Abstract
Background
Non‐surgical treatment for carpal tunnel syndrome is frequently offered to those with mild to moderate symptoms. The effectiveness and duration of benefit from non‐surgical treatment for carpal tunnel syndrome remain unknown.
Objectives
To evaluate the effectiveness of non‐surgical treatment (other than steroid injection) for carpal tunnel syndrome versus a placebo or other non‐surgical, control interventions in improving clinical outcome.
Search methods
We searched the Cochrane Neuromuscular Disease Group specialised register (searched March 2002), MEDLINE (searched January 1966 to February 7 2001), EMBASE (searched January 1980 to March 2002), CINAHL (searched January 1983 to December 2001), AMED (searched 1984 to January 2002), Current Contents (January 1993 to March 2002), PEDro and reference lists of articles.
Selection criteria
Randomised or quasi‐randomised studies in any language of participants with the diagnosis of carpal tunnel syndrome who had not previously undergone surgical release. We considered all non‐surgical treatments apart from local steroid injection. The primary outcome measure was improvement in clinical symptoms after at least three months following the end of treatment.
Data collection and analysis
Three reviewers independently selected the trials to be included. Two reviewers independently extracted data. Studies were rated for their overall quality. Relative risks and weighted mean differences with 95% confidence intervals were calculated for the primary and secondary outcomes in each trial. Results of clinically and statistically homogeneous trials were pooled to provide estimates of the efficacy of non‐surgical treatments.
Main results
Twenty‐one trials involving 884 people were included. A hand brace significantly improved symptoms after four weeks (weighted mean difference (WMD) ‐1.07; 95% confidence interval (CI) ‐1.29 to ‐0.85) and function (WMD ‐0.55; 95% CI ‐0.82 to ‐0.28). In an analysis of pooled data from two trials (63 participants) ultrasound treatment for two weeks was not significantly beneficial. However one trial showed significant symptom improvement after seven weeks of ultrasound (WMD ‐0.99; 95% CI ‐1.77 to ‐ 0.21) which was maintained at six months (WMD ‐1.86; 95% CI ‐2.67 to ‐1.05). Four trials involving 193 people examined various oral medications (steroids, diuretics, nonsteroidal anti‐inflammatory drugs) versus placebo. Compared to placebo, pooled data for two‐week oral steroid treatment demonstrated a significant improvement in symptoms (WMD ‐7.23; 95% CI ‐10.31 to ‐4.14). One trial also showed improvement after four weeks (WMD ‐10.8; 95% CI ‐15.26 to ‐6.34). Compared to placebo, diuretics or nonsteroidal anti‐inflammatory drugs did not demonstrate significant benefit. In two trials involving 50 people, vitamin B6 did not significantly improve overall symptoms. In one trial involving 51 people yoga significantly reduced pain after eight weeks (WMD ‐1.40; 95% CI ‐2.73 to ‐0.07) compared with wrist splinting. In one trial involving 21 people carpal bone mobilisation significantly improved symptoms after three weeks (WMD ‐1.43; 95% CI ‐2.19 to ‐0.67) compared to no treatment. In one trial involving 50 people with diabetes, steroid and insulin injections significantly improved symptoms over eight weeks compared with steroid and placebo injections. Two trials involving 105 people compared ergonomic keyboards versus control and demonstrated equivocal results for pain and function. Trials of magnet therapy, laser acupuncture, exercise or chiropractic care did not demonstrate symptom benefit when compared to placebo or control.
Authors' conclusions
Current evidence shows significant short‐term benefit from oral steroids, splinting, ultrasound, yoga and carpal bone mobilisation. Other non‐surgical treatments do not produce significant benefit. More trials are needed to compare treatments and ascertain the duration of benefit.
Plain language summary
Oral steroids, splinting, ultrasound, yoga and wrist mobilisation provide short‐term relief from carpal tunnel syndrome, but other non‐surgical methods have not been shown to help.
Carpal tunnel syndrome is caused by compression of the median nerve at the wrist, leading to mild to severe pain and pins and needles in the hand. Other Cochrane reviews show benefit from nerve decompression surgery and steroids. This review of other non‐surgical treatments found some evidence of short‐term benefit from oral steroids, splinting/hand braces, ultrasound, yoga and carpal bone mobilisation (movement of the bones and tissues in the wrist), and insulin and steroid injections for people who also had diabetes. Evidence on ergonomic keyboards and vitamin B6 is unclear, while trials so far have not shown benefit from diuretics, non‐steroidal anti‐inflammatory drugs, magnets, laser acupuncture, exercise or chiropractic.
Background
Carpal tunnel syndrome (CTS) is a condition in which the median nerve at the level of the carpal tunnel undergoes irritation, often attributed to compression (Kerwin 1996). Symptoms of CTS include pain in the wrist and hand which can radiate to the forearm (Rempel 1998) and paraesthesiae in the thumb, index, middle and radial half of the ring finger (Szabo 1994). Advanced stages of median nerve compression can result in thenar muscle weakness (Szabo 1994).
Median nerve compression in the carpal tunnel is the most common example of nerve compression in the body (Rosenthal 1987). Carpal tunnel syndrome is said to affect one per cent of the population (Katz 1990; Levine 1993) but higher rates have been identified in populations of certain occupations such as meat packers (Hagberg 1992) and those with medical conditions such as renal failure (Katims 1989). Newport (Newport 2000) suggests that the incidence of CTS is increasing, and that with age expectancy of seventy years, 3.5 per cent of males and 11 per cent of females will be affected by CTS. Other studies have observed certain personal characteristics such as obesity to be associated with increased incidence of CTS (Atroshi 1999). Age and gender have also been found to have an effect upon the incidence of CTS. Females in their fourth and fifth decades suffer CTS four times more commonly than men (Atroshi 1999).
Carpal tunnel syndrome does not follow a predictable course. Some patients experience a deterioration in hand function whilst others describe 'silent' periods and intermittent exacerbation of symptoms (Braun 1989). Some patients have described spontaneous improvement of symptoms without medical treatment (Padua 2001; Futami 1992). The treatment of carpal tunnel syndrome can be categorized into surgical and non‐surgical. Surgical treatment is usually offered to those with severe carpal tunnel syndrome, who have constant symptoms, severe sensory disturbance and/ or thenar motor weakness. Non‐surgical treatments are offered to those who have the intermittent symptoms of mild to moderate carpal tunnel syndrome. Non‐surgical interventions may also be used as a temporary measure while awaiting carpal tunnel release.
Surgery for CTS involves open or endoscopic division of the flexor retinaculum in order to provide greater space for the contents of the carpal canal. Carpal tunnel release is the most common hand and wrist surgery in the USA, where more than 400,000 carpal tunnel releases are performed annually (Concannon 2000). Surgical treatment options for patients with CTS have been examined in other Cochrane reviews: surgical treatment options for CTS (Scholten 2002), and the effect of surgery versus non‐surgical treatment (Verdugo 2002).
Non‐surgical options for the treatment of CTS include many different interventions such as splinting, exercises, yoga, therapeutic ultrasound, activity or ergonomic modification, oral medication and vitamins. Their effectiveness in the management of CTS remain uncertain. As stated above, surgical management of CTS offers relief of symptoms by creating greater space in the carpal canal. Non‐surgical treatments for CTS must address different pathophysiological aspects of CTS in order to be successful. For example, splinting of the affected wrist in a neutral position is recommended in order to maintain the wrist in a position that has the lowest intra‐canal pressure and therefore the least pressure on the median nerve (Gelberman 1984).
Yoga was investigated for the treatment of CTS (Garfinkel 1998) because stretching may relieve compression in the carpal tunnel, better joint posture may decrease nerve compression, and blood flow may be improved to the median nerve. Stretching exercises for CTS have also been prescribed for the same reason and also to mobilise the median nerve within the carpal canal if it is adherent.
Activity modification aims to position the wrist in a neutral position to provide maximum space within the carpal canal, and to avoid forceful and repeated movements that are central to occupations associated with increased risk for carpal tunnel syndrome (Hagberg 1992).
Therapeutic ultrasound is claimed to have an anti‐inflammatory effect and has been applied with the aim of healing the median nerve in cases of CTS (Ebenbichler 1998).
Oral anti‐inflammatory medication aims to reduce swelling in the median nerve and other contents within the carpal canal (Seradge 1994). Vitamins in the B group have also been prescribed to relieve symptoms (Spinner 1995).
Objectives
The objective of this review was to compare the effectiveness of non‐surgical treatment (other than steroid injection) for carpal tunnel syndrome with no treatment, placebo or another non‐surgical treatment for improving clinical outcome.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished studies using or attempting to use a randomised methodology were included. Studies attempting to compare a non‐surgical treatment with no treatment (or a placebo) or with each other were also considered.
Types of participants
All participants with a diagnosis of CTS as defined by the authors of each paper were accepted. Participants who had previous surgery for CTS were excluded.
Types of interventions
All non‐surgical treatments were included, except where steroid injection was the primary treatment under investigation. Steroid injection has been examined in a separate review (Marshall 2001).
Types of outcome measures
Primary outcomes
The primary outcome measure was improvement in clinical symptoms, such as pain and paraesthesiae, at least three months after the end of treatment.
Secondary outcomes
Secondary outcome measures included:
improvement in functional status and/or health‐related quality of life parameters at least three months after treatment;
improvement in objective physical examination measures, such as grip, pinch strength, and sensory perception at least three months after treatment;
improvement in neurophysiological parameters after three months after treatment;
clinical improvement at less than three months of follow‐up;
clinical improvement at one year after treatment;
need for surgical release of the flexor retinaculum during follow‐up.
Search methods for identification of studies
Electronic searches
See: Neuromuscular Disease Review Group search strategy The Cochrane Neuromuscular Disease Group specialised register was searched in June 2001 and March 2002 for randomised controlled trials using 'carpal tunnel syndrome' as the search term. The reference lists of all trials identified by this strategy were also searched.
In addition, a search of additional electronic databases was conducted in June 2001 and March 2002 using MEDLINE (1996 to Week 5 2001), EMBASE (1980 ‐ 2002), CINAHL (1983 ‐ December 2001), AMED (1985 ‐ January 2002), Current Contents (1993 ‐ 2002) and PEDro. The search strategy used for MEDLINE is presented in Appendix 1. This search strategy was adapted as appropriate to search the other electronic databases.
Data collection and analysis
Selection of studies
Three reviewers (DOC, SM, NMW) independently selected the trials to be included in the review. Firstly, each reviewer examined the titles and abstracts of trials identified from the search. The reviewers were blinded with regard to authors, institution and journal of the trials. Secondly, each reviewer read the full text of all studies of possible relevance for independent assessment. The reviewers independently decided which trials fitted the inclusion criteria. Disagreement was resolved by discussion and consensus between the reviewers.
Data extraction and management
Two reviewers (DOC, SM) independently extracted data using specially developed data extraction forms. Information was collected on participants (age, sex, diagnostic criteria used to confirm CTS, severity of symptoms, duration of symptoms, recruitment method, inclusion/exclusion criteria, comorbid conditions, trial setting, allocation procedure, blinding, number of participants or hands randomised), interventions (description of interventions, method of delivery, treatment length, number and explanation for any drop‐outs, crossovers), outcome measures (description of measures used, timing of administration, continuous/dichotomous nature, psychometric properties, references provided), and results (point estimates and measures of variability, frequency counts for dichotomous variables, number of patients or hands). One reviewer (DOC) compiled all comparisons and entered all outcome data into a computerised database (RevMan 4.1). A second reviewer (NMW) performed double‐data entry to ensure accuracy of results. Data were cross‐checked by all of the reviewers. For trials where the required data were not reported, further information was requested from the authors by one of the reviewers (DOC). When unsuccessful, the study was included in the review and fully described, but not included in any meta‐analysis. An entry of this process was made in the notes section of the included studies table.
Assessment of risk of bias in included studies
The methodological quality of the included trials was assessed by two reviewers (DOC, SM) with particular emphasis on selection, performance, attrition and detection bias as advocated by the Cochrane Reviewers' Handbook (Clarke 1999). A descriptive approach to quality assessment was selected rather than use of a scale due to concerns regarding the validity of existing quality scales. Specific considerations for quality assessment of each study included:
Was the process of subject recruitment clearly defined?
Was the assigned treatment adequately concealed prior to allocation?
Were care programmes, other than the trial options, the same?
Were the treatment providers blind to assignment status?
Were the subjects blind to assignment status after allocation?
Were withdrawals of patients equal between study groups and explained?
Were the outcome assessors blinded to the treatment status?
Were the outcome measures appropriate and clearly described?
Each criterion was graded as met, unmet or unclear with the exception of allocation concealment which was scored as adequate (A), unclear (B), inadequate (C) or not used (D). When criteria were scored as unclear, one reviewer (DOC) attempted to obtain further information from the authors of the trial. The overall quality of individual trials was summarised according to the approach outlined in the Cochrane Reviewers' Handbook (Clarke 1999). The risk of bias in a trial was rated as low when all of the criteria were met (A), moderate when one or more criteria were partly met (B), or high when one or more criteria were not met (C). Any disagreement in the individual or summarised quality scoring of trials was discussed by the reviewers to reach a consensus.
The quality of the diagnostic criteria used in the included trials was assessed according to the criteria proposed by Rempel and colleagues (Rempel 1998). The trials were classified into high (A), moderate (B) and low (C) quality based on these criteria.
A ‐ combination of electrodiagnostic findings and symptoms for the diagnosis of CTS;
B ‐ combination of symptoms and physical examination findings for the diagnosis of CTS (in absence of electrodiagnostic findings);
C ‐ symptoms or physical examination findings for the diagnosis of CTS (in absence of electrodiagnostic findings).
Data synthesis
RevMan 4.1 software was used for the statistical analysis. Results were expressed as relative risks with 95 per cent confidence intervals for dichotomous outcomes and weighted mean difference with 95 per cent confidence intervals for continuous outcomes. Results of clinically and statistically homogeneous trials were pooled to provide estimates of the efficacy of various non‐surgical treatments (other than steroid injection) for carpal tunnel syndrome. Clinical homogeneity was satisfied when participants, interventions, outcome measures and timing of outcome measurement were considered to be similar. Statistical homogeneity was assessed with the Chi‐square statistic. Pooled results were analysed using a fixed‐effects or random‐effects model (depending on the level of heterogeneity). Statistical significance was set at p<0.05 for pre‐defined primary and secondary outcome measures. For trials that were clinically heterogeneous or presented insufficient information for pooling, a qualitative analysis was performed. Qualitative analysis reported the findings of the trial as reported by authors and rated the levels of evidence according to the rating system adapted from Tulder and colleagues (Tulder 2002):
Strong evidence ‐ provided by generally consistent findings in multiple RCTs with low bias ratings.
Moderate evidence ‐ provided by generally consistent findings in one RCT with low bias and one or more RCTs with moderate or high bias ratings, or by generally consistent findings in multiple RCTs with moderate or high bias ratings.
Limited evidence ‐ limited evidence, with only one RCT (any bias rating).
Equivocal evidence ‐ conflicting evidence, with inconsistent findings in multiple RCTs.
No evidence ‐ no evidence (no RCTs).
Sensitivity analysis
Sensitivity analyses were performed to assess the effect of methodological quality, quality of diagnostic criteria, severity of CTS symptoms and gender on findings.
Sensitivity analyses were defined for the following subgroups:
1. Methodological quality of trials
Trials rated as A (low risk of bias), B (moderate risk of bias), C (high risk of bias) were distinguished. Sensitivity analyses were performed in which (a) B and C were excluded and (b) C was excluded.
2. Quality of diagnostic criteria
Trials were classified into high (A), moderate (B) and low (C) quality according to criteria proposed by Rempel and colleagues (Rempel 1998) and described above.
Sensitivity analyses were performed in which (a) B and C were excluded and (b) C was excluded.
3. Severity of CTS symptoms in participants according to clinical classification
Participants with early (E), intermediate (I) and advanced (A) (Szabo 1992) CTS were distinguished. Sensitivity analyses were performed in which: (a) I and A were excluded, (b) A was excluded and (c) E was excluded.
4. Gender
Results
Description of studies
See Table of studies
Trials identified
A total of 43 eligible randomised or quasi‐randomised controlled trials were identified. All trials were from the published literature. Twenty‐two of the 43 trials were excluded. Seven of the excluded trials (Bhatia 2000; Bury 1995; Chaise 1994; Cook 1995; Finsen 1999; Hochberg 2001; Provinciali 2000) included participants who underwent carpal tunnel release which was an exclusion criterion for this review. Nine of the excluded trials (Celiker 2002; Dammers 1999; Elbaz 1994; Girlanda 1993; Lucantoni 1992; O'Gradaigh 2000; Ozdogan 1984; Piotrowski 1998; Wong 2001) were concerned with the investigation of steroid injection as the primary treatment, and did not meet our inclusion criteria. Two of the excluded trials (Wolaniuk 1983; Wu 1991) did not measure the primary or secondary outcome measures specified by the review. Two of the excluded trials (Baum 1986; Jarmuzewska 2000) did not examine the efficacy of non‐surgical treatment for CTS. Two of the excluded trials (Bennett 1998; Guy 1988) involved participants not diagnosed with CTS.
Other citations identified by the search strategy included six clinical commentaries on other studies (Abbot 1999; Bonebrake 1994; Deliss 1998; Hafner 1999; Helwig 2000; Sucher 1999) and 10 studies (Bonebrake 1993; Daniel 2000; Ellis 1982; Kruger 1991; Li 1999; Monge 1995; Nathan 2001; Padua 1999; Rozmaryn 1998; Sucher 1994) which were not randomised trials.
Trials included
Twenty‐one of the 43 trials were included. Of these, two trials (Aigner 1999; Koyuncu 1995) were published in languages other than English (one in German and one in Turkish) and were subsequently translated for this review. The 21 trials presented findings in 12 treatment areas: splinting, therapeutic ultrasound, ergonomic keyboards, oral medications, vitamins, exercise, yoga, mobilisation, magnet therapy, chiropractic care, laser acupuncture and insulin injection.
Three of the included trials (Burke 1994; Manente 2001; Walker 2000) were concerned with splinting. Burke and colleagues (Burke 1994) compared the position for wrist splinting (neutral versus 20 degrees extension) in 59 subjects. Manente et al. (Manente 2001) examined the efficacy of wearing a hand brace at night when compared to no treatment for four weeks. Walker et al. (Walker 2000) contrasted full‐time versus night only wearing of a wrist splint for six weeks.
Three of the included trials (Ebenbichler 1998; Koyuncu 1995; Oztas 1998) examined the effect of therapeutic ultrasound. Ebenbichler and colleagues (Ebenbichler 1998) compared pulsed ultrasound therapy versus placebo ultrasound for seven weeks duration. Koyuncu (Koyuncu 1995) compared the delivery of circular ultrasound at two different frequencies (1 and 3MHz) for four weeks. Oztas et al.(Oztas 1998) compared the use of continuous ultrasound at different intensities (1.5, 0.8 and 0.0W/cm2) for two weeks.
Two of the included trials (Rempel 1999; Tittiranonda 1999) studied ergonomic keyboards. Rempel et al. (Rempel 1999) compared an ergonomically adjusted keyboard, using altered force‐displacement key characteristics, with a standard keyboard for 12 weeks. Tittiranonda et al.(Tittiranonda 1999) compared three ergonomic keyboard designs with a standard keyboard for six months.
Six of the included trials (Chang 1998; Herskovitz 1995; Hui 2001; Pal 1988; Spooner 1993; Stransky 1989) studied oral medication or vitamins. Chang and colleagues(Chang 1998) compared the use of diuretic, nonsteroidal anti‐inflammatory and oral steroid treatment with a placebo for four weeks. Herskovitz et al. (Herskovitz 1995) compared the use of prednisone with placebo treatment for two weeks. Hui and colleagues (Hui 2001) compared the efficacy of prednisolone compared with placebo for 10 days. Pal and colleagues (Pal 1988) compared a diuretic (bendrofluazide) with placebo for four weeks. Spooner et al. (Spooner 1993) compared vitamin B6 (pyridoxine) with placebo for 12 weeks, whilst Stransky and colleagues (Stransky 1989) did the same for 10 weeks.
The remaining seven included trials (Akalin 2002; Garfinkel 1998; Tal‐Akabi 2000; Carter 2002; Davis 1998; Aigner 1999; Ozkul 2001) examined various different interventions for CTS. Akalin and colleagues (Akalin 2002) examined the benefit of daily nerve and tendon gliding exercises compared with (wrist splints) for four weeks. Garfinkel et al.(Garfinkel 1998) studied the efficacy of yoga performed twice weekly for eight weeks with wrist splints. Tal‐Akabi et al.(Tal‐Akabi 2000) compared the provision of carpal bone and neurodynamic mobilisation with no treatment for three weeks. The procedure for neurodynamic mobilisation is described as upper limb tension test 2a (ULTT2a) by Butler (Butler 1991). This mobilisation procedure involves movement of the patient's affected upper limb through its passive range of motion. The stages in ULTT2a mobilisation include: Stage 1: the patient starts lying supine on a bed; Stage 2: the clinician passively moves the patient's upper limb into slight glenohumeral abduction and shoulder girdle depression; Stage 3: elbow extension is added; Stage 4: lateral rotation of the whole arm is added; Stage 5: wrist, thumb and finger extension is added; Stage 6: maintenance of other postures and addition of glenohumeral abduction to the end of available range or to the point where symptoms are produced. Carter and colleagues (Carter 2002) compared the effect of wearing a magnetic device over the carpal tunnel versus a placebo device for 45 minutes. Davis et al. (Davis 1998) compared chiropractic care, comprising manual thrusts, massage, ultrasound and wrist splints, with medical management (ibuprofen and wrist splint) for seven weeks. Aigner and colleagues (Aigner 1999) compared soft laser acupuncture treatment with placebo for three weeks. Ozkul et al. (Ozkul 2001) compared the efficacy of weekly injections of insulin into the carpal tunnel with placebo for seven weeks.
Diagnostic criteria
The quality of the diagnostic criteria reported in the included trials was assessed according to the criteria proposed by Rempel (Rempel 1998). Seventeen of the included trials (Aigner 1999; Akalin 2002; Davis 1998; Ebenbichler 1998; Garfinkel 1998; Herskovitz 1995; Hui 2001; Koyuncu 1995; Manente 2001; Ozkul 2001; Oztas 1998; Pal 1988; Spooner 1993; Stransky 1989; Tal‐Akabi 2000; Walker 2000) reported using a combination of electrophysiologic findings and symptoms for the diagnosis of CTS and were graded as high quality (A). Three of the included trials (Burke 1994; Rempel 1999; Tittiranonda 1999) reported using a combination of symptoms and physical examination findings for the diagnosis of CTS and received a moderate quality rating (B). Only one of the included trials (Carter 2002) reported the use of symptoms alone for the diagnosis of CTS and received a low quality rating (C). One difference between the samples in the trials was that some included participants were screened for differential diagnoses to CTS, such as polyneuropathy and cervical disc disease, (Akalin 2002; Chang 1998; Ebenbichler 1998; Herskovitz 1995; Hui 2001; Manente 2001; Ozkul 2001; Rempel 1999; Spooner 1993; Tal‐Akabi 2000; Tittiranonda 1999). Some studies mentioned screening for concurrent conditions that are associated with CTS, such as pregnancy, renal disease, diabetes mellitus, rheumatoid arthritis (Davis 1998; Ebenbichler 1998; Garfinkel 1998; Hui 2001; Manente 2001; Ozkul 2001; Oztas 1998; Pal 1988; Spooner 1993; Tal‐Akabi 2000). One trial (Ozkul 2001) included only participants who had diabetes mellitus and CTS. Only seven studies (Chang 1998; Ebenbichler 1998; Herskovitz 1995; Manente 2001; Ozkul 2001; Pal 1988; Walker 2000) attempted to classify the severity of CTS symptoms in participants. Methods included the use of electrophysiologic findings (Chang 1998; Pal 1988; Walker 2000), duration of symptoms (Ebenbichler 1998; Ozkul 2001) and the use of a previously reported classification system (Manente 2001). One trial (Herskovitz 1995) did not report the method used to classify symptom severity. None of the studies included an equal gender representation while two of the studies (Ozkul 2001; Oztas 1998) only included females. Three of the studies (Burke 1994; Ebenbichler 1998; Stransky 1989) did not publish the gender distribution of participants.
Summary details of the trials are provided in the 'Table of included studies'.
Suitability of trials for meta‐analysis
Data from three trials (Chang 1998; Herskovitz 1995; Hui 2001) could be pooled to provide an estimate of the effect of oral steroid medication for CTS. Each trial examined the change in symptom severity after two weeks of oral steroid treatment using a global symptom score. Two of the trials (Herskovitz 1995; Hui 2001) also evaluated the effects of oral steroid use after treatment cessation (at eight weeks).
Data from two ultrasound treatment trials (Ebenbichler 1998; Oztas 1998) were pooled to provide an estimate of the effect upon symptom severity after two weeks. No other data were statistically pooled. This was because studies were clinically heterogeneous in type and duration of interventions, outcome measures reported or the characteristics of participants. Twelve different types of treatment were identified from the included trials (splinting, ultrasound, ergonomic keyboards, oral medication, vitamins, exercise, yoga, mobilisation, magnet therapy, chiropractic care, laser acupuncture, insulin injection) and duration of treatment varied from 45 minutes (Carter 2002) to six months (Tittiranonda 1999).
Availability of primary and secondary outcome measures
Three of the included trials (Ebenbichler 1998; Ozkul 2001; Pal 1988) assessed our proposed primary outcome of improvement in clinical symptoms after a minimum of three months following treatment end. Ebenbichler et al. (Ebenbichler 1998) reviewed symptom improvement at four months after the end of treatment, Ozkul et al. (Ozkul 2001) recorded a global symptom score at 15 weeks following the end of treatment and Pal and colleagues (Pal 1988) recorded symptom improvement at five months after the end of treatment. Five other trials (Akalin 2002; Carter 2002; Davis 1998; Herskovitz 1995; Hui 2001) measured outcome at a period beyond the end of treatment (eight weeks, two weeks, one month, two weeks and six weeks after the end of treatment respectively). The remaining 13 trials met the secondary outcome of measuring clinical improvement at less than three months of follow‐up and these were included in the analysis. All data, which reported our proposed primary or secondary outcome measures, were entered into RevMan. A table summarising the treatment comparisons (Table 1) is appended to this review. Seven of the trials (Davis 1998; Garfinkel 1998; Koyuncu 1995; Manente 2001; Oztas 1998; Stransky 1989; Walker 2000) reported peripheral nerve conduction findings measured earlier than our proposed timeframe. As this did not meet our protocol, these data were not entered into RevMan or considered in this review.
1. Treatment comparisons.
| Study reference | Baseline treatment | Comparison treatment |
| Aigner 1990 | placebo | laser acupuncture |
| Carter 2002 | placebo | magnet therapy |
| Chang 1998 | placebo | diuretic |
| Chang 1998 | placebo | NSAID |
| Chang 1998 | placebo | oral steroid |
| Ebenichler 1998 | placebo | ultrasound |
| Herskovitz 1995 | placebo | oral steroid |
| Hui 2001 | placebo | oral steroid |
| Ozkul 2001 | placebo | insulin injection |
| Oztas 1998 | placebo | ultrasound |
| Pal 1988 | placebo | diuretic |
| Spooner 1993 | placebo | vitamin B6 |
| Tittiranonda 1999 | placebo | ergonomic keyboard |
| Rempel 1999 | placebo, control | ergonomic keyboard |
| Stransky 1989 | placebo, control | vitamin B6 |
| Akalin 2002 | control | nerve and tendon gliding exercise |
| Manente 2001 | control | neurodynamic mobilisation |
| Tal‐Akabi 2000 | control | neurodynamic mobilisation |
| Tal‐Akabi 2000 | control | carpal bone mobilisation |
| Garfinkel 1998 | control (splint and concurrent tx) | yoga |
| Davis 1998 | control (medical tx) | chiropractic |
| Burke 1994 | splint (in neutral) | splint (in extension) |
| Walker 2000 | splint (full‐time) | splint (night only) |
| Koyuncu 1995 | ultrasound (1 MHz) | ultrasound (3MHz) |
Risk of bias in included studies
See Table of included studies
The overall methodological quality of the included trials was assessed according to the approach outlined by Clarke (Clarke 1999). This summary takes into consideration the potential for selection, performance, attrition and detection bias. The risk of bias was rated as low (A) in three of the included trials (Ebenbichler 1998; Hui 2001; Spooner 1993), as moderate (B) in eight (Aigner 1999; Carter 2002; Chang 1998; Herskovitz 1995; Ozkul 2001; Oztas 1998; Pal 1988; Rempel 1999)and high (C) in 10 (Akalin 2002; Burke 1994; Davis 1998; Garfinkel 1998; Koyuncu 1995; Manente 2001; Stransky 1989; Tal‐Akabi 2000; Tittiranonda 1999; Walker 2000). The most common sources of bias included inadequate or unclear allocation concealment (selection bias) and lack of blinding of subjects or clinicians to treatment (performance bias).
Allocation concealment was rated as adequate (A) in 11 of the included trials (Aigner 1999; Carter 2002; Chang 1998; Davis 1998; Ebenbichler 1998; Garfinkel 1998; Herskovitz 1995; Hui 2001; Manente 2001; Rempel 1999; Spooner 1993). The method of subject allocation was unclear (B) in six of the included trials (Koyuncu 1995; Ozkul 2001; Oztas 1998; Pal 1988; Stransky 1989; Tittiranonda 1999) and attempts to clarify this issue with authors unsuccessful. Allocation concealment was rated as inadequate (C) or not used (D) in four included trials (Akalin 2002; Burke 1994; Tal‐Akabi 2000; Walker 2000). Methods of allocation for these trials comprised random numbers (Akalin 2002), alternating allocation between intervention groups (Burke 1994), pulling names out of a hat (Tal‐Akabi 2000) and using the last digit of subjects' social security number (Walker 2000).
Effects of interventions
Nocturnal hand brace versus control (no treatment)
One trial (Manente 2001) with a high bias rating was identified. It evaluated the short‐term effects of the nocturnal hand brace on symptoms, hand function, patient‐reported change and nerve conduction. A significant effect in favour of nocturnal hand brace use for CTS was demonstrated. The weighted mean difference for improvement in symptoms following two weeks and four weeks of use was ‐1.03 (95% CI ‐1.31 to ‐0.75) and ‐1.07 (95% CI ‐1.29 to ‐0.85) respectively using a 1 to 5 point scale. The weighted mean difference for improvement in hand function following two weeks and four weeks of use was ‐0.52 (95% CI ‐0.79 to ‐0.25) and ‐0.55 (95% CI ‐0.82 to ‐0.28) respectively (1 to 5 point scale). The relative rate of participants reporting overall improvement after four weeks of brace use was 4.00 (95% CI 2.34 to 6.84). In summary, there is limited evidence that a nocturnal hand brace improves symptoms, hand function and overall patient‐reported change in the short‐term (up to four weeks of use).
Wrist splint: full‐time versus night‐only use
One trial (Walker 2000) with a high bias rating was identified. It compared the short‐term effects of full‐time use of a wrist splint with nocturnal use on symptoms, hand function and nerve conduction. No significant difference in symptom or hand function improvement was demonstrated between the groups over the six‐week period. In summary, there is limited evidence that night‐only wrist splint use is equally effective as full‐time wrist splint use in improving short‐term symptoms and hand function .
Wrist splint: neutral versus 20 degree extension angle
One trial (Burke 1994) with a high bias rating compared the short‐term effects of wrist splinting in neutral with splinting in an extended wrist position (20 degrees) on overall, nocturnal and daytime symptoms. A significant effect was demonstrated in favour of the neutral position for wrist splinting in CTS. The relative risk for improvement in overall and nocturnal symptoms at two weeks following fabrication of the neutral wrist splint was 2.43 (95% CI 1.12 to 5.28) and 2.14 (95% CI 0.99 to 4.65) respectively. No effect of wrist position was found for daytime symptoms at two weeks following splint use. In summary, there is limited evidence that neutral wrist splinting results in superior short‐term overall and nocturnal symptom relief (at two weeks) when compared with wrist splinting in extension. Furthermore, limited evidence suggests that short‐term daytime symptom relief is similar for both splint groups.
Ultrasound versus placebo
One trial (Ebenbichler 1998) with a low bias rating and one trial (Oztas 1998) with a moderate bias rating were identified. Although the two trials used different modes of delivery (one pulsed, one continuous, varying frequencies and intensities) they were considered to be sufficiently homogeneous, both clinically and statistically, to pool findings in relation to short‐term symptom relief at two weeks. Statistical homogeneity was demonstrated between the trials (Chi‐square 0.29; df=1; p=0.59). Both evaluated the short‐term effects of ultrasound treatment when compared with a placebo. Long‐term effects were also assessed in one trial (Ebenbichler 1998). Both trials assessed symptoms and nerve conduction, while one trial assessed sensation, grip strength, pinch strength and patient‐reported improvement (Ebenbichler 1998) and the other assessed pain and nocturnal waking (Oztas 1998). No significant improvement in pain, symptoms, or nocturnal waking was demonstrated in favour of therapeutic ultrasound after two weeks of treatment. No significant improvement in peripheral nerve conduction, grip strength or pinch strength assessed at six months was found after seven weeks of ultrasound treatment. However, a significant effect of ultrasound on symptom improvement was demonstrated after seven weeks of treatment and at six months follow‐up (Ebenbichler 1998). The weighted mean difference was ‐0.99 (95% CI ‐1.77 to ‐0.21) and ‐1.86 (95% CI ‐2.67 to ‐1.05) respectively on a 0 to 10 point visual analogue scale (VAS). A significant effect in favour of seven weeks of therapeutic ultrasound was also demonstrated for sensation and self‐reported improvement. The weighted mean difference for sensory improvement at six months was ‐1.18 (95% CI ‐2.02 to ‐0.34) on a 0 to 10 point VAS. The relative risk for self‐reported improvement at six months was 1.91 (95% CI 1.13 to 3.23). In summary, there is moderate evidence that two weeks of ultrasound treatment does not improve short‐term symptoms beyond that achieved with placebo. However, limited evidence does suggest that ultrasound results in superior symptom relief after seven weeks of treatment and beyond a seven week treatment period (assessed at six months) when compared with placebo. There is limited evidence that seven weeks of ultrasound therapy results in better sensory perception and self‐reported improvement when compared to placebo. There is limited evidence that short‐term pain and nocturnal waking are similar between ultrasound and placebo‐treated groups. Furthermore, there is limited evidence that long‐term nerve conduction, grip and pinch strength values are similar for ultrasound and placebo groups.
Ultrasound (various intensities): 1.5 W/cm2 versus 0.8 W/cm2 versus placebo
One trial (Oztas 1998) with a moderate bias rating compared the short‐term effects of continuous ultrasound treatment of different intensities (1.5W/cm2 or 0.8W/cm2) with placebo ultrasound (0.0 W/cm2) on pain, symptoms, nocturnal waking and peripheral nerve conduction. No significant effect of varying intensity of ultrasound delivery was demonstrated for pain, symptoms or nocturnal waking. There is, therefore, limited evidence that continuous ultrasound at 1.5W/cm2 is equally effective in improving short‐term pain, symptoms and nocturnal waking as continuous ultrasound at 0.8W/cm2.
Ultrasound (various frequencies): 1 MHz versus 3 MHz
One trial (Koyuncu 1995) with a high bias rating was identified. It compared the short‐term effects of ultrasound with different frequencies (1 MHz or 3 MHz) on pain, paraesthesia, sensation, grasping ability, provocative tests (Phalen, Tinel) and peripheral nerve conduction. No significant effect of varying frequency of ultrasound delivery was demonstrated for pain, paraesthesia, superficial sensation, large or small object grasping ability, Tinel's sign or Phalen's sign. In summary, there is limited evidence that ultrasound delivery at 1 MHz is similar to ultrasound delivery at 3 MHz for pain, paraesthesia, sensation, grasp and provocative testing measures in the short‐term.
Ergonomic keyboard versus standard keyboard
One trial (Rempel 1999) with a moderate bias rating and one trial (Tittiranonda 1999) with a high bias rating were included. Both trials evaluated the effects of ergonomic keyboard use when compared with a standard keyboard. Outcome measures assessed in both trials included pain, hand function and timed Phalen's test. Phalen's and Tinel's sign (Tittiranonda 1999) and peripheral nerve conduction (Rempel 1999) were also examined. Although the two trials used common outcome measures, one trial (Rempel 1999) reported endpoint scores while the other trial (Tittiranonda 1999) reported change scores for continuous outcomes. Therefore, pooling data for a meta‐analysis was not performed. No significant effect in favour of ergonomic keyboard provision was demonstrated for improving Phalen's or Tinel's sign, timed Phalen's test or peripheral nerve conduction. While findings from one trial (Tittiranonda 1999) demonstrated no significant effect of ergonomic keyboard on pain, the other trial (Rempel 1999) did demonstrate an effect in favour of ergonomic keyboard with a weighted mean difference of ‐2.40 (95% CI ‐4.45 to ‐0.35) on a 0 to 10 point scale. Change scores for pain and hand function reported by (Tittiranonda 1999) demonstrated considerable variability (indicated by large standard deviations). Findings demonstrated no effect of two ergonomic keyboard designs (Protouch Keyboard, Comfort Keyboard System) on hand function, but a significant effect in favour of two other styles (Microsoft Natural Keyboard, Apple Adjustable Keyboard) was demonstrated by (Tittiranonda 1999). Mean differences for these keyboards were 1.92 (95% CI 0.84 to 3.00) and 0.93 (95% CI 0.26 to 1.60) respectively (0 to 10 point scale). In summary, limited evidence suggests that ergonomic and standard keyboards provide similar improvements in Phalen's and Tinel's sign, timed Phalen's test and peripheral nerve conduction. There is equivocal evidence regarding the effect of ergonomic keyboards on pain relief and hand function.
Diuretic treatment versus placebo
Two trials (Chang 1998; Pal 1988) with moderate bias ratings were included. Chang et al. (Chang 1998) evaluated the short‐term effects of diuretic treatment (and other drug treatments) on carpal tunnel symptoms when compared with a placebo. Pal and colleagues (Pal 1988) also evaluated the effects of four weeks of diuretic treatment on carpal tunnel symptoms and median nerve latency when compared to a placebo. No significant effect in favour of diuretic treatment was demonstrated for improving carpal tunnel symptoms. A significant effect of diuretic treatment on peripheral nerve conduction was reported by Pal (Pal 1988), but the actual values of the outcome measures were not published. In summary, limited evidence suggests that diuretic treatment does not improve short‐term symptoms in CTS.
Nonsteroidal anti‐inflammatory treatment versus placebo
One trial (Chang 1998) with a moderate bias rating was included. It evaluated the short‐term effects of nonsteroidal anti‐inflammatory drug (NSAID) treatment (and other oral medications) on carpal tunnel symptoms when compared with a placebo. No significant effect in favour of NSAID treatment was demonstrated for improving carpal tunnel symptoms. In summary, limited evidence suggests that NSAID treatment does not improve short‐term symptoms in CTS.
Oral steroids versus placebo
Two trials (Chang 1998; Herskovitz 1995) with moderate bias ratings and one trial (Hui 2001) with a low bias rating were included. All three trials assessed symptom improvement following short‐term treatment with oral steroids, either using prednisolone (Chang 1998; Hui 2001) or prednisone (Herskovitz 1995). There was a minor variation in treatment length between two of the trials (Herskovitz 1995; Hui 2001) of two weeks and 10 days respectively, but it was felt that this did not pose a significant threat to clinical homogeneity. All three trials were pooled in relation to short‐term symptom improvement after two weeks of treatment. There was no significant statistical heterogeneity between the trials (Chi‐square 0.80; df=2; p=0.67). A significant effect in favour of oral steroids was demonstrated on symptom improvement with two and four weeks of treatment. The pooled weighted mean difference for improvement of symptoms after two weeks of treatment was ‐7.23 (95% CI ‐10.31 to ‐4.14) on a 0 to 50 point scale. This significant positive effect of oral steroids was also demonstrated after four weeks of treatment with weighted mean difference for symptoms of ‐10.8 (95% CI ‐15.26 to ‐6.34) (Chang 1998). However, findings from one of the trials (Herskovitz 1995) demonstrated that the benefit of two weeks of oral steroid treatment on symptoms is lost after an additional two weeks of no treatment. The weighted mean difference for symptoms assessed at two weeks following the end of two‐week treatment period was ‐6.19 (95% CI ‐15.14 to 2.76) (0 to 50 point scale). Two trials (Herskovitz 1995; Hui 2001) examined the effect of oral steroid at six weeks following cessation of treatment. Findings from one trial (Herskovitz 1995) demonstrated no effect, while the other (Hui 2001) found continued benefit from oral steroid use on symptoms at six weeks following treatment cessation. There was no significant heterogeneity between these two trials (Chi‐square 2.11; df=1; p=0.15). The pooled weighted mean difference for improvement of carpal tunnel symptoms at eight weeks (six weeks following treatment end) was ‐6.46 (95% CI ‐11.93 to ‐0.99) (0‐ to 50‐point scale). In summary, there is moderate evidence that oral steroid treatment for two weeks improves short‐term symptoms. Limited evidence suggests that symptom improvement is also achieved with four weeks of oral steroid treatment. There is equivocal evidence regarding the short‐term symptom benefit beyond the end of an oral steroid treatment period.
Diuretic versus nonsteroidal anti‐inflammatory treatment
One trial (Chang 1998) with a moderate bias rating was included. It evaluated the short‐term effects of diuretic treatment (and other oral medications) on carpal tunnel symptoms when compared with NSAID treatment. No significant difference in symptom improvement was demonstrated between the groups following two and four weeks of treatment. In summary, limited evidence suggests that there is no difference in the effect of diuretics and NSAIDs on short‐term CTS symptoms.
Diuretic versus oral steroids
One trial (Chang 1998) with a moderate bias rating was included. It evaluated the short‐term effects of diuretic treatment (and other oral medications) on carpal tunnel symptoms when compared with oral steroids. A significant effect in favour of oral steroids was demonstrated on symptom improvement with two and four weeks of treatment. The weighted mean difference for improvement of symptoms after two weeks of treatment was 7.30 (95% CI 3.43 to 11.17) and after four weeks 11.60 (95% CI 7.25 to 15.95) on a 0 to 50 point scale. In summary, there is limited evidence that short‐term oral steroid treatment improves CTS symptoms significantly more than diuretic treatment.
Nonsteroidal anti‐inflammatory treatment versus oral steroids
One trial (Chang 1998) with a moderate bias rating was included. It evaluated the short‐term effects of NSAID treatment (and other oral medications) on carpal tunnel symptoms when compared with oral steroids. A significant effect in favour of oral steroids was demonstrated on symptom improvement with 2 and 4 weeks of treatment. The weighted mean difference for improvement of symptoms after two weeks of treatment was 9.70 (95% CI 4.85 to 14.55) and after four weeks 14.00 (95% CI 8.57 to 19.43) on a 0 to 50 point scale. In summary, there is limited evidence to suggest that oral steroid use for 2 to 4 weeks significantly improves short‐term symptoms when compared to NSAID treatment.
Vitamin B6 (pyridoxine) versus placebo
One trial (Spooner 1993) with a low bias rating and one trial (Stransky 1989) with a high bias rating were included. Both trials evaluated the medium‐term effects of oral vitamin B6 (pyridoxine) as compared to a placebo. Although the treatment duration differed slightly between trials (12 and 10 weeks respectively), the dosage and delivery methods were identical. All except one outcome measure used between trials were different which prevented pooling of results. The outcome measured in both trials, peripheral nerve conduction, did not meet our outcome criteria and so was not included in analysis. The other outcomes evaluated were nocturnal discomfort, finger swelling, movement discomfort, hand co‐ordination, Phalen's sign and Tinel's sign (Spooner 1993) and symptoms (Stransky 1989). No significant effect of vitamin B6 was demonstrated for improvement in symptoms, nocturnal discomfort, hand co‐ordination, Phalen's sign or Tinel's sign after 10 to 12 weeks of treatment. However, a significant effect in favour of vitamin B6 was demonstrated for finger swelling and movement discomfort after 12 weeks of intervention. The weighted mean difference for finger swelling was ‐1.00 (95% CI ‐1.90 to ‐0.10) and for movement discomfort was ‐1.00 (95% CI ‐1.94 to ‐0.06) on 0 to 4 point scales. There is, therefore, limited evidence that vitamin B6 improves finger swelling and movement discomfort with 12 weeks of treatment. Limited evidence suggests that vitamin B6 does not improve symptoms, nocturnal discomfort, hand co‐ordination, Phalen's sign and Tinel's sign in the short‐term.
Nerve and tendon gliding exercise and neutral wrist splint versus control (neutral wrist splint alone)
One trial (Akalin 2002) with a high bias rating was included. It evaluated the medium‐term effects of performing nerve and tendon gliding exercises and using a wrist splint for 4 weeks on symptoms, hand function, grip strength, pinch strength, two‐point discrimination, Tinel's sign, Phalen's sign and patient satisfaction when compared with wrist splinting alone. No significant effect in favour of nerve and tendon gliding exercises was demonstrated for improving symptoms, hand function, grip strength, pinch strength, Phalen's sign, Tinel's sign or patient satisfaction at eight weeks following the four week exercise program. However, a significant effect of gliding exercises on static two‐point discrimination was demonstrated at eight weeks following treatment end. The weighted mean difference was ‐0.70 millimetres (95% CI ‐1.24 to ‐0.16). In summary, there is limited evidence that nerve and tendon gliding exercises and wrist splinting result in superior static two‐point discrimination compared to wrist splinting alone in the medium‐term. Limited evidence suggests that exercise plus wrist splinting and wrist splinting alone provide similar improvement in symptoms, hand function, grip strength, pinch strength, Phalen's sign, Tinel's sign and patient satisfaction.
Yoga versus wrist splint
One trial (Garfinkel 1998) with a high bias rating was included. It evaluated the short‐term effects of yoga on nocturnal waking, pain, Phalen's sign, Tinel's sign, grip strength and peripheral nerve conduction when compared to a control treatment of wrist splinting. No significant effect in favour of yoga was demonstrated for improving nocturnal waking, Tinel's sign or grip strength after eight weeks of treatment. However, a significant effect of yoga on improving pain and Phalen's sign was demonstrated after eight weeks of treatment. The weighted mean difference for pain was ‐1.40 (95% CI ‐2.73 to ‐0.07) on a 0 to 10 point VAS and the relative risk for Phalen's sign was 5.25 (95% CI 1.28 to 21.47). In summary, there is limited evidence that yoga results in superior short‐term pain relief and improved outcome for Phalen's sign compared to wrist splinting. There is limited evidence that yoga and wrist splinting provide similar short‐term improvement in nocturnal waking, Tinel's sign and grip strength.
Neurodynamic mobilisation versus control (no treatment)
One trial (Tal‐Akabi 2000) with a high bias rating was included. It evaluated the short‐term effect of neurodynamic mobilisation (and another form of mobilisation) on symptoms, pain, hand function, wrist motion, upper limb tension testing and need for surgery when compared to no treatment. The upper limb tension test is a specific tension test which is used to bias the median nerve (previously reported by Butler (Butler 1991)). It is performed to reproduce symptoms or identify changes in existing symptoms. The authors describe the test as involving "slight glenohumeral abduction, shoulder girdle depression, elbow extension, lateral rotation of the whole arm, wrist, thumb and finger extension and finally glenohumeral abduction" (Tal‐Akabi 2000). No significant effect in favour of neurodynamic mobilisation was demonstrated for improving symptoms, pain, hand function, active wrist motion, upper limb tension test or need for surgical release after three weeks of treatment. In summary, limited evidence suggests that neurodynamic mobilisation does not improve short‐term symptoms, pain, hand function, wrist motion, upper limb tension testing nor reduce the likelihood of continuing to carpal tunnel release surgery.
Carpal bone mobilisation versus control (no treatment)
One trial (Tal‐Akabi 2000) with a high bias rating was included. It evaluated the short‐term effect of carpal bone mobilisation (and neurodynamic mobilisation) versus no treatment. No significant effect in favour of carpal bone mobilisation was demonstrated for improving pain, hand function, active wrist motion, upper limb tension test or need for surgical release after three weeks of treatment. However, a significant effect of carpal bone mobilisation on improving symptoms was demonstrated. The weighted mean difference for symptoms was ‐1.43 (95% CI ‐2.19 to ‐0.67) on a 0 to 5 point scale. In summary, limited evidence suggests that carpal bone mobilisation improves symptoms in the short‐term (with three weeks of treatment). Limited evidence also suggests that carpal bone mobilisation does not improve short‐term pain, hand function, wrist motion, upper limb tension test findings or the subsequent need for surgery.
Neurodynamic versus carpal bone mobilisation
One trial (Tal‐Akabi 2000) with a high bias rating was included. It evaluated the short‐term effect of neurodynamic mobilisation as compared to carpal bone mobilisation (and no treatment). No significant difference between the two forms of mobilisation was demonstrated for improving symptoms, pain, hand function, active wrist motion, upper limb tension test or need for surgical release after three weeks of treatment. In summary, limited evidence suggests that there is no significant benefit of neurodynamic over carpal bone mobilisation for improving short‐term CTS outcomes.
Magnet therapy versus placebo
One trial (Carter 2002) with a moderate bias rating was included. It evaluated the short‐term effect of applying a magnetic device over the carpal tunnel (for 45 minutes) on pain compared with a placebo device. No significant effect in favour of magnetic therapy was demonstrated for improving pain directly following treatment or after two weeks. In summary, limited evidence suggests that magnet therapy does not significantly improve short‐term pain relief in CTS.
Chiropractic treatment (manual thrusts, myofascial massage/loading, ultrasound and nocturnal wrist splint) versus medical treatment (ibuprofen and nocturnal wrist splint)
One trial (Davis 1998) with a high bias rating was included. It assessed the effect of chiropractic care (comprising various interventions: manual thrusts, myofascial massage and loading, ultrasound and nocturnal wrist splint) on physical distress, mental distress, vibrometry, hand function, health‐related quality of life and peripheral nerve conduction when compared to medical treatment (ibuprofen and wrist splint). No significant effect of chiropractic care was demonstrated for improving mental distress, vibrometry, hand function or health‐related quality of life after nine weeks of treatment. However, a significant effect favouring medical treatment on improving physical distress was demonstrated. The weighted mean difference was 3.51 (95% CI 0.09 to 6.93) on a 0 to 64 point scale. In summary, there is limited evidence that medical care over nine weeks improves physical distress in the short‐term when compared with chiropractic treatment. Limited evidence also suggests that chiropractic and medical treatment provide similar short‐term improvement in mental distress, vibrometry, hand function and health‐related quality of life.
Laser acupuncture versus placebo
One trial (Aigner 1999) with a moderate bias rating was included. It evaluated the short‐term effect of laser acupuncture applied to various acupuncture points on paraesthesiae and night pain compared with a placebo laser. No significant difference in paraesthesiae or night pain was demonstrated between laser acupuncture and placebo over a three‐week treatment period. In summary, limited evidence suggests that laser acupuncture does not improve short‐term paraesthesiae and night pain in CTS.
Steroid and insulin injections versus steroid and placebo injections
One trial (Ozkul 2001) with a moderate bias rating was included. It evaluated the medium and long‐term effects of steroid injection into the carpal tunnel followed by weekly injections of insulin on symptoms and peripheral nerve conduction when compared with steroid injection into the carpal tunnel followed by weekly placebo injections. A significant effect in favour of steroid plus insulin injections on symptom and nerve conduction values was demonstrated over steroid plus placebo group. The weighted mean difference for each outcome could not be calculated as point estimates and measures of variability were not reported in the published trial. Attempts to obtain the raw data from the authors were unsuccessful. In summary, limited evidence suggests that a steroid injection followed by weekly insulin injections into the carpal tunnel for eight weeks results in superior symptom relief and nerve conduction compared with steroid injection and weekly placebo injections over the same period.
Sensitivity analyses
Sensitivity analyses were performed where data were combined from more than one trial to estimate the effect of non‐surgical treatment for CTS. Pooled weighted mean differences were calculated to provide estimates of the efficacy of ultrasound (Ebenbichler 1998; Oztas 1998) and oral steroid use (Chang 1998; Herskovitz 1995; Hui 2001) on symptoms. No change in the effect of either treatment on symptom improvement was found when the effect of methodological quality and quality of diagnostic criteria was examined. It was not possible to conduct sensitivity analysis to test the effect of symptom severity and gender due to inadequate information.
Discussion
We set out to determine the effectiveness of non‐surgical treatments (other than steroid injection) when compared with no treatment, a placebo, or with other non‐surgical treatments for improving clinical outcome in persons with CTS. Twenty‐one trials which investigated splinting, therapeutic ultrasound, ergonomic keyboards, oral medication, vitamins, exercise, yoga, carpal mobilisation, magnet therapy, chiropractic care, laser acupuncture and insulin injection were included.
Methodological quality of the trials
Between one and three RCTs were found regarding each treatment, providing some moderate but mainly limited evidence that will be discussed below, in order of strongest evidence first. The quality of the trials was mostly moderate or low when the bias scoring approach outlined by Clarke (Clarke 1999) in the Cochrane Reviewers' Handbook was applied. The scoring system disadvantaged trials in which blinding of treatment providers and participants could not be achieved. Several trials, (i.e. splinting, tendon and nerve gliding exercise etc), were unable to blind treatment providers and subjects to the treatment, and these trials received a high bias rating. In contrast trials which could minimise performance bias by double blinding had the potential to be rated as having moderate or low bias. This scoring approach places therapy trials where blinding is not possible at a disadvantage when compared with trials where blinding of intervention is achievable. One element of the studies that was not reviewed was the power of the negative studies to assure that a type II error did not occur due to insufficient sample size.
Quality of diagnostic criteria
The criteria proposed by Rempel and colleagues (Rempel 1998) were used to judge the diagnostic quality in this review. Rempel and colleagues advocate the combination of electrodiagnostic findings and symptoms to diagnose CTS. The American Academies of Neurology, Electrodiagnostic Medicine and Physical Medicine and Rehabilitation (AAN 1993; Jablecki 2002) outline electrodiagnostic studies accepted as appropriate for confirmation of CTS diagnosis. Seventeen of the 21 trials included in this review reported a combination of electrodiagnostic findings and symptoms. A statement by the authors confirming the use of electrodiagnostic testing in combination with the assessment of clinical symptoms was considered to satisfy this criterion.
Outcome measures
We performed a detailed assessment of the outcome measures used in the included trials. The review highlighted a wide variation in the outcome measures assessed, the lack of evidence regarding their reliability, validity and responsiveness in CTS populations, and the varied and predominantly short‐term nature of outcome assessment across trials (i.e. majority of trials only measured outcome at conclusion of treatment). In fact, only three studies used our recommended primary outcome measure of symptom improvement at least three months post intervention. These features meant that pooling of results was rarely possible, interpretation of the clinical significance and accuracy of findings was made difficult, and little information about the medium to long‐term effects of non‐surgical treatments can be concluded. Furthermore, most studies failed to quantify the severity of CTS leaving open the question of whether or not different severities of CTS respond similarly.
Evidence for non‐surgical treatment of CTS
Moderate evidence (consistent findings in more than one RCT) suggests that there is no significant improvement immediately following two weeks of therapeutic ultrasound. This effect was demonstrated by pooling the results from one high quality and one moderate quality trial, both using high quality diagnostic criteria for CTS.
Moderate evidence supports a positive effect on symptoms immediately following oral steroid treatment for a two‐week period. This effect was reached by pooling data from three trials of high and moderate methodological quality, in which good diagnostic criteria were used. Systemic adverse effects from oral steroids are quite common, however these did not appear limiting in these trials using short courses of oral steroids. The weighted mean difference in symptom severity between the oral steroid and placebo groups was demonstrated to be just over seven points on a global symptom score, with 95% confidence limits ranging from 4 to 10 points. The global symptom score is a patient rating of symptom severity across five areas (pain, numbness, paraesthesiae, weakness/clumsiness, nocturnal waking) with the global score ranging from 0 (no symptoms) to 50 (worst symptoms). Unfortunately there is no evidence regarding the reliability, validity and responsiveness of the global symptom score used in the oral steroid trials.
The treatment effects for ultrasound and oral steroid treatment remained consistent when sensitivity analyses were conducted to examine the effect of methodological quality and diagnostic quality.
Limited evidence (findings from one RCT) suggests that therapeutic ultrasound for seven weeks provides a positive short to long‐term effect on symptom severity. This finding is derived from one trial (Ebenbichler 1998) rated as having high methodological and diagnostic quality. The average difference in symptom severity between the ultrasound and placebo groups at six months was reported to be almost two points on a visual analogue scale (95% confidence limits ranging from 1.05 to 2.67). The visual analogue scale is used to quantify symptom severity and ranges from 0 (no symptoms) to 10 (worst symptoms). This difference is likely to be of clinical significance. This treatment also provides a positive effect on sensation and patient‐reported improvement when assessed at six months. The average difference in patient‐reported sensation between the ultrasound and placebo groups was reported to be just over one point on a visual analogue scale, and the relative likelihood that patients receiving ultrasound will report improvement at six months is almost double that of patients in the placebo group.
Limited evidence supports a positive short‐term effect on symptoms following the use of a hand splint for two or four weeks. The average difference in symptom severity between the hand brace and control groups at the end of the treatment period is reported to be approximately one point on the carpal tunnel questionnaire (95% confidence intervals ranging from 0.75 to 1.31). The scale used to quantify symptom severity ranges from 1 point (no symptoms) to 5 points (very severe symptoms). There is evidence of reliability, validity and responsiveness of the questionnaire in CTS populations, and that this difference in symptom severity is likely to be clinically significant (Amadio 1996; Katz 1994; Levine 1993). This finding is derived from one trial (Manente 2001) rated as using high quality diagnostic criteria for CTS but also a high risk of performance bias. There is a relative likelihood that patients using the hand splint will report improvement, almost four times more than patients who receive no treatment.
Limited evidence supports a positive short‐term effect on symptom severity when splinting the wrist in neutral as compared to the wrist in extension. It is twice as likely that patients using the neutral wrist splint will report overall and nocturnal symptom relief after two weeks than patients who receive a wrist splint in extension. This was reported by one trial (Burke 1994) of poor methodological quality and diagnostic criteria of moderate quality. Hence, caution should be used in the interpretation of this finding due to these limitations.
Limited evidence suggests that an eight‐week yoga treatment provides short‐term improvements in pain when compared with the use of a wrist splint. The average difference in pain severity between the yoga and wrist splint groups was 1.4 points on a visual analogue scale (95% confidence intervals ranging from 0.07 to 2.73 points). The visual analogue scale was used to quantify pain severity and ranges from 0 (no pain) to 10 (worst possible pain). This difference is likely to be of clinical significance. This treatment also provides a positive effect on provocative testing when assessed at eight weeks. The relative likelihood that patients receiving yoga treatment will experience an improvement in Phalen's sign is around five times that of patients in the splint group. These findings were derived from one trial (Garfinkel 1998) rated as using high quality diagnostic criteria for CTS but having a high risk of selection, performance and detection bias.
Limited evidence suggests that carpal bone mobilisation over a three‐week period provides positive short‐term benefit on symptoms. The average difference in symptoms between the mobilisation and the control groups was 1.4 points on a visual analogue scale, (95% confidence limits ranging from 0.67 to 2.19). This finding is derived from one trial (Tal‐Akabi 2000) having high quality diagnostic criteria for CTS but having also a high risk of selection and performance bias.
Limited evidence suggests that vitamin B6 for 12 weeks decreases finger swelling and movement discomfort when assessed at the end of treatment. The average difference in symptoms between the vitamin B6 and placebo groups for both outcomes was around one point on a short ordinal scale. The scale rated symptom severity as 0 (none) to 4 (a great deal). It is unclear whether these differences in outcome represent clinically meaningful findings. The validity of these findings might be enhanced if they were converted to dichotomous data. Green and Deeks advise that short ordinal scales should not be treated as continuous variables but instead treated as binary outcomes (Green 2002). The authors reported these outcomes as continuous variables only.
Limited evidence suggests that medical care for nine weeks provides a small but significant benefit in terms of physical distress (function) when compared with chiropractic care. The average difference in physical distress between the medical and chiropractic groups was 3.5 points on a long ordinal scale. Participants' physical distress was measured by their responses to 16 questions about difficulty in daily activities. The physical distress score ranged from 0 (no difficulty) to 64 (extreme difficulty). It is unclear whether the difference between the groups constitutes a clinically significant finding.
Nerve and tendon gliding exercises performed over four weeks in combination with a wrist splint improved two‐point discrimination when assessed at three months, and compared to wrist splinting alone. The average difference in two‐point discrimination between the two groups was 0.70 of a millimetre. This difference was not considered clinically significant. Whilst two‐point discrimination has fair to good reliability in CTS populations (Marx 1998), such a small difference would be likely to be overshadowed by measurement error.
This systematic review was conducted according to the methods stipulated in the protocol. However, future revisions will divide up the content into reviews of related non‐surgical treatments to reduce the overall size of the review and facilitate usefulness for the reader.
The following would enhance future studies:
Use of electrodiagnostic findings (AAN 1993; Jablecki 2002) in combination with symptoms for CTS diagnosis.
Documentation and classification of severity and duration of symptoms of participants.
Short and long‐term assessment of treatment outcome (minimally at the end of treatment and at least three months following the end of treatment; and ideally up to one or two years after treatment).
Use of outcome measures which have been assessed for reliability, validity and sensitivity to change in CTS populations.
Consensus of outcome measurement across trials to facilitate meta‐analysis.
Prospective power analysis to detect clinically meaningful differences in outcome.
Analysis of direct and indirect costs associated with treatment.
Authors' conclusions
Implications for practice.
Moderate evidence shows significant short‐term benefit from oral steroids. Limited evidence shows significant short‐term benefit from splinting, ultrasound, yoga and carpal bone mobilisation. Other non‐surgical treatments do not produce significant benefit.
Implications for research.
More high quality research is needed to strengthen the moderate to limited evidence currently available on non‐surgical treatment. Future research needs to examine the relative contributions of different non‐surgical treatments for CTS, the optimal forms of delivery, the duration of any benefit (both during active treatment and after treatment cessation) and the optimal timing of delivery during the course of CTS. More high quality studies are required to establish better evidence to direct clinical practice.
Feedback
Comment
Summary
Jan M Bjordal
Date received: 09 February 2006
In the results section for ultrasound therapy you state that:"In summary, there is moderate evidence that two weeks of ultrasound treatment does not improve short‐term symptoms beyond that achieved with placebo". Your statement rest upon 2 trials, the moderate bias trial by Oztas, and the low bias(high quality) trial by Ebenbichler. Your statement is contradicted by the Ebenbichler trial report which found significant effects after 2 weeks for the main complaint p = 0.015 and 3 of 6 secondary outcomes.
In the symptoms analysis of ultrasound, I could not find the 2 weeks data you have used in the original Ebenbichler trial report. Where are they taken from? Are they 2 weeks data or data of change from baseline to 2 weeks?
The negative results and possibly reported harm for motor nerve conduction in the Oztas trial, may be due to the high continuous intensities of 0.8 and 1.5 W/cm2, while the Ebenbichler study used an intensity 0.2 W/cm2 when adjusted for pulsed mode. Why do you not make a dose analysis which could show that the different results may arise from different doses; i.e. and simply state that average intensity at 0.2 W/cm2 seems effective, while average intensities of 0.8 and 1.5 are ineffective?
Jan M Bjordal
Reply
Denise O'Connor
Date received: 25 August 2006
Dear Jan Bjordal,
Thank you for your comment regarding our review on non‐surgical treatment (other than steroid injection) for carpal tunnel syndrome, and more specifically, our findings comparing ultrasound vs. placebo.
The outcome data that you refer to in the text and that are displayed in Analysis 04.02 (Comparison: ULTRASOUND vs. PLACEBO; Outcome: symptoms) are endpoint mean and standard deviation values that were provided to us by the trial investigators (personal communication with Gerold Ebenbichler, dated 5 March 2002). We make reference to the use of this data in the notes section of the 'Characteristics of Included Studies' table where we report "Mean and standard deviation values for symptoms, sensation, grip strength, pinch strength and nerve conduction outcomes were provided by authors to facilitate entry into RevMan". We used endpoint data provided by Ebenbichler 1998 in favour of the change scores reported in their publication to facilitate pooling with data from the Oztas 1998 trial (which reported endpoint scores). At the time of publishing the review, the Cochrane Handbook did not advise combining endpoint and change scores in meta‐analyses.
We did not undertake a dose‐response analysis in relation to motor nerve conduction because the primary objective of the review was to compare the effectiveness of non‐surgical treatment with control, placebo or other non‐surgical treatments for CTS and we did not set out a priori to explore the relationship between dose and the size of treatment effect as a secondary aim of the review. However, we intend to investigate this in our next update of the review.
I hope this response has clarified the issues you identified. Thank you for your interest in our review.
Yours sincerely,
Denise O'Connor on behalf of the review team
Contributors
Denise O'Connor
What's new
| Date | Event | Description |
|---|---|---|
| 13 December 2017 | Amended | Update to Published notes |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 1, 2003
| Date | Event | Description |
|---|---|---|
| 13 June 2012 | Amended | Update to Published notes |
| 16 May 2012 | Amended | Update to Published notes |
| 7 December 2011 | Review declared as stable | Information added to Published notes about the updating of this review. |
| 5 May 2008 | Amended | Converted to new review format. |
| 15 August 2005 | Amended | An update to this review is currently in progress and expected to be published in 2006. |
| 28 October 2002 | New citation required and conclusions have changed | Substantive amendment |
Notes
This review will be updated by the publication of new Cochrane reviews and the change in scope of an existing review. When all new titles are published we will withdraw this review from publication. New titles published to date in the Cochrane Library are:
Ergonomic positioning or equipment for treating carpal tunnel syndrome (Issue 1, 2012);
Therapeutic ultrasound for carpal tunnel syndrome (Issue 1, 2012);
Exercise and mobilisation interventions for carpal tunnel syndrome (Issue 6, 2012);
Splinting for carpal tunnel syndrome (Issue 7, 2012); and
Low‐level laser therapy for carpal tunnel syndrome (Issue 8, 2017).
The scope of an existing review of Local corticosteroid injection for carpal tunnel syndrome is to be widened at the next update to include treatment with oral steroids (due to be published in the first half of 2018). A review of acupuncture for the symptoms of carpal tunnel syndrome is in progress and due for publication in early 2018.
Acknowledgements
We would like to thank Malgorzata Bala, Duray Seker, Usha Buenger and other colleagues for their assistance in translating abstracts and papers for this review.
We thank Louisa Dunn, Kate Jewitt, Carolyn Reid and Angela Gunn from the Cochrane Neuromuscular Disease Group for their assistance in devising the search strategy, helping to locate people to translate the non‐English trials and ongoing support for this review.
We thank the trialists (Nicholas Aigner, Elif Akalin, Cheryl Aspy, David Burke, Ming‐Hong Chang, Gerold Ebenbichler, Steven Herskovitz, Neil Lava, Yasar Ozkul, B Pal, David Rempel, Rick Spooner, Amir Tal‐Akabi, Antonino Uncini, William Walker, SM Wong) who corresponded with the principal reviewer to clarify additional information and/or provided additional data for the review.
We thank the following institutions for their support during the review: The School of Occupational Therapy, University of South Australia, Adelaide, AUSTRALIA The Institute for Rehabilitation Research and Development, Ottawa, CANADA
Appendices
Appendix 1. MEDLINE on OVID (1996 to Week 5 2001) search strategy
1 randomized controlled trial.pt. 2 randomized controlled trials/ 3 controlled clinical trial.pt. 4 controlled clinical trials/ 5 random allocation/ 6 double‐blind method/ 7 single‐blind method/ 8 clinical trial.pt. 9 exp clinical trials/ 10 (clin$ adj25 trial$).tw. 11 ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$ or dummy)).tw. 12 placebos/ 13 placebo$.tw. 14 random$.tw. 15 research design/ 16 (clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt. 17 multicenter study.pt. 18 meta analysis.pt. 19 prospective studies/ 20 intervention studies/ 21 cross‐over studies/ 22 meta‐analysis/ 23 (meta?analys$ or systematic review$).tw. 24 control$.tw. 25 or/1‐24 26 human/ 27 25 and 26 28 Carpal tunnel syndrome/dt,rh,th [Drug Therapy, Rehabilitation, Therapy] 29 27 and 28
Data and analyses
Comparison 1. HAND SPLINT (BRACE) VS CONTROL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 2 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 After 4 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Hand function | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 After 2 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 After 4 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Self‐reported improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 After 4 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.

Comparison 1 HAND SPLINT (BRACE) VS CONTROL, Outcome 1 Symptoms.
1.2. Analysis.
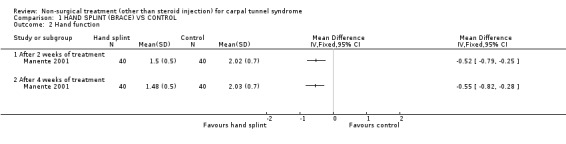
Comparison 1 HAND SPLINT (BRACE) VS CONTROL, Outcome 2 Hand function.
1.3. Analysis.
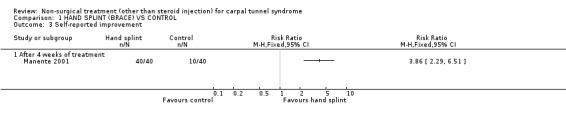
Comparison 1 HAND SPLINT (BRACE) VS CONTROL, Outcome 3 Self‐reported improvement.
Comparison 2. FULLTIME VS NOCTURNAL WRIST SPLINT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 6 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Hand function | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 After 6 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
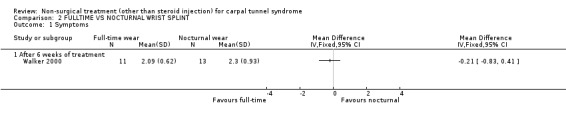
Comparison 2 FULLTIME VS NOCTURNAL WRIST SPLINT, Outcome 1 Symptoms.
2.2. Analysis.
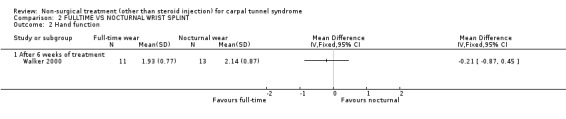
Comparison 2 FULLTIME VS NOCTURNAL WRIST SPLINT, Outcome 2 Hand function.
Comparison 3. NEUTRAL VS EXTENSION WRIST SPLINT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptom relief | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Overall relief after 2 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Nocturnal relief after 2 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Daytime relief after 2 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
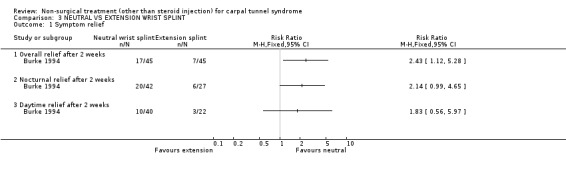
Comparison 3 NEUTRAL VS EXTENSION WRIST SPLINT, Outcome 1 Symptom relief.
Comparison 4. ULTRASOUND VS PLACEBO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 2 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Symptoms | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 After 2 weeks of treatment | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.67, 0.45] |
| 2.2 After 7 weeks of treatment | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐0.99 [‐1.77, ‐0.21] |
| 2.3 At 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.86 [‐2.67, ‐1.05] |
| 3 Nocturnal waking | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 After 2 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Sensation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Grip strength (kg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Pinch strength (kg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Self‐reported improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 At 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Median nerve conduction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Distal motor latency (ms) at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Sensory conduction velocity (m/s) at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.
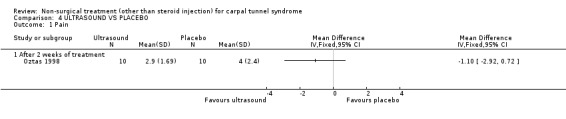
Comparison 4 ULTRASOUND VS PLACEBO, Outcome 1 Pain.
4.2. Analysis.
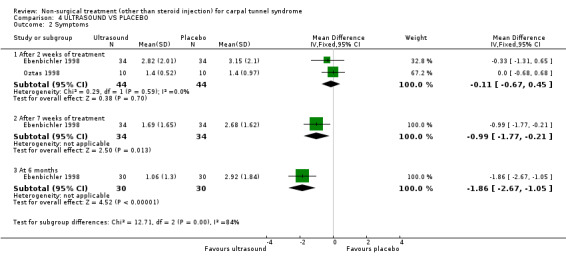
Comparison 4 ULTRASOUND VS PLACEBO, Outcome 2 Symptoms.
4.3. Analysis.
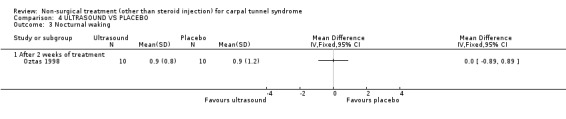
Comparison 4 ULTRASOUND VS PLACEBO, Outcome 3 Nocturnal waking.
4.4. Analysis.

Comparison 4 ULTRASOUND VS PLACEBO, Outcome 4 Sensation.
4.5. Analysis.
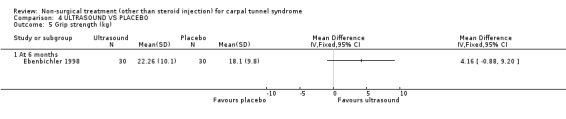
Comparison 4 ULTRASOUND VS PLACEBO, Outcome 5 Grip strength (kg).
4.6. Analysis.
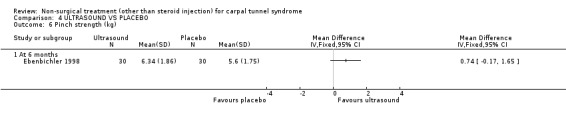
Comparison 4 ULTRASOUND VS PLACEBO, Outcome 6 Pinch strength (kg).
4.7. Analysis.

Comparison 4 ULTRASOUND VS PLACEBO, Outcome 7 Self‐reported improvement.
4.8. Analysis.
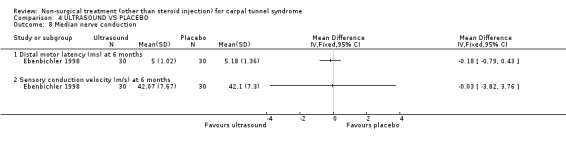
Comparison 4 ULTRASOUND VS PLACEBO, Outcome 8 Median nerve conduction.
Comparison 5. ULTRASOUND VS ULTRASOUND (VARYING INTENSITY).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 2 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 After 2 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Nocturnal waking | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 After 2 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.
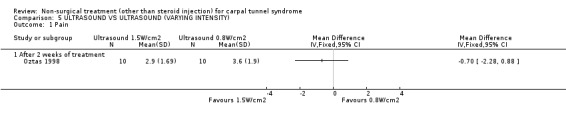
Comparison 5 ULTRASOUND VS ULTRASOUND (VARYING INTENSITY), Outcome 1 Pain.
5.2. Analysis.

Comparison 5 ULTRASOUND VS ULTRASOUND (VARYING INTENSITY), Outcome 2 Symptoms.
5.3. Analysis.
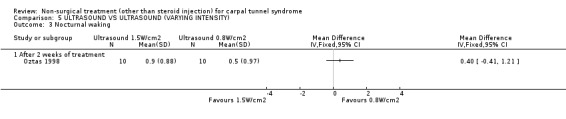
Comparison 5 ULTRASOUND VS ULTRASOUND (VARYING INTENSITY), Outcome 3 Nocturnal waking.
Comparison 6. ULTRASOUND VS ULTRASOUND (VARYING FREQUENCY).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improved pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 4 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Improved paresthesia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 After 4 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Improved superficial sensation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 After 4 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Improved grasp | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Large objects after 4 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Small objects after 4 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Improved Tinel's sign | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 After 4 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Improved Phalen's sign | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 After 4 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
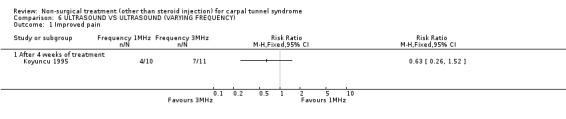
Comparison 6 ULTRASOUND VS ULTRASOUND (VARYING FREQUENCY), Outcome 1 Improved pain.
6.2. Analysis.
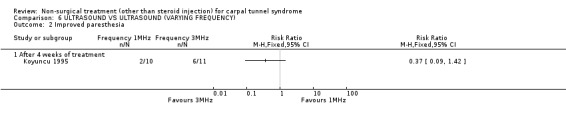
Comparison 6 ULTRASOUND VS ULTRASOUND (VARYING FREQUENCY), Outcome 2 Improved paresthesia.
6.3. Analysis.
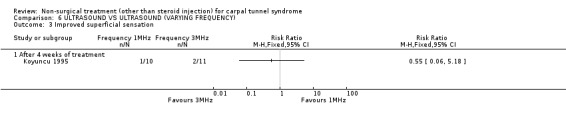
Comparison 6 ULTRASOUND VS ULTRASOUND (VARYING FREQUENCY), Outcome 3 Improved superficial sensation.
6.4. Analysis.
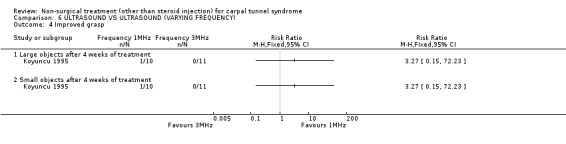
Comparison 6 ULTRASOUND VS ULTRASOUND (VARYING FREQUENCY), Outcome 4 Improved grasp.
6.5. Analysis.
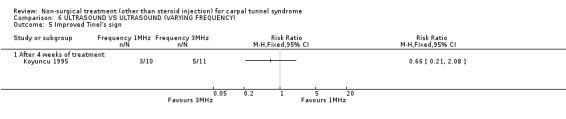
Comparison 6 ULTRASOUND VS ULTRASOUND (VARYING FREQUENCY), Outcome 5 Improved Tinel's sign.
6.6. Analysis.
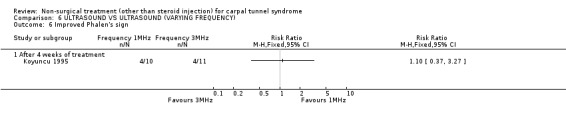
Comparison 6 ULTRASOUND VS ULTRASOUND (VARYING FREQUENCY), Outcome 6 Improved Phalen's sign.
Comparison 7. ERGONOMIC KEYBOARD VS PLACEBO/CONTROL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 After 3 months (Protouch Keyboard) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain (change scores) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 After 6 months (Comfort Keyboard System) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 After 6 months (Microsoft Natural Keyboard) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 After 6 months (Apple Adjustable Keyboard) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Hand function | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 After 3 months (Protouch Keyboard) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Hand function (change scores) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 After 6 months (Comfort Keyboard System) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 After 6 months (Microsoft Natural Keyboard) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 After 6 months (Apple Adjustable Keyboard) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Improved Phalen's sign | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 After 6 months (Microsoft Natural Keyboard) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 After 6 months (Apple Adjustable Keyboard) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Improved Tinel's sign | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 After 6 months (Microsoft Natural Keyboard) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 After 6 months (Apple Adjustable Keyboard) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Phalen test time (seconds) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Right hand after 3 months (Protouch Keyboard) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Left hand after 3 months (Protouch Keyboard) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Right hand after 6 months (Microsoft Natural Keyboard) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Left hand after 6 months (Microsoft Natural Keyboard) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 Right hand after 6 months (Apple Adjustable Keyboard) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.6 Left hand after 6 months (Apple Adjustable Keyboard) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Median nerve conduction: palm‐wrist sensory latency (ms) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Right hand after 3 months (Protouch Keyboard) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Left hand after 3 months (Protouch Keyboard) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
7.1. Analysis.
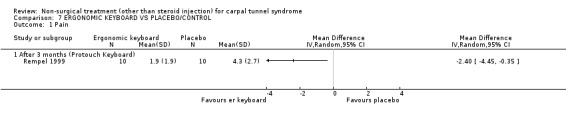
Comparison 7 ERGONOMIC KEYBOARD VS PLACEBO/CONTROL, Outcome 1 Pain.
7.2. Analysis.
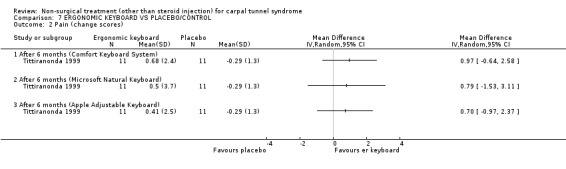
Comparison 7 ERGONOMIC KEYBOARD VS PLACEBO/CONTROL, Outcome 2 Pain (change scores).
7.3. Analysis.
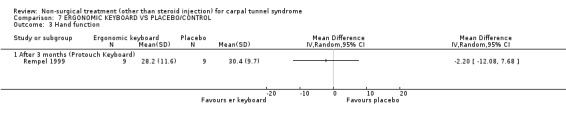
Comparison 7 ERGONOMIC KEYBOARD VS PLACEBO/CONTROL, Outcome 3 Hand function.
7.4. Analysis.
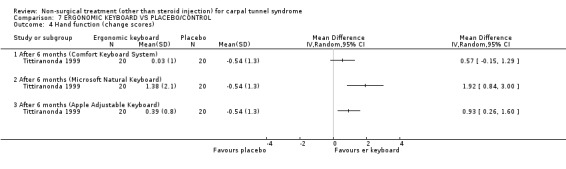
Comparison 7 ERGONOMIC KEYBOARD VS PLACEBO/CONTROL, Outcome 4 Hand function (change scores).
7.5. Analysis.
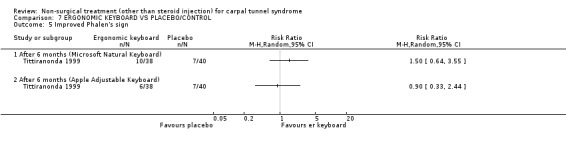
Comparison 7 ERGONOMIC KEYBOARD VS PLACEBO/CONTROL, Outcome 5 Improved Phalen's sign.
7.6. Analysis.

Comparison 7 ERGONOMIC KEYBOARD VS PLACEBO/CONTROL, Outcome 6 Improved Tinel's sign.
7.7. Analysis.
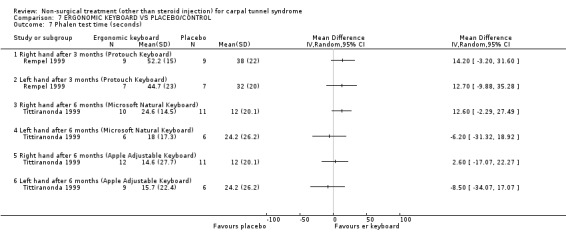
Comparison 7 ERGONOMIC KEYBOARD VS PLACEBO/CONTROL, Outcome 7 Phalen test time (seconds).
7.8. Analysis.
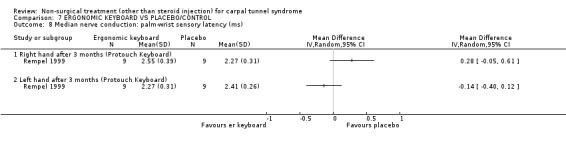
Comparison 7 ERGONOMIC KEYBOARD VS PLACEBO/CONTROL, Outcome 8 Median nerve conduction: palm‐wrist sensory latency (ms).
Comparison 8. DIURETIC VS PLACEBO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 2 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 After 4 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Symptom improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 After 4 weeks of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
8.1. Analysis.
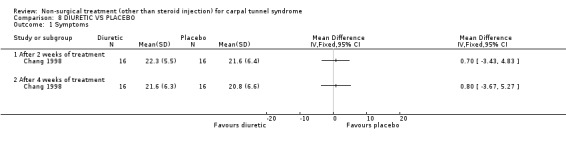
Comparison 8 DIURETIC VS PLACEBO, Outcome 1 Symptoms.
8.2. Analysis.
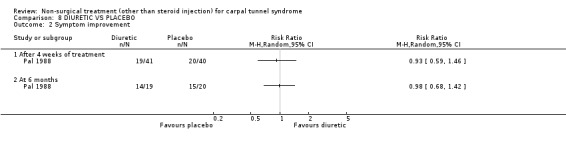
Comparison 8 DIURETIC VS PLACEBO, Outcome 2 Symptom improvement.
Comparison 9. NSAID VS PLACEBO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 2 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 After 4 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
9.1. Analysis.
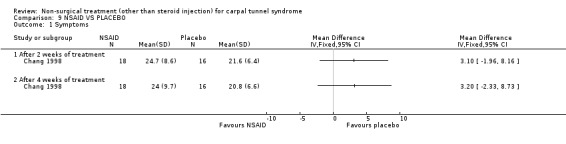
Comparison 9 NSAID VS PLACEBO, Outcome 1 Symptoms.
Comparison 10. ORAL STEROID (PREDNISOLONE OR PREDNISONE) VS PLACEBO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptoms | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 After 2 weeks of treatment | 3 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐7.23 [‐10.31, ‐4.14] |
| 1.2 After 4 weeks of treatment | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐10.8 [‐15.26, ‐6.34] |
| 1.3 At 4 weeks (2 weeks following treatment end) | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐6.19 [‐15.14, 2.76] |
| 1.4 At 8 weeks (6 weeks following treatment end) | 2 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐6.46 [‐11.93, ‐0.99] |
10.1. Analysis.
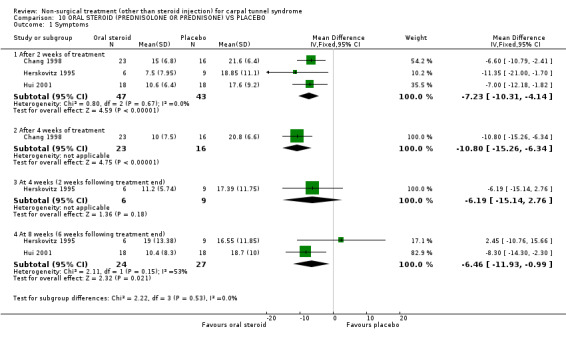
Comparison 10 ORAL STEROID (PREDNISOLONE OR PREDNISONE) VS PLACEBO, Outcome 1 Symptoms.
Comparison 11. DIURETIC VS NSAID.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 2 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 After 4 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
11.1. Analysis.

Comparison 11 DIURETIC VS NSAID, Outcome 1 Symptoms.
Comparison 12. DIURETIC VS ORAL STEROID (PREDNISOLONE).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 2 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 After 4 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
12.1. Analysis.
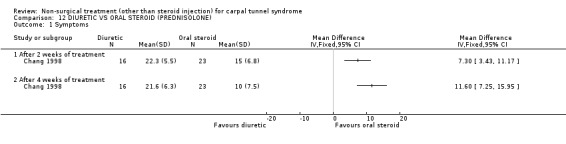
Comparison 12 DIURETIC VS ORAL STEROID (PREDNISOLONE), Outcome 1 Symptoms.
Comparison 13. NSAID VS ORAL STEROID (PREDNISOLONE).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 2 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 After 4 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
13.1. Analysis.
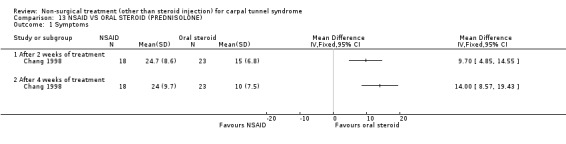
Comparison 13 NSAID VS ORAL STEROID (PREDNISOLONE), Outcome 1 Symptoms.
Comparison 14. VITAMIN B6 (PYRIDOXINE) VS PLACEBO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptom improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 10 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Nocturnal discomfort | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 After 12 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Finger swelling | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 After 12 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Movement discomfort | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 After 12 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Hand co‐ordination | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 After 12 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Improved Phalen's sign | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 After 12 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Improved Tinel's sign | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 After 12 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Median nerve conduction: distal latency (ms) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Palmar after 12 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Motor after 12 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Median nerve conduction: motor amplitude (mV) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 After 12 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Median nerve conduction: motor conduction velocity (m/s) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 After 12 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
14.1. Analysis.
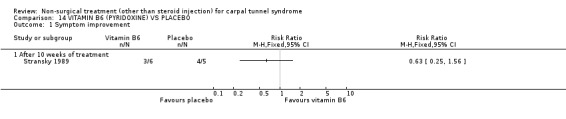
Comparison 14 VITAMIN B6 (PYRIDOXINE) VS PLACEBO, Outcome 1 Symptom improvement.
14.2. Analysis.
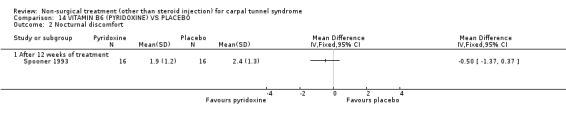
Comparison 14 VITAMIN B6 (PYRIDOXINE) VS PLACEBO, Outcome 2 Nocturnal discomfort.
14.3. Analysis.

Comparison 14 VITAMIN B6 (PYRIDOXINE) VS PLACEBO, Outcome 3 Finger swelling.
14.4. Analysis.
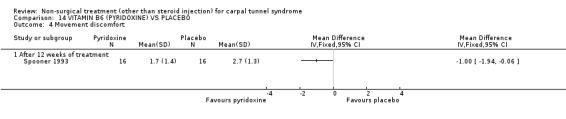
Comparison 14 VITAMIN B6 (PYRIDOXINE) VS PLACEBO, Outcome 4 Movement discomfort.
14.5. Analysis.
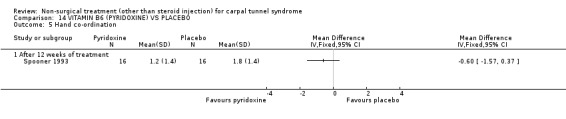
Comparison 14 VITAMIN B6 (PYRIDOXINE) VS PLACEBO, Outcome 5 Hand co‐ordination.
14.6. Analysis.
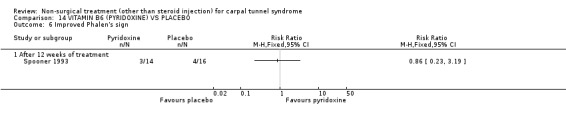
Comparison 14 VITAMIN B6 (PYRIDOXINE) VS PLACEBO, Outcome 6 Improved Phalen's sign.
14.7. Analysis.
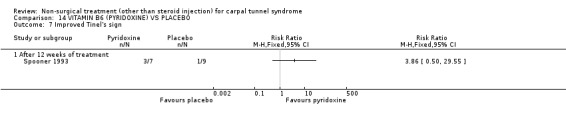
Comparison 14 VITAMIN B6 (PYRIDOXINE) VS PLACEBO, Outcome 7 Improved Tinel's sign.
14.8. Analysis.
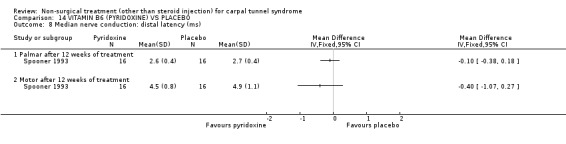
Comparison 14 VITAMIN B6 (PYRIDOXINE) VS PLACEBO, Outcome 8 Median nerve conduction: distal latency (ms).
14.9. Analysis.
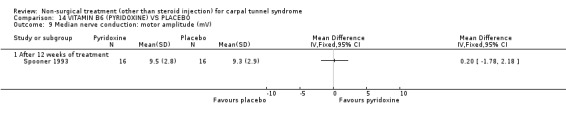
Comparison 14 VITAMIN B6 (PYRIDOXINE) VS PLACEBO, Outcome 9 Median nerve conduction: motor amplitude (mV).
14.10. Analysis.
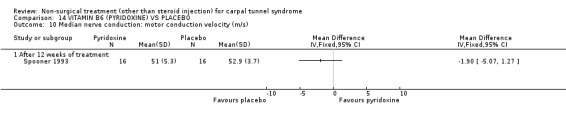
Comparison 14 VITAMIN B6 (PYRIDOXINE) VS PLACEBO, Outcome 10 Median nerve conduction: motor conduction velocity (m/s).
Comparison 15. NERVE AND TENDON GLIDING EXERCISES (PLUS WRIST SPLINT) VS CONTROL (WRIST SPLINT ONLY).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Hand function | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Grip strength (lbs) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Pinch strength (lbs) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Static two‐point discrimination (mm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Positive Phalen's sign | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 At 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Positive Tinel's sign | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 At 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 High patient satisfaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 At 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
15.1. Analysis.
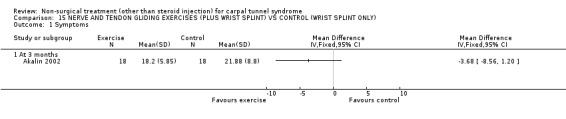
Comparison 15 NERVE AND TENDON GLIDING EXERCISES (PLUS WRIST SPLINT) VS CONTROL (WRIST SPLINT ONLY), Outcome 1 Symptoms.
15.2. Analysis.
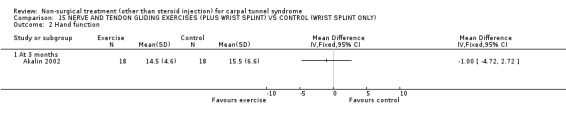
Comparison 15 NERVE AND TENDON GLIDING EXERCISES (PLUS WRIST SPLINT) VS CONTROL (WRIST SPLINT ONLY), Outcome 2 Hand function.
15.3. Analysis.

Comparison 15 NERVE AND TENDON GLIDING EXERCISES (PLUS WRIST SPLINT) VS CONTROL (WRIST SPLINT ONLY), Outcome 3 Grip strength (lbs).
15.4. Analysis.
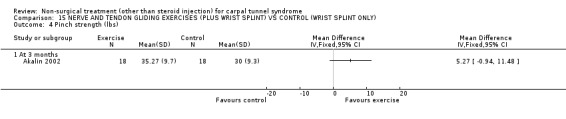
Comparison 15 NERVE AND TENDON GLIDING EXERCISES (PLUS WRIST SPLINT) VS CONTROL (WRIST SPLINT ONLY), Outcome 4 Pinch strength (lbs).
15.5. Analysis.
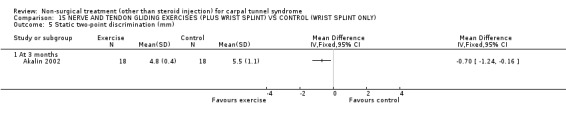
Comparison 15 NERVE AND TENDON GLIDING EXERCISES (PLUS WRIST SPLINT) VS CONTROL (WRIST SPLINT ONLY), Outcome 5 Static two‐point discrimination (mm).
15.6. Analysis.
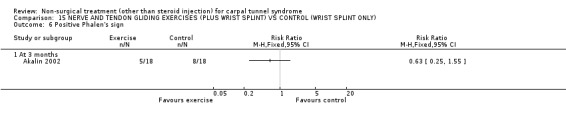
Comparison 15 NERVE AND TENDON GLIDING EXERCISES (PLUS WRIST SPLINT) VS CONTROL (WRIST SPLINT ONLY), Outcome 6 Positive Phalen's sign.
15.7. Analysis.
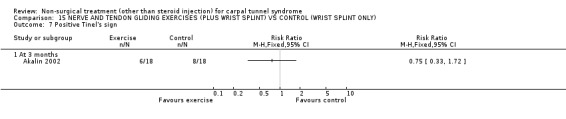
Comparison 15 NERVE AND TENDON GLIDING EXERCISES (PLUS WRIST SPLINT) VS CONTROL (WRIST SPLINT ONLY), Outcome 7 Positive Tinel's sign.
15.8. Analysis.
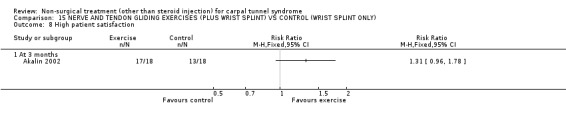
Comparison 15 NERVE AND TENDON GLIDING EXERCISES (PLUS WRIST SPLINT) VS CONTROL (WRIST SPLINT ONLY), Outcome 8 High patient satisfaction.
Comparison 16. YOGA VS WRIST SPLINT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in nocturnal waking | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 8 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 After 8 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Improved Phalen's sign | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 After 8 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Improved Tinel's sign | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 After 8 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Grip strength (mmHg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 After 8 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
16.1. Analysis.
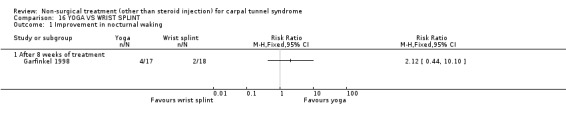
Comparison 16 YOGA VS WRIST SPLINT, Outcome 1 Improvement in nocturnal waking.
16.2. Analysis.
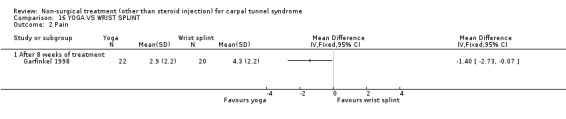
Comparison 16 YOGA VS WRIST SPLINT, Outcome 2 Pain.
16.3. Analysis.
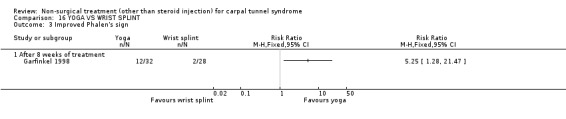
Comparison 16 YOGA VS WRIST SPLINT, Outcome 3 Improved Phalen's sign.
16.4. Analysis.
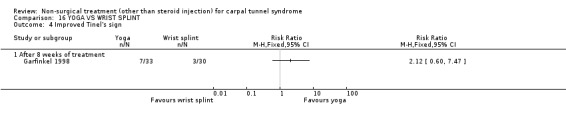
Comparison 16 YOGA VS WRIST SPLINT, Outcome 4 Improved Tinel's sign.
16.5. Analysis.
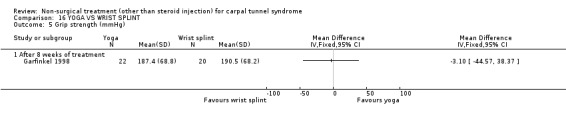
Comparison 16 YOGA VS WRIST SPLINT, Outcome 5 Grip strength (mmHg).
Comparison 17. NEURODYNAMIC MOBILISATION VS CONTROL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 3 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Improved pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 After 3 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Improved hand function | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 After 3 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Active wrist flexion (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 After 3 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Active wrist extension (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 After 3 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Improvement in upper limb tension test (ULTT2a) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 After 3 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Need for surgical release | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 After 3 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
17.1. Analysis.
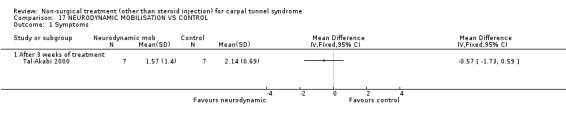
Comparison 17 NEURODYNAMIC MOBILISATION VS CONTROL, Outcome 1 Symptoms.
17.2. Analysis.
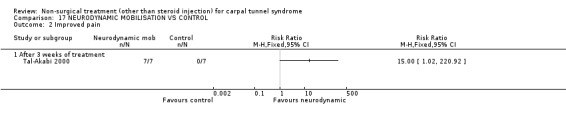
Comparison 17 NEURODYNAMIC MOBILISATION VS CONTROL, Outcome 2 Improved pain.
17.3. Analysis.
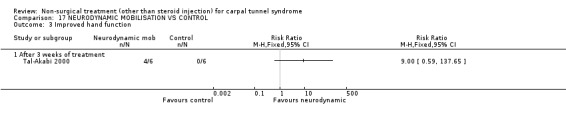
Comparison 17 NEURODYNAMIC MOBILISATION VS CONTROL, Outcome 3 Improved hand function.
17.4. Analysis.
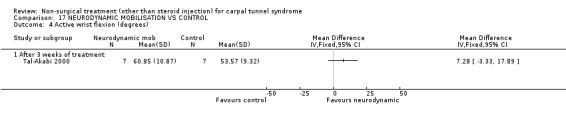
Comparison 17 NEURODYNAMIC MOBILISATION VS CONTROL, Outcome 4 Active wrist flexion (degrees).
17.5. Analysis.
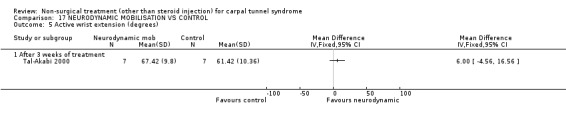
Comparison 17 NEURODYNAMIC MOBILISATION VS CONTROL, Outcome 5 Active wrist extension (degrees).
17.6. Analysis.
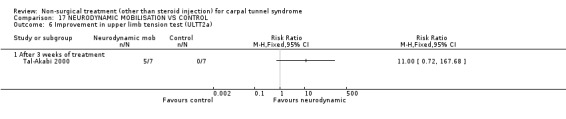
Comparison 17 NEURODYNAMIC MOBILISATION VS CONTROL, Outcome 6 Improvement in upper limb tension test (ULTT2a).
17.7. Analysis.

Comparison 17 NEURODYNAMIC MOBILISATION VS CONTROL, Outcome 7 Need for surgical release.
Comparison 18. CARPAL BONE MOBILISATION VS CONTROL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 3 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Improved pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 After 3 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Improved hand function | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 After 3 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Active wrist flexion (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 After 3 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Active wrist extension (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 After 3 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Improvement in upper limb tension test (ULTT2a) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 After 3 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Need for surgical release | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 After 3 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
18.1. Analysis.
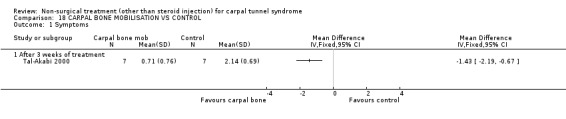
Comparison 18 CARPAL BONE MOBILISATION VS CONTROL, Outcome 1 Symptoms.
18.2. Analysis.
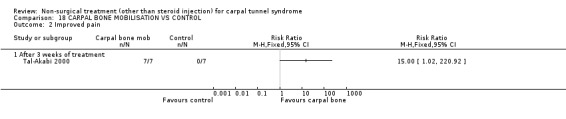
Comparison 18 CARPAL BONE MOBILISATION VS CONTROL, Outcome 2 Improved pain.
18.3. Analysis.
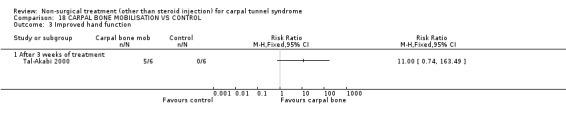
Comparison 18 CARPAL BONE MOBILISATION VS CONTROL, Outcome 3 Improved hand function.
18.4. Analysis.
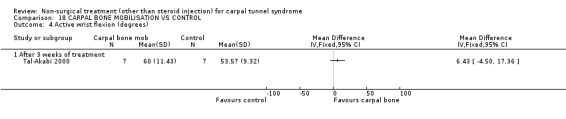
Comparison 18 CARPAL BONE MOBILISATION VS CONTROL, Outcome 4 Active wrist flexion (degrees).
18.5. Analysis.
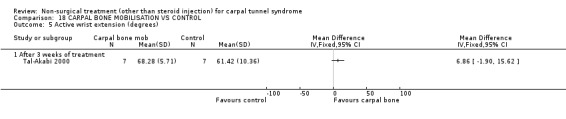
Comparison 18 CARPAL BONE MOBILISATION VS CONTROL, Outcome 5 Active wrist extension (degrees).
18.6. Analysis.
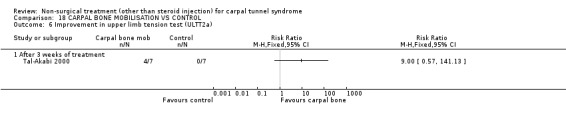
Comparison 18 CARPAL BONE MOBILISATION VS CONTROL, Outcome 6 Improvement in upper limb tension test (ULTT2a).
18.7. Analysis.
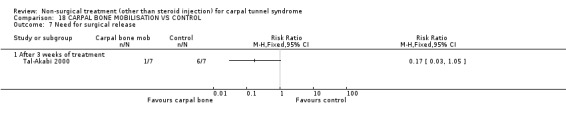
Comparison 18 CARPAL BONE MOBILISATION VS CONTROL, Outcome 7 Need for surgical release.
Comparison 19. NEURODYNAMIC VS CARPAL BONE MOBILISATION.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 3 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Improved pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 After 3 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Improved hand function | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 After 3 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Active wrist flexion (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 After 3 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Active wrist extension (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 After 3 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Improvement in upper limb tension test (ULTT2a) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 After 3 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Need for surgical release | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 After 3 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
19.1. Analysis.
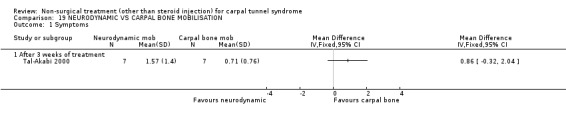
Comparison 19 NEURODYNAMIC VS CARPAL BONE MOBILISATION, Outcome 1 Symptoms.
19.2. Analysis.

Comparison 19 NEURODYNAMIC VS CARPAL BONE MOBILISATION, Outcome 2 Improved pain.
19.3. Analysis.
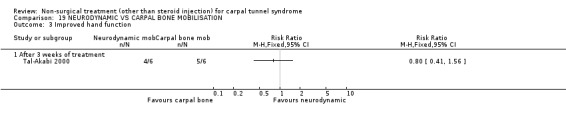
Comparison 19 NEURODYNAMIC VS CARPAL BONE MOBILISATION, Outcome 3 Improved hand function.
19.4. Analysis.
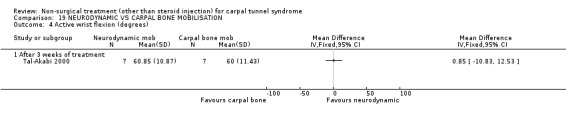
Comparison 19 NEURODYNAMIC VS CARPAL BONE MOBILISATION, Outcome 4 Active wrist flexion (degrees).
19.5. Analysis.

Comparison 19 NEURODYNAMIC VS CARPAL BONE MOBILISATION, Outcome 5 Active wrist extension (degrees).
19.6. Analysis.
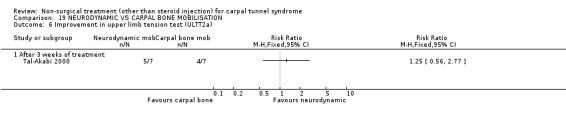
Comparison 19 NEURODYNAMIC VS CARPAL BONE MOBILISATION, Outcome 6 Improvement in upper limb tension test (ULTT2a).
19.7. Analysis.
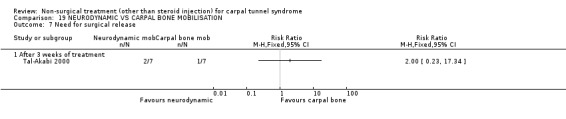
Comparison 19 NEURODYNAMIC VS CARPAL BONE MOBILISATION, Outcome 7 Need for surgical release.
Comparison 20. MAGNET THERAPY VS PLACEBO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 45 minutes of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 2 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
20.1. Analysis.
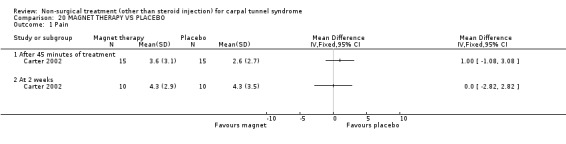
Comparison 20 MAGNET THERAPY VS PLACEBO, Outcome 1 Pain.
Comparison 21. CHIROPRACTIC VS MEDICAL CARE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Physical distress | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 9 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mental distress | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 After 9 weeks of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Vibrometry (db) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Right hand at 13 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Left hand at 13 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Hand function | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 At 13 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Health‐related quality of life (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 At 13 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
21.1. Analysis.
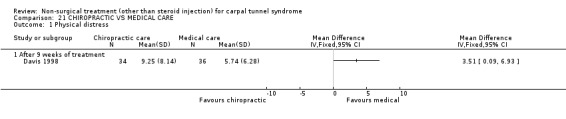
Comparison 21 CHIROPRACTIC VS MEDICAL CARE, Outcome 1 Physical distress.
21.2. Analysis.
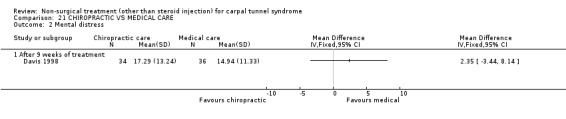
Comparison 21 CHIROPRACTIC VS MEDICAL CARE, Outcome 2 Mental distress.
21.3. Analysis.
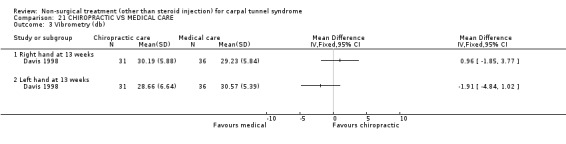
Comparison 21 CHIROPRACTIC VS MEDICAL CARE, Outcome 3 Vibrometry (db).
21.4. Analysis.
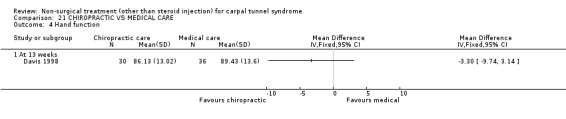
Comparison 21 CHIROPRACTIC VS MEDICAL CARE, Outcome 4 Hand function.
21.5. Analysis.
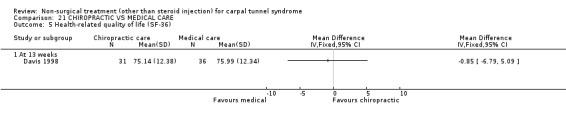
Comparison 21 CHIROPRACTIC VS MEDICAL CARE, Outcome 5 Health‐related quality of life (SF‐36).
Comparison 22. LASER ACUPUNCTURE VS PLACEBO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improved paresthesia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Digit 1 after 3 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Digit 2 after 3 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Digit 3 after 3 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Improved night pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 After 3 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
22.1. Analysis.
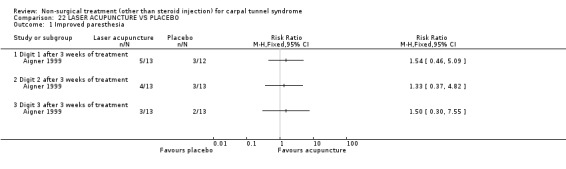
Comparison 22 LASER ACUPUNCTURE VS PLACEBO, Outcome 1 Improved paresthesia.
22.2. Analysis.
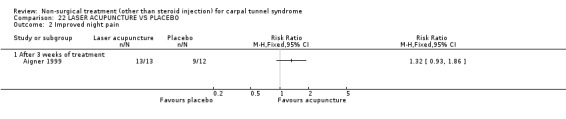
Comparison 22 LASER ACUPUNCTURE VS PLACEBO, Outcome 2 Improved night pain.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aigner 1999.
| Methods | Randomised, double‐blind, placebo‐controlled trial Blinded subjects and assessor Quality score: B Selection bias ‐ in part Performance bias ‐ no Attrition bias ‐ no Detection bias ‐ in part BIAS RATING = MODERATE Quality of diagnostic criteria = A |
|
| Participants | Total n = 26 randomised
Intervention group n = 13
Control group n = 13 20 males; 6 females Mean ± sd age: 54 ± 9 yrs (range 43‐72) Inclusion criteria: 1. CTS with typical complaints 2. Documented electrophysiologic study abnormalities Exclusion criteria: 1. Diabetes mellitus 2. Chronic alcohol intake 3. Previous CTS surgery |
|
| Interventions | Intervention: Laser acupuncture (5mW, 632.8 nm wavelength Helium‐Neon laser) applied to various acupuncture points (P 6, 7, 8, TB 5, SI 6, H7 and ear points 55, 67) for 15 second periods; 2 treatments per week for 3 weeks Placebo: Placebo laser acupuncture (identical machine) applied to same acupuncture points for 15 second periods; 2 treatments per week for 3 weeks |
|
| Outcomes | Outcome assessed at 3 weeks 1. Night pain (rated on ordinal scale 1‐5) 2. Paraesthesiae (rated on ordinal scale 1‐5) |
|
| Notes | Participants were recruited from a surgery wait list and all proceeded to surgery following trial Allocation method and outcome data was clarified in personal communication with authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Akalin 2002.
| Methods | Randomised controlled trial No blinding Quality score: C Selection bias ‐ yes Performance bias ‐ yes Attrition bias ‐ no Detection bias ‐ yes BIAS RATING = HIGH Quality of diagnostic criteria = A |
|
| Participants | Total n = 28 (36 hands) randomised
Intervention group n = 14 (18 hands)
Control group n = 14 (18 hands) 2 males; 26 females Mean ± sd age: Intervention 51.7 ± 5.5 yrs Control 52.2 ± 5.6 yrs Inclusion criteria: 1. Subjective symptoms (history of paraesthesiae or pain in median nerve distribution, nocturnal pain and dysesthesia) 2. Positive Phalen's sign or Tinel's sign 3. Electrophysiologic studies confirmed CTS diagnosis Exclusion criteria: 1. Underlying metabolic disorders (diabetes mellitus, thyroid disease) 2. Rheumatoid arthritis 3. Pregnancy 4. History of steroid injection to carpal tunnel 5. Severe thenar atrophy 6. History of splint use |
|
| Interventions | Intervention: Nerve and tendon gliding exercises performed 5 times daily and use of a custom‐made neutral volar wrist splint for 4 weeks Control: Custom‐made neutral volar wrist splint for 4 weeks Participants in both groups were instructed to wear the splint all night and during the day as much as possible |
|
| Outcomes | Outcome assessed at 12 weeks (8 weeks following end of treatment). Assessment of patient satisfaction occurred between 5 and 11 months post intervention 1. Grip strength (in lbs) (Martin vigorimeter) 2. Pinch strength (in lbs) (Martin vigorimeter) 3. Static two‐point discrimination of the pulps of radial 3 digits (in mm) 4. Tinel's sign (rated as positive or negative) 5. Phalen's sign (rated as positive or negative) 6. Symptoms using carpal tunnel questionnaire (rates 11 items on ordinal scale 1‐5) 7. Hand function using carpal tunnel questionnaire (rates 8 items on ordinal scale 1‐5) 8. Patient satisfaction (rates as excellent, good, fair, poor). Excellent = completely asymptomatic, good = occasional symptoms, fair = frequent symptoms but still some improvement, poor = continuous symptoms |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Burke 1994.
| Methods | Double‐blind clinical trial using alternate allocation (attempted randomisation) Blinded subjects and assessors* No control group Quality score: C Selection bias ‐ yes Performance bias ‐ yes Attrition bias ‐ in part Detection bias ‐ in part BIAS RATING = HIGH Quality of diagnostic criteria = B |
|
| Participants | Total n = 59 (90 hands) randomised
Group 1 n = 45 hands
Group 2 n = 45 hands Inclusion criteria: 1. Clinical diagnosis of CTS (hypesthesia or paraesthesiae in median nerve distribution, weakness or atrophy in abductor pollicis brevis or opponens pollicis) Exclusion criteria: 1. History of CTR surgery 2. Injection at wrist 3. Previous splint use |
|
| Interventions | Group 1: Wrist splint in neutral Group 2: Wrist splint in 20 degrees extension Treatment length and wearing regime not controlled |
|
| Outcomes | Outcome assessed at 2 weeks 1. Symptom relief** (overall, nocturnal, daytime) assessed using ordinal scale (1=not at all, 2=a little, 3=a lot, 4=completely) |
|
| Notes | Age and sex of participants not reported *Confirmed with author in personal communication **Dichotomised by author for analysis into 'a lot/complete relief' and 'none/little relief' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Carter 2002.
| Methods | Randomised, triple‐blind, placebo‐controlled trial Blinded subjects, treaters and assessors (subjects self‐assessed) Quality score: B Selection bias ‐ no Performance bias ‐ no Attrition bias ‐ in part Detection bias ‐ no BIAS RATING = MODERATE Quality of diagnostic criteria = C |
|
| Participants | Total n = 30 randomised
Intervention group n = 15
Control group n= 15 4 males, 26 females Mean ± sd age: Intervention 51 ± 15.5 yrs Control 49 ± 11.7 yrs Inclusion criteria: 1. Presence of chronic wrist pain in the area of the carpal tunnel 2. Willingness to accept randomisation Exclusion criteria: 1. Source of pain attributed to cause other than CTS 2. Use of pain medication within 4 hours of beginning treatment 3. Body mass index > 35 4. Painfree at treatment commencement |
|
| Interventions | Intervention: Magnet therapy by applying a magnetic device over the surface of the carpal tunnel. Device secured with foam and wrist bracelet. Device contained 5 individual magnets with a total magnetic energy of 1000 gauss at the surface of the centre of the magnet. The magnet therapy was delivered for 45 minutes during one session only (subject was seated) Control: Placebo device looked identical to the intervention magnets. Method of delivery and length of treatment for placebo was identical to intervention group |
|
| Outcomes | Outcome assessed at 15 minute intervals during treatment (15, 30, 45 minutes) and at 2 weeks 1. Pain (using pain visual analogue scale) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Chang 1998.
| Methods | Randomised, triple‐blind, placebo‐controlled trial Blinded subjects, treaters and assessors Quality score: B Selection bias ‐ no Performance bias ‐ in part Attrition bias ‐ no Detection bias ‐ in part BIAS RATING = MODERATE Quality of diagnostic criteria = A |
|
| Participants | Total n = 91 randomised
Intervention group 1 n = 20
Intervention group 2 n = 22
Intervention group 3 n = 26
Placebo group n = 23 20 males; 53 females* Mean ± sd age: Intervention 1: 46 ± 5 yrs* Intervention 2: 47 ± 6 yrs* Intervention 3: 45 ± 5 yrs* Placebo: 44 ± 5 yrs* Inclusion criteria: 1. Clinical symptoms and signs of CTS confirmed with electrodiagnostic testing Exclusion criteria: 1. Abnormalities in radial or ulnar nerves on electrodiagnostic testing 2. Severe CTS (fibrillation potentials or reinnervation by needle EMG in abductor pollicis brevis muscle) 3. Clinical or electrodiagnostic evidence of cervical radiculopathy, proximal median neuropathy or polyneuropathy 4. Hypothyroidism, diabetes mellitus, wrist arthritis, pregnancy, vibratory machine use, obesity 5. Cognitive impairment 6. Recent peptic ulcer or history of steroid or NSAID intolerance |
|
| Interventions | Intervention 1: Diuretic treatment with trichlormethiazide, 2 mg daily for 4 weeks Intervention 2: Nonsteroidal anti‐inflammatory drug (NSAID) treatment with tenoxicam‐SR, 20 mg daily for 4 weeks Intervention 3: Oral steroid treatment with prednisolone, 20 mg daily for 2 weeks, followed by 10 mg daily for 2 weeks Placebo: Placebo pill for 4 weeks All treatments consisted of white pills of similar size and shape |
|
| Outcomes | Outcome assessed at 2 and 4 weeks 1. Symptoms using questionnaire (rates pain, numbness, paraesthesiae, weakness/clumsiness, nocturnal wakening on 0‐10 scale and summarises as a global symptom score) |
|
| Notes | *Data only reported for participants completing treatment (n=73) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Davis 1998.
| Methods | Randomised, single‐blind, controlled trial Blinded assessors Quality score: C Selection bias ‐ no Performance bias ‐ yes Attrition bias ‐ no Detection bias ‐ in part BIAS RATING = HIGH Quality of diagnostic criteria = A |
|
| Participants | Total n = 91 randomised
Intervention group n = 45
Control group n = 46 37 males; 54 females Mean ± sd age: Intervention 38 ± 5 yrs Control 36 ± 6 yrs Inclusion criteria: 1. Positive electrodiagnostic testing 2. Positive clinical exam for CTS (pinch/grip strength, Phalen's and Tinel's sign, Semmes‐Weinstein monofilaments) 3. Symptoms of CTS including numbness and tingling 4. Age 21‐45 years Exclusion criteria: 1. Currently prescribed CTS treatment 2. Pending workers' compensation claim 3. Pregnancy 4. Systemic condition (diabetes, thyroid disorder) 5. Prior wrist surgery 6. Use of anti‐inflammatory medication or vitamin B6 supplementation 7. Wrist splint worn on regular basis 8. Electrodiagnostic abnormalities inconsistent with CTS or indicating axonal degeneration |
|
| Interventions | Intervention: Chiropractic treatment consisting of manual thrusts, myofascial massage/loading, ultrasound (over carpal tunnel at 1 MHz, 1.0‐1.5 W/cm2, for 5 minutes), and nocturnal wrist splint. Treatment was provided 3 times per week for 2 weeks, followed by twice per week for 3 weeks, then one treatment per week for 4 weeks*. Content of treatment session was at the discretion of chiropractic physician Control: Medical treatment consisting of ibuprofen (800 mg, 3 times per day for 1 week; 800 mg, 2 times per day for 1 week; 800 mg as required for 7 weeks to a maximum daily dose of 2400 mg) plus nocturnal wrist splint Total treatment length for both groups = 9 weeks |
|
| Outcomes | Outcome assessed at 9 and 13 weeks 1. Nerve conduction: median motor and sensory distal latencies (at 9 weeks only) 2. Physical distress using CTS Outcome Assessment Physical Distress (CTOA‐P) scale (at 9 weeks only) 3. Mental distress using CTS Outcome Assessment Mental Distress (CTOA‐M) scale (at 9 weeks only) 4. Vibrometry (8‐500 Hz) on digit 3 using Total Jetzer Index (at 13 weeks only) 5. Hand function using Hand‐Finger Functioning (HAND) scale (at 13 weeks only) 6. Health‐related quality of life using Short Form 36 (SF36) scale (at 13 weeks only) |
|
| Notes | *Ultrasound was provided for half of the chiropractic treatment visits | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Ebenbichler 1998.
| Methods | Randomised, triple‐blind, placebo‐controlled trial Blinded subjects, treaters and assessors Quality score: A Selection bias ‐ no Performance bias ‐ no Attrition bias ‐ no Detection bias ‐ no BIAS RATING = LOW Quality of diagnostic criteria = A |
|
| Participants | Total n = 45 (90 wrists) randomised
Intervention group n = 45 (45 wrists)
Control group n = 45 (45 wrists) Mean ± sd age: 51 ± 15 yrs Inclusion criteria: 1. Bilateral idiopathic CTS confirmed with electrodiagnostic testing 2. Mild to moderate pain lasting longer than 3 months 3. Informed written consent Exclusion criteria: 1. Secondary entrapment neuropathies 2. Systemic disease 3. Electroneurographic and clinical signs of median nerve axonal degeneration 4. Previous CTR 5. Previous ultrasound treatment 6. History of steroid injection into carpal tunnel 7. Regular analgesic or anti‐inflammatory drug requirements |
|
| Interventions | Intervention: Pulsed ultrasound therapy using 1.0 W/cm2 intensity and 1 MHz frequency, 15 minute session daily, 5 times a week for 2 weeks, followed by twice a week for 5 weeks Control: Placebo ultrasound therapy using 0.0 W/cm2 intensity, 15 minute session daily, 5 times a week for 2 weeks, followed by twice a week for 5 weeks |
|
| Outcomes | Outcome assessed at 2 weeks (after 10 sessions), 7 weeks (at end of treatment) and 6 months after end of treatment 1. Symptoms using 0‐10 visual analogue scale 2. General symptom improvement (ordinal scale 1=free of symptoms, 5=much worse) (at 6 months only) 3. Sensation using sharp pin wheel and visual analogue scale 4. Grip strength in kilograms using Preston dynamometer (at 6 months only) 5. Pinch strength in kilograms using Preston dynamometer (at 6 months only) 6. Nerve conduction: median distal motor latency, sensory nerve action potentials, sensory nerve conduction velocity (at 6 months only) |
|
| Notes | Sex of participants not reported Mean and standard deviation values for symptoms, sensation, grip strength, pinch strength and nerve conduction outcomes were provided by authors to facilitate entry into RevMan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Garfinkel 1998.
| Methods | Randomised, single‐blind, controlled trial Blinded assessors Quality score: C Selection bias ‐ yes Performance bias ‐ yes Attrition bias ‐ yes Detection bias ‐ in part BIAS RATING = HIGH Quality of diagnostic criteria = A |
|
| Participants | Total n = 51 randomised
Intervention group n = 26
Control group n = 25 13 males; 28 females* Mean age: (sd not reported) Intervention 49 yrs Control 49 yrs Inclusion criteria: 1. Presence of 2 or more of the following: positive Tinel's; positive Phalen's; pain in median nerve distribution; sleep disturbance due to hand; numbness/paresthesias in median nerve distribution 2. Abnormal electrophysiological findings 3. Subject agrees not to change medications, receive other new treatments or change work duties during trial Exclusion criteria: 1. Previous surgery for CTS 2. Rheumatoid arthritis or other recognised inflammatory arthritis 3. CTS related to systemic disease (hypothyroidism) 4. Pregnancy |
|
| Interventions | Intervention: Yoga for 1‐1.5 hours twice weekly for 8 weeks Control: Wrist splint to supplement current treatment for 8 weeks |
|
| Outcomes | Outcome assessed at 8 weeks 1. Pain severity using visual analogue scale 2. Nocturnal wakening using ordinal scale (rated as worsened, same, improved) 3. Phalen's sign (rated as worsened, same, improved) 4. Tinel's sign (rated as worsened, same, improved) 5. Grip strength in mmHg using sphygmomanometer cuff (mean of 3 trials) 6. Nerve conduction: median motor and sensory distal latencies (in ms) |
|
| Notes | *1 missing subject for demographic data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Herskovitz 1995.
| Methods | Randomised, triple‐blind, placebo‐controlled trial Blinded subjects, treaters and assessors Quality score: B Selection bias ‐ no Performance bias ‐ in part Attrition bias ‐ no Detection bias ‐ no BIAS RATING = MODERATE Quality of diagnostic criteria = A |
|
| Participants | Total n = 18 randomised
Intervention group n = 8
Placebo group n = 10 3 males; 12 females* Mean age: (sd not stated) Intervention 55 yrs Placebo 46 yrs Inclusion criteria: 1. Symptoms restricted to median nerve distribution (pain, numbness, tingling, nocturnal symptoms) 2. Focal signs and symptoms of CTS confirmed with electrodiagnostic testing 3. Minimal to moderate weakness of thenar muscles 4. 18 years of age or older Exclusion criteria: 1. Clinical or electrophysiologic evidence of cervical radiculopathy, proximal median neuropathy, significant polyneuropathy or marked orthopaedic abnormalities 2. Moderate to severe thenar muscle weakness or atrophy, or EMG evidence of more than mild motor axon degeneration 3. Cognitive impairment 4. Recent peptic ulcer or history of steroid intolerance |
|
| Interventions | Intervention: Prednisone, 20 mg daily for 1 week, followed by 10mg daily for 1 week Placebo: Placebo tablets for 2 weeks |
|
| Outcomes | Outcome assessed at 2, 4 and 8 weeks 1. Symptoms using questionnaire (rates pain, numbness, paresthesia, weakness/clumsiness, nocturnal wakening on 0‐10 scale and summarised as a global symptom score) |
|
| Notes | *Data only reported for participants completing treatment (n=15) Mean and standard deviations for endpoint scores were obtained from the authors in a personal communication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Hui 2001.
| Methods | Randomised, triple‐blind, placebo‐controlled trial Blinded subjects, treaters and assessors Quality score: A Selection bias ‐ no Performance bias ‐ no Attrition bias ‐ no Detection bias ‐ no BIAS RATING = LOW Quality of diagnostic criteria = A |
|
| Participants | Total n = 36 randomised
Intervention group n = 18
Placebo group n = 18 2 males; 34 females Mean ± sd age: Intervention 43 ± 7 yrs Placebo 45 ± 10 yrs Inclusion criteria: 1. Clinical CTS diagnosis, of more than 3 months duration, confirmed with electrodiagnostic testing (prolonged median nerve distal latencies >4ms or median ulnar palmar sensory latency difference >0.5ms) Exclusion criteria: 1. Severe CTS (fibrillation potentials or reinnervation on needle examination of APB) 2. Coexisting disorders which mimic CTS (cervical radiculopathy, peripheral neuropathy) 3. Contraindication to steroid use 4. History of underlying disorders associated with CTS (diabetes mellitus, rheumatoid arthritis) |
|
| Interventions | Intervention: Prednisolone, 25mg per day, for 10 days Placebo: Placebo tablet, once per day, for 10 days Both treatments were given in tablet form, identical in appearance |
|
| Outcomes | Outcome assessed at 2 and 8 weeks 1. Global symptom score (rates 5 categories of symptoms on a 0‐10 scale. Categories include: pain, numbness, paraesthesia, weakness/clumsiness, nocturnal awakening) |
|
| Notes | Median values for symptoms were published by authors. Mean and standard deviation values were obtained from the authors in a personal communication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Koyuncu 1995.
| Methods | Randomised, double‐blind clinical trial Blinded subjects and assessors No control group Quality score: C Selection bias ‐ in part Performance bias ‐ in part Attrition bias ‐ no Detection bias ‐ yes BIAS RATING = HIGH Quality of diagnostic criteria = A |
|
| Participants | Total n = 16 (21 wrists) randomised
Group 1 n = 10 wrists
Group 2 n = 11 wrists 1 male; 15 females Median ± sd age: 49.4 ± 2.7 yrs Inclusion criteria: 1. Clinical diagnosis of CTS based on physical findings and confirmed with electrodiagnostic testing (detail not specified) Exclusion criteria: None stated |
|
| Interventions | Group 1: Circular ultrasound therapy over volar wrist surface using 1.0 W/cm2 intensity and 1MHz frequency, 8 minute session, 5 days per week, for 4 weeks (total of 20 sessions) Group 2: Circular ultrasound therapy over volar wrist surface using 1.0 W/cm2 intensity and 3MHz frequency, 8 minute session, 5 days per week, for 4 weeks (total of 20 sessions) |
|
| Outcomes | Outcome assessed weekly and at end of treatment (4 weeks) 1. Pain using ordinal scale 0‐3 (0=no pain, 1=mild, 2=moderate, 3=severe) 2. Paraesthesiae using ordinal scale 0‐3 (0=none, 1=mild, 2=moderate, 3=severe) 3. Superficial touch sensation using dichotomous scale (0=normal, 1=decreased) 4. Large object grasping using dichotomous scale (0=normal, 1=decreased) 5. Small object grasping using dichotomous scale (0=normal, 1=decreased) 6. Motor nerve distal transmission delay* 7. Sensory nerve transmission delay* 8. Tinel's sign 9. Phalen's sign |
|
| Notes | Attempts to clarify allocation method with authors were unsuccessful *Note. Only median values for neurophysiological endpoints were published by authors. Attempts to obtain mean and standard deviation data were unsuccessful |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Manente 2001.
| Methods | Randomised controlled trial No blinding* Quality score: C Selection bias ‐ no Performance bias ‐ yes Attrition bias ‐ no Detection bias ‐ no BIAS RATING = HIGH Quality of diagnostic criteria = A |
|
| Participants | Total n = 80 randomised
Intervention group n = 40
Control group n = 40 11 males; 69 females Mean ± sd age: Intervention 46 ± 13 yrs Control 50 ± 13 yrs Inclusion criteria: 1. CTS symptoms (pain, numbness, paraesthesiae in median nerve distribution) 2. CTS signs (hypaesthesia in median nerve distribution, thenar atrophy, positive Phalen's) 3. At least one abnormal CTS electrodiagnostic study Exclusion criteria: 1. Previous CTR 2. Rheumatoid arthritis 3. Systemic disease 4. Pregnancy 5. Polyneuropathy |
|
| Interventions | Intervention: Nocturnal hand brace for 4 weeks Control: No treatment for 4 weeks |
|
| Outcomes | Outcome assessed at 2 and 4 weeks 1. Symptoms using carpal tunnel questionnaire (rates 11 items on ordinal scale 1‐5) 2. Hand function using carpal tunnel questionnaire (rates 8 items on ordinal scale 1‐5) 3. Global impression of change (patient‐rated questionnaire) (at 4 weeks only) 4. Nerve conduction: median motor distal latency (ms), median sensory conduction velocity (m/s), sensory nerve action potential amplitude (uV) (at 4 weeks only) |
|
| Notes | *Confirmed with author in personal communication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Ozkul 2001.
| Methods | Randomised, double‐blind, placebo‐controlled trial Blinded subjects and treaters Quality score: B Selection bias ‐ in part Performance bias ‐ no Attrition bias ‐ no Detection bias ‐ no BIAS RATING = MODERATE Quality of diagnostic criteria = A |
|
| Participants | Total n = 50 (72 wrists) randomised
Intervention group n = 25
Placebo group n = 25 50 females Mean ± sd age: Intervention 47 ± 1.3 yrs* Placebo 48 ± 0.9 yrs* Inclusion criteria: 1. Subjects with non‐insulin dependent diabetes mellitus (NIDDM) whose plasma glucose and glycosylated hemoglobin levels were lower than 13.88mM and 8% respectively Exclusion criteria: 1. Thenar atrophy or spontaneous activity (fibrillation and fasciculation potentials, and positive sharp waves) on EMG examination of APB muscle 2. Absence of motor or sensory potentials of the median nerve 3. History of wrist trauma, rheumatic disease, acromegaly, hypothyroidism, pregnancy or prominent orthopaedic abnormalities 4. Various other disorders resembling CTS such as cervical radiculopathy, brachial plexopathy, pronator teres syndrome and polyneuropathy |
|
| Interventions | Intervention: Injection of methylprednisolone (20mg in 1ml) into carpal tunnel, followed after one week by injections of NPH insulin (0.3 ml ‐ 12 U) into the carpal tunnel, once per week for 7 weeks Placebo: Injection of methylprednisolone (20 mg in 1 ml) into carpal tunnel, followed after one week by injections of placebo (0.3 ml ‐ 0.9% saline solution) into the carpal tunnel, once per week for 7 weeks |
|
| Outcomes | Outcome assessed weekly for 8 weeks, then at 15 and 23 weeks 1. Global symptom score** (rates 5 categories of symptoms on a 0‐10 scale. Categories include: pain, numbness, paraesthesia, weakness/clumsiness, nocturnal awakening) 2. Nerve conduction studies** (median nerve motor distal latency, median nerve sensory velocity) |
|
| Notes | *Data only reported for participants completing treatment (n=43) Attempts to clarify allocation method with authors were unsuccessful **Note. Outcome data was not entered into RevMan as values were only reported in graphical form. Differences between groups for symptom and peripheral nerve conduction could not be calculated. Attempts to obtain raw data from authors were unsuccessful |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Oztas 1998.
| Methods | Randomised, single‐blind, placebo‐controlled trial Blinded subjects Quality score: B Selection bias ‐ in part Performance bias ‐ in part Attrition bias ‐ no Detection bias ‐ no BIAS RATING = MODERATE Quality of diagnostic criteria = A |
|
| Participants | Total n = 18 (30 hands) randomised
Intervention group 1 n = 7 (10 hands)
Intervention group 2 n = 9 (10 hands)
Control group n = 9 (10 hands) 18 females Mean ± sd age: 52 ± 7 yrs Inclusion criteria: 1. Clinical diagnosis of CTS confirmed with electrodiagnostic studies 2. Symptom duration greater or equal to 6 months Exclusion criteria: 1. Diabetes mellitus 2. Rheumatic disease 3. Acute trauma 4. Pregnancy 5. Physical or medical therapy in previous month 6. Corticosteroid injection in previous 3 months 7. Serious medical problems interfering with electrodiagnostic studies 8. Medical problems contraindicating use of ultrasound 9. Muscle atrophy, anesthesia or intractable pain due to CTS |
|
| Interventions | Intervention group 1: Continuous ultrasound therapy using 1.5 W/cm2 intensity and 3 MHz frequency, 5 minute session, 5 days per week, for 2 weeks Intervention group 2: Continuous ultrasound therapy using 0.8 W/cm2 intensity and 3 MHz frequency, 5 minute session, 5 days per week, for 2 weeks Control: Placebo treatment using 0.0 W/cm2 intensity without energy emission, 5 minute session, 5 days per week, for 2 weeks |
|
| Outcomes | Outcome assessed at 2 weeks 5 days 1. Pain severity (100mm horizontal visual analogue scale) 2. Symptoms* (nocturnal, day pain, paresthesia on ordinal scale: 0=no symptoms, 1=mild, 2=moderate, 3=severe) 3. Nocturnal waking* (ordinal scale: 0=never wake, 1=awaken 1‐2 times a week, 2= awaken 3‐6 times per week, 3= awaken 7 times or more) 4. Nerve conduction: median motor and sensory distal latencies, median motor forearm conduction velocity, sensory nerve conduction velocity |
|
| Notes | Attempts to clarify allocation method with authors were unsuccessful *Note. These outcomes used short ordinal scales which should be treated as binary data. Authors reported as continuous data. Attempts to obtain raw data from authors were unsuccessful |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Pal 1988.
| Methods | Randomised, double‐blind, placebo‐controlled trial Blinded subjects and assessor Quality score: B Selection bias ‐ in part Performance bias ‐ no Attrition bias ‐ in part Detection bias ‐ in part BIAS RATING = MODERATE Quality of diagnostic criteria = A |
|
| Participants | Total n = 48 randomised
Intervention group n = 23 (41 hands)
Control group n = 25 (40 hands) 5 males; 43 females Mean ± sd age: Intervention 41 ± 13 yrs Control 53 ± 13 yrs Inclusion criteria: 1. CTS confirmed with electrodiagnostic testing Exclusion criteria: 1. Patients with recognised causes of CTS: rheumatoid arthritis, other inflammatory arthropathies, thyroid disease, diabetes mellitus, acromegaly, amyloid disease 2. Pregnancy 3. Recent weight gain 4. Trauma involving the wrist 5. Patients already treated with diuretics 6. Known hypersensitivity to bendrofluazide or other thiazides |
|
| Interventions | Intervention: Diuretic treatment with bendrofluazide, 5mg daily for 4 weeks Placebo: Placebo tablet for 4 weeks |
|
| Outcomes | Outcome assessed at 4 weeks and 6 months** (5 months following end of treatment) 1. Symptom improvement (rated on ordinal scale 0‐5, 0=no improvement at all, 5=full recovery) 2. Nerve conduction* (median motor and sensory distal latencies) |
|
| Notes | Attempts to clarify allocation method with authors were unsuccessful *Note. Nerve conduction data was not entered into RevMan as mean values were published without data for variability (sd). Differences between groups for motor and sensory latencies could not be calculated. Attempts to obtain raw data from authors were unsuccessful **Outcome at 6 months was only assessed for patients who showed improvement at 4 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Rempel 1999.
| Methods | Randomised, triple‐blind, controlled trial Blinded subjects, treaters and assessors Quality score: B Selection bias ‐ in part Performance bias ‐ no Attrition bias ‐ in part Detection bias ‐ no BIAS RATING = MODERATE Quality of diagnostic criteria = B |
|
| Participants | Total n = 25 randomised
Intervention group* n = 10
Control group* n = 10 4 males; 16 females* Mean ± sd age: Intervention 45.3 ± 10.4 yrs* Control 39.9 ± 9.38 yrs* Inclusion criteria: 1. Clinical diagnosis of CTS based on history and physical examination 2. Paraesthesiae, numbness or tingling in at least 2 fingers of median nerve distribution 3. Positive Phalen's or Tinel's sign or thenar atrophy 4. Numbness, tingling or diminished sensation with use of hands or awkward posture 5. Keyboard used greater than or equal to 2 hours per day or greater/equal to 10 hours per week 6. Employed in current job for greater than or equal to 3 months Exclusion criteria: 1. Neck symptoms 2. Acute major trauma to arm or shoulder 3. Evidence of cervical root involvement, thoracic outlet syndrome or pronator teres syndrome on physical examination 4. Prior CTR or surgery to hands, wrists |
|
| Interventions | Intervention: Protouch Keyboard (ergonomically adjusted for force‐displacement characteristics of keys) for 12 weeks Control: MacPro Plus Keyboard (standard keyboard) for 12 weeks |
|
| Outcomes | Outcome assessed at 6 and 12 weeks 1. Pain using visual analogue scale 2. Hand function using ordinal questionnaire (13 items modified from Levine/Pransky scored on ordinal scale 1‐5; summed to provide overall score) 3. Phalen test time (in seconds) 4. Nerve conduction: right and left palm‐wrist median sensory latencies (in msec) (at 12 weeks only) Note: end points are reported for continuous outcomes |
|
| Notes | *Data only reported for participants completing treatment (n=20) Peripheral nerve conduction values for both hands are displayed on RevMan. Mean and standard deviation data for Phalen test time endpoints were provided by the authors in a personal communication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Spooner 1993.
| Methods | Randomised, triple‐blind, placebo‐controlled trial Blinded subjects, treaters and assessors Quality score: A Selection bias ‐ no Performance bias ‐ no Attrition bias ‐ no Detection bias ‐ no BIAS RATING = LOW Quality of diagnostic criteria = A |
|
| Participants | Total n = 35 randomised
Intervention group n = 18
Placebo group n = 17 13 males; 22 females Mean age (sd not reported): 43 yrs Inclusion criteria: 1. At least 1 provocative sign (Phalen's or Tinel's sign) or 2 or more of the following: nocturnal tingling or discomfort; swollen feeling in fingers; tingling following repetitive motion of hands; difficulty with co‐ordinated movements 2. Abnormal electrodiagnostic findings Exclusion criteria: 1. Pregnancy 2. History of alcoholism 3. Significant trauma to forearm 4. Diabetes mellitus 5. Hypothyroidism 6. Rheumatoid arthritis 7. Polyneuropathy |
|
| Interventions | Intervention: 200mg of pyridoxine daily for 12 weeks Placebo: Placebo capsule daily for 12 weeks Both treatments provided via identically‐looking capsules |
|
| Outcomes | Outcome assessed at 6 and 12 weeks 1. Nocturnal discomfort* using 5 point ordinal scale (0‐4) 2. Swelling* using 5 point ordinal scale (0‐4) 3. Movement discomfort* using 5 point ordinal scale (0‐4) 4. Hand co‐ordination* using 5 point ordinal scale (0‐4) 5. Phalen's sign (only at 12 weeks) 6. Tinel's sign (only at 12 weeks) 7. Nerve conduction: median palmar distal latency, median motor distal latency, median motor amplitude, median motor conduction velocity (at 12 weeks only) |
|
| Notes | *These outcomes used short ordinal scales which should be treated as binary data. Authors reported as continuous data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Stransky 1989.
| Methods | Randomised, double‐blind, placebo‐controlled trial Blinded subjects in intervention and placebo groups; blinded assessors* Quality score: C Selection bias ‐in part Performance bias ‐ yes Attrition bias ‐ yes Detection bias ‐ no BIAS RATING = HIGH Quality of diagnostic criteria = A |
|
| Participants | Total n = 15 randomised
Intervention group n = 6
Placebo group n = 5
Control group n = 4 Inclusion criteria: 1. History of CTS confirmed with electrodiagnostic testing |
|
| Interventions | Intervention: 200mg of vitamin B6 daily for 10 weeks Placebo: Dextrose pill daily for 10 weeks Control: No treatment for 10 weeks |
|
| Outcomes | Outcome assessed at 10 weeks 1. Symptoms using questionnaire (rated as improved, worsened) 2. Nerve conduction: median motor and sensory distal latencies |
|
| Notes | *Confirmed with author in personal communication Age and sex of participants not reported and could not be supplied by authors Attempts to clarify allocation method with authors were unsuccessful |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Tal‐Akabi 2000.
| Methods | Randomised, single‐blind, controlled trial Blinded assessors Quality score: C Selection bias ‐ yes Performance bias ‐ yes Attrition bias ‐ no Detection bias ‐ no BIAS RATING = HIGH Quality of diagnostic criteria = A |
|
| Participants | Total n = 21 randomised
Intervention group 1 n = 7
Intervention group 2 n = 7
Control group n = 7 7 males; 14 females Mean ± sd age: 47 ± 15 yrs Inclusion criteria: 1. Positive electrodiagnostic testing 2. Positive Phalen's and Tinel's sign 3. Positive upper limb tension test (ULTT) 2a with a median nerve bias 4. Diagnosis of CTS by surgeon and candidate for decompression Exclusion criteria: 1. Psychosocial problems 2. Diabetes mellitus 3. Herpes zoster 4. Rheumatoid arthritis 5. Pregnancy 6. Hyperthyroidism 7. Known abnormality of nervous system 8. Cervical or thoracic spine origin of symptoms |
|
| Interventions | Intervention group 1: Neurodynamic mobilisation (ULTT2a as described by Butler 1991) for 3 weeks Intervention group 2: Carpal bone mobilisation including posterior‐anterior mobilisation and flexor retinaculum stretch (as described by Maitland 1991) for 3 weeks For both intervention groups, the grade, amplitude and progression of treatment was individualised Control: No treatment for 3 weeks |
|
| Outcomes | Outcome assessed at 3 weeks* 1. Symptoms using a symptom diary with visual analogue scale 2. Pain relief using a short ordinal scale 0‐5 (called the modified pain relief scale); 0= no pain relief, 5= complete pain relief ** 3. Hand function using modified functional box scale (short ordinal scale; 0=able to button/unbutton shirt or grip without any problem, 4=not able to do alone)** 4. Active wrist flexion (in degrees) 5. Active wrist extension (in degrees) 6. ULTT2a (dichotomous score: positive or negative) 7. Need for surgical release (dichotomous score) |
|
| Notes | *Confirmed with principal author in personal communication **Short ordinal scales dichotomised for entry into RevMan 4.1. Pain recoded as 'improved' (score 1‐5) and 'no relief' (score 0); hand function recoded to 'improved' (improvement in score from baseline to week 3) and 'not improved/worsened' (no change or deterioration in score from baseline to week 3). Note, a subject in each group (neurodynamic, carpal bone and control) had normal hand function at baseline and had not changed after 3 weeks of follow‐up). These subjects were not included in the totals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
Tittiranonda 1999.
| Methods | Randomised, single‐blind, placebo‐controlled trial of three ergonomic keyboard designs Blinded assessors Quality score: C Selection bias ‐ in part Performance bias ‐ in part Attrition bias ‐ yes Detection bias ‐ no BIAS RATING = HIGH Quality of diagnostic criteria = B |
|
| Participants | Total n = 80 randomised
Intervention group 1 n = 20
Intervention group 2 n = 20
Intervention group 3 n = 20
Placebo group n = 20 34 males; 46 females Mean ± sd age: Intervention group 1: 45 ± 8 yrs Intervention group 2: 41 ± 10 yrs Intervention group 3: 45 ± 7 yrs Placebo group: 44 ± 8 yrs Inclusion criteria: 1. Medical history and physical examination consistent with CTS 2. Paraesthesia, numbness or tingling on volar surface of digits 1‐3 3. Numbness, tingling or diminished sensation in hands with use or with awkward posture 4. Symptom duration of at least 1 week or having occurred at least 20 times in past year 5. Positive Phalen's or Tinel's sign 6. Full‐time employee in current job for > 3 months 7. Use computer keyboard greater than or equal to 4 hours per day or greater/equal to 20 hours per week Exclusion criteria: 1. Acute major trauma to hand, wrist or shoulder within last year 2. Thoracic outlet, cervical root or pronator teres syndromes on physical exam 3. Previous hand or wrist surgery 4. CTS diagnosis > 2 years prior to assessment date |
|
| Interventions | Intervention group 1: Apple Adjustable keyboard for 6 months Intervention group 2: Comfort Keyboard System for 6 months Intervention group 3: Microsoft Natural Keyboard for 6 months Placebo group: Regular keyboard for 6 months |
|
| Outcomes | Outcome assessed at 6 months 1. Phalen's sign 2. Tinel's sign 3. Phalen test time (in seconds) 4. Pain using visual analogue scale (0=no pain, 10=worst pain) 5. Hand function using questionnaire (11 items modified from Levine/Pransky scored on visual analogue scale) |
|
| Notes | Attempts to clarify allocation method with authors were unsuccessful Note. Change scores are reported for continuous outcomes. Negative values indicate worsening of symptoms or funtion. Attempts to obtain endpoint scores conducive to meta‐analysis were unsuccessful Values for Phalen's sign and Tinel's sign are an aggregate of right and left hands |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Walker 2000.
| Methods | Randomised clinical trial of two wearing regimes for wrist splints No control group No blinding Quality score: C Selection bias ‐ yes Performance bias ‐ yes Attrition bias ‐ no Detection bias ‐ no BIAS RATING = HIGH Quality of diagnostic criteria = A |
|
| Participants | Total n = 21 (30 hands) randomised
Group 1* n = 11 hands
Group 2* n = 13 hands 20 males; 1 female Mean ± sd age: Group 1: 60 ± 9 yrs Group 2: 61 ± 13 yrs Inclusion criteria: 1. Clinical diagnosis of CTS confirmed with electrodiagnostic studies 2. No previous treatment for CTS |
|
| Interventions | Group 1: Full time wear of wrist splint for 6 weeks Group 2: Night only wear of wrist splint for 6 weeks |
|
| Outcomes | Outcome assessed at 6 weeks 1. Symptoms using carpal tunnel questionnaire (rates 11 items on ordinal scale 1‐5) 2. Hand function using carpal tunnel questionnaire (rates 8 items on ordinal scale 1‐5) 3. Nerve conduction: median motor and sensory distal latencies (in ms) |
|
| Notes | *Data only reported for participants completing treatment (n=17 subjects, 24 hands) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbot 1999 | Not a randomised clinical trial. This is a clinical commentary on the Garfinkel 1998 trial. |
| Baum 1986 | Did not examine the efficacy of non‐surgical treatment for carpal tunnel syndrome. |
| Bennett 1998 | Participants were not diagnosed with carpal tunnel syndrome. Participants were diagnosed with fibromyalgia. |
| Bhatia 2000 | Participants underwent carpal tunnel release, which is an exclusion criterion for this review. |
| Bonebrake 1993 | Not a randomised clinical trial. |
| Bonebrake 1994 | Not a randomised clinical trial. This is a clinical commentary on the Bonebrake 1993 study. |
| Bury 1995 | Participants underwent carpal tunnel release, which is an exclusion criterion for this review. |
| Celiker 2002 | Steroid injection was a primary treatment under investigation. To be considered for inclusion in next update of separate review on steroid injection by Marshall 2001. |
| Chaise 1994 | Participants underwent carpal tunnel release, which is an exclusion criterion for this review. |
| Cook 1995 | Participants underwent carpal tunnel release, which is an exclusion criterion for this review. |
| Dammers 1999 | Steroid injection was the primary treatment under investigation. |
| Daniel 2000 | Not a randomised clinical trial. |
| Deliss 1998 | Not a randomised clinical trial. This is a clinical commentary on the Ebenbichler 1998 trial. |
| Elbaz 1994 | Steroid injection was the primary treatment under investigation. |
| Ellis 1982 | Not a randomised clinical trial. |
| Finsen 1999 | Participants underwent carpal tunnel release, which is an exclusion criterion for this review. |
| Girlanda 1993 | Steroid injection was the primary treatment under investigation. |
| Guy 1988 | Participants were not diagnosed with carpal tunnel syndrome. Participants were diagnosed with diabetic neuropathy; participants with symptomatic nerve entrapment syndromes at the time of recruitment were excluded. |
| Hafner 1999 | Not a randomised clinical trial. This is a clinical commentary on the Davis 1998 trial. |
| Helwig 2000 | Not a randomised clinical trial. This is a clinical commentary on the Dammers 1999 trial. |
| Hochberg 2001 | Participants underwent carpal tunnel release, which is an exclusion criterion for this review. |
| Jarmuzewska 2000 | Did not examine the efficacy of non‐surgical treatment. |
| Kruger 1991 | Not a randomised clinical trial. |
| Li 1999 | Not a randomised clinical trial. |
| Lucantoni 1992 | Steroid injection was a primary treatment under investigation. To be considered for inclusion in next update of separate review on steroid injection by Marshall 2001. |
| Monge 1995 | Not a randomised clinical trial. |
| Nathan 2001 | Not a randomised clinical trial. |
| O'Gradaigh 2000 | Steroid injection was the primary treatment under investigation. |
| Ozdogan 1984 | Steroid injection was the primary treatment under investigation. |
| Padua 1999 | Not a randomised clinical trial. |
| Piotrowski 1998 | Steroid injection was the primary treatment under investigation. |
| Provinciali 2000 | Participants underwent carpal tunnel release, which is an exclusion criterion for this review. |
| Rozmaryn 1998 | Not a prospective randomised clinical trial. Outcomes were collected retrospectively from participants' clinical case notes. |
| Sucher 1994 | Not a randomised clinical trial. |
| Sucher 1999 | Not a randomised clinical trial. This is a clinical commentary on the Oztas 1998 trial. |
| Wolaniuk 1983 | Did not measure the primary or secondary outcome measures specified by the review. |
| Wong 2001 | Steroid injection was a primary treatment under investigation. To be considered for inclusion in next update of separate review on steroid injection by Marshall 2001. |
| Wu 1991 | Did not measure the primary or secondary outcome measures specified by the review. Measured neurophysiological parameters at end of treatment only. |
Contributions of authors
The primary reviewer (DOC) co‐ordinated each stage of the review and was responsible for:
the conception and design of the review (in collaboration with NMW and SM);
developing the protocol (in collaboration with SM and NMW);
developing the search strategy in collaboration with the Neuromuscular Disease Review Group;
undertaking the searches for trials;
screening the search results;
organising retrieval of papers;
screening retrieved papers against inclusion/exclusion criteria (independently of, but in addition to SM and NMW);
appraising the quality of papers (independently of, but in addition to SM);
abstracting data from papers (independently of, but in addition to SM);
writing to authors of papers for additional information;
providing additional data about papers;
entering data into RevMan 4.1;
compiling the list of comparisons, table of included and excluded studies, reference lists;
performing analysis of data;
interpreting the findings;
writing the review;
final approval of the version to be published.
The second reviewer (SM) was involved in the following stages of the review:
the design of the review (in collaboration with DOC and NMW);
developing the protocol (in collaboration with DOC and NMW);
screening the search results (independently of, but in addition to DOC and NMW);
screening the retrieved papers against inclusion/exclusion criteria(independently of, but in addition to DOC and NMW);
appraising the quality of the papers (independently of, but in addition to DOC);
abstracting data from papers (independently of, but in addition to DOC);
contributing to the writing of the review.
The third reviewer (NMW) was involved in the following stages of the review:
the conception and design of the review (in collaboration with DOC and SM);
developing the protocol (in collaboration with DOC and SM);
screening the search results (independently of, but in addition to DOC and SM);
screening the retrieved papers against inclusion/exclusion criteria (independently of, but in addition to DOC and SM);
summarising the quality appraisal of the trials (rated independently by DOC and SM);
performing double‐data entry;
contributing to the writing of the review.
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Aigner 1999 {published data only}
- Aigner N, Zoch G, Petje G. Results of laser‐acupuncture in carpal tunnel syndrome: a prospective, randomised and blinded study [Laserakupunktur bei der praoperativen schmerzbekampfung beim karpaltunnelsyndrom: eine prospektiv randomisierte studie]. Deutsche Zeitschrift für Akupunktur 1999;42:70‐5. [Google Scholar]
Akalin 2002 {published data only}
- Akalin E, El O, Peker O, Senocak O, Tamci S, Gulbahar S, Cakmur R, Oncel S. Treatment of carpal tunnel syndrome with nerve and tendon gliding exercises. American Journal of Physical Medicine & Rehabilitation 2002;81(2):108‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Burke 1994 {published data only}
- Burke DT, Burke MM, Stewart GW, Cambre A. Splinting for carpal tunnel syndrome: in search of the optimal angle. Archives of Physical Medicine & Rehabilitation 1994;75:1241‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carter 2002 {published data only}
- Carter R, Hall T, Aspy CB, Mold J. The effectiveness of magnet therapy for treatment of wrist pain attributed to carpal tunnel syndrome. Journal of Family Practice 2002;51:38‐40. [MEDLINE: ] [PubMed] [Google Scholar]
Chang 1998 {published data only}
- Chang MH, Chiang HT, Lee SSJ, Ger LP, Lo YK. Oral drug of choice in carpal tunnel syndrome. Neurology 1998;51:390‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Davis 1998 {published data only}
- Davis PT, Hulbert JR, Kassak KM, Meyer JJ. Comparative efficacy of conservative medical and chiropractic treatments for carpal tunnel syndrome: a randomized clinical trial. Journal of Manipulative & Physiological Therapeutics 1998;21:317‐26. [MEDLINE: ] [PubMed] [Google Scholar]
Ebenbichler 1998 {published data only}
- Ebenbichler GR, Resch KL, Nicolakis P, Wiesinger GF, Uhl F, Ghanem A, Fialka V. Ultrasound treatment for treating the carpal tunnel syndrome: randomised 'sham' controlled trial. British Medical Journal 1998;316:731‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garfinkel 1998 {published data only}
- Garfinkel MS, Singhal A, Katz WA, Allan DA, Reshetar R, Schumacher HR Jr. Yoga‐based intervention for carpal tunnel syndrome: a randomized trial. Journal of the American Medical Association 1998;280:1601‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Herskovitz 1995 {published data only}
- Herskovitz S, Berger AR, Lipton RB. Low‐dose, short‐term oral prednisone in the treatment of carpal tunnel syndrome. Neurology 1995;45:1923‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hui 2001 {published data only}
- Hui ACF, Wong SM, Wong KS, Li E, Kay R, Yung P, Hung LK, Yu LM. Oral steroid in the treatment of carpal tunnel syndrome. Annals of the Rheumatic Diseases 2001;60(8):813‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koyuncu 1995 {published data only}
- Koyuncu H, Unver FN, Sahin U, Togay P. 1Mhz ‐ 3MHz ultrasound applications in carpal tunnel syndrome [Karpal tunel sendromunda 1MHz ‐ 3MHz ultrason uygulamasi]. Fizik Tedavi ve Rehabilitasyon Dergisi 1995;19:141‐5. [Google Scholar]
Manente 2001 {published data only}
- Manente G, Torrieri F, Blasio F, Staniscia T, Romano F, Uncini A. An innovative hand brace for carpal tunnel syndrome: a randomized controlled trial. Muscle & Nerve 2001;24:1020‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ozkul 2001 {published data only}
- Ozkul Y, Sabuncu T, Yazgan P, Nazligul Y. Local insulin injection improves median nerve regeneration in NIDDM patients with carpal tunnel syndrome. European Journal of Neurology 2001;8:329‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oztas 1998 {published data only}
- Oztas O, Turan B, Bora I, Kerim Karakaya M. Ultrasound therapy effect in carpal tunnel syndrome. Archives of Physical Medicine & Rehabilitation 1998;79:1540‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pal 1988 {published data only}
- Pal B, Mangion P, Hossain MA, Wallace AS, Diffey BL. Should diuretics be prescribed for idiopathic carpal tunnel syndrome? Results of a controlled trial. Clinical Rehabilitation 1988;2:299‐301. [Google Scholar]
Rempel 1999 {published data only}
- Rempel D, Tittiranonda P, Burastero S, Hudes M, So Y. Effect of keyboard keyswitch design on hand pain. Journal of Occupational and Environmental Medicine 1999;41:111‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Spooner 1993 {published data only}
- Spooner GR, Desai HB, Angel JF, Reeder BA, Donat JR. Using pyridoxine to treat carpal tunnel syndrome: randomized control trial. Canadian Family Physician 1993;39:2122‐7. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Stransky 1989 {published data only}
- Stransky M, Rubin A, Lava NS, Lazaro RP. Treatment of carpal tunnel syndrome with vitamin B6: a double‐blind study. Southern Medical Journal 1989;82:841‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tal‐Akabi 2000 {published data only}
- Tal‐Akabi A, Rushton A. An investigation to compare the effectiveness of carpal bone mobilisation and neurodynamic mobilisation as methods of treatment for carpal tunnel syndrome. Manual Therapy 2000;5:214‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tittiranonda 1999 {published data only}
- Tittiranonda P, Rempel D, Armstrong T, Burastero S. Effect of four computer keyboards in computer users with upper extremity musculoskeletal disorders. American Journal of Industrial Medicine 1999;35:647‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Walker 2000 {published data only}
- Walker WC, Metzler M, Cifu DX, Swartz Z. Neutral wrist splinting in carpal tunnel syndrome: a comparison of night‐only versus full‐time wear instructions. Archives of Physical Medicine & Rehabilitation 2000;81:424‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbot 1999 {published data only}
- Abbot NC. Yoga‐based intervention for carpal tunnel syndrome: commentary. Focus on Alternative and Complementary Therapies 1999;4:81‐2. [Google Scholar]
Baum 1986 {published data only}
- Baum H, Ludecke DK, Herrmann HD. Carpal tunnel syndrome and acromegaly. Acta Neurochirurgica 1986;83:54‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bennett 1998 {published data only}
- Bennett RM, Clark SC, Walczyk J. A randomized, double‐blind, placebo‐controlled study of growth hormone in the treatment of fibromyalgia. American Journal of Medicine 1998;104:227‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bhatia 2000 {published data only}
- Bhatia R, Field J, Grote J, Huma H. Does splintage help pain after carpal tunnel release?. Journal of Hand Surgery (British Volume) 2000;25:150. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bonebrake 1993 {published data only}
- Bonebrake AR, Fernandez JE, Dahalan JB, Marley RJ. A treatment for carpal tunnel syndrome: results of a follow‐up study. Journal of Manipulative & Physiological Therapeutics 1993;16:125‐39. [MEDLINE: ] [PubMed] [Google Scholar]
Bonebrake 1994 {published data only}
- Bonebrake AR. A treatment for carpal tunnel syndrome: results of follow‐up study. Journal of Manipulative and Physiological Therapeutics 1994;17(8):565‐7. [PubMed] [Google Scholar]
Bury 1995 {published data only}
- Bury TF, Akelman E, Weiss AP. Prospective, randomized trial of splinting after carpal tunnel release. Annals of Plastic Surgery 1995;35:19‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Celiker 2002 {published data only}
- Celiker R, Arslan S, Inanici F. Corticosteroid injection vs nonsteroidal antiinflammatory drug and splinting in carpal tunnel syndrome. American Journal of Physical Medicine & Rehabilitation 2002;81:182‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chaise 1994 {published data only}
- Chaise F, Guest M, Bellemere P, Friol JP, Gaisne E, Lehert P. Efficacy of naftidrofuryl on autonomic symptoms following surgery for carpal tunnel syndrome [Efficacite du naftidrofuryl sur les signes neurovegetatifs survenant dans les suites de la chirurgie du canal carpien]. Annals de Chirurgie de la Main et du Membre Superieur (Annals of Hand Surgery) 1994;13:214‐21. [DOI] [PubMed] [Google Scholar]
Cook 1995 {published data only}
- Cook AC, Szabo RM, Birkholz SW, King EF. Early mobilization following carpal tunnel release: a prospective randomized study. Journal of Hand Surgery (British Volume) 1995;20:228‐30. [DOI] [PubMed] [Google Scholar]
Dammers 1999 {published data only}
- Dammers JW, Veering MM, Vermeulen M. Injection with methylprednisolone proximal to the carpal tunnel: randomised double blind trial. British Medical Journal 1999;319:884‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Daniel 2000 {published data only}
- Daniel ES, Paul S. A comparison study of the volar wrist cock‐up splint and ulnar gutter splint in carpal tunnel syndrome. Occupational Therapy in Health Care 2000;12:79‐93. [DOI] [PubMed] [Google Scholar]
Deliss 1998 {published data only}
- Deliss L. Ultrasound treatment for carpal tunnel syndrome: emphasis must be on return of sensation and function. British Medical Journal 1998;317:601. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbaz 1994 {published data only}
- Elbaz A, Gagnon S, Beaumont P, Morcos R, Proulx S, Page JM, Renaud E, Dumont M. Carpal tunnel syndrome: a double blind study on the effect of steroid injection. Journal of Bone and Joint Surgery (British Volume) 1994;76 Supp I:22. [Google Scholar]
Ellis 1982 {published data only}
- Ellis JM, Folkers K, Levy M, Shizukuishi S, Lewandowski J, Nishii S, Schubert HA, Ulrich R. Response of vitamin B6 deficiency and the carpal tunnel syndrome to pyridoxine. Proceedings of the National Academy of Sciences of the United States of America 1982;79:7494‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Finsen 1999 {published data only}
- Finsen V, Andersen K, Russwurm H. No advantage from splinting the wrist after open carpal tunnel release: a randomized study. Acta Orthopaedica Scandinavica 1999;70(3):288‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Girlanda 1993 {published data only}
- Girlanda P, Dattola R, Venuto C, Mangiapane R, Nicolosi C, Messina C. Local steroid treatment in idiopathic carpal tunnel syndrome: short‐ and long‐term efficacy. Journal of Neurology 1993;240:187‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guy 1988 {published data only}
- Guy RJ, Gilbey SG, Sheehy M, Asselman P, Watkins PJ. Diabetic neuropathy in the upper limb and the effect of twelve months sorbinil treatment. Diabetologia 1988;31:214‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hafner 1999 {published data only}
- Hafner E, Kendall J, Kendall P. Comparative efficacy of conservative medical and chiropractic treatments for carpal tunnel syndrome: a randomized clinical trial [letter; comment]. Journal of Manipulative and Physiological Therapeutics 1999;22:348‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Helwig 2000 {published data only}
- Helwig AL. Treating carpal tunnel syndrome. Journal of Family Practice 2000;49:79‐80. [MEDLINE: ] [PubMed] [Google Scholar]
Hochberg 2001 {published data only}
- Hochberg J. A randomized prospective study to assess the efficacy of two cold‐therapy treatments following carpal tunnel release. Journal of Hand Therapy 2001;14:208‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jarmuzewska 2000 {published data only}
- Jarmuzewska EA, Ghidoni A. Study of onset and progression of peripheral neuropathy and hypertension in NIDDM [Studia dell'esordio e della progressione della neuropatia periferica e dell'ipertensione nei NIDDM]. Minerva Medica 2000;91:1‐15. [MEDLINE: ] [PubMed] [Google Scholar]
Kruger 1991 {published data only}
- Kruger VL, Kraft GH, Deitz JC, Ameis A, Polissar L. Carpal tunnel syndrome: objective measures and splint use. Archives of Physical Medicine & Rehabilitation 1991;72:517‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Li 1999 {published data only}
- Li S, Liu L, Miyazaki M, Warren S. Effectiveness of splinting for work‐related carpal tunnel syndrome: a three‐month follow‐up study. Technology and Disability 1999;11(1/2):51. [Google Scholar]
Lucantoni 1992 {published data only}
- Lucantoni C, Grottoli S, Gaetti R. Comparison between He‐Ne laser therapy and steroid injections in the treatment of idiopathic carpal tunnel syndrome [Confronto tra laserterapia He‐Ne e terapia infiltrativa steroidea nel trattamento della sindrome idiopatica del tunnel carpale [Italian]]. La Riabilitazione 1992;25(4):249‐56. [Google Scholar]
Monge 1995 {published data only}
- Monge L, Mattei M, Dani F, Sciarretta A, Carta Q. Effect of treatment with an aldose reductase inhibitor on symptomatic carpal tunnel syndrome in type 2 diabetes. Diabetic Medicine 1995;12:1097‐1101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nathan 2001 {published data only}
- Nathan PA, Wilcox A, Emerick PS, Meadows KD, McCormack AL. Effects of an aerobic exercise program on median nerve conduction and symptoms associated with carpal tunnel syndrome. Journal of Occupational and Environmental Medicine 2001;43:840‐3. [DOI] [PubMed] [Google Scholar]
O'Gradaigh 2000 {published data only}
- O'Gradaigh D, Merry P. Corticosteroid injection for the treatment of carpal tunnel syndrome. Annals of the Rheumatic Diseases 2000;59:918‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozdogan 1984 {published data only}
- Ozdogan H, Yazici H. The efficacy of local steroid injections in idiopathic carpal tunnel syndrome: a double‐blind study. British Journal of Rheumatology 1984;23:272‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Padua 1999 {published data only}
- Padua L, Padua R, Moretti C, Nazzaro M, Tonali P. Clinical outcome and neurophysiological results of low‐power laser irradiation in carpal tunnel syndrome. Lasers in Medical Science 1999;14:196‐202. [Google Scholar]
Piotrowski 1998 {published data only}
- Piotrowski M, Szczepanski L, Dmoszynska M. Treatment of rheumatic conditions with local instillation of betamethasone and methylprednisolone: comparison of efficacy and frequency of irritative pain reaction [Leczenie chorob tkanek miekkich okolostawowych i zapalen stawow iniekcjami octanu metylprednizolonu (depo‐medrol) i betametazonem (diprophos): porownanie skutecznosci i wystepowania miejscowych odczynow bolowych]. Reumatologia 1998;36:78‐84. [Google Scholar]
Provinciali 2000 {published data only}
- Provinciali L, Giattini A, Splendiani G, Logullo F. Usefulness of hand rehabilitation after carpal tunnel surgery. Muscle & Nerve 2000;23:211‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rozmaryn 1998 {published data only}
- Rozmaryn LM, Dovelle S, Rothman ER, Gorman K, Olvey KM, Bartko JJ. Nerve and tendon gliding exercises and the conservative management of carpal tunnel syndrome. Journal of Hand Therapy 1998;11:171‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sucher 1994 {published data only}
- Sucher BM. Palpatory diagnosis and manipulative management of carpal tunnel syndrome. Journal of the American Osteopathic Association 1994;94(8):647‐63. [MEDLINE: ] [PubMed] [Google Scholar]
Sucher 1999 {published data only}
- Sucher BM. Ultrasound therapy effect in carpal tunnel syndrome [letter; comment]. Archives of Physical Medicine & Rehabilitation 1999;80:1117. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wolaniuk 1983 {published data only}
- Wolaniuk A, Vadhanavikit S, Folkers K. Electromyographic data differentiate patients with the carpal tunnel syndrome when double blindly treated with pyridoxine and placebo. Research Communications in Chemical Pathology & Pharmacology 1983;41:501‐11. [PubMed] [Google Scholar]
Wong 2001 {published data only}
- Wong SM, Hui ACF, Tang A, Ho PC, Hung LK, Wong KS, Kay R, Li E. Local vs systemic corticosteroids in the treatment of carpal tunnel syndrome. Neurology 2001;56:1565‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wu 1991 {published data only}
- Wu SF, Chan RC, Hsu TC. Electrodiagnostic evaluation of conservative treatment in carpal tunnel syndrome. Chinese Medical Journal 1991;48:125‐30. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies awaiting assessment
Toro 1997 {published data only}
- Rioja Toro J, Garcia Rodriguez I, Prada Espinel J, Garcia Caballero M, Arroyo Rodriguez F. Idiopathic carpal tunnel syndrome: efficacy of treatment with iontophoresis‐corticoid versus iontophoresis‐placebo (galvinization) [Sindrome del canal carpiano cronico idiopatico: eficacia del tratamiento de iontoforesis‐corticoide frente a iontoforesis‐placebo (galvanizacion)]. Rehabilitacion 1997;31:118‐26. [Google Scholar]
Additional references
AAN 1993
- American Academy of Neurology, American Association of Electrodiagnostic Medicine, American Academy of Physical Medicine, Rehabilitation. Practice parameter for electrodiagnostic studies in carpal tunnel syndrome. Neurology 1993;43:2404‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Amadio 1996
- Amadio P, Silverstein M, Ilstrup D, Schleck C, Jensen L. Outcome assessment for carpal tunnel surgery: the relative responsiveness of generic, arthritis‐specific, disease‐specific, and physical examination measures. Journal of Hand Surgery (American Volume) 1996;21:338‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Atroshi 1999
- Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosen I. Prevalence of carpal tunnel syndrome in a general population. Journal of the American Medical Association 1999;282:153‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Braun 1989
- Braun RM, Davidson K, Doehr S. Provocative testing in the diagnosis of dynamic carpal tunnel syndrome. Journal of Hand Surgery (American Volume) 1989;14:195‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Butler 1991
- Butler D. Mobilization of the Nervous System. First Edition. Melbourne: Churchill Livingstone, 1991. [Google Scholar]
Clarke 1999
- Clarke M, Oxman AD editors. Cochrane Reviewers' Handbook 4.0 [updated July 1999]. In: The Cochrane Library [database on CDROM]. The Cochrane Collaboration. Oxford: Update Software; 2000, Issue 2 1999.
Concannon 2000
- Concannon MJ, Brownfield ML, Puckett CL. The incidence of recurrence after endoscopic carpal tunnel release. Plastic & Reconstructive Surgery 2000;105:1662‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Futami 1992
- Futami T, Kobayashi A, Wakabayshi N, Kouichi Y, Nakamura K. Natural history of carpal tunnel syndrome. Journal of Japanese Society for Surgery of the Hand 1992;8:410‐2. [Google Scholar]
Gelberman 1984
- Gelberman RH, Szabo RM, Mortenson MM. Carpal tunnel pressures and wrist position in patients with colles fractures. Journal of Trauma, Injury, Infection and Critical Care 1984;24:747‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Green 2002
- Green S, Deeks J. Meta‐analysis of continuous data. Australasian Cochrane Centre: Workshop on Introduction to Analysis 2002; Vol. May 24, Adelaide, South Australia.
Hagberg 1992
- Hagberg M, Morgenstern H, Kelsh M. Impact of occupations and job tasks on the prevalence of carpal tunnel syndrome. Scandinavian Journal of Work, Environment & Health 1992;18:337‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jablecki 2002
- Jablecki CK, Andary MT, Floeter MK, Miller RG, Quartly CA, Vennix MJ, Wilson JR. Practice parameter: electrodiagnostic studies in carpal tunnel syndrome. Report of the American Association of Electrodiagnostic Medicine, American Academy of Neurology, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2002;58:1589‐1592. [DOI] [PubMed] [Google Scholar]
Katims 1989
- Katims JJ, Rouvelas P, Sadler B, Weseley SA. Current Perception Threshold: Reproducibility and comparison with nerve conduction in evaluation of carpal tunnel syndrome. Transactions of the American Society for Artificial Internal Organs 1989;35:280‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Katz 1990
- Katz JN, Stirrat CR, Larson MG, Fossel AH, Eaton HM, Liang MH. A self‐administered hand symptom diagram for the diagnosis and epidemiologic study of carpal tunnel syndrome. Journal of Rheumatology 1990;17:1495‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Katz 1994
- Katz JN, Gelberman RH, Wright EA, Lew RA, Liang MH. Responsiveness of self‐reported and objective measures of disease severity in carpal tunnel syndrome. Medical Care 1994;32:1127‐33. [DOI] [PubMed] [Google Scholar]
Kerwin 1996
- Kerwin G, Williams CS, Seiler JG 3rd. The pathophysiology of carpal tunnel syndrome. Hand Clinics 1996;12:243‐51. [MEDLINE: ] [PubMed] [Google Scholar]
Levine 1993
- Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, Katz JN. A self‐administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. Journal of Bone & Joint Surgery 1993;75:1585‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marshall 2001
- Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome (Cochrane Review). The Cochrane Library 2001, Issue 1. [Google Scholar]
Marx 1998
- Marx RG, Hudak PV, Bombardier C, Graham B, Goldsmith C, Wright JG. The reliability of physical examination for carpal tunnel syndrome. Journal of Hand Surgery (British Volume) 1998;23(4):499‐502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Newport 2000
- Newport ML. Upper extremity disorders in women. Clinical Orthopaedics & Related Research 2000;372:85‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Padua 2001
- Padua L, Padua R, Aprile I, Pasqualetti P, Tonali P. Multiperspective follow‐up of untreated carpal tunnel syndrome: a multicentre study. Neurology 2001;56:1459‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rempel 1998
- Rempel D, Evanoff B, Amadio PC, Krom M, Franklin G, Franzblau A, et al. Consensus criteria for the classification of carpal tunnel syndrome in epidemiologic studies. American Journal of Public Health 1998;88:1447‐51. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenthal 1987
- Rosenthal EA. Tenosynovitis: tendon and nerve entrapment. Hand Clinics 1987;3:585‐607. [MEDLINE: ] [PubMed] [Google Scholar]
Scholten 2002
- Scholten RJPM, Gerritsen AAM, Uitdehaag BMJ, Geldere D, Vet HCW, Bouter LM. Surgical treatment options for carpal tunnel syndrome (Cochrane Review). The Cochrane Library 2002, Issue 4. [DOI] [PubMed] [Google Scholar]
Seradge 1994
- Seradge H, Parker WL. Is present conservative treatment for carpal tunnel syndrome adequate?. Southern Medical Journal 1994;87:S64‐7. [Google Scholar]
Spinner 1995
- Spinner M. Nerve lesions in continuity. In: Hunter JM, Mackin EJ, Callhan AD editor(s). Rehabilitation of the Hand: Surgery and Therapy. St Louis: Mosby, 1995:627‐34. [Google Scholar]
Szabo 1992
- Szabo RM, Madison M. Carpal tunnel syndrome. Orthopedic Clinics of North America 1992;23:103‐109. [MEDLINE: ] [PubMed] [Google Scholar]
Szabo 1994
- Szabo RM, Steinberg DR. Nerve entrapment syndromes in the wrist. Journal of the American Academy of Orthopedic Surgeons 1994;2:115‐23. [DOI] [PubMed] [Google Scholar]
Tulder 2002
- Tulder MW van, Esmail R, Bombardier C, Koes BW. Back schools for non‐specific low back pain (Cochrane Review). The Cochrane Library 2002, Issue 3. [DOI] [PubMed] [Google Scholar]
Verdugo 2002
- Verdugo RJ, Salinas RS, Castillo J, Cea JG. Surgical versus non‐surgical treatment for carpal tunnel syndrome (Cochrane Review). The Cochrane Library 2002, Issue 3. [DOI] [PubMed] [Google Scholar]


