Abstract
Background
Glucocorticoids play a major role in the treatment of acute lymphoblastic leukaemia (ALL). However, supraphysiological doses can suppress the hypothalamic‐pituitary‐adrenal (HPA) axis. HPA axis suppression resulting in reduced cortisol response may cause an impaired stress response and an inadequate host defence against infection, which remain a cause of morbidity and death. Suppression commonly occurs in the first days after cessation of glucocorticoid therapy, but the exact duration is unclear. This review is the second update of a previously published Cochrane review.
Objectives
To examine the occurrence and duration of HPA axis suppression after (each cycle of) glucocorticoid therapy for childhood ALL.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 11), MEDLINE/PubMed (from 1945 to December 2016), and Embase/Ovid (from 1980 to December 2016). In addition, we searched reference lists of relevant articles, conference proceedings (the International Society for Paediatric Oncology and the American Society of Clinical Oncology from 2005 up to and including 2016, and the American Society of Pediatric Hematology/Oncology from 2014 up to and including 2016), and ongoing trial databases (the International Standard Registered Clinical/Social Study Number (ISRCTN) register via http://www.controlled‐trials.com, the National Institutes of Health (NIH) register via www.clinicaltrials.gov, and the International Clinical Trials Registry Platform (ICTRP) of the World Health Organization (WHO) via apps.who.int/trialsearch) on 27 December 2016.
Selection criteria
All study designs, except case reports and patient series with fewer than 10 children, examining effects of glucocorticoid therapy for childhood ALL on HPA axis function.
Data collection and analysis
Two review authors independently performed study selection. One review author extracted data and assessed 'Risk of bias'; another review author checked this information.
Main results
We identified 10 studies (total of 298 children; we identified two studies for this update) including two randomised controlled trials (RCTs) that assessed adrenal function. None of the included studies assessed the HPA axis at the level of the hypothalamus, the pituitary, or both. Owing to substantial differences between studies, we could not pool results. All studies had risk of bias issues. Included studies demonstrated that adrenal insufficiency occurs in nearly all children during the first days after cessation of glucocorticoid treatment for childhood ALL. Most children recovered within a few weeks, but a small number of children had ongoing adrenal insufficiency lasting up to 34 weeks.
Included studies evaluated several risk factors for (prolonged) adrenal insufficiency. First, three studies including two RCTs investigated the difference between prednisone and dexamethasone in terms of occurrence and duration of adrenal insufficiency. The RCTs found no differences between prednisone and dexamethasone arms. In the other (observational) study, children who received prednisone recovered earlier than children who received dexamethasone. Second, treatment with fluconazole appeared to prolong the duration of adrenal insufficiency, which was evaluated in two studies. One of these studies reported that the effect was present only when children received fluconazole at a dose higher than 10 mg/kg/d. Finally, two studies evaluated the presence of infection, stress episodes, or both, as a risk factor for adrenal insufficiency. In one of these studies (an RCT), trial authors found no relationship between the presence of infection/stress and adrenal insufficiency. The other study found that increased infection was associated with prolonged duration of adrenal insufficiency.
Authors' conclusions
We concluded that adrenal insufficiency commonly occurs in the first days after cessation of glucocorticoid therapy for childhood ALL, but the exact duration is unclear. No data were available on the levels of the hypothalamus and the pituitary; therefore, we could draw no conclusions regarding these outcomes. Clinicians may consider prescribing glucocorticoid replacement therapy during periods of serious stress in the first weeks after cessation of glucocorticoid therapy for childhood ALL to reduce the risk of life‐threatening complications. However, additional high‐quality research is needed to inform evidence‐based guidelines for glucocorticoid replacement therapy.
Special attention should be paid to patients receiving fluconazole therapy, and perhaps similar antifungal drugs, as these treatments may prolong the duration of adrenal insufficiency, especially when administered at a dose higher than 10 mg/kg/d.
Finally, it would be relevant to investigate further the relationship between present infection/stress and adrenal insufficiency in a larger, separate study specially designed for this purpose.
Plain language summary
Suppression of the stress system in children who received synthetic stress hormones for acute lymphoblastic leukaemia
Review question
We reviewed the evidence for suppression of the stress system/hypothalamic‐pituitary‐adrenal (HPA) axis (how often does it happen? how long does the suppression persist?) after treatment with synthetic stress hormones/glucocorticoids in children with acute lymphoblastic leukaemia (ALL).
Background
ALL is the most frequent type of cancer among children. Glucocorticoids, such as prednisone and dexamethasone, play a very important role in the treatment of ALL. However, high‐dose glucocorticoids can cause suppression of the stress axis (in medical terms, the hypothalamic‐pituitary‐adrenal (HPA) axis). Suppression of the stress or HPA axis results in inadequate cortisol production. Cortisol is the natural stress hormone found in humans. When this hormone is produced insufficiently, response to stressors (e.g. trauma, surgery, inflammation) may be impaired and defence against infections may be inadequate. Therefore, insufficient production of cortisol remains a cause of morbidity and death in childhood. The occurrence and duration of HPA axis suppression after glucocorticoid therapy for childhood ALL are unclear.
Study characteristics
This systematic review included eight cohort studies and two randomised studies with a total number of 298 patients. All studies assessed adrenal function in paediatric patients treated with glucocorticoids for ALL. The evidence is current to December 2016. None of these studies assessed the HPA axis at the level of the hypothalamus, the pituitary, or both. We could not combine the results of different studies because of heterogeneity.
Key results
Adrenal insufficiency occurred in nearly all children during the first days after completion of glucocorticoid therapy. Most children recovered within a few weeks, but a small number had ongoing adrenal insufficiency lasting up to 34 weeks. Three studies looked into differences in duration of adrenal insufficiency between children who received prednisone and those who were given dexamethasone (two types of glucocorticoids). Two of these three studies found no differences. In the other study, children who received prednisone recovered earlier than those who received dexamethasone. Also, treatment with a certain antifungal drug (fluconazole) seemed to prolong the duration of adrenal insufficiency. Two studies investigated this. Finally, two studies evaluated the presence of infection/stress as a risk factor for adrenal insufficiency. One study found no relationship. The other study reported that increased infection was associated with a longer duration of adrenal insufficiency.
More high‐quality research is needed to define the exact occurrence and duration of HPA axis suppression. Then adequate guidelines for glucocorticoid replacement therapy can be formulated.
Quality of the evidence
All of the included studies had some risk of bias issues.
Background
Of all malignancies in children, acute lymphoblastic leukaemia (ALL) occurs most frequently. In the Netherlands, ALL is newly diagnosed in approximately 120 children annually (DCOG 2014). Treatment and survival rates for childhood ALL have substantially improved over time, and morbidity and mortality due to treatment‐related side effects have become increasingly important. Unfortunately, up to 5% of children die as the result of toxic side effects of treatment, and this percentage is even greater in higher‐risk subgroups. The main cause of this treatment‐related mortality is infection associated with cytotoxic and immunosuppressive drugs (Christensen 2005; Pruckner 2009; Rubnitz 2004; Wheeler 1996). Glucocorticoid therapy is an important contributing factor to the occurrence and severity of infection (Te Poele 2007). Several studies have reported an increase in sepsis and lethal infections among children with ALL when prednisone was substituted for the more potent glucocorticoid dexamethasone (Hurwitz 2000; Igarashi 2005; Te Poele 2007).
Glucocorticoids play a major role in the treatment of ALL, inducing apoptosis of lymphoblastic cells (Planey 2000). Children with ALL receive cyclical courses of high‐dose glucocorticoids such as prednisone (or prednisolone) and dexamethasone. However, supraphysiological doses of glucocorticoids can suppress hypothalamic secretion of corticotrophin‐releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) by the pituitary gland, resulting in secondary adrenal cortex atrophy with delayed recovery of hypothalamic‐pituitary‐adrenal (HPA) axis function. In states of profound or prolonged ACTH deficiency, the adrenal glands may be temporarily unable to generate sufficient cortisol (Henzen 2000; Krasner 1999). Supraphysiological glucocorticoid therapy is the most common cause of secondary adrenal insufficiency (Shulman 2007). The HPA axis plays a major role in the stress response and in host defence against infection. Stressors such as trauma, surgery, or inflammation stimulate the HPA axis, leading to an increase in cortisol production. In turn, increased cortisol levels cause anti‐inflammatory effects and inhibition of pro‐inflammatory cytokines (Hettmannsperger 1992; Nyhlén 2000; Waage 1988). Suppression of the HPA axis resulting in reduced adrenal cortisol production represents an impaired stress response and an inadequate host defence against infection and remains a cause of morbidity and death in childhood (Shulman 2007).
Various tests of HPA axis function have been well established. Morning serum cortisol value reflects basal adrenal function but gives no indication of capacity to respond to stress (Agwu 1999; Schlaghecke 1992). Stimulation tests are used to assess the response of the HPA axis to stress. The insulin tolerance test is considered the most reliable way to evaluate HPA axis function at the level of the pituitary and the adrenal glands, but it is associated with potentially dangerous side effects (Shah 1992). The CRH stimulation test is indicated only for individuals with a central disorder of the HPA axis (Maghnie 2005; Van Tijn 2008). The glucagon test has proved to be a safe and reliable method for testing HPA axis function at the levels of the pituitary and the adrenal glands, but it may induce mild inadvertent side effects (Böttner 2005; Rao 1987; Vanderschueren‐Lodeweyckx 1974). A well‐established alternative method for testing the HPA axis at the level of the adrenal gland without undesirable side effects is the low‐dose (1 µg) ACTH stimulation test, which can detect more subtle degrees of adrenal atrophy caused by central adrenal insufficiency than are detected by the 'normal' ACTH stimulation test (250 µg). Results of the low‐dose ACTH test closely correlate with those of the insulin tolerance test (Abdu 1999; Dickstein 1997; Tordjman 2000).
Several studies have prospectively assessed HPA axis function following treatment with high‐dose glucocorticoids for ALL. Adrenal stimulation tests have been performed and repeated until cortisol levels were normalised. Most children seemed to recover in a few weeks, but prolonged suppression may occur, lasting longer than several months in some cases (Einaudi 2008; Mahachoklertwattana 2004; Rix 2005; Salem 2015; Vestergaard 2011). Children with ALL plus HPA axis suppression with fever or other stressors may benefit from glucocorticoid replacement therapy (e.g. hydrocortisone); therefore, it is important to derive a consistent picture of HPA axis impairment after corticosteroid therapy.
This is the second update of the first Cochrane systematic review of HPA axis function after glucocorticoid therapy for childhood ALL (Gordijn 2012; Gordijn 2015). On the basis of information provided in this review, adequate guidelines for glucocorticoid substitution can be formulated and implemented to reduce risk of infection in childhood ALL.
Objectives
To examine the occurrence and duration of HPA axis suppression after (each cycle of) glucocorticoid therapy for childhood ALL.
Methods
Criteria for considering studies for this review
Types of studies
All study designs except case reports and patient series with fewer than 10 children, examining effects of glucocorticoid therapy for childhood ALL on HPA axis function. This effect can be evaluated both during treatment for ALL (after cessation of a glucocorticoid course) and after all ALL treatment is completed. Review authors resolved disagreements concerning the definition of a cohort study by consensus, with no third party arbitration needed.
Types of participants
Children between the ages of 0 and 18 years who were treated with glucocorticoids for ALL, irrespective of the duration of follow‐up after completion of glucocorticoid therapy. Exclusion criteria consisted of cranial radiotherapy because this treatment may damage the HPA axis, as well as assessment of HPA axis function by a CRH stimulation test only because this test is indicated only for patients with a central disorder of the HPA axis (Maghnie 2005; Van Tijn 2008).
Types of interventions
Glucocorticoid therapy (prednisone, prednisolone, dexamethasone) during treatment for ALL. The intervention was not compared with a control because this option was not available (except in the included randomised controlled trials (RCTs)).
Types of outcome measures
Outcomes reported here were not used as criteria for including studies but are the outcomes of interest within studies identified for inclusion.
Primary outcome
Adrenal insufficiency (occurrence and duration), measured by early‐morning plasma cortisol levels (between 8 and 10 a.m.) or by stimulation tests (e.g. low‐dose ACTH stimulation test, glucagon stimulation test). We used the cutoff limit as defined by the authors of original studies.
Secondary outcomes
To examine whether adrenal insufficiency after administration of glucocorticoids is dependent on:
moment of testing after cessation of glucocorticoid therapy;·
(cumulative) dose of glucocorticoids;
type of glucocorticoid: prednisone, prednisolone, or dexamethasone;
duration of glucocorticoid therapy;
method of cessation of glucocorticoid therapy: abrupt or gradual; or
other possible risk factors (such as fluconazole and presence of infection/stress).
Search methods for identification of studies
See Cochrane Childhood Cancer methods used in reviews (Module CCG). The objective of the literature search was to identify all studies, except case reports and case series, reporting on HPA axis function after glucocorticoid therapy for childhood ALL. Cochrane Childhood Cancer ran searches in the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and Embase; the review authors ran all other searches.
Electronic searches
We searched the following electronic databases: CENTRAL (2016, Issue 11), MEDLINE/PubMed (from 1945 to 12 December 2016), and Embase/Ovid (from 1980 to 12 December 2016). We have provided in appendices the search strategies used for individual electronic databases (using a combination of controlled vocabulary and text words) (Appendix 1; Appendix 2; Appendix 3).
Searching other resources
We located information about trials not registered in CENTRAL, MEDLINE, or Embase, either published or unpublished, by searching the reference lists of relevant articles and review articles. We handsearched conference proceedings of the International Society for Paediatric Oncology (SIOP) and the American Society of Clinical Oncology (ASCO) (from 2005 up to and including 2016). Moreover, for this second review update, we handsearched conference proceedings of the American Society of Pediatric Hematology/Oncology (ASPHO) (from 2014 up to and including 2016). For the search strategy, see Appendix 4.
We scanned the following registers for ongoing trials: the International Standard Randomized Controlled Trial Number (ISRCTN) register (http://www.controlled‐trials.com), the National Institutes of Health (NIH) register (www.clinicaltrials.gov), and the International Clinical Trials Registry Platform (ICTRP) of the World Health Organization (apps.who.int/trialsearch) (all screened 27 December 2016; for the search strategy, see Appendix 5).
We imposed no language restrictions. We will update these searches every two years.
Data collection and analysis
Selection of studies
Two review authors independently selected studies meeting the inclusion criteria. Review authors resolved disagreements by consensus, with no third party arbitration needed. We obtained in full for closer inspection any study that seemed to meet the inclusion criteria upon review of titles or abstracts, or both. We clearly stated reasons for exclusion of any study considered for this review. We have included in the review a flow chart for selection of studies.
Data extraction and management
One review author performed data extraction using standardised forms; another review author checked the data recorded. Review authors were not blinded to journal, authors, or institution. We extracted data on the following categories: study characteristics, children, interventions, outcome measures, length of follow‐up, risk factors, and 'Risk of bias' assessment. We resolved disagreements between review authors by consensus, with no third party arbitration needed.
Assessment of risk of bias in included studies
We based our assessment of risk of bias on previously described checklists for observational studies according to evidence‐based medicine criteria (Grimes 2002; Laupacis 1994). One review author performed the risk of bias assessment of included studies; another review author checked these assessments. We have described in Table 1 the 'Risk of bias' assessment criteria for observational studies. In assessing RCTs, we used 'Risk of bias' items as described in the module of Cochrane Childhood Cancer (Module CCG) and based on recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (see Table 2). We resolved disagreements among review authors by consensus, with no third party arbitration needed.
1. 'Risk of bias' assessment criteria for observational studies.
| Internal validity | External validity | |
| Study group | Selection bias (representative: yes/no):
|
Reporting bias (well defined: yes/no):
|
| Follow‐up | Attrition bias (adequate: yes/no):
|
Reporting bias (well defined: yes/no):
|
| Outcome | Detection bias (blinding: yes/no):
|
Reporting bias (well defined: yes/no):
|
| Risk estimation | Confounding (adjustment for other factors: yes/no):
|
Analysis (well defined: yes/no):
|
ALL: acute lymphoblastic leukaemia.
2. 'Risk of bias' assessment criteria for randomised controlled trials.
|
Selection bias Sequence generation (adequate: yes/no):
Allocation concealment (adequate: yes/no):
|
Performance bias
Blinding of care providers (yes/no):
Blinding of participants (yes/no):
|
Detection bias
Blinding of outcome assessors (yes/no; assessed for each outcome separately):
|
Attrition bias
Incomplete outcome data (adequate: yes/no; assessed for each outcome separately):
|
Reporting bias
Selective outcome reporting (yes/no):
|
Other bias
Other bias (yes/no):
|
Measures of treatment effect
Prevalence of HPA axis suppression at several follow‐up time points and time duration of HPA axis suppression.
Dealing with missing data
We contacted authors of individual studies to ask for clarification of unclear data or to request missing data regarding selection of studies, 'Risk of bias' assessment, or data extraction.
Assessment of heterogeneity
We planned to assess heterogeneity both by visually inspecting forest plots and by performing a formal statistical test for heterogeneity, that is, the I2 statistic. However, because we were not able to pool the results of included studies, this approach was not applicable.
Assessment of reporting biases
We planned to use a funnel plot to quantify the potential presence of publication bias. However, because we were not able to pool the results of included studies, this approach was not applicable.
Data synthesis
We planned to perform analyses using the statistical software Comprehensive Meta‐Analysis (Biostat, Inc., Englewood, NJ, USA) (Biostat, Inc, USA).
Across studies, we planned to conduct a multi‐variate linear meta‐regression analysis model using a backwards selection strategy (P < 0.10) to examine the relation between potential predictive factors and HPA axis suppression.
However, because we were not able to pool the results of included studies, this was not applicable, and we have described the results of individual studies separately.
Sensitivity analysis
We planned to perform a sensitivity analysis for 'Risk of bias' assessment criteria used. However, because we were not able to pool the results of included studies, this was not applicable.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
For the original version of this review (Gordijn 2012), searches run in the electronic databases of CENTRAL, MEDLINE/PubMed, and Embase/Ovid revealed a total of 1388 references. Initial screening of titles and abstracts led to exclusion of 1375 references that clearly did not fulfil all criteria for inclusion of studies in this review. For the remaining 13 references, we examined full‐text articles and deemed that 7 of the 13 were eligible for inclusion in this review. We have provided the reasons for exclusion under Characteristics of excluded studies. Scanning the reference lists of relevant articles and reviews did not reveal additional eligible studies. Scanning the conference proceedings of SIOP and ASCO did not reveal additional eligible studies, and scanning of ongoing trials databases revealed no ongoing studies.
Searches run for the first update (Gordijn 2015) using the electronic databases of CENTRAL, MEDLINE, and Embase, on 16 June 2014, revealed a total of 314 references. Initial screening of titles and abstracts led to exclusion of 310 references that clearly did not fulfil all criteria for inclusion of studies in this review. We examined the full‐text versions of three of the four articles (one full‐text article was not yet available and was placed in the studies awaiting classification list) and determined that one of these was eligible for inclusion in this review. We have provided the reasons for exclusion under Characteristics of excluded studies. Scanning the reference lists of relevant articles and reviews did not reveal additional eligible studies. Scanning the conference proceedings of SIOP and ASCO did not reveal eligible studies, and scanning of ongoing trials databases did not reveal any ongoing studies.
Searches run for the second update in the electronic databases of CENTRAL, MEDLINE, and Embase, on 12 December 2016, revealed a total of 236 references. Initial screening of these titles and abstracts led to exclusion of 235 references that clearly did not fulfil all criteria for inclusion of studies in this review. We examined the full‐text version of one article and determined that this article was eligible for inclusion in the review.
Scanning the reference list of the relevant article did not reveal additional eligible studies.
Scanning the conference proceedings of SIOP, ASCO, and ASPHO revealed one eligible study. The full‐text article for this study was not yet available (see Characteristics of studies awaiting classification) (Schlosser 2016).
Scanning the ongoing trials databases did not reveal any ongoing studies.
For the first update of the review, we placed one study in the list of studies awaiting classification. This study has since been published (Perdomo‐Ramírez 2016). We examined the full‐text article and determined that this study was eligible for inclusion in this review update.
In summary, we included 10 articles in this review. We attempted to contact trial authors to clarify aspects of study design and data analysis. We have summarised characteristics of the included studies in the Characteristics of included studies table. See Figure 1 for a flow diagram showing selection of studies for this systematic review.
1.
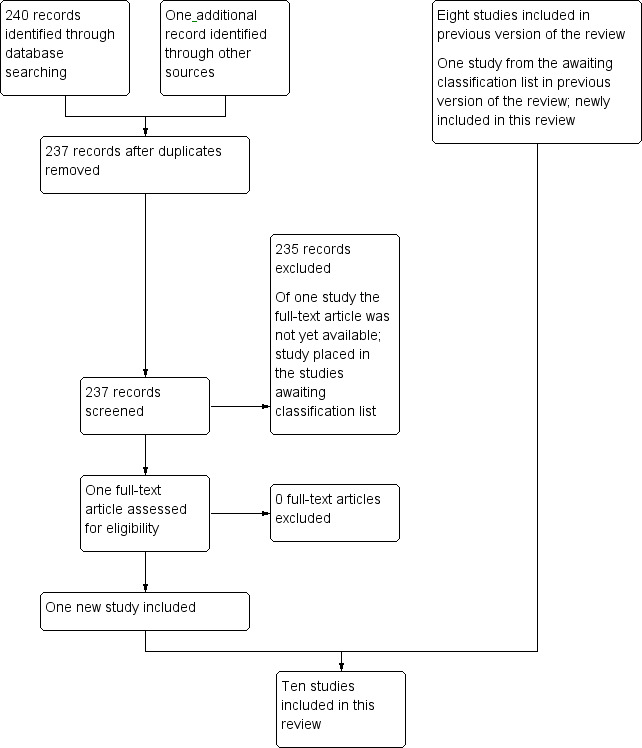
Study flow diagram.
Included studies
All included studies evaluated adrenal function after glucocorticoid therapy for childhood ALL. None of these studies assessed the HPA axis at the level of the hypothalamus, the pituitary, or both. Studies included a total of 298 children. The 10 included studies examined adrenal function after different types, doses, and durations of glucocorticoid therapy and after different methods of cessation of glucocorticoid therapy. Three studies examined effects of dexamethasone on adrenal function (Cunha 2004; Felner 2000; Kuperman 2001). One study examined effects of prednisone on adrenal function (Perdomo‐Ramírez 2016). Six studies evaluated effects of both dexamethasone and predniso(lo)ne on adrenal function (Einaudi 2008; Kuperman 2012; Mahachoklertwattana 2004; Petersen 2003; Rix 2005; Salem 2015). Some investigators measured adrenal function by early‐morning plasma cortisol levels (between 8 and 10 a.m.) (Cunha 2004; Kuperman 2001). Others used the (low‐dose) ACTH stimulation test (Einaudi 2008; Felner 2000; Kuperman 2012; Mahachoklertwattana 2004; Perdomo‐Ramírez 2016; Petersen 2003; Rix 2005; Salem 2015 ). Six studies performed follow‐up tests until normalisation of adrenal function (Einaudi 2008; Felner 2000; Mahachoklertwattana 2004; Perdomo‐Ramírez 2016; Petersen 2003; Salem 2015). Durations of follow‐up for the other four studies were one month, two weeks, two months, and two to seven days, respectively (Cunha 2004; Kuperman 2001; Kuperman 2012; Rix 2005). Eight of the 10 included studies were observational studies (Cunha 2004; Felner 2000; Kuperman 2001; Mahachoklertwattana 2004; Perdomo‐Ramírez 2016; Petersen 2003; Rix 2005; Salem 2015). Two were RCTs evaluating prednisone versus dexamethasone (Einaudi 2008; Kuperman 2012). In one of the RCTs, both treatment groups received prednisone before randomisation (Einaudi 2008).
Excluded studies
We have provided in the Characteristics of excluded studies table information on the eight studies excluded during examination of full‐text articles. The most common reasons for exclusion were cranial irradiation therapy and lack of (adequate) HPA axis function tests.
Risk of bias in included studies
Cohort studies
For evaluation of 'internal validity' of the eight included cohort studies, we assessed risks of selection bias, attrition bias, detection bias, and confounding. Upon obtaining additional information from trial authors, we determined the following. Risk of selection bias (based on representativeness of the study group) was low in four of the eight studies, as the study group consisted of more than 90% of the original cohort (Felner 2000; Kuperman 2001; Petersen 2003; Rix 2005). One study selected an unrepresentative study group of about 30% of the original cohort (Cunha 2004). For the three other studies, neither published articles nor correspondence with trial authors yielded information on selection of children (Mahachoklertwattana 2004; Perdomo‐Ramírez 2016; Salem 2015). Risk of attrition bias (based on completeness of follow‐up) was low in seven of the eight studies, as investigators assessed outcomes for 60% to 90% of the study group at the end date of the study (Cunha 2004; Felner 2000; Kuperman 2001; Mahachoklertwattana 2004; Perdomo‐Ramírez 2016; Petersen 2003; Rix 2005). For the other study, neither the published article nor correspondence with trial authors yielded information on risk of attrition bias (Salem 2015). The authors of two studies reported that the outcome assessor was not blinded (Perdomo‐Ramírez 2016; Rix 2005). None of the other studies provided information on blinding of the outcome assessor to glucocorticoid treatment, so we could not rule out detection bias. Two of the eight included cohort studies addressed risk factors for development or persistence of adrenal insufficiency (Petersen 2003; Salem 2015). Confounding (based on important risk factors and follow‐up taken into account) was not present in these studies.
For evaluation of 'external validity' of the included cohort studies, we assessed risk of reporting bias. Five studies did not define the study group well in terms of treatment protocol and (cumulative) dose, type, duration, and form of cessation of glucocorticoid treatment (Cunha 2004; Mahachoklertwattana 2004; Perdomo‐Ramírez 2016; Petersen 2003; Rix 2005). Two studies did not mention the treatment protocol (Felner 2000; Kuperman 2001). One study did not mention the duration of tapering of glucocorticoid treatment, thus cumulative dose and total duration of glucocorticoid treatment were unclear (Salem 2015). All eight studies defined follow‐up well, as trial authors mentioned both length of follow‐up and frequency of measurement (Cunha 2004; Felner 2000; Kuperman 2001; Mahachoklertwattana 2004; Perdomo‐Ramírez 2016; Petersen 2003; Rix 2005; Salem 2015). For these studies, outcomes were well defined, methods of detection were described, and outcome definitions were objective and precise (Cunha 2004; Felner 2000; Kuperman 2001; Mahachoklertwattana 2004; Perdomo‐Ramírez 2016; Petersen 2003; Rix 2005; Salem 2015). One of the two studies addressing risk factors defined risk estimation (based on calculation of risk ratio, odds ratio, attributable risk, linear or logistic regression model, mean difference, or Chi2 statistic) well (Salem 2015). The other study did not define risk estimation well (Petersen 2003).
See also Table 3.
3. Risk of bias in included observational studies.
| Study | Representative study group | Complete follow‐up assessment | Blinded outcome assessor | Adjustment for important confounders | Well‐defined study group | Well‐defined follow‐up | Well‐defined outcome | Well‐defined risk estimation |
| Cunha 2004 | No, based on additional information provided by trial authors, the study group described did not consist of more than 90% of the original cohort and was not a random sample. | Yes, outcome was assessed for 60% to 90% of the study group at the end date of the study. | Unclear whether outcome assessor was blinded to glucocorticoid treatment | Yes, treatment protocol and (cumulative) dose, type, duration, and form of cessation of glucocorticoid treatment were mentioned. | Yes, length of follow‐up and frequency of measuring were mentioned. | Yes, methods of detection were described, and outcome definition was objective and precise. | ||
| Felner 2000 | Yes, based on additional information provided by trial authors, the study group described consisted of more than 90% of the original cohort. | Yes, outcome was assessed for 60% to 90% of the study group at the end date of the study. | Unclear whether outcome assessor was blinded to glucocorticoid treatment | No, treatment protocol was not mentioned. | Yes, length of follow‐up and frequency of measuring were mentioned. | Yes, methods of detection were described, and outcome definition was objective and precise. | ||
| Kuperman 2001 | Yes, based on additional information provided by trial authors, the study group described consisted of more than 90% of the original cohort. | Yes, outcome was assessed for 60% to 90% of the study group at the end date of the study. | Unclear whether outcome assessor was blinded to glucocorticoid treatment | No, treatment protocol was not mentioned. | Yes, length of follow‐up and frequency of measuring were mentioned. | Yes, methods of detection were described, and outcome definition was objective and precise. | ||
| Mahachoklertwattana 2004 | Unclear whether the study group consisted of more than 90% of the original cohort, or if it was a random sample | Yes, outcome was assessed for 60% to 90% of the study group at the end date of the study. | Unclear whether outcome assessor was blinded to glucocorticoid treatment | Yes, treatment protocol and (cumulative) dose, type, duration, and form of cessation of glucocorticoid treatment were mentioned. | Yes, length of follow‐up and frequency of measuring were mentioned. | Yes, methods of detection were described, and outcome definition was objective and precise. | ||
| Perdomo‐Ramírez 2016 | Unclear whether the study group consisted of more than 90% of the original cohort, or if it was a random sample | Yes, outcome was assessed for 60% to 90% of the study group at the end date of the study. | No, outcome assessor was not blinded to glucocorticoid treatment. This information was based on additional information provided by trial authors. | Yes, treatment protocol and (cumulative) dose, type, and duration of glucocorticoid therapy were mentioned. | Yes, length of follow‐up and frequency of measuring were mentioned. | Yes, methods of detection were described, and outcome definition was objective and precise. | Yes, mean difference was calculated. | |
| Petersen 2003 | Yes, the study group described consisted of more than 90% of the original cohort. | Yes, outcome was assessed for 60% to 90% of the study group at the end date of the study. | Unclear whether outcome assessor was blinded to glucocorticoid treatment. | Yes, important prognostic factors or follow‐up was taken into account. | Yes, treatment protocol and (cumulative) dose, type, and duration of glucocorticoid treatment were mentioned. Information on the method of cessation of glucocorticoid treatment was based on additional information provided by trial authors. | Yes, length of follow‐up and frequency of measuring were mentioned. | Yes, methods of detection were described, and outcome definition was objective and precise. | No, risk ratio, odds ratio, attributable risk, linear or logistic regression model, mean difference, or Chi2 statistic was not calculated. |
| Rix 2005 | Yes, the study group described consisted of more than 90% of the original cohort. | Yes, outcome was assessed for 60% to 90% of the study group at the end date of the study. | No, outcome assessor was not blinded to glucocorticoid treatment. | Yes, treatment protocol and (cumulative) dose, type, and duration of glucocorticoid treatment were mentioned. Information on the method of cessation of glucocorticoid treatment was based on additional information provided by trial authors. | Yes, length of follow‐up and frequency of measuring were mentioned. | Yes, methods of detection were described, and outcome definition was objective and precise. | ||
| Salem 2015 | Unclear whether the study group consisted of more than 90% of the original cohort, or if it was a random sample | Unclear whether outcome was assessed for 60% to 90% at the end date of the study. | Unclear whether outcome assessor was blinded to glucocorticoid treatment | Yes, important prognostic factors or follow‐up was taken into account. | No, duration of tapering of glucocorticoid treatment was not mentioned. | Yes, length of follow‐up and frequency of measuring were mentioned. | Yes, methods of detection were described, and outcome definition was objective and precise. | Yes, mean difference was calculated. |
RCTs
For included RCTs, we evaluated risks of selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. Upon receiving additional information from trial authors, we made the following determinations. We found no selection bias (based on sequence generation and concealment of allocation) in two studies (Einaudi 2008; Kuperman 2012). We could not rule out performance bias (based on blinding of care providers and children) or detection bias (based on blinding of outcome assessors) in one RCT (Einaudi 2008). This same RCT was susceptible to reporting bias, as trial authors did not report all of the study's prespecified primary outcomes (Einaudi 2008). (For assessment of selective outcome reporting bias, we compared the methods and results sections of included RCTs.) We found no performance bias, detection bias, or reporting bias in the other RCT (Kuperman 2012). For both RCTs, we judged that risks of attrition bias (based on completeness of outcome data) and other bias (i.e. based on potential sources of bias related to specific study design, premature termination of the study due to some data‐dependent process, or extreme baseline imbalance) were low (Einaudi 2008; Kuperman 2012). See also Table 4.
4. Risk of bias in included randomised controlled trials.
| Study | Adequate sequence generation? | Adequate allocation concealment? | Blinding? | Incomplete outcome data addressed? | Free of selective reporting? | Free of other bias? |
| Einaudi 2008 | Yes, according to additional information provided by trial authors, the rule for allocating interventions to children was based on some chance (random) process. | Yes, according to additional information provided by trial authors, the randomisation method did not allow investigator and child to know or influence allocation of treatment before eligible children entered the study. | Based on additional information provided by trial authors, care providers, children, and outcome assessors were not blinded. | Yes, no outcome data were missing. | No, "adrenal function completely recovered in the 12 children evaluated with subsequent low‐dose ACTH test (in 4, 3, and 5 patients after 4, 8, and 10 weeks, respectively)". However, it was not reported which of these children received prednisone and which received dexamethasone. Therefore, not all of the study's prespecified primary outcomes were reported. | Yes |
| Kuperman 2012 | Yes, the rule for allocating interventions to children was based on some chance (random) process. | Yes, according to additional information provided by trial authors, the randomisation method did not allow investigator and child to know or influence allocation of treatment before eligible children entered the study. | Yes, based on additional information provided by trial authors, care providers, children, and outcome assessors were all blinded. | Outcomes were assessed for 83% to 93% of the study population. | Yes | Yes |
ACTH: adrenocorticotropic hormone.
Effects of interventions
Adrenal insufficiency (occurrence and duration)
We could extract from all included studies data on the prevalence and duration of adrenal insufficiency after treatment with glucocorticoid therapy for childhood ALL. However, it should be noted that individual studies used different types and (cumulative) doses of glucocorticoids, and we detected differences in the duration and method of cessation of glucocorticoid therapy. Methods of testing adrenal function varied as well. Owing to this heterogeneity, pooling of results was not possible. For more information, see Characteristics of included studies.
ACTH stimulation test
Two studies used the ACTH stimulation test with comparable cutoff limits (stimulated cortisol 18 µg/dL (500 nmol/L)) in measuring adrenal function (Felner 2000; Petersen 2003). Before glucocorticoid therapy, adrenal function was normal in all children in one study (Felner 2000). However, all 10 children (100%) had insufficient cortisol levels one day after abrupt cessation of 28 days of dexamethasone at 6 mg/m2/d. Three out of 10 children (30%) had ongoing adrenal insufficiency after four weeks, but all recovered after eight weeks. Another study assessed two types of glucocorticoid therapy: induction therapy comprising 35 days of prednisolone 60 mg/m2/d with tapering over nine days, and reinduction therapy comprising 21 days of dexamethasone 10 mg/m2/d with tapering over nine days (Petersen 2003). Reinduction therapy followed induction therapy, thus children in the dexamethasone group also received induction therapy with prednisolone. Investigators did not assess HPA axis function before glucocorticoid therapy. After induction therapy (n = 10), 7 out of 10 children (70%) had adrenal insufficiency within the first week. Six children (60%) had ongoing adrenal insufficiency after three weeks, and four children (40%) after seven weeks. These four children remained insufficient at the end of follow‐up, that is, after 10, 11, 11, and 19 weeks, respectively. The last child, who showed no recovery by 19 weeks, received, in addition to induction therapy, two one‐week‐long reinduction courses including prednisolone 60 mg/m2/d during adrenal insufficiency. After completing reinduction therapy (n = 7), five out of seven children (71%) had adrenal insufficiency within the first week. Four children (57%) had ongoing adrenal insufficiency after three weeks, and three children (43%) after seven weeks. These three children remained insufficient at the end of follow‐up, that is, after 16, 33, and 34 weeks, respectively. One of these children, who showed no recovery after 16 weeks, received an additional one‐week‐long reinduction course of prednisolone 60 mg/m2/d during the period of adrenal insufficiency. The other two children, who showed no recovery after 33 and 34 weeks, received three additional one‐week‐long reinduction courses, including prednisolone 60 mg/m2/d, during adrenal insufficiency. See Table 5 for an overview of the prevalence and duration of adrenal insufficiency in studies that used an ACTH suppression test.
5. Prevalence and duration of adrenal insufficiency evaluated by an ACTH stimulation test.
| Felner et al | Therapy: dexamethasone (cumulative dose 168 mg/m2) | |||
| Time after cessation | Before | 1 day | 4 weeks | 8 weeks |
| n insufficient/n total | 0/10 | 10/10 | 3/10 | 0/10 |
| Petersen et al (1) | Therapy: prednisolone (cumulative dose 2257.5 mg/m2)a | |||
| Time after cessation | 1 week | 3 weeks | 7 weeks | End of follow‐up: 10, 11, 11, and 19 weeks, respectively |
| n insufficient/n total | 7/10 | 6/10 | 4/10 | 4/10 |
| Petersen et al (2) | Therapy: dexamethasone (cumulative dose 236.25 mg/m2)b | |||
| Time after cessation | 1 week | 3 weeks | 7 weeks | End of follow‐up: 16, 33, and 34 weeks, respectively |
| n insufficient/n total | 5/7 | 4/7 | 3/7 | 3/7 |
ACTH: adrenocorticotropic hormone. a One child received additional 840 mg/m2 prednisolone during the period of adrenal insufficiency.
b These children received prednisolone 2257.5 mg/m2 as induction therapy before. Three high‐risk patients received an additional 560 mg/m2 prednisolone in advance. Furthermore, owing to persistent adrenal insufficiency, one of these high‐risk children received an additional 420 mg/m2 prednisolone during the period of insufficiency, and the other two high‐risk children received an additional 1260 mg/m2 prednisolone during that period.
Low‐dose ACTH stimulation test with comparable cutoff limits
Five studies used the low‐dose ACTH stimulation test with comparable cutoff limits (stimulated cortisol 18 µg/dL (500 nmol/L)) in measuring adrenal function (Einaudi 2008; Mahachoklertwattana 2004; Perdomo‐Ramírez 2016; Rix 2005; Salem 2015 ).
In one study, all children received induction therapy with prednisolone 40 mg/m2/d (Mahachoklertwattana 2004). This was followed four weeks after completion by maintenance therapy consisting of seven days of dexamethasone 8 mg/m2/d every four weeks. Baseline cortisol levels before induction therapy and two weeks afterwards were not significantly different between adrenal‐suppressed and adrenal‐unsuppressed groups. Eleven out of 24 children (46%) had adrenal insufficiency two weeks after abrupt cessation of 28 days of induction therapy. Nine children (38%) had ongoing adrenal insufficiency after four weeks, seven children (29%) after eight weeks, and three children (13%) after 12 weeks. The last three children remained insufficient at the end of follow‐up at 20 weeks.
Another study (Rix 2005) evaluated three types of glucocorticoid treatment. All children (at standard, intermediate, and high risk) received induction therapy (22 children in total were tested afterwards; for two children, no information was available) comprising 35 days of prednisolone 60 mg/m2/d with tapering over nine days. All children also received seven‐day courses of prednisolone 60 mg/m2/d without tapering (13 children in total were tested afterwards); intermediate‐ and high‐risk children also received 21 days of dexamethasone 10 mg/m2/d with tapering over nine days (seven children in total were tested afterwards). The seven‐day course of prednisolone and the dexamethasone course followed induction therapy. The intermediate‐risk group received the dexamethasone course before the seven‐day course of prednisolone, and the high‐risk group received the dexamethasone course after the seven‐day course of prednisolone. Trial authors provided information showing that 13 children were tested before induction therapy, and all had normal adrenal function. Sixteen out of 22 children (73%) had adrenal insufficiency one day after cessation of induction therapy; one was lost to follow‐up thereafter. Five out of 22 children (23%) were not tested at this time point. Eight children (36%) had ongoing adrenal insufficiency after three days; two were lost to follow‐up afterwards. Seven children (32%) with no confirmed adrenal recovery were not tested at this time point (including the child lost to follow‐up after the first test moment subsequent to cessation of induction therapy). Eight children (36%) had ongoing adrenal insufficiency at the end of five days of follow‐up. Three children (14%) (i.e. the three children lost to follow‐up) with no confirmed adrenal recovery were not tested at this time point. After a seven‐day course of prednisolone, all 13 children (100%) remained insufficient at the end of the follow‐up period of two days. However, two out of 13 children (15%) had insufficient adrenal function before receiving prednisolone therapy. Five out of seven children (71%) underwent a low‐dose ACTH test before receiving dexamethasone therapy; all had sufficient cortisol levels. One day after the dexamethasone course, two out of seven children (29%) had adrenal insufficiency; one was lost to follow‐up thereafter. Five out of seven children (71%) were not tested at this time point. Three children (43%) had ongoing adrenal insufficiency after three days; one was lost to follow‐up afterwards. Two children (29%) (including the child lost to follow‐up) with no confirmed adrenal recovery were not tested at this time point. One child (14%) had ongoing adrenal insufficiency at the end of the follow‐up period of seven days. Two children (29%) (i.e. the two children lost to follow‐up) with no confirmed adrenal recovery were not tested at this time point.
Another study examined two randomised arms of glucocorticoid therapy: 22 days of prednisone 60 mg/m2/d with tapering over nine days (n = 40), and 22 days of dexamethasone 10 mg/m2/d with tapering over nine days (n = 24) (Einaudi 2008). Both groups of children received seven days of prednisone 60 mg/m2/d in advance. At diagnosis, basal cortisol values were within the normal range for all children. In the prednisone arm, 32 out of 40 children (80%) had adrenal insufficiency one day after cessation of glucocorticoid therapy, eight children (20%) had ongoing adrenal insufficiency after 7 to 14 days, and five children (13%) after 28 days. All children (100%) recovered in 10 weeks. In the dexamethasone arm, 20 out of 24 children (83%) had adrenal insufficiency one day after cessation of glucocorticoid therapy. Four children (17%) had ongoing adrenal insufficiency after 7 to 14 days, and three children (13%) after 28 days. All children (100%) recovered in 10 weeks.
In another study, researchers evaluated two types of glucocorticoid treatment during both induction and reinduction phases of treatment (Salem 2015). During the induction phase, children whose condition was diagnosed before 2006 (dexamethasone group) received dexamethasone 6 mg/m2/d for 28 consecutive days with an unknown duration of tapering (n = 20), and children whose condition was diagnosed during and after 2006 (prednisone group) received prednisone 60 mg/m2/d for 28 consecutive days with an unknown duration of tapering (n = 20). During the reinduction phase, the dexamethasone group received dexamethasone 6 mg/m2/d for 21 days with an unknown duration of tapering, and the prednisone group received prednisone 60 mg/m2/d for 21 days with an unknown duration of tapering. Time that passed between induction and reinduction was not mentioned by trial authors. Adrenal function was tested at diagnosis (before start of therapy) and during both induction and reinduction at the following time points: immediately after the last steroid course (week 0), two weeks after completion of glucocorticoid treatment (week 2), four weeks after completion of glucocorticoid treatment (week 4), and every two weeks thereafter until HPA axis recovery was reached. Moreover, adrenal function was tested during infectious events. This study found that children who received dexamethasone had a statistically significantly longer duration of adrenal insufficiency than those who received prednisone (P < 0.005). At diagnosis, adrenal insufficiency was present in 11 out of 40 children (27.5%) ‐ 5 out of 20 (25%) in the dexamethasone group and 6 out of 20 (30%) in the prednisone group. In the dexamethasone group, 50% to 55% of participants had adrenal insufficiency at week 4, and 25% to 35% had ongoing insufficiency at week 6 during both induction and reinduction. All children recovered in 20 weeks. Mean duration to adrenal recovery was 7.20 weeks for the dexamethasone group (during both induction and reinduction). In the prednisone group, 25% to 35% showed adrenal insufficiency at week 2, and 5% to 30% had ongoing adrenal insufficiency at weeks 4 and 6, during both induction and reinduction. All children recovered in 20 weeks. Mean time until adrenal recovery was 3.00 and 4.80 weeks (respectively, during induction and reinduction) in the prednisone group. Trial authors did not provide specific data on participant levels or for each time point.
Another study evaluated 28 days of prednisone 60 mg/m2/d with nine days of tapering (n = 40) (Perdomo‐Ramírez 2016). Researchers included in the study only children without adrenal insufficiency before the start of treatment; therefore, basal cortisol and ACTH levels were normal in all children at the start of therapy. Three days after cessation of glucocorticoid therapy, adrenal insufficiency was present in 29 out of 40 children (72.5%). One of these children died before the second evaluation on day 7 after cessation of glucocorticoid therapy. Of the remaining 39 participants, 11 had adrenal insufficiency on day 7 after cessation (28.2%), and three (7.7%) had ongoing adrenal insufficiency 14 days after cessation of glucocorticoid treatment. All children (100%) recovered 30 days after cessation of glucocorticoid treatment.
Low‐dose ACTH stimulation test with lower cutoff limit
One study used the low‐dose ACTH stimulation test with a lower cutoff limit of 14.2 µg/dL (392 nmol/L) (Kuperman 2012). This cutoff value was determined by a control group of 16 children who underwent the low‐dose ACTH test because they were suspected of having any endocrinopathy other than adrenal insufficiency. Investigators examined two randomised arms of glucocorticoid therapy: 28 days of prednisone 40 mg/m2/d without tapering (n = 16), and 28 days of dexamethasone 6 mg/m2/d without tapering (n = 13). Before the start of glucocorticoid therapy, basal cortisol values were abnormal in three children (one in the prednisone arm and two in the dexamethasone arm). Children in both treatment arms (prednisone and dexamethasone) displayed similar mean peak cortisol levels before treatment. Thereafter, a low‐dose ACTH test was performed weekly for eight weeks. However, not all children underwent the stimulation test every week. Information on individual testing schedules and on adrenal recovery per child was not provided. In the prednisone arm, 7 out of 14 children (50%) had insufficient cortisol levels one week after the start of glucocorticoid therapy. Five children had insufficient cortisol levels in the second, third, and fourth weeks of therapy, out of 14 children (36%), 15 children (33%), and 14 children (36%), respectively. During week 5 ‐ the first week after cessation of glucocorticoid therapy ‐ 3 out of 13 children (23%) had adrenal insufficiency. During the second week after cessation (week 6), 4 out of 14 children (29%) had adrenal insufficiency. During the third week after cessation (week 7), 5 out of 14 children (36%) had adrenal insufficiency. At the end of follow‐up (week 8) ‐ four weeks after cessation of glucocorticoid therapy, 4 out of 15 children (27%) remained insufficient. In the dexamethasone arm, 4 out of 10 children (40%) had insufficient cortisol levels one week after the start of glucocorticoid therapy. During the second week of therapy, two out of seven children (29%) had adrenal insufficiency; in the third week, 5 out of 13 children (38%) were insufficient; and in the last week (week 4), only 1 out of 12 children (8%) had abnormal adrenal function. However, in subsequent weeks after cessation of glucocorticoid therapy, 3 out of 13 children (13%) and 5 out of 11 children (45%) were insufficient during week 5 and week 6, respectively. In week 7, 3 out of 10 (30%) children had adrenal insufficiency. At the end of follow‐up (week 8) ‐ four weeks after cessation of glucocorticoid therapy ‐ 3 out of 12 children (25%) remained insufficient.
See Table 6 for an overview of the prevalence and duration of adrenal insufficiency in studies that used a low‐dose ACTH test.
6. Prevalence and duration of adrenal insufficiency evaluated by a low‐dose ACTH stimulation test.
| Mahachoklertwattana et al | Therapy: prednisolone (cumulative dose 1120 mg/m2)a | ||||
| Time after cessation | 2 weeks | 4 weeks | 8 weeks | 12 weeks | 20 weeks |
| n insufficient/n totalb | 11/24 | 9/24 | 7/24 | 3/24 | 3/24 |
| Rix et al (1) | Therapy: prednisolone (cumulative dose 2257.5 mg/m2) | ||||
| Time after cessation | Before | 1 day | 3 days | 5 days | ‐ |
| n insufficient/n totalb | 0/13 | 16/17 | 8/15 | 8/17 | ‐ |
| Rix et al (2) | Therapy: prednisolone (cumulative dose 420 mg/m2)c | ||||
| Time after cessation | Before | 2 days | ‐ | ‐ | ‐ |
| n insufficient/n totalb | 2/13 | 13/13 | ‐ | ‐ | ‐ |
| Rix et al (3) | Therapy: dexamethasone (cumulative dose 236.25 mg/m2)c | ||||
| Time after cessation | Before | 1 day | 3 days | 7 days | ‐ |
| n insufficient/n totalb | 0/5 | 2/2 | 3/5 | 1/5 | ‐ |
| Einaudi et al (1) | Therapy: prednisone (cumulative dose 1477.5 mg/m2)d | ||||
| Time after cessation | 1 day | 7 to 14 days | 28 days | 42 days | 10 weeks |
| n insufficient/n totalb | 32/40 | 8/32 | 5/8 | 5/5 | 0/5 |
| Einaudi et al (2) | Therapy: dexamethasone (cumulative dose 246.25 mg/m2)d | ||||
| Time after cessation | 1 day | 7 to 14 days | 28 days | 42 days | 10 weeks |
| n insufficient/n totalb | 20/24 | 4/20 | 3/4 | 3/3 | 0/3 |
| Kuperman et al 2012 (1) | Therapy: prednisone (cumulative dose 1120 mg/m²) | ||||
| Time after cessation | Before (4 weeks after start of glucocorticoid treatment) | 1 week | 2 weeks | 3 weeks | 4 weeks |
| n insufficient/n totalb | 5/14 | 3/13 | 4/14 | 5/14 | 4/15 |
| Kuperman et al 2012 (2) | Therapy: dexamethasone (cumulative dose 168 mg/m²) | ||||
| Time after cessation | Before (4 weeks after start of glucocorticoid treatment) | 1 week | 2 weeks | 3 weeks | 4 weeks |
| n insufficient/n totalb | 1/12 | 3/13 | 5/11 | 3/10 | 3/12 |
| Perdomo‐Ramírez et al 2015 | Therapy: prednisone (cumulative dose 1837.5 mg/m2) | ||||
| Time after cessation | Before | 3 days | 1 week | 2 weeks | 4 weeks |
| n insufficient/n totalb | 0/40 | 29/40 | 11/28 | 3/11 | 0/3 |
|
Salem et al 2015 (1) (both induction and reinduction phases) |
Therapy: dexamethasone (cumulative dose unknown) | ||||
| Time after cessation | Before (at diagnosis) | 1 day to 18 weeks | 20 weeks | ||
| n insufficient/n totalb | 5/20 | No data on patient level were available. | All children recovered.e | ||
|
Salem et al 2015 (2) (both induction and reinduction phases) |
Therapy: prednisone (cumulative dose unknown) | ||||
| Time after cessation | Before (at diagnosis) | 1 day to 18 weeks | 20 weeks | ||
| n insufficient/n totalb | 6/20 | No data on patient level were available. | All children recovered.e | ||
ACTH, adrenocortiotropic hormone. a Four weeks after completion of induction therapy, children received maintenance therapy consisting of a 7‐day course of high‐dose dexamethasone 8 mg/m2/d every 4 weeks. Cumulative dose depended on how long the child had been followed up.
b If not all children were tested at all time points, then "n total" = n tested.
c All children first received prednisolone (cumulative dose 2257.5 mg/m2).
d After 7 days of prednisone (60 mg/m2/d, cumulative dose 420 mg/m2).
eFrom 4 weeks after cessation of glucocorticoid therapy, only children who were adrenal insufficient at that time point underwent further low‐dose ACTH testing every 2 weeks until adrenal recovery. Therefore it is unknown how many children were tested at 20 weeks (in both induction and reinduction phases).
Basal morning cortisol values
Two studies used basal morning cortisol values in measuring adrenal function. One study, which included 35 children, found that median basal cortisol levels were inhibited on the eighth day (1.2 µg/dL, range 0.9 to 132.7 µg/dL) and on the 28th day (0.9 µg/dL, range 0.9 to 6.6 µg/dL) of 28 days of dexamethasone 6 mg/m2/d and 48 hours after cessation (over 10 days) of dexamethasone treatment (2.4 µg/dL, range 0.9 to 11.2 µg/dL) compared with pre‐glucocorticoid therapy levels (17.5 µg/dL, range 7.6 to 40.9 µg/dL) (P = 0.01 for the three tests vs pre‐glucocorticoid levels) (Cunha 2004). Median basal cortisol levels one month after cessation of dexamethasone treatment (12.4 µg/dL, range 1.8 to 29.0 µg/dL), although slightly lower, did not show a significant difference compared with pre‐glucocorticoid therapy levels. No data on participant levels were provided. In another study, which included 15 children, mean basal cortisol levels (± standard error of the mean) were significantly (P < 0.05) lower on day 7 (10.8 ± 1.0 µg/dL) and day 14 (11.5 ± 2.0 µg/dL) after abrupt cessation of 42 days of dexamethasone 6 mg/m2/d than at pretreatment (17.8 ± 1.3 µg/dL) (Kuperman 2001). Levels at day 7 and day 14 did not differ significantly. Additional information provided by trial authors revealed that all children (100%) had sufficient basal cortisol levels at diagnosis (> 7 µg/dL), whereas 4 out of 15 children (27%) had insufficient basal cortisol levels seven days after cessation of dexamethasone therapy. One child was lost to follow‐up thereafter. Fourteen days after cessation of dexamethasone therapy, 4 out of 14 children (29%) had insufficient basal cortisol levels. It should be noted that one of them had a sufficient basal cortisol level seven days earlier. See Table 7 for an overview of the prevalence and duration of adrenal insufficiency in studies that used basal morning cortisol values.
7. Prevalence and duration of adrenal insufficiency evaluated by basal morning cortisol values.
| Cunha et al | No data on patient levels were available. | ||
| Time after cessation | |||
| n insufficient/n total | |||
| Kuperman et al 2001 | Therapy: dexamethasone (cumulative dose 252 mg/m2)a | ||
| Time after cessation | Before | 1 week | 2 weeks |
| n insufficient/n total | 0/15 | 4/15 | 4/14 |
a Results based on basal cortisol levels; cutoff level 7 µg/dL = 194 nmol/L.
Risk factors for adrenal insufficiency
Four of the included studies addressed risk factors for development or persistence of adrenal insufficiency (Einaudi 2008; Kuperman 2012; Petersen 2003; Salem 2015).
Type of glucocorticoid
Three studies, including two RCTs, investigated differences between prednisone and dexamethasone in occurrence and/or duration of adrenal insufficiency Einaudi 2008; Kuperman 2012; Salem 2015). In one RCT, all children received prednisone before randomisation (Einaudi 2008). See results above. In summary, investigators in the two RCTs found no differences between prednisone and dexamethasone arms with regard to occurrence and duration of adrenal insufficiency (Einaudi 2008; Kuperman 2012). In the other (observational) study, children who received prednisone recovered earlier than those who received dexamethasone (Salem 2015).
Fluconazole
Two cohort studies evaluated fluconazole therapy as a risk factor for persistence of adrenal insufficiency. Three children in one study received fluconazole (Petersen 2003). Two of these children had ongoing adrenal insufficiency eight months after cessation of glucocorticoid therapy (dexamethasone). The third child recovered after three weeks. In the other study, up to 80% of all children received fluconazole at some point during induction or reinduction therapy (Salem 2015). Half of these children received a dosage above 10 mg/kg/d. These children had a longer duration of adrenal insufficiency than those who received lower doses of fluconazole (< 10 mg/kg/d). Investigators did not further specify this information and did not provide data on participant levels.
Infection/stress
Two studies investigated the presence of infection/stress as a risk factor for adrenal insufficiency (Kuperman 2012; Salem 2015). One of these trialsrandomly assigned children to receive prednisone or dexamethasone (Kuperman 2012). Investigators defined episodes of infection/stress by hospitalisation due to fever with or without neutropenia. In the prednisone group, six episodes of infection/stress occurred in children with adrenal insufficiency, and eight episodes in children with adequate cortisol levels (14 episodes of infection/stress in total). In the dexamethasone group, one episode of infection/stress occurred in a child with an insufficient cortisol level, and 10 episodes in children with adequate cortisol levels (11 episodes of infection/stress in total). Trial authors found no relationship between the presence of infection/stress and adrenal insufficiency. In the other cohort study, children received prednisone or dexamethasone according to their treatment protocol (which differed between children whose condition was diagnosed before 2006 and those whose condition was diagnosed during and after 2006) (Salem 2015). For both dexamethasone and prednisone groups, longer duration of adrenal insufficiency was associated with increased occurrence of infection (P = 0.002 and 0.005, respectively). Investigators did not further specify this information and provided no data on participant levels.
Other outcome measures
Owing to heterogeneity, it was not possible to identify whether adrenal function after administration of glucocorticoids was dependent on the moment of testing; the (cumulative) dose, type, or duration of glucocorticoid therapy; or the method of cessation of glucocorticoid therapy.
Discussion
With improvement in survival among individuals with childhood acute lymphoblastic leukaemia (ALL), treatment‐related side effects have become increasingly relevant. Glucocorticoids play an important role in the treatment of those with childhood ALL, but supraphysiological doses may suppress the hypothalamic‐pituitary‐adrenal (HPA) axis, resulting in an impaired stress response and inadequate defence against infection (Henzen 2000; Krasner 1999). Children with HPA axis suppression may benefit from glucocorticoid replacement therapy (e.g. hydrocortisone) to reduce the risk of life‐threatening complications. HPA axis suppression commonly occurs in the first days after cessation of glucocorticoid therapy, but its exact duration is unclear. Adequate guidelines for glucocorticoid substitution are lacking. This is the second update of the first systematic review conducted to evaluate HPA axis function after treatment with glucocorticoid therapy for childhood ALL (Gordijn 2012; Gordijn 2015).
We identified two new studies published since the last review update (Gordijn 2015). In total, we identified 10 studies evaluating adrenal function after treatment with glucocorticoid therapy for childhood ALL, including two randomised controlled trials (RCTs). None of these studies evaluated the HPA axis at the level of the hypothalamus, the pituitary, or both. Owing to substantial differences in types and (cumulative) doses of glucocorticoids used, in duration and method of cessation of glucocorticoid therapy, and in methods of testing adrenal function, pooling of results was not possible. This systematic review used a very broad search strategy in identifying eligible studies. However, although it is unlikely that eligible studies were missed, it is never possible to rule out reporting bias.
Included studies showed that adrenal insufficiency occurs in almost all patients during the first days after cessation of glucocorticoid therapy for childhood ALL. Most children recovered from adrenal insufficiency within seven weeks. However, a small number of children had ongoing adrenal insufficiency lasting up to 34 weeks. At first impression, no significant differences in the occurrence and duration of adrenal insufficiency between different types, (cumulative) doses, durations, and methods of cessation of glucocorticoid therapy are evident, but because of heterogeneity between studies, we were not able to assess this further. Owing to this limitation and to the small numbers of children enrolled in the included studies (i.e. low power), we can provide no definitive conclusions. The two studies designed as RCTs enabled comparison between two different types of glucocorticoid therapy: prednisone versus dexamethasone (Einaudi 2008; Kuperman 2012). In one of these RCTs, both treatment groups received prednisone before randomisation (Einaudi 2008). The occurrence and duration of adrenal insufficiency did not differ between prednisone and dexamethasone (± prednisone) treatment arms. However, because of the low power of included RCTs, we cannot provide definitive conclusions on this topic. One observational study compared prednisone and dexamethasone as well. This study found that children who received dexamethasone had a longer duration of adrenal insufficiency than those given prednisone (mean duration to recovery 7.20 weeks in the dexamethasone group (during both induction and reinduction) vs 3.00 and 4.80 weeks (respectively, during induction and reinduction) in the prednisone group; P < 0,005) (Salem 2015). However, this comparison involved two (non‐random) groups of children on different ALL treatment protocols. Children whose condition was diagnosed before 2006 received dexamethasone according to the CCG‐1991 protocol, and those whose condition was diagnosed during and after 2006 received prednisone according to the modified BFM‐1990 protocol. Thus, results of comparisons of glucocorticoid therapy reported in this study should be interpreted with caution.
Previous studies demonstrated adrenal suppression after high‐dose fluconazole therapy (Albert 2001; Shibata 2001). Only two of the studies included in this review reported on fluconazole therapy (Petersen 2003; Salem 2015). In one of these studies, two of the three children receiving fluconazole had ongoing adrenal insufficiency eight months after cessation of dexamethasone therapy, whereas the third child recovered after three weeks (Petersen 2003). It should be considered that fluconazole therapy may have influenced the duration of adrenal insufficiency in these children. In the other study, up to 80% of children received fluconazole somewhere during induction or reinduction (Salem 2015). Half of these children received a dose higher than 10 mg/kg/d. Compared with children who received lower doses of fluconazole (< 10 mg/kg/d), these children had a prolonged duration of adrenal insufficiency (not specified). Investigators did not provide data on participant levels. Two of the studies included in this review evaluated the presence of infection and/or stress episodes as a risk factor for adrenal insufficiency (Kuperman 2012; Salem 2015). One study randomly assigned children to receive prednisone or dexamethasone (Kuperman 2012). In the prednisone group, 14 episodes of infection/stress occurred (defined by hospitalisation due to fever with or without neutropenia). Six of these episodes occurred in children with insufficient cortisol levels; the other eight episodes concerned children with adequate cortisol levels. In the dexamethasone group, 11 episodes of infection/stress occurred: one in a child with an insufficient cortisol level, and the other 10 in children with adequate cortisol levels. In conclusion, trial authors found no relationship between the presence of infection/stress and adrenal insufficiency. In the other study, longer duration of adrenal insufficiency was associated with increased infection in both dexamethasone and prednisone groups (P = 0.002 and 0.005, respectively) (Salem 2015). Investigators did not further specify this information. In conclusion, it would be relevant to investigate this relationship further in a larger, separate study specifically designed for this purpose.
Owing to the paucity of RCTs on HPA axis suppression after glucocorticoid therapy in childhood ALL, most of the studies included in this systematic review were uncontrolled studies. We identified only two RCTs. The lack of control groups made it impossible for review authors to evaluate possible causes of HPA axis suppression other than glucocorticoid therapy. Moreover, all of the included studies used biochemical markers to evaluate adrenal insufficiency; only two studies additionally discussed the clinical consequences of adrenal insufficiency (Kuperman 2012; Salem 2015). All included studies had risk of bias issues, but currently they provide the best available evidence on occurrence and duration of adrenal insufficiency after glucocorticoid therapy in childhood ALL.
Authors' conclusions
Implications for practice.
Upon review of currently available evidence, we can conclude that adrenal insufficiency routinely occurs during the first days after cessation of glucocorticoid therapy, but the exact duration of adrenal insufficiency remains unclear. Data on levels of the hypothalamus and the pituitary are not available; therefore, we can draw no conclusions regarding these outcomes. Most children in included studies seemed to recover at between three days and seven weeks. However, a small number had prolonged adrenal insufficiency, persisting up to several months. Clinicians may consider prescribing glucocorticoid replacement therapy (e.g. hydrocortisone) during periods of serious stress in the first weeks after cessation of glucocorticoid therapy for childhood acute lymphoblastic leukaemia (ALL), to reduce the risk of life‐threatening complications. If replacement therapy is indicated, its beneficial effects and side effects should be evaluated. Until results of future adequate studies on the incidence and duration of hypothalamic‐pituitary‐adrenal (HPA) axis suppression become available, clinicians may consider performing an HPA axis stimulation test, for example, two months after cessation of glucocorticoids, to determine whether the HPA axis has recovered, and whether replacement therapy provided during periods of stress can be discontinued. Exclusively morning cortisol levels are inappropriate for evaluation of HPA axis suppression because they reflect only basal cortisol prediction ‐ not the ability of the HPA axis to respond to stress (Agwu 1999).
Special attention should be paid to children receiving fluconazole therapy, and perhaps similar antifungal drugs, as such treatment may prolong the duration of adrenal insufficiency. We can make no definitive conclusions regarding differences in occurrence and duration of adrenal insufficiency in terms of type (predniso(lo)ne vs dexamethasone); (cumulative) dose, duration, and method of cessation (abrupt or gradual) of glucocorticoid therapy; and other risk factors such as infection/stress.
Implications for research.
Studies examining HPA axis suppression after high‐dose glucocorticoid therapy for childhood ALL are scarce, especially RCTs. High‐quality research regarding occurrence and duration of HPA axis suppression after glucocorticoid therapy for childhood ALL is needed to inform adequate evidence‐based guidelines for glucocorticoid replacement therapy. Studies evaluating long‐term effects of glucocorticoid therapy on the HPA axis are also needed. Future studies should focus on identifying differences in effects of type, (cumulative) dose, repeated exposure, duration, and method of cessation of glucocorticoid therapy and other risk factors on occurrence and duration of HPA axis suppression. The number of included children should be sufficient to obtain the power needed for reliable results. Furthermore, an interesting and relevant topic for future research would be the (genetic) susceptibility of individuals to HPA axis suppression after glucocorticoid treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 17 April 2017 | New citation required but conclusions have not changed | Summary of most important changes in this update. • We updated the search for eligible studies to December 2016. • We identified 2 new prospective cohort studies including 80 children and included them in the review. However, the conclusions of this review did not change. • We included a new secondary outcome measure: other possible risk factors (such as fluconazole and presence of infections/stress). |
| 12 December 2016 | New search has been performed | We updated the search for eligible studies to December 2016. |
History
Protocol first published: Issue 10, 2010 Review first published: Issue 5, 2012
| Date | Event | Description |
|---|---|---|
| 6 January 2015 | New citation required but conclusions have not changed | Summary of most important changes in this update. • We updated the search for eligible studies to June 2014. • As Kaplan ‐Meier curves were used for time‐to‐event outcomes, and as time to adrenal recovery was not that certain in the included studies because it depended in part on the moment of performing an adrenal function test, we decided to omit Kaplan ‐Meier curves from this review update. • We identified 1 new randomised controlled trial (RCT) comparing prednisone and dexamethasone and included it in the review. However, the conclusions of this review did not change. |
| 16 June 2014 | New search has been performed | We updated the search for eligible studies to June 2014. |
Acknowledgements
We would like to acknowledge the Editorial Base of Cochrane Childhood Cancer for advice and support provided. We thank Leontien Kremer for assistance in preparing the protocol of this review; Peter van de Ven for statistical advice; and Y. Loke and S. Neggers ‐ peer reviewers for the first version of this review ‐ whose comments have improved this paper. We also would like to thank Anna Font Gonzalez for assistance in translating one of the articles. Maartje S. Gordijn was a co‐author of the protocol for this systematic review, the original version, and the first update; we thank her for her valuable input. Finally, we thank all study authors for providing additional information upon request.
The Editorial Base of Cochrane Childhood Cancer is funded by ‘Stichting Kinderen Kankervrij’ (KiKa), the Netherlands.
Appendices
Appendix 1. Search strategy for the Cochrane Central Register of Controlled Trials (CENTRAL)
1. For children, the following text words were used:
(infant OR infan* OR newborn OR newborn* OR new‐born* OR baby OR baby* OR babies OR neonat* OR child OR child* OR schoolchild* OR schoolchild OR school child OR school child* OR kid OR kids OR toddler* OR adolescent OR adoles* OR teen* OR boy* OR girl* OR minors OR minors* OR underag* OR under ag* OR juvenil* OR youth* OR kindergar* OR puberty OR puber* OR pubescen* OR prepubescen* OR prepuberty* OR pediatrics OR pediatric* OR paediatric* OR peadiatric* OR schools OR nursery school* OR preschool* OR pre school* OR primary school* OR secondary school* OR elementary school* OR elementary school OR high school* OR highschool* OR school age OR schoolage OR school age* OR schoolage* OR infancy)
For the update in 2016 the following search strategy was used: infan* OR newborn* OR new‐born* OR perinat* OR neonat* OR baby OR baby* OR babies OR toddler* OR minors OR minors* OR boy OR boys OR boyfriend OR boyhood OR girl* OR kid OR kids OR child OR child* OR children* OR schoolchild* OR schoolchild OR school child OR school child* OR adolescen* OR juvenil* OR youth* OR teen* OR under*age* OR pubescen* OR pediatrics OR pediatric* OR paediatric* OR peadiatric* OR school OR school* OR prematur* OR preterm*
2. For acute lymphocytic leukemia, the following text words were used:
acute lymphocytic leukemia OR childhood ALL OR Precursor Cell Lymphoblastic Leukemia‐Lymphoma OR Precursor Cell Lymphoblastic Leukemia Lymphoma OR Acute Lymphoblastic Leukemia OR Lymphoblastic Lymphoma OR Acute Lymphocytic Leukemia OR Acute Lymphoid Leukemia OR Lymphoblastic Leukemia OR L1 Lymphocytic Leukemia OR L2 Lymphocytic Leukemia OR ((akut* OR acut*) AND (leukemi* OR leukaemi*) OR (lymphocyt* OR lymphoblast*))
3. For glucocorticoids, the following text words were used:
Steroid OR steroids OR steroid* OR glucocorticoid OR glucocorticoids OR glucocorticoid* OR corticoid OR corticoids OR corticoid* OR adrenal cortex hormones OR prednison OR prednisone OR Dehydrocortisone OR delta‐Cortisone OR Winpred OR ICN Brand of Prednisone OR Cortancyl OR Panafcort OR Aventis Brand of Prednisone OR Cutason OR mibe Brand of Prednisone OR Dacortin OR Merck Brand of Prednisone OR Decortin Brand of Prednisone OR Decortisyl OR Hoechst Brand of Prednisone OR Deltasone OR Pharmacia Brand of Prednisone OR Encortone OR Encorton OR Enkortolon OR Kortancyl OR Liquid Pred OR Meticorten OR Schering‐Plough Brand of Prednisone OR Orasone OR Solvay Brand of Prednisone OR Panasol OR Seatrace Brand of Prednisone OR Predni Tablinen OR Lichtenstein Brand of Prednisone OR Prednidib OR Diba Brand of Prednisone OR Predniment OR Ferring Brand of Prednisone OR Prednison acsis OR acis Brand of Prednisone OR Prednison Galen OR GALENpharma Brand of Prednisone OR Prednison Hexal OR Hexal Brand of Prednisone OR Pronisone OR Rectodelt OR Trommsdorff Brand of Prednisone OR Ultracorten OR Sone OR Fawns & McAllan Brand of Prednisone OR Sterapred OR Merz Brand of Prednisone OR Apo‐Prednisone OR Apotex Brand of Prednisone OR Cortan OR Halsey Drug Brand of Prednisone OR prednisolon OR dexamethason OR dexametasone OR dexamethasone OR Methylfluorprednisolone OR Hexadecadrol OR Maxidex OR Alcon Brand of Dexamethasone OR Dexamethasone Intensol OR Roxane Brand of Dexamethasone OR Decaject OR Merz Brand 1 of Dexamethasone OR Oradexon OR Decameth OR Foy Brand of Dexamethasone OR Decaspray OR Merck Brand of Dexamethasone OR Dexasone OR ICN Brand of Dexamethasone OR Hexadrol OR Millicorten OR Dexpak OR ECR Brand of Dexamethasone OR Decaject‐L.A. OR Decaject L.A. OR Merz Brand 2 of Dexamethasone
4. For HPA function, the following text words were used:
HPA OR HPA axis OR hypothalamic‐pituitary‐adrenal OR hypothalamic‐pituitary‐adrenal axis OR adrenal insufficiency OR adrenal axis OR Hypothalamo‐Hypophyseal System OR hypothalamic insufficiency OR pituitary OR Pituitary‐Adrenal Function Tests OR Pituitary Adrenal Function Tests OR Pituitary‐Adrenal Function Test OR Pituitary‐Adrenal System OR Pituitary Adrenal System OR Pituitary‐Adrenal Systems OR hypothalamus OR hypothalam* OR hypophysis OR hypophys* OR Hypothalamo‐Hypophyseal OR Pituitary Gland OR Pituitary Glands OR Hypothalamic Hormones OR Hypothalamic Pituitary‐Regulating Peptides OR Hypothalamic Pituitary Regulating Peptides OR Hypothalamic Pituitary‐Regulating Hormones OR Hypothalamic Pituitary Regulating Hormones OR Pituitary Hormones OR adrenal glands OR adrenal function test OR adrenal function tests OR adrenal function testing OR adrenal function evaluation OR ACTH stimulation test OR ACTH stimulation tests OR ACTH stimulation testing OR ACTH test OR ACTH tests OR ACTH testing OR ACTH evaluation OR CRH stimulation test OR CRH stimulation tests OR CRH stimulation testing OR CRH test OR CRH tests OR CRH testing OR CRH evaluation OR glucagon stimulation test OR glucagon stimulation tests OR glucagon stimulation testing OR glucagon test OR glucagon tests OR glucagon testing OR glucagon evaluation OR fasting cortisol OR morning cortisol
Final search: 1 and 2 and 3 and 4.
The search was performed in title, abstract, or keywords.
Appendix 2. Search strategy for PubMed
1. For children, the following MeSH headings and text words were used:
infant OR infan* OR newborn OR newborn* OR new‐born* OR baby OR baby* OR babies OR neonat* OR perinat* OR postnat* OR child OR child* OR schoolchild* OR schoolchild OR school child OR school child* OR kid OR kids OR toddler* OR adolescent OR adoles* OR teen* OR boy* OR girl* OR minors OR minors* OR underag* OR under ag* OR juvenil* OR youth* OR kindergar* OR puberty OR puber* OR pubescen* OR prepubescen* OR prepuberty* OR pediatrics OR pediatric* OR paediatric* OR peadiatric* OR schools OR nursery school* OR preschool* OR pre school* OR primary school* OR secondary school* OR elementary school* OR elementary school OR high school* OR highschool* OR school age OR schoolage OR school age* OR schoolage* OR infancy OR schools, nursery OR infant, newborn
For the update in 2016 the following search strategy (Leclercq 2013) was used:
infan* OR newborn* OR new‐born* OR perinat* OR neonat* OR baby OR baby* OR babies OR toddler* OR minors OR minors* OR boy OR boys OR boyfriend OR boyhood OR girl* OR kid OR kids OR child OR child* OR children* OR schoolchild* OR schoolchild OR school child[tiab] OR school child*[tiab] OR adolescen* OR juvenil* OR youth* OR teen* OR under*age* OR pubescen* OR pediatrics[mh] OR pediatric* OR paediatric* OR peadiatric* OR school[tiab] OR school*[tiab] OR prematur* OR preterm*
2. For acute lymphocytic leukemia, the following MeSH headings and text words were used:
acute lymphocytic leukemia OR childhood ALL OR Precursor Cell Lymphoblastic Leukemia‐Lymphoma OR Precursor Cell Lymphoblastic Leukemia Lymphoma OR Leukemia, Lymphoblastic OR Leukemia, Lymphoblastic, Acute OR Leukemia, Lymphocytic, Acute OR Leukemia, Lymphoid, Acute OR Lymphoblastic Leukemia, Acute OR Acute Lymphoblastic Leukemia OR Leukemia, Acute Lymphoblastic OR Lymphoblastic Lymphoma OR Lymphomas, Lymphoblastic OR Lymphocytic Leukemia, Acute OR Acute Lymphocytic Leukemia OR Leukemia, Acute Lymphocytic OR Lymphoma, Lymphoblastic OR Acute Lymphoid Leukemia OR Leukemia, Acute Lymphoid OR Lymphoid Leukemia, Acute OR Lymphoblastic Leukemia OR Leukemia, Lymphocytic, Acute, L1 OR Lymphocytic Leukemia, L1 OR L1 Lymphocytic Leukemia OR Leukemia, L1 Lymphocytic OR Lymphoblastic Leukemia, Acute, Childhood OR Lymphoblastic Leukemia, Acute, L1 OR ALL, Childhood OR Childhood ALL OR Leukemia, Lymphoblastic, Acute, L1 OR Leukemia, Lymphocytic, Acute, L2 OR Lymphocytic Leukemia, L2 OR L2 Lymphocytic Leukemia OR Leukemia, L2 Lymphocytic OR Lymphoblastic Leukemia, Acute, Adult OR Lymphoblastic Leukemia, Acute, L2 OR Leukemia, Lymphoblastic, Acute, L2 OR Leukemia, Lymphoblastic, Acute, Philadelphia‐Positive OR ((akut* OR acut*) AND ((leukemi* OR leukaemi*) OR (lymphocyt* OR lymphoblast*)))
3. For glucocorticoids, the following MeSH headings and text words were used:
Steroid OR steroids OR steroid* OR glucocorticoid OR glucocorticoids OR glucocorticoid* OR corticoid OR corticoids OR corticoid* OR adrenal cortex hormones OR hormones, adrenal cortex OR prednison OR prednisone OR 53‐03‐2 OR Dehydrocortisone OR delta‐Cortisone OR Winpred OR ICN Brand of Prednisone OR Cortancyl OR Panafcort OR Aventis Brand of Prednisone OR Cutason OR mibe Brand of Prednisone OR Dacortin OR Merck Brand of Prednisone OR Decortin Brand of Prednisone OR Decortisyl OR Hoechst Brand of Prednisone OR Deltasone OR Pharmacia Brand of Prednisone OR Encortone OR Encorton OR Enkortolon OR Kortancyl OR Liquid Pred OR Meticorten OR Schering‐Plough Brand of Prednisone OR Orasone OR Solvay Brand of Prednisone OR Panasol OR Seatrace Brand of Prednisone OR Predni Tablinen OR Lichtenstein Brand of Prednisone OR Prednidib OR Diba Brand of Prednisone OR Predniment OR Ferring Brand of Prednisone OR Prednison acsis OR acis Brand of Prednisone OR Prednison Galen OR GALENpharma Brand of Prednisone OR Prednison Hexal OR Hexal Brand of Prednisone OR Pronisone OR Rectodelt OR Trommsdorff Brand of Prednisone OR Ultracorten OR Sone OR Fawns & McAllan Brand of Prednisone OR Sterapred OR Merz Brand of Prednisone OR Apo‐Prednisone OR Apotex Brand of Prednisone OR Cortan OR Halsey Drug Brand of Prednisone OR prednisolon OR 50‐24‐8 OR dexamethason OR dexametasone OR dexamethasone OR 50‐02‐2 OR Methylfluorprednisolone OR Hexadecadrol OR Maxidex OR Alcon Brand of Dexamethasone OR Dexamethasone Intensol OR Roxane Brand of Dexamethasone OR Decaject OR Merz Brand 1 of Dexamethasone OR Oradexon OR Decameth OR Foy Brand of Dexamethasone OR Decaspray OR Merck Brand of Dexamethasone OR Dexasone OR ICN Brand of Dexamethasone OR Hexadrol OR Millicorten OR Dexpak OR ECR Brand of Dexamethasone OR Decaject‐L.A. OR Decaject L.A. OR Merz Brand 2 of Dexamethasone
4. For HPA function, the following MeSH headings and text words were used:
HPA OR HPA axis OR hypothalamic‐pituitary‐adrenal OR hypothalamic‐pituitary‐adrenal axis OR adrenal insufficiency OR adrenal axis OR Hypothalamo‐Hypophyseal System OR hypothalamic insufficiency OR pituitary OR Pituitary‐Adrenal Function Tests OR Function Test, Pituitary‐Adrenal OR Function Tests, Pituitary‐Adrenal OR Pituitary Adrenal Function Tests OR Pituitary‐Adrenal Function Test OR Test, Pituitary‐Adrenal Function OR Tests, Pituitary‐Adrenal Function OR Pituitary‐Adrenal System OR Pituitary Adrenal System OR Pituitary‐Adrenal Systems OR System, Pituitary‐Adrenal OR Systems, Pituitary‐Adrenal OR hypothalamus OR hypothalam* OR hypophysis OR hypophys* OR Hypothalamo‐Hypophyseal OR Pituitary Gland OR Pituitary Glands OR Hypothalamic Hormones OR Hormones, Hypothalamic OR Hypothalamic Pituitary‐Regulating Peptides OR Hypothalamic Pituitary Regulating Peptides OR Peptides, Hypothalamic Pituitary‐Regulating OR Pituitary‐Regulating Peptides, Hypothalamic OR Hypothalamic Pituitary‐Regulating Hormones OR Hormones, Hypothalamic Pituitary‐Regulating OR Hypothalamic Pituitary Regulating Hormones OR Pituitary‐Regulating Hormones, Hypothalamic OR Pituitary Hormones OR Hormones, Pituitary OR adrenal glands OR adrenal function test[all fields] OR adrenal function tests[all fields] OR adrenal function testing [all fields] OR adrenal function evaluation[all fields] OR ACTH stimulation test[all fields] OR ACTH stimulation tests[all fields] OR ACTH stimulation testing [all fields] OR ACTH test[all fields] OR ACTH tests[all fields] OR ACTH testing [all fields] OR ACTH evaluation[all fields] OR CRH stimulation test[all fields] OR CRH stimulation tests[all fields] OR CRH stimulation testing [all fields] OR CRH test[all fields] OR CRH tests[all fields] OR CRH testing [all fields] OR CRH evaluation[all fields] OR glucagon stimulation test[all fields] OR glucagon stimulation tests[all fields] OR glucagon stimulation testing [all fields] OR glucagon test [all fields] OR glucagon tests[all fields] OR glucagon testing [all fields] OR glucagon evaluation[all fields] OR fasting cortisol OR morning cortisol
Final search: 1 and 2 and 3 and 4.
[* = 1 or more characters]
Appendix 3. Search strategy for Embase (Ovid)
1. For children, the following Emtree terms and text words were used:
1. infant/ or infancy/ or newborn/ or baby/ or child/ or preschool child/ or school child/ 2. adolescent/ or juvenile/ or boy/ or girl/ or puberty/ or prepuberty/ or pediatrics/ 3. primary school/ or high school/ or kindergarten/ or nursery school/ or school/ 4. or/1‐3 5. (infant$ or newborn$ or (new adj born$) or baby or baby$ or babies or neonate$ or perinat$ or postnat$).mp. 6. (child$ or (school adj child$) or schoolchild$ or (school adj age$) or schoolage$ or (pre adj school$) or preschool$).mp. 7. (kid or kids or toddler$ or adoles$ or teen$ or boy$ or girl$).mp. 8. (minors$ or (under adj ag$) or underage$ or juvenil$ or youth$).mp. 9. (puber$ or pubescen$ or prepubescen$ or prepubert$).mp. 10. (pediatric$ or paediatric$ or peadiatric$).mp. 11. (school or schools or (high adj school$) or highschool$ or (primary adj school$) or (nursery adj school$) or (elementary adj school) or (secondary adj school$) or kindergar$).mp. 12. or/5‐11 13. 4 or 12
For the update in 2016, the following search strategy was used:
1. infan$.mp. 2. (newborn$ or new‐born$).mp. 3. (perinat$ or neonat$).mp. 4. baby/ 5. (baby or baby$ or babies).mp. 6. toddler$.mp. 7. (minors or minors$).mp. 8. (boy or boys or boyfriend or boyhood).mp. 9. girl$.mp. 10. (kid or kids).mp. 11. child/ 12. (child or child$ or children$).mp. 13. school child/ 14. (schoolchild$ or schoolchild).mp. 15. (school child or school child$).ti,ab. 16. (adolescen$ or youth$ or teen$).mp. 17. (juvenil$ or under$age$).mp. 18. pubescen$.mp. 19. exp pediatrics/ 20. (pediatric$ or paediatric$ or peadiatric$).mp. 21. (school or school$).mp. 22. (prematur$ or preterm$).mp. 23. or/1‐22
2. For acute lymphocytic leukemia, the following Emtree terms and text words were used:
1. acute lymphocytic leukemia.mp. or exp Acute Lymphocytic Leukemia/ 2. (childhood adj ALL). 3. precursor cell lymphoblastic leukemia‐lymphoma.mp. or exp Acute Lymphoblastic Leukemia/ 4. lymphoblastic lymphoma.mp. or exp Lymphoblastoma/ 5. (acute lymphoblastic leukemia or acute lymphoid leukemia).mp. 6. lymphoblastic leukemia.mp. or exp Lymphatic Leukemia/ 7. (L1 lymphocytic leukemia or L2 lymphocytic leukemia).mp. 8. (lymphoblastic lymphoma or lymphoblastic lymphomas).mp. or exp lymphatic leukemia/ 9. childhood acute lymphoblastic leukemia.mp. or exp Childhood Leukemia/ 10. philadelphia positive acute lymphoblastic leukemia.mp. 11. ((akut$ or acut$) and (leukemi$ or leukaemi$ or lymphocyt$ or lymphoblast$)).mp. 12. or/1‐11
3. For glucocorticoids, the following Emtree terms and text words were used:
1. steroid.mp. or exp steroid/ 2. (steroids or steroid$).mp. 3. glucocorticoid.mp. or exp glucocorticoid/ 4. (glucocorticoids or glucocorticoid$).mp. 5. corticoid.mp. or exp corticosteroid/ 6. (corticoids or corticoid$).mp. 7. adrenal cortex hormones.mp. 8. prednison.mp. or exp prednisone/ 9. prednisone.mp. 10. 53‐03‐2.rn. 11. (dehydrocortisone or delta‐cortisone or winpred or cortancyl or panafcort or cutason or dacortin or decortisyl).mp. 12. (deltasone or encortone or encorton or emkortolon or kortancyl or meticorten).mp. 13. (orasone or panasol or predni tablinen or prednidib or predniment or prednison acsis).mp. 14. (prednison galen or prednison hexal or pronisone or rectodelt or ultracorten or sone).mp. 15. (sterapred or apo‐prednisone or cortan or prednisolon).mp. 16. 50‐24‐8.rn. 17. exp dexamethasone/ 18. (dexamethason or dexamethasone or dexametasone).mp. 19. 50‐02‐2.rn. 20. (methylfluorprednisolone or hexadecadrol or maxidex or dexamethasone intensol).mp. 21. (decaject or oradexon or decameth or decaspray or dexasone or hexadrol or millicorten or dexpak or decaject‐la or decaject la).mp. 22. or/1‐21
4. For HPA function, the following Emtree terms and text words were used:
1. exp hypothalamus hypophysis adrenal system/ 2. (HPA or HPA axis).mp. 3. (hypothalamic‐pituitary‐adrenal or hypothalamic‐pituitary‐adrenal axis).mp. 4. adrenal insufficiency.mp. or exp adrenal insufficiency/ 5. adrenal axis.mp. 6. hypothalamo‐hypophyseal system.mp. or exp hypothalamus hypophysis system/ 7. hypothalamic insufficiency.mp. 8. pituitary.mp. 9. (pituitary‐adrenal function tests or pituitary‐adrenal function test).mp. 10. (pituitary adrenal function tests or pituitary adrenal function test).mp. 11. (pituitary adrenal system or pituitary‐adrenal system or pituitary adrenal systems or pituitary‐adrenal systems).mp. 12. exp hypothalamus/ or hypothalamus.mp. 13. hypothalam$.mp. 14. exp hypophysis/ or hypophysis.mp. 15. hypophys$.mp. 16. exp hypothalamus hypophysis system/ or hypothalamo‐hypophyseal.mp. 17. (pituitary gland or pituitary glands).mp. 18. hypothalamic hormones.mp. or exp hypothalamus hormone/ 19. (hypothalamic pituitary‐regulating peptides or hypothalamic pituitary regulating peptides).mp. 20. (hypothalamic pituitary‐regulating hormones or hypothalamic pituitary regulating hormones).mp. 21. pituitary hormones.mp. or exp hypophysis hormone/ 22. adrenal glands.mp. or exp adrenal gland/ 23. adrenal function test.mp. or exp endocrine function test/ 24. (adrenal function test or adrenal function testing or adrenal function evaluation).mp. 25. ACTH stimulation test.mp. or exp corticotropin test/ 26. (ACTH stimulation tests or ACTH stimulation testing).mp. 27. (ACTH test or ACTH tests or ACTH testing or ACTH evaluation).mp. 28. (CRH stimulation test or CRH stimulation tests or CRH stimulation testing).mp. 29. (CRH test or CRH tests or CRH testing or CRH evaluation).mp. 30. (glucagon stimulation test or glucagon stimulation tests or glucagon stimulation testing).mp. 31. (glucagon test or glucagon tests or glucagon testing or glucagon evaluation).mp. 32. (fasting cortisol or morning cortisol).mp. 33. or/1‐32
Final search: 1 and 2 and 3 and 4.
[mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name; $ = 1 or more characters; / = Emtree term; rn = registry number]
Appendix 4. Search strategy for conference proceedings
Search used in International Society of Paediatric Oncology (SIOP) proceedings: (http://www.siop.nl) Separate searches were done for the following search terms: HPA HPA axis adrenal hypothalamus hypothalamic‐pituitary‐adrenal
Search used in American Society of Pediatric Hematology/Oncology (ASPHO) proceedings:
(http://www.aspho.org)
Separate searches were done for the following search terms:
HPA
HPA axis
adrenal
hypothalamus
hypothalamic‐pituitary‐adrenal
Search used in American Society of Clinical Oncology (ASCO) proceedings: (http://www.asco.org) Only annual meetings were searched (since molecular markers, breast, genitourinary, gastrointestinal and prostate specific meetings were not expected to yield results that involved HPA axis suppression during acute lymphoblastic leukemia). Separate searches were done for the following search terms: HPA HPA axis adrenal hypothalamus hypothalamic‐pituitary‐adrenal The search was performed in title field.
Appendix 5. Search strategy for ongoing trials registers
Search used for ongoing trials in the International Standard Randomized Controlled Trial Number (ISRCTN) register (http://www.controlled‐trials.com), the National Institutes of Health (NIH) register (www.clinicaltrials.gov), and the International Clinical Trials Registry Platform of the World Health Organization (WHO) (apps.who.int/trialsearch): Separate searches were done for the following search terms: HPA HPA axis adrenal hypothalamus hypothalamic‐pituitary‐adrenal The search was performed in title field.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cunha 2004.
| Methods | Study type: prospective multi‐centre study Setting: Brazil (University Hospital of Federal University of Minas Gerais, Santa Casa de Misericórdia, and Hospital Felício Rocho, Belo Horizonte). This information was based on additional information provided by trial authors. | |
| Participants | 35 children (median age at diagnosis/first HPA axis function test 6.9 years (range 1.2 to 14.4 years); 17 boys and 18 girls) with ALL | |
| Interventions | Treatment according to the Brazilian Group for Treatment of ALL, 1993 protocol (GBTLI‐93). Specific medication not defined
Type of glucocorticoid therapy: dexamethasone (6 mg/m2/d, twice daily) given for 28 days
Cumulative dexamethasone dose: 183.75 mg/m2
Duration of glucocorticoid therapy: 28 days + 9 days tapering doses (in total, 37 days)
Methods of cessation of glucocorticoid therapy: dose reduction over 10 days (50% each 3 days, with complete withdrawal on the 10th day) No control intervention |
|
| Outcomes | Specific HPA axis function test: ovine CRH stimulus test at 8 a.m. (after an overnight fasting period), including cortisol basal morning value Moment of testing: before introduction of dexamethasone, on 8th and 28th days of dexamethasone use, and 48 hours and 1 month after cessation of dexamethasone. Tests were performed during treatment for ALL. Cutoff limits defined by original studies: baseline cortisol: 5 to 25 µg/dL (138.9 to 694.4 nmol/L); stimulated cortisol levels were compared with levels before treatment. |
|
| Notes | 7 children were lost to follow‐up at testing on the 8th and 28th days of dexamethasone use, 1 more child at 48 hours after cessation of dexamethasone, and 7 more children at 1 month after cessation of dexamethasone. Length of follow‐up after glucocorticoid therapy: 1 month Funding source: Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) Declaration of interest among primary researchers: not mentioned |
|
Einaudi 2008.
| Methods | Study type: multi‐centre RCT Setting: Department of Pediatric Onco‐Hematology, University of Turin, Italy; and Department of Pediatrics, University of Milano‐Bicocca, Hospital of Monza, Italy. This information was based on additional information provided by trial authors. | |
| Participants | 64 children (24 who received dexamethasone: mean age at diagnosis 4 years 11 months (range 1 year 2 months to 12 years 1 month), 11 boys and 13 girls; 40 children who received prednisone: mean age at diagnosis 6 years 9 months (range 1 year 2 months to 17 years 6 months), 18 boys and 22 girls) with ALL. This information was based on additional information provided by trial authors. | |
| Interventions | Treatment according to the AIEOP ALL 2000 study. Induction phase 1A: prednisone (from day 8 randomisation prednisone or dexamethasone), vincristine, daunorubicin, Escherichia coli L‐asparaginase, and IT methotrexate. Induction phase 1B: 6‐mercaptopurine, cyclophosphamide, cytosine‐arabinoside, and IT methotrexate Type of glucocorticoid therapy: induction phase: oral prednisone (60 mg/m2/d, divided into 3 daily doses) given on days 1 to 7. On day 8, children were randomised to receive either dexamethasone (10 mg/m2/d) or prednisone (60 mg/m2/d), both divided into 3 oral doses until day 29. From day 30 onwards, the dose of both corticosteroids was tapered by 50% every 3 days until complete withdrawal over 9 days. Cumulative dose of glucocorticoid therapy: prednisone 420 mg/m2 + dexamethasone 246.25 mg/m2 or prednisone 1477.50 mg/m2 Duration of glucocorticoid therapy: prednisone 7 days + dexamethasone 22 days or prednisone 22 days + 9 days tapering doses (in total 39 days) Method of cessation of glucocorticoid therapy: Dose was tapered by 50% every 3 days, until complete withdrawal over 9 days. | |
| Outcomes | Specific HPA axis function test: basal cortisol between 8 and 9 a.m. at diagnosis and low‐dose ACTH test between 8 and 11 a.m. (1 µg/1.74 m2 of tetracosactrin (Synacthen, Novartis, Basel, Switzerland), basal morning value cortisol, after 30 and 60 minutes) Moment of testing: at diagnosis, basal cortisol was determined. The first low‐dose ACTH stimulation test was performed 24 hours after the last tapering dose of glucocorticoid (on day 39), which was given as a single dose in the morning. Tests were performed during treatment for ALL. Cutoff limits defined by original studies: basal cortisol: 6 to 30 µg/dL (167 to 833 nmol/L). Low‐dose ACTH test: normal response ≥ 18 µg/dL (≥ 500 nmol/L) This study addressed type of glucocorticoid as a risk factor. |
|
| Notes | 0 children were lost to follow‐up. Length of follow‐up after glucocorticoid therapy: Children with suppressed levels underwent further low‐dose ACTH testing between 7 and 14 days from the last glucocorticoid dose and every 2 weeks thereafter until cortisol levels were normalised. Total follow‐up duration was 10 weeks. Funding source: not mentioned Declaration of interest among primary researchers: not mentioned |
|
Felner 2000.
| Methods | Study type: prospective single‐centre study Setting: Children's Medical Center of Dallas (University of Texas Southwestern Medical School). This information was based on additional information provided by trial authors. | |
| Participants | 10 children (mean age at diagnosis 5.3 ± 2.9 years (range 2.0 to 9.9 years); 7 boys and 3 girls) with early B‐cell lineage ALL | |
| Interventions | Induction therapy: dexamethasone, vincristine, L‐asparaginase, and daunorubicin. High‐risk therapy: 1 additional lumbar puncture with IT chemotherapy during induction
Type of glucocorticoid therapy: induction phase: oral dexamethasone (6 mg/m2/d, divided into 2 daily doses) for 28 consecutive days
Cumulative dexamethasone dose: 168 mg/m2
Duration of glucocorticoid therapy: 28 days
Methods of cessation of glucocorticoid therapy: abrupt No control intervention |
|
| Outcomes | Specific HPA axis function test: 250 µg cosyntropin stimulation test (synthetic corticotrophin 1‐24/ACTH test) IV between 8 and 10 a.m. (Cortrosyn, Organon) (basal morning value cortisol and after 45 minutes) Moment of testing: at diagnosis (baseline), 24 hours after completion of the dexamethasone course, and every 4 weeks thereafter until normalisation of adrenal function. Tests were performed during treatment for ALL. Cutoff limits defined by original studies: baseline cortisol: not defined. Low‐dose ACTH test: normal response ≥ 18 µg (≥ 500 nmol/L) |
|
| Notes | 0 children were lost to follow‐up. Length of follow‐up after glucocorticoid therapy: Children with suppressed levels underwent further testing every 4 weeks thereafter until cortisol levels were normalised. Total follow‐up duration was 8 weeks. Funding source: National Institutes of Health grants Declaration of interest among primary researchers: not mentioned |
|
Kuperman 2001.
| Methods | Study type: prospective single‐centre study Setting: Oncology Department of the Children's Institute, Hospital das Clinicas‐Sao Paulo University School of Medicine, Brazil | |
| Participants | 15 children with ALL (age at diagnosis 5 months to 12 years; 5 boys and 10 girls) | |
| Interventions | Dexamethasone, daunomycin, vincristine, L‐asparaginase, and cytosine arabinoside
Type of glucocorticoid therapy: induction phase: oral dexamethasone (6 mg/m2/d, divided into 3 daily doses) for 42 consecutive days
Cumulative dexamethasone dose: 252 mg/m2
Duration of glucocorticoid therapy: 42 days
Methods of cessation of glucocorticoid therapy: abrupt No control intervention |
|
| Outcomes | Specific HPA axis function test: 1 µg/kg ovine CRH stimulation test IV, after an 8‐hour fast, between 8 and 9 a.m. (basal morning value cortisol and after 15, 30, 60, and 90 minutes) Moment of testing: before dexamethasone therapy (baseline), 7 and 14 days after last dose of dexamethasone. It was not defined whether tests were performed during treatment for ALL. Cutoff limits defined by original studies: basal cortisol: 7.0 µg/dL. This information was based on additional information provided by trial authors. Ovine CRH test: Cortisol above 12.8 µg/dL (353.2 nmol/L) was considered normal (basal and peak cortisol levels at 3 different time points were compared with one another). |
|
| Notes | Additional information provided by trial authors revealed that 1 child was lost to follow‐up 14 days after administration of dexamethasone. Length of follow‐up after glucocorticoid therapy: 14 days Funding source: not mentioned Declaration of interest among primary researchers: not mentioned |
|
Kuperman 2012.
| Methods | Study type: randomised double‐blind comparative study Setting: Instituto da Criança (Children’s Institute), São Paulo University Medical School Hospital, Brazil. This information was based on additional information provided by trial authors. |
|
| Participants | 29 children (16 children who received prednisone: mean age at diagnosis 8.0 ± 4.4 years; 3 boys and 13 girls; 13 children who received dexamethasone: mean age at diagnosis 5.3 ± 3.6 years, 9 boys and 4 girls) with ALL. Control group for determining cutoff peak cortisol level: 16 children (mean age at HPA axis function test 8.1 ± 2.7 years; 7 boys and 9 girls) suspected of having any endocrinopathy other than adrenal insufficiency | |
| Interventions | Treatment according to the Brazilian Childhood Leukemia Protocol 99. Standard remission induction phase: vincristine, L‐asparaginase, daunorubicin, methotrexate (IT), cytarabine (IT), and dexamethasone (IT). This information was based on additional information provided by trial authors. Type of glucocorticoid therapy: remission induction phase: Children were randomised to receive prednisone (40 mg/m2/d) or dexamethasone (6 mg/m2/d), both divided into 3 daily doses for 28 days, without tapering. Cumulative dose of glucocorticoid therapy: prednisone 1120 mg/m2 or dexamethasone 168 mg/m2 Duration of glucocorticoid therapy: 28 days Methods of cessation of glucocorticoid therapy: abrupt |
|
| Outcomes | Specific HPA axis function test: low‐dose ACTH test between 8 and 9 a.m. (1.0 µg/m2 of cosyntropin, basal cortisol and after 30 minutes) Moment of testing: intervention groups: before remission induction and subsequently every week after 28 days of glucocorticoid therapy, over a total period of 8 weeks Cutoff limits were defined by the control group: basal cortisol: not defined. Low‐dose ACTH test: normal response ≥ 14.2 µg/dL (≥ 392 nmol/L) after 30 minutes (control group’s mean peak cortisol level minus 1.96 standard deviation) This study addressed type of glucocorticoid and presence of infection/stress (defined as hospitalisation due to fever with or without neutropenia) as risk factors. |
|
| Notes | 2 children were lost to follow‐up at final measurement. Moreover, not all children underwent the low‐dose ACTH test every week after remission induction. Information on individual testing schedules and on adrenal recovery per child was not provided.
Length of follow‐up after glucocorticoid therapy: 8 weeks Funding source: research grant from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) Declaration of interest among primary researchers: Researchers declared that they had no conflicts of interest. |
|
Mahachoklertwattana 2004.
| Methods | Study type: prospective single‐centre study Setting: Department of Pediatrics, Faculty of Medicine, Ramathibodi Hospital, Bangkok, Thailand | |
| Participants | 24 children (median age at diagnosis 3.5 years (range 1 to 14 years); 13 boys and 11 girls) with newly diagnosed ALL | |
| Interventions | According to modification of St. Jude Children's Research Hospital Total XIII Protocol for ALL: standard induction therapy: prednisolone, vincristine, L‐asparaginase, doxorubicin, etoposide, and cytosine arabinoside
Type of glucocorticoid therapy: induction phase: oral prednisolone (40 mg/m2/d, divided into 3 daily doses) for 28 consecutive days. At 4 weeks after completion of induction therapy, children received maintenance therapy consisting of a 7‐day course of high‐dose dexamethasone 8 mg/m2/d, every 4 weeks, in conjunction with other chemotherapeutic agents according to risk classification.
Cumulative prednisolone dose was 1120 mg/m2. Cumulative dexamethasone dose per child depended upon how long the child was followed up. Dexamethasone course (56 mg/m2/d) was administered at 4, 8, 12, and 16 weeks after induction therapy. Maximum cumulative dose of dexamethasone (4 courses) was 224 mg/m2.
Duration of glucocorticoid therapy: 28 days of prednisolone and an additional 7‐day course of dexamethasone
Methods of cessation of glucocorticoid therapy: abrupt No control intervention |
|
| Outcomes | Specific HPA axis function test: serum cortisol level at 8 a.m., at diagnosis (baseline); low‐dose ACTH stimulation test (1 µg cosyntropin (Cortrosyn, Organon, West Orange, NJ)) at 8 a.m. after an overnight fast (basal cortisol and after 30 minutes) Moment of testing: Baseline adrenal function was assessed by determination of serum cortisol level at 8 a.m. before induction therapy. The first low‐dose ACTH stimulation test was performed 2 weeks after discontinuation of prednisolone. Children with adrenal insufficiency underwent repeated ACTH testing 4 weeks after completion of the prednisolone course and every 4 weeks thereafter in the morning of the day on which children were admitted for the next course of maintenance chemotherapy until normalisation. Tests were performed during treatment for ALL. Cutoff limits defined by original studies: basal cortisol: not defined. Low‐dose ACTH test: normal response ≥ 18 µg/dL (≥ 500 nmol/L) |
|
| Notes | 0 children were lost to follow‐up. Length of follow‐up after glucocorticoid therapy: up to 20 weeks Funding source: not mentioned Declaration of interest among primary researchers: not mentioned |
|
Perdomo‐Ramírez 2016.
| Methods | Study type: prospective single‐centre study Setting: Hospital La Misericordia, Bogota, Colombia Information was based on additional information provided by trial authors. |
|
| Participants | 40 children with newly diagnosed ALL (mean age at diagnosis 8.5 years (range 2 to 17 years); 21 boys and 19 girls) | |
| Interventions | According to the Berlin‐Frankfurt‐Münster (BFM) protocol: prednisone, vincristine, daunorubicin, L‐asparaginase Type of glucocorticoid therapy: induction phase: prednisone 60 mg/m2 for 28 consecutive days with 9 days of tapering Cumulative prednisone dose: 1837.5 mg/m2 Duration of glucocorticoid therapy: 28 days Method of cessation of glucocorticoid therapy: Dose was tapered by 50% every 3 days, until complete withdrawal over 9 days. No control intervention |
|
| Outcomes | Specific HPA axis function test: basal cortisol and ACTH levels at baseline; low‐dose ACTH test (1 µg Synacthen) between 7 and 8 a.m. at time points after cessation of glucocorticoid therapy (see below). This information was based on additional information provided by trial authors. Moment of testing: Baseline adrenal function was assessed by basal cortisol and ACTH levels before induction therapy. The first low‐dose ACTH stimulation test was performed 3 days after cessation of prednisone. Children with adrenal insufficiency underwent repeated low‐dose ACTH testing 7, 14, and 30 days after cessation of glucocorticoid therapy until normalisation of adrenal function. Tests were performed during treatment for ALL. Cutoff limits defined by original studies: basal cortisol: 6 to 30 µg/dL, baseline ACTH: 4.4 to 22 pmol/L. Low‐dose ACTH test: normal response ≥ 18 µg/dL (≥ 500 nmol/L) |
|
| Notes | One child died during follow‐up. Length of follow‐up after glucocorticoid therapy: up to 30 days. Funding source: Premio de Investigación (Research award), Josefa Cualla de Barberi Declaration of interest among primary researchers: Researchers declared that they had no conflicts of interest. |
|
Petersen 2003.
| Methods | Study type: prospective single‐centre study Setting: University Hospital, Rigshospitalet, Copenhagen, Denmark | |
| Participants | 17 children (median age at diagnosis 5.4 years (range 2 to 15 years)) with ALL | |
| Interventions | According to risk groups by NOPHO ALL‐1992 or ALL‐2000 protocol. Ten children were studied after receiving prednisolone, weekly vincristine, 4 doses of IT methotrexate, L‐asparaginase, and doxorubicin. Seven additional children were studied following reinduction therapy with dexamethasone, weekly vincristine and daunorubicin, 4 doses of L‐asparaginase, and IT methotrexate.
Type of glucocorticoid therapy: induction phase (n = 10): prednisolone (60 mg/m2/d, in 3 daily doses) during first 5 weeks of induction therapy followed by 9 days of tapering 1‐Week courses of prednisolone (60 mg/m2/d, based on additional information provided by trial authors) were administered every 4 to 10 weeks as part of reinduction therapy, beginning approximately 8 weeks after prednisolone.
Reinduction therapy (n = 7): dexamethasone (10 mg/m2/d, divided into 3 daily doses) for 3 weeks on protocol days 169 to 190 (4 intermediate‐risk patients) or days 246 to 267 (3 high‐risk patients) followed by 9 days of tapering. High‐risk patients received two 1‐week courses of prednisolone (40 mg/m2/d) 4 and 8 weeks before dexamethasone therapy. One‐week courses of prednisolone (60 mg/m2/d) were administered every 4 to 10 weeks as part of reinduction therapy, beginning approximately 11 weeks after dexamethasone therapy.
Cumulative dose: induction therapy: 2100 mg/m2 + 157.5 mg/m2 prednisolone. One child received an additional 840 mg/m2 prednisolone during the period of adrenal insufficiency. Reinduction therapy: 210 mg/m2 + 26.5 mg/m2 dexamethasone. High‐risk patients received 560 mg/m2 prednisolone in advance; 1 high‐risk patient received an additional 420 mg/m2 prednisolone during period of adrenal insufficiency, and 2 high‐risk patients received an additional 1260 mg/m2 prednisolone during period of adrenal insufficiency.
Duration of glucocorticoid therapy: induction therapy: 35 days of prednisolone + 9 days tapering doses. One child received an additional 14 days of prednisolone. Reinduction therapy: 21 days of dexamethasone + 9 days tapering doses. High‐risk patients received 14 days of prednisolone 4 and 8 weeks before dexamethasone therapy. After the dexamethasone course, high‐risk patients also received prednisolone for 1 week (n = 1) or 3 weeks (n = 2).
Methods of cessation of glucocorticoid therapy: 50% each 3 days, over 9 days in total No control intervention |
|
| Outcomes | Specific HPA axis function test: ACTH stimulation test (250 µg tetracosactide (Synacthen, Novartis)) between 8 and 11 a.m. (basal cortisol and after 30 and 60 minutes) Moment of testing: Adrenal function was assessed by an ACTH stimulation test within 2 weeks after discontinuation of glucocorticoid therapy. Testing was repeated every 3 to 5 weeks until recovery or end of follow‐up. Tests were performed during treatment for ALL. Cutoff limits defined by original studies: low‐dose ACTH test: normal response > 500 nmol/L Fluconazole therapy was evaluated as a risk factor for adrenal insufficiency. |
|
| Notes | 0 children were lost to follow‐up. Length of follow‐up after glucocorticoid therapy: fluctuating Funding source: not mentioned Declaration of interest among primary researchers: not mentioned |
|
Rix 2005.
| Methods | Study type: prospective multi‐centre study Setting: Department of Pediatrics, Aalborg University Hospital, and Department of Pediatrics, Aarhus University Hospital, Skejby, Denmark. This information was based on additional information provided by trial authors. | |
| Participants | 24 children (median age at diagnosis 4.5 years (range 1.8 to 14.6 years); 17 boys and 7 girls) with newly diagnosed ALL. 12 had standard‐risk ALL according to Nordic risk criteria, 7 had intermediate‐risk ALL, and 5 had high‐risk ALL. | |
| Interventions | According to NOPHO ALL‐92 protocol:
Type of glucocorticoid therapy: All children received prednisolone (60 mg/m2/d, in 3 daily doses) during first 5 weeks of induction therapy followed by 9 days of tapering. All children received 1‐week courses of prednisolone (60 mg/m2/d) without tapering; children with intermediate‐ and high‐risk criteria received an additional 3‐week course of dexamethasone (10 mg/m2/d) with tapering over a 9‐day period.
Cumulative dose of glucocorticoid therapy: All children received 2100 mg/m2 + 157.5 mg/m2 prednisolone. In addition, they received several courses (not defined) of 420 mg/m2/d prednisolone. Intermediate‐ and high‐risk patients received an additional 210 mg/m2 + 26.25 mg/m2 dexamethasone.
Duration of glucocorticoid therapy: induction therapy: 35 days of prednisolone + 9 days tapering doses. Additional 7‐day courses of prednisolone. Intermediate‐ and high‐risk patients: additional 21 days dexamethasone + 9 days tapering doses
Methods of cessation of glucocorticoid therapy: 50% every 3 days over 9 days in total No control intervention |
|
| Outcomes | Specific HPA axis function test: low‐dose ACTH stimulation test (1 µg tetracosactide (Synacthen, Novartis) between 8 and 10 a.m. (basal cortisol and after 30 minutes)) Moment of testing: for each child: before 5‐week course of prednisolone (weeks 1 to 5) and on days 1, 3, and 5 after tapering was completed. Before 1‐week course of prednisolone (weeks 14 (standard risk), 28 (high risk), and 37 (intermediate risk)) and on day 2 after cessation. Before 3‐week course of dexamethasone (weeks 25 to 27 (intermediate risk) or 36 to 38 (high risk)), before tapering, and on days 1, 3, and 7 after tapering was completed. Tests were performed during treatment for ALL. Cutoff limits defined by original studies: low‐dose ACTH test: normal response > 500 nmol/L |
|
| Notes | Based on additional information provided by trial authors, 5 children were lost to follow‐up. Length of follow‐up after glucocorticoid therapy: varied Funding source: research grants from the Danish Cancer Society Declaration of interest among primary researchers: not mentioned |
|
Salem 2015.
| Methods | Study type: prospective single‐centre study Setting: Pediatric Hematology/Oncology Unit, Children's Hospital, Ain Shams University, Cairo, Egypt |
|
| Participants | 40 children (mean age at diagnosis unknown; 22 girls and 18 boys) with newly diagnosed standard‐risk ALL. | |
| Interventions | Induction and reinduction therapy according to CCG‐1991 protocol (dexamethasone, vincristine, L‐asparaginase, daunomycin) or modified BFM‐1990 protocol (prednisone, vincristine, L‐asparaginase, daunomycin), depending on time of diagnosis (before or during and after the year 2006). 20 children were treated according to CCG‐1991 protocol, and 20 children followed the modified BFM‐1990 protocol. Type of glucocorticoid therapy: induction phase CCG‐1991 protocol: dexamethasone 6 mg/m2 for 28 consecutive days, reinduction phase: dexamethasone 6 mg/m2 for 21 consecutive days, both followed by a tapering phase of unknown duration. Induction phase modified BFM‐1990 protocol: prednisone 60 mg/m2 for 28 consecutive days, reinduction phase: prednisone 60 mg/m2 for 21 consecutive days, both followed by a tapering phase of unknown duration Cumulative dose of glucocorticoid therapy: unknown Duration of glucocorticoid therapy: induction phase 28 days, reinduction phase 21 days, both followed by a tapering phase of unknown duration Methods of cessation of glucocorticoid therapy: tapering, duration unknown |
|
| Outcomes | Specific HPA axis function test: low‐dose ACTH stimulation test (1 µg tetracosactide (Synacthen, Novartis) between 8 and 11 a.m. (basal cortisol and after 30 minutes)) Moment of testing: at diagnosis, and during both induction and reinduction at the following time points: immediately after last steroid course, 2 weeks after end of glucocorticoid treatment, 4 weeks after end of glucocorticoid treatment, and every 2 weeks thereafter until HPA axis recovery was reached. Additionally, adrenal function was tested at the time of an infectious event. Tests were performed during treatment for ALL. Cutoff limits defined by original studies: low‐dose ACTH test: normal response > 18 µg/dL (> 500 nmol/L) Type of glucocorticoid, fluconazole therapy, and presence of infection (study authors stated: The duration and frequency of hospitalisation for septic episodes, number of days with neutropenia > 500/mm³ were recorded. Infectious events were graded from mild to severe according to whether febrile neutropenic patients were managed as outpatients or needed hospitalisation for antimicrobial coverage. We recorded data about whether fluconazole (FCZ) was used or not in the febrile event and the dose given (< 10 mg/kg/d or more)) were evaluated as risk factors for (prolonged) adrenal insufficiency. |
|
| Notes | Children lost to follow‐up: unknown Length of follow‐up after glucocorticoid therapy: up to 20 weeks Funding source: none Declaration of interest among primary researchers: Researchers declared that they had no conflicts of interest. |
|
ACTH: adrenocorticotropic hormone. AIEOP: Associazione Italiana Ematologia e Oncologia. ALL: acute lymphoblastic leukaemia. CRH: corticotrophin‐releasing hormone. HPA: hypothalamic‐pituitary‐adrenal. IT: intrathecal. IV: intravenous. NOPHO: Nordic Society of Pediatric Hematology and Oncology. RCT: randomised controlled trial.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bessho 1984 | HPA axis was not examined. |
| Birkebaek 1998 | No data on intervention (dose, duration, methods of cessation of glucocorticoid therapy) No data on cutoff limits of HPA axis function tests No accurate data on follow‐up period Data on children without cranial irradiation not reported separately |
| Felder‐Puig 2007 | HPA axis function was examined only during glucocorticoid treatment. |
| Felner 2011 | No clinical study (but a response to the article of Vestergaard 2011) |
| Lightner 1981 | Children received cranial irradiation. |
| Pawlaczyk 1993 | Children received cranial irradiation. |
| Silva 2006 | Double publication of Cunha 2004 |
| Vestergaard 2011 | Some of the included children received cranial irradiation. Data on children without cranial irradiation were not reported separately. |
HPA: hypothalamic‐pituitary‐adrenal.
Characteristics of studies awaiting assessment [ordered by study ID]
Schlosser 2016.
| Methods | Study type: retrospective single‐centre study Setting: Children's Hospital of Eastern Ontario, Ottawa, Ontario, Canada |
| Participants | 176 paediatric patients with ALL during maintenance phase of treatment |
| Interventions | Treatment protocol: unknown Type of glucocorticoid therapy: unknown Methods of cessation of glucocorticoid therapy: unknown |
| Outcomes | Specific HPA axis function test: unknown Moment of testing: unknown Cutoff limits defined by original studies: unknown |
| Notes | This study has not been published in full text (search conducted 12 December 2016) but was presented to the ASPHO 2016 conference. It is not yet clear whether this study is eligible for inclusion in this review. |
ALL: acute lymphoblastic leukaemia.
ASPHO: American Society of Pediatric Hematology/Oncology.
HPA: hypothalamic‐pituitary‐adrenal.
Differences between protocol and review
In addition to our primary objective of examining the occurrence of hypothalamic‐pituitary‐adrenal (HPA) axis suppression after treatment with glucocorticoids for childhood acute lymphoblastic leukaemia (ALL), as stated in the protocol, we studied the duration of HPA axis suppression.
One review author performed data extraction and 'Risk of bias' assessment of included studies, and two other review authors independently checked this information. This approach differed from that stated in the protocol, which indicated that two independent review authors would extract data and assess ‘Risk of bias’.
In the second update, we searched an additional conference proceeding (ASPHO) (which is a new policy of Cochrane Childhood Cancer). We also searched an additional ongoing trials register (ICTRP/WHO). Furthermore, Cochrane Childhood Cancer adjusted the search strategy for children; as opposed to the earlier version, this is a validated strategy. We did include a new secondary outcome measure based on advancing knowledge of the topic: other possible risk factors (such as fluconazole and presence of infection/stress). Finally, since performing the first update, Cochrane Childhood Cancer has slightly changed the risk of bias criteria for observational studies: Risk estimation should now be assessed only for studies evaluating risk factors. We made the necessary changes for all included studies.
Contributions of authors
Niki Rensen:
identified studies meeting the inclusion criteria;
searched for unpublished and ongoing studies;
performed data extraction and 'Risk of bias' assessment of included studies;
analysed data and interpreted results of analysis; and
revised the manuscript.
Reinoud Gemke and Gertjan Kaspers:
identified studies meeting the inclusion criteria;
checked data extraction and 'Risk of bias' assessment of included studies;
contributed to interpretation of results; and
critically reviewed the protocol and the manuscript.
Elvira van Dalen:
contributed to analysis of data and interpretation of results; and
critically reviewed the manuscript.
Joost Rotteveel contributed to interpretation of results and critically reviewed the protocol and the manuscript.
All review authors approved the final report.
Sources of support
Internal sources
No sources of support supplied
External sources
Dutch Cancer Society, Netherlands.
‘Stichting Kinderen Kankervrij’ (KiKa), Netherlands.
Declarations of interest
None.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Cunha 2004 {published and unpublished data}
- Cunha CF, Silva IN, Finch FL. Early adrenocortical recovery after glucocorticoid therapy in children with leukemia. Journal of Clinical Endocrinology and Metabolism 2004;89(6):2797‐802. [DOI] [PubMed] [Google Scholar]
Einaudi 2008 {published and unpublished data}
- Einaudi S, Bertorello N, Masera N, Farinasso L, Barisone E, Rizzari C, et al. Adrenal axis function after high‐dose steroid therapy for childhood acute lymphoblastic leukemia. Pediatric Blood & Cancer 2008;50(3):537‐41. [DOI] [PubMed] [Google Scholar]
Felner 2000 {published and unpublished data}
- Felner EI, Thompson MT, Ratliff AF, White PC, Dickson BA. Time course of recovery of adrenal function in children treated for leukemia. Journal of Pediatrics 2000;137(1):21‐4. [DOI] [PubMed] [Google Scholar]
Kuperman 2001 {published data only (unpublished sought but not used)}
- Kuperman H, Damiani D, Chrousos GP, Dichtchekenian V, Manna TD, Filho VO, et al. Evaluation of the hypothalamic‐pituitary‐adrenal axis in children with leukemia before and after 6 weeks of high‐dose glucocorticoid therapy. Journal of Clinical Endocrinology & Metabolism 2001;86(7):2993‐6. [DOI] [PubMed] [Google Scholar]
Kuperman 2012 {published and unpublished data}
- Kuperman H, Filho VO, Cristofani LM, Assis de Almeida MT, Setian N, Damiani D. Evaluation of adrenal reserve in children with acute lymphoblastic leukemia treated with prednisone or dexamethasone. Hormone Research in Paediatrics 2012;78:73‐80. [DOI] [PubMed] [Google Scholar]
Mahachoklertwattana 2004 {published and unpublished data}
- Mahachoklertwattana P, Vilaiyuk S, Hongeng S, Okascharoen C. Suppression of adrenal function in children with acute lymphoblastic leukemia following induction therapy with corticosteroid and other cytotoxic agents. Journal of Pediatrics 2004;144(6):736‐40. [DOI] [PubMed] [Google Scholar]
Perdomo‐Ramírez 2016 {published and unpublished data}
- Perdomo‐Ramírez I, Linares‐Ballesteros A, Acevedo‐Sedano L, Coll‐Barrios M. Hypothalamus‐pituitary‐adrenal axis suppression following induction chemotherapy in children with acute lymphoblastic leukemia [Supresión del eje hipotálamo‐hipófisis‐suprarrenal después de la quimioterapia de inducción en niños con leucemia linfoide aguda]. Iatreia 2016;29(1):18‐26. [Google Scholar]
Petersen 2003 {published and unpublished data}
- Petersen KB, Müller J, Rasmussen M, Schmiegelow K. Impaired adrenal function after glucocorticoid therapy in children with acute lymphoblastic leukemia. Medical and Pediatric Oncology 2003;41(2):110‐4. [DOI] [PubMed] [Google Scholar]
Rix 2005 {published and unpublished data}
- Rix M, Birkebaek NH, Rosthøj S, Clausen N. Clinical impact of corticosteroid‐induced adrenal suppression during treatment for acute lymphoblastic leukemia in children: a prospective observational study using the low‐dose adrenocorticotropin test. Journal of Pediatrics 2005;147(5):645‐50. [DOI] [PubMed] [Google Scholar]
Salem 2015 {published and unpublished data}
- Salem A, Tantawy A, Sedfy H, Laboudy M, Toaima D, Mahmoud N, et al. A prospective study of the hypothalamic‐pituitary‐adrenal axis in children with acute lymphoblastic leukemia receiving chemotherapy. Hematology 2015;20(6):320‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bessho 1984 {published data only}
- Bessho F, Kagawa J, Mizutani S, Egi S, Fujiu M, Kaku H, et al. Effects of antileukemic therapy of endocrine functions and development of children. European Paediatric Haematology and Oncology 1984;1:135‐41. [Google Scholar]
Birkebaek 1998 {published data only (unpublished sought but not used)}
- Birkebaek NH, Fisker S, Clausen N, Tuovinen V, Sindet‐Pedersen S, Christiansen JS. Growth and endocrinological disorders up to 21 years after treatment for acute lymphoblastic leukemia in childhood. Medical and Pediatric Oncology 1998;30(6):351‐6. [DOI] [PubMed] [Google Scholar]
Felder‐Puig 2007 {published data only}
- Felder‐Puig R, Scherzer C, Baumgartner M, Ortner M, Aschenbrenner C, Bieglmayer C, et al. Glucocorticoids in the treatment of children with acute lymphoblastic leukemia and Hodgkin's disease: a pilot study on the adverse psychological reactions and possible associations with neurobiological, endocrine, and genetic markers. Clinical Cancer Research 2007;13(23):7093‐100. [DOI] [PubMed] [Google Scholar]
Felner 2011 {published data only}
- Felner EL. Reducing the risk for adrenal insufficiency in those treated for all: tapering glucocorticoids before abrupt discontinuation. Journal of Pediatric Hematology/Oncology 2011;33:406‐8. [DOI] [PubMed] [Google Scholar]
Lightner 1981 {published data only}
- Lightner ES, Johnson H, Corrigan JJ Jr. Rapid adrenocortical recovery after short‐term glucocorticoid therapy. American Journal of Diseases of Children 1981;135(9):790‐2. [DOI] [PubMed] [Google Scholar]
Pawlaczyk 1993 {published data only}
- Pawlaczyk B, Malecka EH, Krause W. Adrenocortical function and reserve in children treated for acute lymphoblastic leukaemia [Czynnosc i rezerwa korowo‐nadnerczowa u dzieci po leczeniu ostrej bialaczki limfoblastycznej]. Pediatria Polska 1993;68:49‐54. [Google Scholar]
Silva 2006 {published data only}
- Silva IN, Cunha CF, Finch FL, Colosimo EA. Evaluation of hypothalamic‐pituitary‐adrenal axis recovery after corticotherapy by using basal cortisol secretion [Avaliação da recuperação do eixo hipotalâmicohipofisário‐adrenal após corticoterapia por meio do cortisol basal]. Arquivos Brasileiros de Endocrinologia e Metabologia 2006;50(1):118‐24. [DOI] [PubMed] [Google Scholar]
Vestergaard 2011 {published data only}
- Vestergaard TR, Anders J, Lausten‐Thomsen U, Lausen B, Hjalgrim H, Kvist TK, et al. Duration of adrenal insufficiency during treatment for childhood acute lymphoblastic leukemia. Journal of Pediatric Hematology/Oncology 2011;33:442‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Schlosser 2016 {published and unpublished data}
- Schlosser M, Burd D, Ahmet A, Lawrence S, Bassal M. Adrenal suppression in pediatric patients during maintenance treatment for acute lymphoblastic leukemia. Pediatric Blood and Cancer 2016;63(Suppl S1 (29th Annual Meeting of the American Society of Pediatric Hematology/Oncology (ASPHO), 11‐14 May, Minneapolis, MN, USA)):S41. [Google Scholar]
Additional references
Abdu 1999
- Abdu TA, Elhadd TA, Neary R, Clayton RN. Comparison of the low dose short synacthen test (1 microg), the conventional dose short synacthen test (250 microg), and the insulin tolerance test for assessment of the hypothalamo‐pituitary‐adrenal axis in patients with pituitary disease. Journal of Clinical Endocrinology and Metabolism 1999;84(3):838‐43. [DOI] [PubMed] [Google Scholar]
Agwu 1999
- Agwu JC, Spoudeas H, Hindmarsh PC, Pringle PJ, Brook CG. Tests of adrenal insufficiency. Archives of Disease in Childhood 1999;80:330‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Albert 2001
- Albert SG, DeLeon MJ, Silverberg AB. Possible association between high‐dose fluconazole and adrenal insufficiency in critically ill patients. Critical Care Medicine 2001;29(3):668‐70. [DOI] [PubMed] [Google Scholar]
Biostat, Inc, USA
- Borenstein M, Rothstein H. Comprehensive Meta Analysis. Comprehensive Meta‐Analysis. Englewood, New Jersey, USA: Biostat, Inc. , 1999. [Google Scholar]
Böttner 2005
- Böttner A, Kratzsch J, Liebermann S, Keller A, Pfaffle RW, Kiess W, et al. Comparison of adrenal function tests in children ‐ the glucagon stimulation test allows the simultaneous assessment of adrenal function and growth hormone response in children. Journal of Pediatric Endocrinology & Metabolism 2005;18(5):433‐42. [DOI] [PubMed] [Google Scholar]
Christensen 2005
- Christensen MS, Heyman M, Möttönen M, Zeller B, Jonmundsson G, Hasle H. Treatment‐related death in childhood acute lymphoblastic leukaemia in the Nordic countries: 1992‐2001. British Journal of Haematology 2005;131(1):50‐8. [DOI] [PubMed] [Google Scholar]
DCOG 2014
- DCOG. Leukemia [Leukemie]. https://www.skion.nl/voor‐patienten‐en‐ouders/ziektebeelden/542/ziektebeelden/543/leukemie/#ALL 2014 (accessed June 2014).
Dickstein 1997
- Dickstein G, Spigel D, Arad E, Shechner C. One microgram is the lowest ACTH dose to cause a maximal cortisol response. There is no diurnal variation of cortisol response to submaximal ACTH stimulation. European Journal of Endocrinology 1997;137(2):172‐5. [DOI] [PubMed] [Google Scholar]
Grimes 2002
- Grimes DA, Schulz KF. Cohort studies: marching towards outcomes. The Lancet 2002;359(9303):341‐5. [DOI] [PubMed] [Google Scholar]
Henzen 2000
- Henzen C, Suter A, Lerch E, Urbinelli R, Schorno XH, Briner VA. Suppression and recovery of adrenal response after short‐term, high‐dose glucocorticoid treatment. The Lancet 2000;85(2):542‐5. [DOI] [PubMed] [Google Scholar]
Hettmannsperger 1992
- Hettmannsperger U, Detmar M, Owskianowski M, Tenorio S, Kammler HJ, Orfanos CE. Cytokine‐stimulated human dermal microvascular endothelial cells produce interleukin 6 ‐ inhibition by hydrocortisone, dexamethasone, and calcitriol. Journal of Investigative Dermatology 1992;99(5):531‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. www.handbook.cochrane.org.
Hurwitz 2000
- Hurwitz CA, Silverman LB, Schorin MA, Clavell LA, Dalton VK, Glick KM, et al. Substituting dexamethasone for prednisone complicates remission induction in children with acute lymphoblastic leukemia. Cancer 2000;88(8):1964‐9. [PubMed] [Google Scholar]
Igarashi 2005
- Igarashi S, Manabe A, Ohara A, Kumagai M, Saito T, Okimoto Y, et al. No advantage of dexamethasone over prednisolone for the outcome of standard‐ and intermediate‐risk childhood acute lymphoblastic leukemia in the Tokyo Children's Cancer Study Group L95‐14 protocol. American Journal of Clinical Oncology 2005;23(27):6489‐98. [DOI] [PubMed] [Google Scholar]
Krasner 1999
- Krasner AS. Glucocorticoid‐induced adrenal insufficiency. JAMA 1999;282(7):671‐6. [DOI] [PubMed] [Google Scholar]
Laupacis 1994
- Laupacis A, Wells G, Richardson WS, Tugwell P. Users' guides to the medical literature. V. How to use an article about prognosis. Evidence‐Based Medicine Working Group. JAMA 1994;272(3):234‐7. [DOI] [PubMed] [Google Scholar]
Leclercq 2013
- Leclercq E, Leeflang MMG, Dalen EC, Kremer LCM. Validation of search filters for identifying pediatric studies in PubMed. Journal of Pediatrics 2013;162:629‐34. [DOI] [PubMed] [Google Scholar]
Maghnie 2005
- Maghnie M, Uga E, Temporini F, lorgi N, Secco A, Tinelli C, et al. Evaluation of adrenal function in patients with growth hormone deficiency and hypothalamic‐pituitary disorders: comparison between insulin‐induced hypoglycemia, low‐dose ACTH and CRH stimulation tests. European Journal of Endocrinology 2005;152(5):735‐41. [DOI] [PubMed] [Google Scholar]
Module CCG
- Kremer LCM, Leclercq E, Noorman JK, Jellema P, Dalen EC. Cochrane Childhood Cancer Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2017, issue 4:Art. No.: CHILDCA.
Nyhlén 2000
- Nyhlén K, Linden M, Andersson R, Uppugunduri S. Corticosteroids and interferons inhibit cytokine‐induced production of IL‐8 by human endothelial cells. Cytokine 2000;12(4):355‐60. [DOI] [PubMed] [Google Scholar]
Planey 2000
- Planey SL, Litwack G. Glucocorticoid‐induced apoptosis in lymphocytes. Biochemical and Biophysical Research Communications 2000;279(2):307‐12. [DOI] [PubMed] [Google Scholar]
Pruckner 2009
- Pruckner C, Attarbaschi A, Peters C, Dworzak MN, Pötschger U, Urban C, et al. Induction death and treatment‐related mortality in first remission of children with acute lymphoblastic leukemia: a population‐based analysis of the Austrian Berlin‐Frankfurt‐Münster study group. Leukemia 2009;23(7):1264‐9. [DOI] [PubMed] [Google Scholar]
Rao 1987
- Rao RH, Spathis GS. Intramuscular glucagon as a provocative stimulus for the assessment of pituitary function: growth hormone and cortisol responses. Metabolism 1987;36(7):658‐63. [DOI] [PubMed] [Google Scholar]
Rubnitz 2004
- Rubnitz JE, Lensing S, Zhou Y, Sandlund JT, Razzouk BI, Ribeiro RC. Death during induction therapy and first remission of acute leukemia in childhood: the St. Jude experience. Cancer 2004;101(7):1677‐84. [DOI] [PubMed] [Google Scholar]
Schlaghecke 1992
- Schlaghecke R, Kornely E, Santen RT, Ridderskamp P. The effect of long‐term glucocorticoid therapy on pituitary‐adrenal responses to exogenous corticotropin‐releasing hormone. New England Journal of Medicine 1992;326(4):226‐30. [DOI] [PubMed] [Google Scholar]
Shah 1992
- Shah A, Stanhope R, Matthew D. Hazards of pharmacological tests of growth hormone secretion in childhood. BMJ 1992;304(6820):173‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shibata 2001
- Shibata S, Kami M, Kanda Y, Machida U, Iwata H, Kishi Y, et al. Acute adrenal failure associated with fluconazole after administration of high‐dose cyclophosphamide. American Journal of Hematology 2001;66(4):303‐5. [DOI] [PubMed] [Google Scholar]
Shulman 2007
- Shulman DI, Palmert MR, Kemp SF, Lawson Wilkins Drug and Therapeutics Committee. Adrenal insufficiency: still a cause of morbidity and death in childhood. Pediatrics 2007;119(2):484‐94. [DOI] [PubMed] [Google Scholar]
Te Poele 2007
- Poele EM, Bont ES, Marike Boezen H, Revesz T, Bökkerink JP, Bieshuizen A, et al. Dexamethasone in the maintenance phase of acute lymphoblastic leukaemia treatment: is the risk of lethal infections too high?. European Journal of Cancer 2007;43(17):2532‐6. [DOI] [PubMed] [Google Scholar]
Tordjman 2000
- Tordjman K, Jaffe A, Trostanetsky Y, Greenman Y, Limor R, Stern N. Low‐dose (1 microgram) adrenocorticotrophin (ACTH) stimulation as a screening test for impaired hypothalamo‐pituitary‐adrenal axis function: sensitivity, specificity and accuracy in comparison with the high‐dose (250 microgram) test. Clinical Endocrinology 2000;52(5):633‐40. [DOI] [PubMed] [Google Scholar]
Van Tijn 2008
- Tijn DA, Vijlder JJ, Vulsma T. Role of corticotropin‐releasing hormone testing in assessment of hypothalamic‐pituitary‐adrenal axis function in infants with congenital central hypothyroidism. Journal of Clinical Endocrinology & Metabolism 2008;93(10):3794‐803. [DOI] [PubMed] [Google Scholar]
Vanderschueren‐Lodeweyckx 1974
- Vanderschueren‐Lodeweyckx M, Wolter R, Malvaux P, Eggermont E, Eeckels R. The glucagon stimulation test: effect of plasma growth hormone and on immunoreactive insulin, cortisol, and glucose in children. Journal of Pediatrics 1974;85(2):182‐7. [DOI] [PubMed] [Google Scholar]
Waage 1988
- Waage A, Bakke O. Glucocorticoids suppress the production of tumour necrosis factor by lipopolysaccharide‐stimulated human monocytes. Immunology 1988;63(2):299‐302. [PMC free article] [PubMed] [Google Scholar]
Wheeler 1996
- Wheeler K, Chessells JM, Bailey CC, Richards SM. Treatment related deaths during induction and in first remission in acute lymphoblastic leukaemia: MRC UKALL X. Archives of Disease in Childhood 1996;74(2):101‐7. [DOI: 10.1136/adc.74.2.101] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Gordijn 2010
- Gordijn MS, Gemke RJBJ, Dalen EC, Rotteveel J, Kaspers GJL. Hypothalamic‐pituitary‐adrenal (HPA) axis suppression after treatment with glucocorticoid therapy for childhood acute lymphoblastic leukemia. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD008727] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gordijn 2012
- Gordijn MS, Gemke RJBJ, Dalen EC, Rotteveel J, Kaspers GJL. Hypothalamic‐pituitary‐adrenal (HPA) axis suppression after treatment with glucocorticoid therapy for childhood acute lymphoblastic leukaemia. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD008727.pub2] [DOI] [PubMed] [Google Scholar]
Gordijn 2015
- Gordijn MS, Rensen N, Gemke RJBJ, Dalen EC, Rotteveel J, Kaspers GJL. Hypothalamic‐pituitary‐adrenal (HPA) axis suppression after treatment with glucocorticoid therapy for childhood acute lymphoblastic leukaemia. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD008727.pub3] [DOI] [PubMed] [Google Scholar]


