated only mean differences (MD).
6. In the Dealing with missing data, we analyzed all available data, as it was unclear whether missing study data were random or differential.
Contributions of authors
| Roles and responsibilities | |
| Draft the protocol | M Rietberg, J Veerbeek, E van Wegen, R Gosselink, G Kwakkel |
| Develop and run the search strategy | M Rietberg with the assistance of TSC for the Cochrane MS Review Group |
| Obtain copies of studies | M Rietberg, J Veerbeek |
| Select which studies to include (2 people) | M Rietberg, J Veerbeek |
| Extract data from studies (2 people) | J Veerbeek, M Rietberg |
| Enter data into RevMan | J Veerbeek, M Rietberg |
| Carry out the analysis | J Veerbeek, M Rietberg, E van Wegen |
| Interpret the analysis | J Veerbeek, M Rietberg, R Gosselink, G Kwakkel, E van Wegen |
| Draft the final review | M Rietberg, J Veerbeek, E van Wegen |
| Update the review | M Rietberg, E van Wegen |
Declarations of interest
M Rietberg: none known.
J Veerbeek: none known.
R Gosselink: none known.
G Kwakkel: none known.
E van Wegen: none known.
New
References
References to studies included in this review
Fry 2007 {published data only}
- Fry DK, Pfalzer LA, Chokshi AR, Wagner MT, Jackson ES. Randomized control trial of effects of a 10‐week inspiratory muscle training program on measures of pulmonary function in persons with multiple sclerosis. Journal of Neurological Physical Therapy 2007;31(4):162‐72. [DOI] [PubMed] [Google Scholar]
Gosselink 2000 {published data only}
- Gosselink R, Kovacs L, Ketelaer P, Carton H, Decramer M. Respiratory muscle weakness and respiratory muscle training in severely disabled multiple sclerosis patients. Archives of Physical Medicine and Rehabilitation 2000;81(6):747‐51. [DOI] [PubMed] [Google Scholar]
Klefbeck 2003 {published data only}
- Klefbeck B, Hamrah Nedjad J. Effect of inspiratory muscle training in patients with multiple sclerosis. Archives of Physical Medicine and Rehabilitation 2003;84(7):994‐9. [DOI] [PubMed] [Google Scholar]
Mutluay 2007 {published data only}
- Mutluay FK, Demir R, Ozyilmaz S, Caglar AT, Altintas A, Gurses HN. Breathing‐enhanced upper extremity exercises for patients with multiple sclerosis. Clinical Rehabilitation 2007;21(7):595‐602. [DOI] [PubMed] [Google Scholar]
Smeltzer 1996 {published data only}
- Smeltzer SC, Lavietes MH, Cook SD. Expiratory training in multiple sclerosis. Archives of Physical Medicine and Rehabilitation 1996;77(9):909‐12. [DOI] [PubMed] [Google Scholar]
Westerdahl 2016 {published data only}
- Westerdahl E, Wittrin A, Kånåhols M, Gunnarsson M, Nilsagård Y. Deep breathing exercises with positive expiratory pressure in patients with multiple sclerosis ‐ a randomized controlled trial. Clinical Respiratory Journal 2016;10(6):698‐706. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chiara 2006 {published data only}
- Chiara T, Martin AD, Davenport PW, Bolser DC. Expiratory muscle strength training in persons with multiple sclerosis having mild to moderate disability: effect on maximal expiratory pressure, pulmonary function, and maximal voluntary cough. Archives of Physiocal Medicine and Rehabilitation 2006;87(4):468‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiara 2007 {published data only}
- Chiara T, Martin D, Sapienza C. Expiratory muscle strength training: speech production outcomes in patients with multiple sclerosis. Neurorehabilitation and Neural Repair 2007;21(3):239‐49. [DOI] [PubMed] [Google Scholar]
Feltman 2013 {published data only}
- Feltham MG, Collett J, Izadi H, Wade DT, Morris MG, Meaney AJ, et al. Cardiovascular adaptation in people with multiple sclerosis following a twelve week exercise programme suggest deconditioning rather than autonomic dysfunction caused by the disease results from a randomized controlled trial. European Journal of Physical Rehabilitation Medicine 2013;49(6):765‐74. [PubMed] [Google Scholar]
Hansen 2015 {published data only}
- Hansen D, Wens I, Keytsman C, Verboven K, Dendale P, Eijnde BO. Ventilatory function during exercise in multiple sclerosis and impact of training intervention: cross‐sectional and randomized controlled trial. European Journal of Physical Rehabilitation Medicine 2015;51(5):557‐68. [PubMed] [Google Scholar]
Natour 2012 {published data only}
- Natour Y, Marie B, Aljunidy L. The respiratory muscle capabilities of Jordanian patients with multiple sclerosis. Journal of Voice 2012;26(6):e15‐18. [DOI] [PubMed] [Google Scholar]
Olgiati 1989 {published data only}
- Olgiati R, Girr A, Hügi L, Haegi V. Respiratory muscle training in multiple sclerosis: a pilot study. Schweizer Archiv für Neurologie und Psychiatrie 1989;140(1):46‐50. [PubMed] [Google Scholar]
Pfalzer 2011 {published data only}
- Pfalzer L, Fry D. Effects of a 10‐week inspiratory muscle training program on lower‐extremity mobility in people with multiple sclerosis: a randomized controlled trial. International Journal of MS Care 2011;13(1):32‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rampello 2007 {published data only}
- Rampello A, Franceschini M, Piepoli M, Antenucci R, Lenti G, Olivieri D, et al. Effect of aerobic training on walking capacity and maximal exercise tolerance in patients with multiple sclerosis: a randomized crossover controlled study. Physical Therapy 2007;87(5):545‐55. [DOI] [PubMed] [Google Scholar]
Ray 2013 {published data only}
- Ray AD, Udhoji S, Mashtare TL, Fisher NM. A combined inspiratory and expiratory muscle training program improves respiratory muscle strength and fatigue in multiple sclerosis. Archives of Physical Medicine and Rehabilitation 2013;94(10):1964‐70. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00856518 {unpublished data only}
- NCT00856518. Expiratory muscle training for persons with neurodegenerative disease [Expiratory muscle training for persons with neurodegenerative disease]. clinicaltrials.gov/ct2/show/record/NCT00856518?term=NCT00856518&rank=1 (first received 3 March 2009).
NCT02104492 {unpublished data only}
- NCT02104492. Effects of a 12‐week respiratory muscles training program in persons with relapsing‐ remitting multiple sclerosis [Clinical research on the effect of a 12‐week respiratory muscles training program in persons with relapsing‐remitting multiple sclerosis]. clinicaltrials.gov/ct2/show/record/NCT02104492?term=NCT02104492&rank=1 (first received 27 March 2014).
NCT02726672 {unpublished data only}
- NCT02726672. Fatigue and inspiratory muscles training in patients with multiple sclerosis [Fatigue and inspiratory muscles training against resistance in patients with multiple sclerosis with severe disabilities]. clinicaltrials.gov/ct2/show/record/NCT02726672?term=NCT02726672&rank=1 (first received 22 March 2016).
Additional references
Berlowitz 2013
- Berlowitz DJ, Tamplin J. Respiratory muscle training for cervical spinal cord injury. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD008507.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bosnak‐Guclu 2012
- Bosnak‐Guclu M, Gunduz AG, Nazliel B, Irkec C. Comparison of functional exercise capacity, pulmonary function and respiratory muscle strength in patients with multiple sclerosis with different disability levels and healthy controls. Journal of Rehabilitation Medicine 2012;44(1):80‐6. [DOI] [PubMed] [Google Scholar]
EuroQol 1990
- EuroQol Group. EuroQol: a new facility for the measurement of health‐related quality of life. HealthPolicy (New York) 1990;16(3):199–208. [DOI] [PubMed] [Google Scholar]
Ferreira 2016
- Ferreira G, Costa A, Plentz R, Coronel C, Sbruzzi G. Respiratory training improved ventilatory function and respiratory muscle strength in patients with multiple sclerosis and lateral amyotrophic sclerosis: systematic review and meta‐analysis. Physiotherapy 2016;102(3):221‐8. [DOI] [PubMed] [Google Scholar]
Foglio 1994
- Foglio K, Clini E, Facchetti D, Vitacca M, Marangoni S, Bonomelli M, et al. Respiratory muscle function and exercise capacity in multiple sclerosis. European Respiratory Journal 1994;7(1):23‐8. [DOI] [PubMed] [Google Scholar]
Gomes‐Neto 2016
- Gomes‐Neto M, Saquetto MB, Silva CM, Carvalho VO, Ribeiro N, Conceição CS. Effects of respiratory muscle training on respiratory function, respiratory muscle strength, and exercise tolerance in patients post stroke: a systematic review with meta‐analysis. Archives of Physical Medicine and Rehabilitation 2016;97(11):1994‐2001. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of InterventionsVersion 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Koch‐Henriksen 2010
- Koch‐Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurology 2010;9(5):520‐32. [DOI] [PubMed] [Google Scholar]
Laciuga 2014
- Laciuga H, Rosenbek JC, Davenport PW, Sapienza CM. Functional outcomes associated with expiratory muscle strength training: narrative review. Journal of Rehabilitation Research & Development 2014;51(4):535‐46. [DOI] [PubMed] [Google Scholar]
Martín‐Valero 2014
- Martín‐Valero R, Zamora‐Pascual N, Armenta‐Peinado JA. Training of respiratory muscles in patients with multiple sclerosis: a systematic review. Respiratory Care 2014;59(11):1764‐72. [DOI] [PubMed] [Google Scholar]
McDonald 2001
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of Neurology 2001;50(1):121‐7. [DOI] [PubMed] [Google Scholar]
Motl 2011a
- Motl RW, Goldman M. Physical inactivity, neurological disability, and cardiorespiratory fitness in multiple sclerosis. Acta Neurologica Scandinavica 2011;123(2):98‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Motl 2011b
- Motl RW, McAuley E, Wynn D, Suh Y, Weikert M. Effects of change in fatigue and depression on physical activity over time in relapsing‐remitting multiple sclerosis. Psychology, Health & Medicine 2011;16(1):1‐11. [DOI] [PubMed] [Google Scholar]
Neder 1999
- Neder JA, Andreoni S, Lerario MC, Nery LE. Reference values for lung function tests. II. maximal respiratory pressures and voluntary ventilation. Brazilian Journal of Medical and Biological Research 1999;32(6):719‐27. [DOI] [PubMed] [Google Scholar]
Polman 2005
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Annals of Neurology 2005;58(6):840‐6. [DOI] [PubMed] [Google Scholar]
Poser 1983
- Poser C, Paty D, Scheinberg L. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13(3):227‐31. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reyes 2013
- Reyes A, Ziman M, Nosaka K. Respiratory muscle training for respiratory deficits in neurodegenerative disorders: a systematic review. Chest 2013;143(5):1386‐94. [DOI] [PubMed] [Google Scholar]
Rietberg 2010
- Rietberg MB, Wegen EEH, Kwakkel G. Measuring fatigue in patients with multiple sclerosis: reproducibility, responsiveness and concurrent validity of three Dutch self‐report questionnaires. Disability and Rehabilitation 2010;32(22):1870–6. [DOI] [PubMed] [Google Scholar]
Rietberg 2016a
- Rietberg, MB. [Request for additional results [personal communication]]. Email to: R.Gosselink 11‐02‐2016.
Rietberg 2016b
- Rietberg, MB. [Request for additional results [personal communication]]. Email to: B. Klefbeck 11‐02‐2016.
Rietberg 2016c
- Rietberg, MB. [Request for additional results [personal communication]]. Email to: SC Smeltzer 11‐02‐2016.
Schumacher 1965
- Schumacher GA, Beebe GW, Kibler RF, Kurland LT, Kurtzke JF, McDowell F, et al. Problems of experimental trials of therapy in multiple sclerosis. Annals of the New York Academy of Science 1965;31(122):552‐68. [DOI] [PubMed] [Google Scholar]
Smeltzer 1988
- Smeltzer SC, Utell MJ, Rudick RA, Herndon RM. Pulmonary function and dysfunction in multiple sclerosis. Archives of Neurology 1988;45(11):1245‐9. [DOI] [PubMed] [Google Scholar]
Smith Hammond 2001
- Smith Hammond CA, Goldstein LB, Zajac DJ, Gray L, Davenport PW, Bolser DC. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology 2001;56(4):502‐6. [DOI] [PubMed] [Google Scholar]
Stuke 2009
- Stuke K, Flachenecker P, Zettl UK, Elias WG, Freidel M, Haas J, et al. Symptomatology of MS: results from the German MS Registry. Journal of Neurology 2009;256(11):1932‐5. [DOI] [PubMed] [Google Scholar]
Sumelahti 2010
- Sumelahti ML, Hakama M, Elovaara I, Pukkala E. Causes of death among patients with multiple sclerosis. Multiple Sclerosis 2010;16(12):1437‐42. [DOI] [PubMed] [Google Scholar]
Toomey 2012
- Toomey E, Coote SB. Physical rehabilitation interventions in nonambulatory people with multiple sclerosis: a systematic review. International Journal of Rehabilitation Research 2012;35(4):281‐91. [DOI] [PubMed] [Google Scholar]
WHO 2001
- World Health Organization. International Classification of Function, Disability and Health. Geneva: World Health Organization, 2001. [Google Scholar]
Wilson 1984
- Wilson SH, Cooke NT, Edwards RH, Spiro SG. Predicted normal values for maximal respiratory pressures in Caucasian adults and children. Thorax 1984;39(7):535‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]


