Abstract
Background
Acute respiratory failure is a common life‐threatening complication of acute onset neuromuscular diseases, and may exacerbate chronic hypoventilation in patients with neuromuscular disease or chest wall disorders. Standard management includes oxygen supplementation, physiotherapy, cough assistance, and, whenever needed, antibiotics and intermittent positive pressure ventilation. Non‐invasive mechanical ventilation (NIV) via nasal, buccal or full‐face devices has become routine practice in many centres.
Objectives
The primary objective of this review was to compare the efficacy of non‐invasive ventilation with invasive ventilation in improving short‐term survival in acute respiratory failure in people with neuromuscular disease and chest wall disorders. The secondary objectives were to compare the effects of NIV with those of invasive mechanical ventilation on improvement in arterial blood gas after 24 hours and lung function measurements after one month, incidence of barotrauma and ventilator‐associated pneumonia, duration of mechanical ventilation, length of stay in the intensive care unit and length of hospital stay.
Search methods
We searched the following databases on 11 September 2017: the Cochrane Neuromuscular Specialised Register, CENTRAL, MEDLINE and Embase. We also searched conference proceedings and clinical trials registries.
Selection criteria
We planned to include randomised or quasi‐randomised trials with or without blinding. We planned to include trials performed in children or adults with acute onset neuromuscular diseases or chronic neuromuscular disease or chest wall disorders presenting with acute respiratory failure that compared the benefits and risks of invasive ventilation versus NIV.
Data collection and analysis
Two review authors reviewed searches and independently selected studies for assessment. We planned to follow standard Cochrane methodology for data collection and analysis.
Main results
We did not identify any trials eligible for inclusion in the review.
Authors' conclusions
Acute respiratory failure is a life‐threatening complication of acute onset neuromuscular disease and of chronic neuromuscular disease and chest wall disorders. We found no randomised trials on which to elaborate evidence‐based practice for the use of non‐invasive versus invasive mechanical ventilation. For researchers, there is a need to design and conduct new randomised trials to compare NIV with invasive ventilation in acute neuromuscular respiratory failure. These trials should anticipate variations in treatment responses according to disease condition (acute onset versus acute exacerbation on chronic neuromuscular diseases) and according to the presence or absence of bulbar dysfunction.
Plain language summary
Invasive versus non‐invasive ventilation for acute respiratory failure in neuromuscular disease and chest wall disorders
What is the aim of this review?
The aim of this Cochrane review was to look at how the effects of non‐invasive ventilation (NIV) compared to invasive ventilation in the treatment of respiratory failure in people with diseases affecting the nerves, muscles or the chest wall. The review set out to compare the two methods in terms of the effects on short‐term survival, side effects, and the length of hospital stay.
What was studied in the review?
When someone has severe difficulty in breathing, they may need assistance from a machine (ventilator) which is able to move air in and out of the lungs. Invasive and non‐invasive ventilation differ in how the air is delivered to the person. In invasive ventilation, air is delivered via a tube that is inserted into the windpipe through the mouth or sometimes the nose. In NIV, air is delivered through a sealed mask that can be placed over the mouth, nose or the whole face.
Invasive ventilation is the standard treatment used for people with neuromuscular diseases or chest wall disorders who are suffering from acute respiratory failure. However, NIV may offer some advantages such as being able to talk and swallow, and may have fewer risks.
Key Messages
The review authors collected and assessed all the relevant studies to answer this question but did not identify any trials that met the standards needed to be included in the review.
So far, there is no evidence from randomised studies either for or against the routine use of NIV instead of invasive ventilation in people with acute respiratory failure caused by either neuromuscular disease or a chest wall disorder.
However, some evidence from observational studies suggests that NIV should be trialled in all patients except for those with bulbar dysfunction.
How up to date is this review?
The evidence is up to date to 11 September 2017.
Background
Description of the condition
Acute respiratory failure is the most common life‐threatening complication of neuromuscular diseases and chest wall disorders (Carr 2014; Serrano 2010). A recent population‐based study in Northen Ireland found that acute neuromuscular respiratory failure had an incidence rate of about 2.81 (2.12 to 3.66) cases per million person‐years, representing about 1.5% of all non‐surgical admissions to the intensive care unit (ICU), and a mortality rate of about 0.26 (0.08 to 0.60) deaths per million person‐years (Serrano 2010). These epidemiological data are thought likely to be comparable in other European and North American countries.
Acute respiratory failure may occur in people with acute onset neuromuscular diseases such as Guillain‐Barré syndrome and myasthenic crisis, or as an exacerbation of chronic hypoventilation in people with neuromuscular diseases or chest wall disorders such as amyotrophic lateral sclerosis or Duchenne muscular dystrophy. The main mechanisms include: 1) weakness or paralysis of the diaphragm and accessory respiratory muscles resulting in acute alveolar hypoventilation; 2) oropharyngeal weakness with upper airway obstruction; 3) ineffective cough resulting in accumulation of bronchial secretions with pulmonary atelectasis (collapse of segments of the lung due to the interruption of airflow by sputum in the bronchia), hypoxaemia and infection, and 4) bulbar dysfunction with impaired swallowing and aspiration pneumonia (Wijdicks 2017). The decrease in alveolar ventilation and in bronchial secretion clearance reduce the clearance of carbon dioxide (CO2) causing hypercarbia, acidosis and usually moderate hypoxaemia. Patients often present with tachypnoea (fast breathing), tachycardia (fast heart rate), and sometimes with sweating, acute hypertension and altered mental status. There may be a subacute history of headache and daytime somnolence (sleepiness).
Description of the intervention
Treatment of both acute and acute on chronic respiratory muscle failure usually requires referral of the patient to a facility where mechanical ventilation can be implemented, such as an ICU. Management of the patient usually consists of physiotherapy, antibacterial drugs whenever there is a coexisting infection, and cough assistance to improve clearance of bronchial secretions. Patients whose condition is refractory to this initial management, require to be assisted with mechanical ventilation (Goligher 2009).
Assisted mechanical ventilation can be delivered invasively through an orotracheal tube or a tracheostomy, or non‐invasively using a broad variety of patient/ventilator interfaces such as nasal, buccal or full‐face masks.
In acute respiratory failure, mechanical ventilation almost always relies on positive pressure ventilation. The ventilator is set to detect the rapid decrease in airway pressure that occurs at the early phase of inspiration, and then it pushes the gas (usually enriched in oxygen) into the upper airway and the lung. The delivery of gas by the ventilator is regulated either by fixing the volume of gas to be injected (volume control mode) or by fixing a pressure or flow to be achieved in the airway within a predetermined time (pressure control mode). Then, invasive mechanical ventilation commonly uses the volume control mode with tidal volume and respiratory rate titrated to normalise arterial CO2 tension, and titration of the inspired fraction of oxygen to achieve an arterial oxygen saturation of more than 90%. Non‐invasive ventilation commonly uses the pressure control mode.
Over the last decade, non‐invasive mechanical ventilation (NIV) has become routine practice worldwide in the management of acute respiratory failure of various origins (Esteban 2008).
How the intervention might work
Invasive or non‐invasive positive pressure ventilation is expected to compensate respiratory muscle weakness and to allow appropriate recruitment of lung alveoli to restore a normal minute ventilation. In addition, by maintaining a positive pressure, mechanical ventilation prevents the upper airway from collapsing. Subsequently, positive pressure ventilation improves the clearance of CO2 from arterial blood and reverses lungs atelectasis and normalises ventilation/perfusion mismatch.
Theoretically, as compared to NIV, on the one hand, invasive ventilation protects the airways from aspiration pneumonia and may ease the clearance of bronchial secretions. Owing to the necessary leaks associated with non‐invasive interfaces, invasive ventilation also may offer a better control of minute ventilation and of the impermeability of the upper airway. On the other hand, invasive mechanical ventilation may increase the risk of hospital‐acquired pneumonia, of barotrauma, of laryngeal and tracheal stenosis and of weaning failure and prolonged ventilator dependency.
Systematic reviews have shown reduced morbidity and mortality with NIV, as compared to invasive ventilation, in patients with acute cardiogenic pulmonary oedema (Vital 2013), and in patients with acute exacerbation of chronic obstructive pulmonary disease (COPD) with acute hypercarbic respiratory failure (Quon 2008). Likewise, NIV can be used in weaning critically ill patients from invasive ventilation (Burns 2009). NIV has also been proven to be superior to invasive mechanical ventilation in the management of acute respiratory failure in immunosuppressed patients (Hilbert 2001), In contrast, in patients with acute hypoxaemic respiratory failure invasive mechanical ventilation remains the standard treatment (Keenan 2004).
Why it is important to do this review
A Cochrane systematic review suggested favourable benefit to risk ratio of nocturnal mechanical ventilation in patients with chronic hypoventilation related to neuromuscular or chest wall disorders (Annane 2014). There is little information on the benefit to risk ratio of NIV versus invasive ventilation in patients with these conditions who present with acute respiratory failure. Data are scarce regarding the effects of NIV in acute respiratory failure among people with neuromuscular diseases or chest wall disorders. The favourable benefit to risk ratio of NIV versus invasive ventilation demonstrated in people with cardiogenic oedema or acute exacerbation of COPD cannot be extrapolated to neuromuscular patients who often present with cough weakness, bulbar dysfunction and swallowing problems, or facial paresis, which might reduce the efficacy and tolerance of NIV. For example, in patients with amyotrophic lateral sclerosis, bulbar impairment as assessed by the Norris bulbar score was an independent predictor of the inefficacy of NIV (Servera 2015). Recent guidelines have suggested that a trial of NIV should be systematically tested in acute neuromuscular respiratory failure (Davidson 2016). Nevertheless, the same guidelines highlighted that in these patients triggering may be inefficient, bulbar dysfunction and communication difficulties may alter NIV efficacy, and sudden unpredictable deterioration is a common complication of NIV. The guidelines panel also pointed out as the main reason for using NIV as the first‐line treatment is the high risk for extubation failure following invasive mechanical ventilation in these patients. However, in a retrospective cohort of 85 patients admitted to ICU for acute neuromuscular respiratory failure, as compared to invasively ventilated patients, NIV‐treated patients had a shorter length of stay (P = 0.02), but similar functional outcomes such as long‐term ventilatory dependency (Serrano 2010). In another retrospective cohort study of 55 patients admitted to ICU for acute neuromuscular respiratory failure, the need for invasive mechanical ventilation in the acute setting was not associated with an increase risk of long‐term dependency to mechanical ventilation (Carr 2014). Therefore, we intended to systematically search the literature to summarise existing data on NIV versus invasive mechanical ventilation for acute neuromuscular respiratory failure.
Objectives
The primary objective of this review was to compare the efficacy of non‐invasive mechanical ventilation (NIV) to that of invasive ventilation in improving short‐term survival in neuromuscular disease and chest wall disorders.
The secondary objectives were to compare the effects of NIV with those of invasive mechanical ventilation on improvement in arterial blood gas after 24 hours and in lung function measurements after one month, incidence of barotrauma and ventilator‐associated pneumonia, duration of mechanical ventilation, length of stay in the intensive care unit (ICU) and length of hospital stay.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or quasi‐RCTs, with or without blinding.
Types of participants
Children or adults with neuromuscular disease or chest wall disorders with acute respiratory failure. We planned to consider studies of acute respiratory failure in heterogeneous critically ill populations whenever separate data were available for participants with neuromuscular diseases or chest wall disorders.
Acute respiratory failure was defined as a condition characterised by an abrupt loss in lung function resulting in inadequate gas exchange and acute fall in arterial oxygen tension (below 8 kPa [60mmHg]), called hypoxia with normal or low arterial CO2 tension in type 1 respiratory failure, or with increased arterial CO2 tension (> 6.5 kPA [50mmHg]) in type 2 respiratory failure (Leavers 2017).
Types of interventions
All forms of non‐invasive ventilation (using a nasal or facial mask, or mouth piece) compared with invasive ventilation (via endotracheal intubation or tracheostomy).
Types of outcome measures
Primary outcomes
The primary outcome was short‐term (i.e. one‐month) survival after initiation of assisted ventilation.
Secondary outcomes
Improvement in arterial blood gas after 24 hours from initiation of treatment, as demonstrated by increase in partial pressure of oxygen in arterial blood (PaO2) or decrease in partial pressure of carbon dioxide in arterial blood (PaCO2) or increase in PaO2/FiO2, (PaO2/fraction of inspired oxygen) in that order of preference.
Improvement in lung function measurements after one month, as demonstrated by increase in forced vital capacity or improvement in ventilation‐perfusion mismatch, in that order of preference.
Incidence of barotrauma: mechanical ventilation can lead to barotrauma of the lungs. The resultant alveolar rupture can lead to: pneumothorax, pulmonary interstitial emphysema (PIE) and pneumomediastinum.
Duration of mechanical ventilation, length of stay in the ICU and length of hospital stay.
Incidence of ventilator‐associated pneumonia.
Search methods for identification of studies
Electronic searches
We searched the following databases for RCTs and quasi‐RCTs.
Cochrane Neuromuscular Specialised Register (11 September 2017) Appendix 1
Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Register of Studies (CRS Web) (11 September 2017) Appendix 1
MEDLINE (1966 to 11 September 2017) Appendix 2
Embase (1980 to 11 September 2017) Appendix 3
Searching other resources
We searched proceedings of international scientific meetings, i.e. the American Thoracic Society, European Respiratory Society, Chest (1992 to May 2017) and the references list of identified articles. We also searched the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (Appendix 5) and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch/) (Appendix 6) on 24 November 2017. There was no language restriction.
We planned to check bibliographies of trials identified and contact the authors seeking published and unpublished studies.
Data collection and analysis
Selection of studies
Two review authors (FL and DA) independently read the titles and abstracts retrieved by the search to identify trials that met inclusion criteria. The same two review authors retrieved and reviewed independently the full text of potentially relevant articles. Two review authors (FL and DA) decided which trials fit the inclusion criteria. The nine review authors would have resolved any discrepancy by discussion, but there was no disagreement.
This review has a published protocol (Luo 2010). Appendix 7 contains methods for use if the review identified eligible studies.
Results
Description of studies
Results of the search
Database searches retrieved 2123 potentially relevant references of which 1248 remained after removal of duplicates. We assessed 14 full‐text reports for eligibility but none met our inclusion criteria (Figure 1).
1.
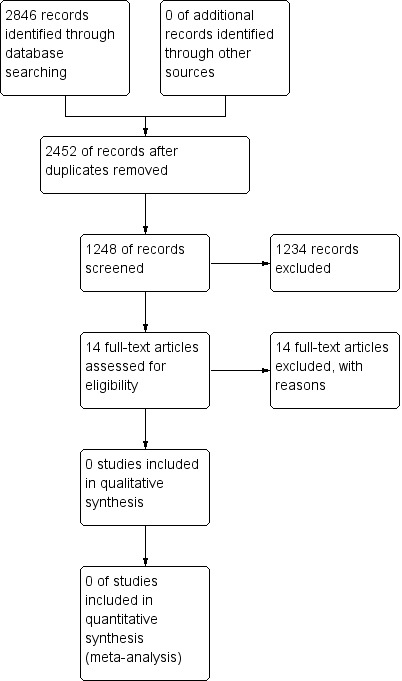
Study flow diagram.
Included studies
No study met the predefined eligibility criteria.
Excluded studies
After screening of titles and abstracts, we selected 14 potentially eligible studies for further assessment. Through the full‐text screening, we excluded these 14 studies for the following reasons.
In 11 trials, both experimental and control interventions were NIV (Aggarwal 2009; Fanfulla 2005; Jackson 2001; Jaye 2009; Chaisson 2006; Tejeda 1997; Toussaint 2003; Chatwin 2008; Nava 2008; Lechtzin 2010; Martin 2000).
One trial excluded people who needed invasive ventilation (Piastra 2006).
One prospective cross‐over study compared the effects of mechanical insufflation‐exsufflation versus suctioning via tracheostomy tubes on respiratory variables in six participants with amyotrophic lateral sclerosis (ALS) (Sancho 2003).
In another trial, there was no description of treatment allocation, and NIV was the only intervention (Reardon 2005).
See Characteristics of excluded studies for details.
Risk of bias in included studies
No studies were eligible for inclusion in the review.
Effects of interventions
No studies were eligible for inclusion in the review.
Discussion
We found no randomised trials that compared the benefits and risks of invasive ventilation with those of non‐invasive ventilation (NIV).
Acute respiratory failure is a frequent and life‐threatening complication in neuromuscular disease or chest wall disorders. In acute respiratory failure, invasive mechanical ventilation is the standard treatment when initial management with oxygen supplementation, physiotherapy, cough assistance, or antibacterial drugs are insufficient to stabilise the patient, although this may have potentially life‐changing consequences for the patient with neuromuscular disease who may become ventilator‐dependent via a tracheostomy.
Over the last decade, NIV has been increasingly used to manage both acute and chronic respiratory failure in a broad variety of conditions. In patients with amyotrophic lateral sclerosis (ALS), long‐term NIV may provide survival benefit and may improve patients' well‐being and quality of life, particularly in those without bulbar impairment (Radunovic 2017). Likewise, in patients with chronic hypoventilation related to neuromuscular or chest wall disorders, long‐term nocturnal mechanical ventilation may improve long‐term survival (Annane 2014). In patients with acute respiratory failure, reduced morbidity and mortality with NIV as compared to invasive ventilation, has been suggested for acute respiratory failure in immunosuppressed patients (Hilbert 2001), acute cardiogenic pulmonary oedema (Vital 2013), acute exacerbation of chronic obstructive pulmonary disease (COPD), and acute hypercarbic respiratory failure (Quon 2008). However, these findings cannot be extrapolated to patients with neuromuscular diseases who also have facial weakness, bulbar disorders, and poor cough, which may substantially reduce the efficacy of NIV.
Most recent guidelines recommend NIV as the first‐line therapeutic approach for the management of acute hypercapnic respiratory failure, whether it is related to neuromuscular disorders or not (Davidson 2016). On the one hand, NIV, by avoiding endotracheal intubation, may reduce the person's exposure time to mechanical ventilation and to ICU, prevent lung infections, barotrauma, tracheal stenosis, and the need for tracheostomy. On the other hand, NIV efficacy may be dramatically reduced by the presence of bulbar dysfunction and by excessive bronchial secretions (Senevirame 2008). NIV may also be associated with sudden deterioration in respiratory function and vital signs that require immediate tracheal intubation, which can be challenging (Garpestad 2007).
We performed a systematic search of the main electronic databases for publications, with no time or language restrictions, and we searched for communications in proceedings of major scientific meetings. Since registry of trials on publicly available databases has been mandatory for close to 15 years and we identified no relevant trial by systematically searching registries, it is unlikely that we have missed any relevant unpublished trials.
Authors' conclusions
Implications for practice.
There is no evidence from randomised trials to support the routine use of non‐invasive ventilation (NIV) instead of invasive ventilation in patients with acute neuromuscular respiratory failure. However, some evidence from observational studies suggests that NIV should be trialled in all patients except those with bulbar dysfunction.
Implications for research.
The benefits and risks of NIV should be compared with those of invasive ventilation in well‐designed randomised controlled trials. In addition, future trials will need to compare different types of patient/ventilator interfaces, i.e. nasal versus full‐face mask versus mouthpiece.
Future trials will need to explore the interaction between patients' responses to these treatments and whether acute neuromuscular respiratory failure occurs in patients with acute onset neuromuscular diseases or in patients with acute exacerbation of neuromuscular or chest wall disorders related to chronic hypoventilation. They also should explore any interaction between patients' responses to NIV or invasive mechanical ventilation and the presence at baseline of bulbar dysfunction.
Acknowledgements
We would like to thank Angela Gunn and Rachel Barton for developing the search strategy and for their helpful comments. We would also like to thank the referees for their comments and feedback during the preparation of this review.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health. Cochrane Neuromuscular is also supported by the MRC Centre for Neuromuscular Diseases and the Motor Neurone Disease Association.
Appendices
Appendix 1. Cochrane Neuromuscular Specialised Register & Cochrane Register of Controlled Trials (CRSweb) search strategy
#1 MeSH DESCRIPTOR Respiratory Insufficiency Explode All [REFERENCE] [STANDARD] #2 MeSH DESCRIPTOR Respiration, Artificial Explode All [REFERENCE] [STANDARD] #3 "respiratory failure" or "respiratory insufficiency" or (artificial NEAR2 respiration) or "mechanical ventilation" [REFERENCE] [STANDARD] #4 tracheotomy or tracheostomy [REFERENCE] [STANDARD] #5 "non invasive ventilation" [REFERENCE] [STANDARD] #6 "noninvasive ventilation" [REFERENCE] [STANDARD] #7 #1 or #2 or #3 or #4 or #5 or #6 [REFERENCE] [STANDARD] #8 "neuromuscular disease*" or "muscular disease"* or "muscular disorders" or muscular atrophy" or myositis or myastheni*" [REFERENCE] [STANDARD] #9 MeSH DESCRIPTOR Motor Neuron Disease Explode All [REFERENCE] [STANDARD] #10 "moto? neuron? disease?" or "moto?neuron? disease?" [REFERENCE] [STANDARD] #11 "charcot disease" [REFERENCE] [STANDARD] #12 "amyotrophic lateral sclerosis" [REFERENCE] [STANDARD] #13 als:ti or als:ab or nmd:ti or mnd:ab [REFERENCE] [STANDARD] #14 MeSH DESCRIPTOR Neuromuscular Diseases Explode All [REFERENCE] [STANDARD] #15 MeSH DESCRIPTOR Muscular Diseases Explode All [REFERENCE] [STANDARD] #16 "neuromuscular disease" or "neuromuscular diseases" or "muscular disease" or "muscular diseases" [REFERENCE] [STANDARD] #17 MeSH DESCRIPTOR Neuromuscular Junction Diseases Explode All [REFERENCE] [STANDARD] #18 MeSH DESCRIPTOR Thoracic Diseases Explode All [REFERENCE] [STANDARD] #19 "neuromuscular junction disease" or "neuromuscular junction diseases" or "chest wall disorder" [REFERENCE] [STANDARD] #20 "thoracic disease" or "thoracic diseases" or "chest wall oscillation" or "chest wall disorders" [REFERENCE] [STANDARD] #21 MeSH DESCRIPTOR Muscular Disorders, Atrophic Explode All [REFERENCE] [STANDARD] #22 MeSH DESCRIPTOR Muscular Atrophy, Spinal Explode All [REFERENCE] [STANDARD] #23 MeSH DESCRIPTOR Muscular Diseases WITH CN [REFERENCE] [STANDARD] #24 "spinal muscular atrophy" or myastheni* [REFERENCE] [STANDARD] #25 (congenital NEAR2 myopathy) or (congenital NEAR2 myopathies) [REFERENCE] [STANDARD] #26 MeSH DESCRIPTOR Myositis Explode All [REFERENCE] [STANDARD] #27 MeSH DESCRIPTOR Neuromuscular Diseases Explode All [REFERENCE] [STANDARD] #28 MeSH DESCRIPTOR Peripheral Nervous System Diseases Explode All [REFERENCE] [STANDARD] #29 "Peripheral Nervous System Disease" or "Peripheral Nervous System Diseases" [REFERENCE] [STANDARD] #30 (inflammatory NEAR3 myopath*):ti or (inflammatory NEAR3 myopath*):ab [REFERENCE] [STANDARD] #31 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 [REFERENCE] [STANDARD] #32 #7 and #31 [REFERENCE] [STANDARD] #33 (#7 and #31) AND (INREGISTER) [REFERENCE] [STANDARD]
Appendix 2. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present> Ovid MEDLINE(R) 1946 to August Week 5 2017 Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (481130) 2 controlled clinical trial.pt. (96855) 3 randomized.ab. (421208) 4 placebo.ab. (196549) 5 drug therapy.fs. (2061444) 6 randomly.ab. (291657) 7 trial.ab. (442554) 8 groups.ab. (1799326) 9 or/1‐8 (4258162) 10 exp animals/ not humans.sh. (4581831) 11 9 not 10 (3680281) 12 neuromuscular disease$.mp. or exp Neuromuscular Diseases/ (290941) 13 exp Motor Neuron Disease/ (24673) 14 (moto$1 neuron$1 disease$1 or moto?neuron$1 disease$).mp. (8263) 15 Amyotrophic Lateral Sclerosis.mp. (23448) 16 (Charcot syndrome or (Lou Gehrig$1 adj5 syndrome$1)).mp. (6) 17 (Charcot disease or (Lou Gehrig$1 adj5 disease$1)).mp. (204) 18 muscular disease$.mp. or exp Muscular Diseases/ (163043) 19 neuromuscular junction disease$.mp. or exp Neuromuscular Junction Diseases/ (18407) 20 exp Thoracic Diseases/ or chest wall disorder$.mp. (29764) 21 chest wall oscillation.mp. or Chest Wall Oscillation/ (319) 22 exp Muscular Disorders, Atrophic/ (26365) 23 exp Muscular Atrophy, Spinal/ (4384) 24 muscular diseases/cn (578) 25 (congenital adj2 myopath$).mp. (1531) 26 exp myositis/ (18715) 27 neuromuscular diseases/ or peripheral nervous system diseases/ (31850) 28 (inflammatory adj3 (myopath$ or neuropath$)).mp. (4971) 29 myastheni$.mp. (18830) 30 or/12‐29 (403408) 31 respiratory failure.mp. or exp Respiratory Insufficiency/ (76920) 32 mechanical ventilation.mp. or exp Respiration, Artificial/ (88677) 33 tracheotomy.mp. or Tracheotomy/ (11331) 34 tracheostomy.mp. or Tracheostomy/ (13855) 35 non invasive ventilation.mp. (1894) 36 noninvasive ventilation.mp. (2925) 37 or/31‐36 (166910) 38 11 and 30 and 37 (1375) 39 remove duplicates from 38 (1301)
Appendix 3. Embase (OvidSP) search strategy
Database: Embase <1980 to 2017 Week 37> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure.sh. (53120) 2 double‐blind procedure.sh. (139921) 3 single‐blind procedure.sh. (29457) 4 randomized controlled trial.sh. (468336) 5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (1420824) 6 trial.ti. (228842) 7 controlled clinical trial/ (448309) 8 or/1‐7 (1709556) 9 exp animal/ or exp invertebrate/ or animal.hw. or non human/ or nonhuman/ (24698928) 10 human/ or human cell/ or human tissue/ or normal human/ (18823835) 11 9 not 10 (5902992) 12 8 not 11 (1519618) 13 limit 12 to (conference abstracts or embase) (1282059) 14 neuromuscular disease.mp. or exp neuromuscular disease/ (163332) 15 neuromuscular disease$.mp. or exp neuromuscular disease/ (164034) 16 motor neuron disease/ or amyotrophic lateral sclerosis/ (36727) 17 (moto$1 neuron$1 disease$1 or moto?neuron$1 disease$1).mp. (12036) 18 ((Lou Gehrig$1 adj5 syndrome$1) or (Lou Gehrig$1 adj5 disease$1)).mp. (199) 19 (Chracot syndrome or Charcot disease).mp. (27) 20 amyotrophic lateral sclerosis.mp. (34020) 21 muscular disease.mp. or exp muscle disease/ (432751) 22 exp muscle disease/ (432677) 23 (muscular disease$ or muscle disease$).mp. (21982) 24 neuromuscular disease/ or exp neuromuscular junction disorder/ (39488) 25 neuromuscular junction disease$.tw. (38) 26 exp thorax disease/ (112344) 27 exp thorax disease/ or chest wall disorder$.mp. (112386) 28 chest wall oscillation/ or chest wall oscillation.tw. (286) 29 or/14‐28 (545673) 30 exp muscle atrophy/ (32022) 31 exp spinal muscular atrophy/ (43474) 32 exp muscular dystrophy/ (37547) 33 muscle disease/cn [Congenital Disorder] (315) 34 (congenital adj2 myopath$).mp. (1611) 35 exp myositis/ (31231) 36 exp neuromuscular disease/ (162473) 37 exp peripheral neuropathy/ (61614) 38 (inflammatory adj3 (myopath$ or neuropath$)).mp. (7071) 39 myastheni$.mp. (23483) 40 or/29‐39 (601892) 41 (respiratory failure or respiratory insufficiency).mp. (80183) 42 exp artificial ventilation/ (161716) 43 (tracheotomy or tracheostomy or mechanical ventilation or (artificial adj1 respiration)).mp. (85870) 44 non invasive ventilation.mp. (4397) 45 noninvasive ventilation.mp. (7643) 46 or/41‐45 (247801) 47 13 and 40 and 46 (1128) 48 remove duplicates from 47 (1089)
Appendix 4. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
Search run on Thu Aug 11 2016 #1 MESH DESCRIPTOR Neuromuscular Diseases EXPLODE ALL TREES5130 #2 MESH DESCRIPTOR Muscular Diseases EXPLODE ALL TREES3898 #3 MESH DESCRIPTOR Neuromuscular Junction Diseases EXPLODE ALL TREES118 #4 MESH DESCRIPTOR Thoracic Diseases EXPLODE ALL TREES131 #5 ("neuromuscular disease" or "neuromuscular diseases" or "muscular disease" or "muscular diseases" or "muscle disease" or "muscle diseases" or myositis):TI,AB,KY895 #6 ("thoracic disease" or "thoracic diseases" or "chest wall disease" or "chest wall diseases" or "chest wall oscillation"):TI,AB,KY151 #7 MESH DESCRIPTOR Motor Neuron Disease EXPLODE ALL TREES346 #8 "motor neuron disease" or "motor neurone disease" or "motoneuron disease" or "motoneurone disease"102 #9 "amyotrophic lateral sclerosis"516 #10 Gehrig NEAR (disease or syndrome)0 #11 MESH DESCRIPTOR Muscular Disorders, Atrophic EXPLODE ALL TREES261 #12 MESH DESCRIPTOR Muscular Atrophy, Spinal EXPLODE ALL TREES32 #13 MESH DESCRIPTOR Muscular Diseases EXPLODE ALL TREES WITH QUALIFIERS CN1 #14 "spinal muscular atrophy"55 #15 (congenital NEAR2 (myopathy or myopathies)):TI,AB,KY1 #16 MESH DESCRIPTOR Myositis EXPLODE ALL TREES93 #17 MESH DESCRIPTOR Peripheral Nervous System Diseases EXPLODE ALL TREES2885 #18 "peripheral nervous system disease" or "peripheral nervous system diseases"425 #19 inflammatory NEAR3 (myopathy or myopathies or neuropathy or neuropathies)79 #20 myastheni*261 #21 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #15 OR #16 OR #17 OR #19 OR #208421 #22 (respiratory failure or respiratory insufficiency):TI,AB,KY2319 #23 MESH DESCRIPTOR Respiratory Insufficiency EXPLODE ALL TREES1695 #24 MESH DESCRIPTOR Respiration, Artificial EXPLODE ALL TREES4619 #25 "mechanical ventilation" or "artificial respiration"3731 #26 (tracheotomy or tracheostomy):TI,AB,KY515 #27 ("non invasive ventilation"):TI,AB,KY337 #28 ("noninvasive ventilation"):TI,AB,KY451 #29 #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #289781 #30 #21 AND #29215 #31 neuromusc:cc6177 #32 #30 not #31122
Appendix 5. US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov
46 studies, search run on 24 November 2017
Advanced search
Condition: (Neuromuscular OR neuromyopathies OR thoracic OR chest wall) and (respiratory failure OR Respiratory Insufficiency)
Intervention: (ventilation OR ventilator)
Appendix 6. World Health Organization (WHO) International Clinical Trials Registry Platform
36 records for 37 trials, search run on 24 November 2017
Neuromuscular AND respiratory failure AND ventilation OR neuromyopathies AND respiratory failure AND ventilation OR thoracic AND respiratory failure AND ventilation OR chest wall AND respiratory failure) AND ventilation OR Neuromuscular AND Respiratory Insufficiency AND ventilation OR neuromyopathies AND Respiratory Insufficiency AND ventilation OR thoracic AND Respiratory Insufficiency AND ventilation OR chest wall AND Respiratory Insufficiency AND ventilation OR Neuromuscular AND respiratory failure AND ventilator OR neuromyopathies AND respiratory failure AND ventilator OR thoracic respiratory failure AND ventilator OR chest wall AND respiratory failure) AND ventilation OR Neuromuscular AND Respiratory Insufficiency AND ventilator OR neuromyopathies AND Respiratory Insufficiency AND ventilator OR thoracic AND Respiratory Insufficiency AND ventilator OR chest wall AND Respiratory Insufficiency AND ventilator
Appendix 7. Additional methods
Data extraction and management
Two review authors will perform data extraction independently and will systematically contact authors of trials to provide missing data. One review author will enter data into the Cochrane authoring and statistical software Reference Manager 5 (RevMan), and other review authors will check the data entry.
Assessment of risk of bias in included studies
Two review authors will assess risk of bias in the included trials independently according to the Cochrane Handbook of Systematic Reviews of Interventions (Chapter 8.5) (Higgins 2011). They will assess the domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias. They will assess trials for each domain as at low, high, or unclear risk of bias. The two review authors will resolve any disagreement by discussion.
Measures of treatment effect
We will express results as risk ratios (RR) with 95% confidence intervals (CI) and number needed to treat for an additional beneficial outcome (NNTB)/number needed to treat for an additional harmful outcome for dichotomous outcomes, and mean difference (MD) and 95% confidence intervals (CIs) for continuous outcomes.
Unit of analysis issues
We planned to take into account:
whether groups or individuals were randomised in each study;
whether individuals underwent more than one intervention; and
whether there were multiple observations for the same outcome.
Dealing with missing data
We will obtain missing or additional data from the authors of included studies where possible.
Assessment of heterogeneity
We will assess heterogeneity using the I² statistic and Chi² test provided by RevMan.
Assessment of reporting biases
We will seek evidence of publication bias using the funnel plot method if sufficient studies are available.
Data synthesis
We planned to use a fixed‐effect model using RevMan and perform a sensitivity analysis using a random‐effects model.
'Summary of findings' table
We will include a 'Summary of findings' table showing the following outcomes if we find eligible trials.
Short‐term (one‐month) survival after initiation of assisted ventilation.
Incidence of barotrauma: pneumothorax, pulmonary interstitial emphysema (PIE), pneumomediastinum.
Duration of mechanical ventilation.
Length of stay in intensive care unit (ICU).
Length of stay in hospital.
Incidence of ventilator‐associated pneumonia.
Subgroup analysis and investigation of heterogeneity
We will compute the primary endpoint in the following subgroups:
acute respiratory failure in acute versus chronic neuromuscular diseases;
children versus adults.
Sensitivity analysis
We will perform sensitivity analyses on the basis of risk of bias of trials.
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aggarwal 2009 | This prospective randomised controlled trial aimed to evaluate if the combination of pressure‐support ventilation (PSV) and automatic tube compensation (ATC) is superior to PSV alone in weaning patients with severe neurotoxic snake envenoming receiving mechanical ventilation. The trial was excluded as it did not compare non‐invasive ventilation (NIV) to invasive ventilation. |
| Chaisson 2006 | This randomised clinical pilot study evaluated the efficacy of high‐frequency chest wall oscillation (HFCWO) administered through the VestTM Airway Clearance System when added to standard care in preventing pulmonary complications and prolonging survival in patients with amyotrophic lateral sclerosis (ALS). The trial was excluded as it did not compare NIV to invasive ventilation, and did not include patients with acute respiratory failure. |
| Chatwin 2008 | This prospective randomised controlled trial evaluated long‐term home mechanical ventilation (HMV) in outpatients and inpatients who had stable neuromuscular and chest wall disease with nocturnal hypoventilation. The trial was excluded as did not compare NIV to invasive ventilation, and did not include patients with acute respiratory failure. |
| Fanfulla 2005 | This trial studied nine inpatients affected by chronic respiratory failure or nocturnal hypoventilation due to miscellaneous neuromuscular disorders who were admitted during a period of clinical stability for a follow‐up control. The trial was excluded as it did not compare NIV to invasive ventilation. |
| Jackson 2001 | This prospective randomised study was performed in 20 amyotrophic lateral sclerosis (ALS) patients who had an forced vital capacity (FVC) of 70% to 100% received non‐invasive positive pressure ventilation (NPPV). The trial was excluded as it did not compare NIV to invasive ventilation. |
| Jaye 2009 | This randomised cross‐over trial compared the efficacy of automatic titration of non‐invasive ventilation (NIV) with conventional NIV in stable neuromuscular and chest wall disorder patients established on long‐term ventilatory support. The trial was excluded as it did not compare NIV to invasive ventilation, and did not include patients with acute respiratory failure. |
| Lechtzin 2010 | This prospective trial compared lung compliance (CL) before and after supramaximal lung inflation via mouthpiece‐delivered positive pressure. The trial was excluded as it did not compare NIV to invasive ventilation. |
| Martin 2000 | This prospective randomised trial compared bilevel positive airway pressure, with usual medical care (UMC) in the therapy of patients with acute respiratory failure (ARF). The trial was excluded as it did not compare NIV to invasive ventilation. |
| Nava 2008 | This randomised cross‐over pilot study investigated the clinical effects of heated humidification (HH) and heat and moisture exchanger (HME) during long‐term non‐invasive mechanical ventilation (NIMV) in stable hypercapnic patients. The trial was excluded as it did not compare NIV to invasive ventilation and did not include patients with acute respiratory failure. |
| Piastra 2006 | This prospective pilot study was carried out on neuromuscular patients admitted to a paediatric intensive care unit (PICU). The trial was excluded as it did not compare NIV to invasive ventilation. |
| Reardon 2005 | This randomised controlled study was conducted to compare efficacy of intrapulmonary percussive ventilation (IPV) with incentive spirometry (IS) in reducing number of days of antibiotic use in adolescents with neuromuscular disease. The trial was exclude as it did not compare NIV to invasive ventilation. |
| Sancho 2003 | This prospective cross‐over study compared the effects of mechanical insufflation‐exsufflation vs. suctioning via tracheostomy tubes on respiratory variables for six patients with amyotrophic lateral sclerosis. The trial was excluded as it did not compare NIV to invasive ventilation. |
| Tejeda 1997 | This short‐term (4h) prospective study assessed whether pressure support ventilation (PSV) could be used as an alternative ventilatory mode to assist‐control (A/C) ventilation in the treatment of respiratory failure. The trial was excluded as it did not compare NIV to invasive ventilation. |
| Toussaint 2003 | This randomised, cross‐over study compared assisted mucus clearance techniques with and without intrapulmonary percussive ventilation (IPV). The trial was excluded as it did not compare NIV to invasive ventilation. |
Differences between protocol and review
We defined acute respiratory failure as a condition characterised by an abrupt loss in lung function resulting in inadequate gas exchange and acute fall in arterial oxygen tension (below 8 kPa [60mmHg]), called hypoxia with normal or low arterial CO2 tension in type 1 respiratory failure, or with increased arterial CO2 tension (> 6.5 kPA [50mmHg]) in type 2 respiratory failure (Leavers 2017). In our protocol we had planned to allow any definition used in individual studies.
We specified one month as the definition of 'short term' for our primary outcome, short‐term survival after initiation of assisted ventilation.
Three rather than two authors independently selected studies.
We changed our planned subgroup analysis for the primary outcome to:
acute respiratory failure in acute versus chronic neuromuscular diseases;
children versus adults.
We specified outcomes for inclusion in a 'Summary of findings' table.
Dr Ji Yang and Dr Yun Zhang withdrew from authorship.
Methods relating to eligible studies were not used as this review had no included studies; we moved these additional methods to Appendix 7. We did not update the methods to reflect all current Cochrane standards (Higgins 2016), as no update is planned.
Contributions of authors
FL and DA screened the literature. FL drafted the review. DA and DO provided critical analysis of the draft. All authors approved the final text.
Sources of support
Internal sources
China Cochrane Centre, China.
External sources
No sources of support supplied
Declarations of interest
FL: none known
DA: none known
DO: DO has received grants from AFRM for a randomised study of non‐invasive ventilation in myotonic dystrophy type 1 and from Phillips for a study of Average Volume Assured Pressure Support (AVAPS) in myotonic dystrophy type 1, and fees from IP Santé à domicile. He has also acted as a medical referee for Foyer ADEP Evry. All the activities mentioned are in the field of care and research conducted in neuromuscular diseases and home ventilation. Outside this work, he received funding from Covidien and from Genzyme to attend the AAN conference in 2012.
LH: none known
MY: none known
MZ: none known
GJL: none known
New
References
References to studies excluded from this review
Aggarwal 2009 {published data only}
- Aggarwal AN, Agarwal R, Gupta D. Automatic tube compensation as an adjunct for weaning in patients with severe neuroparalytic snake envenomation requiring mechanical ventilation: a pilot randomized study. Respiratory Care 2009;54(12):1697‐702. [PubMed] [Google Scholar]
Chaisson 2006 {published data only}
- Chaisson KM, Walsh S, Simmons Z, Vender RL. A clinical pilot study: high frequency chest wall oscillation airway clearance in patients with amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis 2006;7(2):107‐11. [DOI] [PubMed] [Google Scholar]
Chatwin 2008 {published data only}
- Chatwin M, Nickol AH, Morrell MJ, Polkey MI, Simonds AK. Randomised trial of inpatient versus outpatient initiation of home mechanical ventilation inpatients with nocturnal hypoventilation. Respiratory Medicine 2008;102(11):1528‐35. [DOI] [PubMed] [Google Scholar]
Fanfulla 2005 {published data only}
- Fanfulla F, Delmastro M, Berardinelli A, D’Artavilla Lupo N, Nava S. Effects of different ventilator settings on sleep and inspiratory effort in patients with neuromuscular disease. American Journal of Respiratory and Critical Care Medicine 2005;172:619‐24. [DOI] [PubMed] [Google Scholar]
Jackson 2001 {published data only}
- Jackson CE, Rosenfeld J, Moore DH, Bryan WW, Barohn RJ, Wrench M, et al. A preliminary evaluation of a prospective study of pulmonary function studies and symptoms of hypoventilation in ALS/MND patients. Journal of the Neurological Sciences 2001;191(1 ‐ 2):75‐8. [DOI] [PubMed] [Google Scholar]
Jaye 2009 {published data only}
- Jaye J, Chatwin M, Dayer M, Morrell MJ, Simonds AK. Autotitrating versus standard noninvasive ventilation: a randomised crossover trial. European Respiratory Journal 2009;33(3):566‐73. [DOI] [PubMed] [Google Scholar]
Lechtzin 2010 {published data only}
- Lechtzin N, Shade D, Clawson L, Wiener CM. Supramaximal inflation improves lung compliance in subjects with amyotrophic lateral sclerosis. Chest 2006;129(5):1322‐9. [DOI] [PubMed] [Google Scholar]
Martin 2000 {published data only}
- Martin TJ, Hovis JD, Costantino JP, Bierman MI, Donahoe MP, Rogers RM, et al. A randomized, prospective evaluation of noninvasive ventilation for acute respiratory failure. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Pt 1):807‐13. [DOI] [PubMed] [Google Scholar]
Nava 2008 {published data only}
- Nava S, Cirio S, Fanfulla F, Carlucci A, Navarra A, Negri A, et al. Comparison of two humidification systems for long‐term noninvasive mechanical ventilation. European Respiratory Journal 2008;32(2):460‐4. [DOI] [PubMed] [Google Scholar]
Piastra 2006 {published data only}
- Piastra M, Antonelli M, Caresta E, Chiaretti A, Polidori G, Conti G. Noninvasive ventilation in childhood acute neuromuscular respiratory failure: a pilot study. Respiration 2006;73(6):791‐8. [DOI] [PubMed] [Google Scholar]
Reardon 2005 {published data only}
- Reardon CC, Christiansen D, Barnett ED, Cabral HJ. Intrapulmonary percussive ventilation vs incentive spirometry for children with neuromuscular disease. Archives of Pediatrics & Adolescent Medicine 2005;159:526‐31. [DOI] [PubMed] [Google Scholar]
Sancho 2003 {published data only}
- Sancho J, Servera E, Vergara P, Marín J. Mechanical insufflation‐exsufflation vs. tracheal suctioning via tracheostomy tubes for patients with amyotrophic lateral sclerosis: a pilot study. American Journal of Physical Medicine & Rehabilitation 2003;82(10):750‐3. [DOI] [PubMed] [Google Scholar]
Tejeda 1997 {published data only}
- Tejeda M, Boix JH, Alvarez F, Balanzá R, Morales M. Comparison of pressure support ventilation and assist‐control ventilation in the treatment of respiratory failure. Chest 1997;111(5):1322‐5. [DOI] [PubMed] [Google Scholar]
Toussaint 2003 {published data only}
- Toussaint M, Win H, Steens M, Soudon P. Effect of intrapulmonary percussive ventilation on mucus clearance in Duchenne muscular dystrophy patients: a preliminary report. Respiratory Care 2003;48(10):940‐7. [PubMed] [Google Scholar]
Additional references
Annane 2014
- Annane D, Orlikowski D, Chevret S, Chevrolet JC, Raphael JC. Nocturnal mechanical ventilation for chronic hypoventilation in patients with neuromuscular and chest wall disorders. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD001941.pub3] [DOI] [Google Scholar]
Burns 2009
- Burns KE, Adhikari NK, Keenan SP, Meade M. Use of non‐invasive ventilation to wean critically ill adults off invasive ventilation: meta‐analysis and systematic review. BMJ 2009;338:b1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carr 2014
- Carr AS, Hoeritzauer AI, Kee R, Kinney M, Campbell J, Hutchinson A, et al. Acute neuromuscular respiratory failure: a population‐based study of aetiology and outcome in Northern Ireland. Postgraduate Medical Journal 2014;90(1062):201‐4. [DOI] [PubMed] [Google Scholar]
Davidson 2016
- Davidson AC, Banham S, Elliott M, Kennedy D, Gelder C, Glossop A, et al. BTS Standards of Care Committee Member, British Thoracic Society/Intensive Care Society Acute Hypercapnic Respiratory Failure Guideline Development Group, On behalf of the British Thoracic Society Standards of Care Committee. BTS/ICS guideline for the ventilatory management of acute hypercapnic respiratory failure in adults. Thorax 2016;71(Suppl 2):ii1‐35. [DOI] [PubMed] [Google Scholar]
Esteban 2008
- Esteban A, Ferguson ND, Meade MO, Frutos‐Vivar F, Apezteguia C, Brochard L, et al. VENTILA Group. Evolution of mechanical ventilation in response to clinical research. American Journal of Respiratory and Critical Care Medicine 2008;177(2):170‐7. [DOI] [PubMed] [Google Scholar]
Garpestad 2007
- Garpestad E, Brennan J, Hill NS. Noninvasive ventilation for critical care. Chest 2007;132(2):137. [DOI] [PubMed] [Google Scholar]
Goligher 2009
- Goligher E, Ferguson ND. Mechanical ventilation: epidemiological insights into current practices. Current Opinion in Critical Care 2009;15(1):44‐51. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 updated March 2011. The Cochrane Collaboration. Available from www.cochrane‐handbook.org.
Higgins 2016
- Higgins JPT, Lasserson T, Chandler J, Tovey D, Churchill R. Methodological Expectations of Cochrane Intervention Reviews. Cochrane: London 2016.
Hilbert 2001
- Hilbert G, Gruson D, Vargas F, Valentino R, Gbikpi‐Benissan G, Dupon M, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. New England Journal of Medicine 2001;344:481‐7. [DOI] [PubMed] [Google Scholar]
Keenan 2004
- Keenan SP, Sinuff T, Cook DJ, Hill NS. Does noninvasive positive pressure ventilation improve outcome in acute hypoxemic respiratory failure? A systematic review. Critical Care Medicine 2004;32(12):2516‐23. [DOI] [PubMed] [Google Scholar]
Leavers 2017
- Leavers S, Evans T. Acute respiratory failure. In: Warrell DA, Cox TM, Firth JD editor(s). Oxford Textbook of Medicine. Oxford University Press, 2017. [DOI: 10.1093/med/9780199204854.003.1705_update_004] [DOI] [Google Scholar]
Quon 2008
- Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest 2008;133(3):756‐66. [DOI] [PubMed] [Google Scholar]
Radunovic 2017
- Radunovic A, Annane D, Rafiq MK, Brassngton R, Mustfa M. Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database of Systematic Reviews 2017, Issue 10. [DOI: 10.1002/14651858.CD004427.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Senevirame 2008
- Senevirame J, Mandrekar J, Wijdicks EF, Rabinstein AA. Noninvasive ventilation in myasthenic crisis. Archives of Neurology 2008;65(1):54. [DOI] [PubMed] [Google Scholar]
Serrano 2010
- Serrano MC, Rabinstein AA. Causes and outcomes of acute neuromuscular respiratory failure. Archives of Neurology 2010;67(9):1089‐94. [DOI] [PubMed] [Google Scholar]
Servera 2015
- Servera E, Sancho J, Bañuls P, Marín J. Bulbar impairment score predicts noninvasive volume‐cycled ventilation failure during an acute lower respiratory tract infection in ALS. Journal of the Neurological Sciences 2015;358(1‐2):87‐91. [DOI] [PubMed] [Google Scholar]
Vital 2013
- Vital FMR, Ladeira MT, Atallah ÁN. Non‐invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD005351.pub3] [DOI] [PubMed] [Google Scholar]
Wijdicks 2017
- Wijdicks EFM. The neurology of acutely failing respiratory mechanics. Annals of Neurology 2017;81:485‐94. [PUBMED: 28253561] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Luo 2010
- Luo F, Annane D, Orlikowski D, He L, Zhang Y, Yang J, et al. Invasive versus non‐invasive ventilation for acute respiratory failure in neuromuscular disease and chest wall disorders. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD008380] [DOI] [PMC free article] [PubMed] [Google Scholar]


