Abstract
Background
The mean age of women undergoing local treatment for pre‐invasive cervical disease (cervical intra‐epithelial neoplasia; CIN) or early cervical cancer (stage IA1) is around their 30s and similar to the age of women having their first child. Local cervical treatment has been correlated to adverse reproductive morbidity in a subsequent pregnancy, however, published studies and meta‐analyses have reached contradictory conclusions.
Objectives
To assess the effect of local cervical treatment for CIN and early cervical cancer on obstetric outcomes (after 24 weeks of gestation) and to correlate these to the cone depth and comparison group used.
Search methods
We searched the following databases: Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library, 2017, Issue 5), MEDLINE (up to June week 4, 2017) and Embase (up to week 26, 2017). In an attempt to identify articles missed by the search or unpublished data, we contacted experts in the field and we handsearched the references of the retrieved articles and conference proceedings.
Selection criteria
We included all studies reporting on obstetric outcomes (more than 24 weeks of gestation) in women with or without a previous local cervical treatment for any grade of CIN or early cervical cancer (stage IA1). Treatment included both excisional and ablative methods. We excluded studies that had no untreated reference population, reported outcomes in women who had undergone treatment during pregnancy or had a high‐risk treated or comparison group, or both
Data collection and analysis
We classified studies according to the type of treatment and the obstetric endpoint. Studies were classified according to method and obstetric endpoint. Pooled risk ratios (RR) and 95% confidence intervals (CIs) were calculated using a random‐effects model and inverse variance. Inter‐study heterogeneity was assessed with I2 statistics. We assessed maternal outcomes that included preterm birth (PTB) (spontaneous and threatened), preterm premature rupture of the membranes (pPROM), chorioamnionitis, mode of delivery, length of labour, induction of delivery, oxytocin use, haemorrhage, analgesia, cervical cerclage and cervical stenosis. The neonatal outcomes included low birth weight (LBW), neonatal intensive care unit (NICU) admission, stillbirth, perinatal mortality and Apgar scores.
Main results
We included 69 studies (6,357,823 pregnancies: 65,098 pregnancies of treated and 6,292,725 pregnancies of untreated women). Many of the studies included only small numbers of women, were of heterogenous design and in their majority retrospective and therefore at high risk of bias. Many outcomes were assessed to be of low or very low quality (GRADE assessment) and therefore results should be interpreted with caution. Women who had treatment were at increased overall risk of preterm birth (PTB) (less than 37 weeks) (10.7% versus 5.4%, RR 1.75, 95% CI 1.57 to 1.96, 59 studies, 5,242,917 participants, very low quality), severe (less than 32 to 34 weeks) (3.5% versus 1.4%, RR 2.25, 95% CI 1.79 to 2.82), 24 studies, 3,793,874 participants, very low quality), and extreme prematurity (less than 28 to 30 weeks) (1.0% versus 0.3%, (RR 2.23, 95% CI 1.55 to 3.22, 8 studies, 3,910,629 participants, very low quality), as compared to women who had no treatment.
The risk of overall prematurity was higher for excisional (excision versus no treatment: 11.2% versus 5.5%, RR 1.87, 95% CI 1.64 to 2.12, 53 studies, 4,599,416 participants) than ablative (ablation versus no treatment: 7.7% versus 4.6%, RR 1.35, 95% CI 1.20 to 1.52, 14 studies, 602,370 participants) treatments and the effect was higher for more radical excisional techniques (less than 37 weeks: cold knife conisation (CKC) (RR 2.70, 95% CI 2.14 to 3.40, 12 studies, 39,102 participants), laser conisation (LC) (RR 2.11, 95% CI 1.26 to 3.54, 9 studies, 1509 participants), large loop excision of the transformation zone (LLETZ) (RR 1.58, 95% CI 1.37 to 1.81, 25 studies, 1,445,104 participants). Repeat treatment multiplied the risk of overall prematurity (repeat versus no treatment: 13.2% versus 4.1%, RR 3.78, 95% CI 2.65 to 5.39, 11 studies, 1,317,284 participants, very low quality). The risk of overall prematurity increased with increasing cone depth (less than 10 mm to 12 mm versus no treatment: 7.1% versus 3.4%, RR 1.54, 95% CI 1.09 to 2.18, 8 studies, 550,929 participants, very low quality; more than 10 mm to 12 mm versus no treatment: 9.8% versus 3.4%, RR 1.93, 95% CI 1.62 to 2.31, 8 studies, 552,711 participants, low quality; more than 15 mm to 17 mm versus no treatment: 10.1 versus 3.4%, RR 2.77, 95% CI 1.95 to 3.93, 4 studies, 544,986 participants, very low quality; 20 mm or more versus no treatment: 10.2% versus 3.4%, RR 4.91, 95% CI 2.06 to 11.68, 3 studies, 543,750 participants, very low quality). The comparison group affected the magnitude of effect that was higher for external, followed by internal comparators and ultimately women with disease, but no treatment. Untreated women with disease and the pre‐treatment pregnancies of the women who were treated subsequently had higher risk of overall prematurity than the general population (5.9% versus 5.6%, RR 1.24, 95% CI 1.14 to 1.34, 15 studies, 4,357,998 participants, very low quality).
pPROM (6.1% versus 3.4%, RR 2.36, 95% CI 1.76 to 3.17, 21 studies, 477,011 participants, very low quality), low birth weight (7.9% versus 3.7%, RR 1.81, 95% CI 1.58 to 2.07, 30 studies, 1,348,206 participants, very low quality), NICU admission rate (12.6% versus 8.9%, RR 1.45, 95% CI 1.16 to 1.81, 8 studies, 2557 participants, low quality) and perinatal mortality (0.9% versus 0.7%, RR 1.51, 95% CI 1.13 to 2.03, 23 studies, 1,659,433 participants, low quality) were also increased after treatment.
Authors' conclusions
Women with CIN have a higher baseline risk for prematurity. Excisional and ablative treatment appears to further increases that risk. The frequency and severity of adverse sequelae increases with increasing cone depth and is higher for excision than it is for ablation. However, the results should be interpreted with caution as they were based on low or very low quality (GRADE assessment) observational studies, most of which were retrospective.
Plain language summary
Obstetric outcomes after conservative treatment for cervical intraepithelial lesions
The issue Cervical intra‐epithelial neoplasia (CIN) is a pre‐cancerous lesion of the cervix uteri (neck of the womb) caused by human papillomavirus (HPV), which may develop into cervical cancer, if not treated. Local treatment involves destroying or removing the abnormal area of the cervix, leaving most of the cervix, and the uterus in place maintaining the ability to become pregnant in the future, if desired. Certain types of local treatment may also be suitable for very early cervical cancer (stage IA1) if the tumour is very small and very unlikely to have spread beyond the cervix. There are many studies investigating whether the local treatment for CIN and early cervical cancer increases the risk of preterm birth (PTB) in subsequent pregnancies. However, there is no definite conclusion and this creates confusion for both the medical staff and women who may be recommended treatment, but also want to have children in the future.
The aim of the review We aimed to assess whether the local conservative treatment techniques for cervical precancer (CIN) and early cervical cancer increased the risk of complications for mother and baby during pregnancy occurring after treatment, and especially whether treatment is associated with an increase in the risk of PTB. We also studied whether the risk of PTB increases with increasing amount of cervical tissue removed.
Selection criteria We included all studies that investigated the effect of treatment of CIN and early cervical cancer on late pregnancy outcomes (beyond 24 weeks of gestation) in women who had been treated previously for CIN and early cervical cancer, as compared to women who had not been treated. We excluded studies that had no untreated comparison group, reported pregnancy outcomes in women who had undergone treatment during pregnancy, or had a high‐risk treated, comparison group or both.
What are the main findings? We included 69 studies (6,357,823 pregnancies: 65,098 pregnancies of treated and 6,292,725 pregnancies of untreated women). Treatment was associated with an increased risk of PTB before 37 pregnancy weeks, as well as an increased risk of severe PTB (less than 32 to 34 pregnancy weeks), extreme PTB (less than 28 to 30 pregnancy weeks) and pPROM (premature preterm rupture of the membranes) as compared to untreated women. The risk of overall PTB was higher for women treated by excisional methods (where tissue is cut away) than by ablative treatments (where tissue is destroyed instead of being cut away). Multiple treatments, as well as increasing amounts of tissue removed at the time of treatment, were associated with an increased risk of overall PTB. However, women with CIN who were not treated also had a higher risk of overall PTB than the general population. Low birth weight (LBW) < 2500g), neonatal intensive care unit (NICU) admission and perinatal mortality rates were also found to be increased after treatment.
What is the quality of the evidence? Due to the nature of the intervention and outcomes studied, we were only able to include observational studies, of which the majority were retrospective. These types of studies are of low quality with a high level of variability between the studies, therefore the level of evidence for most outcomes can only be considered to be of low or very low quality.
What are the conclusions? Women with CIN have a higher baseline risk for PTB than the general population and the treatment for CIN probably increase this risk further. The risk for PTB is probably higher when excisional techniques are used than for ablative treatments. Also, the risk of PTB appears to increase with multiple treatments and increasing amounts of tissue removed. However, these results should be interpreted with caution due to the low and very low quality of the included studies.
Summary of findings
Background
Description of the condition
Cervical cancer remains the commonest gynaecological malignancy worldwide, and accounts for 7.5% of female cancer deaths. Over half a million new cases are diagnosed each year around the world, with the vast majority occurring in the developing world, where a woman's risk of cervical cancer by age 74 is almost double that in the developed world (1.6% versus 0.9%) (Ferlay 2015).The introduction of cervical screening programmes over the last 20 years has produced a profound decrease in the incidence and mortality from cervical cancer (Arbyn 2009; Quinn 1999). This is due to the treatment of pre‐invasive lesions, cervical intra‐epithelial neoplasia (CIN), detected by screening (IARC 2005).
CIN is an abnormality in the squamous cells of the cervix and, if left untreated, cervical cancer may develop. The condition is asymptomatic and interventions are usually performed only on women with higher grade CIN of grade (CIN 2 or 3). This is because cervical treatment has been correlated with adverse obstetric sequelae (Kyrgiou 2006), while many of the low‐grade lesions (also known as LSIL, low‐grade squamous intra‐epithelial lesions or CIN 1) resolve spontaneously in young individuals (NHS Cervical Screening Programme 2016).
The average age of a woman diagnosed and treated for CIN is between 25 and 30 years of age, although it may occur in women considerably younger (NHS Cervical Screening Programme 2016). As the pre‐cancerous lesions typically occur in young women of reproductive age, the impact of their treatment on the outcomes of subsequent pregnancies has been an area of active research for the past decade. Whilst it is paramount that effective treatment is undertaken, it is also important that this treatment has minimal adverse effects on future fertility and pregnancy outcomes for this young female population.
Description of the intervention
The conservative methods for treatment of CIN are classified into excisional and ablative. These techniques remove or destroy the transformation zone (TZ) containing the abnormal cells whilst preserving cervical function. Excisional methods include cold knife conisation (CKC), laser conisation (LC), needle excision of the transformation zone (NETZ), also known as straight wire excision of the transformation zone (SWETZ), large loop excision of the transformation zone (LLETZ) (Kitchener 1995; Prendiville 1989), also known as loop electrosurgical excisional procedure (LEEP) and Fischer cone biopsy excisor (FCBE). Ablative methods include laser ablation (LA), radical diathermy (RD), cold coagulation (CC) and cryotherapy (CT).
The mean age of women undergoing treatment for pre‐invasive cervical disease is around their 30s and similar to the age of women having their first child (Herbert 2000; Paraskevaidis 1992). Local cervical treatment has been correlated with an increased risk of preterm birth, perinatal morbidity and mortality in a subsequent pregnancy (Albrechtsen 2008; Arbyn 2008; Bruinsma 2011; Kyrgiou 2006; Kyrgiou 2014; Noehr 2009a). The underlying mechanism is unclear; hypotheses include immunomodulation relating to human papillomavirus (HPV) infection affecting parturition pathways, unexplained confounders in women with CIN and acquired ‘mechanical weakness’ secondary to loss of cervical tissue (Kyrgiou 2012).
In England alone in 2013 to 2014, 3.6 million women aged between 25 and 64 years attended for cervical screening and over 23,800 cervical procedures were carried out (CervicalCancerScreening 2015), the vast majority in an outpatient setting. In contrast in the USA, there are approximately 400,000 cases of pre‐invasive disease per year (Henk 2010). The regulations in colposcopy are more liberal than in the UK leading to wide variation in clinical practice. In Germany, treatment for CIN is still commonly performed with the cold knife under general analgesia (Petry 2008). The long‐term sequelae of treatment remains therefore an important international issue to both healthcare professionals and women, whatever the clinical setting.
How the intervention might work
The characteristics of the conservative methods of treatment are well‐described and established in the medical literature (Martin‐Hirsch 2013). LLETZ, LC and LA are usually performed under local anaesthesia in an outpatient setting, while CKC requires general anaesthesia and hospitalisation. Theoretically, the excisional techniques (CKC, LC, NETZ, LLETZ, FCBE) are superior over ablative techniques (LA, RD, CC, CT) as they allow a comprehensive histological evaluation of the excised tissue and the whole TZ with precise evaluation of excision margins. Ablative techniques destroy the TZ epithelium and preclude histological evaluation and, therefore, demand accurate pre‐treatment biopsy at a separate visit. LLETZ is the most favoured technique (Kitchener 1995), by combining all the advantages of the excisional techniques mentioned above together with a relatively shorter duration, low cost, good compliance, simplicity and easier learning curve for practitioners.
The best available evidence suggests that these methods (CKC, LC, LLETZ, LA) present similar low morbidity and are equally successful, in terms of eradicating CIN (Martin‐Hirsch 2013; Nuovo 2000) and in preventing invasive cervical cancer (Chew 1999; Paraskevaidis 1991; Soutter 1997). However, the existing data regarding future fertility and pregnancy outcomes are conflicting.
Why it is important to do this review
Observational studies have indicated that the treatment of CIN could have detrimental effects on fertility and pregnancy outcome; although the conclusions are usually equivocal, perhaps due to the weakness associated with small sample sizes used in the studies. We have not found any published randomised controlled trials (RCTs) comparing pregnancy outcome between treated and untreated women in the literature and because of the pre‐malignant nature of the condition treated, it is perhaps unlikely that one will be ever conducted. Thus, the only available level of evidence on this subject may have to be provided by systematic reviews and meta‐analyses of observational studies.
Media publicity has heightened public awareness that treatment for cervical precancer is associated with an increased reproductive morbidity. There has been a substantial increase in enquiries from patients and clinicians on the risks associated with different treatment techniques and cone depths (Founta 2010; Kyrgiou 2015a), and as to how this risk may be managed and prevented. With a rapidly evolving evidence base and lack of a robust synthesis of the published literature, these questions are becoming increasingly difficult to answer.
Since the first systematic review of the reproductive risk associated with treatment almost a decade ago (Kyrgiou 2006), more than 50 observational studies have been published confirming (Jakobsson 2007; Ortoft 2010) or disputing these associations (Castanon 2012; Reilly 2012); some of these reporting data from large population‐based datasets. Individual attempts to synthesise parts of this rapidly evolving evidence base in small systematic reviews and meta‐analyses reached contradictory conclusions (Arbyn 2008; Bruinsma 2011; Conner 2014; Crane 2003; Danhof 2015; Jin 2014; Kyrgiou 2006; Kyrgiou 2014;) and initiated debates and confusion within the scientific community (Arbyn 2008; Conner 2014; Crane 2003; Danhof 2015; Jin 2014). Whether these discrepancies were due to questionable quality of some of these primary and secondary studies or differences in the explored comparisons (Bruinsma 2011; Conner 2014; Danhof 2015; Jin 2014), the subject is open to a definitive comprehensive high‐quality synthesis of the existing evidence that will be highly informative to women, clinicians and policy makers (Arbyn 2008; Bruinsma 2011; Conner 2014; Danhof 2015; Jin 2014; Kyrgiou 2014). Because many large studies (Albrechtsen 2008; Bruinsma 2007; Castanon 2012; Heinonen 2013; Jakobsson 2007; Jakobsson 2009; Noehr 2009a; Ortoft 2010; Reilly 2012; Shanbhag 2009) have been published since Kyrgiou's first meta‐analysis (Kyrgiou 2006), we decided to update it in order to incorporate the latest studies and pay attention especially to the effect of the comparison group and the depth of the excised cone; two areas on which other meta‐analyses do not emphasise.
Objectives
To assess the effect of local cervical treatment for CIN and early cervical cancer on obstetric outcomes (after 24 weeks of gestation) and to correlate these to the cone depth and comparison group used.
Methods
Criteria for considering studies for this review
Types of studies
We included all studies reporting on late obstetric outcomes (beyond 24 weeks of gestation) in women with one or more previous local cervical treatments for CIN or early invasive disease (stage IA1), as compared to women without treatment. The interventions included any type of conservative treatment, either excisional or ablative (See:Types of interventions).
Studies were included irrespective of the type of untreated control group, which could have been drawn from one of the following sources: a) external group from general population that was mostly matched or adjusted for confounders; b) internal group with self‐matching of the pregnancies for the same women before and after treatment; c) internal group with the pre‐treatment and post‐treatment pregnancies of a given population; d) women attending colposcopy with or without CIN/biopsy but no treatment; e) women with high‐grade disease but no treatment (high‐grade squamous intra‐epithelial lesion (HSIL)). As the studies are non‐randomised, the choice of comparison group can impact on the magnitude of effect of the proposed comparisons. We know that women with CIN may have demographic and behavioural characteristics or even background immunological imbalances that place them at higher baseline risk of adverse reproductive outcomes. The different comparison groups have advantages and disadvantages and subgroup analyses for the different groups will allow better assessment of the true effects of treatment. More details are described in Assessment of risk of bias in included studies.
We excluded studies that did not include an untreated control group, compared different treatment techniques without an untreated control, reported on only fertility or early obstetric outcomes (before 24 weeks of gestation), reported only on obstetric outcomes beyond 24 weeks of gestation that are not listed below (see: Types of outcome measures), compared outcomes for treatments performed during pregnancy, or those that described outcomes in high‐risk women (i.e. women with history of miscarriage or women conceiving through assisted reproductive technology (ART))
Types of participants
We included women who had a pregnancy with or without a previous conservative treatment for CIN/early cervical cancer (stage IA1). We included women irrespective of the grade of the lesion for both squamous and glandular intra‐epithelial neoplasia. There was also no age restriction.
Types of interventions
Any comparison of interventions for treatment of CIN or stage IA1 cervical cancer by conservative methods of either:
excision (cold knife conisation (CKC); laser conisation (LC); needle excision of the transformation zone (NETZ), also known as straight wire excision of the transformation zone (SWETZ); large loop excision of the transformation zone (LLETZ), also known as loop electrosurgical excisional procedure (LEEP); Fischer cone biopsy excisor (FCBE));
ablation (laser ablation (LA); radical diathermy (RD); cold coagulation (CC); cryotherapy (CT)).
In studies that reported on the impact of several treatment techniques, we extracted data for each specific method, where possible. If the outcomes were not reported separately for each technique, we analysed the intervention under broader terms, i.e. excisional treatment not otherwise specified (NOS), ablative treatment NOS and treatment NOS.
Types of outcome measures
Primary outcomes
Maternal outcomes
overall (less than 37 weeks) prematurity (both iatrogenic and spontaneous)
severe (less than 32 to 34 weeks) prematurity
extreme (less than 28 to 30 weeks) prematurity
overall prematurity in singleton and multiple pregnancies
overall prematurity in nulliparous and parous women
overall prematurity for single and multiple cones
overall prematurity for different cone depths and volumes
overall prematurity for different comparison groups
Secondary outcomes
Maternal outcomes
overall spontaneous (i.e. non‐iatrogenic) prematurity
severe spontaneous prematurity
extreme spontaneous prematurity
threatened preterm birth
premature rupture of the membranes
chorioamnionitis
mode of delivery (caesarean section, instrumental deliveries)
length of labour (precipitous, prolonged)
induction of labour or use of oxytocin
haemorrhage (antepartum, postpartum)
analgesia (epidural, pethidine, not otherwise specified)
cervical stenosis
cervical cerclage
Neonatal outcomes
low birth weight (less than 2500 g, less than 2000 g, less than 1500 g, less than 1000 g)
admission to neonatal intensive unit (NICU)
perinatal mortality
stillbirth
Apgar score
In cases of heterogeneity in the cut‐offs used for cone depth and prematurity classification, these were grouped together when possible (i.e. 32 to 34 weeks to include both cut‐offs, 10 to 12 mm cone depth to include studies grouping at both these cut‐offs including or not the values equal to these numbers).
Search methods for identification of studies
The literature searches started from 1948 when the conservative methods of treatment for CIN were introduced into clinical practice and included references published up to June 2017.
Electronic searches
We searched the following electronic databases.
Cochrane Central Register of Controlled Trials (CENTRAL) (CENTRAL, 2017, Issue 5) (Appendix 1).
MEDLINE (1948 to June week 4, 2017) (Appendix 2).
Embase (1980 to 2017, week 26) (Appendix 3).
The searches started from inception to date in order to capture all studies published since the late 1970s. The treatment techniques used predominantly to manage the disease have changed over the years, although there are still clinical indications for the oldest techniques.
We used the 'related articles' feature in MEDLINE to retrieve additional references.
Searching other resources
We searched Metaregister, Physicians Data Query, www.controlled‐trials.com/rct, www.clinicaltrials.gov and www.cancer.gov/clinicaltrials for ongoing studies. We contacted the main investigators of any relevant ongoing trials for further information.
We searched conference proceedings and abstracts through ZETOC (http://zetoc.mimas.ac.uk)) and WorldCat Dissertations. We searched reports of conferences in the following sources.
Annual Meeting of the British Society of Colposcopy and Cervical Pathology.
Annual Meeting of the International Federation of Cervical Pathology and Colposcopy.
Annual Meeting of European Federation of Colposcopy.
Annual Meeding of the American Society of Colposcopy and Cervical Pathology.
In an attempt to identify any articles missed by the initial search or any unpublished data, we handsearched the references of the retrieved articles and meta‐analyses and the proceedings of relevant conferences. We contacted experts in the field, including directors of UK cancer and colposcopy registries, to identify further reports of studies.
We included both published and unpublished studies, if they met the inclusion criteria for the review.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searches to the reference management database Endnote. We also added titles and abstracts retrieved from other sources to Endnote. We removed duplicates and two review authors (MK, AA) independently examined the remaining references. Titles and abstracts retrieved from other sources were also added to the EndNote database. We excluded those studies which clearly did not meet the inclusion criteria and we obtained copies of the full text of potentially relevant references. We assessed the eligibility of retrieved papers independently, compared the results and resolved disagreements by discussion. If necessary, we reached consensus with the involvement of a third review author (MA). We documented reasons for exclusion.
Data extraction and management
We classified the studies according to treatment modality (i.e. CKC, LC, LLETZ, LA etc) and in groups of excisional or ablative techniques.
From each study, we extracted data on the study design and setting, the study population, the interventions examined, the comparison group, the quality of the data and risk of bias and the outcomes assessed. We retrieved from each study and outcome, the number of events in treated and untreated women. If required, we contacted authors to obtain additional data if the numbers provided in the published report did not allow sufficient precision in the data extraction.
More specifically, we extracted the following data.
Author, year of publication, journal and language.
Country.
Setting where the study was conducted (hospital‐based versus population‐based).
Inclusion and exclusion criteria.
Study design, methodology, source of information.
Study population:
total number enrolled and number included in each group;
grade of CIN;
cone size;
single/multiple pregnancy;
single/multiple treatment;
nulliparous/parous women;
-
control for confounding factors:
age;
smoking;
parity;
socio‐economic status;
race;
history of previous preterm birth (PTB);
others.
-
Intervention details:
type of procedure used (excisional or ablative);
specific type of procedure used (excisional: CKC, LC, NETZ, LLETZ, FCBE; ablative: LA, RD, CC, CT).
-
Comparison group:
external untreated comparison group (general population);
-
internal comparison group:
self‐matching: the treated group consisted of only parous women and the pregnancy after treatment was compared to the pregnancy before treatment;
pre‐treatment pregnancies: in some studies, the treated group consisted of both nulliparous and parous women and the comparison group consisted of the pregnancies of the parous women before treatment.
untreated women with colposcopy with or without a biopsy who did not undergo treatment;
untreated women with untreated high‐grade disease.
Risk of bias (Assessment of risk of bias in included studies).
Outcomes reported in each study.
-
Primary outcomes:
overall (less than 37 weeks) prematurity (both iatrogenic and spontaneous);
severe (less than 32 to 34 weeks) prematurity;
extreme (less than 28 to 30 weeks) prematurity;
overall prematurity in singleton and multiple pregnancies;
overall prematurity in nulliparous and parous women;
overall prematurity for single and multiple cones;
overall prematurity for different cone depths and volumes;
overall prematurity for different comparison groups.
-
Secondary outcomes:
maternal outcomes: overall (less than 37 weeks) spontaneous (i.e. non‐iatrogenic) prematurity, severe (less than 32 to 34 weeks) spontaneous prematurity, extreme (less than 28 weeks) spontaneous prematurity, threatened preterm birth, premature rupture of the membranes, chorioamnionitis, mode of delivery (caesarean section, instrumental deliveries), length of labour (precipitous, prolonged), induction of labour or use of oxytocin, haemorrhage (antepartum, postpartum), analgesia (epidural, pethidine, not otherwise specified), cervical stenosis, cervical cerclage;
neonatal outcomes: low birth weight (LBW) (less than 2500 g, less than 2000 g, less than 1500 g, less than 1000 g), perinatal mortality, stillbirth, Apgar score.
-
-
For each reported outcome, we extracted information on:
the outcome definition;
number of participants allocated to each group;
for the dichotomous outcomes of interest: number of adverse pregnancy events in each group (treated and untreated), in order to estimate the risk ratio (RR), and missing participants.
-
Two review authors (MK, AA) abstracted data independently in a data abstraction form specially designed for the review. They resolved differences by discussion or by appeal to a third review author (EP) if necessary.
Assessment of risk of bias in included studies
To assess the risk of bias in included RCTs, we planned to use Cochrane's'Risk of bias' tool, comprising assessments of the following study characteristics: sequence generation; allocation concealment; blinding (of participants, healthcare providers and outcome assessors); incomplete outcome data; selective reporting of outcomes; other possible sources of bias (Higgins 2011).
As RCTs comparing women with CIN with non‐treated are not feasible or ethical due to the pre‐malignant nature of the condition, we anticipated that published evidence might rely only on observational cohort studies. As the comparison groups (treated for CIN with a particular procedure versus non‐treated) are non‐randomised, effects and effect sizes cannot be attributed with certainty to the treatment alone. The differences in the size of the treatment effect across studies may be partly explained by the choice of control population, because women with CIN may have demographic and behavioural characteristics or even background immunological imbalances that place them at higher baseline risk of adverse reproductive outcomes.
It should also be noted that all eligible comparison groups have advantages and limitations. A recent meta‐analysis showed that the use of historical external controls might produce inherent biases that could inflate the contribution of cervical treatment to adverse outcomes, even if the authors control for possible confounders (such as age, parity, smoking etc; Bruinsma 2011). The use of internal controls (pregnancies in the index woman before treatment) is an attractive alternative approach, but even this might be inadequate for confounders that are liable to change with time. Women with mild precancerous lesions that do not warrant excision treatment probably provide the best, although still imperfect, comparator. In contrast, those with high‐grade disease who neglect treatment advice aimed at preventing cancer may have high risk for confounders related to low socioeconomic class that may influence fertility or pregnancy outcomes.
For non‐randomised studies (NRS), we assessed the risk of bias in the following domains: Incomplete outcome Data (Attrition bias) was considered to be present if for more than 20% of the cohort the outcome data were missing or the method to collect outcome data was not systematic; Selective reporting (Reporting bias) was considered to be present if outcome data were not reported stratified according to all included study types; Performance and selection bias was assessed in whether the treatment assignment was reported appropriately, based on the representativeness of both the treatment and comparison groups, i.e. whether the treated cohort indeed represented the population at risk and was not subjected to possible selection bias, whether the comparison groups were drawn from the same source as the treated group and on their comparability, i.e. whether the authors used internal or self‐matched comparison group, or otherwise matched or adjusted for the possible confounders or effect modifiers; no other possible sources of bias were assessed.
We used the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) (GRADE Working Group 2004) approach to assess the quality of evidence provided by the included studies. We used GRADEpro (GRADE profiler) software to generate 'Summary of findings' tables to include an assessment of the more clinically relevant outcomes .
Measures of treatment effect
We calculated risk ratio (RR) and 95% confidence intervals (95% CIs) for each adverse pregnancy outcome in the treated versus untreated women for dichotomous outcomes. We separated studies by general type of treatment (excisional or ablative) and we further grouped them by specific treatment procedure and by specific comparison group. We used a random‐effects model to pool RR (Dersimonian 1986). We used unadjusted data for the analyses.
Unit of analysis issues
In studies with multiple treatment groups, we proportionally divided the ‘shared’ comparison group into the number of treatment groups (i.e. based on the number of treated women for each technique), in order to avoid duplicate inclusion of some untreated women in the same forest plot. We treated comparisons between each treatment group and the split comparison group as independent comparisons.
When more than one comparison group was described in the included studies, the comparison groups were summed together if appropriate (i.e. external, any CIN or HSIL without treatment). If an external and internal self‐matching group was available, only data on the external group were included. In one study (Castanon 2012), with both internal controls, pre‐conisation population was used in preference to self‐matching.
Dealing with missing data
We contacted study authors about inclusion/exclusion criteria and eligibility of their study. We further contacted authors for additional data not presented in the original manuscript i.e. data according to different cone depths.
Assessment of heterogeneity
We assessed inter‐study heterogeneity with Cochran Q test (Cochran 1954), by visual inspection of forest plots, by estimation of the percentage heterogeneity between studies which cannot be ascribed to sampling variation (I2 statistic) (Higgins 2003), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). If there was evidence of substantial heterogeneity, we investigated and reported the possible reasons for this.
Assessment of reporting biases
For the outcomes presented in the 'Summary of findings' tables that included more than 10 studies, we explored potential publication bias graphically by the funnel plot in the Cochrane Review Manager software (Review Manager 2104).
Data synthesis
We pooled the results of the studies in meta‐analyses. For dichotomous outcomes, we calculated RR and 95% CIs and these were then pooled. We analysed the data separately for each treatment modality, in groups of ablative and excisional techniques, and as a whole, irrespective of the type of method used. We further analysed the data according to the cone depth.
Several studies provided separate data for overall and spontaneous PTB (sPTB) (less than37 weeks of gestation). If only data on sPTB were provided, these were also included in the overall PTB analysis (Crane 2006; Noehr 2009a; Ortoft 2010; Poon 2012; Stout 2015). If studies provided data on nulliparous and parous women separately, these were presented together and were also included in the respective forest plots for overall PTB (i.e. Analysis 1.15; Analysis 1.16). This was also the case for single and multiple treatment and singleton and multiple pregnancies .
1.15. Analysis.

Comparison 1 Maternal Outcomes‐PTB, Outcome 15 PTB (<37w)‐Nulliparous women.
1.16. Analysis.
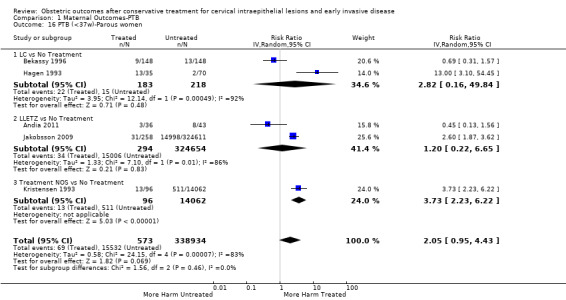
Comparison 1 Maternal Outcomes‐PTB, Outcome 16 PTB (<37w)‐Parous women.
We used random‐effects models with inverse variance weighting for all meta‐analyses (Dersimonian 1986).
If data were not of suitable quality for meta‐analysis, we reported the results as a narrative in the text of the review.
Subgroup analysis and investigation of heterogeneity
Given the non‐randomised nature of the included studies, we assessed whether the choice of comparison group impacts on the risk estimate for each outcome and over‐inflates the effect of treatment that could be partly attributed to other confounders. We therefore distinguished the different untreated comparison groups used across studies and performed analyses for the risk of PTB for each individual comparator (external; internal (self‐matching); internal (pre‐treatment pregnancies); colposcopy but no treatment; HSIL but no treatment). We performed separate analyses according to the comparison group for PTB (less than 37 weeks of gestation).
Furthemore, for the outcome of PTB (less than 37 weeks of gestation), we also performed analyses according to parity (nulliparous and parous women separately), number of treatments (women with single and multiple treatments separately), and number of fetuses (women with singleton and multiple pregnancies separately).
Sensitivity analysis
Finally, we performed meta‐regression analysis to assess the impact of some factors on the risk of PTB (less than37 weeks). These included the year of study (1979 to 1989, 1990 to 1999, 2000 to 2009, 2010 to 2015); type of treatment (excision or ablation); type of comparator (external, internal – pregnancies before treatment, internal – self‐matching, CIN but no treatment, HSIL but no treatment).
Results
Description of studies
The characteristics of the included and excluded studies and the outcomes examined are described in the Characteristics of included studies and in the Characteristics of excluded studies, respectively.
Results of the search
We retrieved 3219 citations from the literature search. Of those, 2849 were excluded based on the title or abstract; 370 were retrieved in full text for evaluation. We identified 69 studies that fulfilled the inclusion criteria and 289 were excluded (of these 289 studies, 242 were reviews/meta‐analyses, conference proceedings, letters or duplicates; the remaining 47 studies are listed in detail in Excluded studies and Characteristics of excluded studies); 12 studies are awaiting classification. No unpublished studies could be identified. The details, including reasons for exclusion, are present in the PRISMA flowchart (Moher 2009; Figure 1).
1.
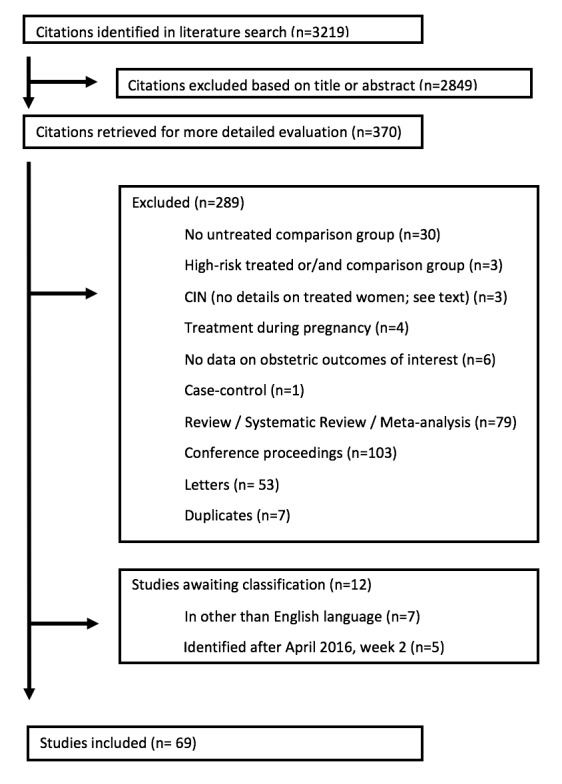
Flow diagram.
Included studies
Sixty‐nine studies fulfilled the inclusion criteria of this systematic review and were also included in the meta‐analysis. We identified no unpublished studies. The detailed characteristics of the included studies are shown in Characteristics of included studies. All studies were cohorts and retrospective, except for five prospective studies (Fischer 2010; Frega 2013; Guo 2013; Poon 2012; Simoens 2012). Fifteen studies were population‐based (the data were drawn from registers, or they included a large number of hospitals covering a large area) (Albrechtsen 2008; Andia 2011; El‐Bastawissi 1999; Frega 2013; Heinonen 2013; Jakobsson 2007; Jones 1979; Kristensen 1985; Kristensen 1993; Larsson 1982; Noehr 2009a; Noehr 2009b; Reilly 2012; Shanbhag 2009; Sjoborg 2007) and the remaining studies were hospital‐based (the data were drawn from hospital records). There were no RCTs.
Seven studies were identified in non‐English language and were not included (He 2007; Kalitsaris 1991; Kasum 1991; Lund 1986; Praest 1979; Spuhler 1995; Zornoza‐Garcia 2009) given the large number of included studies and the low quality of these small studies we considered that their inclusion would not alter the conclusions of the review. In future updates we will consider the inclusion of these reports (Characteristics of studies awaiting classification). Five studies published after April 2016, week 2 are awaiting classification in future updates of this review (Aleman 2016; Bjorge 2016; Brie 2016; Jancar 2016; Zebitay 2017).
Many of the included studies included cohorts treated with a variety of treatment modalities. Specifically, 14 studies examined the impact of cold knife conisation; (CKC) (Bruinsma 2007; Buller 1982; Crane 2006; Ehsanipoor 2014; Guo 2013; Jones 1979; Klaritsch 2006; Kuoppala 1986; Larsson 1982; Ludviksson 1982; Moinian 1982; Ortoft 2010; Sozen 2014; Weber 1979), 10 of laser conisation (LC) (Andersen 1999; Bekassy 1996; Forsmo 1996; Hagen 1993; Lima 2011; Raio 1997; Sadler 2004; Sagot 1995; Simoens 2012; Spitzer 1995), one of needle excision of the transformation zone (NETZ) (Ortoft 2010), 32 of large loop excision of the transformation zone (LLETZ) (Acharya 2005; Andia 2011; Blomfield 1993; Braet 1994; Bruinsma 2007; Crane 2006; Cruickshank 1995; Ehsanipoor 2014; Frega 2013; Frey 2013; Gunasekera 1992; Guo 2013; Haffenden 1993; Heinonen 2013; Himes 2007; Jakobsson 2009; Kitson 2014; Lima 2011; Martyn 2015; Noehr 2009a; Noehr 2009b; Ortoft 2010; Paraskevaidis 2002; Parikh 2008; Poon 2012; Sadler 2004; Samson 2005; Simoens 2012; Stout 2015; Tan 2004; Turlington 1996; Werner 2010), one of Fischer cone biopsy Excisor (FCBE) (Anwar 2016), eight of laser ablation (LA) (Anderson 1984; Bruinsma 2007; Forsmo 1996; Gunasekera 1992; Sadler 2004; Saunders 1986; Spitzer 1995; van Rooijen 1999), one of radical diathermy (RD) (Bruinsma 2007), two of cryotherapy (CT) (Crane 2006; Hemmingsson 1982), 15 of excision not otherwise specified (NOS) (Albrechtsen 2008; Armarnik 2011; Castanon 2012; El‐Bastawissi 1999; Fischer 2010; Jakobsson 2007; Martyn 2015; Miller 2015; Reilly 2012; Shanbhag 2009; Simoens 2012; Sjoborg 2007; van de Vijner 2010; Van Hentenryck 2012; Wuntakal 2013), five of ablation NOS (Ehsanipoor 2014; El‐Bastawissi 1999; Jakobsson 2007; Reilly 2012; Shanbhag 2009) and three of treatment NOS (Kirn 2015; Kristensen 1985; Kristensen 1993).
There were five types of comparison groups: external (general population) (Acharya 2005; Albrechtsen 2008; Andersen 1999; Anderson 1984; Andia 2011; Armarnik 2011; Bekassy 1996; Blomfield 1993; Braet 1994; Castanon 2012; Crane 2006; Cruickshank 1995; Ehsanipoor 2014; El‐Bastawissi 1999; Fischer 2010; Forsmo 1996; Frega 2013; Frey 2013; Gunasekera 1992; Haffenden 1993; Hagen 1993; Heinonen 2013; Jakobsson 2007; Jakobsson 2009; Jones 1979; Kirn 2015; Klaritsch 2006; Kristensen 1985; Kristensen 1993; Kuoppala 1986; Lima 2011; Ludviksson 1982; Miller 2015; Noehr 2009a; Noehr 2009b; Ortoft 2010; Paraskevaidis 2002; Parikh 2008; Poon 2012; Raio 1997; Reilly 2012; Samson 2005; Saunders 1986; Shanbhag 2009; Simoens 2012; Sjoborg 2007; Sozen 2014; Tan 2004; van de Vijner 2010; Van Hentenryck 2012; van Rooijen 1999; Weber 1979; Werner 2010); internal (pre‐treatment pregnancies) (Acharya 2005; Albrechtsen 2008; Andia 2011; Buller 1982; Castanon 2012; Cruickshank 1995; Hemmingsson 1982; Larsson 1982; Moinian 1982; Sagot 1995; Spitzer 1995; Stout 2015; Werner 2010; Wuntakal 2013); internal (self‐matching) (Anwar 2016; Bekassy 1996; Castanon 2012; Jakobsson 2009; Kristensen 1993; Ortoft 2010; Raio 1997; Sjoborg 2007); women who attended colposcopy with or without biopsy who did not undergo treatment (Bruinsma 2007; Castanon 2012; Frey 2013; Guo 2013; Himes 2007; Kitson 2014; Martyn 2015; Miller 2015; Noehr 2009a; Poon 2012; Sadler 2004; Stout 2015; Wuntakal 2013), and women with untreated high‐grade squamous intra‐epithelial lesion (HSIL) (El‐Bastawissi 1999; Ortoft 2010; Shanbhag 2009).
As many studies were old, dating back to the 1980s, we ensured that we avoided overlapping the same patients in different reports, particularly those from the Scandinavian countries. More specifically, seven studies were identified from Denmark. Weber 1979 was an old hospital‐based study (delivery during 1974 to 1975), with no overlapping with the other more recent studies. Kristensen 1985 identified treated women from the registry of a Danish county (1973 to1980), but only women delivering in a specific university hospital (up to 1982) were included. Kristensen 1993 was a population‐based study from the whole of Denmark including only parous women, with their first infant delivered in 1982, and their second during 1982 to 1987 (treatment during 1977 to 1987). There was no overlapping with Kristensen 1985. Andersen 1999 was a hospital‐based study including women treated during 1985 to 1989. There was a negligible degree of overlapping with Kristensen 1993 (for women with delivery in 1982, treatment during 1985 to 1987 and subsequent delivery until 1987). Noehr 2009a was a population‐based study from the whole of Denmark that was comprised of women treated during 1997 to 2005 with subsequent singleton pregnancy at the same time period. There was no overlapping with the previous studies. Noehr 2009b had the same design with Noehr 2009a and the only difference was that only women with twin pregnancies were included. Ortoft 2010 identified treated women from the Danish nationwide pathology database (1989 to 2007), but only women delivering at Aarhus University Hospital until 2007 were included. Approximately 8% of all Danish births take place in this hospital. Women delivering during 1997 to 2005 (nine years) were also included in Noehr 2009a, but women delivering during 1989 to 1996 and 2006 to 2007 (10 years) were not included in Noehr 2009a. Because there was no way to eliminate the overlapping, we decided to include both studies.
Four studies were identified from Finland. Some of the authors in Jakobsson 2007 (population‐based study from the whole of Finland), Jakobsson 2009 (hospital‐based study) and Heinonen 2013 (population‐based study from the whole of Finland) were common, and we carefully avoided duplication. More details about the outcomes extracted from each study are listed in Characteristics of included studies. Kuoppala 1986 was another hospital‐based study from Finland, but there was no overlapping with the aforementioned population‐based studies.
Five studies were identified from Norway. Albrechtsen 2008 was a population‐based study from the whole of Norway (excisional treatment during 1953 to 1979 or 1986 to 2003 and subsequent pregnancy during 1967 to 2003). All participants in Acharya 2005 and Sjoborg 2007 were also included in Albrechtsen 2008, thus we excluded Acharya 2005 and Sjoborg 2007 from the analyses, which also included Albrechtsen 2008. There was no overlapping between Acharya 2005 and Sjoborg 2007. Forsmo 1996 was a hospital‐based study which included women treated with LLETZ or LA during 1983 to 1988. There was overlapping with Albrechtsen 2008 for women treated with LLETZ during 1986 to 1988, but there was no way to eliminate this overlapping. In Hagen 1993, all women had received LLETZ during 1983to 1985 and there was no overlapping with Albrechtsen 2008.
Six studies were identified from Sweden. Five of these studies were hospital‐based (Bekassy 1996; Hemmingsson 1982; Ludviksson 1982; Moinian 1982; van Rooijen 1999). Larsson 1982 identified women from the South Swedish Regional Tumour Registry, but only women delivering in two hospitals were included. One of these hospitals was also included in Bekassy 1996, but the studies took place in different periods with no overlapping.
Although case‐control studies and studies assessing the impact of treatment performed during pregnancy were excluded, we included the study by Ortoft 2010, as only 18 women (2.5%) were treated during pregnancy, and the case‐control study by Castanon 2012, as additional data were provided by the authors.
There was no risk of overlapping for studies from other countries.
Excluded studies
The characteristics of the 47 excluded studies (not including reviews/meta‐analyses, conference proceedings, letters or duplicates) are shown in Characteristics of excluded studies. The PRISMA flowchart is shown in Figure 1 . We excluded studies without an untreated comparison group (Althuisius 2001; Berghella 2004; Berretta 2013; Bull‐Phelps 2007; Chevreau 2017; Conner 2013; Ferenczy 1995; Gordon 1991; Gronroos 1979; Khalid 2012; Kim 2016; Kindinger 2016; Kullander 1971; Leiman 1980; Liu 2014; Liverani 2016; Macvicar 1968; Mariya 2016; Masamoto 2008; Michelin 2009; Monaghan 1982; Nam 2010; Novikova 1994; Patrelli 2008; Radha Bai Prabhu 2010; Rafaeli‐Yehudai 2014; Sangkarat 2014; Shin 2010; Wakita 1990; Wongtiraporn 2014), studies with women treated during pregnancy (Mitsuhashi 2000; Rosen 1991; Seki 2010; Sljivancanin 2013), or late obstetric outcomes (beyond 24 weeks) that we did not study in this meta‐analysis (Ciavattini 2015; Gentry 2000; Kalliala 2012; Naleway 2015; Ricciotti 1995; Spracklen 2013), case‐control studies (Watson 2012), studies with a high‐risk treated and/or comparison group (i.e. previous history of mid trimester loss (Pils 2014), conceived through assisted reproductive technology (ART) (Ciavattini 2014; Pinborg 2015)), and studies assessing the impact of CIN on outcomes without information as to whether treatment was performed (Al‐Halal 2013; Smaldone 2010; Zuo 2011).
Risk of bias in included studies
The included studies were non‐randomised studies (NRS); they were prospective or retrospective cohorts and were therefore at high risk of underlying bias. The included studies varied with regard to design, the data source, the study and comparison populations, the reported outcomes, the length of follow‐up and the matching for possible confounders, as described above.
The summary of the authors' judgements about each 'Risk of bias' item is presented in Figure 2 and the detailed evaluation of 'Risk of bias' domains separately for each included study in Figure 3.
2.
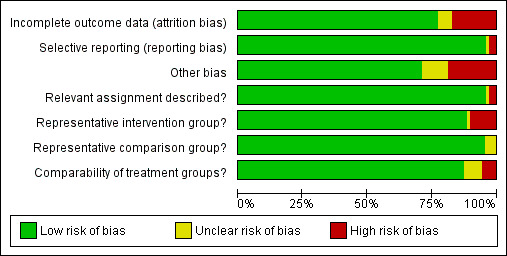
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
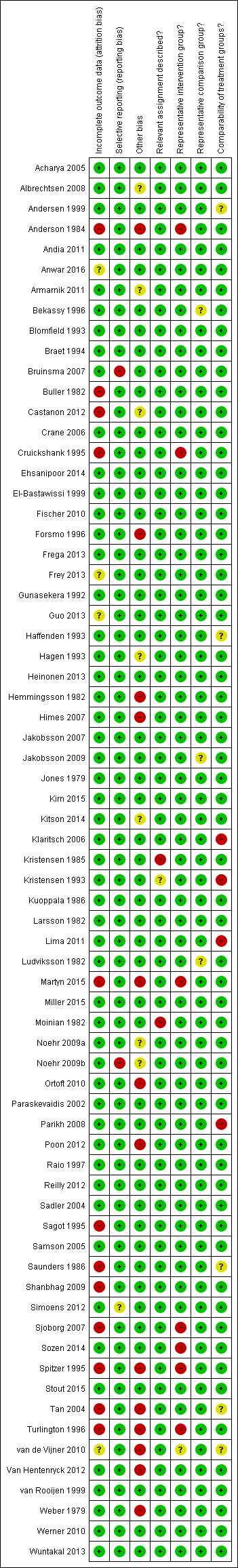
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
A description of the quality of the evidence is provided based on the GRADE assessment on maternal outcomes (Table 1) and on fetal outcomes (Table 2).
Summary of findings for the main comparison. The effect of treatment for CIN on maternal outcomes.
| The effect of treatment for CIN on maternal outcomes | ||||||
| Patient or population: women with known obstetric outcomes Setting: hospitals/clinics Intervention: treatment for CIN before pregnancy Comparison: women with no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with [comparison] | Risk with [intervention] | |||||
| PTB (< 37 w) | Study population | RR 1.75 (1.57 to 1.96) | 5,242,917 (59 observational studies) | ⊕⊝⊝⊝ VERY LOW 1 | ||
| 54 per 1000 | 95 per 1000 (85 to 106) | |||||
| PTB (< 32 to 34 w) | Study population | RR 2.25 (1.79 to 2.82) | 3,793,874 (24 observational studies) | ⊕⊝⊝⊝ VERY LOW 2 | ||
| 14 per 1000 | 32 per 1000 (26 to 40) | |||||
| PTB (< 28 to 30 w) | Study population | RR 2.23 (1.55 to 3.22) | 3,910,629 (8 observational studies) | ⊕⊝⊝⊝ VERY LOW 3 | ||
| 3 per 1000 | 7 per 1000 (5 to 11) | |||||
| PTB (< 37 w) ‐ Repeat cones versus No Treatment | Study population | RR 3.78 (2.65 to 5.39) | 1,317,284 (11 observational studies) | ⊕⊝⊝⊝ VERY LOW 4 | ||
| 41 per 1000 | 156 per 1000 (109 to 222) | |||||
| pPROM (<3 7 w) | Study population | RR 2.36 (1.76 to 3.17) | 477,011 (21 observational studies) | ⊕⊝⊝⊝ VERY LOW 5 | ||
| 34 per 1000 | 80 per 1000 (60 to 108) | |||||
| PTB (< 37 w) ‐ Depth ≤ 10 mm to 12 mm versus No Treatment | Study population | RR 1.54 (1.09 to 2.18) | 550,929 (8 observational studies) | ⊕⊝⊝⊝ VERY LOW 6 | ||
| 34 per 1000 | 53 per 1000 (37 to 75) | |||||
| PTB (< 37 w) ‐ PTB (< 37 w) ‐ Depth ≥10 mm to 12 mm versus No Treatment | Study population | RR 1.93 (1.62 to 2.31) | 552,711 (8 observational studies) | ⊕⊕⊕⊝ LOW 7 | ||
| 34 per 1000 | 66 per 1000 (55 to 79) | |||||
| PTB (< 37w) ‐ PTB (<37w) ‐ Depth ≥15 to 17mm versus No Treatment | Study population | RR 2.77 (1.95 to 3.93) | 544,986 (4 observational studies) | ⊕⊕⊕⊕ VERY LOW 8 | ||
| 34 per 1000 | 94 per 1000 (66 to 134) | |||||
| PTB (< 37 w) ‐ PTB (< 37 w) ‐ Depth ≥ 20 mm versus No Treatment | Study population | RR 4.91 (2.06 to 11.68) | 543,750 (3 observational studies) | ⊕⊕⊕⊕ VERY LOW 9 | ||
| 34 per 1000 | 167 per 1000 (70 to 397) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Low‐quality evidence (based on observational studies only) is downgraded one level because of considerable heterogeneity (I2 90%)
2 Low‐quality evidence (based on observational studies only) is downgraded one level because of considerable heterogeneity (I2 83%) and suspected publication bias
3 Low‐quality evidence (based on observational studies only) is downgraded one level because of considerable heterogeneity (I2 84%)
4 Low‐quality evidence (based on observational studies only) is downgraded one level because of considerable heterogeneity (I2 75%)
5 Low‐quality evidence (based on observational studies only) is downgraded one level because of considerable heterogeneity (I2 79%)
6 Low‐quality evidence (based on observational studies only) is downgraded one level because of substantial heterogeneity (I2 67%)
7 Low‐quality evidence (based on observational studies only); heterogeneity was low (I2 37%)
8 Low‐quality evidence (based on observational studies only) is downgraded one level because of moderate heterogeneity (I2 53%)
9 Low‐quality evidence (based on observational studies only) is downgraded one level because of considerable heterogeneity (I2 77%)
Summary of findings 2. The effect of treatment for CIN on neonatal outcomes.
| The effect of treatment for CIN on neonatal outcomes | ||||||
| Patient or population: women with known obstetric outcomes Setting: hospitals/clinics Intervention: treatment for CIN before pregnancy Comparison: women with no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with No Treatment | Risk with Treatment | |||||
| LBW (< 2500 g) ‐ Treatment versus No Treatment | Study population | RR 1.81 (1.58 to 2.07) | 1,348,206 (30 observational studies) | ⊕⊝⊝⊝ VERY LOW 1 | ||
| 37 per 1000 | 66 per 1000 (58 to 76) | |||||
| NICU Admission ‐ Treatment versus No Treatment | Study population | RR 1.45 (1.16 to 1.81) | 2557 (8 observational studies) | ⊕⊕⊝⊝ LOW 2 | ||
| 89 per 1000 | 130 per 1000 (104 to 162) | |||||
| Perinatal Mortality ‐ Treatment versus No Treatment | Study population | RR 1.51 (1.13 to 2.03) | 1,659,433 (23 observational studies) | ⊕⊕⊝⊝ LOW 3 | ||
| 7 per 1000 | 11 per 1000 (8 to 14) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Low‐quality evidence (based on observational studies only) is downgraded one level because of substantial heterogeneity (I2 63%) 2 Low‐quality evidence (based on observational studies only); there was no heterogeneity (I2 0%) 3 Low‐quality evidence (based on observational studies only); heterogeneity was low (I2 36%)
Allocation
Representativeness of intervention group
Risk of bias due to unrepresentative intervention group was generally considered low. In six studies (Anderson 1984; Cruickshank 1995; Martyn 2015; Sjoborg 2007; Spitzer 1995; Turlington 1996), the representativeness of the intervention group was considered inadequate due to considerable, over 20%, non‐responder rate to follow‐up questionnaires, which might result in selection bias due to women in higher social classes being more prone to answer. In one study (Sozen 2014), the intervention group was considered unrepresentative due to the very small number of participants (15 patients) and in one study (van de Vijner 2010), the representativeness was unclear due to lack of information regarding the number of women providing questionnaire‐based follow‐up data.
Representativeness of comparison group
The representativeness of the comparison group was considered good in all but three studies. In two studies (Bekassy 1996; Jakobsson 2009), the untreated external comparison group was not drawn from the same source as the treated population and in one study (Ludviksson 1982), the source of reference population was unclear.
Comparability of the groups
The comparison group used and the adjustment for possible risk factors are important measures of study quality and risk of bias. Of the studies that used an external comparison group (n = 53, Included studies), 49 matched for known risk factors or performed a regression analysis to control for known confounders. Only four studies did not include any measures to control for confounders (Klaritsch 2006; Kristensen 1993; Lima 2011; Parikh 2008) and were hence considered to be of high risk of bias. Of the remaining 49 studies, 29 studies used matching (Acharya 2005; Andersen 1999; Anderson 1984; Bekassy 1996; Blomfield 1993; Braet 1994; Cruickshank 1995; Fischer 2010; Forsmo 1996; Frega 2013; Gunasekera 1992; Haffenden 1993; Hagen 1993; Jones 1979; Kirn 2015; Kristensen 1985; Kuoppala 1986; Ludviksson 1982; Paraskevaidis 2002; Raio 1997; Samson 2005; Saunders 1986; Simoens 2012; Sozen 2014; Tan 2004; van de Vijner 2010; Van Hentenryck 2012; van Rooijen 1999; Weber 1979), 17 studies used regression analysis (Albrechtsen 2008; Andia 2011; Armarnik 2011; Castanon 2012; Crane 2006; Ehsanipoor 2014; Heinonen 2013; Jakobsson 2007; Jakobsson 2009; Miller 2015; Noehr 2009a; Noehr 2009b; Ortoft 2010; Poon 2012; Reilly 2012; Shanbhag 2009; Werner 2010) and three studies used both matching and regression analysis (El‐Bastawissi 1999; Frey 2013; Sjoborg 2007). In five studies (Andersen 1999; Haffenden 1993; Saunders 1986; Tan 2004; van de Vijner 2010), the risk was considered unclear due to incomplete matching between the two groups.
Of the 13 studies that had an internal comparison group (pre‐treatment pregnancies), two used matching (Larsson 1982; Spitzer 1995) and four studies also performed regression analysis (Albrechtsen 2008; Castanon 2012; Werner 2010; Wuntakal 2013). Of the eight studies that had an internal comparison group (self‐matching), three studies also performed regression analysis (Castanon 2012; Jakobsson 2009; Sjoborg 2007).
Of the 15 studies that used as a comparison group women who attended colposcopy with or without biopsy who did not undergo treatment or untreated HSIL, 10 studies performed regression analysis (Bruinsma 2007; Castanon 2012; El‐Bastawissi 1999; Miller 2015; Noehr 2009a; Ortoft 2010; Poon 2012; Sadler 2004; Shanbhag 2009; Wuntakal 2013), four studies used matching for confounders (Guo 2013; Kitson 2014; Martyn 2015; Stout 2015), and one study (Frey 2013) used both matching and regression analysis. The most common confounding factors that were used in matching or regression analysis were age, parity, smoking, race/ethnicity and social class.
Blinding
Relevant assignment described (Performance bias)
Bias due to unclear or inappropriate treatment assignment was considered low in most studies. In two studies the risk was deemed high, due to the conisation type not being described (Kristensen 1985) and the CKC treatment being described to be more radical than as usual (Moinian 1982). In one study (Kristensen 1993), the risk was unclear as the method of treatment was not specified but could have included three different types of treatment.
Incomplete outcome data
Overall 12/69 studies (17%) were considered to be at high risk of attrition bias due to incomplete outcome data. In 10 studies information about the subsequent pregnancies was missing for more than 20% of the study population (Anderson 1984; Buller 1982; Castanon 2012; Cruickshank 1995; Martyn 2015; Sagot 1995; Sjoborg 2007; Spitzer 1995; Tan 2004; Turlington 1996), in one study (Saunders 1986), the method to retrieve subsequent pregnancy data was deemed non‐systematic, i.e. contacting local general practitioners, and in one study (Shanbhag 2009), the method of CIN treatment was unclear for more than 50% and these women were excluded from all analyses. In four studies (Anwar 2016; Frey 2013; Guo 2013; van de Vijner 2010), the number of women lost to follow‐up and therefore the risk of attrition bias was unclear and for all other studies the risk was deemed to be low.
Selective reporting
Reporting bias due to selective reporting was considered low in almost all included studies. For two studies (Bruinsma 2007; Noehr 2009b), the risk was deemed high due to the results being reported only for the whole treated‐group, not stratified according to the treatment type. For one study (Simoens 2012), the risk was considered to be unclear due to reporting the outcomes only after some, not all included treatments.
Other potential sources of bias
The outcome ascertainment was made with a questionnaire in seven studies (Martyn 2015; Ortoft 2010; Poon 2012; Spitzer 1995; Turlington 1996; van de Vijner 2010; Weber 1979), which might predispose to recall bias and misclassification and the risk of other bias was hence considered high in these studies. A further five studies were as well considered to be of high risk of other bias, due to contradictions between the tables and the text (Himes 2007; Tan 2004; Van Hentenryck 2012), due to including treated women in comparison groups (Forsmo 1996), and marked temporal differences in outcomes between the exposed and unexposed groups (Hemmingsson 1982). The risk of other bias was considered unclear in six studies. In two studies (Albrechtsen 2008; Castanon 2012), some women in the comparison group might have been being treated but the effect of this possible misclassification was deemed unclear, in one study some of the data were discrepant but the possible effect of this again deemed unclear (Armarnik 2011), and in two studies (Noehr 2009a; Noehr 2009b), the LLETZ group might have included women treated with LC as well, but the number was considered negligible and unclear whether it would introduce bias.
We assessed the presence of publication bias for the outcomes presented in the 'Summary of findings' tables if more than 10 studies were included. There was evidence of publication bias only for PTB at < 32 to 34 weeks of gestation. None of the other outcomes showed any evidence of publication bias.
Effects of interventions
MATERNAL OUTCOMES
Preterm birth
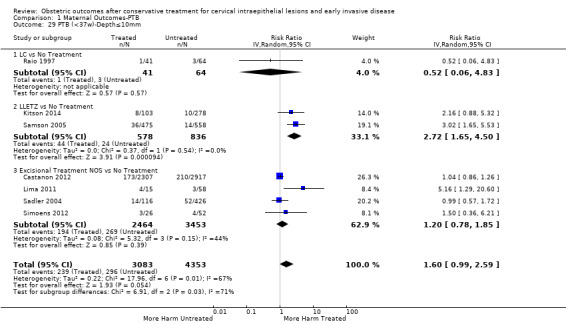
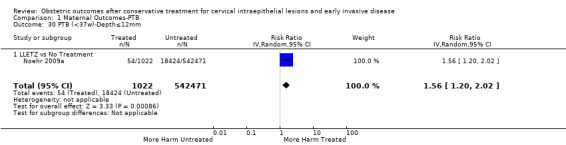
The risk of preterm birth was increased after cervical treatment. This was the case for prematurity rate overall at less than 37 weeks of gestation (Analysis 1.1: 59 studies, 5,242,917 women, 10.7% versus 5.4%, risk ratio (RR) 1.75, 95% confidence interval (CI) 1.57 to 1.96; Analysis 1.2: 59 studies, 5,242,917 women, 10.7% versus 5.4%, RR 1.78, 95% CI 1.60 to 1.98, very low quality of evidence because of considerable (I2 90% and 88%, respectively) heterogeneity (Table 1)); for severe prematurity less than 32 to 34 weeks of gestation (Analysis 1.3: 24 studies, 3,793,874 women, 3.5% versus 1.4%, RR 2.25, 95% CI 1.79 to 2.82; Analysis 1.4: 24 studies, 3,793,874 women, 3.5% versus 1.4%, RR 2.35, 95% CI 1.88 to 2.95, very low quality of evidence because of considerable (I2 83% and 82%, respectively) heterogeneity and suspected publication bias (Table 1)); and extreme prematurity less than 28 to 30 weeks of gestation (Analysis 1.5: 8 studies, 3,910,629 women, 1.0% versus 0.3%, RR 2.23, 95% CI 1.55 to 3.22; Analysis 1.6: 8 studies, 3,910,629 women, 1.0% versus 0.3%, RR 2.43, 95% CI 1.69 to 3.49, very low quality of evidence because of considerable (I2 84% and 82%, respectively) heterogeneity (Table 1)). We further conducted analyses for more specific gestational ages cut‐offs (i.e. 34 weeks or less (Analysis 1.7 (RR 2.59, 95% CI 1.78 to 3.77); Analysis 1.8 (RR 2.56, 95% CI 1.78 to 3.69)), less than 32 to 33 weeks (Analysis 1.9 (RR 2.08, 95% CI 1.55 to 2.79); Analysis 1.10 (RR 2.26, 95% CI 1.70 to 3.01)), less than 30 weeks (Analysis 1.11 (RR 2.86, 95% CI 0.12 to 69.11); Analysis 1.12 (RR 2.86, 95% CI 0.12 to 69.11)), less than 28 weeks (Analysis 1.13 (RR 2.22, 95% CI 1.54 to 3.22); Analysis 1.14 (RR 2.52, 95% CI 1.71 to 3.72)) for broader treatment groups as well as individual techniques. The impact of treatment was not different for nulliparous (Analysis 1.15) and multiparous (Analysis 1.16) women. The effect of multiple treatment on the risk of prematurity was substantially higher than the effect of single treatments (single treatment versus no treatment (Analysis 1.17): 17 studies, 1,367,023 women, 7.5% versus 4.2%, RR 1.75, 95% CI 1.49 to 2.06; repeat treatment versus no treatment (Analysis 1.18): 11 studies, 1,317,284 women, 13.2% versus 4.1%, RR 3.78, 95% CI 2.65 to 5.39, very low quality of evidence because of considerable (I2 75%) heterogeneity (Table 1)). The relative risk of preterm birth for two excisional treatments not otherwise specified (NOS) was as high as 5.48 (95% CI 2.68 to 11.24) and that of two loop excisions as high as 2.81 (95% CI 2.33 to 3.39), as compared to no treatment.
1.1. Analysis.
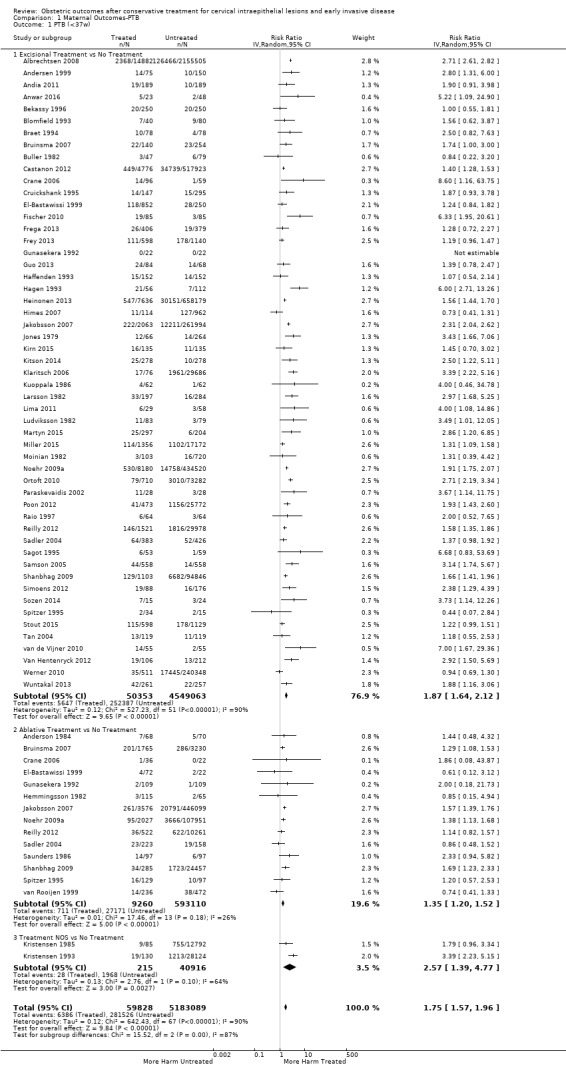
Comparison 1 Maternal Outcomes‐PTB, Outcome 1 PTB (<37w).
1.2. Analysis.
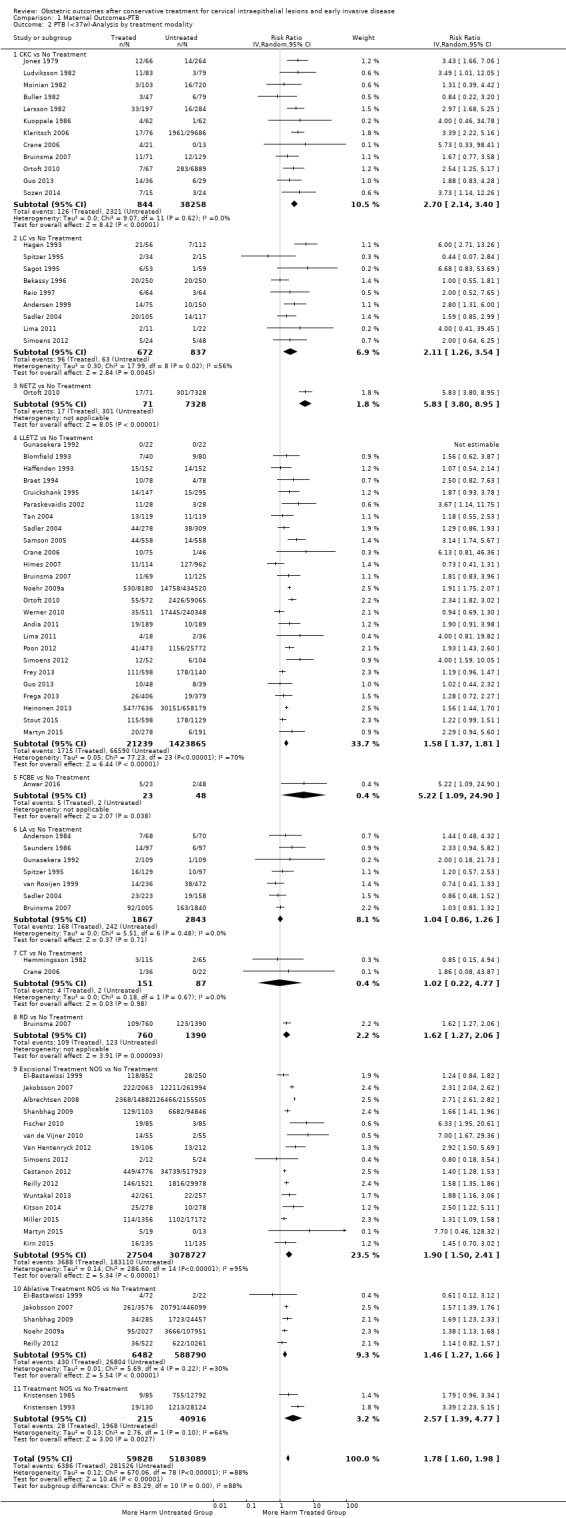
Comparison 1 Maternal Outcomes‐PTB, Outcome 2 PTB (<37w)‐Analysis by treatment modality.
1.3. Analysis.
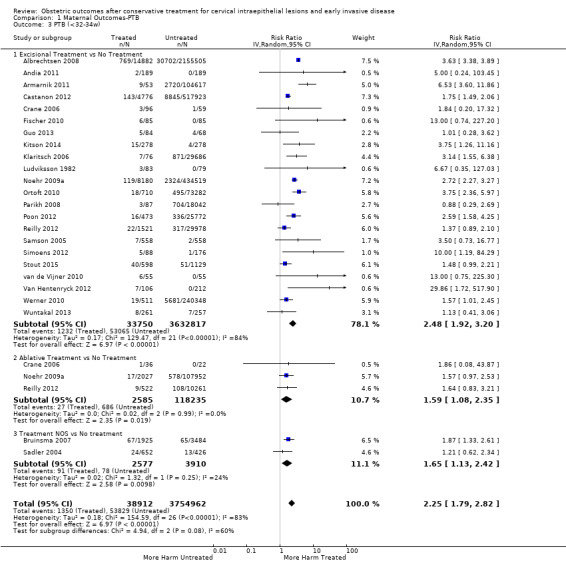
Comparison 1 Maternal Outcomes‐PTB, Outcome 3 PTB (<32‐34w).
1.4. Analysis.

Comparison 1 Maternal Outcomes‐PTB, Outcome 4 PTB (<32‐34w)‐Analysis by treatment modality.
1.5. Analysis.
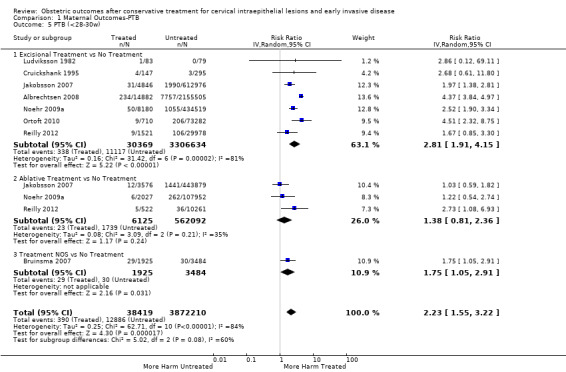
Comparison 1 Maternal Outcomes‐PTB, Outcome 5 PTB (<28‐30w).
1.6. Analysis.
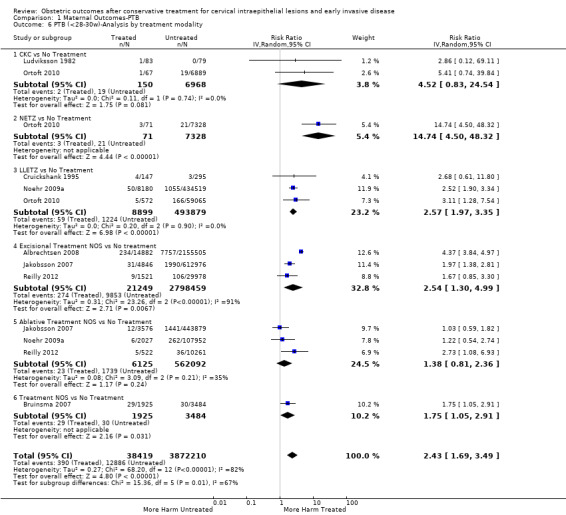
Comparison 1 Maternal Outcomes‐PTB, Outcome 6 PTB (<28‐30w)‐Analysis by treatment modality.
1.7. Analysis.
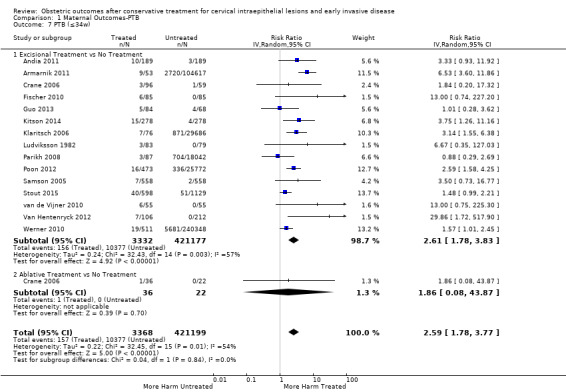
Comparison 1 Maternal Outcomes‐PTB, Outcome 7 PTB (≤34w).
1.8. Analysis.

Comparison 1 Maternal Outcomes‐PTB, Outcome 8 PTB (≤34w)‐Analysis by treatment modality.
1.9. Analysis.
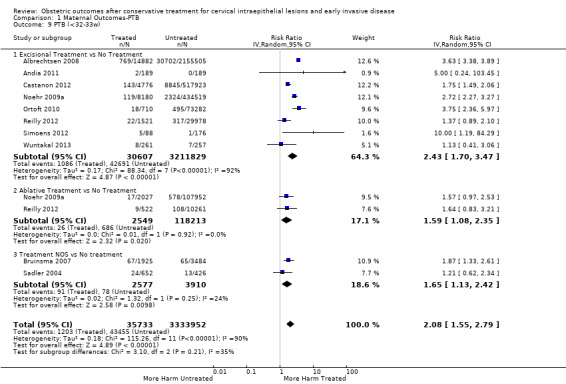
Comparison 1 Maternal Outcomes‐PTB, Outcome 9 PTB (<32‐33w).
1.10. Analysis.

Comparison 1 Maternal Outcomes‐PTB, Outcome 10 PTB (<32‐33w)‐Analysis by treatment modality.
1.11. Analysis.

Comparison 1 Maternal Outcomes‐PTB, Outcome 11 PTB (<30w).
1.12. Analysis.
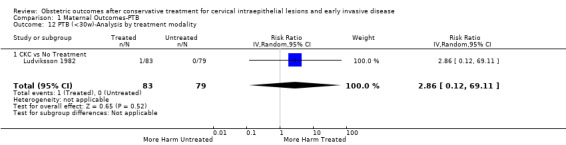
Comparison 1 Maternal Outcomes‐PTB, Outcome 12 PTB (<30w)‐Analysis by treatment modality.
1.13. Analysis.
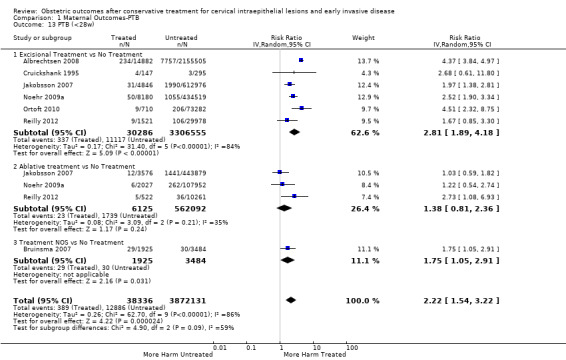
Comparison 1 Maternal Outcomes‐PTB, Outcome 13 PTB (<28w).
1.14. Analysis.
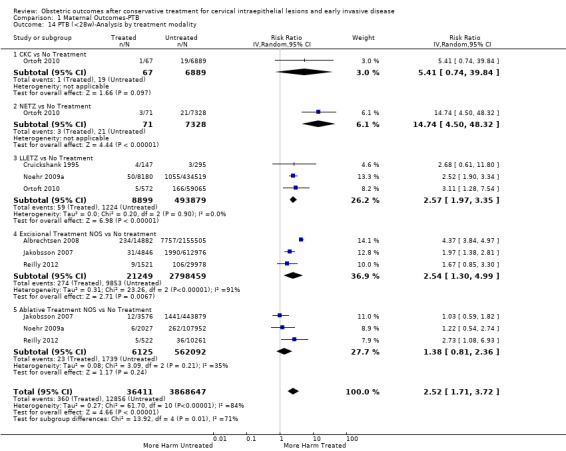
Comparison 1 Maternal Outcomes‐PTB, Outcome 14 PTB (<28w)‐Analysis by treatment modality.
1.17. Analysis.
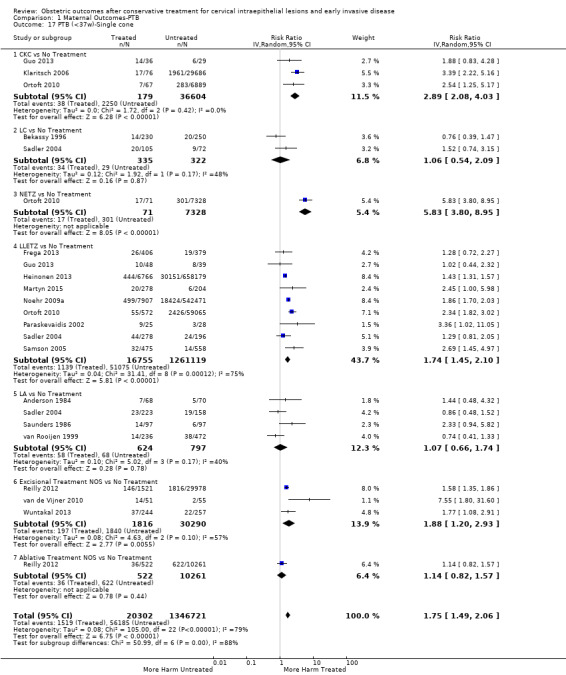
Comparison 1 Maternal Outcomes‐PTB, Outcome 17 PTB (<37w)‐Single cone.
1.18. Analysis.

Comparison 1 Maternal Outcomes‐PTB, Outcome 18 PTB (<37w)‐Repeat cones.
The magnitude of the effect of treatment was higher for excision (Analysis 1.1.1: RR 1.87, 95% CI 1.64 to 2.12) rather than ablation (Analysis 1.1.2: RR 1.35, 95% CI 1.20 to 1.52) and for more radical treatment techniques. The risk of preterm birth at less than 37 weeks of gestation for individual treatment techniques varied: cold knife conisation (CKC) (Analysis 1.2.1: RR 2.70, 95% CI 2.14 to 3.40); laser conisation (LC) (Analysis 1.2.2: RR 2.11, 95% CI 1.26 to 3.54); large loop excision of the transformation zone (LLETZ) (Analysis 1.2.4: RR 1.58, 95% CI 1.37 to 1.81); laser ablation (LA) (Analysis 1.2.6: RR 1.04, 95% CI 0.86 to 1.26); CT (Analysis 1.2.7: RR 1.02, 95% CI 0.22 to 4.77); excision NOS (Analysis 1.2.9: RR 1.90, 95% CI 1.50 to 2.41); ablation NOS (Analysis 1.2.10: RR 1.46, 95% CI 1.27 to 1.66) and treatment NOS (Analysis 1.2.11: (RR 2.57, 95% CI 1.39 to 4.77). Similar trends were noted for severe (Analysis 1.4) and extreme (Analysis 1.6) prematurity. Some, but not all, types of treatments were also associated with an increased risk of preterm birth for women with singleton (Analysis 1.19) or multiple pregnancies (Analysis 1.20; Analysis 1.21; Analysis 1.22), but the results were inconsistent due to the small number of studies.
1.19. Analysis.

Comparison 1 Maternal Outcomes‐PTB, Outcome 19 PTB (<37w)‐Singleton pregnancies.
1.20. Analysis.
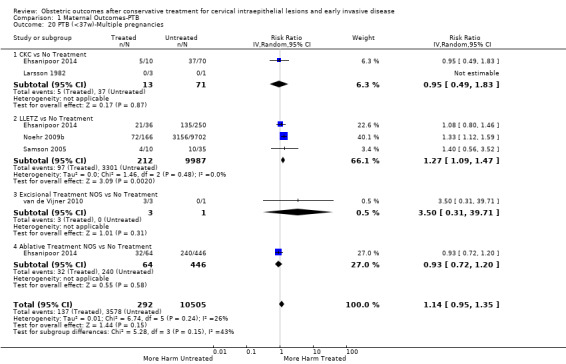
Comparison 1 Maternal Outcomes‐PTB, Outcome 20 PTB (<37w)‐Multiple pregnancies.
1.21. Analysis.

Comparison 1 Maternal Outcomes‐PTB, Outcome 21 PTB (<32‐34w)‐Multiple pregnancies.
1.22. Analysis.
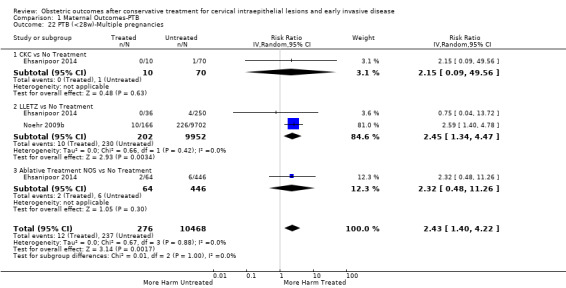
Comparison 1 Maternal Outcomes‐PTB, Outcome 22 PTB (<28w)‐Multiple pregnancies.
Preterm birth — dimensions of excised cone
We further analysed the data on the risk of preterm birth at less than 37 weeks of gestation for different cone depths. The risk for treated versus untreated women was greater for women with cone depth ≤ 10 mm to 12 mm (Analysis 1.23: 8 studies, 550,929 women, 7.1 % versus 3.4%, RR 1.54, 95% CI 1.09 to 2.18, very low quality of evidence because of substantial (I2 67%) heterogeneity (Table 1)), and the magnitude of effect increased with increasing cone depth (≥ 10 mm to 12 mm (Analysis 1.24): 8 studies, 552,711 women, 9.8 % versus 3.4%, RR 1.93, 95% CI 1.62 to 2.31, low quality of evidence (Table 1); ≥ 15 mm to 17 mm (Analysis 1.25): 4 studies, 544,986 women, 10.1 % versus 3.4%, RR 2.77, 95% CI 1.95 to 3.93, very low quality of evidence because of moderate (I2 53%) heterogeneity (Table 1); ≥ 20 mm (Analysis 1.26): 3 studies, 543,750 women, 10.2 % versus 3.4%, RR 4.91, 95% CI 2.06 to 11.68, very low quality of evidence because of considerable (I2 67%) heterogeneity (Table 1)). A similar increasing effect was observed, although based on only one study, with increasing cone volume (less than 6 cc (Analysis 1.27): 1 study, 550 women, 8.1% versus 3.6%, RR 2.25, 95%CI 1.09 to 4.66; > 6 cc (Analysis 1.28): 1 study, 284 women, 50.0% versus 3.6%, RR 13.90, 95% CI 5.09 to 37.98). We have performed additional analyses assessing several more specific cone depths/volumes without merging these in broader groups (Analysis 1.29; Analysis 1.30; Analysis 1.31; Analysis 1.32; Analysis 1.33; Analysis 1.34; Analysis 1.35; Analysis 1.36; Analysis 1.37; Analysis 1.38; Analysis 1.39; Analysis 1.40; Analysis 1.41; Analysis 1.42).
1.23. Analysis.

Comparison 1 Maternal Outcomes‐PTB, Outcome 23 PTB (<37w)‐Depth≤10‐12mm.
1.24. Analysis.
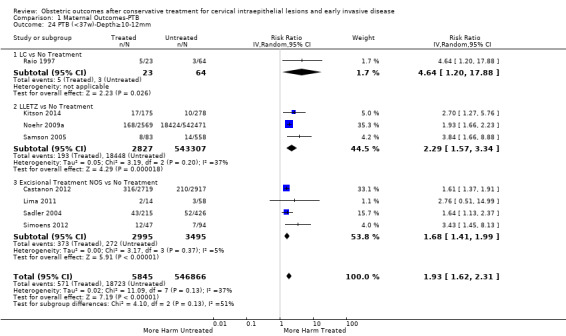
Comparison 1 Maternal Outcomes‐PTB, Outcome 24 PTB (<37w)‐Depth≥10‐12mm.
1.25. Analysis.
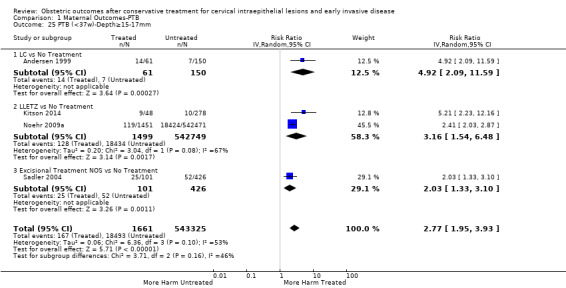
Comparison 1 Maternal Outcomes‐PTB, Outcome 25 PTB (<37w)‐Depth≥15‐17mm.
1.26. Analysis.
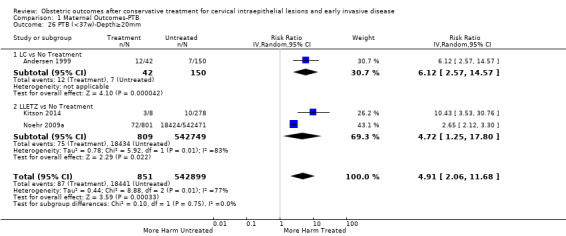
Comparison 1 Maternal Outcomes‐PTB, Outcome 26 PTB (<37w)‐Depth≥20mm.
1.27. Analysis.
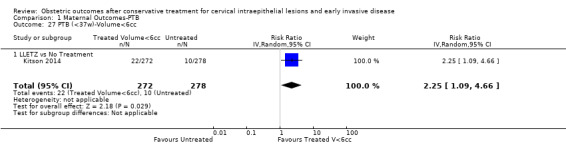
Comparison 1 Maternal Outcomes‐PTB, Outcome 27 PTB (<37w)‐Volume<6cc.
1.28. Analysis.
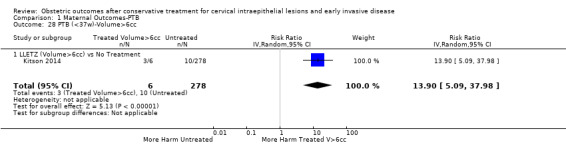
Comparison 1 Maternal Outcomes‐PTB, Outcome 28 PTB (<37w)‐Volume>6cc.
1.29. Analysis.
Comparison 1 Maternal Outcomes‐PTB, Outcome 29 PTB (<37w)‐Depth≤10mm.
1.30. Analysis.
Comparison 1 Maternal Outcomes‐PTB, Outcome 30 PTB (<37w)‐Depth≤12mm.
1.31. Analysis.
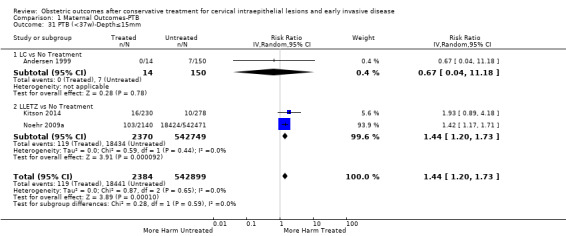
Comparison 1 Maternal Outcomes‐PTB, Outcome 31 PTB (<37w)‐Depth≤15mm.
1.32. Analysis.
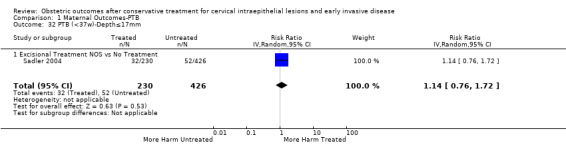
Comparison 1 Maternal Outcomes‐PTB, Outcome 32 PTB (<37w)‐Depth≤17mm.
1.33. Analysis.

Comparison 1 Maternal Outcomes‐PTB, Outcome 33 PTB (<37w)‐Depth≤15‐17mm.
1.34. Analysis.
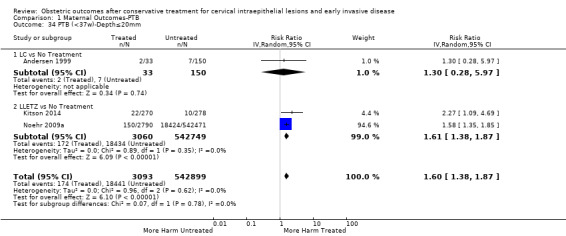
Comparison 1 Maternal Outcomes‐PTB, Outcome 34 PTB (<37w)‐Depth≤20mm.
1.35. Analysis.
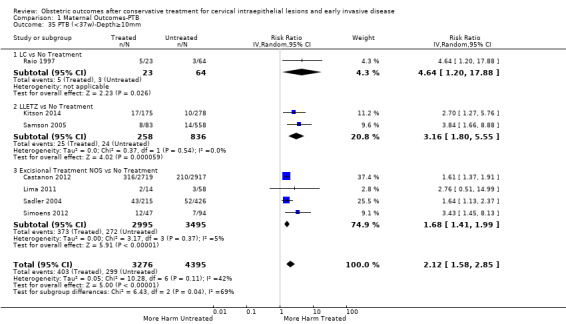
Comparison 1 Maternal Outcomes‐PTB, Outcome 35 PTB (<37w)‐Depth≥10mm.
1.36. Analysis.
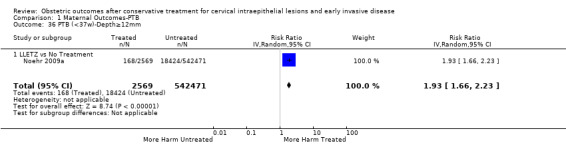
Comparison 1 Maternal Outcomes‐PTB, Outcome 36 PTB (<37w)‐Depth≥12mm.
1.37. Analysis.
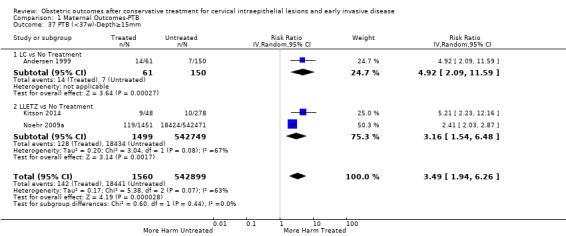
Comparison 1 Maternal Outcomes‐PTB, Outcome 37 PTB (<37w)‐Depth≥15mm.
1.38. Analysis.
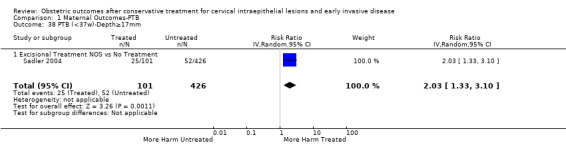
Comparison 1 Maternal Outcomes‐PTB, Outcome 38 PTB (<37w)‐Depth≥17mm.
1.39. Analysis.
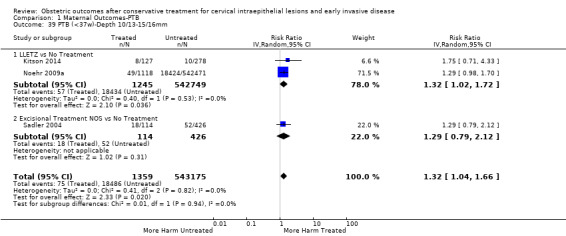
Comparison 1 Maternal Outcomes‐PTB, Outcome 39 PTB (<37w)‐Depth 10/13‐15/16mm.
1.40. Analysis.

Comparison 1 Maternal Outcomes‐PTB, Outcome 40 PTB (<37w)‐Depth 15/16‐19/20mm.
1.41. Analysis.
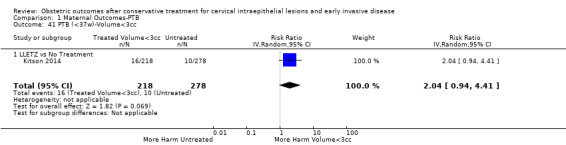
Comparison 1 Maternal Outcomes‐PTB, Outcome 41 PTB (<37w)‐Volume<3cc.
1.42. Analysis.
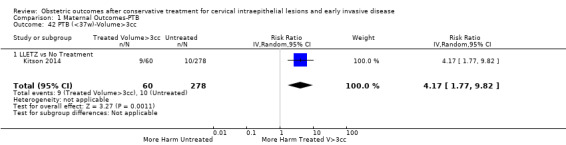
Comparison 1 Maternal Outcomes‐PTB, Outcome 42 PTB (<37w)‐Volume>3cc.
The comparison of treated women for different cone depths revealed that deep excisions increased the risk of preterm birth (less than 37 weeks) as opposed to less deep excisions and the magnitude of the effect increased in longer cones (≥ 10 mm to 12 mm versus ≤ 10 mm to 12 mm (Analysis 1.43): 7 studies, 6359 women, 12.3 % versus 7.8%, RR 1.54, 95% CI 1.31 to 1.80; ≥ 15 mm to 17 mm versus ≤ 15 mm to 17 mm (Analysis 1.44): 4 studies, 4275 women, 10.1 % versus 5.7%, RR 1.82, 95% CI 1.47 to 2.26; ≥ 20 mm versus ≤ 20 mm (Analysis 1.45): 3 studies, 3944 women, 10.2 % versus 5.6%, RR 2.79, 95% CI 1.24 to 6.27). The findings were similar for the comparison of cone volumes (> 3 cc versus less than 3 cc (Analysis 1.46): 1 study, 278 women, 15.0 % versus 7.3%, RR 2.04, 95% CI 0.95 to 4.39; > 6 cc versus less than 6 cc (Analysis 1.47): 1 study, 278 women, 50.0% versus 8.1%, RR 6.18, 95% CI 2.53 to 15.13). Further subgroup analyses of comparisons of more specific cone depths are also shown (Analysis 1.48; Analysis 1.49; Analysis 1.50).
1.43. Analysis.

Comparison 1 Maternal Outcomes‐PTB, Outcome 43 PTB (<37w)‐Depth≥10‐12mm vs ≤10‐12mm.
1.44. Analysis.
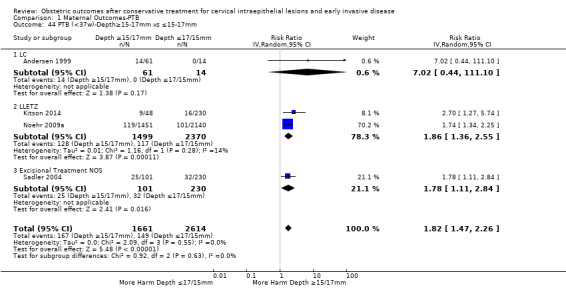
Comparison 1 Maternal Outcomes‐PTB, Outcome 44 PTB (<37w)‐Depth≥15‐17mm vs ≤15‐17mm.
1.45. Analysis.
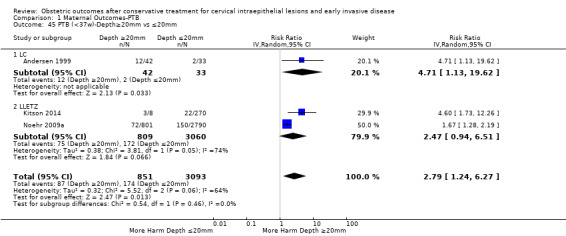
Comparison 1 Maternal Outcomes‐PTB, Outcome 45 PTB (<37w)‐Depth≥20mm vs ≤20mm.
1.46. Analysis.
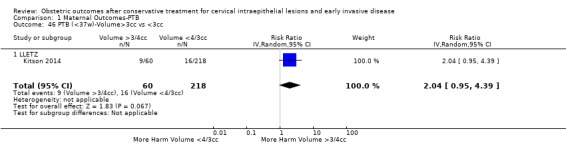
Comparison 1 Maternal Outcomes‐PTB, Outcome 46 PTB (<37w)‐Volume>3cc vs <3cc.
1.47. Analysis.

Comparison 1 Maternal Outcomes‐PTB, Outcome 47 PTB (<37w)‐Volume>6cc vs <6cc.
1.48. Analysis.
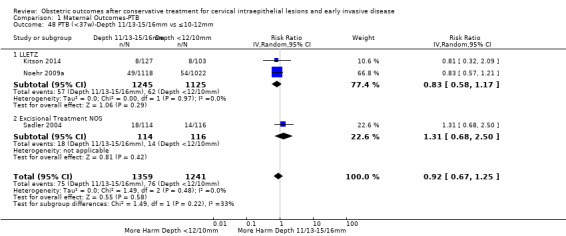
Comparison 1 Maternal Outcomes‐PTB, Outcome 48 PTB (<37w)‐Depth 11/13‐15/16mm vs ≤10‐12mm.
1.49. Analysis.
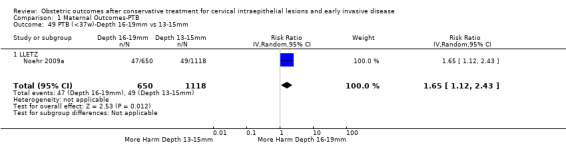
Comparison 1 Maternal Outcomes‐PTB, Outcome 49 PTB (<37w)‐Depth 16‐19mm vs 13‐15mm.
1.50. Analysis.

Comparison 1 Maternal Outcomes‐PTB, Outcome 50 PTB (<37w)‐Depth≥20mm vs 15/16‐19/20mm.
Preterm birth — effect of the comparison group
The impact that the choice of comparison group may have on the magnitude of effect was assessed by additional analysis that classified different studies according to the comparator used. The magnitude of effect was higher when an external comparison group was used (Analysis 1.51: 44 studies, 5,192,047 women, 10.5% versus 5.4%, RR 1.92, 95% CI 1.70 to 2.16), followed by internal comparators (self‐matching (Analysis 1.52): 8 studies, 2987 women, 10.9% versus 7.0%, RR 1.59, 95% CI 1.19 to 2.13; pre‐treatment pregnancies (Analysis 1.53): 13 studies, 83,404 women, 14.1% versus 6.4%, RR 1.39, 95% CI 0.98 to 1.96) and ultimately women with disease but no treatment (Analysis 1.54: 13 studies, 74,958 women, 8.8% versus 6.0%, RR 1.27, 95% CI 1.14 to 1.41). A further analysis of treated women versus those with untreated HSIL was also explored although this included only three studies (Analysis 1.55: 3 studies, 3764 women, 12% versus 7.8%, RR 1.37, 95% CI 0.85 to 2.19). The forest plot where we compared treatment in general to the different comparison groups can be seen in Analysis 1.56. When women with disease but no treatment and the pregnancies of the parous women before treatment were compared to the general population, the risk of preterm birth was higher (Analysis 1.57: 15 studies, 4,357,998 women, 5.9% versus 5.6%, RR 1.24, 95% CI 1.14 to 1.34) based on very low‐quality evidence. These groups were also analysed separately (Analysis 1.57.1; Analysis 1.57.2; Analysis 1.57.3).
1.51. Analysis.

Comparison 1 Maternal Outcomes‐PTB, Outcome 51 PTB (<37w)‐Untreated External Comparison Group.
1.52. Analysis.
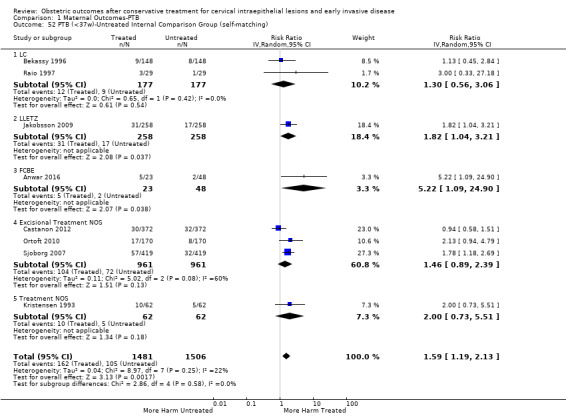
Comparison 1 Maternal Outcomes‐PTB, Outcome 52 PTB (<37w)‐Untreated Internal Comparison Group (self‐matching).
1.53. Analysis.
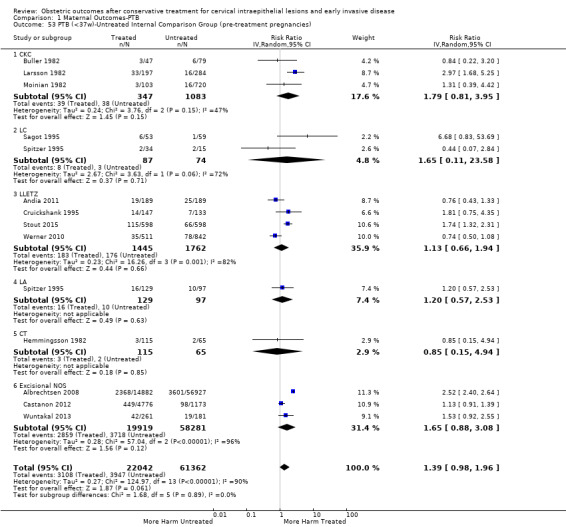
Comparison 1 Maternal Outcomes‐PTB, Outcome 53 PTB (<37w)‐Untreated Internal Comparison Group (pre‐treatment pregnancies).
1.54. Analysis.
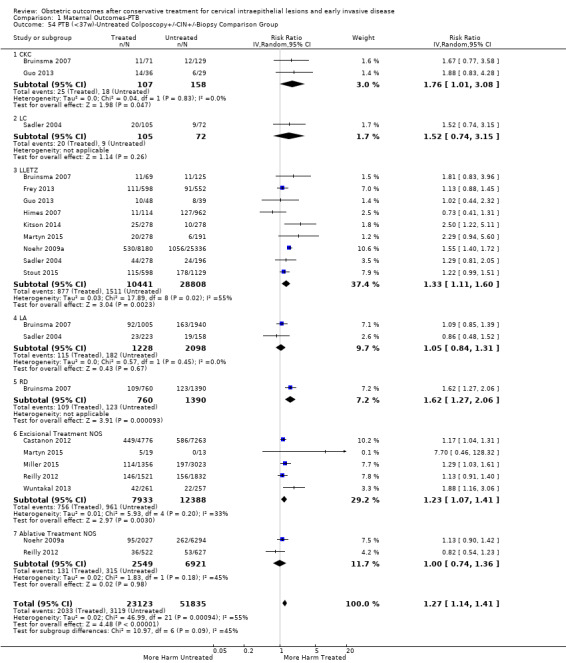
Comparison 1 Maternal Outcomes‐PTB, Outcome 54 PTB (<37w)‐Untreated Colposcopy+/‐CIN+/‐Biopsy Comparison Group.
1.55. Analysis.
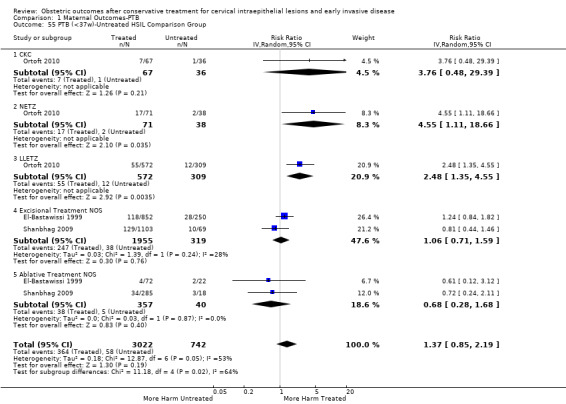
Comparison 1 Maternal Outcomes‐PTB, Outcome 55 PTB (<37w)‐Untreated HSIL Comparison Group.
1.56. Analysis.
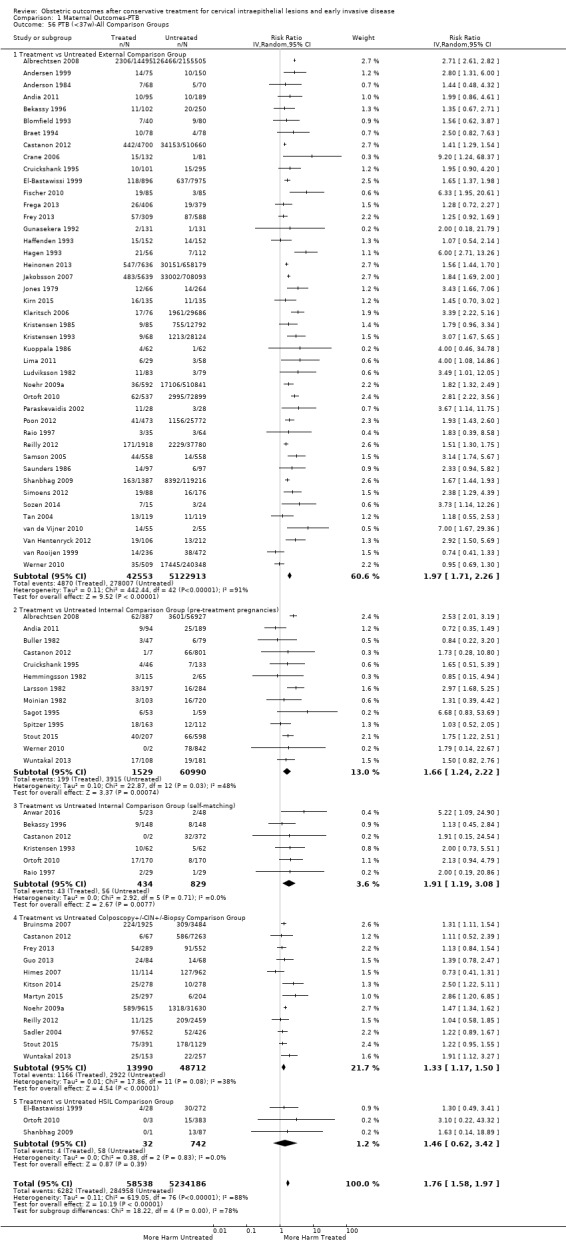
Comparison 1 Maternal Outcomes‐PTB, Outcome 56 PTB (<37w)‐All Comparison Groups.
1.57. Analysis.
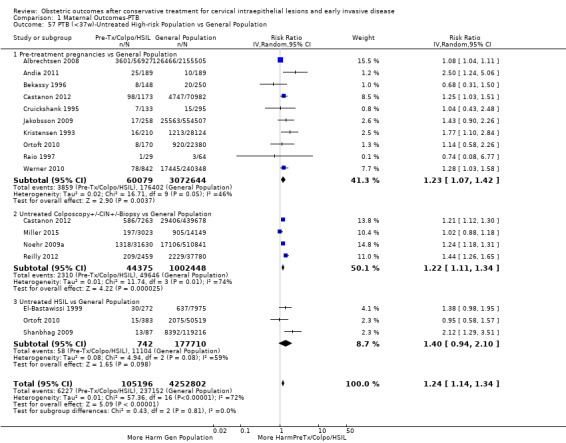
Comparison 1 Maternal Outcomes‐PTB, Outcome 57 PTB (<37w)‐Untreated High‐risk Population vs General Population.
Furthermore, we performed a series of analyses for different cone depths and comparison groups with particularly emphasis on the comparisons of different depths of treatment versus untreated women with CIN (Analysis 1.58; Analysis 1.59; Analysis 1.60; Analysis 1.61; Analysis 1.62; Analysis 1.63; Analysis 1.64; Analysis 1.65; Analysis 1.66; Analysis 1.67; Analysis 1.68; Analysis 1.69; Analysis 1.70; Analysis 1.71; Analysis 1.72; Analysis 1.73; Analysis 1.74; Analysis 1.75). The subgroup analysis of the risk of preterm birth (less than 37 weeks of gestation) according to cone depth when compared to women with CIN but not treated revealed similar direction of effect although for cone depth ≤ 10 mm to 12 mm, the difference was less marked. The number of the studies was however small for many comparisons (treatment versus women with untreated CIN = cone depth ≤ 10 mm to 12 mm (Analysis 1.60): 4 studies, 43,145 women, 7.0% versus 5.0%, RR 1.11, 95% CI 0.85 to 1.43; ≥ 10 mm to 12 mm (Analysis 1.67): 4 studies, 45,275 women, 9.6% versus 5.0%, RR 1.52, 95% CI 1.37 to 1.68; ≥ 15 mm to 17 mm (Analysis 1.69): 3 studies, 33,934 women, 9.6% versus 4.3%, RR 2.30, 95% CI 1.57 to 3.35; ≥ 20 mm (Analysis 1.71): 2 studies, 32,717 women, 9.3% versus 4.2%, RR 4.32, 95% CI 0.93, 20.03) based on low‐ or very low‐quality evidence.
1.58. Analysis.
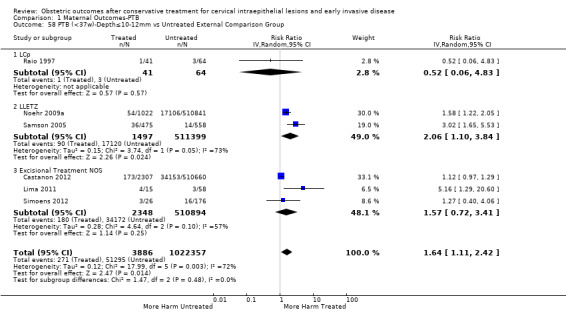
Comparison 1 Maternal Outcomes‐PTB, Outcome 58 PTB (<37w)‐Depth≤10‐12mm vs Untreated External Comparison Group.
1.59. Analysis.
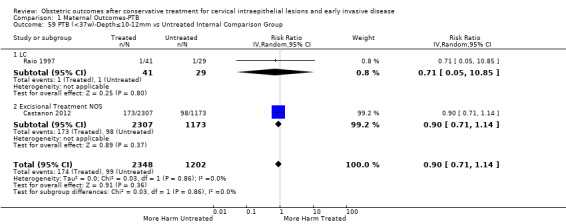
Comparison 1 Maternal Outcomes‐PTB, Outcome 59 PTB (<37w)‐Depth≤10‐12mm vs Untreated Internal Comparison Group.
1.60. Analysis.
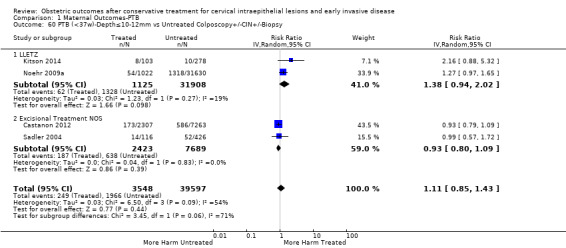
Comparison 1 Maternal Outcomes‐PTB, Outcome 60 PTB (<37w)‐Depth≤10‐12mm vs Untreated Colposcopy+/‐CIN+/‐Biopsy.
1.61. Analysis.

Comparison 1 Maternal Outcomes‐PTB, Outcome 61 PTB (<37w)‐Depth≤15‐17mm vs Untreated External Comparison Group.
1.62. Analysis.
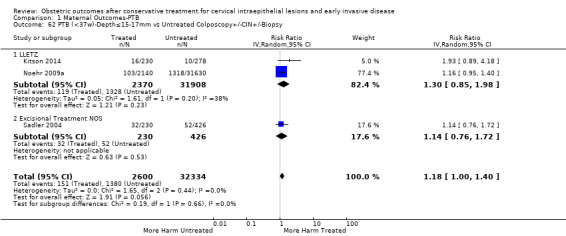
Comparison 1 Maternal Outcomes‐PTB, Outcome 62 PTB (<37w)‐Depth≤15‐17mm vs Untreated Colposcopy+/‐CIN+/‐Biopsy.
1.63. Analysis.
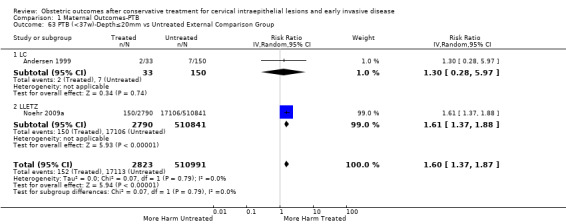
Comparison 1 Maternal Outcomes‐PTB, Outcome 63 PTB (<37w)‐Depth≤20mm vs Untreated External Comparison Group.
1.64. Analysis.
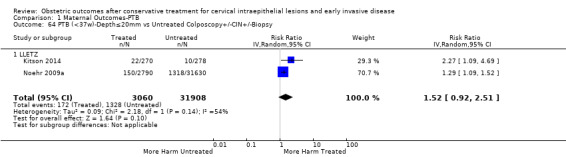
Comparison 1 Maternal Outcomes‐PTB, Outcome 64 PTB (<37w)‐Depth≤20mm vs Untreated Colposcopy+/‐CIN+/‐Biopsy.
1.65. Analysis.
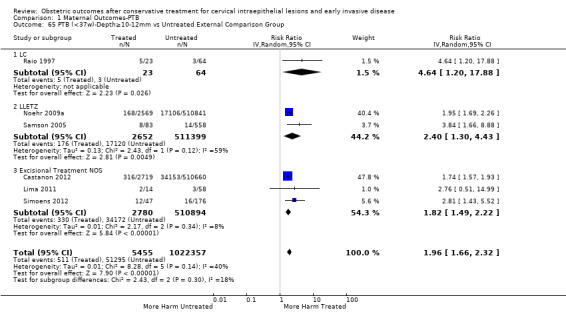
Comparison 1 Maternal Outcomes‐PTB, Outcome 65 PTB (<37w)‐Depth≥10‐12mm vs Untreated External Comparison Group.
1.66. Analysis.

Comparison 1 Maternal Outcomes‐PTB, Outcome 66 PTB (<37w)‐Depth≥10‐12mm vs Untreated Internal Comparison Group.
1.67. Analysis.
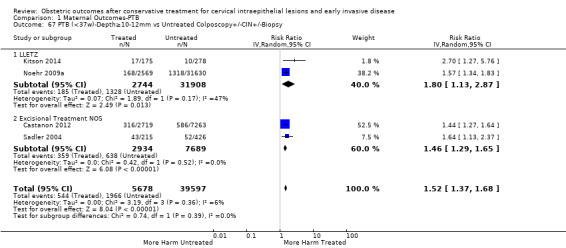
Comparison 1 Maternal Outcomes‐PTB, Outcome 67 PTB (<37w)‐Depth≥10‐12mm vs Untreated Colposcopy+/‐CIN+/‐Biopsy.
1.68. Analysis.
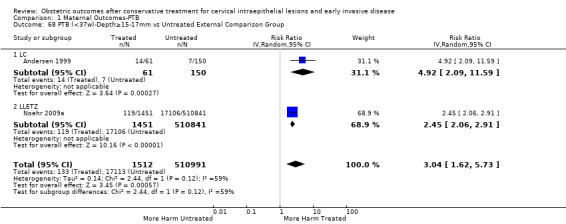
Comparison 1 Maternal Outcomes‐PTB, Outcome 68 PTB (<37w)‐Depth≥15‐17mm vs Untreated External Comparison Group.
1.69. Analysis.
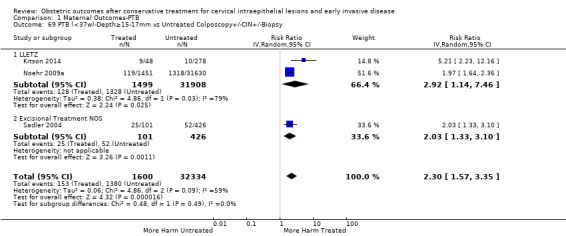
Comparison 1 Maternal Outcomes‐PTB, Outcome 69 PTB (<37w)‐Depth≥15‐17mm vs Untreated Colposcopy+/‐CIN+/‐Biopsy.
1.70. Analysis.
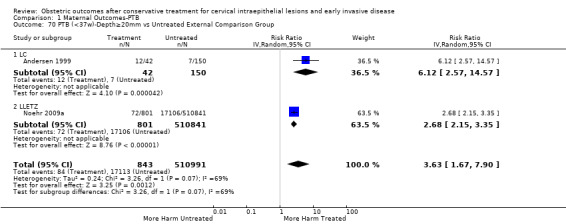
Comparison 1 Maternal Outcomes‐PTB, Outcome 70 PTB (<37w)‐Depth≥20mm vs Untreated External Comparison Group.
1.71. Analysis.
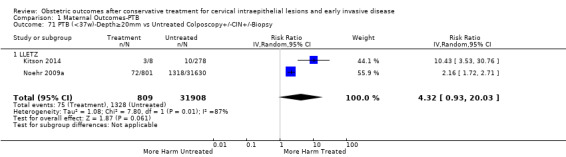
Comparison 1 Maternal Outcomes‐PTB, Outcome 71 PTB (<37w)‐Depth≥20mm vs Untreated Colposcopy+/‐CIN+/‐Biopsy.
1.72. Analysis.
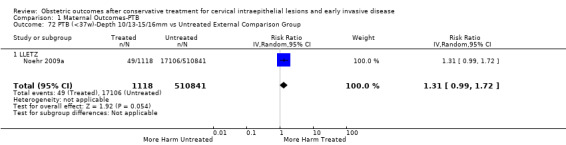
Comparison 1 Maternal Outcomes‐PTB, Outcome 72 PTB (<37w)‐Depth 10/13‐15/16mm vs Untreated External Comparison Group.
1.73. Analysis.
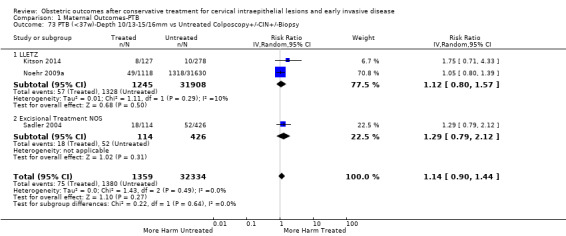
Comparison 1 Maternal Outcomes‐PTB, Outcome 73 PTB (<37w)‐Depth 10/13‐15/16mm vs Untreated Colposcopy+/‐CIN+/‐Biopsy.
1.74. Analysis.
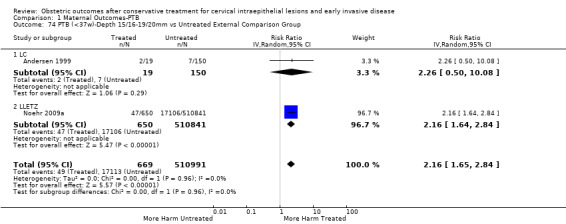
Comparison 1 Maternal Outcomes‐PTB, Outcome 74 PTB (<37w)‐Depth 15/16‐19/20mm vs Untreated External Comparison Group.
1.75. Analysis.
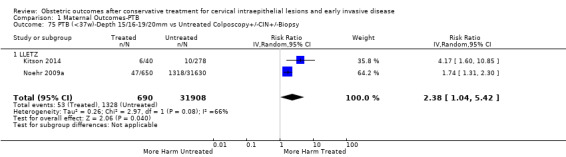
Comparison 1 Maternal Outcomes‐PTB, Outcome 75 PTB (<37w)‐Depth 15/16‐19/20mm vs Untreated Colposcopy+/‐CIN+/‐Biopsy.
Preterm birth ‐ sensitivity and meta‐regression analysis
The mono‐variate meta‐regression analysis suggested that the type of treatment and comparator affected the risk of preterm birth, although the year of study did not. Type of treatment and comparison group remained important factors for risk of preterm birth (PTB) in a multivariate regression analysis. When we performed further meta‐regression restricting only to excisional treatments and using as a comparator women with colposcopy/biopsy, we found that all treatments were associated with an increased risk of PTB (RR 1.34, 95% CI 1.10 to 1.64, for LLETZ; RR 2.3, 95% CI 1.39 to 3.85, for CKC; RR 1.6, 95% CI 0.91 to 2.87, for LC; and RR 4.26, 95% CI 1.96 to 9.33, for needle excision of the transformation zone) (data not shown).
Other Maternal outcomes
Maternal outcomes other than preterm birth were assessed in several studies and many of these were found to be increased after cervical treatment. This increase was more frequent for excisional as opposed to ablative techniques and with increasing treatment radicality, although the number of studies assessing each individual treatment method was frequently small.
Cervical treatment increased the risk of spontaneous overall, severe and extreme preterm birth (less than 37 weeks (Analysis 2.1): 14 studies, 1,024,731 women, 7.0% versus 3.7%, RR 1.76, 95% CI 1.47 to 2.11; less than 32 to 34 weeks (Analysis 2.2): 7 studies, 655,675 women, 1.8% versus 0.6%, RR 2.63, 95% CI 1.91 to 3.62; less than 28 weeks (Analysis 2.3): 2 studies, 626,670 women, 0.6% versus 0.2%, RR 3.18, 95% CI 1.64 to 6.16). The risk of spontaneous preterm birth (less than 37 weeks) was higher for CKC (Analysis 2.1.1: RR 3.53, 95% CI 2.05 to 6.05) followed by excision NOS (Analysis 2.1.7: RR 1.70, 95% CI 1.17 to 2.46), LLETZ (Analysis 2.1.4: RR 1.60, 95% CI 1.22 to 2.08) and ablation NOS (Analysis 2.1.8: RR 1.42, 95% CI 1.20 to 1.70). Needle excision of the transformation zone (NETZ) (Analysis 2.1.3) and LA (Analysis 2.1.5) were only assessed in one study, respectively. There was substantial heterogeneity for the comparisons assessing spontaneous preterm birth at all cut‐offs (P value less than 0.05) based on low‐ or very low‐quality evidence.
2.1. Analysis.
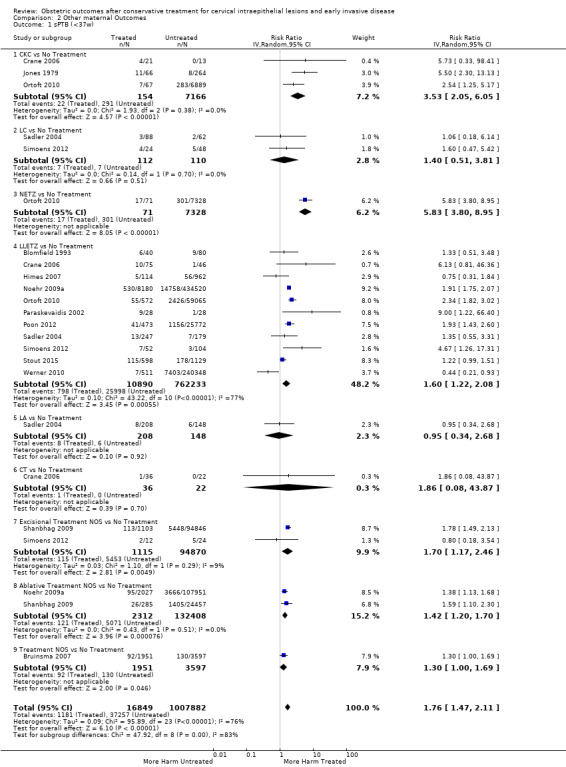
Comparison 2 Other maternal Outcomes, Outcome 1 sPTB (<37w).
2.2. Analysis.
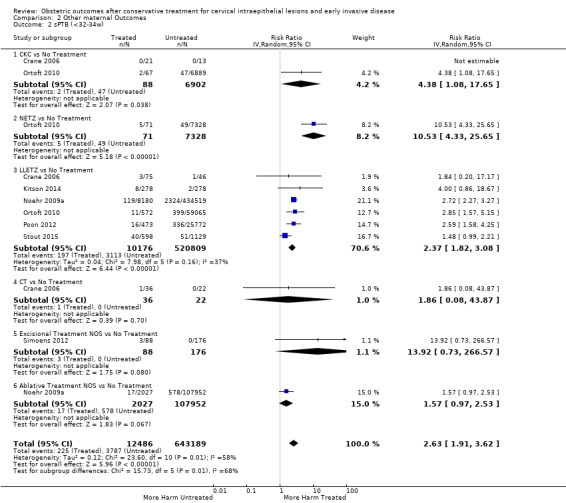
Comparison 2 Other maternal Outcomes, Outcome 2 sPTB (<32‐34w).
2.3. Analysis.
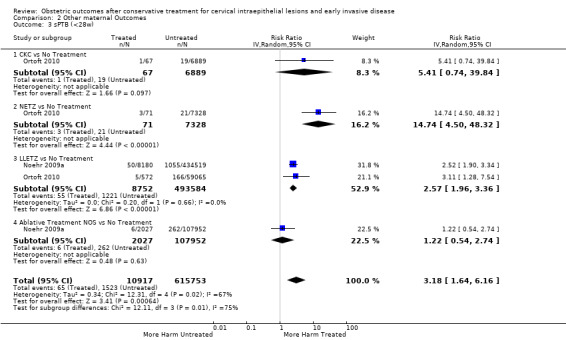
Comparison 2 Other maternal Outcomes, Outcome 3 sPTB (<28w).
The risk of pPROM less than 37 weeks (Analysis 2.4: 21 studies, 477,011 women, 6.1% versus 3.4%, RR 2.36, 95% CI 1.76 to 3.17, very low quality of evidence because of considerable (I2 79%) heterogeneity (Table 1)) was also increased after treatment. The risk of pPROM was higher for CKC (Analysis 2.4.1: RR 4.11, 95% CI 2.05 to 8.25) followed by LLETZ (Analysis 2.4.4: RR 2.15, 95% CI 1.48 to 3.12). NETZ (Analysis 2.4.3) was only assessed in one study and LA (Analysis 2.4.5) did not significantly affect the risk but was only assessed in two studies based on low‐ or very low‐quality evidence. We also included analyses at different gestational cut‐offs (Analysis 2.5; Analysis 2.6)
2.4. Analysis.
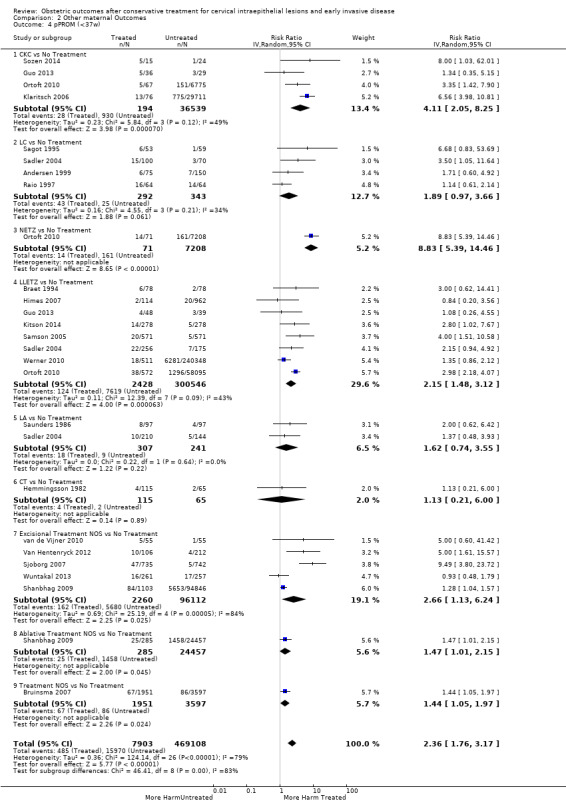
Comparison 2 Other maternal Outcomes, Outcome 4 pPROM (<37w).
2.5. Analysis.
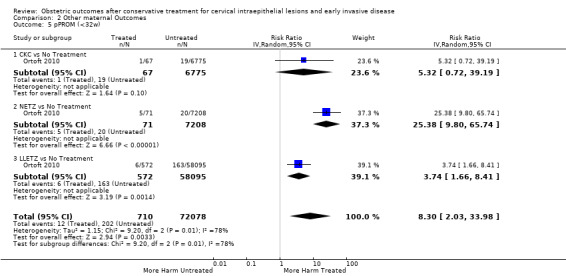
Comparison 2 Other maternal Outcomes, Outcome 5 pPROM (<32w).
2.6. Analysis.
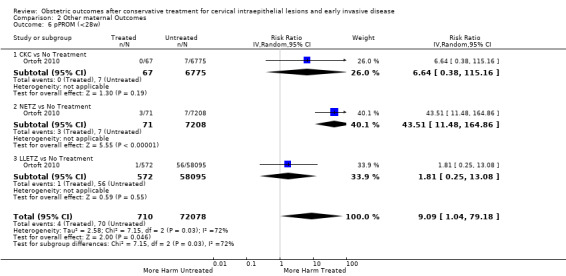
Comparison 2 Other maternal Outcomes, Outcome 6 pPROM (<28w).
Treatment increased the risk of admission for threatened preterm birth (Analysis 2.7; 5 studies, 903 women, 9.1% versus 3.2%, RR 2.44, 95% CI 1.37 to 4.33) and chorioamnionitis (Analysis 2.8; 4 studies, 29,198 women, 3.5% versus 1.1%, RR 3.43, 95% CI 1.36 to 8.64).The mode of delivery (caesarean section (Analysis 2.9; very low quality of evidence because of imprecision and strongly suspected publication bias (Table 2)) or instrumental delivery (Analysis 2.10)), the length of labour (precipitous (Analysis 2.11) or prolonged (Analysis 2.12)), the rate of induction of labour with or without oxytocin to (Analysis 2.13), the use of oxytocin (Analysis 2.14), the use of analgesia (epidural (Analysis 2.15), pethidine (Analysis 2.16) or NOS (Analysis 2.17)), cervical stenosis (Analysis 2.18) or antepartum haemorrhage (Analysis 2.19). was not affected by treatment. The risk of postpartum haemorrhage was increased after treatment, but this outcome included just one study (Analysis 2.20; 1 study, 149 women, 18.7% versus 4.1%, RR 4.60, 95% CI 1.38 to 15.36). The risk of massive obstetric haemorrhage was not increased but there was only one small study (Analysis 2.21). As expected, the rate of cervical cerclage insertion was higher for treated as opposed to non‐treated women (Analysis 2.22; 8 studies, 141,300 women, 4.0% versus 0.7%, RR 14.29, 95% CI 2.85 to 71.65) and more so for CKC (Analysis 2.22.1: RR 31.42, 95% CI 2.32 to 426.22) and excisional treatment NOS (Analysis 2.22.4: RR 42.45, 95% CI 28.99 to 62.16) based on low‐ or very low‐quality evidence.
2.7. Analysis.
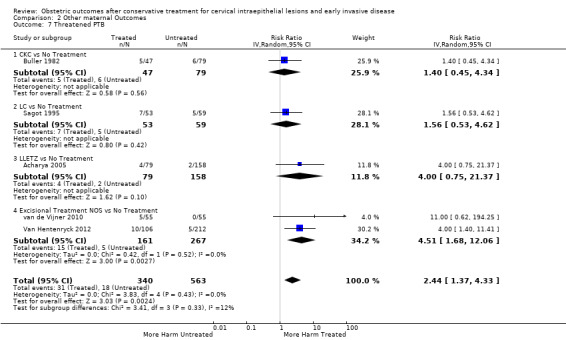
Comparison 2 Other maternal Outcomes, Outcome 7 Threatened PTB.
2.8. Analysis.

Comparison 2 Other maternal Outcomes, Outcome 8 Chorioamnionitis.
2.9. Analysis.

Comparison 2 Other maternal Outcomes, Outcome 9 Caeserean Section.
2.10. Analysis.
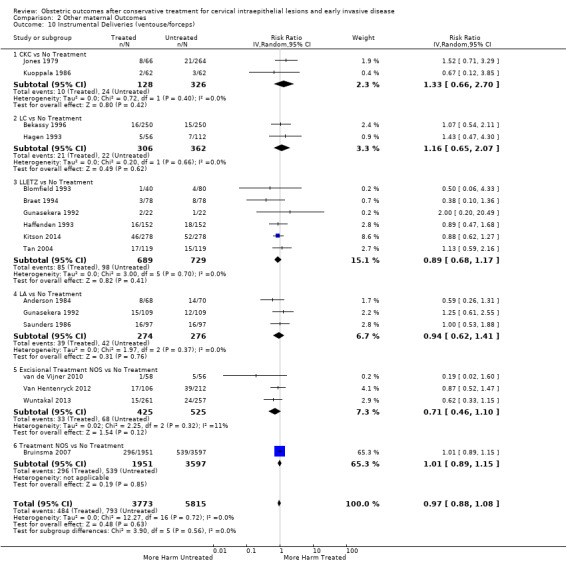
Comparison 2 Other maternal Outcomes, Outcome 10 Instrumental Deliveries (ventouse/forceps).
2.11. Analysis.
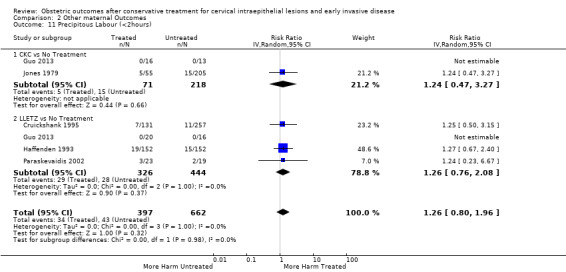
Comparison 2 Other maternal Outcomes, Outcome 11 Precipitous Labour (<2hours).
2.12. Analysis.

Comparison 2 Other maternal Outcomes, Outcome 12 Prolonged labour (>12hours).
2.13. Analysis.
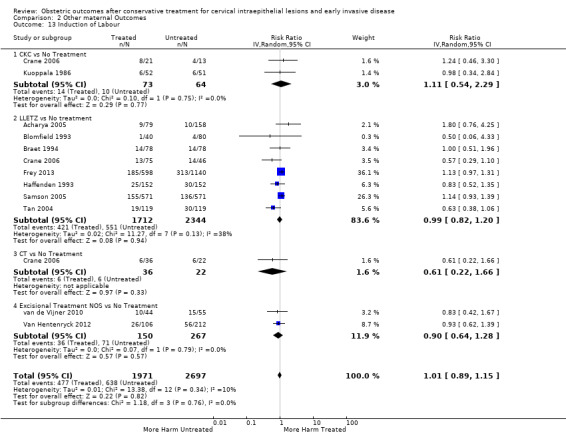
Comparison 2 Other maternal Outcomes, Outcome 13 Induction of Labour.
2.14. Analysis.

Comparison 2 Other maternal Outcomes, Outcome 14 Oxytocin Use.
2.15. Analysis.
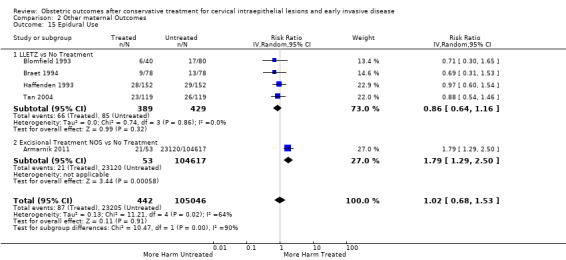
Comparison 2 Other maternal Outcomes, Outcome 15 Epidural Use.
2.16. Analysis.
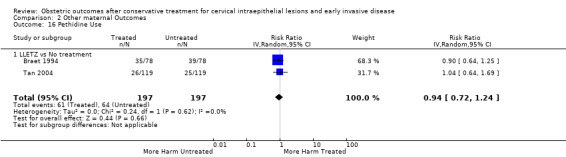
Comparison 2 Other maternal Outcomes, Outcome 16 Pethidine Use.
2.17. Analysis.
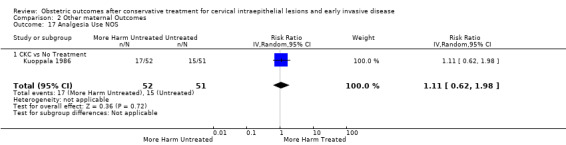
Comparison 2 Other maternal Outcomes, Outcome 17 Analgesia Use NOS.
2.18. Analysis.

Comparison 2 Other maternal Outcomes, Outcome 18 Cervical stenosis.
2.19. Analysis.
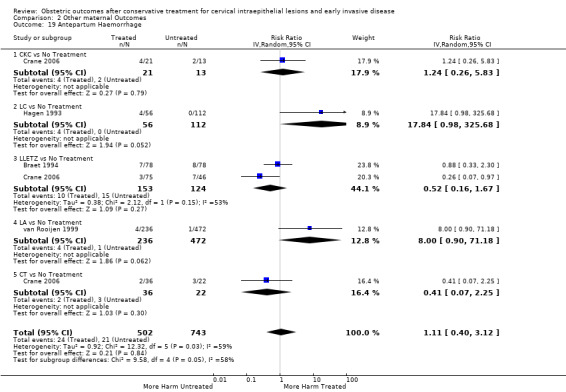
Comparison 2 Other maternal Outcomes, Outcome 19 Antepartum Haemorrhage.
2.20. Analysis.
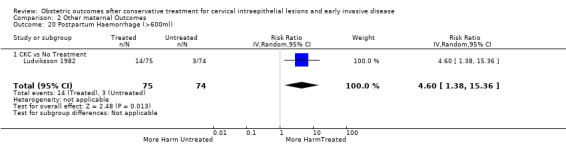
Comparison 2 Other maternal Outcomes, Outcome 20 Postpartum Haemorrhage (>600ml).
2.21. Analysis.
Comparison 2 Other maternal Outcomes, Outcome 21 Massive Obstetric Haemorrhage (>1000ml).
2.22. Analysis.
Comparison 2 Other maternal Outcomes, Outcome 22 Cervical cerclage.
Neonatal outcomes
More than 30 studies assessed one or more neonatal outcomes. Cervical treatment (excisional or ablative) was associated with a higher frequency of adverse neonatal outcomes as opposed to women who had not been treated (comparison group not specified). The association with adverse neonatal events was stronger and more frequent for excisional as opposed to ablative techniques and with increasing treatment radicality, although the number of studies for each individual treatment technique was often limited.
More specifically, cervical treatment overall increased the risk of low birth weight (LBW) for some, but not all, cut‐offs, although the number of studies was small for most cut‐offs (less than 2500 g (Analysis 3.1): 30 studies, 1,348,206 women, 7.9% versus 3.7%, RR 1.81, 95% CI 1.58 to 2.07, very low quality of evidence because of substantial (I2 63%) heterogeneity (Table 2); less than 2000 g (Analysis 3.2): 3 studies, 74,981 women, 4.7% versus 1.1%, RR 2.49, 95% CI 0.97 to 6.36; less than 1500 g (Analysis 3.3)): 5 studies, 76,836 women, 2.0% versus 0.5%, RR 3.00, 95% CI 1.54 to 5.85; less than 1000 g (Analysis 3.4): 2 studies, 2185 women, 1.1% versus 0.3%, RR 2.09, 95% CI 0.06 to 74.71). Treatment also increased the risk of neonatal intensive care unit (NICU) admission (Analysis 3.5: 8 studies, 2557 women, 12.6% versus 8.9%, RR 1.45, 95% CI 1.16 to 1.81, low quality of evidence (Table 2)) and perinatal mortality (Analysis 3.6: 23 studies, 1,659,433 women, 0.9% versus 0.7%, RR 1.51, 95% CI 1.13 to 2.03, low quality of evidence (Table 2)). Subgroup analyses of the perinatal mortality for different gestational ages at delivery was also included (Analysis 3.7; Analysis 3.8; Analysis 3.9). The rate of stillbirth was not affected by treatment (Analysis 3.10), while the analysis on the impact of treatment on the Apgar scores only included a small number of studies (Analysis 3.11; Analysis 3.12; Analysis 3.13).
3.1. Analysis.
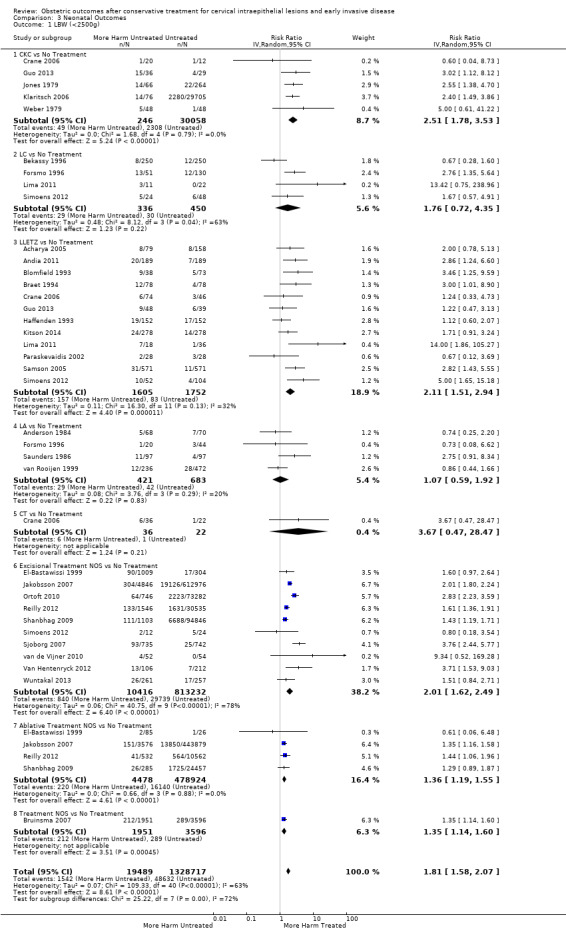
Comparison 3 Neonatal Outcomes, Outcome 1 LBW (<2500g).
3.2. Analysis.
Comparison 3 Neonatal Outcomes, Outcome 2 LBW (<2000g).
3.3. Analysis.
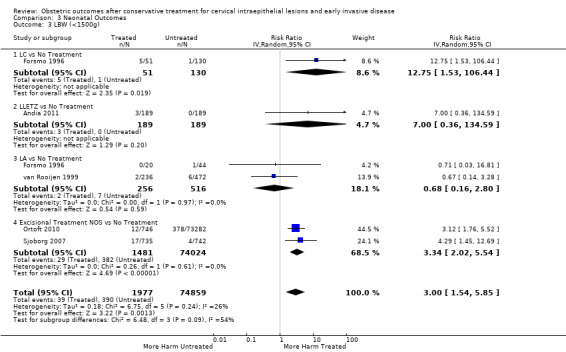
Comparison 3 Neonatal Outcomes, Outcome 3 LBW (<1500g).
3.4. Analysis.
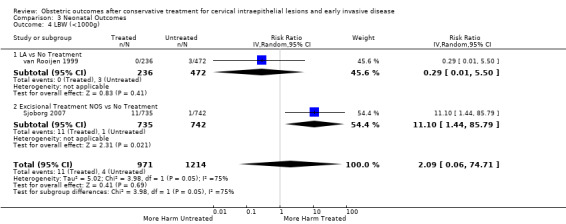
Comparison 3 Neonatal Outcomes, Outcome 4 LBW (<1000g).
3.5. Analysis.
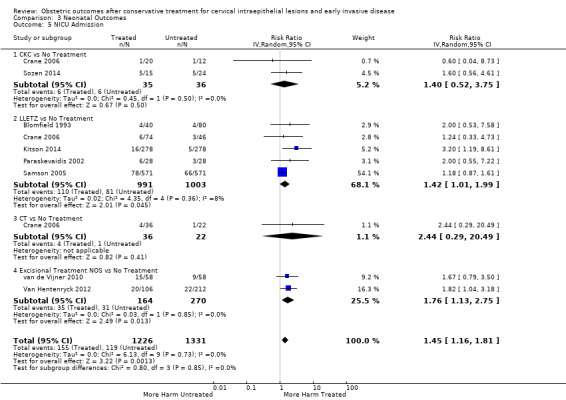
Comparison 3 Neonatal Outcomes, Outcome 5 NICU Admission.
3.6. Analysis.

Comparison 3 Neonatal Outcomes, Outcome 6 Perinatal Mortality.
3.7. Analysis.
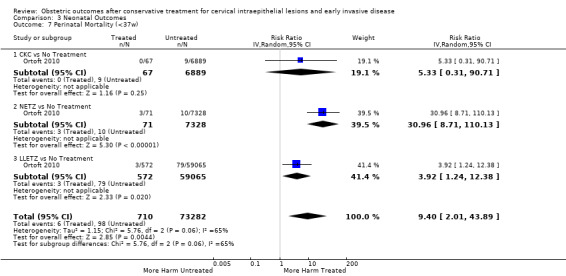
Comparison 3 Neonatal Outcomes, Outcome 7 Perinatal Mortality (<37w).
3.8. Analysis.

Comparison 3 Neonatal Outcomes, Outcome 8 Perinatal Mortality (<32w).
3.9. Analysis.

Comparison 3 Neonatal Outcomes, Outcome 9 Perinatal Mortality (<28w).
3.10. Analysis.
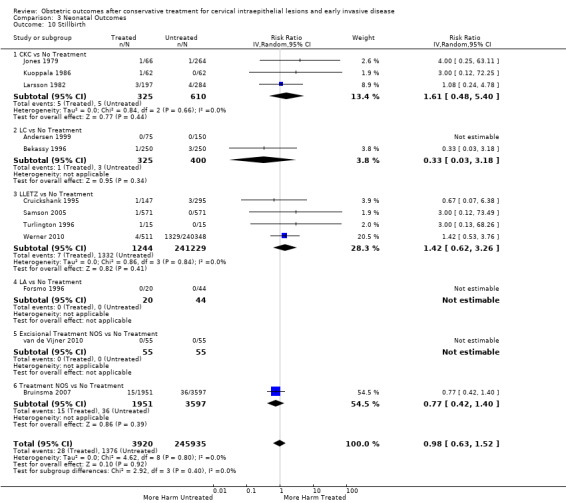
Comparison 3 Neonatal Outcomes, Outcome 10 Stillbirth.
3.11. Analysis.
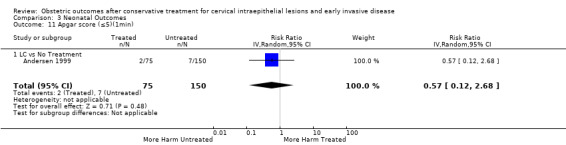
Comparison 3 Neonatal Outcomes, Outcome 11 Apgar score (≤5)(1min).
3.12. Analysis.
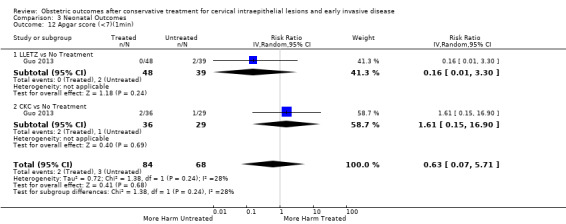
Comparison 3 Neonatal Outcomes, Outcome 12 Apgar score (<7)(1min).
3.13. Analysis.

Comparison 3 Neonatal Outcomes, Outcome 13 Apgar score (<7)(5min).
The rate of neonates born with A birth weight of less than 2500 g was more frequently observed in women treated with CKC (Analysis 3.1.1: 5 studies, 30,304 women, RR 2.51, 95% CI 1.78 to 3.53), LLETZ (Analysis 3.1.3: 12 studies, 3357 women, RR 2.11, 95% CI 1.51 to 2.94), excisional (Analysis 3.1.6: 10 studies, 823,648 women, RR 2.01, 95% CI 1.62 to 2.49) or ablative (Analysis 3.1.7: 4 studies, 483,402 women, RR 1.36, 95% CI 1.19 to 1.55) treatment NOS, but not so for laser ablation (Analysis 3.1.4: RR 1.07, 95% CI 0.59 to 1.92), although only four studies with a total of 1104 participants assessed that comparison. The rate of NICU admission was increased overall (Analysis 3.5), and for LLETZ (Analysis 3.5.2: 5 studies, 1994 women, RR 1.42, 95% CI 1.01 to 1.99) and excisional technique NOS (Analysis 3.5.4: 2 studies, 434 women, RR 1.76, 95% CI 1.13 to 2.75). Perinatal mortality was greater for treated women overall (Analysis 3.6), and for excisional technique NOS (Analysis 3.6.7: 5 studies, 820,028 women, RR 1.85, 95% CI 1.02 to 3.36), but not for the individual techniques, possibly due to the limited number of studies and the low prevalence of the outcome. Subgroup analysis according to the different comparison groups or cone depths was not possible due to the limited number of studies assessing each outcome based on low‐ or very low‐quality evidence.
Discussion
Summary of main results
The results of this systematic review suggest that all types of local cervical treatment technique (excisional or destructive) are associated with an increased risk of preterm birth (PTB) and adverse obstetric sequelae. This is the first systematic review that includes subgroup analyses according to the depth (and volume) of treatment, the number of treatments and the comparison group used.
The knowledge that local treatment for cervical pre‐cancer, particularly excisional treatment, increases the risk of PTB has led to major changes in clinical practice. With a rapidly evolving evidence base and inconsistencies in the published literature (Castanon 2012; Conner 2013; Danhof 2015; Jakobsson 2009; Jin 2014; Reilly 2012), a high‐quality synthesis of the evidence should be available for effective patient counselling at colposcopy and antenatal clinics.
This meta‐analysis documents that any local cervical treatment for cervical pre‐invasive or early invasive disease increases the risk of PTB and adverse sequelae in a subsequent pregnancy, although the impact of small excisions, as opposed to just having the disease, remains uncertain and is likely to be small. Cervical treatment was found to be associated with an increased risk of overall, severe and extreme prematurity, spontaneous PTB, threatened preterm labour, pPROM, chorioamnionitis, low birth weight, neonatal admission and perinatal death. The rate of cervical cerclage was unsurprisingly substantially increased in treated women as opposed to untreated controls. Treatment equally affected outcomes for nulliparous as well as parous, singleton and multiple pregnancies. The analysis suggested that local treatment had no impact on mode of delivery, length of labour, the induction rate, the use of analgesia, the rate of stenosis and haemorrhage .
The magnitude of the effect of treatment was higher for more radical techniques (i.e. cold knife conisation (CKC) > large loop excision of the transformation zone (LLETZ) > laser ablation (LA)) and for excision rather than ablation. Multiple conisations increased four‐fold the risk of PTB compared to untreated controls. Subgroup analyses clearly demonstrated that the risk of PTB directly correlates to the cone dimensions (depth and volume) and progressively increases with increasing cone depth (‘dose‐effect’). Although the risk was increased even for excisions measuring less than 10 mm in depth, this was almost two‐fold higher for excisions of more than 10 mm, three‐fold higher for more than 15 MM to 17 mm and almost five‐fold higher for excisions exceeding 20 mm in depth.
It has been previously suggested that the impact of treatment on the risk of PTB may not be a consequence of treatment but rather a product of other confounders present in women with cervical disease (Castanon 2012; Kyrgiou 2012; Reilly 2012). Our subgroup analyses that stratified the risk to the comparator used, clearly documents that although the risk of PTB is increased after treatment, irrespective of the comparison group used, the choice of comparator may over‐inflate or under‐estimate the effect of treatment. The magnitude of effect was higher when external controls were used, followed by internal control, followed by women who had cervical intra‐epithelial neoplasia (CIN), but who were not treated. The analyses in women with high‐grade squamous intra‐epithelial lesion (HSIL) but no treatment only included three studies and 3764 participants; we were unable to draw any firm conclusions from this comparison. When we assessed the risk of PTB according to the cone depth when compared to women with CIN but no treatment, we noted the same direction of effect. Although the difference in the risk for PTB for small excisions (10 mm to 12 mm or less) as opposed to just having CIN but no treatment did not differ greatly, the number of studies assessing that comparison was however small and firm conclusions cannot be drawn. We also found that women with CIN have a higher baseline risk of prematurity when compared to the general population.
Overall completeness and applicability of evidence
For some of the outcomes of interest, we identified only a small number of studies and a limited number of participants. The cut‐off used for the definitions of severe and extreme prematurity and for different cone depths varied slightly across studies; these were merged in broader groups for the analysis. Individual patient meta‐analysis data is required to more accurately describe the stratified risk of PTB for individual cone depths. The data on the cone dimensions relied on retrospective documentation data recorded in histopathology reports of formalin‐fixed samples with obvious limitations. The formulas used for the volume calculation also varied across studies. Future research should aim to correlate outcomes with prospective precise done depth and cervical measurements.
Both the included and excluded studies demonstrated a wide range of inclusion/exclusion criteria and outcome measures limiting statistical pooling of all the primary studies. There should be agreement amongst academic colposcopists and obstetricians on core research clinical outcome measures in line with the CROWN initiative of the premier reproductive health journals (http://www.crown‐initiative.org/aims‐and‐scope/). This would improve the applicability of findings of primary and secondary research internationally.
Quality of the evidence
This meta‐analysis included a large number of studies (69 cohorts) with sufficient sample size and power to explore several comparisons of treatment techniques and cone depths. We performed analyses for the different comparison groups used across studies and made observations on how this impacts on the results.
However, the results should be interpreted with caution. Due to the pre‐malignant nature of the disease, no randomised controlled trials (RCTs) could be identified. All the included studies were cohorts, in the vast majority retrospective. Such reports are at known risk of recall bias and inadequate adjustment for known and unknown confounders, while some of the outcomes of interest were difficult to measure objectively. Many of the studies relied on data collected from structured interviews and mailed questionnaires and in some of these, the response rate was small, increasing also the risk of incomplete outcome data (attrition). Altogether 13 studies were considered to be of very low quality because of attrition bias or recall bias or small number of participants, but we were able to upgrade 31 studies to moderate quality because they were population‐based, had a large number of participants or were prospective. Still, the quality of the evidence based on the GRADE assessment was low or very low for the assessed maternal or fetal outcomes (Table 1) and low or high for analyses regarding the effect of the cone depth (Table 2). Although the overall number of studies was large, for some outcomes and comparisons the numbers of studies was small and the analyses did not have sufficient sample sizes to support definite conclusions.
Meta‐regression was possible for some, but not all, possible confounders. For many moderators, the data were reported only in a proportion of the included studies. When these studies were not deemed representative of the whole population of studies, we did not perform meta‐regression as this would introduce bias. Sensitivity and subgroups analyses based on the studies’ quality did not change the effect of the meta‐analysis.
Potential biases in the review process
This review relies on non‐randomised studies and therefore at high risk of bias. Many of the included studies were of low or very low quality.
We opted for the use of unadjusted data for the analysis that was recommended during the protocol and early stages of this review. More recent guidance recommends the use of adjusted as opposed to unadjusted data. This is because unadjusted data may over‐inflate or underestimate the effect of treatment due to other confounding factors. Despite existing controversies, it is possible that the use of adjusted data may better control for existing confounders that may affect the magnitude of the effect in observational studies. In future updates of this review adjusted data will be incorporated to meet he more recent recommendations.
There was no evidence of publication bias apart from one outcome (PTB <32 to 34 weeks of gestation).
One of the major strengths of this systematic review is that this provides a full assessment of the up‐to‐date literature for all clinically relevant outcomes. However, the reporting of a large number of outcomes increases the chance of type 1 statistical error (outcomes will be found to be statistically significant just by chance; incorrect rejection of a true null hypothesis).
Agreements and disagreements with other studies or reviews
The knowledge that local treatment for cervical precancer, particularly excisional, increases the risk of PTB has led to major changes in clinical practice. With an increasing evidence base suggesting that this risk is higher for more radical techniques, there has been a tendency to use less aggressive treatments. Although it was previously thought that the various techniques had comparable efficacy (Martin‐Hirsch 2013), evidence from a population‐based study raised concerns that less radical treatment may increase the risk of post‐treatment invasion (Arbyn 2014; Strander 2014). Although the decreased number of hysterectomies may explain this increase, the move to less conservative methods in another plausible explanation. Additionally, since the first documentation of the reproductive risk associated with treatment almost a decade ago (Kyrgiou 2006), subsequent observational studies and even meta‐analyses reached contradictory conclusions (Arbyn 2008; Bruinsma 2011; Conner 2014; Danhof 2015; Jin 2014; Kyrgiou 2014) and initiated debates within the scientific community. With some authors raising concerns that the progressive reduction in the radicality of treatment has led to increased risk of future of invasion (Arbyn 2014; Strander 2014), and others advocating the move to less radical techniques like laser ablation (LA) for the prevention of treatment‐associated future perinatal morbidity and mortality (Paraskevaidis 2007), high‐quality synthesis of the evidence had become an urgent unmet need. Some of the previous small meta‐analyses suffered methodological flaws and attempted analysis of individual treatment techniques or subgroups minimising the validity of their findings in context with the rest of the literature (Conner 2014; Danhof 2015; Jin 2014). All of the published meta‐analyses failed to analyse the data according to major confounders and stratifiers of risk, the comparison group and the depth of the excision. Although Bruinsma and colleagues first approached the comparison group as a possible confounder, data on the depth and dimensions of the treatment were not available (Bruinsma 2011).
Preterm birth is a major cause of neonatal death and disability and represents an enormous cost to health services and society. While pregnant, these women make up a large proportion of preterm clinics referrals. These referrals have increased from almost none in 1999, to more than 40% in 2012 (Kindinger 2016). Ultrasound‐directed surveillance is labour‐intensive, costly, and may be associated with maternal anxiety, more so because 85% of women post‐excision are effectively low risk and will deliver at term (Bruinsma 2011; Kyrgiou 2006).
With rapidly accumulating evidence correlating cervical treatment to adverse reproductive morbidity, quantification of the comparative obstetric morbidity for different treatment techniques and cone depths was required to assist clinicians' decision‐making and counselling. The results of this meta‐analysis will allow clinicians, patients and policy makers to balance the absolute increase in reproductive morbidity with increasing treatment radicality. Patients should be informed that treatment increases the risk of PTB, as opposed to having CIN only, but the absolute increase in risk in small type 1 excisions is likely to be low, if any.
Furthermore, the quantified individual risk stratified by treatment and cone depth could allow obstetricians the selection of those considered to be at high risk of PTB that would benefit from intensive surveillance antenatally and minimise the unnecessary interventions for those at low risk. The antenatal management of women after treatment has been inconsistent and largely unit‐ or clinician‐dependent (Buller 1982). The risks and benefits associated with various interventions in pregnant women with a history of cervical treatment have not been fully assessed in properly designed studies (Kindinger 2016). Future research should assess their value in this distinct clinical group and devise a logical prevention strategy.
Authors' conclusions
Implications for practice.
Women with cervical intra‐epithelial neoplasia (CIN) have a higher baseline risk of preterm birth (PTB) compared to women from the general population. Local cervical treatment for pre‐invasive or early invasive disease further increases the risk, more so for excisional treatment, but also for ablative techniques. The risk of PTB increases with increasing cone depth (and volume) and techniques that remove or destroy larger parts of the cervix. The increase in risk for small excisions, as opposed to having CIN, is likely to be small, if any. However, for many outcomes the quality of evidence was judged to be low or very low, creating uncertainty about the confidence in the effect estimate.
When deciding to treat women of reproductive age, every effort should be made to perform a local treatment that will optimise the chances of a healthy pregnancy without compromising the completeness of the local treatment. Quality assurance in treatment of disease should include audit of dimensions of excisional specimens and persistent disease rates to ensure that treatment depth is kept to acceptable parameters (i.e. at least 8 mm to involved crypts) and that oncological outcomes are not compromised. Clinical experience is important in achieving the optimal balance between complete removal of the lesion and minimum damage of healthy cervical tissue. Unecessary large excisions may lead to severe adverse obstetric outcomes, whilst overly conservative excisions that compromise clearance of the disease may result in high recurrence rates and the need for repeat excision multiplying the frequency and severity of the reproductive sequelae. Clinicians should inform women about the increased risk of adverse reproductive outcomes in a future pregnancy after treatment, particularly for large and repeat treatments. These women may be offered more intensive surveillance when pregnant (Kindinger 2016).
Implications for research.
Future research should use individual patient data and network meta‐analyses methodologies to quantify the oncological and reproductive impact of different treatment techniques. It is also important to investigate why women who have CIN appear to be susceptible to both CIN and PTB, or whether HPV‐induced disease alone is the principal factor in increasing premature delivery. It is likely that a combination of immunological and other factors play a role. The uptake of prophylactic vaccination has been mixed in the developed world and minimal in low‐income countries. The impact of cervical treatment is still going to be relevant for many decades and therefore robust clinical research in this field should remain a priority.
Acknowledgements
We thank Jo Morrison, Clare Jess, Jane Hayes and Andy Bryant of the CGNOC editorial team for their contribution to the editorial process. We thank the peer reviewers for their many helpful suggestions.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
CENTRAL search strategy
#1 MeSH descriptor Uterine Cervical Neoplasms explode all trees #2 cervi* and (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinom*) #3 MeSH descriptor Cervical Intraepithelial Neoplasia explode all trees #4 CIN #5 cervi* and (intraepithel* or epithel* or dysplasia or pre‐cancer* or precancer*) #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Conization explode all trees #8 conisation or conization #9 MeSH descriptor Laser Therapy explode all trees #10 laser #11 MeSH descriptor Cryotherapy explode all trees #12 cryotherapy #13 cold coagulation #14 MeSH descriptor Diathermy explode all trees #15 diatherm* #16 cone biopsy #17 loop #18 LLETZ #19 LEEP #20 ablat* #21 excision* #22 transformation zone #23 CKC or LA or LC or CC or RD or TZ #24 conservative and (method* or treatment* or intervention* or management) #25 (#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24) #26 (#6 AND #25) #27 MeSH descriptor Premature Birth explode all trees #28 preterm or premature #29 MeSH descriptor Infant, Low Birth Weight explode all trees #30 birth weight #31 MeSH descriptor Perinatal Mortality explode all trees #32 perinatal mortality #33 MeSH descriptor Intensive Care, Neonatal explode all trees #34 neonat* and (intensive care) #35 MeSH descriptor Fertility explode all trees #36 fertil* #37 conception #38 MeSH descriptor Pregnancy explode all trees #39 pregnancy #40 gestation* #41 MeSH descriptor Abortion, Spontaneous explode all trees #42 miscarriage* #43 MeSH descriptor Cesarean Section explode all trees #44 cesarean or caesarean #45 MeSH descriptor Obstetric Labor, Premature explode all trees #46 MeSH descriptor Labor, Obstetric explode all trees #47 labor or labour #48 MeSH descriptor Fetal Membranes, Premature Rupture explode all trees #49 pPROM #50 (#27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49) #51 (#26 AND #50)
Appendix 2. MEDLINE search strategy
1 exp Uterine Cervical Neoplasms/ 2 (cervi* and (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinom*)).mp. 3 exp Cervical Intraepithelial Neoplasia/ 4 CIN.mp. 5 (cervi* and (intraepithel* or epithel* or dysplasia or pre‐cancer* or precancer*)).mp. 6 or/1‐5 7 exp Conization/ 8 (conisation or conization).mp. 9 exp Laser Therapy/ 10 laser.mp. 11 exp Cryotherapy/ 12 cryotherapy.mp. 13 cold coagulation.mp. 14 exp Diathermy/ 15 diatherm*.mp. 16 cone biopsy.mp. 17 loop.mp. 18 LLETZ.mp. 19 LEEP.mp. 20 ablat*.mp. 21 excision*.mp. 22 transformation zone.mp. 23 (CKC or LA or LC or CC or RD or TZ).mp. 24 (conservative and (method* or treatment* or intervention* or management)).mp. 25 or/7‐24 26 6 and 25 27 exp Premature Birth/ 28 (preterm or premature).mp. 29 exp Infant, Low Birth Weight/ 30 birth weight.mp. 31 Perinatal Mortality/ 32 perinatal mortality.mp. 33 exp Intensive Care, Neonatal/ 34 (neonatal and intensive care).mp. 35 exp Fertility/ 36 fertil*.mp. 37 conception.mp. 38 exp Pregnancy/ 39 pregnancy.mp. 40 gestation*.mp. 41 exp Abortion, Spontaneous/ 42 miscarriage*.mp. 43 exp Cesarean Section/ 44 (cesarean or caesarean).mp. 45 exp Obstetric Labor, Premature/ 46 exp Labor, Obstetric/ 47 (labor or labour).mp. 48 Fetal Membranes, Premature Rupture/ 49 pPROM.mp. 50 or/27‐49 51 26 and 50
key: mp=title, original title, abstract, name of substance word, subject heading word
Appendix 3. Embase search strategy
1 exp uterine cervix tumor/ 2 (cervi* and (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinom*)).mp. 3 uterine cervix carcinoma in situ/ 4 CIN.mp. 5 (cervi* and (intraepithel* or epithel* or dysplasia or pre‐cancer* or precancer*)).mp. 6 or/1‐5 7 uterine cervix conization/ 8 (conisation or conization).mp. 9 low level laser therapy/ 10 laser.mp. 11 exp cryotherapy/ 12 cryotherapy.mp. 13 cold coagulation.mp. 14 diathermy/ 15 diatherm*.mp. 16 cone biopsy.mp. 17 loop.mp. 18 LLETZ.mp. 19 LEEP.mp. 20 ablat*.mp. 21 excision*.mp. 22 transformation zone.mp. 23 (CKC or LA or LC or CC or RD or TZ).mp. 24 (conservative and (method* or treatment* or intervention* or management)).mp. 25 or/7‐24 26 6 and 25 27 prematurity/ 28 (preterm or premature).mp. 29 exp low birth weight/˜ 30 birth weight.mp. 31 perinatal mortality/ 32 perinatal mortality.mp. 33 newborn intensive care/ 34 (neonat* and intensive care).mp. 35 female fertility/ 36 fertil*.mp. 37 conception/ 38 conception.mp. 39 exp pregnancy/ 40 pregnancy.mp. 41 gestation*.mp. 42 spontaneous abortion/ 43 miscarriage*.mp. 44 cesarean section/ 45 (cesarean or caesarean).mp. 46 premature labor/ 47 (labor or labour).mp. 48 premature fetus membrane rupture/ 49 pPROM.mp. 50 or/27‐49 51 26 and 50
key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name
Appendix 4. List of abbreviations
CENTRAL: Cochrane Central Register of Controlled Trials CC: Cold Coagulation CI: (95%) Confidence Interval CIN: Cervical Intra‐epithelial Neoplasia CKC: Cold Knife Conisation CS: Ceasarean Section CT: Cryotherapy FCBE: Fischer Cone Biopsy Excisor LA: Laser Ablation LBW: Low Birth Weight LEEP: Loop Electrosurgical Excision Procedure LLETZ: Large Loop Excision of the Transformation Zone NETZ: Needle Excision of the Transformation Zone NICU: Neonatal Intensive Care Unit NOS: Not Otherwise Specified pPROM: preterm premature rupture of membranes PTB: Preterm Birth RCT: Randomised Controlled Trial RD: Radical Diathermy RR: Relative Risk SWETZ: Straight Wire Excision of the Transformation Zone TZ: Transformation Zone
Appendix 5. List of definitions
Extreme prematurity: delivery (spontaneous or iatrogenic) at less than 28 to 30 weeks of gestation First trimester miscarriage: miscarriage at less than 12 weeks of gestation Low birth weight: birth weight less than 2500 g Perinatal mortality rate: number of stillbirths and neonatal deaths occurring within seven days after birth (early perinatal mortality) or within seven to 28 days after birth (late perinatal mortality) per 1000 total births Overall prematurity: delivery (spontaneous or iatrogenic) at less than 37 weeks of gestation Preterm prelabour rupture of membranes: rupture of membranes at less than 37 weeks of gestation and prior to initiation of labour Second trimester miscarriage: miscarriage between 12 and 24 weeks of gestation Severe prematurity: delivery (spontaneous or iatrogenic) at less than 32 to 34 weeks of gestation
Data and analyses
Comparison 1. Maternal Outcomes‐PTB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PTB (<37w) | 59 | 5.242917E6 | Risk Ratio (IV, Random, 95% CI) | 1.75 [1.57, 1.96] |
| 1.1 Excisional Treatment vs No Treatment | 53 | 4.599416E6 | Risk Ratio (IV, Random, 95% CI) | 1.87 [1.64, 2.12] |
| 1.2 Ablative Treatment vs No Treatment | 14 | 602370 | Risk Ratio (IV, Random, 95% CI) | 1.35 [1.20, 1.52] |
| 1.3 Treatment NOS vs No Treatment | 2 | 41131 | Risk Ratio (IV, Random, 95% CI) | 2.57 [1.39, 4.77] |
| 2 PTB (<37w)‐Analysis by treatment modality | 59 | 5.242917E6 | Risk Ratio (IV, Random, 95% CI) | 1.78 [1.60, 1.98] |
| 2.1 CKC vs No Treatment | 12 | 39102 | Risk Ratio (IV, Random, 95% CI) | 2.70 [2.14, 3.40] |
| 2.2 LC vs No Treatment | 9 | 1509 | Risk Ratio (IV, Random, 95% CI) | 2.11 [1.26, 3.54] |
| 2.3 NETZ vs No Treatment | 1 | 7399 | Risk Ratio (IV, Random, 95% CI) | 5.83 [3.80, 8.95] |
| 2.4 LLETZ vs No Treatment | 25 | 1.445104E6 | Risk Ratio (IV, Random, 95% CI) | 1.58 [1.37, 1.81] |
| 2.5 FCBE vs No Treatment | 1 | 71 | Risk Ratio (IV, Random, 95% CI) | 5.22 [1.09, 24.90] |
| 2.6 LA vs No Treatment | 7 | 4710 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.86, 1.26] |
| 2.7 CT vs No Treatment | 2 | 238 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.22, 4.77] |
| 2.8 RD vs No Treatment | 1 | 2150 | Risk Ratio (IV, Random, 95% CI) | 1.62 [1.27, 2.06] |
| 2.9 Excisional Treatment NOS vs No Treatment | 15 | 3.106231E6 | Risk Ratio (IV, Random, 95% CI) | 1.90 [1.50, 2.41] |
| 2.10 Ablative Treatment NOS vs No Treatment | 5 | 595272 | Risk Ratio (IV, Random, 95% CI) | 1.46 [1.27, 1.66] |
| 2.11 Treatment NOS vs No Treatment | 2 | 41131 | Risk Ratio (IV, Random, 95% CI) | 2.57 [1.39, 4.77] |
| 3 PTB (<32‐34w) | 24 | 3.793874E6 | Risk Ratio (IV, Random, 95% CI) | 2.25 [1.79, 2.82] |
| 3.1 Excisional Treatment vs No Treatment | 22 | 3.666567E6 | Risk Ratio (IV, Random, 95% CI) | 2.48 [1.92, 3.20] |
| 3.2 Ablative Treatment vs No Treatment | 3 | 120820 | Risk Ratio (IV, Random, 95% CI) | 1.59 [1.08, 2.35] |
| 3.3 Treatment NOS vs No treatment | 2 | 6487 | Risk Ratio (IV, Random, 95% CI) | 1.65 [1.13, 2.42] |
| 4 PTB (<32‐34w)‐Analysis by treatment modality | 24 | 3.793874E6 | Risk Ratio (IV, Random, 95% CI) | 2.35 [1.88, 2.95] |
| 4.1 CKC vs No Treatment | 5 | 36979 | Risk Ratio (IV, Random, 95% CI) | 3.07 [1.72, 5.49] |
| 4.2 NETZ vs No Treatment | 1 | 7399 | Risk Ratio (IV, Random, 95% CI) | 10.53 [4.33, 25.65] |
| 4.3 LLETZ vs No Treatment | 11 | 791554 | Risk Ratio (IV, Random, 95% CI) | 2.13 [1.66, 2.75] |
| 4.4 CT vs No Treatment | 1 | 58 | Risk Ratio (IV, Random, 95% CI) | 1.86 [0.08, 43.87] |
| 4.5 Excisional Treatment NOS vs No Treatment | 9 | 2.830635E6 | Risk Ratio (IV, Random, 95% CI) | 2.94 [1.82, 4.77] |
| 4.6 Ablative Treatment NOS vs No Treatment | 2 | 120762 | Risk Ratio (IV, Random, 95% CI) | 1.59 [1.08, 2.35] |
| 4.7 Treatment NOS vs No treatment | 2 | 6487 | Risk Ratio (IV, Random, 95% CI) | 1.65 [1.13, 2.42] |
| 5 PTB (<28‐30w) | 8 | 3.910629E6 | Risk Ratio (IV, Random, 95% CI) | 2.23 [1.55, 3.22] |
| 5.1 Excisional Treatment vs No Treatment | 7 | 3.337003E6 | Risk Ratio (IV, Random, 95% CI) | 2.81 [1.91, 4.15] |
| 5.2 Ablative Treatment vs No Treatment | 3 | 568217 | Risk Ratio (IV, Random, 95% CI) | 1.38 [0.81, 2.36] |
| 5.3 Treatment NOS vs No Treatment | 1 | 5409 | Risk Ratio (IV, Random, 95% CI) | 1.75 [1.05, 2.91] |
| 6 PTB (<28‐30w)‐Analysis by treatment modality | 8 | 3.910629E6 | Risk Ratio (IV, Random, 95% CI) | 2.43 [1.69, 3.49] |
| 6.1 CKC vs No Treatment | 2 | 7118 | Risk Ratio (IV, Random, 95% CI) | 4.52 [0.83, 24.54] |
| 6.2 NETZ vs No Treatment | 1 | 7399 | Risk Ratio (IV, Random, 95% CI) | 14.74 [4.50, 48.32] |
| 6.3 LLETZ vs No Treatment | 3 | 502778 | Risk Ratio (IV, Random, 95% CI) | 2.57 [1.97, 3.35] |
| 6.4 Excisional Treatment NOS vs No treatment | 3 | 2.819708E6 | Risk Ratio (IV, Random, 95% CI) | 2.54 [1.30, 4.99] |
| 6.5 Ablative Treatment NOS vs No Treatment | 3 | 568217 | Risk Ratio (IV, Random, 95% CI) | 1.38 [0.81, 2.36] |
| 6.6 Treatment NOS vs No Treatment | 1 | 5409 | Risk Ratio (IV, Random, 95% CI) | 1.75 [1.05, 2.91] |
| 7 PTB (≤34w) | 15 | 424567 | Risk Ratio (IV, Random, 95% CI) | 2.59 [1.78, 3.77] |
| 7.1 Excisional Treatment vs No Treatment | 15 | 424509 | Risk Ratio (IV, Random, 95% CI) | 2.61 [1.78, 3.83] |
| 7.2 Ablative Treatment vs No Treatment | 1 | 58 | Risk Ratio (IV, Random, 95% CI) | 1.86 [0.08, 43.87] |
| 8 PTB (≤34w)‐Analysis by treatment modality | 15 | 424567 | Risk Ratio (IV, Random, 95% CI) | 2.56 [1.78, 3.69] |
| 8.1 CKC vs No Treatment | 4 | 30023 | Risk Ratio (IV, Random, 95% CI) | 2.85 [1.50, 5.41] |
| 8.2 LLETZ vs No Treatment | 9 | 289218 | Risk Ratio (IV, Random, 95% CI) | 1.83 [1.41, 2.39] |
| 8.3 CT vs No Treatment | 1 | 58 | Risk Ratio (IV, Random, 95% CI) | 1.86 [0.08, 43.87] |
| 8.4 Excisional Treatment NOS vs No Treatment | 4 | 105268 | Risk Ratio (IV, Random, 95% CI) | 7.30 [4.17, 12.80] |
| 9 PTB (<32‐33w) | 10 | 3.369685E6 | Risk Ratio (IV, Random, 95% CI) | 2.08 [1.55, 2.79] |
| 9.1 Excisional Treatment vs No Treatment | 8 | 3.242436E6 | Risk Ratio (IV, Random, 95% CI) | 2.43 [1.70, 3.47] |
| 9.2 Ablative Treatment vs No Treatment | 2 | 120762 | Risk Ratio (IV, Random, 95% CI) | 1.59 [1.08, 2.35] |
| 9.3 Treatment NOS vs No treatment | 2 | 6487 | Risk Ratio (IV, Random, 95% CI) | 1.65 [1.13, 2.42] |
| 10 PTB (<32‐33w)‐Analysis by treatment modality | 10 | 3.369685E6 | Risk Ratio (IV, Random, 95% CI) | 2.26 [1.70, 3.01] |
| 10.1 CKC vs No Treatment | 1 | 6956 | Risk Ratio (IV, Random, 95% CI) | 4.38 [1.08, 17.65] |
| 10.2 NETZ vs No Treatment | 1 | 7399 | Risk Ratio (IV, Random, 95% CI) | 10.53 [4.33, 25.65] |
| 10.3 LLETZ vs No Treatment | 3 | 502714 | Risk Ratio (IV, Random, 95% CI) | 2.74 [2.30, 3.26] |
| 10.4 Excisional Treatment NOS vs No Treatment | 5 | 2.725367E6 | Risk Ratio (IV, Random, 95% CI) | 2.09 [1.20, 3.63] |
| 10.5 Ablative Treatment NOS vs No Treatment | 2 | 120762 | Risk Ratio (IV, Random, 95% CI) | 1.59 [1.08, 2.35] |
| 10.6 Treatment NOS vs No treatment | 2 | 6487 | Risk Ratio (IV, Random, 95% CI) | 1.65 [1.13, 2.42] |
| 11 PTB (<30w) | 1 | 162 | Risk Ratio (IV, Random, 95% CI) | 2.86 [0.12, 69.11] |
| 11.1 Excisional Treatment vs No Treatment | 1 | 162 | Risk Ratio (IV, Random, 95% CI) | 2.86 [0.12, 69.11] |
| 12 PTB (<30w)‐Analysis by treatment modality | 1 | 162 | Risk Ratio (IV, Random, 95% CI) | 2.86 [0.12, 69.11] |
| 12.1 CKC vs No Treatment | 1 | 162 | Risk Ratio (IV, Random, 95% CI) | 2.86 [0.12, 69.11] |
| 13 PTB (<28w) | 7 | 3.910467E6 | Risk Ratio (IV, Random, 95% CI) | 2.22 [1.54, 3.22] |
| 13.1 Excisional Treatment vs No Treatment | 6 | 3.336841E6 | Risk Ratio (IV, Random, 95% CI) | 2.81 [1.89, 4.18] |
| 13.2 Ablative treatment vs No Treatment | 3 | 568217 | Risk Ratio (IV, Random, 95% CI) | 1.38 [0.81, 2.36] |
| 13.3 Treatment NOS vs No Treatment | 1 | 5409 | Risk Ratio (IV, Random, 95% CI) | 1.75 [1.05, 2.91] |
| 14 PTB (<28w)‐Analysis by treatment modality | 6 | 3.905058E6 | Risk Ratio (IV, Random, 95% CI) | 2.52 [1.71, 3.72] |
| 14.1 CKC vs No Treatment | 1 | 6956 | Risk Ratio (IV, Random, 95% CI) | 5.41 [0.74, 39.84] |
| 14.2 NETZ vs No Treatment | 1 | 7399 | Risk Ratio (IV, Random, 95% CI) | 14.74 [4.50, 48.32] |
| 14.3 LLETZ vs No Treatment | 3 | 502778 | Risk Ratio (IV, Random, 95% CI) | 2.57 [1.97, 3.35] |
| 14.4 Excisional Treatment NOS vs No treatment | 3 | 2.819708E6 | Risk Ratio (IV, Random, 95% CI) | 2.54 [1.30, 4.99] |
| 14.5 Ablative Treatment NOS vs No Treatment | 3 | 568217 | Risk Ratio (IV, Random, 95% CI) | 1.38 [0.81, 2.36] |
| 15 PTB (<37w)‐Nulliparous women | 6 | 245707 | Risk Ratio (IV, Random, 95% CI) | 1.92 [1.23, 2.98] |
| 15.1 LC vs No Treatment | 2 | 267 | Risk Ratio (IV, Random, 95% CI) | 2.18 [1.09, 4.37] |
| 15.2 LLETZ vs No Treatment | 3 | 231344 | Risk Ratio (IV, Random, 95% CI) | 1.51 [0.76, 3.02] |
| 15.3 Treatment NOS versus No Treatment | 1 | 14096 | Risk Ratio (IV, Random, 95% CI) | 3.53 [1.70, 7.33] |
| 16 PTB (<37w)‐Parous women | 5 | 339507 | Risk Ratio (IV, Random, 95% CI) | 2.05 [0.95, 4.43] |
| 16.1 LC vs No Treatment | 2 | 401 | Risk Ratio (IV, Random, 95% CI) | 2.82 [0.16, 49.84] |
| 16.2 LLETZ vs No Treatment | 2 | 324948 | Risk Ratio (IV, Random, 95% CI) | 1.20 [0.22, 6.65] |
| 16.3 Treatment NOS vs No Treatment | 1 | 14158 | Risk Ratio (IV, Random, 95% CI) | 3.73 [2.23, 6.22] |
| 17 PTB (<37w)‐Single cone | 17 | 1.367023E6 | Risk Ratio (IV, Random, 95% CI) | 1.75 [1.49, 2.06] |
| 17.1 CKC vs No Treatment | 3 | 36783 | Risk Ratio (IV, Random, 95% CI) | 2.89 [2.08, 4.03] |
| 17.2 LC vs No Treatment | 2 | 657 | Risk Ratio (IV, Random, 95% CI) | 1.06 [0.54, 2.09] |
| 17.3 NETZ vs No Treatment | 1 | 7399 | Risk Ratio (IV, Random, 95% CI) | 5.83 [3.80, 8.95] |
| 17.4 LLETZ vs No Treatment | 9 | 1.277874E6 | Risk Ratio (IV, Random, 95% CI) | 1.74 [1.45, 2.10] |
| 17.5 LA vs No Treatment | 4 | 1421 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.66, 1.74] |
| 17.6 Excisional Treatment NOS vs No Treatment | 3 | 32106 | Risk Ratio (IV, Random, 95% CI) | 1.88 [1.20, 2.93] |
| 17.7 Ablative Treatment NOS vs No Treatment | 1 | 10783 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.82, 1.57] |
| 18 PTB (<37w)‐Repeat cones | 11 | 1.317284E6 | Risk Ratio (IV, Random, 95% CI) | 3.78 [2.65, 5.39] |
| 18.1 CKC/LA vs No Treatment | 1 | 99 | Risk Ratio (IV, Random, 95% CI) | 12.56 [5.11, 30.87] |
| 18.2 LC/LC vs No Treatment | 1 | 270 | Risk Ratio (IV, Random, 95% CI) | 3.75 [1.70, 8.27] |
| 18.3 LLETZ/LLETZ vs No Treatment | 4 | 1.202174E6 | Risk Ratio (IV, Random, 95% CI) | 2.81 [2.33, 3.39] |
| 18.4 LLETZ/Treatment NOS vs No Treatment | 1 | 298 | Risk Ratio (IV, Random, 95% CI) | 9.40 [3.53, 25.03] |
| 18.5 Excisional Treatment NOS/Excisional Treatment NOS vs No Treatment | 3 | 73651 | Risk Ratio (IV, Random, 95% CI) | 5.48 [2.68, 11.24] |
| 18.6 Treatment NOS/Treatment NOS vs No Treatment | 2 | 40792 | Risk Ratio (IV, Random, 95% CI) | 1.71 [1.10, 2.67] |
| 19 PTB (<37w)‐Singleton pregnancies | 32 | 2.18962E6 | Risk Ratio (IV, Random, 95% CI) | 1.76 [1.57, 1.98] |
| 19.1 CKC vs No Treatment | 6 | 37759 | Risk Ratio (IV, Random, 95% CI) | 2.89 [2.22, 3.77] |
| 19.2 LC vs No Treatment | 4 | 545 | Risk Ratio (IV, Random, 95% CI) | 2.54 [1.24, 5.20] |
| 19.3 NETZ vs No Treatment | 1 | 7399 | Risk Ratio (IV, Random, 95% CI) | 5.83 [3.80, 8.95] |
| 19.4 LLETZ vs No Treatment | 18 | 1.444175E6 | Risk Ratio (IV, Random, 95% CI) | 1.61 [1.39, 1.87] |
| 19.5 LA vs No Treatment | 3 | 3420 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.75, 1.62] |
| 19.6 CT vs No Treatment | 1 | 58 | Risk Ratio (IV, Random, 95% CI) | 1.86 [0.08, 43.87] |
| 19.7 RD vs No Treatment | 1 | 2150 | Risk Ratio (IV, Random, 95% CI) | 1.62 [1.27, 2.06] |
| 19.8 Excisional Treatment NOS vs No Treatment | 7 | 542892 | Risk Ratio (IV, Random, 95% CI) | 1.42 [1.17, 1.72] |
| 19.9 Ablative Treatment NOS vs No Treatment | 2 | 110091 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.56, 2.32] |
| 19.10 Treatment NOS vs No Treatment | 2 | 41131 | Risk Ratio (IV, Random, 95% CI) | 2.57 [1.39, 4.77] |
| 20 PTB (<37w)‐Multiple pregnancies | 5 | 10797 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.95, 1.35] |
| 20.1 CKC vs No Treatment | 2 | 84 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.49, 1.83] |
| 20.2 LLETZ vs No Treatment | 3 | 10199 | Risk Ratio (IV, Random, 95% CI) | 1.27 [1.09, 1.47] |
| 20.3 Excisional Treatment NOS vs No Treatment | 1 | 4 | Risk Ratio (IV, Random, 95% CI) | 3.5 [0.31, 39.71] |
| 20.4 Ablative Treatment NOS vs No Treatment | 1 | 510 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.72, 1.20] |
| 21 PTB (<32‐34w)‐Multiple pregnancies | 3 | 10789 | Risk Ratio (IV, Random, 95% CI) | 1.68 [0.95, 2.98] |
| 21.1 CKC vs No Treatment | 1 | 80 | Risk Ratio (IV, Random, 95% CI) | 3.5 [1.29, 9.52] |
| 21.2 LLETZ vs No Treatment | 3 | 10199 | Risk Ratio (IV, Random, 95% CI) | 1.76 [0.88, 3.50] |
| 21.3 Ablative Treatment NOS vs No Treatment | 1 | 510 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.38, 1.91] |
| 22 PTB (<28w)‐Multiple pregnancies | 2 | 10744 | Risk Ratio (IV, Random, 95% CI) | 2.43 [1.40, 4.22] |
| 22.1 CKC vs No Treatment | 1 | 80 | Risk Ratio (IV, Random, 95% CI) | 2.15 [0.09, 49.56] |
| 22.2 LLETZ vs No Treatment | 2 | 10154 | Risk Ratio (IV, Random, 95% CI) | 2.45 [1.34, 4.47] |
| 22.3 Ablative Treatment NOS vs No Treatment | 1 | 510 | Risk Ratio (IV, Random, 95% CI) | 2.32 [0.48, 11.26] |
| 23 PTB (<37w)‐Depth≤10‐12mm | 8 | 550929 | Risk Ratio (IV, Random, 95% CI) | 1.54 [1.09, 2.18] |
| 23.1 LC vs No Treatment | 1 | 105 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.06, 4.83] |
| 23.2 LLETZ vs No Treatment | 3 | 544907 | Risk Ratio (IV, Random, 95% CI) | 2.01 [1.28, 3.15] |
| 23.3 Excisional Treatment NOS vs No Treatment | 4 | 5917 | Risk Ratio (IV, Random, 95% CI) | 1.20 [0.78, 1.85] |
| 24 PTB (<37w)‐Depth≥10‐12mm | 8 | 552711 | Risk Ratio (IV, Random, 95% CI) | 1.93 [1.62, 2.31] |
| 24.1 LC vs No Treatment | 1 | 87 | Risk Ratio (IV, Random, 95% CI) | 4.64 [1.20, 17.88] |
| 24.2 LLETZ vs No Treatment | 3 | 546134 | Risk Ratio (IV, Random, 95% CI) | 2.29 [1.57, 3.34] |
| 24.3 Excisional Treatment NOS vs No Treatment | 4 | 6490 | Risk Ratio (IV, Random, 95% CI) | 1.68 [1.41, 1.99] |
| 25 PTB (<37w)‐Depth≥15‐17mm | 4 | 544986 | Risk Ratio (IV, Random, 95% CI) | 2.77 [1.95, 3.93] |
| 25.1 LC vs No Treatment | 1 | 211 | Risk Ratio (IV, Random, 95% CI) | 4.92 [2.09, 11.59] |
| 25.2 LLETZ vs No Treatment | 2 | 544248 | Risk Ratio (IV, Random, 95% CI) | 3.16 [1.54, 6.48] |
| 25.3 Excisional Treatment NOS vs No Treatment | 1 | 527 | Risk Ratio (IV, Random, 95% CI) | 2.03 [1.33, 3.10] |
| 26 PTB (<37w)‐Depth≥20mm | 3 | 543750 | Risk Ratio (IV, Random, 95% CI) | 4.91 [2.06, 11.68] |
| 26.1 LC vs No Treatment | 1 | 192 | Risk Ratio (IV, Random, 95% CI) | 6.12 [2.57, 14.57] |
| 26.2 LLETZ vs No Treatment | 2 | 543558 | Risk Ratio (IV, Random, 95% CI) | 4.72 [1.25, 17.80] |
| 27 PTB (<37w)‐Volume<6cc | 1 | 550 | Risk Ratio (IV, Random, 95% CI) | 2.25 [1.09, 4.66] |
| 27.1 LLETZ vs No Treatment | 1 | 550 | Risk Ratio (IV, Random, 95% CI) | 2.25 [1.09, 4.66] |
| 28 PTB (<37w)‐Volume>6cc | 1 | 284 | Risk Ratio (IV, Random, 95% CI) | 13.90 [5.09, 37.98] |
| 28.1 LLETZ (Volume>6cc) vs No Treatment | 1 | 284 | Risk Ratio (IV, Random, 95% CI) | 13.90 [5.09, 37.98] |
| 29 PTB (<37w)‐Depth≤10mm | 7 | 7436 | Risk Ratio (IV, Random, 95% CI) | 1.60 [0.99, 2.59] |
| 29.1 LC vs No Treatment | 1 | 105 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.06, 4.83] |
| 29.2 LLETZ vs No Treatment | 2 | 1414 | Risk Ratio (IV, Random, 95% CI) | 2.72 [1.65, 4.50] |
| 29.3 Excisional Treatment NOS vs No Treatment | 4 | 5917 | Risk Ratio (IV, Random, 95% CI) | 1.20 [0.78, 1.85] |
| 30 PTB (<37w)‐Depth≤12mm | 1 | 543493 | Risk Ratio (IV, Random, 95% CI) | 1.56 [1.20, 2.02] |
| 30.1 LLETZ vs No Treatment | 1 | 543493 | Risk Ratio (IV, Random, 95% CI) | 1.56 [1.20, 2.02] |
| 31 PTB (<37w)‐Depth≤15mm | 3 | 545283 | Risk Ratio (IV, Random, 95% CI) | 1.44 [1.20, 1.73] |
| 31.1 LC vs No Treatment | 1 | 164 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.04, 11.18] |
| 31.2 LLETZ vs No Treatment | 2 | 545119 | Risk Ratio (IV, Random, 95% CI) | 1.44 [1.20, 1.73] |
| 32 PTB (<37w)‐Depth≤17mm | 1 | 656 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.76, 1.72] |
| 32.1 Excisional Treatment NOS vs No Treatment | 1 | 656 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.76, 1.72] |
| 33 PTB (<37w)‐Depth≤15‐17mm | 4 | 545939 | Risk Ratio (IV, Random, 95% CI) | 1.38 [1.17, 1.64] |
| 33.1 LC vs No Treatment | 1 | 164 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.04, 11.18] |
| 33.2 LLETZ vs No Treatment | 2 | 545119 | Risk Ratio (IV, Random, 95% CI) | 1.44 [1.20, 1.73] |
| 33.3 Excisional Treatment NOS vs No Treatment | 1 | 656 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.76, 1.72] |
| 34 PTB (<37w)‐Depth≤20mm | 3 | 545992 | Risk Ratio (IV, Random, 95% CI) | 1.60 [1.38, 1.87] |
| 34.1 LC vs No Treatment | 1 | 183 | Risk Ratio (IV, Random, 95% CI) | 1.30 [0.28, 5.97] |
| 34.2 LLETZ vs No Treatment | 2 | 545809 | Risk Ratio (IV, Random, 95% CI) | 1.61 [1.38, 1.87] |
| 35 PTB (<37w)‐Depth≥10mm | 7 | 7671 | Risk Ratio (IV, Random, 95% CI) | 2.12 [1.58, 2.85] |
| 35.1 LC vs No Treatment | 1 | 87 | Risk Ratio (IV, Random, 95% CI) | 4.64 [1.20, 17.88] |
| 35.2 LLETZ vs No Treatment | 2 | 1094 | Risk Ratio (IV, Random, 95% CI) | 3.16 [1.80, 5.55] |
| 35.3 Excisional Treatment NOS vs No Treatment | 4 | 6490 | Risk Ratio (IV, Random, 95% CI) | 1.68 [1.41, 1.99] |
| 36 PTB (<37w)‐Depth≥12mm | 1 | 545040 | Risk Ratio (IV, Random, 95% CI) | 1.93 [1.66, 2.23] |
| 36.1 LLETZ vs No Treatment | 1 | 545040 | Risk Ratio (IV, Random, 95% CI) | 1.93 [1.66, 2.23] |
| 37 PTB (<37w)‐Depth≥15mm | 3 | 544459 | Risk Ratio (IV, Random, 95% CI) | 3.49 [1.94, 6.26] |
| 37.1 LC vs No Treatment | 1 | 211 | Risk Ratio (IV, Random, 95% CI) | 4.92 [2.09, 11.59] |
| 37.2 LLETZ vs No Treatment | 2 | 544248 | Risk Ratio (IV, Random, 95% CI) | 3.16 [1.54, 6.48] |
| 38 PTB (<37w)‐Depth≥17mm | 1 | 527 | Risk Ratio (IV, Random, 95% CI) | 2.03 [1.33, 3.10] |
| 38.1 Excisional Treatment NOS vs No Treatment | 1 | 527 | Risk Ratio (IV, Random, 95% CI) | 2.03 [1.33, 3.10] |
| 39 PTB (<37w)‐Depth 10/13‐15/16mm | 3 | 544534 | Risk Ratio (IV, Random, 95% CI) | 1.32 [1.04, 1.66] |
| 39.1 LLETZ vs No Treatment | 2 | 543994 | Risk Ratio (IV, Random, 95% CI) | 1.32 [1.02, 1.72] |
| 39.2 Excisional Treatment NOS vs No Treatment | 1 | 540 | Risk Ratio (IV, Random, 95% CI) | 1.29 [0.79, 2.12] |
| 40 PTB (<37w)‐Depth 15/16‐19/20mm | 3 | 543608 | Risk Ratio (IV, Random, 95% CI) | 2.24 [1.73, 2.91] |
| 40.1 LC vs No Treatment | 1 | 169 | Risk Ratio (IV, Random, 95% CI) | 2.26 [0.50, 10.08] |
| 40.2 LLETZ vs No Treatment | 2 | 543439 | Risk Ratio (IV, Random, 95% CI) | 2.53 [1.42, 4.51] |
| 41 PTB (<37w)‐Volume<3cc | 1 | 496 | Risk Ratio (IV, Random, 95% CI) | 2.04 [0.94, 4.41] |
| 41.1 LLETZ vs No Treatment | 1 | 496 | Risk Ratio (IV, Random, 95% CI) | 2.04 [0.94, 4.41] |
| 42 PTB (<37w)‐Volume>3cc | 1 | 338 | Risk Ratio (IV, Random, 95% CI) | 4.17 [1.77, 9.82] |
| 42.1 LLETZ vs No Treatment | 1 | 338 | Risk Ratio (IV, Random, 95% CI) | 4.17 [1.77, 9.82] |
| 43 PTB (<37w)‐Depth≥10‐12mm vs ≤10‐12mm | 7 | 6359 | Risk Ratio (IV, Random, 95% CI) | 1.54 [1.31, 1.80] |
| 43.1 LC | 1 | 64 | Risk Ratio (IV, Random, 95% CI) | 8.91 [1.11, 71.73] |
| 43.2 LLETZ | 2 | 836 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.74, 2.17] |
| 43.3 Excision NOS | 4 | 5459 | Risk Ratio (IV, Random, 95% CI) | 1.55 [1.31, 1.83] |
| 44 PTB (<37w)‐Depth≥15‐17mm vs ≤15‐17mm | 4 | 4275 | Risk Ratio (IV, Random, 95% CI) | 1.82 [1.47, 2.26] |
| 44.1 LC | 1 | 75 | Risk Ratio (IV, Random, 95% CI) | 7.02 [0.44, 111.10] |
| 44.2 LLETZ | 2 | 3869 | Risk Ratio (IV, Random, 95% CI) | 1.86 [1.36, 2.55] |
| 44.3 Excisional Treatment NOS | 1 | 331 | Risk Ratio (IV, Random, 95% CI) | 1.78 [1.11, 2.84] |
| 45 PTB (<37w)‐Depth≥20mm vs ≤20mm | 3 | 3944 | Risk Ratio (IV, Random, 95% CI) | 2.79 [1.24, 6.27] |
| 45.1 LC | 1 | 75 | Risk Ratio (IV, Random, 95% CI) | 4.71 [1.13, 19.62] |
| 45.2 LLETZ | 2 | 3869 | Risk Ratio (IV, Random, 95% CI) | 2.47 [0.94, 6.51] |
| 46 PTB (<37w)‐Volume>3cc vs <3cc | 1 | 278 | Risk Ratio (IV, Random, 95% CI) | 2.04 [0.95, 4.39] |
| 46.1 LLETZ | 1 | 278 | Risk Ratio (IV, Random, 95% CI) | 2.04 [0.95, 4.39] |
| 47 PTB (<37w)‐Volume>6cc vs <6cc | 1 | 278 | Risk Ratio (IV, Random, 95% CI) | 6.18 [2.53, 15.13] |
| 47.1 LLETZ | 1 | 278 | Risk Ratio (IV, Random, 95% CI) | 6.18 [2.53, 15.13] |
| 48 PTB (<37w)‐Depth 11/13‐15/16mm vs ≤10‐12mm | 3 | 2600 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.67, 1.25] |
| 48.1 LLETZ | 2 | 2370 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.58, 1.17] |
| 48.2 Excisional Treatment NOS | 1 | 230 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.68, 2.50] |
| 49 PTB (<37w)‐Depth 16‐19mm vs 13‐15mm | 1 | 1768 | Risk Ratio (IV, Random, 95% CI) | 1.65 [1.12, 2.43] |
| 49.1 LLETZ | 1 | 1768 | Risk Ratio (IV, Random, 95% CI) | 1.65 [1.12, 2.43] |
| 50 PTB (<37w)‐Depth≥20mm vs 15/16‐19/20mm | 3 | 1560 | Risk Ratio (IV, Random, 95% CI) | 1.46 [0.95, 2.23] |
| 50.1 LC | 1 | 61 | Risk Ratio (IV, Random, 95% CI) | 2.71 [0.67, 10.96] |
| 50.2 LLETZ | 2 | 1499 | Risk Ratio (IV, Random, 95% CI) | 1.40 [0.84, 2.36] |
| 51 PTB (<37w)‐Untreated External Comparison Group | 44 | 5.192047E6 | Risk Ratio (IV, Random, 95% CI) | 1.92 [1.70, 2.16] |
| 51.1 CKC | 7 | 37370 | Risk Ratio (IV, Random, 95% CI) | 3.28 [2.44, 4.42] |
| 51.2 LC | 6 | 1126 | Risk Ratio (IV, Random, 95% CI) | 2.39 [1.24, 4.61] |
| 51.3 NETZ | 1 | 7361 | Risk Ratio (IV, Random, 95% CI) | 5.82 [3.79, 8.94] |
| 51.4 LLETZ | 19 | 1.414769E6 | Risk Ratio (IV, Random, 95% CI) | 1.72 [1.48, 2.00] |
| 51.5 LA] | 4 | 1258 | Risk Ratio (IV, Random, 95% CI) | 1.27 [0.67, 2.40] |
| 51.6 CT | 1 | 58 | Risk Ratio (IV, Random, 95% CI) | 1.86 [0.08, 43.87] |
| 51.7 Excisional Treatment NOS | 12 | 3.100025E6 | Risk Ratio (IV, Random, 95% CI) | 1.91 [1.50, 2.44] |
| 51.8 Ablative Treatment NOS | 5 | 588949 | Risk Ratio (IV, Random, 95% CI) | 1.45 [1.26, 1.67] |
| 51.9 Treatment NOS | 2 | 41131 | Risk Ratio (IV, Random, 95% CI) | 2.57 [1.39, 4.77] |
| 52 PTB (<37w)‐Untreated Internal Comparison Group (self‐matching) | 8 | 2987 | Risk Ratio (IV, Random, 95% CI) | 1.59 [1.19, 2.13] |
| 52.1 LC | 2 | 354 | Risk Ratio (IV, Random, 95% CI) | 1.30 [0.56, 3.06] |
| 52.2 LLETZ | 1 | 516 | Risk Ratio (IV, Random, 95% CI) | 1.82 [1.04, 3.21] |
| 52.3 FCBE | 1 | 71 | Risk Ratio (IV, Random, 95% CI) | 5.22 [1.09, 24.90] |
| 52.4 Excisional Treatment NOS | 3 | 1922 | Risk Ratio (IV, Random, 95% CI) | 1.46 [0.89, 2.39] |
| 52.5 Treatment NOS | 1 | 124 | Risk Ratio (IV, Random, 95% CI) | 2.0 [0.73, 5.51] |
| 53 PTB (<37w)‐Untreated Internal Comparison Group (pre‐treatment pregnancies) | 13 | 83404 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.98, 1.96] |
| 53.1 CKC | 3 | 1430 | Risk Ratio (IV, Random, 95% CI) | 1.79 [0.81, 3.95] |
| 53.2 LC | 2 | 161 | Risk Ratio (IV, Random, 95% CI) | 1.65 [0.11, 23.58] |
| 53.3 LLETZ | 4 | 3207 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.66, 1.94] |
| 53.4 LA | 1 | 226 | Risk Ratio (IV, Random, 95% CI) | 1.20 [0.57, 2.53] |
| 53.5 CT | 1 | 180 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.15, 4.94] |
| 53.6 Excisional NOS | 3 | 78200 | Risk Ratio (IV, Random, 95% CI) | 1.65 [0.88, 3.08] |
| 54 PTB (<37w)‐Untreated Colposcopy+/‐CIN+/‐Biopsy Comparison Group | 13 | 74958 | Risk Ratio (IV, Random, 95% CI) | 1.27 [1.14, 1.41] |
| 54.1 CKC | 2 | 265 | Risk Ratio (IV, Random, 95% CI) | 1.76 [1.01, 3.08] |
| 54.2 LC | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 1.52 [0.74, 3.15] |
| 54.3 LLETZ | 9 | 39249 | Risk Ratio (IV, Random, 95% CI) | 1.33 [1.11, 1.60] |
| 54.4 LA | 2 | 3326 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.84, 1.31] |
| 54.5 RD | 1 | 2150 | Risk Ratio (IV, Random, 95% CI) | 1.62 [1.27, 2.06] |
| 54.6 Excisional Treatment NOS | 5 | 20321 | Risk Ratio (IV, Random, 95% CI) | 1.23 [1.07, 1.41] |
| 54.7 Ablative Treatment NOS | 2 | 9470 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.74, 1.36] |
| 55 PTB (<37w)‐Untreated HSIL Comparison Group | 3 | 3764 | Risk Ratio (IV, Random, 95% CI) | 1.37 [0.85, 2.19] |
| 55.1 CKC | 1 | 103 | Risk Ratio (IV, Random, 95% CI) | 3.76 [0.48, 29.39] |
| 55.2 NETZ | 1 | 109 | Risk Ratio (IV, Random, 95% CI) | 4.55 [1.11, 18.66] |
| 55.3 LLETZ | 1 | 881 | Risk Ratio (IV, Random, 95% CI) | 2.48 [1.35, 4.55] |
| 55.4 Excisional Treatment NOS | 2 | 2274 | Risk Ratio (IV, Random, 95% CI) | 1.06 [0.71, 1.59] |
| 55.5 Ablative Treatment NOS | 2 | 397 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.28, 1.68] |
| 56 PTB (<37w)‐All Comparison Groups | 58 | 5.292724E6 | Risk Ratio (IV, Random, 95% CI) | 1.76 [1.58, 1.97] |
| 56.1 Treatment vs Untreated External Comparison Group | 43 | 5.165466E6 | Risk Ratio (IV, Random, 95% CI) | 1.97 [1.71, 2.26] |
| 56.2 Treatment vs Untreated Internal Comparison Group (pre‐treatment pregnancies) | 13 | 62519 | Risk Ratio (IV, Random, 95% CI) | 1.66 [1.24, 2.22] |
| 56.3 Treatment vs Untreated Internal Comparison Group (self‐matching) | 6 | 1263 | Risk Ratio (IV, Random, 95% CI) | 1.91 [1.19, 3.08] |
| 56.4 Treatment vs Untreated Colposcopy+/‐CIN+/‐Biopsy Comparison Group | 12 | 62702 | Risk Ratio (IV, Random, 95% CI) | 1.33 [1.17, 1.50] |
| 56.5 Treatment vs Untreated HSIL Comparison Group | 3 | 774 | Risk Ratio (IV, Random, 95% CI) | 1.46 [0.62, 3.42] |
| 57 PTB (<37w)‐Untreated High‐risk Population vs General Population | 15 | 4.357998E6 | Risk Ratio (IV, Random, 95% CI) | 1.24 [1.14, 1.34] |
| 57.1 Pre‐treatment pregnancies vs General Population | 10 | 3.132723E6 | Risk Ratio (IV, Random, 95% CI) | 1.23 [1.07, 1.42] |
| 57.2 Untreated Colposcopy+/‐CIN+/‐Biopsy vs General Population | 4 | 1.046823E6 | Risk Ratio (IV, Random, 95% CI) | 1.22 [1.11, 1.34] |
| 57.3 Untreated HSIL vs General Population | 3 | 178452 | Risk Ratio (IV, Random, 95% CI) | 1.40 [0.94, 2.10] |
| 58 PTB (<37w)‐Depth≤10‐12mm vs Untreated External Comparison Group | 6 | 1.026243E6 | Risk Ratio (IV, Random, 95% CI) | 1.64 [1.11, 2.42] |
| 58.1 LCp | 1 | 105 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.06, 4.83] |
| 58.2 LLETZ | 2 | 512896 | Risk Ratio (IV, Random, 95% CI) | 2.06 [1.10, 3.84] |
| 58.3 Excisional Treatment NOS | 3 | 513242 | Risk Ratio (IV, Random, 95% CI) | 1.57 [0.72, 3.41] |
| 59 PTB (<37w)‐Depth≤10‐12mm vs Untreated Internal Comparison Group | 2 | 3550 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.71, 1.14] |
| 59.1 LC | 1 | 70 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.05, 10.85] |
| 59.2 Excisional Treatment NOS | 1 | 3480 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.71, 1.14] |
| 60 PTB (<37w)‐Depth≤10‐12mm vs Untreated Colposcopy+/‐CIN+/‐Biopsy | 4 | 43145 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.85, 1.43] |
| 60.1 LLETZ | 2 | 33033 | Risk Ratio (IV, Random, 95% CI) | 1.38 [0.94, 2.02] |
| 60.2 Excisional Treatment NOS | 2 | 10112 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.80, 1.09] |
| 61 PTB (<37w)‐Depth≤15‐17mm vs Untreated External Comparison Group | 2 | 513145 | Risk Ratio (IV, Random, 95% CI) | 1.43 [1.19, 1.73] |
| 61.1 LC | 1 | 164 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.04, 11.18] |
| 61.2 LLETZ | 1 | 512981 | Risk Ratio (IV, Random, 95% CI) | 1.44 [1.19, 1.74] |
| 62 PTB (<37w)‐Depth≤15‐17mm vs Untreated Colposcopy+/‐CIN+/‐Biopsy | 3 | 34934 | Risk Ratio (IV, Random, 95% CI) | 1.18 [1.00, 1.40] |
| 62.1 LLETZ | 2 | 34278 | Risk Ratio (IV, Random, 95% CI) | 1.30 [0.85, 1.98] |
| 62.2 Excisional Treatment NOS | 1 | 656 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.76, 1.72] |
| 63 PTB (<37w)‐Depth≤20mm vs Untreated External Comparison Group | 2 | 513814 | Risk Ratio (IV, Random, 95% CI) | 1.60 [1.37, 1.87] |
| 63.1 LC | 1 | 183 | Risk Ratio (IV, Random, 95% CI) | 1.30 [0.28, 5.97] |
| 63.2 LLETZ | 1 | 513631 | Risk Ratio (IV, Random, 95% CI) | 1.61 [1.37, 1.88] |
| 64 PTB (<37w)‐Depth≤20mm vs Untreated Colposcopy+/‐CIN+/‐Biopsy | 2 | 34968 | Risk Ratio (IV, Random, 95% CI) | 1.52 [0.92, 2.51] |
| 64.1 LLETZ | 2 | 34968 | Risk Ratio (IV, Random, 95% CI) | 1.52 [0.92, 2.51] |
| 65 PTB (<37w)‐Depth≥10‐12mm vs Untreated External Comparison Group | 6 | 1.027812E6 | Risk Ratio (IV, Random, 95% CI) | 1.96 [1.66, 2.32] |
| 65.1 LC | 1 | 87 | Risk Ratio (IV, Random, 95% CI) | 4.64 [1.20, 17.88] |
| 65.2 LLETZ | 2 | 514051 | Risk Ratio (IV, Random, 95% CI) | 2.40 [1.30, 4.43] |
| 65.3 Excisional Treatment NOS | 3 | 513674 | Risk Ratio (IV, Random, 95% CI) | 1.82 [1.49, 2.22] |
| 66 PTB (<37w)‐Depth≥10‐12mm vs Untreated Internal Comparison Group | 2 | 3944 | Risk Ratio (IV, Random, 95% CI) | 2.05 [0.56, 7.48] |
| 66.1 LC | 1 | 52 | Risk Ratio (IV, Random, 95% CI) | 6.30 [0.79, 50.27] |
| 66.2 Excisional Treatment NOS | 1 | 3892 | Risk Ratio (IV, Random, 95% CI) | 1.39 [1.12, 1.73] |
| 67 PTB (<37w)‐Depth≥10‐12mm vs Untreated Colposcopy+/‐CIN+/‐Biopsy | 4 | 45275 | Risk Ratio (IV, Random, 95% CI) | 1.52 [1.37, 1.68] |
| 67.1 LLETZ | 2 | 34652 | Risk Ratio (IV, Random, 95% CI) | 1.80 [1.13, 2.87] |
| 67.2 Excisional Treatment NOS | 2 | 10623 | Risk Ratio (IV, Random, 95% CI) | 1.46 [1.29, 1.65] |
| 68 PTB (<37w)‐Depth≥15‐17mm vs Untreated External Comparison Group | 2 | 512503 | Risk Ratio (IV, Random, 95% CI) | 3.04 [1.62, 5.73] |
| 68.1 LC | 1 | 211 | Risk Ratio (IV, Random, 95% CI) | 4.92 [2.09, 11.59] |
| 68.2 LLETZ | 1 | 512292 | Risk Ratio (IV, Random, 95% CI) | 2.45 [2.06, 2.91] |
| 69 PTB (<37w)‐Depth≥15‐17mm vs Untreated Colposcopy+/‐CIN+/‐Biopsy | 3 | 33934 | Risk Ratio (IV, Random, 95% CI) | 2.30 [1.57, 3.35] |
| 69.1 LLETZ | 2 | 33407 | Risk Ratio (IV, Random, 95% CI) | 2.92 [1.14, 7.46] |
| 69.2 Excisional Treatment NOS | 1 | 527 | Risk Ratio (IV, Random, 95% CI) | 2.03 [1.33, 3.10] |
| 70 PTB (<37w)‐Depth≥20mm vs Untreated External Comparison Group | 2 | 511834 | Risk Ratio (IV, Random, 95% CI) | 3.63 [1.67, 7.90] |
| 70.1 LC | 1 | 192 | Risk Ratio (IV, Random, 95% CI) | 6.12 [2.57, 14.57] |
| 70.2 LLETZ | 1 | 511642 | Risk Ratio (IV, Random, 95% CI) | 2.68 [2.15, 3.35] |
| 71 PTB (<37w)‐Depth≥20mm vs Untreated Colposcopy+/‐CIN+/‐Biopsy | 2 | 32717 | Risk Ratio (IV, Random, 95% CI) | 4.32 [0.93, 20.03] |
| 71.1 LLETZ | 2 | 32717 | Risk Ratio (IV, Random, 95% CI) | 4.32 [0.93, 20.03] |
| 72 PTB (<37w)‐Depth 10/13‐15/16mm vs Untreated External Comparison Group | 1 | 511959 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.99, 1.72] |
| 72.1 LLETZ | 1 | 511959 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.99, 1.72] |
| 73 PTB (<37w)‐Depth 10/13‐15/16mm vs Untreated Colposcopy+/‐CIN+/‐Biopsy | 3 | 33693 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.90, 1.44] |
| 73.1 LLETZ | 2 | 33153 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.80, 1.57] |
| 73.2 Excisional Treatment NOS | 1 | 540 | Risk Ratio (IV, Random, 95% CI) | 1.29 [0.79, 2.12] |
| 74 PTB (<37w)‐Depth 15/16‐19/20mm vs Untreated External Comparison Group | 2 | 511660 | Risk Ratio (IV, Random, 95% CI) | 2.16 [1.65, 2.84] |
| 74.1 LC | 1 | 169 | Risk Ratio (IV, Random, 95% CI) | 2.26 [0.50, 10.08] |
| 74.2 LLETZ | 1 | 511491 | Risk Ratio (IV, Random, 95% CI) | 2.16 [1.64, 2.84] |
| 75 PTB (<37w)‐Depth 15/16‐19/20mm vs Untreated Colposcopy+/‐CIN+/‐Biopsy | 2 | 32598 | Risk Ratio (IV, Random, 95% CI) | 2.38 [1.04, 5.42] |
| 75.1 LLETZ | 2 | 32598 | Risk Ratio (IV, Random, 95% CI) | 2.38 [1.04, 5.42] |
Comparison 2. Other maternal Outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 sPTB (<37w) | 14 | 1.024731E6 | Risk Ratio (IV, Random, 95% CI) | 1.76 [1.47, 2.11] |
| 1.1 CKC vs No Treatment | 3 | 7320 | Risk Ratio (IV, Random, 95% CI) | 3.53 [2.05, 6.05] |
| 1.2 LC vs No Treatment | 2 | 222 | Risk Ratio (IV, Random, 95% CI) | 1.40 [0.51, 3.81] |
| 1.3 NETZ vs No Treatment | 1 | 7399 | Risk Ratio (IV, Random, 95% CI) | 5.83 [3.80, 8.95] |
| 1.4 LLETZ vs No Treatment | 11 | 773123 | Risk Ratio (IV, Random, 95% CI) | 1.60 [1.22, 2.08] |
| 1.5 LA vs No Treatment | 1 | 356 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.34, 2.68] |
| 1.6 CT vs No Treatment | 1 | 58 | Risk Ratio (IV, Random, 95% CI) | 1.86 [0.08, 43.87] |
| 1.7 Excisional Treatment NOS vs No Treatment | 2 | 95985 | Risk Ratio (IV, Random, 95% CI) | 1.70 [1.17, 2.46] |
| 1.8 Ablative Treatment NOS vs No Treatment | 2 | 134720 | Risk Ratio (IV, Random, 95% CI) | 1.42 [1.20, 1.70] |
| 1.9 Treatment NOS vs No Treatment | 1 | 5548 | Risk Ratio (IV, Random, 95% CI) | 1.30 [1.00, 1.69] |
| 2 sPTB (<32‐34w) | 7 | 655675 | Risk Ratio (IV, Random, 95% CI) | 2.63 [1.91, 3.62] |
| 2.1 CKC vs No Treatment | 2 | 6990 | Risk Ratio (IV, Random, 95% CI) | 4.38 [1.08, 17.65] |
| 2.2 NETZ vs No Treatment | 1 | 7399 | Risk Ratio (IV, Random, 95% CI) | 10.53 [4.33, 25.65] |
| 2.3 LLETZ vs No Treatment | 6 | 530985 | Risk Ratio (IV, Random, 95% CI) | 2.37 [1.82, 3.08] |
| 2.4 CT vs No Treatment | 1 | 58 | Risk Ratio (IV, Random, 95% CI) | 1.86 [0.08, 43.87] |
| 2.5 Excisional Treatment NOS vs No Treatment | 1 | 264 | Risk Ratio (IV, Random, 95% CI) | 13.92 [0.73, 266.57] |
| 2.6 Ablative Treatment NOS vs No Treatment | 1 | 109979 | Risk Ratio (IV, Random, 95% CI) | 1.57 [0.97, 2.53] |
| 3 sPTB (<28w) | 2 | 626670 | Risk Ratio (IV, Random, 95% CI) | 3.18 [1.64, 6.16] |
| 3.1 CKC vs No Treatment | 1 | 6956 | Risk Ratio (IV, Random, 95% CI) | 5.41 [0.74, 39.84] |
| 3.2 NETZ vs No Treatment | 1 | 7399 | Risk Ratio (IV, Random, 95% CI) | 14.74 [4.50, 48.32] |
| 3.3 LLETZ vs No Treatment | 2 | 502336 | Risk Ratio (IV, Random, 95% CI) | 2.57 [1.96, 3.36] |
| 3.4 Ablative Treatment NOS vs No Treatment | 1 | 109979 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.54, 2.74] |
| 4 pPROM (<37w) | 21 | 477011 | Risk Ratio (IV, Random, 95% CI) | 2.36 [1.76, 3.17] |
| 4.1 CKC vs No Treatment | 4 | 36733 | Risk Ratio (IV, Random, 95% CI) | 4.11 [2.05, 8.25] |
| 4.2 LC vs No Treatment | 4 | 635 | Risk Ratio (IV, Random, 95% CI) | 1.89 [0.97, 3.66] |
| 4.3 NETZ vs No Treatment | 1 | 7279 | Risk Ratio (IV, Random, 95% CI) | 8.83 [5.39, 14.46] |
| 4.4 LLETZ vs No Treatment | 8 | 302974 | Risk Ratio (IV, Random, 95% CI) | 2.15 [1.48, 3.12] |
| 4.5 LA vs No Treatment | 2 | 548 | Risk Ratio (IV, Random, 95% CI) | 1.62 [0.74, 3.55] |
| 4.6 CT vs No Treatment | 1 | 180 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.21, 6.00] |
| 4.7 Excisional Treatment NOS vs No Treatment | 5 | 98372 | Risk Ratio (IV, Random, 95% CI) | 2.66 [1.13, 6.24] |
| 4.8 Ablative Treatment NOS vs No Treatment | 1 | 24742 | Risk Ratio (IV, Random, 95% CI) | 1.47 [1.01, 2.15] |
| 4.9 Treatment NOS vs No Treatment | 1 | 5548 | Risk Ratio (IV, Random, 95% CI) | 1.44 [1.05, 1.97] |
| 5 pPROM (<32w) | 1 | 72788 | Risk Ratio (IV, Random, 95% CI) | 8.30 [2.03, 33.98] |
| 5.1 CKC vs No Treatment | 1 | 6842 | Risk Ratio (IV, Random, 95% CI) | 5.32 [0.72, 39.19] |
| 5.2 NETZ vs No Treatment | 1 | 7279 | Risk Ratio (IV, Random, 95% CI) | 25.38 [9.80, 65.74] |
| 5.3 LLETZ vs No Treatment | 1 | 58667 | Risk Ratio (IV, Random, 95% CI) | 3.74 [1.66, 8.41] |
| 6 pPROM (<28w) | 1 | 72788 | Risk Ratio (IV, Random, 95% CI) | 9.09 [1.04, 79.18] |
| 6.1 CKC vs No Treatment | 1 | 6842 | Risk Ratio (IV, Random, 95% CI) | 6.64 [0.38, 115.16] |
| 6.2 NETZ vs No Treatment | 1 | 7279 | Risk Ratio (IV, Random, 95% CI) | 43.51 [11.48, 164.86] |
| 6.3 LLETZ vs No Treatment | 1 | 58667 | Risk Ratio (IV, Random, 95% CI) | 1.81 [0.25, 13.08] |
| 7 Threatened PTB | 5 | 903 | Risk Ratio (IV, Random, 95% CI) | 2.44 [1.37, 4.33] |
| 7.1 CKC vs No Treatment | 1 | 126 | Risk Ratio (IV, Random, 95% CI) | 1.40 [0.45, 4.34] |
| 7.2 LC vs No Treatment | 1 | 112 | Risk Ratio (IV, Random, 95% CI) | 1.56 [0.53, 4.62] |
| 7.3 LLETZ vs No Treatment | 1 | 237 | Risk Ratio (IV, Random, 95% CI) | 4.0 [0.75, 21.37] |
| 7.4 Excisional Treatment NOS vs No Treatment | 2 | 428 | Risk Ratio (IV, Random, 95% CI) | 4.51 [1.68, 12.06] |
| 8 Chorioamnionitis | 4 | 29198 | Risk Ratio (IV, Random, 95% CI) | 3.43 [1.36, 8.64] |
| 8.1 CKC vs No Treatment | 1 | 28531 | Risk Ratio (IV, Random, 95% CI) | 2.39 [0.61, 9.43] |
| 8.2 LC vs No Treatment | 1 | 112 | Risk Ratio (IV, Random, 95% CI) | 3.33 [0.14, 80.11] |
| 8.3 LLETZ vs No Treatment | 1 | 237 | Risk Ratio (IV, Random, 95% CI) | 10.00 [1.19, 84.15] |
| 8.4 Excisional Treatment NOS vs No Treatment | 1 | 318 | Risk Ratio (IV, Random, 95% CI) | 3.0 [0.51, 17.68] |
| 9 Caeserean Section | 36 | 272360 | Risk Ratio (IV, Random, 95% CI) | 1.06 [0.98, 1.14] |
| 9.1 CKC vs No Treatment | 6 | 30462 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.91, 1.68] |
| 9.2 LC vs No Treatment | 5 | 1038 | Risk Ratio (IV, Random, 95% CI) | 1.38 [0.90, 2.11] |
| 9.3 LLETZ vs No Treatment | 14 | 5436 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.94, 1.15] |
| 9.4 LA vs No Treatment | 4 | 1258 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.61, 1.20] |
| 9.5 CT vs No Treatment | 2 | 238 | Risk Ratio (IV, Random, 95% CI) | 2.47 [1.02, 6.01] |
| 9.6 Excisional Treatment NOS vs No Treatment | 9 | 203532 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.89, 1.20] |
| 9.7 Ablative Treatment NOS vs No Treatment | 2 | 24848 | Risk Ratio (IV, Random, 95% CI) | 1.38 [0.42, 4.58] |
| 9.8 Treatment NOS vs No Treatment | 1 | 5548 | Risk Ratio (IV, Random, 95% CI) | 1.13 [1.00, 1.27] |
| 10 Instrumental Deliveries (ventouse/forceps) | 16 | 9588 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.88, 1.08] |
| 10.1 CKC vs No Treatment | 2 | 454 | Risk Ratio (IV, Random, 95% CI) | 1.33 [0.66, 2.70] |
| 10.2 LC vs No Treatment | 2 | 668 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.65, 2.07] |
| 10.3 LLETZ vs No Treatment | 6 | 1418 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.68, 1.17] |
| 10.4 LA vs No Treatment | 3 | 550 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.62, 1.41] |
| 10.5 Excisional Treatment NOS vs No Treatment | 3 | 950 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.46, 1.10] |
| 10.6 Treatment NOS vs No Treatment | 1 | 5548 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.89, 1.15] |
| 11 Precipitous Labour (<2hours) | 5 | 1059 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.80, 1.96] |
| 11.1 CKC vs No Treatment | 2 | 289 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.47, 3.27] |
| 11.2 LLETZ vs No Treatment | 4 | 770 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.76, 2.08] |
| 12 Prolonged labour (>12hours) | 7 | 1854 | Risk Ratio (IV, Random, 95% CI) | 1.25 [0.92, 1.69] |
| 12.1 CKC vs No Treatment | 2 | 325 | Risk Ratio (IV, Random, 95% CI) | 1.99 [0.89, 4.45] |
| 12.2 LC vs No Treatment | 1 | 500 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.41, 2.04] |
| 12.3 LLETZ vs No Treatment | 4 | 673 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.55, 1.70] |
| 12.4 LA vs No Treatment | 2 | 356 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.88, 2.26] |
| 13 Induction of Labour | 11 | 4668 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.89, 1.15] |
| 13.1 CKC vs No Treatment | 2 | 137 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.54, 2.29] |
| 13.2 LLETZ vs No treatment | 8 | 4056 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.82, 1.20] |
| 13.3 CT vs No Treatment | 1 | 58 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.22, 1.66] |
| 13.4 Excisional Treatment NOS vs No Treatment | 2 | 417 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.64, 1.28] |
| 14 Oxytocin Use | 6 | 2006 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.64, 1.26] |
| 14.1 CKC vs No Treatment | 1 | 103 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.59, 1.63] |
| 14.2 LLETZ vs No Treatment | 4 | 1804 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.43, 1.34] |
| 14.3 Excisional Treatment NOS vs No Treatment | 1 | 99 | Risk Ratio (IV, Random, 95% CI) | 1.18 [0.67, 2.05] |
| 15 Epidural Use | 5 | 105488 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.68, 1.53] |
| 15.1 LLETZ vs No Treatment | 4 | 818 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.64, 1.16] |
| 15.2 Excisional Treatment NOS vs No Treatment | 1 | 104670 | Risk Ratio (IV, Random, 95% CI) | 1.79 [1.29, 2.50] |
| 16 Pethidine Use | 2 | 394 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.72, 1.24] |
| 16.1 LLETZ vs No treatment | 2 | 394 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.72, 1.24] |
| 17 Analgesia Use NOS | 1 | 103 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.62, 1.98] |
| 17.1 CKC vs No Treatment | 1 | 103 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.62, 1.98] |
| 18 Cervical stenosis | 2 | 680 | Risk Ratio (IV, Random, 95% CI) | 2.26 [0.24, 21.59] |
| 18.1 LC vs No Treatment | 1 | 500 | Risk Ratio (IV, Random, 95% CI) | 3.0 [0.12, 73.29] |
| 18.2 CT vs No Treatment | 1 | 180 | Risk Ratio (IV, Random, 95% CI) | 1.71 [0.07, 41.31] |
| 19 Antepartum Haemorrhage | 4 | 1245 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.40, 3.12] |
| 19.1 CKC vs No Treatment | 1 | 34 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.26, 5.83] |
| 19.2 LC vs No Treatment | 1 | 168 | Risk Ratio (IV, Random, 95% CI) | 17.84 [0.98, 325.68] |
| 19.3 LLETZ vs No Treatment | 2 | 277 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.16, 1.67] |
| 19.4 LA vs No Treatment | 1 | 708 | Risk Ratio (IV, Random, 95% CI) | 8.00 [0.90, 71.18] |
| 19.5 CT vs No Treatment | 1 | 58 | Risk Ratio (IV, Random, 95% CI) | 0.41 [0.07, 2.25] |
| 20 Postpartum Haemorrhage (>600ml) | 1 | 149 | Risk Ratio (IV, Random, 95% CI) | 4.60 [1.38, 15.36] |
| 20.1 CKC vs No Treatment | 1 | 149 | Risk Ratio (IV, Random, 95% CI) | 4.60 [1.38, 15.36] |
| 21 Massive Obstetric Haemorrhage (>1000ml) | 1 | 149 | Risk Ratio (IV, Random, 95% CI) | 3.95 [0.45, 34.48] |
| 21.1 CKC vs No Treatment | 1 | 149 | Risk Ratio (IV, Random, 95% CI) | 3.95 [0.45, 34.48] |
| 22 Cervical cerclage | 8 | 141300 | Risk Ratio (IV, Random, 95% CI) | 14.29 [2.85, 71.65] |
| 22.1 CKC vs No Treatment | 3 | 30744 | Risk Ratio (IV, Random, 95% CI) | 31.42 [2.32, 426.22] |
| 22.2 LC vs No Treatment | 1 | 112 | Risk Ratio (IV, Random, 95% CI) | 6.68 [0.83, 53.69] |
| 22.3 LLETZ vs No Treatment | 1 | 56 | Risk Ratio (IV, Random, 95% CI) | 11.00 [0.64, 189.96] |
| 22.4 Excisional Treatment NOS vs No Treatment | 2 | 104840 | Risk Ratio (IV, Random, 95% CI) | 42.45 [28.99, 62.16] |
| 22.5 Treatment NOS vs No Treatment | 1 | 5548 | Risk Ratio (IV, Random, 95% CI) | 2.16 [1.24, 3.76] |
Comparison 3. Neonatal Outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 LBW (<2500g) | 30 | 1.348206E6 | Risk Ratio (IV, Random, 95% CI) | 1.81 [1.58, 2.07] |
| 1.1 CKC vs No Treatment | 5 | 30304 | Risk Ratio (IV, Random, 95% CI) | 2.51 [1.78, 3.53] |
| 1.2 LC vs No Treatment | 4 | 786 | Risk Ratio (IV, Random, 95% CI) | 1.76 [0.72, 4.35] |
| 1.3 LLETZ vs No Treatment | 12 | 3357 | Risk Ratio (IV, Random, 95% CI) | 2.11 [1.51, 2.94] |
| 1.4 LA vs No Treatment | 4 | 1104 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.59, 1.92] |
| 1.5 CT vs No Treatment | 1 | 58 | Risk Ratio (IV, Random, 95% CI) | 3.67 [0.47, 28.47] |
| 1.6 Excisional Treatment NOS vs No Treatment | 10 | 823648 | Risk Ratio (IV, Random, 95% CI) | 2.01 [1.62, 2.49] |
| 1.7 Ablative Treatment NOS vs No Treatment | 4 | 483402 | Risk Ratio (IV, Random, 95% CI) | 1.36 [1.19, 1.55] |
| 1.8 Treatment NOS vs No Treatment | 1 | 5547 | Risk Ratio (IV, Random, 95% CI) | 1.35 [1.14, 1.60] |
| 2 LBW (<2000g) | 3 | 74981 | Risk Ratio (IV, Random, 95% CI) | 2.49 [0.97, 6.36] |
| 2.1 LC vs No Treatment | 1 | 181 | Risk Ratio (IV, Random, 95% CI) | 4.46 [1.36, 14.59] |
| 2.2 LA vs No Treatment | 2 | 772 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.39, 2.29] |
| 2.3 Excisional Treatment NOS vs No Treatment | 1 | 74028 | Risk Ratio (IV, Random, 95% CI) | 4.60 [3.32, 6.37] |
| 3 LBW (<1500g) | 5 | 76836 | Risk Ratio (IV, Random, 95% CI) | 3.00 [1.54, 5.85] |
| 3.1 LC vs No Treatment | 1 | 181 | Risk Ratio (IV, Random, 95% CI) | 12.75 [1.53, 106.44] |
| 3.2 LLETZ vs No Treatment | 1 | 378 | Risk Ratio (IV, Random, 95% CI) | 7.0 [0.36, 134.59] |
| 3.3 LA vs No Treatment | 2 | 772 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.16, 2.80] |
| 3.4 Excisional Treatment NOS vs No Treatment | 2 | 75505 | Risk Ratio (IV, Random, 95% CI) | 3.34 [2.02, 5.54] |
| 4 LBW (<1000g) | 2 | 2185 | Risk Ratio (IV, Random, 95% CI) | 2.09 [0.06, 74.71] |
| 4.1 LA vs No Treatment | 1 | 708 | Risk Ratio (IV, Random, 95% CI) | 0.29 [0.01, 5.50] |
| 4.2 Excisional Treatment NOS vs No Treatment | 1 | 1477 | Risk Ratio (IV, Random, 95% CI) | 11.10 [1.44, 85.79] |
| 5 NICU Admission | 8 | 2557 | Risk Ratio (IV, Random, 95% CI) | 1.45 [1.16, 1.81] |
| 5.1 CKC vs No Treatment | 2 | 71 | Risk Ratio (IV, Random, 95% CI) | 1.40 [0.52, 3.75] |
| 5.2 LLETZ vs No Treatment | 5 | 1994 | Risk Ratio (IV, Random, 95% CI) | 1.42 [1.01, 1.99] |
| 5.3 CT vs No Treatment | 1 | 58 | Risk Ratio (IV, Random, 95% CI) | 2.44 [0.29, 20.49] |
| 5.4 Excisional Treatment NOS vs No Treatment | 2 | 434 | Risk Ratio (IV, Random, 95% CI) | 1.76 [1.13, 2.75] |
| 6 Perinatal Mortality | 23 | 1.659433E6 | Risk Ratio (IV, Random, 95% CI) | 1.51 [1.13, 2.03] |
| 6.1 CKC vs No Treatment | 7 | 50588 | Risk Ratio (IV, Random, 95% CI) | 1.46 [0.83, 2.57] |
| 6.2 LC vs No Treatment | 3 | 906 | Risk Ratio (IV, Random, 95% CI) | 1.89 [0.26, 13.87] |
| 6.3 NETZ vs No Treatment | 1 | 7399 | Risk Ratio (IV, Random, 95% CI) | 9.99 [3.13, 31.92] |
| 6.4 LLETZ vs No Treatment | 7 | 302271 | Risk Ratio (IV, Random, 95% CI) | 1.53 [0.88, 2.67] |
| 6.5 LA vs No Treatment | 2 | 258 | Risk Ratio (IV, Random, 95% CI) | 3.0 [0.12, 72.74] |
| 6.6 CT vs No Treatment | 2 | 238 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.01, 4.59] |
| 6.7 Excisional Treatment NOS vs No Treatment | 5 | 820028 | Risk Ratio (IV, Random, 95% CI) | 1.85 [1.02, 3.36] |
| 6.8 Ablative Treatment NOS vs No Treatment | 2 | 472197 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.42, 1.13] |
| 6.9 Treatment NOS vs No Treatment | 1 | 5548 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.63, 1.58] |
| 7 Perinatal Mortality (<37w) | 1 | 73992 | Risk Ratio (IV, Random, 95% CI) | 9.40 [2.01, 43.89] |
| 7.1 CKC vs No Treatment | 1 | 6956 | Risk Ratio (IV, Random, 95% CI) | 5.33 [0.31, 90.71] |
| 7.2 NETZ vs No Treatment | 1 | 7399 | Risk Ratio (IV, Random, 95% CI) | 30.96 [8.71, 110.13] |
| 7.3 LLETZ vs No Treatment | 1 | 59637 | Risk Ratio (IV, Random, 95% CI) | 3.92 [1.24, 12.38] |
| 8 Perinatal Mortality (<32w) | 1 | 73992 | Risk Ratio (IV, Random, 95% CI) | 12.81 [2.70, 60.87] |
| 8.1 CKC vs No Treatment | 1 | 6956 | Risk Ratio (IV, Random, 95% CI) | 6.75 [0.39, 117.10] |
| 8.2 NETZ vs No Treatment | 1 | 7399 | Risk Ratio (IV, Random, 95% CI) | 44.23 [11.67, 167.61] |
| 8.3 LLETZ vs No Treatment | 1 | 59637 | Risk Ratio (IV, Random, 95% CI) | 5.43 [1.71, 17.30] |
| 9 Perinatal Mortality (<28w) | 1 | 73992 | Risk Ratio (IV, Random, 95% CI) | 13.76 [2.37, 79.89] |
| 9.1 CKC vs No Treatment | 1 | 6956 | Risk Ratio (IV, Random, 95% CI) | 9.21 [0.51, 164.95] |
| 9.2 NETZ vs No Treatment | 1 | 7399 | Risk Ratio (IV, Random, 95% CI) | 51.61 [13.17, 202.29] |
| 9.3 LLETZ vs No Treatment | 1 | 59637 | Risk Ratio (IV, Random, 95% CI) | 4.49 [1.09, 18.45] |
| 10 Stillbirth | 12 | 249855 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.63, 1.52] |
| 10.1 CKC vs No Treatment | 3 | 935 | Risk Ratio (IV, Random, 95% CI) | 1.61 [0.48, 5.40] |
| 10.2 LC vs No Treatment | 2 | 725 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.03, 3.18] |
| 10.3 LLETZ vs No Treatment | 4 | 242473 | Risk Ratio (IV, Random, 95% CI) | 1.42 [0.62, 3.26] |
| 10.4 LA vs No Treatment | 1 | 64 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Excisional Treatment NOS vs No Treatment | 1 | 110 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.6 Treatment NOS vs No Treatment | 1 | 5548 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.42, 1.40] |
| 11 Apgar score (≤5)(1min) | 1 | 225 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.12, 2.68] |
| 11.1 LC vs No Treatment | 1 | 225 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.12, 2.68] |
| 12 Apgar score (<7)(1min) | 1 | 152 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.07, 5.71] |
| 12.1 LLETZ vs No Treatment | 1 | 87 | Risk Ratio (IV, Random, 95% CI) | 0.16 [0.01, 3.30] |
| 12.2 CKC vs No Treatment | 1 | 65 | Risk Ratio (IV, Random, 95% CI) | 1.61 [0.15, 16.90] |
| 13 Apgar score (<7)(5min) | 2 | 297 | Risk Ratio (IV, Random, 95% CI) | 0.82 [0.19, 3.59] |
| 13.1 CKC vs No Treatment | 1 | 32 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 LLETZ vs No Treatment | 1 | 120 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.16, 5.37] |
| 13.3 CT vs No Treatment | 1 | 58 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.04, 9.28] |
| 13.4 Excisional Treatment NOS vs No Treatment | 1 | 87 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Acharya 2005.
| Methods | Retrospective cohort study Comparison group: A) External ‐ matching for age (+/‐ 3 years), parity, date of delivery, smoking (+/‐ 5 cigarettes per day) and previous obstetric history B) Internal (pre‐treatment pregnancies) Information source ‐ Hospital records of the University Hospital of Northern Norway |
|
| Participants | A) Treated group: 79 women < 45 years who had a LLETZ (December 1995 to December 2000) and subsequently delivered (> 20 weeks) at the University Hospital of Northern Norway. Inclusion criteria: only first pregnancies (> 20 weeks) following LLETZ Exclusion criteria: Women with ectopic pregnancies, miscarriages, TOPs. Untreated group: 158 matched women who were identified using routinely entered data from the birth register. B) Of the 79 women of the treated group, 45 were parous. The last pregnancy before LLETZ of these 45 women can serve as an internal comparison group. |
|
| Interventions | LLETZ | |
| Outcomes | PTB (< 37 weeks); threatened PTL; chorioamnionitis; induction of labour; LBW (< 2500 g); perinatal mortality | |
| Notes | Because all women included in this study have been also included in Albrechtsen 2008, we excluded it from the analyses in which Albrechtsen 2008 has been also included. A total of 428 women < 45 years had LLETZ performed during the study period and 89 of them had a pregnancy after the procedure. Ten women were excluded (three ectopic pregnancies, two TOPs and five miscarriages) from the study. Data from 79 women whose pregnancies progressed > 20 weeks and 158 matched controls were analysed. The histological diagnosis was normal in 3 (3.8%), CIN1 in 5 (6.3%), CIN2 in 18 (22.8%), and CIN3 in 53 (67.1%) of cases. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women having LLETZ and subsequently delivering in a single university hospital between December 1995 to December 2000 |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for date of delivery, age, parity, previous obstetric history and smoking habit |
Albrechtsen 2008.
| Methods | Retrospective cohort study Comparison groups: A) External B) Internal (pre‐treatment pregnancies) Both had regression analysis for age and birth order Information source ‐ Cancer Registry of Norway, Medical Birth Registry of Norway, Central Population Registry, Cause of Death Registry |
|
| Participants | Treated group: all pregnancies proceeding beyond 24 weeks of gestation (n = 14,882) of all women in Norway who delivered during 1967 to 2003 after cervical conisation (CKC, LLETZ, LC) Untreated group: A) all pregnancies proceeding beyond 24 weeks of gestation (n = 2,155,505) of all women in Norway who delivered during 1967 to 2003 without previous cervical conisation B) all pregnancies proceeding beyond 24 weeks of gestation (n = 56,927) of all women in Norway who delivered during 1967 to 2003 before cervical conisation (CKC, LLETZ, LC) Exclusion criteria: women ≥ 45years at the time of cervical conization; women who had their CIN diagnosis during 1980 to 1985 because it is not known if they had a treatment (these women were included in the untreated group) |
|
| Interventions | Excisional NOS (CKC, LC, LLETZ) | |
| Outcomes | PTB (< 37 weeks); PTB (< 33 weeks); PTB (< 28 weeks) | |
| Notes | Since 1953, the cancer registry has collected information on all cancer diagnoses as well as premalignant lesions, including intraepithelial neoplasia with staging.
The compulsory reporting system is based on clinical, pathology, and cytology reports. During 1953 to 1979 and from 1986 onwards, treatment of intraepithelial neoplasia with cervical conisation has also been notified, though without specification of surgical method. During 1980 to 1985, only data on histological diagnoses—that is, the grade of intraepithelial neoplasia— were notified and the researchers excluded these women from the exposed group and included them in the not treated group. 226 pregnancies after treatment were late miscarriages (< 24 weeks). 209 pregnancies before treatment were late miscarriages. 8501 pregnancies of the untreated group were late miscarriages. In this meta‐analysis, these pregnancies were subtracted from the total number. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from national registries; Cancer registry: includes all cancer diagnoses as well as premalignant lesions plus their treatment (during 1980 to 1985 did not include treatment); Birth registry: the proportion of women with missing data on gestational age amounted to 5.3%, while data on birth weight were almost complete. |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Unclear risk | During 1980 to 1985 the Cancer registry included only the grade of CIN and did not include the treatment. The researchers excluded those women from the treated group and included them in the untreated group, even though they might have had treatment before or after pregnancy. Because the population of this study is big enough, it is not estimated that this probable misclassification has affected the results of the study to a significant extent. |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women in Norway who delivered during 1967 to 2003, before or after cervical conisation (a population‐based study) |
| Representative comparison group? | Low risk | A) The untreated external comparison group was drawn from the same source as the treated group B) Internal matching (self‐matching) |
| Comparability of treatment groups? | Low risk | A) Regression analysis for age (at delivery or treatment) and birth order B) Internal matching (self‐matching) |
Andersen 1999.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age and parity Information source ‐ Hospital records of the Aalborg Hospital |
|
| Participants | Treated group ‐ 75 pregnancies (< 27 weeks) of 62 women who had undergone LA before the pregnancy at the Aalborg Hospital (LA during 1985 to 1989) Exclusion: 6 patients with TOP, 3 with miscarriage and 1 with ectopic pregnancy Untreated group ‐ 150 pregnancies of women without previous treatment (the next two women entering the delivery ward who were matched by age and parity) |
|
| Interventions | LC | |
| Outcomes | PTB (≤ 37 weeks); PTB (≤ 37 weeks) (D < 15 mm); PTB (≤ 37 weeks) (D = 15 mm to 20 mm); PTB (≤ 37 weeks) (D > 20 mm); pPROM; CS; perinatal mortality; stillbirth; Apgar score (≤ 5) (1 min); | |
| Notes | From 1985 to 1989, combination LC was performed in 536 patients. After LC, 72 patients became pregnant. After the exclusion of the 10 ineligible women, the remaining 62 patients had 75 pregnancies (> 27 weeks) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All women who had had LC in a single hospital (1985 to 1989) and subsequently had a pregnancy (> 27 weeks) |
| Representative comparison group? | Low risk | The control group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Unclear risk | Matching for age and parity. No matching for smoking, although the intervention group had substantially higher rate of smoking (62.7% vs 27.3%). |
Anderson 1984.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age, race, births and miscarriages/TOP Information source ‐ hospital records (for the ascertainment of the exposure) and postal questionnaires (for the ascertainment of the outcome); additional information from obstetricians who delivered other women |
|
| Participants | Treated group ‐ 68 deliveries of women who had been treated by LA as their initial treatment for CIN at the Samaritan Hospital for women, London, between December 1978 and February 1984, and subsequently had a pregnancy. Women who were treated in the previous 3 months were excluded. Untreated group ‐ 70 deliveries of women without previous treatment who delivered at the St Mary's Hospital, London |
|
| Interventions | LA | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (single cone); CS; instrumental deliveries (forceps); prolonged labour (> 12 hours); LBW (< 2500 g) | |
| Notes | 1013 patients were treated by LA as their initial therapy for CIN at the Samaritan Hospital for Women, London, between December 1978 and February 1984, and were followed up for at least 10 months thereafter. A questionnaire was sent to all the women, apart from those treated in the previous 3 months, asking for information about pregnancies before and after LA. About 25% of the questionnaires were returned by the Post Office as the women had moved away and could not be traced. This proportion is not surprising in a mobile, urban population. Additional information was obtained from obstetricians who delivered other women. In total, the researched found 118 pregnancies in 110 patients. Of these pregnancies, 68 ended to delivery and were included in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25% of the women did not reply to the postal questionnaire because they had moved away and could not be traced. There is a high risk of attrition bias, although this proportion is not surprising in a mobile, urban area. |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | High risk | Recall bias because postal questionnaires were used for the ascertainment of the outcome |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | High risk | 25% of the women did not reply to the questionnaire, because they had moved away. The women who replied are more likely to belong to a higher socioeconomic class. |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for age, race, births and miscarriages/TOP |
Andia 2011.
| Methods | Retrospective cohort study Comparison group: A) External B) Internal (pre‐treatment pregnancies) Both had regression analysis for age, parity and smoking Information source ‐ Databases of the Cervical Pathology Units of the 5 main hospitals of the Basque Country participating in this study (Basurto, Cruces, Donostia, Galdakao, and Txagorritxu); Basque Country Health Service databases |
|
| Participants | Treated group ‐ 189 women who had undergone LLETZ during 1988 to 2007 at the 5 main hospitals of the Basque Country (Basurto, Cruces, Donostia, Galdakao, and Txagorritxu) and subsequently delivered Untreated group ‐ A) 189 women who delivered during 1988 to 2007 without previous treatment and were identified from the Basque Country Health Service databases B) Internal population of women that had pregnancies before LLETZ (n = 189) Inclusion criteria (for both groups): only singletons were included |
|
| Interventions | LLETZ | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (nulliparous); PTB (< 37 weeks) (parous); PTB (< 37 weeks) (singleton pregnancies); PTB (< 35 weeks); PTB (< 32 weeks); CS; LBW (< 2500 g); LBW (1500 g) | |
| Notes | Adjusting for maternal age, parity, and maternal smoking did not affect the results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | A stratified random sampling of the women having LLETZ during 1988 to 2007 at the five main hospitals of the Basque Country and subsequently delivering |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Regression analysis for age, parity, smoking |
Anwar 2016.
| Methods | Retrospective cohort study Comparison group: Internal (self‐matching) Information source ‐ Colposcopy Electronic Data Base (CYRIS) for identification of the treated women & Trust Electronic Pathology Data (WebV) for details of the cone biopsy treatment; Hospital Episode Statistics and electronic maternity data (CMIS) for identification of the women who subsequently achieved pregnancy & obstetric case notes by the local audit department for pregnancy details |
|
| Participants | Treated group ‐ 15 women (23 pregnancies) who underwent electrosurgical cone biopsy using FCBE electrode at Diana Princess of Wales Hospital Grimsby between January 2000 and December 2011 and subsequently delivered at the same hospital before March 2013 Untreated group ‐ The 48 pregnancies of these 15 women before treatment |
|
| Interventions | FCBE (Fischer Cone Biopsy Excisor) | |
| Outcomes | PTB (< 37 weeks) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Small number of included treated women; there is possible bias due to unknown loss to follow‐up of women who delivered at units other than the host institution |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women undergoing treatment and delivering in a single hospital |
| Representative comparison group? | Low risk | Internal comparison group (self‐matching) |
| Comparability of treatment groups? | Low risk | Internal comparison group (self‐matching) |
Armarnik 2011.
| Methods | Retrospective cohort study Comparison group: External ‐ unmatched; regression analysis for age, birth order, year of delivery, smoking and cervical incompetence with cerclage Information source ‐ medical records in combination with a computerised perinatal database (Soroka University Medical Center, Israel) |
|
| Participants | Treated group ‐ 53 deliveries of women who had undergone conisation and then delivered at the Soroka University Medical Centre Untreated group ‐ 104,617 deliveries of women who delivered at the Soroka University Medical Centre without previous conisation Exclusion criteria: multiple gestations; patients lacking prenatal care |
|
| Interventions | Excision NOS (CKC, LC, LLETZ, other) | |
| Outcomes | PTB (< 34 weeks); CS; epidural use; cervical cerclage; perinatal mortality | |
| Notes | Using the delivery record database, 53 deliveries after conisation were found. Using the medical records, 57 deliveries after conisation were found. The discrepancy between these two databases is because the delivery record database had a recording gap. LLETZ was the most common treatment (LLETZ: 18; CKC: 7; LC: 2; other: 14; >1 conisation: 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/57 women (7%) were not included in the analysis, because of a recording gap in the delivery record database |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Unclear risk | Table 1 in page 767: When we add the women according to the conisation type, the sum is 42. When we add the women according to the histology of cervical biopsy or the smoking status during pregnancy, the sum is 40. There is a difference of two women. |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women delivering in a single hospital |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Regression analysis for age, birth order, year of delivery, smoking and cervical incompetence with cerclage |
Bekassy 1996.
| Methods | Retrospective cohort study Comparison group: A) External ‐ matching for age, parity and time of delivery B) Internal (self‐matching) Information source ‐ Anaesthetic records of University Hospital of Lund, Sweden, and National Medical Birth Registry at the National Board of Health and Welfare, Stockholm |
|
| Participants | A) Treated group ‐ 250 women who had undergone LC at University Hospital of Lund, Sweden, and had a subsequent delivery, between January 1980 and June 1988 Untreated group ‐ 250 women selected from the National Medical Birth Registry B) Of the 250 women of the treated group, 148 were parous. For these women, self‐matching was also possible. |
|
| Interventions | LC ('laser miniconisation') | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (nulliparous); PTB (< 37 weeks) (parous); PTB (< 37 weeks) (single cone); PTB (< 37 weeks) (repeat cones); CS; instrumental deliveries (ventouse/forceps); prolonged labour (> 12 hours); cervical stenosis; LBW (< 2500 g); perinatal mortality; stillbirth | |
| Notes | From January 1980 to June 1988, 1485 women between age 16 to 58 were treated by carbon dioxide laser miniconisation because of CIN at University Hospital of Lund. These women were identified retrospectively via certain operation code numbers in the Anaesthetic hospital records. Each woman had also a specific 10‐tailed patient identification number (PIN), which is also used by the National Medical Birth Registry to register births in Sweden. The information of these 1485 women was transferred to a magnetic tape which was then run against data held at the National Medical Birth registry and 250 women having a delivery after treatment (3 had twin pregnancies) were identified. Of these women, 245 delivered at the Department of Obstetrics and Gynaecology, University Hospital of Lund, and the other 45 at 21 different hospitals around Sweden. 20 women had LC twice before pregnancy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from hospital records and national registries |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible women having LC at the University Hospital of Lund between January 1980 to June 1988 |
| Representative comparison group? | Unclear risk | A) The untreated group was not drawn from the same source as the treated group B) Internal comparison group (self‐matching) |
| Comparability of treatment groups? | Low risk | A) Matching for age, parity and time of delivery B) Internal comparison group (self‐matching) |
Blomfield 1993.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age, parity and ethnic group Information source ‐ Computer database of Dudley Road hospital |
|
| Participants | Treated group ‐ 40 women who had undergone LLETZ and were subsequently delivered at Dudley Road Hospital, between January 1989 and January 1992 Untreated group ‐ 80 women without previous treatment delivering immediately before and after the cases at Dudley Road Hospital |
|
| Interventions | LLETZ | |
| Outcomes | PTB (< 37 weeks); sPTB (< 37 weeks); CS; instrumental deliveries (ventouse/forceps); induction of labour; oxytocin use; epidural use; LBW (< 2500 g); NICU admission; perinatal mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from computerised hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible women that had LLETZ at Dudley Road Hospital between January 1982 to January 1992; low risk. However, more than 60% of the women delivering at Dudley Road Hospital are nonwhite |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for age, parity and ethnic group |
Braet 1994.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age (+/‐ 5 years), parity and smoking Information source ‐ Hospital records of Rotherham District General Hospital |
|
| Participants | Treated group ‐ 78 women who had undergone LLETZ in Rotherham District General Hospital between 1 December 1998 and 15 October 1992 and had a viable pregnancy afterwards. Only the first pregnancy after treatment was included. Only singleton pregnancies were included. Untreated group ‐ 78 women who were the next following patients delivered in the same hospital. |
|
| Interventions | LLETZ | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (singleton pregnancies); pPROM; CS; instrumental deliveries (ventouse/forceps); APH; LBW (< 2 500 g); perinatal mortality | |
| Notes | Between 1 December 1988 and 15 October 1992, a total of 1000 women had LLETZ in Rotherham District General Hospital. It was possible to identify 84 viable pregnancies in patients who had undergone the procedure before conception. Of the 84 pregnancies, 5 were second pregnancies after LLETZ and one was a twin pregnancy. The other 78 women were finally included in the treated group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women that had LLETZ in a hospital between 1988 to 1992 |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Patients from the same unit matched for age (+/‐ 5 years), parity and smoking |
Bruinsma 2007.
| Methods | Retrospective cohort study Comparison groups: A) Women with colposcopy before pregnancy, but no treatment B) Women with colposcopy during pregnancy, but no treatment Both had regression analysis for age, illicit drug use during pregnancy, delivery at the RWH, marital status, maternal medical condition, previous TOP, previous miscarriage, previous PTB, previous treatment Information source ‐ Records of the Cervical Dysplasia Clinic of the Royal Women's Hospital (RWH) (for the ascertainment of the exposure); Victorian Perinatal Data Collection Unit (PDCU) (for the ascertainment of the outcomes) |
|
| Participants | Treated group ‐ 1951 women who were referred to the Royal Women's Hospital (RWH) during 1982 to 2000, received treatment for CIN and thereafter had a pregnancy in the state of Victoria during 1983 to 2002. Women with hysterectomy or treatment during pregnancy were excluded. Untreated group ‐ 3597 women who were referred to the RWH during 1982 to 2000 and then delivered in the state of Victoria during 1983 to 2002 without receiving treatment (referral during the index pregnancy:1303; referral before the index pregnancy:2294) Inclusion criteria (for both groups): referral to the RWH either for assessment of an abnormality detected on a routine Pap smear or for evaluation of a cervix that appeared abnormal; only the first pregnancy after the referral/treatment Exclusion criteria (for both groups): missing date of birth; multiple pregnancies; referral to the RWH for assessment of a non‐cervical lesion; women recorded in their clinic record as having no previous children and were 45 years or older at time of initial visit or who indicated that they had children but were older than 40 years at initial visit in 1982 or 41 years in 1983, etc. |
|
| Interventions | CKC, LLETZ, LA, RD | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (singleton pregnancies); PTB (< 32 weeks); PTB (< 28 weeks); sPTB; pPROM; CS; instrumental deliveries (ventouse/forceps); LBW (< 2500 g); perinatal mortality; stillbirth | |
| Notes | Since 1982, the Victorian Perinatal Data Collection Unit (PDCU) has collected data on all births in the state of Victoria greater than or equal to 20 weeks of gestation or 400 g. All women were followed up for at least 2 years (range 2 to 20 years), the median follow‐up time being 9 years for treated women and 10 years for untreated women. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from hospital records |
| Selective reporting (reporting bias) | High risk | All outcomes, except for PTB (< 37 weeks), are presented only for the whole treated group and not separately according to the type of treatment |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women undergoing treatment for CIN during 1982 to 2000 in the largest treatment centre in Victoria |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Regression analysis (for the outcome of PTB) for age, illicit drug use during pregnancy, delivery at the RWH, marital status, maternal medical condition, previous TOP, previous miscarriage, previous PTB, previous treatment |
Buller 1982.
| Methods | Retrospective cohort study Comparison group ‐ Internal (pre‐treatment pregnancies) Information source ‐ Hospital records of University of California Hospital, San Fransisco, and Kaiser Hospital, Honolulu |
|
| Participants | Treated group ‐ 47 deliveries of women who had undergone diagnostic and/or therapeutic conization of the cervix at either the University of California Hospital, California, or Kaiser Hospital, Honolulu, between 1968 and 1978, and had a subsequent delivery. Inclusion criteria: Women of reproductive age (arbitrarily defined as age 39 or less) at the time of surgery. Exclusion criteria: Women with a hysterectomy or a sterilisation procedure and women who were lost to follow‐up. Untreated group ‐ 79 deliveries of the women of the treated group who had also a delivery before the treatment |
|
| Interventions | CKC | |
| Outcomes | PTB (< 37 weeks); Threatened PTL; CS | |
| Notes | 503 underwent diagnostic and/or therapeutic conization of the cervix at either the University of California Hospital, San Fransisco, or Kaiser Hospital, Honolulu, between 1968 and 1978. Of these, 314 were of reproductive age, arbitrarily defined as age 39 or less, at the time of surgery. A hysterectomy or a sterilisation procedure was subsequently performed on 87 of these 314 patients. An additional 61 patients were lost to follow‐up within 12 months of the conisation. Of the remaining 166 patients, 61 patients achieved 88 pregnancies and the other 105 patients did not become pregnancies after conisation. Of the 88 pregnancies, 47 led to a labour. The same women before the treatment had 106 pregnancies (79 led to a labour). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 227 women were eligible for the study. Of these, 61 (26.9%) were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible women that had CKC in two hospitals between 1968 to1978 |
| Representative comparison group? | Low risk | Internal comparison group (pre‐treatment pregnancies) |
| Comparability of treatment groups? | Low risk | Internal comparison group (pre‐treatment pregnancies) |
Castanon 2012.
| Methods |
Castanon 2012 (this is the main study): Retrospective cohort study Comparison groups ‐ A) External (general population) B) Women with punch biopsy but no treatment C) Internal (pre‐treatment pregnancies) D) Internal (self‐matching: same women before and after treatment) Regression analysis for age, parity and study site for some comparison groups, but not for the ones that we used in our meta‐analysis (see "Notes") Information source ‐ Records of the 12 participating NHS hospitals (for the ascertainment of the exposure); hospital episode statistics of inpatient obstetric records for the whole of England (for the ascertainment of the outcomes) Castanon 2014: Case‐control study nested in a retrospective cohort study Matching for age, parity, study site and whether the birth occurred before or after the first colposcopy; regression analysis for age, parity, study site and index of multiple deprivation |
|
| Participants |
Castanon 2012: Study period for the treatment/biopsy: January 1987 to December 2009 Study period for the delivery: April 1998 to April 2010 Location for the treatment/biopsy: one of the 12 participating NHS hospitals Location for the delivery: any NHS hospital Treated group ‐ 4776 deliveries of women who had undergone excisional treatment and subsequently had a pregnancy Untreated group ‐ A) 510,660 deliveries (general population of England) B) 7263 deliveries of women who had undergone punch biopsy but no treatment and subsequently had a pregnancy C) The deliveries (1173) of the treated group before their treatment D) For 372 women who had at least one delivery both before and after the delivery, internal matching (self‐matching) was also possible: the first delivery after treatment was compared with the last delivery before treatment Exclusion criteria (for all groups): Pregnancies with no gestational age recorded, with gestational age > 43 weeks, with gestational age < 20 weeks or with no year of birth recorded as well as multiple pregnancies. Additional exclusion criteria (for the women with treatment or punch biopsy): Women for whom the date of histology was unknown Castanon 2014: Study period for the treatment/biopsy: April 1988 to December 2011 Study period for the delivery: April 1998 to March 2011 Location for the treatment/biopsy: one of the 12 participating NHS hospitals Location for the delivery: any NHS hospital Cases ‐ 768 women with a preterm birth after excisional treatment or punch biopsy. Only the earliest occurring singleton preterm birth (with any parity) in each woman was included Controls ‐ 830 matched women with a term birth after excisional treatment or punch biopsy Inclusion criteria: births at 37 weeks' gestational age, women with incomplete colposcopy records, women for whom the only pathology sample reported was non‐cervical, women who were recorded as being sterilised while pregnant, women with a diagnosis of cervical cancer at any time, women whose pregnancy was at high risk (diabetes mellitus, hypertension, placenta praevia with haemorrhage, supervision of high risk pregnancy, mental disorders, and diseases of the nervous system complicating pregnancy, childbirth, and the puerperium) |
|
| Interventions | Excision NOS (CKC, LC, LLETZ, other) | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (D < 10 mm); PTB (< 37 weeks) (D ≥ 10 mm); PTB (< 37 weeks) (singleton pregnancies); PTB (< 33 weeks) | |
| Notes |
Castanon 2012: In addition to the treated/comparison groups that we used in our meta‐analysis, the authors had a variant of those as well: only the first birth recorded in the dataset for each woman during the study period, after exclusion of antepartum stillbirths and stillbirths of indeterminate timing. For these groups there was regression analysis for age, parity and study site, but no regression analysis for the groups that we selected. However, we selected the latter, because the population is bigger and we had no reason to restrict to the first pregnancy of each woman during the study period. Castanon 2014: this was a case‐control study nested in this retrospective cohort study. The cases were 768 preterm births and the controls were 830 term births, all occurring after excisional treatment or punch biopsy. The main outcome of this study was the depth of the cone in the cases and controls stratified in the following categories: 1 mm to ‐9 mm, 10 mm to 14 mm, 15 mm to 19 mm, ≥ 20mm. The cases and the controls, as a case‐control study, were different from the cases and the controls of all the other studies. We have contacted the investigators of the study and also used the published data to extract the PTB (< 37 weeks) rate for women with excision of < 10 mm in depth and ≥10 mm in a treated group versus women who had punch biopsy but no treatment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the total number of pregnancies (n = 26897) of the women with a pregnancy before or after treatment/punch biopsy, 8050 pregnancies (29.9%) had an unknown gestational age |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Unclear risk | The authors do not have information as to whether the punch biopsy group may have had a history of ablative treatment or whether the treatment group had antenatal interventions when pregnant. There is no evidence of the efficacy of these interventions so it is unclear whether this is a source of bias. |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | Women having treatment at one of the 12 participating NHS hospitals (representation from the whole England) and then delivering at any NHS hospital |
| Representative comparison group? | Low risk | A/B) The untreated group was drawn from the same source as the treated group C/D) Internal controls |
| Comparability of treatment groups? | Low risk | A) General population of the whole England B) Women with punch biopsy (it is considered that possible confounding factors are not different between this group and the treated group and thus, this is one of the best comparison groups, in general) C/D) Internal controls No regression analysis for the comparison groups that we used (see "Notes" above) |
Crane 2006.
| Methods | Retrospective cohort study Comparison groups: A) Untreated women without a history of sPTB (low risk); this is the control group we used for out meta‐analysis B) Untreated women with a history of sPTB (high risk) Both had regressional analysis for maternal age, gestational age at the time of transvaginal ultrasonography, parity, smoking, antepartum bleeding after 20 weeks of gestation and previous sPTB Information source ‐ Hospital records of the Women's Health Centre of the Health Care Corporation of St. John's |
|
| Participants | Treated group ‐ 132 (LLETZ = 75, CKC = 21, CT = 36) pregnant women with singleton gestations from June 2001 to June 2004 at the Women's Health Centre of the Health Care Corporation of St. John's who previously had LLETZ , CKC or cryotherapy Untreated group ‐ A) 81 women without history of sPTB or treatment for cervical dysplasia (low‐risk control group) B) 63 women with a history of sPTB not having had treatment for cervical dysplasia |
|
| Interventions | CKC; LLETZ; CT | |
| Outcomes | sPTB (< 37 weeks); sPTB (< 37 weeks) (singleton pregnancies); sPTB (< 34 weeks); CS; induction of labour; APH; LBW (< 2500 g); NICU admission; perinatal mortality; Apgar score (< 7) (5min) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women delivering in a single hospital during June 2001 to June 2004 |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Regressional analysis for maternal age, gestational age at the time of transvaginal ultrasonography, parity, smoking, antepartum bleeding after 20 weeks of gestation, and sPTB |
Cruickshank 1995.
| Methods | Retrespective cohort study Comparison group: A) External ‐ matching for age, parity, husband's or partner's social class, height and daily cigarette consumption B) Internal (pre‐treatment pregnancies) Information source ‐ Aberdeen Maternity and Neonatal Databank, postal questionnaires |
|
| Participants | A) Treated group ‐ 149 women who had undergone LLETZ between 1989 and 1991. Only the first singleton pregnancies following treatment that progressed to 20 weeks of gestation were included. Multiple pregnancies were excluded. We also excluded 2 miscarriages, giving a total of 147 women. Untreated group ‐ 298 women without previous treatment (two controls for each case). We excluded 3 miscarriages, giving a total of 295 women B) The 147 deliveries of the treated group after LLETZ were compared with the 133 deliveries of the treated group before LLETZ |
|
| Interventions | LLETZ | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (singleton pregnancies); PTB (< 28 weeks); CS; precipitous labour (< 2 hours); stillbirth | |
| Notes | 1000 women who had undergone LLETZ between 1989 and 1991 were identified via Aberdeen Maternity and Neonatal Databank. A postal questionnaire was sent to these women in 1993 and 653 replied. Of these, 149 had a singleton pregnancy after treatment and were included in the treated group. The control group was also pooled from Aberdeen Maternity and Neonatal Databank. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Postal questionnaires were used for the selection of the treated group and many women did not reply (347/1000 = 34.7%) |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | For the treated group, questionnaires were used for the ascertainment of the outcome; there is a risk of recall bias and misclassification of the outcome |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | High risk | Postal questionnaires were used for the selection of the treated group and many women did not reply (34.7%). The women who replied are more likely to have a higher educational level. |
| Representative comparison group? | Low risk | A) The untreated group was drawn from the same source as the treated group B) Internal comparison group (pre‐treatment pregnancies) |
| Comparability of treatment groups? | Low risk | A) Matching for age, parity, husband's or partner's social class, height and daily cigarette consumption B) Internal comparison group (pre‐treatment pregnancies) |
Ehsanipoor 2014.
| Methods | Retrospective cohort study Comparison group: External ‐ unmatched; regression analysis for age, parity, race, history of PTB, history of tobacco use, history of drug use and chorionicity Information source ‐ maternal prenatal records and impatient hospital charts from two community hospitals in California |
|
| Participants | Treated group ‐ 110 women who had a twin pregnancy (≥ 24 weeks of gestation) at two community hospitals in California during 1998 to 2005 and had previously undergone treatment for CIN (CKC = 10, LLETZ = 36, LA/CT = 64) Exclusion:women with colposcopy or biopsy only, pregnancies with major fetal anomalies or intrauterine death, multi‐fetal pregnancy reduction, indicated delivery prior to 34 weeks, twin–twin transfusion syndrome, or cerclage placement Untreated group ‐ 766 women who had a twin pregnancy (≥ 24 weeks of gestation) at two community hospitals in California during 1998 to 2005 with no history of cervical procedures |
|
| Interventions | CKC; LLETZ; Ablation NOS (LA, CT) | |
| Outcomes | PTB (< 37 weeks) (multiple pregnancies); PTB (< 34 weeks) (multiple pregnancies); PTB (< 28 weeks) (multiple pregnancies) | |
| Notes | No woman had more than one twin delivery during the time period specified. If a participant had undergone both an ablative and excisional procedure, she was included in the excisional group. A total of 110 (12.6%) women had undergone a prior procedure for cervical dysplasia. This included 10 with a CKC, 36 with a LEEP, 59 with cryotherapy and 5 had undergone CO2 laser ablation. One of the participants with a CKC also had cryotherapy. One participant had undergone cryotherapy twice and none of the women had more than one excisional procedure. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women having a twin pregnancy in two hospitals in California during 1998 to 2005 |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Regression analysis for age, parity, race, history of PTB, history of tobacco use, history of drug use and chorionicity |
El‐Bastawissi 1999.
| Methods | Retrospective cohort study Comparison groups: A) External from general population ‐ matching for age and country of origin (foreign vs USA) B) Women with Carcinoma in situ (CIS) but no treatment ‐ unmatched Both had regressional analysis for parity, race, maternal smoking, marital status and history of TOPs. Information source ‐ Cancer Surveillance System (a population‐based cancer registry covering 13 counties of western Washington) at the Fred Hutchinson Cancer Research Center in Seattle, Washington, and Birth Certificates from the Department of Health in Washington state |
|
| Participants | Treated group ‐ 1096 women who were less than 50 years old with CIS, were diagnosed between 1984 and 1992, were treated with excisional or ablative therapy and subsequently delivered live singletons between 1984 and 1995 (the women were identified by the Cancer Surveillance System) Untreated group ‐ A) 9201 women (random sample selected from birth certificates, but frequency‐matched for age and the country of origin) without cervical cancer who gave birth during the same years without previous treatment. B) 330 women with untreated CIS Only women (for both the treated and untreated group) residing in the 13 counties of western Washington covered by the Cancer Survellance system were included. Only women who indicated the same father of the index infant and previous children were included. |
|
| Interventions | Excision NOS (CKC, LC, LLETZ); Ablation NOS (LA, CT) | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (singleton pregnancies); CS; LBW (< 2500 g) | |
| Notes | From the 1851 women with CIS, 1539 women had a pregnancy after the CIS diagnosis. Of these women, 212 had no surgical procedure before pregnancy, 227 had D&C or ECC before pregnancy, 85 had cryosurgery or LA before pregnancy, and 1011 had conisation before pregnancy. For 4 women, the procedure (if any) before pregnancy was unknown. From the 1851 women with CIS, 312 were pregnant at the time of the diagnosis. Of these women, 118 had no surgical procedure during pregnancy, 33 had D&C or ECC during pregnancy, 6 had cryosurgery or LA during pregnancy, and 142 had conisation during pregnancy. For 13 women, the procedure (if any) during pregnancy was unknown. It is possible to make the following comparisons in our meta‐analysis: a) Women with CIS and treatment before pregnancy versus women with CIS but no treatment (diagnosis of CIS before pregnancy) b) Women with CIS and treatment before pregnancy versus women with CIS but no treatment (diagnosis of CIS during pregnancy) c) Women with CIS and treatment before pregnancy versus women with CIS but no treatment (diagnosis of CIS before or during pregnancy) d) Women with CIS and treatment before pregnancy versus general population Women that had treatment during pregnancy were excluded according to the exclusion criteria of the systematic review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information on birth weight, gestation length, and delivery method was complete for 98.8%, 83.2%, and 93.8% of women with CIS versus 99.7%, 86.7%, and 94.7% for comparison women, respectively. |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women from 13 counties of western Washington (a population‐based study) |
| Representative comparison group? | Low risk | Both untreated groups were drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for age and country of origin (foreign vs USA). Regressional analysis for parity, race, maternal smoking, marital status and history of TOPs. |
Fischer 2010.
| Methods | Prospective cohort study Comparison group: External ‐ matching for age (+/‐ 5 years), race, the number of prior vaginal deliveries at ≥ 20 weeks and gestational age at the time of cervical sonography (+/‐ 2 weeks) Information source ‐ medical records of one of the southern New Jersey maternal–fetal medicine offices |
|
| Participants | Treated group ‐ 85 pregnant women presenting to one of the southern New Jersey maternal–fetal medicine offices (during 2001 to 2007) with a history of LLETZ (n = 68), CKC (n = 15), or both (n = 2) Unterated group ‐ 85 pregnant women referred from the referred obstetrical ultrasound population (during 2007 to 2008) without previous cervical surgery Exclusion criteria (for both groups): multiple gestations, a clinical history of cervical insufficiency (defined as a history of repeat midtrimester pregnancy loss associated with painless cervical dilatation), presence of a cerclage or planned cerclage, ruptured membranes, or a fetal aneuploidy or major anomaly recognized at the time of cervical sonography |
|
| Interventions | Excision NOS (CKC; LLETZ) | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (singleton pregnancies); PTB (< 34 weeks); CS; cervical cerclage | |
| Notes | No enrolled patients were excluded from analysis after cervical sonography had been performed. The researchers had difficulty finding a matched control for one of the study participants, a 40‐year‐old Caucasian with four previous vaginal deliveries. They finally identified a Filipino gravida who otherwise matched the study patient. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No enrolled patient was excluded from analysis after cervical sonography had been performed |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other source of bias is obvious |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women having a cervical sonography during 2001 to 2007 |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for age (+/‐ 5 years), race, the number of prior vaginal deliveries at ≥ 20 weeks, gestational age at the time of cervical sonography (+/‐ 2 weeks) |
Forsmo 1996.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age (+/‐ 3 years), parity and place of delivery (hospital with perinatal care unit, smaller hospital or local district maternity wards) Information source ‐ Medical records, postal questionnaires |
|
| Participants | Treated group ‐ 71 women who were treated by LC or LA (LC = 51; LA = 20) in the Department of Obstetrics and Gynaecology, University Hospital of Tromso, Norway, during 1983‐88 and had subsequently a delivery (deliveries till June 1992 were included). Only first deliveries after treatment (delivery after 24th week) were included. Only singletons were reported by the women. Control group ‐ 174 women who delivered without previous treatment. |
|
| Interventions | LC; LA | |
| Outcomes | LBW (< 2500 g); LBW (< 2000 g); LBW (< 1500 g); perinatal mortality; stillbirth | |
| Notes | During 1983 to 1988, 356 women were treated for CIN I‐III with laser conisation or ablation in the Department of Obstetrics and Gynecology, University Hospital of Thomso, Norway. Twelve women (3.4%) were lost afterwards. In June 1992, a postal questionnaire was sent to the other 344 women. 319 women (93%) replied. The short questionnaire comprised questions about pregnancy outcome after treatment, birth weights, complications in pregnancy or delivery, and place of delivery. A total of 87 women, all women, reported that they fell pregnant at least once after treatment. Of these women, 71 had a delivery after 24 weeks of gestation. Information about gestational length and verification of birth weight in women with ≤ 2500 g was collected from medical records. Data concerning treatment, diagnosis and parity before pregnancy were previously registered. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 12 women (3.4%) did not receive a postal questionnaire, because they were lost after treatment. Of the other 344 eligible for the study women, 319 (93%) replied. |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | High risk | There are some contradictions: in the table III where the LBW rates are presented, the authors have also included the women of the treated group with miscarriage (n = 11), TOP (n = 3) and ectopic pregnancy (n = 2). In these cases, there is no birth weight to be calculated. It is not clear if there are also miscarriages, ectopic pregnancies and TOPs in the total number of the controls. |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women that had LC or LA at the University Hospital of Tromso between 1983 to 1988 |
| Representative comparison group? | Low risk | The untreated group was drawn from the same area and period although not necessarily from the same hospital |
| Comparability of treatment groups? | Low risk | Matching for age (+/‐ 3 years), parity, place of delivery (hospital with perinatal care unit, smaller hospital or local district maternity wards) |
Frega 2013.
| Methods | Prospective cohort study Comparison group: External ‐ matching for parity (all women were nulliparous) and race (all women were white) Information source ‐ records of university teaching hospitals and country hospitals across Italy |
|
| Participants | Study period ‐ January 2003 to January 2007 Treated group ‐ 475 pregnant women who had previously undergone LLETZ for CIN 2/3; Inclusion criteria: women with only one previous LLETZ, no repeated cervical excisional or ablative treatments and no relapse of CIN for at least 12 months after LLETZ Untreated group ‐ 441 pregnant women with no previous treatment for CIN Inclusion criteria (for both groups): women of age 42 years or younger, women who had spontaneous pregnancy, white women and nulliparous women Exclusion criteria (for both groups): twin pregnancies, any major disease (e.g. cardiovascular disease, diabetes, HIV infection, or hypertension) and alcohol, smoke or substance abuse. |
|
| Interventions | LLETZ | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (nulliparous); PTB (< 37 weeks) (single cone); PTB (< 37 weeks) (singleton pregnancies) | |
| Notes | In the treated group, 69/475 women had a miscarriage (≤ 24 weeks of gestation). In the untreated group, 62/441 women had a miscarriage (≤ 24 weeks of gestation). These women were not in the denominator for the calculation of the PTB rate. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18/493 (3.7%) pregnant women in the treated group were lost to follow‐up; 21/462 (4.5%) pregnant women in the untreated group were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women delivering at the participating hospitals across Italy during 2003 to January 2007 |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for parity (all women were nulliparous) and race (all women were white). The mean age in the treated group was 30.8 vs 31.9 in the untreated group |
Frey 2013.
| Methods | Retrospective cohort study Comparison groups: A) External from women that had cervical smear B) Women with punch biopsy but no treatment Both had matching for age and year of treatment/Pap test/punch biopsy, and regression analysis for age, parity, race, meternal diabetes, maternal BMI, neonate birth weight and prior CS Information source ‐ clinical databases of surgical pathology at the nine participating hospitals (for the ascertainment of the exposure); structured phone interviews and confirmation from medical files after informed consent (for the ascertainment of the outcomes) |
|
| Participants | Treated group ‐ 598 women who had undergone LLETZ at one of the nine participating hospitals during 1996 to 2006 and then had a singleton pregnancy beyond 20 weeks of gestation Untreated group ‐ A) 588 women who had had Pap test only at one of the nine participating hospitals during 1996 to 2006 and then had a singleton pregnancy beyond 20 weeks of gestation B) 552 women who had had punch biopsy but no treatment at one of the nine participating hospitals during 1996 to 2006 and then had a singleton pregnancy beyond 20 weeks of gestation Inclusion criteria: only the first pregnancy after procedure (LLETZ, Pap test, punch biopsy) Exclusion criteria: women in the untreated groups who reported any history of LLETZ or other cervical excisional treatment; women with missing data (pregnancy history, mode of delivery, dates of the cervical procedure/delivery), women for whom medical records were unavailable |
|
| Interventions | LLETZ | |
| Outcomes | PTB (< 37 weeks); CS; induction of labour | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified how many women had missing data on the outcomes of the index pregnancy |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women having LLETZ at nine hospitals during 1996 to 2006 and then delivering |
| Representative comparison group? | Low risk | The untreated groups were drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for age and year of treatment/Pap test/punch biopsy; regression analysis for age, parity, race, meternal diabetes, maternal BMI, neonate birth weight and prior CS |
Gunasekera 1992.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age, parity, race, duration of pregnancy and smoking habit Information source ‐ Hospital records of the Department of Obstetrics and Gynaecology of Watford General Hospital, Hertfordshire |
|
| Participants | Treated group ‐ 140 women who had undergone LA or LLETZ for CIN (LLETZ = 23; LA = 117) at Watford General Hospital and had a subsequent intra‐uterine pregnancy, whose outcome was known. The observation period was February 1987 to January 1991. Untreated group ‐ 140 matched women |
|
| Interventions | LLETZ; LA | |
| Outcomes | PTB (< 37 weeks); CS; instrumental deliveries (forceps); prolonged labour (> 12 hours) | |
| Notes | The majority of patients had been treated with the laser because this method had been in use longer than LLETZ. 3 patients who had been treated with LC were too small a group to analyse. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women who were treated with LA or LLETZ in a single general hospital and had a subsequent pregnancy, between February 1987 to January 1991 |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for age, parity, race, duration of pregnancy and smoking habit |
Guo 2013.
| Methods | Prospective cohort study Comparison group: Women with colposcopic biopsy (CIN1 or less) but no treatment ‐ matching for smoking (all women were non‐smokers) Information source ‐ Records of the First Affiliated Hospital of Zhengzhou University, China |
|
| Participants | Treated group ‐ 84 women who underwent LLETZ or CKC (CCK = 36, LLETZ = 48) at the University Hospital of Zhengzhou during January 2005 to January 2009, wanted thereafter to become pregnant and succeeded in becoming pregnant; Exclusion criteria: women with postoperative infertility, multiple‐time conisation or positive incisal edge Untreated group ‐ 68 women who became pregnant after exclusion of CIN II or above with colposcopic biopsy and did not receive any other surgical procedures Exclusion criteria (for both groups): history of infertility or recurrent miscarriages, evidence of premature delivery, smoking habits |
|
| Interventions | CKC; LLETZ | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (single cone); PTB (< 34 weeks); pPROM; CS; precipitous labour; prolonged labour; LBW (< 2500 g); Aprgar score (< 7) (1min) | |
| Notes | The follow‐up lasted two years. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified how many women were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women undergoing CKC or LLETZ in a single hospital during January 2005 to January 2009 |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Women with colposcopic biopsy (CIN1 or less); matching for smoking (all women were non‐smokers). |
Haffenden 1993.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age and parity Information source ‐ Hospital records of Gloucestershire Royal Hospital |
|
| Participants | Treated group ‐ 152 women who had undergone LLETZ at Gloucestershire Royal Hospital between April 1988 and December 1989 and had a subsequent delivery (delivery after 24 weeks) at the same hospital. Untreated group ‐ 152 women without previous treatment delivering at Gloucestershire Royal Hospital (the next following suitable woman after case). |
|
| Interventions | LLETZ | |
| Outcomes | PTB (< 37 weeks); CS; instrumental deliveries (ventouse/forceps); precipitous labour (< 2 hours); prolonged labour (> 12 hours); induction of labour; oxytocin use; epidural use; LBW (2500 g) | |
| Notes | Between April 1988 and December 1989, 1000 women with cervical smears showing repeatedly borderline changes of dyskaryosis and who had satisfactory colposcopy underwent LLETZ at the Gloustershire Royal Hospital. Pregnancies in this study group which occurred since LLETZ and which resulted in referral to Gloucestershire Royal Hospital were identified. Deliveries after 24 weeks' gestation were matched against a control: the delivery of the next women of the same age and parity at Gloucestershire Royal Hospital. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | The treated group included all women that underwent LLETZ between 1988 and 1989 in a single hospital |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Unclear risk | Matching for age and parity. The intervention group had substantially higher rate of smoking (36% vs 14.4%) |
Hagen 1993.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age (+/‐ 3 years) and parity (equal); regression analysis for maternal height, marital status, level of education, smoking, previous TOP, and, in the index pregnancy, occurrence of gestational hypertension or antepartum haemorrhage and the mode of delivery Information source ‐ Hospital records of University Hospital of Trondheim, Norway |
|
| Participants | Treated group ‐ 56 women who had undergone LLETZ at the Department of Obstetrics and Gynaecology, University Hospital, Trondheim, Norway between 1983 and 1985, were 38 years of age or younger at the time of operation and had been delivered of live infants beyond 22 weeks gestation after the conisation and before 1991 (all infants were singletons). Only the first birth after treatment was included. Untreated group ‐ 112 women without previous treatment delivered at the same hospital (the first two women after each case) |
|
| Interventions | LC | |
| Outcomes | PTB (≤ 37 weeks); PTB (≤ 37 weeks) (nulliparous); PTB (≤ 37 weeks) (parous); PTB (≤ 37 weeks) (singleton pregnancies); CS; instrumental deliveries (ventouse/forceps); APH | |
| Notes | During the three year period from a January 1983 to 31 December 1985, 351 women underwent LC of the cervix. 6 women were lost afterwards. 247 women who were 38 years of age or younger at the time of operation were studied for reproductive events. By 1 January 1991, 79 of these women had become pregnant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from hospital records. Of the 351 who had undergone LLETZ between 1983 to 1985, only 6 (1.71%) were lost after the treatment |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Unclear risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All women that had LLETZ at the University Hospital of Trondheim between 1983 to 1985 |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for age and parity; regression analysis for maternal height, marital status, level of education, smoking, previous TOP, and, in the index pregnancy, occurrence of gestational hypertension or antepartum haemorrhage and the mode of delivery. |
Heinonen 2013.
| Methods | Retrospective cohort study Comparison group: External ‐ unmatched; regression analysis for maternal age, socioeconomic status, marital status, urbanism, time since LLETZ, previous PTBs Information source ‐ Hospital Discharge Register (for the ascertainment of the exposure); Medical Birth Register (for the ascertainment of the outcome) |
|
| Participants | Treated group ‐ 7636 singleton deliveries of women of reproductive age (15 to 49 years) who had undergone LLETZ during 1997 to 2009 and delivered during 1998 to 2009 Untreated group ‐ 658,179 singleton deliveries (1998 to 2009) of women without previous LLETZ |
|
| Interventions | LLETZ | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (single cone); PTB (< 37 weeks) (repeat cones); PTB (< 37 weeks) (singleton pregnancy) | |
| Notes | The 3 studies (Heinonen 2013, Jakobsson 2009, Jakobsson 2007) refer to overlapping populations from the Finnish Register. We considered as primary study the most recent (Heinonen 2013) that was a population‐based study assessing the impact of LLETZ from 1997 to 2009. From this study we extracted PTB (< 37 weeks) rates, overall as well as for single cones, repeat cones and singleton pregnancies. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information was obtained from national registers |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women in Finland undergoing LLETZ during 1997 to 2009 and subsequently delivering during 1998 to 2009 (a population‐based study) |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Regression analysis for maternal age, socioeconomic status, marital status, urbanism, time since LLETZ, previous PTBs |
Hemmingsson 1982.
| Methods | Retrospective cohort study Comparison group: Internal (pre‐treatment pregnancies) Information source ‐ Hospital records of the University Hospital of Uppsala,Sweden |
|
| Participants | Treated group ‐ 115 pregnancies of women who had undergone CT for CIN at the Department of Gynaecological Oncology of the University Hospital of Uppsala between 1973 to 1979 and had a subsequent pregnancy (≥ 28 weeks of gestation). Exclusion criteria: women > 40 years of age at the time of cryotherapy Untreated group ‐ 65 pregnancies before the cryotherapy of the same women |
|
| Interventions | CT | |
| Outcomes | PTB (< 36 weeks); pPROM; CS; cervical stenosis; perinatal mortality | |
| Notes | Almost all women were delivered at the University Hospital of Uppsala. Most pre‐therapy pregnancies were completed during 1973 to 1975 (86%) in contrast with post‐therapy pregnancies, of which 76% occurred in 1976 to 1980. The only difference found in this study about the effect of cryosurgery was an increase in the number of CS in the post‐therapy group.However, the higher CS rate only reflects a general trend towards a higher CS rate in Sweden (in 1973 the CS rate at the department was 6% but rose to 13% in 1980). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | High risk | The CS rate was six times higher in the treated group than in the untreated group. The higher CS rate probably reflects the general trend towards a higher CS rate in Sweden in the last 10 years before the publication. This is discussed by the authors. |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds. |
| Representative intervention group? | Low risk | All eligible for the study women who had undergone cryotherapy for CIN in a single University Hospital between 1973 to 1979 and had a subsequent pregnancy |
| Representative comparison group? | Low risk | Internal comparison group (pre‐treatment pregnancies) |
| Comparability of treatment groups? | Low risk | Internal comparison group (pre‐treatment pregnancies) |
Himes 2007.
| Methods | Retrospective cohort study Comparison group: Women with colposcopic biopsy but no treatment ‐ unmatched; regression analysis for age, race, marital status, payor status, years of education, tobacco use, history of preterm delivery and height of the cone specimen Information source ‐ Hospital records (pathological and obstetric database) |
|
| Participants | Treated group ‐ 114 women who had undergone LLETZ between November 2001 and December 2004 and subsequently delivered a singleton, non‐anomalous pregnancy of at least 20 weeks of gestation at Magee‐Womens Hospital. Exclusion criteria: Women with CKC, women with treatment during pregnancy, women with cervical cerclage Untreated group ‐ 962 women who had undergone colposcopic biopsy between November 2001 and December 2004 and subsequently delivered a singleton, non‐anomalous pregnancy of at least 20 weeks of gestation at Magee‐Womens Hospital. Exclusion criteria: Women with cervical cerclage |
|
| Interventions | LLETZ | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (singleton pregnancies); sPTB (< 37 weeks); pPROM | |
| Notes | The numbers of patients with CKC was small and their exclusion did not change the results. 3 women had conisation during pregnancy and they were excluded. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information was obtained from hospital records (pathological and obstetric database) |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | High risk | Table 2 in page 316 is wrong, but the correct data can be pooled from the text. |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women delivering in a single hospital |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Regression analysis for age, race, marital status, payor status, years of education, tobacco use, history of preterm delivery and height of the cone specimen |
Jakobsson 2007.
| Methods | Retrospective cohort study Comparison group: External ‐ unmatched; regression analysis for age, parity, smoking Information source ‐ Hospital Discharge Register (information on all inpatient episodes in health care facilities since 1967); Finnish Medical Birth Register |
|
| Participants | Treated group ‐ 8422 singleton pregnancies of reproductive‐aged women (15 to 49 years) in Finland who had undergone treatment for CIN during 1986 to 2003 and had a subsequent delivery during 1987 to 2004 (excision:4846; ablation:3576). Exclusion criteria: Women with irrelevant cervical treatments (such as TOPs and excisions of polyps). Untreated group ‐ all singleton pregnancies (1,056,855) of women in Finland who did not have a history of treatment for CIN and delivered during 1987 to 2004. |
|
| Interventions | Excision NOS (CKC, LC, LLETZ); Ablation NOS (LA, CT, electrocoagulation) | |
| Outcomes | PTB (< 37 weeks); PTB (< 28 weeks); LBW (< 2500 g); perinatal mortality | |
| Notes | The 3 studies (Heinonen 2013, Jakobsson 2009, Jakobsson 2007) refer to overlapping populations from the Finnish Register. We considered as primary study the most recent (Heinonen 2013) that was a population‐based study assessing the impact of LLETZ from 1997 to 2009. From Jakobsson 2007, we extracted data on the PTB (< 37 weeks) but after exclusion of all patients that were treated after 1997 because we wanted to avoid duplication with Heinonen 2013. We also proportionally adjusted the control population to avoid duplication. We further analysed PTB (< 28 weeks), LBW (< 2500 g) and perinatal mortality as this data are not provided in any other study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from national registers; It is estimated that 95% of all hospitalizations are registered in the Hospital Discharge Register; Less than 0.1% of all newborns are missing from the Medical Birth Register |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study Finnish women having a singleton delivery between 1987 to 2004 (a population‐based study) |
| Representative comparison group? | Low risk | The control group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | The women in the treated group were slightly older, were more often nulliparous (42.8% vs 30.9%), were twice as often smokers (26.6% vs 15.2%), and had lower socioeconomic status. However, adjusting for age, parity and smoking did not change the results of the study. The researchers were not able to adjust also for the socioeconomic status (they were unable to define the socioeconomic status for all women), but in Finland socioeconomic status and smoking are strongly correlated. |
Jakobsson 2009.
| Methods | Retrospective cohort study Comparison groups: A) External: no matching B) Internal (self‐matching) Both had regression analysis for age, parity, or both Information source ‐ Hospital Discharge Register, Medical Birth Register and hospital records of the Helsinki University Hospital and the Maternity Hospital, Finland |
|
| Participants | A) Treated group ‐ 624 women who had undergone LLETZ for CIN during 1997 to 2003 and subsequently delivered at the Helsinki University Hospital or the Maternity Hospital, Finland, until 2006. Inclusion criteria: only singleton pregnancies. Exclusion criteria: women who were treated during pregnancy; women with a delivery during the study year but before LLETZ; women with a previous CKC; multiple pregnancies. Untreated group ‐ 554,507 women having a singleton delivery during 1997 to 2006 (general population of Finland) B) 258 women of the treated group had also a delivery before LLETZ. For these women internal matching (self‐matching) was possible, in addition to the external comparison group. |
|
| Interventions | LLETZ | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (nulliparous); PTB (< 37 weeks) (parous) | |
| Notes | The 3 studies (Heinonen 2013, Jakobsson 2009, Jakobsson 2007) refer to overlapping populations from the Finnish Register. We considered as primary study the most recent (Heinonen 2013) that was a population‐based study assessing the impact of LLETZ from 1997 to 2009. From Jakobsson 2009 that refers to two hospitals in Southern Finland, we have included as outcomes the PTB (< 37 weeks) for nulliparous as opposed to multiparous women that is not described in the other two cohorts. We also included data in the analysis from this paper on self‐matching (internal controls). These data are not presented in the other two references of the same population. In order to minimise overlap, we have only included data for the internal comparison in the separate forest plot for internal matching but not in the merged one, as it was impossible to discriminate the possible overlap with Heinonen 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information was obtained from national registries and hospital records. For some parameters, there was incomplete data (e.g. for 45.5% of the women, the cone size was unknown and for 7.7% of the women, the CIN diagnosis was unknown). Complete data for the duration of pregnancy. |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women delivering in two hospitals during 1997 to 2006 |
| Representative comparison group? | Unclear risk | A) The external comparison group was not drawn from the same source as the treated group (it was drawn from the national Birth Register covering the whole of Finland, whereas the treated group was drawn from two specific hospitals) B) Internal (self‐matching) |
| Comparability of treatment groups? | Low risk | A) External: Regressional analysis for age, parity, or both. No conspicuous difference between the treated and the external comparison group, regarding socioeconomic class, smoking during pregnancy, alcohol consumption during pregnancy, or substance abuse during pregnancy. B) Internal (self‐matching) |
Jones 1979.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age (+/‐ 5 years), parity, social class, date of delivery (same month or immediately preceding or following month) and singleton birth Information source ‐ Clinical records available from the Cardiff Cervical Cytology Study (for cases), Cardiff Birth Survey (for controls) |
|
| Participants | Treated group ‐ 66 pregnancies of all women from Cardiff who had undergone CKC between February 1965 and April 1974 and subsequently had a singleton pregnancy (up to April 1975) proceeding beyond 28 weeks' gestation. Untreated group ‐ 264 pregnancies of women from Cardiff having a singleton pregnancy without previous treatment. |
|
| Interventions | CKC | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (singleton pregnancies); sPTB (< 37 weeks); CS; instrumental deliveries (ventouse/forceps); precipitous labour (< 2 hours); prolonged labour (> 12 hours); LBW (< 2500 g); perinatal mortality; stillbirth | |
| Notes | Between February 1965 and April 1974, 600 women from Cardiff had CKC. 372 of these women were potentially fertile and up to April 1975, 76 had 91 pregnancies after the CKC. 13 pregnancies aborted spontaneously, 11 were terminated and there was one twin pregnancy. The other 66 singleton pregnancies were included. 10 pregnancies were the second one after the treatment and one pregnancy was the third one after the treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from hospital records and registries |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible women from Cardiff that had CKC between February 1965 to April 1974 (a population‐based study) |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for age (+/‐ 5 years), parity, social class, date of delivery (same month or immediately preceding or following month) and singleton birth |
Kirn 2015.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age, parity and smoking Information source ‐ University Hospital of the Ludwig‐Maximilians University, Munich |
|
| Participants | Treated group ‐ 135 patients who delivered at the University Hospital of the Ludwig‐Maximilians University between 2006 and 2012 and had undergone cervical conisation before giving birth; exclusion criteria: twin pregnancies Untreated group ‐ 135 women who had not undergone cervical conisation before giving birth |
|
| Interventions | Excision NOS | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (singleton); CS | |
| Notes | At first, 144 patients with treatment before pregnancy were identified. However, 3 patients were excluded for having twin pregnancies and six women could not be matched and therefore were also excluded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women delivering in a single university hospital between 2006 to 2012 |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for age, parity and smoking |
Kitson 2014.
| Methods | Retrospective cohort study Comparison group: Women with punch biopsy but no treatment ‐ matching for age, parity and smoking Information source ‐ maternity and colposcopy databases of a large tertiary unit in the North East of England |
|
| Participants | Treated group ‐ 278 women who had undergone LLETZ during 2000 to 2010 and subsequently delivered in a large tertiary unit in the North East of England during 2008 to 2011. Only the first pregnancy after treatment was included. Untreated group ‐ 278 women who delivered in the same unit during the same time period and had had punch biopsy but no treatment before birth Inclusion criteria (for both groups): singleton pregnancies of at least 20 weeks of gestation |
|
| Interventions | LLETZ | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (singleton pregnancies); PTB (< 34 weeks); sPTB; pPROM; CS; instrumental deliveries; LBW (< 2500 g); NICU admission | |
| Notes | 30 women underwent two or more LLETZ procedures. The mean gestational age of these women did not differ from the mean gestational age of the women who underwent only one LLETZ procedure. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Unclear risk | Excised cones were formalin fixed at the time of receipt in the pathology lab which is known to result in tissue retraction and hence a reduction in the measured dimensions |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women delivering in a large tertiary unit during 2008 to 2011 |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source |
| Comparability of treatment groups? | Low risk | Matching for age, parity and smoking |
Klaritsch 2006.
| Methods | Retrospective cohort study Comparison group: External ‐ no matching, no regression analysis Information source ‐ Hospital records |
|
| Participants | Treated group ‐ 76 singleton deliveries of 65 women who delivered at the Department of Obstetrics & Gynaecology, Medical University of Graz, Austria, between 1992 to 2002 and had previously undergone CKC at the same hospital or other hospital. Exclusion criteria: Women with LLETZ, repeated conisation, previous PTB or multiple gestations. No woman had undergone CKC during pregnancy. Untreated group ‐ all singleton deliveries (29711) of the women who delivered at the Department of Obstetrics & Gynaecology, Medical University of Graz, Austria, between 1992 to 2002 and did not have a history of cervical conisation |
|
| Interventions | CKC | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (single cone); PTB (< 37 weeks) (singleton pregnancies); PTB (< 34 weeks); pPROM; CS; chorioamnionitis; LBW (< 2500 g); perinatal mortality | |
| Notes | There were 29,809 singleton deliveries at the University Hospital of Graz between 1992 and 2002. 98 deliveries of 86 women with history of conisation were identified. The researchers excluded 21 women and their 22 deliveries. 16 of them had undergone LLETZ, 2 had had more than one conisation, 1 had had previous PTB, and 2 had multiple gestations. 65 women with a total of 76 deliveries had undergone CKC and were included in the conisation group. For controls the researchers took the remaining 29,711 singleton deliveries in the study period. 53 women had undergone CKC at the University Hospital of Graz, 12 at other hospitals. No woman had undergone CKC during pregnancy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete data in the control group; a small percentage of incomplete data for most outcomes in the untreated group |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women delivering in a single hospital between 1992 to 2002 |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | High risk | Neither matching nor regression analysis; the median age at delivery was 30 years (range 21 to 43) in the treated group vs 28 years (range 14 to 61) in the untreated group; 73.7% multigravidas in the treated group vs 49.9% multigravidas in the untreated group; no significant difference in smoking habits |
Kristensen 1985.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age and parity Information source ‐ Records of the Data Processing Unit at Odense University Hospital/Questionnaires (treated group), Records of Odense University Hospital (untreated group) |
|
| Participants | Treated group ‐ All women who had conisation performed in the county of Funen between April 1973 and December 1980 and had a subsequent pregnancy (before April 1982). 85 pregnancies (proceeding beyond 28 weeks) of 82 women were finally included in the analysis. Untreated group ‐ All singleton deliveries at Odense University Hospital between 1978 and 1982. (Odense University Hospital mainly serves the town of Ostense and the surrounding rural areas) |
|
| Interventions | Treatment NOS | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (singleton pregnancies); LBW (< 2500 g) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The needed information was obtained from hospital records. For women having left the county of Funen, a questionnaire was sent about the outcome of probable pregnancies after treatment; replies were received from all patients. |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | High risk | The type of conisation is not described |
| Representative intervention group? | Low risk | All eligible women from the county of Funen that had treatment for CIN between April 1973 to December 1980 (a population‐based study) |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for age and parity |
Kristensen 1993.
| Methods | Retrospective cohort study Comparison groups: A) External ‐ no matching, no regression analysis B) Internal (self‐matching) Information source ‐ Medical Birth Register, National Register of Hospital Discharges |
|
| Participants | All women with a permanent address in Denmark with singleton pregnancies who gave birth to their first infant in 1982 and second infant during the time period 1982 to 1987. For treated group, treatment took place during 1977 to 1987. A) The first and second delivery of the women whose treatment took place before the first delivery (68 deliveries of 34 women) and the second delivery of the women whose treatment took place between the first and second delivery (62 deliveries of 62 women) were compared with the first and second delivery of the women with no treatment (28124 deliveries of 14062 women) B) For the 62 women whose treatment took place between the first and second delivery, the first delivery was compared with the second delivery (self‐matching) |
|
| Interventions | Treatment NOS (CKC, laser, electrocautery) | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (nulliparous); PTB (< 37 weeks) (parous); PTB (<3 7 weeks) (singleton pregnancies) | |
| Notes | In the cohort of 14,233 women, 170 had cervical conisation: 34 before the first childbirth, 62 between the first and second childbirth, and 74 after the second childbirth. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from national registers |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Unclear risk | The type of conisation is not described as details are not included in the national registry. Treatment may include CKC, laser or electrocautery |
| Representative intervention group? | Low risk | This is a population‐based study and the treated group is representative of the average women who undergoes conisation |
| Representative comparison group? | Low risk | A) The external comparison group was drawn from the same source as the treated group B) Internal comparison group (self‐matching) |
| Comparability of treatment groups? | High risk | A) No matching, no regression analysis B) Internal comparison group (self‐matching) |
Kuoppala 1986.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age, parity, date of delivery (generally same month) and singleton birth Information source ‐ Hospital records of University Central Hospital of Tampere |
|
| Participants | Treated group ‐ Women who had CKC at the University Central Hospital of Tampere in 1962 to 1979 and had a subsequent pregnancy. Finally, 62 pregnancies lasting more than 28 weeks were included in the analysis. Untreated group ‐ 62 pregnancies (> 28 weeks) of women from the labour room register without previous treatment. |
|
| Interventions | CKC | |
| Outcomes | PTB (< 37 weeks); CS; instrumental deliveries (ventouse); induction of labour; oxytocin use; analgesia use NOS; cervical cerclage; perinatal mortality; stillbirth | |
| Notes | The study comprised patients who had cervical conization at the University Central Hospital of Tampere in 1962 to 1979. A total of 317 women had cone biopsy: 77 between them had 98 pregnancies. Of these pregnancies, 36 lasted less than 28 weeks and 62 more than 28 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious. |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible women that had CKC at the Univeristy Hospital of Tampere between 1962 to 1979 |
| Representative comparison group? | Low risk | The untreated group was pooled from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for age, parity, date of delivery and singleton birth |
Larsson 1982.
| Methods | Retrospective cohort study Comparison group: Internal (pre‐treatment pregnancies) with matching for age, parity, socioeconomic status, smoking, surgical interventions and various diseases Information source ‐ South Swedish Regional Tumour Registry, hospital records |
|
| Participants | Treated group ‐ 197 deliveries after CKC Untreated group ‐ 284 deliveries before CKC The CKC took place between 1962 and 1976 |
|
| Interventions | CKC | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (singleton pregnancies); PTB (< 37 weeks) (multiple pregnancies); perinatal mortality; stillbirth | |
| Notes | 988 women had undergone conization because of dysplasia in varying degree or carcinoma in situ over the 15‐year period 1962 to 1976. 197 women became pregnant with a total of 635 pregnancies before and after conisation. 37 of the women had not been pregnant before the conisation. Number of pregnancies before conisation: 341 (284 deliveries). Number of pregnancies after conization: 294 (197 deliveries) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from hospital records and registries |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | The treated group was pooled from the South Swedish Regional Tumour Registry. The treated group is in all likelihood representative of the average women who undergoes CKC (a population‐based study) |
| Representative comparison group? | Low risk | Internal comparison group (self‐matching) |
| Comparability of treatment groups? | Low risk | Internal comparison group (self‐matching) with matching for age, parity, socioeconomic status, smoking, surgical interventions and various diseases |
Lima 2011.
| Methods | Retrospective cohort study Comparison group: External ‐ no matching, no regression analysis Information source ‐ Hospital records of Dr Alfredo Da Costa Maternity, Lisbon |
|
| Participants | Treated group ‐ 29 women who had undergone LLETZ or LC (LC = 11; LLETZ = 18) between 2000 to 2005 at Dr Alfredo da Costa Maternity and had a subsequent delivery at the same hospital. Untreated group ‐ 58 women without previous cervical treatment who delivered at the same hospital during 2000 to 2005 (the immediate matched delivery before and after each case) |
|
| Interventions | LC; LLETZ | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (D≤ 10 mm); PTB (< 37 weeks) (D> 10 mm); CS; LBW (< 2500g); Apgar score (<7) (5min) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious sources of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women delivering in a single hospital during the study period |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | High risk | No matching or regression analysis for possible confounders |
Ludviksson 1982.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age, parity and time of delivery Information source ‐ Hospital records of the Regional Hospital of Orebro |
|
| Participants | Treated group ‐ 83 deliveries of 75 women who were below 35 years of age, underwent a cone biopsy at the Regional Hospital of Orebro between 1964 and 1978 and had later a pregnancy ending in a delivery. Women who had an elective section were excluded. Untreated group ‐ 79 deliveries of 79 women without previous treatment. |
|
| Interventions | CKC | |
| Outcomes | PTB (≤ 3 7weeks); PTB (≤ 33 weeks); PTB (< 30 weeks); PPH (> 600 mL); MOH (1000 mL) | |
| Notes | 780 women below 35 years of age underwent a cone biopsy at the Regional Hospital of Orebro between 1964 and 1978. Of these 780 women, 79 later had a pregnancy ending in a delivery. As elective section was performed in four cases of the conized group, the final number of women in this group was 75 with 83 deliveries, as opposed to 79 in the control group. The cone depth was almost 2 cm in most operations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds. |
| Representative intervention group? | Low risk | All women < 35 years that underwent CKC in a Regional Hospital between 1964 and 1978 |
| Representative comparison group? | Unclear risk | It is unclear how the control group was selected. |
| Comparability of treatment groups? | Low risk | Matching for age, parity and time of delivery |
Martyn 2015.
| Methods | Retrospective cohort study Comparison group: Women with colposcopy but no treatment ‐ matching for age Information source ‐ Records of the National Maternity Hospital (Mediscan database, pathology records and other clinical records) and postal questionnaires |
|
| Participants | Treated group ‐ 297 women who had undergone LLETZ or CKC at the National Maternity Hospital during 2001 to 2007 and subsequently had a pregnancy (≥ 24 weeks of gestation) Untreated group ‐ 204 women who had had colposcopy but no treatment at the National Maternity Hospital during 2001 to 2007 and subsequently had a pregnancy (≥ 24 weeks of gestation) Inclusion criteria (for both groups): women aged 24 to 40 years *This is only a subgroup of the study population. The main outcome of the study was the effect of CKC/LLETZ on subsequent fertility, but we are not studying infertility in this meta‐analysis. Therefore, we restricted the population to the pregnant women with a pregnancy of at least 24 weeks of gestation (see "Notes" below for more details) |
|
| Interventions | LLETZ; Excision NOS (CKC, multiple LLETZ) | |
| Outcomes | PTB ( <37 weeks); PTB (< 37 weeks) (single cone) | |
| Notes | 3590 women aged 24 to 40 years, who had attended the colposcopy services in the National Maternity Hospital between 2001 and 2007, were sent a postal questionnaire about the fertility and the pregnancies, if any, after colposcopy/CKC/LLETZ (LLETZ:1729; CKC:66;colposcopy only:1795). 1355 women (37.7%) replied. Of those who responded, 759 had one LLETZ, 37 had CKC, 22 had more than one LLETZ (in total, 818 had surgery) and 537 women had colposcopy only. i) Of those with surgery, 321 became pregnant: 38 had a gestational age less than 14 weeks and 5 had a gestational age 14 weeks to < 24 weeks. We excluded from our meta‐analysis these women and we included the remaining 278. ii) Of those with colposcopy only, 228 became pregnant: 23 had a gestational age less than 14 weeks and 1 had a gestational age 14 weeks to < 24 weeks. We excluded from our meta‐analysis these women and we included the remaining 204. We included LLETZ and Excision NOS (CKC and multiple LLETZ) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 37.7% of the women responded to the postal questionnaire which was sent to them |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | High risk | Questionnaires were used for the ascertainment of the outcomes; there is a risk of recall bias and misclassification of the outcomes |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | High risk | Only a small percentage of the women responded to the questionnaire which was sent to them. The women who replied are more likely to belong to a higher socioeconomic class. |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for age; Both groups had colposcopy before pregnancy |
Miller 2015.
| Methods | Retrospective cohort study Comparison group: A) External B) Women with prior dysplasia but no treatment Both had regression analysis for age, parity, race/ethnicity, BMI and cervical length during pregnancy Information source ‐ Hospital records of Northwestern Memorial Hospital, Chicago |
|
| Participants | Treated ‐ 1356 women with prior excisional procedure for cervical dysplasia who underwent routine cervical length assessment between 18 to 23 6/7 weeks of gestation from December 2010 through January 2014 at Northwestern Memorial Hospital in Chicago. Untreated ‐ A) 14,149 women with no prior dysplasia and no excisional procedure B) 3023 women with prior dysplasia but no excisional procedure Exclusion criteria: women younger than 18 years old, twin pregnancies, women with unavailable delivery records |
|
| Interventions | Excision NOS | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (singleton) | |
| Notes | At first, 144 women with treatment before pregnancy were identified. However, 3 women were excluded for having twin pregnancies and six women could not be matched and therefore were also excluded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women undergoing routine cervical length assessment in a single hospital during December 2010 to January 2014 |
| Representative comparison group? | Low risk | Both comparison groups were drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Regression analysis for age, parity, race/ethnicity, BMI and cervical length during pregnancy |
Moinian 1982.
| Methods | Retrospective cohort study Comparison group: Internal (pre‐treatment pregnancies) Information source ‐ Hospital records of the East Hospital, Gothenburg |
|
| Participants | Treated group ‐ all viable pregnancies after CKC (103) of 90 women who had CKC at the East Hospital, Gothenburg, between 1968 and 1973 Untreated group ‐ all viable pregnancies before CKC (720) of the same women All miscarriages were excluded |
|
| Interventions | CKC | |
| Outcomes | PTB (< 37 weeks); cervical cerclage | |
| Notes | Radical CKC procedure: if the endocervix was involved, the entire endocervical cancel was excised to the level of the internal os. Between 1968 and 1973, 414 women were treated by cone biopsy at the East Hospital, Gothenburg, Sweden. 324 women had been pregnant before conisation, with 801 pregnancies between them, and after cone biopsy 90 women became pregnant, with 122 pregnancies. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | High risk | Yes, but CKC described as more than usual radical |
| Representative intervention group? | Low risk | All eligible women that had CKC at East Hospital, Gothenburg, between 1968 and 1973 |
| Representative comparison group? | Low risk | Internal comparison group (pre‐treatment pregnancies) |
| Comparability of treatment groups? | Low risk | Internal comparison group (pre‐treatment pregnancies) |
Noehr 2009a.
| Methods | Retrospective cohort study Comparison groups: A) External B) Women with biopsy but no treatment Both were unmatched but had regression analysis for age, year of delivery, smoking during pregnancy and marital status during pregnancy Information source ‐ Medical Birth Registry, National Patient Registry, Danish Registry of Pathology, Danish IVF Registry |
|
| Participants | Treated group ‐ 10,207 deliveries of women who had undergone LLETZ or ablation (LLETZ = 8180; Ablation = 2027) during 1997 to 2005 and had a subsequent singleton delivery (21 to 45 weeks of gestation) during 1997 to 2005. Exclusion criteria: women with previous CKC; deliveries with medical induction before 37 completed weeks of gestation; infants delivered by CS performed before 37 completed weeks of gestation; deliveries subsequent to a collum amputation. Untreated group ‐ A) 510,841 singleton deliveries (21 to 45 weeks of gestation during 1997 to 2005) of women without previous cervical procedure (treatment or biopsy) B) 31,630 singleton deliveries (21 to 45 weeks of gestation during 1997 to 2005) of women without previous treatment but with previous biopsy. |
|
| Interventions | LLETZ; Ablation NOS | |
| Outcomes | sPTB (< 37 weeks); sPTB (< 37 weeks) (D ≤ 12 mm); sPTB (< 37 weeks) (D = 13 mm to 15mm); sPTB (< 37 weeks) (D = 16 mm to 19 mm); sPTB (< 37 weeks) (D ≥ 20 mm); sPTB (< 37 weeks) (single cone); sPTB (< 37 weeks) (repeat cones); sPTB (< 37 weeks) (singleton pregnancies); sPTB (< 32 weeks); sPTB (< 28 weeks) | |
| Notes | Of the 552,678 deliveries in the study, 8180 deliveries were subsequent to LLETZ (1 LLETZ: 7907; 2 LLETZ: 255; ≥ 3 LLETZ: 18). Of these 8180 deliveries, 162 were subsequent to both LLETZ and ablation. The addition of the number of deliveries subsequent to biopsy, ablation, LLETZ and no procedure (table 3 of the article) gives the total number of pregnancies (552,678), thus we concluded that the authors subtracted these 162 deliveries from the ablation group and left them only in the LLETZ group. In this way, we made sure that we will not make a duplicate extraction of the same deliveries. The Nordic Classification of Surgical Procedures has the same code for LLETZ and LC and the authors were not able to separate LLETZ from LC. However, LC has become rare in Denmark since the introduction of LLETZ and the authors included the pregnancies with this code in the LLETZ group. In our meta‐analysis, we made the same. In a second article, Noehr and colleagues 2009 investigated the association between cone depth of the LLETZ and the subsequent risk of sPTB (on the same singleton deliveries as above). Deliveries after LLETZ with no information on cone depth (n = 4302) and deliveries after two or more LLETZ (n = 273) were not included in the cone depth analysis. Nohr and colleagues 2007 is an overlapping study, but it was impossible to extract only the data that was not included in Noehr and colleagues 2009 and we excluded this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information was obtained from national registries |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Unclear risk | The Nordic Classification of Surgical Procedures has the same code for LLETZ and LC and the authors were not able to separate LLETZ from LC. However, LC has become rare in Denmark since the introduction of LLETZ and the authors included all these pregnancies in the LLETZ group. |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study Danish women delivering during 1997 to 2005 (a population‐based study) |
| Representative comparison group? | Low risk | The 2 comparison groups were drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Regression analysis for age, year of delivery, smoking during pregnancy and marital status during pregnancy |
Noehr 2009b.
| Methods | Retrospective cohort study Comparison group: External ‐ unmatched; regression analysis for age, year of delivery, smoking during pregnancy, marital status during pregnancy and IVF Information source ‐ Medical Birth Registry, National Patient Registry, Danish Registry of Pathology, Danish IVF Registry |
|
| Participants | Treated group ‐ 166 deliveries of women who had undergone LLETZ during 1997 to 2005 and had a subsequent twin delivery (21 to 45 weeks of gestation) during 1997 to 2005. Exclusion criteria: women with previous CKC; deliveries with medical induction before 37 completed weeks of gestation; infants delivered by CS performed before 37 completed weeks of gestation; deliveries subsequent to a collum amputation. Untreated group ‐ 9702 twin deliveries (21 to 45 weeks of gestation during 1997 to 2005) of women without previous LLETZ |
|
| Interventions | LLETZ | |
| Outcomes | sPTB (< 37 weeks) (multiple pregnancies); sPTB (< 32 weeks) (multiple pregnancies); sPTB (< 28 weeks) (multiple pregnancies) | |
| Notes | Of the 9868 twin deliveries in the study, 166 were subsequent to LEEP (of which 11 were subsequent to both LEEP and ablation), 43 were subsequent to ablation, 766 were subsequent to biopsy with no additional cervical procedure, and the remaining 8893 deliveries were not preceded by any cervical procedure. Only 4 (2.4%) of the deliveries subsequent to LEEP were preceded by more than one LEEP. The Nordic Classification of Surgical Procedures has the same code for LLETZ and LC and the authors were not able to separate LLETZ from LC. However, LC has become rare in Denmark since the introduction of LLETZ and the authors included all these pregnancies in the LLETZ group. In our meta‐analysis, we made the same. In contrast to another article of Noehr and colleagues 2009 with the same study design but about singletons, this one does not give sPTB rate in the group with no cervical procedure or in the group with biopsy only (gives only adjusted ORs). Therefore, we used the no‐LLETZ group (which may have undergone ablation or biopsy before delivery) as the control group. Nohr 2007 is a duplicate study that includes a proportion of the population that is presented in Noehr 2009. As there is a possibility of substantial overlap the data from this study were not used. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information was obtained from national registries |
| Selective reporting (reporting bias) | High risk | Data are not shown for the group with ablation, for the group with biopsy only and for the group with no cervical procedure |
| Other bias | Unclear risk | The control group is not really untreated because 43 women in it (0.4%) have undergone ablation prior to delivery. The Nordic Classification of Surgical Procedures has the same code for LLETZ and LC and the authors were not able to separate LLETZ from LC. However, LC has become rare in Denmark since the introduction of LLETZ and the authors included the pregnancies with this code in the LLETZ group. |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study Danish women delivering during 1997 to 2005 (a population‐based study) |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Regression analysis for age, year of delivery, smoking during pregnancy, marital status during pregnancy and IVF |
Ortoft 2010.
| Methods | Retrospective cohort study Comparison Groups: A) External B) Women with HSIL but no treatment Both had regression analysis for age, parity, smoking status, educational level and marital status C) Internal (self‐matching) Information source ‐ the pathology database, hospital records, the Aarhus birth cohort and questionnaires |
|
| Participants | Treated group ‐ 758 women who had a conisation between 1989 to 2007 (one conisation = 721; two conisations = 37) and were identified from the Danish nationwide pathology database and subsequently had a pregnancy at the University Hospital of Aarhus until March 2007. Only the first delivery after treatment was included. Untreated group ‐ A) 74,552 deliveries of women without history of conisation or dysplasia who delivered at the University Hospital of Aarhus during the study period B) 390 deliveries of women with CIN not treated with conisation who delivered at the University Hospital of Aarhus during the study period Inclusion criteria (for both groups): singleton deliveries. Exclusion criteria: missing gestational age or missing birth weight (0 in the treated group; 355 in the untreated group; women with preterm induced birth (4 in the treated group; 778 in the untreated group), preterm acute CS before labour (6 in the treated group; 534 in the untreated group) or preterm elective CS (2 in the treated group; 348 in the untreated group) were excluded from the Cox regression. C) Self‐matching for 170 women who had one conisation and had a delivery both before and after the conisation (last child born before versus first child born after the single conisation) |
|
| Interventions | CKC; Electroknife; LLETZ | |
| Outcomes | sPTB (< 37 weeks); sPTB (< 37 weeks) (single cone); sPTB (< 37 weeks) (repeat cones); sPTB (< 37 weeks)(singleton pregnancies); sPTB (< 32 weeks); sPTB (< 28 weeks); pPROM (< 37 weeks); pPROM (< 32 weeks); pPROM (< 28 weeks); LBW (< 2500 g); LBW (< 2000 g); LBW (< 1500 g); perinatal mortality; perinatal mortality (<37 weeks); perinatal mortality (< 32 weeks); perinatal mortality (< 28 weeks) | |
| Notes | Approximately 8% of all Danish births take place at the University Hospital of Aarhus. Most conisation procedures were performed at the University Hospital of Aarhus, but a small number were performed at specialist clinics in Aarhus or at the County Hospital in Odder. From 1989 to 1992, all conisations were performed with the cold‐knife procedure, and from 1992 to 1995 the cold‐knife procedure was only used for high conisations and in pregnant women. After 1995, the cold‐knife procedure was omitted, and instead the electro knife was used when a high conus biopsy was warranted. Of the 710 women with one conisation (excluding preterm induced birth (n = 4), preterm acute CS before labour (n = 5) and preterm elective CS (n = 2)) prior to pregnancy, the conus procedures were distributed as follows: 572 women had LLETZ, 71 women had an electrosurgical needle procedure (electroknife), and 67 had CKC. 18 women had treatment during pregnancy. Because the percentage of the women with treatment during pregnancy was low (2.5%), we decided to include this study, because we estimated that these women would not change the results. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the untreated external/CIN group, 355 deliveries (0.5%) were excluded because of missing gestational age or missing birth weight. In the treated group, no delivery was excluded because of missing data. 14% of the study cohort did not reply to a questionnaire about, inter alia, previous pregnancies. These women were not excluded from the study, but more women might have been eligible for the internal comparison group. |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | High risk | Questionnaires for the outcomes of previous pregnancies; there is a risk of recall bias and misclassification of the outcome when self‐matching was used |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women delivering in a single university hospital during 1989 to 2007 |
| Representative comparison group? | Low risk | A) External ‐ B) women with HSIL but no treatment: The untreated group was drawn from the same source as the treated group C) Internal comparison group (self‐matching) |
| Comparability of treatment groups? | Low risk | A) External B) Women with HSIL but no treatment Both had regression analysis for age, parity,smoking status, educational level and marital status C) Internal comparison group (self‐matching) |
Paraskevaidis 2002.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age, parity, smoking, multiple pregnancies and history of previous PTBs Information source ‐ Hospital records of the University Hospital of Ioannina |
|
| Participants | Treated group ‐ 28 women with stage IA1 cervical carcinoma without vascular or lymph space involvement who were treated with LLETZ between 1990 to 1998 at the University Hospital of Ioannina and had at least one pregnancy beyond 24 weeks following the treatment (by 2001) Untreated group ‐ 28 women who delivered at the same hospital during the same year without previous treatment of the cervix |
|
| Interventions | LLETZ | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (single cone); PTB (< 37 weeks) (repeat cones); sPTB; CS; precipitous labour (< 2 hours); LBW (< 2500 g); NICU admission | |
| Notes | During the period 1990 to 1998, 47 women with microinvasive cervical carcinoma stage IA1 without vascular or lymph space involvement were managed exclusively with LLETZ and had clear excisional margins after a single or repeat cone. Of these women, 28 had at least one pregnancy beyond 24 weeks. Of the remaining 19 women, 12 did not become pregnant by 2001, 6 had first‐trimester miscarriage, and one had an elective TOP. 5 cases had LLETZ performed in two steps in a tophat configuration, because of an endocervically extended lesion. Three women had repeat LLETZ. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study, women having LLETZ in a single university hospital between 1990 to 1998 |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for age, parity, smoking, multiple pregnancies and history of previous PTBs |
Parikh 2008.
| Methods | Retrospective cohort study Comparison group: External ‐ no matching, no regression analysis Information source ‐ Hospital records of St. Luke’s Hospital and Health Network |
|
| Participants | Treated women ‐ 87 women who had ≥1 LLETZ and then conceived, underwent cervical length screening by transvaginal ultrasound during 2001 to 2005 at St. Luke’s Hospital and Health Network and delivered at the same hospital. Exclusion criteria: women with other surgical procedures to the cervix (such as laser, CT, CKC, cerclage); other causes of PTB, such as multiple gestation, major fetal anomaly and preterm induction of labor resulting from maternal or fetal indication; women delivering in other hospitals Untreated group ‐ 18,042 singleton births of women without previous treatment who delivered at the same hospital during 2001 to 2005 |
|
| Interventions | LLETZ | |
| Outcomes | PTB (≤ 34 weeks) | |
| Notes | A total of 97 patients who had undergone LLETZ were identified during the specified time period. Of these, 10 patients were lost to follow‐up because of delivery outside the St. Luke’s Hospital and Health Network. Thus 87 patients were included in the LLETZ group for analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were obtained from hospital records; 10 patients in the treated group (10.3%) were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women delivering in a single hospital during 2001 to 2005 |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | High risk | No matching, no regression analysis |
Poon 2012.
| Methods | Prospective cohort study Comparison group: External ‐ unmatched; regression analysis for parity, race, smoking, cervical length, previous delivery at term, previous PTB, previous miscarriage and previous LLETZ (for the prediction of sPTB) Information source ‐ questionnaires (for the ascertainment of the exposure); maternity records of the King's College Hospital and the University Hospital Lewisham/records of general practitioners (for the ascertainment of the outcomes) |
|
| Participants | Treated group ‐ 473 pregnant women with a prior LLETZ who underwent routine antenatal care at King’s College Hospital or University Hospital Lewisham and had a transvaginal sonographic measurement of the cervical length at 20 to 24 weeks of gestation, during January 1998 to July 2006 Untreated group ‐ 25,722 pregnant women without previous LLETZ who underwent routine antenatal care at King’s College Hospital or University Hospital Lewisham and had a transvaginal sonographic measurement of the cervical length at 20 to 24 weeks of gestation, during January 1998 to July 2006 Inclusion criteria (for both groups): singleton pregnancies Exclusion criteria (for both groups): women with major fetal abnormalities, with painful regular uterine contractions, with a history of ruptured membranes or cervical cerclage in situ, with a cervical length at 20 to 24 weeks of gestation ≤ 15mm and because of this treated with a cervical cerclage or prophylactic progesterone |
|
| Interventions | LLETZ | |
| Outcomes | sPTB (< 37 weeks); sPTB (< 34 weeks) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information was obtained from medical records and from questionnaires which were completed by all patients |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | High risk | Questionnaires were used for the ascertainment of the exposure; there is a risk of recall bias and misclassification of the exposure |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women with antenatal care at two hospitals during January 1998 to July 2006 |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Regression analysis for parity, race, smoking, cervical length, previous delivery at term, previous PTB, previous miscarriage and previous LLETZ (for the prediction of sPTB) |
Raio 1997.
| Methods | Retrospective cohort study Comparison groups: A) External ‐ matching for age (+/‐ 1 year), parity, marital status, social class, smoking habits and previous PTB B) Internal (self‐matching) Information source ‐ Hospital records of the Department of Obstetrics and Gynecology of Munsterlingen, Kantonsspital, Switzerland |
|
| Participants | A) Treated group ‐ 64 women younger than 35 years of age who had undergone LC at the Department of Obstetrics and Gynecology of Munsterlingen, Kantonsspital, Switzerland, from August 1, 1986, to December 31, 1994, and subsequently had a pregnancy by December 31, 1996. Only the first pregnancy after treatment was included. Exclusion criteria: voluntary termination of pregnancy, twin gestation, first‐trimester miscarriage, fetal death, ectopic pregnancy, second‐trimester termination of pregnancy for fetal structural abnormalities, blighted ovum, and cervical incompetence for previous pregnancy. Untreated group ‐ 64 women who were delivered at the same hospital during the study period and had not had any surgical procedure of the cervix. B) 26 women of the treated group were parous. For these women, self‐matching was also possible. |
|
| Interventions | LC | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (singleton pregnancies); PTB (< 37 weeks) (D < 10 mm); PTB (< 37 weeks) (D ≥ 10 mm); pPROM | |
| Notes | The study was conducted at the Department of Obstetrics and Gynecology of Munsterlingen, Kantonsspital, Switzerland, from August 1, 1986, to December 31, 1994. Laser conisation was performed in 228 women younger than 35 years of age with CIN. 26 women were lost after treatment. By December 31, 1996, 117 pregnancies occurred in 78 patients who had undergone laser conisation. If women conceived more than once, only the first subsequent gestation was included in the analysis. 14 women were excluded because of the following reasons: voluntary termination of pregnancy (n = 4), twin gestation (n = 3), first‐trimester miscarriage (n = 2), fetal death (n = 1), ectopic pregnancy (n=1), second‐trimester termination of pregnancy for fetal structural abnormalities (n = 1), blighted ovum (n = 1), and cervical incompetence for previous pregnancy (n = 1). The remaining 64 women with singleton pregnancies were included in the treated group. CIN 3 = 33/64 (51.6%); CIN 2 = 22/64 (34.4%); CIN 1 = 9/64 (14%). Regression analysis for history of PTB, advanced maternal age, smoking, multiparity and cone height: cone height was the only covariate that remained significant. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 228 women who had undergone LC from August 1, 1986, to December 31, 1994, 26 women (11.4%) were lost after treatment. |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious. |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds. |
| Representative intervention group? | Low risk | All eligible women that had LC in a single Swiss canton hospital between August 1986 to December 1994 |
| Representative comparison group? | Low risk | A) The untreated group was pooled from the same source as the treated group B) Internal comparison group (self‐matching) |
| Comparability of treatment groups? | Low risk | A) Matching for age (+/‐ 1 year), parity, marital status, social class, smoking habits and previous PTB B) Internal comparison group (self‐matching) |
Reilly 2012.
| Methods | Retrospective cohort study Comparison groups: A) External B) Women with colposcopy +/‐ punch biopsy but no treatment Both were unmatched but had regression analysis for maternal age at birth, social deprivation, smoking status, time interval between screening/colposcopy/treatment and conception, any history of a previous adverse pregnancy outcome (and gestational age for LBW outcome) Information source ‐ the Cervical Screening Wales programme database, the National Community Child Health Database and All Wales Perinatal Survey |
|
| Participants | Treated group ‐ all women in Wales aged 20 to 39 years who had a first referral to colposcopy and received cervical treatment for CIN between April 2001 ‐ March 2004, and then had a singleton pregnancy of at least 24 weeks until January 2009; n=2202 (single excision:1546; single ablation:534; multiple treatments:82; other:40) Untreated group ‐ A) all women in Waled aged 20 to 39 years who had a negative cervical smear between April 2001to March 2004 with no history of abnormal smears, and then had a singleton pregnancy of at least 24 weeks of gestation until January 2009; n=38983 B) all women in Wales aged 20 to 39 years who had a first referral to colposcopy +/‐ punch biopsy (but no cervical treatment) between April 2001 to March 2004, and then had a singleton pregnancy of at least 24 weeks of gestation until January 2009; n = 2534 Inclusion criteria: only the first pregnancy following the smear, colposcopy, or treatment Exclusion criteria: women who were pregnant at the time of screening/colposcopy/treatment during the study period; women in the colposcopy and treatments groups in the study period who had a history of colposcopy or treatment to the cervix; women who had negative smears up to and during the study period and a subsequent abnormal smear, colposcopy or treatment during the follow‐up period |
|
| Interventions | Single excision NOS (LLETZ, CKC); single ablation NOS (LA, CC, CT); multiple treatments (either multiple excisions, either multiple ablations, or both); other | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (single cone); PTB (< 37 weeks) (repeat cones); PTB (< 37 weeks) (singleton pregnancies); PTB (< 32 weeks); PTB (< 28 weeks); LBW (< 2500 g) | |
| Notes | At the bottom of the table 2 (page 240) it is stated that the percentages are based on the babies for whom the gestational age and the birth weight was known. However, the authors do not give more information about how many babies in each category had a known gestational age and a known birth weight. Thus, i) we calculated the total number of babies with a known gestational age in each category according to the rate of PTB < 37 weeks ii) we calculated the total number of babies with a known birth weight in each category according to the rate of LBW < 2500 g. Because the percentages were rounded, this calculation was not very precise. The addition of the numbers of babies with a known gestational age of all categories diverged from the known number of babies with a known gestational age given at the bottom of the table. The same for the babies with a known birth weight. Because the divergence was small, we decided not to make any other correction. Women that had 'other treatments' were not included in our meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For 225 women (0.1% of the total initial study population) no unique identifier (such as NHS number or date of birth) was available and these women were excluded. 18,512 women (10.6% of the eligible for data linkage women) died in the follow‐up period or were no longer Welsh residents. 2.6% of the babies had an unknown gestational age and 0.4% of the babies had an unknown birth weight. |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women having cervical treatment in Wales (April 2001 to March 2004) and then delivering in Wales (till January 2009) (a population‐based study) |
| Representative comparison group? | Low risk | Both untreated groups were drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Regression analysis for maternal age at birth, social deprivation, smoking status, time interval between screening/colposcopy/treatment and conception, any history of a previous adverse pregnancy outcome (and gestational age for LBW outcome |
Sadler 2004.
| Methods | Retrospective cohort study Comparison group: Women with colposcopy but no treatment ‐ unmatched; regressional analysis for age, ethnicity, socioeconomic status, smoking in pregnancy, previous obstetric history, transfer to the National Women's Hospital and antepartum haemorrhage Information source ‐ linking the colposcopy service database at National Women’s Hospital (NWH), Auckland, for the years 1988 through 1999 with the obstetric database at the same hospital for deliveries from 1989 through 2000. |
|
| Participants | Treated group ‐ 652 women whose pregnancy occurred after treatment with LA, LC, or LLETZ. Inclusion criteria: Women who were seen and/or treated at the colposcopy clinic (1988 to 1999), subsequently carried a singleton pregnancy to at least 20 completed weeks' gestation, and delivered or had postpartum care at the study hospital (1989 to 2000). Only the first qualifying pregnancy per woman was included in the study. Exclusion criteria: Women whose prior treatment status was unknown. Women treated by modes other than LA, LC and LLETZ, and women who had cervical treatments before 1988 Untreated group ‐ 426 women who had a pregnancy following a visit to the colposcopy service but before no cervical treatments that may have been administered. A pregnancy was included only if the visit to the colposcopy clinic or treatment occurred before the first menstrual period was missed (i.e. last menstrual period + 30 days) |
|
| Interventions | LC; LLETZ; LA | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (single cone); PTB (< 37 weeks) (repeat cones); PTB (< 37 weeks) (singleton pregnancies); PTB (< 37 weeks) (D ≤ 10 mm); PTB (< 37 weeks) (D = 11 mm to 16 mm); PTB (< 37 weeks) (D ≥ 17 mm); PTB (< 32 weeks); sPTB (< 37 weeks); pPROM | |
| Notes | From the colposcopy database of 9226 women, 1208 women were found to have had a singleton live birth of at least 20 weeks’ gestation, by linkage with the obstetric records. Of these, 27 were excluded for invalid treatments (13 cryotherapy, 8 Cartier biopsies, and 6 coldknife conisations), 10 for unknown previous treatment status, and 93 because of previous treatment, leaving a cohort of 1078 women who had given birth following treatment or their first encounter at the colposcopy clinic. The clinical records of 1020 women (95%) were abstracted in full. Data were obtained from the database for women whose colposcopy or obstetric records could not be located (4%). In the treated group, 606 women had one treatment before pregnancy, 44 two treatments and two three treatments. The proportion of women in treated vs untreated group with histology: HPV/CIN1 (32.1% vs 46.6%); CIN2/3/AIS (61.7% vs 5.2%); Microinvasion (0.9% vs 0%); No dysplasia/other diagnosis (2% vs 21.1%); none (3.4% vs 27%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from hospital records; small number of incomplete data |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women that had LA, LC or LLETZ at the National Women's Hospital (NWH); NWH is the principal provider of public colposcopy services and inpatient obstetric care to women in the central Auckland area |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Regressional analysis for age, ethnicity, socioeconomic status, smoking in pregnancy, previous obstetric history, transfer to the National Women's Hospital and antepartum haemorrhage. |
Sagot 1995.
| Methods | Retrospectice cohort study Comparison group: Internal (pre‐treatment pregnancies) Information source ‐ Hospital records of the Hospital Mere‐Enfant, Nantes, France |
|
| Participants | Treated group ‐ 54 women (53 pregnancies) under 39 years of age who had undergone LC between 1 July 1982 and 30 June 1992 and had one or more pregnancies after their operation. Untreated group: 38 women of the treated group (59 pregnancies) who had also a pregnancy before treatment Inclusion criteria: Only pregnancies leading to the birth of a child were included. |
|
| Interventions | LC ‐ two different techniques: Before 1986, hand‐held laser (10/54) under GA with 2 stitches, cone‐shaped 1 cm to 2 cm deep, radius 1 cm to 1.5 cm, LA for haemostasis ‐ After 1986, micromanipulator (44/54), less radical, cylinder, 0.8 cm to 1.8 cm deep, radius 0.6 cm to 0.8 cm. | |
| Outcomes | PTB (< 37 weeks); Threatened PTL; pPROM; CS; chorioamnionitis; cervical cerclage | |
| Notes | Between 1 July 1982 and 30 June 1992, 222 women under 39 years of age underwent CO2 laser conisation for CIN. 27 had subsequent hysterectomy or tubal sterilisation, and 48 others could not be recontacted. Thus, 147 women were available for study. Of these 147 women, 54 had a total of 71 pregnancies after the operation. Of these 71 pregnancies, 53 led to the birth of a live child (two sets of twins). These 53 pregnancies were included in the treated group. Of the 54 women which made out the treated group, 38 had also a pregnancy before the operation. These 38 women had a total of 82 pregnancies before the operation. Of these 82 pregnancies, 59 (all monofetal) led to the birth of a live child. These 59 pregnancies were included in the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 222 women who underwent LC between 1 July 1982 and 30 June 1992, 48 (21.6%) could not be recontacted. |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds. |
| Representative intervention group? | Low risk | All women that had LC in a single university hospital in France between July 1982 to June 1992 |
| Representative comparison group? | Low risk | Internal comparison group (pre‐treatment pregnancies) |
| Comparability of treatment groups? | Low risk | Internal comparison group (pre‐treatment pregnancies) |
Samson 2005.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age (+/‐ 1 year), parity (nulliparous/parous), smoking status (yes/no/unknown), year of delivery (+/‐ 1 year) Information source ‐ the Provincial Cytology/Colposcopy Registry (information about all women who have had colposcopy and treatment for CIN in Nova Scotia since 1992) and the Nova Scotia Atlee Perinatal Database (information about all pregnancies and deliveries in Nova Scotia carried beyond 20 weeks of gestation since 1988) |
|
| Participants | Treated group ‐ 571 women who had LLETZ in Halifax County between 1992 and 1999 and then had a subsequent singleton pregnancy of greater than 20 weeks of gestation with delivery at the IWK Health Centre in Halifax, Nova Scotia. Only the first delivery after treatment was included. Untreated group ‐ 571 women from Halifax County with no history of cervical surgery who delivered at the IWK Health Centre beyond 20 weeks of gestation. Each control was randomly selected from a pool that included all who matched to a specific case. Exclusion criteria (for both groups): Women who had known major risk factors for PTB, including previous PTB and multiple gestations. Multiple gestations were analysed separately. Women who had an indicated PTB for maternal or fetal reasons. |
|
| Interventions | LLETZ | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (single cone); PTB (< 37 weeks) (repeat cones); PTB (< 37 weeks) (singleton pregnancies); PTB (< 37 weeks) (multiple pregnancies); PTB (< 34 weeks); PTB (< 34 weeks) (multiple pregnancies); pPROM; CS; induction of labour; oxytocin use; LBW (< 2500 g); NICU admission; perinatal mortality; stillbirth | |
| Notes | Using the Provincial Cytology/Colposcopy Registry database, the authors found that 3056 women had a total of 3315 LLETZ in Halifax County between 1992 and 1999. When the Provincial Cytology/Colposcopy Registry database was linked with the Atlee Database, 1629 women were matched, indicating that they had a pregnancy and delivery at some time in their lives. Of the 1629 matches, 876 (54%) had deliveries only before their LEEP. Of the 753 women with deliveries after their LEEP, 122 (16%) were excluded because they were not Halifax County residents. Additional exclusions were made for indicated preterm delivery, previous preterm delivery, and missing matching variables (parity, age, delivery date), constituting another 50 cases in total. Thus, after appropriate exclusions, there were 581 women who had at least one delivery after their LEEP, 571 singleton, and 10 twin pregnancies. The multiple gestations were excluded from the primary analysis, leaving 571 women in the study group. The authors then retrieved a matched comparison group, consisting of 571 women. In a separate analysis, for the 10 twin pregnancies a matched comparison group was selected in a 5:1 ratio. 5 appropriate matches could not be found for each study patient, resulting in 35 women in the comparison group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The information was obtained from official databases; small number of incomplete data |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women having LLETZ in Halifax County and then delivering at the IWK Health Centre |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for age (+/‐ 1 year), parity (nulliparous/parous), smoking status (yes/no/unknown), year of delivery (+/‐ 1 year) |
Saunders 1986.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age (+/‐5 years), parity, race, year of delivery and singleton pregnancy Information source ‐ Hospital case notes and contact with local general practitioners |
|
| Participants | Treated group ‐ 97 pregnancies of 96 women who had previously undergone LA Exclusion criteria: pregnancies ending in the 1st trimester (miscarriage or TOP); women having CKC before LA Untreated group ‐ 97 pregnancies of women booking around 13 to 16 weeks' gestation, with no history of treatment and with recorded pregnancy outcomes |
|
| Interventions | LA | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (single cone); PTB (< 37 weeks) (repeat cones); pPROM; CS; instrumental deliveries (forceps); LBW (< 2500 g); perinatal mortality | |
| Notes | By consulting hospital case notes and contacting local general practitioners, it was possible to identify 100 pregnancies which had progressed beyond the first trimester in a group of 99 women previously treated with by laser vaporisation cone for CIN. Of the 99 patients, 3 had knife cut cone biopsy followed by laser vaporisation employed at a subsequent date because of recurrent CIN, and because of this were excluded from the main analysis. Thus, 96 patients with 97 pregnancies were included (1 patient had two consecutive normal term deliveries). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A part of the data were obtained from contact with local general practitioners (a non‐systematic approach) |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | Women were selected if they had LA and subsequent fell pregnant at Sheffield |
| Representative comparison group? | Low risk | The untreated population was drawn from the same source as the treated group |
| Comparability of treatment groups? | Unclear risk | Matching for age (+/‐5 years), parity, race, year of delivery and singleton pregnancy. Even though it was found that the percentage of smokers was much higher in the treated that the control group (48% vs 26%), the researchers did not match for smoking. |
Shanbhag 2009.
| Methods | Retrospective cohort study Comparison groups: A) External B) Women with CIN 3 but no treatment Both were unmatched but had regression analysis for maternal age at delivery, smoking, socioeconomic status, year of delivery, birth weight, malpresentation, sPTB and pPROM Information source ‐ Scottish Cancer Registry, Scottish Morbidity Record, National Health Service Scotland Information and Statistics Division |
|
| Participants | Treated group ‐ 1388 women (from the whole Scotland) with CIN 3 diagnosed by histology, treated with excisional (CKC = 2; LC = 4; LLETZ = 1097) or ablative treatment (LA = 84, cold coagulation = 181, diathermy coagulation = 20) and subsequently having a first pregnancy that ended between 1980 to 2005 Untreated group ‐ A) 119216 women (from the whole Scotland) without a record of CIN, whose first pregnancies ended between 1980 to 2005; B) 87 women (from the whole Scotland) with CIN3 diagnosed by histology who had a first pregnancy (that ended between 1980 to 2005) without previously receiving treatment for the CIN 3 Inclusion criteria (for all groups): women who delivered between the ages of 20 and 45 years inclusive, at a gestational age of 24 to 43 weeks, neonatal birth weight more than 350 g, and, in case of CIN 3, women who were diagnosed at the age of 20 or older |
|
| Interventions | Excision NOS (CKC, LC, LLETZ); Ablation (LA, CC, diathermy coagulation) | |
| Outcomes | PTB (< 37 weeks); sPTB (< 37 weeks); pPROM; CS; LBW (< 2500 g); perinatal mortality | |
| Notes | For 1638 women (1638/3113 = 52.6%) with CIN 3, the type of therapy (if any) was not known. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Information was obtained from national registries. For 52.6% of the treated population, the treatment methods (if any) were not known and these were not included in the analysis. |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women in Scotland having a delivery during 1980 to 2005 (a population‐based study) |
| Representative comparison group? | Low risk | Both untreated groups were drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Regression analysis for maternal age at delivery, smoking, socioeconomic status, year of delivery, birth weight, malpresentation, sPTB, pPROM |
Simoens 2012.
| Methods | Prospective cohort study Comparison group: External ‐ matching for admittance in the same maternity ward; regression analysis for age, parity, ethnicity, smoking, education, HIV status Information source ‐ a centralised web‐based database of the four participating Belgian academic hospitals ("Hopital de la Citadelle" and "Centre Hospitalier du Bois de l’Abbaye (CHBAH)" in Liege, "Hopital St. Pierre" and "Hopital Erasme" in Brussels); questionnaires in combination with checking of obstetrical medical files |
|
| Participants | Treated group ‐ 97 women who had undergone excisional or ablative treatment for CIN and then delivered at one of the four participating Belgian academic hospitals during September 2008 to November 2010 Untreated group ‐ 194 women who delivered at one of the four participating Belgian academic hospitals during September 2008 to November 2010 and who never had treatment for CIN or a history of CIN (the next two women after each case meeting these criteria and who delivered in the same maternity ward) Inclusion criteria: only women with a singleton pregnancy Exclusion criteria: women with insufficient data on previous treatment, without a nonexposed cluster, lacking pregnancy outcome data and unexposed women in excess |
|
| Interventions | LC, LLETZ, Excision NOS (CKC, LC, LLETZ) +/‐ Ablation NOS (LA, CC, CT) | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (D ≤ 10 mm); PTB (< 37 weeks) (D> 10 mm); PTB (< 37 weeks) (singleton pregnancies); PTB (< 32 weeks); sPTB (< 37 weeks); sPTB (< 32 weeks); CS; LBW (< 2500 g) | |
| Notes | Of the 97 treated women, 81 received an excisional treatment, eight an ablative treatment and eight an excisional treatment followed by ablation (mixed) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information was obtained from hospital records and questionnaires in combination with checking of medical files |
| Selective reporting (reporting bias) | Unclear risk | The results are presented analytically only for the following 3 treatment categories: i) only excision or excision in combination with ablation ii) LLETZ iii) LC; the remaining treatments only had a small number of patients and were not presented |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women delivering at 4 university hospitals during September 2008 to November 2010 |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for admittance in the same maternity ward; regression analysis for age, parity, ethnicity, smoking, education, HIV status |
Sjoborg 2007.
| Methods | Retrospective cohort study Comparison groups: A) External ‐ matching for age (+/‐ 2 years), parity and plurality B) Internal (self‐matching) Both had regression analysis for smoking, marital status and education Information source ‐ Records of the eight hospitals that participated in this regional multicentre study (Oestfold Hospital Trust, Soerlandet Hospital Trust, Vestfold Hospital Trust, Telemark Hospital Trust, Baerum Hospital, Buskerud Hospital Trust, Ringerike Hospital, Rikshospitalet University Hospital) |
|
| Participants | Treated group ‐ 742 women who had undergone LC or LLETZ (LC: 609; LLETZ: 133) during 1990 to 1999 at one of the eight participating hospitals, were 40 years or younger at the time of operation, subsequently had a pregnancy progressing beyond 16 weeks, and gave their permission to the researchers to collect information about their pregnancy from their medical records in the hospital where they delivered. Only the first pregnancy after treatment was included Untreated group ‐ A) 742 women without previous treatment who delivered at one of the eight participating hospitals (the first subsequent after case delivering woman matched by age, parity and plurality) B) In the treated group, 419 women had delivered before the treatment as well. Thus, it is also possible to compare their first pregnancy after treatment with their first pregnancy before treatment. |
|
| Interventions | Excision NOS (LC, LLETZ) | |
| Outcomes | PTB (< 37 weeks); PTB (< 32 weeks); PTB (< 28 weeks); pPROM; LBW (< 2500 g); LBW (< 1500 g); LBW (< 1000 g); perinatal mortality | |
| Notes | Because all women included in this study have been also included in Albrechtsen 2008, we excluded it from the analyses in which Albrechtsen 2008 has been also included. A regional, multi‐centre, retrospective case‐control study was designed to investigate pregnancy outcome after LC or LLETZ compared to a control group of pregnant women without such a procedure. Women who underwent either LC or LLETZ in the period from January 1, 1990 to December 31, 1999, were investigated for reproductive events. Those who were 40 years of age or younger at the time of operation were contacted in writing with information about the study and a request for permission to collect relevant information from their medical records in the hospitals where they had given birth. A total of 2,393 women were contacted, and 742 women (31%) answered our request. The non‐responding group consisted of both true non‐responders who had given birth after conisation, and patients who had not given birth after conisation. Written consent was not collected for the controls, since their data were extracted anonymously from the birth registries of the participating hospitals. In the external treated group, 7 women had a second‐trimester miscarriage. In the article, in the tables with the results, these women were not included. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The women who had undergone LLETZ or LC were contacted in writing so that the researchers would obtain their consent to collect information about their probable future pregnancies. Only 742 (31%) responded and gave their consent. The non‐responding group consisted of both true non‐responders who had given birth after conisation, and patients who had not given birth after conisation. |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | High risk | The women who had undergone LLETZ or LC were contacted in writing so that the researchers would obtain their consent to collect information about their probable future pregnancies. Only 742 (31%) responded and gave their consent. The non‐responding group consisted of both true non‐responders who had given birth after conisation, and patients who had not given birth after conisation. The women who replied are more likely to have a higher level of education. |
| Representative comparison group? | Low risk | A) The untreated group was drawn from the same source as the treated group B) Internal comparison group (self‐matching) |
| Comparability of treatment groups? | Low risk | A) Matching for age (+/‐2 years), parity and plurality B) Internal comparison group (self‐matching) Both comparison groups had regression analysis for smoking, marital status and education |
Sozen 2014.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age, parity and obstetric history Information source ‐ Records of the Zeynep Kamil Women's Hospital, Istanbul |
|
| Participants | Treated group ‐ 15 women who had undergone CKC at the Zeynep Kamil Women's Hospital during 2005 to 2010 and subsequently had a pregnancy (beyond 20 weeks of gestation) Inclusion ‐ 24 women who had a pregnancy (beyond 20 weeks of gestation) without a history of cervical intervention Exclusion criteria (for both group) ‐ miscarriages |
|
| Interventions | CKC | |
| Outcomes | PTB (< 37 weeks); pPROM; NICU admission | |
| Notes | Among 392 patients who had CKC at Zeynep Kamil Women's Hospital (a tertiary referral teaching institution in Istanbul, Turkey) between the years 2005 to 2010, 22 had a subsequent pregnancy. 7 of those pregnancies resulted in miscarriage and were excluded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | High risk | Very small number of the treated group (n = 15) |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for age, parity and obstetric history |
Spitzer 1995.
| Methods | Retrospective cohort study Comparison group: Internal (pre‐treatment pregnancies) with matching for age and parity Information source ‐ Hospital records of the Colposcopy Clinic at Queens Hospital Centre and every record from the private practice of the authors (for the ascertainment of the exposure); questionnaires by mail, telephone or in person (for the ascertainment of the outcomes) |
|
| Participants | Treated group ‐ 163 livebirths after LC or LA (34 after LC, 129 after LA) Control group ‐ 112 livebirths before LC or LA (15 before LC, 97 before LA) Inclusion criteria: Laser surgery during 1979‐1989. Women under the age of 40 at the time of delivery Exclusion criteria: Pre‐treatment or after‐treatment intervals, during which women were not at risk for pregnancy (e.g. they had tubal ligation or hysterectomy, or their husbands had vasectomies). Pregnancies that were ongoing at the time the women responded to the questionnaire. After‐treatment intervals for which no appropriate pre‐treatment interval was found. |
|
| Interventions | LC; LA | |
| Outcomes | PTB (< 37 weeks) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The researchers used questionnaires to acquire information about previous pregnancies. Of the initial 1069 women fulfilling the inclusion criteria, only 512 (47.9%) responded. |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | High risk | Questionnaires were used for the ascertainment of the outcomes of previous pregnancies. There is a risk of recall bias and misclassification of the outcome. |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | High risk | Almost half of the women did not reply to the questionnaires. The women that replied are more likely to have a higher educational level. |
| Representative comparison group? | Low risk | Internal comparison group (pre‐treatment pregnancies) with matching for age and parity |
| Comparability of treatment groups? | Low risk | Internal comparison group (pre‐treatment pregnancies) with matching for age and parity |
Stout 2015.
| Methods | Retrospective cohort study Comparison groups: A) Women with cervical cytology/punch biopsy ‐ matching for age, hospital site and calendar year of cervical procedure (LLETZ, cervical cytology, punch biopsy) B) Internal (pre‐treatment pregnancies) Information source ‐ electronic pathology databases of several tertiary and community hospitals; structured phone interviews in combination with checking of medical records |
|
| Participants | Treated group ‐ 598 women who had undergone LLETZ at the participating tertiary and community hospitals between 1996 and 2006 and subsequently had a pregnancy Untreated group ‐ A) 1129 women who had undergone cervical cytology (580) or cervical punch biopsy (549) at the participating hospitals during the same period and subsequently had a pregnancy B) Internal (pre‐treatment pregnancies of the women with LLETZ) Inclusion criteria (for all groups):only the first singleton pregnancy beyond 20 weeks of delivery following the cervical procedure Exclusion criteria (for all groups): women with a history of CKC; women without gestational age of delivery recorded; women with a cervical procedure outside the participating hospitals; women with medically indicated PTB (e.g. pre‐eclampsia, intrauterine growth restriction, non‐reassuring fetal testing) |
|
| Interventions | LLETZ | |
| Outcomes | sPTB (< 37 weeks); sPTB (< 37 weeks) (singleton pregnancies); sPTB (< 34 weeks) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The gestation age at delivery was unknown for < 6% of the population |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women having LLETZ at several hospitals during 1996 to 2006 |
| Representative comparison group? | Low risk | A) The untreated group was drawn from the same source as the treated group B) Internal comparison group (pre‐treatment pregnancies) |
| Comparability of treatment groups? | Low risk | A) The untreated group was women with cervical cytology/punch biopsy matched for age, hospital site and calendar year of cervical procedure B) Internal comparison group (pre‐treatment pregnancies) |
Tan 2004.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age and parity Information source ‐ Hospital records of Basildon District Hospital |
|
| Participants | Treated group ‐ 119 women 35 years old or younger who had undergone LLETZ and delivered in Basildon District Hospital, between 1995 and 1998. Only first pregnancies following treatment were included. Control group ‐ 119 women who had not had colposcopy/LLETZ and delivered in Basildon District Hospital between 1995 and 1998. |
|
| Interventions | LLETZ | |
| Outcomes | PTB (< 37 weeks); CS; instrumental deliveries (ventouse/forceps); prolonged labour (> 12 hours); induction of labour; oxytocin use; epidural use; pethidine use | |
| Notes | 168 women 35 years old or younger had undergone LLETZ and delivered in Basildon District Hospital, between 1995 and 1998. Of these, 119 women were included in the treated group and the others were excluded, because their notes could not be retrieved. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 168 women were eligible for the study. Of these, 49 (29.2%) were excluded because their notes could not be retrieved, with no further details given by the authors. |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious. |
| Other bias | High risk | There are some contradictions in the presented data; e.g. although it is stated that there are 14/119 and 11/119 miscarriages in the treated and untreated group, respectively, it later presents the mode of delivery in 119 women in each group |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds. |
| Representative intervention group? | Low risk | All eligible women delivering in a single hospital between 1995 and 1998. |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Unclear risk | Matching for age and parity. Smoking was higher in the treated group (52.3%) than in the untreated group (24.4%). |
Turlington 1996.
| Methods | Retrospective cohort study Comparison group: Women with colposcopically directed biopsy but no treatment ‐ regression analysis for age Information source ‐ Hospital records of New Havover Regional Medical Center, telephone interviews/mail‐in questionnaires (for reproductive history since their visit to the colposcopy clinic) |
|
| Participants | Treated group ‐ 15 women between the ages of 16 and 40 who were treated with LEEP for CIN at New Havover Regional Medical Center, Wilmington, North Carolina, between 1991 and 1992, and then had a pregnancy which led to delivery Untreated group ‐ 15 women of the same range of age who had been seen in the colposcopy clinic during the same period for abnormal smear but who did not receive excisional or ablative therapy, and then had a pregnancy which led to delivery Exclusion criteria: Women who were anatomically unable to become pregnant as a result of permanent sterilization or hysterectomy. |
|
| Interventions | LLETZ | |
| Outcomes | Stillbirth | |
| Notes | Between January 1991 and December 1992, 647 women were evaluated and treated for abnormal cervical cytologic smears in the colposcopy clinic at New Hanover Regional Medical Centerm a 630‐bed hospital in Wilmington, North Carolina, that serves as a referral centre for southeastern North Carolina. Using telephone interviews and mail‐in questionnaires, 158 women between the ages of 16 and 40 who had undergone colposcopy for abnormal smears were contacted and questioned about their reproductive history since their visit to the colposcopy clinic (79 destined for the treated group and 79 destined for the control group). In the treated group, 54 women replied and were included in the analysis. In the control group, 57 women replied and were included in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 158 women surveyed, 111 (70.3%) responded to the questionnaire or telephone interview; 29.7% did not reply. |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | High risk | Questionnaires and telephone interviews were used for the ascertainment of the outcome; there is a risk of recall bias and misclassification of the outcome |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | High risk | Questionnaires or telephone interviews were used for the selection of the treated group; the women who replied are more likely to belong to a higher socioeconomic class |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | The untreated group was taken from women that were seen in the colposcopy clinic; regression analysis for age |
van de Vijner 2010.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age (+/‐2 years), parity and year of delivery (+/‐2 years) Information source ‐ electronic and paper patient files at the University Hospital of Leuven and questionnaires |
|
| Participants | Treated group ‐ 55 pregnancies (beyond 22 weeks of gestation) of 43 women who had undergone LLETZ (40 patients; 50 pregnancies) or LC (3 patients, 5 pregnancies) during 1999 to 2003 at the University Hospital of Leuven and subsequently delivered before 2007. Exclusion criteria: pregnancies that resulted in a miscarriage Untreated group ‐ 55 pregnancies of 54 women who had no intervention on the cervix or were never diagnosed with CIN |
|
| Interventions | Excision NOS (LC, LLETZ) | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (single cone); PTB (< 37 weeks) (repeat cones); PTB (< 37 weeks) (singleton pregnancies); PTB (< 37 weeks) (multiple pregnancies); PTB (< 34 weeks); threatened PTL; pPROM; CS; instrumental delivery (ventouse/forceps); induction of labour; oxytocin use; LBW (< 2500 g); NICU admission; perinatal mortality; stillbirth | |
| Notes | 599 women underwent a conisation of the cervix (LLETZ, laser or cold knife) between 1 January 1999 and 31 December 2003 in the University Hospital in Leuven. In this group, 47 women could be identified who became pregnant after the intervention and delivered before 1 January 2007. The study concerned 72 pregnancies. Seventeen (23.6%) resulted in a miscarriage and were excluded. Finally, the treated group consisted of 55 evolutive pregnancies (delivery after 22 weeks of gestation) in 43 women. There were nine women with two and two women with three subsequent pregnancies. 2 women in the treated group had two conisations (4 pregnancies). In the control group there were 3 twin pregnancies. In the control group there was one twin pregnancy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Questionnaires were sent to 599 women to identify who of them became pregnant after the intervention. The authors do not give information about how many replied; they just write that 47 of them fell pregnant. |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | High risk | In some outcomes the population increases and there is no description where these additional pregnancies come from (e.g. NICU admission 15/58 in the treated group vs 9/58 in the untreated group, although the treated group consists of 55 pregnancies and so does the untreated group). The use of questionnaires for the outcomes increases the risk of recall and misclassification bias. |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Unclear risk | There may be women that did not reply to the questionnaire. This information is not provided by the authors. |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Unclear risk | Matching for age (+/‐2 years), parity and year of delivery (+/‐2 years). The treated group, in comparison with the untreated group, had more sexual partners (4.6 vs 2.5) and smoked more before pregnancy (50% vs 20.4%). Differences in smoking during pregnancy, socioeconomic status and education level were not statistically significant. |
Van Hentenryck 2012.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age at delivery, parity, smoking, history of gestation and HIV status Information source ‐ the electronic databases of the Gynecopathologic and Obstetrics Departments of Erasme University Hospital, Belgium |
|
| Participants | Treated group ‐ 106 deliveries of 88 women who had undergone excisional treatment for CIN at the Erasme University Hospital between 1999 to 2010 and then delivered at the same hospital between 2000 to 2010 (CKC = 14; LC = 33; LLETZ = 40; unknown = 1) Untreated group ‐ 212 deliveries of 176 women who delivered at the Erasme University Hospital between 2000 to 2010 without previous excision (the first preceding and following women after each case fulfilling the matching criteria) |
|
| Interventions | Excision NOC (CKC, LC, LLETZ) | |
| Outcomes | PTB (< 37 weeks); PTB (< 34 weeks); threatened PTL; pPROM; chorioamnionitis; CS; instrumental deliveries (ventouse); induction of labour; LBW (< 2500 g); NICU admission | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | High risk | There were inconsistencies in the numbers reported in the tables and the text |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women undergoing excisional treatment (1999 to 2010) and delivering (2000 to 2010) at a single university hospital |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for age at delivery, parity, smoking, history of gestation, HIV status |
van Rooijen 1999.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age (+/‐ 3 years), parity and year of delivery (same year) Information source ‐ Hospital records of Karolinska Hospital in Stockholm |
|
| Participants | Treated group ‐ 236 women who had undergone LA and delivered in Karolinska Hospital in Stockholm, between 1982 and 1992. Only first delivery after treatment was included. Women who had undergone previous conisation (8) or reoperation with cervical conisation before pregnancy (11) were excluded. Untreated group ‐ 472 women (2 controls for each case) without previous treatment who delivered in the same hospital during the same period. The controls were selected from the birth registry of the hospital. |
|
| Interventions | LA | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (single cone); CS; APH; LBW (< 2500 g); LBW (< 2000 g); LBW (< 1500 g); LBW (< 1000 g) | |
| Notes | During the 11‐year period 1982 to 1992 a total of 1828 women underwent laser vaporization of the uterine cervix at the Department of Obstetrics and Gynaecology, Karolinska Hospital in Stockholm. Until the end of 1992, 298 of these women gave birth to at least one child after operation. 62 of them were excluded from the study because of previous conisation (8), reoperation with cervical conization before pregnancy (11) or because of incomplete data in their charts (43). The remaining 236 women were included in the treated group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 279 women were eligible for the study. Of these, 46 (16.5%) were excluded because of incomplete data in their charts |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women delivering in a single hospital between 1982 to 1992 |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for age (+/‐ 3 years), parity, year of delivery (same year) |
Weber 1979.
| Methods | Retrospective cohort study Comparison group: External ‐ matching for age Information source ‐ interview (about previous CKC, age, previous pregnancies, education, employment and smoking habits), hospital records (about current pregnancy) |
|
| Participants | Treated group ‐ 44 women with CKC in the past were delivered at Fredriksberg Hospital and Rigshospitalet during March 1974 and December 1975. All pregnancies (48) of these women after CKC were included in the treated group (even if these were not the current ones). Untreated group ‐ All pregnancies (48) of the age‐matched women after the date of the CKC of the cases (even if these were not the current ones). The age‐matched women were delivered at the same hospital and had not had treatment for CIN in the past. |
|
| Interventions | CKC | |
| Outcomes | LBW (<2500g) | |
| Notes | During March 1974 and December 1975, 7327 women were delivered at Fredriksberg Hospital and Rigshospitalet. At their first contact with the antenatal clinics all patients were interviewed by specially employed and trained staff. 44 stated a previous conisation. 17 of the 44 women had had one or more pregnancies between the CKC and the current pregnancy. In total, 5 different treated‐control groups were chosen and five different comparisons were described. We decided to include in our analysis only one of them (described above), in order to avoid a duplicate extraction and analysis of the same participants (patients). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All eligible for the study women were interviewed; the information about the outcome was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | High risk | Interviews were used to pool information about previous CKC. There is a risk of recall bias and misclassification of exposure. |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women delivering in two hospitals during March 1974 to December 1975 |
| Representative comparison group? | Low risk | The untreated group was drawn from the same source as the treated group |
| Comparability of treatment groups? | Low risk | Matching for age |
Werner 2010.
| Methods | Retrospective cohort study Comparison groups: A) External B) Internal (pre‐treatment pregnancies) Both had regression analysis for age, parity and race Information source ‐ records of Parkland Health & Hospital System |
|
| Participants | Treated group ‐ 511 women who had undergone LLETZ at the Parkland Hospital during January 1992‐May 2008 and subsequently had a pregnancy at the same hospital Untreated group ‐ A) 240,348 women who delivered at the Parkland Hospital without previous LLETZ B) Internal population of women that had pregnancies before LLETZ (n = 842) Inclusion criteria (for all groups): Only singletons were included |
|
| Interventions | LLETZ | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (singleton pregnancies); sPTB (< 37 weeks); pPROM; perinatal mortality; stillbirth | |
| Notes | Perinatal mortality was defined as the addition of stillbirths and neonatal deaths up to 28 days of life | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women having LLETZ and then delivering in a single hospital during January 1992 to May 2008 |
| Representative comparison group? | Low risk | A) The untreated group was drawn from the same source as the treated group B) Internal comparison group (pre‐treatment pregnancies) |
| Comparability of treatment groups? | Low risk | A) Regression analysis for age, parity and race B) Internal comparison group (pre‐treatment pregnancies) |
Wuntakal 2013.
| Methods | Retrospective cohort study Comparison groups: A) Women with punch biopsy but no treatment B) Internal (pre‐treatment pregnancies) Both had regression analysis for parity, ethnicity and deprivation Information source ‐ the Pathology and Obstetric databases of the Whipps Cross University Hospital |
|
| Participants | Treated group ‐ 261 deliveries of women who had undergone excisional treatment for CIN (LLETZ, LC, CKC) during 1995 to 2009 at the Whipps Cross University Hospital and subsequently delivered at the same hospital Untreated group ‐ A) 257 deliveries of women who had only punch biopsy before delivery B) The treated group had 181 deliveries before the treatment. These deliveries can be compared with the deliveries after the treatment Inclusion criteria (for all groups): singleton pregnancies with a gestational age of 26 to 42 weeks of gestation. Exclusion criteria: antepartum stillbirths |
|
| Interventions | Excision NOS (CKC, LC, LLETZ) | |
| Outcomes | PTB (< 37 weeks); PTB (< 37 weeks) (single cone); PTB (< 37 weeks) (repeat cones); PTB (< 33 weeks); pPROM; CS; instrumental deliveries (ventouse/forceps); LBW (< 2500 g) | |
| Notes | The patients of this study are also included in the paper by Castanon and colleagues BMJ 2012. As this is a duplicate study we only included in the analysis the outcomes that were not presented in Castanon 2012. The outcomes on PTB < 37 weeks and PTB < 33 weeks were not included in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information was obtained from hospital records |
| Selective reporting (reporting bias) | Low risk | No reporting bias is obvious |
| Other bias | Low risk | No other obvious source of bias |
| Relevant assignment described? | Low risk | Yes, treatment performed on clinical grounds |
| Representative intervention group? | Low risk | All eligible for the study women having treatment and delivering in a single university hospital during 1995 to 2009 |
| Representative comparison group? | Low risk | A) The untreated group was drawn from the same source as the treated group B) Internal comparison group (pre‐treatment pregnancies) |
| Comparability of treatment groups? | Low risk | A) Women with punch biopsy only B) Internal comparison group (pre‐treatment pregnancies) Both had regression analysis for parity, ethnicity and deprivation |
APH: antepartum haemorrhage; BMI: body mass index; CIN: cervical intra‐epithelial neoplasia; CKC: cold knife conisation; CS: caesarean Section; CT: cryotherapy; FCBE: Fischer cone biopsy excisor; LA: laser ablation; LBW: low birth weight; LC: laser conisation; LLETZ: large loop excision of the transformation zone; MOH: major obstetric haemorrhage;NICU: neonatal intensive care unit; NOS: not otherwise specified; PPH: Postpartum haemorrhage; pPROM: preterm premature rupture of membranes; PTB: preterm birth; PTL: preterm labour; RD: radical diathermy; sPTB: spontaneous preterm birth; TOP: termination of pregnancy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Halal 2013 | Comparison of women with CIN (+/‐treatment) versus general population. We could not include this in any comparison as we had no information as to whether women with CIN had or not treatment and the increase in the PTB could be attributed to either. |
| Althuisius 2001 | No untreated comparison group |
| Berghella 2004 | No untreated comparison group |
| Berretta 2013 | No untreated comparison group |
| Bull‐Phelps 2007 | No untreated comparison group |
| Chevreau 2017 | No untreated comparison group |
| Ciavattini 2014 | Both the treated and untreated group consisted of a high‐risk population (women that conceived via assisted reproductive technology (ART)) |
| Ciavattini 2015 | This study reports the miscarriage rates in the treated and untreated group but none of the outcomes that we are studying |
| Conner 2013 | No untreated comparison group |
| Ferenczy 1995 | No untreated comparison group |
| Gentry 2000 | This study reports the cervical length before and after LLETZ (self‐matching), but we do not study this outcome |
| Gordon 1991 | No untreated comparison group |
| Gronroos 1979 | No untreated comparison group |
| Kalliala 2012 | This study reports the Incidence of any pregnancy, livebirths, miscarriages, extrauterine pregnancies, molar pregnancies, and terminations of pregnancies (TOPs), but none of the outcomes that we are studying |
| Khalid 2012 | No untreated comparison group |
| Kim 2016 | No untreated comparison group |
| Kindinger 2016 | No untreated comparison group |
| Kullander 1971 | No untreated comparison group |
| Leiman 1980 | No untreated comparison group |
| Liu 2014 | No untreated comparison group |
| Liverani 2016 | No untreated comparison group |
| Macvicar 1968 | No untreated comparison group |
| Mariya 2016 | No untreated comparison group |
| Masamoto 2008 | No untreated comparison group |
| Michelin 2009 | No untreated comparison group |
| Mitsuhashi 2000 | 1) Treatment during pregnancy 2) No untreated comparison group |
| Monaghan 1982 | No untreated comparison group |
| Naleway 2015 | This study reports the percentage of the women that fell pregnant in the treated and untreated group but not the pregnancy outcomes |
| Nam 2010 | No untreated comparison group |
| Novikova 1994 | No untreated comparison group |
| Patrelli 2008 | No untreated comparison group |
| Pils 2014 | The untreated comparison group was high risk: consisted only of women with a history of second‐trimester miscarriage |
| Pinborg 2015 | Both treated and untreated groups consisted of a high‐risk population (women that conceived via assisted reproductive technology (ART)) |
| Radha Bai Prabhu 2010 | No untreated comparison group |
| Rafaeli‐Yehudai 2014 | No untreated comparison group (treated women with cerclage versus treated women without cerclage) |
| Ricciotti 1995 | This study reports the cervical length before and after LLETZ (self‐matching), but we are not studying this outcome |
| Rosen 1991 | Treatment during pregnancy |
| Sangkarat 2014 | No untreated comparison group |
| Seki 2010 | 1) Treatment during pregnancy 2) No untreated comparison group |
| Shin 2010 | No untreated comparison group (treated women with cerclage versus treated women without cerclage) |
| Sljivancanin 2013 | Treatment during pregnancy |
| Smaldone 2010 | Comparison of women with CIN (+/‐treatment) versus general population. We could not include this in any comparison as we had no information as to whether women with CIN had or not treatment and the increase in the PTB could be attributed to either. |
| Spracklen 2013 | Data only on fertility |
| Wakita 1990 | No untreated comparison group |
| Watson 2012 | Case‐control study |
| Wongtiraporn 2014 | No untreated comparison group |
| Zuo 2011 | Comparison of women with CIN (+/‐treatment) versus general population. We could not include this in any comparison as we had no information as to whether women with CIN had or not treatment and the increase in the PTB could be attributed to either. |
CIN: cervical intra‐epithelial neoplasia; LLETZ: large loop excision of the transformation zone; PTB: preterm birth
Characteristics of studies awaiting assessment [ordered by study ID]
Aleman 2016.
| Methods | Retrospective cohort study |
| Participants | Women who had undergone conisation at Antwerp University Hospital and subsequently delivered + control group |
| Interventions | Conisation |
| Outcomes | Rates of adverse obstetric outcomes (such as LBW and CS) after conisation and comparison to the control group |
| Notes | Identified after April 2016, week 2; it will be included in the next version of this meta‐analysis |
Bjorge 2016.
| Methods | Population‐based retrospective cohort study |
| Participants | Women from whole of Norway who had undergone treatment for CIN/early invasive cervical cancer during 1998 to 2014 and subsequently had singleton pregnancy + control group |
| Interventions | Treatment for CIN/early invasive cervical cancer |
| Outcomes | Rates of adverse obstetric outcomes (such as PTB) after treatment and comparison to the control group |
| Notes | Identified after April 2016, week 2; it will be included in the next version of this meta‐analysis |
Brie 2016.
| Methods | Retrospective case‐control study |
| Participants | Women who had undergone conisation in a university hospital between January 2002 to January 2012 and subsequently delivered + control group |
| Interventions | Conisation |
| Outcomes | Rates of adverse obstetric outcomes (such as PTB < 37 weeks, PTB < 32 weeks, PTB < 28 weeks and PROM) after treatment and comparison to the control group |
| Notes | Identified after April 2016, week 2; it will be included in the next version of this meta‐analysis |
He 2007.
| Methods | Retrospective study |
| Participants | Women who underwent conisation from 1999 to 2005 in Peking Union Medical College Hospital |
| Interventions | Conisation |
| Outcomes | Fertility and pregnancy |
| Notes | Article in Chinese ‐ pending translation for inclusion in the next update of this meta‐analysis |
Jancar 2016.
| Methods | Population‐based retrospective cohort study |
| Participants | Women from whole Slovenia who had a singleton pregnancy during 2003 to 2012 and had previously undergone CKC or LLETZ + control group |
| Interventions | CKC, LLETZ |
| Outcomes | Rates of adverse obstetric outcomes (sPTB < 37 weeks, < 34 weeks, < 32 weeks, < 28 weeks) after treatment and comparison to the control group |
| Notes | Identified after April 2016, week 2; it will be included in the next version of this meta‐analysis |
Kalitsaris 1991.
| Methods | Retrospective study |
| Participants | Women who underwent conisation from 1967 to 1989 |
| Interventions | Conisation |
| Outcomes | Percentage of attested postoperative pregnancies |
| Notes | Article in Italian ‐ pending translation for inclusion in the next update of this meta‐analysis |
Kasum 1991.
| Methods | Retrospective |
| Participants | Women with previously made conisation of the uterine cervix |
| Interventions | Conisation |
| Outcomes | Term, deliveries, preterm deliveries and spontaneous abortions |
| Notes | Article in Yogoslavian ‐ pending translation for inclusion in the next update of this meta‐analysis |
Lund 1986.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Article in Norwegian ‐ pending translation for inclusion in the next update of this meta‐analysis |
Praest 1979.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Article in Danish ‐ pending translation for inclusion in the next update of this meta‐analysis |
Spuhler 1995.
| Methods | Retrospective study |
| Participants | Women followed at our colpolaparotomy surgery clinic |
| Interventions | Laser CO2 therapy |
| Outcomes | Pregnancy and delivery |
| Notes | Article in French ‐ pending translation for inclusion in the next update of this meta‐analysis |
Zebitay 2017.
| Methods | Population‐based multicentric trial |
| Participants | Turkish women with singleton pregnancy during 2007 to 2013 who had previously undergone CKC + control group |
| Interventions | CKC |
| Outcomes | Rates of PTB after CKC and comparison to the control group; data about removed volume and height of the removed cone and correlation to PTB |
| Notes | Identified after April 2016, week 2; it will be included in the next version of this meta‐analysis |
Zornoza‐Garcia 2009.
| Methods | Retrospective cohort study |
| Participants | Women treated at the Leon Hospital in Spain, between 1999 and 2007 |
| Interventions | Conisation |
| Outcomes | Conception and pregnancy |
| Notes | Article in Spanish ‐ pending translation for inclusion in the next update of this meta‐analysis |
CIN: cervical intra‐epithelial neoplasia; CKC: cold knife conisation; CS: caesarean section; LBW: low birth weight; LLETZ: large loop excision of the transformation zone; PTB: preterm birth; sPTB: spontaneous preterm birth.
Differences between protocol and review
The original protocol was drafted to analyse fertility, early pregnancy and obstetric outcomes in women with a history of treatment for CIN versus untreated controls. Due to the clinical difference of the outcomes and the large number of studies, interventions and outcomes, it was decided to split the review into two. Fertility and early pregnancy outcomes were removed and published in a prior review (Kyrgiou 2015b).
The current review addresses the impact of conservative treatment on obstetric outcomes. We also included two treatment techniques, called needle excision of the transformation zone (NETZ) or straight wire excision of the transformation zone (SWETZ) and Fischer cone biopsy excisor (FCBE), respectively as they are a variation of large loop excision of the transformation zone (LLETZ)/loop electrosurgical excisional procedure (LEEP). We also extended the inclusion criteria to include women treated for early cervical cancer (stage IA1).
Only studies published in English were included as given the large number of included studies and the low quality of these small studies we considered that their inclusion would not alter the conclusions of the review. In future updates we will consider the inclusion of these reports (Characteristics of studies awaiting classification).
We considered both studies with adjusted or unadjusted data and extracted unadjusted data for the analysis as previously described in our protocol. More recent guidance recommends the use of adjusted data and this will be incorporated in future updates of this review.
Contributions of authors
The study was conceived and designed by MK, MA, PB, and EP. MK, AA, and MP acquired the data, which were collated and analysed by MK, AA, IK, and MA. All review authors drafted and critically revised the manuscript for important intellectual content. MA, PB, and EP are joint senior authors. All authors gave final approval of the version to be published and have contributed to the manuscript.
Sources of support
Internal sources
-
Imperial Healthcare NHS Trust NIHR Biomedical Research Centre, UK.
P45272 (MK and PRB)
External sources
-
The British Society for Colposcopy and Cervical Pathology, UK.
Jordan/Singer Award (P47773) (MK)
-
Genesis Research Trust, UK.
P55549 (MK)
-
Sigrid Jusélius Fellowship, Finland.
P52483 (IK and MK)
-
COHEAHR Network, Other.
grant No 603019 (MA); funded by Seventh Framework Programme of DG Research of the European Commission (Brussels); Institut National du Cancer (Paris) through the COSPCC study (Conséquences obstétricales du (sur)traitement des précurseurs du cancer du col); the European Federation of Colposcopy (Birmingham); and the Joint Action CANCON
-
Imperial College Healthcare Charity, UK.
P47907 (AM and MK)
-
Imperial Healthcare NHS Trust Biomedical Researh Centre, UK.
P45272 (MK, PRB)
-
NIHR RfPB, UK.
(MK, IK, PRB)
Declarations of interest
No known conflict of interest for the published work. Details are included in the individual conflict of interest forms completed by each author.
New
References
References to studies included in this review
Acharya 2005 {published data only}
- Acharya G, Kjeldberg I, Hansen SM, Sørheim N, Jacobsen BK, Maltau JM. Pregnancy outcome after loop electrosurgical excision procedure for the management of cervical intraepithelial neoplasia. Archives of Gynecology and Obstetrics 2005;272(2):109‐12. [DOI] [PubMed] [Google Scholar]
Albrechtsen 2008 {published data only}
- Albrechtsen S, Rasmussen S, Thoresen S, Irgens LM, Iversen OE. Pregnancy outcome in women before and after cervical conisation: population based cohort study. BMJ 2008;337:a1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andersen 1999 {published data only}
- Andersen ES, Pedersen B, Boris J. Pregnancy outcome after combination laser conization: a case‐control study. Journal of Gynecologic Surgery 1999;15(1):7‐12. [Google Scholar]
Anderson 1984 {published data only}
- Anderson MC, Horwell DH, Broby Z. Outcome of pregnancy after laser vaporization conization. Journal of Gynecologic Surgery 1984;1(1):35‐9. [Google Scholar]
Andia 2011 {published data only}
- Andia D, Mozo de Rosales F, Villasante A, Rivero B, Diez J, Perez C. Pregnancy outcome in patients treated with cervical conization for cervical intraepithelial neoplasia. International Journal of Gynaecology and 0bstetrics 2011;112(3):225‐8. [DOI] [PubMed] [Google Scholar]
Anwar 2016 {published data only}
- Anwar A, Igbenehi C, Lindow SW, Noor N, Musa S, Saha, A. Pregnancy outcome after electrosurgical cervical cone biopsy using Fischer cone biopsy excisor. Journal of Maternal‐Fetal & Neonatal Medicine 2016;29(3):477‐81. [DOI] [PubMed] [Google Scholar]
Armarnik 2011 {published data only}
- Armarnik S, Sheiner E, Piura B, Meirovitz M, Zlotnik A, Levy A. Obstetric outcome following cervical conization. Archives of Gynecology and Obstetrics 2011;283(4):765‐9. [DOI] [PubMed] [Google Scholar]
Bekassy 1996 {published data only}
- Bekassy Z, Iosif CS. Laser "miniconisation' and the outcome of subsequent pregnancy. Archives of Gynecology and Obstetrics 1996;258(2):75‐9. [DOI] [PubMed] [Google Scholar]
Blomfield 1993 {published data only}
- Blomfield PI, Buxton J, Dunn J, Luesley DM. Pregnancy outcome after large loop excision of the cervical transformation zone. American Journal of Obstetrics and Gynecology 1993;169(3):620‐5. [DOI] [PubMed] [Google Scholar]
Braet 1994 {published data only}
- Braet PG, Peel JM, Fenton DW. A case controlled study of the outcome of pregnancy following loop diathermy excision of the transformation zone. Journal of Obstetrics and Gynaecology 1994;14(2):79‐82. [Google Scholar]
Bruinsma 2007 {published data only}
- Bruinsma F, Lumley J, Tan J, Quinn M. Precancerous changes in the cervix and risk of subsequent preterm birth. BJOG: an international journal of obstetrics and gynaecology 2007;114(1):70‐80. [DOI] [PubMed] [Google Scholar]
Buller 1982 {published data only}
- Buller RE, Jones HW 3rd. Pregnancy following cervical conization. American Journal of Obstetrics and Gynecology 1982;142(5):506‐12. [DOI] [PubMed] [Google Scholar]
Castanon 2012 {published and unpublished data}
- Castanon A, Brocklehurst P, Evans H, Peebles D, Singh N, Walker P, et al. Risk of preterm birth after treatment for cervical intraepithelial neoplasia among women attending colposcopy in England: retrospective‐prospective cohort study. BMJ 2012;345:e5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon A, Landy R, Brocklehurst P, Evans H, Peebles D, Singh N, et al. for the PaCT Study Group. Risk of preterm delivery with increasing depth of excision for cervical intraepithelial neoplasia in England: nested case‐control study. BMJ 2014;349:g6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crane 2006 {published data only}
- Crane JM, Delaney T, Hutchens D. Transvaginal ultrasonography in the prediction of preterm birth after treatment for cervical intraepithelial neoplasia. Obstetrics and Gynecology 2006;107(1):37‐44. [DOI] [PubMed] [Google Scholar]
Cruickshank 1995 {published data only}
- Cruickshank ME, Flannelly G, Campbell DM, Kitchener HC. Fertility and pregnancy outcome following large loop excision of the cervical transformation zone. British Journal of Obstetrics and Gynaecology 1995;102(6):467‐70. [DOI] [PubMed] [Google Scholar]
Ehsanipoor 2014 {published data only}
- Ehsanipoor RM, Jolley JA, Goldshore MA, Szymanski LM, Haydon ML, Gaffaney CL, et al. The relationship between previous treatment for cervical dysplasia and preterm delivery in twin gestations. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(8):821‐4. [DOI] [PubMed] [Google Scholar]
El‐Bastawissi 1999 {published data only}
- El‐Bastawissi AY, Becker TM, Daling JR. Effect of cervical carcinoma in situ and its management on pregnancy outcome. Obstetrics and Gynecology 1999;93(2):207‐12. [DOI] [PubMed] [Google Scholar]
Fischer 2010 {published data only}
- Fischer RL, Sveinbjornsson G, Hansen C. Cervical sonography in pregnant women with a prior cone biopsy or loop electrosurgical excision procedure. Ultrasound in Obstetrics & Gynecology 2010;36(5):613‐7. [DOI] [PubMed] [Google Scholar]
Forsmo 1996 {published data only}
- Forsmo S, Hansen MH, Jacobsen BK, Oian P. Pregnancy outcome after laser surgery for cervical intraepithelial neoplasia. Acta Obstetricia et Gynecologica Scandinavica 1996;75(2):139‐43. [DOI] [PubMed] [Google Scholar]
Frega 2013 {published data only}
- Frega A, Sesti F, Sanctis L, Pacchiarotti A, Votano S, Biamonti A, et al. Pregnancy outcome after loop electrosurgical excision procedure for cervical intraepithelial neoplasia. International Journal of Gynaecology and Obstetrics 2013;122(2):145‐9. [DOI] [PubMed] [Google Scholar]
Frey 2013 {published data only}
- Frey HA, Stout MJ, Odibo AO, Stamilio DM, Cahill AG, Roehl KA, et al. Risk of cesarean delivery after loop electrosurgical excision procedure. Obstetrics and Gynecology 2013;121(1):39‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gunasekera 1992 {published data only}
- Gunasekera PC, Lewis BV, Phipps JH. Obstetric outcome after cervical surgery for intra‐epithelial neoplasia. Journal of Obstetrics and Gynaecology 1992;12(5):291‐3. [Google Scholar]
Guo 2013 {published data only}
- Guo HJ, Guo RX, Liu Y. Effects of loop electrosurgical excision procedure or cold knife conization on pregnancy outcomes. European Journal of Gynaecological Oncology 2013;34(1):79‐82. [PubMed] [Google Scholar]
Haffenden 1993 {published data only}
- Haffenden DK, Bigrigg A, Codling BW, Read MD. Pregnancy following large loop excision of the transformation zone. British Journal of Obstetrics and Gynaecology 1993;100(11):1059‐60. [DOI] [PubMed] [Google Scholar]
Hagen 1993 {published data only}
- Hagen B, Skjeldestad FE. The outcome of pregnancy after CO2 laser conisation of the cervix. British Journal of Obstetrics and Gynaecology 1993;100(8):717‐20. [DOI] [PubMed] [Google Scholar]
Heinonen 2013 {published data only}
- Heinonen A, Gissler M, Riska A, Paavonen J, Tapper AM, Jakobsson M. Loop electrosurgical excision procedure and the risk for preterm delivery. Obstetrics and Gynecology 2013;121(5):1063‐8. [DOI] [PubMed] [Google Scholar]
Hemmingsson 1982 {published data only}
- Hemmingsson E. Outcome of third trimester pregnancies after cryotherapy of the uterine cervix. British Journal of Obstetrics and Gynaecology 1982;89(8):675‐7. [DOI] [PubMed] [Google Scholar]
Himes 2007 {published data only}
- Himes KP, Simhan HN. Time from cervical conization to pregnancy and preterm birth. Obstetrics and Gynecology 2007;109(2 Pt 1):314‐9. [DOI] [PubMed] [Google Scholar]
Jakobsson 2007 {published data only}
- Jakobsson M, Gissler M, Sainio S, Paavonen J, Tapper AM. Preterm delivery after surgical treatment for cervical intraepithelial neoplasia. Obstetrics and Gynecology 2007;109(2 Pt 1):309‐13. [DOI] [PubMed] [Google Scholar]
Jakobsson 2009 {published data only}
- Jakobsson M, Gissler M, Paavonen J, Tapper AM. Loop electrosurgical excision procedure and the risk for preterm birth. Obstetrics and Gynecology 2009;114(3):504‐10. [DOI] [PubMed] [Google Scholar]
Jones 1979 {published data only}
- Jones JM, Sweetnam P, Hibbard BM. The outcome of pregnancy after cone biopsy of the cervix: a case‐control study. British Journal of Obstetrics and Gynaecology 1979;86(12):913‐6. [DOI] [PubMed] [Google Scholar]
Kirn 2015 {published data only}
- Kirn V, Geiger P, Riedel C, Bergauer F, Friese K, Kainer F, Knabl J. Cervical conisation and the risk of preterm delivery: a retrospective matched pair analysis of a German cohort. Archives of Gynecology and Obstetrics 2015;291(3):599‐603. [DOI] [PubMed] [Google Scholar]
Kitson 2014 {published data only}
- Kitson SJ, Greig E, Michael E, Smith M. Predictive value of volume of cervical tissue removed during LLETZ on subsequent preterm delivery: a cohort study. European Journal of Obstetrics and Gynecology and Reproductive Biology 2014;180:51‐5. [DOI] [PubMed] [Google Scholar]
Klaritsch 2006 {published data only}
- Klaritsch P, Reich O, Giuliani A, Tamussino K, Haas J, Winter R. Delivery outcome after cold‐knife conization of the uterine cervix. Obstetrics and Gynecology 2006;103(2):604‐7. [DOI] [PubMed] [Google Scholar]
Kristensen 1985 {published data only}
- Kristensen GB. The outcome of pregnancy and preterm delivery after conization of the cervix. Archives of Gynecology 1985;236(3):127‐30. [DOI] [PubMed] [Google Scholar]
Kristensen 1993 {published data only}
- Kristensen J, Langhoff‐Ross J, Kristensen FB. Increased risk of preterm birth in women with cervical conization. Obstetrics and Gynecology 1993;81(6):1005‐8. [PubMed] [Google Scholar]
Kuoppala 1986 {published data only}
- Kuoppala T, Saarikoski S. Pregnancy and delivery after cone biopsy of the cervix. Archives of Gynecology 1986;237(3):149‐54. [DOI] [PubMed] [Google Scholar]
Larsson 1982 {published data only}
- Larsson G, Grundsell H, Gullberg B, Svennerud S. Outcome of pregnancy after conization. Acta Obstetricia et Gynecologica Scandinavica 1982;61(5):461‐6. [DOI] [PubMed] [Google Scholar]
Lima 2011 {published data only}
- Lima AF, Francisco C, Julio C, Paula T, Vitorino A, Borrego J. Obstetric outcomes after treatment for cervical intraepithelial neoplasia: six years of experience. Journal of Lower Genital Tract Disease 2011;15(14):276‐9. [DOI] [PubMed] [Google Scholar]
Ludviksson 1982 {published data only}
- Ludviksson K, Sandstrom B. Outcome of pregnancy after cone biopsy‐‐a case‐control study. European Journal of Obstetrics and Gynecology and Reproductive Biology 1982;14(3):135‐42. [DOI] [PubMed] [Google Scholar]
Martyn 2015 {published data only}
- Martyn FM, McAuliffe FM, Beggan C, Downey P, Flannelly G, Wingfield MB. Excisional treatments of the cervix and effect on subsequent fertility: a retrospective cohort study. European Journal of Obstetrics and Gynecology and Reproductive Biology 2015;185:114‐20. [DOI] [PubMed] [Google Scholar]
Miller 2015 {published data only}
- Miller ES, Sakowicz A, Grobman, WA. The association between cervical dysplasia, a short cervix, and preterm birth. American Journal of Obstetrics and Gynecology 2015;213:543 e1‐4. [DOI] [PubMed] [Google Scholar]
Moinian 1982 {published data only}
- Moinian M, Andersch B. Does cervix conization increase the risk of complications in subsequent pregnancies?. Acta Obstetricia et Gynecologica Scandinavica 1982;61(2):101‐3. [DOI] [PubMed] [Google Scholar]
Noehr 2009a {published data only}
- Noehr B, Jensen A, Frederiksen K, Tabor A, Kjaer SK. Depth of cervical cone removed by loop electrosurgical excision procedure and subsequent risk of spontaneous preterm delivery. Obstetrics and Gynecology 2009;114(6):1232‐8. [DOI] [PubMed] [Google Scholar]
- Noehr B, Jensen A, Frederiksen K, Tabor A, Kjaer SK. Loop electrosurgical excision of the cervix and subsequent risk for spontaneous preterm delivery: a population‐based study of singleton deliveries during a 9‐year period. American Journal of Obstetrics and Gynecology 2009;201(1):33.e1‐6. [DOI] [PubMed] [Google Scholar]
- Nohr B, Tabor A, Frederiksen K, Kjaer SK. Loop electrosurgical excision of the cervix and the subsequent risk of preterm delivery. Acta Obstetricia et Gynecologica Scandinavica 2007;86(5):596‐603. [DOI] [PubMed] [Google Scholar]
Noehr 2009b {published data only}
- Noehr B, Jensen A, Frederiksen K, Tabor A, Kjaer SK. Loop electrosurgical excision of the cervix and risk for spontaneous preterm delivery in twin pregnancies. Obstetrics and Gynecology 2009;114(3):511‐5. [DOI] [PubMed] [Google Scholar]
Ortoft 2010 {published data only}
- Ortoft G, Henriksen T, Hansen E, Petersen L. After conisation of the cervix, the perinatal mortality as a result of preterm delivery increases in subsequent pregnancy. BJOG: an international journal of obstetrics and gynaecology 2010;117(3):258‐67. [DOI] [PubMed] [Google Scholar]
Paraskevaidis 2002 {published data only}
- Paraskevaidis E, Koliopoulos G, Lolis E, Papanikou E, Malamou‐Mitsi V, Agnantis NJ. Delivery outcomes following loop electrosurgical excision procedure for micro‐invasive (FIGO stage IA1) cervical cancer. Gynecologic Oncology 2002;86(1):10‐3. [DOI] [PubMed] [Google Scholar]
Parikh 2008 {published data only}
- Parikh R, Horne H, Feinstein SJ, Anasti JN. Cervical length screening in patients who have undergone loop electrosurgical excision procedure. Journal of Reporoductive Medicine 2008;53(12):909‐13. [PubMed] [Google Scholar]
Poon 2012 {published data only}
- Poon LC, Savvas M, Zamblera D, Skyfta E, Nicolaides KH. Large loop excision of transformation zone and cervical length in the prediction of spontaneous preterm delivery. BGOJ: an international journal of obstetrics and gynaecology 2012;119(6):692‐8. [DOI] [PubMed] [Google Scholar]
Raio 1997 {published data only}
- Raio L, Ghezzi F, Naro E, Gomez R, Luscher KP. Duration of pregnancy after carbon dioxide laser conization of the cervix: influence of cone height. Obstetrics and Gynecology 1997;90(6):978‐82. [DOI] [PubMed] [Google Scholar]
Reilly 2012 {published data only}
- Reilly R, Paranjothy S, Beer H, Brooks CJ, Fielder HM, Lyons RA. Birth outcomes following treatment for precancerous changes to the cervix: a population‐based record linkage study. BJOG: an international journal of obstetrics and gynaecology 2012;119(2):236‐44. [DOI] [PubMed] [Google Scholar]
Sadler 2004 {published data only}
- Sadler L, Saftlas A, Wang W, Exeter M, Whittaker J, McCowan L. Treatment for cervical intraepithelial neoplasia and risk of preterm delivery. JAMA 2004;291(17):2100‐6. [DOI] [PubMed] [Google Scholar]
Sagot 1995 {published data only}
- Sagot P, Caroit Y, Winer N, Lopes P, Boog G. Obstetrical prognosis for carbon dioxide laser conisation of the uterine cervix. European Journal of Obstetrics and Gynecology and Reproductive Biology 1995;58(1):53‐8. [DOI] [PubMed] [Google Scholar]
Samson 2005 {published data only}
- Samson SL, Bentley JR, Fahey TJ, McKay DJ, Gill GH. The effect of loop electrosurgical excision procedure on future pregnancy outcome. Obstetrics and Gynecology 2005;105(2):325‐32. [DOI] [PubMed] [Google Scholar]
Saunders 1986 {published data only}
- Saunders N, Fenton DW, Soutter WP, Brown VA, Sharp F, Abernethy F. A case controlled study of the outcome of pregnancy following laser vaporization cone of the cervix. In: Sharp F, Jordan JA editor(s). Gynaecological Laser surgery: Proceedings of the Fifteenth Study Group of the Royal College of Obstetricians and Gynaecologists. Reproductive and Perinatal Medicine (VI). Ithaca, New York: Perinatology Press, 1986:121‐5. [Google Scholar]
Shanbhag 2009 {published data only}
- Shanbhag S, Clark H, Timmaraju V, Bhattacharya S, Cruickshank M. Pregnancy outcome after treatment for cervical intraepithelial neoplasia. Obstetrics and Gynecology 2009;114(4):727‐35. [DOI] [PubMed] [Google Scholar]
Simoens 2012 {published data only}
- Simoens C, Goffin F, Simon P, Barlow P, Antoine J, Foidart JM, et al. Adverse obstetrical outcomes after treatment of precancerous cervical lesions: a Belgian multicentre study. BJOG: an international journal of obstetrics and gynaecology 2012;119(10):1247‐55. [DOI] [PubMed] [Google Scholar]
Sjoborg 2007 {published data only}
- Sjoborg KD, Vistad I, Myhr SS, Svenningsen R, Herzog C, Kloster‐Jensen A, et al. Pregnancy outcome after cervical cone excision: a case‐control study. Acta Obstetricia et Gynecologica Scandinavica 2007;86(4):423‐8. [DOI] [PubMed] [Google Scholar]
Sozen 2014 {published data only}
- Sozen H, Namazov A, Cakir S, Akdemir Y, Vatansever D, Karateke A. Pregnancy outcomes after cold knife conization related to excised cone dimensions. A retrospective cohort study. Journal of Reporoductive Medicine 2014;59(1‐2):81‐6. [PubMed] [Google Scholar]
Spitzer 1995 {published data only}
- Spitzer M, Herman J, Krumholz BA, Lesser M. The fertility of women after laser cervical conisation. Obstetrics and Gynecology 1995;86(4 Pt 1):504‐8. [DOI] [PubMed] [Google Scholar]
Stout 2015 {published data only}
- Stout MJ, Frey HA, Tuuli MG, Cahill AG, Odibo AO, Roehl KA, et al. Loop electrosurgical excision procedure and risk of vaginal infections during pregnancy: an observational study. BJOG : an international journal of obstetrics and gynaecology 2015;122(4):545‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2004 {published data only}
- Tan L, Pepra E, Haloob RK. The outcome of pregnancy after large loop excision of the transformation zone of the cervix. Journal of Obstetrics and Gynaecology 2004;24(1):25‐7. [DOI] [PubMed] [Google Scholar]
Turlington 1996 {published data only}
- Turlington WT, Wright BD, Powell JL. Impact of the loop electrosurgical excision procedure on future fertility. Journal of Reporoductive Medicine 1996;41(11):815‐8. [PubMed] [Google Scholar]
van de Vijner 2010 {published data only}
- Vijver A, Poppe W, Verguts J, Arbyn M. Pregnancy outcome after cervical conisation: a retrospective cohort study in the Leuven University Hospital. BJOG: an international journal of obstetrics and gynaecology 2010;117(3):268‐73. [DOI] [PubMed] [Google Scholar]
Van Hentenryck 2012 {published data only}
- Hentenryck M, Noel JC, Simon P. Obstetric and neonatal outcome after surgical treatment of cervical dysplasia. European Journal of Obstetrics and Gynecology and Reproductive Biology 2012;162(1):16‐20. [DOI] [PubMed] [Google Scholar]
van Rooijen 1999 {published data only}
- Rooijen M, Persson E. Pregnancy outcome after laser vaporization of the cervix. Acta Obstetricia et Gynecologica Scandinavica 1999;78(4):346‐8. [PubMed] [Google Scholar]
Weber 1979 {published data only}
- Weber T, Obel EB. Pregnancy complications following conization of the uterine cervix (II). Acta Obstetricia et Gynecologica Scandinavica 1979;58(4):347‐51. [DOI] [PubMed] [Google Scholar]
Werner 2010 {published data only}
- Werner CL, Lo JY, Heffernan T, Griffith WF, McIntire DD, Leveno KJ. Loop electrosurgical excision procedure and risk of preterm birth. Obstetrics and Gynecology 2010;115(3):605‐8. [DOI] [PubMed] [Google Scholar]
Wuntakal 2013 {published data only}
- Wuntakal R, Castanon A, Sasieni PD, Hollingworth A. Pregnancy outcomes after treatment for cervical intraepithelial neoplasia in a single NHS hospital. International Journal of Gynecological Cancer 2013;23(4):710‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Halal 2013 {published data only}
- Al‐Halal H, Kezouh A, Abenhaim HA. Incidence and obstetrical outcomes of cervical intraepithelial neoplasia and cervical cancer in pregnancy: a population‐based study on 8.8 million births. Archives of Gynecology and Obstetrics 2013;287(2):245‐50. [DOI] [PubMed] [Google Scholar]
Althuisius 2001 {published data only}
- Althuisius SM, Schornagel IJ, Dekker GA, Geijn HP, Hummel P. Loop electrosurgical excision procedure of the cervix and time of delivery in subsequent pregnancy. International Journal of Gynaecology and Obstetrics 2001;72(1):31‐4. [DOI] [PubMed] [Google Scholar]
Berghella 2004 {published data only}
- Berghella V, Pereira L, Gariepy A, Simonazzi G. Prior cone biopsy: prediction of preterm birth by cervical ultrasound. American Journal of Obstetrics and Gynecology 2004;191(4):1393‐7. [DOI] [PubMed] [Google Scholar]
Berretta 2013 {published data only}
- Berretta R, Gizzo S, Dall'Asta A, Mazzone E, Monica M, Franchi L, et al. Risk of preterm delivery associated with prior treatment of cervical precancerous lesion according to the depth of the cone. Disease Markers 2013;35(6):721‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bull‐Phelps 2007 {published data only}
- Bull‐Phelps SL, Garner EI, Walsh CS, Gehrig PA, Miller DS, Schorge JO. Fertility‐sparing surgery in 101 women with adenocarcinoma in situ of the cervix. Gynecologic Oncology 2007;107(2):316‐9. [DOI] [PubMed] [Google Scholar]
Chevreau 2017 {published data only}
- Chevreau J, Mercuzot A, Foulon A, Attencourt C, Sergent F, Lanta S, Gondry J. Impact of age at conization on obstetrical outcome: a case‐control study. Journal of Lower Genital Tract Disease 2017;21(2):97‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ciavattini 2014 {published data only}
- Ciavattini A, Stortoni P, Mancioli F, Puglia D, Tranquilli AL, Liverani CA. The impact of loop electrosurgical excision procedure (LEEP) for CIN 2,3 on spontaneous preterm delivery in twin pregnancies by assisted reproductive technique: preliminary data. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(11):1169‐71. [DOI] [PubMed] [Google Scholar]
Ciavattini 2015 {published data only}
- Ciavattini A, Clemente N, Delli Carpini G, Gentili C, Giuseppe J, Barbadoro P, et al. Loop electrosurgical excision procedure and risk of miscarriage. Fertility and Sterility 2015;103(4):1043‐8. [DOI] [PubMed] [Google Scholar]
Conner 2013 {published data only}
- Conner SN, Cahill AG, Tuuli MG, Stamilio DM, Odibo AO, Roehl KA, et al. Interval from loop electrosurgical excision procedure to pregnancy and pregnancy outcomes. Obstetrics and Gynecology 2013;122(6):1154‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferenczy 1995 {published data only}
- Ferenczy A, Choukroun D, Falcone T, Franco E. The effect of cervical loop electrosurgical excision on subsequent pregnancy outcome: North American experience. American Journal of Obstetrics and Gynecology 1995;172(4 Pt 1):1246‐50. [DOI] [PubMed] [Google Scholar]
Gentry 2000 {published data only}
- Gentry DJ, Baggish MS, Brady K, Walsh PM, Hungler MS. The effects of loop excision of the transformation zone on cervical length: implications for pregnancy. American Journal of Obstetrics and Gynecology 2000;182(3):516‐20. [DOI] [PubMed] [Google Scholar]
Gordon 1991 {published data only}
- Gordon HK, Duncan ID. Effective destruction of cervical intraepithelial neoplasia (CIN) 3 at 100 degrees C using the Semm cold coagulator: 14 years experience. British Journal of Obstetrics and Gynaecology 1991;98(1):14‐20. [DOI] [PubMed] [Google Scholar]
Gronroos 1979 {published data only}
- Gronroos M, Liukko P, Kilkku P, Punnonen R. Pregnancy and delivery after conization of the cervix. Acta Obstetricia et Gynecologica Scandinavica 1979;58(5):477‐80. [DOI] [PubMed] [Google Scholar]
Kalliala 2012 {published data only}
- Kalliala I, Anttila A, Dyba T, Hakulinen T, Halttunen M, Nieminen P. Pregnancy incidence and outcome among patients with cervical intraepithelial neoplasia: a retrospective cohort study. BJOG: an international journal of obstetrics and gynaecology 2012;119(2):227‐35. [DOI] [PubMed] [Google Scholar]
Khalid 2012 {published data only}
- Khalid S, Dimitriou E, Conroy R, Paraskevaidis E, Kyrgiou M, Harrity C, et al. The thickness and volume of LLETZ specimens can predict the relative risk of pregnancy‐related morbidity. BJOG: an international journal of obstetrics and gynaecology 2012;119(6):685‐91. [DOI] [PubMed] [Google Scholar]
Kim 2016 {published data only}
- Kim M, Ishioka S, Endo T, Baba T, Saito T. Obstetrical prognosis of patients with cervical intraepithelial neoplasia (CIN) after "coin‐shaped" conization. Archives of Gynecology and Obstetrics 2016;293(3):651‐7. [DOI] [PubMed] [Google Scholar]
Kindinger 2016 {published data only}
- Kindinger L, Kyrgiou M, MacIntyre D, Cacciatore S, Yulia A, Cook J, et al. Preterm birth prevention post‐conization: a model of cervical length screening with targeted cerclage. PLOS One 2016;11(11):e0163793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kullander 1971 {published data only}
- Kullander S, Sjoberg NO. Treatment of carcinoma in situ of the cervix uteri by conization. A five‐year follow‐up. Acta Obstetricia et Gynecologica Scandinavica 1971;50(2):153‐7. [DOI] [PubMed] [Google Scholar]
Leiman 1980 {published data only}
- Leiman G, Harrison NA, Rubin A. Pregnancy following conization of the cervix: complications related to cone size. American Journal of Obstetrics and Gynecology 1980;136(1):14‐8. [DOI] [PubMed] [Google Scholar]
Liu 2014 {published data only}
- Liu Y, Qiu HF, Tang Y, Chen J, Lv J. Pregnancy outcome after the treatment of loop electrosurgical excision procedure or cold‐knife conization for cervical intraepithelial neoplasia. Gynecological Obstetric Investigation 2014;77(4):240‐4. [DOI] [PubMed] [Google Scholar]
Liverani 2016 {published data only}
- Liverani CA, Giuseppe J, Clemente N, Delli Carpini G, Monti E, Fanetti F, et al. Length but not transverse diameter of the excision specimen for high‐grade cervical intraepithelial neoplasia (CIN 2‐3) is a predictor of pregnancy outcome. European Journal of Cancer Prevention 2016;25(5):416‐22. [DOI] [PubMed] [Google Scholar]
Macvicar 1968 {published data only}
- Macvicar J, Willocks J. The effect of diathermy conization of the cervix on subsequent fertility, pregnancy and delivery. Journal of Obstetrics and Gynaecology of the British Commonwealth 1968;75(3):355‐6. [DOI] [PubMed] [Google Scholar]
Mariya 2016 {published data only}
- Mariya T, Nishikawa A, Sogawa K, Suzuki R, Saito M, Kawamata A, ET AL. Virological and cytological clearance in laser vaporization and conization for cervical intra‐epithelial neoplasia grade 3. Journal of Obstetrics and Gynaecology Research 2016;42:1808‐13. [DOI] [PubMed] [Google Scholar]
Masamoto 2008 {published data only}
- Masamoto H, Nagai Y, Inamine M, Hirakawa M, Okubo E, Ishisoko A, et al. Outcome of pregnancy after laser conization: implications for infection as a causal link with preterm birth. Journal of Obstetrics and Gynaecology Research 2008;34(5):838‐42. [DOI] [PubMed] [Google Scholar]
Michelin 2009 {published data only}
- Michelin MA, Merino LM, Franco CA, Murta EF. Pregnancy outcome after treatment of cervical intraepithelial neoplasia by the loop electrosurgical excision procedure and cold knife conization. Clinical and Experimental Obstetrics & Gynecology 2009;36(1):17‐9. [PubMed] [Google Scholar]
Mitsuhashi 2000 {published data only}
- Mitsuhashi A, Sekiya S. Loop electrosurgical excision procedure (LEEP) during first trimester of pregnancy. International Journal of Gynaecology and Obstetrics 2000;71(3):237‐9. [DOI] [PubMed] [Google Scholar]
Monaghan 1982 {published data only}
- Monaghan JM, Kirkup W, Davis JA, Edington PT. Treatment of cervical intraepithelial neoplasia by colposcopically directed cryosurgery and subsequent pregnancy experience. British Journal of Obstetrics and Gynaecology 1982;89(5):387‐92. [DOI] [PubMed] [Google Scholar]
Naleway 2015 {published data only}
- Naleway AL, Weinmann S, Krishnarajah G, Arondekar B, Fernandez J, Swamy G, et al. Pregnancy after treatment for cervical cancer precursor lesions in a retrospective matched cohort. PLOS One 2015;10(2):e0117525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nam 2010 {published data only}
- Nam KH, Kwon JY, Kim YH, Park YW. Pregnancy outcome after cervical conization: risk factors for preterm delivery and the efficacy of prophylactic cerclage. Journal of Gynecologic Oncology 2010;21(4):225‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Novikova 1994 {published data only}
- Novikova EG, Antoshechkina MA. [Reproductive function after the organ‐preserving treatment of early cancerous pathology of the cervix uteri]. Akusherstvo i ginekologiia (Mosk) 1994;2:44‐7. [PubMed] [Google Scholar]
Patrelli 2008 {published data only}
- Patrelli TS, Anfuso S, Vandi F, Valitutto S, Migliore M, Salvati MA, et al. Preterm delivery and premature rupture of membranes after conization in 80 women. Preliminary data. Minerva Ginecologica 2008;60(4):295‐8. [PubMed] [Google Scholar]
Pils 2014 {published data only}
- Pils S, Eppel W, Seemann R, Natter C, Ott J. Sequential cervical length screening in pregnancies after loop excision of the transformation zone conisation: a retrospective analysis. BJOG : an international journal of obstetrics and gynaecology 2014;121(4):457‐62. [DOI] [PubMed] [Google Scholar]
Pinborg 2015 {published data only}
- Pinborg A, Ortoft G, Loft A, Rasmussen SC, Ingerslev HJ. Cervical conization doubles the risk of preterm and very preterm birth in assisted reproductive technology twin pregnancies. Human Reproduction 2015;30(1):197‐204. [DOI] [PubMed] [Google Scholar]
Radha Bai Prabhu 2010 {published data only}
- Radha Bai Prabhu T. Pregnancy outcome following large loop excision of the transformation zone (LLETZ). Journal of Obstetrics and Gynaecology of India 2010;60(2):149‐51. [Google Scholar]
Rafaeli‐Yehudai 2014 {published data only}
- Rafaeli‐Yehudai T, Kessous R, Aricha‐Tamir B, Sheiner E, Erez O, Meirovitz M, et al. The effect of cervical cerclage on pregnancy outcomes in women following conization. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(15):1594‐7. [DOI] [PubMed] [Google Scholar]
Ricciotti 1995 {published data only}
- Ricciotti HA, Burke L, Kobelin M, Slomovic B, Ludmir J. Ultrasound evaluation of cervical shortening after loop excision of the transformation zone (LETZ). International Journal of Gynaecology and Obstetrics 1995;50(2):175‐8. [DOI] [PubMed] [Google Scholar]
Rosen 1991 {published data only}
- Rosen A, Klein M, Vavra N, Gitsch G, Karasegh S, Schrock A, et al. [PAP IV in pregnancy‐‐a retrospective multicenter study]. Geburtshilfe Frauenheilkunde 1991;51(3):208‐10. [DOI] [PubMed] [Google Scholar]
Sangkarat 2014 {published data only}
- Sangkarat S, Ruengkhachorn I, Benjapibal M, Laiwejpithaya S, Wongthiraporn W, Rattanachaiyanont M. Long‐term outcomes of a loop electrosurgical excision procedure for cervical intraepithelial neoplasia in a high incidence country. Asian Pacific Journal of Cancer Prevention 2014;15(2):1035‐9. [DOI] [PubMed] [Google Scholar]
Seki 2010 {published data only}
- Seki N, Kodama J, Kusumoto T, Nakamura K, Hongo A, Hiramatsu Y. Complications and obstetric outcomes after laser conization during pregnancy. European Journal of Gynaecological Oncology 2010;31(4):399‐401. [PubMed] [Google Scholar]
Shin 2010 {published data only}
- Shin MY, Seo ES, Choi SJ, Oh SY, Kim BG, Bae DS, et al. The role of prophylactic cerclage in preventing pretermdelivery after electrosurgical conization. Journal of Gynecologic Oncology 2010;21(4):230‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sljivancanin 2013 {published data only}
- Sljivancanin D, Kesic V, Tulic L, Dotlic J. [Assessment of the natural course and treatment of premalignant uterine cervical lesions in pregnancy]. Srpski Aarhiv za Celokupno Lekarstvo 2013;141(3‐4):192‐7. [DOI] [PubMed] [Google Scholar]
Smaldone 2010 {published data only}
- Smaldone GM, Krohn MA, McGee EA. Cervical cancer and risk for delivery of small‐for‐gestational age neonates. Journal of Womens Health (Larchmt) 2010;19(5):969‐74. [DOI] [PubMed] [Google Scholar]
Spracklen 2013 {published data only}
- Spracklen CN, Harland KK, Stegmann BJ, Saftlas AF. Cervical surgery for cervical intraepithelial neoplasia and prolonged time to conception of a live birth: a case‐control study. BJOG: an international journal of obstetrics and gynaecology 2013;120(8):960‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wakita 1990 {published data only}
- Wakita K, Izumi T, Kuramoto H, Sasaki N, Shimada N. Pregnancy after laser therapy for the treatment of uterine cervical neoplasia. Journal of Clinical Laser Medicine & Surgery 1990;8(5):71‐6. [DOI] [PubMed] [Google Scholar]
Watson 2012 {published data only}
- Watson LF, Rayner JA, King J, Jolley D, Forster D. Intra‐cervical procedures and the risk of subsequent very preterm birth: a case‐control study. Acta Obstetricia et Gynecologica Scandinavica 2012;91(2):204‐10. [DOI] [PubMed] [Google Scholar]
Wongtiraporn 2014 {published data only}
- Wongtiraporn W, Laiwejpithaya S, Sangkarat S, Benjapibal M, Rattanachaiyanont M, Ruengkhachorn I, et al. Long term outcomes of laser conization for high grade cervical intraepithelial neoplasia in Thai women. Asian Pacific Journal of Cancer Prevention 2014;15(18):7757‐61. [DOI] [PubMed] [Google Scholar]
Zuo 2011 {published data only}
- Zuo Z, Goel S, Carter JE. Association of cervical cytology and HPV DNA status during pregnancy with placental abnormalities and preterm birth. American Journal of Clinical Pathology 2011;136(2):260‐5. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Aleman 2016 {published data only}
- Aleman JM, Arien F, Tjalma WAA. The impact of conisation on pregnancy outcome. European Journal of Gynaecological Oncology 2016;37:786‐91. [PubMed] [Google Scholar]
Bjorge 2016 {published data only}
- Bjorge T, Skare GB, Bjorge L, Trope A, Lonnberg S. Adverse pregnancy outcomes after treatment for cervical intraepithelial neoplasia. Obstetrics and Gynecology 2016;128(6):1265‐73. [DOI] [PubMed] [Google Scholar]
Brie 2016 {published data only}
- Brie C, Turck M, Cheret A, Morello R, Benoist G, Dreyfus M. [Case‐control study of obstetrical outcomes of conisation]. Journal de Gynécologie, Obstétrique et Biologie de la Reproduction [Journal of Gynecology Obstetrics and Human Reproduction] 2016;45(2):192‐7. [DOI] [PubMed] [Google Scholar]
He 2007 {published data only}
- He HJ, Pan LY, Huang HF, Lang JH. [Clinical analysis of the effect of cervical conization on fertility and pregnancy outcome]. Zhonghua Fu Chan Ke Za Zhi 2007;42(8):515‐7. [PubMed] [Google Scholar]
Jancar 2016 {published data only}
- Jancar N, Mihevc Ponikvar B, Tomsic S. Cold‐knife conisation and large loop excision of transformation zone significantly increase the risk for spontaneous preterm birth: a population‐based cohort study. European Journal of Obstetrics and Gynecology and Reproductive Biology 2016;203:245‐9. [DOI] [PubMed] [Google Scholar]
Kalitsaris 1991 {published data only}
- Kalitsaris A, Paschopoulos M, Paraskevaidis E, Dalkalitsis N, Tsanadis G, Adonakis G, et al. [Fertility and pregnancy after conization]. Annali di Ostetricia, Ginecologia, Medicina Perinatale 1991;112(4):257‐61. [PubMed] [Google Scholar]
Kasum 1991 {published data only}
- Kasum M, Kuvacic I. [Pregnancy outcome after conization]. Jugoslavenska Ginekologija i Perinatologija 1991;31:31‐4. [PubMed] [Google Scholar]
Lund 1986 {published data only}
- Lund E, Bjerkedal T. [Cancer in situ of the uterine cervix. Increased perinatal death and prematurity after conization]. Tidsskrift for den Norske Lægeforening 1986;106(7):543‐6. [PubMed] [Google Scholar]
Praest 1979 {published data only}
- Praest J. [Conization of the cervix of the uterus]. Ugeskrift For Laeger 1979;51:3509‐11. [PubMed] [Google Scholar]
Spuhler 1995 {published data only}
- Spuhler S, Outcha Adjahoto E, Raszka K, Grandi P. [Consequences of laser CO2 treatment of cervical intraepithelial neoplasias on the anatomic and functional integrity of the cervix]. Journal de Gynécologie, Obstétrique et Biologie de la Reproduction [Journal of Gynecology, Obstetrics and Biological Reproduction (Paris)] 1995;24(2):127‐33. [PubMed] [Google Scholar]
Zebitay 2017 {published data only}
- Zebitay AG, Gungor ES, Ilhan G, Cetin O, Dane C. Furtuna C, et al. Cervical conization and the risk of preterm birth: a population‐based multicentric trial of Turkish cohort. Journal of Clinical and Diagnostic Research 2017;11(3):QC21–QC24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zornoza‐Garcia 2009 {published data only}
- Zornoza‐Garcia V, Luengo‐Tabernero A, Reyero‐Alvarez MP, Salas‐Valien S, Gonzalez‐Garcia C. [Obstetrics results after conization. Leon Hospital. 1999‐2007 period]. Clínica e Investigación en Ginecología y Obstetricia [Clinical Investigation of Gin Obst 2009;36(1):9‐12. [Google Scholar]
Additional references
Arbyn 2009
- Arbyn M, Raifu AO, Bray F, Weiderpass E, Anttila A. Trends of cervical cancer mortality in the member states of the European Union. European Journal of Cancer 2009;45:2640‐8. [DOI] [PubMed] [Google Scholar]
Arbyn 2014
- Arbyn M, Kyrgiou M, Gondry J, Petry KU, Paraskevaidis E. Long term outcomes for women treated for cervical precancer. BMJ 2014;348:f7700. [DOI] [PubMed] [Google Scholar]
Bruinsma 2011
- Bruinsma FJ, Quinn MA. The risk of preterm birth following treatment for precancerous changes in the cervix: a systematic review and meta‐analysis. British Journal of Obstetrics and Gynaecology 2011;118(9):1031‐41. [DOI] [PubMed] [Google Scholar]
CervicalCancerScreening 2015
- Cervical Cancer Screening 2015. Available from: http://www.cancerscreening.nhs.uk/cervical/.
Chew 1999
- Chew GK, Jandial L, Paraskevaidis E, Kitchener HC. Pattern of CIN recurrence following laser ablation treatment: long‐term follow‐up. International Journal of Gynecological Cancer 1999;9:487‐90. [DOI] [PubMed] [Google Scholar]
Cochran 1954
- Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101‐29. [Google Scholar]
Conner 2014
- Conner SN, Frey HA, Cahill AG, Macones GA, Colditz GA, Tuuli MG. Loop electrosurgical excision procedure and risk of preterm birth: a systematic review and meta‐analysis. Obstet Gynecol 2014;123(4):752‐761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crane 2003
- Crane JM. Pregnancy outcome after loop electrosurgical excision procedure: a systematic review. Obstetrics and Gynecology 2003;102(5 Pt 1):1058‐62. [DOI] [PubMed] [Google Scholar]
Danhof 2015
- Danhof NA, Kamphuis EI, Limpens J, Lonkhuijzen LR, Pajkrt E, Mol BW. The risk of preterm birth of treated versus untreated cervical intraepithelial neoplasia (CIN): a systematic review and meta‐analysis. European Journal of Obstetrics and Gynecology and Reproductive Biology 2015;188:24‐33. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
Dersimonian 1986
- Dersimonian R, Laird NM. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Ferlay 2015
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2015;136(5):E359‐86. [DOI] [PubMed] [Google Scholar]
Founta 2010
- Founta C, Arbyn M, Valasoulis G, Kyrgiou M, Tsili A, Martin‐Hirsch P, et al. Proportion of excision and cervical healing after large loop excision of the transformation zone for cervical intraepithelial neoplasia. BJOG : an international journal of obstetrics and gynaecology 2010;117(12):1468‐74. [DOI] [PubMed] [Google Scholar]
GRADE Working Group 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;7454:1490‐4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henk 2010
- Henk HJ, Insinga RP, Singhal PK, Darkow T. Incidence and costs of cervical intraepithelial neoplasia in a US commercially insured population. Journal of Lower Genital Tract Disease 2010;14(1):29‐36. [DOI] [PubMed] [Google Scholar]
Herbert 2000
- Herbert A. Cervical screening in England and Wales: its effect has been underestimated. Cytopathology 2000;11:471‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org 2011.
IARC 2005
- IARC. Cervix Cancer Screening. IARC Handbooks of Cancer Prevention. International Agency for Researchy in Cancer, Lyon. Vol. 10, IARCPress, Lyon (France), 2005:1‐302. [Google Scholar]
Jin 2014
- Jin G, LanLan Z, Li C, Dan Z. Pregnancy outcome following loop electrosurgical excision procedure (LEEP) a systematic review and meta‐analysis. Archives of Gynecology and Obstetrics 2014;289(1):85‐99. [DOI] [PubMed] [Google Scholar]
Kitchener 1995
- Kitchener HC, Cruickshank ME, Farmery E. The 1993 British Society for Colposcopy and Cervical Pathology/National Coordinating Network United Kingdom Colposcopy Survey. Comparison with 1988 and the response to introduction of guidelines. British Journal of Obstetrics and Gynaecology 1995;102:549‐552. [DOI] [PubMed] [Google Scholar]
Kyrgiou 2012
- Kyrgiou M, Arbyn M, Martin‐Hirsch P, Paraskevaidis E. Increased risk of preterm birth after treatment for CIN. BMJ 2012;345:e5847. [DOI] [PubMed] [Google Scholar]
Kyrgiou 2014
- Kyrgiou M, Mitra A, Arbyn M, Stasinou SM, Martin‐Hirsch P, Bennett P, et al. Fertility and early pregnancy outcomes after treatment for cervical intraepithelial neoplasia: systematic review and meta‐analysis. BMJ 2014;349:g6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kyrgiou 2015a
- Kyrgiou M, Valasoulis G, Stasinou SM, Founta C, Athanasiou A, Bennett P, et al. Proportion of cervical excision for cervical intraepithelial neoplasia as a predictor of pregnancy outcomes. International Journal of Gynaecology and Obstetrics 2015;128(2):141‐7. [DOI] [PubMed] [Google Scholar]
Kyrgiou 2015b
- Kyrgiou M, Mitra A, Arbyn M, Paraskevaidi M, Athanasiou A, Martin‐Hirsch PPL, et al. Fertility and early pregnancy outcomes after conservative treatment for cervical intraepithelial neoplasia. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD008478.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin‐Hirsch 2013
- Martin‐Hirsch PPL, Paraskevaidis E, Bryant A, Dickinson HO. Surgery for cervical intraepithelial neoplasia. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD001318.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic review and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [DOI] [PubMed] [Google Scholar]
NHS Cervical Screening Programme 2016
- NHS Cervical Screening Programme 2016. NHS Cervical Screening Programme 2016: Document 20. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/515817/NHSCSP_colposcopy_management.pdf. 2012.
Nuovo 2000
- Nuovo J, Melnikow J, Willan AR, Chan BK. Treatment outcomes for squamous intraepithelial lesions. International Journal of Gynaecology and Obstetrics 2000;68:25‐33. [DOI] [PubMed] [Google Scholar]
Paraskevaidis 1991
- Paraskevaidis E, Jandial L, Mann EM, Fisher PM, Kitchener HC. Pattern of treatment failure following laser for cervical intraepithelial neoplasia: implications for follow‐up protocol. Obstetrics and Gynecology 1991;78:80‐3. [PubMed] [Google Scholar]
Paraskevaidis 1992
- Paraskevaidis E, Kitchener HC, Miller ID, Mann E, Jandial L, Fisher PM. A population‐based study of microinvasive disease of the cervix ‐ a colposcopic and cytologic analysis. Gynecologic Oncology 1992;45:9‐12. [DOI] [PubMed] [Google Scholar]
Paraskevaidis 2007
- Paraskevaidis E, Kyrgiou M, Martin‐Hirsch P. Have we dismissed ablative treatment too soon in colposcopy practice? ‐ Author's reply. BJOG: an international journal of obstetrics and gynaecology 2007;114(6):774‐5. [DOI] [PubMed] [Google Scholar]
Petry 2008
- Petry KU, Breugelmans JG, Benard S, Lamure E, Littlewood KJ, Hillemanns P. Cost of screening and treatment of cervical dyskaryosis in Germany. European Journal of Gynaecolic Oncology 2008;29(4):345‐9. [PubMed] [Google Scholar]
Prendiville 1989
- Prendiville W, Cullimore J, Norman S. Large loop excision of the transformation zone (LLETZ). A new method of management for women with cervical intraepithelial neoplasia. British Journal of Obstetrics and Gynaecology 1989;96(9):1054‐60. [DOI] [PubMed] [Google Scholar]
Quinn 1999
- Quinn M, Babb P, Jones J, Allen E. Effect of screening on incidence of and mortality from cancer of the cervix in England: evaluation based on routinely collected statistics. BMJ 1999;318:904‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2104 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. Review Manager (RevMan). Version 5.3.5. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, 2014.
Soutter 1997
- Soutter WP, Barros Lopes A, Fletcher A, Monaghan JM, Duncan ID, Paraskevaidis E, et al. Invasive cervical cancer after conservative therapy for CIN. Lancet 1997;349:978‐80. [DOI] [PubMed] [Google Scholar]
Strander 2014
- Strander B, Hallgren J, Sparen P. Effect of ageing on cervical or vaginal cancer in Swedish women previously treated for cervical intraepithelial neoplasia grade 3: population based cohort study of long term incidence and mortality. BMJ 2014;348:f7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Arbyn 2008
- Arbyn M, Kyrgiou M, Simoens C, Raifu AO, Koliopoulos G, Martin‐Hirsch P, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta‐analysis. BMJ 2008;337:a1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kyrgiou 2006
- Kyrgiou M, Koliopoulos G, Martin‐Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta‐analysis. Lancet 2006;367(9509):489‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kyrgiou 2016
- Kyrgiou M, Athanasiou A, Paraskevaidi M, Mitra A, Kalliala I, Martin‐Hirsch P, et al. Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: systematic review and meta‐analysis. BMJ 2016;354:i3633. [DOI] [PMC free article] [PubMed] [Google Scholar]


