Abstract
Background
Long‐term illnesses affect a significant proportion of the population in developed and developing countries. Mobile phone messaging applications, such as Short Message Service (SMS) and Multimedia Message Service (MMS), may present convenient, cost‐effective ways of supporting self‐management and improving patients' self‐efficacy skills through, for instance, medication reminders, therapy adjustments or supportive messages.
Objectives
To assess the effects of mobile phone messaging applications designed to facilitate self‐management of long‐term illnesses, in terms of impact on health outcomes and patients' capacity to self‐manage their condition. Secondary objectives include assessment of: user evaluation of the intervention; health service utilisation and costs; and possible risks and harms associated with the intervention.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL,The Cochrane Library 2009, Issue 2), MEDLINE (OvidSP) (January 1993 to June 2009), EMBASE (OvidSP) (January 1993 to June 2009), PsycINFO (OvidSP) (January 1993 to June 2009), CINAHL (EbscoHOST) (January 1993 to June 2009), LILACS (January 1993 to June 2009) and African Health Anthology (January 1993 to June 2009).
We also reviewed grey literature (including trial registers) and reference lists of articles.
Selection criteria
We included randomised controlled trials (RCTs), quasi‐randomised controlled trials (QRCTs), controlled before‐after (CBA) studies, or interrupted time series (ITS) studies with at least three time points before and after the intervention. We selected only studies where it was possible to assess the effects of mobile phone messaging independent of other technologies or interventions.
Data collection and analysis
Two review authors independently assessed all studies against the inclusion criteria, with any disagreements resolved by a third review author. Study design features, characteristics of target populations, interventions and controls, and results data were extracted by two review authors and confirmed by a third. Primary outcomes of interest were health outcomes as a result of the intervention and capacity to self‐manage long‐term conditions. We also considered patients' and providers' evaluation of the intervention, perceptions of safety, health service utilisation and costs, and potential harms or adverse effects. The included studies were heterogeneous in type of condition addressed, intervention characteristics and outcome measures. Therefore, a meta‐analysis to derive an overall effect size for the main outcome categories was not considered justified and findings are presented narratively.
Main results
We included four randomised controlled trials involving 182 participants.
For the primary outcome of health outcomes, including physiological measures, there is moderate quality evidence from two studies involving people with diabetes showing no statistical difference from text messaging interventions compared with usual care or email reminders for glycaemic control (HbA1c), the frequency of diabetic complications, or body weight. There is moderate quality evidence from one study of hypertensive patients that the mean blood pressure and the proportion of patients who achieved blood pressure control were not significantly different in the intervention and control groups, and that there was no statistically significant difference in mean body weight between the groups. There is moderate quality evidence from one study that asthma patients receiving a text messaging intervention experienced greater improvements on peak expiratory flow variability (mean difference (MD) ‐11.12, 95% confidence interval (CI) ‐19.56 to ‐2.68) and the pooled symptom score comprising four items (cough, night symptoms, sleep quality, and maximum tolerated activity) (MD ‐0.36, 95% CI ‐0.56 to ‐0.17) compared with the control group. However, the study found no significant differences between the groups in impact on forced vital capacity or forced expiratory flow in 1 second.
For the primary outcome of capacity to self‐manage the condition, there is moderate quality evidence from one study that diabetes patients receiving the text messaging intervention demonstrated improved scores on measures of self‐management capacity (Self‐Efficacy for Diabetes score (MD 6.10, 95% CI 0.45 to 11.75), Diabetes Social Support Interview pooled score (MD 4.39, 95% CI 2.85 to 5.92)), but did not show improved knowledge of diabetes. There is moderate quality evidence from three studies of the effects on treatment compliance. One study showed an increase in hypertensive patients' rates of medication compliance in the intervention group (MD 8.90, 95% CI 0.18 to 17.62) compared with the control group, but in another study there was no statistically significant effect on rates of compliance with peak expiratory flow measurement in asthma patients. Text message prompts for diabetic patients initially also resulted in a higher number of blood glucose results sent back (46.0) than email prompts did (23.5).
For the secondary outcome of participants' evaluation of the intervention, there is very low quality evidence from two studies that patients receiving mobile phone messaging support reported perceived improvement in diabetes self‐management, wanted to continue receiving messages, and preferred mobile phone messaging to email as a method to access a computerised reminder system.
For the secondary outcome of health service utilisation, there is very low quality evidence from two studies. Diabetes patients receiving text messaging support made a comparable number of clinic visits and calls to an emergency hotline as patients without the support. For asthma patients the total number of office visits was higher in the text messaging group, whereas the number of hospital admissions was higher for the control group.
Because of the small number of trials included, and the low overall number of participants, for any of the reviewed outcomes the quality of the evidence can at best be considered moderate.
Authors' conclusions
We found some, albeit very limited, indications that in certain cases mobile phone messaging interventions may provide benefit in supporting the self‐management of long‐term illnesses. However, there are significant information gaps regarding the long‐term effects, acceptability, costs, and risks of such interventions. Given the enthusiasm with which so‐called mHealth interventions are currently being implemented, further research into these issues is needed.
Keywords: Humans, Reminder Systems, Self Care, Text Messaging, Asthma, Asthma/therapy, Cell Phone, Chronic Disease, Diabetes Mellitus, Diabetes Mellitus/therapy, Hypertension, Hypertension/therapy, Patient Satisfaction, Randomized Controlled Trials as Topic
Plain language summary
Mobile phone messaging for facilitating self‐management of long‐term illnesses
Many people suffer from long‐term conditions such as asthma or diabetes. To make living with the long‐term illnesses as easy as possible, people have to regularly monitor the symptoms of their conditions and adapt their lifestyles. This review studied whether mobile phone applications such as Short Message Service (SMS) (also known as text messaging) and Multimedia Message Service (MMS) can support people to better manage their long‐term illnesses by sending medication reminders or supportive messages, or by offering a way for people to communicate important information to their healthcare providers and receive feedback.
We found moderate quality evidence that under some conditions these types of applications may indeed have some positive impacts on the health status of patients with diabetes, hypertension and asthma, and on their ability to manage their own condition, although for some outcomes no significant effect was observed. In two studies, there was very low quality evidence that participants evaluated the mobile phone messaging support positively. Also, in two studies, there was very low quality evidence that: there was no difference in health service utilisation by diabetes patients receiving text messaging support and those who did not (one study); and that asthma patients receiving text messages visited the doctor more often but were admitted to hospital less often than those not receiving the messages (one study).
Because of the small number of patients involved in these studies the evidence is not very strong. Furthermore, the usefulness and potential negative consequences of mobile phone messaging over extended periods of use for self‐managing long‐term conditions are not yet known.
Summary of findings
Summary of findings for the main comparison. Mobile phone messaging for facilitating self‐management of long‐term illnesses.
|
Patient or population: Patients with long‐term illnesses
Settings: Outpatient services in Scotland, USA, Spain and Croatia
Intervention: Mobile phone messaging support for self‐management of diabetes, asthma or hypertension Comparison: Usual care, or usual care with self‐management support delivered by email | |||
| Outcomes | Impact | No of Participants (studies) | Quality of the evidence (GRADE) |
| Health outcomes: Glycaemic control (HbA1c) | One study found no statistical difference on glycaemic control between groups receiving the intervention or usual care. The other study found mobile phone messaging no more effective than email reminders in achieving glycaemic control. Overall, mean pooled glycaemic control (HbA1C) for the control groups was 9.9 (SD 1.5). In the text messaging groups this was 0.15 units lower (0.77 lower to 0.47 higher). | 88 (2 studies) | ⊕⊕⊕⊝ moderate1 |
| Health outcomes: Variety of measures | For diabetes and hypertension no statistically significant differences were found between the intervention and control groups on body mass index, weight or blood pressure. For asthma a significant improvement in the text messaging group was found for only 2 out of 4 outcome measures, that is peak expiratory flow variability and pooled symptom score. | 142 (3 studies) |
⊕⊕⊕⊝ moderate2 |
|
Capacity to self‐manage the condition: Management and knowledge of diabetes |
Patients receiving text messaging support showed significantly improved scores on the Self‐Efficacy for Diabetes test and the Diabetes Social Support Interview. It did not, however, result in improved knowledge of diabetes. | 59 (1 study) |
⊕⊕⊕⊝
moderate1 |
|
Capacity to self‐manage the condition: Treatment compliance |
Medication compliance in hypertension patients was 8.9% higher (0.18% higher to 17.62% higher) in the text messaging group as compared with the control group. There were no statistically significant effects on compliance with peak expiratory flow (PEF) measurement for asthma patients, or on self‐reported adherence in young people with diabetes. Text message prompts for diabetes patients initially also resulted in a higher number of blood glucose results (46.0) sent back than email prompts (23.5) did. | 142 (3 studies) |
⊕⊕⊕⊝ moderate2 |
| Participants' evaluation of the intervention | Patients receiving mobile phone messaging support reported improvement in self‐management of diabetes, wanted to continue receiving messages, and preferred mobile phone messaging to email as a method to access the Computerised Automated Reminder Diabetes System. | 72 (2 studies) |
⊕⊝⊝⊝ very low3 |
| Health service utilisation | Diabetes patients receiving text messaging support made a comparable number of clinic visits and calls to an emergency hotline as patients without the support. For asthma patients, the total number of office visits was higher in the text messaging group, whereas the number of hospital admissions was higher for the control group. | 75 (2 studies) |
⊕⊝⊝⊝ very low4 |
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
1 Number of participants is low in both studies on diabetes.
2 All included trials have a low number of participants.
3 The number of participants is low in both included trials. The outcomes are not compared between the intervention and control groups.
4 Both included trials have a low number of participants. The reasons for clinic or clinic visits and hospitalisations were not known, so the causal link between the intervention and the outcome measures is not clear.
Background
Mobile phone messaging is an important means of human communication globally. Mobile phone penetration is rapidly increasing, particularly in the Asia Pacific region, with 90% of the global and 80% of rural population having access to a mobile network in 2010. The number of subscriptions in 2010 reached 5.3 billion, representing a 76.2% global penetration rate (ITU 2010). The penetration rates are 70% to 90% in high‐income countries, with a similar rate of increase across all socio‐economic groups (Atun 2006).
Most digital mobile phones provide Short Message Service (SMS), also known as text messaging, and Multimedia Message Service (MMS) for transmitting graphics, video clips and sound files. SMS, in particular, has rapidly developed into a powerful communication medium, particularly among young adults. The total number of text messages sent globally tripled between 2007 and 2010, from an estimated 1.8 trillion to 6.1 trillion, with about 200,000 messages sent every second (ITU 2010). These short messages, where up to 160 characters of text are sent from the Internet or from a mobile phone to one or several mobile phones, could provide an important, inexpensive medium of communication. The terms text message, text, or txt are more commonly used in North America, the UK, Spain and the Philippines, while in many other countries the term SMS is used. In this review we will use the term ‘text messaging’ to refer to the use of SMS only, distinguishing it from the term ‘mobile phone messaging’, which encompasses both SMS and MMS. Increasingly, the latter term also refers to mobile email and ‘instant messaging’ delivered to the mobile phone.
Text messages, compared to other communication channels, have the advantage of instant transmission and low cost. There is also a smaller chance of being misplaced compared to print materials, and of being invasive to daily lives compared to phone calls (Kaplan 2006). Features such as ubiquity, mobility, direct and instantaneous access and direct communication offer the possibility of using mobile phones for health information transfer (Atun 2006). A literature review on the use of mobile phones in health care has demonstrated the wide application and potential of mobile phones to: increase access to health care; enhance efficiency of service delivery; improve diagnosis, treatment and rehabilitation; and support public health programmes (Atun 2006; Car 2012). Mobile phone messaging has, for example, been used to provide appointment reminders (Bos 2005), to improve patient compliance with medications (Fairley 2003; Marquez Contreras 2004; Vilella 2004), to monitor chronic conditions (Ferrer‐Roca 2004; Kwon 2004; Ostojic 2005) and to provide psychological support (Bauer 2003; Franklin 2006). Mobile phones have also been used in managing communicable diseases and in health promotion programmes (e.g. in smoking cessation (Obermayer 2004; Rodgers 2005)). Furthermore, the use of mobile phones has been shown to improve service utilization among population groups such as teenagers and young adult males who do not typically use health services, by providing the opportunity to remotely access care providers for advice (Atun 2006b). However for older adults, some of whom are less able or willing to use mobile phones, the effect on service utilization could be limited (Atun 2006b).
Challenges in using mobile phone applications in health care include incomplete coverage of mobile networks across regions, lack of standards, and possible information overload (Adler 2007).
This review is part of a series of four reviews which aim to determine the effects of mobile phone messaging in improving the processes of healthcare service delivery and service utilization.
We divided the reviews into four areas based on specific interventions and related outcomes:
Mobile phone messaging for facilitating self‐management of long‐term illnesses (this review);
Mobile phone messaging for communicating results of medical investigations (Gurol‐Urganci 2012);
Mobile phone messaging for preventive health care (Vodopivec‐Jamsek 2012);
Mobile phone messaging reminders for attendance at healthcare appointments (Car 2012);
Description of the condition
Long‐term diseases such as diabetes, asthma, heart disease and human Immunodeficiency virus (HIV) affect people's lives over a long period of time. They usually place a substantial burden on the health, economic status, and quality of life of individuals, families and communities. Given that a significant proportion of healthcare resources is utilised by people with long‐term conditions, policy makers give high priority to the effective management of these conditions. It has been suggested that, in order to improve the quality and effectiveness of long‐term disease management, a systematic approach is needed, comprising proactive healthcare systems and an active role for patients in self‐managing their disease (Yanez‐Cadena 2006). There is strong global interest in the role of self‐management programs in controlling and preventing long‐term disease complications (Bodenheimer 2002; Bodenheimer 2002b; Bodenheimer 2002c; Foster 2007). For instance, the United Kingdom (UK) Department of Health issued a policy document in 2005 recognising support for self‐care as one of the three pillars of the National Health Service (NHS) and social care long‐term conditions model (DoH 2005).
The term 'self‐management' of a long‐term illness refers to the tasks a person can perform to minimise the impact of that illness on his/her health status by him‐/herself, or with the support of a healthcare provider (Clark 1991). These tasks can be classified into medical management, emotional management or role management tasks (Corbin 1988). Typically, self‐management of a long‐term illness requires that a person has the skills to self‐monitor the symptoms and clinical markers of that condition, to understand the associated implications, and to adjust medication, treatment or behaviour accordingly (Barlow 2002; Corben 2005).
Description of the intervention
Communication between patient and healthcare provider plays an important support role in both disease monitoring and education. For example, care providers can send patients reminders to self‐monitor or attend to their care; or patients can send messages to their provider reporting the results from self‐monitoring (DoH 2005). Communication to support self‐management can take a number of forms, such as face‐to‐face conversations, phone conversations or phone messaging. The communication of self‐monitoring results or reminders typically does not require the exchange of lengthy or complex information, and phone messaging therefore presents an interesting new delivery medium for such messages. Relevant interventions provide disease‐related information to patients, support self‐monitoring of illnesses, support adherence to treatment or medications or both, or offer a channel for peer‐to‐peer networking and support through SMS or MMS.
How the intervention might work
Mobile phone messaging interventions can be used to: enhance self‐efficacy (e.g. reminders, feedback on treatment success); provide a form of social support (from peers and health professionals); or establish social networks (support groups, peer‐to‐peer networks). By increasing self‐efficacy (Bandura 1977; Bandura 1982) and providing support mechanisms (Cohen 1985; Cobb 2002; Christakis 2004), these interventions may influence health behaviours and enhance self‐management of long‐term illnesses.
Text messaging can be beneficial in this context by providing patients or their carers with information on their condition, by monitoring of illness, by promoting improved adherence to treatment and/or medications, or as a channel of peer‐to‐peer networking and support. In particular, text messaging can be an important source of support to people in remote locations and to those with mobility issues. Text messaging may facilitate education on self‐management problem solving skills, and in this way enhance patient confidence to carry out the behaviours necessary to reach a desired goal.
For instance, Anhøj and colleagues describe a small study in Denmark in which asthma patients were sent four daily text messages that included a medication reminder as well as requests to send back peak flow measurements, data on sleep loss, and medication dosage (Anhøj 2004). These data were entered in an online asthma diary to facilitate communication between patients and their doctors, and to aid in the development of an individual patient‐based treatment plan. In another small trial of an intervention for asthma patients, patients sent daily text messages with peak flow data to an asthma specialist who once a week adjusted their therapy accordingly (Ostojic 2005). The study concluded that text messaging (when supplemented by a written action plan and standard follow‐up) was a convenient, reliable, affordable, and secure means to help asthma control.
In Ferrer‐Roca 2004, diabetic patients used text messaging to send data on blood glucose levels and body weight to a web‐based database. In response they received help or warning messages if the recorded measurements were out of range for that individual patient, as well as monthly calculated glycosylated haemoglobin results, leading to improved self‐management in elderly persons and teenagers. 'Sweet‐Talk', a text‐messaging support system for paediatric patients with Type 1 diabetes, was also successful in improving self‐efficacy for young people who are harder to reach in healthcare settings (Franklin 2003; Franklin 2006). Similar positive results were recorded for adolescents with type 1 diabetes in Rami 2006, although with some technical problems due to data loss, which resulted in patient dissatisfaction.
Several studies describe the use of text messaging for sending medication or treatment reminders. In a study involving 26 primary healthcare centres in Spain, people with hypertension received medication reminders for compliance with therapy in the form of text messages. However, no significant improvement was observed in the intervention group (Marquez Contreras 2004). In another study, HIV‐infected patients aged 16 to 24 were sent text message reminders for highly active antiretroviral therapy. Although these reminders were found to be helpful, and the level of daily intrusion was seen as acceptable, the study period of 12 weeks was not adequate to assess their full impact (Puccio 2006).
Continuous support from healthcare providers, peers and the community can be critical in conditions with a high risk of relapse, such as bulimia nervosa. In Germany, text messaging was used to send bulimic patients who had finished inpatient treatment, weekly individually‐tailored feedback messages (Bauer 2003). This type of support was found to be well‐accepted, practical and effective. In another site, however, there was limited acceptance of a text message intervention in the after‐care of bulimic patients who had received outpatient psychotherapy (Robinson 2006).
Acceptability and risks of the intervention
Studies in which patients and/or providers rated text messaging for promoting disease self‐management positively, noted features of simplicity and timeliness of the intervention (Ferrer‐Roca 2004; Pinnock 2006). On the other hand, some skepticism was reported regarding clinical benefits, time and cost implications (Pinnock 2006).
Possible disadvantages of using mobile phone messaging include the risk of inaccurate data input (Norwell 2003), lack of understanding or misinterpretation of the information, and difficulties in reading for those with poor vision or literacy problems. Furthermore, mobile phone messaging is intended to support or complement the process of care delivery, rather than to substitute for it. A narrow focus on the technology may result in providers misinterpreting it as an endpoint to their responsibilities within the care delivery process, believing that their work is completed once the message is sent. This may result in inadequate follow‐up of patients after the intervention. Additionally, text messaging cannot capture the verbal and non‐verbal cues that may also influence the interpretation of the message. The psychological and social impacts of using the mobile phone in this way are other key issues.
Having correct patient contact information and securely‐stored health records are essential to meet privacy, confidentiality and data protection requirements. Failures or delays in mobile phone message delivery are rare but possible; however, harm is unlikely as senders are usually notified instantly in cases of a transmission problem. There may be additional monetary and time costs, as backup systems may be needed. Lastly, risks associated with mobile phone messaging in general may apply, for instance increased risk of car accidents as a result of messaging whilst driving.
Why it is important to do this review
Although there is some evidence on the use and effectiveness of mobile phone messaging in healthcare delivery, answers to questions regarding the implementation of these technologies in routine care, such as their impact on patient‐related outcomes or on processes of healthcare delivery, are unclear. Given the topical nature of the subject, we conducted this review to identify answers to these questions and propose directions for future research. This review complements available studies on use of telephone consultations (Car 2003), email (Car 2004; Car 2004b) and personal digital assistants (PDAs) (Baumgart 2005) in health care, and Cochrane reviews by these authors on mobile phone messaging for a range of purposes (Car 2012; Gurol‐Urganci 2012; Vodopivec‐Jamsek 2012).
Objectives
To assess the effects of mobile phone messaging for facilitating self‐management of long‐term illnesses, in terms of impact on health outcomes and patients' capacity to self‐manage their condition. Secondary objectives include assessment of: user evaluation of the intervention; health service utilisation and costs; and possible risks and harms associated with the intervention.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), quasi‐randomised controlled trials (QRCTs), controlled before and after studies (CBAs), and interrupted time series (ITS) with at least three time points before and after the intervention.
We define QRCT as a controlled trial in which the participant allocation is not truly random, such as allocation by date of birth or the order in which participants are included in the study. We included QRCT, CBA and ITS designs because our initial literature searching suggested that only a small number of RCTs on mobile phone messaging interventions exist.
Types of participants
We included all study participants regardless of age, gender and ethnicity, as well as all types and stages of diseases. We included studies in all settings, i.e. primary care settings (services of primary health care), outpatient settings (outpatient clinics), community settings (public health services, anywhere where a person can use a mobile phone) and hospital settings. We did not exclude studies according to the type of healthcare provider (e.g. nurse, doctor, allied staff) involved.
Types of interventions
We included interventions using SMS or MMS to facilitate self‐management of long‐term illnesses, i.e. often slowly‐progressing conditions that affect people's lives over a long period of time. The messaging needed to be between a healthcare provider (either in person or automated) or a 'treatment buddy' (e.g. a lay health worker or peer supporter) and a patient, regardless of who sent the first message. We excluded studies of mobile phone messaging between two healthcare providers.
We excluded studies in which mobile phone messaging was a part of a multifaceted intervention, as it would not be possible to separate the effects of messaging alone.
We aimed to make comparisons between mobile phone messaging and no intervention, as well as with other modes of communication such as face‐to‐face, postal letters, calls to land‐lines or mobile telephones, email or via electronic health records; and if applicable, automated versus personal text messaging.
Types of outcome measures
A number of processes and outcomes may be affected by interventions that aim to enhance and/or facilitate the communication between patients and/or carers, and healthcare providers (individuals or institutions) using mobile phone messaging.
Primary outcomes
Health outcomes as a result of the intervention, including physiological measures, e.g. blood pressure, clinical assessments, biomarker values, self‐reporting of symptom resolution, or quality of life;
Capacity to self‐manage long‐term conditions, including lifestyle modification, understanding of disease, impact on independence and responsibility, self‐esteem and/or creation of a supportive environment;
Secondary outcomes
User (patient, carer or healthcare provider) evaluation of the intervention, including satisfaction, readiness to use, timeliness, availability and/or convenience;
Health service utilisation following the intervention;
Costs (direct and indirect) of the intervention;
User (patient, carer or healthcare provider) perceptions of safety;
Potential harms or adverse effects of the intervention, such as misreading or misinterpretation of data, transmission of inaccurate data, loss of verbal and non‐verbal communication cues, issues of privacy and disclosure, or failure or delay in the message delivery.
Search methods for identification of studies
We used a common search strategy for all four reviews (this review; Car 2012, Gurol‐Urganci 2012; Vodopivec‐Jamsek 2012) and allocated relevant studies to their respective reviews before assessing their risk of bias and extracting data. The search strategies for each database are given in Appendix 1 to Appendix 7.
Electronic searches
We restricted the searches to studies published since 1993 as the first commercial SMS message was sent in December 1992 (Wikipedia 2007). We included LILACS and the African Health Anthology because mobile phone messaging applications are increasingly used in low‐ and middle‐income regions. There were no language restrictions.
One review author (IGU) searched the following electronic databases on October 13, 2008 and updated the search on June 22, 2009:
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 2)
MEDLINE (OvidSP) (1993 to June 22, 2009);
EMBASE (OvidSP) (1993 to June 22, 2009);
PsycINFO (OvidSP) (1993 to June 22, 2009);
CINAHL (EbscoHOST) (1993 to June 22, 2009);
LILACS (1993 to June 22, 2009);
African Health Anthology (1993 to June 22, 2009).
Searching other resources
For grey literature we searched:
Proceedings from AMIA Congresses;
WHO Clinical Trial Search Portal (www.who.int/trialsearch);
Current Controlled Trials (www.controlled‐trials.com);
Dissertation Abstracts International.
We searched the reference lists of included studies to identify additional studies. We contacted study authors for further information on their studies and to enquire whether they were aware of any other published or ongoing studies that would meet our inclusion criteria.
Data collection and analysis
Selection of studies
The selection of studies was done by IGU, TdJ and VVJ. IGU and TdJ independently assessed the relevance of all titles and abstracts identified from the electronic searches. We retrieved full text copies of all articles judged to be potentially relevant from the titles and abstracts. TdJ and VVJ independently assessed these articles for inclusion. IGU checked the final list of included and excluded studies, and any disagreements were resolved by discussion with VVJ, JC, and RA. We also reviewed the reference lists of key publications. Where the description of the intervention was not sufficiently detailed to allow the review authors to judge whether it met the inclusion criteria, we contacted the study authors for further details.
Data extraction and management
We extracted the following data from the included studies, using a modified version of the Cochrane Consumers and Communication Review Group's data extraction template:
General information: title, authors, source, publication status, date published, language, review author information, date reviewed.
Study methods: aims of intervention, aim of study, study design, methods of participant recruitment, inclusion/exclusion criteria, informed consent and ethical approval, funding.
Risk of bias: data depended on the study design (see 'Risk of bias in included studies).
Participants: description, geographic location, setting, number, age, gender, ethnicity, socio‐economic status. If relevant: principal health problem or diagnosis, stage of illness, treatment received.
Providers: description, geographic location, setting, age, gender.
Interventions: description including technical specifications on SMS and handset provider, duration of intervention, purpose of intervention, initiator of intervention, message content, details of control/usual or routine care, co‐interventions.
Outcomes: primary and secondary outcomes as specified above, methods of assessing outcomes, follow up for non‐respondents, timing of outcome assessment, adverse events.
Results: all reported measurements for the primary and secondary outcomes, including multiple timings for measurements, subgroup analyses or results in different measurement scales if applicable.
TdJ and VVJ independently extracted the above data onto a standard form. The forms were then assessed by one review author (IGU) who checked these descriptive data. Any discrepancies between the two data extraction sheets were discussed by two review authors (TdJ and VVJ), and resolved jointly with the two other review authors (IGU and JC). For missing data, we contacted the study authors to obtain the missing information.
Assessment of risk of bias in included studies
We assessed the risk of bias of included studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) which recommends the explicit reporting of sequence generation, allocation concealment, blinding of participants, providers and outcome assessors, incomplete outcome data, selective outcome reporting and other sources of bias for RCTs (see also Ryan 2007).
Had studies using other study designs been identified for inclusion in the review, we would have assessed these using a variation of the above tool.
Two review authors (TdJ and VVJ) independently assessed the risk of bias in the included studies, with any disagreements resolved by discussion and consensus of the team. We used a template to guide the assessment of risk of bias, and judged each domain as 'yes' (indicating a low risk of bias), 'no' (indicating a high risk of bias) or 'unclear' (indicating an uncertain risk of bias).
We have presented the results of the risk of bias assessment in tables, and provided a narrative discussion of risk of bias in individual domains.
Measures of treatment effect
We used risk ratios (RR) as effect measures for dichotomous outcomes and mean differences (MD) for continuous outcomes. RR and MDs have been derived from Manzel‐Haenszel and inverse variance methods respectively. We used a random‐effects model, where possible, to pool the results and reported 95% confidence intervals with all measures of effect.
Unit of analysis issues
We noted the method of randomisation in each included trial, and considered additional issues regarding the assessment of risk of bias of cluster randomised trials as discussed in Chapter 16 of the Cochrane Handbook (Higgins 2011). In the case of repeated measurements, we defined several outcomes based on different periods of follow‐up and performed separate analyses for each outcome. In studies with more than two treatment groups, we made multiple pair‐wise comparisons between all possible pairs of intervention groups.
Dealing with missing data
We contacted the original investigators to request missing data. With incomplete outcome data (such as drop‐outs, loss to follow‐up and withdrawn study participants), we assessed and reported the risk of bias as high risk/unclear/low risk as guided by the Cochrane Handbook (Higgins 2011) and identified the numbers as well as the reasons for incomplete data. As the numbers and reasons for incomplete outcome data in included studies suggested that data were missing at random, we used only available data in the review and did not use imputation methods.
Assessment of heterogeneity
We did not assess heterogeneity due to the small number of studies included.
Assessment of reporting biases
We were unable to assess reporting bias using funnel plots, because of the small number of studies included. Selective outcome reporting was assessed using the Cochrane Risk of Bias assessment tool.
Data synthesis
We conducted a meta‐analysis using Cochrane Review Manager (RevMan) software to calculate an overall effect size for glycaemic control as described in Measures of treatment effect.
For other reported outcomes, due to the heterogeneity in the nature of interventions and outcome measures reported in the studies, it was not appropriate to combine the results of the studies statistically. We present a narrative overview of the findings, including tabular summaries of extracted data. We have structured the narrative primarily according to the intended purpose of the mobile phone messages.
Subgroup analysis and investigation of heterogeneity
We were unable to conduct subgroup analyses by participant age (0 to 18, 18 to 55, over 55) as planned, due to the small number of studies included and the absence of data for subgroups.
Sensitivity analysis
We did not conduct the planned sensitivity analyses due to the small number of studies included. We had aimed to explore the influence of the following factors on effect size:
excluding unpublished studies;
taking account of risk of bias of included studies, as specified above;
excluding any large studies to establish how they impact on the results;
excluding studies using the following filters: criteria used for clinical diagnosis and eligibility for intervention, language of publication, source of funding (industry versus other), country;
the length of the interval between delivery of the intervention and measurement of the effect.
Consumer participation
The draft review was circulated for peer review by consumers in The Cochrane Collaboration. The review received comments from two consumers through the Cochrane Consumers and Communication Review Group's standard editorial process. We also examined whether consumers were involved in the design and implementation of each included study.
Results
Description of studies
Results of the search
Our search (across all four reviews) identified 3937 citations. We excluded 3750 citations that, based on the abstract alone, showed insufficient relevance to the suite of reviews or did not meet the stated study design criteria. After review of the full text of the remaining 187 citations, a further 149 were subsequently rejected from this review for failing to meet the inclusion criteria. In the final selection stage, we excluded 31 of the remaining 38 citations from this review because the interventions were multifaceted interventions (i.e. included technologies or interventions besides the mobile phone messaging intervention), or because the studies lacked a control group (see Characteristics of excluded studies).
Included studies
We included four studies, reported in seven papers, in this review. The papers by Franklin 2003, Franklin 2008 and Waller 2006 provided supplementary information to the paper by Franklin 2006 and describe the same study. It should be noted that these seven included papers were also considered relevant to our complementary review on mobile phone messaging in preventive health care as they describe interventions aimed at secondary or tertiary prevention (Vodopivec‐Jamsek 2012). However, to avoid duplication between the two reviews, these studies are included in this review only. We present key characteristics of the included studies below and in Characteristics of included studies.
Methods
All included studies were randomised controlled trials (RCTs). In three of the studies (Franklin 2006; Hanauer 2009;Ostojic 2005) the unit of randomisation was the individual patient, and in one study a cluster method of randomisation was used, so that all patients recruited by the same investigator were assigned to the same group (Marquez Contreras 2004). We excluded one study arm (Intensive Insulin Therapy + SweetTalk) from Franklin 2006 from our analysis, as the effects of text messaging could not be separated from those of the clinically‐distinct treatment. Study durations were 3 months (Hanauer 2009), 16 weeks (Ostojic 2005), 24 weeks (Marquez Contreras 2004) and 12 months (Franklin 2006). Three studies compared the effects of the text messaging intervention to usual care (Franklin 2006; Marquez Contreras 2004; Ostojic 2005) and one study compared the effects of reminders for self‐monitoring sent by text message to those sent by email (Hanauer 2009).
Participants
Studies included between 16 and 67 participants. They targeted three distinct long‐term illnesses: diabetes (Franklin 2006; Hanauer 2009), hypertension (Marquez Contreras 2004) and asthma (Ostojic 2005). They were set in Scotland, the United States of America, Spain and Croatia, respectively. In all studies participants were ambulatory patients attending primary and/or secondary health facilities. The target group for the intervention varied: the studies on diabetes were targeted to youth and young adults (Franklin 2006; Hanauer 2009); Marquez Contreras 2004 targeted hypertensive patients aged over 18 years; and, though not explicitly targeted to a specific age group, Ostojic 2005 included primarily young adults (mean age 24.6 ± 6.5 years) with a diagnosis of moderate persistent asthma. All four studies included men and women in approximately equal ratios. Franklin 2006 included almost exclusively participants of white ethnicity (97%), living in areas with a below average Carstairs deprivation score (an unweighted combination of four census variables: unemployment, overcrowding, car ownership and social class. Lower scores reflect less deprivation). None of the other studies provided details on any other patient characteristics.
Intervention
Purpose
The purpose of the intervention varied across studies. In two studies patients were sent regular text messages with health information and medication reminders (Franklin 2006; Marquez Contreras 2004). One study involved two‐way communication between patients and an automated system, whereby the system generated reminders for blood glucose monitoring that were sent to the patient at a specified time, either by text message or by email. Patients then returned their blood glucose results to a central system, and in response received automatically generated messages with feedback and, if the blood glucose value was out of the desired range, appropriate care recommendations (Hanauer 2009). In addition, patients could opt to daily receive two random short messages: one with diabetes‐related information and one with unusual fun facts or trivia (unrelated to diabetes). Lastly, Ostojic 2005 involved two‐way communication between patients and healthcare providers whereby patients used text messaging to send daily asthma self‐monitoring results to a central database, and healthcare providers would personally review the results and provide weekly feedback and advice.
Specifications
The text messaging interventions were delivered using different platforms. Marquez Contreras 2004 used a commercial web‐based service (MyAlert, Inc.) to randomly select and send items from a preset list of messages to subscribers. The other studies combined text messaging with purposely‐designed web‐based interfaces to allow analysis and graphical representation of the collected data (Franklin 2006; Ostojic 2005) or to customise the schedule for delivery of the intervention, and view and print data (Hanauer 2009). The software used to support the SweetTalk intervention was developed in close collaboration between users, researchers and software developers in an iterative, user‐centred design (Waller 2006). Collected peak expiratory flow results from asthma patients were analysed with Asthma Center 0.90 software (Polimedika d.o.o., Zagreb, Croatia) (Ostojic 2005).
In three studies patients used their own mobile phones (or those of a partner) to send and receive text messages (Hanauer 2009; Marquez Contreras 2004; Ostojic 2005). In the SweetTalk study (Franklin 2006) all participants were provided a mobile telephone by Orange® for the duration of the study, and given a phone card to the value of 10 UK pounds.
Message content
Only the SweetTalk study reported pilot testing the message content with potential users, and subsequent refinement of the messages (Franklin 2003). It used messages composed in 'textese', a texting language based on abbreviations, slang and phonetic representations commonly used by young people (e.g. 'Don't 4get 2 inject!'). Some of the messages were personalised by tailoring the content to the self‐management goals set by the patients in the diabetes clinic.
Hanauer 2009 and Marquez Contreras 2004 used randomly‐selected standard messages written in conventional language (English and Spanish, respectively). The aim of the messages in Marquez Contreras 2004 was to: provide information on hypertension; promote compliance, good health and dietary habits; and remind patients to take their medication (e.g. 'Try to take your pills exactly as your doctor advised you. This ensures that your treatment will be useful'). Ostojic 2005 does not give any examples of the form and content of reminder messages. Presumably, the messages sent in response to abnormal peak expiratory flow values were personalised.
Timing and frequency
The frequency with which messages were sent and received varied across studies. In Hanauer 2009 participants could set their own schedule for reminders and factoids, and on average received 2.7 messages per day. Asthma patients in Ostojic 2005 were encouraged to submit their daily peak expiratory flow results and received weekly feedback. On average these patients submitted two messages per day and received 1.2 responses per week. The SweetTalk system automatically sent each patient one message daily and generated additional weekly messages to remind participants of their self‐management goals (Franklin 2006). Marquez Contreras 2004 had the lowest frequency of contact as participants received text message reminders no more than twice per week.
Treatment
In addition to the text‐messaging interventions, patients in all studies continued to receive clinical care for their condition. In the SweetTalk study all patients in the included study arms continued with conventional diabetes care delivered by a multidisciplinary team, including routine clinic visits and access to an emergency hotline. All patients enrolled in Hanauer 2009 had access to the Computerized Automated Reminder Diabetes System (CARDS) to support their diabetes management, and were under the care of the diabetes centre in which the study was conducted. Hypertensive patients in Marquez Contreras 2004 whose hypertension was not well controlled with monotherapy were started on a combination of a single‐dose angiotensin II antagonist and a diuretic. Both groups of asthma patients in Ostojic 2005 were treated according to the guidelines of the Global Initiative for Asthma (GINA) and all kept paper asthma diaries.
Outcomes
Outcome measures reported include health outcomes (physiological measures), capacity to self‐manage the condition, user evaluation of the intervention, health service utilisation and costs (direct and indirect) of intervention.
Excluded studies
After review of the full text of the articles, we excluded 31 citations describing 20 individual studies from this review due to possible confounding or lack of appropriate controls (see Characteristics of excluded studies). In 12 studies additional means of data transmission, such as World Wide Web (www), Wireless Application Protocol (WAP) or General Packet Radio Service (GPRS), were used such that the independent effects of text messaging could not be separated from those of the overall intervention (e.g. these were multifaceted interventions); 7 studies had no controls; 1 study did not report any outcome measures after implementation of the intervention.
Ongoing studies
We identified eight trials that may be relevant to this review but for which no data were available at the time of conducting this review (see Characteristics of ongoing studies). Of these, four trials were still recruiting participants at the time of this review (Jackson 2006; Liang 2009, Maurino 2009; Shetty 2008); one was ongoing (Shotan 2006); and three had already been completed but results had not yet been published (Møldrup 2007; Rodríguez‐Idígoras 2003; van Schayk 2005). These eight trials cover a somewhat larger set of conditions than those included in our review to date:
| Ongoing study | Participants |
| Jackson 2006; Møldrup 2007 | People with asthma |
| Liang 2009 | Teenagers with depressive disorder |
| Maurino 2009; van Schayk 2005 | Patients undergoing treatment for schizophrenia |
| Rodríguez‐Idígoras 2003; Shetty 2008 | Type 2 diabetics |
| Shotan 2006 | Patients started on statin treatment after an acute coronary event |
We successfully contacted four of the trial coordinators for additional information (Maurino 2009, Shotan 2006, Rodríguez‐Idígoras 2003, Shetty 2008). The completed study Rodríguez‐Idígoras 2003 included a total of 328 participants. The findings were at the time being written up for publication. The same research group is also in the process of conducting a similar trial in children with type 1 diabetes. Shotan 2006 recruited a total of 120 participants. We did not receive any preliminary findings from the contacted trial coordinators.
These trials will be assessed for inclusion in a future update of this review.
Risk of bias in included studies
Risk of bias in the included studies is summarised in Figure 1 and reported in the table Characteristics of included studies.
1.
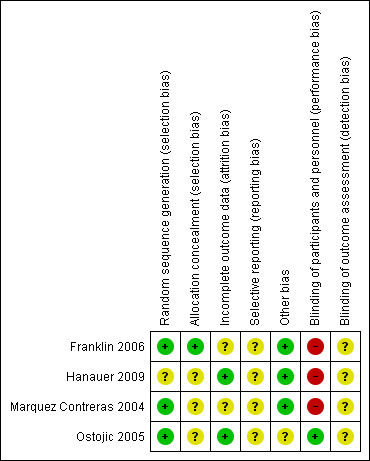
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Three included studies reported the use of adequate sequence generation methods (computer generated random allocation sequences or random number tables); one study did not specify the method of randomisation (Hanauer 2009). In all studies it was unclear whether allocation was concealed. Only one study explicitly addressed the lack of blinding of patients (Ostojic 2005). Though not stated in any of the other studies, we assume that in none of them did blinding of patients, healthcare providers or outcome assessors take place. Because we were not able to review the original study protocols, fully‐informed inferences on potential selective reporting cannot be made. Intervention and control groups were sufficiently comparable in all studies.
Only one of the included studies stated that analysis was done in accordance with the intention‐to‐treat (ITT) principle (Franklin 2006). In this case ITT was interpreted to mean that participants were compared in the groups to which they were originally randomly assigned, regardless of whether they actually received some or all of the intervention. However, patients who withdrew from the study before baseline data were collected or who moved away from the study area were subsequently removed from the analysis. Hanauer 2009 presented data only for participants who had actively engaged with the intervention. In Marquez Contreras 2004 final analysis was performed on only those patients who had received the intervention and for whom all records were available. In Ostojic 2005 all those who were randomised completed the study.
We identified no other potential sources of bias.
Effects of interventions
See: Table 1
We chose to group all outcomes reported in the studies into categories, and each category is reported in a separate table (see Data and analyses). The primary outcomes were grouped as health outcomes (physiological measures) (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4), and effects on capacity to self‐manage the condition (Analysis 2.1; Analysis 2.2) (Table 1). The secondary outcomes were grouped as patients' evaluation of the intervention, and healthcare utilisation (Analysis 3.2; Analysis 3.1) and costs (Table 1). None of the studies reported providers' evaluation of the intervention or perceptions of the intervention's safety. Due to the heterogeneity across studies in clinical disorders, type of interventions, and outcome measures it was rarely possible to quantify differences between groups or to calculate effect sizes across studies.
1.1. Analysis.
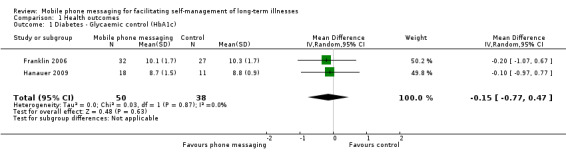
Comparison 1 Health outcomes, Outcome 1 Diabetes ‐ Glycaemic control (HbA1c).
1.2. Analysis.
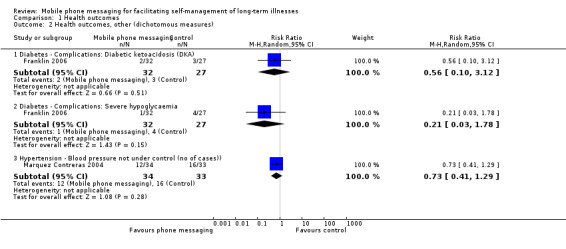
Comparison 1 Health outcomes, Outcome 2 Health outcomes, other (dichotomous measures).
1.3. Analysis.
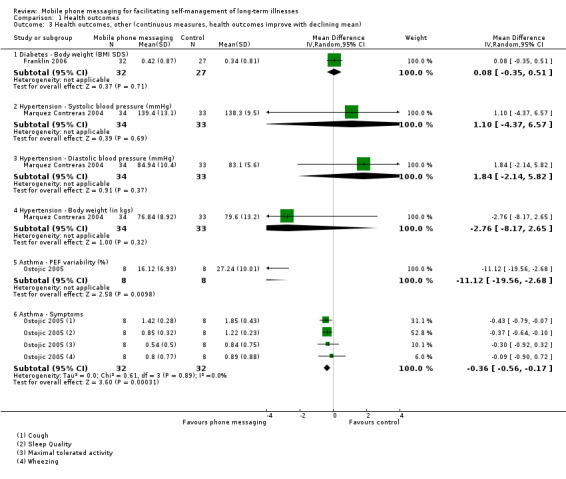
Comparison 1 Health outcomes, Outcome 3 Health outcomes, other (continuous measures, health outcomes improve with declining mean).
1.4. Analysis.
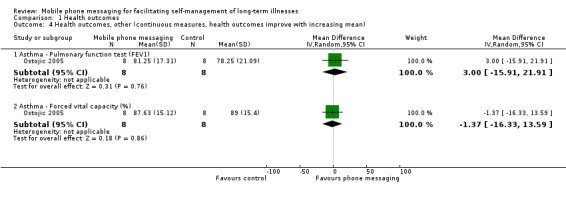
Comparison 1 Health outcomes, Outcome 4 Health outcomes, other (continuous measures, health outcomes improve with increasing mean).
2.1. Analysis.
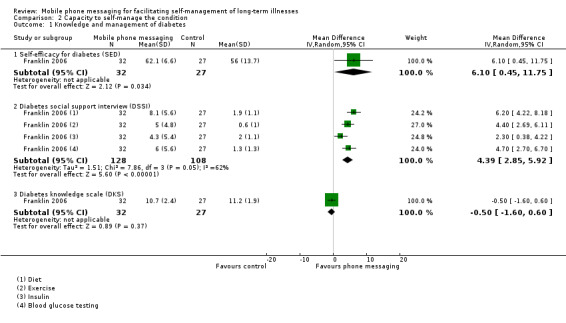
Comparison 2 Capacity to self‐manage the condition, Outcome 1 Knowledge and management of diabetes.
2.2. Analysis.
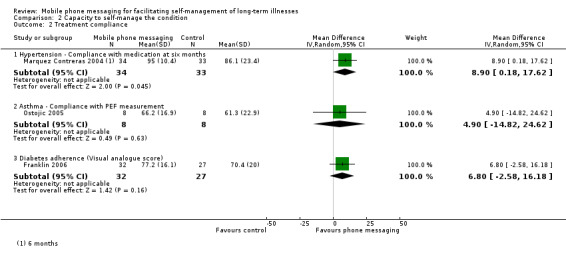
Comparison 2 Capacity to self‐manage the condition, Outcome 2 Treatment compliance.
3.2. Analysis.
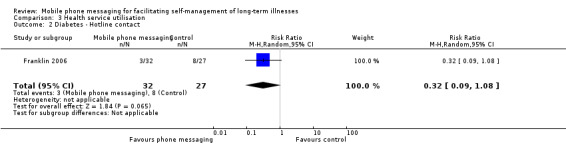
Comparison 3 Health service utilisation, Outcome 2 Diabetes ‐ Hotline contact.
3.1. Analysis.
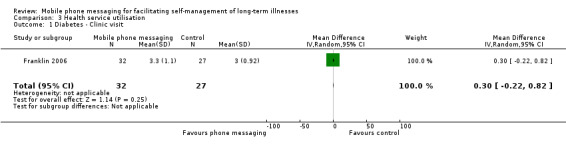
Comparison 3 Health service utilisation, Outcome 1 Diabetes ‐ Clinic visit.
Health outcomes
Diabetes
Improved glycaemic control (measured as per cent glycosylated haemoglobin (HbA1c)) by supporting the self‐management of diabetes was analysed in two studies (Franklin 2006. Hanauer 2009). No significant difference in glycaemic control was found, however, between the intervention and control groups (MD ‐0.15, 95% CI ‐0.77 to 0.47) (Analysis 1.1).
Severe diabetic complications were rare in the one year follow‐up period after the SweetTalk intervention (Franklin 2006), and there were no statistical differences in the occurrence of complications between the control and intervention groups (diabetic ketoacidosis (RR 0.56, 95% CI 0.10 to 3.12); severe hypoglycaemia (RR 0.21, 95% CI 0.03 to 1.78)). Changes in body weight as measured by body mass index standard deviation scores were also not different across the groups (MD 0.08, 95% CI ‐0.35 to 0.51; Analysis 1.3). Because of the small study size of both studies, the quality of the evidence on any of these health outcome measures was considered moderate (Table 1).
Hypertension
Marquez Contreras 2004 compared systolic and diastolic blood pressure in groups of patients with and without text message support, at baseline and 1, 3 and 6 months after initiation of the study. Blood pressure levels at 6 months were comparable in the two groups (systolic blood pressure MD 1.10, 95% CI ‐4.37 to 6.57); diastolic blood pressure MD 1.84, 95% CI ‐2.14 to 5.82; Analysis 1.3). Achievement of good blood pressure control (as defined by having blood pressure less than 140/90 mm Hg in patients without diabetes and 130/85 mm Hg in patients with diabetes) at the end of the study was not statistically different between the control and intervention groups (RR of not achieving blood pressure control 0.73, 95% CI 0.41 to 1.29; Analysis 1.2). The body weight of the participants at 6 months was also comparable between groups (MD ‐ 2.76 (95% CI ‐8.17 to 2.65; Analysis 1.3). As before, however, for all of the reported outcome measures the evidence is considered to be of moderate quality only, due to the small number of patients enrolled in the trial. The observed effect sizes are likely to be affected by further research (Table 1).
Asthma
The aim of Ostojic 2005 was improved asthma control through self‐monitoring and regular personalised feedback. Health measures associated with improved asthma control include pulmonary function test results and occurrence of asthma‐related symptoms. Compared to patients receiving routine care only, patients in the intervention group did not show any improvements in forced expiratory volume in 1 second (FEV1) (MD 3.00, 95% CI ‐15.91 to 21.91) or forced vital capacity (FVC) (MD ‐1.37, 95% CI ‐16.33 to 13.59; Analysis 1.4), but displayed significantly improved (i.e. decreased) peak expiratory flow (PEF) variability (MD ‐11.12, 95% CI ‐19.56 to ‐2.68) (Analysis 1.3). Cough and sleep quality were improved in the study group (P < 0.05) whereas wheezing and maximum tolerated activity showed little to no difference between groups (Analysis 1.3). There was a significant difference between intervention and control groups in the pooled asthma symptom score, favouring the intervention group (MD ‐0.36, 95% CI ‐0.56 to ‐0.17; Analysis 1.3). However, the extremely small sample size of the study means that the quality of the evidence for any of these measures is considered low (Table 1).
Capacity to self‐manage the long‐term illness
Diabetes
The primary aims of Franklin 2006 and Hanauer 2009 was to improve the self‐efficacy of patients with diabetes. Franklin 2006 reported a number of measures relating to patients' ability to monitor and respond to their condition (Analysis 2.1). Compared with the conventional insulin therapy control group, patients additionally receiving SweetTalk showed significantly improved scores on the Self‐Efficacy for Diabetes scale (MD 6.10, 95% CI 0.45 to 11.75). The Diabetes Social Support Interview scores, furthermore, demonstrated SweetTalk had improved patients' perceptions of the quantity of support received on all four self‐management tasks. The overall improvement in the four items of the Diabetes Social Support Interview for the intervention group was higher than for the control group (MD 4.39, 95% CI 2.85 to 5.92). It did not, however, result in improved knowledge of diabetes (MD ‐0.50, 95% CI ‐1.60 to 0.60; Analysis 2.1) or self‐reported adherence (MD 6.80, 95% CI ‐2.58 to 16.18; Analysis 2.2). Hanauer 2009 showed that text message prompts initially resulted in a higher number of blood glucose results sent back (46.0) than email prompts (23.5) did (Table 1).
Hypertension
Reminder messages in the study by Marquez Contreras 2004 were designed to promote improved compliance with drug therapy. The results showed a marginally significant increase in rate of compliance of the intervention group at six months (MD 8.90, 95% CI 0.18 to 17.62) (Analysis 2.2; Table 1).
Asthma
The use of text messaging for transmitting pulmonary function test results did not result in improved compliance with PEF measurement (MD 4.90, 95% CI ‐14.82 to 24.62; Analysis 2.2).
For any of the discussed conditions, the quality of the evidence for impact on the capacity to self‐manage the condition ranges from low to, at best, moderate, because of the small number of participants in the included trials (Table 1).
Participants' evaluation of the intervention
The studies primarily focused on the health and self‐management effects of the interventions, with little to no discussion of patient satisfaction or acceptability of the intervention. Franklin 2006 was the only study to explicitly address these issues. The SweetTalk system was developed in a participatory fashion, involving users, researchers and software developers. Before the trial the system had undergone several rounds of iterative refinement and validation (Franklin 2003, Waller 2006). A user satisfaction survey conducted at the end of the trial (analysing all three arms) showed that 81% of patients felt the system had improved their diabetes self‐management and 90% wanted to continue receiving messages (Table 1). Of the patients in the intervention group, 97% were happy with the frequency of receiving messages, but 20% complained about receiving the same message repeatedly. Subsequent content analysis of the messages suggested that patients generally valued the opportunity to engage in reciprocal communication (Franklin 2008). Hanauer 2009 provided a less explicit evaluation of the acceptability of text messaging to support self‐management of long‐term illnesses by demonstrating that, after an initial period of more frequent use, the number of blood glucose monitoring results submitted by patients declined sharply over the 3‐month duration of the study. Nonetheless, when asked how they would prefer to access the system in future, more people (50%) chose cell phone reminders over email (17%). In fact, two‐ thirds (12 of 18) of the participants assigned to the e‐mail group commented that they would have preferred cell phone reminders.
Health service utilisation and costs (direct and indirect)
Two of the included studies investigated the impact on health services utilisation. Patients on conventional insulin therapy receiving SweetTalk support made a comparable number of clinic visits (MD 0.30, 95% CI ‐0.22 to 0.82) (Analysis 3.1) and calls to an emergency hotline (RR 0.32, 95% CI 0.09 to 1.08) as patients without the support (Franklin 2006). For asthma patients, the total number of office visits was higher in the intervention group (21 versus 15 visits), whereas the number of hospital admissions was higher for the control group (2 versus 7 admissions) (Ostojic 2005; Analysis 3.3). The particulars of hospital admissions in this study, however, are not known, so no unambiguous conclusions can be drawn about the intervention's impact on asthma‐related hospitalisation.
3.3. Analysis.
Comparison 3 Health service utilisation, Outcome 3 Asthma ‐ Utilisation.
| Asthma ‐ Utilisation | |||
|---|---|---|---|
| Study | Outcome | Mobile phone (n=8) | Control (n=8) |
| Ostojic 2005 | Hospitalisations | 2 | 7 |
| Ostojic 2005 | Office visits | 21 | 15 |
In addition to potential impacts on health services utilisation, Ostojic 2005 considered the direct costs of the text messaging intervention for patients and providers. The additional cost in the intervention group of follow‐up per patient, per week, amounted to (Euro) €0.67 to the patient and €1.00 to the physician. Per week patients spent an additional 11.5 minutes on data transmission. Costs to the patient in the study by Marquez Contreras 2004 were covered through sponsorship and receipt of messages was free.
Discussion
Summary of main results
This review draws together the evidence for delivering health interventions that focus on management of long‐term conditions by mobile phone messaging. The results of our review show that interventions delivered through mobile phone messaging had few direct impacts on health outcomes related to the management of long‐term conditions. Studies on diabetes and hypertension did not demonstrate a significant impact from text messaging on health indicators. Only the study on asthma management demonstrated some potential to improve the health condition of patients (Ostojic 2005). In this study text messaging was used as a means to establish interactive 2‐way communication between patients and their physicians, rather than as a 1‐way channel for relaying health education or support messages, as was the case in the other studies. Although at present we have insufficient evidence to further explore this hypothesis, one possible explanation could be that the method of initiation of the interaction (e.g. patient‐ versus provider‐initiated) and the intensity of the communication are contributing factors to the success of text messaging in healthcare delivery. It should also be recognised that the purpose of these text message interventions was not so much to directly affect health status as to achieve behavioural change and improve self‐efficacy. The causal relationship between self‐management capacity and health status may not always be clearly established and, furthermore, the evaluation period for the included studies may have been insufficient to capture causal effects.
The evidence on the effects of text messaging for promoting patients' self‐management of their condition appears to be mixed. In one study, diabetes patients reported that text messaging gave them greater capacity to manage their illness (Franklin 2006). These patients also demonstrated somewhat better therapy adherence compared to patients without text‐message based support. In contrast, studies involving hypertension and asthma patients did not show any effect from text message reminders on rates of compliance with medication or self‐monitoring (Ostojic 2005, Marquez Contreras 2004). These results show that, although in some cases text messaging can be of some use in supporting self‐management, more research is needed into the mechanisms underpinning these effects, and the role of factors such as message content and frequency.
Our review found very little evidence on the acceptability of text messaging in supporting self‐management of long‐term conditions to patients or their care providers. In one study the majority of participants expressed their readiness to continue using the intervention (Franklin 2008). Another study, however, suggested that over time interest in this type of support gradually decreases (Hanauer 2009). Evidently, the short‐ and long‐term acceptability of text messaging in disease self‐management is an area that requires further attention.
Little attention has also been paid to direct and indirect costs associated with text messaging in self‐management of long‐term conditions. Although our review found that, whilst text messaging support may have some potential for reducing severe adverse events leading to hospitalisation, the overall effect on health service utilisation remains unclear. Only one of the included studies attempted to quantify the direct costs to all users and considered it affordable (Ostojic 2005). It should, however, be recognised that such costs may be highly dependent on the nature of the intervention and the size and characteristics of the target group.
Overall completeness and applicability of evidence
We systematically collected and analysed the evidence on the effects of mobile phone messaging in supporting self‐management of long‐term illnesses. However, a number of limitations should be taken into account. Firstly, we have deliberately taken a rather narrow focus: including only those studies in which the intervention is delivered exclusively through text messaging. We excluded studies in which text messages were combined with other forms of data transmission, such as email, Internet or GPRS, as it would be difficult to assess the independent effect of a text message within such complex interventions. This strategy restricted the body of evidence that we were able to build on as we found that many studies in the area of mHealth have relied on multi‐faceted interventions in which text messaging was combined with other technologies. Our review thus contains only a relatively small number of studies, none of which has a sample size larger than 67 participants and one of which had only 16 participants. Combined with the substantial heterogeneity in the selected studies, it is very difficult to assess to what extent our findings have more general relevance. Secondly, the fact that no data have been collected beyond a study period of 12 months means that no conclusions can be drawn about the long‐term effects of text messaging in supporting the self‐management for conditions that are characterised by their protracted, often life‐long, duration. Studies with longer periods of follow‐up are needed.
One other important limitation of this review is that all of the included studies were set in high‐income countries where mobile phone ownership is widespread and data transmission reliable. Although mobile phone ownership and network coverage are on the increase in most parts of the world, particular attention should be given to the suitability of mobile phone based applications in low‐income settings. None of the studies evaluated potential complications from text messaging such as loss or misinterpretation of data. No consideration was given to issues of security and confidentiality. Particularly in low‐income countries where mobile phones are frequently shared between family members, these are important issues that need to be taken into account.
Quality of the evidence
The included studies were of varying methodological quality with most studies providing insufficient information to accurately assess the risk of bias. On the whole, sequence generation for randomisation was considered adequate, but in none of the studies was it clear if, and how, the allocation was concealed. The lack of blinding in all studies can be partly explained by the interactive nature of the interventions, which does not permit the blinding of patients or their healthcare providers. There is, however, a potential for bias from the apparent lack of blinding of outcome assessors.
The individual studies examined a wide variety of outcomes. Health outcomes and measures of the capacity to self‐manage long‐term conditions were mostly evaluated with formally‐validated measures. Nonetheless, the heterogeneity in the outcome measures makes it difficult to draw unambiguous conclusions on the effects of text messaging. Assessment of patient satisfaction or acceptability of the intervention relied on less defined measures, and the generalisability of these findings is questionable. Because of the limited number of studies included in this review and the relatively small number of participants in each of the studies, the quality of the overall evidence is moderate at best, and strong conclusions on the effectiveness of text messaging in supporting self‐management of long‐term conditions cannot be drawn. However, despite these limitations this review provides a useful overview and has exposed important gaps in the current knowledge in this area which merit further research.
Potential biases in the review process
We believe that we have identified all the studies concerning the use of mobile phone messaging to support the self‐management of long‐term and chronic conditions that met our study design criteria (RCT, CBA, ITT) to June 2009. We also successfully contacted four trial coordinators to obtain additional information regarding ongoing trials. However, by excluding studies with possible confounding from other communication and/or data transmission methods, we may have introduced selection bias towards less successful interventions, as there is a likelihood that more complex interventions are more successful at facilitating self‐management.
Agreements and disagreements with other studies or reviews
This review comes in the wake of two other reviews that have a similar focus. Fjeldsoe 2009 reviewed the evidence for behaviour change interventions delivered by text messaging, whereas Krishna 2009 looked more broadly at healthcare delivery via mobile phones in the management and prevention of disease. Both of these reviews feature three out of the four studies we have included, with only Hanauer 2009 being a more recent addition. However, our review differs from these two reviews in several respects. Firstly, Krishna 2009 focuses on all possible fields of application for mobile phones in disease management and prevention rather than on their utility in supporting self‐management of long‐term disease alone. Their conclusions are thus based on an even more heterogeneous set of studies, further complicating the process of deriving robust conclusions. Secondly, the review was not restricted to text messaging applications alone, but also included interventions whereby mobile phones were used for regular phone calls or for data transmission by GPRS (for example to transmit data received from a wireless device), thus including a number of studies which we have excluded from our analysis to minimise possible confounding. Interestingly, Krishna 2009 is overall more positive regarding the impact of mobile phones on health outcomes, compliance with medication and self‐efficacy. A possible explanation could be that interventions that employ a more extensive set of technologies in the communication between patients and healthcare providers are better tailored to patients' needs and preferences than those that exclusively rely on text messaging, thus leading to better outcomes.
The focus of Fjeldsoe 2009 is somewhat closer to that of our review, as it looks specifically at behaviour change interventions, evaluating changes in both preventive health behaviour and behaviour associated with the management of clinical conditions. The review, however, used somewhat less stringent selection criteria, and included studies without a control group. Furthermore, although text messaging had to be the main method of intervention delivery, the review also considered studies in which other technologies were used as adjuncts. Despite the wider scope of the review and the inclusion of less rigorously conducted studies, the conclusions of Fjeldsoe 2009 ‐ that text message‐based interventions can have some positive short‐term behavioural outcomes but that further research into long‐term effects and acceptability is required ‐ are largely in line with those of our review.
Authors' conclusions
Implications for practice.
Although this review found that, in certain cases, mobile phone messaging applications may support the self‐management of long‐term conditions, the evidence base for the implementation of this technology is currently very limited at best. Furthermore, very little is known about long‐term effectiveness, risks and limitations, and consumer satisfaction with the intervention. At least in developed countries, the rapid rise of so‐called 'smartphones' (that is, phones that can connect to the Internet) could mean that simple mobile phone messaging interventions such as those reviewed here will be replaced with more complex interventions that use a combination of web‐based and mobile phone messaging technologies.
Implications for research.
Although mobile phone messaging health interventions have been the subject of many studies, few of these meet the high standards for evidence associated with RCTs. The evidence of this review is based on just four RCTs. However, we also found numerous pilot studies that did not meet our inclusion criteria. Researchers should focus more on validating their findings from such pilot studies through follow‐up studies with adequate research designs (e.g. RCTs, QRCTs, CBAs) and including appropriate controls. These studies should include not only assessment of health outcomes and measures of self‐efficacy but also pay attention to issues of risk and acceptability of the intervention. For interventions that are designed to support the self‐management of long‐term illnesses in particular, more attention should also be paid to their effects over longer periods of time.
Acknowledgements
We are very grateful for the support provided by the Cochrane Consumers and Communication Review Group editorial base, in particular by Sophie Hill and Megan Prictor.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
cellular phone/
text messag$.ab,ti.
texting.ab,ti.
short messag$.ab,ti.
sms.ab,ti.
(multimedia messag$ or multi‐media messag$).ab,ti.
mms.ab,ti.
((cellular phone$ or cell phone$ or mobile phone$) and (messag$ or text$)).ab,ti.
or/1‐8
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized controlled trials.sh.
random allocation.sh.
double blind method.sh.
single blind method.sh.
or/10‐15
animals/ not (human/ and animals/)
16 not 17
clinical trial.pt.
exp clinical trials/
(clin$ adj25 trial$).ti,ab.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab.
placebos.sh.
placebo$.ti,ab.
random$.ti,ab.
research design.sh.
or/19‐26
27 not 17
18 or 28
exp evaluation studies/
follow up studies/
prospective studies/
(control$ or prospectiv$ or volunteer$).tw.
cross over studies/
comparative study/
or/30‐35
experiment$.tw.
(time adj series).tw.
(pre test or pretest or (posttest or post test)).tw.
(pre intervention or preintervention or (post intervention or postintervention)).tw.
(impact$ or intervention$ or chang$ or outcome$).tw.
effect$.tw.
or/37‐42
36 and 43
animals/ not (human/ and animals/
44 not 45
29 or 46
47 and 9
limit 48 to yr="1993 ‐ 2008"
Appendix 2. EMBASE (Ovid) search strategy
mobile phone/
wireless communication/
(cellular phone* or cellular telephon* or cell phone* or mobile phone* or mobile telephon* or wireless phone* or wireless telephon*).ti.
1 or 2 or 3
limit 4 to abstracts
(cellular phone* or cellular telephon* or cell phone* or mobile phone* or mobile telephon* or wireless phone* or wireless telephon*).tw.
(text* or messag* or multimedia or multi‐media or imag* or data or input* or sms or mms).tw.
(5 or 6) and 7
4 not 5
(text messag* or texting or texted).tw.
(short messag* or (sms not (somatostatin* or sphingomyelin*))).tw.
(multimedia messag* or multi‐media messag*).tw.
(mms and (multimedia or multi‐media)).tw.
or/8‐13
Randomized Controlled Trial/
random*.tw.
experiment*.tw.
time series.tw.
(pre test or pretest or post test or posttest).tw.
impact.tw.
intervention*.tw.
chang*.tw.
evaluat*.tw.
effect?.tw.
compar*.tw.
control*.tw.
or/15‐26
nonhuman/
27 not 28
14 and 29
limit 30 to yr="1993‐2009"
Appendix 3. PsycINFO (Ovid) search strategy
(cellular phone* or cellular telephon* or cell phone* or mobile phone* or mobile telephon* or wireless phone* or wireless telephon*).tw.
(text* or messag* or multimedia or multi‐media or imag* or data or input* or sms or mms).tw.
1 and 2
(text messag* or texting or texted).tw.
(short messag* or sms).tw.
(multimedia messag* or multi‐media messag*).tw.
(mms and (multimedia or multi‐media)).tw.
or/3‐7
random*.tw.
experiment*.tw.
trial.tw.
placebo.ab.
groups.ab.
((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).tw.
time series.tw.
time series/
(pre test or pretest or post test or posttest).tw.
(pre intervention or preintervention or post intervention or postintervention).tw.
(cross over or crossover).tw.
latin square.tw.
(prospective* or volunteer*).tw.
impact.tw.
intervention*.tw.
chang*.tw.
evaluat*.tw.
effect?.tw.
compar*.tw.
control*.tw.
treatment effectiveness evaluation/
mental health program evaluation/
exp experimental design/
or/9‐31
limit 32 to human
limit 33 to yr="1993‐2008"
(health* or medic* or telemedic* or patient* or illness* or therap* or psychiatr* or nurs* or remind* or consult*).tw.
("27" or "32" or "33" or "34").cc.
35 or 36
8 and 34
38 and 37
Appendix 4. CENTRAL search strategy
#1 "cellular phone":kw or "mobile phone":kw or ((text next messag*) or texting or texted or (short next messag*) or (sms not (somatostatin* or sphingomyelin*)) or (multimedia next messag*) or (multi‐media next messag*) or (mms and (multimedia or multi‐media)) or (cellular next phone*) or (cellular next telephon*) or (cell next phone*) or (mobile next phone*) or (mobile next telephon*) or (wireless next phone*) or (wireless next telephon*)):ti,ab in Clinical Trials
#2 human*:kw in Clinical Trials
#3 #1 and #2
Appendix 5. CINAHL (EBSCO) search strategy
| S15 | s14 |
| S14 | S10 or S13 |
| S13 | s11 and s12 |
| S12 | PT Research |
| S11 | S3 not S10 |
| S10 | s3 and s9 |
| S9 | S4 or S5 or S6 or S7 or S8 |
| S8 |
pre test or pretest or post test or posttest or pre intervention or preintervention or post intervention or postintervention or time series |
| S7 |
TI ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)) or AB ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)) |
| S6 |
random* or trial or groups or placebo* or experiment* or control* or compar* or intervention* or chang* or evaluat* or impact* or effect? |
| S5 | PT Clinical Trial |
| S4 |
MH Experimental Studies+ or MH Random Assignment or MH Comparative Studies or MH Comparative Studies or MH Crossover Design or MH Placebos or MH Quantitative Studies or MH Quasi‐Experimental Studies+ |
| S3 | S1 or S2 |
| S2 |
cellular phone* or cellular telephon* or cell phone* or mobile phone* or mobile telephon* or wireless phone* or wireless telephon* or text messag* or texting or texted or short messag* or sms or multimedia messag* or multi‐media messag* or (mms and (phone* or telephon* or multimedia or multi‐media or messag*)) |
| S1 | MH Wireless Communications |
Appendix 6. African Health Anthology search strategy
1 ‐ Query 1:
| KEY WORDS/PHRASES |
RANDOM* OR TRIAL* OR CONTROL* OR PROSPECTIV* OR VOLUNTEER* OR EXPERIMENT* OR TIME SERIES OR PRE TEST OR PRETEST OR POST TEST OR POSTTEST OR PRE INTERVENTION OR PREINTERVENTION OR POST INTERVENTION OR POSTINTERVENTION OR IMPACT* OR INTERVENTION* OR CHANG* OR EFFECT* |
| TITLE | PLACEBO OR GROUPS |
| INDEX TERMS | RESEARCH DESIGN OR FOLLOW UP STUDIES OR PROSPECTIVE STUDIES OR CROSS OVER STUDIES OR DRUG THERAPY |
2 ‐ Query 2:
| KEY WORDS/PHRASES |
((TEXT* OR MESSAG* OR MULTIMEDIA OR MULTI‐MEDIA OR IMAG* OR DATA OR INPUT* OR SMS OR MMS) AND (CELLULAR PHONE* OR CELLULAR TELEPHON* OR CELL PHONE* OR MOBILE PHONE* OR MOBILE TELEPHON* OR WIRELESS PHONE* OR WIRELESS TELEPHON*)) OR TEXT MESSAG* OR TEXTING OR TEXTED OR SHORT MESSAG* OR (SMS NOT (SOMATOSTATIN* OR SPHINGOMYELIN*)) OR MULTIMEDIA MESSAG* OR MULTI‐MEDIA MESSAG* OR (MMS AND (MULTIMEDIA OR MULTI MEDIA)) |
| TITLE |
CELLULAR PHONE* OR CELLULAR TELEPHON* OR CELL PHONE* OR MOBILE PHONE* OR MOBILE TELEPHON* OR WIRELESS PHONE* OR WIRELESS TELEPHON* |
| INDEX TERMS | CELLULAR PHONE |
3 ‐ Query 1 and Query 2.
Appendix 7. Search Strategy for LILACS, trial portals and grey literature
cellular phone OR mobile phone OR cellular telephone* OR mobile telephone* OR text messag* OR
texting OR texted OR short messag* OR multimedia messag* OR sms OR mms
Bottom of Form
Data and analyses
Comparison 1. Health outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diabetes ‐ Glycaemic control (HbA1c) | 2 | 88 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.77, 0.47] |
| 2 Health outcomes, other (dichotomous measures) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Diabetes ‐ Complications: Diabetic ketoacidosis (DKA) | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.10, 3.12] |
| 2.2 Diabetes ‐ Complications: Severe hypoglycaemia | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.03, 1.78] |
| 2.3 Hypertension ‐ Blood pressure not under control (no of cases)) | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.41, 1.29] |
| 3 Health outcomes, other (continuous measures, health outcomes improve with declining mean) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Diabetes ‐ Body weight (BMI SDS) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.35, 0.51] |
| 3.2 Hypertension ‐ Systolic blood pressure (mmHg) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐4.37, 6.57] |
| 3.3 Hypertension ‐ Diastolic blood pressure (mmHg) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 1.84 [‐2.14, 5.82] |
| 3.4 Hypertension ‐ Body weight (in kgs) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐2.76 [‐8.17, 2.65] |
| 3.5 Asthma ‐ PEF variability (%) | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐11.12 [‐19.56, ‐2.68] |
| 3.6 Asthma ‐ Symptoms | 1 | 64 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.56, ‐0.17] |
| 4 Health outcomes, other (continuous measures, health outcomes improve with increasing mean) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Asthma ‐ Pulmonary function test (FEV1) | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 3.00 [‐15.91, 21.91] |
| 4.2 Asthma ‐ Forced vital capacity (%) | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐1.37 [‐16.33, 13.59] |
Comparison 2. Capacity to self‐manage the condition.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Knowledge and management of diabetes | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Self‐efficacy for diabetes (SED) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 6.10 [0.45, 11.75] |
| 1.2 Diabetes social support interview (DSSI) | 1 | 236 | Mean Difference (IV, Random, 95% CI) | 4.39 [2.85, 5.92] |
| 1.3 Diabetes knowledge scale (DKS) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.60, 0.60] |
| 2 Treatment compliance | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Hypertension ‐ Compliance with medication at six months | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 8.90 [0.18, 17.62] |
| 2.2 Asthma ‐ Compliance with PEF measurement | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 4.90 [‐14.82, 24.62] |
| 2.3 Diabetes adherence (Visual analogue score) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 6.80 [‐2.58, 16.18] |
Comparison 3. Health service utilisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diabetes ‐ Clinic visit | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.22, 0.82] |
| 2 Diabetes ‐ Hotline contact | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.09, 1.08] |
| 3 Asthma ‐ Utilisation | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Franklin 2006.
| Methods | RCT (3 arms, study duration 12 months) | |
| Participants | Paediatric patients (aged 8 to 18 years) with Type 1 Diabetes Mellitus receiving conventional insulin therapy attending a clinic in Tayside, Scotland. A total of 92 patients were randomised, of which 89 received their allocated interventions and data were analysed for 90 patients (Group 1 n = 27; Group 2 n = 32; Group 3 n = 31). | |
| Interventions | Participants were randomly assigned to one of three groups: 1) Conventional Insulin Therapy (CIT); 2) CIT with SweetTalk intervention; or 3) Intensive Insulin Therapy (IIT) with SweetTalk intervention. We excluded the third arm from this review. Sweet Talk is an automated, scheduled text‐messaging system designed to offer regular support to patients with diabetes to optimise their self‐management and diabetes control. Patients contract personal diabetes self‐management goals during the diabetes consultation and, based on these goals and patients' age, sex and diabetes regimen, SweetTalk schedules the automated delivery of a series of appropriately‐tailored text messages, including a weekly reminder of the goal set in clinic, and a daily message providing tips, information or reminders to reinforce this goal. In addition, patients receive occasional text newsletters regarding topical diabetes issues. |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
Outcome measures were determined at baseline and at the end of the study (12 months). |
|
| Notes | Mobile phones and ongoing technical support for the study were provided by Orange. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated allocation sequence was used to assign participants to one of three groups. |
| Allocation concealment (selection bias) | Low risk | Allocation is said to have been concealed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | At the end of the study (12 months) 4/27 patients were missing from Group 1 (3 discontinued therapy for clinical reasons, 1 withdrew), 6/33 missing from Group 2 (5 discontinued therapy for clinical reasons, 1 moved away), and 5/29 missing from Group 3 (5 discontinued therapy for clinical reasons). The number of patients who discontinued the intervention is comparable in all 3 groups and relatively small. Unlikely to influence results. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available but data presented match the outcome measures described in the methods section. Likely free of selective reporting. |
| Other bias | Low risk | Intervention and control groups were comparable at baseline; no other sources of bias were identified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants was not possible due to nature of the intervention. Blinding of researchers was not discussed, but likely not done. Unlikely to influence outcome measures. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessment. |
Hanauer 2009.
| Methods | RCT (2 arms, study duration 3 months) | |
| Participants | Diabetes patients (aged 12 to 25 yrs) on insulin treatment (n = 40). | |
| Interventions | The Computerized Automated Reminder Diabetes System (CARDS) includes a web‐based module and a messaging/reminder module designed to run autonomously. Participants log into the system via a secure website where they can customize their schedule for reminder messages, and view, edit, and print their blood glucose (BG) diaries. Participants can opt to receive two daily factoids: one related to diabetes education/nutrition and one with trivia. At a pre‐set time, CARDS sends a reminder to check the BG either by cell phone text message (intervention) or by email (control). After a user submits a BG value, regardless of the result, (s)he receives positive feedback. If the submitted BG value is out of range, CARDS provides a warning to take appropriate action according to the healthcare team's recommendations, and then recheck the BG. | |
| Outcomes | Primary outcomes: Number of BG results submitted. Secondary outcomes: HbA1c (%). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomized to receive reminders either via cell phone text messaging or by e‐mail". No further information on the method or randomisation was presented. |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data presented for all patients randomised. Presumably no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available but data presented match the outcome measures described in the methods section. Likely free of selective reporting. |
| Other bias | Low risk | There were no significant differences between the email (control) and cell phone (intervention) groups at baseline; no other sources of bias were identified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants was not possible due to nature of the intervention. Blinding of researchers was not discussed, but likely not done. Unlikely to influence outcome measures. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessment. |
Marquez Contreras 2004.
| Methods | RCT (2 arms, study duration 24 weeks, cluster randomisation) | |
| Participants | Ambulatory hypertension (HT) patients (aged over 18 yrs) whose HT was not well uncontrolled with monotherapy, and who were eligible for treatment with a combination of a single‐dose angiotensin II antagonist and a diuretic (n=67). Excluded were patients: a) on treatment with 2 or more antihypertensive drugs; b) with secondary HT; c) with known contra‐indications for any of the antihypertensive drugs to be used; d) whose clinical condition might have interfered with the study; e) who were participating in other research studies; f) who lived with a person who was being treated with the same antihypertensive drug; or g) who were unable to give their informed consent. |
|
| Interventions | Patients in the intervention group were subscribed to an SMS alerting system programmed to generate random messages. The aim of the messages was to provide information on HT, promote compliance, and good health and dietary habits, and remind patients to take their medication. Two messages were sent per week on randomly chosen weekdays during the 6‐month study period. Receipt of the messages was free to participants in the study and independent of their telephone service operator. | |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Researchers were randomised to 1 of the 2 groups with a random number table. |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | After 24 weeks data for 3/36 patients were missing from the control group and 2/36 missing from the intervention group due to lack of record of the number of tablets consumed. The reasons for loss to follow‐up are similar in both groups and unlikely to affect the results. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available but data presented match the outcome measures described in the methods section. Likely free of selective reporting. |
| Other bias | Low risk | Intervention and control groups were comparable at baseline; no other sources of bias were identified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants was not possible due to nature of the intervention. Blinding of researchers was not discussed, but likely not done. Unlikely to influence outcome measures. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessment. |
Ostojic 2005.
| Methods | RCT (2 arms, study duration 16 weeks) | |
| Participants | Patients with moderate persistent asthma for at least 6 months and being treated with inhaled corticosteroids and long acting beta agonist at a general hospital clinic in Zagreb, Croatia (n=16). | |
| Interventions | Patients in the intervention group were instructed to send their Peak Expiratory Flow (PEF) results daily via text message to a mobile telephone connected to a computer running the Asthma Center 0.90 Software. The software automatically computed maximal, minimal, and mean PEF, PEF variability, and compliance. Patients also received weekly instructions by text message from an asthma specialist on adjustments of therapy and recommended follow‐up based on the PEF values received by text message. Patients in both the intervention and control groups were treated according to GINA guidelines and kept paper asthma diaries. | |
| Outcomes | Pulmonary Function Test results (Forced Expiratory Volume in the first second (FEV1), PEF variability, Forced Vital Capacity); compliance with PEF measurements; asthma symptoms (cough, night symptoms, wheezing, limitation of activity); daily consumption of inhaled medicine and; cost to patient and provider. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised by computer into either the SMS study group or the control group. Although it is not explicitly mentioned, this suggests use of a random number sequence. |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patient withdrew from the study after enrolment. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available but data presented match the outcome measures described in the methods section. Likely free of selective reporting. |
| Other bias | Unclear risk | Intervention and control groups comparable at baseline. However, the study "is limited by the small number of patients and by the particulars of the population studied. The follow‐up period may not have been sufficiently long to reveal all significant differences between the groups." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was not blinded, but this, we believe, has not influenced the outcome. First, compliance was not significantly different in the two groups. Second, the patients in both groups were managed by the same current guidelines." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessment. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anhøj 2004 | No control group |
| Bauer 2003 | No control group |
| Benhamou 2003 | Combines mobile phone and PDA‐based data transmission (not SMS) with SMS based feedback |
| Bjerke 2008 | No control group; qualitative study |
| Carrasco 2008 | Combines mobile phone based (WAP, GPRS and SMS) and Internet‐based data transmission with SMS‐based feedback |
| Chang 2008 | Combines SMS‐based data transmission with regular mobile phone conversation |
| Faridi 2008 | Combines mobile phone‐based data transmission (not SMS) with tailored feedback to patients via SMS |
| Ferrer‐Roca 2004 | No control group |
| Fonseca 2006 | No outcome measures reported after initiation of the study |
| Gray 2006 | Combines SMS‐based data transmission with regular mobile phone conversation |
| Hodgson 2005 | No control group |
| Kim 2005 | Combines PC and mobile phone‐based data transmission with Internet and SMS‐based recommendations |
| Lim 2007 | Combines SMS with Internet‐based data input |
| Manfrida 2007 | No control group; qualitative study |
| Newton 2009 | Combines SMS‐based support with use of open pedometers |
| Rami 2006 | Combines SMS with GPRS data transmission |
| Spaniel 2008 | Combines SMS‐based questionnaire with email alerts and personal follow‐up; No control group |
| van der Meer 2006 | Combines PC or mobile phone‐based data transmission with SMS and Internet‐based feedback |
| Vähätalo 2004 | Combines mobile phone‐based data transmission (not SMS) with SMS‐based feedback |
| Wangberg 2006 | No control group |
Characteristics of ongoing studies [ordered by study ID]
Jackson 2006.
| Trial name or title | Improving childhood asthma management through a telemedicine monitoring network |
| Methods | RCT (study duration 6 months) |
| Participants | Participants (aged 3 to 16 yrs) with established doctor diagnosis of episodic or persistent asthma who have had at least one admission to hospital or one episode of acute care in an emergency department or paediatric clinic or general practitioner for asthma requiring steroid rescue within the previous 12 months. |
| Interventions | Asthma monitoring via mobile phone using SMS |
| Outcomes |
Primary outcomes: Health resource utilisation Secondary outcomes: School days missed (children) and days off work (parents); Use of medications; Health related Quality of Life (QOL) |
| Starting date | September 2006 |
| Contact information | Jackson, M, Department of Respiratory Medicine Royal Children's Hospital, Herston Rd, Herston, Brisbane QLD, Australia. Mary_Jackson@health.qld.gov.au |
| Notes | Recruiting at the time of this review. |
Liang 2009.
| Trial name or title | Using a text‐message system to engage depressed adolescents in cognitive‐behavioral therapy homework. |
| Methods | RCT (study duration 2 month) |
| Participants | Participants (aged 13 to 17 yrs) with major depressive disorder |
| Interventions | Homework will be standardised through a primary tool (DTR) for participants to evaluate and respond in writing to their automatic thoughts. The text‐messaging system allows homework to be submitted directly through a cellular phone, includes text‐messaged homework reminder prompts, and collates all homework for therapists to review with participants during therapy sessions. This is assigned and reviewed weekly for 4 weeks. |
| Outcomes |
Primary outcomes: Therapy homework compliance (% homework completed) Secondary outcomes: Self reported depressive symptoms (Mood Feeling Questionnaire) |
| Starting date | February 2009 |
| Contact information | Liang, HC. University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, United States. liangh2@upmc.edu |
| Notes | Recruiting at the time of this review. |
Maurino 2009.
| Trial name or title | Effect of daily Short Message System (SMS) reminders on medication adherence to oral antipsychotics in patients with schizophrenia. |
| Methods | RCT (study duration 6 months) |
| Participants | Stabilised out‐patients (aged over 18 yrs) with a diagnosis of schizophrenia (DSM‐IV TR criteria) and on oral antipsychotic mono‐therapy. |
| Interventions | Daily SMS medication reminders |
| Outcomes | Self‐reported adherence (Morisky Green Questionnaire); Disease awareness (Scale to Assess Unawareness of Mental Disorder (SUMD) Insight Questionnaire); Clinical Global Impression‐Schizophrenia scale score; EQ‐5D score; Attitude towards compliance (DAI‐10). |
| Starting date | April 2009 |
| Contact information | Maurino, J and Diez, T. AstraZeneca Pharmaceuticals, Spain. jorgealejandro.maurino@astrazeneca.com |
| Notes | Recruiting at the time of this review. |
Møldrup 2007.
| Trial name or title | Assessment of the health‐related effects of compliance optimization in asthma through use of SMS (Short Message System) ‐ a controlled trial. |
| Methods | RCT (study duration 90 days) |
| Participants | Participants (aged 18 to 45 yrs) with asthma. 244 participants enrolled |
| Interventions | SMS compliance and monitoring system for optimised asthma treatment |
| Outcomes | Asthma control; EQ‐5D score; Use of health services; Use of preventive medicine |
| Starting date | November 2007 |
| Contact information | Claus M ldrup, Associate Professor PhD, University of Copenhagen. cm@farma.ku.dk |
| Notes | Study completed May 2008 |
Rodríguez‐Idígoras 2003.
| Trial name or title | Telemedicine Influence in the Follow up of the Type 2 Diabetes Patient |
| Methods | RCT (study duration 12 months) |
| Participants | Participants (aged over 30 yrs) with a diagnosis of type 2 diabetes and on Self‐Monitoring Blood Glucose (SMBG) at least 6 months before |
| Interventions | Participants could send SMBG values to a web page via SMS. The healthcare provider could access this web page to check and, if necessary, return recommendations by SMS |
| Outcomes | HbA1c level |
| Starting date | October 2003 |
| Contact information | Rodríguez‐Idígoras, MI. Málaga Health Department, Junta de Andalucia, Spain. misabel.rodriguez@juntadeandalucia.es |
| Notes | Study completed June 2005. Authors contacted: publication in preparation at the time of this review |
Shetty 2008.
| Trial name or title | Reinforcement of adherence to prescription recommendations in diabetic patients using Short Message Service (SMS) ‐ a pilot study |
| Methods | RCT (study duration 12 months) |
| Participants | Participants (aged 30 to 65 yrs) with type 2 diabetes for a minimum period of 5 years and receiving oral hypoglycaemic agents and/or insulin. |
| Interventions | SMS reminders (once per 3 days) regarding the need for adherence to lifestyle modification and medication. |
| Outcomes | At baseline and at the end of the study, lipids, and renal function test will be done. A validated questionnaire will be used to assess physical activity, diet habits, adherence to drug prescriptions and frequency of monitoring of blood glucose. Body weight, blood pressure, biochemical variables, scores for diet and physical activity and compliance to drugs, will be compared. |
| Starting date | August 2008 |
| Contact information | Shetty, SA. India Diabetes Research Foundation (IDRF) and Dr. A. Ramachandran's Diabetes Hospitals. ramachandran@ardiabetes.org; snehalatha@vsnl.com. |
| Notes | Recruiting at the time of this review. |
Shotan 2006.
| Trial name or title | Short Message Service (SMS) impact on patient compliance receiving long‐term lipid lowering therapy with statins. |
| Methods | RCT (study duration 12 months) |
| Participants | Participants (aged 18 to 80 yrs) discharged from the Intensive Cardiac Care Unit or the Internal Medicine Department following acute coronary syndrome (ACS) events such as unstable angina or acute myocardial infarction who will be prescribed a statin for the first time for preventing further coronary episodes. 120 participants enrolled. |
| Interventions | Daily SMS medication reminders |
| Outcomes |
Primary outcomes: Number of patients who achieve target LDL goals Secondary outcomes: Reductions of total cholesterol, LDL, LDL/HDL and CRP; Increase of HDL; Readmissions due to ACS |
| Starting date | August 2006 |
| Contact information | Shotan, A. Hillel Yaffe medical center. shotan@hy.health.gov.il |
| Notes | Ongoing at the time of this review. |
van Schayk 2005.
| Trial name or title | A non‐interventional naturalistic project to investigate the effect of the use of SMS text service on treatment adherence in patients treated with Seroquel. |
| Methods | Prospective case study |
| Participants | Participants with schizophrenia or participants experiencing a manic episode associated with a bipolar disorder who were being treated with Quetiapine according to the Core Data Sheet and who were on a stable dosing regime. 128 participants enrolled |
| Interventions | Daily SMS text messages to enhance patient adherence with medication |
| Outcomes | Unknown |
| Starting date | September 2005 |
| Contact information | van Schayk, NPJT, AstraZeneca, The Netherlands |
| Notes | Study completed April 2008 |
Differences between protocol and review
Search strategy
We were not able to search the following databases we listed in the protocol:
Proceedings from the MEDNET Congress: We could not access the proceedings.
TrialsCentralTM (www.trialscentral.org): The website for the database was not functional and did not allow for the search of clinical trials.
African Trials Register: The trials in the African Trials Register are collected with a search strategy using the Cochrane Controlled Trials Register and the African Health Anthology (AHA). As we search both original sources, it was not necessary to access the African Trials Register separately.
Health Star: The database ceased to exist as of December 2000, with all peer‐reviewed journal articles transferred to PubMed.
Contributions of authors
Josip Car conceived the review together with Rifat Atun. He is the contact author for the reviews and coordinated all stages of the study, including the protocol stage. He advised on designing the search strategy, screening search results against inclusion criteria, and appraising quality of the papers. He assisted in interpreting the data and contributed to writing the review, providing clinical and consumer perspectives.
Ipek Gurol‐Urganci developed the protocols. She has led the search process and assisted in screening the papers. She collected, analysed and interpreted data and contributed to writing the review, providing methodological and policy perspectives.
Vlasta Vodopivec Jamšek developed the protocols. She has been involved in the screening and quality appraisal process and assisted in undertaking searches. She has collected, analysed and interpreted data and contributed to writing the review, providing clinical and consumer perspectives.
Thyra de Jongh coordinated data extraction and management and was involved in the screening and quality appraisal process. She has collected, analysed and interpreted data and contributed to writing the review, providing a methodological perspective.
Rifat Atun provided general direction for the studies and assisted in writing the review.
Sources of support
Internal sources
-
eHealth Unit, Department of Primary Care and Social Medicine, Imperial College, UK.
salaries, office space
-
Department of Family Medicine, Faculty of Medicine, University of Ljubljana, Slovenia.
salaries, office space
-
Centre for Health Management, Tanaka Business School, Imperial College, UK.
salaries, office space
-
Imperial College London, UK.
salaries, office space
External sources
-
Ministry of Higher Education, Science and Technology, Slovenia.
grant funding
Declarations of interest
None known.
New
References
References to studies included in this review
Franklin 2006 {published data only}
- Franklin V, Waller A, Pagliari C, Greene S. "Sweet Talk": text messaging support for intensive insulin therapy for young people with diabetes. Diabetes Technology and Therapeutics 2003;5(6):991‐6. [DOI] [PubMed] [Google Scholar]
- Franklin VL, Greene A, Waller A, Greene SA, Pagliari C. Patients' engagement with "Sweet Talk" ‐ a text messaging support system for young people with diabetes. Journal of Medical Internet Research 2008;10(2):e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin VL, Waller A, Pagliari C, Greene SA. A randomized controlled trial of Sweet Talk, a text‐messaging system to support young people with diabetes. Diabetic Medicine 2006;23(12):1332‐8. [DOI: 10.1111/j.1464-5491.2006.01989.x] [DOI] [PubMed] [Google Scholar]
- Waller A, Franklin V, Pagliari C, Greene S. Participatory design of a text message scheduling system to support young people with diabetes. Health Informatics Journal 2006;12(4):304‐18. [DOI] [PubMed] [Google Scholar]
Hanauer 2009 {published data only}
- Hanauer DA, Wentzell K, Laffel N, Laffel LM. Computerized Automated Reminder Diabetes System (CARDS): E‐Mail and SMS cell phone text messaging reminders to support diabetes management. Diabetes Technology and Therapeutics 2009;11(2):99‐106. [DOI: 10.1089/dia.2008.0022] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marquez Contreras 2004 {published data only}
- Marquez Contreras E, Figuera von Wichmann M, Gil‐Guillen V, Ylla‐Catala A, Figueras M, Balana M, et al. Effectiveness of an intervention to provide information to patients with hypertension as short text messages and reminders sent to their mobile phone (HTA‐Alert) [Eficacia de una intervención informativa a hipertensos mediante mensajes de alerta en el teléfono móvil (HTA‐ALERT)]. Atencion Primaria 2004;34(8):399‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ostojic 2005 {published data only}
- Ostojic V, Cvoriscec B, Ostojic SB, Reznikoff D, Stipic‐Markovic A, Tudjman Z. Improving asthma control through telemedicine: a study of short‐message service. Telemedicine Journal and e‐Health 2005;11(1):28‐35. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anhøj 2004 {published data only}
- Anhøj J, Moldrup C. Feasibility of collecting diary data from asthma patients through mobile phones and SMS (short message service): response rate analysis and focus group evaluation from a pilot study. Journal of Medical Internet Research 2004;6(4):e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bauer 2003 {published data only}
- Bauer S, Hagel J, Okon E, Meermann R, Kordy H. Experiences with the Short Message Service (SMS) in the aftercare of patients with bulimia nervosa [SMS in der nachstationären Betreuung von Patientinnen mit Bulimia nervosa]. Psychodynamische Psychotherapie 2003;5(3):127‐36. [Google Scholar]
- Bauer S, Percevic R, Okon E, Meermann R, Kordy H. Use of text messaging in the aftercare of patients with bulimia nervosa. European Eating Disorders Review 2003;11(3):279‐90. [Google Scholar]
- Robinson S, Perkins S, Bauer S, Hammond N, Treasure J, Schmidt U. Aftercare intervention through text messaging in the treatment of bulimia nervosa: feasibility pilot. The International Journal of Eating Disorders 2006;39(8):633‐8. [DOI] [PubMed] [Google Scholar]
Benhamou 2003 {published data only}
- Benhamou PY, Hanaire H, Halimi S, Bosson JL. Web‐based follow‐up using cellular phone in type 1 diabetic patients under insulin pump therapy: the PumpNet study. Clinical Trials.gov Accessed from http://clinicaltrials.gov/ct2/show/NCT00324584 2003. [[ClinicalTrials.gov: NCT00324584]] [DOI] [PubMed] [Google Scholar]
- Benhamou PY, Melki V, Boizel R, Perreal F, Quesada JL, Bessieres‐Lacombe S, et al. One‐year efficacy and safety of web‐based follow‐up using cellular phone in type 1 diabetic patients under insulin pump therapy: the PumpNet study. Diabetes and Metabolism 2007;33:220‐6. [[ClinicalTrials.gov: NCT00324584]] [DOI] [PubMed] [Google Scholar]
Bjerke 2008 {published data only}
- Bjerke TN, Kummervold PE, Christiansen EK, Hjortdahl P. "It made me feel connected" ‐‐ an exploratory study on the use of mobile SMS in follow‐up care for substance abusers. Journal of Addictions Nursing 2008;19(4):195‐200. [DOI: 10.1080/10884600802504735]] [DOI] [Google Scholar]
Carrasco 2008 {published data only}
- Carrasco MP, Salvador CH, Sagredo PG, Márquez‐Montes J, González de Mingo MA, Fragua JA, et al. Impact of patient‐general practitioner short‐messages‐based interaction on the control of hypertension in a follow‐up service for low‐to‐medium risk hypertensive patients: a randomized controlled trial. IEEE Transactions on Information Technology in Biomedicine 2008;12(6):780‐91. [DOI: 10.1109/TITB.2008.926429] [DOI] [PubMed] [Google Scholar]
Chang 2008 {published data only}
- Chang LW, Kagaayi J, Nakigozi G, Packer AH, Serwadda D, Quinn TC, et al. Responding to the human resource crisis: peer health workers, mobile phones, and HIV care in Rakai, Uganda. AIDS Patient Care and STDs 2008;22(3):173‐4. [[ClinicalTrials.gov: NCT00675389] [DOI] [PMC free article] [PubMed] [Google Scholar]
Faridi 2008 {published data only}
- Faridi Z, Liberti L, Shuval K, Northrup V, Ali A, Katz DL. Evaluating the impact of mobile telephone technology on type 2 diabetic patients' self‐management: the NICHE pilot study. Journal of Evaluation in Clinical Practice 2008;14(3):465‐9. [DOI] [PubMed] [Google Scholar]
Ferrer‐Roca 2004 {published data only}
- Ferrer‐Roca O, Cardenas A, Diaz‐Cardama A, Pulido P. Mobile phone text messaging in the management of diabetes. Journal of Telemedicine and Telecare 2004;10(5):282‐5. [DOI] [PubMed] [Google Scholar]
Fonseca 2006 {published data only}
- Fonseca JA, Costa‐Pereira A, Delgado L, Fernandes L, Castel‐Branco MG. Asthma patients are willing to use mobile and web technologies to support self‐management. Allergy 2006;61(3):389‐90. [DOI] [PubMed] [Google Scholar]
Gray 2006 {published data only}
- Gray R. Impact of peer health workers and mobile phones on HIV care. [ClinicalTrials.gov: NCT00675389]
Hodgson 2005 {published data only}
- Hodgson Y. Short Message Service as a support tool in medication adherence and chronic disease management. Health Care and Informatics Review Online 2005;9(3). [Google Scholar]
Kim 2005 {published data only}
- Kim HS. A randomized controlled trial of a nurse short‐message service by cellular phone for people with diabetes. International Journal of Nursing Studies 2007;44(5):687‐92. [DOI] [PubMed] [Google Scholar]
- Kim HS. Effects of web‐based diabetic education in obese diabetic patients. Taehan Kanho Hakhoe Chi 2005;3(5):924‐30. [DOI] [PubMed] [Google Scholar]
- Kim HS, Jeong HS. A nurse short message service by cellular phone in type‐2 diabetic patients for six months. Journal of Clinical Nursing 2007;16(6):1082‐7. [DOI] [PubMed] [Google Scholar]
- Kim HS, Kim NC, Ahn SH. Impact of a nurse short message service intervention for patients with diabetes. Journal of Nursing Care Quality 2006;21(3):266‐71. [DOI] [PubMed] [Google Scholar]
- Kim HS, Song MS. Technological intervention for obese patients with type 2 diabetes. Applied Nursing Research 2008;21(2):84‐9. [DOI] [PubMed] [Google Scholar]
- Kim HS, Yoo YS, Shim HS. Effects of an Internet‐based intervention on plasma glucose levels in patients with type 2 diabetes. Journal of Nursing Care Quality 2005;20(4):335‐40. [DOI] [PubMed] [Google Scholar]
- Kim SI, Kim HS. Effectiveness of mobile and internet intervention in patients with obese type 2 diabetes. International Journal of Medical Informatics 2008;77(6):399‐404. [DOI] [PubMed] [Google Scholar]
- Yoon KH, Kim HS. A short message service by cellular phone in type 2 diabetic patients for 12 months. Diabetes Research and Clinical Practice 2008;79(2):256‐61. [DOI] [PubMed] [Google Scholar]
Lim 2007 {published data only}
- Lim FS, Foo M, Kanagalingam D, Lim R, Bahadin J, Tan KL, et al. Enhancing chronic disease management through telecare ‐ the Singapore Health Services experience. Journal of Telemedicine and Telecare 2007;13:73‐6. [DOI: 10.1258/135763307783247257] [DOI] [Google Scholar]
Manfrida 2007 {published data only}
- Manfrida G, Eisenberg E. Scriptavolant! Use and utility of SMS messages in psychotherapy. Terapia Familiare 2007;85:59‐82. [Google Scholar]
Newton 2009 {published data only}
- Newton KH, Wiltshire EJ, Elley CR. Pedometers and text messaging to increase physical activity: randomized controlled trial of adolescents with type 1 diabetes. Diabetes Care 2009;32(5):813‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rami 2006 {published data only}
- Rami B, Popow C, Horn W, Waldhoer T, Schober E. Telemedical support to improve glycemic control in adolescents with type 1 diabetes mellitus. European Journal of Pediatrics 2006;165(10):701‐5. [DOI] [PubMed] [Google Scholar]
Spaniel 2008 {published data only}
- Spaniel F, Vohlídka P, Hrdlicka J, Kozený J, Novák T, Motlová L, et al. ITAREPS: Information Technology Aided Relapse Prevention Programme in Schizophrenia. Schizophrenia Research 2008;98(1‐3):312‐7. [DOI] [PubMed] [Google Scholar]
Vähätalo 2004 {published data only}
- Vähätalo MA, Virtamo HE, Viikari JS, Rönnemaa T. Cellular phone transferred self blood glucose monitoring: prerequisites for positive outcome. Practical Diabetes International 2004;21(5):192‐4. [DOI: 10.1002/pdi.642] [DOI] [Google Scholar]
van der Meer 2006 {published data only}
- Meer V, Stel HF, Bakker MJ, Roldaan AC, Assendelft WJ, Sterk PJ, et al. SMASHING (Self‐Management of Asthma Supported by Hospitals, ICT, Nurses and General practitioners) Study Group. Weekly self‐monitoring and treatment adjustment benefit patients with partly controlled and uncontrolled asthma: an analysis of the SMASHING study. Respiratory Research 2010;10(11):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meer, V. Self‐Management of Asthma Supported by Hospitals, Information and communication technology, Nurses and General practitioners (SMASHING in adults). 2006. [ISRCTN79864465]
Wangberg 2006 {published data only}
- Wangberg SC, Arsand E, Andersson N. Diabetes education via mobile text messaging. Journal of Telemedicine and Telecare 2006;12(Suppl 1):55‐6. [PUBMED: 16884582] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Jackson 2006 {unpublished data only}
- Improving childhood asthma management through a telemedicine monitoring network. Ongoing study September 2006.
Liang 2009 {unpublished data only}
- Using a text‐message system to engage depressed adolescents in cognitive‐behavioral therapy homework.. Ongoing study February 2009.
Maurino 2009 {unpublished data only}
- Effect of daily Short Message System (SMS) reminders on medication adherence to oral antipsychotics in patients with schizophrenia.. Ongoing study April 2009.
Møldrup 2007 {unpublished data only}
- Assessment of the health‐related effects of compliance optimization in asthma through use of SMS (Short Message System) ‐ a controlled trial.. Ongoing study November 2007.
Rodríguez‐Idígoras 2003 {unpublished data only}
- Telemedicine Influence in the Follow up of the Type 2 Diabetes Patient. Ongoing study October 2003.
Shetty 2008 {unpublished data only}
- Reinforcement of adherence to prescription recommendations in diabetic patients using Short Message Service (SMS) ‐ a pilot study. Ongoing study August 2008. [PubMed]
Shotan 2006 {unpublished data only}
- Short Message Service (SMS) impact on patient compliance receiving long‐term lipid lowering therapy with statins.. Ongoing study August 2006.
van Schayk 2005 {unpublished data only}
- A non‐interventional naturalistic project to investigate the effect of the use of SMS text service on treatment adherence in patients treated with Seroquel.. Ongoing study September 2005.
Additional references
Adler 2007
- Adler R. Health care unplugged: the evolving role of wireless technology. http://www.chcf.org/documents/chronicdisease/HealthCareUnpluggedTheRoleOfWireless.pdf. Chronic Health Care Foundation, 2007.
Atun 2006
- Atun RA, Sittampalam S. A review of the characteristics and benefits of SMS in delivering healthcare. The role of mobile phones in increasing accessibility and efficiency in healthcare. Vodafone Group Plc, 2006:18‐28. [Google Scholar]
Atun 2006b
- Atun RA, Gurol‐Urganci I. Analysis of calls to NHS Direct. The role of mobile phones in increasing accessibility and efficiency in healthcare. Vodafone Group Plc, 2006:12‐17. [Google Scholar]
Bandura 1977
- Bandura A. Self‐efficacy: toward a unifying theory of behaviour change. Psychological Review 1977;84:191‐215. [DOI] [PubMed] [Google Scholar]
Bandura 1982
- Bandura A. Self‐efficacy mechanism in human agency. American Psychologist 1982;37:122‐47. [Google Scholar]
Barlow 2002
- Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self‐management approaches for people with chronic conditions: a review. Patient Education and Counseling 2002;48(2):177‐87. [DOI] [PubMed] [Google Scholar]
Baumgart 2005
- Baumgart DC. Personal digital assistants in health care: experienced clinicians in the palm of your hand?. Lancet 2005;366(9492):1210‐22. [DOI] [PubMed] [Google Scholar]
Bodenheimer 2002
- Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA 2002;288(15):1909‐14. [DOI] [PubMed] [Google Scholar]
Bodenheimer 2002b
- Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA 2002;288(14):1775‐9. [DOI] [PubMed] [Google Scholar]
Bodenheimer 2002c
- Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self‐management of chronic disease in primary care. JAMA 2002;288(19):2469‐75. [DOI] [PubMed] [Google Scholar]
Bos 2005
- Bos A, Hoogstraten J, Prahl‐Andersen B. Failed appointments in an orthodontic clinic. American Journal of Orthodontics and Dentofacial Orthopedics 2005;127(3):355‐7. [DOI] [PubMed] [Google Scholar]
Car 2003
- Car J, Sheikh A. Telephone consultations. BMJ 2003;326(7396):966‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Car 2004
- Car J, Sheikh A. Email consultations in health care: 1‐scope and effectiveness. BMJ 2004;329(7463):435‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Car 2004b
- Car J, Sheikh A. Email consultations in health care: 2‐acceptability and safe application. BMJ 2004;329(7463):439‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Car 2012
- Car J, Gurol‐Urganci I, Jongh T, Vodopivec‐Jamsek V, Atun R. Mobile phone messaging reminders for attendance at healthcare appointments. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD007458.pub2] [DOI] [PubMed] [Google Scholar]
Christakis 2004
- Christakis NA. Social networks and collateral health effects. BMJ 2004;329:184‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 1991
- Clark NM, Becker MH, Janz NK, Lorig K, Rakowski W, Anderson L. Self‐management of chronic disease by older adults. Journal of Aging and Health 1991;3(1):3‐27. [Google Scholar]
Cobb 2002
- Cobb S. Social support as a moderator of life stress. Psychosomatic Medicine 2002;38:300‐14. [DOI] [PubMed] [Google Scholar]
Cohen 1985
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological Bulletin 1985;98:310‐57. [PubMed] [Google Scholar]
Corben 2005
- Corben S, Rosen R. Patients' perspectives on the way ahead. Self‐Management for Long‐term Conditions. King's Fund, 2005. [Google Scholar]
Corbin 1988
- Corbin JM, Strauss AL. Unending Work and Care: Managing Chronic Illness at Home. San Francisco, CA: Jossey‐Bass Publishers, 1988. [Google Scholar]
DoH 2005
- Department of Health. Supporting people with long term conditions. An NHS and Social Care Model to support local innovation and integration. (http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4100252; accessed 7 Aug. 2008) 2005.
Fairley 2003
- Fairley CK, Levy R, Rayner CR, Allardice K, Costello K, Thomas C, et al. Randomized trial of an adherence programme for clients with HIV. International Journal of STD & AIDS 2003;14(12):805‐9. [DOI] [PubMed] [Google Scholar]
Fjeldsoe 2009
- Fjeldsoe BS, Marshall AL, Miller YD. Behavior change interventions delivered by mobile telephone short‐message service. American Journal of Preventive Medicine 2009;36(2):165‐73. [DOI: 10.1016/j.amepre.2008.09.040] [DOI] [PubMed] [Google Scholar]
Foster 2007
- Foster G, Taylor SJC, Eldridge SE, Ramsay J, Griffiths CJ. Self‐management education programmes by lay leaders for people with chronic conditions. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD005108.pub2] [DOI] [PubMed] [Google Scholar]
Gurol‐Urganci 2012
- Gurol‐Urganci I, Jongh T, Vodopivec‐Jamsek V, Car J, Atun R. Mobile phone messaging for communicating results of medical investigations. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD007456] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org, 2011. [Google Scholar]
ITU 2010
- International Telecommunications Union. The World in 2010: ICT Facts and Figures. http://www.itu.int/ITU‐D/ict/material/FactsFigures2010.pdf 2010.
Kaplan 2006
- Kaplan WA. Can the ubiquitous power of mobile phones be used to improve health outcomes in developing countries?. Global Health 2006;2:9. [DOI: 10.1186/1744-8603-2-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krishna 2009
- Krishna S, Boren SA, Balas EA. Healthcare via cell phones: a systematic review. Telemedicine and e‐Health 2009;15(3):231‐40. [DOI: 10.1089/tmj.2008.0099] [DOI] [PubMed] [Google Scholar]
Kwon 2004
- Kwon HS, Cho JH, Kim HS, Lee JH, Song BR, Oh JA, et al. Development of web‐based diabetic patient management system using short message service (SMS). Diabetes Research and Clinical Practice 2004;66 Suppl 1:S133‐7. [DOI] [PubMed] [Google Scholar]
Norwell 2003
- Norwell N. Text messaging raises medicolegal issues. BMJ 2003;326(7399):1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Obermayer 2004
- Obermayer JL, Riley WT, Jean‐Mary J. College smoking‐cessation using cell phone text messaging. Journal of American College Health 2004;53(2):71‐8. [DOI] [PubMed] [Google Scholar]
Pinnock 2006
- Pinnock H, Slack R, Pagliari C, Price D, Sheikh A. Professional and patient attitudes to using mobile phone technology to monitor asthma: questionnaire survey. Primary Care Respiratory Journal 2006;15(4):237‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Puccio 2006
- Puccio JA, Belzer M, Olson J, Martinez M, Salata C, Tucker D, et al. The use of cell phone reminder calls for assisting HIV‐infected adolescents and young adults to adhere to highly active antiretroviral therapy: a pilot study. AIDS Patient Care and STDs 2006;20(6):438‐44. [DOI] [PubMed] [Google Scholar]
Robinson 2006
- Robinson S, Perkins S, Bauer S, Hammond N, Treasure J, Schmidt U. Aftercare Intervention Through Text Messaging in the Treatment of Bulimia Nervosa—Feasibility Pilot. International Journal of Eating Disorders Dec 2006;39(8):633‐8. [DOI] [PubMed] [Google Scholar]
Rodgers 2005
- Rodgers A, Corbett T, Bramley D, Riddell T, Wills M, Lin RB, et al. Do u smoke after txt? Results of a randomised trial of smoking cessation using mobile phone text messaging. Tobacco Control 2005;14(4):255‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryan 2007
- Ryan R, Hill S, Prictor M, McKenzie J. Cochrane Consumers and Communication Review Group. Study Quality Guide. http://www.latrobe.edu.au/chcp/cochrane/resources.html 2007.. [Google Scholar]
Vilella 2004
- Vilella A, Bayas JM, Diaz MT, Guinovart C, Diez C, Simo D, et al. The role of mobile phones in improving vaccination rates in travellers. Preventive Medicine 2004;38(4):503‐9. [DOI] [PubMed] [Google Scholar]
Vodopivec‐Jamsek 2012
- Vodopivec‐Jamsek V, Jongh T, Gurol‐Urganci I, Atun R, Car J. Mobile phone messaging for preventive health care. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD007457.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wikipedia 2007
- Wikipedia. Short Message Service. http://en.wikipedia.org/wiki/short_message_service (accessed February 2007).
Yanez‐Cadena 2006
- Yáñez‐Cadena D, Sarria‐Santamera A, García‐Lizana F. [Can we improve management and control of chronic diseases?] [¿Podemos mejorar eltratamiento y el control de las enfermedades crónicas?]. Atención Primaria 2006;37(4):221‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]


