Abstract
Background
Most people who receive a kidney transplant die from either cardiovascular disease or cancer before their transplant fails. The most common reason for someone with a kidney transplant to lose the function of their transplanted kidney necessitating return to dialysis is chronic kidney transplant scarring. Immunosuppressant drugs have side effects that increase risks of cardiovascular disease, cancer and chronic kidney transplant scarring. Belatacept may provide sufficient immunosuppression while avoiding unwanted side effects of other immunosuppressant drugs. However, high rates of post‐transplant lymphoproliferative disease (PTLD) have been reported when belatacept is used in particular kidney transplant recipients at high dosage.
Objectives
1) Compare the relative efficacy of belatacept versus any other primary immunosuppression regimen for preventing acute rejection, maintaining kidney transplant function, and preventing death. 2) Compare the incidence of several adverse events: PTLD; other malignancies; chronic transplant kidney scarring (IF/TA); infections; change in blood pressure, lipid and blood sugar control. 3) Assess any variation in effects by study, intervention and recipient characteristics, including: differences in pre‐transplant Epstein Barr virus serostatus; belatacept dosage; and donor‐category (living, standard criteria deceased, or extended criteria deceased).
Search methods
We searched the Cochrane Renal Group's Specialised Register to 1 September 2014 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review.
Selection criteria
Randomised controlled trials (RCT) that compared belatacept versus any other immunosuppression regimen in kidney transplant recipients were eligible for inclusion.
Data collection and analysis
Two authors independently extracted data for study quality and transplant outcomes and synthesized results using random effects meta‐analysis, expressed as risk ratios (RR) and mean differences (MD), both with 95% confidence intervals (CI). Subgroup analyses and univariate meta‐regression were used to investigate potential heterogeneity.
Main results
We included five studies that compared belatacept and calcineurin inhibitors (CNI) that reported data from a total of 1535 kidney transplant recipients. Of the five studies, three (478 participants) compared belatacept and cyclosporin and two (43 recipients) compared belatacept and tacrolimus. Co‐interventions included basiliximab (4 studies, 1434 recipients); anti‐thymocyte globulin (1 study, 89 recipients); alemtuzumab (1 study, 12 recipients); mycophenolate mofetil (MMF, 5 studies, 1509 recipients); sirolimus (1 study, 26 recipients) and prednisone (5 studies, 1535 recipients).
Up to three years following transplant, belatacept and CNI‐treated recipients were at similar risk of dying (4 studies, 1516 recipients: RR 0.75, 95% CI 0.39 to 1.44), losing their kidney transplant and returning to dialysis (4 studies, 1516 recipients: RR 0.91, 95% CI 0.61 to 1.38), and having an episode of acute rejection (4 studies, 1516 recipients: RR 1.56, 95% CI 0.85 to 2.86). Belatacept‐treated kidney transplant recipients were 28% less likely to have chronic kidney scarring (3 studies, 1360 recipients: RR 0.72, 95% CI 0.55 to 0.94) and also had better graft function (measured glomerular filtration rate (GFR) (3 studies 1083 recipients): 10.89 mL/min/1.73 m², 95% CI 4.01 to 17.77; estimated GFR (4 studies, 1083 recipients): MD 9.96 mL/min/1.73 m², 95% CI 3.28 to 16.64) than CNI‐treated recipients. Blood pressure was lower (systolic (2 studies, 658 recipients): MD ‐7.51 mm Hg, 95% CI ‐10.57 to ‐4.46; diastolic (2 studies, 658 recipients): MD ‐3.07 mm Hg, 95% CI ‐4.83 to ‐1.31, lipid profile was better (non‐HDL (3 studies 1101 recipients): MD ‐12.25 mg/dL, 95% CI ‐17.93 to ‐6.57; triglycerides (3 studies 1101 recipients): MD ‐24.09 mg/dL, 95% CI ‐44.55 to ‐3.64), and incidence of new‐onset diabetes after transplant was reduced by 39% (4 studies (1049 recipients): RR 0.61, 95% CI 0.40 to 0.93) among belatacept‐treated versus CNI‐treated recipients.
Risk of PTLD was similar in belatacept and CNI‐treated recipients (4 studies, 1516 recipients: RR 2.79, 95% CI 0.61 to 12.66) and was no different among recipients who received different belatacept dosages (high versus low dosage: ratio of risk ratios (RRR) 1.06, 95% CI 0.11 to 9.80, test of difference = 0.96) or among those who were Epstein Barr virus seronegative compared with those who were seropositive before their kidney transplant (seronegative versus seropositive; RRR 1.49, 95% CI 0.15 to 14.76, test for difference = 0.73).
The belatacept dose used (high versus low), type of donor kidney the recipient received (extended versus standard criteria) and whether the kidney transplant recipient received tacrolimus or cyclosporin made no difference to kidney transplant survival, incidence of acute rejection or estimated GFR. Selective outcome reporting meant that data for some key subgroup comparisons were sparse and that estimates of the effect of treatment in these groups of recipients remain imprecise.
Authors' conclusions
There is no evidence of any difference in the effectiveness of belatacept and CNI in preventing acute rejection, graft loss and death, but treatment with belatacept is associated with less chronic kidney scarring and better kidney transplant function. Treatment with belatacept is also associated with better blood pressure and lipid profile and a lower incidence of diabetes versus treatment with a CNI. Important side effects (particularly PTLD) remain poorly reported and so the relative benefits and harms of using belatacept remain unclear. Whether short‐term advantages of treatment with belatacept are maintained over the medium‐ to long‐term or translate into better cardiovascular outcomes or longer kidney transplant survival with function remains unclear. Longer‐term, fully reported and published studies comparing belatacept versus tacrolimus are needed to help clinicians decide which patients might benefit most from using belatacept.
Plain language summary
Belatacept for kidney transplant recipients
Kidney transplants can improve the quality and length of life for patients with end‐stage kidney disease (ESKD) compared with chronic dialysis. To prevent a kidney transplant from being rejected by the body, immune‐system suppressing drugs (most commonly a calcineurin inhibitors (CNI)) are used. CNI are associated with high blood pressure, high lipid levels, an increased risk of developing diabetes, and chronic scarring of the kidney transplant. Chronic kidney scarring is the main reason that kidney transplants lose function in people who do not die before their kidney transplant fails. Belatacept might be an alternative immune‐system suppressing drug which prevents rejection but which also causes fewer side‐effects than CNI.
We included five studies that compared belatacept and calcineurin inhibitors (CNI) and enrolled 1535 kidney transplant recipients. We found that belatacept was no different to a CNI at being able to prevent acute rejection and at keeping a transplanted kidney working. Recipients who received belatacept had lower blood pressure, less diabetes and better kidney transplant function than recipients who received a CNI. The chance of dying after a kidney transplant was similar in recipients treated with belatacept and CNI.
Summary of findings
for the main comparison.
| Belatacept versus calcineurin inhibitors (CNI) for kidney transplant recipients | ||||||
|
Patient or population: Kidney transplant recipients Settings: multinational Intervention: Belatacept (any dosage) Comparison: CNI | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| CNI | Belatacept | |||||
| Death | 57.5 per 1000 | 43.0 per 1000 (22.4 to 82.8) | 0.75 (0.39 to 1.44) | 1516 (4) | High quality | |
| Loss of kidney transplant | 59.5 per 1000 | 54.1 per 1000 (36.3 to 82.1) | 0.91 (0.61 to 1.38) | 1516 (4) | High quality | |
| Acute rejection | 164.7 per 1000 | 257.0 per 1000 (140.0 to 471.0) | 1.56 (0.85 to 2.86) | 1516 (4) | High quality | |
| Chronic kidney scarring | 413.3 per 1000 | 297.4 per 1000 (227.2 to 388.2) | 0.72 (0.55 to 0.94) | 1350 (3) | High quality | |
| Malignancy | 37.7 per 1000 | 37.7 per 1000 (21.9 to 64.8) | 1.00 (0.58 to 1.72) | 1516 (4) | Moderate quality | |
| PTLD | 10.9 per 1000 | 30.4 per 1000 (6.6 to 138.0) | 2.79 (0.61 to 12.66) | 1516 (4) | Moderate quality | Very low quality for Epstein Barr virus and belatacept dosage subgroups |
| New onset diabetes | 67.2 per 1000 | 50.0 per 1000 (26.9 to 62.5) | 0.61 (0.40 to 0.93) | 1049 (4) | Moderate quality | |
| Delayed graft function | 321.0 per 1000 | 298.5 per 1000 (253.6 to 349.9) | 0.93 (0.79 to 1.09) | 1209 (2) | High quality | |
| Presence of donor specific antibodies | 18.5 per 1000 | 2.6 per 1000 (0.2 to 24.2) | 0.14 (0.01 to 1.31) | 1209 (2) | Moderate quality | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; MD: Mean Difference. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
PTLD ‐ post‐transplant lymphoproliferative disorder
Background
Description of the condition
Kidney transplantation can offer most people with end‐stage kidney disease (ESKD) improved quality and length of life compared with remaining on chronic dialysis (Wong 2012). Short‐term survival of a kidney transplant has improved significantly over the last 20 years since the introduction of cyclosporin, but medium‐ to long‐term kidney and recipient outcomes have not. One‐year kidney transplant survival in the United States is 95% for recipients of living and 89% for recipients of deceased donor kidneys whereas five‐year kidney transplant survival falls to 80% for recipients of living and 67% for recipients of deceased donor kidneys (SRTR 2010). This trend is also evident in Australia and New Zealand where one‐year first kidney transplant survival for living and deceased donor kidneys is 97% and 92%, falling to 90% and 82% by five years (ANZDATA 2012).
Most people who receive a kidney transplant die with a transplant that is still working. Cardiovascular disease and cancer are the biggest causes of death among people with a kidney transplant. Calcineurin inhibitors (CNI) cause high blood pressure, diabetes and high blood lipid levels, as well as impaired immune system detection of cancer cells and antiviral immune activity (Buell 2005; Meier‐Kriesche 2004; Nankivell 2003; Vanrenterghem 2008; Vincenti 2007). Of those kidney transplant recipients who do not die with their transplant still working, chronic kidney transplant scarring is the biggest cause of losing kidney function (requiring recommencement of dialysis). Chronic kidney transplant scarring (also known as interstitial fibrosis/tubular atrophy or IF/TA) can be caused by toxicity from CNI, but also by ischaemia‐reperfusion injury at around the time of the transplant and immune injury from acute or chronic rejection after the transplant (Nankivell 2003). Avoiding cardiovascular disease, cancer and chronic transplant kidney scarring may improve medium‐ and long‐term recipient and kidney transplant outcomes.
Description of the intervention
Belatacept – a biologic immunosuppressive agent – is an inhibitor of T‐cell co‐stimulation, was approved by the United States Food and Drug Administration (FDA) in June 2011 for use in adult recipients of kidney transplants. It was approved on the basis of results from three randomised controlled trials (RCTs) which enrolled 1425 recipients comparing belatacept to cyclosporin in a regimen with concomitant use of MMF and steroids. In all three studies, belatacept was demonstrated to be non‐inferior to cyclosporin in preventing acute rejection in kidney transplants (BENEFIT Study 2008; BENEFIT‐EXT 2009; Vincenti 2007).
How the intervention might work
A direct antagonist of the ligands CD80 and CD86 present on antigen‐presenting cells, belatacept prevents activation of the T‐cell CD28 receptor. This specificity is designed to eliminate the non‐immunological activity associated with conventional immunosuppressive agents while providing potent inhibition of T‐cell activation and proliferation required for preventing acute rejection (Sayegh 1998).
Why it is important to do this review
CNI avoidance, minimisation and substitution strategies have all been tried in clinical studies in the past (Ekberg 2009; Flechner 2011; Watson 2005). None of these regimens found the optimal balance between sufficient immunosuppression to avoid kidney transplant rejection and the avoidance of unwanted side effects. FDA approval for belatacept use is limited to a low dose regimen in Epstein Barr seropositive adult kidney transplant recipients because early clinical studies reported an increased incidence of PTLD when belatacept was used in Epstein Barr seronegative recipients or in a high dosage regimen (Vincenti 2005). Before belatacept can be used widely, clinicians require a more comprehensive understanding of its benefits and harms to ensure that it is used appropriately and safely for specific kidney transplant recipients (Webster 2009).
This review aimed to synthesise data from RCTs that compared belatacept with other primary maintenance immunosuppression regimens.
Objectives
Compare the relative efficacy of belatacept versus any other primary immunosuppression regimen for preventing acute rejection, maintaining kidney transplant function, and preventing death.
Compare the incidence of several adverse events: PTLD; other malignancies; chronic transplant kidney scarring; infections; change in blood pressure, lipid and blood sugar control.
Assess any variation in effects by study, intervention and recipient characteristics, including: differences in pre‐transplant Epstein Barr virus serostatus; belatacept dosage; and donor‐category (living, standard criteria deceased, or extended criteria deceased).
Methods
Criteria for considering studies for this review
Types of studies
We included all RCT and quasi‐RCT, whether published or available only in abstract form.
Types of participants
We included all adults and children receiving a kidney transplant from a living or deceased donor (standard or extended criteria), in whom belatacept was tested versus any other immunosuppressive agent in a primary induction and maintenance regimen. Kidney transplant recipients in whom belatacept was tested against any other immunosuppressive agent in a secondary immunosuppression regimen were excluded, i.e. when treatment was changed due to acute rejection or chronic kidney scarring or calcineurin‐inhibitor (CNI) toxicity. We also excluded recipients who received another solid organ in addition to a kidney transplant (e.g. kidney and pancreas).
Types of interventions
Belatacept given in combination with any other immunosuppressive co‐intervention in which control recipients received no belatacept, placebo, or another agent that the belatacept arm did not receive
Belatacept given in combination with any other immunosuppressive co‐intervention in which control recipients received a dosage comparison (e.g. high versus low dosage belatacept).
Types of outcome measures
The following binary outcome measures were considered.
-
Recipient and kidney survival
Death (any cause)
Loss of kidney transplant (loss of a kidney transplant requiring return to dialysis)
Surviving with a working transplant
Incidence and grade of acute rejection
Incidence and grade of chronic kidney transplant scarring
Incidence of delayed kidney transplant (graft) function (DGF)
Incidence of malignancies: all‐cause, skin cancers (basal cell carcinoma (BCC), squamous cell carcinoma (SCC)), PTLD
Incidence of infections: cytomegalovirus (CMV viraemia and CMV disease), polyoma virus (BK viraemia and BK virus‐associated nephropathy), pneumonia, tuberculosis (TB), urinary tract infection (UTI)
Incidence of diabetes
Use of antihypertensive agents.
The following continuous outcomes were analysed.
Kidney transplant function: measured glomerular filtration rate (GFR), estimated GFR (eGFR)
Cardiovascular and biochemical measures: mean systolic and diastolic blood pressure (SBP, DBP), total cholesterol, high density lipoprotein (HDL), non‐HDL, low density lipoprotein (LDL), triglycerides (TGs), serum blood glucose
Search methods for identification of studies
Electronic searches
We searched the Cochrane Renal Group's Specialised Register to 1 September 2014 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review.
The Cochrane Renal Group’s Specialised Register contains studies identified from:
Quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of renal‐related journals and the proceedings of major renal conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected renal journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of the Cochrane Renal Group. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about the Cochrane Renal Group.
See Appendix 1 for search terms used in strategies for this review.
Data collection and analysis
Selection of studies
The search strategies described identified eligible studies. Titles and abstracts resulting from the searches were then screened by two authors who independently assessed retrieved abstracts and if necessary the full text of these studies to determine which studies satisfied the inclusion criteria. Disagreement about inclusion was resolved by discussion with a third author. Where more than one report of a study existed, we grouped reports together and used the first complete (index) study publication as the primary data source. We examined any prior or subsequent report for supplementary outcomes or data to ensure the inclusion of all relevant information.
Data extraction and management
Data extraction was carried out independently by two authors using standardised data extraction forms. All data were entered into RevMan 5.2 and additional statistical analyses were done using STATA 11.2 software (Statacorp, TX, USA).
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel
Outcome assessors
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For binary outcomes, results were expressed as risk ratios (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment we calculated the mean difference (MD) with 95% CI.
Dealing with missing data
We first tried to clarify unclear or missing data by directly contacting the author of the study report. For the three largest studies (BENEFIT Study 2008; BENEFIT‐EXT 2009; Vincenti 2005) we held a teleconference with representatives from Bristol Myers Squibb (the manufacturers of belatacept) in an attempt to access unpublished data for particular outcomes in certain patient subgroups. Specifically, we requested data on PTLD stratified by recipient pre‐transplant Epstein Barr virus serostatus and whether recipients were randomised to the high or low dosage belatacept treatment arm.
Assessment of heterogeneity
Heterogeneity amongst studies was analysed using a Cochran Q test (n‐1 degrees of freedom), with an alpha of 0.05 used for statistical significance and with the I² test calculated to measure the proportion of total variation in the estimates of treatment effect that was due to heterogeneity beyond chance (Higgins 2003). I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
We planned to use funnel plots to assess the potential existence of publication and small study biases but this was not informative due to the small number of included studies (Higgins 2011).
Data synthesis
We pooled data from individual studies using a random‐effects model, because the random effects model provides a more conservative estimate of effect in the presence of known or unknown potential heterogeneity (Higgins 2003).
Subgroup analysis and investigation of heterogeneity
Subgroups analyses were pre‐specified and included belatacept dosage (high or low), kidney donor source (living, standard‐ or extended‐criteria deceased), calcineurin inhibitor (tacrolimus or cyclosporine) and pre‐transplant Epstein Barr serostatus (negative or positive). For binary outcomes, we presented results as the ratio of risk ratios (RRR and 95%CI), showing the proportional change in risk for the study or participant character listed versus the reference range comparison. For continuous outcomes, results were presented as the difference in mean differences (DMD and 95%CI). Tests of heterogeneity among pooled subgroup estimates were calculated using univariate random‐effects meta‐regression.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
After searching the Cochrane Renal Group's Specialised Register (to 1 September 2014) we identified 642 reports. After duplicate removal and screening of titles and abstracts 250 full‐text reports were assessed. A further 56 reports were excluded (duplicates (43), study protocols (6), not primary immunosuppression (7)). After we grouped the remaining reports, 13 potential studies (194 reports) were identified. We identified five included studies (174 reports), three excluded studies (15 reports), and five ongoing studied (5 reports) (Figure 1).
1.
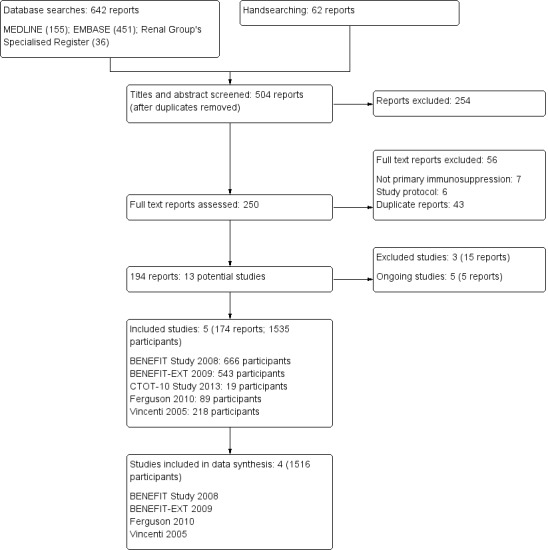
Flow chart showing study selection procedure
Included studies
We included five studies (174 reports published in 10 journals) that involved 1535 randomised kidney transplant recipients. One study (CTOT‐10 Study 2013) met our inclusion criteria but did not contribute data to the meta‐analysis because data for key clinical outcomes were only in abstract form and we were unable to calculate reliable risk ratios at common time‐points. This three arm phase II study compared belatacept maintenance therapy (used at an unknown dosage) with either tacrolimus or no CNI in 19 participants induced with either alemtuzumab or basiliximab. This study was stopped prematurely before one year of follow‐up because of an excess incidence of kidney transplant thrombosis.
Study characteristics
Of the studies included in our meta‐analysis, study participants varied from 89 (Ferguson 2010) to 666 (BENEFIT Study 2008). Four studies (BENEFIT Study 2008; BENEFIT‐EXT 2009; Ferguson 2010; Vincenti 2005) were multinational, multicentre, parallel design RCTs with three treatment arms, conducted in Europe and North America from 2001. Two studies were phase II (Ferguson 2010; Vincenti 2005) and two were phase III (BENEFIT Study 2008; BENEFIT‐EXT 2009) studies. Duration of completed follow‐up varied from one (Ferguson 2010) to five years. Studies that reported five‐year results (BENEFIT Study 2008; BENEFIT‐EXT 2009; Vincenti 2005) were long‐term extensions which only included participants who were still alive with a functioning kidney at three years, and so we only present analyses based on outcomes at three years to avoid the selection bias of these longer‐term analyses. Studies were powered to variably detect non‐inferiority or superiority of belatacept versus CNI for a variety of primary outcomes. Ferguson 2010 and Vincenti 2005 were non‐inferiority studies with primary outcomes of acute rejection at six months. BENEFIT Study 2008 had three co‐primary outcomes, powered to detect superiority in kidney function and non‐inferiority in composite patient and graft survival and incidence of acute rejection. BENEFIT‐EXT 2009 was powered to detect non‐inferiority in composite patient and graft survival, and superiority in kidney function.
Main intervention
Four studies (536 participants; BENEFIT Study 2008; BENEFIT‐EXT 2009; Vincenti 2005; Ferguson 2010) used high dose belatacept regimens and three studies (472 participants; BENEFIT Study 2008; BENEFIT‐EXT 2009; Vincenti 2005) used low dose belatacept. High dose belatacept recipients received 10 mg/kg on days 1 and 5, then every two weeks until week 12, then every four weeks until week 24; and then 5 mg/kg every four weeks thereafter. Low dose belatacept participants received 10 mg/kg on days 1 and 5, then every two weeks until week 4, then every four weeks until week 12 and then 5 mg/kg every four weeks thereafter.
Belatacept was compared with cyclosporin in three studies (478 participants; BENEFIT Study 2008; BENEFIT‐EXT 2009; Vincenti 2005) and tacrolimus in one study (30 participants; Ferguson 2010). CNI formulation was not clearly stated in any study. Cyclosporin target levels varied among studies: 100 to 250 ng/mL for years 1 to 3 (BENEFIT Study 2008); 150 to 300 ng/mL in months 0 to 1, 100 to 250 ng/mL in months 2 to 12 (BENEFIT‐EXT 2009); 150 to 400 ng/mL in months 0 to 1, 150 to 300 ng/mL in months 2 to 12 (Vincenti 2005), and for tacrolimus; 8 to 12 ng/mL until day 30, 5 to 10 ng/mL thereafter (Ferguson 2010).
Co‐interventions
Co‐interventions also varied. Three studies (1415 participants; BENEFIT Study 2008; BENEFIT‐EXT 2009; Vincenti 2005) used basiliximab for induction, and one study (89 participants; Ferguson 2010) used antithymocyte globulin. All participants received perioperative intravenous (IV) methylprednisolone, although durations varied: days 0 and 1 (BENEFIT Study 2008; BENEFIT‐EXT 2009; Vincenti 2005); and days 0, 1 and 2 (Ferguson 2010). All but one study (Ferguson 2010) prescribed maintenance prednisone, tapered variably to no less than 2.5 mg/d by day 15 (BENEFIT Study 2008), 5 mg to 7 mg/d by months 9 to 12 (BENEFIT‐EXT 2009) or no less than 5 mg/d by 6 months (Vincenti 2005). All studies used mycophenolate mofetil (MMF) (1490 participants) at a dose of 2 g/d with reductions allowed for symptomatic or haematological side effects, with the exception of a subgroup of 26 recipients in the Ferguson 2010 study who instead received sirolimus. Studies treated episodes of acute rejection similarly. Acute rejection less severe than Banff 2A criteria received pulsed intravenous methylprednisolone. Banff 2B or worse acute rejection was treated with either corticosteroids or T‐cell depleting therapy at the investigator's discretion in three studies (BENEFIT Study 2008; BENEFIT‐EXT 2009; Ferguson 2010) whereas one study routinely used T‐cell depleting therapy (Vincenti 2005). Three studies universally prescribed anti‐viral prophylaxis for at least 3 months post transplant or upon initiating T‐cell depleting agents and used Pneumocystis jejuni prophylaxis for 6 months (BENEFIT Study 2008; BENEFIT‐EXT 2009; Ferguson 2010). One study did not provide details of their bacterial and viral prophylaxis protocols (Vincenti 2005).
Baseline donor and recipient characteristics
One study (543 participants, BENEFIT‐EXT 2009) exclusively recruited extended criteria deceased donor kidney transplant recipients. The other three studies recruited a total of 973 living donor or standard‐criteria deceased donor kidney recipients (of which at least 488 were living donor and 485 standard‐criteria deceased donor kidney transplant recipients). Recipients of extended criteria donor kidneys were older (mean age 56.2 years) than recipients of standard criteria or living donor kidney transplants (mean age 44.6 years). No study included recipients younger than 18 years. One study (Ferguson 2010) excluded recipients who were seronegative for Epstein Barr virus at baseline, and of the other four studies, one (Vincenti 2005) did not provide details of participants' serostatus. Three studies (BENEFIT Study 2008; BENEFIT‐EXT 2009; Ferguson 2010) included a total of 1176 pre‐transplant Epstein Barr virus seropositive and 136 pre‐transplant seronegative participants. Sensitization (determined by panel reactive antibody titre) did not vary between studies, with most participants being non‐sensitized. Eighteen percent (BENEFIT Study 2008), 20% (BENEFIT‐EXT 2009) and 34% (Ferguson 2010) of participants had diabetes before receiving a kidney transplant. It was unclear how many participants had pre‐existing diabetes in Vincenti 2005. The most common cause of ESKD was glomerulonephritis; 22% (BENEFIT Study 2008), 26% (BENEFIT‐EXT 2009) and 35% (Vincenti 2005) of kidney transplant recipients. Similar proportions of recipients had ESKD caused by diabetes, hypertension, and polycystic kidney disease amongst studies.
Outcome ascertainment and definitions
Data for acute rejection and chronic kidney scarring were obtained from kidney transplant biopsies, scored according to the Banff '97 diagnostic categories for kidney allograft biopsies. Four studies (BENEFIT‐EXT 2009; BENEFIT Study 2008; Ferguson 2010; Vincenti 2005) performed indication biopsies based on protocol‐defined reasons for clinical suspicion of acute rejection. Three studies performed pre‐specified protocol transplant biopsies at 12 months (BENEFIT‐EXT 2009; BENEFIT Study 2008; Vincenti 2005). Severe acute rejection included kidney biopsies scored as Banff IIA or worse. Chronic kidney scarring included mild (affecting < 25% of the kidney cortex), moderate (25 to 50% of the cortex), and severe changes (affecting > 50% of the cortex).
Excluded studies
We excluded three studies (Kamar 2013; Kirk 2012; Rostaing 2011) in which belatacept was not used in a primary immunosuppression regimen, but rather as a switch from conventional immunosuppression at some time distant from kidney transplantation.
Ongoing studies
Five ongoing studies were identified that met inclusion criteria but have not yet published any results (EudraCT2006‐00311417; EudraCT2011‐00616240; EudraCT2013‐00117820; NCT01729494; NTR4242).
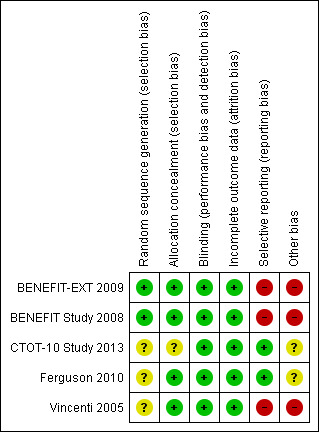
Risk of bias in included studies
Study methodology reporting is summarised in Figure 2.
2.
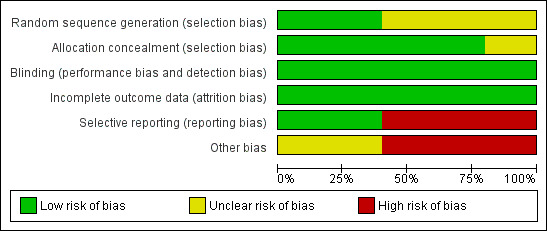
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Of the studies included in meta‐analysis, two reported adequate sequence generation (BENEFIT Study 2008; BENEFIT‐EXT 2009) and two described the process in insufficient detail to allow a confident judgement of the risk of bias (Ferguson 2010; Vincenti 2005). Four studies reported adequate allocation concealment (BENEFIT Study 2008; BENEFIT‐EXT 2009; Ferguson 2010; Vincenti 2005). We deemed no study to be at high risk of selection bias.
Blinding
We judged that all four studies (BENEFIT Study 2008; BENEFIT‐EXT 2009; Ferguson 2010; Vincenti 2005) were at low risk of performance and detection bias despite the blinding of neither participants nor outcome assessors. Belatacept was administered intravenously, and CNI orally with no placebo infusion described in the study protocols. However, the outcomes assessed were objective measures recorded by standardized and validated methods which we thought were unlikely to be influenced by a lack of blinding (e.g. estimated GFR).
Incomplete outcome data
All studies (BENEFIT Study 2008; BENEFIT‐EXT 2009; Ferguson 2010; Vincenti 2005) adequately addressed incomplete outcome data for up to three years post kidney transplant. Long‐term extension data was presented between three and five years following kidney transplant in three studies (BENEFIT‐EXT 2009; BENEFIT Study 2008; Vincenti 2005). We considered that this data was biased by survival of the kidney transplant recipients themselves (and their kidney transplants) to at least three years following transplant and so presented our analyses based on three year outcome data available for the full intention‐to‐treat populations.
Selective reporting
Two studies were at high risk of selective reporting (BENEFIT Study 2008; BENEFIT‐EXT 2009). This arose because key outcomes were not uniformly reported at standardized time‐points amongst studies including for key outcomes such as graft loss, PTLD and diabetes. In addition, for clinical outcomes where we expected that the risk of particular adverse events might vary by specific subgroups of patients (for example the risk of PTLD by a recipient's pre‐transplant Epstein Barr virus status), data was often not published at all or not published in enough detail to contribute to data synthesis. Finally, even after directly requesting missing data from data custodians (BENEFIT‐EXT 2009; BENEFIT Study 2008), we received no data that was not already available in the public domain. The other two studies (Ferguson 2010; Vincenti 2005) provided insufficient information to permit judgement and so remain unclear in their potential for reporting bias.
Other potential sources of bias
Bristol Myers Squibb ‐ the manufacturer of belatacept ‐ funded three of the studies (BENEFIT Study 2008; BENEFIT‐EXT 2009; Vincenti 2005).
Effects of interventions
See: Table 1
Belatacept versus calcineurin inhibitors
Up to three years after a kidney transplant, belatacept and CNI‐treated kidney recipients were at similar risk of dying (Analysis 1.1.1 (4 studies, 1516 recipients): RR 0.75, 95% CI 0.39 to 1.44; I² = 38%), losing their kidney transplant (Analysis 1.1.2 (4 studies, 1516 recipients): RR 0.91, 95% CI 0.61 to 1.38; I² = 0%) and surviving with a functioning kidney transplant (Analysis 1.2 (4 studies 1516 recipients): RR 1.01, 95% CI 0.96 to 1.06; I² = 42%).
1.1. Analysis.
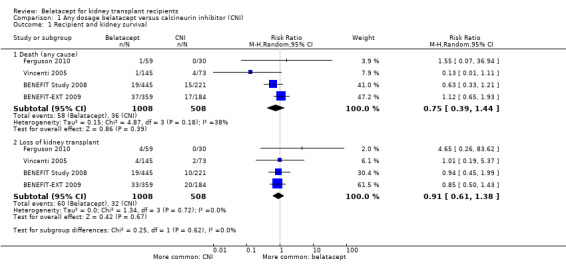
Comparison 1 Any dosage belatacept versus calcineurin inhibitor (CNI), Outcome 1 Recipient and kidney survival.
1.2. Analysis.
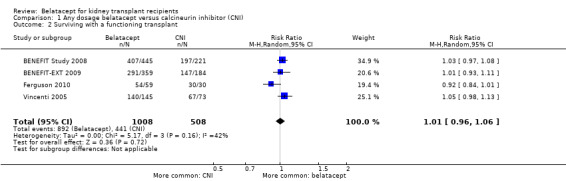
Comparison 1 Any dosage belatacept versus calcineurin inhibitor (CNI), Outcome 2 Surviving with a functioning transplant.
Acute rejection occurred with similar incidence in recipients who received belatacept and CNI (Analysis 1.3.1 (4 studies, 1516 participants): RR 1.56, 95% CI 0.85 to 2.86; I² = 63%) with no difference in the chances of the rejection being severe (Analysis 1.3.2 (2 studies, 194 recipients): RR 1.02, 95% CI 0.76 to 1.37; I² = 34%). Overall, chronic kidney scarring was significantly reduced by 28% (Analysis 1.3.3 (3 studies, 1360 recipients): RR 0.72, 95% CI 0.55 to 0.94; I² = 63%) in belatacept‐treated recipients but not severe chronic rejection (Analysis 1.3.4 (3 studies, 1360 recipients): RR 0.75, 95% CI 0.42 to 1.32; I² = 0%).
1.3. Analysis.
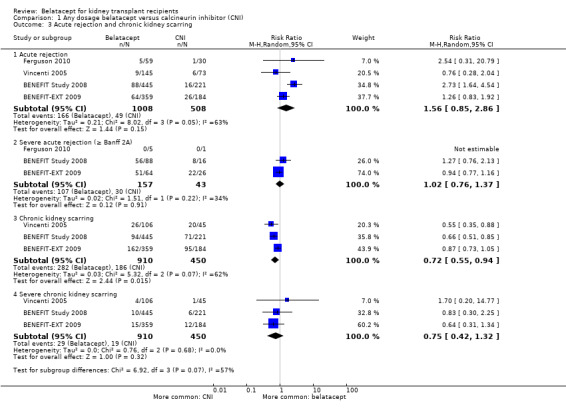
Comparison 1 Any dosage belatacept versus calcineurin inhibitor (CNI), Outcome 3 Acute rejection and chronic kidney scarring.
Belatacept‐treated kidney transplant recipients had better kidney function (Analysis 1.4.1 (measured GFR (3 studies 1083 recipients): MD 10.89 mL/min/1.73 m², 95% CI 4.01 to 17.77; I² = 79%) (Analysis 1.4.2 (eGFR (4 studies, 1143 recipients): MD 9.96 mL/min/1.73 m², 95% CI 3.28 to 16.64; I² = 79%), lower blood pressure (Analysis 1.4.3 (systolic (2 studies, 658 participants): MD ‐7.51 mm Hg, 95% CI ‐10.77 to ‐4.46; I² = 0%) (Analysis 1.4.4 (diastolic (2 studies, 658 recipients): MD ‐3.07 mm Hg, 95% CI ‐4.83 to ‐1.31; I² = 0%) and had a smaller rise in non‐HDL cholesterol (Analysis 1.4.5 (3 studies, 1101 participants): MD ‐12.25 mg/dL, 95% CI ‐17.93 to ‐6.57; I² = 11%) and triglycerides during the study (Analysis 1.4.6 (3 studies, 1101 recipients): MD ‐24.09 mg/dL, 95% CI ‐44.55 to ‐3.64; I² = 69%) than recipients who received a CNI.
1.4. Analysis.
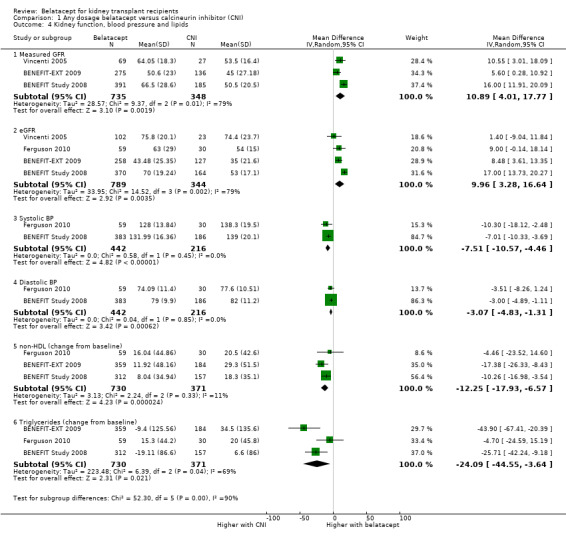
Comparison 1 Any dosage belatacept versus calcineurin inhibitor (CNI), Outcome 4 Kidney function, blood pressure and lipids.
The risk of any malignancy (Analysis 1.5.1 (4 studies, 1516 recipients): RR 1.00, 95% CI 0.58 to 1.72; I² = 0%) and PTLD (Analysis 1.5.2 (4 studies, 1516 recipients): RR 2.79, 95% CI 0.61 to 12.66; I² = 0%) and was similar in both treatment arms.
1.5. Analysis.
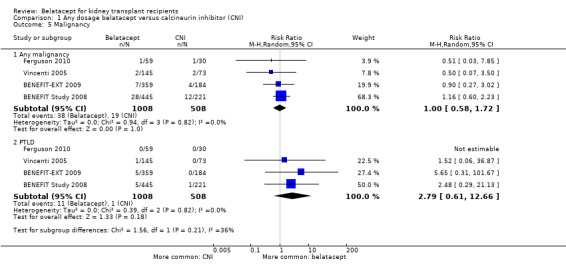
Comparison 1 Any dosage belatacept versus calcineurin inhibitor (CNI), Outcome 5 Malignancy.
Any infection (Analysis 1.6.1 (4 studies, 1516 recipients): RR 0.98, 95% CI 0.87 to 1.09; I² = 49%) and serious infections (Analysis 1.6.2 (2 studies, 1209 recipients): RR 0.96, 95% CI 0.83 to 1.12; I² = 0%) occurred with similar incidence. Specifically, tuberculosis (Analysis 1.6.3 (2 studies, 1209 recipients): RR 3.96, 95% CI 0.72 to 21.67; I² = 0%), CMV disease (Analysis 1.6.4 (2 studies, 1209 recipients): RR 1.51, 95% CI 0.91 to 2.50; I² = 0%), and polyoma‐virus related (BK) nephropathy (Analysis 1.6.5 (1 study, 666 recipients): RR 0.25, 95% CI 0.05 to 1.35) were equally likely in recipients receiving belatacept as those receiving a CNI.
1.6. Analysis.
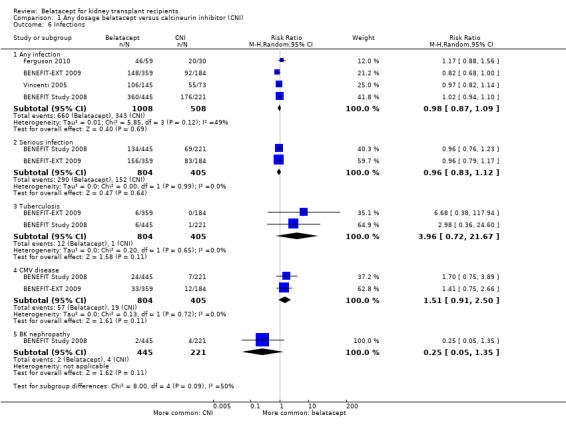
Comparison 1 Any dosage belatacept versus calcineurin inhibitor (CNI), Outcome 6 Infections.
We observed a 39% reduction in the risk of developing new‐onset diabetes (Analysis 1.7.1 (4 studies, 1049 participants): RR 0.61, 95% CI 0.40 to 0.93; I² = 0%) and a 17% reduction in the number of kidney transplant recipients who needed to take one or two blood pressure medicines following their transplant (Analysis 1.7.2 (2 studies, 755 recipients): RR 0.79, 95% CI 0.67 to 0.92; I² = 0%) amongst those who received belatacept versus those who received a CNI. There was no difference between the groups for those patients requiring three or blood pressure medicines (Analysis 1.7.3 (3 studies, 1298 recipients): RR 0.83, 95% CI 0.65 to 1.07; I² = 51%).
1.7. Analysis.
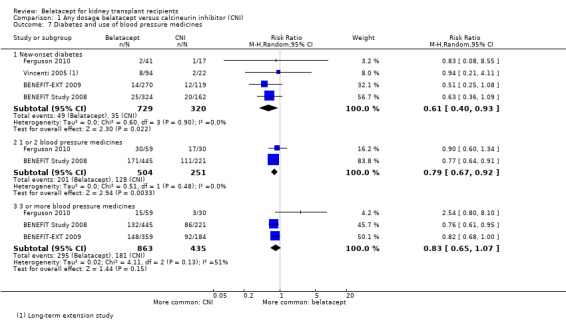
Comparison 1 Any dosage belatacept versus calcineurin inhibitor (CNI), Outcome 7 Diabetes and use of blood pressure medicines.
Presence of new donor‐specific antibodies was no different between recipients of belatacept or a CNI (Table 1 (2 studies, 1209 recipients): RR 0.41, 95% CI 0.01 to 1.31). Delayed graft function occurred at a similar rate in kidney transplant recipients who received belatacept to recipients who received a CNI (Table 1 (2 studies, 1209 recipients): RR 0.93, 95% CI0.79 to 1.09).
High versus low dosage belatacept
Three studies (949 recipients) compared high versus low dosage belatacept (BENEFIT Study 2008; BENEFIT‐EXT 2009; Vincenti 2005).
High and low dosage treated kidney transplant recipients had similar risks of dying (Analysis 2.1.1 (3 studies, 949 recipients): RR 1.24, 95% CI 0.75 to 2.06; I² = 0%), losing their kidney transplant (Analysis 2.1.2 (3 studies, 949 recipients): RR 0.96, 95% CI 0.59 to 1.56; I² = 0%), and surviving with a functioning kidney at up to three years (Analysis 2.2 (3 studies, 949 recipients): RR 0.98, 95% CI 0.95 to 1.02; I² = 0%).
2.1. Analysis.
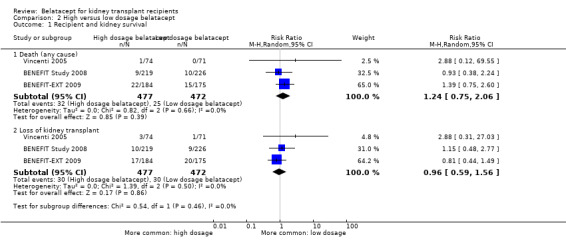
Comparison 2 High versus low dosage belatacept, Outcome 1 Recipient and kidney survival.
2.2. Analysis.
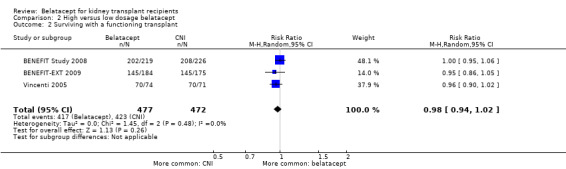
Comparison 2 High versus low dosage belatacept, Outcome 2 Surviving with a functioning transplant.
Acute rejection (Analysis 2.3.1 (3 studies, 949 recipients): RR 1.17, 95% CI 0.88 to 1.55; I² = 0%), severe acute rejection (Analysis 2.3.2 (2 studies, 152 recipients): RR 1.28, 95% CI 0.67 to 2.43; I² = 88%), chronic kidney scarring (Analysis 2.3.3 (3 studies, 910 recipients): RR 0.94, 95% CI 0.74 to 1.20; I² = 27%), and severe chronic kidney scarring (Analysis 2.3.4 (3 studies, 910 recipients): RR 0.77, 95% CI 0.34 to 1.75; I² = 10%) occurred with similar incidence in with either dosage.
2.3. Analysis.
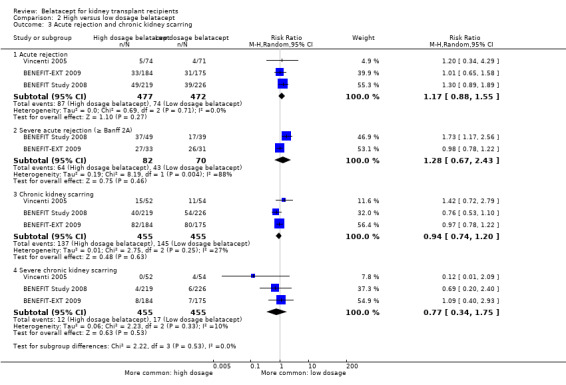
Comparison 2 High versus low dosage belatacept, Outcome 3 Acute rejection and chronic kidney scarring.
There was no difference in kidney transplant function (Analysis 2.4.1 (measured GFR (3 studies, 735 recipients): RR 0.37 mL/min/1.73m², 95% CI ‐3.49 to 4.23; I² = 12%) (Analysis 2.4.2, eGFR (3 studies, 747 recipients): MD 0.13 mL/min/1.73m², 95% CI ‐2.94 to + 3.20; I² = 0%), blood pressure (Analysis 2.4.3 (systolic (2 studies 659 recipients): MD +1.16 mm Hg, CI ‐1.33 to + 3.66; I² = 0%) (Analysis 2.4.4 (diastolic (2 studies 659 recipients): MD 0.00 mm Hg, 95% CI ‐1.52 to +1.52; I² = 0%), change in non‐HDL cholesterol (Analysis 2.4.5 (2 studies, 671 recipients), MD 0.59 mg/dL, 95% CI ‐5.54 to +6.72; I² = 0%), or change in triglycerides during the study (Analysis 2.4.6 (2 studies, 671 recipients): MD 8.82 mg/dL, 95% CI ‐6.63 to 24.28; I² = 0%) in recipients who received high dosage belatacept compared with those who received low dosage belatacept.
2.4. Analysis.
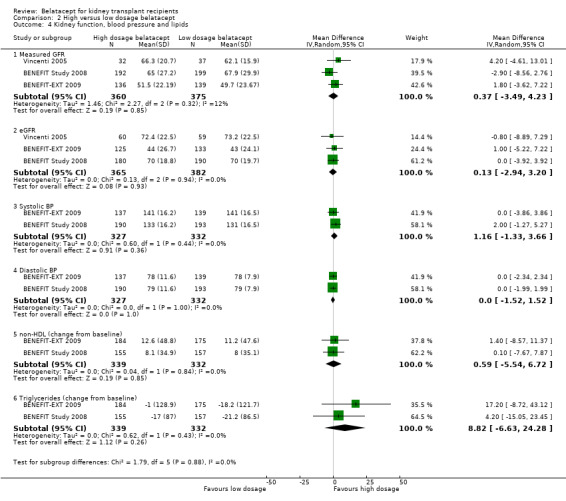
Comparison 2 High versus low dosage belatacept, Outcome 4 Kidney function, blood pressure and lipids.
Any malignancy (Analysis 2.5.1 (3 studies, 949 participants): RR 1.27, 95% CI 0.52 to 3.13; I² = 0%) and PTLD (Analysis 2.5.2 (2 studies, 949 recipients): RR 1.25, 95% CI 0.39 to 3.99; I² = 0%) were equally likely in recipients treated with high dosage belatacept as in recipients treated with low dosage belatacept.
2.5. Analysis.
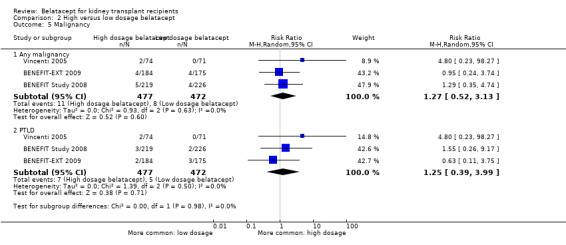
Comparison 2 High versus low dosage belatacept, Outcome 5 Malignancy.
Any infection (Analysis 2.6.1 (3 studies, 949 recipients): RR 0.98, 95% CI 0.92 to 1.04; I² = 0%) and serious infections (Analysis 2.6.2 (2 studies, 804 recipients): RR 0.89, 95% CI 0.73 to 1.09; I² = 17%) occurred with similar incidence. Tuberculosis (Analysis 2.6.3 (2 studies, 804 recipients): RR 0.99, 95% CI 0.23 to 4.17; I² = 31%), CMV disease (Analysis 2.6.4 (3 studies, 949 recipients): RR 1.02, 95% CI 0.74 to 1.40; I² = 0%), and BK nephropathy (Analysis 1.6.5 (1 study, 445 recipients): RR 1.03, 95% CI 0.06 to 16.40) were equally likely in recipients receiving high or low dosage belatacept.
2.6. Analysis.
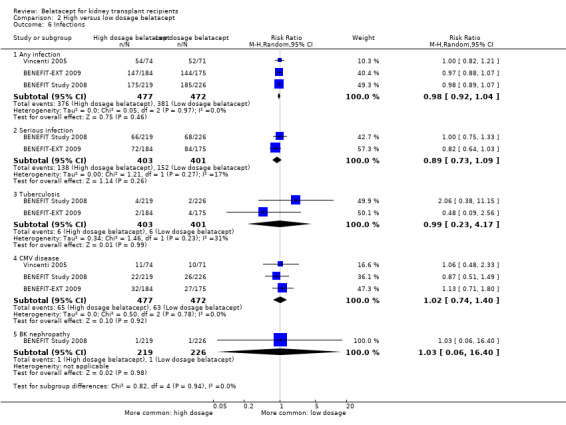
Comparison 2 High versus low dosage belatacept, Outcome 6 Infections.
We observed no difference in the effect of dosage on the incidence of new‐onset diabetes (Analysis 2.7.1 (3 studies, 739 recipients): RR 0.91, 95% CI 0.33 to 2.51; I² = 48%). Use of one or two (Analysis 2.7.2 (1 study, 445 recipients): RR 1.15, 95% CI 0.91 to 1.45) or three or more blood pressure medicines (Analysis 2.7.3 (2 studies, 804 recipients): RR 1.10, 95% CI 0.91 to 1.32; I² = 0%) were similar in the different belatacept dosage groups.
2.7. Analysis.
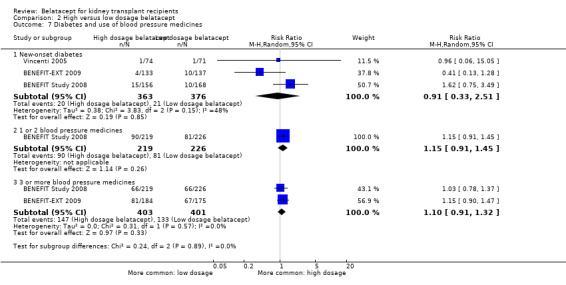
Comparison 2 High versus low dosage belatacept, Outcome 7 Diabetes and use of blood pressure medicines.
Variation in treatment effect by key study intervention and participant characteristics
Subgroup analyses were performed for main clinical outcomes to examine whether key study and participant characteristics modified overall results. Stratified into high and low belatacept dosage groups, there was no difference in treatment effects between groups for comparisons of belatacept versus CNI for recipient and graft survival (RRR 0.99, 95% CI 0.92 to 1.07, test for difference = 0.88), acute rejection (RRR 1.18, 95% 95% CI 0.49 to 2.81, test for difference = 0.71), PTLD (RRR 1.06, 95% CI 0.11 to 9.80, test for difference = 0.96 or eGFR (DMD ‐0.25 mL/min/1.73 m², 95% CI ‐9.18 to +8.69, test for difference = 0.96). The relative effects of belatacept versus CNI did not vary between extended‐criteria versus living or standard‐criteria donor kidney recipients for recipient and graft survival (RRR 1.03, 95% CI 0.88 to 1.21, test for difference = 0.69) or eGFR (DMD ‐1.21 mL/min/1.73 m², 95% CI ‐20.1 to +17.7, test for difference = 0.90). Comparing the relative effects of tacrolimus and cyclosporin against belatacept, there was no difference in recipient and graft survival (RRR 0.89, 95% CI 0.74 to 1.06, test for difference = 0.11), eGFR (DMD ‐0.95, 95% CI ‐19.4 to +17.5, test for difference = 0.92) or acute rejection (RRR 1.71 95% CI 0.01 to 393.06, test for difference = 0.71), though estimates were imprecise for rare events such as acute rejection. Epstein Barr virus serostatus did not appear to affect the relative risk of PTLD (RRR 1.49, 95% CI 0.15 to 14.76, test for difference = 0.73), though again the imprecision of this estimate or effect made meaningful interpretation difficult.
Sensitivity analyses
The small number of included studies precluded meaningful sensitivity analyses.
Reporting bias
We intended to examine funnel plots for asymmetry to detect publication bias, small‐study effects and differences in estimates of effect arising from study methodological quality. However, this was not informative due to the small number of included studies.
Discussion
The availability of new biologic agents like belatacept (which may avoid the unwanted side‐effects of currently used immunosuppression regimens) means that established treatment strategies require re‐evaluation. Transplant clinicians need reliable estimates of the relative benefits and harms of treatment with belatacept, particularly because high rates of PTLD have been reported when belatacept is used at high dosage or in recipients who are Epstein Barr virus seronegative before their kidney transplant.
Summary of main results
Treatment with belatacept was associated with similar rates of death, kidney transplant survival and acute rejection to treatment with a CNI in the first three years following a kidney transplant. Belatacept‐treated kidney transplant recipients had less new‐onset diabetes after transplant, lower blood pressure, better kidney transplant function and less chronic kidney scarring seen on kidney biopsies than CNI‐treated kidney transplant recipients. PTLD occurred at similar rates in recipients treated with high and low dosage belatacept and did not appear to significantly differ in incidence between Epstein Barr virus seropositive and seronegative kidney transplant recipients. Estimates of relative effects in subgroups of patients receiving high versus low dosage belatacept and in subgroups who were Epstein Barr virus seropositive versus seronegative were imprecise because of selective outcome reporting of PTLD. All other malignancies and infections reported occurred at similar rates. The main deficiency in the published data was that outcomes were reported selectively and inconsistently which limited between‐study comparisons and the opportunities for data synthesis.
Overall completeness and applicability of evidence
This is the first synthesis of data from all studies evaluating the benefits and harms of primary immunosuppression with belatacept. We undertook an extensive literature search and sought data from every report of each study, identifying 12% of included reports from hand‐searching of conference abstracts, and contacting triallists and study data custodians directly where data was missing or unclear. We also explicitly examined important subgroup effects to explore potential differences that might arise from immunosuppressive co‐interventions, donor and recipient characteristics. There were data gaps, particularly relating to PTLD, belatacept dosage and Epstein Barr virus serostatus. Despite direct communication with Bristol‐Myers‐Squibb over a number of months and requests for unpublished data (specifically for particular patient subgroups where data was sparse) we did not obtain any new informative data which was not already in the public domain and so the data we present is subject to outcome reporting bias. Further data is being collected in three post‐marketing clinical studies required by the FDA as a condition of belatacept licence approval which may help address some current data deficiencies. The first study is the creation of a belatacept registry which will collect data on the incidence of PTLD. The second study will analyse the pattern of use of belatacept in routine clinical practice using the United Network for Organ Sharing database, whilst the third will use these data to compare rates of PTLD between belatacept and CNI‐based immunosuppression regimens.
Although kidney transplant recipients included in studies were generally representative of the ESKD who receive kidney transplants, there were important exceptions. Recipients aged less than 18 years old were excluded from all studies, immediately limiting the generalisability of our findings to the adult transplant population. Approximately 40% of patients on the United States' active kidney transplant waiting list are sensitised with a PRA of > 20% (OPTN 2013). In our review, recipients were generally non‐sensitized. Sensitized patients spend more time on the kidney transplant waiting list, have an increased risk of acute rejection and are at higher risk of producing donor‐specific antibodies following a kidney transplant. These all increase the risk of death and kidney transplant loss and so the effectiveness of belatacept in recipients with levels of PRA > 20% may not be represented by our data (Gloor 2009; Hidalgo 2009). Thirty percent of standard criteria kidney transplants performed in Australia in 2010 were from living donors (ANZDATA 2012). In our study, 50% of recipients received a standard criteria kidney, but we could not analyse outcomes separately for living and deceased donor kidney recipients by drug treatment allocation groups as only the overall number receiving a living donor or a deceased donor was provided in reports. Donor factors which significantly affect deceased donor transplant outcomes have lesser effects on living donor transplant outcomes (Rao 2009).
The main limitation in applying our findings to contemporary clinical practice is that belatacept still remains relatively untested against tacrolimus. Eighty six percent of new kidney transplant recipients now receive tacrolimus for primary immunosuppression (ANZDATA 2012). Only two percent (30 participants) of kidney transplant recipients included in our meta‐analysis received tacrolimus (Ferguson 2010). Tacrolimus and cyclosporine vary in their effects on blood pressure, lipids, blood sugar regulation and risk of infection with polyoma virus, and so the relative effects of belatacept and CNI we observed may not be replicated when belatacept is used in mainstream clinical practice (Webster 2005).
Quality of the evidence
Overall study quality was good (Figure 2), though selective outcome reporting was suspected in two of the included studies with data for the main safety concern surrounding belatacept – PTLD in different belatacept dosage and Epstein Barr virus serostatus groups – mainly derived from FDA licensing documentation and not the original study reports (Figure 3). Even after directly requesting data for these subgroups from Bristol Myers Squibb, data were not provided. The potential for selective outcome reporting bias remains, and estimates of effect for key clinical outcomes (including PTLD in different patient and dosage groups) remain imprecise. Opportunities for synthesizing data were also limited by incomplete reporting of statistical data for key recipient and patient‐centred outcomes. These included data for blood pressure, physical and mental well‐being scores, symptom distress scores, and health‐related quality of life (Dobbels 2014). The design, conduct and analysis of included studies were frequently difficult to assess because of omission of important methodological detail in the study reports. No single study adequately addressed all domains of the risk of bias assessment (Figure 2) despite using multiple sources of data. It is therefore impossible to exclude the possibility that some internal biases may be present in the results of the meta‐analysis (Begg 1996; Moher 2009).
3.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Authors' conclusions
Implications for practice.
Belatacept is a valid alternative to CNI for the maintenance immunosuppression of kidney transplant recipients, though some uncertainty remains about its effects in specific subgroups of patients. Pragmatically, the balance between benefits and harms of treatment with belatacept might favour treatment of transplant candidates in whom there is concern about the side‐effects of CNI, for example in recipients with pre‐transplant impaired glucose tolerance or poorly controlled hypertension. Until further data becomes available for core outcomes at standardized time‐points, the precision of estimates of effects for some outcomes will remain poor, and so belatacept must be used carefully, and adverse events reported routinely. The medium to long‐term effect of belatacept on the incidence of cancers remains largely unknown and ongoing surveillance using linked kidney and cancer registry data and data from post‐marketing cohort studies must be integrated into our current understanding of benefits and harms when informing clinical decision making.
Implications for research.
There are three main implications for future research. Firstly, triallists should comprehensively report their results according to existing international guidelines, and publishing journals and editors should rigorously enforce these standards (Begg 1996; Masson 2013). The FDA's limited approval to use belatacept only at low dosage in Epstein Barr seropositive kidney transplant recipients means that future studies are unlikely to help clarify the uncertain effects of high dosage belatacept, or the use of belatacept at any dosage in Epstein Barr seronegative recipients. Unpublished study data should be made available for sharing in a data repository, so as to maximise the usefulness and usage of data and promote transparency (Godlee 2012). These initiatives would allow more informative synthesis and between‐study comparisons, and would help address some of the current deficiencies in the literature regarding the effectiveness of belatacept in specific recipient subgroups. Secondly, contemporary studies should reflect contemporary clinical practice, and new studies must test belatacept against tacrolimus. Finally, surrogate markers of definitive endpoints in studies of kidney transplantation remain un‐validated and researchers should conduct longer term studies with sufficient power to demonstrate significant differences in effect for core clinical and patient‐centred outcomes (White 2010).
Acknowledgements
We are grateful to the Cochrane Renal Group for their assistance, in particular, Gail Higgins who carried out the search.
We wish to thank the referees for their comments and feedback during the preparation of this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE (OVID SP) |
|
| EMBASE (OVID SP) |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Any dosage belatacept versus calcineurin inhibitor (CNI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recipient and kidney survival | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Death (any cause) | 4 | 1516 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.39, 1.44] |
| 1.2 Loss of kidney transplant | 4 | 1516 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.61, 1.38] |
| 2 Surviving with a functioning transplant | 4 | 1516 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.96, 1.06] |
| 3 Acute rejection and chronic kidney scarring | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Acute rejection | 4 | 1516 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.85, 2.86] |
| 3.2 Severe acute rejection (≥ Banff 2A) | 3 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.76, 1.37] |
| 3.3 Chronic kidney scarring | 3 | 1360 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.55, 0.94] |
| 3.4 Severe chronic kidney scarring | 3 | 1360 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.42, 1.32] |
| 4 Kidney function, blood pressure and lipids | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Measured GFR | 3 | 1083 | Mean Difference (IV, Random, 95% CI) | 10.89 [4.01, 17.77] |
| 4.2 eGFR | 4 | 1133 | Mean Difference (IV, Random, 95% CI) | 9.96 [3.28, 16.64] |
| 4.3 Systolic BP | 2 | 658 | Mean Difference (IV, Random, 95% CI) | ‐7.51 [‐10.57, ‐4.46] |
| 4.4 Diastolic BP | 2 | 658 | Mean Difference (IV, Random, 95% CI) | ‐3.07 [‐4.83, ‐1.31] |
| 4.5 non‐HDL (change from baseline) | 3 | 1101 | Mean Difference (IV, Random, 95% CI) | ‐12.25 [‐17.93, ‐6.57] |
| 4.6 Triglycerides (change from baseline) | 3 | 1101 | Mean Difference (IV, Random, 95% CI) | ‐24.09 [‐44.55, ‐3.64] |
| 5 Malignancy | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Any malignancy | 4 | 1516 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.58, 1.72] |
| 5.2 PTLD | 4 | 1516 | Risk Ratio (M‐H, Random, 95% CI) | 2.79 [0.61, 12.66] |
| 6 Infections | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Any infection | 4 | 1516 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.87, 1.09] |
| 6.2 Serious infection | 2 | 1209 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.83, 1.12] |
| 6.3 Tuberculosis | 2 | 1209 | Risk Ratio (M‐H, Random, 95% CI) | 3.96 [0.72, 21.67] |
| 6.4 CMV disease | 2 | 1209 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.91, 2.50] |
| 6.5 BK nephropathy | 1 | 666 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.05, 1.35] |
| 7 Diabetes and use of blood pressure medicines | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 New‐onset diabetes | 4 | 1049 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.40, 0.93] |
| 7.2 1 or 2 blood pressure medicines | 2 | 755 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.67, 0.92] |
| 7.3 3 or more blood pressure medicines | 3 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.65, 1.07] |
Comparison 2. High versus low dosage belatacept.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recipient and kidney survival | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Death (any cause) | 3 | 949 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.75, 2.06] |
| 1.2 Loss of kidney transplant | 3 | 949 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.59, 1.56] |
| 2 Surviving with a functioning transplant | 3 | 949 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.94, 1.02] |
| 3 Acute rejection and chronic kidney scarring | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Acute rejection | 3 | 949 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.88, 1.55] |
| 3.2 Severe acute rejection (≥ Banff 2A) | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.67, 2.43] |
| 3.3 Chronic kidney scarring | 3 | 910 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.74, 1.20] |
| 3.4 Severe chronic kidney scarring | 3 | 910 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.34, 1.75] |
| 4 Kidney function, blood pressure and lipids | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Measured GFR | 3 | 735 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐3.49, 4.23] |
| 4.2 eGFR | 3 | 747 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐2.94, 3.20] |
| 4.3 Systolic BP | 2 | 659 | Mean Difference (IV, Random, 95% CI) | 1.16 [‐1.33, 3.66] |
| 4.4 Diastolic BP | 2 | 659 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.52, 1.52] |
| 4.5 non‐HDL (change from baseline) | 2 | 671 | Mean Difference (IV, Random, 95% CI) | 0.59 [‐5.54, 6.72] |
| 4.6 Triglycerides (change from baseline) | 2 | 671 | Mean Difference (IV, Random, 95% CI) | 8.82 [‐6.63, 24.28] |
| 5 Malignancy | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Any malignancy | 3 | 949 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.52, 3.13] |
| 5.2 PTLD | 3 | 949 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.39, 3.99] |
| 6 Infections | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Any infection | 3 | 949 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.04] |
| 6.2 Serious infection | 2 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.73, 1.09] |
| 6.3 Tuberculosis | 2 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.23, 4.17] |
| 6.4 CMV disease | 3 | 949 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.74, 1.40] |
| 6.5 BK nephropathy | 1 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.06, 16.40] |
| 7 Diabetes and use of blood pressure medicines | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 New‐onset diabetes | 3 | 739 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.33, 2.51] |
| 7.2 1 or 2 blood pressure medicines | 1 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.91, 1.45] |
| 7.3 3 or more blood pressure medicines | 2 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.91, 1.32] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
BENEFIT Study 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | High dose belatacept group
Low dose belatacept group
CSA group
Co‐interventions (all groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation by computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded to belatacept dosage allocation but not to whether recipients received belatacept or CNI. No dummy IV infusion for recipients allocated CNI |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT to 3 years following kidney transplant |
| Selective reporting (reporting bias) | High risk | Reports of PTLD by dosage and Epstein Barr virus status from FDA approval reports, not original manuscripts and data not provided despite directly requesting from Bristol Myers Squibb |
| Other bias | High risk | Funded by Bristol Myers Squibb |
BENEFIT‐EXT 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | High dose belatacept group
Low dose belatacept group
CSA group
Co‐interventions (all groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation by computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded to belatacept dosage allocation but not to whether recipients received belatacept or CNI. No dummy IV infusion for recipients allocated CNI |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT to 3 years following kidney transplant |
| Selective reporting (reporting bias) | High risk | Standard deviations missing for continuous outcomes (eGFR). Reports of PTLD by dosage and Epstein Barr virus status from FDA approval reports, not original manuscripts and data not provided despite directly requesting from Bristol Myers Squibb |
| Other bias | High risk | Funded by Bristol Myers Squibb |
CTOT‐10 Study 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Group 1 (control)
Group 2
Group 3
Co‐interventions (all groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in abstracts |
| Allocation concealment (selection bias) | Unclear risk | Not described in abstracts |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes for all enrolled patients described in detail at time of study termination |
| Selective reporting (reporting bias) | Low risk | Adverse events reported fully in keeping with primary outcomes stated in protocol, though only in abstract form |
| Other bias | Unclear risk | Insufficient data published for reliable judgment |
Ferguson 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Belatacept‐MMF group
Belatacept‐SRL group
CNI group
Co‐interventions (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Central, interactive voice response system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded to belatacept dosage allocation but not to whether recipients received belatacept or CNI. No dummy IV infusion for recipients allocated CNI |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT to 3 years |
| Selective reporting (reporting bias) | Low risk | Outcomes reported match protocol |
| Other bias | Unclear risk | Not described |
Vincenti 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | High dose belatacept group
Low dose belatacept group
Cyclosporin group
Co‐interventions (all groups)
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Randomisation performed centrally |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded to dose of belatacept. Unblinded to cyclosporin administration |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT to 3 years. Loss to follow‐up small |
| Selective reporting (reporting bias) | High risk | Epstein Barr virus serostatus not reported (except in FDA report of belatacept approval) |
| Other bias | High risk | Supported by Bristol‐Myers Squibb |
BP ‐ blood pressure; CMV ‐ cytomegalovirus; CNI ‐ calcineurin inhibitor; eGFR ‐ estimated GFR; GFR ‐ glomerular filtration rate; HDL ‐ high‐density lipoprotein; ITT ‐ intention‐to‐treat; LDL ‐ low‐density lipoprotein; MMF ‐ Mycophenolate mofetil; NODAT ‐ new onset diabetes after transplantation; NS ‐ not stated; PRA ‐ panel reactive antibodies; PTLD ‐ post‐transplant lymphoproliferative disorder; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; SRL ‐ sirolimus; TB ‐ tuberculosis; UTI ‐ urinary tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Kamar 2013 | Not primary immunosuppressive regimen; switch from CNI‐based regimen to belatacept |
| Kirk 2012 | Not primary immunosuppressive regimen |
| Rostaing 2011 | Not primary immunosuppressive regimen; switch from CNI‐based regimen to belatacept versus continued CNI at 12 months |
CNI ‐ calcineurin inhibitor
Characteristics of ongoing studies [ordered by study ID]
EudraCT2006‐00311417.
| Trial name or title | A randomized, open‐label, multi‐center, parallel‐group study of belatacept‐based corticosteroid‐free regimens in renal transplant |
| Methods | Open label RCT |
| Participants | Adult recipients of a living or deceased‐donor kidney transplant |
| Interventions | Belatacept versus tacrolimus, both with thymoglobulin and MMF |
| Outcomes | Primary: incidence acute rejection at 6 months Secondary: severity of rejection, death, metabolic/cardiovascular comorbidities |
| Starting date | September 2007 |
| Contact information | Bristol Myers Squibb |
| Notes |
EudraCT2011‐00616240.
| Trial name or title | New‐onset diabetes mellitus after renal transplantation. A multicentre, prospective randomized, open study to evaluate belatacept‐based versus tacrolimus‐based immunosuppression |
| Methods | Open label RCT |
| Participants | Patients aged either > 60, or > 45 at high risk of glucose metabolism disorder after kidney transplant |
| Interventions | Belatacept versus tacrolimus |
| Outcomes | Primary: incidence of glucose metabolism disorder at 6 months. Secondary: acute rejection, graft survival and function |
| Starting date | May 2013 |
| Contact information | Frances Mateos. Hospital Vall d'Hebron. Department of Nephrology |
| Notes |
EudraCT2013‐00117820.
| Trial name or title | Cardiovascular risk prediction and biomarkers in renal transplant recipients treated with belatacept compared to calcineurin inhibitors |
| Methods | Open label RCT |
| Participants | Adult recipients of living or deceased‐donor kidney transplants |
| Interventions | Belatacept versus CNI |
| Outcomes | Primary: cardiovascular risk calculated by risk calculator in transplant recipients Secondary: blood pressure, lipids, glucose, graft function |
| Starting date | October 2013 |
| Contact information | Elin Karlberg, Uppsala University Hospital, Sweden |
| Notes |
NCT01729494.
| Trial name or title | Belatacept early steroid withdrawal trial |
| Methods | Open label RCT |
| Participants | 315, > 18 y, recipient of living or deceased‐donor kidney transplant |
| Interventions | Belatacept versus tacrolimus, both with early steroid withdrawal and either alemtuzumab or ATG induction |
| Outcomes | Primary: composite patient death or graft loss of eGFR < 45 |
| Starting date | September 2012 |
| Contact information | E. Steve Woddle, University of Cincinnati, USA |
| Notes |
NTR4242.
| Trial name or title | Belatacept study (BMS no. 55230) |
| Methods | Open label, unblinded RCT |
| Participants | 40 |
| Interventions | Belatacept versus tacrolimus |
| Outcomes | Primary: CD4+ and CD8+ cell counts Secondary: incidence acute rejection, eGFR (MDRD), infections, malignancy, PTLD |
| Starting date | October 2013 |
| Contact information | DA Hesselink, Erasmus Medical Centre, Rotterdam, The Netherlands |
| Notes |
ATG ‐ antithymocyte globulin; CNI ‐ calcineurin inhibitor; eGFR ‐ estimated glomerular filtration rate; MDRD ‐ Modification of Diet in Renal Disease; MMF ‐ mycophenolate mofetil; PTLD ‐ post‐transplant lymphoproliferative disorder; RCT ‐ randomised controlled trial
Contributions of authors
Draft the protocol: PM
Study selection: PM, LH
Extract data from studies: PM, LH
Enter data into RevMan: PM, LH
Carry out the analysis: PM, AW
Interpret the analysis: PM, AW, JCC,
Draft the final review: PM, LH, ACW, JRC, JCC
Disagreement resolution: AW, JCC
Update the review: PM, LH, AW, JCC
Declarations of interest
Philip Masson: none known
Lorna Henderson: none known
Jeremy R Chapman: none known
Jonathan C Craig: none known
Angela C Webster: none known
New
References
References to studies included in this review
BENEFIT‐EXT 2009 {published data only}
- Bremer S, Vethe NT, Rootwelt H, Jorgensen PF, Stenstrom J, Holdaas H, et al. Mycophenolate pharmacokinetics and pharmacodynamics in belatacept treated renal allograft recipients ‐ a pilot study. Journal of Translational Medicine 2009;7:64. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier B, Grinyo J, Medina‐Pestana J, Vanrenterghem I, Vincente F, Dong Y, et al. 3‐year safety profile of belatacept in kidney transplant recipients from the BENEFIT and BENEFIT‐EXT studies [abstract no: O‐242]. Transplant International 2011;24(Suppl 2):68‐9. [EMBASE: 70527318] [Google Scholar]
- Charpentier B, Medina Pestana JO, Del CR, Rostaing L, Grinyo J, Vanrenterghem Y, et al. Long‐term exposure to belatacept in recipients of extended criteria donor kidneys. American Journal of Transplantation 2013;13(11):2884‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Charpentier B, Vincenti F, Rice K, Campistol J, Duan T, Pupim L, et al. Three‐year outcomes in patients with delayed graft function in phase III studies of belatacept vs cyclosporine in kidney transplantation (BENEFIT and BENEFIT‐EXT) [abstract no: PO27.076]. 24th International Congress of the Transplantation Society (TTS); 2012 Jul 15‐19; Berlin, Germany. 2012. [EMBASE: 71251772]
- Dobbels F, Wong S, Joo S, Kalsekar A. Health‐related quality of life after kidney transplantation: results from belatacept clinical trials [abstract no: 1099]. American Journal of Transplantation 2011;11(Suppl 2):352‐3. [EMBASE: 70406145] [Google Scholar]
- Dobbels F, Wong S, Min Y, Sam J, Kalsekar A. Beneficial effect of belatacept on health‐related quality of life and perceived side effects: results from the BENEFIT and BENEFIT‐EXT trials. Transplantation 2014;98(9):960‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Durrbach A, Citterio F, Mulloy L, David‐Neto E, Russ G, Vitko S, et al. Renal function in patients treated with belatacept‐ or cyclosporine‐based regimens at year 3 in the BENEFIT and BENEFIT‐EXT studies [abstract no: 230]. American Journal of Transplantation 2011;11(Suppl 2):100. [EMBASE: 70405265] [Google Scholar]
- Durrbach A, Citterio F, Mulloy L, David‐Neto E, Russ G, Vitko S, et al. Renal function in patients treated with belatacept‐ or cyclosporine‐based regimens at year 3 in the BENEFIT and BENEFIT‐EXT studies [abstract no: Sa521]. NDT Plus 2011;4(Suppl 2):4.s2.60. [Google Scholar]
- Durrbach A, Citterio F, Mulloy L, David‐Neto E, Russ G, Vitko S, et al. Renal function in patients treated with belatacept‐or cyclosporine‐based regimens at year 3 in the BENEFIT and BENEFIT‐EXT studies [abstract no: P‐080]. Transplant International 2011;24(Suppl 2):249. [EMBASE: 70527977] [Google Scholar]
- Durrbach A, Florman S, Larsen C, Pestana JM, Vanrenterghem Y, Vincente F, et al. Primary outcomes from a randomized, phase III study of belatacept versus cyclosporine in ECD kidney transplants (BENEFIT‐EXT study) [abstract no: 28]. American Journal of Transplantation 2010;10(Suppl 2):7. [EMBASE: 70081054] [Google Scholar]
- Durrbach A, Florman S, Zhang R, Becker T, Grinyo J, Lang P, et al. Four‐year outcomes by donor type from the long‐term extension of the belatacept BENEFIT and BENEFIT‐EXT Studies [abstract no: 1293]. American Journal of Transplantation 2012;12(Suppl 3):407. [EMBASE: 70747260] [Google Scholar]
- Durrbach A, Florman S, Zhang R, Lang P, Lehner F, Massari P, et al. Five‐year outcomes by donor type from the long‐term extension of the belatacept BENEFIT‐EXT Study [abstract no: B933]. American Journal of Transplantation 2013;13(Suppl S5):311. [EMBASE: 71057509] [Google Scholar]
- Durrbach A, Grinyo J, Vanrenterghem Y, Becker T, Florman S, Lang P, et al. Belatacept compared with cyclosporine in renal allograft recipients of extended criteria donor kidneys: 3‐year outcomes from the phase III BENEFIT‐EXT trial [abstract no: F568]. NDT Plus 2011;4(Suppl 2):4.s2.43. [Google Scholar]
- Durrbach A, Larsen C, Medina PJ, Vanrenterghem Y, Vincenti F, Florman S, et al. Belatacept vs cyclosporine in ECD kidney transplants: two year outcomes from the BENEFIT‐EXT study [abstract]. British Transplantation Society (BTS). 14th Annual Congress; 2011 Mar 9‐11; Bournemouth, UK. 2011.
- Durrbach A, Larsen C, Medina‐Pestana J, Jonge H, Vincenti F, Florman S, et al. Belatacept vs cyclosporine in ECD kidney transplants: Two‐year outcomes from the BENEFIT‐EXT study [abstract no: 1394]. Transplantation 2010;90(Suppl):157. [EMBASE: 71531405] [Google Scholar]
- Durrbach A, Larsen C, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, Florman S, et al. Belatacept vs cyclosporine in ECD kidney transplant: two‐year outcomes from the BENEFIT‐EXT study [abstract no: 0089]. Transplant International 2010;23(Suppl 2):17. [Google Scholar]
- Durrbach A, Larsen C, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, Florman S, et al. Belatacept vs cyclosporine in ECD kidney transplants: two‐year outcomes from the BENEFIT‐EXT Study [abstract no: SA‐FC444]. Journal of the American Society of Nephrology 2010;21(Abstracts):101A‐2A. [Google Scholar]
- Durrbach A, Larsen C, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, Florman S, et al. Primary outcomes from a randomised, phase III study of belatacept vs cyclosporine in ECD kidney transplants (BENEFIT‐EXE study) [abstract no: O49]. British Transplantation Society (BTS). 13th Annual Congress; 2010 Mar 17‐19; London, UK. 2010.
- Durrbach A, Larsen C, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, Florman S, et al. Primary outcomes from a randomized, phase 3 study of belatacept vs cyclosporine in ECD kidney transplants (BENEFIT‐EXT study) [abstract no: SA‐FC352]. Journal of the American Society of Nephrology 2009;20:82A‐3A. [Google Scholar]
- Durrbach A, Larsen C, Medina‐Pestana JD, Vanrenterghem Y, Vincenti F, Florman S, et al. Primary outcomes from a randomized, phase III study of belatacept vs cyclosporine in ECD kidney transplants (BENEFIT‐EXT Study) [abstract no: O‐332]. Transplant International 2009;22(Suppl 2):89. [Google Scholar]
- Durrbach A, Larsen C, Medina‐Pestana JD, Vanrenterghem Y, Vincenti F, Florman S, et al. Primary outcomes from a randomized, phase III study of belatacept vs cyclosporine in ECD kidney transplants (BENEFIT‐EXT study) [abstract no: 27]. American Journal of Transplantation 2009;9(Suppl 2):199. [Google Scholar]
- Durrbach A, Larsen CP, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, Florman S, et al. Belatacept vs cyclosporine in ECD kidney transplants: two‐year outcomes from the BENEFIT‐EXT study [abstract no: 143]. American Journal of Transplantation 2010;10(Suppl 4):83. [EMBASE: 70463504] [Google Scholar]
- Durrbach A, Larsen CP, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, Florman S, et al. Belatacept vs cyclsoporine in ECD kidney transplants: two‐year outcomes from the BENEFIT‐EXT study [abstract no: Sa652]. NDT Plus 2010;3(Suppl 3):iii262. [EMBASE: 70484118] [Google Scholar]
- Durrbach A, Medina‐Pestana J, Becker T, Grinyo J, Lang P, Garcia VD, et al. Four‐year outcomes by donor type from the long‐term extension of the belatacept BENEFIT and BENEFIT‐EXT Studies [abstract no: PO27.057]. 24th International Congress of the Transplantation Society (TTS); 2012 Jul 15‐19; Berlin, Germany. 2012.
- Durrbach A, Medina‐Pestana J, Pearson T, Vincenti F, Garcia VD, Campistol J, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT‐EXT study). American Journal of Transplantation 2010;10(3):547‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Durrbach A, Medina‐Pestana J, Rostaing L, Breshahan B, Helderman JH, Rice K, et al. Improving or maintaining renal function with belatacept: 5 year BENEFIT long‐term extension results [abstract no: BO199]. Transplant International 2013;26(Suppl 2):92. [EMBASE: 71359311] [Google Scholar]
- Durrbach A, Medina‐Pestana J, Vanrenterghem Y, Rial M, Charpentier B, Matas A, et al. Improving or maintaining renal function over 5 years with belatacept in recipients of extended‐criteria donor kidneys [abstract no: O067]. Transplant International 2013;26(Suppl 2):44. [EMBASE: 71359105] [Google Scholar]
- Durrbach A, Muehlbacher F, Florman S, Medina PJ, Jones‐Burton C, Charpentier B. Outcomes at 3‐years in EBV+ recipients of UNOS criteria ECD kidneys from a randomized trial (BENEFIT‐EXT) comparing belatacept vs cyclosporine [abstract]. Transplantation 2014;98(Suppl 1):456‐7. [EMBASE: 71545057] [Google Scholar]
- Durrbach A, Muehlbacher F, Florman S, Medina Pestana J, Jones‐Burton C, Charpentier B. Outcomes at 3‐Years in EBV+ recipients of UNOS criteria ECD kidneys from a randomized trial (BENEFIT‐EXT) comparing belatacept vs cyclosporine [abstract no: A202]. American Journal of Transplantation 2014;14(Suppl 3):456. [Google Scholar]
- Florman S, Becker T, Bresnahan B, Chevaile‐Ramos A, Carvalho D, Muehlbacher F, et al. Three year outcomes by donor type in phase III studies of belatacept vs cyclosporine in kidney transplantation (BENEFIT and BENEFIT‐EXT) [abstract no: P145]. Transplant International 2011;24(Suppl 3):51. [EMBASE: 70537445] [Google Scholar]
- Florman S, Becker T, Bresnahan B, Chevaile‐Ramos A, DeCarvalho D, Muehlbacher F, et al. Three‐year outcomes by donor type in phase III studies of belatacept vs cyclosporine in kidney transplantation (BENEFIT & BENEFIT‐EXT) [abstract no: 229]. American Journal of Transplantation 2011;11(Suppl 2):100. [EMBASE: 70405264] [Google Scholar]
- Florman S, Bresnahan B, Chan L, Helderman H, Dong Y, Harler MB, et al. Three year outcomes in Black/African American kidney transplant recipients from the BENEFIT and BENEFIT‐EXT studies [abstract no: 1091]. American Journal of Transplantation 2011;11(Suppl 2):350. [EMBASE: 70406137] [Google Scholar]
- Florman S, Durrbach A, Grinyo J, Medina‐PestanaJ, Carmen Rial M, Vitko S, et al. 4 year results from the long term extension of the belatacept BENEFIT‐EXT study [abstract no: 187]. American Journal of Transplantation 2012;12(Suppl 3):82. [EMBASE: 70746134] [Google Scholar]
- Florman S, Durrbach A, Larsen C, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, et al. Outcomes as a function of donor criteria from a phase III study of belatacept vs cyclosporine in kidney transplantation (BENEFIT‐EXT) [abstract no: 373]. American Journal of Transplantation 2010;10(Suppl 4):150. [Google Scholar]
- Florman S, Durrbach A, Larsen C, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, et al. Outcomes as a function of donor criteria from a phase iii study of belatacept vs cyclosporine in kidney transplantation (BENEFIT‐EXT) [abstract no: 1773]. Transplantation 2010;90(Suppl):342. [EMBASE: 71531743] [Google Scholar]
- Florman S, Durrbach A, Larsen C, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, et al. Outcomes as a function of donor type from a phase III study of belatacept vs cyclosporine in kidney transplantation (BENEFIT‐EXT) [abstract no: F‐PO1997]. Journal of the American Society of Nephrology 2010;21(Abstracts):568A. [Google Scholar]
- Florman S, Pestana J, Rial M, Rostaing L, Grinyo J, Renterghem Y, et al. Long‐term exposure to belatacept in recipients of extended criteria donor kidneys [abstract no: B934]. American Journal of Transplantation 2013;13(Suppl S5):311. [EMBASE: 71057510] [DOI] [PubMed] [Google Scholar]
- Florman S, Rice K, Chan L, Steinberg S, Pearson T, Duan T, et al. Four year outcomes in Black/African American kidney transplant recipients from the long term‐extension of the belatacept BENEFIT and BENEFIT‐EXT studies [abstract no: 1284]. American Journal of Transplantation 2012;12(Suppl 3):404. [EMBASE: 70747251] [Google Scholar]
- Florman S, Rice K, Chan L, Zhang R, Abouljoud M, Steinberg S, et al. Outcomes at five years in Black/African‐American kidney transplant recipients from the long‐term extension of the belatacept BENEFIT and BENEFIT‐EXT studies [abstract no: B935]. American Journal of Transplantation 2013;13(Suppl S5):311. [EMBASE: 71057510] [Google Scholar]
- Grinyo J, Charpentier B, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, Reyes‐Acevedo R, et al. An integrated safety profile analysis of belatacept in kidney transplant recipients. Transplantation 2010;90(12):1521‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grinyo J, Charpentier B, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, Shi R, et al. Safety profile of belatacept in kidney transplant recipients from a pooled analysis of phase II and phase III studies [abstract no: 1417]. Transplantation 2010;90(Suppl):156. [EMBASE: 71531402] [DOI] [PubMed] [Google Scholar]
- Grinyo J, Charpentier B, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, Shi R, et al. Safety profile of belatacept in kidney transplant recipients from a pooled analysis of phase II and phase III studies [abstract no: 144]. American Journal of Transplantation 2010;10(Suppl 4):83. [EMBASE: 70463505] [Google Scholar]
- Grinyo J, Charpentier B, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, Shi R, et al. Safety profile of belatacept in kidney transplant recipients from a pooled analysis of phase II and phase III studies [abstract no: SA‐FC442]. Journal of the American Society of Nephrology 2010;21(Abstracts):101A. [Google Scholar]
- Grinyo J, Charpentier B, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, Shi R, et al. Safety profile of belatacept in kidney transplant recipients from a pooled analysis of phase II and phase III studies [abstract no: Sa676]. NDT Plus 2010;3(Suppl 3):iii270. [EMBASE: 70484142] [Google Scholar]
- Grinyo J, Charpentier B, Zhang R, Abouljoud M, Lehner F, Rial M, et al. Likelihood of improving or sustaining renal function over four years with belatacept or CSA: insights from the BENEFIT‐EXT long‐term extension study [abstract no: SA‐OR115]. Journal of the American Society of Nephrology 2012;23:92A. [Google Scholar]
- Grinyo J, Durrbach A, Rostaing L, Bresnahan B, Helderman J, Rice K, et al. Likelihood of improving or maintaining renal function over five years with belatacept or CsA: insights from the BENEFIT long‐term extension study [abstract no: 492]. American Journal of Transplantation 2013;13(Suppl S5):182. [EMBASE: 71057069] [Google Scholar]
- Grinyo J, Florman S, Medina‐Pestana J, Carmen Rial M, Muehlbacher F, Durrbach A, et al. Long‐term extension of the belatacept BENEFIT‐EXT Study: Results at month 48 [abstract no: PO27.033]. 24th International Congress of the Transplantation Society (TTS); 2012 Jul 15‐19; Berlin, Germany. 2012.
- Grinyo J, Larsen C, Charpentier B, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, et al. One year safety profile of belatacept in kidney transplant patients (BENEFIT & BENEFIT‐EXT) [abstract no:TH‐PO1016]. Journal of the American Society of Nephrology 2009;20:343A. [Google Scholar]
- Grinyo J, Medina‐Pestana J, Becker T, Rial M, Dong Y, Block A, et al. Likelihood of improving or sustaining renal function over three years with belatacept or CsA: Insights from the BENEFIT‐EXT study [abstract no: 188]. American Journal of Transplantation 2012;12(Suppl 3):82. [EMBASE: 70746135] [Google Scholar]
- Grinyo J, Rial M, Alberu J, Steinberg S, Manfro R, Nainan G, et al. Outcomes of switching to belatacept from a calcineurin inhibitor in kidney transplant recipients: 3 year results from the long‐term extension of a phase II study [abstract no: 491]. American Journal of Transplantation 2013;13(Suppl S5):182. [EMBASE: 71057068] [Google Scholar]
- Grinyo J, Vanrenterghem Y, Durrbach A, Rial M, Charpentier B, Matas A, et al. Likelihood of improving or maintaining renal function in recipients of extended‐criteria donor kidneys over five years with belatacept or CsA (BENEFIT‐EXT long‐term extension study) [abstract no: B931]. American Journal of Transplantation 2013;13(Suppl S5):310. [EMBASE: 71057507] [Google Scholar]
- Larsen C, Alberu J, Massari P, Acevedo R, Kamar N, Lin C, et al. 4 year results from the long term extension of the belatacept BENEFIT study [abstract no: 186]. American Journal of Transplantation 2012;12(Suppl 3):82. [EMBASE: 70746133] [Google Scholar]
- Larsen C, Bray R, Gebel H, Ganguly B, Kulbokas E, Brickman D, et al. Evaluation of donor specific antibodies in kidney transplant patients treated with belatacept‐ or cyclosporine‐based immunosuppression in BENEFIT and BENEFIT‐EXT [abstract no: O‐244]. Transplant International 2011;24(Suppl 2):69. [EMBASE: 70527320] [Google Scholar]
- Larsen C, Charpentier B, Grinyo JM, Medina‐Pestana JD, Vanrenterghem Y, Vincenti F, et al. One year safety profile of belatacept in kidney transplant patients from the BENEFIT and BENEFIT‐EXT Studies [abstract no: 1080]. American Journal of Transplantation 2009;9(Suppl 2):495. [Google Scholar]
- Larsen C, Charpentier B, Grinyo JM, Medina‐Pestana JD, Vanrenterghem Y, Vincenti F, et al. One year safety profile of belatacept in kidney transplant patients from the BENEFIT and BENEFIT‐EXT studies [abstract no: O‐335]. Transplant International 2009;22(Suppl 2):90. [Google Scholar]
- Larsen C, Grinyo J, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, Dong Y, et al. 3‐year safety profile of belatacept in kidney transplant recipients from the BENEFIT and BENEFIT‐EXT studies [abstract no: 228]. American Journal of Transplantation 2011;11(Suppl 2):99‐100. [EMBASE: 70405263] [Google Scholar]
- Larsen C, Vincenti F, Grinyo J, Rice K, Steinberg S, Gaite L, et al. Long term belatacept exposure maintains efficacy and safety at 5 years: results from the long‐term extension (LTE) of the belatacept evaluation of nephroprotection and efficacy as first‐line immunosuppression trial (BENEFIT) study [abstract no: B937]. American Journal of Transplantation 2013;13(Suppl S5):312. [EMBASE: 71057513] [DOI] [PubMed] [Google Scholar]
- Larsen C, Vincenti F, Grinyo JM, Charpentier B, Russo GB, Garg P. Limited impact of acute rejection on graft outcomes in belatacept‐treated kidney transplant recipients (BENEFIT/BENEFIT‐EXT) [abstract no: P‐397]. Transplant International 2009;22(Suppl 2):193. [Google Scholar]
- Larsen CP, Grinyo J, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, Breshahan B, et al. Belatacept‐based regimens versus a cyclosporine A‐based regimen in kidney transplant recipients: 2‐year results from the BENEFIT and BENEFIT‐EXT studies. Transplantation 2010;90(12):1528‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lehner F, Budde K, Morales JM, Blancho G, Muehlbacher F, Rice K, et al. Impact of donor age on 3‐year outcomes of extended criteria donor kidney recipients in BENEFIT‐EXT [abstract no: BO197]. Transplant International 2013;26(Suppl 2):92. [EMBASE: 71359309] [Google Scholar]
- Levy A, Johnston K, Kalsekar A, Schnitzler M, L'Italien G, Kasiske B. Modeled long term projections of clinical outcomes from BENEFIT and BENEFIT‐EXT [abstract no: 1292]. American Journal of Transplantation 2012;12(Suppl S3):406‐7. [EMBASE: 70747259] [Google Scholar]
- Medina Pestana J, Grinyo J, Vanrenterghem Y, Becker T, Florman S, Lang P, et al. Belatacept compared with cyclosporine in renal allograft recipients of extended criteria donor kidneys: 3 year outcomes from the phase III BENEFIT‐EXT trial [abstract no: O‐241]. Transplant International 2011;24(Suppl 2):68. [EMBASE: 70527317] [Google Scholar]
- Medina Pestana J, Grinyo J, Vanrenterghem Y, Becker T, Florman S, Lang P, et al. Belatacept compared with cyclosporine in renal allograft recipients of extended criteria donor kidneys: 3‐year outcomes from the phase III BENEFIT‐EXT trial [abstract no: 1088]. American Journal of Transplantation 2011;11(Suppl 2):349. [EMBASE: 70406134] [Google Scholar]
- Medina Pestana J, Grinyo J, Vanrenterghem Y, Becker T, Florman S, Lang P, et al. Belatacept compared with cyclosporine in renal allograft recipients of extended criteria donor kidneys: 3‐year outcomes from the phase III BENEFIT‐EXT trial [abstract no: P143]. Transplant International 2011;24(Suppl 3):51. [EMBASE: 70537443] [Google Scholar]
- Medina‐Pestana J, Campistol J, Carmen Rial M, Duro G, Becker T, Agarwal M, et al. Belatacept preserves renal function and structure at 1 year compared with cyclosporine in extended criteria donor (ECD) kidney transplant patients (BENEFIT‐EXE) [abstract no: O53]. British Transplantation Society (BTS). 13th Annual Congress; 2010 Mar 17‐19; London, UK. 2010.
- Medina‐Pestana J, Grinyo J, Vanrenterghem I, Becker T, Florman S, Lang P, et al. Belatacept compared with ciclosporin in renal allograft recipients of extended criteria donor kidneys: 3‐year outcomes from the phase III BENEFIT‐EXT trial [abstract]. British Transplantation Society (BTS). 15th Annual Congress; 2012 Feb 22‐24; Glasgow, UK. 2012.
- Medina‐Pestana JD, Campistol JM, Carmen Rial M, Garcia VD, Becker T, Agarwal M, et al. Belatacept is associated with preservation of renal function and structure at 1 year compared to cyclosporine in extended criteria donor (ECD) kidney transplant patients (BENEFIT‐EXT Study) [abstract no: O‐333]. Transplant International 2009;22(Suppl 2):89. [Google Scholar]
- Medina‐Pestana JD, Campistol JM, C Rial M, Garcia VD, Becker T, Agarwal M, et al. Belatacept is associated with preservation of renal function and structure at 1 year compared to cyclosporine in extended criteria donor (ECD) kidney transplant patients (BENEFIT‐EXT study) [abstract no: 28]. American Journal of Transplantation 2009;9(Suppl 2):199. [Google Scholar]
- Medina‐Pestana JO, Grinyo JM, Vanrenterghem Y, Becker T, Campistol JM, Florman S, et al. Three‐year outcomes from BENEFIT‐EXT: a phase III study of belatacept versus cyclosporine in recipients of extended criteria donor kidneys. American Journal of Transplantation 2012;12(3):630‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Muehlbacher F, Becker T, Campistol J, Carvalho D, Florman S, Lang P, et al. Donor sub‐type analysis of three year outcomes from a phase III study of belatacept in recipients of extended criteria donor kidneys (BENEFIT‐EXT Trial) [abstract no: RO‐331]. Transplant International 2011;24(Suppl 2):221‐2. [EMBASE: 70527872] [Google Scholar]
- Muehlbacher F, Durrbach A, Florman S, Medina PJ, Harler M, Larsen C. Outcomes at 3‐years in EBV+ recipients of deceased donor kidneys from two randomized trials (BENEFIT and BENEFIT‐EXT) comparing belatacept vs cyclosporine [abstract]. Transplantation 2014;98(Suppl 1):455. [EMBASE: 71545051] [Google Scholar]
- Muehlbacher F, Florman S, Medina‐Pestana J, Rial M, Rostaing L, Grinyo J, et al. Long‐term exposure to belatacept in recipients of extended criteria donor kidneys [abstract no: O066]. Transplant International 2013;26(Suppl 2):44. [EMBASE: 71359104] [DOI] [PubMed] [Google Scholar]
- Muehlbacher F, Florman S, Zhang R, Lang P, Lehner F, Massari P, et al. 5‐year outcomes by donor type from the long‐term extension of the belatacept BENEFIT‐EXT study [abstract no: BO198]. Transplant International 2013;26(Suppl 2):92. [Google Scholar]
- Muelbacher F, Becker T, Campistol J, Carvalho D, Florman S, Lang P. Donor sub‐type analysis of 3 year outcomes from a phase III study of belatacept in recipient of extended criteria donor kidneys (BENEFIT‐EXT trial) [abstract]. British Transplantation Society (BTS). 15th Annual Congress; 2012 Feb 22‐24; Glasgow, UK. 2012.
- Rice K, Chan L, Rostaing L, Bresnahan B, Helderman JH, Harler MB, et al. Likelihood of improving or sustaining renal function over four years with belatacept or CsA: insights from the BENEFIT Long‐Term Extension Study [abstract no: TH‐PO1042]. Journal of the American Society of Nephrology 2012;23:340A. [Google Scholar]
- Rice K, Vanrenterghem Y, Merville P, Muehlbacher F, Zhang R, Duan T, et al. Three year outcomes in elderly kidney transplant recipients treated with belatacept vs cyclosporine in BENEFIT‐EXT [abstract no: 1281]. American Journal of Transplantation 2012;12(Suppl 3):403. [EMBASE: 70747247] [Google Scholar]
- Rostaing L, Neumayer HH, Reyes‐Acevedo R, Bresnahan B, Florman S, Vitko S, et al. Belatacept‐versus cyclosporine‐based immunosuppression in renal transplant recipients with pre‐existing diabetes. Clinical Journal of the American Society of Nephrology: CJASN 2011;6(11):2696‐704. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostaing L, Reyes‐Acevedo R, Neumayer H, Vitko S, Xing J, Thomas D, et al. Outcomes at 3 years in kidney transplant recipients with pre‐transplant diabetes from two phase 3 belatacept studies [abstract no: O‐243]. Transplant International 2011;24(Suppl 2):69. [EMBASE: 70527319] [Google Scholar]
- Rostaing L, Reyes‐Acevedo R, Neumayer H, Vitko S, Xing J, Thomas D, et al. Outcomes at 3 years in kidney transplant recipients with pre‐transplant diabetes from two phase III belatacept studies [abstract]. British Transplantation Society (BTS). 15th Annual Congress; 2012 Feb 22‐24; Glasgow, UK. 2012.
- Rostaing L, Vincenti F, Grinyo J, Rice K, Steinberg S, Gaite L, et al. Long‐term belatacept maintains efficacy & safety: 5‐year BENEFIT long‐term extension (LTE) results [abstract no: O065]. Transplant International 2013;26(Suppl 2):44. [EMBASE: 71359103] [DOI] [PubMed] [Google Scholar]
- Rostaing L, Vincenti F, Neumayer H, Reyes‐Acevedo R, Block A, Russo G, et al. Belatacept demonstrates superior composite patient/graft survival in diabetic kidney transplant recipients vs. CsA: Results from BENEFIT & BENEFIT‐EXT [abstract no: F‐PO2028]. Journal of the American Society of Nephrology 2009;20:573a. [Google Scholar]
- Rostaing L, Vincenti F, Neumayer H, Reyes‐Acevedo R, Block A, Russo GB, et al. Belatacept demonstrates superior composite patient/graft survival in diabetic kidney transplant recipients vs CsA: results from the BENEFIT and BENEFIT‐EXT studies [abstract no: 763]. American Journal of Transplantation 2009;9(Suppl 2):412. [Google Scholar]
- Rostaing L, Vincenti F, Neumayer HH, Reyes‐Acevedo R, Block AJ, Russo GB. Belatacept demonstrates superior composite patient/graft survival in diabetic kidney transplant recipients vs CSA: results from the BENEFIT and BENEFIT‐EXT studies [abstract no: P‐395]. Transplant International 2009;22(Suppl 2):192. [Google Scholar]
- Russ G, Agarwal M, Becker T, Bresnahan B, Campistol JM, Darji P, et al. Belatacept associated with preserved renal function and structure compared with cyclosporine (CSA) in kidney transplant patients [abstract]. Immunology & Cell Biology 2010;88(6):A11‐2. [EMBASE: 70313652] [Google Scholar]
- Russ G, Durrbach A, Larsen CP, Medina‐Pestana J, Vanrenterghem Y, et al. BENEFIT‐EXT study two year outcomes: belatacept vs cyclosporine (CSA) in extended criteria donor (ECD) kidney transplants [abstract no: 2]. Transplantation Society of Australia & New Zealand (TSANZ). 29th Annual Scientific Meeting 2011 Jun 29‐Jul 1, Canberra, Australia. 2011:37.
- Shen J, Gelb JS, Townsend RM, Zhou Z, Pfister M, Harler MB, et al. Rationale for belatacept less intensive regimen in renal transplant recipients [abstract no: 1096]. American Journal of Transplantation 2011;11(Suppl 2):352. [EMBASE: 70406142] [Google Scholar]
- Sherrill B, Wang J, Hall D, Kalsekar A. A Q‐TWiST analysis of belatacept compared with cyclosporine in kidney transplant recipients [abstract no: SA‐OR114]. Journal of the American Society of Nephrology 2012;23:92A. [Google Scholar]
- Vanrenterghem Y, Bresnahan B, Campistol J, Durrbach A, Grinyo J, Neumayer HH, et al. Belatacept‐based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT‐EXT studies). Transplantation 2011;91(9):976‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vanrenterghem Y, Mancilla‐Urrea E, Lang P, Agarwal M, Block A, Xing J. Belatacept is associated with improved cardiovascular and metabolic risk factors compared to cyclosporine in kidney transplant patients (BENEFIT & BENEFIT‐EXE Studies) [abstract no: O52]. British Transplantation Society (BTS). 13th Annual Congress; 2010 Mar 17‐19; London, UK. 2010.
- Vanrenterghem Y, Mancilla‐Urrea E, Lang P, Agarwal M, Block A, Xing J. Belatacept is associated with improved cardiovascular and metabolic risk factors compared to cyclosporine in kidney transplant patients (BENEFIT & BENEFIT‐EXT studies) [abstract no: TH‐PO1018]. Journal of the American Society of Nephrology 2009;20:343a. [Google Scholar]
- Vanrenterghem Y, Mancilla‐Urrea E, Lang P, Agarwal M, Block AJ. Belatacept is associated with improved cardiovascular and metabolic risk factors compared to cyclosporine in kidney transplant patients (BENEFITand BENEFIT‐EXT studies) [abstract no: O‐334]. Transplant International 2009;22(Suppl 2):90. [Google Scholar]
- Vanrenterghem Y, Mancilla‐Urrea E, Lang P, Agarwal M, Block AJ, Xing J. Belatacept is associated with improved cardiovascular and metabolic risk factors compared to cyclosporine in kidney transplant patients (BENEFIT and BENEFIT‐EXT Studies) [abstract no: 1079]. American Journal of Transplantation 2009;9(Suppl 2):495. [DOI] [PubMed] [Google Scholar]
- Vanrenterghem Y, Medina‐Pestana J, Becker T, Rial M, Agarwal M, Lin CS, et al. Likelihood of improving or sustaining renal function over three years with belatacept or CsA: Insights from the BENEFIT‐EXT Study [abstract no: PO27.056]. 24th International Congress of the Transplantation Society (TTS); 2012 Jul 15‐19; Berlin, Germany. 2012.
- Vincenti F, Grinyo J, Larsen C, Medina‐Pestana J, Vanrenterghem Y, Dong Y, et al. 3‐year safety profile of belatacept in kidney transplant recipients from the BENEFIT and BENEFIT‐EXT studies [abstract no: F564]. NDT Plus 2011;4(Suppl 2):4.s2.43. [Google Scholar]
- Vincenti F, Grinyo J, Larsen C, Medina‐Pestana J, Vanrenterghem Y, Duan T, et al. Benefit‐risk profile of the belatacept LI regimen at 2 years in EBV(+) kidney transplant recipients [abstract no: 3370]. Transplantation 2010;90(Suppl):649. [EMBASE: 71532340] [Google Scholar]
- Vincenti F, Rostaing L, Durrbach A, Rice K, Pupim L, Grinyo J. Improving or maintaining renal function over 5 years with belatacept or cyclosporine (CsA): insights from the BENEFIT and BENEFIT‐EXT long‐term extension (LTE) studies [abstract no: SA‐PO1012]. Journal of the American Society of Nephrology 2013;24(Abstracts):860A. [Google Scholar]
- Zhou Z, Shen J, Kaul S, Pfister M, Roy A. Belatacept population pharmacokinetics in renal transplant patients [abstract no: 1505]. American Journal of Transplantation 2010;10(Suppl 4):467. [Google Scholar]
BENEFIT Study 2008 {published data only}
- Blancho G, Budde K, Merville P, Moal M, Rostaing L, Harler M, et al. Outcomes at 3 years in EBV+ European subpopulations from BENEFIT and BENEFIT‐EXT [abstract no: A198]. American Journal of Transplantation 2014;14(Suppl 5):455. [EMBASE: 71545053] [Google Scholar]
- Bluestone JA, Liu W, Yabu JM, Laszik ZG, Putnam A, Belingheri M, et al. The effect of costimulatory and interleukin 2 receptor blockade on regulatory T cells in renal transplantation. American Journal of Transplantation 2008;8(10):2086‐96. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R, Gebel H, Brannon P, Ganguly B, Townsend R, Meier‐Kriesche U, et al. Evaluation of donor‐specific antibodies through 5 years with belatacept in BENEFIT and BENEFIT‐EXT [abstract no: 2163]. American Journal of Transplantation 2014;14(Suppl 3):117. [EMBASE: 71543898] [Google Scholar]
- Bresnahan B, Vincenti F, Grinyo J, Charpentier B, Russo GD, Garg P, et al. Renal benefit of belatacept versus cyclosporine in kidney transplant patients is not impacted by acute rejection (BENEFIT study) [abstract no: 56]. American Journal of Transplantation 2010;10(Suppl 2):14. [EMBASE: 70081079] [Google Scholar]
- Charpentier B, Grinyo J, Medina‐Pestana J, Vanrenterghem I, Vincente F, Dong Y, et al. 3‐year safety profile of belatacept in kidney transplant recipients from the BENEFIT and BENEFIT‐EXT studies [abstract no: O‐242]. Transplant International 2011;24(Suppl 2):68‐9. [EMBASE: 70527318] [Google Scholar]
- Charpentier B, Vincenti F, Rice K, Campistol J, Duan T, Pupim L, et al. Three‐year outcomes in patients with delayed graft function in phase III studies of belatacept vs cyclosporine in kidney transplantation (BENEFIT and BENEFIT‐EXT) [abstract no: PO27.076]. 24th International Congress of the Transplantation Society (TTS); 2012 Jul 15‐19; Berlin, Germany. 2012.
- Dobbels F, Wong S, Joo S, Kalsekar A. Health‐related quality of life after kidney transplantation: Results from belatacept clinical trials [abstract no: 1099]. American Journal of Transplantation 2011;11(Suppl 2):352‐3. [EMBASE: 70406145] [Google Scholar]
- Dobbels F, Wong S, Min Y, Sam J, Kalsekar A. Beneficial effect of belatacept on health‐related quality of life and perceived side effects: results from the BENEFIT and BENEFIT‐EXT trials. Transplantation 2014;98(9):960‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dobbels F, Wong S, You M, Kalsekar A. Patient reports of immunosuppressant related side‐effects after kidney transplantation: results from the belatacept phase III clinical trial (BENEFIT) [abstract no: 1100]. American Journal of Transplantation 2011;11(Suppl 2):353. [EMBASE: 70406146] [Google Scholar]
- Durrbach A, Citterio F, Mulloy L, David‐Neto E, Russ G, Vitko S, et al. Renal function in patients treated with belatacept‐ or cyclosporine‐based regimens at year 3 in the BENEFIT and BENEFIT‐EXT studies [abstract no: 230]. American Journal of Transplantation 2011;11(Suppl 2):100. [EMBASE: 70405265] [Google Scholar]
- Durrbach A, Citterio F, Mulloy L, David‐Neto E, Russ G, Vitko S, et al. Renal function in patients treated with belatacept‐ or cyclosporine‐based regimens at year 3 in the BENEFIT and BENEFIT‐EXT studies [abstract no: Sa521]. NDT Plus 2011;4(Suppl 2):4.s2.60. [Google Scholar]
- Durrbach A, Citterio F, Mulloy L, David‐Neto E, Russ G, Vitko S, et al. Renal function in patients treated with belatacept‐or cyclosporine‐based regimens at year 3 in the BENEFIT and BENEFIT‐EXT studies [abstract no: P‐080]. Transplant International 2011;24(Suppl 2):249. [EMBASE: 70527977] [Google Scholar]
- Durrbach A, Florman S, Zhang R, Becker T, Grinyo J, Lang P, et al. Four‐year outcomes by donor type from the long‐term extension of the belatacept BENEFIT and BENEFIT‐EXT Studies [abstract no: 1293]. American Journal of Transplantation 2012;12(Suppl 3):407. [EMBASE: 70747260] [Google Scholar]
- Durrbach A, Medina‐Pestana J, Becker T, Grinyo J, Lang P, Garcia VD, et al. Four‐year outcomes by donor type from the long‐term extension of the belatacept BENEFIT and BENEFIT‐EXT Studies [abstract no: PO27.057]. 24th International Congress of the Transplantation Society (TTS); 2012 Jul 15‐19; Berlin, Germany. 2012.
- Florman S, Becker T, Bresnahan B, Chevaile‐Ramos A, Carvalho D, Muehlbacher F, et al. Three year outcomes by donor type in phase III studies of belatacept vs cyclosporine in kidney transplantation (BENEFIT and BENEFIT‐EXT) [abstract no: P145]. Transplant International 2011;24(Suppl 3):51. [EMBASE: 70537445] [Google Scholar]
- Florman S, Becker T, Bresnahan B, Chevaile‐Ramos A, DeCarvalho D, Muehlbacher F, et al. Three‐year outcomes by donor type in phase III studies of belatacept vs cyclosporine in kidney transplantation (BENEFIT & BENEFIT‐EXT) [abstract no: 229]. American Journal of Transplantation 2011;11(Suppl 2):100. [EMBASE: 70405264] [Google Scholar]
- Florman S, Bresnahan B, Chan L, Helderman H, Dong Y, Harler MB, et al. Three year outcomes in Black/African American kidney transplant recipients from the BENEFIT and BENEFIT‐EXT studies [abstract no: 1091]. American Journal of Transplantation 2011;11(Suppl 2):350. [EMBASE: 70406137] [Google Scholar]
- Florman S, Rice K, Chan L, Steinberg S, Pearson T, Duan T, et al. Four year outcomes in Black/African American kidney transplant recipients from the long term‐extension of the belatacept BENEFIT and BENEFIT‐EXT studies [abstract no: 1284]. American Journal of Transplantation 2012;12(Suppl 3):404. [EMBASE: 70747251] [Google Scholar]
- Florman S, Rice K, Chan L, Zhang R, Abouljoud M, Steinberg S, et al. Outcomes at five years in Black/African‐American kidney transplant recipients from the long‐term extension of the belatacept BENEFIT and BENEFIT‐EXT studies [abstract no: B935]. American Journal of Transplantation 2013;13(Suppl S5):311. [EMBASE: 71057510] [Google Scholar]
- Furuzawa‐Carballeda J, Lima G, Alberu J, Palafox D, Uribe‐Uribe N, Morales‐Buenrostro LE, et al. Infiltrating cellular pattern in kidney graft biopsies translates into forkhead box protein 3 up‐regulation and p16INK4alpha senescence protein down‐regulation in patients treated with belatacept compared to cyclosporin A. Clinical & Experimental Immunology 2012;167(2):330‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuzawa‐Carballeda J, Lima G, Uribe‐Uribe N, Avila‐Casado C, Mancilla E, Morales‐Buenrostro LE, et al. High levels of IDO‐expressing CD16+ peripheral cells, and Tregs in graft biopsies from kidney transplant recipients under belatacept treatment. Transplantation Proceedings 2010;42(9):3489‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grimbert P, Audard V, Diet C, Matignon M, Plonquet A, Mansour H, et al. T‐cell phenotype in protocol renal biopsy from transplant recipients treated with belatacept‐mediated co‐stimulatory blockade. Nephrology Dialysis Transplantation 2011;26(3):1087‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grinyo J, Abouljoud M, Germain M, Manfro R, Morales J, Legendre C, et al. Likelihood of improving or sustaining renal function over 3 years with belatacept or ciclosporin: insights from the BENEFIT study [abstract]. British Transplantation Society (BTS). 15th Annual Congress; 2012 Feb 22‐24; Glasgow, UK. 2012.
- Grinyo J, Abouljoud M, Germain M, Manfro R, Morales J, Legendre C, et al. Likelihood of improving or sustaining renal function over three years with belatacept or CsA: insights from the BENEFIT study [abstract no: 1087]. American Journal of Transplantation 2011;11(Suppl 2):349. [EMBASE: 70406133] [Google Scholar]
- Grinyo J, Abouljoud M, Germain MJ, Manfro R, Morales J, Legendre C, et al. Improving or sustaining renal function over 3 years with belatacept or cyclosporine A (CsA): insights from the BENEFIT study [abstract no: P‐083]. Transplant International 2011;24(Suppl 2):250. [EMBASE: 70527980] [Google Scholar]
- Grinyo J, Charpentier B, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, Reyes‐Acevedo R, et al. An integrated safety profile analysis of belatacept in kidney transplant recipients. Transplantation 2010;90(12):1521‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grinyo J, Charpentier B, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, Shi R, et al. Safety profile of belatacept in kidney transplant recipients from a pooled analysis of phase II and phase III studies [abstract no: 144]. American Journal of Transplantation 2010;10(Suppl 4):83. [EMBASE: 70463505] [Google Scholar]
- Grinyo J, Charpentier B, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, Shi R, et al. Safety profile of belatacept in kidney transplant recipients from a pooled analysis of phase II and phase III studies [abstract no: SA‐FC442]. Journal of the American Society of Nephrology 2010;21(Abstracts):101A. [Google Scholar]
- Grinyo J, Charpentier B, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, Shi R, et al. Safety profile of belatacept in kidney transplant recipients from a pooled analysis of phase II and phase III studies [abstract no: Sa676]. NDT Plus 2010;3(Suppl 3):iii270. [EMBASE: 70484142] [Google Scholar]
- Grinyo J, Charpentier B, Pestana JOM, Vanrenterghem Y, Vincenti F, Shi R, et al. Safety profile of belatacept in kidney transplant recipients from a pooled analysis of phase II and phase III studies [abstract no: 1417]. Transplantation 2010;90(Suppl):156. [EMBASE: 71531402] [DOI] [PubMed] [Google Scholar]
- Grinyo J, Larsen C, Charpentier B, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, et al. One year safety profile of belatacept in kidney transplant patients (BENEFIT & BENEFIT‐EXT) [abstract no: TH‐PO1016]. Journal of the American Society of Nephrology 2009;20:343A. [Google Scholar]
- Grinyo J, Mondragon‐Ramirez G, Darji P, Bresnahan B, Pearson T, Russo G, et al. Belatacept is associated with preservation or renal function and structure at 1 year compared to cyclosporine in kidney transplant patients (BENEFIT study) [abstract no: O51]. British Transplantation Society (BTS). 13th Annual Congress; 2010 Mar 17‐19; London, UK. 2010.
- Grinyo J, Mondragon‐Ramirez G, Darji P, Bresnahan B, Pearson T, DiRusso G, et al. Belatacept is associated with preservation of renal function and structure at 1 year vs cyclosporine in kidney transplant patients (BENEFIT Study) [abstract no: SA‐FC358]. Journal of the American Society of Nephrology 2009;20:84a. [Google Scholar]
- Grinyo JM, Mondragon‐Ramirez G, Darji P, Bresnahan B, Pearson T, Russo GB, et al. Belatacept is associated with preservation of renal function and structure at 1 year compared to cyclosporine in kidney transplant patients (BENEFIT Study) [abstract no: 236]. American Journal of Transplantation 2009;9(Suppl 2):258‐9. [EMBASE: 70010109] [Google Scholar]
- Grinyo JM, Mondragon‐Ramirez G, Darji P, Bresnahan B, Pearson T, Russo GB, et al. Belatacept is associated with preservation of renal function and structure at 1 year compared to cyclosporine in kidney transplant patients (BENEFIT Study) [abstract no: P‐396]. Transplant International 2009;22(Suppl 2):193. [Google Scholar]
- Kamar N, Ribes D, Lavit M, Esposito L, Lavayssiere L, Cointault O, et al. Pharmacokinetic monitoring of mycophenolate mofetil in de novo kidney transplant patients receiving belatacept or cyclosporine A [abstract no: 783]. American Journal of Transplantation 2007;7(Suppl 2):349. [Google Scholar]
- Kamar N, Ribes D, Lavit M, Esposito L, Lavayssiere L, Cointault O, et al. Pharmacokinetic monitoring of mycophenolate mofetil in de novo kidney‐transplant patients receiving belatacept or cyclosporine A [abstract no: P094]. Transplant International 2007;20(Suppl 2):118. [Google Scholar]
- Larsen C, Bray R, Gebel H, Ganguly B, Kulbokas E, Brickman D, et al. Evaluation of donor specific antibodies in kidney transplant patients treated with belatacept‐ or cyclosporine‐based immunosuppression in BENEFIT and BENEFIT‐EXT [abstract no: O‐244]. Transplant International 2011;24(Suppl 2):69. [EMBASE: 70527320] [Google Scholar]
- Larsen C, Charpentier B, Grinyo JM, Medina‐Pestana JD, Vanrenterghem Y, Vincenti F, et al. One year safety profile of belatacept in kidney transplant patients from the BENEFIT and BENEFIT‐EXT Studies [abstract no: 1080]. American Journal of Transplantation 2009;9(Suppl 2):495. [Google Scholar]
- Larsen C, Charpentier B, Grinyo JM, Medina‐Pestana JD, Vanrenterghem Y, Vincenti F, et al. One year safety profile of belatacept in kidney transplant patients from the BENEFIT and BENEFIT‐EXT studies [abstract no: O‐335]. Transplant International 2009;22(Suppl 2):90. [Google Scholar]
- Larsen C, Grinyo J, Charpentier B, Medina PJ, Kamar N, Vanrenterghem Y, et al. Belatacept vs cyclosporine in kidney transplant recipients: two year outcomes from the BENEFIT study [abstract no: 0076]. Transplant International 2010;23(Suppl 2):17. [Google Scholar]
- Larsen C, Grinyo J, Charpentier B, Medina‐Pestana J, Kamar N, Vanrenterghem Y, et al. Belatacept vs cyclosporine in kidney transplant recipients: Two year outcomes from the BENEFIT study [abstract]. British Transplantation Society (BTS). 14th Annual Congress; 2011 Mar 9‐11; Bournemouth, UK. 2011.
- Larsen C, Grinyo J, Charpentier B, Medina‐Pestana J, Kamar N, Vanrenterghem Y, et al. Belatacept vs cyclosporine in kidney transplant recipients: two‐year outcomes from the BENEFIT Study [abstract no: SA‐FC443]. Journal of the American Society of Nephrology 2010;21(Abstracts):101A. [Google Scholar]
- Larsen C, Grinyo J, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, Dong Y, et al. 3‐year safety profile of belatacept in kidney transplant recipients from the BENEFIT and BENEFIT‐EXT studies [abstract no: 228]. American Journal of Transplantation 2011;11(Suppl 2):99‐100. [EMBASE: 70405263] [Google Scholar]
- Larsen C, Grinyo JM, Charpentier B, Medina‐Pestana J, Kamar N, Vanrenterghem Y, et al. Belatacept vs cyclosporine in kidney transplant recipients: two‐year outcomes from the BENEFIT study [abstract no: 1374]. Transplantation 2010;90(Suppl):158. [Google Scholar]
- Larsen C, Vincenti F, Grinyo JM, Charpentier B, Russo GB, Garg P. Limited impact of acute rejection on graft outcomes in belatacept‐treated kidney transplant recipients (BENEFIT/BENEFIT‐EXT) [abstract no: P‐397]. Transplant International 2009;22(Suppl 2):193. [Google Scholar]
- Larsen C, Vincenti F, Grinyo JM, Charpentier B, Russo GB, Garg P, et al. Renal benefit of belatacept vs cyclosporine in kidney transplant patients is not impacted by acute rejection (BENEFIT study) [abstract no: 100]. American Journal of Transplantation 2009;9(Suppl 2):220. [EMBASE: 70009973] [Google Scholar]
- Larsen CP, Grinyo J, Charpentier B, Medina Pestana J, Kamar N, Vanrenterghem YF, et al. Belatacept vs cyclosporine in kidney transplant recipients: two‐year outcomes from the BENEFIT Study [abstract no: 142]. American Journal of Transplantation 2010;10(Suppl 4):82. [EMBASE: 70463503] [Google Scholar]
- Larsen CP, Grinyo J, Charpentier B, Pestana JM, Kamar N, Vanrenterghem Y, et al. Belatacept vs cyclosporine in kidney transplant recipients: two‐year outcomes from the BENEFIT study [abstract no: Sa653]. NDT plus 2010;3(Suppl 3):iii262. [EMBASE: 70484119] [Google Scholar]
- Larsen CP, Grinyo J, Medina‐Pestana J, Vanrenterghem Y, Vincenti F, Breshahan B, et al. Belatacept‐based regimens versus a cyclosporine A‐based regimen in kidney transplant recipients: 2‐year results from the BENEFIT and BENEFIT‐EXT studies. Transplantation 2010;90(12):1528‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Latek R, Fleener C, Lamian V, Kulbokas E, Davis PM, Suchard SJ, et al. Assessment of belatacept‐mediated costimulation blockade through evaluation of CD80/86‐receptor saturation. Transplantation 2009;87(6):926‐33. [EMBASE: 19300198] [DOI] [PubMed] [Google Scholar]
- Levy A, Johnston K, Kalsekar A, Schnitzler M, L'Italien G, Kasiske B. Modeled long term projections of clinical outcomes from BENEFIT and BENEFIT‐EXT [abstract no: 1292]. American Journal of Transplantation 2012;12(Suppl S3):406‐7. [EMBASE: 70747259] [Google Scholar]
- Muehlbacher F, Durrbach A, Florman S, Medina PJ, Harler M, Larsen C. Outcomes at 3‐years in EBV+ recipients of deceased donor kidneys from two randomized trials (BENEFIT and BENEFIT‐EXT) comparing belatacept vs cyclosporine [abstract]. Transplantation 2014;98(Suppl 1):455. [EMBASE: 71545051] [Google Scholar]
- Muehlbacher F, Durrbach A, Florman S, Medina Pestana J, Harler M, Larsen C. Outcomes at 3‐Years in EBV+ recipients of deceased donor kidneys from two randomized trials (BENEFIT and BENEFIT EXT) comparing belatacept vs cyclosporine [abstract no: A196]. American Journal of Transplantation 2014;14(Suppl 3):455. [Google Scholar]
- Pearson T, Vincenti F, Grinyo J, Charpentier B, Medina‐Pestana J, Rostaing L, et al. Primary outcomes from a randomized, phase III study of belatacept versus cyclosporine in kidney transplant recipients (BENEFIT study) [abstract no: 25]. American Journal of Transplantation 2010;10(Suppl 2):6. [EMBASE: 70081051] [Google Scholar]
- Rostaing L, Neumayer HH, Reyes‐Acevedo R, Bresnahan B, Florman S, Vitko S, et al. Belatacept‐versus cyclosporine‐based immunosuppression in renal transplant recipients with pre‐existing diabetes. Clinical Journal of the American Society of Nephrology: CJASN 2011;6(11):2696‐704. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostaing L, Reyes‐Acevedo R, Neumayer H, Vitko S, Xing J, Thomas D, et al. Outcomes at 3 years in kidney transplant recipients with pre‐transplant diabetes from two phase 3 belatacept studies [abstract no: O‐243]. Transplant International 2011;24(Suppl 2):69. [EMBASE: 70527319] [Google Scholar]
- Rostaing L, Reyes‐Acevedo R, Neumayer H, Vitko S, Xing J, Thomas D, et al. Outcomes at 3 years in kidney transplant recipients with pre‐transplant diabetes from two phase III belatacept studies [abstract]. British Transplantation Society (BTS). 15th Annual Congress; 2012 Feb 22‐24; Glasgow, UK. 2012.
- Rostaing L, Vincenti F, Grinyo J, Rice KM, Bresnahan B, Steinberg S, et al. Long‐term belatacept exposure maintains efficacy and safety at 5 years: Results from the long‐term extension of the BENEFIT Study. American Journal of Transplantation 2013;13(11):2875‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rostaing L, Vincenti F, Neumayer H, Reyes‐Acevedo R, Block A, Russo G, et al. Belatacept demonstrates superior composite patient/graft survival in diabetic kidney transplant recipients vs. CsA: Results from BENEFIT & BENEFIT‐EXT [abstract no: F‐PO2028]. Journal of the American Society of Nephrology 2009;20:573A. [Google Scholar]
- Rostaing L, Vincenti F, Neumayer H, Reyes‐Acevedo R, Block A, Russo GB, et al. Belatacept demonstrates superior composite patient/graft survival in diabetic kidney transplant recipients vs CsA: results from the BENEFIT and BENEFIT‐EXT studies [abstract no: 763]. American Journal of Transplantation 2009;9(Suppl 2):412. [Google Scholar]
- Rostaing L, Vincenti F, Neumayer HH, Reyes‐Acevedo R, Block AJ, Russo GB. Belatacept demonstrates superior composite patient/graft survival in diabetic kidney transplant recipients vs CSA: results from the BENEFIT and BENEFIT‐EXT studies [abstract no: P‐395]. Transplant International 2009;22(Suppl 2):192. [Google Scholar]
- Russ G, Agarwal M, Becker T, Bresnahan B, Campistol JM, Darji P, et al. Belatacept associated with preserved renal function and structure compared with cyclosporine (CSA) in kidney transplant patients [abstract]. Immunology & Cell Biology 2010;88(6):A11‐2. [EMBASE: 70313652] [Google Scholar]
- Shen J, Gelb JS, Townsend RM, Zhou Z, Pfister M, Harler MB, et al. Rationale for belatacept less intensive regimen in renal transplant recipients [abstract no: 1096]. American Journal of Transplantation 2011;11(Suppl 2):352. [EMBASE: 70406142] [Google Scholar]
- Vanrenterghem Y, Bresnahan B, Campistol J, Durrbach A, Grinyo J, Neumayer HH, et al. Belatacept‐based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT‐EXT studies). Transplantation 2011;91(9):976‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vanrenterghem Y, Mancilla‐Urrea E, Lang P, Agarwal M, Block A, Xing J. Belatacept is associated with improved cardiovascular and metabolic risk factors compared to cyclosporine in kidney transplant patients (BENEFIT & BENEFIT‐EXE Studies) [abstract no: O52]. British Transplantation Society (BTS). 13th Annual Congress; 2010 Mar 17‐19; London, UK. 2010.
- Vanrenterghem Y, Mancilla‐Urrea E, Lang P, Agarwal M, Block A, Xing J. Belatacept is associated with improved cardiovascular and metabolic risk factors compared to cyclosporine in kidney transplant patients (BENEFIT & BENEFIT‐EXT studies) [abstract no: TH‐PO1018]. Journal of the American Society of Nephrology 2009;20:343A. [Google Scholar]
- Vanrenterghem Y, Mancilla‐Urrea E, Lang P, Agarwal M, Block AJ. Belatacept is associated with improved cardiovascular and metabolic risk factors compared to cyclosporine in kidney transplant patients (BENEFITand BENEFIT‐EXT studies) [abstract no: O‐334]. Transplant International 2009;22(Suppl 2):90. [Google Scholar]
- Vanrenterghem Y, Mancilla‐Urrea E, Lang P, Agarwal M, Block AJ, Xing J. Belatacept is associated with improved cardiovascular and metabolic risk factors compared to cyclosporine in kidney transplant patients (BENEFIT and BENEFIT‐EXT Studies) [abstract no: 1079]. American Journal of Transplantation 2009;9(Suppl 2):495. [DOI] [PubMed] [Google Scholar]
- Vincente F, Charpentier B, Rostaing L, Reyes‐Acevedo R, Massari P, Vitko S, et al. Long‐term extension of the belatacept BENEFIT Study: Results at month 48 [abstract no: MO05.01]. 24th International Congress of the Transplantation Society (TTS); 2012 Jul 15‐19; Berlin, Germany. 2012.
- Vincenti F, Charpentier B, Rostaing L, Reyes‐Acevedo R, Massari P, Vitko S, et al. Long‐term extension of the belatacept BENEFIT Study: results at month 48 [abstract no: PO27.001]. 24th International Congress of the Transplantation Society (TTS); 2012 Jul 15‐19; Berlin, Germany. 2012.
- Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A phase III study of belatacept‐based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). American Journal of Transplantation 2010;10(3):535‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vincenti F, Grinyo J, Charpentier B, Medina PJ, Rostaing L, Vanrenterghem Y, et al. Primary outcomes from a randomised, phase III study of belatacept vs cyclosporine in kidney transplant recipients (BENEFIT study) [abstract no: O50]. British Transplantation Society (BTS). 13th Annual Congress; 2010 Mar 17‐19; London, UK. 2010.
- Vincenti F, Grinyo J, Charpentier B, Pestana J, Rostaing L, Lin C, et al. Outcomes as a function of donor: recipient characteristics from a phase III study of belatacept vs cyclosporine in kidney transplantation (BENEFIT) [abstract no: 2857]. Transplantation 2010;90(Suppl):175. [Google Scholar]
- Vincenti F, Grinyo J, Charpentier B, Pestana JM, Rostaing L, Vanrenterghem Y, et al. Primary outcomes from a randomized, phase III study of belatacept vs cyclosporine in kidney transplant recipients (BENEFIT study) [abstract no: SA‐FC351]. Journal of the American Society of Nephrology 2009;20:82A. [Google Scholar]
- Vincenti F, Grinyo J, Larsen C, Medina‐Pestana J, Vanrenterghem Y, Dong Y, et al. 3‐year safety profile of belatacept in kidney transplant recipients from the BENEFIT and BENEFIT‐EXT studies [abstract no: F564]. NDT Plus 2011;4(Suppl 2):4.s2.43. [Google Scholar]
- Vincenti F, Grinyo J, Larsen C, Medina‐Pestana J, Vanrenterghem Y, Duan T, et al. Benefit‐risk profile of the belatacept LI regimen at 2 years in EBV(+) kidney transplant recipients [abstract no: 3370]. Transplantation 2010;90(Suppl):649. [EMBASE: 71532340] [Google Scholar]
- Vincenti F, Grinyo JM, Charpentier B, Medina‐Pestana JD, Rostaing L, Vanrenterghem Y, et al. Primary outcomes from a randomized, phase III study of belatacept vs cyclosporine in kidney transplant recipients (BENEFIT study) [abstract no: 4]. American Journal of Transplantation 2009;9(Suppl 2):191. [Google Scholar]
- Vincenti F, Grinyo JM, Charpentier B, Medina‐Pestana JD, Rostaing L, Vanrenterghem Y, et al. Primary outcomes from a randomized, phase III study of belatacept vs cyclosporine in kidney transplant recipients (BENEFIT study) [abstract no: O‐331]. Transplant International 2009;22(Suppl 2):89. [Google Scholar]
- Vincenti F, Larsen C, Alberu J, Garcia V, Rostaing L, Rice K, et al. Three year outcomes from BENEFIT: A phase III study of belatacept vs cyclosporine in kidney transplant recipients [abstract no: O‐240]. Transplant International 2011;24(Suppl 2):68. [EMBASE: 70527316] [Google Scholar]
- Vincenti F, Larsen C, Alberu J, Garcia V, Rostaing L, Rice K, et al. Three year outcomes from BENEFIT: A phase III study of belatacept vs cyclosporine in kidney transplant recipients [abstract no: O57]. Transplant International 2011;24(Suppl 3):21. [EMBASE: 70537323] [Google Scholar]
- Vincenti F, Larsen C, Alberu J, Garcia VD, Rostaing L, Rice K, et al. Three‐year outcomes from BENEFIT: a phase III study of belatacept vs cyclosporine in kidney transplant recipients [abstract no: SaO043]. NDT Plus 2011;4(Suppl 2):4.s2.18. [Google Scholar]
- Vincenti F, Larsen C, Grinyo J, Charpentier B, Russo G, Garg P, et al. Renal benefit of belatacept vs cyclosporine in kidney transplant patients is not impacted by acute rejection (BENEFIT study) [abstract no: TH‐PO1013]. Journal of the American Society of Nephrology 2009;20:342A. [Google Scholar]
- Vincenti F, Larsen CP, Alberu J, Bresnahan B, Garcia VD, Kothari J, et al. Three‐year outcomes from BENEFIT, a randomized, active‐controlled, parallel‐group study in adult kidney transplant recipients. American Journal of Transplantation 2012;12(1):210‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vincenti F, Larsen CP, Alberu J, Garcia VD, Rostaing L, Rice K, et al. Three‐year outcomes from BENEFIT: a phase III study of belatacept vs cyclosporine in kidney transplant recipients [abstract no: 227]. American Journal of Transplantation 2011;11(Suppl 2):99. [EMBASE: 70405262] [Google Scholar]
- Vincenti F, Rostaing L, Durrbach A, Rice K, Pupim L, Grinyo J. Improving or maintaining renal function over 5 years with belatacept or cyclosporine (CsA): insights from the BENEFIT and BENEFIT‐EXT long‐term extension (LTE) studies [abstract no: SA‐PO1012]. Journal of the American Society of Nephrology 2013;24(Abstracts):860A. [Google Scholar]
- Vitalone MJ, Ganguly B, Li L, Hsieh SC, Latek R, Kulbokas E, et al. A comparison of kidney toxicity‐related gene expression, chronic allograft injury and renal function in belatacept versus cyclosporine‐treated patients [abstract no: CO53.02]. 24th International Congress of the Transplantation Society (TTS); 2012 Jul 15‐19; Berlin, Germany. 2012.
- Zhou Z, Shen J, Kaul S, Pfister M, Roy A. Belatacept population pharmacokinetics in renal transplant patients [abstract no: 1505]. American Journal of Transplantation 2010;10(Suppl 4):467. [Google Scholar]
CTOT‐10 Study 2013 {published data only}
- Newell K, Mannon R, Mehta A, Tomlanovich S, Morrison Y, Ikle D, et al. Long‐term calcineurin inhibitor (CNI) and corticosteroid (CS) avoidance using belatacept: the CTOT‐10 experience [abstract no: 15]. American Journal of Transplantation 2013;13(Suppl 5):34. [EMBASE: 71056592] [Google Scholar]
- Suessmuth Y, Stempora L, Johnson B, Cheeseman J, Morrison Y, Bridges N, et al. Comparison of baseline and day 28 post transplant renal allograft samples from the CTOT‐10 belatacept study [abstract no: A630]. American Journal of Transplantation 2013;13(Suppl S5):225. [EMBASE: 71057206] [Google Scholar]
Ferguson 2010 {published data only}
- Ferguson R, Grinyo J, Vincenti F, Kaufman DB, Woodle ES, Marder BA, et al. Immunosuppression with belatacept‐based, corticosteroid‐avoiding regimens in de novo kidney transplant recipients. American Journal of Transplantation 2011;11(1):66‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ferguson R, Vincenti F, Kaufman D, Woodle ES, Marder BA, Citterio F, et al. Immunosuppression with belatacept‐based, CNI‐avoiding and steroid‐avoiding regimens vs a tacrolimus‐based, steroid‐avoiding regimen in kidney transplant patients: results of a 1‐year, randomized study [abstract no: 1436]. Transplantation 2010;90(Suppl):156. [EMBASE: 71531403] [Google Scholar]
- Ferguson R, Vincenti F, Kaufman DB, Woodle ES, Marder BA, Citterio F, et al. Immunosuppression with belatacept‐based, CNI‐avoiding and steroid‐avoiding regimens vs a tacrolimus‐based, steroid‐avoiding regimen in kidney transplant patients: results of a 1‐year, randomized study [abstract no: 372]. American Journal of Transplantation 2010;10(Suppl 4):150. [EMBASE: 70463733] [Google Scholar]
- Grinyo J, Marder B, Harler M, Vincenti F, Citterio F, Nori U, et al. Outcomes at 4 years in a randomized trial evaluating belatacept‐based regimens with simultaneous CNI and steroid‐avoidance in kidney transplant [abstract no: 2162]. American Journal of Transplantation 2014;14(Suppl 3):116. [EMBASE: 71543897] [Google Scholar]
- Grinyo J, Marder B, Harler M, Vincenti F, Citterio F, Nori U, et al. Outcomes at 4 years in a randomized trial evaluating belatacept‐based regimens with simultaneous CNI and steroid‐avoidance in kidney transplant [abstract]. Transplantation 2014;98(Suppl 1):116‐7. [EMBASE: 71543897] [Google Scholar]
- Grinyo J, Marks W, Vincenti F, Kaufman C, Marder B, Woodle S, et al. Immunosuppression with belatacept‐based, CNI free, steroid avoiding regimens in kidney transplant recipients: 6 month, interim results [abstract no: O‐336]. Transplant International 2009;22(Suppl 2):90‐1. [Google Scholar]
- Grinyo JM, Marks W, Vincenzi F, Kaufman DB, Marder BA, Woodle S, et al. Immunosuppression with belatacept‐based, CNI‐free, steroid‐avoiding regimens in kidney transplant recipients: 6 month, interim results [abstract no: LB02]. American Journal of Transplantation 2009;9(Suppl 2):382. [EMBASE: 70010532] [DOI] [PubMed] [Google Scholar]
- Woodle E, Marder B, Harler M, Vincenti F, Citterio F, Nori U, et al. Renal function at 4 years in a phase 2 randomized trial evaluating belatacept‐based regimens with simultaneous calcineurin inhibitor and corticosteroid avoidance in kidney transplant recipients [abstract no: 2157]. American Journal of Transplantation 2014;14(Suppl 3):114‐5. [EMBASE: 71543892] [Google Scholar]
- Woodle E, Marder B, Harler M, Vincenti F, Citterio F, Nori U, et al. Renal function at 4 years in a phase 2 randomized trial evaluating belatacept‐based regimens with simultaneous calcineurin inhibitor and corticosteroid avoidance in kidney transplant recipients [abstract]. Transplantation 2014;98(Suppl 1):114‐5. [EMBASE: 71543892] [Google Scholar]
Vincenti 2005 {published data only}
- Blancho G, Nashan B, Vincenti F, Charpentier B. Selective co‐stimulation blockade with belatacept (LEA29Y) shows improved preservation of renal function at 12 months vs cyclosporine [abstract]. Transplant International 2005;18(Suppl 1):22. [Google Scholar]
- Bluestone JA, Liu W, Yabu JM, Laszik ZG, Putnam A, Belingheri M, et al. The effect of costimulatory and interleukin 2 receptor blockade on regulatory T cells in renal transplantation. American Journal of Transplantation 2008;8(10):2086‐96. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier B, Grannas G, Pearson T, Soulillou J, Vanrenterghem Y, Friend P, et al. Co‐stimulation blockade with LEA29Y in renal transplant: improved renal function and cv/metabolic profile at 6 months compared with cyclosporine [abstract]. Transplantation 2004;78(2 Suppl):84. [Google Scholar]
- Charpentier B, Larsen C, Grinyo J, Muehlbacher F, Blancho G, Grannas G, et al. Final results from the long term extension (LTE) of the belatacept phase 2 study in kidney transplantation [abstract no: 2164]. American Journal of Transplantation 2014;14(Suppl 3):117. [EMBASE: 71543899] [Google Scholar]
- Charpentier B, Larsen C, Grinyo J, Muehlbacher F, Blancho G, Grannas G, et al. Final results from the long‐term extension (LTE) of the belatacept phase 2 study in kidney transplantation [abstract]. Transplantation 2014;98(Suppl 1):117. [EMBASE: 71543899] [Google Scholar]
- Chavez H, Beaudreuil S, Abbed K, Taoufic Y, Kriaa F, Charpentier B, et al. Absence of CD4CD25 regulatory T cell expansion in renal transplanted patients treated in vivo with Belatacept mediated CD28‐CD80/86 blockade. Transplant Immunology 2007;17(4):243‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Friend P, Mühlbacher F, Charpentier B, Larsen C, Agarwal M, Vincenti F. Long‐term safety of belatacept: 5 year results of a phase II study [abstract no: O46]. British Transplantation Society (BTS). 12th Annual Congress; 2009 Apr 21‐24; Liverpool, UK. 2009.
- Grannas G, Richter N, Klempnauer J, Lehner F. 10 years' experience with belatacept (Nulojix) [abstract no: PO27.012]. 24th International Congress of the Transplantation Society (TTS); 2012 Jul 15‐19; Berlin, Germany. 2012.
- Grimbert P, Audard V, Diet C, Matignon M, Plonquet A, Mansour H, et al. T‐cell phenotype in protocol renal biopsy from transplant recipients treated with belatacept‐mediated co‐stimulatory blockade. Nephrology Dialysis Transplantation 2011;26(3):1087‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grinyo J, Charpentier B, Pestana JM, Vanrenterghem Y, Vincenti F, Reyes‐Acevedo R, et al. An integrated safety profile analysis of belatacept in kidney transplant recipients. Transplantation 2010;90(12):1521‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grinyo J, Halloran P, Vanrenterghem Y, Pearson T, Nashan B, Sen K, et al. Belatacept (LEA29Y) as part of a CNI‐free regimen in recipients of renal allografts with higher risk of poor long‐term function and graft loss: comparison with cyclosporine A [abstract no: 946]. American Journal of Transplantation 2005;5(Suppl 11):397. [Google Scholar]
- Hirose K, Posselt AM, Stock PG, Hirose R, Vincenti F. Treatment of kidney transplant patients with the novel co‐stimulatory blocker LEA29y (BMS‐224818) and antiil2 receptor antibody does not impede the development of regulatory t cells [abstract]. American Journal of Transplantation 2004;4(Suppl 8):442. [CENTRAL: CN‐00509233] [Google Scholar]
- Larsen C, Charpentier B, Wekerle T, Blancho G, Zhou W, Levy E, et al. Calcineurin inhibitor‐free immunosuppression with belatacept (LEA29Y) in renal transplant: phase II 12‐month results [abstract no: 535]. American Journal of Transplantation 2005;5(Suppl 11):293. [CENTRAL: CN‐00764038] [Google Scholar]
- Latek R, Fleener C, Lamian V, Kulbokas E, Davis PM, Suchard SJ, et al. Assessment of belatacept‐mediated costimulation blockade through evaluation of CD80/86‐receptor saturation. Transplantation 2009;87(6):926‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Muehlbacher F, Charpentier B, Larsen C, Agarwal M, Vincenti F. Long‐term safety of belatacept: 6 year results of a phase II study [abstract no: 621]. Transplantation 2008;86(2 Suppl):218. [Google Scholar]
- Nashan B, Grinyo J, Vincenti F, Halloran P, Hagerty D, Zhou W, et al. Co‐stimulation blockade with LEA29y in renal transplant: improved renal function and cv/metabolic profile at 6 months compared with cyclosporine [abstract]. American Journal of Transplantation 2004;4(Suppl 8):441. [CENTRAL: CN‐00509129] [Google Scholar]
- Shen J, Gelb JS, Townsend RM, Zhou Z, Pfister M, Harler MB, et al. Rationale for belatacept less intensive regimen in renal transplant recipients [abstract no: 1096]. American Journal of Transplantation 2011;11(Suppl 2):352. [EMBASE: 70406142] [Google Scholar]
- Vincenti F. Costimulation blockade with belatacept in renal transplantation [abstract]. 8th International Conference on New Trends in Immunosuppression and Immunotherapy; 2008 Feb 14‐17; Berlin, Germany. 2008.
- Vincenti F, Blancho G, Durrbach A, Friend P, Grinyo J, Halloran PF, et al. Five‐year safety and efficacy of belatacept in renal transplantation. Journal of the American Society of Nephrology 2010;21(9):1587‐96. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenti F, Durbach A, Larsen C, Muhlbacher F, Nashan B, LEA29Y SG. Study design and baseline characteristics of a multiple dose, randomized, controlled open‐label study comparing a costimulation blocker based regimen of BMS‐224818 (LEA29Y) vs. cyclosporine in renal transplant [abstract]. American Journal of Transplantation 2003;3(Suppl 5):473. [EMBASE: CN‐00448208] [Google Scholar]
- Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, et al. Costimulation blockade with belatacept in renal transplantation. New England Journal of Medicine 2005;353(8):770‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vincenti F, Larsen C, Grinyo J, Muhlbacher F, Blancho G, Vanrenterghem Y, et al. A decade of treatment with belatacept in renal transplantation: final results from the long‐term extension (LTE) of the phase 2 study [abstract no: SA‐PO1013]. Journal of the American Society of Nephrology 2013;24(Abstracts):860A. [Google Scholar]
- Vincenti F, Muehlbacher F, Nashan B, Larsen C, Atillasoy E, Natarajan K, et al. Co‐stimulation blockade with LEA29y in a calcineurin inhibitor free maintenance regimen in renal transplant: 6‐month efficacy and safety [abstract]. American Journal of Transplantation 2004;4(Suppl 8):442. [Google Scholar]
- Wekerle T, Lang P, Larsen C, Muelbacher F. Selective co‐stimulation blockade with belatacept (LEA29Y) results in decreased incidence of chronic allograft nephropathy in recipients of suboptimal renal allografts, compared with cyclosporine [abstract no: PO‐274]. Transplant International 2005;18(Suppl 1):116. [Google Scholar]
- Zhou Z, Shen J, Kaul S, Pfister M, Roy A. Belatacept population pharmacokinetics in renal transplant patients [abstract no: 1505]. American Journal of Transplantation 2010;10(Suppl 4):467. [Google Scholar]
References to studies excluded from this review
Kamar 2013 {published data only}
- Kamar N, Rial M, Alberu J, Steinberg S, Manfro R, Nainan G, et al. Three‐years outcomes after switching to belatacept from calcineurin inhibitor in stable kidney transplant recipients [abstract]. Transplant International 2013;26(Suppl 3):22. [EMBASE: 71356163] [Google Scholar]
- Kamar N, Rial M, Alberu J, Steinberg SM, Manfro R, Nainan G, et al. 3‐year outcomes after switching to belatacept from a calcineurin inhibitor in stable kidney transplant recipients [abstract]. Transplant International 2013;26(Suppl 2):44. [EMBASE: 71359102] [Google Scholar]
Kirk 2012 {published data only}
- Kirk A, Meas S, Xu H, Mehta A, Guasch A, Cheeseman J, et al. Kidney transplantation using alemtuzumab induction and belatacept/sirolimus maintenance therapy [abstract no: 56]. American Journal of Transplantation 2011;11(Suppl 2):45. [EMBASE: 70405091] [Google Scholar]
- Kirk A, Mehta A, Guasch A, Mead S, Xu H, Cheeseman J, et al. Kidney transplantation using alemtuzumab induction and belatacept/sirolimus maintenance therapy [abstract no: 560]. American Journal of Transplantation 2012;12(Suppl 3):197. [EMBASE: 70746514] [Google Scholar]
Rostaing 2011 {published data only}
- Grinyo J, Alberu J, Contieri FL, Manfro RC, Mondragon G, Nainan G, et al. Improvement in renal function in kidney transplant recipients switched from cyclosporine or tacrolimus to belatacept: 2‐year results from the long‐term extension of a phase II study. Transplant International 2012;25(10):1059‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grinyo J, Nainan G, Carmen RM, Steinberg S, Vincenti F, Dong Y, et al. Renal function at 2 years in kidney transplant recipients switched from ciclosporin or tacrolimus to belatacept: results from the long‐term extension of a phase II study [abstract]. British Transplantation Society (BTS). 15th Annual Congress; 2012 Feb 22‐24; Glasgow, UK. 2012.
- Grinyo J, Nainan G, Carmen RM, Steinberg S, Vincenti F, Dong Y, et al. Renal function at 2 years in kidney transplant recipients switched from cyclosporine or tacrolimus to belatacept: results from the long term extension of a phase II study [abstract no: O‐245]. Transplant International 2011;24(Suppl 2):70. [DOI] [PubMed] [Google Scholar]
- Grinyo J, Nainan G, Carmen Rial M, Steinberg S, Vincenti F, Dong Y, et al. Renal function at 2 years in kidney transplant recipients switched from cyclosporine or tacrolimus to belatacept: results from the long‐term extension of a phase II study [abstract no: 567]. NDT Plus 2011;4(Suppl 2):4.s2.43. [DOI] [PubMed] [Google Scholar]
- Grinyo J, Nainan G, Rial M, Steinberg S, Vincenti F, Dong Y, et al. Renal function at 2 years in kidney transplant recipients switched from cyclosporine or tacrolimus to belatacept: Results: from the long‐term extension of a phase II study [abstract no: 226]. American Journal of Transplantation 2011;11(Suppl 2):99. [EMBASE: 70405261] [DOI] [PubMed] [Google Scholar]
- Rostaing L, Massari P, Garcia VD, Mancilla‐Urrea E, Nainan G, Rial MC, et al. Switching from calcineurin inhibitor‐based regimens to a belatacept‐based regimen in renal transplant recipients: a randomized phase II study. Clinical Journal of the American Society of Nephrology: CJASN 2011;6(2):430‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostaing L, Nainan G, C Rial C, Steinberg S, Vincenti F, Shi R, et al. Switch from a CNI‐ to a belatacept‐based immunosuppressive regimen in kidney transplant recipients is safe and results in better renal function: 12 month results from a phase II study [abstract no: 166]. American Journal of Transplantation 2010;10(Suppl 4):90. [EMBASE: 70463527] [Google Scholar]
- Rostaing L, Nainan G, Carmen Rial M, Steinberg S, Vincenti F, Shi R, et al. Switch from a CNI‐ to a belatacept‐based immunosuppressive regimen in kidney transplant recipients is safe and results in better renal function: 12 month results from a phase II study [abstract no: OSu022]. NDT Plus 2010;3(Suppl 3):iii285. [EMBASE: 70484172] [Google Scholar]
- Rostaing L, Nainan G, Rial MC, Steinberg S, Vincenti F, Shi R, et al. Switch from a CNI to a belatacept‐based immunosuppressive regimen in kidney transplant recipients is safe and results in better renal function: 12 month results from a phase II study [abstract no: 1446]. Transplantation 2010;90(Suppl):157. [EMBASE: 71531404] [Google Scholar]
- Shen J, Lee S, Townsend R, Zhou Z, Roy A, Thomas D, et al. Pharmacokinetics (PK) and pharmacodynamics (PD) of belatacept in kidney transplant recipients (KTR) after conversion from CNI based regimens [abstract no: 939]. American Journal of Transplantation 2012;12(Suppl 3):301‐2. [Google Scholar]
- Walker RG, Rostaing L, Nainan G, C Rial M, Steinberg S, Vincenti F, et al. A switch to belatacept‐based immunosuppressive regimen in kidney transplant recipients from calcineurin inhibitors (CNI) has a favourable safety profile and results in improved renal function: 12‐month results from a phase II study [abstract no: 4]. Transplantation Society of Australia & New Zealand (TSANZ). 29th Annual Scientific Meeting 2011 June 29‐Jul 1, Canberra, Australia. 2011:39.
References to ongoing studies
EudraCT2006‐00311417 {published data only}
- EUCTR2006‐003114‐17. A randomized, open‐label, multicenter, parallel‐group study of belatacept‐based corticosteroid‐free regimens in renal transplant. www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number (accessed 4 June 2014).
EudraCT2011‐00616240 {published data only}
- Moreso F. New‐onset diabetes mellitus after renal transplantation. a multicentre, prospective, randomized, open study to evaluate belatacept‐based versus tacrolimus‐based immunosuppression. www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number (accessed 4 June 2014).
EudraCT2013‐00117820 {published data only}
- Karlberg E. Cardiovascular (CV) risk prediction and CV biomarkers in renal transplant recipients treated with belatacept compared to calcineurin inhibitors (CNI). Open randomized 12 month study. www.clinicaltrialsregister.eu/ctr‐search/search?query=2013‐001178‐20 (accessed 4 June 2014).
NCT01729494 {published data only}
- Woodle S. Randomized, open label, multicenter study of belatacept‐based early steroid withdrawal regimen with alemtuzumab or RATG induction compared to tacrolimus‐based early steroid withdrawal regimen with RATG induction in renal transplantation [Belatacept early steroid withdrawal trial]. www.clinicaltrials.gov/ct2/show/NCT01729494 (accessed 26 May 2014).
NTR4242 {published data only}
- Hesselink DA. Immune monitoring to characterize t‐cell responses of kidney transplant patients during costimulation blockade by belatacept. www.trialregister.nl/trialreg/admin/rctview asp?TC=4242 (accessed 4 June 2014).
Additional references
ANZDATA 2012
- Clayton P, Hurst K, McDonald S, Chadban S. ANZDATA Registry Report 2012. Chapter 8: Transplantation. www.anzdata.org.au/anzdata/AnzdataReport/35thReport/2012c08_transplants_v1.5.pdf (accessed 11 November 2014):2‐27.
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. JAMA 1996;276(8):637‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Buell 2005
- Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation 2005;80(2 Suppl):S254‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dobbels 2014
- Dobbels F, Wong S, Min Y, Sam J, Kalsekar A. Beneficial effect of belatacept on health‐related quality of life and perceived side effects: results from the BENEFIT and BENEFIT‐EXT trials. Transplantation 2014;98(9):960‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ekberg 2009
- Ekberg H, Bernasconi C, Tedesco‐Silva H, Vitko S, Hugo C, Demirbas A, et al. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. American Journal of Transplantation 2009;9(8):1876‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Flechner 2011
- Felchner SM, Glyda M, Cockfield S, Grinyo J, Legendre C, Russ G, et al. The ORION study: comparison of two sirolimus‐based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients. American Journal of Transplantation 2011;11(8):1633‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gloor 2009
- Gloor JM, Winters JL, Cornell LD, Fix LA, DeGoey SR, Knauer RM, et al. Baseline donor‐specific antibody levels and outcomes in positive crossmatch kidney transplantation. American Journal of Transplantation 2010;10(3):582‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Godlee 2012
- Godlee F, Groves T. The new BMJ policy on sharing data from drug and device trials. BMJ 2012;345:e37888. [DOI] [PubMed] [Google Scholar]
Hidalgo 2009
- Hidalgo LG, Campbell PM, Sis B, Einecke G, Mengel M, Chang J, et al. De novo donor‐specific antibody at the time of kidney transplant biopsy associates with microvascular pathology and late graft failure. American Journal of Transplantation 2009;9(11):2532‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Masson 2013
- Masson P, Duthie FA, Ruster LP, Kelly PJ, Merrifield A, Craig JC, et al. Consistency and completeness of reported outcomes in randomized trials of primary immunosuppression in kidney transplantation. American Journal of Transplantation 2013;13(11):2892‐901. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meier‐Kriesche 2004
- Meier‐Kriesche HU, Schold JD, Srinvias TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. American Journal of Transplantation 2004;4(3):378‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Annals of Internal Medicine 2009;151(4):264‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nankivell 2003
- Nankivell B, Borrows R, Fung C, O'Connell P, Allen R, Chapman J. The natural history of chronic allograft nephropathy. New England Journal of Medicine 2003;349(24):2326‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
OPTN 2013
- Organ Procurement & Transplantation Network. optn.transplant.hrsa.gov/converge/data/ (accessed 11 November 2014).
Rao 2009
- Rao PS, Ojo A. The alphabet soup of kidney transplantation: SCD, DCD, ECD‐‐fundamentals for the practicing nephrologist. Clinical Journal of The American Society of Nephrology: CJASN 2009;4(11):1827‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sayegh 1998
- Sayegh M, Turka L. The role of T‐cell costimulatory activation pathways in transplant rejection. New England Journal of Medicine 1998;338(25):1813‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SRTR 2010
- Scientific Registry of Transplant Recipients. 2010 Annual Report Data. srtr.transplant.hrsa.gov/annual_reports/2010/chapter_index.htm (accessed 11 November 2014).
Vanrenterghem 2008
- Vanrenterghem Y, Claes K, Montagnino G, Fieuws S, Maes B, Villa M, et al. Risk factors for cardiovascular events after successful renal transplantation. Transplantation 2008;85(2):209‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vincenti 2007
- Vincenti F, Friman S, Scheurmann E, Rostaing L, Jenssen T, Campistol J, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. American Journal of Transplantation 2007;7(6):1506‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Watson 2005
- Watson CJ, Firth J, Williams P, Bradley J, Pritchard N, Chaudhry A, et al. A randomized controlled trial of late conversion from CNI‐based to sirolimus‐based immunosuppression following renal transplantation. American Journal of Transplantation 2005;5(10):2496‐503. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Webster 2005
- Webster AC, Taylor RR, Chapman JR, Craig JC. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003961.pub2] [DOI] [PubMed] [Google Scholar]
Webster 2009
- Webster AC, Wong G, Craig JC, Chapman JR. Managing cancer risk and decision making after kidney transplantation. American Journal of Transplantation 2008;8(11):2185‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
White 2010
- White CA, Siegal D, Akbari A, Knoll GA. Use of kidney function end points in kidney transplant trials: a systematic review. American Journal of Kidney Diseases 2001;56(6):1140‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wong 2012
- Wong G, Howard K, Chapman JR, Chadban S, Cross N, Tong A, et al. Comparative survival and economic benefits of deceased donor kidney transplantation and dialysis in people with varying ages and co‐morbidities. PloS ONE 2012;7(1):e295291. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Masson 2013a
- Masson P, Henderson L, Chapman JR, Craig JC, Webster A. Belatacept for kidney transplant recipients. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD010699] [DOI] [PMC free article] [PubMed] [Google Scholar]


