Abstract
Background
Nephrolithiasis, or urinary stone disease, in children causes significant morbidity, and is increasing in prevalence in the North American population. Therefore, medical and dietary interventions (MDI) for recurrent urinary stones in children are poised to gain increasing importance in the clinical armamentarium.
Objectives
To assess the effects of medical and dietary interventions (MDI) for the prevention of idiopathic urinary stones in children aged from one to 18 years.
Search methods
We searched multiple databases using search terms relevant to this review, including studies identified from the Cochrane Central Register of Controlled Trials (CENTRAL, 2017, Issue 1), MEDLINE OvidSP (1946 to 14 February 2017), Embase OvidSP (1980 to 14 February 2017), International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov. Additionally, we handsearched renal‐related journals and the proceedings of major renal conferences, and reviewed weekly current awareness alerts for selected renal journals. The date of the last search was 14 February 2017. There were no language restrictions.
Selection criteria
Randomized controlled trials of at least one year of MDI versus control for prevention of recurrent idiopathic (non‐syndromic) nephrolithiasis in children.
Data collection and analysis
We used standard methodologic procedures expected by Cochrane. Titles and abstracts were identified by search criteria and then screened for relevance, and then data extraction and risk of bias assessment were carried out. We assessed the quality of evidence using GRADE.
Main results
The search identified one study of 125 children (72 boys and 53 girls) with calcium‐containing idiopathic nephrolithiasis and normal renal morphology following initial treatment with shockwave lithotripsy (SWL). Patients were randomized to oral potassium citrate 1 mEq/kg per day for 12 months versus no specific medication or preventive measure with results reported for a total of 96 patients (48 per group). This included children who were stone‐free (n = 52) or had residual stone fragments (n = 44) following SWL.
Primary outcomes:
Medical therapy may lower rates of stone recurrence with a risk ratio (RR) of 0.19 (95% confidence interval (CI) 0.06 to 0.60; low quality evidence). This corresponds to 270 fewer stone recurrences per 1000 (133 fewer to 313 fewer) children. We downgraded the quality of evidence by two levels for very serious study limitations related to unclear allocation concealment (selection bias) and a high risk of performance, detection and attrition bias. While the data for adverse events were incomplete, they reported that six of 48 (12.5%) children receiving potassium citrate left the trial because of adverse effects. This corresponds to a RR of 13.0 (95% CI 0.75 to 224.53; very low quality evidence); an absolute effect size estimate could not be generated. We downgraded the quality of evidence for study limitations and imprecision.
We found no information on retreatment rates.
Secondary outcomes:
We found no evidence on serum electrolytes, 24‐hour urine collection parameters or time to new stone formation.
We were unable to perform any preplanned secondary analyses.
Authors' conclusions
Oral potassium citrate supplementation may reduce recurrent calcium urinary stone formation in children following SWL; however, our confidence in this finding is limited. A substantial number of children stopped the medication due to adverse events. There is no trial evidence on retreatment rates. There is a critical need for additional well‐designed trials in children with nephrolithiasis.
Plain language summary
Medical and dietary interventions for preventing recurrent urinary stones in children
Review question
We performed this review to find out whether medicines or diet changes are better than no intervention at preventing children (up to 18 years of age) who had been treated for kidney stones from getting kidney stones again.
Background
Many children form kidney stones for unclear reasons and require treatment. Changes in what they eat and drink or medicines (or both) may help lower the risk of these children to get kidney stones again but we do not know how well this works and what the side effects are.
Study characteristics
We examined research published up to 14 February 2017. We looked for studies of boys and girls from age one to 18 years who sometime before had problems with kidney stones and who were assigned to a different diet or a medicine (or both) to stop the stones from coming back for at least 12 months. We were most interested in whether the stones returned, how many side effects there were and if children had to have more treatments for kidney stones.
Key results
We only found one small study with 125 children (72 boys and 53 girls) who had been treated with waves similar to those that carry sound, so‐called shock waves to treat their kidney stones. These children formed kidney stones for unknown reasons and had otherwise normal kidneys. Fifty‐two children had no more stones and 44 children still had small stone pieces left when they started the study. One group was given a medicine by mouth called potassium citrate; the other group was given no special medicine. The children were on this study for about two years.
The study reported on the findings in 96 children; 48 in each group. Based on this study, we found that this medicine may result in stones coming back less often. However, we are not sure about this finding because the study was not of good quality and small. One in eight patients stopped the medicine because of side effects. We did not find any information on how often children had to be treated for stones again.
Quality of the evidence
The evidence quality for stones coming back less often was low and that for side effects very low. We found no evidence on how often children had to be treated for stones again.
Summary of findings
Summary of findings for the main comparison. Potassium citrate compared to no intervention for preventing urinary stones in children.
| Patient or population: children with idiopathic urinary calculi treated with shockwave lithotripsy Setting: likely outpatient Intervention: potassium citrate Comparison: no intervention | |||||
| Outcomes | No of participants (studies) | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with no intervention | Risk difference with medical or dietary interventions | ||||
| Proportion of participants who developed a new urinary stone follow‐up: mean 24.4 months | 96 (1 RCT) | ⊕⊕⊝⊝ Low1 | RR 0.19 (0.06 to 0.60) | Study population | |
| 333 per 1000 | 270 fewer per 1000 (133 fewer to 313 fewer) | ||||
|
Proportion of participants with adverse events while undergoing intervention follow‐up: mean 24.4 months |
96 (1 RCT) | ⊕⊝⊝⊝ Very low1,2,3 | RR 13.00 (0.75 to 224.53) | Study population | |
| ‐ | ‐ | ||||
| Proportion of participants undergoing retreatment for urinary stones | no information found | NA | NA | NA | NA |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; NA: not applicable (since no information found). | |||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded by two levels for study limitations: almost all domains were unclear or high risk of bias.
2Downgraded by two levels for imprecision: very rare event resulting in very wide confidence interval.
3No event in control arm.
Background
Description of the condition
Although more frequent in adults, urinary tract stones (urolithiasis) occur in children, with reported incidence of 2% to 3% and a boy:girl ratio of 1:1 (Malek 1975; Pietrow 2002). Urinary tract stones in children account for 1/1000 to 1/7600 hospital admissions, according to data from the 1970s to 1990s (Nimkin 1992; Troup 1972; Walther 1980). However, more recent reports suggest that urinary calculi are being recognized at an increasing frequency in children (Dwyer 2012; Trinchieri 1996; VanDervoort 2007), and by 2010 it was reported that up to one in every 685 pediatric hospital admissions occurred for an indication of nephrolithiasis (Bush 2010).
In the US, there are over two million outpatient visits annually for renal colic (Pearle 2005). Studies have also found an 86% increase in emergency room visits secondary to kidney stones in children from 1999 to 2008 (Kairam 2013). There is a 27% to 50% risk of developing a recurrent stone within the first five years of the initial stone episode and approximately 70% of children who form stones will continue with recurrent stone disease during childhood and adulthood (Kocvara 1999; Milliner 1993). Thus, most of these children develop chronic, painful and debilitating stone disease that erratically interrupts both their education, and the work‐life of their parents. Additionally, the monetary cost of urinary stone disease is very high, at an estimated annual cost of two billion dollars (Pearle 2005). Because of these factors, prevention of future episodes is critical.
Excluding genetic and secondary causes of stone disease (i.e. medication induced or intestinal malabsorption), there are many nutritional risk factors for the formation of idiopathic urinary stones, as the composition of urine is largely determined by diet (Heilberg 2013). For example, high dietary sodium intake decreases proximal tubular sodium reabsorption, which reduces renal calcium reabsorption causing hypercalciuria secondary to increased calcium excretion (Heilberg 2013). High animal protein intake, a source of purines, contributes to hyperuricosuria, as well as leading to hypercalciuria secondary to increased bone resorption and lower tubular calcium reabsorption (Heilberg 2013). In contrast, dietary modifications are also protective against stone disease. Citrates increase the solubility of stone‐forming calcium salts and inhibit calcium oxalate crystal growth as well as induce a systemic alkalization which reduces calcium excretion (Heilberg 2013). In addition, as metabolic acidosis results in increased bone resorption, increased citrate intake may also improve bone mineral density (Arrabal‐Polo 2013). Potassium may also be protective as decreased potassium intake increases calcium excretion and citrate reabsorption, and ingested potassium will accompany organic ions such as citrate that will be metabolized to bicarbonate (Saxena 2010). Finally, low urinary volume is a well‐known risk factor for stone disease and increased water intake results in excretion of a less saturated and higher volume of urine, which benefits all types of stone‐formers (Borghi 1996).
After the initial acute stone event, long‐term management of urinary stone disease focuses on the prevention of recurrent or new urinary stones. Urinary stones may be composed of a variety of constituents including calcium oxalate, calcium phosphate, uric acid and struvite. The prevention of recurrent stones focuses on improving the balance between the crystal‐forming and crystal‐inhibiting substances in the urine (Fink 2013). The most basic interventions are dietary and include increasing water intake, restricting consumption of animal protein and salt, and increasing ingestion of fiber, calcium and potassium (Kocvara 1999). Pharmacologic interventions include thiazide diuretics, potassium salts, allopurinol and phosphate.
Description of the intervention
Prevention of urinary stones revolves around improving the concentration of various substances in the urine to prevent the precipitation of salts which form into stones. This can be achieved with a variety of dietary and pharmacologic interventions. Simply increasing water intake causes an increase in urine volume (Escribano 2014). This should in turn decrease the concentration of calcium, oxalate, phosphorus and uric acid in the urine, and thus subsequently reduce the saturation of the salts that form stones (Borghi 1996). An increase in urine volume to more than 2.5 L/day has been previously shown in adults to decrease the time to, and total number of, stone recurrences (Borghi 1996).
With regard to specific diets, it has been hypothesized that excess animal protein intake increases urinary calcium, oxalate and uric acid, and decreases urinary citrate (Dussol 2008). In addition, a high fiber diet also may decrease urinary calcium (Dussol 2008). Oversaturation of urine with calcium is one of the most important risk factors for calcium nephrolithiasis and paradoxically, studies have shown that diets low in calcium actually have increased risks for stone disease possibly secondary to an increase in urinary oxalate (Heilberg 2013). As expected, urinary oxalate excretion increases as dietary oxalate intake increases and thus a low oxalate diet is also thought to be protective (Heilberg 2013). High sodium intake reduces renal tubular calcium reabsorption and thus increases calcium excretion and the risk for stone formation (Heilberg 2013). Citrate is protective against the formation of urinary tract stones because urinary citrate increases the solubility of stone‐forming calcium salts and so inhibits calcium oxalate crystal growth (Heilberg 2013).
There are also a variety of medications that can alter the urinary parameters and are hypothesized to prevent urinary stones. Thiazide diuretics cause enhanced calcium reabsorption in the distal renal tubule and decrease the urinary concentration of calcium (Escribano 2009). Because urinary citrate inhibits stone formation, the administration of citrate salts is also hypothesized to prevent recurrent stone disease. Allopurinol, a xanthine oxidase inhibitor which reduces uric acid synthesis and lowers urinary uric acid, is effective in reducing recurrence of uric acid and calcium stones which form via heterogeneous nucleation (Escribano 2009). Finally, neutral phosphates, sodium/potassium phosphate or both may be used as urinary chelators to reduce stone formation.
How the intervention might work
The intervention would be geared toward children who have already been diagnosed with a first‐time episode of idiopathic urinary stone formation, who then receive oral medical therapy or dietary modification with a goal of preventing recurrent stone formation. Oral medical therapy intervention could consist of potassium citrate supplementation, thiazide diuretic administration, allopurinol or neutral phosphates. Dietary modification could comprise of increased daily fluid volume intake, reducing animal protein, a high fiber diet, increased oral citrate intake or modification of oral oxalate load.
Why it is important to do this review
To date, there have been few small studies that have explored the use of any pharmacologic or dietary interventions for prevention of recurrent urinary stone disease in children. Yet, urinary stone disease is a major public health issue; one in 33 children will be affected. Given the increasing incidence of urinary tract stones in children, any treatments aimed at preventing recurrent disease will diminish pain and distress, surgical interventions and medical health costs. There is a paucity of rigorous systematic review literature on this topic that focus on patient‐important outcomes and evaluate using GRADE criteria. It is anticipated that the current review will stimulate further studies in interventions for the prevention of urinary tract stones in children.
Objectives
To assess the effects of medical and dietary interventions (MDI) for the prevention of idiopathic urinary stones in children aged from one to 18 years.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was by alternation, use of alternate medical records, date of birth or other predictable methods) of children treated with medical or dietary interventions for the prevention of recurrent urinary stones.
Types of participants
Inclusion criteria
Boys or girls aged from one to 18 years.
Participants with a history of urinary stones as documented on imaging by ultrasound; kidney, ureter and bladder (KUB) radiography; or computer tomography (CT) scan.
Exclusion criteria
Pregnancy.
Primary bladder stones.
Genetically defined metabolic stone diseases (e.g. cystinuria, primary hyperoxaluria, inherited disorders of uric acid metabolism, adenine phosphoribosyl transferase (APRT) deficiency, or genetic disorders which may cause hypercalciuria such as Dent disease, or ).
People with secondary causes of calcium oxalate stones (e.g. inflammatory bowel disease).
People on diet/medications predisposing to stones (e.g. ketogenic diet, topiramate, acetazolamide).
People with known anatomic abnormalities predisposing to urinary stone formation (e.g. congenital ureteropelvic junction obstruction).
Hyperparathyroidism.
If we found studies of both adults and children, we planned to include these studies if the number of children was at least 10 and we were able to obtain separate results for this group, either from the published report or from the authors.
Types of interventions
We included interventions with a minimum duration of treatment of 12 months. The type of intervention targeted was for idiopathic stone formers, as metabolic stone formers due to known genetic traits were excluded. Examples of pharmacologic interventions included thiazide diuretics, citrate salts, medications that reduce uric acid such as allopurinol, and consumption of calcium supplements before meals. Examples of dietary interventions may have included low protein diets, low sodium diets, low oxalate diets and high citrate diets (e.g. the lemonade diet (Seltzer 1996)).
We included the following comparisons.
Any pharmacologic intervention versus placebo.
Any pharmacologic intervention versus no treatment.
Any pharmacologic intervention versus standard of care (hydration).
Comparison of any two pharmacologic interventions.
Comparison of any amount, frequency, duration or mode of administration of a type of pharmacologic intervention.
Any dietary intervention versus placebo.
Any dietary intervention versus no treatment.
Any dietary intervention versus standard of care (hydration).
Comparison of any dietary and pharmacologic intervention.
Types of outcome measures
Primary outcomes
Proportion of participants who developed a new urinary stone (recurrence rate/participant/year) after initiation of intervention as documented by ultrasound, X‐ray or CT imaging.
Proportion of participants with adverse events while undergoing medical or dietary intervention for recurrent idiopathic stone disease (e.g. hypokalemia, hyperkalemia, allergic reaction such as skin rash, abdominal pain, nausea, vomiting, hypotension and elevations in serum uric acid).
Proportion of participants undergoing retreatment for urinary stones.
Secondary outcomes
Difference in serum electrolytes (calcium, sodium, potassium, phosphate and uric acid) between treatment and control groups.
Twenty‐four‐hour urinary collection parameters (calcium, oxalate, citrate, uric acid, pH, sodium, potassium) between treatment and control groups as well as creatinine (mg/kg/24 hours) to affirm adequate collection.
Time to new stone formation.
Main outcomes for 'Summary of findings' table
Proportion of participants who developed a new urinary stone (recurrence rate/participant/year) after initiation of intervention as documented by ultrasound, X‐ray or CT imaging.
Proportion of participants with adverse events while undergoing intervention.
Proportion of participants undergoing retreatment for urinary stones.
Search methods for identification of studies
We performed a comprehensive search with no restriction on the language of publication or publication status.
Electronic searches
We searched multiple databases using search terms relevant to this review, including studies identified from the following sources:
Cochrane Central Register of Controlled Trials (CENTRAL) 2017, Issue 1;
MEDLINE OvidSP (1946 to 14 February 2017);
Embase OvidSP (1980 to 14 February 2017);
Weekly current awareness alerts for selected journals (Journal of Urology, Journal of Pediatric Urology);
International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
See Appendix 1 for search terms and search strategies.
Searching other resources
The authors screened and handsearched abstract proceedings (from 2013 to January 2017) to help identify unpublished studies (American Urological Association and Society of Pediatric Urology national meeting abstracts).
The authors sent letters seeking information about unpublished or incomplete studies to investigators and companies known to be involved in previous studies.
Data collection and analysis
Selection of studies
One review author used EndNote reference management software to identify and remove potential duplicate records. Two review authors (LB and AK) independently assessed the titles, abstract, or both, of records identified in the search against the predefined inclusion and exclusion criteria to determine which studies should be assessed further. Two review authors (LB and AK) investigated all potentially relevant records as full text, map records to studies and classified studies as included studies, excluded studies, studies awaiting classification or ongoing studies in accordance with the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We were to resolve any discrepancies through discussion or arbitration by a third review author (GG). Where resolution of a disagreement was not possible, we designated the study as 'awaiting classification' and contacted the study authors for clarification. We documented reasons for exclusion of studies that may have reasonably been expected to be included in the review in the Characteristics of excluded studies table. We presented an adapted PRISMA flow diagram showing the process of study selection (Liberati 2009).
Data extraction and management
Two review authors (AK and LB) independently extracted data using a standard data extraction form. Studies reported in non‐English language journals were to be translated before assessment. Where more than one publication of one study existed, reports were to be grouped together and the publication with the most complete data would have been used in the analyses. Where relevant outcomes were only published in earlier versions, we used these data. Any discrepancies between published versions were highlighted.
We developed a dedicated data abstraction form which underwent pilot testing. For studies that fulfilled inclusion criteria, two review authors (LB and AK) independently abstracted the following information, which are provided in the Characteristics of included studies table:
study design;
study dates (if dates were not available then this was reported as such);
study settings and country;
participant inclusion and exclusion criteria;
participant details, baseline demographics;
number of participants by study and by study arm;
details of relevant experimental and comparator interventions such as dose, route, frequency and duration;
definitions of relevant outcomes, method and timing of outcome measurement, and any relevant subgroups.
study funding sources;
declarations of interest by primary investigators.
We extracted outcomes data relevant to this Cochrane Review as needed for calculation of summary statistics and measures of variance. For dichotomous outcomes, we attempted to obtain numbers of events and totals for population of a two by two table, as well as summary statistics with corresponding measures of variance. For continuous outcomes, we attempted to obtain means and standard deviations (SD) or data necessary to calculate this information.
We resolved any disagreements by discussion (LB and AK).
We were to provide information, including trial identifier, about potentially relevant ongoing studies in a Characteristics of ongoing studies table.
We attempted to contact authors of included or excluded studies to obtain key missing data as needed.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary study, we maximized yield of information by mapping all publications to unique studies and collating all available data. We used the most complete dataset aggregated across all known publications. In cases of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (AK and LB) independently assessed the risk of bias of each included study on a per‐outcome basis. We resolved disagreements by consensus, or, if required, by consulting a third review author (GG). We assessed risk of bias using the Cochrane tool for assessing risk of bias (Higgins 2011b). We assessed the following ’Risk of bias’ domains:
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective reporting (reporting bias);
other sources of bias.
We judged the domains as 'low risk,' 'high risk' or 'unclear risk,' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b; see Appendix 2).
We presented a 'Risk of bias' summary figure to illustrate these findings (Figure 1).
1.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study (single study).
For performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessment), we evaluated the risk of bias separately for each outcome, and grouped outcomes according to whether they were measured subjectively or objectively when reporting our findings in the 'Risk of bias' tables.
We defined the following endpoint as subjective outcomes: proportion of participants with adverse events while undergoing intervention.
We defined the following endpoints as objective: proportion of participants who develop a new urinary stone (recurrence rate/participant/year) after initiation of intervention as documented by ultrasound, X‐ray or CT imaging and proportion of participants undergoing retreatment for urinary stones.
We assessed attrition bias (incomplete outcome data) on an outcome‐specific basis, and presented the judgment for each outcome separately when reporting our findings in the 'Risk of bias' table.
Measures of treatment effect
For dichotomous outcomes (recurrence rate of urinary stones, adverse effects, retreatment), we expressed results as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (serum electrolytes, urinary parameters, time to formation of new stone), we used the mean difference (MD) if similar scales were used, or the standardized mean difference (SMD) if different scales were used. For continuous variables missing SD, we imputed SDs from published statistics that enabled calculation or estimation of the SD such as P values or standard error.
Unit of analysis issues
The unit of analysis was the individual participant. We were to handle trials with more than two intervention groups for inclusion in the review in accordance with guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. email or letter to corresponding author) and any relevant information obtained in this manner was included in the review. Evaluation of important numerical data such as screened, randomized participants and intention‐to‐treat, as‐treated and per‐protocol population was to be carefully performed. Attrition rates, for example, dropouts, losses to follow‐up and withdrawals, were investigated. Issues of missing data and imputation methods (e.g. last observation carried forward) were critically appraised (Higgins 2011a).
Assessment of heterogeneity
Heterogeneity was to be analyzed using a Chi² test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). I² values of 25%, 50% and 75% correspond to moderate, substantial and considerable levels of heterogeneity. However, this could not be performed due to an insufficient number of studies with exactly similar inclusion criteria.
Assessment of reporting biases
We had planned that if sufficient RCTs were identified, examination for reporting bias was to be undertaken using a funnel plot (Higgins 2011a). This could not be performed because there were insufficient studies identified (only a single study was ultimately included) to enable meaningful funnel plot construction.
Data synthesis
We performed statistical analyses according to the statistical guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). For dichotomous outcomes, we used the Mantel‐Haenszel method; for continuous outcomes, we used the inverse variance method; and for time‐to‐event outcomes, we used the generic inverse variance method. We used Review Manager 5 to perform analysis (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity; therefore, where sufficient data were available, we planned to perform the following predefined subgroup analyses.
type of urinary stone;
participant age.
Heterogeneity among participants could be related to age; some studies may include children and adults as well as results pertaining to different chemical stone compositions. In the studies that included both children and adults in which data were not reported separately, we planned to contact study investigators for child‐specific subgroup data. If the participants were analyzed separately by age (18 and less versus greater than 18 years), we planned to include only child‐specific subgroup data.
Sensitivity analysis
We planned to perform sensitivity analyses based on risk of bias by excluding studies judged to be at 'high risk' or 'unclear risk' of bias for the particular outcome.
'Summary of findings' table
We presented the overall quality of the evidence for each outcome according to the GRADE approach, which considers five criteria related to internal validity (risk of bias, inconsistency, imprecision, publication bias) and external validity (such as directness of results) (Guyatt 2008). For each comparison, two review authors (AK and LB) independently rated the quality of evidence for each outcome as 'high,' 'moderate,' 'low' or 'very low' using GRADEpro GDT. We resolved any discrepancies by consensus, or, if needed, by arbitration by a third review author (GG). For each comparison, we present a summary of the evidence for the main outcomes in a 'Summary of findings' table, which provides key information about the best estimate of the magnitude of the effect in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of the overall confidence in effect estimates for each outcome (Guyatt 2011; Schünemann 2011). Given the low number of included studies, meta‐analysis was not possible, thus we presented results in a narrative 'Summary of findings' table with the only comparison being that between medical intervention and placebo, as relevant to the single study included in the review.
Results
Description of studies
Results of the search
We searched the databases up to 14 February 2017. Our search revealed 523 hits (see Figure 2). We screened 10 records after we applied initial inclusion criteria. Upon scrutinizing these abstracts, we found one study that fulfilled the inclusion criteria and that was relevant to the intended subject. We contacted experts in the field of urinary stone disease, but this effort failed to contribute any relevant studies, published or unpublished.
2.
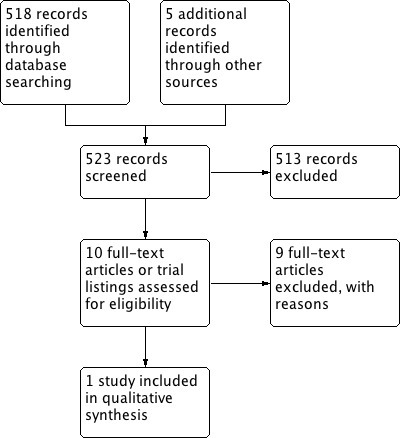
Study flow diagram.
Included studies
The search identified one eligible study (see Characteristics of included studies table) (Sarica 2006).
This randomized study evaluated and analyzed 125 children in a Turkish population for the preventive effect of potassium citrate therapy on stone recurrence following initial shockwave lithotripsy (SWL) for idiopathic pediatric calcium nephrolithiasis. The total study population consisted of 72 boys and 53 girls aged four to 14 years with a mean age of 6.6 years. Results were only reported on 96 patients; 48 patients per treatment arm.
All SWL treatments were performed under general anesthesia. Four weeks after SWL, children becoming stone free (n = 52) and children in whom a stone less than 5 mm persisted (n = 44) were randomized independently into two groups, oral potassium citrate therapy 1 mEq/kg per day for 12 months or no specific treatment.
Mean follow‐up was 24.4 months. Sarica 2006 reported the proportion of participants who developed a new urinary stone and adverse events. Sonographic examination with abdominal X‐ray was performed to determine stone growth or stone formation during follow‐up.
Sarica 2006 did not report funding sources and conflicts of interests.
Excluded studies
We identified nine studies or trials that were fully screened and subsequently excluded (see Characteristics of excluded studies table).
Five had the wrong study design (Gheissari 2012; NCT00120731; NCT02289755; Ogûz 2013; Tekin 2002). Of the remaining studies we excluded, two had the wrong population (Choi 2011; IRCT2014041217234N1), and two had the wrong intervention (Naseri 2011; Yousefichaijan 2015).
Risk of bias in included studies
See Characteristics of included studies table, Figure 1 and Figure 3.
3.
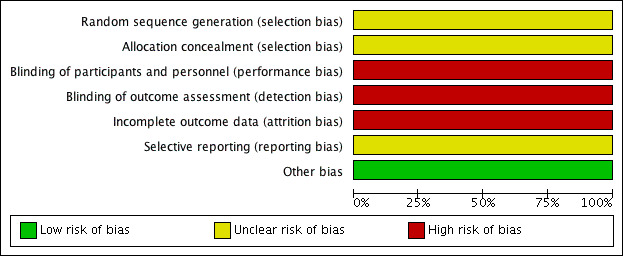
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies (single study).
Allocation
Random sequence generation
Sarica 2006 did not elaborate the method of random sequence generation. Therefore, the risk of bias was unclear.
Allocation concealment
Sarica 2006 did not elaborate whether allocation concealment was utilized, and if so, what method was used. Therefore, the risk of bias due to lack of allocation concealment was unclear.
Blinding
Sarica 2006 did not use blinding. Therefore, the risk of bias was high for both performance and detection bias for all outcomes.
Incomplete outcome data
Only 96/125 randomized participants were included in the analysis of stone recurrence; therefore, the risk of attrition bias was high. We did not assess the risk of attrition bias for other outcomes since they were not reported.
Selective reporting
For Sarica 2006, there was no protocol available; therefore, the risk of bias from selective reporting was unclear.
Other potential sources of bias
We detected no other potential threats to validity.
Effects of interventions
See: Table 1
Any pharmacologic intervention versus control
We included one study with 96 participants (n = 48 per group) that compared oral potassium citrate therapy versus control (Sarica 2006). See Table 1.
Primary outcomes
1. Proportion of participants who developed a new urinary stone
Oral potassium citrate compared to no intervention may reduce the rate of recurrent stones (RR 0.19, 95% CI 0.06 to 0.60). This would correspond to 270 fewer stone recurrences per 1000 children (133 fewer to 313 fewer). We rated the quality of evidence as low, downgrading for study limitations.
2. Proportion of participants with adverse events
The included trial did not explicitly report adverse events. However, it did provide information that 6/48 (12.5%) participants withdrew from the study due to nausea or abdominal pain. This corresponded to a RR of 13.0 (95% CI 0.75 to 224.53; very low quality evidence); an absolute effect size estimate could not be generated. We downgraded the quality of evidence for study limitations and imprecision.
3. Proportion of participants undergoing retreatment for urinary stones
The included trial did not report retreatment rates.
Secondary outcomes
1. Difference in serum electrolytes
The included trial did not report serum electrolytes.
2. Twenty‐four‐hour urinary collection parameters
The included study did not report 24‐hour urinary collection parameters.
3. Time to new stone formation
The included study did not report time to new stone formation.
Subgroup and sensitivity analysis
We were unable to perform any of the planned secondary analyses due to paucity of included studies or lack of relevant data in the included study.
Other comparisons
We found no studies comparing any pharmacologic intervention versus placebo or standard of care (hydration); any two pharmacologic interventions; any amount, frequency, duration or mode of administration of a type of pharmacologic intervention; any dietary intervention versus placebo, no treatment or standard of care (hydration); or any dietary and pharmacologic intervention.
Discussion
Summary of main results
We included one study with 125 participants that compared oral potassium citrate to no treatment. The study population consisted of 72 boys and 53 girls, and the mean age was 6.6 years. The mean follow‐up was 24.4 months and results were limited to 96 children; 48 per arm. Oral potassium citrate compared to no intervention may reduce the rate of recurrent stones. It may also increase the risk of adverse effects and treatment withdrawals but we are uncertain about this finding. We were unable to perform any of the preplanned secondary analyses.
We were unable to identify any evidence on the effectiveness of other pharmacologic or dietary therapies.
Overall completeness and applicability of evidence
The single study included in this review examined a population of children (following SWL for first urinary stone occurrence) that was not fully representative of people seen in clinical practice. Children with recurrent idiopathic stone formation encountered in real‐world practice may have been managed by a variety of ways including expectant medical management with medical expulsive therapy, ureteroscopic treatment or invasive surgical therapy (e.g. percutaneous nephrolithotomy), in comparison with SWL alone.
The mean age of participants included in the review for the primary intervention was 6.6 years. While it is the authors' judgment that the limited findings in Sarica 2006 may be broadly applicable to children of other ages, we found no evidence objectively demonstrating these findings in older children.
The single included study did not explicitly report on adverse event rates but provided information on the number of participants who withdrew due to adverse events. There may have been additional adverse events that did not lead to study withdrawal that were not captured.
Regarding the predefined primary outcomes of this review, only urinary stone recurrence rate was fully reported. The primary outcome of stone retreatment rate was not reported, and of the secondary outcomes, only select urinary chemical parameters were reported with respect to the first primary intervention of pharmacologic therapy using oral potassium citrate.
Given that the single included study addressed only one of two primary interventions and reported only two of three primary outcomes, the overall completeness and applicability of the evidence contained in this review was judged to be low.
Quality of the evidence
The quality of evidence for the single comparison and the two outcomes was low and very low. We downgraded for study limitations (unclear allocation concealment, lacking of blinding, and risk of performance and selection bias and attrition bias) and imprecision. As a result, our confidence in the effect estimate is limited or very limited; the true effect may be substantially different or is likely substantially different from the reported estimate of the effect.
Potential biases in the review process
To reduce the potential for publication bias using Cochrane methodology, we performed an extensive literature search without language or publication status restrictions, and additionally searched trial registries for unpublished, planned or ongoing studies. It is possible that additional studies may have been conducted but not yet published, or that additional studies have been published but not yet identified. However, we made every reasonable attempt to contact the authors of unpublished trials and to contact known experts in the field. Should any such studies be identified, we will include them in updates of this review.
We considered only prospective RCTs for inclusion in this review. Although some literature has suggested that there may not be significant differences in the risk estimates of outcome events from RCTs versus observational studies (Golder 2011), it is generally recognized that, especially when expected outcome event rates are low or the cohort size is small, randomized prospective studies offer a superior ability to discriminate effects, and that while studies of other designs (such as cohort, case‐control and other observational studies) may offer valuable information, their review was beyond the scope of this article.
Another potential bias in the review was the decision to include studies only where there was a duration of intervention of a minimum of 12 months. This stipulation resulted in the exclusion of several studies. The review authors weighed this and considered that a minimum duration of intervention of 12 months was appropriate based on both clinical experience and published literature. For example, in the adult idiopathic stone literature, large series report stone recurrence rates in the order of a 31% cumulative event rate at 10 years within a population (Rule 2014). Tasian 2017 reported that in a cohort of children with prior urinary stones, at 12 months following the initial presentation the stone recurrence rate is in the order of 25%. This implies that studies where the duration of intervention spans only weeks or months will have insufficient follow‐up to discriminate meaningful differences between interventions based on the low expected event rate during the follow‐up period. It was for this reason that the cutoff of a minimum of 12 months' duration of intervention was chosen for this review.
Agreements and disagreements with other studies or reviews
We found no other systematic studies or reviews that exactly align with the objectives of this review, and that have similar inclusion criteria.
Authors' conclusions
Implications for practice.
There is low quality evidence that oral potassium citrate therapy may reduce the idiopathic urinary stone recurrence rates among children aged about six years old who have already undergone shockwave lithotripsy for an initial symptomatic stone event. Adverse events leading to treatment withdrawals appear increased, but we are uncertain of this finding.
Implications for research.
Additional high‐quality, adequately powered trials of sufficient duration and follow‐up, reporting on outcomes of stone recurrence and adverse events, following initial stone events treated by multiple modalities, are required to elucidate the comparisons put forth in this review. Given the recent remarkable increase in the prevalence of pediatric urinary stone disease in North America (see Description of the condition), such a study would be both timely and relevant. Adequate clinical follow‐up in such a study would encompass the time in which a significant number of recurrent stone events would be expected to accrue; likely at least 12 months based on stone recurrence rates reported in Tasian 2017.
Further trials should address the following, including the primary outcomes of the present study:
stone recurrence rates, stratified by radiographic versus symptomatic recurrence;
adequate clinical follow‐up duration;
accounting of adverse effects of therapy;
stone retreatment rates.
Such a study would best be completed by multi‐center collaboration, and we propose this as the next logical step for the urologic research community to undertake on this important topic.
Acknowledgements
We would like to thank the referees for their comments and feedback during the preparation of this review, and acknowledge Cochrane Urology Group for their support.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| Embase |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomized sequence. |
Low risk of bias: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgment of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: insufficient information about the sequence generation process to permit judgment. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment. |
Low risk of bias: randomization method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomization; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: used an open random allocation schedule (e.g. a list of random numbers); assignment envelopes used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: randomization stated but no information on method used. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study. |
Low risk of bias: no blinding or incomplete blinding, but review authors judged that outcome was not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: no blinding or incomplete blinding, and outcome was likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that blinding could have been broken, and outcome is likely to be influenced by lack of blinding. | |
| Unclear: insufficient information to permit judgment. | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: no blinding of outcome assessment, but review authors judged that outcome measurement was not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that blinding could have been broken. |
| High risk of bias: no blinding of outcome assessment, and outcome measurement was likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that blinding could have been broken, and outcome measurement was likely to be influenced by lack of blinding. | |
| Unclear: insufficient information to permit judgment. | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: no missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data were imputed using appropriate methods. |
| High risk of bias: reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; 'as‐treated' analysis done with substantial departure of the intervention received from that assigned at randomization; potentially inappropriate application of simple imputation. | |
| Unclear: insufficient information to permit judgment. | |
|
Selective reporting Reporting bias due to selective outcome reporting. |
Low risk of bias: study protocol was available and all of the study's prespecified (primary and secondary) outcomes that were of interest in the review were reported in the prespecified way; study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon). |
| High risk of bias: not all of the study's prespecified primary outcomes were reported; ≥ 1 primary outcomes was reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified; ≥ 1 reported primary outcomes were not prespecified (unless clear justification for their reporting was provided, such as an unexpected adverse effect); ≥ 1 outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis; study report failed to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: insufficient information to permit judgment. | |
|
Other bias Bias due to problems not covered elsewhere in this table. |
Low risk of bias: study appeared free of other sources of bias. |
| High risk of bias: had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; was claimed to have been fraudulent; had some other problem. | |
| Unclear: insufficient information to assess whether an important risk of bias existed; insufficient rationale or evidence that an identified problem would introduce bias. |
Data and analyses
Comparison 1. Potassium citrate versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stone recurrence and regrowth | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.06, 0.60] |
| 2 Adverse events | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 13.00 [0.75, 224.53] |
1.1. Analysis.

Comparison 1 Potassium citrate versus no treatment, Outcome 1 Stone recurrence and regrowth.
1.2. Analysis.
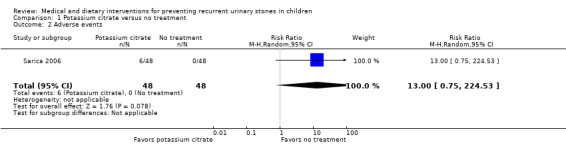
Comparison 1 Potassium citrate versus no treatment, Outcome 2 Adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Sarica 2006.
| Methods |
Design: randomized trial. Setting/country: single center/Turkey. Dates when study was conducted: not reported. |
|
| Participants |
Inclusion criteria: children with calcium‐containing idiopathic nephrolithiasis and normal renal morphology following initial treatment with shockwave lithotripsy. Exclusion criteria: children with anatomic abnormalities, previous stone surgery or urinary tract infection, renal tubular acidosis, renal functional disorders, cystinuria or any other evident metabolic abnormality (primary or secondary hyperoxaluria, hyperparathyroidism, etc.). Total number of participants randomly assigned: 125 (58 boys, 38 girls). Experimental group:
Control group:
|
|
| Interventions | Experimental group:
Control group:
Follow‐up (mean): 24.4 months. |
|
| Outcomes |
|
|
| Notes | Funding source and conflicts of interests: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method unspecified. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method unspecified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and researchers not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants and researchers not blinded. |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | High (23%) attrition rate in study cohort. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available for review; therefore, risk of bias from selective reporting was unclear. |
| Other bias | Low risk | Apparently free of other problems that could put it at a risk of bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Choi 2011 | Wrong population (not all children included in the cohort had a primary stone event). |
| Gheissari 2012 | Wrong study design (non‐randomized). |
| IRCT2014041217234N1 | Wrong population (children with small stone, not recurrent). |
| Naseri 2011 | Wrong intervention (duration of intervention < 12 months). |
| NCT00120731 | Wrong study design (single arm). |
| NCT02289755 | Wrong study design (single arm). |
| Ogûz 2013 | Wrong study design (non‐randomized retrospective). |
| Tekin 2002 | Wrong study design (non‐randomized). |
| Yousefichaijan 2015 | Wrong intervention (duration of intervention < 12 months). |
Differences between protocol and review
This review was based on a published protocol (Grimsby 2014), with differences as described here.
In addition to the previously published methodology, the review included plans for GRADE assessment and for preparation of a 'Summary of Findings' tables using GRADEpro GDT software. GRADE assessment is reported in the review, as well as within the 'Summary of findings' table alongside the reportable primary outcome for the main comparison estimable from the available literature.
Due to a combination of low quality available evidence and overall limited numbers of available studies, only one full prospective randomized study was included. There were no high quality descriptive studies available, only low quality descriptive studies by GRADE criteria; therefore, these descriptive studies were not included as planned.
The Newcastle‐Ottawa scoring scale was not utilized for assessment of risk of bias, as the review included no non‐randomized studies (Wells 2012).
Several data collection and analysis steps were not performed since we found no relevant data. For measurement of treatment effect, only dichotomous outcome information was available from the single included study, so no analysis of continuous outcomes data was performed. Similarly, no special analysis was undertaken for unit of analysis issues, as this was not relevant to the single included study, neither was an assessment of heterogeneity of data undertaken. Likewise, data synthesis, subgroup and sensitivity analysis steps were omitted since only one study met inclusion criteria.
We dropped the secondary outcome of number of retreatment per year predefined in the protocol as it was too similar to the primary outcome of retreatment to add value. However, there was no evidence for either outcome.
Contributions of authors
Draft the protocol: GG, LB.
Study selection: HM, AK.
Extract data from studies: AK.
Enter data into RevMan: AK.
Carry out the analysis: AK.
Interpret the analysis: AK, LB.
Draft the final review: AK.
Disagreement resolution: LB, GG.
Update the review: AK.
Sources of support
Internal sources
Departmental, USA.
External sources
No sources of support supplied
Declarations of interest
AK: none known.
GG: none known.
HM: none known.
LB none known.
New
References
References to studies included in this review
Sarica 2006 {published data only}
- Sarica K, Erturhan S, Yurtseven C, Yagci F. Effect of potassium citrate therapy on stone recurrence and regrowth after extracorporeal shockwave lithotripsy in children. Journal of Endourology / Endourological Society 2006;20(11):875‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Choi 2011 {published data only}
- Choi JN, Lee JS, Shin JI. Low‐dose thiazide diuretics in children with idiopathic renal hypercalciuria. Acta Paediatrica 2011;100(8):71‐4. [DOI] [PubMed] [Google Scholar]
Gheissari 2012 {published data only}
- Gheissari A, Ziaee A, Farhang F, Farhang F, Talaei Z, Merrikhi A, et al. Evaluating the effectiveness of adding magnesium chloride to conventional protocol of citrate alkali therapy in children with urolithiasis. International Journal of Preventive Medicine 2012;3(11):791‐7. [PMC free article] [PubMed] [Google Scholar]
IRCT2014041217234N1 {unpublished data only}
- IRCT2014041217234N1. Comparing effectiveness of combination of magnesium oxide and potassium citrate with potassium citrate in children with urolithiasis. www.irct.ir/searchresult.php?keyword=&id=17234&number=1&prt=6562&total=10&m=1 Date first received: 22 December 2014.
Naseri 2011 {published data only}
- Naseri M, Sadeghi R. Role of high‐dose hydrochlorothiazide in idiopathic hypercalciuric urolithiasis of childhood. Iranian Journal of Kidney Diseases 2011;5(3):162‐8. [PubMed] [Google Scholar]
NCT00120731 {unpublished data only}
- NCT00120731. Effects of potassium citrate in urine of children with elevated calcium in urine and kidney stones. clinicaltrials.gov/ct2/show/study/NCT00120731 Date first received: 19 July 2005.
NCT02289755 {unpublished data only}
- NCT02289755. Evaluating ALLN‐177 for reducing urinary oxalate excretion in calcium oxalate kidney stone formers with hyperoxaluria. clinicaltrials.gov/ct2/show/study/NCT02289755 Date first received: 13 November 2014.
Ogûz 2013 {published data only}
- Ogûz U, Unsal A. The efficacy of medical prophylaxis in children with calcium oxalate urolithiasis after percutaneous nephrolithotomy. Journal of Endourology / Endourological Society 2013;27(1):92‐5. [DOI] [PubMed] [Google Scholar]
Tekin 2002 {published data only}
- Tekin A, Tekgul S, Atsu N, Bakkaloglu M, Kendi S. Oral potassium citrate treatment for idiopathic hypocitruria in children with calcium urolithiasis. Journal of Urology 2002;168(6):2572‐4. [DOI] [PubMed] [Google Scholar]
Yousefichaijan 2015 {published data only}
- Yousefichaijan P, Cyrus A, Dorreh F, Rafeie M, Sharafkhah M, Frohar F, et al. Oral zinc sulfate as adjuvant treatment in children with nephrolithiasis: a randomized, double‐blind, placebo‐controlled clinical trial. Iranian Journal of Pediatrics 2015;25(6):e1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Arrabal‐Polo 2013
- Arrabal‐Polo MA, Arrabal‐Martin M, Arias‐Santiago S, Garrido‐Gomez J, Poyatos‐Andujar A, Zuluaga‐Gomez A. Importance of citrate and the calcium: citrate ratio in patients with calcium renal lithiasis and severe lithogenesis. BJU International 2013;111(4):622‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Borghi 1996
- Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5‐year randomized prospective study. Journal of Urology 1996;155(3):839‐43. [MEDLINE: ] [PubMed] [Google Scholar]
Bush 2010
- Bush NC, Xu L, Brown BJ, Holzer MS, Gingrich B, Schuler B, et al. Hospitalizations for pediatric stone disease in United States, 2002‐2007. Journal of Urology 2010;183(3):1151‐6. [DOI] [PubMed] [Google Scholar]
Dussol 2008
- Dussol B, Iovanna C, Rotily M, Morange S, Leonetti F, Dupuy P, et al. A randomized trial of low‐animal‐protein or high‐fiber diets for secondary prevention of calcium nephrolithiasis. Nephron Clinical Practice 2008;110(3):c185‐94. [PUBMED: 18957869] [DOI] [PubMed] [Google Scholar]
Dwyer 2012
- Dwyer ME, Krambeck AE, Bergstralh EJ, Milliner DS, Lieske JC, Rule AD. Temporal trends in incidence of kidney stones among children: a 25‐year population based study. Journal of Urology 2012;188(1):247‐52. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
EndNote [Computer program]
- Clarivate Analytics. EndNote. Version 7.6. Clarivate Analytics, 2016.
Escribano 2009
- Escribano J, Balaguer A, Pagone F, Feliu A, Roque I, Figuls M. Pharmacological interventions for preventing complications in idiopathic hypercalciuria. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004754.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Escribano 2014
- Escribano J, Balaguer A, Roque M, Figuls I, Feliu A, Ferre N. Dietary interventions for preventing complications in idiopathic hypercalciuria. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD006022.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fink 2013
- Fink HA, Wilt TJ, Eidman KE, Garimella PS, MacDonald R, Rutks IR, et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Annals of Internal Medicine 2013;158(7):535‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Golder 2011
- Golder S, Loke YK, Bland M. Meta‐analyses of adverse effects data derived from randomised controlled trials as compared to observational studies: methodological overview. PLoS Medicine 2011;8(5):e1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 17 February 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Grimsby 2014
- Grimsby G, Mayo H, Jacobs MA, Baker LA. Medical and dietary interventions for preventing recurrent urinary stones in children. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD011252] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Schünemann HJ, et al. GRADE: what is "quality of evidence" and why is it important to clinicians?. BMJ (Clinical Research Ed.) 2008;336(7651):995‐8. [DOI: 10.1136/bmj.39490.551019.BE] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI: 10.1016/j.jclinepi.2010.04.026] [DOI] [PubMed] [Google Scholar]
Heilberg 2013
- Heilberg IP, Goldfarb DS. Optimum nutrition for kidney stone disease. Advances in Chronic Kidney Disease 2013;20(2):165‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kairam 2013
- Kairam N, Allegra JR, Eskin B. Rise in emergency department visits of pediatric patients for renal colic from 1999 to 2008. Pediatric Emergency Care 2013;29(4):462‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kocvara 1999
- Kocvara R, Plasgura P, Petrik A, Louzensky G, Bartonickova K, Dvoracek J. A prospective study of nonmedical prophylaxis after a first kidney stone. BJU International 1999;84(4):393‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malek 1975
- Malek RS, Kelalis PP. Pediatric nephrolithiasis. Journal of Urology 1975;113(4):545‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Milliner 1993
- Milliner DS, Murphy ME. Urolithiasis in pediatric patients. Mayo Clinic Proceedings 1993;68(3):241‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nimkin 1992
- Nimkin K, Lebowitz RL, Share JC, Teele RL. Urolithiasis in a children's hospital: 1985‐1990. Urologic Radiology 1992;14(3):139‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pearle 2005
- Pearle MS, Calhoun EA, Curhan GC, Urologic Diseases of America Project. Urologic diseases in America project: urolithiasis. Journal of Urology 2005;173(3):848‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pietrow 2002
- Pietrow PK, Pope JC 4th, Adams MC, Shyr Y, Brock JW 3rd. Clinical outcome of pediatric stone disease. Journal of Urology 2002;167(2 Pt 1):670‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rule 2014
- Rule AD, Lieske JC, Li X, Melton LJ, Krambeck AE, Bergstralh EJ. The ROKS nomogram for predicting a second symptomatic stone episode. Journal of the American Society of Nephrology : JASN 2014;25(12):2878‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saxena 2010
- Saxena A, Sharma RK. Nutritional aspect of nephrolithiasis. Indian Journal of Urology 2010;26(4):523‐30. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Seltzer 1996
- Seltzer MA, Low RK, McDonald M, Shami GS, Stoller ML. Dietary manipulation with lemonade to treat hypocitraturic calcium nephrolithiasis. Journal of Urology 1996;156(3):907‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Tasian 2017
- Tasian GE, Kabarriti AE, Kalmus A, Furth SL. Kidney stone recurrence among children and adolescents. Journal of Urology 2017;197(1):246‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trinchieri 1996
- Trinchieri A. Epidemiology of urolithiasis. Archivio Italiano di Urologia, Andrologia 1996;68(4):203‐49. [MEDLINE: ] [PubMed] [Google Scholar]
Troup 1972
- Troup CW, Lawnicki CC, Bourne RB, Hodgson NB. Renal calculus in children. Journal of Urology 1972;107(2):306‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
VanDervoort 2007
- VanDervoort K, Wiesen J, Frank R, Vento S, Crosby V, Chandra M, et al. Urolithiasis in pediatric patients: a single center study of incidence, clinical presentation and outcome. Journal of Urology 2007;177(6):2300‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Walther 1980
- Walther PC, Lamm D, Kaplan GW. Pediatric urolithiases: a ten‐year review. Pediatrics 1980;65(6):1068‐72. [MEDLINE: ] [PubMed] [Google Scholar]
Wells 2012
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 20 May 2014).


