Abstract
Background
Mobile phone messaging, such as Short Message Service (SMS) and Multimedia Message Service (MMS), has rapidly grown into a mode of communication with a wide range of applications, including communicating the results from medical investigations to patients. Alternative modes of communication of results include face‐to‐face communication, postal messages, calls to landlines or mobile phones, through web‐based health records and email. Possible advantages of mobile phone messaging include convenience to both patients and healthcare providers, reduced waiting times for health services and healthcare costs.
Objectives
To assess the effects of mobile phone messaging for communicating results of medical investigations, on people's healthcare‐seeking behaviour and health outcomes. Secondary objectives include assessment of participants' evaluation of the intervention, direct and indirect healthcare costs and possible risks and harms associated with the intervention.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2009, Issue 2), MEDLINE (OvidSP) (January 1993 to June 2009), EMBASE (OvidSP) (January 1993 to June 2009), PsycINFO (OvidSP) (January 1993 to June 2009), CINAHL (EbscoHOST) (January 1993 to June 2009), LILACS (January 1993 to June 2009) and African Health Anthology (January 1993 to June 2009). We also reviewed grey literature (including trial registers) and reference lists of articles.
Selection criteria
We included randomised controlled trials (RCTs), quasi‐randomised controlled trials (QRCTs), controlled before‐after (CBA) studies, or interrupted time series (ITS) studies with at least three time points before and after the intervention. We included studies assessing mobile phone messaging for communicating results of medical tests, between a healthcare provider or 'treatment buddy' and patient. We only included studies in which it was possible to assess the effects of mobile phone messaging independent of other technologies or interventions.
Data collection and analysis
Two review authors independently assessed all studies against the inclusion criteria, with any disagreements resolved by a third review author. Study design features, characteristics of target populations, interventions and controls, and results data were extracted by two review authors and confirmed by a third. Primary outcomes of interest were health outcomes and healthcare utilisation as a result of the intervention. We also considered patients' and providers' evaluation of the intervention, perceptions of safety, costs and potential harms or adverse effects of the intervention.
Main results
We included one randomised controlled trial involving 2782 participants. The study investigated the effects of mobile phone messaging in alleviating anxiety in women waiting for prenatal biochemical screening results for Down syndrome, by providing fast reporting of results before a follow‐up appointment. The study measured health outcomes using the Spielberger State‐Trait Anxiety Inventory (STAI), which includes a scale (20 to 80 points, higher score indicates higher anxiety) to describe how the respondent feels at a particular moment in time (state anxiety). The study, which was at high risk of bias, found that women who had received their test result early by text message had a mean anxiety score 2.48 points lower than women who had not yet received their result (95% CI ‐ 8.79 to 3.84). Women with a serum‐negative test result receiving their result early had a mean anxiety score 5.3 points lower (95% CI ‐ 5.99 to ‐4.61) than women in the control group. Women with a serum‐positive test result receiving their result early by text message had a mean anxiety score 1.2 points higher (95% CI ‐ 3.48 to 5.88) than women in the control group.The evidence was of low quality due to high risk of bias in the included study, and the fact that the evidence comes from one study only. The study did not report on other outcomes of interest, such as patient satisfaction, adverse events or cost.
Authors' conclusions
We found very limited evidence of low quality that communicating results of medical investigations by mobile phone messaging may make little or no difference to women's anxiety overall or in women with positive test results, but may reduce anxiety in women with negative test results. However, with only one study included in this review, this evidence is insufficient to inform recommendations at this time. More research is needed on the effectiveness and user evaluation of these interventions. In particular, more research should be conducted into the potential risks and limitations of these interventions.
Keywords: Female, Humans, Pregnancy, Cell Phone, Text Messaging, Anxiety, Anxiety/psychology, Down Syndrome, Down Syndrome/diagnosis, Prenatal Diagnosis, Prenatal Diagnosis/psychology, Randomized Controlled Trials as Topic
Plain language summary
Mobile phone messaging for communicating results of medical investigations
Mobile phones offer a way to communicate information quickly through simple, short text messages. This review studied whether mobile phone applications such as Short Message Service (SMS) and Multimedia Message Service (MMS) can be useful to send information to patients about their test results. We also looked at possible risks of communicating in this way. Our review found only one study evaluating the use of mobile phone messaging for communicating results of medical investigations. This study was at high risk of bias. The study suggested that the early communication of an antenatal screen test result by text messaging would not result in a difference in the anxiety scores of all pregnant women (irrespective of the test result) or when their test result is positive, however may reduce anxiety in pregnant women when their test result is negative. The usefulness of mobile phone messaging in other situations, or potential negative consequences, are not yet known.
Summary of findings
Summary of findings for the main comparison. Mobile phone messaging for communicating results of medical investigations.
| Early communication of result from prenatal serum screening for Down syndrome by mobile phone messaging compared to standard care | ||||
| Patient or population: Pregnant women undergoing prenatal serum screening for Down syndrome Settings: One district general hospital in Taiwan Intervention: The test result from prenatal screening is communicated early, i.e. before the scheduled clinic appointment, by mobile phone messaging Comparison: The test result from prenatal screening is communicated directly only at the time of the scheduled clinic appointment | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | |||
| Standard care | Mobile phone messaging | |||
| State anxiety score before scheduled clinic appointment (that is, when the intervention group had already received the test result by SMS) | ||||
| Overall effect (i.e. irrespective of screening result) | The mean anxiety score for the control group was 39.2 (SD 10.2). | The overall mean state anxiety score in the intervention group was 2.48 lower (8.79 lower to 3.84 higher). | 2782 (1 study) | ⊕⊕⊝⊝ low1 |
| Serum‐negative group | The mean anxiety score for the control group was 39.1 (SD 10.1). | The mean state anxiety score for the serum‐negative group in the intervention group was 5.30 lower (5.99 to 4.61 lower). | 2673 (1 study) | ⊕⊕⊝⊝ low1 |
| Serum‐positive group | The mean anxiety score for the control group was 42.9 (SD 11.5). | The mean state anxiety score for the serum‐positive group in the intervention group was 1.20 higher (3.48 lower to 5.88 higher). | 109 (1 study) | ⊕⊕⊝⊝ low1 |
| Other outcomes | ||||
| Health‐seeking behaviour | Not measured | |||
| Patient's evaluation of the intervention (including perceptions of safety) | Not measured | |||
| Harms & adverse effects | Not measured | |||
| Costs | Not measured | |||
| *The basis for the assumed risk (the mean control group risk across studies) is provided above. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
1 High or unclear risk in three of the six risk of bias domains (serious limitations in study design)
Background
Mobile phone messaging is an important means of human communication globally. Mobile phone penetration is rapidly increasing particularly in the Asia‐Pacific region, with 90% of the global and 80% of rural population having access to a mobile network in 2010. The number of subscriptions in 2010 reached 5.3 billion, representing a 76.2% global penetration rate (ITU 2010). The penetration rates are 70% to 90% in high‐income countries, with a similar rate of increase across all socio‐economic groups (Atun 2006).
Most digital mobile phones provide Short Message Service (SMS), also known as text messaging, and Multimedia Message Service (MMS) for transmitting graphics, video clips and sound files. SMS, in particular, has rapidly developed into a powerful communication medium, particularly among young adults. The total number of text messages sent globally tripled between 2007 and 2010, from an estimated 1.8 trillion to 6.1 trillion, with about 200,000 messages sent every second (ITU 2010). These short messages, where up to 160 characters of text are sent from the Internet or from a mobile phone to one or several mobile phones, could provide an important, inexpensive medium of communication. The terms text message, text, or txt are more commonly used in North America, the UK, Spain and the Philippines, while in many other countries the term SMS is used. In this review we will use the term ‘text messaging’ to refer to the use of SMS only, distinguishing it from the term ‘mobile phone messaging’, which encompasses both SMS and MMS. Increasingly, the latter term also refers to mobile e‐mail and ‘instant messaging’ delivered to the mobile phone.
Text messages, compared to other communication channels, have the advantage of instant transmission and low cost. There is also a smaller chance of being misplaced compared to print materials, and of being invasive to daily lives compared to phone calls (Kaplan 2006). Features such as ubiquity, mobility, direct and instantaneous access and direct communication offer the possibility of using mobile phones for health information transfer (Atun 2006). A literature review on the use of mobile phones in health care has demonstrated the wide application and potential of mobile phones to: increase access to health care; enhance efficiency of service delivery; improve diagnosis, treatment and rehabilitation; and support public health programmes (Atun 2006; Car 2008b). Mobile phone messaging has, for example, been used to provide appointment reminders (Bos 2005), to improve patient compliance with medications (Fairley 2003; Marquez Contreras 2004; Vilella 2004), to monitor chronic conditions (Ferrer‐Roca 2004; Kwon 2004; Ostojic 2005) and to provide psychological support (Bauer 2003; Franklin 2003). Mobile phones have also been used in managing communicable diseases (e.g. in contact tracing and partner notification for sexually transmitted illnesses (Newell 2001)) and in health promotion programmes (e.g. in smoking cessation (Obermayer 2004; Rodgers 2005)). Furthermore, the use of mobile phones has been shown to improve service utilization among population groups such as teenagers and young adult males who do not typically use health services, by providing the opportunity to remotely access care providers for advice (Atun 2006b). However, for older adults, some of whom are less able or willing to use mobile phones, the effect on improved service utilization could be limited (Atun 2006b).
Challenges in using mobile phone applications in health care include incomplete coverage of mobile networks across regions, lack of standards, and possible information overload (Adler 2007).
This review is part of a series of four reviews which aim to determine the effects of mobile phone messaging in improving the processes of healthcare service delivery and service utilization.
We divided the reviews into four areas based on the specific interventions and related outcomes:
Mobile phone messaging for communicating results of medical investigations (this review);
Mobile phone messaging for preventive health care (Vodopivec‐Jamsek 2008);
Mobile phone messaging reminders for attendance at healthcare appointments (Car 2008);
Mobile phone messaging for facilitating self‐management of long‐term illnesses (de Jongh 2008).
Description of the condition
In this review, we include all conditions that may require medical investigation. Medical investigations can be defined as tests used for screening, diagnosis and monitoring of disease. Some examples of medical investigations are: blood, urine and stool tests; medical imaging; and medical radiology.
Description of the intervention
For communicating results of medical investigations, seven possible modes of communication are: face‐to‐face, postal message, call to landline, call to mobile, via web‐based electronic health records (EHR), email and SMS/MMS. Basic characteristics and a comparison with alternative modes of communication are outlined in Table 2 (adopted from Atun 2006). Although the most common route of communication is from the health provider to the patient, other routes are possible to enhance access, such as from a laboratory to the health professional in a rural clinic.
1. Characteristics of communication modes.
| Face‐to‐face | Postal Letter | Call to Landline | Call to Mobile | Web Based (EHR) | E‐mail | SMS / MMS | |
| Immediacy | Slow: Requires a visit to provider | Slow: 2 days | Immediate: If person at home. Return call may be necessary | Immediate: If person answers (more likely than landline) Return call may be necessary | Immediate: | Immediate Or stored | Immediate Or stored |
| Privacy and Confidentiality | High: Personal communication | High: Personally addressed | Low: Confidentiality prevents message being left as others may answer or retrieve it | High: Personal device enables possibility of message being left | Moderate: Personal / public device? | Moderate: Personal / public device? | High if Personal device. |
| Likelihood of misinterpretation | Low | Moderate | Low: Patient can request immediate clarification | Low: Patient can request immediate clarification | Moderate | Moderate | Moderate |
| Delivery confirmation | N/A | Yes: at significant expense | Unnecessary if call answered. No if message left | Unnecessary if call answered. No if message left | N/A | Yes | Yes |
| Cost | High | Moderate | Low | Moderate | Low | Low | Low |
Some applications of SMS/MMS technology reported to date in high‐income countries include: communicating the results of in‐vitro diagnostic tests, such as blood or microbiology tests (Bradbeer 2003); and radiological imaging such as breast cancer screening (Lamont 2005). In low‐income countries the applications are potentially more diverse, as there are greater barriers to accessing healthcare facilities. Some applications reported to date include sending results to clinics in rural areas more efficiently, and expediting the communication of occupational health examination results of foreign workers to their employers (Atun 2006).
How the intervention might work
Effective communication involves accurate and timely transmission of result to correct recipients, securing privacy and confidentiality, and using strategies to minimise misunderstanding or misinterpretation of the results. The healthcare provider should also ensure that appropriate follow‐up actions are taken once the result is known, such as further investigation, change of treatment, or setting a new date for review and an explanation of what the result means.
Traditional approaches to communicating results of medical investigations and diagnoses to patients often require patients to visit the healthcare provider and collect the results in person. In circumstances where visiting the healthcare provider is inconvenient for the patient, for example, because there are significant transportation costs or the patient's health status is poor, SMS/MMS interventions are likely to result in reduced waiting times and cost‐savings for patients and healthcare providers, increased convenience and satisfaction, and an improved access to services (Lovitt 2005; Pal 1998). Sending results by SMS/MMS is faster than by other means and adheres to privacy and confidentiality requirements if the mobile phone is a personal device and the contact details in the health records are accurate.
Acceptability and risks of the intervention
One study related to patient preferences regarding notification of test results identified privacy, responsive and interactive feedback, convenience, timeliness, and provision of details as the main issues (Baldwin 2005). Preferences for particular modes of communication were mixed (Baldwin 2005; Lin 2005; Meza 2000; Schofield 1994). With regard to newer technologies, studies have reported positive responses to communicating test results using web messaging and electronic health records (Hassol 2004; Kleiner 2002; Liederman 2003; Lin 2005; Ralston 2007). Studies in which patients and/or providers rated text messaging for promoting disease self‐management positively, noted features of simplicity and timeliness of the intervention (Ferrer‐Roca 2004; Pinnock 2006). On the other hand, some skepticism was reported regarding clinical benefits, time and cost implications (Pinnock 2006). In addition, participants' perceptions of personal invasion and behavioural control may be affected by inappropriate SMS initiation methods, and the intervention may have the opposite effect of that intended.
Possible risks of using mobile phone messaging include the risk of inaccurate data input (Norwell 2003), lack of understanding or misinterpretation of the information, and difficulties in reading for those with poor vision or problems with literacy. Furthermore, mobile phone messaging is intended to support or complement the process of care delivery rather than to substitute for it. A possible risk of a narrow focus on the technology is that providers may misinterpret it as an endpoint to their responsibilities within the care delivery process, believing that their work is completed once the message is sent. This may result in inadequate follow‐up of patients after the intervention. Additionally, text messaging cannot capture the verbal and non‐verbal cues that may also influence the interpretation of the message. Physicians sending abnormal test results in particular may fail to immediately fulfil patient needs in term of explanation of the implications of the results, prognosis and treatment options. Patient safety may also be compromised after receiving information on abnormal results if the information is not acted upon appropriately. The psychological and social impacts of using the mobile phone in this way are other key issues.
Having correct patient contact information and securely stored health records are essential to meet privacy, confidentiality and data protection requirements. Failures or delays in message delivery are rare but possible; however, harm is unlikely as senders are usually notified instantly in cases of a transmission problem. There may be additional monetary and time costs, as backup systems may be needed. Lastly, risks associated with mobile phone messaging in general may apply, for instance increased risk of car accidents as a result of messaging whilst driving.
Why it is important to do this review
Although there is some evidence on the use and effectiveness of mobile phone messaging in healthcare delivery, answers to questions regarding the implementation of these technologies in routine care, such as their impact on patient‐related outcomes or on processes of healthcare delivery, are unclear. Given the topical nature of the subject, we conducted this review to identify answers to these questions and propose directions for future research. This review complements available studies on the use of telephone consultations (Car 2003), email (Car 2004; Car 2004b) and personal digital assistants (PDAs) (Baumgart 2005) in health care, as well as forthcoming Cochrane reviews on mobile phone messaging for a range of purposes (Car 2008; de Jongh 2008; Vodopivec‐Jamsek 2008).
Objectives
To assess the effects of mobile phone messaging for communicating results of medical investigations on people's healthcare‐seeking behaviour and health outcomes. Secondary objectives include assessment of participants' evaluation of the intervention, direct and indirect healthcare costs and possible risks and harms associated with the intervention.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), quasi‐randomised controlled trials (QRCTs), controlled before and after studies (CBAs), and interrupted time series (ITS) with at least three time points before and after the intervention.
We define a QRCT as a controlled trial in which the participant allocation is not truly random, such as allocation by date of birth or the order in which participants are included in the study. We included QRCT, CBA and ITS designs because our initial literature searching suggested that only a small number of RCTs on mobile phone messaging interventions exist.
Types of participants
We included all study participants regardless of age, gender and ethnicity, as well as all types and stages of diseases. We included studies in all settings, i.e. primary care settings (services of primary health care), outpatient settings (outpatient clinics), community settings (public health services, anywhere where a person can use a mobile phone) and hospital settings. We did not exclude studies according to the type of healthcare provider (e.g. nurse, doctor, allied staff).
Types of interventions
We included interventions using SMS or MMS for communicating results of medical tests, regardless of the purpose of the test (screening, diagnostic, guide to treatment, monitoring etc.). The messaging needed to be between a healthcare provider (either in person or automated) or a 'treatment buddy' (e.g. a lay health worker or peer supporter) and a patient, regardless of who sent the first message.
We excluded studies of mobile phone messaging to people other than those who were notified of their medical investigations, or messaging between two healthcare providers. We also excluded studies in which mobile phone messaging was a part of a multifaceted intervention, as it would not be possible to separate the effects of messaging alone.
We aimed to make comparisons between mobile phone messaging and no intervention, as well as other modes of communication such as face‐to‐face, postal letters, calls to landline or mobile telephones, email or via electronic health records; and if applicable, automated versus personal text messaging.
Types of outcome measures
A number of processes and outcomes may be affected by interventions that aim to enhance and/or facilitate the communication between patients and/or carers, and healthcare providers (individuals or institutions) using mobile phone messaging. We sought all relevant outcomes relating to the following categories:
Primary outcomes
Healthcare‐seeking behaviour in response to the intervention, including utilisation of, and time to contact, health provider;
Health outcomes as a result of the intervention, including physiological measures, clinical assessments, biomarker values, self‐reporting of symptom resolution, and quality of life.
Secondary outcomes
User (patient, carer or healthcare provider) evaluation of the intervention, including satisfaction, readiness to use, timeliness, availability and/or convenience;
User (patient, carer or healthcare provider) perceptions of safety;
Potential harms or adverse effects of the intervention, such as misreading or misinterpretation of the test results, transmission of inaccurate data, loss of verbal and non‐verbal communication cues, issues of privacy and disclosure, or failure or delay in the message delivery;
Healthcare costs (direct and indirect) of the intervention.
Search methods for identification of studies
We used a common search strategy for all four reviews (Car 2008; de Jongh 2008; Vodopivec‐Jamsek 2008), and allocated relevant studies to their respective reviews before assessing their risk of bias and extracting data. A study may be relevant to, and included in, more than one review.
The search strategies for each database are given in Appendix 1 to Appendix 7.
Electronic searches
We restricted the searches to studies published since 1993 as the first commercial SMS message was sent in December 1992 (Wikipedia 2007). We included LILACS and the African Health Anthology because mobile phone messaging applications are increasingly used in low‐ and middle‐income regions. There were no language restrictions.
One review author (IGU) searched the following electronic databases on October 13, 2008 and updated the search on June 22, 2009:
The Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2009, issue 2);
MEDLINE (OvidSP) (January 1993 to June 22, 2009);
EMBASE (OvidSP) (January 1993 to June 22, 2009);
PsycINFO (OvidSP) (January 1993 to June 22, 2009);
CINAHL (EbscoHOST) (January 1993 to June 22, 2009);
LILACS (January 1993 to June 22, 2009);
African Health Anthology (January 1993 to June 22, 2009).
Searching other resources
For grey literature we searched:
Proceedings from AMIA Congresses;
WHO Clinical Trial Search Portal (www.who.int/trialsearch);
Current Controlled Trials (www.controlled‐trials.com);
Dissertation Abstracts International.
We searched the reference lists of included studies to identify additional studies. We contacted study authors for further information on their studies and to enquire whether they were aware of any other published or ongoing studies that would meet our inclusion criteria.
Data collection and analysis
Selection of studies
The selection of studies was done by IGU, TdJ and VVJ. IGU and TdJ independently assessed the relevance of all titles and abstracts identified from the electronic searches. We retrieved full text copies of all articles judged to be potentially relevant from the titles and abstracts. TdJ and VVJ independently assessed these articles for inclusion. IGU checked the final list of included and excluded studies, and any disagreements were resolved by discussion with VVJ, JC, and RA. Where the description of the intervention was not sufficiently detailed to allow the review authors to judge whether it met the inclusion criteria, we contacted the study authors for further details.
Data extraction and management
We sought to extract the following data from the included study, using a modified version of the Cochrane Consumers and Communication Review Group's data extraction template:
General information: title, authors, source, publication status, date published, language, review author information, date reviewed.
Study methods: aims of intervention, aim of study, study design, methods of participant recruitment, inclusion/exclusion criteria, informed consent and ethical approval, funding.
Risk of bias: data depended on the study design (see 'Assessment of risk of bias in included studies').
Participants: description, geographic location, setting, number, age, gender, ethnicity, socio‐economic status. If relevant: principal health problem or diagnosis, stage of illness, treatment received.
Providers: description, geographic location, setting, age, gender.
Interventions: description including technical specifications on SMS and handset provider, duration of intervention, purpose of intervention, initiator of intervention, message content, details of control/usual or routine care, co‐interventions.
Outcomes: primary and secondary outcomes as specified at Types of outcome measures, methods of assessing outcomes, follow up for non‐respondents, timing of outcome assessment, adverse events.
Results: all reported measurements for the primary and secondary outcomes, including multiple timings for measurements, subgroup analyses or results in different measurement scales if applicable.
Two review authors (TdJ, VVJ) independently extracted the above data onto a standard form. The forms were then assessed by one review author (IGU) who checked these data. Any discrepancies between the two data extraction sheets were discussed by two review authors (TdJ, VVJ) and resolved jointly with the two other review authors (IGU and JC). For missing data, we contacted the study authors to obtain the missing information.
Assessment of risk of bias in included studies
We assessed the risk of bias of included study in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) which recommends the explicit reporting of sequence generation, allocation concealment, blinding of participants, providers and outcome assessors, incomplete outcome data, selective outcome reporting and other sources of bias for RCTs.
Had studies using other study designs been identified for inclusion in the review, we would have assessed these using a variation of the above tool.
Two review authors (TdJ, VVJ) independently assessed the risk of bias in the included study, with any disagreements resolved by discussion and consensus of the team. We used a template to guide the assessment of risk of bias, and judged each domain as 'yes' (indicating a low risk of bias), 'no' (indicating a high risk of bias) or 'unclear' (indicating an uncertain risk of bias).
We have presented the results of the risk of bias assessment in a table, and provided a narrative discussion of risk of bias in individual domains.
Measures of treatment effect
We used risk ratios (RR) as effect measures for dichotomous outcomes and mean differences (MD) for continuous outcomes. RR and MDs have been derived from Manzel‐Haenszel and inverse variance methods respectively. We used a random‐effects model, where possible, to pool the results and reported 95% confidence intervals with all measures of effect.
Unit of analysis issues
We noted the method of randomisation in the included trial. We would have considered additional issues regarding the assessment of risk of bias of cluster randomised trials as discussed in Chapter 16 of the Cochrane Handbook (Higgins 2011). In the case of repeated measurements, we would have defined several outcomes based on different periods of follow‐up and performed separate analyses for each outcome. In studies with more than two treatment groups, we would have made multiple pair‐wise comparisons between all possible pairs of intervention groups.
Dealing with missing data
We contacted the original investigators to request missing data. With incomplete outcome data (such as drop‐outs, loss to follow‐up and withdrawn study participants), we assessed and reported the risk of bias as high/unclear/low as guided by the Cochrane Handbook (Higgins 2011) and identified the numbers as well as the reasons for incomplete data. As the numbers and reasons for incomplete outcome data in the included study suggested that data were missing at random, we used only available data in the review and did not use imputation methods.
Assessment of reporting biases
We were unable to assess reporting bias statistically or using funnel plots, as we included only one study. We assessed selective outcome reporting using the Cochrane Risk of Bias assessment tool.
Data synthesis
As only one study was included, we present a narrative overview of the findings, including tabular summaries of extracted data. Methods for combining results statistically have been retained (see Appendix 8) for potential use in future updates of this review.
Subgroup analysis and investigation of heterogeneity
We conducted a post‐hoc subgroup analysis according to differences in outcomes for participants who received positive versus negative results from their medical investigations.
We were unable to conduct subgroup analyses by participant age (0 to 18, 18 to 55, over 55), as planned, because only one study was included.
Sensitivity analysis
We did not conduct the planned sensitivity analyses as only one study was included. We had aimed to determine the influence of the following factors on effect size:
excluding unpublished studies;
taking into account of risk of bias of included studies, as specified above;
excluding any large studies to establish how they impact on the results;
excluding studies using the following filters: criteria used for clinical diagnosis and eligibility for intervention, language of publication, source of funding (industry versus other), country;
the length of the interval between delivery of the intervention and measurement of the effect.
Consumer participation
The draft review was circulated for peer review by consumers in The Cochrane Collaboration. The review received comments from two consumers through the Cochrane Consumers and Communication Review Group's standard editorial process. We also examined whether consumers were involved in the design and implementation of each included study.
Results
Description of studies
Results of the search
Our search (across all four reviews) identified 3937 citations. We excluded 3750 citations that, based on the abstract alone, showed insufficient relevance to the suite of reviews or did not meet the stated study design criteria. After review of the full text of the remaining 187 citations, a further 184 were subsequently rejected from this review for failing to meet the inclusion criteria. In the final selection stage, 2 of the remaining 3 citations were excluded from this review as both were observational studies, one with an historical control group. We did not identify any ongoing studies relevant to this review.
Included studies
Only one study was included in this review (Cheng 2008). We present key characteristics of this study below and in the Characteristics of included studies table.
Methods
The included study was a randomised controlled trial (RCT). The unit of randomisation was the individual participant. Study duration was 24 months from January 2005 to December 2006. The study compared the effects of the text messaging intervention to usual care.
Participants
Cheng 2008 was set in a department of gynaecology and obstetrics in a Taiwanese general hospital. Participants were pregnant women of all ages who could speak Chinese and who were attending a routine second‐trimester Down syndrome screening. Among 3691 potential participants, 3178 gave consent to participate in the study. Of these, 88% (n = 2782) completed anxiety questionnaires at all three measurement points: (1) before the prenatal screening, (2) after screening but before the clinic appointment, and (3) three days after the clinic appointment. No further information was provided on the 396 women who did not complete all the questionnaires. The study found no significant differences between the intervention and control groups regarding age, marital status, parity, education, occupation, total family income, proportion of planned/unplanned pregnancies, previous pregnancy with congenital abnormality, or gestational age at serum screening.
Interventions
Purpose
The purpose of the intervention was to provide fast communication of results via text messaging before the follow‐up appointment, with the purpose to alleviate anxiety in women waiting to receive results of prenatal screening for Down syndrome.
Specifications
An Internet Service Provider (ISP) handled the transmission of screening results data from a modified web server to the mobile phones of the intervention recipients.
Message content
The study does not report the exact content or format of the text message used. The authors state that it was derived from the data that contained the results of the screening. The study did not provide detailed information on the timing of the intervention relative to either the initial screening or the follow‐up appointment.
Outcomes
Cheng 2008 measured participant anxiety levels (1) before the prenatal screening, (2) after screening but before the clinic appointment, and (3) three days after the clinic appointment. Anxiety levels were measured with the Spielberger State‐Trait Anxiety Inventory (STAI), which includes two scales to describe how the respondent feels at a particular moment in time (state‐anxiety) and how the respondent generally feels (trait‐anxiety). The scales ranged from 20 to 80 points, with higher scores indicating higher anxiety.
Risk of bias in included studies
Risk of bias in the included study is summarised in Figure 1.
1.
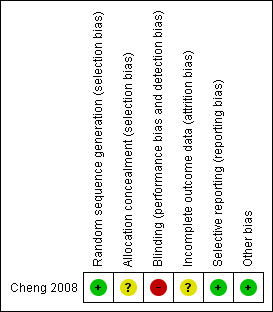
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The study reported the use of an adequate sequence generation method (computer generated random allocation sequences). Although not stated in the study, we assume that no blinding of participants, healthcare providers or outcome assessors took place.
The authors do not mention whether allocation concealment methods were used. This could have introduced a source of bias.
Because we were not able to review the original study protocol, we cannot fully exclude the possibility of selective reporting. However, this would appear to be unlikely as the study objective was narrowly formulated as investigating the effects of early communication of test results on the women's anxiety in the period between screening and a clinic appointment. This outcome measure was fully reported. Intervention and control groups were reported to be comparable on patient characteristics such as women’s age, marital status, parity, education, occupation, and total family income, although the supporting data for this claim were not reported. In addition to the state anxiety scores measured before the clinic appointment (that is, the variable of interest), trait and state anxiety scores were measured both before the prenatal test and three days after the final clinic appointment when both groups of women had received the full test results. There were no significant differences on these scores between the groups.
Analysis did not follow intention‐to‐treat (ITT) principles as data were analysed only for women who had completed all three questionnaires. The study did not report reasons for loss to follow‐up, or discuss how this could have influenced outcomes.
We contacted the study authors for further information, but did not get a response.
Effects of interventions
See: Table 1
In Cheng 2008, when measured before a scheduled clinic appointment, women who had received their test result early by text message had a mean anxiety score 2.48 points lower than women who had not yet received their result (95% CI ‐ 8.79 to 3.84) Analysis 1.1). However, of the total participants (n = 2782), those women with a serum‐negative test result receiving their result early had a mean anxiety score 5.3 points lower (95% CI ‐ 5.99 to ‐4.61) than women in the control group. Those women with a serum‐positive test result receiving their result early by text message had a mean anxiety score 1.2 points higher (95% CI ‐ 3.48 to 5.88) than women in the control group (Table 1).The evidence was of low quality due to high risk of bias in the included study, and the fact that the evidence comes from one study only.
1.1. Analysis.
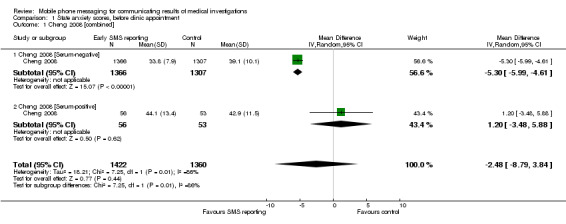
Comparison 1 State anxiety scores, before clinic appointment, Outcome 1 Cheng 2008 [combined].
The study did not evaluate other important outcomes, such as healthcare‐seeking behaviour in response to the intervention, patient satisfaction or cost.
Discussion
Summary of main results
Our review found only one study assessing the effects of mobile phone messaging for communicating results of medical investigations. In this study (Cheng 2008) SMS was used to provide pregnant women the results from prenatal screening for Down's syndrome before their next clinic appointment. Across all women, the study showed that early reporting of test results by SMS may have made little or no difference to anxiety. The intervention may have reduced anxiety in pregnant women who received a serum‐negative result, but may have made little or no difference to anxiety in women who received a serum‐positive result.
Overall completeness and applicability of evidence
We systematically collected and analysed the evidence to date on the potential for mobile phone messaging in communicating results of medical investigations. Only one study met the inclusion criteria. In selecting studies for inclusion we have deliberately taken a rather narrow focus: only those studies where the intervention is delivered exclusively through mobile phone messaging were eligible. Studies in which mobile phone messages were combined with other forms of data transmission, such as e‐mail, Internet or General Packet Radio Service (GPRS), were excluded, as it would have been difficult to assess the independent effect of the text message within such complex interventions. However, this strategy restricted the body of evidence we were able to identify, as we found that many studies in the area of mobile health have relied on multifaceted interventions in which text messaging was combined with other technologies.
Quality of the evidence
The study we included was of low methodological quality with high risk of bias. The review's results, therefore, do not provide a robust foundation upon which to build recommendations for the use of mobile phone messaging to communicate the results of medical investigations.
Potential biases in the review process
We believe that we identified all studies concerning the use of mobile phone messaging for communicating results of medical investigations that met our study design criteria (RCT, QRCT, CBA, ITS) up to June 2009. However, by excluding studies with possible confounding from other communication and/or data transmission methods, we may have introduced selection bias towards less successful interventions, as more complex interventions may be more effective at communicating results of medical investigations.
Agreements and disagreements with other studies or reviews
This review comes in the wake of two other reviews that analyse text messaging interventions. Fjeldsoe 2009 reviewed the evidence for behavioural change interventions delivered by SMS, whereas Krishna 2009 more broadly looked at healthcare delivery via mobile phones in the management and prevention of disease. Neither of the studies commented on the interventions for communicating results of medical investigations.
Authors' conclusions
Implications for practice.
Reliable conclusions on the effects of text messaging in communicating results of medical investigations cannot be drawn, based on the one study we found.The low quality evidence from this study suggests that sending negative (clear) screening results by text message may reduce anxiety, but that sending positive (concerning) results by text message may make little or no difference to anxiety. Health service providers may wish to consider the implications of these findings when implementing new approaches to communicating test results to patients.
Implications for research.
This review shows that there is currently insufficient evidence regarding the benefits and risks associated with mobile phone messaging for communicating results of medical investigations. Evidence is limited to one randomised controlled trial.
Future research should utilise randomised controlled trials to ensure robustness and minimise bias and should report on intermediate indicators such as health‐seeking behaviour (which correlate with health outcomes), patients’ evaluation of the intervention, costs, economic benefits, and potential adverse effects. The latter may be particularly important in instances where mobile phone messaging is used to communicate test results which are potentially upsetting to the patient, e.g. when patients test positive for certain conditions.
As the timing of the message along the care pathway (for example at screening stage or for control of established disease), frequency of the messaging, the message content and the length of message, as well as mode of communication, can affect outcomes, future studies should clearly describe the intervention with reference to the message attributes.
Acknowledgements
We would like to acknowledge the very helpful support of the Cochrane Consumers and Communication Review Group editors and staff, particular Dr. Megan Prictor and Dr. Sophie Hill, at different stages of this review.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
cellular phone/
text messag$.ab,ti.
texting.ab,ti.
short messag$.ab,ti.
sms.ab,ti.
(multimedia messag$ or multi‐media messag$).ab,ti.
mms.ab,ti.
((cellular phone$ or cell phone$ or mobile phone$) and (messag$ or text$)).ab,ti.
or/1‐8
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized controlled trials.sh.
random allocation.sh.
double blind method.sh.
single blind method.sh.
or/10‐15
animals/ not (human/ and animals/)
16 not 17
clinical trial.pt.
exp clinical trials/
(clin$ adj25 trial$).ti,ab.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab.
placebos.sh.
placebo$.ti,ab.
random$.ti,ab.
research design.sh.
or/19‐26
27 not 17
18 or 28
exp evaluation studies/
follow up studies/
prospective studies/
(control$ or prospectiv$ or volunteer$).tw.
cross over studies/
comparative study/
or/30‐35
experiment$.tw.
(time adj series).tw.
(pre test or pretest or (posttest or post test)).tw.
(pre intervention or preintervention or (post intervention or postintervention)).tw.
(impact$ or intervention$ or chang$ or outcome$).tw.
effect$.tw.
or/37‐42
36 and 43
animals/ not (human/ and animals/
44 not 45
29 or 46
47 and 9
limit 48 to yr="1993 ‐ 2008
Appendix 2. EMBASE (Ovid) search strategy
1. mobile phone/
2. wireless communication/
3. (cellular phone* or cellular telephon* or cell phone* or mobile phone* or mobile telephon* or wireless phone* or wireless telephon*).ti.
4. 1 or 2 or 3
5. limit 4 to abstracts
6. (cellular phone* or cellular telephon* or cell phone* or mobile phone* or mobile telephon* or wireless phone* or wireless telephon*).tw.
7. (text* or messag* or multimedia or multi‐media or imag* or data or input* or sms or mms).tw.
8. (5 or 6) and 7
9. 4 not 5
10. (text messag* or texting or texted).tw.
11. (short messag* or (sms not (somatostatin* or sphingomyelin*))).tw.
12. (multimedia messag* or multi‐media messag*).tw.
13. (mms and (multimedia or multi‐media)).tw.
14. or/8‐13
15. Randomized Controlled Trial/
16. random*.tw.
17. experiment*.tw.
18. time series.tw.
19. (pre test or pretest or post test or posttest).tw.
20. impact.tw.
21. intervention*.tw.
22. chang*.tw.
23. evaluat*.tw.
24. effect?.tw.
25. compar*.tw.
26. control*.tw.
27. or/15‐26
28. nonhuman/
29. 27 not 28
30. 14 and 29
31. limit 30 to yr="1993‐2009"
Appendix 3. PsycINFO (Ovid) search strategy
1. (cellular phone* or cellular telephon* or cell phone* or mobile phone* or mobile telephon* or wireless phone* or wireless telephon*).tw.
2. (text* or messag* or multimedia or multi‐media or imag* or data or input* or sms or mms).tw.
3. 1 and 2
4. (text messag* or texting or texted).tw.
5. (short messag* or sms).tw.
6. (multimedia messag* or multi‐media messag*).tw.
7. (mms and (multimedia or multi‐media)).tw.
8. or/3‐7
9. random*.tw.
10. experiment*.tw.
11. trial.tw.
12. placebo.ab.
13. groups.ab.
14. ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).tw.
15. time series.tw.
16. time series/
17. (pre test or pretest or post test or posttest).tw.
18. (pre intervention or preintervention or post intervention or postintervention).tw.
19. (cross over or crossover).tw.
20. latin square.tw.
21. (prospective* or volunteer*).tw.
22. impact.tw.
23. intervention*.tw.
24. chang*.tw.
25. evaluat*.tw.
26. effect?.tw.
27. compar*.tw.
28. control*.tw.
29. treatment effectiveness evaluation/
30. mental health program evaluation/
31. exp experimental design/
32. or/9‐31
33. limit 32 to human
34. limit 33 to yr="1993‐2008"
35. (health* or medic* or telemedic* or patient* or illness* or therap* or psychiatr* or nurs* or remind* or consult*).tw.
36. ("27" or "32" or "33" or "34").cc.
37. 35 or 36
38. 8 and 34
39. 38 and 37
Appendix 4. CENTRAL search strategy
#1 "cellular phone":kw or "mobile phone":kw or ((text next messag*) or texting or texted or (short next messag*) or (sms not (somatostatin* or sphingomyelin*)) or (multimedia next messag*) or (multi‐media next messag*) or (mms and (multimedia or multi‐media)) or (cellular next phone*) or (cellular next telephon*) or (cell next phone*) or (mobile next phone*) or (mobile next telephon*) or (wireless next phone*) or (wireless next telephon*)):ti,ab in Clinical Trials
#2 human*:kw in Clinical Trials
#3 #1 and #2
Appendix 5. CINAHL (EBSCO) search strategy
| S15 | s14 |
| S14 | S10 or S13 |
| S13 | s11 and s12 |
| S12 | PT Research |
| S11 | S3 not S10 |
| S10 | s3 and s9 |
| S9 | S4 or S5 or S6 or S7 or S8 |
| S8 | pre test or pretest or post test or posttest or pre intervention or preintervention or post intervention or postintervention or time series |
| S7 | TI ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)) or AB ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)) |
| S6 | random* or trial or groups or placebo* or experiment* or control* or compar* or intervention* or chang* or evaluat* or impact* or effect? |
| S5 | PT Clinical Trial |
| S4 | MH Experimental Studies+ or MH Random Assignment or MH Comparative Studies or MH Comparative Studies or MH Crossover Design or MH Placebos or MH Quantitative Studies or MH Quasi‐Experimental Studies+ |
| S3 | S1 or S2 |
| S2 | cellular phone* or cellular telephon* or cell phone* or mobile phone* or mobile telephon* or wireless phone* or wireless telephon* or text messag* or texting or texted or short messag* or sms or multimedia messag* or multi‐media messag* or (mms and (phone* or telephon* or multimedia or multi‐media or messag*)) |
| S1 | MH Wireless Communications |
Appendix 6. African Health Anthology search strategy
1 ‐ Query 1:
| KEY WORDS/PHRASES | RANDOM* OR TRIAL* OR CONTROL* OR PROSPECTIV* OR VOLUNTEER* OR EXPERIMENT* OR TIME SERIES OR PRE TEST OR PRETEST OR POST TEST OR POSTTEST OR PRE INTERVENTION OR PREINTERVENTION OR POST INTERVENTION OR POSTINTERVENTION OR IMPACT* OR INTERVENTION* OR CHANG* OR EFFECT* |
| TITLE | PLACEBO OR GROUPS |
| INDEX TERMS | RESEARCH DESIGN OR FOLLOW UP STUDIES OR PROSPECTIVE STUDIES OR CROSS OVER STUDIES OR DRUG THERAPY |
2 ‐ Query 2:
| KEY WORDS/PHRASES | ((TEXT* OR MESSAG* OR MULTIMEDIA OR MULTI‐MEDIA OR IMAG* OR DATA OR INPUT* OR SMS OR MMS) AND (CELLULAR PHONE* OR CELLULAR TELEPHON* OR CELL PHONE* OR MOBILE PHONE* OR MOBILE TELEPHON* OR WIRELESS PHONE* OR WIRELESS TELEPHON*)) OR TEXT MESSAG* OR TEXTING OR TEXTED OR SHORT MESSAG* OR (SMS NOT (SOMATOSTATIN* OR SPHINGOMYELIN*)) OR MULTIMEDIA MESSAG* OR MULTI‐MEDIA MESSAG* OR (MMS AND (MULTIMEDIA OR MULTI‐MEDIA)) |
| TITLE | CELLULAR PHONE* OR CELLULAR TELEPHON* OR CELL PHONE* OR MOBILE PHONE* OR MOBILE TELEPHON* OR WIRELESS PHONE* OR WIRELESS TELEPHON* |
| INDEX TERMS | CELLULAR PHONE |
3 ‐ Query 1 and Query 2.
Appendix 7. Search Strategy for LILACS, trial portals and grey literature
“cellular phone” OR “mobile phone” OR cellular telephone* OR mobile telephone* OR text messag* OR texting OR texted OR short messag* OR multimedia messag* OR sms OR mms
Appendix 8. Data synthesis methods
We will consider whether it is appropriate to combine the studies quantitatively once we have completed the search. The decision is likely to rest on the diversity of interventions and outcome measures used in the studies. Studies will be classified on the following issues:
Study design: RCTs, QRCTs, CBAs, ITS;
Outcome measures used, as described at Types of outcome measures.
If quantitative analysis is undertaken, the meta‐analysis will depend on the outcomes reported. For continuous data, where outcomes have been measured in a standard way across studies, we will report the SMD and confidence intervals (Alderson 2002b). For dichotomous data, when outcomes have been measured in a standard way, we will report the RR. In such cases, a cautious approach will be taken to combining results, and the rationale will be detailed. We will conduct statistical analysis according to the guidelines in the Cochrane Handbook (Higgins 2011).
Data and analyses
Comparison 1. State anxiety scores, before clinic appointment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cheng 2008 [combined] | 1 | 2782 | Mean Difference (IV, Random, 95% CI) | ‐2.48 [‐8.79, 3.84] |
| 1.1 Cheng 2008 [Serum‐negative] | 1 | 2673 | Mean Difference (IV, Random, 95% CI) | ‐5.30 [‐5.99, ‐4.61] |
| 1.2 Cheng 2008 [Serum‐positive] | 1 | 109 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐3.48, 5.88] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cheng 2008.
| Methods | Study design: RCT | |
| Participants | Taiwan. Department of Gynecology and Obstetrics in one district general hospital. 3691 women who could speak Chinese and who agreed to undergo a Down syndrome screening test were eligible to participate. 3178 gave consent. 2782 completed the questionnaires on all three occasions. Baseline comparability: Age, marital status, parity, education, occupation, and total family income, planned/unplanned pregnancy, previous pregnancy with congenital abnormality, gestational age at which the serum screening was done [as stated in the publication, no data provided]. |
|
| Interventions | All pregnant women were given appointments for regular clinical follow‐up after the serum screening during which the information regarding results was communicated. SMS group: Women received fast reporting of the screening results via SMS before the routine appointment. Control group: Fast reporting by SMS was not provided, women were informed about their results during the clinic follow‐up. |
|
| Outcomes | Anxiety levels using the Spielberger State‐Trait Anxiety Inventory measured before prenatal screening, before clinic appointment and three days after clinic appointment. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random number system. |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No information of blinding of providers or researchers provided. Blinding of participants not possible due to nature of intervention. Unlikely to influence outcome measures. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 396 women did not complete the questionnaires on all occasions. No further data are provided on whether the missing information is balanced across the intervention and control groups. |
| Selective reporting (reporting bias) | Low risk | Protocol is not available. However, the outcome measure reported (state anxiety before the clinic appointment) is consistent with the objective of the study. |
| Other bias | Low risk | The authors state that the groups were comparable at baseline on demographic variables, although no supporting data are provided. The intervention groups are comparable on trait and state anxiety scores measured at baseline and 3 days after the clinic appointment. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Lim 2008 | Study design: Cohort study with historical control |
| Menon‐Johansson 2006 | Study design: Observational study |
Differences between protocol and review
Search strategy
We were not able to search the following databases we listed in the protocol:
Proceedings from the MEDNET Congresses: We could not access the proceedings.
TrialsCentralTM (www.trialscentral.org): The website for the data base was not functional and did not allow for the search of clinical trials.
African Trials Register: The trials in the African Trials Register are collected with a search strategy using the Cochrane Controlled Trials Register and the African Health Anthology (AHA). As we searched both original sources, it was not necessary to access the African Trials Register separately.
Health Star: The database ceased to exist as of December 2000, with all peer‐reviewed journal articles transferred to PubMed.
Subgroup analysis
We conducted a post‐hoc subgroup analysis according to differences in outcomes for participants who received positive versus negative results from their medical investigations.
We were unable to conduct subgroup analyses by participant age (0 to 18, 18 to 55, over 55), as planned, because only one study was included.
Contributions of authors
Josip Car conceived the review together with Rifat Atun. He is the contact author for the reviews and coordinated all stages of the study, including the protocol stage. He advised on designing the search strategy, screening search results against inclusion criteria, and appraising quality of the papers. He assisted in interpreting the data and contributed to writing the review, providing clinical and consumer perspectives and will be responsible for leading the update of this review.
Ipek Gurol‐Urganci developed the protocols. She has lead the search process and assisted in screening the papers. She collected, analysed and interpreted data and contributed to writing the review, providing methodological and policy perspectives.
Vlasta Vodopivec Jamek developed the protocols. She has been involved in the screening and quality appraisal process and assisted in undertaking searches. She has collected, analysed and interpreted data and contributed to writing the review, providing clinical and consumer perspectives.
Thyra de Jongh coordinated data extraction and management and was involved in the screening and quality appraisal process. She has collected, analysed and interpreted data and contributed to writing the review, providing a methodological perspective.
Rifat Atun provided general direction for the studies and assisted in writing and revising the review.
Sources of support
Internal sources
-
eHealth Unit, Department of Primary Care and Social Medicine, Imperial College, UK.
salaries, office space
-
Centre for Health Management, Tanaka Business School, Imperial College, UK.
salaries, office space
-
Department of Family Medicine, Faculty of Medicine, University of Ljubljana, Slovenia.
salaries, office space
-
Imperial College London, UK.
salaries, office space
-
London School of Hygiene and Tropical Medicine, Health Services Research Unit, UK.
salaries, office space
External sources
-
Ministry of Higher Education, Science and Technology, Slovenia.
grant funding
Declarations of interest
JC is an adviser to iPLATO a health text‐messaging company. iPLATO had no connection to this review.
New
References
References to studies included in this review
Cheng 2008 {published data only}
- Cheng PJ, Wu TL, Shaw SW, Chueh HY, Lin CT, Hsu JJ, et al. Anxiety levels in women undergoing prenatal maternal serum screening for Down syndrome: the effect of a fast reporting system by mobile phone short‐message service. Prenatal Diagnosis 2008;28(5):417‐21. [DOI: 10.1002/pd.1988] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Lim 2008 {published data only}
- Lim EJ, Haar J, Morgan J. Can text messaging results reduce time to treatment of Chlamydia trachomatis?. Sexually Transmitted Infections 2008;84(7):563‐4. [DOI] [PubMed] [Google Scholar]
Menon‐Johansson 2006 {published data only}
- Menon‐Johansson AS, McNaught F, Mandalia S, Sullivan AK. Texting decreases the time to treatment for genital Chlamydia trachomatis infection. Sexually Transmitted Infections 2006;82(1):49‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Adler 2007
- Adler R. Health Care Unplugged: The Evolving Role of Wireless Technology. Chronic Health Care Foundation, 2007. [Google Scholar]
Atun 2006
- Atun RA, Sittampalam S. A review of the characteristics and benefits of SMS in delivering healthcare. In: Atun RA, et al. editor(s). The role of mobile phones in increasing accessibility and efficiency in healthcare. Vodafone Group PLC, 2006. [Google Scholar]
Atun 2006b
- Atun RA, Gurol‐Urganci I. Analysis of calls to NHS Direct. In: Atun RA, et al. editor(s). The Role of Mobile Phones in Increasing Accessibility and Efficiency in Healthcare. Vodafone Group PLC, 2006. [Google Scholar]
Baldwin 2005
- Baldwin DM, Quintela J, Duclos C, Staton EW, Pace WD. Patient preferences for notification of normal laboratory test results: a report from the ASIPS Collaborative. BMC Family Practice 2005;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bauer 2003
- Bauer S, Percevic R, Okon E, Meermann R, Kordy H. Use of text messaging in the aftercare of patients with bulimia nervosa. European Eating Disorders Review 2003;11(3):279‐90. [Google Scholar]
Baumgart 2005
- Baumgart DC. Personal digital assistants in health care: experienced clinicians in the palm of your hand?. Lancet 2005;366(9492):1210‐22. [DOI] [PubMed] [Google Scholar]
Bos 2005
- Bos A, Hoogstraten J, Prahl‐Andersen B. Failed appointments in an orthodontic clinic. American Journal of Orthodontics and Dentofacial Orthopedics 2005;127(3):355‐7. [DOI] [PubMed] [Google Scholar]
Bradbeer 2003
- Bradbeer C, Mears A. STI services in the United Kingdom: how shall we cope?. Sexually Transmitted Infections 2003;79(6):435‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Car 2003
- Car J, Sheikh A. Telephone consultations. BMJ 2003;326(7396):966‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Car 2004
- Car J, Sheikh A. Email consultations in health care: 1‐scope and effectiveness. BMJ 2004;329(7463):435‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Car 2004b
- Car J, Sheikh A. Email consultations in health care: 2‐acceptability and safe application. BMJ 2004;329(7463):439‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Car 2008
- Car J, Gurol‐Urganci I, Jongh T, Vodopivec‐Jamsek V, Atun R. Mobile phone messaging reminders for attendance at scheduled healthcare appointments. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007458] [DOI] [Google Scholar]
Car 2008b
- Car J, Ng C, Atun R, Card A. SMS text message healthcare appointment reminders in England. Journal of Ambulatory Care Management 2008 Jul‐Sep;31(3):216‐9. [DOI] [PubMed] [Google Scholar]
de Jongh 2008
- Jongh T, Gurol‐Urganci I, Vodopivec‐Jamsek V, Car J, Atun R. Mobile phone messaging telemedicine for facilitating self management of long‐term illnesses. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007459] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fairley 2003
- Fairley CK, Levy R, Rayner CR, Allardice K, Costello K, Thomas C, et al. Randomized trial of an adherence programme for clients with HIV. International Journal of STD & AIDS 2003;14(12):805‐9. [DOI] [PubMed] [Google Scholar]
Ferrer‐Roca 2004
- Ferrer‐Roca O, Cardenas A, Diaz‐Cardama A, Pulido P. Mobile phone text messaging in the management of diabetes. Journal of Telemedicine and Telecare 2004;10(5):282‐5. [DOI] [PubMed] [Google Scholar]
Fjeldsoe 2009
- Fjeldsoe BS, Marshall AL, Miller YD. Behavior change interventions delivered by mobile telephone short‐message service. American Journal of Preventive Medicine 2009;36(2):165‐73. [DOI] [PubMed] [Google Scholar]
Franklin 2003
- Franklin V, Waller A, Pagliari C, Greene S. "Sweet Talk": text messaging support for intensive insulin therapy for young people with diabetes. Diabetes Technology & Therapeutics 2003;5(6):991‐6. [DOI] [PubMed] [Google Scholar]
Hassol 2004
- Hassol A, Walker JM, Kidder D, Rokita K, Young D, Pierdon S, et al. Patient experiences and attitudes about access to a patient electronic health care record and linked web messaging. Journal of the American Medical Informatics Association 2004;11:505‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org, 2011. [Google Scholar]
ITU 2010
- International Telecommunications Union. The World in 2010: ICT Facts and Figures. http://www.itu.int/ITU‐D/ict/material/FactsFigures2010.pdf 2010.
Kaplan 2006
- Kaplan WA. Can the ubiquitous power of mobile phones be used to improve health outcomes in developing countries?. Global Health 2006;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kleiner 2002
- Kleiner KD, Akers R, Burke BL, Werner EJ. Parent and physician attitudes regarding electronic communication in pediatric practices. Pediatrics 2002;109:740‐4. [DOI] [PubMed] [Google Scholar]
Krishna 2009
- Krishna S, Boren SA, Balas EA. Healthcare via cell phones: a systematic review. Telemedicine and e‐Health 2009 Apr;15(3):231‐40. [DOI] [PubMed] [Google Scholar]
Kwon 2004
- Kwon HS, Cho JH, Kim HS, Lee JH, Song BR, Oh JA, et al. Development of web‐based diabetic patient management system using short message service (SMS). Diabetes Research and Clinical Practice 2004;66 Suppl 1:S133‐7. [DOI] [PubMed] [Google Scholar]
Lamont 2005
- Lamont M. Text messaging and breaking bad news. BMJ 2005;330:1217. [Google Scholar]
Liederman 2003
- Liederman EM, Morefield CS. Web messaging: a new tool for patient‐physician communication. Journal of the American Medical Informatics Association 2003;10:260‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2005
- Lin CT, Wittevrongel L, Moore L, Beaty BL, Ross SE. An Internet‐based patient‐provider communication system: randomized controlled trial. Journal of Medical Internet Research 2005;7:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lovitt 2005
- Lovitt C. Patient choice?. BMJ 2005;330:1217. [Google Scholar]
Marquez Contreras 2004
- Marquez Contreras E, Figuera von Wichmann M, Gil‐Guillen V, Ylla‐Catala A, Figueras M, Balana M, et al. [Effectiveness of an intervention to provide information to patients with hypertension as short text messages and reminders sent to their mobile phone (HTA‐Alert)]. Atencion Primaria 2004;34(8):399‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meza 2000
- Meza JP, Webster DS. Patient preferences for laboratory test results notification. American Journal of Managed Care 2000;6:1297‐300. [PubMed] [Google Scholar]
Newell 2001
- Newell A. A mobile phone text message and Trichomonas vaginalis. Sexually Transmitted Infections 2001;77(3):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Norwell 2003
- Norwell N. Text messaging raises medicolegal issues. BMJ 2003;326(7399):1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Obermayer 2004
- Obermayer JL, Riley WT, Jean‐Mary J. College smoking‐cessation using cell phone text messaging. Journal of American College Health 2004;53(2):71‐8. [DOI] [PubMed] [Google Scholar]
Ostojic 2005
- Ostojic V, Cvoriscec B, Ostojic SB, Reznikoff D, Stipic‐Markovic A, Tudjman Z. Improving asthma control through telemedicine: a study of short‐message service. Telemedicine Journal and e‐Health 2005;11(1):28‐35. [DOI] [PubMed] [Google Scholar]
Pal 1998
- Pal B, Taberner DA, Readman LP, Jones P. Why do outpatients fail to keep their clinic appointments? Results from a survey and recommended remedial actions. International Journal of Clinical Practice 1998;52(6):436‐7. [PubMed] [Google Scholar]
Pinnock 2006
- Pinnock H, Slack R, Pagliari C, Price D, Sheikh A. Professional and patient attitudes to using mobile phone technology to monitor asthma: questionnaire survey. Primary Care Respiratory Journal 2006;15(4):237‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ralston 2007
- Ralston JD, Carrell D, Reid R, Anderson M, Moran M, Hereford J. Patient web services integrated with a shared medical record: patient use and satisfaction. Journal of the American Medical Informatics Association 2007;14:798‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodgers 2005
- Rodgers A, Corbett T, Bramley D, Riddell T, Wills M, Lin RB, et al. Do u smoke after txt? Results of a randomised trial of smoking cessation using mobile phone text messaging. Tobacco Control 2005;14(4):255‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schofield 1994
- Schofield MJ, Sanson‐Fisher R, Halpin S, Redman S. Notification and follow‐up of Pap test results: current practice and women's preferences. Preventive Medicine 1994;23:276‐83. [DOI] [PubMed] [Google Scholar]
Vilella 2004
- Vilella A, Bayas JM, Diaz MT, Guinovart C, Diez C, Simo D, et al. The role of mobile phones in improving vaccination rates in travelers. Preventive Medicine 2004;38(4):503‐9. [DOI] [PubMed] [Google Scholar]
Vodopivec‐Jamsek 2008
- Vodopivec‐Jamsek V, Jongh T, Gurol‐Urganci I, Atun R, Car J. Mobile phone messaging for preventive health care. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007457] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wikipedia 2007
- Wikipedia. Short Message Service. http://en.wikipedia.org/wiki/short_message_service (accessed February 2007).


