Abstract
The purpose of this study is to examine the treatment of noncancer musculoskeletal pain in different clinical settings by assessing patient demographics, pain diagnoses, opioid analgesic monitoring, and alternative treatments.
Data was collected in a retrospective chart review involving 300 randomly selected charts with an active musculoskeletal diagnosis based on the 10th revision of the International Statistical Classification of Diseases and Related Health Problems codes. The population consisted of primary care outpatient clinic and emergency department encounters during the timeframe of January 1, 2016 to March 31, 2016 in a predominantly rural community in Michigan. Variables included prescription medications, musculoskeletal conditions, and prescription drug monitoring modalities. Statistical analysis was accomplished using means, standard deviations, proportions, 2-sample proportional tests, multivariable logistic regression, and multinomial regression models.
Opioid prescribing was observed in 64% of outpatient and 68.9% of emergency department encounters. Back pain was the most common problem with 61.9% patients prescribed opioids having at least 1 diagnosis of back pain. Patients on opioids were older (mean age 58) than patients taking nonopioids (mean age 50). For every year of increasing age, there is a 3.1% increase in the odds of an opioid being prescribed (odds ratio 1.03, confidence interval 1.012–1.049, P = .001). Documentation was extremely low with only 15.2%, 1.5%, and 1.5% of patient charts prescribed opioids demonstrating documentation of urine drug screens, pain agreements, and review of a state prescription drug monitoring program, respectively.
Despite drug monitoring recommendations, low rates of monitoring were observed. Back pain was the largest contributing pain location and had higher opioid use compared to other sites. Many patients had additional pain medications being concurrently prescribed with opioids suggesting that musculoskeletal pain is not often controlled by a single medication type. Reported alcohol abuse, active tobacco use, and illicit substance use can serve as predictors when assessing patients for pain management options. The use of alternative measures and integrative treatment modalities (which saw low utilization in this study) should be implemented as either primary or supplementary therapy as a way to reduce the pharmacologic burden on the patient.
Keywords: musculoskeletal conditions, nonopioids, opioid monitoring, opioids
1. Introduction
The United States (U.S.) makes up approximately 4% of the world's population and uses over 80% of the world's opioids.[1] An estimated 91.8 million Americans used prescription opioids in 2015[2]; this equates to over one third of the American population accounting for the majority of opioid use. Additionally, the amount of prescription painkillers dispensed in the U.S. has increased fourfold from 1999 to 2013. In concordance with this, the number of deaths from opioids has also quadrupled in this same timeframe.[3] In response to these worrisome statistics, the Centers for Disease Control and Prevention (CDC) released specific opioid prescribing guidelines in 2016.[4]
A recent study by Han et al using data from the U.S. Department of Health and Human Services estimated that in 2015, 11.5 million of adults misused opioids and 1.9 million had an opioid use disorder.[2] The Food and Drug Administration released a revision to the previous guidelines for health care clinicians which broadens it to include information on aspects of pain management. This includes adapting principles on acute and chronic pain management, opioid and nonopioid (ie, medications that work through pathways other than opioid receptors, henceforth called “nonopioids”) analgesic treatments for pain, and nonpharmacologic treatments for pain.[1]
Other organizations have also recommended guidelines regarding opioid prescribing such as the CDC, American College of Physicians, and the American Academy of Family Physicians which recommend assessing urine drug screens (UDS) and a state-wide Prescription Drug Monitoring Program (PDMP) as a part of ongoing treatment plans for patients receiving chronic opioid therapy.[4–6] PDMPs in the U.S. are typically state-wide electronic databases which track prescriptions of controlled substances and provide key information such as prescriber names, number of prescribers being seen by the patient, which specific medications are being prescribed, as well as when, and at which pharmacies, the prescriptions are being filled. These databases can allow providers to access and monitor prescribing histories to identify drug-seeking behaviors but also to assist in preventing coprescribing of controlled substances with possible harmful side effect profiles. In Michigan, where the study was completed, the PDMP in use is designated as the Michigan automated prescription system (MAPS).
Despite increased opioid use, there is little available evidence that supports using opioids for maintenance of pain over longer periods of time. Additionally, there is a lack of significant evidence for improved physical function when using opioids to treat chronic noncancer pain (CNCP).[7–9] On the contrary, it has been found that opioids taken chronically for CNCP and chronic musculoskeletal (MSK) pain, as well as higher medication dosing, predict poorer functional outcomes overall. Particularly, this has been exhibited in various measures such as lower rates of returning to work and increased healthcare utilization.[10,11]
Up to this point, there has been insufficient data looking at specific opioid prescribing patterns and whether effective monitoring is being done in rural communities. Based on clinical experience and what has been seen in national trends, we hypothesized that opioid prescribing for CNCP and acute or chronic MSK pain in this area would be high while monitoring of these patients would be low. This study aims to address this by quantifying opioid prescribing in a predominantly rural area of Michigan while also serving as a community needs assessment. Additional aims included identifying differences in opioid versus nonopioid (which includes analgesics other than opioids and nonpharmacologic treatment) interventions, identifying prescribing differences based on care setting, demographic differences in treatment, likelihood of additional referral, and the use of monitoring in either outpatient clinics or in the emergency department (ED).
2. Methods
2.1. Abstraction methods and study population
This study consisted of a cross-sectional chart review of outpatient data collected from an electronic medical record (EMR) database within a 3-month timeframe from January to March 2016. This 3-month window was an arbitrary timeframe selection felt to be long enough to include a wide variety of clinical encounters and therefore an adequate representative sample. Though this timeframe has not been used in previous cross-sectional studies, it served its purpose as a needs assessment on regional prescribing trends in a Michigan community.
On initial abstraction over 20,000 individual codes, as outlined by the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10), were gathered. A sample of 300 patient records was then randomly selected from this initial pool. This study size was chosen because it was deemed reasonable to allow for 2 medical students to collect the necessary data in a 1-month timeframe. Patient encounters specifically reviewed in the EMR were based on the ICD-10 codes of specific medical conditions including an ICD-10 diagnosis of acute or chronic MSK conditions, and diagnosis of a CNCP condition. The secondary outcomes of interest included whether the state PDMP system (MAPS) was checked and which types of additional medications were used to treat pain. To evaluate the use of prescription monitoring, the records were reviewed for documentation of opioid risk screening and whether pain agreements (a non-legally binding agreement to adhere to clinician-given guidelines) were completed, along with a comparison of the clinical care setting where the treatment was rendered (outpatient clinic vs ED). Reviewing charts from both the ED and outpatient settings was done to include more diverse types of clinical encounters and assess opioid prescribing where acute and chronic pain are often treated despite their differing clinical goals. This study was approved by the Institutional Review Board (IRB) and performed in concordance with the Declarations of Helsinki and Taipei by the World Medical Association.
2.2. Description of variables
Variables abstracted included opioid prescriptions versus any other pain relievers prescribed including nonsteroidal anti-inflammatory drugs (NSAIDs), gabapentin, pregabalin, acetaminophen (APAP), muscle relaxants, and topical analgesics. Opioid medications for this study included hydrocodone, oxycodone, codeine, tramadol, morphine, hydromorphone, and fentanyl. Specific sociodemographic factors evaluated included age, gender, ethnicity, and body mass index (BMI). BMI was included to evaluate the assumption that higher BMIs are associated with increased MSK morbidity, especially lower extremity joints. Smoking status was focused on active smoking regardless of packs per day smoked or pack-year history. Patient-reported alcohol use and prior or active substance abuse was recorded to determine the use of opioids in those with other substance use (or prior history of it). For this study, alcohol abuse was cumulatively defined as greater than 2 drinks a day for men and greater than 1 drink per day for women. Substance abuse was defined as current or past use of marijuana, cocaine, opioid use inconsistent with prescribed directions, illegally acquired prescription opioids, heroin, and phencyclidine which were determined based on patient self-reporting or presence on a drug screen at time of prescription. Presence of opioids on UDS was not considered as patients with current prescriptions would be expected to test positive for opioids. Instead, this could only be determined based on patient self-report documented in their chart. Use of medicinal marijuana was added for completeness even though medical marijuana use is legal in Michigan. Referrals to rehabilitative therapy (for example physical therapy, occupational therapy, physical medicine, and rehabilitation consultation) were also collected to evaluate utilization of nonpharmacological intervention.
2.3. Inclusion criteria
Microsoft Excel (Microsoft, Redmond, Washington) was used to randomize the list by using the “randomization” function to assign each code a random number between 0 and 1, then using the “sort” function to sort the list by the random numbers, thereby resulting in a randomized list. The patient identification codes were then screened using the “remove duplicates” function to ensure that the encounters used were from 300 unique patients. To be considered, the patient needed to have an acute or chronic MSK pain condition ICD-10 code associated with an encounter during the aforementioned timeframe. For reference, all musculoskeletal codes are written in the convention of “M” followed by a numeric code unique to that diagnosis. For example, M17.11 is the code for unilateral primary osteoarthritis of the right knee. ICD-10 Codes utilized are as follows: M15-M19.99 = inflammatory arthropathies and osteoarthritis; M20-M29.99 = arthralgias, stiffness, other joint disorders; M30-M39.99 = systemic connective tissue disorders (see exclusion criteria below); M40-M49.99 = spondylopathies and deforming dorsopathies; M50-M59.99 = dorsalgia and intervertebral disc disorders; M60-M69.99 = disorders of muscles, synovium, and tendons; M70-M79.99 = soft tissue disorders including bursopathies, enthesopathies, and other soft tissue disorders not specified otherwise (Table 1).[12]
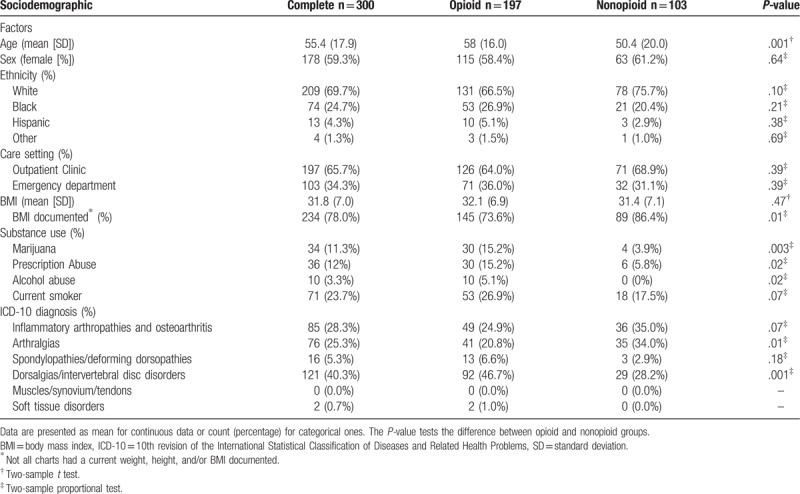
Table 1.
Descriptive statistics for the complete, opioid only, and nonopioid only sample.
2.4. Exclusion criteria
No exclusions were made based on any demographic information such as age, gender, or race/ethnicity. Patients with ICD-10 codes in the range of M30.00-M39.99, which consists of autoimmune and systemic connective tissue disorders such as systemic lupus erythematosus and systemic sclerosis, were excluded from the analysis. This was due to their unique autoimmune etiologies and different therapeutic strategies compared to the other conditions considered (ie, the use of immunosuppressant drugs for systemic disease control compared to symptomatic pain relief as seen with other MSK pain diagnoses). Any pain medications, levels of pain, and other therapies given to a patient postoperatively were excluded. Postoperative physical therapy was excluded as well. Similarly, postoperative pain was excluded because of the difficulty of elucidating if the pain was from the surgery or the MSK condition necessitating the surgery.
2.5. Medical record review/abstraction
The medical record review was conducted by 2 trained medical student abstractors using a standardized checklist and a data abstraction sheet for documenting all variables of interest. All of which was in adherence to IRB-approved methodology. To assess inter-rater reliability, the clinically trained third-year medical student (also trained in medical record abstraction) reviewed 50 randomly selected medical records overlapping with the total 300 of charts reviewed. The 2nd abstractor reviewed the presence or absence of each variable listed in the medical record abstraction tool using the same methods. The mean Kappa score across all variables obtained for 50 randomly selected medical records was 0.73, indicating substantial agreement between the chart reviewers.[13]
Selection bias was addressed by using a completely random sample from the entire initial pool which included all of the MSK diagnosis codes from the clinical settings of interest during the selected timeframe.
2.6. Statistical analysis
Statistical analysis was done using the statistical software SPSS Statistics version 23.0 (IBM, Armonk, NY). Descriptive statistics were provided including mean, standard deviation (SD), and proportions. The 2-sample proportional test was adopted to examine whether the proportions of patients with a certain trait in 1 group were different from that in another group. For instance, whether the proportion of Caucasian patients in the opioid group is different from the proportion of Caucasian patients in the nonopioid group. Of note, it was deemed that the independent variables, as seen in Tables 1 and 5, occurred without rare outcomes and therefore allowed for use of 2-sample t tests and proportional calculations. Multivariable logistic regression models were adopted for the response variables of opioid use and UDS monitoring. A multinomial regression model examined referrals as related to the various independent variables examined. Logistic regression typically requires a large sample size; however, without rare outcomes for each of the independent variables, the sample size of 300 is sufficient. The sample size of 300 also fulfilled the assumption requirement of multinomial regression. For all 3 models, these independent variables included ethnicity, gender, smoking status, alcohol abuse, other substance use, care setting, and BMI. All of the analytical results were considered to be significant when P-values were less than or equal to .05.
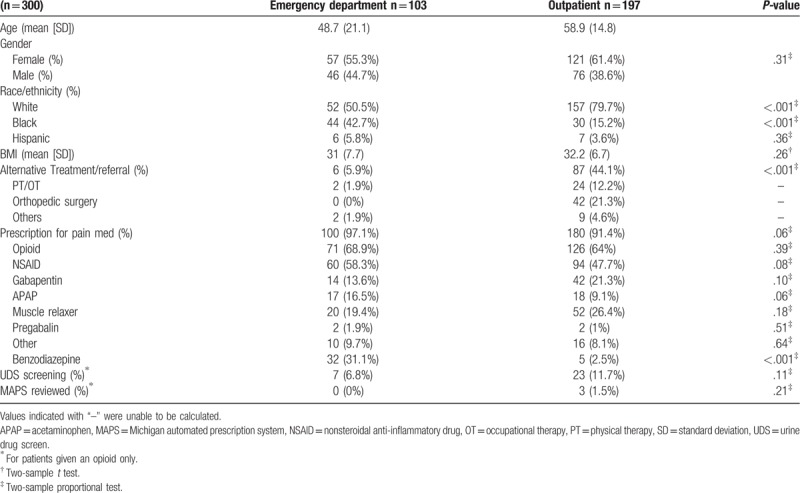
Table 5.
Descriptive statistics for samples from outpatient and emergency department. Data are presented as mean (standard deviation) for continuous data or count (percentage) for categorical ones. The P-value tests the difference between the 2 care-setting groups.
2.7. Role of the funding source
Funding was through the Blue Cross Blue Shield of Michigan Foundation Student Award Program, which did not influence the design, conduct, or reporting of this study.
3. Results
3.1. Sociodemographic factors
The sociodemographic factors of the 300 patients are described in Table 1. The mean age of patients given an opioid prescription was older at 58 (SD = 16), when compared to patients prescribed a nonopioid analgesic who had a mean age of 50.4 (SD = 20). This difference was statistically significant (P = .001). When comparing ethnicity, there was a higher proportion of Caucasians given nonopioids, while a slightly higher proportion of African-Americans and Hispanics were prescribed opioids. However, these differences were not statistically significant (P = .10, .21, and .38, respectively). Obesity was another common factor with the mean BMI of the entire sample being 31.8 (SD = 7.0) without any significant difference between the 2 populations.
Patients prescribed an opioid had significantly higher reported marijuana (15.2% for opioid vs 3.9% nonopioid, P = .003) and prescription abuse (15.2% opioid vs 5.8% nonopioid, P = .02). Similarly, smokers made up a higher proportion of patients given opioids (26.9%) compared to those given nonopioids (17.5%) but this difference was not statistically significant (P = .07). Additionally, 10 patients receiving opioids had reported alcohol abuse (as reported in chart or, as defined earlier, by consuming more than 14 drinks per week for men or more than 7 per week for women). This was significantly higher than patients treated with nonopioids meeting this criteria, which was zero (P = .02).
Regarding diagnosis, the largest group of ICD diagnoses was M50-M59.99, which includes most types of back pain except spondylopathies and deforming dorsopathies, making up 40.3% of the study population. The spine was the single most common site for MSK pain with 45.6% of patients having at least 1 diagnosis of back pain (M40-M59.99). This group also made up a significantly larger proportion of the opioid-prescribed population with 46.7% (M50-M59.99) compared to 28.2% of patients given non-opioids (P = .001). Both ranges of M15-M19.99 (inflammatory arthropathies and osteoarthritis) and M20-M29.99 (arthralgias, stiffness, other joint disorders) showed proportionally more nonopioid treatment which was a nonsignificant (P = .07) and a significant difference (P = .01), respectively.
Besides looking at the patient characteristics, another aim was to evaluate possible differences seen between care settings, namely outpatient encounters and encounters in the ED. Considering care setting, 65.7% of patients were treated in a primary care outpatient setting with the other 34.3% in the ED. When comparing the types of pharmacologic treatments given for MSK pain (opioid vs nonopioid) at each care setting, differences were not significant (P = .39) (Table 1).
3.2. Multivariable logistic regression model and multinomial regression model
To better correlate sociodemographic factors with clinical ones, we performed a multivariable logistic regression for opioid prescribing and UDS assessment as well as a multinomial regression model for referral types. These variables were adjusted for ethnicity, gender, smoking status, alcohol abuse, substance use, care setting, BMI, pain agreement, and age. Results are outlined in Table 2 given as odds ratios (OR) with 95% confidence intervals (CI) and with key findings bolded.
Table 2.
Multivariable logistic regression models based on sociodemographic factors on prescription type (opioid), and urine drug screening. Multinomial regression model based on sociodemographic factors on referral type.
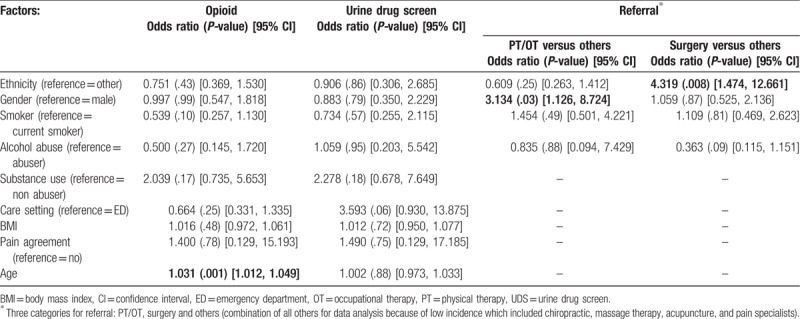
First, for every year of increasing age, there is a 3.1% increase in the odds of an opioid being prescribed (OR 1.031, CI 1.012–1.049, P = .001). For female patients, referral to physical therapy/occupational therapy (PT/OT) (instead of other referral types) is 3.1 times the estimated odds as compared to referrals for male patients (OR 3.134, CI 1.126–8.724, P = .03). Finally, for Caucasian patients, a referral to surgery is 4.3 the estimated odds as compared to referrals to surgery for minority patients (OR 4.319, CI 1.474–12.661, P = .008). No referrals were given to other alternative pain management including chiropractic, massage therapy, or acupuncture, though only 2 patients were referred to a pain specialist from the entire cohort (all included in “Other,” Table 2).
3.3. Anatomic locations of pain in patients treated with opioids
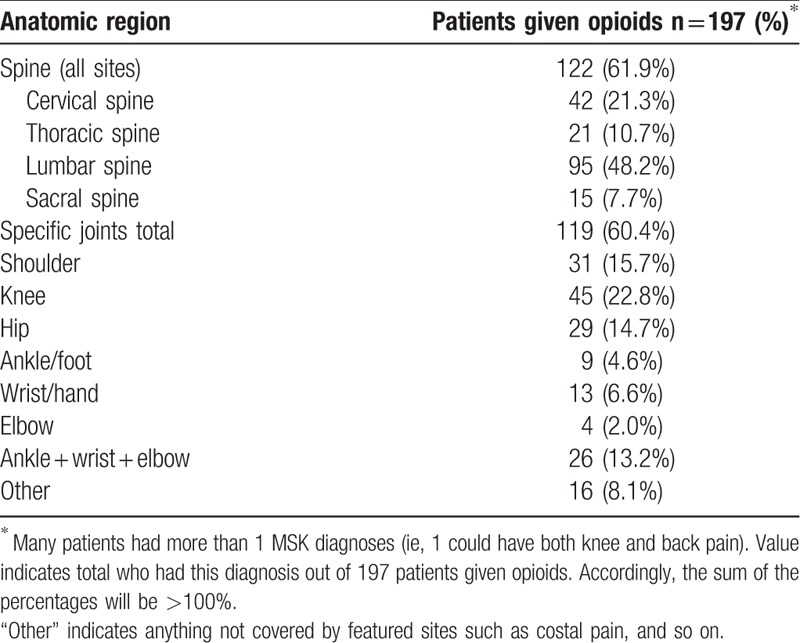
Anatomic locations were evaluated based on the ICD-10 diagnoses associated with each patient encounter in order to better understand which pain sites were being treated with opioids. As seen in Table 3, which only included patients with an opioid prescribed, there was a higher prevalence of spine involvement with 122 patients having a current spine pain diagnosis while all other joint diagnoses totaled 119 (60.4%). The lumbar spine was by far the most common site for opioid-treated MSK pain (48.2% of opioid patients had a lumbar back pain diagnosis) compared to any other anatomic site. Knee pain was the second highest pain diagnosis treated with opioids at 22.8%, followed closely by the cervical spine at 21.3%. The rest given in decreasing order are: shoulder, hip, thoracic spine, “other,” sacral spine, wrist/hand, ankle/foot, and elbow (Table 3).
Table 3.
Anatomical pain sites of patients given opioids.
3.4. Description of patients given opioids and clinical interventions used
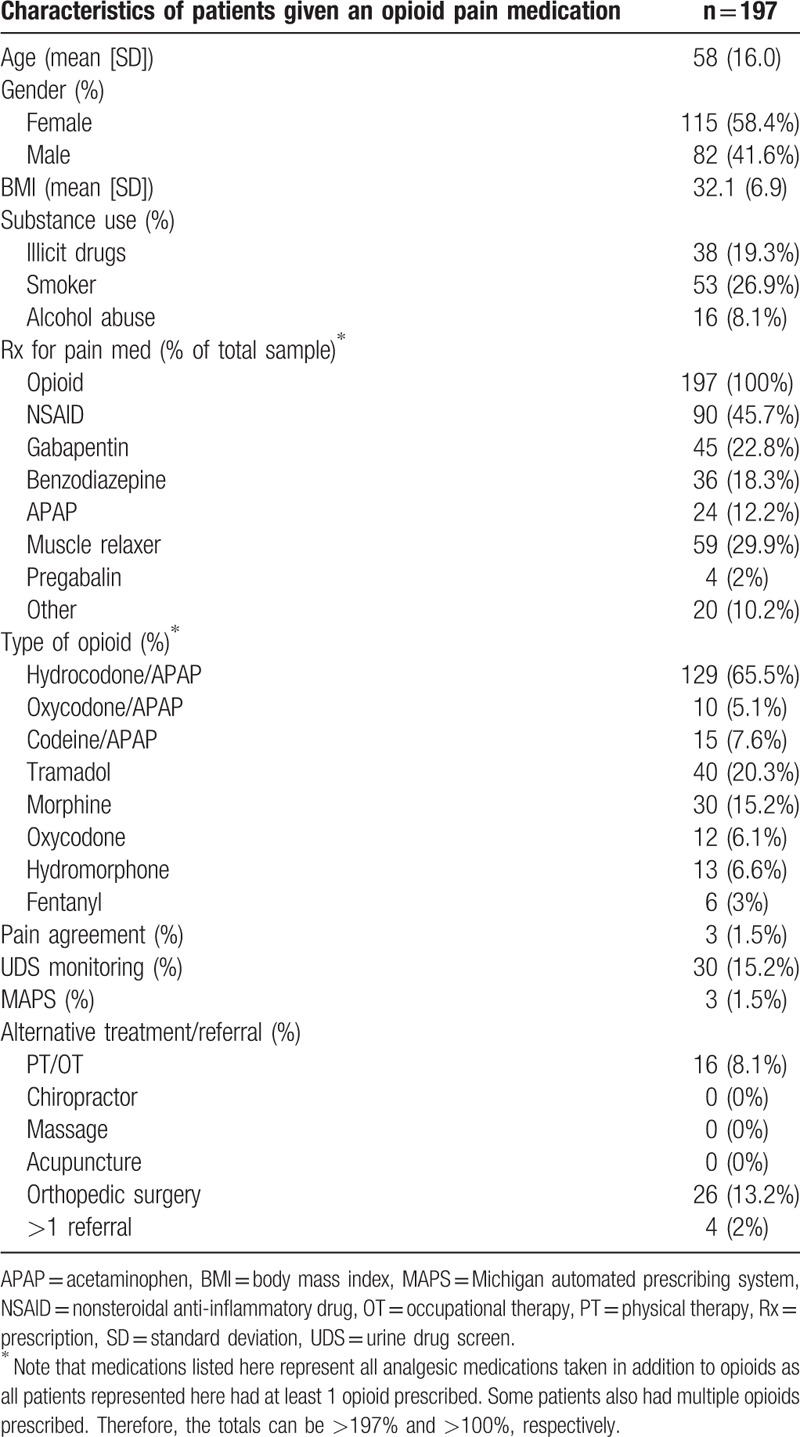
Besides 197 patients having a prescription for an opioid, many patients were on other concurrent nonopioid analgesic medications for pain. The 3 most common pain medications being taken in combination with opioids were NSAIDs (45.7%), muscle relaxants (29.9%), and gabapentin (22.8%). Others included benzodiazepines (which can be considered a muscle relaxant and anxiolytic), APAP, pregabalin, and others (such as topical analgesics like capsaicin ointment).
Hydrocodone/APAP was given more than any other opioid, making up 65.5% of all opioids prescribed. Following hydrocodone, in decreasing order of occurrence were tramadol, morphine, codeine, hydromorphone, oxycodone, oxycodone/APAP, and fentanyl. Even though the overall prescribing of opioids was high, the documented use of screening and monitoring modalities was extremely low. Only 15.2%, 1.5%, and 1.5% of patient these encounters showed documentation of a UDS, pain agreement, and a MAPS assessment, respectively.
Treatment modalities besides pharmacologic therapy also had low rates of utilization/referral. The most common referral was to orthopedic surgery with 13.2% of patient charts showing evidence of referral. Only 8.1% of the patients were referred to rehabilitative therapy during the study timeframe. In cases where more than 1 referral was given, it was a combination of orthopedic surgery and PT/OT (Table 4).
Table 4.
Description of pain medications and other interventions for patients with opioids prescribed.
3.5. Comparison of care setting
For treatment location of MSK pain, there was a higher proportion of African-Americans treated in the ED than in an outpatient setting, making up 42.7% of ED visits as compared to 15.2% of outpatient encounters (P < .001). Conversely, Caucasians were treated more often in an outpatient clinic setting rather than in the ED for MSK pain (P < .001), making up 79.7% and 50.5% of the total encounters, respectively.
The ED had a higher proportion of patients given opioid pain medications with 68.9% compared to outpatient clinics at 64%, although this difference was not significant (P = .39). The ED also saw higher prescribing rates for most of the medications, although these were not statistically significant differences. The only exception being a significantly higher proportion of benzodiazepine use at 31.1% (P < .001). While the ED had a higher proportion of pain being treated with medication at 97.1% of cases, outpatient settings had significantly higher proportions of alternative treatment referral with 44.1% versus 5.9% (P < .001). Orthopedic surgery referral was the most common totaling 42 new or active referrals, all of which were in the outpatient setting. This was followed by PT/OT with 26 total referrals (24 from outpatient and 2 from the ED). Very few referrals to other types of alternative treatments were given regardless of medication type or treatment setting. Only 2 patients from the entire cohort were referred to a pain specialist and no referrals were given to alternative pain management including chiropractic, massage therapy, or acupuncture (all of which are included in “others” in Table 5). On investigation of patient monitoring, only 7 patients (6.8%) in the ED had a UDS assessed, while 23 patients (11.7%) had UDS monitoring in the outpatient clinic setting during our timeframe. The use of MAPS was even lower with 0 ED encounters showing recorded evidence of a MAPS assessment and only 3 outpatient encounters had documentation of MAPS assessment.
4. Discussion
The results of this study reveal concerning prescribing trends along with a lack of monitoring for co-occurring substance abuse or confirmation of opioid prescription usage in a predominantly rural community. It was found that 65.7% of patients had an opioid prescription associated with their MSK diagnosis. This amount of prescribing is observed even in the absence of substantial data that opioids are effective in the treatment of CNCP and MSK pain.[7–9] This high usage of opioids for MSK pain was met with extremely low occurrences of monitoring despite recommendations that patients taking opioids (especially chronically) should be monitored.[4,5,14]
Although their use is recommended by multiple professional societies,[4–6] PDMP effectiveness in altering opioid prescribing and related health outcomes has seen mixed results since their inclusion in healthcare systems. On one hand, it can be said that using these monitoring methods may reduce reliance on physician judgment or bias.[4–6] Additionally, some studies have shown associations between PDMP implementation and mandatory usage with decreases in both opioid-related deaths and in numbers of opioids prescribed to Medicaid enrollees.[15,16] However, other studies have shown that PDMP usage has not made significant decreases in opioid use, such as overall prescribing rates or adverse outcomes, when compared to non-use of PDMPs.[17–19] The same can be said when comparing different levels of requirements for PDMP usage (ie, mandatory enrollment).[20] Another study on opioid prescribing in the ED found that automated queries by a PDMP did not reduce prescribing,[21] but a different study found that implementation of state-wide mandatory PDMP usage did have an impact on reducing controlled substance prescribing.[22]
Despite uncertainty about their true effectiveness, there are benefits to using a PDMP when it is used as intended. Since clinicians have access to information such as prescription amounts, dosages, and previous prescribers, they can use it to avoid prescribing of multiple opioids (or other controlled substances contraindicated with opioids), better clarify patient history, prevent “doctor shopping” where patients try to obtain more opioids through multiple prescribers, and prevent diversion of opioids (ie, given or sold to others).[23–25] In a recent study, Walker et al quantified possible prescription and patient characteristics which correlated to higher possibilities of “shopping” behavior. These included higher numbers of practices and pharmacies used, higher numbers of self-paid prescription fills, higher numbers of opioids dispensed, and even the use of immediate-release opioids instead of extended-release/long-acting opioids.[25] Utilization of a PDMP as well as having an astute clinical suspicion for these potential warning signs can be key in identifying patients who may be at a high risk of overdose or diversion.
As of 2015, even though 49 states have existing PDMPs, the median PDMP registration rate for prescribers who issue at least 1 controlled substance prescription was 35%.[23] Additionally, a 2014 survey found that among all physicians surveyed, 72% were aware of their state's PDMP but only 53% reported using one of the programs.[26] Therefore, this study further validates previous studies that have shown that prescribers of opioids are not appropriately monitoring medication use. It is possible that the low rates of PDMP utilization seen in this study was a result of clinicians not documenting their use of MAPS before prescribing a medication. However, few studies have addressed this issue, and due to the nature of retrospective analysis and reliance on the record-keeping of others, there is no way to confirm or deny this. On this issue, one may consider the common adage of medical record keeping, that if something is not documented, it did not happen. Regardless, the findings suggest that there was little evidence of PDMP usage.
UDS monitoring has also been found to be an effective way to monitor for abuse and its utility improved when coupled with reports on patient behavior.[14] As with PDMP usage, UDS was used very little. Only 30 of the 197 patients receiving opioids had a UDS done at the time of encounter or a recent one within the study timeframe. Some patients being monitored on a longer timeframe may be missed by a 3-month window but this does not explain lack of use in acute settings or for new prescriptions. A lack of UDS reporting in patient charts by clinicians cannot explain these low utilization numbers observed either because it would be recorded in the EMR automatically upon ordering. Regardless of care setting, health care providers should diligently monitor patients who are being prescribed opioids.
Back pain as a whole was found to be the largest contributing pain location, which is consistent with the existing literature.[27,28] For patients with an active opioid prescription, the lumbar spine was the single most prevalent pain location. Similarly, spine pain of any location was more prevalent than any other anatomic site with approximately one-half of patients prescribed an opioid having an ICD-10 diagnosis of back pain. This was found to be a significant difference compared to the nonopioid group. In fact, spine pain was more prevalent than all other sites combined. These findings agree with other studies which found that 59% of chronic pain patients using opioids reported back pain, while opioids were the most frequently prescribed medication for lower back pain.[29,30]
Use of opioids was common in both clinical settings assessed, with the ED having a slightly higher proportion. This finding differs from data reported in a previous study which observed fewer Medicare claims for schedule II opioids from the ED setting.[31] Pletcher et al found that the number of ED visits in the U.S. for all types of pain which resulted in an opioid prescription increased from 23% of visits in 1993 up to 37% in 2005, showing a definite trend in increasing opioid use in the ED.[32] This finding is contrary to evidence demonstrating that primary care physicians are the primary source of opioid prescriptions.[31,33] However, this study has limitations since the level of acuity was not assessed for the encounters in the ED. Another point is that opioid prescriptions given in the ED are commonly for short periods of time, covering only a couple of days. This is often done to provide pain relief to patients until they can be managed as an outpatient. Although this may seem harmless, it can also be a concern for exposing previously opioid-naïve patients to opioids or contributing to diversion as noted previously. Therefore, it is prudent to recommend use of opioids only if absolutely necessary to put patient and public safety first.
Hydrocodone was the most prescribed opioid by far, contributing to 65.5% of all opioids we found. This finding is consistent with trends observed by the U.S. Drug Enforcement Administration in recent national datasets which reported hydrocodone as the most used opioid.[34] Many patients had additional pain medications being used concurrently with opioids as well. This finding suggests that MSK pain is often not completely controlled by a single medication type, further supporting our idea that the use of alternative measures should be implemented as either primary therapy, adjuvant therapy, or as a way to reduce the pharmacologic burden on the patient.
Regarding demographics, patients prescribed opioids were older, had a higher prevalence of reported alcohol abuse, active tobacco use, and had higher illicit substance use with higher reported (either self-reported or found on UDS) marijuana and prescription abuse. Unfortunately, these sociodemographic factors, except for older age, have been found to be risk factors for opioid misuse.[35–38] Based on these findings, clinicians could potentially use these factors as warning signs when assessing patients for pain management options. Identifying potential risk factors should raise suspicion for the possibility of misuse and warrant diligent monitoring of opioids in these patients.
A significant difference was found when comparing pain treatment location by ethnicity. African-Americans made up a larger proportion of patients seen in the ED compared to outpatient clinics. Based on recent studies, the implementation of the affordable care act (ACA) in 2012 provided health care insurance to over 30 million people who were previously uninsured, but 1 out of 5 African-Americans and 1 out of 3 Hispanic persons with a chronic disease still lacked coverage and access to healthcare.[39,40] However, implementation of the ACA has also been too short to assess its definitive effects.[39] Therefore, access to care is a prevalent issue and may partially explain the care setting disparity found in this cross-sectional study even though it was not directly assessed.
4.1. Limitations
There are important limitations to disclose about this study. First, this study was conducted using EMR data from a single healthcare system which may limit generalizability of study findings. Second, since this study was done in a single, predominantly rural region, the data may not be generalizable to other demographic regions. Third, severity/acuity of cases was not considered largely in part to inconsistent reporting of patient self-reported scores on a pain scale. Although, due to the subjectivity of this type of pain assessment, we felt it was more important to rely on what medications were prescribed as a way to reflect prescriber judgment on pain severity. Lastly, the 3-month timeframe may be a limitation as it could potentially miss longitudinal pain management screenings such as UDS assessments or pain agreements. As stated previously, this study was primarily meant to be a cross-sectional study acting as a needs assessment for a specific community. Despite these limitations, the study confers useful information on how the observed opioid prescribing trends warrant more prudent monitoring and consideration in all clinical settings.
5. Conclusion
Ideally, finding ways to decrease the amount of opioids prescribed while improving patient outcomes should be the goal for all clinicians, regardless of care setting. By taking the factors investigated here, and incorporating their evaluation into clinical decision-making, clinicians may change their approach to MSK pain. It is possible that removing, reducing, or not prescribing opioids (as first-line therapy) should be some of the first steps in forming a comprehensive pain management plan. In such a plan we believe that opioids should be reserved for last-line use only after combinations of other treatments have failed. This study lends to an important paradox about the increased use of opioids in the outpatient setting and the limited amount of monitoring done. Notably, clinicians evaluating patients in the ED or in outpatient clinics who do prescribe opioids for CNCP should ensure that consistent and diligent use of appropriate monitoring is conducted by assessing a UDS, reviewing and documenting the findings from the PDMP, and documenting a pain agreement (especially with chronic prescribing of opioids during outpatient clinic encounters).
One possible direction for opioid alternatives is to look towards integrative treatment modalities such as exercise therapy, acupuncture, and physical therapy, of which there were low rates of utilization found in this study. There is some evidence that both exercise alone, and exercise in conjunction with education, may prevent low back pain (the single greatest contributing diagnosis identified in this study) for up to 1 year.[41]
Based on the findings of our study, it is recommended that clinicians leverage the use of integrative modalities to either treat chronic pain or use them as adjunct to achieve optimal patient benefits. These strategies enhance overall functioning as a means to decrease opioid prescribing, although this approach may only be feasible for clinicians treating patients with MSK pain in an outpatient setting. In the future, we hope that the continued publicity and awareness of the national opioid crisis will lead to a change of opinion regarding opioid prescribing in the minds of both practitioners and the general public. With this study, we hope to provide additional evidence for validating the concerns on opioid safety and prescribing patterns, and ultimately assist in the continued changes being made in healthcare. It is our sincere hope and expectation that with increased awareness and policy changes, the use of opioids prescribed will decrease while safety monitoring will increase. Indeed, further studies will be necessary in the future to objectively measure the effectiveness of these changes.
Author contributions
Conceptualization: Derek Paul Richard Pierce, Juliette Perzhinsky.
Data curation: Derek Paul Richard Pierce, Brett Pierce.
Formal analysis: Chin-I Cheng.
Funding acquisition: Derek Paul Richard Pierce.
Investigation: Derek Paul Richard Pierce, Juliette Perzhinsky.
Methodology: Juliette Perzhinsky.
Project administration: Juliette Perzhinsky.
Resources: Juliette Perzhinsky.
Software: Chin-I Cheng.
Supervision: Juliette Perzhinsky.
Writing – original draft: Derek Paul Richard Pierce, Juliette Perzhinsky.
Writing – review and editing: Derek Paul Richard Pierce, Brett Pierce, Juliette Perzhinsky, Chin-I Cheng.
Derek Paul Richard Pierce orcid: 0000-0002-4475-9057.
Footnotes
Abbreviations: ACA = affordable care act, APAP = acetaminophen, BMI = body mass index, CDC = Centers for Disease Control and Prevention, CI = confidence interval, CNCP = chronic noncancer pain, ED = emergency department, EMR = electronic medical record, ICD-10 = 10th revision of the International Statistical Classification of Diseases and Related Health Problems, IRB = Institutional Review Board, MAPS = Michigan automated prescription system, MSK = musculoskeletal, NSAID = nonsteroidal anti-inflammatory drug, OR = odds ratio, OT = occupational therapy, PDMP = prescription drug monitoring program, PT = physical therapy, SD = standard deviation, U.S. = United States, UDS = urine drug screen.
This research was supported by a Student Award Program grant from the Blue Cross and Blue Shield of Michigan Foundation.
The views in the article do not represent that of Central Michigan University, CMU Health, nor the Veterans Health Administration.
The authors have no conflicts of interest to disclose.
References
- [1].Stone AB, Wick EC, Wu CL, et al. The US opioid crisis: a role for enhanced recovery after surgery. Anesth Analg 2017;125:1803–5. [DOI] [PubMed] [Google Scholar]
- [2].Han B, Compton WM, Blanco C, et al. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 National Survey on Drug Use and Health. Ann Intern Med 2017;167:293–301. [DOI] [PubMed] [Google Scholar]
- [3].Data Overview [Internet]. Centers for Disease Control and Prevention; Jan 12, 2016 [Updated July 18, 2017]. Available at: http://www.cdc.gov/drugoverdose/data/index.html [Accessed on February 7, 2016].
- [4].Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep 2016;65:1–49. [DOI] [PubMed] [Google Scholar]
- [5].Berland D, Rodgers P. Rational use of opioids for management of chronic nonterminal pain. Am Fam Physician 2012;86:252–8. [PubMed] [Google Scholar]
- [6].Kinsman D. ACP Supports CDC Guideline for Prescribing Opioids for Chronic Pain [Internet]. American College of Physicians; Mar 15, 2016. Available at: https://www.acponline.org/acp-newsroom/acp-supports-cdc-guideline-for-prescribing-opioids-for-chronic-pain [Accessed on August 8, 2017].
- [7].Franklin GM. Opioids for chronic noncancer pain: a position paper of the American Academy of Neurology. Neurology 2014;83:1277–84. [DOI] [PubMed] [Google Scholar]
- [8].Noble M, Tregear SJ, Treadwell JR, et al. Long-term opioid therapy for chronic noncancer pain: a systematic review and meta-analysis of efficacy and safety. J Pain Symptom Manage 2008;35:214–28. [DOI] [PubMed] [Google Scholar]
- [9].Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain 2004;109:207–9. [DOI] [PubMed] [Google Scholar]
- [10].Kidner CL, Mayer TG, Gatchel RJ. Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. J Bone Joint Surg Am 2009;91:919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Eriksen J, Sjørgen P, Bruera E, et al. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain 2006;125:172–9. [DOI] [PubMed] [Google Scholar]
- [12].Diseases of the Musculoskeletal System and Connective Tissue M00-M99. [Internet] (n.d.). Available at: http://www.icd10data.com/ICD10CM/Codes/M00-M99 [Accessed on June 30, 2016].
- [13].Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med 2005;37:360–3. [PubMed] [Google Scholar]
- [14].Katz NP, Sherburne S, Beach M, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg 2003;97:1097–102. [DOI] [PubMed] [Google Scholar]
- [15].Patrick SW, Fry CE, Jones TF, et al. Implementation of prescription drug monitoring programs associated with reductions in opioid-related death rates. Health Aff (Millwood) 2016;35:1324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wen H, Schackman BR, Aden B, et al. States with prescription drug monitoring mandates saw a reduction in opioids prescribed to medicaid enrollees. Health Aff (Millwood) 2017;36:733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Deyo RA, Hallvik SE, Hildebran C, et al. Association of prescription drug monitoring program use with opioid prescribing and health outcomes: a comparison of program users and nonusers. J Pain 2017;19:166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Paulozzi LJ, Kilbourne EM, Desai HA. Prescription drug monitoring programs and death rates from drug overdose. Pain Med 2011;12:747–54. [DOI] [PubMed] [Google Scholar]
- [19].Brady JE, Wunsch H, DiMaggio C, et al. Prescription drug monitoring and dispensing of prescription opioids. Public Health Rep 2014;129:139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lin HC, Wang Z, Boyd C, et al. Associations between statewide prescription drug monitoring program (PDMP) requirement and physician patterns of prescribing opioid analgesics for patients with non-cancer chronic pain. Addict Behav 2018;76:348–54. [DOI] [PubMed] [Google Scholar]
- [21].Sun BC, Charlesworth CJ, Lupulescu-Mann N, et al. Effect of automated prescription drug monitoring program queries on emergency department opioid prescribing. Ann Emerg Med 2017;71:337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Suffoletto B, Lynch M, Pacella CB, et al. The impact of a statewide mandatory prescription drug monitoring program on opioid prescribing by emergency medicine providers across 15 hospitals in a single health system. J Pain 2018;19:430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Haffajee RL, Jena AB, Weiner SG. Mandatory use of prescription drug monitoring programs. JAMA 2015;313:891–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].State Prescription Drug Monitoring Programs [Internet]. Drug Enforcement Administration Diversion Control Division; June 2016. Available at: https://www.deadiversion.usdoj.gov/faq/rx_monitor.htm [Accessed on December 29, 2017].
- [25].Walker AM, Weatherby LB, Cepeda MS, et al. Possible opioid shopping and its correlates. Clin J Pain 2017;33:976–82. [DOI] [PubMed] [Google Scholar]
- [26].Rutkow L, Turner L, Lucas E, et al. Most primary care physicians are aware of prescription drug monitoring programs, but many find the data difficult to access. Health Aff (Millwood) 2015;34:484–92. [DOI] [PubMed] [Google Scholar]
- [27].Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med 2008;9:803–12. [DOI] [PubMed] [Google Scholar]
- [28].Health, United States, 2016: With Chartbook on Long-term Trends in Health [Internet]. Hyattsville (MD): National Center for Health Statistics; 2016 [Updated December 13, 2017]. Available at: https://www.cdc.gov/nchs/hus/index.htm [Accessed on August 27, 2017]. [PubMed]
- [29].Hudson TJ, Edlund MJ, Steffick DE, et al. Epidemiology of regular prescribed opioid use: results from a national, population-based survey. J Pain Symptom Manage 2008;36:280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ivanova JI, Birnbaum HG, Schiller M, et al. Real-world practice patterns, health-care utilization, and costs in patients with low back pain: the long road to guideline-concordant care. Spine J 2011;11:622–32. [DOI] [PubMed] [Google Scholar]
- [31].Chen JH, Humphreys K, Shah NH, et al. Distribution of opioids by different types of medicare prescribers. JAMA Intern Med 2016;176:259–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pletcher MJ, Kertesz SG, Kohn MA, et al. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA 2008;299:70–8. [DOI] [PubMed] [Google Scholar]
- [33].Volkow ND, McLellan AT. Opioid abuse in chronic pain – misconceptions and mitigation strategies. N Engl J Med 2016;374:1253–63. [DOI] [PubMed] [Google Scholar]
- [34].Drug and Chemical Information: Hydrocodone. [Internet] Drug Enforcement Administration Office of Diversion Control; October 2014. Available at: https://www.deadiversion.usdoj.gov/drug_chem_info/ [Accessed on December 24, 2017].
- [35].Ives TJ, Chelminski PR, Hammett-Stabler CA, et al. Predictors of opioid misuse in patients with chronic pain: a prospective cohort study. BMC Health Serv Res 2006;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rice JB, White AG, Birnbaum HG, et al. A model to identify patients at risk for prescription opioid abuse, dependence, and misuse. Pain Med 2012;13:1162–73. [DOI] [PubMed] [Google Scholar]
- [37].White AG, Birnbaum HG, Schiller M, et al. Analytic models to identify patients at risk for prescription opioid abuse. Am J Manag Care 2009;15:897–906. [PubMed] [Google Scholar]
- [38].Yoon JH, Lane SD, Weaver MF. Opioid analgesics and nicotine: more than blowing smoke. J Pain Palliat Care Pharmacother 2015;29:281–9. [DOI] [PubMed] [Google Scholar]
- [39].Blumenthal DB, Abrams M, Nuzum R. The affordable care act at 5 years. N Engl J Med 2015;372:2451–8. [DOI] [PubMed] [Google Scholar]
- [40].Torres H, Poorman E, Tadepalli U, et al. Coverage and access for Americans with chronic disease under the affordable care act: a quasi-experimental study. Ann Intern Med 2017;166:472–9. [DOI] [PubMed] [Google Scholar]
- [41].Steffens D, Maher CG, Pereira LS, et al. Prevention of low back pain a systematic review and meta-analysis. JAMA Intern Med 2016;176:199–208. [DOI] [PubMed] [Google Scholar]


